Abstract
The global spread of highly pathogenic avian influenza (HPAI) A (H5N1) clade 2.3.4.4b virus since 2021 necessitates a re-evaluation of the role of vaccination in controlling HPAI outbreaks among poultry, which has been controversial because of the concern of silent spread with viral mutation and spillover to human. We systematically reviewed and meta-analyzed all existing data from experimental challenge trials to assess the efficacy of HPAI vaccines against mortality in specific pathogen free (SPF) chickens, with evaluation of the certainty of evidence (CoE) using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. Out of 223 screened publications, 46 trials met our eligibility criteria. Inactivated vaccines showed an efficacy of 95% (risk ratio [RR] = 5% [95% CI: 1% to 17%], I2 = 0%, CoE high) against homologous strains and an efficacy of 78% (RR = 22% [95% CI: 14% to 37%], I2 = 18%, CoE high) against heterologous strains (test for subgroup difference p = 0.02). Live recombinant vaccines exhibited the highest efficacy at 97% (RR = 3% [95% CI: 1% to 13%], I2 = 0%, CoE high). Inactivated recombinant vaccines had an overall efficacy of 90% (RR = 10% [95% CI: 6% to 16%], I2 = 47%, CoE high). Commercial vaccines showed an overall efficacy of 91% (RR = 9% [95% CI: 5% to 17%], I2 = 23%, CoE high), with 96% efficacy (RR = 4% [95% CI: 1% to 21%], I2 = 0%, CoE high) against homologous strains and 90% efficacy (RR = 10% [95% CI: 5% to 20%], I2 = 31%, CoE moderate) against heterologous strains. Our systematic review offers an updated and unbiased assessment of vaccine efficacy against HPAI-related mortality, providing timely and crucial information for re-evaluating the role of vaccination in poultry avian influenza control policy amist the global HPAI outbreak post-2021.
Keywords: Highly pathogenic avian influenza, HPAI, Vaccine, Vaccine efficacy, Meta-analysis
1. Introduction
Since 2021, the highly pathogenic avian influenza (HPAI) A (H5N1) clade 2.3.4.4b virus, has spread globally [[1], [2], [3]], resulting in significant morbidity and mortality among domestic poultry [4] and affecting the supply chain of poultry products as well as human food safety [[5], [6], [7]]. This situation necessitated a re-evaluation of the role of vaccination in controlling avian influenza among poultry [8]. At the 90th General Session of the World Organization for Animal Health (WOAH) on May 25, 2023, the challenges and unsustainability of relying solely on conventional biosecurity measures and mass culling as control strategies were recognized [9]. In response, France has implemented a pilot vaccination program targeting approximately 64 million commercial ducks, set to begin on October 1, 2023 [10].
Until now, the use of vaccination as a strategy against HPAI in poultry has remained highly controversial. This is primarily due to concerns that vaccination might complicate surveillance efforts by masking HPAI-related mortality [11,12], leading to silent spread [[13], [14], [15]] and viral mutation [[16], [17], [18]] with the potential risk of spillover to humans and the danger of a new wave of global pandemic. However, the primary data on the efficacy of vaccination in domestic poultry comprise of challenge tests with very small sample sizes. To date, data on vaccine efficacy against HPAI have not been synthesized according to modern methodological standards for evidence-based veterinary medicine.
To inform policymaking, we systematically reviewed and meta-analyzed all existing data from experimental challenge trials to assess the efficacy of HPAI vaccines against mortality in specific pathogen free (SPF) chickens, with evaluation of the certainty of evidence (CoE) using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [19].
2. Methods
2.1. Protocol and registration
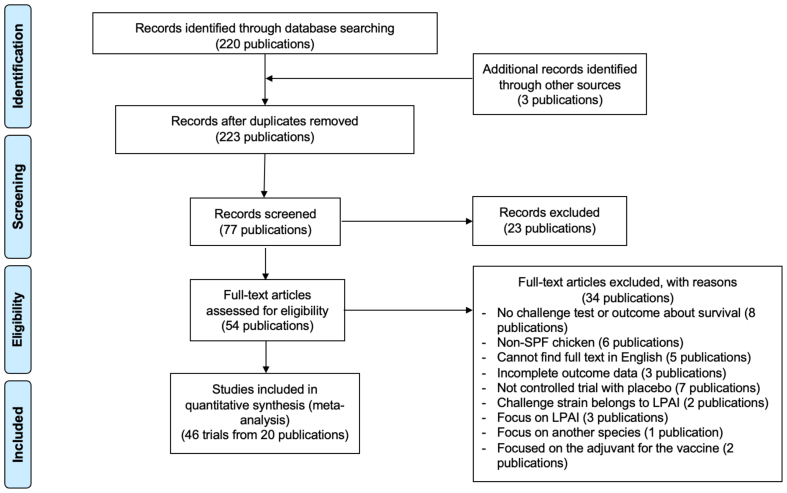
This study adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA). A flow chart illustrating the study's design and methodology is provided in Fig. 1.
Fig. 1.
PRISMA flow diagram. (SPF: Specific Pathogen Free; LPAI: Low Pathogenic Avian Influenza).
