Abstract
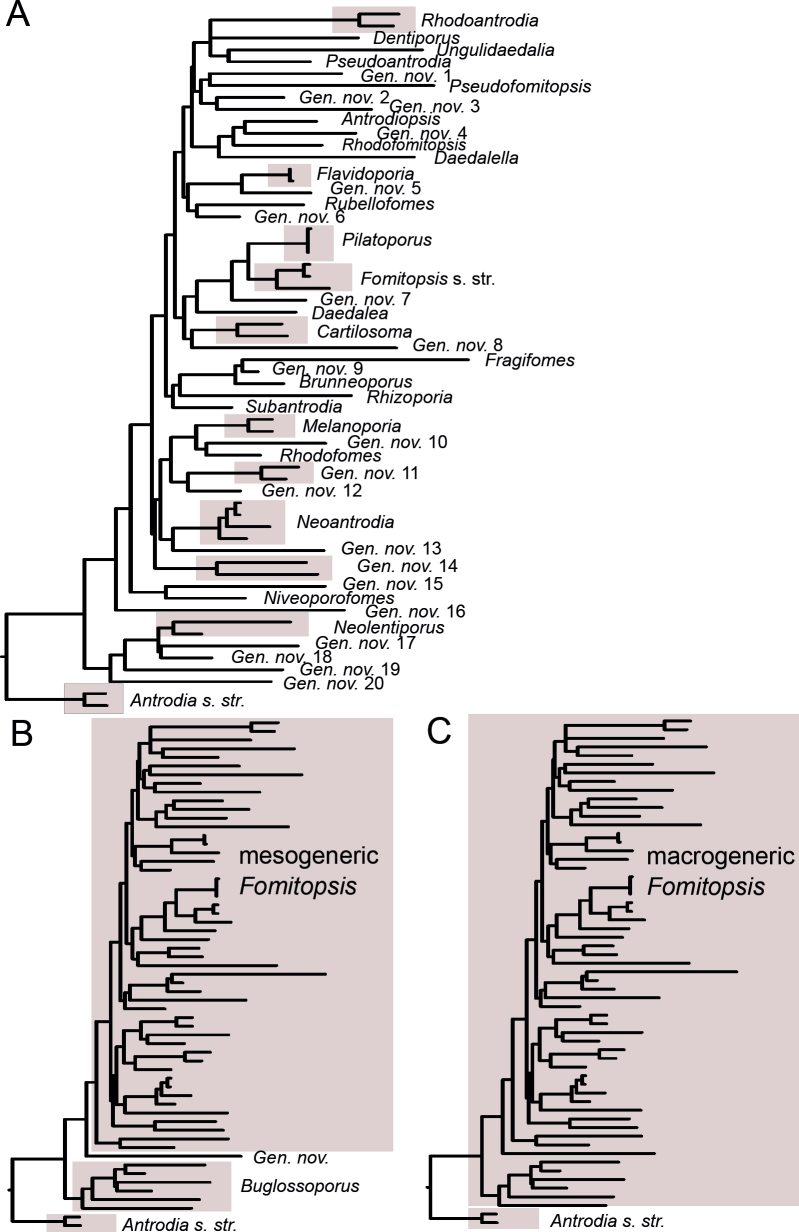
Based on seven- and three-gene datasets, we discuss four alternative approaches for a reclassification of Fomitopsidaceae (Polyporales, Basidiomycota). After taking into account morphological diversity in the family, we argue in favour of distinguishing three genera only, viz. Anthoporia, Antrodia and Fomitopsis. Fomitopsis becomes a large genus with 128 accepted species, containing almost all former Fomitopsis spp. and most species formerly placed in Antrodia, Daedalea and Laccocephalum. Genera Buglossoporus, Cartilosoma, Daedalea, Melanoporia, Neolentiporus, alongside twenty others, are treated as synonyms of Fomitopsis. This generic scheme allows for morphologically distinct genera in Fomitopsidaceae, unlike other schemes we considered. We provide arguments for retaining Fomitopsis and suppressing earlier (Daedalea, Caloporus) or simultaneously published generic names (Piptoporus) considered here as its synonyms. Taxonomy of nine species complexes in the genus is revised based on ITS, ITS + TEF1, ITS + TEF1 + RPB1 and ITS + TEF1 + RPB2 datasets. In total, 17 species are described as new to science, 26 older species are reinstated and 26 currently accepted species names are relegated to synonymy. A condensed identification key for all accepted species in the genus is provided.
Taxonomic novelties: New species: Fomitopsis algumicola Grebenc & Spirin, F. caseosa Vlasák & Spirin, F. cupressicola Vlasák, J. Vlasák Jr. & Spirin, F. derelicta Vlasák & Spirin, F. dollingeri Vlasák & Spirin, F. fissa Vlasák & Spirin, F. lapidosa Miettinen & Spirin, F. lignicolor Vlasák & Spirin, F. maculosa Miettinen & Spirin, F. pannucea Runnel & Spirin, F. perhiemata Viner & Spirin, F. purpurea Spirin & Ryvarden, F. retorrida Spirin & Kotiranta, F. solaris Rivoire, A.M. Ainsworth & Vlasák, F. tristis Miettinen & Spirin, F. tunicata Miettinen & Spirin, F. visenda Miettinen & Spirin. New combinations: Fomitopsis aculeata (Cooke) Spirin & Miettinen, F. aethalodes (Mont.) Spirin, F. alaskana (D.V. Baxter) Spirin & Vlasák, F. albidoides (A. David & Dequatre) Bernicchia & Vlasák, F. amygdalina (Berk. & Ravenel) Spirin & Vlasák, F. angusta (Spirin & Vlasák) Spirin & Vlasák, F. atypa (Lév.) Spirin & Vlasák, F. caespitosa (Murrill) Spirin & Miettinen, F. calcitrosa (Spirin & Miettinen) Spirin & Miettinen, F. circularis (B.K. Cui & Hai J. Li) Spirin, F. concentrica (G. Cunn.) M.D. Barrett, F. cyclopis (Miettinen & Spirin) Miettinen & Spirin, F. dickinsii (Berk. ex Cooke) Spirin, F. elevata (Corner) Spirin & Miettinen, F. eucalypti (Kalchbr.) Spirin, F. ferrea (Cooke) Spirin & Viner, F. flavimontis (Vlasák & Spirin) Vlasák & Spirin, F. foedata (Berk.) Spirin & Miettinen, F. gilvidula (Bres.) Spirin & Miettinen, F. glabricystidia (Ipulet & Ryvarden) Miettinen & Ryvarden, F. globispora (Ryvarden & Aime) Spirin, F. hartmannii (Cooke) M.D. Barrett & Spirin, F. hyalina (Spirin, Miettinen & Kotir.) Spirin & Miettinen, F. hypoxantha (Bres.) Spirin & Miettinen, F. incana (Lév.) Spirin & V. Malysheva, F. infirma (Renvall & Niemelä) Miettinen & Niemelä, F. juniperina (Murrill) Spirin & Vlasák, F. kuzyana (Pilát ex Pilát) Spirin & Vlasák, F. leioderma (Mont.) Spirin & Vlasak, F. leucaena (Y.C. Dai & Niemelä) Spirin & Miettinen, F. luzonensis (Murrill) Spirin & Miettinen, F. maculatissima (Lloyd) Spirin, F. madronae (Vlasák & Ryvarden) Vlasák & Ryvarden, F. malicola (Berk. & M.A. Curtis) Spirin, F. marchionica (Mont.) Spirin & Miettinen, F. marianii (Bres.) Spirin, Vlasák & Cartabia, F. mellita (Niemelä & Penttilä) Niemelä & Miettinen, F. microcarpa (B.K. Cui & Shun Liu) Spirin, F. micropora (B.K. Cui & Shun Liu) Spirin, F. modesta (Kuntze ex Fr.) Vlasák & Spirin, F. monomitica (Yuan Y. Chen) Spirin & Viner, F. morganii (Lloyd) Spirin & Vlasák, F. moritziana (Lév.) Spirin & Miettinen, F. neotropica (D.L. Lindner, Ryvarden & T.J. Baroni) Vlasák, F. nigra (Berk.) Spirin & Miettinen, F. nivosella (Murrill) Spirin & Vlasák, F. oboensis (Decock, Amalfi & Ryvarden) Spirin, F. oleracea (R.W. Davidson & Lombard) Spirin & Vlasák, F. philippinensis (Murrill) Spirin & Vlasák, F. primaeva (Renvall & Niemelä) Miettinen & Niemelä, F. psilodermea (Berk. & Mont.) Spirin & Vlasák, F. pulverulenta (Rivoire) Rivoire, F. pulvina (Pers.) Spirin & Vlasák, F. pulvinascens (Pilát ex Pilát) Niemelä & Miettinen, F. quercina (L.) Spirin & Miettinen, F. ramentacea (Berk. & Broome) Spirin & Vlasák, F. renehenticii (Rivoire, Trichies & Vlasák) Rivoire & Vlasák, F. roseofusca (Romell) Spirin & Vlasák, F. sagraeana (Mont.) Vlasák & Spirin, F. sandaliae (Bernicchia & Ryvarden) Bernicchia & Vlasák, F. sclerotina (Rodway) M.D. Barrett & Spirin, F. serialiformis (Kout & Vlasák) Vlasák, F. serialis (Fr.) Spirin & Runnel, F. serrata (Vlasák & Spirin) Vlasák & Spirin, F. squamosella (Bernicchia & Ryvarden) Bernicchia & Ryvarden, F. stereoides (Fr.) Spirin, F. subectypa (Murrill) Spirin & Vlasák, F. substratosa (Malençon) Spirin & Miettinen, F. tropica (B.K. Cui) Spirin, F. tumulosa (Cooke) M.D. Barrett & Spirin, F. tuvensis (Spirin, Vlasák & Kotir.) Spirin & Vlasák, F. uralensis (Pilát) Spirin & Miettinen, F. ussuriensis (Bondartsev & Ljub.) Spirin & Miettinen, F. variiformis (Peck) Vlasák & Spirin, F. yunnanensis (M.L. Han & Q. An) Spirin, Daedaleopsis candicans (P. Karst.) Spirin, Megasporoporia eutelea (Har. & Pat.) Spirin & Viner, Neofomitella hemitephra (Berk.) M.D. Barrett, Pseudophaeolus soloniensis (Dubois) Spirin & Rivoire, P. trichrous (Berk. & M.A. Curtis) Vlasák & Spirin. New synonyms: Antrodia bondartsevae Spirin, A. huangshanensis Y.C. Dai & B.K. Cui, A. taxa T.T. Chang & W.N. Chou, A. wangii Y.C. Dai & H.S. Yuan, Antrodiella subnigra Oba, Mossebo & Ryvarden, Antrodiopsis Audet, Boletus quercinus Schrad., Brunneoporus Audet, Buglossoporus Kotl. & Pouzar, Buglossoporus eucalypticola M.L. Han, B.K. Cui & Y.C. Dai, Caloporus P. Karst., Cartilosoma Kotlaba & Pouzar, Coriolus clemensiae Murrill, C. cuneatiformis Murrill, C. hollickii Murrill, C. parthenius Hariot & Pat., C. rubritinctus Murrill, Daedalea Pers., Daedalea allantoidea M.L. Han, B.K. Cui & Y.C. Dai, D. americana M.L. Han, Vlasák & B.K. Cui, D. radiata B.K. Cui & Hai J. Li, D. rajchenbergiana Kossmann & Drechsler-Santos, D. sinensis Lloyd, Daedalella B.K. Cui & Shun Liu, Dentiporus Audet, Flavidoporia Audet, Fomes subferreus Murrill, Fomitopsis cana B.K. Cui, Hai J. Li & M.L. Han, F. caribensis B.K. Cui & Shun Liu, F. cystidiata B.K. Cui & M.L. Han, F. ginkgonis B.K. Cui & Shun Liu, F. iberica Melo & Ryvarden, F. incarnata K.M. Kim, J.S. Lee & H.S. Jung, F. subfeei B.K. Cui & M.L. Han, F. subtropica B.K. Cui & Hai J. Li, Fragifomes B.K. Cui, M.L. Han & Y.C. Dai, Leptoporus epileucinus Pilát, Melanoporia Murrill, Neoantrodia Audet, Neolentiporus Rajchenb., Nigroporus macroporus Ryvarden & Iturr., Niveoporofomes B.K. Cui, M.L. Han & Y.C. Dai, Pilatoporus Kotl. & Pouzar, Piptoporus P. Karst., Polyporus aurora Ces., P. durescens Overh. ex J. Lowe, P. griseodurus Lloyd, Poria incarnata Pers., Pseudoantrodia B.K. Cui, Y.Y. Chen & Shun Liu, Pseudofomitopsis B.K. Cui & Shun Liu, Ranadivia Zmitr., Rhizoporia Audet, Rhodofomes Kotl. & Pouzar, Rhodofomitopsis B.K. Cui, M.L. Han & Y.C. Dai, Rhodofomitopsis pseudofeei B.K. Cui & Shun Liu, R. roseomagna Nogueira-Melo, A.M.S. Soares & Gibertoni, Rubellofomes B.K. Cui, M.L. Han & Y.C. Dai, Subantrodia Audet, Trametes fulvirubida Corner, T. lignea Murrill, T. lusor Corner, T. pseudodochmia Corner, T. subalutacea Bourdot & Galzin, T. supermodesta Ryvarden & Iturr., T. tuberculata Bres., Tyromyces multipapillatus Corner, T. ochraceivinosus Corner, T. palmarum Murrill, T. singularis Corner, T. squamosellus Núñez & Ryvarden, Ungulidaedalea B.K. Cui, M.L. Han & Y.C. Dai. Lectotypes: Hexagonia sulcata Berk., Polyporus castaneae Bourdot & Galzin, Poria incarnata Pers., Trametes subalutacea Bourdot & Galzin, Ungulina substratosa Malençon. Neotypes: Agaricus soloniensis Dubois, Boletus pulvinus Pers.
Citation: Spirin V, Runnel K, Vlasák J, Viner I, Barrett MD, Ryvarden L, Bernicchia A, Rivoire B, Ainsworth AM, Grebenc T, Cartabia M, Niemelä T, Larsson K-H, Miettinen O (2024). The genus Fomitopsis (Polyporales, Basidiomycota) reconsidered. Studies in Mycology 107: 149–249. doi: 10.3114/sim.2024.107.03
Keywords: brown-rot fungi, new taxa, phylogeny, polypores, taxonomy
INTRODUCTION
The transition from the traditional morphology-based classification to one supported by molecular phylogenetics heralded the beginning of a new era in fungal taxonomy. Within the past two decades the new methods (phylogenetic analyses) and evidence (genetic markers) have largely replaced the morphology-based phenetic systematics that guided fungal taxonomy for centuries. The challenge now lies in connecting the old and new types of evidence. As a rule, contemporary phylogenetic studies maintain safe links to traditional morphology-based systems, but often with little critical overview. This becomes evident when reviewing modern genus-level taxonomy wherein many newly detected smallscale clades in old morphology-based genera have been raised to generic rank. This approach fulfils some of the criteria necessary for solid genus delimitation while overlooking others. Notably, whereas the criterion of monophyly (cf. Vellinga et al. 2015) is fulfilled, alternative formal classifications to the multiple new genera are rarely discussed. This may be due to limitations in the data: such discussions would require analysis of the deeper nodes of the phylogenetic clades, which is difficult because it requires both extensive global sampling and the use of multiple genetic markers. Further complication is that the type material, a benchmark in the morphology-based systematics, is often very old and difficult (or impossible) to sequence and connect to recent material. This could be partly compensated with critical morphological analysis but is sufficiently carried out in a few recent studies only. Combined, these issues have brought about many cases of unstable phylogeny. It is particularly problematic when associated with well-known fungal groups that perform crucial functions in ecosystems and serve as flagships in fungal conservation.
Polypores constitute one such well-known group among the wood-inhabiting basidiomycetes. Before the advent of modern DNA methods, taxonomy of wood-inhabiting basidiomycetes in general and polypores in particular relied on two main cornerstones: the type of wood decay and anatomical traits of basidiocarps. Depending on wood-decay characteristics, these fungi were traditionally divided in two groups – brown- and white-rot producing species. The type of rot was recognized as a taxonomically significant character sufficient for rearranging morphologically similar species into separate genera or supra-generic units after the studies by Nobles (1958, 1971), David (1980) and Gilbertson (1980). In addition to wood-decay features, hyphal structure (hyphal system) has been regarded as one of the most profound characters for taxonomy of polypores (Corner 1935, 1953, Cunningham 1954). The morphology-based genus division of brown-rot polypores, that until recently prevailed in taxonomic literature, widely corresponded to Corner’s concept of hyphal systems: species with monomitic hyphal structure (all hyphae more or less uniform, clamped) were placed in the genus Postia (= Oligoporus), species with dimitic structure (possessing fibrous, thick-walled and nonclamped ‘skeletal’ hyphae alongside thin-walled and clamped ones) referred to Antrodia, and predominantly trimitic species (with branched ‘binding’ hyphae, in addition to the two previous hyphal types) gathered in Fomitopsis. Additionally, a few genera of brown-rot polypores had been characterized via hymenophore configuration (Daedalea), specific basidiocarp colour (Melanoporia), basidiospore morphology (Sarcoporia, Jahnoporus), presence of peculiar cystidia (Amylocystis, Auriporia) or absence of clamp connections (Laetiporus, Pycnoporellus). In general, hyphal structure was regarded as correlating with basidiocarp type and, consequently, with the life strategy of a species: monomitic taxa possess ephemeral, usually soft basidiocarps, dimitic ones have sturdier, seasonal fruitbodies, while trimitic taxa produce tough, as a rule perennial basidiocarps (Ryvarden 1991).
The first attempts to reconsider this simplistic approach were undertaken by Kotlaba and Pouzar (1957, 1990, 1993, 1998) in several publications dealing with the genus Fomitopsis. They stressed the high morphological diversity of Fomitopsis spp. and argued for further splitting of this genus based on consistency and pigmentation of basidiocarps, as well as thickness of the basidiospore wall. Ryvarden (1991) criticized their reclassification, pointing out the lack of data regarding the value of the aforementioned characters in dismantling Fomitopsis sensu lato. Most of the polypore manuals published between 1990 and 2010 treated Fomitopsis in a wide sense (e.g., Ryvarden & Gilbertson 1993, Bernicchia 2005, Niemelä 2005) while a few authors supported ideas by Kotlaba and Pouzar (Vampola 1996, Spirin & Zmitrovich 2003, Spirin et al. 2006). Eventually, it became apparent that no good solution could be proposed for a redefinition of Fomitopsis and allied genera with the use of traditional criteria only.
Applying the molecular phylogenetic methods generated considerable turbulence in the generic definition of Fomitopsis but has not yet resulted in solutions that would satisfy the taxonomic community as well as the broader audience. Kim et al. (2005) were the first to show that Fomitopsis is polyphyletic by applying DNA methods to investigate the phylogenetic relationships of this genus and other brown-rot polypores. Ortiz-Santana et al. (2013) obtained the same result with a much broader set of taxa. Neither of these studies, however, resulted in taxonomic conclusions. Justo et al. (2017) proposed a comprehensive family-level reclassification of the Polyporales based on a three-gene dataset. They reinstated the family Fomitopsidaceae to encompass Fomitopsis spp. (except F. officinalis considered incertae sedis), Antrodia sensu stricto (as redefined by Spirin et al. 2013a), most of the residual Antrodia species (after Ortiz-Santana et al. 2013, Spirin et al. 2013b, 2016), and Anthoporia (Karasiński & Niemelä 2016). The three included Fomitopsis species formed a strongly supported clade with residual Antrodia spp., as well as Daedalea quercina (generic type of Daedalea) and Piptoporus betulinus (generic type of Piptoporus). Nevertheless, Justo et al. (2017) refrained from giving an explicit interpretation for delimiting genera in the Fomitopsidaceae. A year before, Han et al. (2016) detected representatives of Fomitopsis in nine different clades, of which eight belonged to Fomitopsidaceae sensu Justo et al., which were described or re-introduced as separate genera. These results were based on a six-gene dataset including sequences of 26 Fomitopsis sensu lato species, i.e. nearly half of all species accepted in the genus at that time. Audet (2017) and Zmitrovich (2018) proposed eight new genera to name unranked clades containing residual Antrodia/Daedalea spp. in the Fomitopsidaceae, basing their suggestions on already published data. Finally, Liu et al. (2022) published an all-encompassing revision of brown-rot taxa in the Polyporales grounded on a partial seven-gene dataset. Their study resulted in introducing four more genera in the family. However, there is still a need for studies of deep nodes in the Fomitopsidaceae with a sufficiently wide taxon sampling and, consequently, there has been a lack of discussions regarding other options for translating the phylogenetic trees into formal taxonomic units.
In this study, we aimed to redefine the genus Fomitopsis by combining thorough type studies with multigene analyses in the Fomitopsis clade containing residual Antrodia spp., along with Daedalea/Piptoporus/Fomitopsis spp. in the Fomitopsidaceae sensu Justo et al. (2017). First, we compiled comprehensive multigene datasets to show the position and limits of Fomitopsidaceae within Polyporales, and among other brown-rotting families. Simultaneously, we revised 133 type specimens and sequenced at least two genetic markers for 80 species assigned to the genus Fomitopsis below. As Fomitopsis sensu lato encompasses dozens of described species all over the world, we aimed for a wide geographic coverage, to include many species not hitherto sequenced.
Our earlier studies in brown-rot polypores showed that an apparent morphological dissimilarity does not necessarily imply evolutionary remoteness. When redefining the genus Antrodia, we showed it embraces not only sturdy dimitic poroid species but also monomitic or nearly monomitic poroid and corticioid taxa; the latter ones were previously classified among widely different genera, i.e. Postia and Phlebia (Spirin et al. 2013a, Runnel et al. 2019). The same is true for Fomitopsis as redefined in the present study: we show it should encompass several members of such traditional polypore genera as Dichomitus, Gloeophyllum, Junghuhnia, Laccocephalum, Nigroporus, Skeletocutis, Trametes, and Tyromyces, which were not included in phylogenetic studies of brown-rot polypores before. We assign 128 species to the genus Fomitopsis and describe seventeen species as new. Twenty-six names in use are proposed as synonyms of already described taxa.
MATERIAL AND METHODS
Morphological study
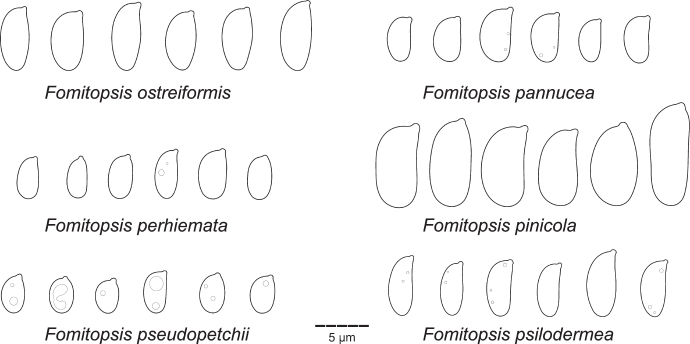
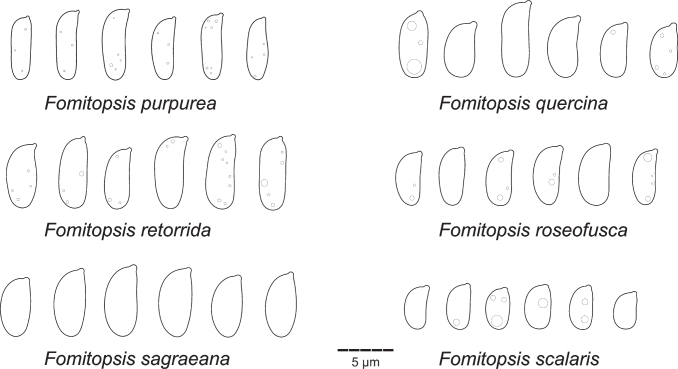
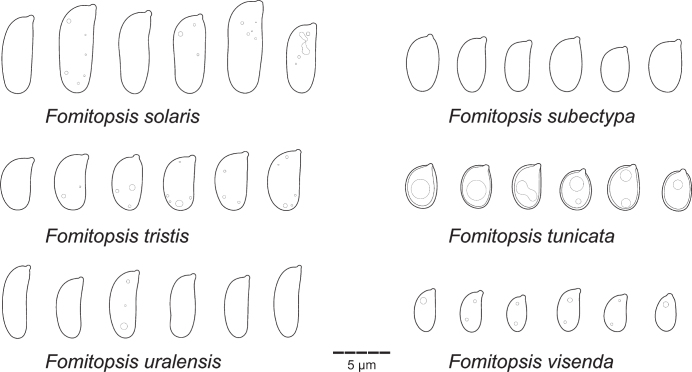
Type specimens and other collections from herbaria H, O, S, PC, K, PRM, TUF, LY, LE, UPS, L, HUBO, NY, BPI, MICH, FH, GB, W, LJF, MCF, MJ, C, MPU, MG, BO, BORH, DNA, MEL, and PERTH were studied. Herbarium acronyms are given according to Thiers (2021). Morphological study (i.e., pore and spore measuring techniques) follows Miettinen et al. (2018). All structures were measured from microscopic mounts in Cotton Blue (henceforth CB), using phase contrast and oil immersion lenses (Leitz Diaplan microscope, ×1 250 magnification). In total, 20–30 basidiospores, 20 subhymenial/tramal and subicular/context hyphae (skeletal hyphae for dimitic species, generative hyphae for monomitic ones), and at least 10 basidia were measured per specimen studied. The following abbreviations are used in morphological descriptions: L – mean basidiospore length, W – mean basidiospore width, Q’ – length / width ratio, Q – mean length/width ratio, n – number of measurements per specimens measured.
While describing hyphal structures in Fomitopsis spp., we apply the term ‘monomitic’ to the species having clamps throughout and ‘dimitic’ to those with unclamped, aseptate, thick-walled (skeletal) hyphae, regardless of the degree of branching in the skeletal hyphae. We follow proposals of Rajchenberg (1986) and Hattori (2005) and avoid describing hyphal structure in the genus as trimitic because all species treated below do not have differentiated binding hyphae characteristic for the representatives of Polyporaceae (e.g., Funalia, Trametes, Ganoderma) (see Results for further discussion on this subject). Basidiospores are described as thin-walled if the spore wall is visible in phase contrast as an outer contour, having a distinct wall if the wall thickness is at the measurable minimum (i.e. 0.1–0.2 μm thick), and slightly thick-walled if the spore wall is 0.3–0.4 μm thick.
DNA isolation and sequencing
DNA extraction, PCR, and sequencing of the target loci for this study (ITS, LSU, RPB1 and TEF1) followed protocols described by Spirin et al. (2013b), Tamm and Põldmaa (2013), and Liimatainen and Ainsworth (2018). The ITS region was amplified using primers ITS1F and ITS4B (Gardes & Bruns 1993), and/or ITS2, ITS4, ITS5 (White et al. 1990), 58A1F (Martin & Rygiewicz 2005), LR22 (Vilgalys lab, Duke University) (https://sites.duke.edu/vilgalyslab/files/2017/08/rDNA-primers-for-fungi.pdf)), and the D1–D2 domains of the LSU region using primers CTB6 (Garbelotto et al. 1997) and LR7 (Vilgalys & Hester 1990). The area between conserved domains A and C of RPB1 (c. 1 400 bp) was amplified using primers RPB1gAf and RPB1-fCr (Stiller & Hall 1997, Matheny et al. 2002). The TEF1 region was amplified using primers EF983F and EF2218R (Matheny et al. 2007). Amplification products were sequenced at the Genomics Laboratory of the Biology Centre, Academy of Sciences of the Czech Republic (České Budějovice, Czech Republic), Macrogen Europe (Netherlands), Eurofins Genomics (Germany), and the Jodrell Laboratory, Royal Botanic Gardens (Kew, UK). Deciphering and assembling of chromatograms were performed as described in Viner et al. (2021). Data for the studied specimens and the GenBank accession numbers of ITS, LSU, RPB1, RPB2 and TEF1 sequences are presented in Table 1 and Suppl. Table S1.
Table 1.
DNA sequences used in the genus/species-level phylogenies (newly generated sequences are given in bold face).
| Species | Specimen (culture)/repository | Origin (ISO code) | GenBank/UNITE accession number | Reference(s) | ||||
|---|---|---|---|---|---|---|---|---|
| LSU | ITS | RPB1 | RPB2 | TEF1 | ||||
| Amylocystis lapponica | FP-105131-Sp (CFMR) | US-CO | KY948879 | KY948805 | KY948973 | – | – | Justo et al. (2017) |
| Amyloporia carbonica | Zabel-40-GLN (CFMR) | US-NY | KC585065 | KC585243 | KY949013 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| A. sinuosa | FP-105386-Sp (CFMR) | US-NH | KC585066 | KC585244 | KY949018 | – | – | Ortiz-Santana etal. (2013); Justo et al. (2017) |
| A. xantha | DAOM 16570 | CA-BC | KC585076 | KC585254 | KY949016 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Anthoporia albobrunnea | Spirin 4665 (H) | RU-LEN | KY948880 | KY948808 | KY949020 | – | – | Justo et al. (2017) |
| Antrodia griseoflavescens | Spirin 11175(H) | RU-LEN | MK119762 | MK119762 | MK134850 | – | – | Runnel et al. (2019) |
| A. heteromorpha | HHB-140162 (CFMR) | US-AK | KC585279 | KC585279 | KY949010 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| A. mappa | Spirin 4605 (H) | RU-VLG | MK119770 | MK119770 | MK134844 | – | – | Runnel et al. (2019) |
| A. multiformis | Vlasak 1209/76 (PRM) | US-AZ | KT381618 | KT381618 | MK134846 | – | – | Kout et al. (2017); Runnel et al. (2019) |
| A. serpens | Vampola 20.09.1990 (MJ) | SK | KC543143 | KC543143 | KY949012 | – | – | Spirin et al. (2013a); Justo et al. (2017) |
| A. tenerifensis | Kout 1412/2 (PRM) | ES | KY446066 | KY446066 | MK134848 | – | – | Kout et al. (2017); Runnel et al. (2019) |
| Crustoderma corneum | HHB-5685-Sp (CFMR) | US-MT | KC585143 | KC585318 | KY949037 | – | – | Ortiz-Santana et al. (2013); Justo at al (2017) |
| Dacryobolus karstenii | Miettinen 18685 (H) | US-WA | KY948900 | KY948743 | KY948955 | – | 00789530 | Justo et al. (2017) |
| Daedalea ryvardeniana | FLOR41052 | BR | – | OP526845 | – | – | – | Cristaldo et al. (2022) |
| D. ryvardeniana | URM80515 | BR | – | OP526846 | – | – | – | Cristaldo et al. (2022) |
| Fibroporia gossypium | Rajchenberg 11443 | AR | KY948897 | KY948811 | KY949029 | – | – | Justo et al. (2017) |
| F. vaillantii | FP-90877-R (CFMR) | US-NJ | KC585170 | KC585345 | KY949035 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Fomitopsis abieticola | Cui 10521 (BJFC) | ON | – | MN 148231 | – | – | MN161746 | Liu et al. (2021) |
| F. abieticola | Cui 10532 (BJFC) | ON | – | MN 148230 | – | MN 158174 | MN161745 | Liu et al. (2021) |
| F. aculeata | CSIRO (M) E7436 | ID-KI | – | AJ536655 | – | – | – | GenBank |
| CSIRO (M) E7381 | ID-KI | – | AJ542522 | – | – | – | GenBank | |
| CSIRO (M) E7393 | ID-KI | – | AJ542530 | – | – | – | GenBank | |
| Cui 8624 (BJFC) | ON | – | JQ314348 | – | – | – | Li & Cui (2013) | |
| Cui 8487 (BJFC) | ON | – | JQ314349 | – | – | – | Li & Cui (2013) | |
| Yuan 3629 (BJFC) | ON | – | JQ314350 | – | – | – | Li & Cui (2013) | |
| Miettinen 8674 (H) | ID-RI | ON970637 | ON970637 | – | – | – | This study | |
| F. aethalodes | Campi 70 (FACEN 004306) | PY | – | OP526843 | – | – | – | Cristaldo et al. (2022) |
| F. afficana | Kout 1408/K9 (H) | TH | ON924667 | ON994669 | OP022430 | – | – | This study |
| Mossebo 13* (MUCL42384) | CM | – | DQ491422 | – | – | – | Kim et al. (2008) | |
| isolate 6565 | IN | – | MG430333 | – | – | – | GenBank | |
| isolate 6566 | IN | – | MG430334 | – | – | – | GenBank | |
| isolate 6537 | IN | – | MG430342 | – | – | – | GenBank | |
| Miettinen 15198 (H) | ID-RI | – | ON970652 | – | – | – | This study | |
| F. albidoides | Bernicchia 5672 | IT | KC543147 | KC543147 | – | – | – | Spirin et al. (2013a) |
| Bernicchia 7224 (HUBO) | IT | – | KC543114 | OP022431 | – | – | Spirin et al. (2013a); this study | |
| F. algumicola | MCF MAK 01/2095 | MK | – | FM872461 | – | – | – | GenBank |
| MCF MAK 07/8020 | MK | – | FM872462 | – | – | – | GenBank | |
| MCF MAK xx/7771 | MK | – | FM872463 | – | – | – | GenBank | |
| MCF MAK 00/4578 | MK | – | FM872464 | – | – | – | GenBank | |
| MCF MAK 07/8293 | MK | – | FM872465 | – | – | – | GenBank | |
| F. amygdalina | Vlasak 1707/9J (H) | CR | MN318452 | MN318452 | – | – | – | GenBank |
| F atypa | TFRI 781 | TW | – | EU232191 | – | – | – | GenBank |
| TFRI 782 | TW | – | EU232192 | – | – | – | GenBank | |
| Cui 8355 (BJFC) | CN | – | KC907398 | – | – | – | GenBank | |
| Dai 7857 (BJFC) | CN | – | KC907399 | – | – | – | GenBank | |
| Cui 8515 (BJFC) | CN | – | KP171204 | – | – | – | Han et al. (2015) | |
| Cui 10151 (BJFC) | CN | – | KP171205 | – | – | – | Han et al. (2015) | |
| Cui 10124 (BJFC) | CN | – | KR605291 | – | – | – | Han et al. (2016) | |
| ZD16091109 | CN | – | MN523241 | – | – | – | GenBank | |
| Dunaev 271.2019 (H) | IN | – | ON970658 | – | – | – | This study | |
| Ryvarden 17588 (0) | TH | – | ON994670 | – | – | – | This study | |
| F. betulina | L-15603-Sp (CFMR) | US-NY | KC585202 | KC585373 | KY949005 | – | – | Justo et al. (2017) |
| CIRM-BRFM1772 | – | – | CIRM-BRFM1772 | – | CIRM-BRFM1772 | CIRM-BRFM1772 | JGI | |
| F. caespitosa | Cui 10140 (BJFC) | CN | – | JQ067651 | – | – | KR610699 | Li et al. (2013); Han et al. (2016) |
| Cui 10154 (BJFC) | CN | – | JQ067652 | – | – | – | Li et al. (2013) | |
| Cui 10181 (BJFC) | CN | – | JQ067653 | – | – | KR610700 | Li et al. (2013); Han et al. (2016) | |
| Miettinen 5486 (H) | ID-RI | – | KC595913 | – | – | – | Ortiz-Santana et al. (2013) | |
| Miettinen 8737 (H) | ID-RI | – | KC595910 | – | – | – | Ortiz-Santana et al. (2013) | |
| Miettinen 13019(H) | ID-SB | – | KC595911 | – | – | – | Ortiz-Santana et al. (2013) | |
| Miettinen 13076 (H) | ID-SB | – | KC595912 | – | – | – | Ortiz-Santana et al. (2013) | |
| Dunaev w/n (H) | LK | – | ON994671 | – | – | – | This study | |
| KUN 1123 (H) | MY | – | ON970655 | – | – | – | This study | |
| KUN 2874 (H) | MY | – | ON970656 | – | – | – | This study | |
| Miettinen 8823.3 (H) | ID-RI | – | ON970632 | – | – | – | This study | |
| F. cajanderi | CBS 127.24 | US | – | DQ491407 | – | – | – | Kim et al. (2008) |
| HOU 10773 | RU-PRI | – | DQ491413 | – | – | – | Kim et al. (2008) | |
| Cui 9888 (BJFC) | CN | – | KC507156 | – | – | – | Han & Cui (2015) | |
| Spirin LE213630 | RU-NIZ | – | KC595915 | – | – | – | Ortiz-Santana et al. (2013) | |
| Vlasak 0410/14 (JV) | US-VA | – | KR605768 | – | – | – | Han et al. (2016) | |
| Spirin 4089 (H) | RU-KHA | – | ON994672 | – | – | – | This study | |
| F. calcitrosa | FP-133692 (CFMR) | US-OR | KC585127 | KC585303 | KY948998 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
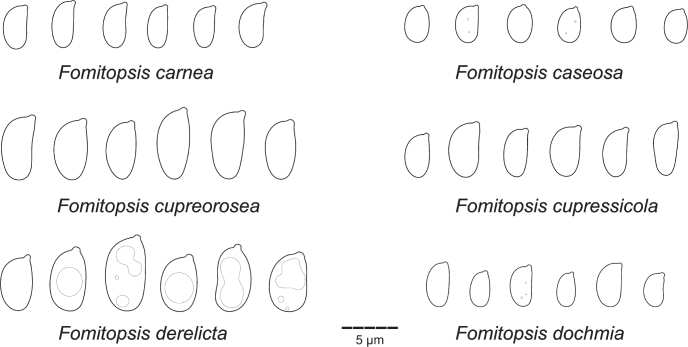
| F. carnea | Miettinen 13120.1 (H) | ID-SB | – | KC595916 | – | – | – | Ortiz-Santana et al. (2013) |
| BBH 40441 | TH | – | KX421865 | – | – | – | GenBank | |
| Miettinen 15115.2 (H) | ID-RI | ON924668 | ON924668 | – | – | – | This study | |
| Dai 18551 (BJFC) | MA | – | MW377283 | – | – | – | Liu et al. (2022) | |
| Dai 18562 (BJFC) | MA | – | MW377284 | – | – | – | Liu et al. (2022) | |
| Dai 17823 (BJFC) | SG | – | MW377285 | – | – | – | Liu et al. (2022) | |
| F. aff. carnea | UOCMINNPM18 | LK | – | KP780437 | – | – | – | GenBank |
| F. caseosa | Vlasak 1504/28 (PRM) | CR | ON994673 | ON994673 | – | – | – | This study |
| F. cellularis | Vlasak 1504/36J (H) | CR | ON924669 | ON994674 | OP022433 | – | – | This study |
| F. circularis | Cui 8488 (BJFC) | CN | – | JQ314351 | – | – | – | Li & Cui (2013) |
| Cui 10134 (BJFC) | CN | – | JQ314352 | – | – | – | Li & Cui (2013) | |
| Cui 10125 (BJFC) | CN | – | JQ780411 | – | – | – | GenBank | |
| Dai 13062 (BJFC) | CN | – | KP171200 | – | – | – | Han et al. (2016) | |
| F. concentrica | Barrett F197/11 (PERTH) | AU | ON691651 | ON691651 | ON667940 | – | – | This study |
| F. condensa | Vlasak 1312/E15 (PRM) | CR | KT156690 | KT156690 | OP022434 | – | – | Vlasák et al. (2016); this study |
| F. cupreorosea | AN49 | BR | – | MF589756 | – | – | – | Soares et al. (2017) |
| NM692 | BR | – | MF589757 | – | – | – | Soares et al. (2017) | |
| NM710 | BR | – | MF589758 | – | – | – | Soares et al. (2017) | |
| JMB34 | BR | – | MF589760 | – | – | – | Soares et al. (2017) | |
| NM731 | BR | – | MF589761 | – | – | – | Soares et al. (2017) | |
| PS2013-01 | BR | – | MF772343 | – | – | – | Soares et al. (2017) | |
| Kout 0610/K4 (H) | BZ | – | ON994675 | – | – | – | This study | |
| Vlasak 1908/81 (H) | GF | – | ON994676 | – | – | – | This study | |
| F. cupressicola | Dollinger 778 (H) | US-FL | – | ON994677 | – | – | – | This study |
| Vlasak 1706/7J (H) | US-NJ | – | ON994678 | – | – | – | This study | |
| Vlasak 1706/9J (H) | US-NJ | – | ON994679 | – | – | – | This study | |
| Vlasak 1710/21 | US-NJ | – | ON994680 | – | – | – | This study | |
| F. derelicta | Ryvarden 45191 (CFMR) | BZ | – | FJ403211 | – | – | – | Lindner et al. (2011) |
| Vlasak 2104/2J (H) | US-TX | – | ON994681 | – | – | – | This study | |
| F. dickinsii | NBRC4979 | JP | – | AB733158 | – | – | – | GenBank |
| NBRC31163 | JP | – | AB733161 | – | – | – | GenBank | |
| strain 027 | CN | – | EU661878 | – | – | – | GenBank | |
| Ryvarden 21710 | CN | – | FJ403210 | – | – | – | Lindner et al. (2011) | |
| strain xsd08139 | CN | – | FJ481049 | – | – | – | GenBank | |
| strain dd08028 | CN | – | FJ810145 | – | – | – | GenBank | |
| strain dd08028 | CN | – | FJ810167 | – | – | – | GenBank | |
| strain dd08076 | CN | – | FJ810173 | – | – | – | GenBank | |
| strain dd08089 | CN | – | FJ810178 | – | – | – | GenBank | |
| isolate 143 | CN | – | JN182911 | – | – | – | GenBank | |
| Cui 6825 (BJFC) | CN | – | JQ314353 | – | – | – | Li & Cui (2013) | |
| HE2730 | CN | – | KC505574 | – | – | – | GenBank | |
| KUC20130903A-13 | KR | – | KJ668556 | – | – | – | GenBank | |
| Yuan 2685 (BJFC) | CN | – | KP171201 | – | – | – | Han et al. (2015) | |
| Yuan 2707 (BJFC) | CN | – | KP171202 | – | – | – | Han et al. (2015) | |
| Yuan 1090 (BJFC) | CN | – | KR605790 | – | – | – | Han et al. (2016) | |
| 450526MF0259 | CN | – | MG712332 | – | – | – | GenBank | |
| MHHNU8317 | CN | – | MK172824 | – | – | – | GenBank | |
| strain KMCC04903 | KR | – | MN823154 | – | – | – | GenBank | |
| F. dochmia | Dunaev 711.2019(H) | IN | – | ON970657 | – | – | OP019459 | This study |
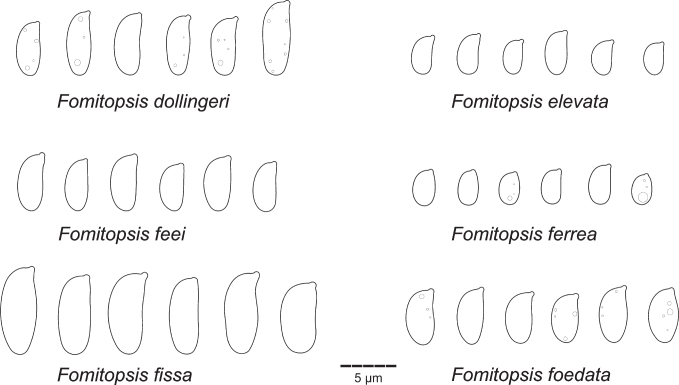
| F. dollingeri | Dollinger 56 (H) | US-FL | ON924670 | ON994682 | OP022432 | – | – | This study |
| F. elevata | Miettinen 8692 (H) | ID-RI | – | ON994683 | – | – | – | This study |
| Miettinen 20529 (H) | ID-RI | ON924671 | ON994684 | – | – | – | This study | |
| F. eucalypti | Schigel 5234 (H) | AU | – | KC595917 | – | – | – | Ortiz-Santana et al. (2013) |
| Cui 16748 (BJFC) | AU | – | MW377280 | – | – | – | Liu et al. (2022) | |
| Cui 16773 (BJFC) | AU | – | MW377281 | – | – | – | Liu et al. (2022) | |
| Cui 16786 (BJFC) | AU | – | MW377282 | – | – | – | Liu et al. (2022) | |
| F. eucalypticola | Cui 16594 (BJFC) | AU | – | MK852560 | – | – | MK900483 | Liu et al. (2019) |
| Cui 16595 (BJFC) | AU | – | MK852561 | – | – | – | Liu et al. (2019) | |
| Cui 16598 (BJFC) | AU | – | MK852562 | – | – | MK900484 | Liu et al. (2019) | |
| F. feel | Ryvarden 37603 (0) | VE | – | KC844850 | – | – | – | Han & Cui (2015) |
| Oinonen 60119006 (H) | BR | – | KC844851 | – | – | – | Han & Cui (2015) | |
| URM 86162 | BR | – | KX423689 | – | – | – | Soares et al. (2017) | |
| F. ferrea | Dunaev 26.1.2019 (H) | IN | – | ON970659 | – | – | OP019460 | This study |
| F. fissa | Vlasak 0407/13J (H) | US-CA | ON924673 | ON994685 | OP022435 | – | OP215805 | This study |
| F. flabellata | URM 89405 | BR | – | KX423688 | – | – | – | Soares et al. (2017) |
| F. foedata | Uotila 42928 (H) | AU | ON924672 | KF999924 | – | – | – | Han & Cui (2015); this study |
| Cui 16794 (BJFC) | AU | – | MK461952 | – | – | – | Yuan et al. (2020) | |
| Cui 16803 (BJFC) | AU | – | MK461953 | – | – | – | Yuan et al. (2020) | |
| Cui 16807 (BJFC) | AU | – | MK461954 | – | – | – | Yuan et al. (2020) | |
| Miettinen 11466 (H) | ID-PA | ON970630 | ON970630 | – | – | – | This study | |
| F. fragills | Cui 10919 (BJFC) | CN | KF937286 | KF937286 | – | – | – | Han et al. (2016) |
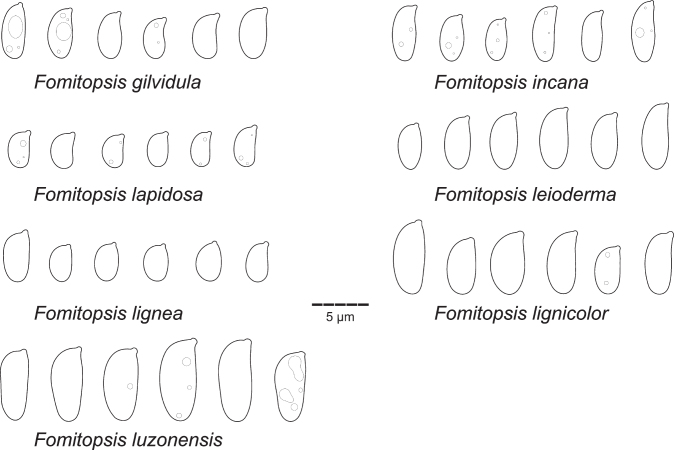
| F. gilvidula | Miettinen 20535 (H) | ID-RI | 0N924674 | 0N994686 | – | – | – | this study |
| F. glabrocystidia | Ipulet 378 (0) | UG | ON970633 | ON970633 | 0Q789528 | – | – | this study |
| F. globispora | C5 | – | – | JX434660 | – | – | – | GenBank |
| Fomitopsis sp. S-20 | MX | – | KR135353 | – | – | – | GenBank | |
| Aime 3413(0) | BZ | – | KC017760 | – | – | – | GenBank | |
| F. hartmannii | Dai 13660 (BJFC) | CN | – | KR605808 | – | – | – | Han et al. (2016) |
| Nunez 554 (0) | JP | OQ701091 | – | – | – | – | This study | |
| Nunez 679 (0) | JP | 0N924675 | 0N994687 | – | – | – | This study | |
| F. hengduanensis | Cui 16259 (BJFC) | CN | – | MN148232 | – | MN158175 | MN161747 | Liu et al. (2021) |
| Cui 17056 (BJFC) | CN | – | MN148233 | – | MN158176 | MN161748 | Liu et al. (2021) | |
| F. hyalina | Spirin 2772 (H) | RU-NIZ | JQ700283 | JQ700283 | KY949007 | – | – | Spirin et al. (2013b); Justo et al. (2017) |
| F. hypoxantha | Cui 8951 (BJFC) | CN | – | KC507164 | – | – | – | GenBank |
| Dai 5983 (H) | CN | – | KC595924 | – | – | – | Ortiz-Santana et al. (2013) | |
| Cui 8969 (BJFC) | CN | – | KR605785 | – | – | – | Han et al. (2016) | |
| KA12-1397 | KR | – | KR673596 | – | – | – | Kim et al. (2015) | |
| Zhao 2241 | CN | – | MH 114658 | – | – | – | GenBank | |
