Abstract
A growing body of literature describes the use of buprenorphine for the treatment of chronic pain in people with sickle cell disease. The experiences of people with sickle cell disease who have tried buprenorphine are not yet reported. This qualitative descriptive study was conducted to explore perspectives on buprenorphine for chronic pain in sickle cell disease. We interviewed 13 participants with sickle cell disease who had been prescribed buprenorphine and had a clinic visit between December 1, 2020 and April, 2022 in our Sickle Cell Center for Adults. Interviews were recorded, transcribed, and analyzed using thematic analysis. Eleven out of 13 participants were taking buprenorphine at the time of the interview, with mean treatment duration of 33 months (SD 18, range 7 to 78 months). Five major themes were identified: 1) dissatisfaction with full agonist opioids; 2) navigating uncertainty with autonomy in deciding to try buprenorphine; 3) functional and relational changes after starting buprenorphine, 4) enduring systemic barriers to pain treatment, and 5) trusting treatment relationships are necessary when approaching patients about buprenorphine. The experience of adulthood living with sickle cell disease before and after starting buprenorphine is qualitatively different with significant improvements in social functioning.
Perspective
This study examined the experience of adults with sickle cell disease and chronic pain transitioning from full agonist opioids to buprenorphine. It is the first qualitative study of buprenorphine in people with sickle cell disease, contributing to a small but growing literature about buprenorphine and sickle cell disease.
Keywords: Sickle cell disease, Buprenorphine, Chronic Pain, Opioids
Introduction
Pain is the hallmark symptom of sickle cell disease (SCD). In addition to acute pain crises, 54% of adults with SCD experience chronic pain defined as pain on more than half of days, in addition to episodic acute pain crises.[1] Opioids are the mainstay of SCD pain treatment and 23–27% of adults with SCD are prescribed daily opioid therapy.[2, 3] Chronic opioid therapy is linked to central sensitization, depression, greater acute care utilization, increased pain, and worse quality of life.[4, 5] Opioid use also contributes to stigmatization of patients with SCD, particularly as the opioid epidemic rages.[6–8]
Research on alternative chronic pain treatment strategies in SCD is motivated by concerns about the inadequacies of treating chronic SCD pain with high doses of full agonist opioids.[9–14] One such alternative, buprenorphine, is a high affinity partial mu opioid receptor agonist and kappa opioid receptor antagonist approved for treating both opioid use disorder and chronic pain.[15] Buprenorphine offers a favorable side effect profile due to its partial agonist properties, including lower physical dependence and a ceiling effect on respiratory depression that decreases risk of overdose deaths.[16] For example, patients with chronic non-cancer pain who transitioned to buprenorphine reported improvement in pain and quality of life.[17] Initial, limited studies describing the use of buprenorphine in people with SCD suggest safety and efficacy.[9–14] In the largest report to date, 36 patients treated at our Center had a 72% reduction in acute care utilization after switching to buprenorphine, which was prescribed in the context of comprehensive sickle cell care.[10] In this care model, adults with SCD receive longitudinal expert care informed by hematologists with expertise in SCD and psychiatrists with expertise in SCD and complex chronic pain who supervise advanced practice providers delivering daily care to outpatients, inpatients and patients seen for acute SCD complications at our infusion center[18]. Decisions about offering buprenorphine to patients are made during multi-disciplinary meetings which also include our Center social worker. These decisions occur in tandem with individualized recommendations designed to optimize SCD treatment with, for example, hydroxyurea or chronic red blood cell transfusion therapy.
Little is known about how adults with SCD decide to try buprenorphine nor about how their experience taking buprenorphine compares to their experience with full agonist therapy. Buprenorphine is already being prescribed for patients with SCD and because it is an FDA-approved medicine, rigorous randomized controlled trials which would capture patient reported outcomes are unlikely to be performed. Qualitative data is needed to inform the use of buprenorphine as a treatment for chronic pain in people with SCD and may be used to design standardized patient reported outcome measures for people taking buprenorphine.
The purpose of this qualitative study of adults with SCD with current or historic buprenorphine use was to understand their perspective on buprenorphine and to gather information about which adults with SCD might be considered buprenorphine candidates.
Methods
Recruitment and sampling
This study was approved by the Johns Hopkins Institutional Review Board. Participants were recruited from the Sickle Cell Center for Adults between December 2020 and April 2022. The inclusion criteria were: age 18 years or older, diagnosis of SCD, taking buprenorphine now or in the past, English fluency, and capacity to consent to the study. Research staff contacted eligible participants to describe the study and invite participation during routine follow up visits in the clinic, by telephone, or text message between December 2020 and April 2022. Oral informed consent was obtained from interested, eligible participants. A convenience sampling approach was used. The research protocol followed the COREQ framework for conducting qualitative research and reporting.[19]
Data collection
Participants completed a one-on-one audio recorded telephone interview with a research team member, a male M.D. in his third year of a combined Medicine-Pediatrics Residency Program with clinical experience caring for people with SCD and prescribing buprenorphine (PL). He did not have a treatment relationship with any of the participants. Interviews were supervised by LHP, a female M.D., M.H.S. with training in qualitative research methods, along with clinical expertise in the management of children and adults with SCD. She did not have a treatment relationship with any of the participants. LHP informed interview guide development and met with the interviewer (PL) following interviews. The interviewer followed a semi-structured interview guide, querying participants on their decision to try buprenorphine, experience with buprenorphine compared to their previous pain medication regimen, and advice to other people with SCD considering this therapy (Supplement 1). The semi-structured interview guide was not modified through the interview process. Interviews were conducted between December 2020 and April 2022. Mean interview duration was 35 minutes (SD 12.5 minutes). Participants received $35 compensation for their time. Demographic, disease characteristics, and opioid prescribing details including age, gender identity, race, genotype, and baseline daily opioid dosage were obtained by chart review.
