Abstract
Previously we showed that infection of human type II airway epithelial (A549) cells with purified respiratory syncytial virus (pRSV) induced interleukin-8 transcription by a mechanism involving cytokine-inducible cytoplasmic-nuclear translocation of the RelA transcription factor. In unstimulated cells, RelA is tethered in the cytoplasm by association with the IκB inhibitor and can be released only following IκB degradation. In this study, we examined the spectrum of IκB isoform expression and kinetics of proteolysis of the isoforms in A549 cells following pRSV infection. In contrast to the rapid and robust activation of RelA DNA binding that peaked within 15 min of treatment produced by the prototypic activator tumor necrosis factor alpha (TNF-α), pRSV produced a weaker increase in RelA binding that began at 3 h and did not peak until 24 h after infection. A549 cells expressed the IκB inhibitory subunits IκBα, IκBβ, and p105; however, following either stimulus, only the IκBα and IκBβ steady-state levels declined in parallel with the increase in RelA DNA-binding activity. The >120-min half-life of IκBα in control cells was shortened to 5 min in TNF-α-stimulated cells and to 90 min in pRSV-infected cells. Although IκBα was resynthesized within 30 min following recombinant human TNFα treatment due to a robust 25-fold increase of IκBα mRNA expression (the RelA:IκBα positive feedback loop), following pRSV infection, there was no reaccumulation of IκBα protein, as infected cells produced only a 3-fold increase in IκBα mRNA at 24 h, indicating the RelA:IκBα positive feedback loop was insufficient to restore control IκBα levels. IκBα proteolysis induced by TNF-α occurred through the 26S proteasome, as both 26S proteasome activity and IκBα proteolysis were blocked by specific inhibitors lactacystin, MG-132, and ZLLF-CHO. Although total proteasome activity in 24-h pRSV-infected lysates increased twofold, its activity was >90% inhibited by the proteasome inhibitors; surprisingly, however, IκBα proteolysis was not. We conclude that RSV infection produces IκBα proteolysis through a mechanism primarily independent of the proteasome pathway.
The enveloped negative-sense RNA virus respiratory syncytial virus (RSV) is the major cause of bronchiolitis and pneumonia in infants and young children (20). In addition to producing persistent airway hyperreactivity in previously healthy children, RSV infection is a major cause of morbidity in children with preexisting pulmonary or heart disease (18, 28, 48). RSV replicates primarily in the respiratory epithelial cell. The infected airway epithelial cell is an important initiator in the response to RSV infection, by synthesizing and secreting potent cytokines that are involved in the immune and inflammatory responses in the airway mucosa (16, 41).
Recent studies have demonstrated that the cytokines interleukin-1 (IL-1), IL-6, IL-8, IL-11, RANTES, macrophage inflammatory protein-1α, monocyte chemotactic protein-1, granulocyte/macrophage stimulatory factor, tumor necrosis factor alpha (TNF-α), and the soluble TNF receptor are produced by RSV-infected respiratory epithelial cells and may play important roles in the development of inflammation in vivo (2, 4, 16, 17, 30, 38). Of particular relevance to mononuclear inflammation, IL-8 gene expression is activated at the level of transcription through the effects of RSV-inducible transcription factors nuclear factor-κB (NF-κB) and NF-IL6 (4, 13, 17, 25, 29). From these studies, NF-κB emerges as the primary activator of IL-8 transcription whose binding is absolutely required for inducible expression (5, 13). Indeed, NF-κB translocation appears to be essential for activity not only of IL-8 but also of a genetic network including the cytokines IL-1, IL-6, and IL-11 in the RSV-infected epithelium (4). NF-κB constitutes a family of cytokine-inducible transcription factors that include the potent RelA (p65) transactivator, as well as the RelB, c-Rel, NF-κB1 (p50), and NF-κB2 (p52) (the last two being encoded by the proteolytically processed precursors p105 and p100, respectively [40]) subunits. Inducible NF-κB subunits interact with cytoplasmic inhibitors, collectively known as IκBs, through motifs contained within a conserved NH2-terminal Rel homology domain (3). IκB subunits, responsible for cytoplasmic retention and inactivation of NF-κB DNA-binding activity, consist predominantly of four isoforms, IκBα, IκBβ, IκBγ, and the NF-κB1 precursor, p105, that are expressed and regulated in a cell specific fashion (21, 23, 44).
NF-κB-inducing signals control its cytoplasmic-nuclear abundance by a mechanism involving proteolytic degradation of the IκB inhibitor. Once liberated, free cytoplasmic NF-κB passes through the nuclear pore complex and enters the nucleus (translocates), to bind and activate target genes. Stimulation of cells with the prototypical activator TNF-α results in rapid serine phosphorylation of the IκBα NH2 terminus (6, 45), an event coupled to the rapid polyubiquitination and proteolysis of phospho-IκB (IκBαP) through the 26S proteasome (1, 10). The role of the 26S proteasome has been implicated by the ability of the proteasome-specific calpain inhibitor I, Z-LLF-CHO, or MG-132 to block IκB proteolysis in TNF-α-stimulated cells (1, 5, 10, 12, 37, 46).
Although we have shown that pRSV infection increases the nuclear abundance of NF-κB (RelA) in A549 cells, its mechanism of activation is unexplored. Therefore, we systematically investigated the mechanism and kinetics of NF-κB activation in pRSV-infected airway epithelial cells in comparison to those of TNF-α. We show herein that in contrast to the rapid activation of RelA produced by TNF-α, pRSV infection produces a gradual increase in RelA binding peaking at 24 h. For both activators TNF-α and pRSV, IκBα and IκBβ proteolysis occurred in parallel with the increases in RelA DNA-binding activity. Although specific proteasome inhibitors significantly block 26S protease activity and IκBα proteolysis by TNF-α, they do not completely prevent IκBα proteolysis by pRSV infection. These data indicate that a major aspect of IκBα proteolysis occurs via a proteasome-independent mechanism in virus-infected epithelium.
MATERIALS AND METHODS
Cell culture and treatment.
Human A549 pulmonary type II epithelial cells (American Type Culture Collection, Manassas, Va.) were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum as previously described (17, 25). Logarithmically growing A549 cells were either infected with sucrose gradient-purified Long strain A2 RSV (pRSV) at a multiplicity of infection (MOI) of 1 (17, 25) or stimulated with 20 ng of recombinant human TNF-α (rhTNF-α; Calbiochem) per ml and incubated for various times at 37°C. After stimulation, cell pellets were washed in phosphate-buffered saline and fractionated into cytoplasmic and nuclear extracts by using hypotonic lysis and high-salt extraction (17, 25). The protein contents of cellular extracts were measured by a spectrophotometric (Bradford) assay using bovine serum albumin as a standard (Bio-Rad protein detection reagent). Where indicated, the protein synthesis inhibitor cycloheximide (CHX) was added to culture medium at a final concentration of 50 μg/ml. The proteasome inhibitors lactacystin (a gift of E. J. Corey, Harvard University), carbobenzoxyl-leucinyl-leucinyl-leucinal-H (MG-132; Sigma Aldrich, St. Louis, Mo.), and benzyloxycarbonyl-Leu-Leu-phenylalaninal (Z-LLF-CHO; a gift of M. Suto, Signal Pharmaceuticals) were added directly to the culture medium at the indicated concentrations.
