Abstract
Background
Adenoid tissue is a first‐line host defense secondary lymphoid organ, especially in childhood. The endoplasmic reticulum (ER) is required to maintain balanced cellular activity. With impaired ER functions, protein accumulation occurs, resulting in ER stress, which plays a role in the etiopathogenesis of many diseases.
Objective
We aimed to investigate the relationship between ER stress and adenoid tissue disorders, thereby elucidating the mechanisms of immunity‐related diseases.
Methods
Fifty‐four pediatric patients (>3 years old) who underwent adenoidectomy for chronic adenoiditis (CA) or adenoid hypertrophy (AH) were enrolled in this prospective, parallel‐group clinical study. Adenoids were divided into two groups (CA or AH) based on their size and evaluated for ER stress pathway and apoptosis pathway markers by Real‐time PCR and Western blot analysis.
Results
ER stress pathway markers significantly differed between the CA and AH groups. Children with CA had higher ER stress marker levels than the AH group (p < .001 for ATF‐4, ATF‐6, and GRP78, and p < .05 for EDEM1, CHOP, EIF2AK3, ERNI, and GRP94). Apoptosis pathway marker levels (BAX and BCL‐2) were not different between groups.
Conclusions
ER stress contributes to the etiopathogenesis of adenoid tissue diseases and the pathogenesis of adenoid tissue disorders, which are part of the immune response. These results may guide the development of new and alternative treatments for immune system disorders.
Keywords: adenoid, endoplasmic reticulum stress, genetics, immune system, immunology, unfolded protein response
Short abstract
Adenoid tissue is a first‐line host defense secondary lymphoid organ, especially in childhood. The endoplasmic reticulum (ER) is required to maintain balanced cellular activity. With impaired ER functions, protein accumulation occurs, resulting in ER stress, which plays a role in the etiopathogenesis of many diseases. We investigated the relationship between ER stress and adenoid tissue disorders. ER stress contributes to the etiopathogenesis of adenoid tissue diseases and the pathogenesis of adenoid tissue disorders, which are part of the immune response. These results may guide the development of new and alternative treatments for immune system disorders.
1. INTRODUCTION
Adenoid tissue at the entry to the oropharynx comprises secondary lymphoid structures such as the tonsils. 1 Adenoids are important effector organs that form the first line of immune defense along with tonsils against upper respiratory tract allergens and pathogens and contribute to mucosal and systemic adaptive immunity. 2 Adenoids have a specific lymphoepithelial tissue and contain epithelial cells, lymphocytes, macrophages, plasma cells, and dendritic cells, which are important for an effective immune response. 3
Histomorphological and functional changes in the immunological barrier of adenoid tissue can be triggered by recurrent or chronic respiratory tract infections or by allergens. 4 , 5
Although there are no definitive accepted diagnostic criteria for chronic adenoiditis (CA), CA occurs with long‐term local infection of the adenoid tissue and is caused by infected adenoids. The presence of purulent nasal discharge, malodorous breath, prolonged expectoration, cough, prolonged throat‐clearing attempts, and a foreign body sensation in the pharynx and nasopharyngeal inhalation help make the diagnosis. 6 Other symptoms, such as prolonged nasal congestion, rhinokinesmus, sneezing, dry throat, and headache, may also occur in CA. In CA, physical examination and nasal endoscopy may detect marked retropharyngeal folliculitis and cobblestone‐like changes, sticky mucinous or purulent secretions, and mucosal edema on the adenoid surface, while the size of the adenoids may be hypertrophic, normal, or atrophic. 6
Adenoid hypertrophy (AH) is a condition in which the size of the adenoids increases to the point of obstruction in the upper airway. Although AH can be caused by recurrent and chronic inflammation, its exact cause is unknown, and diet, genetic, and humoral factors are thought to play a role in etiology. 7 , 8
The impact of AH and CA on the composition of immune cells is not completely known. Surgical removal of adenoids, which consists of a large amount of lymphoid tissue, may provide an accessible source to investigate the interplay between foreign pathogens and allergens and the human immune system. 9 Moreover, adenoids can be a suitable in vivo model for investigating inflammatory processes and mechanisms of infection in lymphoid tissues, similar to tonsil tissue. 9
The endoplasmic reticulum (ER) is an organelle that controls the content, structure, folding, and release of proteins and is critical in maintaining protein homeostasis. It is also essential for many cellular activities in common calcium, lipid, and carbohydrate metabolism. 10 , 11 When ER functional deterioration occurs, unfolded or misfolded proteins accumulate in the ER lumen, causing ER stress. 12 ER stress has an important contribution to the pathogenesis of many diseases and pathological conditions such as cancer, inflammatory and autoimmune diseases, proinflammatory cytokine expression, and neurodegenerative diseases. Factors that determine whether the effect of ER stress is favorable or unfavorable are the intensity and duration of an agent and the type of cell, but the most important underlying mechanism is the unfolded protein response (UPR), which is characterized by three signal pathways, and it can reverse homeostasis or stimulate cellular apoptosis. 13 The UPR regulates intracellular metabolic oxidative stress and inflammation pathways. 14 Although the relationship between ER stress and immune responses is clear, the causality relationship between them remains unknown. 13 Additionally, mechanisms coordinating the UPR signal cascades with immunity remain unclear. Further studies are required to determine the role of ER stress and UPR in the immune system, its mechanisms, and relevant signal molecules. 13 In chronic inflammation and hypertrophy in lymphoid organs, the regulation of immune responses may be affected by ER stress and UPR. However, although the effect of ER stress on the immune system has been studied in several organs to date, it has not been investigated in the adenoid tissue, a secondary lymphoid organ and a part of the immune system. Therefore, we hypothesized that the ER stress response plays a role in the pathophysiological process of adenoid tissue diseases, which involve the differentiation and maturation of immune cells.
