Abstract
Aims
Recent trial data demonstrate beneficial effects of active rhythm management in patients with atrial fibrillation (AF) and support the concept that a low arrhythmia burden is associated with a low risk of AF-related complications. The aim of this document is to summarize the key outcomes of the 9th AFNET/EHRA Consensus Conference of the Atrial Fibrillation NETwork (AFNET) and the European Heart Rhythm Association (EHRA).
Methods and results
Eighty-three international experts met in Münster for 2 days in September 2023. Key findings are as follows: (i) Active rhythm management should be part of the default initial treatment for all suitable patients with AF. (ii) Patients with device-detected AF have a low burden of AF and a low risk of stroke. Anticoagulation prevents some strokes and also increases major but non-lethal bleeding. (iii) More research is needed to improve stroke risk prediction in patients with AF, especially in those with a low AF burden. Biomolecules, genetics, and imaging can support this. (iv) The presence of AF should trigger systematic workup and comprehensive treatment of concomitant cardiovascular conditions. (v) Machine learning algorithms have been used to improve detection or likely development of AF. Cooperation between clinicians and data scientists is needed to leverage the potential of data science applications for patients with AF.
Conclusions
Patients with AF and a low arrhythmia burden have a lower risk of stroke and other cardiovascular events than those with a high arrhythmia burden. Combining active rhythm control, anticoagulation, rate control, and therapy of concomitant cardiovascular conditions can improve the lives of patients with AF.
Keywords: Atrial fibrillation, Artificial intelligence, Biomarkers, Heart failure, Atrial cardiomyopathy, Cognitive function, Dementia, Outcomes, Quality of care, Cost, Research, Rhythm management, Catheter ablation, Anticoagulation, Bleeding, Research priorities, Technology, Stroke, Integrated care, Screening, AFNET, EHRA, Guidelines, Consensus statement
Graphical Abstract
Graphical Abstract.
What’s new?
Recent evidence suggests important improvements to the management of patients with one atrial fibrillation (AF).
Active rhythm management should be part of the default initial treatment for patients with AF.
Patients with device-detected AF have a low burden of AF and a low risk of stroke. Anticoagulation prevents some strokes and also increases major but non-lethal bleeding.
More research is needed to improve stroke risk prediction in patients with AF, especially in those with a low AF burden. Biomolecules, genetics, and imaging can support this.
In summary, combining active rhythm control, anticoagulation, rate control, and therapy of concomitant cardiovascular conditions can improve the lives of patients with AF.
Introduction
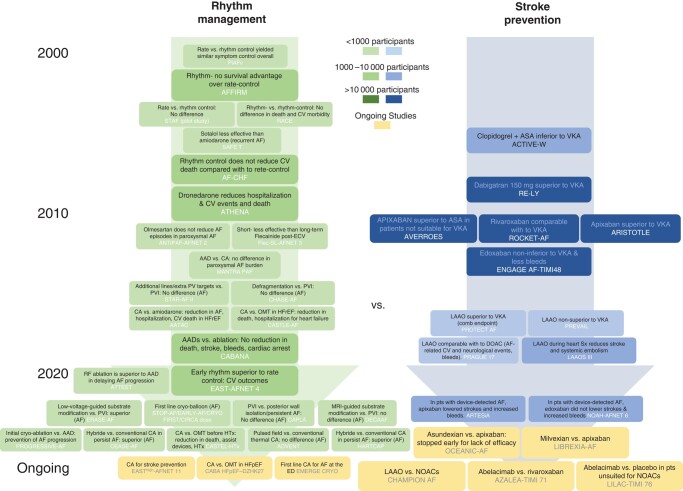
The year 2023 is the first year since 2011 in which three hot line presentations of clinical trials in patients with atrial fibrillation (AF) were presented at the annual congress of the European Society of Cardiology (ESC) and simultaneously published in the New England Journal of Medicine (CASTLE-HTx, ADVENT, and NOAH-AFNET 6).1–3 In November 2023, ARTESiA was presented and published.4 Unlike in 2011, when the focus was on anticoagulation,5–7 two of the trials presented at ESC evaluated AF ablation,2,3 the most effective method for active rhythm management. The two other large trials, although primarily assessing the efficacy and safety of anticoagulation in patients with device-detected AF, found a low stroke risk in a population with risk factors and a very low AF burden, highlighting the possible role of arrhythmia burden for stroke risk.1,4 This shift from evaluating the effect of anticoagulation towards evaluating active rhythm control management in clinical trials highlights the recent growth in this clinical area (Figure 1). From 11–13 September 2023, experts from academia and industry met for the 9th AFNET/EHRA consensus conference of the Atrial Fibrillation NETwork (AFNET) and the European Heart Rhythm Association (EHRA) to discuss these recent findings. To acknowledge the 20th anniversary of AFNET, the 9th AFNET/EHRA consensus conference was held in Münster, Germany, the home of AFNET.
Figure 1.
Timeline of landmark trials in atrial fibrillation management on active rhythm management (left) and stroke prevention (right) from 2000 until today. The studies are colour-coded based on their size (from light to dark: <1000, 1000–10 000, and >10 000 participants), and ongoing studies are shown in orange. AAD, antiarrhythmic drugs; AF, atrial fibrillation; CA, catheter ablation; CV, cardiovascular; ECV, electrical cardioversion; ED, emergency department; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; Htx, heart transplantation; LAAO, left atrial appendage occlusion; OMT, optimal medical treatment; NOACs, novel oral anticoagulant drugs; PVI, pulmonary vein isolation; VKA, vitamin K antagonist.
Methods
The 9th AFNET/EHRA consensus conference brought together 83 international interdisciplinary experts including arrhythmia and heart failure specialists, pharmacologists, basic and translational scientists, general practitioners, neurologists, nurse practitioners, epidemiologists, clinical trialists, and health economists in Münster, Germany on 11–13 September 2023. The conference started with four sessions of expert talks summarizing recent developments in the field. Thereafter, the participants split into six breakout groups to discuss specific topics. Each break-out group summarized their thoughts and statements on posters and presented them to the plenary. These were discussed and adapted in poster walk-through sessions. The consensus summarized here integrates this iterative, intensive dialogue in each group and in the plenum, using formal and informal feedback. Refinement of the consensus and integration of new data4,8,9 was done during the writing process. Details of the methodology have been described before.10–13
Active rhythm management: from symptom control to outcome reduction
Atrial fibrillation guidelines recommend active rhythm control to improve symptoms in patients with AF. Since the release of the 2020 ESC AF guidelines, new data indicate that patients with recent onset AF and stroke risk factors14 and those with heart failure with reduced ejection fraction have better cardiovascular outcomes on rhythm control therapy.3,15,16 This evidence supports the use of early rhythm control irrespective of symptoms. The trials do not show safety signals associated with rhythm control. The safety of modern rhythm control is confirmed in analyses of large electronic health records.17–19 Furthermore, the risk of stroke is low in patients with risk factors and a very low burden of device-detected AF (see ‘Atrial fibrillation burden in patients with electrocardiogram-diagnosed atrial fibrillation and in patients with device-detected atrial fibrillation’ section). This suggests that a reduction in arrhythmia burden could explain the outcome-reducing effect of rhythm control therapy. The concept of AF burden reduction as a component of treating patients with AF has been highlighted in the recent 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of AF.20 Taking this in context with earlier trials such as ATHENA,21,22 the group sees a paradigm shift that moves rhythm control from a symptom-improving ‘lifestyle therapy’ to an outcome-reducing treatment to reduce stroke, heart failure, and, to a lesser extent, acute coronary syndrome and cardiovascular death.
Identification of patients suitable for rhythm control
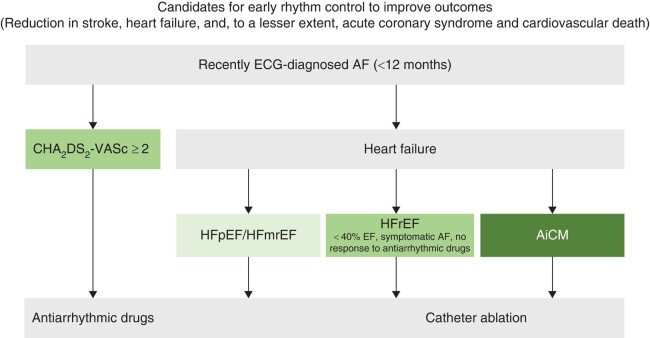
Currently, only a small minority of patients with AF are treated with rhythm control therapy. Based on the outcome-reducing effects of early rhythm control and AF ablation, patients with AF should, by default, undergo at least one attempt of active rhythm control (Figure 2). This may include a ‘diagnostic cardioversion’ to unmask AF-related symptoms and arrhythmia-induced cardiomyopathy. Left ventricular dysfunction should probably encourage rhythm control.3,15,16,23 Early rhythm control reduced outcomes in patients with heart failure.23 Atrial fibrillation ablation reduced cardiovascular events compared with medical therapy in two randomized trials, CASTLE-AF and CASTLE-HTx,3,15 and in a pre-specified sub-analysis of the CABANA trial.24 A few patients, who experience a good symptom control by rate control alone and in whom preventing cardiovascular events is no longer relevant (for example due to limited life expectancy or advanced age), may opt to not receive rhythm control therapy.
Figure 2.
Candidates for early rhythm control to improve outcome. Colours represent the quality and availability of data (from dark for best quality and availability of data to light for the worst quality and availability of data). AF, atrial fibrillation; AiCM, arrhythmia induced cardiomyopathy; HFrEF, heart failure with reduced ejection fraction; HFpEF/HFmrEF, heart failure with preserved or mid-range ejection fraction; RF, risk factors.
Role of atrial fibrillation ablation for delivering early rhythm control
The outcome-reducing effect of early rhythm control was achieved using antiarrhythmic drugs in most patients.25 Attaining sinus rhythm was the main mediator of outcome reduction in EAST-AFNET 4.26 The EAST-AFNET 4 trial also showed that early and systematic rhythm control is effective across AF patterns, including paroxysmal AF, persistent AF, and first-diagnosed AF.27 Antiarrhythmic drugs remain a key component of rhythm control therapy. Atrial fibrillation ablation reduced symptoms,28 psychological distress,29 and arrhythmia burden30 more than antiarrhythmic drug therapy. Ongoing and planned trials are evaluating whether AF ablation can also reduce cardiovascular events [CABA-HFPEF DZHK27 trial (NCT05508256), EASThigh-AFNET 11, and others].
Improving atrial fibrillation ablation
Pulmonary vein isolation (PVI) remains the main target for AF ablation. The STAR-AF II,31 CAPLA,32 and DECAAF II33 studies showed that empiric placement of additional ablation lines or magnetic resonance–guided ablation of fibrotic areas does not improve AF rhythm outcome after AF ablation compared with PVI alone. Several smaller recent trials comparing additional AF ablation targets to PVI only, including ERASE-AF,34 showed a mix of neutral outcomes and improved prevention of recurrent AF. Additional studies, such as COAST AF (NCT03347227) and STAR-AF III (NCT04428944), will further evaluate additional ablation strategies on top of PVI. Recent randomized trials evaluating hybrid AF ablation combining surgical and endocardial ablation approaches, including CEASE-AF35 (71.6% vs. 39.2%) and HARTCAP36 (89% vs. 41%), showed good sinus rhythm maintenance without increased procedural complications in patients with persistent AF who have more recurrences of AF after PVI.37,38
Pulsed field ablation (PFA), a non-thermal energy source, conceptually targets cardiomyocytes and may spare other cell types. This conceptual advantage does not translate into better rhythm control in the ADVENT trial.2 So far, there are very few reports of oesophageal complications or phrenic nerve injuries persisting past hospital discharge, comparable with cryo-balloon-based PVI,39 and major complications (pericardial tamponade, stroke, and stroke resulting in death) appear low at 1.6%.40 More data are collected to define the efficacy and safety of PFA as an energy source for AF ablation.