| F. incana | Dai 13612A (BJFC) | CN | – | KR605795 | – | – | – | Han et al. (2016) |
| LE 313649 | IN | 0N787634 | 0N787633 | – | – | – | This study | |
| F. juniperina | FP 105489-Sp (CFMR) | US-MD | – | KC585282 | – | – | – | Ortiz-Santana et al. (2013) |
| FP71540 (CFMR) | US-MD | – | KC585283 | – | – | – | Ortiz-Santana et al. (2013) | |
| SRM 403 (CFMR) | US-NE | KC585109 | KC585285 | KY948991 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) | |
| FP97452-T (CFMR) | ET | – | AY966454 | – | – | – | GenBank | |
| WM-284-T (CFMR) | US-PA | – | EU232212 | – | – | – | GenBank | |
| E. Larsson 15-11 (GB) | ES | – | KC543112 | – | – | – | Spirin et al. (2013a) | |
| Dai 17104 (BJFC) | UZ | – | KX958182 | – | – | – | Yuan et al. (2017) | |
| Dai 17105 (BJFC) | UZ | – | KX958183 | – | – | – | Yuan et al. (2017) | |
| Vlasak 0310/1J (H) | US-PA | – | MG787606 | – | – | – | GenBank | |
| CBS 117.40 | US-MD | – | MH856056 | – | – | – | Vu et al. (2019) | |
| CBS 639.75 | TZ | – | MH860961 | – | – | – | Vu et al. (2019) | |
| CBS 105824 | ET | – | ON970645 | – | – | – | This study | |
| H7068514 | US-AR | – | 0N994688 | – | – | – | This study | |
| Vlasak 0709/154(H) | US-VA | – | 0N994689 | – | – | – | This study | |
| Vlasak 1209/14 (H) | US-AZ | – | ON994690 | – | – | – | This study | |
| Vlasak 1711/13J (H) | US-AZ | – | 0N994691 | – | – | – | This study | |
| F. kenyensis | 0 F-915372 | KE | – | KP171196 | – | – | – | Han et al. (2016) |
| F. kesiyae | Cui 16437 (BJFC) | VN | – | MN148234 | – | MN158177 | MN161749 | Liu et al. (2021) |
| Cui 16466 (BJFC) | VN | – | MN148235 | – | MN158178 | MN161750 | Liu et al. (2021) | |
| F. kuzyana | Rivoire 3574 (LY) | FR | – | ON994693 | OP022437 | – | – | Spirin et al. (2016); this study |
| Spirin LE208476 | RU-NIZ | JQ700282 | JQ700282 | KY948992 | – | – | Spirin et al. (2013b); Justo et al. (2017) | |
| Spirin 6771 (H) | RU-NIZ | – | ON994692 | OP022436 | – | – | Spirin et al. (2016); this study | |
| F. lapidosa | Miettinen 21981 (H) | ID-PB | ON924676 | ON994694 | – | – | – | This study |
| F. leioderma | Vlasak 1908/82 (H) | GF | – | ON994695 | – | – | – | This study |
| F. lignea | Ryvarden 41624 (0) | JM | – | ON994754 | – | – | – | This study |
| F. lignicolor | Vlasak 1312/A4 (H) | CR | ON924677 | KT156689 | OP022438 | – | – | Vlasák et al. 2016; this study |
| Kout 0402/M 1 | VE | – | OQ673257 | – | – | – | This study | |
| F. lilacinogilva | CBS 236.87 | CR | – | DQ491400 | – | – | – | Kim et.al. (2008) |
| CBS 422.84 | AU | – | DQ491403 | – | – | – | Kim et.al. (2008) | |
| Schigel 5193 (H) | AU | – | KR605773 | – | – | – | Han et.al. (2016) | |
| F. luzonensis | Miettinen 14311 (H) | ID-PB | KC595920 | KC595920 | KY949006, OP022439 | – | 1 | Ortiz-Santana et.al. (2013); Justo et.al. (2017); this study |
| BCC233382 | TH | – | FJ372684 | – | – | – | Rungjindamai et al. (2009) | |
| Miettinen 11573 (H) | ID-PB | – | KC595918 | – | – | – | Ortiz-Santana et al. (2013) | |
| Miettinen 14417 (H) | ID-PB | – | KC595919 | – | – | – | Ortiz-Santana et al. (2013) | |
| Vlasak 0509/52-X (JV) | CN | – | KR605779 | – | – | KR610686 | Han et al. (2016) | |
| Miettinen 5678 (H) | ID-RI | ON970642 | ON970642 | – | – | – | This study | |
| Miettinen 11224 (H) | ID-RI | – | ON994696 | OP022440 | – | OP215806 | This study | |
| Miettinen 13163 (H) | ID-RI | ON970638 | ON970638 | – | – | OP019457 | This study | |
| Miettinen 13162 (H) | ID-RI | ON970639 | ON970639 | – | – | – | This study | |
| Miettinen 14261 (H) | ID-PB | ON970640 | ON970640 | – | – | – | This study | |
| Miettinen 15222 (H) | ID-RI | – | ON970646 | – | – | – | This study | |
| Miettinen 23504 (H) | ID-RI | – | ON970650 | – | – | – | This study | |
| F. maculatissima | Rajchenberg 158 (BAFC) | AR | AF518632 | – | – | – | – | GenBank |
| CIEFAP92 | AR | – | JX090121 | – | – | – | GenBank | |
| CIEFAP93 | AR | – | JX090122 | – | – | – | GenBank | |
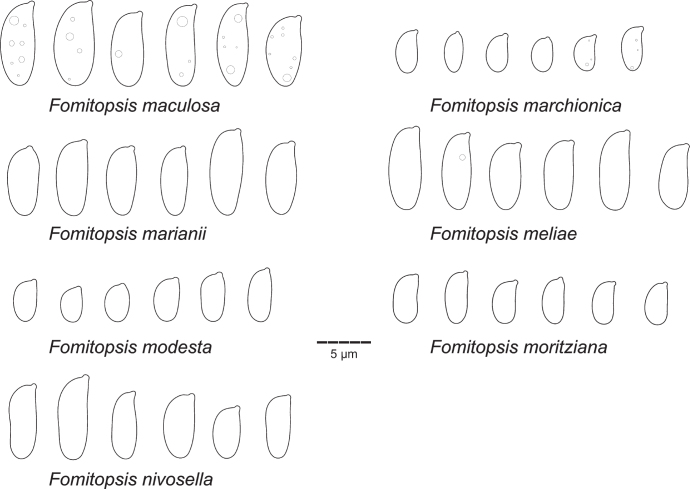
| F. maculosa | Miettinen 12230 (H) | ID-BA | OQ701093 | OQ701093 | – | – | – | This study |
| Miettinen 12233.1 (H) | ID-BA | OQ701092 | OQ701092 | – | – | – | This study | |
| F. madronae | Vlasak 0709/117 (PRM) | US-OR | JN592494 | JN592494 | OP022441 | – | – | Vlasák et al. (2012); this study |
| JLF 3745 | US-OR | – | MK991847 | – | – | – | GenBank | |
| F. marchionica | Miettinen 11454 (H) | ID-PA | – | ON994699 | – | – | – | This study |
| F. marianii | ATCC 62978 | JP | – | MJIM01000216 | MJIM01000024 | – | MJIM01000001 | GenBank |
| Betak 10/743 (JV) | CZ | ON924678 | ON754045 | OP022442 | – | OP215807 | This study | |
| Spirin LE209783 | RU-NIZ | – | JQ700276 | – | – | – | Spirin et al. (2013b) | |
| Dai 6613(H) | CN | – | JQ700277 | – | – | – | Spirin et al. (2013b) | |
| Cui 5525 (BJFC) | CN | – | JQ837942 | – | – | – | Cui (2013) | |
| Cui 7597 (BJFC) | CN | – | KP171213 | – | – | KR610687 | Han et al. (2015, 2016) | |
| Cui 7615 (BJFC) | CN | – | KR605780 | – | – | KR610688 | Han et al. (2016) | |
| MS 48 (CFMR) | US-WI | – | KC585352 | – | – | – | Ortiz-Santana et al. (2013) | |
| Vampola s.n. (H) | cz | – | KC595930 | – | – | – | Ortiz-Santana et al. (2013) | |
| Fo6 | IT | – | KF615756 | – | – | – | Roccotelli et al. (2014) | |
| TYP-6137 (CFMR) | JP | – | KJ995920 | – | – | – | GenBank | |
| CF010808 | AU | – | KR605770 | – | – | KR610675 | Han et al. (2016) | |
| CF10810 | PT | – | KR605771 | – | – | – | Han et al. (2016) | |
| OF10811 | IT | – | KR605772 | – | – | – | Han et al. (2016) | |
| Cui 16871 (BJFC) | US-PR | – | MK852559 | – | – | MK900482 | Liu et al. (2019) | |
| Cui 17170 (BJFC) | CN | – | MK852563 | – | – | MK900485 | Liu et al. (2019) | |
| Cui 17171 (BJFC) | CN | – | MK852564 | – | – | MK900486 | Liu et al. (2019) | |
| Dollinger 836 (H) | US-FL | ON924679 | ON754046 | OP022443 | – | OP215808 | This study | |
| Gilbertson 14757 (0) | US-LA | – | ON754047 | – | – | OP215809 | This study | |
| MJ 4157 | SK | – | ON754048 | OP022444 | – | OP215810 | This study | |
| MJ 4158 | HR | – | ON754049 | – | – | OP215811 | This study | |
| MJ 4606 | CZ | – | ON754050 | – | – | OP215812 | This study | |
| Rivoire 6563 (LY) | FR | – | ON754051 | OP022445 | – | OP215813 | This study | |
| Spirin 5175(H) | RU-KHA | – | ON754052 | – | – | – | This study | |
| Spirin 5176 (H) | RU-KHA | – | ON754053 | – | – | OP215814 | This study | |
| Spirin 5302 (H) | RU-KHA | – | ON754054 | – | – | OP215815 | This study | |
| Spirin 9267 (H) | RU-NIZ | – | ON754055 | – | – | – | This study | |
| Spirin 10503 (H) | RU-NIZ | – | ON754056 | – | – | – | This study | |
| Spirin 10575 (H) | RU-NIZ | – | ON754057 | – | – | – | This study | |
| Spirin 11249 (H) | RU-NIZ | – | ON754058 | – | – | OP215816 | This study | |
| F. massoniana | Cui 2848 (BJFC) | CN | – | MN148236 | – | – | MN161751 | Liu et al. (2021) |
| Cui 9058 (BJFC) | CN | – | MN148237 | – | – | MN161752 | Liu et al. (2021) | |
| Cui 11288 (BJFC) | CN | – | MN148238 | – | MN158179 | MN161753 | Liu et al. (2021) | |
| Cui 11304 (BJFC) | CN | – | MN148239 | – | – | MN161754 | Liu et al. (2021) | |
| F. meliae | FP-105065 (CFMR) | US-MS | – | KC585350 | – | – | – | Ortiz-Santana et al. (2013) |
| SRM 209 (CFMR) | US-NE | – | KC585351 | – | – | – | Ortiz-Santana et al. (2013) | |
| Roberts GA863 (K) | VG | – | KR605775 | – | – | KR610682 | Han et al. (2016) | |
| Ryvarden 16863 (O) | CO | – | KR605776 | – | – | KR610681 | Han et al. (2016) | |
| Vlasak 1109/40J (H) | US-TX | – | KY264030 | – | – | 1 | Vlasák & Dollinger (2017); this study | |
| isolate FM1C20 | US-AZ | – | MW221272 | – | – | MW590292 | GenBank | |
| isolate FM1C7 | US-AZ | – | MW567238 | – | – | MW590290 | GenBank | |
| isolate FM1C11 | US-AZ | – | MW567239 | – | – | MW590291 | GenBank | |
| isolate FM1C21 | US-AZ | – | MW567240 | – | – | MW590293 | GenBank | |
| isolate FM1C22 | US-AZ | – | MW567241 | – | – | MW590294 | GenBank | |
| isolate FM1C30 | US-AZ | – | MW567242 | – | – | MW590295 | GenBank | |
| isolate FM1C33 | US-AZ | – | MW567243 | – | – | MW590296 | GenBank | |
| Dollinger 989 (H) | US-FL | – | ON994700 | – | – | OP215817 | This study | |
| Dollinger 991 (H) | US-FL | – | ON994701 | – | – | OP215818 | This study | |
| Hormia2109 (H) | PE | – | ON994702 | – | – | – | This study | |
| Vlasak 1511/24J (JV) | VG | – | ON994703 | – | – | OP215819 | This study | |
| Vlasak 1612/22J (JV) | GP | – | ON994704 | OP022446 | – | OP215820 | This study | |
| Vlasak 1704/61 (H) | CR | – | ON994705 | OP022447 | – | OP215821 | This study | |
| Vlasak 1704/78J (H) | CR | – | ON994706 | – | – | OP215822 | This study | |
| Vlasak 1712/27 (JV) | MQ | – | ON994707 | – | – | – | This study | |
| Vlasak 1808/33 (JV) | GF | – | ON994708 | – | – | – | This study | |
| Vlasak 1808/81 (JV) | GF | – | ON994709 | – | – | – | This study | |
| Vlasak 1808/82 (JV) | GF | – | ON994710 | – | – | – | This study | |
| F. mellita | Spirin 3315(H) | RU-LEN | KC543139 | KC543139 | KY948994 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| F. minutispora | Rajchenberg 10661 (BAFC) | AR | KR605716 | KR605777 | – | – | – | Han et al. (2016) |
| F. minutula | Spirin 2680 (H) | RU-NIZ | KC595898 | KC595898 | KY948993 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| F. modesta | FLOR70928 | BR | – | OP526847 | – | – | – | Cristaldo et al. (2022) |
| FLOR70929 | BR | – | OP526848 | – | – | – | Cristaldo et al. (2022) | |
| Vlasak 1312/A7-J (JV) | CR | – | ON994711 | – | – | – | This study | |
| Vlasak 1407/90 (H) | CR | – | ON994712 | – | – | – | This study | |
| Vlasak 1504/21 (H) | CR | – | ON994713 | – | – | – | This study | |
| Vlasak 1808/87 (H) | GF | – | ON994714 | – | – | – | This study | |
| F. monomitica | Dai 10630 (BJFC) | CN | – | KY421732 | – | – | – | Chen &Wu (2017) |
| Dai 16894 (BJFC) | CN | – | KY421733 | – | – | – | Chen &Wu (2017) | |
| Viner KUN2550 (H) | RU-PRI | – | ON970661 | – | – | – | This study | |
| F. moritziana | Miettinen 11662 (H) | MA | – | ON970653 | – | – | – | This study |
| F. mounceae | AFTOL-770 | US | AY684164 | AY854083 | AY864874 | AY786056 | AY885152 | GenBank |
| F. mounceae | 32TT (CFMR) | US-WA | – | KF169621 | – | KF169690 | KF178346 | Haight et al. (2016) |
| F. mounceae | CS-1 (CFMR) | US-OR | – | KF169622 | – | KF169691 | KF178347 | Haight et al. (2016) |
| DR-301 (CFMR) | US-MI | – | KF169623 | – | KF169692 | KF178348 | Haight et al. (2016) | |
| DR-366 (CFMR) | US-MI | – | KF169624 | – | KF169693 | KF178349 | Haight et al. (2016) | |
| DR-472 (CFMR) | US-MI | – | KF169625 | – | KF169694 | KF178350 | Haight et al. (2016) | |
| JAG-08-19 (CFMR) | US-ID | – | KF169626 | – | KF169695 | KF178351 | Haight et al. (2016) | |
| JAG-08-20 (CFMR) | US-ID | – | KF169627 | – | KF169696 | KF178352 | Haight et al. (2016) | |
| JAG-08-25 (CFMR) | US-ID | – | KF169628 | – | KF169697 | KF178353 | Haight et al. (2016) | |
| JEH-78 (CFMR) | CA-AB | – | KF169629 | – | KF169698 | KF178354 | Haight et al. (2016) | |
| JEH-82 (CFMR) | CA-AB | – | KF169630 | – | KF169699 | KF178355 | Haight et al. (2016) | |
| JEH-86 (CFMR) | CA-AB | – | KF169631 | – | KF169700 | KF178356 | Haight et al. (2016) | |
| JEH-146 (CFMR) | US-WI | – | KF169632 | – | KF169701 | KF178357 | Haight et al. (2016) | |
| JEH-147 (CFMR) | US-WI | – | KF169633 | – | KF169702 | KF178358 | Haight et al. (2016) | |
| KM-1 (CFMR) | US-OR | – | KF169634 | – | KF169703 | KF178359 | Haight et al. (2016) | |
| LT-5 (CFMR) | US-AK | – | KF169635 | – | KF169704 | KF178360 | Haight et al. (2016) | |
| MJL-112-Sp (CFMR) | US-NY | – | KF169636 | – | KF169705 | KF178361 | Haight et al. (2016) | |
| FP-105760-T (CFMR) | US-ID | – | KF169637 | – | KF169706 | KF178362 | Haight et al. (2016) | |
| FP-133890-T (CFMR) | US-MT | – | KF169638 | – | KF169707 | KF178363 | Haight et al. (2016) | |
| FP-125086-T (CFMR) | US-NH | – | KF169639 | – | KF169708 | KF178364 | Haight et al. (2016) | |
| MB_03_036 (CFMR) | US-CA | – | MH086259 | – | MK208855 | MK236353 | Haight et al. (2019) | |
| Niemela 2530 (H) | CA-QC | – | MN 148240 | – | – | MN161755 | Liu et al. (2021) | |
| Ahti 60351 (H) | CA | – | MN 148241 | – | – | MN161756 | Liu et al. (2021) | |
| Miettinen 18782 (H) | US-ID | – | MN148242 | – | – | MN161757 | Liu et al. (2021) | |
| Spirin 8367 (H) | US-WA | – | MN148243 | – | – | MN161758 | Liu et al. (2021) | |
| F. neotropica | DLC04-74 (CFMR) | BZ | – | FJ403216 | – | – | – | Lindner et al. (2011) |
| DLC04-80 (CFMR) | BZ | – | FJ403217 | – | – | – | Lindner et al. (2011) | |
| DLC04-100 (CFMR) | BZ | – | FJ403218 | – | – | – | Lindner et al. (2011) | |
| DLC04-174 (CFMR) | BZ | – | FJ403219 | – | – | – | Lindner et al. (2011) | |
| Vlasak 1312/E18-J (JV) | CR | – | KT156688 | – | – | – | Vlasák et al. (2016) | |
| F. nigra | Davidson-Wester (CBS 341.63) | US-DC | KC543172 | KC543172 | OP114093 | – | – | Spirin et al. (2013a); this study |
| Vlasak 1410/10 J (JV) | US-PA | – | KT156694 | OP022448 | – | – | Vlasák et al. (2016); this study | |
| F. niveomarginata | Cui 10108 (BJFC) | CN | KR605717 | KR605778 | – | – | – | Han et al. (2016) |
| F. nivosella | PCO43 | CO | – | HQ248222 | – | – | – | GenBank |
| Ryvarden 41410 (0) | VE | – | KF937292 | – | – | KR610669 | Han et al. (2016) | |
| Overholts 4215 (BPI) | US-OH | – | KF937293 | – | – | – | Han et al. (2016) | |
| de Jesus OF 10833 | BR-AM | – | ON994715 | – | – | – | This study | |
| de Meijer 3465 (0) | BR-PR | – | ON994716 | – | – | – | This study | |
| Kout 1807/19 (H) | US-PR | – | ON994717 | – | – | OP215823 | This study | |
| F. ochracea | DLL-3 (CFMR) | US-MN | – | KF169588 | – | KF169657 | KF178313 | Haight et al. (2016) |
| DLL-4 (CFMR) | US-MN | – | KF169589 | – | KF169658 | KF178314 | Haight et al. (2016) | |
| FP-125083-T (CFMR) | US-NH | – | KF169590 | – | KF169659 | KF178315 | Haight et al. (2016) | |
| HHB-17661 (CFMR) | US-AK | – | KF169591 | – | KF169660 | KF178316 | Haight et al. (2016) | |
| HHB-19667 (CFMR) | US-TN | – | KF169592 | – | KF169661 | KF178317 | Haight et al. (2016) | |
| HHB-19670 (CFMR) | US-TN | – | KF169593 | – | KF169662 | KF178318 | Haight et al. (2016) | |
| HHB-19692 (CFMR) | US-TN | – | KF169594 | – | KF169663 | KF178319 | Haight et al. (2016) | |
| HHB-3331-Sp (CFMR) | US-MI | – | KF169595 | – | KF169664 | KF178320 | Haight et al. (2016) | |
| JEH-12E (CFMR) | US-AK | – | KF169597 | – | KF169666 | KF178322 | Haight et al. (2016) | |
| JEH-12F (CFMR) | US-AK | – | KF169598 | – | KF169667 | KF178323 | Haight et al. (2016) | |
| JEH-13A(CFMR) | US-AK | – | KF169599 | – | KF169668 | KF178324 | Haight et al. (2016) | |
| JEH-13D (CFMR) | US-AK | – | KF169601 | – | KF169670 | KF178326 | Haight et al. (2016) | |
| JEH-37 (CFMR) | US-AK | – | KF169602 | – | KF169671 | KF178327 | Haight et al. (2016) | |
| JEH-79 (CFMR) | CA-AB | – | KF169604 | – | KF169673 | KF178329 | Haight et al. (2016) | |
| JEH-80 (CFMR) | CA-AB | – | KF169605 | – | KF169674 | KF178330 | Haight et al. (2016) | |
| JEH-81 (CFMR) | CA-AB | – | KF169606 | – | KF169675 | KF178331 | Haight et al. (2016) | |
| JEH-83 (CFMR) | CA-AB | – | KF169607 | – | KF169676 | KF178332 | Haight et al. (2016) | |
| JEH-85 (CFMR) | CA-AB | – | KF169608 | – | KF169677 | KF178333 | Haight et al. (2016) | |
| JEH-87 (CFMR) | CA-BC | – | KF169610 | – | KF169679 | KF178334 | Haight et al. (2016) | |
| JEH-88 (CFMR) | CA-AB | – | KF169611 | – | KF169680 | KF178336 | Haight et al. (2016) | |
| JEH-91 (CFMR) | CA-AB | – | KF169612 | – | KF169681 | KF178337 | Haight et al. (2016) | |
| KTS-28 (CFMR) | US-VT | – | KF169613 | – | – | KF178338 | Haight et al. (2016) | |
| LT-12 (CFMR) | US-AK | – | KF169614 | – | KF169683 | KF178339 | Haight et al. (2016) | |
| LT-18 (CFMR) | US-AK | – | KF169616 | – | KF169685 | KF178341 | Haight et al. (2016) | |
| LT-19 (CFMR) | US-AK | – | KF169617 | – | KF169686 | KF178342 | Haight et al. (2016) | |
| LT-17 (CFMR) | US-AK | – | KF169618 | – | KF169687 | KF178343 | Haight et al. (2016) | |
| TRTC48800 | CA-NL | – | KF169619 | – | KF169688 | KF178344 | Haight et al. (2016) | |
| CFMR: PEL-Lk-6-1 | US-MN | – | KF169620 | – | KF169689 | KF178345 | Haight et al. (2016) | |
| Miettinen 18568 (H) | US-WA | – | MN148244 | – | – | MN161759 | Liu et al. (2021) | |
| Miettinen 18673 (H) | US-WA | – | MN148245 | – | – | MN161760 | Liu et al. (2021) | |
| Spirin 8165 (H) | US-WA | – | MN148246 | – | – | MN161761 | Liu et al. (2021) | |
| Vlasak 1811/13J (JV) | US-UT | ON924680 | ON994718 | – | – | OP215824 | This study | |
| F. oleracea | HHB-5988-Sp (CFMR) | US-AZ | KC585117 | KC585293 | KY948987 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| RLG-3818-Sp (CFMR) | US-NC | – | EU232198 | – | – | – | GenBank | |
| FP-48282-R (CFMR) | US-MI | – | KC585290 | – | – | – | Ortiz-Santana et al. (2013) | |
| FP-70890-Sp (CFMR) | US-GA | – | KC585291 | – | – | – | Ortiz-Santana et al. (2013) | |
| HHB-3543-Sp (CFMR) | US-MI | – | KC585292 | – | – | – | Ortiz-Santana et al. (2013) | |
| Mad-497 (CFMR) | US-TX | – | KC585295 | – | – | – | Ortiz-Santana et al. (2013) | |
| MD-177 (CFMR) | US-WI | – | KC585296 | – | – | – | Ortiz-Santana et al. (2013) | |
| CBS 388.51 | US-MI | – | MH856911 | – | – | – | Vu et al. (2019) | |
| strain KRT_lso_3 | US-KY | – | MN430923 | – | – | – | GenBank | |
| Vlasak 1009/31 (H) | US-PA | – | ON994719 | – | – | – | This study | |
| F. ostreiformis | Dai 9611 (BJFC) | CN | – | JX435776 | – | – | – | Li et al. (2013) |
| Cui 6239 (BJFC) | CN | – | JX435777 | – | – | – | Li et al. (2013) | |
| Miettinen 10071 (H) | CN | – | KC595914 | OP022449 | – | OP215825 | Ortiz-Santana et al. (2013); this study | |
| Dai 10035 (BJFC) | CN | – | KR605774 | – | – | KR610683 | Han et al. (2016) | |
| Dammrich 2003 (H) | LK | ON970641 | ON970641 | – | – | – | This study | |
| Miettinen 8854.1 (H) | ID-RI | ON970636 | ON970636 | – | – | – | This study | |
| Miettinen 11629 (H) | ID-PB | – | ON994720 | OP022450 | – | OP215826 | This study | |
| Miettinen 21986 (H) | ID-RI | – | ON970649 | – | – | – | This study | |
| Miettinen 23532 (H) | SG | – | ON970651 | – | – | – | This study | |
| F. palustris | Dollinger 782 (PRM) | US-FL | – | ON994721 | OP022451 | – | OP215827 | This study |
| Lowe 4092 (PRM) | US-GE | – | ON994722 | – | – | OP215828 | This study | |
| Ryvarden 44439 (0) | BZ | – | ON994723 | – | – | – | This study | |
| Vlasak 1904/1J (H) | US-FL | – | ON994724 | OP022452 | – | – | This study | |
| F. pannucea | Runnel 824 (TUF) | GF | UDB035620 | UDB035620 | UDB07672434 | – | – | This study |
| F. perhiemata | Viner 2021/35 (H) | RU-KDA | – | ON970660 | – | – | – | This study |
| F. philippinensis | JZ36 | IN | – | MG719293 | – | – | – | GenBank |
| Kout 1408/K2 (H) | TH | – | ON994725 | – | – | – | This study | |
| Ryvarden 17840 (CBS 426.84) | TH | – | DQ491401 | – | – | – | Kim et al. (2008) | |
| F. pinicola | GR9-4 | SE | MPVS00000000 | MPVS00000000 | MPVS01000315 | MPVS01000002 | MPVS01000007 | GenBank |
| LT-323 (CFMR) | EE | – | KF169651 | – | KF169720 | KF178376 | Haight et al. (2016) | |
| LT-319 (CFMR) | EE | – | KF169652 | – | KF169721 | KF178377 | Haight et al. (2016) | |
| FCUG2056 | SE | – | KF169653 | – | KF169722 | KF178378 | Haight et al. (2016) | |
| Kotiranta 19330 (H) | RU-SVE | – | KF169654 | – | KF169723 | KF178379 | Haight et al. (2016) | |
| TS-Fp-24 (CFMR) | RU-MOW | – | KF169655 | – | KF169724 | KF178380 | Haight et al. (2016) | |
| FCUG2034 | SE | – | KF169656 | – | KF169725 | KF178381 | Haight et al. (2016) | |
| Kotiranta 15815(H) | RU | – | KU171406 | – | MK236364 | MK236361 | Haight et al. (2019) | |
| Kotiranta 27183 (H) | RU-CHU | – | ON994726 | – | – | OP215829 | This study | |
| Spirin 4111 (H) | RU-KHA | – | ON994727 | – | – | OP215830 | This study | |
| Vlasak 1905/1 (H) | CZ | ON924681 | ON994728 | – | – | OP215831 | This study | |
| F. pseudopetchii | Miettinen 14284 (H) | ID-PB | OQ701094 | OQ701094 | – | – | – | This study |
| Miettinen 14373.1 (H) | ID-PB | OQ701097 | – | – | – | – | This study | |
| F. psilodermea | Vlasak 1504/34 (H) | CR | – | MG787587 | – | – | – | GenBank |
| F. pulvina | CBS 858.72 | DE | – | DQ491419 | – | – | – | Kim et al. (2008) |
| LE287547 | RU | – | KM411464 | – | – | – | GenBank | |
| Vlasak 0906/15J (JV) | US-PA | ON924682 | KR605800 | OP022453 | – | – | Han et al. (2016); this study | |
| Vlasak 1406/1 (PRM) | CZ | – | KR605801 | – | – | – | Han et al. (2016) | |
| Rivoire 2030 (LY) | FR | – | KR605799 | – | – | – | Han et al. (2016) | |
| Bigelow w/n | US-NJ | – | MT939446 | – | – | – | GenBank | |
| F. pulvinascens | Pennanen 1532 (H) | Fl | JC700286 | JC700286 | KY948995 | – | – | Spirin et al. (2013b); Justo et al (2017) |
| F. purpurea | Ryvarden 10118(0) | TZ | – | KF999921 | – | – | – | Han & Cui (2015) |
| 0 F915519 | TZ | – | KC507155 | – | – | – | Han & Cui (2015) | |
| F. quercina | HHB-8735 (CFMR) | US-WI | – | FJ403214 | – | – | – | Lindner et al. (2011) |
| Miettinen 12662 (H) | Fl | – | JX109855 | – | – | – | Binder et al. (2013) | |
| FP103364-T (CFMR) | US-GA | – | KC585335 | – | – | – | Ortiz-Santana et al. (2013) | |
| FP125063-T (CFMR) | US-NH | – | KC585336 | – | – | – | Ortiz-Santana et al. (2013) | |
| OKM-3802-Sp (CFMR) | US-MD | – | KC585337 | – | – | – | Ortiz-Santana et al. (2013) | |
| FP56429 (CFMR) | US-PE | KY948883 | KY948809 | KY948989 | – | – | Justo et al. (2017) | |
| JLF3791 | US-OR | – | MH277957 | – | – | – | GenBank | |
| Russell MycoMap 7714 | US-IN | – | MK532776 | – | – | – | GenBank | |
| Grootmyers4.X.2015 | US-IN | – | MK607490 | – | – | – | GenBank | |
| JLF1738 | US-OR | – | MK991837 | – | – | – | GenBank | |
| FFUI-4 | NG | – | MN596945 | – | – | – | GenBank | |
| K(M)250617 | GB | – | MZ159711 | – | – | – | GenBank | |
| Vlasak 0404/2-J (H) | US-GA | – | ON994729 | – | – | – | This study | |
| F. ramentacea | Spirin 2540 (H) | RU-NIZ | KC595903 | KC595903 | KY949002 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Marstad 274-09 (0) | NO | – | KC543138 | – | – | – | Spirin et al. (2013a) | |
| Kout 1001/1 (H) | CZ | – | ON994730 | – | – | OP215832 | This study | |
| David 3182 (LY) | FR | – | ON994731 | – | – | OP215833 | This study | |
| F. renehenticii | Rivoire 4111 (LY) | FR | – | KM068100 | – | – | OP215834 | Rivoire et al. (2015); this study |
| Bernicchia 8142 (HUBO) | IT | – | KM068101 | – | – | OP215835 | Rivoire et al. (2015); this study | |
| PRM 951454 | CZ | – | MK558724 | – | – | – | Zibarova et al. (2019) | |
| PRM 944766 | CZ | – | MK558725 | – | – | – | Zibarova et al. (2019) | |
| Kout 1709/7 (KBI) | CZ | – | MK558726 | – | – | – | Zibarova et al. (2019) | |
| PRM 951086 | CZ | – | MK558727 | – | – | – | Zibarova et al. (2019) | |
| Rivoire 3059 (LY) | FR | – | ON994732 | – | – | OP215836 | This study | |
| Rivoire 6274 (LY) | FR | – | ON994733 | – | – | – | This study | |
| Rivoire 7347 (LY) | FR | – | ON994734 | – | – | – | This study | |
| F. renehenticii x Solaris | Rivoire 6356 (LY) | FR | – | ON994735 | – | – | OP215837 | This study |
| F. retorrida | Kotiranta 28979 (H) | RU-SAK | ON924683 | ON994736 | OP022454 | – | – | This study |
| F. rosea | RLG-6954 (CFMR) | US-AZ | KC585181 | KC585353 | KY949003 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| DLL2009-166 (CFMR) | US-MN | – | JQ673050 | – | – | – | Brazee et al. (2012) | |
| Oivanen 118(H) | Fl | – | KC595923 | – | – | – | Ortiz-Santana et al. (2013) | |
| Vlasak 1110/9 (JV) | CZ | – | KR605783 | – | – | – | Han et al. (2016) | |
| FIAPH485-13 | Fl | – | ON970648 | – | – | – | This study | |
| F. roseofusca | Ryvarden 42363 (0) | VE | ON970634 | ON970634 | – | – | – | This study |
| Vlasak 1908/83 (H) | GF | ON924684 | ON994737 | OR703809 | – | – | This study | |
| F. sagraeana | Vlasak 1512/3J (H) | CR | ON924685 | ON994738 | OP022455 | – | – | This study |
| CBS 424.84 | MX | – | DQ491402 | – | – | – | Kim et al. (2008) | |
| Kout 0610/K9 (JV) | MX | – | KF999922 | – | – | – | Han & Cui (2015) | |
| Lopez 1324 (0 14115) | CR | – | KF999923 | – | – | – | Han & Cui (2015) | |
| Vlasak 1412/5J (H) | CR | – | ON994739 | – | – | – | This study | |
| Vlasak 1512/2 J (H) | CR | – | ON994740 | – | – | – | This study | |
| Vlasak 0904/39 (H) | US-FL | – | ON994741 | – | – | – | This study | |
| Vlasak 1707/27J (H) | US-FL | – | ON994742 | – | – | – | This study | |
| F. sandaliae | HUBO 7083 | IT | JN592495 | JN592495 | – | – | – | Vlasák et al. (2012) |
| F. scalaris | Vlasak 1808/50 (JV) | GF | ON924686 | ON994743 | – | – | – | This study |
| Vlasak 1909/66 (H) | GF | – | ON994744 | OP022456 | – | – | This study | |
| F. schrenkii | FP-58527 (CFMR) | US-SD | – | FP-58527 SS1 | – | AEHC02000126 | AEHC02000044 | JGI; GenBank |
| FP-105881-R (CFMR) | US-CO | – | KF169641 | – | KF160710 | KF178366 | Haight et al. (2016) | |
| JEH-121A (CFMR) | US-NM | – | KU169355 | – | MK208856 | MK236354 | Haight et al. (2019) | |
| JEH-142-ss12 (CFMR) | US-NM | – | KF169642 | – | KF160711 | KF178367 | Haight et al. (2016) | |
| JEH-142-ss14 (CFMR) | US-NM | – | KF169643 | – | KF160712 | KF178368 | Haight et al. (2016) | |
| JEH-142-ss5 (CFMR) | US-NM | – | KF169644 | – | KF160713 | KF178369 | Haight et al. (2016) | |
| JEH-142-ss6 (CFMR) | US-NM | – | KF169645 | – | KF160714 | KF178370 | Haight et al. (2016) | |
| JEH-144 (CFMR) | US-NM | – | KU169364 | – | MK208857 | MK236355 | Haight et al. (2019) | |
| JEH-150 (CFMR) | US-SD | – | KU169365 | – | MK208858 | MK236356 | Haight et al. (2019) | |
| JEH-152 (CFMR) | US-SD | – | KU169367 | – | MK208859 | MK236357 | Haight et al. (2019) | |
| JW24-525-0-sap (CFMR) | US-CO | – | KF169646 | – | KF160715 | KF178371 | Haight et al. (2016) | |
| JW24-549B-1-sap (CFMR) | US-CO | – | KF169647 | – | KF160716 | KF178372 | Haight et al. (2016) | |
| JW18-240-1-sap (CFMR) | US-CO | – | KF169648 | – | KF160717 | KF178373 | Haight et al. (2016) | |
| J. J. Worrall w/n (CFMR) | US-CO | – | KF169649 | – | KF160718 | KF178374 | Haight et al. (2016) | |
| RLG-10752-Sp (CFMR) | US-AZ | – | KF169650 | – | KF160719 | KF178375 | Haight et al. (2016) | |
| Ahonen 58 (H) | US | – | MN148248 | – | – | MN161763 | Liu et al. (2021) | |
| Vlasak 1209/61J (BJFC) | US-AZ | – | MN148247 | – | MN158180 | MN161762 | Liu et al. (2021) | |
| F. sclerotina | Syme 2967 (PERTH) | AU | ON691652 | ON691652 | ON667941 | – | – | This study |
| F. serialiformis | FP-105717 (CFMR) | US-MD | KC585126 | KC585302 | KY949000 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| F. serialis | Sig1Antser10 (0) | NO | Sig1Antser10 | Sig1Antser10 | Sig1Antser10 | – | – | JGI |
| Vlasak 1509/5 (JV) | CZ | – | KT995120 | OP022457 | – | – | Spirin et al. (2017); this study | |
| F. Solaris | isolate IBL104f | PL | – | KT334658 | – | – | – | GenBank |
| MR 11937 | AR | – | JF713082 | – | – | – | Rajchenberg et al. (2011) | |
| Boertmann 2013-501446 (C) | DK | – | ON994745 | – | – | OP215838 | This study | |
| K(M) 84579 | GB | – | ON808966 | – | – | – | This study | |
| K(M)85251 | GB | – | ON808967 | – | – | – | This study | |
| K(M)137688 | GB | – | ON808968 | – | – | – | This study | |
| K(M)153625 | GB | – | ON808965 | – | – | – | This study | |
| K(M)169193 | GB | – | ON808964 | – | – | – | This study | |
| K(M) 180117 | GB | – | ON808969 | – | – | – | This study | |
| K(M)191007 | JE | – | ON808963 | – | – | – | This study | |
| Niemela 3058 (H) | CA-NT | – | ON994746 | – | – | OP215839 | This study | |
| Niemela 3061 (H) | CA-NT | – | ON994747 | – | – | – | This study | |
| Rivoire 4732 (LY) | FR | – | ON994748 | – | – | OP215840 | This study | |
| Zmitrovich 2008 (H) | IL | – | ON994749 | – | – | OP215841 | This study | |
| F. spraguei | Ryvarden 46569 (H) | US-TN | – | ON970644 | – | – | – | This study |
| Vlasak 1608/14J (JV) | US-NY | ON924687 | ON994750 | OP022458 | – | – | This study | |
| CBS 365.64 | US-OH | – | D0491406 | – | – | – | Kim et al. (2008) | |
| Rivoire 4638 (LY) | FR | – | KR605784 | – | – | – | Han et al. (2016) | |
| TENN069504 | US-TN | – | KY777368 | – | – | – | GenBank | |
| TENN065680 | US-TN | – | MG663257 | – | – | – | GenBank | |
| Mushroom Observer 247114 | US-NC | – | MK571181 | – | – | – | GenBank | |
| S.D. Russell Mycomap 6609 | US-IN | – | MK560112 | – | – | – | GenBank | |
| S.D. Russell Mycomap 73 | US-IN | – | MK575221 | – | – | – | GenBank | |
| S.D. Russell Mycomap 1318 | US-IN | – | MK575222 | – | – | – | GenBank | |
| S.D. Russell Mycomap 105 | US-IN | – | MK575223 | – | – | – | GenBank | |
| W. Langer 348 | US-IN | – | OM972619 | – | – | – | GenBank | |
| F. squamosella | Bernicchia 7690-03 (HUBO) | IT | ON924688 | ON994751 | OP022459 | – | – | This study |
| F. stereoides | 0 10551 | ET | – | FJ403215 | – | – | – | Lindner etal. (2011) |
| F. subectypa | Vlasak 0312/24.7 J (JV) | US-FL | – | KP171197 | – | – | – | Han et al. (2015) |
| Vlasak 0904/19 (H) | US-FL | – | KP171198 | – | – | – | Han et al. (2015) | |
| Vlasak 0904/20 (BJFC) | US-FL | – | KP171199 | – | – | – | Han et al. (2015) | |
| Vlasak 1408/3 (JV) | CR | – | ON994752 | – | – | – | This study | |
| Vlasak 1704/107 J (JV) | CR | – | ON994753 | – | – | – | This study | |
| F. subpinicola | Cui 9836 (BJFC) | CN | – | MN148249 | – | – | MN161764 | Liu et al. (2021) |
| Cui 9819 (BJFC) | CN | – | MN148250 | – | – | MN161765 | Liu et al. (2021) | |
| Dai 11101 (BJFC) | CN | – | MN148251 | – | MN158182 | MN161766 | Liu et al. (2021) | |
| Dai 11206 (BJFC) | CN | – | MN148252 | – | MN158183 | MN161767 | Liu et al. (2021) | |
| Dai 13480 (BJFC) | CN | – | MN148253 | – | MN158184 | MN161768 | Liu et al. (2021) | |
| Yuan 4912 (BJFC) | CN | – | MN148254 | – | – | MN161769 | Liu et al. (2021) | |
| F. substratosa | Cui 9229 (BJFC) | CN | – | KR605789 | – | – | – | Han et al. (2016) |
| F. sulcata | Kout 1408/K5 (H) | TH | OR702646 | OR702647 | – | – | – | This study |
| F. tianshanensis | Wei 1568 (BJFC) | CN | – | MN 148255 | – | – | MN161770 | Liu et al. (2021) |
| Wei 1473a (BJFC) | CN | – | MN 148256 | – | – | MN161771 | Liu et al. (2021) | |
| Wei 1462a (BJFC) | CN | – | MN 148257 | – | – | MN161772 | Liu et al. (2021) | |
| Cui 16821 (BJFC) | CN | – | MN148258 | – | – | MN161773 | Liu et al. (2021) | |
| Cui 16823 (BJFC) | CN | – | MN148259 | – | – | MN161774 | Liu et al. (2021) | |
| Cui 16825 (BJFC) | CN | – | MN148260 | – | – | MN161775 | Liu et al. (2021) | |
| Cui 16828 (BJFC) | CN | – | MN148261 | – | – | MN161776 | Liu et al. (2021) | |
| F. tristis | Miettinen 14263(H) | ID-PB | OQ701095 | OQ701095 | – | – | – | This study |
| F. tropica | Dai 13434 (BJFC) | CN | KX958185 | KX958181 | – | – | – | Yuan et al. (2017) |
| F. tumulosa | CBS 332.49 | AU | MH856544 | MH856544 | – | – | – | Vu et al. (2019) |
| F. tunicata | Miettinen 8579 (H) | ID-RI | ON970631 | ON970631 | OQ789527 | – | – | This study |
| Miettinen 13050 (H) | ID-SB | ON970635 | ON970635 | OQ789527 | – | – | This study | |
| F. uralensis | Cui 3705 (BJFC) | CN | – | FJ627258 | – | – | – | GenBank |
| Dai 6082 (BJFC) | CN | – | JO837943 | – | – | – | Cui (2013) | |
| Dai 6118 (BJFC) | CN | – | KC951178 | – | – | – | Cui & Dai (2013) | |
| Cui 10277 (BJFC) | CN | – | KX958178 | – | – | – | Yuan et al. (2017) | |
| Spirin 5399 (H) | RU-KHA | ON970647 | ON970647 | – | – | OP019458 | This study | |
| Spirin 10946 (H) | RU-KHA | – | ON994756 | – | – | OP215842 | This study | |
| F. ussuriensis | SNU m-05072501 | KR | – | DQ491409 | – | – | – | Kim et al. (2008) |
| SFC04010313 | KR | – | DQ491411 | – | – | – | Kim et al. (2008) | |
| BCRC 35447 | TW | – | EU232200 | – | – | – | GenBank | |
| KUC20130725-21 | KR | – | KJ668548 | – | – | – | GenBank | |
| Dai 3023 (H) | CN-JL | ON970643 | ON970643 | – | – | – | This study | |
| F. yunnanensis | Zhao 4566 (SWFC) | CN | MT497887 | MT497885 | – | – | – | Han et al. (2020) |
| F. variiformis | FP-104442 (CFMR) | US-CO | KC585134 | KC585309 | KY948997 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| F. visenda | Miettinen 6223 (H) | ID-JA | OQ701096 | OQ701096 | – | – | – | This study |
| Fomitopsis sp. Darwin (M.D. Barrett F17/09) | Barrett F17/09 (DNA D0268068) | AU | ON691653 | ON691653 | ON667941 | – | – | This study |
| Laetiporus sulphureus | CT-1 | US-CT | EU402532 | EU402565 | KY949025 | – | – | Lindner & Banik (2008); Justo et al. (2017) |
| Laricifomes officinalis | Kotiranta 27358 (H) | RU-SA | ON924689 | ON994757 | OP022460 | – | – | This study |
| Megasporoporia eutelea | Razmadze 2202/9 (H) | OM | – | ON970654 | – | – | – | This study |
| Postia balsamea | FP-135372-Sp (CFMR) | GB | KC585187 | KC585358 | KY948974 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Postia cyanescens | Kinnunen 5087 (H) | PL | KY948885 | KY948814 | KY948978 | – | – | Justo et al. (2017) |
| Pseudoantrodia monomitica | Dai 10828 (BJFC) | CN | – | MG787601 | – | – | – | Liu et al. (2022) |
| P. monomitica | Dai 13381 (BJFC) | CN | – | MG787602 | – | – | – | Liu et al. (2022) |
| Pseudophaeolus trichrous | Vlasak 2203/5 (H) | CR | OQ660486 | ON994758 | OP022461 | – | – | This study |
| Pycnoporellus fulgens | CA-20(Ta) | US-CA | KC585218 | KC585385 | KY949040 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Rhodonia placenta | Dietz7E | US-CA | KC585223 | KC585390 | KY949028 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Sarcoporia polyspora | L-14910-Sp (CFMR) | US-NY | KC585226 | KC585393 | KY949022 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Skeletocutis chrysella | L-15957-Sp (CFMR) | US-NY | – | – | KY948982 | – | – | Justo et al. (2017) |
| Miettinen 9472 (H) | Fl | FN907916 | FN907916 | – | – | – | Miettinen & Larsson (2011) | |
| Sparassis radicata | OKM4756 (CFMR) | US-ID | KF053407 | KC987580 | KY949023 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
| Trametes junipericola | PRM842893 | MA | – | ON994759 | – | – | – | This study |
| Wolfiporia dilatohypha | CS635913 | US-KY | KC585234 | KC585400 | KY949026 | – | – | Ortiz-Santana et al. (2013); Justo et al. (2017) |
Taxon sampling for phylogenetic analyses
We compiled 22 datasets for phylogenetic analyses (Table 2). Datasets were aligned in the online version of MAFFT v. 7 (Katoh et al. 2019) using the E- or G-INS-I algorithm or PRANK (Löytynoja & Goldman 2010) followed by manual adjustment in AliView (Larsson 2014) or PhyDE (Müller et al. 2010). Each marker was aligned separately. GBlocks (Castresana 2000) followed by manual curation was used to select those regions of the sequences that were confidently aligned to be included in the family- and genus-level analyses. The number of included taxa, as well as length of aligned datasets with and without the excluded regions are given in Table 2 and Suppl. Table S1.