EMSA.
Electrophoretic mobility shift assay (EMSA) was performed with a duplex oligonucleotide corresponding to nucleotides −96 to −69 of the human IL-8 gene promoter: 5′-GATCCATCAGTTGCAAATCGTGGAATTTCCTCTCTA-3′ 3′- GTAGTCAACGTTTAGCACCTTAAAGGAGAGATCTAG-5′
DNA-binding reactions were carried out in a mixture of 20 μg of nuclear protein, 12 mM HEPES (pH 7.9), 40 mM KCl, 120 mM NaCl, 0.2 mM EDTA, 0.2 mM EGTA, 0.4 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 5% glycerol, 1.5 μg of poly(dA-dT), and 20,000 cpm of 32P-labeled duplex DNA. The reaction mixture was incubated at 22°C for 15 min and then fractionated by 6% nondenaturing polyacrylamide gel electrophoresis (PAGE). The gel was dried and exposed for autoradiography using Kodak X-AR film at −70°C.
Western immunoblotting and coimmunoprecipitation.
For Western immunoblotting, indicated amounts of nuclear or cytoplasmic extracts were mixed with Laemmli buffer, boiled at 100°C for 5 min, fractionated on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) as described elsewhere (5, 17, 25). Membranes were washed, blocked in 8% nonfat dry milk, and probed with affinity-purified rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.) for RelA, c-Rel, and NF-κB1 (in nuclear fractions) or IκBα, IκBβ, and IκBγ (in cytoplasmic fractions). After incubation with the secondary donkey anti-rabbit immunoglobulin G antibody-horseradish peroxidase conjugate (Amersham International), immunocomplexes were detected by enhanced chemiluminescence (ECL) reaction (ECL kit; Amersham) as specified by the manufacturer. For the two-step coimmunoprecipitation-Western immunoblot assay, A549 whole-cell extract (WCE) was made by adding radioimmunoprecipitation assay buffer (150 mM NaCl–1.0% Nonidet P-40–0.5% deoxycholate–0.1% SDS–50 mM Tris-Cl [pH 8.0] containing 1 μg of pepstatin A, 1 μg of leupeptin, and 10 μg of aprotinin per ml, 1 mM dithiothreitol, and 1 mM PMSF) to A549 monolayers. Equal amounts of WCE (2.5 mg of protein) were incubated with 12 μl of affinity-purified RelA antibody (Santa Cruz Biotechnology) in 1 ml of TST buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 150 mM NaCl 0.05% Triton X-100) for 2 h at 4°C. Then protein A-agarose was added, and the mixture was incubated for another 2 h. The protein A-agarose immune complex was precipitated by centrifugation. After five washes with TST buffer, the precipitates were boiled in Laemmli buffer and immunoblotted with indicated antibodies as described above. Where indicated, anti-RelA and IκBα antibodies were preadsorbed by incubating them with 20-fold excess peptide at 4°C overnight.
Northern blotting.
Forty micrograms total cellular RNA was extracted from A549 cells by acid guanidinium-phenol extraction (RNAzol B; Tel-Test Inc., Friendswood, Tex.), fractionated by electrophoresis on a 1.2% agarose-formaldehyde gel, capillary transferred to a nitrocellulose membrane (MSI, Westboro, Mass.), and prehybridized as described previously (47). The membrane was hybridized with 106 cpm of 32P-IκBα cDNA probe per ml at 50°C overnight in 5% SDS hybridization buffer (47). The membrane was washed with a buffer containing 5% SDS and 1× SSC (0.15 M NaCl, 0.015 M sodium citrate) for 20 min at room temperature followed by 30 min at 50°C. The membrane was exposed to XAR film (Kodak) for 24 to 48 h. For β-actin Northern blotting, the same membrane was stripped and probed with 32P-labeled β-actin cDNA. Bands in the autoradiogram were quantitated by exposure to a Molecular Dynamics PhosphorImager cassette.
26S proteasome assay.
Proteasome activity in fresh cytoplasmic extracts was measured as described previously (37). Two hundred micrograms of cytoplasmic protein was added to assay buffer (20 mM Tris-Cl [pH 8.0], 1 mM ATP, and 2 mM MgCl2) in the presence of the synthetic fluorogenic substrate Suc-Leu-Leu-Val-Tyr-7-amido-4-methyl coumarin (final concentration, 60 μM; Sigma Aldrich, St. Louis, Mo.) in a final volume of 1 ml. The tubes were incubated at 30°C for 30 min, after which the reaction was terminated by the addition of 1 ml of cold ethanol and the lysate was spun at 12,000 × g for 10 min at 4°C. Fluorescence in the supernatant was measured in a fluorometer at 440-nm emission (Iex, 380 nm). For each assay, a standard curve was measured with known dilutions of 7-amino-4 methylcoumarin (AMC; Sigma Aldrich).
RESULTS
Comparative kinetics of RelA activation.
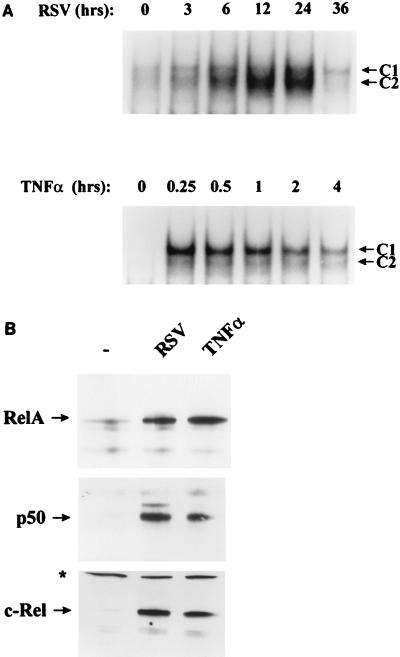
Previously we have shown that both RSV and TNF-α induce the binding of two sequence-specific complexes in EMSA, C1 and C2, to the IL-8-inducible enhancer (located at nucleotides −96 to −69 relative to the cap site [17]). C1 and C2 both contain the RelA transactivator, as indicated by a strong supershift produced in the presence of specific NH2- and COOH-directed RelA antibodies (17), and are due to a heterotypic association with c-Rel and NF-κB1 transcription factors, producing detectable differences in their migration on nondenaturing PAGE (5). We therefore used the presence of C1 and C2 in EMSA to compare the kinetics of RelA activation in response to pRSV infection to that produced by rhTNF-α treatment. pRSV infection induced C1/C2 complex binding in a time-dependent manner; binding was first faintly detected 3 h after the addition of pRSV and peaked at 2.6-fold 24 h postinfection, with C2 predominating (Fig. 1A, top panel). At 36 h, an apparent decrease in C1/C2 complex binding was observed (at a time when the cells are fragile and beginning to lose viability). In contrast, the addition of 20 ng of rhTNF-α per ml produced a much more rapid activation of C1/C2 DNA binding; maximal C1/C2 induction occurred at 15 min (at an 8.12-fold activation), with the C1 complex predominating. C1/C2 binding progressively declined thereafter (even in the continued presence of ligand), and in data not shown, returned to nearly control levels after 8 h of rhTNF-α treatment.
FIG. 1.
