Abstract
The ciliary body, located at the junction of the choroid and iris, is crucial in the development of the embryonic eye. Notch2 signalling, Wnt signalling, transforming growth factor β (TGF-β) signalling, and Pax6 signalling are critical for coordinating the ciliary body formation. These signalling pathways are coordinated with each other and participate in the ciliary body development, ensuring the precise formation and optimal functioning of the eye structure. Although rare, ciliary body hypoplasia, ciliary tumours, and genetic-related iritis indicate the intricate nature of ciliary body development. Given the ciliary body's important biological significance and potential medical relevance, we aim to provide a comprehensive overview of the developmental molecular mechanisms governing ciliary body formation and function. Here, we focus on the intricate signalling pathways governing ciliary body development and corresponding genetic ciliary diseases.
Keywords: Ciliary body, Developmental signalling pathways, Genetic diseases
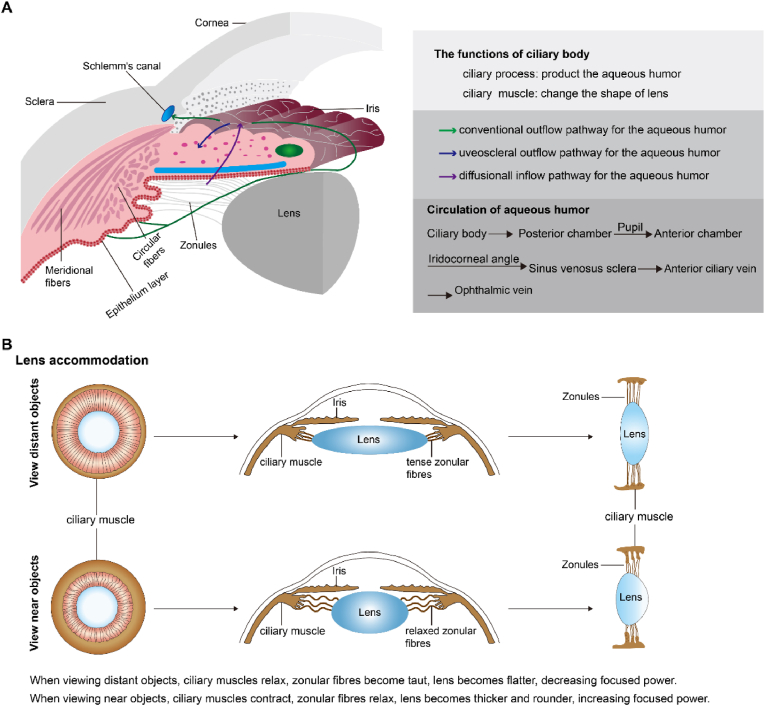
The ciliary body consists of three key components: the ciliary epithelium, ciliary processes, and ciliary muscle (Fig. 1) (Fernández-Vigo et al., 2022). The ciliary epithelium consists of two epithelial layers, including the pigmented layer and the nonpigmented layer. The outer layer is the pigmented epithelium, adjacent to the stroma, and its processes contain cuboidal cells rich in melanin granules. The inner layer is formed by the nonpigmented layer, which contributes to the production of the aqueous humour. These layers are essential for regulating intraocular pressure (IOP) and nourishing the cornea, lens, and vitreous. The stability of IOP underlies the maintenance of uninterrupted embryonic eye development and adult eye function (Skalicky, 2016). The ciliary processes are attached to the lens by the fibrous zonular fibres and are thus participate in the accommodation reflex. More importantly, the ciliary processes secrete aqueous humour because of the specialized highly vascularized epithelium. When viewing objects at different distances, the ciliary muscle controls the shape of the lens and regulates the flow of aqueous humour into Schlemm's canal. This muscle works with the dilatator pupillae and sphincter pupillae muscles to control the size of the pupil (Knaus et al., 2021). These structures are involved in various functions and physiological processes within the ciliary body to maintain ocular health and promote optimal vision.
Fig. 1.
The structure and function of the ciliary body. Adapted from Fernández-Vigo et al. (2022).
During eye development, the ciliary epithelium is derived from the optic vesicle, which folds to form the ciliary processes, and the mesenchymal cells differentiate into the connective tissue of the ciliary body (Zhao et al., 2002). Systematic and comprehensive signalling mechanisms regulate ciliary developmental processes and coordinate cell proliferation and shape morphological features. Precise spatiotemporal control of signalling pathway activation is essential for ciliary body formation during development and for intraocular homeostasis (Pang et al., 2021). Signalling pathways are the key biological mechanisms that govern diverse cellular communications from the exterior of the cell to intracellular mediators. Dysregulation or disruption of these pathways can result in abnormalities of the ciliary body, impacting eye structure and normal function.
In this review, we summarize the molecular developmental mechanisms governing the formation and function of the ciliary body to underscore the fundamental importance of these pathways. Furthermore, we systematically elucidate the pathogenesis of genetic diseases associated with the ciliary body, thereby illuminating the underlying molecular mechanisms involved.
1. Ciliary body development
1.1. Notch2 signalling
Notch2 signalling is a pivotal molecule in the intricate orchestration of ciliary body development (Fig. 2). Its dynamic distribution and homeostasis are rigorously governed to safeguard the precise progression of ciliary body development and function (Bao & Cepko, 1977). Aberrant regulation of the Notch2 pathway, caused by abnormal epigenetic modifications, posttranslational alterations, gene overexpression or mutations, results in anomalous ciliary body development and related complications.
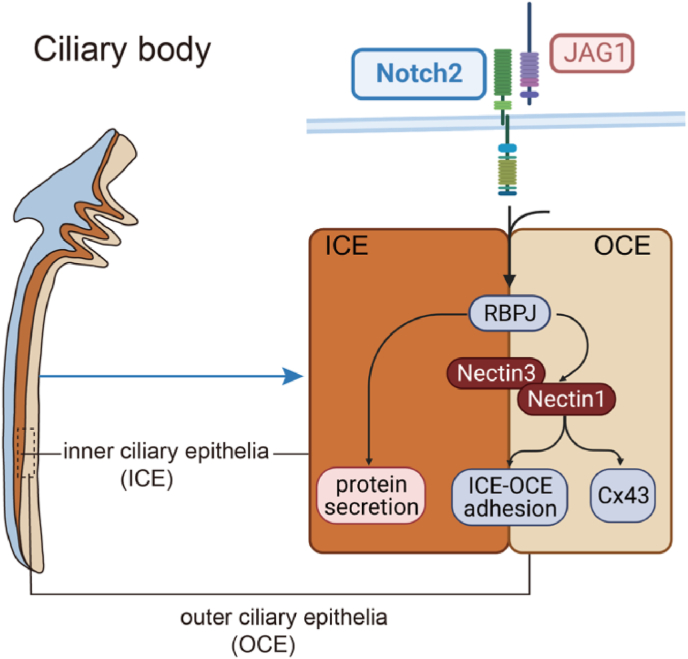
Fig. 2.