Our purpose was to explore the effect of ER stress, apoptosis, and UPR pathways on the pathogenesis of adenoid tissue diseases and to improve novel approaches that focus on UPR pathways, which may be used to treat diseases related to immune system disorders.
Our primary outcome was to investigate whether ER stress and apoptosis pathways play a role in the etiopathogenesis of CA and AH, which are adenoid tissue disorders and affect immune system function.
2. MATERIALS AND METHODS
2.1. Study design
This prospective, parallel‐group clinical study was supported by the Scientific Research Projects Coordination Unit of Selcuk University (Project number: 19401030) and approved by the Selcuk University Clinical Studies Ethics Committee (Ref no. 2018/18) in September 2018. This study was followed the ethical standards in the Declaration of Helsinki, and each patient's parents provided informed consent. This study was registered in the Clinical Trial Registry at Clinicaltrials.gov (identifier no. NCT04583631), and followed the relevant requirements of the CONSORT Statement. 15 All human adenoid samples used in this study were obtained from 54 patients who underwent adenoidectomy between November 2018 and August 2019 at the Department of Otorhinolaryngology, Selcuk University Faculty of Medicine Hospital, Konya, Turkey.
2.2. Study population and protocol
Based on our previous studies, we assumed that there should be at least 21 patients in each group to detect a difference between the groups. 5 , 16 Considering possible drop‐outs, 25 patients were planned to be included in each group. The main indications for adenoidectomy were AH and CA. Thus, 54 patients with a clinical diagnosis of CA and AH and 3 to 9 years old were included in this study. Patients with a systemic disease or another ear, nose, and throat disease, craniofacial and/or congenital abnormalities, and bleeding disorders were excluded from the study. An acute exacerbation of CA was controlled with antibiotics and reevaluated at least 2 weeks later, so none of the patients have postnasal drip, rhinorrhea, or fever, which are also contraindications for elective surgical procedures. None of the patients had a history of intranasal steroid use within the past month and non‐steroid anti‐inflammatory drug or antibiotic use within the 2 weeks. Adenoid tissue was evaluated using a 2.5‐mm diameter endoscope (Karl Storz, Tuttlingen, Germany). The boundaries of the choana and adenoid tissue were visualized, and the ratio of the size of the adenoid tissue to the diameter of the choana was classified as more than 50% and less than 50%. 17
2.3. Chronic adenoidits group (n = 25)
Patients with and without hypertrophy were identified, and those who had purulent rhinorrhea symptoms, foul‐smelling breath, postnasal drainage, exudate, and an adenoid‐to‐choana ratio below 50% were placed into the CA group.
2.4. Adenoid hypertrophy (n = 29)
In addition to symptoms such as purulent rhinorrhea, foulsmelling breath, and postnasal drainage, patients who snored, open‐mouth breathing, hyponasal speech symptoms, the presence of obstructive adenoids that did not respond to oral antibiotics, and those with an adenoid‐to‐choana ratio over 50% were placed into the AH group.
2.5. Adenoidectomy procedure
The adenoidectomy procedure was performed under general anesthesia using a curettage technique, and adenoid tissues were placed into TRIzol tubes and sent to the medical genetics department for the investigation of ER stress and apoptosis. Consultants from the medical genetics department (NK, TD) were unaware of patients' clinical differences and similarities thus, were blinded to study groups. ER stress and apoptosis were evaluated in all adenoid tissue using real‐time polymerase chain reaction (PCR) and western blotting.
2.6. Measurements
Activating transcription factor (ATF)‐4, ATF‐6, α‐mannosidase‐like protein 1 (EDEM1), CHOP, glucose‐regulated protein 78 kDa (GRP78), EIF2AK3, ER to nucleus signaling 1 (ERN1), and glucose‐regulated protein 94 kDa (GRP94) protein expression in the ER stress pathway and BAX and BCL‐2 protein expression in the apoptosis pathway were compared between the CA and AH groups using real‐time PCR and western blot methods.
2.7. Real‐time PCR analysis
Total RNA extraction from tissue samples obtained from patients was performed using TRIzol® reagent (Invitrogen, Waltham, MA, USA) in accordance with a previously described protocol. 18 Tissue pieces were frozen in liquid nitrogen before extraction. cDNA synthesis was performed using the Transcriptor High‐Fidelity cDNA Synthesis kit (Roche, Basel, Switzerland) with oligo (dT) and random primers in accordance with the manufacturer's instructions. Oligonucleotide primers were designed using the IDT DNA primer request tool (Biomers Inc., Ulm, Germany). Primer sequences are presented in Table 1. All PCR reactions were performed using the LightCycler® 480 Instrument II (Roche, Penzberg, Germany) real‐time PCR via SYBR Green Master Mix (Bio‐Rad Hercules, CA, USA). Before the genes of interest were subjected to PCR, primer optimization was performed. Housekeeping genes such as 18S rRNA, 28S rRNA, β‐actin (ACTB), β2‐microglobulin (β2M), and glyceraldehyde‐3‐ phosphate dehydrogenase (GAPDH) were examined for normalization. ACTB levels of these were found to be more stable than the other genes, and thus, ACTB gene expression was used in subsequent experiments to normalize the gene expression results.
TABLE 1.