Analysis
Interviews were audio recorded and transcribed by a medical transcriptionist. Transcripts were analyzed using NVIVO software.[20] Qualitative analysis occurred iteratively using thematic analysis with inductive coding.[21] Two study members (PL, ME) individually coded the first three transcripts using inductive coding, and created a codebook with the senior investigator (LHP), resolving coding discrepancies by discussion until consensus was achieved. Subsequent transcripts were coded using the codebook. Categories were later grouped into themes and subthemes. Figures were developed to visually present the themes in synthesized, conceptual terms.
Results
Participant characteristics
We contacted 30 eligible participants, 11 during routine follow up visits and 19 by telephone. All 11 patients recruited during follow up visits completed interviews, and 2 additional participants were recruited by telephone. The 17 eligible participants contacted by telephone who did not complete interviews either declined to participate (n=5) or could not be reached (n=12). A total of 13 participants were consented and completed interviews (Table 1). They were nine women and four men, aged 26 to 60 years. Participants’ full agonist opioid regimens before buprenorphine initiation ranged from 60 to 500 oral morphine milligram equivalents daily. At the time of interview, most participants (n=11) were taking buprenorphine. One participant stopped buprenorphine after developing wheezing. The other described buprenorphine was ineffective for their frequent acute pain crises. Quotes from these two participants in the manuscript are identified. The average duration of buprenorphine treatment was 33 months (SD 18, range 7 to 78 months). Mean interview duration was 35 minutes (SD 12.5 minutes).
Table 1.
Participant demographic, disease, and opioid characteristics.
| Age | Gender Identity | Race | Genotype | Baseline Oral Morphine Equivalents | Months taking buprenorphine |
|---|---|---|---|---|---|
| 26 | Woman | Black | HgbS Beta+ | 170 | 37 months |
| 26 | Woman | Black | HgbSS | 60 | 7 months (stopped before interview) |
| 34 | Man | Black | HgbS Beta0 | 156 | 27 months |
| 34 | Woman | Black | HgbSS | 740 | 52 months |
| 35 | Man | Black | HgbSS | 126 | 29 months |
| 42 | Man | Black | HgbSS | 500 | 18 months |
| 42 | Woman | Black | HgbSS | 237 | 38 months |
| 43 | Woman | Black | HgbSS | 96 | 40 months |
| 46 | Woman | Black | HgbS Beta0 | 90 | 3 weeks (stopped before interview) |
| 56 | Woman | Black | HgbSS | 156 | 35 months |
| 57 | Man | Black | HgbSS | 450 | 78 months |
| 58 | Woman | Black | HgbSC | 186 | 11 months |
| 60 | Woman | Black | Hgb SS | 150 | 20 months |
Interview results
Five themes emerged from the interviews: 1) switching to buprenorphine is motivated by dissatisfaction with full agonist opioid treatment; 2) deciding to try buprenorphine involves navigating uncertainty with autonomy; 3) flourishing of functioning and relationships after starting buprenorphine, 4) enduring social and structural barriers to optimized pain management, and 5) approaching other patients with SCD about buprenorphine should occur in the context of trusting treatment relationships.
I. “What I’m doing now isn’t working”: Readiness for change
Participants described that full agonist opioids had negative effects on their mental state, sense of well-being, and ability to function (Table 2). A 34-year-old woman said, “I just really felt like it was a fog. I was always either sleeping or wanting to go to sleep or tired.” A 57-year-old man said, “I was groggy, I was mean. I was miserable. Always fussing. Wasn’t pleasant. It wasn’t a good look.” A 43-year-old woman described the intrusion and conflict she experienced with chronic, high dose full agonist opioid,
It’s a very big dependency being on [full agonist opioids]. And it’s very hard for you to [get] your mind off. “I don’t need this. I don’t need this,” when your body is telling you, “I need it. I need it. I need it.
Participants were motivated to try buprenorphine due to unresolved pain with full agonist opioid therapy. A 26-year-old woman who had stopped taking buprenorphine before participating said, “[Before buprenorphine] I thought about my current level of pain and how desperate I was to really try anything that could take care of that.” Many described full agonist opioid therapy as ineffective. A 34-year-old woman said, “I was on a lot of opioids... a really high dose, and I was still uncomfortable. And I just didn’t have a good quality of life.”
Table 2.
“What I’m doing now isn’t working”: reflections on the negative effects and uncontrolled pain with full agonist opioid therapy
| Negative effects of full agonist opioids | A [full agonist opioid], it slows your thought process. You know what I'm saying? It slows your brain or something – 43-year-old woman |
| One of the problems that I had is that I had a lot of nausea and cramping [on full agonist opioids]. And so, I finally was like, you know, I don’t like this feeling, so let’s switch and try to do something different. – 35-year-old man | |
| I was just fed up with having to take so many pills and being out of it, and I felt like it made me depressed to have to take so much medication. – 42-year-old woman | |
| I became medically addicted to [full agonist opioids] because I've been taking that ever since I was a child… I could not stand it. I couldn’t take it anymore. I mean over 30 years, 40 years, really, of being on it, I probably became immune to it…and my body was dealing with the side effects of telling me it was time to take it. – 60-year-old woman | |
| Uncontrolled pain on full agonist opioids | I was always in pain. I was always checking the clock to when I could take the next pain medication. I was always tired. It wasn’t doing anything for my pain. – 34-year-old woman |
| I had to carry them everywhere with me. And they weren’t particularly effective. It was more or less short-term pain management. Usually after two hours I’d end up in pain again and obviously severely limited the activities I could take part in. – 34-year-old man | |
| I was in pain every day. And it was severe pain. It wasn’t just a little bit of aches here and there. It was bad pain that was keeping me in the house all day. – 26-year-old woman |
II. “You have the right to say yes or no”: Navigating uncertainty with autonomy
When deciding to try buprenorphine, participants navigated ambivalence, fear and hope (Figure 1). A 34-year-old woman described her uncertainty,
What if it doesn’t work? Should I take a chance on it? What’s the worst thing that can happen? I was so used to the [full agonist opioids] that it was kind of hard to give it up because at least I know this will work for 15 minutes. I don’t know what buprenorphine is going to do because I never tried that.