A working model for the Notch2 signalling in the regulation of ciliary body development. NOTC-RBPJ signalling directly controls vitreous protein secretion in the inner ciliary epithelia (ICE). Nectin1 is primarily expressed in the outer ciliary epithelia (OCE), and works with Nectin3 to mediate ICE-OCE adhesion for driving ciliary morphogenesis. Besides, Nectin1 forms a direct bond with aquaporin (connexin 43, Cx43) to regulate the aqueous humour secretion.
Notch2 controls bone morphogenetic protein (BMP) signalling through interactions with classical ligands such as Jagged canonical Notch ligand 1 (JAG1) (Zhou et al., 2013). Inactivation of the Notch2-JAG1 signalling leads to the absence of ciliary body tissue, indicating the common involvement of these proteins in changing the morphology of the ciliary body cell layer. Loss of canonical Notch signalling results in normal iris development but the absence of the ciliary body (Sarode et al., 2014). Furthermore, the inactivation of the recombining binding protein J-kappa (RBPJ), a transcription factor downstream of the Notch2 signalling pathway, triggers the degeneration of ocular tissues and results in ciliary body hypoplasia.
NOTCH-RBPJ signalling pathway directly controls vitreous protein secretion in the inner ciliary epithelium (ICE). The Notch2 signalling pathway is essential for regulating the nectin cell adhesion molecule (Nectin1) and Nectin3. Nectin1 is predominantly expressed in the outer ciliary epithelium (OCE), whereas the closely related protein Nectin3 is expressed in both ICE and OCE. Nectin1 works with Nectin3 to manage ICE-OCE adhesion and drive ciliary body development. In addition, Nectin1 binds directly to aquaporin (connexin 43, Cx43), which regulates aqueous humour secretion and contributes to the maintenance of vitreous and IOP stability (Pang et al., 2021). Finally, the Notch2 signalling pathway affects the morphology of the ciliary body through cell adhesion, contributing to the blood-aqueous barrier establishment when interacting with the endothelial cells. The blood-aqueous barrier substances transport, preserves epithelial integrity and regulates the aqueous humour secretion (Ragg et al., 2019). Destruction of the blood-aqueous barrier can cause inflammation and poor vision, and more severely, lead to vision loss or blindness (Aghaei et al., 2021). Thus, Notch2 signalling pathway regulates ciliary body epithelial formation, maintains protein secretion, and ensures the stability of the IOP.
1.2. Wnt signalling
The Wnt pathway is critical in the ciliary body cell differentiation and maturation of ciliary body cells through the precise regulation of downstream targets (Fig. 3). The Wnt/Frizzled-mediated signalling is categorized as the canonical (known as the Wnt/β-catenin pathway), or noncanonical (also referred to as the β-catenin-independent pathway) (Caracci et al., 2021; Mi et al., 2022). In the canonical Wnt pathway, Dishevelled, a principal component of the Wnt signalling pathway, interacts with the receptor Frizzled and prevents the constitutive proteolytic destruction of β-catenin (Mahoney et al., 2022).
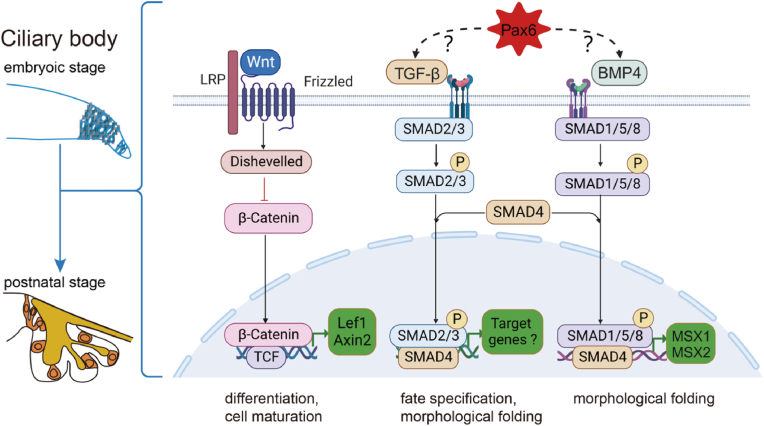
Fig. 3.
A working model for the Wnt, TGF-β, and Pax6 signalling in the regulation of ciliary body development. From embryonic to postnatal stages, the Wnt signalling pathway is mainly involved in cell differentiation and cell maturation in the ciliary body. The TGF-β and Bmp4 signalling pathways principally contribute to fate specification and morphological folding. However, the specific regulatory mechanism of Pax6 on TGF-β and Bmp4 is currently unclear.
Inactivation of β-catenin in vivo is associated with a reduced ciliary margin, ciliary body size and ciliary margin-specific gene expression. Pharmacological and genetic stabilization of β-catenin has been reported to lead to ectopic ciliary body gene expression while inhibiting neural retina development. Conversely, conditional inactivation of β-catenin disrupts ciliary body development, indicating that β-catenin signalling promotes the development of the ciliary body and peripheral eye fates (Liu et al., 2007). The identification maintenance of retinal progenitor cells is disrupted by Wnt2b, which causes retinal cells to transform into peripheral cells in the ciliary body or iris (Cho & Cepko, 2006; Kitamoto & Hyer, 2010). Additionally, Forkhead Box G1 (Foxg1), a new molecular player in ciliary margin specification, is recognized to inhibit the Wnt/β-catenin pathway, impeding the development of ciliary body tissue (Fotaki et al., 2013; Robertson et al., 2023). Lymphoid enhancer-binding factor 1 (Lef1), a transcriptional regulator of the Wnt signalling pathway, is expressed in ciliary body marginal zone, and its expression decreases towards the central retina (Kubo et al., 2003). Expression of a Lef1-engrailed fusion protein was associated with localized dysplasia of the iris, followed by ciliary dysplasia, suggesting that canonical Wnt signalling is instrumental in determining the characteristics of the ciliary body and iris (Cho & Cepko, 2006). Germline deletion of Axin-related protein 2 (Axin2), another downstream effector of the Wnt signalling, causes severe defects in the eyes, e.g., coloboma, microphthalmia, expanded ciliary margin and lens defects (Alldredge & Fuhrmann, 2016; Wang et al., 2023). The Wnt signalling pathway is important for maintaining genetic stability and orchestrating cellular developmental processes, including proliferation, maturation and differentiation.