New antiarrhythmic drugs
Despite the advances in ablation therapy, there remains an unmet need for effective and safe antiarrhythmic drugs. Such compounds will need to demonstrate improvements compared with existing drugs that show good efficacy and safety when used in appropriate patients.14 The development of antiarrhythmic agents has declined over the last decades,41 but several promising compounds targeting ion channels are currently in clinical development (Table 1). Small conductance Ca2+-activated K+ (SK) channels are up-regulated in patients with AF.42 In a Phase 2 proof-of-concept study, a relatively selective SK-channel blocker successfully met efficacy and safety endpoints for pharmacological cardioversion of patients with recent-onset AF.43 A Phase 1 study for a second-generation oral lead compound (AP31969) for sinus rhythm maintenance is leading to the planning of a Phase 2 study in patients with implantable loop recorders. HSY244 is a novel antiarrhythmic drug with the undisclosed mechanism of action and has been evaluated concerning efficacy for cardioversion of AF. The programme was terminated in 2023 based on business decisions (NCT04582409). HBI-3000 is a multi-channel blocker, which was well tolerated in the Phase 1 clinical trial and is currently investigated in a Phase 2 trial for acute intravenous cardioversion of patients with recent-onset AF (NCT04680026). An oral multi-channel amiodarone analogue with a relatively short elimination half-life, known as budiodarone, was successfully investigated in PASCAL, a Phase 2 study in patients with recurrent AF documented with pacemakers, and awaits further development. Additional, ongoing work aims to develop inhalable formulations of antiarrhythmic drugs and the repurposing of drugs approved for other indications (e.g. oral doxapram, colchicine, and metformin and injection of botulinum toxin type A into epicardial fat pads) as antiarrhythmic drug therapy in patients with AF (Table 1). Ranolazine is approved as an antianginal agent in Europe, and in the USA, it is also approved for the management of long QT3 syndrome. It is a late sodium current inhibitor with a minor inhibitory effect on the HERG current. It is being used, often in combination with amiodarone, for the suppression of AF recurrences.
Table 1.
New antiarrhythmic drugs and new formulations of existing antiarrhythmic drugs in development
| Novel antiarrhythmic agents | ||||
|---|---|---|---|---|
| Agent (developer) | Main antiarrhythmic target | Indication | Formulation | Current clinical status |
| AP30663 | Small conductance calcium-activated potassium (SK) channel blocker | Cardioversion of recent-onset AF | Intravenous | Phase 2 completed (NCT04571385) |
| AP31969 | Small conductance calcium-activated potassium (SK) channel blocker | Sinus rhythm maintenance | Oral | Phase 1 ongoing |
| HSY244 | Undisclosed | Cardioversion of recent-onset AF | Intravenous | Phase 2 terminated (business decision) (NCT04582409) |
| HBI-3000 (sulcardine) |
Multi-channel blocker | Cardioversion of recent-onset AF (>2 and <72 h) | Intravenous | Phase 2 ongoing (NCT04680026) |
| Budiodarone | Multi-channel blocker | Sinus rhythm maintenance | Oral | Phase 2 completed (PASCAL) |
| Botulinum toxin A | Cholinergic neurotransmission blocker | Prevention of postoperative AF | Injection around ganglionated plexuses | Phase 2 (NCT01842529; NOVA) |
| Reformulation of already approved AADs | ||||
|---|---|---|---|---|
| Antiarrhythmic drug | Main antiarrhythmic target | Indication | Reformulation | Current clinical status |
| Flecainide | Sodium channel blocker | Cardioversion of recent-onset symptomatic AF | Inhalation solution | Phase 2 terminated (NCT05039359; RESTORE-1) Phase 3 currently on hold (NCT03539302; INSTANT) |
| Repurposing of already approved medications | ||||
|---|---|---|---|---|
| Agent (developer) | Main antiarrhythmic target | Indication | Formulation | Current clinical status |
| Bucindolol | Beta 1 adrenergic blockade and receptor reduction | Sinus rhythm maintenance | Oral | Phase 2 completed (NCT01970501; GENETIC-AF) |
| Doxapram | TASK 1 inhibitor | Cardioversion of recent-onset AF | Intravenous | Phase 2 ongoing (EudraCT 2018-002979-17; DOCTOS) |
| Ranolazine | Late sodium channel blocker with minor effect on HERG channel | Sinus rhythm maintenance following cardioversion Reduction in AF burden in paroxysmal AF: Ranolazine and dronedarone given alone and in combination |
Oral Oral |
Phase 2 completed (NCT01534962; RAFFAELLO) Phase 2 completed (NCT01522651) |
Published results of completed studies are explained in more details, including references, in the text.
Rate control drugs and ablate and pace
Rate control therapy remains an important component of rate and rhythm management in patients with AF.10 The concept of rate control, enabling better cardiac function by slowing and regularizing ventricular rate during episodes of AF, remains unchanged. Almost all rhythm control trials are conducted against a background therapy of rate control,14 typically using beta-blockers, calcium channel antagonists, and digitalis glycosides.44,45 Medical rate control therapy should be part of active rhythm management in patients with AF, considering the rate-controlling effects of several antiarrhythmic drugs, including amiodarone, dronedarone, propafenone, and sotalol. The recent APAF-CRT trial showed that there is a role for rate control using a pace-and-ablate strategy in symptomatic heart failure patients with permanent AF to improve clinical outcome.46 Ablate-and-pace therapy should be considered when rhythm control therapy is unsuccessful.47 After AV-node ablation, patients become pacemaker dependent, which may lead to pacing-induced cardiomyopathy.48 Technical improvements increased the interest in conduction system pacing (CSP).49 Conduction system pacing might be the most appropriate pacing mode for avoiding the development of pacing-induced cardiomyopathy50 and is already used in patients treated with ablate-and-pace rate control.51 Outcome studies are planned or ongoing (CONDUCT-AF, LBBAP-AFHF, RAFT-P&A, and others) and reviewed elsewhere.52
Practical considerations and summary
Most patients with AF should undergo at least one attempt of active rhythm management during the first year after AF is diagnosed. In patients with AF and concomitant heart failure with reduced ejection fraction, rhythm management should be introduced as a fifth pillar on top of the established ‘fantastic 4’ [an angiotensin receptor–neprilysin inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist, and a sodium-glucose co-transporter (SGLT) 2 inhibitor] for comprehensive heart failure management strategies.53 In light of the emerging role of AF therapy in heart failure patients, electrophysiologists with knowledge in rhythm control, AF ablation, and ablate-and-pace therapies should be an integral part of heart failure teams.
Knowledge gaps and opportunities
Quantification of arrhythmia burden, number, and duration of recurrent episodes is needed to better understand the emerging link between AF burden and cardiovascular events. The outcome-reducing effects of rhythm control therapy may be mediated by reducing AF burden and attaining sinus rhythm. This questions the relevance of the primary outcome of older rhythm control trials and time to the first AF recurrence.3,15,16
Antiarrhythmic drugs and AF ablation exert synergistic rhythm-controlling effects.54,55 More research is needed evaluating the effectiveness of antiarrhythmic drugs in the context of AF ablation.
Most of the clinical trials evaluating rhythm control therapy so far were conducted in relatively young patients with AF. The outcome-reducing effect of early rhythm control therapy, in contrast, was most pronounced in patients with AF and a high comorbidity burden (CHA₂DS₂-VASc score 4 or more).23 There is a clear unmet need to evaluate rhythm control, including AF ablation, in patients with AF and a high comorbidity burden.
More research is needed to define the best methodology to deliver rhythm control therapy for all, integrating innovations in antiarrhythmic drug therapy and AF ablation.
Future research in the field of rate control should focus on patient selection and timing of ablate-and-pace therapy. Studies that include multiple arms with AF ablation compared with AV-node ablation with CSP are necessary to guide clinical practice.
Quantification of AF burden in drug trials, especially in trials of heart failure and in trials of new antiarrhythmic drugs, is needed to determine their effect on the association of AF burden reduction and prevention of AF-related outcomes.
Atrial fibrillation burden in patients with electrocardiogram-diagnosed atrial fibrillation and in patients with device-detected atrial fibrillation
Longer rhythm monitoring durations lead to a higher likelihood of detecting rare and short AF episodes, thereby increasing the number of patients with AF.56 This has been conceptually described in earlier iterations of the AFNET/EHRA consensus conference.57 Intermittent electrocardiogram (ECG) recordings or 24-h ambulatory ECG monitors detect fewer patients with AF than continuous rhythm monitoring, mainly diagnosing AF in patients with a high arrhythmia burden.56,58,59 The growing availability of consumer electronics capable of detecting and quantifying arrhythmia episodes will make such information more widely available in the near future.60–62
Device-detected atrial fibrillation and electrocardiogram-documented atrial fibrillation
This group, including investigators from NOAH-AFNET 6 and ARTESiA, recommends to use the term ‘device-detected AF’ in preference to ‘atrial high-rate episodes’ and ‘sub-clinical AF’. This recommendation is based on the following observations: A careful, core-lab–based analysis of all episodes leading to inclusion into NOAH-AFNET 6 revealed that 97% of these episodes showed all signs of AF.63 Despite small differences in sensitivity and specificity, the algorithms for recognition of device-detected AF are mature and have been validated and refined over time.64 The differences in outcomes, e.g. the lower rate of stroke, between device-detected AF and ECG-documented AF is unlikely to be related to differences in the signals recorded during an episode. The term device-detected AF has been used by others after the publication of NOAH-AFNET 6 and ARTESiA9,65 and can simplify thinking and discussion around this phenomenon.
It remains unclear which patient and what AF burden merits oral anticoagulation to prevent AF-related complications such as death, stroke, thromboembolism, and other morbidities or mortality. At present, the distinction between ECG-documented AF and device-detected AF draws a boundary that has developed historically. Electrocardiogram-documented AF selects patients with a high AF burden. In the absence of documented ECG-diagnosed AF, randomized clinical trials performed so far have failed to demonstrate a benefit of therapy with oral anticoagulation, including patients with embolic stroke of undetermined source,66,67 patients with heart failure,68,69 or patients with atrial cardiomyopathy, but without AF.70 Existing evidence that shows the effectiveness of oral anticoagulation in patients with paroxysmal and persistent AF is based on trials enrolling patients with ECG-diagnosed AF (often requiring at least two AF ECG documentations within 1 year as an inclusion criterion, which likely represents a high AF burden). The STROKESTOP study randomized (1:1) > 75-year-olds to be invited to screening for AF by a handheld ECG 2×/day for 2 weeks or to a control group.71 Treatment with oral anticoagulants upon ECG documentation of AF reduced the primary combined endpoint of ischaemic or haemorrhagic stroke, systemic embolism, bleeding leading to hospitalization, and all-cause death {hazard ratio 0.96 [95% confidence interval (CI) 0.92–1.00]; P = 0.045}. Studies initiating anticoagulation based on AF detection by continuous rhythm monitoring by implantable cardiac monitors in the LOOP study72 or based on device-detected AF found by 24/7 rhythm monitoring via implantable cardiac devices in NOAH-AFNET 61 and ARTESiA4 found a low event rate without anticoagulation, including a rate of stroke of 1%/year.9 A sub-analysis from NOAH-AFNET 6 suggests that the low rate of stroke without anticoagulation extends to patients with long episodes of only device-detected AF ≥ 24 h.8 The absolute treatment effects were the prevention of three strokes and an increase of seven to 16 major bleeds per 1000 patient-years.9 These effects are probably not sufficient to recommend anticoagulation in patients with device-detected AF. Refined classifications of AF patterns73 and ideally AF burden should be incorporated in outcome trials investigating the efficacy of modern rhythm control strategies.