NF-κB activation and translocation in RSV-infected and rhTNF-α-stimulated A549 cells. (A) NF-κB binding to the IL-8 promoter in EMSA. A549 cells were infected with pRSV (MOI of 1) or stimulated with 20 ng of rhTNF-α per ml for different periods of time. Shown is an autoradiogram of EMSA using a nuclear extract binding the IL-8 TNF-responsive element (nucleotides −96 to −69; see Materials and Methods), with the autoradiographic exposure of the pRSV time course 1.5 times longer than that for the rhTNF-α time course (to demonstrate relative C1/C2 induction patterns). C1 and C2 are the inducible NF-κB complexes predominantly containing RelA (17). For pRSV, activation relative to control binding is 1.23-fold (3 h), 1.74-fold (6 h), 1.9-fold (12 h), 2.6-fold (24 h), or 1-fold (36 h). For rhTNF-α, C1/C2 activation is 8.12-fold (0.25 h), 7.9-fold (0.5 h), 7.3-fold (1 h), 6.3-fold (2 h), or 5.2-fold (4 h). (B) Nuclear abundance of NF-κB family members measured by Western immunoblotting. Nuclear extracts (216 μg) from control, pRSV-infected (24 h), and rhTNF-α-treated (15 min) cells were fractionated and probed with anti-RelA, -NF-κB1, and -c-Rel antibodies. The levels of the 65-kDa RelA, 50-kDa NF-κB1, and 75-kDa c-Rel proteins were increased following either pRSV or rhTNF-α treatment. ∗, nonspecific protein detected by c-Rel antibody, confirming equivalent loading.
To determine if there were qualitative differences in the pattern of NF-κB subunit induction, changes in nuclear abundance were compared by Western immunoblotting (Fig. 1B). Compared with controls, nuclei from pRSV-infected and rhTNF-α-treated cells both show similar inductions of RelA, NF-κB1, and c-Rel proteins. The slight differences in C1/C2 binding patterns may be the consequence of subtle quantitative differences in relative abundance of the NF-κB subunits and will require further investigation. These data indicate marked differences in the kinetics and pattern of NF-κB activation between TNF-α signalling pathways and pRSV infection.
IκB isoform expression in type II pulmonary epithelial cells and inducible changes in cytoplasmic abundance.
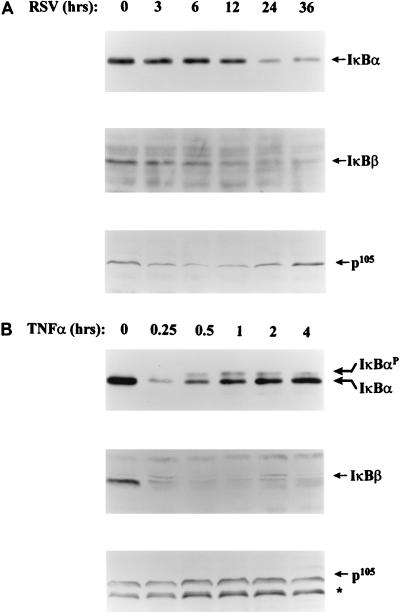
In resting cells, NF-κB is inactivated by complexing with cell-type-restricted IκBα, IκBβ, IκBγ, and the p105 NF-κB1 inhibitors (21, 44). Western immunoblots of A549 cytoplasmic extracts (Fig. 2) revealed expression of 37-kDa IκBα, 49-kDa IκBβ, and the p105 NF-κB1 precursor, as assessed by determining the appropriate molecular weight and ability to block staining upon preadsorption of the antibody with the appropriate peptide (reference 21 and data not shown]. We noted that a greater amount of cytoplasmic lysate was needed to detect these latter subunits, probably indicating either that the IκBα isoform is more abundantly expressed or that the antibodies have significant differences in affinity for the target antigen (see Discussion). We were unable to detect the alternatively spliced NF-κB1 COOH terminus, IκBγ, even though its precursor, p105, was expressed. To determine the effect of RSV infection on IκB abundance, A549 cells were infected with pRSV at an MOI of 1 for various times prior to cytoplasmic extraction and Western immunoblotting (Fig. 2A). A faintly detectable decrease in IκBα could be discerned at 3 h, and the level continued to decrease to 24 h, when 45% of the control signal remained. A similar pattern of proteolysis was observed for the IκBβ isoform. Finally, a transient decrement of p105 could be detected at 6 h, which gradually returned to control values by 24 h. The decline in IκBα and -β, but not p105, abundance exactly parallels the pattern of pRSV-induced nuclear C1/C2 DNA binding to the IL-8-inducible enhancer in EMSA (cf. Fig. 1) and is consistent with the requirement for IκB proteolysis in NF-κB translocation.
FIG. 2.
Expression and regulation of IκB isoforms. (A) Kinetics of IκB proteolysis following pRSV infection. A549 cells were infected with pRSV for 0 to 36 h, and cytoplasmic extracts were prepared. Results of Western immunoblotting using specific antibodies for IκBα, IκBβ, and p105 are shown. For IκBα, 200 μg of cytoplasmic protein was loaded for each time point; for IκBβ, 750 μg was loaded; and for p105, 500 μg was loaded. Relative to control levels, IκBα declined to 93% ± 3.4% (3 h), 84% ± 6% (6 h), 82% ± 3.6% (12 h), 46% ± 3.3% (24 h), or 44% ± 10.2% (36 h). No IκBγ isoform was detectable. (B) Kinetics of IκB proteolysis following rhTNF-α stimulation. A549 cells were stimulated with rhTNF-α for 0 to 4 h, and IκB isoforms were detected by Western immunoblotting. Proteins were normalized by using the Bio-Rad protein detection reagent, and equivalent loading was confirmed by staining a parallel SDS-PAG with Coomassie brilliant blue. A phosphorylated form of IκBα (IκBαP), migrating more slowly, was produced by rhTNF-α stimulation. ∗, an 85-kDa protein that specifically cross-reacts with anti-p105 antibody whose presence occurred in late passage A549 cells and probably represents p84NF-κB1, an alternatively spliced product of the p105 gene (19). Relative to control values, IκBα decreased 26% ± 2.6% (0.25 h), 54% ± 4.6% (0.5 h), 73% ± 3% (1 h), 80% ± 4% (2 h), and 85% ± 5% (4 h).
By contrast, in response to rhTNF-α, IκBα was rapidly and more completely proteolysed than that produced at any time by pRSV infection (Fig. 2B). For example, IκBα was 26% of control values at 15 min. In contrast to pRSV infection, IκBα reaccumulated during the course of the experiment, simultaneously with the appearance of a slower-migrating form, representing a serine-phosphorylated IκBα form (6). As with the changes observed in IκBα abundance, a rapid IκBβ proteolysis occurred within 15 min, but in contrast, IκBβ did not reaccumulate. The steady-state levels of p105 isoforms remained unchanged after rhTNF-α treatment. Like the effect of pRSV, the pattern of IκBα/β proteolysis in rhTNF-α-stimulated cells exactly matched the kinetics of nuclear C1/C2 DNA-binding activation (cf. Fig. 1).
pRSV infection induces changes in abundance of RelA-associated IκBα.
