Abstract
The phylogenetic position of several clitocyboid/pleurotoid/tricholomatoid genera previously considered incertae sedis is here resolved using an updated 6-gene dataset of Agaricales including newly sequenced lineages and more complete data from those already analyzed before. Results allowed to infer new phylogenetic relationships, and propose taxonomic novelties to accommodate them, including up to ten new families and a new suborder. Giacomia (for which a new species from China is here described) forms a monophyletic clade with Melanoleuca (Melanoleucaceae) nested inside suborder Pluteineae, together with the families Pluteaceae, Amanitaceae (including Leucocortinarius), Limnoperdaceae and Volvariellaceae. The recently described family Asproinocybaceae is shown to be a later synonym of Lyophyllaceae (which includes also Omphaliaster and Trichocybe) within suborder Tricholomatineae. The families Biannulariaceae, Callistosporiaceae, Clitocybaceae, Fayodiaceae, Macrocystidiaceae (which includes Pseudoclitopilus), Entolomataceae, Pseudoclitocybaceae (which includes Aspropaxillus), Omphalinaceae (Infundibulicybe and Omphalina) and the new families Paralepistaceae and Pseudoomphalinaceae belong also to Tricholomatineae. The delimitation of the suborder Pleurotineae (= Schizophyllineae) is discussed and revised, accepting five distinct families within it, viz. Pleurotaceae, Cyphellopsidaceae, Fistulinaceae, Resupinataceae and Schizophyllaceae. The recently proposed suborder Phyllotopsidineae (= Sarcomyxineae) is found to encompass the families Aphroditeolaceae, Pterulaceae, Phyllotopsidaceae, Radulomycetaceae, Sarcomyxaceae (which includes Tectella), and Stephanosporaceae, all of them unrelated to Pleurotaceae (suborder Pleurotineae) or Typhulaceae (suborder Typhulineae). The new family Xeromphalinaceae, encompassing the genera Xeromphalina and Heimiomyces, is proposed within Marasmiineae. The suborder Hygrophorineae is here reorganized into the families Hygrophoraceae, Cantharellulaceae, Cuphophyllaceae, Hygrocybaceae and Lichenomphaliaceae, to homogenize the taxonomic rank of the main clades inside all suborders of Agaricales. Finally, the genus Hygrophorocybe is shown to represent a distinct clade inside Cuphophyllaceae, and the new combination H. carolinensis is proposed.
Taxonomic novelties: New suborder: Typhulineae Vizzini, Consiglio & P. Alvarado. New families: Aphroditeolaceae Vizzini, Consiglio & P. Alvarado, Melanoleucaceae Locq. ex Vizzini, Consiglio & P. Alvarado, Paralepistaceae Vizzini, Consiglio & P. Alvarado, Pseudoomphalinaceae Vizzini, Consiglio & P. Alvarado, Volvariellaceae Vizzini, Consiglio & P. Alvarado, Xeromphalinaceae Vizzini, Consiglio & P. Alvarado. New species: Giacomia sinensis J.Z. Xu. Stat. nov.: Cantharellulaceae (Lodge, Redhead, Norvell & Desjardin) Vizzini, Consiglio & P. Alvarado, Cuphophyllaceae (Z.M. He & Zhu L. Yang) Vizzini, Consiglio & P. Alvarado, Hygrocybaceae (Padamsee & Lodge) Vizzini, Consiglio & P. Alvarado, Lichenomphaliaceae (Lücking & Redhead) Vizzini, Consiglio & P. Alvarado. New combination: Hygrophorocybe carolinensis (H.E. Bigelow & Hesler) Vizzini, Consiglio & P. Alvarado. New synonyms: Sarcomyxineae Zhu L. Yang & G.S. Wang, Schizophyllineae Aime, Dentinger & Gaya, Asproinocybaceae T. Bau & G.F. Mou. Incertae sedis taxa placed at family level: Aphroditeola Redhead & Manfr. Binder, Giacomia Vizzini & Contu, Hygrophorocybe Vizzini & Contu, Leucocortinarius (J.E. Lange) Singer, Omphaliaster Lamoure, Pseudoclitopilus Vizzini & Contu, Resupinatus Nees ex Gray, Tectella Earle, Trichocybe Vizzini. New delimitations of taxa: Hygrophorineae Aime, Dentinger & Gaya, Phyllotopsidineae Zhu L. Yang & G.S. Wang, Pleurotineae Aime, Dentinger & Gaya, Pluteineae Aime, Dentinger & Gaya, Tricholomatineae Aime, Dentinger & Gaya. Resurrected taxa: Fayodiaceae Jülich, Resupinataceae Jülich.
Citation: Vizzini A, Alvarado P, Consiglio G, Marchetti M, Xu J (2024). Family matters inside the order Agaricales: systematic reorganization and classification of incertae sedis clitocyboid, pleurotoid and tricholomatoid taxa based on an updated 6-gene phylogeny. Studies in Mycology 107: 67–148. doi: 10.3114/sim.2024.107.02
Keywords: Agaricales, Agaricanae, incertae sedis taxa, multi-locus, new taxa, phylogeny, taxonomy
INTRODUCTION
Tricholomatineae is one of the suborders in which order Agaricales is currently divided. It names a lineage whose monophyletic status is significantly supported by phylogenomic and multilocus phylogenetic analyses (Dentinger et al. 2016, Zhao et al. 2017, Varga et al. 2019, Ke et al. 2020, Olariaga et al. 2020, Sánchez-García et al. 2020, Wang et al. 2023b). This suborder corresponds to the Tricholomatoid clade as delimited by Binder et al. (2010), which was also detected before them by other authors (Moncalvo et al. 2002, Matheny et al. 2006, Garnica et al. 2007). It currently contains about thirty genera, including ectomycorrhizal and nonectomycorrhizal groups (Sánchez-García et al. 2014, Sánchez-García 2016). In addition to the Tricholomataceae (Sánchez-García et al. 2014), it currently encompasses another 10 families: Asproinocybaceae (Bau & Mou 2021), Biannulariaceae (Vizzini et al. 2020a), Callistosporiaceae (Vizzini et al. 2020a), Clitocybaceae (Alvarado et al. 2015, Vizzini et al. 2020b), Entolomataceae (Kluting et al. 2014), Fayodiaceae (Moncalvo et al. 2002), Lyophyllaceae (Hofstetter et al. 2014, Bellanger et al. 2015), Macrocystidiaceae (Dentinger et al. 2016), Omphalinaceae (Vizzini et al. 2020b), and Pseudoclitocybaceae (Alvarado et al. 2018a).
Still, many white-spored clitocyboid and tricholomatoidlooking genera cannot be easily classified within any of these families, and even their position inside suborder Tricholomatineae cannot be confirmed with phylogenetic analyses because of the incomplete data available from some of them (mostly ribosomal DNA sequences). For example, the classification of Asproinocybe, Aspropaxillus, Dendrocollybia, Giacomia, Hertzogia, Hygrophorocybe, Infundibulicybe, Lepistella, Leucocortinarius, Notholepista, Omphaliaster, Omphalina, Paralepista, Paralepistopsis, Pseudoclitopilus, Pseudoomphalina, Resupinatus, Rimbachia, Ripartites, Trichocybe or Tricholosporum is not fully clear (Vizzini et al. 2010, 2012a, b, 2020, Hofstetter et al. 2014, Sánchez-García et al. 2014, 2016, 2017, Vizzini 2014a, Angelini et al. 2017, Alvarado et al. 2018a, b, He et al. 2019, Raj et al. 2019, Varga et al. 2019, Kalichman et al. 2020, Olariaga et al. 2020, He & Yang 2022, Wiest 2022).
The classification of these incertae sedis lineages requires the reconstruction of the phylogeny of the entire order Agaricales. DNA-based studies of the evolutionary history and taxonomy of Agaricales can be classified in different stages, depending on the scope and the sources of information employed:
Early works: the first sequence-based phylogenetic analyses of fungi were not specifically focused on the internal structure of Agaricales, but instead addressed fungal classification at higher ranks and/or investigated the origin of specific morphological types (Swann & Taylor 1993, 1995a, b, Gargas et al. 1995, Hibbett et al. 1997, Bruns et al. 1998, Pine et al. 1999, Thorn et al. 2000, Hibbett & Donoghue 2001, Hibbett & Thorn 2001, Binder & Hibbett 2002, Hibbett & Binder 2002). These works were based on too scarce information, often coming from a single ribosomal DNA (rDNA) gene region obtained from distant and highly diverse groups.
Mainly LSU-based works: the internal structure of order Agaricales was specifically addressed at first employing sequences of nuclear rDNA, typically the 28S or large subunit (LSU). These works (Moncalvo et al. 2000, 2002, Bodensteiner et al. 2004, Binder et al. 2005, 2006, Walther et al. 2005) successfully obtained significant support for multiple clades inside Agaricales, helping to delimit the phylogenetic concept of classical families. However, the relationships between these families were rarely resolved with this approach, and sometimes results varied if different datasets were employed.
Multigene works: the addition of more information coming from protein-coding genes greatly improved the outcome of phylogenetic analyses of Agaricales. A relationship between the amount of information and the significance of results seems plausible. For example, the use of LSU, SSU (the 18S nrDNA or small subunit), RPB1 (DNA-directed RNA polymerase II, largest subunit) and RPB2 (DNA-directed RNA polymerase II, second largest subunit) (Matheny et al. 2006) allowed to produce a seminal reconstruction of the structure of Agaricales, obtaining statistical support for multiple major clades (now suborders). However, a more limited analysis using only LSU and RPB1 (Garnica et al. 2007) led to good support values for most suborders, excepting Tricholomatineae and Marasmiineae, while Hygrophorineae could not be separated from the pteruloid lineages. The analysis of additional information, coming from LSU, SSU, RPB1, RPB2 and TEF1 (translation elongation factor 1-alpha) sequences (Matheny et al. 2007) suggested some changes to the previous results (i.e., in the position of Pluteus and Amanita) but the new dataset also contained a different selection of taxa. A too diverse dataset could be the cause behind the lack of support of most suborders of Agaricales in the analysis of Binder et al. (2010), a work focused on the closely related order Amylocorticiales which included also sequences of Boletales, Atheliales and Jaapiales, as well as other orders as outgroups. The phylogenies in Zhao et al. (2017) and He et al. (2019) used even larger datasets containing all lineages of Basidiomycotina and some Ascomycotina, and both failed to obtain significant support for most suborders and families of Agaricales. On the other hand, Olariaga et al. (2020) employed a dataset filling an important gap in the diversity of this order, that of typhuloid fungi, obtaining good support for most suborders, but missed important lineages from some of them (i.e., Giacomia, Hohenbuehelia, Limacella, Mycena, Resupinatus, Volvariella). The most recent study of Agaricales following the multigene phylogenetic approach is that of Sheikh et al. (2022), which analyzed a large dataset of LSU, SSU, RPB1 and RPB2 sequences of multiple species of Ascomycotina, Basidiomycotina and Mucoromycotina. While support values cannot be directly checked in the published figures, the position of several clades does not fit with that in previous works, i.e., Amanitaceae (nested in Agaricineae), Hypsizygus (nested in Pluteineae), Cantharocybe and Tricholomopsis (nested in Pleurotineae), Sarcomyxaceae (sister to Hygrophorineae), or Typhulaceae and Phyllotopsidineae (related and sister to Marasmiineae).
Phylogenomic works: Next generation sequencing (NGS) of entire genomes provides a much larger amount of information than Sanger sequencing of individual target regions. The first attempts to build a genome database of fungi (Grigoriev et al. 2014) were followed by the first phylogenomic analysis of Agaricales (Dentinger et al. 2016), that employed 208 different loci. The result was the proposal of a new taxonomic arrangement dividing Agaricales into seven distinct suborders, which matched more or less the clades found in previous phylogenetic studies based on 5–6 loci. Later, Ke et al. (2020) incorporated additional information from genomes produced by multiple researchers, as well as those of five bioluminiscent species of Mycenaceae obtained by them. After the analysis of 360 loci, they produced a phylogeny consistent with that of Dentinger et al. (2016), but unfortunately important clades were not included (i.e., Hygrophorineae, Clavariineae, Phyllotopsidineae, Tricholomatineae). Li et al. (2021) built a phylogeny of the kingdom Fungi based on sequences of 290 loci obtained from genomic data of 1 679 taxa (89 Agaricales), obtaining significant support for the suborders Agaricineae, Pluteineae and Tricholomatineae, but apparently merging Hygrophorineae and the family Clavariaceae, as well as Pleurotineae and Pterulaceae. Recently, Wang et al. (2023b) further improved the resolution of phylogenomic studies by sequencing 38 new genomes, from which 555 genes were compared with those of the other sequenced Agaricales. As a result, ten suborders were recognized (after separating Phyllotopsidineae and Sarcomyxineae from Pleurotineae), but some families did not nest inside any of them (i.e., Mycenaceae and Typhulaceae).
In the present study, new sequences from some of the aforementioned incertae sedis taxa (Tables 1–3, S1, Figs 6–8) were produced in order to resolve their most probable phylogenetic position after the analysis of an updated 6-gene dataset of Agaricales. Additional sequences from other clades were produced as well to create a representative background for phylogenetic analysis. Results are compared with those published in previous works and different taxonomic decisions are taken accordingly.
Table 1.
Taxa, vouchers, and GenBank accessions numbers of the DNA sequences used in the Agaricales-wide phylogenetic analysis inferred from a six-gene dataset (5.8S, LSU, SSU, RPB1, RPB2 and TEF1). Sequences in bold were generated in this study.
| Group | Species | Voucher | LSU | RPB2 | SSU | TEF1 | ITS | RPB1 |
|---|---|---|---|---|---|---|---|---|
| Agaricineae | Agaricus bisporus | AFTOL-ID 448, RWK1885 | AY635775 | genome | AY787216 | GU187673 | DQ404388 | — |
| Apioperdon pyriforme | AFTOL-ID 480, DSH 96-054 | AF287873 | AY218495 | AF026619 | AY883426 | AY854075 | AY860523 | |
| Bolbitius vitellinus | AFTOL-ID 730, MTS5020 | AY691807 | — | AY705955 | DQ408148 | DQ200920 | DQ435802 | |
| Conocybe lactea | AFTOL-ID 1675, CUWPBM2706 + NL1012 | DQ457660 | DQ470834 | DQ437683 | JX968427 | DQ486693 | DQ447893 | |
| Coprinopsis cinereus | A43mut B43mut pab1-1 #326 | AF041494 | genome | genome | genome | genome | genome | |
| Coprinus comatus | AFTOL-ID 626, ECV3198 | AY635772 | AY780934 | AY665772 | AY881026 | AY854066 | AY857983 | |
| Cortinarius iodes | AFTOL-ID 285, PBM2426 | AY702013 | AY536285 | AY771605 | AY881027 | AF389133 | AY857984 | |
| Crepidotus cf. applanatus | WTUPBM717 | AY380406 | AY333311 | AY705951 | DQ028581 | DQ202273 | AY333303 | |
| Crucibulum laeve | CBS:166.37 | MH867376 | genome | genome | genome | genome | genome | |
| Cyathus striatus | NPCB87405 | genome | genome | genome | genome | genome | genome | |
| Cystoderma amianthinum | HKAS: 107327 + AFTOL-ID 1553 | MW258914 | MW289806 | DQ440632 | MW324496 | MW258862 | MW289817 | |
| Echinoderma flavidoasperum | KUN-HKAS:87905 | MN810098 | MN820969 | — | MN820903 | MN810147 | — | |
| Floccularia luteovirens | FLZJUC10 | genome | genome | genome | genome | genome | genome | |
| Hebeloma velutipes | AFTOL-ID 980, PBM2277 | AY745703 | DQ472718 | AY752972 | GU187707 | AY818351 | DQ447904 | |
| Hydnangium cameurn | Trappe31123 | KU685892 | KU686038 | — | KU686144 | KU685741 | — | |
| Inocybe myriadophylla | AFTOL-ID 482, V19652F | AY700196 | AY803751 | AY657016 | DQ435791 | DQ221106 | DQ447916 | |
| Laccaria bicolor | S238N-H82 | genome | XM001873347 | genome | XM001873179 | JX312964 | XM001881359 | |
| Macrolepiota dolichaula | AFTOL-ID 481, HKAS:38718 | DQ411537 | DQ385886 | AY771602 | DQ435785 | DQ221111 | DQ447920 | |
| Mythicomyces corneipes | AFTOL-ID 972, PBM1210 | AY745707 | DQ408110 | DQ092917 | DQ029197 | DQ404393 | DQ447929 | |
| Parasola conopilea | ZRL20151990 + LO186-02 | DQ389725 | KY419025 | KY418946 | KJ732832 | LT716064 | — | |
| Pholiota gummosa | TENN:074768, HMJAU:37426, ET34-ET8 | MN251152 | MN329726 | — | MN311973 | MN209769 | — | |
| Romagnesiella clavus | AMB:15091, ALV16952 + LIPPAM06090110 | MK353795 | MK359092 | MK353799 | — | EF051060 | — | |
| Squamanita schreieri | ZT:Myc2185 | MW258904 | MW289801 | MW258882, MW258931 | MW324510 | MW258852 | — | |
| Tubaria confragosa | AFTOL-ID 498, PBM2105 | AY700190 | DQ408113 | AY665776 | — | DQ267126 | DQ447944 | |
| Clavariineae | Camarophyllopsis hymenocephala | AFTOL-ID 1892, DJL98-081505 | DQ457679 | DQ472726 | DQ444862 | — | DQ484066 | DQ516070 |
| Ceratellopsis acuminata | CBS:146691 + S.Huhtinen 15/07 - EPITYPE | NG_075348 | MT242330 | NG_070864 | MT242352 | MT232347 | MT242316 | |
| Clavaria inaequalis | AFTOL-ID 984, CUW:MB04-016 | AY745693 | DQ385880 | DQ437680 | DQ029198 | DQ202267 | DQ447890 | |
| Clavaria zollingeri | AFTOL-ID 563, TENN:58652 | AY639882 | AY780940 | AY657008 | AY881024 | AY854071 | AH014578 | |
| Ramariopsis kunzei | GG141104 | EF561638 | GU187807 | GU187647 | GU187745 | GU187552 | GU187479 | |
| Hygrophorineae | Ampulloclitocybe clavipes | AFTOL-ID 542, PBM2474 | AY639881 | AY780937 | AY771612 | AY881022 | AY789080 | AY788848 |
| Cantharocybe gruberi | AFTOL-ID 1017 | DQ234540 | DQ385879 | DQ234546 | DQ059045 | DQ200927 | — | |
| Chromosera cyanophylla | AFTOL-ID 1684, WTUPBM1577 | DQ457655 | KF381509 | DQ435813 | — | DQ486688 | — | |
| Chrysomphalina grossula | OSC:113683 | EU652373 | DQ470832 | AY752969 | — | EU644704 | DQ516072 | |
| Cuphophyllus aurantius | CFMRPR6601 | KF291100 | KF291102 | KF291101 | — | KF291099 | — | |
| Cuphophyllus sp. | KUN-HKAS:105671, JSP346 | MW763000 | MW789179 | — | — | MW762875 | MW789163 | |
| Gloioxanthomyces nitidus | GDGM:41710 | MG712282 | MG711911 | — | — | MG712283 | — | |
| Hygroaster albellus | AFTOL-ID 1997 | EF551314 | KF381510 | KF381532 | — | KF381521 | — | |
| Hygrocybe coccinea | AFTOL-ID 1715, WTUPBM915 | DQ457676 | DQ472723 | — | GU187705 | DQ490629 | DQ447910 | |
| Hygrophorocybe nivea | LPA:SMGC2020121621 | OR863514 | OR828267 | OR863576 | OR828325 | OR863446 | — | |
| Hygrophorus aurantiosquamosus | KUN-HKAS:112569 | MW763001 | MW789180 | — | MW773440 | MW762876 | MW789164 | |
| Hygrophorus pudorinus | AFTOL-ID 1723, CUWPBM2721 | DQ457678 | DQ472725 | DQ444861 | GU187710 | DQ490631 | DQ447912 | |
| Lichenomphalia umbellifera | CFMR:J.Geml2 + GAL9547 | GU811045 | KF381515 | KF381538 | GU811010 | GU810969 | — | |
| Neohygrocybe ingrata | TENN:DJL05TN62 | KF381558 | KF381516 | KF381539 | — | KF381525 | — | |
| Neohygrocybe ovina | Rhosisaf ABS + K:M187568, GEDC0877 | KF291234 | KF291236 | KF291230 | — | KF291233 | — | |
| Porpolomopsis aff. calyptrifbrmis | TENN:DJL05TN80 | KF291247 | KF291249 | KF291248 | — | KF291246 | — | |
| Porpolomopsis lewelliniae | CORTTJB10034 | KF291239 | KF291241 | KF291240 | — | KF291238 | — | |
| Pseudoarmillariella ectypoides | AFTOL-ID 1557, PBM1588 | DQ154111 | DQ474127 | DQ465341 | GU187733 | DQ192175 | DQ516076 | |
| Spodocybe rugosiceps | KUN-HKAS:112563 - TYPE | MW763013 | MW789192 | — | MW789160 | MW762888 | MW789176 | |
| Marasmiineae | Anthracophyllum archeri | AFTOL-ID 973, PBM2201 | AY745709 | DQ385877 | DQ092915 | DQ028586 | DQ404387 | DQ435799 |
| Armillaria mellea | AFTOL-ID 449, PBM2470 | AY700194 | AY780938 | AY787217 | AY881023 | AY789081 | AY788849 | |
| Baeospora myosura | AFTOL-ID 1799, CUWPBM2748 | DQ457648 | — | DQ435796 | GU187762 | DQ484063 | DQ435801 | |
| Cheimonophyllum candidissimum | AFTOL-ID 1765, WTUPBM2411 | DQ457654 | DQ470831 | DQ435812 | GU187760 | DQ486687 | DQ447888 | |
| Dictyopanus pusillus | LMB36 | genome | genome | genome | genome | genome | genome | |
| Favolaschia claudopus | BBC-V001 23/10/2022 | OR863498 | OR828255 | OR863564 | OR828316 | OR863428 | — | |
| Flammulina velutipes | AFTOL-ID 558, TENN:52002 | AY639883 | AY786055 | AY665781 | AY883423 | AY854073 | AY858966 | |
| Gymnopus contrarius | AFTOL-ID 1758, CUWPBM2711 | DQ457670 | DQ472716 | DQ440643 | GU187700 | DQ486708 | DQ447902 | |
| Heimiomyces aff. tenuipes | McAdoo725 | OR863508 | OR828263 | OR863572 | OR828321 | OR863439 | — | |
| Hemimycena lactea | OULU:GAJ15636 | OR863509 | OR828264 | OR863573 | OR828322 | OR863440 | — | |
| Megacollybia platyphylla | AFTOL-ID 560, TENN:59432 | AY635778 | DQ385887 | AY786053 | DQ435786 | DQ249275 | DQ447923 | |
| Mycena chlorophos | 110903 Hualien Pintung | genome | genome | genome | genome | genome | genome | |
| Mycena citricolor | CBS:193.57 | MH869233 | genome | genome | genome | genome | genome | |
| Mycena galopus | ATCC:62051 | genome | genome | genome | genome | genome | genome | |
| Mycena indigotica | 171206 Taipei | genome | genome | genome | genome | genome | genome | |
| Mycena kentingensis | 111111 Pintung | genome | genome | genome | genome | genome | genome | |
| Mycena sanguinolenta | 160909 Yilan | genome | genome | genome | genome | genome | genome | |
| Mycetinis alliaceus | AFTOL-ID 556, TENN:55620 | AY635776 | AY786060 | AY787214 | AY883431 | AY854076 | — | |
| Panellus luminescens | KLU:M1278, ACL205 | KJ206955 | KJ406362 | — | — | KJ206979 | — | |
| Panellus stypticus | CORT11CA052 | KR869943 | KC816996 | — | KC816902 | — | — | |
| Phloeomana gracilis | AFTOL-ID 1732, CUW:PBM2715 | DQ457671 | DQ472719 | DQ440644 | GU187709 | DQ490623 | DQ447905 | |
| Porotheleum fimbriatum | AFTOL-ID 1725, CBS:788.86 | DQ457673 | DQ472721 | DQ444854 | — | DQ490626 | DQ447907 | |
| Rhodocollybia maculata | AFTOL-ID 540, PBM2481 | AY639880 | AY787220 | AY752966 | DQ061279 | DQ404383 | DQ447936 | |
| Roridomyces sp. | KLU:M1292, ACL273 | KJ206958 | KJ406372 | — | — | — | — | |
| Xeromphalina Campanella | TENN:F069178 + GLM 46039 | KM011910 | KP835655 | — | — | KP835678 | DQ067940 | |
| Xerula radicata | AFTOL-ID 561, TENN:59235 | AY645051 | AY786067 | AY654884 | DQ029194 | DQ241780 | DQ447946 | |
| Outgroup | Amylocorticium cebennense | CFMR:HHB-2808 | GU187561 | GU187770 | GU187612 | GU187675 | GU187505 | GU187439 |
| Ceraceomyces borealis | CFMR:L-8014 | GU187570 | GU187782 | GU187624 | GU187686 | GU187512 | — | |
| Suillus pictus | AFTOL-ID 717, MB 03-002 | AY684154 | AY786066 | AY662659 | AY883429 | AY854069 | AY858965 | |
| Phyllotopsidineae | Aphanobasidium pseudotsugae | CFMR:HHB-822 | GU187567 | GU187781 | GU187620 | GU187695 | GU187509 | GU187455 |
| Aphroditeola sp. | HRL1230 | OR863490 | OR828247 | OR863558 | OR828309 | OR863420 | — | |
| Aphroditeola sp. | TRgmb00556 | OR863491 | OR828248 | OR863559 | OR828310 | OR863421 | — | |
| Aphroditeola sp. | TRgmb00561 | OR863492 | OR828249 | OR863560 | — | OR863422 | — | |
| Cristinia sp. | CFMR:FP100305 | GU187585 | — | GU187637 | GU187718 | GU187526 | GU187470 | |
| Lindtneria flava | K:M143556 | KM086909 | — | — | KM087001 | KM086815 | — | |
| Macrotyphula fistulosa | S:IO.14.214, UPS:IO.14.214 + 10.15.123 | KY224088 | MT242336 | MT232495 | MT242354 | MT232352 | MT242317 | |
| Macrotyphula juncea | 10.14.177 | MT232306 | MT242337 | — | MT242355 | MT232353 | — | |
| Macrotyphula phacorrhiza | S:IO.14.200 | MT232314 | — | MT232505 | MT242366 | MT232363 | — | |
| Phyllotopsis sp. | AFTOL-ID 773, MB35 | AY684161 | AY786061 | AY707090 | DQ059047 | DQ404382 | DQ447933 | |
| Pleurocybella porrigens | UPS:F611822 + AFTOL-ID 2001, JFA12544 + TUB:012154 | EF537894 | MT242339 | GU187660 | GU187740 | MT232355 | DQ067994 | |
| Pterula echo | AFTOL-ID 711, DJM302S58 | AY458123 | GU187805 | DQ092911 | GU187743 | DQ494693 | — | |
| Pterula echo | ZRL20151311 | KY418881 | KY419026 | KY418947 | KY419076 | LT716065 | KY418979 | |
| Pterula gracilis | S:IO.14.142 | MT232310 | — | MT232498 | — | MT232356 | — | |
| Radulomyces molaris | ARAN:Fungi2003 | MT232311 | MT242340 | MT232499 | MT242359 | — | MT242320 | |
| Sarcomyxa edulis | HMJAU7066 | GQ219739 | genome | genome | genome | genome | genome | |
| Sarcomyxa serotina | AFTOL-ID 536, PBM2519 | AY691887 | DQ859892 | U59088 | GU187754 | DQ494695 | DQ447938 | |
| Stephanospora caroticolor | IOC-137/97+ TUB:019072 | AF518652 | — | AF518591 | GU187747 | AJ419224 | KF211335 | |
| Tectella patellaris | McAdoo991 | OR863548 | OR828299 | OR863602 | OR828350 | OR863481 | — | |
| Tricholomopsis decora | AFTOL-ID 537, PBM2482 | AY691888 | DQ408112 | DQ092914 | DQ029195 | DQ404384 | DQ447943 | |
| Tricholomopsis osiliensis | ZRL20151760 | KY418884 | KY419029 | KY418949 | KY419079 | — | — | |
| Pleurotineae | Auriculariopsis ampla | NL-1724 | OL957174 | genome | genome | genome | OL957174 | — |
| Fistulina antarctica | AFTOL-ID 1335, CBS:701.85 | AY293181 | DQ472713 | AY293131 | GU187698 | DQ486702 | — | |
| Flagelloscypha sp. | PMI 526 | genome | genome | genome | genome | genome | genome | |
| Hohenbuehelia atrocoerulea | AMB: 18080 | KU355389 | KU355418 | — | KU355439 | KU355304 | — | |
| Hohenbuehelia faerberioides | Mertens | MG553645 | MW240980 | — | MW240984 | MG553638 | — | |
| Hohenbuehelia grisea | MCVE:27293 | KU355394 | — | — | KU355447 | KU355329 | — | |
| Hohenbuehelia tremula | AFTOL-ID 1503, PBM2301 | — | — | DQ440645 | — | DQ182504 | — | |
| Hohenbuehelia tremula | DAOM:180808 | KU355405 | KU355434 | — | KU355465 | KU355357 | OR828361 | |
| Hohenbuehelia unguicularis | Z+ZT1112 | KU355408 | — | — | KU355467 | KU355361 | — | |
| Lachnella villosa | CBS:609.87, AFTOL-ID 525 + CCJ1547 | DQ097347 | — | AY705959 | GU187721 | DQ097362 | DQ068007 | |
| Pleurotus citrinopileatus | Hfpri PC 051Y1-BHFW01000088 | genome | genome | genome | genome | genome | genome | |
| Pleurotus dryinus | AMB:18868 | OR863538 | OR828286 | OR863593 | OR828338 | OR863471 | OR828363 | |
| Pleurotus fuscosquamulosus | A. Baglivo 13-07-2014 | — | OR828287 | — | OR828339 | OR863472 | — | |
| Pleurotus ostreatus | AFTOL-ID 564, TENN:53662 | AY645052 | AY786062 | AY657015 | AY883432 | AY854077 | AY862186 | |
| Pleurotus salmoneostramineus | NBRC-31859 | genome | genome | genome | genome | genome | genome | |
| Pleurotus tuber-regium | ACCC:50657-18 | genome | genome | genome | genome | genome | genome | |
| Porodisculus orientalis | G0896 + SNU-m 030828-101 | MK278522 | EU423191 | — | — | EU423186 | — | |
| Resupinatus applicatus | AMB:18098 | MH430596 | — | — | MH449588 | MH137821 | — | |
| Resupinatus europaeus | AMB: 18078 | KU355409 | — | — | KU355468 | KU355368 | — | |
| Resupinatus griseopallidus | AMB:18277 | MH165881 | — | — | — | MH137823 | — | |
| Resupinatus kavinae | AMB:19612 | OR863543 | OR828293 | — | OR828344 | OR863477 | — | |
| Resupinatus niger | AMB: 18095 | KU355413 | — | — | KU355470 | KU355371 | — | |
| Resupinatus rouxii | Z+ZT971 | MH190787 | — | — | MH449590 | MH137828 | — | |
| Resupinatus striatulus | JA:Cussta8634 | MH430597 | — | — | MH449591 | MH137829 | — | |
| Resupinatus vetlinianus | TENNP69285, TFB14587 | KP987309 | — | — | — | KP026243 | — | |
| Schizophyllum radiatum | AFTOL-ID 516, CBS:301.32 | MH866782 | DQ484052 | AY705952 | — | MH855328 | DQ447939 | |
| Pluteineae | Amanita brunnescens | AFTOL-ID 673, PBM2429 | AY631902 | AY780936 | AY707096 | AY881021 | AY789079 | AY788847 |
| Amanita phalloides | HKAS:75773 + TUB:011556 | JX998060 | KJ466612 | — | JX998000 | JX998031 | DQ067953 | |
| Amanita subglobosa | HKAS:58837 | JN941152 | JQ031121 | JN941126 | KJ482004 | JN943177 | JN994123 | |
| Catatrama costaricensis | DAOM:211663 | KT833804 | KT833819 | — | KT833834 | — | — | |
| Giacomia mirabilis | AMB:19297 | JQ639154 | OR828261 | OR863570 | — | JQ639153 | — | |
| Giacomia mirabilis | ANGE1598 | OR863505 | OR828262 | OR863571 | OR828320 | OR863436 | OR828360 | |
| Giacomia sinensis | HMJU:265 - TYPE | MZ435884 | MZ441372 | MZ435869 | MZ441376 | MZ435888 | MZ441380 | |
| Giacomia sinensis | HMJU:268 | MZ435885 | MZ441373 | MZ435870 | MZ441377 | MZ435889 | MZ441381 | |
| Leucocortinarius bulbiger | AMB: 19593 | OR864301 | OR828271 | OR863581 | OR828326 | — | — | |
| Leucocortinarius bulbiger | TUB:011568 | DQ071745 | — | — | — | — | DQ068019 | |
| Limacella glioderma | HKAS:90169 + ZLYD 72 | KT833808 | KT833823 | — | KT833836 | MH508658 | DQ067952 | |
| Limacellopsis asiatica | HKAS:101436 | MH486964 | MH486357 | — | MH509184 | — | — | |
| Limacellopsis guttata | MB100157 | KT833813 | KT833828 | — | KT833841 | — | — | |
| Limnoperdon incarnatum | IFO:30398 | AF426958 | — | AF426952 | — | DQ097363 | — | |
| Limnoperdon sp. | CBS:160.95 | OR863524 | OR828272 | OR863582 | OR828327 | OR863457 | — | |
| Melanoleuca aff. graminicola | AMB:19613 | OR863528 | OR828276 | OR863586 | OR828331 | OR863461 | — | |
| Melanoleuca communis | ZRL20151882 | KY418885 | KY419030 | KY418950 | KY419080 | LT716069 | — | |
| Melanoleuca exscissa | AMB:19614 | OR863530 | OR828278 | OR863588 | OR828332 | OR863463 | — | |
| Melanoleuca exscissa | BRNM781061 + LAS97-019 | — | LT594191 | — | LT594175 | LT594122 | JX429104 | |
| Melanoleuca friesii | AMB: 18865 | OR863531 | OR828279 | OR863589 | OR828333 | OR863464 | — | |
| Melanoleuca microcephala | HMJAS:00138 + BRNM:817787 | MK660045 | MW488179 | — | MW488164 | MW491334 | — | |
| Melanoleuca rasilis | BRNM751967, G0924 | MK278374 | LT594187 | — | LT594171 | LT594154 | — | |
| Melanoleuca tristis | AMB:18866 | OR863532 | OR828280 | OR863590 | OR828334 | OR863465 | OR828362 | |
| Melanoleuca verrucipes | WTUPBM2289, AFTOL-ID 818 | DQ457687 | DQ474119 | DQ457645 | GU 187726 | DQ490642 | DQ447924 | |
| Pluteus cervinus | AMB:18870 | OR863539 | OR828288 | OR863594 | OR828340 | OR863473 | OR828364 | |
| Pluteus hongoi | ZRL20151600 | KY418878 | KY419023 | — | KY419074 | LT716062 | — | |
| Pluteus multiformis | PL40, AC4249, AH:40107 - TYPE | MK278503 | LR697101 | — | LR697100 | HM562201 | — | |
| Pluteus romellii | AFTOL-ID 625, ECV3201 | AY634279 | AY786063 | AY657014 | AY883433 | AY854065 | AY862187 | |
| Pluteus romellii | AMB:18871 | OR863540 | OR828289 | OR863595 | OR828341 | OR863474 | — | |
| Pluteus variabilicolor | AMB:18872 | OR863541 | OR828290 | OR863596 | OR828342 | OR863475 | OR828365 | |
| Pluteus variabilicolor | AMB:18873 | OR863542 | OR828291 | OR863597 | OR828343 | OR863476 | OR828366 | |
| Saproamanita thiersii | SKay4041 | HQ593114 | genome | genome | genome | HQ625010 | genome | |
| Volvariella aff. nigrovolvacea | AMB:18775 | OR863550 | OR828301 | OR863604 | OR828352 | OR863483 | OR828367 | |
| Volvariella aff. pusilia | AMB:19290 | OR863552 | OR828303 | OR863606 | OR828354 | OR863485 | OR828368 | |
| Volvariella aff. pusilia | K:M145618 | OR863551 | OR828302 | OR863605 | OR828353 | OR863484 | — | |
| Volvariella bombycina | AMB:19312 | OR863553 | OR828304 | OR863607 | OR828355 | OR863486 | — | |
| Volvariella caesiotincta | AMB:19319 | OR863554 | OR828305 | — | OR828356 | OR863487 | — | |
| Volvariella volvacea | PDD:96362, JAC12235 | MN738572 | genome | genome | genome | genome | genome | |
| Volvopluteus earlei | AGMT-71 | OR863556 | OR828307 | OR863609 | OR828358 | MK204989 | — | |
| Volvopluteus gloiocephalus | AFTOL-ID 890 | AY745710 | — | DQ089020 | — | DQ494701 | DQ447945 | |
| Volvopluteus gloiocephalus | NTNU:27884555 | OR863557 | OR828308 | OR863610 | OR828359 | OR863489 | — | |
| Zhuliangomyces illinitus | HKAS:90168 | KT833814 | KT833829 | — | KT833842 | MH508659 | — | |
| Tricholomatineae | Asproinocybe sinensis | HMJAU:59026 | OK377051 | OK625401 | OK377040 | OK625331 | OK377048 | OK625398 |
| Aspropaxillus giganteus | AMB:18857 | OR863493 | OR828250 | OR863561 | OR828311 | OR863423 | — | |
| Atractosporocybe inornata | HKAS:105578 + TO:AV201012d | MZ714592 | MZ681898 | KJ681075 | MZ681877 | MZ714587 | MZ681888 | |
| Bonomyces sinopicus | KATO:Fungi-3689 | MG696627 | MG702595 | MG696623 | MG702592 | MG696619 | — | |
| Callistosporium graminicolor | AFTOL-ID 978, WTU:PBM2341 | AY745702 | — | AY752974 | GU187761 | DQ484065 | GU187493 | |
| Catathelasma ventricosum | DAOM:225247 | MN017477 | MN018851 | MN017585 | MN026906 | MN017537 | KP255480 | |
| Clitocella fallax | CBS:129.63 | AF223166 | EF421018 | — | EF421089 | AF357017 | EF421051 | |
| Clitocybe dealbata | IE-BSG HC95.cp3 | AF223175 | DQ825407 | DQ825431 | EF421080 | AF357061 | DQ825414 | |
| Clitocybe ditopa | AMB:19311 | OR863496 | OR828253 | OR863563 | OR828314 | OR863426 | — | |
| Clitocybe nebularis | AFTOL-ID 1495, WTU:PBM2259 + CBS:362.65 | DQ457658 | EF421011 | DQ437681 | EF421081 | AF357063 | DQ825415 | |
| Clitocybe subditopoda | AFTOL-ID 533, PBM2489 | AY691889 | AY780942 | AY771608 | DQ408150 | DQ202269 | DQ447892 | |
| Clitolyophyllum akcaabatense | P. Alvarado 5836 | OR863497 | OR828254 | — | OR828315 | OR863427 | — | |
| Clitopaxillus alexandri | TO:AV45634 | MG321393 | MG334546 | MG321329 | MG334537 | MG321345 | — | |
| Clitopilopsis hirneola | CORTTB8490 +CORTREH8490 | GU384611 | GU384646 | — | KC816820 | — | — | |
| Clitopilus pallidogriseus | MENoordeloos2004032 + CORTE652 | GQ289216 | GQ289283 | — | KC816875 | — | — | |
| Collybia tuberosa | AFTOL-ID 557, TENN:53540 | AY639884 | AY787219 | AY771606 | AY881025 | AY854072 | AY857982 | |
| Entoloma prunuloides | AFTOL-ID 523, TJB4765 | AY700180 | DQ385883 | AY665784 | DQ457633 | DQ206983 | DQ447898 | |
| Entoloma undatum | HKAS:115925 | MZ853561 | MZ852824 | — | — | MZ855875 | MZ852812 | |
| Fayodia bisphaerigera | OW241-19 | OR863499 | OR828256 | — | OR828317 | OR863429 | — | |
| Gamundia sp. | YM18172 | OR863500 | OR828257 | OR863565 | OR828318 | OR863430 | — | |
| Gamundia striatula | JL45-18 | OR863501 | OR828258 | OR863566 | OR828319 | OR863431 | — | |
| Harmajaea harperi | LIP:0401361 | MG321399 | MG334549 | MG321333 | MG334541 | MG321366 | — | |
| Hertzogia martiorum | AMB:18863 | OR863510 | OR828265 | OR863574 | OR828323 | OR863441 | — | |
| Hypsizygus ulmarius | DUKE:JM/HW | AF042584 | EF420996 | — | EF421062 | EF421105 | EF421030 | |
| Infundibulicybe geotropa | AMB:18861 | OR863518 | OR828268 | OR863577 | — | OR863450 | — | |
| Infundibulicybe gibba | AFTOL-ID 1508, CUW:JCS0704B | DQ457682 | DQ472727 | DQ115780 | GU187759 | DQ490635 | DQ447913 | |
| Lepista glaucocana | AMB: 18862 | OR863520 | — | OR863579 | — | OR863452 | — | |
| Lepista Irina | AFTOL-ID 815, WTUPBM2291 | DQ234538 | DQ385885 | AY705948 | DQ028591 | DQ221109 | DQ447919 | |
| Lepista ricekii | AMB: 18864 | OR863521 | OR828270 | OR863580 | — | OR863453 | — | |
| Lepista saeva | TENN:066100, ADW0097 | KJ417193 | KJ424376 | KJ417159 | — | KJ137270 | — | |
| Leucocybe candicans | AFTOL-ID 541, PBM2476 | AY645055 | DQ385881 | AY771609 | DQ408149 | DQ202268 | DQ447891 | |
| Lyophyllum semitale | HC85/13 | AF042581 | EF421002 | — | EF421068 | AF357049 | EF421036 | |
| Lyophyllum turcicum | GB:0065321 | OR863525 | OR828273 | OR863583 | OR828328 | OR863458 | — | |
| Macrocystidia cucumis | JX.1294733#45 | OR863527 | OR828275 | OR863585 | OR828330 | OR863460 | — | |
| Macrocystidia sp. | Kekki3956 | OR863526 | OR828274 | OR863584 | OR828329 | OR863459 | — | |
| Musumecia bettlachensis | TO:HG2284 | JF926521 | KJ681060 | KJ681069 | KJ681082 | JF926520 | — | |
| Nolanea sericea | VHAs03/02 | DQ367423 | DQ367435 | DQ367421 | DQ367428 | DQ367430 | DQ825424 | |
| Notholepista fistulosa | HKAS:115934 | OK104059 | OK105137 | — | OK105127 | OK104077 | OK105132 | |
| Notholepista fistulosa | HMJU:288 | MZ435886 | MZ441374 | MZ435871 | MZ441378 | MZ435890 | MZ441382 | |
| Notholepista fistulosa | HMJU:592 | MZ435887 | MZ441375 | MZ435872 | MZ441379 | MZ435891 | MZ441383 | |
| Notholepista subzonalis | GB:0087013 | KJ417208 | KJ424385 | KJ417167 | — | KP453695 | — | |
| Omphaliaster borealis | TROM:43 | OR863533 | OR828281 | — | OR828335 | OR863466 | — | |
| Omphalina pyxidata | AMB:19294 | OR863534 | OR828282 | OR863591 | — | OR863467 | — | |
| Omphalina pyxidata | AMB:19295 | OR863535 | OR828283 | OR863592 | — | OR863468 | — | |
| Paralepista flaccida | KUN-HKAS:115937 | MZ853571 | MZ681894 | — | MZ857193 | MZ855885 | MZ857194 | |
| Paralepista flaccida | TO:AV20140410 | OR863536 | OR828284 | — | OR828336 | OR863469 | — | |
| Paralepistopsis amoenolens | AMB:18867 | OR863537 | OR828285 | — | OR828337 | OR863470 | — | |
| Pogonoloma spinulosum | K:M107286 | KJ417238 | KJ424401 | KU058571 | — | KP453705 | KU139037 | |
| Pseudoclitocybe cyathiformis | AFTOL-ID 1998, WTUJFA12811 + GLM46020 | EF551313 | GU187815 | GU187659 | GU 187742 | GU187553 | DQ067939 | |
| Pseudoclitopilus rhodoleucus | GB:0110967, TK03/203 | KJ417218 | KJ424393 | KU058577 | — | KP453696 | KU139046 | |
| Pseudoclitopilus rhodoleucus | KUN-HKAS:105563 | MZ714594 | MZ681899 | — | MZ681878 | MZ714588 | MZ681889 | |
| Pseudolaccaria pachyphylla | GB:0066637, LAS07/012 | KU058542 | KU139006 | KU058579 | — | KU058504 | KU139048 | |
| Pseudoomphalina kalchbrenneri | GB:0066625, LAS06/037 | KU058541 | KU139005 | KU058578 | — | KU058503 | KU139047 | |
| Pseudoomphalina umbrinopur-purascens | LSS20181215-2 | MK424271 | OR828292 | OR863598 | — | MK424270 | — | |
| Pseudotricholoma metapodium | AH22102006-K | KJ417219 | KJ424394 | KJ417171 | — | KJ417308 | KU139049 | |
| Rhizocybe alba | KUN-HKAS:55110 | MZ675571 | MZ681893 | — | MZ681871 | MZ675560 | MZ681882 | |
| Rhodophana stangliana | KUN-HKAS:115926 | MZ853562 | MZ852825 | — | MZ852801 | MZ855876 | MZ852813 | |
| Ripartites odorus | F. Di Rita 08-12-2018 | MN595290 | OR828295 | OR863599 | OR828346 | MN595290 | — | |
| Ripartites odorus | T. Clements 248705 | OR863544 | OR828294 | — | OR828345 | MK559718 | — | |
| Ripartites sp. | Kekki2112 | OR863545 | OR828296 | OR863600 | OR828347 | OR863478 | — | |
| Ripartites tricholoma | Kekki1910 | OR863546 | OR828297 | OR863601 | OR828348 | OR863479 | — | |
| Ripartites tricholoma | KUN-HKAS77956 | MZ675573 | — | — | MZ681873 | MZ675562 | MZ681884 | |
| Singerocybe umbilicata | KUN-HKAS:105572 | MZ714591 | MZ681896 | — | MZ681875 | MZ714585 | MZ681886 | |
| Trichocybe puberula | Ferisin11.3.2016-03 | OR863549 | OR828300 | OR863603 | OR828351 | OR863482 | — | |
| Tricholoma viridiolivaceum | TENN:063670, PDD:97890, PBM3093 | JF706317 | JF706319 | JF706318 | — | JF706316 | KU139072 | |
| Tricholomella constricta | HC84/75 | AF223188 | DQ825412 | DQ825434 | AF357036 | DQ825422 | ||
| Tricholosporum goniospermum | AR122, TUR:A209107 | MW367864 | KU559863 | KU559865 | — | KU559848 | — | |
| Tricholosporum guangxiense | HMJAU:59028 | OK377056 | OK625403 | OK377043 | OK625333 | OK377047 | — | |
| Typhulineae | Typhula erythropus | S:IO.14.123, UPS:IO.14.123 | KY224096 | MT242343 | — | MT242362 | MT232359 | — |
| Typhula gyrans | S:IO.14.103, UPS:IO.14.103 | KY224097 | MT242344 | — | MT242363 | MT232360 | MT242323 | |
| Typhula incarnata | S:IO.14.92, UPS:IO.14.92 +CBS:35979 | MT232313 | MT242346 | MT232504 | MT242365 | MT232362 | MT242325 | |
| Typhula sclerotioides | S:IO.14.22 | MT232317 | MT242349 | MT232507 | MT242369 | MT232365 | MT242327 |
Table 3.
Taxa, vouchers, and GenBank/Unite accessions numbers of the DNA sequences used in the Cuphophylloideae-wide phylogenetic analysis inferred from a four-gene dataset (ITS, LSU, RPB2 and TEF1). Sequences in bold were generated in this study.
| Species | Herbarium | ITS | LSU | RPB2 | TEF1 |
|---|---|---|---|---|---|
| Ampulloclitocybe clavipes | KUN-HKAS:54426 | MW616462 | MW600481 | MW656471 | MW656461 |
| TENN:DJL06TN40 | FJ596912 | KF381542 | KF407938 | — | |
| WTU:PBM2474, AFTOL-ID 542 | AY789080 | AY639881 | AY780937 | AY881022 | |
| Amylocorticium cebennense | CFMR:HHB-2808 | GU187505 | GU187561 | GU187770 | GU187675 |
| Cantharocybe brunneovelutina | CFMR:DJLBZ1883 - TYPE | NR_160458 | NG_068731 | — | — |
| Cantharocybe gruberi | AH:24539 | JN006422 | JN006420 | — | — |
| WTU:PBM510, AFTOL-ID 1017 | DQ200927 | DQ234540 | DQ385879 | DQ059045 | |
| Cantharocybe virosa | HKAS:79012 | — | KF303143 | — | — |
| TENN:063483 | KX452405 | JX101471 | — | — | |
| Ceraceomyces borealis | CFMR:L-8014 | — | GU187570 | GU187782 | GU187686 |
| Cuphophyllus acutoides var. pallidus | CFMR:TN-257 | KF291096 | KF291097 | — | — |
| Cuphophyllus aff. pratensis | WTU:PBM2752, AFTOL-ID 1682 | DQ486683 | DQ457650 | — | — |
| Cuphophyllus aurantius | CFMR:PR-6601 | KF291099 | KF291100 | KF291102 | — |
| Cuphophyllus cinerellus | GB:0156961, EL30-16 | MK573935 | MN430913 | MN556847 | — |
| Cuphophyllus esteriae | TU:117603 | MK547063 | MN430911 | MN556855 | — |
| Cuphophyllus flavipes | TUR:A-199692, Campo131027 | MN453872 | MN430919 | MN556851 | — |
| Cuphophyllus fornicatus | CFMR:D.Boertmann 2009/94 | KF291123 | KF291124 | — | — |
| Cuphophyllus hygrocyboides | GB:0156992, EL177-13 | MK573937 | MN430917 | MN534321 | — |
| Cuphophyllus lamarum | TU:117564 | MK547062 | MN430915 | MN556853 | — |
| Cuphophyllus pratensis | CFMR:DJL-Scot-8 | KF291057 | KF291058 | — | — |
| Lueck7 | — | KP965789 | — | — | |
| Cuphophyllus sp. | KUN-HKAS:105671 | MW762875 | MW763000 | MW789179 | — |
| Hygrophorocybe aff. carolinensis | UCSC:F0690 | OR863442 | OR863511 | OR828266 | OR828324 |
| Hygrophorocybe aff. carolinensis (as Clitocybe carolinensis) | TENN:021888 - TYPE | NR_119886 | — | — | — |
| Hygrophorocybe nivea | AMB:19292 | OR863444 | OR863512 | — | — |
| AMB:19293 | OR863445 | OR863513 | — | — | |
| LPA:SMGC2020121621 | OR863446 | OR863514 | OR828267 | OR828325 | |
| TO:AV20100811 | OR863448 | OR863516 | — | — | |
| TO:AV20112411 | OR863449 | OR863517 | — | — | |
| Hygrophorocybe nivea (as Clitocybe alni-glutinosae) | IB:19960896 - TYPE | UDB023989 | — | — | — |
| Hygrophorocybe nivea (as Clitocybe hypotheja) | MCVE:530 - TYPE | OR863443 | — | — | — |
| Spodocybe bispora | KUN-HKAS:112564 | MW762882 | MW763007 | MW789186 | MW773446 |
| KUN-HKAS:73310 - TYPE | MW762880 | MW763005 | MW789184 | MW773444 | |
| Spodocybe cf. trulliformis (as Clitocybe cf. trulliformis) | G0460, DB1302 | — | MK277728 | — | — |
| Spodocybe collina | AMB:19296 | OR863480 | OR863547 | OR828298 | OR828349 |
| WU:0018453, G0342 | — | MK277717 | — | — | |
| Spodocybe herbarum (as Clitocybe herbarum) | G0171, NL-2261 | — | MK277719 | — | — |
| Spodocybe rugosiceps | KUN-HKAS:112563 - TYPE | MW762888 | MW763013 | MW789192 | MW789160 |
| KUN-HKAS:71071 | MW762886 | MW763011 | MW789190 | MW773449 | |
| Spodocybe sp. | KUN-HKAS:112560 | MW762889 | MW763014 | MW789193 | MW789161 |
| KUN-HKAS:112565 | MW762890 | MW763015 | MW789194 | MW789162 |
Fig. 6.
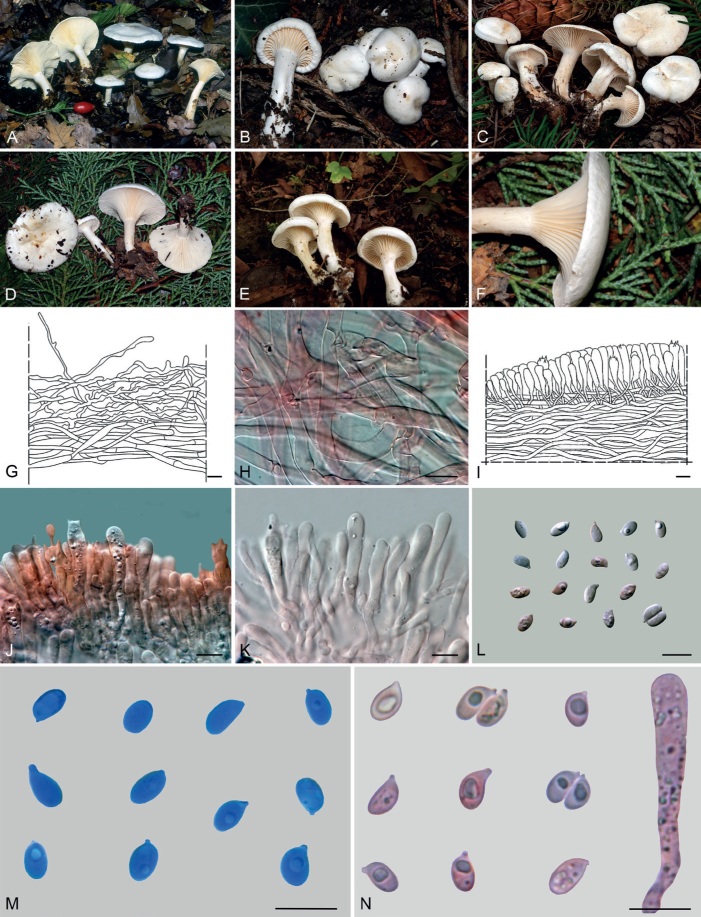
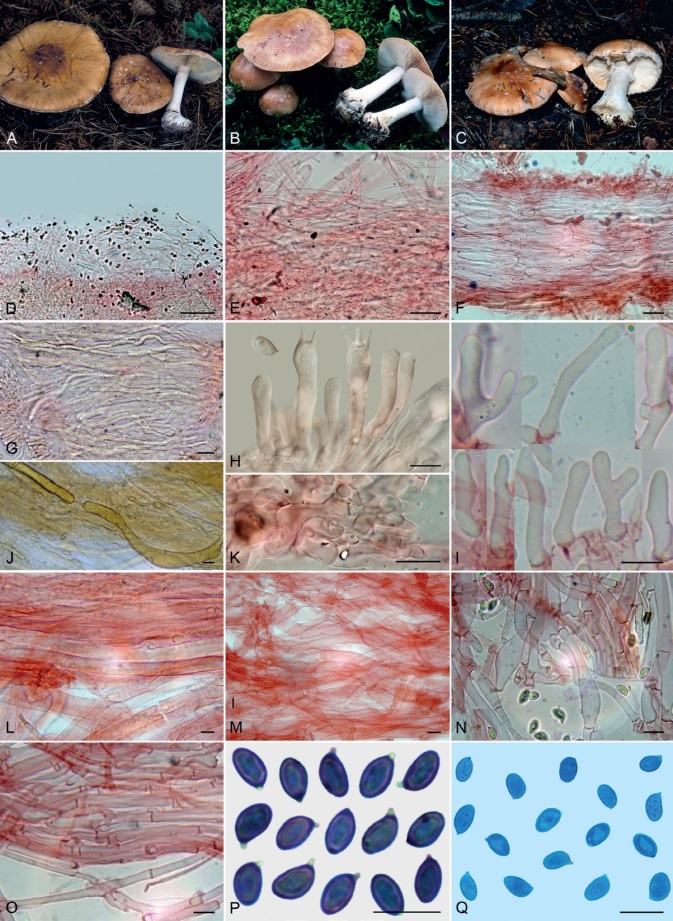
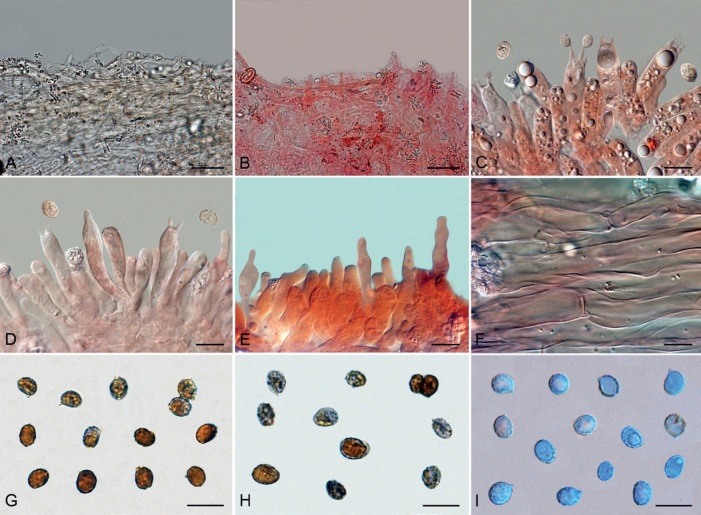
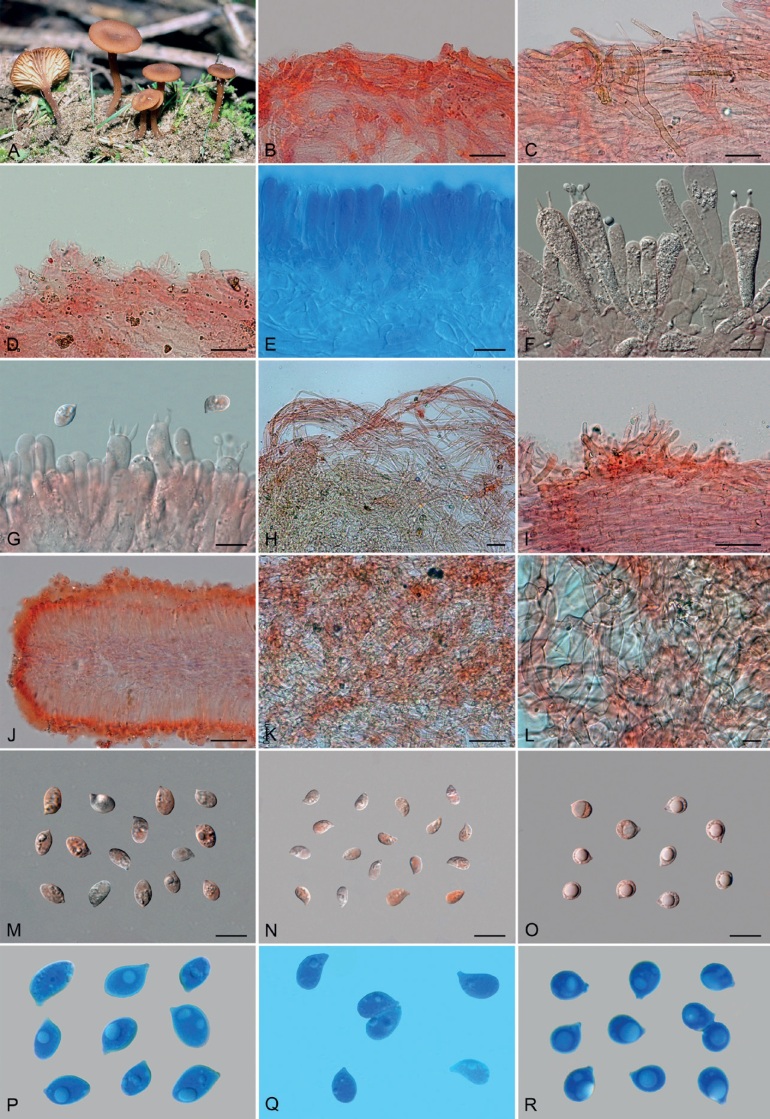
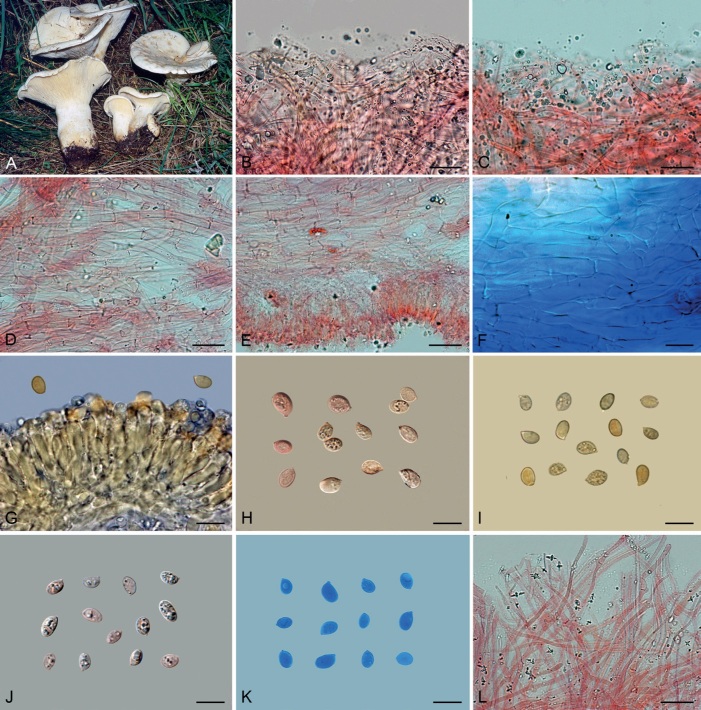
Basidiomes of taxa within Agaricales sequenced in the present work. A. Aphroditeola olida (HRL1230). B. Aspropaxillus giganteus (AMB:18858). C. Clitocybe ditopa (AMB:19311). D. Favolaschia claudopus (B. Child-Villiers 23-10-22). E. Fayodia bisphaerigera (OW241-19). F. Gamundia sp. (YM18172). G. Giacomia mirabilis [ANGE1598 (TO)]. H. Giacomia sinensis (HMJU:265 holotype). I. Heimiomyces aff. tenuipes (McAdoo 725). J. Hemimycena lactea (OULU:GAJ15636). K. Hertzogia martiorum (AMB:18863). L. Hygrophorocybe nivea (AMB:19292). M. Hygrophorocybe aff. carolinensis (UCSC:F0690). N. Infundibulicybe gibba (AMB:19313). O. Lepista glaucocana (AMB:18862). Photographs A by R. Lebeuf, B, C, K, L, N, O by G. Consiglio, D by B. Child-Villiers, E by Ø. Weholt, F by Y. Mourgues, G by C. Angelini, H by J. Xu, I by W. McAdoo, J by S. Huhmarniemi, M by C. Schwarz.
Fig. 8.
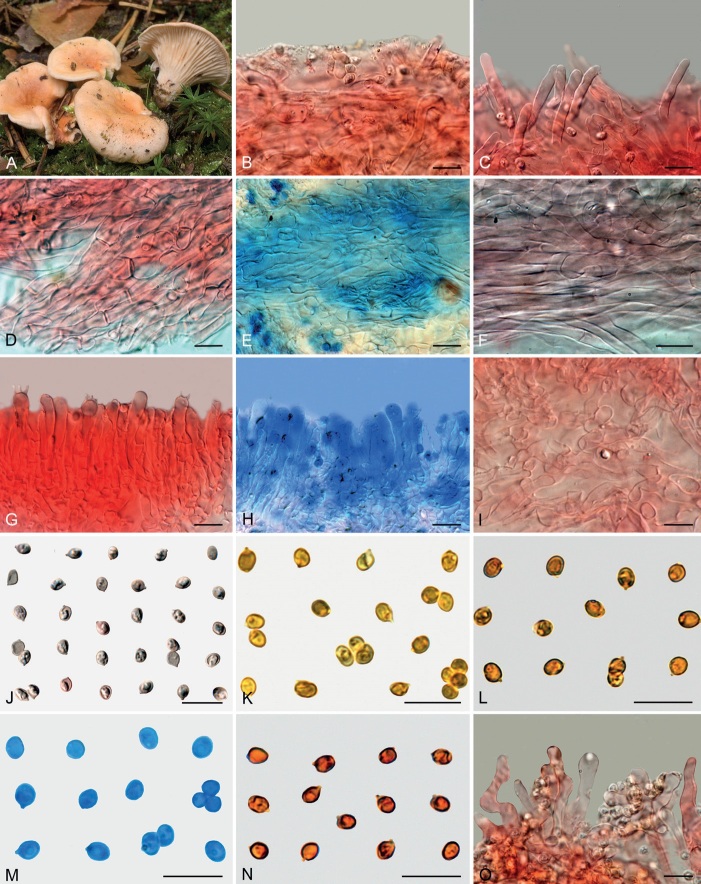
Basidiomes of taxa within Agaricales sequenced in the present work. A. Pseudoomphalina umbrinopurpurascens (LSS20181215-2). B. Resupinatus applicatus (AMB:18098). C. Ripartites odorus (F. Di Rita 08-12-2018). D. Ripartites tricholoma (Kekki1910). E. Spodocybe collina (AMB:19296). F. Tectella patellaris (McAdoo991). G. Trichocybe puberula (Ferisin11.3.2016-03). H. Volvariella bombycina (AMB:19312). I. Volvariella aff. nigrovolvacea (AMB:18775). J. Volvariella aff. pusilla (AMB:19290). K. Volvopluteus earlei (AGMT-71). L. Volvopluteus gloiocephalus (NTNU:27884555). Photographs A by L. Sánchez, B, E, H–J by G. Consiglio, C by M. Atzeni, D by T. Kekki, F by W. McAdoo, G by G. Ferisin, K by F. Giannoni, L by P.G. Larssen.
