Abstract
Evidence from Europe shows that perioperative chemotherapy may be beneficial for the treatment of locally advanced gastric cancer, but reliable and robust data is lacking. To rectify this, the phase 3 RESONANCE trial investigated the efficacy and safety of S-1 plus oxaliplatin (SOX) as a perioperative chemotherapy regimen for gastric cancer. This randomized, open-label trial enrolled patients from 19 medical centers with stage II/III resectable gastric cancer who were centrally randomly assigned to either perioperative chemotherapy (PC) arm or adjuvant chemotherapy (AC) arm. Patients in the PC arm received two to four cycles of SOX followed by surgery and four to six cycles of SOX. Patients in the AC arm received upfront surgery and eight cycles of SOX. 386 patients in each group were enrolled and 756 (382 in PC and 374 in AC) were included in the mITT population. The three-year DFS rate was 61.7% in the PC arm and 53.8% in the AC arm (log-rank p = 0.019). The R0 resection rate in the PC arm was significantly higher than that in the AC arm (94.9% vs. 83.7%, p < 0.0001). There was no difference between two arms in surgical outcomes or postoperative complications. Safety-related data were like the known safety profile. In conclusion, from a clinical perspective, this trial indicated a trend towards higher three-year disease-free survival rate with perioperative SOX in stage II/III resectable gastric cancer with well-tolerated toxicity compared to adjuvant SOX, which might provide a theoretical basis for applying perioperative SOX in advanced gastric cancer patients. (ClinicalTrials.gov NCT01583361)
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01536-7.
Keywords: Gastric cancer, Perioperative, Adjuvant, Chemotherapy, S-1, Oxaliplatin
To the Editor.
Curative resection is the mainstay for resectable gastric cancer [1]. To further improve survival, multidisciplinary strategies such as perioperative chemotherapy and postoperative chemotherapy have been assessed. The MAGIC study, FNCLCC/FFCD 9703 study, and FLOT4 study have established the rationale for perioperative chemotherapy in western countries, showing better overall survival in perioperative settings than surgery only [2–4]. In contrast, the ACTS-GC trial and CLASSIC trial have solidified adjuvant chemotherapy as a standard treatment in East Asia [5, 6]. Despite these advances, current evidence does not suggest a preferred therapeutic strategy or an optimal chemotherapy regimen. Several studies have shown that the S-1 plus oxaliplatin chemotherapy (SOX) was efficient and well tolerated [7–10]. However, there remains a scarcity of direct comparisons between perioperative and adjuvant chemotherapy using SOX. Therefore, the randomized RESONANCE trial was conducted to compare perioperative with adjuvant SOX chemotherapy in patients with locally advanced gastric cancer. Study Methods were contained in Additional file 1.
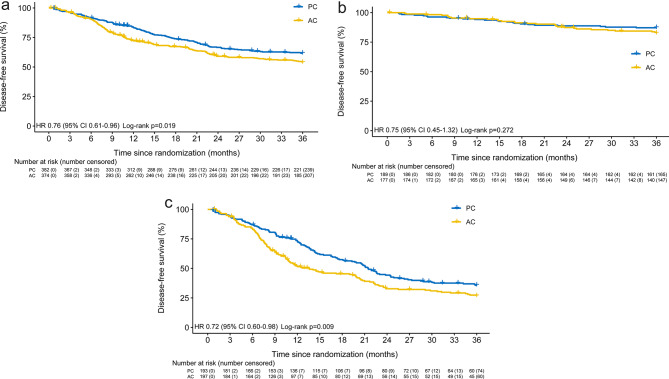
Between Sep 1, 2012, and Jul 1, 2019, 772 patients from 19 medical centers were enrolled and randomly assigned to perioperative chemotherapy (PC) arm or adjuvant chemotherapy (AC) arm (Additional file 2: Fig. S1, Table S1). 382 in PC arm receiving preoperative chemotherapy and 374 in AC arm receiving surgical resection formed the modified intention-to-treat (mITT) population (Additional file 2: Table S2). The three-year disease-free survival (DFS) rate was 61.7% (95%CI 56.8-66.6%) in PC group and 53.8% (95%CI 48.8-58.9%) in AC group. The hazard ratio (HR) was 0.76 (95%CI 0.61–0.96) and log-rank p = 0.019 (Fig. 1A). Subgroup analysis revealed a significant difference in DFS between the two groups among stage III patients, rather than among stage II patients (Fig. 1B and C, Additional file 2: Fig. S2). In the per-protocol population, which consisted of patients who received surgery and preoperative and postoperative chemotherapy in PC group or postoperative chemotherapy in AC group, the three-year DFS rate was 63.0% (95%CI 58.1-67.9%) in PC group and 55.5% (95%CI 50.3-60.7%) in AC group (HR 0.77, 95%CI 0.61–0.96, p = 0.026) (Additional file 2: Fig. S3).
