Abstract
During 25 surveys of global Phytophthora diversity, conducted between 1998 and 2020, 43 new species were detected in natural ecosystems and, occasionally, in nurseries and outplantings in Europe, Southeast and East Asia and the Americas. Based on a multigene phylogeny of nine nuclear and four mitochondrial gene regions they were assigned to five of the six known subclades, 2a–c, e and f, of Phytophthora major Clade 2 and the new subclade 2g. The evolutionary history of the Clade appears to have involved the pre-Gondwanan divergence of three extant subclades, 2c, 2e and 2f, all having disjunct natural distributions on separate continents and comprising species with a soilborne and aquatic lifestyle and, in addition, a few partially aerial species in Clade 2c; and the post-Gondwanan evolution of subclades 2a and 2g in Southeast/East Asia and 2b in South America, respectively, from their common ancestor. Species in Clade 2g are soilborne whereas Clade 2b comprises both soil-inhabiting and aerial species. Clade 2a has evolved further towards an aerial lifestyle comprising only species which are predominantly or partially airborne. Based on high nuclear heterozygosity levels ca. 38 % of the taxa in Clades 2a and 2b could be some form of hybrid, and the hybridity may be favoured by an A1/A2 breeding system and an aerial life style. Circumstantial evidence suggests the now 93 described species and informally designated taxa in Clade 2 result from both allopatric non-adaptive and sympatric adaptive radiations. They represent most morphological and physiological characters, breeding systems, lifestyles and forms of host specialism found across the Phytophthora clades as a whole, demonstrating the strong biological cohesiveness of the genus. The finding of 43 previously unknown species from a single Phytophthora clade highlight a critical lack of information on the scale of the unknown pathogen threats to forests and natural ecosystems, underlining the risk of basing plant biosecurity protocols mainly on lists of named organisms. More surveys in natural ecosystems of yet unsurveyed regions in Africa, Asia, Central and South America are needed to unveil the full diversity of the clade and the factors driving diversity, speciation and adaptation in Phytophthora.
Taxonomic novelties: New species: Phytophthora amamensis T. Jung, K. Kageyama, H. Masuya & S. Uematsu, Phytophthora angustata T. Jung, L. Garcia, B. Mendieta-Araica, & Y. Balci, Phytophthora balkanensis I. Milenković, Ž. Tomić, T. Jung & M. Horta Jung, Phytophthora borneensis T. Jung, A. Durán, M. Tarigan & M. Horta Jung, Phytophthora calidophila T. Jung, Y. Balci, L. Garcia & B. Mendieta-Araica, Phytophthora catenulata T. Jung, T.-T. Chang, N.M. Chi & M. Horta Jung, Phytophthora celeris T. Jung, L. Oliveira, M. Tarigan & I. Milenković, Phytophthora curvata T. Jung, A. Hieno, H. Masuya & M. Horta Jung, Phytophthora distorta T. Jung, A. Durán, E. Sanfuentes von Stowasser & M. Horta Jung, Phytophthora excentrica T. Jung, S. Uematsu, K. Kageyama & C.M. Brasier, Phytophthora falcata T. Jung, K. Kageyama, S. Uematsu & M. Horta Jung, Phytophthora fansipanensis T. Jung, N.M. Chi, T. Corcobado & C.M. Brasier, Phytophthora frigidophila T. Jung, Y. Balci, K. Broders & I. Milenković, Phytophthora furcata T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, Phytophthora inclinata N.M. Chi, T. Jung, M. Horta Jung & I. Milenković, Phytophthora indonesiensis T. Jung, M. Tarigan, L. Oliveira & I. Milenković, Phytophthora japonensis T. Jung, A. Hieno, H. Masuya & J.F. Webber, Phytophthora limosa T. Corcobado, T. Majek, M. Ferreira & T. Jung, Phytophthora macroglobulosa H.-C. Zeng, H.-H. Ho, F.-C. Zheng & T. Jung, Phytophthora montana T. Jung, Y. Balci, K. Broders & M. Horta Jung, Phytophthora multipapillata T. Jung, M. Tarigan, I. Milenković & M. Horta Jung, Phytophthora multiplex T. Jung, Y. Balci, K. Broders & M. Horta Jung, Phytophthora nimia T. Jung, H. Masuya, A. Hieno & C.M. Brasier, Phytophthora oblonga T. Jung, S. Uematsu, K. Kageyama & C.M. Brasier, Phytophthora obovoidea T. Jung, Y. Balci, L. Garcia & B. Mendieta-Araica, Phytophthora obturata T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, Phytophthora penetrans T. Jung, Y. Balci, K. Broders & I. Milenković, Phytophthora platani T. Jung, A. Pérez-Sierra, S.O. Cacciola & M. Horta Jung, Phytophthora proliferata T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, Phytophthora pseudocapensis T. Jung, T.-T. Chang, I. Milenković & M. Horta Jung, Phytophthora pseudocitrophthora T. Jung, S.O. Cacciola, J. Bakonyi & M. Horta Jung, Phytophthora pseudofrigida T. Jung, A. Durán, M. Tarigan & M. Horta Jung, Phytophthora pseudoccultans T. Jung, T.-T. Chang, I. Milenković & M. Horta Jung, Phytophthora pyriformis T. Jung, Y. Balci, K.D. Boders & M. Horta Jung, Phytophthora sumatera T. Jung, M. Tarigan, M. Junaid & A. Durán, Phytophthora transposita T. Jung, K. Kageyama, C.M. Brasier & H. Masuya, Phytophthora vacuola T. Jung, H. Masuya, K. Kageyama & J.F. Webber, Phytophthora valdiviana T. Jung, E. Sanfuentes von Stowasser, A. Durán & M. Horta Jung, Phytophthora variepedicellata T. Jung, Y. Balci, K. Broders & I. Milenković, Phytophthora vietnamensis T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, Phytophthora ×australasiatica T. Jung, N.M. Chi, M. Tarigan & M. Horta Jung, Phytophthora ×lusitanica T. Jung, M. Horta Jung, C. Maia & I. Milenković, Phytophthora ×taiwanensis T. Jung, T.-T. Chang, H.-S. Fu & M. Horta Jung.
Citation: Jung T, Milenković I, Balci Y, Janoušek J, Kudláček T, Nagy ZÁ, Baharuddin B, Bakonyi J, Broders KD, Cacciola SO, Chang T-T, Chi NM, Corcobado T, Cravador A, Đorđević B, Durán A, Ferreira M, Fu C-H, Garcia L, Hieno A, Ho H-H, Hong C, Junaid M, Kageyama K, Kuswinanti T, Maia C, Májek T, Masuya H, Magnano di San Lio G, Mendieta-Araica B, Nasri N, Oliveira LSS, Pane A, Pérez-Sierra A, Rosmana A, Sanfuentes von Stowasser E, Scanu B, Singh R, Stanivuković Z, Tarigan M, Thu PQ, Tomić Z, Tomšovský M, Uematsu S, Webber JF, Zeng H-C, Zheng F-C, Brasier CM, Horta Jung M (2024). Worldwide forest surveys reveal forty-three new species in Phytophthora major Clade 2 with fundamental implications for the evolution and biogeography of the genus and global plant biosecurity. Studies in Mycology 107: 251–388. doi: 10.3114/sim.2024.107.04
Keywords: allopatric speciation, biodiversity, breeding systems, Gondwana, Laurasia, lifestyle, new taxa, phylogeny, sympatric species radiation
INTRODUCTION
The oomycete genus Phytophthora currently includes eight obligate biotrophic and 210 culturable necrotrophic or hemibiotrophic described species which are soil-, water- or airborne plant pathogens causing some of the most damaging diseases of horticultural and agricultural crops, forests and other natural ecosystems (Erwin & Ribeiro 1996, Yang et al. 2017, Jung et al. 2018a, 2022, Brasier et al. 2022, Chen et al. 2022, Abad et al. 2023a). Recently Abad et al. (2023a) have consolidated the formal taxonomy of the genus by designating lectotypes, epitypes or neotypes for numerous species, validating other species and providing additional taxonomic descriptions. Phytophthora is monophyletic and currently resolves into 15 major phylogenetic clades with numerous subclades (Yang et al. 2017, Brasier et al. 2022, Chen et al. 2022, Abad et al. 2023a). In addition, phylogenetic and phylogenomic studies demonstrated that the 20 genera of obligate biotrophic downy mildews are residing as two separate clades within the genus Phytophthora as a result of a paraphyletic evolutionary jump followed by rapid global radiation driven by specialization to non-woody host plants (Cooke et al. 2000, Thines & Choi 2016, Jung et al. 2017a, McCarthy & Fitzpatrick 2017, Bourret et al. 2018, Fletcher et al. 2018, 2019, Scanu et al. 2021, Brasier et al. 2022, Abad et al. 2023a).
At the time of the first genus-wide molecular phylogeny (Cooke et al. 2000) Phytophthora comprised around 50 known taxa, including seven species in major Clade 2: P. botryosa, P. capsici, P. citricola, P. citrophthora, P. colocasiae, P. inflata and P. multivesiculata. Phytophthora inflata was later declared a lost and invalid species (Jung & Burgess 2009). Soon, however, it was estimated that the genus might comprise as many as 600 species (Brasier 2009) and subsequent surveys in natural ecosystems, nurseries and plantations, together with a revision of the ‘P. citricola complex’ using multigene phylogenetic analyses, enlarged Clade 2 to 34 described species. These reside in five evolutionary divergent subclades, Clades 2a–2e (Aragaki & Uchida 2001, Maseko et al. 2007, Reeser et al. 2007, Abad et al. 2008, 2011, 2023a, Hong et al. 2009, 2011, Jung & Burgess 2009, Scott et al. 2009, Bezuidenhout et al. 2010, Rea et al. 2010, Vettraino et al. 2011, Ginetti et al. 2014, Henricot et al. 2014, Ann et al. 2015, Man In’t Veld et al. 2015, Brazee et al. 2017, Crous et al. 2017, 2020, Ruano-Rosa et al. 2018, Albuquerque Alves et al. 2019, Burgess et al. 2020, Bose et al. 2021a, Dang et al. 2021, Decloquement et al. 2021, Chen et al. 2022). Half of these species, including P. acaciae, P. acaciivora, P. amaranthi, P. botryosa, P. capsici, P. citricola, P. citrophthora, P. colocasiae, P. frigida, P. gloveri (previously P. glovera; Abad et al. 2011, 2023a), P. meadii, P. mekongensis, P. mengei, P. multibullata, P. oleae, P. theobromicola, P. tropicalis and P. ×vanyenensis cause severe root rots, bark cankers, fruit rots or leaf blights on tropical and subtropical crops, tree crops and plantation trees (Erwin & Ribeiro 1996, Aragaki & Uchida 2001, Drenth & Guest 2004, Maseko et al. 2007, Abad et al. 2011, Hong et al. 2009, Lamour 2013, Ann et al. 2015, Crous et al. 2017, Ruano-Rosa et al. 2018, Albuquerque Alves et al. 2019, Burgess et al. 2020, Dang et al. 2021, Decloquement et al. 2021, Brasier et al. 2022, Chen et al. 2022). Others, such as P. acerina, P. aysenensis, P. elongata, P. multivora, P. pini, P. plurivora and P. siskiyouensis are primarily pathogens of forest trees and shrubs causing root rots and bark cankers often resulting in decline and dieback (Reeser et al. 2007, Jung & Burgess 2009, Scott et al. 2009, Rea et al. 2010, Hong et al. 2011, Ginetti et al. 2014, Milenković et al. 2018, Jung et al. 2018a, Corcobado et al. 2020, Crous et al. 2020). Many of the aforementioned species plus P. bishii (previously P. bisheria; Abad et al. 2008, 2023a), P. capensis, P. emzansi, P. multivesiculata, P. occultans, P. pachypleura and P. terminalis also cause severe losses of a wide range of host plants in nurseries and ornamental plantings (Ilieva et al. 1998, Abad et al. 2008, Donahoo & Lamour 2008a, Moralejo et al. 2008, Leonberger et al. 2013, Pérez-Sierra & Jung 2013, Bienapfl & Balci 2014, Henricot et al. 2014, Prigigallo et al. 2015, Man In’t Veld et al. 2015, Jung et al. 2016, Frankel et al. 2020, Mora-Sala et al. 2022). In contrast, P. himalsilva, P. insulinativitatica and several informally designated Clade 2 taxa have only been obtained from soil around apparently healthy vegetation in natural forests in Nepal, Christmas Island, the Cocos Islands, Taiwan and Papua New Guinea (Vettraino et al. 2011, Jung et al. 2017b, 2020, Burgess et al. 2021, Dang et al. 2021). Also, several species behaving as aggressive pathogens in invasive situations including P. bishii, P. citrophthora and P. plurivora in Europe and North America, P. multivora in Australia, Europe and North America, and P. elongata in Australia have been found in ‘healthy’ natural ecosystems in East Asia (P. bishii, P. citrophthora, P. elongata, P. plurivora; Brasier et al. 2010, Bennett et al. 2017, Jung et al. 2017b, 2020) and South Africa (P. multivora; Oh et al. 2013, Tsykun et al. 2022).
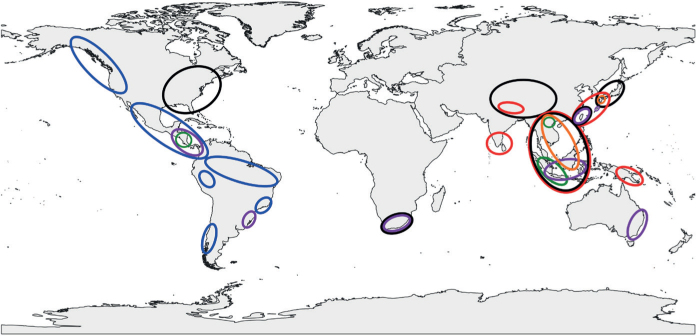
Since 1998, we have conducted 25 Phytophthora surveys in natural and managed ecosystems across the Americas, Europe, Southeast and East Asia intending to better understand the diversity of the genus, its evolutionary history and the potential scale of the biosecurity threat posed to forests globally by scientifically ‘unknown’ tree pathogens (cf. Brasier 2008, Jung et al. 2016). Remarkably, these surveys have produced, amongst others, ca. 1 000 Phytophthora isolates preliminarily identified as belonging to 43 putative new Clade 2 taxa. Several of these have been informally taxonomically designated e.g. as P. citricola VII, P. taxon occultans-like, P. taxon ×botryosa-like and P. taxon ×meadii-like (Jung et al. 2017b), P. taxon botryosa-like 2, P. taxon meadii-like 1 and 2, P. taxon multivesiculata-like 1 and P. taxon tropicalis-like 2 (Jung et al. 2020), P. valdiviana nom. prov. (Jung et al. 2018b) and P. ×citrophthora-related1, P. ×citrophthora-related2 and P. ×citrophthora2 (Van Poucke et al. 2021). In this study, we used morphological and physiological criteria together with DNA sequence data from nine nuclear and four mitochondrial gene regions to characterise the 43 putative new taxa and compare them with each other and to previously known Clade 2 species. Here we describe each of them as new species and discuss the implications of our findings for the evolution and biogeography of the Clade and global biosecurity.
TERMINOLOGY
Use of the terms Phytophthora taxon x and Phytophthora sp. x
The informal term ‘Phytophthora taxon x’ (cf. Brasier et al. 2003) was developed to cover situations where it was clear that a novel entity of some taxonomic level had been identified, but formal description was likely to be delayed pending further analysis to determine the level of taxonomic distinction (e.g. species, subspecies, variety etc; cf. Brasier & Rayner 1987) and because of the often considerable time required to produce the appropriate publication. This situation has arisen more frequently as more and more novel Phytophthora taxa are being discovered.
In this context, we do not concur with the use of the informal terminology ‘Phytophthora sp. x’. A putative new taxon is not a species (or a ‘sp.’) until its correct hierarchical status has been determined (as far as is reasonable), and its proposed name has been formally designated under the ICNafp (International Code of Nomenclature for algae, fungi, and plants; https://www.iapt-taxon.org) guidelines. On this basis, we consider that informal use of the term ‘Phytophthora sp. x’ in the case of a putative but only partially characterised new taxon is essentially prejudicial to its eventual ranking. We have therefore confined ourselves to using the term “Phytophthora taxon x” throughout this manuscript.
Subclade phraseology
Naming of the subclades follows Yang et al. (2017), Chen et al. (2022) and Abad et al. (2023a). For brevity, throughout much of the text the main phylogenetic subclades 2a–2e of Phytophthora Clade 2, technically ‘Clade 2 subclade a’, ‘Clade 2 subclade b’ etc., are referred to as Clade 2a, Clade 2b and so on. Following Abad et al. (2023a), the monospecific lineage of P. oleae is named as subclade 2d whereas subclade 2e of Abad et al. (2023a) is divided into two subclades (as previously in Yang et al. 2017 and Chen et al. 2022) named here 2e and 2f. A new subclade is designated as 2g.
Definitions of ‘homothallism’, ‘heterothallism’ and sterility
Homothallism and heterothallism are somewhat archaic, quasimorphological terms used more to describe whether gametangia are formed in single or paired Phytophthora cultures rather than the biological strategy this represents. We prefer a more Darwinian process-related definition that implies the organism’s underlying breeding system or breeding strategy.
However, because historically these terms have been used routinely in species descriptions, we have also used them occasionally here, but with the following qualification. By homothallic we mean intrinsically self-fertile in a single culture and therefore often inbreeding, but this process does not preclude outbreeding in nature as a result of the fusion between antheridia and oogonia of different genotypes of the species. We will more often refer to these taxa in the text as ‘self-fertile’. By heterothallic we mean that two mating or compatibility types (A1 and A2) are typically required to initiate gametogenesis between bisexual, largely self-incompatible individuals; but while this process promotes outcrossing, once initiated it can also lead to a significant frequency of self-fertilisation (selfing). We will refer to this breeding system in the text as A1/A2 outcrossing, or just ‘A1/A2’. By sterile we mean an apparent lack of the intrinsic ability to form gametangia whether in a single culture or pairings with A1 or A2 isolates; but this does not exclude the possibility that an isolate or taxon may act as a ‘silent’ A1 or A2, inducing gametangial formation by selfing in an A2 or an A1 isolate of another species (cf. P. gonapodyides, Brasier et al. 2003).
MATERIAL AND METHODS
Phytophthora isolates
Details of all isolates used in the phylogenetic, morphological and temperature-growth studies are given in Table S1. Sampling and isolation methods from forest soil and river systems were described by Jung et al. (1996, 2017b, 2018b, 2020) and Pérez-Sierra et al. (2022). Necrotic baiting leaves or naturally fallen leaves collected from streams or the forest ground were plated onto selective PARPNH-agar (Jung et al. 1996, 2020). The isolates of the 43 new Clade 2 species were recovered from streams, rhizosphere soil and necrotic leaves in Valdivian rainforests in Chile; tropical or subtropical montane cloud forests in Nicaragua, Panama and Vietnam; tropical submontane to montane forests in Sumatra, Java, Sulawesi and Hainan island; tropical lowland rainforests in Nicaragua, Panama, Kalimantan, Sulawesi, Sumatra and Vietnam; subtropical monsoon forests in Japan, Taiwan and Vietnam; subtropical forests in Louisiana, USA; warm-temperate forests in Japan; cool-temperate forests in Bosnia-Herzegovina, Serbia and Japan; and Mediterranean forests in Italy and Portugal (Table S1). Isolates were also obtained from nursery plants, ornamental or horticultural plantings and amenity trees in Croatia, Germany, Hungary, the UK, Morocco and Sumatra (Table S1). In addition, for comparative studies isolates of 11 described Clade 2 species were sourced from the culture collections of the authors (Table S1) while isolates from another seven described Clade 2 species were obtained between 2013 and 2020: P. acaciivora from the effluent of an Acacia and Eucalyptus nursery in Sumatra; P. citrophthora from rhizosphere soil and streams in temperate forests in Japan and Serbia, a subtropical monsoon forest in Taiwan and Mediterranean forests in Portugal; P. colocasiae from necrotic taro leaves in a tropical lowland rainforest in Sumatra; P. pini from rhizosphere soil and streams in temperate forests in Bosnia-Herzegovina and Serbia, subtropical forests in Louisiana, USA, and amenity plantings in Croatia, Germany and Slovakia; P. siskiyouensis from a bleeding bark canker of Alnus cordata in the UK; P. tropicalis from streams and naturally fallen leaves in tropical lowland and hill forests in Java, Sumatra, Nicaragua and Panama, and a subtropical monsoon forest in Taiwan; and P. ×vanyenensis from rhizosphere soil and streams in tropical lowland rainforests in Java, Sulawesi and Sumatra (Table S1). For all isolates, single hyphal tip cultures were produced under the stereomicroscope at ×20 from the margins of fresh cultures on V8-juice agar (V8A; 16 g agar, 3 g CaCO3, 100 mL Campbell’s V8 juice, 900 mL distilled water; Jung et al. 1999). Stock cultures were maintained on V8A and carrot juice agar (CA; 20 g agar, 3 g CaCO3, 100 mL organic carrot juice from the company DM in Karlsruhe, Germany, 900 mL distilled water; Scanu et al. 2014) at 10 °C in the dark. All isolates of the 43 new Phytophthora spp. are preserved in the culture collection maintained at Mendel University in Brno. Ex-type cultures were deposited at the CBS culture collection (CBS) at the Westerdijk Institute, Utrecht, Netherlands (Table S1). Dried V8A cultures of the 43 ex-type isolates were deposited as holotypes in the CBS Fungarium (CBS H; also at the Westerdijk Institute).
DNA isolation, amplification and sequencing
For all Phytophthora isolates from Clade 2 obtained in this study or sourced from the culture collections of the authors, for the ex-epitype isolate CBS 147289 of P. infestans from Clade 1c and the ex-type isolate CBS 111772 of P. pseudosyringae from Clade 3, DNA was extracted from ca. 15–100 mg of mycelium scraped from 1–3-wk-old V8A cultures, placed into 2 mL homogenisation tubes (Lysis Matrix A; MP Biomedicals, Irvine, USA) and disrupted using a Precellys Evolution instrument (Bertin Technologies, Montigny-le-Bretonneux, France) until the mixture was homogenous. DNA was purified using the Monarch Genomic DNA Purification Kit (New England Biolabs, Ipswich, USA) and treated with RNase A following the manufacturer´s protocol for tissue samples. DNA was eluted with 100 μL of pre-warmed elution buffer and preserved at −80 °C for long-term storage.
Nine nuclear gene regions, i.e., the internal transcribed spacer region (ITS1–5.8S–ITS2) of the ribosomal RNA gene (ITS), the 5’ terminal domain of the large subunit (28S-LSU) of the nuclear ribosomal RNA, heat shock protein 90 (hsp90), β-tubulin (βtub), 60S ribosomal protein L10 (rpl10), TIGA gene fusion protein (genes encoding triose-phosphate isomerase (TPI) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) fused and forming a single transcriptional unit: tigA), translation elongation factor 1 alpha (tef-1α), enolase (enl), Ras-like GTP-binding protein YPT1 (ras-ypt1), and the four mitochondrial genes cytochrome-c oxidase 1 (cox1) and 2 (cox2), subunit 1 of NADH dehydrogenase (nadh1), and 40S ribosomal protein S10 (rps10) were amplified and sequenced (Table S2). The PCR amplifications were performed using a LightCycler 480 II instrument (Roche, Basel, Switzerland) or Eppendorf Mastercycler nexus GSX1 (Eppendorf, Hamburg, Germany). Table S2 provides a comprehensive overview of the PCR conditions and the primers used. All primers were synthesized by Elizabeth Pharmacon spol. s.r.o. (Brno, Czech Republic). Their annealing temperatures were estimated using a Tm calculator (http://tmcalculator.neb.com/#!/main) and adjusted empirically, according to observed PCR amplification rates.
The PCR products were visualised by gel electrophoresis (300 V; 5 min) using 2 % agarose gel stained by DNA Stain G (SERVA, Heidelberg, Germany). All amplicons were purified and sequenced in both directions by Eurofins Genomics GmbH (Cologne and Ebersberg, Germany) using the amplification primers, except for the LSU and tigA amplicons which required each two additional primers (Table S2). Electropherograms were quality-checked and forward and reverse reads were compiled using Geneious Prime® v. 2022.0.2 (Biomatters Ltd., Auckland, New Zealand). Pronounced double peaks were considered as heterozygous positions and labelled according to the IUPAC (International Union of Pure and Applied Chemistry; https://iupac.org) coding system. All sequences generated in this study were deposited in GenBank and accession numbers are given in Table S3.
Phylogenetic analysis
For phylogenetic analyses, the sequences obtained in this study were complemented with publicly available sequences of isolates from Phytophthora Clade 2 sourced from the GenBank Nucleotide Collection, many of them identified selected using the IDphy Phytophthora online resource (https://idtools.org/phytophthora; Abad et al. 2023b), and GenBank Whole-Genome Shotgun contigs (Table S3). The sequences of all loci used in the analyses were aligned using the MAFFT v. 7 (Katoh & Standley 2013) plugin within the Geneious Prime® v. 2023.3.1 software (https://www.geneious.com) by the E-INS-I strategy (ITS) or the G-INS-I strategy (all other loci). The ITS alignments in this study were manually edited and adjusted.
The phylogenetic structure of Clade 2 was studied using a 13-partition (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of 91 type and other key isolates from the 43 new and 36 previously described species and two informally designated taxa within Clade 2 with P. infestans from Clade 1c and P. pseudosyringae from Clade 3 as outgroup taxa.
The relative phylogenetic positions of the 43 new Clade 2 species within their respective subclades were studied using six separate 13-partition (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) datasets for subclades 2a (120 isolates from 6 new and 11 known species and 5 informally designated taxa); 2b (104 isolates from 9 new and 9 known species and 3 informally designated taxa); 2c (106 isolates from 15 new and 9 known species); 2e (39 isolates from 6 new and 5 known species and 1 informally designated taxon); 2f (26 isolates from 3 new and 1 known species and 1 informally designated taxon); and 2g (13 isolates from 4 new species). In all analyses, P. infestans from Clade 1c and P. pseudosyringae from Clade 3 were used as outgroup taxa.
For Maximum Likelihood (ML) analyses best-fit substitution models were selected using PartitionFinder v. 2 (Lanfear et al. 2016) based on the corrected Akaike Information Criterion (AICc). Each locus was considered to be a separate partition. All 84 available evolutionary models were used, including those with base frequencies estimated by maximum likelihood (the parameter models = allx;). The phylogeny was reconstructed with RAxML-NG v. 1.1.0 (Kozlov et al. 2019). The MRE-based bootstopping test was applied to determine the necessary number of bootstrap replicates. The bootstopping method based on the extended majority rule (MRE) (also known as greedy consensus) (Pattengale et al. 2010) was implemented to automatically determine a sufficient number of bootstrap replicates with the cut-off value being set to 0.03 (the option--bs-cutoff 0.03). To calculate branch support values, the Transfer Bootstrap Expectation method (TBE; Lemoine et al. 2018) was selected. The presented ML trees represent the best-scoring trees with the TBE support values mapped onto them.
Bayesian Inference (BI) analyses were performed using BEAST v. 2 (Bouckaert et al. 2014). For all BI analyses Metropolis coupled MCMC (MC3) implemented in the CoupledMCMC package (Müller & Bouckaert 2020) was used with four chains – three heated and one cold. The chain length was always set to 20 M, except for the whole Clade dataset and the Clade 2a dataset where it was 40 M, and every 5 000th state was sampled. The target switch probability was set to the recommended value of 0.234 (Kone & Kofke 2005, Atchadé et al. 2011). Site models for individual partitions were automatically selected by model averaging implemented in the bModelTest package (Bouckaert & Drummond 2017). For all analyses, the optimised relaxed clock (Douglas et al. 2021), a performance-optimised version of the uncorrelated log-normal relaxed molecular clock model (Drummond et al. 2006), was used. The unit of branch lengths of the sampled trees was set to be substitutions per site. Parameter estimates were summarized with TreeAnnotator v. 2.6.0 (part of BEAST v. 2) and mapped onto the 50 % majority-rule consensus tree created by SumTrees v. 4.4.0 (Sukumaran & Holder 2015) from the Python library DendroPy v. 4.4.0 (Sukumaran & Holder 2010). The edge lengths of the summarizing tree were calculated as mean lengths for the corresponding edges in the input set of trees. The option ‘forcerooted’ was set for SumTrees telling the program to treat all the trees as rooted. The posterior estimates of the parameters were summarised with Tracer (Rambaut et al. 2018). The quality of the parameter estimates was assessed based on visual analysis of the trace plots and ESS values. The minimum ESS value for the parameter estimate to be considered properly sampled was 200 (standard setting). The likelihood and most of the other parameters of all the final trees were higher than 200. In all BI analyses a 25 % burn-in was used.
Phylogenetic trees were visualised in TreeGraph2 v. 2.15.0-887 beta (Stöver & Müller 2010) and/or MEGA 11 v. 11.0.11 (Tamura et al. 2021) and edited in figure editor programs. All datasets and trees deriving from BI and ML analyses were deposited in the Mendeley Data Repository, V1, (doi: 10.17632/8r5ww3w7mn.1).
Morphology of asexual and sexual structures
Morphological features of sporangia, oogonia, oospores, antheridia, chlamydospores, hyphal swellings and aggregations of all isolates of the 43 new species and selected isolates of related species from Clade 2 were compared with each other.
To induce the formation of sporangia, two 12–15 mm square discs were cut from the growing edge of a 3–7-d-old colony on V8A and flooded in a 90-mm-diam Petri dish with non-sterile soil extract (50 g of filtered oak forest soil in 1 000 mL of distilled water, filtered after 24 h) just above the surface of the aerial mycelium (Jung et al. 1996). The Petri dishes were incubated at 20 °C and natural daylight near a window and the soil extract changed after ca. 6 h. Shape, type of apex, caducity and special features of sporangia and the formation of hyphal swellings and aggregations were recorded after 24–48 h. For each isolate 50 sporangia were measured at ×400 using a compound microscope (Zeiss Imager.Z2), a digital camera (Zeiss Axiocam ICc3) and a biometric software (Zeiss ZEN).
The formation of chlamydospores, gametangia (oogonia and antheridia) and their characteristic features were examined on V8A (self-fertile or ‘homothallic’ species) or on clarified carrot agar prepared from fresh grated organic carrots (fgCA) (A1/A2 outcrossing or ‘heterothallic’ species; Brasier 1967) after 21–30 d growth at 20 ºC in the dark. Self-sterile isolates of P. botryosa, P. calidophila, P. citrophthora, P. multiplex, P. pseudocitrophthora, P. pseudofrigida, P. obovoidea, P. pyriformis, P. tropicalis, P. variepedicellata, P. vietnamensis, P. ×australasiatica, P. ×taiwanensis and P. ×vanyenensis were paired on V8A with A1 and A2 mating type tester strains of P. cinnamomi (A1: TW12; A2: MP74), P. meadii (A1: MYA-4042; A2: MYA-4043) and P. acaciivora (A1 isolate SU1735; only used for pairings with P. pseudofrigida) and examined after 4 wk incubation at 20 °C in the dark to determine their mating type (Jung et al. 2017c). Then for those species with the presence of both mating types isolates from opposite mating types were paired with each other while isolates from species with a lack of one mating type were paired with a P. meadii tester strain of opposite mating type using a polycarbonate membrane (Whatman Nuclepore™ Track-Etched Membranes, Sigma-Aldrich, St. Louis, MO, USA) test (Scanu et al. 2021). For each isolate each 50 oogonia, oospores and antheridia chosen at random were measured under a compound microscope at ×400 as described before. The oospore wall index was calculated according to Dick (1990). In addition, if present, the diameters of 50 chlamydospores per isolate were measured.
Colony morphology, growth rates and cardinal temperatures
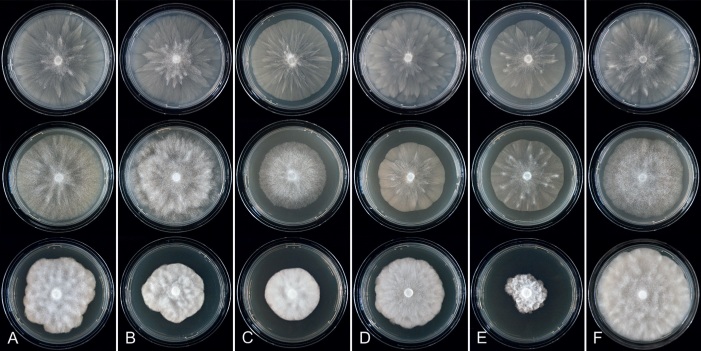
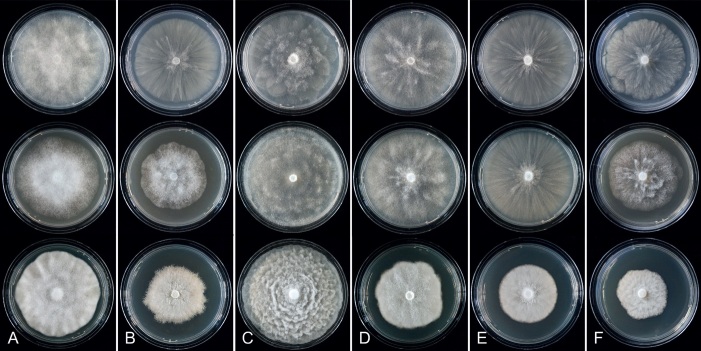
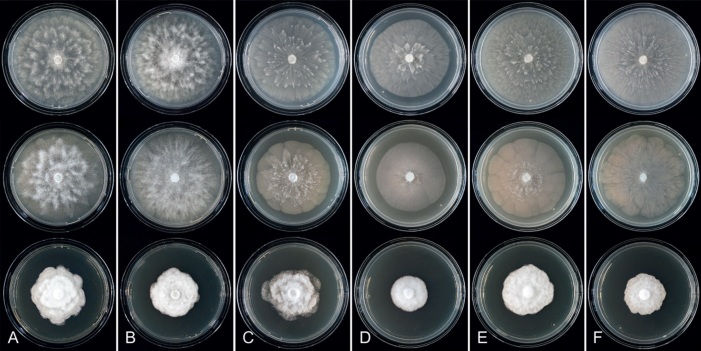
Colony growth patterns of all 43 new Clade 2 species, one new informally designated Clade 2 taxon and 17 described Clade 2 species, i.e., P. acaciivora, P. acerina, P. botryosa, P. citrophthora, P. colocasiae, P. elongata, P. meadii, P. mekongensis, P. mengei, P. multivora, P. occultans, P. pachypleura, P. pini, P. plurivora, P. siskiyouensis, P. tropicalis and P. ×vanyenensis, were described from 7-d-old cultures grown at 20 °C in the dark on V8A, CA and potato-dextrose agar (PDA; HiMedia, Mumbai, India). Colony morphologies were described according to patterns observed previously (Erwin & Ribeiro 1996, Jung & Burgess 2009, Jung et al. 2011, 2017c, d, 2021, 2022).
For temperature-growth relationships, representative isolates of all 43 new Clade 2 species, one new informally designated Clade 2 taxon and the 17 described Clade 2 species used for the colony morphologies (Table S1) were sub-cultured onto 90-mm-diam V8A plates and incubated for 24 h at 20 °C to stimulate onset of growth (Jung et al. 2002). Then three replicate plates per isolate were transferred to 10, 15, 20, 25, 27.5, 30, 32.5 and 35 °C. Radial growth was recorded after 4–14 d, before colonies reached the margin of the Petri dishes, along two lines intersecting the centre of the inoculum at right angles and the mean growth rates (mm/d) were calculated. Plates showing no growth at 25, 27.5, 30, 32.5 or 35 °C were returned to 20 °C to determine the lethal temperatures.
RESULTS
Phylogenetic results
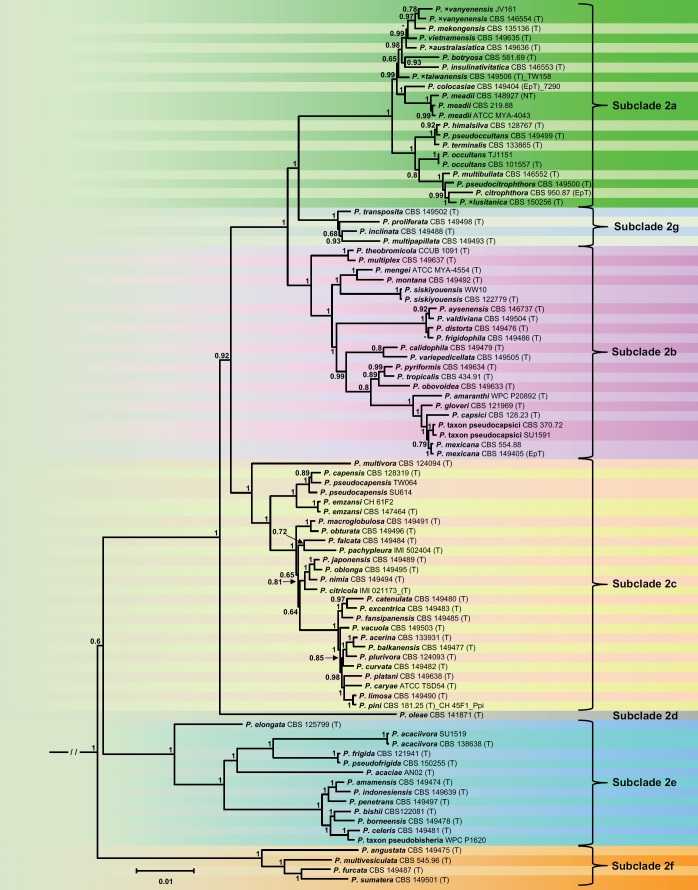
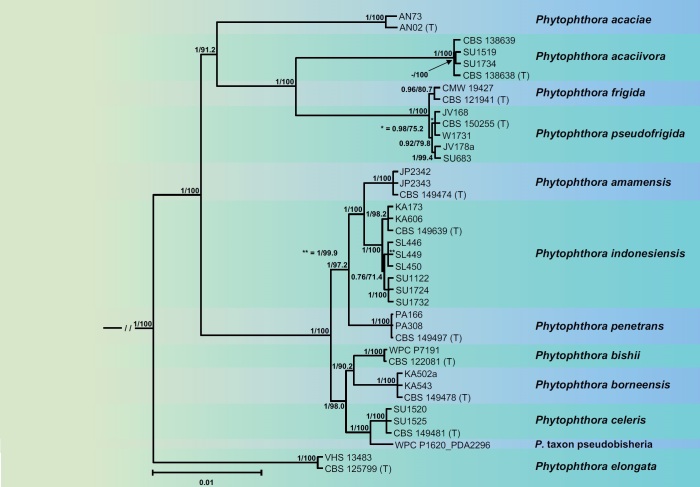
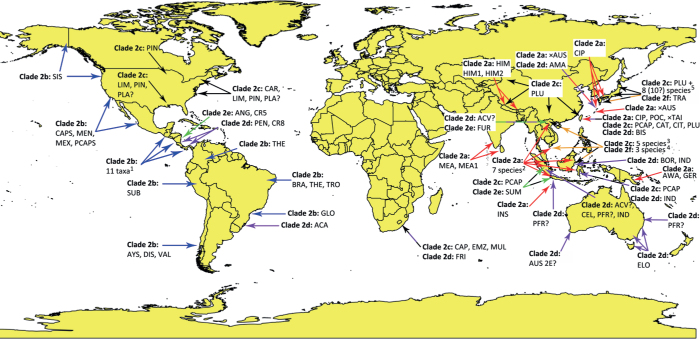
The phylogenetic structure of Clade 2 was studied using a 13-partition (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of 91 type and other key isolates from the 43 new and 36 previously described species and three informally designated taxa within Clade 2 with P. infestans from Clade 1c and P. pseudosyringae from Clade 3 as outgroup taxa. Being largely similar, the topologies of the ML bootstrap best tree and the 50 % majority consensus rule tree derived from the BI analysis differed mainly in the relative positions of Clades 2e and 2f, and the relative positions of several taxa within Clades 2a, 2b and 2c. In contrast to the BI tree, the ML tree showed two polytomies in Clade 2c and each one polytomy in Clades 2a and 2b. Therefore, the BI tree is presented here (Fig. 1), and the ML tree is given as Fig. S1. In both analyses, the deeper phylogeny resolved six discrete clusters corresponding to known Clades 2a–2f and a new subclade designated here as Clade 2g (Fig. 1). The phylogenetic positions of most Phytophthora taxa were well or fully supported. In the BI analysis, the evolutionary history of the clade is characterised by the early divergence of Clade 2f followed by subsequent divergences of Clades 2e, 2d, 2c and 2b and more recently the splitting between Clades 2g and 2a (Fig. 1). The deeper phylogeny was generally well supported except for the relative position of Clade 2e which, hence, remains ambiguous (Fig. 1). The ML analysis revealed a similar evolutionary history except for Clade 2e diverging earlier than Clade 2e which was well-supported (Fig. S1).
Fig. 1.
Fifty percent majority rule consensus phylogram derived from Bayesian inference analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora major Clade 2. Bayesian posterior probabilities are indicated but not shown below 0.60. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT), (NT) and (T) denote ex-epitype, ex-neotype and ex-type isolates. Scale bar indicates 0.01 expected changes per site per branch.
The relative phylogenetic positions of the 43 new Clade 2 species within their respective subclades were studied using six separate 13-partition (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) datasets for subclades 2a (120 isolates from 6 new and 11 known species and 5 informally designated taxa), 2b (104 isolates from 9 new and 9 known species and 3 informally designated taxa), 2c (106 isolates from 15 new and 9 known species), 2e (39 isolates from 6 new and 5 known species and 1 informally designated taxon), 2e (26 isolates from 3 new and 1 known species and 1 informally designated taxon) and 2g (13 isolates from 4 new species). In all analyses, P. infestans from Clade 1c and P. pseudosyringae from Clade 3 were used as outgroup taxa.
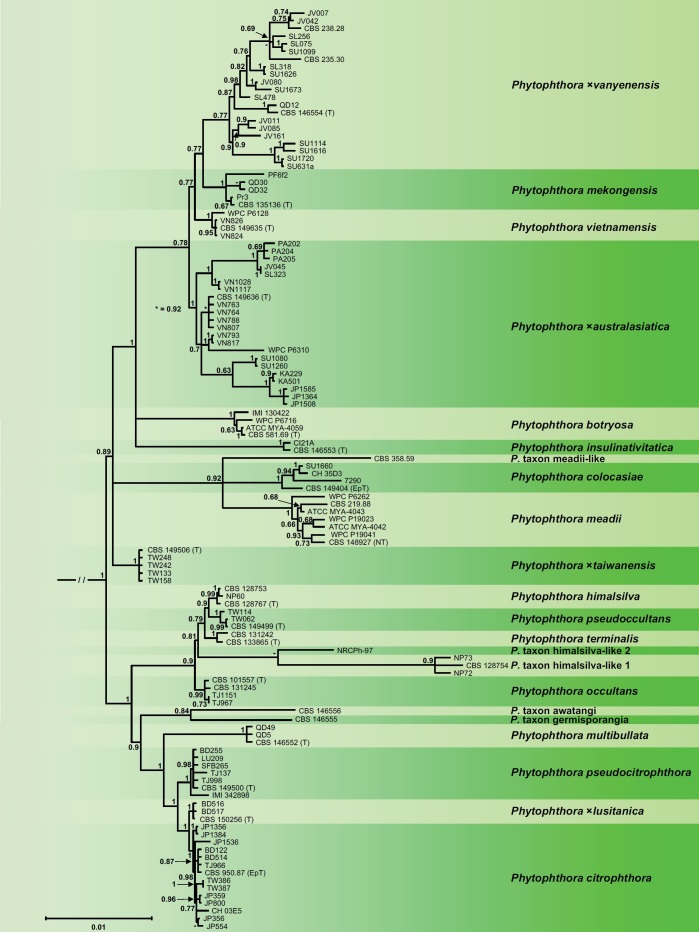
Within Clade 2a both BI and ML analyses resolved 22 discrete lineages. The ML bootstrap best tree and the 50 % majority rule consensus tree derived from the BI analysis showed partly different topologies but had similar support values for most nodes. Since the ML analysis failed to resolve the deeper phylogeny and the relative positions of P. mekongensis, P. vietnamensis and the hybrid species P. ×vanyenensis (Fig. S2) the BI tree is presented here (Fig. 2). The BI analysis grouped the 22 taxa in two large clusters (Fig. 2). One cluster comprised a subcluster of five self-fertile (homothallic) taxa, i.e., P. himalsilva, P. occultans, P. pseudoccultans, P. terminalis and P. taxon himalsilva-like 1, and P. taxon himalsilva-like 2 with unknown breeding system. Phytophthora himalsilva from Nepal and P. pseudoccultans from Taiwan proved to be closely related sister species with P. terminalis residing in a weakly supported, hence, ambiguous basal position to them. The separation between P. taxon himalsilva-like 1 and P. taxon himalsilva-like 2 had no support, probably because for the latter only four gene regions were available. Phytophthora occultans appeared in a fully supported basal position of this subcluster. The second subcluster contained the self-sterile (heterothallic) P. taxon awatangi and P. taxon germisporangia from Papua New Guinea which diverged first; the A1/A2 (‘heterothallic’) P. multibullata from Vietnam; and three sterile species, i.e., the sister species P. citrophthora and P. ×lusitanica together with P. pseudocitrophthora, the latter grouping in a basal position.
Fig. 2.
Fifty percent majority rule consensus phylogram derived from Bayesian inference analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2a. Bayesian posterior probabilities are indicated but not shown below 0.60. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT), (NT) and (T) denote ex-epitype, ex-neotype and ex-type isolates. Scale bar indicates 0.01 expected changes per site per branch.
In the second large cluster, the deeper phylogeny was characterised by three polytomies (trifurcations). The first one was well supported and comprised the A1/A2 hybrid species P. ×taiwanensis, a second well-supported polytomy containing the predominantly A1/A2 P. meadii, P. colocasiae and P. taxon meadii-like, and a third fully supported polytomy containing the A1/A2 P. botryosa and P. insulinativitatica and a cluster of four species. Within the latter, the A1/A2 P. ×australasiatica resided in a basal position to a subcluster which comprised the sterile P. vietnamensis and the A1/A2 P. mekongensis and P. ×vanyenensis. However, the nodes separating the four species were only weakly supported (BI posterior probabilities 0.77–0.78) and their relative phylogenetic positions within the subcluster remain ambiguous (Fig. 2). This was most likely caused by the high number of isolates from the hybrid species P. ×australasiatica and P. ×vanyenensis with numerous heterozygous positions in the nine nuclear gene regions, and partially by the presence of ancestors and descendants in the dataset (see below). Isolates of P. ×australasiatica showed considerable genetic variation and were grouped separately according to their origin from Japan, Panama, different regions of Vietnam and different islands of Indonesia. Isolate WPC P6310 from T. cacao in Indonesia, previously designated as P. taxon P6310 (Yang et al. 2017), resided within P. ×australasiatica (Fig. 2; Table S1). Phytophthora ×vanyenensis showed considerable genetic variation both within and between regions (Fig. 2; Table S1). Isolates CBS 235.30 from Sulavesi and CBS 238.28 of unknown origin, both previously designated as P. botryosa, resided within P. ×vanyenensis. Isolates QD30 and QD32 from Cinnamomum cassia plantations in the North of Vietnam, previously designated as P. ×vanyenensis (Dang et al. 2021), grouped with P. mekongensis isolates from Citrus plantations in the South of Vietnam (Fig. 2). The three polytomies in Clade 2a could indicate true species radiations or phylogenetic conflicts caused by reticulation events, recombination events or homoplasy (Bandelt et al. 1999, Posada et al. 2001, Cassens et al. 2005), or the presence of ancestors and descendents in the dataset as shown recently for the different lineages of P. ramorum (Jung et al. 2021). Across the alignments of 8 754 nuclear and 3 174 mitochondrial characters pairwise sequence differences between the Clade 2a taxa were 0.1–3.9 % and 0–6.3 %, respectively.
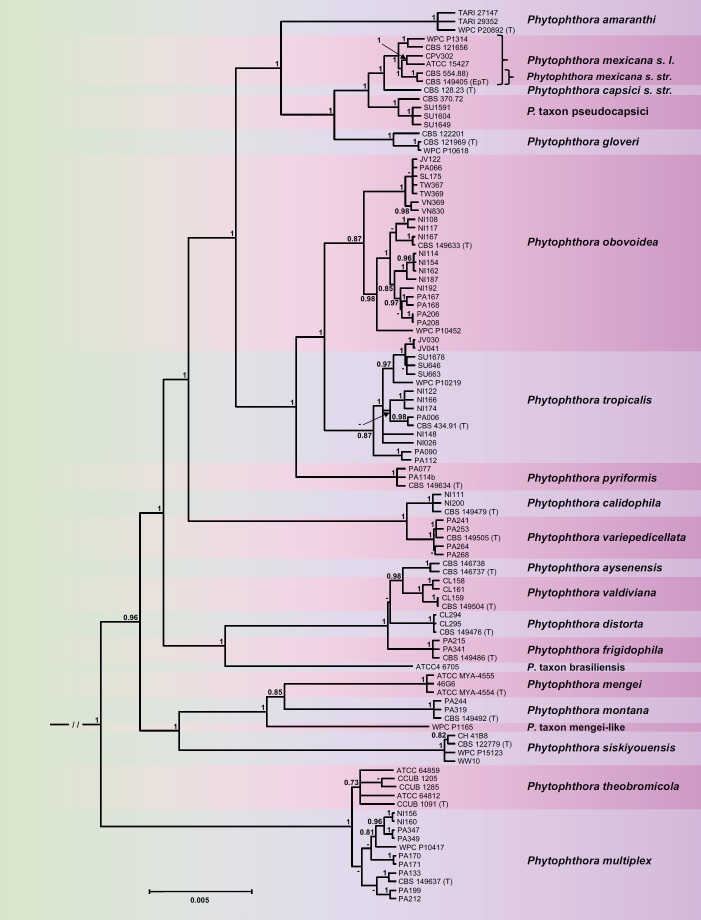
For Clade 2b the topologies of the 50 % majority rule consensus trees derived from the BI and ML (bootstrap best tree) analyses were mostly similar with several exceptions given below. Similar to the Clade 2a analyses, within species with highly heterozygous and diverse nuclear genes and potential interspecific hybridisation or introgression, i.e., P. multiplex, P. obovoidea and P. tropicalis, the ML analysis had lower power of resolution than the BI analysis resulting in polytomies and a transitional placement of individual isolates (Figs 3, S3). The BI tree is presented here (Fig. 3) and the ML tree is given as supplementary material (Fig. S3). Within Clade 2b, the phylogenetic analyses revealed 21 discrete lineages (Figs 3, S3) unambiguously corresponding to the nine described species P. amaranthi, P. aysenensis, P. capsici, P. gloveri, P. mengei, P. mexicana, P. siskiyouensis, P. theobromicola and P. tropicalis, nine new species, i.e., P. calidophila, P. distorta, P. frigidophila, P. montana, P. multiplex, P. obovoidea, P. pyriformis, P. valdiviana and P. variepedicellata, and three undescribed taxa, i.e., the informally designated P. taxon brasiliensis and P. taxon mengei-like and P. taxon pseudocapsici designated here. The evolutionary history of the subclade is characterised by the fully supported early divergence of the lineage leading to the extant Central and South American sister species P. multiplex and P. theobromicola from a large cluster comprising all other known Clade 2b taxa (Figs 3, S3). Within the ‘main’ Clade 2b cluster, a group of self-fertile (homothallic) species from Central and North America, including the two sister species P. mengei and P. montana, P. taxon mengei-like and P. siskiyouensis, reside in a basal position. The long branch length of P. siskiyouensis indicates long-term isolation and evolution. All nodes were well supported (Fig. 3, S3). The next separated cluster contained three self-fertile species from Chile, i.e., P. aysenensis, P. distorta and P. valdiviana, the self-fertile P. frigidophila from Panama and the distinct P. taxon brasiliensis in a basal position. All lineages were fully supported except P. distorta which was distinct but whose relative position within the cluster could not be unambiguously resolved (Figs 3, S3). An ensuing divergence of the self-sterile (heterothallic) Central American sister species P. calidophila and P. variepedicellata from a cluster including the P. capsici and P. tropicalis subclusters was also well supported (Figs 3, S3). Within the P. capsici subcluster the self-fertile P. amaranthi from Taiwan apparently diverged first followed by the splitting between the self-fertile P. gloveri from Brazil and a cluster of three fully supported A1/A2 lineages. One well-supported lineage comprises the ex-epitype (CBS 149405) and isolate CBS 554.88 of P. mexicana from Texas and Mexico, respectively, and several isolates from Mexico or the USA previously designated as P. capsici (CBS 121656, WPC P1314, CPV302) or P. aff. capsici (ATCC 15427; Yang et al. 2017), designated here as P. mexicana sensu lato; another with full support in the BI analysis but lower support (78.3 %) in the ML analysis the ex-type isolate of P. capsici (CBS 128.23) from New Mexico designated here as P. capsici sensu stricto; and the third P. taxon pseudocapsici newly designated here, fully supported and comprising three isolates obtained from Sumatra during this study. Clustering in the BI analysis with P. taxon pseudocapsici isolate CBS 370.72 from New Mexico previously designated as P. capsici grouped in the ML analysis between P. taxon pseudocapsici and P. capsici s. str. (Figs 3, S3). Within the P. tropicalis cluster, P. pyriformis from Panama resides in a well-supported basal position to the sister species P. obovoidea and P. tropicalis both of which showed considerable intraspecific variation. Phytophthora obovoidea contained one lineage with wide distribution in Java, Panama, Sulawesi, Taiwan and Vietnam, a more diverse group with three lineages from Nicaragua and Panama, and an isolate from southern California (WPC P10452) in a basal position (Figs 3, S3). Within P. tropicalis, in the BI analysis two isolates from Panama grouped in a basal position to a fully supported polytomy containing a group of isolates with Western Pacific distribution (Java, Sumatra, Tahiti), another group comprising isolates from Central America (Nicaragua, Panama) and the ex-type isolate from Hawaii, and two distinct isolates from Nicaragua (Fig. 3). In the ML analysis P. tropicalis is characterised by a weakly supported polytomy comprising a small cluster with the ex-type isolate and isolate PA006 from Panama; another cluster with five isolates from Nicaragua and Panama; a cluster with the Western Pacific isolates and isolate NI148 from Nicaragua in a basal position; and the distinct Nicaraguan isolate NI026 (Fig. S3). The polytomies in both analyses could result from adaptive or non-adaptive radiation or indicate phylogenetic conflicts possibly due to the presence of ancestral and descending lineages in the dataset. Within P. obovoidea and P. tropicalis, most nodes had higher support values in the BI analysis (Figs 3, S3). Across the alignments of 8 736 nuclear characters and 3 156 mitochondrial characters pairwise sequence differences between the Clade 2b taxa were 0.1–4.8 % and 0.1–4.8 %, respectively.
Fig. 3.
Fifty percent majority rule consensus phylogram derived from Bayesian inference analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2b. Bayesian posterior probabilities are indicated but not shown below 0.60. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT) and (T) denote ex-epitype and ex-type isolates, respectively. Scale bar indicates 0.005 expected changes per site per branch.
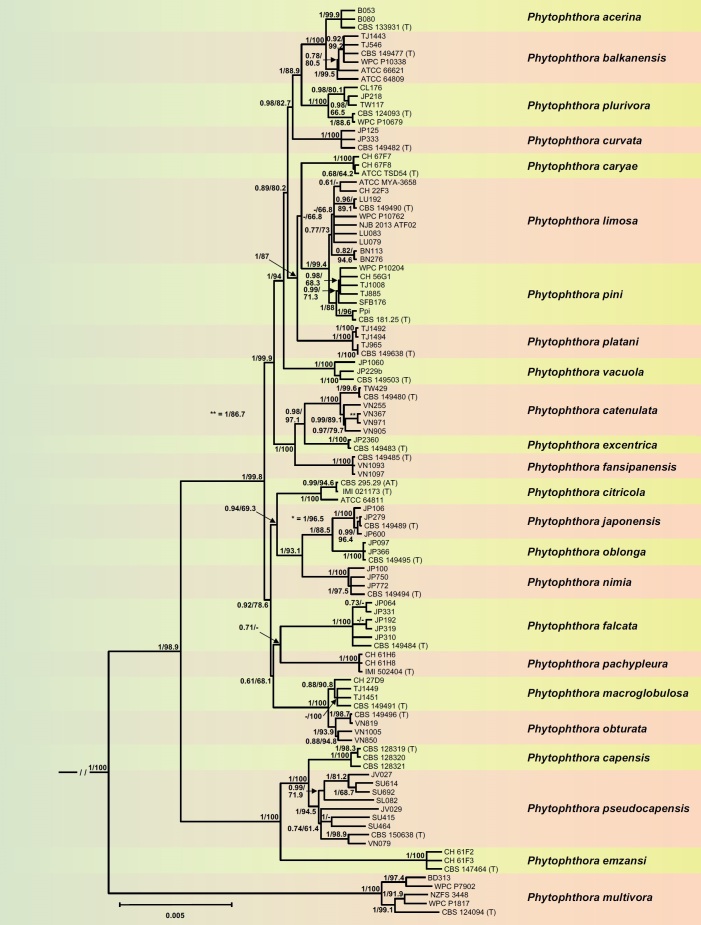
For the largest of the subclades, Clade 2c, both the BI and ML analyses produced trees with largely similar topology and strong support for the deeper phylogeny and most other nodes. The BI tree is presented here with both Bayesian Posterior Probability values and Maximum Likelihood bootstrap values included (Fig. 4). The phylogenetic analyses demonstrated 24 discrete lineages unambiguously corresponding to nine described species, i.e., P. acerina, P. capensis, P. caryae, P. citricola, P. emzansi, P. multivora, P. pachypleura, P. pini and P. plurivora, and 15 new species, i.e., P. balkanensis, P. catenulata, P. curvata, P. excentrica, P. falcata, P. fansipanensis, P. japonensis, P. limosa, P. macroglobulosa, P. nimia, P. platani, P. oblonga, P. obturata, P. pseudocapensis and P. vacuola. The overall structure of Clade 2c is characterised by the early divergence of P. multivora (Fig. 4), which lies in a distinct basal position, followed by the divergence of a small cluster comprising the basal P. emzansi and P. capensis, both from South Africa, and P. pseudocapensis, a new genetically diverse sister species of P. capensis that is widely distributed across Java, Sumatra, Sulawesi, Taiwan and Vietnam. Within P. pseudocapensis the isolates sampled from Taiwan and Vietnam grouped separately from those obtained from the different Indonesian islands, which were intermingled (Fig. 4). All lineages in this cluster received strong support values. The ‘main’ Clade 2c cluster comprised two separate and fully supported lineages. One lineage contained in the BI analysis two fully supported subclusters represented by eight species from East and Southeast Asia. Within one subcluster P. macroglobulosa from the Chinese Hainan Island and P. obturata from Northern Vietnam constituted closely related and fully supported sister species. Phytophthora pachypleura and P. falcata from Japan also grouped in a sister position but with lower support (0.71). Both species resided at the ends of relatively long branches indicating long-term isolation (Fig. 4). Within this subcluster the relative positions to each other of the species pairs P. macroglobulosa/P. obturata and P. falcata/P. pachypleura could not be resolved unambiguously as shown by low support values (Fig. 4). The ML tree differed from the BI tree in the relative position of P. pachypleura which formed a weakly supported polytomy with the P. macroglobulosa - P. obturata - P. falcata subcluster and the other subcluster. The latter comprised the ex-type and authentic type isolates of P. citricola from Japan and Taiwan together with a South African P. citricola isolate grouping in a basal position to P. nimia and the two sister species P. japonensis and P. oblonga, all from Japan. All nodes in this subcluster were well supported in both analyses (Fig. 4).
Fig. 4.
Fifty percent majority rule consensus phylogram derived from Bayesian inference analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2c. Bayesian posterior probabilities and Maximum Likelihood bootstrap values (in %) are indicated but not shown below 0.60 and 60 %, respectively. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (AT) and (T) denote ex-authentic type and ex-type isolates, respectively. Scale bar indicates 0.005 expected changes per site per branch.
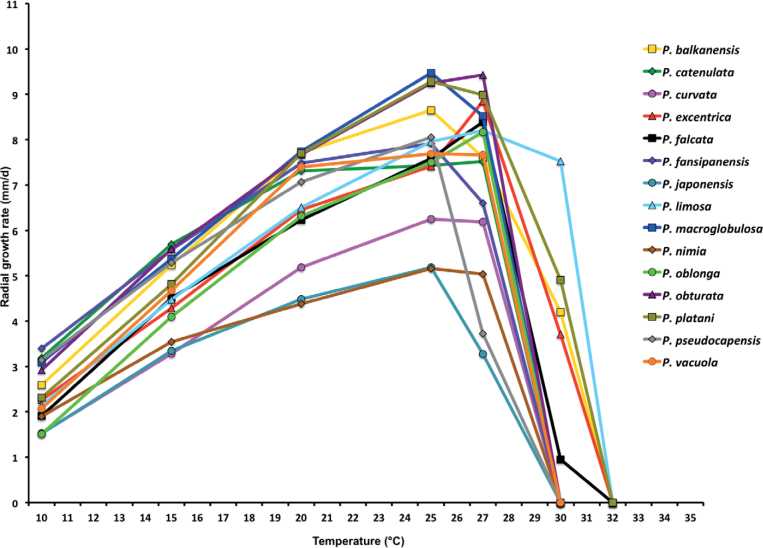
Within the other lineage of the ‘main’ Clade 2c cluster, a group of species from Southeast and East Asia apparently diverged first. These included the sister species P. catenulata and P. excentrica together with P. fansipanensis which resides in a basal position to them. The Taiwanese and Vietnamese populations of P. catenulata were closely related but grouped separately. All nodes received high support values (Fig. 4). The Japanese P. vacuola diverged from a cluster containing two well-supported subclusters. One subcluster comprised the sister species P. acerina and P. balkanensis with P. plurivora grouping basal to them and P. curvata from Japan basal to the whole subcluster. The three isolates of P. acerina from Italy were probably clonal whereas P. balkanensis showed some intraspecific variability: isolates from several Balkan countries (TJ546, TJ1443, CBS 149477) and Ireland (WPC P10338) grouping separately from those from California (ATCC 64809) and Taiwan (ATCC 66621). The P. plurivora isolates from Chile, Europe, Japan, Taiwan and New Zealand also showed intraspecific variability. All lineages in this subcluster were well supported (Fig. 4). In the second subcluster P. platani isolates from Italy and the UK resided in the BI analysis in a strongly supported basal position to three species whose isolates came either from North America or partly from Europe: the sister species P. pini and P. limosa, and P. caryae (Fig. 4). Phytophthora caryae lay in a weakly supported and hence ambiguous basal position to the latter species, probably because only 5–9 of the 13 gene regions in the individual isolates were available (Table S3). In the ML analysis, P. caryae and P. platani grouped in sister position to each other but with weak support (66.8 %). In both analyses isolates previously designated as P. taxon citricola III and P. taxon 22F3 (Yang et al. 2017) grouped within P. limosa, and isolate CH 56G1 previously designated as P. taxon pini-like grouped within P. pini. Isolates of P. limosa from Bosnia-Herzegovina clustered separately from US isolates but the node received only weak support. The separation between P. limosa and P. pini was, however, fully supported (Fig. 4). Across the alignments of 8 740 nuclear characters and 3 156 mitochondrial characters pairwise sequence differences between the Clade 2c taxa were 0.1–3.2 % and 0.2–4.7 %, respectively.
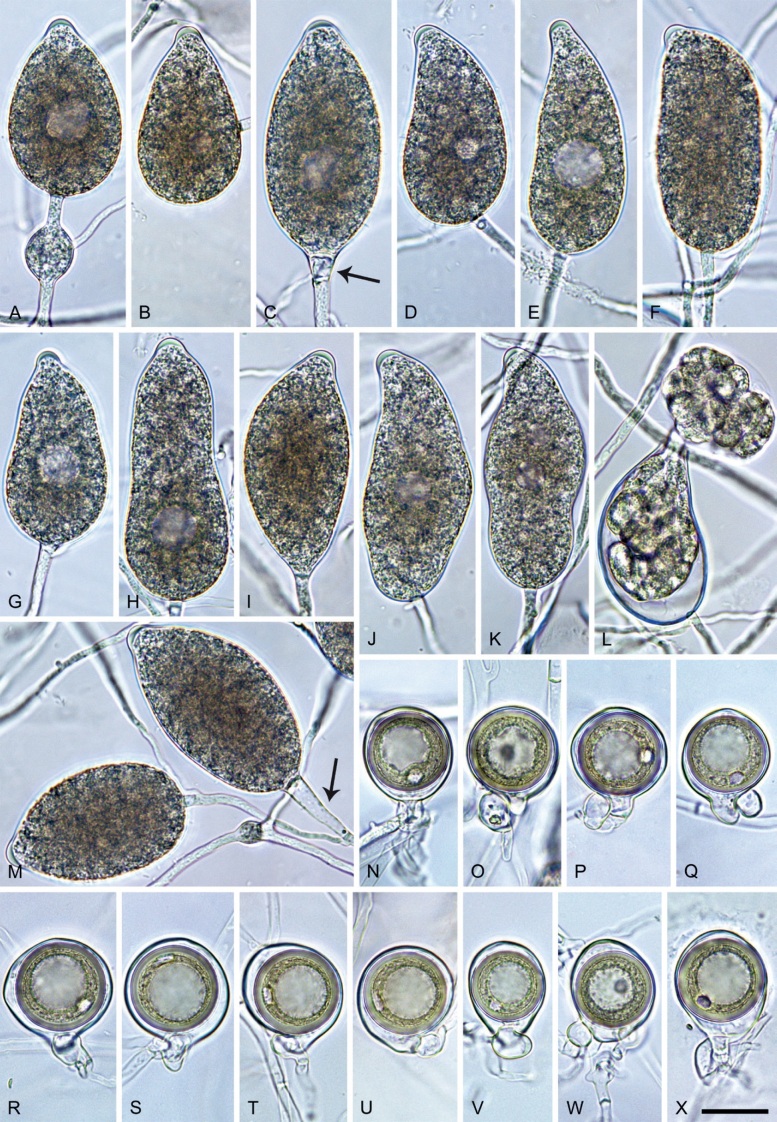
With Clade 2e both the BI and ML analyses produced trees with similar topology and strong support for all nodes. The BI tree is presented here with both Bayesian Posterior Probability values and Maximum Likelihood bootstrap values included (Fig. 5). Both BI and ML analyses revealed 12 discrete and fully supported lineages within Clade 2e unambiguously corresponding to the five described species P. acaciae, P. acaciivora, P. bishii, P. elongata and P. frigida; the six new species P. amamensis, P. borneensis, P. celeris, P. indonesiensis, P. penetrans and P. pseudofrigida; and the informally designated P. taxon pseudobisheria (Fig. 5). The overall structure of Clade 2e showed P. elongata residing in a distinct basal position to a large cluster which was characterised by the early divergence between a smaller cluster of A1/A2 species, comprising P. acaciae in a basal position, P. acaciivora and the closely related sister species P. frigida and P. pseudofrigida, and a larger cluster of self-fertile taxa with two fully supported subclusters. One subcluster comprised the sister species P. amamensis from the Japanese Amami Island and P. indonesiensis with P. penetrans from Panama residing in a basal position to them. The other one contained two pairs of sister taxa, P. bishii - P. borneensis and P. celeris - P. taxon pseudobisheria, respectively. It is noteworthy that within P. indonesiensis the populations from three different Indonesian islands formed three distinct subclusters, with the intermediate Kalimantan population grouping in a fully supported basal position to the Sumatra and Sulawesi populations (Fig. 5). Across the nuclear 8 754-character alignment and the mitochondrial 3 153-character alignment the Clade 2e taxa showed pairwise sequence differences of 0.4–6.2 % and 0.3–4.4 % (0.1 % for P. frigida vs. P. pseudofrigida), respectively.
Fig. 5.
Fifty percent majority rule consensus phylogram derived from Bayesian inference analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2e. Bayesian posterior probabilities and Maximum Likelihood bootstrap values (in %) are indicated but not shown below 0.70 and 70 %, respectively. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (T) denotes ex-type isolates. Scale bar indicates 0.01 expected changes per site per branch.
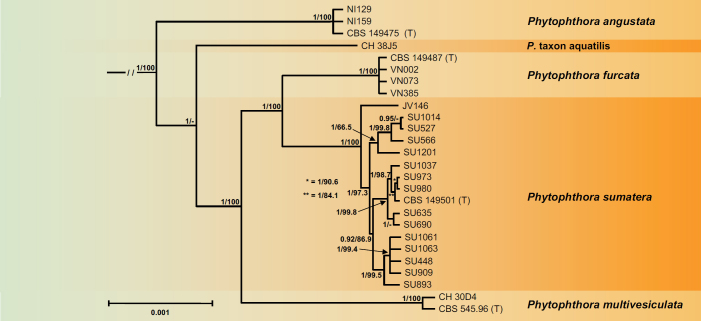
For Clade 2f both the BI and ML analyses produced trees with similar topology and strong support for all nodes. The BI tree is presented here with both Bayesian Posterior Probability values and Maximum Likelihood bootstrap values included (Fig. 6). The overall structure of Clade 2f is characterised by the early divergence of P. angustata from Nicaragua (Fig. 6). This is followed by the separation of the North American P. taxon aquatilis from a cluster comprising P. furcata from Vietnam and P. sumatera in a sister position to each other, and the globally distributed P. multivesiculata which occurs in a fully supported basal position to the latter two species. Phytophthora sumatera showed high intraspecific genetic variability with isolate JV146 from Java grouping basal to three fully supported separate subclusters from Sumatra (Fig. 6). Across the nuclear 8 720-character alignment and the mitochondrial 3 153-character alignment pairwise sequence differences between the Clade 2f taxa were 0.6–2% and 1.5–3.6%, respectively.
Fig. 6.
Fifty percent majority rule consensus phylogram derived from Bayesian inference analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2f. Bayesian posterior probabilities and Maximum Likelihood bootstrap values (in %) are indicated but not shown below 0.80 and 60 %, respectively. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (T) denotes ex-type isolates. Scale bar indicates 0.001 expected changes per site per branch.
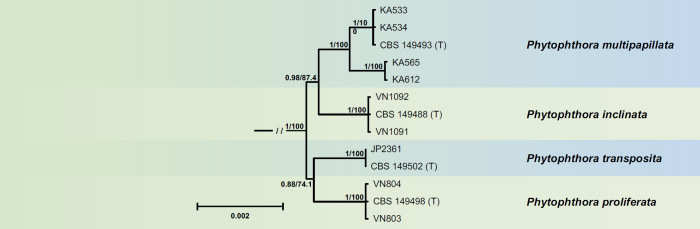
Currently comprising the four new species P. inclinata, P. multipapillata, P. proliferata and P. transposita, the new Clade 2g is the smallest subclade apart from the monospecific lineage of P. oleae (Figs 1, 7). Both BI and ML analyses revealed two pairs of sister species, i.e., P. inclinata from the Vietnamese Côn Lôn Island and P. multipapillata from Borneo; and P. proliferata from Cuc Phuong National Park in the North of Vietnam and P. transposita from the Japanese Kyushu Island. The populations of P. multipapillata from two different locations in East Kalimantan were grouped separately. All lineages were strongly supported. The nuclear 8 725-character alignment and the mitochondrial 3 156-character alignment of the four species showed sequence differences of 0.6–0.8 % and 1.1–1.5 %, respectively.
Fig. 7.
Fifty percent majority rule consensus phylogram derived from Bayesian inference analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2g. Bayesian posterior probabilities and Maximum Likelihood bootstrap values (in %) are indicated but not shown below 0.80 and 70 %, respectively. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (T) denotes ex-type isolates. Scale bar indicates 0.002 expected changes per site per branch.
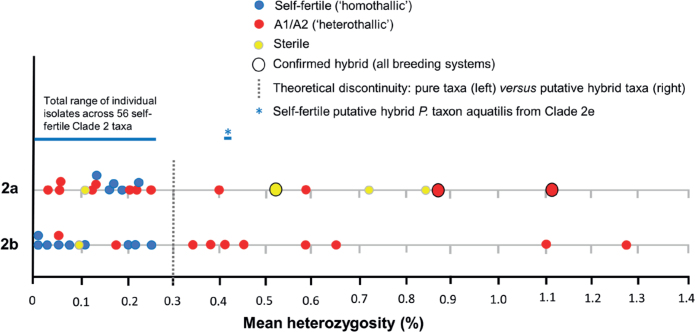
Nuclear gene heterozygosity. In contrast to the homozygous mitochondrial (cox1, cox2, nadh1, rps10) alignments, the nine-loci nuclear (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) alignments of all subclades contained numerous heterozygous positions. Among the 87 Clade 2 taxa with available data mean heterozygosity varied widely from close to zero to 1.27 % (Table 1). Further, within subclades 2a and 2b wide variations in heterozygosity also occurred (Table 1) according to the breeding system (Table 2) and even within some species. Relationships between these characters and interspecific hybridity were also detected. These aspects are considered in detail under the Notes on the relationship between level of heterozygosity, breeding system and interspecific hybridity below. Together with information published elsewhere (Van Poucke et al. 2021), they formed the basis for deciding whether a new species would be formally designated a hybrid.
Table 1.
Range of mean heterozygosity across nine nuclear genes (LSU, ITS, ßtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) for taxa within the Clade 2 subclades.
| Subclade | No. of taxa | Heterozygosity (%) | |
|---|---|---|---|
| Range | Mean | ||
| 2a | 21 | 0.03–1.09 | 0.34 |
| 2b | 21 | 0.0–1.27 | 0.31 |
| 2c | 24 | 0.03–0.18 | 0.09 |
| 2d | 1 | n/a | 0 |
| 2e | 11 | 0.02–0.06 | 0.12 |
| 2f | 5 | 0.01–0.42 | 0.1 |
| 2g | 4 | 0.07–0.09 | 0.08 |
| All subclades | 87 | 0.0–1.27 | 0.14 |
n/a = not applicable.
Table 2.
Mean heterozygosity levels across nine nuclear genes (LSU, ITS, ßtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) versus breeding systems across the subclades of Phytophthora Clade 2.
| Subclade | Breeding system1,2 | ||
|---|---|---|---|
| Self-fertile | A1/A2 | Sterile | |
| 2a | 0.18 (5) | 0.28 (12) | 0.55 (4) |
| 2b | 0.10 (10) | 0.54 (10) | 0.09 (1) |
| 2c | 0.09 (24) | n/a | n/a |
| 2d | 0 (1) | n/a | n/a |
| 2e | 0.07 (8) | 0.24 (3) | n/a |
| 2f | 0.02 (4)3 | n/a | n/a |
| P. taxon aquatilis | 0.42 (1) | n/a | n/a |
| 2g | 0.08 (4) | n/a | n/a |
| All subclades | 0.09 (56)3
0.1 (57) |
0.40 (25) | 0.46 (5) |
1 See Terminology.
2 No. of taxa in parentheses.
3 Excluding P. taxon aquatilis.
Taxonomy
Clade 2a
For all Clade 2a species included in this study colony morphologies on CA, PDA and V8A and temperature-growth relations on V8A are presented in Figs 8–12. Morphological and physiological characters and morphometric data of the six newly described and 11 known species and five informally designated taxa in Clade 2a are given in the comprehensive Tables S4–S6. The known species P. citrophthora and P. meadii were recently validated by Abad et al. (2023a) and taxonomic re-descriptions given based on a newly designated epitype (P. citrophthora) and a newly designated neotype (P. meadii). However, morphometric data and growth rates from different studies often show considerable differences. To enable detailed comparisons with new species from Clade 2a, for both P. citrophthora and P. meadii isolates from different parts of the world were included in our morphological and temperature-growth studies and taxonomic descriptions without nomenclatural act are given below.
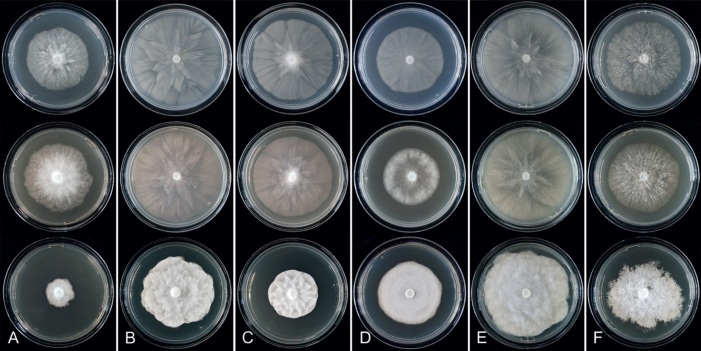
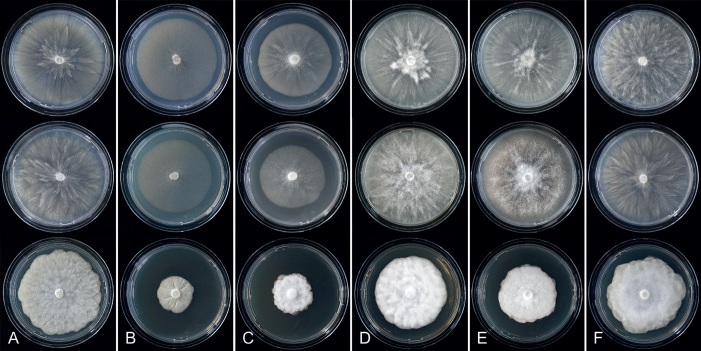
Fig. 8.
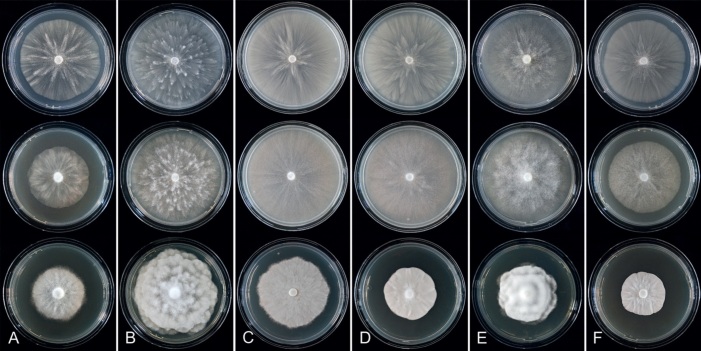
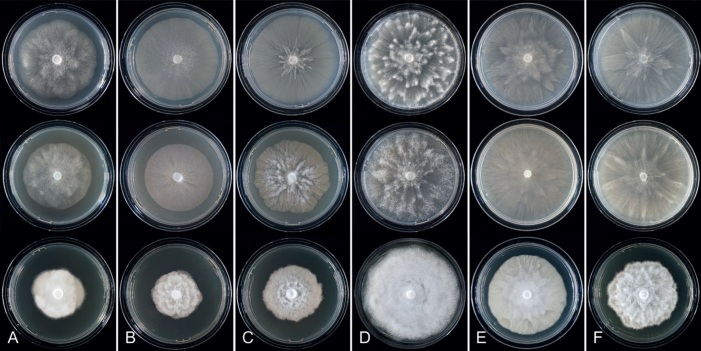
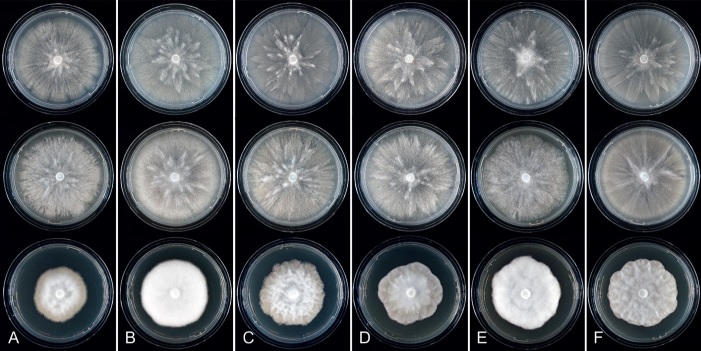
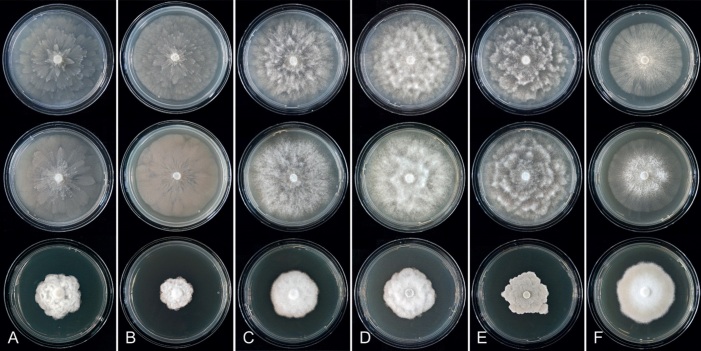
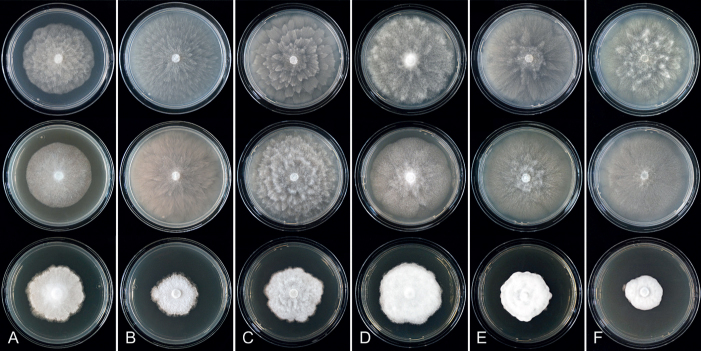
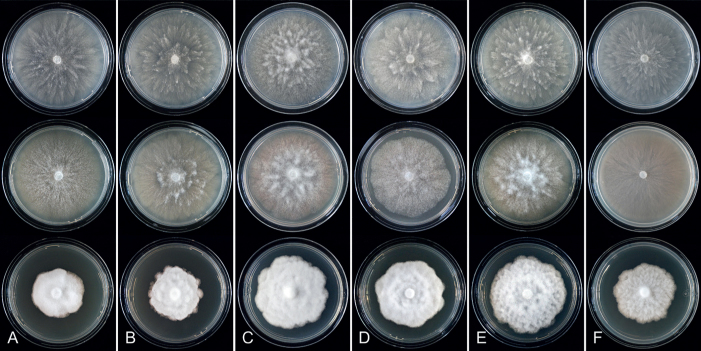
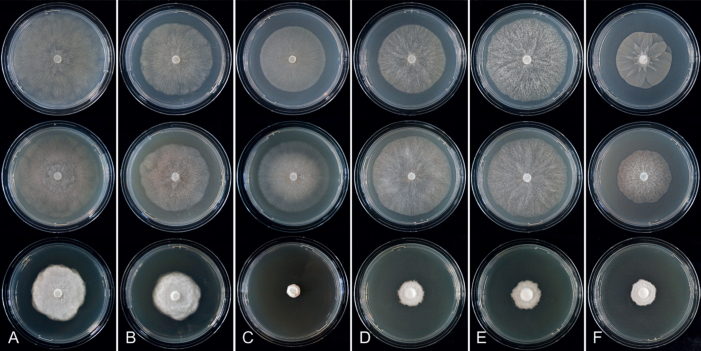
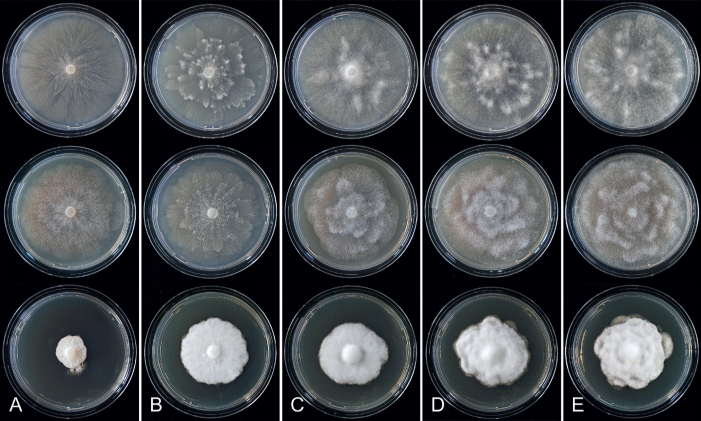
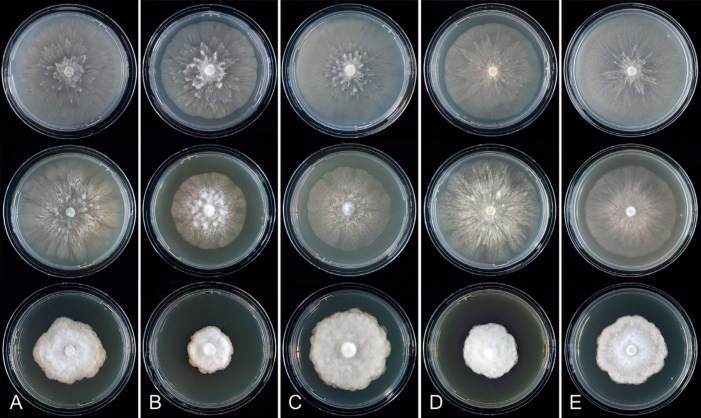
Colony morphology of Phytophthora species from subclade 2a after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora botryosa (MYA-4059). B, C. Phytophthora citrophthora (B. TW386; C. JP554). D. Phytophthora colocasiae (SU1665). E, F. Phytophthora meadii (E. MYA-4043; F. MYA-4042).
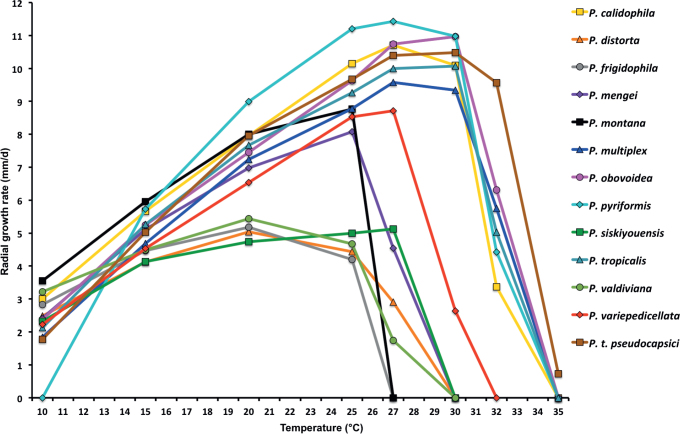
Fig. 12.
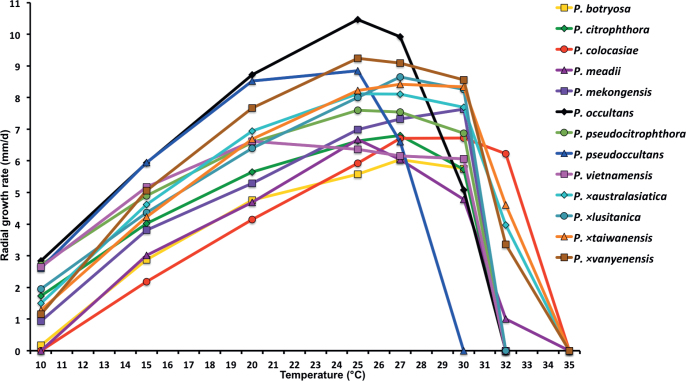
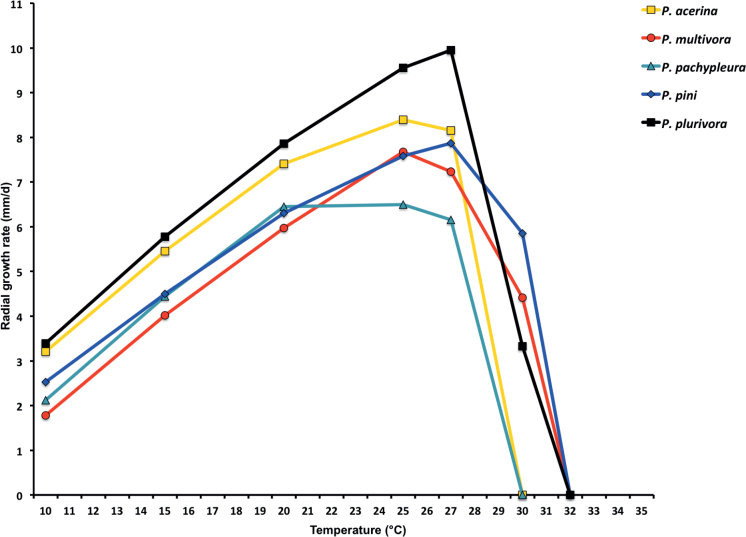
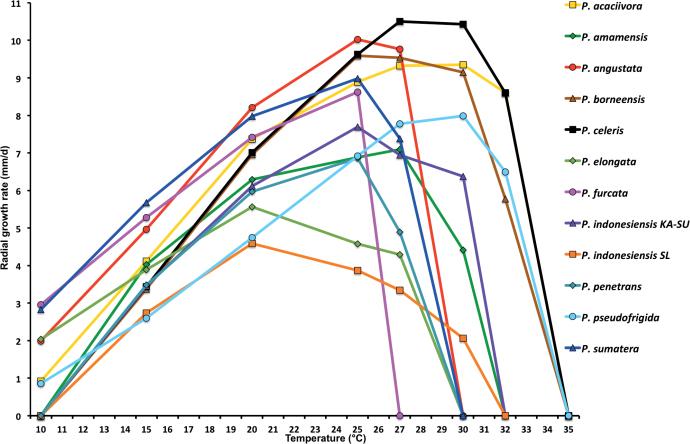
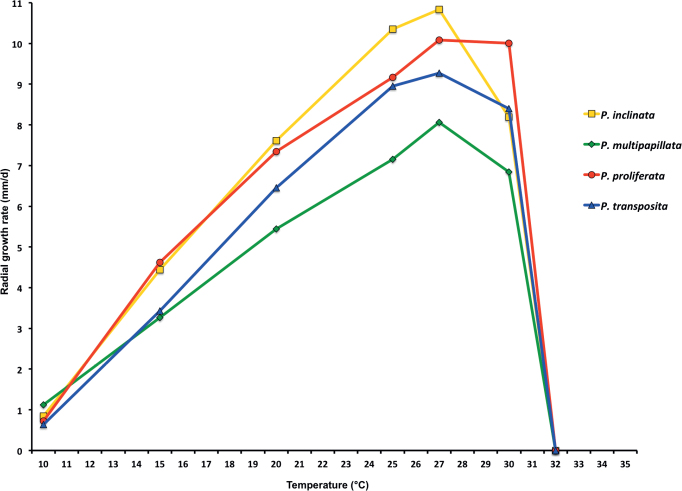
Mean radial growth rates of seven known and five new Phytophthora species from subclade 2a on V8-agar at different temperatures: P. botryosa (2 isolates); P. citrophthora (13 isolates); P. colocasiae (3 isolates); P. meadii (2 isolates); P. mekongensis (2 isolates); P. occultans (2 isolates); P. pseudocitrophthora (9 isolates); P. pseudoccultans (3 isolates); P. vietnamensis (5 isolates); P. ×australasiatica (28 isolates); P. ×lusitanica (4 isolates); P. ×taiwanensis (14 isolates); P. ×vanyenensis (33 isolates).