Continuous (e.g. by implantable loop recorders) or semi-continuous (e.g. by wearable digital devices) long-term rhythm monitoring can provide information on AF burden, number of AF episodes, and duration of longest AF episode.74 The average burden of device-detected AF in the absence of ECG-documented AF is low in patients with multiple comorbidities (0.13% median AF burden in LOOP75). Recent data from the RACE V registry suggest that paroxysmal AF has a higher burden that can be further differentiated into subgroups.76,77 While continuous rhythm monitoring is the preferred method to evaluate AF burden, serial longer-term monitor10,78 or even long-term intermittent monitoring by recording one to three short-term handheld ECGs per day provides effective, albeit less precise alternative methods.79 So far, these rhythm monitoring methods have mainly been used in research settings.80–82
Practical considerations and summary
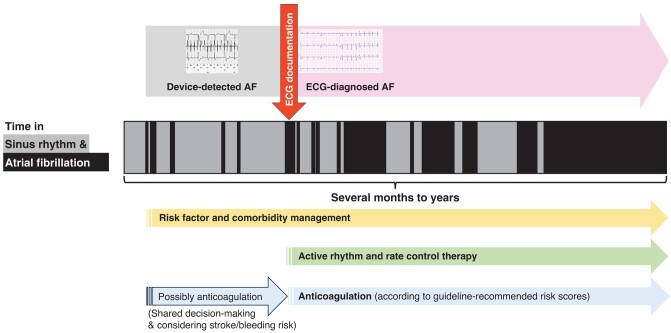
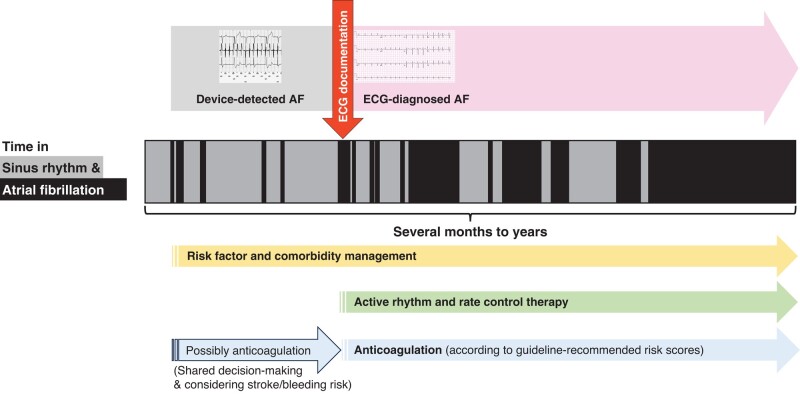
This group believes that ECG-diagnosed AF should be differentiated from device-detected AF in clinical care (Figure 3). The biological rationale is most likely a higher arrhythmia burden in patients with ECG-documented AF.43 Electrocardiogram-documented AF remains a reason to initiate anticoagulation,10 provide active rhythm management (see above), and treat cardiovascular comorbidities. Detection of device-detected AF by implanted devices should, in contrast, trigger ECG monitoring to diagnose AF. Treatment of concomitant conditions that can lead to AF can be intensified upon detection of device-detected AF to prevent AF progression. The outcomes of NOAH-AFNET 61 and ARTESiA4 with a small reduction of ischaemic stroke in the context of a low overall rate of stroke and with an expected increase in major but non-fatal bleedings provide solid information for a shared decision process on anticoagulation in selected patients with device-detected AF.
Figure 3.
Device-detected compared with electrocardiogram-diagnosed atrial fibrillation in relation to atrial fibrillation progression (which can take several months to years) from very low to very high burden. Electrocardiogram documentation can be achieved using single-lead electrocardiograms or 6- to 12-lead electrocardiograms using accepted definitions of atrial fibrillation. Electrocardiogram documentation of atrial fibrillation is a simple, clinically operable method to identify patients with a high arrhythmia burden. AF, atrial fibrillation; ECG, electrocardiogram.
Knowledge gaps and hurdles
The amount of AF that distinguishes low and high burden of AF, the interaction of arrhythmia burden with comorbidities, and the best methods to quantify AF burden require more research. To estimate this, research needs to include quantification of arrhythmia burden in patients with paroxysmal AF with an assessment of the extent of AF burden reduction achieved by active rhythm management and how this relates to prevention of AF-related outcomes.83
Reducing AF burden is an emerging therapeutic goal in patients with AF based on this consensus document. Similar thoughts can be found in the recently published ACC/AHA/HRS AF guidelines.20 The best methods to reduce AF burden in patients with different AF patterns and clinical situations need to be determined.
Uncertainty also remains about the best management of patients with arrhythmias detected by wearables and handheld devices. These devices semi-continuously monitor rhythm, enable an estimation of arrhythmia burden, and are used by increasing numbers of individuals.10,74,84
This group believes that quantifiable markers for AF-related disease processes are needed to enable this research. Further research is needed to explore the interaction between the number and severity of stroke risk factors, arrhythmia burden, and individual risk.
Another important line of research should describe the range of arrhythmia progression and regression patterns found in patients and factors identifying patients who are unlikely to experience progression to ECG-documented AF.
Based on the NOAH-AFNET 6 sub-study in patients with device-detected AF episodes ≥ 24 h,8 it remains unclear whether detection of device-detected AF episodes ≥ 24-h duration is equivalent to progression to ‘clinical’ AF.
The clinical relevance and utility of AF patterns and AF progression and regression detected by long-term rhythm monitoring need to be better understood to guide personalized treatments for AF.
Finally, more precise methods are needed to identify patients with device-detected AF at risk of stroke.
Improved stroke prevention
The current clinical assessment of stroke risk using the CHA₂DS₂-VASc score85 is limited by several factors, including the following:
modest predictive ability of contemporary risk prediction scores with the potential for over-/under-treatment due to imprecise risk estimation86 and variable stroke rates across different populations (leading to inaccurate assessment of risk/benefit)87,88;
the emergence of newer therapeutic interventions, such as early rhythm control therapy,14 left atrial appendage removal or closure,89 and others65 that reduce stroke risk without having systemic antithrombotic effects; and
the emergence of ‘lower risk’ AF populations not considered by traditional risk prediction schema, illustrated by patients with device-detected AF who show a relatively low stroke risk despite older age and multiple comorbidities.
These recent developments reinforce earlier calls90 for improved and dynamic risk stratification schemes to re-evaluate the decision to use anticoagulants. Atrial fibrillation burden, concentrations of circulating biomolecules, and cardiovascular imaging parameters (e.g. atrial cardiomyopathy) have shown potential to improve and refine stroke risk prediction. At the same time, direct evidence is accumulating that AF therapy not only reduces stroke but also reduces heart failure events and cardiovascular death.3,14
Clinical risk factors
Clinical stroke risk factors, summarized as the CHA₂DS₂-VASc score, are clinically used to start oral anticoagulation in patients with AF. Consideration of additional clinical features such as chronic kidney disease, tobacco use, ventricular hypertrophy,91 hypertrophic cardiomyopathy, amyloid, and other inherited cardiac conditions may offer further discriminative ability.
Genetic risk
Initiated by the pioneering work in the population of Iceland,92 a large body of data science now provides robust risk scores for AF and stroke based on genetic information.93,94 These scores allow us to quantify AF and stroke risk with a five-fold range between the lowest-risk and highest-risk sub-populations.93,94 Genetic risk alleles have been associated with recurrent AF on rhythm control therapy,95,96 and AF risk scores can be used to predict the effectiveness of early rhythm control therapy.97 Stroke risk can be refined by using genetic risk scores for stroke98,99 and especially genetic changes related to both stroke and AF.100 Recent data suggest that the genetic risk for AF overlaps with the genetic risk for heart failure, especially when rare variants are considered.
Atrial fibrillation burden
As discussed above, AF burden emerges as a promising modulator of stroke risk. Early rhythm management reduces cardiovascular events, including a numerical 30% reduction in ischaemic stroke, in anticoagulated patients.14 This effect is of a comparable magnitude to surgical removal of the left atrial appendage during open heart surgery.89
Biomolecules
Circulating biomolecules play an important role in the diagnosis and management of patients with cardiovascular disease.101 Several biomolecules have shown an independent added value for risk stratification in patients with AF.86 The biomarker-based ABC-AF stroke and bleeding risk scores [Age, Biomarkers (N-terminal pro-B-type natriuretic peptide, troponin, haemoglobin, and GDF-15), Clinical history of stroke/TIA or bleeding in Atrial Fibrillation] improve prediction of stroke and bleeding.86 Newer biomolecules that can be accurately quantified include fibroblast growth factor 23 and bone morphogenetic protein 10 (BMP10).102 Elevated concentrations of these biomolecules are associated with prevalent102 and recurrent AF103,104 and with AF-related outcomes.105
Imaging
Atrial cardiomyopathy summarizes the histologic and anatomical disease processes that may lead to the development of AF, contribute to its recurrence and progression, and potentially enhance the risk of AF-related cardiovascular events. Left atrial size, a simple integral of atrial cardiomyopathy, has been variably associated with stroke and systemic embolism. Anticoagulation did not prevent strokes in patients with atrial cardiomyopathy, but without AF (ARCADIA),70 adding to the evidence that AF is a required interacting factor for atrial cardiomyopathy to create a stroke risk. Atrial fibrosis, which can be visualized using late gadolinium enhancement cardiac magnetic resonance imaging, has been associated with an increased risk for major adverse cardiovascular and cerebrovascular events in patients with AF, primarily driven by increased risk for the occurrence of stroke or transient ischaemic attack.106 More recently, echocardiographic parameters of left atrial function, including left atrial strain and left atrial appendage flow velocity, have been proposed as refined methods to quantify atrial cardiomyopathy and as risk modulators in patients with AF.
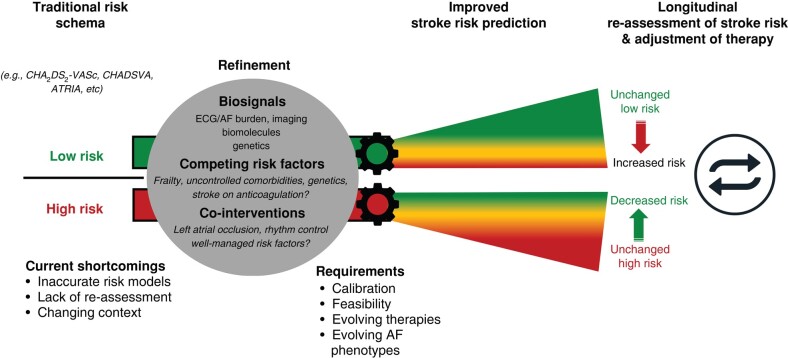
Longitudinal reassessment of risk and adjustment of therapy
Most patients with ECG-documented AF should be on oral anticoagulation to reduce their risk of stroke. Atrial fibrillation is a dynamic disease, progressing and regressing from self-terminating to sustained arrhythmia episodes.107 Stroke risk increases with age or in the context of disease progression and new comorbidities. Stroke risk will decrease with early rhythm control,14 especially when sinus rhythm is attained,26 with better treatment of concomitant cardiovascular conditions, or with spontaneous regression of AF burden.