Fig. 1.
Kaplan-Meier estimates of disease-free survival for mITT patients (A), stage II patients (B), and stage III patients (C). HR, hazard ratio; PC, perioperative chemotherapy; AC, adjuvant chemotherapy
In the PC arm, 157 patients (41.1%) completed eight cycles of perioperative chemotherapy, while 68 (19.2%) in the AC group completed eight cycles of postoperative chemotherapy, which was significantly lower than that of the PC group (p < 0.001) (Additional file 2: Table S3). Preoperative chemotherapy resulted in pathological complete response (pCR) in 23.6% of patients in the PC arm. Additionally, post-hoc re-evaluation by the third party yielded a pCR rate of 22.3%.
No significant difference was found in terms of surgical time, blood loss, gastrectomy, number of dissected lymph nodes, and lymphadenectomy (Additional file 2: Table S4). The R0 resection rate of the PC group was 94.9%, which was higher than that of 83.7% in the AC group. The stratified analysis revealed higher R0 resection rates in the PC arm compared to the AC arm for stage IIIC patients or patients with tumors located in the esophagogastric junction (Additional file 2: Fig. S4).
Postoperative complications occurred in 68 patients (18.1%) in the PC arm and 73 (19.5%) in the AC arm. No significant difference in postoperative hospital stays or the rate of complication was found between the two arms (Additional file 2: Table S5, Table S6). Adverse events (AE) are listed in Table 1. The most common hematological and non-hematological AE were thrombocytopenia and fatigue, respectively. Neutropenia was the most frequent AE in all observed grade 3/4 AE. Two patients from PC group and one patient from AC group died from thrombotic event, cardiovascular event and abdominal infection, respectively.
Table 1.
Adverse events
| PC arm | AC arm (N = 354) | P value (PC-post vs. AC) |
||||||
|---|---|---|---|---|---|---|---|---|
| Preoperative (N = 382) | Postoperative (N = 364) | |||||||
| All | Grade 3/4 | All | Grade 3/4 | All | Grade 3/4 | All | Grade 3/4 | |
| Serious adverse events | 8(2.1%) | 3(0.8%) | 12(3.3%) | 6(1.6%) | 18(5.1%) | 11(3.1%) | 1.000 | 1.000 |
| Hematological | ||||||||
| Anemia | 251(65.7%) | 30(7.9%) | 191(52.5%) | 25(6.9%) | 201(56.8%) | 23(6.5%) | 0.246 | 0.842 |
| Leukopenia | 242(63.4%) | 16(4.2%) | 184(50.5%) | 19(5.2%) | 190(53.7%) | 14(4.0%) | 0.402 | 0.418 |
| Neutropenia | 209(54.7%) | 75(19.6%) | 173(47.5%) | 54(14.8%) | 162(45.8%) | 67(18.9%) | 0.636 | 0.143 |
| Thrombocytopenia | 292(76.4%) | 40(10.5%) | 250(68.7%) | 32(8.8%) | 243(68.6%) | 28(7.9%) | 0.991 | 0.670 |
| Non-hematological | ||||||||
| Anorexia | 267(69.9%) | 18(4.7%) | 219(60.2%) | 16(4.4%) | 231(65.3%) | 9(2.5%) | 0.159 | 0.176 |
| Diarrhea | 180(47.1%) | 12(3.1%) | 156(42.9%) | 9(2.5%) | 130(36.7%) | 11(3.1%) | 0.093 | 0.605 |
| Fatigue | 288(75.4%) | 20(5.2%) | 247(67.9%) | 12(3.3%) | 245(69.2%) | 14(4.0%) | 0.697 | 0.637 |
| Mucositis | 108(28.3%) | 2(0.5%) | 89(24.5%) | 1(0.3%) | 105(29.7%) | 3(0.8%) | 0.116 | 0.303 |
| Nausea | 261(68.3%) | 8(2.1%) | 201(55.2%) | 5(1.4%) | 191(54.0%) | 11(3.1%) | 0.734 | 0.116 |
| Neuropathy | 187(49.0%) | 14(3.7%) | 157(43.1%) | 15(4.1%) | 144(40.7%) | 9(2.5%) | 0.505 | 0.239 |
| Vomitting | 121(31.7%) | 6(1.6%) | 94(25.8%) | 8(2.2%) | 104(29.4%) | 8(2.3%) | 0.287 | 0.955 |
Chemotherapy population (patients who received at least one cycle of chemotherapy). Data are n (%). PC, perioperative chemotherapy; AC, adjuvant chemotherapy; PC-post, adverse events observed in postoperative chemotherapy in the PC group