Primers information used in the present study.
| Gene | Sequence |
|---|---|
| EDEM1 | |
| Forward primer | CGGACGAGTACGAGAAGCG |
| Reverse primer | CGTAGCCAAAGACGAACATGC |
| ATF4 | |
| Forward primer | ATGACCGAAATGAGCTTCCTG |
| Reverse primer | GCTGGAGAACCCATGAGGT |
| ATF6 | |
| Forward primer | TCCTCGGTCAGTGGACTCTTA |
| Reverse primer | CTTGGGCTGAATTGAAGGTTTTG |
| GRP78 | |
| Forward primer | CATCACGCCGTCCTATGTCG |
| Reverse primer | CGTCAAAGACCGTGTTCTCG |
| CHOP | |
| Forward primer | GGAAACAGAGTGGTCATTCCC |
| Reverse primer | CTGCTTGAGCCGTTCATTCTC |
| EIF2AK3 (PERK) | |
| Forward primer | GGAAACGAGAGCCGGATTTATT |
| Reverse primer | ACTATGTCCATTATGGCAGCTTC |
| ERN1 | |
| Forward primer | CACAGTGACGCTTCCTGAAAC |
| Reverse primer | GCCATCATTAGGATCTGGGAGA |
| GRP94 | |
| Forward primer | GCTGACGATGAAGTTGATGTGG |
| Reverse primer | CATCCGTCCTTGATCCTTCTCTA |
| BAX | |
| Forward primer | CCCGAGAGGTCTTTTTCCGAG |
| Reverse primer | CCAGCCCATGATGGTTCTGAT |
| BCL2 | |
| Forward primer | GGTGGGGTCATGTGTGTGG |
| Reverse primer | CGGTTCAGGTACTCAGTCATCC |
2.8. Western blot analysis
Antibodies and western blotting ATF‐6 (D4Z8V) rabbit anti‐human monoclonal antibody (mAb; #65880), ATF‐4 (D4B8) rabbit anti‐human mAb (#11815), GRP78 (C50B12) rabbit anti‐human mAb (#3177), CHOP (D46F1) rabbit anti‐human mAb (#5554), PERK (C33E10) rabbit antihuman mAb (#3192), ERN1 (IRE1α) (14C10) rabbit antihuman mAb (#3294), GRP94 (D6X2Q) XP® rabbit antihuman mAb (#20292), BAX (D2E11) rabbit antihuman mAb (#5023), BCL‐2 (D55G8) rabbit antihuman mAb (#4223), and β‐actin (13E5) rabbit anti‐human mAb (#4970) were obtained from Cell Signaling Technology Co (Leiden, The Netherlands). Western blotting analyses were performed in accordance with protocols that were previously described in the literature. 19 ACTB levels were used for normalization in western blot analysis. Changes in protein levels were determined after this normalization.
2.9. Statistical analysis
PCR primers were configured using IDT PrimerQuest software (Integrated DNA Technologies, Coralville, IA, USA). Image‐based data were resolved using ImageJ software (Oxford Instruments, Abingdon, UK). The statistical significance was explored using GraphPad Prism V6 software (GraphPad Software Inc., La Jolla, CA, USA). Expression results were calculated using the 2ΔΔct method. All technical and biological experiments were performed with at least three replicates (N ≥ 3). The SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used to analyze the study data. The data were first examined for a normal distribution based on skewness and kurtosis values (which were between 0.846 and 0.924) and Q_Q plot graphics. The data showed a normal distribution. Based on these results, an independent group t‐test was used to compare the ER stress and apoptosis protein levels in the CA and AH groups. The level of significance was defined as p < .05.
3. RESULTS
Fifty‐four patients were included in the study (28 men and 26 women; mean age, 5.64 years). Twenty‐five patients were included in the CA group (mean age, 6.02 years), and 29 were included in the AH group (mean age, 4.91 years). There was no difference in demographic characteristics between groups. ΔCT values for the protein levels were compared in the CA and AH groups for the ER stress pathway and apoptosis pathway using real‐time PCR. In the CA group, ER stress protein levels were significantly greater than those in the AH group (p < .001 for ATF‐4, ATF‐6, and GRP78, and p < .05 for EDEM1, CHOP, EIF2AK3, ERNI, and GRP94). Additionally, no differences were found between the groups for the apoptosis proteins (i.e., BAX and BCL‐2; Table 2, Figure 1).
TABLE 2.
Comparison between adenoid hypertrophy and chronic adenoiditis groups in terms of ΔCT values of endoplasmic reticulum stress and apoptosis pathways genes.
| Adenoid hypertrophy (n = 29) | Chronic adenoiditis (n = 25) | t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| ER stress pathway | ||||||
| ATF4 ΔCT | 6.82 | 2.91 | 10.15 | 1.44 | −5.445 | <.001 |
| ATF6 ΔCT | 2.91 | 1.92 | 6.91 | 1.89 | −7.680 | <.001 |
| EDEM1 ΔCT | 3.24 | 2.77 | 4.58 | 1.50 | −2.169 | .035* |
| CHOP ΔCT | −2.32 | 1.46 | −0.74 | 2.91 | −2.562 | .020* |
| GRP78 ΔCT | 1.48 | 1.34 | 5.43 | 1.62 | −9.776 | <.001 |
| EIF2AK3 ΔCT | −2.60 | 2.08 | −1.37 | 2.14 | −2.139 | .037* |
| ERN1 ΔCT | 1.68 | 2.79 | 3.47 | 2.19 | −2.583 | .013* |
| GRP94 ΔCT | −1.53 | 2.10 | −0.33 | 1.92 | −2.187 | .033* |
| Apoptosis pathway | ||||||
| BAX ΔCT | 3.48 | 1.65 | 2.94 | 1.30 | 1.312 | .195** |
| BCL‐2 ΔCT | 2.57 | 1.89 | 3.63 | 2.08 | −1.968 | .054** |
p < .05.
p > .05.
FIGURE 1.