Participants described fear that buprenorphine would not control pain. A 57-year-old man worried that buprenorphine might not be “enough to hold my pain down”. A 35-year-old man said, “initially the only fear was whether it was going to be able to manage my pain as well as what I had already been used to.” Participants also felt skeptical; a 43-year-old woman said bluntly, “I thought, ‘This shit is not going to work, I’ll be in too much pain.’”
Figure 1.
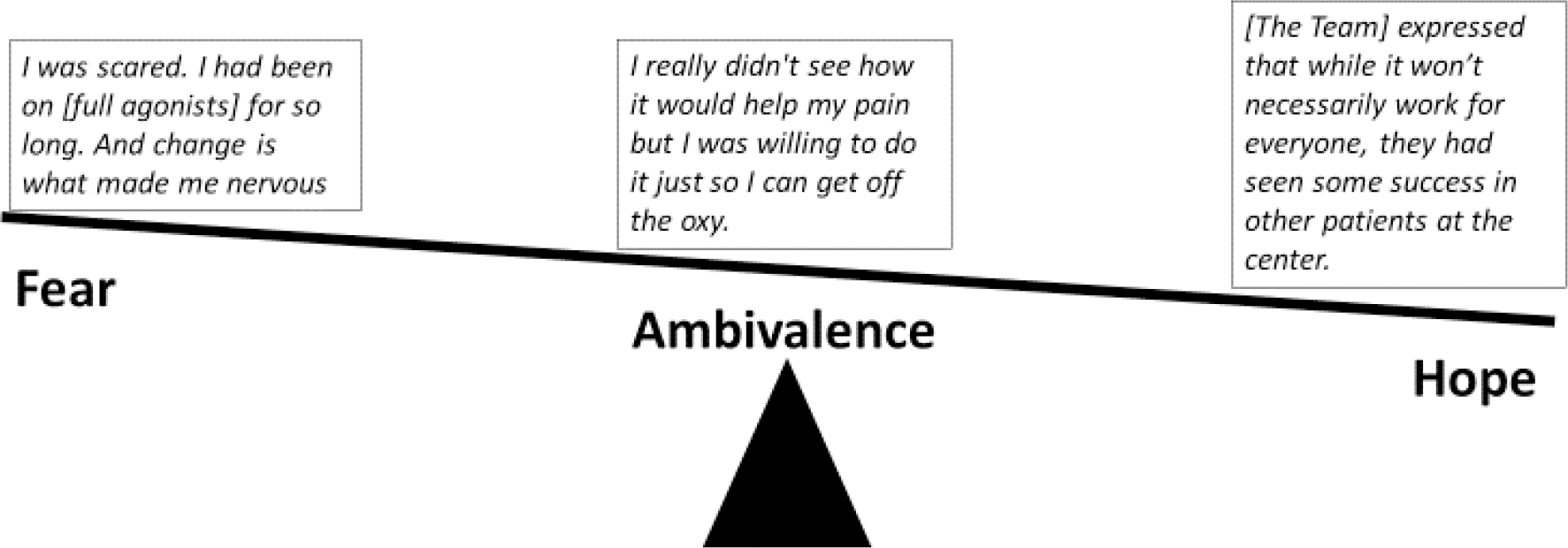
What tips the scale? Navigating the fear, ambivalence and hope associated with trying a new approach to opioid therapy.
To address their uncertainties and concerns, participants gathered information from their treatment team, by talking to others with SCD, conducting independent research, and in discussion with their families.
I would Google... I had joined the sickle cell page to see what other people do who took medication and see, were they responsive and how it affected them. – 46-year-old woman who stopped buprenorphine before participation
[Clinicians] just continually talk to me all the time, that’s how I decided to just give it a chance. Of course, with prayer and talking to my family. – 34-year-old woman
Retaining autonomy was central to participants decision making process.
Everything they offered me was optional. You can take this or you can’t take this... not a “you take it or else.” ... you have the right to say yes or no. - 26-year-old woman
Give them the facts and allow them to decide for themselves if they want to try [buprenorphine]. – 42-year-old woman
III. “Things just started changing for me”: Relationship changes
After starting buprenorphine, participants described transformed relationships to pain medications and SCD care overall, to their sense of well-being, and to their level of functioning in multiple domains of life (Figure 2). Most, but not all, described strong feelings that buprenorphine was effective for pain.
If you was in pain ... a few minutes after you take that strip, I swear... it works. And this is coming from a person who has been in a lot of damn pain. – 43-year-old woman
Pain medication became part of a medication routine instead of an intrusion into daily life.
Back when I was taking [full agonist] opioids, it was at the forefront of your thoughts, you can’t leave the house without medication, you have to take it everywhere... but taking buprenorphine, it can just kind of fade into the background. – 34-year-old man
SCD treatment focus shifted. A 57-year-old man reported fewer acute care visits after starting buprenorphine, “I just been functioning real good. No hospital stays. And I was in the hospital every other month.” Another participant, a 42-year-old woman, reported fewer “instances where I need to go to the infusion center or the emergency room or have to be hospitalized.” Participants felt more able to engage with their lives. A 58-year-old woman said, “the buprenorphine, it works so differently. My mind is clear.” A 42-year-old man reflected, “I’m more aware, I’m more focused, I’m more involved.” One 57-year-old man explained,
I can function, I can really focus and not be dozing off and not looking in the space and not in a subdued world...I’m more productive...I’m more active, I’m not just laying around groggy.
Interpersonal relationships flourished. Participants described increased engagement with others in their familial, communal, and work-place roles (Table 3). A 42-year-old woman said “I’m able to work now and do more with my children and clean up my house and do things that I wasn’t able to do before.” Multiple participants described changes in their ability to parent after initiating buprenorphine. A 35-year-old father said,
...having to take [full agonist opioid] ... prevented me from being able to care for [my children] properly. [With buprenorphine] I can be more engaged with them. I can be ... overall a better parent, and I think they noticed that as well. Because they ask me to play with them a lot more. They ask me to do things and be involved as much as possible.
Figure 2.