The results of our study have suggested a tendency towards higher three-year disease-free survival rate with perioperative SOX for patients with resectable stage II/III gastric cancer compared to the adjuvant SOX. The results of the subgroup analysis provide compelling evidence supporting the recommendation in the Chinese guidelines for administering neoadjuvant chemotherapy in stage III patients [11]. The limitations of this study include potential deviations in stage or response evaluation, the absence of using Lauren’s classification and microsatellite instability status, and the uneven number of enrolled cases across different centers. Despite these, we believed that this study might provide a theoretical basis for applying perioperative SOX as a standard cure in Chinese advanced gastric cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the patients, their families, and the institutions involved in this study. We also acknowledge Infinity Scope Inc. for their support.
Abbreviations
- SOX
S-1 plus oxaliplatin
- PC
Perioperative chemotherapy
- AC
Adjuvant chemotherapy
- DFS
Disease-free survival
- mITT
Modified intention-to-treat
- HR
Hazard ratio
- pCR
Pathological complete response
Author contributions
LC supervised the study. LC, BW, YT, and Xinxin W proposed the concept and designed the trial. ZL, YX, YY, Zhongtao Z, YS, HL, KL, LZ, Zhichao Z, YZ, YH, FL, Xin W, PL, HH, GL, XS, JJ, YT, and ZX provided administrative support, acquired, and input the data. Xinxin W, CL, SL, BW, ZL did the data validation and statistical analysis. SL, Xinxin W, CL, ZL and LC wrote and revised the manuscript. All authors finally approved the manuscript and had final responsibility for the decision to submit for publication. All authors read and approved the final manuscript. Xinxin W, CL, BW, SL and ZL contributed equally to this work.
Funding
None.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All patients gave their written informed consent. The study was approved by the Ethics Committee of the Chinese PLA General Hospital in Beijing on February 28th, 2012.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinxin Wang, Canrong Lu, Bo Wei, Shuo Li, and Ziyu Li have contributed equally to this work.
References
- 1.Li GZ, Doherty GM, Wang J. Surgical Management of Gastric Cancer: a review. JAMA Surg. 2022;157(5):446–54. doi: 10.1001/jamasurg.2022.0182. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 4.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 5.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 6.Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–96. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Chen L. [Efficacy and safety of SOX regimen as neoadjuvant chemotherapy for advanced gastric cancer] Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14(2):104–6. [PubMed] [Google Scholar]
- 8.Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92. doi: 10.1016/S1470-2045(21)00297-7. [DOI] [PubMed] [Google Scholar]
- 9.Iwatsuki M, Orita H, Kobayashi K, Hidaka S, Arigami T, Kusumoto T, et al. Phase II study of S-1 and oxaliplatin as neoadjuvant chemotherapy for locally advanced adenocarcinoma of the gastric or esophagogastric junction: KSCC1601. Gastric Cancer. 2022;25(1):180–7. doi: 10.1007/s10120-021-01218-0. [DOI] [PubMed] [Google Scholar]
- 10.Honma Y, Yamada Y, Terazawa T, Takashima A, Iwasa S, Kato K, et al. Feasibility of neoadjuvant S-1 and oxaliplatin followed by surgery for resectable advanced gastric adenocarcinoma. Surg Today. 2016;46(9):1076–82. doi: 10.1007/s00595-015-1276-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang FH, Zhang XT, Tang L, Wu Q, Cai MY, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun (Lond) 2024;44(1):127–72. doi: 10.1002/cac2.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.