2.2. Literature search
The literature search was conducted using the PubMed, Embase, and Science Citation Index (Web of Science) databases. To be relevant for contemporary vaccine technology in the poultry farming, our literature search covered the period from January 1, 2010, to September 5, 2023. All selected studies were in English. The search strategy utilized the following query: ((poultry) OR (chicken) OR (layer)) AND ((avian influenza [Title]) OR (HPAI [Title]) OR (LPAI [Title]) OR (bird flu [Title]) OR (avian flu [Title])) AND ((vaccine [Title])) AND ((efficacy [Title]) OR (protect [Title]) OR (immunization [Title])) (Appendix Table 1).
The inclusion criteria for study selection were defined as follows:
-
1.
Studies must include a challenge test as part of their experimental design.
-
2.
The target population for the challenge test should be SPF or serum-antibody-negative (SAN) chickens.
-
3.
The challenge test must utilize an HPAI strain that causes a mortality rate of 90% or higher in the sham-inoculated control group.
Exclusion criteria were carefully defined to ensure the meta-analysis remained focused and coherent. The following study types were excluded:
-
1.
In vitro studies.
-
2.
Studies involving non-chicken animal or human subjects.
-
3.
Research using a vaccine seed or challenge strain classified as low pathogenic avian influenza (LPAI).
This deliberate exclusion of certain study types was intended to enhance the homogeneity and reliability of the evidence, thereby strengthening the validity of our conclusions.
2.3. Quality of studies
Two authors (IST and BYP) independently searched the literature, applied the criteria to screen all identified literature, and assessed the risk of bias in each study using the Cochrane Collaboration tool [20]. Any discrepancies encountered at each stage of the study selection process were resolved by discussing with an independent third reviewer (CTF). This approach ensures methodological rigor in the evaluation of study quality.
2.4. Data extraction
The assessment of vaccine efficacy against mortality was based on the count of fatal cases. In cases where the primary literature lacked explicit operational definitions, the analysis defaulted to evaluating mortality at a seven-day interval post-challenge. Vaccines in this study were methodically categorized into four principal groups according to their manufacturing technologies: inactivated vaccines, recombinant vaccines, DNA vaccines, and virus-like particle (VLP) vaccines. Notably, even if a vaccine is inactivated but derived from a mixed or “reassorted” virus, it is classified as a recombinant vaccine. For recombinant vaccines, only data related to hybrid recombinant formulations were included in our analysis, while data on parental viral strains were deliberately excluded. In cases examining vaccine adjuvants, both groups - those receiving adjuvant-containing vaccines and those receiving adjuvant-free vaccines - were included in the experimental group for analysis. Where studies used different concentrations of a vaccine, the analysis employed data from the highest concentration administered. In the subgroup analysis focusing on the relationship between the vaccine seed strain and the challenge virus, strains were categorized as “homologous” if they shared the same HA (including HA1 and HA2) and NA proteins and belonged to the same “clade.” Conversely, strains were deemed “heterologous” if there was a difference in HA or NA proteins or if there was a discrepancy in the “clade” classification between the vaccine seed and the challenge virus.
2.5. Statistical analysis
Statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), with the “meta” package. We conducted a random-effects meta-analysis using the inverse-variance method to estimate the effects between experimental and control groups, while correcting for confounding variables. The primary outcome measure was the risk ratio (RR) of post-challenge mortality in vaccinated versus unvaccinated groups. Vaccine efficacy was calculated as 1 minus the pooled RR. To assess the robustness of our findings, subgroup analyses were conducted. For evaluating potential publication bias, both funnel plot asymmetry and Begg's test were utilized. Statistical significance was set at a p-value threshold of less than 0.05.
For evaluating potential publication bias, both funnel plot asymmetry and Begg's test were utilized. The p-value of the Q test was used to test the presence of heterogeneity. The heterogeneity index (I2) was used to measure the extent of heterogeneity. I2 values at 25%, 50%, and 75% imply low, medium, and high heterogeneity [21]. Statistical significance was set at a p-value threshold of less than 0.05.
2.6. Grade of evidence
For the assessment of the CoE using the GRADE approach [19], we employed the GRADEpro tool (available at https://gradepro.org/). This tool evaluates the confidence level in the effect estimates, based on several critical factors, including risk of bias, inconsistency, indirectness, imprecision, and publication bias. Additionally, considerations such as the presence of a large effect, and plausible confounding that would change the effect were also integral to evaluating the confidence level in the effect estimates. The definitions for the levels of certainty are as follows:
-
•
High CoE: further research is very unlikely to change our confidence in the estimate of effect.
-
•
Moderate CoE: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
•
Low CoE: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
•
Very low CoE: any estimate of effect is very uncertain.
The results of our assessment are illustrated in Appendix Fig. 1.
3. Results
3.1. Study selection and characteristics
We identified a total of 223 publications, of which 220 were sourced from database searches and 3 through other methods. After duplicate removal, 77 publications were screened, and 54 underwent full-text assessment. Ultimately, our dataset included data from 46 trials across 20 experimental studies [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]], as detailed in Appendix Table 2. These trials collectively involved 863 SPF chickens. Of these, 596 were administered vaccinations. It is important to note that within some publications, multiple trials utilized the same placebo groups, resulting in the total number of subjects in the placebo control groups being 267.