1.3. TGF-β signalling
Transforming growth factor-beta (TGF-β), a secreted cytokine, is considered essential for embryogenesis and adult tissue homeostasis in mammalian development and disease (Barcellos-Hoff, 2022). The TGF-β superfamily, which includes TGF-βs and Bmp, participates in the increased diversity and complexity of multicellular animal evolution (Karampetsou et al., 2022; Sader & Roy, 2022).
TGF-β is predominantly expressed in optic cup rim and ciliary epithelial cells, exerting regulatory control over ciliary body cell development through the Smad pathway (Helbig et al., 1991; Igarashi et al., 2021). The absence of TGF-β2 is characterized by abnormal eye development, resulting in hypoplasia of the anterior segment of the eye, highlighting the critical role of TGF-β2 in the ciliary body formation (Saika et al., 2001; Wilson, 2021). Moreover, Bmp4, a growth factor that belongs to the TGF-β superfamily, is involved in the folding of ciliary body morphology through the downstream pSmad1/5/8 signalling (Zhao et al., 2002). The specification of the ciliary body may occur during the optic vesicle stages as a result of overlapping signals of fibroblast growth factor (FGF) and BMP. This finding is in accordance with the developmental stages of the neural retina and pigmented epithelium (Da Silva et al., 2007).
Msh homeobox (Msx) has emerged as a potential effector molecule of Bmp signalling (Ramos & Robert, 2005). Specifically, Msx1 is initially expressed in the optic cup inner layer around embryonic 12.5 (E12.5), peaking at E16.0 (Belanger et al., 2017). It is involved in the morphological folding processes during ciliary body development. Furthermore, developmental overexpression of Msx2 results in the downregulation of Bmp4 and the upregulation of Bmp7 in the developing murine optic vesicles (Wu et al., 2003). These findings collectively suggest a reciprocal relationship between Bmp and Msx, where Bmp directly regulates Msx expression to facilitate the ciliary body formation, and Msx expression mediates Bmp signalling.
1.4. Pax6 signalling
Pax6 is a strongly specialized embryonic transcription factor that contributes to the growth of the visual system, central nervous system, and endocrine system (Ochi et al., 2022). The expression of Pax6 affects the asymmetric transient dorsal region during the growth process of the pituitary. The expression is extinguished before the ventral-dorsal appearance of specific cell types. Any disruptions in expression or transcription levels lead to developmental disorders during embryonic development (Thompson et al., 2021).
PAX6-associated foveal hypoplasia is often associated with anterior segment abnormalities, including ciliary body abnormalities, which severely impair vision (Yu et al., 2023). Specifically, Pax6 is highly expressed in the distal optic cup, weakly expressed in the proximal retina, and absent in pigmented cells. The unique expression pattern of Pax6 suggested that this gene is responsible for regulating optic cup cell differentiation and ciliary body morphogenesis (Davis et al., 2009). The recognition of cells with retinal progenitor cell characteristics in the ciliary margin of adults informed researchers of their progenitor and differentiation potential. Pigmented ciliary epithelial cells express Pax6 in adult mammalian eyes, which is required for the proliferation and expansion of retinal stem cells (Xu et al., 2007).
Rare quiescent cells characterized as stem cells have been separated from the ocular ciliary body of adult mammals. Upon stimulation by growth factors, a subset of ciliary body epithelial cells undergoes a remarkable transformation, reacquiring embryonic characteristics such as Nestin expression. Additionally, these cells expressed CyclinD1 and Ki67, which are associated with cell cycle entry, as well as the retinal progenitor homeodomain transcription factors Pax6 and Chx10, which may suggest that consistently expressed Pax6 is essential for maintaining stem cell properties in ciliary pigment cells (Abdouh & Bernier, 2006; Froen et al., 2011). Mutations in Pax6 in animal models have shown that Pax6 profoundly impacts gene regulation during ciliary body development. For example, complete deletion of Pax6 is responsible for ciliary body and iris defects, subsequently affecting the lens and vitreous. Conversely, overexpression of Pax6 inhibits ciliary body cell differentiation and results in hypertrophy of the iris sphincter muscle, causing circular constriction of the pupil (Davis et al., 2009). In addition, the Bmp4 and TGF-β2 genes are the direct downstream targets of Pax6 (Wang et al., 2017).
In summary, the Wnt, TGF-β, and Pax6 signalling pathways participate in ciliary body developmental processes, including cell differentiation, maturation, fate specification, and morphological fold formation (Fig. 3).
2. Ciliary body hereditary diseases
The intricate development of the ciliary body is governed by a network of signalling pathways, including Wnt, TGF-β, and Pax6 pathways, which govern cell differentiation pathways, maturation, fate specification, and forming morphological folds within the ciliary body. However, disruptions in these pathways can lead to a spectrum of hereditary diseases affecting the ciliary body. Such conditions include hypoplasia, characterized by underdevelopment of the ciliary body, and tumorous growths within ciliary. The interplay between these developmental pathways and the manifestation of ciliary diseases underscores the fundamental importance of understanding the molecular mechanisms guiding ciliary body development.
2.1. Ciliary body hypoplasia
Ciliary body hypoplasia is related to incomplete closure of the optic fissure, which is manifested by the failure of opposing neuroepithelia along the entire proximal-distal axis of the ventral optic cup (Patel & Sowden, 2019; Weigele & Bohnsack, 2020). Developmental failure contributes to visual impairment and is closely related to various intricate ocular phenotypes, including anterior segment dysgenesis (ASD), microphthalmia, and aniridia (Fig. 4) (Chan et al., 2020; Patel et al., 2020; Stahnke et al., 2018).