MATERIALS AND METHODS
Morphological studies
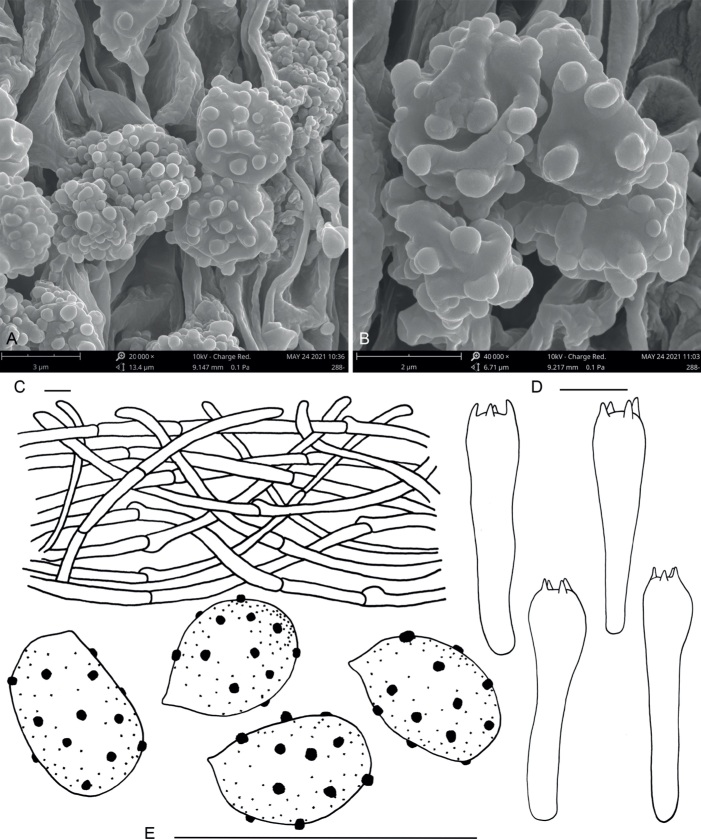
Macroscopic morphological features were studied in fresh specimens. Colour codes follow Kornerup & Wanscher (1978). The following abbreviations are employed: L = number of lamellae reaching the stipe, l = number of lamellulae between each pair of lamellae. Microscopic structures were examined in dried material using different mounting media: water, L4 (Clémençon 1972), Melzer’s reagent, ammoniacal Congo red, phloxine, Cresyl blue and Cotton blue. Dried pieces of the samples were rehydrated in water and mounted in L4. All microscopic measurements were carried out with a Nikon Eclipse 80i microscope, using immersion oil at ×1 000. Spore measurements were taken by capturing images of a single visual field with multiple spores (obtained from lamellar squashes of exsiccate material of mature specimens) which were then measured using the DS-L1 Nikon camera control unit. Spore dimensions do not include the hilar appendix, and are reported as follows: (minimum–) average minus standard deviation of length–average of length–average plus standard deviation of length (−maximum) × (minimum–) average minus standard deviation of width–average of width–average plus standard deviation of width (−maximum); Q (ratio length/width) = (minimum–) average minus standard deviation–average–average plus standard deviation (−maximum); V (volume, μm3) = (minimum–) average minus standard deviation–average–average plus standard deviation (−maximum). The approximate spore volume was calculated as that of an ellipsoid (Gross 1972, Meerts 1999). The notation [n/m/p] indicates that measurements were made on ‘n’ randomly selected spores from ‘m’ basidiomes of ‘p’ collections. The width of the basidia was measured at the widest part, and the length was measured from the apex (sterigmata excluded) to the basal septum. Microscopy images were taken using a Nikon DS 5M digital connected to the microscope with both bright field and interferential contrast optics. Macro- and microchemical testing of pigments were performed using basic solutions (5 % KOH and 10 % ammonia, separately). In some cases, basidiospores were observed under the scanning electron microscope (SEM), using the following procedure: lamellae were attached to specimen holders by carbon tape, coated with platinum-palladium using a Hitachi MC 1000 Ion Sputter Coater and examined with a FEI Quanta 200 FE-SEM operated at 5–10 kV as in Xu et al. (2019). For nomenclatural matters, reference was made to the Shenzhen Code (Turland et al. 2018).
DNA extraction, amplification, and sequencing
Total DNA was extracted from dry specimens (Table 1) employing a modified protocol based on Murray & Thompson (1980). Amplification reactions (Mullis & Faloona 1987) included 35 cycles with an annealing temperature of 54 ºC. The primers ITS1F and ITS4 (White et al. 1990, Gardes & Bruns 1993) were employed to amplify the internal transcribed spacer region 1, 5.8S rDNA and internal transcribed spacer region 2 (ITS), LR0R and LR5 (Vilgalys & Hester 1990, Cubeta et al. 1991) were used for the 28S rDNA region (LSU), NS19b and NS41 (Hibbett 1996) for the 18S rDNA (SSU), EF1-728F, EF1-983F, EF1-1567R and EF1-2218R (Carbone & Kohn 1999, Rehner & Buckley 2005) for the translation elongation factor-1a (TEF1) gene, bRPB2-6F2 (reverse of bRPB26R2), and bRPB2-7R2 for the DNA-directed RNA polymerase II second largest subunit (RPB2) gene (Matheny et al. 2007), as well as RPB1-Af (Stiller & Hall 1997) and RPB1-Cr (Matheny et al. 2002) for DNA-directed RNA polymerase II largest subunit (RPB1) gene. The PCR products were checked in 1 % agarose gels, and amplicons were sequenced with one or both PCR primers. Sequences were corrected to remove reading errors in chromatograms using MEGA v. 6.0 (Tamura et al. 2013).
Phylogenetic analyses
Three different datasets were built from sequences produced in the present work and others downloaded from public databases (Tables 1–3). Dataset 1 (Agaricales) aimed to resolve the phylogenetic relationships of the incertae sedis lineages studied with the different suborders of Agaricales. It included sequences of six different loci (5.8S, LSU, SSU, RPB1-exons, RPB2-exons, TEF1-exons) from the main lineages analyzed by Matheny et al. (2006), Varga et al. (2019), Ke et al. (2020), Olariaga et al. (2020) and Sánchez-García et al. (2020). Sequences of Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales, Binder et al. 2010, Hodkinson et al. 2014, Zhao et al. 2017), as well as Suillus pictus (Boletales, Hodkinson et al. 2014, He et al. 2019) were employed as outgroup taxa. Dataset 2 (Hygrophorineae) aimed to provide a more accurate view of the major clades within suborder Hygrophorineae. This dataset included sequences of LSU, RPB2-exons (from which a small region of up to 57 bp with multiple insertions/deletions of codons was removed), and TEF1-exons from all specimens of Hygrophorineae in Dataset 1 (as well as A. cebennense and C. borealis as outgroups) plus additional lineages known to belong in this suborder (Lodge et al. 2014, He & Yang 2021). Finally, Dataset 3 (Cuphophylloideae) aimed to focus on species included in this subfamily, and employed sequences of ITS, LSU, RPB2-exons, and TEF1-exons (using A. cebennense and C. borealis again as outgroup taxa). Another two datasets of Agaricales including taxa of suborder Clavariineae (Dataset 4) or the family Cyphellopsidaceae (Dataset 5) were built too (same loci as Dataset 1), but they failed to produce significant support for several major clades of Agaricales, probably due to the insufficient information available from the linages included or missing lineages in the diversity analyzed. As a result, the phylogenetic trees obtained from them are provided as Supplementary Figs S1, S2 and their sequences are included in Table 1. Alignments of Datasets 1–5 are available online (https://figshare.com/; Dataset 1 – Agaricales: 10.6084/m9.figshare.24999359, Dataset 2 – Hygrophorineae: 10.6084/m9.figshare.24999371, Dataset 3 – Cuphophylloideae: 10.6084/m9.figshare.24999362, Dataset 4 – Clavariineae: 10.6084/m9.figshare.24999365, Dataset 5 – Cyphellopsidaceae: 10.6084/m9.figshare.24999368. Sequences newly generated in this study and their GenBank (http://www.ncbi.nlm.nih.gov) accession numbers are shown in Tables 1–3 and Supplementary Table S1.
BLASTn (Altschul et al. 1990) was used to select related homologous sequences from the International Nucleotide Sequence Database Collaboration public database (INSDC, Arita et al. 2021) and UNITE (Nilsson et al. 2018). All sequences employed are listed in Table 1. Sequences were first aligned in MEGA v. 6.0 with its ClustalW application and then realigned manually as needed to establish positional homology. Dataset 1 (Agaricales) included the following partitions (variable sites/total sites/sequences): 53/158/209 (5.8S), 489/864/221 (LSU), 413/1 683/166 (SSU), 440/654/112 (RPB1), 629/999/203 (RPB2), and 548/960/183 (TEF1). Dataset 2 (Hygrophorineae) included the following partitions (variable sites/total sites/sequences): 416/792/100 (LSU), 344/660/63 (RPB2), and 171/472/26 (TEF1). Dataset 3 (Cuphophylloideae) included the following partitions (variable sites/total sites/sequences): 677/1 101/35 (ITS), 292/792/38 (LSU), 277/660/22 (RPB2), and 155/472/14 (TEF1). Aligned loci also were subjected to MrModeltest v. 2.3 (Nylander 2004) in PAUP v. 4.0b10 (Swofford 2003). Model GTR+G+I was selected and implemented in all partitions in MrBayes v. 3.2.6 (Ronquist et al. 2012), where a Bayesian analysis was performed (each locus analyzed in a different partition, two simultaneous runs, four chains, temperature set to 0.2, sampling every 1 000th generation) until the average split frequencies between the simultaneous runs fell below 0.01 after 16.39 M (Agaricales), 2.01 M (Hygrophorineae) and 0.25 M (Cuphophylloideae) generations, respectively. Finally, a full search for the best-scoring maximum likelihood tree was performed in RAxML v. 8.2.12 (Stamatakis 2014) using the standard search algorithm (same partitions, GTRCAT model, 2 000 bootstrap replications). All the analyses were run through the CIPRES Science Gateway platform (Miller et al. 2010). The significance threshold was set above 0.95 for posterior probability (PP) and 70 % bootstrap proportions (BP).
RESULTS
DNA phylogeny
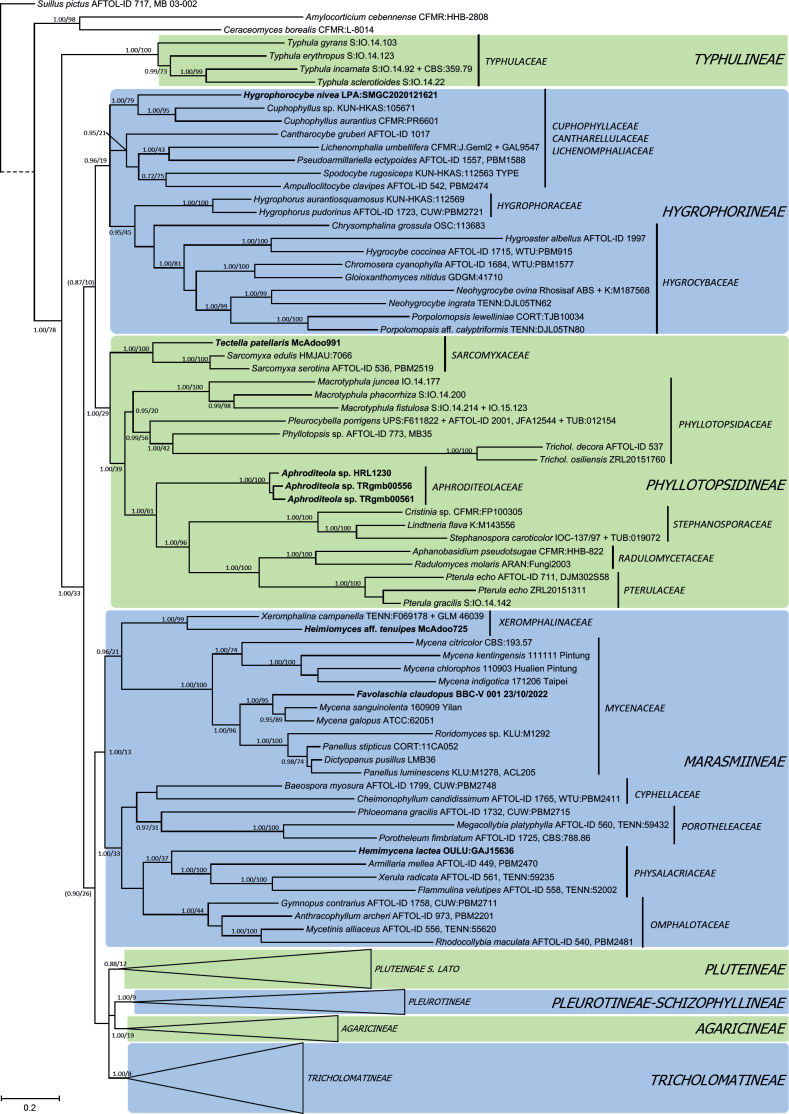
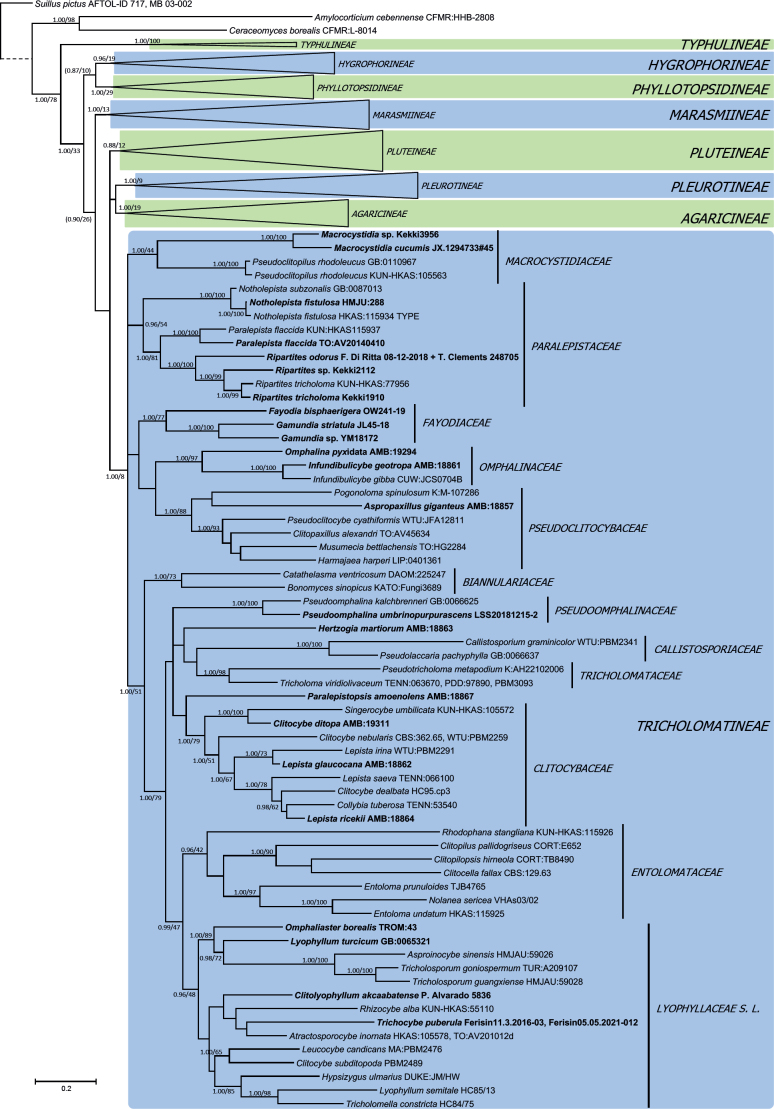
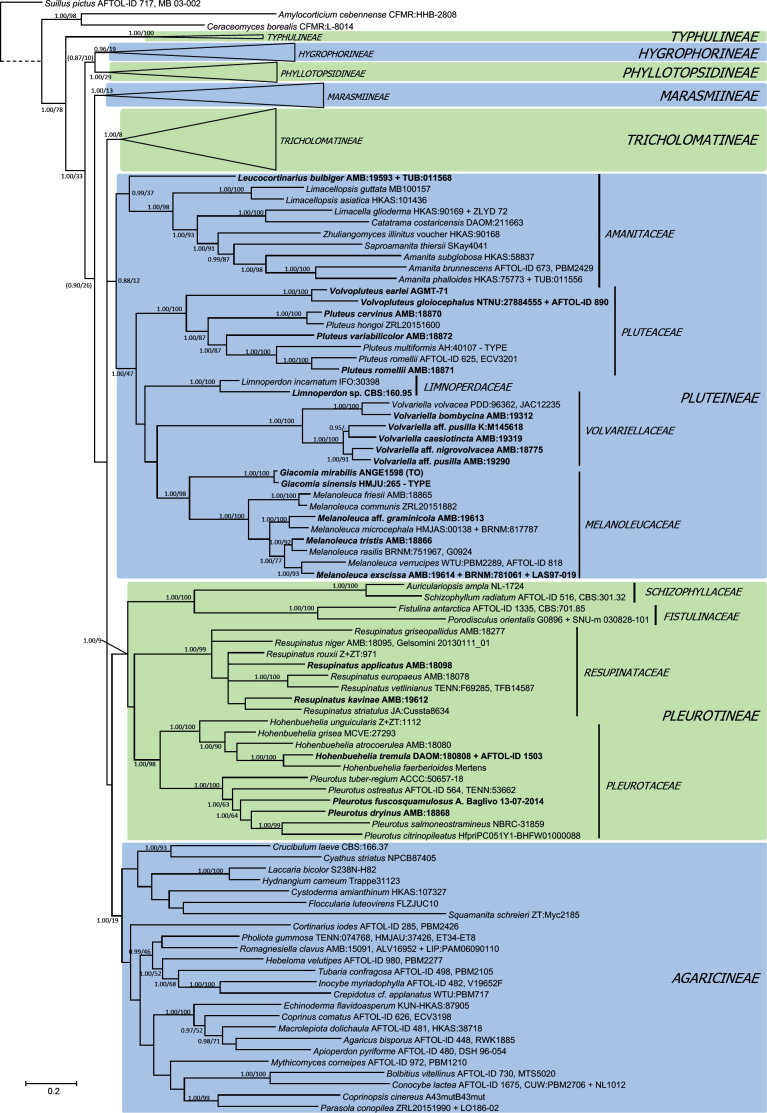
Bayesian analysis of Dataset 1, order Agaricales (Figs 1–3), significantly supported the following hypotheses: 1) family Typhulaceae has a basal position to the remaining suborders analyzed; 2) seven main clades with a significant monophyletic origin were found, matching suborders Agaricineae, Pleurotineae (including Schizophyllineae), Pluteineae, Hygrophorineae, Marasmiineae, Phyllotopsidineae (including Aphroditeola and Sarcomyxineae) and Tricholomatineae; 3) suborder Pleurotineae also encompasses the families Fistulinaceae and Schizophyllaceae, and so it could be considered a synonym of Schizophyllineae; 4) suborder Pluteineae includes Amanitaceae and Leucocortinarius (PP 0.99), as well as the families Pluteaceae, Limnoperdaceae, a strongly supported clade (1.00 PP, 98 % BP) consisting of Melanoleuca and Giacomia, and another including Volvariella; 5) suborder Tricholomatineae has at least twelve families: Macrocystidiaceae (type Macrocystidia, probably related to Pseudoclitopilus); Omphalinaceae (including Infundibulicybe and Omphalina), Pseudoclitocybaceae (including Aspropaxillus), Fayodiaceae (here including Fayodia and Gamundia, but probably also Caulorhiza, Conchomyces and Myxomphalia according to Moncalvo et al. 2002); Biannulariaceae, Callistosporiaceae, Tricholomataceae, Clitocybaceae, Lyophyllaceae sensu lato, Entolomataceae, as well as the unclassified lineages of Neohygrocybe/Pseudoomphalina, Paralepistopsis, Hertzogia and the clade formed by Notholepista, Ripartites, and Paralepista; 6) family Clitocybaceae includes the genera Clitocybe sensu stricto, Lepista, Singerocybe, Collybia sensu lato (He et al. 2023), and the lineage of C. ditopa; 7) family Lyophyllaceae sensu lato is integrated by Lyophyllaceae sensu stricto as well as the so-called hemilyophylloid lineages (Binder et al. 2010, Hofstetter et al. 2014), including the genera Asproinocybe/Tricholosporum (family Asproinocybaceae), Atractosporocybe, Clitolyophyllum, Leucocybe, Omphaliaster, Rhizocybe, Trichocybe, and several species whose generic status needs to be reviewed; 8) family Mycenaceae is part of the suborder Marasmiineae, where it is sister to the significant clade formed by Xeromphalina and Heimiomyces; 9) the previous concepts of the genera Mycena and Hemimycena are polyphyletic; 10) genus Hygrophorocybe is nested inside suborder Hygrophorineae.
Fig. 1.
Bayesian inference phylogram built with nucleotide sequence data of six loci (5.8S, LSU, SSU, RPB1-exons, RPB2-exons and TEF1-exons) of the main lineages inside order Agaricales (focused on suborders Hygrophorineae, Marasmiineae and Phyllotopsidineae), rooted with Suillus pictus (Boletales), Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main suborders are shown in color boxes, while family names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study. The dashed branch was shortened for graphic presentation.
Fig. 3.
Bayesian inference phylogram built with nucleotide sequence data of six loci (5.8S, LSU, SSU, RPB1-exons, RPB2-exons and TEF1-exons) of the main lineages inside order Agaricales (focused on suborder Tricholomatineae), rooted with Suillus pictus (Boletales), Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main suborders are shown in color boxes, while family names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study. The dashed branch was shortened for graphic presentation.
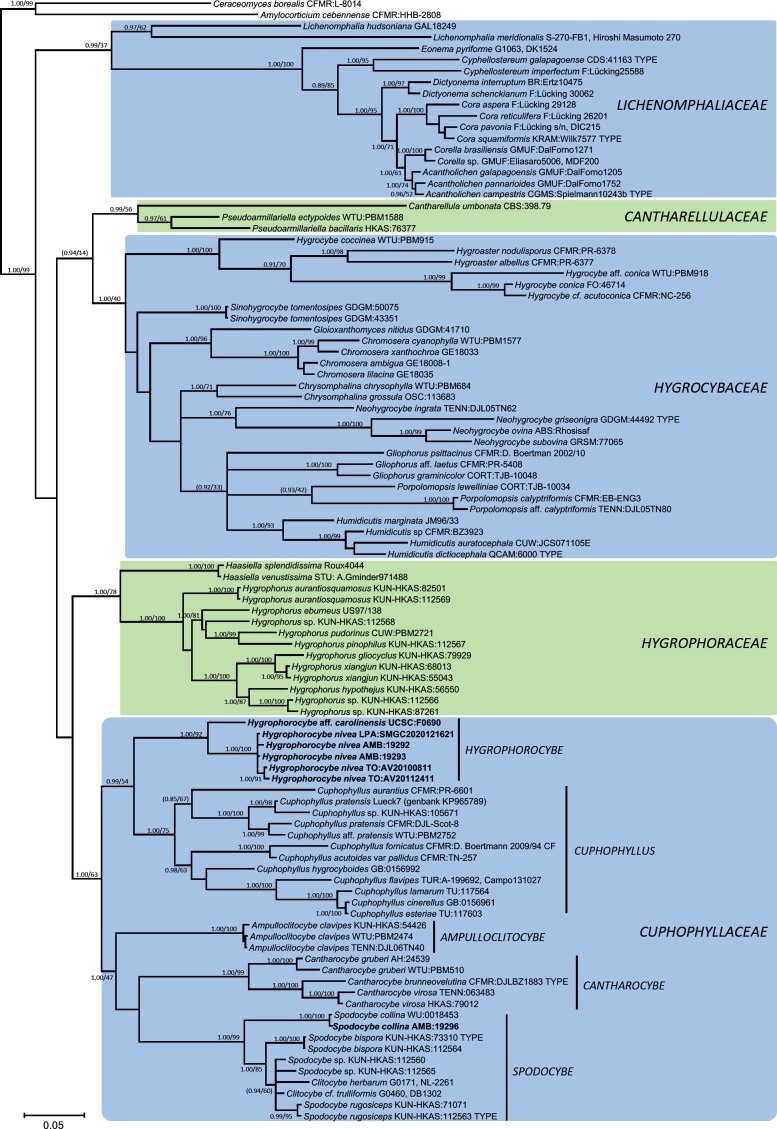
The Bayesian analysis of Dataset 2, the extended dataset of suborder Hygrophorineae (Fig. 4), supported the following five major monophyletic clades: 1) subfamily Lichenomphalioideae; 2) subfamily Hygrocyboideae, 3) tribe Cantharelluleae, 4) subfamily Hygrophoroideae, and 5) subfamily Cuphophylloideae, which includes the genera Ampulloclitocybe, Cantharocybe, Cuphophyllus, Hygrophorocybe (including Clitocybe aff. carolinensis) and Spodocybe.
Fig. 4.
Bayesian inference phylogram built with nucleotide sequence data of three loci (LSU, RPB2-exons and TEF1-exons) of the main lineages inside suborder Hygrophorineae rooted with Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main families are shown in color boxes, while generic names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study.
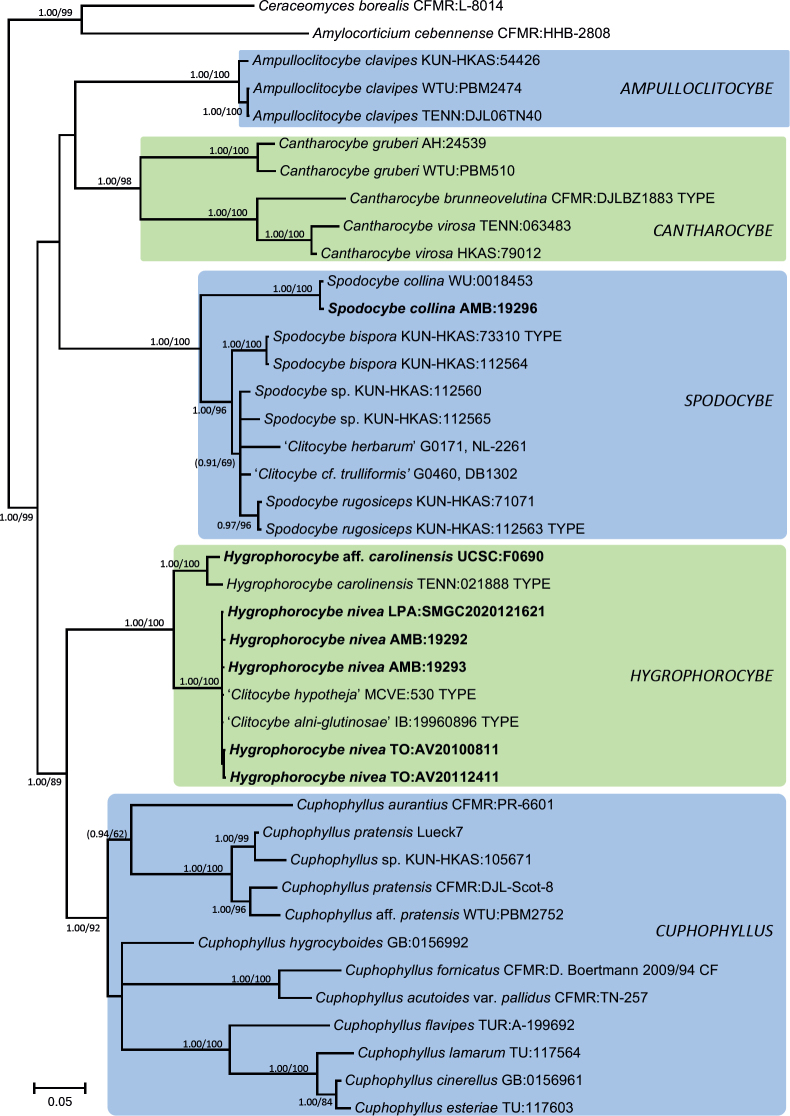
The Bayesian analysis of Dataset 3, the extended dataset of subfamily Cuphophylloideae (Fig. 5), supported the same hypotheses as Fig. 4. The analysis of ITS rDNA allowed also to infer that the holotype collections of Clitocybe alni-glutinosae and C. hypotheja are identical to that of Hygrophorocybe nivea and confirmed that the holotype of C. carolinensis belongs to another clade of Hygrophorocybe.
Fig. 5.
Bayesian inference phylogram built with nucleotide sequence data of four loci (ITS, LSU, RPB2-exons and TEF1-exons) of the main lineages inside family Cuphophyllaceae rooted with Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main genera are shown in color boxes. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study.
The taxo nomy of all these lineages is updated below in accordance with the results obtained from phylogenetic analysis.
Taxonomy
Agaricales Underw., Moulds, mildews, and mushrooms: 97. 1899.
Synonyms: Agarics, Euagarics, Euagarics clade, Euagaricoid clade sensu Hibbett et al. (1997), Pine et al. (1999), Moncalvo (2000, 2002), Hibbett & Binder (2001), Hibbett & Thorn (2001), Redhead et al. (2002a, b), Bodensteiner et al. (2004), Larsson et al. (2004), Binder et al. (2005), Walther et al. (2005), Wilson & Desjardin (2005), Garnica et al. (2007).
Type: Agaricus L., Species Plantarum 2: 1171. 1753.
Representative suborders: Agaricineae, Clavariineae, Hygrophorineae, Marasmiineae, Phyllotopsidineae, Pluteineae, Pleurotineae, Tricholomatineae, and Typhulineae.
Notes: There is no known morphological synapomorphy that unites the order Agaricales. This lineage evolved into several basidiome types, from resupinate (corticioid) to conchate, cyphelloid, stereoid, clavarioid, agaricoid (pileostipitate, with open or enclosed hymenophore), and gasteroid/sequestrate (epigeous or hypogeous). Pileostipitate forms with protective veils (universal and partial) and lamellate hymenophore are the most frequent, but hymenophores can also be smooth, wrinkled, odontoid or poroid. The sequestrate forms show locules, and a columella (vestigial structure of the stipe) can be present, reduced, or absent. The hyphal system is mainly monomitic, with or without clamp connections, rarely dimitic or sarcodimitic. Basidia are holobasidiate, chiastic, usually sterigmate, ballistosporic (when the hymenophore is very early exposed to air) or statismosporic (in gasteroid/sequestrate epigeous to hypogeous forms). Basidiospores are extremely diverse with regards to their shape, wall thickness, colour in mass (white, pink, brown, purplebrown, black), ornamentation, and reactivity, sometimes being dextrinoid/amyloid (Melzer’s reagent), metachromatic (Cresyl blue), or cyanophilous (Cotton blue). Cystidia, pseudocystidia, setae and other sterile elements (acanthocytes, stephanocytes) may be present in the hymenium, pileus and stipe surface and basal mycelium. An asexual morph phase is sometimes present, conidiogenesis mainly thallic, rarely blastic. Dolipores are usually provided with perforate parenthesomes. For a delimitation of Agaricales see Matheny et al. (2006), Dentinger et al. (2016), (Agerer 2018), He et al. (2019), and Olariaga et al. (2020). Agaricales species are mostly ectomycorrhizal (mainly associated with the roots of conifers and dicotyledons), saprotrophic (decaying leaf litter, plant debris, and decaying wood, and include coprophilous, humicolous, and lignicolous species), or parasitic (red algae, plants, including some important phytopathogens), while endophytic and lichenized lifestyles are less frequent (Hibbett & Thorn 2001, Oberwinkler 2012, Agerer 2018). A few species are nematode-trapping or form mutualistic symbiosis with ants and termites (Money 2016, Agerer 2018, Kalichman et al. 2020). The vast majority of Agaricales is terrestrial, found in almost any habitat, from woods and grasslands to deserts and dunes (Kusuma et al. 2021), only a few taxa are known for freshwater (Desjardin et al. 1995, Frank et al. 2010, Abdel-Aziz 2016) or marine environments (Hibbett & Binder 2001, Binder et al. 2006, Jones et al. 2015, 2019, Abdel-Wahab et al. 2019). Lignicolous Agaricales are mainly associated with white rot (Worrall et al. 1997). Brown rot is a rare feeding strategy in Agaricales, associated with small genera such as Hypsizygus and Ossicaulis (Redhead & Ginns 1985). An unusual intermediate wood decomposition type was recently detected in the polyporoid Fistulina and corticioid Cylindrobasidium (Floudas et al. 2015). Enzymes secreted by Agaricales fungi responsible for wood rot are highly relevant to carbon and nutrient cycling in nature play important roles in maintaining environmental balance (Yang et al. 2017, Ruiz-Dueñas et al. 2020, Floudas et al. 2020, Sánchez-Ruiz et al. 2021). Toxic secondary metabolites as amatoxins, psilocybin, muscarine and oxazole compounds which can lead to poisoning in humans are produced mainly by taxa in Agaricineae, Pluteineae and Tricholomatineae (Enjalbert et al. 2002, Sgambelluri et al. 2014, Lee et al. 2018, Luo et al. 2018, Reynolds et al. 2018, Lüli et al. 2019, Sarawi et al. 2022, He et al. 2023). By now, about 25 400 species have been ascribed to the order Agaricales (Bánki et al. 2023), which contains 684 genera, including at least nine extinct (fossil) taxa (Hibbett et al. 2003, Poinar 2016, Cai et al. 2017, Heads et al. 2017), clustered in 45 families (Catalogue of Life, https://www.catalogueoflife.org/).
Agaricales is sister to Amylocorticiales (Binder et al. 2010, Hodkinson et al. 2014, Dentinger et al. 2016, Zhao et al. 2017, He et al. 2019, Sánchez-García et al. 2020, Li et al. 2021, Liu et al. 2023) together forming the superorder Agaricanae (Agerer 2018). Amylocorticiales consists mainly of lignicolous saprotrophic fungi with predominantly resupinate, rarely effuse-reflexed, cupulate or flabellate basidiomes with smooth, wrinkled to tuberculate, rarely tubulose hymenophore (Binder et al. 2010, Garnica et al. 2021). Agaricanae and “Boletanae ad int.” (composed of Boletales and Atheliales; Agerer 2018) form the subclass Agaricomycetidae of the class Agaricomycetes. Jaapiales was proposed in Agaricomycetidae based on multigene data (Binder et al. 2010), but later placed outside it by Li et al. (2021) based on genomic data.
Agaricineae Fr. [as ‘Agaricini’], Syst. orb. veg. (Lundae) 1: 65 (1825) emend. Aime et al., Biol. J. Linn. Soc. 117: 27. 2016.
Representative families: Agaricaceae (including Coprinaceae, Lycoperdaceae, Podaxaceae and Tulostomataceae), Bolbitiaceae, Cortinariaceae, Crassisporiaceae, Crepidotaceae, Galeropsidaceae, Hydnangiaceae, Hymenogastraceae (including Chromocyphellaceae), Inocybaceae, Mythicomycetaceae, Nidulariaceae, Psathyrellaceae, Squamanitaceae, Strophariaceae, and Tubariaceae.
Notes: Saprotrophic (Singer 1986), some associated with rodent latrines (ammonia-fungi or post-putrefaction fungi, Hymenogastraceae, Psathyrellaceae, Sagara 1975, 1995, Sagara et al. 2000, Suzuki 2009), ECM (ectomycorrhizal) forming (Cortinariaceae, Hydnangiaceae, Hymenogastraceae and Inocybaceae; Rinaldi et al. 2008, Tedersoo et al. 2010, Tedersoo & Smith 2013, Soop et al. 2016), arbutoid mycorrhizas forming (Smith & Read 2008, Kühdorf et al. 2016), leaf cutting ants associated (Leucoagaricus, Fisher et al. 1994, Araújo et al. 2022, Urrea-Valencia et al. 2023), nematode hunters (nematophagy, Vizzini 2008) or mycoparasitic (Squamanita, Dissoderma, Psathyrella epimyces; Redhead et al. 1994, Liu et al. 2021, Saar et al. 2022). A previously unknown ectomycorrhizal relationship between poplar roots and Bovista limosa (Agaricaceae) was recently described by Xiao et al. (2023a). The Agaricineae emend. Aime et al. represents one of the seven suborders of Agaricales identified by Dentinger et al. (2016) using a phylogenomic approach and later confirmed in different works, i.e., Varga et al. (2019), Olariaga et al. (2020), Li et al. (2021), Wang et al. (2023b). This suborder corresponds to the “Agaricoid” clade found in previous works (Matheny et al. 2006, 2015, Garnica et al. 2007, Binder et al. 2010, Kohler et al. 2015). The present results agree with recent studies focusing on relationships at the family level within Agaricineae, i.e., Matheny et al. (2015), Vizzini et al. (2019a) or Liu et al. (2021). Many species in Agaricineae show pigmented and/or thick-walled spores (Matheny et al. 2006, 2015, Garnica et al. 2007, Vizzini et al. 2019a). Although species producing dark-pigmented spores (dark-pigmented agarics) are present in a few other lineages (e.g., Melanomphalia, Hygrophorineae, Lichenomphaliaceae, Aime et al. 2005 or Ripartites, Tricholomatineae, Paralepistaceae, see below), the overwhelming majority of these have evolved within Agaricineae. The presence of basidiospores with a thickened, dark-pigmented wall, and occasionally also germ pores, is probably indicative of adaptations to survive harsh conditions in specialised environments (e.g., dung, burnt sites) (Watling 1988, Garnica et al. 2007, Halbwachs et al. 2015, Halbwachs & Bässler 2021). As pigmentation and thick walls are necessary, for example, to the basidiospores of coprophilous species to survive harsh conditions in digestive systems of animals but reduce their germination capability, the germ pore is suggested facilitating germination providing a preferential thin-walled spot where the germ tube can force its way through the tough spore wall (Watling 1988, Halbwachs et al. 2015, Halbwachs & Bässler 2021). In the taxa with asexual morphs, thallic conidiogenesis is the most frequent (Watling 1979, Pantidou et al. 1983, Buchalo 1988, Walther et al. 2005, Walther & Weiß 2006).
Clavariineae Olariaga et al., Stud. Mycol. 96: 171. 2020.
Type: Clavaria Vaill. ex L., Species Plantarum 2: 1182. 1753.
Representative family: Clavariaceae.
Notes: Suborder Clavariineae is characterized by clavarioid basidiomes (Ceratellopsis, Clavaria, Clavicorona, Clavulinopsis, Hirticlavula, Holocoryne, Ramariopsis), more rarely agaricoid, gymnocarpic with waxy hygrophoroid decurrent lamellae (Camarophyllopsis, Hodophilus, Lamelloclavaria), hydnoid (Mucronella) or corticioid (Hyphodontiella). Hyphal system monomitic, or more rarely dimitic. Basidiospores colourless, usually thin-walled, smooth or ornamented, usually with multiguttulate contents, sometimes with amyloid or dextrinoid reactions. Basidia claviform, with up to four sterigmata, occasionally sometimes with a loop-like (medallion) basal clamp (Clavaria subgen. Holocoryne). Cystidia usually absent. Pileipellis either a hymeniderm or a trichoderm with rounded terminal elements in genera with pileostipitate basidiomes. Clamp connections present or absent, sometimes restricted to basidia. Saprotrophic on dead wood, herbaceous plants, or leaves, or biotrophic with grasses and bryophyte gametophytes (Birkebak 2015, Birkebak et al. 2013, 2016). The presence of TEF1 intron 21 (numbering according to Matheny et al. 2007), absent in the rest of the Agaricales (Matheny et al. 2007), seems so far restricted to some genera of Clavariaceae (Camarophyllopsis, Clavaria, Clavulinopsis; absent in Ceratellopsis).
The traditional concept of the family Clavariaceae as circumscribed by Corner (1950, 1970), Thind (1961), Parmasto (1965), Jülich (1984), and Petersen (1988) was later expanded upon by several authors based on the results of DNA-based phylogenetic analyses. The family Clavariaceae was first shown to have affinities with the Agaricales by Pine et al. (1999) using nuclear and mitochondrial rDNA loci. Clavaria fusiformis (now Clavulinopsis) was apparently near to the /tricholomopsis clade and sister to the /hemimycena clade in Moncalvo et al. (2002). Clavaria, Clavulinopsis, Mucronella and Ramariopsis were found to be monophyletic by Larsson et al. (2004) and Dentinger & McLaughlin (2006). Matheny et al. (2006) demonstrated that the gilled pileate-stipitate genus Camarophyllopsis belongs in the Clavariaceae, and not inside the Hygrophoraceae as suggested by Hesler & Smith (1963), Arnolds (1974a, b, 1986), Kühner 1980, Singer (1986), Kovalenko (1989), Young (1999, 2005), and Boertmann (2002). The resupinate wood-inhabiting genus Hyphodontiella was shown to belong in the Clavariaceae too by Larsson (2007). Kautmanová et al. (2012a) included in their phylogenetic analysis of Clavariaceae the type of Clavaria (C. fragilis), confirming the previous assumptions. The genera Hodophilus (gilled) and Clavicorona (clavarioid) were also shown to be members of the Clavariaceae by Birkebak et al. (2013); and so was the clavarioid Hirticlavula by Petersen et al. (2014), the clavarioid Holocoryne, the gilled Lamelloclavaria by Birkebak et al. (2016), and the clavarioid Ceratellopsis emended by Olariaga et al. (2020).
As regards the phylogenetic placement of the family, members of Clavariaceae were considered incertae sedis for a long time by Pine et al. (1999), Moncalvo et al. (2002), Larsson et al. (2004), Larsson (2007), and in Lodge et al. (2014). In subsequent analyses, Clavariaceae was found to be an early diverging basal clade of Agaricales, either showing an isolated position (e.g., Binder et al. 2010, Sánchez-García et al. 2020, Olariaga et al. 2020) or related to Atheliaceae p.p. (Plicaturopsidoid clade, Matheny et al. 2006), or Hygrophoraceae (Ryberg & Matheny 2011, Dentinger et al. 2016, Varga et al. 2019). Based on a more diverse dataset, Olariaga et al. (2020) established suborder Clavariineae inside the order Agaricales, although most species were represented only by ribosomal DNA (LSU) sequences. Suborder Clavariineae was removed from the present analysis to avoid adding too much phylogenetic noise from a clade already shown to be basal to the remaining ones (Olariaga et al. 2020, Wang et al. 2023b), and because of the incomplete information available in databases (i.e., multigene data of Hirticlavula or Hyphodontiella are not available). However, additional analyses (Supplementary Fig. S1) agree to place Clavariineae in an early branching (basal) clade not related to any other in the dataset employed.
Hygrophorineae Aime et al., Biol. J. Linn. Soc. 117: 26. 2016.
Synonyms: Hygrophorales Bon, Flore mycologique d’Europe 1: 87. 1990.
Hygrophorales Locq., Mycol. Gén. Struct. (Paris): 98. 1984, nom. inval., Art. 39.1 (Shenzhen).
Type: Hygrophorus Fr., Fl. Scan.: 339. 1836. [1835].
Representative families: Cantharellulaceae, Cuphophyllaceae, Hygrocybaceae, Hygrophoraceae, and Lichenomphaliaceae.
Notes: Hygrophorineae is characterized by basidiomes primarily agaricoid, hymenophore predominantly lamellate, occasionally smooth, wrinkled or forked, often pigmented with L-DOPA betalains or carotenoids, and waxy; spore deposit white or rarely lightly pigmented (ochraceous, salmon, green); hyphae monomitic, usually with clamp connections; cystidia normally absent; basidia normally 2–4-sporic, mean ratio of basidia to basidiospore length 3–7; basidiospores colourless, predominantly inamyloid; terricolous, lignicolous, bryicolous, pteridicolous, saprotrophic, rarely parasitic on mosses, or symbiotic and then lichen-forming with cyanobacteria and/or green algae or ectomycorrhizal.
To treat the major monophyletic clades within Hygrophorineae (Fig. 4) at the same level as those of the other suborders, the main clades (four subfamilies and one tribe) recovered by Lodge et al. (2014) and He & Yang (2021) within Hygrophoraceae are here upgraded to the rank of independent families. As a result, five families are recognized in the present work inside Hygrophorineae (see below).
Hygrophoraceae Lotsy, Vortr. Bot. Stammesgesch. 1: 705. 1907. Perhaps based on Hygrophorées Roze, Bull. Soc. Bot. France 23: 110. 1876, nom. inval., Art. 32.1(b); see Art. 18.4 (Shenzhen).
Representative genera: Haasiella and Hygrophorus.
Notes: Hygrophoraceae is characterized by gymnocarpous or secondarily mixangiocarpous basidiomes; lamellae subdecurrent to deeply decurrent; trama inamyloid; hymenophoral trama divergent (hyphae diverging from a central strand), or bidirectional (horizontal hyphae that are parallel to the lamellar edge present, sometimes woven through vertically oriented, regular or subregular generative hyphae that are confined or not to a central strand) and a pachypodial structure below the active hymenium; basidiospores thin- or thick-walled, inamyloid, metachromatic or not, colorless or lightly pigmented (ochraceous, salmon, green); pigments muscaflavin (betalain) or carotenoids; habit terricolous (ectomycorrhizal, Hygrophorus) or xylophagous (saprotrophic, Haasiella) (Tedersoo et al. 2010, Seitzman et al. 2011, Lodge et al. 2014, Feng & Yang 2019). The phylogenetic affinities of Haasiella with Hygrophorus (Fig. 4) had already been previously highlighted by Vizzini et al. (2012b), Lodge et al. (2014), He & Yang (2021) and Wang et al. (2023a). The genus Aeruginospora (typified with A. singularis) is probably closely related to Haasiella based on their morphology: a similar basidiome form, bidirectional hymenophoral trama, a thickening hymenium forming a pachypodial structure, and spores that are thick-walled, pigmented, and with a red metachromatic endosporium (Lodge et al. 2014).
Hygrocybaceae (Padamsee & Lodge) Vizzini, Consiglio & P. Alvarado, stat. nov. & comb. nov. MycoBank MB 851141.
Synonym: Hygrophoraceae subfamily Hygrocyboideae Padamsee & Lodge, Fungal Diversity 64: 19. 2013. [2014].
Type: Hygrocybe (Fr.) P. Kumm., Führ. Pilzk. (Zwickau): 111. 1871. Synonym: Hygrophorus subg. Hygrocybe Fr., Summa veg. Scand., Section Post. (Stockholm): 308. 1849.
Representative genera: Chromosera, Chrysomphalina, Gliophorus, Gloioxanthomyces, Humidicutis, Hygrocybe (Hygroaster included), Neohygrocybe, Porpolomopsis, and Sinohygrocybe.
Notes: The circumscription of the family Hygrocybaceae is similar to those outlined by Lodge et al (2014), Wang et al. (2018) and He & Yang (2021) for Hygrophoraceae subfamily Hygrocyboideae except for the position of Chrysomphalina (typified with C. chrysophylla), which was included in Hygrophoraceae subfamily Hygrophoroideae by these latter authors and recently by Wang et al. (2023a). Our analysis (Fig. 4) indicated Chrysomphalina as part of Hygrocybaceae. Prior to the first sequencing and phylogenetic analyses of Haasiella, Redhead et al. (2002a) postulated a close relationship between this genus and Chrysomphalina based on pigments and micromorphology, although Kost (1986) disagreed based on the micromorphology. Clémençon (1982) combined Chrysomphalina grossula into Camarophyllus (subg. Aeruginospora) owing to the similar structure of their hymenophoral trama. Romagnesi (1996) included Haasiella and Phyllotopsis along with the type, Chrysomphalina, in his tribe Chrysomphalineae (invalidly published before that as tribe Paracantharelleae, Romagnesi 1995) due to the presence of carotenoid pigments in all of them. Hygrocybaceae is characterized by basidiomes with colors usually bright, rarely dull; lamellae, usually thick, yielding a waxy substance when crushed, rarely absent; true veils lacking, rarely with false peronate veils formed by fusion of the gelatinous ixocutis of the pileus and stipe, and fibrillose partial veils formed by hyphae emanating from the lamellar edge and stipe apex; basidiospores thin-walled, guttulate, colourless (though species with black staining basidiomes may have fuscous inclusions), smooth or ornamented by conical spines, inamyloid, usually acyanophilous; basidia guttulate, mono- or dimorphic; pleurocystidia absent; pseudocystidia sometimes present; true cheilocystidia usually absent but cystidia-like hyphoid elements emanating from the lamellar context or cylindric or strangulated ixo-cheilocystidia embedded in a gelatinous matrix sometimes present; hymenophoral trama inamyloid, regular or subregular but not highly interwoven, divergent or pachypodial; comprised of long or short hyphal segments with oblique or perpendicular cross walls, often constricted at the septations, usually thin-walled but hyphae of the central mediostratum sometimes slightly thickened. Pileipellis structure a cutis, disrupted cutis, ixocutis, ixotrichodermium or trichodermium, but never hymeniform; pigments muscaflavin or carotenoids; clamp connections present or absent; habit terrestrial, rarely on wood or arboreal, often associated with mosses, growing in grasslands or forests; possibly biotrophic (Seitzman et al. 2011) but not known to form ectomycorrhizae with woody plants.
Lichenomphaliaceae (Lücking & Redhead) Vizzini, Consiglio & P. Alvarado, stat. nov. & comb. nov. MycoBank MB851143.
Synonyms: Hygrophoraceae subfamily Lichenomphalioideae Lücking & Redhead, Fungal Diversity 64: 68. 2013. [2014].
Arrheniaceae Locq., Mycol. Gén. Struct. (Paris): 109. 1984, nom. inval., Art. 39.1 (Shenzhen).
Type: Lichenomphalia Redhead et al., Mycotaxon 83: 38. 2002.
Representative genera: Acantholichen, Arrhenia, Cora, Corella, Cyphellostereum, Dictyonema, Eonema, and Lichenomphalia.
Notes: The present work does not include collections of Arrhenia, but other studies suggest that this genus belongs in Lichenomphaliaceae (Lodge et al 2014, Wang et al. 2018, He & Yang 2021). The delimitation of this family corresponds quite well to subfamily Lichenomphalioideae (in Lodge et al. 2014) except for its tribe Cantharelluleae which is here raised to family rank. Lichenomphaliaceae is characterized by basidiomes omphalinoid, pleurotoid, stereoid-corticioid or lentoid-cyphelloid, rarely absent, usually fuscous, green or colourless, rarely orange or yellow; hymenium lamellate, cantharelloid, merulioid (wrinkled) or smooth; basidiospores inamyloid; basidia elongated or not; clamp connections present or absent; L-DOPA betalains and apparently also carotenoid pigments absent; habit primarily bryophilous or phycophilous, often lichenized, rarely parasitic, or saprobic. The basidiolichens are mainly distributed in five orders of Agaricomycetes, viz. Agaricales, Atheliales, Lepidostromatales, Cantharellales and Corticiales (Oberwinkler 2012, Hodkinson et al. 2014, Lücking et al. 2017, Masumoto & Degawa 2020, Zhang et al. 2022). Among the Agaricales, virtually all lichenized species are accommodated within the Lichenomphaliaceae with the only striking exception of Omphalina licheniformis which belongs to Omphalinaceae (Tricholomatineae, Zhang et al. 2022). Some species of Collybiopsis (Omphalotaceae, Marasmiineae) are associated to the green alga Coccomyxa and suspected to be lichenized (Lepp 2011a, b, Cooper & Leonard 2013).
Cantharellulaceae (Lodge et al.) Vizzini, Consiglio & P. Alvarado, stat. nov. & comb. nov. MycoBank MB851144.
Synonym: Hygrophoraceae subfamily Lichenomphalioideae, tribe Cantharelluleae Lodge et al., Fungal Diversity 64: 74. 2013. [2014].
Type: Cantharellula Singer, Rev. Mycol. (Paris) 1: 281. 1936.
Representative genera: Cantharellula and Pseudoarmillariella.
Notes: Cantharellulaceae is characterized by basidiomes clitocyboid or omphalinoid; pileus convex, depressed or infundibuliform; lamellae decurrent, repeatedly forked, sometimes staining reddish brown; stipe central or eccentric; basidiospores smooth, with a length usually at least twice the diameter, colourless, distinctly amyloid, acyanophilous; basidia with basal clamp connections, about 4 times the length of the basidiospores; cheilocystidia and pleurocystidia absent; pileipellis hyphae with cytoplasmatic pigments, with or without encrusting pigments; hymenophoral trama partly gelatinized at the lamellar edge, tridirectional, with a subregular or regular central strand, lateral strands with frequent hyphae parallel to the lamellar edge woven through a few vertically oriented hyphae, and abundant generative hyphae arranged predominantly in parallel to the basidia and giving rise to the subhymenial cells, but obliquely angled (divergent) at the lamellar edge; subhymenium subramose or pachypodial, composed of short- or long-celled hyphal segments predominantly parallel and oriented in the same direction as the basidia, but a few highly curved and intertwined; forming a weak hymenial palisade via proliferation of basidia from subhymenial cells (thickening hymenium); L-DOPA betalains and apparently also carotenoid pigments absent; bryophilous or lignicolous. The forked lamellae, long, smooth, amyloid spores, thickening hymenium and subhymenium, and tridirectional hymenophoral trama is a unique combination of characters within Hygrophorineae shared by Cantharellula and Pseudoarmillariella (Singer 1956, 1986, Norvell et al. 1994, Redhead et al. 2002a, Yang et al. 2013, Lodge et al. 2014). Singer (1936) established the genus Cantharellula to accommodate Merulius umbonatus where then he transferred Agaricus ectypoides (Singer 1942a). Later, he classified both species in Cantharellula subg. Pseudoarmillariella (Singer 1948), and finally raised Cantharellula subg. Pseudoarmillariella to genus rank around P. ectypoides (Singer 1956). Two species were recognized within Cantharellula by Lodge et al. (2014), viz. the type C. umbonata (with a wide distribution) and C. infundibuliformis, another species from Argentina by now lacking sequence data. Several species of Cantharellula were recently moved to other genera: Cantharellula foetida to Pseudoclitocybe by Cooper (2014); C. humicola to Corneriella and C. umbrosa to Pseudotricholoma by Sánchez-García et al. (2014); and C. intermedia to Pseudoomphalina by Voitk et al. (2020a). Regarding Pseudoarmillariella, three are the species known, viz. the type P. ectypoides (present in North and Central America, Singer 1986, Norvell et al. 1994, Lodge et al. 2014), P. fistulosa (New Zealand, Stevenson 1964, Horak 1971), and P. bacillaris (China, Yuan & Sun 2007, as Cantharellus melanoxeros; Yang et al. 2013). DNA sequences of P. ectypoides and P. bacillaris are present in public databases (Yang et al. 2013, Lodge et al. 2014). Pseudoarmillariella differs from Cantharellula in the presence of encrusting pigments on the pileipellis hyphae, presence of bright ochraceous pigments in the hymenophore (Norvell et al. 1994, Singer 1986, Lodge et al. 2014), and in growing on fallen rotting gymnospermous wood (Stevenson 1964, Singer 1986, Yang et al. 2013) (while C. umbonata is associated with mosses; Lawrey et al. 2009, Lodge et al. 2014).
The systematic position of these two genera has been debated for a long time, being unclear until recently. Singer (1942, 1948, 1986) recognized the close relationship between C. umbonata and P. ectypoides and placed them together with other amyloid spored genera in the Tricholomataceae, tribe Leucopaxilleae. Moncalvo et al. (2002) were the first to provide molecular evidence of the sister relation between the lineages of Cantharellula and Pseudoarmillariella, a clade apparently close to the Arrhenia group on the basis of nrLSU sequences. Lodge et al. (2006) were the first to obtain a significant support for the Cantharelluleae clade using an extended four-gene dataset, while Matheny et al. (2006) and Lawrey et al. (2009) obtained significant evidence for the placement of P. ectypoides and Lichenomphalia umbellifera inside the family Hygrophoraceae. Lodge et al. (2014), employing an extended multigene dataset, placed Cantharellula and Pseudoarmillariella in a new tribe, Cantharelluleae, within subfamily Lichenomphalioideae. Because of the peculiar morphological traits of this tribe and the taxonomic treatment of the other clades of suborder Hygrophorineae, it is here raised to the family rank (see above).
Cuphophyllaceae (Z.M. He & Zhu L. Yang) Vizzini, Consiglio & P. Alvarado, stat. nov. & comb. nov. MycoBank MB 851145.
Synonym: Hygrophoraceae subfamily Cuphophylloideae Z.M. He & Zhu L. Yang, MycoKeys 79: 138. 2021.
Type: Cuphophyllus (Donk) Bon, Doc. Mycol. 14(no. 56): 10. 1985. [1984].
Synonym: Hygrocybe subgen. Cuphophyllus Donk, Beih. Nova Hedwigia 5: 45. 1962.
Representative genera: Ampulloclitocybe, Cantharocybe, Cuphophyllus, Hygrophorocybe, and Spodocybe (Fig. 8E).
Notes: The family corresponds well to the Cuphophylloid grade in Lodge et al. (2014) and Wang et al. (2018), and to Hygrophoraceae subfamily Cuphophylloideae (He & Yang 2021) plus Hygrophorocybe. Cuphophyllaceae is characterized by basidiomes mostly clitocyboid, rarely omphalinoid or mycenoid, pileus convex, applanate to funnel-shaped; surface usually dry, smooth, lubricous or rarely viscid; lamellae decurrent to long decurrent; veils absent; basidiospores ellipsoid, oblong or subglobose, thin-walled, smooth (light microscopy), cyanophilous or not and inamyloid; pileipellis usually a cutis, sometimes ixocutis or trichoderm; hymenophoral trama regular, subregular, interwoven or bidirectional; clamp connections usually present; L-DOPA betalains and apparently also carotenoid pigments absent; terricolous, rarely on wood, widespread in temperate and tropical regions. Most species are presumably saprotrophic (Lodge et al. 2014, He & Yang 2021). Ampulloclitocybe clavipes (Merlini et al. 2000), and C. virgineus (Farrell et al. 1977), have been successfully cultured on agar media – a trait shared, for example, with saprotrophic species of the related suborder Phyllotopsidineae (see below) such as Aphroditeola (Redhead 2013), Phyllotopsis nidulans (Jayasinghe & Parkinson 2008), Sarcomyxa serotina (Kim et al. 2012), Tricholomopsis rutilans (Murphy & Mitchell 2001), and Macrotyphula spp. (Dentinger & McLaughlin 2006). Some species of Cuphophyllus have a biotrophic mode of nutrition, but the nature of the fungus-plant association is largely unknown (Griffith et al. 2002, Seitzman et al. 2011, Halbwachs et al. 2018). Cuphophyllus virgineus was shown to be a root endophyte of Plantago lanceolata and vertically transmitted via seeds (Tello et al. 2014).
The genus Cuphophyllus is characterized by lamellae mostly arcuate-decurrent, subdecurrent or decurrent, rarely sinuate, usually thick near the pileus, often forked or veined, usually distant, usually brittle, often acquiring a chalky opaque appearance, basidiospores frequently broadly ellipsoid, subglobose or globose, sometimes ellipsoid or oblong, smooth also under SEM, acyanophilous, basidia long typically 7−8 (rarely 5−6) times the length of the basidiospores, hymenophoral trama usually highly interwoven (rarely subregular), with or without a regular or subregular central strand; cystidia absent; hyphae predominantly or partly interwoven, usually with dissolved pigments, sometimes with intraparietal and encrusting pigments (Lodge et al. 2014, Voitk et al. 2020b). Ampulloclitocybe (= Clavicybe) differs from Cuphophyllus in having not forked, thin and close lamellae, basidia less than 5 times the length of the basidiospores, a bidirectional hymenophoral trama, subparallel rather than interwoven pileipellis hyphae, and basidiospores appearing smooth with light microscopy but minutely roughened-rugose when viewed under SEM (scanning electron microscope) (Pegler & Young 1971, Bon 1997, Redhead et al. 2002a, Harmaja 2002, Lodge et al. 2014). It is known to produce a coprine-like (antabuse-like) aldehyde dehydrogenase inhibitor (Cochran & Cochran 1978, Yamaura et al. 1986) as well as a tyrosine kinase inhibitor named clavilactone (Cassinelli et al. 2000). Cantharocybe differs in having a regular hymenophoral trama, basidia to basidiospore length less than 5 and presence of cheilo- and caulocystidia (Bigelow & Smith 1973, Ovrebo et al. 2011, Kumar & Manimohan 2013, Lodge et al. 2014, Hosen et al. 2016, Kumla et al. 2018, Parnmen et al. 2020, Hussain et al. 2021). Spodocybe (Fig. 8E) is characterized by a rugose to felty-squamulose grey-brown pileus depressed (funnel-shaped) at maturity, usually with farinaceous odours, the ratio of basidia to basidiospore length less than 5, and subregular hymenophoral trama (Bon 1997, as Clitocybe, He & Yang 2021, Xiao et al. 2023b, Xu et al. 2023). Finally, Hygrophorocybe has thin lamellae, cyanophilous basidiospores, and a subregular hymenophoral trama (Romagnesi 1974, Bellú 1996, Bon 1997, as Clitocybe, Vizzini 2014a, and see below).
Hygrophorocybe Vizzini & Contu, Index Fungorum 161: 1. 2014.
Type: Hygrophorocybe nivea (Velen.) Vizzini & Contu, in Vizzini, Index Fungorum 161: 1. 2014.
Representative species: Hygrophorocybe nivea, H. carolinensis.
Notes: Bon (1997) included H. nivea in Clitocybe subgenus Hygroclitocybe (typified with C. clavipes, therefore a synonym of Ampulloclitocybe) because of the hygrophoroid long basidia, hymenophoral trama not strictly regular, and vacuolar pigment. This extremely artificial subgenus also included species of the unrelated genus Infundibulicybe (Harmaja 2003, Omphalinaceae, Tricholomatineae), so Vizzini (2014b) proposed a new genus, Hygrophorocybe, to accommodate C. nivea based on its morphology and unpublished rDNA data. Its position was considered uncertain by He et al. (2019), as DNA sequences were not yet available in public databases. In the present work, phylogenetic analyses based on sequences of rDNA and protein-coding genes suggest that Hygrophorocybe belongs in suborder Hygrophorineae (family Hygrophoraceae in Lodge et al. 2014) (Fig. 1), family Cuphophyllaceae (Figs 4, 5), being a sister lineage of Cuphophyllus. Cuphophyllus differs from Hygrophorocybe in its thick lamellae, acyanophilous basidiospores, interwoven (rarely almost subregular) hymenophoral trama, with or without a regular or subregular central strand, and very long basidia relative to spore length (usually 7–8, rarely 5–6 times spore length) (Bon 1985, 1990a, Lodge et al. 2014, Voitk et al. 2020b). Based on the original description by Maire (1928), Clitocybe chudacae, a species first collected in Algeria, should be considered a later synonym of C. nivea. Due to their white basidiomes with hygrophoroid habit, long basidia and large spores, two species from North America, Clitocybe hygrophoroides (Bigelow 1965, 1982) and C. variabilis (Murrill 1913, Smith 1944, Bigelow 1982, Gregory 2007) are here thought to be putative members of Hygrophorocybe too.
Hygrophorocybe nivea (Velen.) Vizzini & Contu, in Vizzini, Index Fungorum 161: 1. 2014. Figs 6L, 9.
Fig. 9.
Hygrophorocybe nivea. A–F. Basidiomes (A. AMB:19293; B. TO:AV20100811; C. TUR-A 216591; D, F. TO:AV20112411; E. LPA SMGC2020121621). G. Pileipellis (TO: AV20112411). H. Elements of the pileipellis (AMB:19293). I. Hymenium and hymenophoral trama (TO:AV20112411). J, K. Hymenium (J. AMB:19293; K. AMB:19292). L, M. Basidiospores (AMB:19293). N. Basidiospores and young basidium (AMB:19292). Mounting media were Congo Red in ammonia (J–L, N), and Cotton Blue (M). Scale bars: G–N = 10 μm. Photographs A by G. Consiglio, B, D, F by A. Vizzini, C by M. Carbone, E by V.J. Escobio García, H, J–N by M. Marchetti; drawings by L. Panno.
Basionym: Clitocybe nivea Velen., České Houby (Praze) 2: 255. 1920.
Synonyms: Clitocybe hypotheja Bellù, Rivista Micol. 39(2): 106. 1996.
Clitocybe alni-glutinosae Contu & Ruggero, in Contu, Rivista Micol. 41(4): 349. 1999 [1998].
? Clitocybe chudacae Maire, Bull. Trimestriel Soc. Mycol. France 42: 38. 1928.
Description: Habit clitocyboid/hygrophoroid. Pileus 10–80(−120) mm diam, broadly convex at first then expanding, becoming plane or obtusely umbonate, rarely infundibuliform when old; margin incurved and inrolled at first, minutely ribbed (as in Tricholoma stiparophyllum); surface dry, smooth, glabrous, pruinose to velutinous, non-hygrophanous, sometimes rivulose to cracked, whitish, sometimes with light ochre-yellow (3A6-8, 4A7-8) tinges at centre. Lamellae decurrent to long decurrent, subdistant to distant, L = 35–45, l = 1–2(−3), narrow, 2–3(−4) mm broad, often forked or anastomosing, pinkish-cream (5A4-6) to yellowish (4A5-6) at maturity (yellowish in dried basidiomes), easily separable from the pileus context. Stipe (20)–30–60(−80) × 5–8(−12) mm, central, equal, or tapered upward, curved at times; interior solid, stuffed, finally hollow; surface smooth, with thin fibrillose coating, glabrescent; concolorous with the pileus. Context thick and firm at pileus centre (up to 15 mm thick), white. Odour none or very faintly fragrant; taste mild. Spore deposit whitish to light pinkish cream. Basidiospores (5.3–)6.4–7.2–7.9(−9.9) × (3.2–)3.9–4.2–4.6(−5.1) μm [208/6/6], Q= (1.34–)1.53–1.71–1.88(−2.19), V= (33.0–)51.7–68.3–84.8(−121) μm3, ovoid to ellipsoid, sometimes nearly oblongcylindrical, smooth, wall thin or somewhat thickened, inamyloid, cyanophilous. Basidia hygrophoroid, 30–45(−50) × 6.5–8.5 μm, 4–5(−6) times spore length, mostly 4-sporic, but sometimes 1and 2-sporic, subclavate or narrowly clavate; sterigmata up to 6 μm long, sometimes delimited by secondary septa, clamped; sclerobasidia (crassobasidia) occasionally present; basidioles sometimes irregular in shape, clamped. Subhymenium consisting of short to elongated intertwined elements. Hymenial cystidia not observed. Hymenophoral trama subregular, unidirectional of subparallel hyphae; hyphae 1.5–8 μm diam, mostly cylindrical, some inflated, smooth, pale yellow in KOH, clamped. Pileipellis a cutis, loosely woven; hyphae 3–10 μm diam, cylindrical, some pileocystidioid, most smooth, with rare and short diverticula, some finely encrusted, yellow in KOH, clamped. Subpellis consisting of cylindrical or inflated, 4–15 μm wide hyphae, smooth, refractive, yellow in KOH, clamped. Stipitipellis arranged as a cutis of parallel, cylindrical (2–)2.5–4(−5) µm wide hyphae. Caulocystidia present often in tufts, mostly at stipe apex, 15–40(−50) × (2–)3–4(−5) µm, thin-walled, cylindrical, sinuous, with rounded apex, often trapping mature basidiospores fallen from the overlying hymenium. Stipititrama non-sarcodimitic, composed of colourless 4–7(−8) µm wide cylindrical hyphae (wall up to 0.5 µm thick). Thromboplerous hyphae present. Clamp connections present, very common in the pileipellis and stipitipellis. Hyphal system monomitic.
Habitat and distribution: Scattered or gregarious, in soil or needle litter, usually under coniferous trees, Picea, Pinus spp., Pseudotsuga menziesii, Cryptomeria japonica, Chamaecyparis lawsoniana, Cedrus atlantica (Cavet & Martin 1998) but also in mixed forest of broad-leaved trees; alpine and Mediterranean areas; autumn.
Materials examined: Italy, Emilia-Romagna, Ronchi (Monterenzio, BO), in a mixed forest of Quercus pubescens and Q. cerris, 5 Nov. 1992 and 1 Nov. 1994, G. Consiglio & G. Spisni (AMB:19314 and AMB:19293); Tudiano (Grizzana Morandi, BO), in a mixed forest of broad-leaved trees, 9 Oct. 1998, G. Consiglio & G. Spisni (AMB:19315); Puzzola (Grizzana Morandi, BO), in a mixed forest of broad-leaved trees, 13 Nov. 1999, G. Consiglio, G. Perdisa & G. Spisni (AMB:19316); Eremo di Zena (S. Lazzaro di Savena, BO), in a mixed forest of broad-leaved trees, 8 Dec. 1999, G. Consiglio & G. Bordoni (AMB:19317); Val Serena (San Benedetto Val Di Sambro, BO), under Picea abies, 30 Oct. 2001, G. Consiglio & E. Franceschini (AMB:19292); Lombardia, Brallo di Pregola (PV), Cima Colletta, 1493 m a.s.l., among litter in an artificial planting of Pseudotsuga menziesii, 15 Oct. 2022, M. Carbone (TUR-A 216591); Piemonte, Torino, Parco Leopardi, on Chamaecyparis lawsoniana litter, 8 Nov. 2010, A. Vizzini (TO:AV20100811); Torino, Parco della Rimembranza (Parco della Maddalena), on Cryptomeria japonica litter, 24 Nov. 2011, A. Vizzini (TO:AV20112411). Spain, Canary Islands, El Hierro, under Myrica faya and Erica arborea, 16 Dec. 2020, V. Escobio García (LPA:SMGC2020121621).