The evaluated vaccines in this study comprised 26 trials of recombinant vaccines, 16 trials of inactivated vaccines, two trials of VLP vaccines, and two trials of DNA vaccines. Geographically, 30 trials were conducted in countries with established mass vaccination programs, specifically China, Egypt, Indonesia, and Vietnam [42]. Two trials were conducted in Belgium, a country at the early stages of implementing a vaccination program. The remaining 14 trials were conducted in various other countries, including South Korea, the United States, Australia, the Netherlands, Taiwan, and South Africa. Temporally, 10 trials were published in 2010, eight in 2021, and seven in 2017, with the remaining studies spread across the years 2011 to 2022. The risk of bias is judged to be low for the majority of the trials (Appendix Table 3).
3.2. Vaccine efficacy and grading of evidence
The overall RR for mortality outcomes, calculated from 46 trials across 20 experimental studies, was 0.11, with I2 of 36% (CoE moderate). Consequently, the overall efficacy of the vaccine in reducing mortality was estimated to be 89%, using the formula: 1 – pooled RR = 1–0.11 (Appendix Fig. 2). A subgroup analysis was conducted to determine the efficacy of all vaccines based on the alignment between the strain used in the vaccine seed and the challenge virus. This analysis showed a vaccine efficacy of 91% (I2 = 0%, CoE high) against homologous strains and a vaccine efficacy of 87% (I2 = 50%, CoE moderate) against heterologous strains (Appendix Fig. 2). Additionally, subgroup analysis was performed based on the country. In countries with a mass vaccination policy, the overall vaccine efficacy against mortality was 0.90 with an I2 of 0% in China, 0.81 with an I2 of 0% in Egypt, 0.84 with an I2 of 55% in Indonesia, and 0.86 with an I2 of 0% in Vietnam (Appendix Fig. 3). Publication bias assessment, as shown by a funnel plot in Appendix Fig. 4, resulted in a Begg's test p-value of 0.411, indicating no significant publication bias. Leave-one-out sensitivity analyses produced results similar to the main analysis when stratified by strains (Appendix Fig. 5).
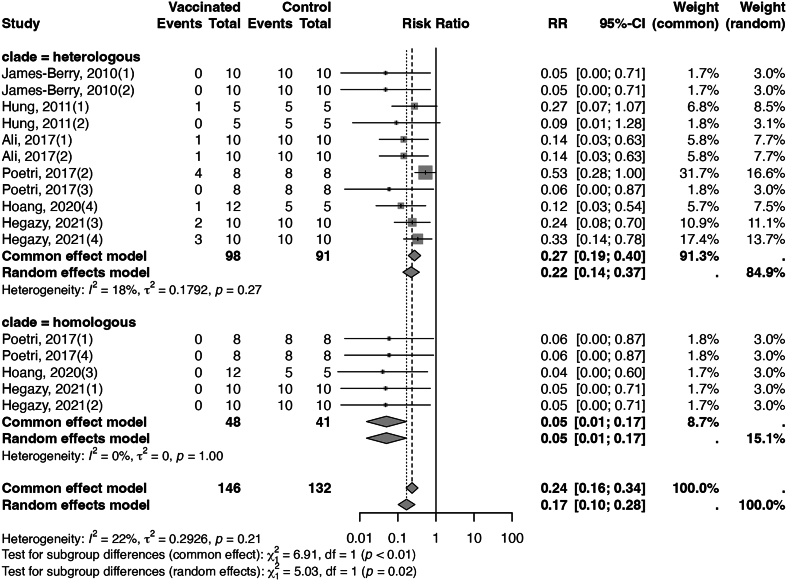
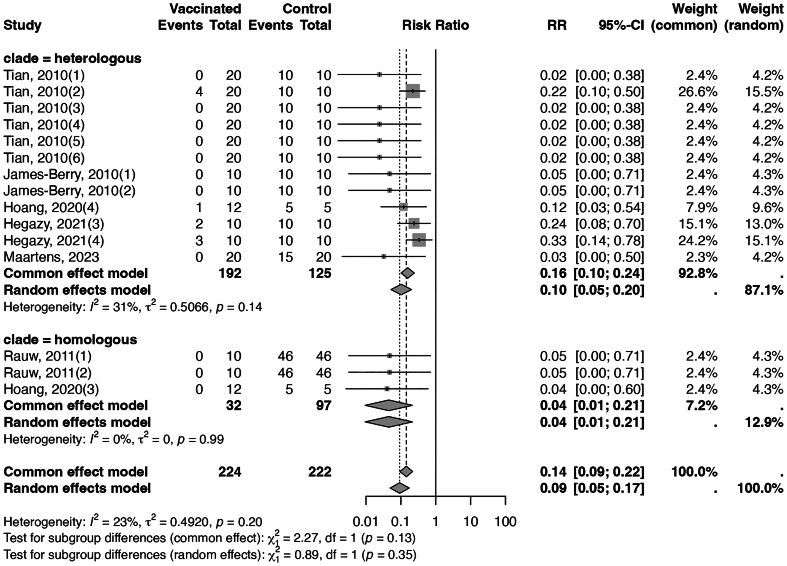
Fig. 2 presents a forest plot for inactivated vaccines, showing a significantly higher efficacy against homologous strains than heterologous strains (95% with I2 of 0%, CoE high, versus 78% with I2 of 18%, CoE high, test for subgroup difference p = 0.02). Additionally, the analysis indicated that booster doses might be superior to a single dose regimen, exhibiting an efficacy rate of 88% and no heterogeneity (I2 = 0%), compared to an 81% efficacy rate with an I2 of 30%, although the test for subgroup difference did not reach statistical significance (p = 0.40, Appendix Fig. 6). Moreover, challenge tests conducted three weeks or longer post-vaccination demonstrated a higher efficacy rate of 84% with an I2 of 30%, as opposed to a 78% efficacy rate with an I2 of 0% for tests performed less than three weeks, (test for subgroup difference p = 0.64, Appendix Fig. 7). The assessment of publication bias, illustrated by a funnel plot in Appendix Fig. 8, resulted in a Begg's test p-value of 0.726, indicating no significant publication bias. Finally, leave-one-out sensitivity analyses produced results consistent with the main analysis, as seen in Appendix Fig. 9.