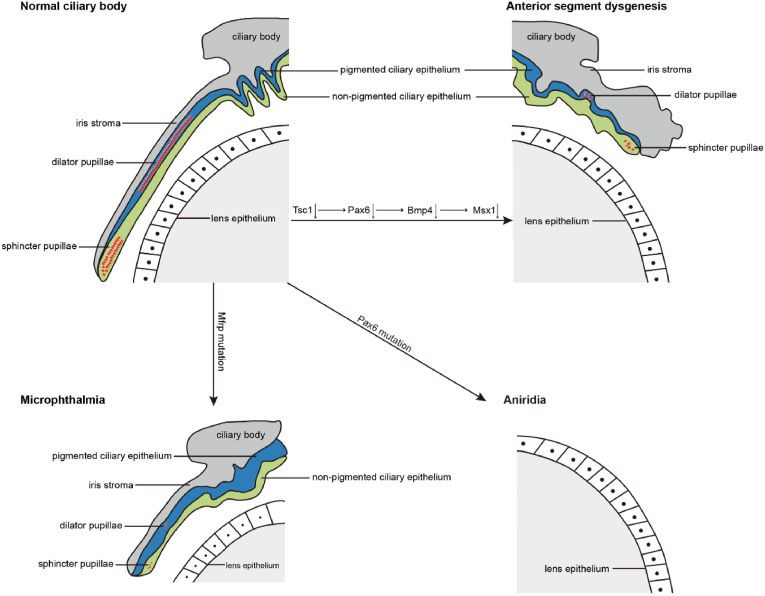
Fig. 4.
Schematic of ciliary body hypoplasia. Adapted from Hägglund et al. (2017). Conditional deletion of the Tsc1 gene causes a reduction in the downstream target genes Pax6, Bmp4, and Msx1, which further contributes to the development of anterior segment dysgenesis. Mutations in Pax6 contribute to aniridia, and mutations in Mfrp contribute to microphthalmia.
ASD is a complex developmental syndrome affecting the tissues of the anterior segment, and is generally caused by ocular and systemic dysplasia induced by inherited genetic mutations (Kaushik et al., 2022a). The tuberous sclerosis complex 1-ablated (Tsc1-ablated) mouse model has been proven to be an effective method for revealing complex genotypic and phenotypic heterogeneity. Downregulation of Pax6, Bmp4, and Msx1 expression and decreased progenitor cell proliferation led to the conditional deletion of Tsc1 in the ciliary margin. Subsequently, the ciliary body and iris fail to develop (Hägglund et al., 2017).
Aniridia, a rare genetic eye disorder, arises from an embryonic anomaly affecting the development of the neural ectoderm and mesoderm. Aniridia leads to congenital iris hypoplasia or the complete absence of the ciliary body and iris and approximately 50% of individuals with aniridia also develop glaucoma (Guo et al., 2022; Munoz-Negrete et al., 2021). Research has provided insights into the ocular conditions of patients with aniridia, revealing severe ciliary body defects. These anomalies have been correlated with the Pax6 gene mutation at chromosome 11p13 (Chen et al., 2020; Nylander et al., 2022). Therefore, mutations in the Pax6 gene are believed to underlie the genetic basis of aniridia and associated ciliary body defects.
Kaushik et al. (2022b), in a study of 124 children with primary aphakia, coupled with ciliary body and aqueous humour dysfunctions, reported that the cornea is highly susceptible to surgical incision and infections. The membrane frizzled-related protein (Mfrp) is selectively expressed in the ciliary body and retinal pigment epithelium. Mfrp mutation has been shown to be directly associated with ciliary body hypoplasia and microphthalmia, together with retinitis pigmentosa (Metlapally et al., 2008).
Malfunction or abnormality of the ciliary body during the developmental stage further exacerbates ASD, aniridia and microphthalmia to a certain extent. Exploring potential target genes in the signalling pathway regulating optic chiasm closure may contribute to understanding the pathogenic mechanisms of ciliary developmental disorders.
2.2. Uveitis
Uveitis, one of the leading causes of blindness, is a condition manifested by inflammation of the portion of the uvea consisting of the iris, ciliary body, and choroid (Nakayama et al., 2023). Anterior uveitis encompasses inflammation of the iris or ciliary body, causing symptoms such as redness, pain, and sensitivity to light in the eye (Harthan et al., 2016; Jiang et al., 2021).
RNA sequencing of the ciliary body revealed the involvement of the IL15 receptor α (IL15RA) in immune-mediated uveitis (Lou et al., 2022). RNA biomarkers specific to uveitis subtypes further support the role of IL15RA in the immunopathogenesis of uveitis (Rosenbaum et al., 2021). Additionally, a study of lipopolysaccharide-stimulated ciliary explants demonstrated that the endotoxin receptor proteins Toll-like receptor 4 (TLR4) and CD14 are highly expressed in human ciliary nonpigmented epithelial cells. Notably, TLR4 significantly inhibited the secretion of tumour necrosis factor by the ciliary body (Brito et al., 2004). These findings reveal the coexpression pattern of TLR4 and CD14 in the ciliary body, which potentially indicates the mechanisms contributing to the development of uveitis.
2.3. Congenital glaucoma
Congenital glaucoma is triggered by obstructed aqueous humour drainage because of abnormal development of the trabecular meshwork and anterior chamber angle, which commonly occurs before the age of three years (Badawi et al., 2019). Increased IOP is a significant risk factor, and is usually triggered by impaired circulation of aqueous humour secreted by the ciliary body (Chong et al., 2023).
In a study involving 114 participants, Chen et al. (2022) reported that individuals with primary closed-angle glaucoma exhibit a thinner thickness of ciliary body and a more anterior positioning of the ciliary process, which could explain the disrupted equilibrium of aqueous humour circulation. Notably, the ciliary muscle ameliorates the aqueous humour circulation disorders by facilitating the drainage of aqueous humour into the Schlemm's canal, suggesting that the ciliary muscle is a potential target for therapies focused on ciliary muscle paralysis and glaucoma treatment (Safwat et al., 2020). Additionally, RNA sequencing of the single cells has revealed the involvement of the angiopoietin receptor TEK and intercellular cell adhesion molecule-1 (ICAM-1) in ciliary body cell interactions, which are strongly related to the risk factors for primary congenital glaucoma (Kabra et al., 2017; Lou et al., 2022).
Al Nosair et al. (2017) have illuminated that congenital glaucoma is not only linked to anomalies in the anterior chamber angle but also intricately connected to dysplasia affecting the iris, ciliary body, and scleral spicules. The presence of ciliary hypoplasia adds to this complexity, leading to hindered drainage of aqueous humour, ultimately culminating in elevated IOP. Therefore, abnormal tissue development is extremely important in the pathogenesis of congenital glaucoma.
2.4. Ciliary body tumours
Ciliary body tumours, which are rare but highly genetically related, constitute a significant category of tumours affecting the anterior eye segment. It is crucial to have a comprehensive understanding of the clinical presentations, imaging characteristics, and genetic attributes of ciliary body developmental disorders. A detailed classification and overview of ciliary body tumours can be found in Table 1.