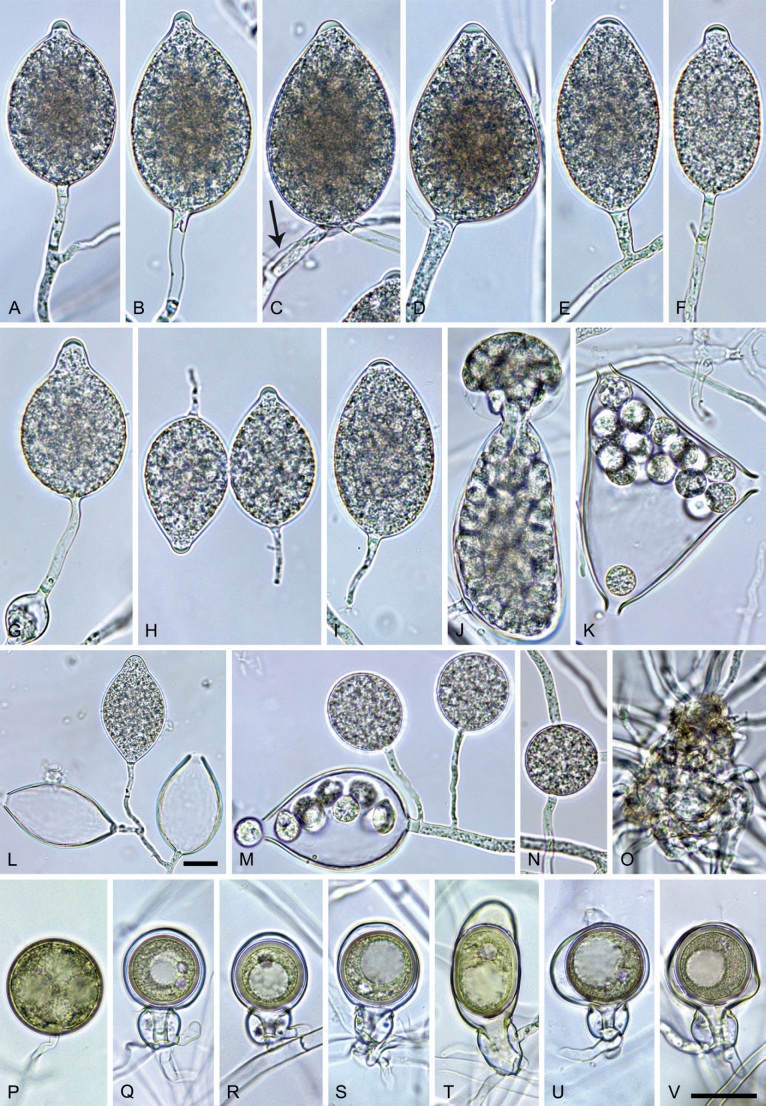
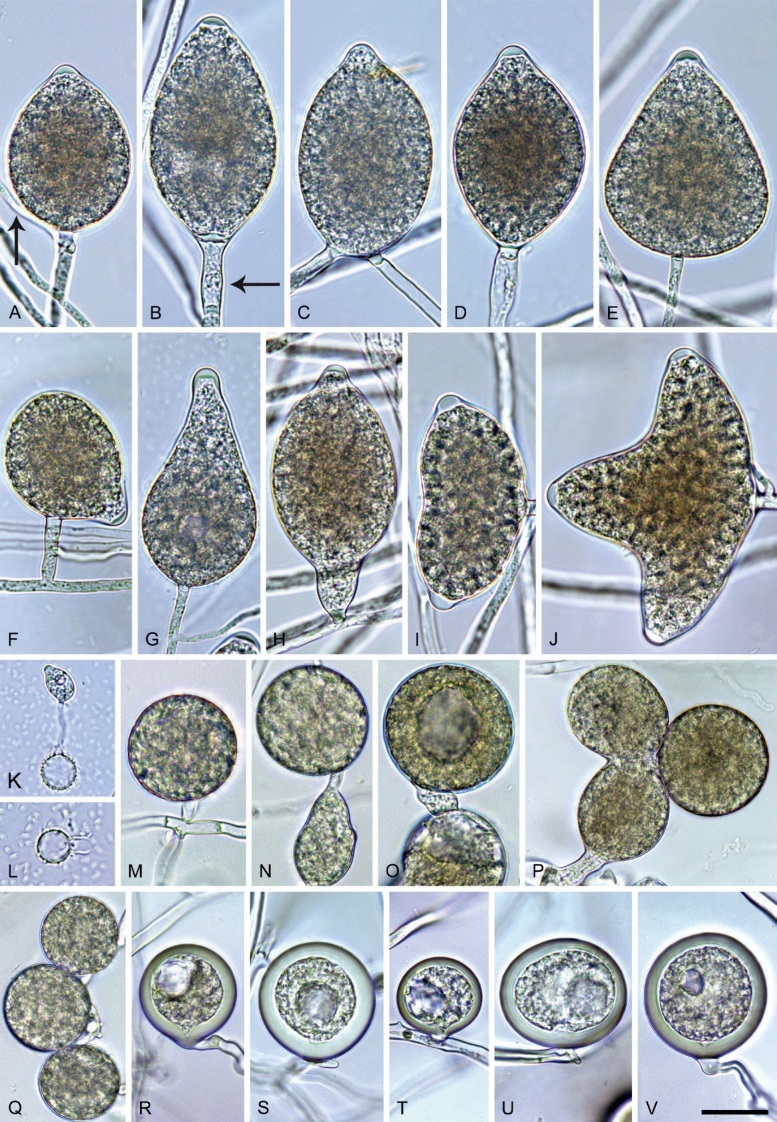
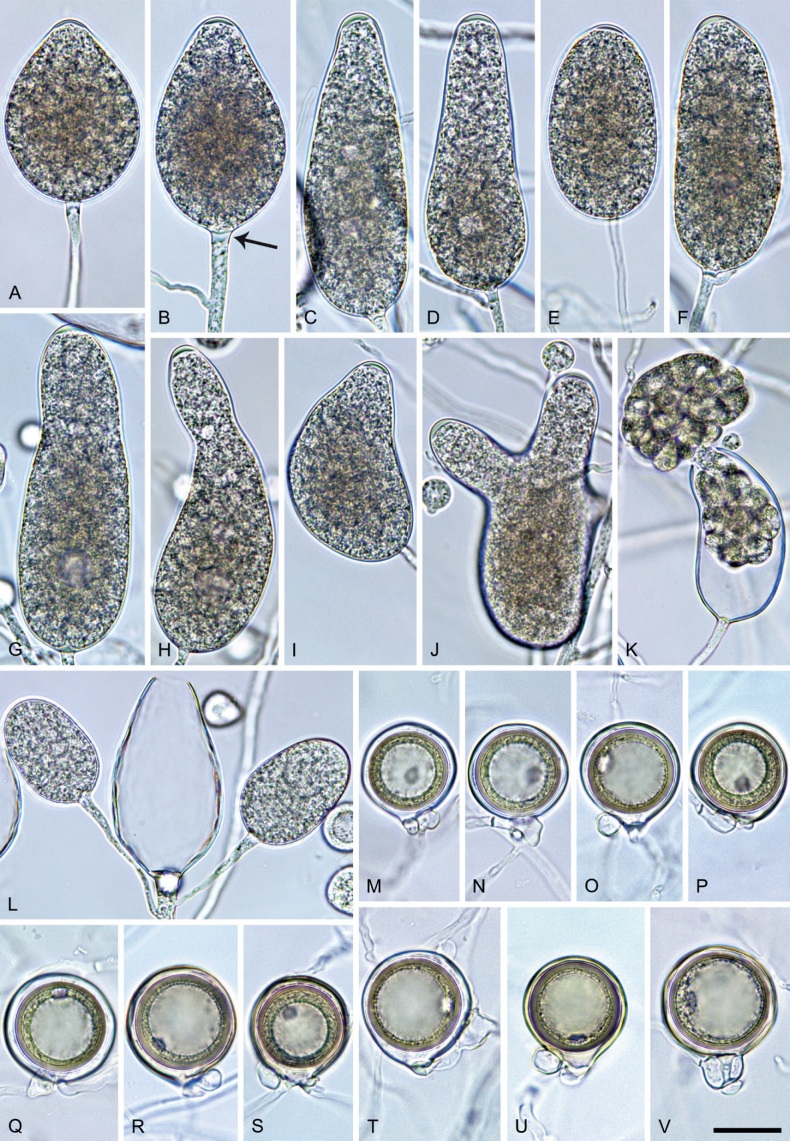
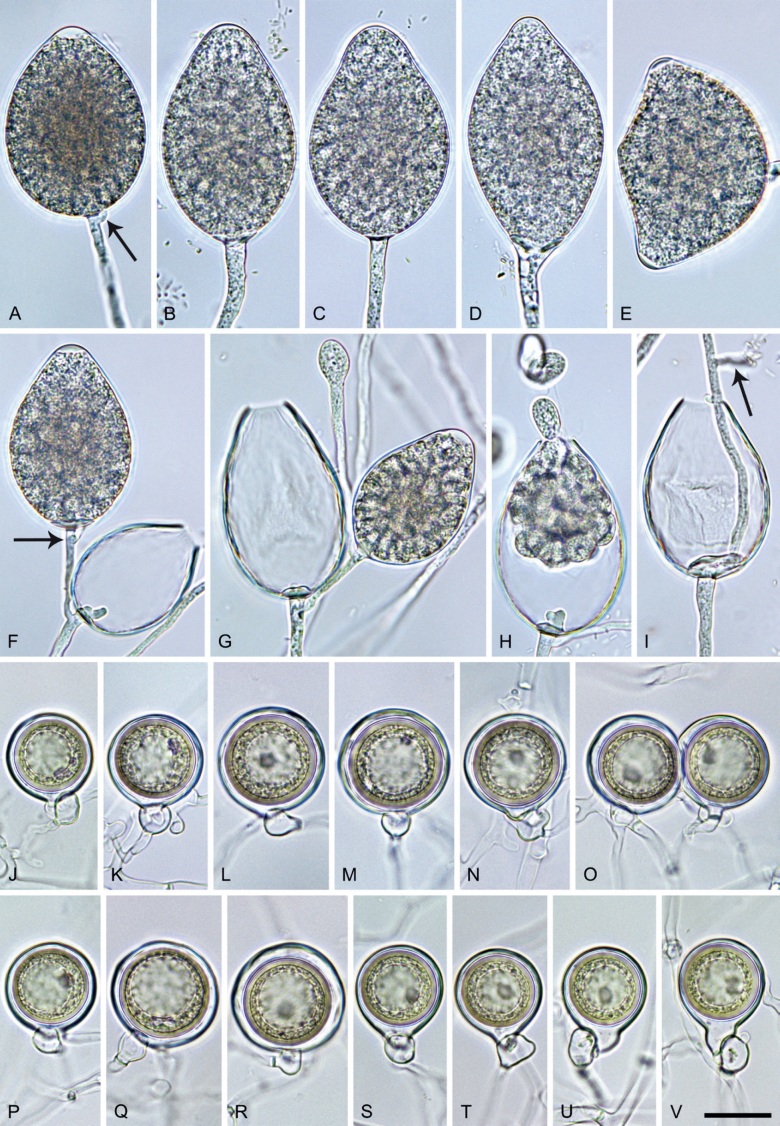
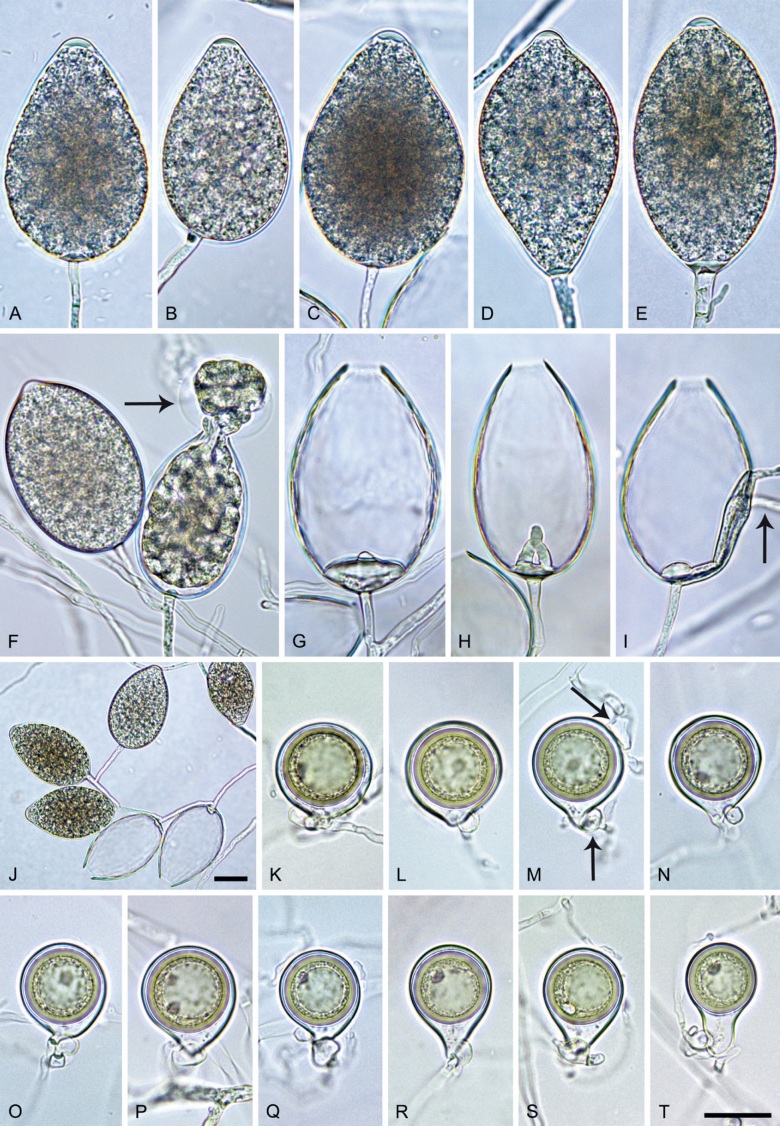
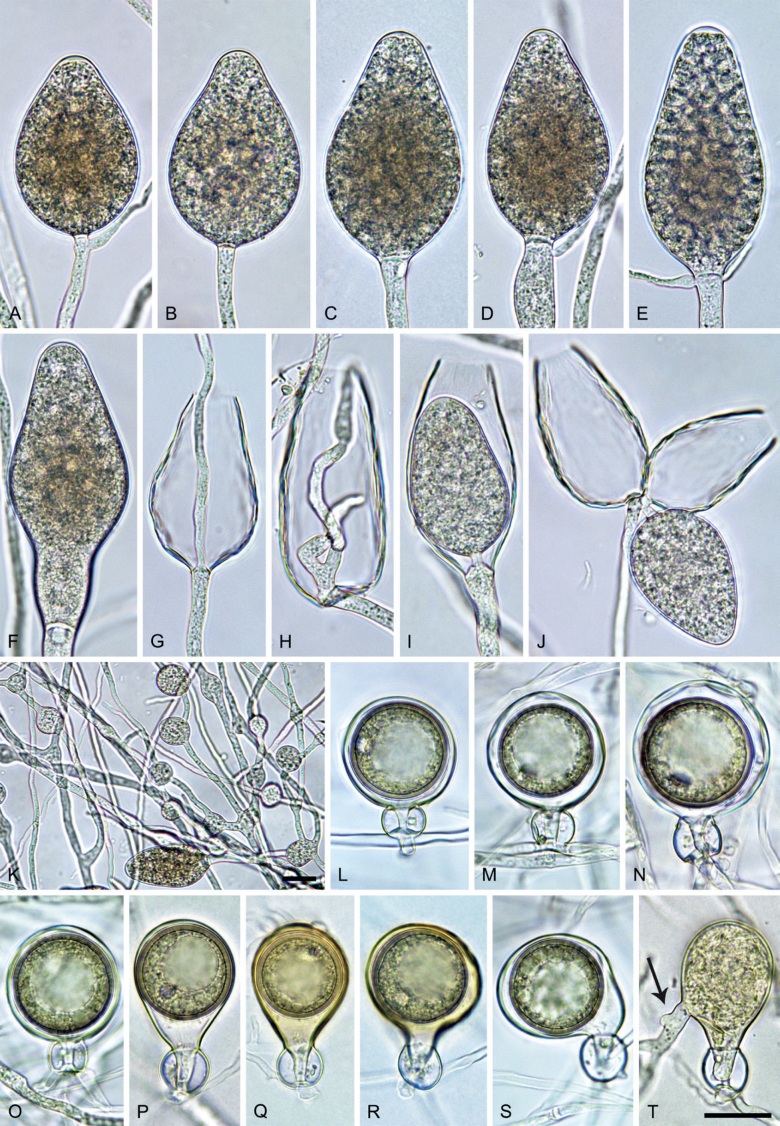
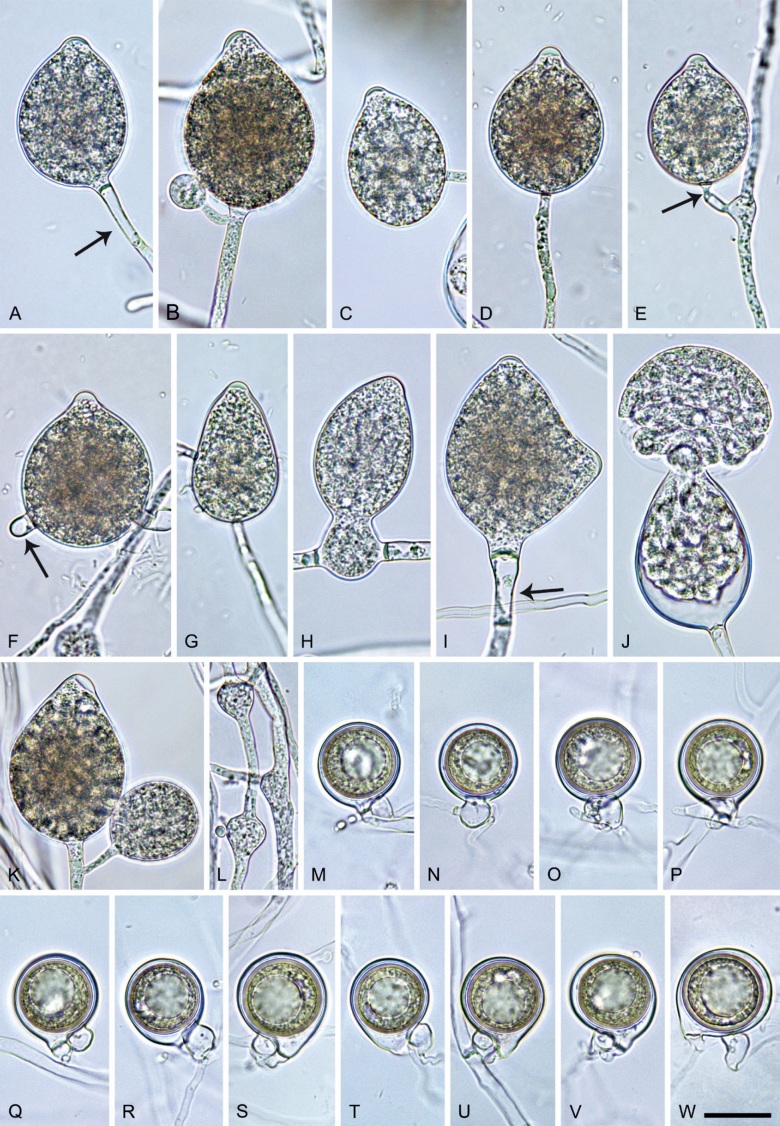
Phytophthora citrophthora (R.E. Sm. & E.H. Sm.) Leonian, Amer. J. Bot. 12 (7): 445. 1925. [MycoBank MB 251464]. Fig. 13.
Fig. 13.
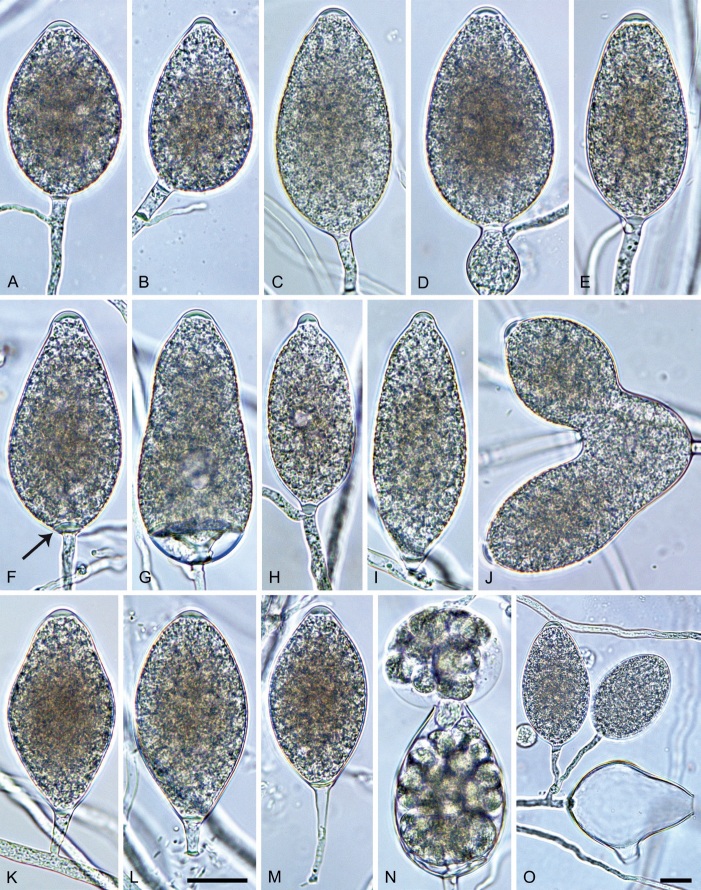
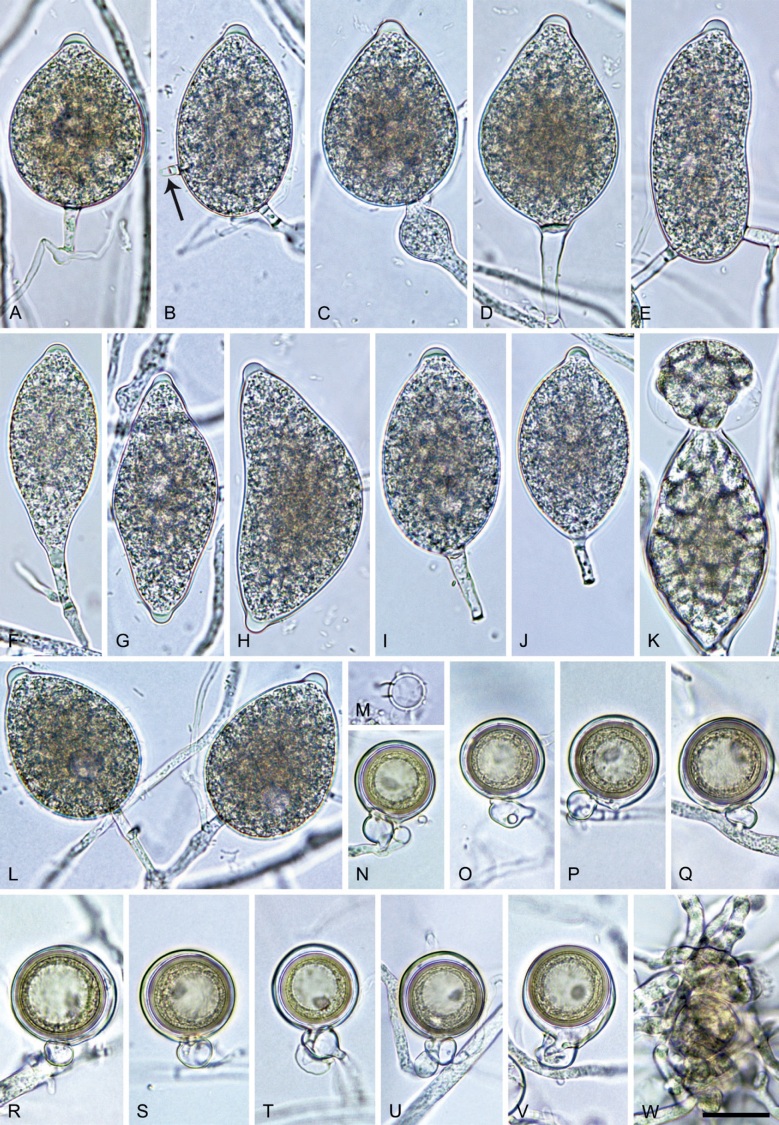
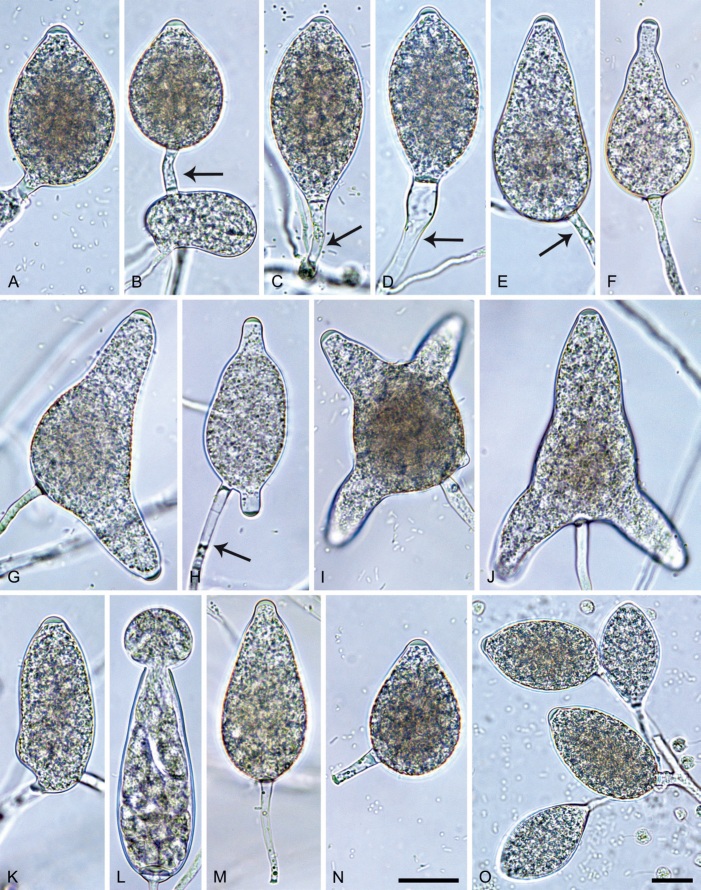
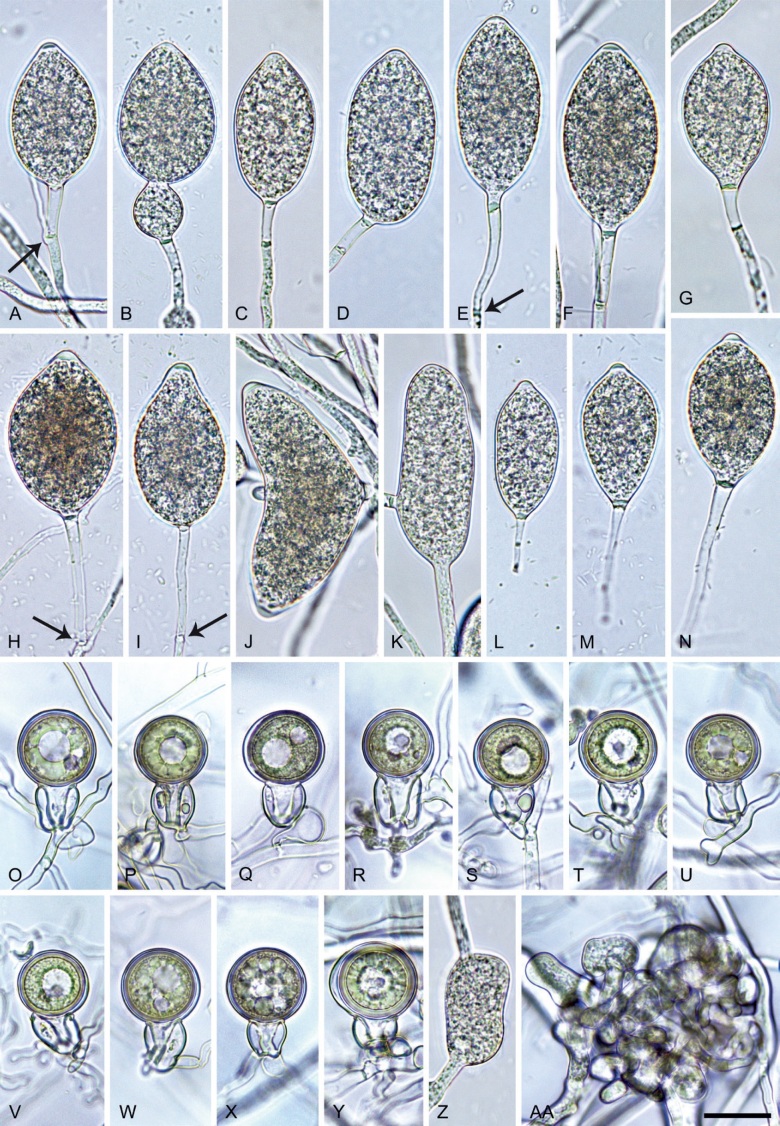
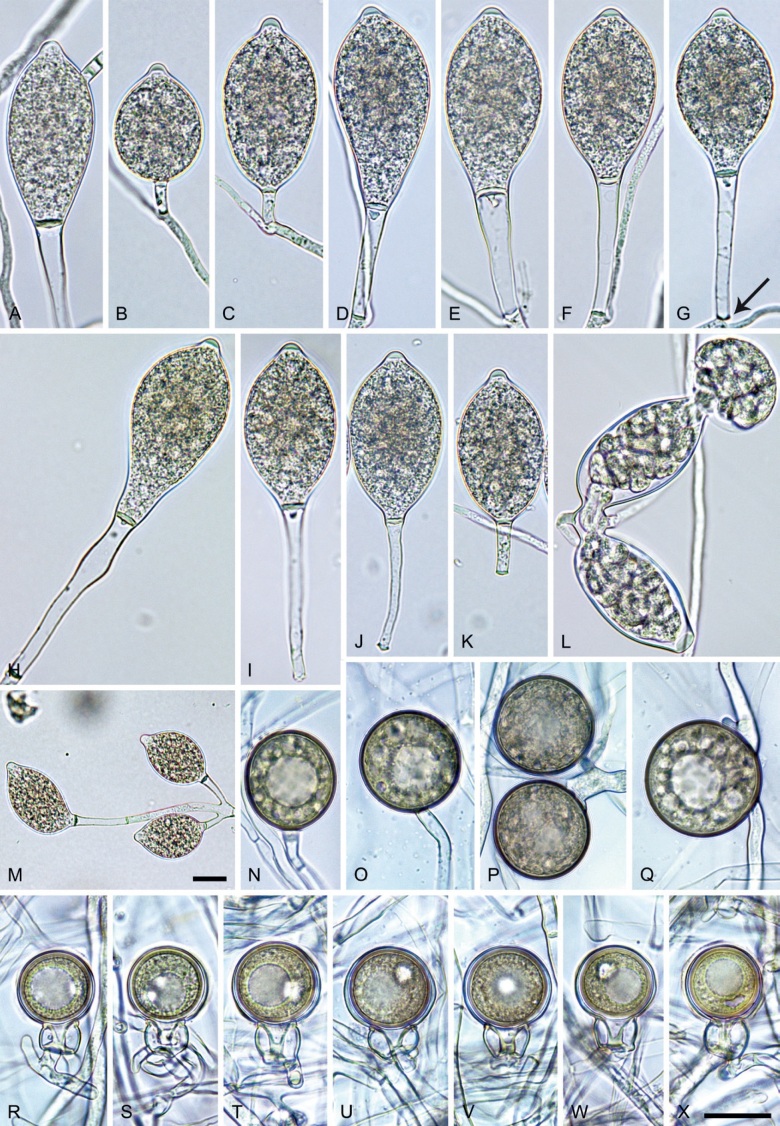
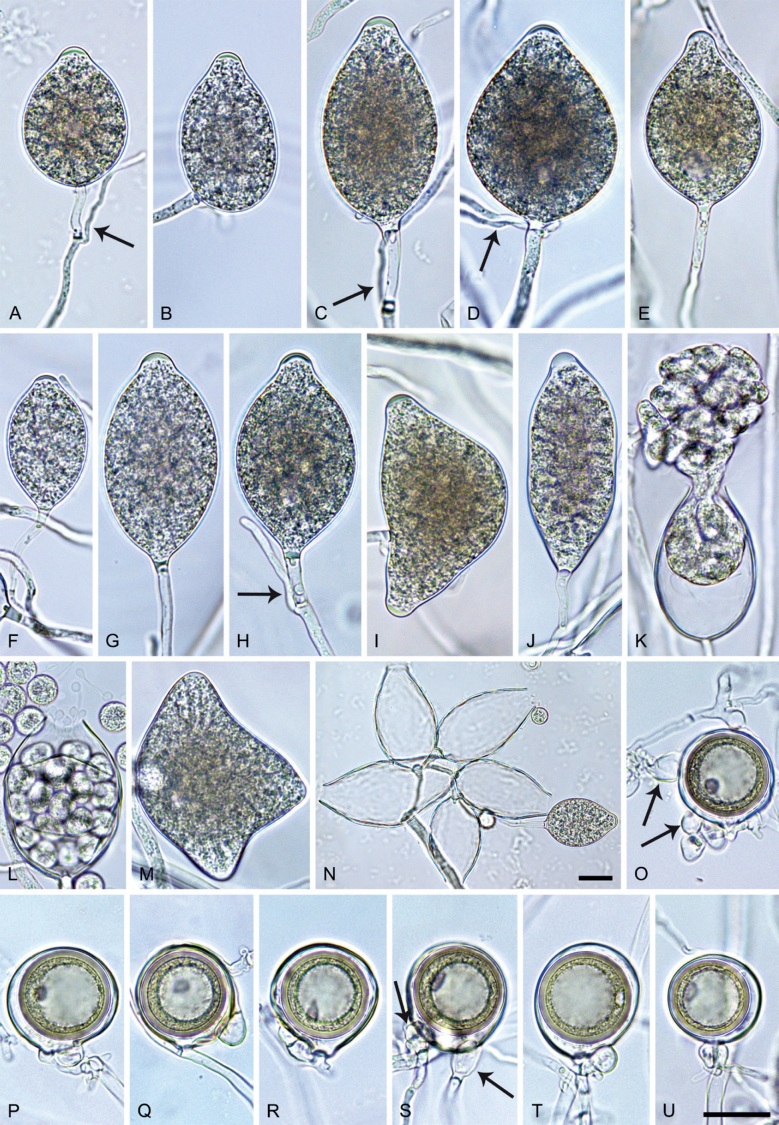
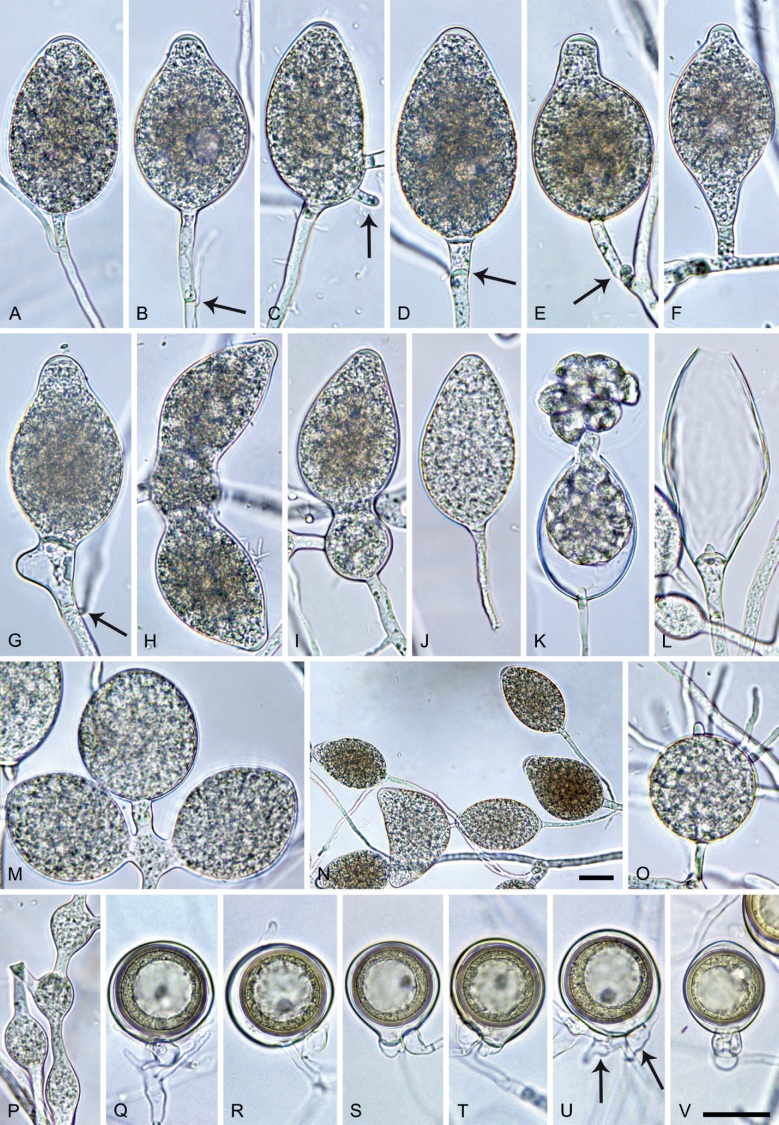
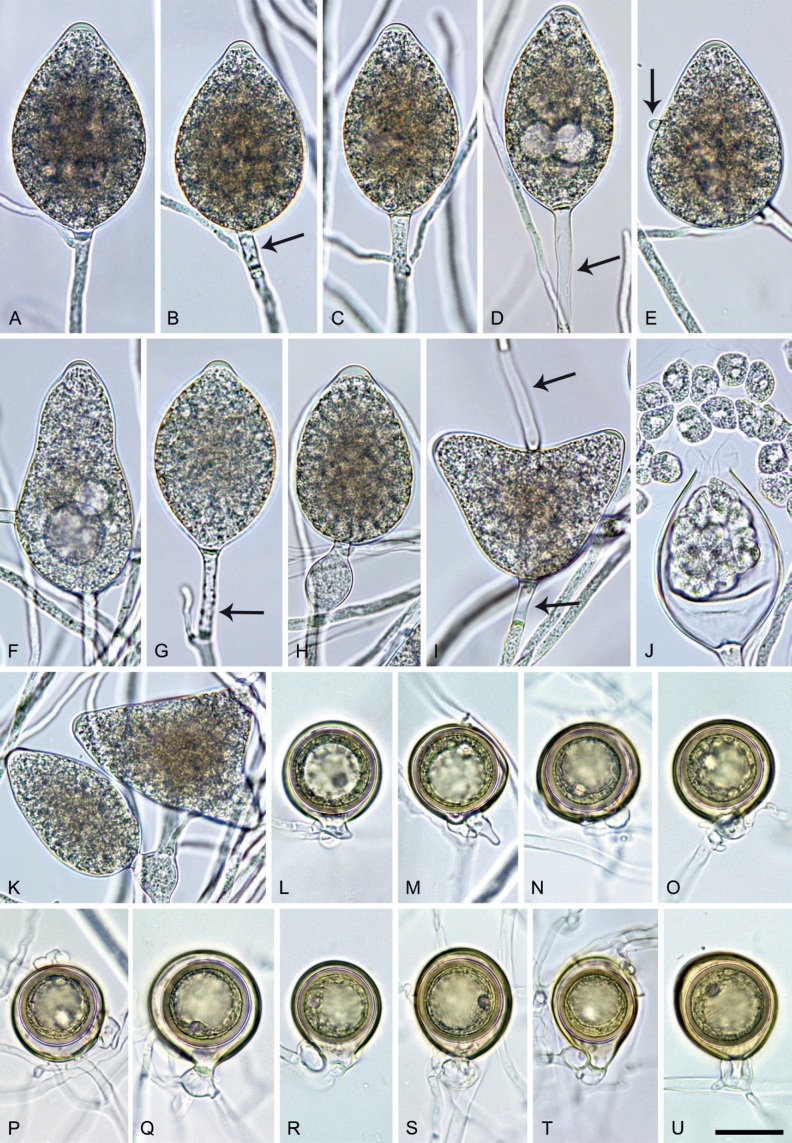
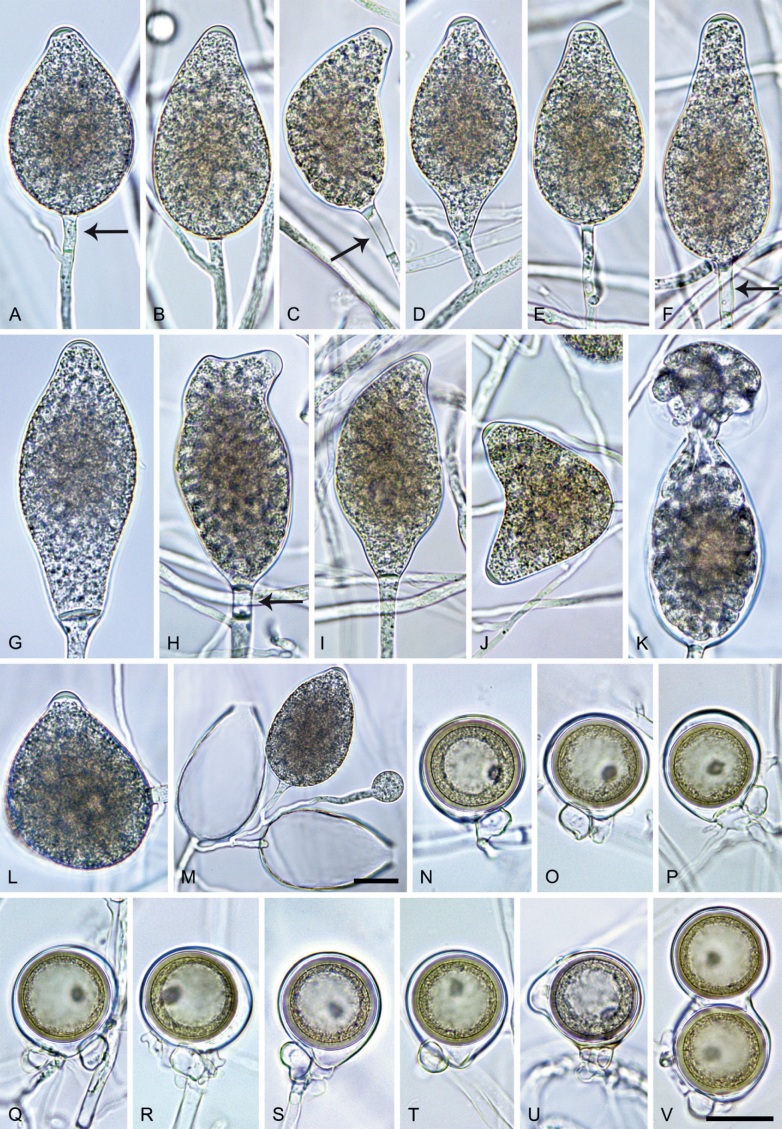
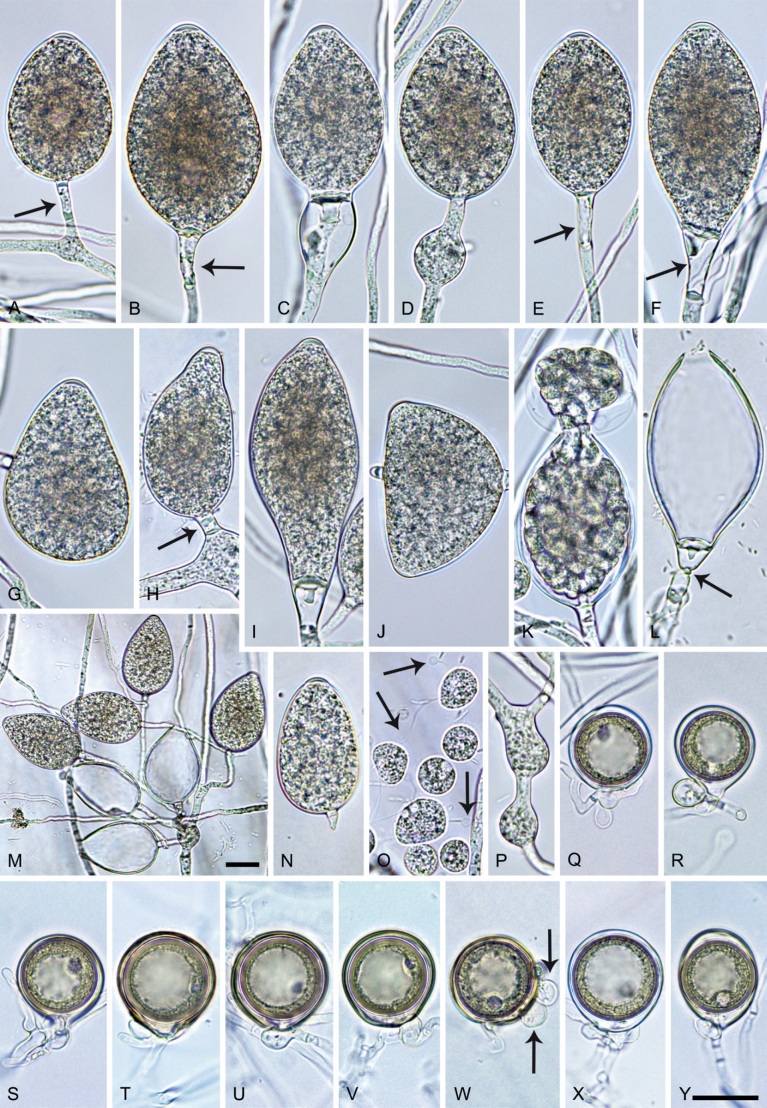
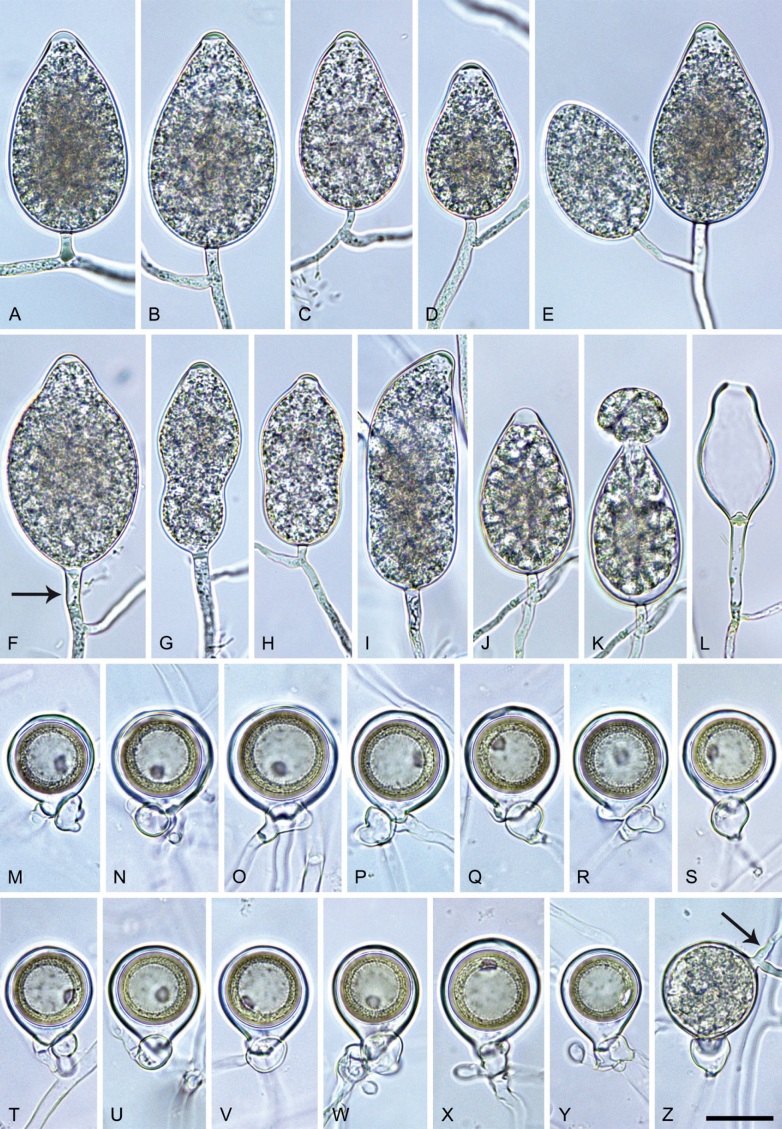
Phytophthora citrophthora. A–M. Semipapillate and papillate sporangia formed on V8-agar in soil extract. A–I, K. Ovoid, limoniform, obpyriform or ellipsoid persistent sporangia. A, B, D, E, G, H. External proliferation. F. Conspicuous basal plug (arrow). G. Cytoplasm not filling the sporangium. J. Bilobed persistent sporangium. L, M. Caducous limoniform sporangia with varying pedicel length. N. Ovoid sporangium releasing zoospores. O. Dense sporangial sympodium. Images: A, N. BD122; B. JP1356; C–G, J, L, M, O. BD786; H, I. JP554; K. TW386. Scale bars = 20 µm; L applies to A–N.
Typus: USA, California, La Habra isolated from Citrus sp., collection date and collector unknown, isolated by P. Oudemans (epitype CBS 950.87 preserved in a metabolically inactive state, designated in Abad et al. 2023a, MBT 10008019, ex-epitype living culture CBS 950.87 = CPHST BL60 = ATCC 52231 = WPC P0479).
Morphological structures on V8A: Sporangia infrequently observed on solid agar and abundantly produced in non-sterile soil extract; borne predominantly terminally (95.6 %) on unbranched long or short sporangiophores or in dense or lax sympodia of 2–4 sporangia (Fig. 12O), or infrequently sessile (1.8 %; Fig. 13I) or intercalary (2.6 %); mostly ovoid, broad-ovoid or elongated-ovoid (54.7 %; Fig. 13A–E, N) or limoniform to elongated-limoniform (25.1 %; Fig. 13I, K–M, O), less frequently distorted with often two or sometimes three apices (9.3 %; Fig. 13J), obpyriform to elongated-obpyriform (6.1 %; Fig. 13F, G), ellipsoid to elongated-ellipsoid (2.5 %; Fig. 13H, O), obovoid (1 %), ampulliform (0.8 %) or pyriform (0.5 %); lateral attachment of sporangiophores (17.5 %; Fig. 13B), a conspicuous basal plug (45.2 %; Fig. 13E–H) and pedicels of variable length (av. 24.9 ± 15.9 µm; range 2.5–78.4 µm; Fig. 13B, C, K–M) commonly observed; rarely caducous (Fig. 13L, M); sporangia occasionally too big for the available cytoplasm and hence, not filled completely in the basal part, often with an additional strong plug below the cytoplasm (6.8 %; Fig. 13G); sporangial apices on solid agar exclusively papillate; in water mainly semipapillate (61.1 %; Fig. 13A, C–E, I–M, O) or less frequently papillate or semipapillate to papillate (36 %; Fig. 13B, F–H, M), occasionally nonpapillate (2.9 %); sporangial proliferation exclusively external (Fig. 13A, B, D, E, G, H, O); sporangial dimensions averaging 66.0 ± 8.3 × 36.2 ± 4.5 µm (overall range 46.5–110.2 × 23.7–48.5 µm; range of isolate means 62.5–73.9 × 30.8–39.7 µm) with a length/breadth ratio of 1.84 ± 0.31 (overall range 1.31–3.86); sporangial germination indirectly with zoospores discharged through an exit pore 3.7–9.3 µm wide (av. 5.9 ± 1.0 µm) (Fig. 13N, O). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 11.2 ± 1.3 µm) on encystment; cysts usually germinating directly forming a hypha or a microsporangium or infrequently indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings sometimes formed in water on sporangiophores, often close to the sporangial base, subglobose to globose, limoniform or irregular (Fig. 13D); diam 14.8 ± 4.1 µm (range 7.0–20.3 µm). Chlamydospores not observed. Hyphal aggregations commonly formed. Oogonia not observed in single culture; in mating tests with A1 and A2 mating type isolates of P. meadii two of the nine tested isolates (TW386 and TW387) stimulated the production of oogonia in the A2 isolate MYA-4043 of P. meadii and, hence, were of silent A2 mating type. The other seven isolates were sterile.
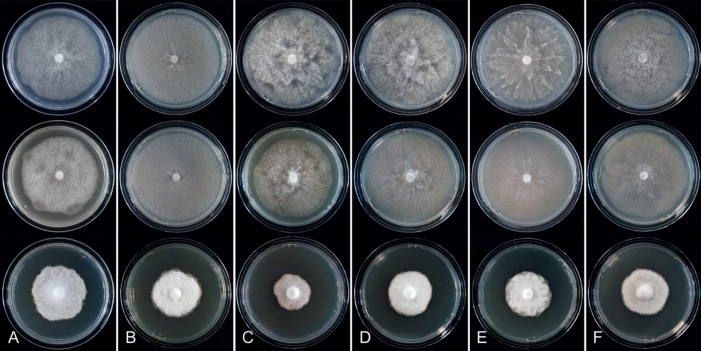
Culture characteristics: Colonies on V8A and CA mostly submerged with scanty aerial mycelium, stellate or faintly stellate; on PDA dense felty-cottony, with rosaceous or stellate patterns (Fig. 8).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 6.8 ± 0.58 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 12), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 5.6 ± 0.53 mm/d, 4.7 ± 0.49 mm/d and 2.6 ± 0.48 mm/d, respectively.
Materials examined: Japan, Kyushu, isolated from necrotic baiting leaves floating in forest streams; May 2017, T. Jung, A. Hieno & K. Kageyama (JP359, JP554); isolated from a naturally fallen necrotic leaf of Neolitsea sericea collected from the forest ground, Feb. 2017, H. Masuya (JP976); Amami-Ōshima, isolated from a naturally fallen tree leaf floating in a stream running through a subtropical lowland forest, Nov. 2018, T. Jung & M. Horta Jung (JP1356); Okinawa, isolated from a naturally fallen tree leaf floating in a stream running through a subtropical lowland forest, Nov. 2018, T. Jung & S. Uematsu (JP1536). Portugal, Tavira, isolated from necrotic baiting leaves floating in the Rio Séqua, Sep. 2012, T. Jung & M. Horta Jung (BD513, BD514); isolated from naturally fallen fruits of Citrus sinensis floating in the Rio Séqua running through Citrus orchards, Sep. 2011, T. Jung & M. Horta Jung (BD786, BD787, BD788). Spain, Galicia, isolated from rhizosphere soil of planted Citrus sinensis, before 2013, O. Aguin (TJ966 = EFA-16). Taiwan, Fushan, isolated from rhizosphere soil of Quercus tarokoensis, 2013, T. Jung, T.-T. Chang & M. Horta Jung (TW386, TW387).
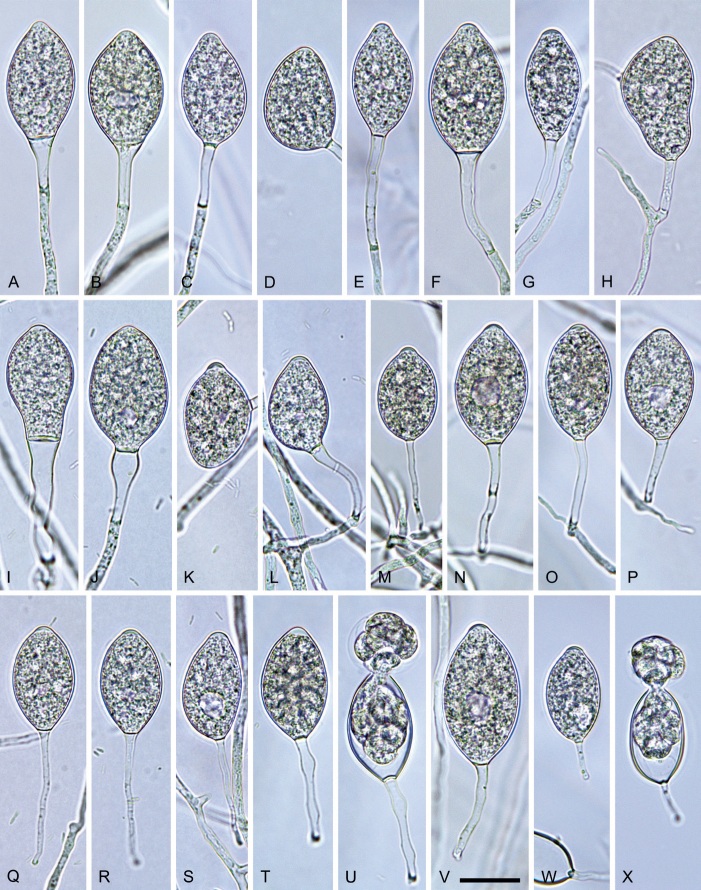
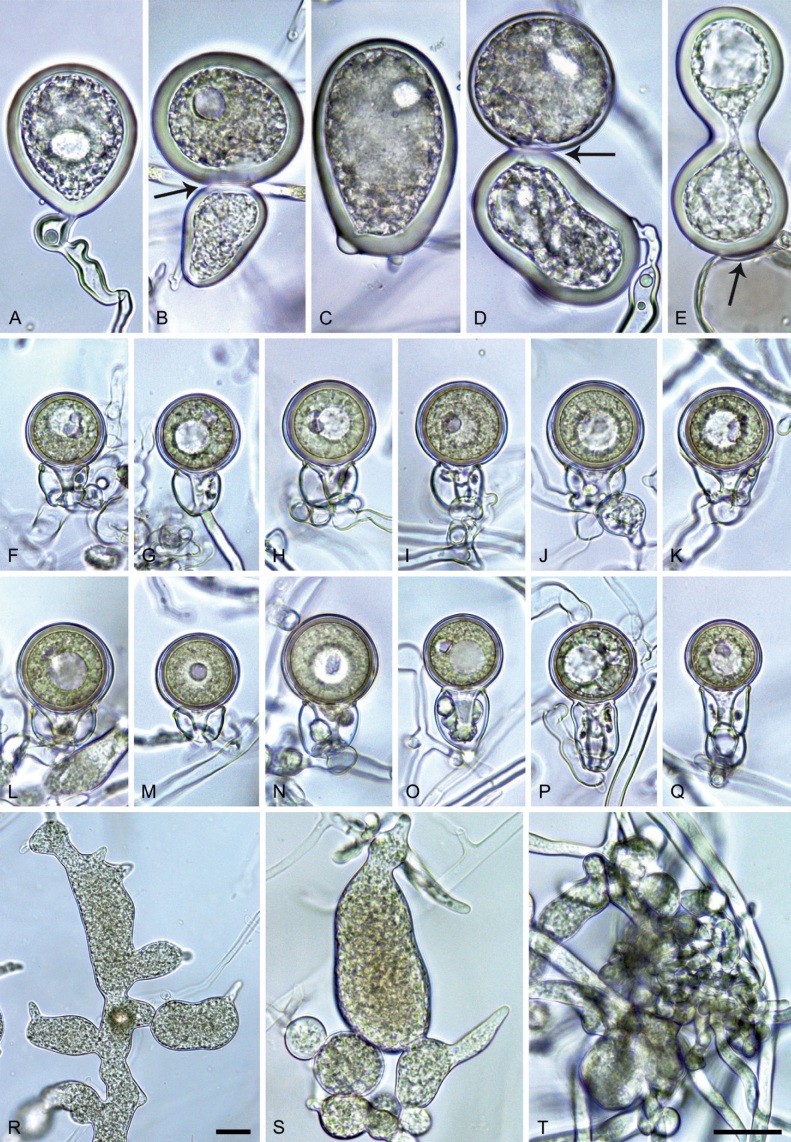
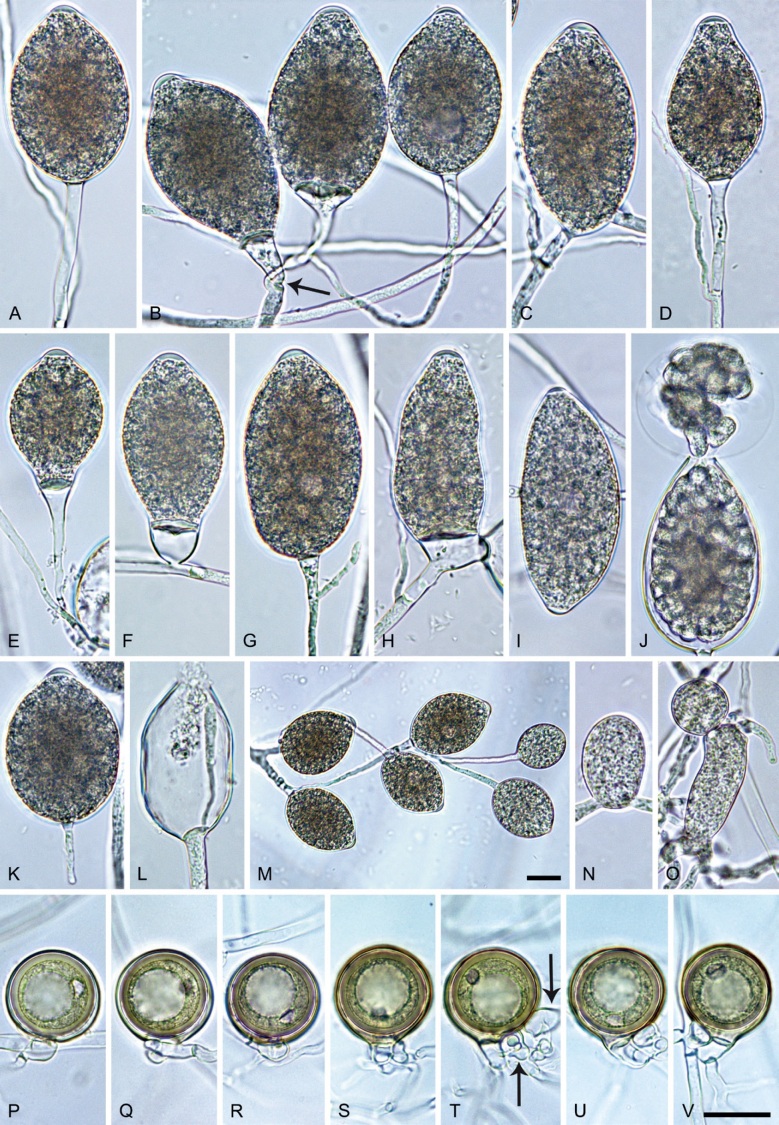
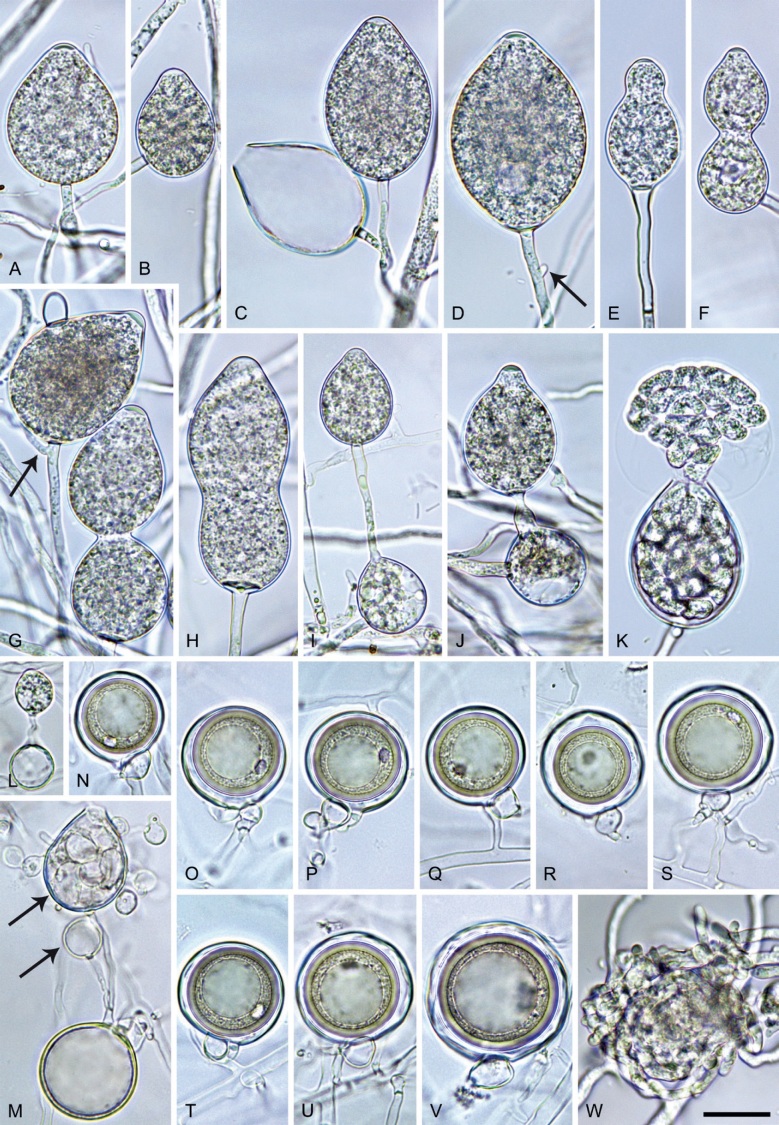
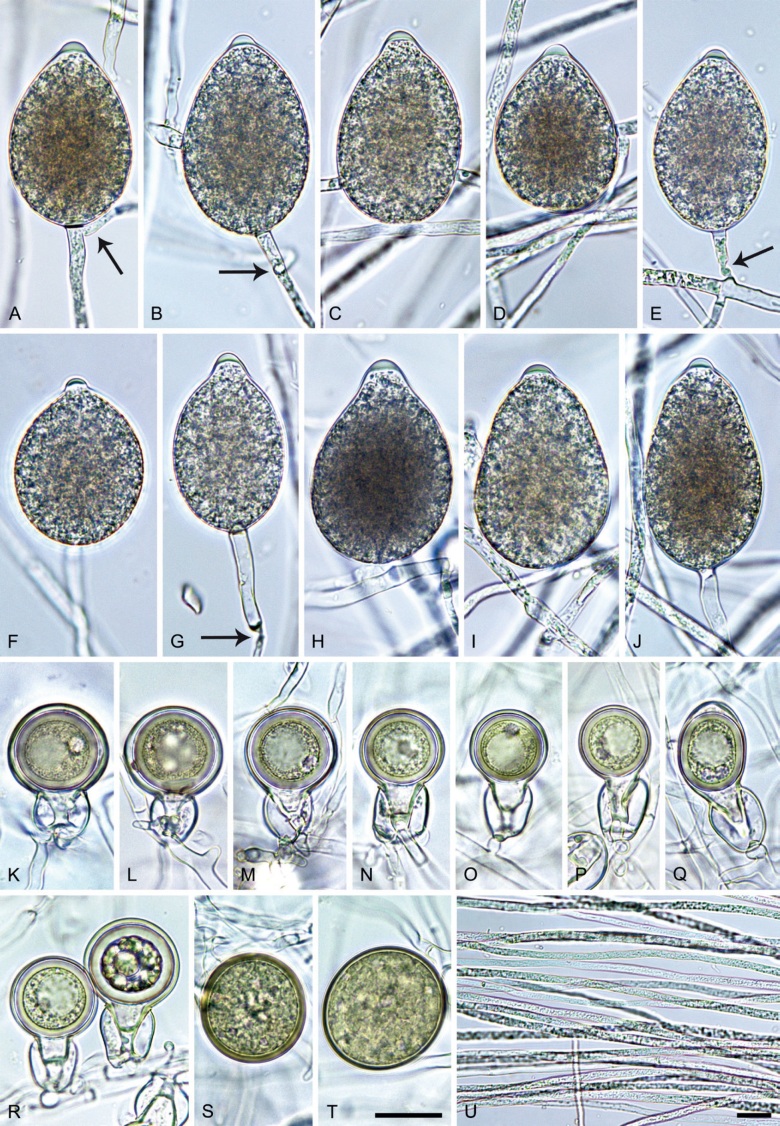
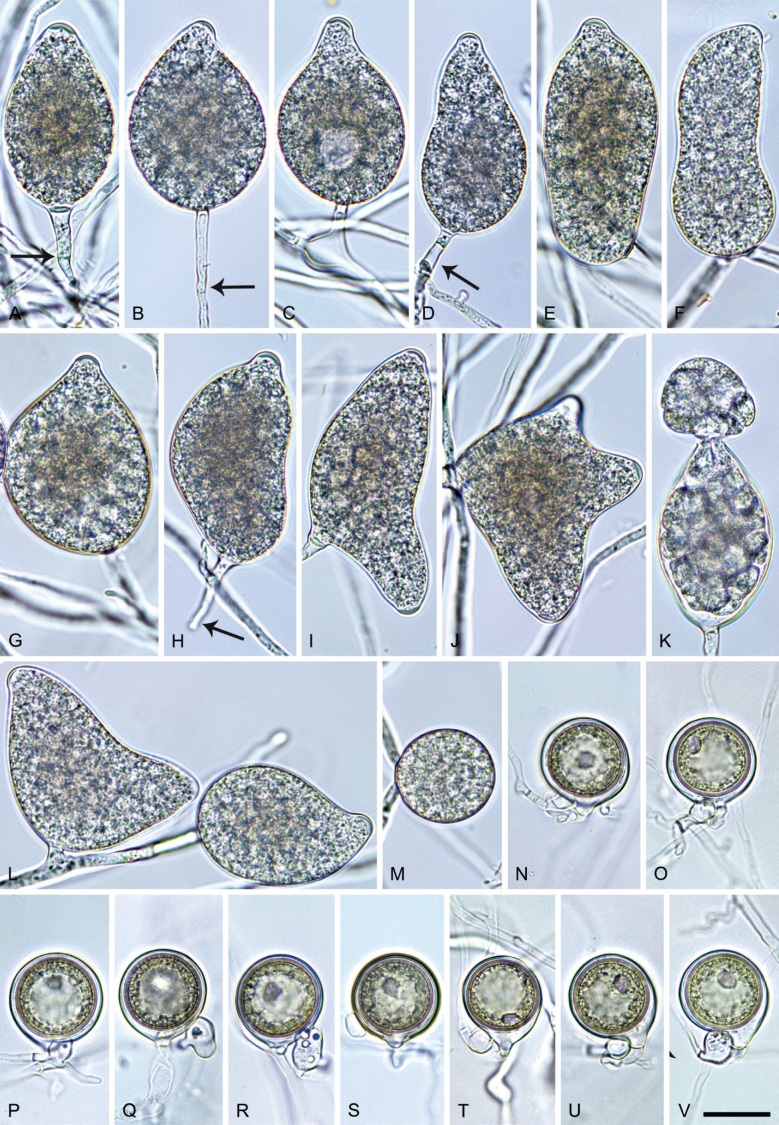
Phytophthora meadii McRae, J. Bombay Nat. Hist. Soc. 25: 760. 1918. [MycoBank MB 120866]. Fig. 14.
Fig. 14.
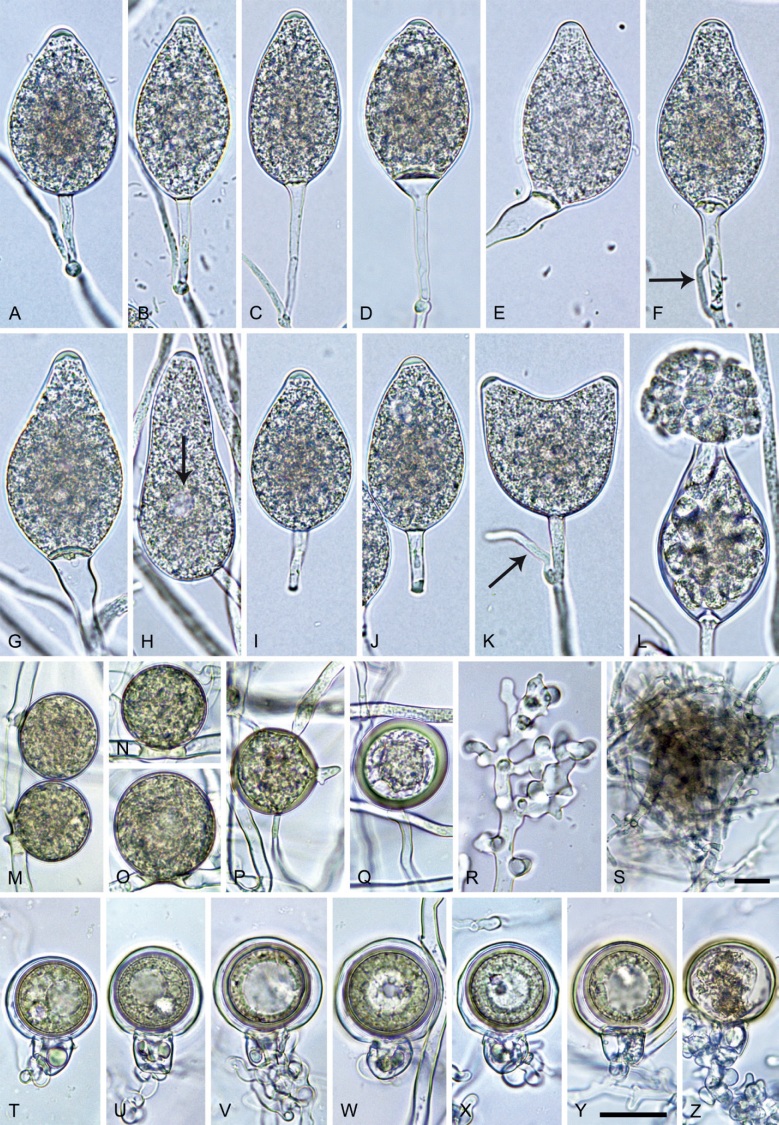
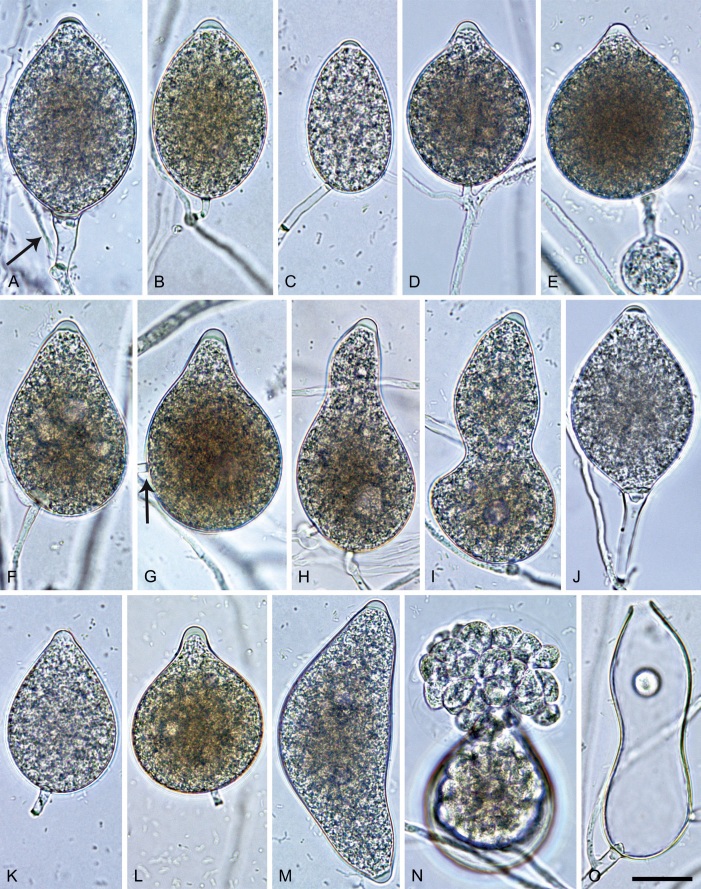
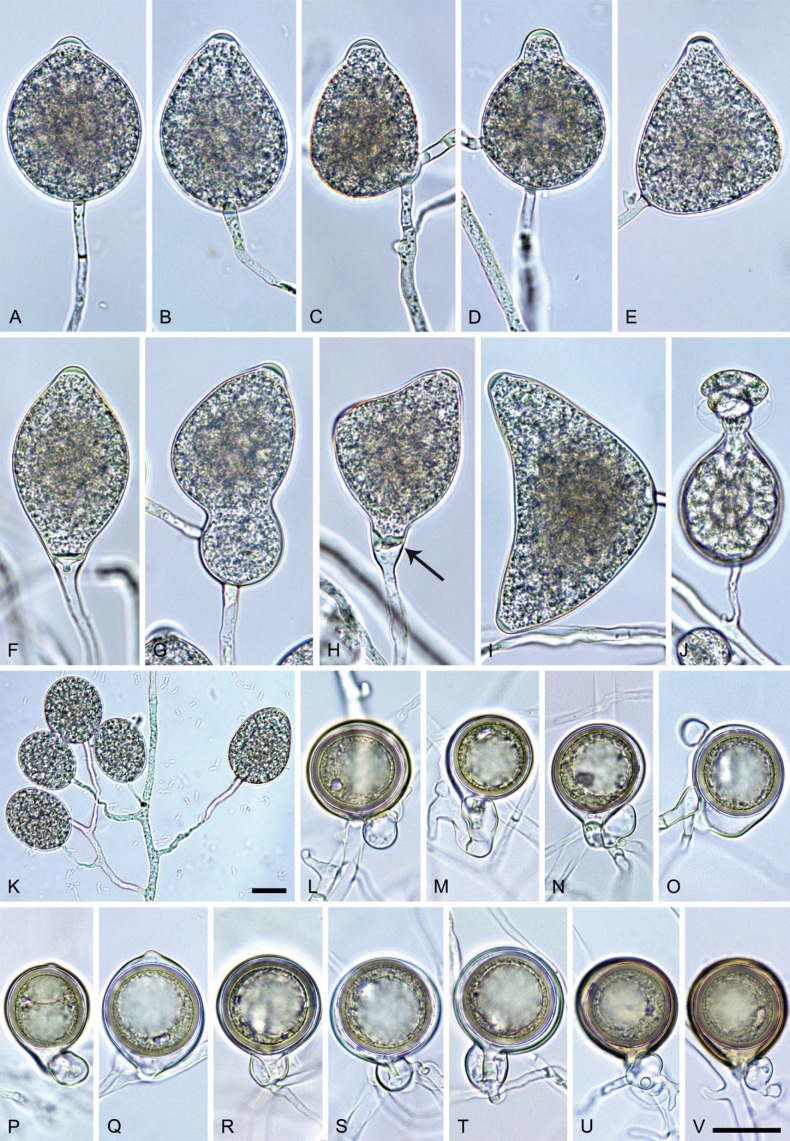
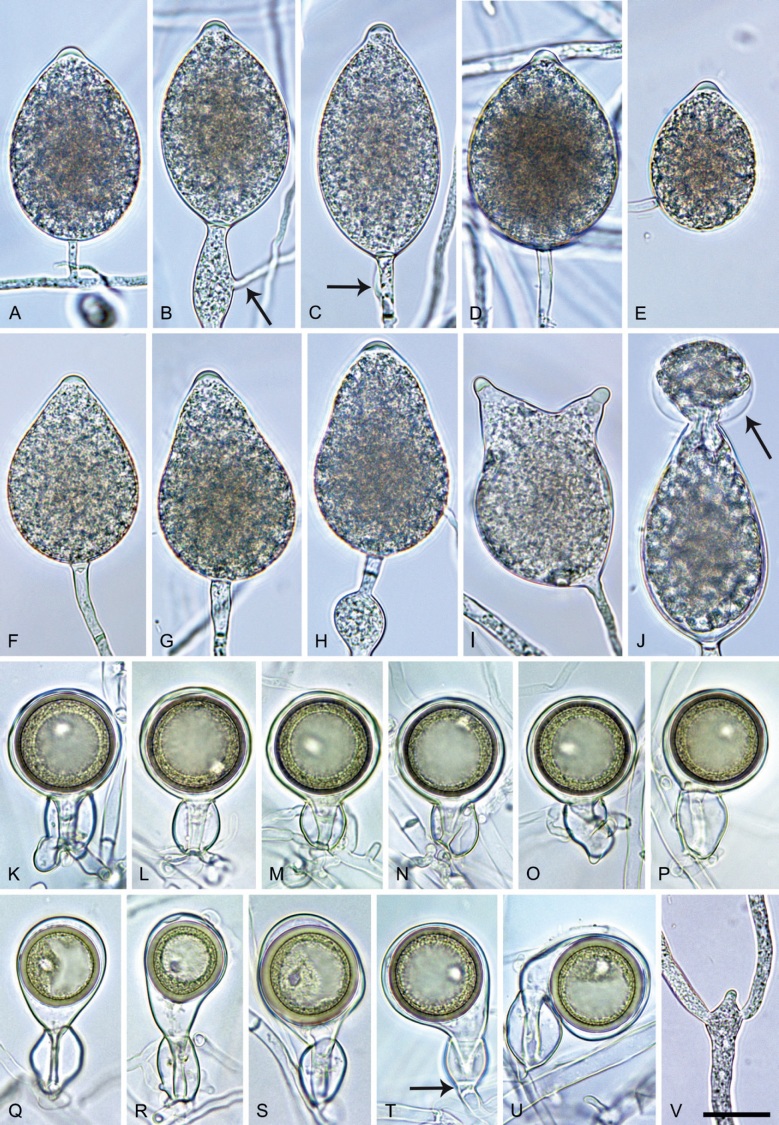
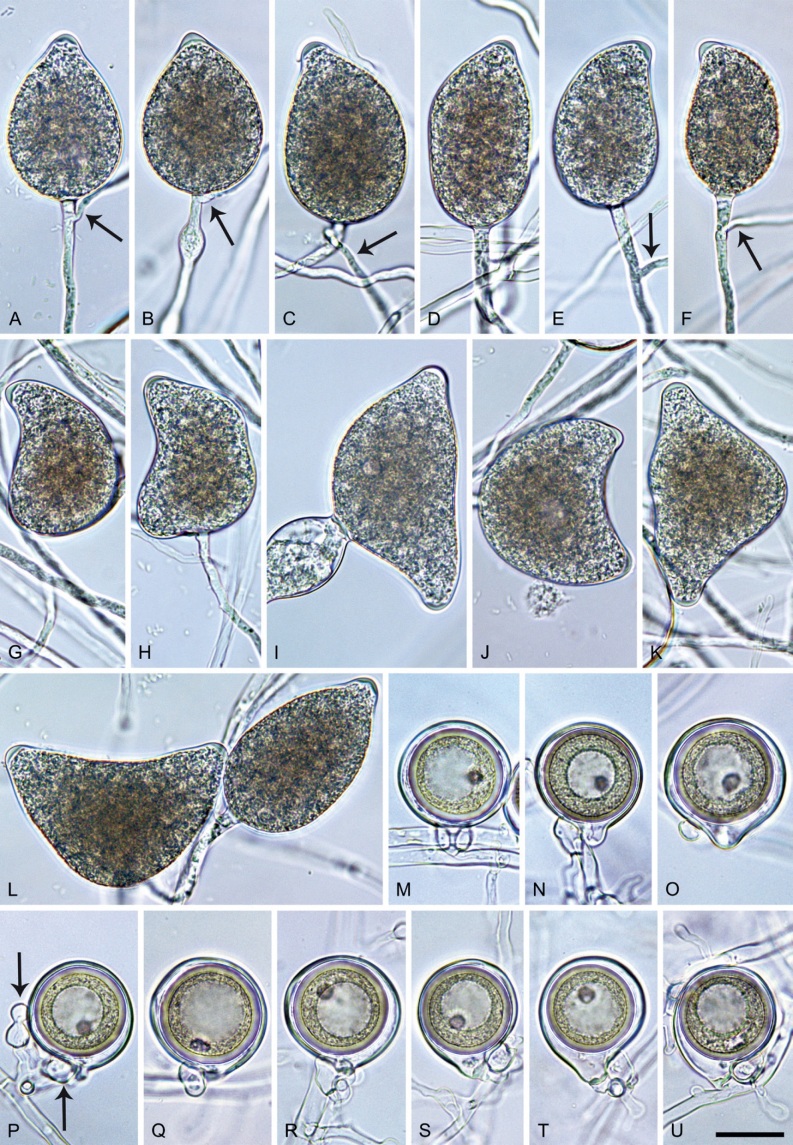
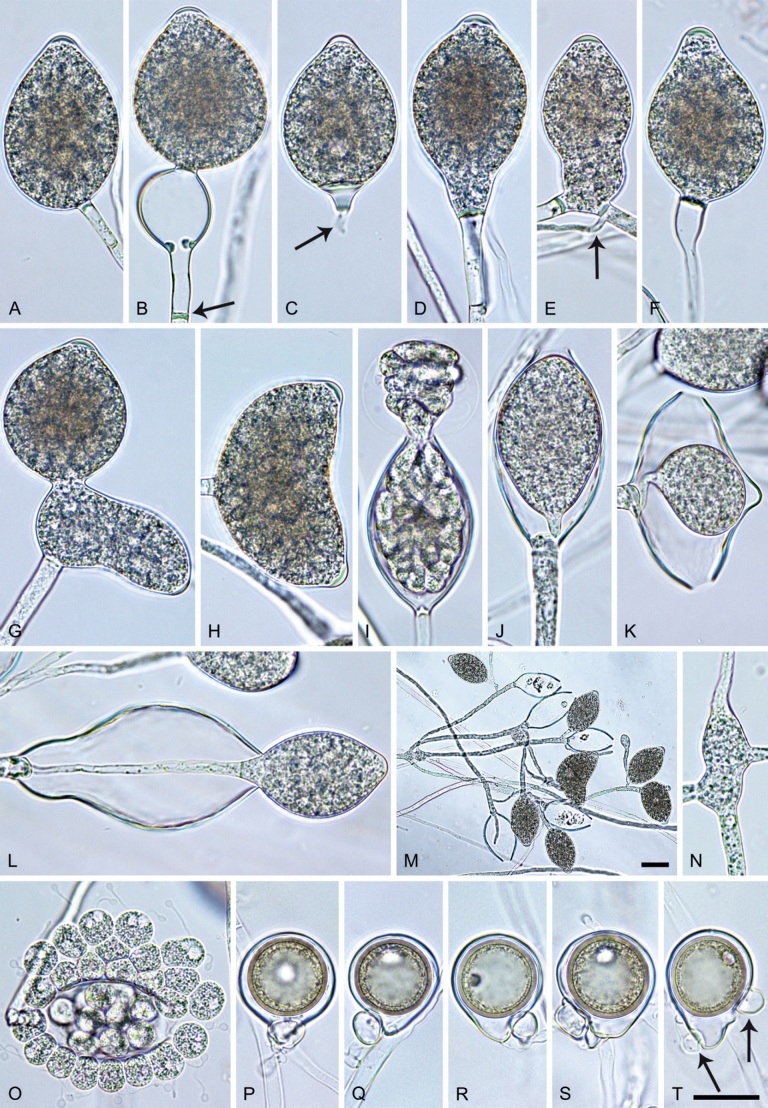
Phytophthora meadii. A–L. Sporangia formed on V8-agar (V8A) in soil extract. A–D, F–J. Ovoid, obpyriform and ellipsoid sporangia with semipapillate or papillate apices. A–D, F, G, I, J. Medium-length to long pedicels. E. Nonpapillate obpyriform sporangium. A–C, F, K. External proliferation (arrows). H. Vacuole (arrow). I, J. Caducous sporangia. K. Bilobed semipapillate sporangium. L. Zoospore releases. M–Q. Chlamydospores formed in solid carrot agar (fgCA). R. Coralloid hypae in solid V8A. S. Hyphal aggregation in solid V8A. T–Y. Oogonia with slightly aplerotic to aplerotic oospores and amphigynous unicellular antheridia, formed in mating test in solid fgCA. Z. Aborted oogonium. Images: A–C, E–R. MYA-4043; D, S. MYA-4042; T–Z. MYA-4042 × MYA-4043. Scale bars = 20 µm; Y applies to A–R, T–Z.
Neotypus: India, Kerala State, Palapilly Region isolated from Hevea brasiliensis, 2001, unknown collector (neotype CBS H-25073 designated in Abad et al. 2023a, MBT 10008019, dried culture on V8A; ex-neotype living culture CBS 148927 = NRRL 64146 = WPC P19007).
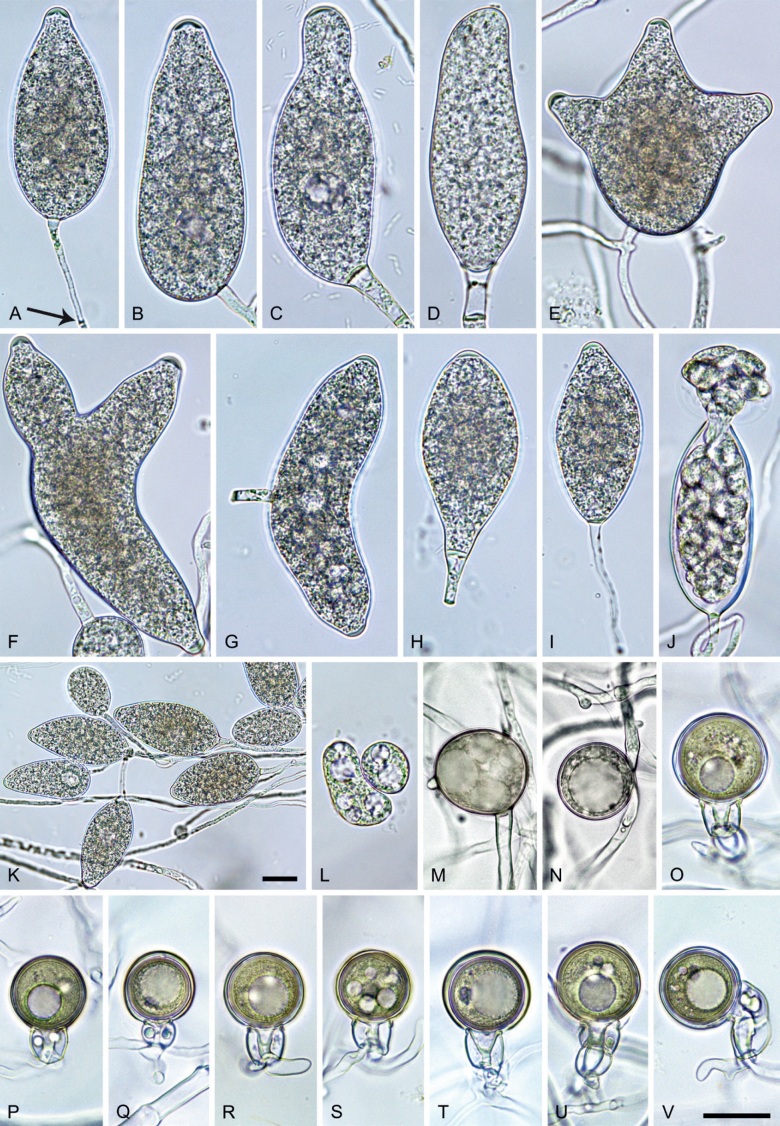
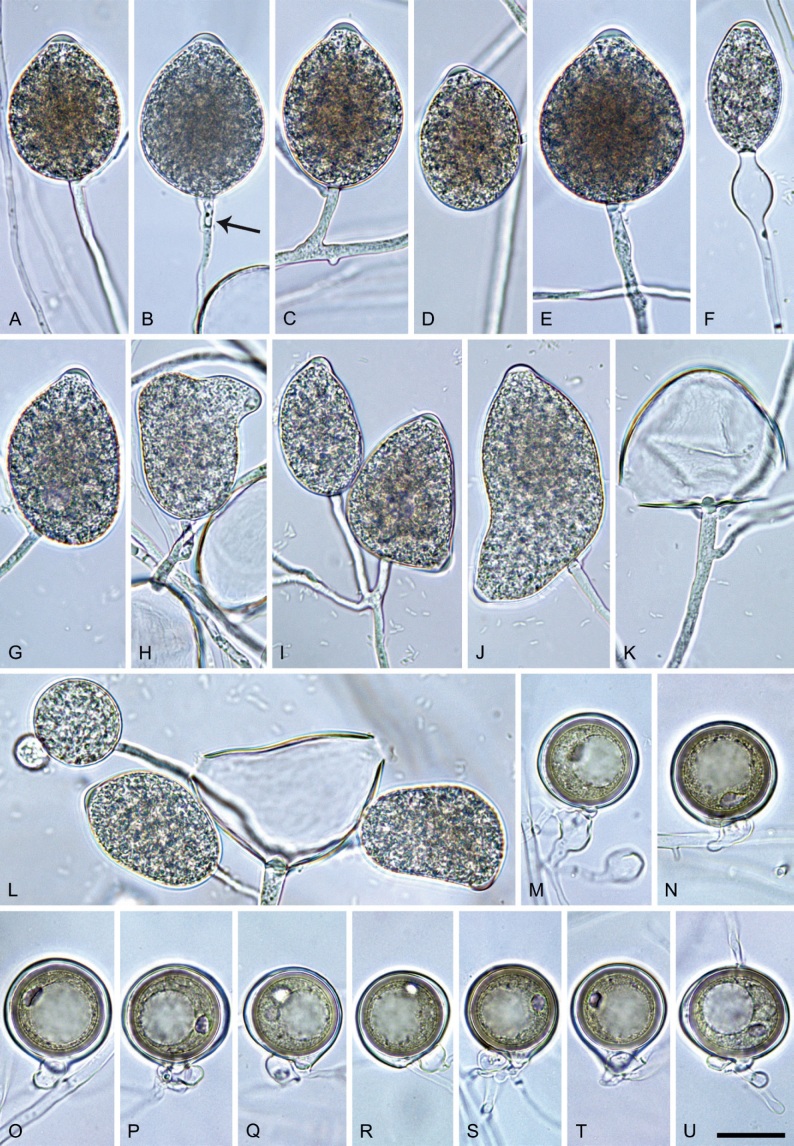
Morphological structures on V8A: Sporangia infrequently observed on solid agar and abundantly produced in non-sterile soil extract; borne almost exclusively terminally (99.2 %) on unbranched long or short sporangiophores or in dense or lax sympodia of 2–6 sporangia, or rarely intercalary (0.8 %; Fig. 14H); mostly ovoid to elongated ovoid (47.7 %; Fig. 14A–C, I, J) or obpyriform to elongated-obpyriform (28 %; Fig. 14E–H, L), less frequently distorted with often two or sometimes three apices (14.6 %; Fig. 14K), limoniform to elongated-limoniform (4.5 %), ellipsoid (2.5 %; Fig. 14D), pyriform (1.5 %) or subglobose; lateral attachment of sporangiophores (11.4 %; Fig. 14E, H), a widening of the sporangiophore towards the sporangial base (14.4 %; Fig. 14E, G) and vacuoles (7.3 %; Fig. 14H) commonly observed; sporangia frequently too big for the available cytoplasm and hence, not filled completely in the basal part, often with a strong plug below the cytoplasm (17.7 %; Fig. 14D, F, G); mostly (65.8 %) with pedicels of variable length (av. 22.7 ± 9.2 µm; range 7.3–46.4 µm; Fig. 14A–D, F, I, J) and caducous (Fig. 14I, J), but 34.2 % of sporangia without pedicel and persistent (Fig. 14K); sporangial apices on solid agar exclusively papillate, but in water mainly semipapillate to shallow papillate (77.2 %; Fig. 14A–D, G–K) with a smooth transition between both forms or less frequently nonpapillate and mostly pointed (22.8 %; Fig. 14E, F); sporangial proliferation exclusively external (Fig. 14A–C, F, K); sporangial dimensions averaging 44.5 ± 5.6 × 30.3 ± 4.9 µm (overall range 29.4–59.7 × 17.5–43.5 µm; range of isolate means 43.5–45.5 × 27.1–33.5 µm) with a length/breadth ratio of 1.5 ± 0.28 (overall range 1.13–2.45); sporangial germination indirectly with zoospores discharged through an exit pore 4.0–10.5 µm wide (av. 6.4 ± 1.2 µm) (Fig. 14L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.4 ± 0.7 µm) on encystment; cysts germinating directly forming a hypha or a microsporangium. Hyphal swellings are sometimes formed in water on sporangiophores, usually close to the sporangial base, subglobose to globose or limoniform. Chlamydospores infrequently formed in solid agar in single cultures, but abundantly produced in mating tests; globose to subglobose, borne intercalary or sessile (Fig. 14M–Q), sometimes catenulate (Fig. 14M) or with radiating hyphae (Fig. 14P); dimensions 28.0 ± 3.3 µm (overall range 23.1–34.3 µm); with relatively thin (Fig. 14M–O) or thick wall (Fig. 14P, Q) averaging 1.11 ± 1.0 µm (range 0.35–3.5 µm); often golden-brown (Fig. 14M–P). Hyphae in solid agar often coralloid and swollen (Fig. 14R). Hyphal aggregations are commonly formed (Fig. 14S). Oogonia not observed in single cultures, but commonly produced in mating tests between A1 and A2 mating type isolates (‘heterothallic’ breeding system); mostly sessile with short thin stalks and rounded base, globose to slightly subglobose (Fig. 14T–Y), often slightly excentric (30 %; Fig. 14V–X); wall predominantly smooth (87.5 %; Fig. 14T–V, Y) or occasionally slightly wavy (12.5 %; Fig. 14W, X); oogonial diam 31.7 ± 4.2 µm (overall range 21.5–40.5 µm); slightly aplerotic to aplerotic (Fig. 14T–Y). Oospores globose, usually with one large lipid globule (Fig. 14U–Y) or infrequently with multiple smaller globules (6 %; Fig. 14T); diam 27.1 ± 3.6 µm (overall range 18.3–34.0 µm) wall thickness 1.46 ± 0.24 µm (overall range 1.13–2.07 µm), oospore wall index 0.29 ± 0.03; high abortion rate of 66 % after 4 wk (Fig. 14Z). Antheridia exclusively amphigynous and cylindrical or subglobose, sometimes curved, and unicellular (Fig. 14T–Z); dimensions 15.2 ± 2.3 × 13.4 ± 1.3 µm.
Culture characteristics: Colonies on V8A and CA submerged to appressed with scanty aerial mycelium, radiate to stellate or faintly radiate on V8A, and stellate or radiate on CA; on PDA dense felty-cottony and petaloid or dense felty with a stoloniferous pattern (Fig. 8).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 6.67 ± 2.85 mm/d radial growth, maximum 32.5 °C, minimum >10–<15 °C (Fig. 12), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 4.68 ± 1.0 mm/d, 5.18 ± 0.7 mm/d and 4.78 ± 0.97 mm/d, respectively.
Materials examined: India, Karnataka, Beligundi, isolated from a leaf of Coorg orange (Citrus reticulata), 1992, S.D. Sawant (MYA-4043 = NRRL 64250 = IMI 403509 = WPC P10191 = CH 22G5 = MEGp75 = TJ1112); Nittur village isolated from a leaf of Coorg orange, 1992, S.D. Sawant (MYA-4042 = WPC P10190 = CH 22G4 = MEGp74 = TJ1149).