Notes: Clitocybe nivea was briefly described for the first time from specimens collected in some areas of the Czech Republic (Velenovský 1920) as a large and fleshy species with a pileus 3–10 cm diam, convex, white, felted-cottony at the margin; lamellae at first white then with pinkish hues; large ellipsoid spores, 6–9 µm; and growing gregarious in coniferous (spruce) litter. After the original description, the species fell into oblivion until Romagnesi (1974) resurrected the name for collections found under Picea in southern France. The species was later found again in France (e.g., Bon 1997, Cavet & Martin 1998, Eyssartier & Roux 2011), Italy (e.g., Bellú 1996, Consiglio 1997, Contu 1998, Migliozzi & Camboni 1999, Mua & Sanna 2006), Spain (e.g., Tabarés 1996, Bañares & Beltrán 2009), Cyprus (under Pinus brutia; Loizides 2021), and Turkey (Oğuzhan Kaygusuz, pers. comm.). Its white hygrophoroid basidiomes (pileus convex and long decurrent subdistant lamellae), lamellae with pinkish cream to yellowish tones when mature and large basidiospores (on average over 7 μm in length) have a useful diagnostic value. Clitocybe hypotheja (1996) is probably a later synonym of H. nivea, at least based on the ITS rDNA sequence available from the holotype (Fig. 5). Clitocybe alni-glutinosae, described also from Sardinia (Italy) (Contu 1998), was thought to differ from H. nivea because of its small-sized basidiomes (pileus 10–30 mm diam, stipe 15–25 × 2–5 mm), faint odour, less spaced, narrower and less decurrent lamellae, slightly smaller basidia (30–37.5 × 6–7.5 μm), absence of pileocystidioid elements, and growth under Alnus glutinosa. The ITS rDNA sequence (UDB023989) available from the holotype of C. alni-glutinosae (IB19960896) suggests that this species is also conspecific with H. nivea (Fig. 5).
Hygrophorocybe carolinensis (H.E. Bigelow & Hesler) Vizzini, Consiglio & P. Alvarado, comb. nov. MycoBank MB 851148. Fig. 6M.
Basionym: Clitocybe carolinensis H.E. Bigelow & Hesler, J. Elisha Mitchell Sci. Soc. 76: 156. 1960.
Notes: The ITS rDNA sequence (NR_119886) obtained by Schoch et al. (2014) from the holotype of C. carolinensis (TENN:021888; North Carolina, Bigelow & Hesler 1960, Bigelow 1982) is very similar (97.47 %) to that of a recently collected specimen (UCSC:F-0690, USA: Santa Cruz County, CA, mixed evergreen forest dominated by Coast Redwood, leg. Christian Schwarz, Fig. 5) sequenced in the present work, which nests inside Hygrophorocybe based on multigene data (Figs 4, 5). While both collections could belong to distinct species, the results support the combination of C. carolinensis into Hygrophorocybe. Hygrophorocybe carolinensis, a species originally described from pine woods in USA (North Carolina), is characterized by a grey pileus surface, close and narrow lamellae, smaller basidiospores, 5–7 × 2.5–4 μm, and shorter basidia, 19–35 × 4–7 μm (Bigelow & Hesler 1960, Bigelow 1982).
Marasmiineae Aime et al., Biol. J. Linn. Soc. 117: 26. 2016.
Type: Marasmius Fr., Fl. Scan.: 339. 1836 [1835].
Representative families: Cyphellaceae, Cystostereaceae, Marasmiaceae, Mycenaceae, Omphalotaceae, Physalacriaceae, Porotheleaceae, and Xeromphalinaceae.
Notes: In the present phylogeny (Fig. 1), suborder Marasmiineae sensu lato (including Mycenaceae, Xeromphalina and Heimiomyces) received high support in the Bayesian analysis (1.0 PP). To ascertain the limits of the Marasmiaceae (not included in the present analysis) multigene data from additional genera other than Marasmius are necessary. Species of Marasmiineae are characterized by basidiomes mostly gymnocarpic, agaricoid (pileistipitate with central stipe), pleurotoid, rarely corticioid, gasteroid or cyphelloid, often gracile, slender in stature; hymenophore smooth, wrinkled, lamellate to rarely poroid; lamellae or tubes, when present, not free; hyphal system monomitic to sarcodimitic; basidiospores are colourless, usually smooth, without a germ pore, amyloid or not; basidia mostly 4-sporic, usually ballistosporic; cystidia often present; pileipellis very diverse, ranging from a cutis to a hymeniderm; clamp connections present or absent. Most species are litter saprobes, with some rare pathogenic species of economically important plants, e.g., Moniliophthora perniciosa, Paramarasmius palmivorus (Sena et al. 2014, Antonín et al. 2022). Some species of Collybiopsis (= Marasmiellus) are presumably lichenized (Singer 1970, 1973a, Kantvilas & May 1995, Kantvilas & Jarman 2006, Lepp 2011a, b, Oberwinkler 2012, Cooper & Leonard 2013, Lücking et al. 2017, Hubregtse 2019). Extensive research has shown that several Mycena species are essential for stimulating germination and the early stages of protocorm development in the myco-heterotrophic Gastrodia elata and other orchids (Park & Lee 2013, Liu et al. 2022). Harder et al. (2023) argued that Mycena species, usually considered saprotrophic fungi, can be opportunist-generalist plant root invaders. A few species of Marasmiineae are known to reproduce predominantly by conidia, e.g., blastic conidiogenesis in Baeospora spp. (Walther et al. 2005, Hutchison et al. 2012) and Hemimycena conidiogena (Moreau et al. 2005), as well as rhexolytic thallic conidiation in Flammulina, Marasmius puerariae, Mycena citricolor, Moniliophthora roreri, M. perniciosa (Delgado & Cook 1976, Ingold 1980, Petersen 1995, Petersen et al. 1999, Redhead et al. 2000a, Kirschner et al. 2013, Díaz-Valderrama & Aime 2016). Other taxa reproduce by means of vegetative rhizomorphs, e.g., Armillaria spp., Brunneocorticium spp., Crinipellis spp., Gymnopus spp., Marasmius spp., Rhizomarasmius spp. (Yafetto 2018). Rhizomorphs of Marasmiineae are not always restricted to a subterranean habit; in moist tropical rainforests, they are also found in the tree canopy or subcanopy as a dense tangle of black and brown wiry webs. These aerial rhizomorphs trap falling leaf litter for subsequent nutrient exploitation (Hedger 1990) and, interestingly, they are used by birds as construction material in nests (Aubrecht et al. 2013, César et al. 2018, 2020, Koch et al. 2018, 2020, Elliott et al. 2019, Bach et al. 2022).
The evolution of reduced astipitate and cyphelloid forms has occurred multiple times in Agaricales (Bodensteiner et al. 2004, Agerer 2018, Consiglio et al. 2021, Vizzini et al. 2022) and they are striking cases of parallel evolutionary reduction of complex fungal morphology. Most of them are present in Marasmiineae.
All the bioluminescent fungi known so far are Basidiomycota, with the remarkable exception of a Xylaria hypoxylon collection and undetermined species of Xylariaceae (Ascomycota, Sordariomycetes, Xylariales; Foerster et al. 1965, Seas-Carvajal & Avalos 2013). Within Basidiomycota, all the bioluminescent taxa (with the possible exception of the recently described cyphelloid genus Eoscyphella included in Cyphellopsidaceae by Silva-Filho et al. 2023, a family considered affiliated to Pleurotineae in the present analysis, Supplementary Fig. S2, see below) belong to suborder Marasmiineae, occurring in four major lineages: the Omphalotus lineage (Omphalotaceae), Armillaria lineage (Physalacriaceae), Lucentipes lineage (Porotheleaceae), and the mycenoid lineages (mostly Mycenaceae) (Desjardin et al. 2008, Oliveira et al. 2012, Chew et al. 2015, Kotlobay et al. 2018, Antonín et al. 2019, Cortés-Pérez et al. 2023). Ke et al. (2020) showed that bioluminescence in Marasmiineae evolved from a common ancestor 160 M years ago.
Xeromphalinaceae Vizzini, Consiglio & P. Alvarado, fam. nov. MycoBank MB 851150.
Diagnosis: It is characterized by basidiomes omphalinoid, marasmioid to collybioid, gymnocarpic, pileus usually brightly coloured, lamellae broadly adnate to decurrent, never purely white; stipe usually central, rarely eccentric, dry, with yellowish-brownish basal tomentum and rhizomorphs usually present. Taste mild to bitter. Spore deposit white. Basidiospores ellipsoid, broadly ellipsoid, oblong, cylindrical, or slightly allantoid, colourless, thin-walled, smooth, amyloid, acyanophilous; cheilocystidia always present; pleurocystidia absent or present; pileocystidia present, often of two types i) thin-walled and unbranched, ii) thin- to slightly thick-walled and often branched or coralloid (circumcystidia). Hyphae neither amyloid nor dextrinoid. Stipe context sarcodimitic. Clamp connections present. Saprotrophic, on conifers, less frequently also on broadleaved wood, sometimes in Sphagnum bogs or in forest litter. Known from temperate zones of both hemispheres and alpine zones of tropical regions.
Type: Xeromphalina Kühner & Maire, in Konrad & Maublanc, Icon. Select. Fung. 6: 236. (1934) [as ‘Xeromphalia’, orth. cons.]; see also Kühner & Maire, Bull. Trimestriel Soc. Mycol. France 50: 18. 1934.
Synonyms: Valentinia Velen., Novitates Mycologicae Novissimae: 38. 1939. (fide Kühner 1979b).
Omphalopsis Earle, Bull. New York Bot. Gard. 5: 425. 1909, [nom. illegit., non Omphalopsis Grev. 1863 (Algae)].
Representative genera: Heimiomyces (Fig. 6I) and Xeromphalina.
Notes: Xeromphalinaceae differs from its sister family Mycenaceae by an omphalinoid or collybioid to marasmioid habit, basidiomes with bright yellow-brown, rusty-yellow to rusty-brown tinges, a yellowishbrown tomentum at stipe base with radiating hairs, abundant encrusting epiparietal pigment on pileipellis elements, non-dextrinoid trama hyphae, sarcodimitic structure of stipe trama and common presence of highly structured armillaria-like rhizomorphs (Miller 1968, Klán 1984, Singer 1986, Redhead 1987, 1988, Rizzo et al. 1990, Watling & Turnbull 1998, Antonín & Noordeloos 2004, Aldrovandi et al. 2015). Xeromphalina, typified by X. campanella, was segregated from the heterogeneous Marasmius and Omphalia to accommodate omphaloid species with a stipe always (yellow) rusty-brown, hispidulo-tomentose at the base, a golden-coloured basal mycelium, and amyloid basidiospores (Kühner & Maire in Konrad & Maublanc 1934, Kühner & Maire 1934). Other differential characteristics highlighted by subsequent authors are the presence of rhizomorphs in many species, incrusting extracellular yellow-brown pigment in the pileipellis elements, abundant caulocystidia, cheilocystidia more or less fusoid, thin-walled, usually without prolongations, and clamp connections in all tissues (Miller OK 1968, Singer 1965, 1986, Horak 1979a, Klán 1984, Redhead 1988, Maas Geesteranus & Horak 1995, Moreno & Heykoop 1996, Watling & Turnbull 1998, Antonín 2000a, b, Antonín & Noordeloos 2004, Noordeloos 2008, 2012, Esteve-Raventós et al. 2010, Aldovrandi et al. 2015, Liu & Bau 2018). Xeromphalina species form exocarpic, apertopileate and amphiblemate basidiomes (Clémençon 2005). Mating systems are tetrapolar in all 12 species studied (Johnson & Petersen 1997). In spite of the few known taxa (about 32 species, Agerer 2018), Xeromphalina is a rather well-studied genus in Europe, where it has been the object of several monographic works at a local and continental scale (Klán 1984, Gulden 1992, Moreno & Heykoop 1996, Watling & Turnbull 1998, Bon 1999, Antonín 2000a, b, Ludwig 2001a, b, Antonín & Noordeloos 2004, Noordeloos 2008, 2012, Esteve-Raventós et al. 2010). Monographic studies on North American species have been made by Smith (1953), Miller (1968), Redhead (1988) and Aldovrandi et al. (2015), who dealt also with the northern Eurasian taxa. Additional works studied species of Xeromphalina in South America (Singer 1965, Redhead & Halling 1987), Papua New Guinea and New Caledonia (Maas Geesteranus & Horak 1995), and Asia (Horak 1979a, Liu & Bau 2018).
Outdated classifications based on morphological characters placed Xeromphalina in various families. Kühner (1980) included it in the tribe Marasmieae of the Marasmiaceae; Jülich (1981) in the Mycenaceae; Klán (1984) in the Tricholomataceae; Singer (1986) in the tribe Myceneae of the Tricholomataceae; and Redhead (1987, 1988) in the Xerulaceae. Antonín & Noordeloos (2004), Noordeloos (2008, 2012) and Agerer (2018) agreed to classify Xeromphalina close to mycenoid fungi, while Kühner’s point of view was followed by Bon (1999). The rDNA phylogeny in Moncalvo et al. (2002) contains a significantly distinct xeromphalinoid clade consisting of Xeromphalina and Heimiomyces, among the core of the white-spored euagarics. Xeromphalina campanella, type of the genus, was found to be nested inside the Hygrophoroid clade by Matheny et al. (2006), close to the gilled genera Sarcomyxa and Phyllotopsis, as well as the morphologically distinct members of the families Pterulaceae and Typhulaceae. Not much later, Garnica et al. (2007) found that Xeromphalina was sub-significantly related to a clade containing Lachnella villosa, Fistulina hepatica and Schizophyllum commune. In the phylogeny obtained by Binder et al. (2010), Xeromphalina appears (without support) near Mycena galericulata and M. plumbea, all of them considered members of a broad Hygrophoroid clade, and therefore it was considered an incertae sedis lineage at the base of the Hygrophoroid clade by Ovrebo et al. (2011) and Lodge et al. (2014). Finally, Olariaga et al. (2020) found that Xeromphalina was significantly related to a clade including Marasmiineae and Schizophyllineae, apparently representing an early diverging lineage of this group; it is considered as incertae sedis in Sánchez-García et al. (2020).
Singer (1942) segregated Heimiomyces (type H. rheicolor) from Xeromphalina, but later Smith (1953), Singer (1962, 1965, Singer 1986), Miller (1968), Redhead (1988) and Ramírez et al. (2013) considered it a subgenus or section of Xeromphalina. On the other hand, Horak (1968, 1979) recognized Heimiomyces as an independent genus, and his point of view was followed by Klán (1984), Maas Geesteranus & Horak (1995), Corner (1996) and Desjardin & Perry (2017). The independent status of Heimiomyces seems molecularly supported in Moncalvo et al. (2002), Esteve-Raventós et al. (2010) and Sánchez-García et al. (2020). It seems that species of Heimiomyces differ from those of Xeromphalina by their collybioid/marasmioid habit, viz. adnate to adnexed or broadly emarginate lamellae and not depressed pileus (papillate, umbonate), stipe entirely pruinose-velvety, a duplex pileus trama (the upper half gelatinized and the lower half of thick-walled glassy hyphae), and cheilocystidia with numerous rod-like projections. Heimiomyces species are common pantropical taxa (Singer 1965) that had also been recorded in North America (Smith 1953, Singer 1965, Miller 1968, Horak 1979a, Redhead 1988, Maas Geesteranus & Horak 1995, Corner 1996, Ramírez et al. 2013, Desjardin & Perry 2017).
Phyllotopsidineae Zhu L. Yang & G.S. Wang, Mycology, 2023 DOI: 10.1080/21501203.2023.2263031, hic emend.
Synonym: Sarcomyxineae Zhu L. Yang & G.S. Wang, Mycology, 2023 DOI: 10.1080/21501203.2023.2263031
Type: Phyllotopsis E.-J. Gilbert & Donk ex Singer 1936.
Emended circumscription of the family: Basidiomes are very variable, primarily clavarioid to pleurotoid/tricholomatoid (Pterulaceae, Sarcomyxaceae, Phylloporopsis, Pleurocybella, Tricholomopsis), polyporoid (Radulotubus), corticioid/resupinate (Radulomycetaceae partim, Stephanosporaceae partim), pustulose (Bulbillomyces), sequestrate-hypogeous (Stephanosporaceae partim), rarely agaricoid (Aphroditeola) or maybe cyphelloid (Cyphelloporia and Rectipilus, unverified), often tough textured; hyphal system often dimitic, hyphae colourless, non-amyloid; basidia clavate, 2–4 sporic; basidiospores colourless, inamyloid or amyloid, clamp connections often present. They show a diverse spectrum of trophic strategies ranging from saprotrophism (on ground or wood), parasitism (as plant pathogens, e.g., Pterulicium xylogenum, Acharya 2010), symbiotic lifestyle (ant mutualisms, Myrmecopterula velohortorum, M. nudihortorum, Dentinger et al. 2009, Leal-Dutra et al. 2020).
Representative families: Aphroditeolaceae, Phyllotopsidaceae (including Tricholomopsis), Pterulaceae, Radulomycetaceae, Sarcomyxaceae (including Tectella), and Stephanosporaceae.
Notes: The suborder Phyllotopsidineae is here emended to include also Sarcomyxaceae, in accordance with the phylogenetic results obtained. Six families are recognized within suborder Phyllotopsidineae: Aphroditeolaceae fam. nov., Phyllotopsidaceae, Pterulaceae, Radulomycetaceae, Sarcomyxaceae and Stephanosporaceae (Larsson 2007a, Lebel et al. 2015, Liu et al. 2016, Zhao C-L et al. 2016, Leal-Dutra et al. 2020, Karasiński et al. 2023). Basidiome shape is very diverse and there are no obvious synapomorphic traits shared by all Phyllotopsidineae. The group is mostly delimited on a molecular basis, roughly corresponding to the ‘lower’ Hygrophoroid clade identified by Lodge et al. (2014), and most lineages of the Pleurotineae sensu Olariaga et al. (2020) excepting Pleurotus and Typhulaceae. Wang et al. (2023b) separated Phyllotopsidineae from Pleurotineae after their phylogenomic study showed that both clades were not related, a result found also in the present work with a multigene phylogeny including an extended dataset with important lineages of Phyllotopsidineae and Pleurotineae not present in the previous works. Pterulaceae and Radulomycetaceae are recently split sister families (Leal-Dutra et al. 2020) constituted by a complex of taxa with clavarioid, corticioid or polyporoid basidiome types. All species of Pterulaceae and Radulomycetaceae show a distinct phenolic or naphthalene odour when fresh, as described for Pterula multifida (Corner 1950, 1970). It was suggested by Olariaga et al. (2020) that such an odour, produced by an unidentified volatile metabolite, may be a synapomorphic character of these two families. In the present work, the family Aphroditeolaceae is introduced, and the family Sarcomyxaceae (suborder Sarcomyxineae in Wang et al. 2023b) is significantly linked to Phyllotopsidineae for the first time. The addition of sequences of Aphroditeola and Tectella (and other lineages in the remaining suborders) might be the cause of the different result, suggesting that gaps in the diversity analyzed could affect the phylogenetic (and phylogenomic) results. Karasiński et al. (2023), recovered a significant relationship between Phyllotopsidaceae and a clade formed by the cyphelloid genera Cyphelloporia and Rectipilus, but the correct classification of these genera needs to be further explored analyzing multigene data in the context of the entire suborder Phyllotopsidineae. A new family name might be necessary to accommodate them.
Sarcomyxaceae Olariaga et al., Stud. Mycol. 96: 177. 2020.
Synonym: Tectellaceae Locq., Mycologie générale et structurale: 109. 1984, nom. inval., Art. 36.1 (Shenzhen).
Type: Sarcomyxa P. Karst., Meddn Soc. Fauna Flora fenn. 18: 62. 1891.
Representative genera: Sarcomyxa and Tectella (Fig. 8F).
Notes: The family is here characterized by basidiomes pleurotoid, with a gelatinous layer in the pileus, lamellae slightly decurrent, crowded, usually forked. Stipe lateral to reduced. Partial veil present or absent. Spore deposit white. Basidiospores cylindrical to allantoid, amyloid. Basidia (2–)4-sporic, clamped. Cheilo- and pleurocystidia fusiform to clavate, more or less thick-walled. Thick-walled hyphae present in almost all tissues of basidiomes. Pileipellis and part of trama gelatinised. Clamp connections present. Saprotrophic, lignicolous. The family was originally established by Olariaga et al. (2020), who classified it within Pleurotineae, to accommodate Sarcomyxa, a genus containing only two cryptic species (S. serotina and S. edulis, once considered to belong to the genus Panellus) with pleurotoid basidiome, gelatinised pileipellis, fusiform to clavate cheilo- and pleurocystidia, thick-walled hyphae in the context, and amyloid basidiospores (Horak 1968, Jin et al. 2001, Dai et al. 2003, Knudsen & Vesterholt 2012, Kunze et al. 2012, Saito et al. 2014, Læssøe & Petersen 2019, Olariaga et al. 2020, Tian et al. 2021, Cai et al. 2023). Two new species, S. baishanzuensis and S. ochracea, have been recently described from China (Cai et al. 2023).
The present analysis (Fig. 1) suggests that the genus Tectella (represented by ITS, LSU, SSU, RPB2 and TEF1 sequences obtained from a Northamerican collection of T. patellaris) is part of the family Sarcomyxaceae. Tectella, typified by T. operculata (= T. patellaris) was considered an incertae sedis genus based on rDNA alone (Aime 2001, Jin et al. 2001, Moncalvo et al. 2002, Cifuentes et al. 2003). Bodensteiner et al. (2004) found that T. patellaris is subsignificantly related to Panellus serotinus (51 BP) within Agaricales (Euagarics), but not close to the type of the morphologically similar genus Panellus, P. stipticus, which is nested within Mycenaceae (Marasmiineae) (Moncalvo et al. 2002, Bodensteiner et al. 2004, Binder et al. 2005, Saito et al. 2014, Ke et al. 2020, Tian et al. 2021, Zhang & Dai 2021, Zhang et al. 2022, present work Fig. 1). Matheny et al. (2006) found that Sarcomyxa, Xeromphalina, Pterulaceae (Pterula and Phyllotopsis) and Typhulaceae formed a significantly monophyletic group nested inside the Hygrophoroid clade, but this result could be due to an incomplete dataset lacking representative lineages and/or sufficient DNA data from them.
Tectella patellaris (Fig. 8F) mainly differs from Sarcomyxa because of its pseudostipitate basidiome which displays a partial veil at least in young stages (e.g., Earle 1909, Pilát 1935, Horak 1968, Miller 1970, Candoussau et al. 1974, Perrin 1979, Reijnders 1983, Singer 1986, Cavet & Moreau 1994, Cucchi 1997, Elborne & Læssøe 2008, 2012, Schmitt & Heseler 2009a, b, Seok et al. 2011, Trnkoczy 2011, Jančovičová et al. 2012, Læssøe & Petersen 2019). Tectella patellaris occurs in Europe (Elborne & Læssøe 2012), Asia (Seok et al. 2011) and North America (Miller 1970). The species was classified into various genera such as Panellus (Subgen. Mitellus, Burdsall & Miller 1975), Panus (where it was originally described, Fries 1838, Kühner 1980), Pleurotus and Pocillaria (Pilát 1935, Kirk 2012), Velopanus (Singer 1936b, nom. prov.), as well as in the families Favolaschiaceae (Elborne & Læssøe 2008, 2012), Mycenaceae (Elborne & Læssøe 2012), Pleurotaceae (e.g., Kühner 1980, Roux 1997, 2006) or Tricholomataceae sensu lato (e.g., Moser 1978, Singer 1986, Hansen & Knudsen 1992). The status of other species of Tectella proposed on the basis of morphology, such as T. luteohinnulea (Stevenson 1964) from New Zealand and T. phellodendri (Singer 1942b) from Asia (Khabarovsk, Russian Far East), needs to be checked with molecular tools.
Phyllotopsidaceae Locquin ex Olariaga et al., Stud. Mycol. 96: 175. 2020.
Type: Phyllotopsis E.-J. Gilbert & Donk ex Singer, Beih. Bot. Centralbl., Abt. 2 56: 143. 1936.
Representative genera: Conoloma, Macrotyphula, Phyllotopsis, Pleurocybella, Tricholomopsis and maybe also Bulbillomyces, Cyphelloporia and Rectipilus.
Notes: The family is characterized here by basidiomes pleurotoid, tricholomatoid, clavarioid (typhuloid) and sometimes arising from a sclerotium, corticioid, or cyphelloid (Cyphelloporia, Rectipilus, unverified). Spore deposit white to salmon pink, pale ochre. Hyphal system monomitic. Basidiospores colourless, cylindrical, allantoid or subglobose, smooth, without iodine reactions. Cheilocystidia sometimes present in pleurotoid genera. Clamp connections present, rarely absent. Saprotrophic, usually lignicolous. Macrotyphula, Phyllotopsis and Pleurocybella were first found to be closely related by Dentinger & McLaughlin (2006), and later Olariaga et al. (2020) established the new family Phyllotopsidaceae for this monophyletic group. Wang et al. (2023b) added a new monospecific genus, Conoloma, which differs from the allied Tricholomopsis mainly by its pileus with a mucronate umbo, a fibrillose annuliform zone on the stipe apex, and smaller cheilocystidia. No obvious synapomorphic characters could be identified between the typhuloid Macrotyphula and the pleurotoid Phyllotopsis and Pleurocybella (Moncalvo et al. 2002). All three genera contain saprotrophic species, mostly lignicolous, and possess clamp connections (Singer 1986, Watling & Gregory 1989, Knudsen 2008a, 2012a, Vesterholt 2008b, 2012b, Knudsen & Shiryaev 2012). A corticioid sample identified as Bulbillomyces farinosus seems related to Macrotyphula in some works (Karasiński et al. 2023), but another candidate lineage for this name exists in the Polyporales (Larsson 2007b, Justo et al. 2017). The classification of the monospecific genus Bulbillomyces needs to be confirmed by selecting an epitype, and so its position inside Phyllotopsidaceae is by now doubtful.
The present analysis (Fig. 1) also supports that the genus Tricholomopsis could be part of the family Phyllotopsidaceae, sister to a clade formed by the gilled genera Pleurocybella and Phyllotopsis, as previously suggested by other works, some of them including the type species T. rutilans (Garnica et al. 2007, Binder et al. 2010, Lodge et al. 2014, Sánchez-García et al. 2020). In the molecular phylogeny produced by Moncalvo et al. (2002), Tricholomopsis, represented by the LSU nrDNA sequences of T. rutilans and Collybia aurea (later combined in Tricholomopsis by Desjardin & Perry 2017), was placed in one phylogenetic clade together with Clavaria fusiformis and Marasmius rhyssophyllus. In a multigene analysis (RPB1, RPB2, ncRNA) another representative species, T. decora, nested inside the Pluteoid clade, outside of any conventional families, close to Amanitaceae (Matheny et al. 2006). In Garnica et al. (2007), T. rutilans formed a clade with Macrotyphula fistulosa, Pleurocybella porrigens, Phyllotopsis nidulans, sister to Hygrophorus chrysodon, Lentaria albovinacea and Sarcomyxa serotina. In Binder et al. (2010) T. decora is placed in the Hygrophoroid clade (minus Pterulaceae) together with Phyllotopsis sp., Pleurocybella porrigens, Macrotyphula fistulosa, Typhula phacorrhiza, and Sarcomyxa serotina). He et al. (2019) considered Tricholomopsis an incertae sedis genus inside Agaricales. Wang et al. (2023b) found also that Tricholomopsis is nested inside Phyllotopsidaceae after analyzing genome data of multiple species in this genus.
The genus Tricholomopsis includes lignicolous species that cause white rot of conifers (Singer 1986, Smith, 1960, Murphy & Mitchell 2001, Vauras 2009, Razaq et al. 2012, Wang et al. 2023b), as well as very few species reported to be bambusicolus, Pteridiumassociated or terrestrial (Dennis 1951, Hongo 1959, 1960, Olariaga et al. 2015). Species of Tricholomopsis have tricholomatoid, brightly colored (mostly yellow) basidiomes, a tomentose or finely fibrillosesquamulose pileus (but basidiome collybioid, pileus smooth, hygrophanous and shallowly depressed or umbilicate in age in the pantropical T. aurea, Desjardin & Perry 2017), adnate-sinuate lamellae, whitish spore deposit, smooth, inamyloid and broadly ellipsoid basidiospores, large cheilocystidia, absent or sparse pleurocystidia, a trichoderm to cutis type pileipellis, and clamp connections (Smith 1960, Bon 1984, Singer 1986, Boekhout & Noordeloos 1999, Vesterholt 2008c, Holec 2009, 2012a, 2012b, Vauras 2009, Holec & Kolařík 2011, 2012, Vauras et al. 2012, Cooper & Park 2016, Olariaga et al. 2015, Agerer 2018, Holec et al. 2019, Hosen et al. 2020, Mao et al. 2021, Jayawardena et al. 2022, Wang et al. 2023b). The genus was traditionally considered a member of the Tricholomataceae (e.g., Kühner 1980, Bon 1984, 1991, Singer 1986, Boekhout & Noordeloos 1999, Holec 2012a, Agerer 2018). Phyllotopsis nidulans, type of Phyllotopsis, shows the same yellow lamellae (due to carotenoids in Phyllotopsis, Fiasson 1969, Arpin & Fiasson 1971, Kost 1986, but yet undetermined in Tricholomopsis, Gill & Steglich 1987), squamulose-hirsute pileus and growth on dead wood, as most Tricholomopsis species, but its basidiomes are conchate, shell to kidney-shaped and sessile, its basidiospores are reniform, allantoid, cheilocystidia absent or acicular-filiform and spore deposit yellowish-pink to pale ochre (Pilát 1935, Domański 1969, Singer 1986, Hrouda 2001, Knudsen 2008a, 2012a, Agerer 2018).
Aphroditeolaceae Vizzini, Consiglio & P. Alvarado, fam. nov. MycoBank MB 851151.
Diagnosis: Basidiomes pileostipitate, pileus depressed to infundibuliform, hymenophore folded with folds dichotomously forked, cystidia absent, basidiospores colourless, faintly amyloid and indextrinoid, clamp connections present, terricolous, in forests.
Type: Aphroditeola Redhead & Manfr. Binder, Index Fungorum 15: 1. 2013.
Representative genus: Aphroditeola.
Notes: The genus Aphroditeola was established in a brief note in Index Fungorum (Redhead 2013) to accommodate Cantharellus olidus. This species is traditionally characterized by small and pink cantharelloid to omphalinoid basidiomes with fruity-sweetish to floral fragrant odour (described as candy-like, Hebeloma sacchariolenslike, cinnamony or pink bubble gum-like), dichotomously forked hymenophoral folds, smooth, colourless inamyloid and indextrinoid basidiospores, absence of hymenial cystidia, presence of clamp connections, in vitro pinkish to reddish-orange mycelium, and growing on coniferous litter (Smith 1944, Petersen 1976, Fries 1979, Kuyper 1995a, Knudsen & Taylor 2008, 2012 as Hygrophoropsis, Redhead 2013). Basidiospores are reported as non-amyloid by nearly all authors except Petersen (1976) who found a faint amyloidity in the holotype. All the collections examined in the present work showed weakly amyloid basidiospores (see below). Recently, Aphroditeola was found to be associated to the white females of the cereal cyst nematode (CCN, Heterodera avenae) in China (Hu et al. 2020). Traditionally, Cantharellus olidus was classified in Hygrophoropsis (Métrod 1949, Kuyper 1995a, Knudsen & Taylor 2008, 2012, Kibby 2012) as part of the family Hygrophoropsidaceae, a lineage shown to be nested within the order Boletales (Moncalvo et al. 2002, Binder & Hibbett 2006). However, Hygrophoropsis produces saprotrophic lignicolous and brown-rotting basidiomes with strongly dextrinoid spores (e.g., Kuyper 1995a, Gminder 2001, Watling & Hills 2005, Knudsen & Taylor 2008, 2012, Kibby 2012, Sesli 2014). Bigelow (in Bigelow & Barr 1962) examined type material of Cantharellus morganii, concluding that it was conspecific with C. olidus and C. rosellus. Phylogenetically, Aphroditeola was previously thought to be close to the family Hygrophoraceae, inside the Hygrophoroid clade (= Hygrophorineae sensu Dentinger et al. 2016) (Lodge et al. 2014, Lavorato et al. 2015, He & Yang 2021). However, Sánchez-García et al. (2017) found that Aphroditeola is related to Stephanosporaceae, Radulomycetaceae and Pterulaceae. According to the present analysis (Fig. 1) Aphroditeola represents an independent evolutionary line inside suborder Phyllotopsidineae, sister to a clade formed by Stephanosporaceae, Radulomycetaceae and Pterulaceae. This clade is, in turn, sister to Phyllotopsidaceae.
Aphroditeola olida (Quél.) Redhead & Manfr. Binder, Index Fungorum 15: 1 (2013). Figs 6A, 10.
Fig. 10.
Aphroditeola olida. A. Basidiomes (TRgmb00561). B, C. Pileipellis (B. TRgmb00561; C. TRgmb00556). D. Subpellis (pileitrama) (TRgmb00561). E, F. Hymenophoral trama (E. TRgmb00561; F. TRgmb00556). G, H. Hymenium (G. TRgmb00556; H. TRgmb00561). I. Subhymenium (TRgmb00561). J–N. Basidiospores (J–M. TRgmb00561; N. TRgmb00556). O. Caulocystidia (TRgmb00561). Mounting media were Melzer’s reagent (K, L, N), Congo Red in ammonia (B–D, F, G, I, O), and Cotton Blue (E, H, M). Scale bars: B–O = 10 μm. Photographs A by M. Floriani, B–O by M. Marchetti.
Basionym: Cantharellus olidus Quél., in Cooke & Quélet, Clavis syn. Hymen. Europ. (London): 148. 1878.
Synonyms: Merulius olidus (Quél.) Kuntze, Revis. gen. pl. (Leipzig) 2: 862. 1891.
Clitocybe olida (Quél.) Konrad, Bull. Trimestriel Soc. Mycol. France 45: 60. 1929.
Hygrophoropsis olida (Quél.) Métrod, Schweiz. Z. Pilzk. 14(3): 15. 1949.
Cantharellus morganii Peck [as ‘morgani’], Bot. Gaz. 7(4): 43. 1882.
Merulius morganii (Peck) Kuntze, Revis. gen. pl. (Leipzig) 2: 862. 1891.
Clitocybe morganii (Peck) H.E. Bigelow, Rhodora 64: 129. 1962.
Hygrophoropsis morganii (Peck) H.E. Bigelow, Beih. Nova Hedwigia 51: 66. 1975.
Cantharellus rosellus Peck, Rep. (Annual) New York State Mus. Nat. Hist. 42: 120. 1889.
Merulius rosellus (Peck) Kuntze, Revis. gen. pl. (Leipzig) 3(3): 494. 1898.
Description: Pileus 10–40 mm, convex when young, then plane with a depressed centre, finally funnel-shaped, margin typically incurved, irregularly undulating, lobate to subcrenulate, not hygrophanous, not translucently striate, surface smooth to finely tomentose, fleshpinkish (7A5-6) to ochre-orange (5A7-8, 6A7-8) or pale buff (7A7-8) (like Hydnum rufescens), paler at margin. Lamellae crowded, L = 35–50, l = 1–2, very narrow (thin), long-decurrent, repeatedly forked, intervenose in age, sometimes foldlike, whitish to pale pinkish buff (7A3-4), with an obtuse, entire, concolorous edge. Stipe 15–35 × 2–5 mm, slightly eccentric, solid, somewhat broadened at apex (up to 8 mm), conic, attenuated towards the base, very finely pruinose-fibrillose then polished, concolorous with the pileus or paler. Context thin, whitish to very pale pinkish buff (7A2-3) at stipe base. Odour evident, strong, fragrant, sweetish, of cinnamom candy, as in Entoloma ameides, Hebeloma sacchariolens; taste sharp but soon mild, hard to distinguish because of the odour. Spore deposit whitish. Basidiospores (3.34–)3.53–3.78–4.03(−4.37) × (2.62–)2.75–2.93–3.11(−3.55) µm [90/2/1], Q = (1.11–)1.19–1.29–1.40(−1.57), V= (12–)14.56–17.07–19.58(−26.5) µm3, ellipsoid to broadly ellipsoid, thin-walled, smooth, colourless, often mono- to multiguttulate, apiculus prominent, up to 1 µm long, abrupt, wall cyanophilous, weakly amyloid, non- to very weakly dextrinoid. Basidia (18–)20–35 × 5–7(−7.2) µm, clavate, 4-sporic, rarely 2-sporic, sterigmata up to 4 µm long, minutely guttulate, often with basal clamp connection. Subhymenium thickening, formed by short, intertwined, 2–4 µm wide elements. Hymenial cystidia absent. Hymenophoral trama irregular of subcylindrical, tightly interwoven hyphae, 3–8 µm wide, thin- to moderately thick-walled (and then wall up to 1(−1.5) µm thick), colourless to pale yellowish (pigment parietal and intracellular), dextrinoid. Pileipellis an ixocutis of repent, subparallel to subintricate hyphae with several single or agglutinated ascending elements, cylindrical to claviform, 5–8(−10) µm wide, with pale yellowish intracellular pigment. Subpellis (pileitrama) formed by cylindrical to claviform 4–8(−10) µm wide, colourless or pale yellowish hyphae. Stipitipellis made up of loose, sub-intertwined, 4–8 µm wide cylindrical colourless hyphae, with abundant clamp connections, with reclining or emerging terminal elements together with up to 60–80 µm long cystidioid hyphae aggregating dense deposits of basidiospores; present at the base of the cystidioid elements also subglobose to claviform cells. Stipititrama regular to subirregular made up of cylindrical, 3–8(−12) µm wide hyphae, sometimes enlarged at the septa, colourless to slightly yellow, with wall up to 1 µm thick. Thromboplerous hyphae not observed. Clamp connections present in all tissues.
Habitat and distribution: Scattered or gregarious, in soil or needle litter, usually under coniferous trees. Europe, Asia and North America.
Materials examined: Canada, Quebec, MRC Lac-Saint-Jean-Est, Alma, on fir’s litter, coniferous forest, 11 Sep. 2012, R. Lebeuf, HRL1230. Italy, Trentino-Alto Adige, Loc. Brusoladi (Valfloriana, TN), 1 160 m asl, Lat.: 46,2512° N - Long.: 11,3609° E, mixed coniferous forest with a prevalence of Picea abies and Pinus sylvestris, 7 Aug. 2011, M. Floriani & L. Eccher (TRgmb00561); Loc. Castelìr (Predazzo, TN), 1 550 m asl, Lat.: 46,31428° N - Long.: 11,69167° E, subalpine coniferous forest with a prevalence of Picea abies, 24 Aug. 2004, M. Floriani, M. Donini et al. (TR gmb00556).
Notes: According to the present results (and additional unpublished data), genus Aphroditeola is probably composed of at least three distinct species with a phylogenetic similarity between them ranging from 94 % to 96.5 % in ITS rDNA (and an intraspecific variability between 0 % and 1.5 %). Two of these lineages are present in North America and Europe, while the only known sample of the third one comes from Europe. No diagnostic traits have been identified to discriminate these clades from one another, so by now they are considered cryptic species. Whether the holotypes of Cantharellus olidus, C. morganii and C. rosellus belong in the same or different clades should be further investigated to take the appropriate taxonomic decisions at species level.
Pleurotineae Aime et al., Biol. J. Linn. Soc. 117(1): 26. 2016, hic emend.
Synonyms: Schizophyllineae Aime et al., Biol. J. Linn. Soc. 117(1): 26. 2016.
Schizophyllales Nuss., Hoppea 39: 179. 1980.
Type: Pleurotus (Fr.) P. Kumm., Führ. Pilzk. (Zerbst): 24. 1871.
Emended circumscription of the family: Basidiomes pileate, conchate, spathulate, tongue-shaped, resupinate to cyphelloid/cupulate; dry or viscid; pileus convex to plane-depressed, urniform to reniform, bell-shaped, cupulate solitary or forming a pore-like compound structure on a subiculum or disc; stipe present (lateral to eccentric, rarely central), rudimentary or absent; hymenophore lamellate, smooth, tubular (tubes separate but closely packed or coherent), merulioid, or folded (pseudolamellate), lamellae usually decurrent or attenuating towards the centre; veils usually absent, rarely present; spore deposit white to cream, pale ochre; hymenophoral trama irregular to subregular, immersed or not in a gelatinous matrix; hyphal system monomitic or dimitic, hyphae colourless, inamyloid, non-dextrinoid, with or without clamp connections; acantophyses sometimes present (Porodisculus, Pseudofistulina, Resupinatus); context gelatinous or with a distinct gelatinous layer or non-gelatinous; basidiospores colourless, or occasionally yellow-brown, smooth, thin-walled, inamyloid, non-dextrinoid, acyanophilous; basidia clavate or subclavate, 4- or 2-sporic holobasidia; hymenial cystidia and pileocystidia absent or present; pileipellis a cutis or a trichoderm of smooth or irregularly ramified and with gnarled outgrowing hyphae; asexual morph phase usually present in Hohenbuehelia (Nematoctonus), Fistulina (Confistulina), rarely in Pleurotus (Antromycopsis in Pleurotus subgenus Coremiopleurotus). Lignicolous, herbicolous, saprotrophic, or parasitic on angiosperms and gymnosperms, producing a white rot or showing an intermediate behaviour between white-rot and brown-rot fungi (Fistulinaceae and Schizophyllaceae); nematode trapping via toxin droplets (Pleurotus) or adhesive knobs (Hohenbuehelia).
Representative families: Fistulinaceae, Pleurotaceae, Resupinataceae, Schizophyllaceae, and presumably Cyphellopsidaceae Jülich.
Notes: The first phylogenomic study focused on Agaricales (Dentinger et al. 2016) found a close relationship between Pleurotus ostreatus (Pleurotaceae) and Pterula multifida (Pterulaceae), coining the suborder Pleurotineae for them. Pleurotus appeared related to Pterula too in the phylogenomic analysis by Varga et al. (2019). It appeared as an incertae sedis lineage basal to the rest of Agaricales in the phylogenomic study by Ke et al. (2020), but Pterula was absent from this analysis. In the multigene analysis by Olariaga et al. (2020), Pleurotineae sensu Dentinger et al. (2016) was emended to include also Phyllotopsidaceae, Sarcomyxaceae, Radulomycetaceae, Stephanosporaceae and Typhulaceae. In the phylogenomic work by Li et al. (2021), Pleurotus and Pterula are again close to each other, but their monophyletic origin lacks significant support. In contrast with the previous phylogenetic and phylogenomic works, Wang et al. (2023b) found that Phyllotopsidineae and Pleurotineae were not directly related after analyzing more information (555 genes) from a more diverse dataset of Agaricales. The present analysis (Fig. 2), based on multigene sequence data from several species of Pleurotus, Hohenbuehelia and Resupinatus, confirms this result, but it also suggests a monophyletic origin of Pleurotineae and Schizophyllineae (Auriculariopsis, Fistulina, Porodisculus, Schizophyllum). Wang et al. (2023b) did not find a direct relation between Pleurotineae and Schizophyllineae, but this could be due to the lack of important lineages in their analysis (i.e., basal species of Pleurotus, Hohenbuehelia, Resupinatus).
Fig. 2.
Bayesian inference phylogram built with nucleotide sequence data of six loci (5.8S, LSU, SSU, RPB1-exons, RPB2-exons and TEF1-exons) of the main lineages inside order Agaricales (focused on suborders Agaricineae, Pleurotineae and Pluteineae), rooted with Suillus pictus (Boletales), Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main suborders are shown in color boxes, while family names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study. The dashed branch was shortened for graphic presentation.
The family Cyphellopsidaceae Jülich 1982 [= Digitatisporaceae Jülich 1982, nom. inval., Art. 36.1; Niaceae Jülich 1982, nom. inval., Art. 36.1; Lachnellaceae Boud. 1907, as ‘Lachnellacées’, nom. inval., Art. 32.1(c), see Art. 18.4 (Shenzhen)] encompasses the genera Calathella, Dendrothele, Digitatispora, Eoscyphella, Flagelloscypha, Halocyphina, Lachnella, Merismodes (including also Cyphellopsis and Maireina, Silva-Filho et al. 2023), Nia, Peyronelina, and Woldmaria (Binder et al. 2001, 2066, Bodensteiner et. 2004, Matheny et al. 2006, Yamaguchi et al. 2009, Henkel et al. 2010, Læssøe et al. 2016, Azevedo et al. 2018, Abdel-Wahab et al. 2019, Silva-Filho et al. 2023). It seemed related to Schizophyllaceae (but often lacking statistical support) in Binder et al. (2001, 2005, 2010), Matheny et al. (2006, significant support, inside the Marasmioid clade), Garnica et al. (2007, subsignificant support), Yamaguchi et al. (2009), and Henkel et al. (2010). However, in other works, i.e., Bodensteiner et al. (2004) and Olariaga et al. (2020), this clade seems to be far from the Schizophylloid clade (/Schizophyllineae). Wang et al. (2023b) found a significant relation between Cyphellopsidaceae (as Niaceae) and Schizophyllaceae and Fistulinaceae (as Schizophyllineae) with a phylogenomic approach, although they only included a single species of this family (Flagelloscypha sp.). Additional analyses conducted in the present work (Dataset 5: 10.6084/m9.figshare.24999368) seem to confirm that the family Cyphellopsidaceae is closely related to Schizophyllaceae and Fistulinaceae inside suborder Pleurotineae (Supplementary Fig. S2), but the resulting phylogeny lost support for other major nodes, maybe because the species analyzed do not represent properly the biodiversity in these lineages and/or the DNA markers employed contain incomplete or insufficient information. The clade of Henningsomyces sensu stricto (Bodensteiner 2004) has been recently found to be apparently related to the family Cyphellopsidaceae too (Karasiński et al. 2023), but its status needs to be confirmed analyzing multigene or genomic data. Regardless of its actual classification, this clade will probably need its own family name to reflect the great genetic distance from the other families of Agaricales.
Pleurotaceae Kühner, Bull. Mens. Soc. Linn. Lyon 49: 784. 1980.
Type: Pleurotus (Fr.) P. Kumm., Führ. Pilzk. (Zerbst): 24. 1871.
Representative genera: Hohenbuehelia and Pleurotus (Fig. 7L, M).
Fig. 7.
Basidiomes of taxa within Agaricales sequenced in the present work. A. Lepista ricekii (AMB:18864). B. Leucocortinarius bulbiger (AMB:19593). C. Lyophyllum turcicum (GB:0065321). D. Macrocystidia cucumis (JX.1294733#45). E. Macrocystidia sp. (Kekki3956). F. Melanoleuca friesii (AMB:18865). G. Melanoleuca tristis (AMB:18866). H. Notholepista fistulosa (HMJU:288). I. Omphalina pyxidata (AMB:19295). J. Paralepista flaccida (TO:AV20140410). K. Paralepistopsis amoenolens (AMB:18867). L. Pleurotus dryinus (AMB:18868). M. Pleurotus fuscosquamulosus (A. Baglivo 13-07-2014). N. Pluteus romellii (AMB:18871). O. Pluteus variabilicolor (AMB:18872). Photographs A, B, F, G, I, K, L, N, O by G. Consiglio, C by L. Stridvall, D by T. Vuorinen, E by Tapio Kekki, H by J. Xu, J by A. Vizzini, M by A. Baglivo.
Notes: The family Pleurotaceae is characterized by its basidiomes pileate, conchate, spathulate; dry or viscid; pileus convex to plane-depressed, urniform to reniform; stipe present (lateral to eccentric, rarely central), rudimentary or absent; hymenophore lamellate, lamellae usually decurrent or attenuating towards the centre; veils absent, rarely present; spore deposit white to cream; hymenophoral trama irregular to subregular, immersed or not in a gelatinous matrix; hyphal system monomitic or dimitic, hyphae colourless, inamyloid, non-dextrinoid, with clamp connections; context gelatinous or with a distinct gelatinous layer or non-gelatinous; basidiospores colourless, smooth, thin-walled, inamyloid, non-dextrinoid, acyanophilous; basidia clavate or subclavate, 2- or 4-sporic holobasidia; hymenial cystidia and pileocystidia absent or present; pileipellis a cutis or a trichoderm of smooth hyphae; asexual morph phase usually present in Hohenbuehelia (Nematoctonus), rarely in Pleurotus (Antromycopsis in Pleurotus subgenus Coremiopleurotus). Lignicolous, herbicolous, saprotrophic, or parasitic on angiosperms and gymnosperms, producing a white rot; nematode trapping via toxin droplets (Pleurotus) or adhesive knobs (Hohenbuehelia). The family was established by Kühner (1980) with very broad limits, for all subcoriaceous, white-spored taxa with a pleurotoid habit (viz. Pleurotus, Lentinus, Panus, Phyllotopsis, Sarcomyxa). However, Singer (1986) did not recognize Pleurotaceae and placed Pleurotus in tribe Lentineae of Polyporaceae and Hohenbuehelia within tribe Resupinateae of Tricholomataceae. Pleurotaceae (including Pleurotus and Hohenbuehelia) was early recognized as a monophyletic lineage by Moncalvo et al. (2000, 2002) and Thorn et al. (2000, 2005) based on rDNA data, a conclusion confirmed later by multigene analyses (Binder et al. 2005, Matheny et al. 2006, McDonald 2015, Petersen et al. 2015, Varga et al. 2019, Olariaga et al. 2020, Sánchez-García et al. 2020). Members of Hohenbuehelia possess a thick gelatinous zone, metuloid cystidia, a nematoctonus-like asexual morph, and capture nematodes predominantly by means of adhesive knobs (Thorn & Barron 1986, Thorn et al. 2000, Consiglio & Setti 2018, Consiglio et al. 2018). Species of Pleurotus lack a gelatinous zone (although some species have a gelatinized pileipellis), metuloid cystidia and a nematoctonus-like asexual morph, and they capture nematodes by non-adhesive droplets containing the biotoxic volatile ketone, 3-octanone, named toxocysts by Clémençon (2004) (Hilber 1982, Barron & Thorn 1987, Thorn et al. 2000, Satou et al. 2008, Marlin et al. 2019, Lee et al. 2023). Nematophagous fungi can be found also in suborder Agaricineae, i.e., Crepidotus (Crepidotaceae, Senn-Irlet 1994, Senn-Irlet & Scheidegger 1994), Stropharia (Strophariaceae, Luo et al. 2006), and Coprinus comatus (Agaricaceae, Luo et al. 2004, 2007). Unlike Pleurotus, the Conocybe lactea mycelium does not locate and colonize immobilized nematodes and (or) consume them as a nutrient source. Toxin droplets exuded by its hyphae probably act only as antifeedant compounds, by repelling or killing fungus-feeding nematodes (Hutchison et al. 1996, Hallen et al. 2003).
Resupinataceae Jülich, Biblioth. Mycol. 85: 388. 1982. [1981].
Synonym: Tricholomataceae tribe Resupinateae Singer, Sydowia 2: 30. 1948.
Type: Resupinatus Nees ex Gray, Nat. Arr. Brit. Pl. (London) 1: 617. 1821. (Fig. 8B).
Synonyms: Phyllotus P. Karst., Bidr. Känn. Finl. Nat. Folk 32: XIV. 1879.
Stigmatolemma Kalchbr., Grevillea 10(no. 55): 104. 1882.
Asterotus Singer, Mycologia 35: 161. 1943.
Rhodocyphella W.B. Cooke, Beih. Sydowia 4: 105. 1961.
Lignomyces R.H. Petersen & Zmitr., Mycologia 107: 1046. 2015.
Notes: Traditionally, the genus Resupinatus, typified with R. applicatus (Fig. 8B), has always been considered closely related to Hohenbuehelia (Singer 1948, 1975, 1986, Kühner 1980) from which it differs because it lacks metuloid cystidia (except R. niger), the Nematoctonus asexual morph, nematophagy, the presence of ramified hyphae in the pileipellis (Rameales type), which in some species is repeatedly dichotomously branched (asterostromelloid), and diverticulate coralloid cheilocystidia (Singer 1986, Consiglio & Setti 2017, 2018). In addition, Resupinatus has been shown to possess a peculiar hymenophore development: while in most other species of the Agaricales lamellae production is usually completed within the mushroom primordium, and lamellae only become larger (either wider, longer, or both) during development, in Resupinatus they increase in number during the maturation of the basidiome (Reijnders 1948, 1963, Moore 1987). The similarities between the two genera were so striking as to induce Kühner (1980) to consider Hohenbuehelia a subgenus of Resupinatus. Singer (1948) established the tribe Resupinateae of Tricholomataceae to include Resupinatus and Hohenbuehelia because they share a gelatinous layer in their pileus context and colourless inamyloid spores. Later, Singer (1962) included in the tribe also the reduced genera Asterotus and Stigmatolemma based on micromorphological characters, but Asterotus was later considered a posterior synonym of Resupinatus, and reduced to a subgenus of Resupinatus (Singer 1973b, 1975). Kühner (1980) included only Resupinatus (with Hohenbuehelia as a subgenus) inside the tribe Resupinatae of Pleurotaceae. Jülich (1981) established the family Resupinataceae for Resupinatus. Finally, Singer (1986) widened the concept of his tribe Resupinateae (Tricholomataceae) to include the lamellate genera Agaricochaete, Hohenbuehelia and Resupinatus, and the cyphelloid genera Aphyllotus, Stigmatolemma, and Stromatocyphella.
With the aid of molecular phylogenetic studies, Asterotus, Lignomyces, Rhodocyphella, Stigmatolemma, and Stromatocyphella were shown to be later synonyms of Resupinatus (Thorn et al. 2000, 2005, McDonald 2015, Consiglio & Setti 2018, McDonald & Thorn 2019), while Aphyllotus was moved to Marasmiaceae (McDonald 2015, based only on morphology). However, the classification of Resupinatus (tribe Resupinateae sensu stricto) has always been very uncertain. Most works failed to obtain significant support for any phylogenetic relationship of Resupinatus, i.e., Moncalvo et al. (2000) placed Resupinatus near Phyllotopsis and Pleurocybella within the euagarics clade; Thorn et al. (2000) near the Tricholomataceae sensu lato (Tricholomatineae); Moncalvo et al. (2002) close to Marasmiaceae; Bodensteiner et al. (2004) as part, together with Stigmatolemma, of the /resupinatus clade in the euagarics clade; Binder et al. (2005) as sister to Arrhenia in the core euagarics clade; Thorn et al. (2005) placed it close to the /hemimycena clade and the /phyllotopsis clade; Matheny et al. (2006) as part of the Pleurotaceae (together with Cantharocybe gruberi) within the Pluteoid clade; McDonald (2015) close to the Pleurotaceae, Entolomataceae, and Tricholomataceae; Petersen et al. (2015) as incertae sedis within Agaricales (sister with no support to Phyllotopsis); Liu et al. (2016) as sister to Mycena spp. in Tricholomataceae sensu lato in the ITS analysis and sister to Arthromyces in the LSU analysis; Consiglio & Setti (2017), as sister to Pleurotaceae; Varga et al. (2019), within Marasmiineae; Sánchez-García et al. (2020), within Marasmiineae, close to Phloeomana and Hemimycena (Cyphellaceae, Marasmiineae, see Vizzini et al. 2022); Karasiński et al. (2023) as incertae sedis in Cyphellaceae, Marasmiineae (LSU-based analysis). The present analysis (Fig. 2) suggests that Resupinatus is an independent clade within Pleurotineae. The important morphological differences with the family Pleurotaceae, and the lack of a significant support for their monophyletic origin excluding the family Schizophyllaceae, are the basis to propose restoring the family name Resupinataceae to accommodate this clade.
Schizophyllaceae Quél., Fl. Mycol. France (Paris): 365. 1888.
Synonyms: Auriculariopsidaceae Jülich, Biblioth. Mycol. 85: 355. 1982. [1981].
Schizophyllaceae Roze [as ‘Schizophyllées’], Bull. Soc. Bot. France 23: 108 (1876), nom. inval., Art. 32.1(c), 32.1(b); see Art. 18.4 (Shenzhen); see Donk (1964).
Type: Schizophyllum Fr. [as ‘Schizophyllus’, orth. cons.], Observ. Mycol. (Havniae) 1: 103. 1815.
Representative genera: Auriculariopsis and Schizophyllum.
Notes: The family Schizophyllaceae is characterized by its basidiomes typically astipitate, gymnocarpic, resupinate to cyphelloid/cupulate. Hymenophore smooth or folded (pseudolamellate). Hyphal system monomitic to dimitic, hyphae usually presenting clamp connections, non-amyloid, and often immersed in a gelatinous matrix. Basidia usually 4-sporic and basidiospores colourless or occasionally yellow-brown, non-dextrinoid, inamyloid. Cystidia usually absent. In culture, Schizophyllum commune and Auriculariopsis ampla are characterized by clamped hyphae with spines (Essig 1922, Nobles 1948, Watling & Sweeney 1971, Nuss 1980, Stalpers 1988, Nakasone 1996). Lignicolous, often bark-specialized, pioneer colonizers of dead plant debris, intermediate behaviour between white-rot and brown-rot fungi (see below), with a suggested weak phytopathogenic potential in Schizophyllum (Takemoto et al. 2010, Rezgui et al. 2018). Schizophyllum commune is also known to be an opportunistic pathogen in humans due to its peculiar wood-degrading enzymes and toxic metabolites (Viswanathan et al. 2019, Tam et al. 2022).
The first to find a significant phylogenetic relationship between Fistulina and Schizophyllum were Hibbett et al. (1997), a result confirmed later by Moncalvo et al. (2002), Bodensteiner et al. (2004) and Henkel et al. (2010), suggesting that the families Schizophyllaceae and Fistulinaceae have a monophyletic origin. Taxa belonging to Schizophyllaceae and Fistulinaceae clustered together within or as a basal clade of the Marasmioid clade (Marasmiineae) in several works (Matheny et al. 2006, Maynard et al. 2019, Olariaga et al. 2020, Ke et al. 2020, Li et al. 2021, Wang et al. 2023b), or else as a distinct incertae sedis clade (Yamaguchi et al. 2009, Binder et al. 2010, Floudas et al. 2015, Dentinger et al. 2016, Almási et al. 2019, Varga et al. 2019, Sánchez-García et al. 2020). In the phylogenomic work by Dentinger et al. (2016) the new suborder Schizophyllineae was established for the clade containing Schizophyllum commune and Fistulina hepatica. Wang et al. (2023b) showed that this clade contains at least the families Schizophyllaceae, Fistulinaceae and Cyphellopsidaceae (as Niaceae). In the present analysis (Fig. 2) suborder Schizophyllineae seems to have a monophyletic origin with the families Pleurotaceae and Resupinataceae, and consequently Schizophyllineae and Pleurotineae are here considered synonyms. Since both were proposed in the same work (Dentinger et al. 2016), priority is given here to Pleurotineae. Comparative genomic and transcriptomic analyses of Schizophyllaceae (Auriculariopsis ampla and Schizophyllum commune) and Fistulinaceae (Fistulina hepatica) suggest that these fungi have peculiar plant cell wall-degrading enzymes transitional between those of white rot species and less efficient wood-degraders such as brown rot or mycorrhizal fungi (Floudas et al. 2015, Almási et al. 2019, Veloz Villavicencio et al. 2020).
Fistulinaceae Lotsy, Vortr. Bot. Stammesgesch. 1: 695, 704. 1907.
Synonym: Fistulinaceae Maire, Bull. Soc. Mycol. France 18 (Suppl.): 111. 1902, nom. inval., Art. 32.1(b); see Art. 18.4 (Shenzhen).
Type: Fistulina Bull., Hist. Champ. Fr. (Paris) 1(2): 313. 1791.
Synonyms: Agarico-carnis Paulet, Traité champ. (Paris) 2: 97. 1793.
Buglossus Wahlenb., Fl. Upsal.: 459. 1820.
Hypodrys Pers., Mycol. Eur. (Erlanga) 2: 148. 1825.
Confistulina Stalpers, Canad. J. Bot. 61: 1660. 1983.
Representative genera: Fistulina, Porodisculus, and Pseudofistulina.
Notes: The family Fistulinaceae is characterized by its basidiomes gymnocarpic, large (20–300 mm wide), pileate, non-stipitate, or pileate-stipitate, pileus circular, tongue- or kidney-shaped, base often attenuate, or flabelliform, lobate or tiny (1–4 mm), pendant, soft or resinous hard when dried, horny, very dense, surface velutinous or tufted by hairs to pruinose, reddish or brownish; stipe absent or present, solitary to confluent, lateral or eccentric, sometimes rooting, minutely velutinous; hymenophore tubular, tubes independent, separate but closely packed or coherent, fused (polyporoid), whitish to yellowish or reddish brown, particularly after bruising. Hyphal system monomitic, pileipellis a trichoderm or an interwowen cutis. Hyphae simple or as acanthophyses, thin- to thick-walled, colourless, inamyloid, non-dextrinoid. Clamp connections present or absent. Cystidia absent or rarely present as pileocystidia-like hyphal ends or as hymenial elements. Basidia clavate or suburniform, sterigmata 4-sporic. Basidiospores subglobose or ellipsoid, cylindrical to allantoid, smooth, colourless to pale cream, thin- or thick-walled, inamyloid, non-dextrinoid. Pileipellis and stipitipellis sometimes with coralloid brown hyphae. Lignicolous, terricolous, saprotrophic, or parasitic on wood or roots. Asexual morph phase sometimes present (Confistulina). Inducing wood decay in living trees (Fistulina and Pseudofistulina; Gilbertson & Ryvarden 1986, Guzmán 1987, Schwarze et al. 2000, González et al. 2021).
Phylogenetically, Fistulina, with its pileate basidiomes, tongueor kidney-shaped gelatinous soft pileus and separate tubes (Song et al. 2015, Sun et al. 2019, González et al. 2021, Zhou et al. 2022), is closely related to Porodisculus (Bodensteiner et al. 2004, Binder et al. 2005, Henkel et al. 2010, Song et al. 2015, Sun et al. 2019, González et al. 2021, Zhou et al. 2022) and Pseudofistulina (González et al. 2021, Zhou et al. 2022). Porodisculus is characterized by tiny and pendant basidiomes, resinous hard when dried, coherent tubes, and coralloid brown hyphae (acanthophyses) in pileipellis and stipitipellis (Gilbertson & Ryvarden 1987, Ginns 1997, Lee & Jung 2008, Chuzho & Dkhar 2020). Pseudofistulina has pileate-stipitate basidiomes, fleshy coriaceus, soft, stipe solitary to somewhat confluent, lateral or eccentric, often rooting, separate tubes, and slightly amyloid acanthophyses in pileipellis and hymenium (Wright 1961, Fidalgo & Fidalgo 1962, Burdsall 1971, Gilbertson & Ryvarden 1986, Guzmán 1987).
Pluteineae Aime et al., Biol. J. Linn. Soc. 117: 27. 2016.
Synonym: Pluteales Kühner, Bull. Mens. Soc. Linn. Lyon 49(Num. Spéc.): 357. 1980.
Type: Pluteus Fr., Fl. Scan.: 338. 1836.
Representative families: Amanitaceae, Limnoperdaceae, Melanoleucaceae, Pluteaceae, and Volvariellaceae.
Notes: Five families are here recognized within suborder Pluteineae: Amanitaceae (including Leucocortinarius), Limnoperdaceae, Melanoleucaceae, Pluteaceae, and Volvariellaceae. There is no obvious morphological synapomorphy that unites the Pluteineae. Basidiomes are pileostipitate (agaricoid) (with open or enclosed hymenophore) or gasteroid/sequestrate (angiocarpic development, epigeous or hypogeous), mostly fleshy, heterogeneous (context of the pileus not continuous with the context of the stipe and consequently pileus and stipe separable from each other) and with free lamellae (homogeneous with adnate to subdecurrent lamellae in Leucocortinarius and Melanoleucaceae). Hyphae monomitic; clamp connections present or absent; non-amyloid, sometimes slightly metachromatic. Basidia ballistosporic or statismosporic. Basidiospores colourless or with pink/red tinges, smooth or verrucose, without a germ pore, amyloid or inamyloid, cyanophilous (Cotton blue) or not, slightly metachromatic (Cresyl blue) in Leucocortinarius. Hymenial cystidia often present. Hymenophoral tramal regular, bilateral, or inverse. Pileipellis typically a cutis or a trichoderm except for the hymenodermic/cellulodermic structure found in some species of Pluteus. Protective veils are often present (monovelangiocarpic to bivelangiocarpic development). Most Amanitaceae are ectomycorrhizal, except species of Saproamanita, and the remaining families include mostly terricolous or lignicolous saprotrophic taxa, rarely mycoparasitic (i.e., Volvariella surrecta). In the present analysis, the family Amanitaceae is only subsignificantly related to the remaining families of Pluteineae (0.88 PP), but this seems to be caused by insufficient data from some lineages (i.e., Leucocortinarius), as their exclusion from the analyses resulted in full support for a monophyletic origin of Amanitaceae and the remaining Pluteineae (i.e., Supplementary Fig. S1).
Amanitaceae E.-J. Gilbert, Iconogr. Mycol., Suppl. I (Milan) 27: 63. 1940.
Synonym: Torrendiaceae Jülich, Biblioth. Mycol. 85: 392. 1982. [1981].
Type: Amanita Pers., Tent. disp. meth. fung. (Lipsiae): 65. 1797.
Representative genera: Amanita, Catatrama, Leucocortinarius, Limacella, Limacellopsis, Saproamanita and Zhuliangomyces (= Myxoderma Kühner 1926 sensu Cui et al. 2018 and Yang et al. 2018; nom. illegit., non Myxoderma Schmidle 1901, Cyanophyta).
Notes: For general features of the family and generic delimitation see Redhead et al. (2016), Cui et al. (2018), and Yang et al. (2018). Basidiomes are predominantly agaricoid (but Amanita also includes angiocarpic/sequestrate species formerly classified in Torrendia and Amarrendia, see Justo et al. 2010), mono- to bivelangiocarpic, with or without a schizohymenial development of the hymenophore (Reijnders 1963, Bas 1969), pileus convex to applanate with margin smooth or radially sulcate, dry or greasy, sometimes glutinous, covered or not with remnants of the universal veil; lamellae usually free, rarely subfree or adnate (Leucocortinarius); stipe, when present, cylindrical, with or without a basal inflation or a bulb, with or without a partial veil as an annulus, dry or slimy, base with or without remnants of the universal veil which can form a saccate volva; spore deposit white, greenish to light pink; basidiospores globose to ellipsoid or cylindrical, colourless, mostly thin-walled, predominantly smooth, rarely echinulate-verrucose, amyloid or not, dextrinoid or not, cyanophilous or not, metachromatic in Leucocortinarius, usually binucleate; basidia usually 4-sporic, ballistosporic or statismosporic; hymenophoral trama bilateral or irregular to regular (Leucocortinarius); hymenial cystidia usually absent but spheropeduncolate elements present on the lamella edge in Amanita, resembling cheilocystidia, rare cheilocystidia in Leucocortinarius; pileipellis a cutis, ixocutis, ixotrichoderm or ixopalisadoderm; stipe trama longitudinally acrophysalidic (heteromerous) or not; clamp connections present or absent. Species are terricolous, mainly symbiotic mutualists forming ectomycorrhizas with angiospermous and gymnospermous trees (Trappe 1962, Singer 1986, Cuvelier 1990, Mleczko 2004, Daniele et al. 2005, Agerer 2006, Rinaldi et al. 2008, Bai et al. 2009, Niazi et al. 2009, Tedersoo et al. 2010) and/or arbutoid mycorrhizas with Ericaceae (Molina & Trappe 1982, Smith & Read 2008), or saprotrophic (Saproamanita, Limacella sensu lato and Catatrama, Wolfe et al 2012, Hess & Pringle 2014, Redhead et al. 2016, Li et al. 2020).
Leucocortinarius (J.E. Lange) Singer, Lloydia 8(3): 141. 1945.
Synonym: Cortinarius subgen. Leucocortinarius J.E. Lange, Dansk Bot. Ark. 8(no. 7): 6. 1935.
Type: Leucocortinarius bulbiger (Alb. & Schwein.) Singer, Lloydia 8: 141. 1945. Figs 7B, 11.
Fig. 11.