Fig. 2.
Forest plot of inactivated vaccines illustrating efficacy in mortality across different stains. (“clade = homogenous” meant the vaccine seed and the challenging strain belonged to the same clade, and “clade = heterologous” meant the vaccine seed and the challenging strain belonged to different clades.)
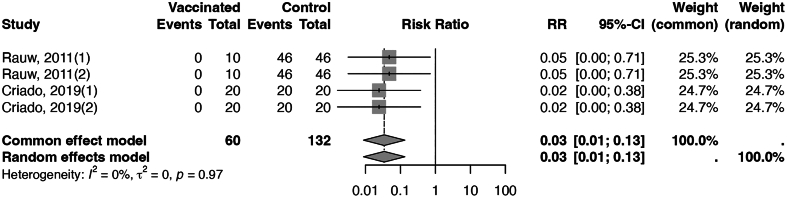
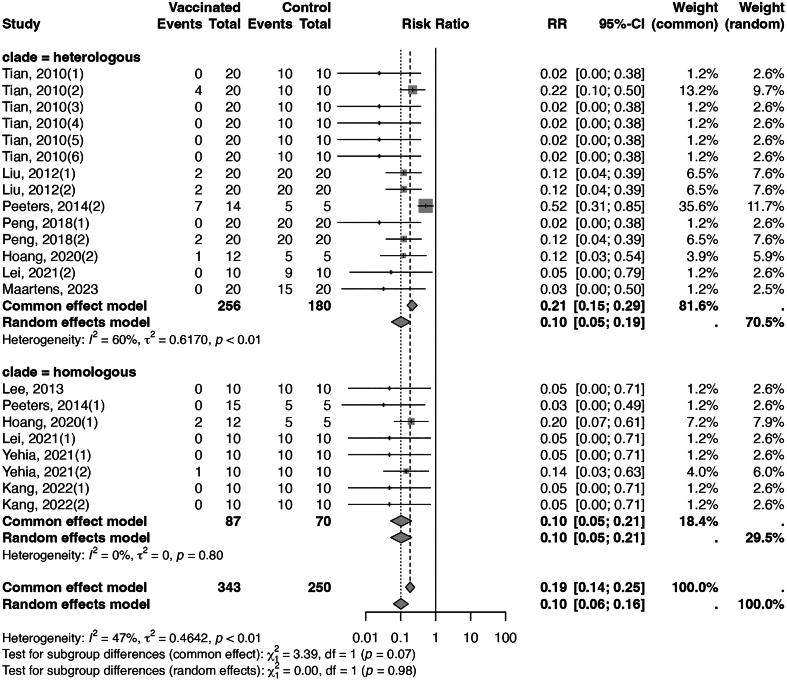
Within the category of recombinant vaccines, live recombinant vaccines have demonstrated considerable effectiveness in preventing mortality, achieving an efficacy of 97% with an I2 of 0% (Fig. 3), and the CoE was moderate. In contrast, inactivated recombinant vaccines have shown an overall efficacy of 90%, with medium heterogeneity (I2 = 47%, Fig. 4). Stratifying the data, based on the alignment between the vaccine seed strain and the challenge virus, yielded an efficacy of 90% (I2 = 0%, CoE high) against homologous strains and an efficacy of 90% (I2 = 60%, CoE moderate) against heterologous strains. The assessment of publication bias for both live and inactivated recombinant vaccines was conducted using funnel plots (Appendix Figs. 10 and 11). The funnel plot for inactivated recombinant vaccines showed minimal asymmetry; however, the p-value of Begg's test for inactivated recombinant vaccines was 0.782, indicating no substantial evidence of publication bias. Sensitivity analyses for these two types of vaccines yielded results consistent with the main analysis (Appendix Figs. 12 and 13).
Fig. 3.
Forest plot of live recombinant vaccines illustrating efficacy in reducing mortality.
Fig. 4.
Forest plot of inactivated recombinant vaccines illustrating efficacy in reducing mortality across different strains. (“clade = homogenous” meant the vaccine seed and the challenging strain belonged to the same clade, and “clade = heterologous” meant the vaccine seed and the challenging strain belonged to different clades.)
In commercially available vaccines that have been broadly manufactured, the overall vaccine efficacy was estimated to be 91%, with an I2 of 23%. Notably, vaccines utilizing a homologous strain (with an efficacy of 96%, an I2 = 0%, and CoE moderate; Fig. 5) demonstrated superior efficacy in reducing morbidity compared to those using a heterologous strain (with an efficacy of 90%, an I2 = 31%, and moderate CoE) (test for subgroup difference p = 0.35). Furthermore, the analysis indicated that booster doses, with an efficacy rate of 96% and no heterogeneity (I2 = 0%), were more effective than a single-dose regimen, which showed a 90% efficacy rate with an I2 of 30%, although the difference did not reach statistical significance, as shown in Appendix Fig. 14. The evaluation of publication bias for commercial vaccines, conducted using a funnel plot, yielded a Begg's test p-value of 0.7057, suggesting no significant evidence of publication bias (Appendix Fig. 15). Sensitivity analyses also yielded results similar to those of the main analysis (Appendix Fig. 16).