Table 2.
Datasets compiled for the present study.
| Dataset | Figure number | Number of sequences | Alignment length | Number of parsimony informative sites |
|---|---|---|---|---|
| ITS + LSU + RPB1 brown-rot Polyporales | 1 | 58 | 1 913 | 338 |
| Seven-gene Fomitopsidaceae | 2 | 50 | 1 997 | 170 |
| ITS + LSU + RPB1 Fomitopsis | 3, 4 | 59 | 1 997 | 231 |
| ITS Daedalea clade | 5 | 88 | 438 | 136 |
| ITS Pilatoporus clade | 6 | 106 | 401 | 58 |
| ITS + TEF1 F. marianii complex | 7 | 28 | 1 046 | 58 |
| ITS + TEF1 F. meliae complex | 8 | 23 | 1 016 | 139 |
| ITS + TEF1 + RPB1 Pilatoporus clade | 9 | 12 | 2 408 | 261 |
| ITS + TEF1 + RPB2 F. pinicola clade | 10 | 75 | 1 244 | 92 |
| ITS F. feei clade | 11 | 60 | 481 | 174 |
| ITS F. rosea clade | 12 | 25 | 488 | 71 |
| TEF1 F. ramentacea clade | 13 | 13 | 526 | 18 |
| ITS + TEF1 F. ramentacea clade | 14 | 13 | 1 072 | 50 |
| ITS F. spraguei clade | 15 | 21 | 548 | 23 |
| Seven-gene Polyporales | S1 | 124 | 3 990 | 190 |
| ITS + LSU Fomitopsis | S2 | 65 | 1 218 | 121 |
| TEF1 F. marianii complex | S3 | 22 | 452 | 40 |
| TEF1 F. meliae complex | S4 | 22 | 427 | 67 |
| ITS + TEF1 F. pinicola clade | S5 | 104 | 711 | 50 |
| ITS F. ramentacea clade | S6 | 35 | 546 | 34 |
| ITS F. juniperina clade | S7 | 21 | 574 | 29 |
| ITS Buglossoporus clade | S8 | 20 | 680 | 168 |
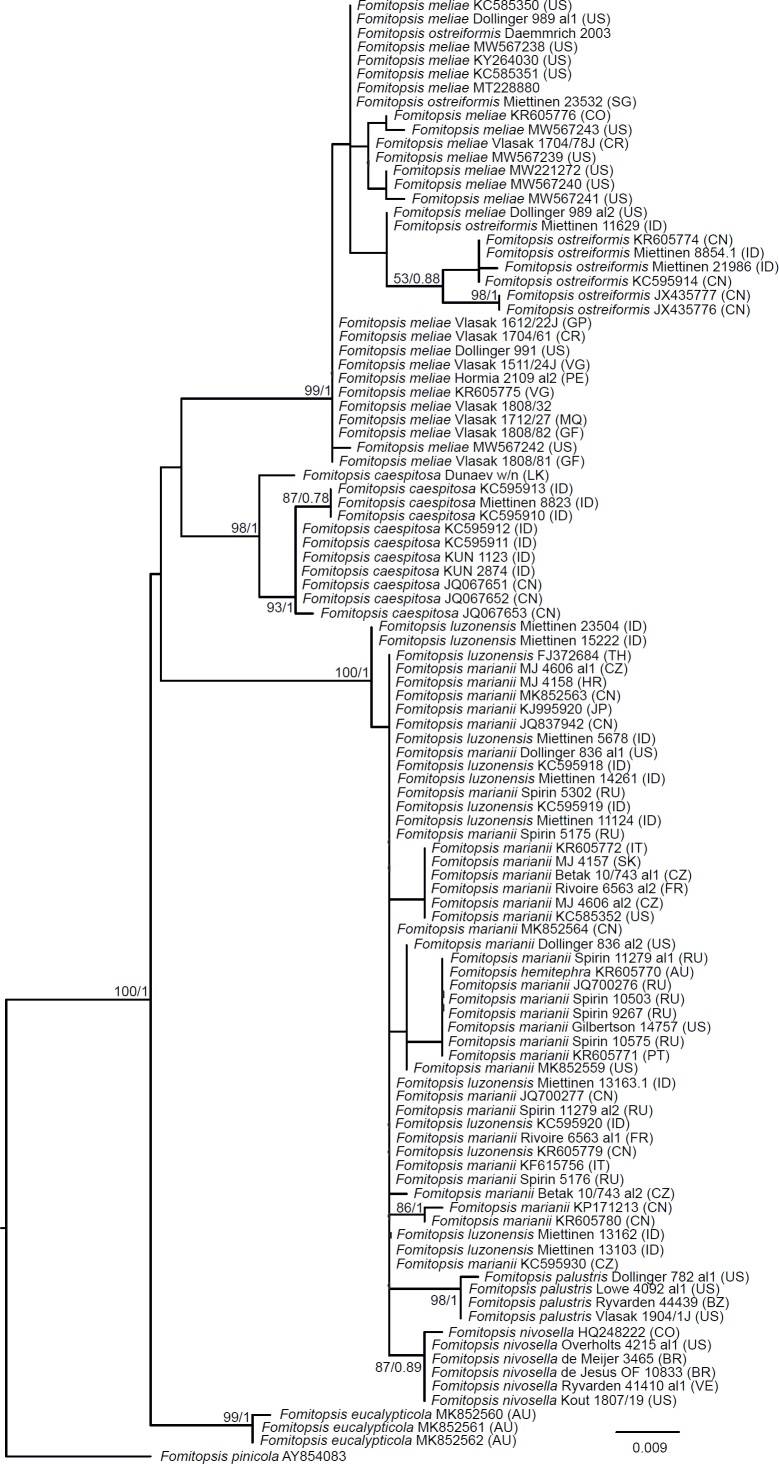
The first and second datasets used seven markers: nrDNA 18S (SSU), ITS, 28S (LSU), mtDNA 16S (mtSSU), RPB1, RPB2 and TEF1. Taxa were selected to this dataset after Liu et al. (2022) and other available sequences including genomes, so that at least LSU and two additional markers were present. In the first dataset (the seven-gene Polyporales dataset), we aimed to cover all families in the Polyporales with emphasis on the brown-rotters. Outgroups were Thelephora (Thelephorales) and Heterobasidion (Russulales), in accordance to Miyauchi et al. (2020). For Pseudophaeolus soloniensis, we excluded mtSSU (GenBank OM039220) from the analysis, since it is nearly identical with Fomitopsis pinicola and has either a wrong identity or is in severe phylogenetic conflict with the rest of the data. The final dataset represented 124 species (Suppl. Table S2). In the second dataset (the seven-gene Fomitopsidaceae dataset), we focused on the Fomitopsidaceae and allied taxa subsampling from the first dataset. The outgroup for the Fomitopsidaceae included Amyloporia, Rhodonia, and Ryvardenia, and was selected based on the results of the analyses of the seven-gene Polyporales dataset. The final dataset represented 50 species.
The seven-gene Polyporales and seven-gene Fomitopsidaceae datasets, while maximizing the number of markers, had a significant amount of missing data (Suppl. Table S2). In the seven-gene Polyporales dataset, in total 160 out of 875 single markers (18%) were lacking across all taxa and all markers. In the seven-gene Fomitopsidaceae dataset, in total 94 out of 350 single markers (27%) were lacking across all taxa and all markers. For this reason, we compiled additional datasets with fewer markers but a full matrix of high-quality markers.
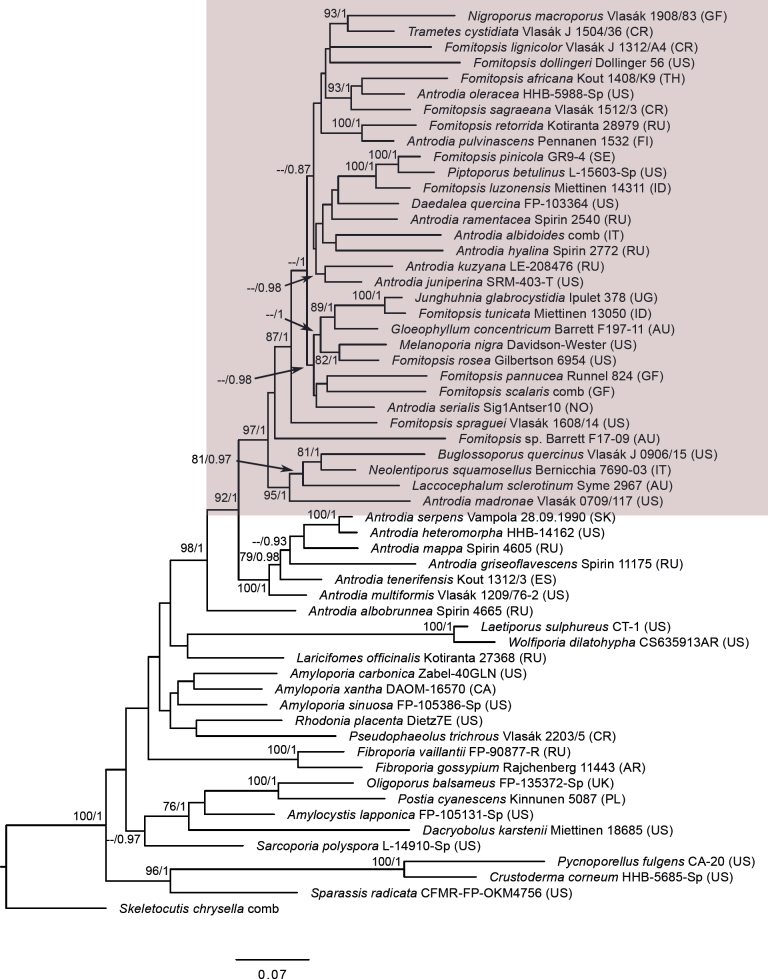
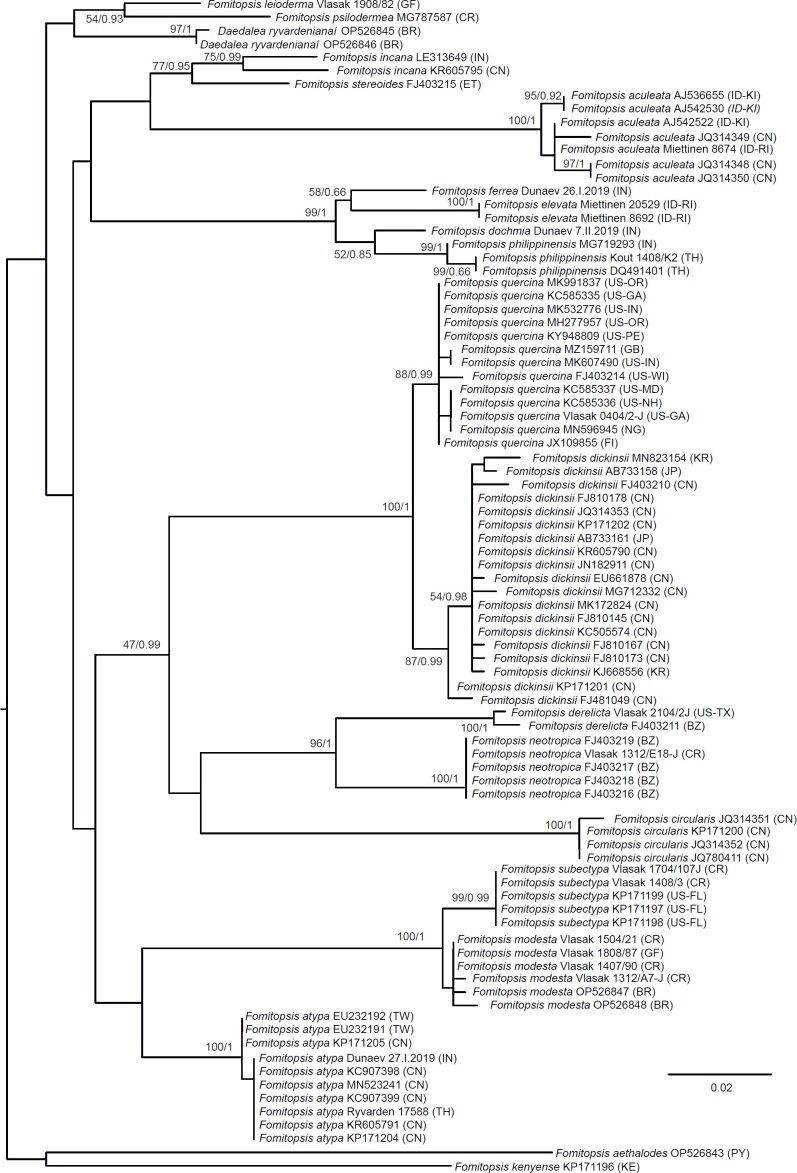
The third dataset (the three-gene brown-rotter dataset), had the aim of showing the position and limits of Fomitopsidaceae among other brown-rotting families in the so-called antrodia clade in the Polyporales (Ortiz-Santana et al. 2013, Justo et al. 2017). It contained a selection of combined ITS + LSU + RPB1 sequences representing all major groups in this clade including Fomitopsidaceae, amyloporia/fibroporia clade, Dacryobolaceae and Laetiporaceae. Skeletocutis chrysella; a representative of the neighbouring ‘Skeletocutis/Tyromyces’ clade (Justo et al. 2017), was used as an outgroup. The final dataset represented 58 species (32 taxa from Fomitopsidaceae, six taxa from the neighbouring Antrodia sensu stricto, Antrodia (Anthroporia) albobrunnea and two to four taxa from each of the other larger groups in the antrodia clade).
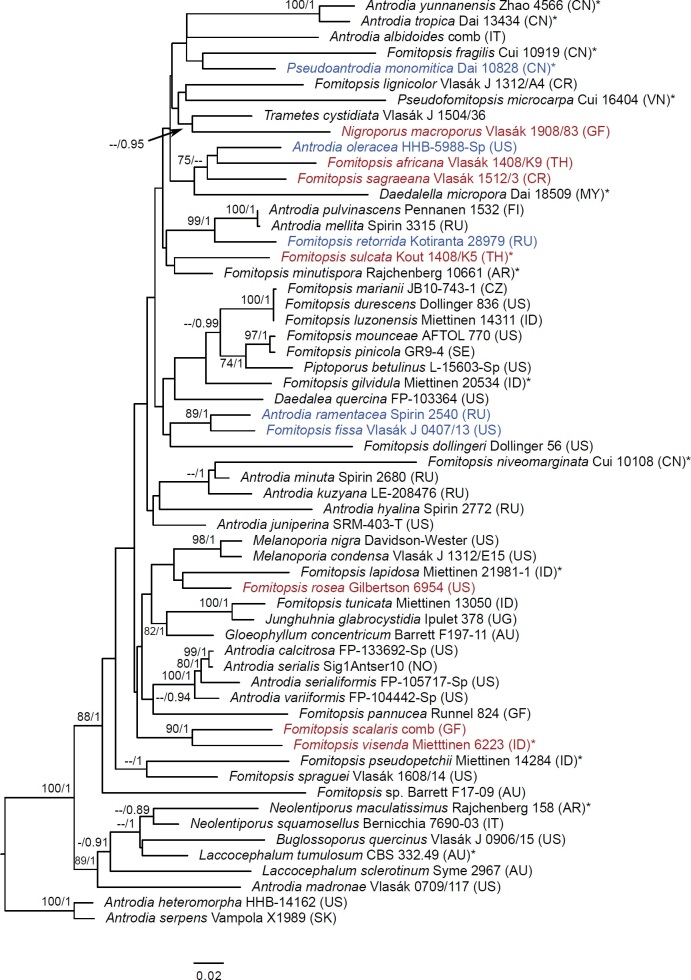
The fourth and fifth datasets assessed phylogenetic relationships of species in the ‘Daedalea – Fomitopsis clade’. Both datasets consisted of sequences representing the members of Fomitopsis sensu lato in Fomitopsidaceae, Daedalea and Piptoporus spp. and residual Antrodia spp. (after Han et al. 2016, Justo et al. 2017, Runnel et al. 2019 and Liu et al. 2022). Two representatives of the closely related Antrodia sensu stricto (Runnel et al. 2019) were used as an outgroup. The fourth dataset (the Fomitopsis ITS+LSU+RPB1 dataset), mainly contained combined ITS+LSU+RPB1 sequences (in total, 44), but in addition 15 samples represented only by ITS+LSU were included to position the key taxa in Fomitopsis clade that lacked the RPB1 marker. The total number of sequences in the second dataset was 59. The fifth dataset (Fomitopsis ITS+LSU dataset) was limited to the combined ITS+LSU region and included five additional taxa that were only represented with ITS sequences (totalling 65 taxa).
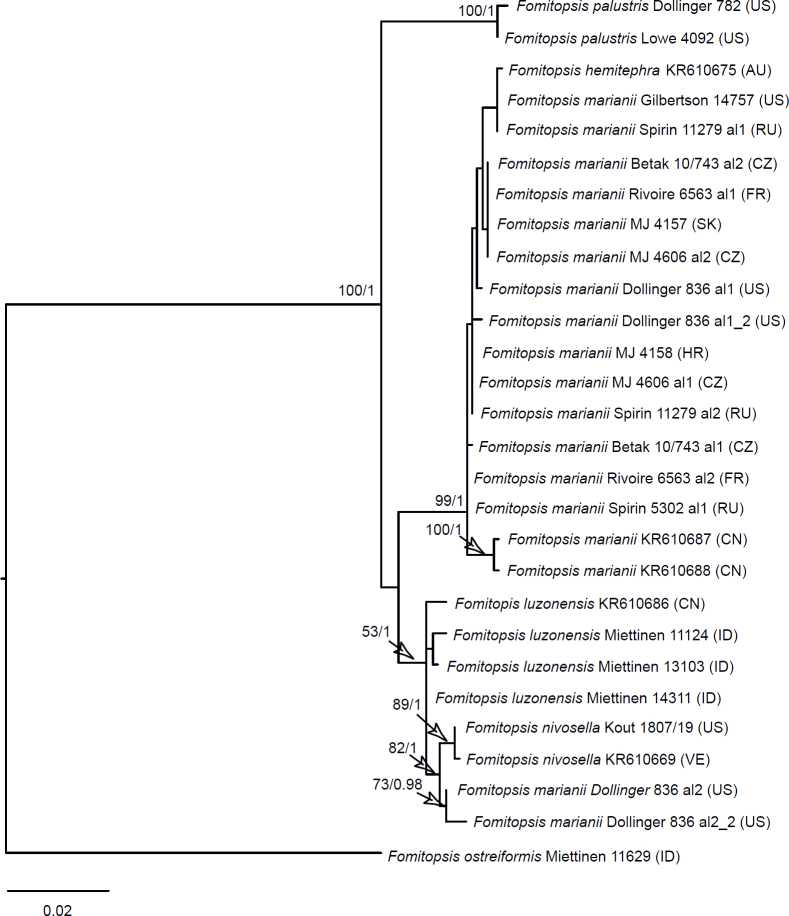
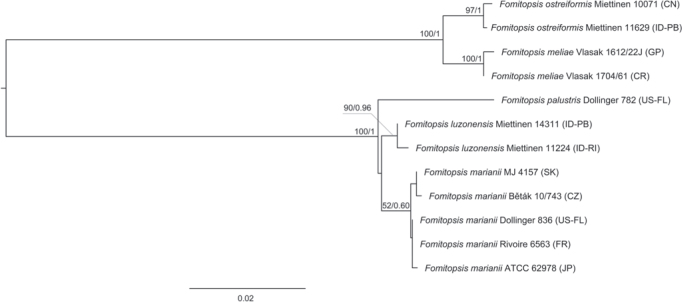
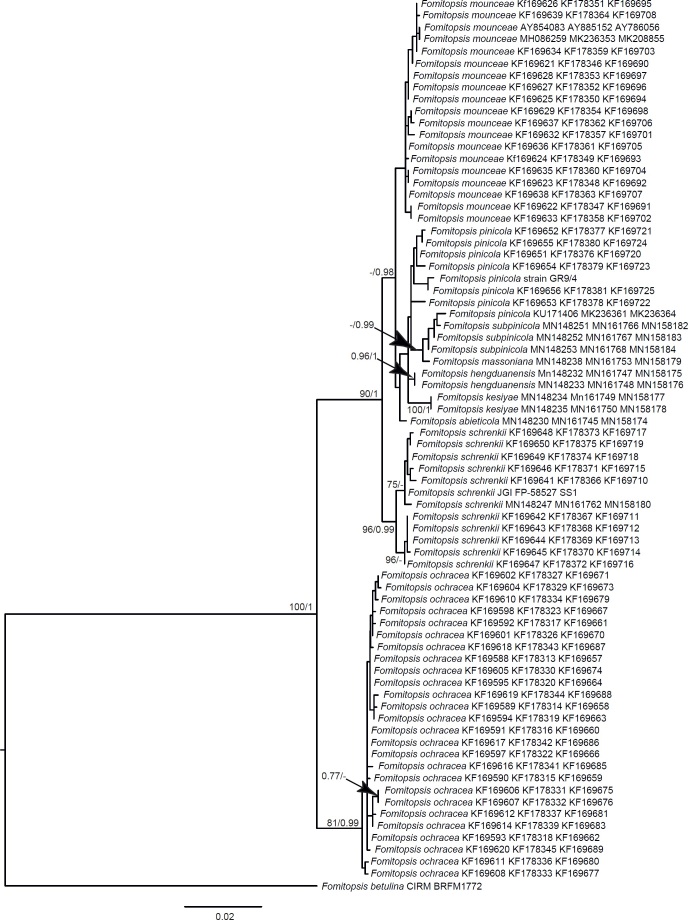
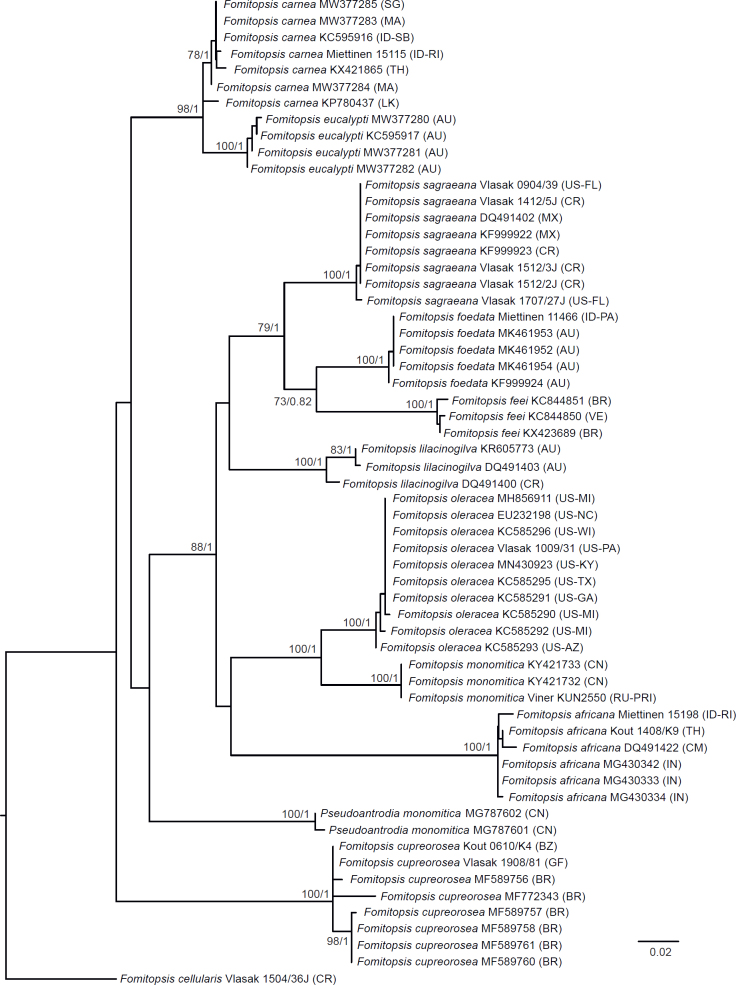
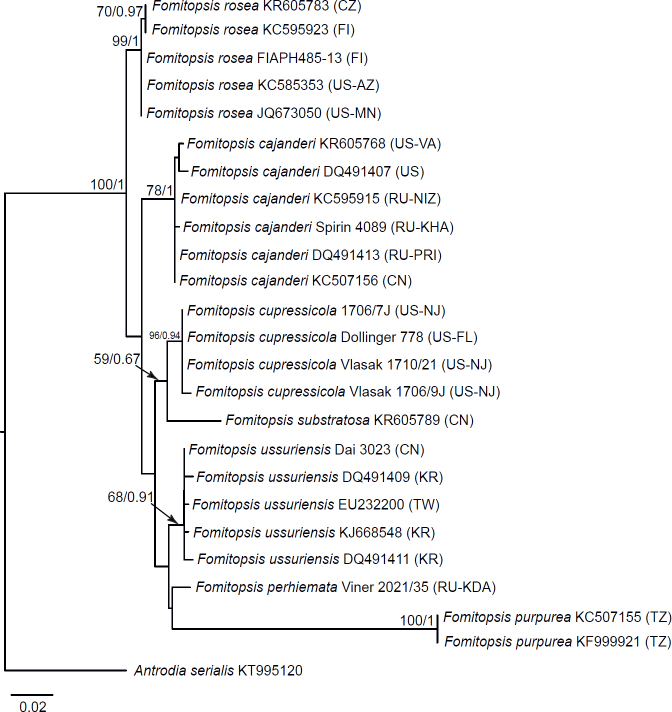
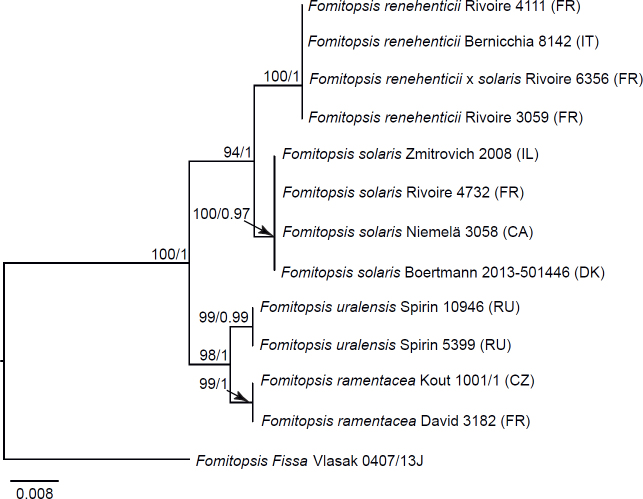
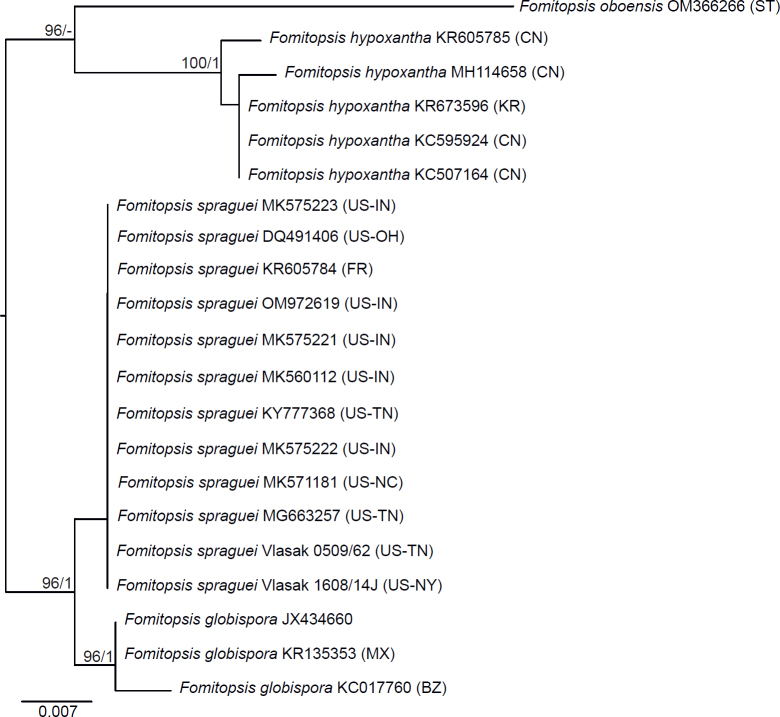
Seventeen additional datasets were compiled to justify species concepts in several lineages within the Daedalea – Fomitopsis group (Table 2, Suppl. Table S2). In each case, the outgroup selection was guided by the three-gene phylogenies of the brown-rot Polyporales and Fomitopsis clade (see Figs 1 and 3).
Fig 1.
Phylogenetic placement of the Daedalea – Fomitopsis clade (box) within the “antrodia clade” (including all taxa shown other than outgroups) based on Maximum Likelihood of the ITS + LSU + RPB1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
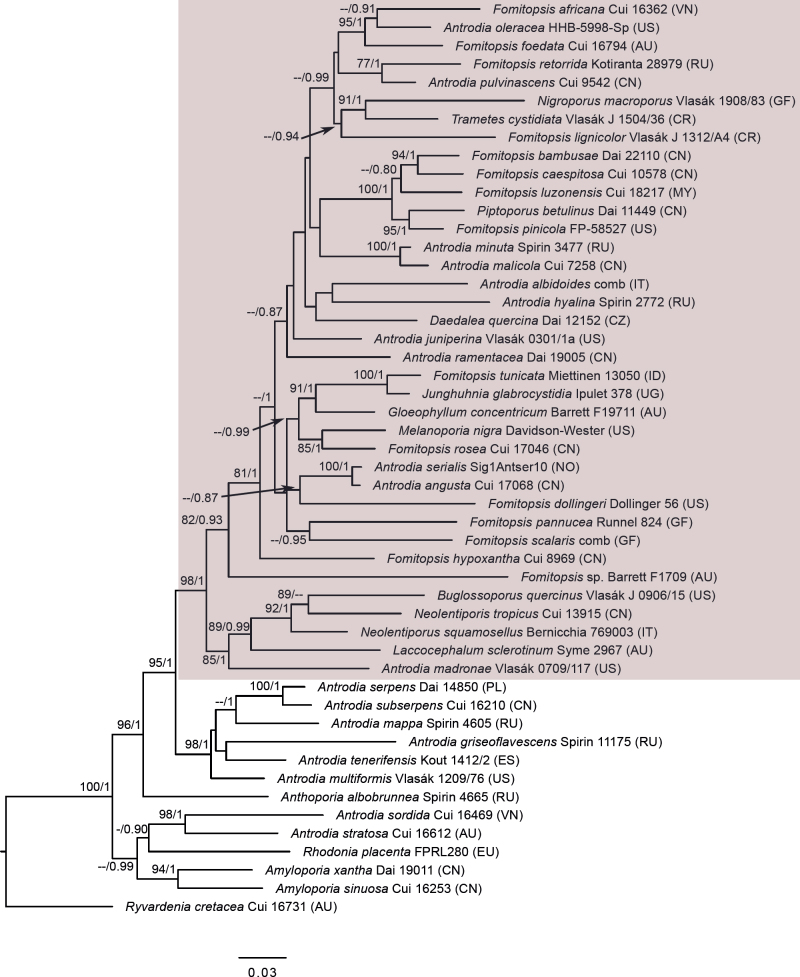
Fig. 3.
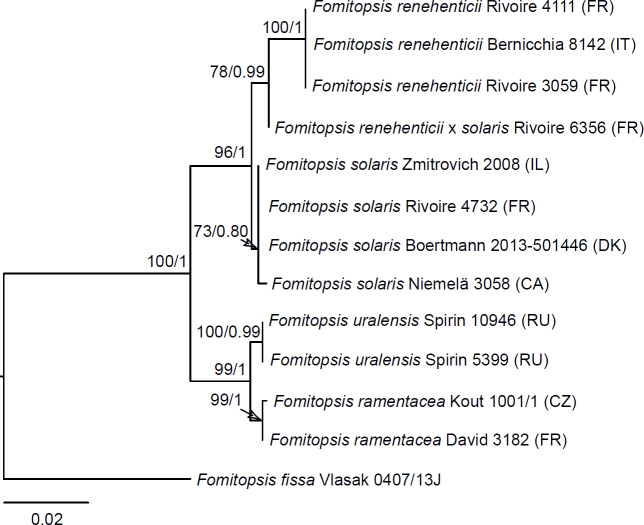
Phylogenetic relationships of species in the Daedalea – Fomitopsis clade based on Maximum Likelihood of the ITS + LSU + RPB1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The species represented with ITS + LSU only are marked with an asterisk (*). The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin. Names of pink-coloured perennial species are given in red, those of monomitic species are in blue.