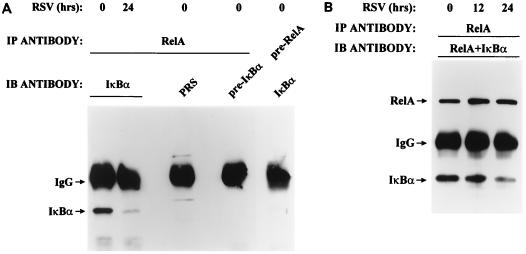
These data indicated that proteolysis of IκB subunits was temporally linked to RelA DNA-binding activation. To demonstrate whether IκBα associates with RelA and, if so, whether this association was lost as a result of pRSV infection, we used a two-step immunoprecipitation-Western immunoblot assay (21). In this assay, uninfected and infected A549 whole-cell lysate was prepared and RelA was immunoprecipitated under native conditions. RelA-associated IκBα was then detected in the immunocomplexes by Western immunoblotting using anti-IκBα antibody (Fig. 3A). IκBα immunostaining was not observed when preadsorbed RelA antibody was used in the primary immunoprecipitation or when either preadsorbed IκBα or preimmune serum was used in the secondary Western immunoblot analysis. Only when both anti-RelA (in the primary immunoprecipitation) and anti-IκBα (in the Western immunoblot analysis) antibodies were used sequentially could IκBα be detected. Thus, RelA is associated with IκBα in uninfected A549 cells.
FIG. 3.
Effect of pRSV infection on RelA-associated IκBα. (A) Determination of coimmunoprecipitation assay specificity. RSV-infected (0 or 24 h) WCEs were immunoprecipitated (IP) either with anti-RelA antibody or with preadsorbed RelA antibody (pre-RelA), and the immunoprecipitate was used for the detection of IκBα by Western blotting (IB) using anti-IκBα antibody, preimmune serum (PRS), or preadsorbed IκBα antibody (pre-IκBα). IgG, immunoglobulin G. (B) Degradation of RelA-associated IκBα by RSV infection. Uninfected or pRSV-infected A549 WCE (for 0, 12, or 24 h) was immunoprecipitated with RelA antibody. The immunoprecipitate was subjected to Western immunoblotting with both RelA and IκBα antibodies (Materials and Methods). After 24 h of pRSV infection, RelA-associated IκBα was significantly decreased. For the different time points, the IκBα/RelA ratio was 1.63 (0 h), 1.2 (12 h), and 0.9 (24 h), indicating that IκBα association with RelA falls following pRSV infection.
Further, the abundance of RelA-associated IκBα was significantly reduced upon RSV infection (compare 0- and 24-h pRSV infection in Fig. 3A). To control for a potential artifact of unequal RelA recovery, the two-step immunoprecipitation-Western immunoblot assay was repeated, the immunoblot being stained with both anti-IκBα and anti-RelA antibodies (Fig. 3B). For each lane, the ratio of IκBα to RelA signal was quantitated to give a relative measure of the IκBα associated with RelA. The IκBα/RelA ratio fell from 1.63 in control lysates to 1.2 at 12 h and by 24 h was 0.9. Therefore, the abundance of RelA-associated IκBα was unambiguously diminished following pRSV infection. We have been unable to detect significant amounts of IκBβ and p105 with RelA (data not shown), possibly because of their low abundance of expression or their preference for other members of the NF-κB family. Together these data indicate that the abundance of IκBα and its association with RelA fall upon pRSV infection.
Quantitation of IκBα protein t1/2 after pRSV infection.
To more precisely demonstrate that the decrease in IκBα abundance after pRSV infection occurred as a consequence of proteolysis, we directly measured the IκBα half-life (t1/2) in control, rhTNF-α-treated and 24-h pRSV-infected A549 cells. For this, changes in the abundance of IκBα were measured in the presence of the protein synthesis inhibitor CHX, a standard technique for determination of rapid IκBα turnover (26, 27). Relative changes in IκBα abundance were determined by comparing Western immunoblot signals by using identical amounts of cytoplasmic proteins under conditions where the changes in signal were linear to input protein (Fig. 4A). The time course of IκBα disappearance in the absence of new protein synthesis is shown for control, pRSV-infected, and rhTNF-α-treated cells (Fig. 4B; quantitation in Fig. 4C). In control cells, IκBα was stable, with a t1/2 that exceeded 120 min. By contrast, the turnover of IκBα was very rapid in rhTNF-α-stimulated cells, with an estimated t1/2 of less than 5 min. At 24 h of pRSV infection, the IκBα t1/2 was 90 min, an intermediate value that was shorter than control measurements yet markedly longer than the t1/2 seen after rhTNF-α treatment. To exclude the effects of potential artifacts of CHX on IκB turnover, relative differences in IκBα t1/2 were confirmed by using [35S]methionine pulse-chase-labeled cells (not shown). These data provide direct evidence that pRSV infection produces IκBα proteolysis and this is, in part, the mechanism for its disappearance during pRSV infection.
FIG. 4.
Turnover rates of IκBα. (A) Quantitation of Western immunoblot analysis. Indicated concentrations of cytoplasmic lysates (Cyto. Ext.; from control cells) were fractionated by SDS-PAGE, and levels of IκBα were detected by Western blotting using ECL. IκBα bands were quantitated by scanning densitometry (in arbitrary units [AU]) as 102,667 AU (400 μg of extract), 81,071 AU (200 μg), 54,839 AU (100 μg), 35,708 AU (50 μg), 7,503 AU (25 μg), and 3,891 AU (12.5 μg). Between 12.5 and 200 μg of protein, a linear dose-response profile was obtained (r2 = 0.93). (B) pRSV- and rhTNF-α-induced changes in IκBα levels. CHX-treated uninfected (control), pRSV-infected, and rhTNF-α-treated A549 cells were harvested at the indicated times. The autoradiogram shows a Western blot of IκBα. For pRSV-infected cells, cells infected for 24 h were used. (C) Quantitation of t1/2 (graphical representation of IκBα degradation taken from Fig. 5A). IκBα signals were scanned, quantitated, and expressed as percentage of initial signal (0-h cycloheximide). Each point in the curve is mean ± standard error of three independent experiments.
pRSV infection produces a delayed (and blunted) RelA:IκBα positive feedback loop.
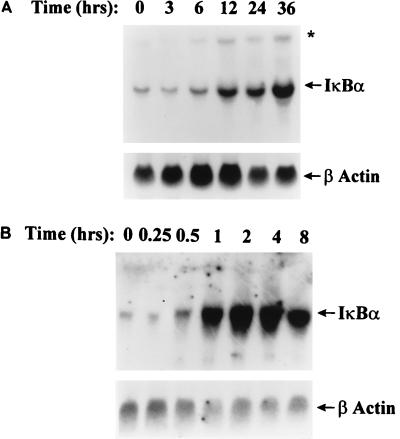
Infection with pRSV produced a disappearance of IκBα in A549 cells without appreciable resynthesis, in spite of only weakly inducing IκBα turnover (Fig. 2A). This was somewhat surprising because others have shown that in response to nuclear RelA translocation, a rapid activation of the iκbα gene expression results in IκBα protein resynthesis and termination of nuclear RelA activation (21, 43). Indeed, this phenomenon occurred in A549 cells upon rhTNF-α administration because 30 min following rhTNF-α treatment, cytoplasmic IκBα reaccumulated; this IκBα resynthesis temporally coincided with termination of nuclear C1/C2 DNA binding (Fig. 1 and 2B). To determine whether the RelA:IκBα positive feedback loop was operative in pRSV-infected cells, we compared the magnitude and kinetics of expression of IκBα mRNA to those produced by rhTNF-α (Fig. 5). Upon pRSV infection, IκBα mRNA abundance increased 3-fold at 24 h and 5.5-fold at 36 h relative to control (uninfected) cells. By contrast, rhTNF-α produced a 25-fold increase in IκBα mRNA by 2 h, which subsequently fell to a 13-fold increase at 8 h (the IκBα mRNA increase preceded increases in cytoplasmic IκBα protein abundance [cf. Fig. 2B and 5B]). Notably, levels induced by pRSV were significantly less than those produced by rhTNF-α treatment. These data indicate that the RelA:IκBα positive feedback loop was less strongly activated by pRSV infection and is inadequate to produce significant reaccumulation of the protein.