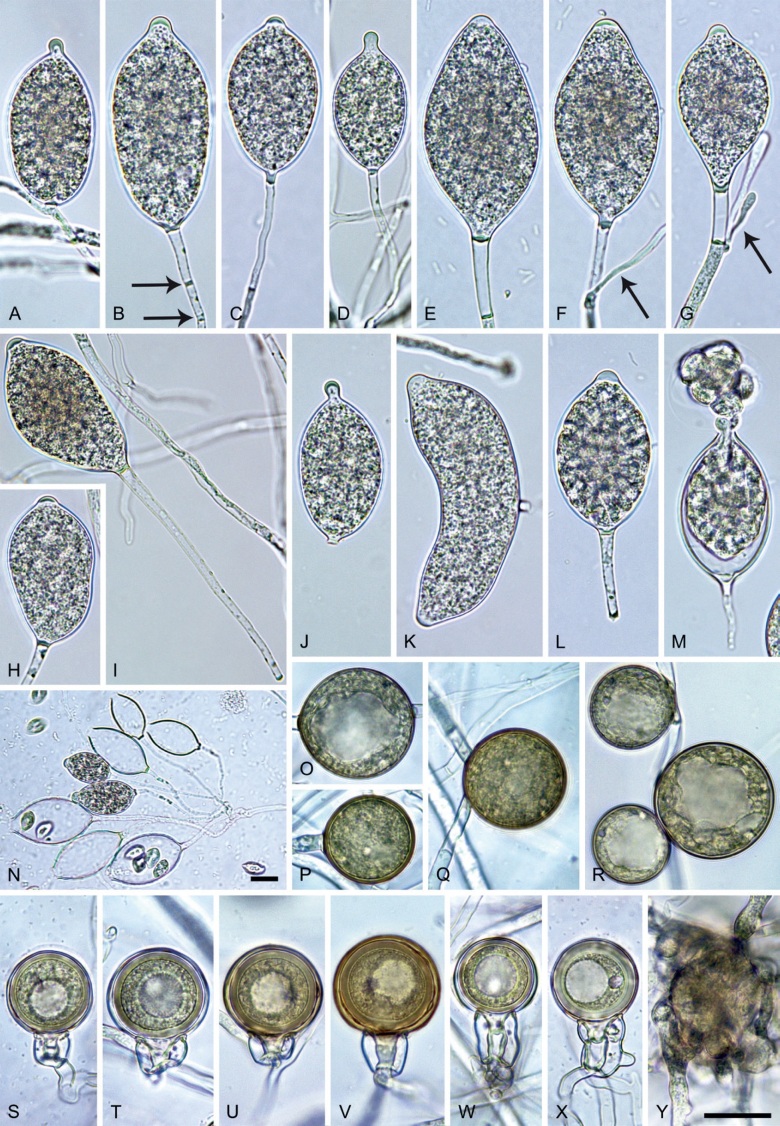
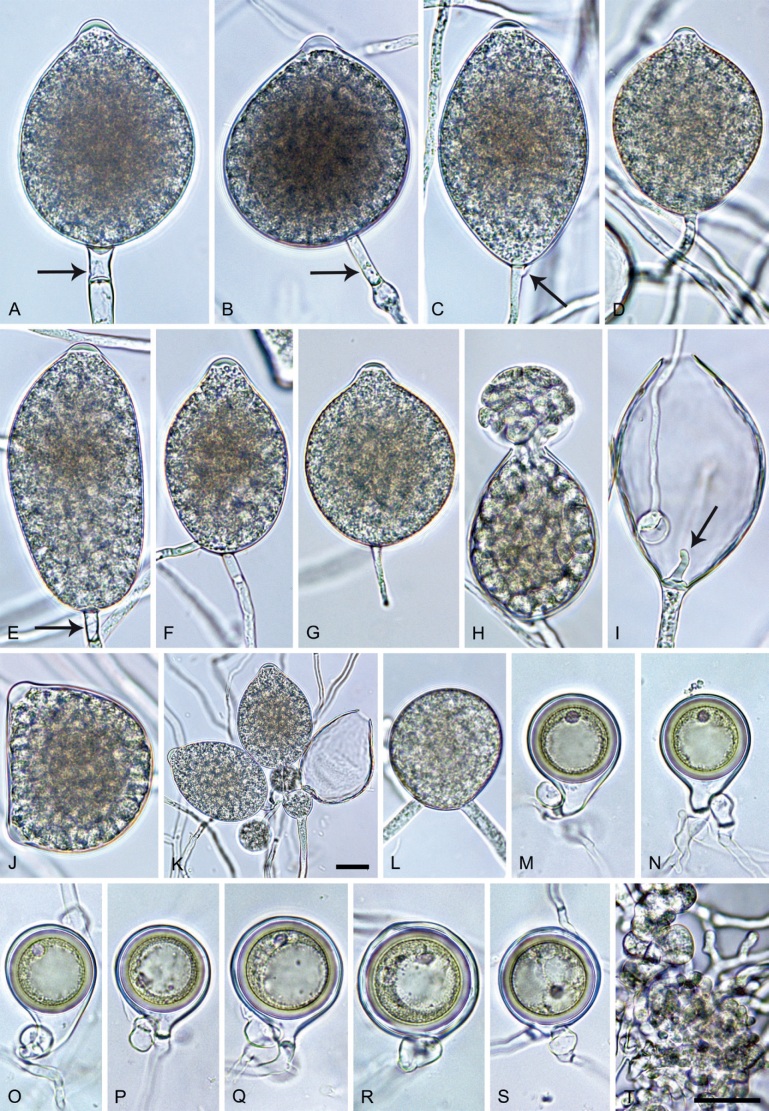
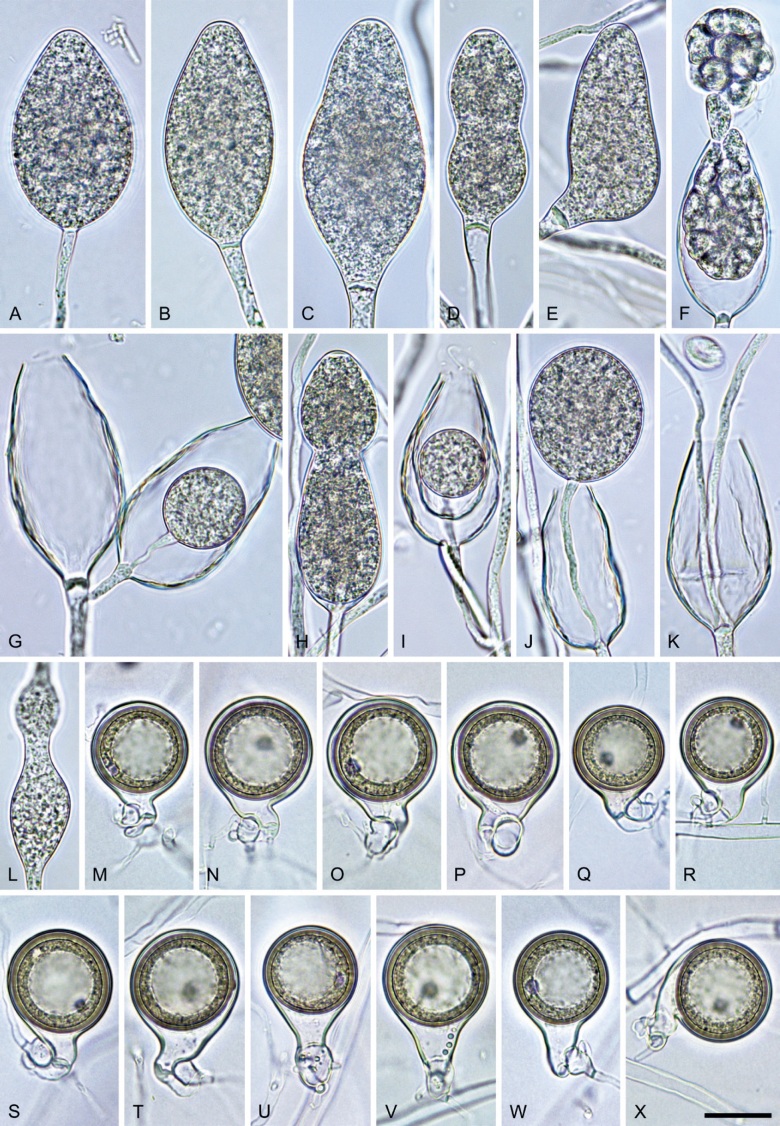
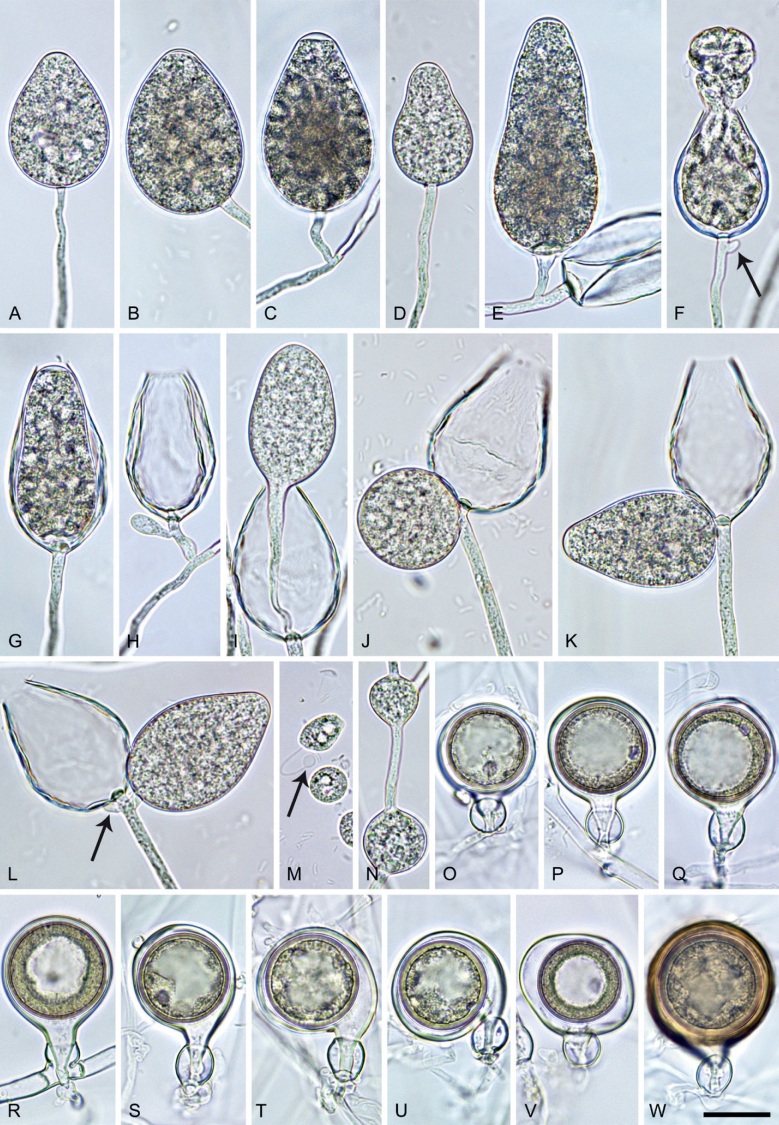
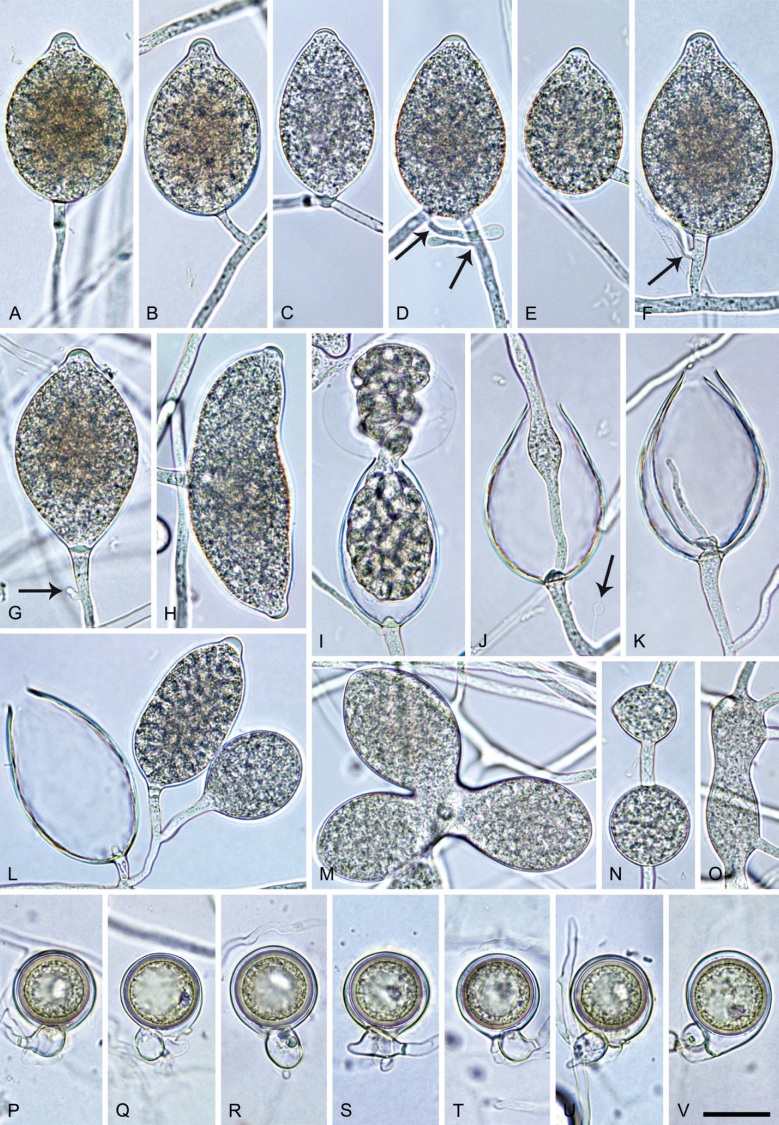
Phytophthora pseudocitrophthora T. Jung, S.O. Cacciola, J. Bakonyi & M. Horta Jung, sp. nov. MycoBank MB 847265. Fig. 15.
Fig. 15.
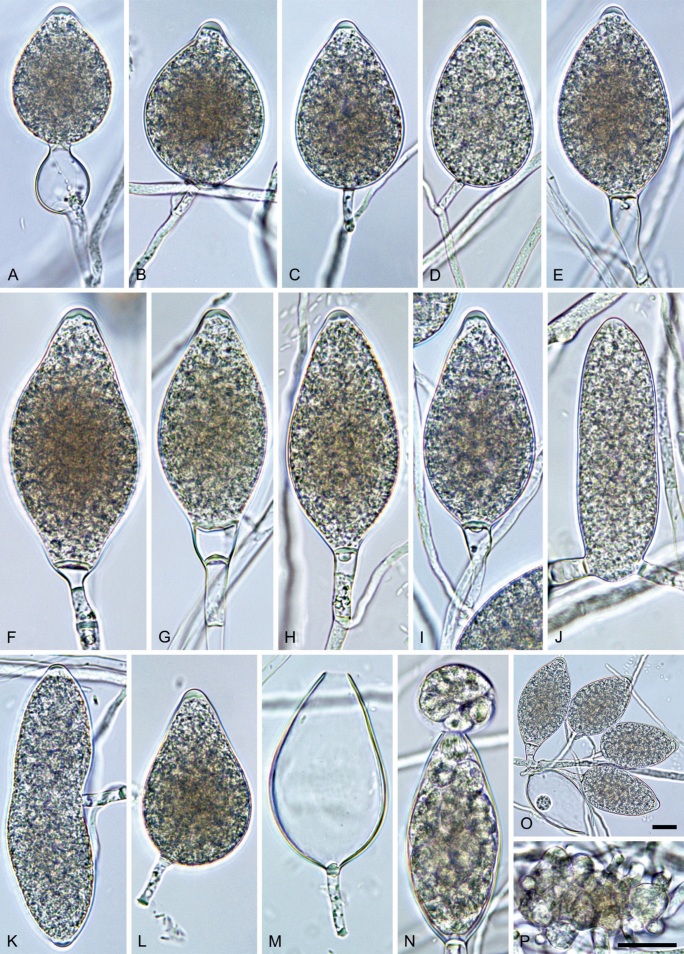
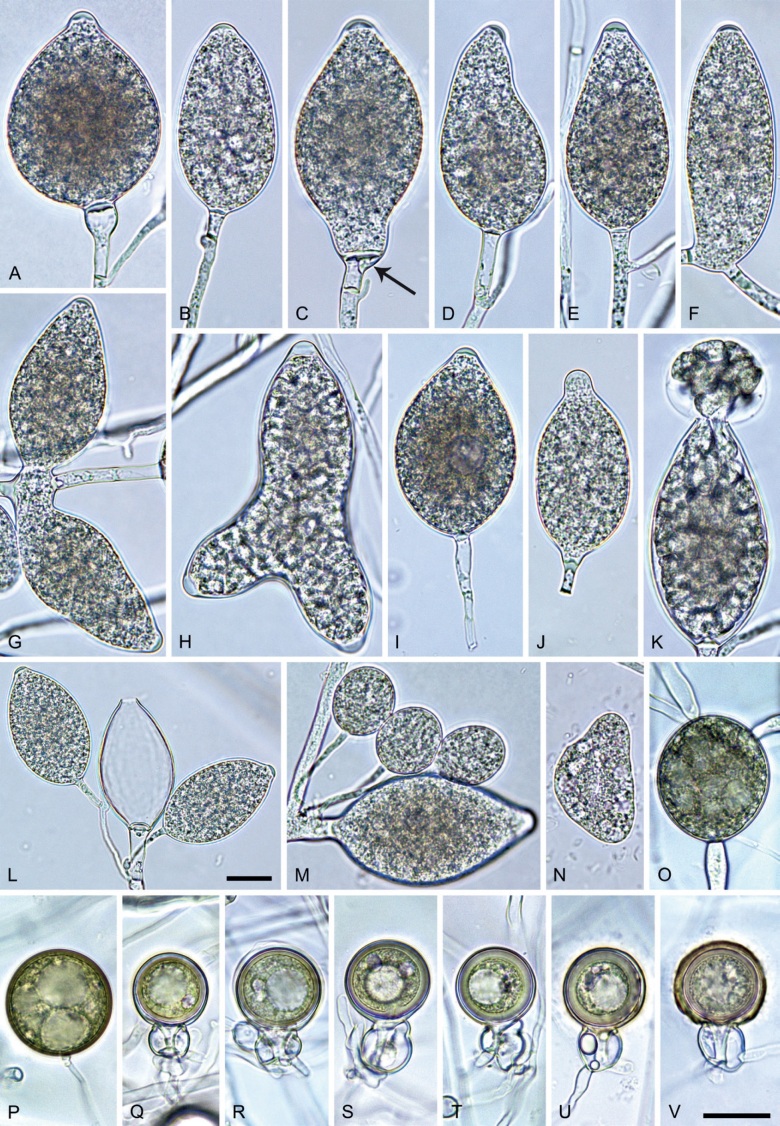
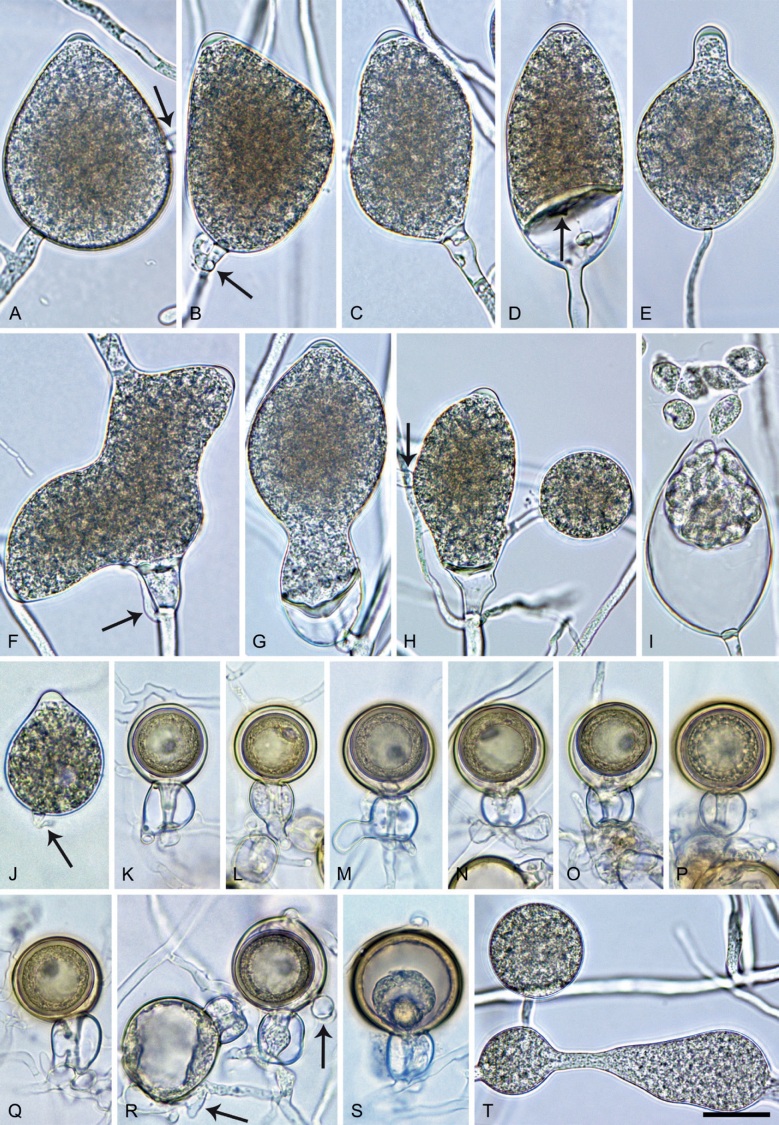
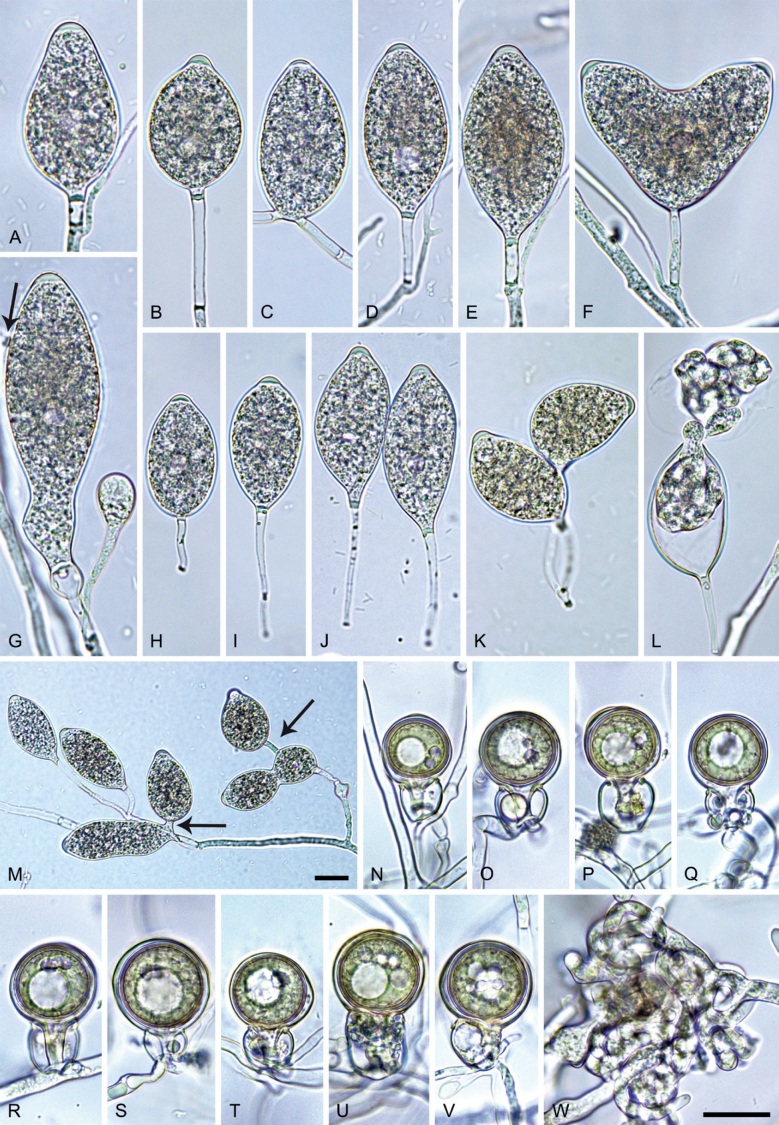
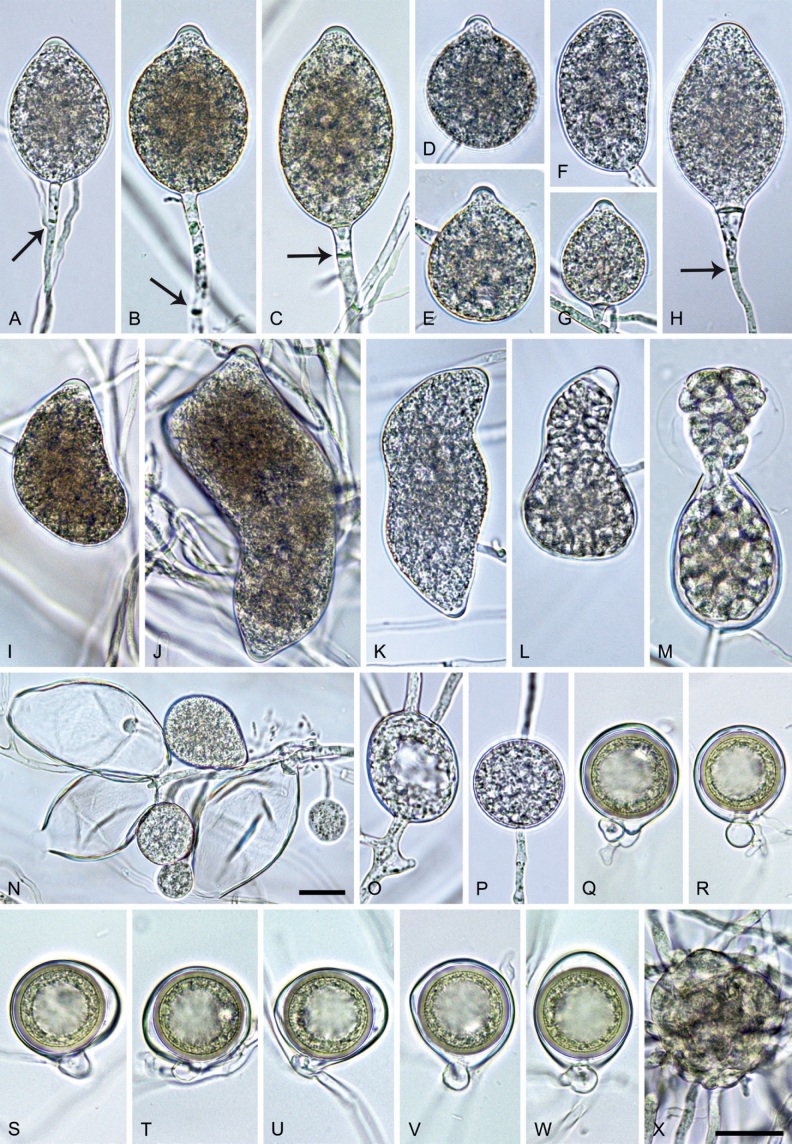
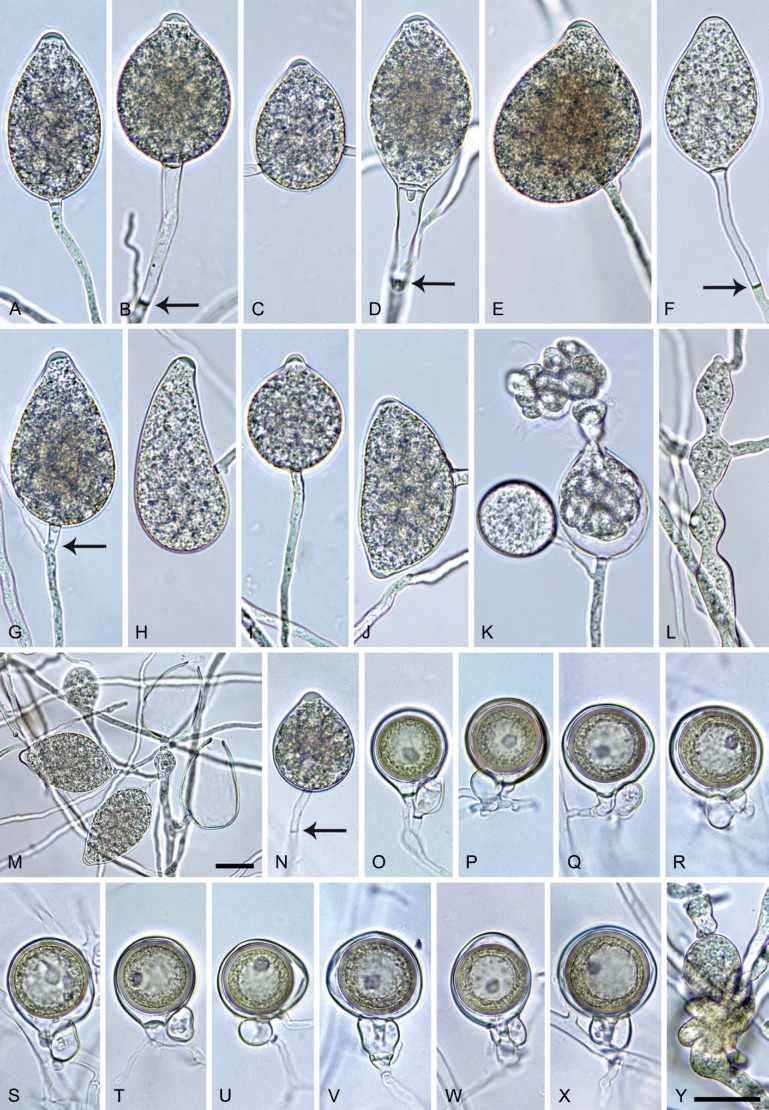
Phytophthora pseudocitrophthora. A–O. Sporangia formed on V8-agar (V8A) in soil extract. A–I. Ovoid, limoniform or obpyriform sporangia with papillate apices and medium-length to long pedicels. A–C, E, H. External proliferation. F, G. Cytoplasm not completely filling the sporangia. J. Ellipsoid, intercalary nonpapillate sporangium. K. Distorted sporangium with two semipapillate apices. L, M. Caducous sporangia. N. Zoospore release. O. Dense sporangial sympodium. P. Hyphal aggregation formed in solid V8A. Images: A, G–J, M, N, P. Ex-type CBS 149500; B–D. BD505; E. TJ998; F. TJ133; K. TJ995; L. TJ065; O. TJ480. Scale bars = 20 µm; P applies to A–N, P.
Etymology: The name refers to the morphological similarity and phylogenetic relatedness to P. citrophthora.
Typus: Italy, Sicily, Pantalica Nature Reserve, isolated from rhizosphere soil of Platanus orientalis in a riparian forest, May 2013, T. Jung & S.O. Cacciola (holotype CBS H-25124, dried culture on V8A, ex-holotype living culture CBS 149500 = TJ798).
Morphological structures on V8A: Sporangia infrequently observed on solid agar and abundantly produced in non-sterile soil extract; borne predominantly terminally (99.1 %) on unbranched long or short sporangiophores or in dense or lax sympodia of 2–8 sporangia (Fig. 15O), or rarely intercalary (0.8 %; Fig. 15J); mostly ovoid, broad-ovoid or elongated-ovoid (63.7 %; Fig. 15A–E, H, M, O) or limoniform to elongated-limoniform (20 %; Fig. 15F, G, N, O), less frequently distorted with often two or sometimes three apices (7.4 %; Fig. 15K), obpyriform to elongated-obpyriform (6.2 %; Fig. 15I, L), ellipsoid to elongated-ellipsoid (1.2 %; Fig. 15J), subglobose, pyriform, obturbinate, obovoid or ampulliform (each 0.3 %); lateral attachment of sporangiophores (23.1 %; Fig. 15B, D, L) and a conspicuous basal plug (42.9 %; Fig. 15E–I, M, N) commonly observed; sporangiophores sometimes widening towards the sporangial base (6.9 %; Fig. 15E); sporangia occasionally too big for the available cytoplasm and hence, not filled completely in the basal part, often with an additional strong plug below the cytoplasm (4.9 %; Fig. 15F, G); predominantly (86.3 %) with pedicels of variable length (av. 24.8 ± 13.6 µm; range 4.4–90.1 µm; Fig. 15A–M, O); infrequently caducous (Fig. 15L, M); sporangial apices on solid agar exclusively papillate; in water mainly papillate (75.9 %; Fig. 15A–I, L, O) or less frequently semipapillate (14.6 %; Fig. 15K) or nonpapillate and mostly pointed (9.5 %; Fig. 15J); sporangial proliferation exclusively external (Fig. 15A–E, K, O); sporangial dimensions averaging 61.6 ± 7.6 × 36.0 ± 4.0 µm (overall range 39.3–89.4 × 20.5–45.5 µm; range of isolate means 54.4–71.5 × 32.8–39.8 µm) with a length/breadth ratio of 1.73 ± 0.26 (overall range 1.12–2.68); sporangial germination indirectly with zoospores discharged through an exit pore 3.7–7.3 µm wide (av. 5.3 ± 0.7 µm) (Fig. 15M, N). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.7 ± 0.9 µm) on encystment; cysts usually germinating directly forming a hypha or a microsporangium or infrequently indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings sometimes formed in water on sporangiophores, often close to the sporangial base, subglobose to globose, limoniform or irregular (Fig. 15A); diam 13.0 ± 6.1 µm (range 5.6–21.7 µm). Chlamydospores not observed. Hyphal aggregations commonly formed (Fig. 15P). Oogonia not observed in single culture; in mating tests with A1 and A2 mating type isolates of P. meadii one of the 10 tested isolates (TJ133) stimulated the production of a few oogonia in the A1 isolate MYA-4042 of P. meadii and, hence, was a silent A2 mating type, whereas the other nine isolates were sterile.
Culture characteristics: Colonies on V8A mostly submerged with scanty aerial mycelium, stellate to radiate; on CA submerged to appressed with limited aerial mycelium and faint radiate pattern; on PDA dense felty-cottony and mostly appressed, with a radiate pattern (Fig. 9).
Fig. 9.
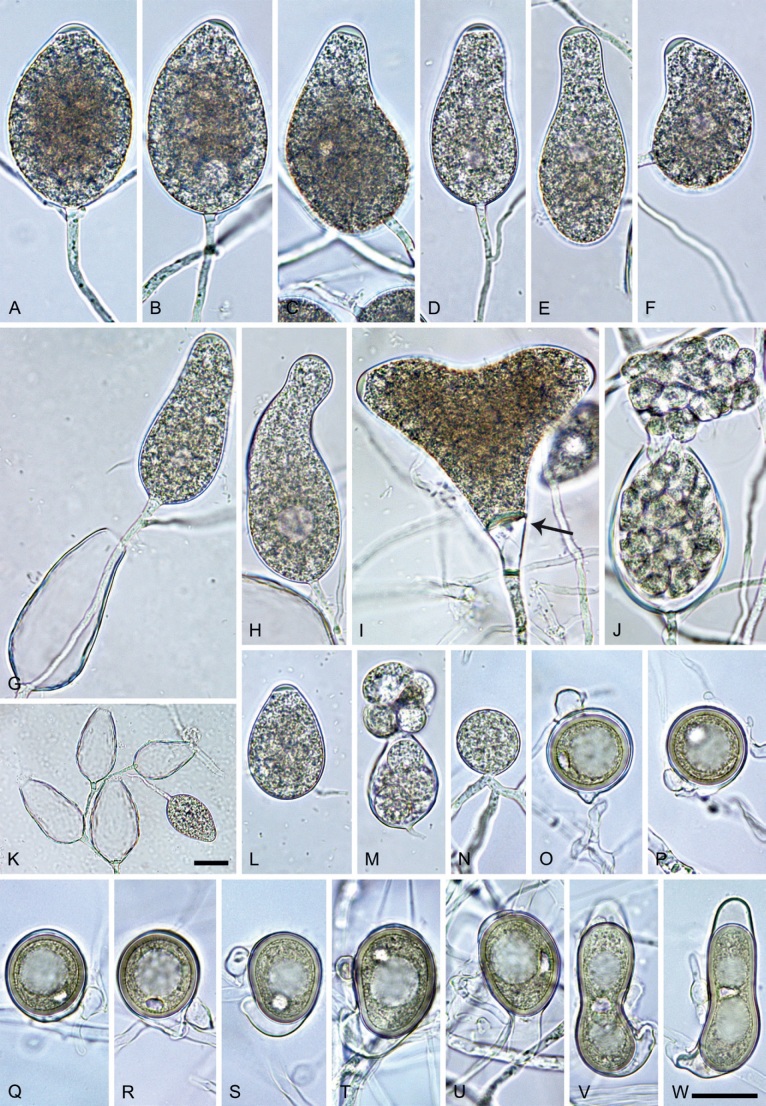
Colony morphology of Phytophthora species from subclade 2a after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora mekongensis (ex-type CBS 135136). B. Phytophthora occultans (TJ967). C, D. Phytophthora pseudocitrophthora (C. TJ133; D. ex-type CBS 149500). E. Phytophthora pseudoccultans (ex-type CBS 149499). F. Phytophthora vietnamensis (ex-type CBS 149635).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 7.59 ± 1.15 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 12), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 6.59 ± 0.59 mm/d, 5.62 ± 0.17 mm/d and 3.45 ± 0.45 mm/d, respectively.
Additional materials examined: Hungary, Répceszemere, isolated from Syringa vulgaris, Sep. 2008, J. Bakonyi (TJ133 = JA149); Gencsapáti, isolated from Abies procera, Aug. 2009, J. Bakonyi (TJ137 = JA333). Morocco, Marrakech, isolated from rhizosphere soil of planted Citrus limon in a garden, Jan. 2012, T. Jung (TJ995); isolated from rhizosphere soil of planted Myrthus communis in a garden, Jan. 2012, T. Jung (TJ998). Portugal, Parque Natural Sintra-Cascais, isolated from a baiting leaf of Quercus suber floating in a stream running through planted forests, Mar. 2015, T. Jung & C. Maia (BD255); Tavira, isolated from a naturally fallen fruit of Citrus sinensis floating in the Rio Séqua river running through Citrus orchards, Sep. 2011, T. Jung & M. Horta Jung (BD505). Serbia, Fruska Gora National Park, isolated from rhizosphere soil of Quercus petraea in a planted forest, Apr. 2012, I. Milenković (SFB265). Spain, Mallorca, unknown, 2010, E.M. Moralejo (TJ065 = P300 = P4142); Valencia, isolated from rhizosphere soil of planted Pistacia lentiscus, 2010, A. Pérez-Sierra (TJ480 = 917b). USA, Louisiana, Little Bayou Sara, isolated from a naturally fallen necrotic leaf floating in a forest stream, Mar. 2020, T. Corcobado & T. Majek (LU209).
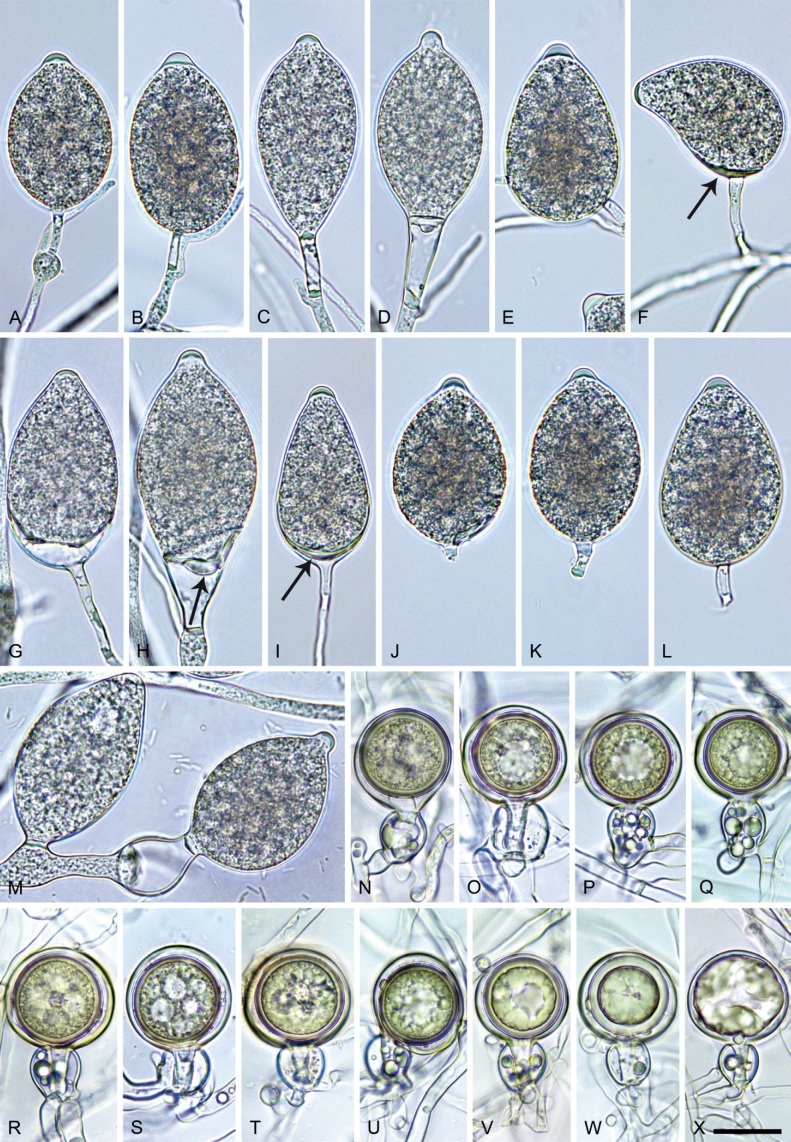
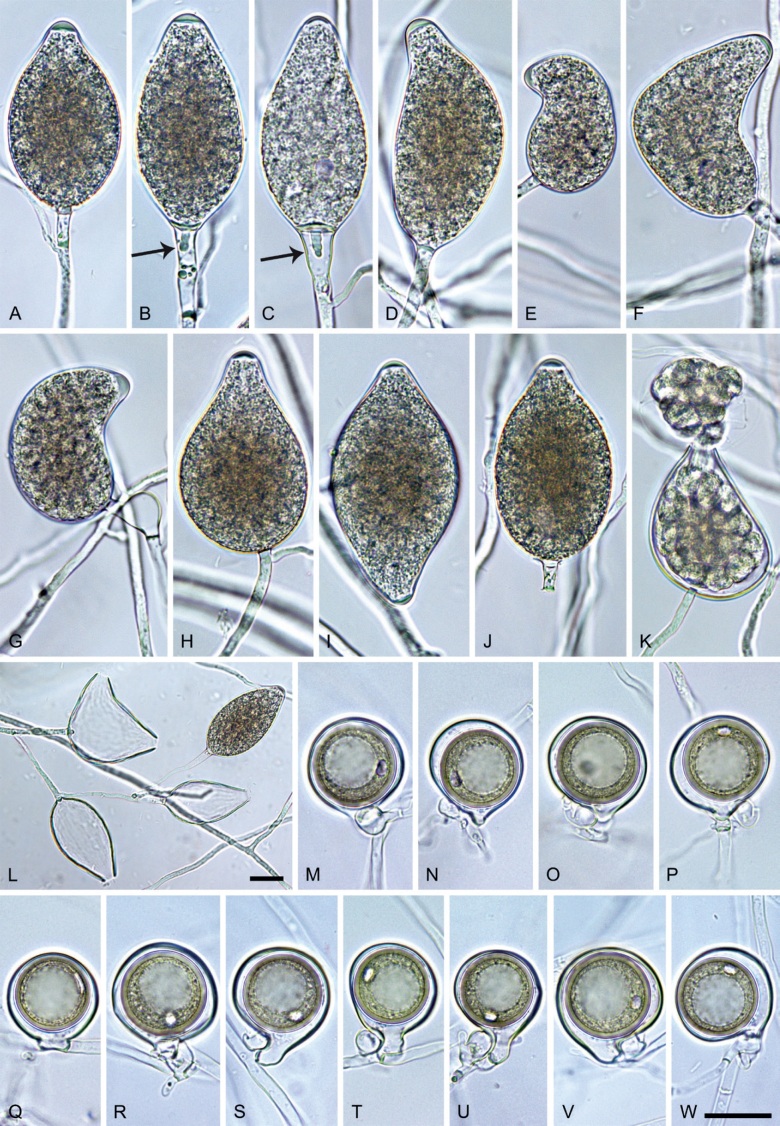
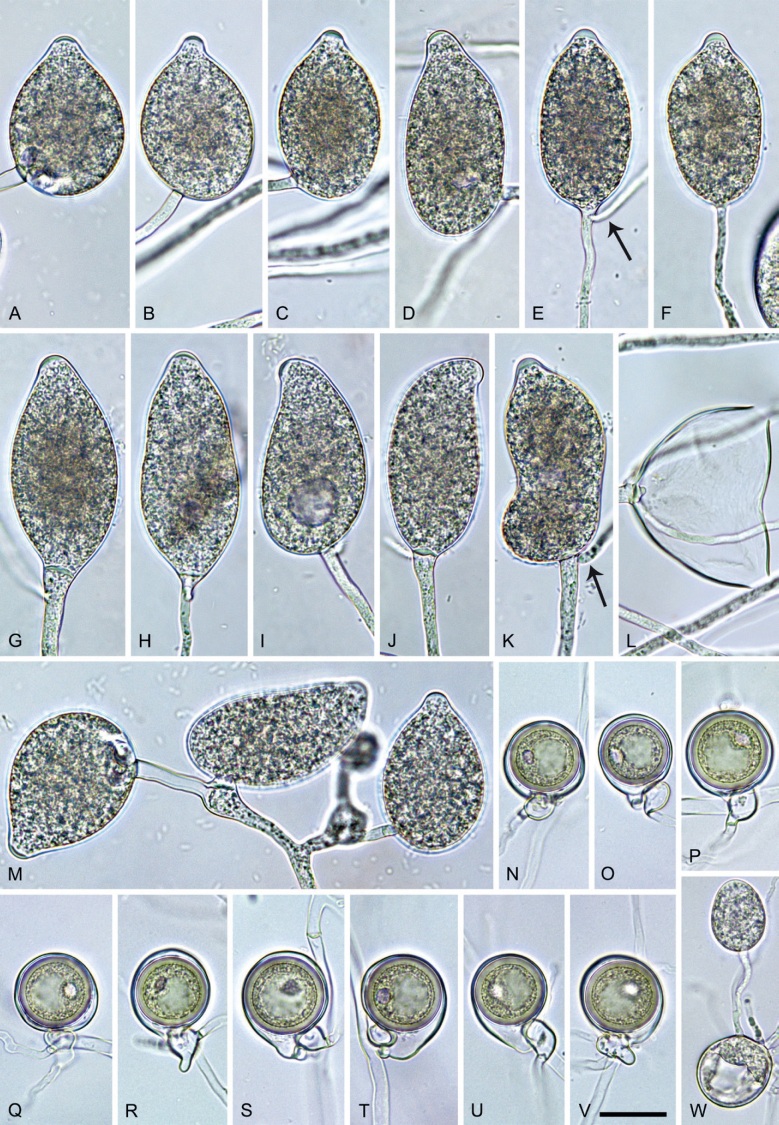
Phytophthora pseudoccultans T. Jung, T.-T. Chang, I. Milenković & M. Horta Jung, sp. nov. MycoBank MB 847272. Fig. 16.
Fig. 16.
Phytophthora pseudoccultans. A–L. Sporangia formed on V8-agar (V8A) in soil extract. A–J, L. Papillate sporangia. A–F, I–L. Ovoid, obpyriform, ellipsoid and limoniform sporangia. A, B, D-F, I, J, L. Pedicels. B. Hyphal extension (arrow). E. Intercalary sporangium. G, H. Distorted, bipapillate sporangia. I, J. Caducous sporangia. K. Zoospore release. L. Beginning sporangial sympodium. M. Empty zoospore cyst after releasing a secondary zoospore (diplanetism). N–V. Oogonia with near-plerotic to slightly aplerotic oospores produced in solid V8A. N–S. Paragynous antheridia. T–V. Amphigynous antheridia. W. Hyphal aggregation formed in solid V8A. Images: A–E, G–I, L, M, O–T, W. Ex-type CBS 149499; F, J, K, N, U, V. TW062. Scale bar = 20 µm; W applies to A–W.
Etymology: The name refers to the morphological similarity to P. occultans.
Typus: Taiwan, Fushan, isolated from a baiting leaf floating in a tributary of Ha-pen River running through subtropical Castanopsis-Machilus forest, Mar. 2013, T. Jung & T.-T. Chang (holotype CBS H-25123, dried culture on V8A, ex-holotype living culture CBS 149499 = TW044).
Morphological structures on V8A: Sporangia rarely observed on solid agar but produced abundantly in non-sterile soil extract; typically borne terminally (94 %) in dense or lax sympodia of 2–4 sporangia (Fig. 16L) or on unbranched long or short lateral sporangiophores, or intercalary (6 %; Fig. 16E); apices papillate (Fig. 16A–J, L); mostly ovoid, broad-ovoid or elongated ovoid (62.1 %; Fig. 16A–C, I, L), less frequently distorted and often bi- or tripapillate (18.4 %; Fig. 16G, H), limoniform to elongated-limoniform (10.2 %; Fig. 16F, J), obpyriform to broad-obpyriform (4.5 %; Fig. 16D), ellipsoid to elongated-ellipsoid (2 %; Fig. 16E), obovoid (2 %) or pyriform (0.8 %); lateral attachment of the sporangiophore (35 %; Fig. 16B, C, G–I) common; mostly with pedicels (69.9 %; Fig. 16A, B, D, F, I, J) averaging 17.3 ± 8.1 µm in length (range 4.3–38.1 µm) and caducous (Fig. 16I, J); infrequently with a vacuole (Fig. 16L); sporangial proliferation exclusively external (Fig. 16A, F, L); sporangial dimensions averaging 57.9 ± 6.3 ´ 36.0 ± 3.6 µm (overall range 42.6–77.9 ´ 28.2–45.1 µm; range of isolate means 56.9–58.9 ´ 35.3–36.6 µm) with a length/breadth ratio of 1.62 ± 0.23 (overall range 1.24–2.47); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.8–7.8 µm wide (av. 6.6 ± 0.8 µm) (Fig. 16K). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.4 ± 0.7 µm) on encystment; cysts mostly germinating directly or by releasing a secondary zoospore (= diplanetism; Fig. 16M). Hyphal swellings infrequently produced in water on sporangiophores and hyphae; globose to subglobose or limoniform, often close to the sporangial base (Fig. 16C); dimensions 10.8 ± 2.3 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), mostly sessile without stalk or on short thin stalks, with rounded base, smooth-walled, globose to slightly subglobose (93 %; Fig. 16N–U) or sometimes slightly elongated (7 %; Fig. 16V); oogonial diam 28.8 ± 2.6 µm (overall range 22.4–35.3 µm; range of isolate means 27.8–29.7 µm); nearly plerotic to slightly aplerotic (Fig. 16N–V). Oospores globose with a large lipid globule (Fig. 16N–V); diam 24.3 ± 2.4 µm (overall range 18.8–33.3 µm; range of isolate means 23.5–25.2 µm) wall thickness 1.37 ± 0.22 µm (overall range 1.02–2.48 µm), oospore wall index 0.3 ± 0.04; abortion rate 9–21 % (av. 15 %) after 4 wk. Antheridia predominantly paragynous and club-shaped, ovoid or subglobose (77 %; Fig. 16N–S) or less frequently amphigynous, unicellular and club-shaped to cylindrical (23 %; Fig. 16T–V); sometimes two antheridia attached to one oogonium (4 %); dimensions 11.4 ± 2.3 × 8.7 ± 1.6 µm.
Culture characteristics: Colonies on V8A mostly submerged to appressed with limited aerial mycelium and a radiate pattern; on CA shallow cottony with submerged margin and a faint radiate pattern; on PDA cottony and petaloid (Fig. 9).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 8.85 ± 0.71 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 12), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 8.53 ± 2.04 mm/d, 6.05 ± 0.43 mm/d and 4.09 ± 0.28 mm/d, respectively.
Additional materials examined: Taiwan, Fushan, isolated from a baiting leaf floating in Cu-keng River, Mar. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW062); Tunyuan, isolated from rhizosphere soil of Quercus variabilis in montane, temperate, seasonally dry Quercus–Pinus forest, Mar. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW114).
Phytophthora vietnamensis T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, sp. nov. MycoBank MB 847280. Fig. 17.
Fig. 17.
Phytophthora vietnamensis. A–O. Sporangia formed on V8-agar (V8A) in soil extract. A–J, L. Ovoid, obpyriform, ampulliform or limoniform sporangia with papillate or semipapillate apices. A–C, J–L. Short to long pedicels. A, B, D, F, O. External proliferation (arrow in A). C, H, O. Intercalary sporangia. G. Lateral attachment (arrow). K–M. Caducous sporangia. K. Nonpapillate apex. M. Bipapillate sporangium. N. Zoospore release. O. Ampulliform sporangium after zoospore release. Images: A, C–E, I–K, M, O. Ex-type CBS 149635; B, F–H, L, N. CBS 149635. Scale bar = 20 µm; O applies to A–O.
Etymology: The name refers to the origin of all known isolates in Vietnam.
Typus: Vietnam, Sapa, Xin Chài Mountain, isolated from rhizosphere soil of Alnus nepalensis in montane, temperate Alnus forest, Mar. 2016, T. Jung & N.M. Chi (holotype CBS H-25190, dried culture on V8A, ex-holotype living culture CBS 149635 = VN368).
Morphological structures on V8A: Sporangia infrequently observed on solid agar but abundantly produced in non-sterile soil extract; borne mostly terminally (91.6 %) on unbranched long or short sporangiophores (Fig. 17B, I) or in dense or lax sympodia of 2–5 sporangia, or less frequently intercalary (8.4 %; Fig. 17C, H, O); mostly ovoid to broad-ovoid (52.2 %; Fig. 17A–E, K) or obpyriform, broad-obpyriform or elongated-obpyriform (28.4 %; Fig. 17F–H, L), less frequently distorted with often two or sometimes three apices (9.6 %; Fig. 17M), limoniform to elongated-limoniform (5.6 %; Fig. 17J), ampulliform (3.6 %; Fig. 17I, O) or obovoid (0.6 %); sporangial apices predominantly papillate (92.6 %; Fig. 16B, D–I, L, M), sometimes semipapillate (4.8 %; Fig. 17A, C, J) or nonpapillate and pointed (2.6 %; Fig. 17K); frequently curved (20.8 %; Fig. 17G–I); lateral attachment of sporangiophores (54.4 %; Fig. 17E–G, K, L), small vacuoles (32.9 %; Fig. 17F–I) and a conspicuous basal plug (22.8 %; Fig. 17A, F, J, O) commonly observed; predominantly (72.8 %) with short- to medium-length pedicels (9.7 ± 4.3 µm; range 2.1–34.2 µm; Fig. 17A–C, J–L); often caducous (Fig. 17K–M); sporangial proliferation exclusively external (Fig. 17A, B, D, F, O); sporangial dimensions averaging 63.3 ± 7.2 × 42.4 ± 4.5 µm (overall range 42.3–100.1 × 29.3–54.1 µm; range of isolate means 61.8–67.2 × 40.8–44.5 µm) with a length/breadth ratio of 1.51 ± 0.28 (overall range 1.13–2.83); sporangial germination indirectly with zoospores discharged through an exit pore 4.0–9.2 µm wide (av. 6.1 ± 0.7 µm) (Fig. 17N, O). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.2 ± 1.1 µm) on encystment; cysts germinating directly forming a hypha or a microsporangium. Hyphal swellings sometimes formed in water on sporangiophores, often close to the sporangial base, subglobose to globose, limoniform or irregular (Fig. 17E); diam 13.6 ± 4.7 µm (range 5.8–23.7 µm). Chlamydospores not observed. Oogonia not observed in single cultures or in mating tests with A1 and A2 mating type isolates of P. meadii (sterile breeding system).
Culture characteristics: Colonies on V8A mostly submerged with scanty aerial mycelium and a radiate pattern; on CA submerged to appressed with limited aerial mycelium and faint radiate pattern; on PDA dense-felty and appressed with limited aerial mycelium and a radiate pattern (Fig. 9).
Cardinal temperatures and growth rates: On V8A optimum 20 °C with 6.62 ± 0.8 mm/d radial growth but growing only slightly slower at 25, 27.5 and 30 °C, maximum 30–<32.5 °C, minimum <10 °C (Fig. 12), lethal temperature 32.5–<35 °C. On CA and PDA at 20 °C 4.48 ± 0.12 mm/d and 2.72 ± 0.08 mm/d, respectively.
Additional materials examined: Vietnam, Sapa, Xin Chài Mountain, isolated from rhizosphere soil of Alnus nepalensis in montane, temperate Alnus forest, Mar. 2016, T. Jung, M. Horta Jung & N.M. Chi (VN824, VN825, VN826, VN827).
Phytophthora ×australasiatica T. Jung, N.M. Chi, M. Tarigan & M. Horta Jung, sp. nov. MycoBank MB 847287. Fig. 18.
Fig. 18.
Phytophthora ×australasiatica. A–M. Sporangia formed on V8-agar (V8A) in soil extract. A–I. Papillate or semipapillate sporangial apices. A–F, I–M. Ovoid, obpyriform, ellipsoid or limoniform sporangia. A–C, I, J, L. Medium-length to long pedicels. F, G. Intercalary insertion. G. Bilobed sporangium. H. Trilobed sporangium before zoospore release. A, C–E, K–M. External proliferation. I, J. Caducous sporangia. J. Nonpapillate apex. K. Zoospore release. L. Sporangial sympodium. N. Large multinucleate zoospore. O, P. Chlamydospores formed in carrot agar (fgCA). Q–V. Oogonia with plerotic oospores and amphigynous unicellular antheridia, formed in fgCA in mating tests. Images: A, C, G, J, M, N, P. Ex-type CBS 149636; B. SU658; D, E. JP1585; F. KA501; H. PA205; K, O. PA201; I, L. SU1087; Q, R. JP1538 × JP1364; S, T, V. CBS 149636 × VN1028; U. SL323 × VN1028. Scale bars = 20 µm; V applies to A–K, M–V.
Etymology: The name refers to the hybrid status and the origin of most known isolates in the south of Asia (australis Latin = southern).
Typus: Vietnam, Cuc Phuong National Park, isolated from mixed rhizosphere soil of Allophylus cobbe, Ficus sp. and Homalium sp. in tropical lowland rainforest, Mar. 2016, T. Jung, & N.M. Chi (holotype CBS H-25191, dried culture on V8A, ex-holotype living culture CBS 149636 = VN812).
Morphological structures on V8A: Sporangia infrequently observed on solid agar and abundantly produced in non-sterile soil extract; borne mostly terminally (96.7 %) in dense or lax sympodia of 2–6 sporangia (Fig. 18L, M) or rarely on unbranched long or short sporangiophores, less frequently intercalary or sessile (3.3 %; Fig. 18F, G); mostly ovoid, broad-ovoid or elongated ovoid (61.8 %; Fig. 18A, B, I, K, L), less frequently distorted with often two or sometimes three apices or bilobed (16.2 %; Fig. 18G, H), limoniform to elongated limoniform (9 %; Fig. 18C, M), obpyriform to elongated-obpyriform (7.1 %; Fig. 18D, E, J), ellipsoid to elongated ellipsoid (2.6 %; Fig. 18F), obovoid (2.3 %), ampulliform (0.5 %), pyriform (0.3 %), mouse-shaped (0.1 %) or sickle-shaped (0.1 %); lateral attachment of sporangiophores (25.6 %; Fig. 18A, B, D, I, J) and a conspicuous basal plug (48.8 %; Fig. 18A, C, G, J–L) commonly observed; sometimes with a vacuole (Fig. 18I) or with sporangiophores widening towards the sporangial base (0.5 %; Fig. 18A); predominantly (74.8 %) with pedicels of variable length (av. 16.4 ± 9.4 µm; range 2.0–70.4 µm; Fig. 18A–D, I, J, L) and often caducous (Fig. 18I, J), but 25.2 % of sporangia without pedicel and persistent (Fig. 18E, F, M); sporangial apices on solid agar mostly papillate, but in water variable ranging from papillate (30.1 %; Fig. 18A, E) and semipapillate (68.7 %; Fig. 18B–D, F, G, I, L) to nonpapillate (1.2 %; Fig. 18J); sporangial proliferation exclusively external (Fig. 18A, C–E, K–M), sometimes with 2 or 3 sporangiophores arising from the same point close to a sporangial base (Fig. 18M); sporangial dimensions averaging 62.4 ± 8.6 × 36.9 ± 5.0 µm (overall range 31.0–100.8 × 20.2–69.0 µm; range of isolate means 49.1–75.0 × 29.1–41.2 µm) with a length/breadth ratio of 1.7 ± 0.23 (overall range 0.67–3.7); sporangial germination indirectly with zoospores discharged through an exit pore 3.4–10.4 µm wide (av. 6.0 ± 0.9 µm) (Fig. 18K). Zoospores limoniform to reniform whilst motile (Fig. 18N), becoming spherical (av. diam = 10.3 ± 0.9 µm) on encystment; sometimes unusually large and multinucleate (Fig. 18N); cysts germinating directly by forming a hypha or a microsporangium or infrequently indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings rarely formed in water on sporangiophores, subglobose to globose, limoniform or irregular; diam 13.7 ± 4.6 µm. Chlamydospores rarely produced in single culture but commonly produced in mating tests between A1 and A2 mating type isolates; globose (85 %) or subglobose (15 %), borne intercalary, sessile or terminal, with one or multiple lipid globules (Fig. 18O, P), sometimes with radiating hyphae (15 %; Fig. 18O); diam 36.9 ± 4.7 µm (range 28.0–47.6 µm); walls often turning golden-brown during maturation (Fig. 18P); wall diam 1.1 ± 0.5 µm (range 0.4–2.1 µm). Oogonia not observed in single culture, but commonly produced in mating tests between A1 and A2 mating type isolates (‘heterothallic’ breeding system; 35 tested isolates from Japan, Java, Kalimantan, Panama, Sulawesi, Sumatra and Vietnam belonging to the A1 mating type and 6 isolates from Japan and Vietnam to the A2 mating type); globose to slightly subglobose, smooth-walled (Fig. 18Q–U) or rarely with a thicker, slightly wavy wall (Fig. 18V), mostly sessile with short to medium-sized, often thin stalks and rounded base (76.9 %; Fig. 18S–V), less frequently with short tapering base (23.1 %; Fig. 18Q, R); sometimes oogonial wall turning golden-brown during maturation (Fig. 18V); oogonial diam 27.1 ± 2.9 µm (overall range 16.1–38.4 µm; range of means in different mating combinations 25.0–31.2 µm); nearly plerotic to plerotic (Fig. 18Q–V). Oospores globose with one large lipid globule (Fig. 18Q–V); diam 24.5 ± 2.7 µm (overall range 14.8–34.6 µm; range of means in different mating combinations 22.2–28.4 µm); wall thickness 1.22 ± 0.28 µm (overall range 0.6–2.59 µm), oospore wall index 0.27 ± 0.05; high abortion rate of 76.6 % (48–66 %) after 4 wk. Antheridia exclusively amphigynous, cylindrical, subglobose or irregular, and unicellular (Fig. 18Q–V); dimensions 15.2 ± 2.5 × 14.1 ± 1.7 µm.
Culture characteristics: Colonies variable between isolates; on V8A submerged to appressed with limited aerial mycelium and radiate or stellate patterns; on CA either submerged to appressed with limited aerial mycelium and radiate to striate pattern, or appressed with limited woolly mycelium and faint radiate pattern or uniform; on PDA either appressed, dense felty and uniform with stoloniferous margin or woolly-cottony and petaloid (Fig. 10).
Fig. 10.
Colony morphology of Phytophthora species from subclade 2a after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A–D. Phytophthora ×australasiatica (A. ex-type CBS 149636; B. JP1364; C. PA205; D. SU1084). E. Phytophthora ×lusitanica (ex-type CBS 150256). F. Phytophthora ×taiwanensis (ex-type CBS 149506).
Cardinal temperatures and growth rates: On V8A optimum at 25 °C (9 tested isolates) or 27.5 °C (21 isolates) with 8.12 ± 1.32 and 8.11 ± 1.44 mm/d radial growth, respectively; maximum 30–<32.5 °C (10 isolates) or 32.5–<35 °C (18 isolates); minimum <10 °C (Fig. 12), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 6.64 ± 0.89 mm/d, 4.98 ± 0.53 mm/d and 3.84 ± 0.58 mm/d, respectively.
Additional materials examined: Indonesia, Java, Bandung area, isolated from a naturally fallen, necrotic leaf of an unidentified tree species collected from the ground in a tropical rainforest, Feb. 2019, T. Jung & M. Junaid (JV045); Kalimantan, Balikpapan area, isolated from a naturally fallen, necrotic leaf of an unidentified tree species floating in a stream running through a lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (KA229); isolated from rhizosphere soil of Gmelina sp. in a lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (KA501); Sulawesi, Palanro, isolated from rhizosphere soil of Mangifera indica and Szygium sp. in a tropical lowland rainforest, May 2019, T. Jung & M. Junaid (SL323); Sumatra, Padang area, isolated from naturally fallen, necrotic leaves of unidentified tree species floating in a stream running through a tropical rainforest, Sep. 2018, T. Jung, M. Tarigan & T. Corcobado (SU637, SU658); isolated from rhizosphere soil of unidentified tree species in a tropical rainforest, Sep. 2018, T. Jung, M. Tarigan & T. Corcobado (SU1080, SU1084, SU1087, SU1093, SU1260). Japan, Amami-Ōshima, Sumiyou, isolated from a naturally fallen, necrotic leaf of an unidentified tree species floating in a stream running through a subtropical monsoon forest, Nov. 2018, T. Jung, K. Kageyama & M. Horta Jung (JP1364); Okinawa, isolated from naturally fallen, necrotic leaves of unidentified tree species floating in streams running through subtropical monsoon forests, Nov. 2018, T. Jung, A. Hieno, H. Masuya & S. Uematsu (JP1508, JP1538, JP1585). Panama, Herrera, El Montoso Reserva Forestal, isolated from naturally fallen, necrotic leaves of unidentified tree species collected from the ground in a tropical lowland rainforest, Nov. 2019, K. Broders & Y. Balci (PA201, PA202, PA203, PA205, PA330, PA332, PA334). Vietnam, Cuc Phuong National Park, isolated from mixed rhizosphere soil of Allophylus cobbe, Ficus sp. and Homalium sp. in a tropical lowland rainforest, Mar. 2016, T. Jung, N.M. Chi & M. Horta Jung (VN763, VN764, VN788, VN793, VN807, VN808, VN809, VN810, VN811, VN813, VN814, VN815, VN816, VN817, VN1086, VN1087, VN1088); Côn Đảo National Park, Côn Lôn Island, isolated from rhizosphere soil of Canarium album, Hopea sp. and Leucaena leucocephala in a tropical lowland rainforest, April 2017, N.M. Chi (VN1028, VN1116, VN1117, VN1118, VN1119).
Phytophthora ×lusitanica T. Jung, M. Horta Jung, C. Maia & I. Milenković, sp. nov. MycoBank MB 849628. Fig. 19.
Fig. 19.
Phytophthora ×lusitanica. A–O. Semipapillate to papillate sporangia formed on V8-agar flooded in extract. A–F, K–O. Ovoid, obovoid, limoniform or obpyriform sporangia. B–E, H, M, N. Medium-length to long pedicels (arrows). B. Hyphal swelling. C, D. External proliferation. G–J. Distorted sporangia with 2–4 apices. K. Intercalary sporangium. L. Zoospore release. M, N. Caducous sporangia. O. Dense sporangial sympodium. Images: A–C, G, I, K, N, O. Ex-type CBS 150256; D. BD517; E, F, H, J, L, M. BD516. Scale bars = 20 µm; N applies to A–N.
Etymology: The name refers to the hybrid status and the origin of all known isolates from Portugal (lusitanica Latin = from Lusitania which is the old Roman name for Portugal).
Typus: Portugal, Algarve, Tavira, isolated from a baiting leaf floating in Rio Séqua, Jul. 2011, T. Jung & M. Horta Jung (holotype CBS H-25293, dried culture on V8A, ex-holotype living culture CBS 150256 = BD518).
Morphological structures on V8A: Sporangia infrequently observed on solid agar and abundantly produced in non-sterile soil extract; borne predominantly terminally (99 %) on unbranched long or short sporangiophores (Fig. 19F, H) or in dense or lax sympodia of 2–5 sporangia (Fig. 19O), or rarely intercalary (1 %; Fig. 19K); mostly ovoid, broad-ovoid or elongated-ovoid (40.9 %; Fig. 19A, B, L, N, O), distorted with often two or three or rarely four apices (22.6 %; Fig. 19G–J) or obpyriform to elongated-obpyriform (18.5 %; Fig. 19E, F, M), less frequently limoniform to elongated-limoniform (9 %; Fig. 19C, O), obovoid (5 %; Fig. 19D) or ampulliform (1 %); lateral attachment of sporangiophores (38 %; Fig. 19A, E, G, H, N) and pedicels (51.3 %) of variable length (av. 18.8 ± 10.7 µm; range 3.2–50.6 µm; Fig. 19B–E, H, M, N) common; infrequently caducous (1.9 %; Fig. 19M, N); seldom with a conspicuous basal plug (1 %; Fig. 19L); sporangiophores sometimes widening towards the sporangial base (1.9 %; Fig. 19D); in water semipapillate to papillate (Fig. 19A–K, M–O); sporangial proliferation exclusively external (Fig. 19C, D, O); sporangial dimensions averaging 53.9 ± 8.6 × 32.5 ± 4.7 µm (overall range 34.1–79.8 × 22.5–48.3 µm; range of isolate means 48.1–61.5 × 29.9–37.5 µm) with a length/breadth ratio of 1.67 ± 0.27 (overall range 1.15–2.43); sporangial germination indirectly with zoospores discharged through an exit pore 4.5–7.5 µm wide (av. 5.9 ± 0.6 µm) (Fig. 19L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.6 ± 0.9 µm) on encystment. Hyphal swellings occasionally formed in water on sporangiophores, often close to the sporangial base, subglobose to globose, limoniform, ovoid or irregular (Fig. 19B); diam 11.6 ± 3.2 µm (range 5.3–18.3 µm). Chlamydospores or hyphal aggregations not observed. Oogonia not observed in single culture; in mating tests with A1 and A2 mating type isolates of P. meadii all four tested isolates stimulated abundant production of oogonia in the A1 isolate MYA-4042 of P. meadii and, hence, were a silent A2 mating type.
Culture characteristics: Colonies on V8A and CA mostly submerged with scanty aerial mycelium and radiate pattern; on PDA dense felty with limited aerial mycelium, irregular margins and a faint petaloid pattern (Fig. 10).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 8.65 ± 1.17 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 12), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 6.4 ± 1.04 mm/d, 4.55 ± 0.57 mm/d and 2.55 ± 0.47 mm/d, respectively.
Additional materials examined: Portugal, Algarve, Tavira, isolated from a baiting leaf floating in Rio Séqua, Jul. 2011, T. Jung & M. Horta Jung (BD515, BD516, BD517).
Phytophthora ×taiwanensis T. Jung, T.-T. Chang, H.-S. Fu & M. Horta Jung, sp. nov. MycoBank MB 847288. Fig. 20.
Fig. 20.
Phytophthora ×taiwanensis. A–K. Sporangia formed on V8-agar in soil extract. A–D, H–K. Elongated ovoid, obpyriform, pyriform, limoniform or ellipsoid sporangia. E–G. Distorted bi- or trilobed sporangia. A–C, E–I, L. Semipapillate to papillate apices. D. Nonpapillate apex. A, C, D, G–J. Medium-length to long pedicels (arrow in A). B, E, F. External proliferation. G, H. Caducous sporangia. J. Zoospore release. K. Dense sporangial sympodium. L. Multinucleate zoospores. M, N. Chlamydospores formed in carrot agar (fgCA). O–V. Oogonia with near-plerotic to plerotic oospores and amphigynous antheridia, formed in fgCA in polycarbonate membrane mating tests. O, U, V. Bicellular antheridia. P–R. Unicellular antheridia. Images: A, B, E–G, I–K, G, M–O, Q, T–V. Ex-type CBS 149506; C, D. TW158; H, P, R, S. TW133; L. TW172. Scale bars = 20 µm; V applies to A–J, L–V.
Etymology: The name refers to the hybrid status and the origin of all known isolates in Taiwan.
Typus: Taiwan, Hualien County, isolated from rhizosphere soil of Styrax suberifolia in a subtropical, evergreen lowland monsoon forest, Aug. 2013, T. Jung & T.-T. Chang (holotype CBS H-25130, dried culture on V8A, ex-holotype living culture CBS 149506 = TW161).
Morphological structures on V8A: Sporangia infrequently observed on solid agar and abundantly produced in non-sterile soil extract; borne almost exclusively terminally (99.4 %) in dense or lax sympodia of 2–6 sporangia (Fig. 20K) or less frequently on unbranched long or short sporangiophores, or rarely intercalary or sessile (0.6 %); ovoid to elongated-ovoid (31.7 %; Fig. 20A, K), obpyriform to elongated-obpyriform (19.3 %; Fig. 20B, C), limoniform to elongated-limoniform (18.2 %; Fig. 20I, K), distorted with often two or sometimes three or four apices (16.5 %; Fig. 20E–G), ellipsoid to elongated ellipsoid (8.4 %; Fig. 20D, J), less frequently mouse-shaped (3.1 %) or pyriform (2.8 %; Fig. 20H); lateral attachment of sporangiophores (16.6 %; Fig. 20B, C, E), vacuoles (14.4 %; Fig. 20B, C, K) and a curved apex (11.9 %; Fig. 20D) commonly observed; sometimes sporangiophores widening towards the sporangial base (1.2 %); predominantly (80.4 %) with pedicels of variable length (av. 15.4 ± 5.9 µm; range 4.8–58.0 µm; Fig. 20A, C, D, G–I) and often caducous (Fig. 20G–I), but 19.6 % of sporangia without pedicel and persistent (Fig. 20B, E); sporangial apices on solid agar exclusively papillate, but in water variable ranging from papillate (40.7 %; Fig. 20A, E, F) and semipapillate (50.1 %; Fig. 20B, C, G–I, K) to nonpapillate (10.2 %; Fig. 20D); sporangial proliferation exclusively external (Fig. 20B, E, F, K); sporangial dimensions averaging 61.9 ± 11.0 × 31.9 ± 5.0 µm (overall range 23.7–123.1 × 17.3–52.1 µm; range of isolate means 45.1–79.5 × 26.5–42.3 µm) with a length/breadth ratio of 1.95 ± 0.32 (overall range 1.21–3.38); sporangial germination indirectly with zoospores discharged through an exit pore 3.4–9.9 µm wide (av. 6.3 ± 1.2 µm) (Fig. 20J). Zoospores limoniform to reniform whilst motile (Fig. 20L), becoming spherical (av. diam = 11.7 ± 1.2 µm) on encystment; sometimes with multiple nuclei (Fig. 20L); cysts germinating directly by forming a hypha or a microsporangium. Hyphal swellings rarely formed in water on sporangiophores, subglobose to globose, limoniform or irregular; diam 12.6 ± 2.0 µm. Chlamydospores rarely produced in single culture but infrequently produced in mating tests with tester strains of P. meadii; globose to subglobose, borne intercalary, sessile or terminal, with one or multiple lipid globules (Fig. 20M, N); diam 30.8 ± 3.5 µm (range 27.3–34.3 µm). Oogonia not observed in single culture, but commonly produced in mating tests with the A2 mating type isolate MYA-4043 of P. meadii (‘heterothallic’ breeding system; all 18 tested isolates belonging to the A1 mating type); globose to slightly subglobose, smooth-walled, mostly sessile with short to medium-sized, often thin stalks and rounded base (87.4 %; Fig. 20O–S, U, V), less frequently with short tapering base (12.6 %; Fig. 20T); sometimes appearing comma-shaped due to an angle between the oogonial stalk and the bearing hypha (Fig. 20V); oogonial diam 25.6 ± 2.1 µm (overall range 20.0–30.9 µm; range of means in different mating combinations 23.2–26.3 µm); nearly plerotic to plerotic (Fig. 20O–V). Oospores globose, usually with one large lipid globule (Fig. 20O–R, T–V) or infrequently with multiple smaller globules (4.4 %; Fig. 20S); diam 22.9 ± 1.9 µm (overall range 18.1–29.1 µm; range of means in different mating combinations 20.6–23.8 µm); wall thickness 1.19 ± 0.21 µm (overall range 0.72–2.13 µm), oospore wall index 0.28 ± 0.04; high abortion rate of 67 % (42–96 %) after 4 wk. Antheridia exclusively amphigynous, cylindrical or subglobose, predominantly unicellular (95.5; Fig. 20P–T), rarely bicellular and slightly curved (4.5 %; Fig. 20O, U, V); dimensions 13.3 ± 2.0 × 12.5 ± 1.5 µm.
Culture characteristics: Colonies variable between isolates; on V8A submerged to appressed with limited aerial mycelium and radiate or stellate patterns; on CA either submerged to appressed with limited aerial mycelium and radiate to striate pattern, or appressed with limited woolly mycelium and faint radiate pattern or uniform; on PDA either appressed, dense felty and uniform with stoloniferous margin or woolly-cottony and petaloid (Figs 10, 11).
Fig. 11.
Colony morphology of Phytophthora species from subclade 2a after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A, B. Phytophthora ×taiwanensis (A. TW168; B. TW248). C–F. Phytophthora ×vanyenensis (C. JV014; D. JV009; E. SL318; F. SU631a).
Cardinal temperatures and growth rates: On V8A optimum at 25 °C (4 isolates), 27.5 °C (4 isolates) or 30 °C (6 isolates) with 8.23 ± 0.95, 8.42 ± 1.17 and 8.33 ± 1.17 mm/d radial growth, respectively; maximum 30–<32.5 °C (2 isolates) or 32.5–<35 °C (12 isolates); minimum <10 °C (Fig. 12), lethal temperature 32.5 °C (1 isolate), 35 °C (10 isolates) or >35 °C (3 isolates). At 20 °C on V8A, CA and PDA 6.68 ± 0.85 mm/d, 4.98 ± 0.62 mm/d and 4.68 ± 0.78 mm/d, respectively.
Additional materials examined: Taiwan, Hualien County, isolated from rhizosphere soil of S. suberifolia in a subtropical, evergreen lowland monsoon forest, Aug. 2013, T. Jung, T.-T. Chang & M. Horta Jung (TW158, TW162, TW163); isolated from rhizosphere soil of Cinnamomum camphora in a subtropical, evergreen lowland monsoon forest, Aug. 2013, T. Jung, T.-T. Chang & M. Horta Jung (TW165, TW167, TW168, TW172, TW173, TW379); isolated from mixed rhizosphere soil of a subtropical, evergreen lowland monsoon forest, Aug. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW248, TW249); isolated from a baiting leaf floating in Xiao-Qingshui Creek running through a subtropical, evergreen lowland monsoon forest, Aug. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW133, TW135); Taitung County, isolated from rhizosphere soil of Trema orientalis in a subtropical, evergreen lowland monsoon forest, Aug. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW242, TW243, TW244).
Notes on Clade 2a taxa: Across the nuclear (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) 8 754-character alignment and the mitochondrial (cox1, cox2, nadh1 and rps10) 3 174-character alignment pairwise sequence differences between the 11 known and six newly described Phytophthora species and five informally designated taxa in Clade 2a were 0.1–3.9 % and 0–6.3 %, respectively. In addition, the six new and the seven known Clade 2a species examined (P. botryosa, P. citrophthora, P. colocasiae, P. meadii, P. mekongensis, P. occultans and P. ×vanyenensis) developed distinctive colony morphologies on V8A, CA and PDA at 20 °C (Figs 8–11). In addition, the five new species can be separated from each other and from other Clade 2a species by a combination of morphological (Figs 13–22) and physiological characters (Fig. 12) of which the most discriminating are highlighted in bold in Tables S4–S6.
Fig. 22.
Phytophthora ×vanyenensis. A–M. Sporangia formed on V8-agar (V8A) in soil extract. A–I. Ovoid, ellipsoid or obpyriform sporangia with papillate or semipapillate apices. A–C, F–I. Medium length to long pedicels. A, C, D, F. External proliferation (arrow in C). C. Intercalary sporangium. H, I. Caducous sporangia. J, M. Zoospore release. K. Distorted sporangium with three apices and zoospore cysts. L. Sympodium with a nonpapillate sporangium. M. Beginning sporangial sympodium. N. Hyphal swelling in water. O. Hyphal aggregation in V8A. P. Chlamydospore in carrot agar (fgCA). Q–V. Oogonia with oospores and amphigynous unicellular antheridia formed in fgCA in mating tests. T–V. Elongated or excentric oogonia. Images: A, B, K. SU1615; C, O. SU631a; D, E, I, L, M. SL318; F, G. JV102; H, P. JV042; J. SU1055; N. SU1099; Q–S, U. SL075 × SL478; T. JV004 × CBS 235.30; V. SL075 × SL327. Scale bars = 20 µm; V applies A–K, M–V.
The ex-type isolate CBS 581.69 and key isolate MYA-4059 of P. botryosa produced larger sporangia than originally reported by Chee (1969) (39.1 × 22.6 vs. 28 × 15 µm). Furthermore, they were only partly caducous and had variable apices (12 % papillate, 42 % semipapillate, 36 % nonpapillate) while those in the original description were fully caducous and exclusively papillate (Table S4; Fig. 21). These discrepancies most likely reflect differences between sporangia formed in water (this study) and sporangia produced on solid agar (Chee 1969).
Fig. 21.
Phytophthora botryosa. A–F. Ovoid, limoniform, pyriform and ellipsoid sporangia with medium-length to long pedicels formed on V8-agar in soil extract. A–F, I, J, N, P, S, V. Nonpapillate sporangia. G, H, L, M, O, Q, R, W. Semipapillate sporangia. K, T. Papillate sporangia. H. Intercalary sporangium with external proliferation. P–X. Caducous sporangia. U. Zoospore release. Images: A, D, H–L, P–U. Ex-type CBS 581.69; B, C, E–G, M–O, V–X. MYA-4059. Scale bar = 20 µm; V applies to A–X.
Phytophthora meadii key isolates MYA-4043 and MYA-4042 used in the present study originate from India like the isolates McRae (1918) based the original description on and like the ex-neotype isolate CBS 148127 designated by Abad et al. (2023a). The sporangia formed by isolates MYA-4043 and MYA-4042 (44.5 × 30.3 µm; Table S5) were on average slightly larger than those reported by Oudemans & Coffey (1991) (38.5 × 24.7 µm; Table S4) for seven isolates and considerably larger than in the original description by McRae (1918) (20–44 × 16–29 µm) and those reported by Abad et al. (2023a) (17–44 × 15–29 μm) for the ex-neotype isolate CBS 148927. However, sporangial l/b ratios and pedicel lengths were comparable between this study and Oudemans & Coffey (1991). The breeding system of P. meadii is still a conundrum. While McRae (1918) described P. meadii as ‘homothallic’ later studies found evidence of heterothallism (Peries & Dantanarayana 1965; Rajalakshmy et al. 1985, Sansome et al. 1990, Abad et al. 2023a). In a mating study, 45 of 50 Indian rubber isolates were described as ‘heterothallic’ (A1/A2) with 31 and 14 belonging to the A1 and A2 mating type, respectively; two isolates were described as ‘homothallic’ (possibly weakly self-fertile A2s as described in Sansome et al. 1990), and three isolates were sterile (Rajalakshmy et al. 1985). Sizes and morphological features of oogonia produced in a mating test between isolates A1 and A2 isolates MYA-4042 and MYA-4043 were comparable to those reported by Rajalakshmy et al. (1985) and Erwin & Ribeiro (1996), and their optimum and maximum temperatures for growth were in accordance with Erwin & Ribeiro (1996) (Tables S4, S5). Sansome et al. (1990) described both diploidy and polyploidy in A1 and A2 isolates of P. meadii from Sri Lanka, the former associated with meiotic instability, sterility and smaller oogonia.
Despite being one of the oldest known and most widespread Phytophthora species P. citrophthora is still poorly characterised, probably reflecting the erroneous inclusion of other species with diverging characters during the pre-molecular era. Using isozyme patterns Mchau & Coffey (1994) provided a redescription of the species based on a worldwide collection of 77 isolates. However, their isozyme analysis demonstrated that Indonesian and Brazilian isolates from cocoa (Theobroma cacao) trees constituted distinct electrophoretic subgroups CTR2 and CTR3 which were phylogenetically separate from the largest electrophoretic subgroup CTR1 from diverse hosts and geographical locations. Recently, the Brazilian cocoa isolates have been described as P. theobromicola residing within Clade 2b (Decloquement et al. 2021). As the morphometric and physiological data provided by Mchau & Coffey (1994) were not assigned to their subgroups they cannot be used for species comparisons. The morphological characters, the morphometric data and the cardinal temperatures for growth of the 13 P. citrophthora isolates in this study were largely in agreement with the data given by Abad et al. (2023a) for the ex-epitype isolate CBS 950.87 except for the production of chlamydospores by the ex-epitype. In the present study “P. citrophthora-like” isolates from a wide range of hosts, ecosystems and locations formed three separate phylogenetic groups. The group containing the ex-epitype isolate of P. citrophthora from Citrus in California, Citrus isolates from Portugal and Spain and a range of isolates from natural ecosystems in warm-temperate and subtropical regions of Japan and Taiwan is considered to be the original P. citrophthora. Another group, comprising isolates from diverse hosts in nurseries and gardens in Europe and Morocco and streams in Portugal, Serbia and Louisiana, USA, is described here as P. pseudocitrophthora. The third group, a hybrid between P. citrophthora as the maternal parent and another Clade 2a species as the paternal parent, was only obtained from the Rio Séqua in the South of Portugal and is described here as P. ×lusitanica. The sporangia of the three species are similar, with a low degree of caducity in most isolates (Tables S4–S6; Figs 13, 15, 19). However, the proportions of papillate, semipapillate and nonpapillate sporangia differ considerably between P. citrophthora (36 %, 61.1 % and 2.9 %, respectively) and P. pseudocitrophthora (75.9 %, 14.6 % and 9.5 %, respectively) while the sporangia of P. ×lusitanica are mostly a transition between semipapillate and papillate (Figs 12, 14, 18). Furthermore, in P. citrophthora sporangia are on average slightly longer (66.0 µm) than in P. pseudocitrophthora (61.6 µm) and much longer than in P. ×lusitanica (53.9 µm) with a higher l/b ratio (1.84 vs. 1.73 vs. 1.67), form frequently larger sympodia (2–8 vs. 2–4 vs. 2–4 sporangia) and have a higher frequency of pedicels (86 % vs. 37 % vs. 51.3 %). In addition, P. ×lusitanica has higher proportions of sporangia with two, three or four apices (22.6 %) than P citrophthora (9 %) and P. pseudocitrophthora (7.4 %). Phytophthora citrophthora, P. pseudocitrophthora and P. ×lusitanica are self-sterile, failing to produce oogonia in mating tests with A1 and A2 tester strains of P. cinnamomi and P. meadii. However, two of the 13 tested isolates of P. citrophthora and all four tested isolates of P. ×lusitanica are silent A1s and one of the nine tested isolates of P. pseudocitrophthora is a silent A2 mating type. The large subgroup CTR1 in Mchau & Coffey (1994) was also self-sterile, with 15 of 56 isolates being silent A2s. Phytophthora peudocitrophthora has a slightly lower optimum temperature of growth than P. citrophthora and P. ×lusitanica (25 vs. 27.5 vs. 27.5 °C) and shows on average slightly faster growth between 10 and 30 °C than P. citrophthora whereas P. ×lusitanica grows faster than the other two species between 25 and 30 °C (Tables S4–S6; Fig. 12). Like subgroup CTR1 of Mchau & Coffey (1994), none of the 28 isolates of P. citrophthora, P. pseudocitrophthora and P. ×lusitanica examined in this study produced chlamydospores.
Crous et al. (2017) reported that no gametangia were produced in mating tests between P. mekongensis and A1 and A2 tester strains of P. nicotianae and P. citrophthora and concluded that P. mekongensis has a sterile breeding system. However, in this study, the ex-type isolate CBS 135136 and isolate PF6f2 of P. mekongensis both produced abundant oogonia when mated with A1 isolate MYA-4042 of P. meadii demonstrating it has an A1/A2 breeding system and that they are all of A2 mating type. Since the abortion rate of these selfed oogonia was 100 % and no A1 isolates of P. mekongensis are currently known it remains unclear whether the A1/A2 system is fully functional.
Phytophthora pseudoccultans can be discriminated from P. occultans by having on average considerably larger sporangia (57.9 × 36.0 vs. 47 × 30 µm) which are exclusively papillate, the production of hyphal swellings, a lower maximum temperature for growth (27.5–<30 vs. 30–<32.5 °C) and by showing slower growth between 20 and 27.5 °C (Man In’t Veld et al. 2015; Table S5; Fig. 12). Phytophthora terminalis differs from P. pseudoccultans by its considerably smaller sporangia (41.7 × 30.4 µm) and sporangial l/b ratio (1.37 vs. 1.62) (Man In’t Veld et al. 2015; Table S5).
Phytophthora vietnamensis can be distinguished from all other self-sterile species in Clade 2a by having a fully sterile breeding system and a low optimum temperature for growth of 20 °C (Tables S4–S6, Fig. 12). Phytophthora ×australasiatica can be easily discriminated from other self-sterile species in Clade 2a by having considerably larger sporangia than P. botryosa, P. insulinativitatica, P. meadii, P. mekongensis and P. multibullata and larger oogonia than P. multibullata (Crous et al. 2017, Dang et al. 2021; Tables S4–S6). Both P. ×australasiatica and P. ×taiwanensis differ from P. citrophthora, P. pseudocitrophthora, P. vietnamensis and P. ×lusitanica by having functional A1/A2 breeding systems (Tables S4–S6; Figs 13, 15, 17–20); from the exclusively semipapillate and host specific P. colocasiae by producing on average 30 % and 41 % papillate sporangia, respectively, and by their different and wider host ranges (Erwin & Ribeiro 1996; Tables S4, S6); from P. ×vanyenensis by having on average higher sporangial l/b ratios and larger chlamydospores, and the high proportion of excentric and elongated oogonia in P. ×vanyenensis (Table S6; Figs 18, 20, 22); and from P. multibullata by their considerably larger sporangia and faster growth (Dang et al. 2021; Tables S5, S6). Phytophthora ×australasiatica can be distinguished from P. ×taiwanensis by its higher proportion of ovoid sporangia (62 vs. 32 %), its lower sporangial l/b ratio (1.7 vs. 1.95) and by not producing bicellular antheridia (Table S6; Figs 18, 20). The morphological data and cardinal temperatures of the 35 isolates of P. ×vanyenensis in this study largely concurred with the original description (Dang et al. 2021). However, in the present study, P. ×vanyenensis produced chlamydospores (which were particularly abundant in mating tests) and exhibited a considerably faster growth rate (Table S6).
Several new Clade 2a species described here were previously known under informal names, i.e., P. pseudoccultans as P. taxon occultans-like (Jung et al. 2017b), P. vietnamensis as P. taxon meadii-like 1 (Jung et al. 2020), P. ×australasiatica as P. taxon botryosa-like 2 and P. taxon meadii-like 2 (Jung et al. 2020) and P. ×citrophthora-related2 (Van Poucke et al. 2021), P. ×lusitanica as P. ×citrophthora2 (Van Poucke et al. 2021), and P. ×taiwanensis as P. taxon ×botryosa-like and P. taxon ×meadii-like (Jung et al. 2017b) and P. ×citrophthora-related1 (Van Poucke et al. 2021).
Clade 2b
For all Clade 2b species included in this study colony morphologies on CA, PDA and V8A and temperature-growth relations on V8A are presented in Figs 23–26. Morphological and physiological characters and morphometric data of the nine known and nine newly described species and three informally designated taxa in Clade 2b are given in the comprehensive Tables S7–S9. The known species P. tropicalis was included in the morphological and temperature-growth studies and a taxonomic description is given below to enable detailed comparisons with new closely related species from Clade 2b.
Fig. 23.
Colony morphology of Phytophthora species from subclade 2b after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora calidophila (ex-type CBS 149479). B. Phytophthora distorta (ex-type CBS 149476). C. Phytophthora frigidophila (ex-type CBS 149486). D. Phytophthora mengei (TJ1109). E. Phytophthora montana (ex-type CBS 149492). F. Phytophthora pyriformis (PA214).
Fig. 26.
Mean radial growth rates of five known and nine new Phytophthora species from subclade 2b on V8-agar at different temperatures: P. calidophila (8 isolates); P. distorta (4 isolates); P. frigidophila (4 isolates); P. mengei (3 isolates); P. montana (5 isolates); P. multiplex (11 isolates); P. obovoidea (22 isolates); P. pyriformis (6 isolates); P. siskiyouensis (1 isolate); P. tropicalis (12 isolates); P. valdiviana (5 isolates); P. variepedicellata (5 isolates); P. taxon pseudocapsici (6 isolates).
Phytophthora calidophila T. Jung, Y. Balci, L. Garcia & B. Mendieta-Araica, sp. nov. MycoBank MB 847289. Fig. 27.
Fig. 27.
Phytophthora calidophila. A–N. Sporangia formed on V8-agar (V8A) in soil extract. A–I, K–N. Ovoid, ellipsoid, obovoid, obpyriform, pyriform and limoniform sporangia. A–I, L–N. Medium-length to long pedicels (arrows in E, H, I pointing at basal pedicel septum). A–G, I–M. Nonpapillate to semipapillate apices. H, M. Papillate apices. A, F, H. External proliferation (arrow in A). J. Bipapillate sporangium. K. Intercalary sporangium. L–N. Caducous sporangia. O–Y. Oogonia with near-plerotic to plerotic oospores and amphigynous unicellular antheridia, formed in carrot agar in polycarbonate membrane mating tests. Z. Hyphal swelling in water. AA. Hyphal aggregation in V8A. Images: A–G, I, K, M, O, R–T, X, Y, AA. Ex-type CBS 149479; H, J, L, N, P, Q, U–W, Z. NI111. Scale bar = 20 µm; AA applies to A–AA.
Etymology: The name refers to the high maximum temperature for growth and the fast growth at 30 °C (calidus Latin = warm, hot; philos Greek = friend, loving).
Typus: Nicaragua, Matagalpa, Selva Negra, isolated from a necrotic lesion on a naturally fallen leaf of a non-identified tree species collected from the ground in a lower montane tropical forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (holotype CBS H-25101, dried culture on V8A, ex-holotype living culture CBS 149479 = NI112).