There is a residual risk of ischaemic stroke despite anticoagulant therapy (1–2%/year in the pivotal randomized controlled trials), calling for augmented therapy.65,108,109 Patients experiencing a stroke on anticoagulation can potentially benefit from a call to A-C-T-I-O-N to improve outcome.110 Sub-optimal treatment of comorbidities and treatment with anticoagulation and low, untested, and non-approved doses111 may contribute to stroke and cardioembolism.109 The effects of LAAOS III89 and EAST-AFNET 414 highlight the potential of treating atrial causes of stroke in patients with AF experiencing a stroke on anticoagulation. Whether novel FXIa inhibitors,112,113 a distinct new class of drugs under investigation for thrombosis prevention, improve outcomes in patients with AF will be evaluated in ongoing registration trials. Phase 3 trial of asundexian in patients with AF has recently been stopped early due to lack of efficacy.114 Trials with other compounds are ongoing.
Practical considerations and summary
Guideline-recommended risk prediction schemes are useful to guide the initial decision for oral anticoagulation. Genetic risk scores, imaging, and circulating biomolecules may be able to refine this initial assessment. Longitudinal assessment of dynamic risk modulators integrating AF burden, atrial myopathy, and circulating biomolecules of cardiovascular and inflammatory origin can improve risk prediction. Such dynamic risk assessment can result in intensified and combination therapies and in de-escalation of therapy (Figure 4).
Figure 4.
Refined risk assessment resulting in improved stroke risk prediction compared with traditional risk schemas. A longitudinal re-assessment of stroke risk may trigger adjustment of therapy (intensified therapies or de-escalation of therapy). AF, atrial fibrillation; ECG, electrocardiogram.
Knowledge gaps and research opportunities
Research is needed to evaluate novel quantitative risk predictors, including AF-burden, circulating biomolecules, imaging, and genetic markers and their effect on improving prediction of stroke risk, and prediction of the risk of other AF-related complications such as heart failure and cardiovascular death.
Randomized clinical trials to prospectively evaluate biomarker-based risk scores for therapy selection and proteomic screening to better understand the pathophysiology of AF complications are ongoing.
Evaluation of organ-specific atrial (e.g. BMP10) and cerebral health (e.g. neurofilament light chain polypeptide) biomolecules is ongoing with promising initial results. Biomolecules may also provide quantitative proxies for cardiac and atrial fibrosis.
In addition to new randomized trials, individual participant data meta-analysis will generate adequate power to assess the risks and benefits of anticoagulation in patients at different risks.115 This is currently addressed in a collaborative effort of the AF SCREEN and the AFFECT-EU consortia as well as in COMBINE-AF.116
Stroke risk also appears low after AF ablation.117–124 Randomized studies such as OCEAN125 (NCT02168829) will determine whether the stroke risk after successful AF ablation is sufficiently reduced to withhold oral anticoagulation. REACT-AF (NCT05836987) will assess smartwatch-guided anticoagulation.
Risk factor and comorbidity management for secondary prevention of atrial fibrillation
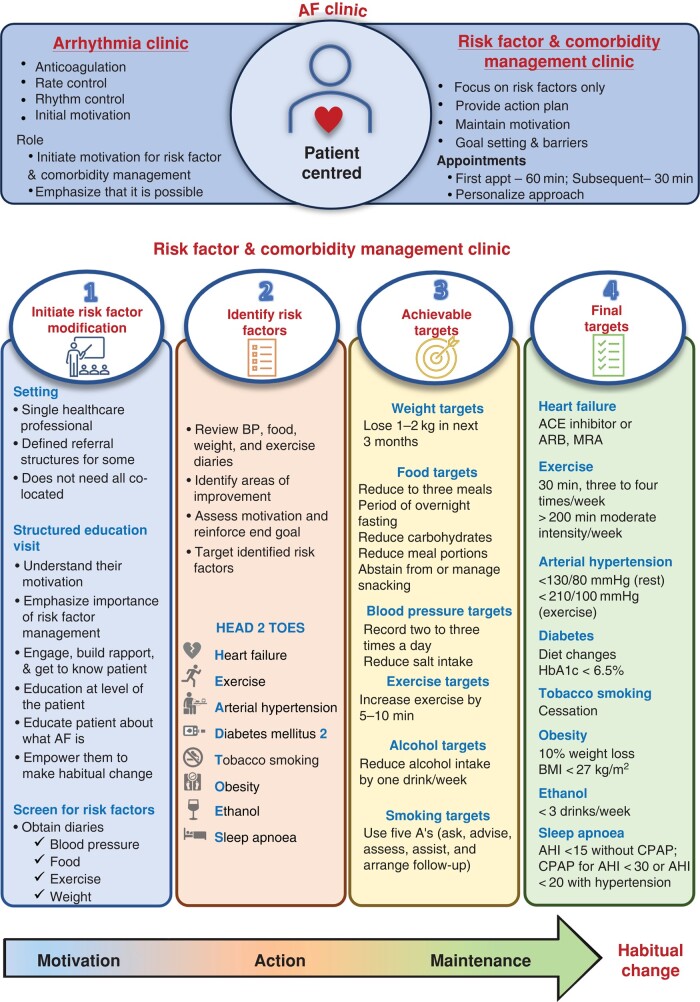
A healthy lifestyle and effective treatment of concomitant cardiovascular conditions, often embedded in integrated care pathways, improve maintenance of sinus rhythm and quality of life,126–130 in addition to the outcome-reducing effect in larger populations.131,132 Managing individual risk factors in isolation, such as excessive alcohol consumption, can improve AF outcomes.133 Similarly, behavioural weight loss126,127,130 and bariatric surgery can prevent AF outcomes in severely obese individuals.134–138 Weight loss–inducing glucagon-like peptide (GLP)-1 receptor antagonists, e.g. orforglipron, semaglutide, and tirzepatide, can reduce cardiovascular events and may reduce AF in obese populations.139–142 While regional patterns of care will vary, integrated risk factor and comorbidity management clinics and specialists can improve the prevention and treatment of concomitant conditions in patients with AF.127,130 The concept of risk factor and comorbidity management for secondary prevention of AF can be exemplified by the ‘Adelaide’ model: In Adelaide, the risk factor and comorbidity management clinic are separated from the AF clinic,143 while both services share a unified messaging emphasizing the importance of treatment of concomitant cardiovascular conditions. This risk factor and comorbidity management clinic have a single healthcare professional who uses academic detailing and structured education visits to build rapport, educate, engage and empower individuals to make informed decisions, set achievable goals, and monitor progress towards habitual behavioural change. Comorbidity treatment and risk factor modification can follow the ‘HEAD 2 TOES’ acronym (Figure 5),144 enhanced by treatment of coronary artery disease and valvular disease.145 While a single healthcare professional (not a ‘village’ of co-located healthcare professionals) primarily manages most aspects of risk factor and comorbidity management, appropriate referrals may be used as required and available. Remote consulting and digital health approaches incorporated in such referral structures may not replace but will support these inter-disciplinary referral structures for the management of comorbidities in patients treated in established AF clinics.146,147 Importantly, genetic testing may be valuable for identifying underlying conditions in young patients without apparent identifiable factors, which may have not yet manifested as cardiomyopathies (see prior sections).148 Pharmacological treatment of type-2 diabetes with SGLT2 inhibitors (Odds ratio 0.83, 95% CI 0.68–1.01)149 or GLP-1 receptor agonists (Relative risk 0.86, 95% CI 0.76–0.97),150 hypertension, vascular disease, and importantly of heart failure will have AF-reducing effects in addition to the outcome-reducing effects of these medications,151,152 including treatment with SGLT2 inhibitors153–157 and with finerenone.158
Figure 5.
A risk factor and comorbidity management clinic according to the ‘Adelaide’ model: the risk factor and comorbidity management clinic is separated from the atrial fibrillation clinic and has a single healthcare professional who (i) initiates risk factor modification, (ii) identifies risk factors according to HEAD 2 TOES, (iii) sets achievable goals, and (iv) monitors progress towards habitual change. AF, atrial fibrillation; BMI, body mass index.
Practical considerations and summary
The presence of AF, probably including device-detected AF, should trigger treatment of concomitant cardiovascular conditions. To improve universal access and adoption of these treatments,159 the participants of the 9th AFNET/EHRA consensus conference propose to implement integrative risk management clinics to improve this treatment domain in patients with AF.
Knowledge gaps and hurdles
Lifestyle improvement interventions and pharmacological treatments need to be tested at scale.160 A large randomized controlled study spanning different geographies and healthcare models focusing on hard endpoints such as mortality, stroke, and hospitalization and equally cost-effectiveness measures such as quality-adjusted life-year is needed.161
Local institutional infrastructures and funding models have been identified as barriers to implementing risk factor and comorbidity management clinics in a recent survey.159 The H2020 consortium EHRA-PATHS (EU grant agreement ID: 945260) aims to develop new systematic care pathways for the management of AF-related comorbidities across Europe.162
Little is known about the direct antiarrhythmic properties of novel heart failure medications: Their antiarrhythmic mechanisms are not well understood and their effect on AF and AF-related outcomes requires robust quantification. Prospective trials are needed and ongoing.
A comparative study of GLP-1 receptor therapy and surgical and and behavioural weight loss is needed to determine their relative antiarrhythmic effectiveness, safety, and cost-effectiveness.
Furthermore, whether phenotyping of patients with AF may allow appropriate characterization of AF and identification of possible underlying causes that have specific treatment (e.g. hypertrophic cardiomyopathy—myosin inhibitors) requires further research.
Artificial intelligence in the detection and management of atrial fibrillation and stroke
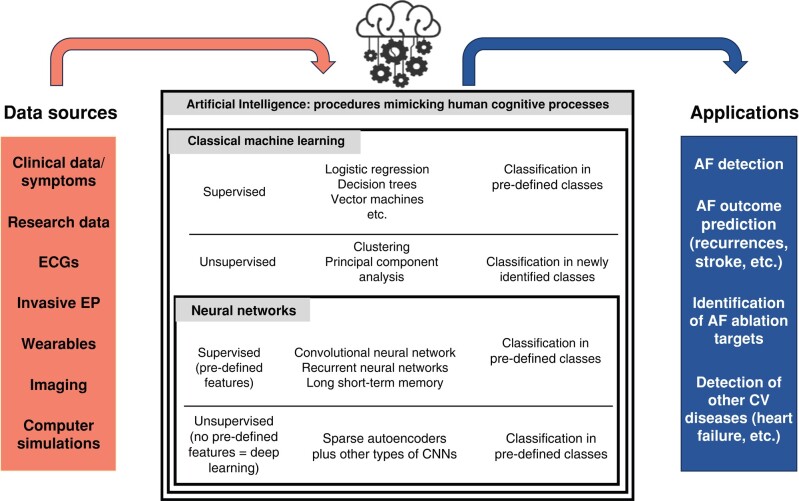
Since the 6th AFNET/EHRA consensus conference, artificial intelligence (AI) and modern data science techniques have been a topic during each AFNET/EHRA consensus conference.10,12,163 There has been progress in the research implementation of AI and the provision of explainable AI to improve stroke prevention, rhythm management, and comorbidity management.164,165 Artificial intelligence consists of supervised and unsupervised methodologies. In supervised learning, the output or target is defined (e.g. recognition of a sinus rhythm or AF on the ECG). The learning process uses labelled data sets to solve classification and regression or prediction problems. In unsupervised learning, there is no prediction of any output or need for labelled data.165 Data are sub-divided into classes that were not pre-specified and that are agnostic to the purpose of the investigation. An important domain of AI is machine learning, of which deep learning is an important sub-domain.164 Deep learning is typically a feedforward artificial neural network, where each node is an activation function that can produce an output signal if the sum of the inputs exceeds a certain threshold level.165 These techniques are often used for classification purposes based on unspecified features extracted from imaging data or ECGs. In Table 2 and Figure 6, the various groups of AI techniques, their dominant features and outputs, and potential applications are summarized.