Leucocortinarius bulbiger. A–C. Basidiomes (A. JV13908F TUR-A; B, JV25486F TUR-A; C, AMB:19592). D. Pileipellis (AMB:19593). E. Subpellis (AMB:19593). F. Hymenophoral trama (JV13908F). G. Thromboplerous hyphae of the hymenophoral trama (GC96078). H. Hymenium (basidia) (AMB:19593). I. Cheilocystidioid elements (JV13908F TUR-A). J. Thromboplerous hypha of the stipitipellis (B JV25486F TUR-A). K. Subhymenium (GC96078). L. Stipititrama (JV13908F TUR-A). M. Bulb trama (JV13908F TUR-A). N. Elements of the universal veil (JV13908F TUR-A). O. Elements of the partial veil (JV13908F TUR-A). P, Q. Basidiospores (P. JV13908F TUR-A; Q. AMB:19593). Mounting media were Melzer’s reagent (J), Congo Red in ammonia (D–I, K–O), Cotton Blue (Q), and Cresyl Blue (P). Scale bars: D, E = 30 μm; F–Q = 10 μm. Photographs A, B by J. Vauras, C by G. Consiglio, D, E, G, H, K, Q by M. Marchetti, F, I, J, L–P by E. Campo.
Basionym: Agaricus bulbiger Alb. & Schwein., Consp. fung. (Leipzig): 150. 1805.
Synonyms: Armillaria bulbigera (Alb. & Schwein.) P. Kumm., Führ. Pilzk. (Zerbst): 135. 1871.
Gyrophila bulbigera (Alb. & Schwein.) Quél., Enchir. fung. (Paris): 9. 1886.
Mastoleucomyces bulbiger (Alb. & Schwein.) Kuntze, Revis. gen. pl. (Leipzig) 2: 861. 1891.
Cortinellus bulbiger (Alb. & Schwein.) Pat., Essai Tax. Hyménomyc. (Lons-le-Saunier): 161. 1900.
Tricholoma bulbigerum (Alb. & Schwein.) Ricken, Die Blätterpilze: 331. 1914.
Cortinarius bulbiger (Alb. & Schwein.) J.E. Lange, Dansk Bot. Ark. 8(no. 7): 13. 1935.
Agaricus malleipes Lasch, Linnaea 4: 519. 1829.
Agaricus cupreus Secr., Mycographie Suisse 1: 70. 1833 (nom. inval.), Art. 34.1 (Shenzhen).
Description: Habit cortinarioid (phlegmacioid), bivelangiocarpic, basidiome homogeneous non-schizohymenial. Pileus 30–120(−150) mm diam, convex, with incurved margin when young, later plane, smooth, slightly gelatinous, orange brown to reddish brown (6A5-8), vinaceous brown (7A7-8), cracking as it ages; when young covered with white veil remnants (mainly as patches). Lamellae moderately crowded, adnate-emarginate to subfree, white, becoming cream (4A4-6) with age. Stipe 30–90 × 6–20 mm, usually tapering towards the apex, base swollen and usually abruptly bulbous with a rounded to flattened marginate 20–40 mm wide bulb, white, staining somewhat brownish (5B5-7) when mature; partial veil present, coarsely fibrillose (not cobweb-like as in Cortinarius sensu lato), forming both a fragile cottony-ring-like zone at stipe apex and abundant remnants on pileus margin; universal veil white, on pileus disk and bulb rim (margin). Context white, odour agreable, taste mild. Spore deposit white to pale cream. Basidiospores (5.6–)6.6–7.1–7.7(−9.4) × (3.4–) 4.2–4.6–4.9(−5.3) µm [166/4/4], Q = (1.29–)1.44–1.57–1.70(−2.06), V = (41.5–)63.4–78.1–92.8(−119) µm3, ellipsoid to broadly ellipsoid (rounded apex) or subamygdaliform (slightly tapered apex), smooth, thick-walled and with weak suprahilar depression and prominent apiculus, containing numerous greenish guttulae or a single large central guttula, wall slightly congophilous, cyanophilous in Cotton blue and weakly metachromatic in Cresyl blue (Fig. 11P), inamyloid and indextrinoid. Basidia 30–45(−50) × 7–9(−10.5) µm, long claviform, most 4-sporic, rarely 2-sporic, sterigmata up to 4–5 µm long, with minute greenish internal droplets. Cheilocystidioid elements present in some collections, (18–)20–27(−32) × (4–)4.2–5(−5.5) µm, colourless, thin-walled, cylindrical to fusoid, sometimes bifid, with basal clamp connection. Hymenophoral trama regular, consisting of subparallel, colourless to light yellow, weakly metachromatic, thin-walled hyphae, often slightly constricted at septa, in the medium stratum voluminous, (5–)6–14(−25) µm wide, in the lateral stratum 3–7 µm wide; thromboplerous hyphae abundant, 4–10 µm wide, with grey yellowish to greenish content. Subhymenium thin, of 3–6 μm wide, short and often intertwined hyphal elements. Partial veil formed by parallel-oriented, thin-walled and clamped cylindrical hyphae, 2–5 µm wide, with a faint yellowish parietal pigment and slightly metachromatic. Universal veil constituted by a network of 3–6 µm wide clamped hyphae, cylindrical, sometimes spindle-shaped, enlarged in the median part up to 13 µm, faintly coloured by a yellowish parietal pigment and weakly metachromatic; rare 3–8 µm wide thromboplerous hyphae are present. Pileipellis an ixotrichocutis (transition between cutis and trichoderm) of congophobic, 2–4(−5) µm wide, loose, and sinuous ascending clamped hyphae with spaced septa, often forming tufts, some with a gold yellow vacuolar pigment, immersed in a gelatinized matrix and arising from deeper layers of horizontal 3–7 µm wide congophilous hyphae, some with minute brownish granular epiparietal pigments. Subpellis of subparallel, up to 10 µm wide colourless hyphae; nodulose 4–10 µm wide thromboplerous hyphae present. Stipitipellis consisting of a cutis of thin-walled, 4–10 µm wide colourless hyphae, slightly metachromatic, with long, just emerging terminal elements with obtuse apex and clamp connections on each septum; 5–15 µm wide thromboplerous hyphae abundant. Stipititrama (both the cylindrical portion and the bulb) not acrophysalidic, characterized by 5–25(−30) µm wide colourless and slightly metachromatic clamped hyphae, subparallel oriented in the cylindrical portion, somewhat intertwined in the bulb; abundant nodulose, 5–25 µm wide thromboplerous hyphae. Clamp connections and thromboplerous hyphae frequent in all parts of the basidiome. Trama hyphae neither amyloid nor dextrinoid but weakly metachromatic.
Habitat and distribution: Terricolous, usually associated with coniferous trees, especially Picea, Abies, and Pinus sylvestris, usually on calcareous soil, Europe, Asia, and North America. Late summer to autumn. It is ectomycorrhizal (Singer & Morello 1960, Trappe 1962, Newton & Haigh 1998, Bai et al. 2009, Zheng et al. 2016).
Materials examined: Finland, Etelä-Häme, Hattula commune, Retulansaari, S side of the road, pastured meadows with abundant Juniperus communis and scattered Pinus sylvestris, under P. sylvestris, 12 Aug. 1998, J. Vauras, 13908F (TUR-A); Etelä-Häme, Tammela, Mustiala, Syrjänharju, near and S of Toivonsilta, fairly rich forest with mainly Picea abies, by walking trail, 19 Sep. 2007, J. Vauras, 25486F (TUR-A). Italy, Castel Lamberto (BZ), under Picea abies, 5 Sep. 1996, G. Consiglio (AMB:19592); Laghestel, Baselga di Piné (TN) under Picea abies, 28 Sep. 2005, G. Consiglio (AMB:19593).
Notes: By now, the genus Leucocortinarius is presumably monospecific. The only other species described in Leucocortinarius is L. castulifer (≡ Armillariella castulifera), a very rare taxon (there are three samples at PC from the 1970s and 1980s) no longer found by other authors after its description. According to the original reports (Romagnesi 1978, 1980) it seems to differ from L. bulbiger only in a more consistent and striated ring. Agaricus bulbiger was described as characterized by an orange cinnamomeus reddish pileus with white veil remnants, white emarginate-adnexed lamellae, stipe with a marginate depressed bulb and a white annulus at apex, white spore deposit, and occurring in deciduous thorn-thickets among leaves and mosses, everywhere solitary or somewhat gregarious (Albertini & Schweinitz 1805). It was later transferred into numerous genera until Lange (1935) established Cortinarius subg. Leucocortinarius for it. This subgenus was later raised to the rank of genus by Singer (1945). The taxon has been considered to be either akin to pale-spored genera of Tricholomataceae sensu lato (Armillaria, Tricholoma; e.g., Kummer 1871, Quélet 1886, Kuntze 1891, Ricken 1914, Singer 1945, Kühner & Romagnesi 1953, Romagnesi 1978, 1980, Bon 1987a, 2004, Courtecuisse & Duhem 1994, Consiglio & Papetti 2001, Ludwig 2001a, b, Boccardo et al. 2008, Frøslev 2012, Agerer 2018, Læssøe & Petersen 2019, Kalichman et al. 2020, Kibby 2020) or to ochre-spored Cortinarius and allied taxa (e.g., Patouillard 1900, Lange 1935, Konrad & Maublanc 1952, Singer 1951, 1962, 1975, 1986, Singer & Morello 1960, Horak 1968, 2005, Moser 1978, Kühner 1980, Jülich 1981, Reijnders & Stalpers 1992, Watling & Gregory 1994, Bas & Kuyper 1995, Eyssartier & Roux 2011).
Leucortinarius shares with most tricholomataceous fungi the white spore deposit, non-free lamellae, smooth basidiospores, green reaction of basidiomes surfaces with TL-4 (Romagnesi 1978, 1980) and with some species of Cortinarius sensu lato the bivelangiocarpic development (Reijnders 1979), the presence of a marginate bulb and of binucleate basidiospores (Kühner 1945, 1980, Singer 1951, 1986). Macromorphologically, Leucocortinarius bears a superficial resemblance to some bulbous-based webcaps (Cortinarius sect. Scauri species) but its spore deposit is white rather than rusty brown; more specifically it resembles a pale-spored form of Cortinarius multiformis (now included in Thaxterogaster emend. Niskanen & Liimat. subgen. Multiformes, Liimatainen et al. 2022).
Molecular phylogenetic analyses based only on one or a few markers (mainly ribosomal DNA) and/or a limited taxon sampling did not provide a satisfactory answer about the classification of this taxon within the Agaricales (He et al. 2019 considered it as incertae sedis) i.e., Garnica et al. (2007), Varga et al. (2019) and Kalichman et al. (2020) placed it in Tricholomataceae sensu lato; Saar et al. (2009) as close (wihout support) to Hygrocybe coccinea; Zheng et al. (2016) as sister to Hebeloma cylindrosporum and Sánchez-García et al. (2020) as sister to Amanitaceae, the latter a conclusion supported also by the present analysis (Fig. 2).
Limnoperdaceae G.A. Escobar, Mycologia 68: 878. 1976.
Type: Limnoperdon G.A. Escobar, Mycologia 68: 875. 1976.
Representative genus: Limnoperdon.
Notes: This monogeneric and by now monospecific family (type L. incarnatum) is characterized by a reduced minute basidiome (0.3–1.5 mm diam) at first cupulate (cyphelloid) then sequestrate (enclosed), puffball-like, with a 1-loculate gleba lined up by a hymenium, at maturity opening by a pore through which a sporecontaining drop extrudes, statismosporic (gasteroid) basidia, basidiospores symmetrical, thin- to thick walled, in mass pink/redcoloured, inamyloid, thin-walled hyphae with clamp connections, absence of hymenial cystidia, and growing on plant material (leaves, twigs) exposed to estuarian or fresh water (Escobar et al. 1976, Escobar & McCabe 1979, McCabe 1979, Webster & Descals 1981, Michaelides & Kendrick 1982, Nakagiri & Ito 1991, Webster et al. 1993, Voglmayr 1994, Abdel-Aziz 2016). It has been reported from Japan, Austria, Canada, USA, Egypt, South Africa, and Argentina (Webster et al. 1993, Voglmayr 1994, Donoghue & Alverson 2000, Hibbett & Binder 2001, Bärlocher et al. 2008, Abdel-Aziz 2016, Agerer 2018). Hibbett & Binder 2001 placed Limnoperdon in the core Euagaricoid clade; Bodensteiner et al. (2004) as sister (without support) of a clade with Melanoleuca and Pluteus; Binder et al. (2005) as basal to core euagarics and close to Pluteus and Entoloma; Matheny et al. (2006) as sister to Pluteaceae in the Pluteoid clade; Justo et al. 2011 as part of the Pluteoid clade (consisting also of Pluteus, Volvariella gloiocephala-group = Volvopluteus, Amanitaceae, Melanoleuca, and Macrocystidia) with moderate to high statistical support in all the analyses; and Sánchez-García et al. (2020, suppl. mat.) as sister to a clade encompassing Melanoleuca, Pluteus, and Volvopluteus. The present analysis includes for the first-time sequences of protein-coding genes of Limnoperdon sp. (aff. incarnatum) (voucher CBS:160.95, WU 12660, Austria, studied in Voglmayr 1994), suggesting an ancient origin of this evolutive lineage and its treatment at the rank of family within Pluteineae. The closest ITS sequence to that obtained from CBS:160.95 is that of the ex-type strain of L. incarnatum, DSM1832 (UDB034350), but both are only 90 % similar, while their LSU is 99.3 % identical. Such a great distance between their ITS sequences seems to point to the existence of a reproductive barrier between these samples, but a larger sampling is necessary to draw reliable conclusions. Other sequences identified as Limnoperdon spp. in public databases seem to represent at least two other distinct species. Unfortunately, many of these sequences were obtained from environmental samples, so their taxonomic status cannot be resolved yet.
Melanoleucaceae Locq. ex Vizzini, Consiglio & P. Alvarado, fam. nov. MycoBank MB 851152.
Synonym: Melanoleucaceae Locq. Mycol. gén. struct. (Paris): 145. 1984, nom. inval., Art. 39.1 (Shenzhen).
Diagnosis: Basidiomes pileostipitate, large collybioid to tricholomatoid, homogeneous (context of the pileus continuous with the context of the stipe and consequently pileus and stipe not separable from each other), lamellae adnexed to subdecurrent, partial veil absent or present. Spore deposit white to pale cream. Hyphal system monomitic. Hymenophoral trama subregular. Basidiospores colourless, subglobose to ellipsoid, decorated with rounded to acute-blunt amyloid verrucae, cyanophilous or not. Basidia sometimes containing micro-type siderophilous granules (Melanoleuca). Cheilocystidia usually present. Pleurocystidia usually absent or rare. Pileipellis typically a cutis or a trichoderm. Clamp connections present or absent. Terricolous, probably saprotrophic, although Giacomia mirabilis cannot be easily cultured in vitro (Moser 1963), suggesting an ectomycorrhizal lifestyle.
Type: Melanoleuca Pat., Cat. Rais. Pl. Cellul. Tunisie (Paris) (7): 22. 1897.
Representative genera: Giacomia (Figs 6G, 12–14) and Melanoleuca (7F–G).
Fig. 12.
Giacomia mirabilis. A–F. Basidiomes (A. TUR-A 209709; B. AMB:18860; C. TUR-A 195638; D. AMB:19594; E. G. f. nigrescens AMB:19297; F. TO:AV20231010). G. Pileus margin (TUR-A 209709). H, I. Stipe apex (H. AMB:19595; I. TUR-A 195638). Photographs A, C, G, I by E. Campo; B, E by G. Consiglio; D by C. Feltrin; F by J. Ferrari; H by E. Zanella.
Fig. 14.
Giacomia sinensis (HMJU:265 holotype). A. Basidiome. B. Basidiospores (Melzer’s reagent). C. Basidiospores (SEM). D. Pileipellis. E. Basidia. F. Basidiospores. Scale bars: B, D–F = 10 μm. Photographs and drawings by J. Xu.
Notes: The present analysis supports for the first time that Giacomia and Melanoleuca have a monophyletic origin inside suborder Pluteineae (Fig. 2), a result not found in previous studies based only on ribosomal DNA data of Giacomia, which placed this genus in an uncertain position within the Tricholomatineae (Vizzini et al. 2012a, Sánchez-García et al. 2014, Sánchez-García 2016, Angelini et al. 2017, He et al. 2019). However, Varga et al. (2019) suggested that Giacomia is not related to suborder Tricholomatineae. On the other hand, the phylogenetic proximity of Melanoleuca, Pluteus and allied taxa had already been highlighted by Moncalvo et al. (2002), Matheny et al. (2006), Garnica et al. (2007), Justo et al. (2011), and Sánchez-García et al. (2020, suppl. mat.). Melanoleuca is a cosmopolitan genus with more than 440 species epithets (Index Fungorum, https://www.indexfungorum.org/names/Names.asp) corresponding to ca. 60 confirmed species (Agerer 2018, He et al. 2019). Morphologically, Melanoleuca is characterized by collybioid to tricholomatoid gymnocarpic basidiomes, convex to slightly depressed pileus often with a low central umbo, emarginate, adnate, or shortly decurrent (with tooth) lamellae, pileipellis in the form of cutis to trichoderm, cyanophilous, amyloid ornamented basidiospores, basidiospores with a well-delimited suprahilar plage (Singer 1986, Bon 1991), basidia with siderophilous granules of the micro type, the frequent presence of long, thin- to thick-walled and often muricate cheilocystidia (macrocystidia), pleurocystidia (if present) similar to cheilocystidia, and the absence of clamp connections (Singer 1972, Clémençon 1978, 2004, Kühner 1978, Singer 1986, Boekhout 1988, 1999a, Bon 1991, Watling & Turnbull 1998, Garnica et al. 2007, Vesterholt 2008a, 2012a, Vizzini et al. 2011a, Xu et al. 2019, Antonín et al. 2022).
Giacomia Vizzini & Contu, Mycosphere 3: 84. 2012.
Type: Giacomia mirabilis (Bres.) Vizzini & Contu
Notes: Giacomia differs from Melanoleuca because of the presence of clamp connections, basidia without inner siderophilous granules, evanescent veil at stipe apex, not well differentiated and not muricate hymenial cystidia, and basidiospores without a well-delimited suprahilar plage (Vizzini et al. 2011a and see below). Until the present work, Giacomia was considered a monospecific genus. Hereafter, a second species is formally described and a full description of the very rare G. mirabilis is provided.
Giacomia mirabilis (Bres.) Vizzini & Contu, Mycosphere 3: 84. 2012. Figs 6G, 12–13.
Fig. 13.
Giacomia mirabilis. A, B. Pileipellis (A. AMB:18860; B. AMB:19297). C. Basidia and basidiospores (AMB:18860). D. Hymenium (AMB:19297). E. Cheilocystidia (AMB:18860). F. Elements of the hymenophoral trama (AMB:19297). G–I. Basidiospores (G, I. AMB:18860; H. AMB:19297). Mounting media were water (A), Melzer’s reagent (G, H), Congo Red in ammonia (B–F), and Cotton Blue (I). Scale bars: A, B = 30 μm; C–I = 10 μm. Photographs M. Marchetti.
Basionym: Tricholoma mirabile Bres., Fungi Tridentini 1(2): 16. 1881.
Synonyms: Melanoleuca mirabilis (Bres.) Singer, Lloydia 5: 121. 1942.
Leucopaxillus mirabilis (Bres.) Konrad & Maubl., Encyclop. Mycol. 20: 191. 1952.
Tricholoma mirabile Bres. var. nigrescens Bres., Icon. Mycol. 2: 92. 1927.
Melanoleuca nigrescens (Bres.) Bon, Doc. Mycol. 9: 47. 1978.
Leucopaxillus mirabilis var. nigrescens (Bres.) Fontenla & Para, Rivista Micol. 50: 233. 2007.
Giacomia mirabilis f. nigrescens (Bres.) Vizzini & Contu, Mycosphere 3: 84. 2012.
Leucopaxillus amarus f. phaeopus J. Favre & Poluzzi, Vita Helvetica 71: 74. 1949.
Leucopaxillus phaeopus (J. Favre & Poluzzi) Bon, Bull. Trimestriel Féd. Mycol. Dauphiné-Savoie 27: 29. 1987.
Leucopaxillus mirabilis var. paxilloides M.M. Moser ex Bon, Doc. Mycol. 9(no. 33): 22. 1978, nom. inval., Art. 39.1 (Shenzhen).
Description: Habit tricholomatoid to leucopaxilloid. Pileus (30–)40–100(−120) mm diam, at first convex, then expanded and depressed at centre, sometimes with an obtuse flat umbo; surface dry, not or slightly hygrophanous, minutely tomentose to fibrillose, dark brown (4E6-8), grey brown (7D3-5), reddish brown (5B6-8), blackish brown (4F2-8, 5F3-6), but also ochre-brown (6C5-8) or yellowish-cream (4A6-8, 4B7-8), sometimes paler at centre; margin paler, initially inrolled and often wavy when old, sometimes costate to furrowed, typically minutely hairy with white hairs up to 8 mm long (Fig. 12G), single or in tufts (it looks like Pogonoloma spinulosum). Lamellae arcuate, decurrent to adnexed with tooth, crowded (L = 45–70), with lamellulae [l = (1–)2–5], white then cream, sometimes brownish at the edge and face level near the stipe (as in Lactarius lignyotus), with an entire edge. Stipe (30–)40–60(−70) × (6–)8–12(−15) mm, cylindric, terete, sometimes attenuated towards the base, dry, fibrillose-squamulose, ochre-brown, reddish-brown, blackishbrown, white to cream toward the base. The stipe apex can appear in two ways: concolorous with the rest of the stipe and exhibiting brown reticulating lines which appear to be extensions of the lamellae (Fig. 12H), or with an evident white belt (band) delimited by a hoary ring-like zone (Fig. 12I). Context whitish, ochre-brownish under the cortical parts. Odour subfarinaceous, sometimes subspermatic, herbaceous. Taste mild, sometimes slightly bitterish after a long time of mastication. Spore deposit whitish to pale cream. Basidiospores (5.39–)6.11–6.68–7.25(−8.73) × (3.8–)4.52–5.0–5.48(−5.96) μm [99/3/3], Q = (1.1–)1.21–1.33–1.45(−1.75), V = (50.4–)68.3–90.2–112.0(−139.7) μm3, broadly ellipsoid to ellipsoid, sometimes in profile adaxially flattened, colourless, thin-walled, pluriguttulate, with a well-developed hilar appendix, warty, amyloid, cyanophilous, with irregularly distributed, rounded to acute-blunt verrucae, without a suprahilar plage. Basidia (25.0–)29.13–34.87(−37.0) × (5.52–)6.42–8.38(−9.64) μm, Q = (3.14–)3.83–4.91(−5.43), 4-sporic, rarely 2-sporic, clavate, with up to 6 μm long sterigmata, not siderophilous, with numerous inner refractive droplets. Hymenophoral trama regular to subregular, consisting of parallel cylindrical hyphae, (3.0–)5.0–15.0(−20.0) μm wide, colourless, thin-walled, sometimes with extracellular refractive crystalline deposits. Subhymenium 20–30 μm thick, of 2–4 μm wide, short and often intertwined hyphal elements. Cheilocystidia 20–40 × (2–)3.5–7 μm, sometimes very rare, versiform, polymorphic, cylindrical, lageniform, sinuous, diverticulate, forked, subcapitate, colourless, thin-walled, without inner content. Pleurocystidia absent. Pileipellis a cutis of parallel to intertwined cylindrical hyphae, 2–8 μm wide, terminal elements slightly gelatinized and sometimes ascendant and with enlarged apex, thin-walled; pigment yellowish, intracellular and parietal; grey-yellowish thromboplerous hyphae (oleiferous hyphae sensu Clémençon 2004) common; polymorphic, extracellular refractive crystalline deposits present on superficial hyphae. Subcutis consisting of cylindrical, 4–10 μm wide thin-walled hyphae. Stipitipellis of yellow-brown cylindrical hyphae 2–6 μm wide, thin- to thick-walled, intertwined, irregular-nodose, some terminal elements ascendant (emerging, repent), with abundant refractive crystalline deposits. Stipititrama of colourless, parallel, thin-walled, cylindrical, 5–10 μm wide hyphae. Clamp connections abundant in all tissues.
Habitat and distribution: Gregarious, growing with coniferous trees, often with Picea abies (spruce), Pinus sylvestris (Scots pine) or Abies cephalonica (Pantidou 1973, Pantidou 1990, Zervakis et al. 1998). Restricted to alpine habitats with calcareous soils. So far known from northeast Italy, France, Germany, Switzerland, Austria, Spain, and Greece (Bresadola 1927, Moser 1963, Kühner 1977, Bon 1991, Breitenbach & Kränzlin 1991, Moser & Jülich 1993, Ballarà 1997, Zervakis et al. 1998, Consiglio & Contu 2000, Karasch & Hahn 2009).
Materials examined: Giacomia mirabilis var. mirabilis. Italy, Friuli-Venezia Giulia, Prato Carnico (UD), Località Pradibosco, 1 200 m asl, forest of Fagus sylvatica, Picea abies and Abies alba, among the Petasites, at the edge of a ski run, 5 Sep. 2020, C. Angelini, ANGE1598 (TO); Udine, Villa Santina, Invillino, among grass at the border of a path, near Corylus avellana and Pinus sylvestris, 2 Nov. 2011, E. Campo (TUR-A 195638); Auronzo di Cadore (BL), Ponte Malon, under Picea abies, 5 Sep. 2020, E. Campo (TUR-A 209709); Trentino-Alto Adige, Bolzano, San Vigilio di Marebbe, Villa Romantica, in a grassy meadow, among apple and plum trees, 5 Sep. 2017, C. Feltrin (AMB:19594); Trento, Predaia, Sette Larici, forest of Pinus pinaster, 28 Sep. 2018, A. Marangon (AMB: 19595); Trento, Terzolas, Le Tovare, mixed forest with Abies alba and Fagus sylvatica, 6 Sep. 2007, G. Consiglio (AMB:18860); Braies (BZ), Braies Vecchia, 1 200 m asl, on the edge of a forest of Picea abies and Pinus sylvetris, 10 Oct. 2022, R.J. Ferrari (TO:AV20231010). Giacomia mirabilis f. nigrescens. Italy, Trentino-Alto Adige, Trento, Brez, Traversara, forest of Picea abies, 18 Oct. 1987, M. Donini (AMB:19596); Trento, Sarnonico, Regole di Malosco, forest of Picea abies, 17 Sep. 1994, G. Consiglio (AMB:19297).
Notes: Giacomia mirabilis was originally described as Tricholoma mirabile (Bresadola 1881), then combined in Melanoleuca (Singer 1942a) and Leucopaxillus (Konrad & Maublanc 1952), and finally separated from Leucopaxillus sensu lato in the monotypic genus Giacomia on the basis of molecular studies (Vizzini et al. 2012a). It is a rare striking European taxon that can be easily recognized in the field due to its dark brown pileus and stipe, a hairy pileus margin, a wrinkled stipe apex with a thin ring formed by an araneous partial veil, abundant polymorphic cheilocystidia, and basidiospores varying in size, shape and ornaments (Moser 1963, Kühner 1950, 1977, Bon 1978, 1987, 1991, Marchand 1986, Breitenbach & Kränzlin 1991, Moser & Jülich 1993, Consiglio & Contu 2000, Ludwig 2001a, b). When present, basidiospore ornaments consist in isolated hemispherical warts, like those of Melanoleuca cognata (Pegler & Young 1973). Giacomia mirabilis cannot be easily cultured in vitro (Moser 1963), suggesting an ectomycorrhizal lifestyle. Due to its peculiar features, Bon (1991) classified this species in the monospecific subsection Mirabilini (characterized by the presence of a stipe with an areaneous ring-like veil) of sect. Mirabiles (characterized by the presence of cheilocystidia) of Leucopaxillus. Leucopaxillus mirabilis var. nigrescens differs only in having a darker pileus (Bresadola 1927, Bon 1991, Fontenla & Para 2007). The ITS sequences obtained in the present work from samples of L. mirabilis var. mirabilis [ANGE1598 (TO), AMB:18860, AMB:19297, AMB:19595, TO:AV20231010, TUR-A 195638, TUR-A 209709] and L. mirabilis var. nigrescens (AMB: 19297, AMB:19596) (Supplementary Table S1) are 99.9 % identical, supporting the infraspecific rank of this taxon, which is here considered as a colour form. Phylogenetic results (Fig. 2) suggest that Giacomia mirabilis is not closely related to Leucopaxillus (Tricholomataceae, Tricholomatineae), but to the genus Melanoleuca (Pluteineae), forming a monophyletic clade with it. The great genetic distance and the important morphological differences between these genera discourage to merge them, and so the current taxonomical arrangement is not changed.
Giacomia mirabilis is a morphologically variable species: colours range from almost whitish to blackish brown; spores can be smooth to coarsely warty, globose to ellipsoid; cheilocystidia are also polymorphic, sometimes scarce or even completely absent. It displays a hoary belt at the apex of the stipe. The taste is mild or sometimes slightly bitterish after some time. It grows in calcareous soils under alpine conifers, especially Picea sp. and Pinus sylvestris. After checking multiple collections, the remnants of a cortiniform veil reported previously in the upper part of the stipe (Kühner 1977, Bon 1991, Vizzini et al. 2012a) were occasionally observed, and some specimens present a distinctly delimited 5–10 mm broad, white, and almost smooth zone (like that observed in Tricholoma ustaloides, Christensen & Heilmann-Clausen 2013, Halama et al. 2016). Leucopaxillus phaeopus (= L. amarus f. phaeopus) is said to differ from G. mirabilis because of its parietal and incrusting pigments (intracellular in Giacomia mirabilis), and the absence of a pseudoanular zone at the apex of the stipe (Bon 1987b, Ballarà 1997, Consiglio & Contu 2000, Lavorato & Contu 2001). Based on the original description and colour illustration (Favre & Poluzzi 1949), and the fact that all collections studied present both intracellular and parietal pigmentation, we agree with Ludwig (2001a) who considered L. phaeopus a synonym of G. mirabilis.
Giacomia sinensis J.Z. Xu, sp. nov. MycoBank MB 851153. Figs 6H, 14.
Etymology: sinensis (Latin) meaning ‘from China’, the country where the holotype collection was found.
Diagnosis: Giacomia sinensis differs from G. mirabilis by its brownish orange and creamy white pileus, a white stipe, a trichodermal pileipellis, and absence of cheilocystidia.
Type: China, Qinghai Prov., Haixi Mongolian and Tibetan Autonomous Prefecture, Halihatu National Forest Park, 37°2′9″ N, 98°39′39″ E, on the ground covered with moss under Picea crassifolia, 7 Aug. 2018, J.Z. Xu (holotype designated here HMJU:265).
Description: Pileus 26–32 mm diam, almost applanate, brownish orange (6C6) in the centre, sometimes slightly pinkish-shaded become paler towards the margin, margin light beige to creamy white (6A2, 5A2, 5A3), surface smooth, dry, with brownish orange (6C6) spots, margin involute, tomentose. Lamellae adnate with decurrent tooth, 0.15–0.25 mm wide, moderately crowded, white, with 1–2 tiers of lamellulae intercalated, edges entire, even. Stipe 36–44 × 5–6 mm wide, central, cylindrical, surface white, slightly fibrillose, with residual arachnoid cortina. Context white, odour and taste not distinct. Spore deposit white. Basidiospores (6.3–)6.9–7.3–7.7(−8.2) × (5.1–)5.4–5.7–6.1(−6.4) μm [40/2/2], Q = (1.14–)1.21–1.28–1.34(−1.41), V = (86.7–)107–127–147(−172) μm3, ellipsoid, surface verruculose, warts hemispherical, up to 0.2 μm high, amyloid, not or slightly cyanophilous. Basidia (25.5–)25.9–38.9(−44.1) × (4.4–)6.7–11.1(−11.3) μm, clavate, 4-sporic, sterigmata up to 2–5 μm long. Hymenial cystidia not observed. Hymenophoral trama subregular, parallel, colourless, hyphae cylindrical, 2–11 μm wide, thin-walled. Pileipellis an intricate trichoderm, composed of cylindrical hyphae, hyphae 2–12 μm wide, thin-walled. Stipitipellis a cutis, composed of parallel, cylindrical, repent, colourless hyphae, 2–13 μm wide, thin-walled. Clamp connections present.
Habitat and distribution: Single on the ground covered with mosses under Picea crassifolia. So far only known from China.
Additional materials examined: China, Gansu Prov., Zhangye City, Sunan Yugu Autonomous County, on soil or moss in mixed forests, 9 Aug. 2018, J.Z. Xu (HMJU:268).
Notes: Giacomia sinensis is a distinctive species found on the ground covered with mosses under Picea crassifolia (endemic tree species in China). It is mainly characterized by its tricholomatoid basidiomes with a brownish orange pileus with a light beige to creamy white margin, a white stipe with a residual arachnoid cortina, verruculose spores and pileipellis of an intricate trichoderm. Giacomia sinensis is phylogenetically related to G. mirabilis, which also presents a hairy pileus margin and basidiospores ornamented with hemispherical warts, but G. mirabilis has a larger pileus, abundant cheilocystidia and pileipellis as a cutis, while G. sinensis lacks cheilocystidia and has a pileipellis as an intricate trichoderm. Additionally, G. mirabilis produces a dark brown stipe, but the stipe of G. sinensis is white (see above).
Pluteaceae Kotl. & Pouzar, Česká Mykol. 26(4): 218. 1972.
Type: Pluteus Fr., Fl. Scan.: 338. 1836.
Representative genera: Pluteus (including Chamaeota) (Fig. 7N–O) and Volvopluteus (Fig. 8K–L).
Notes: Based on the present phylogenetic analysis (Fig. 2), this family is restricted here to the genera Pluteus and Volvopluteus. Pluteus also includes species with partial veil traditionally classified in the genus Chamaeota (Minnis et al. 2006, Justo et al. 2011a, b, Vizzini & Ercole 2011). The family is characterized by basidiomes pileostipitate, heterogeneous, pileus dry or viscid, lamellae free, stipe central, partial veil absent or present and forming an annulus, universal veil absent or present and then forming a persistent volva at stipe base; spore deposit pinkish to pinkish brown or reddish; basidiospores subglobose to ovoid, or ellipsoid, exceptionally triangular, smooth, colourless, usually exceeding 11 μm in length in the taxa with saccate volva (Volvopluteus), inamyloid, non-dextrinoid or slightly dextrinoid; hymenial cystidia normally present as cheilo- and pleurocystidia, apex sometimes digitate or with hooks; hymenophoral trama inverse; pileipellis an (ixo)cutis or (ixo)trichoderm, trichopalisade or hymeniderm; clamp connections predominantly absent, rarely present. Pluteus and Volvopluteus species grow mostly on wood or other decaying plant material (sawdust, straw, wood chips). Volvopluteus (type V. gloiocephalus) shows a unique combination of characters that separate it from Pluteus and Volvariella sensu stricto (Volvariellaceae), such as an average basidiospore length >11 μm, a pileipellis arranged as an ixocutis, composed of relatively thin hyphae (on average <15 μm wide), embedded in a very thick gelatinous matrix (Justo et al. 2011a, b, Giannoni et al. 2018, Montoya et al. 2021). The presence of a volva can be used as an additional character to separate Volvopluteus and Pluteus, although inconspicuous (but constant) ‘volva-like’ remnants have been described also for P. stephanobasis, a species of Pluteus sect. Pluteus (Singer 1958).
Volvariellaceae Vizzini, Consiglio & P. Alvarado, fam. nov. MycoBank MB 851154.
Diagnosis: Basidiomes pileostipitate, heterogeneous, pluteoid (lamellae free, context of the pileus discontinuous with the context of the stipe, stipe usually longer than pileus diameter), with a saccate universal veil (volva) at the stipe base, and a pink to pinkish brown spore deposit. Basidiospores usually < 11 μm in length, non-amyloid, non-dextrinoid, cyanophilous. Hymenial cystidia present in most species. Hymenophoral trama inverse. Pileipellis usually not strongly gelatinized. Species of Volvariella grow as soil or litter saprotrophs, directly on wood, or more rarely as parasites on basidiomes of other mushrooms, e.g., V. surrecta on basidiomes of Clitocybe nebularis.
Type: Volvariella Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 6: 119. 1898. [1899].
Representative genus: Volvariella (Fig. 8H–J).
Notes: Volvariella (typified with V. argentina = V. pusilla following Shaffer 1962) was traditionally considered a member of the Pluteaceae due to its heterogeneous basidiomes (the pileus separates easily from the stipe) with lamellae that are free from the stipe; pink or pinkish brown spore deposit; basidiospores smooth, inamyloid, non-dextrinoid, cyanophilous; and inverse hymenophoral trama (Kotlaba & Pouzar 1972, Singer 1986, Justo et al. 2011a), differing from the other genera of the family by the presence of a sac-like volva. The genus Volvariella includes about 50–57 species worldwide (Agerer 2018, He et al. 2019). Taxonomic reviews of Volvariella that include molecular data have been published for different areas of Eurasia (e.g., Vizzini et al. 2011b, Senthilarasu et al. 2012, Li et al. 2009, Xu et al. 2015, Malysheva et al. 2019, 2022, 2023, Kaygusuz et al. 2020, Niego et al. 2021, Kumla et al. 2022, Malysheva & Popov 2022, Haqnawaz et al. 2023), Africa (Daniëls et al. 2015), and South America (Menolli & Capelari 2008, with molecular data in Justo et al. 2011a). Apparently, the genus Volvariopsis (type V. volvacea) is phylogenetically distinct from Volvariella sensu stricto, but putatively intermediate lineages between them (Malysheva et al. 2019, Chattopadhyay et al. 2022, Kumla et al. 2022) need to be analyzed to confirm its most suitable taxonomic status. The volvariella-like habit and inverse hymenophoral trama seem to have arisen at least twice independently within the Pluteineae, viz. in Volvopluteus and Volvariella.
Tricholomatineae Aime et al., Biol. J. Linn. Soc. 117(1): 27. 2016.
Synonym: Tricholomatales Kühner, Bull. Mens. Soc. Linn. Lyon 49(Num. Spéc.): 677. 1980.
Type: Tricholoma (Fr.) Staude, Schwämme Mitteldeutschl. 1: xxviii, 125. 1857, nom. cons., see Art. 14 (Shenzhen).
Representative families: Biannulariaceae, Callistosporiaceae, Clitocybaceae, Entolomataceae, Fayodiaceae, Lyophyllaceae (including Asproinocybaceae), Macrocystidiaceae, Omphalinaceae, Paralepistaceae, Pseudoclitocybaceae, Pseudoomphalinaceae, and Tricholomataceae.
Orphaned genera: Hertzogia, Paralepistopsis.
Notes: Suborder Tricholomatineae is characterized by basidiomes predominantly agaricoid (pileostipitate), mostly fleshy, homogeneous (context of the pileus continuous with the context of the stipe), mostly with adnate, subdecurrent or decurrent lamellae. Hyphal system monomitic or rarely sarcodimitic; clamp connections present or absent; pigments often encrusting. Basidiospores colourless or with pink tinges and then often angular in at least one plane or longitudinally striate, smooth, or verrucose, without a germ pore, thin-walled, immediately or latently amyloid or inamyloid, cyanophilous or acyanophilous. Basidia mostly 4-sporic, usually ballistosporic, with or without siderophilic granulations. Pileipellis usually a cutis or trichoderm (rarely hymenidermic in a few Entolomataceae and Lyophyllaceae). Protective veils rarely present (monovelangiocarpic development with partial veil). Tricholomatineae contains mushrooms with a wide spectrum of trophic roles ranging from ectomycorrhizal symbionts (Sánchez-García & Matheny 2017), wood and soil saprobes, necrotrophism (Collybia), mycoparasites (some Entolomataceae), and obligate insect-associated species (Termitomyces and allied genera of the termitomycetoid clade; van de Peppel et al. 2021, 2022). Asexual morph sometimes present on basidiome surface (some Lyophyllaceae and Dendrocollybia) This suborder corresponds well to the Tricholomatoid clade of Matheny et al. (2006), except for the exclusion of Mycenaceae and inclusion of Infundibulicybe, as well as to the Tricholomatoid clade of Binder et al. (2010). Tricholomatineae was established by Dentinger et al. (2016) (69 % BP) and a well-supported clade corresponding to the Tricholomatineae was recovered by Varga et al. (2019) (99 % BP), Olariaga et al. (2020) (1 PP, 54 % BP), Li et al. (2021) (98 % BP) and in the present work (1 PP).
Biannulariaceae Jülich, Biblioth. Mycol. 85: 356. 1982. [1981].
Synonyms: Tricholomataceae tribe Biannularieae Singer ex Bas, Persoonia 14: 235. 1990.
Catathelasmataceae Wasser, Agarikovye griby SSSR (Kiev): 29. 1985.
Type: Catathelasma Lovejoy, Bot. Gaz. 50: 383. 1910.
Representative genera: Bonomyces, Catathelasma and Cleistocybe.
Notes: The molecular circumscription of the limits of the family was first carried out by Vizzini et al. (2020a). It is characterized by basidiomes with tricholomatoid, clitocyboid or pleurotoid habit; lamellae adnate, adnexed, sinuate, emarginate to decurrent; partial veil present (simple or double) or inconsistent and reduced to a granular, pseudoanular zone at the apex of the stipe; odour usually farinaceous; spore deposit white; basidiospores ellipsoid to fusoid, cylindrical, colourless, smooth, inamyloid or amyloid, acyanophilous or cyanophilous; basidia lacking siderophilous inner bodies; cheilocystidia present or absent, pleurocystidia absent or if present then only as pseudocystidia; hymenophoral trama regular to bilateral becoming regular, pileipellis a cutis, an ixocutis or a cutis becoming a trichoderm, context (pileitrama and stipititrama) formed by densely arranged slender hyphae which give rise to a dry and fibrous consistency, clamp connections present. Specimens grow on soil or rotten wood, saprotrophic or ectomycorrhizal in conifer forests. They can be found in Europe, Asia, North and Central America. Bonomyces was recently monographed by Alvarado et al. (2018b), Cleistocybe by Ammirati et al. (2007) and Wu et al. (2018), and Catathelasma by Vizzini et al. (2020a). Recently, the newly discovered species Bonomyces squamulosus, B. pseudoarnoldii and Cleistocybe vernaloides have been described from China (Wu et al. 2018, He & Yang 2022, Mao et al. 2022).
Callistosporiaceae Vizzini et al., Fungal Diversity 101: 223. 2020.
Type: Callistosporium Singer, Mycologia 36: 363. 1944.
Representative genera: Anupama, Callistosporium (including Pleurocollybia), Guyanagarika, Macrocybe, Pseudolaccaria, and Xerophorus.
Notes: The molecular circumscription of the limits of the family was first carried out by Vizzini et al. (2020a). It is distinguished by basidiomes with tricholomatoid, collybioid or pleurotoid habit, veils absent; lamellae adnate, adnexed, sinuate, emarginate to decurrent; spore deposit white, basidiospores ellipsoid, colourless, smooth, inamyloid or amyloid (Pseudolaccaria), cyanophilous or acyanophilous; basidia lacking siderophilous inner bodies; cheilocystidia present or absent, pleurocystidia absent or if present then only as pseudocystidia; hymenophoral trama regular to slightly bilateral becoming regular, pileipellis a cutis, ixocutis or cutis becoming a trichoderm, clamp connections usually absent, very rarely present (in Xerophorus, partim; in Callistosporium imbricatum at the base of basidia). Specimens are found growing on soil or rotten wood, saprotrophic or ectomycorrhizal (Sánchez-García et al. 2016, Raj et al. 2019, Vizzini et al. 2020a).
Clitocybaceae Vizzini et al., Index Fungorum 462: 1. 2020.
Synonyms: Agaricinées tribe Clitocybeae Fayod ex Lotsy, Vorträge Bot. St. Gesch. 1: 711. 1907.
Tricholomataceae subfamily Clitocyboideae (Fayod) M. Bon, Doc. Mycol. 78: 37. 1990.
Clitocybaceae Roze [as ‘Clitocybées’], Bull. Soc. Bot. France, Act. Bot. 23: 112. 1876 [nom. inval., Art. 32.1I, see Art. 18.4 (Shenzhen)], van Overeem, Bull. Jard. Bot. Buitenzorg, ser. 3, 9: 21. 1927 [correctly spelled but nom. inval., Art. 32.1(c)].
Lepistaceae Locq., Mycol. Gén. Struct. (Paris): 139. 1984, nom. inval., Art. 39.1 (Shenzhen).
Lepistamycetidae Locq., Mycol. Gén. Struct. (Paris): 97. 1984, nom. inval., Art. 39.1 (Shenzhen).
Type: Clitocybe (Fr.) Staude, Schwämme Mitteldeutschl.: xxviii, 122. 1857.
Representative genera: Clitocybe (Fig. 6C), Collybia, Lepista (= Rhodopaxillus) (Figs 6O, 7A), Singerocybe, Dendrocollybia (this latter according to Mou & Bau 2021, He & Yang 2022 and He et al. 2023 on multigene analyses; Sánchez-García & Matheny 2017 and Sánchez-García et al. 2020 on ribosomal DNA markers), and Pseudolyophyllum (He et al. 2023). Leucocalocybe was first considered an independent genus inside Clitocybaceae (Yu et al. 2011, Sánchez-García et al. 2020), but recently downgraded to a subgenus of Collybia sensu lato (He et al. 2023). Based only on ribosomal DNA markers, Lepistella (Varga et al. 2019), and Paralepistopsis (Varga 2019, Sánchez-García et al. 2020 but see below) might belong here.
Notes: The family Clitocybaceae was first referred to as the “Clitocybées” by Roze (1876), including 24 species of the genus Clitocybe; however, this name was not validly published since it contains a French termination instead of a Latin termination (Shenzhen, 2018: Art. 18.4). In 1927, Van Overeem (1927) published the name “Clitocybaceae” as a nomen nudum (Art. 38.1). This name was informally used for this clade in some works (Cooper 2016, Kalichman et al. 2020, Kibby 2020), but it was only recently validly published by Vizzini et al. (2020b). It corresponds to “Agaricinés” tribe Clitocybeae (Fayod 1889, originally including Clitocybe, Lepista, Nyctalis and Laccaria) and to Tricholomataceae subfamily Clitocyboideae (Bon 1990b, 1997, including Clitocybe and Armillaria). It can be loosely circumscribed because of the clitocyboid, lepistoid or collybioid habit, veils absent (gymnocarpic development), lamellae adnexed, sinuate, emarginate to decurrent, removable (separable) or not from the pileus context, spore deposit white, cream to pale pink or pinkish buff, basidiospores colourless, smooth to warty, verrucose (verruculose to spiny) inamyloid, cyanophilous or acyanophilous, basidia clavate, usually 4-sporic, not hygrophoroid, lacking siderophilous inner bodies, hymenophoral trama regular to subregular composed of parallel cylindrical hyphae, cheilocystidia absent or present, thin-walled, pleurocystidia absent, pileipellis a cutis or trichoderm, with (Singerocybe) or without swollen elements (vesicles), clamp connections present or absent. Stipe sometimes with conidiogenous branches (i.e., Tilachlidiopsis asexual morph in Dendrocollybia). Presence of muscarine (most species of Collybia sensu lato, He et al. 2023). Habitat on soil, litter, or associated with dead wood (Lepistella) or with basidiomes of other species (mycosaprobic) and then usually forming small sclerotia (Collybia, Dendrocollybia); saprotrophic or mycosaprobic/mycoparasitic.
Entolomataceae Kotl. & Pouzar, Česká Mykol. 26(4): 218. 1972.
Type: Entoloma (Fr.) P. Kumm., Führ. Pilzk. (Zerbst): 23. 1871.
Representative genera: Calliderma, Clitocella, Clitopiloides, Clitopilopsis, Clitopilus, Entocybe, Entoloma (including Richoniella and Rhodocybella), Fibropilus, Lulesia, Rhodocybe, and Rhodophana.
Notes: The molecular circumscription of the limits of the family and intergeneric relationships were studied by Co-David et al. (2009), Baroni et al. (2011, 2020), Baroni & Matheny (2011), Kinoshita et al. (2012), and Kluting et al. (2014). Basidiomes are pileostipitate with a very diverse habit: mycenoid, clitocyboid, collybioid, omphalinoid, tricholomatoid, or rarely secotioid; lamellae adnate, adnexed, emarginate, occasionally almost free, subdecurrent, decurrent; stipe usually central and well-developed, reduced or absent; veils absent; spore deposit pinkish or rarely greyish; basidiospores angular, rhomboid, ribbed with longitudinal ribs, with pustules, bumps or verrucae, rarely reticulate, thin- to thick-walled, inamyloid, non-dextrinoid, usually cyanophilous, colourless on light microscopy, typically binucleate; basidia not siderophilous or with micro type siderophilic granules; hymenial cystidia absent, or as cheilocystidia, occasionally as pleurocystidia or pseudocystidia; hymenophoral trama regular to subregular, pileipellis a cutis or a trichoderm, sometimes a hymeniderm, colourless or with wall, encrusting or cytoplasmatic pigment, clamp connections absent or present. The family encompasses mainly saprotrophic species; mycoparasites are present in Clitopilus, Entoloma, and Rhodophana (Noordeloos 1988, 1993, Czederpiltz et al. 2001, Koch & Herr 2021); the ECM taxa are restricted to the Rhodopolioid clade of Entoloma (Sánchez-García & Matheny 2017, Brandrud et al. 2018) and often associate with Quercus, Salix, Alnus, Populus, and exceptionally with Pyrus, usually with an incomplete Hartig net (Linkins & Antibus 1982, Loree et al. 1989, Læssøe & Rosendahl 1994, Agerer 1997, 1998, Smith et al. 2007, Shishikura et al. 2021).
The genera recognized in Entolomataceae by Kluting et al. (2014) and Baroni et al. (2020) were recently supplemented with Lulesia by Varga et al. (2019) based on a single LSU sequence. This genus, typified with L. densifolia was placed by Singer (1970, 1986) in subtribe Omphalineae (tribe Clitocybeae, family Tricholomataceae). Lulesia is apparently different from the rest of the genera of the Entolomataceae due to its withish to cream lamellae without pink tinges when mature, and whitish spore deposit, but at least two of the three known species have basidiospores appearing slightly angular and nodulose on light microscopy (Singer 1970, 1986, Lechner et al. 2006). Macrocystidia and Rhodotus had been classified within or close to Entolomataceae on account of their pink spores (e.g., Kühner 1980, Romagnesi 1992), but molecular phylogenetic studies place them outside this family (Moncalvo et al. 2000, 2002, Walther et al. 2005, Matheny et al. 2006, Garnica et al. 2007, Petersen & Hughes 2010, Varga et al. 2019, Sánchez-García et al. 2020).
Fayodiaceae Jülich, Biblioth. Mycol. 85: 367. 1982. [1981].
Synonyms: Marasmiaceae Tribu Fayodieae Kühner, Bull. Mens. Soc. linn. Lyon 49(3): 770. 1980.
Tricholomataceae, Subfamily Clitocyboideae, tribe Omphalineae, subtribe Heterosporulae Bon, Doc. Mycol. 26 (102): 19. 1996.
Fayodiamycetidae Locq., Mycol. Gén. Struct. (Paris): 96. 1984, nom. inval., Art. 39.1 (Shenzhen).
Fayodiales Locq., Mycol. Gén. Struct. (Paris): 126. 1984, nom. inval., Art. 39.1 (Shenzhen).
Type: Fayodia Kühner, Bull. Soc. linn. Lyon 9: 68. 1930.
Representative genera: Caulorhiza, Conchomyces, Fayodia (Fig. 6E), Gamundia (Fig. 6F), Myxomphalia.
Notes: The family Fayodiaceae was established by Jülich (1981) based on morphological features: omphalinoid basidiomes (rarely collybioid or mycenoid); basidiospores colourless, globose or ellipsoid, thin-walled, and smooth, or somewhat thick-walled and with a complex wall structure consisting of a spiny endosporium overlain by a smooth episporium, amyloid in most taxa; 2–4-sporic basidia, thin-walled hymenial cystidia; hymenophoral trama regular, pileipellis of smooth repent, rarely diverticulate hyphae, clamp connections present; terricolous, saprotrophic. Jülich recognized only Fayodia and Myxomphalia within the family and suggested (following previous indications by Smith & Reid 1962) a possible affinity with the Cribbeaceae, where the same spore wall structure is present. Cribbea species were found to belong to the family Physalacriaceae (Marasmiineae) with affinities to Xerula and Oudemansiella by Lebel & Catcheside (2009). Before the publication of the family Fayodiaceae, Kühner (1980) had placed Fayodia and allied genera in tribu Fayodieae of Marasmiaceae together with Delicatula, Clitocybula, Hydropus and Megacollybia, with affinity to Collybia. Other authors considered Fayodiaceae a synonym of Tricholomataceae sensu lato (Pouzar 1985, Korf 1988), a synonym of tribe Myceneae of the Tricholomataceae (Kuyper 1995b, c, Singer 1986), or an independent tribe Fayodieae of subfamily Clitocyboideae inside Tricholomataceae (Bon 1997). The first pioneering molecular works focusing on the Agaricales (mainly based on nrLSU sequences alone, i.e., Moncalvo et al. 2002), found an incertae sedis /fayodioid clade consisting of Gamundia leucophylla, Caulorhiza hygrophoroides, Conchomyces bursaeformis, Myxomphalia maura, and Fayodia gracilipes. In a combined analysis of LSU and RPB1 sequences of a smaller dataset of Agaricales, Garnica et al. (2007) found a relationship between F. gracilipes, Leucocortinarius bulbiger and Infundibulicybe geotropa. In the present analysis (Fig. 3) Fayodia bisphaerigera and Gamundia striatula formed an independent clade within suborder Tricholomatineae. Accordingly, the family name Fayodiaceae is here applied for this clade.
The genera Fayodia, Myxomphalia and Gamundia (= Heterosporula; = Stachyomphalina) were sometimes considered subgenera of a single genus Fayodia sensu lato (e.g., Singer 1986) but important authors as Bigelow (1979), Kühner (1980), Bon (1997), Kuyper (1995b, c), or Antonín & Noordeloos (2004) separated them. Fayodia (typified by F. bisphaerigera) is characterized by a very distinct, two-layered basidiospore wall consisting of a non-amyloid, echinulate-verruculose epispore and a smooth amyloid perispore, 2-sporic basidia, and a dry, non-gelatinized pilei- and stipitipellis (Kühner 1930, 1938a, 1973, 1980, Besson 1969, Pegler & Young 1971, Bigelow 1979, 1983, Emmett 1993, Kuyper 1995b, Antonín 2004, Antonín & Noordeloos 2004, Clémençon 2004). Gamundia (typified by G. pseudoclusilis = G. striatula) is distinguished by its verruculose-echinulate, thin-walled, non-amyloid basidiospores and gelatinized pileipellis (Bigelow 1979, 1983, Kuyper 1995c, Antonín 2004, Antonín & Noordeloos 2004, Musumeci et al. 2010). Bon (1996) accommodated Gamundia in the subtribe Heterosporulae of tribe Omphalineae within subfamily Clitocyboideae, Tricholomataceae. Finally, Myxomphalia (typified by M. maura) has thick-walled, smooth to minutely verruculose (when observed under SEM) amyloid spores and gelatinized pilei- and stipitipellis (Bigelow 1979, Weholt 1988, Antonín 1999, Antonín & Noordeloos 2004).
Lyophyllaceae Jülich, Biblioth. Mycol. 85: 378. 1982. [1981].
Synonyms: Tricholomataceae Tribe Lyophylleae Kühner, Bull. Mens. Soc. Linn. Lyon 7(7): 209. 1938.
Tricholomataceae subfamily Lyophylloideae Kühner ex Bon, Doc. Mycol. 3(12): 7. 1974.
Asproinocybaceae T. Bau & G.F. Mou, J. Fungi 7(12, no. 1086): 7. 2021.
Type: Lyophyllum P. Karst., Acta Soc. Fauna Flora fenn. 2(no. 1): 29. 1881. [1881-1885].
Representative genera: Arthromyces, Asproinocybe, Asterophora, Atractosporocybe, Australocybe, Blastosporella, Calocybe (including Rugosomyces), Calocybella, Clitolyophyllum, Fibulochlamys, Gerhardtia, Hypsizygus, Leucocybe, Lyophyllopsis, Lyophyllum (Fig. 7C), Myochromella, Nigrocarnea, Omphaliaster, Ossicaulis, Phaeotephrocybe, Praearthromyces, Sagaranella, Sphagnurus, Termitomyces, Tephrocybe, Tephrocybella, Tephroderma, Trichocybe (Fig. 8G), Tricholomella, Tricholosporum, and Tricholyophyllum.
Notes: The family Lyophyllaceae is characterized by basidiomes with an extremely diverse habit: mycenoid, collybioid, clitocyboid or tricholomatoid; hymenophore lamellate, lamellae attached, adnate, emarginate or exceptionally almost free; partial veil absent or rarely present, general veil exceptionally present; stipe central to eccentric, cylindrical to distinctly rooting (pseudorhiza, termitomycetoid clade sensu van de Peppel et al. 2021, 2022); spore deposit white to pale cream; hyphal system monomitic, hyphae with or without clamp connections; basidia usually with siderophilous granules (macro- and oligo-type, Clémençon 1978, 1986a, b, 2004) or without them; basidiospores colourless, with distinct apiculus, predominantly smooth, but also verrucose, warty, undulate, echinulate, tuberculate to stellate (Asproinocybe), or cruciform to stauriform (Tricholosporum), thin- to slightly thick-walled, neither amyloid nor dextrinoid, cyanophilous or not; no other part of the basidiome is amyloid, dextrinoid or cyanophilous; cystidia absent or present as cheilocystidia, rarely as pleurocystidia; hymenophoral trama regular to subregular; pileipellis ranging from a cutis, trichoderm, celluloderm, sometimes a conioderm of chlamydospores (Asterophora) or arthroconidia (Arthromyces, Blastosporella, Nigrocarnea, Praearthromyces). Lyophyllaceae display broad ecological strategies with frequent transitions between them, ranging from terricolous saprotrophs (most of the species), plant decayers (Hypsizygus, Ossicaulis), parasite species (Asterophora, Sphagnurus), symbiotic species of insects (termitomycetoid clade, Termitomyces, insect-associated mutualistic genus, or Arthromyces and Blastosporella, insect-faecal associated genera) to ectomycorrhizal species with angiospermous and gymnospermous trees (Lyophyllum decastes species complex, Lyophyllum shimeji and L. decastes) (Singer 1986, Agerer & Beenken 1998, Yamada et al. 2001a, b, Tedersoo et al. 2010, Larsson & Sundberg, 2011, Hofstetter et al. 2014, van de Peppel 2021, 2022). The presence of an asexual morph phase seems to be quite common in the family Lyophyllaceae. The genera Calocybe, Fibulochlamys, Gerhardtia, Hypsizygus, Ossicaulis, Sagaranella, Sphagnurus, and Termitomyces have asexual morph life cycles at the vegetative mycelium stage, viz. arthroconidia production on hyphal strands at the stipe base, and mycelium with both schizolytic and rhexolytic secession (Clémençon 1968, Brunner & Miller 1988, Nagasawa & Arita 1988, Walther et al. 2005, Madrid et al. 2010, Endo et al. 2019, 2022). In addition, Asterophora, Arthromyces, Blastosporella, Nigrocarnea and Praearthromyces produce arthroconidia or chlamydospores on the basidiomes (Thompson 1936, Corner 1966, Singer 1986, Redhead & Seifert 2001, Baroni et al. 2007, van de Peppel 2021, 2022).
Historically, the tribe Lyophylleae was circumscribed by Kühner (1938b) based on the shared synapomorphic character of basidia with inner siderophilous (carminophilous) granules upon acetate carmine staining. He included in the tribe the genera Calocybe, Lyophyllum (type), Nyctalis (current name Asterophora) and Tephrophana (now Tephrocybe). Later, Bon (1974) upgraded the tribe to subfamily rank as Lyophylloideae, and finally Jülich (1981) established the new family Lyophyllaceae. Molecular phylogenetic studies provided support to the monophyletic status of the traditional concept of Lyophyllaceae (e.g., Hofstetter et al. 2002, Moncalvo et al. 2002 as Lyophylleae group; Matheny et al. 2006, Baroni et al. 2011, Sánchez-García et al. 2014).
In the present analysis (Fig. 3), Aspropaxillaceae is part of the Lyophyllaceae, and a sister relationship (0.99 PP, 47 % BP) between Lyophyllaceae sensu lato and Entolomataceae was found, as previously highlighted by Hofstetter et al. (2002), Matheny et al. (2006), Sánchez-García et al. (2016, 2020), Sánchez-García & Matheny (2017), Raj et al. (2019), and He & Yang (2022). Whereas Baroni et al. (2011) recovered a sister relationship of Lyophyllaceae with Entoloma sensu lato and a paraphyletic Entolomataceae, Sánchez-García et al. (2014) found that Lyophyllaceae was basal to Tricholomataceae sensu stricto and Entolomataceae, and Hofstetter et al. (2014) retrieved Entolomataceae at the base of a clade formed by Lyophyllaceae and Tricholomataceae. Morphological affinities between the families Lyophyllaceae and Entolomataceae were already pointed out by Kühner & Romagnesi (1953) and Clémençon (1978). They share the same diverse habit and pileipellis structure of the basidiomes, the non-free lamellae, the regular to subregular hymenophoral trama, often cyanophylous basidiospores, and basidia often containing siderophilous granules (macro-type in Lyophyllaceae, micro- and crypto-type in Entolomataceae; Clémençon 1978, 2004). Species with collybioid habit and basidiospores ± angular in polar view are present both in Lyophyllaceae (Calocybella and Gerhardtia, white-spored) and Entolomataceae (Rhodocybe sensu lato, pinkspored) (Kluting et al. 2014, Vizzini et al. 2017, Endo et al. 2019, 2022). The macro-type of siderophilous granulation seems to be restricted to Lyophyllaceae sensu stricto (= sensu Matheny et al. 2006, excluding the hemilyophylloid clade). It was found in three closely related species of Rhodocybe (Entolomataceae) and this lead Clémençon (1968) to combine two of them into Lyophyllum (L. suburens and L. leucopaxilloides), a proposal first rejected by Singer (1975) and only partially accepted later by him (Singer 1986), placing them in Lyophyllum subg. Lyophyllopsis Gerhardt with great uncertainty. The two species were finally transferred to the genus Gerhardtia by Contu & Consiglio (2004).
Macrocystidiaceae Kühner, Bull. Mens. Soc. Linn. Lyon 48(3): 172. 1979.
Type: Macrocystidia Joss., Bull. Trimestriel Soc. Mycol. France 49: 373, 376. 1934 [1933].
Synonym: Macrocystis R. Heim, Encyclop. Mycol., 1 Le Genre Inocybe (Paris): 71 .1931., nom. illegit., Art. 53.1, non Macrocystis C. Agardh 1820 (Algae)].
Representative genera: Macrocystidia (Fig. 7D, E) and Pseudoclitopilus.
Notes: Macrocystidia, typified by M. cucumis, is characterized by gymnocarpic mycenoid to collybioid basidiomes, almost free lamellae, a usually strong rancid-farinaceous to fish-like odour, brownish pink spore deposit, regular to subregular hymenophoral trama, presence of lageniform to fusiform, thin-walled cystidia (as hymenial cystidia, pileo- and caulocystidia), basidiospores ellipsoid, thick-walled (multilayered), cyanophilous, inamyloid and mononucleate, pileipellis arranged as a cutis, presence of clamp connections; presence of conidiogenous hyphae in the mycelium, mostly fragmenting into up to eight conidia; terricolous, saprotrophic (Heim 1931, Josserand 1933, Capellano 1976, Kühner 1979a, 1980, Noordeloos 1995a, Walther et al. 2005, Knudsen 2012, Læssøe & Petersen 2019).
The present analysis (Fig. 3) supports that Macrocystidiaceae belongs to an independent evolutive lineage within Tricholomatineae, where it is significantly related to Pseudoclitopilus (1.00 BPP). Pseudoclitopilus, typified by P. rhodoleucus, is a genus recently segregated from Leucopaxillus sensu lato (Vizzini et al. 2012a), characterized by its agaricoid basidiomes resembling Clitopilus prunulus or Hygrophorus karstenii due to their strongly decurrent lamellae with pinkish tinges (especially in young stages), and a white, somewhat hygrophanous pileus, veils absent, white spore deposit, basidiospores with amyloid warts, cystidia and pseudocystidia absent, pileipellis a cutis of repent to interwoven, cylindrical hyphae, clamp connections present, and no sarcodimitic texture in any part of the basidioma. Specimens of Pseudoclitopilus occur on the ground, never on wood (Szemere 1966, Pegler & Young 1973, Trimbach 1978, Verde & Calonge 1980, Fanelli 1984, Bon 1991, Watling & Turnbull 1998, Winterhoff 1998, Consiglio & Contu 2000, Anon. 2001, Markones 2003, Christensen 2008, 2012, Vizzini et al. 2012a). Two species are known so far, P. rhodoleucus and P. salmonifolius, both very rare. Pseudoclitopilus salmonifolius differs mainly in having shorter basidiospores (4.5–6 µm vs. 6–8 µm in P. rhodoleucus) (Moser 1979, Bidaud 1993). All previous molecular works placed Pseudoclitopilus in an uncertain position (Sánchez-García et al. 2014, 2016, 2020, Sánchez-García & Matheny 2017, Alvarado et al. 2018a, b, He et al. 2019, 2023, Raj et al. 2019, Vizzini et al. 2020a, He & Yang 2022). While Macrocystidia and Pseudoclitopilus share a gymnocarpic agaricoid basidiome, a cutis-like pileipellis, presence of clamp connections, and a terricolous growth, no other synapomorphic trait could be found. As a result, the inclusion of Pseudoclitopilus in Macrocystidiaceae needs to be confirmed with additional data from other species.
Omphalinaceae Vizzini et al., Index Fungorum 462: 1. 2020. Fig. 15.
Fig. 15.
Omphalinaceae. A. Omphalina pyxidata basidiomes (AMB:19294). B–D. Pileipellis (B. Infundibulicybe gibba AMB:19313; C. I. geotropa AMB:18861; D. O. pyxidata AMB:19295). E. Hymenium and subhymenium (I. gibba AMB:19313). F, G. Hymenium (F. I. geotropa AMB:18861; G. O. pyxidata AMB:19295). H. Stipitipellis (I. geotropa AMB:18861). I. Stipitipellis and caulocystidia (O. pyxidata AMB:19295). J, K. Hymenophoral trama (J. O. pyxidata AMB:19295; K. I. gibba AMB:19313). L. Elements of the hymenophoral trama (I. gibba AMB:19313). M–R. Basidiospores (M, P. O. pyxidata AMB:19295; N, Q. I. gibba AMB:19313; O, R. I. geotropa AMB:18861). Mounting media were Congo Red in ammonia (B–D, F–O), and Cotton Blue (E, P–R). Scale bars: B–D, H–K = 30 μm, E–G, L–R = 10 μm. Photographs A by G. Consiglio, B–R by M. Marchetti.
Synonyms: Tricholomataceae tribe Clitocybeae, subtribe Omphalininae Singer, Fl. Neotrop. Monogr. 3: 5. 1970. [as ‘Omphalinae’]
Tricholomataceae tribe Omphalineae (Singer) Bon, Doc. Mycol. 24(93): 40. 1995.
Type: Omphalina Quél., Enchir. Fung.: 42. 1886.
Representative genera: Infundibulicybe (Figs 6N, 15B, C, E, F, H, K, L, N, O, Q, R) and Omphalina (Figs 7I, 15A, D, G, I, J, M, P).
Notes: Omphalinaceae is characterized by basidiomes with an omphalinoid or clitocyboid habit, veils absent (gymnocarpic development), pileus usually depressed at centre, dry, often with ochre, reddish brown, rusty, vinaceous brown or orangish brown tinges; lamellae decurrent; spore deposit white to cream; basidiospores colourless, smooth-walled (except I. trachyspora), inamyloid, acyanophilous (cyanophobic wall but cyanophilic cytoplasm); basidia clavate, usually 4-sporic, not hygrophoroid, lacking siderophilous inner bodies; hymenophoral trama subregular or irregular of intertwined, interwoven hyphae; hymenial cystidia usually absent, rarely present, thin-walled; pileipellis arranged as a cutis to a trichoderm; pigments intracellular and wall encrusting; clamp connections present; habitat on soil, litter, or associated with bryophites (bryophilous); usually saprotrophic but, recently, Zhang et al. (2022) described O. licheniformis from China, the first example of a lichenized omphalinoid fungus outside genus Lichenomphalia (Lichenomphaliaceae, Hygrophorineae); Northern Hemisphere, mostly temperate to boreal.
