Abstract
Background:
Myocardial fibrosis, as diagnosed on cardiac magnetic resonance imaging (cMRI) by late gadolinium enhancement (LGE), is associated with adverse outcomes in adults with hypertrophic cardiomyopathy (HCM), but its prevalence and magnitude in children with HCM have not been established. We investigated: 1) the prevalence and extent of myocardial fibrosis as detected by LGE cMRI; 2) the agreement between echocardiographic and cMRI measurements of cardiac structure; and 3) whether serum concentrations of N-terminal pro hormone B-type natriuretic peptide (NT-proBNP) and cardiac troponin-T are associated with cMRI measurements.
Methods:
A cross-section of children with HCM from 9 tertiary-care pediatric heart centers in the U.S. and Canada were enrolled in this prospective NHLBI study of cardiac biomarkers in pediatric cardiomyopathy (ClinicalTrials.gov Identifier: NCT01873976). The median age of the 67 participants was 13.8 years (range 1-18 years). Core laboratories analyzed echocardiographic and cMRI measurements, and serum biomarker concentrations.
Results:
In 52 children with non-obstructive HCM undergoing cMRI, overall low levels of myocardial fibrosis with LGE >2% of left ventricular (LV) mass were detected in 37 (71%) (median %LGE, 9.0%; IQR: 6.0%, 13.0%; range, 0% to 57%). Echocardiographic and cMRI measurements of LV dimensions, LV mass, and interventricular septal thickness showed good agreement using the Bland-Altman method. NT-proBNP concentrations were strongly and positively associated with LV mass and interventricular septal thickness (P<0.001), but not LGE.
Conclusions:
Low levels of myocardial fibrosis are common in pediatric patients with HCM seen at referral centers. Longitudinal studies of myocardial fibrosis and serum biomarkers are warranted to determine their predictive value for adverse outcomes in pediatric patients with HCM.
Keywords: Pediatric hypertrophic cardiomyopathy, cardiac magnetic resonance imaging, late gadolinium enhancement, myocardial fibrosis, myocardial stress, left ventricular mass
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is characterized by hypertrophy of the left ventricular (LV) myocardium without dilation of the LV or evidence of other cardiac or systemic diseases capable of causing LV hypertrophy. (1,2) Isolated HCM in children may be idiopathic or the result of a known disease-causing mutation and can also occur in the context of various genetic or metabolic syndromes. The disease increases the risk of heart failure (HF), ventricular arrhythmias, and sudden cardiac death (SCD). (2) The incidence of SCD in children and adolescents with HCM is about 6 cases per 100,000 population annually. (3-6)
In adults with HCM, risk factors for SCD and criteria for consideration of primary implantable cardioverter defibrillator (ICD) placement include a maximal interventricular septal thickness >30 mm, a history of non-sustained ventricular tachycardia, unexplained syncope, LV systolic dysfunction, LV apical aneurysm, and/or a family history of SCD secondary to HCM. (2) In addition, extensive myocardial fibrosis, as assessed by late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (cMRI), has been adopted as a supportive criterion for defibrillator placement as primary prevention in adults because myocardial fibrosis may be a substrate for arrhythmias and HF. (7-12) Accordingly, several studies have tested serum cardiac and collagen metabolism biomarkers that may relate to myocardial fibrosis, as well as N-terminal-proB-type natriuretic peptide (NT-proBNP) and cardiac troponin-T, in patients with HCM to establish their value in detecting and monitoring myocardial fibrosis, myocardial stress and injury. (13-15)
Recent data suggest that many of the classical risk factors accepted in adult patients are important in children, but there is still no consensus on the importance of LGE on cMRI as a risk factor in children with HCM. Among 1,085 children with HCM enrolled in the NHLBI-supported Pediatric Cardiomyopathy Registry between 1990 and 2006, identified risk factors for death or transplant were age at diagnosis of <1-year, HF, or a mixed cardiomyopathy phenotype (HCM with dilated or restrictive features), diminished LV fractional shortening, and increased LV posterior wall thickness in diastole measured by echocardiography. (5) That study was conducted before the availability of LGE imaging. The importance of prevalence and degree of myocardial fibrosis in children with HCM are not well established. A primary a priori aim of the NHLBI-funded Cardiac Biomarkers in Pediatric Cardiomyopathy prospective study (ClinicalTrials.gov Identifier: NCT01873976) from the NHLBI-sponsored Pediatric Cardiomyopathy Registry (ClinicalTrials.gov Identifier: NCT00005391) was to determine the prevalence of myocardial fibrosis as assessed by LGE in children with HCM, to determine whether echocardiographic and cMRI measurements for interventricular septal thickness and LV dimensions agree, and whether serum concentrations of NT-proBNP and cardiac troponin-T are associated with cMRI measurements of LV hypertrophy, mass, or myocardial fibrosis. This paper addresses this primary study aim.
METHODS
Study Design
In this cross-sectional study, we enrolled children with HCM seen at 9 tertiary pediatric heart centers in the U.S. and Canada (Clinical Trial Registration: NCT01873976). Eligibility was based on strict echocardiographic or cMRI criteria, as described by Everitt et al. (16) Briefly, patients were eligible for enrollment if 1) they were alive and <21 years old, 2) had been diagnosed with idiopathic or familial HCM or HCM from a known-disease causing mutation, and 3) had undergone cMRI imaging within 2 months of enrollment and had a blood specimen and echocardiogram acquired within 6 months of the cMRI. Children were excluded if they had syndromes associated with cardiomyopathy (e.g., Noonan syndrome or phenocopies, glycogen storage disease, metabolic disease, mitochondrial disease); a history of rheumatic fever; a history of radiation treatment, chemotherapy treatment, iron overload, uremia, or other potential toxic exposures; a history of congenital heart disease, ischemic coronary artery disease, or Kawasaki disease; a history of autoimmune disease or immunodeficiency; or a history of systemic or pulmonary hypertension or pulmonary vascular disease. (16) Infants of mothers with diabetes were also excluded.
The Institutional Review Boards at all centers participating in the study approved the protocol, with informed consent obtained from parents or legal guardians and assent from participants when appropriate.
Clinical Data Collection
Study data were abstracted from the electronic health record at each site by a specially trained data collection team. Data were entered into the study database through a web-based system designed by the study’s data coordinating center at the New England Research Institutes, Inc. (HealthCore, Inc.) in Watertown, MA. Data elements included demographics, age at HCM diagnosis, cardiac medications used at enrollment, results of clinical genetic testing, and surgical history.