Table 2.
Various groups of artificial intelligence
|
Supervised machine learning
Decision trees, support vector machines, random forest, boosted trees, etc. |
Unsupervised machine learning
Clustering, anomaly detection, dimensionality reduction, principal component analysis |
Supervised deep learning
Convolutional neural networks, recurrent neural networks, transformers, etc. |
Unsupervised deep learning
Autoencoders or generative adversarial networks |
|
| Feature | Input: quantified pre-defined individual features incl. annotation in training set
|
Input: quantified individual features
|
Input: pre-defined individual features, and/or raw signals or images, incl. annotation in training set
|
Input: raw signals or images
|
| Output | Classification based on pre-defined features in pre-defined classes | Automatized classification in unknown number of not pre-defined classes
|
Classification based on pre-defined and/or extracted features in pre-defined classes
|
Classification based on raw signals or image analysis in which unidentified features might carry diagnostic or predictive information. Generation of synthetic signal or images. |
Figure 6.
Various groups of artificial intelligence techniques using different data sources, their dominant features and outputs, and potential applications. AF, atrial fibrillation; CV, cardiovascular; CNN, convolutional neural network; ECG, electrocardiogram.
A growing clinical and consumer use of AI is the automated detection of AF episodes in ECG and sensor recordings (e.g. photoplethysmography and gyroscopes)61,74,166–168; AI models can also enhance AF prediction based on ECG during sinus rhythm,169,170 chest X-ray,171 or facial photoplethysmography signals using a digital camera.172 Deep-learning models have been used for the prediction of recurrent AF on rhythm control therapy.173,174 In addition, ECG analysis using AI has been applied to guide the identification of patients with low ejection fraction175 and predict ischaemic stroke risk in AF patients.176
Explainable artificial intelligence
One of the rate-limiting factors for further implementation of AI in AF research and clinical practice is the black box nature of AI methods. The reliance on non-transparent AI algorithms raises concerns regarding understanding the systems’ output and also about the responsibility and accountability for these outputs. To overcome this limitation, much effort has recently been invested in ‘explainable AI’ technology. Visualization techniques like attention maps, saliency maps, or heatmaps can highlight input variables and their effect direction within a structured data set, underscoring their importance and their influence on model decisions. Highlighting significant segments in ECGs or visualizing decision-making in structured data sets can provide insights that can be interpreted based on mechanistic understanding.170,177–180 Additionally, the availability of generic frameworks enables the visualization of decisions from various deep neural networks, making them applicable to multiple data sources, including structured data sets.181
Knowledge gaps and research opportunities
Artificial intelligence approaches have become essential tools for researchers to integrate data of a distinct nature such as genetics, cardiac tissue structure including atrial fat, biomolecules, information on comorbidities, and transcriptome data. This strategy requires sharing of multi-modal data coming from different centres, processing of data through the complex steps of regulatory, interoperability, annotation, pseudonymization, and then centralizing data in a data hub to generate and use algorithms.182 This is the goal of the European H2020 consortium MAESTRIA (EU grant agreement ID: 965286), which was created in 2021 bringing together 18 academic and private partners including AFNET.
For primary prevention strategy to prevent the development of AF and reduction of AF burden, AI can help to integrate information from multi-dimensional clinical parameters to create biomarkers that can inform on AF risk and risk of AF progression.183
Whether the use of AI-generated risk markers can guide AF therapy184,185 requires clinical evaluation, e.g. in the EU-funded MAESTRIA consortium in its prospective AFNET-10 cohort of patients with different types of AF.
In addition, federated learning techniques offer opportunities for model development independent from the logistic challenges of data transfer, which warrants further investigations.
Summary
The conference attendees identified several changes in the management of patients with AF supported by good evidence:
Active rhythm control therapy combined with rate control should be part of the default initial management of most patients with AF.
Biomolecules, genetics, and imaging parameters may help to refine the risk of stroke and other AF-related complications and to identify patient groups with likely therapy failure.
The stroke rate in patients with device-detected AF is low. Oral anticoagulation can reduce this low rate of stroke and induce major bleeding in patients with device-detected AF. More research is needed to identify patients with device-detected AF at high risk of stroke.
The presence of AF should trigger treatment of concomitant cardiovascular conditions, as already implemented for patients with coronary artery disease. The evidence for such measures drawn from research in patients with AF data sets supports wide-spread implementation.
Detection of AF and other chronic cardiovascular diseases is one of the first applications of unsupervised and supervised data science techniques. Iterative cooperation between clinicians and data scientists is needed to leverage the potential of data science and artificial explainable intelligence applications for patients with AF.
Acknowledgements
We wish to thank all participants of the 9th AFNET/EHRA consensus conference and the staff of AFNET, EHRA, and ESC for the excellent organization of the conference.
Contributor Information
Dominik Linz, Department of Cardiology, Maastricht University Medical Center, Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Jason G Andrade, Division of Cardiology, Vancouver General Hospital, Vancouver, Canada; Montreal Heart Institute, Montreal, Canada.
Elena Arbelo, Institut Clínic Cardiovascular, Hospital Clinic, Universitat de Barcelona, Barcelona, Catalonia, Spain; Institut d’Investigació August Pi i Sunyer (IDIBAPS), Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart—ERN GUARD-Heart.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Polyclinic of Modena, Modena, Italy.
Guenter Breithardt, Department of Cardiovascular Medicine, University Hospital, Münster, Germany; Atrial Fibrillation NETwork (AFNET), Muenster, Germany.
A John Camm, Cardiology Clinical Academic Group, Molecular and Clinical Sciences Institute, St. George's University of London, London, UK.
Valeria Caso, Stroke Unit, Santa Maria della Misericordia Hospital, University of Perugia, Perugia, Italy.
Jens Cosedis Nielsen, Department of Cardiology, Aarhus University Hospital and Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Mirko De Melis, Bakken Research Center, Maastricht, The Netherlands.
Tom De Potter, Cardiovascular Center, OLV Hospital, Aalst, Belgium.
Wolfgang Dichtl, Department of Internal Medicine III, Cardiology and Angiology, Medical University Innsbruck, Innsbruck, Austria.
Søren Zoega Diederichsen, Department of Cardiology, Copenhagen University Hospital—Rigshospitalet, Copenhagen, Denmark.
Dobromir Dobrev, Institute of Pharmacology, Faculty of Medicine, University Hospital Essen, University Duisburg-Essen, Essen, Germany.
Nicolas Doll, Department of Cardiac Surgery, Schüchtermann-Klinik, Bad Rothenfelde, Germany.
David Duncker, Hannover Heart Rhythm Center, Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany.
Elke Dworatzek, Pfizer Pharma GmbH, Berlin, Germany.
Lars Eckardt, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Department of Cardiology II—Electrophysiology, University Hospital Münster, Münster, Germany.
Christoph Eisert, Preventicus GmbH, Jena, Germany.
Larissa Fabritz, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; University Center of Cardiovascular Science, UHZ, UKE, Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site: Hamburg/Kiel/Lübeck, Hamburg, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston, Birmingham, UK.
Michal Farkowski, Department of Cardiology, Ministry of Interior and Administration, National Medical Institute, Warsaw, Poland.
David Filgueiras-Rama, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; Centro Nacional de Investigaciones Cardiovasculares (CNIC), Novel Arrhythmogenic Mechanisms Program, Madrid, Spain; Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), Cardiovascular Institute, C/ Profesor Martín Lagos, Madrid, Spain.
Andreas Goette, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Department of Cardiology and Intensive Care Medicine, St Vincenz-Hospital Paderborn, Paderborn, Germany.
Eduard Guasch, Institut d’Investigació August Pi i Sunyer (IDIBAPS), Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; Clinic Barcelona, University of Barcelona, Barcelona, Spain.
Guido Hack, Bristol-Myers Squibb GmbH & Co. KGaA, Munich, Germany.
Stéphane Hatem, IHU ICAN, Hospital Pitié-Salpêtrière, Paris, France.
Karl Georg Haeusler, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Department of Neurology, Universitätsklinikum Würzburg (UKW), Würzburg, Germany.
Jeff S Healey, Division of Cardiology, McMaster University, Hamilton, Ontario, Canada; Population Health Research Institute, Hamilton, Ontario, Canada.
Hein Heidbuechel, Antwerp University Hospital, Cardiovascular Sciences, University of Antwerp, Antwerp, Belgium.
Ziad Hijazi, Antwerp University Hospital, Cardiovascular Sciences, University of Antwerp, Antwerp, Belgium; Department of Medical Sciences, Cardiology, Uppsala University, Uppsala, Sweden; Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden.
Lucas H Hofmeister, Global Medical Affairs Bayer AG, Berlin, Germany.
Leif Hove-Madsen, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain; Biomedical Research Institute Barcelona (IIBB-CSIC), Barcelona, Spain; IR Sant Pau, Hospital de Sant Pau, Barcelona, Spain.
Thomas Huebner, Preventicus GmbH, Jena, Germany.
Stefan Kääb, European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart—ERN GUARD-Heart; Department of Medicine I, University Hospital, LMU Munich, Munich, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site Munich, Munich Heart Alliance, Munich, Germany.
Dipak Kotecha, Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston, Birmingham, UK; NIHR Birmingham Biomedical Research Centre, University Hospitals Birmingham NHS Trust, Birmingham, UK.
Katarzyna Malaczynska-Rajpold, Lister Hospital, East and North Hertfordshire NHS Trust, Stevenage, UK; Royal Brompton Hospital, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
José Luis Merino, La Paz University Hospital, IdiPaz, Autonomous University of Madrid, Madrid, Spain.
Andreas Metzner, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Hamburg, Germany.
Lluís Mont, Institut Clínic Cardiovascular, Hospital Clinic, Universitat de Barcelona, Barcelona, Catalonia, Spain; Institut d’Investigació August Pi i Sunyer (IDIBAPS), Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain.
Ghulam Andre Ng, Department of Cardiovascular Sciences, University of Leicester, Leicester, UK; NIHR Leicester Biomedical Research Centre, Leicester, UK.
Michael Oeff, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Cardiology Department, Medizinische Hochschule Brandenburg, Brandenburg/Havel, Germany.
Abdul Shokor Parwani, Department of Cardiology, Deutsches Herzzentrum der Charité (CVK), Berlin, Germany.
Helmut Puererfellner, Cardiological Department, Ordensklinikum Linz Elisabethinen, Linz, Austria.
Ursula Ravens, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Institute of Experimental Cardiovascular Medicine, University Clinic Freiburg, Freiburg, Germany.
Michiel Rienstra, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Prashanthan Sanders, Centre for Heart Rhythm Disorders, University of Adelaide and Royal Adelaide Hospital, Adelaide, Australia.
Daniel Scherr, Division of Cardiology, Medical University of Graz, Graz, Austria.
Renate Schnabel, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site: Hamburg/Kiel/Lübeck, Hamburg, Germany; Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Hamburg, Germany.
Ulrich Schotten, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Departments of Physiology, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, The Netherlands.
Christian Sohns, Herz- und Diabeteszentrum Nordrhein-Westfalen, Universitätsklinik der Ruhr-Universität Bochum, Klinik für Elektrophysiologie—Rhythmologie, Bad Oeynhausen, Germany.
Gerhard Steinbeck, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Center for Cardiology at Clinic Starnberg, Starnberg, Germany.
Daniel Steven, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Heart Center, Department of Electrophysiology, University Hospital Cologne, Cologne, Germany.
Tobias Toennis, German Centre for Cardiovascular Research (DZHK), Partner Site: Hamburg/Kiel/Lübeck, Hamburg, Germany; Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Hamburg, Germany.