FIG. 5.
RelA:IκBα positive feedback loop. (A) Induction of IκBα mRNA by pRSV infection. IκBα and β-actin mRNA levels were detected by Northern blotting in pRSV-infected cells for indicated times between 0 and 36 h (top). IκBα cDNA hybridized with 1.8- and 2.7-kb (∗) mRNA species (the latter representing a form containing a longer 3′ untranslated region), both of which were increased in parallel by RSV infection. Compared to its level at 0 h, IκBα mRNA was increased 2.5-fold at 12 h, 3-fold at 24 h, and 5.5-fold at 36 h. (B) Induction of IκBα mRNA by rhTNF-α. A549 cells were stimulated with rhTNF-α for indicated times between 0 and 8 h (top). Relative to the control, in rhTNF-α-treated cells, IκBα mRNA increased 13-fold (1 h), 25-fold (2 h), 20-fold (4 h), and 13.2-fold (8 h).
Distinct mechanisms for intracellular IκBα proteolysis.
A large body of work has indicated that the ubiquitin-26S proteasome pathway is a major, if not the sole, mechanism for inducible proteolysis of the IκBα inhibitor (1, 12, 33). However, our data indirectly argued for differences in the proteolytic pathway between rhTNF-α stimulation and viral infection. The distinct kinetics of proteolysis and the absence of phosphorylated IκBα isoforms in cells infected with pRSV (Fig. 2) prompted us to further examine the proteolytic mechanism(s) involved. We therefore examined whether selective 26S proteasome inhibitors could block IκBα proteolysis in pRSV-infected A549 cells. These inhibitors included lactacystin, a Streptomyces metabolite that is a potent and irreversible inhibitor of the mammalian subunit X (MB1) of the proteasome and has been shown to be a highly selective cell permeable proteasome inhibitor without measurable inhibition of protein kinase C, thrombin, plasminogen activator, or cytoplasmic calpains (references 12 and 31 and our unpublished data), and the peptide aldehydes Z-LLF-CHO and MG-132, which are highly potent inhibitors of proteasome activity in cells (10, 38).
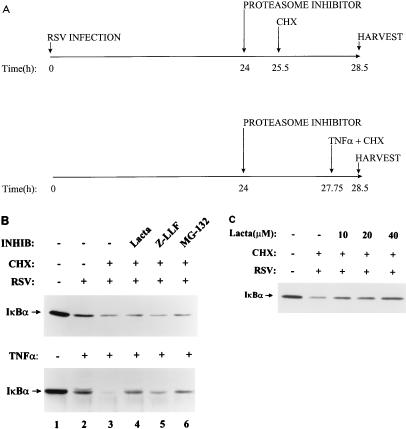
A549 cells were separately preincubated with effective doses of proteasome inhibitors and subsequently stimulated with 20 ng of rhTNF-α per ml. To isolate the effect of proteolysis without the confounding phenomenon of IκBα resynthesis, these studies were done in the presence of the protein synthesis inhibitor CHX (schematically diagrammed in Fig. 6A). rhTNF-α produced complete proteolysis of IκBα when protein resynthesis was blocked (Fig. 6B, bottom; compare lane 1 with lane 3). Concomitant administration of lactacystin, Z-LLF-CHO, or MG-132 significantly blocked rhTNF-α-induced IκBα proteolysis (compare lanes 4, 5, and 6 with lane 3). In these blots, the IκBαP isoform is easily detected. In contrast, equivalent doses and incubation times of proteasome inhibitors had no detectable effect on IκBα degradation by pRSV infection (Fig. 6B, top; compare lanes 4, 5, and 6 with lane 3) and the IκBαP form was not detected. To exclude a major contribution of the proteasome, the experiment was repeated with pRSV-infected cells treated with lactacystin at concentrations of 10 to 40 μM (Fig. 6C), well above its effective dose of 1 μM (31). At 40 μM, well above the saturation dose required for inhibiting the rhTNF-α effect (10 μM), a minor inhibition by lactacystin was seen.
FIG. 6.
Effect of 26S proteasome inhibitors on IκBα proteolysis. (A) Experimental strategy showing time line of sequence of CHX or protease inhibitor addition and cell harvest. Due to the slower turnover of IκBα protein in pRSV-infected cells, 3 h of CHX treatment was used to detect proteolysis. To control for potential lability of the inhibitor, the rhTNF-α experiments were designed so that protease inhibitors were added for the same length of time. (B) Western immunoblot of IκBα proteolysis. The upper section shows the effect of proteasome inhibitors on pRSV-induced IκBα proteolysis. Cell infected with pRSV for 24 h were incubated with the indicated proteasome inhibitors (INHIB) (10 μM lactacystin [Lacta], 10 μM Z-LLF-CHO [Z-LLF], and 25 μM MG-132) for 1.5 h prior to adding CHX (50 μg/ml). Cytoplasmic IκBα was detected by Western blotting. Relative to uninfected cells (lane 1, taken as 100%), pRSV-infected CHX treatment reduced IκBα to 37.5% ± 7.9% of the initial signal; the addition of lactacystin (lane 4; 36.9% ± 4.5% of control values) or Z-LLF (lane 5; 32.7% ± 6.5% of control) had no effect in three separate experiments (not significant); the addition of MG-132 (lane 6) also had no effect (38.2% ± 5.9% of control values, n = 3, not significant). The lower section shows the effect of proteasome inhibitors on rhTNF-α-induced IκBα proteolysis. To control for potential lability of proteasome inhibitors, in this experiment, before the brief rhTNF-α treatment (0.75 h), cells were preincubated with the inhibitors for 3.75 h (the same duration as for the pRSV treatment). Cytoplasmic IκBα was detected by Western blotting. Relative to control cells (lane 1, taken as 100%), rhTNF-α–CHX treatment reduced IκBα to 17.5% ± 1.0%, lactacystin produced an increase in IκBα abundance to 60% ± 1.4% of the control value (n = 3, P < 0.0003), Z-LLF also increased IκBα abundance to 38.7% ± 0.14% of the control value (n = 3, P < 0.0103), and MG-132 increased IκBα abundance to 45.7% ± 0.17 (n = 3, P < 0.0024). Similar results were obtained with proteasome inhibitors added immediately prior to rhTNF-α stimulation. (C) Dose response of IκBα proteolysis to increasing concentrations of proteasome inhibitor. Increasing concentrations of lactacystin (indicated at the top) were used to block pRSV-inducible IκBα proteolysis. Relative to control cells, IκBα abundance decreased 41% (0 μM lactacystin), 54% (10 μM lactacystin), 51% (20 μM lactacystin), and 67% (40 μM lactacystin).