Fig. 5.
Forest plot of commercial vaccines illustrating efficacy in reducing mortality across different stains. (“clade = homogenous” meant the vaccine seed and the challenging strain belonged to the same clade, and “clade = heterologous” meant the vaccine seed and the challenging strain belonged to different clades.)
4. Discussion
This systematic review and meta-analysis provide updated, high-quality evidence on the estimated efficacy of vaccines in reducing mortality caused by HPAI. Our results show that HPAI vaccines have an efficacy against mortality ranging from 78% to 97%, depending on vaccine platforms and match (or mismatch) between vaccine strains and challenge strains. This protective effect was particularly marked when the vaccine strain and the challenging strain belonged to the same clade, a trend most notable in inactivated vaccines and current commercial vaccines.
Previous studies showed that vaccinated chickens challenged with the HPAI virus can still transmit it to both vaccinated and unvaccinated poultry [43]. This phenomenon, known as “silent spread,” is a major concern in the widespread implementation of vaccination in poultry. However, our findings indicate that vaccine efficacy is not guaranteed to be 100%, regardless of the platform, interval, dosage, or strains involved. Therefore, the excess mortality rate from HPAI in vaccinated flocks may still be detectable, exceeding the normal weekly mortality rates for layers (less than 0.1% [44]) and broilers (less than 1% [45]) even under the best-case scenario with a 3% mortality rate from live recombinant vaccines or with booster doses in commercial vaccines. These results suggest that massive vaccination may not mask the surveillance of HPAI and highlight the importance of maintaining biosecurity measures. However, further field epidemiological studies are necessary to resolve these uncertainties.
A finding with important implications is the large difference in vaccine efficacy against homologous HPAI strains versus heterologous HPAI strains for inactivated vaccines (95% vs 78%, p = 0.02) and commercial vaccines (96% vs 90%, p = 0.35). Therefore, the rapid evolution of HPAI viruses can compromise the effectiveness of vaccines. A 2017 study [46] assessing vaccine efficacy in Indonesia highlighted significant inconsistencies, implying a reduction in vaccine efficacy due to the evolution of the dominant HPAI strain. Ongoing updates in epidemiological surveys and the development of new vaccines are therefore essential. Interestingly, this trend was not observed in recombinant vaccines, suggesting that the efficacy of recombinant vaccines might be less likely to be affected by genetic variations.
Another important finding is that challenge tests conducted with a mean interval of two weeks show a vaccine efficacy lower but comparable to that after the three-week post-vaccination period recommended by WOAH [47] for optimal immune response development (78% vs. 84%, p = 0.64). These findings underscore the necessity for further research into the onset of vaccine efficacy, the trajectory of neutralizing antibody titers post-vaccination, and the potential for emergency use of vaccines.
Although a previous meta-analysis summarized the efficacy of vaccination using data published before 2010 [48], it did not employ the Cochrane systematic review methodology to assess the quality of evidence, and the main finding suffered from publication bias. A systematic review and meta-analysis on the efficacy of commercial HPAI vaccines in Indonesia before 2017 [46], which also did not assess the quality of evidence, revealed that LPAI vaccines are ineffective against HPAI virus and that high heterogenicity in efficacy exists among HPAI vaccines probably because of rapid viral evolution. Another systematic review and meta-analysis [49], which also did not assess the quality of evidence, investigated the correlation between the standardized mean difference in survival and the HA1 amino acid sequence similarity of the challenge strain (or hemagglutination inhibition [HI] titer against the challenge strain). However, it did not provide a summary of the vaccine's effect on HPAI-associated mortality in terms of the absolute reduction in death percentage. Our systematic review offers an updated and unbiased assessment of vaccine efficacy against HPAI-related mortality, providing timely and crucial information for re-evaluating the role of vaccination in poultry avian influenza control policy amidst the global HPAI outbreak post-2021.
This study has some limitations. The efficacy assessments in this meta-analysis were conducted exclusively on SPF White Leghorn chickens, rather than directly on commercial layers or broilers, which limits the generalizability of the results to these groups. Additionally, the lack of specific-pathogen maternal antibodies in the study subjects hinders understanding of how such antibodies might influence vaccine efficacy. Lastly, the heterogeneity observed in some results could be due to varying operational procedures across studies, such as differences in vaccination schedules, challenge test timings, and vaccine dosages.
5. Conclusions
In summary, this meta-analysis offers an updated and unbiased assessment evalution of avian influenza vaccine efficacy in poultry, demonstrating an efficacy range of 78% to 97%, depending on vaccine platforms and match (or mismatch) between vaccine strains and challenge strains. Our results show that vaccination, which does not completely prevent HPAI-related mortality among poultry, needs to be a part of a comprehensive new global avian influenza control strategy. Adoption of vaccination in poultry farming may dramatically decrease economic loss from mass culling of poultry populations and thus enhance food security to the human population. Our findings also indicate that current concerns for potential risk for human health from silent transmission with mutation could be overstated as vaccination is unlikely to mask the outbreak of HPAI. These findings are vital for shaping global vaccine strategies and policies, providing timely and crucial information for re-evaluating the role of vaccination in poultry avian influenza control policy amidst the global HPAI outbreak post-2021.