The family is currently thought to include the type genus Omphalina, as well as Infundibulicybe (Vizzini et al. 2020b, Zhang et al. 2022). Infundibulicybe differs from Omphalina mainly because of its larger basidiomes (pileus 20–200 mm diam, stipe 5–20 mm wide), thicker context, pileus non-hygrophanous, not translucently striate, hymenophoral trama regular in young stages, subirregular to irregular only in aged basidiomes (Harmaja 2003, Vizzini et al. 2011c), and not strictly moss-associated (non-bryophilous). Historically, Singer (1943) and Bigelow (1974, 1985) were the first to recognize morphological affinities between Clitocybe sect. Pyxidatae and sect. Infundibuliformes. They believed sect. Pyxidatae derived from section Infundibuliformes due to the shared presence of abundant brownish encrusting pigment. Phylogenetic evidence of a shared monophyletic origin between Infundibulicybe and Omphalina was first obtained by Moncalvo et al. (2000, 2002), and then confirmed by Vizzini et al. (2011a, 2012a, b), Vizzini & Ercole 2012, Lodge et al. 2014, Varga et al. (2019), and Sánchez-García et al. (2016, 2017, 2020). The lineage was then formally accommodated in the family Omphalinaceae by Vizzini et al. (2020b), and recent phylogenetic analyses by He & Yang (2022) and Zhang et al. (2022) also support this decision.
Omphalina Quél., Enchir. fung. (Paris): 42. 1886.
Synonyms: Agaricus ** Pyxidatae Fr., Epicr. syst. mycol. (Upsaliae): 122. 1838. [1836–1838].
Clitocybe subg. Infundibuliformes (Fr.) Bigelow, sect. Pyxidatae (Fr.) Bigelow, Bull. Soc. Linn Lyon (Num. Spéc.): 39. 1974.
Omphalina sect. Pyxidatae (Fr.) Bon, Doc. Mycol. 22(86): 40. 1992. Omphalia (Fr.) Gray, Nat. Arr. Brit. Pl. (London) 1: 611. 1821 [nom. illegit., Art. 53.2; non Omphalea L. 1759, nom. cons. (Euphorbiaceae)]
Type: O. pyxidata (Bull.) Quél. [lectotype, proposed by Redhead (1993), recommended by Gams (1995), accepted, and published in Greuter et al. (2000)].
Notes: Omphalina was originally upgraded to genus by Quélet (1886) from a previous undetermined rank in Fries (1838), although no type was indicated. Redhead (1993) proposed the lectotype O. pyxidata (accepted in Greuter et al. 2000, p. 192). Recent molecular analyses showed that the classical concept of Omphalina, mainly based on morphology (Bigelow 1970, Lamoure 1974, 1975, Clémençon 1982, Norvell et al. 1994, Kuyper 1995d, Bon 1997) includes both non-lichenized and lichenized omphalinoid genera phylogenetically nested inside different clades of the order Agaricales (Moncalvo et al. 2002, Redhead et al. 2002a, Matheny et al. 2006, Lawrey et al. 2009, Lodge et al. 2014, Varga et al. 2019, Chalange & Moreau 2023), as well as Hymenochaetales (Redhead et al. 2002b, Larsson et al. 2006, Korotkin et al. 2018, Olariaga et al. 2020). The non-lichenized omphalinoid bryophilous species of Omphalina with a greyish, blackish, bluish, brown-grey or whitish pileus and stipe, as well as concolorous hymenia were transferred to an emended genus Arrhenia inside suborder Hygrophorineae (Redhead et al. 2002a, Barrasa & Rico 2003, Barrasa et al. 2003, Lodge et al. 2014, Blanco-Dios 2019). Omphalina was restricted to the usually bryophilous species phylogenetically related to O. pyxidata (the conserved lectotype of Omphalina, Redhead 1993, Moncalvo et al. 2000, 2002, Redhead et al. 2002a), which nest inside suborder Tricholomatineae. Species in this lineage typically display reddish brown, rusty, vinaceous brown or orange brown tinges on the pileus and stipe, a paler, non-concolorous hymenophore, and no hymenial cystidia (Redhead et al. 2002a). This reduced concept of Omphalina contains more or less the same species as the Omphalina complex/stirp as delimited by Lamoure (1974, 1982), Clitocybe sect. Pyxidatae by Bigelow (1974, 1985), as well as Omphalina sect. Pyxidatae by Bon (1997). Lamoure (1974) recognized six different taxa based on morphological data and compatibility tests. Micromorphological features, such as non-amyloid spores, subregular to irregular hymenophoral trama and pileipellis with incrusting pigment, are shared by all members of the genus. Vizzini et al. (2012b) described a molecularly confirmed collection of O. pyxidata with well-developed hymenial cystidia as well as pileo-, and caulocystidia.
Infundibulicybe Harmaja, Ann. Bot. Fenn., 40(3): 215. 2003.
Type: I. gibba (Pers.) Harmaja, Ann. Bot. Fenn., 40(3): 217. 2003.
Notes: Infundibulicybe is one of the multiple genera proposed to accommodate deviant lineages originally classified within Clitocybe sensu lato, now limited to the species phylogenetically related to the currently accepted lectotype, C. nebularis (Redhead et al. 2002a, b, Harmaja 2003, Vizzini et al. 2010, Vizzini & Ercole 2012, Musumeci & Contu 2014, Vizzini 2014a, b, Sesli et al. 2016, Alvarado et al. 2015, 2018a, b, He et al. 2023). The genus corresponds to Clitocybe sect. Infundibuliformes sensu Harmaja (1969), and to Clitocybe subg. Infundibuliformes, sect. Infundibuliformes, subsect. Infundibuliformes (Bigelow 1985), and sect. Clitocybe sensu Bon 1997, but not sect. Infundibuliformes sensu Bigelow (1968), whose concept is too broad and includes multiple unrelated taxa. Infundibulicybe is characterized by: (i) basidiospores smooth (except for Infundibulicybe trachyspora which exhibits minutely warted basidiospores on both light and SEM microscopy) that do not adhere to form tetrads (ii) all or most of the basidiospores have lacrymoid appearance, confluent base and a cyanophobic spore wall, and (iii) mycelia unable to reduce nitrates (Schwöbel 1984, Harmaja 2003, Vizzini et al. 2011c, Zhao et al. 2016, He & Yang 2023). The independence of Infundibulicybe from Clitocybe has been confirmed by phylogenetic analyses based on DNA sequences and shown to belong in suborder Tricholomatineae (e.g., Binder et al. 2010, Sánchez-García et al. 2016, 2017, 2020, Alvarado et al. 2018a, b, Varga et al. 2019, Vizzini et al. 2020a, He & Yang 2022). Twenty-two species of Infundibulicybe were recognized worldwide by He et al. (2019) but multiple new taxa have been described afterwards from Asia (Ishaq et al. 2019, Ali et al. 2020, Xu et al. 2022). Species of Infundibulicybe are distributed mainly in temperate, boreal, and alpine regions of the Northern Hemisphere (Harmaja 2003, Kirk et al 2008, Vizzini et al. 2011c, Zhao et al. 2016, Ishaq et al. 2019, Ali et al. 2020, Xu et al. 2022, He & Yang 2023).
Paralepistaceae Vizzini, Consiglio & P. Alvarado, fam. nov. MycoBank MB 851155.
Diagnosis: Basidiomes clitocyboid, spore deposit white, cream, or sordid ochre, basidiospores subglobose to broadly ellipsoid with pustulose-aculeate, inamyloid or amyloid, cyanophilous or acyanophilous ornamentation, hymenial cystidia usually absent, hymenophoral trama regular, clamp connections present, terricolous, among litter, presumably saprotrophic.
Type: Paralepista Raithelh., Die Gattung Clitocybe (Stuttgart) 1: 17. 1981.
Representative genera: Notholepista (Figs 7H, 16), Paralepista (Fig. 7J), and Ripartites (Figs 8C, D).
Fig. 16.
Notholepista fistulosa (HMJU:288). A, B. Basidiospores (SEM). C. Pileipellis. D. Basidia. E. Basidiospores. Scale bars: C–E = 10 μm. Photographs and drawings by J. Xu.
Notes: The three genera inside Paralepistaceae (Notholepista, Paralepista and Ripartites) share a group of morphological features, namely, small to medium-sized basidiomes, clitocyboid habit (centrally depressed pileus and sub-decurrent to long decurrent lamellae), usually detachable lamellae, a regular hymenophoral trama, presence of clamp connections, and subglobose to broadly ellipsoid mononucleate basidiospores with pustulose-aculeate ornamentation (Huijsman 1960, Pegler & Young 1974, Kühner 1976, 1980, Breitenbach & Kränzlin 1991, Noordeloos 1995b, Vizzini & Ercole 2012, Vizzini et al. 2012a, Bau et al. 2013, Læssøe & Petersen 2019, He & Yang 2022). However, the spores of Notholepista are amyloid and warted while those of Paralepista and Ripartites are inamyloid and spiny (Pegler & Young 1974, Vizzini & Ercole 2012, Vizzini et al. 2012a, Bau et al. 2013, Læssøe & Petersen 2019). Ripartites shows a sordid brown spore deposit (Huijsman 1960, Singer 1986, Noordeloos 1995b, Bon 1997, Antonini et al. 1998, Bau et al. 2013) while that of Notholepista and Paralepista is white or whitish with cream-ochre hues, respectively (Bon 1991, 1997, Raithelhuber 2004, Vizzini & Ercole 2012, Vizzini et al. 2012a, He & Yang 2022). The presence in the same family of genera with amyloid and non-amyloid basidiospores has been already reported in suborder Tricholomatineae, i.e., Biannulariaceae, Callistosporiaceae, Pseudoclitocybaceae, and Tricholomataceae (Sánchez-García et al. 2014, 2017, Alvarado et al. 2018a, Vizzini et al. 2020a, c), and in Tricholoma most species have been found to be latently amyloid (Vizzini et al. 2020c). The presence, within the same genus, of species with amyloid and non-amyloid basidiospores is also well known, e.g., Dermoloma (Tricholomataceae, Sánchez-García et al. 2021), or Amanita (Amanitaceae, Pluteineae, Neville & Poumarat 2004, Cui et al. 2018). Similarly, pale-spored and dark-spored species can be found within the same family in suborders Agaricineae (Agaricaceae, Agaricus, Coprinus, Matheny et al. 2006, Dentinger et al. 2016) and Hygrophorineae (Lichenomphaliaceae, Melanomphalia, Aime et al. 2005).
On a morphological basis, affinities between members of these three genera had already been recognized previously. Historically, Ripartites was first thought by some authors to be close to the ochre-spored Galera, Conocybe and Hebeloma (Heim 1969), or to belong to Crepidotaceae (Singer 1951), or Paxillaceae (Boletales) (e.g., Fries 1821, Quélet 1886, Machol & Singer 1971, Jülich 1981, Singer 1986) due to its spore deposit colour. Other authors classified it very generically in the Agaricales (Reijnders & Stalpers 1992) or inside Tricholomataceae sensu lato, close to Lepista (Kühner & Romagnesi 1953, Harmaja 1974a, Pegler & Young 1974, Kühner 1976, 1980, Noordeloos 1995b, Bon 1997), where Kühner (1976) even established a separate tribe Lepisteae for both genera. Besson (1970), Harmaja (1974a), Kühner (1976, 1980) and Pegler & Young (1969, 1971, 1974) recognized strong affinities between Ripartites and the L. inversa species complex (Lepista sect. Gilva = sect. Inversae) because of their basidiospores showing the same electron microscopy ultrastructure, with a strong cyanophilous perispore.
Notholepista Vizzini & Contu, Mycosphere 3: 84. 2012.
Type: Notholepista subzonalis (Peck) Vizzini & Contu, Mycosphere 3: 85. 2012.
Notes: The type species, N. subzonalis, resembles Paralepista gilva macroscopically, developing clitocyboid basidiomes with a yellow to orange pileus with distinct drop-like blotches (Bon 1978, 1997, Læssøe & Petersen 2019). In addition, basidiospores of Notholepista under SEM (Fig. 16A, B) are very similar to those of Paralepista and Ripartites species presented in literature (e.g, Pegler & Young 1969, 1971, 1974, Besson 1970, Kühner 1980, Bigelow 1981). A full description of new records of the recently described species Notholepista fistulosa is provided below. ITS sequences in public databases (KP453712, MF686504) obtained from specimens identified as Leucopaxillus pulcherrimus (FH:00301901 and TENN:070768, respectively) seem to be closely related to those of N. subzonalis and N. fistulosa, suggesting that this species should be transferred to Notholepista as well.
Notholepista fistulosa Z.M. He & Zhu L. Yang, Mycol. Progr. 21(2, no. 26): 9. 2022. Figs 7H, 16.
Description: Pileus 20–36 mm diam, centre depressed, slightly infundibuliform, dark orange (6A7, 6A8), sometimes chrome yellow (5A6, 5A8) at margin, surface hygrophanous, smooth, margin slightly involute. Lamellae decurrent, almost crowded, 0.7–1 mm wide, white, with 2–3 tiers of lamellulae intercalated, edges entire, even. Stipe 24–38 × 3–4 mm wide, central, cylindrical, concolorous with pileus or a little paler (6A7, 6A8), surface smooth, sometimes tomentose at the base, solid. Context dark orange (6A7, 6A8), odour and taste not distinct. Basidiospores (4.2–)4.7–5.0–5.4(−5.8) × (3.1–)3.4–3.7–4.1(−4.8) μm [40/2/2], Q= (1.15–)1.26–1.36–1.46(−1.60), V= (20.7–)28.0–37.6–47.1(−69.3) μm3 broadly ellipsoid to ellipsoid, surface verruculose, warts hemispherical, up to 0.5 μm high, amyloid, cyanophobic. Basidia (26.7–)28.4–38.1(−39.1) × (5.9–)6.0–9.0(−9.2) μm, clavate, sometimes cylindrical, 4-sporic, sterigmata up to 0.5 μm long. Cystidia not observed. Hymenophoral trama regular, parallel, colourless, hyphae cylindrical, 2–17 μm wide, thin-walled. Pileipellis an intricate trichoderm, composed of dense cylindrical hyphae, hyphae 3–14 μm wide, thin-walled. Stipitipellis a cutis, composed of parallel, cylindrical, repent, colourless hyphae, 3–18 μm wide. Clamp connections present.
Habitat and distribution: Scattered on soil in mixed forests dominated by Pinus koraiensis. So far only known from China.
Materials examined: China, Heilongjiang Prov., Yichun City, Liangshui National Nature Reserve, 47°10′50″ N, 128°53′20″ E, on soil under mixed forests dominated by Pinus koraiensis, 10 Sep. 2017, J.Z. Xu (HMJU:288, HMJU:592).
Notes: Notholepista fistulosa is a conspicuous species found on the ground in mixed forests. It is characterized by its small, entirely dark orange basidiomes, slightly infundibuliform pileus, decurrent lamellae, verruculose spores and pileipellis arranged as a cutis. The type species of genus Notholepista, N. subzonalis, resembles N. fistulosa because of their similar infundibuliform pileus with hygrophanous surface, solid stipe, verruculose amyloid spores and lack of cystidia. However, they differ in some features: basidiomes of N. fistulosa (pileus 20–36 mm diam, stipe 24–38 × 3–4 mm) are smaller than those of N. subzonalis (pileus 50–100 mm diam, stipe 20–50 × 10–20 mm); the pileus margin of N. fistulosa is almost entire, while that of N. subzonalis is frequently incised or wavy; and finally, N. fistulosa has a pileipellis arranged as an intricate trichoderm, while N. subzonalis has it arranged as a cutis (Singer & Smith 1943, 1947 as L. pulcherrimus; Bigelow 1965, 1985).
Pseudoclitocybaceae Vizzini et al., Fungal Diversity 90: 112. 2018.
Type: Pseudoclitocybe (Singer) Singer, Mycologia 48: 725. 1956.
Representative genera: Aspropaxillus, Clitopaxillus, Harmajaea, Musumecia, Pogonoloma, and Pseudoclitocybe.
Notes: The family Pseudoclitocybaceae shares several morphological traits with Tricholomataceae and Clitocybaceae, being loosely characterized by often large- to medium-sized basidiomes (50–150 mm), clitocyboid or tricholomatoid, decurrent to subdecurrent or uncinate lamellae; a tendency of most species to turn yellowish to dirty brown when drying; basidia small (mostly < 35 × 8 μm), without siderophilic granulations, hymenial cystidia usually absent; spores usually smooth, acyanophilous, often amyloid, with broad, truncate and prominent apiculus; context homomorphous with cylindrical hyphae >4 μm wide (sarcodimitic in Pogonoloma), mixed or not with thromboplerous hyphae, hymenophoral trama regular to subregular with usually long, parallel or subparallel hyphae, loop-like (medallion) clamp connections usually present at least in mycelium, and pileipellis as a dry or weakly gelatinized cutis or a trichocutis. They are presumably saprotrophic species (uncertain for Pogonoloma), occurring worldwide, mostly in temperate to boreal regions (Alvarado et al. 2018a).
Aspropaxillus Kühner & Maire, Bull. Soc. Mycol. France 50: 13. 1934.
Synonyms: Leucopaxillus sect. Aspropaxilli (Kühner & Maire) Singer & A.H. Sm., Pap. Michigan Acad. Sci. 28: 96. 1943. [1942].
Clitocybe subgen. Aspropaxillus (Kühner & Maire) Konr. & Maubl., Rév. Hymén. France: 339. 1936.
Clitocybe sect. Clitocybe subsect. Aspropaxillus (Kühner & Maire) H.E. Bigelow, Beih. Nova Hedwigia 72: 55. 1982.
Leucopaxillus subgen. Aspropaxillus (Kühner & Maire) Bon, Doc. Mycol. 20(79): 57. 1990.
Type: Aspropaxillus giganteus (Sowerby) Kühner & Maire, Bull. Soc. Mycol. France 50: 13. 1934.
Notes: The genus Aspropaxillus was established to accommodate the large clitocyboid species morphologically similar to Leucopaxillus but producing smooth amyloid spores (Kühner & Maire 1934). Morphologically, Aspropaxillus is close to Pogonoloma sensu stricto (Kühner 1980, Singer 1986, Bon 1991, Sánchez-García et al. 2014, Alvarado et al. 2018a), which differs mainly in a tricholomatoid habit (convex pilus and non-decurrent lamellae), lamellae not easily separable from the context, and sarcodimitic context. Known species of Aspropaxillus are characterized by large basidiomes with pale coloured pileus, decurrent lamellae, smooth amyloid basidiospores, presence of clamp connections, absence of hymenial cystidia (Vizzini et al. 2012a) (Kühner & Maire 1934, Singer & Smith 1943, Singer 1989, Bon 1991, Dhancholia et al. 1991, Noordeloos 1995c, Consiglio & Contu 2000, Riva 2001, Christensen 2008,2012, Vizzini et al. 2012a). They are all terricolous and presumably saprotrophic.
Aspropaxillus giganteus (Sowerby) Kühner & Maire, Bull. Soc. Mycol. France 50: 13. 1934. Figs 6B, 17.
Fig. 17.
Aspropaxillus giganteus. A. Basidiomes (AMB:18857). B, C. Pileipellis (B. AMB:19305; C. AMB:18858). D. Subpellis (AMB:18857). E, F. Hymenophoral trama (AMB:18858). G. Hymenium (AMB:18858). H–K. Basidiospores (H, I, K. AMB:18858; J. AMB:19305). L. Stipitipellis (AMB:18857). Mounting media were Melzer’s reagent (G, I), Congo Red in ammonia (B–E, H, J, L), and Cotton Blue (F, K). Scale bars: B–E, L = 30 μm; F–K = 10 μm. Photographs A by G. Consiglio, B–L by M. Marchetti.
Basionym: Agaricus giganteus Sowerby, Col. fig. Engl. Fung. Mushr. (London) 3(no. 19): tab. 244 1803.
Synonyms: Clitocybe gigantea (Sowerby) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 88. 1872.
Paxillus giganteus (Sowerby) Fr., Hymenomyc. eur. (Upsaliae): 401. 1874.
Omphalia geotropa var. gigantea (Sowerby) Quél., Enchir. fung. (Paris): 23. 1886.
Leucopaxillus giganteus (Sowerby) Singer, Schweiz. Z. Pilzk. 17: 14. 1939.
Clitocybe candida Bres., Fung. trident. 1(2): 16. 1882.
Leucopaxillus candidus (Bres.) Singer, Rev. Mycol. (Paris) 4: 68. 1939.
Aspropaxillus candidus (Bres.) M.M. Moser, Kl. Krypt.-Fl. Mitteleuropa - Die Blätter- und Bauchpilze (Agaricales und Gastromycetes) (Stuttgart) 2: 66. 1953.
Leucopaxillus septentrionalis Singer & A.H. Sm., Mycologia 39(6): 726. 1948. [1947].
Clitocybe septentrionalis (Singer & A.H. Sm.) H.E. Bigelow, Canad. J. Bot. 37(5): 772. 1959.
Aspropaxillus septentrionalis (Singer & A.H. Sm.) Vizzini, Mycosphere 3(1): 83. 2012.
Description: Pileus 80–250(−450) mm; at first convex, then flat, eventually developing a central depression and becoming somewhat funnel-shaped to subinfundibuliform; dry, not hygrophanous, smooth, subtomentose, velutinous at centre; the margin inrolled at first, later wavy and sometimes obscurely lined/ribbed; fragile in age; whitish at first, buff to tan (4A2-5, 5A2-4) at maturity. Lamellae running down the stipe, deeply arcuate decurrent; very crowded; easily detachable from the context, whitish or buff (5A2-3), becoming light brown (5A5) in age; some forking, with entire, concolorous edge. Stipe 40–80(−100) × 20–45(−60) mm, more or less equal; dry; whitish, with tiny fibers that darken in age; base with abundant white mycelium. Context whitish; rather thick, turning brownish in places in old basidiomes, proportionally thin in age. Odour and taste: taste pleasant, foul or mealy; odor similar. Spore deposit white. Basidiospores (5.6–) 6.2–7.0–7.7(−9.1) × (3.6–)4.1–4.7–5.3(−6.2) μm [113/3/3], Q= (1.24–)1.36–1.49–1.63(−1.85), V= (43.6–)54.1–83.6–113(−182) μm3, broadly ellipsoid to ellipsoid, smooth, colourless, thin-walled, congophilous, acyanophilous, amyloid, with prominent apiculus, mono-pluriguttulate. Basidia 25–35(−40) × 6.5–8.5(−9) μm, clavate to slightly suburniform, sometimes with median constriction or with a long curved basal portion, 4-sporic, rarely 2-sporic, sterigmata 3–4(−5) µm long, with basal clamp connection, with numerous inner refractive droplets. Hymenophoral trama regular, consisting of parallely oriented cylindrical hyphae, septa very close together, thin-walled, colourless, 5–10(−15) μm wide, with extracellular refractive and polymorphic crystalline deposits and thromboplerous hyphae. Subhymenium textura intricata type, elements short and 2–4 µm wide. Hymenial cystidia absent. Pileipellis a transition between a cutis and a trichoderm (or trichocutis), formed of subparallel to intertwined cylindrical hyphae, 2–6 µm wide, slightly immersed in a gelatinous matrix, colourless to pale yellowish, extracellular refractive and polymorphic crystalline deposits abundant; subpellis composed of colourless, cylindrical, 5–12 µm wide hyphae, sometimes enlarged at septa. Stipitipellis composed of tufts of hyphae in gelled matrix with long, straight to intertwined (tangled) articles, 3–7 µm wide, often with a wall up to 0.8(−1.0) µm thick, yellowish-cream, with copious presence of extracellular polymorphic crystalline deposits. Stipititrama composed of subirregular up to intertwined, cylindrical, 6–12(−14) µm wide hyphae, with up to 0.8(−1.0) µm thick, slightly yellowish wall. Clamp connections present in all parts of the basidiome, voluminous, some of the medallion (loop-like) type.
Habitat and distribution: Gregarious, terricolous, saprotrophic, often forming large fairy rings, in grasslands, rarely in open forests. Found in Europe, Asia, and North America.
Materials examined: Italy, Trentino-Alto Adige, Regole di Fondo (TN), under Picea abies, 17 Sep. 1994, G. Consiglio (AMB:18857); Bellamonte (TN), under Picea abies, 26 Aug. 2015, G. Consiglio (AMB:19305); Abruzzo, Rocca di mezzo (AQ), under Picea abies, 18 Sep. 1998, G. Consiglio (AMB:18858).
Notes: Currently, six species of Aspropaxillus have been described (Agerer 2018, He et al. 2019), distributed in the northern Hemisphere (Europe, Asia, and North America, Vizzini et al. 2012a) but A. candidus from Europe and A. septentrionalis from USA would appear to be later synonyms of A. giganteus according to the phylogenetic analyses by Angelini et al. (2017) and Harada et al. (2021). Aspropaxillus giganteus usually produces fairy rings in acid grasslands, its mycelium is easily cultured in vitro, and is considered as surely non-mycorrhizal (Kaiser 1998, Kohzu 1999, Yamada et al. 2001a, b, Barros et al. 2006, Harada et al. 2021).
Pseudoomphalinaceae Vizzini, Consiglio & P. Alvarado, fam. nov. MycoBank MB 851156.
Diagnosis: Basidiomes omphalinoid or rarely clitocyboid, spore deposit white, basidiospores smooth, amyloid, hymenial cystidia usually absent, rarely as very slender, cylindrical to filiform (hyphal) pseudoparaphysoid-like sterile elements, hymenophoral trama irregular to interwoven, pileipellis a cutis with encrusting pigment, clamp connections present and abundant, terricolous, saprotrophic.
Type: Pseudoomphalina (Singer) Singer, Mycologia 48(5): 725. 1956 (Fig. 8A).
Representative genus: Pseudoomphalina (including Neohygrophorus)
Notes: Pseudoomphalina, typified with P. kalchbrenneri, is mainly characterized by its clitocyboid/omphalinoid habit, presence of clamp connections, interwoven hymenophoral trama, and amyloid spores (Singer 1986, Consiglio et al. 2006, Contu 2010, Lavorato et al. 2015, Voitk et al. 2020a). Originally proposed as a subgenus of Cantharellula (Singer 1948), it was later upgraded to genus (Singer 1956). However, Kühner (1980) included Pseudoomphalina as part of Aspropaxillus inside tribe Clitocybeae of Tricholomataceae, and Bon (1997) considered it a member of tribe Fayodiae inside subfamily Clitocybeae of Tricholomataceae together with Clitocybula, Fayodia, Myxomphalia, and Pseudoclitocybe. Based on rDNA data, Lavorato et al. (2015) found Pseudoomphalina kalchbrenneri and allied species (Pseudoomphalina clade) in an uncertain position within the Tricholomatoid clade (suborder Tricholomatineae) and treated the genus Neohygrophorus as a later synonym of Pseudoomphalina. Neohygrophorus (typified with N. angelesianus) and Pseudoomphalina share the clitocyboid/omphalinoid habit, hyphae with clamp connections, interwoven hymenophoral trama and amyloid spores but Neohygrophorus differs in the grey-violaceous pigments turning red in alkali solutions and in the absence of filiform, hyphal sterile elements in hymenium and stipitipellis (Singer 1986, Redhead et al. 2000b, Consiglio et al. 2006, Contu 2010, Lavorato et al. 2015, Voitk et al. 2020a). Pseudoomphalina umbrinopurpurascens seems to fill the morphological gap between these two genera, as it has the peculiar violaceous pigments of Neohygrophorus (even though they are not reddening in alkaline solutions) and the filiform cystidia of Pseudoomphalina (Contu 2010, Lavorato et al. 2015, Rubio & Sánchez 2019). Lavorato et al. (2015) also introduced the new genus Pseudolaccaria to accommodate the phylogenetically distant Pseudoomphalina pachyphylla which is nested inside the Callistosporioid clade (now Callistosporiaceae, Vizzini et al. 2020a). The analyses by Sánchez-García et al. (2016, 2017, 2020), Alvarado et al. (2018a, b), Vizzini et al. (2020a) and He & Yang (2022) confirmed the affiliation of Pseudoomphalina to the Tricholomatineae but without showing significant phylogenetic affinities with other clades. The present analysis (Fig. 3) supports that the clade of Pseudoomphalina represents a distinct evolutionary lineage within suborder Tricholomatineae and, hence, a new family for it is proposed here.
Tricholomataceae R. Heim ex Pouzar, Česká Mykol. 37(3): 175. 1983, nom. cons., see Art. 14.
Synonyms: Tricholomataceae subfamily Tricholomatoideae see Bon, Doc. Mycol. 3(12): 5. 1974.
Leucopaxillaceae Jülich, Biblioth. Mycol. 85: 376. 1982. [1981].
Tricholomataceae Roze [as ‘Tricholomées’], Bull. Soc. Bot. France, Act. Bot. 23: 112. 1876, nom. inval., Art. 32.1(c), see Art. 18.4 (Shenzhen).
Agaricinès Tribe Tricholomées (as Tricholomés) Fayod, Ann. Sci. Nat., Bot., Série 7, 9: 346. 1889, nom. inval., Art. 32.1(c), see Art. 18.4 (Shenzhen).
Dermolomataceae Bon, Bull. Fed. Hist. Nat. Mycol. 1: 10. 1979 [nom. inval., Art. 33.2; nom. rejic., Art. 14 (Shenzhen)].
Type: Tricholoma (Fr.) Staude, Schwämme Mitteldeutschl. 1: xxviii, 125. 1857, nom. cons., see Art. 14 (Shenzhen).
Representative genera: Albomagister, Dennisiomyces, Dermoloma, Corneriella, Leucopaxillus, Porpoloma sensu stricto, Pseudobaeospora, Pseudotricholoma, and Tricholoma.
Notes: A major clade, named Tricholomataceae sensu stricto, which encompasses seven monophyletic subclades corresponding to the genera Leucopaxillus, Tricholoma, Pseudotricholoma, Porpoloma sensu stricto, Dennisiomyces, Corneriella, and Albomagister, was first recovered by Sánchez-García et al. (2014) based on a multigene analysis. A similar phylogeny was reconstructed by Corriol & Jargeat (2019) who reported the first collection of Dennisiomyces in Europe. Later, Pseudobaeospora and Dermoloma were also placed in Tricholomataceae sensu stricto (Desjardin et al. 2014, Sánchez-García & Matheny 2017, Sánchez-García et al. 2021, He & Yang 2022). Only Porpoloma sensu stricto and Tricholoma are considered ECM-forming genera without doubt (Kühner 1980, Garrido 1988, Tedersoo et al. 2010, Sánchez-García et al. 2014, Sánchez-García & Matheny 2017). The family Tricholomataceae is characterized by a tricholomatoid or rarely tricholomatoid-collybioid habit; pileus conical, convex, plano-convex to applanate, smooth, tomentose, or scaly, dry or viscid, rarely hygrophanous; lamellae adnate, adnexed, sinuate-emarginate to decurrent; spore deposit pure white, rarely pale cream; basidiospores subglobose, ellipsoid or ellipsoidoblong, colourless, thin-walled, without a germ pore, smooth or verrucose, positive reacting to Melzer’s reagent (immediately amyloid, latently amyloid or dextrinoid, Vizzini et al. 2020c); basidia without siderophilous granulation; hymenial cystidia present or absent as cheilocystidia, pleurocystidia present in some groups; hymenophoral trama regular; pileipellis a cutis, ixocutis or trichoderm; clamp connections present or absent; ectomycorrhizal or saprotrophic on soil, humus and debris in forests and grass-lands; mainly found in temperate regions of the northern and southern hemispheres, but also in the tropics.
Orphaned incertae sedis genera inside Tricholomatineae
Paralepistopsis Vizzini, Mycotaxon 120: 257. 2012.
Type: Paralepistopsis amoenolens (Malençon) Vizzini, Mycotaxon 120: 257. 2012. (Fig. 7K).
Basionym: Clitocybe amoenolens Malençon, Trav. Inst. Sci. Chérifien, Sér. Bot. Biol. Veg. 33: 141. 1975.
Notes: The two species of Paralepistopsis known so far, P. acromelalga and P. amoenolens are characterized by clitocyboid habit (decurrent and crowded lamellae), confluent pileus and stipe and pileus colours (ochre-orange tinges) reminiscent of Paralepista or Infundibulicybe; lamellae easily separating from the pileus context; whitish to cream spore deposit; smooth cyanophilous broadly ellipsoid basidiospores often arranged in tetrads in dried specimens and rarely exceeding 5(−6) μm in length; basidia reaching 35–40 μm in length; (rare) shortly diverticulate hyphae in the pileipellis, thromboplerous hyphae rare to abundant; presence of toxic compounds, acromelic acids, viz. powerful neurotoxic aminoacids responsible for erythromelalgic poisoning (Nakamura et al. 1987, Saviuc et al. 2001, 2002, Leonardi et al. 2002, Marinetti & Recchia 2005) and structurally homologous with kainic acid (a strong agonist of non-N-methyl-D-aspartate glutamate receptor subtypes) and domoic acid; and saprotrophic growth on soil (Moreau et al. 2001, Vizzini & Ercole 2012). Paralepista differs in having conspicuously ornamented basidiospores (Raithelhuber 1995, 2004, Consiglio & Contu 2003, Vizzini & Ercole 2012); Infundibulicybe is distinguished by its smooth lacrymoid spores with confluent bases and not arranged in tetrads, and cyanophobic spore walls (Harmaja 2003). Other morphologically look alike genera as Cleistocybe and Catathelasma are distinguished from Paralepistopsis mainly by the presence of a partial veil, divergent to interwoven hymenophoral trama, and larger cyanophobic basidiospores (Ammirati et al. 2007, Vizzini et al. 2020a); additionally, Catathelasma basidiospores are amyloid (Singer 1986, Vizzini et al. 2020a).
Paralepistopsis amoenolens was first described employing specimens found in Morocco (Malençon & Bertault 1975), but it was later found also in southern and southwestern Europe (mainly France, Italy, and Spain). This species is delimited by a unique combination of macro-/micromorphological and chemical features, such as a convex pileus with orange tinges, a strong aromatic, floral odour reminiscent of Tricholoma caligatum, Inocybe corydalina, Lepista irina, and Entoloma ameides [caused by volatile metabolites as methyl-(E)-cinnamate, methylbenzoate, (E)nerolidol, and methylanthranilate, Fons et al. (2006)], basidiospores (3.8–)4.0–5.4(−5.6) × (2.3–)3.2–4.0(−4.3) µm, thromboplerous hyphae very common, and the presence of the toxic metabolite acromelic acid A (Bon 1987c, Poumarat & Neville 1993, Moreau et al. 2001, Leonardi et al. 2002, Bessard et al. 2004, Martínez et al. 2010, Vizzini & Ercole 2012, Vizzini 2014c). The Asiatic species Paralepistopsis acromelalga differs from P. amoenolens in a darker pileus and stipe, a pileus that soon becomes depressed, a different odour, thromboplerous hyphae occurring only rarely, smaller basidiospores (Ichimura 1918, Romagnesi 1989, Guez 1990, Miyauchi 1998, Moreau et al. 2001), and a more complex metabolite pattern (presence of acromelic acids A–E with 19 other toxins among which clitidine; Konno et al. 1983, 1988, Fushiya et al. 1990, 1992, Saviuc & Danel 2006, Wurita et al. 2019). In accordance with these differences, P. acromelalga was shown to be related but phylogenetically distinct from P. amoenolens with rDNA sequences, both species clustering together near Cleistocybe (Vizzini & Ercole 2012). However, based on the same rDNA information, Sánchez-García & Matheny (2017), Varga et al. (2019) and Sánchez-García et al. (2020) found that P. amoenolens is sister or embedded inside Clitocybaceae. In the present multigene analysis (Fig. 3) P. amoenolens seems to be sister (with no significant support) to Clitocybaceae.
Since Singer (1986) transferred P. acromelalga to the heterogeneous genus Neoclitocybe based on the presence of rare diverticulate hyphae in the pileipellis, the status of this genus should be addressed too after studying authentic material of the type species, N. byssiseda, to check for a putative synonymy with Paralepistopsis. Based on their small basidiospores and the Paralepista-like habit, Clitocybe gilvaoides and C. gracilis (part of Clitocybe sect. Gilvaoideae) from the coniferous forests of North America and Scandinavia (Harmaja 1969, Bigelow 1985) may also belong to Paralepistopsis, but modern specimens are needed to perform molecular and biochemical studies to test it.
Hertzogia R. Wiest, Bull. Soc. Mycol. Strasbourg 121: 33. 2022.
Type: Hertzogia martiorum (J. Favre) R. Wiest, Bull. Soc. Mycol. Strasbourg 121: 33. 2022. (Fig. 6K).
Basionym: Clitocybe martiorum J. Favre, Schweiz. Z. Pilzk. 34(11): 169. 1956.
Synonym: Lepista martiorum (J. Favre) Bon, Doc. Mycol. 22(no. 88): 46. 1993.
Notes: Wiest (2022) proposed the new monospecific genus Hertzogia to accommodate Clitocybe martiorum, a species with an uncertain position within Tricholomatineae in the present work (Fig. 3). It is characterized by pileostipitate clitocyboid basidiomes with convex pileus, lamellae adnate to subdecurrent, more or less separable from the pileus context; spore deposit pinkish; basidiospores small (less than 6 μm in length), appearing smooth on light microscopy, cyanophilous, inamyloid, hymenial cystidia absent, clamp connections present, terricolous, presumably saprotrophic. Favre (1956) already reported that the classification of C. martiorum seemed uncertain due to its pinkish spore deposit, presence of clamp connections, and its lamellae easily separable from pileus context. He observed that it resembles Rhodopaxillus (= Lepista) but its smooth basidiospores led him to classify it inside Clitocybe. The species is not covered in Harmaja’s milestone monograph (1969). Later, Clémençon (1984) placed C. martiorum within Clitocybe sect. Roseospora, and finally Bon (1993, 1997), after discussing the subtle boundaries between Clitocybe and Lepista, proposed to include it in Lepista because of the lamellae detachable from the pileus and strongly cyanophilous basidiospores with minute ornamentations under SEM. Raithelhuber (2004) reported it as Clitocybe (Lepista?) martiorum.
Typhulineae Vizzini, Consiglio & P. Alvarado, subord. nov. MycoBank MB 851161.
Type: Typhula (Pers.: Fr.) Fr. Obs. Mycol. 2: 296. 1818: Fr., Syst. Mycol. 1: 494. 1821, nom. cons. prop., see Olariaga et al. (2022) and Stalpers et al. (2021).
Basionym: Clavaria [unranked] Typhula Pers.: Fr., Syn. Meth. Fung. 1: XVIII. 1801.
Synonyms: Pistillaria Fr., Syst. Mycol. (Lundae) 1: 464, 496. 1821.
Phacorhiza Pers., Mycol. Eur. 1: 192. 1822.
Cnazonaria Corda in J. Sturm, Deutschl. Fl., Pilze 2: 55. 1829.
Scleromitra Corda in Sturm, Deutschl. Fl., 3 Abt., 2: 59. 1829.
Pistillina Quél., Compt. Rend. Assoc. Franç. Avancem. Sci. 9: 671. 1881. [“1880”].
Sphaerula Pat., Tab. Anal. Fung. 1: 27. 1883.
Phaeotyphula Henn., Bot. Jahrb. Syst. 28(3): 320. 1900.
Gliocoryne Maire, Bull. Soc. Bot. France 55: 121. 1909.
Dacryopsella Höhn., Anz. Kaiserl. Akad. Wiss. Wien, Math. - Naturwiss. Kl., Abt. 1, 124: 50. 1915.
Sclerotiomyces Woron., Ann. Mycol. 24(3/4): 233 (1926)
Tygervalleyomyces Crous, Persoonia 39: 387. 2017.
Representative family: Typhulaceae Jülich, Biblioth. Mycol. 85: 393. 1982. [“1981”].
Representative genus: Typhula. The monospecific genus Lutypha (Khurana et al. 1977), morphologically assignable to this suborder, has not yet been sequenced.
Diagnosis: Basidiomes clavarioid, simple or branched, solitary or densely gregarious, usually erect, exceptionally prostrate, cylindrical to slightly flattened or apically with an inflated clavate, cylindrical or subglobose head, stipe (when differentiated) filiform and sterile, usually arising from a sclerotium. Hymenium not thickening, covering the inflated portion completely or leaving the lower or upper portion free. Spore deposit white. Hyphal system monomitic. Cystidia occasionally present as caulocystidia. Basidia 2–4-sporic, basidiospores colourless, smooth, thin-walled, amyloid or inamyloid, non-dextrinoid. Clamp connections present or absent. Dolipores with continuous parenthosomes. Lignicolous, herbicolous, saprotrophic, or parasitic.
Notes: Two genera, Typhula and Macrotyphula, were traditionally accepted in Typhulaceae (e.g., Berthier 1976, Jülich 1984, Petersen 1988, Knudsen & Shiryaev 2012). Typhula [type T. incarnata Lasch, in Fries, Epicr. syst. mycol. (Upsaliae): 585 (1838) [1836–1838], typ. cons. prop., see Olariaga et al. (2022)] includes phytopathogenic species with small basidiomes (usually under 10 mm long) that often arise from sclerotia and have amyloid spores (Berthier 1976, Jülich 1984). Additionally, an asexual morph forming cylindrical conidia with a truncate base may be present in some species of Typhula (Tygervalleyomyces, Berthier 1976, Crous et al. 2017, Olariaga et al. 2020). The genus Typhula comprises about 90 currently accepted species (Olariaga et al. 2022), including several important cold-adapted plant-pathogenic fungi, with T. incarnata and T. ishikariensis complex, T. variabilis and T. japonica of special economic importance (Vergara et al. 2004, Hoshino et al. 2022, 2023). These species are causal agents of “grey snow mould”, also called “Typhula blight”, producing considerable damage to turf grass and cereal crops (e.g., Matsumoto 1992, 2009, Hsiang & Wu 2000, Hoshino et al. 2009, 2023, Ikeda et al. 2015, 2016). The clade of Typhula sensu stricto was shown to be independent from all other suborders of Agaricales by Olariaga et al. (2020), and later confirmed by the phylogenomic analysis of Wang et al. (2023b). The analyses conducted in the present work using a more diverse dataset (Fig. 1, Suppl. Figs S1, S2), agree with the previous results, and so the lineage of Typhula is here accommodated in its own suborder, Typhulineae.
DISCUSSION
In the present work, the phylogenetic affinities of several incertae sedis genera are resolved using an extended dataset with newly sequenced lineages of Agaricales and more complete data than those analyzed in previous works. Sequences of TEF1, RPB1 and RPB2 genes were obtained from multiple genera previously represented mainly by ribosomal DNA markers (i.e., Aphroditeola, Aspropaxillus, Clitolyophyllum, Fayodia, Gamundia, Heimiomyces, Hemimycena, Hertzogia, Leucocortinarius, Limnoperdon, Melanoleuca, Omphaliaster, Omphalina sensu stricto, Paralepistopsis, Pseudoomphalina, Resupinatus, Ripartites, Tectella, Trichocybe, Volvopluteus), as well as lineages not present before in public databases (Giacomia, Hygrophorocybe), increasing the number and representativeness of informative positions in the analyses (Frøslev et al. 2005, Matheny et al. 2006, Schoch et al. 2009). Incomplete information coming from a limited number of species and/or genes could be the cause of some of the differences between the present results and previous multigene studies (i.e., Olariaga et al. 2020). The present phylogeny is based on less information from each species compared with phylogenomic approaches (i.e., Dentinger et al. 2016, Ke et al. 2020, Wang et al. 2023b), but adds important lineages not present in these works, which could help to resolve ancient nodes. The diversity analyzed could play an important role on the results, maybe like that of the amount of information analyzed from each lineage, at least at the scale of the present study. Philippe et al. (2011) discussed that ancient evolutionary events are difficult to be resolved by phylogenetic studies, even with extensive genomic data, producing erroneous (but statistically supported) inferences in case the diversity analyzed does not represent the evolutionary history of the ingroups properly. This could be a problem also in studies using a phylogenomic backbone constraint for deep nodes (i.e., Varga et al. 2019). The analysis of a more representative dataset filling diversity gaps should help to reduce the phylogenetic noise produced by the accumulation of multiple mutations and other sources of homoplasies.
During the preliminary analyses made for the present work, Bayesian results were greatly influenced too by the identity of the taxa analyzed, leading to the complete failure to reach convergence in a reasonable timeframe, or important changes in the resulting topology and statistical support of several major nodes occurring when some lineages were excluded from the analyses or represented by too few species. The information available from each lineage seemed to affect results critically too, producing artifactual support values between lineages with incomplete information (i.e., those represented by ribosomal DNA data alone) and unrelated clades. These problems could be caused by the Markov chains falling into suboptimal states that cannot be overcome without restarting the analysis from another random state. In turn, the maximum likelihood analysis (ML) was found to be more conservative than Bayesian inference, producing low support values for many deep nodes of the present phylogeny. This probably indicates that the information contained in the 6-gene dataset is barely enough to represent the evolutionary history of the lineages studied, probably due to the great amount of phylogenetic ‘noise’ introduced by homoplasies. Genome sequencing projects (i.e., Li et al. 2018, Ruiz-Dueñas et al. 2020, Wang et al. 2023b), greatly increase the amount of information available, but still some important phylogenetic gaps need to be filled to analyze truly representative information. Genomic studies including lineages that apparently fill such gaps in the present work would be necessary to test if the present phylogenetic backbone is right or not.
Based on the phylogenetic results obtained in the present work (Figs 1–3, Suppl. Figs S1, S2), it is here hypothesized that the order Agaricales contains at least nine suborders: Agaricineae, Clavariineae, Hygrophorineae, Marasmiineae, Phyllotopsidineae (= Sarcomyxineae), Pleurotineae (= Schizophyllineae), Pluteineae, Tricholomatineae and Typhulineae. Many clitocyboid/pleurotoid/tricholomatoid incertae sedis genera analyzed nest inside Tricholomatineae, but results led to the creation of ten new families throughout the entire order Agaricales. The main results are discussed below.
HYGROPHORINEAE
Matheny et al. (2006) identified the Hygrophoroid clade as the one formed by the families Hygrophoraceae, Pterulaceae and Typhulaceae, as well as the genera Sarcomyxa and Xeromphalina. A similar relation was also found between some of these lineages by Garnica et al. (2007) analyzing LSU and RPB1 sequences. However, in Matheny et al. (2007), Pterulaceae and Tricholomopsis were found to be independent from the family Hygrophoraceae, and Binder et al. (2010) did not find either a significant similarity between Hygrophoraceae and these clades using an extended dataset. Lodge et al. (2014) reviewed the entire group (as family Hygrophoraceae), which they divided into three subfamilies (Hygrophoroideae, Hygrocyboideae and Lichenomphalioideae), as well as a basal ‘Cuphophylloid’ grade. Later, He & Yang (2021) found significative support for a subfamily Cuphophylloideae. These subfamilies, as well as the tribe Cantharelluleae of Lichenomphalioideae (first recognized by Lodge et al. 2014), are upgraded to the rank of independent families in the present work. This decision was taken on the basis of a 3-gene dataset including a quite diverse, but still not complete, subset of genera of Hygrophorineae. Additional lineages need to be sequenced to get a more accurate phylogeny of this suborder, and ultimately, a phylogenomic analysis should be conducted to confirm the present decisions. The genera Macrotyphula, Phyllotopsis, Pleurocybella, Sarcomyxa, Tricholomopsis and Typhula were also considered basal to the Hygrophoroid group (but without a significant support) by Lodge et al. (2014), but they were accommodated (except for Typhula) in the suborder Phyllotopsidineae by Wang et al. (2023b), a result confirmed in the present work. A subsignificant relationship between Hygrophorineae and Phyllotopsidineae (PP 0.87) was found (Fig. 1), suggesting that both suborders could have a monophyletic origin, a result already observed by Wang et al. (2023b). The genome-based phylogenies by Dentinger et al. (2016) and Li et al. (2021), placed Hygrophoraceae and Clavariaceae in a monophyletic group, named Hygrophorineae by Dentinger et al. (2016), but Olariaga et al. (2020) found no significant similarities between an extended dataset of Clavariaceae (Ceratellopsis acuminata, Clavaria zollingeri, Clavulinopsis laeticolor, Hodophilus hymenocephalus, Mucronella calva, and Ramariopsis kunzei) and Hygrophoraceae (Ampulloclitocybe clavipes, Cantharocybe gruberi, Hygrocybe coccinea, Hygrophorus pudorinus, and Pseudoarmillariella ectypoides), and therefore, they proposed the new suborder Clavariineae to accommodate Clavariaceae, a result confirmed by Wang et al. (2023b) with an extended phylogenomic dataset, and the present work (Suppl. Fig. S1).
MARASMIINEAE
Suborder Marasmiineae was formally proposed by Dentinger et al. (2016) for the clade containing families Cyphellaceae, Marasmiaceae, Omphalotaceae, Physalacriaceae and the hydropoid clade (now Porotheleaceae, Antonín et al. 2019, Vizzini et al. 2019b, 2022, Matheny et al. 2020a, Consiglio et al. 2021), as well as the Mycenaceae (with a lower statistical support). This decision was later confirmed by Varga et al. (2019), Ke et al. (2020) and Olariaga et al. (2020). In some studies, Marasmiineae (or the Marasmioid clade) included also Schizophyllineae, either nested inside (Thorn et al. 2005, Matheny et al. 2006, Varga et al. 2019, Olariaga et al. 2020, Sánchez-García et al. 2020, Li et al. 2021), or sister to (Ke et al. 2020, Wang et al. 2023b) the core lineage of Marasmiineae. Regarding the family Mycenaceae, it was found to be unrelated to Marasmiineae in Moncalvo et al. (2002) and Thorn et al. (2005); nested in the Tricholomatoid clade in Matheny et al. (2006) and Sánchez-García et al. (2020); nested in the Marasmioid clade / Marasmiineae but without support in Binder et al. (2010) and Dentinger et al. (2016); and finally significantly related to Marasmiineae (Mycena luteopallens, Varga et al. 2019, Ke et al. 2020, Li et al. 2021, Wang et al. 2023b). Interestingly, some phylogenomic studies found a significant monophyletic origin between Mycenaceae and the remaining families of Marasmiineae (Ke et al. 2020), while others found a monophyletic origin between Mycenaceae and Schizophyllineae instead (Li et al. 2021, Wang et al. 2023b), a difference maybe caused by the representativeness of the diversity analyzed.
The present results (Fig. 1) suggest that the sister families Mycenaceae and Xeromphalinaceae are significantly related to Marasmiineae. A phylogenomic analysis including Xeromphalina and Heimiomyces would be useful to confirm this hypothesis. The family Mycenaceae includes the genera Mycena (apparently polyphyletic), Favolaschia (Fig. 6D), Panellus (including Dictyopanus) and Roridomyces. The analysis of a specimen identified as Hemimycena lactea (type of the genus) (OULU:GAJ15636, Fig. 6J) suggested that this species is not related to H. gracilis, but this problem should be addressed separately to take reliable taxonomic decisions. The polyphyletic status of Hemimycena had already been suggested by the molecular works of Dima (in Lehmann & Lüderitz 2018), Bau et al. (2021) and Vizzini et al. (2022). In these works, most species clustered together with Phloeomana (type P. speirea, Cyphellaceae) (Lehmann & Lüderitz 2018, Vizzini et al. 2022), a genus corresponding to species in Mycena, section Hiemales (Maas Geesteranus 1992, Ronikier & Aronsen 2007, Redhead 2013, Aronsen & Læssøe 2016, Robich 2016, Holec & Kolařík 2017, Lehmann & Lüderitz 2018), characterized by greyish brownish mycenoid to omphalinoid basidiomes, non-amyloid spores, cheilocystidia of shape slightly different from that of basidia, hyphae of pileipellis with ramified digitations, usually smooth stipe hyphae with scattered caulocystidia, growing on bark and dead wood. However, the core clade of Hemimycena (= Hemimycena sensu stricto, where the type, H. lactea is included) occupied an isolated position and is distantly related to Cyphellaceae (Lehmann & Lüderitz 2018). To better understand the status of Hemimycena versus Phloemana, additional species should be analyzed, and other markers (protein-coding genes) sequenced.
PLEUROTINEAE AND PHYLLOTOPSIDINEAE
Dentinger et al. (2016) found that Pleurotus ostreatus and Pterula multifida formed a significantly supported clade, which they called suborder Pleurotineae (type Pleurotaceae). Later, Olariaga et al. (2020) defended a monophyletic origin between two species of Pleurotus (one of them represented only by 18S and 5.8S rDNA sequences), and the families Phyllotopsidaceae, Pterulaceae, Radulomycetaceae, Sarcomyxaceae, Stephanosporaceae, and Typhulaceae. Wang et al. (2023b) produced genomic data of these families and concluded that they are not directly related to Pleurotineae (represented only by two species of Pleurotus), accommodating them in the suborder Phyllotopsidineae. This result is confirmed in the present work using a more diverse dataset of Pleurotineae including 19 species of the genera Pleurotus, Hohenbuehelia and Resupinatus. These pleurotoid genera were sometimes merged in different ways (Pilát 1935, Coker, 1944, Kühner 1980) or at least classified together inside the family Pleurotaceae (Singer 1962a, 1975, 1986, Barron 1986). In the present work, two distinct families are recognized: Pleurotaceae (Pleurotus and Hohenbuehelia) and Resupinataceae (Resupinatus), on account of the multiple differences between both clades (i.e., habit, pileipellis structure, nematophagy) and the lack of a significant support for their monophyletic origin. The genera Auriculariopsis, Fistulina, Porodisculus and Schizophyllum were considered by Dentinger et al. (2016), Olariaga et al. (2020) and Wang et al. (2023b) as part of the Schizophyllineae, but this suborder is apparently related to Pleurotineae in the present work (Figs 1, 2, Suppl. Fig. S2). As a result, both suborders are considered synonyms here, giving priority to Pleurotineae. A phylogenomic analysis including representatives of the genera Hohenbuehelia and Resupinatus would be useful to confirm this hypothesis.
PLUTEINEAE
The lineage of Pluteineae was first found to be independent from all others in Moncalvo et al. (2000) as clade G (Amanita, Limacella and Pluteus) and Moncalvo et al. (2002) (/pluteus, /melanoleuca plus Amanitaceae group including Amanita, Limacella and Catatrama) but without a formal clade designation. A broad nonsignificant Pluteoid clade containing Pleurotaceae, Pluteaceae, Amanitaceae, Lymnoperdaceae, Melanoleuca and Tricholomopsis was reported by Matheny et al. (2006) and Binder et al. (2010) with Pluteus, Volvopluteus, Melanoleuca and Amanita. Other studies failed to obtain significant similarities between Amanitaceae and Pluteaceae (Bodensteiner et al. 2004, Sheikh et al. 2022) or Volvariella and Pluteaceae (Moncalvo et al. 2002, Justo et al. 2011). Pioneer phylogenomic analyses at Mycocosm (Grigoriev et al. 2014) suggested a close relation of V. volvacea with Pluteus cervinus and various species of Amanita. Based on genomic data, Dentinger et al. (2016) established Pluteineae for the clade consisting of Amanita muscaria, Aspidella thiersii (Saproamanita) and Volvariella volvacea, a result reproduced later by Ke et al. (2020). Employing a phylogenomic backbone, Varga et al. (2019) found a nearly significant support for the same clade including also Pluteus cervinus. In the six-gene analysis by Olariaga et al. (2020) a monophyletic clade formed by Amanita brunnescens, Pluteus romellii and Lachnella villosa was found (0.98 PP). In the phylogeny by Sánchez-García et al. (2020, suppl. mat.), a clade including a collection named “Volvariella media”, Pluteus species, Volvopluteus gloiocephalus, Melanoleuca species and Limnoperdon incarnatum was recovered, but Volvariella species (V. perciliata, V. bombycina, V. volvacea, V. surrecta, V. hypopithys, V. taylorii and V. caesiocincta) were found sister to Lachnellaceae. In the phylogenomic analysis by Li et al. (2021), eight species of Amanita sensu lato (six Amanita and two Saproamanita) were found to be sister (98 % BP) to V. volvacea and P. cervinus. The phylogenomic analysis performed by Wang et al. (2023b) found that Amanita, Pluteus and Volvariella have a monophyletic origin. In the present analysis (Fig. 2) Pluteineae consisted in two major clades: the family Amanitaceae plus Leucocortinarius (0.99 PP) and a clade formed by Limnoperdaceae, Melanoleucaceae, Pluteaceae and Volvariellaceae (1 PP). While the Bayesian support for the entire suborder (0.88 PP) is subsignificant, this is probably due to the existence of gaps in the diversity analyzed and/or a low ratio signal/noise in the sequences included in the analysis. Analyses lacking Leucocortinarius produced a significant support for the rest of Pluteineae (i.e., Supplementary Fig. S1). Genomic studies of Leucocortinarius are necessary to confirm it truly belongs to the family Amanitaceae.
Amanitaceae
The widening of the limits of the Amanitaceae to accommodate Leucocortinarius is supported by several shared morphological features. Leucortinarius and most species of Amanitaceae have a bivelangiocarpic development, a bulbous stipe base, white spore deposit, binucleate basidiospores, and an ectomycorrhizal trophic status (Bas 1969, Reijnders 1979, Kühner 1980, Singer 1986, Neville & Poumarat 2004, Cui et al. 2018). A non-schizohymenial development of the hymenophore and non-acrophysalidic stipititrama are also known in other genera of Amanitaceae (Limacella sensu lato, Catatrama, Franco-Molano 1991, Vrinda et al. 2000, Wartchow et al. 2007). Non-free lamellae might also be present in some species of Amanita and Limacella (Neville & Poumarat 2004, Hahn & Lohmeyer 2010, Cui et al. 2018). Furthermore, genera with free lamellae and others with attached to subfree lamellae (homogeneous versus heterogeneous basidiome texture) do coexist in the same family or different families of the suborders Pluteineae and Agaricineae (Singer 1986, Moncalvo et al. 2002, Matheny et al. 2006, 2015). In Leucocortinarius, only the presence of a regular hymenophoral trama (vs. an inverse one) and metachromatic basidiospores constitute truly new characters for the family. Sporal metachromacy, highlighted in Leucocortinarius for the first time in the present work, is not shared either by any other member of the Pluteineae. The character is sparingly present in other gilled agaricoid genera, being so far known only in the Tribe Leucocoprineae (Macrolepiota, Leucoagaricus, Leucocoprinus, Chlorophyllum, Singer 1986) of the Agaricaceae in the Agaricineae (Matheny et al. 2006, Vellinga 2004, Vellinga et al. 2011), in Haasiella and Aeruginospora of the Hygrophoraceae in the Hygrophorineae (Lodge et al. 2014, Vizzini et al. 2012b) and in Tricholoma cookeanum (Bon 1984, Bon 1991) of the Tricholomataceae sensu stricto in the Tricholomatineae (Matheny et al. 2006, Sánchez-García et al. 2014, Sánchez-García 2016, Sánchez-García & Matheny 2017).
Melanoleucaceae
The phylogenetic position of Giacomia, a name resulting from the fragmentation of the classical concept of Leucopaxillus/Porpoloma (Vizzini et al. 2012a, 2016, Sánchez-García et al. 2014), has always been uncertain (He et al. 2019, Kalichman et al. 2020). According to previous studies based on ribosomal DNA data (Sánchez-García et al. 2014, Sánchez-García 2016, Angelini et al. 2017), Giacomia does not belong to the families Tricholomataceae, Entolomataceae or Lyophyllaceae, while others (Varga et al. 2019) suggest that it is not even related to suborder Tricholomatineae. In the preliminary analyses of the present work (data not shown), Giacomia and Melanoleuca appeared significantly related to Limnoperdon (represented only by rDNA sequences of the ex-type strain in public databases), but the addition of newly obtained sequences of protein-coding genes (RPB2, TEF1) did not support that hypothesis anymore. As a result, Melanoleuca and Giacomia are here accommodated in a new family, Melanoleucaceae (not “Melanoleucaceae” Locq., Loquin 1984, invalid Art. 39), which is related to Limnoperdaceae (Escobar et al. 1976), Pluteaceae and Volvariellaceae, as well as Amanitaceae inside suborder Pluteineae. Both Melanoleuca and Limnoperdon were already found to be related to genus Pluteus (Moncalvo et al. 2002, Bodensteiner et al. 2004, Matheny et al. 2006, Garnica et al. 2007, Binder et al. 2010, Zhao et al. 2017). In addition, genus Volvariella was found to be related to Pluteus in most previous phylogenies of order Agaricales, forming the so-called Pluteoid clade, loosely characterized by the presence of hymenial cystidia and salmon pink to reddish brown spores with complex spore walls (Matheny et al. 2006). Suborder Pluteineae (type Pluteaceae) was created by Dentinger et al. (2016) for the families Pluteaceae and Amanitaceae after observing a significant similarity between 208 genes of one species of Volvariella and two Amanitaceae.
TRICHOLOMATINEAE
Clitocybaceae
Matheny et al. (2006), Binder et al. (2010), Vizzini et al. (2011d), Vizzini & Ercole (2012), Raj et al. (2019), and Olariaga et al. (2020) recovered significant support for a monophyletic origin of Clitocybe, Collybia sensu stricto and Lepista, and Alvarado et al. (2015, 2018a, b), Sánchez-García et al. (2016), Sesli et al. (2016), Sánchez-García & Matheny (2017), Vizzini et al. (2020a), Mou & Bau (2021), He & Yang (2022) and He et al. (2023) found also that genus Singerocybe is nested inside it. Similar results were obtained for Leucocalocybe (Yu et al. 2011, Sánchez-García et al. 2020), Dendrocollybia (Sánchez-García & Matheny 2017, Sánchez-García et al. 2020, Mou & Bau 2021, He & Yang 2022, He et al. 2023), as well as Lepistella and Paralepistopsis (Varga et al. 2019). In the present analyses, Paralepistopsis was not significantly similar to the other genera of Clitocybaceae, but its phylogenetic position should be confirmed with data from additional species other than the type and/or genomic data. While Clitocybe sensu stricto (type C. nebularis), Lepista and Singerocybe seem to represent independent clades within Clitocybaceae, the relationships between the other genera need to be re-examined with a more complete dataset. Unlike most other genera in Clitocybaceae, Singerocybe has a distinct epicutis presenting vesicles (Harmaja 1974b, Qin et al. 2014), evidencing that this feature can vary among the different lineages of the family (i.e., textura epidermoidea in Dendrocollybia, Hughes et al. 2001). The position of Clitocybe ditopa in the present phylogeny suggests that this species could belong to a different genus too, in accordance with its own deviant morphological traits (subglobose spores < 4 µm long), but this issue will be treated in a different work. The phylogenetic placement of Clitocybe sensu stricto is also debatable, because the currently accepted lectotype species, C. nebularis, is not related to the bulk of muscarine-producing species, which are closer to Collybia sensu stricto (He et al. 2023). The status of other putatively related genera, i.e., Lepistella, Leucocalocybe, Pseudolyophyllum, or Rubeolarius, needs to be specifically addressed too in order to propose an integral taxonomic solution for the family.
Lyophyllaceae
Binder et al. (2010) found multiple clitocyboid lineages sister to the main core clade of Lyophyllaceae, and Hofstetter et al. (2014) named them the “hemilyophylloid” clade, which included Clitocybe candicans, C. connata, C. subditopoda, and Hypsizygus ulmarius. Hypsizygus ulmarius and C. connata (as Lyophyllum connatum) are two species traditionally classified in tribe Lyophylleae (e.g., Kühner & Romagnesi 1953, Moser 1978, Singer 1986) or family Lyophyllaceae (Bon 1999, Consiglio & Contu 2002, Kalamees 2004, Horak 2005) because they show siderophilous granulation in their basidia. However, these two species exhibit granules of the oligo-type (Clémençon 1978, 1986a, b, 2004) compared to those of the Lyophyllaceae sensu stricto which are of the macro-type. In Ossicaulis, which is part of the core Lyophyllaceae in several molecular analyses (e.g., Hofstetter et al. 2014, Bellanger et al. 2015, Sánchez-García et al. 2020), granulation was reported to be absent by Singer (1947) but found by Hofstetter et al. (2014) as very small granules seen in phase contrast that can easily escape attention when observed in bright field microscopy. However, granules are absent from the basidia of other ‘hemilyophylloids’, such as C. candicans and C. cf. subditopoda (Hofstetter et al. 2014). Alvarado et al. (2015) proposed three new generic names for clitocyboid lineages inside the ‘hemilyophylloid’ clade: Leucocybe for C. connata and C. candicans, Atractosporocybe for C. inornata, and Rhizocybe for C. vermicularis and related taxa. Subsequently, other clitocyboid taxa lacking siderophilous basidia were shown to nest within this clade, namely Tephroderma (Musumeci & Contu 2014), and Clitolyophyllum (Sesli et al. 2016). Consequently, it must be accepted that Lyophyllaceae sensu lato includes both species with siderophilic granules (macro-type and oligo-type) and without them.
In the present work, two incertae sedis genera were confirmed to belong inside Lyophyllaceae sensu lato (but outside Lyophyllaceae sensu stricto, therefore ‘hemilyophylloids’), Omphaliaster and Trichocybe, after the analysis of rDNA and protein-coding genes. Omphaliaster borealis (type of the genus) is a species with a troubled systematic history. Originally described as Omphalia asterospora (Lange 1930), it was then combined in Clitocybe (Moser 1953), Hygroaster (Singer 1962b), Rhodocybe (Lange & Sivertsen 1966), Omphaliaster (Lamoure 1971), Omphalina (Kühner 1980), and Austroclitocybe (Raithelhuber 1983). Omphaliaster borealis was found to form, together with Dendrocollybia racemosa, a clade sister to a clade consisting of Collybia-Clitocybe group and Lyophylleae group by Moncalvo et al. (2002). Yu et al. (2011) recovered it as incertae sedis in Tricholomatineae and Sánchez-García et al. (2020) as part of a clade including also Clitocybe subditopoda, Hypsizygus, Tephroderma, Clitolyophyllum, Clitocybe sp. and Leucocybe. The genus Omphaliaster, which contains six species so far (Agerer 2018), is characterized by basidiomes omphalinoid, no clamp connections, pileipellis a cutis with encrusting pigments, no hymenial cystidia, basidia non-siderophilic, basidiospores subglobose, warty, or with a few conical projections (bumped), inamyloid, acyanophilous, colourless, spore deposit white, terricolous, saprotrophic (Lamoure 1971, Einhellinger 1977, Kuyper 1995e, Bon 1997, Bresinsky 2008, Vašutová et al. 2013). Because of its ornamented but non-amyloid basidiospores Omphaliaster was included together with Gamundia within Tricholomataceae, subfamily Clitocyboideae, tribe Omphalineae, subtribe Heterosporulae by Bon (1997). The monospecific genus Trichocybe was erected by Vizzini et al. (2010) to include the peculiar species Clitocybe puberula (type of Clitocybe sect. Puberulae). It is distinguished by habit clitocyboid, pileipellis a plagiotrichoderm, basidiospores inamyloid, acyanophilous, basidia non-siderophilic, clamp connections present, hymenial cystidia present, lignicolous, saprotrophic, and growth in spring (Kuyper 1983, Vizzini et al. 2010, Ferisin et al. 2021). Considered as an incertae sedis genus (Vizzini et al. 2010, Qin et al. 2014, He et al. 2019, Kalichman et al. 2020), the present analysis indicated it as part of the hemilyophylloid clade (Fig. 3). Finally, multigene data obtained from specimen GB:0065321 of Lyophyllum turcicum suggest that this species is not related to Lyophyllaceae sensu stricto, but to Asproinocybe, Tricholosporum and Omphaliaster, and might need to be combined into a different genus. On the other hand, specimen GB:0065321 was originally identified as Lyophyllum putidum (= Tephrocybe putida) by the collectors, so a putative synonymy between both epithets should be explored too.