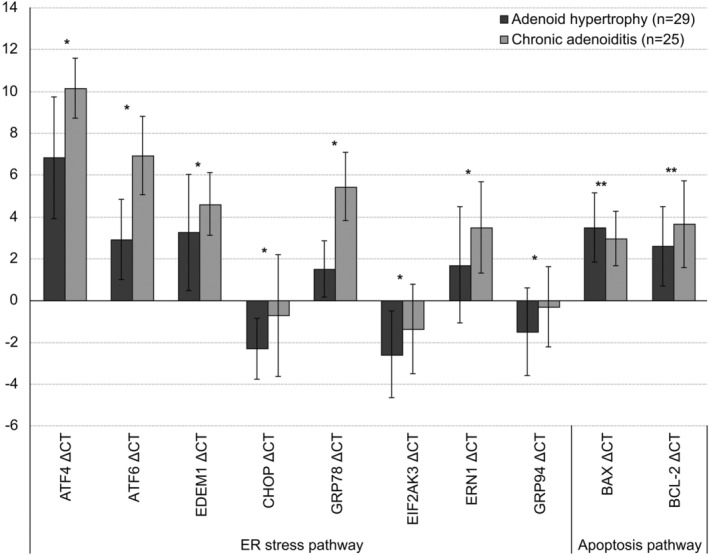
Levels of endoplasmic reticulum (ER) stress and apoptosis markers in the adenoid hypertrophy group and the chronic adenoiditis group based on of the real‐time polymerase chain reaction analysis results.
Western blot was performed to verify the results of normalized gene expression analysis, and ATF‐4, ATF‐6, CHOP, and GRP78 protein levels in the CA group were 2.48, 4.17, 2.78, and 5.25‐times greater, respectively, than those in the AH group. No significant difference was found in normalized apoptosis proteins and the other protein levels based on of the western blot analysis between the two groups. These results were consistent with the real‐time PCR results (Figure 2).
FIGURE 2.
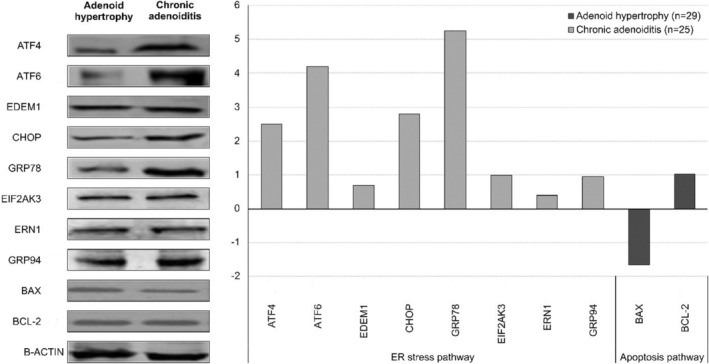
Western blot analysis of the endoplasmic reticulum stress and apoptosis markers.
4. DISCUSSION
The two most common indications for adenoidectomy, which is one of the most common surgical procedures performed in childhood, are CA and AH. 20 CA and AH are histopathologically similar, and both diseases are considered to be reactive lymphoid hyperplasia. Besides genetic susceptibility, the immunological parameters, local lymphocyte dysfunction, exposure to cigarette smoke, allergens, and recurrent respiratory infections are thought to play a role in the etiology of these two pathologic conditions. 21 CA often results from bacterial biofilm development, making it a source of recurrent upper respiratory tract infections in children. It is also thought that allergic processes contribute to the development of CA and AH. 22 Additionally, chronic irritation caused by stomach acid in the pharynx due to gastroesophageal reflux, especially in infants and young children, may play a role in CA and AH. 23 Also, viral upper respiratory tract infections occur six to eight times a year in children, and 5%–13% of these are known to cause bacterial superinfection, leading to adenoiditis and sinusitis. 24 Therefore, adenoiditis often occurs with rhinosinusitis and adenotonsillar diseases, making it difficult to determine the exact incidence, prevalence, and histopathological processes. However, adenoiditis in children is often accompanied by rhinosinusitis, suggesting that we can predict the incidence of adenoiditis from the incidence of sinusitis. 25
In a previous study in which AH histopathology was compared with that of CA, apoptosis was shown to play a critical role in the pathogenesis of adenoid diseases. However, the etiopathogenesis of CA and AH remains unclear. 5 Reacting to ER dysfunction is an important characteristic of whole cells. However, under severe and prolonged ER stress conditions, the proapoptotic process may become predominant and ER stress may cause cell apoptosis. 26 The role of ER stress, which is a significant mechanism of apoptotic cell death, has not been investigated in association with CA and AH etiopathogenesis. 27 The role of ER stress in adenoid tissue disease pathogenesis was demonstrated in this study. Although ER stress‐related apoptosis dysregulation has been reported to contribute to the pathogenesis of several human diseases, the role of the ER stress response has only been investigated in some mucosal immune cells in vivo. 28 Adenoid and tonsil tissues are located at the entry point of the respiratory and digestive systems. They are secondary lymphoid organs that perform cellular and humoral immune functions, which form the first line of defense against antigens entering the body from the respiratory and digestive systems from childhood to adolescence. 29 The role of ER stress in the etiopathogenesis of adenoid tissue disorders, an essential component of the mucosal immune system, was shown for the first time in the current study. In our study, ATF‐4, ATF‐6, EDEM1, GRP78, EIF2AK3, ERN1, and GRP94 protein expression was significantly higher in the CA group than in the AH group. However, BAX and BCL‐2 proteins, which are markers of apoptosis, were not different between the groups. This may be because prolonged exposure to stress causes an imbalance in apoptosis signaling pathways.