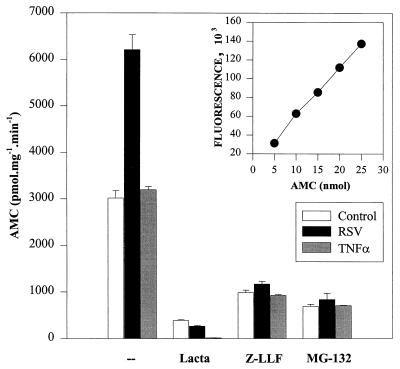
To unambiguously interpret the effect of the proteasome inhibitors on inducible degradation of IκBα, we directly measured 26S proteasome activity in control cells and in cells either after rhTNF-α stimulation or 24 h after pRSV infection (Fig. 7). For this, a standard assay monitoring hydrolysis of the fluorogenic substrate Suc-Leu-Leu-Val-Tyr-AMC was performed with cytoplasmic A549 cell lysates. Figure 7 demonstrates that the fluorescence emission intensity at 440 nm (Iex, 380 nm) is linear over 5 to 25 nm of product, a range used in the measurement of the A549 cell lysates. In control and rhTNF-α-treated cells, cell extracts hydrolyzed 3,019 ± 161.6 and 3,199 ± 67.8 pmol of Suc-Leu-Leu-Val-Tyr-AMC substate · mg−1 · min−1 (30°C) respectively. Surprisingly, compared to the control, pRSV infection increased total proteasome activity twofold to 6,210 ± 322.68 pmol · mg−1 · min−1 (30°C) (P < 0.0001, Student’s t test). The irreversible inhibitor lactacystin inhibited proteasome activity in all samples to less than 12% of control values. Z-LLF-CHO and MG-132 also inhibited activities of the proteasome to similar degrees in all samples but were less potent than lactacystin. These data provide direct evidence that although proteasome activity is increased by pRSV infection, the inhibitors lactacystin, Z-LLF-CHO, and MG-132 block its activity to the same extent as for the control or rhTNF-α-treated cells yet are largely ineffective in preventing pRSV-induced IκBα proteolysis. We therefore conclude that although the proteasome is involved in rhTNF-α-induced IκBα proteolysis, a distinct proteolytic pathway is involved in pRSV-induced IκBα proteolysis.
FIG. 7.
26S proteasome activity after treatment and in the presence of proteasome inhibitors. Control, pRSV-infected, or rhTNF-α-treated A549 cells were incubated in the absence or presence of 10 μM lactacystin (Lacta), 10 μM Z-LLF-CHO (Z-LLF), or 25 μM MG-132 for 4 h. After 3.5 h of preincubation, cells were stimulated with rhTNF-α for 0.5 h. Proteasome activities were measured in the cytoplasmic extracts, using the fluorogenic substrate Suc-Leu-Leu-Val-Tyr-AMC (37). Results are expressed as the amount of AMC formed by the enzymatic cleavage of substrate. The inset represents a standard curve for AMC drawn by measuring the fluorescence of known quantities of AMC.
DISCUSSION
The airway epithelium, forming a physical barrier between the extracellular environment and the intracellular milieu, represents the major target for RSV replication. Through its ability to activate the expression and subcellular abundance of transcription factors controlling cytokine gene expression, the virally infected epithelium initiates a cytokine cascade responsible for initiating the host response, including recruitment of inflammatory mononuclear cells into the peribronchial mucosa. Although a number of studies have reported the activation of NF-κB family members in respiratory epithelial cells following pRSV infection in vitro (4, 13, 17, 29), a systematic evaluation of the expression and regulation of tissue-specific IκB isoforms has not been reported. In this report, we show that the IκBα, IκBβ, and p105 are expressed and differentially regulated by ligand (TNF-α) and infectious (pRSV) NF-κB inducers. Surprisingly, although the proteasome is responsible for IκBα proteolysis in many cell lines, including TNF-α-mediated activation in A549 cells, we provide evidence for an independent proteolytic pathway involved in IκBα turnover following RSV infection.
Kinetics and magnitude of NF-κB activation following pRSV infection.
We and others have demonstrated an increase in NF-κB DNA-binding activity following pRSV infection (4, 13, 17, 29) and a simultaneous increase in the nuclear abundance of the potent transcriptional activator RelA (17). In this study, using side-by-side comparisons with the effects of rhTNF-α, a slower (and lower level of) NF-κB activation is seen following pRSV infection, where a 2.2-fold increase in NF-κB DNA-binding activity occurs over a 3- to 24-h period postinfection. Moreover, subtle differences in C1/C2 complex binding can also be seen, indicating quantitative effects in the NF-κB induction profile. Other investigators, using nonpurified viral preparations, have shown a biphasic effect for NF-κB activation (14), where an initial transient, replication-independent increase in NF-κB binding is seen 30 to 90 min postinfection, followed by a nadir and a gradual reaccumulation of NF-κB after 24 h. As a marker for NF-κB activation, the early 2-h peak is associated with a burst of IL-8 gene expression (14). Taking equivalent time points (in data not shown), we do not see a consistent biphasic NF-κB activation pattern, nor is there a biphasic profile of IL-8 expression in highly purified preparations of RSV (this study and reference 17). It should be emphasized that the highly purified RSV preparations lack any measurable NF-κB-activating cytokine such as IL-1, known to be produced in abundance in pRSV-infected A549 cells (34), which may explain the discrepancy in our observations. We recognize, of course, that during RSV infection in vivo, both cytokine and direct viral effects act in concert and are likely to be operative in NF-κB induction. Nevertheless, our studies on RelA activation and IκB proteolysis in vitro are relevant only for the later, viral replication-dependent, phase of NF-κB activation.
Expression and regulation of distinct IκB isoforms.
We have previously shown the expression of the IκBα, -β, and -γ but not p105 isoforms in hepatocytes (21), and in this study, IκBα, IκBβ, and p105, but not IκBγ, were expressed in airway epithelial cells. Encoded by separate genes and containing differences in the number of ankyrin repeat domains, IκBα and -β are widely expressed, binding to and inactivating the NF-κB members RelA and c-Rel with slightly different affinities (8, 11, 22, 44). In our detection system, the level of IκBβ expression is so low that RelA-associated IκBβ is not detectable by coimmunoprecipitation, leading us to conclude that IκBα abundance is likely to be the major determinant of RelA subcellular localization in these cells. It still remains formally possible that there is a significant difference in the affinities of the two IκB antibodies; additional studies will be required to unambiguously clarify this issue. Similarly, p105, the unprocessed precursor of the 50-kDa NF-κB1, also contains ankyrin repeats and can function in cytoplasmic retention/inactivation of RelA. However, p105 is also expressed at low levels and is largely inert to stimulation, indicating that 105 processing is also irrelevant to the mechanism of NF-κB activation by these agents.