Cardiac Magnetic Resonance Image Acquisition and Measurements
Results of cMRI were collected and analyzed in the core MRI laboratory at Washington University School of Medicine in St. Louis, MO. (16) All scans were performed on 1.5 T-conventional, whole-body standard medical MRI scanners with phased-array coils. The cardiac protocol included scout scans for localization; horizontal and vertical long- and short-axis; and multiphase, gated cine imaging using a steady-state free-precession (SSFP) sequence for function and anatomy at the highest optimal flip angle. In addition, LGE, phase-sensitive, inversion-recovery, and gradient-echo images were obtained after intravenous administration of a gadolinium-based contrast agent, (gadopentetic acid [Magnevist], gadoversetamide [OptiMARK], gadodiamide [Omniscan], gadoterate meglumine [Dotarem], or gadobenate dimeglumine [MultiHance]).
Gadolinium-enhanced images were acquired 7 to 10 minutes after administering the intravenous gadolinium bolus. Inversion times were between 200 and 350 ms, set to null signal from the normal myocardium. All long-axis images were acquired at a 6-mm slice thickness. Short-axis images were, in general, acquired at a 6-mm slice thickness (0 gap) in children <6 years old, and at an 8-mm slice thickness (0 gap) in children >6 years old. All cMRI images were de-identified and interpreted at a core laboratory by a single trained cardiac radiologist (PKW). Only the age of the child was known at the time of interpretation. Left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), interventricular septal thickness, LV mass, and %LGE were measured using Medis QMass software (Leiden, Netherlands). Late gadolinium enhancement in the myocardium was defined as 6 standard deviations above the signal in the normal myocardium. (17, 18) No adjustment or erasure was made of identification of high myocardial signal potentially caused by artifact.
Echocardiographic Image Acquisition and Measurements
Echocardiograms were acquired at each study site according to the study’s echocardiographic imaging protocol, which consisted of complete two-dimensional, transthoracic echocardiographic and Doppler evaluations, a complete assessment of any anatomic abnormalities, valve dysfunction, and intracardiac thrombi, and standard short- and long-axis views of the LV to assess regional wall motion. (16)
The study sites provided age, height, weight, and blood pressure data. Echocardiograms were uploaded in the DICOM (Digital Imaging and Communications in Medicine) format using a commercial, HIPAA-compliant image transfer service (AMBRA, Inc) to the study’s core echocardiography laboratory at Boston Children’s Hospital. Images were de-identified during import to the core lab server, and all analyses were performed by a single trained cardiologist (FIL) using custom core-lab designed software that archived all measurements as a non-destructive overlay and the derivation of all calculated variables, as specified by the American Society of Echocardiography guidelines. (19) Body surface area (BSA) was calculated according to the formula of Haycock et al., and Z-scores were calculated relative to age or BSA as described elsewhere. (20, 21) The cardiologist measuring the echocardiograms was blinded to the cMRI results.
Serum Biomarker Concentrations
Serum concentrations of NT-proBNP and cardiac troponin-T were measured. Blood was drawn within 2 months of the cMRI study. Samples were sent by express mail to the Study Biological Specimen Repository (Wayne State University, Detroit, MI). Blood samples were processed centrally and stored at −80°C until batch analysis occurred at the Diabetes Research Institute Immunoassay and Chemistry CLIA-approved Core Laboratory (University of Miami Miller School of Medicine, Miami, FL).
Statistical Methods
All data were analyzed by the Data Coordinating Center at the New England Research Institutes with SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). Normally distributed data are summarized with means and standard deviations, and skewed data are summarized with medians and interquartile ranges. Distributions were examined using histograms and Q-Q plots, and log-transformation was used to achieve normal distributions. Pearson correlation analysis was used to determine relationships between cMRI parameters, age at diagnosis, and disease duration. Within this analysis, LV mass index was developed using a scaling factor of 1.33, as per previously published pediatric analysis of structural measurements adjusting for growth. (21, 22)
Bland-Altman analysis was used to determine the agreement between echocardiographic and cMRI measurements. Values near the line of equality or within the confidence interval of the mean difference indicate good agreement and minimal bias. Associations between cMRI and biomarker measures were assessed with multivariable linear regression models, adjusted for age at diagnosis, time since diagnosis, race, and ethnicity. Linearity was assessed with residual plots. Alpha was set at 0.05, and all tests were twotailed. List-wise deletion or complete-case analysis was used by removing observations with missing values.
Sources of Funding
The Pediatric Cardiomyopathy Registry is supported by grants to S.E.L. from the NHLBI (HL53392, HL111459, and HL109090), in part from the National Institutes of Health (HL139968, HL137558, and CA211996), the CCF and the KJRF. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI, NIH, CCF or KJRF.
RESULTS
Patient Characteristics
The median age of the 67 participants was 12.1 years (range, birth to 18 years) at the time of HCM diagnosis, and 13.8 years (range, 1 to 18 years) at study enrollment. Children were predominantly boys (78%) and White (82%; Table 1). Most patients (57%) were on a beta-blocker at time of enrollment and 10% were on a calcium channel blocker.
Table 1.
Characteristics of 67 Children with Hypertrophic Cardiomyopathy in a Study to Determine the Prevalence of Myocardial Fibrosis with Late Gadolinium-Enhancement
| Patient Characteristic | Values |
|---|---|
| Age at Enrollment, median (IQR), years | 13.8 (11.1 to 15.3) |
| Age at Diagnosis, median (IQR), years | 12.1 (8.8 to 14.3) |
| Males, N (%) | 52 (78) |
| Ethnicity, N (%) | |
| Not Hispanic | 63 (94) |
| Hispanic | 4 (6) |
| Race, N (%) | |
| White | 55 (82) |
| Black or African American | 6 (9) |
| American Indian or Alaskan Native | 1 (1.5) |
| Asian | 1 (1.5) |
| Multi-racial | 3 (4.5) |
| Unknown | 1 (1.5) |
| Cardiac medications at enrollment, N (%) | |
| Beta-Blocker | 38 (57) |
| Calcium-Channel Blocker | 7 (10) |
Cardiac Imaging Measurements
Of the 67 participants with cMRI scans, 63 (94%) had images suitable for measuring LV wall-thickness and chamber volumes and function; 52 (78%) had LGE images suitable for calculating the extent of myocardial fibrosis. Reasons for excluding cMRI images from further analysis were lack of or incomplete LGE imaging, artifacts caused by excess respiratory motion, and/or poor reduction of background signal (nulling) of the normal myocardium on LGE images to accentuate areas of abnormal tissue. Cardiac MRIs were obtained at clinical discretion, typically with consideration of ICD placement and/or heart transplantation.
In our cohort, 48 of 52 (92%) children had detectable LGE, and 37 of 52 (71%) had %LGE greater than 2% of the LV mass. Median %LGE was 9.0% (IQR: 6.0%, 13.0%) with a range of 0% to 57% (Table 2). Children <5 years old had the highest %LGE with a median %LGE of 11.5% (IQR: 9.0%, 13.0%). Children between 11 and 15 years old had the lowest %LGE, with a median %LGE of 8.0% (IQR: 5.0%, 14.0%). Overall, when we excluded LGE <2%, similar levels of myocardial fibrosis were measured across all age groups.