Funding statement
This study is supported by Population Health Research Centre from Featured Areas Research Centre Programme within the framework of the Higher Education Sprout Project by the Taiwan Ministry of Education (grant number NTU-112L9004) and Taiwan National Science and Technology Council (grant number NSC-112-2314-B-002-216-MY3). The publication of this article is supported by Infectious Diseases Research and Education Center, Ministry of Health and Welfare and National Taiwan University (Taipei, Taiwan). The funders have no role in study design, data collection and analysis, preparation of manuscript, or decision to submission.
CRediT authorship contribution statement
IShin Tseng: Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Bing-Yi Pan: Data curation, Writing – review & editing. Yen-Chen Feng: Supervision, Writing – review & editing. Chi-Tai Fang: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
All authors declare that they have no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2024.100714.
Appendix A. Supplementary data
Supplementary material
Data availability
All data analyzed in this study are included in the published article, including supplementary material.
References
- 1.Wille M., Barr I.G. Resurgence of avian influenza virus. Science. 2022;376(6592):459–460. doi: 10.1126/science.abo1232. https://www.science.org/doi/10.1126/science.abo1232. [DOI] [PubMed] [Google Scholar]
- 2.Xie R., Edwards K.M., Wille M., Wei X., Wong S.S., Zanin M., Dhanasekaran V. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature. 2023:1–8. doi: 10.1038/s41586-023-06631-2. https://www.nature.com/articles/s41586-023-06631-2 [DOI] [PubMed] [Google Scholar]
- 3.Sun Y., Zhang T., Zhao X., Qian J., Jiang M., Jia M., et al. High activity levels of avian influenza upwards 2018–2022: A global epidemiological overview of fowl and human infections. One Health. 2023;100511 doi: 10.1016/j.onehlt.2023.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J., Zeng X., Cui P., Yan C., Chen H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microb. Infect. 2023;12(1):2155072. doi: 10.1080/22221751.2022.2155072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chmielewski R., Swayne D.E. Avian influenza: public health and food safety concerns. Annu. Rev. Food Sci. Technol. 2011;2:37–57. doi: 10.1146/annurev-food-022510-133710. [DOI] [PubMed] [Google Scholar]
- 6.Nurzijah I., Elbohy O.A., Kanyuka K., Daly J.M., Dunham S. Development of plant-based vaccines for prevention of avian influenza and Newcastle disease in poultry. Vaccines. 2022;10(3):478. doi: 10.3390/vaccines10030478. https://www.mdpi.com/2076-393X/10/3/478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harder T.C., Buda S., Hengel H., Beer M., Mettenleiter T.C. Poultry food products—a source of avian influenza virus transmission to humans? Clin. Microbiol. Infect. 2016;22(2):141–146. doi: 10.1016/j.cmi.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Exotic & Emerging Avian Viral Diseases Research, Evaluation of Currently Available Vaccines for the Control of H5N1 Highly Pathogenic Avian Influenza. U.S. Department of Agriculture, Agricultural Research Service; 2024. https://www.ars.usda.gov/research/project/?accnNo=441991 (accessed November 16, 2023) [Google Scholar]
- 9.World Organization for Animal Health 90th General Session of the World Assembly of Delegates. 2023. https://www.woah.org/en/event/90th-general-session-of-the-world-assembly-of-delegates/ (accessed November 16, 2023)
- 10.Ministère de l'Agriculture et de la Souveraineté alimentaire Tout ce qu'il faut savoir sur le Plan d'action vaccination IAHP en France, La stratégie de vaccination choisie et les raisons de ce choix. 2023. https://agriculture.gouv.fr/tout-ce-quil-faut-savoir-sur-le-plan-daction-vaccination-iahp-en-france (accessed November 16, 2023)
- 11.Swayne D.E., Spackman E. Vaccines and Diagnostics for Transboundary Animal Diseases. VOL. 135. Karger Publishers; 2013. Current status and future needs in diagnostics and vaccines for high pathogenicity avian influenza; pp. 79–94. [DOI] [PubMed] [Google Scholar]
- 12.Swayne D.E., Spackman E., Pantin-Jackwood M. Success factors for avian influenza vaccine use in poultry and potential impact at the wild bird–agricultural interface. EcoHealth. 2014;11:94–108. doi: 10.1007/s10393-013-0861-3. [DOI] [PubMed] [Google Scholar]
- 13.Peyre M., Fusheng G., Desvaux S., Roger F. Avian influenza vaccines: a practical review in relation to their application in the field with a focus on the Asian experience. Epidemiol. Infect. 2009;137(1):1–21. doi: 10.1017/S0950268808001039. [DOI] [PubMed] [Google Scholar]