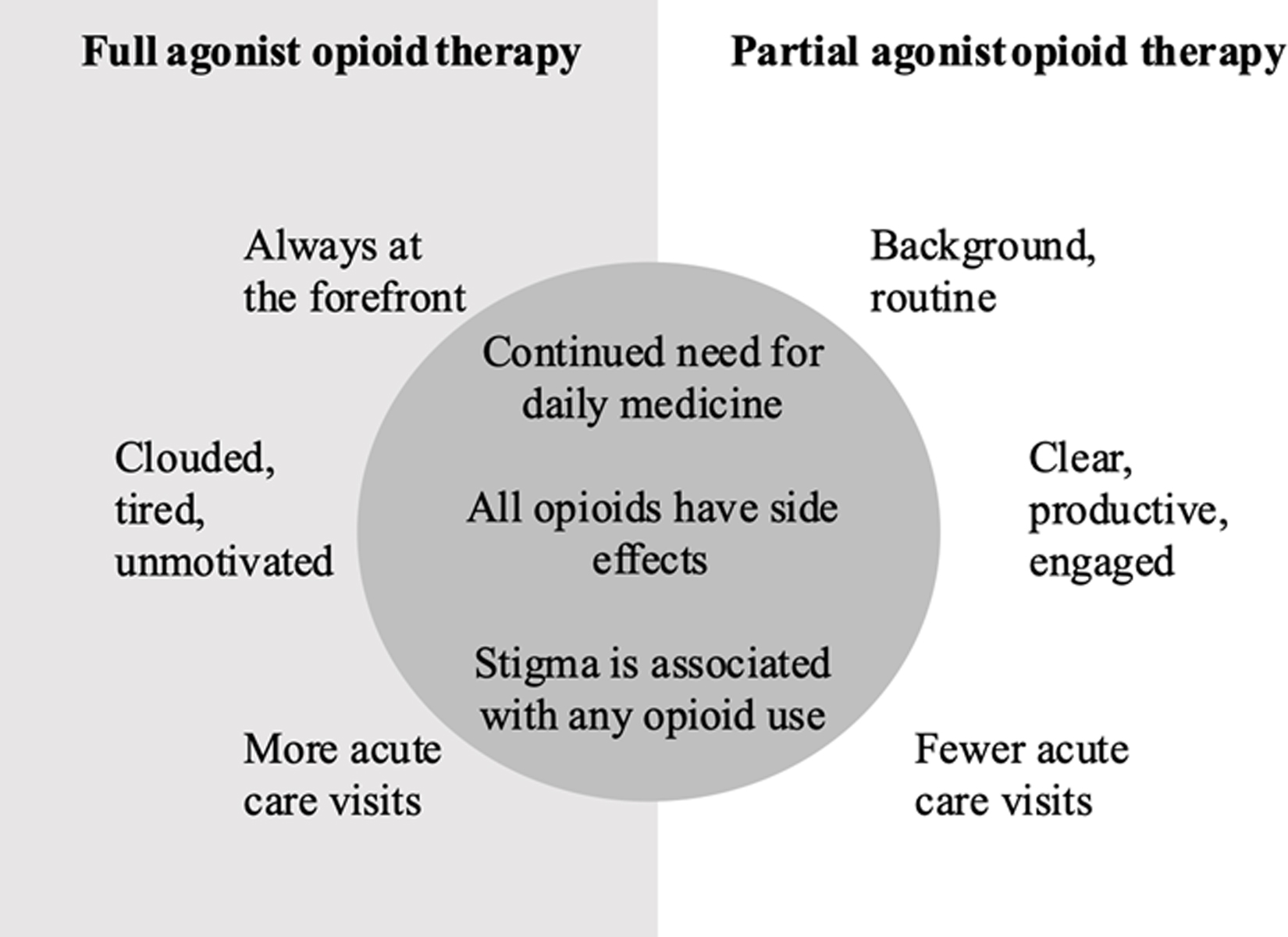
“It works so differently” and “I don’t see no difference”: Participants described mixed experiences with full agonist opioids and the partial agonist buprenorphine for chronic pain in sickle cell disease.
Table 3:
“I can do more”: Improvement in functioning in multiple domains of life with buprenorphine
| Daily life | I can do more. I can get up and go. With taking [full agonist opioids] and stuff, I mean, you can get up and go do stuff, but you’re groggy. - 43-year-old woman |
| With [buprenorphine], I was able to do everything that I wanted to do as far as go to the stores or going shopping or do things around the house. I was able to get it done and keep it moving. I had a lot more energy. I’m not saying that the buprenorphine gave me that, but me just not being on the [full agonist opioids] allowed me to be more active. – 34-year-old woman | |
| Parenting | With my children, I can do a lot more with them. I’m able to help with homework and go to school and pick up my son like I need to. – 42-year-old woman |
| Being able to be more active and more playful with my children. So being able to... go out for walks or go to the playground and running around with them, I’m able to do that more. – 35-year-old man | |
| And I don’t be coming to the infusion center all the time no more. I can go and do stuff with my kids now. – 43-year-old woman | |
| Social life | I’m out of the house, whereas the [full agonist opioids] kept me in the house … I can’t believe I went to a movie and it was absolutely wonderful being able to go out with a friend. – 58-year-old woman |
| I can actually make my mom dinner … or talk to my friends for longer than a few minutes or hang out with my family. – 26-year-old woman | |
| Driving | I don’t doze at the wheel no more, I used to doze at the wheel sometimes. So, there’s a difference. I’m more aware, I’m more alert. – 42-year-old man |
| I’m able to drive and go out the house. We do more errands because I don’t have to worry about being drowsy while driving as well. – 35-year-old man | |
| With [full agonist opioids] my reaction time wouldn’t be as on point. I wouldn’t think about getting behind the wheel myself, especially with my children. But now with the [buprenorphine], because it doesn’t make me as out of it, I will be able to drive a car. – 42-year-old woman |
IV. “Not an entirely new experience”: Unchanged dimensions of life and care
Despite many benefits, participants identified that some social experiences and structural barriers persisted after switching to buprenorphine (Figure 2). Stigma associated with taking opioids endured (Table 4), as did difficulties with pain management in the emergency department and the need to navigate prior authorizations and take a daily pain medicine.