Phylogenetic analyses
We conducted Maximum Likelihood (ML) and Bayesian inference (BI) for all datasets. The ITS and LSU regions and introns and exons of protein coding markers were partitioned separately. ModelTest-NG (Darriba et al. 2020) was used to select models for the order- and family-level analyses, using AICc as the selection criterion. The same models were used in ML and BI analyses (Suppl. Table S1). For these datasets, we used RAxML-NG v. 1.1.0 (Kozlov et al. 2019) to search for the highest likelihood tree with 50 parsimony and 50 random starting trees, coupled with 1 000 bootstrap replicates. MrBayes v. 3.2.7a was run with three parallel runs 8 chains each for 10–40 million generations, sampling every 10 000 generations (Suppl. Table S1). Good convergence (SD of split frequencies < 0.01) was reached in all cases except seven-gene Polyporales dataset (SD still over > 0.2 after 40 million generations) and the Fomitopsis ITS+LSU dataset (moderate convergence of SD ≈0.02). These computations were run at the CSC – IT Centre for Science (Espoo, Finland) computing environment.
For the phylogenetic analyses of species complexes within Fomitopsis, we used IQ-TREE v. 1.2.2 (Nguyen et al. 2015) with 1 000 bootstrap (bs) replicates and the ‘best-fitted model’ for searching the ML tree. MrBayes v. 3.2.6 (Ronquist et al. 2012) was used for BI analyses at the CIPRES Science Gateway (Miller et al. 2010). Nucleotide substitution models for the species level BI datasets were chosen with TOPALI v. 2.5 (Milne et al. 2008) based on the Bayesian information criterion (BIC). Two parallel MCMC analyses were run, each consisting of four chains, initiated from random starting trees. The analyses were run for 3–10 million generations, sampling every 1 000 generations (Suppl. Table S2). The first 25% of the trees were discarded as the burn-in ensuring the average standard deviation of split frequencies had reached < 0.01 for all datasets, after which posterior probability (pp) values were calculated. Analyses for the Buglossoporus clade, three-gene Fomitopsis palustris group and F. juniperina group datasets were conducted as described for the family-level analyses. Results were also analysed in Tracer v. 1.6.0 (Rambaut et al. 2018). Posterior probabilities were calculated from the remaining trees in all cases.
The final alignments for all datasets as well as the input data for the phylogenetic analyses (nexus blocks) and phylograms are stored in the PlutoF web platform, at https://dx.doi.org/10.15156/BIO/2483943.
RESULTS
Phylogenetic placement and structure of the Fomitopsidaceae
We undertook an order-wide, seven-gene analysis of the Polyporales with a focus on brown-rot fungi, at most parts mirroring the one of Liu et al. (2022). We used this dataset to assess the monophyly and placement of the Fomitopsidaceae (sensu Justo et al. 2017). The Bayesian analysis of this dataset could not be concluded satisfactorily, since runs of the Bayesian analysis (Suppl. Fig. S1) did not converge. We suggest this is partly due to missing marker data and availability of only short fragments for some markers (RPB2, TEF1), but notwithstanding these issues this set of markers is probably still too small to recover the complex branching history in the order. We studied topologies of consensus trees of different runs and high-probability stationary states individually as well as the ML tree (Suppl. Fig. S1), and while they differ in the case of many deeper nodes without support, they agreed on some aspects.
Monophyly of brown-rot fungi was never supported, but the recognized families Dacryobolaceae, Laetiporaceae and Sparassidaceae appeared with varying support. Fomitopsidaceae was consistently present in all the consensus trees (ML bs = 95%, overall Bayesian pp = 0.9). In all analyses Amyloporia was placed as its sister clade, except in some runs when it was the sister of a Fomitopsidaceae – Laricifomes – Ryvardenia clade. Monophyly of Amyloporia – Fibroporia clade earlier introduced by Justo et al. (2017) was not supported. A big clade of brown-rot fungi with Fomitopsidaceae, Amyloporia, Laricifomes – Ryvardenia, Fibroporia, Pseudophaeolus, Sparassis, Crustoderma – Pycnoporellus and Laetiporaceae was also consistent in all the trees, albeit not always with good support (bs = 34%, pp = 0.88).
Next, we conducted a partially overlapping analysis of a three-gene brown-rotter dataset, which represents a sample of brown-rot families in the antrodia clade, but with no missing marker data. This analysis showed that Fomitopsidaceae (sensu Justo et al. 2017) resolves as highly supported (bs = 98%, pp = 1) (Fig. 1). Based on these results, we are fairly confident in the circumscription of Fomitopsidaceae. Its closest relatives include other dimitic brown-rot polypores traditionally placed in Antrodia or Fomitopsis, such as Amyloporia. Additionally, our data assert that Fomitopsis officinalis should be excluded from Fomitopsidaceae and treated under Laricifomes, for which it is the type species (Fig. 1), in accordance with the previous results of Liu et al. (2022).
The three-gene brown-rotter analysis split Fomitopsidaceae in three well-supported clades (Fig. 1): (1) Antrodia albobrunnea clade (= Anthoporia) represented by a single species; (2) Antrodia sensu stricto clade as redefined by Runnel et al. (2019) (bs = 100%, pp = 1) with several poroid and one corticioid species; (3) a large Daedalea – Fomitopsis clade (bs = 97%, pp = 1). Our third phylogeny, based on the seven-gene dataset of Fomitopsidaceae and satellite taxa (Fig. 2), corroborates monophyly of the Daedalea – Fomitopsis clade (bs = 98%, pp = 1). Justo et al. (2017) first recognized the Daedalea – Fomitopsis group as a strongly supported clade and our expanded datasets confirm that result.
Fig 2.
Phylogenetic placement of the Daedalea – Fomitopsis clade (box) within the Fomitopsidaceae and allied taxa (including all taxa shown other than outgroups) based on Maximum Likelihood of the ITS + LSU + SSU + mtSSU + RPB1 + RPB2 + TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Three subclades can be defined in the Daedalea – Fomitopsis clade, detected in our seven-gene analyses (Suppl. Fig. S1, Fig. 2) and the three-gene brown-rotter analysis (Fig. 1):
-
-
the Buglossoporus subclade containing the effusedreflexed Antrodia madronae, stipitate terrestrial sclerotiaforming Laccocephalum spp. and semistipitate wood-inhabiting Neolentiporus squamosellus and Buglossoporus quercinus;
-
-
a single-species lineage represented by an undescribed species, Fomitopsis sp. Darwin (M.D. Barrett F17/09) from tropical Australia, which is unusual in Fomitopsis sensu lato in having stipitate to substipitate basidiocarps, and lacking clamps at most septa (scattered clamps only);
-
-
the Fomitopsis subclade containing the rest (vast majority) of the species.
The Bayesian inference (BI) topology makes it possible to consider the Fomitopsis spraguei complex as a separate lineage although this option is not supported in the maximum likelihood (ML) topology (Fig. 1 – represented by F. spraguei, Fig. 2 – by F. hypoxantha).
Our second three-gene dataset focussed on the Daedalea – Fomitopsis clade (Fig. 3). This dataset had higher coverage of Fomitopsis than the previous analyses but was missing some RPB1 marker data. Among these taxa, sixteen species are either types of genera or closest relatives of the type species. Most of these genera have been introduced in the last seven years after splitting Fomitopsis and Antrodia sensu lato (Han et al. 2016, Audet 2017, Liu et al. 2022). The analyses based on this extended dataset supported the division of the Daedalea – Fomitopsis clade to only two subclades (Buglossoporus and Fomitopsis, the latter containing also Fomitopsis sp. Darwin), but the overall topology was broadly similar to the three- and seven-gene datasets described above. Importantly, the Daedalea – Fomitopsis subclade was divided into about 40 supported clades or orphan species, whose relations were unresolved.
Of the taxa that lacked the RPB1 sequence, Fomitopsis niveomarginata formed a long distinct branch within the Daedalea – Fomitopsis clade in the phylogenetic tree representing the Daedalea – Fomitopsis three-gene dataset (Fig. 3) and the corresponding ITS+LSU dataset (Suppl. Fig. S2). Adding F. niveomarginata to the phylogenetic analyses causes a slight change in the inner structure of the Daedalea – Fomitopsis clade (Fig. 1 vs. Fig. 3). As a result, the inner structure of the largest subclade containing this species (i.e. the Fomitopsis subclade) loses support for the most part.
Alternative treatments of genus level taxonomy in Fomitopsidaceae
Taxonomically, the outcome of our phylogenetic analyses can be interpreted in four possible ways:
The microgeneric approach. Each strongly supported terminal lineage in the Daedalea – Fomitopsis group is accepted as a separate genus. This approach was first applied by Han et al. (2016) who advocated splitting Fomitopsis sensu lato into several smaller genera. However, more extensive taxon sampling makes maintaining this approach problematic for two main reasons. First, even smaller genera become more ill-defined after adding more Fomitopsis sensu lato/Daedalea/residual Antrodia spp. to the dataset. For example, Antrodia oleracea makes Rhodofomitopsis polyphyletic and Rubellofomes does not recover as a single clade (Figs 2, 3). Second, at least 22 new genera in Daedalea – Fomitopsis subclade would be necessary (Fig. 4, Suppl. Fig. S2). Of these, at least fifteen would be monotypic, in addition to five single-species genera introduced in the previous publications (Han et al. 2016, Audet 2017). Any reliable morphological definition of these potential genera is eventually impossible (we discuss this in more detail below). If applied further in Fomitopsidaceae, it would be natural to split Antrodia sensu stricto to many genera as well, causing similar problems.
The mesogeneric approach. The Daedalea – Fomitopsis clade is split in two to three genera. Both the analyses of the three-gene brown-rotter dataset and the dataset explicitly focussing on the Daedalea – Fomitopsis clade (Figs 2, 3) show three strongly supported subclades which could be interpreted as separate genera: Buglossoporus, Fomitopsis and a single-species genus for the Australian Fomitopsis sp. Darwin (M.D. Barrett F17/09). Being its sister, the latter could also be included in a monophyletic Fomitopsis (two-genera approach). The main problem with such splitting of the Daedalea – Fomitopsis clade is that it could be based only on calculation of support values but would not allow for morphologically identifiable genera. For instance, the Buglossoporus subclade encompasses mainly sappy, semistipitate or stipitate polypores with rather sparse skeletal hyphae in tube trama, but it also contains Antrodia madronae. The latter species, as well as its closest relative A. sandaliae, are morphologically much more similar to some residual Antrodia spp. in the Fomitopsis subclade (e.g., A. infirma, A. oleracea, A. tuvensis) than to Buglossoporus quercinus and its satellites, and therefore they blur any presumed differences between the Buglossoporus subclade and the other lineages in the Daedalea – Fomitopsis clade. For the time being, we do not consider the mesogeneric approach useful.
The macrogeneric approach. The whole Daedalea – Fomitopsis group is treated as one genus, but Anthoporia and Antrodia sensu stricto (sensu Runnel et al. 2019) are retained. Despite their outwardly diverse habit, members of the Daedalea – Fomitopsis group share several principal anatomical traits presented below that separate them from Anthoporia and Antrodia sensu stricto. For the present, we see this approach as the most reasonable taxonomic solution.
The monogeneric approach – one family, one genus. All of Fomitopsidaceae are placed in one genus, including Antrodia sensu stricto and Anthoporia. Equating genera and families is a reasonable solution when genetic and morphological variation is small, typically in the case of small isolated genera (e.g., Ischnoderma). In contrast, the family Fomitopsidaceae shows significant genetic variation and can be divided into morphologically identifiable clades (at least Anthoporia, Antrodia and Fomitopsis as discussed below). Fusing Antrodia and Fomitopsis would remove issues with genus identification within the Fomitopsidaceae, but Antrodia sensu lato species are found in other families of the Polyporales, too. This solution would thus not allow easy identification of genera either.
Fig 4.
Three generic concepts for delimiting genera in the Daedalea – Fomitopsis clade. A. Microgeneric approach. B. Mesogeneric approach. C. Macrogeneric approach (accepted in the present study). For details about topology, see Fig. 2.
Morphological evidence for maintaining the Daedalea – Fomitopsis clade as one genus
If interpreted taxonomically as one genus, the Daedalea – Fomitopsis clade can be separated from two other genera of Fomitopsidaceae, i.e. Anthoporia and Antrodia sensu stricto, by the combination of the following features:
Branched and coloured skeletal hyphae. In dimitic species of the Daedalea – Fomitopsis clade skeletal hyphae are occasionally branched and quite often coloured (brownish to brown or more rarely reddish). Rajchenberg (1986) and Hattori (2005) described and discussed them as a characteristic feature of Daedalea spp. We were able to demonstrate the presence of occasionally branched skeletals in residual Antrodia spp. outside Antrodia sensu stricto (Spirin et al. 2016, 2017) and now confirm it for the rest of the dimitic species in the whole Daedalea – Fomitopsis group. In contrast, all poroid species in Antrodia sensu stricto possess unbranched, as a rule hyaline vegetative hyphae (Spirin et al. 2013a, Kout et al. 2017). The single corticioid representative of the latter genus with monomitic basidiocarps, A. griseoflavescens, produces skeletal hyphae in culture (Hallenberg 1990). In turn, Anthoporia albobrunnea has partly pigmented generative hyphae in the context and uppermost tube trama while skeletal hyphae of this species are unbranched and hyaline (Karasiński & Niemelä 2016).
Relatively small basidia and basidiospores. Basidia and basidiospores in the Daedalea – Fomitopsis group are on average smaller than in Antrodia sensu stricto, in almost all species not exceeding 25 μm and 10 μm long, respectively, when considered in combination (both characters found in the same species). Only six of the 128 members of the Daedalea – Fomitopsis clade treated below, i.e. Antrodia albidoides, A. madronae, A. variiformis, Neolentiporus maculatissimus, N. squamosellus and Laccocephalum tumulosum, are exceptions to this rule, i.e. their basidia and basidiospores exceed the aforementioned limits. Of them, the three latter species have stipitate, soft basidiocarps strikingly different from those ones of Antrodia sensu stricto. In turn, A. albidoides possesses well-developed skeletocystidia (if compared with representatives of Antrodia sensu stricto) and its basidiospores show no arcuate depression at the apiculus so characteristic of Antrodia sensu stricto (Spirin et al. 2013a). Antrodia variiformis produces encrusted cystidioles and distinctly coloured hyphae in the uppermost part of context (Spirin et al. 2017); these features rule out Antrodia sensu stricto. Antrodia madronae is a soft, fleshy polypore with hyphae easily dissolving in both KOH and IKI (Vlasák et al. 2012). None of those features fit Antrodia sensu stricto. Nevertheless, these exceptional cases indicate that the length of basidia and basidiospores have limited value as a discriminating character and can be used only in combination with two other morphological traits discussed here. Basidia of Anthoporia albobrunnea are small, comparable with many species of the Daedalea – Fomitopsis complex, but its basidiospores are strikingly different, allantoid to narrowly cylindrical, mostly evenly curved. They are reminiscent more of Amyloporia and Postia than other members of the Fomitopsidaceae.
The presence of skeletocystidia and hyphidia. Skeletocystidia and hyphidia are characteristic for truly dimitic representatives of both the Daedalea – Fomitopsis clade and Antrodia sensu stricto but they are absent in Anthoporia. In Antrodia sensu stricto, skeletocystidia occur only occasionally as poorly differentiated blunt endings of skeletal hyphae, and at least some hyphidia are bi- or trifurcate (Spirin et al. 2013a, Runnel et al. 2019). In the Daedalea – Fomitopsis group, all truly dimitic species with pore size equal to dimitic Antrodia sensu stricto species (i.e. 0.5–3 per mm) possess well-developed skeletocystidia, which are either apically clearly inflated or acute. Only a single representative of the Daedalea – Fomitopsis clade, the small-pored Antrodia serialiformis, has more or less regularly branched hyphidia similar to those in Antrodia sensu stricto (Spirin et al. 2017), while they are normally unbranched in all other species of the clade.
We illustrate with further examples why the macrogeneric approach is preferable to the microgeneric one. Han et al. (2016) divided the pink-coloured species of Fomitopsis sensu lato between two genera, Rhodofomes (the Fomitopsis rosea complex) and the newly erected Rhodofomitopsis (the Fomitopsis feei complex). They justified this rearrangement via phylogenetic analyses and indicated the absence of cystidia and cystidioles in Rhodofomitopsis spp. as the only morphological feature separating them from Rhodofomes and similarly coloured Rubellofomes and Daedalea spp. Our observations suggest this is true only for F. feei sensu stricto (as redefined below) while eight other representatives of the F. feei complex have cystidioles and one of them (F. cupreorosea) produces easily detectable skeletocystidia. Moreover, our data show the pink-coloured species of Fomitopsis sensu lato belong to at least nine different lineages in the Daedalea – Fomitopsis group (Fig. 3, Suppl. Fig. S2). We have not found unique morphological traits to justify their acceptance as separate genera. Of course, separation into nine genera is not totally elusive if based on phylogenetic evidence only. However, implementing it would cause a cascade splitting in other parts of the Daedalea – Fomitopsis complex.
The case of Fomitopsis dochmia is even more striking. According to our results, its current concept covers six species (F. dochmia sensu stricto, F. elevata, F. ferrea, F. lapidosa, F. lignea, and F. philippinensis) belonging to two very distantly related lineages within the Daedalea – Fomitopsis group (Figs 3, 5, Suppl. Fig. S2). Morphological differences between them are minimal, however, and these species can be separated with some confidence based only on macroscopic traits of basidiocarps and geographic distribution. Fomitopsis caseosa, F. niveomarginata and F. pseudopetchii can provide another example of the utmost morphological similarity of phylogenetically distant species (Fig. 3, Suppl. Fig. S2).
Fig 5.
Phylogenetic relationships of species in the Fomitopsis (Daedalea) quercina clade based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
The merits of the mesogeneric approach, i.e. interpreting the Buglossoporus and Fomitopsis subclades as separate genera, deserve further comments. In all published phylogenetic analyses these two lineages are well supported, and at first this division appears to be supported by morphology, making the mesogeneric approach appealing. In fact, Buglossoporus as a genus was included in the earliest versions of this manuscript. Morphologically, all Fomitopsidaceae species with the combination of stipitate, fleshy basidiocarps and nearly monomitic tube trama are found in the Buglossoporus subclade. Two of the ten species we have included in the analyses are effused-reflexed or totally resupinate (Antrodia madronae, A. sandaliae). These species also have a partly monomitic hyphal structure. Additionally, A. madronae has unusually large basidia for Fomitopsis, like several other members of the Buglossoporus subclade. There is no character that is unique for the subclade, however. There are monomitic or nearly monomitic species in several lineages of the Fomitopsis subclade (e.g., A. infirma, A. monomitica, A. oleracea, A. ramentacea). Large hymenial cells are also found in the A. ramentacea complex and a few other species (A. albidoides, A. tuvensis, A. variiformis).
Here, we have defined the large Fomitopsis clade through a combination of characters. Applying the same scheme to recognize Buglossoporus looks problematic. Antrodia sandaliae is difficult to place in Buglossoporus even with a combination of characters. A separate genus for it would cause a cascade of splitting, which in essence would mark a return to the microgeneric approach. When two of the ten species do not fit comfortably in a genus, we feel it better to include the whole Buglossoporus subclade in one genus with the members of the Fomitopsis subclade. Surely, a large portion of species diversity remains undiscovered. Considering the degree of morphological plasticity both in the Fomitopsis and Buglossoporus subclades, that diversity might still significantly alter our understanding of morphological variation. With a deeper understanding of diversity and morphological variation, the mesogeneric approach might become a good option, but for now we consider it is suboptimal to the macrogeneric approach.
Arguments for choosing Fomitopsis as a generic name for the Daedalea – Fomitopsis clade
If the one-genus concept for the whole Daedalea – Fomitopsis group is accepted, then the oldest generic name available for it is Daedalea, introduced by Persoon (1801) and typified with Agaricus quercinus (= Daedalea quercina). The three next names in line are Caloporus (typified with Poria incarnata, see further remarks under Fomitopsis marianii) (Karsten 1881a), Fomitopsis (typified with Boletus pinicola = Fomitopsis pinicola) and Piptoporus (typified with Boletus betulinus = Piptoporus betulinus) (Karsten 1881b).
We argue for retaining Fomitopsis and suppressing other older (Daedalea, Caloporus) or simultaneously published (Piptoporus) names for the following reasons. First, of the 128 species accepted here in the Daedalea – Fomitopsis generic unit, 33 were already combined or described in Fomitopsis and only 18 in Daedalea. Therefore, applying Fomitopsis will require fewer new combinations than maintaining Daedalea. Second, using Daedalea will necessitate a species epithet change for at least ten species, which would be highly undesirable in the case of two common and well-known species, F. pinicola and P. betulinus (now Fomitopsis betulina). Boletus marginatus for the first species and Agarico-pulpa pseudoagaricon for the second one are available as replacing names, if combination were to be made in Daedalea. Both names are obscure and almost unknown to current mycologists. If Fomitopsis is accepted, only three species (Antrodia minuta, Daedalea africana and Trametes cystidiata) will need new species names. Additionally, two common and well-known polypores, D. quercina and Antrodia serialis, will change their generic affiliation but they will hold their species epithets. Finally, the family name Fomitopsidaceae is in use and retaining the name Fomitopsis in use makes it easier to connect the family name with species. For these reasons, we see more advantages in keeping Fomitopsis as a generic epithet for the Daedalea – Fomitopsis group. A corresponding conservation proposal has been submitted to the Nomenclatural Committee for Fungi.
Reassessing selected species complexes in Fomitopsis
We prepared seventeen additional datasets to reconsider species limits in nine selected groups within the genus.
1. Fomitopsis (Daedalea) quercina clade (= Daedalea sensu stricto as redefined by Lindner et al. 2011, Li et al. 2013 and Han et al. 2016). The ML and BI analyses of the ITS dataset resulted in nearly identical topologies with seventeen strongly supported lineages taxonomically interpreted as separate species (Fig. 5). Four of them, Daedalea dickinsii, D. neotropica and D circularis (recombined in Fomitopsis below), as well as Fomitopsis derelicta sp. nov., are closely related and morphologically similar to F. quercina. Fomitopsis derelicta is a newly described member of this complex occurring in subtropical – tropical areas of North America. At the same time, F. quercina is shown to be widely distributed in Europe and temperate – warm temperate forests of North America (from Oregon to Georgia). The ITS sequence of Daedalea pseudodochmia published by Lindner et al. (2011) (GenBank FJ403210) is based on a misidentification and belongs to the East Asian D. dickinsii. Our data show that Daedalea pseudodochmia is not related to the F. quercina complex and its phylogenetic position in the genus Fomitopsis deserves further study because it is so far represented by the single ITS sequence (Suppl. Fig. S2). Hattori (2005) argued that D. pseudodochmia is the same species as Trametes incana. This viewpoint is not supported by our data. We assert that Trametes incana is a member of F. quercina complex closely related to Daedalea stereoides and it is conspecific with the recently described Daedalea allantoidea. In turn, D. pseudodochmia is treated below as Fomitopsis moritziana (see Taxonomy section).
Fomitopsis dochmia is proven to be a polyphyletic species complex containing at least six species, of which four (F. dochmia s. str., F. elevata, F. ferrea and F. philippinensis) form a strongly supported lineage in the F. quercina clade. The remaining two (F. lapidosa, F. lignea) do not belong to the F. quercina clade. ITS sequences of Trametes modesta (= Daedalea modesta) are split among two distantly related lineages within the F. quercina clade, in good accordance with their geographic origin and, to a lesser extent, morphological evidence. Sequences of specimens collected in Central and South America represent T. modesta sensu stricto which turns out to be a close relative of Trametes subectypa (= Daedalea americana), another species from subtropical North America. In turn, the Southeast Asian specimens of T. modesta belong to yet another species, for which Polyporus atypus is available as the oldest name. Finally, Polyporus leiodermus, Polystictus aculeatus, Polyporus psilodermeus and Daedalea ryvardeniana are three more members of the F. quercina group; according to our data, they are not closely related to other species in the clade (Fig. 5). Two species, D. africana (= Fomitopsis kenyensis, nom. nov.) and D. aethalodes, ascribed to Daedalea clade by Han et al. (2016), Liu et al. (2022) and Cristaldo et al. (2022), are most distant from the rest of species, and therefore their sequences were used as an outgroup.
2. Fomitopsis (Pilatoporus) palustris complex. Kotlaba and Pouzar (1990) argued that F. palustris and its siblings should be treated as members of the separate genus Pilatoporus. However, this option was not supported by phylogenetic studies (Kim et al. 2005, Ortiz-Santana et al. 2013, Han et al. 2016), which have shown that F. palustris and its sibs are closely related to the generic type of Fomitopsis, F. pinicola. Our results (Figs 3, 4) do not preclude from recognizing Pilatoporus as a separate taxonomic unit, would the microgeneric approach be applied to the reclassification of Fomitopsidaceae.
Members of the F. palustris group are widely distributed from temperate to tropical zones, and many competing species names, both historical and recently introduced, are available for them. The species concepts proposed here are based on morphological studies of type specimens and newly collected sequenced specimens. They are mostly backed by DNA data, but not fully. This is mainly due to lack of reliable differences between sister species and a rather high variation within the ITS region in some of them.
We could recognize eight species in the complex: F. caespitosa (= F. subtropica), F. eucalypticola, F. luzonensis (= F. ostreiformis sensu auct., non sensu typi), F. marianii (= F. iberica), F. meliae, F. nivosella (= F. durescens), F. ostreiformis sensu typi (= F. cana) and F. palustris. ITS-based phylogeny divided the F. palustris complex in four strongly supported lineages: F. eucalypticola, F. meliae – F. ostreiformis lineage, F. caespitosa and a large group containing F. luzonensis, F. marianii, F. nivosella and F. palustris (henceforth F. marianii lineage) (Fig. 6). Further phylogenetic analyses of the F. marianii lineage were based on TEF1 and combined ITS + TEF1 (+ RPB1) datasets.
Fig 6.
Phylogenetic relationships of species in Pilatoporus (Fomitopsis palustris) group based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Both the TEF1 only and the ITS + TEF1 phylogeny split the F. marianii lineage in three strongly supported terminal clades, i.e. Fomitopsis marianii, F. luzonensis – F. nivosella, and F. palustris (Fig. 7, Suppl. Fig. S3). While the F. palustris clade is limited to the single North American species, F. palustris, easily identifiable due to its peculiar morphology (small basidiocarps and rather large basidia and basidiospores) and host species (restricted to warm temperate – subtropical Pinus spp.), the species subdivision of the two other clades is much more problematic. No reliable sequence differences were found between sequences of F. marianii from the temperate – subtropical northern hemisphere and a single specimen of F. hemitephra from New Zealand. However, this sequenced specimen of F. hemitephra seems to be either misidentified or contaminated, since F. hemitephra is a white rot fungus here referred to Neofomitella in the Polyporaceae (see remarks under excluded taxa). Neither the ML nor BI topology show F. luzonensis and F. nivosella forming distinct clades, despite small but stable differences in both ITS and TEF1 regions (3 and 3–4 bp, respectively). For now, we treat them as two different species due to rather clear morphological differences (summarized in Table 3) and different geographic distribution.
Fig 7.
Phylogenetic relationships of species in the Fomitopsis marianii complex based on Maximum Likelihood of the ITS + TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Table 3.
Morphological, ecological and geographic traits in Fomitopsis palustris complex.
| Species | Geographic distribution/host | Macroscopic characters | Tramal skeletal hyphae | Basidia | Basidiospores |
|---|---|---|---|---|---|
| F. caespitosa | East Asia, Oceania, South America; angiosperms | usually effusedreflexed with large resupinate parts; pores 7–11 per mm | rather densely interwoven, 3.5–7 μm in diam, approaching dissepiment edges | 9–12 × 4–5 μm | broadly cylindrical to ellipsoid, 3.0–4.2 × 1.9–2.9 μm; L = 3.58, W = 2.13, Q = 1.68 |
| F. eucalypticola (fide Liu et al. 2019) | Oceania; angiosperms (Eucalyptus spp.) | sessile or effusedreflexed, pores 3–5 per mm | interwoven, 1.5–3 μm in diam | 15–23 × 7–10.5 μm | cylindrical to ellipsoid, 5.8–9.1 × 2.7–5.0 μm; L = 7.52, W = 3.42, Q = 2.23 |
| F. luzonensis | South-East Asia; angiosperms | sessile or effusedreflexed, pores 8–10 per mm | rather densely interwoven, 2–4.5 μm in diam, approaching dissepiment edges | 10–16.5 × 4.5–6.5 μm | cylindrical to fusiform, 5.1–8.1 × 2.0–3.1 μm; L = 6.04, W = 2.66, Q = 2.27 |
| F. marianii | Eurasia, North America, Oceania (?); angiosperms, very rarely conifers | usually sessile or effused-reflexed, pores 4–6 per mm | rather loosely interwoven, 2–5 μm in diam, absent at dissepiment edges | 12.5–21.5 × 4.5–7 μm | cylindrical to fusiform, 5.1–8.7 × 2.0–3.2 μm; L = 6.45, W = 2.60, Q = 2.48 |
| F. meliae | North America (subtropics – tropics), South America; angiosperms | usually sessile or effused-reflexed, pores 4–7 per mm | rather loosely interwoven, 3–5 μm in diam, approaching dissepiment edges | 11–17.5 × 4.5–6 μm | cylindrical to fusiform, 4.2–7.2 × 2.1–2.9 μm; L = 5.62, W = 2.40, Q = 2.34 |
| F. nivosella | North and South America; angiosperms | sessile or effusedreflexed, pores 5–6 per mm | rather loosely interwoven, 2–4 μm in diam, approaching dissepiment edges | 11–17 × 4–7 μm | cylindrical to fusiform, 5.0–8.0 × 1.8–3.0 μm; L = 6.60, W = 2.50, Q = 2.64 |
| F. ostreiformis | East Africa, South-East Asia; angiosperms | sessile, effusedreflexed or totally resupinate, pores 5–8 per mm | rather loosely interwoven, 3–4.5 μm in diam, approaching dissepiment edges | 10–18 × 4.5–6 μm | cylindrical to fusiform, 4.2–7.3 × 2.1–3.0 μm; L = 5.61, W = 2.55, Q = 2.20 |
| F. palustris | North America; conifers (Pinus spp.) | sessile, pores 5–7 per mm | loosely interwoven, 3–5 μm in diam, approaching dissepiment edges | 14–22 × 5.5–7 μm | cylindrical to fusiform, 6.2–8.2 × 2.4–3.7 μm; L = 7.11, W = 2.95, Q = 2.41 |
The specimen Dollinger 836 (designated as F. marianii) poses one more problem for the species: polymorphism in its ITS and TEF1 sequences can be interpreted as containing two alleles, one similar to F. nivosella and the other identical with F. marianii. The RPB1 sequence does not show this pattern and corresponds to F. marianii, thus excluding that this specimen would represent a firstgeneration hybrid between the two species/populations. With only one specimen showing this pattern, no far-reaching conclusions about species delimitation can be drawn, but it indicates there may still be gene flow between the parental species / populations.
The TEF1 and ITS + TEF1 phylogenies of the F. meliae – F. ostreiformis lineage show sequences of the latter species clustering together although they do not form a clade clearly delimited from F. meliae (Fig. 8, Suppl. Fig. S4). In the ITS + TEF1 + RPB1 phylogram, where these species are represented by two specimens each, they do receive support (Fig. 9). We retain F. meliae and F. ostreiformis as independent species, based on geographic distribution (subtropical – tropical North America vs. paleotropics), morphological differences (commented under F. meliae and summarized in Table 3), and the minor DNA differences. It is evident that the whole group deserves a thorough revision with a much more extensive sampling and the use of additional genetic markers. We consider it quite possible that such a sampling may result in fewer species in this complex (notwithstanding diversity yet undiscovered).
Fig 8.
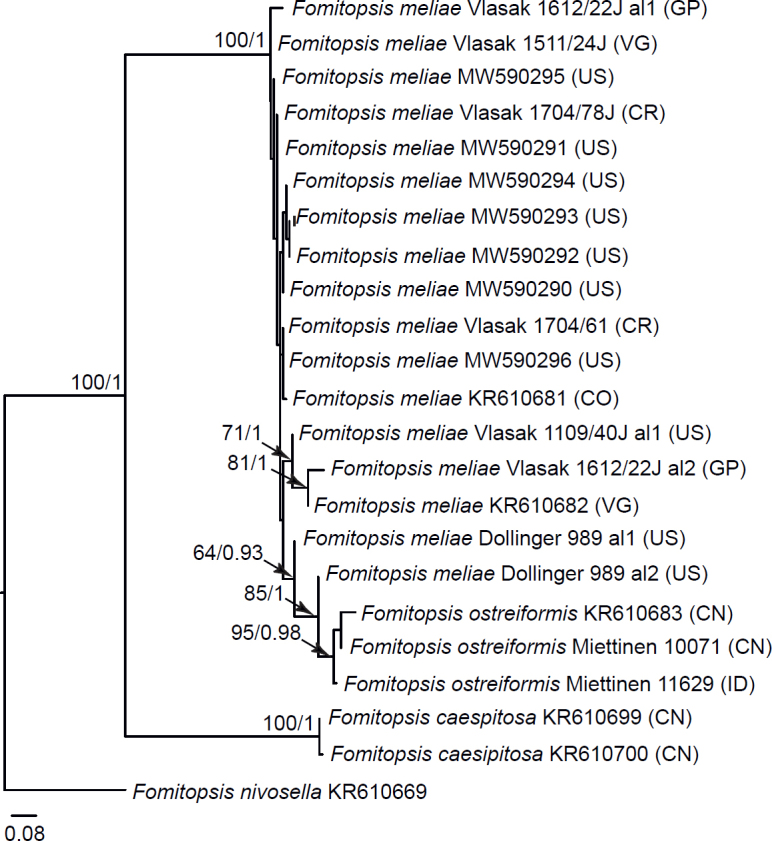
Phylogenetic relationships of species in the Fomitopsis meliae complex based on Maximum Likelihood of the ITS + TEF1 dataset. Numbers on nodes represent bootstrap values > 70 % and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Fig 9.
Phylogenetic relationships of species in the Pilatoporus (Fomitopsis palustris) group based on Maximum Likelihood of the ITS + TEF1 + RPB1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin. The tree is midpoint-rooted.
3. Fomitopsis pinicola complex. Haight et al. (2019) and Liu et al. (2021) have recently described eight new species from North America and Asia in addition to the previously introduced F. pinicola and F. ochracea (Ryvarden & Stokland 2008). Here, we compiled the most comprehensive dataset for this group published so far, including newly produced sequences and all credible sequences from Haight et al. (2019) and Liu et al. (2021). Our phylogenetic analyses of the ITS + TEF1 and ITS + TEF1 + RPB2 datasets do not fully support any of the eight newly described species (Fig. 10, Suppl. Fig. S5). Fomitopsis ochracea was resolved at the deepest split of the F. pinicola complex, but even recognition of this species as a separate lineage is not supported unambiguously in our phylogenetic analyses. However, morphological differences and previous mating tests (Mounce & Macrae 1938, Högberg et al. 1999) convinced us to recognize this species. Before division beyond the two species F. pinicola and F. ochracea is adopted, we call for convincing evidence for their existence through much more solid specimen and gene sampling. For a detailed discussion, consult the Taxonomy section (under F. pinicola).
Fig 10.
Phylogenetic relationships of species in the Fomitopsis pinicola complex based on Maximum Likelihood of the ITS + TEF1 +RPB2 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
4. Fomitopsis feei clade (= Rhodofomitopsis sensu B.K. Cui et al. in Yuan et al. 2020). Morphologically, the group is highly diverse, encompassing totally effused, soft, monomitic and sturdy, perennial polypores with sessile caps. This makes a morphological definition of the clade eventually impossible. Monomitic porioid species are divided in two groups. One of them contains sequences of Antrodia oleracea from North America and A. monomitica from East Asia, and another consists of Pseudoantrodia monomitica (Fig. 11). Eight remaining species represent sturdy, pileate Fomitopsis spp. and they all are redescribed below. ITS sequences of F. africana, F. carnea and F. lilacinogilva reveal a considerable variation (up to 10 bp in the two latter species), and the taxonomy of these taxa certainly deserves further study.
Fig 11.
Phylogenetic relationships of species in the Fomitopsis feei clade based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Trametes marchionica, for a long time considered a synonym of F. feei, is morphologically highly similar to the representatives of the F. feei complex but phylogenetically very distant from them, here recovered sister to D. pseudodochmia (Suppl. Fig. S2). Therefore, we transfer it to Fomitopsis as a good species and reintroduce it below.
5. Fomitopsis rosea clade (= Rhodofomes). Kotlaba & Pouzar (1990) introduced Rhodofomes with F. rosea as the type and the sole species. Han et al. (2016) accepted the genus and expanded it with four more species. We compiled an ITS dataset for the F. rosea complex and our analyses confirm seven species in this group (Fig. 12). Of them, F. cupressicola from North America, F. perhiemata from Caucasus and F. purpurea from East Africa are described as new. Nigroporus ussuriensis and Fomitopsis incarnata are proven to be conspecific, and a new combination, F. ussuriensis, is proposed below. Morphological traits of F. rosea and all other pink-coloured species in the genus are summarized in Table 4.
Fig 12.
Phylogenetic relationships of species in the Fomitopsis rosea clade based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Table 4.
Morphological, ecological and geographic traits in pink-coloured Fomitopsis spp.1.
| Species | Geographic distribution/host | Macroscopic characters | Tramal skeletal hyphae | Skeletocystidia | Basidiospores |
|---|---|---|---|---|---|
| F. africana | Africa, South-East Asia; angiosperms | basidiocarps sessile, hymenophore indistinctly stratified; pores 7–8 per mm | loosely interwoven, brownish, 3–5 μm in diam | not differentiated | cylindrical-subfusiform, 5.8–7.8 × 2.5–3.4 μm; L = 6.53, W = 2.98, Q = 2.21 |
| F. cajanderi | Holarctic; conifers | basidiocarps sessile or rarely effused-reflexed, hymenophore indistinctly stratified; pores 5–7 per mm | interwoven, reddish-brown, 2.5–4 μm in diam | not differentiated | narrowly cylindrical to subfusiform, 4.2–6.1 × 1.8–2.1 μm; L = 5.24, W = 1.95, Q = 2.69 |
| F. carnea | South-East Asia; angiosperms | basidiocarps sessile, hymenophore indistinctly stratified; pores 7–9 per mm | densely interwoven, hyaline, yellowish or brownish, 3–4 μm in diam | not differentiated | cylindrical-subfusiform to narrowly ellipsoid, 2.9–4.5 × 1.9–2.3 μm; L = 3.63, W = 2.07, Q = 1.75 |
| F. cupreorosea | Neotropics; angiosperms, very rarely conifers | basidiocarps sessile, hymenophore indistinctly stratified; pores 3–5 per mm | densely interwoven, brownish to pinkishbrown, 3–4.5 μm in diam | abundant, normally blunt, slightly projecting | cylindrical-subfusiform, 4.6–6.6 × 2.1–3.0 μm; L = 5.36, W = 2.52, Q = 2.12 |
| F. cupressicola | North America (eastern part); conifers | basidiocarps sessile, hymenophore indistinctly stratified; pores 6–8 per mm | densely interwoven, brown; 2–3.5 μm in diam | poorly differentiated, obtuse, embedded or slightly projecting | cylindrical to narrowly ellipsoid, 3.7–5.2 × 1.9–2.8 μm; L = 4.31, W = 2.23, Q = 1.96 |
| F. eucalypti | Oceania; angiosperms | sessile or effusedreflexed, hymenophore indistinctly stratified; pores 5–7 per mm | densely interwoven, yellowish, 3–4.5 μm in diam | not differentiated | narrowly ellipsoid to cylindrical, 4.0–6.8 × 2.1–3.0 μm; L = 4.81, W = 2.56, Q = 1.90 |
| F. feei | South America; angiosperms | sessile, hymenophore indistinctly stratified; pores 6–7 per mm | densely interwoven, brownish to reddish-brown, 3–4 μm in diam | not differentiated | cylindrical-subfusiform, 4.0–6.2 × 2.1–2.9 μm; L = 4.82, W = 2.37, Q = 2.05 |
| F. flabellata (fide Tibpromma et al. 2017) | South America; angiosperms | sessile, hymenophore one-layered, pores 3–4 per mm | interwoven, 3–5 μm in diam | not differentiated | cylindrical, 4–5 × 2–2.5 μm |
| F. foedata | Oceania; angiosperms | sessile, hymenophore indistinctly stratified; pores 6–8 per mm | densely interwoven, yellowish to reddish-brown, 3–4 μm in diam | not differentiated | cylindrical-subfusiform, 4.0–5.9 × 2.1–3.0 μm; L = 5.05, W = 2.62, Q = 1.95 |
| F. lilacinogilva | Europe, North America, Oceania; angiosperms | sessile, hymenophore indistinctly stratified; pores 3–5 per mm | interwoven, brownish to reddish-brown, 3–4.5 μm in diam | not differentiated | cylindrical-subfusiform, 5.2–9.2 × 2.6–3.2 μm; L = 6.73, W = 2.97, Q = 2.27 |
| F. marchionica | Oceania; angiosperms | sessile, hymenophore indistinctly stratified; pores 5–7 per mm | densely interwoven, yellowish to brownish, 3–4 μm in diam | not differentiated | cylindrical-subfusiform, 3.2–4.2 × 1.9–2.2 μm; L = 3.63, W = 2.08, Q = 1.75 |
| F. perhiemata | Europe; angiosperms | effused-reflexed, hymenophore indistinctly stratified; pores 5–7 per mm | densely interwoven, brown, 3–4 μm in diam | not differentiated | cylindrical to narrowly ellipsoid, 4.1–5.2 × 2.1–3.0 μm; L = 4.50, W = 2.40, Q = 1.89 |
| F. purpurea | Africa; angiosperms | sessile, hymenophore clearly stratified; pores 6–7 per mm | interwoven, brownish to reddish-brown, 2.5–3.5 μm in diam | not differentiated | cylindrical-subfusiform, 5.0–6.7 × 2.0–2.2 μm; L = 5.79, W = 2.10, Q = 2.77 |
| F. rosea | Holarctic; conifers, very rarely angiosperms | sessile, hymenophore indistinctly stratified; pores 3–5 per mm | interwoven, brownish to reddish-brown, 3–3.8 μm in diam | not differentiated | cylindrical, 5.0–6.6 × 2.2–2.7 μm; L = 5.80, W = 2.40, Q = 2.40 |
| F. roseofusca | South America; angiosperms | sessile, hymenophore clearly stratified; pores 1–2 per mm | densely interwoven, brownish to brown, 3–5 μm in diam | abundant, acute, slightly projecting | cylindrical-subfusiform, 4.8–6.2 × 1.8–2.8 μm; L = 5.41, W = 2.40, Q = 2.32 |
| F. sagraeana | North America (subtropical – tropical); angiosperms | sessile, hymenophore indistinctly stratified; pores 6–8 per mm | densely interwoven or subparallel, brownish to reddish-brown, 2.5–4 μm in diam | not differentiated | cylindrical-subfusiform, 4.9–7.3 × 2.6–3.9 μm; L = 5.81, W = 2.99, Q = 1.96 |
| F. scalaris | South America; angiosperms | sessile, hymenophore clearly stratified; pores 6–8 per mm | densely interwoven, brownish to brown, 2–4 μm in diam | poorly differentiated, obtuse, embedded or slightly projecting | cylindrical, 3.1–5.0 × 2.0–2.3 μm; L = 3.70, W = 2.12, Q = 1.75 |
| F. substratosa | North Africa and South-East Asia; conifers | sessile to effusedreflexed, hymenophore clearly stratified; pores 4–7 per mm | densely interwoven, brownish to brown, 2.5–4 μm in diam | not differentiated | cylindrical to narrowly ellipsoid, 3.8–5.1 × 2.0–2.4 μm; L = 4.24, W = 2.16, Q = 1.97 |
| F. ussuriensis | East Asia; angiosperms, very rarely conifers | sessile to effusedreflexed or resupinate, hymenophore clearly stratified; pores 6–7 per mm | densely interwoven, brownish to brown, 2.5–3 μm in diam | not differentiated | cylindrical, 3.7–4.7 × 1.9–2.2 μm; L = 4.07, W = 2.02, Q = 2.02 |
1 Species occasionally possessing pinkish tints in tubes which quickly disappear in herbarium specimens (e.g., F. atypa, F. gilvidula, F. ostreiformis, F. sulcata) or violet-black spots on pileal surface (F. neotropica) are not included.