Table 1.
Classification and characteristics of ciliary body tumour.
| Tumour Types | Classification | Clinical Manifestations | Affected Population | Treatment |
|---|---|---|---|---|
| medulloepithelioma | malignant | tissues arranged in nests or cords; cells arranged in single or compound layers, glandular tubular or finger-like | children | local excision or enucleation |
| melanoma | malignant | dark brown lump, marked cellular heterogeneity, and melanin granules in the cytoplasm | middle-aged | for large tumour, enucleation; for medium-sized, 125I; for small, monitoring |
| melanocytoma | benign | large and polygonal cells; pigment-rich granules in cytoplasm | middle-aged | local excision of a larger lump |
| adenoma of non-pigmented ciliary epithelium | benign hyperplasia | Irregularly striated or nested structures, powder-stained basement membrane-like material | middle-aged | local excision of the lump |
| adenocarcinoma of the non-pigmented ciliary body epithelium | malignant | interstitial fibrous tissue hyperplasia intracellular vacuoles and marked cellular heterogeneity | middle-aged and elderly | early stage, local excision; advanced, local radiotherapy |
| leiomyoma | benign | cells are arranged in small bundles and weaves, swirling structures, and localized calcium deposits | childbearing age woman | larger tumour, enucleation; localized symptoms, tumour excision feasible |
Abbreviations: NSE, Neuron-specific enolase; CK, Cytokeratin pan; SMA, smooth muscle actin.
Medulloepithelioma of the ciliary body originates from the nonpigmented ciliary epithelium, and primarily develops in children (He et al., 2023). Ciliary body tumours are pinkish-white and may appear as chalky calcified opacities when located in the ciliary body through slit-lamp microscopic examination (Rehman et al., 2021). Initial clinical symptoms often include reduced vision, increased IOP, angle closure, and eye redness. Visual impairment in affected individuals can be attributed to factors such as cataracts, lens subluxation, lens coloboma, retrolental membranes, or neovascular glaucoma (Tadepalli et al., 2019). Medulloepithelioma of the ciliary body occurs in approximately 3% of DICER-1-positive patients. DICER-1 is a member of the ribonuclease III family that is inherited in an autosomal dominant manner (Cai et al., 2017).
Ciliary body uveal melanoma, a rare type of cancer that occurs in the eye, accounts for approximately 3–5% of all uveal melanoma and is characterized as an immunogenic cancer with diverse initial clinical manifestations (Pasarica et al., 2022). Notably, ciliary body melanoma is linked to strong risk cytogenetics and a rich microvasculature, creating an environment conducive to tumour growth and metastasis. Recent genetic testing revealed a pathogenic heterozygous mutation in BRCA1-associated protein 1 (BAP1) and offers valuable insights into the underlying pathogenic mechanisms of ciliary melanoma (Carrera et al., 2020). A retrospective case-control study revealed that GNAQ/GNA11 mutations were found in 91% (32) of these patients (patients with melanocytoma (n = 16) and melanoma (n = 19) of the anterior uvea), suggesting the anterior segment uveal melanocytomas did not display oncogenic alterations beyond GNAQ/GNA11 (Solomon et al., 2022). Given the rarity of ciliary melanoma, clinical diagnosis and intervention are of utmost importance, and these procedures could be life-saving.
3. Conclusions and perspectives
Ciliary body development is a complex and multistep process that requires coordinated variation in cell proliferation and differentiation, maturation and folding. This process involves multiple signalling pathways that regulate ciliary morphogenesis. Disruption of any of these signalling pathways can lead to impaired development of the ciliary body, further contributing to eye abnormalities.
Genetic ciliary body diseases are characterized by developmental anomalies and unique molecular hereditary features. An in-depth study of the mechanisms of ciliary body development is vital for understanding the complexity of the ciliary body and would provide valuable avenues for the diagnosis and treatment of genetic diseases. Currently, investigations into the mechanisms governing ciliary body development are needed to determine the clinical application of treatments for ciliary body diseases.
Author contributions
Baige Li was the major contributor in reviewing the literature, writing the manuscript, and creating descriptive figures. Ting Xie, Scott Nawy, and Yin Shen critically reviewed and revised the manuscript. All the authors read and approved the final manuscript.
Declaration of competing interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This work was supported by National Key R&D Program of China (2017YFE0103400), NSFC (81470628, 81900874).
References
- Abdouh M., Bernier G. In vivo reactivation of a quiescent cell population located in the ocular ciliary body of adult mammals. Experimental Eye Research. 2006;83(1):153–164. doi: 10.1016/j.exer.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Aghaei H., Kheirkhah A., Es'Haghi A., Aghamirsalim M.R., Asgari S., Kordamiri M.M. Disruption of blood-aqueous barrier in dry eye disease. Ocular Surface. 2021;19:266–269. doi: 10.1016/j.jtos.2020.10.002. [DOI] [PubMed] [Google Scholar]
- Al Nosair G., Khandekar R., Al-Shamrani M., Edward D.P. Ciliary body location in eyes with and without primary congenital glaucoma. Canadian Journal of Ophthalmology. 2017;52(6):578–582. doi: 10.1016/j.jcjo.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Alldredge A., Fuhrmann S. Loss of Axin2 causes ocular defects during mouse eye development. Investigative Ophthalmology & Visual Science. 2016;57(13):5253–5262. doi: 10.1167/iovs.15-18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A.H., Al-Muhaylib A.A., Al Owaifeer A.M., Al-Essa R.S., Al-Shahwan S.A. Primary congenital glaucoma: An updated review. Saudi J. Ophthalmol. 2019;33(4):382–388. doi: 10.1016/j.sjopt.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z.Z., Cepko C.L. The expression and function of Notch pathway genes in the developing rat eye. Journal of Neuroscience. 1977;17(4):1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M.H. The radiobiology of TGFβ. Seminars in Cancer Biology. 2022;86(Pt 3):857–867. doi: 10.1016/j.semcancer.2022.02.001. [DOI] [PubMed] [Google Scholar]