Table 2.
Percent of Late Gadolinium Enhancement Detected in Children with Hypertrophic Cardiomyopathy
| Age group, years |
%LGE | %LGE >2% |
LV Mass adjusted for BSA, gni/m2 |
LV Mass to LVEDV (gm/ml) |
LV Mass to LVESV (gm/ml) |
Interventricular septal thickness adjusted for BSA, mm/m2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (25%, 75%) |
N | Median (25%, 75%) |
N | Median (25%, 75%) |
N | Median (25%, 75%) |
N | Median (25%, 75%) |
N | Median (25%, 75%) |
|
| Overall | 5 | 6.0 (2.0, | 3 | 9.0 | 5 | 94.3 | 5 | 1.3 | 5 | 4.9 | 63 | 11.6 (6.8,16.2) |
| Birth to 5 | 6 | 11.5 (9.0,13.0) | 6 | 11.5(9.0,13.0) | 6 | 94.3(78.0,138.3) | 6 | 1.3(1.1,1.4) | 6 | 5.4(2.7,6.8) | 10 | 18.4 (11.5,24.9) |
| 6 to 10 | 13 | 7.0 (3.0, 8.0) | 10 | 7.0(6.0,9.0) | 13 | 81.2(74.9,89.2) | 13 | 1.2(0.9,1.3) | 13 | 5.0(3.4,6.5) | 14 | 13.7 (12.0,16.2) |
| 11 to 15 | 28 | 4.5 (1.0, 9.5) | 18 | 8.0(5.0,14.0) | 28 | 104.3(68.7,121.9) | 27 | 1.4(0.9,1.6) | 27 | 4.6(3.0,7.8) | 33 | 10.2 (6.6,13.5) |
| 16+ | 5 | 7.0 (1.0,11.0) | 3 | 11.0(7.0,14.0) | 5 | 77.6(74.9,114.8) | 5 | 1.3(0.9,1.4) | 5 | 4.2(2.8,5.2) | 6 | 7.6 (5.6,10.2) |
Abbreviations: BSA, body surface area; LV, left ventricle; LVEDV, left ventricular end- diastolic volume; LVESV, left ventricular end-systolic volume; %LGE, percent late gadolinium enhancement.
Further analysis of LV mass and interventricular septal thickness adjusted for BSA showed a greater interventricular septal thickness in children <5 years old (median, 18.4 mm/m2; IQR: 11.5 mm/m2, 24.9 mm/m2). Interventricular septal thickness and LV mass adjusted for BSA were strongly correlated (r = 0.70, P<0.001, Table 3). Neither interventricular septal thickness nor LV mass adjusted for BSA had any significant correlation with %LGE (or %LGE >2%), duration of disease, or age at diagnosis. Across all age groups, only 2 children had LGE solely at right/left ventricular hingepoints, also associated with thickened myocardium in this region. These were not present in patients with < 2% LGE.
Table 3.
Pearson Correlation (Correlation P-value) Between Cardiac Parameters, Age at Diagnosis and Disease Duration (Defined as the Time from Date of Diagnosis to Date of Cardiac Magnetic Resonance Imaging).
| %L GE |
Interventricular Septal Thickness (mm) |
LV Mass / BSA (gm/m2’66) |
LV Mass / BSA1’33* (gm/m2’66) |
LV Mass /LVEDV (gm/ ml) |
LV Mass /LVESV (gm/ ml) |
Age at Diagnosis |
Disease Duration (years) |
|
|---|---|---|---|---|---|---|---|---|
| %LGE | 1.0 | |||||||
| Interventricula r Septal Thickness (mm) | 0.02 (0.8 7) | 1.0 | ||||||
| LV Mass / BSA (gm/m2) | 0.27 (0.0 5) | 0.730 (<0.001) | 1.0 | |||||
| LV Mass / BSA1’33* (gm/m2) | 0.28 (0.0 5) | 0.70 (<0.001) | 0.98 (<0.001) | 1.0 | ||||
| LV Mass/LVEDV < 2% LGE. (gm/ml) | 0.16 (0.2 6) | 0.76 (<.0001) | 0.91 (<.0001) | 0.91 (<.0001) | 1.0 | |||
| LV Mass/LVESV (gm/ml) | 0.05 (0.7 2) | 0.67 (<.0001) | 0.66 (<.0001) | 0.67 (<.0001) | 0.74 1.0 (<.00 01) | 1.0 | ||
| Age at Diagnosis (years) | - 0.13 (0.3 4) | 0.08 (0.55) | 0.02 (0.88) | -0.10 (0.47) | 0.004 (0.98) | -0.03 (0.83) | 1.0 | |