Asproinocybaceae
The family Asproinocybaceae was established by Mou & Bau (2021) to accommodate the genera Asproinocybe (typified with A. lactifera) and Tricholosporum (typified with T. goniospermum). Asproinocybe has irregularly turberculate to stellate spores and laticiferous hyphae, while Tricholosporum has cruciform to stauriform spores and lacks laticiferous hyphae (Singer 1986, Guzmán et al. 1990, 2004, Roux et al. 2000, Angelini et al. 2014, Lebel et al. 2020). Both genera have tricholomatoid basidiomes with distinctive purplish, violaceous, or lilac-vinaceous tinges, and non siderophilous basidia (Angelini et al. 2014). Asproinocybe was recently reported to be an ectomycorrhizal genus (Kumar & Atri 2021). Historically, Singer (1986) placed Asproinocybe as an independent genus in tribe Tricholomateae of Tricholomataceae (a decision later followed by Guzmán et al. 1990, 2004, Angelini et al. 2014, Xu et al. 2018, Lebel et al. 2020), but considered Tricholosporum a later synonym of Tricholoma, placing T. goniospermum and allied species in Tricholoma section Iorigida. This view was followed by some authors such as Bohus (1982, 1985), Bon (1984, 1991), Alessio (1986), Hongo (1988), and Bon & Braiotta (1989), but more recently other authors considered Tricholosporum an independent genus (Baroni 1982, Halling & Franco Molano 1996, Reid et al. 1998, Bohus et al. 1999, Contu & Mua 2000, Boisselet & Moreau 2008, Riva 2008, Fernandez Vicente et al. 2010, Christensen & Heilmann-Clausen 2013, Angelini et al. 2014, Lebel et al. 2020).
The classification of Tricholosporum and Asproinocybe seemed doubtful after independent analyses using ITS and LSU data linked these genera to different families of Tricholomatineae (Heaton & Kropp 2013, Liu et al. 2016, Sánchez-García & Matheny 2017, Lebel et al. 2020, Ralaiveloarisoa et al. 2020, Sánchez-García et al. 2020). Using information from ITS, LSU, SSU and RPB2, T. goniospermum appeared loosely related to several families of the Tricholomatoid clade (Angelini et al. 2017). Mou & Bau (2021) conducted an analysis using sequences of ITS, LSU, SSU, RPB1, RPB2 and TEF1, and concluded that Asproinocybe and Tricholosporum formed an independent clade sister to Callistosporiaceae, for which they established the new family Asproinocybaceae. A similar result was later obtained by He & Yang (2022) and He et al. (2023). However, these works did not include a truly representative sample of the biodiversity of Lyophyllaceae sensu lato, missing many lineages of the Hemilyophylloid group. In the present analysis multigene sequences of two such species, ‘Lyophyllum’ turcicum and Omphaliaster borealis, were added, showing that Asproinocybaceae is not significantly different from Lyophyllaceae. These two species were selected among many others in the Hemilyophylloid clade sequenced by the present authors (unpublished data) to clarify the position of Asproinocybaceae.
Asproinocybe and Tricholosporum share many features with the rest of Lyophyllaceae, such as a tricholomatoid habit, veils absent; lamellae adnate, adnexed, sinuate, emarginate to decurrent; spore deposit white, spores thin-walled, cyanophilous or acyanophilous; hymenophoral trama regular; and pileipellis arranged as a cutis. All species of Asproinocybe and Tricholosporum present purplish, violaceous, or lilac-vinaceous tinges, a coloration present also in some species of Calocybe (e.g., C. onychina, C. favrei or C. hypoxantha). Also, most species in the Hemilyophylloid clade have non siderophilic basidia, similar to those of Asproinocybaceae (Lamoure 1971, Vašutová et al. 2013). Finally, inequilateral (asymmetrically rhomboidal, triangular…), goniosporous, or bumped basidiospores similar to those of Asproinocybe and Tricholosporum are also present in several species of Calocybella, Gerhardtia, Lyophyllum, and Sagaranella (Hongo & Clémençon 1983, Clémençon 1986b, Hofstetter et al. 2002, 2014, Mešić & Tkalčec 2009, Vizzini et al. 2015, 2017, Li et al. 2017, Endo et al. 2019, 2022, Latha et al. 2020, Mu et al. 2021, Wei et al. 2023). Other families containing both genera with inequilateral or bumped basidiospores and genera with smooth and regular spores are known in Agaricales, e.g., Tetrapyrgos in Marasmiaceae (Horak 1983, Honan et al. 2015, Desjardin et al. 2017, Komura et al. 2020), Rhodotus in Physalacriaceae (Moncalvo et al. 2000, 2002, Tang et al. 2014), Mycenella in Cyphellaceae (Boekhout 1985, 1999b, Komorowska 2005, Malysheva & Morozova 2005, Vizzini et al. 2022), Inocybe sensu stricto in Crepidotaceae-Inocybaceae (Horak 1979b, 1987, Matheny et al. 2020b), Clavaria in Clavariaceae (Petersen 1988, Geesink & Bas 1992, Kautmanová et al. 2012a, b, Olariaga et al. 2015, Franchi & Marchetti 2021), and Catatrama in Amanitaceae (Franco-Molano 1991, Cui et al. 2018, Yang et al. 2018). Historically, Kühner & Romagnesi (1953) had already underlined morphological affinities between Tricholoma goniospermum and Lyophyllum sensu lato, and invalidly transferred Tricholoma cossonianum to Lyophyllum (nom. inval., Art. 41.5, Shenzhen), a species described from Algeria with lilac lamellae and subtriangular basidiospores (Maire 1926), based on a doubtful presence of siderophilic granules inside the basidia. Later, Moreau & Contu (2007), after studying the type and recent collections of Tricholoma cossonianum, and finding them devoid of siderophilic basidia, combined it in Tricholosporum. The rDNA sequences (ITS: MW367842, MW367843; LSU: MW367863) of two Tricholoma cossonianum collections from Sardinia and Tuscany recently deposited in GenBank (Puddu et al., unpubl. data) seem to confirm this decision.
Macrocystidiaceae
Since its creation, the classification of the genus Macrocystidia has long been debated. Singer (1951, 1962, 1964) first placed Macrocystidia in the family Tricholomataceae, tribe Marasmieae, subtribe Macrocystidiinae, close to Flammulina, but he later moved it to Tricholomataceae tribe Clitocybeae (Singer 1975) or Tricholomataceae tribe Tricholomateae, subtribe Omphalineae, close to Lactocollybia (Singer 1970, 1986). Kühner (1979a) established the independent family Macrocystidiaceae because of the peculiar features of the genus, and later, due to the pink spore deposit, included it within his concept of order Pluteales (corresponding to the ‘Hyporhodiales’ in Romagnesi 1992), representing an evolutionary link between the Pluteaceae and Rhodophyllaceae (now Entolomataceae), because of the similar spore structure as in Pluteus and same hymenophoral trama as in the Rhodophyllaceae (Kühner 1980, 1984). Kühner (1980, 1984) predicted also a closer phylogenetic relationship between Macrocystidiaceae and Pluteaceae based on their similar features (almost free lamellae, pinkish spore deposit and smooth complex cyanophilous spore walls) but distinguished the former by the noninverse hymenophoral trama. While some authors accepted the status of Macrocystidiaceae (i.e., Jülich 1981, Knudsen 2008b, 2012b), Bas (1988a, b) highlighted the similarities in pileipellis structure and cystidia between Macrocystidia and Flammulina, suggesting that Macrocystidia belongs in Tricholomataceae, and more specifically inside Tricholomataceae tribe Macrocystidieae (Bas 1990, Noordeloos 1995a). Finally, other authors (Agerer 2018) included Macrocystidia inside Marasmiaceae. Most molecular works including Macrocystidia were not able to resolve its phylogenetic position within Agaricales (Moncalvo et al. 2002, Matheny et al. 2006), probably due to the lack of sequences from protein-coding genes. Walther et al. (2005) found a significant relationship with Ripartites, and more recently, Sánchez-García et al. (2020) found Macrocystidia nested in a clade consisting of Ripartites and Paralepista. In the phylogenomic works by Dentinger et al. (2016), Varga et al. (2019) and Wang et al. (2023b), Macrocystidia was found to be related to the remaining families of Tricholomatineae. In the present work, this hypothesis is supported, and Pseudoclitopilus is accepted as a member of Macrocystidiaceae, although additional information probably needs to be analyzed to confirm this conclusion.
Omphalinaceae
The genus Infundibulicybe was proposed by Harmaja (2003) for Clitocybe gibba and allied species because of their morphological differences with genus Clitocybe sensu stricto. Infundibulicybe did not show significant phylogenetic similarities with any lineage of the order Agaricales analyzed in the phylogeny of Matheny et al. (2006), but later Binder et al. (2010) provided the first evidence supporting that Infundibulicybe belongs in the Tricholomatoid clade (suborder Tricholomatineae), where it sometimes clustered with Pseudoclitocybe and/or Omphalina sensu stricto (Lutzoni 1997, Lutzoni & Pagel 1997, Moncalvo et al. 2000, 2002, Binder et al. 2010, Vizzini et al. 2010, 2011a, 2012a, Yu et al. 2011, Vizzini & Ercole 2012, Vizzini et al. 2012c, Sánchez-García et al. 2016, Sánchez-García & Matheny 2017, Olariaga et al. 2020, He & Yang 2022), as well as some species of Rimbachia (Varga et al. 2019, Sánchez-García et al. 2020), and Macrocystidia (Dentinger et al. 2016). The present analysis supports a close relationship between Infundibulicybe and Omphalina sensu stricto, suggesting that Infundibulicybe should be classified inside the family Omphalinaceae. Both Infundibulicybe and Omphalina display cream-reddish brown tinges in their pileus and stipe, and develop long-decurrent lamellae, lacrymoid spores, a similar hymenophoral trama (first subregular, then irregular to interwoven), and strongly encrusting pigment (Harmaja 2003, Elborne 2008, 2012, Vesterholt 2008d, 2012c, Vizzini et al. 2011c, 2012c). Omphalinaceae probably also contains some species of Rimbachia (i.e., R. bryophila, Sánchez-García et al. 2020; R. arachnoidea, Gaya et al. unpubl. pers. comm.), a hypothesis apparently confirmed by unpublished results of the present authors. However, the taxonomic status of Rimbachia sensu stricto needs to be addressed before drawing conclusions, as other species of this genus seem related to Hygrophorineae (i.e., R. neckerae, Varga et al. 2019). DNA sequences from the type, R. paradoxa, would be necessary to this end.
Paralepistaceae
The traditional concept of genus Lepista, viz. clitocyboid fungi with lamellae usually detachable from the context, a white to pinkish yellow spore deposit, and inamyloid cyanophilous ornamented (verruculose to spiny) basidiospores (Singer 1986, Bon 1997, Consiglio & Contu 2003) was first shown to be polyphyletic by Vizzini & Ercole (2012). Species of Lepista subg. Paralepista (= Lepista sect. Gilva, = Lepista sect. Inversae), a group typified with L. inversa, were found to be unrelated to Lepista sensu stricto (lectotype Lepista densifolia) and all other taxa in the Tricholomatineae by Vizzini & Ercole (2012), and consequently, the genus name Paralepista (Raithelhuber 1981) was accepted for Lepista flaccida, L. gilva and allies. These species show very crowded decurrent lamellae and whitish spore deposit with subglobose to largely ellipsoidal spores (Bigelow 1985, Bon 1997, Raithelhuber 2004). Later, Sánchez-García et al. (2016, 2020) indicated a close relationship (without significant statistical support) between Paralepista and Ripartites. The enigmatic genus Ripartites, typified with R. tricholoma, had also an uncertain systematic placement until the molecular works of Walther et al. (2005), where it seemed significantly related to the ochre-reddish spored species Macrocystidia cucumis, and Garnica et al. (2007) where it nested in a clade consisting of the white-spored Catathelasma and Pseudoclitocybe, as well as Infundibulicybe, Fayodia and Leucocortinarius. Later, a significant similarity between sequences of Paralepista, Ripartites and Notholepista was found by Sánchez-García & Matheny (2017) and He & Yang (2022). Sánchez-García et al. (2020) recovered a significant clade composed of Ripartites, Macrocystidia and Paralepista. The classification of Notholepista (typified with N. subzonalis), a genus resulting from the fragmentation of Leucopaxillus sensu lato, has been unclear since its creation (Vizzini et al. 2012a, Sánchez-García et al. 2014, 2016, Angelini et al. 2017, Alvarado et al. 2018a, b, Mou et al. 2021). In the present work, significant support was obtained in the Bayesian analysis (0.96 PP) (and subsignificant in the ML analysis, 54 % BP) for a clade consisting of Notholepista, Paralepista and Ripartites.
Pseudoclitocybaceae
In the present work, Aspropaxillus is classified in the family Pseudoclitocybaceae based on results from a phylogenetic analysis of rDNA and protein-coding genes (Fig. 3). This result agrees with the phylogeny inferred from rDNA data alone published by He & Yang (2022). The classification of Aspropaxillus has been uncertain since its creation by Kühner & Maire (1934). Singer & Smith (1943) reduced it to a section of Leucopaxillus, while Kühner (1979b, 1980) considered Aspropaxillus (including also Pseudoclitocybe and Pseudoomphalina) a subgenus of Clitocybe, as previously proposed by Konrad & Maublanc (1936). Bigelow (1982) made it a subsection within his very broad concept of genus Clitocybe, and finally Bon (1990c, 1991) combined Aspropaxillus again as a subgenus of Leucopaxillus. However, based on its deviant ITS and LSU rDNA sequences, Aspropaxillus was resurrected by Vizzini et al. (2012a) as an independent incertae sedis genus within the Tricholomatoid clade (suborder Tricholomatineae). In the ITS analysis published by these latter authors, Aspropaxillus was sister (PP = 0.95; BP = 67 %) to Pogonoloma, and both genera were related to a clade formed by Pseudoclitocybe and Musumecia. This decision was later confirmed by Sánchez-García et al. (2014) and Angelini et al. (2017). The multigene analyses by Sánchez-García et al. (2016, 2020), Sánchez-García & Matheny (2017), Raj et al. (2019) and He & Yang (2022) also retrieved a significant relationship between Aspropaxillus, Pogonoloma and Pseudoclitocybe (the type of family Pseudoclitocybaceae, Alvarado et al. 2018a). The present analyses confirm these results with additional data coming from non-ribosomal genes.
TYPHULINEAE
The first phylogenetic inferences on Typhulaceae made by Dentinger & McLaughlin (2006), Matheny et al. (2006), Binder et al. (2010), and Lodge et al. (2014) were mainly based on Typhula phacorrhiza which is now considered a species of Macrotyphula (family Phyllotopsidaceae, Olariaga et al. 2020). Macrotyphula (type M. fistulosa) differs from Typhula (type Typhula incarnata, Olariaga et al. 2020, 2022) in having large, yellow-brown long clavarioid basidiomata (30–300 mm) that never arise from sclerotia, and non-amyloid spores (Petersen 1972, 1988, Berthier 1976, Jülich 1984). Olariaga & Salcedo (2012) first reduced Macrotyphula to a later synonym of Typhula based on morphological data, but it was later shown (Olariaga et al. 2020) to represent an independent lineage nested inside the family Phyllotopsidaceae, which the authors classified inside the suborder Pleurotineae. However, Wang et al. (2023b) showed that they are not related and erected the suborder Phyllotopsidineae for the former. In the present work, this result is confirmed including additional lineages of Pleurotineae (Hohenbuehelia, Resupinatus) and Phyllotopsidineae (Aphroditeolaceae), and a more complete sampling of the other suborders of Agaricales. The family Typhulaceae consistently formed an independent clade, apparently basal to all other suborders (except Clavariineae), that is here accommodated in the new suborder Typhulineae.
CONCLUDING REMARKS
The taxonomic decisions taken in the present work are mainly the result of Bayesian analysis of an updated dataset built to fill important gaps in the diversity of Agaricales observed in previous works. The new arrangement at suborder level differs in some ways from those based on phylogenomic studies of this group, and, ultimately, genomic data of the newly sequenced lineages would be necessary to test the present hypotheses. The status of some important missing lineages (i.e., the clades of Henningsomyces and Rectipilus) still needs to be addressed, as they might represent new families or even new suborders. Also, some lineages of clitocyboid and tricholomatoid fungi inside Tricholomatineae are still considered incertae sedis (i.e., Hertzogia, Paralepistopsis), and therefore need more research to be satisfactorily classified.
Table 2.
Taxa, vouchers, and GenBank accessions numbers of the DNA sequences used in the Hygrophorineae-wide phylogenetic analysis inferred from a three-gene dataset (LSU, RPB2 and TEF1). Sequences in bold were generated in this study.
| Species | Herbarium | LSU | RPB2 | TEF1 |
|---|---|---|---|---|
| Acantholichen campestris | CGMS:Gumboski1043b, Spielmann10243b - TYPE | NG_070392 | KT429818 | — |
| Acantholichen galapagoensis | GMUF:DalForno1204 | KT429799 | KT429811 | — |
| Acantholichen pannarioides | GMUF:DalForno1752, MDF352 | KT429807 | KT429817 | — |
| Ampulloclitocybe clavipes | KUN-HKAS:54426 | MW600481 | MW656471 | MW656461 |
| TENN:DJL06TN40 | KF381542 | KF407938 | — | |
| WTU:PBM2474, AFTOL-ID 542 | AY639881 | AY780937 | AY881022 | |
| Amylocorticium cebennense | CFMR:HHB-2808 | GU187561 | GU187770 | GU187675 |
| Cantharellula umbonata | CBS:398.79 | MH872990 | — | — |
| Cantharocybe brunneovelutina | CFMR:DJL-BZ-1883 - TYPE | NG_068731 | — | — |
| Cantharocybe gruberi | AH:24539 | JN006420 | — | — |
| WTU:PBM510, AFTOL-ID 1017 | DQ234540 | DQ385879 | DQ059045 | |
| Cantharocybe virosa | HKAS:79012 | KF303143 | — | — |
| TENN:063483 | JX101471 | — | — | |
| Ceraceomyces borealis | CFMR:L-8014 | GU187570 | GU187782 | GU187686 |
| Chromosera ambigua | GE18008-1 | MK645587 | MK645593 | — |
| Chromosera cyanophylla | WTU:PBM1577, AFTOL-ID 1684 | DQ457655 | KF381509 | — |
| Chromosera lilacina | GE18035 | MK645591 | MK645597 | — |
| Chromosera xanthochroa | GE18033 | MK645590 | MK645596 | — |
| Chrysomphalina chrysophylla | WTU:PBM684, AFTOL-ID 1523 | DQ457656 | — | — |
| Chrysomphalina grossula | OSC:113683 | EU652373 | — | — |
| Cora aspera | F:Lücking 29128, DIC110 | KF443257 | KF443267 | — |
| Cora pavonia | F:Lücking s/n, DIC215 | KF443261 | KF443275 | — |
| Cora reticulifera | F:Lücking 26201, DIC119 | KF443262 | KF443269 | — |
| Cora squamiformis | KRAM:Wilk7577 - TYPE + Wilk7562 | NG_060405 | KF443273 | — |
| Corella brasiliensis | GMUF:DalForno1271, MDF017 | KF443255 | KF443276 | — |
| Corella sp. | GMUF:Eliasaro5006, MDF200 | KY861725 | — | — |
| Cuphophyllus acutoides var. pallidus | CFMR:TN-257 | KF291097 | — | — |
| Cuphophyllus aff. pratensis | WTU:PBM2752, AFTOL-ID 1682 | DQ457650 | — | — |
| Cuphophyllus aurantius | CFMR:PR6601 | KF291100 | KF291102 | — |
| Cuphophyllus cinerellus | GB:0156961, EL30-16 | MN430913 | MN556847 | — |
| Cuphophyllus esteriae | TU:117603 | MN430911 | MN556855 | — |
| Cuphophyllus flavipes | TUR:A-199692, Campo131027 | MN430919 | MN556851 | — |
| Cuphophyllus fornicatus | CFMR:D. Boertmann 2009/94 | KF291124* | — | — |
| Cuphophyllus hygrocyboides | GB:0156992, EL177-13 | MN430917 | MN534321 | — |
| Cuphophyllus lamarum | TU:117564 | MN430915 | MN556853 | — |
| Cuphophyllus pratensis | CFMR:DJL-Scot-8 | KF291058 | — | — |
| Lueck7 | KP965789 | — | — | |
| Cuphophyllus sp. | KUN-HKAS:105671 | MW763000 | MW789179 | — |
| Cyphellostereum galapagoense | CDS:41163 - TYPE | NG_068806 | — | — |
| Cyphellostereum imperfectum | F:Lücking25588, DIC115a | KF443243 | KF443277 | — |
| Dictyonema interruptum | BR:Ertz10475, DIC065 | EU825967 | KF443282 | — |
| Dictyonema schenckianum | F:Lücking30062, DIC113 | KF443251 | KF443285 | — |
| Eonema pyriforme | G1063, DK1524 | MK278075 | — | — |
| Gliophorus aff. laetus | CFMR:PR-5408, SAC-PR-9901 | KF291070 | — | — |
| Gliophorus graminicolor | CORT:TJB-10048 | KF381545 | KF407936 | — |
| Gliophorus psittacinus | CFMR:D. Boertman 2002/10, DEN25 | KF291076 | KF291078 | — |
| Gloioxanthomyces nitidus | GDGM:41710 | MG712282 | MG711911 | — |
| Haasiella splendidissima | Roux4044 | JN944401 | — | — |
| Haasiella venustissima | STU: A.Gminder971488 | KF291093 | — | — |
| Humidicutis auratocephala | CUW:JCS071105E, AFTOL-ID 1727 | DQ457672 | DQ472720 | — |
| Humidicutis dictiocephala | QCAM:6000 - TYPE | NG_066384 | — | — |
| Humidicutis marginata | JM96/33 | AF042580 | — | — |
| Humidicutis sp. | CFMR:BZ3923, D.J.Lodge DJL-BZ-3 | KF291111 | — | — |
| Hygroaster albellus | CFMR:PR-6377, AFTOL-ID 1997 | EF551314 | — | — |
| Hygroaster nodulisporus | CFMR:PR-6378, AFTOL-ID 2020 | EF561625 | — | — |
| Hygrocybe aff. conica | WTU:PBM918, AFTOL-ID 729 | AY684167 | AY803747 | — |
| Hygrocybe cf. acutoconica | CFMR:NC-256, DJL04NC2 | KF291118 | KF291120 | — |
| Hygrocybe coccinea | WTU:PBM915, AFTOL-ID 1715 | DQ457676 | DQ472723 | GU187705 |
| Hygrocybe conica | FO:46714 | DQ071739 | — | — |
| Hygrophorocybe aff. carolinensis | UCSC:F0690 | OR863511 | OR828266 | OR828324 |
| Hygrophorocybe nivea | AMB:19292 | OR863512 | — | — |
| AMB:19293 | OR863513 | — | — | |
| LPA:SMGC2020121621 | OR863514 | OR828267 | OR828325 | |
| TO:AV20100811 | OR863516 | — | — | |
| TO:AV20112411 | OR863517 | — | — | |
| Hygrophorus aurantiosquamosus | KUN-HKAS:112569 | MW763001 | MW789180 | MW773440 |
| KUN-HKAS:82501 | MW600482 | MW656472 | MW656462 | |
| Hygrophorus eburneus | US97/138 | AF430279 | — | — |
| Hygrophorus gliocyclus | KUN-HKAS:79929 | MW600485 | MW656475 | MW656465 |
| Hygrophorus hypothejus | KUN-HKAS:56550 | MW762775 | MW656476 | — |
| Hygrophorus pinophilus | KUN-HKAS:112567 | MW763003 | MW789182 | MW773442 |
| Hygrophorus pudorinus | CUW:PBM2721, AFTOL-ID 1723 | DQ457678 | DQ472725 | GU187710 |
| Hygrophorus sp. | KUN-HKAS:112566 | MW763002 | MW789181 | MW773441 |
| KUN-HKAS:112568 | MW763004 | MW789183 | MW773443 | |
| KUN-HKAS:87261 | MW600487 | MW656477 | MW656466 | |
| Hygrophorus xiangjun | KUN-HKAS:55043 | MW600484 | MW656474 | MW656464 |
| KUN-HKAS:68013 | MW600483 | MW656473 | MW656463 | |
| Lichenomphalia hudsoniana | GAL18249 | JQ065875 | — | — |
| Lichenomphalia meridionalis | S-270-FB1, Hiroshi Masumoto 270 | LC428307 | — | — |
| Neohygrocybe griseonigra | GDGM:44492 - TYPE | NG_067810 | — | — |
| Neohygrocybe ingrata | TENN:DJL05TN62 | KF381558 | KF381516 | — |
| Neohygrocybe ovina | ABS:Rhosisaf | KF291234 | KF291236 | — |
| Neohygrocybe subovina | GRSM:77065, DJL04TN16 | KF291141 | — | — |
| Porpolomopsis aff. calyptriformis | TENN:DJL05TN80 | KF291247 | KF291249 | — |
| Porpolomopsis calyptriformis | CFMR:EB-ENG-3 | KF291243 | KF291245 | — |
| Porpolomopsis lewelliniae | CORT:TJB-10034 | KF291239 | KF291241 | — |
| Pseudoarmillariella bacillaris | HKAS:76377 | KC222316 | — | — |
| Pseudoarmillariella ectypoides | WTU:PBM1588, AFTOL-ID 1557 | DQ154111 | DQ474127 | GU187733 |
| Sinohygrocybe tomentosipes | GDGM:43351 - TYPE | NG_064497 | MG696905 | — |
| GDGM:50075 | MG696902 | MG696906 | — | |
| Spodocybe bispora | KUN-HKAS:112564 | MW763007 | MW789186 | MW773446 |
| KUN-HKAS:73310 - TYPE | MW763005 | MW789184 | MW773444 | |
| Spodocybe cf. trulliformis | G0460, DB1302 | MK277728 | — | — |
| Spodocybe collina | AMB:19296 | OR863547 | OR828298 | OR828349 |
| WU:0018453 | MK277717 | — | — | |
| Spodocybe herbarum | G0171, NL-2261 | MK277719 | — | — |
| Spodocybe rugosiceps | KUN-HKAS:112563 - TYPE | MW763013 | MW789192 | MW789160 |
| KUN-HKAS:71071 | MW763011 | MW789190 | MW773449 | |
| Spodocybe sp. | KUN-HKAS:112560 | MW763014 | MW789193 | MW789161 |
| KUN-HKAS:112565 | MW763015 | MW789194 | MW789162 |
Acknowledgments
We owe a particular debt of gratitude towards the following friends and colleagues who have collaborated, at various titles, in accomplishing the present article, apologizing, right from the start, for possible, unintentional omissions:
Claudio Angelini (Pordenone, Italy), Manuel Atzeni (Monteriggioni, Siena, Italy), Arturo Baglivo (Lecce, Italy), Rafael Blasco (Zaragoza, Spain), Emanuele Brugaletta (Ragusa, Italy), Emanuele Campo (Sacile, Pordenone, Italy), Matteo Carbone (Genova, Italy), Ottorino Chiarello (Cornedo Vicentino, Vicenza, Italy), Beatrice Child-Villiers (Jersey Island, UK), Teresa Clements (Arizona, USA), Marco Contu (Olbia, Sassari, Italy), Régis Courtecuisse (Université de Lille 2, Lille, France), Adriano De Angelis (Urbino, Pesaro-Urbino, Italy), Donatella De Giorgi (Lecce, Italy), Federico Di Rita (Sapienza Università di Roma, Italy), Marco Donini (Trento, Italy), Vicente José Escobio García (Canary Islands, Spain), Guillaume Eyssartier (Paris, France), Cesare Feltrin (Montegalda, Vicenza, Italy), Giuliano Ferisin (Cervignano del Friuli, UD, Italy), Renato Jonny Ferrari (San Lorenzo di Sebato, BZ, Italy), Marco Floriani (Pergine Valsugana, TN, Italy), Enzo Franceschini (Bologna, Italy), Gianfranco Gasparini (Vicenza, Italy), Antonio Gennari (Arezzo, Italy), Francesco Giannoni (Marina di Pietrasanta, Lucca, Italy), Andreas Gminder (Goslar, Germany), Seppo Huhmarniemi (Finland), Tapio Kekki (Rovaniemi, Finland), Oğuzhan Kaygusuz (Isparta, Turkey), Perry Gunnar Larssen (Ålesund, Norway), Ellen Larsson (University of Gothenburg, Sweden), Leonardo La Spina (Mascali, Catania, Italy), Renée Lebeuf (St. Casimir, PQ, Canada), Jostein Lorås (Nord University, Norway), Marco Maletti (Pesaro, Pesaro-Urbino, Italy), Antonio Marangon (Brogliano, Vicenza, Italy), Giorgio Marasca (Pergine Valsugana, Trento, Italy), William McAdoo (Washington, USA). Mario Melis (Cagliari, Italy), Stefano Morini (Imola, Bologna, Italy), Yannick Mourgues (Saint Germain du Teil, France), Alberto Mua (Quartu S. Elena, Cagliari, Italy), Claudio Orlandini (Carpi, Modena, Italy), Fabio Padovan (Belluno, Italy), Luigi Panno (Varese, Italy), Guido Perdisa (Casalecchio di Reno, Bologna, Italy), Luigi Perrone (Roma, Italy), Luciano Polidori (Fano, Ancona, Italy), Florentino Rodriguez (Asturias, Spain), Leandro Sánchez (Montgat, Spain), Christian Schwarz (Santa Cruz, CA, USA), Ledo Setti (Suzzara, Mantova, Italy), Carmelina Signorino (Mascali, Catania, Italy), Giampaolo Simonini (Reggio Emilia, Italy), Gastone Spisni (Castenaso, Bologna, Italy), Jukka Vauras (Turku, Finlandia), Gianfranco Visentin (Boara Polesine, Rovigo, Italy), Tupu Vuorinen (Finland), Øyvind Weholt (Torp, Norway), Evelina Zanella (Altavilla Vicentina, Vicenza, Italy), Adler Zuccherelli (Ravenna, Italy).
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supplementary Material: https://studiesinmycology.org/
Bayesian inference phylogram built with nucleotide sequence data of six loci (5.8S, LSU, SSU, RPB1, RPB2 and TEF1) of the main lineages inside order Agaricales (including taxa of the suborder Clavariineae), rooted with Suillus pictus (Boletales), Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main suborders are shown in color boxes, while family names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study. The dashed branch was shortened for graphic presentation.
Bayesian inference phylogram built with nucleotide sequence data of six loci (5.8S, LSU, SSU, RPB1, RPB2 and TEF1) of the main lineages inside order Agaricales (including taxa of Cyphellopsidaceae), rooted with Suillus pictus (Boletales), Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main suborders are shown in color boxes, while family names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study. The dashed branch was shortened for graphic presentation.
Data of the collections used for phylogenetic analyses and/or morphological studies with details of their host, location, collector, and GenBank accessions numbers.
REFERENCES
- Abdel-Aziz FA. (2016). Freshwater fungi from the River Nile, Egypt. Mycosphere 7(6): 741–756. [Google Scholar]
- Abdel-Wahab MA, Jones EBG, Abdel-Aziz FA. et al. (2019). Nia lenicarpa sp. nov. (Niaceae, Agaricales) from Red Sea mangroves in Saudi Arabia with comments on Nia vibrissa. Phytotaxa 406: 157–168. [Google Scholar]
- Acharya S. (2010). New report of death terror Pterulicium xylogenum in edible bamboo of Tripura. Journal of Pure and Applied Microbiology 4: 891–893. [Google Scholar]
- Agerer R. (2006). Fungal relationships and structural identity of their ectomycorrhizae. Mycological Progress 5: 67–107. [Google Scholar]
- Agerer R. (1997). Entoloma sinuatum (Bull.: Fr.) Kummer + Salix spec. Descriptions of Ectomycorrhizae 2: 13–18. [Google Scholar]
- Agerer R. (1998). Entoloma sinuatum. In: Colour Atlas of Ectomycorrhizae (Agerer R. ed): plate 117. Einhorn, Schwäbisch Gmünd, Germany. [Google Scholar]
- Agerer R. (2018). Subphylum Agaricomycotina Doweld. In: Begerow D, Agerer R, McTaggart A, Basidiomycota and Entorhizomycota. A. Engler’s Syllabus of Plant Families, part 1/3 (Frey W. ed). Borntraeger, Stuttgart, Germany: 130–444. [Google Scholar]
- Agerer R, Beenken L. (1998). Lyophyllum decastes (Fr.) Sing. + Quercus robur L. Descriptions of Ectomycorrhizae 3: 43–47. [Google Scholar]
- Aime MC. (2001). Biosystematic Studies in Crepidotus and the Crepidotaceae (Basidiomycetes, Agaricales). Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology, Blacksburg, Virginia, https://vtechworks.lib.vt.edu/handle/10919/27546 [Google Scholar]
- Aime MC, Vilgalys R, Miller OK., Jr (2005). The Crepidotaceae (Basidiomycota, Agaricales): Phylogeny and taxonomy of the genera and revision of the family based on molecular evidence. American Journal of Botany 92: 74–82. [DOI] [PubMed] [Google Scholar]
- Albertini JB, Schweinitz LD., von (1805). Conspectus Fungorum in Lusatiae superioris agro Niskiensi crescentium, e methodo Persooniana. Sumtibus Kummerianis, Lipsiae, Germany. [Google Scholar]
- Aldrovandi MS, Johnson JE, O’Meara B. et al. (2015). The Xeromphalina campanella/kauffmanii complex: species delineation and biogeographical patterns of speciation. Mycologia 107: 1270–1284. [DOI] [PubMed] [Google Scholar]
- Alessio CL. (1986). La variabilità in Tricholoma goniospermum Bres. specie rara ed endemica. Micologia Italiana 15: 30–36. [Google Scholar]
- Ali M, Khan J, Bashir H. et al. (2020). Infundibulicybe macrospora sp. nov., a new and noteworthy species from Himalayan moist temperate forests of Pakistan. Phytotaxa 452: 268–277. [Google Scholar]
- Almási É, Sahu N, Krizsán K. et al. (2019). Comparative genomics reveals unique wood-decay strategies and fruiting body development in the Schizophyllaceae. New Phytologist 224: 902–915. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W. et al. (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Alvarado P, Moreno G, Vizzini A. et al. (2015) Atractosporocybe, Leucocybe and Rhizocybe: three new clitocyboid genera in the Tricholomatoid clade (Agaricales) with notes on Clitocybe and Lepista. Mycologia 107: 123–136. [DOI] [PubMed] [Google Scholar]
- Alvarado P, Moreau P-A, Dima B. et al. (2018a). Pseudoclitocybaceae fam. nov. (Agaricales, Tricholomatineae), a new arrangement at family, genus and species level. Fungal Diversity 90: 109–133. [Google Scholar]
- Alvarado P, Moreau PA, Sesli E. et al. (2018b). Phylogenetic studies on Bonomyces (Tricholomatineae, Agaricales) and two new combinations from Clitocybe. Cryptogamie, Mycologie 39: 149–168. [Google Scholar]
- Ammirati JF, Parker AD, Matheny PB. (2007). Cleistocybe, a new genus of Agaricales. Mycoscience 48: 282–289. [Google Scholar]
- Angelini C, Contu M, Vizzini A. (2014). Tricholosporum caraibicum (Basidiomycota, Tricholomataceae), a new species from the Dominican Republic. Mycosphere 5: 430–439. [Google Scholar]
- Angelini P, Arcangeli A, Bistocchi G. et al. (2017). Tricholosporum goniospermum, genetic diversity and phylogenetic relationship with the Tricholomatineae [formerly tricholomatoid clade]. Sydowia 69: 9–18. [Google Scholar]
- Anon (2001). Fungal portraits: No. 6. Leucopaxillus rhodoleucus. Field Mycology 2: 39. [Google Scholar]
- Antonín V. (1999). Notes on the genus Fayodia s.l. (Tricholomataceae) - 1. Type studies of European Myxomphalia species. Mycotaxon 73: 325–334. [Google Scholar]
- Antonín V. (2000a). Xeromphalina brunneola (Tricholomataceae), a new member of the European mycoflora. Czech Mycology 52: 237–242. [Google Scholar]
- Antonín V. (2000b). The first European record of Xeromphalina campanelloides (Tricholomataceae) from Austria. Österreichische Zeitschrift für Pilzkunde 9: 111–114. [Google Scholar]
- Antonín V. (2004). Notes on the genus Fayodia s.l. (Tricholomataceae) - II. Type studies of European species described in the genera Fayodia and Gamundia. Persoonia 18: 341–364. [Google Scholar]
- Antonín V, Noordeloos ME. (2004). A monograph of the genera Hemimycena, Delicatula, Fayodia, Gamundia, Myxomphalia, Resinomycena, Rickenella and Xeromphalina in Europe. IHW Verlag, Frankfurt, Germany. [Google Scholar]
- Antonín V, Borovička J, Holec J. et al. (2019). Taxonomic update of Clitocybula sensu lato with a new generic classification. Fungal Biology 123: 431–447. [DOI] [PubMed] [Google Scholar]
- Antonín V, Ďuriška O, Jančovičová S. et al. (2022a). Multilocus phylogeny and taxonomy of European Melanoleuca subgenus Melanoleuca. Mycologia 114: 114–143. [DOI] [PubMed] [Google Scholar]
- Antonín V, Hosaka K, Kolařík M. (2022b). Taxonomy and phylogeny of Paramarasmius gen. nov. and Paramarasmius mesosporus, a worldwide distributed fungus with a strict ecological niche. Plant Biosystems 157: 286–293. [Google Scholar]
- Antonini D, Antonini M, Caroti V. et al. (1998). Indagini preliminari su alcuni macromiceti della lecceta di Lucese (Parco delle Alpi Apuane). Atti della Società Toscana di Scienze naturali, Mem. Serie B 105: 1–7. [Google Scholar]
- Araújo S, Seibert J, Ruani A. et al. (2022). The symbiotic fungus Leucoagaricus gongylophorus (Möller) Singer (Agaricales, Agaricaceae) as a target organism to control leaf-cutting ants. Insects 13: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Karsch-Mizrachi I, Cochrane G. (2021). The international nucleotide sequence database collaboration. Nucleic Acids Research 49: D121–D124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnolds E. (1974a). Taxonomie en Floristiek van Hygrophorus subgenera Hygrotrama, Cuphophyllus en Hygrocybe in Nederland. Doctoraalverslag Instituut voor Systematische Plantkunde, Rijksuniversiteit Utrecht. [Google Scholar]
- Arnolds E. (1974b). De oecologie en geografische verspreiding van Hygrophorus subgenera Hygrotrama, Cuphophyllus en Hygrocybe in Nederland. Doctoraalverslag Rijksherbarium Leiden. [Google Scholar]
- Arnolds E. (1986). Notes on Hygrophoraceae IX. Camarophyllopsis Herink, an older name for Hygrotrama Sing. Mycotaxon 25: 639–644. [Google Scholar]
- Aronsen A, Læssøe T. (2016). The Genus Mycena s.l. Fungi of Northern Europe (Vol. 5). Narayana Press, Gylling, Denmark. [Google Scholar]
- Arpin N, Fiasson JL. (1971). The pigments of Basidiomycetes: their chemotaxonomic interest. In: Evolution in the higher Basidiomycetes: an international symposium (Petersen RH. ed). Univ. Tennessee Press, Knoxville, USA: 63–98. [Google Scholar]
- Aubrecht G, Huber W, Weissenhofer A. (2013). Coincidence or benefit? The use of Marasmius (horse-hair fungus) filaments in bird nests. Avian Biology Research 6: 26–30. [Google Scholar]
- Azevedo E, Barata M, Caeiro MF. (2018). Morphological and phylogenetic analyses of Nia vibrissa, a marine Basidiomycota collected in Portuguese waters. Regional Studies in Marine Science 23: 53–59. [Google Scholar]
- Bach RAK, Brann M, Aime MC. (2022). Viability of fungal rhizomorphs used in bird nest construction in tropical rainforests. Symbiosis 87: 175–179. [Google Scholar]
- Bai SL, Li GL, Liu Y. et al. (2009). Ostryopsis davidiana seedlings inoculated with ectomycorrhizal fungi facilitate formation of mycorrhizae on Pinus tabulaeformis seedlings. Mycorrhiza 19: 425–434. [DOI] [PubMed] [Google Scholar]
- Ballarà J. (1997). Leucopaxillus phaeopus. Pl. 777. Bolets de Catalunya, XVI Collecció, Societat Catalana de Micologia, Barcelona, Spain. [Google Scholar]
- Bañares Á, Beltrán E. (2009). Estudio micológico del Parque Nacional de Garajonay (La Gomera, Islas Canarias). Agaricales s.l. I. Anales del Jardín Botánico de Madrid 66: 47–61. [Google Scholar]
- Bánki O, Roskov Y, Döring M. et al. (2023). Catalogue of Life Checklist (Version 2023-04-19). Catalogue of Life. 10.48580/dfry (accessed 30 April 2023). [DOI] [Google Scholar]
- Bärlocher F, Seena S, Wilson K. et al. (2008). Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshwater Biology 53: 368–379. [Google Scholar]
- Baroni TJ. (1982). Tricholosporum and notes on Omphaliaster and Clitocybe. Mycologia 74: 865–871. [Google Scholar]
- Baroni TJ, Matheny PB. (2011). A re-evaluation of gasteroid and cyphelloid species of Entolomataceae from Eastern North America. Harvard Papers in Botany 16: 293–310. [Google Scholar]
- Baroni TJ, Franco-Molano AE, Lodge J. et al. (2007). Arthromyces and Blastosporella, two new genera of conidia-producing lyophylloid agarics (Agaricales, Basidiomycota) from neotropics. Mycological Research 111: 572–580. [DOI] [PubMed] [Google Scholar]
- Baroni TJ, Hofstetter V, Largent DL. et al. (2011). Entocybe is proposed as a new genus in the Entolomataceae (Agaricomycetes, Basidiomycota) based on morphological and molecular evidence. North American Fungi 6: 1–19. [Google Scholar]
- Baroni TJ, Angelini C, Bergemann SE. et al. (2020). Rhodocybe-Clitopilus clade (Entolomataceae, Basidiomycota) in the Dominican Republic: new taxa and first reports of Clitocella, Clitopilus, and Rhodocybe for Hispaniola. Mycological Progress 19: 1083–1099. [Google Scholar]
- Barrasa JM, Rico VJ. (2003). The non-omphalinoid species of Arrhenia in the Iberian Peninsula. Mycologia 95: 700–713. [DOI] [PubMed] [Google Scholar]
- Barrasa JM, Rico VJ, Villareal M. (2003). Arrhenia eburnea, sp. nov. from Spain. Mycotaxon 88: 113–118. [Google Scholar]
- Barros L, Baptista P, Ferreira ICFR. (2006). Influence of the culture medium and pH on the growth of saprobic and ectomycorrhizal mushroom mycelia. Minerva Biotecnologica 18: 165–170. [Google Scholar]
- Bas C. (1969). Morphology and subdivision of Amanita and a monograph on its section Lepidella. Persoonia 5: 285–579. [Google Scholar]
- Bas C. (1988a). Orders and families in agarics and boleti. In: Flora agaricina neerlandica Vol. 1 (Bas C, Kuyper THW, Noordeloos M.E. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 40–49. [Google Scholar]
- Bas C. (1988b). Preliminary keys to orders and families of agarics and boleti as conceived in this work and as occurring in the Netherlands and adjacent regions (cyphelloid genera excepted). In: Flora agaricina neerlandica Vol. 1 (Bas C, Kuyper ThW, Noordeloos M.E. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 73–74. [Google Scholar]
- Bas C. (1990). Notulae ad Floram agaricinam neerlandicam-XVII. On tribus names in the family Tricholomataceae sensu lato. Persoonia 14: 233–235. [Google Scholar]
- Bas C, Kuyper ThW. (1995). Tricholomataceae R. Heim ex Pouz. (continuation). In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 23–30. [Google Scholar]
- Bau T, Na Q, Liu L. (2021). A monograph of Mycenaceae (Agaricales) in China. Science Press, Beijing. [Google Scholar]
- Bau T, Liu Y, Jin X. (2013). Two new records of the genus Ripartites from China. Journal of Northeast Forestry University 41: 122–123. [Google Scholar]
- Bellanger JM, Moreau PA, Corriol G. et al. (2015). Plunging hands into the mushroom jar: a phylogenetic framework for Lyophyllaceae (Agaricales, Basidiomycota). Genetica 143: 169–194. [DOI] [PubMed] [Google Scholar]
- Bellú F. (1996). Alcune specie mediterranee di recente identificazione con particulare riguardo al genere Clitocybe. Rivista di Micologia 39: 99–114. [Google Scholar]
- Berthier J. (1976). Monographie des Typhula Fr., Pistillaria Fr. et genres voisins. Bulletin mensuel de la Société linnéenne de Lyon. Special issue. Terreaux Freres, Villeurbanne, France. [Google Scholar]
- Bessard J, Saviuc P, Chane-Yene Y. et al. (2004). Mass spectrometric determination of acromelic acid A from a new poisonous mushroom: Clitocybe amoenolens. Journal of Chromatography A 1055: 99–107. [DOI] [PubMed] [Google Scholar]
- Besson M. (1969). La paroi sporique de Fayodia bisphaerigera (Lange) Kühner en microscopic électronique et photonique. Comptes rendus hebdomadaires des séances de l’Académie des sciences, Paris 268 (D): 3167–3169. [Google Scholar]
- Besson M. (1970). Ultrastructure de la paroi sporique de quelques Agaricales à ornements cyanophiles non amyloïdes. Comptes Rendus de l’Académie des Sciences de Paris, série D 251: 1508–1510, pl. 1–11. [Google Scholar]
- Bidaud A. (1993). Leucopaxillus salmonifolius Moser & Lamoure. Bulletin trimestriel de la Fédération mycologique Dauphiné-Savoie 130: 33–34. [Google Scholar]
- Bigelow HE. (1965). The genus Clitocybe in North America: Section Clitocybe. Lloydia 28: 139–180. [Google Scholar]
- Bigelow HE. (1968). The genus Clitocybe in North America. II. Section Infundibuliformes. Lloydia 31: 43–62. [Google Scholar]
- Bigelow HE. (1970). Omphalina in North America. Mycologia 62: 1–32. [Google Scholar]
- Bigelow HE. (1974). The Clitocybe pyxidata group. In: Travaux mycologiques dédiés à R. Kühner. Numéro spécial du Bulletin de la Société Linnéenne de Lyon, 43e année: 39–46. [Google Scholar]
- Bigelow HE. (1975). Studies in the Tricholomataceae: Hygrophoropsis, Cantharellula, Myxomphalia, Omphaliaster. Beihefte zur Nova Hedwigia 51: 61–77. [Google Scholar]
- Bigelow HE. (1979). Notes on Fayodia ss. lato. Mycotaxon 9: 38–47. [Google Scholar]
- Bigelow HE. (1981). Spore ornamentation in the Tricholomataceae. I. Mycologia 73: 128–140. [Google Scholar]
- Bigelow HE. (1982). North American species of Clitocybe. Part I. Beihefte zur Nova Hedwigia 72: 1–280. [Google Scholar]
- Bigelow HE. (1983). Spore ornamentation in the Tricholomataceae. II. Sydowia 36: 11–18. [Google Scholar]
- Bigelow HE. (1985). North American species of Clitocybe. Part II. Beihefte zur Nova Hedwigia 81: 281–471. [Google Scholar]
- Bigelow HE, Hesler LR. (1960). Clitocybe in Tennessee and North Carolina. Journal of the Elisha Mitchell Scientific Society 76: 155–167. [Google Scholar]
- Bigelow HE, Smith AH. (1973). Cantharocybe, a new genus of Agaricales. Mycologia 65: 485–488. [Google Scholar]
- Binder M, Hibbett DS. (2002). Higher-level phylogenetic relationships of Homobasidiomycetes (mushroom-forming fungi) inferred from four rDNA regions. Molecular Phylogenetics and Evolution 22: 76–90. [DOI] [PubMed] [Google Scholar]
- Binder M, Hibbett DS, Molitoris HP. (2001). Phylogenetic relationships of the marine gasteromycete Nia vibrissa. Mycologia 93: 679–688. [Google Scholar]
- Binder M, Hibbett DS. (2006). Molecular systematics and biological diversification of Boletales. Mycologia 98: 971–981. [DOI] [PubMed] [Google Scholar]
- Binder M, Hibbett DS, Larsson K-H. et al. (2005). The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Systematics and Biodiversity 3: 113–157. [Google Scholar]
- Binder M, Hibbett DS, Wang Z. et al. (2006). Evolutionary relationships of Mycaureola dilseae (Agaricales), a basidiomycete pathogen of a subtidal rhodophyte. American Journal of Botany 93: 547–556. [DOI] [PubMed] [Google Scholar]
- Binder M, Larsson K-H, Matheny PB. et al. (2010). Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae were dominated by corticioid forms. Mycologia 102: 865–880. [DOI] [PubMed] [Google Scholar]
- Birkebak JM. (2015). Systematics and diversification patterns of morphologically and ecologically diverse lineages of Agaricomycetes: Clavariaceae and Cantharellales. PhD dissertation, University of Tennessee. https://trace.tennessee.edu/utk_graddiss/3542 [Google Scholar]
- Birkebak JM, Mayor JR, Ryberg M. et al. (2013). A systematic, morphological and ecological overview of the Clavariaceae (Agaricales). Mycologia 105: 896–911. [DOI] [PubMed] [Google Scholar]
- Birkebak JM, Adamčík S, Looney BP. et al. (2016). Multilocus phylogenetic reconstruction of the Clavariaceae (Agaricales) reveals polyphyly of agaricoid members. Mycologia 108: 860–868. [DOI] [PubMed] [Google Scholar]
- Blanco-Dios JB. (2019). Notes on the genus Arrhenia (I): Arrhenia pontevedrana, sp. nov. and A. subglobisemen (Agaricales, Basidiomycota), from the northwest of the Iberian Peninsula. Studies in Fungi 4: 185–191. [Google Scholar]
- Boccardo F, Traverso M, Vizzini A. et al. (2008). Funghi d’Italia. Zanichelli, Bologna, Italy. [Google Scholar]
- Bodensteiner P, Binder M, Moncalvo JM. et al. (2004). Phylogenetic relationships of cyphelloid homobasidiomycetes. Molecular Phylogenetics and Evolution 33: 501–515. [DOI] [PubMed] [Google Scholar]
- Boekhout T. (1985). Notulae ad Floram Agaricinam Neerlandicam – IX. Mycenella. Persoonia 12: 427–440. [Google Scholar]
- Boekhout T. (1988). Notulae ad Floram Agaricinam Neerlandicam – XVI. New taxa, new combinations in Melanoleuca Pat. and notes on rare species in the Netherlands. Persoonia 13: 397–431. [Google Scholar]
- Boekhout T. (1999a). Melanoleuca Pat. In: Flora agaricina neerlandica Vol. 4 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 153–165. [Google Scholar]
- Boekhout T. (1999b). Mycenella (J. E. Lange) Singer. In: Flora agaricina neerlandica Vol. 4 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 173–177. [Google Scholar]
- Boekhout T, Noordeloos ME. (1999). Tricholomopsis Sing. In: Flora agaricina neerlandica Vol. 4 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 151–152. [Google Scholar]
- Boertmann D. (2002). Index Hygrocybearum. A catalogue to names and potential names in tribus Hygrocybeae Kühner (Tricholomatales, Fungi). Bibliotheca Mycologica 192: 1–168. [Google Scholar]
- Boisselet P, Moreau PA. (2008). Tricholosporum tetragonosporum (Maire) Contu & Mua récolté en Ardèche. Bulletin mycologique et botanique Dauphiné-Savoie 189: 17–21. [Google Scholar]
- Bohus G. (1982). Some results of systematical and ecological research on Agaricales, IX. Studia Botanica Hungarica 16: 41–47. [Google Scholar]
- Bohus G. (1985). Tricholoma sectio Iorigida Sing. in Europe and North Africa. Agarica 6: 381–386. [Google Scholar]
- Bohus G, Vasas G, Locsmandi CS. (1999). Two new fungus species from Hungary (Basidiomycetes, Agaricales). Annales Historico-Naturales Musei Nationalis Hungarici 91: 37–44. [Google Scholar]
- Bon M. (1974). La famille des “Tricholomataceae” Sing. Documents Mycologiques 3(12): 5–8. [Google Scholar]
- Bon M. (1978). Tricholomataceae de France et d’Europe occidentale (Leucopaxilloideae). Documents Mycologiques 9(33): 1–79. [Google Scholar]
- Bon M. (1983). Tricholomataceae de France et d’Europe Occidentale (6 ème partie: tribu Clitocybeae Fay.) Clé monographique. Documents Mycologiques 13(51): 1–53. [Google Scholar]
- Bon M. (1985). [1984] Le genre Cuphophyllus (Donk) st. nov. Documents Mycologiques 14(56): 9–12. [Google Scholar]
- Bon M. (1984). Les Tricholomes de France et d’Europe occidentale. Encyclopédie Mycologique 36. Le Chevalier, Paris, France. [Google Scholar]
- Bon M. (1987a). The mushrooms and toadstools of Britain and Northwestern Europe. Hodder & Stoughton, H Fournier. [Google Scholar]
- Bon M. (1987b). Agaricomycètes étudiés au symposium de Val di Rabbi (Italie, Septembre 1983). Documents Mycologiques 18(69): 27–34. [Google Scholar]
- Bon M. (1987c). Quelques espèces intéressantes étudiées au stage F.M.D.S. de St-Germain-Mt-d’Or. Bulletin Trimestriel de la Fédération Mycologique Dauphiné-Savoie 105: 28–30. [Google Scholar]
- Bon M. (1990a). Les Hygrophores. Flore mycologique d’Europe 1. Documents Mycologiques Mémoire Hors Série 5. CRDP de l’Académie d’Amiens, Amiens, France. [Google Scholar]
- Bon M. (1990b). Novitates–3. Combinaisons nouvelles et validations. Documents Mycologiques 20(78): 37–40. [Google Scholar]
- Bon M. (1990c). Taxons nouveaux et validations. Documents Mycologiques 20(79): 57–62. [Google Scholar]
- Bon M. (1991). Tricholomataceae (Fayod) Heim (1ère partie) (Tricholomoideae et Leucopaxilloideae). Les Tricholomes et Ressemblants. Flore mycologique d’Europe 2. Documents Mycologiques Mémoire Hors Série 2. CRDP de l’Académie d’Amiens, Amiens, France. [Google Scholar]
- Bon M. (1993). Agaricomycètes rares ou intéressants de Nord-Picardie. Documents Mycologiques 22(88): 41–51. [Google Scholar]
- Bon M. (1996). Novitates - Tricholomataceae. Sous-famille Clitocyboideae. Documents Mycologiques 26(102): 17–19. [Google Scholar]
- Bon M. (1997). Les Clitocybes, Omphales et ressemblants. Flore Mycologique d’Europe 4. Documents Mycologiques Mémoire Hors Série 4. CRDP de l’Académie d’Amiens, Amiens, France. [Google Scholar]
- Bon M. (1999). Les Collybio-Marasmïoïdes et ressemblants. Flore Mycologique d’Europe 5. Documents Mycologiques Mémoire Hors Série 5. CRDP de l’Académie d’Amiens, Amiens, France. [Google Scholar]
- Bon M. (2004). Champignons de France et d’Europe occidentale. Flammarion, France. [Google Scholar]
- Bon M, Braiotta G. (1989). Tricholoma goniospermum forma tetragonosporum (Maire) Bon, comb. nov. Documents Mycologiques 19(75): 49–52. [Google Scholar]
- Brandrud TE, Bendiksen E, Jordal JB. et al. (2018). Entoloma species of the rhodopolioid clade (subgenus Entoloma; Tricholomatinae, Basidiomycota) in Norway. Agarica 38: 21–46. [Google Scholar]
- Breitenbach J, Kränzlin F. (1991). Champignons de Suisse. Tome 3. Boletes et Champignons à lames 1ère partie. Éditeur Mykologia, Luzern, Switzerland. [Google Scholar]
- Bresadola G. (1882). Fungi Tridentini. Novi vel nondum delineati, descripti et iconibus illustrati. 1(2). Zippei J. Ed., Trento, Italy: 15–26. [Google Scholar]
- Bresadola G. (1927). Iconographia Mycologica, vol. 2. Società Botanica Italiana – sezione Lombarda, Milano, Italy: 51–100. [Google Scholar]
- Bresinsky A. (2008). Beiträge zu einer Mykoflora Deutschlands (2): Die Gattungen Hydropus bis Hypsizygus. Regensburger mykologische Schriften 15: 1–304. [Google Scholar]
- Brunner IL, Miller OK., Jr (1988). Calocybe fallax: its ecology and cultural behavior. Mycotaxon 33: 41–50. [Google Scholar]
- Bruns TD, Szaro TM, Gardes M. et al. (1998). A sequence database for the identification of ectomycorrhizal basidiomycetes by phylogenetic analysis. Molecular Ecology 7: 257–272. [Google Scholar]
- Buchalo AS. (1988). Higher edible basidiomycetes in pure culture. Naukova Dumka, Kiev, Ukraine. [Google Scholar]
- Burdsall HH., Jr (1971). Notes on some lignicolous Basidiomycetes of the Southeastern United States. Journal of the Elisha Mitchell Scientific Society 87: 239–245. [Google Scholar]
- Burdsall HH, Jr, Miller OK., Jr (1975). A reevaluation of Panellus and Dictyopanus. In: Studies of higher Fungi (Bigelow NE, Thiers HD. eds). Beihefte zur Nova Hedwigia 51: 79–91. [Google Scholar]
- Cai C, Leschen R, Hibbett D. et al. (2017). Mycophagous rove beetles highlight diverse mushrooms in the Cretaceous. Nature Communications 8: 14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Lüli YJ, Kost G. et al. (2020). Tricholyophyllum brunneum gen. et. sp. nov. with bacilliform basidiospores in the family Lyophyllaceae. Mycosystema 39: 1728–1740. [Google Scholar]
- Cai Q, Lin W-F, Liu J-W. et al. (2023). Two new species of Sarcomyxa (Agaricales) from China. Mycological Progress 22: 38. [Google Scholar]
- Candoussau F, Josserand M, Bon M. (1974). Récolte de Tectella (Panus, Panellus) patellaris (Fr.) Earle dans les Pyréneés-atlantiques. Documents Mycologiques 4(15): 37–41. [Google Scholar]
- Capellano A. (1976). Position systématique du genre Macrocystidia à la lumière des résultats sur l’architecture de sa paroi sporique en microscopie photonique et électronique. Bulletin de la Société Mycologique de France 92: 221–228. [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Cassinelli G, Lanzi C, Pensa T. et al. (2000). Clavilactones, a novel class of tyrosine kinase inhibitors of fungal origin. Biochemical Pharmacology 59: 1539–1547. [DOI] [PubMed] [Google Scholar]
- Cavet J, Martin M. (1998). Première contribution à la connaissance de la flore mycologique du Parc départemental de Bron-Parilly (Rhône) Première partie. Bulletin Mensuel de la Société Linnéenne de Lyon 67: 103–112. [Google Scholar]
- Cavet J, Moreau P-A. (1994). Une Pleurotacée pas très courante: Tectella patellaris (Fr.) Murr. Bulletin de la Fédération Mycologique Dauphiné-Savoie 135: 32–34. [Google Scholar]
- César E, Bandala VM, Montoya L. et al. (2018). A new Gymnopus species with rhizomorphs and its record as nesting material by birds (Tyrannideae) in the subtropical cloud forest from eastern Mexico. MycoKeys 42: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- César E, Montoya L, Bandala VM. et al. (2020). Three new marasmioidgymnopoid rhizomorph-forming species from Mexican mountain cloud forest relicts. Mycological Progress 19: 1017–1029. [Google Scholar]
- Chalange R, Moreau P-A. (2023). Deux taxons rares, Omphalina praticola et Omphalina cyathella, reclasses dans le genre Pulverulina. Bulletin de la Société Mycologique de France 139: 87–105. [Google Scholar]
- Chattopadhyay P, Talukdar M, Beypih J. et al. (2022). A new species of Volvariella (Agaricales, Basidiomycota) from West Bengal, India. Phytotaxa 567: 46–48. [Google Scholar]
- Chew ALC, Desjardin DE, Tan YS. et al. (2015). Bioluminescent fungi from Peninsular Malaysia - a taxonomic and phylogenetic overview. Fungal Diversity 70: 149–187. [Google Scholar]
- Christensen M. (2008). Leucopaxillus Boursier. In: Funga Nordica–Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 409–411. [Google Scholar]
- Christensen M. (2012). Leucopaxillus Boursier. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 474–476. [Google Scholar]
- Christensen M, Heilmann-Clausen J. (2013). The genus Tricholoma. Fungi of Northern Europe. 4. Narayana Press, Gylling, Denmark. [Google Scholar]
- Chuzho K, Dkhar MS. (2020). Pholiota polychroa and Porodisculus orientalis: two new additions to wood-rotting fungi of India. Studies in Fungi 5: 447–451. [Google Scholar]
- Cifuentes J, Petersen RH, Hughes K. (2003). Campanophyllum. A new genus for an old species name. Mycological Progress 2: 285–295. [Google Scholar]
- Clémençon H. (1968). Beiträge zur Kenntnis der Gattungen Lyophyllum und Calocybe (Agaricales, Basidiomycetes). III. Zwei Chlamydosporen bildende Lyophyllum-Arten. Nova Hedwigia 26: 417–427. [Google Scholar]
- Clémençon H. (1972). Zwei verbesserte Präparierlösungen für die microskopische Untersuchung von Pilze. Zeitschrift für Pilzkunde 38: 49–53. [Google Scholar]
- Clémençon H. (1978). Siderophilous granules in the basidia of Hymenomycetes. Persoonia 10: 83–96. [Google Scholar]
- Clémençon H. (1982). Kompendium der Blätterpilze Europäische omphalinoide Tricholomataceae. Zeitschrift für Mykologie 48: 195–237. [Google Scholar]
- Clémençon H. (1984). Kompendium der Blätterpilze. Clitocybe. Beihefte zur Zeitschrift für Mykologie 5: 1–68. [Google Scholar]
- Clémençon H. (1986a). Computer aided taxonomy of the staining species of Lyophyllum (Agaricales, Basidiomycetes). La famiglia delle Tricholomataceae. Atti del Convegno Internazionale 10–15 settembre 1984, Borgo Val di Taro, Italy. Centro Studi per la Flora Mediterranea: 35–61 [Google Scholar]
- Clémençon H. (1986b). Schwärzende Lyophyllum-Arten Europas. Zeitschrift für Mykologie 52: 61–84. [Google Scholar]
- Clémençon H. (2004) Cytology and plectology of the Hymenomycetes. Bibliotheca Mycologica 199: 1–488. [Google Scholar]
- Clémençon H. (2005). Basidiome development of Xeromphalina campanella (Tricholomatales, Basidiomycetes). Persoonia 18: 449–470. [Google Scholar]
- Cochran KW, Cochran MW. (1978). Clitocybe clavipes: antabuse-like reaction to alcohol. Mycologia 70: 1124–1126. [PubMed] [Google Scholar]
- Co-David D, Langeveld D, Noordeloos ME. (2009). Molecular phylogeny and spore evolution of Entolomataceae. Persoonia 23: 147–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio G. (1997). Contributo alla conoscenza dei macromiceti della regione Emilia-Romagna. IX. Genere Clitocybe. Rivista di Micologia 40: 321–337. [Google Scholar]
- Consiglio G, Contu M. (2000). Il genere Leucopaxillus Boursier in Italia, con brevi note sulle rimanenti specie europee. Bollettino dell’Associazione Micologica Ecologica Romana 51: 3–36. [Google Scholar]
- Consiglio G, Papetti C. (2001). Atlante fotografico dei Funghi d’Italia - Vol. 2. Associazione Micologica Bresadola, Centro Studi Micologici, Trento/Vicenza, Italy. [Google Scholar]
- Consiglio G, Contu M. (2002). Il Genere Lyophyllum P. Karst. emend. Kühner, in Italia. Rivista di Micologia 45: 99–181. [Google Scholar]
- Consiglio G, Contu M. (2003). Il genere Lepista in Italia. Rivista di Micologia 46: 131–176. [Google Scholar]
- Consiglio G, Contu M, Caroti V. (2006). Il genere Pseudoomphalina in Italia. I. Nuova descrizione di alcune specie note. Bollettino dell’Associazione Micologica ed Ecologica Romana 68: 5–33. [Google Scholar]
- Consiglio G, Setti L. (2017). Prolegomena to the systematics of family Pleurotaceae in the monograph “I Generi Hohenbuehelia e Resupinatus in Europa”. Rivista di Micologia 60: 237–253. [Google Scholar]
- Consiglio G, Setti L. (2018). The genera Hohenbuehelia and Resupinatus in Europe. Monografie di Pagine di Micologia. Vol. 3. Associazione Micologica Bresadola, Centro Studi Micologici, Trento/Vicenza, Italy. [Google Scholar]
- Consiglio G, Setti L, Thorn RG. (2018). New species of Hohenbuehelia, with comments on the Hohenbuehelia atrocoerulea - Nematoctonus robustus species complex. Persoonia 41: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio G, Vizzini A, Cooper J. et al. (2021). The agaricoid members of the genus Porotheleum (Porotheleaceae, Agaricales), Porotheleum emend., Porotheleaceae s. stricto, and new genera for Agaricus floccipes and Mycena subalpina. Rivista di Micologia 64: 152–190. [Google Scholar]
- Contu M. (1998). Osservazioni sul genere Clitocybe - II. Tre nuovi taxa dalla Sardegna. Rivista di Micologia 41: 349–355. [Google Scholar]
- Contu M. (2010). Tipificazione e posizione tassonomica di Clitocybe umbrino-purpurascens (Basidiomycota, Agaricomycetes). Bollettino dell’Associazione Micologica ed Ecologica Romana 80: 3–12. [Google Scholar]
- Contu M, Mua A. (2000). Il genere Tricholosporum Guzmán (Basidiomycotina, Agaricomycetes) in Europa. Rivista di Micologia 43: 249–257. [Google Scholar]
- Contu M, Consiglio G. (2004). Il genere Gerhardtia (Lyophyllaceae) in Europa, con osservazioni sulle rimanenti specie conosciute. Micologia e Vegetazione Mediterranea 19: 151–162. [Google Scholar]
- Cooper J. (2014). Nomenclatural novelties. Index Fungorum 193: 1. [Google Scholar]
- Cooper J. (2016). Mycological Notes 36. New Zealand Clitocybaceae. https://www.funnz.org.nz/sites/default/files/2016-MycNotes36-Clitocybaceae.pdf
- Cooper JA, Leonard PL. (2013). Three new species of foetid Gymnopus in New Zealand. MycoKeys 7: 31–44. [Google Scholar]
- Cooper JA, Park D. (2016). The fungal genus Tricholomopsis (Agaricales) in New Zealand, including Tricholomopsis scabra sp. nov. Phytotaxa 288: 69–76. [Google Scholar]
- Corner EJH. (1950). A monograph of Clavaria and allied genera. Oxford University Press, London, UK. [Google Scholar]
- Corner EJH. (1966). A monograph of cantharelloid fungi. Oxford University Press, London, UK. [Google Scholar]
- Corner EJH. (1970) Supplement to a monograph of Clavaria and allied genera. Beihefte zur Nova Hedwigia 33: 1–299. [Google Scholar]
- Corner EJH. (1996). The agaric genera Marasmius, Chaetocalathus, Crinipellis, Heimiomyces, Resupinatus, Xerula and Xerulina in Malesia. Nova Hedwigia Beiheft 111. Cramer, Berlin/Stuttgart, Germany. [Google Scholar]
- Corriol G, Moreau PA. (2007). Agaricus (Annularia) fenzlii redécouvert dans les Pyrénées. Notes sur le genre Chamaeota en Europe. Persoonia 19: 233–250. [Google Scholar]
- Corriol G, Jargeat P. (2019). Morphological and phylogenetical description of Dennisiomyces fibrillosus sp. nov. (Agaricales, Tricholomataceae) from the Pyrenees and the first record of the genus in Europe. Phytotaxa 405: 226–236. [Google Scholar]
- Cortés-Pérez A, Guzmán-Dávalos L, Ramírez-Cruz V. et al. (2023). New species of bioluminescent Mycena Sect. Calodontes (Agaricales, Mycenaceae) from Mexico. Journal of Fungi 9: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtecuisse R, Duhem B. (1994). Guide des Champignons de France et d’Europe. Delachaux et Niestlé, Lausanne, Switzerland. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI. et al. (2017). Fungal Planet description sheets: 625–715. Persoonia 39: 270–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeta MA, Echandi E, Abernethy T. et al. (1991). Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 81: 1395–1400. [Google Scholar]
- Cucchi I. (1997). Tectella patellaris (Fries) Murrill, Klebriger Schleierseitling. Schweizerische Zeitschrift für Pilzkunde 75: 115–117, 119. [Google Scholar]
- Cui Y-Y, Cai Q, Tang L-P. et al. (2018). The family Amanitaceae: molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Diversity 91: 5–230. [Google Scholar]