The last time I went to the emergency room they actually mismanaged my care specifically because...I was taking buprenorphine ... so when you go to an emergency room where they’re arguing, where they’re not versed about sickle cell, you have to tell the physician how to handle your care, help to kind of justify why you’re taking the medications – 34-year-old man
At the pharmacy, they give me more problems with taking this medicine...I got to get a pre-authorization every three months. - 42-year-old man
I don’t see no difference because both of them is a dependency no matter how you look at it...It’s just switching from one medicine to another...It ain’t like I can go a day without taking buprenorphine. – 42-year-old man
Finally, buprenorphine does not eliminate pain. A 57-year-old man said, “I still get pain. Now, don’t get me wrong. When it come, it come.”
Table 4.
“Already stigmatized”: Navigating stigma associated with sickle cell disease and buprenorphine.
| Sickle Cell Disease Stigma | Buprenorphine Stigma |
|---|---|
| People with sickle cell disease are already stigmatized as being chronic drug users. – 46-year-old woman who stopped buprenorphine before participation | [Buprenorphine] has a stigma behind it already… I was more concerned about getting rid of the pain than I was about the stigma... I thought the stigma was purely from people not knowing what it was. – 26-year-old woman |
| Sickle cell patients do encounter a lot of stigma as being drug-seekers or as faking their pain and things like that… I’ve overheard conversations between doctors and nurses wondering whether I’m faking my pain just because I’m using the coping mechanisms I’ve developed; listening to music or distracting myself playing games on my phone. - 35-year-old man | I worried about…the stigma of being on [buprenorphine]…they told me that it was for people that use street drugs… I don’t like telling people that I take bup because they automatically think that you're taking it because you were using other drugs… But there's a stigma if you take drugs, period. – 42-year-old woman |
| Even back when I was still on full opioid medication, I would still be approached with caution and scrutiny in the emergency room if I didnť go to a hospital that was versed in sickle cell patients. So yeah, it was a concern. A bit more heightened but iťs not an entirely new experience. – 34-year-old man | I was concerned about the stigma associated with [buprenorphine]. If I go to an emergency hospital, they may not know… why I’m taking buprenorphine. And I’m usually undergoing a crisis, so I’m not particularly talkative or want to give an explanation of why I’m taking a certain type of drug. – 34-year-old man |
One participant, a 26-year-old who described buprenorphine as ineffective for acute pain crises, explained she stopped taking it because “I was constantly going back and forth between the buprenorphine and [full agonist opioids], I don’t feel like I got the full experience of what the buprenorphine would have been like.”
V. “Know who your patient is”: Approaching patients about buprenorphine
Participants thought patients on daily opioids and/or with frequent acute care visits should be offered buprenorphine.
If they’re like me... getting tired of taking so many pills throughout the day and also if they’re having to go to the infusion center a lot or the emergency room a lot, it makes a difference. – 42-year-old woman
If a doctor notices that a patient is running out of medication more often than not or landing in the emergency room because of pain, yeah, they’re definitely candidates. – 34-year-old man
Participants described the need for trust between patient and clinicians when recommending buprenorphine.
If you know who your patient is and what their goals and dreams are, I think that’s a better way to attempt to prescribe something to someone. - 34-year-old woman
Participants recognized buprenorphine as a component of comprehensive SCD care.
It’s not just the [buprenorphine] that has made a difference, I’m also on monthly blood exchanges, which helps out a lot, and...helps to cut down on the sickle cell crisis. - 42-year-old woman
Finally, for others like them, participants recommended counselling on what to expect during the transition to buprenorphine.
It’s not necessarily going to be an easy transition. It’s not something that’s going to happen overnight...be realistic as far as that it’s not necessarily going to be an easy process or a quick process to get where you want to be. – 35-year-old man
Discussion
This study examined the experience of adults with SCD transitioning from full agonist opioids to buprenorphine and is the first qualitative study of buprenorphine in people with SCD, contributing to a small but growing literature about buprenorphine and SCD.[9–14] After starting buprenorphine, participants reported improvements in daily functioning and relationships. Yet buprenorphine is not a panacea for all SCD-related pain challenges. Participants continued to face barriers to optimal pain management including difficulties refilling prescriptions and with emergency department care. Participants stressed the need for decision-making autonomy regarding whether to initiate buprenorphine and the need for trusting treatment relationships when making this treatment change.
Adults with SCD report markedly reduced quality of life[22]. Buprenorphine resulted in functional improvements for most participants suggesting that chronic, high dose full agonist opioid therapy is a risk factor for poor quality of life for some adults with SCD. Isolating the effect of full agonist opioid therapy on quality of life in SCD is complex because the relationship is confounded by comorbid complex chronic pain. [5, 23] Yet all participants in this study had chronic pain and, despite continued chronic pain, the majority described stark differences in their lives when taking full agonist opioids compared to buprenorphine. When taking full agonist opioids, participants described living in “a subdued world,” “still uncomfortable,” and feeling “fed up” with taking so many pills. They articulated an internal conflict between wanting to stop full agonist opioids, but fearing cravings, pain, and withdrawal. Many described that this conflict resolved when they started taking buprenorphine and pain treatment moved “to the background”. Participants’ report marked improvement in social relationships when switching from full agonist opioids to buprenorphine. This finding suggests that full agonist opioid therapy exacerbates SCD-associated social isolation and that buprenorphine improves this experience. Social relationships are protective against the negative effects of chronic pain.[24] Buprenorphine might protect against chronic pain in part by enabling improvements in social relationships, a possibility that needs further study. Such research could also help unconfound the relationship of poor quality of life, chronic pain, and high dose full agonist opioid therapy.