| Disease Duration | 0.06 (0.6) | -0.07 (0.58) | 0.04 (0.76) | 0.04 (0.76) | -0.07 (0.64) | -0.08 (0.6) | −0.64*(<0.001) | 1.0 |
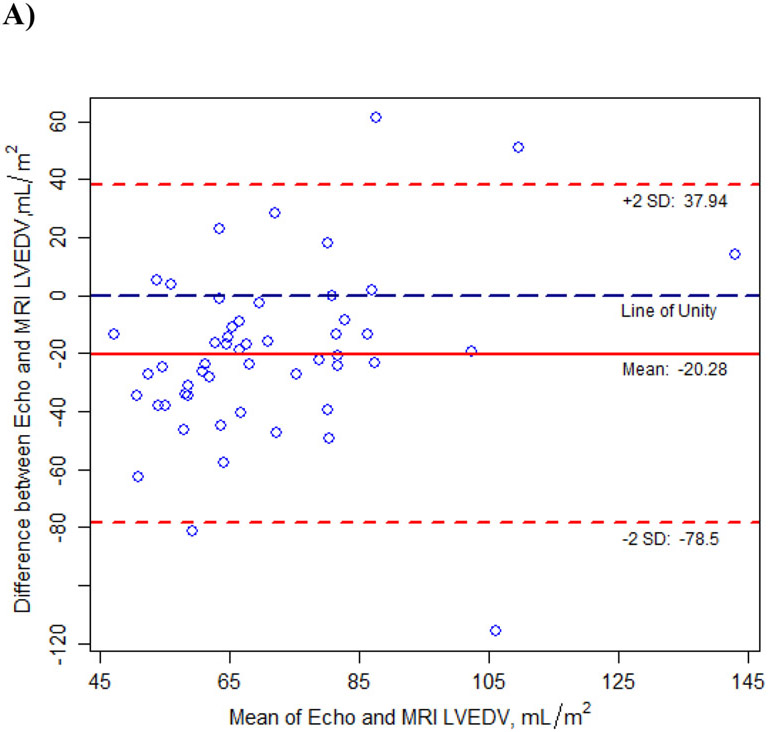
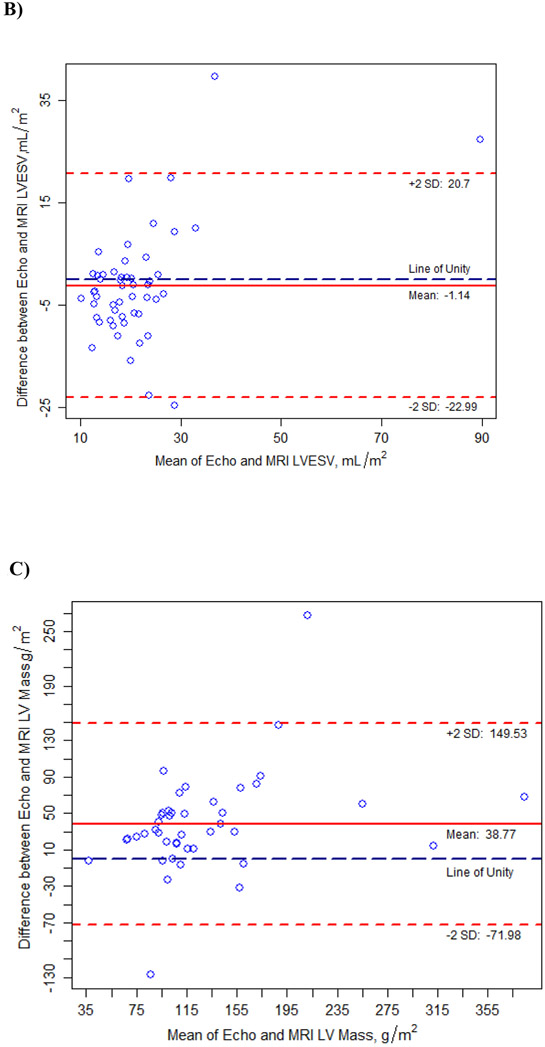
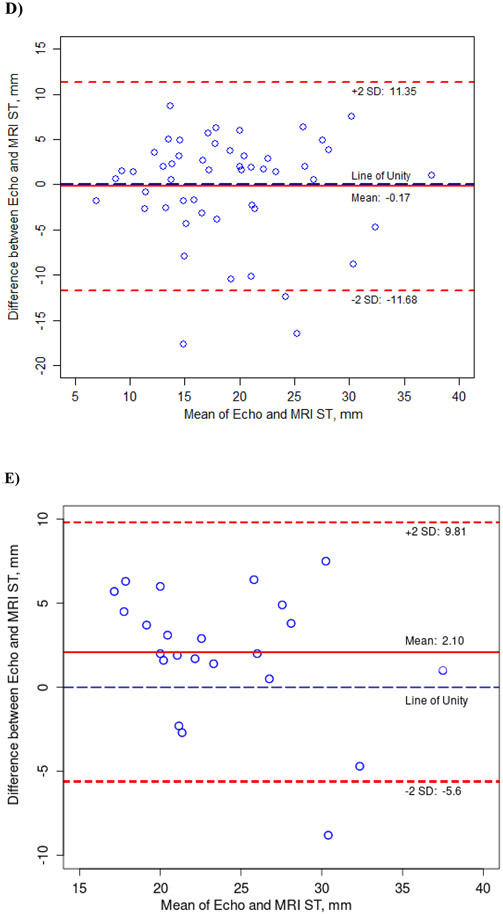
Fifty-five children had evaluable cMRI images and corresponding echocardiographic images acquired within the defined time window. Median time between the cMRI and the echocardiogram was 29 days (IQR: 1 day, 56 days). Bland-Altman analysis indicated good agreement between echocardiographic and cMRI measurements of cardiac structure (LVEDV, LVESV, LV mass, and interventricular septal thickness) within the 95% confidence interval (Figure 1; Supplemental Table). No patient had LV outflow tract obstruction (peak gradient of > 30 mm Hg) as assessed by cMRI or echocardiography at rest.
Figure 1.
Agreement Between Cardiac Measurements from Transthoracic Echocardiograms and Cardiac Magnetic Resonance Images (cMRI) in Children with Hypertrophic Cardiomyopathy, as Determined with the Bland-Altman Method.
All measurements were adjusted for BSA except interventricular septal thickness. A) Left ventricular end-diastolic volume (LVEDV), B) Left ventricular end-systolic volume (LVESV), C) Left ventricular (LV) mass, D) Interventricular septal thickness (ST), E) Interventricular septal thickness (ST) >20 mm by echocardiogram.
Association of Serum Biomarker Concentrations with Cardiac Imaging Measurements
None of the serum biomarkers were significantly associated with %LGE (Table 4). The serum concentration of NT-proBNP was associated (P<0.001) with cMRI measures of LV mass and interventricular septal thickness, and serum cardiac troponin-T concentrations were marginally associated with LV mass adjusted for BSA (P=0.05). A 1%-increase in the average concentration of NT-proBNP (pg/mL) was associated with an increase of 0.20% in the average LV mass (P<0.001) and with a 0.19% increase in the average interventricular septal thickness (P<0.001). A 1%-increase in serum cardiac troponin-T (ng/mL) was marginally associated with an increase of 0.18% in the average LV mass (P=0.05).
Table 4.
Association between Serum Biomarker Concentrations and Cardiac Dimensions as Measured by Cardiac Magnetic Resonance Imaging, Adjusted for Age at Diagnosis, Time Since Diagnosis, Race, and Ethnicity*†
| Log of %LGE | Log of LV Mass (BSA-adjusted) |
Log of LV Mass/ LVEDV |
Log of LV Mass / LVESV |
Log Interventricular Septal Thickness |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum Biomarker |
N | β (SE) |
P- value |
N | β (SE) |
P- value |
N | β (S E) |
P- value |
N | β (S E) |
P- value |
N | β (S E) |
P- value |
| NT-proBN p, pg/mL | 44 | 0.09(0.09) | 0.31 | 4 8 | 0.20(0.03) | <0.001 | 47 | 0.21(0.03) | <0.001 | 47(0.04) | 0.16 | 0.001 | 5 9 | 0.19(0.02) | <0.001 |
| Cardiac troponi n-T, ng/mL | 44 | 0.03(0.20) | 0.90 | 48 | 0.18(0.09) | 0.05 | 47 | 0.12(0.09) | 0.21 | 47 | 0.13(0.11) | 0.22 | 59 | 0.03(0.08) | 0.69 |
Abbreviations: LGE, late gadolinium enhancement; LV, left ventricle; BSA, body surface area; NT-proBNP, N-terminalpro B-type natriuretic peptide.