Morphological structures on V8A: Sporangia abundantly produced in non-sterile soil extract; borne terminally in lax sympodia of 2–4 sporangia or on unbranched sporangiophores (99.2 %; Fig. 27A–I) or rarely intercalary (0.8 %; Fig. 27K); ovoid, broad-ovoid or elongated-ovoid (38.0 %; Fig. 27A, B, H), ellipsoid to elongated-ellipsoid (16.6 %; Fig. 27C–E, K, L), pyriform or elongated-pyriform (15.4 %; Fig. 27M), limoniform to elongated-limoniform (14.2 %; Fig. 27F, N), obovoid (10.8 %; Fig. 27G) or less frequently obpyriform to elongated-obpyriform (2.6 %; Fig. 27I) or distorted, often with two apices (2.4 %; Fig. 27J); apices papillate (37 %; Fig. 27H, J, N), semipapillate (38 %; Fig. 27A, F, I, L, M) or nonpapillate and often pointed (25 %; Fig. 27B–E, G, K); lateral attachment of sporangiophores (5.6 %; Fig. 27D) or a swelling on the sporangiophore close to the sporangial base (2.4 %; Fig. 27B) infrequently observed; predominantly caducous (82 %; Fig. 27L–N) with pedicel length ranging from 8.0 to 52.4 µm (av. 24.3 ± 11.6 µm) (Fig. 27A, C–I, L–N); sporangial proliferation exclusively external (Fig. 27A, F, H); sporangial dimensions averaging 49.2 ± 5.6 × 27.5 ± 2.8 µm (overall range 32.0–66.9 × 20.7–33.8 µm; range of isolate means 47.5–51.0 × 26.3–28.5 µm) with a length/breadth ratio of 1.79 ± 0.18 (overall range 1.31–2.55; sporangial germination indirectly with zoospores discharged through an exit pore 2.4–6.3 µm wide (av. 4.2 ± 0.9 µm). Zoospores limoniform to reniform whilst motile becoming spherical (av. diam = 9.3 ± 1.3 µm) on encystment. Hyphal swellings subglobose, limoniform or irregular, infrequently formed in water on sporangiophores (Fig. 27B) or in solid agar on hyphae (Fig. 27Z), 11.6 ± 2.8 µm. Chlamydospores not observed. Oogonia not observed in single cultures, but abundantly produced by all eight tested isolates in polycarbonate mating tests with the A2 mating type isolate PA172 of P. multiplex (A1/A2 or ‘heterothallic’ breeding system; all tested isolates mating type A1); mostly sessile or terminal on short to medium-length, sometimes curved lateral hyphae, smooth-walled, globose to slightly subglobose (Fig. 27O–Y), sometimes slightly excentric (4 %; Fig. 27Y), with a short or medium-length tapering (57.4 %; Fig. 27O, R–T, W–Y) or non-tapering stalk (42.6 %; Fig. 27P, Q, U, V) which is sometimes inclined (Fig. 27U, V); oogonial diam 25.2 ± 2.8 µm (overall range 18.6–33.2 µm; range of isolate means 23.6–27.6 µm); nearly plerotic to plerotic (Fig. 27O–Y). Oospores globose with one or, less frequently, several medium-large lipid globules (Fig. 27O–Y); mean diam 22.6 ± 2.8 µm (overall range 10.5–30.9 µm; range of isolate means 21.1–24.8 µm); wall thickness 1.28 ± 0.16 µm (overall range 0.87–1.91 µm), oospore wall index 0.3 ± 0.04; abortion 32–56 % (av. 41 %) after 4 wk. Antheridia exclusively amphigynous and cylindrical or subglobose, sometimes asymmetric, unicellular (Fig. 27O–Y); dimensions 15.3 ± 2.8 × 13.9 ± 1.5 µm. Hyphal aggregations observed in all isolates (Fig. 27A, B).
Culture characteristics: Colonies on V8A and CA submerged to appressed with limited aerial mycelium and radiate patterns; dense-felty and shallow cottony on PDA with a rosaceous pattern (Fig. 23).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 10.7 ± 0.3 mm/d radial growth, maximum 32.5–<35 °C, minimum <10 °C (Fig. 26), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 7.96 ± 0.47 mm/d, 5.73 ± 0.14 mm/d and 5.33 ± 0.15 mm/d, respectively.
Additional materials examined: Nicaragua, Matagalpa, Selva Negra, isolated from necrotic lesions on naturally fallen leaves of a non-identified tree species collected from the ground in a lower montane tropical forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI11, NI198, NI199, NI200, NI201, NI202, NI203).
Phytophthora distorta T. Jung, A. Durán, E. Sanfuentes von Stowasser & M. Horta Jung, sp. nov. MycoBank MB 847290. Fig. 28.
Fig. 28.
Phytophthora distorta. A–J. Sporangia formed on V8-agar (V8A) in soil extract. A–I, J. Semipapillate to papillate sporangia. A, D, E, G, I, J. Ovoid, obpyriform and ampulliform sporangia. B, C, F, H. Distorted sporangia. A–C, F, H. External proliferation (arrows in B, F). A, F, H. Intercalary (arrows in A, H). C, F, J. Medium-length pedicels (arrow in J). D, G. Cytoplasm partially filling the sporangia. I. Zoospore release. J. Caducous sporangium. K–S. Oogonia with near-plerotic or aplerotic oospores and amphigynous antheridia, formed in solid V8A. R. Additional paragynous antheridia (arrows); left oogonium aborted. S. Aborted oogonium. T. Catenulate hyphal swellings in water. Images: A, C–F, H, J–T. Ex-type CBS 149476; B, G. CL296; I. CL295. Scale bar = 20 µm; T applies to A–T.
Etymology: The name refers to the distorted asymmetric shapes of many sporangia.
Typus: Chile, Valdivian region, isolated from a baiting leaf floating in the Valdivia River, Nov. 2014, T. Jung, A. Durán & E. Sanfuentes von Stowasser (holotype CBS H-25098, dried culture on V8A, ex-holotype living culture CBS 149476 = CL181).
Morphological structures on V8A: Sporangia occasionally formed in solid agar but abundantly produced in non-sterile soil extract; borne terminally (61.9 %) in dense or lax sympodia of 2–5 sporangia or on long or short unbranched sporangiophores, or intercalary (38.1 %; Fig. 28A, F, H); shapes distorted-asymmetric often with two or three apices (43.9 %; Fig. 28B, C, F–H), ovoid, broad ovoid or elongated ovoid (38.6 %; Fig. 28A, D, I, J), less frequently obpyriform to elongated obpyriform (7.5 %; Fig. 28E), limoniform (4.0 %), ellipsoid (1.5 %), obovoid (1.5 %), pyriform (1 %), turbinate (1 %) or ampulliform (1 %); lateral attachment of the sporangiophore (47 %; Fig. 28B, C, G, I, J) and pedicels (34 %; Fig. 28C, F, J) common; sporangia sometimes too big for the available cytoplasm and hence, not filled completely in the basal part, with a strong plug below the cytoplasm (15.2 %; Fig. 28D, G, H); apices papillate (33.7 %; Fig. 28E, H, J) or more frequently semipapillate (66.3 %; Fig. 28A–D, F, G) with a smooth transition between both forms; sporangial dimensions averaging 57.0 ± 8.0 × 40.1 ± 4.8 µm (overall range 41.1–97.0 × 26.2–51.7 µm; range of isolate means 53.4–61.1 × 37.8–41.2 µm) with a length/breadth ratio of 1.43 ± 0.2 (overall range 1.04–2.39); pedicel length 17.1 ± 15.3 µm (range 1.8–80.1 µm); sporangia formed on solid agar occasionally caducous; in water caducity not observed; sporangial proliferation exclusively external (Fig. 28A–C, F–H); sporangial germination indirectly with zoospores discharged through an exit pore of 4.0–8.9 µm (av. 6.4 ± 1.0 µm) (Fig. 28I). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.6 ± 1.1 µm) on encystment; cysts germinating mostly directly by producing hyphae or indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings commonly produced on sporangiophores and hyphae, globose to subglobose, pyriform, limoniform or irregular, often catenulate (Fig. 28T). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), on short, thin and mostly non-tapering stalks (Fig. 28K–S); smooth-walled, globose to subglobose with round base (Fig. 28K–S), sometimes slightly bend to comma-shaped (2 %; Fig. 28Q); av. oogonial diam 29.0 ± 2.5 µm with an overall range of 21.6–34.4 µm and a range of isolate means of 27.4–30.6 µm; predominantly slightly aplerotic to aplerotic (92 %; Fig. 28K–O, R) or infrequently nearly plerotic (8 %; Fig. 28P, Q). Oospores globose to subglobose with a large lipid globule, often turning golden-brown during maturation (Fig. 28K–R); av. diam 24.8 ± 1.8 µm with an overall range of 19.1–29.6 µm and a range of isolate means of 23.2–26.1 µm; wall diam 1.89 ± 0.22 µm (overall range 1.49–2.46 µm) and oospore wall index 0.39 ± 0.03; abortion 28–54 % (av. 41.8 %; Fig. 28R, S) after 4 wk. Antheridia amphigynous, cylindrical or subglobose, unicellular (Fig. 28K–S), sometimes with finger-like projections (Fig. 28M); occasionally with an additional paragynous antheridium (Fig. 28R); dimensions 16.8 ± 2.2 × 14.0 ± 1.5 µm.
Culture characteristics: Colonies on V8A and CA submerged and faintly striate; on PDA dense-felty with radiating raised lobes separated by trenches (Fig. 23).
Cardinal temperatures and growth rates: On V8A optimum 20.0 °C with 5.04 ± 0.54 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 26), lethal temperature 30 °C. On CA and PDA at 20 °C 4.55 ± 0.11 mm/d and 2.48 ± 0.33 mm/d, respectively.
Additional materials examined: Chile, Valdivian region, isolated from a baiting leaf floating in the Valdivia River, Nov. 2014, T. Jung, A. Durán & E. Sanfuentes von Stowasser (CL294, CL295, CL296).
Phytophthora frigidophila T. Jung, Y. Balci, K. Broders & I. Milenković, sp. nov. MycoBank MB 847291. Fig. 29.
Fig. 29.
Phytophthora frigidophila. A–M. Ovoid, pyriform, limoniform, obpyriform and mouse-shaped sporangia formed on V8-agar in soil extract. A–C, E–L. Papillate or semipapillate apices. A–L. Pedicels with variable length. A–D. External proliferation. F, H, I. Thick plugs below cytoplasm (arrows). G–I. Sporangia partially filled with cytoplasm. J–L. Caducous sporangia. M. Sympodium with nonpapillate (left) and papillate sporangium and hyphal swelling. N–W. Oogonia with aplerotic oospores and amphigynous unicellular antheridia, formed in solid carrot agar. V, W. Aborted oospores. X. Oogonium aborted before oospore formation. Images: A, E–G, I–W. Ex-type CBS 149486; B–D, H, X; PA215A. Scale bar = 20 µm; X applies to A–X.
Etymology: The name refers to the low cardinal temperatures for growth (frigidus Latin = cold, cool; philos Greek = friend, loving).
Typus: Panama, Volcano Baru, isolated from necrotic lesion on a naturally fallen leaf of a non-identified tree species collected from the ground in a tropical cloud forest, Nov. 2019, K.D. Broders & Y. Balci (holotype CBS H-25108, dried culture on V8A, ex-holotype living culture CBS 149486 = PA213A).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne mostly terminally (95.4 %; ) in dense or lax sympodia of 2–4 sporangia (Fig. 29M) or on unbranched sporangiophores or less frequently intercalary (3.2 %; Fig. 29E) or sessile (1.4 %; Fig. 29M); sporangia predominantly papillate (85.8 %; Fig. 29A, B, E, H–M), infrequently semipapillate (10.2 %; Fig. 29C, F, G) or nonpapillate (4 %; Fig. 29D, M), often with a pointed apex; mostly with pedicels (65.2 %; Fig. 29A–D, F–L) averaging 18.3 ± 13.7 µm in length (range 2.5–62.0 µm) and caducous (Fig. 29J–L); predominantly ovoid, broad ovoid or elongated ovoid (61.6 %; Fig. 29A, B, E, G, J–M), less frequently limoniform to elongated-limoniform (14.4 %; Fig. 29D, H, M), obpyriform to elongated-obpyriform (11.6 %; Fig. 29I), pyriform (3.6 %; Fig. 29C), ampulliform (2.8 %), ellipsoid (1.6 %), mouse-shaped (2 %; Fig. 29F), obovoid (1.2 %) or subglobose (1.2 %); bi- or tripapillate sporangia not observed; sporangia sometimes too big for the available cytoplasm and, hence, not filled completely in the basal part, with or without a strong plug below the cytoplasm (11.9 %; Fig. 29G–I); lateral attachment of the sporangiophore (17.6 %; Fig. 29F, G) and a reinforcement of the sporangial base by a laminar basal plug (16.4 %; Fig. 29F) common; sporangial dimensions averaging 55.9 ± 7.8 × 33.5 ± 3.0 µm (overall range 36.6–83.0 × 24.5–42.2 µm; range of isolate means 52.2–58.6 × 32.5–34.3 µm) with a length/breadth ratio of 1.68 ± 0.25 (overall range 1.24–3.07); sporangial proliferation exclusively external (Fig. 29A–D, M); sporangial germination indirectly with zoospores discharged through an exit pore of 4.3–9.4 µm (av. 5.5 ± 0.9 µm). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.7 ± 0.9 µm) on encystment; cysts germinating mostly directly by producing hyphae or indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings frequently formed on sporangiophores, usually close to the sporangial base, ovoid, subglobose or limoniform (Fig. 29A, M), dimensions 14.3 ± 4.5 µm (range 8.0–23.3 µm). Chlamydospores not observed. Oogonia produced in single culture (‘homothallic’ breeding system), predominantly close to the edge of the Petri dish; mostly sessile or terminal on short to medium-length, sometimes curved lateral hyphae, smooth-walled, globose to subglobose (85.5 %; Fig. 29N– T, V–X), excentric and/or comma-shaped (14.5 %; Fig. 29U), with a short or medium-length, mostly non-tapering stalk (Fig. 29N–X); av. oogonial diam 30.7 ± 4.1 µm with an overall range of 20.0–40.9 µm and a range of isolate means of 31.2–34.9 µm; predominantly slightly aplerotic to aplerotic (88.5 %; Fig. 29O–W) or infrequently nearly plerotic (11.5 %; Fig. 29N). Oospores globose to subglobose with one medium-large or several small lipid globules (Fig. 29N–U); av. diam 25.5 ± 3.4 µm with an overall range of 20.5–35.1 µm and a range of isolate means of 23.7–27.6 µm; wall diam 1.57 ± 0.2 µm (overall range 1.13–2.06 µm) and oospore wall index 0.33 ± 0.04; abortion rate after 4 wk very high, av. 94 % (range 85–99 %) with oogonia aborting after (Fig. 29V, W) or before oospore formation (Fig. 29X). Antheridia amphigynous, cylindrical, limoniform, subglobose or irregular, unicellular (Fig. 29N–X); 16.8 ± 2.5 × 15.2 ± 2.5 µm.
Culture characteristics: Colonies on V8A and CA submerged to appressed with scanty aerial mycelium, radiate on V8A and faintly radiate on CA; on PDA dense felty-cottony with a petaloid pattern (Fig. 23).
Cardinal temperatures and growth rates: On V8A optimum 20 °C with 5.18 ± 0.02 mm/d radial growth, maximum 25–<27.5 °C, minimum <10 °C (Fig. 26), lethal temperature 32.5–<35 °C. On CA and PDA at 20 °C 4.05 ± 0.14 mm/d and 2.51 ± 0.21 mm/d, respectively.
Additional materials examined: Panama, Volcano Baru, isolated from necrotic lesions on naturally fallen leaves of a non-identified tree species collected from the ground in a tropical cloud forest, Nov. 2019, K.D. Broders & Y. Balci (PA338, PA339, PA340); isolated from necrotic lesions on naturally fallen leaves of a non-identified tree species floating in a stream running through a tropical cloud forest, Nov. 2019, K.D. Broders & Y. Balci (PA215, PA341, PA342, PA343).
Phytophthora montana T. Jung, Y. Balci, K. Broders & M. Horta Jung, sp. nov. MycoBank MB 847292. Fig. 30.
Fig. 30.
Phytophthora montana. A–K. Sporangia formed on V8-agar in soil extract. A–I. Semipapillate apices. A–G, J. Ovoid, Obpyriform, obturbinate, limoniform and ampulliform sporangia. H. Distorted sporangium. I. Bilobed sporangium. A, C. With pedicels. C, D, G. Intercalary sporangia. C, E. External proliferation. J. Zoospore release. K. Dense sympodium with immature sporangia. L–V. Oogonia with near-plerotic to plerotic oospores, formed in carrot agar. L–Q, U, V. Paragynous antheridia. O. Two antheridia. O–Q. Slightly elongated oogonia. R–T. Amphigynous antheridia. S, T. Slightly bend to comma-shaped oogonia. Images: A–C, E, G–I, K–N, P, T–V. Ex-type CBS 149492; D, F, J, O, Q–S; PA319. Scale bars = 20 µm; V applies to A–J, L–V.
Etymology: The name refers to the mountainous habitat of the known isolates (montana Latin = mountainous).
Typus: Panama, Volcano Baru, isolated from a necrotic lesion on a naturally fallen leaf of a non-identified tree species collected from the ground in a tropical cloud forest, Nov. 2019, K.D. Broders & Y. Balci (holotype CBS H-25115, dried culture on V8A, ex-holotype living culture CBS 149492 = PA243).
Morphological structures on V8A: Sporangia occasionally observed in solid agar but abundantly produced in non-sterile soil extract; borne mostly terminally (86.2 %) in dense or lax sympodia of 2–9 sporangia (Fig. 30K) or on unbranched sporangiophores, or less frequently intercalary (13.8 %; Fig. 30C, D, G); non-caducous with semipapillate apices (Fig. 30A–I); predominantly ovoid, broad ovoid or elongated-ovoid (66.8 %; Fig. 30A–C, J), less frequently limoniform to elongated-limoniform (9.3 %; Fig. 30F), distorted and often with two apices (7.7 %; Fig. 30H, I), obturbinate (6.6 %; Fig. 30E), obpyriform to elongated-obpyriform (3.2 %; Fig. 30D), ellipsoid to elongated-ellipsoid (2.8 %), obovoid (1.6 %), ampulliform (0.8 %; Fig. 30G), subglobose (0.8 %) or mouse-shaped (0.4 %); lateral attachment of the sporangiophore (38.8 %; Fig. 30E, H), a conspicuous basal plug (65.6 %; Fig. 30B, C, F–H) and pedicels (19.1 %; Fig. 30A, C) common; pedicel length variable, averaging 25.3 ± 18.1 µm (range 4.8–87.9 µm); sporangial dimensions averaging 54.1 ± 6.1 × 37.0 ± 3.5 µm (overall range 37.2–78.8 × 25.1–44.2 µm; range of isolate means 51.2–57.7 × 35.4–38.0 µm) with a length/breadth ratio of 1.47 ± 0.19 (overall range 1.12–2.56); sporangial proliferation exclusively external (Fig. 30C, E, K); sporangial germination indirectly with zoospores discharged through an exit pore of 3.7–8.7 µm (av. 5.8 ± 0.8 µm; Fig. 30J). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.8 ± 0.7 µm) on encystment; cysts germinating directly. Hyphal swellings infrequently formed on sporangiophores, ovoid, subglobose or limoniform, dimensions 17.7 ± 5.6 µm (range 11.6–29.1 µm). Chlamydospores not observed. Oogonia produced in single culture (‘homothallic’ breeding system); mostly sessile or terminal on short to medium-length, sometimes curved lateral hyphae, smooth-walled (98 %; Fig. 30L–P, R–V) or rarely with a nipple-like wart at the apex (Fig. 30Q), globose to subglobose (88 %; Fig. 30L–N, R–V) or slightly elongated (12 %; Fig. 30O–Q), sometimes slightly bend (19.5 %; Fig. 30M, T), with a rounded (75.5 %; Fig. 30L, R–U) or short tapering base (24.5 %; Fig. 30M–Q, V); av. oogonial diam 28.0 ± 3.0 µm with an overall range of 17.8–36.6 µm and a range of isolate means of 27.2–28.5 µm; nearly plerotic to plerotic (66.5 %; Fig. 30M–R, U, V) or slightly aplerotic to aplerotic (33.5 %; Fig. 30L, S, T). Oospores globose to subglobose with one or sometimes two medium-large or large lipid globules (Fig. 30L–V), turning golden-brown during maturation (Fig. 30U, V; av. diam 24.9 ± 2.6 µm with an overall range of 15.7–31.3 µm and a range of isolate means of 24.4–25.3 µm; wall diam 1.34 ± 0.2 µm (overall range 0.72–1.94 µm) and oospore wall index 0.29 ± 0.03; abortion rate after 4 wk 56 % (range 28–76 %). Antheridia paragynous, club-shaped, ovoid or subglobose (82 %; Fig. 30L–Q, U, V) or less frequently amphigynous, cylindrical and unicellular (18 %; Fig. 30R–T); sometimes a second paragynous antheridium attached to the oogonial wall (Fig. 30O); dimensions 12.2 ± 2.1 × 9.0 ± 1.3 µm.
Culture characteristics: Colonies on V8A and CA submerged to appressed with limited aerial mycelium, striate to radiate on V8A and radiate on CA; on PDA appressed, dense felty-cottony and uniform to faintly radiate (Fig. 23).
Cardinal temperatures and growth rates: On V8A optimum 25.0 °C with 8.77 ± 0.46 mm/d radial growth, maximum 25–<27.5 °C, minimum <10 °C (Fig. 26), lethal temperature 30 °C. At 20 °C on V8A, CA and PDA 8.01 ± 0.17 mm/d, 5.93 ± 0.07 mm/d and 3.61 ± 0.09 mm/d, respectively.
Additional materials examined: Panama, Volcano Baru, isolated from necrotic lesions on naturally fallen leaves of a non-identified tree species collected from the ground in a tropical cloud forest, Nov. 2019, K.D. Broders & Y. Balci (PA244, PA318, PA319, PA320).
Phytophthora multiplex T. Jung, Y. Balci, K. Broders & M. Horta Jung, sp. nov. MycoBank MB 847300. Figs 31, 32.
Fig. 31.
Phytophthora multiplex. A–J. Sporangia formed on V8-agar (V8A) in soil extract. A, B, D–J. Papillate or semipapillate sporangia. A–H. Ovoid, limoniform, obturbinate and obpyriform sporangia. A, B, G, J. External proliferation (arrow in A). B. Medium-length pedicel (arrow). C. Nonpapillate intercalary sporangium. I, J. Distorted, bi- and tripapillate sporangia before zoospore release. K. Zoospore cyst germinating by forming a microsporangium. L. Empty cyst after release of secondary zoospore (diplanetism). M–Q. Thin-walled globose chlamydospores and swellings formed in V8A. R–V. Extremely thick-walled globose to subglobose chlamydospores formed in carrot agar in mating tests. Images: A, C, D, F, J, M, N, P, Q. Ex-type CBS 149637; B. PA038; E, H. NI160; G, I, K, L. PA212; O. PA133; R–V. Ex-type CBS 149637 × PA172. Scale bar = 20 µm; V applies to A–V.
Fig. 32.
Phytophthora multiplex. A–E. Extremely thick-walled chlamydospores with variable shapes formed in solid carrot agar (fgCA) in mating tests. D, E. New chlamydospore arising (arrow) from other chlamydospores. F–Q. Globose to subglobose, smooth-walled oogonia with near-plerotic to plerotic oospores, formed in fgCA in mating tests. F–O. Unicellular amphigynous antheridia. P, Q. Bicellular amphigynous antheridia. R, S. Catenulate irregular hyphal swellings formed in V8A. T. Dense hyphal aggregation in V8A. Images: A–D, F–O. Ex-type CBS 149637 × PA172; E, P, Q. PA133 × PA172; R. Ex-type CBS 149637; S, T. PA133. Scale bars = 20 µm; T applies A–Q, S, T.
Etymology: The name refers to the diverse set of morphological structures (multiplex Latin = diverse).
Typus: Panama, Parque National Sobernia, isolated from a necrotic lesion on a naturally fallen leaf of a non-identified tree species in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (holotype CBS H-25192, dried culture on V8A, ex-holotype living culture CBS 149637 = PA061).
Morphological structures on V8A: Sporangia common abundantly produced in non-sterile soil extract; borne terminally (97.2 %) on unbranched long or short sporangiophores (Fig. 31F) or in dense or lax sympodia of 2–4 sporangia, or infrequently intercalary (1.6 %; Fig. 31C) or sessile (1.2 %; Fig. 31H); apices mostly papillate (89.9 %; Fig. 31A, D, E, G, I, J) or infrequently semipapillate (8.3 %; Fig. 31B, F, H) or nonpapillate and pointed (1.8 %; Fig. 31C); non-caducous, mostly ovoid, broad-ovoid or elongated ovoid (54.4 %; Fig. 31A–C, F), distorted and often bi- or tripapillate (18 %; Fig. 31I, J), limoniform to elongated-limoniform (16.7 %; Fig. 31D, H) or less frequently obpyriform to elongated obpyriform (5.6 %; Fig. 31G), pyriform (3.2 %), obturbinate (1.3 %; Fig. 31E), ellipsoid (0.6 %) or subglobose (0.2 %); lateral attachment of sporangiophores (13.2 %; Fig. 31F), pedicels (26.9 %; Fig. 31B) and vacuoles (11.8 %; Fig. 31G) commonly observed; pedicel length ranging from 3.1 to 82.6 µm (av. 20.7 ± 12.9 µm); sporangial proliferation exclusively external (Fig. 31A, B, G, J); sporangial dimensions averaging 60.0 ± 8.8 × 37.0 ± 5.1 µm (overall range 20.8–81.9 × 21.2–52.9 µm; range of isolate means 42.6–68.3 × 29.8–41.0 µm) with a length/breadth ratio of 1.63 ± 0.2 (overall range 0.71–2.45; sporangial germination indirectly with zoospores discharged through an exit pore 2.3–9.8 µm wide (av. 5.3 ± 1.0 µm). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.0 ± 1.0 µm) on encystment; cysts germinating directly forming a hypha or a microsporangium or indirectly by releasing a secondary zoospore (diplanetism) (Fig. 31K, L). Hyphal swellings infrequently formed in water on sporangiophores, subglobose to globose or limoniform, 5.3 ± 1.0 µm; frequently formed on solid agar, globose to subglobose, limoniform, irregular or coralloid (Fig. 31N, Fig. 32R, S). Chlamydospores formed in solid agar infrequently in single culture and abundantly in mating tests between A1 and A2 mating type isolates; borne terminally, intercalary or sessile (Fig. 31M–O, Q–V, Fig. 32A, C), sometimes catenulate (Fig. 31Q), or by emergence from another chlamydospore (Fig. 31P, Fig. 32B, D, E); globose to subglobose (68.4 %; Fig. 31M–V, Fig. 32B, D), pyriform (10 %), ellipsoid (8.3 %; Fig. 32C, D), irregular (8.3 %; Fig. 32B), ampulliform (3.3 %; Fig. 32E) or obovoid (1.7 %; Fig. 32A); often containing lipid globules (51.6 %; Fig. 31O, R–U, Fig. 32E); dimensions 47.9 ± 17.1 × 42.6 ± 8.9 µm (overall range 25.2–128.9 × 23.1–65.8 µm); with thin (Fig. 31M–Q) or thick wall (Fig. 31R–V, Fig. 32A–E), on average 4.0 ± 1.7 µm thick (range 0.7–8.4 µm). Oogonia not observed in single culture, but abundantly produced in mating tests between the two A2 mating type isolates PA172A and PA161 and the 10 A1 isolates (‘heterothallic’ breeding system); with short stalks, terminal on short to medium-length, sometimes curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose with a rounded or short-tapering base (Fig. 32F–Q); oogonial diam 26.6 ± 2.7 µm (overall range 18.6–35.5 µm; range of means in different mating combinations 25.5–28.7 µm); plerotic or nearly plerotic (Fig. 32F–Q). Oospores globose with a medium-sized lipid globule (Fig. 32F–Q); mean diam 23.8 ± 2.5 µm (overall range 17.8–33.0 µm; range of means in different mating combinations 22.9–26.0 µm); wall thickness 1.34 ± 0.21 µm (overall range 0.86–2.17 µm), oospore wall index 0.3 ± 0.04; abortion 24–52 % (av. 40.3 %) after 4 wk. Antheridia exclusively amphigynous and cylindrical or subglobose, unicellular (96.7 %: Fig. 32F–O) or infrequently bicellular with the basal cell being smaller than the upper cell (3.3 %; Fig. 32P, Q); dimensions 17.3 ± 2.9 × 15.3 ± 1.6 µm. Hyphal aggregations observed in all isolates (Fig. 32T).
Culture characteristics: Colonies on V8A and CA appressed with limited aerial mycelium, chrysanthemum-like on V8A, and radiate on CA; dense felty-cottony on PDA with a faint petaloid pattern (Fig. 25).
Fig. 25.
Colony morphology of Phytophthora species from subclade 2b after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora siskiyouensis (WW10). B. Phytophthora valdiviana (ex-type CBS 149504). C. Phytophthora variepedicellata (ex-type CBS 149505). D. Phytophthora multiplex (isolate PA171). E, F. Phytophthora taxon pseudocapsici (E. CH 26G2; F. SU1649).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 9.58 ± 1.04 mm/d radial growth, maximum 32.5–<35 °C (10 isolates) or 30–32.5 °C (1 isolate), minimum <10 °C (Fig. 26), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 7.24 ± 0.72 mm/d, 5.17 ± 0.4 mm/d and 5.12 ± 0.51 mm/d, respectively.
Additional materials examined: Nicaragua, Diriomo, Mombacho volcano, isolated from naturally fallen necrotic leaves of unidentified rainforest trees collected from the ground in a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI156, NI160). Panama, Parque National Sobernia, isolated from necrotic lesions on naturally fallen leaves of nonidentified tree species in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA038, PA065, PA133); Parque National de Campana, isolated from necrotic lesions on naturally fallen leaves of non-identified tree species in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA170, PA171, PA172); El Montoso, Reserva Forestal, isolated from necrotic lesions on naturally fallen leaves of non-identified tree species in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA199, PA212).
Phytophthora obovoidea T. Jung, Y. Balci, L. Garcia & B. Mendieta-Araica, sp. nov. MycoBank MB 847293. Fig. 33.
Fig. 33.
Phytophthora obovoidea. A–N. Sporangia formed on V8-agar in soil extract. A–J, L–N. Obovoid, ovoid, limoniform and pyriform sporangia. A–D, F–J, L. Papillate or semipapillate apices. E, K. Nonpapillate apices. B–M. Short to very long pedicels. B. Double pedicel (arrows). F, G. External proliferation (arrows). I–M. Caducous sporangia. K. Bilobed sporangium. N. Dense sporangial sympodium. O–R. Globose thick-walled chlamydospores in carrot agar (fgCA). O, Q. Intercalary. P. Terminal. R. Catenulate. S–X. Globose oogonia with plerotic or near-plerotic oospores and amphigynous antheridia, formed in fgCA in mating tests. X. Bicellular antheridium. Y. Dense hyphal aggregation in V8A. Images: A–D, J, K, M, P–R. Ex-type CBS 149633; E, F, H, I. NI187; G. VN828; L, O, Y. PA212; N. VN369; S–X. TW367 × ex-type CBS 149633. Scale bars = 20 µm; Y applies to A–M, O–Y.
Etymology: The name refers to the obovoid shape of many sporangia.
Typus: Nicaragua, Diriomo, Mombacho volcano, isolated from a naturally fallen necrotic leaf of an unidentified rainforest tree collected from the ground in a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (holotype CBS H-25188, dried culture on V8A, ex-holotype living culture CBS 149633 = NI168).
Morphological structures on V8A: Sporangia common on solid agar and abundantly produced in non-sterile soil extract; borne predominantly terminally (97.6 %) in dense or lax sympodia of 2–10 sporangia (Fig. 33N) or sometimes on unbranched long or short sporangiophores (Fig. 33D), less frequently intercalary (1.2 %) or sessile (1.2 %; Fig. 33A); ovoid, broad-ovoid or elongated ovoid (38.7 %; Fig. 33A, J, L–N), obovoid (25.1 %; Fig. 33B, C, H, I), limoniform to elongated-limoniform (22.8 %; Fig. 33E, F, N), or less frequently ellipsoid (5.2 %; Fig. 33D), distorted, often with two apices (3.6 %; Fig. 33K), pyriform (3.2 %; Fig. 33G), obpyriform to elongated obpyriform (1 %) or ampulliform (0.4 %); usually with pedicels of variable length ranging from 3.2 to 142.1 µm (av. 30.2 ± 17.9 µm) (83.6 %; Fig. 33B–M) and caducous (Fig. 33I–M); lateral attachment of sporangiophores rare (0.2 %; Fig. 33H); apices papillate (77.4 %; Fig. 33A, B, D, G, I, J), less frequently semipapillate (16.5 %; Fig. 33C, F, H) or nonpapillate (6.1 %; Fig. 33E, K); sporangial proliferation exclusively external (Fig. 33F, G, N); sporangial dimensions averaging 51.5 ± 7.0 × 30.2 ± 3.4 µm (overall range 24.3–89.2 × 19.7–44.9 µm; range of isolate means 46.8–59.8 × 27.2–35.4 µm) with a length/breadth ratio of 1.71 ± 0.2 (overall range 0.81–3.19; sporangial germination indirectly with zoospores discharged through an exit pore 1.9–9.8 µm wide (av. 4.8 ± 1.1 µm) (Fig. 33M). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.2 ± 0.8 µm) on encystment; cysts germinating directly forming a hypha or a microsporangium or indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings infrequently formed in water on sporangiophores, subglobose to globose or limoniform, 12.1 ± 3.3 µm. Chlamydospores commonly formed in solid agar; borne terminally, intercalary or sessile (Fig. 33O–R), sometimes catenulate (Fig. 33R); globose to subglobose (95.2 %; Fig. 33O–R), ovoid (3.2 %) or ellipsoid (1.6 %), usually containing one or more vacuoles and turning golden-brown during maturation (Fig. 33O–R); diam 29.8 ± 4.2 µm (overall range 20.3–39.8 µm); wall 1.35 ± 0.34 µm thick (range 0.84–2.8 µm). Oogonia not observed in single cultures, but abundantly produced in mating tests between A1 and A2 mating type isolates (‘heterothallic’ breeding system); globose to slightly subglobose with a rounded base and a short, sometimes slightly tapering stalk, sessile or terminal on short to medium-length, sometimes curved lateral hyphae, smooth-walled (Fig. 33S–X); oogonial diam 27.6 ± 3.2 µm (overall range 20.0–40.6 µm; range of means in different mating combinations 24.9–29.6 µm); plerotic or nearly plerotic (Fig. 33S–X). Oospores globose with a medium-sized or large lipid globule (Fig. 33S–X); mean diam 23.9 ± 2.7 µm (overall range 17.5–36.5 µm; range of means in different mating combinations 21.9–25.4 µm); wall thickness 1.48 ± 0.17 µm (overall range 1.17–2.3 µm), oospore wall index 0.33 ± 0.04; abortion 32–56 % (av. 41.3 %) after 4 wk. Antheridia exclusively amphigynous and cylindrical or subglobose, unicellular (95.3 %; Fig. 33S–W) or infrequently bicellular (3.3 %; Fig. 33X); basal septum of the oogonial stalks mostly inside the antheridium (Fig. 33S, T, V–X); dimensions 14.4 ± 2.7 × 14.5 ± 1.6 µm. Hyphal aggregations observed in all isolates (Fig. 33Y).
Culture characteristics: Colonies on V8A submerged to appressed with limited aerial mycelium and radiate to stellate patterns; on CA appressed with limited or woolly aerial mycelium and a radiate pattern; on PDA dense felty-cottony or woolly with a faint petaloid pattern or uniform (Fig. 24).
Fig. 24.
Colony morphology of Phytophthora species from subclade 2b after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A–C. Phytophthora obovoidea (A. ex-type CBS 149633; B. PA208; C. VN830). D–F. Phytophthora tropicalis (D. PA112; E. SU663a; F. TW343).
Cardinal temperatures and growth rates: On V8A optimum 30 °C with 10.98 ± 0.99 mm/d radial growth but growing only slightly slower at 27.5 °C (10.74 ± 0.91 mm/d), maximum 32.5–<35 °C, minimum <10 °C (Fig. 26), lethal temperature 35 °C (13 isolates) or >35 °C (9 isolates). At 20 °C on V8A, CA and PDA 7.45 ± 0.95 mm/d, 5.29 ± 0.46 mm/d and 3.77 ± 0.56 mm/d, respectively.
Additional materials examined: Indonesia, Java, Bandung area, isolated from a naturally fallen necrotic leaf of an unidentified rainforest tree floating in a stream below waterfall Tilu Leuwi Opat, Feb. 2019, T. Jung, M. Tarigan & L. Oliveira (JV122); Sulawesi, Gowa district, isolated from a naturally fallen necrotic leaf of an unidentified tree floating in a stream running through a submontane rainforest, Jun. 2019, T. Jung, M. Junaid & M. Horta Jung (SL175). Nicaragua, Diriomo, Mombacho volcano, isolated from naturally fallen necrotic leaves of unidentified rainforest trees collected from the ground in a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI154, NI162, NI167); Rivas, Maderas Volcano, isolated from a naturally fallen necrotic leaf of an unidentified rainforest tree collected from the ground in a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI187); Matagalpa, Selva Negra, isolated from naturally fallen necrotic leaves of unidentified rainforest trees collected from the ground in a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI108, NI114, NI117). Panama, Parque National Sobernia, isolated from a naturally fallen necrotic leaf of an unidentified tree collected from the ground in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA066); Parque National de Campana, isolated from naturally fallen necrotic leaves of unidentified trees collected from the ground in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA167, PA168); El Montoso Reserva Forestal, isolated from naturally fallen necrotic leaves of unidentified trees in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA206, PA208, PA211). Taiwan, Taichung County, isolated from baiting leaves floating in tributaries of Da-jia River, Sep. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW343, TW367, TW369). Vietnam, Sapa, Xin Chài Mountain, isolated from rhizosphere soil of Alnus nepalensis in a montane, temperate Alnus forest, Mar. 2016, T. Jung, M. Horta Jung & N.M. Chi (VN369, VN802, VN828, VN829, VN830).
Phytophthora pyriformis T. Jung, Y. Balci, K.D. Boders & M. Horta Jung, sp. nov. MycoBank MB 847297. Fig. 34.
Fig. 34.
Phytophthora pyriformis. A–M. Sporangia formed on V8-agar in soil extract. A–K, M. Pyriform, ovoid, obovoid, limoniform and ellipsoid sporangia with medium-length to long pedicels. A. Nonpapillate, intercalary sporangium. B–H, J, K. Papillate apices. B–G. External proliferation. J. Semipapillate apex. I–K. Caducous sporangia. L. Bilobed intercalary sporangium releasing zoospores. M. Lax sporangial sympodium. N–Q. Globose to subglobose, thick-walled chlamydospores in carrot agar (fgCA). N–P. Terminal. Q. Sessile. R–X. Globose oogonia with near-plerotic to plerotic oospores and unicellular amphigynous antheridia, formed in fgCA in polycarbonate membrane mating tests. Images: A, C–F, H, J, K, N–X. Ex-type CBS 149634; B, G, M. PA114; I. PA077; L. PA066. Scale bars = 20 µm; X applies to A–L, N–X.
Etymology: The name refers to the pyriform shape of many sporangia.
Typus: Panama, Parque National Sobernia, isolated from a naturally fallen necrotic leaf of an unidentified tree collected from the ground in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (holotype CBS H-25189, dried culture on V8A, ex-holotype living culture CBS 149634 = PA106).
Morphological structures on V8A: Sporangia common on solid agar and abundantly produced in non-sterile soil extract; borne predominantly terminally (99.4 %) in dense or lax sympodia of 2–6 sporangia (Fig. 34M) or on unbranched long or short sporangiophores, less frequently intercalary (0.6 %; Fig. 34A); mostly pyriform to elongated-pyriform (33.5 %; Fig. 34A, D, E, H), limoniform to elongated-limoniform (27.1 %; Fig. 34F, G, I, J, M) or obovoid to elongated-obovoid (16.5 %; Fig. 34C), less frequently ovoid, broad-ovoid or elongated ovoid (14.8 %; Fig. 34B, M), ellipsoid (4 %; Fig. 34K), distorted, usually with two or sometimes three apices (2.3 %; Fig. 32L), subglobose (1.2 %) or obpyriform to elongated-obpyriform (0.6 %); almost exclusively with pedicels of variable length ranging from 6.5 to 156.7 µm (av. 62.5 ± 27.3 µm) (99.1 %; Fig. 34A–K, M) and caducous (Fig. 34G, I–K); most sporangia with a conspicuous basal plug, often protruding into the sporangiophore (93.5 %; Fig. 34A, C–J, M); lateral attachment of sporangiophores rare (2 %); apices mostly papillate (90.8 %; Fig. 34B–H, J, K) or infrequently semipapillate (6.2 %; Fig. 34I) or nonpapillate (3 %; Fig. 34A), often pointed; sporangial proliferation exclusively external (Fig. 34B–G, M); sporangial dimensions averaging 54.2 ± 7.3 × 30.2 ± 3.1 µm (overall range 31.1–77.0 × 20.6–39.3 µm; range of isolate means 48.3–57.6 × 27.3–32.2 µm) with a length/breadth ratio of 1.8 ± 0.19 (overall range 0.97–2.89); sporangial germination indirectly with zoospores discharged through an exit pore 2.8–9.1 µm wide (av. 4.0 ± 0.7 µm) (Fig. 34L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.6 ± 0.7 µm) on encystment; cysts germinating directly forming a hypha. Hyphal swellings rarely formed in water on sporangiophores, subglobose to globose, limoniform or ellipsoid. Chlamydospores commonly formed in solid agar; borne terminally on long or short lateral hyphae or sessile (Fig. 34N–Q), less frequently intercalary; globose to subglobose ( 95.2 %; Fig. 34N–Q), ellipsoid (4.7 %) or ovoid (3.3 %), usually containing one or more vacuoles and turning golden- to dark-brown during maturation (Fig. 34N–Q); diam 29.7 ± 3.7 µm (overall range 21.2–39.4 µm); wall 1.31 ± 0.24 µm thick (range 0.84–2.0 µm). Oogonia not observed in single culture, but abundantly produced in mating tests with A2 mating type isolates NI168, PA047 and PA168 of P. obovoidea (‘heterothallic’ breeding system; all tested isolates belonging to the A1 mating type); globose to slightly subglobose with a rounded base and a short, sometimes slightly tapering stalk, sessile or terminal on short to medium-length, sometimes curved lateral hyphae, smooth-walled (Fig. 34R–X); oogonial diam 25.5 ± 2.6 µm (overall range 19.4–32.3 µm; range of means in different mating combinations 22.4–27.9 µm); plerotic or nearly plerotic (Fig. 34R–X). Oospores globose with a medium-sized or large lipid globule (Fig. 34R–X); mean diam 22.7 ± 2.5 µm (overall range 16.7–32.2 µm; range of means in different mating combinations 19.8–25.2 µm); wall thickness 1.37 ± 0.16 µm (overall range 0.92–1.86 µm), oospore wall index 0.32 ± 0.04; abortion 16–44 % (av. 23.7 %) after 4 wk. Antheridia exclusively amphigynous, cylindrical or subglobose and unicellular (Fig. 34R–X); dimensions 14.2 ± 2.1 × 14.6 ± 1.5 µm. Hyphal aggregations not observed.
Culture characteristics: Colonies on V8A and CA submerged to appressed with limited aerial mycelium and radiate to stellate patterns; on PDA dense felty-cottony with a petaloid pattern (Fig. 23).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 11.43 ± 0.53 mm/d radial growth but growing only slightly slower at 25 and 30 °C with 11.21 ± 0.34 and 10.98 ± 0.78 mm/d, maximum 32.5–<35 °C, minimum >10–15 °C (Fig. 26), lethal temperature 35 °C (2 isolates) or >35 °C (4 isolates). At 20 °C on V8A, CA and PDA 9.0 ± 0.65 mm/d, 5.76 ± 0.04 mm/d and 4.85 ± 0.23 mm/d, respectively.
Additional materials examined: Panama, Parque National Sobernia, isolated from naturally fallen necrotic leaves of an unidentified tree species collected from the ground in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA076, PA077, PA079, PA104, PA113, PA114).
Phytophthora tropicalis Aragaki & J.Y. Uchida, Mycologia 93: 139. 2001. [MycoBank MB 467732]. Fig. 35.
Fig. 35.
Phytophthora tropicalis. A–L. Sporangia with medium-length to long pedicels, formed on V8-agar (V8A) in soil extract. A–C, E–L. Limoniform, pyriform, ovoid and obpyriform sporangia. D. Bilobed sporangium. A–D, K, L. External proliferation. A, L. Dense sporangial sympodia. A, C–J. Semipapillate and papillate apices. B. Nonpapillate apex. H–J. Caducous sporangia. K. Zoospore release. M–O. Thick-walled chlamydospores in V8A. M, O. Intercalary. N. Catenulate. P. Exteremely thick-walled sessile chlamydospore formed in carrot agar (fgCA) in mating test. Q–U. Globose oogonia with near-plerotic to plerotic oospores and amphigynous antheridia, formed in fgCA in mating tests. U. Aborted oospore. V. Hyphal aggregation in V8A. Images: A–C, F, H, J, L, M. NI122; D, G, K, V. NI174; E. NI148; I. NI166; N–P. SU663 × NI174; Q, U. PA112 × NI122; R–T. SU663 × NI174. Scale bars = 20 µm; U applies to A–K, M–U.
Typus: USA, Hawaii, Keaau, isolated from an inflorescence of Macadamia integrifolia, Jan. 1975, M. Aragaki (holotype CBS 434.91 preserved in a metabolically inactive state, ex-holotype living culture CBS 434.91 = ATCC 76651 = MYA-4218 = NRRL 64471 = WPC P10329).
Morphological structures on V8A: Sporangia common on solid agar and abundantly produced in non-sterile soil extract; borne predominantly terminally (99.6 %) in dense or lax sympodia of 2–9 sporangia (Fig. 35A, L) or sometimes on unbranched long or short sporangiophores (Fig. 35G), rarely intercalary (0.4 %); limoniform to elongated-limoniform (50.4 %; Fig. 35A, C, E, I, K, L), ovoid, broad-ovoid or elongated ovoid (21.7 %; Fig. 35A, F, J), less frequently pyriform (7.1 %; Fig. 35B, H), obovoid (6.9 %), distorted, often with two apices (5 %; Fig. 35D), obpyriform to elongated obpyriform (4.4 %; Fig. 35G) or ellipsoid (4.3 %); predominantly (98.6 %) with pedicels of variable length ranging from 2.9 to 100.1 µm (av. 33.9 ± 18.8 µm) (Fig. 35A–L) and caducous (Fig. 35G–J); lateral attachment of sporangiophores rare (0.1 %); apices fluent transition from semipapillate to papillate (95.1 %; Fig. 35A–J, L), infrequently nonpapillate and often pointed (4.9 %; Fig. 35B); sporangial proliferation exclusively external (Fig. 35A–D, K, L); sporangial dimensions averaging 55.8 ± 6.9 × 31.1 ± 3.8 µm (overall range 26.3–87.7 × 21.0–39.6 µm; range of isolate means 52.6–59.2 × 26.0–33.9 µm) with a length/breadth ratio of 1.81 ± 0.24 (overall range 0.89–2.67); sporangial germination indirectly with zoospores discharged through an exit pore 2.6–8.0 µm wide (av. 4.8 ± 1.0 µm) (Fig. 35K). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.6 ± 0.9 µm) on encystment; cysts germinating directly forming a hypha or a microsporangium or indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings infrequently formed in water on sporangiophores, subglobose to globose, limoniform or irregular, 12.1 ± 3.1 µm. Chlamydospores commonly formed in solid agar; borne terminally, intercalary or sessile (Fig. 35M–P), sometimes catenulate (Fig. 35N); globose to subglobose ( 85 %; Fig. 35M, N, P), ovoid (3 %) or ellipsoid- to pyriform-elongated (12 %; Fig. 35O), usually containing one or more lipid globules and turning golden-brown during maturation (Fig. 35M, N); diam 29.8 ± 4.2 µm (overall range 20.3–39.8 µm); wall 1.48 ± 0.5 µm thick (range 0.84–4.8 µm). Oogonia not observed in single culture, but abundantly produced in mating tests between A1 and A2 mating type isolates (‘heterothallic’ breeding system); globose to slightly subglobose (97 %) or rarely slightly excentric (3 %) with a rounded base and a short, sometimes slightly tapering stalk, sessile or terminal on short to medium-length, sometimes curved lateral hyphae, smooth-walled (Fig. 35Q–U); oogonial diam 27.3 ± 3.2 µm (overall range 20.6–35.6 µm; range of means in different mating combinations 24.8–30.2 µm); plerotic or nearly plerotic (Fig. 35Q–T). Oospores globose with a medium-sized or less frequently a large lipid globule (Fig. 35Q–T); mean diam 23.6 ± 2.2 µm (overall range 18.1–31.6 µm; range of means in different mating combinations 22.4–25.2 µm); wall thickness 1.35 ± 0.15 µm (overall range 0.91–1.75 µm), oospore wall index 0.31 ± 0.03; abortion 30–40 % (av. 35 %; Fig. 35U) after 4 wk. Antheridia exclusively amphigynous and cylindrical or subglobose, unicellular (Fig. 35Q–U); basal septum of the oogonial stalks often inside the antheridium (Fig. 35S–U); dimensions 13.0 ± 2.8 × 12.8 ± 2.2 µm. Hyphal aggregations observed in all isolates (Fig. 35V).
Culture characteristics: Colonies on V8A and CA submerged to appressed with limited aerial mycelium and stellate to radiate patterns; on PDA dense felty-cottony or woolly with a faint petaloid pattern or uniform (Fig. 24).
Cardinal temperatures and growth rates: On V8A optimum 30 °C with 10.08 ± 1.27 mm/d radial growth but growing only slightly slower at 27.5 °C (10.0 ± 0.82 mm/d), maximum 32.5–<35 °C, minimum <10 °C (Fig. 26), lethal temperature 35 °C (3 isolates) or >35 °C (9 isolates). At 20 °C on V8A, CA and PDA 7.7 ± 0.9 mm/d, 5.56 ± 0.42 mm/d and 4.51 ± 0.41 mm/d, respectively.
Materials examined: Indonesia, Java, Bandung area, isolated from naturally fallen necrotic leaves of unidentified trees floating in streams running through montane rainforests, Feb. 2019, T. Jung & M. Tarigan (JV030, JV041); Sumatra, Western Sumatra, isolated from naturally fallen necrotic leaves of unidentified trees floating in streams running through lowland and montane rainforests, Sep. 2018 and Feb. 2019, T. Jung, M. Tarigan & L. Oliveira (SU646, SU663, SU1678). Nicaragua, Driamba, isolated from a naturally fallen necrotic leaf of an unidentified tree floating in a stream running through a lowland rainforest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI026); Diriomo, Mombacho Volcano, isolated from naturally fallen necrotic leaves of unidentified rainforest trees floating in a stream running through a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI148, NI166, NI174). Panama, Parque National Sobernia, isolated from naturally fallen necrotic leaves of unidentified trees collected from the ground in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (PA006, PA090, PA112).
Phytophthora valdiviana T. Jung, E. Sanfuentes von Stowasser, A. Durán & M. Horta Jung, sp. nov. MycoBank MB 847298. Fig. 36.
Fig. 36.
Phytophthora valdiviana. A–J. Semipapillate and papillate sporangia formed on V8-agar (V8A) in soil extract. A–H, J. Ovoid, obpyriform and limoniform sporangia. I. Bipapillate sporangium. C, D, F–H. Short to medium-length pedicels. B, C. External proliferation (arrows). H. With hyphal swelling. J. Zoospore release into short-lived vesicle (arrow). K–U. Oogonia with slightly aplerotic to near-plerotic oospores and amphigynous antheridia, formed in V8A. K–P. Globose to subglobose oogonia. Q–S. Elongated oogonia with tapering funnel-like bases. T, U. Elongated comma-shaped oogonia. T. Bicellular antheridium with small basal cell (arrow). V. Sympodial hyphal branching. Images: A, D–G, J, K–P, T; Ex-type CBS 149504; B, C, I, R, S. CL158; H, V. CL159; Q, U. CL161. Scale bar = 20 µm; V applies to A–V.
Etymology: The name refers to the origin of all known isolates in the Valdivian region of Chile.
Typus: Chile, Valdivian region, Parque Oncol, isolated from a baiting leaf floating in a stream running through a Valdivian rainforest, Nov. 2014, T. Jung, E. Sanfuentes von Stowasser & A. Durán (holotype CBS H-25128, dried culture on V8A, ex-holotype living culture CBS 149504 = CL157).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract, mostly inside the colonies rather than at the growing margins; borne terminally on mostly long or short unbranched sporangiophores or less frequently in dense or lax sympodia of 2–4 sporangia; papillate (51.5 %; Fig. 36A, D, E, I) or semipapillate (48.5 %; Fig. 36B, C, F–H), often with a pointed apex non-caducous, predominantly ovoid, broad ovoid or elongated ovoid (70 %; Fig. 36A–F, J), less frequently obpyriform to elongated obpyriform (15.2 %; Fig. 36G, H), distorted and often with two or three apices (6.8 %; Fig. 36I), ellipsoid (3.2 %), limoniform (2.8 %), subglobose (1.2 %) or ampulliform (0.8 %); lateral attachment of the sporangiophore (25.2 %; Fig. 36E, G–I) and pedicels (55.4 %; Fig. 36C, D, F–H) common; sporangial dimensions averaging 61.4 ± 10.4 × 38.4 ± 6.4 µm (overall range 31.0–101.0 × 17.8–54.2 µm; range of isolate means 58.0–65.3 × 35.3–43.4 µm) with a length/breadth ratio of 1.62 ± 0.28 (overall range 0.92–3.15); pedicel length 14.1 ± 6.1 µm (range 2.5–33.2 µm); sporangial proliferation exclusively external (Fig. 36B, C); sporangial germination indirectly with zoospores discharged through an exit pore of 3.8–9.4 µm (av. 6.9 ± 1.1 µm) into a short-lived vesicle (Fig. 36J). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 11.8 ± 2.4 µm) on encystment; cysts germinate mostly directly by producing hyphae or indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings infrequently produced on sporangiophores, usually close to the sporangial base, ovoid, subglobose or limoniform (Fig. 36B, H). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), (Fig. 36K–U); smooth-walled, globose to subglobose (68 %; Fig. 36K–P) or elongated (32 %; Fig. 36Q–U), often slightly bend to comma-shaped (29.6 %; Fig. 36P, T, U), with round (62.4 %; Fig. 36K–P) or long tapering, often funnel-like bases (37.6 %; Fig. 36Q–U) which sometimes become very thin (Fig. 36Q); av. oogonial diam 33.2 ± 3.5 µm with an overall range of 20.0–40.9 µm and a range of isolate means of 31.2–34.9 µm; slightly aplerotic to plerotic (Fig. 36K–P, R–U) or infrequently aplerotic (Fig. 36Q). Oospores globose to subglobose with a large lipid globule (Fig. 36K–U); av. diam 30.3 ± 2.9 µm with an overall range of 19.2–37.1 µm and a range of isolate means of 28.6–32.0 µm; wall diam 2.42 ± 0.3 µm (overall range 1.72–3.26 µm) and oospore wall index 0.41 ± 0.05; abortion 2–12 % (av. 6.2 %) after 4 wk. Antheridia amphigynous, cylindrical, limoniform, subglobose or irregular, predominantly unicellular (Fig. 36K–S, U) or rarely bicellular with the basal cell being very small (Fig. 33T), 19.0 ± 2.7 × 15.2 ± 1.5 µm. Hyphae often branch sympodially in a monochasium or dichasium with the bearing hypha ending with a short tip (Fig. 36V).
Culture characteristics: Colonies on V8A and CA submerged to appressed, faintly striate to radiate on V8A and faintly radiate on CA; on PDA dense felty-cottony with a petaloid pattern (Fig. 25).
Cardinal temperatures and growth rates: On V8A optimum 20.0 °C with 5.43 ± 0.38 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 26), lethal temperature 30–32.5 °C. On CA and PDA at 20 °C 3.84 ± 0.35 mm/d and 2.86 ± 0.15 mm/d, respectively.
Additional materials examined: Chile, Valdivian region, Parque Oncol, isolated from a baiting leaf floating in a stream running through a Valdivian rainforest, Nov. 2014, T. Jung, E. Sanfuentes von Stowasser & A. Durán (CL158, CL159, CL160, CL161).
Phytophthora variepedicellata T. Jung, Y. Balci, K. Broders & I. Milenković, sp. nov. MycoBank MB 847299. Fig. 37.
Fig. 37.
Phytophthora variepedicellata. A–M. Sporangia with variable pedicel lengths, formed on V8-agar (V8A) in soil extract. A–E, H–M. Obpyriform, ovoid, limoniform, ellipsoid and mouse-shaped sporangia. C. Intercalary sporangium. F. Bilobed sporangium. G. Intercalary (arrow) distorted sporangium. A, D–G. External proliferation. H–L. Caducous sporangia. L. Zoospore release. M. Dense sporangial sympodium. N–V. Globose to subglobose oogonia with near-plerotic to plerotic oospores, formed in carrot agar in polycarbonate membrane mating tests. N–U. Unicellular amphigynous antheridia. V. Paragynous antheridium. W. Hyphal aggregation in V8A. Images: A, D, E, G, J, M, N, Q–S, V, W. Ex-type CBS 149505; B, C, H, I. PA241; F, K. PA268; L, O, P, T, U. PA264; Scale bars = 20 µm; W applies to A–L, N–W.
Etymology: The name refers to the variable length of the sporangial pedicels.
Typus: Panama, Volcano Baru, isolated from a necrotic lesion on a naturally fallen leaf of a non-identified tree species collected from the ground in a tropical cloud forest, Nov. 2019, K.D. Broders & Y. Balci (holotype CBS H-25129, dried culture on V8A, ex-holotype living culture CBS 149505 = PA254).
Morphological structures on V8A: Sporangia common on solid agar and abundantly produced in non-sterile soil extract; borne terminally in dense or lax sympodia of 2–8 sporangia (98.4 %; Fig. 37K, M) or rarely intercalary (1.6 %; Fig. 37C, M); apices semipapillate (59.5 %; Fig. 37A, C–I, K) or papillate (40.5 %; Fig. 37B, J), sometimes slightly asymmetric or curved (9.2 %; Fig. 37K); mostly ovoid or elongated ovoid (59.6 %; Fig. 37B, C, M), limoniform to elongated-limoniform (19.4 %; Fig. 37D, E, J, M) or less frequently distorted usually with two apices (6.5 %; Fig. 37F, G); obpyriform to elongated obpyriform (5.3 %; Fig. 37A), pyriform or elongated-pyriform (3.9 %), ellipsoid (2.3 %; Fig. 37H), obovoid (1.3 %; Fig. 37I), ampulliform (1.2 %; Fig. 37M) or mouse-shaped (0.5 %; Fig. 37K); lateral attachment of sporangiophores (26.3 %; Fig. 37K, M), small vacuoles (43.7 %; Fig. 37D, F–H, J) and a conspicuous basal plug (95.9 %; Fig. 37B, D, E, G, I, J, L) common; mostly caducous with variable pedicel length ranging from 4.0 to 75.0 µm (96.7 %; av. 19.2 ± 10.0 µm) (Fig. 37H–L), sometimes two sporangia are shed together (Fig. 37K); sporangial proliferation exclusively external (Fig. 37A, D–G, K, M); sporangial dimensions averaging 42.4 ± 8.5 × 24.7 ± 3.2 µm (overall range 20.2–88.7 × 18.6–43.3 µm; range of isolate means 36.3–56.9 × 22.9–27.0 µm) with a length/breadth ratio of 1.72 ± 0.27 (overall range 0.63–2.64; sporangial germination indirectly with zoospores discharged through an exit pore 3.3–7.2 µm wide (av. 4.9 ± 0.8 µm) (Fig. 37L). Zoospores limoniform to reniform whilst motile, sometimes with ring-like flagella ends, becoming spherical (av. diam = 9.5 ± 1.2 µm) on encystment. Hyphal swellings rarely formed on sporangiophores, subglobose, limoniform or deltoid (Fig. 37M), 13.3 ± 1.9 µm. Chlamydospores not observed. Oogonia not observed in single cultures, but abundantly produced by all seven tested isolates in polycarbonate membrane mating tests with the A1 mating type isolate PA133 of P. multiplex (‘heterothallic’ breeding system; all tested isolates mating type A2); with short stalks, terminal on short to medium-length, sometimes curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose with a rounded base (Fig. 37N–V); oogonial diam 25.8 ± 2.8 µm (overall range 21.0–37.3 µm; range of isolate means 25.0–27.1 µm); nearly plerotic to plerotic (Fig. 37N–V). Oospores globose with a large lipid globule (Fig. 37N–V); mean diam 22.6 ± 2.3 µm (overall range 17.0–30.0 µm; range of isolate means 21.9–23.9 µm); wall thickness 1.32 ± 0.24 µm (overall range 0.78–2.15 µm), oospore wall index 0.31 ± 0.05; abortion 40–80 % (av. 56 %) after 4 wk. Antheridia almost exclusively amphigynous and cylindrical or subglobose, unicellular (Fig. 37N–U), but a few paragynous club-shaped antheridia were rarely observed (Fig. 37V); dimensions of amphigynous antheridia 17.6 ± 3.8 × 15.5 ± 1.9 µm. Hyphal aggregations observed in all isolates (Fig. 37W).
Culture characteristics: Colonies on V8A and CA submerged to appressed, radiate with a stellate centre and scanty aerial mycelium on V8A, and radiate with limited aerial mycelium on CA; dense-felty on PDA with a petaloid centre and a uniform margin (Fig. 25).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 8.72 ± 0.55 mm/d radial growth, maximum 27.5–30 °C (2 isolates) or 30–<32.5 °C (3 isolates), minimum <10 °C (Fig. 26), lethal temperature 32.5 °C. At 20 °C on V8A, CA and PDA 6.54 ± 0.68 mm/d, 4.7 ± 0.28 mm/d and 4.2 ± 0.63 mm/d, respectively.
Additional materials examined: Panama, Volcano Baru, isolated from necrotic lesions on naturally fallen leaves of a non-identified tree species collected from the ground in a tropical cloud forest, Nov. 2019, K.D. Broders & Y. Balci (PA241, PA242, PA253, PA264, PA267, PA268).
Notes on Clade 2b taxa: Across the nuclear (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) 8 736-character alignment and the mitochondrial (cox1, cox2, nadh1 and rps10) 3 156-character alignment pairwise sequence differences between the nine known and nine newly described species and three informally designated taxa in Clade 2b were 0.1–4.8 % and 0.1–4.8 %, respectively. In addition, the 13 Clade 2b taxa examined developed distinctive colony morphologies on V8A, CA and PDA at 20 °C (Figs 23–25). In addition, the nine new species are separated from each other and the other Clade 2b species by a combination of morphological (Figs 27–37) and physiological (Fig. 26) characters of which the most discriminating are highlighted in bold in Tables S7–S9.
Full sexual sterility clearly separates P. theobromicola from all other Clade 2b species (Tables S7–S9). Nine of the 18 Clade 2b species are self-fertile (‘homothallic’) and can be discriminated by a combination of morphological and physiological characters.
Phytophthora distorta can be distinguished from P. amaranthi by having a much lower degree of caducity (ca. 1.0 vs. 20–30 %), much thinner oospore walls (1.89 vs. 3.6 µm) and lower optimum and maximum temperatures for growth (20 and 27.5–30 °C vs. 24 and 32 °C) (Ann et al. 2016; Table S7); from P. aysenensis by producing both papillate and semipapillate sporangia which are on average considerably larger and sometimes caducous, the presence of hyphal swellings and by having predominantly aplerotic oospores with considerably thinner walls (1.89 vs. 3.2 µm) (Crous et al. 2020; Table S7; Fig. 28); from P. gloveri by the production of papillate sporangia, occasional caducity, on average smaller oogonia with almost exclusively amphigynous antheridia, the occurrence of hyphal swellings and a lower optimum temperature for growth (20 vs. 25 °C) (Abad et al. 2011; Table S7); from P. mengei, which has exclusively semipapillate persistent sporangia, by producing a considerable proportion of papillate sporangia, by occasional sporangial caducity, larger oogonia with almost exclusively aplerotic oospores, different antheridial insertion (amphigynous vs. paragynous) and slower growth between 15 and 27.5 °C (Hong et al. 2009; Tables S7, S8; Fig. 26); from P. montana by the production of papillate sporangia, occasional sporangial caducity, the absence of tapering oogonial bases, the much lower frequency of excentric, elongated or comma-shaped oogonia (2 vs. 19.5 %), mostly aplerotic oospores, lower optimum (20 vs. 25 °C) and slightly higher maximum temperature (27.5–30 vs. 25–27.5 °C) for growth and slower growth between 10 and 25 °C (Tables S7, S8; Figs 26, 28, 30); from P. siskiyouensis by the production of papillate sporangia, often in sympodia, the absence of paragynous antheridia, and having considerably lower optimum temperature for growth (20 vs. 27.5 °C) (Reeser et al. 2007; Tables S7, S8; Fig. 26); and from the co-occurring P. valdiviana by occasional sporangial caducity and by having a much higher proportion of distorted sporangia with often two apices (44 vs. 7 %), exclusively rounded non-tapering oogonial bases, much lower frequency of excentric, elongated or comma-shaped oogonia (2 vs. 32 %) and almost exclusively aplerotic oospores; (Tables S7, S9; Figs 28, 36). Phytophthora frigidophila differs from P. distorta by the absence of distorted sporangia with two apices, its much lower proportion of semipapillate sporangia (10.2 vs. 66.3 %), much higher oospore abortion rate (94 vs. 46 %) and slightly lower maximum temperature for growth (25–27.5 vs. 27.5–30 °C) (Table S7; Figs 26, 28, 29). Its low optimum temperature for growth of 20 °C separates P. frigidophila from P. amaranthi, P. gloveri, P. mengei, P. montana and P. siskiyouensis (Abad et al. 2011, Ann et al. 2016; Tables S7, S9; Fig. 26). In addition, P. frigidophila can be easily distinguished from all other seven self-fertile Clade 2b species by its predominant sporangial caducity (Reeser et al. 2007, Hong et al. 2009, Abad et al. 2011, Ann et al. 2016, Crous et al. 2020; Tables S7–S9; Figs 28–30, 36).
The production of dense sympodia with up to nine sporangia discriminates P. montana from P. distorta, P. frigidophila, P. mengei, P. siskiyouensis and P. valdiviana. In addition, P. montana has a lower proportion of pedicellate sporangia (19 %) than P. distorta (34 %), P. frigidophila (65 %) and P. valdiviana (55 %), and its lack of sporangial caducity distinguish it from P. amaranthi, P. distorta, P. frigidophila and P. siskiyouensis (Reeser et al. 2007, Hong et al. 2009, Ann et al. 2016; Tables S7–S9). Phytophthora valdiviana differs from all other self-fertile Clade 2b species except P. montana by its high proportion of oogonia with tapering bases (37.6 %) and elongated or comma-like shapes (32 %) (Reeser et al. 2007, Hong et al. 2009, Abad et al. 2011, Ann et al. 2016, Crous et al. 2020; Tables S7–S9; Figs 28–30, 36). Furthermore, it can easily be distinguished from P. amaranthi, P. gloveri, P. mengei, P. montana and P. siskiyouensis by its lower optimum temperature for growth of 20 °C (Abad et al. 2011, Ann et al. 2016; Tables S7–S9; Fig. 26).
Eight species and P. taxon pseudocapsici from Clade 2b share an A1/A2 (‘heterothallic’) breeding system and can be discriminated by a combination of morphological and physiological characters. The morphometric data, morphological characters and cardinal temperatures of the 12 isolates of P. tropicalis examined in this study were largely congruent with the original description by Aragaki & Uchida (2001) except for the sporangial apices which were a blurred transition between semipapillate and papillate (95.1 %) or nonpapillate (4.9 %) rather than exclusively papillate, and having on average considerably shorter sporangial pedicels (34 vs. 87 µm) in this study (Table S9; Fig. 35). It is noteworthy, that all 12 isolates examined in this study had a functional A1/A2 breeding system (six isolates A1, six isolates A2) in contrast to the 53 isolates examined in the study of Aragaki & Uchida (2001) of which eight were found to be A1, one A2 and 44 isolates were fully sterile. For comparisons with the five newly described A1/A2 Clade 2b species, the P. tropicalis data from the present study will be used.
The P. capsici - P. mexicana species complex remains unresolved and further multigene phylogenetic and phenotypic studies of a high number of isolates, representing a wide range of host plants, geographical locations and all electrophoretic types of the isozyme study of Mchau & Coffey (1995), will be needed to define and characterise the species in the complex. In the isozyme population study of Mchau & Coffey (1995), the ex-type isolate of P. capsici and ex-epitype isolate WPC P646 of P. mexicana and a high number of isolates designated as P. capsici grouped as electrophoretic types ET4 and ET15, respectively, within the diverse isozyme group CapA comprising 18 different electrophoretic types. In our multigene phylogenetic analysis, the P. capsici ex-type resided separately from a group including P. mexicana and several isolates designated as P. capsici or P. aff. capsici and another distinct group designated here as P. taxon pseudocapsici which included isolates from Sumatra and one old isolate from Mexico (CBS 370.72 = WPC P6190), previously designated as P. capsici in Mchau & Coffey (1995), where it belonged to ET4 like the P. capsici ex-type, and in the multigene phylogeny of Yang et al. (2017). Either the whole complex has to be considered as one genetically and phenotypically highly variable species which would then be named P. capsici, which due to its earlier description in 1922 would have priority over P. mexicana (described in 1923); or many isolates currently designated as P. capsici would have to be re-designated as P. mexicana or described as one or two separate species more closely related to P. mexicana than P. capsici, and P. taxon pseudocapsici be erected to species status. For the phenotypic comparisons of the new A1/A2 Clade 2b species with known species, the data of CapA from Mchau & Coffey (1995) but without isolates WPC P646 and WPC P6190 will be used for P. capsici.
Phytophthora calidophila can be discriminated from P. multiplex, P. obovoidea, P. pyriformis and P. tropicalis by producing a comparatively high proportion of sporangia with semipapillate (38 %) or nonpapillate (25 %) apices (Tables S7–S9; Figs 27, 31, 33–35). In addition, P. calidophila differs from P. capsici in having considerably shorter pedicels (on average 24.3 vs. 78.4 µm), smaller oogonia and an inability to grow at 35 °C (Mchau & Coffey 1995; Table S7; Fig. 24); from P. mexicana s. str., P. multiplex and P. theobromicola by producing caducous sporangia (Erwin & Ribeiro 1996; Decloquement et al. 2021; Tables S7–S9); from P. multiplex, P. pyriformis and P. tropicalis by having a much higher proportion of oogonia with tapering bases (Tables S7–S9; Figs 27, 32, 34, 35); and from P. variepedicellata by its high proportion of oogonia with tapering bases (57.4 vs. 0 %) and its higher maximum temperature (32.5–<35 vs. 27.5–32.5 ° C) (Tables S7, S9; Figs 26, 27, 37). Phytophthora obovoidea, previously informally designated as P. taxon tropicalis-like 2 (Jung et al. 2020), can be distinguished from P. calidophila by producing chlamydospores and dense sympodia with up to 10 sporangia and by almost exclusively having rounded oogonial bases (Tables S7, S8; Figs 27, 33); from P. pyriformis by having a lower proportion of papillate (77 vs. 91 %), pyriform (3 vs. 34 %) and pedicellate sporangia (84 vs. >99 %), on average shorter pedicel length (30.2 vs. 62.5 µm), often higher numbers of sporangia per sympodium (max. 10 vs. max. 6) and higher optimum temperature for growth (30 vs. 27.5 °C); from P. tropicalis by producing mostly papillate sporangia (77.4 %) with a higher proportion of obovoid shapes (25 vs. 7 %) and a lower frequency of pedicels (84 vs. 99 %), and slightly higher optimum temperature for growth (30 vs. 27.5–30 °C); and from P. variepedicellata by having larger sporangia (52 vs. 42 µm) which are more often papillate (77 vs. 41 %), the production of chlamydospores and higher optimum and maximum temperatures for growth (Tables S7–S9; Figs 26, 33–35, 37). Phytophthora taxon pseudocapsici has considerably longer sporangia than both P. capsici and P. mexicana (58 vs. 46 and 45 µm) and differs from P. mexicana by having caducous sporangia (Tables S7–S9; Mchau & Coffey 1995, Erwin & Ribeiro 1996). Phytophthora pyriformis is differentiated from all other Clade 2b species by its inability to grow at 10 °C (Fig. 26), and from all other A1/A2 Clade 2b species by having the highest proportion of pyriform sporangia (33 %) and the fastest growth at 25 °C (Tables S7–S9; Figs 26, 27, 31, 33–35, 37); and from all other A1/A2 Clade 2b species except P. capsici by having on average the longest sporangial pedicels (Tables S7–S9; Figs 27, 31, 33–35, 37). Phytophthora variepedicellata has on average the smallest sporangia of all A1/A2 Clade 2b species. In addition, its high proportion (59.5 %) of semipapillate sporangia separates P. variepedicellata from P. obovoidea, P. pyriformis, P. tropicalis and P. multiplex (Tables S7–S9; Figs 31, 33–35, 37).
A lack of sporangial caducity discriminates P. mexicana, P. multiplex and P. theobromicola from the caducous airborne P. calidophila, P. capsici, P. obovoidea, P. pyriformis, P. tropicalis, P. variepedicellata and P. taxon pseudocapsici (Erwin & Ribeiro 1996, Decloquement et al. 2021; Tables S7–S9; Figs 27, 31, 33–35, 37). In addition, P. multiplex differs from its closest relative, the sterile P. theobromicola, by its functional A1/A2 breeding system with both A1 and A2 isolates; in producing on average larger chlamydospores (48 vs. 30 µm) and larger sporangia (60 × 37 vs. 52 × 35.5 µm) with a slightly higher l/b ratio (1.63 vs. 1.5), mostly in dense sympodia with up to 14 sporangia instead of simple sporangiophores as in P. theobromicola, and by having a higher optimum temperature for growth (27.5 vs. 20–25 °C) (Decloquement et al. 2021; Tables S8, S9; Figs 31, 32).
Clade 2c
For all Clade 2c species included in this study colony morphologies on CA, PDA and V8A and temperature-growth relations on V8A are presented in Figs 38–43. Morphological and physiological characters and morphometric data of the nine known and 15 newly described species in Clade 2c are given in the comprehensive Tables S10–S13.
Fig. 38.
Colony morphology of Phytophthora species from subclade 2c after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora acerina (ex-type CBS 133931). B. Phytophthora balkanensis (ex-type CBS 149477). C. Phytophthora catenulata (ex-type CBS 149480). D. Phytophthora curvata (ex-type CBS 149482). E. Phytophthora excentrica (ex-type CBS 149483). F. Phytophthora falcata (ex-type CBS 149484).
Fig. 43.
Mean radial growth rates of five known Phytophthora species from subclade 2c on V8-agar at different temperatures: P. acerina (1 isolate); P. multivora (1 isolate); P. pachypleura (1 isolate); P. pini (7 isolates); P. plurivora (2 isolates).
Phytophthora balkanensis I. Milenković, Ž. Tomić, T. Jung & M. Horta Jung, sp. nov. MycoBank MB 847307. Fig. 44.
Fig. 44.
Phytophthora balkanensis. A–N. Persistent sporangia formed on V8-agar (V8A) in soil extract. A–H, J–L, N. Ovoid, obpyriform, limoniform and ellipsoid sporangia. A–I. Sporangia with flat semipapillate apices. A, C, D, H. External proliferation (arrows). A, C, E–H. Medium-length to long pedicels. I. Bilobed sporangium. J. Swollen apex before zoospore release. K, L. Zoospore release. L. Ring-like zoospore flagella ends. M. Trilobed nonpapillate sporangium. N. Dense sporangial sympodium. O–U. Subglobose, slightly elongated or slightly excentric oogonia with near-plerotic to slightly aplerotic oospores, formed in V8A. O–T. Paragynous antheridia. O, S. Two antheridia (arrows). U. Amphigynous antheridium. Images: A, D, L, R, T, U. TJ546; B, E–K, N, O–Q, S. Ex-type CBS 149477; C, M. BN117. Scale bars = 20 µm; U applies to A–M, O–U.
Etymology: The name refers to the occurrence in natural forests in the Balkans.
Typus: Bosnia and Herzegovina, Teslić municipality, isolated from rhizosphere soil of Alnus glutinosa in a riparian forest, May 2019, I. Milenković (holotype CBS H-25099, dried culture on V8A, ex-holotype living culture CBS 149477 = BN286).
Morphological structures on V8A: Sporangia rarely observed on solid agar but produced abundantly in non-sterile soil extract; typically borne terminally in dense or lax sympodia of 2–8 sporangia (Fig. 44N) or on unbranched sporangiophores (Fig. 44E); non-caducous, predominantly ovoid, broad-ovoid or elongated ovoid (60.7 %; Fig. 44A–D, K, L, N), less frequently ellipsoid to elongated-ellipsoid (17.1 %; Fig. 44F, G, J), limoniform to elongated limoniform (9.7 %; Fig. 44H), distorted and often with two or three apices (9 %; Fig. 44I, M, N), obpyriform to elongated-obpyriform (2.1 %; Fig. 44E), obovoid (1 %) or pyriform (0.4 %); apices predominantly semipapillate (98 %; Fig. 44A–I) or rarely nonpapillate (2 %; Fig. 44M); lateral attachment of the sporangiophore (6.8 %; Fig. 44B) and pedicels (28 %; Fig. 44A–C, F–H, J) commonly observed; sporangial proliferation exclusively external (Fig. 44A, C, D, H, N); sporangial dimensions averaging 55.5 ± 7.4 × 34.7 ± 4.7 µm (overall range 37.2–79.5 × 23.0–53.2 µm; range of isolate means 50.7–59.7 × 33.2–37.8 µm) with a length/breadth ratio of 1.61 ± 0.18 (overall range 1.2–2.16); pedicel length 21.2 ± 11.9 µm (range 2.2–53.6 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.9–11.4 µm wide (av. 7.9 ± 1.2 µm) (Fig. 44K, L). Zoospores limoniform to reniform whilst motile, sometimes with ring-like flagella ends (Fig. 44L), becoming spherical (av. diam = 10.7 ± 0.8 µm) on encystment; cysts germinating directly. Hyphal swellings rarely produced in water on sporangiophores and hyphae; globose to subglobose or limoniform. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose (87 %; Fig. 44O–R, U), sometimes slightly excentric (Fig. 44P, U), or slightly elongated (13 %; Fig. 44S, T), with a rounded (89.5 %; Fig. 44O–Q, S–U) or a short tapering base (10.5 %; Fig. 44R); oogonial diam 30.1 ± 3.0 µm (overall range 17.3–35.9 µm; range of isolate means 28.9–31.0 µm); slightly aplerotic to aplerotic (64 %; Fig. 44P, R–U) or nearly plerotic (36 %; Fig. 44O, Q). Oospores globose with a large lipid globule (Fig. 44O–U); diam 26.5 ± 2.5 µm (overall range 14.4–31.6 µm; range of isolate means 25.6–27.2 µm) wall thickness 1.55 ± 0.27 µm (overall range 0.69–2.39 µm), oospore wall index 0.31 ± 0.04; abortion rate 2–18 % (av. 8 %) after 4 wk. Antheridia predominantly paragynous and club-shaped, ovoid or subglobose (99.5 %; Fig. 44O–T) or rarely amphigynous and cylindrical (0.5 %; Fig. 44U); infrequently two antheridia attached to one oogonium (6.4 %; Fig. 44O, S); dimensions 12.3 ± 2.7 × 8.2 ± 1.4 µm.
Culture characteristics: Colonies on V8A and CA appressed with limited aerial mycelium, chrysanthemum-like on V8A and faintly radiate on CA; on PDA dense-felty to felty-cottony with a faint petaloid pattern (Fig. 38).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 8.65 ± 0.17 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 42), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 7.71 ± 0.2 mm/d, 5.96 ± 0.13 mm/d and 2.89 ± 0.23 mm/d, respectively.
Fig. 42.
Mean radial growth rates of 15 new Phytophthora species from subclade 2c on V8-agar at different temperatures: P. balkanensis (8 isolates); P. catenulata (10 isolates); P. curvata (3 isolates); P. excentrica (2 isolates); P. falcata (7 isolates); P. fansipanensis (5 isolates); P. japonica (4 isolates); P. limosa (5 isolates); P. macroglobulosa (4 isolates); P. nimia (4 isolates); P. oblonga (4 isolates); P. obturata (8 isolates); P. platani (11 isolates); P. pseudocapensis (11 isolates); P. vacuola (2 isolates).
Additional materials examined: Bosnia and Herzegovina, Teslić municipality, isolated from rhizosphere soil of Alnus glutinosa in a riparian forest, May 2019, I. Milenković (BN289); Ozren Mountain, isolated from a small stream running through a mixed oak forest, May 2019, I. Milenković (BN117). Serbia, Željin Mountain, isolated from a small stream running through a natural Fagus sylvatica forest, May 2017, I. Milenković (SFB880). Croatia, Zagreb, isolated from nursery-grown Rubus fruticosus seedling, Mar. 2017, Ž. Tomić (TJ546).
Phytophthora catenulata T. Jung, T.-T. Chang, N.M. Chi & M. Horta Jung, sp. nov. MycoBank MB 847308. Fig. 45.
Fig. 45.
Phytophthora catenulata. A–N. Sporangia formed on V8-agar (V8A) in soil extract. A–G, I–L, N. Ovoid, obpyriform, limoniform, ampulliform and ellipsoid sporangia. A–I. Flat semipapillate apices. A, B, D, E, G, J. Pedicels (arrows in D, E). A, B, D, E, G, I. External proliferation (arrows in B, G). C. Intercalary sporangium with hyphal appendix (arrow). H. Bilobed intercalary sporangium. J. Caducous sporangium. K. Zoospore release. M. Developing sporangial sympodium. N. Sporangial sympodium. O, P. Hyphal swellings in water. O. Radiating hyphae. P. Catenulate. Q–V. Oogonia with near-plerotic to aplerotic oospores, formed in V8A. Q–U. Paragynous antheridia. V. Amphigynous antheridium. Images: A, C, E, H, K, N–P, Q, R, V. Ex-type CBS 149480; B, D, F, G, J, L, M, S–U. VN367; I. VN1011. Scale bars = 20 µm; V applies to A–M, O–V.
Etymology: The name refers to the catenulate swellings formed in water on sporangiophores and hyphae.
Typus: Taiwan, Tunyuan, isolated from rhizosphere soil of Quercus variabilis in a montane, temperate, seasonally dry Quercus–Pinus forest, Mar. 2013, T. Jung, T.-T. Chang & M. Horta Jung (holotype CBS H-25102, dried culture on V8A, ex-holotype living culture CBS 149480 = TW115).
Morphological structures on V8A: Sporangia rarely observed on solid agar but produced abundantly in non-sterile soil extract; typically borne terminally (96.4 %) in dense or lax sympodia of 2–8 sporangia (Fig. 45M, N) or rarely on unbranched sporangiophores, or intercalary (3.6 %; Fig. 45C, H, I); predominantly ovoid, broad-ovoid or elongated ovoid (75.4 %; Fig. 45A–D, J, K, N), less frequently limoniform to elongated limoniform (10.9 %; Fig. 45F, L), ellipsoid to elongated-ellipsoid (5.5 %; Fig. 45N), obpyriform to elongated-obpyriform (4.3 %; Fig. 45E, G), obovoid (2 %), subglobose (0.5 %), ampulliform (0.4 %; Fig. 45I), sickle-shaped (0.4 %), distorted and usually with two apices (0.4 %; Fig. 45H) or pyriform (0.2 %); apices predominantly semipapillate (97 %; Fig. 45A–I) or rarely nonpapillate (3 %; Fig. 45J); lateral attachment of the sporangiophore (15.3 %), pedicels (34 %; Fig. 45A, B, D, E, G, J, L), an asymmetric apex (13.6 %; Fig. 45E) and a conspicuous basal plug protruding into the empty sporangium (28.5 %; Fig. 45K, L) commonly observed; sporangial caducity rare (<1 %; Fig. 45J); sporangial proliferation almost exclusively external (Fig. 45A, B, D, E, G, I, N) or infrequently by a sporangiophore emerging laterally from a sporangium (Fig. 45I); sporangial dimensions averaging 57.4 ± 6.8 × 35.7 ± 4.3 µm (overall range 34.0–85.5 × 18.7–47.3 µm; range of isolate means 53.6–64.4 × 32.5–39.4 µm) with a length/breadth ratio of 1.62 ± 0.19 (overall range 1.14–2.54); pedicel length 15.3 ± 8.7 µm; sporangial germination usually indirectly with zoospores discharged through an exit pore 4.5–15.6 µm wide (av. 8.4 ± 1.2 µm) (Fig. 45K, L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. Diam = 10.3 ± 1.3 µm) on encystment; mostly germinating directly although diplanetism occurred in all isolates. Hyphal swellings abundantly produced in water on sporangiophores and hyphae; globose to subglobose, often with radiating hyphae (Fig. 45O), or limoniform to deltoid and often catenulate (Fig. 45P); dimensions 13.9 ± 4.5 µm (range 6.1–29.7 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae, smooth-walled, globose to slightly subglobose (86.7 %; Fig. 45Q–U), sometimes slightly excentric (Fig. 45R, U), or slightly elongated (13.3 %; Fig. 45V), with a rounded (93.7 %; Fig. 45Q, R, U, V) or a short tapering base (6.3 %; Fig. 45S, T); oogonial diam 28.2 ± 2.4 µm (overall range 20.9–36.9 µm; range of isolate means 27.2–29.9 µm); nearly plerotic to plerotic (60.5 %; Fig. 45Q, S, T, V) or slightly aplerotic to aplerotic (39.5 %; Fig. 45R, U). Oospores globose with a large lipid globule (Fig. 45Q–V); diam 25.1 ± 1.9 µm (overall range 19.3–33.3 µm; range of isolate means 24.0–26.5 µm) wall thickness 1.63 ± 0.31 µm (overall range 0.9–2.5 µm), oospore wall index 0.34 ± 0.06; abortion rate 2–6 % (av. 4 %) after 4 wk. Antheridia predominantly paragynous and club-shaped, ovoid or subglobose (95.8 %; Fig. 45Q–U) or rarely amphigynous and cylindrical (4.2 %; Fig. 45V); sometimes two antheridia attached to one oogonium (Fig. 45U); dimensions 12.4 ± 2.5 × 7.3 ± 1.3 µm.
Culture characteristics: Colonies on V8A and CA mostly submerged to appressed, chrysanthemum-like to radiate on V8A and faintly petaloid on CA; on PDA dense-felty with irregular submerged margins and a petaloid pattern (Fig. 38).
Cardinal temperatures and growth rates: Optimum 27.5 °C with 7.52 ± 0.9 mm/d radial growth on V8A, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 7.31 ± 0.7 mm/d, 4.33 ± 0.3 mm/d and 3.14 ± 0.38 mm/d, respectively.
Additional materials examined: Taiwan, Tunyuan, isolated from rhizosphere soil of Q. variabilis in a montane, temperate, seasonally dry Quercus–Pinus forest, Mar. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW429, TW430). Vietnam, Sapa, Xin Chài Mountain, isolated from rhizosphere soil of Alnus nepalensis in a montane, temperate Alnus forest, Mar. 2016, T. Jung, M. Horta Jung & N.M. Chi (VN367, VN1103, VN1104, VN1105); Fansipan Mountain, isolated from naturally fallen leaves floating in streams running through a montane evergreen cloud forest, Mar. 2017, T. Jung, B. Scanu & N.M. Chi (VN905, VN971, VN1011); Sau Chua Mountain, isolated from a naturally fallen leaf floating in a stream running through a montane Chamaecyparis forest, Mar. 2017, T. Jung, C.M. Brasier & N.M. Chi (VN966).
Phytophthora curvata T. Jung, A. Hieno, H. Masuya & M. Horta Jung, sp. nov. MycoBank MB 847309. Fig. 46.
Fig. 46.
Phytophthora curvata. A–L. Semipapillate to papillate sporangia without pedicels, formed on V8-agar (V8A) in soil extract. A–H, J, L. Ovoid, ellipsoid, mouse- and sickle-shaped sporangia. C–J. Curved apices. A–F. External proliferation (arrows). I–L. Bilobed sporangia. I. Hyphal swelling close to sporangium base. L. Beginning sporangial sympodium. M–U. Oogonia with aplerotic to near-plerotic oospores, formed in V8A. M–R. Globose to subglobose oogonia. S–U. Comma-shaped oogonia. M. Amphigynous antheridium. N–U. Paragynous antheridia. Images: A–U. Ex-type CBS 149482. Scale bar = 20 µm; U applies to A–U.
Etymology: The name refers to the high proportion of curved sporangial shapes.
Typus: Japan, Shikoku Island, Ichinomata, isolated from rhizosphere soil of a mature Abies firma tree in a warm-temperate submontane mixed forest, May 2017, T. Jung & A. Hieno (holotype CBS H-25104, dried culture on V8A, ex-holotype living culture CBS 149482 = JP126).
Morphological structures on V8A: Sporangia rarely observed on solid agar but produced abundantly in non-sterile soil extract; typically borne terminally in dense or lax sympodia of 2–4 sporangia (Fig. 46L) or less frequently on unbranched short or long sporangiophores; non-caducous, ovoid, broad-ovoid or elongated ovoid (41 %; Fig. 46A–C, L), mouse-shaped (24 %; Fig. 46E–H), distorted and often with two apices (23 %; Fig. 46I–L) or less frequently obpyriform to elongated-obpyriform (6 %), ellipsoid (4 %; Fig. 46D) and subglobose (2 %); apices papillate (48 %; Fig. 46B, E, G, J) or semipapillate (52 %; Fig. 46A, C, D, H, I, K, L); curved apices (37.4 %; Fig. 46C–I) and lateral attachment of the sporangiophore (16 %; Fig. 46G, I, L) commonly observed; sporangial proliferation exclusively external (Fig. 46A–C, E, F, H, L); sporangial dimensions averaging 54.5 ± 5.7 × 33.6 ± 4.0 µm (overall range 42.2–64.4 × 24.5–41.7 µm; range of isolate means 52.6–56.1 × 32.6–35.2 µm) with a length/breadth ratio of 1.63 ± 0.15 (overall range 1.24–2.04); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.8–7.6 µm wide (av. 6.2 ± 0.8 µm). Zoospores limoniform to reniform whilst motile, becoming spherical (av. Diam = 10.4 ± 1.9 µm) on encystment. Hyphal swellings infrequently produced in water on sporangiophores; limoniform or subglobose, usually close to the sporangial base (Fig. 46B, I); dimensions 9.4–30.6 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae, smooth-walled, globose to slightly subglobose (62 %; Fig. 46M–R), or comma-shaped (38 %; Fig. 46S–U), with a round (54 %; Fig. 46M, P–R) or a short tapering, often curved base (46 %; Fig. 46N, O, S–U); oogonial diam 27.3 ± 4.5 µm (overall range 18.3–33.1 µm; range of isolate means 25.5–28.6 µm); nearly plerotic to plerotic (54.4 %; Fig. 46N, P, Q, S, U) or slightly aplerotic to aplerotic (45.6 %; Fig. 46M, O, R, T). Oospores globose with a large lipid globule (Fig. 46M, O, Q, R, T); diam 22.3 ± 4.0 µm (overall range 15.7–28.5 µm; range of isolate means 21.5–23.6 µm); wall thickness 1.69 ± 0.32 µm (overall range 0.96–2.46 µm), oospore wall index 0.38 ± 0.04; abortion rate 2–7 % (av. 3.7 %) after 4 wk. Antheridia almost exclusively paragynous and club-shaped, ovoid, globose to subglobose or irregular (99.3 %; Fig. 46N–U), or rarely amphigynous, unicellular and cylindrical (Fig. 46M); sometimes two antheridia attached to the same oogonium (Fig. 46P); dimensions 12.3 ± 1.9 ´ 9.0 ± 1.6 µm.
Culture characteristics: Colonies on V8A appressed with limited aerial mycelium, chrysanthemum-like to radiate; on CA submerged and uniform to faintly radiate; on PDA woolly and uniform (Fig. 38).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 6.25 ± 0.22 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 32.5 °C. At 20 °C on V8A, CA and PDA 5.18 ± 0.17 mm/d, 4.48 ± 0.25 mm/d and 2.29 ± 0.06 mm/d, respectively.
Additional materials examined: Japan, Shikoku Island, Ichinomata, isolated from rhizosphere soil of a mature A. firma tree in a warm-temperate submontane mixed forest, May 2017, T. Jung, A. Hieno & H. Masuya (JP125, JP333).
Phytophthora excentrica T. Jung, S. Uematsu, K. Kageyama & C.M. Brasier, sp. nov. MycoBank MB 847310. Fig. 47.
Fig. 47.
Phytophthora excentrica. A–N. Sporangia formed on V8-agar (V8A) in soil extract. A–I, L–N. Ovoid, subglobose, ellipsoid, limoniform, mouse-shaped and obpyriform sporangia. A–G, I, K. Semipapillate apices. H. Nonpapillate apex. J. Papillate apex. A–C, H. Medium-length to long pedicels (arrows in B, C, H). A, M, N. External proliferation (arrow in A). J, K. Distorted with two apices. M. Zoospore release. N. Dense sporangial sympodium. O, P. Intercalary hyphal swellings in water. Q–W. Oogonia with near-plerotic to plerotic oospores and paragynous antheridia, formed in V8A. Q, R. Subglobose oogonia. S–V. Excentric oogonia. W. Elongated oogonium. X. Hyphal aggregation in V8A. Images: A–X. Ex-type CBS 149483. Scale bars = 20 µm; X applies to A–M, O–X.
Etymology: The name refers to the common production of excentric and asymmetric oogonia and sporangia.
Typus: Japan, Shikoku Island, Ichinomata, isolated from rhizosphere soil of Torreya nucifera in a warm-temperate submontane forest, May 2017, T. Jung & S. Uematsu (holotype CBS H-25105, dried culture on V8A, ex-holotype living culture CBS 149483 = JP461).
Morphological structures on V8A: Sporangia produced abundantly in non-sterile soil extract; typically borne terminally (93.8 %) in dense or lax sympodia of 2–8 sporangia (Fig. 47N) or on unbranched short (Fig. 47G) or long sporangiophores, less frequently intercalary (6.2 %); non-caducous, predominantly ovoid, broad-ovoid or elongated ovoid (76 %; Fig. 47A–C, E, G, M, N), less frequently distorted and often with two apices (8 %; Fig. 47J, K), obpyriform to elongated-obpyriform (4 %; Fig. 47L), limoniform to elongated limoniform (4 %; Fig. 47H, N), subglobose (3 %; Fig. 47D), ellipsoid (2 %; Fig. 47F), mouse-shaped (2 %; Fig. 47I) or obovoid (1 %); apices semipapillate (81.2 %; Fig. 47A–G, I, K) or papillate (13.1 %; Fig. 47J) with a smooth transition between both forms, or infrequently nonpapillate (5.7 %; Fig. 47H); lateral attachment of the sporangiophore (46 %; Fig. 47D–F, I–M), asymmetric shapes of the apex or the whole sporangium (25 %; Fig. 47I–L), pedicels (34 %; Fig. 47A–C, H), and conspicuous basal plugs, sometimes protruding backwards into the sporangiophore (24 %; Fig. 47C) common; sporangial proliferation exclusively external (Fig. 47A–C, M, N); sporangial dimensions averaging 56.9 ± 4.9 × 38.0 ± 3.4 µm (overall range 46.7–72.5 × 31.2–45.1 µm; range of isolate means 55.7–58.1 × 37.4–38.6 µm) with a length/breadth ratio of 1.5 ± 0.18 (overall range 1.2–2.22); pedicel length 16.7 ± 16.9 µm (range 5.7–62.5 µm); sporangial germination indirectly with zoospores discharged through an exit pore 5.3–9.0 µm wide (av. 7.3 ± 0.8 µm). Zoospores limoniform to reniform whilst motile, becoming spherical (av. Diam = 11.0 ± 1.4 µm) on encystment; cysts usually germinate directly. Hyphal swellings commonly produced in water on sporangiophores and hyphae; globose to subglobose, ellipsoid or limoniform, sometimes with radiating hyphae (Fig. 47O, P); dimensions 24.3 ± 9.4 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae or sessile (Fig. 47Q–W), smooth-walled, globose to subglobose (47 %; Fig. 47Q, R), slightly to markedly excentric (44 %; Fig. 47S–V) or less frequently elongated (9 %; Fig. 47W), mostly with a round (82 %; Fig. 47R–U, W) or a short-tapering base (18 %; Fig. 47Q, V); oogonial diam 30.6 ± 3.5 µm (overall range 17.5–37.9 µm; range of isolate means 29.9–31.3 µm); nearly plerotic to plerotic (96 %; Fig. 47Q–W) or rarely aplerotic (4 %). Oospores globose with a large lipid globule (Fig. 47Q–W); diam 26.6 ± 3.1 µm (overall range 15.2–32.4 µm; range of isolate means 26.2–27.0 µm); wall thickness 1.64 ± 0.27 µm (overall range 0.87–2.22 µm), oospore wall index 0.33 ± 0.03; abortion rate 5 % after 4 wk. Antheridia almost exclusively paragynous and club-shaped, ovoid, globose to subglobose or irregular (Fig. 47Q–W); dimensions 11.8 ± 2.9 × 8.1 ± 1.8 µm. Hyphal aggregations commonly produced (Fig. 47X).