Stylianos Tzeis, Cardiology Department, Mitera Hospital, Athens, Greece.
Isabelle C van Gelder, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Roderick H van Leerdam, Royal Philips, Amsterdam, The Netherlands.
Kevin Vernooy, Department of Cardiology, Maastricht University Medical Center, Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands.
Manish Wadhwa, Medical Office, Philips Ambulatory Monitoring and Diagnostics, San Diego, CA, USA.
Reza Wakili, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; Department of Medicine and Cardiology, Goethe University, Frankfurt, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site Rhine-Main, Germany.
Stephan Willems, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site: Hamburg/Kiel/Lübeck, Hamburg, Germany; Asklepios Hospital St. Georg, Department of Cardiology and Internal Care Medicine, Faculty of Medicine, Semmelweis University Campus, Hamburg, Germany.
Henning Witt, Pfizer Pharma GmbH, Berlin, Germany.
Stef Zeemering, Departments of Physiology, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, The Netherlands.
Paulus Kirchhof, Atrial Fibrillation NETwork (AFNET), Muenster, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site: Hamburg/Kiel/Lübeck, Hamburg, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston, Birmingham, UK; Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, Hamburg, Germany.
Funding
The 9th AFNET/EHRA consensus conference was co-financed by AFNET, EHRA, and the MAESTRIA consortium (EU grant agreement ID: 965286). Industry participants paid an attendance fee for the conference and provided an industry perspective during the discussions at the meeting but had no involvement in the writing process.
Data availability
All data used for this report are publicly available, and their sources are cited. For further information, please contact info@kompetenznetz-vorhofflimmern.de.
References
- 1. Kirchhof P, Toennis T, Goette A, Camm AJ, Diener HC, Becher N et al. Anticoagulation with edoxaban in patients with atrial high-rate episodes. N Engl J Med 2023;389:1167–79. [DOI] [PubMed] [Google Scholar]
- 2. Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660–71. [DOI] [PubMed] [Google Scholar]
- 3. Sohns C, Fox H, Marrouche NF, Crijns H, Costard-Jaeckle A, Bergau L et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med 2023;389:1380–9. [DOI] [PubMed] [Google Scholar]
- 4. Healey JS, Lopes RD, Granger CB, Alings M, Rivard L, McIntyre WF et al. Apixaban for stroke prevention in subclinical atrial fibrillation. N Engl J Med 2024;390:107–17. [DOI] [PubMed] [Google Scholar]
- 5. Connolly S, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 7. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 8. Becher N, Toennis T, Bertaglia E, Blomstrom-Lundqvist C, Brandes A, Cabanelas N et al. Anticoagulation with edoxaban in patients with long atrial high-rate episodes >/=24 hours. Eur Heart J 2024;45:837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McIntyre WF, Benz AP, Becher N, Healey JS, Granger CB, Rivard L,et al. Direct oral anticoagulants for stroke prevention in patients with device-detected atrial fibrillation: a study-level meta-analysis of the NOAH-AFNET 6 and ARTESiA trials. Circulation 2023. doi: 10.1161/CIRCULATIONAHA.123.067512 [DOI] [PubMed] [Google Scholar]
- 10. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CM et al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fabritz L, Crijns H, Guasch E, Goette A, Hausler KG, Kotecha D et al. Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: the 7th AFNET/EHRA consensus conference. Europace 2021;23:329–44. [DOI] [PubMed] [Google Scholar]
- 12. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A et al. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA consensus conference. Europace 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 13. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace 2007;9:1006–23. [DOI] [PubMed] [Google Scholar]
- 14. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 15. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 16. Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns H et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J 2022;43:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HT et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ 2021;373:n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kany S, Cardoso VR, Bravo L, Williams JA, Schnabel R, Fabritz L et al. Eligibility for early rhythm control in patients with atrial fibrillation in the UK Biobank. Heart 2022;108:1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dickow J, Kirchhof P, Van Houten HK, Sangaralingham LR, Dinshaw LHW, Friedman PA et al. Generalizability of the EAST-AFNET 4 trial: assessing outcomes of early rhythm-control therapy in patients with atrial fibrillation. J Am Heart Assoc 2022;11:e024214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM et al. ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2024;;149:e1–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–78. [DOI] [PubMed] [Google Scholar]
- 22. Connolly SJ, Crijns HJ, Torp-Pedersen C, van Eickels M, Gaudin C, Page RL et al. Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation 2009;120:1174–80. [DOI] [PubMed] [Google Scholar]
- 23. Rillig A, Borof K, Breithardt G, Camm AJ, Crijns H, Goette A et al. Early rhythm control in patients with atrial fibrillation and high comorbidity burden. Circulation 2022;146:836–47. [DOI] [PubMed] [Google Scholar]
- 24. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021;143:1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metzner A, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns H et al. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST—AFNET 4 trial. Europace 2022;24:552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns H et al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST-AFNET 4 trial. Eur Heart J 2022;43:4127–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goette A, Borof K, Breithardt G, Camm AJ, Crijns H, Kuck KH et al. Presenting pattern of atrial fibrillation and outcomes of early rhythm control therapy. J Am Coll Cardiol 2022;80:283–95. [DOI] [PubMed] [Google Scholar]
- 28. Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kenneback G et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Kaisey AM, Parameswaran R, Bryant C, Anderson RD, Hawson J, Chieng D et al. Atrial fibrillation catheter ablation vs medical therapy and psychological distress: a randomized clinical trial. JAMA 2023;330:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2023.;388:105–16. [DOI] [PubMed] [Google Scholar]
- 31. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 32. Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi S et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the CAPLA randomized clinical trial. JAMA 2023;329:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher L et al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huo Y, Gaspar T, Schönbauer R, Wójcik M, Fiedler L, Roithinger FX et al. Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evidence 2022;1:EVIDoa2200141. [DOI] [PubMed] [Google Scholar]
- 35. Doll N, Weimar T, Kosior DA, Bulava A, Mokracek A, Monnig G et al. Efficacy and safety of hybrid epicardial and endocardial ablation versus endocardial ablation in patients with persistent and longstanding persistent atrial fibrillation: a randomised, controlled trial. EClinicalMedicine 2023;61:102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Heijden CAJ, Weberndörfer V, Vroomen M, Luermans JG, Chaldoupi SM, Bidar E et al. Hybrid ablation versus repeated catheter ablation in persistent atrial fibrillation: a randomized controlled trial. JACC Clin Electrophysiol 2023;9:1013–23. [DOI] [PubMed] [Google Scholar]
- 37. Boersma L, Andrade JG, Betts T, Duytschaever M, Purerfellner H, Santoro F et al. Progress in atrial fibrillation ablation during 25 years of Europace journal. Europace 2023;25:euad244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey DJ, Pathak RK et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2018;20:f366–76. [DOI] [PubMed] [Google Scholar]
- 39. Tilz RR, Schmidt V, Purerfellner H, Maury P, Chun K, Martinek M et al. A worldwide survey on incidence, management, and prognosis of oesophageal fistula formation following atrial fibrillation catheter ablation: the POTTER-AF study. Eur Heart J 2023;44:2458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner A et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saljic A, Heijman J, Dobrev D. Recent advances in antiarrhythmic drug therapy. Drugs 2023;83:1147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heijman J, Zhou X, Morotti S, Molina CE, Abu-Taha IH, Tekook M et al. Enhanced Ca(2+)-dependent SK-channel gating and membrane trafficking in human atrial fibrillation. Circ Res 2023;132:e116–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holst AG, Tomcsanyi J, Vestbjerg B, Grunnet M, Sorensen US, Diness JG et al. Inhibition of the K(Ca)2 potassium channel in atrial fibrillation: a randomized Phase 2 trial. Nat Med 2024;30:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363–73. [DOI] [PubMed] [Google Scholar]
- 45. Kotecha D, Bunting KV, Gill SK, Mehta S, Stanbury M, Jones JC et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324:2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J 2021;42:4731–9. [DOI] [PubMed] [Google Scholar]
- 47. Rijks JHJ, Lankveld T, Manusama R, Broers B, Stipdonk A, Chaldoupi SM et al. Left bundle branch area pacing and atrioventricular node ablation in a single-procedure approach for elderly patients with symptomatic atrial fibrillation. J Clin Med 2023;12:4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 49. Jastrzębski M, Kiełbasa G, Cano O, Curila K, Heckman L, De Pooter J et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J 2022;43:4161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palmisano P, Ziacchi M, Dell'Era G, Donateo P, Ammendola E, Aspromonte V et al. Ablate and pace: comparison of outcomes between conduction system pacing and biventricular pacing. Pacing Clin Electrophysiol 2023;46:1258–68. [DOI] [PubMed] [Google Scholar]
- 51. Kircanski B, Boveda S, Prinzen F, Sorgente A, Anic A, Conte G et al. Conduction system pacing in everyday clinical practice: EHRA physician survey. Europace 2023;25:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tung R, Burri H. Role of conduction system pacing in ablate and pace strategies for atrial fibrillation. Eur Heart J Suppl 2023;25:G56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 54. Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J 2014;35:3356–64. [DOI] [PubMed] [Google Scholar]
- 55. Duytschaever M, Demolder A, Phlips T, Sarkozy A, El Haddad M, Taghji P et al. PulmOnary vein isolation with vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J 2018;39:1429–37. [DOI] [PubMed] [Google Scholar]
- 56. Aguilar M, Macle L, Deyell MW, Yao R, Hawkins NM, Khairy P et al. Influence of monitoring strategy on assessment of ablation success and postablation atrial fibrillation burden assessment: implications for practice and clinical trial design. Circulation 2022;145:21–30. [DOI] [PubMed] [Google Scholar]