- 14.Poetri O.N., Van Boven M., Claassen I., Koch G., Wibawan I.W., Stegeman A., Bouma A. Silent spread of highly pathogenic avian influenza H5N1 virus amongst vaccinated commercial layers. Res. Vet. Sci. 2014;97(3):637–641. doi: 10.1016/j.rvsc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Islam A., Munro S., Hassan M.M., Epstein J.H., Klaassen M. The role of vaccination and environmental factors on outbreaks of high pathogenicity avian influenza H5N1 in Bangladesh. One Health. 2023;17 doi: 10.1016/j.onehlt.2023.100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian H., Cui Y., Dong L., Zhou S., Li X., Huang S., et al. Spatial, temporal and genetic dynamics of highly pathogenic avian influenza A (H5N1) virus in China. BMC Infect. Dis. 2015;15(1):1–15. doi: 10.1186/s12879-015-0770-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin X., Deng G., Zeng X., Cui P., Hou Y., Liu Y., Chen H. Genetic and biological properties of H7N9 avian influenza viruses detected after application of the H7N9 poultry vaccine in China. PLoS Pathog. 2021;17(4) doi: 10.1371/journal.ppat.1009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Y., Liu H., Liu D., Liu W., Luo T., Li J. Hemagglutinin Gene Variation Rate of H9N2 Avian Influenza Virus by Vaccine Intervention in China. Viruses. 2022;14(5):1043. doi: 10.3390/v14051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schünemann H.J., Brennan S., Akl E.A., Hultcrantz M., Alonso-Coello P., Xia J., et al. The development methods of official GRADE articles and requirements for claiming the use of GRADE–A statement by the GRADE guidance group. J. Clin. Epidemiol. 2023;159:79–84. doi: 10.1016/j.jclinepi.2023.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Method. 2006;11(2):193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y., Zhang H., Wang G., Zhang P., Tian G., Bu Z., Chen H. Protective efficacy of H7 subtype avian influenza DNA vaccine. Avian Dis. 2010;54(1 Suppl):290–293. doi: 10.1637/8723-032409-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 23.Tian G., Zeng X., Li Y., Shi J., Chen H. Protective efficacy of the H5 inactivated vaccine against different highly pathogenic H5N1 avian influenza viruses isolated in China and Vietnam. Avian Dis. 2010;54(s1):287–289. doi: 10.1637/8707-031709-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 24.James-Berry C.M., Middleton D., Mansfield J.P., Fenwick S.G., Ellis T.M. Use of a tetanus toxoid marker to allow differentiation of infected from vaccinated poultry without affecting the efficacy of a H5N1 avian influenza virus vaccine. Vet. Rec. 2010;167(18):695–699. doi: 10.1136/vr.c5798. [DOI] [PubMed] [Google Scholar]
- 25.Rauw F., Palya V., Van Borm S., Welby S., Tatar-Kis T., Gardin Y., Van den Berg T. Further evidence of antigenic drift and protective efficacy afforded by a recombinant HVT-H5 vaccine against challenge with two antigenically divergent Egyptian clade 2.2. 1 HPAI H5N1 strains. Vaccine. 2011;29(14):2590–2600. doi: 10.1016/j.vaccine.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 26.Hung L.H., Tsai P.C., Wang C.H., Li S.L., Huang C.C., Lien Y.Y., Chaung H.C. Immunoadjuvant efficacy of plasmids with multiple copies of a CpG motif coadministrated with avian influenza vaccine in chickens. Vaccine. 2011;29(29–30):4668–4675. doi: 10.1016/j.vaccine.2011.04.104. [DOI] [PubMed] [Google Scholar]
- 27.Park J.K., Lee D.H., Youn H.N., Kim M.S., Lee Y.N., Yuk S.S., Song C.S. Protective efficacy of crude virus-like particle vaccine against HPAI H5N1 in chickens and its application on DIVA strategy. Influenza Other Respir. Viruses. 2013;7(3):340–348. doi: 10.1111/j.1750-2659.2012.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M., Liu C.G., Zhang Y., Shi W.L., Wang W., Liu Y.Y. Efficacy of a high-yield attenuated vaccine strain wholly derived from avian influenza viruses by use of reverse genetics. Vet. Microbiol. 2012;161(1–2):43–48. doi: 10.1016/j.vetmic.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Lee D.H., Park J.K., Kwon J.H., Yuk S.S., Erdene-Ochir T.O., Jang Y.H., Song C.S. Efficacy of single dose of a bivalent vaccine containing inactivated Newcastle disease virus and reassortant highly pathogenic avian influenza H5N1 virus against lethal HPAI and NDV infection in chickens. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peeters B., Tonnis W.F., Murugappan S., Rottier P., Koch G., Frijlink H.W., Hinrichs W.L. Pulmonary immunization of chickens using non-adjuvanted spray-freeze dried whole inactivated virus vaccine completely protects against highly pathogenic H5N1 avian influenza virus. Vaccine. 2014;32(48):6445–6450. doi: 10.1016/j.vaccine.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 31.Ali Z.M., Hassan M.A.E.M., Hussein H.A., Ahmed B.M., El Sanousi A.A.E.G. Protective efficacy of combined trivalent inactivated ISA 71 oil adjuvant vaccine against avian influenza virus subtypes (H9N2 and H5N1) and Newcastle disease virus. Vet. World. 2017;10(10):1212. doi: 10.14202/vetworld.2017.1212-1220. https://10.14202/vetworld.2017.1212-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pushko P., Tretyakova I., Hidajat R., Zsak A., Chrzastek K., Tumpey T.M., Kapczynski D.R. Virus-like particles displaying H5, H7, H9 hemagglutinins and N1 neuraminidase elicit protective immunity to heterologous avian influenza viruses in chickens. Virology. 2017;501:176–182. doi: 10.1016/j.virol.