Culture characteristics: Colonies on V8A and CA mostly submerged with scanty aerial mycelium, chrysanthemum-like on V8A and petaloid with large petals on CA; on PDA dense felty-cottony with a faint petaloid pattern (Fig. 38).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 8.85 ± 0.14 mm/d radial growth, maximum 30 °C, minimum <10 °C (Fig. 42), lethal temperature 30–<32.5 °C. At 20 °C on V8A, CA and PDA 6.46 ± 0.15 mm/d, 4.98 ± 0.04 mm/d and 3.32 ± 0.09 mm/d, respectively.
Additional materials examined: Japan, Shikoku Island, Ichinomata, isolated from rhizosphere soil of T. nucifera in a warm-temperate submontane forest, May 2017, T. Jung & S. Uematsu (JP2360).
Phytophthora falcata T. Jung, K. Kageyama, S. Uematsu & M. Horta Jung, sp. nov. MycoBank MB 847311. Fig. 48.
Fig. 48.
Phytophthora falcata. A–L. Sporangia formed on V8-agar (V8A) in soil extract. A–J. Semipapillate and papillate apices. A–H, J–L. Ovoid, obpyriform, mouse- and sickle-shaped sporangia. A–C, J. Pedicels with variable lengths. B, C. conspicuous basal plug protruding into the pedicel (arrows). A–C, H. External proliferation. I. Distorted sporangium with two apices. J. Caducous sporangium. K. Zoospore release. L. Sporangial sympodium. M–W. Globose, subglobose and excentric oogonia with near-plerotic to aplerotic oospores and paragynous antheridia, formed in V8A. Q. Intercalary oogonium. S–U. Tapering oogonia bases. W. Comma-shaped oogonium. Images: A–D, F, J, L–Q, T, U. Ex-type CBS 149484; E, JP310; G–I, K. JP719; R. JP253. S, V, W. JP807. Scale bars = 20 µm; W applies to A–K, M–W.
Etymology: The name refers to the common production of curved and often sickle-shaped sporangia in all known isolates (falcata Latin = sickle-shaped).
Typus: Japan, Shikoku Island, Ichinomata, isolated from a baiting leaf floating in a stream running through a diverse warm-temperate forest, May 2017, T. Jung & K. Kageyama (holotype CBS H-25106, dried culture on V8A, ex-holotype living culture CBS 149484 = JP065).
Morphological structures on V8A: Sporangia rarely observed on solid agar but produced abundantly in non-sterile soil extract; typically borne terminally (99.2 %) in dense or lax sympodia of 2–5 sporangia (Fig. 48L) or less frequently on unbranched short or long sporangiophores, or rarely intercalary (0.8 %); mostly ovoid, broad-ovoid or elongated ovoid (44.8 %; Fig. 48A–C, J, L) or curved, sickle-shaped or mouse-shaped (34.2 %; Fig. 48D–G, K), less frequently obpyriform to elongated-obpyriform (10.6 %; Fig. 48H), distorted and often with two or three apices (4.2 %; Fig. 48I, L), limoniform to elongated limoniform (2.4 %), ampulliform (2 %) or ellipsoid to elongated-ellipsoid (1.8 %); apices mostly semipapillate (87.2 %; Fig. 48B, C, E, F, H–J) or less frequently papillate (12.8 %; Fig. 48A, D, G) with a smooth transition between both forms; lateral attachment of the sporangiophore (18 %; Fig. 48E, G–I, K), pedicels (51.6 %; Fig. 48A–C, G, J), and conspicuous basal plugs, often protruding backwards into the sporangiophore (44.8 %; Fig. 48B, C) common; sporangiophores sometimes widening towards the sporangial base (2 %; Fig. 48C); predominantly non-caducous, but caducity occasionally (<1 %) observed in all isolates (Fig. 48J); sporangial proliferation exclusively external (Fig. 48A–C, H, L); sporangial dimensions averaging 58.8 ± 7.1 × 31.6 ± 4.6 µm (overall range 40.0–79.5 × 17.7–45.3 µm; range of isolate means 54.9–64.9 × 29.6–34.9 µm) with a length/breadth ratio of 1.88 ± 0.25 (overall range 1.33–2.8); pedicel length 23.1 ± 12.2 µm (range 1.7–66.9 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.1–8.7 µm wide (av. 6.1 ± 0.9 µm). Zoospores limoniform to reniform whilst motile, becoming spherical (av. Diam = 10.4 ± 1.4 µm) on encystment; cysts usually germinate directly. Hyphal swellings infrequently produced in water on sporangiophores and hyphae; globose to subglobose or limoniform (Fig. 48G); dimensions 13.5 ± 0.8 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae or sessile (Fig. 48M–P, R–W), or occasionally intercalary (Fig. 48Q), smooth-walled, globose to slightly subglobose (87 %; Fig. 48M–V), or comma-shaped (13 %; Fig. 48W), often excentric (23.5 %; Fig. 48N, P, R, V, W) with a round (54 %; Fig. 48M–R, W) or a tapering, often curved base (46 %; Fig. 48S–V); oogonial diam 28.3 ± 2.8 µm (overall range 18.8–35.4 µm; range of isolate means 27.4–29.6 µm); slightly aplerotic to aplerotic (68.5 %; Fig. 48N, P–S, W) or nearly plerotic (31.5 %; Fig. 48M, O, T, U). Oospores globose with a large lipid globule (Fig. 48M–W); diam 23.8 ± 2.5 µm (overall range 16.1–30.0 µm; range of isolate means 23.0–25.0 µm); wall thickness 1.47 ± 0.2 µm (overall range 0.87–2.07 µm), oospore wall index 0.33 ± 0.03; abortion rate 0–1 % (av. 0.5 %) after 4 wk. Antheridia almost exclusively paragynous and club-shaped, ovoid, globose to subglobose or irregular (Fig. 48M–P, R–W), sometimes with finger-like projections (Fig. 48R, U); dimensions 10.8 ± 2.0 × 8.1 ± 1.4 µm.
Culture characteristics: Colonies on V8A and CA mostly submerged to appressed with scanty aerial mycelium, radiate on V8A and petaloid on CA; on PDA dense-felty with a faint petaloid pattern (Fig. 38).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 8.39 ± 0.49 mm/d radial growth, maximum 27.5–<30 °C (5 isolates) or 30 °C (2 isolates), minimum <10 °C (Fig. 42), lethal temperature 30–<32.5 °C. At 20 °C on V8A, CA and PDA 6.23 ± 0.36 mm/d, 5.29 ± 0.1 mm/d and 2.47 ± 0.21 mm/d, respectively.
Additional materials examined: Japan, Honshu Island, Takayama, isolated from a baiting leaf floating in a stream running through a montane riparian Alnus irsute forest, May 2017, T. Jung & A. Hieno (JP253); isolated from rhizosphere soil of A. irsute in two montane riparian forests, May 2017, T. Jung, C.M. Brasier & J.F. Webber (JP319, JP807); Kyushu Island, Takakuma, isolated from a naturally fallen leaf floating in a stream running through a warm-temperate Castanopsis forest, May 2017, T. Jung & S. Uematsu (JP310): Shikoku Island, Ichinomata, isolated from rhizosphere soil of a mature Abies firma tree in a warm-temperate submontane mixed forest, May 2017, T. Jung & H. Masuya (JP331); isolated from a naturally fallen leaf floating in a stream running through a submontane Quercus-Abies-Tsuga forest, May 2017, T. Jung & M. Horta Jung (JP719).
Phytophthora fansipanensis T. Jung, N.M. Chi, T. Corcobado & C.M. Brasier, sp. nov. MycoBank MB 847312. Fig. 49.
Fig. 49.
Phytophthora fansipanensis. A–K. Sporangia formed on V8-agar (V8A) in soil extract. A–I, J, K. Ovoid, obpyriform and limoniform sporangia. A–G, I, K. Semipapillate apices. A–C, E, G. External proliferation. B, D, F, I. Medium-length to long pedicels (arrows). H. Swollen apex before zoospore release and hyphal swelling. I. Bilobed intercalary sporangium. J. Zoospore release. K. Sympodium with ovoid and trilobed sporangia and hyphal swelling. L–U. Oogonia with near-plerotic to aplerotic oospores, formed in V8A. L–T. Paragynous antheridia. L–Q. Globose to subglobose oogonia. R–U. Slightly elongated oogonia. U. Amphigynous antheridium. Images: A–C, G, J–P, U. Ex-type CBS 149485; D, F, I. VN1095; E, H, Q–T. VN1097. Scale bar = 20 µm; U applies to A–U.
Etymology: The name refers to the origin of the first isolates from a forest stream at the Fansipan Mountain in Vietnam.
Typus: Vietnam, Sapa, Fansipan Mountain, isolated from a baiting leaf floating in a stream running through a montane evergreen cloud forest, Mar. 2017, T. Jung & N.M. Chi (holotype CBS H-25107, dried culture on V8A, ex-holotype living culture CBS 149485 = VN970).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally in lax or dense sympodia of 2–5 sporangia (Fig. 49K) or less frequently on unbranched sporangiophores (Fig. 49D, H) or rarely intercalary (1.5 %; Fig. 49F, I); sporangia semipapillate (Fig. 49A–G, I, K) non-caducous, predominantly ovoid, broad ovoid or elongated ovoid (80.4 %; Fig. 49A–E, H, J, K), less frequently limoniform (8.6 %; Fig. 49G), obpyriform to elongated obpyriform (5.6 %; Fig. 49F), distorted and often with two or three apices (2.4 %; Fig. 49I, K), ellipsoid (1.5 %), obovoid (0.5 %), sickle-shaped (0.5 %) or ampulliform (0.5 %); special features like lateral attachment of the sporangiophore (24.2 %; Fig. 49E, K) and pedicels (34.8 %; Fig. 49B, D, G, I), vacuoles (Fig. 49D–F) and swellings close to the sporangial base (Fig. 49H, K) common; small hyphal appendices (Fig. 49E) occasionally observed; sporangial dimensions averaging 53.3 ± 5.0 × 34.5 ± 3.5 µm (overall range 40.3–65.0 × 26.8–42.9 µm; range of isolate means 52.6–54.9 × 34.1–34.8 µm) with a length/breadth ratio of 1.55 ± 0.15 (overall range 1.27–2.18); pedicel length 13.1 ± 6.3 µm (range 3.5–37.8); sporangial proliferation exclusively external (Fig. 49A–C, E, G, K); sporangial germination indirectly with zoospores discharged through an exit pore of 5.4–10.4 µm (av. 7.2 ± 0.8 µm; Fig. 47J). Zoospores limoniform to reniform whilst motile (Fig. 49J), becoming spherical (av. Diam = 10.2 ± 1.1 µm) on encystment; cysts germinating directly by producing hyphae. Hyphal swellings on sporangiophores common, liminiform, ovoid or subglobose (Fig. 49H, K), 14.9 ± 5.2 µm (7.9–27.9 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), on short, often curved stalks (74 %; Fig. 49L, M, Q–U) or sessile (26 %; Fig. 49N–P); smooth-walled, globose to subglobose (80 %; Fig. 49L–Q) or slightly elongated (20 %; Fig. 49R–U), with a round (72.7 %; Fig. 49L–P, S, U) or tapering base (27.3 %; Fig. 49Q, R, T); av. Diam 28.0 ± 2.2 µm with an overall range of 21.3–32.4 µm and a range of isolate means of 27.3–28.4 µm; slightly aplerotic to aplerotic (84 %; Fig. 49N–Q, S–U) or nearly plerotic to plerotic (16.0 %; Fig. 49L, M, R). Oospores globose with a large lipid globule (Fig. 49L–U), turning golden-brown during maturation (Fig. 49N–U); av. Diam 23.3 ± 1.4 µm with an overall range of 18.8–27.9 µm and a range of isolate means of 23.3–23.5 µm; wall diam 1.64 ± 0.19 µm (overall range 1.2–2.27 µm) and oospore wall index 0.37 ± 0.03; abortion rate after 4 wk very low (1–2 %; av. 1.3 %). Antheridia 1-celled, mostly paragynous and club-shaped, ovoid or subglobose (96 %; Fig. 49L–T) or infrequently amphigynous and cylindrical (4 %; Fig. 49U); 11.5 ± 2.3 × 7.4 ± 1.1 µm.
Culture characteristics: Colonies on V8A and CA appressed to submerged with a chrysanthemum-like pattern on V8A and a chrysanthemum-like to stellate pattern on CA; and petaloid and felty-cottony on PDA (Fig. 39).
Fig. 39.
Colony morphology of Phytophthora species from subclade 2c after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora fansipanensis (ex-type CBS 149485). B. Phytophthora japonensis (ex-type CBS 149489). C, D. Phytophthora limosa (C. ex-type CBS 149490; D. LU083). E. Phytophthora macroglobulosa (ex-type CBS 149491). F. Phytophthora multivora (ex-type CBS 124094).
Cardinal temperatures and growth rates: Optimum 25.0 °C with 7.92 ± 0.28 mm/d radial growth on V8A, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 7.49 ± 0.14 mm/d, 5.93 ± 0.15 mm/d and 3.0 ± 0.12 mm/d, respectively.
Additional materials examined: Vietnam, Sapa, Fansipan Mountain, isolated from baiting leaves floating in a stream running through a montane evergreen cloud forest, Mar. 2017, T. Jung, N.M. Chi & T. Corcobado (VN1093, VN1095, VN1097, VN1099).
Phytophthora japonensis T. Jung, A. Hieno, H. Masuya & J.F. Webber, sp. nov. MycoBank MB 847314. Fig. 50.
Fig. 50.
Phytophthora japonensis. A–L. Sporangia formed on V8-agar (V8A) in soil extract. A–J, L. Semipapillate apices. A–C, G–L. External proliferation. A–G, I, L. Ovoid and ellipsoid sporangia. B. Medium-length pedicel. F. Long pedicel and hyphal swelling. H. Distorted sporangium with laterally displaced apex. I–L. Distorted sporangia with two apices. I, L. Dense sporangial sympodia. M–U. Globose to subglobose oogonia with near-plerotic to plerotic oospores and paragynous antheridia formed in solid V8A. Q, R, U. Slightly excentric oogonia. Images: A–C, H, K, L, N, O, R–U. Ex-type CBS 149489; D, G, I, J, P. JP542; E, F, M, Q. JP279. Scale bar = 20 µm; U applies to A–U.
Etymology: Name refers to the distribution in several Japanese islands.
Typus: Japan, Kyushu Island, Aya, isolated from a baiting leaf floating in a stream running through a warm-temperate mixed forest, May 2017, T. Jung & A. Hieno (holotype CBS H-25112, dried culture on V8A, ex-holotype living culture CBS 149489 = JP467).
Morphological structures on V8A: Sporangia produced abundantly in non-sterile soil extract; typically borne terminally (98 %) in dense or lax sympodia of 2–8 sporangia (Fig. 50I, K, L) or less frequently on unbranched long or short sporangiophores (Fig. 50E), or rarely intercalary (2 %); predominantly ovoid, broad-ovoid or elongated ovoid (70.6 %; Fig. 50A–E, G, I) or distorted and often with two apices (24.1 %; Fig. 50H–L), infrequently ellipsoid or elongated-ellipsoid (2.7 %; Fig. 50F, L), ), obpyriform (1.4 %), limoniform (0.8 %) or obovoid (0.4 %); apices semipapillate, sometimes curved or laterally displaced (17 %; Fig. 50H, I, L); non-caducous; a conspicuous basal plug (15.6 %; Fig. 50K, L), pedicels (11.3 %; Fig. 50B) and small swellings close to the sporangial base (2 %; Fig. 50F) infrequently observed; sporangial proliferation exclusively external (Fig. 50A–C, G–L); sporangial dimensions averaging 55.0 ± 5.8 × 36.8 ± 3.8 µm (overall range 39.1–69.3 × 27.0–47.1 µm; range of isolate means 51.8–56.8 × 36.0–37.5 µm) with a length/breadth ratio of 1.5 ± 0.13 (overall range 1.24–1.89); pedicel length 11.3 ± 6.2 µm (range 5.2–22.0 µm); sporangial germination indirectly with zoospores discharged through an exit pore 4.5–9.9 µm wide (av. 6.9 ± 1.0 µm) (Fig. 50K, L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. Diam = 10.4 ± 1.0 µm) on encystment; cysts germinating directly. Hyphal swellings rarely produced in water on sporangiophores, globose to subglobose, ovoid or limoniform (Fig. 50F). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short, sometimes curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose (Fig. 50M–U), sometimes slightly excentric (16.7 %; Fig. 50Q, R, U), usually with a rounded (86 %; Fig. 50M–S, U) or infrequently a short tapering base (14 %; Fig. 50T); oogonial diam 28.9 ± 2.6 µm (overall range 17.3–33.6 µm; range of isolate means 28.4–29.3 µm); nearly plerotic to plerotic (Fig. 50M–U). Oospores with a large lipid globule, globose (Fig. 50M–T) or rarely subglobose (Fig. 50U); diam 25.2 ± 2.4 µm (overall range 15.5–29.8 µm; range of isolate means 24.8–25.6 µm) wall thickness 1.56 ± 0.23 µm (overall range 1.06–2.24 µm), oospore wall index 0.33 ± 0.04; abortion rate 1–2 % (av. 1.3 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ovoid or subglobose (Fig. 50M–U); dimensions 11.1 ± 2.0 × 8.0 ± 1.5 µm.
Culture characteristics: Colonies on V8A appressed with scanty aerial mycelium and a chrysanthemum-like pattern; on CA submerged to appressed with a faint petaloid pattern; on PDA dense-felty with irregular margins and a faint petaloid pattern (Fig. 39).
Cardinal temperatures and growth rates: On V8A optimum at 25 °C with 5.18 ± 0.09 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 4.48 ± 0.02 mm/d, 4.07 ± 1.67 mm/d and 1.92 ± 0.28 mm/d, respectively.
Additional materials examined: Japan, Kyushu Island, Aya, isolated from rhizosphere soil of Machilus thunbergii and Castanopsis cuspidata in a warm-temperate Fagaceae-Lauraceae forest, May 2017, T. Jung & A. Hieno (JP542, JP744); Kyushu Island, Takakuma, isolated from rhizosphere soil of Lithocarpus edulis in a warm-temperate Fagaceae-Lauraceae forest on volcanic soil, May 2017, T. Jung & J.F. Webber (JP279); isolated from a baiting leaf floating in a stream running through a warm-temperate Fagaceae-Lauraceae forest on volcanic soil, May 2017, T. Jung & M. Horta Jung (JP541); Shikoku Island, Satayama, isolated from a baiting leaf floating in a stream running through a warm-temperate Fagaceae-Lauraceae forest, May 2017, T. Jung & H. Masuya (JP600); Shikoku Island, Ichinomata, isolated from a baiting leaf floating in a stream running through a mixed Quercus-Torreya-Tsuga forest, May 2017, T. Jung & H. Masuya (JP106, JP107).
Phytophthora limosa T. Corcobado, T. Majek, M. Ferreira & T. Jung, sp. nov. MycoBank MB 847315. Fig. 51.
Fig. 51.
Phytophthora limosa. A–M. Sporangia formed on V8-agar (V8A) in soil extract. A–G, I, J–M. Ovoid, limoniform, obpyriform and mouse-shaped sporangia. A, B, D–G, J, L, M. Semipapillate apices. A, C, E, F, H. Medium-length pedicels (arrows). A, B, D, E, H, L. External proliferation. B. Swollen apex before zoospore release. H, I. Papillate apices. H. Distorted sporangium with displaced apex. J. Bilobed sporangium. K. Zoospore release. M. Dense sympodium. N–V. Subglobose to globose, excentric or elongated oogonia with plerotic to aplerotic oospores, formed in V8A. N–T, U. Paragynous antheridia. U. Amphigynous antheridium. V. Elongated oogonium with two oospores. Images: A–C, E, F, I, J, L, N, P, Q, S–V. Ex-type CBS 149490; D, G, H, LU079; K, M. BN113; O, R. LU083. Scale bars = 20 µm; V applies to A–L, N–V.
Etymology: The name refers to the occurrence in muddy soils (limosa Latin = muddy).
Typus: USA, Louisiana, Clark Creek, isolated from riverbank soil in a mixed subtropical forest, March 2020, T. Corcobado & T. Majek (holotype CBS H-25113, dried culture on V8A, ex-holotype living culture CBS 149490 = LU185).
Morphological structures on V8A: Sporangia rarely observed on solid agar but produced abundantly in non-sterile soil extract; borne terminally in dense or lax sympodia of 2–4 sporangia (Fig. 51M); non-caducous, ovoid, broad-ovoid or elongated ovoid (55.4 %; Fig. 51A, B, K–M), mouse- or sickle-shaped (15.5 %; Fig. 51C), distorted with often two apices (9.6 %; Fig. 51H, J), limoniform to elongated limoniform (8.2 %; Fig. 51D, G, I), obpyriform to elongated-obpyriform (7.2 %; Fig. 51E, F), ellipsoid to elongated-ellipsoid (3.8 %), pyriform (0.2 %) or obovoid (0.1 %); apices predominantly semipapillate (81.1 %; Fig. 51A–F, J, L, M), less frequently papillate (16.2 %; Fig. 51H, I) or rarely nonpapillate (2.7 %; Fig. 51G), frequently curved or laterally displaced (18.6 %; Fig. 51B, C, H, I); lateral attachment of the sporangiophore (10 %; Fig. 51L) and pedicels (35.6 %; Fig. 51A, C, F, H) commonly observed; sporangial proliferation exclusively external (Fig. 51A, B, D, E, H, L, M) with sometimes two new sporangiophores arising from the same node (Fig. 51L); sporangial dimensions averaging 52.7 ± 7.0 × 31.9 ± 3.1 µm (overall range 37.3–72.2 × 21.9–39.8 µm; range of isolate means 49.2–56.6 × 31.2–32.4 µm) with a length/breadth ratio of 1.66 ± 0.19 (overall range 1.25–2.6); pedicel length 23.9 ± 12.3 µm (range 8.1–57.9 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.9–11.2 µm wide (av. 6.9 ± 1.4 µm) (Fig. 51K). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 11.4 ± 1.9 µm) on encystment; cysts germinating directly. Hyphal swellings infrequently produced in water on sporangiophores; limoniform or ellipsoid, 14.1 ± 5.2 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose (64 %; Fig. 51N, O, Q, S, T), excentric (21 %; Fig. 51R, U), or elongated (15 %; Fig. 51P, V), with a rounded (84 %; Fig. 51N–R, U) or a short tapering base (16 %; Fig. 51S, T); oogonial diam 30.5 ± 3.3 µm (overall range 18.8–39.7 µm; range of isolate means 29.5–31.2 µm); nearly plerotic to plerotic (75.7 %; Fig. 51N, O, S–V) or slightly aplerotic to aplerotic (24.3 %; Fig. 51P–R); in rare cases two oospores inside of one oogonium (Fig. 51V). Oospores globose with a large lipid globule (Fig. 51N–V); diam 27.2 ± 3.1 µm (overall range 16.3–36.5 µm; range of isolate means 26.1–28.1 µm) wall thickness 1.52 ± 0.27 µm (overall range 0.72–2.31 µm), oospore wall index 0.3 ± 0.04; abortion rate 0–17 % (av. 7.3 %) after 4 wk. Antheridia almost exclusively paragynous and club-shaped, ovoid or subglobose (99.5 %; Fig. 51N–T, V) or rarely amphigynous and cylindrical (0.5 %; Fig. 51U); rarely two antheridia attached to one oogonium (Fig. 51Q); dimensions 12.4 ± 2.2 × 9.3 ± 1.6 µm.
Culture characteristics: Colonies on V8A and CA appressed with limited aerial mycelium, chrysanthemum-like on V8A and faintly radiate on CA; on PDA dense-felty and uniform (Fig. 39).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 8.2 ± 0.83 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 42), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 6.51 ± 0.45 mm/d, 5.93 ± 0.12 mm/d and 3.04 ± 0.17 mm/d, respectively.
Additional materials examined: USA, Louisiana, Clark Creek, isolated from riparian soil of a streambank in a mixed subtropical forest, March 2020, T. Corcobado & T. Majek (LU192); Louisiana, JC “Sonny” Gilbert, isolated from naturally fallen Magnolia leaves floating in a stream running through a mixed subtropical forest, March 2020, T. Corcobado & T. Majek (LU079, LU083). Bosnia-Herzegovina, Ozren Mountain, isolated from a necrotic leaf floating in a forest stream, Apr. 2019, I. Milenković (BN113); Teslić, isolated from rhizosphere soil of a riparian Alnus glutinosa tree, May 2019, I. Milenković (BN276, BN277).
Phytophthora macroglobulosa H.-C. Zeng, H.-H. Ho, F.-C. Zheng & T. Jung, sp. nov. MycoBank MB 847316. Fig. 52.
Fig. 52.
Phytophthora macroglobulosa. A–L. Sporangia formed on V8-agar (V8A) in soil extract. A–J. Semipapillate apices. A–I, K, L. Ovoid, ellipsoid, obpyriform and mouse-shaped, often elongated sporangia. B, D, F, L. External proliferation. B. Thick basal plug (arrow). F–H. Sporangia with vacuoles. J. Distorted multilobed sporangium. K. Zoospore release. L. Dense sympodium. M–V. Globose to subglobose oogonia with slightly aplerotic to near-plerotic oospores, formed in solid V8A. M–U. Oogonia with smooth walls and paragynous antheridia. V. Oogonium with slightly wavy wall and amphigynous antheridium. Images: A, M, N, P, Q. TJ1449. B–L, O, R–V Ex-type CBS 149491; Scale bar = 20 µm; V applies to A–V.
Etymology: The name refers to the large size of the lipid globule in most oospores.
Typus: China, Hainan Island, Wuzhi Mountain, isolated from soil in a tropical montane forest, 1999, H.-C. Zeng, H.-H. Ho & F.-C. Zheng (holotype CBS H-25114, dried culture on V8A, ex-holotype living culture CBS 149491 = TJ917 = FFM 2 2-2).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally on unbranched sporangiophores (Fig. 52A, E) or in dense or lax sympodia of 2–4 sporangia (Fig. 52L); non-caducous, predominantly ovoid, broad ovoid or elongated ovoid (77.2 %; Fig. 52A–D, L), less frequently ellipsoid to elongated-ellipsoid (9.4 %; Fig. 52E, F), obpyriform to elongated obpyriform, sometimes asymmetric (6.4 %; Fig. 52G, H), distorted with usually two or three apices (3 %; Fig. 52J), mouse-shaped (3 %; Fig. 52I, K), limoniform (0.6 %) or obovoid (0.4 %); apices semipapillate (Fig. 52A–J); vacuoles (18.2 %; Fig. 52F–H) common and pedicels (6.5 %) infrequently observed; sporangial dimensions averaging 61.2 ± 6.5 × 39.1 ± 3.9 µm (overall range 42.6–79.3 × 28.5–47.6 µm; range of isolate means 59.4–64.5 × 38.4–39.4) with a length/breadth ratio of 1.58 ± 0.19 (overall range 1.25–2.29); pedicel length 22.5 ± 14.9 µm (range 4.2–49.0 µm); sporangial proliferation exclusively external (Fig. 52B, D, F, L); sporangial germination indirectly with zoospores discharged through an exit pore of 6.6–10.8 µm (av. 8.5 ± 0.9 µm) (Fig. 52K). Zoospores limoniform to reniform whilst motile, becoming spherical, ovoid or sometimes irregular on encystment; cysts with an av. diam of 11.0 ± 1.8 µm, germinating mostly directly by producing hyphae or sometimes indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings on sporangiophores are rare, ovoid, limoniform or subglobose. Hyphal aggregations are infrequently produced. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), on short, often curved stalks (82 %) or sessile (18 %); globose to subglobose with round bases (94 %; Fig. 52M–T, V) or short tapering bases (6 %; Fig. 52U) and smooth or slightly wavy walls; av. diam 31.5 ± 2.8 µm with an overall range of 21.9–38.7 µm (range of isolate means 31.1–32.1 µm); slightly aplerotic to aplerotic (59 %; Fig. 52M–O, Q–T) or nearly plerotic (41 %; Fig. 52P, U, V). Oospores globose with a large lipid globule, often almost entirely filling the oospore (Fig. 52O, R, T–V); av. diam 26.9 ± 2.4 µm with an overall range of 18.5–32.3 µm (range of isolate means 26.5–27.3 µm); wall diam 1.58 ± 0.21 µm (overall range 1.13–2.22 µm) and oospore wall index 0.31 ± 0.03; abortion 22 % (6–34 %) after 4 wk. Antheridia almost exclusively paragynous (99.3 %), 1-celled and club-shaped, ovoid, limoniform or subglobose (Fig. 52M–U) or rarely amphigynous (Fig. 52V); 11.9 ± 2.3 × 7.7 ± 1.4 µm.
Culture characteristics: Colonies appressed with limited aerial mycelium and petaloid pattern on V8A and CA; appressed and dense-felty with a petaloid pattern on PDA (Fig. 39).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 9.47 ± 0.16 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 32.5–<35 °C. At 20 °C on V8A, CA and PDA 7.74 ± 0.04 mm/d, 5.93 ± 0.2 mm/d and 2.66 ± 0.24 mm/d, respectively.
Additional materials examined: China, Hainan Island, Wuzhi Mountain, isolated from soil in a tropical montane forest, 1999, H.-C. Zeng, H.-H. Ho & F.-C. Zheng (TJ1449, TJ1451).
Phytophthora nimia T. Jung, H. Masuya, A. Hieno & C.M. Brasier, sp. nov. MycoBank MB 847318. Fig. 53.
Fig. 53.
Phytophthora nimia. A–M. Sporangia formed on V8-agar (V8A) in soil extract. A–I, K. Semipapillate apices. A–G, J–M. Ovoid, ellipsoid, obpyriform and pyriform sporangia. A–E, G, H, M. External proliferation. A–E, G. Medium-length to long pedicels. B. Dense sympodium; one sporangium shedding from sporangiophore (arrow). C–I. Intercalary sporangia. F, H. Thick plugs below cytoplasm. H, I. Distorted sporangia. I. Two apices. J. Zoospore release. K. Caducous sporangium. L. Internal extended proliferation. M. Dense sympodium. N, O. Hyphal swellings on V8A in soil extract. P–V. Globose to subglobose oogonia with near-plerotic to plerotic oospores and paragynous antheridia, formed in V8A. T. Oogonium with two antheridia. Images: A, B, G, H, K, L, O, Q, T–V. Ex-type CBS 149494; C, M, N. JP772; D–F, R, S. JP750; I, J, P. JP100. Scale bars = 20 µm; V applies to A–L, N–V.
Etymology: The name refers to the infrequent occurrence of sporangia which are too big for the available cytoplasm and reduce the effective volume by the production of big plugs inside the sporangia (nimia Latin = too big).
Typus: Japan, Kyushu Island, Takakuma, isolated from rhizosphere soil of Castanopsis sieboldii in a warm-temperate Fagaceae-Lauraceae forest on volcanic soil, May 2017, T. Jung & H. Masuya (holotype CBS H-25117, dried culture on V8A, ex-holotype living culture CBS 149494 = JP490).
Morphological structures on V8A: Sporangia produced abundantly in non-sterile soil extract; typically borne terminally in dense or lax sympodia of 2–8 sporangia (97.7 %; Fig. 53B, M), or less frequently intercalary (1.8 %; Fig. 53C, H) or sessile (0.5 %; Fig. 53F); predominantly ovoid, broad-ovoid or elongated ovoid (75.1 %; Fig. 53A, B, J, K, M), less frequently ellipsoid to elongated-ellipsoid (8.5 %; Fig. 53C, G, L), obpyriform to elongated obpyriform (7 %; Fig. 53D), limoniform or elongated limoniform (6 %; Fig. 53B), pyriform to elongated pyriform (2.5 %; Fig. 53E, F) or distorted often with two apices (0.9 %; Fig. 53H, I); apices semipapillate (Fig. 53A–I, K, M); predominantly persistent but a few caducous sporangia (<1 %; Fig. 53B, K) were present in most isolates; infrequently sporangia too big for the available cytoplasm resulting in the production of big conspicuous plugs inside the sporangia to reduce the effective sporangial volume (3.7 %; Fig. 53B, F, H); lateral attachment of the sporangiophore (6 %;) pedicels (49 %; Fig. 53A–E, K) and a widening of the sporangiophore towards the sporangium (4.5 %; Fig. 53B, E) commonly observed; sporangial proliferation almost exclusively external (Fig. 53A–E, G, H, M), internal extended proliferation rarely occurring in several isolates (Fig. 53L); sporangial dimensions averaging 54.2 ± 6.7 × 35.0 ± 5.0 µm (overall range 30.0–72.6 × 22.4–62.3 µm; range of isolate means 52.2–56.8 × 33.9–36.1 µm) with a length/breadth ratio of 1.56 ± 0.2 (overall range 0.68–2.53); pedicel length 21.7 ± 15.9 µm (range 3.8–91.1 µm); sporangial germination indirectly with zoospores discharged through an exit pore 4.4–9.4 µm wide (av. 6.8 ± 0.8 µm) (Fig. 53J, L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 11.2 ± 1.5 µm) on encystment; cysts usually germinate directly but diplanetism occurs in all isolates. Hyphal swellings infrequently produced in water on sporangiophores and hyphae; globose to subglobose, ovoid, ellipsoid or allantoid (Fig. 53N, O). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, sometimes curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose (Fig. 53P–V), predominantly with a rounded (90.7 %; Fig. 53P–U) or a very short tapering base (9.3 %; Fig. 53V); oogonial diam 27.5 ± 2.6 µm (overall range 19.3–34.8 µm; range of isolate means 26.4–28.2 µm); nearly plerotic to plerotic (Fig. 53P–V). Oospores globose with a large lipid globule (Fig. 53P–V); diam 23.9 ± 2.3 µm (overall range 16.8–30.8 µm; range of isolate means 23.4–24.5 µm) wall thickness 1.62 ± 0.21 µm (overall range 1.08–2.17 µm), oospore wall index 0.35 ± 0.03; abortion rate 1–6 % (av. 3 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ellipsoid or subglobose (Fig. 53P–V); sometimes two antheridia attached to one oogonium (0.7 %; Fig. 53T); dimensions 11.5 ± 2.1 × 8.0 ± 1.6 µm.
Culture characteristics: Colonies on V8A rosaceous with limited aerial mycelium; on CA uniform and appressed with scanty aerial mycelium; on PDA uniform with irregular submerged margins, dense-felty appressed with limited aerial mycelium in the centre (Fig. 40).
Fig. 40.
Colony morphology of Phytophthora species from subclade 2c after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora nimia (ex-type CBS 149494). B. Phytophthora oblonga (ex-type CBS 149495). C. Phytophthora obturata (ex-type CBS 149496). D. Phytophthora pachypleura (ex-type IMI 502404). E, F. Phytophthora platani (E. ex-type CBS 149638; F. TJ1492).
Cardinal temperatures and growth rates: On V8A optimum at 25 °C with relatively slow radial growth of 5.15 ± 0.08 mm/d, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 4.38 ± 0.26 mm/d, 4.27 ± 0.18 mm/d and 3.58 ± 0.1 mm/d, respectively.
Additional materials examined: Japan, Kyushu Island, Takakuma, isolated from rhizosphere soil of C. sieboldii in a warm-temperate Fagaceae-Lauraceae forest on volcanic soil, May 2017, T. Jung & H. Masuya (JP750, JP772); Shikoku Island, Satayama, isolated from the rhizosphere of a mature M. thunbergii tree in a warm-temperate Fagaceae-Lauraceae forest, May 2017, T. Jung & A. Hieno (JP100).
Phytophthora oblonga T. Jung, S. Uematsu, K. Kageyama & C.M. Brasier, sp. nov. MycoBank MB 847306. Fig. 54.
Fig. 54.
Phytophthora oblonga. A–K. Sporangia formed on V8-agar (V8A) in soil extract. A–F, I. Semipapillate, often curved apices. G, H. Nonpapillate apices, A–G, J, K. Ovoid, obpyriform and mouse-shaped sporangia. A, B, D, H, K. External proliferation. D, I. Medium-length pedicels. D–F, H. Sporangia with vacuoles. G. Internal extended proliferation. H. Distorted sporangium with curved nonpapillate apex. I. Bilobed sporangium with thick basal plug (arrow) below the cytoplasm. J. Zoospore release. K. Dense sympodium. L, M. Ovoid, caducous sporangia with pedicels, formed on solid V8A. M. Zoospore release. N. Hyphal swelling on V8A in soil extract. O–W. Oogonia with near-plerotic to plerotic oospores and paragynous antheridia, formed in V8A. O–R. Globose to subglobose oogonia. S–W. Elongated oogonia. Images: A, D, E, G, H, K, M, O, P, S, V. Ex-type CBS 149495; B, C, I, L, W. JP366; F, J, N, Q, R, T, U. JP097. Scale bars = 20 µm; W applies to A–J, L–W.
Etymology: The name refers to the production of elongated oogonia and oospores (oblonga Latin = elongated).
Typus: Japan, Shikoku Island, Satayama, isolated from the rhizosphere of a mature Machilus thunbergii tree in a warm-temperate Fagaceae-Lauraceae forest, May 2017, T. Jung & S. Uematsu (holotype CBS H-25118, dried culture on V8A, ex-holotype living culture CBS 149495 = JP367).
Morphological structures on V8A: Sporangia produced infrequently on solid agar and abundantly in non-sterile soil extract; typically borne terminally (99.3 %) in dense or lax sympodia of 2–8 sporangia (Fig. 54K) or rarely intercalary (0.7 %); predominantly ovoid, broad-ovoid or elongated ovoid (80.3 %; Fig. 54A, B, G, J–M), less frequently obpyriform or elongated obpyriform (10 %; Fig. 54C–E), distorted and mostly with two apices (3.8 %; Fig. 54H, I), limoniform or elongated limoniform (1.8 %), mouse-shaped (1.7 %; Fig. 54F), ellipsoid or elongated-ellipsoid (1.4 %) or obovoid (1 %); apices usually semipapillate (92 %; Fig. 54A–F, I, L) or infrequently nonpapillate (8 %; Fig. 54G, H), sometimes curved or asymmetric (19.3 %; Fig. 54B, C, F–H); sporangia formed in water predominantly (>99 %) persistent but sporangia formed on solid agar almost exclusively caducous (>99 %; Fig. 54L, M); lateral attachment of the sporangiophore (12.3 %; Fig. 54C, E, F, L, M), pedicels (13.3 %; Fig. 54D, I, L, M), vacuoles (24.4 %; Fig. 54B, D–F, H) and a conspicuous basal plug (33.3 %; Fig. 54I) commonly observed; sporangial proliferation mostly external (Fig. 54A, B, D, G, H, K) or infrequently internal in an extended way (Fig. 54G); sporangial dimensions averaging 56.1 ± 6.6 × 37.2 ± 4.7 µm (overall range 38.4–72.1 × 23.8–48.6 µm; range of isolate means 53.1–58.9 × 34.8–39.8 µm) with a length/breadth ratio of 1.52 ± 0.15 (overall range 1.2–2.02); pedicel length 15.1 ± 7.1 µm (range 7.3–32.9 µm); sporangial germination indirectly with zoospores discharged through an exit pore 5.6–11.6 µm wide (av. 8.3 ± 1.1 µm) (Fig. 54G, J, K, M). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 11.6 ± 1.4 µm) on encystment; cysts germinating directly. Hyphal swellings rarely produced in water on sporangiophores and hyphae; globose to subglobose or limoniform (Fig. 54N). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, sometimes curved lateral hyphae or sessile, smooth-walled, globose to subglobose (48.1 %) with a rounded (85.2 %; Fig. 54O–Q) or a very short tapering base (14.8 %; Fig. 54R), or slightly elongated to tube-like or ampulliform elongated, usually with a tapering base (51.9 %; Fig. 54S–W); often comma-shaped (26 %; Fig. 54S, T, V, W); oogonial diam 26.6 ± 3.3 µm (overall range 15.0–38.9 µm; range of isolate means 24.9–28.6 µm); oogonial length ranging from 18.7 to 77.6 µm; nearly plerotic to plerotic (Fig. 54O–W). Oospores globose to subglobose with 1 large lipid globule (Fig. 54O–R) or slightly elongated to tube-like or ampulliform elongated with 1 or 2 large lipid globules (Fig. 54S–W); diam 23.6 ± 2.6 µm (overall range 12.3–30.5 µm; range of isolate means 23.0–24.8 µm), wall thickness 1.47 ± 0.2 µm (overall range 0.82–2.36 µm), oospore wall index 0.33 ± 0.03; abortion rate 1–8 % (av. 4.4 %) after 4 wk. Antheridia almost exclusively paragynous and club-shaped, ellipsoid or subglobose (99.5 %; Fig. 54O–W) or rarely amphigynous and cylindrical (0.5 %); sometimes two antheridia attached to one oogonium (0.4 %); dimensions 11.5 ± 2.1 × 7.6 ± 1.5 µm.
Culture characteristics: Colonies on V8A and CA submerged to suppressed with scanty aerial mycelium; on V8A chrysanthemum-like to radiate and on CA stellate; on PDA uniform with irregular submerged margins, dense-felty appressed (Fig. 40).
Cardinal temperatures and growth rates: On V8A optimum at 27.5 °C with 8.17 ± 0.11 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 6.32 ± 0.17 mm/d, 5.71 ± 0.03 mm/d and 2.83 ± 0.11 mm/d, respectively.
Additional materials examined: Japan, Shikoku Island, Satayama, isolated from the rhizosphere of a mature M. thunbergii tree in a warm-temperate Fagaceae-Lauraceae forest, May 2017, T. Jung & S. Uematsu (JP366); isolated from the rhizosphere of a mature C. sieboldii tree in a warm-temperate Fagaceae-Lauraceae forest, May 2017, T. Jung & K. Kageyama (JP097).
Phytophthora obturata T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, sp. nov. MycoBank MB 847301. Fig. 55.
Fig. 55.
Phytophthora obturata. A–N. Sporangia formed on V8-agar (V8A) in soil extract. A–J, N. Semipapillate apices. A–I, K–N. Ovoid, ellipsoid, limoniform and obpyriform sporangia. A, B, E, F, H, I, K, L, N. Short to medium-length pedicels (arrows). C, E, I, K. Thick plugs below cytoplasm. D. Hyphal swelling. F, M. External proliferation. J. Distorted sporangium with two apices and hyphal extension. K. Zoospore release. L. After zoospore release, with constriction of pedicel (arrow). M. Dense sympodium. N. Caducous sporangium. O. Zoospores with ring-like flagella ends (arrows). P. Catenulate hyphal swellings on V8A in soil extract. Q–Y. Globose, subglobose or elongated oogonia with plerotic to slightly aplerotic oospores and paragynous antheridia formed in V8A. W. Two antheridia (arrows). Images: A, B, E, H, J, M, N–S, X, Y. Ex-type CBS 149496; C, D, F, G, K, T–W. VN850; I, L. VN1005. Scale bars = 20 µm; Y applies to A–L, N–Y.
Etymology: The name refers to the conspicuous basal plug which is formed in many sporangia and often protrudes backwards into the sporangiophore (obturata Latin = plugged).
Typus: Vietnam, Ba Vì National Park, isolated from rhizosphere soil of Meliosma arnottiana in a subtropical humid evergreen forest, Mar. 2016, T. Jung & N.M. Chi (holotype CBS H-25119, dried culture on V8A, ex-holotype living culture CBS 149496 = VN528).
Morphological structures on V8A: Sporangia produced abundantly in non-sterile soil extract; typically borne terminally in dense or lax sympodia of 2–9 sporangia (Fig. 55M) or less frequently on unbranched long or short sporangiophores (Fig. 55A, H), or intercalary (2.5 %); predominantly ovoid, broad-ovoid or elongated ovoid (64.1 %; Fig. 55A–D, G, K, M, N), less frequently ellipsoid or elongated-ellipsoid (14.5 %; Fig. 55E), limoniform or elongated limoniform (11.5 %; Fig. 55F, L), distorted and usually with two apices (4.2 %; Fig. 55J), obpyriform (3 %; Fig. 55H), ampulliform (1.5 %), obovoid (1 %) or pyriform to elongated pyriform (0.2 %; Fig. 55I); apices semipapillate (Fig. 55A–J, M, N); usually persistent but a few caducous sporangia (0.2 %; Fig. 55N) and sporangia with a constriction of the sporangiophore enabling caducity (3.7 %; Fig. 55L) were present in all isolates; a conspicuous basal plug often protruding backward into the sporangiophore (55.7 %; Fig. 55C, F, I, L), lateral attachment of the sporangiophore (9.8 %; Fig. 55G, H), pedicels (49.6 %; Fig. 55A, B, E, F, H, I, K, L, N), slightly asymmetric shapes (11.6 %; Fig. 55G–I), a widening of the sporangiophore towards the sporangial base (1.2 %; Fig. 55C) and small sporangiophore swellings close to the sporangial base (0.7 %; Fig. 55D) commonly observed; sporangial proliferation exclusively external (Fig. 55F, M); sporangial dimensions averaging 53.9 ± 6.5 × 34.0 ± 4.3 µm (overall range 34.0–84.1 × 20.4–57.5 µm; range of isolate means 49.4–57.0 × 30.4–37.6 µm) with a length/breadth ratio of 1.6 ± 0.2 (overall range 0.97–2.48); pedicel length 15.6 ± 9.4 µm (range 1.5–62.5 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 3.7–10.3 µm wide (av. 7.5 ± 1.0 µm) (Fig. 55K, L). Zoospores limoniform to reniform whilst motile, often with flagella ends forming a ring (Fig. 55O); becoming spherical (av. diam = 11.0 ± 1.6 µm) on encystment; cysts mostly germinating directly although diplanetism occurred in all isolates. Hyphal swellings produced in water on sporangiophores and hyphae; globose to subglobose, ovoid, limoniform, deltoid or irregular (Fig. 55D, H, M, P), sometimes catenulate (Fig. 55P); dimensions 13.8 ± 4.7 µm (range 6.4–37.9 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, sometimes curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose (82.3 %; Fig. 55Q–W) or slightly elongated (17.7 %; Fig. 55X, Y), usually with a rounded (84.6 %; Fig. 55Q, S, U–X) or a short tapering base (15.4 %; Fig. 55R, T, Y); oogonial diam 26.0 ± 2.9 µm (overall range 17.4–35.1 µm; range of isolate means 22.7–27.1 µm); nearly plerotic to plerotic (96.4 %; Fig. 55Q–W) or slightly aplerotic (3.6 %; Fig. 55X, Y). Oospores globose with a large lipid globule (Fig. 55Q–Y); diam 23.7 ± 2.5 µm (overall range 15.6–32.2 µm; range of isolate means 21.0–24.8 µm) wall thickness 1.81 ± 0.24 µm (overall range 1.1–2.48 µm), oospore wall index 0.39 ± 0.04; abortion rate 1–6 % (av. 2.3 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ovoid or subglobose (Fig. 55Q–Y); sometimes two antheridia attached to one oogonium (Fig. 55W); dimensions 12.1 ± 2.4 × 6.9 ± 1.0 µm.
Culture characteristics: Colonies on V8A submerged to appressed with a chrysanthemum-like pattern; on CA appressed with scanty aerial mycelium and a chrysanthemum-like pattern; on PDA dense-felty with irregular margins and a faint petaloid pattern (Fig. 40).
Cardinal temperatures and growth rates: On V8A optimum at 25 °C (2 isolates) or 27.5 °C (6 isolates) with 9.43 ± 0.59 mm/d radial growth at 27.5 °C, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 7.67 ± 0.34 mm/d, 5.69 ± 0.11 mm/d and 3.6 ± 0.14 mm/d, respectively.
Additional materials examined: Vietnam, Ba Vì National Park, isolated from rhizosphere soil of M. arnottiana in a subtropical humid evergreen forest, Mar. 2016, T. Jung, M. Horta Jung & N.M. Chi (VN819, VN820, VN821, VN822, VN823); Sapa, Sau Chua Mountain, isolated from naturally fallen leaves floating in a stream running through a montane Chamaecyparis forest, Mar. 2017, T. Jung, B. Scanu & N.M. Chi (VN850, VN1005, VN1013).
Phytophthora platani T. Jung, A. Pérez-Sierra, S.O. Cacciola & M. Horta Jung, sp. nov. MycoBank MB 847302. Fig. 56.
Fig. 56.
Phytophthora platani. A–M. Sporangia formed on V8-agar (V8A) in soil extract. A–F, I, J, L, M. Ovoid, pyriform, ampulliform, obpyriform and limoniform sporangia. A–G. Semipapillate apices. A–D. Short to long pedicels (arrows in B, C). B. Pedicel swelling. C. Caducous sporangium. E. Intercalary sporangium with external proliferation (arrow). G. Ovoid sporangium arising from distorted sporangium. H. Bipapillate sporangium. I. Zoospore release. J, K. Internal nested proliferation. L. Internal extended proliferation. M. Dense compound sympodium. N. Hyphal swelling on V8A in soil extract. O. Zoospores with ring-like flagella ends. P–T. Subglobose to elongated oogonia with near-plerotic to slightly aplerotic oospores and paragynous antheridia formed in V8A. T. Two antheridia (arrows). Images: A, E, Q. APS_272a; B, D, F, G, J, M–O, R, T. Ex-type CBS 149638; C, H, I, K, L, P, S. VN965. Scale bars = 20 µm; T applies to A–L, N–T.
Etymology: The name refers to the isolation of all known isolates from necrotic tissue and rhizosphere soil of Platanus trees.
Typus: Italy, Sicily, Pantalica Nature Reserve, isolated from the rhizosphere of Platanus orientalis in a riparian forest, May 2013, T. Jung & S.O. Cacciola (holotype CBS H-25121, dried culture on V8A, ex-holotype living culture CBS 149638 = TJ812).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally in lax or dense sympodia of 2–15 sporangia (Fig. 56M) or less frequently on unbranched sporangiophores or intercalary (2.9 %; Fig. 56E); sporangia almost exclusively semipapillate (99 %; Fig. 56A–G, M) or rarely papillate (1 %; Fig. 56H), mostly ovoid, broad ovoid or elongated ovoid (54.3 %; Fig. 56A–C, G), less frequently limoniform to elongated-limoniform (18.9 %; Fig. 56I, L), obpyriform to elongated obpyriform (6.5 %; Fig. 56F), pyriform (4.3 %; Fig. 56D), distorted and often with two or three apices (8.4 %; Fig. 56G, H, K), ellipsoid (3.8 %; Fig. 56J), obovoid (3.2 %) or ampulliform (0.6 %; Fig. 56E); special features like lateral attachment of the sporangiophore (22.3 %; Fig. 56A, B, F, G), pedicels (41.1 %; Fig. 56A–D) and an often conspicuous basal plug (82.9 %; Fig. 56C, D, I, K, L) common; swellings close to the sporangial base (0.9 %; Fig. 56B) infrequently observed; almost exclusively non-caducous but a few caducous sporangia breakingoff at a constriction of the sporangiophore (0.9 %; Fig. 56C) were present in all isolates; proliferation external (Fig. 56E, M) and less frequently internal in a nested and extended way (Fig. 56J–M), or rarely by emergence from the wall of a mature sporangium (Fig. 56G); sporangial dimensions averaging 53.6 ± 8.1 × 34.0 ± 4.6 µm (overall range 29.3–91.6 × 21.1–56.3 µm; range of isolate means 48.7–63.8 × 30.1–37.6 µm) with a length/breadth ratio of 1.59 ± 0.22 (overall range 1.15–2.57); pedicel length 20.8 ± 11.3 µm (range 1.4–62.6 µm); sporangial germination indirectly with zoospores discharged through an exit pore of 4.0–10.4 µm (av. 6.7 ± 1.1 µm; Fig. 56I–L). Zoospores limoniform, subglobose or reniform whilst motile, often with ring-like flagella ends (Fig. 56O), becoming spherical (av. diam = 10.5 ± 1.0 µm) on encystment; cysts predominantly germinating directly by producing hyphae or less frequently by releasing a secondary zoospore (diplanetism). Hyphal swellings commonly produced in water, close to the sporangial base or at sporangiophore nodes; limoniform, subglobose, deltoid or irregular (Fig. 56M, N), 15.2 ± 2.9 µm (range 11.0–22.4 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), on short, often curved stalks (87.6 %; Fig. 56R–T) or sessile (12.4 %; Fig. 56P, Q); smooth-walled, globose to subglobose with a short tapering base (80.4 %; Fig. 56P–R) or slightly elongated (19.6 %; Fig. 56S, T), sometimes slightly excentric (13.6 %; Fig. 56S); av. diam 30.5 ± 2.6 µm with an overall range of 16.2–39.7 µm and a range of isolate means of 29.2–31.1 µm; slightly aplerotic to aplerotic (61.2 %; Fig. 56P, Q, S) or nearly plerotic to plerotic (38.8 %; Fig. 56R, T). Oospores globose or subglobose with a large lipid globule (Fig. 56P–T); av. diam 26.3 ± 2.3 µm with an overall range of 14.5–34.5 µm and a range of isolate means of 25.2–27.3 µm; wall diam 1.4 ± 0.22 µm (overall range 0.69–3.53 µm) and oospore wall index 0.29 ± 0.04; abortion rate after 4 wk 1–13 % (av. 5 %). Antheridia 1-celled, almost exclusively paragynous and club-shaped, ovoid or subglobose (99.8 %; Fig. 56P–T) or rarely amphigynous and cylindrical (0.2 %); sometimes two antheridia attached to one oogonium (Fig. 56T); 12.3 ± 2.4 × 9.1 ± 1.8 µm.
Culture characteristics: Colonies on V8A and CA appressed to submerged with scanty aerial mycelium and a stellate pattern on V8A and a faint radiate pattern on CA; dense felty-cottony with a faint petaloid on PDA (Fig. 40).
Cardinal temperatures and growth rates: On V8A optimum 25.0 °C with 9.28 ± 0.37 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 42), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 7.73 ± 0.36 mm/d, 5.82 ± 0.28 mm/d and 2.38 ± 0.17 mm/d, respectively.
Additional materials examined: Italy, Sicily, Pantalica Nature Reserve, isolated from the rhizosphere of P. orientalis in a riparian forest, May 2013, T. Jung & S.O. Cacciola (TJ1446, TJ1447, TJ1448); Sicily, Irminio Nature Reserve, isolated from the rhizosphere of P. orientalis in a riparian forest, May 2013, T. Jung & S.O. Cacciola (TJ965, TJ1349, TJ1350, TJ1351, TJ1352). UK, London, isolated from rhizosphere soil, a necrotic root and a bark canker on a branch of a roadside Platanus × acerifolia tree, Mar. 2020, A. Pérez-Sierra (TJ1492 = APS_272a, TJ1493 = APS_272b, TJ1494 = APS_272c).
Phytophthora pseudocapensis T. Jung, T.-T. Chang, N.M. Chi & M. Horta Jung, sp. nov. MycoBank MB 850536. Fig. 57.
Fig. 57.
Phytophthora pseudocapensis. A–N. Structures formed on V8-agar (V8A) in soil extract. A–K, M. Sporangia. A–I, K, M. Ovoid, limoniform, obpyriform, mouse-shaped and subglobose sporangia. A, D, H, J. Semipapillate apices. B, C, E, G, I. Papillate apices. B, D, F, G. Short to long pedicels (arrows). B, D, E, G, K, M. External proliferation. C. Intercalary sporangium. F. Nonpapillate apex. J. Two apices. K. Zoospore release. L. Catenulate hyphal swellings. M. Dense sympodium. N. Ovoid papillate sporangium with medium-length pedicel (arrow) formed on solid V8A. O–X. Globose, subglobose, slightly excentric or elongated oogonia with near-plerotic to slightly aplerotic oospores, formed in V8A. O–V. Paragynous antheridia. W, X. Amphigynous antheridia. Y. Hyphal aggregation in V8A. Images: A, B, D, F, H, L, O, P, T, U, X. Ex-type CBS 150638; C, G, P, S. SU415; E, M. JV027; J, K, Q, R, V, W. TW064; I, N. VN079; X. JV029. Scale bars = 20 µm; Y applies to A–L, N–Y.
Etymology: The name refers to the morphological similarity and phylogenetic relatedness to P. capensis.
Typus: Taiwan, Fushan, isolated from a baiting leaf floating in a tributary of Ha-pen River running through a subtropical Castanopsis-Machilus forest, Mar. 2013, T. Jung & T.-T. Chang (holotype CBS H-25296, dried culture on V8A, ex-holotype living culture CBS 150638 = TW045).
Morphological structures on V8A: Sporangia produced infrequently on solid agar and abundantly in non-sterile soil extract; typically borne terminally (96 %) in dense or lax sympodia of 2–6 sporangia (Fig. 57M) or rarely on unbranched sporangiophores, or intercalary (4 %; Fig. 57D); non-caducous, predominantly ovoid, broad-ovoid or elongated ovoid (68.2 %; Fig. 57A–C, K, M, N), less frequently obpyriform to elongated-obpyriform (18.6 %; Fig. 57E–H), limoniform to elongated limoniform (5.1 %; Fig. 57D), distorted and usually with two apices (2.5 %; Fig. 57J), subglobose (2.5 %; Fig. 57I), ellipsoid to elongated-ellipsoid (1.8 %), mouse-shaped (0.5 %; Fig. 57H), sickle-shaped (0.4 %) or pyriform (0.4 %); apices papillate (47.8 %; Fig. 57B, C, E, G, I), semipapillate (46.3 %; Fig. 57A, D, H, J, M) or infrequently nonpapillate (5.9 %; Fig. 57F); lateral attachment of the sporangiophore (26.8 %; Fig. 57E, G, H) and pedicels (46.3 %; Fig. 57B, C, F, G, N) common; a conspicuous basal plug (5.2 %; Fig. 57D) infrequently observed; sporangial proliferation exclusively external (Fig. 57B, D, E, G, K, M); sporangial dimensions averaging 55.9 ± 7.9 × 37.4 ± 4.5 µm (overall range 36.3–93.2 × 23.9–50.3 µm; range of isolate means 46.1–63.5 × 34.0–42.1 µm) with a length/breadth ratio of 1.5 ± 0.21 (overall range 1.09–2.51); pedicel length 33.0 ± 7.7 µm (5.3–86.8 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.2–8.2 µm wide (av. 6.0 ± 0.7 µm) (Fig. 57K). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.2 ± 10.8 µm) on encystment. Hyphal swellings infrequently produced in water on sporangiophores; subglobose to limoniform, sometimes catenulate (Fig. 57L, M); dimensions 13.6 ± 2.0 µm (range 11.9–16.8 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length lateral hyphae, smooth-walled, globose to slightly subglobose (60 %; Fig. 57O–R, T, X), slightly excentric to excentric (28.6 %; Fig. 57S, U, V), or slightly elongated (11.4 %; Fig. 57W), with a rounded (77.8 %; Fig. 57P–R, U–X) or a short tapering base (22.2 %; Fig. 57O, S, T); oogonial diam 26.3 ± 2.6 µm (overall range 19.3–35.7 µm; range of isolate means 24.1–29.6 µm); nearly plerotic to plerotic (62.5 %; Fig. 57O, P, T, X) or slightly aplerotic to aplerotic (37.5 %; Fig. 57Q–S, U–W). Oospores globose with a large lipid globule (Fig. 57O–X); diam 22.5 ± 2.2 µm (overall range 16.8–29.6 µm; range of isolate means 20.7–25.2 µm) wall thickness 1.13 ± 0.22 µm (overall range 0.7–1.8 µm), oospore wall index 0.27 ± 0.05; abortion rate 10–35 % (av. 19.2 %) after 4 wk. Antheridia predominantly paragynous and club-shaped, ovoid or subglobose (86.6 %; Fig. 57O–V) or less frequently amphigynous and cylindrical (13.4 %; Fig. 57W, X); dimensions 13.0 ± 2.8 × 9.0 ± 1.7 µm. Hyphal aggregations observed in all isolates (Fig. 57Y).
Culture characteristics: Colonies on V8A and CA appressed with limited aerial mycelium, radiate to stellate on V8A and radiate on CA; on PDA dense-felty with a petaloid pattern (Fig. 41).
Fig. 41.
Colony morphology of Phytophthora species from subclade 2c after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora pini (isolate SFB183). B. Phytophthora plurivora (ex-type CBS 124093). C–E. Phytophthora pseudocapensis (C. ex-type CBS 150638; D. JV027; E. VN079). F. Phytophthora vacuola (ex-type CBS 149503).
Cardinal temperatures and growth rates: Optimum 25 °C with 8.1 ± 0.44 mm/d radial growth on V8A, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 7.1 ± 0.51 mm/d, 5.38 ± 0.31 mm/d and 4.84 ± 0.31 mm/d, respectively.
Additional materials examined: Taiwan, Fushan, isolated from a baiting leaf floating in Cu-keng River, Mar. 2013, T. Jung, M. Horta Jung & T.-T. Chang (TW064). Indonesia, Java, Bandung, isolated from naturally fallen necrotic leaves of unidentified tree species floating in streams running through tropical montane rainforests, Feb. 2019, T. Jung, M. Tarigan & M. Junaid (JV027, JV029); Sulawesi, Pali, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical montane rainforest, May 2019, T. Jung, M. Junaid & M. Horta Jung (SL082); Sumatra, Lake Toba, isolated from naturally fallen necrotic leaves of unidentified tree species floating in streams running through tropical montane rainforests, Aug. 2018, T. Jung, M. Tarigan & I. Milenković (SU415, SU464, SU614); Sumatra, Padang, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical hill rainforest, Sep. 2018, T. Jung, M. Tarigan & T. Corcobado (SU692). Vietnam, Sapa, Fansipan Mountain, isolated from a baiting leaf floating in a stream running through a montane evergreen cloud forest, Mar. 2016, T. Jung, N.M. Chi & M. Horta Jung (VN079); isolated from a baiting leaf floating in a stream running through a montane evergreen cloud forest, Mar. 2016, T. Jung, N.M. Chi & C.M. Brasier (VN974).
Phytophthora vacuola T. Jung, H. Masuya, K. Kageyama & J.F. Webber, sp. nov. MycoBank MB 847305. Fig. 58.
Fig. 58.
Phytophthora vacuola. A–M. Ovoid, ellipsoid, obpyriform, limoniform and mouse-shaped, mostly elongated sporangia formed on V8-agar (V8A) in soil extract. A, C, D, I. Semipapillate apices. B, E–H, J, K, M. Papillate apices. D–F, I–L. Curved apices. A. Hyphal swelling. A, C, E, G, H, J, K. Sporangia with vacuoles. A, F, G, M. External proliferation. C. Short pedicel (arrow). L. Zoospore release. M. Sympodium and medium-length pedicel (arrow). N–X. Oogonia with smooth or wavey walls and plerotic to slightly aplerotic oospores, formed in V8A. N. Amphigynous antheridium. O–X. Paragynous antheridia. N–Q. Subglobose to globose oogonia. R–X. Excentric, comma-shaped or elongated oogonia. Images: A–K, M, P–U, X. Ex-type CBS 149503; L, O. JP229b; N, V, W, JP1060. Scale bar = 20 µm; X applies to A–X.
Etymology: The name refers to the common production of sporangia with vacuoles.
Typus: Japan, Honshu Island, Takayama, isolated from a baiting leaf floating in a stream running through a montane riparian Quercus crispula forest, May 2017, T. Jung & K. Kageyama (holotype CBS H-25127, dried culture on V8A, ex-holotype living culture CBS 149503 = JP229).
Morphological structures on V8A: Sporangia produced abundantly in non-sterile soil extract; typically borne terminally (99 %) on unbranched long or short sporangiophores or in dense or lax sympodia of 2–4 sporangia (Fig. 58M) or rarely intercalary (1 %); mostly ovoid, broad-ovoid or elongated ovoid (58 %; Fig. 58A–C, M) or less frequently mouse-shaped (13.5 %; Fig. 58D, E, J, L), obpyriform to elongated-obpyriform (11.5 %; Fig. 58G, H), ellipsoid or elongated-ellipsoid (7.5 %; Fig. 58F), limoniform or elongated limoniform (5.5 %; Fig. 58I), ampulliform (2.5 %; Fig. 58K) or distorted (1.5 %); sporangia with more than one apex not observed; apices often curved or laterally displaced (36.8 %; Fig. 58D–F, I–L), predominantly papillate (77.3 %; Fig. 58B, E–H, J, K, M) or less frequently semipapillate (22.7 %; Fig. 58A, C, D, I) with a smooth transition between both forms; non-caducous; vacuoles (30.4 %; Fig. 58A, C, E, G, H, J, K), lateral attachment of the sporangiophore (24 %; Fig. 58B, D, J, L) commonly observed; infrequently with pedicels (6 %; Fig. 58C, M) of 21.4 ± 7.5 µm (range 16.0–26.7 µm) length; sporangial proliferation exclusively external (Fig. 58A, F, G, M); sporangial dimensions averaging 55.5 ± 6.7 × 35.9 ± 3.6 µm (overall range 38.9–90.9 × 24.3–43.2 µm; range of isolate means 54.9–56.1 × 34.8–37.1 µm) with a length/breadth ratio of 1.55 ± 0.21 (overall range 1.27–2.42); sporangial germination indirectly with zoospores discharged through an exit pore 5.0–8.4 µm wide (av. 6.8 ± 0.9 µm) (Fig. 58L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.7 ± 1.1 µm) on encystment; cysts germinating directly. Hyphal swellings produced infrequently in water on sporangiophores and hyphae; globose to subglobose, ovoid or limoniform (Fig. 57A). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, sometimes curved lateral hyphae or sessile, with smooth (Fig. 58N, O, Q, S–X) or slightly wavy wall (Fig. 58P, R); globose to slightly subglobose (58 %; Fig. 58N–S), elongated (22 %; Fig. 58V–X) or comma-shaped (20 %; Fig. 58T, U), often slightly excentric (29 %; Fig. 58R–T); with a rounded (88 %; Fig. 58N–Q, S, W, X) or less frequently a short tapering base (12 %; Fig. 58R, T–V); oogonial diam 27.9 ± 2.9 µm (overall range 21.4–36.1 µm; range of isolate means 26.6–29.2 µm); nearly plerotic to plerotic (53 %; Fig. 58N– Q, V–X) or slightly aplerotic (47 %; Fig. 58R–X). Oospores globose with a large lipid globule (Fig. 58N–X); diam 23.9 ± 2.3 µm (overall range 18.2–30.4 µm; range of isolate means 22.9–25.0 µm) wall thickness 1.73 ± 0.28 µm (overall range 1.23–2.48 µm), oospore wall index 0.37 ± 0.04; abortion rate 8–12 % (av. 10 %) after 4 wk. Antheridia predominantly paragynous and club-shaped, ellipsoid or subglobose (98 %; Fig. 58O–X), occasionally amphigynous and cylindrical (2 %; Fig. 58N); dimensions 12.0 ± 2.2 × 8.7 ± 1.5 µm.
Culture characteristics: Colonies on V8A and CA appressed to submerged with scanty aerial mycelium, radiate to stellate on V8A and faintly radiate on CA; on PDA felty-cottony with a faint stellate pattern (Fig. 41).
Cardinal temperatures and growth rates: On V8A optimum at 25 °C with 7.69 ± 1.44 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 42), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 7.39 ± 0.96 mm/d, 4.93 ± 0.06 mm/d and 2.99 ± 0.88 mm/d, respectively.
Additional materials examined: Japan, Honshu Island, Takayama, isolated from a baiting leaf floating in a stream running through a montane Q. crispula forest, May 2017, T. Jung & K. Kageyama (JP229b); Honshu Island, Appi, isolated from the rhizosphere of an Alnus hirsuta tree in a riparian forest, Jun. 2017, T. Jung & H. Masuya (JP1060).
Notes on Clade 2c taxa: Across the nuclear (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) 8 740-character alignment and the mitochondrial (cox1, cox2, nadh1 and rps10) 3 156-character alignment pairwise sequence differences between the nine known and 15 newly described Phytophthora species in Clade 2c were 0.1–3.2 % and 0.2–4.7 %, respectively. The 15 new and the five known Clade 2c species examined (viz. P. acerina, P. multivora, P. pachypleura, P. pini and P. plurivora) developed distinctive colony morphologies on V8A, CA and PDA at 20 °C (Figs 38–41). In addition, the 15 new species are separated from each other and other Clade 2c species by a combination of morphological (Figs 44–58) and physiological (Figs 42, 43) characters of which the most discriminating are highlighted in bold in Tables S10–S13.
The seven isolates of P. pini examined in this study showed several morphological and morphometric differences to the redescription of this species by Hong et al. (2011), most notably larger sporangia (55.6 × 35.4 vs. 47.4 × 31.5 µm), the occurrence of papillate (5.7 %) and nonpapillate (1.7 %) sporangia and aplerotic oospores (38 %), lower maximum (30–<32.5 vs. 35 °C) and slightly higher optimum temperature (27.5 vs. 25 °C) for growth (Table S13). For the following phenotypic comparisons within Clade 2c species, the P. pini data from this study will be used.
The sporangia of the majority of species in Clade 2c have exclusively semipapillate apices, i.e., P. acerina, P. capensis, P. caryae, P. citricola, P. fansipanensis, P. japonensis, P. macroglobulosa, P. multivora, P. nimia, P. obturata, P. pachypleura and P. plurivora, or predominantly semipapillate apices, i.e., P. balkanensis, P. catenulata, P. emzansi, P. excentrica, P. falcata, P. limosa, P. oblonga, P. pini and P. platani (Jung & Burgess 2009, Scott et al. 2009, Ginetti et al. 2014, Henricot et al. 2014, Brazee et al. 2017; Tables S10–S13; Figs 44, 45, 47–56). However, several species are clearly distinguished by producing, in addition to semipapillate sporangia, a significant proportion of either papillate sporangia, i.e., P. curvata (48 %), P. excentrica (13 %), P. falcata (13 %), P. limosa (16.2 %) and P. vacuola (77.3 %), or nonpapillate sporangia, i.e., P. emzansi (10 %), P. excentrica (6 %) and P. oblonga (8 %). Phytophthora pseudocapensis is characterised by having high proportions of both papillate (47.8 %) and semipapillate (46.3 %) sporangia and, in addition, also produces nonpapillate sporangia (5.9 %). In contrast, sporangial apices are exclusively papillate in P. capensis and predominantly papillate in P. vacuola (77.3 %) (Bezuidenhout et al. 2010, Bose et al. 2021a; Tables S10–S13; Figs 46–48, 51, 54, 57, 58).
High sporangial l/b ratios separate P. citricola (1.73 ± 0.28), P. emzansi (1.8–1.9), P. falcata (1.88 ± 0.25), P. limosa (1.76 ± 0.19), P. multivora (1.7 ± 0.22) and P. pachypleura (1.82 ± 0.05) from the majority of Clade 2c species which tend, on average, to form more squat sporangia (Tables S10–S13). In water the sporangia of most Clade 2c species, except P. curvata, are partially pedicellate with the proportion of pedicels ranging widely from only 6–16.5 % in P. japonensis, P. macroglobulosa, P. oblonga and P. vacuola to 42.7–51.6 % in P. falcata, P. nimia, P. obturata, P. pini, P. platani and P. pseudocapensis (Tables S10–S13; Figs 44–58). In contrast to the majority of Clade 2c species with exclusively persistent sporangia, P. catenulata, P. falcata, P. nimia, P. oblonga, P. obturata and P. platani show a low level of caducity (<1 %) in water (Tables S10–S13; Figs 45, 48, 53–56). It is noteworthy that P. oblonga commonly forms sporangia on solid agar which are predominantly caducous (Fig. 54).
Apart from P. capensis (Bezuidenhout et al. 2010), all species from Clade 2c show external sporangial proliferation and form sympodia. Phytophthora acerina, P. emzansi, P. macroglobulosa and P. pachypleura can be discriminated by forming only simple lax sympodia whereas the other Clade 2c species form both lax and dense, sometimes compound sympodia (Bezuidenhout et al. 2010, Ginetti et al. 2014, Henricot et al. 2014; Tables S10–S13; Figs 44–58). In several species, including P. balkanensis, P. catenulata, P. excentrica, P. japonensis, P. nimia, P. oblonga and P. obturata, the sympodia can contain up to 8 or 9 sporangia. In P. platani sympodia with up to 15 sporangia have been observed (Fig. 56). Internal sporangial proliferation occurs only in three Clade 2c species, clearly discriminating them from all other 2c species. Phytophthora nimia and P. oblonga show only occasional internal extended proliferation (Figs 53, 54) whereas in P. platani both extended and nested internal proliferation are relatively frequent (Fig. 56).
Moreover, the occurrence of some sporangial and hyphal features or their comparatively high proportions discriminate several Clade 2c species from other 2c species, e.g. the common occurrence of catenulate hyphal swellings in P. catenulata; high proportions of curved, mouse- or sickle-shaped sporangia in P. curvata (24 %), P. falcata (34.2 %) and P. limosa (15.5 %), distorted sporangia with often two or three apices in P. curvata (23 %) and P. japonensis (24.1 %) (Tables S10–S12), or ellipsoid sporangia in P. pachypleura (27 %; Henricot et al. 2014); the high frequency of curved or asymmetric sporangial apices in P. curvata (34.7 %), and P. vacuola (38.6 %) (Figs 46, 58); in P. obturata the high proportion (55.7 %) of sporangia with a conspicuous basal plug, often protruding backwards into the sporangiophore (Fig. 55); and the high proportion (30.4 %) of sporangia containing vacuoles in P. vacuola (Fig. 58).
All known and new species in Clade 2c are intrinsically self-fertile. Comparatively high proportions of oogonia with tapering instead of rounded bases distinguish P. caryae (53 %), P. curvata (46 %), P. falcata (46 %), P. oblonga (59 %) and P. platani (80.4 %) from other Clade 2c species (Brazee et al. 2017; Tables S10–S13; Figs 44–58). Likewise, a high proportion of excentric, elongated or comma-shaped oogonia discriminate P. curvata (38 %), P. excentrica (53 %), P. falcata (36.5 %), P. limosa (36 %), P. oblonga (51.9 %), P. platani (33.2 %) and P. vacuola (42 %) from other Clade 2c species (Tables S10–S13; Figs 44–58). Phytophthora oblonga differs from all other species by having particularly elongated oogonia (up to 77.6 µm; Fig. 54). The oospore wall index, i.e., the proportion of the oospore volume occupied by the oospore wall, is particularly high in P. pachypleura (0.71; Henricot et al. 2014), P. capensis (0.56; Bezuidenhout et al. 2010) and P. multivora (0.52; Scott et al. 2009), and moderately high in P. obturata (0.39), P. curvata (0.38), P. acerina (Ginetti et al. 2014), P. fansipanensis and P. vacuola (all 0.37) (Tables S10–S13). Relatively high abortion rates are typical for P. acerina (38.5 %; Ginetti et al. 2014), P. caryae (46 %; Brazee et al. 2017), P. emzansi (42–46 %; Bezuidenhout et al. 2010) and P. macroglobulosa (22 %). The latter species is also characterised by having particularly large lipid globules, sometimes filling the oospore almost entirely (Fig. 52). Phytophthora emzansi is the only species in Clade 2c with exclusively amphigynous antheridia (Bezuidenhout et al. 2010) while the antheridia in all other Clade 2c species are predominantly paragynous and only infrequently amphigynous, i.e., P. pseudocapensis (13.4 %), P. catenulata (4.2 %) and P. fansipanensis (4 %); predominantly paragynous and rarely (<1 %) amphigynous, i.e., P. balkanensis, P. citricola (Jung & Burgess 2009), P. curvata, P. excentrica, P. falcata, P. limosa, P. multivora (Scott et al. 2009), P. oblonga, P. pini, P. platani and P. plurivora (Jung & Burgess 2009); or exclusively paragynous (all other Clade 2c species) (Tables S10–S13).
Except for P. emzansi which grows optimally at 20 °C, all species from Clade 2c have an optimum temperature for growth of 25 or 27.5 °C (Jung & Burgess 2009, Bezuidenhout et al. 2010, Brazee et al. 2017, Bose et al. 2021a; Tables S10–S13; Figs 42, 43). Phytophthora limosa from subtropical forests in Louisiana, USA and P. transposita from a warm-temperate forest in Kyushu, Japan show fast growth at 30 °C, discriminating them from P. balkanensis, P. excentrica, P. falcata, P. multivora, P. pini, P. platani and P. plurivora with moderate to slow growth at 30 °C and all other Clade 2c species which have maximum temperatures below 30 °C (Tables S10–S13; Figs 42, 43).
Several new Clade 2c species described here were previously known under informal names, i.e., P. balkanensis as P. citricola E (Jung & Burgess 2009); P. catenulata as P. citricola VII (Jung et al. 2017b, 2020); P. fansipanensis as P. citricola VIII and P. obturata as P. citricola IX (Jung et al. 2020); P. macroglobulosa as P. sp. citricola-like VIII and P. limosa as P. sp. 22F3 (Yang et al. 2017). Isolates of P. pseudocapensis were previously designated as P. capensis (Jung et al. 2017b, 2020).
Clade 2e
For all Clade 2e species included in this study, colony morphologies on CA, PDA and V8A and temperature-growth relations on V8A are presented in Figs 59–62. Morphological and physiological characters and morphometric data of the five known and six newly described species and one informally designated taxon in Clade 2e are given in the comprehensive Tables S14 and S15.
Fig. 59.
Colony morphology of Phytophthora species from subclade 2e after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora acaciivora (isolate SU1734). B. Phytophthora amamensis (ex-type CBS 149474). C, D. Phytophthora borneensis (C. ex-type CBS 149478; D. KA595). E. Phytophthora celeris (SU1509). F. Phytophthora elongata (ex-type CBS 125799).
Fig. 62.
Mean radial growth rates of three known and eight new Phytophthora species on V8-agar at different temperatures: From subclade 2e P. acaciivora (4 isolates); P. amamensis (4 isolates); P. borneensis (7 isolates); P. celeris (6 isolates); P. elongata (5 isolates); P. frigida (5 isolates); P. indonesiensis (9 isolates from Kalimantan and Sumatra; 3 isolates from Sulawesi); P. penetrans (5 isolates). From subclade 2f, P. angustata (6 isolates); P. furcata (6 isolates); P. sumatera (9 isolates).
Phytophthora amamensis T. Jung, K. Kageyama, H. Masuya & S. Uematsu, sp. nov. MycoBank MB 847319. Fig. 63.
Fig. 63.
Phytophthora amamensis. A–L. Ovoid, obpyriform, ampulliform and ellipsoid sporangia formed on V8-agar (V8A) in soil extract. A–M. A. Papillate apex. B–E, G–I. Ssemipapillate apices. B–H, J–L. External proliferation. F. Nonpapillate sporangium with medium-length pedicel (arrow). I. Curved apex. J. Swollen apex before zoospore release. K. Zoospore release. L. After zoospore release, with conspicuous basal plug and long pedicel. M–Y. Subglobose oogonia with slightly aplerotic to aplerotic oospores and paragynous antheridia formed in V8A. Z. Oogonium with paragynous antheridium and oospore germinating (arrow). Images: A, B, D, E, G, L, M–P, S, V, X, Z. Ex-type CBS 149474; C, H, Q, R, W. JP2342; F, I–K. T, U, Y. JP2341. Scale bar = 20 µm; Z applies to A–Z.
Etymology: The name refers to the origin of the first isolates on the Japanese island of Amami-Ōshima.
Typus: Japan, Amami-Ōshima, Kasari, isolated from a naturally fallen tree leaf floating in a stream running through a subtropical lowland forest, Nov. 2018, T. Jung & K. Kageyama (holotype CBS H-25096, dried culture on V8A, ex-holotype living culture CBS 149474 = JP1340).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally, on unbranched sporangiophores or in lax sympodia of 2–3 sporangia (Fig. 63E), or infrequently on short lateral hyphae (Fig. 63A); non-caducous, predominantly ovoid to elongated ovoid (56.5 %; Fig. 63A, B, E, J) or obpyriform to elongated-obpyriform (39 %; Fig. 63C–F, L), ampulliform (2.5 %; Fig. 63G, H), elongated-ellipsoid (1.5 %; Fig. 63I) or limoniform (0.5 %); a curved, laterally displaced apex (12 %; Fig. 63I) or a pedicel (10 %; Fig. 63F, L) sometimes, and a conspicuous basal plug (47 %; Fig. 63F, L) commonly observed; apices predominantly shallow semipapillate (89.6 %; Fig. 63B–E, G–I), less frequently papillate (5.4 %; Fig. 63A) or nonpapillate (5 %; Fig. 63F) with a smooth transition between all types; sporangial proliferation exclusively external (Fig. 63B–H, J–L); sporangial dimensions averaging 56.5 ± 7.5 × 30.6 ± 3.6 µm (overall range 39.5–75.5 × 22.6–42.0 µm; range of isolate means 53.0–59.1 × 29.2–31.9 µm) with a length/breadth ratio of 1.85 ± 0.18 (overall range 1.41–2.82); pedicel length varying from 4.3 to 89.4 µm (av. 29.0 ± 21.0 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.5–7.8 µm wide (av. 6.0 ± 0.7 µm) (Fig. 63K, L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.2 ± 0.8 µm) on encystment. Hyphal swellings and chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), sessile or terminal on short to medium-length, often curved lateral hyphae, smooth-walled, globose to subglobose (64 %; Fig. 63M–P, R–T, Z) or elongated (36 %; Fig. 63Q, U–Y), with a nearly round base (26 %; Fig. 63M–O, Z) or a tapering base (74 %; Fig. 63P–Y); oogonial diam 26.4 ± 2.3 µm (overall range 17.5–31.6 µm; range of isolate means 25.5–27.2 µm); slightly aplerotic to aplerotic (Fig. 63M–Y). Oospores globose with a large lipid globule (Fig. 63M–Y); diam 22.5 ± 1.9 µm (overall range 16.4–27.8 µm; range of isolate means 22.1–23.1 µm) wall thickness 1.49 ± 0.21 µm (overall range 0.54–2.2 µm), oospore wall index 0.35 ± 0.03; abortion 8–35 % (av. 21 %) after 4 wk; after 2 mo at 20 °C many oospores germinating with 1 or 2 hyphae (Fig. 63Z). Antheridia exclusively paragynous and club-shaped, ovoid, subglobose or irregular (Fig. 62M–Z); 13.4 ± 2.2 × 10.5 ± 1.9 µm.
Culture characteristics: Colonies on V8A and CA mostly submerged to appressed and faintly radiate to striate; on PDA dense-felty, uniform with irregular submerged margins (Fig. 59).
Cardinal temperatures and growth rates: Optimum 27.5 °C with 7.1 ± 0.07 mm/d radial growth on V8A, maximum 30–<32.5 °C, minimum >10–15 °C (Fig. 62), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 6.29 ± 0.09 mm/d, 5.28 ± 0.13 mm/d and 3.24 ± 0.07 mm/d, respectively.
Additional materials examined: Japan, Amami-Ōshima, Kasari, isolated from naturally fallen tree leaves floating in a stream running through a subtropical lowland forest, Nov. 2018, T. Jung & K. Kageyama (JP2341, JP2342, JP2343).
Phytophthora borneensis T. Jung, A. Durán, M. Tarigan & M. Horta Jung, sp. nov. MycoBank MB 847320. Fig. 64.
Fig. 64.
Phytophthora borneensis. A–I. Sporangia formed on V8-agar (V8A) in soil extract. A–D, F–I. Ovoid and obpyriform sporangia. A, F–H. External proliferation (arrows in A, F). A. Swollen apex before zoospore release. B–D. Nonpapillate apices. E, F. Semipapillate apices. E. Distorted sporangium with two apices. F, G. Sympodia. F–I. Conspicuous basal plugs. H. Zoospore release. I. Internal extended proliferation with new sporangiophore branching outside the sporangium (arrow). J–V. Globose to subglobose oogonia with near-plerotic to aplerotic oospores and paragynous antheridia, formed in V8A. J–R. With round bases. S–V. With tapering bases. Images: A, E, J, P, Q, S–U. KA595a; B–D, G–I, K–O, R, V. Ex-type CBS 149478; F. KA532. Scale bar = 20 µm; V applies to A–V.
Etymology: The name refers to the origin of the first isolates in Borneo.
Typus: Indonesia, Kalimantan, Balikpapan, isolated from rhizosphere soil of Horsfieldia grandis in a tropical lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (holotype CBS H-25100, dried culture on V8A, ex-holotype living culture CBS 149478 = KA527).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally, on unbranched sporangiophores or in dense or lax sympodia of 2–4 sporangia (Fig. 64F, G); non-caducous, mostly ovoid, broad-ovoid or elongated ovoid (57.5 %; Fig. 64A, B, F–I) or obpyriform (33.5 %; Fig. 64C), less frequently ellipsoid (4.5 %), obovoid (2 %), limoniform (1.5 %; Fig. 64D) or distorted, usually with two apices (1 %; Fig. 64E); sporangiophores sometimes slightly laterally attached to the sporangia (18.5 %; Fig. 64A, H); conspicuous basal plugs, often covering the sporangial base and protruding into the sporangium, (62.5 %; Fig. 64D, F–I) common; pedicels infrequently (12.3 %) observed; sporangial apices semipapillate (57 %; Fig. 64A, E, F) or nonpapillate (43 %; Fig. 64B–D) and sometimes pointed (Fig. 64D); sporangial proliferation mostly external (Fig. 64A, F–H) or sometimes internal in an extended way (Fig. 64I); sporangial dimensions averaging 60.9 ± 11.4 × 38.4 ± 5.5 µm (overall range 33.9–83.4 × 24.4–53.5 µm; range of isolate means 54.9–70.6 × 35.6–41.5 µm) with a length/breadth ratio of 1.58 ± 0.17 (overall range 1.15–2.22); pedicel length 16.2 ± 5.6 µm (range 8.2–29.7 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 3.5–11.6 µm wide (av. 7.1 ± 1.3 µm) (Fig. 64F–I). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.9 ± 1.1 µm) on encystment. Hyphal swellings and chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, thin, sometimes (22 %) curved lateral hyphae, smooth-walled, globose to slightly subglobose (Fig. 64J–V), mostly with a rounded (81 %; Fig. 64J–R) or sometimes with a tapering base (19 %; Fig. 64S–V); oogonial wall predominantly smooth (Fig. 64J–L, N–P, S–V) or less frequently slightly wavy (Fig. 64M, Q, R); oogonial diam 26.2 ± 2.8 µm (overall range 17.8–36.3 µm; range of isolate means 24.2–28.7 µm); slightly aplerotic to aplerotic (63 %; Fig. 64J–R) or nearly plerotic (37 %; Fig. 64S–V). Oospores globose with a large lipid globule (Fig. 64J–V); mean diam 22.3 ± 2.3 µm (overall range 12.9–27.8 µm; range of isolate means 20.7–24.2 µm); wall thickness 1.62 ± 0.25 µm (overall range 0.87–2.06 µm), oospore wall index 0.35 ± 0.03; abortion 23–50 % (av. 41 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ovoid, or subglobose (Fig. 64J–V); 10.5 ± 2.1 × 7.8 ± 1.1 µm.
Culture characteristics: Colonies stellate to petaloid and appressed with limited aerial mycelium on V8A; faintly radiate to uniform and mostly submerged on CA; and uniform and felty-cottony on PDA (Fig. 59).
Cardinal temperatures and growth rates: Optimum 25–27.5 °C with 9.6 ± 0.13 and 9.5 ± 0.42 mm/d radial growth on V8A at 25 and 27.5 °C, respectively, maximum 32.5–<35 °C, minimum >10–15 °C (Fig. 62), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 6.96 ± 0.22 mm/d, 5.4 ± 0.19 mm/d and 3.02 ± 1.08 mm/d, respectively.
Additional materials examined: Indonesia, Kalimantan, Balikpapan, isolated from rhizosphere soil of H. grandis in a tropical lowland rainforest, Feb. 2019, T. Jung, M. Tarigan & M. Junaid (KA528, KA529, KA530, KA531); isolated from rhizosphere soil of Gmelina uniflora in a tropical lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (KA502a); isolated from rhizosphere soil of Macaranga sp. in a tropical lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (KA543, KA595a).
Phytophthora celeris T. Jung, L. Oliveira, M. Tarigan & I. Milenković, sp. nov. MycoBank MB 847321. Fig. 65.
Fig. 65.
Phytophthora celeris. A–K. Ovoid, limoniform, obpyriform and ampulliform sporangia formed on V8-agar (V8A) in soil extract. A–G, J. Semipapillate apices. C, D, G. External proliferation (arrows in D, G). C. Sympodium; mature sporangium with short pedicel. E. Long pedicel. G. Ovoid sporangium with papillate apex and hyphal extension. H, I. Nonpapillate sporangia. I, J. Sporangiophores with swellings. K. Zoospore release. L. Zoospore cyst germinating by forming a microsporangium. M. Two-mo-old oogonium with oospore germinating by forming two sporangia (arrows). N–V. Globose to subglobose oogonia with smooth or wavey walls, slightly aplerotic to aplerotic oospores and paragynous antheridia, formed in V8A. W. Hyphal aggregation formed in V8A. Images: A, E–G, I, J, L–S, W. Ex-type CBS 149481; B. SU1505; C, D, K, SU1535; H, SU1525; T–V. SU1511. Scale bar = 20 µm; W applies to A–W.
Etymology: The name refers to the fast growth in culture (celeris Latin = fast).
Typus: Indonesia, Sumatra, Kerinci, isolated from a nursery effluent, Nov. 2018, T. Jung & M. Tarigan (holotype CBS H-25103, dried culture on V8A, ex-holotype living culture CBS 149481 = SU1500).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally on mostly long unbranched sporangiophores or less frequently in lax sympodia of 2–4 sporangia (Fig. 65C); sporangia shallow semipapillate (77 %; Fig. 65A–G, J), less frequently nonpapillate (19 %; Fig. 65H, I) or rarely papillate (4 %; Fig. 65G), non-caducous, predominantly ovoid, broad ovoid or elongated ovoid (75.6 %; Fig. 65A–C, G, I, K), less frequently obpyriform to elongated obpyriform (10.4 %; Fig. 65E, J), limoniform (7.6 %; Fig. 65D), ampulliform (3.8 %; Fig. 65F–H), ellipsoid (1.8 %) or obovoid (0.8 %); lateral attachment of the sporangiophore (28 %; Fig. 65B, F, G) and pedicels (35.6 %; Fig. 65C, E) common; sporangial dimensions averaging 42.6 ± 12.6 × 27.1 ± 5.4 µm (overall range 13.7–112.0 × 9.4–40.0 µm; range of isolate means 34.1–55.4 × 24.2–29.1 µm) with a length/breadth ratio of 1.58 ± 0.37 (overall range 1.13–3.11); pedicel length 41.1 ± 20.0 µm (range 7.3–96.4 µm); sporangial proliferation exclusively external (Fig. 65C, D, G); sporangial germination indirectly with zoospores discharged through an exit pore of 3.6–10.1 µm (av. 6.8 ± 1.2 µm) into a short-lived vesicle (Fig. 65K). Zoospores limoniform to reniform whilst motile (Fig. 65K), becoming spherical (av. diam = 11.0 ± 1.8 µm) on encystment; cysts germinating directly by producing hyphae or microsporangia (Fig. 65L) or indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings on sporangiophores common, ovoid or subglobose (Fig. 65I, J). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), on short, thin and often curved stalks (Fig. 65M–V); globose to subglobose with round non-tapering bases (Fig. 65N–V) and smooth (Fig. 65M–Q) or slightly wavy walls (Fig. 65R–V); av. diam 29.3 ± 2.2 µm with an overall range of 23.0–36.2 µm and a range of isolate means of 28.7–31.1 µm; slightly aplerotic (Fig. 65N–Q, T) to aplerotic (Fig. 65R, S, U, V). Oospores globose with a large lipid globule (Fig. 65N–V); in 2-mo-old cultures many oospores germinate by producing 1 or 2 sporangia (Fig. 65M); av. diam 24.1 ± 2.1 µm with an overall range of 17.2–33.6 µm and a range of isolate means of 22.3–25.7 µm; wall diam 1.46 ± 0.15 µm (overall range 1.22–1.84 µm) and oospore wall index 0.32 ± 0.03; abortion 12–40 % (av. 28 %) after 4 wk. Antheridia paragynous, 1-celled, and club-shaped, ovoid or subglobose (Fig. 65M–V); 12.2 ± 2.2 × 9.3 ± 1.4 µm. Hyphal aggregations common in all isolates (Fig. 65W).
Culture characteristics: Colonies are stellate and appressed with limited aerial mycelium on V8A; faintly striate to radiate and mostly submerged on CA; and faintly petaloid and felty-cottony on PDA (Fig. 59).
Cardinal temperatures and growth rates: On V8A optimum 27.5–30 °C with 10.5 ± 0.3 mm/d and 10.43 ± 0.63 mm/d radial growth, respectively, maximum 32.5–<35 °C, minimum >10–15 °C (Fig. 62), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 7.01 ± 0.22 mm/d, 5.35 ± 0.57 mm/d and 2.68 ± 0.12 mm/d, respectively.
Additional materials examined: Indonesia, Sumatra, Kerinci, isolated from a nursery effluent, Nov. 2018, T. Jung, M. Tarigan, L. Oliveira & I. Milenković (SU1505, SU1511, SU1520, SU1525, SU1535).
Phytophthora indonesiensis T. Jung, M. Tarigan, L. Oliveira & I. Milenković, sp. nov. MycoBank MB 847323. Fig. 66.
Fig. 66.
Phytophthora indonesiensis. A–K. Sporangia formed on V8-agar (V8A) in soil extract. A–I, K. Ovoid, limoniform, obovoid, ellipsoid and obpyriform sporangia. A, C–G. Semipapillate apices. B. Papillate apex. A, B, E, F. Medium-length pedicels (arrows in A, B, E). C, H, K. External proliferation (arrow in C). F. Intercalary sporangium. G. Caducous sporangium. H. Zoospore release. I. After zoospore release, with conspicuous protruding basal plug (arrow) and germinating zoospore cyst. J. Distorted sporangium with two apices. K. Dense sporangial sympodium and globose hyphal swelling. L. Intercalary hyphal swelling on V8A in soil extract. M–S. Oogonia with plerotic to aplerotic oospores, tapering or round bases and paragynous antheridia, formed in V8A. T. Hyphal aggregation formed in V8A. Images: A, B, I, P–R, T. SL449; C, J. SL446; D–H, K, L–O, S. Ex-type CBS 149639; Scale bars = 20 µm; T applies to A–J, L–T.