- 57. Kirchhof P, Bax J, Blomstrom-Lundquist C, Calkins H, Camm AJ, Cappato R et al. Early and comprehensive management of atrial fibrillation: executive summary of the proceedings from the 2nd AFNET-EHRA consensus conference ‘research perspectives in AF’. Eur Heart J 2009;30:2969–2977c. [DOI] [PubMed] [Google Scholar]
- 58. Charitos EI, Ziegler PD, Stierle U, Robinson DR, Graf B, Sievers HH et al. Atrial fibrillation burden estimates derived from intermittent rhythm monitoring are unreliable estimates of the true atrial fibrillation burden. Pacing Clin Electrophysiol 2014;37:1210–8. [DOI] [PubMed] [Google Scholar]
- 59. Kirchhof P, Bax J, Blomstrom-Lundquist C, Calkins H, Camm AJ, Cappato R et al. Early and comprehensive management of atrial fibrillation: proceedings from the 2nd AFNET/EHRA consensus conference on atrial fibrillation entitled ‘research perspectives in atrial fibrillation’. Europace 2009;11:860–85. [DOI] [PubMed] [Google Scholar]
- 60. Fabritz L, Connolly DL, Czarnecki E, Dudek D, Guasch E, Haase D et al. Smartphone and wearable detected atrial arrhythmias in older adults: results of a fully digital European case finding study. Eur Heart J Digit Health 2022;3:610–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan W et al. Screening for atrial fibrillation in older adults at primary care visits: VITAL-AF randomized controlled trial. Circulation 2022;145:946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kirchhof P, Schotten U, Zapf A. Anticoagulation with Edoxaban in patients with atrial high-rate episodes. Reply. N Engl J Med 2023;389:2302–3. [DOI] [PubMed] [Google Scholar]
- 64. Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018;39:1407–15. [DOI] [PubMed] [Google Scholar]
- 65. Seiffge DJ, Cancelloni V, Räber L, Paciaroni M, Metzner A, Kirchhof P,et al. Secondary stroke prevention in people with atrial fibrillation: treatments and trials. Lancet Neurol 2024;23:404–417. [DOI] [PubMed] [Google Scholar]
- 66. Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med 2019;380:1906–17. [DOI] [PubMed] [Google Scholar]
- 67. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378:2191–201. [DOI] [PubMed] [Google Scholar]
- 68. Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M et al. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 2018;379:1332–42. [DOI] [PubMed] [Google Scholar]
- 69. Vassiliki’ Coutsoumbas G, Di Pasquale G. Ischaemic stroke in the absence of documented atrial fibrillation: is there who could benefit from anticoagulant therapy? Eur Heart J Suppl 2022;24:I89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kamel H, Longstreth WTJr, Tirschwell DL, Kronmal RA, Marshall RS, Broderick JP. Apixaban to prevent recurrence after cryptogenic stroke in patients with atrial cardiopathy: the ARCADIA randomized clinical trial. JAMA 2024;331:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–506. [DOI] [PubMed] [Google Scholar]
- 72. Svendsen JH, Diederichsen SZ, Hojberg S, Krieger DW, Graff C, Kronborg C et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (the LOOP study): a randomised controlled trial. Lancet 2021;398:1507–16. [DOI] [PubMed] [Google Scholar]
- 73. Charitos EI, Purerfellner H, Glotzer TV, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol 2014;63:2840–8. [DOI] [PubMed] [Google Scholar]
- 74. Hermans ANL, Gawalko M, Dohmen L, van der Velden RMJ, Betz K, Duncker D et al. Mobile health solutions for atrial fibrillation detection and management: a systematic review. Clin Res Cardiol 2022;111:479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Diederichsen SZ, Haugan KJ, Brandes A, Lanng MB, Graff C, Krieger D et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol 2019;74:2771–81. [DOI] [PubMed] [Google Scholar]
- 76. De With RR, Erküner Ö, Rienstra M, Nguyen BO, Körver FWJ, Linz D et al. Temporal patterns and short-term progression of paroxysmal atrial fibrillation: data from RACE V. Europace 2020;22:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. van de Lande ME, Rama RS, Koldenhof T, Arita VA, Nguyen BO, van Deutekom C et al. Time of onset of atrial fibrillation and atrial fibrillation progression data from the RACE V study. Europace 2023;25:euad058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Svennberg E, Caiani EG, Bruining N, Desteghe L, Han JK, Narayan SM et al. The digital journey: 25 years of digital development in electrophysiology from an Europace perspective. Europace 2023;25:euad176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hermans ANL, Gawalko M, Pluymaekers N, Dinh T, Weijs B, van Mourik MJW et al. Long-term intermittent versus short continuous heart rhythm monitoring for the detection of atrial fibrillation recurrences after catheter ablation. Int J Cardiol 2021;329:105–12. [DOI] [PubMed] [Google Scholar]
- 80. Kirchhof P, Andresen D, Bosch R, Borggrefe M, Meinertz T, Parade U et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet 2012;380:238–46. [DOI] [PubMed] [Google Scholar]
- 81. Goette A, Schon N, Kirchhof P, Breithardt G, Fetsch T, Hausler KG et al. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol 2012;5:43–51. [DOI] [PubMed] [Google Scholar]
- 82. Kuck KH, Hoffmann BA, Ernst S, Wegscheider K, Treszl A, Metzner A et al. Impact of complete versus incomplete circumferential lines around the pulmonary veins during catheter ablation of paroxysmal atrial fibrillation: results from the gap-atrial fibrillation-German atrial fibrillation competence network 1 trial. Circ Arrhythm Electrophysiol 2016;9:e003337. [DOI] [PubMed] [Google Scholar]
- 83. Schwennesen HT, Andrade JG, Wood KA, Piccini JP. Ablation to reduce atrial fibrillation burden and improve outcomes: JACC review topic of the week. J Am Coll Cardiol 2023;82:1039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar P et al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PubMed] [Google Scholar]
- 85. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 86. Hijazi Z, Lindback J, Oldgren J, Benz AP, Alexander JH, Connolly SJ et al. Individual net clinical outcome with oral anticoagulation in atrial fibrillation using the ABC-AF risk scores. Am Heart J 2023;261:55–63. [DOI] [PubMed] [Google Scholar]
- 87. Quinn GR, Severdija ON, Chang Y, Singer DE. Wide variation in reported rates of stroke across cohorts of patients with atrial fibrillation. Circulation 2017;135:208–19. [DOI] [PubMed] [Google Scholar]
- 88. Lip GYH, Proietti M, Potpara T, Mansour M, Savelieva I, Tse HF et al. Atrial fibrillation and stroke prevention: 25 years of research at EP Europace journal. Europace 2023;25:euad226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. [DOI] [PubMed] [Google Scholar]
- 90. Lubitz SA, Benjamin EJ, Ruskin JN, Fuster V, Ellinor PT. Challenges in the classification of atrial fibrillation. Nat Rev Cardiol 2010;7:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kirchhof P, Breithardt G, Camm AJ, Crijns HJ, Kuck KH, Vardas P et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the early treatment of atrial fibrillation for stroke prevention trial. Am Heart J 2013;166:442–8. [DOI] [PubMed] [Google Scholar]
- 92. Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448:353–7. [DOI] [PubMed] [Google Scholar]
- 93. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. O’Sullivan JW, Shcherbina A, Justesen JM, Turakhia M, Perez M, Wand H et al. Combining clinical and polygenic risk improves stroke prediction among individuals with atrial fibrillation. Circ Genom Precis Med 2021;14:e003168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shoemaker MB, Bollmann A, Lubitz SA, Ueberham L, Saini H, Montgomery J et al. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2015;8:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shoemaker MB, Husser D, Roselli C, Al Jazairi M, Chrispin J, Kuhne M et al. Genetic susceptibility for atrial fibrillation in patients undergoing atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2020;13:e007676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kany S, Al-Taie C, Roselli C, Pirruccello JP, Borof K, Reinbold C et al. Association of genetic risk and outcomes in patients with atrial fibrillation: interactions with early rhythm control in the EAST-AFNET4 trial. Cardiovasc Res 2023;119:1799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018;50:524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mishra A, Malik R, Hachiya T, Jurgenson T, Namba S, Posner DC et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature 2022;611:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet 2018;50:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hijazi Z, Oldgren J, Siegbahn A, Wallentin L. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem 2017;63:152–64. [DOI] [PubMed] [Google Scholar]
- 102. Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J 2019;40:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Reyat JS, Chua W, Cardoso VR, Witten A, Kastner PM, Kabir SN et al. Reduced left atrial cardiomyocyte PITX2 and elevated circulating BMP10 predict atrial fibrillation after ablation. JCI Insight 2020;5:e139179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chua W, Khashaba A, Canagarajah H, Blankenberg S, Cosedis Nielsen J, di Biase L et al. Disturbed atrial metabolism, shear stress, and cardiac load after AF ablation: AXAFA biomolcules study. Europace 2024;26:euae028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hijazi Z, Benz AP, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW et al. Bone morphogenetic protein 10: a novel risk marker of ischaemic stroke in patients with atrial fibrillation. Eur Heart J 2023;44:208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. King JB, Azadani PN, Suksaranjit P, Bress AP, Witt DM, Han FT et al. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol 2017;70:1311–21. [DOI] [PubMed] [Google Scholar]
- 107. Heijman J, Linz D, Schotten U. Dynamics of atrial fibrillation mechanisms and comorbidities. Annu Rev Physiol 2021;83:83–106. [DOI] [PubMed] [Google Scholar]
- 108. Meinel TR, Branca M, De Marchis GM, Nedeltchev K, Kahles T, Bonati L et al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol 2021;89:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tutuncu S, Olma M, Kunze C, Dietzel J, Schurig J, Fiessler C et al. Off-label-dosing of non-vitamin K-dependent oral antagonists in AF patients before and after stroke: results of the prospective multicenter Berlin Atrial Fibrillation Registry. J Neurol 2022;269:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Karl Georg H. Ischaemic stroke in atrial fibrillation patients while on oral anticoagulation-a call for A-C-T-I-O-N. Eur Heart J 2023;44:1815–7. [DOI] [PubMed] [Google Scholar]
- 111. Paciaroni M, Agnelli G, Caso V, Silvestrelli G, Seiffge DJ, Engelter S et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants for stroke prevention. Stroke 2019;50:2168–74. [DOI] [PubMed] [Google Scholar]
- 112. Galli M, Laborante R, Ortega-Paz L, Franchi F, Rollini F, D'Amario D et al. Factor XI inhibitors in early clinical trials: a meta-analysis. Thromb Haemost 2023;123:576–84. [DOI] [PubMed] [Google Scholar]
- 113. Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015;372:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bayer . OCEANIC-AF Study Stopped Early Due to Lack of Efficacy. https://www.bayer.com/media/en-us/oceanic-af-study-stopped-early-due-to-lack-of-efficacy/ (12 December 2023, date last accessed).