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poetri O.N., Van Boven M., Koch G., Stegeman A., Claassen I., Wisaksana I.W., Bouma A. Different cross protection scopes of two avian influenza H5N1 vaccines against infection of layer chickens with a heterologous highly pathogenic virus. Res. Vet. Sci. 2017;114:143–152. doi: 10.1016/j.virol.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Peng C., Hou G., Li J., Wang S., Wang Y., Cheng S., Jiang W. Protective efficacy of an inactivated chimeric H7/H5 avian influenza vaccine against highly pathogenic avian influenza H7N9 and clade 2.3. 4.4 H5 viruses. Vet. Microbiol. 2018;223:21–26. doi: 10.1016/j.vetmic.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Criado M.F., Bertran K., Lee D.H., Killmaster L., Stephens C.B., Spackman E., et al. Efficacy of novel recombinant fowlpox vaccine against recent Mexican H7N3 highly pathogenic avian influenza virus. Vaccine. 2019;37(16):2232–2243. doi: 10.1016/j.vaccine.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Hoang H.T.T., Nguyen C.H., Nguyen N.T.T., Pham A.D., Nguyen H.T.T., Le T.H., et al. Immunization with the H5N1 recombinant vaccine candidate induces high protection in chickens against Vietnamese highly pathogenic avian influenza virus strains. Vaccines. 2020;8(2):159. doi: 10.3390/vaccines8020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei H., Lu X., Li S., Ren Y. High immune efficacy against different avian influenza H5N1 viruses due to oral administration of a Saccharomyces cerevisiae-based vaccine in chickens. Sci. Rep. 2021;11(1):8977. doi: 10.1038/s41598-021-88413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yehia N., AbdelSabour M.A., Erfan A.M., Ali Z.M., Soliman R.A., Samy A., et al. Selenium nanoparticles enhance the efficacy of homologous vaccine against the highly pathogenic avian influenza H5N1 virus in chickens. Saudi J. Biol. Sci. 2022;29(4):2095–2111. doi: 10.1016/j.sjbs.2021.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegazy A.M., Yehia N., Hassan A.F., El-Saadony M.T., Aboelenin S.M., Soliman M.M., Tolba H.M. The potency of newly development H5N8 and H9N2 avian influenza vaccines against the isolated strains in laying hens from Egypt during 2019. Saudi J. Biol. Sci. 2021;28(9):5310–5316. doi: 10.1016/j.sjbs.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang Y.M., Cho H.K., An S.J., Kim H.J., Lee Y.J., Kang H.M. Updating the national antigen bank in korea: protective efficacy of synthetic vaccine candidates against h5nx highly pathogenic avian influenza viruses belonging to clades 2.3. 2.1 and 2.3. 4.4. Vaccines. 2022;10(11):1860. doi: 10.3390/vaccines10111860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maartens L.H., Frizzo da Silva L., Dawson S., Love N., Erasmus B.J. The efficacy of an inactivated avian influenza H5N1 vaccine against an African strain of HPAI H5N8 (clade 2.3. 4.4 B) Avian Pathol. 2023;52(3):176–184. doi: 10.1080/03079457.2023.2181145. [DOI] [PubMed] [Google Scholar]
- 42.Swayne D.E., Pavade G., Hamilton K., Vallat B., Miyagishima K. Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Revue Scientifique et Technique-OIE. 2011;30(3):839. doi: 10.20506/rst.30.3.2081. [DOI] [PubMed] [Google Scholar]
- 43.Savill N.J., St Rose S.G., Keeling M.J., Woolhouse M.E. Silent spread of H5N1 in vaccinated poultry. Nature. 2006;442(7104):757. doi: 10.1038/442757a. [DOI] [PubMed] [Google Scholar]
- 44.Fulton R.M. Causes of normal mortality in commercial egg-laying chickens. Avian Dis. 2017;61(3):289–295. doi: 10.1637/11556-120816-RegR. [DOI] [PubMed] [Google Scholar]
- 45.Poulsen L.L., Thøfner I., Bisgaard M., Christensen J.P., Olsen R.H., Christensen H. Longitudinal study of transmission of Escherichia coli from broiler breeders to broilers. Vet. Microbiol. 2017;207:13–18. doi: 10.1016/j.vetmic.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Villanueva-Cabezas J.P., Coppo M.J., Durr P.A., McVernon J. Vaccine efficacy against Indonesian highly pathogenic avian influenza H5N1: systematic review and meta-analysis. Vaccine. 2017;35(37):4859–4869. doi: 10.1016/j.vaccine.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 47.World Organization for Animal Health OIE Terrestrial Manual, Chapter 3.3.4. Avian Influenza (Including Infection with High Pathogenicity Avian Influenza Viruses) 2024. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm version adopted in May 2021. (accessed July 10, 2023)
- 48.Hsu S.M., Chen T.H.H., Wang C.H. Efficacy of avian influenza vaccine in poultry: a meta-analysis. Avian Dis. 2010;54(4):1197–1209. doi: 10.1637/9305-031710-Reg.1. [DOI] [PubMed] [Google Scholar]
- 49.Mo J., Spackman E., Swayne D.E. Prediction of highly pathogenic avian influenza vaccine efficacy in chickens by comparison of in vitro and in vivo data: A meta-analysis and systematic review. Vaccine. 2023 doi: 10.1016/j.vaccine.2023.07.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
All data analyzed in this study are included in the published article, including supplementary material.