Etymology: The name refers to the distribution of this species across the Indonesian archipelago.
Typus: Indonesia, North Kalimantan, Malinau, isolated from a naturally fallen tree leaf floating in a tributary of Sesayap River in a tropical lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (holotype CBS H-25111, dried culture on V8A, ex-holotype living culture CBS 149639 = KA174).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne mostly terminally on unbranched sporangiophores or in dense or lax sympodia of 2–6 sporangia (Fig. 66K), or sometimes intercalary (Fig. 66F); sporangia semipapillate (88.2 %; Fig. 66A, C–G, K), often with a pointed apex (Fig. 66D, G), or less frequently papillate (11.8 %; Fig. 66B); usually non-caducous but a few caducous sporangia occur in all isolates (Fig. 66G); predominantly ovoid, broad ovoid or elongated ovoid (76.7 %; Fig. 66A, B, H, K), less frequently obpyriform, broad-obpyriform or elongated obpyriform (14 %; Fig. 66F, G), limoniform (3.5 %; Fig. 66C, I), obovoid (2.8 %; Fig. 66D), subglobose (2.2 %), ellipsoid (0.4 %; Fig. 66E), ampulliform (0.2 %) or distorted with two apices (0.2 %; Fig. 65J); slightly asymmetric shapes (26.7 %; Fig. 66B, E, I), a conspicuous protruding basal plug (25.1 %; Fig. 66I), lateral attachment of the sporangiophore (33.6 %; Fig. 66B, J) and medium-length pedicels (32.7 %; Fig. 66A, B, E–G) common; sporangial dimensions averaging 60.8 ± 8.6 × 42.5 ± 5.4 µm (overall range 36.8–85.6 × 26.6–83.5 µm; range of isolate means 52.0–68.0 × 37.2–46.7 µm) with a length/breadth ratio of 1.44 ± 0.19 (overall range 0.62–2.3); pedicel length 22.2 ± 13.9 µm (range 3.4–77.8 µm); sporangial proliferation exclusively external (Fig. 66C, H, K); sporangial germination indirectly with zoospores discharged through an exit pore of 4.8–9.9 µm (av. 7.4 ± 0.9 µm) into a short-lived vesicle (Fig. 66H). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 11.0 ± 1.8 µm; Fig. 66I) on encystment; cysts germinating directly by producing hyphae (Fig. 66I). Hyphal swellings on sporangiophores ovoid or subglobose (Fig. 66K, L), 14.7 ± 0.6 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system); sessile or on short, often curved stalks (Fig. 66M–S); globose to subglobose (66 %; Fig. 66P–S) or elongated (34 %; Fig. 66M–O), with short or long tapering, sometimes funnel-shaped or curved bases (46 %; Fig. 66M–Q) or round non-tapering bases (54 %; Fig. 66R, S), and smooth (Fig. 66M–Q, S) or, less frequently, slightly wavy walls (Fig. 66R); av. diam 26.7 ± 3.8 µm with an overall range of 15.0–43.4 µm and a range of isolate means of 23.8–29.7 µm; plerotic or near-plerotic (82 %; Fig. 66M–Q, S) or less frequently aplerotic (18 %; Fig. 66R). Oospores globose with a large lipid globule (Fig. 66M–R) or rarely with multiple smaller lipid globules (Fig. 66S); av. diam 23.5 ± 3.2 µm with an overall range of 13.0–35.9 µm and a range of isolate means of 21.3–26.0 µm; wall diam 1.72 ± 0.35 µm (overall range 0.96–2.7 µm) and oospore wall index 0.38 ± 0.05; abortion rate 3–9 % (av. 7 %) after 4 wk. Antheridia paragynous, 1-celled, and club-shaped, ovoid, subglobose or irregular (Fig. 66M–S); 11.5 ± 6.0 × 8.1 ± 1.4 µm. Hyphal aggregations common in all isolates (Fig. 66T).
Culture characteristics: Colony morphologies varying between isolates from different islands; isolates from Kalimantan and Sumatra faintly radiate and largely submerged to appressed on V8A and CA, and faintly petaloid and dense-felty appressed on PDA; isolates from Sulawesi faintly striate to uniform and submerged on V8A and CA, and uniform, dense-felty with very slow growth on PDA (Fig. 60).
Fig. 60.
Colony morphology of Phytophthora species from subclade 2e after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A–C. Phytophthora indonesiensis (A. ex-type CBS 149639; B. SL446; C. SU1131). D–F. Phytophthora pseudofrigida (D. ex-type CBS 150255; E. JV168; F. JV165).
Cardinal temperatures and growth rates: On V8A isolates from Kalimantan and Sumatra optimum at 25.0 °C with 7.7 ± 0.8 mm/d radial growth, maximum 30.0–<32.5 °C, minimum >10–15 °C (Fig. 62), lethal temperature 35 °C; at 20 °C on V8A, CA and PDA 6.12 ± 0.14 mm/d, 4.6 ± 0.33 mm/d and 3.85 ± 0.59 mm/d, respectively. Isolates from Sulawesi optimum at 20 °C with radial growth of 4.6 ± 0.17 mm/d on V8A, 4.52 ± 0.1 mm/d on CA and 0.82 ± 0.02 mm/d on PDA; maximum 30.0–<32.5 °C, minimum >10–15 °C (Fig. 62), lethal temperature 35 °C.
Additional materials examined: Indonesia, North Kalimantan, Malinau, isolated from naturally fallen leaves floating in a tributary of Sesayap River in a tropical lowland rainforest, Feb. 2019, T. Jung, M. Tarigan & M. Junaid (KA173, KA606); West Sumatra, Padang, isolated from rhizosphere soil of an unidentified tree in a tropical hill rainforest, Sep. 2018, T. Jung, L. Oliveira & I. Milenković (SU1122, SU1131, SU1724, SU1726, SU1728, SU1732); South Sulawesi, Lembang, isolated from rhizosphere soil of Cinnamomum iners in a tropical mountain rainforest, May 2019, T. Jung & M. Junaid (SL446, SL449, SL450).
Phytophthora penetrans T. Jung, Y. Balci, K. Broders & I. Milenković, sp. nov. MycoBank MB 847324. Fig. 67.
Fig. 67.
Phytophthora penetrans. A–J. Ovoid, obpyriform, pyriform and limoniform sporangia formed on V8-agar (V8A) in soil extract. A–E, J. Semipapillate apices. A, E, G, H. Medium-length pedicels. D–F, J. External proliferation. F. Sympodium with immature sporangium and sporangium releasing zoospores into short-lived vesicle (arrow). G–I. After zoospore release, with large basal plugs. H. Basal plug with two protruding tips. I. Internal extended proferation with new sporangiophores penetrating the sporangial wall (arrow). J. Dense sympodium. K–T. Smooth-walled oogonia with mostly tapering bases, near-plerotic to plerotic oospores and paragynous antheridia formed in V8A. M. Two antheridia (arrows). Images: A–C, E, G, I, K, R–T. Ex-type CBS 149497; D, F, J, P, Q. PA166; H, L–O, PA309. Scale bars = 20 µm; T applies to A–I, K–T.
Etymology: The name refers to the occasional penetration of the sporangial wall by sporangiophores in internally proliferating sporangia.
Typus: Panama, Parque Nacional de Campana, isolated from necrotic lesion on a naturally fallen leaf of a non-identified tree species in a tropical lowland rainforest, Nov. 2019, K.D. Broders & Y. Balci (holotype CBS H-25120, dried culture on V8A, ex-holotype living culture CBS 149497 = PA165).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally, usually in dense or lax sympodia of 2–6 sporangia (Fig. 67J); non-caducous, predominantly ovoid, broad-ovoid or elongated ovoid (70.5 %; Fig. 67A, B, F–J), less frequently obpyriform (22.2 %; Fig. 67C), limoniform (3.3 %; Fig. 67E, J), ellipsoid (3 %; Fig. 67F) or obovoid (1 %; Fig. 67D); lateral attachment of the sporangiophore (16.5 %; Fig. 67B), medium-length pedicels (32.8 %; Fig. 67A, E, G, H) and a conspicuous thick basal plug (84.5 %; Fig. 67A, C, E, G–I), frequently protruding with one or two long tips into the sporangium (Fig. 67H), commonly observed; apices semipapillate (Fig. 67A–E, J); sporangial proliferation mostly external (Fig. 67D–F, J) or infrequently internal in an extended way with 1–2 sporangiophores arising from beside the sporangial basal plug and often penetrating the lateral wall of the empty sporangium (Fig. 67H, I); sporangial dimensions averaging 56.7 ± 9.8 × 36.8 ± 4.3 µm (overall range 37.4–94.2 × 26.9–50.1 µm; range of isolate means 50.0–69.2 × 34.2–41.3 µm) with a length/breadth ratio of 1.53 ± 0.15 (overall range 1.22–2.28); pedicel length 21.9 ± 15.8 µm (range 3.0–89.0 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 5.3–10.8 µm wide (av. 7.7 ± 1.0 µm) (Fig. 67F–I). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.4 ± 1.0 µm) on encystment. Hyphal swellings on sporangiophores subglobose, limoniform or irregular, 17.4 ± 5.5 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae, smooth-walled, globose to slightly subglobose (94.8 %; Fig. 67K–S), sometimes slightly excentric (Fig. 67K, O), or elongated (5.2 %; Fig. 67K), often with a short or long tapering base (77 %; Fig. 67L–N, P–T); oogonial diam 29.3 ± 3.0 µm (overall range 18.1–38.5 µm; range of isolate means 27.5–31.8 µm); slightly aplerotic to aplerotic (68 %; Fig. 67K–P) or nearly plerotic (32 %; Fig. 67Q–T). Oospores globose with a large lipid globule (Fig. 67K–T); diam 25.4 ± 2.6 µm (overall range 15.7–32.8 µm; range of isolate means 24.0–27.7 µm) wall thickness 2.0 ± 0.3 µm (overall range 1.3–3.1 µm), oospore wall index 0.41 ± 0.04; abortion rate 2–46 % (av. 20 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ovoid or subglobose (Fig. 67K–T); sometimes two antheridia attached to one oogonium (Fig. 67M); 11.5 ± 2.1 × 7.8 ± 1.3 µm.
Culture characteristics: Colonies on V8A and CA mostly submerged to appressed, stellate on V8A and faintly radiate on CA; on PDA dense-felty, radiate, often with irregular sectors growing from the margin with submerged margins (Fig. 61).
Fig. 61.
Colony morphology of Phytophthora species after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora penetrans (ex-type CBS 149497) from subclade 2e. B–E. From subclade 2f. B. Phytophthora angustata (ex-type CBS 149475). C. Phytophthora furcata (ex-type isolate CBS 149487). D, E. Phytophthora sumatera (D. ex-type CBS 149501; E. JV146).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 6.86 ± 0.28 mm/d radial growth, maximum 27.5–<30 °C, minimum >10–15 °C (Fig. 62), lethal temperature 30–32.5 °C. At 20 °C on V8A, CA and PDA 5.97 ± 0.15 mm/d, 5.14 ± 0.13 mm/d and 1.76 ± 0.09 mm/d, respectively.
Additional materials examined: Panama, Parque Nacional de Campana, isolated from necrotic lesions on naturally fallen leaves of unidentified tree species in tropical lowland forests, Nov. 2019, K.D. Broders & Y. Balci (PA166, PA304, PA306, PA308, PA309, PA311, PA313).
Phytophthora pseudofrigida T. Jung, A. Durán, M. Tarigan & M. Horta Jung, sp. nov. MycoBank MB 849627. Fig. 68.
Fig. 68.
Phytophthora pseudofrigida. A–L. Papillate sporangia formed on V8-agar (V8A) in soil extract. A–F. Ovoid sporangia. G–J. Obpyriform sporangia. A, B. External proliferation (arrows). B, C. Intercalary sporangia. D, F, H, I. Laterally attached sporangiophores. E, G. Sporangiophore constrictions (arrows). G. Long pedicel. K–T. Structures formed in carrot agar in polycarbonate membrane mating tests. K–R. Oogonia with tapering bases, near-plerotic to plerotic oospores and amphigynous antheridia. P. Bicellular antheridium. Q. Elongated oogonium and oospore. R. Oogonium on the right with aborted oospore. S, T. Thick-walled chlamydospores. U. Unbranched hyphae in V8A. Images: A, B, D–F, I, J, K–M, O, Q, S–U. Ex-type CBS 150255; C, G, H, N, JV168; P, R. SU1280. Scale bars = 20 µm; T applies to A–T.
Etymology: The name refers to the phylogenetic relatedness and morphological similarities to P. frigida.
Typus: Indonesia, Sumatra, Lake Toba, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical montane rainforest, Aug. 2018, T. Jung & M. Tarigan (holotype CBS H-25294, dried culture on V8A, ex-holotype living culture CBS 150255 = SU588).
Morphological structures on V8A: Sporangia infrequently observed in solid agar and abundantly produced in non-sterile soil extract; borne predominantly terminally (87 %) on long or short sporangiophores (Fig. 68A, D–G, J), mostly unbranched or infrequently in lax sympodia of 2–3 sporangia, or intercalary (13.3 %; Fig. 68B, C); sporangia exclusively papillate (Fig. 68A–J), non-caducous, predominantly ovoid, broad ovoid or elongated ovoid (74.7 %; Fig. 68A–G), less frequently obpyriform to elongated obpyriform (19.1 %; Fig. 68H–J), subglobose (2.6 %), limoniform (2.4 %), or mouse-shaped (1.2 %); lateral attachment of the sporangiophore frequent (55.4 %; Fig. 68B, D, F, H, I), pedicels (12 %; Fig. 68G) or a constriction of the sporangiophore (4.8 %; Fig. 68E, G) infrequent; sporangial dimensions averaging 60.3 ± 7.2 × 39.9 ± 4.7 µm (overall range 28.7–74.2 × 23.8–49.0 µm; range of isolate means 58.6–61.3 × 38.3–41.3 µm) with a length/breadth ratio of 1.52 ± 0.16 (overall range 1.19–2.07); pedicel length 20.9 ± 14.8 µm (range 4.0–50.0 µm); sporangial proliferation exclusively external (Fig. 68A, B, D); sporangial germination indirectly with zoospores discharged through an exit pore of 5.3–9.6 µm (av. 7.1 ± 1.3 µm) into a short-lived vesicle. Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.6 ± 0.9 µm) on encystment. Hyphal swellings not observed. Chlamydospores globose to subglobose, 31.5–43.6 µm (av. diam 37.6 ± 6.0 µm), with golden-brown thick walls (wall diam 2.3 ± 0.8 µm) (Fig. 68S, T). Oogonia not observed in single cultures, but abundantly produced by all five tested isolates in polycarbonate membrane mating tests with the A2 mating type isolate SU1735 of P. acaciivora (A1/A2 or ‘heterothallic’ breeding system; all tested isolates mating type A1); predominantly globose to subglobose (86 %; Fig. 68K–P, R) or infrequently elongated (14 %; Fig. 68Q), with smooth walls and almost exclusively (99 %) tapering bases (Fig. 68K–V); av. diam 25.1 ± 2.9 µm with an overall range of 19.2–31.6 µm and a range of isolate means of 24.9–25.3 µm; predominantly plerotic (76 %; Fig. 68M–R) or less frequently aplerotic (Fig. 68K, L). Oospores globose (Fig. 68K–P, R) or rarely elongated (Fig. 68Q) with a large lipid globule; av. diam 21.6 ± 2.2 µm with an overall range of 16.8–26.6 µm and a range of isolate means of 21.1–22.4 µm; wall diam 1.61 ± 0.31 µm (overall range 0.84–2.29 µm) and oospore wall index 0.38 ± 0.05; abortion 68–83 % (av. 76 %; Fig. 68R) after 4 wk. Antheridia amphigynous, 1-celled (Fig. 68L, M, O, Q, R) or 2-celled with the basal cell being significantly smaller (Fig. 68K, N, P), and cylindrical to subcylindrical (Fig. 68K–R); 18.0 ± 2.2 × 15.5 ± 1.6 µm. Hyphae often with long unbranched sections (Fig. 68U).
Culture characteristics: Colonies are submerged to appressed with very limited aerial mycelium on V8A and CA, striate or stellate on V8A and faintly striate or chrysanthemum-like on CA; and uniform, appressed and felty-cottony on PDA (Fig. 60).
Cardinal temperatures and growth rates: On V8A optimum 30 °C with 8.0 ± 0.76 mm/d radial growth but with almost similar growth of 7.9 ± 0.61 mm/d at 27.5 °C, maximum 32.5–<35 °C, minimum <10 °C (Fig. 62), lethal temperature 35 °C. At 20 °C on V8A, CA and PDA 4.74 ± 0.77 mm/d, 4.07 ± 0.63 mm/d and 1.44 ± 0.07 mm/d, respectively.
Additional materials examined: Indonesia, Java, Bandung area, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical montane Pinus merkusii forest, Mar. 2019, T. Jung, M. Tarigan & L. Oliveira (JV168); Java, Papandayan Mountain, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical montane forest, Mar. 2019, T. Jung, M. Tarigan & L. Oliveira (JV178a); Sumatra, Padang, isolated from naturally fallen necrotic leaves floating in a forest stream in a tropical hill rainforest, Sep. 2018, T. Jung, M. Tarigan, T. Corcobado & I. Milenković (SU683, SU1280).
Notes on Clade 2e taxa: Across the nuclear (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) 8 754-character alignment and the mitochondrial (cox1, cox2, nadh1 and rps10) 3 153-character alignment the five known and six newly described Phytophthora species from Clade 2e showed pairwise sequence differences of 0.4–6.2 % and 0.1–4.4 %, respectively. The six new and the two known Clade 2e species examined (viz. P. acaciivora and P. elongata) developed distinctive colony morphologies on V8A, CA and PDA at 20 °C (Figs 59–61). It should be noted that colony morphologies of both P. pseudofrigida and P. indonesiensis are highly variable (Fig. 60). In addition, the six new species can be discriminated from each other and other Clade 2e species by a combination of morphological (Figs 63–68) and physiological characters (Fig. 62) of which the most discriminating are highlighted in bold in Tables S14 and S15.
The four Indonesian isolates of P. acaciivora examined in this study differed from the original description of this species which was based on three Vietnamese isolates by having higher optimum (27.5–30 vs. 25 °C) and lower maximum (32.5–35 vs. >37.5 °C) temperatures for growth (Burgess et al. 2020; Table S14; Fig. 62).
The five isolates of P. pseudofrigida from Java and Sumatra examined in this study showed considerable morphological and morphometric differences from its sister species P. frigida (Maseko et al. 2007), most notably much larger sporangia (60.3 × 39.9 vs. 33 × 27 µm), larger chlamydospores (37.6 vs. 25 µm), smaller oogonia (25 vs. 33 µm), lack of sporangial caducity and a higher optimum temperature for growth (30 vs. 25 °C) (Table S14; Figs 62, 68). Phytophthora acaciae, P. acaciivora, P. frigida and P. pseudofrigida are distinguished from the other Clade 2e species by their exclusively papillate sporangia (Maseko et al. 2007, Albuquerque Alves et al. 2019, Burgess et al. 2020; Tables S14, S15; Fig. 68). In contrast, the sporangia of P. bishii, P. elongata, P. penetrans and P. taxon AUS 2E (previously P. taxon elongata-like) are exclusively semipapillate (Abad et al. 2008, Rea et al. 2010; Tables S14, S15; Fig. 67). Three new Clade 2e species produce predominantly semipapillate sporangia and varying proportions of papillate (P. amamensis 5.4 %; P. celeris 4 %, P. indonesiensis 11.8 %) or nonpapillate sporangia (P. amamensis 5 %, P. celeris 19 %) (Tables S14, S15; Figs 63, 65, 66). Phytophthora borneensis can be discriminated from all other Clade 2e species by producing semipapillate (57 %) and nonpapillate (43 %) sporangia in almost similar proportions (Tables S14, S15; Fig. 64). Phytophthora celeris differs from P. acaciae, P. acaciivora and the other five new Clade 2e species by having considerably smaller sporangia (on average 42.6 vs. 51–60.3 µm). In addition, with 41.1 ± 20.0 µm the pedicels of P. celeris are on average considerably longer than in all other Clade 2e species (av. 11.3–29.0 µm) (Tables S14, S15). Phytophthora amamensis and P. acaciivora have mostly slender sporangia with high sporangial l/b ratios (1.85 and 1.73, respectively) separating them from all other species with more squat sporangia (Tables S14, S15). Two new species from Indonesia, P. borneensis and P. indonesiensis, are discriminated from the other four new species by the occasional production of sporangia with two apices (Figs 63–68). Phytophthora borneensis, P. indonesiensis and P. penetrans share the production of large conspicuous basal plugs which often protrude into the sporangium, but the frequency differs between these three species ranging from 25.1 % in P. indonesiensis to 62.5 % in P. borneensis and 84.5 % in P. penetrans. The latter species also differs from the other two species by frequently forming two long tips protruding from the basal plug into the sporangium. The occurrence of internal extended proliferation differentiates P. borneensis and P. penetrans from all other species in Clade 2e which exclusively show external sporangial proliferation (Maseko et al. 2007, Abad et al. 2008, Albuquerque Alves et al. 2019, Burgess et al. 2020; Tables S14, S15; Figs 63–68). Phytophthora penetrans differs from P. borneensis and all currently known Phytophthora species by producing internally proliferating sporangiophores which occasionally penetrate the lateral wall of the empty sporangium (Fig. 67). The frequent formation of dense hyphal aggregations distinguishes P. celeris and P. indonesiensis from the other Clade 2e species (Maseko et al. 2007, Abad et al. 2008, Albuquerque Alves et al. 2019, Burgess et al. 2020; Tables S14, S15; Figs 63–68).
The A1/A2 breeding systems of P. acaciae, P. acaciivora, P. frigida and P. pseudofrigida readily discriminate them from all other species in Clade 2e which are intrinsically self-fertile (Maseko et al. 2007, Albuquerque Alves et al. 2019, Burgess et al. 2020; Tables S14, S15). Phytophthora bishii differs from the other self-fertile species by producing on average larger oogonia (Abad et al. 2008) whereas P. elongata can easily be identified because of its long oogonial stalks (Rea et al. 2010; Tables S14, S15). High proportions of oogonia with tapering bases distinguish P. amamensis (74 %) and P. penetrans (77 %) from P. borneensis (19 %), P. celeris (0 %) and P. indonesiensis (46 %) (Tables S14, S15; Figs 63–67). Comparatively high frequencies of slightly elongated oogonia also differentiate P. amamensis (36.0 %) and P. indonesiensis (34 %) from P. penetrans (5.2 %), P. borneensis and P. celeris (both 0 %) (Tables S14, S15; Figs 63–67). In P. indonesiensis most oospores (82 %) are near-plerotic to plerotic whereas in the other four new self-fertile Clade 2e species most oospores are slightly aplerotic or aplerotic (Tables S14, S15; Figs 63–67).
At 35 °C P. acaciae has the highest maximum temperature for growth followed by P. acaciivora, P. borneensis, P. celeris and P. pseudofrigida (all 32.5–<35 °C). At 20 °C P. elongata and montane isolates of P. indonesiensis have the lowest optimum temperatures within Clade 2e, followed by P. acaciae (24 °C), P. frigida, P. penetrans, lowland isolates of P. indonesiensis and P. taxon AUS 2E (all 25 °C), P. bishii (26 °C), P. borneensis (25–27.5 °C), P. amamensis (27.5 °C), P. acaciivora and P. celeris (27.5–30 °C) and P. pseudofrigida (30 °C) (Maseko et al. 2007, Abad et al. 2008, Rea et al. 2010, Albuquerque Alves et al. 2019, Burgess et al. 2020; Tables S14, S15; Fig. 62).
Clade 2f
For all Clade 2f species included in this study colony morphologies on CA, PDA and V8A and temperature-growth relations on V8A are presented in Figs 61 and 62, respectively. Morphological and physiological characters and morphometric data of one known and the three newly described species and one informally designated taxon in Clade 2f are given in the comprehensive Table S16.
Phytophthora angustata T. Jung, L. Garcia, B. Mendieta-Araica & Y. Balci, sp. nov. MycoBank MB 847326. Fig. 69.
Fig. 69.
Phytophthora angustata. A–K. Ovoid, limoniform, ampulliform and club-shaped sporangia formed on V8-agar (V8A) in soil extract. A–E. Nonpapillate sporangia. E–G. Conspicuous basal plugs. F. Zoospore release. G. External and internal nested proliferation. H. Internal nested proliferation with new ampulliform sporangium protruding out of the old sporangium. I. Internal nested proliferation. J. Internal extended proliferation. K. Internal extended proliferation with two new sporangiophores. L. Catenulate hyphal swellings on V8A in soil extract. M–X. Smooth-walled oogonia with tapering bases, near-plerotic to plerotic oospores and paragynous antheridia formed in V8A. X. Comma-shaped oogonium. Images: A, D, E, G, I, J, L, M–R, T. Ex-type CBS 149475; B, NI159; C, F. NI164; H, K. NI124; S, W, X. NI165; U, V. NI129. Scale bar = 20 µm; X applies to A–X.
Etymology: The name refers to the tapering oogonial bases (angustata Latin = tapering).
Typus: Nicaragua, Diriomo, Mombacho Volcano, isolated from a naturally fallen necrotic leaf of an unidentified rainforest tree collected from the ground in a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (holotype CBS H-25097, dried culture on V8A, ex-holotype living culture CBS 149475 = NI121).
Morphological structures on V8A: Sporangia not observed on solid agar but abundantly produced in non-sterile soil extract; borne terminally in dense or lax sympodia of 2–4 sporangia (Fig. 69G) or on unbranched long or short sporangiophores (Fig. 69A, B); non-caducous, mostly ovoid or elongated ovoid (83.7 %; Fig. 69A, B, F–H, I, K), less frequently limoniform or elongated-limoniform (7.4 %; Fig. 69C), obpyriform to elongated-obpyriform (3.9 %), ampulliform (2.3 %; Fig. 69D, H), ellipsoid to elongated ellipsoid (1.4 %) or club-shaped (1.3 %; Fig. 69E); lateral attachment of the sporangiophore (18.7 %; Fig. 69B, E) and a conspicuous basal plug (34.7 %; Fig. 69E–G, K) common; apices nonpapillate (Fig. 69A–E, H); sporangial proliferation external (Fig. 69G) and more frequently internal in a nested way (Fig. 69G–I), sometimes with the new sporangium partly protruding out of the old sporangium (Fig. 69H), or in an extended way (Fig. 69J, K), sometimes with multiple sporangiophores inside the empty proliferating sporangium (Fig. 69K); sporangial dimensions averaging 65.9 ± 6.6 ´ 35.6 ± 2.5 µm (overall range 50.7–98.0 × 27.3–41.8 µm; range of isolate means 61.9–69.4 × 33.9–36.7 µm) with a length/breadth ratio of 1.85 ± 0.16 (overall range 1.53–2.83); sporangial germination indirectly with zoospores discharged through an exit pore 7.6–14.8 µm wide (av. 10.6 ± 1.6 µm) (Fig. 69F, G, I–K). Zoospores limoniform to reniform whilst motile, sometimes with ring-like flagella ends, becoming spherical (av. diam = 10.7 ± 0.6 µm) on encystment; cysts germinating directly or less frequently indirectly by releasing a secondary zoospore (diplanetism). Hyphal swellings infrequently formed in water, globose to subglobose, limoniform or irregular, sometimes catenulate (Fig. 69L), av. diam 12.5 ± 0.3 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short or medium-length, often curved lateral hyphae; predominantly globose to subglobose (65.9 %; Fig. 69M–S), elongated (27.3 %; Fig. 69T–W) or comma-shaped (6.8 %; Fig. 69X), with short (34 %; Fig. 69M–S) or long tapering, often (38 %) funnel-like bases (66 %; Fig. 69T–W); smooth-walled (Fig. 69M–X); oogonial diam 31.1 ± 2.3 µm (overall range 25.5–38.2 µm; range of isolate means 29.6–32.7 µm); nearly plerotic to plerotic (Fig. 69M–X). Oospores globose with a large lipid globule (Fig. 69M–X); diam 29.3 ± 2.2 µm (overall range 23.9–35.3 µm; range of isolate means 27.8–30.8 µm) wall thickness 2.05 ± 0.28 µm (overall range 1.29–3.0 µm), oospore wall index 0.36 ± 0.04; abortion rate 3–17 % (av. 8.7 %) after 4 wk. Antheridia almost exclusively (>99 %) paragynous, club-shaped, subglobose, limoniform or irregular (Fig. 69M–X); dimensions 12.8 ± 1.9 × 8.7 ± 1.2 µm.
Culture characteristics: Colonies on V8A and CA submerged to appressed with scanty aerial mycelium, chrysanthemum-like to stellate on V8A and petaloid to stellate on CA; on PDA dense-cottony with a faint radiate pattern (Fig. 61).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 10.02 ± 0.23 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 62), lethal temperature 32.5 °C. At 20 °C on V8A, CA and PDA 8.21 ± 0.48 mm/d, 5.36 ± 0.06 mm/d and 3.65 ± 0.27 mm/d, respectively.
Additional materials examined: Nicaragua, Diriomo, Mombacho Volcano, isolated from naturally fallen necrotic leaves of unidentified rainforest trees collected from the ground in a tropical cloud forest, Nov. 2017, Y. Balci, L. Garcia & B. Mendieta-Araica (NI124, NI129, NI157, NI159, NI165).
Phytophthora furcata T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, sp. nov. MycoBank MB 847327. Fig. 70.
Fig. 70.
Phytophthora furcata. A–K. Structures formed on V8-agar (V8A) in soil extract. A–J. Ovoid, obpyriform and limoniform sporangia. A–D, F. Nonpapillate sporangia. A, D, E. External proliferation. D. Widening sporangiophore. E. Swollen apex before zoospore release. G, H. Internal extended proliferation. H. Sporangiophore furcating inside empty sporangium. I. Internal nested proliferation. J. Dense sympodium. K. Catenulate hyphal swellings and sporangium. L–S. Oogonia with aplerotic to near plerotic oospores and amphigynous antheridia, formed in V8A. L–O. Globose to subglobose oogonia with round bases. M, N. Wavy oogonial walls. P–S. Elongated, excentric or comma-shaped oogonia with tapering bases. T. Oogonium with germinating oospore. Images: A, G, L, Q. VN002; B, D, F, I–K, M–P, S, T. Ex-type CBS 149487; C, VN073; E, H, R. VN384. Scale bars = 20 µm; T applies to A–J, L–T.
Etymology: The name refers to the frequent furcation of sporangiophores inside proliferating empty sporangia (furcata Latin = furcated).
Typus: Vietnam, Sapa, Fansipan Mountain, isolated from rhizosphere soil of Meliosma henryi and Neolitsea merilliana in a montane evergreen cloud forest, Mar. 2016, T. Jung, N.M. Chi & M. Horta Jung (holotype CBS H-25109, dried culture on V8A, ex-holotype living culture CBS 149487 = VN035).
Morphological structures on V8A: Sporangia infrequently observed on solid agar but abundantly produced in non-sterile soil extract; borne terminally in dense or lax sympodia of 2–5 sporangia (Fig. 70J) or on unbranched long or short sporangiophores; non-caducous, mostly ovoid or elongated ovoid (70.7 %; Fig. 70A–D, G, J), less frequently limoniform or elongated-limoniform (14.3 %; Fig. 70F), obpyriform to elongated-obpyriform (10.7 %; Fig. 70E), ellipsoid to elongated ellipsoid (2.7 %) or mouse-shaped (1.6 %); lateral attachment of the sporangiophore (6.7 %; Fig. 70H) and a conspicuous basal plug (10 %; Fig. 70F) occasionally observed; apices almost exclusively nonpapillate (99.7 %; Fig. 70A–D, F, J) or rarely shallow semipapillate (0.3 %), sometimes curved (9.7 %; Fig. 70I); sporangial proliferation both external (Fig. 70A, D, E, J) and internal in a nested (Fig. 70I) or extended way (Fig. 70G, H), often with the sporangiophore furcating inside the empty proliferating sporangium (Fig. 70H); sporangial dimensions averaging 56.4 ± 8.6 × 32.8 ± 3.4 µm (overall range 27.4–99.2 × 17.1–41.6 µm; range of isolate means 49.8–61.3 × 29.8–35.0 µm) with a length/breadth ratio of 1.72 ± 0.22 (overall range 1.32–2.63); sporangial germination indirectly with zoospores discharged through an exit pore 6.5–16.0 µm wide (av. 11.6 ± 1.8 µm) (Fig. 70G–J). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.4 ± 0.8 µm) on encystment. Hyphal swellings abundantly formed in water, globose to subglobose, limoniform or irregular, usually catenulate (Fig. 70K), av. diam 13.6 ± 5.3 µm (range 6.5–58.1 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), sessile, predominantly globose to subglobose (59.7 %; Fig. 70L–O), elongated (15.7 %; Fig. 70P, Q), slightly excentric (19.3 %; Fig. 70R, S) or comma-shaped (5.3 %; Fig. 70S), with a rounded base and short thin stalk (34 %; Fig. 70L–O) or with a long tapering, frequently (24 %) funnel-like base (66 %; Fig. 70P–T); oogonial wall smooth (Fig. 70L, O–T) or slightly wavy (Fig. 70M, N); oogonial diam 31.4 ± 3.1 µm (overall range 19.8–40.4 µm; range of isolate means 29.6–33.0 µm); slightly aplerotic to aplerotic (50.7 %; Fig. 70M, N, R, S) or nearly plerotic to plerotic (49.3 %; Fig. 70L, O–Q). Oospores globose with a large lipid globule (Fig. 70L–S); diam 28.5 ± 2.6 µm (overall range 16.1–36.1 µm; range of isolate means 27.3–29.8 µm) wall thickness 1.94 ± 0.28 µm (overall range 0.91–2.79 µm), oospore wall index 0.36 ± 0.04; abortion rate 16–37 % (av. 25.2 %) after 4 wk; after 2 mo at 20 °C most oospores germinating (Fig. 70T). Antheridia exclusively amphigynous and unicellular, subglobose to cylindrical (Fig. 70L–T); dimensions 15.0 ± 1.8 × 13.3 ± 1.4 µm.
Culture characteristics: Colonies on V8A and CA appressed with limited aerial mycelium, stellate on V8A and petaloid on CA; on PDA uniform, dense-cottony and dome-shaped (Fig. 61).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 8.62 ± 0.41 mm/d radial growth, maximum 25–<27.5 °C, minimum <10 °C (Fig. 62), lethal temperature 27.5 °C. At 20 °C on V8A, CA and PDA 7.42 ± 0.18 mm/d, 4.91 ± 0.18 mm/d and 3.48 ± 0.25 mm/d, respectively.
Additional materials examined: Vietnam, Sapa, Fansipan Mountain, isolated from rhizosphere soil of M. henryi and N. merilliana in a montane evergreen cloud forest, Mar. 2016, T. Jung, N.M. Chi & M. Horta Jung (VN002, VN036, VN384, VN385); isolated from a baiting leaf floating in a stream running through a montane evergreen cloud forest, Mar. 2016, T. Jung, N.M. Chi & M. Horta Jung (VN073).
Phytophthora sumatera T. Jung, M. Tarigan, I. Milenković & A. Durán, sp. nov. MycoBank MB 847328. Fig. 71.
Fig. 71.
Phytophthora sumatera. A–N. Structures formed on V8-agar (V8A) in soil extract. A–L. Ovoid and obpyriform sporangia. A, B, D, E, K, L. Nonpapillate apices. C, G. Swollen apices before zoospore release. C, E, F, H, J–L. External proliferation (arrows in F, L). F. Zoospore release. G, H. Internal nested proliferation. I. Internal extended proliferation. J, K. Dense sympodia with sessile sporangia. M. Zoospores with ring-like flagella ends (arrow). N. Catenulate hyphal swellings. O–W. Oogonia with near-plerotic to aplerotic oospores, tapering bases and amphigynous antheridia formed in V8A. O–R, W. Subglobose to globose oogonia. S–U. Comma-shaped oogonia. V. Excentric oogonium. Images: A, D, F, G, I, L, N, P, R, U, W. Ex-type CBS 149501; B, J, SU1061; C, E, H, K, O, S, T, JV146; M, Q. SU973; V. SU1037. Scale bar = 20 µm; W applies to A–W.
Etymology: The name refers to the origin of all known isolates in Sumatra (Sumatera is the Indonesian name for Sumatra).
Typus: Indonesia, Sumatra, Lake Toba, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical montane rainforest, Aug. 2018, T. Jung, M. Tarigan & I. Milenković (holotype CBS H-25125, dried culture on V8A, ex-holotype living culture CBS 149501 = SU521).
Morphological structures on V8A: Sporangia abundantly produced in non-sterile soil extract; borne terminally on unbranched long or short sporangiophores (Fig. 71A, C, D, G) or in dense sympodia of 2–4 sporangia (Fig. 71E, J–L); non-caducous, predominantly ovoid or elongated ovoid (80.7 %; Fig. 71A–C, F, I–L), less frequently obpyriform to elongated-obpyriform (17.4 %; Fig. 71D, E, G), ellipsoid or elongated ellipsoid (1.3 %), limoniform or elongated-limoniform (0.3 %) or ampulliform (0.3 %); lateral attachment of the sporangiophore (11.7 %; Fig. 71B) and a conspicuous, mostly non-protruding basal plug (24.7 %; Fig. 71E, G, J–L) commonly observed; apices exclusively nonpapillate (Fig. 71A, B, D, E, K, L), appearing semipapillate immediately before zoospore release (Fig. 71C, G); sporangial proliferation both external (Fig. 71E, F, H, J–L) and internal in a nested (Fig. 71G, H) or extended way (Fig. 71I); sporangial dimensions averaging 48.5 ± 8.6 × 32.2 ± 4.1 µm (overall range 34.5–77.5 × 22.3–48.7 µm; range of isolate means 42.1–60.3 × 29.2–35.8 µm) with a length/breadth ratio of 1.51 ± 0.16 (overall range 1.2–2.23); sporangial germination indirectly with zoospores discharged through an exit pore 5.5–13.2 µm wide (av. 8.5 ± 1.2 µm) (Fig. 71F–L). Zoospores limoniform to reniform whilst motile, sometimes with ring-like flagella-ends (Fig. 71M), becoming spherical (av. diam = 10.7 ± 0.9 µm) on encystment; Cysts germinating directly or by releasing a secondary zoospore (diplanetism). Hyphal swellings frequently formed in water, globose to subglobose, limoniform, ovoid or irregular, often catenulate (Fig. 71N), av. diam 15.4 ± 3.6 µm (range 7.0–27.0 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), sessile or on short to medium-length lateral hyphae; globose to subglobose (56.5 %; Fig. 71O, R, W), comma-shaped (33.5 %; Fig. 71S–U) or excentric and/or elongated (10 %; Fig. 71V) with a short tapering or more frequently a long tapering, sometimes (18 %) funnel-like base (Fig. 71O–W); oogonial wall smooth (Fig. 71O, P, R–V) or slightly wavy (Fig. 71Q, W); oogonial diam 32.1 ± 3.9 µm (overall range 23.3–41.6 µm; range of isolate means 30.2–34.2 µm); slightly aplerotic to aplerotic (56.5 %; Fig. 71S–W) or nearly plerotic to plerotic (43.5 %; Fig. 71O–R). Oospores globose with a large lipid globule (Fig. 71O–W); diam 27.7 ± 3.4 µm (overall range 19.4–38.7 µm; range of isolate means 25.9–28.8 µm) wall thickness 1.36 ± 0.2 µm (overall range 0.78–2.02 µm), oospore wall index 0.27 ± 0.04; abortion rate 6–21 % (av. 15.8 %) after 4 wk; sometimes turning golden-brown to dark-brown during maturation (Fig. 71W). Antheridia exclusively amphigynous and unicellular, subglobose to cylindrical (Fig. 71O–W), sometimes intercalary (Fig. 71R); dimensions 13.8 ± 1.8 × 12.2 ± 1.2 µm.
Culture characteristics: Colonies on V8A and CA appressed with limited aerial mycelium, radiate on V8A and petaloid on CA; on PDA felty-cottony and dome-shaped with petaloid pattern and submerged margin (Fig. 61).
Cardinal temperatures and growth rates: On V8A optimum 25 °C with 8.98 ± 0.44 mm/d radial growth, maximum 27.5–<30 °C, minimum <10 °C (Fig. 62), lethal temperature 32.5 °C. At 20 °C on V8A, CA and PDA 7.98 ± 0.36 mm/d, 5.72 ± 0.09 mm/d and 3.57 ± 0.44 mm/d, respectively.
Additional materials examined: Indonesia, Java, Bandung area, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical montane Pinus merkusii forest, Mar. 2019, T. Jung, M. Tarigan & L. Oliveira (JV146); Sumatra, Lake Toba, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical montane rainforest, Aug. 2018, T. Jung, M. Tarigan & I. Milenković (SU566); isolated from rhizosphere soil of an unidentified tree species in a tropical montane rainforest, Aug. 2018, T. Jung, M. Tarigan & I. Milenković (SU909, SU973, SU1037); isolated from rhizosphere soil of a declining Eucalyptus sp. in a montane forest plantation, Aug. 2018, T. Jung, M. Tarigan & I. Milenković (SU1061); Sumatra, Padang, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical lowland rainforest, Sep. 2018, T. Jung, M. Tarigan & L. Oliveira (SU635); Sumatra, Gulung Talang, isolated from a naturally fallen necrotic leaf of an unidentified tree species floating in a stream running through a tropical submontane rainforest, Sep. 2018, T. Jung, M. Tarigan & L. Oliveira (SU690).
Notes on Clade 2f taxa: Across the nuclear (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) 8 720-character alignment and the mitochondrial (cox1, cox2, nadh1 and rps10) 3 153-character alignment pairwise sequence differences between P. multivesiculata, the informally designated Phytophthora taxon aquatilis and the three new Phytophthora species from Clade 2f taxa were 0.6–2 % and 1.5–3.6 %, respectively. Phytophthora angustata, P. furcata, previously informally designated as P. taxon multivesiculata-like 1 (Jung et al. 2020), and P. sumatera developed distinctive colony morphologies on V8A, CA and PDA at 20 °C (Fig. 61). In addition, they are distinguished from each other and other Clade 2f species by a combination of morphological (Figs 69–71) and physiological characters (Fig. 62) of which the most discriminating are highlighted in bold in Table S16.
The sporangia of the three new Clade 2f species are exclusively (P. angustata, P. sumatera) or almost exclusively (P. furcata) nonpapillate whereas those of P. multivesiculata are both nonpapillate and semipapillate (Ilieva et al. 1998; Figs 69–71). Phytophthora taxon aquatilis exclusively forms semipapillate sporangia (Hong et al. 2012). Their larger average sporangial dimensions discriminate P. angustata (65.9 × 35.6 µm) and P. furcata (56.4 × 32.8 µm) from P. multivesiculata (45 × 33 µm), P. sumatera (48.5 × 32.2 µm) and P. taxon aquatilis (45.9 × 29.7 µm). Furthermore, the sporangia of P. angustata and P. furcata have higher l/b ratios (1.85 and 1.72, respectively) than those of P. multivesiculata (1.43), P. sumatera (1.51) and P. taxon aquatilis (1.6). According to Hong et al. (2012) the latter taxon differs from all other Clade 2f species by the caducity of its sporangia, although sporangia reported as being caducous in Fig. 7 of Hong et al. (2012) do not show caducity, and the absence of internal sporangial proliferation. In contrast, P. angustata, P. furcata, P. multivesiculata and P. sumatera exclusively produce persistent sporangia with internal nested and extended proliferation (Ilieva et al. 1998; Table S16; Figs 69–71).
All five taxa in Clade 2f are intrinsically self-fertile. Phytophthora multivesiculata and P. taxon aquatilis produce on average much larger oogonia (41 and 38.2 µm) than the three new Clade 2f species (31.1–32.1 µm). The three new species are also distinguished from P. taxon aquatilis by having a comparatively high frequency of elongated, excentric or comma-shaped oogonia (34–44 %) and exclusively (P. angustata, P. sumatera) or predominantly (P. furcata) tapering oogonial bases (Ilieva et al. 1998; Hong et al. 2012; Table S16). In P. angustata and P. taxon aquatilis oospores are almost exclusively plerotic (Hong et al. 2012; Fig. 69) whereas P. furcata and P. sumatera produce both plerotic and aplerotic oospores in almost equal proportions (Table S16; Figs 70, 71). In P. multivesiculata oospores are mostly aplerotic (Ilieva et al. 1998). After 4 wk at 20 °C the oospore abortion rate is higher in P. furcata (25.2 %) than in P. angustata (8.7 %) and P. sumatera (15.8 %). The almost exclusively paragynous attachment of their antheridia discriminate P. angustata and P. taxon aquatilis from P. furcata and P. sumatera which have exclusively amphigynous antheridia, and P. multivesiculata with 95 % amphigynous and 5 % paragynous antheridia. Phytophthora multivesiculata and the three new Clade 2f species share the abundant production of catenulate hyphal swellings in water whereas P. taxon aquatilis does not form hyphal swellings (Ilieva et al. 1998, Hong et al. 2012; Table S16; Figs 69–71).
With 25 °C P. angustata, P. furcata and P. sumatera have a higher optimum temperature for growth than P. multivesiculata and P. taxon aquatilis (both 20 °C). In contrast, the maximum temperature for growth is higher in P. multivesiculata (30 °C) and P. taxon aquatilis 30–<35 °C) than in P. angustata, P. sumatera (both 27.5–<30 °C) and P. furcata (25–<27.5 °C) (Hong et al. 2012; Table S16; Fig. 62). Phytophthora multivesiculata shows the slowest growth of all Clade 2f species (Hong et al. 2012; Table S16).
Clade 2g
For all known Clade 2g species, colony morphologies on CA, PDA and V8A and temperature-growth relations on V8A are presented in Figs 72 and 73, respectively. Morphological and physiological characters and morphometric data of the four newly described species in Clade 2g are given in the comprehensive Table S17.
Fig. 72.
Colony morphology of Phytophthora species from subclade 2g after 7 d growth at 20 ºC on V8-agar, carrot juice agar and potato-dextrose agar (from top to bottom). A. Phytophthora inclinata (ex-type CBS 149488). B, C. Phytophthora multipapillata (B. ex-type CBS 149493; C. KA565). D. Phytophthora proliferata (ex-type CBS 149498). E. Phytophthora transposita (ex-type CBS 149502).
Fig. 73.
Mean radial growth rates of four new Phytophthora species from subclade 2g on V8-agar at different temperatures: P. inclinata (5 isolates); P. multipapillata (7 isolates); P. proliferata (4 isolates); P. transposita (2 isolates).
Phytophthora inclinata N.M. Chi, T. Jung, M. Horta Jung & I. Milenković, sp. nov. MycoBank MB 847313. Fig. 74.
Fig. 74.
Phytophthora inclinata. A–L. Structures formed on V8-agar (V8A) in soil extract. A–K. Sporangia. A–H, J, K. Ovoid, obpyriform and ampulliform sporangia. A–C, H, I. Semipapillate apices. A, I. Pedicels (arrows). B, K. Inclined apices and external proliferation. D–G, K. Papillate apices. E. Constricted sporangiophore. F. Hyphal extension (arrow). G, H. Intercalary sporangia. J. Zoospore release. L. Catenulate hyphal swellings. M–W. Oogonia with near-plerotic to slightly aplerotic oospores and paragynous antheridia formed in V8A. M–V. Subglobose to globose oogonia. S–V. Tapering curved bases. W. Comma-shaped oogonium. Images: A, D, E–G, K–O, Q, S–U, W. Ex-type CBS 149488; B, C, I, J, P, R. VN1092; H, V. VN1091. Scale bar = 20 µm; W applies to A–W.
Etymology: The name refers to the occurrence of sporangial apices with inclined papillae.
Typus: Vietnam, Côn Đảo National Park, Côn Lôn Island, isolated from rhizosphere soil of Ailanthus triphysa and Chukrasia tabularis in a tropical lowland rainforest, Apr. 2017, N.M. Chi (holotype CBS H-25110, dried culture on V8A, ex-holotype living culture CBS 149488 = VN1023).
Morphological structures on V8A: Sporangia rarely observed on solid agar but produced abundantly in non-sterile soil extract; typically borne terminally (94.5 %) on unbranched short or long sporangiophores (Fig. 74D, E) or in dense or lax sympodia of 2–4 sporangia (Fig. 74B, K), or less frequently intercalary (4.5 %; Fig. 74G, H) or sessile (1 %); predominantly ovoid, broad-ovoid or elongated ovoid (77.5 %; Fig. 74A–E, J, K), less frequently obpyriform to elongated-obpyriform (7.6 %; Fig. 74G), subglobose (5.5 %; Fig. 74F), limoniform to elongated limoniform (4.5 %), distorted and often with two apices (3.5 %; Fig. 74I), ellipsoid (0.5 %), obovoid (0.5 %) or ampulliform (0.4 %; Fig. 74H); apices semipapillate (51.5 %; Fig. 74A–C, H, I) or papillate (48.5 %; Fig. 74D–G, K); lateral attachment of the sporangiophore (39 %; Fig. 74A, C, F), pedicels (21 %; Fig. 74A, E, I), an asymmetric apex, often with inclined papilla or semipapilla (27 %; Fig. 74B, K) and short hyphal appendices (Fig. 74F) commonly observed; usually non-caducous, but a few sporangia with constrictions of the sporangiophore (Fig. 74E), potentially enabling caducity, present in all isolates; sporangial proliferation exclusively external (Fig. 74B, K); sporangial dimensions averaging 51.8 ± 6.6 × 36.1 ± 5.0 µm (overall range 34.2–84.6 × 24.7–57.6 µm; range of isolate means 44.6–57.8 × 33.0–38.7 µm) with a length/breadth ratio of 1.45 ± 0.21 (overall range 1.06–2.37); pedicel length 18.7 ± 13.2 µm (range 2.9–78.0 µm); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.9–8.8 µm wide (av. 6.6 ± 0.7 µm). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.0 ± 0.9 µm) on encystment; cysts mostly germinating directly although diplanetism was infrequently observed in all isolates. Hyphal swellings abundantly produced in water on sporangiophores and hyphae; globose to subglobose, limoniform, deltoid or irregular and often catenulate (Fig. 74L); dimensions 13.7 ± 3.1 µm (range 7.9–22.7 µm). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae, smooth-walled, globose to slightly subglobose (94.5 %; Fig. 74M–V), or slightly elongated (5.5 %; Fig. 74W), with a round (64.5 %; Fig. 74M–O, Q, R, V, W) or a short tapering, often curved base (35.5 %; Fig. 74P, S–U); sometimes comma-shaped (9.9 %; Fig. 74V, W); oogonial diam 25.4 ± 1.9 µm (overall range 14.6–29.8 µm; range of isolate means 24.7–25.7 µm); nearly plerotic to plerotic (81 %; Fig. 74M–U) or slightly aplerotic (19 %; Fig. 74V, W). Oospores globose with a large lipid globule (Fig. 74M–W); diam 22.5 ± 1.8 µm (overall range 12.3–26.6 µm; range of isolate means 22.3–22.8 µm); wall thickness 1.38 ± 0.22 µm (overall range 0.76–2.09 µm), oospore wall index 0.32 ± 0.05; abortion rate 0–3 % (av. 1.3 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ovoid, globose to subglobose or irregular (Fig. 74M–W), sometimes with finger-like projections (Fig. 74O, P); dimensions 11.4 ± 1.8 × 8.6 ± 1.4 µm.
Culture characteristics: Colonies on V8A and CA mostly submerged to appressed with scanty aerial mycelium, chrysanthemum-like to stellate on V8A and radiate on CA; on PDA dense-felty with irregular margins and a faint petaloid pattern (Fig. 72).
Cardinal temperatures and growth rates: Optimum 27.5 °C with 10.83 ± 0.15 mm/d radial growth on V8A, maximum 30–<32.5 °C, minimum slightly below 10 °C (Fig. 73), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 7.61 ± 0.14 mm/d, 5.38 ± 0.31 mm/d and 3.43 ± 0.06 mm/d, respectively.
Additional materials examined: Vietnam, Côn Đảo National Park, Côn Lôn Island, isolated from rhizosphere soil of A. triphysa and C. tabularis in a tropical lowland rainforest, April 2017, N.M. Chi (VN1089, VN1090, VN1091, VN1092).
Phytophthora multipapillata T. Jung, M. Tarigan, I. Milenković & M. Horta Jung, sp. nov. MycoBank MB 847317. Fig. 75.
Fig. 75.
Phytophthora multipapillata. A–L. Sporangia formed on V8-agar (V8A) in soil extract. A–H, L. Ovoid, obpyriform, obovoid and mouse-shaped sporangia. A–D, F, G, I, J, L. Semipapillate apices. E, H. Papillate apices. A, B, D, H. Medium-length pedicels (arrows). H, I. External proliferation. F. Intercalary sporangium. H. Caducous sporangium. I, J, L. Sporangia with multiple apices. K. Zoospore release. L. Sympodium. M. Intercalary hyphal swelling on V8A in soil extract. N–V. Oogonia with slightly aplerotic to plerotic oospores and paragynous antheridia formed in V8A. N–S. Globose to subglobose oogonia. T. Slightly elongated oogonium. U, V. Comma-shaped oogonia with tapering bases. Images: A, C, E–H, M, N, P–S, V. Ex-type CBS 149493; B, D, I, K, L, O, T, U. KA565; J. KA534; Scale bar = 20 µm; V applies to A–V.
Etymology: The name refers to the frequent production of bi- or tripapillate sporangia (multi Latin = many).
Typus: Indonesia, Kalimantan, Balikpapan, isolated from rhizosphere soil of Paraserianthes falcataria in a tropical lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (holotype CBS H-25116, dried culture on V8A, ex-holotype living culture CBS 149493 = KA532).
Morphological structures on V8A: Sporangia produced abundantly in non-sterile soil extract; typically borne terminally (88.5 %) on unbranched long or short sporangiophores (Fig. 75A, B) or in dense or lax sympodia of 2–5 sporangia (Fig. 75L), or intercalary (2.5 %) or sessile (6.5 %; Fig. 75F); mostly ovoid, broad-ovoid or elongated ovoid (58.7 %; Fig. 75A, B, G, K, L), distorted with two or three apices (22.3 %; Fig. 75I, J, L) or less frequently obpyriform to elongated-obpyriform (5.5 %; Fig. 75C, D, F), mouse-shaped (5 %; Fig. 75H), obovoid or elongated obovoid (4 %; Fig. 75E), ellipsoid or elongated-ellipsoid (2.5 %), limoniform or elongated limoniform (2 %); apices often asymmetric or curved (24.5 %; Fig. 75B, C, F, I, L), semipapillate and often pointed (84.5 %; Fig. 75A–D, G, I, J, L), less frequently papillate (14.5 %; Fig. 75E, H) or nonpapillate (1 %; Fig. 75F); usually persistent but a few caducous sporangia (<1 %; Fig. 75H) were present in all isolates; lateral attachment of the sporangiophore (27 %; Fig. 75D, E, G, H) and pedicels (46 %; Fig. 75A, B, D, H) commonly observed; sporangial proliferation exclusively external (Fig. 75H, L); sporangial dimensions averaging 60.4 ± 6.2 × 33.9 ± 4.0 µm (overall range 42.5–75.0 × 22.6–43.1 µm; range of isolate means 57.3–62.6 × 33.2–35.4 µm) with a length/breadth ratio of 1.8 ± 0.2 (overall range 1.22–2.6); pedicel length 30.2 ± 12.9 µm (range 6.3–55.6 µm); sporangial germination indirectly with zoospores discharged through an exit pore 4.6–8.1 µm wide (av. 6.3 ± 0.8 µm) (Fig. 75K). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 10.5 ± 0.8 µm) on encystment; cysts germinating directly. Hyphal swellings produced in water on sporangiophores and hyphae; globose to subglobose, ovoid, limoniform or deltoid (Fig. 75C, M). Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, sometimes curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose (79.6 %; Fig. 75N–S), slightly elongated (2.4 %; Fig. 75T) or comma-shaped (18 %; Fig. 75U, V), usually with a rounded (90.4 %; Fig. 75N–S) or a short tapering base (9.6 %; Fig. 75T–V); oogonial diam 27.2 ± 1.4 µm (overall range 22.9–31.2 µm; range of isolate means 26.8–27.7 µm); slightly aplerotic (53.6 %; Fig. 75N–Q) or nearly plerotic to plerotic (46.4 %; Fig. 75R–V). Oospores globose with a large lipid globule (Fig. 75N–V); diam 24.0 ± 1.2 µm (overall range 20.3–29.6 µm; range of isolate means 23.8–24.4 µm) wall thickness 1.18 ± 0.17 µm (overall range 0.69–1,7 µm), oospore wall index 0.27 ± 0.03; abortion rate 1–14 % (av. 6.4 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ellipsoid or subglobose (Fig. 75N–V), occasionally with finger-like projections (12.8 %; Fig. 75P); sometimes two antheridia attached to one oogonium; dimensions 13.5 ± 3.0 × 8.3 ± 1.2 µm.
Culture characteristics: Colonies on V8A and CA appressed with limited aerial mycelium, chrysanthemum-like to stellate on V8A and faintly radiate on CA; on PDA felty-cottony with a faint petaloid pattern (Fig. 72).
Cardinal temperatures and growth rates: On V8A optimum at 27.5 °C with 8.06 ± 0.43 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 73), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 5.44 ± 0.26 mm/d, 4.66 ± 0.27 mm/d and 3.43 ± 0.81 mm/d, respectively.
Additional materials examined: Indonesia, Kalimantan, Balikpapan, isolated from rhizosphere soil of P. falcataria in a tropical lowland rainforest, Feb. 2019, T. Jung & M. Tarigan (KA533, KA534); isolated from rhizosphere soil of Diospyros pilosanthera in a tropical lowland rainforest, Feb. 2019, T. Jung, M. Tarigan & M. Junaid (KA565, KA612, KA613, KA614).
Phytophthora proliferata T. Jung, N.M. Chi, I. Milenković & M. Horta Jung, sp. nov. MycoBank MB 847303. Fig. 76.
Fig. 76.
Phytophthora proliferata. A–M. Sporangia formed on V8-agar (V8A) in soil extract. A–G, I–L. Ovoid, obpyriform and limoniform sporangia. A, B, G, L. Papillate apices. C–F, H. Semipapillate apices. A, D, F, G, K, L. External proliferation (arrows in D, F, G). C. Sessile sporangium. D. Intercalary sporangium. H. Bilobed sporangium. I. Zoospore release. J. Internal extended proliferation and zoospore flagella with ring-like end (arrow). K. Internal nested and extended proliferation. L. Dense sympodium. M. Immature trilobed sporangium. N, O. Hyphal swellings on V8A in soil extract. N. Catenulate. P–V. Oogonia with slightly aplerotic to plerotic oospores and paragynous antheridia formed in V8A. P–T. Globose to subglobose oogonia. U, V. Slightly elongated oogonia. Images: A, G, O, VN804; B, C–E, H, I, J, K, L, M, R–V. Ex-type CBS 149498; F, N, P, Q. VN803. Scale bar = 20 µm; V applies to A–V.
Etymology: The name refers to the regular external and internal nested and extended proliferation of sporangia.
Typus: Vietnam, Cuc Phuong National Park, isolated from rhizosphere soil of Anogeissus acuminata and Taxotrophis macrophylla in a tropical lowland rainforest, Mar. 2016, T. Jung, & N.M. Chi (holotype CBS H-25122, dried culture on V8A, ex-holotype living culture CBS 149498 = VN686).
Morphological structures on V8A: Sporangia not observed in solid agar but abundantly produced in non-sterile soil extract; borne terminally, in dense or lax sympodia of 2–5 sporangia (Fig. 76L) or on short lateral hyphae (Fig. 76B, F, J), or less frequently intercalary (2 %; Fig. 76D) or sessile (0.5 %; Fig. 76C); non-caducous, predominantly ovoid, broad-ovoid or elongated ovoid (69 %; Fig. 76A–E, I–L), less frequently limoniform or elongated-limoniform (11.5 %; Fig. 76G), ellipsoid (10.5 %; Fig. 76L), subglobose (3 %), distorted and often with two or three apices (2 %; Fig. 76H, M), obovoid (1.5 %), obpyriform (1.5 %; Fig. 76F), pyriform (0.5 %) or ampulliform (0.5 %); lateral attachment of the sporangiophore common (31.5 %; Fig. 76B, E); pedicels infrequent (4 %), 15.1 ± 7.5 µm (range 3.6–50.5 µm); apices papillate (44 %; Fig. 76A, B, G, L) or semipapillate (56 %; Fig. 76C–F, H); sporangial proliferation external (Fig. 76D, F, G, I, K, L) or less frequently internal in an extended (Fig. 76J, K) or nested way (Fig. 76K); sporangial dimensions averaging 49.1 ± 6.8 × 31.3 ± 4.2 µm (overall range 29.6–67.5 × 20.3–42.2 µm; range of isolate means 46.4–52.3 × 28.3–34.9 µm) with a length/breadth ratio of 1.58 ± 0.19 (overall range 1.17–2.26); sporangial germination indirectly with zoospores discharged through an exit pore 4.6–8.9 µm wide (av. 6.6 ± 0.9 µm) (Fig. 76I–L). Zoospores limoniform to reniform whilst motile, sometimes with ring-like flagella ends (Fig. 76J), becoming spherical (av. diam = 10.8 ± 1.3 µm) on encystment. Hyphal swellings infrequently formed in water, globose to subglobose, limoniform or irregular, sometimes catenulate (Fig. 76N, O), av. diam 10.1 ± 2.8 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, often curved lateral hyphae, smooth-walled, globose to slightly subglobose (88 %; Fig. 76P–T) or slightly elongated (12 %; Fig. 76U, V), usually with a round (72.5 %; Fig. 76P–U) or less frequently with a tapering base (27.5 %; Fig. 76V); oogonial diam 26.5 ± 2.0 µm (overall range 15.1–31.0 µm; range of isolate means 26.2–27.1 µm); nearly plerotic to plerotic (97 %; Fig. 76P–U), rarely slightly aplerotic (3 %; Fig. 76V). Oospores globose with a large lipid globule (Fig. 76P–V); diam 23.7 ± 1.8 µm (overall range 13.0–28.1 µm; range of isolate means 22.9–24.2 µm) wall thickness 1.61 ± 0.25 µm (overall range 0.92–2.25 µm), oospore wall index 0.35 ± 0.04; abortion rate 2–19 % (av. 10.5 %) after 4 wk. Antheridia exclusively paragynous and club-shaped, ovoid or globose to subglobose (Fig. 76P–V); 13.5 ± 2.3 × 9.3 ± 1.2 µm.
Culture characteristics: Colonies on V8A and CA mostly submerged to appressed, stellate on V8A and radiate on CA; on PDA felty-cottony with a faint petaloid pattern (Fig. 72).
Cardinal temperatures and growth rates: On V8A optimum 27.5 °C with 10.1 ± 0.17 mm/d radial growth, maximum 30–<32.5 °C, minimum slightly below 10 °C (Fig. 73), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 7.34 ± 0.13 mm/d, 5.47 ± 0.2 mm/d and 3.06 ± 0.14 mm/d, respectively.
Additional materials examined: Vietnam, Cuc Phuong National Park, isolated from rhizosphere soil of A. acuminata and T. macrophylla in a tropical lowland rainforest, Mar. 2016, T. Jung, M. Horta Jung & N.M. Chi (VN803, VN804, VN805, VN806).
Phytophthora transposita T. Jung, K. Kageyama, C.M. Brasier & H. Masuya, sp. nov. MycoBank MB 847304. Fig. 77.
Fig. 77.
Phytophthora transposita. A–M. Sporangia formed on V8-agar (V8A) in soil extract. A–J, M. Ovoid, obovoid, limoniform, obpyriform and mouse-shaped sporangia. A–D, G–J, M. Semipapillate apices. E, F, K. Papillate apices. D, I, J. Curved, laterally displaced apices. A–D, I, M. Laterally attached sporangiophores. E, G, I–M. External proliferation (arrows in E, K). K. Distorted sporangium. L. Sporangium with two apices, after zoospore release. M. Dense sympodium with semipapillate and nonpapillate sporangia and hyphal swellings. N–V. Oogonia with near-plerotic to plerotic oospores and paragynous antheridia formed in V8A. N–R. Subglobose to globose oogonia. S–V. Comma-shaped oogonia with tapering bases. W. Oospore germinating by producing a sporangium. Images: A–C, E, H, J–O, Q, R, U–W. CBS 149502; D, F, G, I, P, S, T. JP2361. Scale bar = 20 µm; V applies to A–W.
Etymology: The name refers to the high proportion of sporangia with laterally displaced sporangiophore attachment and laterally displaced or curved apices (transposita Latin = displaced).
Typus: Japan, Kyushu Island, Aya, isolated from rhizosphere soil of Zanthoxylum ailanthoides in a warm-temperate mixed forest, May 2017, T. Jung & K. Kageyama (holotype CBS H-25126, dried culture on V8A, ex-holotype living culture CBS 149502 = JP583).
Morphological structures on V8A: Sporangia produced abundantly in non-sterile soil extract; typically borne terminally (98 %) in dense or lax sympodia of 2–5 sporangia (Fig. 77M) or less frequently on unbranched long or short sporangiophores (Fig. 77F), or intercalary (2 %); non-caducous, predominantly ovoid, broad-ovoid or elongated ovoid (64 %; Fig. 77A–E, J, M), less frequently obpyriform or elongated-obpyriform (11 %; Fig. 77H), distorted and often with two apices (7.6 %; Fig. 77K, L), limoniform or elongated limoniform (6 %; Fig. 77G), mouse-shaped (5 %; Fig. 77I, M), obovoid (4 %; Fig. 77F) or ampulliform (2.4 %); apices semipapillate (50 %; Fig. 77A–D, G–I, M), papillate (32.6 %; Fig. 77E, F, K) or nonpapillate (17.4 %; Fig. 77J, M), often curved or laterally displaced (28.3 %; Fig. 77B–D, I–K, M); lateral attachment of the sporangiophore (49.1 %; Fig. 77A–D, I, M) and vacuoles (21.7 %; Fig. 77D, I, K) commonly observed; empty sporangia often with a conspicuous protruding basal plug (40 %; Fig. 77L); rarely (4 %) with short pedicels < 10 µm (Fig. 77M); sporangial proliferation exclusively external (Fig. 77E, J–M); sporangial dimensions averaging 61.0 ± 8.5 × 31.5 ± 3.1 µm (overall range 48.1–79.8 × 25.5–37.9 µm; range of isolate means 60.4–61.6 × 30.7–32.3 µm) with a length/breadth ratio of 1.96 ± 0.32 (overall range 1.4–2.74); sporangial germination usually indirectly with zoospores discharged through an exit pore 4.6–6.7 µm wide (av. 5.6 ± 0.6 µm) (Fig. 77L). Zoospores limoniform to reniform whilst motile, becoming spherical (av. diam = 11.0 ± 1.0 µm) on encystment. Hyphal swellings rarely produced in water on sporangiophores; globose to subglobose, ovoid, limoniform or irregular (Fig. 77M); dimensions 10.9–23.6 µm. Chlamydospores not observed. Oogonia abundantly produced in single culture (‘homothallic’ breeding system), terminal on short to medium-length, sometimes curved lateral hyphae or sessile, smooth-walled, globose to slightly subglobose (72 %; Fig. 77N–R) or slightly comma-shaped (28 %; Fig. 77S–V), with a rounded (54 %; Fig. 77N–R) or a tapering base (46 %; Fig. 77S–V); oogonial diam 24.8 ± 1.3 µm (overall range 22.1–27.3 µm; range of isolate means 24.2–25.4 µm); nearly plerotic to plerotic (98 %; Fig. 77N–V) or slightly aplerotic (2 %). Oospores globose with a large lipid globule (Fig. 77N–V); diam 21.1 ± 1.1 µm (overall range 18.4–22.7 µm; range of isolate means 20.8–21.4 µm), wall thickness 1.52 ± 0.19 µm (overall range 1.07–2.02 µm), oospore wall index 0.37 ± 0.03; abortion rate 7 % after 4 wk; after 2 mo at 20 °C many oospores germinating by producing one or multiple sporangia (Fig. 77W). Antheridia exclusively paragynous and club-shaped, ovoid or subglobose (Fig. 77N–V), sometimes with finger-like projections (Fig. 77R, V); dimensions 12.6 ± 2.5 × 7.9 ± 1.3 µm.
Culture characteristics: Colonies on V8A submerged to appressed with limited aerial mycelium and a radiate pattern; on CA submerged to appressed with scanty aerial mycelium and a striate pattern; on PDA appressed, felty-woolly in the centre and dense-felty at the irregular margins, without pattern (Fig. 72).
Cardinal temperatures and growth rates: On V8A optimum at 27.5 °C with 9.28 ± 0.18 mm/d radial growth, maximum 30–<32.5 °C, minimum <10 °C (Fig. 73), lethal temperature 32.5–35 °C. At 20 °C on V8A, CA and PDA 6.46 ± 0.17 mm/d, 4.82 ± 0.07 mm/d and 3.77 ± 0.07 mm/d, respectively.
Additional materials examined: Japan, Kyushu Island, Aya, isolated from rhizosphere soil of Z. ailanthoides in a warm-temperate mixed forest, May 2017, T. Jung & K. Kageyama (JP2361).
Notes on Clade 2g taxa: Across the nuclear (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1) 8 725-character alignment and the mitochondrial (cox1, cox2, nadh1 and rps10) 3 156-character alignment the four new species from Clade 2g showed sequence differences of 0.6–0.8 % and 1.1–1.5 %, respectively. The four new Clade 2g species developed distinctive colony morphologies on V8A, CA and PDA at 20 °C (Fig. 72). In addition, they are separated from each other by a combination of morphological (Figs 74–77) and physiological (Fig. 73) characters of which the most discriminating are highlighted in bold in Table S17.
Three of the four species in Clade 2g, i.e., P. inclinata, P. proliferata and P. transposita, share having high proportions of both semipapillate (50–56 %) and papillate (33–48.5 %) sporangia while in P. multipapillata sporangia are predominantly semipapillate (84.5 %) and less frequently papillate (14.5 %) or nonpapillate (1 %) (Table S17; Figs 74–77). Phytophthora transposita differs from the other three Clade 2g species by having a comparatively high proportion of nonpapillate sporangia (17 %).
High sporangial l/b ratios separate P. multipapillata (1.8 ± 0.2) and P. transposita (1.96 ± 0.32) from P. inclinata (1.45 ± 0.21) and P. proliferata (1.58 ± 0.19) (Table S17). Furthermore, in P. multipapillata 46 % of sporangia are pedicellate and occasionally show caducity (<1 %) whereas in the other three species sporangia are exclusively persistent with much lower proportions of pedicels (4–21 %) (Table S17; Figs 74–77). In addition, P. multipapillata has higher proportions of sporangia with more than one apex than the other Clade 2g species (22 % vs. 2–8 %) (Table S17). Phytophthora proliferata differs from the other three species by having internal nested and extended sporangial proliferation (Table S17; Figs 74–77).
All four species in Clade 2g are intrinsically self-fertile with exclusively paragynous antheridia and low oospore abortion rates. Phytophthora multipapillata differs from the other three species by having a considerably smaller proportion of oogonia with tapering bases (9.6 vs. 27.5–46 %) and a lower oospore wall index (0.27 vs. 0.32–0.37) (Table S17; Figs 74–77).
The four Clade 2g species have identical optimum (27.7 °C) and maximum temperatures (<32.5 °C) for growth but their growth rates differ with P. multipapillata being the slowest species between 20 and 30 °C and P. inclinata and P. proliferata showing the fastest growth at 27.5 and 30 °C, respectively (Fig. 73).
Several new Clade 2g species described here were previously known under informal names, i.e., P. inclinata as P. citricola XI and P. proliferata as P. citricola X (Jung et al. 2020).
Notes on geographical distribution and host and habitat associations of Clade 2 taxa
Apart from P. celeris, which was detected together with P. acaciivora in effluents from an Acacia and eucalypt nursery in Sumatra, and several isolates of P. pseudocitrophthora from nursery plants in Hungary and Spain all the newly described Clade 2 species were isolated either (i) from forest streams (P. amamensis, P. balkanensis, P. catenulata, P. distorta, P. falcata, P. fansipanensis, P. frigidophila, P. furcata, P. indonesiensis, P. japonensis, P. limosa, P. obovoidea, P. obturata, P. pseudocapensis, P. pseudocitrophthora, P. pseudofrigida, P. pseudoccultans, P. sumatera, P. vacuola, P. valdiviana, P. ×australasiatica, P. ×lusitanica, P. ×taiwanensis); (ii) from rhizosphere soil of forest trees (P. balkanensis, P. borneensis, P. catenulata, P. curvata, P. excentrica, P. falcata, P. furcata, P. inclinata, P. indonesiensis, P. japonensis, P. limosa, P. macroglobulosa, P. multipapillata, P. nimia, P. oblonga, P. obovoidea, P. obturata, P. platani, P. proliferata, P. pseudocitrophthora, P. pseudoccultans, P. sumatera, P. transposita, P. vacuola, P. vietnamensis, P. ×australasiatica, P. ×taiwanensis); or (iii) from naturally fallen leaves under the forest canopy (P. angustata, P. calidophila, P. frigidophila, P. montana, P. multiplex, P. obovoidea, P. penetrans, P. pyriformis, P. variepedicellata, P. ×australasiatica). On a purely visual basis, no above-soil stem or foliar symptoms were observed in associated vegetation. It should be noted that, besides being found in the rhizosphere of healthy Platanus orientalis trees in two riparian forests in Sicily, P. platani has also been isolated from aerial bark cankers and necrotic roots of a declining planted mature Platanus × acerifolia tree in London, UK.
Clade 2a taxa
Clade 2a now comprises 22 taxa, i.e., 11 known and six newly described species and five informally designated taxa, with the majority distributed along a belt stretching from South Asia via Southeast Asia to the subtropical islands of the Japanese archipelago (Figs 78, 79).
Fig. 78.
Natural global biogeography of Phytophthora Clade 2. Colours indicate the subclades: Clade 2a = red; Clade 2b = blue; Clade 2c = black; Clade 2e = purple; Clade 2f = green; Clade 2g = orange.
Fig. 79.
Natural global biogeography of Phytophthora Clade 2. Colours of arrows indicate the subclades: Clade 2a = red; Clade 2b = blue; Clade 2c = black; Clade 2e = purple; Clade 2f = green; Clade 2g = orange. Species codes: ACA = P. acaciae; ACV = P. acaciivora; AMA = P. amamensis; ANG = P. angustata; AUS2E = P. taxon AUS 2E; AYS = P. aysenensis; BAL = P. balkanensis; BIS = P. bishii; BOR = P. borneensis; BRA = P. taxon brasiliensis; CAP = P. capensis; CAPS = P. capsici; CAR = P. caryae; CAT = P. catenulata; CEL = P. celeris; CIP = P. citrophthora; CIT = P. citricola; CR5 = P. taxon Costa Rica 5; CR8 = P. taxon Costa Rica 8; DIS = P. distorta; ELO = P. elongata; EMZ = P. emzansi; FRI = P. frigida; FUR = P. furcata; GLO = P. gloveri; HIM = P. himalsilva; HIM1 = P. taxon himalsilva-like 1; HIM2 = P. taxon himalsilva-like 2; IND = P. indonesiensis; INS = P. insulinativitatica; LIM = P. limosa; MEA = P. meadii; MEA1 = P. taxon meadii-like; MEN = P. mengei; MEX = P. mexicana; MUL = P. multivora; PCAP = P. pseudocapensis; PCAPS = P. taxon pseudocapsici; PEN = P. penetrans; PFR = P. pseudofrigida; PIN = P. pini; PLA = P. platani; PLU = P. plurivora; POC = P. pseudoccultans; SIS = P. siskiyouensis; SUB = P. taxon subnubulis; SUM = P. sumatera; THE = P. theobromicola; TRA = P. transposita; TRO = P. tropicalis; VAL = P. valdiviana; ×AUS = P. ×australasiatica; ×TAI = P. ×taiwanensis. Diversity hotspots: 1 = P. calidophila, P. capsici, P. frigidophila, P. mengei, P. montana, P. multiplex, P. obovoidea, P. pyriformis, P. tropicalis, P. variepedicellata, P. taxon mengei-like. 2 = P. botryosa, P. colocasiae, P. mekongensis, P. multibullata, P. vietnamensis, P. ×australasiatica, P. ×vanyenensis. 3 = P. catenulata, P. fansipanensis, P. macroglobulosa, P. obturata, P. pseudocapensis. 4 = P. inclinata, P. multipapillata, P. proliferata. 5 = P. citricola, P. curvata, P. excentrica, P. falcata, P. japonensis, P. nimia, P. oblonga, P. vacuola and most likely P. acerina, P. balkanensis and P. pachypleura.
Both mating types of P. meadii are widespread across the southern part of the Indian subcontinent and Sri Lanka where it has been recognized as the main cause of bark cankers, pod rot and abnormal leaf fall of introduced rubber (Hevea brasiliensis) trees and also damages other crops, including areca nut (Areca catechu), Citrus and vanilla (Peries & Dantanarayana 1965, Peries & Fernando 1966, Rajalakshmy et al. 1985, Erwin & Ribeiro 1996, Bai & Thomas 2000, Drenth & Guest 2004, Krishnan et al. 2019, Patil et al. 2022). Records of P. meadii from elsewhere (cf. Erwin & Ribeiro 1996, Drenth & Guest 2004) lack molecular verification and, hence, could be of other morphologically similar species (e.g. P. insulinativitatica, P. mekongensis, P. multibullata, P. vietnamensis, P. ×australasiatica, P. ×taiwanensis and P. ×vanyenensis).
Phytophthora colocasiae may be host-specific, causing the economically important taro (Colocasia esculenta) leaf blight. It is distributed from India throughout Southeast Asia and Papua New Guinea to Japan and Hawaii. Exclusive findings of A1 mating type isolates in India (Nath et al. 2013) and, in an early study, Hawaii (Ko 1979), and of A2 isolates in Taiwan, Vietnam, Papua New Guinea and several Pacific islands (Tyson & Fullerton 2007, Lin & Ko 2008, Shrestha et al. 2014) and, in a recent study, also Hawaii (Ann et al. 1986) suggest that P. colocasiae is introduced in these regions, probably on plant material and adhering soil (Zentmyer 1988). Recently, Feng et al. (2022) reported self-sterile A1 or A2 mating type isolates and self-fertile A2 and A1A2 isolates from Japan but in a highly unbalanced ratio of 3:63:32:2. In contrast, Zhang et al. (1994) found a more balanced A1: A2: A0 (A0 = sterile) ratio of 49:36:15 in 280 P. colocasiae isolates sampled across Hainan Island, South China, and suggested Hainan and adjacent regions in Southeast Asia to be within the centre of origin of the species. This is supported by the isolation of P. colocasiae from leaf spots on wild taro plants growing in riparian lowland rainforest in Sumatra in the present study.
Phytophthora taxon meadii-like (isolate CBS 358.59, erroneously designated in the CBS collection as P. palmivora, in the WPC collection as P. meadii and at GenBank (accession no. MH760162 as P. colocasiae) was only isolated from H. brasiliensis in Sri Lanka.
Phytophthora botryosa, only known from and possibly native to Malaysia, Thailand and Vietnam where both mating types co-occur, poses a serious threat to the introduced H. brasiliensis, currently the only known host (Chee 1969, Erwin & Ribeiro 1996, Drenth & Guest 2004, Krishnan et al. 2019).
Phytophthora vietnamensis (previously P. taxon meadii-like 1) occurs in a montane Alnus nepalensis forest in the North of Vietnam, close to the border with China (Jung et al. 2020 and this study) and in a Elettaria cardamomum plantation in India (isolate WPC P6128 designated as P. meadii in Blair et al. 2008; see Fig. 2).
Phytophthora mekongensis and P. multibullata are currently only known from plantations of Citrus grandis (P. mekongensis) and the introduced Cinnamomum cassia (both species) in Vietnam (Crous et al. 2017, Puglisi et al. 2017, Dang et al. 2021, this study), and it seems likely that they are native to the natural forests surrounding the affected plantations. For P. multibullata this is supported by the co-occurrence of both mating types in the affected plantations (Dang et al. 2021).
Phytophthora ×vanyenensis, which was first described from Vietnamese C. cassia plantations (Dang et al. 2021), and P. ×australasiatica overlap widely, being found so far from northern Vietnam to Java, Sumatra and Sulawesi. Both species co-occur at several sites in Indonesia. Their functional A1/A2 breeding systems, with both mating types present at several sites, suggest they are native to the region. In addition, P. ×australasiatica was found in native forests on Kalimantan, on the subtropical Amami and Okinawa islands in the Ryukyu Islands chain between Taiwan and southwest Japan, and in a tropical lowland rainforest in Panama. Since no other Clade 2a species has been found in our surveys in Panama and Nicaragua or - apart from the globally distributed P. citrophthora - elsewhere in Central or South America, P. ×australasiatica has probably been introduced from Asia to Panama.
Phytophthora insulinativitatica was described from a tropical rainforest on Christmas Island ca. 350 km south of Java and Sumatra (Dang et al. 2021), where the occurrence of both mating types (and its unique phylogenetic position within Clade 2a; Fig. 2) indicates it might be indigenous to this remote outpost. One isolate of P. insulinativitatica was recently obtained from a tiny coral island belonging to the remote Cocos Islands atoll in the Indian Ocean (Burgess et al. 2021), ca. 1 000 km west of Christmas Island and southwest of Java and Sumatra.
Phytophthora citrophthora was commonly isolated from numerous streams in native healthy forests on the subtropical Japanese islands of Kyushu, Amami and Okinawa and in Taiwan. Its centre of origin lays therefore most probably within the subtropical regions of East Asia. In contrast, in Europe, North and South America, South Africa and India P. citrophthora is considered an introduced invasive pathogen causing severe diseases of Citrus spp. and a wide range of other host plants in horticultural and ornamental nurseries and plantings (Rojic & Cancino 1975, MacDonald et al. 1994, Mchau & Coffey 1994, Erwin & Ribeiro 1996, Ferguson & Jeffers 1999, Themann et al. 2002, Schwingle et al. 2007, Alvarez et al. 2008, Donahoo & Lamour 2008a, Savita & Nagpal 2012, Moralejo et al. 2009, Pane et al. 2009, Salamone et al. 2011, Leonberger et al. 2013, Bienapfl & Balci 2014, Meitz-Hopkins et al. 2014, Parke et al. 2014, Prigigallo et al. 2015, Jung et al. 2016, Burgess et al. 2021, Mora-Sala et al. 2022). However, many previous records of P. citrophthora may be of the morphologically similar and closely related P. pseudocitrophthora.
Phytophthora pseudocitrophthora was discovered in this study on Citrus limon and a range of other host plants in nurseries and plantings in Europe and Morocco. It was isolated previously from a citrus tree in California (isolate WPC P10142, designated as P. citrophthora). It was also found in forest streams in Portugal, Louisiana and Connecticut (isolate NJB2013-HR-27; Brazee et al. 2016), USA, and in the rhizosphere of riparian Platanus orientalis in Sicily, possibly a result of ornamental and forest outplanting in the catchment areas. Phytophthora pseudocitrophthora was also isolated from soil in a remote, montane broadleaved forest in Nepal (isolate PCRh7-1; Vettraino et al. 2011), from Prunus persica in China (isolate WPC P3243) and from Malus pumila in South Korea (isolate CBS 111339) (see Table S1). It seems likely to be native to East Asia.
The only known isolates of P. ×lusitanica are from the Rio Séqua in the South of Portugal where its relatives P. citrophthora and P. pseudocitrophthora were also recovered. Since it is a hybrid with P. citrophthora as its maternal parent P. ×lusitanica was most likely introduced from East Asia.
Phytophthora himalsilva and P. taxon himalsilva-like 1 (previously P. himalsilva in Vettraino et al. 2011, and P. aff. himalsilva in Yang et al. 2017) are plausibly endemic to a remote area of western Nepal, occurring naturally in the rhizosphere of several tree species in healthy, mixed sub-tropical forests (Vettraino et al. 2011).
Phytophthora taxon himalsilva-like 2 (isolate NRCPh-97, designated as P. citrophthora in Das et al. 2016), a sister taxon of P. taxon himalsilva-like 1, was only recorded from a citrus nursery in East Sikkim, India close to Nepal.
Phytophthora pseudoccultans and P. ×taiwanensis are only known from rhizosphere soil and streams in healthy subtropical forests of Taiwan where they are most likely endemic.
Phytophthora occultans and P. terminalis are exclusively known as invasive pathogens in horticultural and ornamental nurseries and plantings in Europe and, in case of P. occultans, also in the USA (Reeser et al. 2012, Nechwatal et al. 2014, Man In’t Veld et al. 2015, this study). Since their closest relatives P. himalsilva, P. pseudoccultans, P. taxon himalsilva-like 1 and P. taxon himalsilva-like 2 are native to South and Southeast Asia, it seems likely that P. occultans and P. terminalis also originate from these regions.
Phytophthora taxon awatangi and P. taxon germisporangia are only known from disturbed rainforests in Papua New Guinea (Dang et al. 2021).
Clade 2b taxa
Clade 2b now comprises 22 taxa, i.e., nine known and nine new species and the undescribed P. taxon brasiliensis, P. taxon mengei-like, P. taxon pseudocapsici and P. taxon subnubulis, of which 21 taxa (95.5 %) are distributed in the Americas from Alaska to the Valdivian region in Chile.
Phytophthora siskiyouensis has the most northerly known distribution of all Clade 2 taxa and occurs commonly in forest streams in Alaska and Oregon occasionally causing bark cankers in Alnus rubra and Notholithocarpus densiflorus and shoot blight of Umbellularia californica (Reeser et al. 2007, 2011, Sims et al. 2015a, b). It is also associated with bark cankers and mortality of introduced European Alnus glutinosa trees in Victoria, Australia (Smith et al. 2006, Burgess et al. 2021) and planted riparian Alnus incana trees in the UK (Pérez-Sierra et al. 2015), in both cases certainly introduced.
Phytophthora mengei is distributed from California to Guatemala causing root rot and bark cankers on cultivated avocado (Persea americana) with high disease incidences since the early 20th Century (Hong et al. 2009, Bezuidenhout et al. 2010). It has only once been recorded from another continent and another host species, i.e., from Vigna unguiculata in Queensland, Australia (ITS GenBank accession no. GU258661.1; erroneously submitted as P. siskiyouensis; Burgess et al. 2021). It is therefore most probably endemic to Central America, perhaps as a relatively benign pathogen on coevolved wild avocados and other neotropical Persea species. This is supported by the probably endemic co-occurrence of its closest relative, P. montana, and two other new Clade 2b species, P. frigidophila and P. variepedicellata, in a remote natural cloud forest near the peak of the Barú Volcano in Panama. All three species were exclusively found in naturally fallen tree leaves of this cloud forest together with the recently described aerial oomycete Synchrospora medusiformis (Jung et al. 2023) and a yet undescribed aerial Phytophthora species from Clade 8 (Y. Balci, K. Broders & T. Jung unpublished results) which is related to the undescribed Phytophthora madida nom. prov. recently found in Chile (Jung et al. 2018b).
Phytophthora taxon mengei-like, which grouped in the multigene phylogeny in a basal position to the sister species P. mengei and P. montana, is another avocado pathogen only known from Guatemala (isolate WPC P1165; Bezuidenhout et al. 2010) where it is most likely endemic.
Phytophthora calidophila was obtained only from a montane rainforest in Nicaragua where it co-occurred with P. obovoidea. The latter species is widespread in both montane and lowland rainforests across Nicaragua and Panama and was also found scattered in Java, Sulawesi, Taiwan and Vietnam. The co-occurrence of both mating types in the same stands and high genetic variability indicates that Nicaragua and Panama may lie within the origin of P. obovoidea, whereas all isolates from Southeast Asia were clonal (Fig. 3) and exclusively A1s.
Phytophthora tropicalis, the sister species of P. obovoidea, is a globally distributed, often damaging pathogen with a wide host range including many tropical crops and ornamentals (Aragaki & Uchida 2001, Gerlach & Schubert 2001, Drenth & Guest 2004, Cerqueira et al. 2006, Leahy 2006, Pane et al. 2009, Uchida & Kadooka 2013, Jung et al. 2016, Luongo et al. 2016, Chávez-Ramírez et al. 2021). Under its previous designation as P. capsici electrophoretic type CAP2, P. tropicalis was shown to cause pod rot of cocoa in the Americas, whereas in tropical regions of Africa and Asia the disease is mainly caused by P. megakarya, P. palmivora and occasionally other Phytophthora species (Brasier and Griffith 1979, Mchau & Coffey 1995, Aragaki & Uchida 2001, MacMahon & Purwantara 2004; Chávez-Ramírez et al. 2021). In the present surveys, both mating types of P. tropicalis were common in tropical rainforests in Nicaragua and Panama, sometimes co-occurring with its closest relative P. obovoidea. From this and its widespread distribution across Central and South America P. tropicalis is most likely native to this region and has spread with imported tropical crops and ornamentals to other continents. Before being formally described by Aragaki & Uchida (2001) P. tropicalis isolates were commonly identified as P. capsici and even now many sequences submitted to GenBank as P. capsici are of P. tropicalis (see appendix S3 in Burgess et al. 2021).
In contrast to P. obovoidea and P. tropicalis, their closest known relative P. pyriformis appears to have a limited distribution and has only been recovered, together with P. obovoidea, from one lowland rainforest in Panama.
Phytophthora capsici is a harmful pathogen with global distribution in agricultural zones and a wide host range of mainly non-woody host plants (Mchau & Coffey 1995, Erwin & Ribeiro 1996, Drenth & Guest 2004, Roberts et al. 2005, Truong et al. 2010, Granke et al. 2012, Sanogo & Bosland 2013, Enzenbacher et al. 2015, Kong et al. 2022, Quesada-Ocampo et al. 2023, Sanogo et al. 2023). A potential origin of P. capsici within Central America and the southern USA is indicated by its particularly wide host range among Fabaceae, Cucurbitaceae and Solanaceae species native to Central America; its wide distribution in Central America and the southern USA, where it was first described from Capsicum annuum in New Mexico by Leonian in 1922; the fact that it has never been recovered in any of the numerous Phytophthora surveys of natural streams and forests across Europe, Asia, Australia and South Africa (Jung et al. 1996, 2013a, 2017b, 2019, 2020, Oh et al. 2013, Dunstan et al. 2016, O’Hanlon et al. 2016, Burgess et al. 2017, 2021, Corcobado et al. 2020, 2023, Seddaiu et al. 2020); the frequent occurrence of both mating types in the same horticultural field; and the probable geographic origin of many Clade 2b relatives in Central America.
Phytophthora mexicana, first described in 1923 as a pathogen of tomato (Solanum lycopersicum) fruits in Mexico (Erwin & Ribeiro 1996), is a cryptic species with only two known living isolates in culture collections (WOC P646 = CBS 554.88 = ATCC 46731 = IMI 92550 = CPHST BL 24 from Mexico and ex-epitype CBS 149405 = CPHST BL 199 = French Ph213 = S972 from the USA; Abad et al. 2023a, Table S1). However, in the multigene phylogenetic analyses of this study, several other isolates previously designated as P. capsici (CBS 121656, WPC P1314, CPV302; Table S1) or P. aff. capsici (ATCC 15427; Yang et al. 2017), all originating from Mexico or the USA, grouped with P. mexicana (Fig. 3). An origin of P. mexicana in Mexico and the southern USA is most likely.
Several isolates obtained in the present study from naturally fallen leaves in three streams running through agricultural areas in Sumatra and isolate CBS 370.72 from New Mexico, previously designated as P. capsici (Yang et al. 2017), grouped in our multigene phylogeny together separately from P. capsici and P. mexicana (Fig. 3) and were, hence, designated as P. taxon pseudocapsici. Although an origin in Southeast Asia cannot be ruled out, it seems more likely that P. taxon pseudocapsici, like its close relatives P. capsici and P. mexicana, is native to Central America and the southern USA.
Phytophthora multiplex, a new probable hybrid species with P. theobromicola as maternal parent, was found widespread in tropical lowland rainforests in Nicaragua and Panama and was also recorded from Costa Rica (isolate WPC P10417; Fig. 3). Along with many other Clade 2b taxa it is probably endemic to Central America.
The recently described P. theobromicola, in the past erroneously identified as P. citrophthora (Mchau & Coffey 1994), is currently known only for causing black pod disease of cocoa in Brazil (Decloquement et al. 2021) and Colombia (Ramírez Martínez et al. 2021; Table S1).
The currently undescribed P. taxon brasiliensis, previously known as P. capsici isozyme group CAP3 and electrophoretic type ET26 (Mchau & Coffey 1995), and sometimes designated as Phytophthora cf. capsici FM-2011, is frequently associated with pod rot of cocoa in Brazil (e.g. WPC isolates P0622, P0630, P15128 and P15130; isolate LT29; isolate Cp-1 designated as P. capsici by Bowers et al. 2007; see Table S1) and was also found in the rhizosphere of the native Brazilian tree Caesalpinia echinata (isolate WPC P6907). Its phylogenetic position as basal taxon of a cluster comprising P. frigidophila from Panama and three Chilean species, i.e., P. aysenensis, P. distorta and P. valdiviana (Fig. 3), supports an origin of P. taxon brasiliensis in Brazil.
Phytophthora gloveri, is exclusively known as the causal agent of root rot and stunting of the introduced Virginian tobacco (Nicotiana tabacum) in southeastern Brazil (Abad et al. 2011) where it is most likely endemic thriving on native South American tobacco species.
Phytophthora taxon subnubulis (isolate L5395) was isolated in 2008 from Capsicum pubescens in the montane Oxapampa region of Peru (Hu et al. 2020, Winkworth et al. 2022) where it is most likely endemic.
Phytophthora aysenensis (Crous et al. 2020), P. distorta and P. valdiviana have all been found exclusively in the cool-temperate Aysen and Valdivia regions in southern Chile where they are endemic.
Phytophthora amaranthi is the only known Clade 2b species that has not been recorded from the Americas. Appearing suddenly in 2007 in central Taiwan and subsequently spreading rapidly through the entire production area of the native Asian edible amaranth (Amaranthus tricolor) resulting in substantial economic losses, P. amaranthi appears to be a typical exotic, introduced pathogen (Ann et al. 2016). Since many species of the cosmopolitan genus Amaranthus are native to North, Central and South America, it seems probable that P. amaranthi, like its closest relatives P. capsici, P. gloveri and P. mexicana, originates from the Americas.
Clade 2c taxa
With nine known and 15 new species, Clade 2c is currently the largest of the seven subclades.
Phytophthora multivora is widely distributed in Australia, where it was first described, and in South Africa and Europe. It has also been reported from the Canary Islands, North America and New Zealand, but only once from Asia on nursery stock in Japan (Scott et al. 2009, Hansen et al. 2012, Hüberli et al. 2013, Mrázková et al. 2013, Szabó et al. 2013, Bienapfl & Balci 2014, Scott & Williams 2014, Puno et al. 2015, Rahman et al. 2015, Scarlett et al. 2015, Jung et al. 2016, Burgess et al. 2017, 2021, Garbelotto et al. 2018, Rodríguez-Padrón et al. 2018, Migliorini et al. 2019, Rooney-Latham et al. 2019, Sims & Garbelotto 2021). Considered to be in equilibrium with the native flora in South Africa (Oh et al. 2013, Tsykun et al. 2022), P. multivora is an emerging aggressive pathogen with a particularly wide host range elsewhere (Scott et al. 2009, 2012, Mrázková et al. 2013, Szabó et al. 2013, Bienapfl & Balci 2014, Migliorini et al. 2019, Jung et al. 2016, Garbelotto et al. 2018, Rodríguez-Padrón et al. 2018, Rooney-Latham et al. 2019, Sims & Garbelotto 2021). A recent analysis of a global population sample of P. multivora confirmed South Africa as its centre of origin (Tsykun et al. 2022).