This study, our previous work (21), and work of others (10) have shown that IκBα and -β proteolysis is rapid (within 15 min) and coordinated, rather than sequential (44) in response to TNF-α. Following pRSV infection, IκBα and -β disappear in parallel but over a much longer period of time, requiring 24 h for peak effect. Over the duration of this experiment, the magnitude of IκBα proteolysis is consistently less than that produced by rhTNF-α, and the degree of protein turnover is less rapid (t1/2 of 90 min for pRSV and <5 min for rhTNF-α). At this time period and MOI of initial infection, however, >90% of the cells are infected by RSV. These data account for the weaker NF-κB DNA-binding activation following pRSV infection and indirectly argue that the mechanisms for activation of IκB proteolysis may differ between the two stimuli.
The RelA:IκBα positive feedback loop in pRSV infection.
Hormonal stimuli producing IκBα proteolysis and RelA translocation activate an autoregulatory pathway (RelA:IκBα positive feedback loop [24, 42]). In this pathway, nuclear RelA binds reiterated NF-κB sites to activate robust expression of the IκBα gene promoter; IκBα resynthesis then recaptures RelA and terminates its activity. We show that in rhTNF-α-treated A549 cells, the IκBα gene is robustly stimulated, resulting in a 25-fold increase in steady-state levels of mRNA (Fig. 4B). Therefore, the IκBα gene is highly inducible in A549 cells. By contrast, pRSV infection stimulates only a 5.5-fold increase in IκBα mRNA, an amount insufficient to replace proteolyzed IκBα. This phenomenon may be due to the relatively weak NF-κB activation induced by pRSV infection, since the rate of IκBα proteolysis is much less than that produced by rhTNF-α, and thus a greater turnover rate of IκBα protein cannot account for the insufficient RelA:IκBα positive feedback loop. We do not think that insufficient IκB gene expression can be ascribed to a nonspecific toxic effect of viral replication because (i) β-actin mRNA continues to be expressed (Fig. 5A); (ii) increases in IκBα, as well as other proteins (25), continue to be observed in cells 24 to 36 h postinfection (Fig. 5A); and (iii) IL-8 mRNA continues to increase in abundance during this time (17).
Nonproteasome pathway involved in pRSV-induced IκBα proteolysis.
The multicatalytic proteinase complex (proteasomes) are high-molecular-mass (∼700-kDa) proteases involved in turnover of unstable cellular proteins, cell cycle progression, and major histocompatibility complex class I processing and have more recently been implicated in the inducible degradation and processing of NF-κB family members including p105 and IκBα (1, 12, 33, 37; reviewed in reference 32). TNF-induced IκBα proteolysis is mediated through a two-step process whereby the IκBα NH2 terminus is inducibly phosphorylated at serine residues 32 and 36 by a ubiquitously expressed kinase complex, targeting the newly phosphorylated IκBα (IκBαP) for polyubiquitination and proteolysis (7, 36). That the proteasome pathway mediates IκBαP degradation comes from the use of selective proteasome inhibitors such as lactacystin, Z-LLF-CHO, and MG-132, all of which are potent inhibitors of its inducible proteolysis (10, 12, 33). Our studies show that addition of proteasome inhibitors results in IκBαP accumulation (Fig. 6) and significant inhibition of TNF-α-inducible proteolysis under conditions where we demonstrate directly a nearly complete inhibition of proteasome activity. It is important to note that total proteasome activity is unaffected by rhTNF-α treatment. This observation strongly argues that a prior step, e.g., phosphorylation-ubiquitination, is the rate-limiting event in IκBα proteolysis.
We were surprised to observe an increase in total proteasome activity in A549 cells following pRSV infection. Although modulators of 26S proteasome activity, including both activators and inhibitors of purified proteasome activity in vitro (9, 35, 39), have been described, changes in total proteasome activity in response to respiratory viral infection have not been reported to our knowledge. This activity may be a homeostatic response for the cell, e.g., to turn over viral proteins or enhance major histocompatibility complex class I expression and viral antigen presentation (15).
A surprising and novel finding in this study is the independence of pRSV-induced IκBα proteolysis from proteasome activity. Under conditions where proteasome activity is 90% inhibited by three different proteasome inhibitors (to values less than those measured in control cells), IκB proteolysis continues to occur in pRSV-infected cells. The nature of the pRSV-inducible IκB protease will be of interest to characterize. In data not shown, the activity is not inhibited by serine protease inhibitors (PMSF), cysteine protease inhibitors (E64 and E64C), or the calpain inhibitor calpeptin and presently is enigmatic. This activity could represent a cytoplasmic or lysosomal protease or an as yet uncharacterized protease encoded by the RSV genome (the kinetics of IκB proteolysis coincides with the pattern of RSV protein expression). We think it unlikely that pRSV induces a proteasome activity with unique substrate specificity that is not assayed by the fluorogenic peptide and whose activity is not affected by conventional proteasome inhibitors. Finally, we note that there is no detectable accumulation of IκBαP isoforms in pRSV-infected cells. Thus, it may be possible that inducible phosphorylation is not required for IκBα proteolysis following pRSV infection.
In summary, we describe a central role for IκBα proteolysis in control of NF-κB (RelA) activation following pRSV infection. Although in response to the cytokine TNF-α, IκBα is only transiently proteolyzed via the proteasome and subsequently reaccumulates as a consequence of the RelA:IκBα positive feedback loop, in the pRSV-infected cell, significant differences are observed. In spite of a slower rate of IκBα proteolysis, IκBα is not significantly resynthesized, and its proteolysis occurs independently of proteasome activity. Identification of the IκBα proteolytic pathway activated by RSV infection may result in efficacious therapies to prevent airway hyperreactivity in acutely infected individuals and reduce morbidity in children with cardiopulmonary disease.
ACKNOWLEDGMENTS
We thank E. J. Corey, Harvard University, for the gift of lactacystin and Mark Suto, Signal Pharmaceuticals, for the gift of Z-LLF-CHO.