- Belanger M.C., Robert B., Cayouette M. Msx1-Positive progenitors in the retinal ciliary margin give rise to both neural and non-neural progenies in mammals. Developmental Cell. 2017;40(2):137–150. doi: 10.1016/j.devcel.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Brito B.E., Zamora D.O., Bonnah R.A., Pan Y.Z., Planck S.R., Rosenbaum J.T. Toll-like receptor 4 and CD14 expression in human ciliary body and TLR-4 in human iris endothelial cells. Experimental Eye Research. 2004;79(2):203–208. doi: 10.1016/j.exer.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Cai S.Y., Zhao W., Nie X.L., Abbas A., Fu L.B., Bihi S., Feng G.S., Liu T.Y., Lv Y.Q., Ma X.L., Peng X.X. Multimorbidity and genetic characteristics of DICER1 syndrome based on systematic review. J. Pediatr. Hematol. Oncol. 2017;39(5):355–361. doi: 10.1097/MPH.0000000000000715. [DOI] [PubMed] [Google Scholar]
- Caracci M.O., Avila M.E., Espinoza-Cavieres F.A., López H.R., Ugarte G.D., De Ferrari G.V. Wnt/β-catenin-dependent transcription in autism spectrum disorders. Frontiers in Molecular Neuroscience. 2021;14 doi: 10.3389/fnmol.2021.764756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera W.M., Denny M.R., Seider M.I. Crystalline lens resorption caused by ciliary body melanoma. Case Reports in Oncology. 2020;13(2):497–500. doi: 10.1159/000507509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B.H.C., Moosajee M., Rainger J. Closing the gap: Mechanisms of epithelial fusion during optic fissure closure. Frontiers in Cell and Developmental Biology. 2020;8 doi: 10.3389/fcell.2020.620774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.Y., He N., Yan Y.J., Fan X., Wu L.L. Ultrasound biomicroscopic imaging demonstrate thinner ciliary body thickness in eyes with angle closure. International Journal of Ophthalmology. 2022;15(9):1476–1482. doi: 10.18240/ijo.2022.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.D., Yang T., Zhu S.Q. Recurrent PAX 6 mutation in a Chinese family with congenital aniridia, progressive cataracts and mental retardation. European Journal of Ophthalmology. 2020;30(1):181–188. doi: 10.1177/1120672118810998. [DOI] [PubMed] [Google Scholar]
- Cho S.H., Cepko C.L. Wnt2b/β-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133(16):3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Chong R.S., Li H.T., Cheong A.J.Y., Fan Q., Koh V., Raghavan L., Nongpiur M.E., Cheng C.Y. Mendelian randomization implicates bidirectional association between myopia and primary open-angle glaucoma or intraocular pressure. Ophthalmology. 2023;130(4):394–403. doi: 10.1016/j.ophtha.2022.11.030. [DOI] [PubMed] [Google Scholar]
- Da Silva M.R.D., Tiffin N., Mima T., Mikawa T., Hyer J. FGF-mediated induction of ciliary body tissue in the chick eye. Developmental Biology. 2007;304(1):272–285. doi: 10.1016/j.ydbio.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Yoffe C., Raviv S., Antes R., Berger J., Holzmann S., Stoykova A., Overbeek P.A., Tamm E.R., Ashery-Padan R. Pax6 dosage requirements in iris and ciliary body differentiation. Developmental Biology. 2009;333(1):132–142. doi: 10.1016/j.ydbio.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Fernández-Vigo J.I., Kudsieh B., Shi H., De-Pablo-Gómez-de-Liaño L., Fernández-Vigo J.A., García-Feijóo J. Diagnostic imaging of the ciliary body: Technologies, outcomes, and future perspectives. European Journal of Ophthalmology. 2022;32(1):75–88. doi: 10.1177/11206721211031409. [DOI] [PubMed] [Google Scholar]
- Fotaki V., Smith R., Pratt T., Price D.J. Foxg1 is required to limit the formation of ciliary margin tissue and Wnt/β-catenin signalling in the developing nasal retina of the mouse. Developmental Biology. 2013;380(2):299–313. doi: 10.1016/j.ydbio.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froen R.C., Johnsen E.O., Petrovski G., Berényi E., Facskó A., Berta A., Nicolaissen B., Moe M.C. Pigment epithelial cells isolated from human peripheral iridectomies have limited properties of retinal stem cells. Acta Ophthalmologica. 2011;89(8):e635–e644. doi: 10.1111/j.1755-3768.2011.02198.x. [DOI] [PubMed] [Google Scholar]
- Guo R.R., Zhang X.T., Liu A.H., Ji J., Liu W. Novel clinical presentation and PAX6 mutation in families with congenital aniridia. Frontiers of Medicine. 2022;9 doi: 10.3389/fmed.2022.1042588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägglund A.C., Jones I., Carlsson L. A novel mouse model of anterior segment dysgenesis (ASD): Conditional deletion of Tsc1 disrupts ciliary body and iris development. Dis. Model. Mech. 2017;10(3):245–257. doi: 10.1242/dmm.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harthan J.S., Opitz D.L., Fromstein S.R., Morettin C.E. Diagnosis and treatment of anterior uveitis: Optometric management. Clinical Ophthalmology. 2016;8:23–35. doi: 10.2147/OPTO.S72079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Pei C., Ge X., Ma J.M., Hu Y.G. Analysis of clinical and pathological features of ciliary body medulloepithelioma. International Journal of Ophthalmology. 2023;16(3):382–387. doi: 10.18240/ijo.2023.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig H., Kittredge K.L., Coca-Prados M., Davis J., Palestine A.G., Nussenblatt R.B. Mammalian ciliary-body epithelial cells in culture produce transforming growth factor-beta. Graefes Archive for Clinical and Experimental Ophthalmology. 1991;229(1):84–87. doi: 10.1007/BF00172268. [DOI] [PubMed] [Google Scholar]