Phytophthora capensis and P. emzansi were first described from South Africa (Bezuidenhout et al. 2010, Bose et al. 2021a). Phytophthora emzansi is exclusively known from South Africa where it was isolated independently in the Western Cape Province from plantations of the endemic shrub Agathosma betulina (Bezuidenhout et al. 2010); from Eerste River and the rhizosphere of the native tree Afrocarpus falcata in the afrotemperate Knysna Forest, and Podocarpus elongata in Kirstenbosch National Botanical Garden (Bose et al. 2021a); and in the province KwaZulu-Natal from soil samples in the Ingeli Forest (Oh et al. 2013). Its distinct position in the phylogenetic analyses with P. capensis as next - though distant - relative (Fig. 4) and its exclusively amphigynous antheria, unique within Clade 2c, indicate long-term isolated evolution of P. emzansi, further supporting an origin in South Africa. Phytophthora capensis was isolated from the cultivated endemic shrubs Olea capensis and Curtisia dentate in the Western Cape Province and was later found in soil samples and streams of two forests and a botanical garden in Eastern Cape Province and KwaZulu-Natal (Bezuidenhout et al. 2010, Oh et al. 2013). This distribution, long-term isolation indicated by a particularly long branch length in the multigene phylogeny (Fig. 4) and lack of any records from elsewhere suggest that P. capensis is native to South Africa.
In contrast, its sister species, P. pseudocapensis, has a wide distribution in natural montane forest streams in Java, Sumatra, Sulawesi (this study) and Vietnam (Jung et al. 2020, designated as P. capensis) and submontane streams in Taiwan (Jung et al. 2017c, designated as P. capensis) with high genetic variability within and between different populations (Fig. 4) suggesting Southeast Asia as the taxons’ centre of origin.
Phytophthora plurivora is almost exclusively known from the Northern Hemisphere where it has the widest distribution of all Clade 2c species commonly occurring in natural ecosystems, nurseries and planting sites across Europe and also reported from many natural ecosystems, nurseries and restoration sites in North America (Jung 2009, Jung & Burgess 2009, Nechwatal et al. 2011, Orlikowski et al. 2011, Reeser et al. 2011, Hansen et al. 2012, Milenković et al. 2012, Jung et al. 2013a, 2016, 2018a, 2019, Mrázková et al. 2013, Szabó et al. 2013, Bienapfl & Balci 2014, Jankowiak et al. 2014, Brazee et al. 2016, Parke et al. 2014, O’Hanlon et al. 2016, Rooney-Latham et al. 2019, Corcobado et al. 2020, Frankel et al. 2020, Matsiakh et al. 2020, Seddaiu & Linaldeddu 2020, Seddaiu et al. 2020, Rossmann et al. 2021, Mora-Sala et al. 2022). There are currently only four records from the Southern Hemisphere, from the Valdivia River in Chile (Jung et al. 2018b), from soil of an Austrocedrus chilensis forest in Patagonia, Argentina (Vélez et al. 2020), from Juglans regia in New Zealand (isolate WPC P10679 = ICMP 14152 = NZFS 2671; Table S1) and from the rhizosphere of a non-native Quercus robur tree in the Blue Mountains Botanic Garden in New South Wales, Australia (Laurence et al. 2023). From a microsatellite study of exclusively European and North American isolates, Schöbel et al. (2014) suggested a European origin for P. plurivora. However, in both Europe and North America P. plurivora clearly behaves as an introduced aggressive pathogen, with a particularly wide host range in native plant species causing severe root rot, bark cankers and mortality in nurseries, out-plantings and natural forests and acting as one of the main drivers of current oak and beech declines across Europe (Jung 2009, Jung & Burgess 2009, Nechwatal et al. 2011, Orlikowski et al. 2011, Milenković et al. 2012, Jung et al. 2013a, 2016, 2018a, 2019, Mrázková et al. 2013, Szabó et al. 2013, Bienapfl & Balci 2014, Jankowiak et al. 2014, Corcobado et al. 2020, Frankel et al. 2020, Linaldeddu et al. 2020, Seddaiu & Linaldeddu 2020, Seddaiu et al. 2020, Mora-Sala et al. 2022). Over the past decade P. plurivora has been recovered from soil around multiple hardwood tree genera in healthy, natural temperate forests in a remote region of western Nepal (Vettraino et al. 2011), and in Yunnan and Taiwan (Huai et al. 2013, Jung et al. 2017c). It has also been obtained, together with the introduced P. cactorum and eight other Phytophthora spp., from declining Malus sieversii forests in the temperate Tian Shan mountains in Xinjiang, Northwest China (Liu et al. 2018, Xu et al. 2019). Further, our recent surveys demonstrated an almost ubiquitous distribution of P. plurivora in native forests and streams across the Japanese main island of Honshu without any evident association with disease symptoms in the native vegetation. These recent observations form a strong argument for long-term coevolution between P. plurivora and the Asian flora, and hence an origin for P. plurivora in temperate Asia.
Phytophthora curvata, P. excentrica, P. falcata, P. japonensis, P. nimia, P. oblonga and P. vacuola are seven new endemic Clade 2c species found in the present study in the rhizosphere and streams of native healthy forests across three main islands of Japan, i.e., Honshu, Shikoku and Kyushu. While P. falcata occurred on all three islands, other species were found on two (P. japonensis, P. nimia) or only one island (P. curvata, P. excentrica, P. oblonga, P. vacuola). The comparatively small island of Shikoku (ca. 18 000 km2) showed the highest Clade 2c diversity, with six new species detected.
Located in a transitional zone between Southeast Asia and East Asia, Taiwan not only shares the occurrence of P. citricola and P. plurivora with Japan (Erwin & Ribeiro 1996, Jung & Burgess 2009, Jung et al. 2017c) but also the new Clade 2c species P. pseudocapensis and P. catenulata (previously P. citricola VII) with Vietnam (Jung et al. 2017c, 2020, this study).
Phytophthora fansipanensis and P. obturata are two new endemic Clade 2c species with apparently local vicariant distributions in Vietnam. Phytophthora fansipanensis was found in cloud forests on the Fansipan Mountain close to the border with Yunnan, China; P. obturata in a subtropical evergreen submontane forest in Ba Vì National Park near Hanoi and a montane Chamaecyparis forest on Sau Chua Mountain near Sapa (Jung et al. 2020, this study).
Phytophthora macroglobulosa is currently known only from a tropical montane forest on Hainan Island (under its previous designation as P. citricola; Zeng et al. 2009) which is also located on the Asian continental shelf and, hence, was connected to Vietnam during the Pleistocene where its sister species P. obturata thrives in ca. 400 km distance in Ba Vì National Park.
Another five Clade 2c species have been recorded in Europe in addition to the introduced P. multivora and P. plurivora. Phytophthora acerina was first described as causing bark cankers, dieback and mortality on native Acer pseudoplatanus trees in a managed forest in Northwestern Italy (Ginetti et al. 2014). The pathogen has subsequently been found associated with declining riparian Alnus glutinosa trees in Sardinia (Seddaiu & Linaldeddu 2020), with root rot and sudden death of olive trees in Northeastern Italy (Linaldeddu et al. 2020) and with declining Quercus suber trees in a botanical garden in Northern Italy (T. Jung & M. Horta Jung unpubl. results). Due to its high aggressiveness to several major European tree species and its sudden appearance in different regions of Italy P. acerina is most likely a recent introduction to Europe. ITSbased records from a stream in Tennessee in 2010 (designated as P. citricola; GenBank accession no. MF959536.1) and in 2015 from rhizosphere soil of Populus fremontii in California (GenBank accession no. MG707815.1) indicate a scattered distribution in the USA. In New South Wales, Australia, P. acerina was isolated in 2017 from a non-native horticultural Prunus dulcis tree (isolate VPRI 44117; Burgess et al. 2021). The oldest known record of P. acerina from 2003 also came from a non-native horticultural Prunus persica tree in Taiwan (GenBank ITS accession no. GU111596.1; submitted as P. citricola), indicating pathogen spread via the international nursery trade. Since P. acerina was recently reported to cause root and collar rot, decline and mortality of native Metasequoia glyptostroboides trees in Central China (Liu et al. 2022) an origin somewhere in East Asia seems likely.
Phytophthora balkanensis, previously informally designated P. citricola E (Jung & Burgess 2009) and as isozyme subgroup and electrophoretic type CIT2-4 (Oudemans et al. 1994) was first isolated in 1986 from horticultural Rubus idaeus plants in Ireland (Jung & Burgess 2009) and later from nursery stock in Italy (Prigigallo et al. 2015) and Croatia (this study). It was also recovered in the present study from soil and streams of beech, oak and alder forests in Bosnia-Herzegovina and Serbia. Outside Europe there are only two known records of P. balkanensis, from R. idaeus and irrigation water in California (isolates WPC P1321 = ATCC 64809, P1803, P1804) and from Fragaria sp. in Taiwan (isolates WPC P6624 = ATCC 66621, P6625, P6627) (Oudemans et al. 1994, Bezuidenhout et al. 2010, Brazee et al. 2017). With a close phylogenetic relationship P. acerina and P. balkanensis have probably evolved in the same biogeographic region, most likely in East Asia along with their relatives P. plurivora and P. curvata (Fig. 4).
Phytophthora pachypleura and P. platani, are currently known exclusively from Europe. Phytophthora pachypleura is plausibly a recently introduced pathogen spreading within the European nursery trade on its only known host Aucuba japonica (Henricot et al. 2014, Ginetti et al. 2015). In our phylogenetic analyses, it formed together with the Japanese P. falcata one of three closely related East and Southeast Asian clusters and, hence, is certainly originating in this region.
Phytophthora platani was first isolated in 2013 from the rhizosphere of healthy Platanus orientalis trees in two natural riparian forests in Sicily, Italy. More recently it was found causing root rot and aerial bark cankers of a mature Platanus × acerifolia tree in London, UK, to which it was also pathogenic on inoculation (A. Pérez-Sierra and T. Jung, unpublished results). Whether P. platani is specific to Platanus trees remains, however, unknown. Its close phylogenetic relationship to a cluster of three species with probable North American origin, i.e., P. caryae, P. limosa and P. pini, indicates that P. platani may be also native to North America which, coincidentally, harbours seven endemic Platanus species.
Phytophthora pini, first described by Leonian (1925) from roots of Pinus resinosa in Minnesota, is widely distributed in nurseries, plantings, streams, forests and agricultural run-off areas across the USA (Ghimire et al. 2011, Hong et al. 2011, Reeser et al. 2011, Bienapfl & Balci 2014, Knaus et al. 2015, Sims et al. 2015b, Brazee et al. 2016, 2017, Yang & Hong 2016, Rooney-Latham et al. 2019). It is also being spread widely on ornamental Rhododendron and conifer nursery stock in the USA (Hong et al. 2011, Knaus et al. 2015) and Europe (Lilja et al. 2011, Jung et al. 2016). Like P. balkanensis, P. pini also has a scattered distribution in nursery outplantings in Europe (Lilja et al. 2011, Jung et al. 2016, this study) and in semi-natural ecosystems in the Balkans where it has been found in forest streams and the rhizosphere of riparian poplar plantations (Milenković et al. 2018) and a Fraxinus angustifolia tree (this study) in Serbia; and in forest streams and the rhizosphere of riparian Alnus glutinosa trees in Bosnia-Herzegovina (this study). Recently it was recorded from Rhododendron pulchrum plants in Nanjing, China (Xu et al. 2021). Since its sister species P. limosa (including the informally designated P. taxon citricola III and P. citricola taxon 22F3; Hong et al. 2011, Brazee et al. 2017, Yang et al. 2017) has so far been found mainly in forests and waterbodies in the USA it seems likely that both species are native to North America. Indeed, the high aggressiveness of P. pini and P. limosa (as P. citricola III) to the common European beech tree, Fagus sylvatica, planted as an ornamental in North American parks and in pathogenicity tests (Jung et al. 2005, Weiland et al. 2010, Kenaley et al. 2014), is a strong argument against a European origin of both these pathogens.
Phytophthora caryae was first described from streams in the eastern USA (Brazee et al. 2017). Its basal position to P. limosa and P. pini in the multigene phylogeny of this study (Fig. 4) supports a North American origin of P. caryae. The only other reports of P. caryae to date are metagenomic records from two forests of Nothofagus pumilio and A. chilensis in Patagonia (Vélez et al. 2020).
Clade 2d
The only known member of this subclade, P. oleae, is exclusively known from one olive tree plantation in southern Italy where it appeared suddenly in 2015 causing excessive fruit rot (Ruano-Rosa et al. 2018). This strongly suggests a recent introduction. The origin of P. oleae and, hence, of Clade 2d remains unknown.
Clade 2e taxa
Clade 2e now comprises the five previously known species P. acaciae, P. acaciivora, P. bishii, P. elongata and P. frigida, six new species, P. amamensis, P. borneensis, P. celeris, P. indonesiensis, P. penetrans and P. pseudofrigida, and the informally designated P. taxon pseudobisheria, P. taxon AUS 2E and P. taxon Costa Rica 8.
Phytophthora acaciae, P. acaciivora and P. frigida were all first described from plantations of exotic Acacia and eucalypt species suggesting their spread via the international nursery trade: P. acaciae in Brazil recorded from Acacia mearnsii native to southeastern Australia (Albuquerque Alves et al. 2019); P. acaciivora in Vietnam from A. mangium native to Maluku, New Guinea and northeastern Queensland (Burgess et al. 2020); and P. frigida in Brazil from A. mearnsii (Albuquerque Alves et al. 2016; Dos Santos 2016) and in South Africa both from A. decurrens, native to eastern New South Wales and Eucalyptus smithii native to southeastern Australia (Maseko et al. 2007).
However, while P. acaciae may have been introduced to Brazil on A. mearnsii, there are currently no records of it from elsewhere, and the co-occurrence of both mating types in Brazil indicates that P. acaciae might be indigenous to the area, naturally associated with native neotropical plant species e.g., from the Acacieae genera Acaciella or Mariosousa. The discovery of the new Clade 2e species P. penetrans in a healthy tropical lowland rainforest in Panama and the occurrence of its closest relative, the undescribed P. taxon Costa Rica 8, in a rainforest in Costa Rica (ITS GenBank accession no. KC479193), increase the possibility of a neotropical origin for P. acaciae.
Phytophthora frigida has also been recovered from a stream in Sicily, Italy (Jung et al. 2019) where it was certainly introduced. Burgess et al. (2017, 2021) considered P. frigida native to Australia based on metagenomic records from soil samples in New South Wales and Western Australia (Burgess et al. 2017) and on isolations from native forest soils in Queensland and New South Wales (Scarlett et al. 2015, Burgess et al. 2021). However, isolate W1731 from New South Wales resided in the multigene phylogeny of this study within P. pseudofrigida casting doubt on the presence of P. frigida in Eastern Australia. Therefore, it seems more likely that P. frigida is native to South Africa which is also supported by its wide distribution in both commercial and native forests, the presence of both mating types and the aggressiveness to the non-native trees A. decurrens and E. smithii (Maseko et al. 2007, Bose et al. 2018, 2021b).
The recoveries in the present study of P. pseudofrigida from four natural streams running through a tropical hill rainforest in Western Sumatra, a montane rainforest in Northern Sumatra and two montane rainforests in Western Java suggest an origin of this new species in Sundaland. This is further supported by the co-occurrence of another new Clade 2e species, P. indonesiensis, in the rhizosphere of the same hill rainforest in Western Sumatra where P. pseudofrigida was isolated from a forest stream. Also, by our detection of the close relative of P. pseudofrigida, P. acaciivora, and another new Clade 2e species, P. celeris, in the effluents of an A. crassicarpa and eucalypt nursery in Central Sumatra established on a site previously inhabited by a natural lowland rainforest. However, the possibility of an Eastern Australian origin of P. pseudofrigida cannot be excluded. Since all isolates from Indonesia and the isolate from New South Wales belonged to the A1 mating type further surveys are needed to find the A2 mating type and clarify the centre of origin. Phytophthora indonesiensis also occurred in the rhizosphere of a montane tropical rainforest in Sulawesi and a stream running through a lowland rainforest in Northeast Kalimantan. In our multigene phylogeny, the populations from the three Indonesian islands grouped separately with the Kalimantan isolates being basal to the isolates from Sumatra and Sulawesi indicating local evolution and adaptation. This is supported by the considerably lower optimum growth temperature of the montane Sulawesi isolates (Fig. 62). Another new Clade 2e species from Indonesia, P. borneensis, was found only in the rhizosphere of a lowland rainforest in East Kalimantan.
Phytophthora bishii thrives on species of Rosaceae in the global plant trade: on Fragaria × ananassa in the USA; Rosa sp. in the Netherlands; Rubus idaeus in Australia (Abad et al. 2008) and Spain (ITS GenBank accession nos. MN134587.1 and MN549026.1). The findings of P. bishii on the Southeast Asian Zingiberaceae species Etlingera elatior planted in Brazil (ITS GenBank accession no. JF917303.1) and in the rhizosphere of a remote, montane natural Chamaecyparis obtusa forest in Taiwan (Brasier et al. 2010) and its close phylogenetic relationship to P. borneensis (Fig. 4) suggest a Southeast or East Asian origin. The informally designated P. taxon pseudobisheria is only known from ornamental plants of Rhododendron and the North American Rhus aromatica in North Carolina and Pennsylvania, respectively (Yang et al. 2017; Table S1), and its origin remains unknown although its close relationship to P. celeris (Fig. 4) suggests it might be native to Southeast Asia, too.
The new species P. amamensis, sister species to P. indonesiensis (Fig. 4), has to date been found only in a subtropical monsoon forest on Amami Island in the Ryukyu archipelago, Japan (this study), which suggests that this species may have an East Asian origin.
Phytophthora penetrans is currently only known from a natural, healthy lowland rainforest in Panama suggesting it might be native there and possibly elsewhere in Central America. This is supported by the occurrence of another Clade 2e taxon, the informally designated P. taxon Costa Rica 8 (ITS GenBank accession no. KC479193), in a tropical rainforest of the neighboring country Costa Rica.
Phytophthora elongata was first described in Western Australia where it was introduced through mine site rehabilitation plantings. It subsequently spread as an aggressive invasive pathogen on a range of native woody plants and along riparian corridors (Rea et al. 2010, Jung et al. 2018a). Based on metabarcoding and isolation records from soils of healthy natural ecosystems in New South Wales, Victoria and Tasmania (Dunstan et al. 2016, Burgess et al. 2017, 2021), an origin of P. elongata in Southeastern Australia has been proposed (Burgess et al. 2017, 2021). There are only two records of P. elongata outside of Australia, as an introduced pathogen on ornamental Buxus, Ilex and Rhododendron in nurseries in Maryland, USA (Bienapfl & Balci 2014) and from natural mangrove leaf litter in a coastal area of the Philippines (Bennett et al. 2017). Further surveys of ecosystems across Southeast Asia and Southeastern Australia are needed to clarify its origin. A close relative of P. elongata, the undescribed P. taxon AUS 2E (previously P. taxon elongata-like; Rea et al. 2010), has also been found widespread in Western Australia where it might be endemic (Burgess et al. 2021).
Clade 2f taxa
Currently, Clade 2f comprises P. multivesiculata, the informally designated P. taxon aquatilis and P. taxon Costa Rica 5, and the three new species P. angustata, P. furcata and P. sumatera.
Phytophthora multivesiculata was first described as causing necroses on stem bases and leaves of Cymbidium orchids in the Netherlands (Ilieva et al. 1998), and was subsequently isolated from Cymbidium plants in New Zealand (Hill 2004), Vietnam (ITS GenBank accession no. DQ835678.1, submitted in 2006; Chern et al. 2011), New South Wales (Cunnington et al. 2009), Taiwan (Chern et al. 2011) and Hawaii (ITS GenBank accession no. GU258925.1; isolate WPC-P7254), and from Cymbidium species and hybrids and the indigenous orchid Ansellia africana in South Africa (Bose & Hammerbacher 2023). This is consistent with its being spread very effectively via the international orchid trade. Its hosts and lack of sporangial caducity suggest a co-evolution with the terrestrial (rather than the epiphytic) Cymbidium species native to subtropical or tropical montane forests in South and Southeast Asia.
An Asian origin for P. multivesiculata is further supported by the natural distributions of its phylogenetic relatives P. furcata, exclusively found in a stream and rhizosphere soil of a cloud forest at the Fansipan Mountain in the North of Vietnam; and P. sumatera which occurs commonly in streams and rhizosphere soil of montane and lowland rainforests in Northern and Western Sumatra and Java.
Phytophthora angustata was only found in a tropical lower montane rainforest at the Mombacho Volcano in Nicaragua, where it is most probably endemic. Interestingly, the undescribed P. taxon Costa Rica 5, for which only an ITS sequence is available (ITS GenBank accession KC479200), was detected in a lowland rainforest of the neighboring country Costa Rica.
Phytophthora taxon aquatilis is currently known only from a single isolate obtained from a stream in Virginia, USA. Its origin remains unknown (Hong et al. 2012). However, since the three Clade 2f species with a probable origin in Asia, P. furcata, P. multivesiculata and P. sumatera, produce almost exclusively amphigynous antheridia whereas the antheridia in P. angustata and P. taxon aquatilis are almost exclusively paragynous (Hong et al. 2012), an origin for P. taxon aquatilis in Central or North America seems more likely.
Clade 2g taxa
Comprising four new species from Southeast and East Asia the new Clade 2g is currently the second-smallest of the subclades. Phytophthora proliferata and P. inclinata thrive in the rhizosphere of healthy tropical lowland rainforests in the Vietnamese Cuc Phuong National Park and on Côn Lôn island situated 50 km off the Vietnamese coast on the Asian continental shelf, respectively (Jung et al. 2020, this study).
Phytophthora multipapillata has only been recovered from two healthy tropical lowland rainforest sites in East Kalimantan. In the multigene phylogenetic analyses, the two populations grouped separately suggesting potential local evolution and adaptation. Despite extensive surveys, P. multipapillata could not be obtained from the adjacent islands of Java, Sumatra and Sulawesi and could, hence, be endemic to the island of Borneo. Noteworthy, its closest relative is P. inclinata from Côn Lôn Island which was connected to Borneo via a land bridge during the Pleistocene.
Phytophthora transposita was found in the rhizosphere of healthy Zanthoxylum ailanthoides trees in a warm-temperate natural forest on the Japanese Kyushu Island where it is most likely endemic.
Summary: natural biogeography of Clade 2 and its subclades
Based mainly on the indirect evidence of endemism of the taxa presented above the main natural distribution of the subclades is summarized in Fig. 78. Terminology follows the recent update of Wallace’ s biogeographic regions by Holt et al. (2013).
Clade 2a is native to the Oriental (= Indo-Malayan), Sino-Japanese and Oceanian biogeographic regions ranging from Nepal and India in the West to Japan and Papua New Guinea in the East and Christmas Island in the South. Multiple diversity hotspots occur in the Himalayas, Indochina, Sundaland, Taiwan and Japan (Figs 78, 79). The aerial Clade 2a species P. ×australasiatica and P. ×vanyenensis range from Sundaland across the Strait of Makassar to Sulawesi, which is the westernmost island of the transitional biogeographic region of Wallacea (Richardson et al. 2012).
Clade 2b is exclusively native to the Americas ranging from boreal Alaska in the North along the temperate and subtropical western regions of the Nearctic via a diversity hotspot of ten species in the transitional tropical Panamanian biogeographic region (= WWF Central America bioregion) and the tropical and temperate regions of eastern South America to the cool-temperate Valdivian and Aysen regions of Chile in the Southwest of the Neotropic realm (Figs 78, 79).
Clade 2c has a disjunct natural distribution in five biogeographic regions. Its main distribution stretches across the Oriental and Sino-Japanese regions from Nepal in the West via Indochina and Sundaland to Taiwan and Japan in the East, with a hotspot of nine species in the Japanese archipelago and another of five species in Southeast Asia and one species, P. pseudocapensis, occurring also in Sulawesi. The presence of P. plurivora in the Tien Shan mountains of Xinjiang, China is the only known natural distribution of a Clade 2c species in the Palearctic region. An isolated natural Clade 2c centre, comprising P. capensis, P. emzansi and P. multivora, lies in South Africa at the southernmost range of the Afrotropical biogeographic region. Another four Clade 2c species are probably endemic to the Midwest, Atlantic and southern regions of the USA in the eastern part of the Nearctic realm (Figs 78, 79).
The origin of the monospecific Clade 2d is unknown.
The main concentration of Clade 2e, including eight of the 11 known and new species plus Phytophthora taxon AUS 2E, stretches along an arc from Tasmania, Victoria, New South Wales and Queensland in the Southeast and East of the Australasian region via Sulawesi in Wallacea, Java, through Sumatra and Kalimantan in the South of the Oriental region to Taiwan and Amami Island in the Southeast of the Sino-Japanese region (Figs 78, 79). A smaller Clade 2e hotspot, currently comprising P. penetrans and the undescribed P. taxon Costa Rica 8, lies in the Panamanian biogeographic region. Phytophthora acaciae appears to be endemic to the southeastern Neotropical region while P. frigida most likely originates from South Africa (Figs 78, 79).
With P. furcata in Vietnam, P. sumatera in Java and Sumatra and a supposed origin of P. multivesiculata somewhere in Southeast Asia, Clade 2f has one centre in the Oriental biogeographic region while a second centre, comprising P. angustata and the undescribed P. taxon Costa Rica 5, lays in the Panamanian region (Figs 78, 79).
Clade 2g has a centre of diversity in the Oriental biogeographic region where three of the four known species occur naturally along a transect from northern Vietnam to Borneo, whereas P. transposita is native to Kyushu in the East of the Sino-Japanese region (Figs 78, 79).
Notes on the evolutionary history of Clade 2
Both multigene molecular phylogenetic analyses (Figs 1, S1) indicate an early divergence of Clades 2e and 2f followed by the subsequent divergences of Clades 2d, 2c and 2b, and most recently the splitting of Clades 2a and 2g. However, due to inconsistent results between the BI and ML analyses, it remains unresolved whether Clade 2e or 2f diverged first. The disjunct natural distributions of Clade 2c, Clade 2e and Clade 2f on separate continents (Figs 78, 79) can only be explained if these subclades evolved before the break-up of the ancient supercontinent Pangea and/or by intercontinental migrations.
In this regard, East Asia and North America were connected via the Beringia land bridges intermittently from the late Cretaceous until the early Pliocene ca. 70–5.3 Mya (Gladenkov et al. 2002, Brikiatis 2016) and temporarily during the glacial periods of the Pleistocene (Vila et al. 2011, Elias & Brigham-Grette 2013). The timing and duration of North Atlantic land bridges is still under debate. While they were long believed to have existed more or less continuously until the early Oligocene ca. 30 Mya (McKenna 1983, Tiffney 1985, Davis et al. 2002), recent evidence indicates that North America, Greenland and Europe were mainly connected via the De Geer and Thulean routes which existed intermittently between 68 and 56 Mya (Brikiatis 2016). The Isthmus of Panama closed completely at ca. 3.5 Mya, fusing the Laurasian North American and the Gondwanan South American Plates, but a volcanic arc in the Panamanian region was already beginning to block the Central American Seaway ca. 12–6 Mya (Woodburne 2010, Bacon et al. 2015, Bogarín et al. 2016). Africa and Asia have been almost continuously connected from 19 Mya via the Gomphotherium landbridge and later the Isthmus of Suez (Harzhauser et al. 2007). Intercontinental migrations and interchange account for the relatedness of the East Asian and North American floras (Qian 2002, Lang et al. 2007, Baskin & Baskin 2016, Wen 1999, Wen et al. 2010, 2016) and for the circum-Pacific or Holarctic distributions of many animal (Morley 2003, Sharma & Giribet 2012, Van Damme & Sinev 2013, Toussaint et al. 2017, Kim et al. 2018) and plant families and genera, including the Fagaceous genera Castanea, Fagus and Quercus (Denk & Grimm 2009, Hubert et al. 2014).
While a potential for intercontinental Phytophthora migration is demonstrated by the distribution of P. uniformis from the otherwise exclusively Eurasian Clade 7a in Alaska and the Pacific Northwest (Adams et al. 2010, Aguayo et al. 2013, Jung et al. 2017a, b), the fact that there are no intermediate native records for Clades 2e and 2f between their centres in East or Southeast Asia and those in Central America and South Africa (only Clade 2e) argues against their intercontinental migration. In addition, New Guinea, the Moluccas and Sulawesi have never been connected by land bridges, while Western Sulawesi and Sundaland have been separated since ca. 45 Mya when rifts opened the Strait of Macassar (MacKinnon et al. 1997, Whitten et al. 2002, Hall 2017, Michaux 2010, Lohmann et al. 2011, Gower et al. 2012), thus preventing potential migration between the extant Clade 2e areas in eastern Australia and those in Asia.
Regarding Clade 2c, during the warmer climates of the late Cretaceous and Paleogene (ca. 70–23 Mya) the boreotropical vegetation and the Beringia land bridge may have allowed intermittent migration and interchange between the extant native centres in East Asia and North America. However, the Clade 2c subcentre in South Africa is separated from the other Clade 2c areas in Asia and North America by vast distances including semi-arid or arid environments and oceans which would have been major obstacles to migration.
Overall, it seems most likely that Clades 2c, 2e and 2f separated before Pangea split into Gondwana and Laurasia (ca. 200 Mya). The disjunct distributions of Clades 2c, 2e and 2f and the often vicariant distributions of species within the subclade centres are most probably a product of fundamentally allopatric, non-adaptive radiation processes due to migration across geographic barriers, the eruption of new geographic barriers (e.g. mountain uplift and the separation of islands) and gradual macroclimatic changes, leading to geographic isolation and accumulating genetic drift (Brasier 1986, Kozak et al. 2006, Rundell & Price 2009). Suggested examples of fundamentally allopatric speciation are, within Clade 2f, the distributions of P. angustata in Nicaragua, P. furcata in northern Vietnam and P. sumatera in Sundaland; within Clade 2e the distributions of P. acaciae in Brazil, P. penetrans in Panama, P. amamensis on Amami Island, P. elongata in southeastern Australia, P. borneensis in East Kalimantan and P. celeris in Central Sumatra; within Clade 2c the centres in North America, South Africa, Southeast and East Asia, and within Southeast Asia the vicariant distributions of P. fansipanensis on the Fansipan Mountain, P. obturata in Ba Vì National Park and on Sau Chua Mountain, and P. macroglobulosa on Hainan Island; and within Clade 2g, similar to Clade 2c, the centre in Southeast Asia and the occurrence of P. transposita in Japan, and within Southeast Asia the vicariant distributions of P. proliferata in Cuc Phuong National Park, P. inclinata on Côn Lôn Island and P. multipapillata in East Kalimantan.
Within the Japanese archipelago, the isolated occurrence of P. vacuola in montane Quercus and Alnus forests in Central Honshu might also reflect allopatric speciation. The co-occurrence of multiple Clade 2c species within other Japanese ecosystems, however, is more likely to result from sympatric events i.e., from local adaptive radiation associated with different host plants, microclimates, soil types and microbiomes (Mayr 1942, Brasier 1986, Givnish 1997, 2015, Rundell & Price 2009, Jung et al. 2017b). Examples include P. curvata, P. excentrica, P. falcata and P. japonensis in a mixed Quercus-Abies-Torreya-Tsuga forest in Ichinomata on Shikoku Island; P. japonensis, P. nimia and P. oblonga in a warm-temperate forest in Satayama on Shikoku Island; and P. falcata, P. japonensis and P. nimia in a warm-temperate forest in Takakuma on Kyushu Island.
The more recently diverged Clades 2a, 2b and 2g, in contrast to Clades 2c, 2e and 2f, are exclusively native to Asia (2a, 2g) or the Americas (2b), indicating that Clade 2b and the common ancestor of Clades 2a and 2g most likely diverged after the splitting of Pangea into Gondwana and Laurasia ca. 175 Mya. The absence of native Clade 2a and 2g species in the Americas and the fact that all known and new species from these subclades most likely originate in subtropical or tropical regions between India and Japan make a migration from the Americas via Beringia highly unlikely, further supporting the hypothesis that Clades 2a and 2g evolved from their common ancestor in South, Southeast or East Asia.
Clade 2b, on the other hand, most probably evolved in South America since the Valdivian rainforests and Nothofagus forests in South Chile, where the P. aysenensis - P. distorta - P. valdiviana complex originates, became isolated by the development of arid and semi-arid environments as a consequence of the Andean uplift between 23 and 12 Mya (Antonelli & Sanmartín 2011, Tecklin et al. 2011, Turchetto-Zolet et al. 2013), i.e., long before the complete closure of the Isthmus of Panama ca. 3.5 Mya enabled the migration of soilborne organisms between North and South America. During its subsequent spread to Central and North America as part of the Great American Biotic Interchange (Rull 2011, Bacon et al. 2015) Clade 2b probably underwent many allopatric and adaptive radiation events, discussed below. Equally, the possibility of some prior exchange of airborne Phytophthora species along the volcanic arc that formed in the Panamanian region ca. 12–6 Mya (cf. Woodburne 2010, Bacon et al. 2015, Bogarín et al. 2016) cannot be ruled out.
Since none of the 22 taxa now known from Clade 2b is native to Africa the subclade most probably evolved in South America after its splitting from Africa ca. 140 Mya. However, the possibility that unknown native Clade 2b species exist somewhere in the vast unsurveyed regions between South Africa and the Mediterranean regions of North Africa cannot be ruled out.
Notes on the drivers of radiation, diversity and endemism within Clade 2
In the Pleistocene recurrent lowering of global sea levels during glaciation periods created temporary land bridges connecting the islands of the Sundaland and Con Dao archipelagos in Southeast Asia and the Japanese archipelago in East Asia with each other and, together with other islands on the Asian continental shelf such as Hainan and Taiwan, with the Asian mainland. These periods resulted in gateways for migration and interchange, whereas the intervening warm-humid interglacial periods resulted in isolation and increased hybridisation, speciation and radiation events (Chang-Fu et al. 1994, Chung-Fu 1994, Gower et al. 2012). Further, on the Asian mainland and adjacent islands, the climate during the glacial periods was cooler and considerably drier than today. This lowered altitudinal zones and forced thermophilic species to retreat into lowland refugia isolated by mountain ranges; and montane and hydrophilic species into “mountain island” refugia surrounded by drier savannahs or grasslands, resulting in disjunct post-Pleistocene populations and radiations (Heaney 1991, Laumonier 1997, MacKinnon et al. 1997, Whitten et al. 1997, 2002, Hope 2001, Hope et al. 2004, Cannon et al. 2009, Tian et al. 2010, Wurster et al. 2010, Ye et al. 2016).
These complex processes are considered mainly responsible for the species richness and the high degree of endemism of the flora and fauna of these regions (Chang-Fu et al. 1994, Chung-Fu 1994, Laumonier 1997, MacKinnon et al. 1997, Whitten et al. 1997, 2002, Axelrod et al. 1998, Averyanov et al. 2003, Roos et al. 2004, Van Welzen & Slik 2009, Lohman et al. 2011, Van Welzen et al. 2011, Richardson et al. 2012, Li et al. 2013), including the disjunct distribution and high phenotypic diversity of the eight known Asian lineages of P. ramorum from Clade 8c (Jung et al. 2021). Accordingly, in East and Southeast Asia, the populations of Phytophthora in general (Zeng et al. 2009, Jung et al. 2017a, c, 2020, 2022) and of Clade 2 are very diverse, exhibiting a high degree of endemism. The Japanese archipelago harbours at least 13 native species from Clades 2a, 2c, 2e and 2g of which nine (69.2 %) are most probably endemic (P. amamensis, P. curvata, P. excentrica, P. falcata, P. japonensis, P. nimia, P. oblonga, P. transposita, P. vacuola). In Taiwan two of the eight (25 %) native species from Clades 2a, 2c and 2e are probably endemic (P. pseudoccultans, P. ×taiwanensis). Sundaland is within the origin of 11 species from Clades 2a, 2c, 2e, 2f and 2g of which five (45.5 %) are most likely endemic (P. borneensis, P. celeris, P. indonesiensis, P. multipapillata, P. sumatera). Currently the second highest recorded degree of probable endemism regarding Clade 2 in Asia is in Vietnam, with eight of the 14 (57.1 %) native species from Clades 2a, 2c, 2e, 2f and 2g (P. fansipanensis, P. furcata, P. inclinata, P. mekongensis, P. multibullata, P. obturata, P. proliferata, P. vietnamensis).
In Central America 10 of the 14 (71.4 %) native taxa from Clades 2b, 2e and 2f are probable endemics (P. angustata, P. calidophila, P. frigidophila, P. montana, P. multiplex, P. penetrans, P. pyriformis, P. variepedicellata, P. taxon Costa Rica 5 and 8). This is consistent with the role of allopatric speciation and adaptive radiation events during the range expansion of Clade 2b to Central America hypothesised above. Further, as in Southeast and East Asia Central America experienced glacial-interglacial alternation during the Pleistocene with recurrent mean cooling of 5–9 °C and rainfall reductions of 30–50 %, resulting in lowering of altitudinal zones, retraction of rainforest species into isolated refugia surrounded by drier forest and savannah vegetation and, at transitional altitudes, mingling of thermophilic lowland and cooler montane species (Bush & Colinvaux 1990, Piperno 2006, Bush et al. 2009, Woodburne 2010).
Today tropical cloud forests form an archipelago of “mountain islands” across Central America with extremely high levels of local endemism (Myers 1969, Luna Vega et al. 1999, Rovito et al. 2015). Accordingly, five of the ten putatively endemic Clade 2 species in the area are restricted to individual tropical cloud forests (P. angustata, P. calidophila, P. frigidophila, P. montana, P. variepedicellata). Indeed, the co-occurrence of P. frigidophila, P. montana and P. variepedicellata in the remote cloud forest near the peak of Volcano Baru in Panama may constitute a textbook example of highly localised sympatric radiation driven by adaptation to different host plants or plant tissues. The highest degree of endemism in Clade 2, however, is found in the South of Chile where all three native Clade 2b species recorded, P. aysenensis, P. distorta and P. valdiviana, are probable endemics. This is most likely a consequence of the millions of years of isolation of the perhumid Gondwanan Valdivian rainforests and Southern beech forests by the Pacific Ocean to the west and semi-arid environments to the north and east (Hueck 1966, Armesto et al. 1995, Seibert 1996, Hinojosa et al. 2006, Antonelli & Sanmartín 2011, Tecklin et al. 2011, Turchetto-Zolet et al. 2013). It is notable that in Clade 10 the four species from the P. kernoviae complex, P. chilensis, P. kernoviae, P. pseudochilensis and P. pseudokernoviae, are also considered endemic to the Valdivian rainforests (Jung et al. 2022).
Notes on the evolution of lifestyle and adaptive traits within Clade 2
In the early-diverged subclade 2f, all five known taxa are self-fertile (‘homothallic’), produce persistent sporangia lacking pedicels (except for P. taxon aquatilis) with nonpapillate or less frequently shallow semipapillate apices and internal nested and extended proliferation (except for P. taxon aquatilis) (Ilieva et al. 1998, Hong et al. 2012, this study). These traits probably reflect a more primitive or archaic soil- and waterborne lifestyle focussed on continuous spread and infection with a high dependence on zoospore inoculum and survival in unfavourable environmental conditions via regularly produced oospores; together with potentially constrained genetic variability due to a high frequency of inbreeding, a strategy probably suited to a more uniform environment (cf. Brasier 1986, Brasier et al. 2003, Jung et al. 2011). With optimum growth temperatures of 25 °C and maximum temperatures below 30 °C, P. angustata, P. furcata and P. sumatera are probably adapted to the rhizosphere and streams of montane tropical cloud and rainforests. Having a lower temperature optimum of 20 °C and higher maximum temperatures of 30 and 30–35 °C, P. multivesiculata and P. taxon aquatilis may be adapted to a temperate or upper montane tropical climate (Ilieva et al. 1998, Hong et al. 2012).
Since all Clade 2f species and the majority of Clade 2e species share persistent, nonpapillate to shallow semipapillate sporangia, self-fertility and soil- or waterborne lifestyle it can be assumed that they inherited this more primitive evolutionary state from the last common ancestor of all extant Clade 2 species.
In the other early-diverged subclade, 2e, sporangial traits, the breeding system and cardinal temperatures show considerably higher variability than in Clade 2f. A majority of eight Clade 2e species are intrinsically self-fertile (‘homothallic’) (Tables S14, S15). However, each species in the cluster of P. acaciae, P. acaciivora, P. frigida and P. pseudofrigida has an A1/A2 and therefore possibly more outcrossing-orientated breeding system that may enhance genetic variability and consequently greater potential for adaptation to changing host populations or environmental conditions (cf. Brasier 1986). Phytophthora acaciae, P. frigida and P. pseudofrigida also produce asexual chlamydospores, which will further ensure their survival in unfavourable conditions. Chlamydospores are not produced by any of the self-fertile species from Clade 2e, nor any other subclade. Apart from P. bishii (Abad et al. 2008), ten of the 11 species with available data produce pedicellate sporangia. While eight of the pedicellate species lack sporangial caducity the sporangia of P. frigida are reported to be caducous (Maseko et al. 2007). Phytophthora indonesiensis produces a small proportion (<1 %) of caducous sporangia, potentially enabling, in addition to a predominantly soilborne lifestyle, infection of some aerial plant tissues in its tropical rainforest habitats. Sporangial apices in Clade 2e range from exclusively papillate (P. acaciae, P. acaciivora, P. frigida, P. pseudofrigida) and exclusively semipapillate (P. bishii, P. elongata, P. penetrans, P. taxon AUS 2E) to predominantly semipapillate (P. amamensis, P. celeris, P. indonesiensis) but with a small proportion of papillate (P. amamensis, P. celeris, P. indonesiensis) and nonpapillate sporangia (P. amamensis, P. celeris) (Abad et al. 2008, Rea et al. 2010, Albuquerque Alves et al. 2019, Burgess et al. 2020, this study). Only P. borneensis produces almost equal proportions of nonpapillate and semipapillate sporangia.
With maximum temperatures for growth between 32.5 and 35 °C P. acaciae, P. acaciivora, P. borneensis, P. celeris and P. pseudofrigida appear adapted to their natural tropical lowland rainforest habitats (Albuquerque Alves et al. 2019, Burgess et al. 2020, this study). Phytophthora indonesiensis isolates from montane rainforests in Sulawesi have significantly lower optimum temperatures than those from lowland rainforests in Borneo and Sumatra (20 vs. 25 °C), suggesting local adaptation. With optimum and maximum temperatures of 20 and <30 °C respectively the soilborne P. elongata is probably adapted to the temperate climate in southeastern Australia.
The next diverged monospecific lineage of P. oleae (Clade 2d) is also characterised by self-fertility and persistent semipapillate sporangia consistent with a soilborne lifestyle in a uniform stable environment.
In the fourth diverged subclade, 2c, sporangial apices are highly variable. Twenty-one of the 24 known and new species have either exclusively semipapillate sporangia or predominantly semipapillate sporangia with a proportion of papillate sporangia (P. emzansi, P. excentrica, P. falcata, P. limosa, P. pini, P. platani) and/or nonpapillate sporangia (P. balkanensis, P. catenulata, P. emzansi, P. excentrica, P. limosa, P. oblonga, P. pini) (Bezuidenhout et al. 2010, Bose et al. 2021a, this study). Phytophthora capensis is reported to have exclusively papillate sporangia (Bezuidenhout et al. 2010) while P. vacuola has predominantly papillate sporangia. Phytophthora curvata and P. pseudocapensis are unique within Clade 2c showing almost equal proportions of papillate and semipapillate sporangia.
Sixteen of the 17 Clade 2c species examined for pedicels to date have varying proportions of pedicellate sporangia, ranging from 6–11 % in P. vacuola and P. japonensis to 49–52 % in P. falcata, P. nimia and P. obturata. Phytophthora curvata is the only species not producing pedicels. While 18 species are probably exclusively soil- and waterborne, the other six species, i.e., P. catenulata, P. falcata, P. nimia, P. oblonga, P. obturata and P. platani, produce up to 1 % caducous sporangia in water, possibly an adaptation to a partially aerial lifestyle. Indeed P. platani has been isolated from cankers of Platanus × acerifolia 18 m above ground (this study). Further in five of these species (P. catenulata, P. nimia, P. oblonga, P. obturata, P. platani) the sporangia are commonly formed in large sympodia of up to 8–15 sporangia, also consistent with a partially aerial lifestyle; and (unlike other Clade 2c species) P. oblonga commonly produces sporangia on solid agar which are exclusively caducous. It is also notable that, as with all five known species in Clade 2f, internal sporangial proliferation in P. platani occurs both in a nested and extended way, whereas in P. nimia and P. oblonga it is solely extended. Since none of the other 20 Clade 2c species and only two species from Clade 2e (P. borneensis, P. penetrans) show internal proliferation, the internal proliferation observed in these species has probably evolved both independently and convergently.
As also with Clades 2d, 2f and 2g but in contrast to the other three subclades, all 24 known and new Clade 2c species are intrinsically self-fertile (‘homothallic’). The probable natural habitats of 19 taxa are known. Thirteen have maximum temperatures for growth between 27.5 and 30 °C and, hence, are adapted for a soilor waterborne lifestyle in montane tropical or subtropical forests (P. catenulata, P. fansipanensis, P. macroglobulosa, P. obturata, P. pseudocapensis) or temperate forests (P. capensis, P. curvata, P. emzansi, P. falcata, P. japonensis, P. nimia, P. oblonga, P. vacuola) (Bose et al. 2021a, this study). The other six species have maximum temperatures between 30 and 32.5 °C consistent with their natural habitats in the soil and streams of subtropical or tropical lowland to lower montane forests (P. citricola, P. limosa, P. multivora, P. pini) and/or warm-temperate forests (P. excentrica, P. pini, P. plurivora).
The 19 known and new species with available data in the more recently divergent Clade 2b exhibit a highly variable range of sporangial traits, breeding systems, cardinal temperatures and lifestyles, including the emergence of a more aerial lifestyle. Four species have exclusively semipapillate persistent (P. gloveri, P. mengei, P. montana) or predominantly persistent (P. siskiyouensis) sporangia in concordance with a predominantly soilborne lifestyle (Reeser et al. 2007, Hong et al. 2009, Abad et al. 2011, this study). In contrast, nine species produce exclusively papillate (P. aysenensis, P. capsici, P. mexicana) or predominantly papillate (P. frigidophila, P. multiplex, P. obovoidea, P. pyriformis, P. theobromicola, P. tropicalis) sporangia. These are mostly caducous in five species (P. capsici, P. frigidophila, P. obovoidea, P. pyriformis, P. tropicalis), consistent with an aerial or partially aerial lifestyle (Mchau & Coffey 1995, Erwin & Ribeiro 1996, Aragaki & Uchida 2001, Crous et al. 2020, Decloquement et al. 2021, this study). High proportions of both papillate and semipapillate sporangia occur in four species. Two of these have persistent or predominantly persistent sporangia consistent with a soil- and waterborne lifestyle (P. distorta, P. valdiviana) and two partly caducous or mostly caducous sporangia suggesting a partially (P. amaranthi; Ann et al. 2016) or predominantly aerial (P. variepedicellata; this study) lifestyle. Phytophthora calidophila and P. taxon pseudocapsici are distinct from all other Clade 2b species in producing high proportions of papillate, semipapillate and nonpapillate sporangia which are mostly caducous.
Within Clade 2b, a firm relationship is apparent between lifestyle and breeding system. Among the nine self-fertile and mainly inbreeding species eight have persistent (P. aysenensis, P. glovera, P. mengei, P. montana, P. valdiviana) or predominantly persistent sporangia (P. amaranthi, P. distorta, P. siskiyouensis) and a soilborne or predominantly soilborne lifestyle (Reeser et al. 2007, Hong et al. 2009, Abad et al. 2011, Ann et al. 2016, Crous et al. 2020, this study). Only one self-fertile species, P. frigidophila, has an aerial lifestyle. The only known sterile species, P. theobromicola, also has persistent sporangia and a soilborne lifestyle (Decloquement et al. 2021). In contrast, apart from P. multiplex, seven of the nine taxa with an A1/A2 and therefore potentially more outcrossing breeding system (P. capsici, P. calidophila, P. obovoidea, P. pyriformis, P. tropicalis, P. variepedicellata) have caducous sporangia and an aerial or predominantly aerial lifestyle as leaf, fruit and shoot pathogens (Mchau & Coffey 1995, Erwin & Ribeiro 1996, Aragaki & Uchida 2001, this study). This may facilitate their ability to respond to changing host populations and environmental conditions.
In the A1/A2 Clade 2b species, production of chlamydospores is common (P. multiplex, P. obovoidea, P. pyriformis, P. tropicalis, P. variepedicellata) or infrequent (P. capsici, P. mexicana) (Mchau & Coffey 1995, Erwin & Ribeiro 1996, Aragaki & Uchida 2001, this study). Together with the formation of dense hyphal aggregations (P. calidophila, P. multiplex, P. obovoidea, P. pyriformis, P. tropicalis, P. variepedicellata, P. taxon pseudocapsici), this may further enhance survival during adverse environmental conditions.
Available data on maximum temperatures for growth of 17 Clade 2b taxa are highly variable in range but appear generally consistent with their known natural habitats: 25–27.5 °C in P. frigidophila and P. montana from the cloud forest at 2 400 m altitude at Volcano Baru in Panama; 27.5–30 °C in P. variepedicellata from the same cloud forest at Volcano Baru, P. distorta and P. valdiviana from the cool-temperate Valdivian rainforests, P. siskiyouensis from the cool-temperate Pacific Northwest and subboreal Alaska and P. mengei with currently unknown natural habitat; (30–)32–<35 °C in P. multiplex, P. pyriformis, P. theobromicola and P. tropicalis from tropical lowland rainforests (Decloquement et al. 2021, this study), P. calidophila from a tropical lower montane forest, P. obovoidea from tropical lowland and montane forests, and P. amaranthi and P. mexicana with probable but unknown natural subtropical/tropical habitats (Ann et al. 2016, this study); and >35 °C in P. capsici and P. taxon pseudocapsici with unknown natural subtropical/tropical habitats. It is noteworthy that all eight Clade 2b species with aerial or partially aerial lifestyle in tropical lowland and lower montane forests (P. calidophila, P. obovoidea, P. pyriformis, P. tropicalis) or unknown natural habitats in tropical or subtropical regions (P. amaranthi, P. capsici, P. mexicana, P. taxon pseudocapsici) have maximum growth temperatures above 32 °C; probably an adaptation to hot daytime temperatures in infected aerial plant tissues.
Within Clade 2g, P. inclinata and P. proliferata have almost equal proportions of papillate and semipapillate sporangia whereas P. multipapillata produces predominantly semipapillate sporangia with a smaller proportion of papillate and a few nonpapillate sporangia. Phytophthora transposita is both unusual and currently unique in Clade 2g producing a mixture of papillate, semipapillate and nonpapillate sporangia. Proportions of pedicellate sporangia vary from 4 % in P. proliferata and P. transposita to 46 % in P. multipapillata. While P. inclinata, P. multipapillata and P. transposita have exclusively persistent sporangia without internal proliferation P. proliferata exhibits rare caducity, possibly enabling a partially aerial lifestyle, and regularly shows internal nested and extended proliferation.
All Clade 2g species share optimum and maximum temperatures of 27.5 and 30–32.5 °C, respectively, consistent with their natural habitats in the rhizosphere of subtropical or tropical lowland to lower montane forests.
The phenotypic and behavioural resemblances between Clade 2g and Clade 2c, i.e., self-fertility (‘homothallism’) with paragynous antheridia, variable sporangial traits and a soilborne or predominantly soilborne lifestyle are probably best explained by convergent evolution. However, it cannot be ruled out that these traits occurred in the various common ancestors of Clade 2c and the 2b-2g-2a cluster; Clade 2b and the Clade 2g-2a cluster; and Clades 2g and 2a.
In Clade 2a, the evolution towards an aerial lifestyle appears even more advanced than in its sister Clade, 2b. In 11 of the 20 known and newly described taxa with available data the sporangia formed in water are exclusively or predominantly caducous (P. colocasiae, P. insulinativitatica, P. mekongensis, P. pseudoccultans, P. vietnamensis, P. ×australasiatica, P. ×taiwanensis, P. ×vanyenensis, P. taxon awatangi, P. taxon germisporangia) while the other nine have partial (P. botryosa, P. himalsilva, P. meadii, P. occultans, P. terminalis, P. taxon himalsilva-like 1) or rare (P. citrophthora, P. multibullata, P. pseudocitrophthora, P. ×lusitanica) sporangial caducity (Erwin & Ribeiro 1996, Vettraino et al. 2011, Man In’t Veld et al. 2015, Crous et al. 2017, Dang et al. 2021, this study). The sporangial apices in water are variable ranging from exclusively (P. himalsilva, P. mekongensis, P. pseudoccultans) or predominantly (P. pseudocitrophthora, P. vietnamensis) papillate and exclusively (P. colocasiae) or predominantly (P. citrophthora, P. meadii) semipapillate to nearly similar proportions of papillate and semipapillate (P. insulinativitatica, P. multibullata, P. occultans, P. terminalis, P. ×australasiatica, P. ×taiwanensis, P. ×vanyenensis) or a transition between semipapillate and papillate (P. ×lusitanica) (Erwin & Ribeiro 1996, Vettraino et al. 2011, Man In’t Veld et al. 2015, Crous et al. 2017, Dang et al. 2021, this study).
In water, P. botryosa produces similar proportions of semipapillate and nonpapillate sporangia and only 12 % papillate sporangia. With P. meadii, 23 % of sporangial apices are nonpapillate whereas smaller proportions (up to 10 %) of nonpapillate sporangia are found in P. citrophthora, P. pseudocitrophthora, P. vietnamensis, P. ×australasiatica, P. ×taiwanensis and P. ×vanyenensis.
Eleven of the 20 Clade 2a taxa with available data (P. botryosa, P. colocasiae, P. insulinativitatica, P. meadii, P. mekongensis, P. multibullata, P. ×australasiatica, P. ×taiwanensis, P. ×vanyenensis, P. taxon awatangi, P. taxon germisporangia) have A1/A2 and therefore potentially more outcrossing orientated breeding system. With the exceptions of P. mekongensis and P. taxon germisporangia, they also produce chlamydospores as an alternative survival structure (Erwin & Ribeiro 1996, Crous et al. 2017, Dang et al. 2021, this study). A similar trend occurs in Clade 2b, and appears to be associated with an exclusively or partially aerial lifestyle and adaptation to a more heterogeneous environment. Five taxa are self-fertile and lack chlamydospores (P. himalsilva, P. occultans, P. pseudoccultans, P. terminalis, P. taxon himalsilva-like 1) (Vettraino et al. 2011, Man In’t Veld et al. 2015, this study). Another four species (P. citrophthora, P. pseudocitrophthora, P. vietnamensis, P. ×lusitanica; Erwin & Ribeiro 1996, this study) appear to be sterile or have ‘silenced’ their sexual reproduction systems. In the tropical A1/A2 (‘heterothallic’) P. colocasiae, around one third of isolates examined by Feng et al. (2022) in Japan were self-fertile, indicating a high frequency of secondary self-fertility (‘secondary homothallism’, Brasier 1992), probably giving P. colocasiae added genetic and adaptive flexibility. Similarly, self-fertile genotypes have also been reported for P. colocasiae isolates from subtropical Taiwan (Ko 1979, Ann et al. 1986).
Maximum temperatures for growth in Clade 2a vary between 27.5 and 35 °C, consistent with their natural habitat. Phytophthora colocasiae, P. insulinativitatica, P. meadii, P. mekongensis, P. taxon awatangi, P. taxon germisporangia and the majority of isolates of the hybrid species P. ×australasiatica, P. ×taiwanensis and P. ×vanyenensis have maximum temperatures for growth above 32.5 °C (Dang et al. 2021, this study) consistent with their infecting aerial plant tissues in tropical lowland rainforests or subtropical lowland monsoon forests. Maximum growth temperatures between 30 and 32.5 °C are found in three taxa from upper montane tropical forests (P. himalsilva, P. vietnamensis, P. taxon himalsilva-like 1), two species from subtropical regions (P. citrophthora, P. pseudocitrophthora), P. multibullata from submontane tropical forests and the tropical P. botryosa (Vettraino et al. 2011, Dang et al. 2021, this study), and P. occultans and P. ×lusitanica with unknown natural habitats (Man In’t Veld et al. 2015, this study). Phytophthora pseudoccultans from montane monsoon forests in Taiwan and P. terminalis with unknown natural habitat have the lowest maximum temperatures of all known and new Clade 2a taxa, at 27.5–30 and 26–28 °C, respectively (Man In’t Veld et al. 2015, this study).
In part because of our baiting mainly the forest soil and water environment the natural and potential host ranges of the 43 Clade 2 species described here are mostly unknown. Even for the many previously known Clade 2 species only one or a few host species are known, and these are confined to non-natural or introduced situations. Thus, based on a comprehensive literature review of 34 described Clade 2 species, Brasier et al. (2022) demonstrated that in terms of records on crops, ornamentals or forest trees, mainly in the context of the introduction of the host or pathogen, their host ranges varied from very wide (>20 host species, 13.9 %) to medium-wide (6−20 host species, 19.4 %) to narrow (1−5 host species, 66.7 %), most being in the narrow range. Disease types ranged from root rots (72.2 % of 34 species), stem cankers on woody hosts (52.7 %) and leaf/shoot blights, bud rots or fruit rots (38.9 %) to declines and diebacks of forest ecosystems (25 %). Furthermore, 14 of the 34 species (41.8 %) were recorded as thriving as saprotrophs in water bodies (Brasier et al. 2022). This compares with 27 of the 43 new Clade 2 species described here (63 %) being recovered from forest streams.
Notes on the relationship between level of heterozygosity, breeding system and interspecific hybridity across the subclades
Heterozygosity data for all taxa examined based on nine nuclear genes and the relationship between mean heterozygosity and breeding system, whether intrinsically self-fertile (‘homothallic’), A1/A2 (‘heterothallic’) or sterile (see Terminology section) are shown in Tables 1–4, S18.
Table 4.
Relationship between level of heterozygosity across nine nuclear genes (LSU, ITS, ßtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1), breeding system and interspecific hybridity in Phytophthora subclade 2b.
| Taxon | No. of isolates | Mean nuclear heterozygosity (%) | Nuclear heterozygosity range (%) | Breeding system | Hybrid status1 |
|---|---|---|---|---|---|
| P. amaranthi2, 8 | 3 | 0 | 0.0–0.0 | Self-fertile | Pure |
| P. aysenensis3, 8 | 2 | 0 | 0.0–0.0 | Self-fertile | Pure |
| P. gloveri | 3 | 0.01 | 0.0–0.03 | Self-fertile | Pure |
| P. capsici s. str.4, 9 | 1 | 0.05 | n/a | A1/A2 | Pure |
| P. montana 8 | 3 | 0.05 | 0.05–0.05 | Self-fertile | Pure |
| P. mengei | 3 | 0.07 | 0.07–0.07 | Self-fertile | Pure |
| P. theobromicola 5 | 4 | 0.09 | 0.0–0.27 | Sterile | Pure |
| P. t. mengei-like6 | 1 | 0.1 | n/a | Self-fertile | Pure |
| P. siskiyouensis | 3 | 0.11 | 0.0–0.17 | Self-fertile | Pure |
| P. taxon brasiliensis7 | 1 | 0.17 | n/a | A1/A2 | Pure |
| P. distorta 8 | 3 | 0.2 | 0.2–0.2 | Self-fertile | Pure |
| P. valdiviana | 4 | 0.21 | 0.21–0.22 | Self-fertile | Pure |
| P. frigidophila 8 | 3 | 0.25 | 0.25–0.25 | Self-fertile | Pure |
| P. mexicana s. l. | 5 | 0.34 | 0.22–0.51 | A1/A2 | Ambiguous |
| P. tropicalis | 15 | 0.38 | 0.16–0.61 | A1/A2 | Ambiguous |
| P. variepedicellata 8 | 5 | 0.41 | 0.39–0.42 | A1/A2 | Probable |
| P. multiplex | 10 | 0.45 | 0.31–0.52 | A1/A2 | Probable |
| P. obovoidea | 21 | 0.59 | 0.27–1.12 | A1/A2 | Ambiguous |
| P. taxon pseudocapsici | 4 | 0.65 | 0.46–0.72 | A1 /A2 | Probable |
| P. calidophila 8 | 3 | 1.1 | 1.1–1.1 | A1/A2 | Probable |
| P. pyriformis 8 | 3 | 1.27 | 1.26–1.28 | A1/A2 | Probable |
1 ‘Pure’: isolates assumed to be representative of a near pure species.
Ambiguous: wide range of nuclear heterozygosity levels suggesting that the studied isolates are a mixture of individuals ranging from a near ‘pure’ non-hybrid status to various hybrid recombinants or introgressants.
Probable: high level of nuclear heterozygosity consistent with all studied isolates being some form of interspecific hybrid; in addition the following can apply: mitochondrial genes show high similarity to one or more other Clade 2a taxa; different mitochondrial genes show high similarity to different other Clade 2a taxa; multiple mitochondrial genotypes.
2 LSU, enl and tef-1α not available.
3 hsp90, tigA, rpl10, tef-1α, enl and ras-ypt1 not available.
4 tigA and enl not available.
5 LSU, tigA, rpl10, enl and ras-ypt1 not available.
6 LSU, hsp90, tigA, rpl10, enl and ras-ypt1 not available.
7 ras-ypt1 not available.
8 Isolates studied could be genetically identical or closely related due to local asexual reproduction.
9 Low heterozygosity could reflect this being only a single isolate of an otherwise widely distributed, competently A1/A2 outcrossing species.
n/a = not applicable.
Across all subclades the A1/A2 and sterile taxa showed markedly higher levels of mean nuclear heterozygosity, at 0.4 % and 0.46 % respectively, than the self-fertile taxa at 0.09 % (Table 2). Indeed, across the 56 self-fertile taxa (excluding P. taxon aquatilis with 0.42 %) mean heterozygosity ranged from only ca. zero to 0.25 (Tables 2–4, S18; see also horizontal bar insert in Fig. 80). Against this benchmark the 25 A1/A2 taxa with suitable data showed a much wider range at zero to 1.27 %, as did the five sterile taxa at 0.09–0.84 % (Tables 2–4, S18; Fig. 80). The A1/A2 and sterile groups therefore included both species with low heterozygosity levels comparable to the self-fertile species, and species with much higher heterozygosity levels (Fig. 80).
Fig. 80.
Distribution of mean nuclear gene heterozygosity versus breeding system for the individual taxa in Phytophthora Clades 2a and 2b.
Almost all the A1/A2 and sterile taxa occur in Clades 2a and 2b (the exceptions being three A1/A2 taxa in Clade 2e). When the 2a and 2b taxa are ranked for heterozygosity levels (Tables 3–4; Fig. 80), not unexpectedly a relationship is indicated between mean heterozygosity, breeding system and species hybridity. Thus, there appears to be a discontinuity between taxa below and above ca. 0.3 % mean heterozygosity. Further, all those >0.3 % are either of A1/A2 type or are sterile.
Table 3.
Relationship between level of heterozygosity across nine nuclear genes (LSU, ITS, ßtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1), breeding system and interspecific hybridity in Phytophthora subclade 2a.
| Taxon | No. of isolates | Mean nuclear heterozygosity (%) | Nuclear heterozygosity range (%) | Breeding system | Hybrid status1 |
|---|---|---|---|---|---|
| P. taxon meadii-like2 | 1 | 0.03 | n/a | A1/A2 | Pure |
| P. taxon awatangi3 | 1 | 0.05 | n/a | A1/A2 | Pure |
| P. insulinativitatica 3 | 2 | 0.06 | 0.04–0.08 | A1/A2 | Pure |
| P. citrophthora | 10 | 0.11 | 0.01–0.14 | Sterile | Pure |
| P. taxon germisporangia3 | 1 | 0.12 | n/a | A1/A2 | Pure |
| P. botryosa | 4 | 0.13 | 0.11–0.24 | A1/A2 | Pure |
| P. pseudoccultans | 3 | 0.13 | n/a | Self-fertile | Pure |
| P. himalsilva | 6 | 0.16 | 0.15–0.17 | Self-fertile | Pure |
| P. taxon himalsilva-like 14 | 1 | 0.17 | n/a | Self-fertile | Pure |
| P. occultans | 3 | 0.19 | 0.18–0.22 | Self-fertile | Pure |
| P. meadii | 5 | 0.2 | 0.14–0.33 | A1/A2 | Pure |
| P. colocasiae | 3 | 0.22 | 0.0–0.29 | A1/A2 | Pure |
| P. terminalis | 1 | 0.23 | n/a | Self-fertile | Pure |
| P. multibullata 3 | 3 | 0.25 | 0.16–0.44 | A1/A2 | Ambiguous |
| P. ×vanyenensis | 12 | 0.4 | 0.19–0.78 | A1/A2 | Ambiguous |
| P. ×lusitanica5 | 3 | 0.52 | 0.51–0.53 | Sterile | Confirmed |
| P. mekongensis | 5 | 0.59 | 0.50–0.71 | A1/A2 | Probable |
| P. pseudocitrophthora | 7 | 0.72 | 0.56–0.79 | Sterile | Probable |
| P. vietnamensis 5 | 3 | 0.84 | 0.84–0.84 | Sterile | Probable |
| P. ×australasiatica | 18 | 0.87 | 0.65–1.11 | A1/A2 | Confirmed |
| P. ×taiwanensis | 5 | 1.09 | 0.89–1.14 | A1/A2 | Confirmed |
1 ‘Pure’: isolates assumed to be representative of a near pure species.
Ambiguous: wide range of nuclear heterozygosity levels suggesting that the studied isolates are a mixture of individuals ranging from a near ‘pure’ non-hybrid status to various hybrid recombinants or introgressants.
Probable: high level of nuclear heterozygosity consistent with all studied isolates being some form of interspecific hybrid; in addition the following can apply: mitochondrial genes show high similarity to one or more other Clade 2a taxa; different mitochondrial genes show high similarity to different other Clade 2a taxa; multiple mitochondrial genotypes.
Confirmed: based on nuclear genotyping by sequencing (Van Poucke et al. 2021); in addition high level of nuclear heterozygosity and identity of mitochondrial genes with other (maternal) Clade 2a species.
2 LSU, hsp90, tigA, rpl10 and tef-1α not available
3 LSU, tigA, rpl10, tef-1α, enl, ras-ypt1 not available.
4 ras-ypt1 not available.
5 Isolates studied could be genetically identical or closely related due to local asexual reproduction.
Previously, isolates representing three of the taxa in the Clade 2a >0.3 % cluster have been designated as some form of species hybrid based on nuclear genotyping by sequencing: isolate BD518, the ex-type of P. ×lusitanica; isolate VN763 of P. ×australasiatica; and five isolates of P. ×taiwanensis (Van Poucke et al. 2021). This conclusion of hybridity is further supported here by their respective high mean levels of nuclear heterozygosity at 0.52 %, 0.89 % and 1.09 %. Also, two of them exhibit a high level of mitochondrial gene identity with another Clade 2a taxon, suggesting possible mitochondrial parentage: P. ×lusitanica with P. citrophthora; and P. ×australasiatica with P. ×vanyenensis (Table S19). The taxa P. ×lusitanica, P. ×australasiatica and P. ×taiwanensis have therefore been formally designated in this study as hybrids and are shown in Table 3 and Fig. 80 as confirmed hybrids.
From this it could be conjectured that most if not all of the taxa in the <0.3 % clusters may be ‘pure’ species, and they are speculatively suggested to be such in Tables 3 and 4; whereas those in the >0.3 % clusters (in addition to P. ×lusitanica, P. ×australasiatica and P. ×taiwanensis) could again be some form of hybrids, whether full allopolyploids, hybrid introgressants or mixed populations of parental species and hybrids. In P. ×vanyenensis, for example, designated as a hybrid previously (Dang et al. 2021), isolates are shown here to have a very wide range in heterozygosity from 0.19−0.78 % (12 isolates, Table 3) and could conceivably be a mixture of a parental species, hybrids and introgressants, as in a hybrid swarm. Indeed, isolate CBS 235.30 of P. ×vanyenensis, reported by Van Poucke et al. (2021) not to be a hybrid, has the second lowest score among the isolates for nuclear heterozygosity (0.26 %). It might therefore be genetically closer to a parent species of a hybrid population. Phytophthora ×vanyenensis and other taxa in the >0.3 % clusters with a relatively broad spread of heterozygosity values, i.e., P. obovoidea and P. tropicalis, are therefore speculatively listed as ambiguous in Tables 3 and 4. Taxa above 0.3 % exhibiting more consistent heterozygosity values, such as P. calidophila, P. mekongensis, P. multiplex, P. pseudocitrophthora, P. pyriformis, P. vietnamensis and P. taxon pseudocapsici, are listed as probable hybrids (Tables 3, 4).
A further indication of the potential hybridity of the taxa >0.3 % in Clades 2a and 2b comes from close matches in mitochondrial gene profiles (Tables S19, S20). Among the Clade 2a taxa listed as probable hybrids in Table S20, P. mekongensis shows high mitochondrial similarity with P. ×vanyenensis; P. vietnamensis with P. ×vanyenensis; and P. pseudocitrophthora with P. occultans (Table S19). Phytophthora pseudocitrophthora may be identical with the hybrid P. occultans × citrophthora-related (isolate CBS 111726) informally designated by Van Poucke et al. (2021). In Clade 2b, P. calidophila, P. multiplex and P. taxon pseudocapsici, all listed as probable hybrids (Table 4), show high mitochondrial similarity or full identity to P. variepedicellata, P. theobromicola and P. capsici, respectively (Table S20); and P obovoidea and P. tropicalis, both listed as ambiguous for hybridity (Table 4), also show high mitochondrial similarity (Table S20).
In Clade 2f, P. taxon aquatilis, described as homothallic (self-fertile) by Hong et al. (2012), is a unique outlier in the collective heterozygosity profile of all self-fertile Clade 2 taxa (Fig. 80) with a mean nuclear heterozygosity level of 0.42 % (Tables 2, S18). Phytophthora taxon aquatilis could therefore also be some form of hybrid. Overall, we consider the higher heterozygosity levels of the taxa listed either as probable hybrids or as ambiguous in Tables 3 and 4 (together with P. taxon aquatilis in Clade 2f), plus in some cases mitochondrial co-similarity with other taxa, to be highly indicative of hybridity. However, we consider a higher burden of proof is required.
With the exceptions of P. ×lusitanica, P. pseudocitrophthora and P. vietnamensis with sterile or silenced breeding systems, the other confirmed or probable hybrids, i.e., P. calidophila, P. mekongensis, P. multiplex, P. pyriformis, P. ×australasiatica and P. ×taiwanensis, and the three ambiguous species P. obovoidea, P. tropicalis and P. ×vanyenensis exhibit apparently functional A1/A2 breeding systems which, in combination with their novel genomes, may have facilitated their survival and success in competition with their parent species. Whether the confirmed hybrid P. ×lusitanica and probable hybrids P. pseudocitrophthora and P. vietnamensis have become sterile as a result of becoming unbalanced allopolyploids needs investigation.
Further, among the putatively ‘pure’ (i.e., non-hybrid) self-fertile taxa in the Clade 2a and 2b <0.3 % clusters the mean heterozygosity ranges of the self-fertile and A1/A2 taxa are remarkably similar, at ca. 0–0.25 % and 0.03–0.22 %, respectively (Fig. 80). This raises the possibility that relative proportions of sexual outbreeding versus inbreeding in each system are also similar. In the self-fertile (and generally assumed more typically inbreeding) species, outbreeding can occur via the fusion of antheridia and oogonia (and subsequently of gametes) between adjacent, genetically dissimilar genotypes, with probably no barrier to this process. In the A1/A2 system, in addition to outcrossing, inbreeding can occur as a result of (i) selfing induced by the interaction of adjacent A1s and A2s and (ii) selfing of individual A1s or A2s in response to other environmental stimuli (Brasier 1971, 1978, 1992, Reeves & Jackson 1974, Zentmyer 1979, Jayasekera et al. 2007, Jung et al. 2013b). For both breeding systems, frequencies of inbreeding versus outbreeding in native (non-introduced) natural populations also need further investigation.
DISCUSSION
This paper is primarily the product of worldwide surveys searching for endemic species of Phytophthora in comparatively underexplored forests and natural ecosystems, and to a lesser extent Phytophthora species that were introduced into nurseries and plantations (cf. Zeng et al. 2009, Brasier et al. 2010, Vettraino et al. 2011, Jung et al. 2016, 2017b, 2018b, 2019, 2020, 2021, 2022, Puglisi et al. 2017, Milenković et al. 2018). These surveys had three main objectives. Firstly, to identify the geographic origins of Phytophthoras which have been highly damaging when introduced into non-native ecosystems, such as P. cinnamomi, P. lateralis, P. ramorum and P. ×cambivora (cf. Brasier et al. 2010, 2012, Jung et al. 2021, Shakya et al. 2021, Mullet et al. 2023) to enhance understanding of their natural breeding systems, host-pathogen relationships and containment by competitors and to potentially obtain new insights for resistance breeding. Secondly, to enhance understanding of the biogeography of the genus, including the origins and spread of its main clades (Jung et al. 2017c, 2022). And thirdly, to improve knowledge of Phytophthora diversity to better inform and manage international forest biosecurity, including measures aimed at managing pathways of introduction.
In 2009, when only 90 Phytophthora species had been formally described, the total number of Phytophthora species worldwide was estimated to lie between 200 and 600 (Brasier 2009). Later, Scott et al. (2019) estimated between 274 and 378 species. This study, presenting 43 new species and designating three other new taxa, doubles the number of taxa in Phytophthora Clade 2 alone from 47 to 93: 79 species and 14 informally designated taxa. It also brings the number of currently known Phytophthora species across all twelve clades to around 260 (based on 218 described and accepted species in Brasier et al. 2022, Chen et al. 2022, Jung et al. 2022 and Abad et al. 2023a). Moreover, the number of described Phytophthora species continues to increase steadily.
The 43 new Clade 2 species are identified based on unique combinations of morphological characters (gametangia, sporangia, chlamydospores, hyphal swellings and aggregations), colony morphology and temperature-growth relations, and a distinct position in the multigene phylogeny. They are distributed across five of the six previously known phylogenetic subclades, 2a–c, e, f, and a new subclade 2g. These now comprise 22 taxa in Clade 2a (6 new and 11 known species plus P. taxon awatangi, P. taxon germisporangia, P. taxon himalsilva-like 1 and 2 and P. taxon meadii-like); 22 in Clade 2b (9 new and 9 known species plus P. taxon brasiliensis, P. taxon mengei-like, P. taxon pseudocapsici and P. taxon subnulis), 24 in Clade 2c (15 new and 9 known species); 14 in Clade 2e (6 new and 5 known species plus P. taxon AUS 2E, P. taxon pseudobisheria and P. taxon Costa Rica 8); six in Clade 2f (3 new and 1 known species plus P. taxon aquatilis and P. taxon Costa Rica 5); four (all new species) in Clade 2g; and one (P. oleae) in Clade 2d. The now substantially revised molecular phylogeny of Clade 2 (Fig. 1) indicates early divergence of subclades 2f and 2e was followed next by a divergence of subclade 2d, then the emergence of subclades 2c and 2b and more recently a separation of subclades 2a and 2g.
We also show here that the six subclades tend to be geographically differentiated (Fig. 78). The three earlier diverged subclades (2e, 2f and 2c) are intercontinental, occurring both in the Americas and in Asia. Clade 2f is present in Southeast Asia and Central America; Clade 2e is circumpacific (Australia, Sundaland, Taiwan, Amami Island, Central America, eastern South America) and most likely also native to South Africa (P. frigida); and Clade 2c occurs in eastern North America, Southeast and East Asia and South Africa. This endemism across several continents is probably best explained by the evolution of these subclades on the ancient supercontinent Pangea before it split into Gondwana and Laurasia. Nonetheless, subsequent intercontinental migrations, possibly via ancient land bridges such as Beringia, the Isthmus of Panama and the North Atlantic land bridges, cannot be ruled out (cf. Notes on the evolutionary history of Clade 2, above). Emergence before the fragmentation of Pangea would put the age of these three subclades at ~175 Mya or more.
The three most recently diverged subclades, however, appear confined to separate continental regions consistent with their diverging after the splitting of Pangea: 2a and 2g to South and Southeast Asia and 2b to the Americas. Since 13 of the 17 known Clade 2a species and all four known Clade 2g species have been found between India and Japan it is most likely that these subclades evolved within this area, as previously suggested for Clade 2a by Dang et al. (2021). The fact that none of the 22 known Clade 2b taxa are native to Africa suggests this subclade probably evolved in South America after its splitting from Africa ca. 140 Mya (cf. Notes on the evolutionary history of Clade 2, above).
Several hotspots of subclade diversity were highlighted by this study (Fig. 79; and see Notes on the geographical distribution of Clade 2, above). Vietnam together with Indochina as a whole constitutes a hotspot of Clade 2a diversity, with at least seven species. The native forests of Taiwan are another 2a hotspot, with three species including two new probable endemics. Central America is the centre of diversity of Clade 2b, hosting 11 of the 22 known and new taxa. The cool-temperate Aysen and Valdivia regions in southern Chile constitute another 2b hotspot, with three endemic species. Clade 2c has its main centres of diversity in East and Southeast Asia, with currently 18 confirmed taxa including a plethora of 15 new taxa. Eight 2c taxa including seven new endemics have now been identified in Southeast Asia alone, and the Japanese archipelago hosts at least ten 2c species including eight endemics. The Indonesian archipelago is a hotspot of Clade 2e diversity, with in total five species. Clade 2f has one possible centre in Asia, comprising three species, and a second centre with two species in the Central American Panamanian region. Currently, in the second-least numerous subclade 2g one centre three of the four known species are in a focus between Vietnam and Borneo and the other occurs in Kyushu, Japan.
Some of these diversity hotspots probably reflect allopatric speciation. For example, the different clusters of Clade 2c species in North America, South Africa, Southeast Asia and East Asia seem likely to result from geographic isolation followed by local radiations. The vicariant distributions of the Clade 2c taxa P. fansipanensis on the Fansipan Mountain, P. obturata in Ba Vì National Park and on Sau Chua Mountain (all in Vietnam); P. macroglobulosa on the Chinese Hainan Island; and of the Clade 2g taxa P. proliferata in Cuc Phuong National Park in northern Vietnam, P. inclinata on Côn Lôn Island and P. multipapillata on Borneo, suggest allopatric speciation resulting from local geographic barriers. Other hotspots may reflect local sympatric speciation, for example, the co-occurrence of the Clade 2c species P. curvata, P. excentrica, P. falcata and P. japonica in mixed sub-tropical Quercus-Abies-Torreya-Tsuga-forests on Shikoku Island, Japan. However, without evidence of the extent of local adaptation of these taxa, in particular their host ranges and their levels of reproductive isolation, it is only possible to speculate.
Interestingly, Clade 2 shows both striking biogeographical similarities and some contrasts to Phytophthora Clade 10. Both clades occur naturally, and sometimes co-occur, in northern Indochina, Amami Island, Java, Sulawesi, Eastern Australia, South Africa, the Valdivian region in southern Chile and the Southeast and East of the USA (Hong et al. 2011, Oh et al. 2013, Yang et al. 2016, Brazee et al. 2017, Jung et al. 2018b, 2020, 2022; Bose et al. 2021a; this study). However, none of the known Clade 10 species is native to western North America, Central America or the east and southeast of South America (Jung et al. 2022), whereas this vast region is naturally inhabited by at least 19 Clade 2 taxa. Clade 10 is also apparently absent from Taiwan and the main islands of Japan, where at least 18 species from Clade 2 are probably native. Further, whereas Clade 2 species do not appear to be native to Europe, Clade 10 has radiated in Europe, occurring as four extant native species in boreal, subboreal and temperate regions (P. gallica, P. scandinavica, P. subarctica and P. ukrainensis; Jung et al. 2022). The absence of Clade 2 in Europe could indicate that it never colonised the region. Indeed, none of the 93 known Clade 2 taxa is adapted to particularly low temperatures and only the North American P. siskiyouensis is native to subboreal regions. If Clade 2 was originally present in Europe it may have become extinct during the long Pleistocene glaciations (cf. Huntley 1993, Latham & Ricklefs 1993, Svenning 2003).
High genomic, phenotypic and behavioural flexibility, in particular an ability to switch between different breeding systems, spore types and lifestyles, is a salient characteristic of the genus Phytophthora that is considered to account for its long-term evolutionary success and for its species being highly successful pathogens when introduced into novel ecosystems (Brasier et al. 2022). This must also have enabled the Clade 2 subclades to adapt to such a very wide variety of environments and hosts worldwide. Indeed, the current 79 well-characterised species and the five informally designated taxa with suitable data (P. taxon aquatilis, P. taxon AUS 2E, P. taxon awatangi, P. taxon germisporangia, P. taxon pseudocapsici) display most of the breeding systems, gametangial and sporangial forms, cardinal temperatures, ‘lifestyles’ and host and substrate characteristics of the genus as a whole (cf. Brasier et al. 2022; Tables 5–7, S4–S17; and see Notes on adaptive traits of Clade 2, above), although no very low temperature-tolerant species have been identified.
Table 5.
Breeding system frequency across the subclades of Phytophthora Clade 2.
| Subclade | No. of taxa | Proportion of taxa (of these % forming chlamydospores) | ||
|---|---|---|---|---|
| Self-fertile | A1/A2 | Sterile | ||
| 2a | 19 | 21 (0) | 58 (83) | 21 (0) |
| 2b1 | 18 | 50 (0) | 44 (63) | 6 (100) |
| 2c | 24 | 100 (0) | 0 | 0 |
| 2d | 1 | 100 (0) | 0 | 0 |
| 2e2 | 12 | 67 (0) | 33 (75) | 0 |
| 2f3 | 5 | 100 (0) | 0 | 0 |
| 2g | 4 | 100 (0) | 0 | 0 |
1 No suitable data available for P. taxon brasiliensis, P. taxon pseudocapsici, P. taxon mengei-like and P. taxon subnulis.
2 No data available for P. taxon pseudobisheria and P. taxon Costa Rica 8.
3 No data available for P. taxon Costa Rica 5.
Table 7.
Frequency of sporangial types across the subclades of Phytophthora Clade 2.
| Subclade | No. of taxa | Proportion of taxa (approx.; %) | |||
|---|---|---|---|---|---|
| Mainly papillate to semipapillate1 | Non-papillate to semi-papillate, non-caducous | ||||
| Fully or partly caducous | Non- or rarely caducous | Internal proliferation | No internal proliferation | ||
| 2a | 19 | 79 | 212 | 0 | 0 |
| 2b3 | 19 | 534 | 47 | 0 | 0 |
| 2c | 24 | 0 | 1005 | 0 | 0 |
| 2d | 1 | 0 | 100 | 0 | 0 |
| 2e6 | 11 | 97 | 918 | 0 | 0 |
| 2f9 | 4 | 0 | 0 | 100 | 0 |
| 2g | 4 | 0 | 10010 | 0 | 0 |
1 Proportion of non-papillate sporangia <20 %.
2 All four species (P. citrophthora, P. multibullata, P. pseudocitrophthora, P. ×lusitanica) show rare caducity.
3 No data available for P. taxon brasiliensis, P. taxon mengei-like and P. taxon subnulis.
4 In P. calidophila 25 % non-papillate sporangia.
5 In six species (= 25 %) <1 % of sporangia caducous; in three species (= 12.5 %) internal proliferation occurring.
6 No data available for P. taxon pseudobisheria and P. taxon Costa Rica 8.
7 P. frigida is reported to be caducous (Maseko et al. 2007).
8 In P. indonesiensis <1 % of sporangia caducous; in two species internal proliferation occurring.
9 No suitable data available for P. taxon aquatilis and P. taxon Costa Rica 5.
10 In P. multipapillata (= 25 % of species) <1 % of sporangia caducous; in P. proliferata (= 25 % of species) internal proliferation occurring.
This phenotypic and behavioural breadth can almost be said of the taxa within Clade 2b alone. Breeding systems of the twenty 2b taxa with suitable data range from intrinsically self-sterile (‘homothallic’) to A1/A2 (‘heterothallic’) to sexually sterile. Antheridia produced by different taxa are either paragynous, amphigynous or a mixture of both (Tables 5, 6, S7–S9). Sporangiophores formed range from simple to lax or dense sympodia; sporangia range from predominantly papillate and semipapillate to a proportion of nonpapillate, and from caducous to partially caducous to non-caducous (Tables 7, S7–S9). Optimal temperatures for growth in subclade 2b, and indeed in Clade 2 as a whole, range from 20–30 °C and maximum temperatures from 25–35 °C (Tables S7–S9, Fig. 62), and are generally consistent with the climate a species will experience in its native habitat (cf. Notes on the evolution of lifestyle and adaptive traits in Clade 2, above).
Table 6.
Frequency of paragynous and amphigynous antheridial types among the self-fertile (‘homothallic’) and the A1/A2 Phytophthora taxa in the Clade 2 subclades.
| Subclade | Self-fertile | A1/A2 | ||||
|---|---|---|---|---|---|---|
| No. of taxa | Proportion of taxa (approx.; %) | No. of taxa | Proportion amphigynous (%) | |||
| Paragynous | Paragynous and amphigynous | Amphigynous | ||||
| 2a1 | 4 | 0 | 100 | 0 | 11 | 100 |
| 2b2 | 9 | 11 | 44.5 | 44.5 | 9 | 100 |
| 2c | 24 | 33 | 633 | 4 | 0 | n/a |
| 2d | 1 | 100 | 0 | 0 | 0 | n/a |
| 2e4 | 8 | 100 | 0 | 0 | 4 | 100 |
| 2f5 | 5 | 0 | 606 | 40 | 0 | n/a |
| 2g | 4 | 100 | 0 | 0 | 0 | n/a |
n/a = not applicable.
1 P. citrophthora, P. pseudocitrophthora, P. vietnamensis and P. ×lusitanica have sterile breeding systems.
2 No data available for P. taxon brasiliensis, P. taxon mengei-like and P. taxon subnulis; P. theobromicola has a sterile breeding system.
3 Typically only 0.2–5 % amphigynous types.
4 No data available for P. taxon pseudobisheria and P. taxon Costa Rica 8.
5 No data available for P. taxon Costa Rica 5.
6 Very wide range, from 5–99 % paragynous and from 1–95 % amphigynous types, respectively.
Since many of the Clade 2 species examined here were obtained via soil and water baiting in the absence of evident symptoms on local non-woody plants or trees, one can only infer their lifestyles indirectly from their properties in vitro and by analogy with similar but behaviourally better-understood taxa in this and other Phytophthora clades (cf. Brasier et al. 2022). On this basis, trends towards ‘lifestyle specialisation’ within the different subclades can be suggested. In Clade 2a, compared to the other subclades more of the species (~58 %) have an A1/A2 system implying potentially greater outcrossing (Table 5), and 2a also has by far the highest frequency of species with fully or partly caducous sporangia (~79 %; Table 7). This suggests a trend towards being adapted for more heterogeneous environments and for aerial dispersal and infection. As three species fitting this pattern, i.e., P. botryosa, P. colocasiae and P. meadii, are damaging pathogens of tree crops, this combination of characters may also indicate a higher biosecurity risk. Clade 2b is more variable than Clade 2a in many characteristics but also has a relatively high frequency of outcrossing taxa (8 species = 44 %). Within the latter, a degree of specialisation is again indicated, as six of them (75 %) produce caducous sporangia. In contrast, among the ten self-fertile Clade 2b species the majority (70 %) are non-caducous or rarely caducous. Therefore, two somewhat divergent ‘lifestyle specialisation’ trends may have occurred in Clade 2b. While the more numerically limited Clade 2e also has a mixture of taxa with self-fertile (~67 %) and A1/A2 (~33 %) systems, in this case only one of the known taxa, the A1/A2 P. frigida, is caducous (Maseko et al. 2007). Clade 2c is particularly distinctive. All 24 species are self-fertile, and all have non-caducous or ‘rarely caducous’ sporangia. This suggests adaptation for survival in more uniform environments in association with a mainly soil-inhabiting and root-infecting lifestyle. If correct, then this evolutionary ‘strategy’ may have been so successful that it has been conserved throughout the migration and radiation of Clade 2c into North America, southern Africa and East and Southeast Asia. Its success is also indicated by the later diverged Clade 2g whose known members are in all aspects effectively ‘Clade 2c look-alikes’. The five known taxa in Clade 2f are also intrinsically self-fertile, but form sporangia that, while non-caducous, are mostly nonpapillate and internally proliferating. Currently, this is the only Clade 2 subclade in which this type of sporangium, possibly an adaptation for rapid sequential production of sporangia in water, occurs. This subclade could therefore be adapted to relatively environmentally stable riparian conditions. Overall, the above trends are an indication of considerable lifestyle fluidity across Clade 2 as a whole, probably comparable in breadth to that in Phytophthora Clade 10 (see Jung et al. 2022).
Regarding the species with an A1/A2 breeding system in Clade 2, certain lifestyle features stand out. First, most of these species, together with one sterile species (P. theobromicola), were found to produce chlamydospores, but no chlamydospores were formed by any of the self-fertile species (Tables 5, S4–S17). This suggests chlamydospores are an important mode of survival for some outcrossing taxa under adverse environmental conditions or when the other compatibility type is ‘absent’. Second, the antheridia formed by the A1/A2 species are consistently amphigynous, whereas those formed by the self-fertile taxa vary (Table 6). This tight association of amphigyny with the A1/A2 breeding system is found across the genus, but the reason has yet to be determined. It could, for example, be associated with delaying or controlling the mixing of cytoplasm between divergent genotypes (cf. Brasier 1983). Some of the species in Clade 2 that produce amphigynous antheridia but are self-fertile and potentially more inbreeding (notably subclade 2b, Table 6) may have evolved from outcrossing species, i.e. they may be ‘secondarily homothallics’ (cf. Brasier 1992).
Thirdly, it is also notable that Clade 2a has a relatively high frequency of sterile species (4 species, ~21 %). These include the economically important P. citrophthora and P. pseudocitrophthora, some isolates of which are silent A1s or A2s; P. ×lusitanica with all known isolates being silent A2s; and P. vietnamensis (which was initially recorded here as a silent A1 but later lost the ability to induce gametangial formation in P. meadii A2; Table S4, this study). In addition, two informally described outcrossing Clade 2a taxa, P. taxon germisporangia and P. taxon awatangi, are exclusively of A1 and A2 type, respectively (Dang et al. 2021). Collectively, this suggests the sterility of some Clade 2a taxa has resulted from the degeneration or loss of a previously functioning A1/A2 system, perhaps due to changes in environmental conditions favouring clonal or asexual spread, such as colonisation of a novel host or another disturbance event (cf. Brasier & Hansen 1992, Brasier 1995). This may also apply to P. tropicalis (Clade 2b), in which both outcrossing and sterile isolates occur (Aragaki & Uchida 2001).
Furthermore, within Clade 2a, some single isolates of the Clade 2a taxa P. colocasiae and P. meadii are not only of A1 or A2 type but are also partially self-fertile (Erwin & Ribeiro 1996, Feng et al. 2022). This may occur when an A1 or an A2 isolate carries an extra chromosome of the ‘opposite’ compatibility type as a result of meiotic non-disjunction (cf. Sansome 1980); or, in the case of P. meadii, a consequence of ‘A1 + A2’ polyploidy (Sansome et al. 1990). Either way, these features reflect the potentially high inbreeding versus outcrossing flexibility of the A1/A2 compatibility system (Brasier 1992).
Interspecific hybridisation has the potential to generate novel variation and rapid evolution in oomycetes and fungi (Brasier 2000a, 2001, Schardle & Craven 2003, Stuckenbrock 2016) and is being increasingly recognised in both true fungi (Newcombe et al. 2000, Gonthier et al. 2007, Paoletti et al. 2006, Brasier et al. 2021) and Phytophthoras (Brasier et al. 2004, Man in’ t Veld et al. 2012, Bertier et al. 2013, Nagel et al. 2013, Burgess 2015, Jung et al. 2017b, c, 2018b, 2020, Van Poucke et al. 2021, Mullet et al. 2023) including the destructive alder dieback pathogen, P. ×alni (Brasier et al. 1999, Husson et al. 2015). Introductions and other anthropogenic disturbance events may create opportunities for hybridisation between previously geographically isolated taxa (Brasier 2001). In this study Clade 2a species P. ×lusitanica, P. ×australasiatica, and P. ×taiwanensis were previously identified as species hybrids based on nuclear genotyping by sequencing (Van Poucke et al. 2021). This is further supported here by their high mean nuclear heterozygosity levels (0.52, 0.89 and 1.09 %) and, in the case of P. ×lusitanica and P. ×australasiatica, a high level of mitochondrial gene identity with another Clade 2a taxon. They were considered confirmed hybrids in this study and formally designated as such.
Another eight taxa in Clade 2a, five in Clade 2b and one in Clade 2f are also proposed here to be some form of hybrid, based on a nuclear heterozygosity level above 0.3 % and in some cases a high level of mitochondrial gene identity with another Clade 2a taxon. Where their nuclear heterozygosity levels were relatively consistent, they were considered probable hybrids. Where their levels were relatively wide-ranging, they were considered ambiguous hybrids. For example, twelve isolates of P. ×vanyenensis (identified as a hybrid by Dang et al. 2021) exhibited a very wide range in heterozygosity from 0.19–0.78 % and might conceivably represent a hybrid swarm including less heterozygous individuals or introgressants closer to a parent species (cf. Brasier et al. 2021). The same may apply to P. tropicalis and several other taxa. However, because of the often limited sample sizes, lack of evidence of nuclear gene homology with more than one species and uncertainty as to whether only limited introgression, full hybridity or a combination of these could be involved, we consider that with the ‘probable’ and ‘ambiguous’ taxa stronger proof of hybridity is required. In particular evidence of comparative genome size and the frequency and distribution of multi-allelic loci (cf. Van Poucke et al. 2021); and where possible clear evidence of direct association with one or more parent species and some indication of whether hybridity is ancient or ongoing.
The increasing emergence or detection of hybridisation processes including hybrid swarms in Phytophthoras is likely to challenge the utility of species concepts used in classical taxonomic and biosecurity protocols.
If confirmed, the overall number of hybrid taxa in Clades 2a and 2b could be remarkably high: eight out of 21 taxa (38.1 %) in each subclade. This could conceivably reflect high levels of recent anthropogenic disturbance in the geographic areas they inhabit (cf. Brasier 2001). Further, all the putative and confirmed hybrid taxa in Clades 2a and 2b have an A1/A2 compatibility system or are sexually sterile, suggesting the A1/A2 system favours interspecific hybridisation, and that sterility may be a regular product of it; and all are aerial pathogens, suggesting the ‘aerial lifestyle’ may also favour hybridisation. No hybrids were detected in Clades 2c, 2d, 2e and 2g. and, apart from P. taxon aquatilis from Clade 2f, (suggested here as a possible hybrid) the other 56 self-fertile taxa examined across all seven subclades exhibited a nuclear heterogeneity level <0.3 % and none were suspected to be hybrids.