The UPR, which develops in cells in response to ER stress, also participates developing essential immune system cells such as plasma cells, dendritic cells, and eosinophils. 30 Many recent studies on various types of immune cells demonstrate the role played by ER stress in many immunity processes, including differentiation, immunity activation, and cytokine expression. 13 Because adenoids are composed of lymphoepithelial tissue and contain lymphocytes, macrophages, and dendritic cells, the UPR mechanism likely plays a role in ER stress. 4
Epithelial cells covering mucosal surfaces such as the intestine, stomach, and pulmonary surfaces are congenital regulators of the adaptive immune response. 31 Intestinal ischemia–reperfusion injury was shown to induce UPR activation, particularly in Peneth cells, and apoptosis is induced in these cells in association with ER stress. 32 Additionally, Peneth cell apoptosis induced by ER stress contributes to bacterial translocation and systemic inflammation. Another study showed that epithelial stem cells with ER stress lose their regeneration capacity. 33
Protein folding disorders that occur in response to ER stress may play a role in the pathogenesis of neuronal dysfunction, neuronal cell death, and all neuronal diseases. 34 For example, increased production of β‐amyloid and its accumulation are considered to be a trigger for the neurodegenerative processes in Alzheimer's disease. When β‐amyloid accumulation occurs, CHOP expression was reported to increase in brain cells, and treatment with CHOP antisense RNA increased neuronal survival. 35 An ongoing state of ER stress will cause an increase in the transcription of CHOP, an important molecule in the apoptotic signaling pathway, which can subsequently trigger apoptosis. 36 It has also been previously shown that activation of the PERK‐eIF2α‐ATF‐4 pathway leads to increased CHOP expression. 37 In a study conducted by Van De Beek et al. in patients with X‐linked adrenoleukodystrophy, which is a neurodegenerative disease characterized by the accumulation of very long chain fatty acids in plasma and tissues, it was shown that saturated fatty acids induce ER stress in fibroblasts, with the expression of ER stress markers EDEM1 GADD34 and CHOP. 12 In addition, the endoplasmic reticulum stress pathway plays a role in the pathophysiology of congenital lipodystrophy associated with muscle dysfunction. 11
Parkinson mimetic drugs imitate Parkinson's disease, which has a pathology that is associated with the presence of intracytoplasmic inclusion bodies in dopaminergic neurons and potentiates ER stress in dopaminergic neurons. At the same time a mutation in CHOP expression decreases the apoptosis induced by dopaminergic drugs. 38 Pancreatic cell apoptosis plays a role in the pathogenesis of diabetes. Beta cells in the pancreas are exposed to marked ER stress because they have to participate strongly in protein release, and insulin demand is high. Therefore, beta cells are thought to have the weakest effect against ER stress and ER stress‐mediated apoptosis that occurs in beta cells, which may play a role in the development of diabetes. 39 Additionally, CHOP expression caused by ER stress in diabetes was reported to activate proinflammatory mechanisms, further enhancing systemic and local ER stress and thereby contributing to kidney damage. 40 CHOP protein expressed in macrophages in association with long‐term ER stress causes calcium release, activates the apoptotic Fas receptor, and decreases anti‐apoptotic BCL‐2 protein levels, thereby increasing macrophage apoptosis. 41
In another study, CHOP expression was shown to mediate cholesterol accumulation and apoptosis in macrophages, contributing to atherosclerosis pathogenesis. 42 CHOP protein, which is expressed via ER stress, was also shown to play a part in the pathogenesis of chronic myocardial ischemia, cardiac hypertrophy, and heart failure. 43 An in vitro study on neonatal mice using the hypoxia/reperfusion model showed that up‐regulation of calpain due to ischemia induces apoptosis by activating ER stress in mouse cardiomyocytes. 44 In another experimental study, by using a calpain inhibitor against cardiac lipotoxicity induced by palmitate, ER stress was inhibited in cardiomyocytes, lipotoxicity‐induced apoptosis, and proinflammatory cytokine expression was reduced, and heart damage was prevented. 45
Additionally, ER stress has been shown to play a role in both the apoptosis of alveolar epithelial cells and the etiopathogenesis of lung fibrosis in patients with idiopathic pulmonary fibrosis. 46 Our study also demonstrated that ER stress plays a role in the etiopathogenesis of AH. Repeated stimulation by pathogenic agents activates monocytes and macrophages in the adenoid tissue. The released cytokines also cause endothelial cell and fibroblast proliferation and induce immunity. However, over time, immunologically active tissue is replaced by fibrotic tissue. 5
In our recent study investigating the role of ER stress and apoptosis in the etiopathogenesis of chronic tonsillitis and tonsillar hypertrophy in children, ER stress gene expression levels, except for EDEM1, were higher in the chronic tonsillitis group than in the tonsillar hypertrophy group. Additionally, a significant difference was found between the groups in BAX and BCL‐2 gene expression levels. This result suggests that the apoptosis pathway gene expression levels of the tonsillar hypertrophy group were significantly higher than those in the chronic tonsillitis group, in contrast to the ER stress gene expression levels. However, western blot analysis showed that normalized ATF‐4, ATF‐6, CHOP, GRP78, and ERN1 protein expression levels were higher in the chronic tonsillitis group than in the tonsillar hypertrophy group. No difference was found between these groups in the western blot analysis for BAX and BCL‐2 levels. 28 These results suggested that ER stress might play a role in the pathogenesis of chronic tonsillitis and that apoptosis may play a role in the pathogenesis of tonsillar hypertrophy.