Four patients with 0% LGE of LV mass were excluded because zero cannot be log-transformed.
Four patients with LAMP2 mutations were excluded from the association analysis.
DISCUSSION
In this study, we found a high prevalence of myocardial fibrosis overall as detected by LGE in children with HCM, particularly in children <5 years old. Additionally, while the interventricular septal thickness and LV mass adjusted for BSA were strongly correlated, neither of these parameters had a significant correlation with %LGE, duration of disease (the clinical HCM phenotype), or age at HCM diagnosis. We also found that echocardiography and cMRI show good agreement in measurements of LV dimensions and interventricular septal thickness. Increases in serum NT-proBNP and cardiac troponin-T concentrations were associated with increasing LV mass. Increased concentrations of serum NT-proBNP were also associated with increases in interventricular septal thickness.
Myocardial Fibrosis
In our cohort, LGE was detected in most children (48/52, 92%) with 34 of 52 children (71%) having >2% LGE of LV mass. Cardiac MRI has the advantage over echocardiography of detecting myocardial fibrosis through LGE, and histologic analyses have confirmed a strong relationship between myocardial fibrosis and %LGE. (23) As suggested in the literature, we used a signal intensity value of 6 SDs above that in the normal myocardium on the most visibly normal slice as the threshold of LGE. (17) However, possible artifacts (a high signal secondary to a motion artifact, for instance) might be included objectively as LGE in an otherwise normal examination, especially at the LV apex. (24, 25)
Few studies have reported the prevalence of myocardial fibrosis in children with HCM Using cMRI, Chaowu et al. found myocardial fibrosis in 52 (73%) of 71 children with HCM, although they did not report %LGE data. (26) In their analysis 2 years later, 11 of the 71 children with HCM had received a cardioverter defibrillator or had undergone heart transplantation, and children with any level of LGE had more adverse outcomes than those without any LGE (P=0.03). El Saiedi el al., also demonstrated similar levels of myocardial fibrosis in their cohort of 40 children with HCM with 82% of children having detectable LGE with average 9.7±9% LGE of LV mass. (27) Another recent study by Raja et al., found myocardial fibrosis in 70 of 155 (46%) children with HCM, with a median %LGE of 3.3% of LV mass (IQR: 0.8%, 7.1%). (28) After a median of 2.5 years, the median %LGE increased from 2.9% (IQR, 0.8%, 3.2%) to 4.3% (IQR: 2.9%, 6.8%; P=0.02). These cohorts, as well as our cohort, illustrate the presence of LGE in children <5 years of age. Such a high prevalence of myocardial fibrosis in children this young with non-obstructive HCM is surprising, given that the duration of disease is shorter in children than in adults. However, our analysis also illustrates that %LGE is not strongly correlated with age at diagnosis or the duration of a clinically detected HCM phenotype. Our study, as well as the other studies discussed, may reflect a selection bias as well for young children with clinically advanced disease who warrant sedation for cardiac MRI and, therefore, LGE assessment.
Overall, as discussed, our study demonstrates presence of myocardial fibrosis like other pediatric cohorts as described. As low levels of LGE (<2%) may also convey background artifact, we also examined our parameters relative to LGE >2% of LV mass. (24, 25) As such, we still identified a large prevalence of myocardial fibrosis in our cohort (71%) but no major differences were seen in further analyses using only children with LGE >2% of %LGE relative to cardiac MRI parameters or serum biomarkers (NT-proBNP and cardiac troponin-T). At this time, we are not aware of other published pediatric cohort analyses to which our results between LGE <2% of LV mass and LGE >2% of LV mass can be compared. Additionally, LGE was solely present at the right/left ventricular hingepoints in only 2 patients. In both patients the myocardium was also thickened and LGE was >2%. As such, this LGE appears to be more related to fibrosis found in HCM rather than mild fibrosis associated with right ventricular strain.
The 2020 AHA/ACC Guidelines for Hypertrophic Cardiomyopathy have updated recommendations for ICD placement with consideration for extensive LGE on cMRI (>15% LGE of LV mass) in adults, in addition to other previously described risk factors. (2) Similar to adult analyses, pediatric cohorts of HCM are under investigation for risk factors for SCD. In particular, the HCM Risk-Kids study by Norrish et al., developed a risk prediction model for SCD in children with HCM with variables including unexplained syncope, interventricular septal thickness, left atrial diameter, LV outflow tract gradient, and non-sustained ventricular tachycardia. (29) Using this model, a newer investigation by Grosse-Wortmann et al., demonstrated LGE to be an independent risk factor for SCD in their analysis of 720 pediatric patients with HCM. (30)
In addition to defining myocardial fibrosis as %LGE measured with cMRI, alternative methods are being explored in adults for measuring myocardial fibrosis in patients with marked nephropathy to avoid the side effects of gadolinium. Native T1 mapping and diffusion-weighted cMRI have both detected myocardial fibrosis. (31, 32) Multiple cMRI methods, including feature-tracking of standard cine images, have the potential to characterize myocardial strain, which decreases as myocardial hypertrophy and myocardial fibrosis increase. (33, 34) Finally, new data suggest that myocardial T2* values are associated with an increased interventricular septum and LV mass indexed by body surface area in a subset of patients with LGE, providing another potential cMRI imaging biomarker for risk stratification. (35) These studies offer promising alternatives for assessing myocardial fibrosis in patients with HCM without the use of contrast agents.