- Igarashi N., Honjo M., Yamagishi R., Kurano M., Yatomi Y., Igarashi K., Kaburaki T., Aihara M. Crosstalk between transforming growth factor β-2 and Autotaxin in trabecular meshwork and different subtypes of glaucoma. J. Biomed. Sci. 2021;28(1):47. doi: 10.1186/s12929-021-00745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Li Z.H., Tao T.Y., Duan R.P., Wang X.G., Su W.R. TNF-α in uveitis: From bench to clinic. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.740057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabra M., Zhang W., Rathi S., Mandal A.K., Senthil S., Pyatla G., Ramappa M., Banerjee S., Shekhar K., Marmamula S., Mettla A.L., Kaur I., Khanna R.C., Khanna H., Chakrabarti S. Angiopoietin receptor TEK interacts with CYP1B1 in primary congenital glaucoma. Human Genetics. 2017;136(8):941–949. doi: 10.1007/s00439-017-1823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karampetsou M., Vekrellis K., Melachroinou K. The promise of the TGF-β superfamily as a therapeutic target for Parkinson's disease. Neurobiology of Disease. 2022;171 doi: 10.1016/j.nbd.2022.105805. [DOI] [PubMed] [Google Scholar]
- Kaushik S., Dubey S., Choudhary S., Ratna R., Pandav S.S., Khan A.O. Anterior segment dysgenesis: Insights into the genetics and pathogenesis. Indian Journal of Ophthalmology. 2022;70(7):2293–2303. doi: 10.4103/ijo.IJO_3223_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S., Snehi S., Kaur S., Kaur A., Choudhary S., Thattaruthody F., Pandav S.S. Primary aphakia: Clinical recognition is the key to diagnosis. J. Aapos. 2022;26(6):298.e1–298.e5. doi: 10.1016/j.jaapos.2022.07.012. [DOI] [PubMed] [Google Scholar]
- Kitamoto J., Hyer J. The expression of Wnt2b in the optic cup lip requires a border between the pigmented and nonpigmented epithelium. Molecular Vision. 2010;16:2701–2717. [PMC free article] [PubMed] [Google Scholar]
- Knaus K.R., Hipsley A., Blemker S.S. The action of ciliary muscle contraction on accommodation of the lens explored with a 3D model. Biomechanics and Modeling in Mechanobiology. 2021;20(3):879–894. doi: 10.1007/s10237-021-01417-9. [DOI] [PubMed] [Google Scholar]
- Kubo F., Takeichi M., Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130(3):587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Liu H., Xu S.B., Wang Y.P., Mazerolle C., Thurig S., Coles B.L.K., Ren J.C., Taketo M.M., van der Kooy D., Wallace V.A. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Developmental Biology. 2007;308(1):54–67. doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Lou B.S., Zeng L., Gao X.B., Qian X.B., Li J.J., Gu X.Y., Liu Z., Liu K.L., Chen X., Lin X.F., Zhang F. A single-cell transcriptomic atlas of the human ciliary body. Cellular and Molecular Life Sciences. 2022;79(10):528. doi: 10.1007/s00018-022-04559-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney J.P., Bruguera E.S., Vasishtha M., Killingsworth L.B., Kyaw S., Weis W.I. PI(4,5)P2-stimulated positive feedback drives the recruitment of Dishevelled to Frizzled in Wnt-β-catenin signaling. Science Signaling. 2022;15(748) doi: 10.1126/scisignal.abo2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlapally R., Li Y.J., Tran-Viet K.N., Bulusu A., White T.R., Ellis J., Kao D., Young T.L. Common MFRP sequence variants are not associated with moderate to high hyperopia, isolated microphthalmia, and high myopia. Molecular Vision. 2008;14:387–393. [PMC free article] [PubMed] [Google Scholar]
- Mi Y.H., Zhong L., Lu S.J., Hu P., Pan Y., Ma X.L., Yan B.H., Wei Z.H., Yang G.M. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. Journal of Ethnopharmacology. 2022;290 doi: 10.1016/j.jep.2022.115066. [DOI] [PubMed] [Google Scholar]
- Munoz-Negrete F.J., Teus M.A., Garcia-Feijoo J., Canut M.I., Rebolleda G. Aniridic glaucoma: An update. Archivos de la Sociedad Espanola de Oftalmologia. 2021;96(S1):52–59. doi: 10.1016/j.oftale.2020.11.011. [DOI] [PubMed] [Google Scholar]
- Nakayama L.F., Ribeiro L.Z., Dychiao R.G., Zamora Y.F., Regatieri C.V.S., Celi L.A., Silva P., Sobrin L., Belfort R. Artificial intelligence in uveitis: A comprehensive review. Survey of Ophthalmology. 2023;68(4):669–677. doi: 10.1016/j.survophthal.2023.02.007. [DOI] [PubMed] [Google Scholar]
- Nylander J., Dugan S.P., Lam J., Elner V.M., Gappy C., Demirci H. A case of ciliary body cyst with extrascleral extension. J. Aapos. 2022;26(3):152–155. doi: 10.1016/j.jaapos.2022.01.007. [DOI] [PubMed] [Google Scholar]
- Ochi S., Manabe S., Kikkawa T., Osumi N. Thirty years' history since the discovery of Pax6: From central nervous system development to neurodevelopmental disorders. International Journal of Molecular Sciences. 2022;23(11):6115. doi: 10.3390/ijms23116115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J., Le L., Zhou Y., Tu R.J., Hou Q., Tsuchiya D., Thomas N., Wang Y.F., Yu Z.L., Alexander R., Thexton M., Lewis B., Corbin T., Durnin M., Li H., Ashery-Padan R., Yan D.Y., Xie T. NOTCH signaling controls ciliary body morphogenesis and secretion by directly regulating nectin protein expression. Cell Reports. 2021;34(2) doi: 10.1016/j.celrep.2020.108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasarica M.A., Curca P.F., Dragosloveanu C.D.M., Tataru C.I., Manole I.R., Murgoi G.E., Grigorescu A.C. Underlying ciliary body uveal melanoma in a patient with chronic lymphocytic leukemia presenting for hyphema. Diagnostics. 2022;12(6):1312. doi: 10.3390/diagnostics12061312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Anderson G., Galea G.L., Balys M., Sowden J.C. A molecular and cellular analysis of human embryonic optic fissure closure related to the eye malformation coloboma. Development. 2020;147(24) doi: 10.1242/dev.193649. [DOI] [PubMed] [Google Scholar]
- Patel A., Sowden J.C. Genes and pathways in optic fissure closure. Seminars in Cell & Developmental Biology. 2019;91:55–65. doi: 10.1016/j.semcdb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Ragg S., Key M., Rankin F., WuDunn D. The effect of molecular weight on passage of proteins through the blood-aqueous barrier. Investigative Ophthalmology & Visual Science. 2019;60(5):1461–1469. doi: 10.1167/iovs.19-26542. [DOI] [PubMed] [Google Scholar]