ATF‐4 protein is a protective gene and transcription activator that regulates the adaptation of cells to pathological conditions such as ER stress, oxidative stress, and anoxic disorder. 47 It has been suggested that ATF‐4 is crucial to avoid p53‐induced apoptosis in anterior lens epithelial cells. 48 Additionally, ATF‐4 has been shown to have pro‐apoptotic and antiproliferative functions during mammary gland development. 49
When the ATF‐6 gene is activated, the transcription factor that enables UPR target gene activation during ER stress is encoded. The ATF‐6 gene functions as an ER stress sensor/transducer after ER stress‐induced proteolysis. Acupuncture has previously been shown to alleviate cerebral ischemia–reperfusion injury by suppressing ER stress, autophagy, and apoptosis. This positive effect of acupuncture occurs by suppressing ER stress in the steps activating PERK, IRE1, and ATF‐6. 50
Misfolded proteins are cleared by ER‐associated degradation (ERAD) in the ER. EDEM1, which enhances ER degradation, is thought to be a gene‐encoding protein involved in the ERAD pathway substrate signaling or recognition. Among its related pathways are the calnexin/calreticulin cycle and photodynamic therapy‐induced UPR. 51
During ER protein quality control and ER‐transmembrane signaling molecule activation, the main ER chaperone protein GRP78 has a critical role. GRP78 plays a role in the UPR stage similar to GRP94, an important molecular chaperone and heat shock protein. It has been shown that the production of intracellular reactive oxygen species is inhibited, cell survival is increased, and apoptosis is inhibited by regulating stress‐related signaling pathways with GRP78. GRP78 regulates the balance between apoptosis and survival in cancer cells. GRP94 is associated with various signaling pathways that prevent apoptosis and promote cell proliferation. 52 , 53
ERN1 mediates the activation of responses that decide what to do to cells under ER stress. Proximal sensors such as ERN1 and PERK regulate the capacity of ER to fold newly synthesized proteins and to eliminate misfolded/unfolded proteins. Additionally, ER stress contributes to apoptosis through the activation of pro‐apoptotic pathways such as GRP78, IRE‐1, XBP‐1, and CHOP. 54
5. STRENGTHS AND LIMITATIONS
Ours is the first study to show the relationship between ER stress, apoptosis, and pathology in adenoid tissue, which plays an active role in the immune system, especially in childhood. However, a limitation of our study was that we did not compare the CA and AH tissue samples with tissues from a control group of participants without adenoid tissue pathology. However, although such a comparison would further strengthen our study, it is not possible ethically and legally. Also, there is no universally accepted difference between CA and AH; they may overlap and intertwine in some patients. This may cause the clinician to act biasedly by taking into account the most prominent complaint of the patients' parents when making a diagnosis in patients with coexistent CA and AH.
6. CONCLUSIONS
The findings obtained in this study suggest that ER stress is the main promoter and compensator in the etiopathogenesis of diseases of the adenoid tissue, which has strategic functions in the immune system from childhood to adolescence. Therefore, suppression of ER stress may be a new strategy to treat diseases associated with the immune system. However, future clinical studies are required to demonstrate the potential of the ER stress pathway as a therapeutic target. Additionally, studies to clarify the functioning of the secondary lymphoid organs, which form the body's primary line of defense from childhood to adolescence, will guide the development of additional treatments against immune system diseases.
FUNDING INFORMATION
The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article. This study was supported by the Scientific Research Projects Coordination Unit of Selcuk University (Project number: 19401030).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
We sincerely thank Prof. Neslihan Saltali from Necmettin Erbakan University for her help with the statistical analysis. We also thank anesthesia technicians Muradiye Karadeniz and Esra Acar for their assistance in collecting adenoid tissues.
Onal M, Elsurer C, Duran T, et al. Possible role of endoplasmic reticulum stress in the pathogenesis of chronic adenoiditis and adenoid hypertrophy: A prospective, parallel‐group study. Laryngoscope Investigative Otolaryngology. 2024;9(2):e1240. doi: 10.1002/lio2.1240
The adenoid tissue samples were collected at the Selcuk University Faculty of Medicine, Department of Otorhinolaryngology. The adenoid tissue samples were evaluated at the Selcuk University Faculty of Medicine, Department of Medical Genetics.
REFERENCES
- 1. Casselbrant ML. What is wrong in chronic adenoiditis/tonsillitis anatomical considerations. Int J Pediatr Otorhinolaryngol. 1999;49(Suppl 1):S133‐S135. doi: 10.1016/s0165-5876(99)00147-0 [DOI] [PubMed] [Google Scholar]
- 2. Brandtzaeg P. Immunology of tonsils and adenoids: everything the ENT surgeon needs to know. Int J Pediatr Otorhinolaryngol. 2003;67:S69‐S76. doi: 10.1016/j.ijporl.2003.08.018 [DOI] [PubMed] [Google Scholar]
- 3. Brodsky L, Koch RJ. Anatomic correlates of normal and diseased adenoids in children. Laryngoscope. 1992;102(11):1268‐1274. doi: 10.1288/00005537-199211000-00013 [DOI] [PubMed] [Google Scholar]
- 4. Brambilla I, Pusateri A, Pagella F, et al. Adenoids in children: advances in immunology, diagnosis, and surgery. Clin Anat. 2014;27(3):346‐352. doi: 10.1002/ca.22373 [DOI] [PubMed] [Google Scholar]