Because of the retrospective nature of this study five different gadolinium-based magnetic resonance imaging contrast agents were used across participating centers. Although, theoretically, the varying contrast agents used could provide some small differences in %LGE, all agents are commonly used in the practice of LGE cMRI and demonstrate similar washout after 5-10 minutes. (36) All agents used, except for gadobenate dimeglumine (MultiHance), have similar relaxivities. (37) The relaxivity or signal of gadobenate dimeglumine is approximately 1.5 times that of the others. (37) In practice, however, all are treated the same in terms of analysis and predictive value. Some prefer to use agents other than gadobenate dimeglumine because of its prolonged half-life in the blood pool secondary to a small intravascular component, and the possibility that small subendocardial infarctions might be missed because of similar signal to the blood pool; however, that was not a concern in this study. (38)
Echocardiography versus Cardiac Magnetic Resonance Imaging
Transthoracic echocardiography remains the most common imaging modality for routinely monitoring patients with HCM. Although cardiac measurements are typically less costly and easier to obtain with echocardiography than with cMRI, they might not be as accurate. A recent study of 19 children with HCM found that measurements made on echocardiograms over-estimated LV wall thickness when compared to those made on cMRI images in certain segments, such as the LV basal anterolateral and apical segments, and underestimated measurements of the mid-ventricular inferior and inferoseptal segments. (39) Some studies in adults have also found discrepancies between echocardiographic and cMRI measurements of interventricular septal thickness and have related these discrepancies to poor acoustic windows, focal LV hypertrophy, LV trabeculations, and the inclusion of the right ventricular myocardium, papillary muscle, and apical septal bundle in the images. (40)
In this study, we found good agreement between echocardiographic and cMRI measurements using the Bland-Altman method, with most data near the line of unity and within the 95% Cl. In particular, interventricular septal thickness between echocardiographic and cMRI measures reveals on average no significant difference between the two imaging modalities. However, as noted earlier, cMRI measures are thought to be more accurate with regards to the interventricular septal thickness. (39, 40). Given these results, cMRI may provide additional clarity in the interventricular septal thickness measurement when considering ICD placement based upon the accepted threshold of 30 mm interventricular septal thickness (or massive LVH by Z-score). (2) Recent guidelines question reliance on absolute interventricular thickness (30 mm) as an indication for pediatric implantable cardioverter-defibrillator placement; rather using age-related septal thickness Z-scores may be preferable (2). In investigating the range of interventricular thickness in this pediatric cohort by echocardiogram, median septal thickness measured 17.0 mm (IQR: 14.0 mm, 22.0 mm) with a median Z-score of+10.4 (IQR: +5.3, +18.1), consistent with significant age-related hypertrophy (Supplemental Table). As noted in Figure 1D, there is good agreement overall between echocardiographic and cMRI measures for septal thickness (Mean difference -0.2 mm; 95% CI: -11.7 mm, 11.4 mm). Given this distribution, we further examined interventricular septal thickness by echocardiogram ≥ 20 mm (Figure 1E). As Figure 1E illustrates, our cohort has 23 children with an interventricular septal thickness >20 mm by echocardiogram with the mean of differences of 2.1 mm (95% CI: -5.6 mm, 9.8 mm). As such, when interventricular septal thickness is <20 mm by echocardiogram, it is more likely to correlate with interventricular septal thickness by cMRI and echocardiogram may be sufficient for the routine surveillance of LV dimensions and interventricular septal thickness.
Associations of Serum Biomarkers with Cardiac Magnetic Resonance Imaging
We found that NT-proBNP concentrations were associated with interventricular septal thickness and LV mass and that serum cardiac troponin-T concentrations were also associated with LV mass, as measured by cMRI. Concentrations of serum NT-proBNP can predict HF in patients with HCM and, in children with HCM, NT-proBNP concentrations previously have been correlated with non-invasive measurements of disease severity and may increase with increased filling pressures in the heart. (41-43) Similarly, serum cardiac troponin-T concentrations have been used to evaluate myocardial injury, especially in adults with HF. (44-46) Recent analysis of an NHLBI supported registry of adults with HCM found that serum concentrations of B-type natriuretic peptide (BNP) and highly sensitive cardiac troponin-T were significantly associated with LGE as measured on cMRI. (47) Studies of the association between imaging characteristics and biomarkers may clarify the usefulness of these biomarkers for disease surveillance in pediatric HCM.
Strengths and Limitations of the Study
Strengths of the study include the prospective identification of eligible cases and the central measurement of cMRIs, echocardiograms, and select serum biomarkers (NT-proBNP and cardiac troponin-T). Children were selected with clinical eligibility criteria and may not be representative of all children with HCM. Children included in this study are from major tertiary pediatric heart centers in the U.S. and Canada. Additionally, because many children under 5 years of age require sedation for cMRI, our results likely reflect more severe cases in which cMRI was clinically warranted at the discretion of providers. Because adolescents generally do not require sedation for cMRI, they notably comprise the largest subset of our analysis. Given our study design, we also were not able to relate our data to genetic information and therefore could not make genotype-phenotype correlations. Additionally, we have insufficient data for left atrial dimensions, which is also a key variable in assessment of children with HCM. Our study also is not designed to correlate cMRI data with risk for arrhythmia or ICD placement as most devices were placed for primary prevention. Finally, as this is not a longitudinal study, we lack sufficient data with regard to outcomes for the parameters studied.
Conclusions
In children with HCM, cMRI measurements of %LGE detected a high prevalence but overall low levels of myocardial fibrosis. Echocardiography and cMRI provided similar measurements of cardiac structure, LV mass, and interventricular septal thickness, suggesting that cMRI need not be used to confirm interventricular septal thickness alone, unless further myocardial characterization and measurement of the extent of myocardial fibrosis are required. Finally, increases in serum NT-proBNP and cardiac troponin-T concentrations may reflect increases in LV mass and interventricular septal thickness; however, no biomarkers were associated with the presence or absence, or the level of myocardial fibrosis.
Recently published HCM guidelines (48) have discouraged serial evaluations of HCM in children < 12 years of age. However, the possibility of attenuation of HCM with therapies instituted early in the course of HCM has been demonstrated in the VANISH trial and in prior work in which we have been part of in genotype positive-phenotype negative children with hypertrophic cardiomyopathy (49,50). Longitudinal studies of the rates of change in myocardial fibrosis, LV mass and dimensions, and serum NT-proBNP and cardiac troponin-T concentrations and the rates of SCD or heart transplantation are warranted to determine the predictive value of each of these variables as well as evaluating the impact of future attenuating therapies on the natural history of HCM.
Supplementary Material
Acknowledgments
We thank the participating centers for patient recruitment and follow-up data collection. We also thank the Children’s Cardiomyopathy Foundation (CCF) and the Kyle John Rymiszewski Foundation (KJRF) for their ongoing support of the Pediatric Cardiomyopathy Registry’s HCM research efforts.