- Ramos C., Robert B. msh/Msx gene family in neural development. Trends Genet. 2005;21(11):624–632. doi: 10.1016/j.tig.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Rehman O., Narang S., Nayyar S., Aggarwal P. Unusual case of intraocular medulloepithelioma in an adult male. Rom. J. Ophthalmol. 2021;65(3):296–299. doi: 10.22336/rjo.2021.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson F.L., O'Duibhir E., Gangoso E., Bressan R.B., Bulstrode H., Marqués-Torrejón M.A., Ferguson K.M., Blin C., Grant V., Alfazema N., Morrison G.M., Pollard S.M. Elevated FOXG1 in glioblastoma stem cells cooperates with Wnt/(3-catenin to induce exit from quiescence. Cell Reports. 2023;42(6) doi: 10.1016/j.celrep.2023.112561. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J.T., Harrington C.A., Searles R.P., Fei S.S., Zaki A., Arepalli S., Paley M.A., Hassman L.M., Vitale A.T., Conrady C.D., Keath P., Mitchell C., Watson L., Planck S.R., Martin T.M., Choi D. Identifying RNA biomarkers and molecular pathways involved in multiple aubtypes of uveitis. American Journal of Ophthalmology. 2021;226:226–234. doi: 10.1016/j.ajo.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sader F., Roy S. Tgf-β superfamily and limb regeneration: Tgf-β to start and Bmp to end. Developmental Dynamics. 2022;251(6):973–987. doi: 10.1002/dvdy.379. [DOI] [PubMed] [Google Scholar]
- Safwat A.M.M., Hammouda L.M., El-Zembely H.I., Omar I.A.N. Evaluation of ciliary body by ultrasound bio-microscopy after trans-scleral diode cyclo-photocoagulation in refractory glaucoma. European Journal of Ophthalmology. 2020;30(6):1335–1341. doi: 10.1177/1120672119899904. [DOI] [PubMed] [Google Scholar]
- Saika S., Saika S., Liu C.Y., Azhar M., Sanford L.P., Doetschman T., Gendron R.L., Kao C.W.C., Kao W.W.Y. TGFβ2 in corneal morphogenesis during mouse embryonic development. Developmental Biology. 2001;240(2):419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Sarode B., Nowell C.S., Ihm J., Kostic C., Arsenijevic Y., Moulin A.P., Schorderet D.F., Beermann F., Radtke F. Notch signaling in the pigmented epithelium of the anterior eye segment promotes ciliary body development at the expense of iris formation. Pigment Cell Melanoma Res. 2014;27(4):580–589. doi: 10.1111/pcmr.12236. [DOI] [PubMed] [Google Scholar]
- Skalicky S.E. Springer; Singapore: 2016. The ciliary body and aqueous fluid formation and drainage. [DOI] [Google Scholar]
- Solomon D.A., Ramani B., Eiger-Moscovich M., Milman T., Uludag G., Crawford J.B., Phan I., Char D.H., Shields C.L., Eagle R.C., Bastian B.C., Bloomer M.M., Pekmezci M. Iris and ciliary body melanocytomas are defined by solitary GNAQ mutation without additional oncogenic alterations. Ophthalmology. 2022;129(12):1429–1439. doi: 10.1016/j.ophtha.2022.07.002. [DOI] [PubMed] [Google Scholar]
- Stahnke T., Erbersdobler A., Knappe S., Guthoff R.F., Kilangalanga N.J. Management of congenital clinical anophthalmos with orbital cyst: A kinshasa case report. Case Rep. Ophthalmol. Med. 2018;2018 doi: 10.1155/2018/5010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadepalli S.H., Shields C.L., Shields J.A., Honavar S.G. Intraocular medulloepithelioma - a review of clinical features, DICER 1 mutation, and management. Indian Journal of Ophthalmology. 2019;67(6):755–762. doi: 10.4103/ijo.IJO_845_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B., Chen Y., Davidson E.A., Garcia-Milian R., Golla J.P., Apostolopoulos N., Orlicky D.J., Schey K., Thompson D.C., Vasiliou V. Impaired GSH biosynthesis disrupts eye development, lens morphogenesis and PAX6 function. Ocular Surface. 2021;22:190–203. doi: 10.1016/j.jtos.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Shan X.H., Gregory-Evans C.Y. A mouse model of aniridia reveals the in vivo downstream targets of Pax6 driving iris and ciliary body development in the eye. Biochimica et Biophysica Acta, Molecular Basis of Disease. 2017;1863(1):60–67. doi: 10.1016/j.bbadis.2016.10.018. [DOI] [PubMed] [Google Scholar]
- Wang C.B., Zang K., Tang Z.X., Yang T., Ye X.Y., Dang Y.Y. Hordenine activated dermal papilla cells and promoted hair regrowth by activating Wnt signaling pathway. Nutrients. 2023;15(3):694. doi: 10.3390/nu15030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigele J., Bohnsack B.L. Genetics underlying the interactions between neural crest cells and eye development. Journal of Developmental Biology. 2020;8(4):26. doi: 10.3390/jdb8040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.E. TGF beta-1,-2 and-3 in the modulation of fibrosis in the cornea and other organs. Experimental Eye Research. 2021;207 doi: 10.1016/j.exer.2021.108594. [DOI] [PubMed] [Google Scholar]
- Wu L.Y., Li M., Hinton D.R., Guo L., Jiang S.Y., Wang J.T., Zeng A., Xie J.B., Snead M., Shuler C., Maxson R.E., Liu Y.H. Microphthalmia resulting from Msx2-induced apoptosis in the optic vesicle. Investigative Ophthalmology & Visual Science. 2003;44(6):2404–2412. doi: 10.1167/iovs.02-0317. [DOI] [PubMed] [Google Scholar]
- Xu S.B., Sunderland M.E., Coles B.L.K., Kam A., Holowacz T., Ashery-Padan R., Marquardt T., McInnes R.R., van der Kooy D. The proliferation and expansion of retinal stem cells require functional Pax6. Developmental Biology. 2007;304(2):713–721. doi: 10.1016/j.ydbio.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.P., Jia H.Y., Ma Q., Zhang R.R., Jiao Y.H. A novel missense variant expands the phenotype and genotype of PAX6-associated foveal hypoplasia accompanied by various manifestations of anterior segment dysgenesis. BMC Ophthalmology. 2023;23(1):349. doi: 10.1186/s12886-023-03054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.L., Chen Q., Hung F.C., Overbeek P.A. BMP signaling is required for development of the ciliary body. Development. 2002;129(19):4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Tanzie C., Yan Z.P., Chen S.Y., Duncan M., Gaudenz K., Li H., Seidel C., Lewis B., Moran A., Libby R.T., Kiernan A.E., Xie T. Notch2 regulates BMP signaling and epithelial morphogenesis in the ciliary body of the mouse eye. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):8966–8971. doi: 10.1073/pnas.1218145110. [DOI] [PMC free article] [PubMed] [Google Scholar]