Participants readily identified opioids as a source of conflict and stigma. Adults with SCD are stigmatized by both interpersonal prejudice and structural racism.[25] Disease-specific discrimination is related to pain management that often requires opioids. Feeling stigmatized is associated with worse patient reported outcomes of disease severity and higher acute care utilization.[26] Participants considered full agonist opioids and buprenorphine stigmatizing medicines because they are both prescribed to people with opioid use disorder. They identified that taking buprenorphine did not resolve certain interpersonal and structural care barriers, noting persistent challenges with managing pain in the emergency department, obtaining medication refills, waiting for prior authorization to obtain pain medications and identifying pharmacies that dispense needed medications. However, buprenorphine may ultimately moderate some of these experiences. For example, the emergency department is a well-recognized site of stigmatizing and unpredictable interactions for adults with SCD[27], a reality that leads many affected people to try to avoid this care site.[28] Since buprenorphine reduces acute care utilization, it reduces emergency department encounters for people with SCD. Successful treatment with buprenorphine may thus help address disease severity by reducing stigmatizing experiences associated with SCD and need for care.
As the avoidance of unpredictable emergency department encounters suggests, trust is a key factor in the quality of SCD pain management.[29] Trust as a component of a therapeutic alliance is particularly salient when accepting a new treatment like buprenorphine. Participants revealed aspects of the decision-making process that engendered trust. These included having pre-existing treatment relationships and being offered decisional autonomy for deciding to start buprenorphine. Patients and their families value shared-decision making approaches to opioid management in SCD.[30] While shared decision making cannot completely resolve the inherently asymmetric power dynamic between patients who wish to direct their own care and clinicians who retain prescribing control of opioids, some elements of shared-decision making may help address this inherent inequality.[31] People cared for at our Center are counseled about buprenorphine by members of their longitudinal care team in the context of an established treatment relationship. Participants in this study valued this approach. Ensuring adults with SCD have access to comprehensive SCD care is necessary to ensure that offering buprenorphine to patients can honor their needs for decisional autonomy and trust.[32, 33]
Most, but not all, participants elected to continue buprenorphine therapy and most, but not all, endorsed benefit from treatment. Participants thought they met the clinical profile of a “buprenorphine candidate” and suggested that buprenorphine be offered to the subset of patients on high dose chronic opioid treatment with frequent hospitalizations. Perhaps uncoincidentally, quantitative data from our center matches participants’ qualitative descriptions. An earlier report from our Center identified that buprenorphine was initiated in people with SCD with mean acute care visit rate of 10.50 every 6-months and baseline full agonist opioid dose 158.15 +/− 109.89 oral morphine equivalents per day.[10] This group constitutes a small proportion of adults with SCD as the overwhelming majority of patients have little hospital use and low opioid dosages.[3] Participants defined other not yet considered characteristics required to try buprenorphine including being “severely limited,” by full agonist opioids and “desperate” to try something else. They also described feeling that full agonist opioid therapy was not helping with pain and feeling ready for change. These perspectives regarding the decision to try buprenorphine may help clinicians develop a language for discussing buprenorphine treatment with patients and for identifying people who are cognitively prepared for change. Further studies are needed to define which people with SCD are candidates for buprenorphine therapy.
This study illustrates the need for further studies that identify facilitators and barriers to continued buprenorphine use. David et al identified that 13.9% of patients discontinued buprenorphine within 6 months of induction[10]. In the present study 15.4% had discontinued by the time of interview. In this study, the two participants no longer taking buprenorphine were taking the lowest daily oral morphine equivalents. Future studies will help establish whether higher daily opioid doses are associated with treatment continuation. Participants described the transition from full agonist to partial agonist opioid therapy as challenging. One of the two participants who discontinued buprenorphine described difficulty transitioning between full agonist opioids for acute pain and buprenorphine for chronic pain management. Discontinuing buprenorphine may reflect inadequate pain control with therapy. However, it may also reflect the failure of our treatment system to successfully manage buprenorphine induction and reinduction. Research that defines the optimal sites and systems for buprenorphine induction and reinduction along with rigorous studies of patient reported outcomes are needed.
In this study, participants attributed functional improvements to discontinuing full agonist opioids, starting buprenorphine, and other dimensions of SCD treatment. Our comprehensive approach for patients with complex chronic pain includes intensifying routine outpatient care, using disease modifying therapy to treat SCD, optimizing non-opioid pharmacotherapy to reduce pain[18, 34–39], deploying a multidisciplinary team including psychiatrists with expertise in SCD and chronic pain,[40, 41] and using individualized acute care plans for the emergency department.[42, 43] Not all children or adults with SCD have access to comprehensive SCD care centers.[44, 45] Buprenorphine alone cannot substitute for the complex and chronic care needs of people with SCD and may be less successful or altogether inappropriate if offered without opportunities for expert SCD care.
This study has limitations. All participants are from a single SCD Center and four of the study authors (EP, LHP, CPC, SL) provide care in this Center, so perspectives on our own care model influenced analysis. However, the primary coders (PL and ME) were trainees who do not work in the Center and therefore brought an outside perspective. The convenience sampling approach had potential for self-selection bias and participants with stronger opinions, positive or negative, may have been more likely to participate. The study was conducted during the COVID-19 pandemic, which reduced the number of in person clinic appointments. This may have affected recruitment as in-person recruitment was strongest for this study. During the pandemic the Sickle Cell Center’s urgent care system remained intact and the Center rapidly implemented effective telemedicine.[46] More counseling regarding buprenorphine occurred by telemedicine, but the induction process continued in person.[10] It is possible that the social reorganization imposed by the pandemic affected participants’ perception of improvements in functioning. However, the pandemic likely increased the isolation of adults with SCD and yet, participants described less isolation and more outward focused behavior with buprenorphine. In our experience, buprenorphine is associated with a reduction in clinic visits. The inability to reach 12 of the eligible patients may reflect the reality that some buprenorphine-treated people with SCD have markedly reduced interest or need for certain kinds of interactions with the SCD Center. Retrospective interviewing is a limitation of this study in as much as recall bias may inform assessments. Prospective research involving repeated qualitative measures as people switch from full agonist opioids to buprenorphine may offer additional insights.