- Cuvelier J-J. (1990). Caractérisation des ectomycorhizes de Betula pendula (I): Cortinarius armillatus, Dermocybe phoenicea et Amanita muscaria. Belgian Journal of Botany 123: 73–91. [Google Scholar]
- Czederpiltz DLL, Volk TJ, Burdsall HH. (2001). Field observations and inoculation experiments to determine the nature of the carpophoroids associated with Entoloma abortivum and Armillaria. Mycologia 93: 841–851. [Google Scholar]
- Dai Y-C, Niemelä T, Qin G-F. (2003). Changbai wood-rotting fungi 14. A new pleurotoid species Panellus edulis. Annales Botanici Fennici 40: 107–112. [Google Scholar]
- Daniele G, Becerra A, Crespo E. (2005). Amanita muscaria (Basidiomycota) y su association micorricica con Cedrus deodara (Pinaceae) en las sierras de Córdoba, Argentina. Boletín de la Sociedad Argentina de Botánica 40: 45–49. [Google Scholar]
- Daniëls PP, Hama O, Fernández A. et al. (2015). First records of some Asian macromycetes in Africa. Mycotaxon 130: 337–359. [Google Scholar]
- Delgado JC, Cook AA. (1976). Arthroconidia formation in cultures of Marasmius perniciosus. Phytopathology 66: 717–718. [Google Scholar]
- Dennis RWG. (1951). Some agaricaceae of Trinidad and Venezuela. Leucosporae. Part. 1. Transactions of the British Mycological Society 34: 411–482. [Google Scholar]
- Dentinger BTM, McLaughlin DJ. (2006). Reconstructing the Clavariaceae using nuclear large subunit rDNA sequences and a new genus segregated from Clavaria. Mycologia 98: 746–762. [DOI] [PubMed] [Google Scholar]
- Dentinger BTM, Lodge DJ, Munkacsi AB. et al. (2009). Phylogenetic placement of an unusual Coral Mushroom challenges the classic hypothesis of strict coevolution in the Apterostigma pilosum Group Ant-Fungus mutualism. Evolution 63: 2172–2178. [DOI] [PubMed] [Google Scholar]
- Dentinger BTM, Gaya E, O’Brien H. et al. (2016). Tales from the crypt: genome mining from fungarium specimens improves resolution of the mushroom tree of life. Biological Journal of the Linnean Society 117: 11–32. [Google Scholar]
- Desjardin DE, Oliveira AG, Stevani CV. (2008). Fungi bioluminescence revisited. Photochemical & Photobiological Sciences 7: 170–182. [DOI] [PubMed] [Google Scholar]
- Desjardin DE, Hemmes DE, Perry BA. (2014). A ruby-colored Pseudobaeospora species is described as new from material collected on the island of Hawaii. Mycologia 106: 456–463. [DOI] [PubMed] [Google Scholar]
- Desjardin DE, Perry BA. (2017). The gymnopoid fungi (Basidiomycota, Agaricales) from the Republic of São Tomé and Príncipe, West Africa. Mycosphere 8: 1317–1391. [Google Scholar]
- Desjardin DE, Perry BA, Shay JE. et al. (2017). The type species of Tetrapyrgos and Campanella (Basidiomycota, Agaricales) are redescribed and epitypified. Mycosphere 8(8): 977–985. [Google Scholar]
- Dhancholia S, Bhatt JC, Pant SK. (1991). Studies on some Himalayan agarics. Acta Botanica Indica 19: 104–109. [Google Scholar]
- Díaz-Valderrama JR, Aime MC. (2016). The cacao pathogen Moniliophthora roreri (Marasmiaceae) produces rhexolytic thallic conidia and their size is influenced by nuclear condition. Mycoscience 57: 208–216. [Google Scholar]
- Di Rita F, Atzeni M, Tudino F. (2020). The history of conifers in central Italy supports long-term persistence and adaptation of mesophilous conifer fungi in Arbutus-dominated shrublands. Review of Palaeobotany and Palynology 282: 104300. [Google Scholar]
- Domański S. (1969). Wood-inhabiting fungi in the Białowieża virgin forests in Poland. XII. Phyllotopsis nidulans (Pers. ex Fr.) Sing. Acta Mycologica 5: 161–172. [Google Scholar]
- Donk MA. (1964). A conspectus of the families of Aphyllophorales. Persoonia 3: 199–324. [Google Scholar]
- Donoghue MJ, Alverson WS. (2000). A new age of discovery. Annals of the Missouri Botanical Garden 87: 110–116. [Google Scholar]
- Earle FS. (1909). The genera of the north american gill fungi. Bulletin of the New York Botanical Garden 5: 373–451. [Google Scholar]
- Einhellinger A. (1977). Die Pilze in primären und sekundären Pflanzengesellschaften oberbayerischer Moore. 2. Berichte der Bayerischen Botanischen Gesellschaft zur Erforschung der Heimischen Flora 48: 61–146. [Google Scholar]
- Elborne SA. (2008). Omphalina Quél. In: Funga Nordica –Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 235–237. [Google Scholar]
- Elborne SA. (2012). Omphalina Quél. In: Funga Nordica –Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 485–487. [Google Scholar]
- Elborne SA, Læssøe T. (2008). Tectella Earle. In: Funga Nordica – Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 390. [Google Scholar]
- Elborne SA, Læssøe T. (2012). Tectella Earle. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 445. [Google Scholar]
- Elliott TF, Jusino MA, Trappe JM. et al. (2019). A global review of the ecological significance of symbiotic associations between birds and fungi. Fungal Diversity 98: 161–194. [Google Scholar]
- Emmett EE. (1993). British Mycena species – 6. Some Mycena “lookalikes”. Mycologist 7: 176–180. [Google Scholar]
- Endo N, Ushijima S, Nagasawa E. et al. (2019). Taxonomic reconsideration of Tricholoma foliicola (Agaricales, Basidiomycota) based on basidiomata morphology, living culture characteristics, and phylogenetic analyses. Mycoscience 60: 323–330. [Google Scholar]
- Endo N., Takahashi M, Nagamune K. et al. (2022). Description of a new species of Gerhardtia (Lyophyllaceae, Agaricales) from Japan based on morphological and molecular phylogenetic analyses and live culture characteristics. Mycoscience 63: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert F, Rapior S, Nouguier-Soulé J. et al. (2002). Treatment of amatoxin poisoning: 20-year retrospective analysis. Journal of Toxicology: Clinical Toxicology 40: 715–757. [DOI] [PubMed] [Google Scholar]
- Escobar GA, McCabe DE. (1979). Limnoperdon, a cyphellaceous fungus with gasteroid basidia? Mycotaxon 9: 48–50. [Google Scholar]
- Escobar GA, McCabe DE, Harpel CW. (1976). Limnoperdon, a floating gasteromycete isolated from marshes. Mycologia 68: 874–880. [Google Scholar]
- Essig FM. (1922). The morphology, development, and economic aspects of Schizophyllum commune Fries. University of California publications in Botany 7: 447–498. [Google Scholar]
- Esteve-Raventós F, Moreno G, Manjón JL. et al. (2010). Xeromphalina setulipes (hygrophoroid clade, Agaricales), a new Mediterranean species. Mycological Progress 9: 575–583. [Google Scholar]
- Eyssartier G, Roux P. (2011). Le guide des champignons–France et Europe. Editions Belin, Paris, France. [Google Scholar]
- Fanelli AL. (1984). Una Tricholomatacea non comune: Leucopaxillus rhodoleucus (Romell) Kühner. Micologia Italiana 13: 16–20. [Google Scholar]
- Farrell IWV, Thalier V, Turner JL. (1977). Natural acetylenes. Part 52. Polyacetylenic acids and aromatic aldehyds from cultures of the fungus Camarophyllus virgineus (Wulfen ex Fr.) Kummer. Journal of the Chemical Society, Perkin Transactions 116: 1886–1888. [Google Scholar]
- Favre J. (1956). Agaricales nouvelles ou peu connues. Schweizerische Zeitschrift für Pilzkunde 34: 169–172. [Google Scholar]
- Favre J, Poluzzi C. (1949). Unsere Pilze. Vita Helvetica 2: 71–76. [Google Scholar]
- Fayod V. (1889). Prodrome d’une histoire naturelle des Agaricinées. Annales des Sciences Naturelles Botanique, Série 7, 9: 181–411. [Google Scholar]
- Feng B, Yang ZL. (2019). Ectomycorrhizal symbioses: Diversity of mycobionts and molecular mechanisms that entail the development of ectomycorrhizae. Scientia Sinica Vitae 49: 436–444. (in Chinese). [Google Scholar]
- Ferisin G, Faggotto U, Bizio E. et al. (2021). I funghi di Bosco di Sotto Grotta di Farra d’Isonzo (GO). Bollettino del Centro Micologico Friuliano 2020-2021: 32–48. [Google Scholar]
- Fernandez Vicente J, Iglesias P, Hidalgo F. et al. (2010). Aportaciones al conocimiento micologico de la Isla de La Palma II y una nueva especie de Tricholosporum. Errotari 7: 84–131. [Google Scholar]
- Fiasson JL. (1969). Recherches chimiotaxinomiques sur les champignons. Les caroténoïdes de Phyllotopsis nidulans (Pers. ex Fr.) Sing. (Agaricales). Comptes Rendus Hebdomadaires des Seances de l’Académie des Sciences de Paris série D Sciences Naturelles 268: 786–788. [Google Scholar]
- Fidalgo O, Fidalgo K. (1962). A new genus based on Fistulina brasiliensis. Mycologia 54: 342–352. [Google Scholar]
- Fisher PJ, Stradling DJ, Pegler DN. (1994). Leucoagaricus basidiomata from a live nest of the leaf-cutting ant Atta cephalotes. Mycological Research 98: 884–888. [Google Scholar]
- Floudas D, Held BW, Riley R. et al. (2015). Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii. Fungal Genetics and Biology 76: 78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floudas D, Bentzer J, Ahrén D. et al. (2020). Uncovering the hidden diversity of litter-decomposition mechanisms in mushroom-forming fungi. ISME Journal 14: 2046–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster GE, Behrens PQ, Airth RL. (1965). Bioluminescence and other characteristics of Collybia velutipes. American Journal of Botany 52: 487–495. [PubMed] [Google Scholar]
- Fons F, Rapior S, Fruchier A. et al. (2006). Volatile composition of Clitocybe amoenolens, Tricholoma caligatum and Hebeloma radicosum. Cryptogamie Mycologie 27: 45–55. [Google Scholar]
- Fontenla R, Para R. (2007). Osservazioni sul Genere Melanoleuca Studio dei typi – I. Rivista di Micologia 50: 221–236. [Google Scholar]
- Franchi P, Marchetti M. (2021). I Funghi Clavarioidi in Italia. Associazione micologica Bresadola, Trento, Italy. [Google Scholar]
- Franco-Molano AE. (1991). Catatrama (Tricholomataceae), a new genus from Costa Rica. Mycologia 83: 501–505. [Google Scholar]
- Frank JL, Coffan RA, Southworth D. (2010). Aquatic gilled mushrooms: Psathyrella fruiting in the Rogue River in Southern Oregon. Mycologia 102: 93–107. [DOI] [PubMed] [Google Scholar]
- Fries EM. (1821). Systema Mycologicum I. E. Berling, Lundiae, Sweden. [Google Scholar]
- Fries EM. (1838). Epicrisis Systematis Mycologici, seu synopsis Hymenomycetum. e Typographia Academica Redemtor Gleerup Lundae, Upsaliae, Sweden. [Google Scholar]
- Fries N. (1979). Germination of spores of Cantharellus cibarius. Mycologia 71: 216–219. [Google Scholar]
- Frøslev TG, Matheny PB, Hibbett DS. (2005). Lower-level relationships in the mushroom genus Cortinarius (Basidiomycota, Agaricales): a comparison of RPB1, RPB2, and ITS phylogenies. Molecular Phylogenetics and Evolution 37: 602–618. [DOI] [PubMed] [Google Scholar]
- Fushiya S, Sato S, Kazasawa T. et al. (1990). Acromelic acid C. A new toxic constituent of Clitocybe acromelalga: an efficient isolation of acromelic acids. Tetrahedron Letters 31: 3901–3904. [Google Scholar]
- Fushiya S, Sato S, Kera Y. et al. (1992). Isolation of acromelic acids D and E from Clitocybe acromelalga. Heterocycles 34: 1277–1280. [Google Scholar]
- Gams W. (1995). Report of the Committee for Fungi: 5. Taxon 44: 411–414. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for Basidiomycetes—application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Gargas A, DePriest PT, Grube M. et al. (1995). Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny. Science 268: 1492–1495. [DOI] [PubMed] [Google Scholar]
- Garnica S, Weiß M, Walther G. et al. (2007). Reconstructing the evolution of agarics from nuclear gene sequences and basidiospore ultrastructure. Mycological Research 111: 1019–1029. [DOI] [PubMed] [Google Scholar]
- Garnica S, Sandoval-Leiva P, Riess K. (2021). Phylogenetic relationships in the genus Podoserpula and description of P. aliweni, a new species from Chile. Mycologia 113: 1110–1121. [DOI] [PubMed] [Google Scholar]
- Garrido N. (1988). Agaricales s.l. und ihre Mykorrhizen in den Nothofagus Waldern Mittelchiles. Bibliotheca Mycologica 120: 1–528. [Google Scholar]
- Geesink J, Bas C. (1992). Clavaria stellifera, spec. nov. Persoonia 14: 671–673. [Google Scholar]
- Giannoni F, Pera U, Della Maggiora M. (2018). Volvopluteus earlei, an uncommon species throughout Europe, new for Tuscany. Micologia Toscana 0: 11–25. [Google Scholar]
- Gilbertson RL, Ryvarden L. (1986). North American Polypores. 1. Abortiporus–Lindtneria. Fungiflora, Oslo, Norway. [Google Scholar]
- Gilbertson RL, Ryvarden L. (1987). North American Polypores. 2. Megasporoporia–Wrightoporia. Fungiflora, Oslo, Norway. [Google Scholar]
- Gill M, Steglich W. (1987). Pigments of fungi (Macromycetes). Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products, vol. 51. Springer, Vienna, Austria. [DOI] [PubMed] [Google Scholar]
- Ginns P. (1997). Porodisculus pendulus: Systematics, cultural characters and Canadian records. Canadian Journal of Botany 75: 220–227. [Google Scholar]
- Gminder A. (2001). Hygrophoropsis (Schröter) Maire. In: Die Großpilze Baden – Württembergs. Band 3. Ständerpilze: Blätterpilze I. (Krieglsteiner GJ. ed). Eugen Ulmer Verlag, Stuttgart, Germany: 273–276. [Google Scholar]
- González GC, Barroetaveña C, Visnovsky SB. et al. (2021). A new species, phylogeny, and a worldwide key of the edible wood decay Fistulina (Agaricales). Mycological Progress 20: 733–746. [Google Scholar]
- Gregory DC. (2007). The Genus Clitocybe of California. A thesis submitted to the faculty of San Francisco State University in partial fulfillment of the requirements for the degree Master of Science in Biology: Ecology and Systematic Biology. San Francisco, California, USA. [Google Scholar]
- Greuter W, McNeill J, Barrie FR. et al. (2000). International Code of Botanical Nomenclature (St. Louis Code). Regnum Vegetabile 138. Koeltz Scientific Books, Königstein, Germany. [Google Scholar]
- Griffith GW, Easton GL, Jones AW. (2002). Ecology and diversity of waxcap (Hygrocybe spp.) fungi. Botanical Journal of Scotland 54: 7–22. [Google Scholar]
- Grigoriev IV, Nikitin R, Haridas S. et al. (2014). MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Research 42 (Database issue): D699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G. (1972). Kernzahl und sporenvolumen bei einigen Hymenogasterarten. Zeitschrift für Pilzkunde 38: 109–158. [Google Scholar]
- Guez D. (1990). Aperçu sur la flore mycologique du Japon. Bulletin trimestriel de la Fédération Mycologique Dauphiné-Savoie 116: 12–16. [Google Scholar]
- Guzmán G. (1987). Distribución y etnomicología de Pseudofistulina radicata en Mesoamérica, con nuevas localidades en México y su primer registro en Guatemala. Revista Mexicana de Micologia 3: 29–38. [Google Scholar]
- Guzmán G, Montoya L, Bandala VM. (1990). Observations on the genera Asproinocybe and Tricholosporum, and description of a new species of Tricholosporum (Agaricales, Tricholomataceae). Mycotaxon 38: 485–495. [Google Scholar]
- Guzmán G, Ramírez-Guillén F, Contu M. et al. (2004). New records of Asproinocybe and Tricholosporum (Agaricales, Tricholomataceae). Documents Mycologiques 33(131): 23–28. [Google Scholar]
- Hahn CH, Lohmeyer TR. (2010). Betrachtungen zur Gattung Amanita: Lamellenansatz und Sporenpulverfarbe. Mycologia Bavarica 11: 37–42. [Google Scholar]
- Halama M, Ważny R, Czosnykowska-Łukacka M. et al. (2016). Tricholoma ustaloides (Agaricales, Basidiomycota) in Poland. Polish Botanical Journal 61: 173–180. [Google Scholar]
- Hallen HE, Watling R, Adams GC. (2003). Taxonomy and toxicity of Conocybe lactea and related species. Mycological Research 107: 969–979. [DOI] [PubMed] [Google Scholar]
- Halbwachs H, Brandl R, Bässler C. (2015). Spore wall traits of ectomycorrhizal and saprotrophic agarics may mirror their distinct lifestyles. Fungal Ecology 17: 197–204. [Google Scholar]
- Halbwachs H, Easton G, Bol R. et al. (2018). Isotopic evidence of biotrophy and unusual nitrogen nutrition in soil-dwelling Hygrophoraceae: Hygrophoraceae 13C/15N natural abundance. Environmental Microbiology 20: 3573–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbwachs H, Bässler C. (2021). Functional traits of stipitate basidiomycetes. In: Encyclopedia of Mycology, vol. 1 (Zaragoza Ó, Casadevall A. eds). Elsevier B.V., Amsterdam, The Netherlands: 361–377. [Google Scholar]
- Halling R, Franco-Molano AE. (1996). Agaricales from Costa Rica: new taxa with ornamented spores. Mycologia 88: 666–670. [Google Scholar]
- Haqnawaz M, Khan Z, Niazi AR. et al. 2023. Volvariella variicystidiosa sp. nov. (Agaricaceae, Basidiomycota) from Punjab, Pakistan. Phytotaxa 578: 189–198. [Google Scholar]
- Harada E, Tada N, Kamei I. et al. (2021). Development of cultivation method for Leucopaxillus giganteus fruiting bodies in Cryptomeria japonica artificial forests. Journal of Forest Research 26(5): 386–394. [Google Scholar]
- Harder CB, Hesling E, Botnen SS. et al. (2023). Mycena species can be opportunist-generalist plant root invaders. Environmental Microbiology 25: 1875–1893. [DOI] [PubMed] [Google Scholar]
- Harmaja H. (1969). The genus Clitocybe (Agaricales) in Fennoscandia. Karstenia 10: 5–168. [Google Scholar]
- Harmaja H. (1974). A revision of the generic limits between Clitocybe and Lepista. Karstenia 14: 82–91. [Google Scholar]
- Harmaja H. (2002). Amylolepiota, Clavicybe and Cystodermella, new genera of the Agaricales. Karstenia 42: 39–48. [Google Scholar]
- Harmaja H. (2003). Notes on Clitocybe s. lato (Agaricales). Annales Botanici Fennici 40: 213–218. [Google Scholar]
- He MQ, Zhao RL, Hyde KD. et al. (2019). Notes, outline and divergence times of Basidiomycota. Fungal Diversity 99: 105–367. [Google Scholar]
- He Z-M, Chen Z-H, Bau T. et al. (2023). Systematic arrangement within the family Clitocybaceae (Tricholomatineae, Agaricales): phylogenetic and phylogenomic evidence, morphological data and muscarine-producing innovation. Fungal Diversity 123: 1–47. [Google Scholar]
- He Z-M, Yang ZL. (2021). A new clitocyboid genus Spodocybe and a new subfamily Cuphophylloideae in the family Hygrophoraceae (Agaricales). MycoKeys 79: 129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z-M, Yang ZL. (2022). The genera Bonomyces, Harmajaea and Notholepista from Northwestern China: two new species and a new record. Mycological Progress 21: 26. [Google Scholar]
- He Z-M, Yang ZL. (2023). A contribution to the knowledge of the genus Infundibulicybe (Tricholomatineae, Agaricales) in China: Two new species and five redescribed taxa. Mycologia 115: 693–713. [DOI] [PubMed] [Google Scholar]
- Heads SW, Miller AN, Crane J. et al. (2017). The oldest fossil mushroom. PLoS ONE 12: e0178327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton K, Kropp B. (2013). Phylogenetic placement of the genus Tricholosporum by DNA sequencing. Biological Posters 2013, Paper 126. https://digitalcommons.usu.edu/biology_posters/126
- Heim R. (1931). Le genre Inocybe. Encyclopédie Mycologique 1, Lechevalier, Paris, France. [Google Scholar]
- Heim R. (1969). Champignons d’Europe. N. Boubée et Cie, Paris, France. [Google Scholar]
- Henkel TW, Smith ME, Aime MC. (2010). Guyanagaster, a new wooddecaying sequestrate genus of Agaricales from the Guiana Shield. American Journal of Botany 97: 1474–1484. [DOI] [PubMed] [Google Scholar]
- Hesler LR, Smith AH. (1963). North American species of Hygrophorus. Univ. Tennessee Press, Knoxville. USA. [Google Scholar]
- Hess J, Pringle A. (2014). The natural histories of species and their genomes: asymbiotic and ectomycorrhizal Amanita fungi. Advances in Botanical Research 70: 235–257. [Google Scholar]
- Hibbett DS. (1996). Phylogenetic evidence for horizontal transmission of group I introns in the nuclear ribosomal DNA of mushroom-forming fungi. Molecular Biology and Evolution 13: 903–917. [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Pine EM, Langer E. et al. (1997). Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proceedings of the National Academy of Sciences USA 94: 12002–12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett DS, Donoghue MJ. (2001). Analysis of character correlations among wood decay mechanisms, mating systems, and substrate ranges in homobasidiomycetes. Systematic Biology 50: 215–242. [PubMed] [Google Scholar]
- Hibbett DS, Binder M, Wang Z. et al. (2003). Another fossil Agaric from Dominican amber. Mycologia 95: 685–687. [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Binder M. (2001). Evolution of marine mushrooms. Biological Bulletin 201: 319–322. [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Binder M. (2002). Evolution of complex fruiting-body morphologies in homobasidiomycetes. Proceedings of the Royal Society London B, Biological Sciences 269: 1963–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett DS, Thorn RG. (2001). Homobasidiomycetes. In: The Mycota, Vol. VII. Part B., Systematics and Evolution (McLaughlin DJ, McLaughlin EG, Lemke PA. eds). Springer-Verlag, Berlin, Germany: 121–168. [Google Scholar]
- Hilber O. (1982). Die Gattung Pleurotus (Fr.) Kummer unter besonderer Berücksichtigung des Pleurotus eryngii – Formenkomplexes. Bibliotheca Mycologica 87. J. Cramer, Vaduz, Liechtenstein. [Google Scholar]
- Hodkinson BP, Moncada B, Lücking R. (2014). Lepidostromatales, a new order of lichenized fungi (Basidiomycota, Agaricomycetes), with two new genera, Ertzia and Sulzbacheromyces, and one new species, Lepidostroma winklerianum. Fungal Diversity 64: 165–179. [Google Scholar]
- Hofstetter V, Clémençon H, Vilgalys R. et al. (2002). Phylogenetic analyses of the Lyophylleae (Agaricales, Basidiomycota) based on nuclear and mitochondrial rDNA sequences. Mycological Research 106: 1043–1059. [Google Scholar]
- Hofstetter V, Redhead SA, Kauff F. et al. (2014). Taxonomic revision and examination of ecological transitions of the Lyophyllaceae (Basidiomycota, Agaricales) based on a multigene phylogeny. Cryptogamie, Mycologie 35: 399–425. [Google Scholar]
- Holec J. (2009). Valid publication of the name Tricholomopsis flammula (Fungi, Basidiomycota, Tricholomataceae), a species clearly separated from T. rutilans. Journal of the National Museum (Prague), Natural History Series 178: 7–13. [Google Scholar]
- Holec J. (2012a). Tricholomopsis Singer. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 510–511. [Google Scholar]
- Holec J. (2012b). Tricholomopsis osiliensis (Basidiomycota, Agaricales), recently described from Estonia, found in Slovakia. Czech Mycology 64: 93–100. [Google Scholar]
- Holec J, Kolařík M. (2011). Tricholomopsis flammula (Basidiomycota, Agaricales)-molecular taxonomy, delimitation, variability and ecology. Mycological Progress 10: 93–99. [Google Scholar]
- Holec J, Kolařík M. (2012). Tricholomopsis in Europe - Phylogeny, key, and notes on variability. Mycotaxon 121: 81–92. [Google Scholar]
- Holec J, Kolařík M. (2017). First report of Mycena clavata (Fungi, Agaricales) in the Czech Republic including notes on its taxonomy, phylogenetic position and ecology. Czech Mycology 69: 1–14. [Google Scholar]
- Holec J, Kunca V, Kolařík M. (2019). Tricholomopsis badinensis sp. nov. and T. sulphureoides - two rare fungi of European old-growth forests. Mycological Progress 18: 321–334. [Google Scholar]
- Honan AH, Desjardin DE, Perry BA. et al. (2015). Towards a better understanding of Tetrapyrgos (Basidiomycota, Agaricales): new species, type studies, and phylogenetic inferences. Phytotaxa 231: 101–132. [Google Scholar]
- Hongo T. (1959). Notes on Japanese larger fungi (14). Journal of Japanese Botany 34: 239–346. [Google Scholar]
- Hongo T. (1960). Notes on Japanese larger fungi (15). Journal of Japanese Botany 35: 83–90. [Google Scholar]
- Hongo T. (1988). On the genus Tricholoma of Japan. Transactions of the Mycological Society of Japan 29: 441–447. [Google Scholar]
- Hongo T, Clémençon H. (1983). A new species of Lyophyllum from Japan. Mycologia Helvetica 1: 43–46. [Google Scholar]
- Horak E. (1968). Synopsis generum Agaricalium (Die Gattungen der Agaricales). Schweizerischen Naturforschenden Gesellschaft: Beiträge zur Kryptogamenflora der Schweiz, Bd. XIII. Büchler & Co AG, Wabern-Bern, Switzerland. [Google Scholar]
- Horak E. (1971). A contribution towards the revision of the Agaricales (fungi) from New Zealand. New Zealand Journal of Botany 9: 403–462. [Google Scholar]
- Horak E. (1979a). Xeromphalina and Heimiomyces in Indomalaya and Australasia. Sydowia 32: 131–153. [Google Scholar]
- Horak E. (1979b). Astrosporina (Agaricales) in Indomalaya and Australia. Persoonia 10: 157–205. [Google Scholar]
- Horak E. (1983). Neufunde und Bemerkungen zu einem emendierten Gattungskonzept von Pterospora Métrod (Agaricales). Sydowia 36: 125–138. [Google Scholar]
- Horak E. (1987). Astrosporina in the Alpine Zone of the Swiss National Park (SNP) and adjacent Regions. In: Arctic and Alpine Mycology II. (Laursen GA, Ammirati JF, Redhead SA. eds). Environmental Science Research, vol. 34. Springer, Boston, MA, USA: 205–234. [Google Scholar]
- Horak E. (2005). Röhrlinge und Blätterpilze in Europa. Spektrum, Elsevier, Heidelberg, Germany. [Google Scholar]
- Hosen MI, Li T-H, Lodge DJ. et al. (2016) The first ITS phylogeny of the genus Cantharocybe (Agaricales, Hygrophoraceae) with a new record of C. virosa from Bangladesh. MycoKeys 14: 37–50. [Google Scholar]
- Hosen MI, Xu J-Y, Li T. et al. (2020). Tricholomopsis rubroaurantiaca, a new species of Tricholomataceae from southern China. Mycoscience 61: 342–347. [Google Scholar]
- Hoshino T, Xiao N, Tkachenko OB. (2009). Cold adaptation in the phytopathogenic fungi causing snow molds. Mycoscience 50: 26–38. [Google Scholar]
- Hoshino T, Tkachenko OB, Tojo M. et al. (2022). Taxonomic revision of the Typhula ishikariensis complex. Mycoscience 63: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Yajima Y, Degawa Y. et al. (2023). Life cycle plasticity in Typhula and Pistillaria in the arctic and the temperate zone. Microorganisms 11: 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrouda P. (2001). Pleurotoid fungi of the family Polyporaceae in the Czech Republic and Slovakia. Czech Mycology 53: 29–87. [Google Scholar]
- Hsiang T, Wu C. (2000). Genetic relationships of pathogenic Typhula species assessed by RAPD, ITS-RFLP and ITS sequencing. Mycological Research 104: 16–22. [Google Scholar]
- Hu J, Hussain M, Zhang X. et al. (2020). Abundant and diverse fungal microbiota inhabit the white females and brown cysts of the cereal cyst nematode. Applied Soil Ecology 147: 103372. [Google Scholar]
- Hubregtse J. (2019). Fungi in Australia, Rev. 2.2. E-published by the Field Naturalists Club of Victoria Inc., Blackburn, Victoria, Australia. http://www.fncv.org.au/fungi-in-australia/ [Google Scholar]
- Huijsman HSC. (1960). Observations sur le genre Ripartites. Persoonia 1: 335–339. [Google Scholar]
- Hussain S, Afshan NuS, Khan J. et al. (2021). Cantharocybe habibii: a new species in the genus Cantharocybe. Plant Systematics and Evolution 307: 49. [Google Scholar]
- Hutchison LJ, Madzia SE, Barron GL. (1996). The presence and antifeedant function of toxin-producing secretory cells on hyphae of the lawninhabiting agaric Conocybe lactea. Canadian Journal of Botany 74: 431–434. [Google Scholar]
- Hutchison LJ, Kropp BR, Hausner G. (2012). Baeospora occidentalis, a new snowbank agaric from western North America. Mycoscience 53: 139–143. [Google Scholar]
- Ichimura T. (1918). A new poisonous mushroom. Botanical Gazette (Tokyo) 65: 109–111. [Google Scholar]
- Ikeda S, Hoshino T, Matsumoto N. et al. (2015). Taxonomic reappraisal of Typhula variabilis, Typhula laschii, Typhula intermedia, and Typhula japonica. Mycoscience 56: 549–559. [Google Scholar]
- Ikeda S, Hoshino T, Matsumoto N. et al. (2016). Rot diseases of carrot and rapeseed caused by Typhula species under snow in Hokkaido, Japan. Journal of General Plant Pathology 82: 286–291. [Google Scholar]
- Ingold CT. (1980). Mycelium, oidia and sporophore initials in Flammulina velutipes. Transactions of the British Mycological Society 75: 107–116 [Google Scholar]
- Ishaq M, Khan MB, Ullah S. et al. (2019). Infundibulicybe kotanensis sp. nov. (Tricholomataceae), a new species from Buner, Pakistan. Phytotaxa 418: 195–202. [Google Scholar]
- Jančovičová S, Glejdura S, Kunca V. (2012). Tectella patellaris (Agaricales) recorded in Slovakia. Catathelasma 14: 15–22. [Google Scholar]
- Jayasinghe BATD, Parkinson D. (2008) Actinomycetes as antagonists of litter decomposer agarics. Applied Soil Ecology 38: 109–118. [Google Scholar]
- Jayawardena RS, Hyde KD, Wang S. et al. (2022). Fungal diversity notes 1512–1610: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 117: 1–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Hughes KW, Petersen RH. (2001). Phylogenetic relationships of Panellus (Agaricales) and related species based on morphology and ribosomal large subunit DNA sequences. Mycotaxon 79: 7–21. [Google Scholar]
- Johnson JE, Petersen RH. (1997). Mating systems in Xeromphalina species. Mycologia 89: 393–399. [Google Scholar]
- Jones EBG, Suetrong S, Sakayaroj J. et al. (2015). Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Diversity 73: 1–72. [Google Scholar]
- Jones EBG, Pang KL, Abdel-Wahab MA. et al. (2019). An online resource for marine fungi. Fungal Diversity 96: 347–433. [Google Scholar]
- Josserand M. (1933). Notes critiques sur quelques champignons de la région lyonnaise. Bulletin de la Société Mycologique de France 49: 340–376. [Google Scholar]
- Jülich W. (1981). Higher Taxa of Basidiomycetes. J. Cramer, Vaduz, Liechtenstein. [Google Scholar]
- Jülich W. (1984). Die Nichtblätterpilze, Gallertpilze und Bauchpilze. Aphyllophorales, Heterobasidiomycetes, Gastromycetes. In: Kleine Kryptogamenflora begründet, IIb/1 (Gams H. ed). Gustav Fischer Verlag, Jena, Germany. [Google Scholar]
- Justo A, Morgenstern I, Hallen-Adams HE. et al. (2010) Convergent evolution of sequestrate forms in Amanita under Mediterranean climate conditions. Mycologia 102(3): 675–688. [DOI] [PubMed] [Google Scholar]
- Justo A, Vizzini A, Minnis AM. et al. (2011a) Phylogeny of the Pluteaceae (Agaricales, Basidiomycota): taxonomy and character evolution. Fungal Biology 115: 1–20. [DOI] [PubMed] [Google Scholar]
- Justo A, Minnis AM, Ghignone S. et al. (2011b). Species recognition in Pluteus and Volvopluteus (Pluteaceae, Agaricales): morphology, geography and phylogeny. Mycological Progress 10: 453–479. [Google Scholar]
- Justo A, Miettinen O, Floudas D. et al. (2017). A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biology 121: 798–824. [DOI] [PubMed] [Google Scholar]
- Kaiser P. (1998). Relations of Leucopaxillus giganteus, basidiomycete of fairy rings, with soil microflora and grassland plants. Cryptogamie, Mycologie 19: 45–61. [Google Scholar]
- Kalamees K. (2004). Palearctic Lyophyllaceae (Tricholomatales) in Northern and Eastern Europe and Asia. Scripta Mycologica 18: 1–135. [Google Scholar]
- Kalichman J, Kirk PM, Matheny PB. (2020). A compendium of generic names of agarics and Agaricales. Taxon 69: 425–447. [Google Scholar]
- Kantvilas G, May TW. (1995). Additional Lichen Records from Australia. 26. Marasmiellus affixus (Berk.) Singer, an overlooked basidiolichen. Australasian Lichenological Newsletter 37: 32–34. [Google Scholar]
- Kantvilas G, Jarman S. (2006). Recovery of lichens after logging: preliminary results from Tasmania’s wet forests. The Lichenologist 38: 383–394. [Google Scholar]
- Karasch P, Hahn Ch. (2009). Rote Liste gefährdeter Großpilze Bayerns. Bayerisches Landesamt für Umwelt, Augsburg, Germany. https://www.lfu.bayern.de/natur/rote_liste_grosspilze/doc/roteliste_grosspilze.pdf [Google Scholar]
- Karasiński D, Nagy LG, Szarkándi JG. et al. (2023). Cyphelloporia bialoviesensis (Fungi, Agaricales) - a new genus and species for a giant cyphelloid fungus from Białowieża virgin forest in Poland. Phytotaxa 589: 119–136. [Google Scholar]
- Kautmanová I, Adamčík S, Lizoň P. et al. (2012a). Revision of taxonomic concept and systematic position of some Clavariaceae species. Mycologia 104: 521–539. [DOI] [PubMed] [Google Scholar]
- Kautmanová I, Tomšovský M, Dueñas M. et al. (2012b). European species of Clavaria (Agaricales, Agaricomycetes) with dark basidiomata - a morphological and molecular study. Persoonia 29: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaygusuz O, Knudsen H, Türkekul I. et al. (2020) Volvariella turcica, a new species from Turkey, and a multigene phylogeny of Volvariella. Mycologia 112: 577–587. [DOI] [PubMed] [Google Scholar]
- Ke HM, Lee HH, Lin CI. et al. (2020). Mycena genomes resolve the evolution of fungal bioluminescence. Proceedings of the National Academy of Sciences USA 117: 31267–31277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana IPS, Thind KS, Berthier J. (1977). Lutypha sclerotiophila gen. et sp.nov. (Aphyllophorales) from India. Transactions of the British Mycological Society 68: 478–483. [Google Scholar]
- Kibby G. (2012). The Hygrophoropsis aurantiaca complex. Field Mycology 13: 43–50. [Google Scholar]
- Kibby G. (2020). Mushrooms and toadstools of Britain & Europe. Volume 2 Agarics – part 1. Kibby G. Pixart printing, Italy. [Google Scholar]
- Kim JH, Lee JS, Lee KR. et al. (2012) Immunomodulating and antitumor activities of Panellus serotinus polysaccharides. Mycobiology 40: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Sasaki H, Nara K. (2012). Multiple origins of sequestrate basidiomes within Entoloma inferred from molecular phylogenetic analyses. Fungal Biology 116: 1250–1262. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW. et al. (2008). Dictionary of the fungi. Oxford University Press, Wallingford, UK. [Google Scholar]
- Kirschner R, Lee IS, Chen CJ. (2013). Ovularia puerariae Sawada is the hyphomycetous anamorph of a new Marasmius species on living leaves of kudzu (Pueraria montana, Fabaceae). Mycologia 105: 781–792. [DOI] [PubMed] [Google Scholar]
- Klán J. (1984). The genus Xeromphalina (Tricholomataceae) in Europe. Ceskä Mykologie 38: 205–217. [Google Scholar]
- Kluting KL, Baroni TJ, Bergemann SE. (2014). Toward a stable classification of genera within the Entolomataceae: a phylogenetic re-evaluation of the Rhodocybe-Clitopilus clade. Mycologia 106: 1127–1142. [DOI] [PubMed] [Google Scholar]
- Knudsen H. (2008a). Phyllotopsis Singer. In: Funga Nordica–Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 243. [Google Scholar]
- Knudsen H. (2008b). Macrocystidia Earle. In: Funga Nordica–Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 312–313. [Google Scholar]
- Knudsen H. (2012a). Phyllotopsis Singer. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 321. [Google Scholar]
- Knudsen H. (2012b). Macrocystidia Earle. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 366. [Google Scholar]
- Knudsen H, Shiryaev AG. (2012). Typhulaceae Jülich. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 298–304. [Google Scholar]
- Knudsen H, Taylor A. (2008). Hygrophoropsis (J. Schröt.) Maire. In: Funga Nordica –Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 151–152. [Google Scholar]
- Knudsen H, Taylor A. (2012). Hygrophoropsis (J. Schröt.) Maire. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 195–197. [Google Scholar]
- Koch RA, Lodge JD, Sourell S. et al. (2018). Tying up loose threads: revised taxonomy and phylogeny of an avian-dispersed Neotropical rhizomorph-forming fungus. Mycological Progress 17: 989–998. [Google Scholar]
- Koch RA, Liu J, Brann M. et al. (2020). Marasmioid rhizomorphs in bird nests: species diversity, functional specifcity, and new species from the tropics. Mycologia 112: 1086–1103. [DOI] [PubMed] [Google Scholar]
- Koch RA, Herr JR. (2021). Transcriptomics reveals the putative mycoparasitic strategy of the mushroom Entoloma abortivum on species of the mushroom genus Armillaria. MSystems 6: e00544–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Kuo A, Nagy LG. et al. (2015). Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics 47: 410–415. [DOI] [PubMed] [Google Scholar]
- Kohzu A, Yoshioka T, Ando T. et al. (1999). Natural 13C and 15N abundance of field-collected fungi and their ecological implications. New Phytologist 144: 323–330. [Google Scholar]
- Komorowska H. (2005). The genus Mycenella (Agaricales, Tricholomataceae) in Poland. Polish Botanical Journal 50: 83–92. [Google Scholar]
- Komura DL, Oliveira JJSD, Moncalvo J. et al. (2020). Six new species of Tetrapyrgos (Basidiomycota, Agaricales, Marasmiaceae) from the Brazilian Amazon. Phytotaxa 440: 193–214. [Google Scholar]
- Konno K, Shirahama H, Matsumoto T. (1983). Isolation and structure of acromelic acid A and B. New kainoids of Clitocybe acromelalga. Tetrahedron Letters 24: 939–942. [Google Scholar]
- Konno K, Hashimoto K, Ohfune Y. et al. (1988). Acromelic acids A and B. Potent neuroexcitatory amino acids isolated from Clitocybe acromelalga. Journal of the American Chemical Society 110: 4807–4815. [Google Scholar]
- Konrad P, Maublanc A. (1934). Icones Selectae Fungorum 6: 1–558. [Google Scholar]
- Konrad P, Maublanc A. (1936). Révision des Hyménomycètes de France et des pays limitrophes. P. Lechevalier, Paris, France. [Google Scholar]
- Konrad P, Maublanc A. (1952). Les Agaricales, vol. 2. Encyclopédie Mycologique 20. P. Lechevalier, Paris, France. [Google Scholar]
- Korf RP. (1988). Reports (N.S. 1) of the Committee for Fungi and Lichens on Proposals to Conserve and/or Reject Names. Taxon 37: 450–463. [Google Scholar]
- Kornerup A, Wanscher JH. (1978). Methuen handbook of colour. 3rd edn. Methuen Ltd., London, UK. [Google Scholar]
- Korotkin HB, Swenie RA, Miettinen O. et al. (2018). Stable isotopic analyses reveal previously unknown trophic mode diversity in the Hymenochaetales. American Journal of Botany 105: 1–9. [DOI] [PubMed] [Google Scholar]
- Kost G. (1986). Morphologie, anatomie und systematic carotinoidhaltiger blätterpilze. Berichte der Deutschen Botanischen Gesellschaft 99: 43–58. [Google Scholar]
- Kotlába F, Pouzar Z. (1972). Taxonomic and nomenclatural notes on some Macromycetes. Ceská Mykologie 26: 217–222. [Google Scholar]
- Kotlobay AA, Sarkisyan KS, Mokrushina YA. et al. (2018). Genetically encodable bioluminescent system from fungi. Proceedings of the National Academy of Sciences USA 115: 12728–12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko A. (1989). Definitorium fungorum URSS. Ordo Hygrophorales. Nauka 37, Leningrad, URSS. [Google Scholar]
- Kühdorf K, Münzenberger B, Begerow D. et al. (2016). Arbutoid mycorrhizas of the genus Cortinarius from Costa Rica. Mycorrhiza 26: 497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühner R. (1930). Un nouveau groupe d’Agarics leucosporés. Bulletin Mensuel de la Société linnéenne de Lyon 9: 67–72. [Google Scholar]
- Kühner R. (1938a). Le genre Mycena. P. Lechevalier, Paris, France. [Google Scholar]
- Kühner R. (1938b). Utilisation du carmin acétique dans la classification des Agarics leucosporés. Bulletin Mensuel de la Société linnéenne de Lyon 7: 204–211. [Google Scholar]
- Kühner R. (1945). Le problème de la filiation des Agaricales à la lumière de nouvelles observations d’ordre cytologique sur les agaricales leucosporées. Bulletin Mensuel de la Société linnéenne de Lyon 14: 160–169. [Google Scholar]
- Kühner R. (1950). Tricholoma mirabile Bres. et le parti qu’on peut tirer des exsiccata d’agarics. Bulletin mensuel de la Société linnéenne de Lyon 19: 215–220. [Google Scholar]
- Kühner R. (1973). Architecture de la paroi sporique des hyménomycetes et de ses différenciations. Persoonia 7: 217–248. [Google Scholar]
- Kühner R. (1976). Agaricales de la zone alpine. Lépistée. Bulletin de la Société mycologique de France 92: 5–32. [Google Scholar]
- Kühner R. (1977) Étude morphologique, anatomique et cytologique des carpophores de l’agaricale Leucopaxillus mirabilis (Bres.) Kuhn.-Romagn. Bulletin Mensuel de la Société linnéenne de Lyon 46: 6–10. [Google Scholar]
- Kühner R. (1978). Agaricales de la zone alpine. Genre Melanoleuca Pat. Bulletin de la Société linnéenne de Lyon 47: 12–52. [Google Scholar]
- Kühner R. (1979a). Les grandes lignes de la classification des Agaricales, Plutéales, Tricholomatales (suite). Bulletin Mensuel de la Société linnéenne de Lyon 48: 145–176. [Google Scholar]
- Kühner R. (1979b). Les grandes lignes de la classification des Agaricales, Plutéales, Tricholomatales (suite). Bulletin Mensuel de la Société linnéenne de Lyon 48: 273–304. [Google Scholar]
- Kühner R. (1980). Les Hyménomycètes agaricoïdes (Agaricales, Tricholomatales, Plutéales, Russulales). Etude générale et classification. Bulletin de la Société linnéenne de Lyon, numéro spécial 49. Société linnéenne de Lyon, Lyon, France. [Google Scholar]
- Kühner R. (1984). Some mainlines of classification in the gill fungi. Mycologia 76: 1059–1074. [Google Scholar]
- Kühner R, Maire R. (1934). Étude de la réaction de la membrane sporique à l’ iode dans les divers genres d’Agarics leucosporés. Bulletin Trimestriel de la Société Mycologique de France 50: 9–24 [Google Scholar]
- Kühner R, Romagnesi H. (1947). Caractères et affinités du Tricholoma guttatum au sens de Lange. Bulletin Mensuel de la Société linnéenne de Lyon 7: 134–137. [Google Scholar]
- Kühner R, Romagnesi H. (1953). Flore anaytique des champignons supérieurs (Agarics, Bolets, Chanterelles). Masson, Paris, France. [Google Scholar]
- Kumar TKA, Manimohan P. (2013). Molecular phylogeny reveals Megacollybia virosa is a Cantharocybe. Mycotaxon 124: 231–238. [Google Scholar]
- Kumar J, Atri NS. (2021). Characterisation and identification of ectomycorrhizae formed by the species of Asproinocybe (Tricholomataceae) and Inocybe (Inocybaceae) with the roots of the tropical sal tree Shorea robusta (Dipterocarpaceae). Ukrainian Botanical Journal 78: 112–122. [Google Scholar]
- Kumla J, Suwannarach N, Lumyong S. (2018). Cantharocybe virosa, first record of the genus in Thailand. Mycotaxon 133: 481–185. [Google Scholar]
- Kumla J, Suwannarach N, Wannathes N. et al. (2022). Survey of Volvariella (Agaricales, Basidiomycota) including two new species, V. neovolvacea and V. thailandensis, from Northern Thailand. Diversity 14: 161. [Google Scholar]
- Kummer P. (1871). Der Führer in die Pilzkunde. Verlag von E. Luppe‘s Buchhandlung, Zerbst, Germany. [Google Scholar]
- Kuntze O. (1891). Revisio generum plantarum 2. A. Felix, Leipzig, Germany: 375–1011. [Google Scholar]
- Kunze A, Neubauer C, Förster H. (2012). Die Mär vom giftigen Muschelseitling und eine Frage des guten Geschmacks. Der Tintling 74: 41–48. [Google Scholar]
- Kusuma HI, Harnelly E, Thomy Z. (2021). Agaricales: the most dominated macroscopic fungi in Tahura Pocut Meurah Intan forest park. Journal of Physics: Conference Series 1882: 012096. [Google Scholar]
- Kuyper TW. (1983). A new species of Clitocybe. Sydowia 36: 173–175. [Google Scholar]
- Kuyper ThW. (1986). Generic delimitation in European omphalinoid Tricholomataceae. In: La Famiglia delle Tricholomataceae, Atti del Centro Studi per la Flora Mediterranea. Borgo Val di Taro (Italy) 6: 83–104. [Google Scholar]
- Kuyper ThW. (1995a). Hygrophoropsis (Schroet.) Maire. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 64–66. [Google Scholar]
- Kuyper ThW. (1995b). Fayodia Kühner. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 153–155. [Google Scholar]
- Kuyper ThW. (1995c). Gamundia Raithelhuber. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 155–156. [Google Scholar]
- Kuyper ThW. (1995d). Genus Omphalina Quél. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 78–88. [Google Scholar]
- Kuyper ThW. (1995e): Omphaliaster Lamoure. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 78. [Google Scholar]
- Læssøe T, Rosendahl S. (1994). Rhodocybe stangliana, a parasite on other Agarics. Mycological Research 98: 88–90. [Google Scholar]
- Læssøe T, Petersen JH. (2008). Svampelivet på ækvator. Svampe 58: 1–52. [Google Scholar]
- Læssøe T, Davey ML, Petersen JH. (2016). A new species of Maireina on Filipendula ulmaria. Karstenia 56: 39–46. [Google Scholar]
- Læssøe T, Petersen JH. (2019). Fungi of temperate Europe. Princeton University Press, Princeton, USA. [Google Scholar]
- Lamoure D. (1971). Agaricales de la zone alpine. Rhodocybe borealis Lange & Skifte, et sa position systematique. Svensk Botanisk Tidskrift 65: 278–282. [Google Scholar]
- Lamoure D. (1974). Agaricales de la zone alpine. Genre Omphalina (1ère partie). Travaux scientifiques du Parc National de la Vanoise 5: 149–164. [Google Scholar]
- Lamoure D. (1975). Agaricales de la zone alpine. Genre Omphalina (2e partie). Travaux scientifiques du Parc National de la Vanoise 6: 153–166. [Google Scholar]
- Lamoure D. (1982). Alpine and circumpolar Omphalina species. In: Arctic and Alpine Mycology 1 (Laursen GA, Ammirati JF. eds). University of Washington Press, Seattle, USA: 201–215. [Google Scholar]
- Lange JE. (1930). Studies in the agarics of Denmark. Part VIII. Omphalia, Pleurotus, Clitocybe. Dansk Botanisk Arkiv 6: 1–61. [Google Scholar]
- Lange JE. (1935). Studies in the agarics of Denmark. Part X. Cortinarius. Dansk Botanisk Arkiv 8: 1–51. [Google Scholar]
- Lange M, Sivertsen S. (1966). Some species of Lyophyllum, Rhodocybe, and Fayodia with rough spores. Nomenclature and taxonomic position. Botanisk Tidsskrift 62: 197–211. [Google Scholar]
- Larsson E, Sundberg H. (2011). Lyophyllum shimeji, a species associated with lichen pine forest in northern Fennoscandia. Mycoscience 52: 289–295. [Google Scholar]
- Larsson KH. (2007a). Re-thinking the classification of corticioid fungi. Mycological Research 111: 1040–1063. [DOI] [PubMed] [Google Scholar]
- Larsson KH. (2007b). Molecular phylogeny of Hyphoderma and the reinstatement of Peniophorella. Mycological Research 111: 186–195. [DOI] [PubMed] [Google Scholar]
- Larsson KH, Larsson E, Kõljalg U. (2004). High phylogenetic diversity among corticioid basidiomycetes. Mycological Research 108: 983–1002. [DOI] [PubMed] [Google Scholar]
- Larsson KH, Parmasto E, Fischer M. et al. (2006) Hymenochaetales: a molecular phylogeny for the hymenochaetoid clade. Mycologia 98: 926–936. [DOI] [PubMed] [Google Scholar]
- Latha KPD, Raj KNA, Manimohan P. (2020). Diversity of the genus Calocybella Vizzini, Consiglio & Setti in Kerala State, India. Cryptogamie, Mycologie 41: 147–156. [Google Scholar]
- Lavorato C, Contu M. (2001). Due interessanti Agaricales raccolte in Svizzera. Rivista di Micologia 44: 347–351. [Google Scholar]
- Lawrey JD, Lücking R, Sipman HJM. et al. (2009). High concentration of basidiolichens in a single family of agaricoid mushrooms (Basidiomycota: Agaricales: Hygrophoraceae). Mycological Research 113: 1154–1171. [DOI] [PubMed] [Google Scholar]
- Leal-Dutra CA, Griffith GW, Neves MA. et al. (2020). Reclassification of Pterulaceae Corner (Basidiomycota: Agaricales) introducing the antassociated genus Myrmecopterula gen. nov., Phaeopterula Henn. And the corticioid Radulomycetaceae fam. Nov. SIM 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel T, Catcheside PS. (2009). The truffle genus Cribbea (Physalacriaceae, Agaricales) in Australia. Australian Systematic Botany 22: 39–55. [Google Scholar]
- Lebel T, Castellano MA, Beever RE. (2015). Cryptic diversity in the sequestrate genus Stephanospora (Stephanosporaceae: Agaricales) in Australasia. Fungal Biology 119: 201–228. [DOI] [PubMed] [Google Scholar]
- Lebel T, Syme K, Barrett MD. et al. (2020). Two new species of Asproinocybe (Tricholomataceae) from Australasia. Muelleria 38: 77–85. [Google Scholar]
- Lechner BE, Wright JE, Popoff E. (2006). New taxa and new records of fungi for Argentina from Iguazú National Park, Misiones. Fungal Diversity 21: 131–139. [Google Scholar]
- Lee JS, Jung HS. (2008). Porodisculus orientalis sp. nov. (Schizophyllaceae, Agaricales) from East Asia. Mycotaxon 104: 215–222. [Google Scholar]
- Lee M, Dukan E, Milne I. (2018). Amanita muscaria (Fly Agaric): from a shamanistic hallucinogen to the search for acetylcholine. Journal of the Royal College of Physicians of Edinburgh 48: 85–91. [DOI] [PubMed] [Google Scholar]
- Lee C-H, Lee Y-Y, Chang Y-C. et al. (2023). A carnivorous mushroom paralyzes and kills nematodes via a volatile ketone. Science Advances 9: eade4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Lüderitz M. (2018). Die “Gattung” Hemimycena in Schleswig-Holstein. Fungi Cimbricae I. Kiel/Eutin, Germany. [Google Scholar]
- Leonardi M, Ciulli G, Pacioni G. et al. (2002). Una intossicazione collettiva da Clitocybe amoenolens riconducibile alla sindrome acromelalgica. Micologia e Vegetazione Mediterranea 17: 133–142. [Google Scholar]
- Lepp H. (2011a). Is Marasmiellus affixus a Lichen? Fungimap Newsletter 44: 14. [Google Scholar]
- Lepp H. (2011b). Case Studies. Marasmiellus affixus. https://www.anbg.gov.au/lichen/case-studies/marasmiellus-affixus.html
- Li Q, He X, Ren Y. et al. (2020). Comparative mitogenome analysis reveals mitochondrial genome differentiation in ectomycorrhizal and asymbiotic Amanita species. Frontiers in Microbiology 11:1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T-H, Chen X-L, Shen Y-H. et al. (2009). A white species of Volvariella (Basidiomycota, Agaricales) from southern China. Mycotaxon 109: 255–261. [Google Scholar]
- Li T, Li T-H, Wang C. et al. (2017). Gerhardtia sinensis (Agaricales, Lyophyllaceae), a new species and newly recorded genus for China. Phytotaxa 332: 172–180. [Google Scholar]
- Li Y, Steenwyk JL, Chang Y. et al. (2021). A genome-scale phylogeny of the kingdom Fungi. Current Biology 31: 1653–1665.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Nakasone KK, Chen C-C. et al. (2022). Taxonomy and phylogeny of Cystostereaceae (Agaricales, Basidiomycota): A new genus, five new species, and three new combinations. Journal of Fungi 8: 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liimatainen K, Kim JT, Pokorny L. et al. (2022). Taming the beast: a revised classification of Cortinariaceae based on genomic data. Fungal Diversity 112: 89–170. [Google Scholar]
- Linkins AE, Antibus RK. (1982). Mycorrhizae of Salix rotundifolia in coastal arctic tundra. In: Arctic and alpine mycology (Laursen GA, Ammirati JF. eds). University of Washington Press, Seattle, USA: 509–531. [Google Scholar]
- Liu J-W, Ge Z-W, Horak E. et al. (2021). Squamanitaceae and three new species of Squamanita parasitic on Amanita basidiomes. SIM 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L-N, Bau T. (2018). Two new Xeromphalina (Mycenaceae, Agaricales) species with eccentric stipes from subtropical China. Phytotaxa 379: 277–286. [Google Scholar]
- Liu L-N, Zhou G-Y, Shen A-R. et al. (2022). Mycena subpiligera sp. nov., a symbiotic species from China associated with the seed germination of Gastrodia elata. Mycobiology 50: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-L, Wu F, He S-H. (2016). Lindtneria asiae-orientalis sp. nov. (Stephanosporaceae, Basidiomycota) from China based on morphological and molecular characters. Phytotaxa 260: 283–290. [Google Scholar]
- Liu S-L, Wei H-W, Zhou L-W. (2023). Xenasmatellales ord. nov. and Xenasmatellaceae fam. nov. for Xenasmatella (Agaricomycetes, Basidiomycota). Mycology 14: 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu P, Hu H. et al. (2016). Tricholosporum, a newly recorded genus of Agaricomycetes in China. Phytotaxa 289: 263–270. [Google Scholar]
- Lodge DJ, Matheny PB, Cantrell SA. et al. (2006). Delineating the Hygrophoraceae: character myths vs. gene trees. Inoculum 57: 27; poster (uploaded to the following website 17 Apr. 2013) http://www.aber.ac.uk/waxcap/links/index.shtml [Google Scholar]
- Lodge DJ, Padamsee M, Matheny PB. et al. (2014). Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales). Fungal Diversity 64: 1–99. [Google Scholar]
- Loizides M. (2021). Basidiomycete diversity within Calabrian pine (Pinus brutia) ecosystems on the island of Cyprus. Mycotaxon 136: 543. [Google Scholar]
- Loree MAJ, Lumme I, Niemi M. et al. (1989). Inoculation of willows (Salix spp.) with ectomycorrhizal fungi on mined boreal peatland. Plant and Soil 116: 229–238. [Google Scholar]
- Lücking R, Hodkinson BP, Leavitt SD. (2017). The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota - Approaching one thousand genera. Bryologist 119: 361–416. [Google Scholar]
- Ludwig E. (2001a). Pilzkompendium. 1: Abbildungen. Die kleineren Gattungen der Makromyzeten mit lamelligem Hymenophor aus den Ordnungen Agaricales, Boletales und Polyporales. IHW-Verlag, Eching, Germany. [Google Scholar]
- Ludwig E. (2001b). Pilzkompendium. 1: Beschreibungen. Die kleineren Gattungen der Makromyzeten mit lamelligem Hymenophor aus den Ordnungen Agaricales, Boletales und Polyporales. IHW-Verlag, Eching, Germany. [Google Scholar]
- Lüli Y, Cai Q, Chen Z-H. et al. (2019). Genome of lethal Lepiota venenata and insights into the evolution of toxin-biosynthetic genes. BMC Genomics 20: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Mo M, Huang X. et al. (2004). Coprinus comatus: A basidiomycete fungus forms novel spiny structures and infects nematode. Mycologia 96: 1218–1224. [PubMed] [Google Scholar]
- Luo H, Li X, Li G. et al. (2006). Acanthocytes of Stropharia rugosoannulata function as a nematode-attacking device. Applied and Environmental Microbiology 72: 2982–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Liu Y, Fang L. et al. (2007). Coprinus comatus damages nematode cuticles mechanically with spiny balls and produces potent toxins to immobilize nematodes. Applied and Environmental Microbiology 73: 3916–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Cai Q, Lüli YJ. et al. (2018). The MSDIN family in amanitinproducing mushrooms and evolution of the prolyl oligopeptidase genes. SIM 9: 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzoni F. (1997). Phylogeny of lichen- and nonlichen-forming omphalinoid mushrooms and the utility of testing for combinability among multiple data sets. Systematic Biology 46: 373–406. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Pagel M. (1997). Accelerated evolution as a consequence of transitions to mutualism. Proceedings of the National Academy of Sciences USA 94: 11422–11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzoni F, Vilgalys R. (1995). Omphalina (Basidiomycota, Agaricales) as a model system for the study of coevolution in lichens. Cryptogamic Botany 5: 71–81. [Google Scholar]
- Maas Geesteranus RA. (1992) Mycenas of the Northern Hemisphere I. Studies in Mycenas and other papers. Mycenas of the Northern Hemisphere II. Conspectus of the Mycenas and other papers. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen Series C. Biological and medical sciences, vol. 90. North–Holland, Amsterdam, The Netherlands. [Google Scholar]
- Maas Geesteranus RA, Horak E. (1995). Mycena and related genera from Papua New Guinea and New Caledonia. Bibliotheca Mycologica 159: 143–229. [Google Scholar]
- Machol RE, Singer R. (1971). Bayesian analysis of generic relations in Agaricales. Nova Hedwigia 21: 753–787. [Google Scholar]
- Madrid H, Cano J, Stchigel A. et al. (2010). Ramophialophora humicola and Fibulochlamys chilensis, two new microfungi from soil. Mycologia 102: 605–612. [DOI] [PubMed] [Google Scholar]
- Maire R. (1926). Études mycologiques (fascicule 2). Bulletin de la Société Mycologique de France 40: 293–317. [Google Scholar]
- Maire R. (1928). Diagnoses des Champignons inédits de l’Afrique du Nord. Bulletin trimestriel de la Société mycologique de France 44: 37–56. [Google Scholar]
- Malençon G, Bertault R. (1975). Flore des champignons supérieurs du Maroc, tome 2. Travaux de l’Institut scientifique chérifien et de la Faculté des sciences. Série Botanique et biologique végétale 33: 1–540. [Google Scholar]
- Malysheva E, Morozova O. (2005). New data on the genus Mycenella (Agaricales) in Russia. Mikologiya I Fitopatologiya 39: 39–49. [Google Scholar]
- Malysheva E, Popov E. (2022). Observations on Pluteaceae in Vietnam. 3. One new species of Volvariella. Phytotaxa 545: 278–286. [Google Scholar]
- Malysheva E, Malysheva V, Alexandrova A. et al. (2019). Observations on Pluteaceae in Vietnam. 1. New species and new records of Volvariella. Phytotaxa 408: 233–254. [Google Scholar]
- Malysheva E, Malysheva V, Alexandrova A. et al. (2020). Observations on Pluteaceae in Vietnam. 2. One new record and ten new species of Pluteus. Phytotaxa 461: 79–107. [Google Scholar]
- Malysheva E, Popov E, Morozova O. et al. (2023). Observations on Pluteaceae in Vietnam: four new species and new records of Pluteus. Journal of Fungi 9: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao N, Xu Y-Y, Fan L. (2021). Three new species of Tricholomopsis (incertae sedis) from North China based on morphological and molecular data. Phytotaxa 507: 155–166. [Google Scholar]
- Mao N, Lv J-C, Zhao T-Y. et al. (2022). Bonomyces pseudoarnoldii (Biannulariaceae, Agaricales), a new species from China revealed by morphology, and multilocus phylogenetic analysis. Phytotaxa 545: 69–78. [Google Scholar]
- Markones R. (2003). The first Bavarian record of Leucopaxillus rhodoleucus. Mycologia Bavarica 6: 37–40. [Google Scholar]
- Marchand A. (1986). Champignons du Nord et du Midi. Volume 9, Les tricholomes. Société Mycologique des Pyrénées Méditerranéennes, Perpignan, France. [Google Scholar]
- Marinetti V, Recchia G. (2005). Nuovi casi di sindrome acromelalgica in Abruzzo. Bollettino del Gruppo Micologico G. Bresadola – Nuova Serie 48: 39–43. [Google Scholar]
- Marlin M, Wolf A, Alomran M. et al. (2019). Nematophagous Pleurotus species consume some nematode species but are themselves consumed by others. Forests 10: 404. [Google Scholar]
- Martínez F, Martínez R, Meléndez A. et al. (2010). Clitocybe amoenolens. Primera cita para España. Boletín de la Sociedad Micológica de Madrid 34: 103–112. [Google Scholar]
- Masumoto H, Degawa Y. (2020). Bryoclavula phycophila gen. et sp. nov. belonging to a novel lichenized lineage in Cantharellales (Basidiomycota). Mycological Progress 19: 705–714. [Google Scholar]
- Matheny PB, Liu Y-J, Ammirati JF. et al. (2002). Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Curtis JC, Hofstter V. et al. (2006). Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 982–995. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Wang Z, Binder M. et al. (2007). Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Molecular Phylogenetics and Evolution 43: 430–451. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Moreau P-A, Vizzini A. et al. (2015). Crassisporium and Romagnesiella: two new genera of dark-spored Agaricales. Systematics and Biodiversity 13: 28–41. [Google Scholar]
- Matheny PB, Hughes KW, Kalichman J. et al. (2020a). Pulverulina, a new genus of Agaricales for Clitocybe ulmicola. Southeastern Naturalist 19: 447–459. [Google Scholar]
- Matheny PB, Hobbs AM, Esteve-Raventós F. (2020b). Genera of Inocybaceae: New skin for the old ceremony. Mycologia 112: 83–120. [DOI] [PubMed] [Google Scholar]
- Matsumoto N. (1992). Evolutionary ecology of the pathogenic species of Typhula. Transactions of the Mycological Society of Japan 33: 269–285. [Google Scholar]
- Matsumoto N. (2009). Snow Molds: a group of fungi that prevail under snow. Microbes and Environments 24: 14–20. [DOI] [PubMed] [Google Scholar]
- Maynard DS, Bradford MA, Kovey KR. et al. (2019). Consistent tradeoffs in fungal trait expression across broad spatial scales. Nature Microbiology 4: 846–853. [DOI] [PubMed] [Google Scholar]
- McCabe DE. (1979). Synchronous production and developmental history of sporocarps of Limnoperdon incarnatum. Mycologia 71: 899–907. [Google Scholar]
- McDonald JV. (2015). Morphological and molecular systematics of Resupinatus (Basidiomycota). Thesis. The University of Western Ontario, London, Canada. [Google Scholar]
- McDonald JV, Thorn RG. (2019). Nomenclatural novelties. Index Fungorum 425: 1. [Google Scholar]
- Meerts P. (1999). The evolution of spores in agarics: do big mushrooms have big spores? Journal of Evolutionary Biology 12: 161–165. [Google Scholar]
- Menolli N, Jr, Capelari M. (2008). Records and two new species of Volvariella (Pluteaceae, Agaricales) from Brazil. Mycotaxon 106: 385–398. [Google Scholar]
- Merlini L, Nasini G, Scaglioni L. et al. (2000). Structure elucidation of clavilactone D: an inhibitor of protein kinases. Phytochemistry 53: 1039–1041. [DOI] [PubMed] [Google Scholar]
- Mešić A, Tkalčec Z. (2009). Studies on Croatian Basidiomycota 1: Gerhardtia piperata (Agaricales). Mycotaxon 110: 413–421. [Google Scholar]
- Métrod G. (1949). Les Clitocybes. Revue de Mycologie 14 (Suppl.): 4–37. [Google Scholar]
- Michaelides J, Kendrick B. (1982). The bubble-trap propagules of Beverwykella, Helicoon and other aero-aquatic fungi. Mycotaxon 14: 247–260. [Google Scholar]
- Migliozzi V, Camboni M. (1999). La micoflora del litorale romano. 1ª Contributo. Bollettino del Gruppo Micologico G. Bresadola – Nuova Serie 42: 15–45. [Google Scholar]
- Miller OK., Jr (1968). A revision of the genus Xeromphalina. Mycologia 60: 156–188. [Google Scholar]
- Miller OK., Jr (1970). The genus Panellus in North America. Michigan Botanist 9: 17–30. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA: 1–8. [Google Scholar]
- Mleczko P. (2004). Amanita citrina (Schaeff.) S.F. Gray + Pinus sylvestris L. Descriptions of Ectomycorrhizae 7/8: 1–10. [Google Scholar]