Abbreviations and Acronyms:
- BNP
B-type natriuretic peptide
- BSA
body surface area
- CLIA
Clinical Laboratory Improvement Amendments
- cMRI
cardiac magnetic resonance imaging
- HF
heart failure
- HCM
hypertrophic cardiomyopathy
- IQR
interquartile range
- ICD
implantable cardioverter defibrillator
- LGE
late gadolinium enhancement
- LV
left ventricle
- LVEDV
left ventricular end-diastolic volume
- LVESV
left ventricular end-systolic volume
- NHLBI
National Heart, Lung, and Blood Institute
- NT-proBNP
N-terminal pro hormone B-type natriuretic peptide
- SCD
sudden cardiac death
Footnotes
Declaration of Interests:
E Pahl is a consultant for Tenaya Therapeutics. JW Rossano is a consultant for Bayer, Abiomed, Novartis, Cytokinetics, and Myokardia. PK Woodard has a research agreement/funding with Siemens Medical Systems and research funding from Bayer. B Feingold is a consultant to Stealth Biotherapeutics. SE Lipshultz has had consultant agreements with Tenaya Therapeutics and Bayer, and has served on an advisory board for Myokardia. He is also the chairman of the medical advisory board of the CCF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. [DOI] [PubMed] [Google Scholar]
- 2.Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J Am Coll Card. 2020;76:e159–e240. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation. 1995;92:785–789. [DOI] [PubMed] [Google Scholar]
- 4.Colan SD, Lipshultz SE, Lowe AM, Sleeper LA, Messere J, Cox GF, Lurie PR, Orav EJ, Towbin JA. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: Findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115:773–781. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Orav EJ, Wilkinson JD, Towbin JA, Messere JE, Lowe AM, Sleeper LA, Cox GF, Hsu DT, Canter CE, Hunter JA, Colan SD. Risk stratification at diagnosis for children with hypertrophic cardiomyopathy: An analysis of data from the Pediatric Cardiomyopathy Registry. Lancet. 2013;382:1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron BJ, Maron MS. The 20 advances that have defined contemporary hypertrophic cardiomyopathy. Trends Cardiovasc Med. 2015;25:54–64. [DOI] [PubMed] [Google Scholar]
- 7.Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y. Prognostic value of LGECMR in HCM: A meta-analysis. JACC Cardiovasc Imaging. 2016;9:1392–1402. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Maron MS. LGE means better selection of HCM patients for primary prevention implantable defibrillators. JACC: Cardiovascular Imaging. 2016;9:1403–1406. [DOI] [PubMed] [Google Scholar]
- 9.Weissler-Snir A, Adler A, Williams L, Gruner C, Rakowski H. Prevention of sudden death in hypertrophic cardiomyopathy: Bridging the gaps in knowledge. Eur Heart J. 2017;38:1728–1737. [DOI] [PubMed] [Google Scholar]
- 10.Maron MS. The role of cardiovascular magnetic resonance in sudden death risk stratification in hypertrophic cardiomyopathy. Card Electrophysiol Clin. 2015;7:187–193. [DOI] [PubMed] [Google Scholar]
- 11.Geske JB, Ommen SR. Role of imaging in evaluation of sudden cardiac death risk in hypertrophic cardiomyopathy. Curr Opin Cardiol. 2015;30:493–499. [DOI] [PubMed] [Google Scholar]
- 12.Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: A meta-analysis. Heart. 2015;101:1406–1411. [DOI] [PubMed] [Google Scholar]
- 13.Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. New Engl J Med. 2010;363:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho JE, Shi L, Day SM, Colan SD, Russell MW, Towbin JA, Sherrid MV, Canter CE, Jefferies JL, Murphy A, Taylor M, Mestroni L, Cirino AL, Sleeper LA, Jarolim P, Lopez B, Gonzalez A, Diez J, Orav EJ, Ho CY. Biomarkers of cardiovascular stress and fibrosis in preclinical hypertrophic cardiomyopathy. Open Heart. 2017;4:e000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernlund E, Gyllenhammar T, Jablonowski R, Carlsson M, Larsson A, Ärnlöv J, Liuba P. Serum biomarkers of myocardial remodeling and coronary dysfunction in early stages of hypertrophic cardiomyopathy in the young. Pediatr Cardiol. 2017;38:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everitt MD, Wilkinson JD, Shi L, Towbin JA, Colan SD, Kantor PF, Canter CE, Webber SA, Hsu DT, Pahl E, Addonizio LJ, Dodd DA, Jefferies JL, Rossano JW, Feingold B, Ware SM, Lee TM, Godown J, Simpson KE, Sleeper LA, Czachor JD, Razoky H, Hill A, Westphal J, Molina KM, Lipshultz SE. Cardiac biomarkers in pediatric cardiomyopathy: Study design and recruitment results from the Pediatric Cardiomyopathy Registry. Prog Pediatr Cardiol. 2019;53:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiewak M, Malek LA, Misko J, Chojnowska L, Milosz B, Klopotowski M, Petryka J, Dabrowski M, Kepka C, Ruzyllo W. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol. 2010;74 e149–153. [DOI] [PubMed] [Google Scholar]
- 18.Galati G, Pasquale F, Leone O, Olivotto I, Grigioni F, Pilato E, Biagini E, Cecchi F, Rapezzi C. Accuracy of LGE-CMR compared with histometric quantification of myocardial fibrosis in transplanted hearts of end-stage HCM. Eur Heart J 2017;38:ehx504.P4497. [Google Scholar]
- 19.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the pediatric measurements writing group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495; quiz 576-577. [DOI] [PubMed] [Google Scholar]
- 20.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. [DOI] [PubMed] [Google Scholar]
- 21.Sluysmans T, Colan SD. Structural measurements and adjustment for growth. In: Lai WW, Mertens LL, Cohen MS, Geva T, ed. Echocardiography in pediatric and congenital heart disease. West Sussex, UK: Wiley-Blackwell; 2016. [Google Scholar]
- 22.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. [DOI] [PubMed] [Google Scholar]
- 23.Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:2260–2264. [DOI] [PubMed] [Google Scholar]
- 24.Harrigan CJ, Peters DC, Gibson CM, Maron BJ, Manning WJ, Maron MS, Appelbaum E. Hypertrophic cardiomyopathy: Quantification of late gadolinium enhancement with contrast-enhanced cardiovascular mr imaging. Radiology. 2011;258:128–133. [DOI] [PubMed] [Google Scholar]
- 25.Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016;9:e004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaowu Y, Shihua Z, Jian L, Li L, Wei F. Cardiovascular magnetic resonance characteristics in children with hypertrophic cardiomyopathy. Circ Heart Fail. 2013;6:1013–1020. [DOI] [PubMed] [Google Scholar]
- 27.El Saiedi S, Behairy NH, Kharabish A, Esmail R, Seliem ZS, Shafik M, El Mozy W. Delayed myocardial enhancement in pediatric hypertrophic cardiomyopathy: Correlation with LV function, echocardiography, and demographic parameters. Pediatr Cardiol. 2017;38:1024–1031. [DOI] [PubMed] [Google Scholar]