- 115. AF SCREEN and AFFECT-EU Collaborators . Protocol for a systematic review and individual participant data meta-analysis of randomized trials of screening for atrial fibrillation to prevent stroke. Thromb Haemost 2023;123:366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Carnicelli AP, Hong H, Giugliano RP, Connolly SJ, Eikelboom J, Patel MR et al. Individual patient data from the pivotal randomized controlled trials of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation (COMBINE AF): design and rationale: from the COMBINE AF (a collaboration between multiple institutions to better investigate non-vitamin K antagonist oral anticoagulant use in atrial fibrillation) investigators. Am Heart J 2021;233:48–58. [DOI] [PubMed] [Google Scholar]
- 117. Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran S et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006;114:759–65. [DOI] [PubMed] [Google Scholar]
- 118. Themistoclakis S, Corrado A, Marchlinski FE, Jais P, Zado E, Rossillo A et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010;55:735–43. [DOI] [PubMed] [Google Scholar]
- 119. Hunter RJ, McCready J, Diab I, Page SP, Finlay M, Richmond L et al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 2012;98:48–53. [DOI] [PubMed] [Google Scholar]
- 120. Bunch TJ, May HT, Bair TL, Weiss JP, Crandall BG, Osborn JS et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm 2013;10:1272–7. [DOI] [PubMed] [Google Scholar]
- 121. Saliba W, Schliamser JE, Lavi I, Barnett-Griness O, Gronich N, Rennert G. Catheter ablation of atrial fibrillation is associated with reduced risk of stroke and mortality: a propensity score-matched analysis. Heart Rhythm 2017;14:635–42. [DOI] [PubMed] [Google Scholar]
- 122. Srivatsa UN, Danielsen B, Amsterdam EA, Pezeshkian N, Yang Y, Nordsieck E et al. CAABL-AF (California Study of Ablation for Atrial Fibrillation): mortality and stroke, 2005 to 2013. Circ Arrhythm Electrophysiol 2018;11:e005739. [DOI] [PubMed] [Google Scholar]
- 123. Joza J, Samuel M, Jackevicius CA, Behlouli H, Jia J, Koh M et al. Long-term risk of stroke and bleeding post-atrial fibrillation ablation. J Cardiovasc Electrophysiol 2018;29:1355–62. [DOI] [PubMed] [Google Scholar]
- 124. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Verma A, Ha ACT, Kirchhof P, Hindricks G, Healey JS, Hill MD et al. The optimal anti-coagulation for enhanced-risk patients post-catheter ablation for atrial fibrillation (OCEAN) trial. Am Heart J 2018;197:124–32. [DOI] [PubMed] [Google Scholar]
- 126. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–60. [DOI] [PubMed] [Google Scholar]
- 127. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R et al. PREVEntion and regReSsive effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. Europace 2018;20:1929–35. [DOI] [PubMed] [Google Scholar]
- 128. Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 129. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 130. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 131. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation 2020;141:e750–72. [DOI] [PubMed] [Google Scholar]
- 132. Global Cardiovascular Risk C, Magnussen C, Ojeda FM, Leong DP, Alegre-Diaz J, Amouyel P et al. Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med 2023;389:1273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 2020;382:20–8. [DOI] [PubMed] [Google Scholar]
- 134. Donnellan E, Aagaard P, Kanj M, Jaber W, Elshazly M, Hoosien M et al. Association between pre-ablation glycemic control and outcomes among patients with diabetes undergoing atrial fibrillation ablation. JACC Clin Electrophysiol 2019;5:897–903. [DOI] [PubMed] [Google Scholar]
- 135. Donnellan E, Wazni O, Kanj M, Hussein A, Baranowski B, Lindsay B et al. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol 2019;12:e007598. [DOI] [PubMed] [Google Scholar]
- 136. Donnellan E, Wazni OM, Elshazly M, Kanj M, Hussein AA, Baranowski B et al. Impact of bariatric surgery on atrial fibrillation type. Circ Arrhythm Electrophysiol 2020;13:e007626. [DOI] [PubMed] [Google Scholar]
- 137. Donnellan E, Wazni OM, Kanj M, Baranowski B, Cremer P, Harb S et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace 2019;21:1476–83. [DOI] [PubMed] [Google Scholar]
- 138. Donnellan E, Wazni OM, Kanj M, Elshazly M, Hussein AA, Patel DR et al. Impact of risk-factor modification on arrhythmia recurrence among morbidly obese patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol 2020;31:1979–86. [DOI] [PubMed] [Google Scholar]
- 139. Wharton S, Blevins T, Connery L, Rosenstock J, Raha S, Liu R et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N Engl J Med 2023;389:877–88. [DOI] [PubMed] [Google Scholar]
- 140. Dandona P, Chaudhuri A, Ghanim H. Semaglutide in early type 1 diabetes. N Engl J Med 2023;389:958–9. [DOI] [PubMed] [Google Scholar]
- 141. Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–15. [DOI] [PubMed] [Google Scholar]
- 142. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023;389:2221–32. [DOI] [PubMed] [Google Scholar]
- 143. Middeldorp ME, Ariyaratnam J, Lau D, Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart 2020;106:325–32. [DOI] [PubMed] [Google Scholar]
- 144. Elliott AD, Middeldorp ME, Van Gelder IC, Albert CM, Sanders P. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol 2023;20:404–17. [DOI] [PubMed] [Google Scholar]
- 145. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–61. [DOI] [PubMed] [Google Scholar]
- 146. Verhaert DVM, Betz K, Gawalko M, Hermans ANL, Pluymaekers N, van der Velden RMJ et al. A VIRTUAL sleep apnoea management pathway for the work-up of atrial fibrillation patients in a digital remote infrastructure: VIRTUAL-SAFARI. Europace 2022;24:565–75. [DOI] [PubMed] [Google Scholar]
- 147. van der Velden RMJ, Hereijgers MJM, Arman N, van Middendorp N, Franssen FME, Gawalko M et al. Implementation of a screening and management pathway for chronic obstructive pulmonary disease in patients with atrial fibrillation. Europace 2023;25:euad193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yoneda ZT, Anderson KC, Ye F, Quintana JA, O'Neill MJ, Sims RA et al. Mortality among patients with early-onset atrial fibrillation and rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol 2022;7:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Aziri B, Begic E, Jankovic S, Mladenovic Z, Stanetic B, Kovacevic-Preradovic T et al. Systematic review of sodium-glucose cotransporter 2 inhibitors: a hopeful prospect in tackling heart failure-related events. ESC Heart Fail 2023;10:1499–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Scheen AJ. Antidiabetic agents and risk of atrial fibrillation/flutter: a comparative critical analysis with a focus on differences between SGLT2 inhibitors and GLP-1 receptor agonists. Diabetes Metab 2022;48:101390. [DOI] [PubMed] [Google Scholar]
- 151. Coats AJS, Heymans S, Farmakis D, Anker SD, Backs J, Bauersachs J,et al. Atrial disease and heart failure: the common soil hypothesis proposed by the Heart Failure Association of the European Society of Cardiology. Eur Heart J 2022;43:863–867. [DOI] [PubMed] [Google Scholar]
- 152. Mohammad Z, Ahmad J, Sultan A, Penagaluri A, Morin D, Dominic P. Effect of sacubitril-valsartan on the incidence of atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol 2023;34:1037–42. [DOI] [PubMed] [Google Scholar]
- 153. Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail 2022;10:184–97. [DOI] [PubMed] [Google Scholar]
- 154. Butt JH, Kondo T, Jhund PS, Comin-Colet J, de Boer RA, Desai AS et al. Atrial fibrillation and dapagliflozin efficacy in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol 2022;80:1705–17. [DOI] [PubMed] [Google Scholar]
- 155. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. [DOI] [PubMed] [Google Scholar]
- 156. Filippatos G, Farmakis D, Butler J, Zannad F, Ferreira JP, Ofstad AP et al. Empagliflozin in heart failure with preserved ejection fraction with and without atrial fibrillation. Eur J Heart Fail 2023;25:970–7. [DOI] [PubMed] [Google Scholar]
- 157. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–28. [DOI] [PubMed] [Google Scholar]
- 158. Filippatos G, Bakris GL, Pitt B, Agarwal R, Rossing P, Ruilope LM et al. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J Am Coll Cardiol 2021;78:142–52. [DOI] [PubMed] [Google Scholar]
- 159. Lee G, Baker E, Collins R, Merino JL, Desteghe L, Heidbuchel H. The challenge of managing multimorbid atrial fibrillation: a pan-European European Heart Rhythm Association (EHRA) member survey of current management practices and clinical priorities. Europace 2022;24:2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gessler N, Willems S, Steven D, Aberle J, Akbulak RO, Gosau N et al. Supervised obesity reduction trial for AF ablation patients: results from the SORT-AF trial. Europace 2021;23:1548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pathak RK, Evans M, Middeldorp ME, Mahajan R, Mehta AB, Meredith M et al. Cost-effectiveness and clinical effectiveness of the risk factor management clinic in atrial fibrillation: the CENT study. JACC Clin Electrophysiol 2017;3:436–47. [DOI] [PubMed] [Google Scholar]
- 162. Heidbuchel H, Van Gelder IC, Desteghe L. ESC and EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: the Horizon 2020 EHRA-PATHS project. Eur Heart J 2022;43:1450–2. [DOI] [PubMed] [Google Scholar]
- 163. Fabritz L, Crijns H, Guasch E, Goette A, Haeusler KG, Kotecha D et al. Dynamic risk assessment to improve quality of care in patients with atrial fibrillation. The 7th AFNET/EHRA consensus conference. Europace 2021;23:329–44. [DOI] [PubMed] [Google Scholar]
- 164. Isaksen JL, Baumert M, Hermans ANL, Maleckar M, Linz D. Artificial intelligence for the detection, prediction, and management of atrial fibrillation. Herzschrittmacherther Elektrophysiol 2022;33:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Benjamins JW, van Leeuwen K, Hofstra L, Rienstra M, Appelman Y, Nijhof W et al. Enhancing cardiovascular artificial intelligence (AI) research in the Netherlands: CVON-AI consortium. Neth Heart J 2019;27:414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Noseworthy PA, Attia ZI, Behnken EM, Giblon RE, Bews KA, Liu S et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: a prospective non-randomised interventional trial. Lancet 2022;400:1206–12. [DOI] [PubMed] [Google Scholar]
- 167. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 168. Hermans ANL, Isaksen JL, Gawalko M, Pluymaekers N, van der Velden RMJ, Snippe H et al. Accuracy of continuous photoplethysmography-based 1 min mean heart rate assessment during atrial fibrillation. Europace 2023;25:835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
- 170. Khurshid S, Friedman S, Reeder C, Di Achille P, Diamant N, Singh P et al. ECG-based deep learning and clinical risk factors to predict atrial fibrillation. Circulation 2022;145:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Matsumoto T, Ehara S, Walston SL, Mitsuyama Y, Miki Y, Ueda D. Artificial intelligence-based detection of atrial fibrillation from chest radiographs. Eur Radiol 2022;32:5890–7. [DOI] [PubMed] [Google Scholar]
- 172. Yan BP, Lai WHS, Chan CKY, Au ACK, Freedman B, Poh YC et al. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol 2020;5:105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Nuñez-Garcia JC, Sánchez-Puente A, Sampedro-Gómez J, Vicente-Palacios V, Jiménez-Navarro M, Oterino-Manzanas A et al. Outcome analysis in elective electrical cardioversion of atrial fibrillation patients: development and validation of a machine learning prognostic model. J Clin Med 2022:11:2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tang S, Razeghi O, Kapoor R, Alhusseini MI, Fazal M, Rogers AJ et al. Machine learning-enabled multimodal fusion of intra-atrial and body surface signals in prediction of atrial fibrillation ablation outcomes. Circ Arrhythm Electrophysiol 2022;15:e010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Yao X, Rushlow DR, Inselman JW, McCoy RG, Thacher TD, Behnken EM et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med 2021;27:815–9. [DOI] [PubMed] [Google Scholar]
- 176. Jung S, Song MK, Lee E, Bae S, Kim YY, Lee D et al. Predicting ischemic stroke in patients with atrial fibrillation using machine learning. Front Biosci (Landmark Ed) 2022;27:80. [DOI] [PubMed] [Google Scholar]
- 177. Wouters PC, van de Leur RR, Vessies MB, van Stipdonk AMW, Ghossein MA, Hassink RJ et al. Electrocardiogram-based deep learning improves outcome prediction following cardiac resynchronization therapy. Eur Heart J 2023;44:680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Cohen-Shelly M, Attia ZI, Friedman PA, Ito S, Essayagh BA, Ko WY et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur Heart J 2021;42:2885–96. [DOI] [PubMed] [Google Scholar]
- 179. Ayano YM, Schwenker F, Dufera BD, Debelee TG. Interpretable machine learning techniques in ECG-based heart disease classification: a systematic review. Diagnostics (Basel) 2022;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. van de Leur RR, Bos MN, Taha K, Sammani A, Yeung MW, van Duijvenboden S et al. Improving explainability of deep neural network-based electrocardiogram interpretation using variational auto-encoders. Eur Heart J Digit Health 2022;3:390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ribeiro MT, Singh S, Guestrin C. “Why should i trust you?” Explaining the predictions of any classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, KDD 2016, San Francisco, CA, USA.
- 182. Steffens S, Schröder K, Krüger M, Maack C, Streckfuss-Bömeke K, Backs J,et al. The challenges of research data management in cardiovascular science: a DGK and DZHK position paper-executive summary. Clin Res Cardiol 2023. doi: 10.1007/s00392-023-02303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Linz D, Verheule S, Isaacs A, Schotten U. Considerations for the assessment of substrates, genetics and risk factors in patients with atrial fibrillation. Arrhythm Electrophysiol Rev 2021;10:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lebert J, Ravi N, Fenton FH, Christoph J. Rotor localization and phase mapping of cardiac excitation waves using deep neural networks. Front Physiol 2021;12:782176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Liao S, Ragot D, Nayyar S, Suszko A, Zhang Z, Wang B et al. Deep learning classification of unipolar electrograms in human atrial fibrillation: application in focal source mapping. Front Physiol 2021;12:704122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for this report are publicly available, and their sources are cited. For further information, please contact info@kompetenznetz-vorhofflimmern.de.