- Molina R, Trappe JM. (1982). Lack of mycorrhizal specificity in the ericaceous hosts Arbutus menziesii and Arctostaphylos uva-ursi. New Phytologist 90: 495–509. [DOI] [PubMed] [Google Scholar]
- Minnis AM, Sundberg WJ, Methven AS. et al. (2006). Annulate Pluteus species: a study of the genus Chamaeota in the United States. Mycotaxon 96: 31–39. [Google Scholar]
- Miyauchi S. (1998). Comparison Clitocybe acromelalga with Clitocybe sp. collected in France. Rapport of the Nagaoka University of Technology, sect. Bioengineering Kamitomioka 1603-1, Nagaoka, Japan. [Google Scholar]
- Moncalvo JM, Lutzoni FM, Rehner SA. et al. (2000). Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Systematic Biology 49: 278–305. [DOI] [PubMed] [Google Scholar]
- Moncalvo JM, Vilgalys R, Redhead SA. et al. (2002) One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400. [DOI] [PubMed] [Google Scholar]
- Money NP. (2016). Fungal Diversity. In: The Fungi, 3rd edn (Watkinson SC, Boddy L, Money NP. eds). Elsevier, Academic Press, Amsterdam, The Netherlands: 1–36. [Google Scholar]
- Monti G, Marchetti M, Gorrieri L. et al. (2001). Funghi di ambienti dunali. Indagine degli ecosistemi dunali del Parco naturale Migliarino San Rossore Massaciuccoli. Ente Parco Regionale Migliarino San Rossore Massaciuccoli, Italy. [Google Scholar]
- Montoya L, Bandala VM, Esqueda M. (2021). Volvopluteus canalipes comb. nov. (Pluteaceae) from the Sonoran Desert of Mexico. Phytotaxa 505: 275–285. [Google Scholar]
- Moore D. (1987). The formation of agaric gills. Transactions of the British Mycological Society 89: 105–108. [Google Scholar]
- Moreau P-A, Courtecuisse R, Guez D. et al. (2001). Analyse taxinomique d’une espèce toxique: Clitocybe amoenolens Malençon. Cryptogamie, Mycologie 22: 95–117. [Google Scholar]
- Moreau P-A, Vila J, Pérez-De-Gregorio MÀ. et al. (2005). Hemimycena conidiogena, a new cistophilous basidiomycete. Mycotaxon 91: 323–332. [Google Scholar]
- Moreau PA, Contu M. (2007). Une espèce remarquable de l’Étage thermoméditerranéen de Corse et de Sardaigne: Tricholosporum cossonianum (Maire) comb. nov. Bulletin Semestriel de la Fédération des Associations Mycologiques Méditerranéennes 32: 41–52. [Google Scholar]
- Moreno G, Heykoop M. (1996). Xeromphalina junipericola sp. nov. (Tricholomataceae, Agaricales) from Spain. Zeitschrift fur Mykologie 62: 37–41. [Google Scholar]
- Moser MM. (1953). Kleine Kryptogamenflora von Mitteleuropa - Die Blätterund Bauchpilze (Agaricales und Gastromycetes) 2 (Gams H. ed). G. Fischer. Stuttgart, Germany. [Google Scholar]
- Moser MM. (1963). Zur variabilität von Leucopaxillus mirabilis (Bres.) Mos. Schweizerische Zeitschrift für Pilzkunde 41: 181–194. [Google Scholar]
- Moser MM. (1978). Röhrlinge und Blätterpilze. 4. Aufl. Kleine Kryptogamenflora Mitteleuropas. Bd. 2b/2 (Gams H. ed). G. Fischer. Stuttgart, Germany. [Google Scholar]
- Moser MM. (1979). Über einige neue oder seltene Agaricales-Arten aus dem Pieniny und aus Biesczciade, Polen. Beihefte zur Sydowia 8: 268–275. [Google Scholar]
- Moser MM, Jülich W. (1993). Farbatlas der Basidiomyceten. III. Leucopaxillus 1. G. Fischer, Stuttgart, New York. [Google Scholar]
- Mou G-F, Bau T. (2021). Asproinocybaceae fam. nov. (Agaricales, Agaricomycetes) for accommodating the genera Asproinocybe and Tricholosporum, and description of Asproinocybe sinensis and Tricholosporum guangxiense sp. nov. Journal of Fungi 7: 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu M, Huang HY, Huang T. et al. (2021). Gerhardtia yunnanensis (Agaricales, Lyophyllaceae), a new species from southwest China. Phytotaxa 484: 217–226. [Google Scholar]
- Mua A, Sanna M. (2006). Contributo alla conoscenza del genere Clitocybe in Sardegna. Bollettino del Gruppo Micologico G. Bresadola – Nuova Serie 49: 113–124. [Google Scholar]
- Mullis KB, Faloona FA. (1987) Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods in Enzymology 155: 335–350. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Mitchell DT. (2001). Interactions between Tricholomopsis rutilans and ectomycorrhizal fungi in paired culture and in association with seedlings of lodgepole pine and Sitka-spruce. Forest Pathology 31: 331–344. [Google Scholar]
- Murray MG, Thompson WF. (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research 8: 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrill WA. (1913). The Agaricaceae of the Pacific Coast: IV. New Species of Clitocybe and Melanoleuca. Mycologia 5: 206–223. [Google Scholar]
- Musumeci E, Contu M, Vizzini A. (2010). Gamundia nivea sp. nov. (Basidiomycota, Agaricomycetes) from central Europe (France). Nordic Journal of Botany 28: 428–431. [Google Scholar]
- Musumeci E, Contu M. (2014). Tephroderma (Agaricomycetidae, Tricholomatoid clade), un nuovo genere di basidiomiceti lamellati dalla Francia. Bolletino dell’ Associazione Mycologica ed Ecologica Romana 91: 20–30. [Google Scholar]
- Nagasawa E, Arita I. (1988). A note on Hypsizygus ulmarius and Hypsizygus marmoreus in Japan. Reports of the Tottori Mycological Institute 26: 71–78. [Google Scholar]
- Nakagiri A, Ito T. (1991). Basidiocarp development of the cyphelloid gasteroid aquatic basidiomycetes Halocyphina villosa and Linmoperdon incarnatum. Canadian Journal of Botany 69: 2320–2327. [Google Scholar]
- Nakamura K, Shoyama F, Toyama J. et al. (1987). Empoisonnement par le Dokou-sassa-ko (Clitocybe acromelalga). Japanese Journal of Toxicology 1: 35–39. [Google Scholar]
- Nakasone KK. (1996). Morphological and molecular studies on Auriculariopsis albomellea and Phlebia albida and a reassessment of A. ampla. Mycologia 88: 762–775. [Google Scholar]
- Neville P, Poumarat S. (2004). Amaniteae: Amanita, Limacella & Torrendia. Edizioni Candusso, Alassio, Italy. [Google Scholar]
- Newton AC, Haigh JM. (1998). Diversity of ectomycorrhizal fungi in Britain: a test of the species-area relationship, and the role of host specificity. New Phytologist 138: 619–627. [Google Scholar]
- Niazi AR, Iqbal SH, Khalid AN. (2009). Ectomycorrhizae between Amanita rubescens and Himalayan spruce (Picea smithiana) from Pakistan. Mycotaxon 107: 73–80. [Google Scholar]
- Niego AGT, Sysouphanthong P, Thongklang N. et al. (2021). A new species of Volvariella and the first record of Volvariella pulla (Agaricales: incertae sedis) from Thailand. Phytotaxa 480: 237–250. [Google Scholar]
- Nilsson RH, Larsson K-H, Taylor AFS. et al. (2018). The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Research 47: D259–D264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles MK. (1948). Studies in forest pathology. VI. Identification of cultures of wood-rotting fungi. Canadian Journal of Research, Sect. C, Botanical Science 26: 281–431. [DOI] [PubMed] [Google Scholar]
- Noordeloos ME. (1988). Clitopilus Kumm. In: Flora agaricina neerlandica Vol. 1 (Bas C, Kuyper THW, Noordeloos M.E. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 82–85. [Google Scholar]
- Noordeloos ME. (1993). Studies in Clitopilus (Basidiomycetes, Agaricales) in Europe. Persoonia 15: 241–248. [Google Scholar]
- Noordeloos ME. (1995a). Macrocystidia Joss. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 174–175. [Google Scholar]
- Noordeloos ME. (1995b). Ripartites P. Karst. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 94–96. [Google Scholar]
- Noordeloos ME. (1995c). Leucopaxillus Boursier. In: Flora agaricina neerlandica Vol. 3 (Bas C, Kuyper ThW, Noordeloos ME. et al., eds). AA Balkema, Rotterdam/Brookfield, The Netherlands: 76–77. [Google Scholar]
- Noordeloos M.E. (2008) Xeromphalina Kühner & Maire. In: Funga Nordica –Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 240–242. [Google Scholar]
- Noordeloos M.E. (2012) Xeromphalina Kühner & Maire. In: Funga Nordica –Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 446–447. [Google Scholar]
- Norvell LL, Redhead SA, Ammirati JF. (1994). Omphalina sensu lato in North America 1–2. 1: Omphalina wynniae and the genus Chrysomphalina. 2: Omphalina sensu Bigelow. Mycotaxon 50: 379–407. [Google Scholar]
- Nuss I. (1980). Untersuchungen zur systematischen Stellung der Gattung Polyporus. Hoppea - Denkschriften der Regensburgischen Botanischen Gesellschaft 39: 127–198. [Google Scholar]
- Nylander JAA. (2004). MrModelTest v. 2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Oberwinkler F. (2012). Basidiolichens. In: The Mycota, Second Edition, Vol. IX. Fungal Associations (Hock B. ed). Springer, Berlin, Heidelberg, New York: 341–362. [Google Scholar]
- Olariaga I, Salcedo I. (2012). New combinations and notes in clavarioid fungi. Mycotaxon 121: 37–44. [Google Scholar]
- Olariaga I, Laskibar X, Holec J. (2015). Molecular data reveal cryptic speciation within Tricholomopsis rutilans: description of T. pteridiicola sp. nov. associated with Pteridium aquilinum. Mycological Progress 14: 21. [Google Scholar]
- Olariaga I, Salcedo I, Daniëls PP. et al. (2015). Taxonomy and phylogeny of yellow Clavaria species with clamped basidia - Clavaria flavostellifera sp. nov. and the typification of C. argillacea, C. flavipes and C. sphagnicola. Mycologia 107: 104–122. [DOI] [PubMed] [Google Scholar]
- Olariaga I, Huhtinen S, Læssøe T. et al. (2020). Phylogenetic origins and family classification of typhuloid fungi, with emphasis on Ceratellopsis, Macrotyphula and Typhula (Basidiomycota). Studies in Mycology 96: 155–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olariaga I, Huhtinen S, Læssøe T. et al. (2022). (2874–2877) Proposals to conserve the names Typhula with a conserved type, Macrotyphula against Sclerotium, and Phyllotopsidaceae against Sclerotiaceae, and to reject the name Sclerotium fulvum (Basidiomycota: Agaricales). Taxon 71: 468–470. [Google Scholar]
- Oliveira AG, Desjardin D, Perry BA. et al. (2012). Evidence that a single bioluminescent system is shared by all known bioluminescent fungal lineages. Photochemical & Photobiological Sciences 11: 848–852. [DOI] [PubMed] [Google Scholar]
- Overeem C., van (1927). Fragmente aus: Die Nutzpilze von Niederländisch-Indiens. Bulletin du Jardin Botanique de Buitenzorg 3 sér., 9: 8–22. [Google Scholar]
- Ovrebo CL, Baroni TJ. (2007). New taxa of Tricholomataceae and Entolomataceae (Agaricales) from Central America. Fungal Diversity 27: 157–170. [Google Scholar]
- Ovrebo CL, Lodge DJ, Aime MC. (2011). A new Cantharocybe from Belize with notes on the type of Cantharocybe gruberi. Mycologia 103: 1102–1109. [DOI] [PubMed] [Google Scholar]
- Pantidou M. (1973). Fungus-host index for Greece. Benaki Phytopathological Institute, Kiphissia, Greece. [Google Scholar]
- Pantidou M. (1990). Mushrooms from the Greek forests. Museum Goulandri of Natural History, Kiphissia: [in Greek]. [Google Scholar]
- Pantidou M, Watling R, Gonou Z. (1983). Mycelial characters, anamorphs, and teleomorphs in genera and species of various families of Agaricales in culture. Mycotaxon 17: 409–432. [Google Scholar]
- Park EJ, Lee WY. (2013). In vitro symbiotic germination of myco-heterotrophic Gastrodia elata by Mycena species. Plant Biotechnology Reports 7: 185–191. [Google Scholar]
- Parmasto E. (1965). Key to the Clavariaceae of USSR. The Academy of Sciences of the USSR, Leningrad, USSR: [In Russian]. [Google Scholar]
- Parnmen S, Nooron N, Leudang S. et al. (2019). Phylogenetic evidence revealed Cantharocybe virosa (Agaricales, Hygrophoraceae) as a new clinical record for gastrointestinal mushroom poisoning in Thailand. Toxicological Research 36: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patouillard NT. (1900). Essai taxonomique sur les familles et les genres des Hyménomycètes. Series: Université de Paris, École supérieure de Pharmacie, Lucien Declume, Lons-Le-Saunier, France. [Google Scholar]
- Pegler DN, Young TWK. (1969). Ultrastructure of basidiospores in Agaricales in relation to taxonomy and spore discharge. Transactions of the British Mycological Society 52: 491–496. [Google Scholar]
- Pegler DN, Young TWK. (1971). Basidiospore morphology in the Agaricales. Beihefte zur Nova Hedwigia 35: 1–210. [Google Scholar]
- Pegler DN, Young TWK. (1973). Basidiospore form in the British Leucopaxilleae. Kew Bulletin 28: 365–379. [Google Scholar]
- Pegler DN, Young TWK. (1974). Basidiospore form in the British species of Lepista and Ripartites (Agaricales). Kew Bulletin 29: 659–667. [Google Scholar]
- Perrin J. (1979). Tectella (Panellus, Panus) patellaris (Fries) Earle. Bulletin de la Société Mycologique de France 95: 133–135, Atlas, Pl 217. [Google Scholar]
- Petersen JH, Davey ML, Læssøe T. (2014). Hirticlavula elegans, a new clavarioid fungus from Scandinavia. Karstenia 54: 1–8. [Google Scholar]
- Petersen RH. (1972). Notes on clavarioid fungi. XII. Miscellaneous notes on Clavariadelphus, and a new segregate genus. Mycologia 64: 137–152. [Google Scholar]
- Petersen RH. (1976). Notes on Cantharelloid Fungi. VII. The taxa described by Charles H. Peck. Mycologia 68: 304–326. [Google Scholar]
- Petersen RH. (1988). The clavarioid Fungi of New Zealand. DSIR Bull. 236. Lubrecht & Cramer Ltd, Wellington, New Zealand. [Google Scholar]
- Petersen RH. (1995). There’s more to a mushroom than meets the eye: mating studies in the Agaricales. Mycologia 87: 1–17. [Google Scholar]
- Petersen RH, Hughes KW, Redhead SA. et al. (1999). Mating systems in the Xerulaceae (Agaricales, Basidiomycotina): Flammulina. Mycoscience 40: 411–426. [Google Scholar]
- Petersen RH, Hughes KW. (2010). The Xerula/Oudemansiella complex (Agaricales). Nova Hedwigia Beihefte Series Volume: 137. J. Cramer/Gebrüder Borntraeger Verlag, Stuttgart, Germany. [Google Scholar]
- Petersen RH, Psurtseva N, Zmitrovich I. et al. (2015). Lignomyces, a new genus of pleurotoid Agaricomycetes. Mycologia 107: 1045–1054. [DOI] [PubMed] [Google Scholar]
- Pilát A. (1935). Pleurotus Fr. – hlíva. In: Atlas hub evropských, Vol. 2. (Kavina K, Pilát A. eds). Praha. Czech Republic. [Google Scholar]
- Pine EM, Hibbett DS, Donoghue MJ. (1999). Phylogenetic relationships of cantharelloid and clavarioid Homobasidiomycetes based on mitochondrial and nuclear rDNA sequences. Mycologia 91: 944–963. [Google Scholar]
- Poinar G., Jr (2016). A gilled mushroom, Gerontomyces lepidotus gen. et sp. nov. (Basidiomycota: Agaricales), in Baltic amber. Fungal Biology 120: 1090–1093. [DOI] [PubMed] [Google Scholar]
- Poumarat S, Neville P. (1993). Espèce de la zone de Quercus ilex au Maroc, montagnarde en France, Clitocybe amoenolens Malençon. Bulletin Semestriel de la Fédération des Associations Mycologiques Méditerranéennes 4: 16–19. [Google Scholar]
- Pouzar Z. (1985). Proposals for the conservation of five family names of fungi. Taxon 34: 709–712. [Google Scholar]
- Qin J, Feng B, Yang ZL. et al. (2014). The taxonomic foundation, species circumscription and continental endemisms of Singerocybe: evidence from morphological and molecular data, Mycologia 106: 1015–1026. [DOI] [PubMed] [Google Scholar]
- Quélet L. (1886). Enchiridion fungorum in Europa Media et præsertim in Gallia vigentium (Manuel des Champignons trouvés en Europe Centrale, et particulièrement en France), O. Doin, Lutetiae (Paris), France. [Google Scholar]
- Raithelhuber JH. (1981). Die Gattung Clitocybe, Teil 1. Metrodiana-Verlag, Stuttgart, Germany. [Google Scholar]
- Raithelhuber JH. (1983). Über die Nomenklatur einiger Argentinischer Blätterpilze. Metrodiana Sonderheft 2: 1–24. [Google Scholar]
- Raithelhuber JH. (1995). Trichterlinge mitteleuropas. Metrodiana 22: 52–94. [Google Scholar]
- Raithelhuber JH. (2004). Mitteleuropäische Trichterlinge. Gattungen Clitocybe, Pseudolyophyllum und Paralepista. JH. Raithelhuber Außenseiterverlag, Stuttgart, Germany. [Google Scholar]
- Raj KNA, Latha KPD, Leelavathy KM. et al. (2019). Anupama: a new genus of Biannulariaceae (Agaricales) from tropical India. Mycological Progress 18: 659–669. [Google Scholar]
- Ralaiveloarisoa AB, Liimatainen K, Ralimanana H. et al. (2020). Two new species of Hygroaster from Madagascar. Mycological Progress 19: 1293–1300. [Google Scholar]
- Ramírez NA, Niveiro N, Popoff OF. (2013). Agaricales del Chaco Oriental 1: Primer registro de Tetrapyrgos nigripes (Marasmiaceae) y Xeromphalina tenuipes (Mycenaceae) para la región. Boletín de la Sociedad Argentina de Botánica 48: 381–386. [Google Scholar]
- Razaq A, Khalid AN, Ilyas S. (2012). Tricholomopsis flammula Métrod ex Holec (Basidiomycota, Agaricales), an addition to the mushroom flora of Pakistan based on molecular identification. Pakistan Journal of Botany 44: 413–416. [Google Scholar]
- Redhead SA. (1987). The Xerulaceae (Basidiomycetes), a family with sarcodimitic tissues. Canadian Journal of Botany 65: 1551–1562. [Google Scholar]
- Redhead SA. (1988). Notes on the genus Xeromphalina (Agaricales, Xerulaceae) in Canada: biogeography, nomenclature, taxonomy. Canadian Journal of Botany 66: 479–507. [Google Scholar]
- Redhead SA. (2013). Nomenclatural novelties. Index Fungorum 15: 1–2. [Google Scholar]
- Redhead SA, Ginns JH. (1985). A reappraisal of agaric genera associated with brown rots of wood. Transactions of the Mycological Society of Japan 26: 349–381. [Google Scholar]
- Redhead SA, Halling RE. (1987). Xeromphalina nubium sp. nov. (Basidiomycetes) from Cerro de la Neblina, Venezuela. Mycologia 79: 383–386. [Google Scholar]
- Redhead SA. (1993). Proposal (1069) to conserve Omphalina with O. pyxidata as conserved type-an alternative to Proposals (927) and (1068). Taxon 42: 452. [Google Scholar]
- Redhead SA, Ammirati JF, Walker GR. et al. (1994). Squamanita contortipes, the rosetta stone of a mycoparasitic agaric genus. Canadian Journal of Botany 72: 1812–1824. [Google Scholar]
- Redhead SA, Seifert KA, Vilgalys R. et al. (2000a). Rhacophyllus and Zerovaemyces-teleomorphs or anamorphs? Taxon 49: 789–798. [Google Scholar]
- Redhead SA, Ammirati JF, Norvell LL. et al. (2000b). Notes on western north American snowbank fungi. Mycotaxon 76: 321–328. [Google Scholar]
- Redhead SA, Seifert KA. (2001). Asterophora Ditmar ex Link 1809 versus Nyctalis Fries 1825, and the status of Ugola Adanson 1763. Taxon 50: 243–268. [Google Scholar]
- Redhead SA, Lutzoni F, Moncalvo JM. et al. (2002a). Phylogeny of agarics: partial systematics solutions for core omphalinoid genera in the Agaricales (euagarics). Mycotaxon 83: 19–57. [Google Scholar]
- Redhead SA, Moncalvo JM, Vilgalys R. et al. (2002b). Phylogeny of agarics: partial systematics solutions for bryophilous omphalinoid agarics outside of the Agaricales (euagarics). Mycotaxon 82: 151–168. [Google Scholar]
- Redhead SA, Vizzini A, Drehmel DC. et al. (2016). Saproamanita, a new name for both Lepidella E.-J. Gilbert and Aspidella E.-J. Gilbert (Amaniteae, Amanitaceae). SIM 7: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner SA, Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Reid DA, Eicker A, Clémençon H. et al. (1998). South African fungi 7: Tricholosporum laeteviolaceum sp. nov., a representative of a genus new to Southern Africa. Mycotaxon 69: 409–418. [Google Scholar]
- Reijnders AFM. (1948). Études sur le developpement et l’organisation histologique des carpophores dans les Agaricales. Recueil Travaux Botanique de la Neerlande 41: 213–396. [Google Scholar]
- Reijnders AFM. (1963). Les problèmes du developpement des carpophores dans les Agaricales et quelques groupes voisins. Junk, The Hague, The Netherlands. [Google Scholar]
- Reijnders AFM. (1979). On carpophore-development in the genera Cortinarius, Dermocybe and Leucocortinarius. Sydowia Beihefte 8: 335–348. [Google Scholar]
- Reijnders AFM. (1983). Le developpement de Tectella patellaris (Fr.) Murr. et la nature des basidiocarpes cupuliformes. Bulletin Trimestriel de la Société Mycologique de France 99: 109–126. [Google Scholar]
- Reijnders AFM, Stalpers JA. (1992). The development of the hymenophoral trama in the Aphyllophorales and the Agaricales. Studies in Mycology 34: 1–109. [Google Scholar]
- Reynolds HT, Vijayakumar V, Gluck-Thaler E. et al. (2018). Horizontal gene cluster transfer increased hallucinogenic mushroom diversity. Evolution Letters 2: 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezgui A, Vallance J, Ben Ghnaya-Chakroun A. et al. (2018). Study of Lasidiodiplodia pseudotheobromae, Neofusicoccum parvum and Schizophyllum commune, three pathogenic fungi associated with the Grapevine Trunk Diseases in the North of Tunisia. European Journal of Plant Pathology 152: 127–142. [Google Scholar]
- Ricken A. (1915). Die Blätterpilze. Band. 1 (Agaricaceae). Deutschlands und der angrenzenden Länder, besonders Oesterreichs und der Schweiz. TO Weigel, Leipzig, Germany. [Google Scholar]
- Rinaldi AC, Comandini O, Kuyper TW. (2008). Ectomycorrhizal fungal diversity: seperating the wheat from the chaff. Fungal Diversity 33: 1–45. [Google Scholar]
- Riva A. (2001). Genere Leucopaxillus Boursier 1925, Subgenere Aspropaxillus Kühn. & R. Maire 1934. Discussione su qualche dubbio ingiustificato. Rivista di Micologia 44: 43–50. [Google Scholar]
- Riva A. (2008). Tricholoma goniospermum. Primo ritrovamento per il territorio svizzero. Schweizerische Zeitschrift für Pilzkunde 86: 34–36. [Google Scholar]
- Rizzo DM, Smereka KJ, Harrington TC. (1990). Notes on the rhizomorphs and mating systems of Xeromphalina campanella. Mycologia 82: 651–655. [Google Scholar]
- Robich G. (2016). Mycena d’Europa. Vol. II. Associazione Micologica Bresadola, Trento, Italy. [Google Scholar]
- Romagnesi H. (1974). Quelques espèces et variétés méconnues d’Agaricales. Bulletin de la Société mycologique de France 90: 161–169. [Google Scholar]
- Romagnesi H. (1978). Quelques espèces méconnues ou nouvelles de Macromycetes – VI. Bulletin Trimestriel de la Société Mycologique de France 94: 97–109. [Google Scholar]
- Romagnesi H. (1980). Armillaria decorosa (Peck) Smith-Walters, espèce américaine nouvelle pour l’Europe et la tribu des Cystodermateae Singer emend. Bulletin Trimestriel de la Société Mycologique de France 96: 145–154. [Google Scholar]
- Romagnesi H. (1989). Curiosité mycologique: un champignon tortionnaire japonais: Clitocybe acromelalga Ichimura. Bulletin de la Société Mycologique de France 105: 131–132. [Google Scholar]
- Romagnesi H. (1992). Sur l’ordre des Pluteales Kühner. Persoonia 14: 357–359. [Google Scholar]
- Romagnesi H. (1995). Prodrome à une flore analytique des hyménomycètes agaricoïdes III. Fam. Cantharellaceae Schroeter. Documents Mycologiques 25(97-100): 417–424. [Google Scholar]
- Romagnesi H. (1996). Validations. Bulletin de la Société Mycologique de France 112: 134–135. [Google Scholar]
- Ronikier A, Aronsen A. (2007). Type study of Mycena phaeophylla reveals its conspecificity with M. clavata. Mycologia 99: 924–935. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Mark P. et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C, Botha AJ, Eicker A. (2000). Distinct basidiospore development in Tricholosporum laeteviolaceum. Mycological Research 104: 1379–1383. [Google Scholar]
- Roux P. (1997). Les champignons pleurotoïdes. Bulletin de la Fédération mycologique Dauphiné-Savoie 145: 4–55. [Google Scholar]
- Roux P. (2006). Mille et un champignons. Éd. Roux, Sainte-Sigolène. France. [Google Scholar]
- Roze E. (1876). Catalogue des Agaricinées observées aux environs de Paris. Bulletin de la Société Botanique de France 23: 108–115. [Google Scholar]
- Rubio E, Sánchez L. (2019). Primer registro ibérico de un hongo Mediterráneo escasamente citado: Pseudoomphalina umbrinopurpurascens (Maire) Contu. Revista Catalana de Micologia 40: 71–75. [Google Scholar]
- Ruiz-Dueñas FJ, Barrasa JM, Sánchez-García M. et al. (2020). Genomic analysis enlightens Agaricales lifestyle evolution and increasing peroxidase diversity. Molecular Biology and Evolution 38: 1428–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg M, Matheny PB. (2011). Incomplete taxon sampling and diversification in a large clade of mushroom-forming fungi. Evolution 65: 1862–1878. [DOI] [PubMed] [Google Scholar]
- Saar I, Põldmaa K, Kõljalg U. (2009). The phylogeny and taxonomy of genera Cystoderma and Cystodermella (Agaricales) based on nuclear ITS and LSU sequences. Mycological Progress 8: 59–73. [Google Scholar]
- Saar I, Thorn RG, Nagasawa E. et al. (2022). A phylogenetic overview of Squamanita, with descriptions of nine new species and four new combinations. Mycologia 114: 769–797. [DOI] [PubMed] [Google Scholar]
- Sagara N. (1975). Ammonia fungi: a chemoecological grouping of terrestrial fungi. Contributions from the Biological Laboratory, Kyoto University 24: 205–276. [Google Scholar]
- Sagara N. (1995). Association of ectomycorrhizal fungi with decomposed animal wastes in forest habitats: a cleaning symbiosis? Canadian Journal of Botany 73 (suppl. 1): S1423–S1433. [Google Scholar]
- Sagara N, Hongo T, Murakami Y. et al. (2000). Hebeloma radicosoides sp. nov., an agaric belonging to the chemoecological group ammonia fungi. Mycological Research 104: 1017–1024. [Google Scholar]
- Saito T, Tonouchi A, Harada Y. (2014). Biological characteristics and molecular phylogeny of Sarcomyxa edulis comb. nov. and S. serotina. Japanese Journal of Mycology 55: 19–28. [Google Scholar]
- Sánchez-García M. (2016). Systematics, diversity and evolution of the suborder Tricholomatineae (Agaricales). PhD dissertation, University of Tennessee, USA. http://trace.tennessee.edu/utk_graddiss/3663 [Google Scholar]
- Sánchez-García M, Matheny PB. (2017). Is the switch to an ectomycorrhizal state an evolutionary key innovation in mushroom-forming fungi? A case study in the Tricholomatineae (Agaricales). Evolution 71: 51–65. [DOI] [PubMed] [Google Scholar]
- Sánchez-García M, Matheny PB, Palfner G. et al. (2014). Deconstructing the Tricholomataceae (Agaricales) and introduction of the new genera Albomagister, Corneriella, Pogonoloma and Pseudotricholoma. Taxon 63: 993–1007. [Google Scholar]
- Sánchez-García M, Henkel TW, Aime MC. et al. (2016). Guyanagarika, a new ectomycorrhizal genus of Agaricales from the Neotropics. Fungal Biology 120: 1540–1553. [DOI] [PubMed] [Google Scholar]
- Sánchez-García M, Ryberg M, Khan FK. et al. (2020). Fruiting body form, not nutritional mode, is the major driver of diversification in mushroom-forming fungi. Proceedings of the National Academy of Sciences USA 117: 32528–32534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-García M, Adamčíková K, Moreau PA. et al. (2021). The genus Dermoloma is more diverse than expected and forms a monophyletic lineage in the Tricholomataceae. Mycological Progress 20: 11–25. [Google Scholar]
- Sánchez-Ruiz MI, Ayuso-Fernández I, Rencoret J. et al. (2021). Agaricales mushroom lignin peroxidase: from structure-function to degradative capabilities. Antioxidants 10: 1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarawi S, Shi Y-N, Lotz-Winter H. et al. (2022). Occurrence and chemotaxonomical analysis of amatoxins in Lepiota spp. (Agaricales). Phytochemistry 195: 113069. [DOI] [PubMed] [Google Scholar]
- Saviuc PF, Danel VC, Moreau PA. et al. (2001). Erythromelalgia and mushroom poisoning. Clinical Toxicology 39: 403–407. [DOI] [PubMed] [Google Scholar]
- Saviuc PF, Danel VC, Moreau PA. et al. (2002). Érythermalgie soudaine: cherchez le champignon! La Revue de Médecine Interne 23: 394–399. [DOI] [PubMed] [Google Scholar]
- Saviuc PF, Danel VC. (2006). New syndromes in mushroom poisoning. Toxicological Reviews 25: 199–209. [DOI] [PubMed] [Google Scholar]
- Satou T, Kaneko K, Li W. et al. (2008). The toxin produced by Pleurotus ostreatus reduces the head size of nematodes. Biological & Pharmaceutical Bulletin 31: 574–576. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Sung GH, Lopez-Giraldez F. et al. (2009). The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58: 224–239. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Robbertse B, Robert V. et al. (2014). Finding needles in haystacks: linking scientific names, reference specimens and molecular data for Fungi. Database (Oxford) 2014: bau061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JA, Heseler U. (2009a). Der Schleierseitling Panellus patellaris: Selten, übersehen oder in Ausbreitung begriffen? http://tintling.com/inhalt/2009/panellus_patellaris.htm [accessed 18 July 2023].
- Schmitt JA, Heseler U. (2009b). Der Schleierseitling Panellus patellaris: Selten, übersehen oder in Ausbreitung begriffen? Der Tintling 14: 14–26. [Google Scholar]
- Schwarze F, Baum S, Fink S. (2000). Dual modes of degradation by Fistulina hepatica in xylem cell walls of Quercus robur. Mycological Research 104: 846–852. [Google Scholar]
- Schwöbel H. (1984). Trichterlinge aus dem C. gibba-Formenkreis. Beiträge zur Kenntnis der Pilze Mitteleuropas 1: 5–10. [Google Scholar]
- Seas-Carvajal C, Avalos G. (2013). Distribution of bioluminescent fungi across old-growth and secondary tropical rain forest in Costa Rica. Revista de Biología Tropical 61: 531–537. [DOI] [PubMed] [Google Scholar]
- Seitzman BH, Ouimette A, Mixon RL. et al. (2011). Conservation of biotrophy in Hygrophoraceae inferred from combined stable isotope and phylogenetic analysis. Mycologia 103: 280–290. [DOI] [PubMed] [Google Scholar]
- Sena K, Alemanno L, Gramacho KP. (2014). The infection process of Moniliophthora perniciosa in cacao. Plant Pathology 63: 1272–1281. [Google Scholar]
- Senn-Irlet B. (1994). Culture morphology of Crepidotus species. Mycotaxon 52: 59–75. [Google Scholar]
- Senn-Irlet B, Scheidegger C. (1994). Stephanocysts in Crepidotus applanatus. Mycological Research 98: 419–422. [Google Scholar]
- Senthilarasu G, Sharma R, Singh S. (2012). A new species of Volvariella from India. Mycotaxon 119: 467–476. [Google Scholar]
- Seok SJ, Jung YA, Jin YJ. et al. (2011). Tectella patellaris from Korea. Mycobiology 39: 303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesli E. (2014). Studies on new fungal records for Turkish Mycota from Trabzon. Turkish Journal of Botany 38: 608–616. [Google Scholar]
- Sesli E, Vizzini A, Contu M. (2016). Clitolyophyllum akcaabatensis gen. nov., sp. nov. (Agaricales, Tricholomatineae), a new fan shaped clitocybioid agaric from Turkey. Canadian Journal of Botany 94: 73–80. [Google Scholar]
- Sgambelluri RM, Epis S, Sassera D. et al. (2014). Profiling of amatoxins and phallotoxins in the genus Lepiota by liquid chromatography combined with UV absorbance and mass spectrometry. Toxins 6: 2336–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer R. (1962). Synonyms, new combinations, and new species in Volvariella (Agaricales). Mycologia 54: 563–572. [Google Scholar]
- Sheikh S, Khan FK, Bahram M. et al. (2022). Impact of model assumptions on the inference of the evolution of ectomycorrhizal symbiosis in fungi. Scientific Reports 12: 22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishikura M, Takemura Y, Sotome K. et al. (2021). Four mycelial strains of Entoloma clypeatum species complex form ectomycorrhiza-like roots with Pyrus betulifolia seedlings in vitro, and one develops fruiting bodies 2 months after inoculation. Mycorrhiza 31: 31–42. [DOI] [PubMed] [Google Scholar]
- Silva-Filho AGS, Mombert A, Nascimento CC. et al. (2023). Eoscyphella luciurceolata gen. and sp. nov. (Agaricomycetes) shed light on Cyphellopsidaceae with a new lineage of bioluminescent fungi. Journal of Fungi 9: 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. (1936a). Notes sur quelques Basidiomycetes. II. Revue de Mycologie (Paris) 1: 279–293. [Google Scholar]
- Singer R. (1936b). Studien zur Systematik der Basidiomyceten. Beihefte zum Botanischen Centralblatt 56: 137–174. [Google Scholar]
- Singer R. (1942a). Type studies on agarics. Lloydia 5: 97–135. [Google Scholar]
- Singer R. (1942b). Das System der Agaricales. II. Annales Mycologici 40: 1–132. [Google Scholar]
- Singer R. (1943). Das System der Agaricales. III. Annales Mycologici 41: 1–189. [Google Scholar]
- Singer R. (1945). New genera of fungi. Lloydia 8: 139–144. [Google Scholar]
- Singer R. (1947). New genera of fungi III. Mycologia 39: 77–79. [PubMed] [Google Scholar]
- Singer R. (1948). Diagnoses fungorum novorum Agaricalium. Sydowia 2: 26–42. [Google Scholar]
- Singer R. (1951). The Agaricales in modern taxonomy. Lilloa 22: 5–832. [Google Scholar]
- Singer R. (1956). New genera of fungi. VII. Mycologia 48: 719–727. [PubMed] [Google Scholar]
- Singer R. (1958). Monographs of South American Basidiomycetes, especially those of the East Slope of the Andes and Brazil 1: The genus Pluteus in South America. Lloydia 21: 195–299. [Google Scholar]
- Singer R. (1962a). The Agaricales in modern taxonomy. 2nd ed. J. Cramer, Weinheim, Germany. [Google Scholar]
- Singer R. (1962b). Diagnoses fungorum novorum Agaricalium II. Sydowia 15: 45–83. [Google Scholar]
- Singer R. (1964). Oudemansiellinae, Macrocystidiinae, Pseudohiatulinae in South America: Monographs of South American Basidiomycetes, especially those of the east slope of the Andes and Brazil. VIII. Darwiniana 13: 145–190. [Google Scholar]
- Singer R. (1965). Monographs of South American Basidiomycetes, especially those of the East slope of the Andes and Brazil. X. Xeromphalina. Boletín de la Sociedad Argentina de Botánica 10: 302–310. [Google Scholar]
- Singer R. (1970). Omphalinae (Clitocybeae-Tricholomataceae Basidiomycetes). Flora Neotropica 3: 1–84. [Google Scholar]
- Singer R. (1972). Cyanophilous spore walls in the Agaricales and agaricoid Basidiomycetes. Mycologia 64: 822–829. [PubMed] [Google Scholar]
- Singer R. (1973a). A Monograph of the Neotropical Species of Marasmiellus. Beihefte zur Nova Hedwigia 44: 1–339. [Google Scholar]
- Singer R. (1973b). Diagnoses Fungorum Novorum Agaricalium III. J. Cramer, Vaduz, Liechtenstein. [Google Scholar]
- Singer R. (1975). The Agaricales in modern taxonomy. 3rd ed. J. Cramer, Vaduz, Liechtenstein. [Google Scholar]
- Singer R. (1986). The Agaricales in modern taxonomy. 4th ed. Koeltz Scientific Books, Koenigstein, Germany. [Google Scholar]
- Singer R. (1989). New taxa and new combinations of Agaricales (Diagnoses fungorum novorum Agaricalium 4). Fieldiana, Botany 21: 1–133. [Google Scholar]
- Singer R, Smith AH. (1943). A monograph on the genus Leucopaxillus Boursier. Papers of the Michigan Academy of Science, Arts & Letters 28: 85–132. [Google Scholar]
- Singer R, Smith AH. (1947). Additional notes on the genus Leucopaxillus. Mycologia 39: 725–736. [Google Scholar]
- Singer R, Morello JH. (1960). Ectotrophic forest tree mycorrhizae and forest communities. Ecology 41: 549–551. [Google Scholar]
- Smith AH. (1944). Unusual North American Agarics. The American Midland Naturalist 32: 669–698. [Google Scholar]
- Smith AH. (1953). New and rare agarics from the Douglas Lake region and Tahquamenon Falls State Park, Michigan, and an account of the North American species of Xeromphalina. Papers of the Michigan Academy of Science, Arts & Letters 38: 53–87. [Google Scholar]
- Smith AH. (1960). Tricholomopsis (Agaricales) in the Western hemisphere. Brittonia 12: 41–70. [Google Scholar]
- Smith AH, Reid DA. (1962). A new genus of the Secotiaceae. Mycologia 54: 98–104. [Google Scholar]
- Smith ME, Douhan GW, Rizzo DM. (2007). Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytologist 174: 847–863. [DOI] [PubMed] [Google Scholar]
- Smith S, Read D. (2008). Mycorrhizal Symbiosis. 3rd Edition. Academic Press, London, UK. [Google Scholar]
- Song J, Han ML, Cui BK. (2015). Fistulina subhepatica sp. nov. from China inferred from morphological and sequence analyses. Mycotaxon 130: 47–56. [Google Scholar]
- Soop K, Dima B, Szarkándi J. et al. (2016). Psathyloma, a new genus in Hymenogastraceae described from New Zealand. Mycologia 108: 397–404. [DOI] [PubMed] [Google Scholar]
- Stalpers JA. (1988). Auriculariopsis and the Schizophyllales. Persoonia 13: 495–504. [Google Scholar]
- Stalpers JA, Redhead SA, May TW. et al. (2021). Competing sexualasexual generic names in Agaricomycotina (Basidiomycota) with recommendations for use. SIM 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. (1964). The Agaricales of New Zealand. V. Tricholomataceae. Kew Bulletin 19(1): 1–59. [Google Scholar]
- Stiller JW, Hall BD. (1997). The origin of red algae: implications for plastid evolution. Proceedings of the National Academy of Sciences USA 94: 4520–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Liu S, Cui BK. (2019). Morphological and phylogenetic analyses reveal a new species of Fistulina (Fistulinaceae, Agaricales) from Australia. Phytotaxa 420: 233–240. [Google Scholar]
- Suzuki A. (2009). Propagation strategy of ammonia fungi. Mycoscience 50: 39–51. [Google Scholar]
- Swann EC, Taylor JW. (1993). Higher taxa of basidiomycetes: an 18S rRNA gene perspective. Mycologia 85: 923–936. [Google Scholar]
- Swann EC, Taylor JW. (1995a). Phylogenetic perspectives on basidiomycete systematics: Evidence from the 18S rRNA gene. Canadian Journal of Botany 73, n. Suppl. 1 Sect. E-H: S862–S868. [Google Scholar]
- Swann EC, Taylor JW. (1995b). Toward a phylogenetic systematics of the Basidiomycota: integrating yeasts and filamentous basidiomycetes using 18S RRNA gene sequences. Studies in Mycology 38: 147–161. [Google Scholar]
- Swofford DL. (2003). PAUP*. Phylogenetic analysis using parsimony (*and other methods) v. 4.0b10. Sinauer Associates, Sunderland, MS, USA. [Google Scholar]
- Szemere L. (1966). Leucopaxillus rhodoleucus (Romell) Kühner. Česká Mykologie 20: 30–31. [Google Scholar]
- Tabarés M. (1996). Clitocybe nivea. In: Bolets de Catalunya, XV Colleció, làm. 704. Societat Catalana de Micologia, Barcelona, Spain. [Google Scholar]
- Takemoto S, Nakamura H, Imamura Y. et al. (2010). Schizophyllum commune as a ubiquitous plant parasite. Japan Agricultural Research Quarterly (JARQ) 44: 357–364. [Google Scholar]
- Tam SY, Uchida K, Enomoto H. et al. (2022). A new metabolite, mannogeranylnerol, specifically produced at body temperature by Schizophyllum commune, a causative fungus of human mycosis. The Journal of Antibiotics 75: 243–246. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L-P, Hao Y-J, Cai Q. et al. (2014). Morphological and molecular evidence for a new species of Rhodotus from tropical and subtropical Yunnan, China. Mycological Progress 13: 45–53. [Google Scholar]
- Tedersoo L, May TW, Smith ME. (2010). Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20: 217–263. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Smith ME. (2013). Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biology Reviews 27: 83–99. [Google Scholar]
- Tello SA, Silva-Flores P, Agerer R. et al. (2014). Hygrocybe virginea is a systemic endophyte of Plantago lanceolata. Mycological Progress 13: 471–475. [Google Scholar]
- Thind KS. (1961). The Clavariaceae of India. Indian Council of Agric. Res., New Delhi, India. [Google Scholar]
- Thompson GE. (1936). Nyctalis parasitica and N. asterophora in culture. Mycologia 28: 222–227. [Google Scholar]
- Thorn RG, Moncalvo J-M, Reddy CA. et al. (2000). Phylogenetic analyses and the distribution of nematophagy support a monophyletic Pleurotaceae within the polyphyletic pleurotoid-lentinoid fungi. Mycologia 92: 241–252. [Google Scholar]
- Thorn RG, Moncalvo J-M, Redhead SA. et al. (2005). A new poroid species of Resupinatus from Puerto Rico, with a reassessment of the cyphelloid genus Stigmatolemma. Mycologia 97: 1140–1151. [DOI] [PubMed] [Google Scholar]
- Tian F, Li C, Li Y. (2021). Genomic analysis of Sarcomyxa edulis reveals the basis of its medicinal properties and evolutionary relationships. Frontiers in Microbiology 12: 652324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe JM. (1962). Fungus associates of ectotrophic mycorrhizae. Botanical Review 28: 538–606. [Google Scholar]
- Trimbach J. (1978). Matériel pour une “checklist” des alpes maritimes (suite). Documents Mycologiques 8(29): 39–53. [Google Scholar]
- Trnkoczy A. (2011). Tectella patellaris (Fr.) Murrill (84928). http://mushroomobserver.org/observer/show_observation/84928 [accessed 18 July 2023].
- Turland NJ, Wiersema JH, Barrie FR. et al. (eds.) (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten, Germany. [Google Scholar]
- Urrea-Valencia S, Bizarria R, Júnior, Kooij PW. et al. (2023). Unraveling fungal species cultivated by lower attine ants. Mycological Progress 22: 66. [Google Scholar]
- van de Peppel LJJ, Nieuwenhuis M, Auxier B. et al. (2021). Ancestral predisposition toward a domesticated lifestyle in the termite-cultivated fungus Termitomyces. Current Biology 31: 4413–4421. [DOI] [PubMed] [Google Scholar]
- van de Peppel LJJ, Aime MC, Læssøe T. et al. (2022). Four new genera and six new species of lyophylloid agarics (Agaricales, Basidiomycota) from three different continents. Mycological Progress 21: 85. [Google Scholar]
- Varga T, Krizsán K, Földi C. et al. (2019). Megaphylogeny resolves global patterns of mushroom evolution. Nature Ecology & Evolution 3: 668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vašutová M, Dvořák D, Beran M. (2013). Rare macromycetes from raised bogs in the Hrubý Jeseník Mts. (Czech Republic). Czech Mycology 65: 45–67. [Google Scholar]
- Vauras J. (2009). Tricholomopsis osiliensis, a new agaric species from Estonia. Folia Cryptogamica Estonica 45: 87–89. [Google Scholar]
- Vauras J, Saar I, Voitk A. (2012). Comparison of European Tricholomopsis osiliensis with putative Tricholomopsis sulfureoides of Newfoundland. Omphalina 3: 9–11. [Google Scholar]
- Velenovský J. (1920). České Houby 2. České Botanické Společnosti, Praha, Czech Republic: 201–424. [Google Scholar]
- Vellinga EC. (2004). Genera in the family Agaricaceae: evidence from nrITS and nrLSU sequences. Mycological Research 108: 354–377. [DOI] [PubMed] [Google Scholar]
- Vellinga EC, Sysouphanthong S, Hyde KD. (2011). The family Agaricaceae: phylogenies and two new white-spored genera. Mycologia 103: 494–509. [DOI] [PubMed] [Google Scholar]
- Veloz Villavicencio E, Mali T, Mattila HK. et al. (2020). Enzyme activity profiles produced on wood and straw by four Fungi of different decay strategies. Microorganisms 8: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde L, Calonge FD. (1980). El género Leucopaxillus Bousier (Basidiomycetes) en España. Boletín de la Sociedad Micológica Castellana 5: 47–54. [Google Scholar]
- Vergara GV, Bughrara SS, Jung G. (2004). Genetic variability of grey snow mould (Typhula incarnata). Mycological Research 108: 1283–1290. [DOI] [PubMed] [Google Scholar]
- Vesterholt J. (2008a). Melanoleuca Pat. In: Funga Nordica–Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 347–352. [Google Scholar]
- Vesterholt J. (2008b). Pleurocybella Singer. In: Funga Nordica–Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 243. [Google Scholar]
- Vesterholt J. (2008c). Tricholomopsis Singer. In: Funga Nordica–Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 334–335. [Google Scholar]
- Vesterholt J. (2008d). Infundibulicybe Harmaja. In: Funga Nordica–Agaricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 310–312. [Google Scholar]
- Vesterholt J. (2012a). Melanoleuca Pat. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 347–352. [Google Scholar]
- Vesterholt J. (2012b). Pleurocybella Singer. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 363. [Google Scholar]
- Vesterholt J. (2012c). Infundibulicybe Harmaja. In: Funga Nordica–Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (Knudsen H, Vesterholt J. eds). Nordsvamp, Copenhagen, Denmark: 467–469. [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan K, Kumaresan V, Sannasimuthu A. et al. (2019). Resolving the pathogenicity factors of a novel opportunistic fungus Schizophyllum commune at molecular level. Molecular Biology Reports 46: 3877–3886. [DOI] [PubMed] [Google Scholar]
- Vizzini A. (2008). Ecology of the genus Crepidotus. In: Il genere Crepidotus in Europa (Consiglio G, Setti L. auth.). Associazione Micologica Bresadola, Trento, Italy: 43–49. [Google Scholar]
- Vizzini A. (2014a). Nomenclatural novelties. Index Fungorum 161: 1. [Google Scholar]
- Vizzini A. (2014b). Nomenclatural novelties. Index Fungorum 159: 1. [Google Scholar]
- Vizzini A. (2014c). Nuove segnalazioni di Clitocybe amoenolens (Agaricales, sindrome acromelelgica) per l’Italia e note sulla sua distribuzione e posizione tassonomica. Atti Quinto convegno internazionale di micotossicologia (5° C.I.M.T.). Milano, 3–4 dicembre 2012 (paper): 147–157. Pagine di Micologia 37: 237–244. [Google Scholar]
- Vizzini A, Ercole E. (2011). A new annulate Pluteus variety from Italy. Mycologia 103: 904–911. [DOI] [PubMed] [Google Scholar]
- Vizzini A, Ercole E. (2012). Paralepistopsis gen. nov. and Paralepista (Basidiomycota, Agaricales). Mycotaxon 120: 253–267. [Google Scholar]
- Vizzini A, Musumeci E, Murat C. (2010). Trichocybe, a new genus for Clitocybe puberula (Agaricomycetes, Agaricales). Fungal Diversity 42: 97–105. [Google Scholar]
- Vizzini A, Para R, Fontenla R. et al. (2011a). A preliminary ITS phylogeny of Melanoleuca (Agaricales) with special reference to European taxa. Mycotaxon 118: 361–381. [Google Scholar]
- Vizzini A, Contu M, Justo A. (2011b). Additional records of Volvariella dunensis (Basidiomycota, Agaricales): morphological and molecular characterization. Mycotaxon 117: 37–43. [Google Scholar]
- Vizzini A, Contu M, Musumeci E. et al. (2011c). A new taxon in the Infundibulicybe gibba complex (Basidiomycota, Agaricales, Tricholomataceae) from Sardinia (Italy). Mycologia 103: 203–208. [DOI] [PubMed] [Google Scholar]
- Vizzini A, Contu M, Ercole E. (2011d). Musumecia gen. nov. in the tricholomatoid clade (Basidiomycota, Agaricales) related to Pseudoclitocybe. Nordic Journal of Botany 29: 734–740. [Google Scholar]
- Vizzini A, Ercole E, Contu M. (2012a). A contribution to the ITS-LSU phylogeny of the genus Leucopaxillus (/tricholomatoid clade, Agaricales), with three new genera and notes on Porpoloma. Mycosphere 3: 79–90. [Google Scholar]
- Vizzini A, Consiglio G, Setti L. et al. (2012b). The phylogenetic position of Haasiella (Basidiomycota, Agaricomycetes) and the relationships between H. venustissima and H. splendidissima. Mycologia 104: 777–784. [DOI] [PubMed] [Google Scholar]
- Vizzini A, Curti M, Contu M. et al. (2012c). A new cystidiate variety of Omphalina pyxidata (Basidiomycota, tricholomatoid clade) from Italy. Mycotaxon 120: 361–371. [Google Scholar]
- Vizzini A, Consiglio G, Setti L. et al. (2015). Calocybella, a new genus for Rugosomyces pudicus (Agaricales, Lyophyllaceae) and emendation of the genus Gerhardtia. SIM 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzini A, Consiglio G, Ercole E. et al. (2016). Pseudoporpoloma, a new genus for Agaricus pes-caprae (Agaricales, Tricholomataceae). Phytotaxa 243: 271–280. [Google Scholar]
- Vizzini A, Angelini C, Ercole E. (2017). Is the species diversity in the lyophylloid genera Calocybella and Gerhardtia (Agaricales, Basidiomycota) underestimated? Two new species from the Dominican Republic. Phytotaxa 291: 241–252. [Google Scholar]
- Vizzini A, Consiglio G, Marchetti M. (2019a). Mythicomycetaceae fam. nov. (Agaricineae, Agaricales) for accommodating the genera Mythicomyces and Stagnicola, and Simocybe parvispora reconsidered. Fungal Systematics and Evolution 1: 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzini A, Picillo B, Perrone L. et al. (2019b). Chrysomycena perplexa gen. et sp. nov. (Agaricales, Porotheleaceae), a new entity from the Lazio region”. Rivista Micologica Romana, Bollettino dell’Associazione Micologica Ecologica Romana 107: 96–107. [Google Scholar]
- Vizzini A, Consiglio G, Marchetti M. et al. (2020a). Insights into the Tricholomatineae (Agaricales, Agaricomycetes): a new arrangement of Biannulariaceae and Callistosporium, Callistosporiaceae fam. nov., Xerophorus stat. nov., and Pleurocollybia incorporated into Callistosporium. Fungal Diversity 101: 211–259. [Google Scholar]
- Vizzini A, Consiglio G, Marchetti M. (2020b). Nomenclatural novelties. Index Fungorum 462: 1. [Google Scholar]
- Vizzini A, Consiglio G, Setti L. (2020c). Testing spore amyloidity in Agaricales under light microscope: the case study of Tricholoma. SIM 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzini A, Consiglio G, Marchetti M. et al. (2022). New data in Porotheleaceae and Cyphellaceae: epitypification of Prunulus scabripes Murrill, the status of Mycopan Redhead, Moncalvo & Vilgalys and a new combination in Pleurella Horak emend. Mycological Progress 21: 44. [Google Scholar]
- Voglmayr H. (1994). Limnoperdon incarnatum, ein aero-aquatischer Gasteromyzet neu für Europa. Österreichische Zeitschrift für Pilzkunde 3: 71–76. [Google Scholar]
- Voitk A, Saar I, Lebeuf R. et al. (2020a). The Pseudoomphalina kalchbrenneri complex in North America. Botany 98: 91–101. [Google Scholar]
- Voitk A, Saar I, Lodge DJ. et al. (2020b), New species and reports of Cuphophyllus from northern North America compared with related Eurasian species. Mycologia 112: 438–452. [DOI] [PubMed] [Google Scholar]
- Vrinda KB, Pradeep CK, Mathew S. et al. (2000). Catatrama (Tricholomataceae), a genus new to India. Persoonia 17: 495–496. [Google Scholar]
- Walther G, Garnica S, Weiß M. (2005). The systematic relevance of conidiogenesis modes in the gilled Agaricales. Mycological Research 109: 525–544. [DOI] [PubMed] [Google Scholar]
- Walther G, Weiß M. (2006). Anamorphs of the Bolbitiaceae (Basidiomycota, Agaricales). Mycologia 98: 792–800. [DOI] [PubMed] [Google Scholar]
- Wang C-Q, Zhang M, Li T-H. et al. (2018). Additions to tribe Chromosereae (Basidiomycota, Hygrophoraceae) from China, including Sinohygrocybe gen. nov. and a first report of Gloioxanthomyces nitidus. MycoKeys 38: 59–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Q, Zhang M, He X-L. et al. (2023a). Species diversity of Hygrophorus in China and a phylogenetic study of the genus. Mycosphere 14: 1742–1834. [Google Scholar]
- Wang G-S, Cai Q, Hao Y-J. et al. (07 Nov 2023b): Phylogenetic and taxonomic updates of Agaricales, with an emphasis on Tricholomopsis. Mycology, DOI: 10.1080/21501203.2023.2263031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Guo H, Li J. et al. (2019). Evaluation of five regions as DNA barcodes for identification of Lepista species (Tricholomataceae, Basidiomycota) from China. PeerJ 7: e7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartchow F, Putzke J, Cavalcanti MAQ. (2007). Catatrama costaricensis (Agaricales): a strange lepiotoid fungus is found in South America. Mycotaxon 101: 35–39. [Google Scholar]
- Watling R. (1979). The morphology, variation, and ecological significance of anamorphs in the Agaricales. In: The Whole Fungus 2 (Kendrick WB. ed). National Museum of Canada, Ottawa, Canada: 453–472. [Google Scholar]
- Watling R. (1988). Larger fungi and some of Earth’s major catastrophies. In: Fungi and Ecological Disturbance (Boddy L, Watling R, Lyon AJE. eds). The Royal Society of Edinburgh, Edinburgh, UK: 49–59. [Google Scholar]
- Watling R, Sweeney J. (1971). Observations on Schizophyllum commune Fries. Sabouraudia 12: 214–226. [PubMed] [Google Scholar]
- Watling R, Gregory NM. (1989). Crepidotaceae, Pleurotaceae and other pleurotoid agarics. British Fungus Flora, Agarics and Boleti. Vol. 6. Royal Botanic Garden Edinburgh, Edinburgh, UK. [Google Scholar]
- Watling R, Gregory NM. (1994). Cortinariaceae p.p. British Fungus Flora, Agarics and Boleti. Vol. 7. Royal Botanic Garden Edinburgh, Edinburgh, UK. [Google Scholar]
- Watling R, Turnbull E. (1998). Cantharellaceae, Gomphaceae and amyloidspored and xeruloid members of Tricholomataceae (excl. Mycena). British Fungus Flora, Agarics and Boleti. Vol. 8. Royal Botanic Garden Edinburgh, Edinburgh, UK. [Google Scholar]
- Watling R, Hills AE. (2005). Boletes and their allies (revised and enlarged edition). British Fungus Flora, Agarics and Boleti. Vol. 8. Royal Botanic Garden Edinburgh, Edinburgh, UK. [Google Scholar]
- Webster J, Descals H. (1981). Morphology, distribution, and ecology of conidial fungi in freshwater habitats. In: Biology of conidial fungi 1 (Cole GT, Kendrick B. eds). Academic Press, New York, USA: 295–355. [Google Scholar]
- Webster J, de Kock AN, Eicker A. (1993). Limnoperdon incarnatum, a Gasteromycete from submerged twigs in South Africa. South African Journal of Botany 59: 519–521. [Google Scholar]
- Weholt Ø. (1988). A study of Myxomphalia with relation to European species and collects of Myxomphalia Hora found in Norwegian herbaria. Agarica 9: 71–101. [Google Scholar]
- Wei S-W, Lu B-Y, Wang Y. et al. (2023). Morphology and phylogeny of lyophylloid mushrooms in China with description of four new species. Journal of Fungi 9: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S. et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ. et al., eds). Academic Press, New York, USA: 315–322. [Google Scholar]
- Wiest R. (2022). Hertzogia gen. nov., un nouveau genre clitocyboïde. Bulletin de la Société Mycologique de Strasbourg 121: 29–35. [Google Scholar]
- Wilson AW, Desjardin DE. (2005). Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade). Mycologia 97: 667–679. [DOI] [PubMed] [Google Scholar]
- Winterhoff W. (1998). Leucopaxillus rhodoleucus in Robinienwäldern. Boletus 22: 49–51. [Google Scholar]
- Wolfe BE, Tulloss RE, Pringle A. (2012). The irreversible loss of a decomposition pathway marks the single origin of an ectomycorrhizal symbiosis. PLoS ONE 7: e39597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall JJ, Anagnost SE, Zabel RA. (1997). Comparison of wood decay among diverse lignicolous fungi. Mycologia 89: 199–219. [Google Scholar]
- Wright JE. (1961). Del género Fistulina en el hemisferio occidental. Boletín de la Sociedad Argentina de Botánica 9: 217–228. [Google Scholar]
- Wu H-M, Luo J-Q, Wang K. et al. (2018). A new species of Cleistocybe (Agaricales, Basidiomycota) from the Tibetan Plateau, China. Phytotaxa 336: 286–292. [Google Scholar]
- Wurita A, Hasegawa K, Konno K. et al. (2019). Quantification of clitidine in caps and stems of poisonous mushroom Paralepistopsis acromelalga by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Forensic Toxicology 37: 378–386. [Google Scholar]
- Xiao Y, Liu C, Hu N. et al. (2023a). Contributions of ectomycorrhizal fungi in a reclaimed poplar forest (Populus yunnanensis) in an abandoned metal mine tailings pond, southwest China. Journal of Hazardous Materials 448: 130962. [DOI] [PubMed] [Google Scholar]
- Xiao Y-Q, Li H-J, Li S-N. et al. (2023b). Spodocybe umbilicata (Hygrophoraceae, Agaricales), a newly discovered species from subtropical China. Phytotaxa 620: 273–282. [Google Scholar]
- Xu J-Z, Li T-H, Shen Y-H. et al. (2015). Volvariella rava sp. nov. from southern China. Mycotaxon 130: 857–865. [Google Scholar]
- Xu J-Z, Zhang C-L, Kasuya T. et al. (2018). A new species of Tricholosporum (Agaricales, Tricholomataceae) from Liaoning Province of China. Phytotaxa 374: 63–70. [Google Scholar]
- Xu J-Z, Yu X-D, Lu M-Z. et al. (2019). Phylogenetic analyses of some Melanoleuca species (Agaricales, Tricholomataceae) in Northern China, with descriptions of two new species and the identification of seven species as a first record. Frontiers in Microbiology 10: 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J-Z, Zhao W, Yu X-D. et al. (2022). Infundibulicybe trachyspora, a new species from Northeastern China based on morphology and molecular phylogeny. Current Microbiology 79: 130. [DOI] [PubMed] [Google Scholar]
- Xu J-Z, Jiang Y, Wang T. et al. (2023). Morphological characteristics and phylogenetic analyses revealed four new species of Agaricales from China. Frontiers in Microbiology 14: 1118525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yafetto L. (2018). The structure of mycelial cords and rhizomorphs of fungi: A mini-review. Mycosphere 9: 984–998. [Google Scholar]
- Yamada A, Ogura T, Ohmasa M. (2001a). Cultivation of mushrooms of edible ectomycorrhizal fungi associated with Pinus densiflora by in vitro mycorrhizal synthesis: I. Primordium and basidiocarp formation in open-pot culture. Mycorrhiza 11: 59–66. [Google Scholar]
- Yamada A, Ogura T, Ohmasa M, (2001b). Cultivation of mushrooms of edible ectomycorrhizal fungi associated with Pinus densiflora by in vitro mycorrhizal synthesis: II. Morphology of mycorrhizas in open-pot soil. Mycorrhiza 11: 67–81. [Google Scholar]
- Yamaguchi K, Degawa Y, Nakagiri A. (2009). An aero-aquatic fungus, Peyronelina glomerulata, is shown to have teleomorphic affinities with cyphelloid basidiomycetes. Mycoscience 50: 156–164. [Google Scholar]
- Yamaura Y, Fukuhara M, Kawamata S. et al. (1986). Effects of Clitocybe clavipes extract on the components and enzymes related to ethanol metabolism in mice. Journal of the Food Hygienic Society of Japan (Shokuhin Eiseigaku Zasshi) 27: 522–527. [Google Scholar]
- Yang R-H, Li Y, Tang LH. et al. (2017). Genome-wide comparison of lignocellulose degradation enzymes in Agaricales. Mycosystema 36: 705–717. [Google Scholar]
- Yang Z-L, Cai Q, Cui Y-Y. (2018). Phylogeny, diversity and morphological evolution of Amanitaceae. Biosystematics and Ecology Series 34: 359–380. [Google Scholar]
- Yang Z-L, Feng B, Hao Y-J. (2013). Pseudoarmillariella bacillaris, a new species with bacilliform basidiospores in Asia. Mycosystema 32: 127–132. [Google Scholar]
- Young AM. (1999). The Hygrocybeae (Fungi, Basidiomycota, Agaricales, Hygrophoraceae) of the Lane Cove Bushland Park, New South Wales. Austrobaileya 5: 535–564. [Google Scholar]
- Young AM. (2005). Fungi of Australia, Hygrophoraceae. Australian Biological Resources Study, Canberra, CSIRO Publishing, Melbourne, Australia. [Google Scholar]
- Yu X-D, Deng H, Yao Y-J. (2011). Leucocalocybe, a new genus for Tricholoma mongolicum (Agaricales, Basidiomycota). African Journal of Microbiology Research 5: 5750–5756. [Google Scholar]
- Yuan MS, Sun PQ. 2007. Pictorial book of mushrooms of China. Sichuan Science and Technology Press, Chengdu, China: (in Chinese). [Google Scholar]
- Zervakis G, Dimou D, Balis C. (1998). A check-list of the Greek macrofungi including hosts and biogeographic distribution: I. Basidiomycotina. Mycotaxon 66: 273–336. [Google Scholar]
- Zhang Q-Y, Dai Y-C. (2021). Two new species of Panellus (Agaricales, Basidiomycota) from China. Mycological Progress 20: 51–60. [Google Scholar]
- Zhang Q-Y, Liu H-G, Papp V. et al. (2022). Taxonomy and multi-gene phylogeny of poroid Panellus (Mycenaceae, Agaricales) with the description of five new species from China. Frontiers in Microbiology 13: 928941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhu X, Vizzini A. et al. (2022). New insights into lichenization in Agaricomycetes based on an unusual new Basidiolichen species of Omphalina s. str. Journal of Fungi 8: 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C-L, Chen H, He S-H. et al. (2016). Radulotubus resupinatus gen. et sp. nov. with a poroid hymenophore in Pterulaceae (Agaricales, Basidiomycota). Nova Hedwigia 103: 265–278. [Google Scholar]
- Zhao Q-I, Hao Y-J, Liu J-K. et al. (2016). Infundibulicybe rufa sp. nov. (Tricholomataceae), a reddish-brown species from southwestern China. Phytotaxa 266: 134–140. [Google Scholar]
- Zhao R-L, Li G-J, Sánchez-Ramírez S. et al. (2017). A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Diversity 84: 43–74. [Google Scholar]
- Zheng R, Wang J, Liu M. et al. (2016). Molecular cloning and functional analysis of two phosphate transporter genes from Rhizopogon luteolus and Leucocortinarius bulbiger, two ectomycorrhizal fungi of Pinus tabulaeformis. Mycorrhiza 26: 633–644. [DOI] [PubMed] [Google Scholar]
- Zhou M, Liu Z-B, Lim YW. et al. (2022). Two new species of Fistulina (Agaricales, Basidiomycota) from the Northern Hemisphere. Frontiers in Microbiology 13: 1063038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bayesian inference phylogram built with nucleotide sequence data of six loci (5.8S, LSU, SSU, RPB1, RPB2 and TEF1) of the main lineages inside order Agaricales (including taxa of the suborder Clavariineae), rooted with Suillus pictus (Boletales), Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main suborders are shown in color boxes, while family names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study. The dashed branch was shortened for graphic presentation.
Bayesian inference phylogram built with nucleotide sequence data of six loci (5.8S, LSU, SSU, RPB1, RPB2 and TEF1) of the main lineages inside order Agaricales (including taxa of Cyphellopsidaceae), rooted with Suillus pictus (Boletales), Amylocorticium cebennense and Ceraceomyces borealis (Amylocorticiales) as outgroups. The main suborders are shown in color boxes, while family names are shown next to vertical bars. Nodes were annotated with Bayesian PP (left) and ML BP (right) values, with the significance threshold considered as Bayesian PP >0.95 and/or ML BP >70 %. Subsignificant support values were annotated in parentheses. Boldface names represent samples sequenced for this study. The dashed branch was shortened for graphic presentation.
Data of the collections used for phylogenetic analyses and/or morphological studies with details of their host, location, collector, and GenBank accessions numbers.