- 28.Raja AA, Farhad H, Valente AM, Couce J-P, Jefferies JL, Bundgaard H, Zahka K, Lever H, Murphy AM, Ashley E, Day SM, Sherrid MV, Shi L, Bluemke DA, Canter CE, Colan SD, Ho CY, Towbin JA, Russell M, Patel A, Kim B, Taylor M, Mestroni L. Prevalence and progression of late gadolinium enhancement in children and adolescents with hypertrophic cardiomyopathy. Circulation. 2018;138:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norrish G, Ding T, Field E, Ziolkowska L, Olivotto I, Limongelli G, Anastasakis A, Weintraub R, Biagini E, Ragni L, Prendiville T, Duignan S, McLeod K, Ilina M, Fernández A, Bökenkamp R, Baban A, Kubuš P, Daubeney PEF, Sarquella-Brugada G, Cesar S, Marrone C, Bhole V, Medrano C, Uzun O, Brown E, Gran F, Castro FJ, Stuart G, Vignati G, Barriales-Villa R, Guereta LG, Adwani S, Linter K, Bharucha T, Garcia-Pavia P, Rasmussen TB, Calcagnino MM, Jones CB, De Wilde H, Toru-Kubo J, Felice T, Mogensen J, Mathur S, Reinhardt Z, OMahony C, Elliott PM, Omar RZ, Kaski JP. Development of a novel risk prediction model for sudden cardiac death in childhood hypertrophic cardiomyopathy (HCM Risk-Kids). JAMA Cardiol. 2019;4:918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grosse-Wortmann L, Wal Lvd, House AV, Benson L, Chan R. Abstract 14760: Myocardial scarring by magnetic resonance predicts sudden cardiac death in pediatric patients with hypertrophic cardiomyopathy. Circulation. 2020;142:A14760. [Google Scholar]
- 31.Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals. 2016;29:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen C, Lu M, Fan Z, Bi X, Kellman P, Zhao S, Li D. Contrast-free detection of myocardial fibrosis in hypertrophic cardiomyopathy patients with diffusion-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu HY, Chen J, Yang ZG, Li R, Shi K, Zhang Q, Liu X, Xie LJ, Jiang L, Guo YK. Early marker of regional left ventricular deformation in patients with hypertrophic cardiomyopathy evaluated by MRI tissue tracking: The effects of myocardial hypertrophy and fibrosis. J Magn Reson Imaging. 2017;46:1368–1376. [DOI] [PubMed] [Google Scholar]
- 34.Wu CW, Wu R, Shi RY, An DA, Chen BH, Jiang M, Bacyinski A, Rahim A, Deen JM, Hu J, Han TT, Xu JR, Wu LM. Histogram analysis of native t(1) mapping and its relationship to left ventricular late gadolinium enhancement, hypertrophy, and segmental myocardial mechanics in patients with hypertrophic cardiomyopathy. J Magn Reson Imaging. 2019;49:668–677. [DOI] [PubMed] [Google Scholar]
- 35.Gastl M, Gotschy A, von Spiczak J, Polacin M, Bönner F, Gruner C, Kelm M, Ruschitzka F, Alkadhi H, Kozerke S, Manka R. Cardiovascular magnetic resonance t2* mapping for structural alterations in hypertrophic cardiomyopathy. Eur J Radiol Open. 2019;6:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng H, Grosse-Wortmann L Gadolinium in pediatric cardiovascular magnetic resonance: what we know and how we practice. J Cardiovasc Magn Reson. 2012;14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Y, Goerner FL, Snyder C, Morelli JN, Hao D, Hu D, Li X, Runge VM. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330–338. [DOI] [PubMed] [Google Scholar]
- 38.Yamrozik J, Rathi VK, Williams RB, Meister J, Rayarao G, Vido DA, Doyle M, Biederman RWW. Can a higher relaxivity contrast agent hinder subendocardial infarct detection in viability imaging? J Cardiovasc Magn Reson 2010;12:T11. [Google Scholar]
- 39.Windram JD, Dragelescu A, Benson L, Forsey J, Shariat M, Yoo SJ, Mertens L, Wong D, Grosse-Wortmann L. Myocardial dimensions in children with hypertrophic cardiomyopathy: A comparison between echocardiography and cardiac magnetic resonance imaging. Can J Cardiol. 2016;32:1507–1512. [DOI] [PubMed] [Google Scholar]
- 40.Hindieh W, Weissler-Snir A, Hammer H, Adler A, Rakowski H, Chan RH. Discrepant measurements of maximal left ventricular wall thickness between cardiac magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10:e006309. [DOI] [PubMed] [Google Scholar]
- 41.Kaski JP, Tomé-Esteban MT, Mead-Regan S, Pantazis A, Marek J, Deanfield JE, McKenna WJ, Elliott PM. B-type natriuretic peptide predicts disease severity in children with hypertrophic cardiomyopathy. Heart. 2008;94:1307–1311. [DOI] [PubMed] [Google Scholar]
- 42.Öner T, Özdemir R, Hazan F, Karadeniz C, Doksoz Ö, Yilmazer MM, Meşe T, Tavli V. The association between brain natriuretic peptide and tissue doppler parameters in children with hypertrophic cardiomyopathy. Bosn J Basic Med Sci. 2016;16:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyaji Y, Iwanaga Y, Nakamura T, Yasuda M, Kawamura T, Miyazaki S. Interrelationship between the myocardial mass, fibrosis, BNP, and clinical outcomes in hypertrophic cardiomyopathy. Intern Med. 2016;55:1261–1268. [DOI] [PubMed] [Google Scholar]
- 44.Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc Res. 2017;113:1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JAC, de Lemos JA, Bertoni A, deFilippi CR. High-sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (multi-ethnic study of atherosclerosis). Circulation. 2017;135:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagarajan V, Hernandez AV, Tang WH. Prognostic value of cardiac troponin in chronic stable heart failure: A systematic review. Heart. 2012;98:1778–1786. [DOI] [PubMed] [Google Scholar]
- 47.Neubauer S, Kolm P, Ho CY, Kwong RY, Desai MY, Dolman SF, Appelbaum E, Desvigne-Nickens P, DiMarco JP, Friedrich MG, Geller N, Harper AR, Jarolim P, Jerosch-Herold M, Kim DY, Maron MS, Schulz-Menger J, Piechnik SK, Thomson K, Zhang C, Watkins H, Weintraub WS, Kramer CM. Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM Registry. J Am Coll Cardiol. 2019;74:2333–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Rowin EJ, Maron MS, Sherrid MV. Diagnosis and evaluation of hypertrophic cardiomyopathy. JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:372–389. [DOI] [PubMed] [Google Scholar]
- 49.Ho CY, Day SM, Axelsson A, Russell MW, Zahka K, Lever HM, Pereira AC, Colan SD, Margossian R, Murphy AM, Canter C, Bach RG, Wheeler MT, Rossano JW, Owens AT, Bundgaard H, Benson L, Mestroni L, Taylor MRG, Patel AR, Wilmot I, Thrush P, Vargas JD, Soslow JH, Becker JR, Seidman CE, Lakdawala NK, Cirino AL; VANISH Investigators, Burns KM, McMurray JJV, MacRae CA, Solomon SD, Orav EJ, Braunwald E. Valsartan in early-stage hypertrophic cardiomyopathy; a randomized phase 2 trial. Nat Med. 2021;27:1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho CY, Lakdawala NK, Cirino AL, Lipshultz SE, Sparks E, Abbasi SA, Kwong RY, Antman EM, Semsarian C, Gonzalez A, Lopez B, Diez J, Orav EJ, Colan SD, Seidman CE. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: A pilot randomized trial to modify disease expression. JACC Heart Fail. 2015;3:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.