6. Fomitopsis (Antrodia) ramentacea clade. Kotlaba & Pouzar (1958) studied anatomy of Trametes subsinuosa (= Polyporus ramentaceus) and found that it is a monomitic species with variably thick-walled hyphae (see further notes on miticity in this complex in the next paragraph). This was the main reason for them to place this species in the newly established genus Cartilosoma. The genus was accepted in some recent publications (Rivoire et al. 2015, Rivoire 2020) while other authors (e.g., Donk 1966, David & Dequatre 1985, Ryvarden 1991) treated P. ramentaceus as a member of Antrodia. David and Dequatre (1985) studied cultural characters of Antrodia ramentacea and concluded it is a species complex. They introduced Antrodia subramentacea as a morphologically indistinguishable but culturally incompatible twin of A. ramentacea. Based on DNA data and morphological evidence, Rivoire et al. (2015) described another species in the complex, Cartilosoma renehenticii.
Here we show that Cartilosoma is a part of the redefined Fomitopsis (Figs 2, 3). Three datasets focussing on the A. ramentacea group (ITS only, TEF1 only and combined ITS + TEF1) resulted in nearly the same topologies allowing the recognition of four species: A. ramentacea (= A. subramentacea), Skeletocutis uralensis (= Antrodia huangshanensis), C. renehenticii, and Fomitopsis solaris, sp. nov. The only exception somehow blurring the species subdivision proposed here is the specimen Rivoire LY-BR 6356 (designated as F. renehenticii × solaris in Figs 13, 14 and Suppl. Fig. S6). This specimen is morphologically identical to C. renehenticii and its TEF1 sequence shows no differences from the latter species (Fig. 13). In contrast, its ITS sequence is identical to F. solaris (Suppl. Fig. S6). Whether this is a case of incomplete lineage sorting or hybridization between the two species is impossible to tell with our data.
Fig 13.
Phylogenetic relationships of species in the Fomitopsis (Antrodia) ramentacea clade based on Maximum Likelihood of the TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Fig 14.
Phylogenetic relationships of species in the Fomitopsis (Antrodia) ramentacea clade based on Maximum Likelihood of the ITS + TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
7. Fomitopsis spraguei clade (= Niveoporofomes sensu Decock et al. 2022). In addition to three species, F. spraguei, Niveoporofomes globisporus and N. oboensis, recently recognized via morphology and ITS sequences (Decock et al. 2022), we were able to detect and name one more species in this complex, Fomitopsis hypoxantha (Fig. 15).
Fig 15.
Phylogenetic relationships of species in Fomitopsis spraguei group based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin. The tree is midpoint-rooted.
8. Fomitopsis (Antrodia) juniperina complex. Based on morphological study and ITS dataset, we could recognize only two species in this group, i.e. F. juniperina and the newly described F. algumicola (Suppl. Fig. S7). Further remarks are given under the latter species and Antrodia uzbekistanica (in excluded taxa).
9. Buglossoporus clade. This group contains 10 species of which eight were included in the general phylogenies of the brown-rot Polyporales and Fomitopsidaceae (Figs 1, 2; Suppl. Figs. S1, S2). The ITS dataset was supplemented by sequences of Antrodia sandaliae and Polyporus amygdalinus to reveal their phylogenetic position in the clade (Suppl. Fig. S8).
Taxonomy
Fomitopsis P. Karst., Meddeland. Soc. Fauna Fl. Fenn. 6: 9. 1881, nom. cons.
Basidiocarps annual, seasonal or perennial, pileate, effusedreflexed or resupinate, or stipitate in a few species, variably coloured. Hymenophore poroid, with angular or regular pores 0.5–10 per mm (in one species concentrically lamellate). Hyphal structure dimitic or (rarely) monomitic; generative hyphae clamped (clamps rare amongst mostly simple septa in one species), skeletal hyphae occasionally branched, in many species lightly tinted. Cystidioles, hymenial cystidia or skeletocystidia present in many taxa. Hyphidia occasionally present, as a rule simple. Basidia clavate, four-spored, normally under 25 µm long; basidia and basidioles becoming slightly thick-walled in senescent hymenium. Basidiospores hyaline, thin-walled or with a distinct wall (in two species slightly thick-walled), cylindrical to ellipsoid, rarely broadly ellipsoid or subglobose, 3–16 × 2–5.5 µm, inamyloid, with occasional small oil droplets, apiculus rather small. Causing brown rot of dead wood of angiosperms and conifers.
Generic type: Boletus pinicola Sw. (= Fomitopsis pinicola (Sw.) P. Karst.).
Synonyms:
Antrodiopsis Audet (type Poria oleracea R.W. Davidson & Lombard) Brunneoporus Audet (type Trametes malicola Berk. & M.A. Curtis) Buglossoporus Kotl. & Pouzar (type Boletus quercinus Schrad.) Caloporus P. Karst. (type Poria incarnata Pers.) Cartilosoma Kotlaba & Pouzar (type Trametes subsinuosa Bres.) Daedalea Pers. (type Agaricus quercinus L.) Daedalella B.K. Cui & Shun Liu (type Daedalella micropora B.K. Cui & Shun Liu)
Dentiporus Audet (type Antrodia albidoides A. David & Dequatre) Flavidoporia Audet (type Poria pulvinascens Pilát) Fragifomes B.K. Cui, M.L. Han & Y.C. Dai (type Fomitopsis niveomarginata L.W. Zhou & Y.L. Wei)
Melanoporia Murrill (type Polyporus niger Berk.) Neoantrodia Audet (type Polyporus serialis Fr.) Neolentiporus Rajchenb. (type Polyporus maculatissimus Lloyd) Niveoporofomes B.K. Cui, M.L. Han & Y.C. Dai (type Polyporus spraguei Berk. & M.A. Curtis)
Pilatoporus Kotl. & Pouzar (type Polyporus palustris Berk. & M.A. Curtis)
Piptoporus P. Karst. (type Boletus betulinus Bull.) Pseudoantrodia B.K. Cui, Y.Y. Chen & Shun Liu (type Pseudoantrodia monomitica B.K. Cui, Y.Y. Chen & Shun Liu) Pseudofomitopsis B.K. Cui & Shun Liu (type Pseudofomitopsis microcarpa B.K. Cui & Shun Liu)
Ranadivia Zmitr. (type Daedalea allantoidea M.L. Han, B.K. Cui & Y.C. Dai)
Rhizoporia Audet (type Antrodia hyalina Spirin, Miettinen & Kotir.) Rhodofomes Kotl. & Pouzar (type Boletus roseus Alb. & Schwein.)
Rhodofomitopsis B.K. Cui, M.L. Han & Y.C. Dai (type Polyporus feei Fr.)
Rubellofomes B.K. Cui, M.L. Han & Y.C. Dai (type Fomitopsis cystidiata B.K. Cui & M.L. Han)
Subantrodia Audet (type Agaricus juniperinus Murrill) Ungulidaedalea B.K. Cui, M.L. Han & Y.C. Dai (type Fomitopsis fragilis B.K. Cui & M.L. Han)
As redefined here, the genus Fomitopsis encompasses poroid species only (in two species, A. albidoides and Gloeophyllum concentricum, hymenophore is nearly irpicoid or lamellate), although of a highly diverse habit. Some species formerly addressed to Antrodia sensu lato (e.g., Antrodia infirma, A. oleracea, A. primaeva) produce rather ephemeral, fleshy, predominantly effused basidiocarps. A few sessile or stipitate species previously treated under Laccocephalum and Pilatoporus also possess short-living, sappy basidiocarps easily damaged by insects. However, most Fomitopsis spp. produce long-living seasonal or truly perennial, sturdy basidiocarps. Many of them are able to produce pilei, even though the basidiocarp shape is exceptionally flexible in many species, varying from sessile caps to fully effused. Of macroscopic characters, consistency and colour(s) of basidiocarps, as well as colour change in aged or dried specimens, ability to produce stratified hymenophore and pore size and shape are the most important traits for the species recognition in Fomitopsis.
Anatomically, the genus is much less diverse than it might be expected from the macroscopic range of variation. A small group of Antrodia sensu lato spp. has monomitic hyphal structure. In two species, A. monomitica and A. oleracea, all hyphae are highly uniform, predominantly thin-walled, while in six other species (members of Antrodia ramentacea complex, plus F. fissa and F. retorrida) hyphae possess variably thickened walls and are often densely glued together (Fig. 16A, B). In the A. ramentacea complex, some contextual hyphae are very thick-walled, having a capillary lumen only, and they might be interpreted as skeletal hyphae. However, they have rare septa with clamps (observable in CB and phase contrast), and therefore they likely represent sclerified generative hyphae. The rest of Fomitopsis spp. are dimitic, bearing variably branched skeletal hyphae, and clamped generative hyphae (absent only in Fomitopsis sp. Darwin (M.D. Barrett F17/09)). Some species with rather soft, as a rule short-living basidiocarps have skeletal hyphae located only in context (e.g., Buglossoporus quercinus, Laccocephalum hartmannii) while in others they also occur in tube trama (e.g., Antrodia primaeva, Fomitopsis palustris group). In both cases, variable transitions between sclerified, richly branched generative hyphae and sparsely branched, unclamped skeletal hyphae can be observed, and generative hyphae are either dominating in both context and tubes or present in the same proportion as skeletal hyphae (Fig. 16C). A vast majority of Fomitopsis spp. have leathery or corky, persistent basidiocarps consisting mainly of skeletals, which are usually coloured and occasionally branched. In many dimitic species, obtuse or acute endings of skeletal hyphae enter the hymenial layer; we call these structures skeletocystidia (Fig. 16D). Basidia in all Fomitopsis spp. treated below are clavate and four-spored, in senescent hymenium often slightly thick-walled and at least partly glued together. Basidiospores vary from narrowly cylindrical to subglobose, and they normally do not exceed 10 μm long, except in the Buglossoporus subclade and a few other species mentioned above.
Fig 16.
Anatomical structures (subicular and subhymenial hyphae, hymenial cells and basidiospores) of Fomitopsis spp. F. oleracea (Miettinen 17902), F. fissa (holotype), F. dollingeri (holotype), F. cellularis (Vlasák 1504/36J). Scale bar = 10 μm.
Morphological diversity in the redefined Fomitopsis makes it hardly distinguishable from other poroid brown-rot genera of Polyporales. Nevertheless, it is worth mentioning here at least some most important traits differentiating them. Differences between Fomitopsis and two other genera of Fomitopsidaceae, Antrodia and Anthoporia, were discussed above. The Laetiporaceae (as redefined by Justo et al. 2017) encompass sappy pileate (Laetiporus) and resupinate (Wolfiporia, Macrohyporia, Melanoporella) polypores which are totally devoid of clamps. Basidiospores of Laetiporaceae are ellipsoid or subglobose, bearing a prominent apiculus and usually having oil-rich contents. The genus Pseudophaeolus (incertae sedis, see notes under excluded taxa and also Fig. 1 and Suppl. Fig. S1) is morphologically highly similar to Laetiporus although it has constantly clamped generative hyphae. Both Laetiporaceae and Pseudophaeolus possess unusually wide (reaching 10–30 μm diam), sclerified hyphae, in the context and (in most taxa) tubes. No such structures are known in Fomitopsidaceae.
Wide, variably thick-walled hyphae are characteristic for Laricifomes officinalis (= Fomitopsis officinalis) and Gilbertsonia angulipora which form an isolated monophyletic lineage among the brown-rot Polyporales (Ortiz-Santana et al. 2013). Unlike Laetiporaceae and Pseudophaeolus, basidiocarps of L. officinalis and G. angulipora have a characteristic chalky consistency. Two species of Ryvardenia are chalky when dried, too, and they also have wide sclerified hyphae in the context (Rajchenberg 1994). However, the basidiospores of Ryvardenia are slightly thick-walled while they are thin-walled in Laricifomes and Gilbertsonia. The phylogenetic position of Ryvardenia among brown-rot polypores was recently clarified by Liu et al. (2022) (see also Suppl. Fig. S1). Three Fomitopsis spp. with chalky basidiocarps, i.e. F. caseosa, F. niveomarginata and F. pseudopetchii, differ from Laricifomes, Gilbertsonia and Ryvardenia in having a dark-coloured cap surface, much narrower hyphae and distinctly smaller basidia and basidiospores.
Some Antrodia sensu lato species in the Fibroporia – Amyloporia clade (as defined by Justo et al. 2017), i.e. Antrodia sinuosa and members of the Antrodia crassa complex, have a certain resemblance to Fomitopsis spp. Crumbling basidiocarps consisting of easily breaking, often twisted skeletal hyphae are characteristic for A. crassa and its siblings. In most cases, these hyphae are either amyloid or quickly swelling and partly dissolving in KOH. Moreover, microscopic mounts of species from the A. crassa complex are usually full of resinous droplets (Spirin et al. 2015a). All these features certainly rule out Fomitopsis. Antrodia sinuosa is a much more difficult case because it has slightly coloured, occasionally branched skeletal hyphae and small basidia reminiscent of those in many Fomitopsis spp. However, basidiospores in A. sinuosa are allantoid or narrowly cylindrical and evenly curved; this spore shape is unknown in Fomitopsis. Narrowly cylindrical, curved basidiospores are characteristic for Taiwanofungus spp. as well. Additionally, representatives of the latter genus have brownish skeletal hyphae showing slight amyloid reaction (Wu et al. 2004). According to phylogenetic data, Taiwanofungus is not or very distantly related to Fomitopsidaceae (Ortiz-Santana et al. 2013, Liu et al. 2022; see also Suppl. Fig. S1).
In many respects, monomitic Fomitopsis spp. approach Rhodonia (Amyloporia clade) and some Postia spp. (Dacryobolaceae). Sappy, effused basidiocarps of Rhodonia spp. resemble A. infirma and A. primaeva. However, the two latter species have on average larger, slightly thick-walled basidia and longer, often subfusiform basidiospores. The majority of Postia sensu lato species have allantoid or narrowly cylindrical, curved basidiospores, and therefore they can be differentiated from Fomitopsis spp. based on this character alone. A few species morphologically similar to monomitic Fomitopsis species (e.g., Postia amara, Postia sequoiae) possess unusually wide context hyphae (6–10 μm diam) occasionally bearing double clamps (Gilbertson & Ryvarden 1987); this feature is unknown in Fomitopsis. Laterally stipitate, fleshy basidiocarps of Jahnoporus spp. (Dacryobolaceae) resemble the former species of Laccocephalum (i.e. L. hartmannii and L. tumulosum) transferred to Fomitopsis in this study. However, they can be easily separated due to their different hyphal structure: Jahnoporus spp. are thoroughly monomitic (Spirin et al. 2015b) while L. hartmannii and sibs have true skeletal hyphae at least in the context.
The species below are divided between those that are accepted and those that are excluded or insufficiently known. They are presented in alphabetical order in both sections. For each species, the basionym and type specimen/illustration are indicated. Synonymy is reduced to the newly detected synonyms and taxonomically important names. Further, either a new description (in almost all cases, based on type material and newly sequenced specimens) or a reference to the most modern and reliable source is provided. All additional information is placed under remarks.
Accepted species
Fomitopsis aculeata (Cooke) Spirin & Miettinen, comb. nov. MycoBank MB 844877. Figs 17, 18.
Fig 17.
Basidiocarps of Fomitopsis spp. A. Fomitopsis aculeata (Miettinen 8647). B. F. angusta (Spirin 10725). C. F. caespitosa (Miettinen 10227). D. F. cajanderi (Miettinen 22477). E. F. carnea (Miettinen 23610). F. F. caseosa (holotype). G. F. castanea (Spirin 5144). H. F. dickinsii (Niemelä 6435).
Fig 18.
Basidiospores of Fomitopsis spp. F. aculeata (Miettinen 8674); F. africana (Kout 1408/K9); F. algumicola (holotype); F. amygdalina (Vlasák 1707/9J); F. atypa (Ryvarden 17588); F. caespitosa (Miettinen 8737). Scale bar = 5 μm.
Basionym: Polystictus aculeatus Cooke, Grevillea 14 (71): 85. 1886.
Typus: Indonesia, Java, Tjikoya [Cikoya], rotten wood, 1843, Zollinger 2055 (holotype PC!).
Synonym: Daedalea radiata B.K. Cui & Hai J. Li, Mycoscience 54: 65. 2013.
Typus: China, Yunnan, Mengla, Wangtianshu, hardwood, 16 Sep. 2007, Yuan 3629* (holotype IFP 13864).
Description: Basidiocarps seasonal, dimidiate or effused-reflexed, projecting up to 2 cm, effused parts up to 8 cm in widest dimension. Upper surface first pale ochraceous, later brownish to brown, hirsute, indistinctly zonate. Pileal edge sharp to rather blunt, concolourous with pileal surface, first sterile, up to 1 mm wide, then fertile. Pore surface first cream-coloured to pale ochraceous, then brownish; pores angular or sinuous, partly fusing together 4–5(−6) per mm, with rather thick, entire or serrate dissepiments. Section: context leathery, ochraceous to brownish, up to 1 mm thick; tubes leathery, one-layered, concolourous with hymenial surface, up to 2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, interwoven, occasionally branched, (3.9–)4.0–5.2(−5.4) μm diam (n = 20/1), lumen varying from rather wide to capillary, side branches 2.5–4 μm diam, generative hyphae hyaline, slightly to distinctly thick-walled, 3–4 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to rusty brown, densely interwoven, predominantly dichotomously branched, sometimes twisted and irregularly inflated, (3.0–)3.2–5.0(−5.2) μm diam (n = 40/2), lumen mostly capillary to indistinct, side branches richly ramified, 1.5–2.5 μm diam, generative hyphae rare, thin- to slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Skeletocystidia present as slightly swollen (up to 4 μm diam), acute or blunt apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles abundant, tapering, often with a long hyphoid neck, 12–17 × 3–3.5 μm. Hyphidia abundant, simple or rarely bifurcate, 1.8–2.2 μm diam at the apex, projecting up to 10 μm. Basidia clavate, (10.7–)10.8–15.2(−19.2) × (4.4–) 4.8–5.3(−5.4) μm (n = 20/1), in senescent hymenium slightly thick-walled and often glued together. Basidiospores thin-walled or with a distinct wall, cylindrical-subfusiform, (4.0–)4.1–5.3(−5.4) × (2.0–) 2.1–2.6(−2.7) μm (n = 30/1), L = 4.81, W = 2.26, Q = 2.13, often with one or a few large oil droplets.
Specimens examined: Indonesia, Riau, Indragiri Hulu, Bukit Aluran Babi, primary rainforest slope, dicot (fallen tree crown), 27 Jun. 2004, Miettinen 8674* (H); Sumatera Barat, Padang, Limau Manis, hilly primary forest, dicot, 11 Jul. 2008, Miettinen 12960 (H); ibid., 16 Jul. 2008, Miettinen 13128.2 (ANDA, H).
Notes: This species was originally described as Polyporus aculeatus Lév. (Léveillé 1846). However, this name was illegitimate due to the existence of P. aculeatus Mont. (now a member of Hexagonia) described six years earlier (Montagne 1840). Cooke (1886) moved Léveillé’s species to Polystictus and thus mechanically validated it.
Fomitopsis aculeata is distributed in Southeast Asia. It is a distinctive species due to its hirsute, brownish upper surface reminiscent of some Funalia spp. Members of the latter genus differ from F. aculeata in having harder basidiocarps with trimitic hyphal structure and broader skeletal hyphae, as well as much larger basidiospores. Three sequences of this species in GenBank (AJ542530, AJ536655, AJ542522) are mislabelled as Funalia trogii. Another genus that comes into mind macroscopically is Gloeophyllum whose representatives are darker-coloured and trimitic and they have distinctly longer basidiospores. See further notes to F. gilvidula.
Fomitopsis aethalodes (Mont.) Spirin, comb. nov. MycoBank MB 844878.
Basionym: Trametes aethalodes Mont., Ann. Sci. Nat., Bot. 4 (5): 370. 1856.
Typus: Brazil, [no locality and collecting date], Weddell (lectotype PC!) (selected by Ryvarden 1982: 76).
Description: Rajchenberg (1986, as Daedalea aethalodes).
Notes: Rajchenberg (1986) provided a description of morphological and cultural characters of T. aethalodes. He proved it is a brown-rot fungus and therefore combined it in Daedalea. Cristaldo et al. (2022) published first DNA sequences of this species and confirmed that it belongs to the Daedalea clade of Fomitopsis (see also Fig. 5). We studied the type material and concluded that D. aethalodes is morphologically most similar to F. aculeata redescribed above.
Fomitopsis africana Mossebo & Ryvarden, Sydowia 49 (2): 148. 1997. Fig 18.
Typus: Cameroon, Mfoundi, Yaoundé, International Institute of Tropical Agriculture, Eucalyptus sp., 2 Jul. 1996, Mossebo 13* (holotype O).
Description: Basidiocarps perennial, sessile, conchate or ungulate, projecting up to 3 cm. Upper surface first cream-to wood-coloured, sometimes with faint pinkish tint, smooth, later grey to greyish-brown, matt. Pileal edge sharp to rather blunt, concolourous with hymenial surface, fertile. Pore surface pinkish-grey to greyish, sometimes with vinaceous-brown stains; pores roundish to angular, 7–8 per mm, with thick, entire dissepiments. Section: crust (present in old basidiocarps) tough, blackish brown, matt, up to 0.2 mm thick, context soft corky, wood-coloured to brownish, up to 5 mm thick; tubes soft corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 8 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, yellowish to brownish, interwoven, occasionally branched, (3.9–)4.2–6.0(−6.2) μm diam (n = 20/1), lumen mostly narrow to indistinct, side branches 2.5–3.5 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae dominating, brownish, loosely interwoven, occasionally branched, (2.9–)3.0–5.0(−5.2) μm diam (n = 40/2), lumen wide to capillary, side branches 1.5–2.5 μm diam, generative hyphae thin- to slightly thick-walled, 2–4 μm diam. Subhymenium partly distinct, up to 15 μm thick. Cystidioles infrequent to rather common, tapering, 10–14 × 3.5–5 μm. Basidia clavate, (10.2–)10.6–14.8(−14.9) × (4.8–)5.0–6.0(−6.2) μm (n = 20/2), in senescent hymenium slightly thick-walled. Basidiospores with a distinct wall, cylindrical-subfusiform, (5.7–)5.8–7.8(−8.1) × (2.4–)2.5–3.4(−3.6) μm (n = 60/2), L = 6.49–6.57, W = 2.97–2.98, Q = 2.19–2.22.
Specimens examined: Indonesia, Riau, Kampar, Serapung, dicot, seasonally flooding village backyard, 6 Dec. 2011, Miettinen 15198* (BO, H). Thailand, Sukhotai, Khiri Mat, Ramkamhaeng, 12 Aug. 2014, Kout 1408/K9* (JV, TUF).
Notes: Fomitopsis africana was originally described from Cameroon (Mossebo & Ryvarden 1997) and then sequenced by Kim et al. (2008). Here we report it from Southeast Asia (Thailand and Sumatra, Indonesia). Two GenBank sequences of ‘Fomitopsis sp.’ from India (KJ670294, MG430346) belong to F. africana. The ITS sequence from the type collection differs in 4 bp from the Southeast Asian ones; for now, it is impossible to decide whether this difference is inter- or infraspecific. Phylogenetically, F. africana is related to the F. feei complex. Differences between F. africana and other members of this group are discussed under F. carnea.
Fomitopsis alaskana (D.V. Baxter) Spirin & Vlasák, comb. nov. MycoBank MB 844879.
Basionym: Trametes alaskana D.V. Baxter, Pap. Mich. Acad. Sci. 27: 150. 1942.
Typus: USA, Alaska, Cordova, Picea sitchensis, 21 Aug. 1933, Baxter 2-2048 (holotype MICH 12309!).
Description and phylogenetic data: Spirin et al. (2017, as Antrodia alaskana).
Fomitopsis albidoides (A. David & Dequatre) Bernicchia & Vlasák, comb. nov. MycoBank MB 844880.
Basionym: Antrodia albidoides A. David & Dequatre, Mycol. Helv. 1 (6): 361. 1986.
Typus: France, Var, Île de Port-Cros, Phillyrea latifolia, 31 Oct. 1977, David 3495 (holotype LY AD-3495!).
Synonyms:
Antrodia subalbidoides A. David & Dequatre, Mycol. Helv. 1 (6): 362. 1986.
Typus: France, Var, Le Brusc, Phillyrea angustifolia, 1984, David 4591 (holotype LY AD-4591!).
Antrodia macrospora Bernicchia & De Dominicis, Polyporaceae sensu lato in Italy: 74. 1990.
Typus: Italy, Tuscany, Grosseto, Riserva di Burano, P. angustifolia, 29 Sep. 1989, Bernicchia 5116 (holotype HUBO!).
Description and phylogenetic data: Spirin et al. (2013b, as A. albidoides), see also Figs 1 and 2.
Specimens examined: Italy, Sardinia, Nuoro, Oliena, Strada per Valle di Lanaittu, Phillyrea sp., 20 Nov. 1999, Bernicchia 7244* (HUBO); Tuscany, Grosseto, Riserva di Burano, P. angustifolia, 27 Oct. 1992, Bernicchia 5672* (H ex HUBO).
Fomitopsis algumicola Grebenc & Spirin, sp. nov. MycoBank MB 844881. Fig 18.
Typus: North Macedonia, Resen, Golem Grad Island, 40.87° 20.99°, Juniperus excelsa (fallen log), 9 Oct. 2000, Karadelev (holotype MCF MAK 00/4578*, isotype H7200206).
Etymology: Algumicola (Lat., noun) – derived from “algum”, the biblical name which seemingly refers to a juniper.
Description: Basidiocarps perennial, effused, up to 15 cm in widest dimension, on sloping substrate with nodulose parts projecting up to 3 cm. Margin sharply delimited, partly detaching, cream-coloured or greyish to ochraceous-brownish, up to 0.5 mm wide, often covered by incomplete pores. Pore surface first cream-coloured to pale ochraceous, then ochraceous-brownish to dark brown, uneven; pores angular to lacerate, 0.5–1.5 per mm, 10–14 per cm, with rather thick, entire or serrate dissepiments. Section: context leathery, cream- to wood-coloured, up to 5 mm thick; tubes leathery, one-layered, concolourous with hymenial surface, up to 15 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, interwoven, occasionally branched, (3.2–)3.4–4.3(−4.8) μm diam (n = 20/1), lumen mostly capillary to indistinct, side branches 1.5–2.5 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae dominating, brownish, interwoven, occasionally branched, (3.8–)3.9–6.3(−8.0) μm diam (n = 60/3), lumen mostly narrow to indistinct, side branches 1.5–2.5 μm diam, generative hyphae thinto slightly thick-walled, 2–4 μm diam. Subhymenium distinct, 20–30 μm thick. Skeletocystidia occasionally present, broadly clavate, up to 7–8 μm diam at the apex. Cystidioles rare, tapering, 15–20 × 3–4 μm; hyphidia rarely present, simple, slightly projecting, 2–2.5 μm diam at the apex. Large rhomboid crystals often present among subhymenial and tramal hyphae, up to 20 μm in widest dimension. Basidia clavate, (13.0–)13.2–22.8(−23.1) × (5.1–)5.2–6.4(−6.7) μm (n = 30/3), in senescent hymenium slightly thick-walled and glued together. Basidiospores with a distinct wall, broadly cylindrical to subfusiform, occasionally slightly concave at the ventral side, (5.1–)5.2–7.6(−9.8) × (2.3–)2.4–3.3(−3.8) μm (n = 90/3), L = 6.09–6.63, W = 2.83–3.07, Q = 1.98–2.23.
Specimens examined: North Macedonia, Kavadartsi, Tikveš, Tikveška Klisura, Juniperus excelsa, 25 Jul. 1990, Karadelev (H 7042099); Petrovec, Katlanovo, J. excelsa, [no collection date], Karadelev & Rusevska (MCF MAK xx/7771*); Kozhle, J. excelsa, 25 Mar. 2001, Karadelev (MCF MAK 01/2095*); ibid., 19 Oct. 2007, Karadelev & Rusevsca (MCF MAK 07/8020*); Valandovo, Chalakli, J. excelsa, 26 Oct. 2007, Karadelev & Theiss (MCF MAK 07/8293*).
Notes: Fomitopsis algumicola is a close relative of F. juniperina distributed in the Balkans (North Macedonia) and inhabiting wood of Juniperus excelsa (Suppl. Fig. S7). The latter species differs from F. algumicola in having larger pores, 0.5–1 per mm, 8–11 per cm, which could be almost lamellate in fully developed specimens, as well as a usual presence of truly pileate parts. Distribution areas of F. algumicola and F. juniperina seem not to coincide.
Fomitopsis amygdalina (Berk. & Ravenel) Spirin & Vlasák, comb. nov. MycoBank MB 844882. Fig 18.
Basionym: Polyporus amygdalinus Berk. & Ravenel, Ann. Mag. Nat. Hist. 2 (12): 432. 1853.
Typus: USA, South Carolina, Newhope, Quercus sp., [no collection date], Ravenel 1153 (isotype NY00730503!).
Description: Basidiocarps annual, spathulate or effused-reflexed, projecting up to 6 cm. Upper surface orange to reddish brownish, azonate, finely velutinous, indistinctly furrowed. Pileal edge rather blunt, concolourous with cap surface, fertile, somewhat undulating. Pore surface pale ochraceous to brownish, slightly concave; pores angular, (4)5–7 per mm, with thin, even or serrate dissepiments. Section: context soft, cream-coloured, up to 25 mm thick; tubes soft, easily cut by a razor blade, one-layered, distinctly paler (cream-coloured) than hymenial surface, up to 3 mm thick, turning reddish-brown after bruising. Smell faint, pleasant (fruit-like) (dry specimen). Hyphal structure monomitic in tube trama and dimitic in context; hyphae clamped. Context dimitic; skeletal hyphae hyaline to yellowish, interwoven, dichotomously branched, (4.2–)4.3–9.2(−10.4) μm diam (n = 40/2), lumen mostly indistinct, side branches 3–4 μm diam, generative hyphae infrequent, hyaline, thin-walled, 4–8 μm diam, occasionally with yellowish cyanophilous contents. Trama monomitic; hyphae mostly hyaline, rarely yellowish or greenish, thin-walled, subparallel, (2.3–)2.8–4.2(−4.8) μm diam (n = 40/2), intermixed with hyaline or coloured (greenish or brownish), irregularly inflated hyphae 3–5 μm diam. Cystidia and cystidioles absent. Basidia clavate, hyaline or yellowish greenish, (15.3–) 16.0–37.0(−38.0) × (5.0–)5.6–7.8(−8.2) μm (n = 25/2), sometimes pleural. Basidiospores thin-walled, fusiform, longest spores somewhat sigmoid, (5.8–)5.9–9.2(−10.1) × (2.5–)2.6–4.0(−4.1) μm (n = 62/2), L = 6.79–7.54, W = 3.03–3.36, Q = 2.25, cytoplasm often guttulate.
Specimen examined: Costa Rica, Puntarenas, Monteverde, Santa Elena, hardwood, Jul. 2017, Vlasák Jr. 1707/9-J* (JV, TUF).
Notes: Berkeley & Curtis (1853) introduced P. amygdalinus based on a single collection from South Carolina. The identity of the species remained obscure until Lowe & Pegler (1973) studied microscopic traits of the authentic specimen and argued it should be treated under Tyromyces. Ryvarden (1977) accepted P. amygdalinus as a member of Dichomitus. Finally, Gilbertson & Ryvarden (1987) placed it in the synonymy of P. virgatus although the latter species was described from Cuba 16 yr later. We restudied the type of P. amygdalinus. It is still in a good condition and characterized by monomitic tubes and dimitic context. Skeletal hyphae are wide, occasionally branched, usually subsolid; they show neither the cyanophilous reaction nor dichotomous branching so characteristic of Dichomitus and Polyporus spp. Some tramal hyphae become brownish or greenish in KOH, and this feature, combined with longclavate basidia and rather long fusiform basidiospores, pointed to Fomitopsis pulvina (= Buglossoporus quercinus, see below) as a potential closest relative of P. amygdalinus. This suggestion was confirmed by a DNA study of a recent specimen from mountain forest in Costa Rica which we found to be conspecific with the type of P. amygdalinus (Suppl. Fig. S8). Therefore, P. amygdalinus is treated here as a member of Fomitopsis.
Basidiocarps of F. amygdalina are strikingly lightweight and show a certain macroscopic similarity to F. pulvina; the colour change after bruising typical to the latter species was also noticed in fresh material of F. amygdalina. Microscopically, these species are almost indistinguishable except that tapering, narrow cystidia are abundant in the hymenium of F. pulvina but not detected in F. amygdalina. Among macroscopic traits, tube layer thickness can be used as a character separating the two species: the tubes of F. amygdalina are considerably shorter than the pileal context while they are of approximately the same thickness in F. pulvina. Additionally, the pores of F. amygdalina are smaller than in F. pulvina, 5–7 vs. 3–4 per mm although they strongly shrink after drying in both species. So far, F. amygdalina is known only from the locus classicus and a new locality in Costa Rica.
Fomitopsis angusta (Spirin & Vlasák) Spirin & Vlasák, comb. nov. MycoBank MB 844883. Fig 17.
Basionym: Antrodia angusta Spirin & Vlasák, Mycologia 109: 223. 2017.
Typus: Russia, Primorie, Krasnoarmeiskii Dist., Valinku, Picea ajanensis, 29 Aug. 2013, Spirin 6479* (holotype H!).
Description and phylogenetic information: Spirin et al. (2017, as A. angusta).
Fomitopsis atypa (Lév.) Spirin & Vlasák, comb. nov. MycoBank MB 844884. Fig 18.
Basionym: Polyporus atypus Lév., Ann. Sci. Nat., Bot. 3 (2): 184. 1844.
Typus: Indonesia, Java, ‘ad truncos’, [no collection date and collector] (holotype PC!).
Synonyms:
Coriolus cuneatiformis Murrill, Bull. Torrey Bot. Club 34: 467. 1907.
Typus: Philippines, Luzon, Lanao River, dead wood, Dec. 1903, Williams (holotype NY 00704951!).
Coriolus clemensiae Murrill, Bull. Torrey Bot. Club 35: 394. 1908.
Typus: Philippines, Mindanao, Lake Lanao, Camp Keithley, dead wood, Sep.–Oct. 1907, Clemens (holotype NY 00704945!).
Coriolus rubritinctus Murrill, Bull. Torrey Bot. Club 35: 396. 1908.
Typus: Philippines, Mindoro, Mt. Halcon, dead wood, Nov. 1906, Merrill 6117 (holotype NY 00704983!).
Coriolus parthenius Hariot & Pat., Bull. Mus. Natn. Hist. Nat. 15: 90. 1909.
Typus: Gabon, Cap Lopez, 11 Jul. 1902, Chevalier 11478 (lectotype FH, isolectotype PC!) (selected by Ryvarden 1983: 28).
Antrodia taxa T.T. Chang & W.N. Chou, Mycol. Res. 103: 622. 1999.
Typus: Taiwan, Kaohsiung, Liu Kuei, Taxus mairei, Nov. 1996, Chang (holotype TFRI 781*).
Description: Basidiocarps short-living perennial, sessile, often with a contracted base (fan-shaped), projecting up to 4.5 cm. Upper surface first cream coloured to beige, felty, later pale ochraceous, usually with numerous narrow concentric zones, glabrous, sometimes radially wrinkled. Pileal edge sharp, concolourous with cap surface, first sterile, up to 1 mm wide, then fertile. Pore surface cream-coloured or beige to pale ochraceous, sometimes with a faint pinkish tint, flat or concave; pores roundish to angular, 5–7 per mm, with thin, entire or serrate dissepiments. Section: context corky, cream- to wood-coloured, normally less than 2 mm thick; tubes corky, one-layered, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae yellowish to brownish, densely interwoven or in subparallel bundles, occasionally or richly branched, (3.0–) 3.6–7.2(−7.3) μm diam (n = 100/5), lumen mostly rather wide, side branches 1.5–3 μm diam, generative hyphae infrequent, hyaline, thin- to moderately thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae dominating, hyaline or yellowish to brownish, densely interwoven, occasionally branched, (2.8–)2.9–5.3(−5.4) μm diam (n = 160/8), lumen mostly narrow to indistinct, side branches 1.5–2.5 μm diam, generative hyphae rather rare, thinto slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Cystidioles abundant to rather rare, tapering, 11–16 × 2.5–3.5 μm; hyphidia occasionally present, simple or bi-trifurcate, 1–2 μm diam at the apex. Basidia clavate, (10.3–)11.8–16.2(−16.3) × (4.3–)4.6–5.7(−5.9) μm (n = 20/2), occasionally pleural. Basidiospores with a distinct wall, broadly cylindrical to ellipsoid, some slightly concave at the ventral side, (3.0–)3.2–4.9(−5.0) × (1.9–)2.0–2.8(−2.9) μm (n = 80/3), L = 3.89–4.39, W = 2.20–2.47, Q = 1.67–1.83.
Specimens examined: India, Kerala, Idukki, Munnar, Pambumkayam, fallen log, 27 Jan. 2019, Dunayev* (TUF). Indonesia, Sumatera Barat, Pesisir Selatan, Gunung Sako, dicot, hilly primary forest, 27 Apr. 2002, Miettinen 6403 (BO, H). Thailand, Cangwat Chiang Mai, 15 Feb. 1979, Ryvarden 17588* (O F508226); ibid., 18 Feb. 1979, Ryvarden 18005 (O 12727).
Notes: Fomitopsis atypa is a sibling species of F. modesta distributed in the paleotropics. The two species can be distinguished primarily due to their ITS sequences and distribution areas. Morphological differences are subtle: F. atypa has pores on average slightly wider than F. modesta, and its basidiospores are slightly narrower, occasionally with a somewhat concave ventral side.
Fomitopsis avellanea (Bres.) Ryvarden, Mycotaxon 33: 304. 1988.
Basionym: Trametes avellanea Bres., Krypt. Exs. Mus. Palat. Vindob. 20: 157. 1910.
Typus: Madagascar, [no locality indicated], ‘ad truncos’, [unkown collection date], Sikora (isotype H!).
Description: Ryvarden (1988a).
Notes: No phylogenetic data are so far available for this species. Fomitopsis avellanea is known only from the type specimen from Madagascar. Macroscopically, it looks like a representative of the F. feei complex although the specimen is completely sterile. New collections are highly desirable to infer the relationships of F. avellanea with other Fomitopsis spp.
Fomitopsis bambusae Y.C. Dai, Meng Zhou & Yuan Yuan, MycoKeys 82: 186. 2021.
Typus: China, Hainan, Haikou, Jinniuling Park, Bambusa sp., 18 Nov. 2020, Dai 22116* (holotype BJFC 36008).
Description and phylogenetic data: Zhou et al. (2021).
Fomitopsis betulina (Bull.) B.K. Cui, M.L. Han & Y.C. Dai, Fungal Diversity 80: 359. 2016.
Basionym: Boletus betulinus Bull., Herbier de la France 7: t. 312. 1788.
Typus: Plate 1254 in Flora Danica 21, 1799 (iconotype) (selected by Ryvarden 1991: 202).
Synonym: Piptoporus betulinus (Bull.) P. Karst., Meddeland. Soc. Fauna Fl. Fenn. 6: 9. 1881.
Description: Ryvarden et al. (2017, as Piptoporus betulinus).
Notes: Phylogenetic data are available in this paper (Figs 1, 3, Suppl. Fig. S2), in accordance with Kim et al. (2005) and Ortiz-Santana et al. (2013).
Fomitopsis caespitosa (Murrill) Spirin & Miettinen, comb. nov. MycoBank MB 844885. Figs 17, 18.
Basionym: Trametes caespitosa Murrill, Bull. Torrey Bot. Club 34: 473. 1907.
Typus: Philippines, Luzon, Bataan, Mt. Mariveles, dead wood, Nov. 1904, Elmer 6951 (holotype NY00705017!).
Synonyms:
Tyromyces multipapillatus Corner, Beih. Nova Hedwigia 96: 180. 1989.
Typus: Solomon Islands, Ysabel, San Jorge, 25 Sep. 1965, Corner (holotype E00159594).
Tyromyces ochraceivinosus Corner, Beih. Nova Hedwigia 96: 182. 1989.
Typus: Singapore, ‘Thompson Road, 9th mile’, 23 May 1943, Corner (holotype E00159597).
Fomitopsis subtropica B.K. Cui & Hai J. Li, Mycol. Progr. 12: 710. 2013.
Typus: China, Guangdong, Guangzhou, Tianluhu, Castanopsis sp., 19 Aug. 2011, Cui 10154* (holotype BJFC).
Antrodiella subnigra Oba, Mossebo & Ryvarden, Syn. Fung. 40: 97. 2020.
Typus: Cameroon, Mfoundi, Yaoundé, unknown dead hardwood, 25 Oct. 2018, Mossebo 1597 (isotype O F-76329!).