- 5. Önal M, Yılmaz T, Bilgiç E, Müftüoğlu S, Sözen T, Bajin MD. Possible role of apoptosis in pathogenesis of adenoid hypertrophy and chronic adenoiditis: prospective case‐control study. Auris Nasus Larynx. 2015;42(6):449‐452. doi: 10.1016/j.anl.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 6. Wang H. Chronic adenoiditis. J Int Med Res. 2020;48(11):300060520971458. doi: 10.1177/0300060520971458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bicknell PG. Role of adenotonsillectomy in the management of pediatric ear, nose and throat infections. Pediatr Infect Dis J. 1994;13(Suppl 1):S75‐S79. doi: 10.1097/00006454-199401001-00016 [DOI] [PubMed] [Google Scholar]
- 8. Yilmaz T, Ceylan M, Akyön Y, Ozçakýr O, Gürsel B. Helicobacter pylori: a possible association with otitis media with effusion. Otolaryngol Head Neck Surg. 2006;134(5):772‐777. doi: 10.1016/j.otohns.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 9. Bowers I, Shermetaro C. Adenoiditis. StatPearls [Internet]. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 10. Li R, Wang Y, Chen P, Meng J, Zhang H. Inhibiting endoplasmic reticulum stress by activation of G‐protein‐coupled estrogen receptor to protect retinal astrocytes under hyperoxia. J Biochem Mol Toxicol. 2021;35(2):e22641. doi: 10.1002/jbt.22641 [DOI] [PubMed] [Google Scholar]
- 11. de Melo A, Campos JT, Dantas de Medeiros JL, Cardoso de Melo ME, et al. Endoplasmic reticulum stress and muscle dysfunction in congenital lipodystrophies. Biochim Biophys Acta Mol basis Dis. 2021;1867(6):166120. doi: 10.1016/j.bbadis.2021.166120 [DOI] [PubMed] [Google Scholar]
- 12. van de Beek MC, Ofman R, Dijkstra I, et al. Lipid‐induced endoplasmic reticulum stress in X‐linked adrenoleukodystrophy. Biochim Biophys Acta Mol basis Dis. 2017;1863(9):2255‐2265. doi: 10.1016/j.bbadis.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 13. So JS. Roles of endoplasmic reticulum stress in immune responses [published correction appears in Mol Cells. 2019 Jun 30;42(6):501]. Mol Cells. 2018;41(8):705‐716. doi: 10.14348/molcells.2018.0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519‐529. doi: 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 15. Schulz KF, Altman DG, Moher D, CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Önal M, Yılmaz T, Bilgiç E, Müftüoğlu SF, Kuşçu O, Günaydın RÖ. Apoptosis in chronic tonsillitis and tonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2015;79(2):191‐195. doi: 10.1016/j.ijporl.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 17. Caylakli F, Hizal E, Yilmaz I, Yilmazer C. Correlation between adenoid‐nasopharynx ratio and endoscopic examination of adenoid hypertrophy: a blind, prospective clinical study. Int J Pediatr Otorhinolaryngol. 2009;73(11):1532‐1535. doi: 10.1016/j.ijporl.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 18. Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. 2010;2010(6) pdb.prot5439. doi: 10.1101/pdb.prot5439 [DOI] [PubMed] [Google Scholar]
- 19. Nami B, Ghasemi‐Dizgah A, Vaseghi A. Overexpression of molecular chaperons GRP78 and GRP94 in CD44(hi)/CD24(lo) breast cancer stem cells. Bioimpacts. 2016;6(2):105‐110. doi: 10.15171/bi.2016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yilmaz T, Koçan EG, Besler HT, Yilmaz G, Gürsel B. The role of oxidants and antioxidants in otitis media with effusion in children. Otolaryngol Head Neck Surg. 2004;131(6):797‐803. doi: 10.1016/j.otohns.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 21. Koch RJ, Brodsky L. Qualitative and quantitative immunoglobulin production by specific bacteria in chronic tonsillar disease. Laryngoscope. 1995;105(1):42‐48. doi: 10.1288/00005537-199501000-00011 [DOI] [PubMed] [Google Scholar]
- 22. Cho KS, Kim SH, Hong SL, et al. Local atopy in childhood adenotonsillar hypertrophy. Am J Rhinol Allergy. 2018;32(3):160‐166. doi: 10.1177/1945892418765003 [DOI] [PubMed] [Google Scholar]
- 23. Niu X, Wu ZH, Xiao XY, Chen X. The relationship between adenoid hypertrophy and gastroesophageal reflux disease: a meta‐analysis. Medicine (Baltimore). 2018;97(41):e12540. doi: 10.1097/MD.0000000000012540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Academy of Pediatrics . Subcommittee on Management of Sinusitis and Committee on quality improvement. Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108(3):798‐808. doi: 10.1542/peds.108.3.798 [DOI] [PubMed] [Google Scholar]
- 25. Zuliani G, Carron M, Gurrola J, et al. Identification of adenoid biofilms in chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol. 2006;70(9):1613‐1617. doi: 10.1016/j.ijporl.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Liu J. Tanshinone I induces cell apoptosis by reactive oxygen species‐mediated endoplasmic reticulum stress and by suppressing p53/DRAM‐mediated autophagy in human hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2020;48(1):488‐497. doi: 10.1080/21691401.2019.1709862 [DOI] [PubMed] [Google Scholar]
- 27. Tagawa Y, Hiramatsu N, Kasai A, et al. Induction of apoptosis by cigarette smoke via ROS‐dependent endoplasmic reticulum stress and CCAAT/enhancer‐binding protein‐homologous protein (CHOP). Free Radic Biol Med. 2008;45(1):50‐59. doi: 10.1016/j.freeradbiomed.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 28. Onal M, Kocak N, Duymus F, et al. Relationship of endoplasmic reticulum stress with the etiopathogenesis of chronic tonsillitis and tonsillar hypertrophy in pediatric patients: a prospective, parallel‐group study. Mol Biol Rep. 2021;48(7):5579‐5586. doi: 10.1007/s11033-021-06579-4 [DOI] [PubMed] [Google Scholar]
- 29. Onal M, Onal O, Turan A. Can secondary lymphoid organs exert a favorable effect on the mild course of COVID‐19 in children? Acta Otolaryngol. 2021;141(1):83‐84. doi: 10.1080/00016489.2020.1814965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107‐138. doi: 10.1146/annurev-immunol-032414-112116 [DOI] [PubMed] [Google Scholar]