In this first study to describe the perspectives of adult patients with SCD regarding buprenorphine use, subjects reported improvement in pain and functioning with transition to buprenorphine as part of a larger SCD treatment paradigm. Buprenorphine does not altogether resolve the need for emergency department care. Ongoing education and partnership with emergency department clinicians is needed to ensure individualized care plans can be executed for all people with SCD, including those taking buprenorphine. Future prospective multi-center studies are needed to identify patients that most benefit from buprenorphine, determine optimal process of buprenorphine induction, and quantify benefits to functioning and symptoms.
Supplementary Material
Acknowledgements:
Thank you to the people with sickle cell disease cared for at the Johns Hopkins Sickle Cell Center for Adults whose courage to try new therapies and grace in the face of an unprecedented, global pandemic continues to inspire us. We extend our particular thanks to the patients who contributed to this study and the advance practice provider team who diligently cared for them. With appreciation to Dr. Maidah Raja who helped prepare and submit this manuscript. Thanks also to Michelle Eakin, PhD, MA for her mentorship in executing this study.
Disclosures:
There is no funding to report for this study. SL receives research funding from Imara, Novartis, Global Blood Therapeutics, Takeda, CSL-Behring, HRSA, PCORI and MD CHRC; consultancy for Bluebird bio, Novo Nordisk, Pfizer and Magenta; owns stock in Pfizer and Teva. LHP is funded through NIH/NHLBI K23HL146841 and NIH/NHLBI U01 HL156620–01, the American Society of Hematology, Doris Duke Charitable Foundation Grant #2020147, and the Mellon Foundation and Alexion and is a consultant for Global Blood Therapeutics and Novo Nordisk. PL, CPC ME, and EP have no disclosures to report.
References
- 1.Smith WR, Penberthy LT, Bovbjerg VE, et al. , Daily assessment of pain in adults with sickle cell disease. Ann Intern Med, 2008. 148(2): p. 94–101. [DOI] [PubMed] [Google Scholar]
- 2.Han J, Zhou J, Saraf SL, Gordeuk VR, Calip GS, Characterization of opioid use in sickle cell disease. Pharmacoepidemiol Drug Saf, 2018. 27(5): p. 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince EJ, Pecker LH, Lanzkron S, Carroll CP, The Complex Association of Daily Opioid Dose with Visits for Pain in Sickle Cell Disease: Tolerance or Treatment-Refractory Pain? Pain Med, 2023. 24(6): p. 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll CP, Lanzkron S, Haywood C Jr., et al. , Chronic Opioid Therapy and Central Sensitization in Sickle Cell Disease. Am J Prev Med, 2016. 51(1 Suppl 1): p. S69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karafin MS, Singavi A, Hussain J, et al. , Predictive factors of daily opioid use and quality of life in adults with sickle cell disease. Hematology, 2018. 23(10): p. 856–863. [DOI] [PubMed] [Google Scholar]
- 6.Labbe E, Herbert D, Haynes J, Physicians’ attitude and practices in sickle cell disease pain management. J Palliat Care, 2005. 21(4): p. 246–51. [PubMed] [Google Scholar]
- 7.Sinha CB, Bakshi N, Ross D, Krishnamurti L, Management of Chronic Pain in Adults Living With Sickle Cell Disease in the Era of the Opioid Epidemic: A Qualitative Study. JAMA Netw Open, 2019. 2(5): p. e194410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulgin D, Asnani M, Vorderstrasse A, Royal C, Pan W, Tanabe P, Stigma and quality of life in adults with sickle cell disease in Jamaica and the United States. Psychol Health Med, 2023. 28(5): p. 1133–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David M, Carroll C, Lauriello A, Salzberg B, Lanzkron S, Assessing the Safety and Efficacy of Converting Adults with Sickle Cell Disease from Full Agonist Opioids to Buprenorphine. Blood, 2018. 132: p. 856. [Google Scholar]
- 10.David MS, Jones J, Lauriello A, et al. , Converting adults with sickle cell disease from full agonist opioids to buprenorphine: A reliable method with safety and early evidence of reduced acute care utilization. Am J Hematol, 2022. 97(11): p. 1435–1442. [DOI] [PubMed] [Google Scholar]
- 11.Buchheit BM, Joslin T, Turner HN, Wong TE, Ambulatory microdose induction of buprenorphine-naloxone in two adolescent patients with sickle cell disease. Pediatr Blood Cancer, 2021. 68(1): p. e28766. [DOI] [PubMed] [Google Scholar]
- 12.Irwin M, Gunther W, Keefer P, et al. , Buprenorphine for Chronic Pain in a Pediatric Patient With Sickle-Cell Disease. J Pain Symptom Manage, 2021. 62(5): p. 1086–1091. [DOI] [PubMed] [Google Scholar]
- 13.Leyde S, Suen L, Pratt L, DeFries T, Transition from Oxycodone to Buprenorphine/Naloxone in a Hospitalized Patient with Sickle Cell Disease: A Case Report. J Gen Intern Med, 2022. 37(5): p. 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osunkwo I, Veeramreddy P, Arnall J, et al. , Use of Buprenorphine/Naloxone in Ameliorating Acute Care Utilization and Chronic Opioid Use in Adults with Sickle Cell Disease. Blood, 2019. 134: p. 790. [Google Scholar]
- 15.Chen KY, Chen L, Mao J, Buprenorphine-naloxone therapy in pain management. Anesthesiology, 2014. 120(5): p. 1262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tompkins DA, Smith MT, Mintzer MZ, Campbell CM, Strain EC, A double blind, within subject comparison of spontaneous opioid withdrawal from buprenorphine versus morphine. J Pharmacol Exp Ther, 2014. 348(2): p. 217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldman S, van Beek M, van Rijswijk S, et al. , Effects of opioid rotation to buprenorphine/naloxone on pain, pain thresholds, pain tolerance, and quality of life in patients with chronic pain and opioid use disorder. Pain, 2022. 163(5): p. 955–963. [DOI] [PubMed] [Google Scholar]
- 18.Lanzkron S, Little J, Wang H, et al. , Treatment of Acute Pain in Adults With Sickle Cell Disease in an Infusion Center Versus the Emergency Department : A Multicenter Prospective Cohort Study. Ann Intern Med, 2021. 174(9): p. 1207–1213. [DOI] [PubMed] [Google Scholar]