Description: Basidiocarps annual, effused-reflexed, solitary or in imbricate groups, projecting up to 2 cm, occasionally totally resupinate and then up to 6 cm in widest dimension. Upper surface smooth or scrupose, greyish-ochraceous to mouse-grey, sometimes with brownish flecks, azonate. Pileal edge sharp, concolourous with cap surface, usually sterile, margin of resupinate parts cream-coloured to pale ochraceous, compact, adnate or partly detaching, sharply delimited. Pore surface ivory to ochraceous, sometimes with brownish stains; pores roundish to angular, (6–)7–11(−12) per mm, with thin or rather thick, entire dissepiments. Section: context soft corky, white to cream-coloured or pale ochraceous, up to 2 mm thick; tubes corky, one-layered, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline to pale ochraceous, densely interwoven, occasionally branched, (3.4–)4.2–7.8(−8.2) μm diam (n = 60/3), lumen varying from narrow to capillary or almost invisible, side branches 2–3.5 μm diam, generative hyphae rare, hyaline, thin- or slightly thick-walled, 3–4 μm diam. Trama dimitic; skeletal hyphae hyaline to pale ochraceous, interwoven to subparallel, some flexuous, occasionally branched, (3.3–)3.4–6.8(−7.6) μm diam (n = 100/5), lumen capillary to invisible, generative hyphae thin- or slightly thick-walled, 2–3 μm diam, in some places forming subhymenial layer up to 10–15 μm thick, dissepiment edges dimitic, consisting of thin-walled generative hyphae and skeletal hyphae with a wide lumen, apically swollen up to 8 μm diam. Stellate or prismatic crystals occasionally present, incrusting hymenial cells or covering hyphal tips at the dissepiment edges. Cystidioles rare, gradually tapering to the apex, 11–14 × 3–4.5 μm. Basidia short-clavate, (8.8–)8.9–12.0(−14.3) × (3.8–)4.0–4.7(−4.8) μm (n = 30/3), in older hymenium partly glued together and covered by amorphous grainy substance. Basidiospores thin-walled or with a distinct wall, broadly cylindrical to ellipsoid, longest spores subfusiform, (2.7–)3.0–4.2(−4.6) × (1.8–)1.9–2.9(−3.0) μm (n = 150/5), L = 3.35–3.88, W = 2.04–2.25, Q = 1.51–1.83.
Specimens examined: Brazil, Para, Paragominas, Mina da Hydro Paragominas, fallen deciduous trunk, 27 Nov. 2014, Runnel 1434* (MG211370). China, Yunnan, Xishuangbanna, 9 Aug. 2005, Miettinen 10120 (H); uprooted tree, 13 Aug. 2005, Miettinen 10227.4 (H); dicot, 15 Aug. 2005, Miettinen 10322 (H). Indonesia, Papua, Jayapura, Cyclop Mountains, steep slope of natural forest, Pometia pinnata, 28 Jan. 2007, Miettinen 11547* (MAN, H); Papua Barat, Manokwari, Amberbaken, Saukorem, dry riverbed in a seaside forest, Terminalia catappa, 31 Oct. 2010, Miettinen 14238 (MAN, H); secondary forest, Pometia pinnata 31 Oct. 2010, Miettinen 14372 (MAN, H); Riau, Indragiri Hulu, Bukit Aluran Babi, dicot, 29 Jun. 2004, Miettinen 8737* (H); felled tree, 2 Jul. 2004, Miettinen 8823* (BO, H); Kampar, Hutan desa Serapung, fallen branch of Syzygium, 4 Dec. 2011, Miettinen 15149.1 (H); Pekanbaru, Rumbai, logged over natural forest, dicot, 2 Apr. 2002, Miettinen 5486* (BO, H); ibid., 18 Jul. 2004, Miettinen 8967 (H); Sumatera Barat, Padang, Limau Manis, dicot, logged over natural forest, 13 Jul. 2008, Miettinen 13019* (ANDA, H); ibid., 15 Jul. 2008, Miettinen 13076* (ANDA, H); Pesisir Selatan, Muara Sako, cinnamon plantation, Durio zibethinus, 25 Apr. 2002, Miettinen 6221 (BO, H). Malaysia, Sabah, Ranau, Poring, felled tree (Sapindales?), 19 Jun. 2013, Miettinen 16417 (SNP, H); Sarawak, Kuching, Bako, fallen logs, 9–13 Feb. 2017, Dunayev KUN 1123*, 2874* (BORH, H). Sri Lanka, Peradeniya, Kandy Royal Bot. Garden, hardwood, 31 Jan. 2013, Dunayev* (TUF).
Notes: Fomitopsis caespitosa is widely distributed in Southeast Asia. It is morphologically most similar to F. luzonensis and F. ostreiformis which have the same geographic distribution. The three species share greyish, tough basidiocarps varying from sessile-imbricate to completely resupinate. Of them, F. caespitosa possesses the widest tramal skeletal hyphae and smallest basidiospores (see Table 3). Hattori (2003) reported this species (as Antrodia multipapillata (Corner) T. Hattori) as common in temperate forests of Japan. Here we report it for the first time from Africa (Cameroon) and South America (Brazil).
Fomitopsis cajanderi (P. Karst.) Kotl. & Pouzar, Česká Mykol. 11 (3): 157. 1957. Fig 17.
Basionym: Fomes cajanderi P. Karst., Öfversigt af Finska Vetenskaps-Societetens Förhandlingdar 46 (11): 8. 1904.
Typus: Russia, Yakutia, Zhigansk, Agrafena, Larix gmelinii, 1 Aug. 1901, Cajander (herb. Karsten 4320) (holotype H 7044546!).
Description: Carranza-Morse & Gilbertson (1986).
Specimens examined: Russia, Khabarovsk Reg., Solnechnyi Dist., Igdomi, Picea ajanensis, 4 Aug. 2011, Spirin 3760 (H); Gorin, Larix gmelinii, 12 Aug. 2011, Spirin 4089* (H); Krasnoyarsk Reg., Turukhansk Dist., Lebed’, coniferous wood, 23 Aug. 2013, Kotiranta 26443* (H).
Notes: Fomitopsis cajanderi is a member of the F. rosea complex (Kim et al. 2008; see also Fig. 12) distributed in the continental parts of Eurasia and in the cold temperate – boreal area in North America. The differences between F. cajanderi and other pink-coloured representatives of the genus are summarized in Table 4.
Fomitopsis calcitrosa (Spirin & Miettinen) Spirin & Miettinen, comb. nov. MycoBank MB 844886.
Basionym: Antrodia calcitrosa Spirin & Miettinen, Mycologia 109: 223. 2017.
Typus: USA, Washington, Pend Oreille Co., Muskegon Lake, Picea engelmannii, 16 Oct. 2014, Spirin 8610* (holotype H!).
Description and phylogenetic data: Spirin et al. (2017, as A. calcitrosa); see also Fig. 3.
Fomitopsis carnea (Blume & T. Nees) Imazeki, Bull. Tokyo Sci. Mus. 6: 92. 1943. Figs 17, 19.
Fig 19.
Basidiospores of Fomitopsis spp. F. carnea (Miettinen 13120.1); F. caseosa (holotype); F. cupreorosea (Kout 0610/K4); F. cupressicola (holotype); F. derelicta (holotype); F. dochmia (Dunaev 7.II.2019). Scale bar = 5 μm.
Basionym: Polyporus carneus Blume & T. Nees, Nova Acta Phys.-Med. Acad. Caes. Leop.-Carol. Nat. Cur. 13: 14. 1826.
Typus: Tab. III (‘Polyporus carneus’) in Nova Acta Phys.-Med. Acad. Caes. Leop.-Carol. Nat. Cur. 13, 1826 (iconotype designated here, MycoBank MBT 10008288). Indonesia, Riau, Kampar Peninsula, logged-over peatswamp forest, Shorea (?), 3 Dec. 2011, Miettinen 15115.2* (H) (epitype designated here, MycoBank MBT 10008289).
Synonym: Polyporus aurora Ces., Atti Accad. Sci. Fis. Mat. Napoli 8: 5. 1879.
Typus: Malaysia, Sarawak, [no collection date], Beccari (isotype BPI US0319608!).
Description: Basidiocarps perennial, sessile, often with a contracted base (fan-shaped), producing large imbricate groups, sometimes fusing together, projecting up to 7 cm. Upper surface smooth, pinkish grey to pinkish buff, sulcate and radially striate. Pileal edge sharp, concolourous with hymenial surface, usually sterile, up to 2 mm wide. Pore surface pink to pinkish grey, usually concave; pores roundish to angular, (6–)7–9 per mm, with thin or moderately thickened, entire dissepiments. Section: context corky, pink or pinkish brownish, normally less than 5 mm thick; tubes corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae yellowish to brownish, densely interwoven, occasionally branched, (3.1–)3.3–5.0(−5.1) μm diam (n = 40/2), lumen normally capillary to indistinct, side branches 1.5–2 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 2.5–4 μm diam. Trama dimitic; skeletal hyphae dominating, hyaline to yellowish or brownish, densely interwoven, occasionally branched, (2.8–)2.9–4.2(−4.3) μm diam (n = 80/4), lumen varying from rather wide to capillary, generative hyphae rare, thin- to slightly thick-walled, 1–2 μm diam. Subhymenium indistinct. Cystidioles abundant to rare, tapering, 8–11 × 3–4 μm. Basidia short-clavate, (8.2–)8.3–13.3(−14.0) × (4.2–)4.7–5.1(−5.2) μm (n = 20/2), in senescent hymenium partly glued together. Basidiospores thin-walled or with a distinct wall, cylindrical-subfusiform to narrowly ellipsoid, occasionally slightly curved, (2.8–)2.9–4.5(−4.7) × (1.8–) 1.9–2.3(−2.4) μm (n = 150/5), L = 3.29–3.92, W = 2.01–2.11, Q = 1.57–1.86.
Specimens examined: Indonesia, Riau, Indragiri Hulu, Bukit Aluran Bab, selectively logged primary rainforest, dicot, 28 Jun. 2004, Miettinen 8700 (H); primary rainforest with Dipterocarpaceae and Dyera costulata, dicot (fallen tree crown), 29 Jun. 2004, Miettinen 8720 (H); half-opened forest, Pentadesmon (uprooted tree), 30 Jun. 2004, Miettinen 8765.1 (H); Kampar, Balung, heavily logged over natural forest, cut bolt of Mangifera, 24 Dec. 2006, Miettinen 11249 (BO, H); Sumatera Barat, Padang, Limau Manis, fallen dicot tree crown, 16 Jul. 2008, Miettinen 13120.1* (H). Singapore, Botanical Garden, dicot, 6 Jan. 2007, Miettinen 11321.1 (H); ibid., 20 Mar. 2020, Miettinen 23637 (SING, H); Shorea (?), 8 Jul. 2013, Miettinen 16605 (H); Bukit Timah, park, cut bolt of dicot, 19 Mar. 2020, Miettinen (SING, H). Thailand, Chanwat Chiang Doo, Doo Chiang Doo Nat. Park, 22 Feb. 1979, Ryvarden 17979 (O 17742, H).
Notes: Polyporus carneus was originally described from Java (Blume & Nees von Esenbeck 1826). The lack of any surviving type material evidently contributed to the numerous misinterpretations of this species. Here we apply the name P. carneus to a relative of F. feei widely distributed in Southeast Asia and macroscopically fitting the protologue. An epitype is selected to support our solution.
Phylogenetically, F. carnea is closest to F. eucalypti from Australia which has larger pores and basidiospores. Fomitopsis foedata, distributed in Oceania, differs from F. carnea in having thinner, flexible basidiocarps, wider pores and larger basidiospores. Fomitopsis africana is one more species of the F. feei complex occurring in the same geographic area; it has softer and paler basidiocarps than those of F. carnea, as well as wider and more loosely arranged tramal skeletal hyphae, and larger basidiospores. Another morphologically similar but phylogenetically distant species, F. marchionica, has more robust basidiocarps with a densely zonate upper surface and wider pores. The latter species is distributed in Oceania (see below). Two previously published sequences of F. carnea came from specimens collected in Tanzania (Han & Cui 2015). They belong to F. purpurea, a representative of the F. rosea complex, which we describe below.
The identity of F. carnea from outside the Malay Archipelago deserves further clarification. In our analysis, we detected the ITS sequence of ‘F. feei’ (GenBank KP780437) from Sri Lanka as sister to F. carnea sequences from Indonesia (Fig. 11). Differences between them were up to 10 bp, and therefore the Sri Lankan specimen may represent a separate species. In the latter case, Polyporus rubidus could be a suitable name for it.
Fomitopsis caseosa Vlasák & Spirin, sp. nov. MycoBank MB 844887. Figs 17, 19.
Typus: Costa Rica, Puntarenas, Golfito, Playa Cacao, abandoned farm, 8.640403°-83.192987°, on a dead stump with a violet core wood (Peltogyne?), 19 Apr. 2015, Vlasák 1504/28* (holotype PRM956227).
Etymology: Caseosus (Lat., adj.) – cheese-like.
Description: Basidiocarps perennial, sessile, ungulate, projecting up to 5 cm. Upper surface brown to brownish-black, smooth, distinctly sulcate, matt. Pileal edge blunt, ochraceous-brownish, sterile, up to 1 mm wide. Pore surface cream-coloured, concave; pores roundish or elongated, 8–9 per mm, with moderately thickened, entire dissepiments. Section: crust brownish-black, matt, 0.5–1 mm thick, context soft corky, brownish, up to 6 mm thick; tubes chalk-like, easily crumbling, clearly stratified, up to 45 mm thick, with up to 10 annual layers 2–4 mm thick, concolourous with hymenial surface, separated by brownish layers of sterile tissue 0.5–2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline to yellowish or brownish, densely interwoven, occasionally branched, (3.2–)3.3–4.2(−5.0) μm diam (n = 20/1), lumen mostly capillary to indistinct, side branches 2–3 μm diam, generative hyphae rare, hyaline, slightly or distinctly thick-walled, 3–4 μm diam. Trama dimitic; skeletal hyphae rather sparse, hyaline, loosely interwoven, occasionally branched, some twisted or irregularly inflated, (2.2–)2.6–3.3(−3.4) μm diam (n = 20/1), lumen varying from wide to capillary or indistinct, generative hyphae abundant, thin- to slightly thick-walled, some sclerified, 2–4 μm diam. Subhymenium not differentiated. Cystidioles abundant, tapering, 9–14 × 3–5 μm. Basidia clavate, (7.1–)7.3–9.7(−9.8) × (3.9–)4.0–4.2(−4.8) μm (n = 12/1), in senescent hymenium slightly thick-walled and partly glued together. Basidiospores with a distinct wall, broadly cylindrical to narrowly ellipsoid, (2.8–)2.9–3.3(−3.7) × 1.9–2.1(−2.2) μm (n = 20/1), L = 3.14, W = 2.01, Q = 1.57.
Specimen examined: Costa Rica, Puntarenas, Golfito, Playa Cacao, the same host as the holotype, 16 Apr. 2017, Vlasák 1704/10 (JV).
Notes: Superficially, F. caseosa is most similar to the Southeast Asian F. pseudopetchii, which has ungulate basidiocarps with a well-developed crust, clearly stratified tubes and small pores 7–10 per mm. Its basidiospore dimensions are very close to those of F. caseosa (see below). In contrast to F. caseosa, the crust in the well-developed basidiocarps of F. pseudopetchii is shining, dark reddish-brown to almost black, reminiscent of some Ganoderma spp., while context and tubes are uniformly cream- to wood-coloured. DNA sequences of F. caseosa and F. pseudopetchii show that these species are not closely related (Suppl. Fig. S2).
Fomitopsis castanea Imazeki, Bull. Gov. Forest Exp. Stn. Tokyo 42: 1. 1949. Fig 17.
Typus: Japan, Kitasaku, Nagano, 9 Oct. 1947, Imazeki (holotype TFM F-346).
Synonyms:
Phellinus quercinus Bondartsev & Ljub., Nov. Syst. Pl. non Vasc. 2: 141. 1965.
Typus: Russia, Primorie, Shkotovo Dist., Peishula, Quercus mongolica, 14 May 1950, Ljubarksy (holotype LE 22516!).
Melanoporia castanea (Imazeki) T. Hatt. & Ryvarden, Mycotaxon 50: 29. 1994.
Description: Núñez & Ryvarden (2001, as M. castanea).
Specimen examined: Russia, Khabarovsk Reg., Khabarovsk Dist., Ilga, Quercus mongolica, 11 Aug. 2012, Spirin 5144* (H).
Fomitopsis cellularis Vlasák & Spirin, nom. nov. MycoBank MB 844888. Fig 16.
Basionym: Trametes cystidiata I. Lindblad & Ryvarden, Mycotaxon 71: 353. 1999.
Typus: Costa Rica, Guanacaste, Pitilla, dead deciduous tree, 23 Mar. 1997, Garcia 99* (isotype O F-450190!).
Etymology: Cellularis (Lat., adj.) – in reference to cellular hymenophore.
Description: Lindblad & Ryvarden (1999, as T. cystidiata).
Specimens examined: Costa Rica, Guanacaste, Guanacaste Nat. Park., Pitilla, dead deciduous wood, 1 Feb. 1997, Lindblad 2655 (O 19098, H); ibid., 3 Feb. 1997, Lindblad 2689 (O 19016, H); Puntarenas, Piedras Blancas Nat. Park, Rio Bonito, 9 May 2000, Fletes 1522 (O 14134, H); ibid., 20 Apr. 2015 Vlasák Jr. 1504/36J* (JV, TUF).
Notes: Originally described from Costa Rica as a member of Trametes, this species proved here to be a member of Fomitopsis (Fig. 1). However, it cannot be combined in Fomitopsis because the epithet Fomitopsis cystidiata is preoccupied by another species described from China (Han et al. 2014) (see under F. sulcata). Therefore, a new name is introduced for it. Lindblad & Ryvarden (1999) depicted cystidia in the type specimen of F. cellularis as finely encrusted; however, they were smooth in all other specimens studied by us.
Fomitopsis circularis (B.K. Cui & Hai J. Li) Spirin, comb. nov. MycoBank MB 844889 Basionym: Daedalea circularis B.K. Cui & Hai J. Li, Mycoscience 54: 63. 2013.
Typus: China, Yunnan, Mengla, Wangtianshu, hardwood, 2 Nov. 2009, Cui 8488* (holotype BJFC 6977).
Description and phylogenetic data: Li & Cui (2013, as D. circularis); see also Fig. 5.
Fomitopsis concentrica (G. Cunn.) M.D. Barrett, comb. nov. MycoBank MB 844890.
Basionym: Gloeophyllum concentricum G. Cunn., Bull. N.Z. Dept. Sci. Industr. Res. 164: 262. 1965.
Typus: Australia, Queensland, Cape York Peninsula, Lower Archer River, Mar. 1933, L. & G. Thomson (holotype PDD 12262).
Synonym: Ischnoderma concentricum (G. Cunn.) Corner, Beih. Nova Hedwigia 96: 80. 1989.
Description: Cunningham (1965, as G. concentricum).
Specimen examined: Australia, Western Australia, Theda Station, Kimberley region, Eucalyptus tetrodonta (recently fallen branch), 18 May 2011, Barrett F197/11* (PERTH).
Notes: Gloeophyllum concentricum is morphologically apomorphic, combining a compound-imbricate basidiocarp, composed of few to many thin overlapping pilei, and concentrically arranged lamellae. Its placement in Gloeophyllum was immediately criticized as “open to serious question” by Reid (1967), due to macroscopic and spore differences. It was later unsatisfactorily moved to Ischnoderma by Corner (1989). While reviewing Cunningham’s types, Buchanan & Ryvarden (1988) accepted the species in Gloeophyllum despite its lack of cystidia typical of that genus, justified by the existence of ‘some’ Gloeophyllum that lack cystidia (although examples were not mentioned). Examination of material in situ by one of us (MB) demonstrated that the species produces a brown hear-rot, most commonly on Eucalyptus but occasionally on other angiosperms. Gloeophyllum concentricum lacks cystidia typical of Gloeophyllum. DNA data confirm that it belongs within the concept of Fomitopsis accepted here, strongly supported as sister to a clade containing F. tunicata and F. glabricystidia (Figs 1, 3).
Fomitopsis condensa Ryvarden & Vlasák, nom. nov. MycoBank MB 844891.
Basionym: Melanoporia condensa Ryvarden & Vlasák, Syn. Fung. 35: 28. 2016. (invalid under Code Art. 8.1).
Typus: Costa Rica, Monteverde, Santa Elena, hardwood, 30 Dec. 2013, Vlasák Jr. 1312/E-15-J* (holotype PRM 933857!).
Description: Vlasák et al. (2016, as M. condensa).
Notes: Melanoporia condensa was described based on material from Costa Rica (Vlasák et al. 2016) although invalidly because the holotype was indicated as stored in three different herbaria. Here we formally validate this species in the genus Fomitopsis and designate its holotype. It is closely related to other species formerly included in Melanoporia (Fig. 3).
Fomitopsis cupreorosea (Berk.) J. Carranza & Gilb., Mycotaxon 25: 476. 1986. Fig 19.
Basionym: Polyporus cupreoroseus Berk., Hooker’s J. Bot. 8: 233. 1856.
Typus: Brazil, Panurè, dead trunks, Feb. 1853, Spruce 184 (lectotype K, isolectotype PC!) (selected by Ryvarden 1984: 337).
Description: Basidiocarps perennial, sessile, often with a contracted base (fan-shaped), projecting up to 10 cm. Upper surface first pinkish-ochraceous, often with a brass tint, smooth, even or indistinctly zonate, later pinkish brownish, frequently zonate and radially wrinkled or furrowed, with a silky lustre. Pileal edge sharp to rather blunt, concolourous with hymenial surface, first sterile, up to 2 mm wide, then fertile. Pore surface deep pink to pinkish-ochraceous, flat or concave; pores roundish to angular or sinuous, 3–5 per mm, with thin or moderately thickened, entire or serrate dissepiments. Section: context corky, deep pink or pinkish brownish, normally less than 3 mm thick; tubes corky, one-layered, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline to yellowish or brownish, densely interwoven or in subparallel bundles, occasionally branched, (3.4–)3.7–5.3(−6.3) μm diam (n = 20/1), lumen normally wide, side branches 1.5–3 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 2.5–4 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to pinkish brown, densely interwoven, occasionally branched, some twisted, (2.8–)2.9–4.7(−5.1) μm diam (n = 40/2), lumen mostly narrow to capillary, generative hyphae rather rare, thinto slightly thick-walled, 2–3.5 μm diam. Subhymenium indistinct. Skeletocystidia present as swollen (up to 5.5 μm diam) apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles infrequent, tapering, 12–15 × 3–5 μm. Basidia clavate, (10.8–)11.3–17.2(−18.9) × (4.8–)5.0–6.0(−6.3) μm (n = 20/2), occasionally pleural, in senescent hymenium slightly thick-walled and partly glued together. Basidiospores with a distinct wall, cylindrical-subfusiform, (4.4–)4.6–6.6(−6.7) × (2.0–)2.1–3.0(−3.1) μm (n = 90/3), L = 5.03–5.75, W = 2.40–2.76, Q = 1.83–2.29.
Specimens examined: Belize, Cayo, Five Sisters, hardwood, 19 Nov. 2001, Ryvarden 44394 (O 17632); Stann Creek, Cockscomb Basin, 30 Oct. 2006, Kout 0610/K4* (JV, H). Brazil, Bahia, Santa Terezinha, Serra do jibóia, angiosperm tree (fallen trunks), 11 Jan. 2006, Oinonen 60111009 & 60111014 (H). French Guiana, Roura, Camp Cayman, 31 Aug. 2019, Vlasák 1908/81* (JV, H); Favard, 17 Oct. 2013, Runnel 654* (TUF130110).
Notes: Fomitopsis cupreorosea is a member of the F. feei complex. It is distributed in tropical forests of Central and South America. Fomitopsis cupreorosea is easily distinguishable versus other related species due to its large, irregular pores. ITS sequences of F. cupreorosea show considerable variation, but our material is too limited to conclude if several sibling species are hidden under this name.
Fomitopsis cupressicola Vlasák, J. Vlasák Jr. & Spirin, sp. nov. MycoBank MB 844892. Fig 19.
Typus: USA, New Jersey, Burlington Co., Batsto Village, 39.68764°-74.66108°, Chamaecyparis thyoides, 1 Jun. 2017, Vlasák Jr. 1706/9-J* (holotype H7200201).
Etymology: Cupressicola (Lat., noun) – in reference to Cupressaceae.
Description: Basidiocarps perennial, sessile, conchate, projecting up to 4 cm, often fusing together. Upper surface first pinkish greyish to pinkish brownish, smooth, later dark brown to almost black, frequently zonate, matt. Pileal edge sharp to rather blunt, concolourous with hymenial surface, sterile, up to 2 mm wide. Pore surface pinkish-grey to pinkish brownish, concave; pores roundish to angular, 6–8 per mm, with thin or thickened, entire or slightly uneven dissepiments. Section: crust (present in old basidiocarps) exceptionally tough, blackish brown, matt, up to 0.3 mm thick, context hard corky, brown, up to 2 mm thick; tubes corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 7 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brown, densely interwoven, mostly unbranched, (2.8–)2.9–3.7(−3.8) μm diam (n = 20/1), lumen narrow to indistinct, generative hyphae rare, hyaline, thick-walled, 2–2.5 μm diam. Trama dimitic; skeletal hyphae dominating, brown, densely interwoven, rarely branched, (2.0–)2.1–3.6(−3.8) μm diam (n = 60/3), lumen mostly narrow to indistinct, generative hyphae rather rare, thin- to slightly thick-walled, 2–3.5 μm diam. Subhymenium partly distinct, up to 10 μm thick. Skeletocystidia present as slightly swollen (3–4 μm diam) apices of tramal skeletal hyphae, embedded or slightly projecting above hymenium. Cystidioles rather common, tapering, 11–16 × 3–5 μm. Basidia clavate, (11.0–)11.1–15.4(−16.0) × (4.8–)4.9–6.1(−6.2) μm (n = 20/2), occasionally pleural, in senescent hymenium slightly thick-walled. Basidiospores with a distinct wall, cylindrical to narrowly ellipsoid, longest spores subfusiform or rarely lacrymoid, (3.6–)3.7–5.2(−5.4) × (1.8–)1.9–2.8(−2.9) μm (n = 120/4), L = 3.93–4.74, W = 2.02–2.34, Q = 1.69–2.36.
Specimens examined: Dominican Republic, La Vega, Cordillera Central, Pinus occidentalis, 18 Feb. 2001, Paino-Perdomo et al. 703 (O 14114, H). USA, Florida, Manatee Co., Braden River, at the base of an old unknown tree, 8 May 2016, Dollinger 778* (JV, H); New Jersey, Burlington Co., Batsto Village, Chamaecyparis thyoides, 1 Jun. 2017, Vlasák Jr. 1706/7-J* (JV, H).
Notes: Fomitopsis cupressicola is a member of the F. rosea complex. It inhabits wood of gymnosperms (predominantly Cupressaceae) in the eastern part of North America. Morphologically, F. cupressicola is most similar to F. cajanderi and F. substratosa. The first species is distributed in boreal zone of Eurasia and North America. It differs from F. cupressicola in having lighter-coloured, pinkish hymenial surface, as well as longer and narrower basidiospores, (4.1–)4.2–6.1(−6.2) × (1.7–)1.8–2.1(−2.2) μm (n = 90/3), L = 5.13–5.35, W = 1.94–1.96, Q = 2.62–2.77. No verified records of F. cajanderi from Cupressaceae are known to us. As accepted here, F. substratosa (= F. subfeei) is a species distributed in the northern part of Africa and Southeast Asia. It differs from F. cupressicola in having larger pores and narrower basidiospores (see below).
Fomitopsis cyclopis (Miettinen & Spirin) Miettinen & Spirin, comb. nov. MycoBank MB 844893.
Basionym: Antrodia cyclopis Miettinen & Spirin, Mycol. Progr. 15 (51): 5. 2016.
Typus: Indonesia, Papua, Kabupaten Jayapura, Sentani, Cyclops Mountains, angiosperm branch, 29 Aug. 2004, Miettinen 9166.1* (isotype H!).
Description and phylogenetic information: Spirin et al. (2016, as A. cyclopis).
Fomitopsis derelicta Vlasák & Spirin, sp. nov. MycoBank MB 844894. Fig 19.
Typus: USA, Texas, Brewster Co., Big Bend, 29.30°–103.356°, Quercus sp., 3 Apr. 2021, Vlasak Jr. 2104/2J* (holotype H7200200).
Etymology: Derelictus (Lat., adj.) – forsaken, desolate.
Description: Basidiocarps perennial, sessile, dimidiate, projecting up to 2.5 cm. Upper surface greyish-ochraceous to brownish, irregularly nodulose, azonate. Pileal edge blunt, concolourous with pileal surface, sterile, up to 5 mm wide. Pore surface pale ochraceous or greyish to brownish, flat or slightly convex; pores angular to sinuous or labyrinthine, (0.5–)1–1.5(−2) per mm, 8–9 per cm, with thick, entire or uneven dissepiments. Section: context fibrous-corky, ochraceous to brownish, up to 15 mm thick; tubes corky, one-layered, concolourous with hymenial surface, up to 10 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, interwoven, occasionally branched, (3.9–)4.0–5.8(−6.0) μm diam (n = 20/1), lumen usually wide, side branches 2–3.5 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae dominating, hyaline to brownish, interwoven, occasionally branched, (2.1–)2.3–4.1(−4.2) μm diam (n = 20/1), lumen mostly capillary to indistinct, side branches 1.5–2.5 μm diam, generative hyphae thin- to slightly thick-walled, 2–3.5 μm diam. Subhymenium sometimes distinct, up to 20 μm thick. Skeletocystidia present as swollen (up to 6 μm diam), acute or blunt apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles occasionally present, tapering, sometimes sinuous, 16–28 × 3–4 μm. Basidia long clavate, occasionally pleural, (23–)25–32(−35) × (5.8–)6.0–7.2(−7.3) μm (n = 20/1), in senescent hymenium slightly thick-walled. Basidiospores with a distinct wall, narrowly ellipsoid to ellipsoid or occasionally sigmoid, (4.6–)4.8–6.8(−7.0) × (2.7–)2.8–3.9(−4.0) μm (n = 32/1), L = 5.60, W = 3.33, Q = 1.69.
Notes: Fomitopsis derelicta is a close relative of F. quercina distributed in subtropical – tropical areas of North America. The species is so far known from two collections – one from the south-western part of Texas, USA and another one from Belize, both from Quercus spp. The latter specimen (CFMR BZ-2779) was reported as Daedalea cf. quercina by Lindner et al. (2011). Macroscopically, F. derelicta differs from F. quercina in having more regular and smaller pores (0.3–1 per mm, 5–8 per cm in F. quercina). In the microscope, the basidiospore shape and size are the main distinguishing characters between the two species. Basidiospores of F. quercina are broadly cylindrical or subfusiform and on average narrower than in F. derelicta, (4.1–)4.7–6.8(−7.2) × (2.2–)2.3–3.1(−3.2) μm (n = 60/2), L = 5.52–5.55, W = 2.78–2.80, Q = 1.99–2.00.
According to available sequences and our own data, F. quercina is widely distributed in temperate – warm temperate forests of North America, and the southernmost record of this species known to us came from Georgia (Fig. 5). Historical specimens of F. quercina from the south-western part of USA should be critically checked to re-define its distribution area on the continent. Another North-American species from this complex is F. neotropica (= Daedalea neotropica, see below). It can be easily differentiated from F. derelicta and F. quercina in having much smaller pores, 3–5 per mm, and violet stains on pileal and pore surfaces. The distribution area of F. neotropica seems to be limited to tropical forests of Central America (Lindner et al. 2011, Vlasák et al. 2016).
Fomitopsis dickinsii (Berk. ex Cooke) Spirin, comb. nov. MycoBank MB 844895. Fig 17.
Basionym: Trametes dickinsii Berk. ex Cooke, Grevillea 19: 100. 1891.
Typus: Japan, [no locality indicated], on trunks, herb. Berkeley (lectotype K) (selected by Ryvarden 1988b: 49).
Description: Hattori & Ryvarden (1994, as Daedalea dickinsii).
Specimens examined: China, Jilin, Antu Co., Changbaishan Nat. Res., Quercus mongolica (fallen log), 20 Sep. 1998, Niemelä 6435 (H). Russia, Khabarovsk Reg., Khabarovsk Dist., Ilga, Q. mongolica (fallen log), 10 Aug. 2012, Spirin 5094 (H).
Notes: Lindner et al. (2011) provided sequences for this species. Our results confirm its affinity with other species until recently addressed to Daedalea (Fig. 5).
Fomitopsis dochmia (Berk. & Broome) Ryvarden, Norw. J. Bot. 19: 231. 1972. Fig 19.
Basionym: Polyporus dochmius Berk. & Broome, J. Linn. Soc., Bot. 14: 50. 1875.
Typus: Sri Lanka, Central Province, Dec. 1868, Berkeley’s herbarium #970 (lectotype K, isolectotype BPI!) (selected by Ryvarden 1984: 338).
Description: Basidiocarps perennial, sessile, conchate, projecting up to 10 cm. Upper surface first reddish-brown, then darkening to almost black, matt, with distinct annual zones, densely longitudinally cracking, growing margin ochraceous brown. Pileal edge sharp or rather blunt, concolourous with hymenial surface, sterile, up to 1 mm wide. Pore surface pinkish- to reddish-brown, concave; pores roundish, 8–10 per mm, with thick, entire dissepiments. Section: crust dark brown to black, tough, up to 0.5 mm thick, context corky, ochraceous or brownish, up to 5 mm thick; tubes corky, stratified, concolourous with hymenial surface, up to 15 mm thick, sterile tissue often present between annual layers. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, yellowish- to reddish brownish, densely interwoven, occasionally branched, (3.2–)3.3–5.8(−6.0) μm diam (n = 40/2), lumen mostly narrow to indistinct, side branches 2–3 μm diam, generative hyphae rare, hyaline, thin- or slightly thick-walled, 2–3.5 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to reddish-brown, densely interwoven, occasionally branched, (2.8–)2.9–5.0(−5.2) μm diam (n = 40/2), lumen mostly capillary to indistinct, side branches 2–2.5 μm diam, generative hyphae rare, hyaline, thin- or slightly thick-walled, 1.5–3 μm diam. Subhymenium indistinct. Skeletocystidia present as more or less blunt apices of tramal skeletal hyphae, 3–4 μm diam, slightly projecting above hymenium. Cystidioles tapering, 12–20 × 3–4 μm, sometimes with a long hyphoid neck. Hyphidia occasionally present, simple or bifurcate, 1.5–2 μm diam. Basidia clavate, occasionally pleural, rare, 10.4–17.2 × 3.3–5.1 μm (n = 8/1). Basidiospores thin-walled or with a distinct wall, cylindrical to broadly cylindrical, occasionally slightly curved, (3.0–)3.1–4.1(−4.2) × (1.8–)1.9–2.3(−2.4) μm (n = 30/1), L = 3.58, W = 2.10, Q = 1.71.
Specimen examined: India, Kerala, Idukki, Munnar, Kannan Devan Hills, fallen log, 7 Feb. 2019, Dunayev* (TUF).
Notes: To date, F. dochmia was treated as a widely distributed tropical species with a dark, characteristically cracking pileal surface and rather light-coloured, stratified tubes (Carranza-Morse & Gilbertson 1986, Ryvarden 2015). Our data reveal the presence of at least six species covered by the present concept of F. dochmia in two phylogenetically distant lineages in Fomitopsis. Here we reinstate F. dochmia based on the type from Sri Lanka and a newly collected and sequenced specimen from the southern part of India. As accepted here, F. dochmia is most similar to F. philippinensis. The latter species seemingly has a more eastern distribution than F. dochmia, and it is recognizable due to larger pores and strongly fading upper surface of older basidiocarps. Fomitopsis ferrea, originally described from Sri Lanka and up to now considered a synonym of F. dochmia, has somewhat larger pores and lighter-coloured pileal tissues than those of F. dochmia. Another look-alike from Southeast Asia is F. elevata (see remarks below). Phylogenetically, these four species form a natural lineage in the genus (Fig. 5).
Two more species, F. lapidosa and F. lignea, are superficially very similar but not closely related to the F. dochmia clade. The first species is strongly reminiscent of F. ferrea and F. elevata but it has more lightweight basidiocarps and smaller pores than those two species. Fomitopsis lapidosa is so far known only from New Guinea. In turn, F. lignea possesses somewhat larger pores than F. dochmia, and it is distributed in the Caribbean. Anatomical differences between all these species are minimal if any.
Fomitopsis dollingeri Vlasák & Spirin, sp. nov. MycoBank MB 844896. Figs 16, 20.
Fig 20.
Basidiospores of Fomitopsis spp. F. dollingeri (holotype); F. elevata (Miettinen 20529); F. feei (Oinonen 60119006); F. ferrea (Dunaev 26.I.2019); F. fissa (holotype); F. foedata (Miettinen 11466). Scale bar = 5 μm.
Typus: USA, Florida, Collier Co., Copeland, Fahkahatchee Strand, 26.0516°–81.3885°, dry hardwood branch, 15 Jun. 2014, Dollinger 56* (holotype H7200204).
Etymology: After Neil Dollinger, a collector of this species.
Description: Basidiocarps annual, effused, up to 4 cm in widest dimension. Margin adnate, compact, cream-coloured, later ochraceous-brownish, up to 1 mm wide. Hymenial surface even, pale cream-coloured; pores angular, 4–6 per mm, dissepiments uneven. Section: subiculum compact, white, hardly visible, 0.05–0.07 mm thick; tubes soft leathery, white or pale cream-coloured, 0.5–1.5 mm thick. Hyphal structure dimitic; hyphae clamped. Subiculum dimitic; skeletal hyphae hyaline, sparsely arranged, interwoven, occasionally branched, (1.8–)2.1–3.2(−3.3) μm diam (n = 20/1), lumen wide to rather narrow, generative hyphae dominating, hyaline, thin-walled, 2–3.5 μm diam. Trama dimitic; skeletal hyphae hyaline, sparse, interwoven, occasionally branched, (1.7–)1.8–3.4(−3.7) μm diam (n = 40/2), lumen mostly wide, generative hyphae dominating, hyaline, thin-walled, 2–3 μm diam; dissepiment edges completely monomitic. Cystidioles rather abundant in senescent hymenium, bullet-shaped to fusiform, 12.5–20 × 3–5 μm. Rhomboid or stellate crystals occasionally present in tube trama, up to 10 μm in longest dimension. Basidia clavate, (13.2–)13.3–17.8(−20.8) × (5.2–)5.3–6.2(−6.3) μm (n = 20/2). Basidiospores thin-walled, broadly cylindrical to narrowly ellipsoid, longest spores subfusiform, (5.1–)5.2–7.2(−7.3) × (2.1–)2.2–3.1(−3.2) μm (n = 60/2), L =5.86–6.36, W = 2.45–2.81, Q = 2.27–2.40.
Specimen examined: USA, Florida, Manatee Co., Parrish, Rye Preserve, hardwood, 27 Mar. 2016, Dollinger 700* (H, JV).
Notes: The species was found twice in Florida, USA, inhabiting dry and still corticated branches of angiosperms. It keys out as Antrodia oleracea if existing manuals of poroid fungi (Lowe 1966, Gilbertson & Ryvarden 1986) are used for identification. However, A. oleracea (= Fomitopsis oleracea below) is a completely monomitic species with easily crumbling basidiocarps, and it inhabits decorticated wood of Quercus spp. (see description below).
Fomitopsis elevata (Corner) Spirin & Miettinen, comb. nov. MycoBank MB 844897. Figs 20, 21.
Fig 21.
Basidiocarps of Fomitopsis spp. A. F. elevata (Miettinen 8692). B. F. foedata (Miettinen 11466). C. F. gilvidula (Miettinen 20535). D. F. lapidosa (holotype). E. F. leioderma (Vlasák 1908/82). F. F. luzonensis (Miettinen 14311). G. F. maculosa (holotype). H. F. marianii (Spirin 10575).
Basionym: Trametes elevata Corner, Beih. Nova Hedwigia 97: 98. 1989.
Typus: Malaysia, Pahang, Sungei Cheka, ‘on the trunk of a small tree’, 14 Jun. 1931, Corner (holotype E00604887).
Description: Basidiocarps perennial, sessile, conchate, sometimes with a contracted base (fan-shaped), solitary or partly fusing together, projecting up to 4 cm. Upper surface blackish brown to bluish-black, with several indistinct annual zones, sometimes longitudinally cracking, growing margin much paler, wood-coloured to ochraceous-brownish, more or less evenly coloured. Pileal edge sharp or rather blunt, concolourous with hymenial surface, first sterile, up to 1 mm wide, then fertile. Pore surface cream-coloured or beige to pale ochraceous, sometimes with scattered blackish-blue stains, even or slightly concave; pores roundish, 6–7 per mm, with very thick, entire dissepiments. Section: crust black, tough, up to 0.5 mm thick, context corky, wood-coloured to pale ochraceous, up to 10 mm thick; tubes corky, indistinctly stratified, concolourous with hymenial surface, up to 5 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, yellowish, densely interwoven, occasionally branched, (3.2–)3.3–5.1(−5.3) μm diam (n = 20/1), lumen capillary to indistinct, side branches 2–3 μm diam, generative hyphae rare, hyaline, thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae dominating, yellowish, densely interwoven, occasionally branched, (3.0–)3.1–4.6(−4.8) μm diam (n = 40/2), lumen mostly capillary to indistinct, side branches 2–3 μm diam, generative hyphae rare, slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. No differentiated skeletocystidia seen. Cystidioles abundant, tapering, 10.5–16 × 2.5–3.5 μm. Basidia clavate, (9.3–)10.0–15.1(−15.2) × (4.0–)4.2–4.9(−5.0) μm (n = 12/2), in senescent hymenium partly glued together. Basidiospores thin-walled, cylindrical, often slightly curved, (3.0–)3.1–4.7(−5.1) × (1.7–)1.8–2.2(−2.3) μm (n = 60/2), L = 3.60–4.18, W = 1.96–2.05, Q = 1.76–2.13.
Specimens examined: Indonesia, Riau, Indragiri Hulu, Bukit Aluran Bab, selectively logged primary rainforest, dicot (fallen log), 28 Jun. 2004, Miettinen 8692* (BO, H); Kampar, Balung, logged-over natural forest, dicot, 24 Dec. 2006, Miettinen 11306.4 (BO, H), Koto Lamo, dicot, 23 Oct. 2016, Miettinen 20529* (BO, H).
Notes: The species is closely related and morphologically exceptionally similar to F. ferrea. It produces on average less robust basidiocarps than the latter species and possesses indistinctly stratified tubes (vs. clearly stratose in F. ferrea). To date, F. elevata has been found in Malaysia and Sumatra, Indonesia. See further notes under F. ferrea and F. philippinensis.
Fomitopsis eucalypti (Kalchbr.) Spirin, comb. nov. MycoBank MB 844898.
Basionym: Polyporus eucalypti Kalchbr. in von Thümen, Grevillea 4 (30): 73. 1875.