The many characteristics shared across the eleven major Phytophthora clades define them as a strongly biologically cohesive and as a relatively tight evolutionary unit (Brasier et al. 2022). Within the Phytophthora phylogenetic tree however, the 20 downy mildew (DM) genera form two distinct clades, rendering Phytophthora paraphyletic (Cooke et al. 2000, Runge et al. 2011, Jung et al. 2017a, Bourret et al. 2018, Scanu et al. 2021, Brasier et al. 2022, Abad et al. 2023a) and the DMs polyphyletic. Although proposals have been made to split Phytophthora into multiple genera (Runge et al. 2011, Crous et al. 2021), the high diversity revealed here within Clade 2 alone adds further support to the conclusion that no major morphological or behavioural synapomorphies exist in the individual clades (or groups of clades) that justify naming them as separate genera (Brasier et al. 2022). In much the same vein, a century ago Leonian (1925), when he transferred P. citrophthora (Clade 2a) from the genus Pythiacystis to Phytophthora, stated “This organism is so obviously a Phytophthora species that the genus Pythiacystis is no longer tenable”.
Both accurate taxonomy and a correspondingly accurate understanding of the scale of pathogen diversity are needed to underpin modern plant biosecurity. However, pathogen outbreak data indicate these aspirations currently fall well short of global biosecurity needs. For example, the number of forest epidemics and declines associated with Phytophthora species globally has increased rapidly from ca five in the 1960s to 41 in 2022, leading to considerable economic and environmental costs (Brasier et al. 2022). It is now widely recognised that such outbreaks are often directly or indirectly attributable to importation of exotic pathogens via the international trade in live plants or parts of plants, in particular rooted plants for planting, with disease often amplified in nurseries and spread by subsequent outplanting of infested stock (Jung & Blaschke 2004, Brasier 2008, Jung 2009, Dehnen-Schmutz et al. 2010, Drew et al. 2010, Liebhold et al. 2012, Santini et al. 2013, Jung et al. 2016, 2018a, Frankel et al. 2020, Riddell et al. 2020; Sims & Garbelotto 2021, Rossmann et al. 2021). The recent arrival of the NA1 lineage of P. ramorum in North America (Mascheretti et al. 2009) and of the EU2 in Northern Ireland (Van Poucke et al. 2012) are probably linked to specialist trade in Asian plants. Findings that European and North American nurseries are infested with numerous exotic Phytophthora species further highlight the risks (e.g., MacDonald et al. 1994, Themann et al. 2002, Schwingle et al. 2007, Moralejo et al. 2009, Leonberger et al. 2013, Bienapfl & Balci 2014, Parke et al. 2014, Jung et al. 2016, Rooney-Latham et al. 2019, Rossmann et al. 2021, Mora-Sala et al. 2022).
Of further concern is the fact that under current agreements biosecurity protocols are heavily dependent on lists of specifically named threat organisms. In Europe, for example, extensive lists of organisms of concern are collated from scientific journals, the internet and elsewhere by national and trans-national plant health agencies (cf. https://gd.eppo.int/; https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/). Many, perhaps most of these listed organisms have come to attention because they have already spread beyond their natural range and are causing damage to vulnerable hosts. Unfortunately, numerous highly damaging forest disease outbreaks over the past century have been caused by pathogens virtually unknown to science before their introduction (Brasier 2008, Roy et al. 2014, Jung et al. 2018a). Prominent examples in the early to mid-20th century were the Dutch elm disease and Chestnut blight pathogens (e.g., Gibbs & Wainhouse 1986, Anagnostakis 1987, Brasier 2000b) and among Phytophthoras, P. cinnamomi, P. ×cambivora and P. lateralis (e.g., Day 1938, Tucker & Milbrath 1942, Crandall & Gravatt 1945, Moreau & Moreau 1952, Hansen et al. 2000, Hardham 2005, Jung et al. 2018a, Mullet et al. 2023). More recently, despite advances in taxonomy and molecular detection, these episodes have continued, including outbreaks caused by the European ash dieback pathogen Hymenoscyphus pseudoalbidus and the previously ‘unknown’ Phytophthoras P. kernoviae, P. multivora, P. pinifolia, P. plurivora and P. ramorum (e.g., Rizzo et al. 2002, Brasier et al. 2005, Durán et al. 2008, Jung & Burgess 2009, Scott et al. 2009, Santini et al. 2013, Gross et al. 2014, Jung et al. 2016, 2018a, b, 2021, Migliorini et al. 2019, Corcobado et al. 2020, Shakya et al. 2021, Brasier et al. 2022).
In practice, there is a critical lack of data on the scale of unknown pathogen threats. This amounts to a major forest biosecurity evidence gap. To offset this, forest biosecurity practitioners need to be more involved in active scientific intelligence gathering (Brasier 2005), i.e., in proactively assessing both the scale and the quality of the unknown threats. The 43 previously unknown Phytophthora Clade 2 species uncovered by the present surveys indicate the numerical scale of the risks. At least 41 of them (95 %) were found in ‘healthy’ forests with no noticeable disease symptoms, but owing to the sampling protocols used their native hosts are largely unknown. Any one of them could potentially be a threat to naïve or non-coevolved hosts elsewhere in the world. This applies also to the many previously unknown Phytophthoras being discovered in other clades (e.g., Hong et al. 2008, 2010, 2012, Yang & Hong 2013, Scanu et al. 2015, 2021, Yang et al. 2013, 2014a, b, c, 2016, Jung et al. 2017b–d, 2018b, 2020, 2022, Burgess et al. 2018, Bose et al. 2021a, Chen et al. 2022).
To assess the quality of the risks posed by these ‘new’ Clade 2 Phytophthoras (and those in other clades) we suggest the following. Firstly, their pathogenicity and potential host ranges be tested on a standard range of well-characterised, non-coevolved hosts (e.g., Rhododendron, Eucalyptus, Fagus, Quercus, Castanea and Chamaecyparis spp.), with well characterised Phytophthora species as controls. In this regard, in one recent pilot test roots of Fagus sylvatica and Castanea sativa were highly susceptible to 11 and seven exotic Clade 2 species, respectively (T. Corcobado, T. Majek and T. Jung, unpubl. results). A greater emphasis might be placed on testing A1/A2 species which, theoretically at least, could be more adaptable if introduced together; although even highly specialised self-fertile species can be extremely dangerous when introduced elsewhere (e.g., P. lateralis on Chamaecyparis spp.; Hansen et al. 2000). Secondly, now that the geographic origins of some damaging Phytophthora species are beginning to be identified through surveys and subsequent population studies (e.g., P. cinnamomi in mixed forests in Taiwan and Vietnam, Shakya et al. 2021; P. kernoviae in Valdivian rainforests in Chile, Jung et al. 2018b, 2022; P. lateralis in Taiwanese cedar forests, Brasier et al. 2010, 2012; P. multivora in South Africa, Tsykun et al. 2022; P. ramorum in east Asian laurasilva forests, Jung et al. 2021; and P. ×cambivora in mixed broadleaved forests in Japan, Mullet et al. 2023), these developments need to be consolidated by research aimed at understanding their role in the ecosystem including their natural hosts and the mechanisms of host resistance.
The results of this study further emphasise the fact that, for Phytophthora at least, the number of scientifically unknown taxa is probably very high, with possibly as many as ~200 to 400 species still to be discovered (Brasier 2009). Therefore, with the continued reliance on lists of named threat organisms, global biosecurity will continue to be on the back foot until measures are more focused on regulating the highest risk pathways of plant movement, such as traded plants, plant collecting and even informal plant-associated tourism (cf. Liebhold et al. 2012, Brockerhoff et al. 2014).
CONCLUSIONS
Comprising 79 described species and 14 informally designated taxa, Clade 2 is currently the largest of the 12 recognised Phytophthora clades.
The natural biogeography of Clade 2 includes southeastern and eastern Australia; a large belt stretching from India and Nepal via China and Southeast Asia to Taiwan and the Japanese archipelago; South Africa; the Americas extending from Alaska to the south of Chile, the southeast of Brazil and eastern North America from the Atlantic and the Gulf of Mexico to the US Midwest. Particularly diverse Clade 2 hotspots are found in East and Southeast Asia and Central America.
The evolutionary history of Clade 2 appears to have involved pre-Gondwanan divergence of three of the extant subclades 2c, 2e and 2f, all with disjunct natural distributions on separate continents. They comprise species with a soilborne and aquatic lifestyle and, in Clade 2c, a few partially aerial species. Three other extant subclades, 2a, 2b and 2g, appear to be a result of the post-Gondwanan divergence in Southeast/East Asia and South America respectively. Clade 2g has a soilborne root-infecting lifestyle whereas Clade 2b comprises both soil-inhabiting and aerially disseminated species. Clade 2a has evolved further towards an aerial lifestyle, comprising species which are predominantly or partially airborne. Evidence suggests the currently 93 described and informally designated Clade 2 taxa have resulted from both allopatric non-adaptive and sympatric adaptive radiations. In Clades 2a and 2b over 30 % of the taxa may be hybrids and the hybridity appears to be associated with an A1/A2 breeding system and an aerial lifestyle. Overall, Clade 2 exhibits much of the diversity in breeding systems, morphologies and lifestyles seen across the genus as a whole (cf. Brasier et al. 2022).
The large number of previously unknown Phytophthora species being uncovered in surveys of underexplored ecosystems underlines the risk of relying on lists of known organisms in international biosecurity protocols. Pathogenicity and host range tests of many new taxa are needed to assess the level of risk they pose to global forest biosecurity.
Acknowledgments
The authors are grateful to the Project Phytophthora Research Centre Reg. No. CZ.02.1.01/0.0/0.0/15_003/0000453 co-financed by the Czech Ministry for Education, Youth and Sports and the European Regional Development Fund, the Portuguese Science and Technology Foundation (FCT) for co-financing the European BiodivERsA project RESIPATH: Responses of European Forests and Society to Invasive Pathogens (BIODIVERSA/0002/2012) and for financing the Exploratory Project EXPL/AGR-FOR/1304/2012 ‘Screening of Asian oak species for potential resistance to Phytophthora spp.’ (QuerResist), the European Union’s Horizon 2020 research and innovation programme for financing under grant agreement No. 63564 project POnTE: Pest Organisms Threatening Europe, to the Japanese Society for the promotion of science, KAKEN No. 18H02245, and to Phytophthora Research and Consultancy for co-funding the expeditions to Japan and Taiwan. Travel and subsistence support for C.M.B. was provided by Brasier Consultancy. DNA sequencing of Hungarian, Taiwanese and Chilean isolates was partly supported by the Hungarian Scientific Research Fund (OTKA) grant K101914. This work was in part supported by the U.S. Department of Agriculture, Agricultural Research Service. The authors also thank the administration of the company ARAUCO as the owner of Parque Oncol in Chile, the administration of the company APRIL in Indonesia and the administration of Hoang-Lien National Park in Vietnam for the permission to take samples in their forests. We are grateful to Dr. Atsushi Sakai (FFPRI, Shikoku Research Centre) for his invaluable assistance during the survey in Shikoku, Japan, and to Aneta Bačová, Anna Hýsková, Henrieta Ďatková and Milica Raco (all Mendel University in Brno, Czech Republic), Mariela González and Sebastian Fajardo (both previously Universidad de Concepción), and Diána Seress (HUN-REN CAR, Plant Protection Institute, Hungary) for much appreciated technical support.
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supplementary Material: https://studiesinmycology.org/
Fifty percent majority rule consensus phylogram derived from maximum likelihood analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora major Clade 2. Maximum Likelihood bootstrap values (in %) are indicated but not shown below 60 %. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT), (NT) and (T) denote ex-epitype, ex-neotype and ex-type isolates. Scale bar indicates 0.02 expected changes per site per branch.
Fifty percent majority rule consensus phylogram derived from maximum likelihood analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2a. Bootstrap values (in %) are indicated but not shown below 60 %. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT), (NT) and (T) denote ex-epitype, ex-neotype and ex-type isolates. Scale bar indicates 0.01 expected changes per site per branch.
Fifty percent majority rule consensus phylogram derived from maximum likelihood analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2b. Bootstrap values (in %) are indicated but not shown below 60 %. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT) and (T) denote ex-epitype and ex-type isolates, respectively isolates. Scale bar indicates 0.01 expected changes per site per branch.
Details of isolates from Phytophthora major Clades 2, 3 and 7 included in the phylogenetic, morphological, growth-temperature and biogeography studies. GenBank accession numbers are given in Table S3.
Overview of PCR conditions and details of primers used for amplification and sequencing of Phytophthora isolates.
GenBank accession numbers of Phytophthora isolates included in the phylogenetic, morphological and growth-temperature studies (sequences obtained in the present study are printed in italics).
Morphological characters and dimensions (mean ± SD; µm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2a (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; µm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2a (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; µm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2a (3rd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2b (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2b (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2b (3rd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (3rd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (4th set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclades 2d (only P. oleae) and 2e (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora taxa from subclade 2e (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora taxa from subclade 2f. Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2g. Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Heterozygosity across nine nuclear gene regions (LSU, ITS, ßtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1), breeding systems and hybrid status of 46 Phytophthora taxa in subclades 2c, 2d, 2e, 2f and 2g.
Pairwise sequence identities (%) for the mitochondrial cox1, cox2, nadh1 and rsp10 genes between eight Phytophthora Clade 2a species with ambiguous, probable or confirmed hybrid status and their closest matches.
Pairwise sequence similarities (%) for the mitochondrial cox1, cox2, nadh1 and rsp10 genes between six Phytophthora Clade 2b taxa with ambiguous or probable hybrid status and their closest matches.
REFERENCES
- Abad ZG, Abad JA, Coffey MD. et al. (2008). Phytophthora bisheria sp. nov., a new species identified in isolates from the Rosaceous raspberry, rose and strawberry in three continents. Mycologia 100: 99–110. [DOI] [PubMed] [Google Scholar]
- Abad ZG, Burgess TI, Bourret T. et al. (2023a). Phytophthora: taxonomic and phylogenetic revision of the genus. Studies in Mycology 106: 259–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad ZG, Burgess TI, Redford AJ. et al. (2023b). IDphy: An international online resource for molecular and morphological identification of Phytophthora based on type specimens. Plant Disease 107: 987–998. [DOI] [PubMed] [Google Scholar]
- Abad ZG, Ivors K, Gallup CA. et al. (2011). Morphological and molecular characterization of Phytophthora glovera sp. nov. from tobacco in Brazil. Mycologia 103: 341–350. [DOI] [PubMed] [Google Scholar]
- Adams GC, Catal M, Trummer L. (2010). Distribution and severity of alder Phytophthora in Alaska. In: Proceedings of the Sudden Oak Death Fourth Science Symposium (Frankel SJ, Kliejunas T, Palmieri KM. eds). USDA Forest Service, Albany, California, USA. General Technical Report PSW-GTR-229: 29–49. [Google Scholar]
- Aguayo J, Adams GC, Halkett F. et al. (2013). Strong genetic differentiation between North American and European populations of Phytophthora alni subsp. uniformis. Phytopathology 103: 190–199. [DOI] [PubMed] [Google Scholar]
- Albuquerque Alves TC, Tessmann DJ, Ivors KL. et al. (2016). First report of gummosis caused by Phytophthora frigida on Black wattle in Brazil. Plant Disease 100: 2336. [Google Scholar]
- Albuquerque Alves TC, Tessmann DJ, Ivors KL. et al. (2019). Phytophthora acaciae sp. nov., a new species causing gummosis of Black wattle in Brazil. Mycologia 111: 445–455. [DOI] [PubMed] [Google Scholar]
- Alvarez LA, Vicent A, De la Roca E. et al. (2008). Branch cankers on citrus trees in Spain caused by Phytophthora citrophthora. Plant Pathology 57: 84–91. [DOI] [PubMed] [Google Scholar]
- Anagnostakis SL. (1987). Chestnut blight: The classical problem of an introduced pathogen. Mycologia 79: 23–37. [Google Scholar]
- Ann P-J, Huang J-H, Tsai J-N. et al. (2016). Morphological, molecular and pathological characterization of Phytophthora amaranthi sp. nov. from amaranth in Taiwan. Journal of Phytopathology 164: 94–101. [Google Scholar]
- Ann PJ, Kao CW, Ko WH. (1986). Mating-type distribution of Phytophthora colocasiae in Taiwan. Mycopathologica 93: 193–194. [Google Scholar]
- Antonelli A, Sanmartín I. (2011). Why are there so many plant species in the Neotropics? Taxon 60: 403–414. [Google Scholar]
- Aragaki M, Uchida JY. (2001). Morphological distinctions between Phytophthora capsici and P. tropicalis sp. nov. Mycologia 93: 137–145. [Google Scholar]
- Armesto J, Villagrán C, Arroyo MTK. (1995). Ecología de los bosques nativos de Chile. Santiago: Editorial Universitaria. [Google Scholar]
- Averyanov LV, Loc PK, Hiep NT. et al. (2003). Phytogeographic review of Vietnam and adjacent areas of Eastern Indochina. Komarovia 3: 1–83. [Google Scholar]
- Axelrod DI, Al-Shehbaz I, Raven PH. (1998). History of the modern flora of China. In: Floristic characteristics and diversity of east Asian plants (Zhang AL, Wu SG. eds). China Higher Education Press/Springer, Beijing: 43–55. [Google Scholar]
- Bacon CD, Silvestro D, Jaramillo C. et al. (2015). Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proceedings of the National Academy of Sciences of the USA 112: 6110–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai RS, Thomas J. (2000). Phytophthora rot - a new disease of vanilla (Vanilla planifolia Andrews) in India. Journal of Spices and Aromatic Crops 9: 73–75. [Google Scholar]
- Bandelt H-J, Forster P, Röhl A. (1999). Median-joining networks for inferring intraspecies phylogenies. Molecular Biology and Evolution 16: 37–48. [DOI] [PubMed] [Google Scholar]
- Baskin JM, Baskin CC. (2016). Origins and relationships of the mixed mesophytic forest of Oregon-Idaho, China, and Kentucky: Review and synthesis. Annals of the Missouri Botanical Garden 101: 525–552. [Google Scholar]
- Bennett RM, Dedeles GR, Thines M. (2017). Phytophthora elongata (Peronosporaceae) is present as an estuarine species in Philippine mangroves. Mycosphere 8: 959–967. [Google Scholar]
- Bertier L, Leus L, D’hondt L. et al. (2013). Host adaptation and speciation through hybridization and polyploidy in Phytophthora. PLoS ONE 8: e85385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezuidenhout CM, Denman S, Kirk SA. et al. (2010). Phytophthora taxa associated with cultivated Agathosma, with emphasis on the P. citricola complex and P. capensis sp. nov. Persoonia 25: 32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienapfl JC, Balci Y. (2014). Movement of Phytophthora spp. in Maryland’s nursery trade. Plant Disease 98: 134–144. [DOI] [PubMed] [Google Scholar]
- Bilodeau GJ, Martin FN, Coffey MD. et al. (2014). Development of a multiplex assay for genus- and species-specific detection of Phytophthora based on differences in mitochondrial gene order. Phytopathology 104: 733–748. [DOI] [PubMed] [Google Scholar]
- Blackwell E. (1949). Terminology in Phytophthora. The Commonwealth Mycological Institute, Kew, Surrey, UK. Mycological Papers 30: 24. [Google Scholar]
- Blair JE, Coffey MD, Park S-Y. et al. (2008). A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology 45: 266–277. [DOI] [PubMed] [Google Scholar]
- Bogarín D, Pupulin F, Smets E. et al. (2016). Evolutionary diversification and historical biogeography of the Orchidaceae in Central America with emphasis on Costa Rica and Panama. Lankesteriana 16: 189–200. [Google Scholar]
- Bose T, Hammerbacher A. (2023). The first report of Phytophthora multivesiculata causing black rot of Cymbidium and Ansellia africana from South Africa. Plant Disease 107: 588. [DOI] [PubMed] [Google Scholar]
- Bose T, Hulbert JM, Burgess TI. et al. (2021a). Two novel Phytophthora species from the southern tip of Africa. Mycological Progress 20: 755–767. [Google Scholar]
- Bose T, Wingfield MJ, Roux J. et al. (2018). Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecology 36: 17–25. [Google Scholar]
- Bose T, Wingfield MJ, Roux J. et al. (2021b). Phytophthora species associated with roots of native and non-native trees in natural and managed forests. Microbial Ecology 81: 122–133. [DOI] [PubMed] [Google Scholar]
- Bouckaert R, Drummond A. (2017). bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evolutionary Biology 17: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert R, Heled J, Kühnert D. et al. (2014). BEAST 2: A software platform for bayesian evolutionary analysis. PLoS Computational Biology 10: e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret TB, Choudhury RA, Mehl HK. et al. (2018). Multiple origins of downy mildews and mito-nuclear discordance within the paraphyletic genus Phytophthora. PLoS ONE 13: e0192502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JH, Martin FN, Tooley PW. et al. (2007). Genetic and morphological diversity of temperate and tropical isolates of Phytophthora capsici. Phytopathology 97: 492–503. [DOI] [PubMed] [Google Scholar]
- Brasier CM. (1967). Physiology of reproduction in Phytophthora. PhD Thesis, University of Hull, UK. [Google Scholar]
- Brasier CM. (1971). Induction of sexual reproduction in single A2 isolates of Phytophthora species by Trichoderma viride. Nature 231: 283. [Google Scholar]
- Brasier CM. (1978). Stimulation of oospore formation in Phytophthora by antagonistic species of Trichoderma and its ecological implications. Annals of Applied Biology 89: 135–139. [Google Scholar]
- Brasier CM. (1983). Problems and prospects in Phytophthora research. In: Phytophthora: Its biology, taxonomy, ecology and pathology (Erwin DC, Bartnicki-Garcia S, Tsao PH. eds). American Phytopathological Society, St. Paul, Minnesota, USA: 353–364. [Google Scholar]
- Brasier CM. (1986). The dynamics of fungal speciation. In: Evolutionary biology of the fungi (Rayner ADM, Brasier CM, Moore D. eds). Cambridge University Press, Cambridge, UK: 231–260. [Google Scholar]
- Brasier CM. (1990). China and the origins of Dutch elm disease: An appraisal. Plant Pathology 39: 5–16. [Google Scholar]
- Brasier CM. (1992). Evolutionary biology of Phytophthora. Part I: Genetic system, sexuality and the generation of variation. Annual Review of Phytopathology 30: 153–171. [Google Scholar]
- Brasier CM. (1995). Episodic selection as a force in fungal microevolution with special reference to clonal speciation and hybrid introgression. Canadian Journal of Botany 73: 1213–1221. [Google Scholar]
- Brasier CM. (2000a). Rise of the hybrid fungi. Nature 405: 134–135. [DOI] [PubMed] [Google Scholar]
- Brasier CM. (2000b). Intercontinental spread and continuing evolution of the Dutch elm disease pathogens. In: The elms: Breeding, conservation and disease management (Dunne CP, ed.). Kluwer Academic Publishers, Boston, USA: 61–72. [Google Scholar]
- Brasier CM. (2001). Rapid evolution of introduced plant pathogens via interspecific hybridization. Bioscience 51: 123–133. [Google Scholar]
- Brasier CM. (2005). Preventing invasive pathogens: deficiencies in the system. The Plantsman (new series) 4: 54–57. [Google Scholar]
- Brasier CM. (2008). The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology 57: 792–808. [Google Scholar]
- Brasier CM. (2009). Phytophthora biodiversity: How many Phytophthora species are there? In: Phytophthoras in Forests and Natural Ecosystems: Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09 (Goheen EM, Frankel SJ. eds). USDA Forest Service, Pacific Southwest Research Station, Albany, California. General Technical Report PSW-GTR-221: 101–115. [Google Scholar]
- Brasier CM, Griffin MJ. (1979). Taxonomy of Phytophthora palmivora on cocoa. Transactions of the British Mycological Society 72: 111–143. [Google Scholar]
- Brasier CM, Rayner ADM. (1987). Whither terminology below the species level in the fungi? In: Evolutionary biology of the fungi (Rayner ADM, Brasier CM, Moore D. eds). Cambridge University Press, Cambridge, UK: 379–388. [Google Scholar]
- Brasier CM, Webber J. (2010). Sudden larch death. Nature 466: 824–825. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Beales PA, Kirk SA. et al. (2005). Phytophthora kernoviae sp. nov. an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis of ornamentals in Britain. Mycological Research 109: 853–859. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Cooke DEL, Duncan JM. (1999). Origins of a new Phytophthora pathogen through interspecific hybridisation. Proceedings of the National Academy of Sciences of the USA 96: 5878–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier CM, Cooke DEL, Duncan JM. et al. (2003). Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides – P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycological Research 107: 277–290. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Franceschini S, Forster J. et al. (2021). Enhanced outcrossing, directional selection and transgressive segregation drive evolution of novel phenotypes in hybrid swarms of the Dutch Elm Disease pathogen Ophiostoma novo-ulmi. Journal of Fungi 7: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier CM, Franceschini S, Vettraino AM. et al. (2012). Four phenotypically and phylogenetically distinct lineages in Phytophthora lateralis. Fungal Biology 116: 1232–1249. [DOI] [PubMed] [Google Scholar]
- Brasier CM, Kirk SA, Delcan J. et al. (2004). Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycological Research 108: 1172–1184. [DOI] [PubMed] [Google Scholar]
- Brasier C, Scanu B, Cooke D. et al. (2022). Phytophthora: an ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus 13: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier CM, Vettraino AM, Chang TT. et al. (2010). Phytophthora lateralis discovered in an old growth Chamaecyparis forest in Taiwan. Plant Pathology 59: 595–603. [Google Scholar]
- Brazee NJ, Wick RL, Hulvey JP. (2016). Phytophthora species recovered from the Connecticut River Valley in Massachusetts, USA. Mycologia 108: 6–19. [DOI] [PubMed] [Google Scholar]
- Brazee NJ, Yang X, Hong CX. (2017). Phytophthora caryae sp. nov., a new species recovered from streams and rivers in the eastern United States. Plant Pathology 66: 805–817. [Google Scholar]
- Brikiatis L. (2016). Late Mesozoic North Atlantic land bridges. Earth-Science Reviews 159: 47–57. [Google Scholar]
- Brockerhoff EG, Kimberley M, Liebhold AM. et al. (2014). Predicting how altering propagule pressure changes establishment rates of biological invaders across species pools. Ecology 95: 594–601. [DOI] [PubMed] [Google Scholar]
- Burgess TI. (2015). Molecular characterization of natural hybrids formed between five related indigenous Clade 6 Phytophthora species. PLoS ONE 10: e0134225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TI, Simamora AV, White D. et al. (2018). New species from Phytophthora Clade 6a: evidence for recent radiation. Persoonia 41: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TI, Dang QN, Le BV. et al. (2020). Phytophthora acaciivora sp. nov. associated with dying Acacia mangium in Vietnam. Fungal Systematics and Evolution 6: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TI, White D, McDougall KM. et al. (2017). Distribution and diversity of Phytophthora across Australia. Pacific Conservation Biology 23: 1–13. [Google Scholar]
- Burgess TI, Edwards J, Drenth A. et al. (2021). Current status of Phytophthora in Australia. Persoonia 47: 151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush MB, Colinvaux PA. (1990). A pollen record of a complete glacial cycle from lowland Panama. Journal of Vegetation Science 1: 105–118. [Google Scholar]
- Bush MB, Correa-metrio AY, Hodell DA. et al. (2009). Re-evaluation of climate change in lowland Central America during the Last Glacial Maximum using new sediment cores from Lake Petén Itzá, Guatemala. In: Past Climate Variability in South America and Surrounding Regions (Vimeux F, Sylvestre F, Khodri M. eds). Developments in Paleoenvironmental Research 14, Springer, Dordrecht: 113–128. [Google Scholar]
- Cannon CH, Morley RJ, Bush AB. (2009). The current refugial rainforests of Sundaland are unrepresentative of their biogeographic past and highly vulnerable to disturbance. Proceedings of the National Academy of Sciences of the USA 106: 11188–11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassens I, Mardulyn P, Milinkovitch MC. (2005). Evaluating intraspecific “network” construction methods using simulated sequence data: Do existing algorithms outperform the Global Maximum Parsimony approach? Systems Biology 54: 363–372. [DOI] [PubMed] [Google Scholar]
- Cerqueira AO, Luz EDMN, De Souza JT. (2006). First record of Phytophthora tropicalis causing leaf blight and fruit rot on breadfruit in Brazil. Plant Pathology 55: 296. [Google Scholar]
- Chang-Fu H, Chung-Fu S, Kuoh-Cheng Y. (1994). Introduction to the flora of Taiwan, 3: floristics, phytogeography, and vegetation. In: Flora of Taiwan. 2nd edition. Editorial Committee of the Flora of Taiwan; Second Edition, Taipei, Republic of China: 7–18. https://tai2.ntu.edu.tw/ebooks/FlTaiwan2nd/1. [Google Scholar]
- Chávez-Ramírez B, Rodríguez-Velázquez ND, Chávez-Sánchez ME. et al. (2021). Morphological and molecular identification of Phytophthora tropicalis causing black pod rot in Mexico. Canadian Journal of Plant Pathology 43: 670–679. [Google Scholar]
- Chee KH. (1969). Variability of Phytophthora species from Hevea brasiliensis. Transactions of the British Mycological Society 52: 425–436. [Google Scholar]
- Chen Q, Bakhshi M, Balci Y. et al. (2022). Genera of phytopathogenic fungi: GOPHY 4. Studies in Mycology 101: 417–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern L-L, Ann P-J, Wang I-T. (2011). Cymbidium black rot caused by an aberrant strain of Phytophthora multivesiculata in Taiwan. Plant Pathology Bulletin 20: 1–10. [Google Scholar]
- Chung-Fu S. (1994). Introduction to the flora of Taiwan, 2: geotectonic evolution, paleogeography, and the origin of the flora. In: Flora of Taiwan. 2nd edition. Editorial Committee of the Flora of Taiwan; Second Edition, Taipei, Republic of China: 3–7. https://tai2.ntu.edu.tw/ebooks/FlTaiwan2nd/1. [Google Scholar]
- Cooke DEL, Drenth A, Duncan JM. et al. (2000). A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genetics and Biology 30: 17–30. [DOI] [PubMed] [Google Scholar]
- Corcobado T, Cech TL, Brandstetter M. et al. (2020). Decline of European beech in Austria: involvement of Phytophthora spp. and contributing biotic and abiotic factors. Forests 11: 895. [Google Scholar]
- Corcobado T, Cech TL, Daxer A. et al. (2023). Phytophthora, Nothophytophthora and Halophytophthora diversity in rivers, streams and riparian alder ecosystems of Central Europe. Mycological Progress 22: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall BS, Gravatt GF, Ryan MM. (1945). Root disease of Castanea species and some coniferous and broadleaf nursery stocks, caused by Phytophthora cinnamomi. Phytopathology 35: 162–180. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI. et al. (2017). Fungal Planet description sheets: 558–624. Persoonia 38: 240–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Chooij MJ. et al. (2020). Fungal Planet description sheets: 1042–1111. Persoonia 44: 301–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Rossman AY, Aime MC. et al. (2021). Names of phytopathogenic fungi: A practical guide. Phytopathology 111: 1500–1508. [DOI] [PubMed] [Google Scholar]
- Cunnington JH, de Alwis SK, Priest M. (2009). Some notes on Phytophthora syringae and P. multivesiculata in Australia. Australasian Plant Disease Notes 4: 42–43. [Google Scholar]
- Dang QN, Pham TQ, Arentz F. et al. (2021). New Phytophthora species in clade 2a from the Asia-Pacific region including a re-examination of P. colocasiae and P. meadii. Mycological Progress 20: 111–129. [Google Scholar]
- Das AK, Nerkar S, Kumar A. et al. (2016). Detection, identification and characterization of Phytophthora spp. infecting Citrus in India. Journal of Plant Pathology 98: 55–69. [Google Scholar]
- Davis CC, Bell CD, Mathews S. et al. (2002). Laurasian migration explains Gondwanan disjunctions: Evidence from Malpighiaceae. Proceedings of the National Academy of Sciences of the USA 99: 6833–6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day WR. (1938). Root-rot of sweet chestnut and beech caused by species of Phytophthora: 1. Cause and symptoms of disease: its relation to soil conditions. Forestry 12: 101–116. [Google Scholar]
- Dehnen-Schmutz K, Holdenrieder O, Jeger MJ. et al. (2010). Structural change in the international horticultural industry: some implications for plant health. Scientia Horticulturae 125: 1–15. [Google Scholar]
- Decloquement J, Ramos-Sobrinho R, Elias SG. et al. (2021). Phytophthora theobromicola sp. nov.: A new species causing black pod disease on cacao in Brazil. Frontiers in Microbiology 12: 537399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk T, Grimm GW. (2009). The biogeographic history of beech trees. Review of Palaeobotany and Palynology 158: 83–100. [Google Scholar]
- Dick MA, Dobbie K, Cooke DEL. et al. (2006). Phytophthora captiosa sp. nov. and P. fallax sp. nov. causing crown dieback of Eucalyptus in New Zealand. Mycological Research 110: 393–404. [DOI] [PubMed] [Google Scholar]
- Dick MW. (1990). Keys to Pythium. University of Reading Press, Reading, UK. [Google Scholar]
- Donahoo RS, Lamour KH. (2008a). Characterization of Phytophthora species from leaves of nursery woody ornamentals in Tennessee. HortScience 43: 1833–1837. [Google Scholar]
- Donahoo RS, Lamour KH. (2008b). Interspecific hybridization and apomixis between Phytophthora capsici and Phytophthora tropicalis. Mycologia 100: 911–920. [DOI] [PubMed] [Google Scholar]
- Dos Santos AF. (2016). Phytophthora frigida. Forest Phytophthoras 6: 10. 5399/osu/fp.6.1.3887. http://journalsoregondigital.org/index.php/ForestPhytophthora/article/view/3887/3710 [Google Scholar]
- Dos Santos AF, Tessmann DJ, Alves TCA. et al. (2011). Root and crown rot of Brazilian pine (Araucaria angustifolia) caused by Phytophthora cinnamomi. Journal of Phytopathology 159: 194–196. [Google Scholar]
- Douglas J, Zhang R, Bouckaert R. (2021). Adaptive dating and fast proposals: revisiting the phylogenetic relaxed clock model. PLoS Computational Biology 17(2): e1008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth A, Guest DI. (eds) (2004). Diversity and management of Phytophthora in Southeast Asia. Australian Centre for International Agricultural Research, Canberra, Australia. [Google Scholar]
- Drew J, Anderson N, Andow D. (2010). Conundrums of a complex vector for invasive species control: a detailed examination of the horticultural industry. Biological Invasions 12: 2837–2851. [Google Scholar]
- Drummond AJ, Ho SYW, Phillips MJ. et al. (2006). Relaxed phylogenetics and dating with confidence. PLoS Biology 4: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan WA, Howard K, Hardy GEStJ. et al. (2016). An overview of Australia’s Phytophthora species assemblage in natural ecosystems recovered from a survey in Victoria. IMA Fungus 7: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán A, Gryzenhout M, Slippers B. et al. (2008). Phytophthora pinifolia sp. nov. associated with a serious needle disease of Pinus radiata in Chile. Plant Pathology 57: 715–727. [Google Scholar]
- Elias S, Brigham-Grette J. (2013). Late Pleistocene glacial events in Beringia. In: Encyclopedia of Quaternary Science (Elias S. ed.). 2nd edition. Elsevier, Amsterdam, Netherlands: 191–201. [Google Scholar]
- Enzenbacher TB, Naegele RP, Hausbeck MK. (2015). Susceptibility of greenhouse ornamentals to Phytophthora capsici and P. tropicalis. Plant Disease 99: 1808–1815. [DOI] [PubMed] [Google Scholar]
- Erwin DC, Ribeiro OK. (1996). Phytophthora diseases worldwide. APS Press, St. Paul, Minnesota, USA. [Google Scholar]
- Feng W, Hieno A, Otsubo K. et al. (2022). Emergence of self-fertile Phytophthora colocasiae is a possible reason for the widespread expansion and persistence of taro leaf blight in Japan. Mycological Progress 21: 49–58. [Google Scholar]
- Ferguson AJ, Jeffers SN. (1999). Detecting multiple species of Phytophthora in container mixes from ornamental crop nurseries. Plant Disease 83: 1129–1136. [DOI] [PubMed] [Google Scholar]
- Fletcher K, Klosterman SJ, Derevnina L. et al. (2018). Comparative genomics of downy mildews reveals potential adaptations to biotrophy. BMC Genomics 19: 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher K, Gil J, Bertier LD. et al. (2019). Genomic signatures of heterokaryosis in the oomycete pathogen Bremia lactucae. Nature Communications 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster ZSL, Albornoz FL, Fieland VJ. et al. (2022). A new oomycete metabarcoding method using the rps10 gene. Phytobiomes Journal 6: 214–226. [Google Scholar]
- Frankel SJ, Conforti C, Hillman J. et al. (2020). Phytophthora introductions in restoration areas: Responding to protect California native flora from human-assisted pathogen spread. Forests 11: 1291. [Google Scholar]
- Garbelotto MM, Frankel S, Scanu B. (2018). Soil- and waterborne Phytophthora species linked to recent outbreaks in northern California restoration sites. California Agriculture 72: 208–216. [Google Scholar]
- Garbelotto MM, Lee HK, Slaughter G. et al. (1997). Heterokaryosis is not required for virulence of Heterobasidion annosum. Mycologia 89: 92–102. [Google Scholar]
- Gerlach WWP, Schubert R. (2001). A new wilt of Cyclamen caused by Phytophthora tropicalis in Germany and the Netherlands. Plant Disease 85: 334. [DOI] [PubMed] [Google Scholar]
- Ghimire SR, Richardson PA, Kong P. et al. (2011). Distribution and diversity of Phytophthora species in nursery irrigation reservoir adopting water recycling system during winter months. Journal of Phytopathology 159: 713–719. [Google Scholar]
- Gibbs JN. (1978). Intercontinental epidemiology of Dutch elm disease. Annual Revue of Phytopathology 16: 287–307. [Google Scholar]
- Gibbs JN, Wainhouse D. (1986). Spread of forest pests and pathogens in the northern hemisphere. Forestry 59: 141–153. [Google Scholar]
- Ginetti B, Moricca S, Squires JN. et al. (2014). Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in Northern Italy. Plant Pathology 63: 858–876. [Google Scholar]
- Ginetti B, Ragazzi A, Carmignani S. et al. (2015). Collar rot and crown wilting by Phytophthora pachypleura on Aucuba japonica in Italian nurseries. Plant Disease 99: 1860. [Google Scholar]
- Givnish TJ. (1997). Adaptive radiation and molecular systematics: aims and conceptual issues. In: Molecular evolution and adaptive radiation (Givnish TJ, Systma KJ. eds). Cambridge University Press, New York, USA: 1–54. [Google Scholar]
- Givnish TJ. (2015). Adaptive radiation versus ‘radiation’ and ‘explosive diversification’: why conceptual distinctions are fundamental to understanding evolution. New Phytologist 207: 297–303. [DOI] [PubMed] [Google Scholar]
- Gladenkov AX, Oleinik AE, Marincovich L. et al. (2002). A refined age for the earliest opening of Bering Strait. Palaeogeography, Palaeoclimatology, Palaeoecology 183: 321–328. [Google Scholar]
- Gonthier P, Nicolotti G, Linzer R. et al. (2007). Invasion of European pine stands by a North American forest pathogen and its hybridization with a native interfertile taxon. Molecular Ecology 16: 1389–1400. [DOI] [PubMed] [Google Scholar]
- Gower DJ, Johnson KG, Richardson JE. et al. (eds) (2012). Biotic evolution and environmental change in Southeast Asia. The Systematics Association Special Volume Series, Cambridge University Press, New York, USA. [Google Scholar]
- Graham JH, Menge JA. (2000). Phytophthora-induced diseases. In: Compendium of Citrus Diseases. 2nd edition. APS Press, St Paul, MN, USA: 12–15. [Google Scholar]
- Granke L, Quesada-Ocampo L, Hausbeck M. (2013). Phytophthora capsici in the Eastern USA. In: Phytophthora a global perspective (Lamour K. ed.). CABI, Wallingford, UK: 96–103. [Google Scholar]
- Granke LL, Quesada-Ocampo L, Lamour K. et al. (2012). Advances in research on Phytophthora capsici on vegetable crops in the United States. Plant Disease 96: 1588–1600. [DOI] [PubMed] [Google Scholar]
- Gross A, Holdenrieder O, Pautasso M. et al. (2014). Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Molecular Plant Pathology 15: 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. (2017). Southeast Asia: New views of the geology of the Malay archipelago. Annual Review of Earth and Planetary Sciences 45: 331–358. [Google Scholar]
- Hansen EM, Goheen DJ, Jules ES. et al. (2000). Managing Port-Orford-cedar and the introduced pathogen Phytophthora lateralis. Plant Disease 84: 4–14. [DOI] [PubMed] [Google Scholar]
- Hansen EM, Reeser PW, Sutton W. (2012). Phytophthora beyond agriculture. Annual Review of Phytopathology 50: 359–378. [DOI] [PubMed] [Google Scholar]
- Hardham AR. (2005). Phytophthora cinnamomi. Molecular Plant Pathology 6: 589–604. [DOI] [PubMed] [Google Scholar]
- Harzhauser M, Kroh H, Mandic O. et al. (2007). Biogeographic responses to geodynamics: A key study all around the Oligo–Miocene Tethyan Seaway. Zoologischer Anzeiger 246: 241–256. [Google Scholar]
- Heaney LR. (1991). A synopsis of climatic and vegetational change in Southeast Asia. Climatic Change 19: 53–61. [Google Scholar]
- Henricot B, Pérez-Sierra A, Jung T. (2014). Phytophthora pachypleura sp. nov., a new species causing root rot of Aucuba japonica and other ornamentals in the United Kingdom. Plant Pathology 63: 1095–1109. [Google Scholar]
- Hill C. (2004). First report of Phytophthora multivesiculata on Cymbidium orchids in New Zealand. Australasian Plant Pathology 33: 603–604. [Google Scholar]
- Hinojosa LF, Armesto JJ, Villagrán C. (2006). Are Chilean coastal forests pre-Pleistocene relicts? Evidence from foliar physiognomy, palaeoclimate, and phytogeography. Journal of Biogeography 33: 331–341. [Google Scholar]
- Holt B, Lessard JP, Borregaard MK. (2013). An update of Wallace’s zoogeographic regions of the world. Science 339: 74–78. [DOI] [PubMed] [Google Scholar]
- Hong CX, Gallegly ME, Browne GT. et al. (2009). The avocado subgroup of Phytophthora citricola constitutes a distinct species, Phytophthora mengei sp. nov. Mycologia 101: 833–840. [DOI] [PubMed] [Google Scholar]
- Hong CX, Gallegly ME, Richardson PA. et al. (2011). Phytophthora pini Leonian resurrected to distinct species status. Mycologia 103: 351–360. [DOI] [PubMed] [Google Scholar]
- Hong C, Gallegly ME, Richardson PA. et al. (2008). Phytophthora irrigata, a new species isolated from irrigation reservoirs and rivers in Eastern United States of America. FEMS Microbiology Letters 285: 203–211. [DOI] [PubMed] [Google Scholar]
- Hong CX, Gallegly ME, Richardson PA. et al. (2010). Phytophthora hydropathica, a new pathogen identified from irrigation water, Rhododendron catawbiense and Kalmia latifolia. Plant Pathology 59: 913–921. [Google Scholar]
- Hong CX, Richardson PA, Hao W. et al. (2012). Phytophthora aquimorbida sp. nov. and Phytophthora taxon ‘aquatilis’ recovered from irrigation reservoirs and a stream in Virginia, USA. Mycologia 104: 1097–1108. [DOI] [PubMed] [Google Scholar]
- Hope G. (2001). Environmental change in the late Pleistocene and later Holocene at Wanda Site, Soroako, South Sulawesi, Indonesia. Palaeogeography, Palaeoclimatology, Palaeoecology 171: 129–145. [Google Scholar]
- Hope G, Kershaw AP, van der Kaars S. et al. (2004). History of vegetation and habitat change in the Austral-Asian region. Quaternary International 118–119: 103–126. [Google Scholar]
- Hopple JS, Vilgalys R. (1994). Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 86: 96–107. [Google Scholar]
- Hu J, Shrestha S, Zhou Y. et al. (2020). Dynamic extreme aneuploidy (DEA) in the vegetable pathogen Phytophthora capsici and the potential for rapid asexual evolution. PLoS ONE 15: e0227250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai WX, Tian G, Hansen EM. et al. (2013). Identification of Phytophthora species baited and isolated from forest soil and streams in northwestern Yunnan province, China. Forest Pathology 43: 87–103. [Google Scholar]
- Hüberli D. (1995). Analysis of variability among isolates of Phytophthora cinnamomi Rands from Eucalyptus marginata Donn ex Sm. and E. calophylla R. Br. based on cultural characteristics, sporangia and gametangia morphology, and pathogenicity. Bachelor thesis. Murdoch University, Murdoch, Australia. [Google Scholar]
- Hüberli D, Hardy GEStJ, White D. et al. (2013). Fishing for Phytophthora from Western Australia’s waterways: a distribution and diversity survey. Australasian Plant Pathology 42: 251–260. [Google Scholar]
- Hubert F, Grimm GW, Jousselin E. et al. (2014). Multiple nuclear genes stabilize the phylogenetic backbone of the genus Quercus. Systematics and Biodiversity 12: 405–423. [Google Scholar]
- Hueck K. (1966). Die Wälder Südamerikas (‘The forests of South America’). Gustav Fischer, Stuttgart, Germany. [Google Scholar]
- Huntley B. (1993). Species-richness in north-temperate zone forests. Journal of Biogeography 20: 163–180. [Google Scholar]
- Husson C, Aguayo J, Revellin C. et al. (2015). Evidence for homoploid speciation in Phytophthora alni supports taxonomic reclassification in this species complex. Fungal Genetics and Biology 77: 12–21. [DOI] [PubMed] [Google Scholar]
- Ilieva E, Man In’t Veld WA, Veenbaas-Rijks W. et al. (1998). Phytophthora multivesiculata, a new species causing rot in Cymbidium. European Journal of Plant Pathology 104: 677–684. [Google Scholar]
- Jankowiak R, Stępniewska H, Bilański P. et al. (2014). Occurrence of Phytophthora plurivora and other Phytophthora species in oak forests of southern Poland and their association with site conditions and the health status of trees. Folia Microbiologica 59: 531–542. [DOI] [PubMed] [Google Scholar]
- Jayasekera AU, McComb JA, Shearer BL. et al. (2007). In planta selfing and oospore production of Phytophthora cinnamomi in the presence of Acacia pulchella. Mycological Research 111: 355–362. [DOI] [PubMed] [Google Scholar]
- Jung T. (2009). Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. Forest Pathology 39: 73–94. [Google Scholar]
- Jung T, Burgess TI. (2009). Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 22: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Blaschke M. (2004). Phytophthora root and collar rot of alders in Bavaria: distribution, modes of spread and possible management strategies. Plant Pathology 53: 197–208. [Google Scholar]
- Jung T, Nechwatal J. (2008). Phytophthora gallica sp. nov., a new species from rhizosphere soil of declining oak and reed stands in France and Germany. Mycological Research 112: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Jung T, Balci Y, Broders KD. et al. (2023). Synchrospora gen. nov., a new Peronosporaceae genus with aerial lifestyle from a natural cloud forest in Panama. Journal of Fungi 9: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Blaschke H, Neumann P. (1996). Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. European Journal of Forest Pathology 26: 253–272. [Google Scholar]
- Jung T, Chang TT, Bakonyi J. et al. (2017b). Diversity of Phytophthora species in natural ecosystems of Taiwan and association with disease symptoms. Plant Pathology 66: 194–211. [Google Scholar]
- Jung T, Colquhoun IJ, Hardy GEStJ. (2013b). New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomi in different natural ecosystems in Western Australia. Forest Pathology 43: 266–288. [Google Scholar]
- Jung T, Durán A, Sanfuentes von Stowasser E. et al. (2018b). Diversity of Phytophthora species in Valdivian rainforests and association with severe dieback symptoms. Forest Pathology 48: e12443. [Google Scholar]
- Jung T, Hansen EM, Winton L. et al. (2002). Three new species of Phytophthora from European oak forests. Mycological Research 106: 397–411. [Google Scholar]
- Jung T, Horta Jung M, Cacciola SO. et al. (2017d). Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 8: 219–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Horta Jung M, Scanu B. et al. (2017c). Six new Phytophthora species from ITS Clade 7a including two sexually functional heterothallic hybrid species detected in natural ecosystems in Taiwan. Persoonia 38: 100–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Horta Jung M, Webber JF. et al. (2021). The destructive tree pathogen Phytophthora ramorum originates from the Laurosilva forests of East Asia. Journal of Fungi 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Hudler GW, Jensen-Tracy SL. et al. (2005). Involvement of Phytophthora spp. in the decline of European beech in Europe and the USA. Mycologist 19: 159–166. [Google Scholar]
- Jung T, La Spada F, Pane A. et al. (2019). Diversity and distribution of Phytophthora species in protected natural areas in Sicily. Forests 10: 259. [Google Scholar]
- Jung T, Milenković I, Corcobado T, Májek T. et al. (2022). Extensive morphological and behavioural diversity among fourteen new and seven described species in Phytophthora Clade 10 and its evolutionary implications. Persoonia 49: 1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Nechwatal J, Cooke DEL. et al. (2003). Phytophthora pseudosyringae sp. nov., a new species causing root and collar rot of deciduous tree species in Europe. Mycological Research 107: 772–789. [DOI] [PubMed] [Google Scholar]
- Jung T, Orlikowski L, Henricot B. et al. (2016). Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. Forest Pathology 46: 134–163. [Google Scholar]
- Jung T, Pérez–Sierra A, Durán A. et al. (2018a). Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia 40: 182–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Scanu B, Bakonyi J. et al. (2017a). Nothophytophthora gen. nov., a new sister genus of Phytophthora from natural and semi-natural ecosystems. Persoonia 39: 143–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Scanu B, Brasier CM. et al. (2020). A survey in natural forest ecosystems of Vietnam reveals high diversity of both new and described Phytophthora taxa including P. ramorum. Forests 11: 93. [Google Scholar]
- Jung T, Stukely MJC, Hardy GEStJ. et al. (2011). Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: evolutionary and ecological implications. Persoonia 26: 13–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Vettraino AM, Cech TL. et al. (2013a). The impact of invasive Phytophthora species on European forests. In: Phytophthora a global perspective (Lamour K. ed.). CABI, Wallingford, UK: 146–158. [Google Scholar]
- Katoh K, Standley DM. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenaley SC, Rose C, Sullivan PJ. et al. (2014). Bleeding canker of European beech in southeastern New York State: Phytophthora species, spatial analysis of disease, and periodic growth of affected trees. Journal of Environmental Horticulture 32: 113–125. [Google Scholar]
- Kim S, de Medeiros BAS, Byun B-K. et al. (2018). West meets East: How do rainforest beetles become circum-Pacific? Evolutionary origin of Callipogon relictus and allied species (Cerambycidae: Prioninae) in the New and Old Worlds. Molecular Phylogenetics and Evolution 125: 163–176. [DOI] [PubMed] [Google Scholar]
- Knaus B, Fieland V, Graham K. et al. (2015). Diversity of foliar Phytophthora species on Rhododendron in Oregon nurseries. Plant Disease 99: 1326–1332. [DOI] [PubMed] [Google Scholar]
- Ko WH. (1979). Mating-type distribution of Phytophthora colocasiae on the island of Hawaii. Mycologia 71: 434–437. [Google Scholar]
- Kone A, Kofke DA. (2005). Selection of temperature intervals for paralleltempering simulations. Journal of Chemical Physics 122: 1–2. [DOI] [PubMed] [Google Scholar]
- Kong JC, Thanh TAV, Yeo FKS. et al. (2022). Characterisation of Phytophthora capsici causing foot rot of black pepper (Piper nigrum L.) in Julau, Sarawak. Archives of Phytopathology and Plant Protection 55: 1686–1712. [Google Scholar]
- Kozak KH, Weisrock DR, Larson R. (2006). Rapid lineage accumulation in a non-adaptive radiation: phylogenetic analysis of diversification rates in eastern North American woodland salamanders (Plethodontidae: Plethodon). Proceedings of the Royal Society B 273: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov A, Darriba D, Flouri T. et al. (2019). RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35: 4453–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Joseph L, Roy CB. (2019). An insight into Hevea - Phytophthora interaction: The story of Hevea defense and Phytophthora counter defense mediated through molecular signalling. Current Plant Biology 17: 33–41. [Google Scholar]
- Kroon LPNM, Bakker FT, van den Bosch GBM. et al. (2004). Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genetics and Biology 41: 766–782. [DOI] [PubMed] [Google Scholar]
- Lamour K. (ed.) (2013). Phytophthora a global perspective. CABI, Wallingford, UK: 96 pp. [Google Scholar]
- Lanfear R, Frandsen P, Wright A. et al. (2016). PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. [DOI] [PubMed] [Google Scholar]
- Lang P, Dane F, Kubisiak TL. et al. (2007). Molecular evidence for an Asian origin and a unique westward migration of species in the genus Castanea via Europe to North America. Molecular Phylogenetics and Evolution 43: 49–59. [DOI] [PubMed] [Google Scholar]
- Latham RE, Ricklefs RE. (1993). Continental comparison of temperatezone tree species richness. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives (Ricklefs RE, Schluter D. eds). The University of Chicago Press, Chicago: 294–314. [Google Scholar]
- Laumonier Y. (1997). The vegetation and physiography of Sumatra. Geobotany book series vol. 22, Springer Science and Business Media, Dordrecht, Germany: 223 pp. [Google Scholar]
- Leahy RM. (2006). Phytophthora Blight of Pothos. Plant Pathology Circular No. 401, Florida Department of Agriculture & Conservation Services, Division of Plant Industry: 4 pp. [Google Scholar]
- Legeay J, Husson C, Boudier B. et al. (2020). Surprising low diversity of the plant pathogen Phytophthora in Amazonian forests. Environmental Microbiology 22: 5019–5032. [DOI] [PubMed] [Google Scholar]
- Lemoine F, Domelevo Entfellner J, Wilkinson E. et al. (2018). Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 556: 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonberger AJ, Speers C, Ruhl G. et al. (2013). A survey of Phytophthora spp. in Midwest nurseries, greenhouses, and landscapes. Plant Disease 97: 635–640. [DOI] [PubMed] [Google Scholar]
- Leonian LH. (1925). Physiological studies on the genus Phytophthora. American Journal of Botany 12: 444–498. [Google Scholar]
- Liebhold AM, Brockerhoff EG, Garrett LJ. et al. (2012). Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Frontiers in Ecology and Environment 10: 135–143. [Google Scholar]
- Li C, Hongjin D, Hua P. (2013). Diversity and distribution of higher plants in Yunnan, China. Biodiversity Science 21: 359–363. [Google Scholar]
- Lilja A, Rytkönen A, Hantula J. (2011). Introduced pathogens found on ornamentals, strawberry and trees in Finland over the past 20 years. Agricultural and Food Science 20: 74–85. [Google Scholar]
- Lin MJ, Ko WH. (2008). Occurrence of isolates of Phytophthora colocasiae in Taiwan with homothallic behavior and its significance. Mycologia 100: 727–734. [DOI] [PubMed] [Google Scholar]
- Linaldeddu BT, Bregant C, Montecchio L. et al. (2020). First report of Phytophthora acerina, P. pini, and P. plurivora causing root rot and sudden death of olive trees in Italy. Plant Disease 103: 996. [Google Scholar]
- Liu AH, Shang J, Zhang JW. et al. (2018). Canker and fine-root loss of Malus sieversii (Ldb.) Roem. Caused by Phytophthora plurivora in Xinjiang Province in China. Forest Pathology 48: e12462. [Google Scholar]
- Liu D-X, Zhao W-X, Xia J-P. et al. (2022). First report of root rot caused by Phytophthora acerina on Metasequoia glyptostroboides in China. Plant Disease 106: 2270. [DOI] [PubMed] [Google Scholar]
- Lohman DJ, de Bruyn M, Page T. et al. (2011). Biogeography of the Indo-Australian Archipelago. Annual Review of Ecology, Evolution, and Systematics 42: 205–226. [Google Scholar]
- Loyd AL, Benson DM, Ivors KL. (2014). Phytophthora populations in nursery irrigation water in relationship to pathogenicity and infection frequency of Rhododendron and Pieris. Plant Disease 98: 1213–1220. [DOI] [PubMed] [Google Scholar]
- Luna Vega I, Ayala OA, Organista DE. et al. (1999). Historical relationships of the Mexican cloud forests: a preliminary vicariance model applying Parsimony Analysis of Endemicity to vascular plant taxa. Journal of Biogeography 26: 1299–1305. [Google Scholar]
- Luongo L, Vitale S, Galli M. et al. (2016). Morphological and molecular identification of Phytophthora tropicalis as causal agent of crown and root rot on Albizia julibrissin. Journal of Phytopathology 164: 959–966. [Google Scholar]
- MacDonald JD, Ali-Shtayeh MS, Kabashima J. et al. (1994). Occurrence of Phytophthora species in recirculated nursery irrigation effluents. Plant Disease 78: 607–611. [Google Scholar]
- MacKinnon K, Hatta G, Halim H. et al. (1997). The Ecology of Kalimantan. Ecology of Indonesia Series, vol. III, Oxford University Press, Oxford, UK. [Google Scholar]
- MacMahon PJ, Purwantara A. (2004). Phytophthora on cocoa. In: Diversity and Management of Phytophthora in Southeast Asia (Drenth A, Guest DI. eds). Australian Centre for International Agricultural Research, Canberra, Australia: 104–115. [Google Scholar]
- Man in't Veld WA, Rosendahl KCHM, Hong C. (2012). Phytophthora x serendipita sp. nov. and P. x pelgrandis, two destructive pathogens generated by natural hybridization. Mycologia 104: 1390–1396. [DOI] [PubMed] [Google Scholar]
- Man in't Veld WA, Rosendahl KCHM, van Rijswick PCJ. et al. (2015). Phytophthora terminalis sp. nov. and Phytophthora occultans sp. nov., two invasive pathogens of ornamental plants in Europe. Mycologia 107: 54–65. [DOI] [PubMed] [Google Scholar]
- Marks GC, Smith IW. (1991). The cinnamon fungus in Victorian forests. History, distribution, management and control. Lands and Forests Bulletin No. 31, Department of Conservation and Environment, Melbourne, Australia: 33 pp. [Google Scholar]
- Martin FN, Tooley PW. (2003). Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 95: 269–284. [PubMed] [Google Scholar]
- Mascheretti S, Croucher PJ, Vettraino A. et al. (2008). Reconstruction of the sudden oak death epidemic in California through microsatellite analysis of the pathogen Phytophthora ramorum. Molecular Ecology 11: 2755–2768. [DOI] [PubMed] [Google Scholar]
- Maseko B, Burgess TI, Coutinho TA. et al. (2007). Two new Phytophthora species from South African Eucalyptus plantations. Mycological Research 111: 1321–1338. [DOI] [PubMed] [Google Scholar]
- Matsiakh I, Kramarets V, Cleary M. (2020). Occurrence and diversity of Phytophthora species in declining broadleaf forests in western Ukraine. Forest Pathology 51: e12662. [Google Scholar]
- Mayr E. (1942). Systematics and the origin of species. Columbia University Press, New York. [Google Scholar]
- McCarthy CGP, Fitzpatrick DA. (2017). Phylogenomic reconstruction of the oomycete phylogeny derived from 37 genomes. mSphere 2: e00095–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchau GRA, Coffey MD. (1994). An integrated study of morphology and isozyme patterns found within a worldwide collection of Phytophthora citrophthora and a redescription of the species. Mycological Research 98: 1291–1299. [Google Scholar]
- Mchau GRA, Coffey MD. (1995). Evidence for the existence of two subpopulations in Phytophthora capsici and a redescription of the species. Mycological Research 99: 89–102. [Google Scholar]
- McKenna MC. (1983). Cenozoic paleogeography of North Atlantic land bridges. In: Structure and development of the Greenland-Scotland Ridge (Bott MHP, Saxov S, Talwani M. et al., eds). Plenum Press, New York: 351–399. [Google Scholar]
- McRae W. (1918). Phytophthora meadii n. sp. on Hevea brasiliensis. Memoirs of the Department of Agriculture in India. Botanical Series 9: 219–273. [Google Scholar]
- Meitz-Hopkins JC, Pretorius MC, Spies CFJ. (2014). Phytophthora species distribution in South African citrus production regions. European Journal of Plant Pathology 138: 733–749. [Google Scholar]
- Michaux B. (2010). Biogeology of Wallacea: geotectonic models, areas of endemism, and natural biogeographical units. Biological Journal of the Linnean Society 101: 193–212. [Google Scholar]
- Migliorini D, Khdiar MY, Rodríguez-Padrón C. et al. (2019). Extending the host range of Phytophthora multivora, a pathogen of woody plants in horticulture, nurseries, urban environments and natural ecosystems. Urban Forestry & Urban Greening 46: 126460. [Google Scholar]
- Milenković I, Keča N, Karadžić D. et al. (2012). Incidence of Phytophthora species in beech stands in Serbia. Folia Forestalia Polonica, series A 54: 223–232. [Google Scholar]
- Milenković I, Keča N, Karadžić D. et al. (2018). Isolation and pathogenicity of Phytophthora species from poplar plantations in Serbia. Forests 9: 330. [Google Scholar]
- Miyasaka SC, Lamour K, Shintaku M. et al. (2013). Taro leaf blight caused by Phytophthora colocasiae. In: Phytophthora a global perspective (Lamour K. ed.). CABI, Wallingford, UK: 104–112. [Google Scholar]
- Moralejo E, Pérez-Sierra A, Alvarez LA. et al. (2009). Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathology 58: 100–110. [Google Scholar]
- Mora-Sala B, León M, Pérez-Sierra A. et al. (2022). New reports of Phytophthora species in plant nurseries in Spain. Pathogens 11: 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Moreau M. (1952). Etude mycologique de la maladie de l’encre du chêne. Revue de Pathologie Vegetale 31: 201–231. [Google Scholar]
- Morley JR. (2003). Interplate dispersal paths for megathermal angiosperms. Perspectives in Plant Ecology, Evolution and Systematics 6: 5–20. [Google Scholar]
- Mrázková M, Černý K, Tomšovský M. et al. (2013). Occurrence of Phytophthora multivora and Phytophthora plurivora in the Czech Republic. Plant Protection Science 49: 155–164. [Google Scholar]
- Müller N, Bouckaert R. (2020). Adaptive parallel tempering for BEAST 2. bioRxiv 10.1101/603514. [Google Scholar]
- Mullet MS, Van Poucke K, Haegemann A. et al. (2023). Phylogeography and population structure of the global, wide host-range hybrid pathogen Phytophthora ×cambivora. IMA Fungus 14: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CW. (1969). The ecological geography of cloud forests in Panama. American Museum Novitates 2396: 1–52. [Google Scholar]
- Nagel JH, Gryzenhout M, Slippers B. et al. (2013). Characterization of Phytophthora hybrids from ITS clade 6 associated with riparian ecosystems in South Africa and Australia. Fungal Biology 117: 329–347. [DOI] [PubMed] [Google Scholar]
- Nath VS, Senthil M, Hedge VM. et al. (2013). Genetic diversity of Phytophthora colocasiae isolates in India based on AFLP analysis. 3 Biotech 3: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechwatal J, Hahn J, Schönborn A. et al. (2011). A twig blight of understorey European beech (Fagus sylvatica) caused by soilborne Phytophthora spp. Forest Pathology 41: 493–500. [Google Scholar]
- Nechwatal J, Schubert R, Gerlach WWP. (2014). A presumably exotic Phytophthora species causing dieback of Buxus sempervirens in Germany and Romania. Journal of Plant Diseases and Protection 121: 193–201. [Google Scholar]
- Newcombe G, Stirling B, McDonald S. et al. (2000). Melampsora x columbiana, a natural hybrid of M. medusae and M. occidentalis. Mycological Research 104: 261–274. [Google Scholar]
- O’Hanlon R, Choiseul J, Corrigan M. et al. (2016). Diversity and detections of Phytophthora species from trade and nontrade environments in Ireland. Bulletin OEPP/EPPO Bulletin 46: 594–602. [Google Scholar]
- Oh E, Gryzenhout M, Wingfield BD. et al. (2013). Surveys of soil and water reveal a goldmine of Phytophthora diversity in South African natural ecosystems. IMA Fungus 4: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlikowski LB, Ptaszek M, Rodziewicz A. et al. (2011). Phytophthora root and collar rot of mature Fraxinus excelsior in forest stands in Poland and Denmark. Forest Pathology 41: 510–519. [Google Scholar]
- Oudemans P, Coffey MD. (1991). A revised systematics of twelve papillate Phytophthora species based on isozyme analysis. Mycological Research 95: 1025–1046. [Google Scholar]
- Oudemans P, Förster H, Coffey MD. (1994). Evidence for distinct isozyme subgroups within Phytophthora citricola and close relationships with P. capsici and P. citrophthora. Mycological Research 98: 189–199. [Google Scholar]
- Pane A, Cacciola SO, Scibetta S. et al. (2009). Four Phytophthora species causing foot and root rot of apricot in Italy. Plant Disease 93: 844. [DOI] [PubMed] [Google Scholar]
- Paoletti M, Buck KW, Brasier CM. (2006). Selective acquisition of novel mating type and vegetative incompatibility genes via interspecies gene transfer in the globally invading eukaryote Ophiostoma novo-ulmi. Molecular Ecology 14: 249–263. [DOI] [PubMed] [Google Scholar]
- Parke JL, Knaus BJ, Fieland VJ. et al. (2014). Phytophthora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology 104: 1052–1062. [DOI] [PubMed] [Google Scholar]
- Pattengale ND, Alipour M, Bininda-Emonds ORP. et al. (2010). How many bootstrap replicates are necessary? Journal of Computational Biology 17: 337–354. [DOI] [PubMed] [Google Scholar]
- Patil B, Hedge V, Sridhara S. et al. (2022). Multigene phylogeny and haplotype analysis reveals predominance of oomycetous fungus, Phytophthora meadii (McRae) associated with fruit rot disease of arecanut in India. Saudi Journal of Biological Sciences 29: 103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sierra A, Jung T. (2013). Phytophthora in woody ornamental nurseries. In: Phytophthora a global perspective (Lamour K. ed.). CABI, Wallingford, UK: 166–177. [Google Scholar]
- Pérez-Sierra A, Kalantarzadeh M, Sancisi-Frey S. et al. (2015). Phytophthora siskiyouensis causing stem lesions and cankers on Alnus incana. New Disease Reports 31: 17. [Google Scholar]
- Pérez-Sierra A, Horta Jung M, Jung T. (2022). Survey and monitoring of Phytophthora species in natural ecosystems: Methods for sampling, isolation, purification, storage, and pathogenicity tests. In: Plant Pathology. Methods in Molecular Biology, vol. 2536 (Luchi N. ed.). Humana, New York, NY, USA: 23–49. [DOI] [PubMed] [Google Scholar]
- Peries OS, Dantanarayana DM. (1965). Compatibility and variation in Phytophthora cultures from Hevea brasiliensis in Ceylon. Transactions of the British Mycological Society 48: 631–637. [Google Scholar]
- Peries OS, Fernando TM. (1966). Studies on the biology of Phytophthora meadii. Transactions of the British Mycological Society 49: 311–325. [Google Scholar]
- Piperno DR. (2006). Quaternary environmental history and agricultural impact on vegetation in Central America. Annals of the Missouri Botanical Garden 93: 274–296. [Google Scholar]
- Posada D, Crandall KA. (2001). Intraspecific gene genealogies: Trees grafting into networks. Trends in Ecology and Evolution 16: 37–45. [DOI] [PubMed] [Google Scholar]
- Prigigallo MI, Mosca S, Cacciola SO. et al. (2015). Molecular analysis of Phytophthora diversity in nursery-grown ornamental and fruit plants. Plant Pathology 64: 1308–1319. [Google Scholar]
- Puglisi I, De Patrizio A, Schena L. et al. (2017). Two previously unknown Phytophthora species associated with brown rot of Pomelo (Citrus grandis) fruits in Vietnam. PLoS ONE 12: e0172085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puno VI, Laurence MH, Guest DI. et al. (2015). Detection of Phytophthora multivora in the Wollemi Pine site and pathogenicity to Wollemia nobilis. Australasian Plant Pathology 44: 205–215. [Google Scholar]
- Qian H. (2002). Floristic relationships between Eastern Asia and North America: Test of Gray’s hypothesis. The American Naturalist 160: 317–332. [DOI] [PubMed] [Google Scholar]
- Quesada-Ocampo LM, Parada-Rojas CH, Hansen Z. et al. (2023). Phytophthora capsici: Recent progress on fundamental biology and disease management 100 years after its description. Annual Review of Phytopathology 61: 185–208. [DOI] [PubMed] [Google Scholar]
- Rahman MZ, Uematsu S, Suga H. et al. (2015). Diversity of Phytophthora species newly reported from Japanese horticultural production. Mycoscience 56: 443–459. [Google Scholar]
- Rajalakshmy VK, Joseph A, Arthassery S. (1985). Occurrence of two mating groups in Phytophthora meadii causing abnormal leaf fall disease of rubber in South India. Transactions of the British Mycological Society 85: 723–725. [Google Scholar]
- Rambaut A, Drummond A, Xie D. et al. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez Martínez J, Cárdenas Toquica M, Guevara-Suarez M. et al. (2021). Oomycete species associated with Theobroma cacao crops in Colombia. Plant Pathology 70: 1695–1707. [Google Scholar]
- Rea AJ, Jung T, Burgess TI. et al. (2010). Phytophthora elongata sp. nov. a novel pathogen from the Eucalyptus marginata forest of Western Australia. Australasian Plant Pathology 39: 477–491. [Google Scholar]
- Reeser PW, Hansen EM, Sutton W. (2007). Phytophthora siskiyouensis, a new species from soil, water, myrtlewood (Umbellularia californica) and tanoak (Lithocarpus densiflorus) in southwestern Oregon. Mycologia 99: 639–643. [DOI] [PubMed] [Google Scholar]
- Reeser PW, Sutton W, Hansen EM. et al. (2011). Phytophthora species in forest streams in Oregon and Alaska. Mycologia 103: 22–35. [DOI] [PubMed] [Google Scholar]
- Reeser PW, Sutton W, Hansen EM. et al. (2012). A new Phytophthora species in ITS Clade 2 killing Ceanothus grown for rehabilitation of disturbed forest sites. In: Phytophthora in Forests and Natural Ecosystems, Proceedings of the 6th Meeting of IUFRO Working Party 7.02.09 (Jung T, Brasier CM, Sánchez ME. et al., eds). University of Córdoba, Spain: 104. [Google Scholar]
- Reeves RJ, Jackson RM. (1974). Stimulation of sexual reproduction in Phytophthora by damage. Transactions of the British Mycological Society 84: 303–310. [Google Scholar]
- Reyes-Tena A, Huguet-Tapia JC, Lamour KH. et al. (2019). Genome sequence data of six isolates of Phytophthora capsici from Mexico. Molecular Plant-Microbe Interactions 32: 1267–1269. [DOI] [PubMed] [Google Scholar]
- Richardson JE, Costion CM, Muellner AN. (2012). The Malesian floristic interchange: plant migration patterns across Wallace’s Line. In: Biotic evolution and environmental change in Southeast Asia (Gower DJ, Johnson KG, Richardson JE. et al., eds). The Systematics Association, Special Volume 82, Cambridge University Press, New York, USA: 138–163. [Google Scholar]
- Riddell CE, Dun HF, Elliot M. et al. (2020). Detection and spread of Phytophthora austrocedri within infected Juniperus communis woodland and diversity of co-associated Phytophthoras as revealed by metabarcoding. Forest Pathology 50: e12602. [Google Scholar]
- Rizzo DM, Garbelotto M, Davidson JM. et al. (2002). Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Disease 86: 205–214. [DOI] [PubMed] [Google Scholar]
- Roberts PD, Urs RR, French-Monar RD. et al. (2005). Survival and recovery of Phytophthora capsici and oomycetes in tailwater and soil from vegetable fields in Florida. Annals of Applied Biology 146: 351–359. [Google Scholar]
- Robideau GP, De Cock AW, Coffey MD. et al. (2011). DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Molecular Ecology Resources 11: 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Padrón C, Siverio F, Pérez-Sierra A. et al. (2018). Isolation and pathogenicity of Phytophthora species and Phytopythium vexans recovered from avocado orchards in the Canary Islands, including Phytophthora niederhauserii as a new pathogen of avocado. Phytopathologia Mediterranea 57: 89–106. [Google Scholar]
- Rojic SV, Cancino EL. (1975). Phytophthora cinnamomi Rands and Ph. citrophthora (Smith et Smith) Leonian, causal agents of walnut (Juglans regia) collar rot in Chile. Investigación agrícola 3: 201. [Google Scholar]
- Rooney-Latham S, Blomquist CL, Kosta KL. et al. (2019). Phytophthora species are common on nursery stock grown for restoration and revegetation purposes in California. Plant Disease 103: 448–455. [DOI] [PubMed] [Google Scholar]
- Roos MC, Keßler PJA, Gradstein SR. et al. (2004). Species diversity and endemism of five major Malesian islands: diversity–area relationships. Journal of Biogeography 31: 1893–1908. [Google Scholar]
- Rossmann S, Lysøe E, Skogen M. et al. (2021). DNA metabarcoding reveals broad presence of plant pathogenic oomycetes in soil from internationally traded plants. Frontiers in Microbiology 12: 637068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovito SM, Vásquez-Almazán CR, Papenfuss CJ. et al. (2015). Biogeography and evolution of Central American cloud forest salamanders (Caudata: Plethodontidae: Cryptotriton), with the description of a new species. Zoological Journal of the Linnean Society 175: 150–166. [Google Scholar]
- Roy BA, Alexander HM, Davidson J. et al. (2014). Increasing forest loss worldwide from invasive pests requires new trade regulations. Frontiers in Ecology and the Environment 12: 457–465. [Google Scholar]
- Ruano-Rosa D, Schena L, Agosteo GE. et al. (2018). Phytophthoraytophthora oleae sp. nov. causing fruit rot of olive in southern Italy. Plant Pathology 67: 1362–1373. [Google Scholar]
- Rull V. (2011). Neotropical biodiversity: timing and potential drivers. Trends in Ecology and Evolution 26: 508–513. [DOI] [PubMed] [Google Scholar]
- Rundell RJ, Price TD. (2009). Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends in Ecology and Evolution 24: 394–399. [DOI] [PubMed] [Google Scholar]
- Runge F, Telle S, Ploch S. et al. (2011). The inclusion of downy mildews in a multi-locus-dataset and its reanalysis reveals a high degree of paraphyly in Phytophthora. IMA Fungus 2: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone A, Scarito G, Pane A. et al. (2011). Root and basal stem rot of rose caused by Phytophthora citrophthora in Italy. Plant Disease 95: 358. [DOI] [PubMed] [Google Scholar]
- Sanogo S, Bosland PW. (2013). Biology and management of Phytophthora capsici in the South-western USA. In: Phytophthora a global perspective (Lamour K. ed.). CABI, Wallingford, UK: 87–95. [Google Scholar]
- Sanogo S, Lamour K, Kousik S. et al. (2023). Phytophthora capsici, 100 years later: Research mile markers from 1922 to 2022. Phytopathology 113: 921–930. [DOI] [PubMed] [Google Scholar]
- Sansome ER. (1980). Reciprocal translocation heterozygosity in heterothallic species of Phytophthora and its significance. Transactions of the British Mycological Society 74: 175–185. [Google Scholar]
- Sansome ER, Brasier CM, Hamm PB. (1990). Phytophthora meadii may be a species hybrid. Mycological Research 95: 273–277. [Google Scholar]
- Santini A, Ghelardini L, De Pace C. et al. (2013). Biogeographic patterns and determinants of invasion by alien forest pathogens in Europe. New Phytologist 197: 238–250. [DOI] [PubMed] [Google Scholar]
- Savita GSV, Nagpal A. (2012). Citrus diseases caused by Phytophthora species. GERF Bulletin of Biosciences 3: 18–27. [Google Scholar]
- Scanu B, Hunter GC, Linaldeddu BT. et al. (2014). A taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. Forest Pathology 44: 1–20. [Google Scholar]
- Scanu B, Jung T, Masigol H. et al. (2021). Phytophthora heterospora sp. nov., a new pseudoconidia-producing sister species of P. palmivora. Journal of Fungi 7: 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu B, Linaldeddu BT, Deidda A. et al. (2015). Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 10: e0143234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu B, Webber JF. (2016). Dieback and mortality of Nothofagus in Britain: ecology, pathogenicity and sporulation potential of the causal agent Phytophthora pseudosyringae. Plant Pathology 65: 26–36. [Google Scholar]
- Scarlett K, Daniel R, Shuttleworth LA. et al. (2015). Phytophthora in the Gondwana Rainforests of Australia World Heritage Area. Australasian Plant Pathology 44: 335–348. [Google Scholar]
- Schardle CL, Craven KD. (2003). Interspecific hybridization in plant-associated fungi and oomycetes: a review. Molecular Ecology 12: 2861–2873. [DOI] [PubMed] [Google Scholar]
- Schöbel CN, Stewart J, Gruenwald NJ. et al. (2014). Population history and pathways of spread of the plant pathogen Phytophthora plurivora. PLoS ONE 9: e85368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingle BW, Smith JA, Blanchette RA. (2007). Phytophthora species associated with diseased woody ornamentals in Minnesota nurseries. Plant Disease 91: 97–102. [DOI] [PubMed] [Google Scholar]
- Scott P, Williams N. (2014). Phytophthora diseases in New Zealand forests. New Zealand Journal of Forestry 59: 14–21. [Google Scholar]
- Scott P, Bader MK-F, Burgess T. et al. (2019). Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environmental Science & Policy 101: 175–182. [Google Scholar]
- Scott PM, Burgess TI, Barber PA. et al. (2009). Phytophthora multivora sp. nov., a new species recovered from declining Eucalyptus, Banksia, Agonis and other plant species in Western Australia. Persoonia 22: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PM, Jung T, Shearer BL. et al. (2012). Pathogenicity of Phytophthora multivora to Eucalyptus gomphocephala and Eucalyptus marginata. Forest Pathology 42: 289–298. [Google Scholar]
- Seddaiu S, Brandano A, Ruiu PA. et al. (2020). An overview of Phytophthora species inhabiting declining Quercus suber stands in Sardinia (Italy). Forests 11: 971. [Google Scholar]
- Seddaiu S, Linaldeddu BT. (2020). First report of Phytophthora acerina, P. plurivora, and P. pseudocryptogea associated with declining common alder trees in Italy. Plant Disease 104: 1874. [Google Scholar]
- Seibert P. (1996). Farbatlas Südamerika - Landschaften und Vegetation (‘Colour atlas South America - landscapes and vegetation’). Eugen Ulmer, Stuttgart, Germany: 288 pp. [Google Scholar]
- Shakya SK, Grünwald NJ, Fieland VJ. et al. (2021). Phylogeography of the wide-host range panglobal plant pathogen Phytophthora cinnamomi. Molecular Ecology 30: 5164–5178. [DOI] [PubMed] [Google Scholar]
- Sharma PP, Giribet G. (2012). Out of the Neotropics: Late Cretaceous colonization of Australasia by American arthropods. Proceedings of the Royal Society B 279: 3501–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Hu J, Fryxell RT. et al. (2014). SNP markers identify widely distributed clonal lineages of Phytophthora colocasiae in Vietnam, Hawaii and Hainan Island, China. Mycologia 106: 676–685. [DOI] [PubMed] [Google Scholar]
- Shrestha SK, Zhou Y, Lamour K. (2013). Oomycetes baited from streams in Tennessee 2010–2012. Mycologia 105: 1516–1523. [DOI] [PubMed] [Google Scholar]
- Sims LL, Goheen E, Kanaskie A. et al. (2015a). Alder canopy dieback and damage in Western Oregon riparian ecosystems. Northwest Science 89: 34–46. [Google Scholar]
- Sims LL, Garbelotto M. (2021). Phytophthora species repeatedly introduced in Northern California through restoration projects can spread into adjacent sites. Biological Invasions 23: 2173–2190. [Google Scholar]
- Sims LL, Sutton W, Reeser P. et al. (2015b). The Phytophthora species assemblage and diversity in riparian alder ecosystems of western Oregon, USA. Mycologia 107: 889–902. [DOI] [PubMed] [Google Scholar]
- Smith IW, Cunnington J, Pascoe I. (2006). Another new? species of Phytophthora on alder ‘down under’ (Australia). In: Progress in research on Phytophthora siseases of forest yrees (Brasier CM, Jung T, Osswald W. eds), Forest Research, Farnham, Hampshire, UK: Poster 30. [Google Scholar]
- Spies CFJ, Meitz-Hopkins JC, Langenhoven SD. et al. (2014). Two clonal lineages of Phytophthora citrophthora from citrus in South Africa represent a single phylogenetic species. Mycologia 106: 1106–1118. [DOI] [PubMed] [Google Scholar]
- Stöver BC, Müller KF. (2010). TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckenbrock EH. (2016). The role of hybridization in the evolution and emergence of new fungal plant pathogens. Phytopathology 106: 104–112. [DOI] [PubMed] [Google Scholar]
- Studholme DJ, Panda P, Sanfuentes von Stowasser E. et al. (2019). Genome sequencing of oomycete isolates from Chile supports the New Zealand origin of Phytophthora kernoviae and makes available the first Nothophytophthora sp. genome. Molecular Plant Pathology 20: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran J, Holder MT. (2010). DendroPy: A Python library for phylogenetic computing. Bioinformatics 26: 1569–1571. [DOI] [PubMed] [Google Scholar]
- Sukumaran J, Holder MT. (2015). SumTrees: Phylogenetic tree summarization. https://github.com/jeetsukumaran/DendroPy [accessed 18 Oct 2023].
- Svenning J-C. (2003). Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecology Letters 6: 646–653. [Google Scholar]
- Szabó I, Lakatos F, Sipos G. (2013). Occurrence of soilborne Phytophthora species in declining broadleaf forests in Hungary. European Journal of Plant Pathology 137: 159–168. [Google Scholar]
- Tamura K, Stecher G, Kumar S. (2021). MEGA11: Molecular Evolutionary Genetics Analysis version 11. Molecular Biology and Evolution 38: 3022–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecklin D, DellaSala DA, Luebert F. et al. (2011). Valdivian temperate rainforests of Chile and Argentina. In: Temperate and boreal rainforests of the world (DellaSala DA. ed.). Island Press, Washington, Covelo, London: 132–153. [Google Scholar]
- Tian S, López-Pujol J, Wang H-W. et al. (2010). Molecular evidence for glacial expansion and interglacial retreat during Quaternary climatic changes in a montane temperate pine (Pinus kwangtungensis Chun ex Tsiang) in southern China. Plant Systematics and Evolution 284: 219–229. [Google Scholar]
- Themann K, Werres S, Lüttmann R. et al. (2002). Observations of Phytophthora spp. in water recirculation systems in commercial hardy ornamental nursery stock. European Journal of Plant Pathology 108: 337–343. [Google Scholar]
- Thines M, Choi Y-J. (2016). Evolution, diversity and taxonomy of the Peronosporaceae, with focus on the genus Peronospora. Phytopathology 106: 6–18. [DOI] [PubMed] [Google Scholar]
- Tiffney BH. (1985). The Eocene North Atlantic land bridge: Its importance in tertiary and modern phytogeography of the northern hemisphere. Journal of the Arnold Arboretum 66: 243–273. [Google Scholar]
- Toussaint EFA, Hendrich L, Hájek J. et al. (2017). Evolution of Pacific Rim diving beetles sheds light on Amphi-Pacific biogeography. Ecography 40: 500–510. [Google Scholar]
- Truong NV, Liew ECY, Burgess LW. (2010). Characterisation of Phytophthora capsici isolates from black pepper in Vietnam. Fungal Biology 114: 160–170. [DOI] [PubMed] [Google Scholar]
- Tsykun T, Prospero S, Schoebel CN. et al. (2022). Global invasion history of the emerging plant pathogen Phytophthora multivora. BMC Genomics 23: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchetto-Zolet AC, Pinheiro F, Salgueiro F. et al. (2013). Phylogeographical patterns shed light on evolutionary process in South America. Molecular Ecology 22: 1193–1213. [DOI] [PubMed] [Google Scholar]
- Tucker CM, Milbrath JA. (1942). Root rot of Chamaecyparis caused by a species of Phytophthora. Mycologia 34: 94–103. [Google Scholar]
- Tyson JL, Fullerton RA. (2007). Mating types of Phytophthora colocasiae from the Pacific region, India and South-east Asia. Australasian Plant Disease Notes 2: 111–112. [Google Scholar]
- Uchida J, Kadooka CY. (2013). Distribution and biology of Phytophthora tropicalis. In: Phytophthora a global perspective (Lamour K. ed.). CABI, Wallingford, UK: 178–186. [Google Scholar]
- Van Damme K, Sinev AY. (2013). Tropical Amphi-Pacific disjunctions in the Cladocera (Crustacea: Branchiopoda). Journal of Limnology 72(s2): 209–244. [Google Scholar]
- Van Poucke K, Franceschini S, Webber JF. et al. (2012). Discovery of a fourth evolutionary lineage of Phytophthora ramorum: EU2. Fungal Biology 116: 1178–1191. [DOI] [PubMed] [Google Scholar]
- Van Poucke K, Haegeman A, Goedefroit T. et al. (2021). Unravelling hybridization in Phytophthora using phylogenomics and genome size estimation. IMA Fungus 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Welzen PC, Parnell JAN, Slik JWF. (2011). Wallace’s Line and plant distributions: two or three phytogeographical areas and where to group Java? Biological Journal of the Linnean Society 103: 531–545. [Google Scholar]
- Van Welzen PC, Slik JWF. (2009). Patterns in species richness and composition of plant families in the Malay Archipelago. Blumea 54: 166–171. [Google Scholar]
- Vélez ML, La Manna L, Tarabini N. et al. (2020). Phytophthora austrocedri in Argentina and co-inhabiting Phytophthoras: Roles of anthropogenic and abiotic factors in species distribution and diversity. Forests 11: 1223. [Google Scholar]
- Vettraino AM, Brasier CM, Brown AV. et al. (2011). Phytophthora himalsilva sp. nov. an unusually phenotypically variable species from a remote forest in Nepal. Fungal Biology 115: 275–287. [DOI] [PubMed] [Google Scholar]
- Vettraino AM, Franceschini S, Vannini A. (2010). First report of Buxus rotundifolia decline caused by Phytophthora citrophthora (R. & E. Sm.) Leonian in Italy. Plant Disease 94: 272. [DOI] [PubMed] [Google Scholar]
- Vila R, Bell CD, Macniven R. et al. (2011). Phylogeny and palaeoecology of Polyommatus blue butterflies show Beringia was a climate-regulated gateway to the New World. Proceedings of the Royal Society B 278: 2737–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Presser JW, Goss EM. (2016). Nuclear DNA content of the hybrid plant pathogen Phytophthora andina determined by flow cytometry. Mycologia 108: 899–904. [DOI] [PubMed] [Google Scholar]
- Waterhouse GM. (1963). Key to the species of Phytophthora de Bary. Mycological Papers 92: 1–22. [Google Scholar]
- Weiland JE, Nelson AH, Hudler GW. (2010). Aggressiveness of Phytophthora cactorum, P. citricola I, and P. plurivora from European beech. Plant Disease 94: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Wen J. (1999). Evolution of Eastern Asian and Eastern North American disjunct distributions in flowering plants. Annual Review of Ecology and Systematics 30: 421–455. [Google Scholar]
- Wen J, Ickert-Bond S, Nie Z-L. et al. (2010). Timing and modes of evolution of eastern Asian-North American biogeographic disjunctions in seed plants. In: Darwin’s Heritage Today: Proceedings of the Darwin 2010 Beijing International Conference (Long M, Gu H, Zhou Z. eds). Higher Education Press, Bejing, China: 252–269. [Google Scholar]
- Wen J, Nie Z-L, Ickert-Bond SM. (2016). Intercontinental disjunctions between eastern Asia and western North America in vascular plants highlight the biogeographic importance of the Bering land bridge from late Cretaceous to Neogene. Journal of Systematics and Evolution 54: 469–490. [Google Scholar]
- White TJ, Bruns T, Lee S. et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications (Innes MA, Gelfand DH, Sninsky JJ. et al., eds). Academic Press, San Diego, California, USA: 315–322. [Google Scholar]
- Whitten T, Damanik SJ, Anwar J. et al. (1997). The Ecology of Sumatra. Ecology of Indonesia Series, Vol. I, Oxford University Press, Oxford, UK. [Google Scholar]
- Whitten T, Mustafa M, Henderson GS. (2002). The Ecology of Sulawesi. Ecology of Indonesia Series, Vol. IV, Oxford University Press, Oxford, UK. [Google Scholar]
- Winkworth RC, Neal G, Ogas RA. et al. (2022). Comparative analyses of complete Peronosporaceae (Oomycota) mitogenome sequences – insights into structural evolution and phylogeny. Genome Biology and Evolution 14: evac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburne MO. (2010). The Great American Biotic Interchange: Dispersals, tectonics, climate, sea level and holding pens. Journal of Mammalian Evolution 17: 245–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster CM, Bird MI, Bull ID. et al. (2010). Forest contraction in north equatorial Southeast Asia during the last glacial maximum. Proceedings of the National Academy of Sciences of the USA 107: 15508–15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang X, Li Y. et al. (2019). Phytophthora species from Xinjiang Wild apple forests in China. Forests 10: 927. [Google Scholar]
- Xu J, Yang X, Li Y. et al. (2021). First report of Phytophthora pini causing foliage blight and shoot dieback of Rhododendron pulchrum in China. Plant Disease 105: 1229. [DOI] [PubMed] [Google Scholar]
- Yang X, Hong C. (2016). Diversity and populations of Phytophthora, Phytopythium and Pythium species recovered from sediments in an agricultural run-off sedimentation reservoir. Plant Pathology 65: 1118–1125. [Google Scholar]
- Yang X, Hong C. (2013). Phytophthora virginiana sp. nov., a high-temperature tolerant species from irrigation water in Virginia. Mycotaxon 126: 167–176. [Google Scholar]
- Yang X, Hong C. (2018). Differential usefulness of nine commonly used genetic markers for identifying Phytophthora species. Frontiers in Microbiology 9: 2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Balci Y, Brazee NJ. et al. (2016). A unique species in Phytophthora clade 10, Phytophthora intercalaris sp. nov., recovered from stream and irrigation water in the eastern USA. International Journal of Systematic and Evolutionary Microbiology 66: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Copes WE, Hong CX. (2013). Phytophthora mississippiae sp. nov., a new species recovered from irrigation reservoirs at a plant nursery in Mississippi. Journal of Plant Pathology and Microbiology 4: 180. [Google Scholar]
- Yang X, Copes WE, Hong CX. (2014a). Two novel species representing a new clade and cluster of Phytophthora. Fungal Biology 118: 72–82. [DOI] [PubMed] [Google Scholar]
- Yang X, Gallegly ME, Hong CX. (2014b). A high-temperature tolerant species in clade 9 of the genus Phytophthora: P. hydrogena sp. nov. Mycologia 106: 57–65. [DOI] [PubMed] [Google Scholar]
- Yang X, Richardson PA, Hong C. (2014c). Phytophthora ×stagnum nothosp. nov., a new hybrid from irrigation reservoirs at ornamental plant nurseries in Virginia. PLoS ONE 9: e103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Tyler BM, Hong C. (2017). An expanded phylogeny for the genus Phytophthora. IMA Fungus 8: 355–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Chen P, Bu W. (2016). Terrestrial mountain islands and Pleistocene climate fluctuations as motors for speciation: A case study on the genus Pseudovelia (Hemiptera: Veliidae). Scientific Reports 6: 33625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H-C, Ho H-H, Zheng F-C. (2009). A survey of Phytophthora species on Hainan Island of South China. Journal of Phytopathology 157: 33–39. [Google Scholar]
- Zentmyer GA. (1979). Stimulation of sexual reproduction in the A2 mating type of Phytophthora cinnamomi by a substance in avocado roots. Phytopathology 69: 1129–1131. [Google Scholar]
- Zentmyer GA. (1988). Origin and distribution of four species of Phytophthora. Transactions of the British Mycological Society 91: 367–378. [Google Scholar]
- Zhang KM, Zheng FC, Li YD. et al. (1994). Isolates of Phytophthora colocasiae from Hainan Island in China: evidence suggesting an Asian origin of this species. Mycologia 86: 108–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fifty percent majority rule consensus phylogram derived from maximum likelihood analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora major Clade 2. Maximum Likelihood bootstrap values (in %) are indicated but not shown below 60 %. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT), (NT) and (T) denote ex-epitype, ex-neotype and ex-type isolates. Scale bar indicates 0.02 expected changes per site per branch.
Fifty percent majority rule consensus phylogram derived from maximum likelihood analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2a. Bootstrap values (in %) are indicated but not shown below 60 %. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT), (NT) and (T) denote ex-epitype, ex-neotype and ex-type isolates. Scale bar indicates 0.01 expected changes per site per branch.
Fifty percent majority rule consensus phylogram derived from maximum likelihood analysis of a concatenated thirteen-locus (LSU, ITS, βtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1, cox1, cox2, nadh1, rps10) dataset of Phytophthora Clade 2b. Bootstrap values (in %) are indicated but not shown below 60 %. Phytophthora infestans and P. pseudosyringae from Clades 1c and 3, respectively, were used as outgroup taxa (not shown). (EpT) and (T) denote ex-epitype and ex-type isolates, respectively isolates. Scale bar indicates 0.01 expected changes per site per branch.
Details of isolates from Phytophthora major Clades 2, 3 and 7 included in the phylogenetic, morphological, growth-temperature and biogeography studies. GenBank accession numbers are given in Table S3.
Overview of PCR conditions and details of primers used for amplification and sequencing of Phytophthora isolates.
GenBank accession numbers of Phytophthora isolates included in the phylogenetic, morphological and growth-temperature studies (sequences obtained in the present study are printed in italics).
Morphological characters and dimensions (mean ± SD; µm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2a (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; µm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2a (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; µm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2a (3rd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2b (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2b (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2b (3rd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (3rd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2c (4th set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclades 2d (only P. oleae) and 2e (1st set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora taxa from subclade 2e (2nd set). Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora taxa from subclade 2f. Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Morphological characters and dimensions (mean ± SD; μm), cardinal temperatures (°C) and temperature-growth relations (mm/d) on V8-juice agar of Phytophthora species from subclade 2g. Most discriminating characters are highlighted in bold. Percentages in brackets are ranges of isolate means. — means character not observed, n/a = not available.
Heterozygosity across nine nuclear gene regions (LSU, ITS, ßtub, hsp90, tigA, rpl10, tef-1α, enl, ras-ypt1), breeding systems and hybrid status of 46 Phytophthora taxa in subclades 2c, 2d, 2e, 2f and 2g.
Pairwise sequence identities (%) for the mitochondrial cox1, cox2, nadh1 and rsp10 genes between eight Phytophthora Clade 2a species with ambiguous, probable or confirmed hybrid status and their closest matches.
Pairwise sequence similarities (%) for the mitochondrial cox1, cox2, nadh1 and rsp10 genes between six Phytophthora Clade 2b taxa with ambiguous or probable hybrid status and their closest matches.