- 19.Booth A, Hannes K, Harden A, Noyes J, Harris J, Tong A, COREQ (Consolidated Criteria for Reporting Qualitative Studies), in Guidelines for Reporting Health Research: A User’s Manual. 2014. p. 214–226. [Google Scholar]
- 20.NVivo. 2020, QSR International Pty Ltd. [Google Scholar]
- 21.Braun V, Clarke V, Using thematic analysis in psychology. Qualitative Research in Psychology, 2006. 3(2): p. 77–101. [Google Scholar]
- 22.Treadwell MJ, Anie KA, Quality of Life in Sickle Cell Disease: What Matters. Hematol Oncol Clin North Am, 2022. 36(6): p. 1137–1149. [DOI] [PubMed] [Google Scholar]
- 23.Smith WR, McClish DK, Dahman BA, et al. , Daily home opioid use in adults with sickle cell disease: The PiSCES project. J Opioid Manag, 2015. 11(3): p. 243–53. [DOI] [PubMed] [Google Scholar]
- 24.Sturgeon JA, Zautra AJ, Social pain and physical pain: shared paths to resilience. Pain Manag, 2016. 6(1): p. 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haywood C Jr., Lanzkron S, Hughes MT, et al. , A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med, 2011. 26(5): p. 518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bediako SM, Lanzkron S, Diener-West M, Onojobi G, Beach MC, Haywood C Jr., The Measure of Sickle Cell Stigma: Initial findings from the Improving Patient Outcomes through Respect and Trust study. J Health Psychol, 2016. 21(5): p. 808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A PG, O’Conor KJ, Lanzkron S, et al. , Do Words Matter? Stigmatizing Language and the Transmission of Bias in the Medical Record. J Gen Intern Med, 2018. 33(5): p. 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crego N, Masese R, Bonnabeau E, et al. , Patient Perspectives of Sickle Cell Management in the Emergency Department. Crit Care Nurs Q, 2021. 44(2): p. 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elander J, Beach MC, Haywood C Jr., Respect, trust, and the management of sickle cell disease pain in hospital: comparative analysis of concern-raising behaviors, preliminary model, and agenda for international collaborative research to inform practice. Ethn Health, 2011. 16(4–5): p. 405–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips S, Schlenz AM, D’Alton S, Johnson M, Kanter J, Patient and Family Opioid Decision-Making for Pain Management in Sickle Cell Disease: A Qualitative Study. J Pain, 2023. 24(7): p. 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galasinski D, Ziolkowska J, Elwyn G, Epistemic justice is the basis of shared decision making. Patient Educ Couns, 2023. 111: p. 107681. [DOI] [PubMed] [Google Scholar]
- 32.Phillips S, Chen Y, Masese R, et al. , Perspectives of individuals with sickle cell disease on barriers to care. PLoS One, 2022. 17(3): p. e0265342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai P, Little J, Kanter J, Bridges K, Andemariam B, Lanzkron S, Kneeling Was the First Step for Sickle Cell Disease. Ann Intern Med, 2021. 174(7): p. 1004–1005. [DOI] [PubMed] [Google Scholar]
- 34.Carroll CP, Haywood C Jr., Lanzkron SM, Examination of the Patient and Hospitalization Characteristics of 30-Day SCD Readmissions. South Med J, 2016. 109(9): p. 583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charache S, Barton FB, Moore RD, et al. , Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore), 1996. 75(6): p. 300–26. [DOI] [PubMed] [Google Scholar]
- 36.Miller ST, Wright E, Abboud M, et al. , Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr, 2001. 139(6): p. 785–9. [DOI] [PubMed] [Google Scholar]
- 37.Brandow AM, Carroll CP, Creary S, et al. , American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv, 2020. 4(12): p. 2656–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Akbar M, Weinsheimer N, Gantz S, Schiltenwolf M, Longitudinal observation of changes in pain sensitivity during opioid tapering in patients with chronic low-back pain. Pain Med, 2011. 12(12): p. 1720–6. [DOI] [PubMed] [Google Scholar]
- 39.Compton P, Halabicky OM, Aryal S, Badiola I, Opioid Taper is Associated with Improved Experimental Pain Tolerance in Patients with Chronic Pain: An Observational Study. Pain Ther, 2022. 11(1): p. 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balsamo L, Shabanova V, Carbonella J, et al. , Improving Care for Sickle Cell Pain Crisis Using a Multidisciplinary Approach. Pediatrics, 2019. 143(5). [DOI] [PubMed] [Google Scholar]
- 41.Kroenke K, Bair MJ, Damush TM, et al. , Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA, 2009. 301(20): p. 2099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnamurti L, Smith-Packard B, Gupta A, Campbell M, Gunawardena S, Saladino R, Impact of individualized pain plan on the emergency management of children with sickle cell disease. Pediatr Blood Cancer, 2014. 61(10): p. 1747–53. [DOI] [PubMed] [Google Scholar]
- 43.Schefft MR, Swaffar C, Newlin J, Noda C, Sisler I, A novel approach to reducing admissions for children with sickle cell disease in pain crisis through individualization and standardization in the emergency department. Pediatr Blood Cancer, 2018. 65(10): p. e27274. [DOI] [PubMed] [Google Scholar]
- 44.Kanter J, Smith WR, Desai PC, et al. , Building access to care in adult sickle cell disease: defining models of care, essential components, and economic aspects. Blood Adv, 2020. 4(16): p. 3804–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulbert ML, Manwani D, Meier ER, et al. , Consensus definition of essential, optimal, and suggested components of a pediatric sickle cell disease center. Pediatr Blood Cancer, 2023. 70(1): p. e29961. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Xue W, Boorman EP, et al. , Accessible Care with High Patient Satisfaction: Telemedicine Use in Sickle Cell Disease. Telemed J E Health, 2023. 29(7): p. 1068–1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