Typus: Australia, Queensland, Rockhampton, Eucalyptus sp., Thozet (comm. Müller, ex herb. M.C. Cooke) (holotype K!).
Description: Basidiocarps perennial, sessile or effused-reflexed, often gregarious, projecting up to 4 cm, resupinate part up to 5 cm diam. Upper surface pinkish ochraceous to brown, first felty, indistinctly zonate and radially striate, then almost smooth and fading to pale ochraceous. Pileal edge sharp, concolourous with hymenial surface, usually sterile, up to 1 mm wide. Pore surface pinkish to pinkish-greyish, flat or concave, often fading to almost white; pores roundish to angular, 5–7 per mm, with thin or moderately thickened, entire dissepiments. Section: context corky, pinkish brownish, up to 3 mm thick; tubes corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae yellowish, densely interwoven, occasionally branched, (3.8–)4.0–5.7(−6.0) μm diam (n = 20/1), lumen narrow to indistinct, side branches 2–3.5 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 2.5–4 μm diam. Trama dimitic; skeletal hyphae dominating, yellowish or brownish to almost hyaline, densely interwoven, occasionally branched, (3.0–)3.2–4.7(−5.0) μm diam (n = 80/4), lumen narrow to indistinct, generative hyphae rare, thinto slightly thick-walled, 2–3.5 μm diam. Subhymenium indistinct. Cystidioles abundant to rather rare, tapering, 10–14 × 3–4.5 μm. Hyphidia occasionally present, usually simple, 1.8–2.2 μm diam. Basidia clavate, (11.4–)11.8–15.6(−15.8) × (4.9–)5.0–6.2(−6.8) μm (n = 40/4), occasionally pleural, in senescent hymenium partly glued together. Basidiospores with a distinct wall, narrowly ellipsoid to cylindrical-subfusiform, rarely slightly curved, (3.9–)4.0–6.8(−7.2) × (2.0–)2.1–3.0(−3.1) μm (n = 90/3), L = 4.45–5.14, W = 2.50–2.59, Q = 1.73–2.02.
Specimens examined: Australia, Queensland, Cairns, Kuranda, angiosperm, 24 Aug. 2006, Schigel 5227 & 5234* (H); Lake Barrine, angiosperm, 19 Aug. 2006, Schigel 5196 & 5204 (H); Lamington Nat. Park, rotting logs, 11 Jul. 2001, Young & Fechner (O 18278, H); ibid., 7 Nov. 2001, Young & Fechner (O 17406, H).
Notes: Polyporus eucalypti was described from Queensland, Australia (Thümen 1875). It was placed to the synonyms of F. feei by Lloyd (1915). Here F. eucalypti is reinstated as a member of the F. feei complex distributed in the northern part of Australia. It differs from F. foedata occurring in the same geographic area in having smaller and thinner, often effused-reflexed basidiocarps with a paler hymenophore. Pores of F. eucalypti are on average larger than in F. foedata. Microscopically, these species are hardly distinguishable, except skeletal hyphae which are less intensively coloured in F. eucalypti than in F. foedata.
Fomitopsis eucalypticola B.K. Cui & Shun Liu, Mycol. Progr. 18: 1323. 2019.
Typus: Australia, Victoria, Dandenong Ranges, Eucalyptus sp., 12 May 2018, Cui 16598* (holotype BJFC).
Description and phylogenetic data: Liu et al. (2019); see also Fig. 6.
Fomitopsis feei (Fr.) Kreisel, Monografias Ciencias Universidad de Habana 16: 83. 1971. Fig 20.
Basionym: Polyporus feei Fr., Linnaea 5: 518. 1830.
Typus: Brazil, [no locality indicated], 1826, comm. Fee (lectotype PC 0705341) (selected by Soares et al. 2017: 79).
Synonyms:
Polyporus sordidus Lév., Ann. Sci. Nat., Bot. 3 (2): 192. 1844.
Typus: Guadeloupe, [no locality and collection date indicated], L’Herminier (holotype PC!).
Rhodofomitopsis roseomagna Nogueira-Melo, A.M.S. Soares & Gibertoni, Phytotaxa 331 (1): 78. 2017.
Typus: Brazil, Pernambuco, Jaqueira, Mata Caranha, hardwood, Sep. 2012, Nogueira-Melo 379* (holotype URM 86162).
Description: Basidiocarps perennial, sessile, often with a contracted base (fan-shaped), occasionally effused-reflexed, projecting up to 5 cm. Upper surface pinkish ochraceous to purplish brownish, later fading to pale ochraceous or light greyish, indistinctly zonate and radially furrowed. Pileal edge sharp, concolourous with hymenial surface, usually sterile, up to 2 mm wide. Pore surface deep pink to pinkish brownish, flat or concave; pores roundish to angular, 6–7(−8) per mm, with thin or moderately thickened, entire dissepiments. Section: context corky, deep pink or pinkish brownish, normally less than 2 mm thick; tubes corky, one-layered or indistinctly stratified, concolourous with hymenial surface, usually up to 2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, densely interwoven, occasionally branched, (3.1–)3.2–6.1(−6.3) μm diam (n = 20/1), lumen normally wide, side branches 2–3 μm diam, generative hyphae rather abundant, hyaline, slightly thick-walled, 4–6 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to reddish-brown, densely interwoven, occasionally branched, (2.8–)2.9–4.1(−4.2) μm diam (n = 40/2), lumen varying from rather wide to capillary, generative hyphae rare, thin- to slightly thick-walled, 2–2.5 μm diam. Subhymenium indistinct. Cystidioles not seen. Basidia short-clavate, (9.4–)10.1–12.8(−13.1) × (4.3–)4.5–6.1(−6.2) μm (n = 20/2), occasionally pleural, in senescent hymenium partly glued together. Basidiospores thin-walled, cylindrical-subfusiform, rarely narrowly ellipsoid, (3.9–)4.0–6.2(−6.7) × (2.0–)2.1–2.9(−3.0) μm (n = 120/4), L = 4.58–5.00, W = 2.33–2.43, Q = 1.98–2.14.
Specimens examined: Brazil, Bahia, Itaberaba, Serra do Orobó, hardwood, 19 Jan. 2006, Oinonen 60119006* (H); Paraná, Iguaçu, decayed manufactured wood, 3 Jan. 1993, de Meijer 2404 (O 13835). Venezuela, Bolívar, Las Nieves, hardwood, 12 Jun. 1995, Ryvarden 37603* (O 10804); ibid., Ryvarden 37901 (O 10802).
Notes: Fomitopsis feei was treated as a species widely distributed in tropical areas of the world. At least five other names were listed among its synonyms (Ryvarden 1991). Of them, Polyporus sagraeanus, P. eucalypti, P. foedatus and Trametes marchionica are reinstated here as separate species, while the identity of P. rubidus remains uncertain (see remarks to F. carnea). Therefore, the name F. feei is retained for the species distributed in the tropical zone of South America, from where it was originally described. Another American representative of the F. feei complex, F. sagraeana, is distributed in the northern and western parts of the Caribbean. It has on average smaller pores and larger basidiospores than F. feei sensu stricto. The distribution areas of the two species seem not to overlap.
Fomitopsis ferrea (Cooke) Spirin & Viner, comb. nov. MycoBank MB 844910. Fig 20.
Basionym: Fomes ferreus Cooke, Grevillea 14 (69): 21. 1885.
Typus: Sri Lanka, Sabaragamuwa, Ratnapura, Adam’s Peak, dead wood, Sep. 1844, Gardner 104 & 106 (syntypes K, isosyntype PC!).
Description: Basidiocarps perennial, sessile, conchate, projecting up to 11 cm. Upper surface first greyish brown, indistinctly striate, then darkening to almost black, in older basidiocarps partly eroded and somewhat notched, with distinct annual zones, densely longitudinally cracking, growing margin wood-coloured to greyish. Pileal edge sharp or rather blunt, concolourous with hymenial surface, sterile, up to 1 mm wide. Pore surface wood-coloured or pale ochraceous, occasionally with brownish or blackish stains, concave; pores roundish, 6–8(−10) per mm, with thick, entire dissepiments. Section: crust black, tough, up to 0.5 mm thick, context woody-hard, ochraceous or brownish, up to 7 mm thick; tubes woody-hard, stratified, older layers brownish to brown, sometimes with blackish-blue flecks, newer layers concolourous with pore surface, up to 25 mm thick, darker-coloured sterile tissue often present between annual layers. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, brownish to brown, densely interwoven, occasionally branched, (2.8–)3.0–5.8(−6.3) μm diam (n = 40/2), lumen mostly narrow to capillary, side branches 1.5–2.5 μm diam, generative hyphae rare, hyaline, thin- or slightly thick-walled, 2–4 μm diam. Trama dimitic; skeletal hyphae dominating, yellowish or brownish, densely interwoven, occasionally branched, (2.3–)2.7–5.2(−5.8) μm diam (n = 40/2), lumen mostly capillary to indistinct, side branches 1.5–3 μm diam, generative hyphae rare, thin- or slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. No differentiated skeletocystidia seen. Cystidioles tapering, 8–12.5 × 3–3.5 μm, sometimes with a short side outgrowth. Hyphidia occasionally present, simple or bifurcate, 1–1.5 μm diam. Basidia rare, clavate, 9.2–10.5 × 3.2–4.0 μm (n = 3/1). Basidiospores thin-walled or with a distinct wall, cylindrical to broadly cylindrical or narrowly ellipsoid, (2.8–)2.9–4.1(−4.2) × (1.8–)1.9–2.3 μm (n = 22/1), L = 3.31, W = 2.05, Q = 1.61.
Specimen examined: India, Kerala, Idukki, Munnar, Anakkulam, fallen log, 26 Jan. 2019, Dunayev* (TUF).
Notes: Fomitopsis ferrea is a close relative of F. dochmia distributed in the same geographic region (Sri Lanka and the southern part of India). It can be separated from F. dochmia due to its somewhat larger pores, as well as lighter-coloured context and tubes. See further remarks under F. dochmia.
Fomitopsis fissa Vlasák & Spirin, sp. nov. MycoBank MB 844911. Figs 16, 20.
Typus: USA, California, Santa Cruz, Henry Cowell Redwoods St. Park, 37.05377°–122.09830°, dry hardwood branch, 8 Jul. 2004, Vlasák Jr. 0407/13-J* (holotype H7200197).
Etymology: Fissus (Lat., adj.) – cracked.
Description: Basidiocarps annual, effused, up to 5 cm in widest dimension. Margin adnate, compact, white, up to 2 mm wide. Hymenial surface even, often cracking, white to pale cream-coloured; pores angular, 4–5 per mm, dissepiments uneven. Section: subiculum compact, white, hardly visible, 0.05–0.1 mm thick; tubes soft leathery, white or pale cream-coloured, 0.1–0.5 mm thick. Hyphal structure monomitic; hyphae clamped. Subicular hyphae hyaline, interwoven to subparallel, very thick-walled, (1.8–)2.2–4.2(−4.3) μm (n = 20/1). Tramal hyphae hyaline, thin- to distinctly thick-walled, partly glued together, (2.2–)2.3–3.5(−3.8) μm (n = 20/1). Subhymenium not differentiated. Cystidioles absent. Basidia clavate, (14.2–)17.3–24.3(−24.7) × (6.2–)6.4–7.2(−8.7) μm (n = 20/1). Basidiospores with a distinct wall, cylindrical, occasionally slightly curved, longest spores subfusiform, (6.3–)6.4–8.0(−8.7) × (2.6–)2.7–3.1(−3.2) μm (n = 30/1), L = 7.24, W = 2.84, Q = 2.56.
Notes: Fomitopsis fissa is phylogenetically closely related to the members of the F. ramentacea complex. Macroscopically, it is strikingly different from them, possessing soft, irregularly cracking basidiocarps with non-gelatinized hymenophore and rather small pores. However, such anatomical traits as monomitic structure with variably thick-walled hyphae, rather long basidia and long cylindrical basidiospores, as well as the lack of cystidioles point to F. ramentacea and its relatives. Fomitopsis fissa is so far recorded only in the type locality in California, USA.
Fomitopsis flabellata A.M.S. Soares & Gibertoni, Fungal Diversity 83: 208. 2017.
Typus: Brazil, Amapá, Porto Grande, hardwood, Oct. 2014, Soares 1794* (holotype URM 89405).
Description and phylogenetic data: Tibpromma et al. (2017).
Fomitopsis flavimontis (Vlasák & Spirin) Vlasák & Spirin, comb. nov. MycoBank MB 844912.
Basionym: Antrodia flavimontis Vlasák & Spirin, Mycologia 109: 223. 2017.
Typus: USA, Wyoming, Park Co., Yellowstone Nat. Park, Pinus contorta, Jul. 2013, J. Vlasák Jr. 1307/17* (holotype H!).
Description and phylogenetic data: Spirin et al. (2017, as A. flavimontis).
Fomitopsis foedata (Berk.) Spirin & Miettinen, comb. nov. MycoBank MB 844913. Figs 20, 21.
Basionym: Polyporus foedatus Berk., J. Linn. Soc., Bot. 16 (1): 41. 1877.
Typus: Australia, Queensland, Cape York, Somerset, 1–8 Sep. 1874, ‘HMS Challendger Expedition’ (lectotype K(M) 264881!) (selected by Cunningham 1965: 171).
Synonym: Rhodofomitopsis pseudofeei B.K. Cui & Shun Liu, Fungal Diversity 104: 138. 2020.
Typus: Australia, Queensland, Cairns, Mt. Whitfield Conservation Park, angiosperm, 7 May 2018, Cui 16794* (holotype BJFC).
Description: Basidiocarps perennial, sessile, often with a contracted base, more rarely effused-reflexed, projecting up to 7 cm, occasionally fusing together. Upper surface first pinkish brown to light brown, felty, then dark brown almost black, smooth and indistinctly zonate. Pileal edge sharp to rather blunt, concolourous with hymenial surface, sterile, up to 2 mm wide. Pore surface pink to pinkish grey, usually concave; pores roundish to angular, 6–8 per mm, with thin or moderately thickened, entire or serrate dissepiments. Section: context leathery, pinkish brownish, up to 10 mm thick; tubes soft-corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae yellowish to brownish, rather loosely interwoven, occasionally branched, (3.0–)3.3–5.8(−6.4) μm diam (n = 40/2), lumen varying from wide to narrow, side branches 2–3 μm diam, generative hyphae rare, hyaline, thin- to slightly thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae dominating, yellowish to reddish-brown, rather densely interwoven, occasionally branched, (2.8–)2.9–4.0(−4.1) μm diam (n = 60/3), lumen capillary to indistinct, generative hyphae rare, thin-walled, 2–3.5 μm diam. Subhymenium indistinct. Cystidioles tapering, 8.5–14.5 × 3.5–5.0 μm. Hyphidia occasionally present, as a rule simple, 2–2.5 μm diam. Basidia clavate, (10.3–)10.8–15.4(−17.8) × (5.0–)5.1–6.2(−6.3) μm (n = 30/2). Basidiospores with a distinct wall, narrowly ellipsoid to cylindrical-subfusiform, (3.8–)4.0–5.9(−6.2) × (2.0–)2.1–3.0(−3.1) μm (n = 50/2), L = 4.85–5.24, W = 2.50–2.73, Q = 1.93–1.96.
Specimens examined: Australia, New South Wales: Buladelah, Duck Creek Road, rotting log, 22 Apr. 1990, Streimann 43973 (H); Kurrajong, dead wood, 1 Jun. 1985, Coveny 108 (O 17744, H); Northern Territory, Darwin, Bicentennial Park, angiosperm, 6 Jun. 1999, Uotila 42928* (H); Queensland, Cairns, Kuranda, angiosperm, 24 Aug. 2006, Schigel 5219 (H). Indonesia, Papua, Merauke, Wasur NP, dry natural forest, Acacia (?), 21 Jan. 2007, Miettinen 11466* (MAN, H).
Notes: The thin, flexible basidiocarps of F. foedata are reminiscent more of F. cajanderi than of other species of the F. feei complex where it belongs to (Fig. 11). The species is distributed in New Guinea and the northern part of Australia. Its differences from other similar-looking species from Australia, F. eucalypti and F. lilacinogilva, are discussed under those species.
Fomitopsis fragilis B.K. Cui & M.L. Han, Mycol. Progr. 13: 909. 2014.
Typus: China, Hainan, Ledong, Jianfengling, hardwood, 7 Nov. 2012, Cui 10919* (holotype BJFC).
Description and phylogenetic information: Han et al. (2014).
Fomitopsis gilvidula (Bres.) Spirin & Miettinen, comb. nov. MycoBank MB 844914. Figs 21, 22.
Fig 22.
Basidiospores of Fomitopsis spp. F. gilvidula (Miettinen 12905); F. incana (LE313649); F. lapidosa (holotype); F. leioderma (Vlasák 1908/82); F. lignea (lectotype of Fomes subferreus); F. lignicolor (holotype); F. luzonensis (Miettinen 14311). Scale bar = 5 μm.
Basionym: Daedalea gilvidula Bres., Hedwigia 51: 320. 1912.
Typus: Philippines, Sibuyan, Guiting-Guiting, fallen logs, Apr. 1910, Elmer 12327, 12391 (syntypes S F14744!, NY00704678!, NY00704685!).
Synonyms:
Trametes tuberculata Bres., Ann. Mycol. 10: 505. 1912.
Typus: Indonesia, Java, Tjibodas, fallen log, [no collection date], Höhnel (holotype S F14738!).
Trametes lusor Corner, Beih. Nova Hedwigia 97: 114. 1989.
Typus: Malaysia, Johor, Gunong Panti, 26 Sep. 1966, Corner (holotype E00430842).
Description: Basidiocarps perennial, sessile, often with a contracted base (fan-shaped) or effused-reflexed, projecting up to 5 cm. Upper surface first pale ochraceous to fawn, smooth or finely pubescent, indistinctly zonate, later greyish to brownish, smooth, more or less clearly and densely zonate, occasionally with a silky lustre. Pileal edge sharp, concolourous with pileal surface, sterile, up to 1 mm wide. Pore surface first cream-coloured to pale ochraceous or pinkish, then discolouring to greyish or almost white, flat or concave; pores angular or sinuous, (1–)1.5–3 per mm, sometimes strongly elongated (almost lamellate) close to the margin, with rather thick, entire or uneven dissepiments. Section: context hard leathery, ochraceous to brownish, up to 2 mm thick; tubes hard leathery, one-layered, concolourous with hymenial surface, up to 2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, interwoven to subparallel, occasionally branched, (3.2–)3.8–5.4(−5.8) μm diam (n = 60/3), lumen varying from rather wide to capillary, side branches 2–3.5 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to rusty brown, densely interwoven, occasionally branched, sometimes twisted and irregularly inflated, (3.0–)3.2–4.8(−5.3) μm diam (n = 80/4), lumen mostly narrow to capillary, generative hyphae rather rare, thin- to slightly thick-walled, 2–3 μm diam. Subhymenium sometimes distinct, up to 20 μm thick. Skeletocystidia present as swollen (up to 5.5 μm diam), acute or blunt apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles abundant, tapering, often with a long hyphoid neck, 11–20.5 × 3–5 μm. Basidia clavate to long clavate, (11.8–)12.3–20.8(−22.3) × (4.1–)4.2–5.7(−5.8) μm (n = 30/3), in senescent hymenium slightly thick-walled and often glued together. Basidiospores thin-walled or with a distinct wall, cylindrical-subfusiform or narrowly ellipsoid, (3.8–)3.9–5.3(−5.8) × (2.0–)2.1–2.9(−3.0) μm (n = 110/4), L = 4.40–4.91, W = 2.26–2.38, Q = 1.87–2.11.
Specimens examined: Indonesia, Riau, Kampar, Balung, logged over natural forest, dicot, 25 Dec. 2006, Miettinen 11290.1 (BO, H); Kampar, Koto Lamo, dicot, 23 Oct. 2016, Miettinen 20535* (BO, H); Sumatera Barat, Padang, Limau Manis, primary forest, Fagaceae, 10 Jul. 2008, Miettinen 12905 (ANDA, H); dicot, 11 Jul. 2008, Miettinen 12957.1 (ANDA, H); dicot, 16 Jul. 2008, Miettinen 13128.1 (ANDA, H). Malaysia, Penang, Pulau Pinang, Pantai Aceh Nat. Park, fallen tree (Shorea?), 1 Dec. 2006, Miettinen 11154.3 (BORH, H).
Notes: Fomitopsis gilvidula seems to be widely distributed in the tropical zone of Southeast Asia. Morphologically, it is most close to F. aculeata, with which it occasionally occurs side by side. Fomitopsis gilvidula differs from F. aculeata in having lighter-coloured basidiocarps with larger pores and a smooth or felty surface. Under the microscope, it can be differentiated from the latter species in having a well-developed subhymenium and longer hymenial cells. Another similar species from the same geographic area is F. incana (see below). Despite their certain morphological similarity, F. gilvidula, F. aculeata and F. incana are not closely related (Suppl. Fig. S2). Two GenBank sequences of an unidentified polypore from East Kalimantan (AJ542523, 542524) belong to F. gilvidula.
Fomitopsis glabricystidia (Ipulet & Ryvarden) Miettinen & Ryvarden, comb. nov. MycoBank MB 844915.
Basionym: Junghuhnia glabricystidia Ipulet & Ryvarden, Syn. Fung. 20: 93. 2005.
Typus: Uganda, Kabarole, Kibali Nat. Park, Ngogo, rotten hardwood log, 6 Jun. 2002, Ipulet 378* (holotype O!).
Description: Ipulet & Ryvarden (2005, as J. glabrocystidia).
Notes: The species was originally described in Junghuhnia due to the presence of abundant thick-walled skeletocystidia (Ipulet & Ryvarden 2005). They are, however, not encrusted as in typical representatives of the latter genus, and the hyphae are broad and show no sign of a cyanophilous reaction. DNA data undoubtedly place this species in Fomitopsis as redefined here (Figs 1, 3). See further remarks under F. tunicata.
Fomitopsis globispora (Ryvarden & Aime) Spirin, comb. nov. MycoBank MB 844916.
Basionym: Trametes globispora Ryvarden & Aime, Syn. Fung. 26: 28. 2009 (as ‘globospora’).
Typus: Belize, Cayo, Maya Mts., Doyle’s Delight, dead hardwood log, 23 Aug. 2007, Aime 3413* (holotype LSUM).
Description: Ryvarden et al. (2009, as T. globospora).
Note: Using sequence data, Decock et al. (2022) confirmed the close relationship of this species with F. spraguei (see also Fig. 15).
Fomitopsis hartmannii (Cooke) M.D. Barrett & Spirin, comb. nov. MycoBank MB 844917.
Basionym: Polyporus hartmannii Cooke, Grevillea 12: 14. 1883.
Typus: Australia, Queensland, Toorvoomba [Toowoomba], [no collection date], Hartmann (lectotype K(M) 252596!) (selected by Ryvarden 1988b: 50).
Synonyms:
Piptoporus ulmi Bondartsev & Ljub., Bot. Mat. Otd. Spor. Rast. 14: 198. 1961.
Typus: Russia, Primorie, Ussuriisk Dist., Ussuri Nat. Res., Ulmus sp., 11 Aug. 1945, Vassiljeva (holotype LE 22548!).
Polyporus sublignosus J.D. Zhao & X.Q. Zhang, Acta Mycol. Sin. 10: 269. 1991.
Typus: China, Hainan, Bawangling, ‘on the ground’, 18 Apr. 1977, Han 803 (holotype HMAS 54101).
Tyromyces squamosellus Núñez & Ryvarden, Fungal Diversity 3: 117. 1999.
Typus: Japan, Ibaraki, Ogawa, dead hardwood, 11 Aug. 1994, Núñez 554* (holotype O!).
Buglossoporus eucalypticola M.L. Han, B.K. Cui & Y.C. Dai, Fungal Diversity 80: 351. 2016.
Typus: China, Hainan, Danzhou, Eucalyptus sp., 15 Jun. 2014, Dai 13660* (holotype BJFC 17399).
Description: Núñez & Ryvarden (1995, as Laccocephalum hartmannii).
Specimen examined: Japan, Shikoku, Ehime Pref., Takanawa Mt., 7 Sep. 1994, Okino (coll. number Núñez 679*) (O 11264, H).
Notes: We studied the type material of P. hartmannii and could not find substantial morphological differences between it and specimens collected in East Asia. Nevertheless, the identity of P. hartmannii sensu typi should be re-established based on sequenced material from the eastern part of Australia.
Fomitopsis hyalina (Spirin, Miettinen & Kotir.) Spirin & Miettinen, comb. nov. MycoBank MB 844918.
Basionym: Antrodia hyalina Spirin, Miettinen & Kotir., Mycol. Progr. 12: 56. 2013.
Typus: Russia, Nizhny Novgorod Reg., Lukoyanov Dist., Razino, Populus tremula, 5 Aug. 2008, Spirin 2772* (holotype H!).
Description and phylogenetic data: Spirin et al. (2013b, as A. hyalina); see also Figs 1 and 3.
Fomitopsis hypoxantha (Bres.) Spirin & Miettinen, comb. nov. MycoBank MB 848358.
Basionym: Polyporus hypoxanthus Bres., Ann. Mycol. 10: 494. 1912.
Typus: Indonesia, Java, Tjibodas, 1907, Höhnel (isotype BPI US021111!).
Description: Basidiocarps annual or short-living perennial, sessile, ungulate, broadly attached, projecting up to 6 cm. Upper surface first cream-coloured to pale ochraceous, azonate, glabrous, later covered by brown to blackish brown crust, smooth, not cracking. Pileal edge normally blunt, concolourous with pore surface, fertile. Pore surface first cream- or pale wood-coloured, then brownish, flat or slightly concave; pores angular, 4–6 per mm, with thin, entire or serrate dissepiments. Section: crust (present in mature basidiocarps) tough, dark brown, matt, up to 1 mm thick, context fibrous to soft corky, cream-coloured, up to 5 mm thick; tubes rather soft, fragile, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 1 cm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, hyaline or yellowish, interwoven, occasionally branched, (3.3–)3.7–5.2(−5.3) μm diam (n = 20/1), lumen mostly capillary to indistinct, generative hyphae rare, hyaline, thin- to slightly thick-walled, 2–4 μm diam. Trama dimitic; skeletal hyphae hyaline to brownish, interwoven, easily crumbling, occasionally branched, (2.7–)2.8–4.2(−4.3) μm diam (n = 40/2), lumen rather wide to capillary or indistinct, side branches 2–3 μm diam, generative hyphae rather abundant, hyaline, thin- to slightly thick-walled, 2–3.5 μm diam, in juvenile tubes producing subhymenial layer up to 20 μm thick. Resinous droplets abundant among tramal and subhymenial tissues of senescent basidiocarps. Cystidioles rather abundant, tapering, 14–20 × 3.5–7 μm. Basidia clavate, (13.0–)14.0–18.9(−21.0) × (5.4–)5.8–8.3(−8.6) μm (n = 20/2), senescent basidia slightly thick-walled. Basidiospores thin-walled or with a distinct wall, broadly ellipsoid to ellipsoid, (4.8–)4.9–6.3(−6.7) × (3.8–)3.9–5.3(−6.0) μm (n = 80/3), L = 5.27–5.63, W = 4.47–4.56, Q = 1.16–1.27.
Specimens examined: China, Hubei, Lichuan, Xiaohe, Castanea sp. (living tree), 27 Sep. 2004, Dai 5983* (H). Indonesia, Java, Tjibodas Bot. Gdn, 26 Jan. 1995, Núñez 707 (O 10876, H).
Notes: Fomitopsis hypoxantha is reinstated here as the South-East Asian relative of F. spraguei (Fig. 15). We failed to find any certain morphological differences between the two species, due to a huge morphological variability of F. spraguei. At the moment, F. hypoxantha can be separated from F. spraguei based on geographic distribution (South-East Asia vs. temperate Europe and North America) and genetic distance.
Fomitopsis incana (Lév.) Spirin & V. Malysheva, comb. nov. MycoBank MB 844920. Fig 22.
Basionym: Trametes incana Lév., Ann. Sci. Nat., Bot. 3 (2): 196. 1844.
Typus: Philippines, Luzon, Manila, ‘ad truncos’, Nov. 1836, Gaudichaud (holotype PC!, isotype BPI US0247025!).
Synonym: Daedalea allantoidea M.L. Han, B.K. Cui & Y.C. Dai, Fungal Diversity 80: 357. 2016.
Typus: China, Yunnan, Jinghong, hardwood, 22 Oct. 2013, Dai 13612A* (holotype BJFC 15075).
Description: Basidiocarps seasonal, effused-reflexed, projecting up to 2 cm. Upper surface first cream-coloured, smooth, even or indistinctly zonate, later pale ochraceous to brownish. Pileal edge sharp, concolourous with hymenial surface, sterile, up to 0.5 mm wide. Pore surface first cream-coloured, then pale ochraceous or greyish, flat or concave; pores roundish to angular, some strongly elongated, (2–)3–4 per mm, with thin, entire or serrate dissepiments. Section: context leathery, almost white to cream-coloured or pale ochraceous, up to 1.5 mm thick; tubes leathery, one-layered, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae yellowish to brownish, densely interwoven, richly branched, (3.0–)3.1–4.1(−4.3) μm diam (n = 20/1), lumen mostly capillary to indistinct, side branches 2–3 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 3–4 μm diam. Trama dimitic; skeletal hyphae dominating, hyaline to yellowish or brownish, densely interwoven, occasionally branched, sometimes twisted, (3.0–)3.1–5.0(−5.2) μm diam (n = 40/2), lumen varying from rather wide to indistinct, generative hyphae rather rare, thin- to slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Skeletocystidia present as blunt or sharp apices of tramal skeletal hyphae, 3–4 μm diam, slightly projecting above hymenium. Cystidioles abundant, tapering, 9–13 × 3–4 μm. Hyphidia present, mostly simple, scattered among basidia, 1.5–2 μm diam at the apex. Basidia clavate, (10.8–)10.9–15.0(−22.0) × (4.1–)4.2–5.1(−5.2) μm (n = 20/1), in senescent hymenium slightly thick-walled. Basidiospores thin-walled or with a distinct wall, cylindrical-subfusiform, sometimes slightly curved, (3.8–)3.9–5.2(−5.7) × (2.0–)2.1–2.8(−2.9) μm (n = 30/1), L = 5.23, W = 2.50, Q = 2.12.
Specimen examined: India, Maharashtra, Pune, Purandar, decayed tree trunk, 2017, Ranadive (LE 313649*).
Notes: The identity of T. incana has been interpreted in the literature in two different ways. Hjortstam & Ryvarden (1984) believed it represents the same species as D. dickinsii (= F. dickinsii above) widely distributed in warm-temperate forests of East Asia. On the contrary, Hattori (2005) accepted T. incana as a separate species of Daedalea and stated it is identical with Trametes pseudodochmia (= F. moritziana below). We restudied the type of T. incana and cannot accept either of these opinions. The remaining material in PC and BPI comprises tiny pieces, evidently of a resupinate part of a basidiocarp, glued on the paper and subsequently soaked in mercury. Nevertheless, it is evident these remains belonged to a light-coloured, leathery polypore 2–3 mm thick, with angular pores 3–4 per mm. The basidiocarp consistency and thickness certainly preclude F. dickinsii and F. moritziana, since both are robust and much sturdier species. Although extremely vague, the protologue (Léveillé 1844) describes the whole polypore as white (but the species epithet refers to light grey), and this description does not accord with F. moritziana which has a reddish-brown to almost black cap surface. In turn, F. dickinsii is a temperate species not known in Southeast Asia (Hattori 2005). Microscopically, the type of T. incana is characterized by dimitic hyphal structure with hyaline or brownish, occasionally branched skeletal hyphae (3.2–)3.3–5.0(−5.2) μm (n = 20/1) diam. Hymenial cells are totally collapsed but a few turgid spores were observed in phase contrast illumination, 5.1–5.2 × 2.3–2.8 μm (n = 3/1). After comparing the type of T. incana with a newly collected and sequenced specimen from India, we concluded they are identical and conspecific with D. allantoidea recently described from China. Therefore, a new combination, F. incana, is proposed here.
Fomitopsis incana is morphologically similar to F. gilvidula, distributed in the same geographic region. The latter species differs from F. incana in having slightly larger, in older basidiocarps clearly elongated pores, warmer-coloured basidiocarps and darker, brownish to rusty-brown skeletal hyphae. Another macroscopically similar species, F. malicola (= Antrodia malicola, see below) has larger basidiospores than F. incana, (6.1–)6.2–10.2(−10.6) × (2.6–) 2.7–4.0(−4.1) µm (Spirin et al. 2016).
Fomitopsis infirma (Renvall & Niemelä) Miettinen & Niemelä, comb. nov. MycoBank MB 844921.
Basionym: Antrodia infirma Renvall & Niemelä, Karstenia 32: 35. 1992.
Typus: Finland, Pohjois-Karjala, Lieksa, Patvinsuo, Pinus sylvestris, 14 Sep. 1989, Penttilä 1235 (holotype H!).
Description: Renvall & Niemelä (1992, as A. infirma).
Note: For phylogenetic placement, see Spirin et al. (2013b).
Fomitopsis juniperina (Murrill) Spirin & Vlasák, comb. nov. MycoBank MB 844922.
Basionym: Agaricus juniperinus Murrill, Bull. Torrey Bot. Club 32 (2): 85. 1905.
Typus: USA, Kansas, Rooks Co., Rockport, Juniperus sp., 1894, Bartholomew (holotype NY 00774779!).
Synonym: Antrodia juniperina (Murrill) Niemelä & Ryvarden, Trans. Br. Mycol. Soc. 65: 427. 1975.
Description: Niemelä & Ryvarden (1975, as Antrodia juniperina).
Specimens examined: Spain, Madrid, Tamajon, Juniperus turifera, 1 Mar. 2011, E. Larsson 15-11* (GB, H). USA, Arizona, Cochise Co., Coronado Nat. Forest, Juniperus monosperma, 23 Aug. 1958, Lowe 9090 (H); Coconino Co., Sedona, Juniperus sp., Nov. 2017, Vlasák Jr. 1711/13-J* (JV, H); Santa Cruz Co., Madera Canyon, Juniperus deppeana, 1 Sep. 2012, Vlasák 1209/14* (JV); Arkansas, Searcy Co., Harriet, Shepherd of the Ozarks, Juniperus sp., 25 Oct. 2013, Cho (H7068514*); Georgia, Stephens Co., Toccoa, Juniperus virginiana, 18 Aug. 1950, Lowe 3969 (H ex SYRF); Pennsylvania, Montgomery Co., Landsdale, J. virginiana, Oct. 2003, Vlasák Jr. 0310/1-J* (JV, H); South Carolina, Pickens Co., Clemson, Juniperus sp., 5 Sep. 1950, Lowe 4480 (H ex SYRF); Virginia, Prince William Co., Woodbridge, 21 Sep. 2007, Vlasák 0709/154* (JV, H); Shenandoah Co., Edinburg, J. virginiana, 15 Sep. 1939, Hepting & Roth 68635 (H ex SYRF).
Note: See remarks to F. algumicola and Antrodia uzbekistanica.
Fomitopsis kenyensis Spirin & Ryvarden, nom. nov. MycoBank MB 848359.
Basionym: Daedalea africana Ryvarden & I. Johans., A preliminary polypore flora of East Africa: 304. 1980.
Typus: Kenya, Kwale, Shimba Hills, Makadara, 14 Feb. 1973, Ryvarden 16485 (holotype O!).
Etymology: Kenyensis (Lat., adj.) – in reference to Kenya where the holotype of this species was collected.
Notes: Han et al. (2016) published sequences of a paratype of D. africana (O 15372) and detected that it belongs to Daedalea clade (see also Fig. 5). Due to the existence of F. africana (see above), a new name for D. africana is necessary to place it in Fomitopsis.
Fomitopsis kuzyana (Pilát ex Pilát) Spirin & Vlasák, comb. nov. MycoBank MB 844923.
Basionym: Trametes kuzyana Pilát ex Pilát, Acta Mus. Nat. Pragae 9 (B), 2 (1): 104. 1953.
Typus: Ukraine, Zakarpats’ka Reg., Trebušany (Dilove), Mt. Menchul, Fagus sylvatica, Aug. 1934, Pilát (holotype PRM 108267!).
Synonym: Antrodia submalicola A. David & Dequatre, Cryptog. Mycol. 5 (4): 299 (1985).
Typus: France, Gard, La Valbonne, Combe du Ruisseau de l’Arnave, Populus sp., 23 Aug. 1983, Callac (holotype LY AD-4497!).
Description and phylogenetic data: Spirin et al. (2016, as Antrodia kuzyana); see also Figs 1 and 3.
Fomitopsis labyrinthica Bernicchia & Ryvarden, Mycol. Helv. 8 (2): 6. 1996.
Typus: Italy, Forlì Cesena: Casentinesi, Sasso Fratino, Abies alba, 18 Oct. 1995, Bernicchia 6497 (holotype HUBO!).
Synonym: Antrodia kmetii Vlasák, Cryptog. Mycol. 34 (3): 206. 2013.
Typus: Slovakia, Banská Bystrica: Badín, Abies alba, 12 Oct. 1993, Vlasák 9310/14* (PRM 861180!, holotype).
Description and phylogenetic data: Vlasák et al. (2013, as A. kmetii).
Specimens examined: Romania, Braşov, Şinca, A. alba, 14 Sep. 2021, Spirin 14932 & 14960 (H). Slovenia, Kočevje, Borovec pri Kočevski Reki, Krokar Forest Reserve, A. alba, 18 Aug. 2021, Spirin 14700 & Grebenc (H, LJF).
Fomitopsis lapidosa Miettinen & Spirin, sp. nov. MycoBank MB 844924. Figs 21, 22.
Typus: Indonesia, Papua Barat, Manokwari, Gunung Meja, −0,84929° 134,07458° (± 50 m), secondary forest with abundant Pometia pinnata, by the forest road, on a man-made stump (dicot, decay stage 2, 45 cm in diameter), 8 Nov. 2018, Miettinen 21981* (holotype MAN, isotypes H7200141, BO).
Etymology: Lapidosus (Lat., adj.) – stone-hard.
Description: Basidiocarps perennial, sessile, conchate, solitary or partly fusing together, projecting up to 5 cm. Upper surface blackish brown to black, with several distinct annual zones, accidentally longitudinally cracking, growing margin much paler, wood-coloured to pale ochraceous, indistinctly zonate and occasionally radially striate. Pileal edge sharp or rather blunt, concolourous with hymenial surface, first sterile, up to 3 mm wide, then fertile. Pore surface cream-coloured or beige to pale ochraceous, slightly concave; pores roundish, 8–10 per mm, with rather thick, entire dissepiments. Section: crust black, tough, up to 1 mm thick, context corky, cream-coloured to pale ochraceous, up to 3 mm thick; tubes corky, indistinctly stratified, concolourous with hymenial surface, up to 2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, hyaline to yellowish, densely interwoven, rarely branched, (3.2–)3.3–5.0(−5.1) μm diam (n = 20/1), lumen varying from wide to capillary, generative hyphae hyaline, thin- to slightly thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae dominating, hyaline to yellowish, densely interwoven, rarely branched, (2.0–)2.3–3.3(−3.7) μm diam (n = 20/1), lumen mostly capillary to indistinct, generative hyphae rare, thin- to slightly thick-walled, 1.5–2 μm diam. Subhymenium indistinct. Cystidioles rare, tapering, 10–14 × 2.8–3.2 μm. Basidia clavate, (10.1–)10.2–15.0(−18.7) × (3.8–)3.9–4.7(−4.8) μm (n = 20/1), in senescent hymenium partly glued together. Basidiospores thin-walled or with a distinct wall, cylindrical, sometimes slightly curved, rarely narrowly ellipsoid, (2.9–)3.0–3.8(−4.1) × (1.8–)1.9–2.1 μm (n = 30/1), L = 3.22, W = 1.98, Q = 1.63.
Specimen examined: Indonesia, Papua, Jayapura, Waena, fallen log, 2006, anonymous collector (H).
Notes: Fomitopsis lapidosa is introduced here based on two collections from New Guinea, Indonesia. Morphologically, it is most similar to F. ferrea and F. elevata and differs from them in having more lightweight (although exceptionally tough) basidiocarps and considerably smaller pores. No reliable microscopic traits were detected to separate these three species. Phylogenetically, F. lapidosa is the closest relative of the Caribbean F. lignea and thus it is very distant from the F. dochmia group.
Fomitopsis leioderma (Mont.) Spirin & Vlasak, comb. nov. MycoBank MB 844925. Figs 21, 22.
Basionym: Polyporus leiodermus Mont., Ann. Sci. Nat., Bot. 4 (1): 134. 1854.
Typus: French Guiana, [no locality indicated], ‘ad cortices’, [no collection date], Leprieur 855 (syntype PC!).
Description: Basidiocarps seasonal, sessile, often with a contracted base (fan-shaped), projecting up to 2 cm. Upper surface first cream-coloured, smooth, even or indistinctly zonate, later pale ochraceous to brownish, with a silky lustre. Pileal edge sharp, concolourous with hymenial surface, sterile, up to 1 mm wide. Pore surface first cream-coloured, then pale ochraceous, flat or concave; pores angular or sinuous, 3–4 per mm, with thin, entire or serrate dissepiments. Section: context leathery, cream-coloured to pale ochraceous, up to 1 mm thick; tubes leathery, one-layered, concolourous with hymenial surface, up to 1 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, interwoven, occasionally branched, (3.8–)3.9–5.0(−5.2) μm diam (n = 20/1), lumen varying from rather wide to capillary, side branches 2–3 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae dominating, brownish, densely interwoven, occasionally branched, (2.9–)3.0–4.3(−4.8) μm diam (n = 40/2), lumen mostly narrow to indistinct, generative hyphae rather rare, thin- to slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Skeletocystidia present as swollen (up to 5.5 μm diam) apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles abundant, tapering, 11.5–17 × 3–4.5 μm. Basidia clavate, (10.0–)10.2–16.2(−17.9) × (4.3–)4.4–5.8(−6.1) μm (n = 20/2), occasionally pleural, in senescent hymenium slightly thick-walled. Basidiospores thin-walled or with a distinct wall, cylindrical, sometimes slightly curved, longest spores subfusiform, (4.1–)4.2–6.2(−6.8) × (2.0–)2.1–2.9(−3.0) μm (n = 30/1), L = 5.23, W = 2.50, Q = 2.12.
Specimen examined: French Guiana, Roura, Camp Cayman, dead log, 31 Aug. 2019, Vlasák 1908/82* (JV, H).
Notes: Montagne (1854) described P. leiodermus based on two collections by Leprieur from French Guiana. We were able to locate and study one of them. This specimen is morphologically identical to one recent sequenced collection from the same area, and it certainly represents a good species from the Daedalea clade (Fig. 5). Ryvarden (1982) included P. leiodermus in the synonymy of Trametes modesta (Kuntze ex Fr.) Ryvarden (see below as F. modesta). The latter species differs from P. leiodermus in having much smaller, more regular pores and shorter basidiospores.