- 31. Coleman OI, Haller D. ER stress and the UPR in shaping intestinal tissue homeostasis and immunity. Front Immunol. 2019;10:2825. Published 2019 Dec 4. doi: 10.3389/fimmu.2019.02825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grootjans J, Hodin CM, de Haan JJ, et al. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology. 2011;140(2):529‐539.e3. doi: 10.1053/j.gastro.2010.10.040 [DOI] [PubMed] [Google Scholar]
- 33. van Galen P, Kreso A, Mbong N, et al. The unfolded protein response governs integrity of the haematopoietic stem‐cell pool during stress. Nature. 2014;510(7504):268‐272. doi: 10.1038/nature13228 [DOI] [PubMed] [Google Scholar]
- 34. Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP‐induced apoptosis in ER stress. Acta Biochim Biophys Sin Shanghai. 2015;47(2):146‐147. doi: 10.1093/abbs/gmu128 [DOI] [PubMed] [Google Scholar]
- 35. Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066‐3077. doi: 10.1101/gad.1250704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai TC, Lai KH, Su JH, Wu YJ, Sheu JH. 7‐Acetylsinumaximol B induces apoptosis and autophagy in human gastric carcinoma cells through mitochondria dysfunction and activation of the PERK/eIF2α/ATF4/CHOP signaling pathway. Mar Drugs. 2018;16(4):104. doi: 10.3390/md16040104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cai Z, Shen L, Ma H, et al. Involvement of endoplasmic reticulum stress‐mediated C/EBP homologous protein activation in Coxsackievirus B3‐induced acute viral myocarditis. Circ Heart Fail. 2015;8(4):809‐818. doi: 10.1161/CIRCHEARTFAILURE.114.001244 [DOI] [PubMed] [Google Scholar]
- 38. Lee JH, Won SM, Suh J, et al. Induction of the unfolded protein response and cell death pathway in Alzheimer's disease, but not in aged Tg2576 mice. Exp Mol Med. 2010;42(5):386‐394. doi: 10.3858/emm.2010.42.5.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silva RM, Ries V, Oo TF, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95(4):974‐986. doi: 10.1111/j.1471-4159.2005.03428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li SY, Gilbert SA, Li Q, Ren J. Aldehyde dehydrogenase‐2 (ALDH2) ameliorates chronic alcohol ingestion‐induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol. 2009;47(2):247‐255. doi: 10.1016/j.yjmcc.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191(6):1113‐1125. doi: 10.1083/jcb.201006121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yao S, Zong C, Zhang Y, et al. Activating transcription factor 6 mediates oxidized LDL‐induced cholesterol accumulation and apoptosis in macrophages by up‐regulating CHOP expression. J Atheroscler Thromb. 2013;20(1):94‐107. doi: 10.5551/jat.13425 [DOI] [PubMed] [Google Scholar]
- 43. Srinivasan K, Sharma SS. Augmentation of endoplasmic reticulum stress in cerebral ischemia/reperfusion injury associated with comorbid type 2 diabetes. Neurol Res. 2011;33(8):858‐865. doi: 10.1179/1743132811Y.0000000015 [DOI] [PubMed] [Google Scholar]
- 44. Zheng D, Wang G, Li S, Fan GC, Peng T. Calpain‐1 induces endoplasmic reticulum stress in promoting cardiomyocyte apoptosis following hypoxia/reoxygenation. Biochim Biophys Acta. 2015;1852(5):882‐892. doi: 10.1016/j.bbadis.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li S, Zhang L, Ni R, et al. Disruption of calpain reduces lipotoxicity‐induced cardiac injury by preventing endoplasmic reticulum stress. Biochim Biophys Acta. 2016;1862(11):2023‐2033. doi: 10.1016/j.bbadis.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baek HA, Kim DS, Park HS, et al. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol. 2012;46(6):731‐739. doi: 10.1165/rcmb.2011-0121OC [DOI] [PubMed] [Google Scholar]
- 47. Ameri K, Lewis CE, Raida M, Sowter H, Hai T, Harris AL. Anoxic induction of ATF‐4 through HIF‐1‐independent pathways of protein stabilization in human cancer cells. Blood. 2004;103(5):1876‐1882. doi: 10.1182/blood-2003-06-1859 [DOI] [PubMed] [Google Scholar]
- 48. Hettmann T, Barton K, Leiden JM. Microphthalmia due to p53‐mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB‐2 transcription factor. Dev Biol. 2000;222(1):110‐123. doi: 10.1006/dbio.2000.9699 [DOI] [PubMed] [Google Scholar]
- 49. Hoshiya Y, Gupta V, Kawakubo H, et al. Mullerian inhibiting substance promotes interferon gamma‐induced gene expression and apoptosis in breast cancer cells. J Biol Chem. 2003;278(51):51703‐51712. doi: 10.1074/jbc.M307626200 [DOI] [PubMed] [Google Scholar]
- 50. Sun X, Liu H, Sun Z, et al. Acupuncture protects against cerebral ischemia‐reperfusion injury via suppressing endoplasmic reticulum stress‐mediated autophagy and apoptosis. Mol Med. 2020;26(1):105. doi: 10.1186/s10020-020-00236-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cormier JH, Tamura T, Sunryd JC, Hebert DN. EDEM1 recognition and delivery of misfolded proteins to the SEL1L‐containing ERAD complex. Mol Cell. 2009;34(5):627‐633. doi: 10.1016/j.molcel.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee AS. Glucose‐regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14(4):263‐276. doi: 10.1038/nrc3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee JH, Yoon YM, Lee SH. GRP78 regulates apoptosis, cell survival and proliferation in 5‐fluorouracil‐resistant SNUC5 colon cancer cells. Anticancer Res. 2017;37(9):4943‐4951. doi: 10.21873/anticanres.11904 [DOI] [PubMed] [Google Scholar]
- 54. Banerjee A, Ahmed H, Yang P, Czinn SJ, Blanchard TG. Endoplasmic reticulum stress and IRE‐1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment. Oncotarget. 2016;7(27):41432‐41444. doi: 10.18632/oncotarget.9180 [DOI] [PMC free article] [PubMed] [Google Scholar]