This project was supported by grants from the NIAID (1 R01 AI40218-01A1 to A.R.B. and AI/HL 15939-14A1 to R.P.G.), Child Health and Human Development (R30HD 27841), and NIEHS (P30 ES06676 to R. S. Lloyd, UTMB). A.R.B. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent IκBα phosphorylation marks the NF-κB inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold R, Humbert B, Werchaus H, Gallati H, Konig W. Interleukin-8, interleukin-6, and soluble tumor necrosis factor receptor type I released from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82:126–133. [PMC free article] [PubMed] [Google Scholar]
- 3.Beg A A, Baldwin A S., Jr The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 4.Bitko V, Velazquez A, Yank L, Yang Y, Barik S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology. 1997;232:369–378. doi: 10.1006/viro.1997.8582. [DOI] [PubMed] [Google Scholar]
- 5.Brasier A R, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo R. A promoter recruitment mechanism for TNFα-induced IL-8 transcription in type II pulmonary epithelial cells: dependence on nuclear abundance of RelA, NF-κB1 and c-Rel transcription factors. J Biol Chem. 1998;273:3551–3561. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 6.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 7.Chen J Z, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 8.Chu Z-L, McKinsey T A, Liu L, Qi X, Ballard D W. Basal phosphorylation of the PEST domain in IκBβ regulates its functional interaction with the c-Rel proto-oncogene product. Mol Cell Biol. 1996;16:5974–5984. doi: 10.1128/mcb.16.11.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu-Ping M, Willy P J, Slaughter C A, DeMartino G N. PA28, an activator of the 20S proteasome, is inactivated by proteolytic modification at its carboxy terminus. J Biol Chem. 1993;268:22514–22519. [PubMed] [Google Scholar]
- 10.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobrzanski P, Ryseck R-P, Bravo R. Differential interactions of Rel-NF-κB complexes with IκBα determine pools of constitutive and inducible NF-κB activity. EMBO J. 1994;13:4608–4616. doi: 10.1002/j.1460-2075.1994.tb06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler M A, Wernke-Dollries K, Stark J M. Inhibition of viral replication reverses respiratory syncytial virus-induced NF-κB activation and interleukin-8 gene expression in A549 cells. J Virol. 1996;70:9079–9082. doi: 10.1128/jvi.70.12.9079-9082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiedler M A, Wernke-Dollries K, Stark J M. Mechanism of RSV-induced IL-8 gene expression in A549 cells before viral replication. Am J Physiol. 1996;271:L963–L971. doi: 10.1152/ajplung.1996.271.6.L963. [DOI] [PubMed] [Google Scholar]
- 15.Garofalo R, Mei F, Espejo R, Ye G, Haeberle H, Baron S, Ogra P L, Reyes V E. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-β and IL-1α. J Immunol. 1997;157:2506–2513. [PubMed] [Google Scholar]
- 16.Garofalo R, Ogra P L. Mechanisms of mucosal immunopathology in respiratory syncytial virus infection. In: Kagnoff M, Kiyono H, editors. Essentials of mucosal immunology. San Diego, Calif: Academic Press; 1996. pp. 405–420. [Google Scholar]
- 17.Garofalo R, Sabry M, Jamaluddin M, Yu R K, Casola A, Ogra P L, Brasier A R. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol. 1996;70:8773–8781. doi: 10.1128/jvi.70.12.8773-8781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groothuis J R, Gutierre K M, Lauer B A. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics. 1988;82:199–203. [PubMed] [Google Scholar]
- 19.Grumont R J, Fecondo J, Gerondakis S. Alternate RNA splicing of murine nfkb1 generates a nuclear isoform of the p50 precursor NF-κB1 that can function as a transactivator of NF-κB-regulated transcription. Mol Cell Biol. 1994;14:8460–8470. doi: 10.1128/mcb.14.12.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall C B, McCarthy C A. Respiratory syncytial virus. In: Mandel G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingston; 1995. pp. 1501–1519. [Google Scholar]
- 21.Han Y, Brasier A R. Mechanism for biphasic RelA:NF-κB1 nuclear translocation in tumor necrosis factor α-stimulated hepatocytes. J Biol Chem. 1997;272:9823–9830. doi: 10.1074/jbc.272.15.9825. [DOI] [PubMed] [Google Scholar]
- 22.Hatada E N, Naumann M, Scheidereit C. Common structural constituents confer IκB activity to NF-κB p105 and IκB/MAD-3. EMBO J. 1993;12:2781–2788. doi: 10.1002/j.1460-2075.1993.tb05939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue J, Kerr L D, Kakizuka A, Verma I M. IκB-γ, a 70 kDa protein identical to the C-terminal half of p110:NF-κB: a new member of the IκB family. Cell. 1992;68:1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 24.Itoh N, Kazantsev A G, Baldwin A S., Jr Three NF-κB sites in the IκB-α promoter are required for induction of gene expression by TNFα. Nucleic Acids Res. 1994;22:3787–3792. doi: 10.1093/nar/22.18.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamaluddin M, Garofalo R, Ogra P L, Brasier A R. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J Virol. 1996;70:1554–1563. doi: 10.1128/jvi.70.3.1554-1563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanno T, Brown K, Franzoso G, Siebenlist U. Kinetic analysis of human T-cell leukemia virus type I Tax-mediated activation of NF-κB. Mol Cell Biol. 1994;14:6443–6451. doi: 10.1128/mcb.14.10.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacoste J, Petropoulos L, Pepin N, Hiscott J. Constitutive phosphorylation and turnover of IκBα in human T-cell leukemia virus type I-infected and Tax-expressing T cells. J Virol. 1995;69:564–569. doi: 10.1128/jvi.69.1.564-569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald N E, Hall C B, Suffin S C. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982;307:397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- 29.Mastronarde J G, He B, Monick M M, Mukaida N, Matsushima K, Hunninghake G W. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-κB and NF-IL-6. J Infect Dis. 1996;174:262–267. doi: 10.1093/infdis/174.2.262. [DOI] [PubMed] [Google Scholar]
- 30.Noah T L, Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol. 1993;265:L472–L478. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- 31.Omura S, Fujimoto T, Otoguro K, Matsuzaki K, Moriguchi R, Tanaka H, Sasaki Y. Lactacystin: a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. J Antibiot. 1990;44:113–118. doi: 10.7164/antibiotics.44.113. [DOI] [PubMed] [Google Scholar]
- 32.Pahl H, Baeuerle P A. Control of gene expression by proteolysis. Curr Opin Cell Biol. 1996;8:340–347. doi: 10.1016/s0955-0674(96)80007-x. [DOI] [PubMed] [Google Scholar]
- 33.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 34.Patel J A, Kunimoto M, Sim T C, Garofalo R, Eliott T, Baron S, Ruuskanen O, Chonmaitree T, Ogra P L, Schmalstieg F C., Jr IL-1α mediates enhanced expression of ICAM-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol. 1995;13:602–609. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- 35.Realini C, Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Molecular cloning and expression of a gamma-interferon-inducible activator of the multicatalytic protease. J Biol Chem. 1994;269:20727–20732. [PubMed] [Google Scholar]
- 36.Regnier C H, Song H, Gao X, Goeddel D V, Gao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 37.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 38.Saito T, Deskin R W, Casola A, Haeberle H, Olzewska B, Ernst P B, Alam R, Ogra P L, Garofalo R. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 39.Seeger M, Ferrell K, Frank R, Dubiel W. HIV-1 tat inhibits the 20S proteasome and its 11S regulator-mediated activation. J Biol Chem. 1997;272:8145–8148. doi: 10.1074/jbc.272.13.8145. [DOI] [PubMed] [Google Scholar]
- 40.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 41.Stadnyk A W. Cytokine production by epithelial cells. FASEB J. 1994;8:1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 42.Sun S C, Ganchi P A, Ballard D W, Greene W C. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 43.Sun S C, Ganchi P A, Beraud C, Ballard D W, Greene W C. Autoregulation of the NF-κB transactivator RelA (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc Natl Acad Sci USA. 1994;91:1346–1350. doi: 10.1073/pnas.91.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 45.Traenckner E BM, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2882. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinitsky A, Michaud C, Powers J, Orlowski M. Inhibition of the chymotrypsin-like activity of the pituitary multicatalytic proteinase complex. Biochemistry. 1992;31:9421–9428. doi: 10.1021/bi00154a014. [DOI] [PubMed] [Google Scholar]
- 47.Virca G D, Northemann W, Shiels B R, Widera G, Broome S. Simplified northern blot hybrdization using 5% sodium dodecyl sulfate. BioTechniques. 1990;8:370–371. [PubMed] [Google Scholar]
- 48.Webb M C, Henry R L, Milner A D. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child. 1985;60:1064–1067. doi: 10.1136/adc.60.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]