Fomitopsis leucaena (Y.C. Dai & Niemelä) Spirin & Miettinen, comb. nov. MycoBank MB 844926.
Basionym: Antrodia leucaena Y.C. Dai & Niemelä, Ann. Bot. Fenn. 39 (4): 259. 2002.
Typus: China, Jilin, Antu Co., Changbaishan Nat. Res., Populus davidiana, 14 Sep. 1998, Dai 2190a & Niemelä* (holotype H!).
Description and phylogenetic data: Spirin et al. (2013b, 2017, as A. leucaena).
Fomitopsis lignea (Berk.) Ryvarden, Norw. J. Bot. 19: 231. 1972. Fig. 22.
Basionym: Polyporus ligneus Berk., Ann. Mag. Nat. Hist. 3: 387. 1839.
Typus: St. Vincent and the Grenadines, St. Vincent, ‘Polyporus fasciatus Swz. Fl.’ (lectotype K, isolectotype BPI US0231445!) (selected by Ryvarden 1976: 95).
Synonym: Fomes subferreus Murrill, North American Flora 9 (2): 97. 1908.
Typus: Cuba, Pinar del Rio, Herradura, dead wood, 7–12 Mar. 1905, Earle & Murrill 184 (lectotype NY00780693!) (selected by Ryvarden 1985: 176).
Description: Basidiocarps perennial, sessile, dimidiate, projecting up to 9 cm. Upper surface blackish brown to black, with a few indistinct annual zones, accidentally longitudinally cracking. Pileal edge sharp or rather blunt, concolourous with hymenial surface, first sterile, up to 1 mm wide, then fertile. Pore surface cream-coloured or beige to pale ochraceous, flat or concave; pores roundish to angular, 5–7 per mm, with rather thick, entire or minutely serrate dissepiments. Section: context corky, cream-coloured to brownish, up to 10 mm thick; tubes corky, indistinctly stratified, concolourous with hymenial surface, up to 10 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae yellowish to brownish, interwoven, occasionally branched, (3.0–)3.2–6.4(−7.0) μm diam (n = 40/2), lumen capillary to indistinct, side branches 2–4 μm diam, generative hyphae rare, hyaline, thin- to slightly thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae dominating, yellowish to brownish, densely interwoven, occasionally branched, sometimes twisted, (2.7–)2.8–4.4(−4.9) μm diam (n = 40/2), lumen mostly narrow to indistinct, generative hyphae thin- to slightly thick-walled, 1.5–3 μm diam. Subhymenium indistinct. Cystidioles infrequent, tapering, sometimes with a long hyphoid neck, 9.5–14 × 2.5–3.5 μm. Basidia clavate, (8.1–)8.2–13.0(−13.2) × (3.2–)3.7–4.3(−4.4) μm (n = 16/2), in senescent hymenium glued together. Basidiospores with a distinct wall, narrowly ellipsoid to broadly cylindrical, (2.8–)2.9–4.1(−4.3) × (1.8–)1.9–2.3(−2.4) μm (n = 40/2), L = 3.34–3.44, W = 2.07–2.08, Q = 1.61–1.66.
Specimens examined: Belize, Cayo, Blue Hole Nat. Park, angiosperm, 18 Nov. 2001, Ryvarden 44326 (O 17633); Orange Walk, La Milpa, dead hardwood, 24 Oct. 2002, Ryvarden 45153 (O 18199). Cuba, Oriente, Jaguey, fallen log, Maxon 4234 (Kryptogamae exsiccatae #1908, H ex W). Jamaica, Trelawny, Crowlands, dead wood, 10 Apr. 1999, Ryvarden 41624* (O 10794). Panama, Veraguas, Montijo, Coiba Nat. Park, dead wood, 21 Nov. 1996, Núñez 1265 (O 10792).
Notes: Fomitopsis lignea is reinstated here as a species closely related to F. lapidosa. It was previously known as Fomes suferreus and usually treated as a synonym of F. dochmia (Lowe 1957, Carranza-Morse & Gilbertson 1986). Despite their high morphological similarity, these species are not closely related. Fomitopsis lignea is distributed in the Caribbean, and all older records of F. dochmia from this area are highly likely to belong to this species. See further notes under F. dochmia and F. lapidosa. Ryvarden (2015) misapplied F. lignea to another species, described here as F. lignicolor.
Fomitopsis lignicolor Vlasák & Spirin, sp. nov. MycoBank MB 848360. Fig 22.
Typus: Costa Rica, Puntarenas, Tarcoles, Carara Nat. Park, fallen log, 18 Apr. 2015, Vlasák 1504/15* (holotype PRM933860, isotype H).
Etymology: Lignicolor (Lat., adj.) – wood-coloured.
Description: Basidiocarps annual or short-living perennial, sessile, ungulate, broadly attached, projecting up to 7 cm. Upper surface first ivory to pale ochraceous, azonate, felty, later covered by brown to blackish brown crust, smooth, not cracking. Pileal edge blunt, concolourous with pore surface, fertile. Pore surface first ivory or pale wood-coloured, then brownish, flat or slightly convex; pores roundish, 3–4 per mm, with thin, entire dissepiments. Section: crust (present in mature basidiocarps) tough, black, matt, up to 1 mm thick, context corky, ochraceous or brownish, up to 15 mm thick, sharply delimited from tubes; tubes tough, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 1 cm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, brownish, interwoven, occasionally branched, (2.6–)2.7–4.2(−4.4) μm diam (n = 20/1), lumen capillary to indistinct, generative hyphae rare, hyaline, thin-walled, 2.5–4.0 μm diam. Trama dimitic; skeletal hyphae brownish, tightly interwoven, sometimes twisted, rarely branched, (2.3–)2.6–3.4(−3.7) μm diam (n = 40/2), lumen rather wide to capillary, generative hyphae hyaline, thin-walled, 2.0–3.5 μm diam, often producing distinct subhymenial layer up to 20–30 μm thick. Resinous droplets abundant among tramal and subhymenial tissues of senescent basidiocarps. Cystidioles rare, tapering, 12–16 × 4–5.4 μm. Basidia short-clavate, (9.4–)9.6–13.7(−13.8) × (4.1–)4.3–5.8(−6.3) μm (n = 20/1), senescent basidia slightly thick-walled. Basidiospores thin-walled or with a distinct wall, cylindrical to subfusiform, (4.1–)4.2–7.1(−7.2) × (2.0–)2.1–2.9(−3.0) μm (n = 60/2), L = 5.05–5.78, W = 2.41–2.45, Q = 2.12–2.38.
Specimens examined: Costa Rica, Puntarenas, Tarcoles, Carara Nat. Park, fallen log, Dec. 2013, J. Vlasak Jr. 1312/A-4 (JV). Panama, Colón, Barro Colorado, 20 Feb. 1985, Grimaldi (O 10814). Venezuela, Aragua, Romerito, Henri Pittier Nat. Park, Feb. 2004 Kout 0402/M* (H, JV).
Notes: First DNA sequences, as well as coloured photographs of this species were published by Vlasák et al. (2016) (as F. lignea). According to our data, F. lignicolor occupies an isolated position within the genus (Figs 1, 3). Short-lived basidiocarps with rather pale-coloured, not stratified tubes and a pronounced crust make this species easily distinguishable from other representatives of the genus. Fomitopsis lignicolor is so far found only in the Caribbean.
Fomitopsis lilacinogilva (Berk.) J.E. Wright & J.R. Deschamps, Rev. Invest. Agropec. INTA, Serie 5, Pat. Veg. 12 (3): 143. 1975. Basionym: Polyporus lilacinogilvus Berk., Ann. Mag. Nat. Hist. 3: 324. 1839.
Typus: Australia, Tasmania, charred wood, [no collection date indicated], Gunn (lectotype K, isolectotype PC!) (selected by Ryvarden 1976: 95).
Description: Basidiocarps perennial, sessile, often with a contracted base, projecting up to 5 cm. Upper surface greyish-ochraceous to pinkish brown, first adpressed-hirsute to strigose, indistinctly zonate, then almost smooth, radially furrowed, often with a silky lustre. Pileal edge sharp, concolourous with hymenial surface, usually sterile, up to 2 mm wide. Pore surface pinkish to pinkish brown, usually concave; pores angular, 3–5 per mm, with thin or moderately thickened, entire or serrate dissepiments. Section: context corky, pinkish to pale ochraceous or brownish, up to 7 mm thick; tubes corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 7 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish to almost hyaline, rather loosely interwoven, occasionally branched, (4.0–)4.1–6.7(−7.2) μm diam (n = 20/1), lumen mostly wide, side branches 2.5–4 μm diam, generative hyphae hyaline, slightly thick-walled, 4–7 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to reddish-brown, interwoven, occasionally branched, (2.8–)3.0–4.6(−5.0) μm diam (n = 60/3), lumen rather wide to capillary, generative hyphae rather rare, thin- to slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Cystidioles uncommon, tapering, 14–21 × 3.5–6 μm. Basidia clavate, (13.1–)13.8–18.2(−20.2) × (5.1–)5.4–7.0(−7.2) μm (n = 20/2), occasionally pleural, in senescent hymenium partly glued together. Basidiospores thin-walled or with a distinct wall, cylindrical-subfusiform, (5.1–)5.2–9.2(−9.4) × (2.3–)2.6–3.2(−3.3) μm (n = 60/2), L = 6.71–6.76, W = 2.92–3.02, Q = 2.24–2.29.
Specimens examined: Australia, Queensland, Cairns, Lake Barrine, angiosperm, 19 Aug. 2006, Schigel 5193* (H); Victoria, Grampians Nat. Park, Halls Gap, charred wood, 5 Mar. 2003, Hausknecht 12/03 (O 19243); Western Australia, Perth, Byford Maryedal Creek, charred wood, 8 Sep. 1964, Smith (O 10818).
Notes: Fomitopsis lilacinogilva is a member of the F. feei complex distributed in Oceania. It differs from other species of this group from the same region (F. eucalypti, F. foedata and F. marchionica) in having distinctly larger pores and longer basidiospores, as well as by the roughly hirsute pileal surface of its vigorously growing basidiocarps. The identity of F. lilacinogilva from the neotropics deserves further study. The single available ITS sequence from Costa Rica (GenBank DQ491400) shows a 10 bp difference vs. sequences from Australian collections, and therefore it may belong to an undescribed sister species of F. lilacinogilva.
Fomitopsis luzonensis (Murrill) Spirin & Miettinen, comb. nov. MycoBank MB 844927. Figs 21, 22.
Basionym: Trametes luzonensis Murrill, Bull. Torrey Bot. Club 34: 474. 1907.
Typus: Philippines, Luzon, Bataan, Mt. Mariveles, dead sticks, Nov. 1904, Elmer 6932 (holotype NY00705039!).
Description: Basidiocarps annual, sessile or effused-reflexed, often fusing together in large imbricate groups, projecting up to 5 cm. Upper surface first cream coloured to pale ochraceous, smooth or scrupose, later greyish ochraceous to mouse grey, sometimes with a brownish tint, azonate. Pileal edge sharp to rather blunt, concolourous with cap surface, usually fertile. Pore surface pale ochraceous to grey, sometimes with brownish stains, as a rule concave; pores roundish to angular, 8–10 per mm, with thin, entire dissepiments. Section: context tough, pale cream-coloured to pale ochraceous, up to 5 mm thick; tubes tough, one-layered, concolourous with hymenial surface, up to 5 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline, densely interwoven or rarely in subparallel bundles, occasionally branched, (2.8–)2.9–6.0(−6.1) μm diam (n = 60/3), lumen narrow to almost invisible, side branches 2–3 μm diam, generative hyphae rather abundant to rare, hyaline, thin- or moderately thick-walled, 3–6 μm diam. Trama dimitic; skeletal hyphae hyaline to yellowish, interwoven, some flexuous, occasionally branched, (2.0–)2.2–4.2(−4.7) μm diam (n = 100/5), lumen wide to capillary, generative hyphae thin- or slightly thick-walled, 2–3 μm diam, dissepiment edges dimitic, consisting of thin-walled generative hyphae and winding skeletals with a wide lumen. Subhymenium usually indistinct. Cystidioles frequent to rare, distinctly tapering to the apex (mostly bottle-shaped), 9–15 × 3.5–7 μm. Basidia short-clavate, (9.4–)9.8–16.3(−17.2) × (4.3–)4.6–6.4(−6.7) μm (n = 34/3), often glued together in a dense palisade layer. Basidiospores thin-walled, cylindrical to fusiform, (5.0–)5.1–8.1(−8.3) × 2.0–3.1(−3.2) μm (n = 120/4), L = 5.94–6.15, W = 2.39–2.89, Q = 2.06–2.56.
Specimens examined: Indonesia, Jawa Barat, Bogor, dicot stump, 8 May 2016, Miettinen 19950 (BO, H); Nusa Tenggara Barat, Lombok Utara, Gili Meno, Casuarina, 6 Jan. 2012, Miettinen 15300 (BO, H); Papua Barat, Manokwari, Cocos nucifera, 1 Feb. 2007, Miettinen 11573* (MAN, H); Aman, Senna, 9 Nov. 2018, Miettinen 21985 (MAN, H); Amberbaken, Wefiani-Wasarak, C. nucifera, 31 Oct. 2010, Miettinen 14261* (MAN, H); Saukorem, dicot, 1 Nov. 2010, Miettinen 14311* (MAN, H); ibid., 4 Nov. 2010, Miettinen 14417* (MAN, H); Riau, Pelalawan, Kampar Peninsula, Serkap, dicot, 16 Dec. 2006, Miettinen 11224* (BO, H); Tasik Besar Wildlife Reserve, 14 Dec. 2006, Miettinen 11184 (BO, H); Pekanbaru, Sukajadi, Filicum decipiens (?) 20 Jul. 2008, Miettinen 13162* (ANDA, H); C. nucifera, 20 Jul. 2008, Miettinen 13163* (ANDA, H); C. nucifera, 30 Nov. 2011, Miettinen 15089 (BO, H); Pometia pinnata, 16 Mar. 2020, Miettinen 23504 (BO, H); Jalan Sudirman, Arecaceae, 6 Jul. 2013, Miettinen 16598 (BO, H); Siak, Tasik Besar Wildlife Reserve, dicot, 10 Apr. 2002, Miettinen 5678* (BO, H); Yogyakarta, Sleman, Pekembinangun, Bambusoidae, 19 Dec. 2011, Miettinen 15222* (BO, H).
Notes: Fomitopsis luzonensis is the correct name for the Southeast Asian species formerly mislabelled as F. ostreiformis (Rungjindamai et al. 2009, Li et al. 2013, Ortiz-Santana et al. 2013). It is one of the most common brown rot fungi in insular Southeast Asia. The latter species represents the same taxon as F. cana (see F. osteiformis below). The two species are morphologically highly similar and they occur in the same habitats. Fomitopsis luzonensis has smaller pores and on average longer basidiospores than F. ostreiformis. Moreover, its basidiocarps are normally sessile-pileate (in contrast to the usually resupinate or effused-reflexed ones of F. ostreiformis), and they are sturdier and heavier than those of F. ostreiformis. Small-pored and hard basidiocarps differentiate F. luzonensis from the phylogenetically closely related F. marianii and F. nivosella (see Table 3).
Fomitopsis maculatissima (Lloyd) Spirin, comb. nov. MycoBank MB 844928.
Basionym: Polyporus maculatissimus Lloyd, Mycol. Writ. 7 (66): 1113. 1922.
Typus: Australia, Tasmania, [no collection date], Rodway (lectotype BPI 304927) (selected by Ryvarden 1990: 92).
Synonym: Neolentiporus maculatissimus (Lloyd) Rajchenberg, Nordic J. Bot. 15: 106. 1995.
Description: Rajchenberg (1995a, as Neolentiporus maculatissimus).
Notes: For phylogenetic placement, see Fig. 3 and Suppl. Figs S2 and S8.
Fomitopsis maculosa Miettinen & Spirin, sp. nov. MycoBank MB 844929. Figs 21, 23.
Fig 23.
Basidiospores of Fomitopsis spp. F. maculosa (holotype); F. marchionica (Miettinen 11454); F. marianii (Spirin 10503); F. meliae (Dollinger 989); F. modesta (Vlasák 1504/52); F. moritziana (Miettinen 11662); F. nivosella (3 left – O F10833, 3 right – holotype). Scale bar = 5 μm.
Typus: Indonesia, Bali, Bedugul, Mt. Tapak, Cagar Alam Batu Karu, -8.27669° 115.14604° (± 50 m), natural (primary) forest slope, plenty of Podocarpus, on a fallen branch (decay stage 2,5 cm in diameter), 25 Dec. 2007, Miettinen 12233.1* (holotype BO, isotype H7200142).
Etymology: Maculosus (Lat., adj.) – stained, spotted.
Description: Basidiocarps effused, first orbicular, gregarious, then fusing together and up to 3 cm in widest dimension. Margin adnate, compact, first violet, fading to cream-coloured, later ochraceous to brownish, up to 1 mm broad. Hymenial surface even or nodulose, violet, quickly fading to greyish to ochraceous-brownish; pores angular to lacerate, 3–5 per mm, dissepiments thin, uneven. Section: subiculum compact, cream-coloured, hardly visible, 0.05–0.1 mm thick; tubes soft leathery, concolourous with hymenial surface, 0.5–2 mm thick. Hyphal structure dimitic; hyphae clamped. Subiculum dimitic; skeletal hyphae hyaline to brownish, subparallel, occasionally branched, (3.7–)3.8–5.8(−6.2) μm diam (n = 20/1), lumen narrow to capillary, side branches 2.5–3.5 μm diam, generative hyphae hyaline, slightly thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae hyaline, loosely interwoven, brownish, sometimes covered by small needle-like crystals or cemented by brownish amorphous substance, occasionally branched, (3.0–)3.1–5.2(−5.8) μm diam (n = 40/2), lumen rather wide to capillary, side branches 2–3.5 μm diam, generative hyphae abundant, hyaline, slightly thick-walled, 3–5 μm diam. Subhymenium distinct, up to 25 μm thick. Cystidioles abundant, tapering to the apex, 13–19 × 3–5 μm. Basidia clavate, (15.2–)17.2–22.7(−28.0) × (5.8–)6.0–7.2(−7.3) μm (n = 20/2), in older hymenium often covered by amorphous brownish matter. Basidiospores with a distinct wall, broadly cylindrical to subfusiform, (6.2–)6.4–8.6(−8.7) × (2.7–)2.8–3.9(−4.1) μm (n = 60/2), L =7.41–7.49, W = 3.20–3.22, Q = 2.33–2.34, often with numerous oil droplets.
Specimen examined: Indonesia, Bali, Bedugul, Mt. Tapak, montain rainforest, fallen branch, 25 Dec. 2007, Miettinen 12230* (BO, H).
Notes: Fomitopsis maculosa is the closest relative of Antrodia tropica (= Fomitopsis tropica below) recently described from the southern part of China (Cui 2013). It differs from the latter in having rather abundant, brownish skeletal hyphae and wider basidiospores. Fomitopsis maculosa is so far only known from the type locality in Indonesia.
Fomitopsis madronae (Vlasák & Ryvarden) Vlasák & Ryvarden, comb. nov. MycoBank MB 844930.
Basionym: Antrodia madronae Vlasák & Ryvarden, Mycotaxon 119: 220. 2012.
Typus: USA, Oregon, Josephine Co., Oregon Caves, Arbutus menziesii, 14 Sep. 2007, Vlasák Jr. 0709/117-J* (holotype PRM 899296!, isotype H7200207).
Description and phylogenetic placement: Vlasák et al. (2012, as A. madronae); see also Figs 1, 3 and Suppl. Fig. S8.
Fomitopsis maire (G. Cunn.) P.K. Buchanan & Ryvarden, Mycotaxon 31: 15. 1988.
Basionym: Laricifomes maire G. Cunn., Bull. N.Z. Dept. Sci. Industr. Res. 164: 262. 1965.
Typus: New Zealand, Auckland, Waitakere Ranges, Anawhata, Nestegis cunninghamii, Aug. 1947, Dingley (holotype PDD38093, isotype H7004760!).
Description: Buchanan & Ryvarden (1988).
Notes: Morphologically, F. maire is very similar to F. sulcata from which it differs mainly in having considerably smaller pores (3–4 per mm in the type of F. maire vs. 0.3–1 per mm in F. sulcata). The phylogenetic position of F. maire can be established only after getting sequenced material from the type locality.
Fomitopsis malicola (Berk. & M.A. Curtis) Spirin, comb. nov. MycoBank MB 844931.
Basionym: Trametes malicola Berk. & M.A. Curtis, J. Acad. Nat. Sci. Philad. 3: 209. 1856.
Typus: USA, Pennsylvania, Bethlehem, Malus sp., [no collection date], Schweinitz 366 (lectotype K(M) 180488!) (selected by Ryvarden 1984: 347).
Synonym: Antrodia malicola (Berk. & M.A. Curtis) Donk, Persoonia 4: 339. 1966.
Description and phylogenetic data: Spirin et al. (2016, as Antrodia malicola).
Fomitopsis marchionica (Mont.) Spirin & Miettinen, comb. nov. MycoBank MB 844932. Fig 23.
Basionym: Trametes marchionica Mont., Voyage au Pôle Sud et dans l’Océanie, Botanique 1: 204. 1845.
Typus: Marquesas Islands, Nuku Hiva, 1841, Hombron 1 (holotype PC!).
Description: Basidiocarps perennial, sessile, often with a contracted base (fan-shaped), solitary or imbricate groups, sometimes fusing together, projecting up to 10 cm. Upper surface smooth, pinkish buff to brownish, covered by numerous darker zones. Pileal edge sharp, concolourous with hymenial surface or slightly darker, usually sterile, up to 2 mm wide. Pore surface first pink to pinkish grey, then fading to almost white, in senescent basidiocarps brownish, concave; pores roundish to angular, 5–7 per mm, with moderately thickened, entire dissepiments. Section: context corky, pink or pinkish brownish, in older basidiocarps brownish, up to 5 mm thick; tubes corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae yellowish to brownish, densely interwoven, richly branched, (3.3–)3.8–6.0(−6.2) μm diam (n = 40/2), lumen wide to capillary or indistinct, side branches 2–4 μm diam, generative hyphae rare, hyaline, slightly to distinctly thick-walled, 3.5–5 μm diam. Trama dimitic; skeletal hyphae dominating, yellowish to brownish, densely interwoven, occasionally branched, (2.8–)2.9–4.2(−4.3) μm diam (n = 20/2), lumen predominantly narrow to indistinct, generative hyphae rather rare, thin- to slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Cystidioles infrequent, tapering, 8–11 × 2.5–4 μm. Basidia short-clavate, (8.3–) 8.7–10.8(−12.0) × (4.2–)4.6–5.4(−5.8) μm (n = 20/1), in senescent hymenium partly glued together. Basidiospores with a distinct wall, cylindrical-subfusiform to narrowly ellipsoid, occasionally slightly curved, (3.1–)3.2–4.2(−4.3) × (1.8–)1.9–2.2(−2.3) μm (n = 30/1), L = 3.63, W = 2.08, Q = 1.75.
Specimen examined: Indonesia, Papua, Merauke, Wasur NP, dry natural forest, fallen dicot tree, 21 Jan. 2007, Miettinen 11454* (MAN, H).
Notes: To date, T. marchionica has been treated as a synonym of F. feei (Ryvarden 1982). The type material studied by us was compared with a newly collected and sequenced specimen from New Guinea, and no essential differences were detected. We therefore consider them conspecific. It is still possible, however, that the type specimen from Marquesas Islands and our New Guinean specimen belong to different species. Solving this problem is possible only after getting new material from the locus classicus. As defined here, F. marchionica differs from the similar-looking members of the F. feei complex in having robust but rather thin basidiocarps with a densely zonate upper surface (see also Table 3). Phylogenetically, it is not closely related to the F. feei group (Suppl. Fig. S2).
Fomitopsis marianii (Bres.) Spirin, Vlasák & Cartabia, comb. nov. MycoBank MB 844933. Figs 21, 23.
Basionym: Polyporus marianii Bres., Nuovo G. Bot. Ital. 7: 313. 1900.
Typus: Italy, Lazio, Latina, Terracina, Quercus cerris, [no collection date], Mariani (lectotype S F14590!) (selected by Ryvarden 1988a: 315).
Synonyms:
Poria incarnata Pers., Ann. Bot. 11: 30. 1794.
Typus: No locality indicated (probably Germany), [no collection date and collector], herb. Persoon (lectotype designated here L 0115607!, MycoBank MBT 10008290).
Trametes lignea Murrill, North American Flora 9: 44. 1907.
Typus: Nicaragua, [no locality indicated], on dead timber, Feb. 1891, C.L. Smith (holotype NY 00705034!).
Trametes subalutacea Bourdot & Galzin, Bull. Soc. Mycol. France 41: 165. 1925.
Typus: France, Aveyron, Saint-Sernin-sur-Rance, Alnus glutinosa, 8 Aug. 1905, Galzin 1229 (herb. Bourdot 3967) (lectotype selected here PC!, MycoBank MBT 10008291).
Leptoporus epileucinus Pilát, Acta Mus. Nat. Pragae 9 (B), 2 (1): 105. 1953.
Typus: Ukraine, Zakarpats’ka Reg., Kobylecká Polana, Fagus sylvatica, Jul. 1929, Pilát (holotype PRM 486790!).
Fomitopsis iberica Melo & Ryvarden, Bolm. Soc. Broteriana 62: 228. 1989.
Typus: Portugal, Ribatejo, Chamusca, Chouto, Quercus suber, 13 Nov. 1986, Melo, Correira & Cardoso 3141 (holotype LISU, isotype H!).
Antrodia bondartsevae Spirin, Mikol. Fitopatol. 36: 33. 2002.
Typus: Russia, Nizhny Novgorod Reg., Sharanga Dist., Kilemary, Tilia cordata, 24 Aug. 2000, Spirin* (holotype LE 209783!).
Antrodia wangii Y.C. Dai & H.S. Yuan, Mycosystema 25: 372. 2006.
Typus: China, Beijing, Xianshan, Prunus sp., 25 Jul. 2005, Dai 6613* (isotype H!).
Fomitopsis caribensis B.K. Cui & Shun Liu, Mycol. Progr. 18: 1322. 2019.
Typus: Puerto Rico, Rio Abajo, hardwood, 16 Jul. 2018, Cui 16871* (holotype BJFC).
Fomitopsis ginkgonis B.K. Cui & Shun Liu, Mycol. Progr. 18: 1325. 2019.
Typus: China, Hubei, Huanggang, Yiaihu, Ginkgo biloba, 20 Oct. 2018, Cui 17170* (holotype BJFC).
Description: Basidiocarps annual, sessile or effused-reflexed, usually fusing together in large groups, projecting up to 7 cm, rarely totally resupinate, up to 8 cm in widest dimension. Upper surface first cream coloured to pale ochraceous, azonate, finely velutinous, later greyish-ochraceous to brownish, smooth or finely striate, often with a few indistinct brownish zones. Pileal edge sharp, concolourous with cap surface, fertile, sometimes incurving during drying. Pore surface cream-coloured or pale ochraceous, sometimes with brownish stains, as a rule concave; pores angular to lacerate, 4–6 per mm, with thin, serrate or dentate dissepiments. Section: context soft, white to cream-coloured, up to 25 mm thick; tubes soft, easily cut by a razor blade, one-layered, concolourous with hymenial surface, up to 10 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline, densely interwoven or in subparallel bundles, richly branched, (3.0–)3.1–7.3(−7.8) μm diam (n = 120/7), lumen narrow to almost invisible, side branches 2–3 μm diam, generative hyphae abundant to rare, hyaline, thin- or moderately thick-walled, 2–4.5 (−5) μm diam. Trama dimitic; skeletal hyphae hyaline, interwoven, some flexuous, occasionally branched, (2.0–)2.2–4.8(−4.9) μm diam (n = 190/9), lumen wide to capillary, generative hyphae thin- or slightly thick-walled, 2–4 μm diam, in some parts dominating and producing distinct subhymenial layer up to 35–50 μm thick, dissepiment edges monomitic. Small angular or rhomboid crystals abundant among tramal tissues, up to 5–7 μm in longest dimension. Cystidioles frequent, tapering, 12–21 × 3–6 μm. Basidia clavate, often with a tapering basal part, (12.0–)12.4–21.3(−25.2) × (4.4–)4.7–7.0(−7.2) μm (n = 51/5), sometimes pleural. Basidiospores thin-walled, cylindrical to fusiform, longest spores sigmoid, (5.0–)5.1–8.7(−9.4) × 2.0–3.2(−3.3) μm (n = 331/11), L = 5.81–7.11, W = 2.32–2.90, Q = 2.31–2.84.
Specimens examined: Croatia, Primorje-Gorski Kotar, Rab, Pinus sp., 23 Sep. 1994, Varjů (MJ4158*). Czech Republic, Brno, Lanžhot, Ranšpurk, Fraxinus angustifolia, 19 Jun. 1999, Vampola (MJ4606*); Zlín, Buchlovice, Holý Kopec, Fagus sylvatica, 2 Aug. 2010, Běták 10/743* (JV). France, Aveyron, St. Sernin, Alnus glutinosa, 12 Aug. 1906, Galzin 1753 (herb. Bourdot 5999) (PC); Salles-la-Source, manufactured wood, 24 Oct. 2016, Rivoire (LY-BR 6563*). Italy, Lombardy, Varese, Via Tasso, Cedrus deodara, 8 Jun. 2019, Cartabia 104* (H); Villa Toeplitz, F. sylvatica, 3 Jul. 2019, Cartabia 129 (H); Villa Mylius, C. deodara, 23 May 2020, Cartabia (H); Inarzo, Betula pendula, 10 Jun. 2019, Cartabia 105 (H); Viggiù, C. deodara, 23 Jun. 2020, Cartabia (H). Russia, Khabarovsk Reg., Khabarovsk Dist., Ilga, Tilia amurensis, 15 Aug. 2012, Spirin 5302* (H); Pravaya Ilga, T. amurensis, 11 Aug. 2012, Spirin 5175* & 5176* (H); Nizhny Novgorod Reg., Bogorodsk Dist., Podvyazie, T. cordata, 30 Jul. 2005, Spirin 2306 (H); Lukoyanov Dist., Panzelka, T. cordata, 9 Aug. 2016, Spirin 10575* (H); Razino, T. cordata, 15 Aug. 2015, Spirin 9267* (H); ibid., 7 Aug. 2016, Spirin 10503* (H); ibid., 29 Jul. 2017, Spirin 11249* (H); B. pendula, 31 Jul. 2017, Spirin 11281 (H). USA, Louisiana, Baton Rouge, Louisiana State University Campus, Ligustrum sp., 6 Jul. 1986, Gilbertson 14757* (O 16323). Slovakia, Trnava, Lakšárska Nová Ves, Pinus sylvestris, 18 Jun. 1994, Kukulka (MJ4157*). Slovenia, Ljubljana, Mala ulica, angiosperm stump, 31 Jul. 2020, Spirin 14034 (H, LJF).
Notes: Fomitopsis marianii is a macroscopically extremely flexible species, with basidiocarps varying from robust pileate to effused-reflexed with thin flabelliform caps or even totally resupinate. This is the reason why the species was described several times in different genera. The oldest available name for it is Poria incarnata. Although sterile, the single authentic specimen designated here as a lectotype of P. incarnata is certainly conspecific with F. marianii. Ryvarden (1991) selected another specimen as the lectotype of P. incarnata. That one was collected by Lindblad in Sweden and it is conspecific with Steccherinum collabens (= Junghuhnia collabens). However, this solution cannot be accepted for two reasons. First, Lindblad’s specimen was evidently collected after Fries compiled his sanctioning treatment. Therefore, it could serve only as a neotype, not as a lectotype. However, neotype selection is precluded if the authentic material suitable for lectotypification is still available (Code Art. 9.13). Second, if accepted, the typification of P. incarnata with Lindblad’s gathering will necessitate a name change for S. collabens. This would be highly undesirable because the latter one is an important indicator species of old-growth spruce-dominated forests in Europe, and it is well known not only to professional mycologists but also to amateurs. Nevertheless, P. incarnata cannot be combined in Fomitopsis because of the presence of Fomitopsis incarnatus (see synonymy under F. ussuriensis). Polyporus marianii is next in line and it is combined here in Fomitopsis. We also studied the type material of Fomitopsis epileucina and F. iberica treated as two independent species by Ryvarden & Gilbertson (1993) and in other subsequent manuals. We concluded they represent the same species and are conspecific with F. marianii.
Fomitopsis marianii is widely distributed in temperate forests of Eurasia, and it seems to be not rare in temperate – subtropical areas of the United States. In North America, it was often mixed up with other similar-looking species, in particular, with F. palustris and F. nivosella (= F. durescens, see below). The first species produces rather small, sessile basidiocarps with somewhat smaller pores, and it is restricted to Pinus spp. in warm temperate – subtropical areas of North America. Moreover, it has on average larger basidia and basidiospores than F. marianii. The only available genome of F. palustris (ATCC 62978) in fact belongs to F. marianii. In turn, F. nivosella can be differentiated from F. marianii due to slightly smaller and more regular pores with mostly entire orifices, as well as more abundant tramal skeletal hyphae reaching dissepiment edges which are monomitic in F. marianii. The name Fomitopsis nivosa was misapplied in North and South America either to F. marianii or to F. nivosella. Liu et al. (2019) introduced two new species, F. caribensis and F. ginkgonis. ITS and partial TEF1 sequences of these species submitted to GenBank show no differences from F. marianii, and no unique morphological traits are mentioned in their protologues. Therefore, we treat them as later synonyms of F. marianii.
Fomitopsis meliae (Underw.) Gilb., Mycotaxon 12 (2): 385. 1981. Fig 23.
Basionym: Polyporus meliae Underw., Bull. Torrey Bot. Club 24: 85. 1897.
Typus: USA, Alabama, Auburn, Melia azedarach, 10 Oct. 1895, Underwood (holotype NY 00730808!).
Synonyms:
Trametes submurina Murrill, North American Flora 9: 43. 1907.
Typus: Jamaica, Kingston, Hope Gardens, on old log, 16 Nov. 1902, Earle 483 (holotype NY 00705075!).
Trametes subnivosa Murrill, North American Flora 9: 43. 1907.
Typus: USA, Louisiana, [no locality], Jan. 1887, Langlois (holotype NY 00705078!).
Description: Basidiocarps annual, sessile or effused-reflexed, often fusing together in large imbricate groups, projecting up to 4 cm, very rarely resupinate. Upper surface first cream-coloured to greyish, azonate, felty, later mouse-grey, occasionally with ochraceous or brownish tints, smooth or indistinctly wrinkled, rarely indistinctly zonate. Pileal edge sharp to rather blunt, concolourous with pore surface, up to 1 mm wide, sterile. Pore surface cream-coloured or pale ochraceous, then greyish, sometimes with brownish flecks, as a rule concave; pores roundish to angular, 4–7 per mm, with thin, entire or serrate dissepiments. Section: context soft corky, cream-coloured to greyish, up to 8 mm thick; tubes rather soft, easily cut by a razor blade, one-layered, concolourous with hymenial surface, up to 6 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline, rarely greyish or brownish, mostly loosely interwoven, occasionally branched, (3.0–)3.3–7.7(−8.2) μm diam (n = 120/6), with a variably pronounced lumen, side branches 1.5–3 μm diam, generative hyphae thin- to moderately thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae hyaline or greyish, loosely interwoven, some flexuous, occasionally branched, (2.7–)2.8–4.9(−5.0) μm diam (n = 140/7), swelling in KOH and CB, lumen rather wide to capillary, side branches 1.5–2.5 μm diam, generative hyphae thin- or slightly thick-walled, 2–4 μm diam, sometimes producing distinct subhymenial layer up to 35–40 μm thick. Rhomboid or cubical crystals occasionally present among context and tramal tissue, solitary or in large groups, up to 13 μm in widest dimension. Cystidioles infrequent, tapering, 12–21 × 3.5–6.0 μm. Basidia clavate, often with a tapering basal part, (10.2–)11.1–17.6(−18.0) × (4.6–)4.7–6.1(−6.2) μm (n = 40/5). Basidiospores thin-walled, cylindrical to fusiform, longest spores sigmoid, (4.1–) 4.2–7.2(−7.4) × (2.0–)2.1–2.9(−3.0) μm (n = 180/6), L = 4.87–6.33, W = 2.23–2.55, Q = 2.19–2.50.
Specimens examined: Colombia, Chocó, Turbo, 1 Jul. 1978, Ryvarden 16893 (O 10826). Costa Rica, Puntarenas, Golfito, 20 Apr. 2017, Vlasák 1704/78-J* (JV, TUF); Playa Nicuesa, 19 Apr. 2017, Vlasák 1704/61* (JV, TUF); San José, Cerro Vueltas, 18 Jul. 2001, Ryvarden 43883 (O 18733). French Guiana, Remire-Montjoly, Lac du Rorota, 23 Aug. 2018, Vlasák 1808/33* (JV); Roura, Sentier Molokoï, 26 Aug. 2018, Vlasák 1808/81* & 1808/82* (JV). Martinique, Le Vauclin, Grand Macabou, 25 Dec. 2017, Vlasák 1712/27* (JV). Peru, Alto Amazonas, Yurimaguas, decaying trunk, 30 Jun. 1984, Hormia 2109* (H). Puerto Rico, Luquillo, Luquillo Beach, C. nucifera, 12 Jan. 2014, Miettinen 18134 (H). USA, Florida, Miami – Dade Co., Miami Beach, 14 Oct. 2016, Dollinger 989* & 991* (JV, H); Texas, Austin, Eanes Cree, felled tree, 15 Aug. 2013, Miettinen 16679 (H); Victoria Co., Coleto Creek, Prunus sp., 26 Sep. 2011, Vlasák Jr. 1109/40-J* (JV, H). US Virgin Islands, Saint John, 28 Nov. 2015, Vlasák Jr. 1511/24-J* (JV).
Notes: Rather small, greyish, imbricate or effused-reflexed basidiocarps, often fusing together in large groups help in macroscopic identification of F. meliae. The species is widely distributed in the warm-temperate – tropical areas of North America, with a few verified records from the north-eastern part of South America. Microscopically, it is distinguishable from the similarly looking species occurring in the same geographic region (i.e. F. marianii, F. nivosella and F. palustris) primarily due to greyish skeletal hyphae and rather short basidiospores.
The taxonomic status of F. meliae from the neotroics vs. F. ostreiformis from the paleotropics deserves further studies. These species did not resolve as two distinct clades in our phylogenetic analyses. The main reason seems to be an unusually high variability of ITS sequences (Fig. 8, and, to a lesser extent, TEF1 region, Suppl. Fig. S4) in F. meliae blurring its differences from F. ostreiformis. Nevertheless, we keep them as two separate species because of their distinct distribution areas and small but constant morphological differences. Although being rather small-sized, F. meliae produces more robust and thicker (up to 1.5 cm thick) basidiocarps than F. ostreiformis, and they are normally pileate. In F. ostreiformis, basidiocarps are thinner (up to 0.6 cm thick but usually less) and they often show a strong tendency to effused growth. Moreover, the grey colouration of basidiocarps (especially tubes) is much more pronounced in F. ostreiformis, often approaching the colours of Bjerkandera adusta. Greyish tints are often present in F. meliae, too, but they are detectable mainly in the pileal surface. Microscopically, the two species are rather similar, although skeletal hyphae in F. meliae are hyaline or greyish, in contrast to the normally brownish skeletals of F. ostreiformis. Furthermore, cystidioles of F. meliae have sturdy walls while they are often collapsing at the very apex in F. ostreiformis (this feature is still observable in the type material of the latter species although it is over 150 years old).
Fomitopsis mellita (Niemelä & Penttilä) Niemelä & Miettinen, comb. nov. MycoBank MB 844934.
Basionym: Antrodia mellita Niemelä & Penttilä, Ann. Bot. Fenn. 29: 56. 1992.
Typus: Finland, Pohjois-Karjala, Lieksa, Patvinsuo Nat. Park, Populus tremula, 21 Sep. 1989, Penttilä 1350 (holotype H!).
Description and phylogenetic placement: Niemelä & Penttilä (1992) and Spirin et al. 2013a (as A. mellita); see also Fig. 3.
Fomitopsis microcarpa (B.K. Cui & Shun Liu) Spirin, comb. nov. MycoBank MB 848361.
Basionym: Pseudofomitopsis microcarpa B.K. Cui & Shun Liu, Fungal Diversity 118: 58. 2022.
Typus: Vietnam, Dong Nai, Thac Mai, Tan Phu Forest Enterprise, angiosperm, 14 Oct. 2017, Cui 16404* (holotype BJFC).
Description and phylogenetic placement: Liu et al. (2022, as P. microcarpa); see also Fig. 3.
Fomitopsis micropora (B.K. Cui & Shun Liu) Spirin, comb. nov. MycoBank MB 848362.
Basionym: Daedalella micropora B.K. Cui & Shun Liu, Fungal Diversity 118: 50. 2022.
Typus: Malaysia, Selangor, Taman Botani Negara Shah Alam, angiosperm, 12 Apr. 2018, Dai 18509* (holotype BJFC).
Description and phylogenetic placement: Liu et al. (2022, as D. micropora); see also Fig. 3.
Fomitopsis minutispora Rajchenb., Mycotaxon 54: 441. 1995.
Typus: Argentina, Neuquén, Lago Queñi, Nothofagus sp., 27 Apr. 1994, Rajchenberg 10851 (holotype BAFC 33360).
Description: Rajchenberg (1995b).
Notes: We used the sequences of Han et al. (2016); for phylogenetic placement, see Fig. 3 and Suppl. Fig. S2.
Fomitopsis minutula Spirin, nom. nov. MycoBank MB 844935. Synonym: Antrodia minuta Spirin, Mycotaxon 101: 150. 2007.
Typus: Russia, Nizhny Novgorod Reg., Lukoyanov Dist., Razino, Populus tremula, 24 Sep. 2000, Spirin 1725 (holotype H!).
Description and phylogenetic data: Spirin et al. (2016, as A. minuta); see also Fig. 3.
Note: The new species epithet is introduced here to avoid homonymy with Fomitopsis minuta Aime & Ryvarden (see under Excluded taxa).
Fomitopsis modesta (Kuntze ex Fr.) Vlasák & Spirin, comb. nov. MycoBank MB 844936. Figs 23, 24.
Fig 24.
Basidiocarps of Fomitopsis spp. A. F. modesta (Vlasák 1808/87). B. F. moritziana (Miettinen 11662). C. F. ochracea (Miettinen 18568). D. F. ostreiformis (Miettinen 23532). E. F. perhiemata (holotype). F. F. pinicola (Niemelä 9424). G. F. pseudopetchii (Miettinen 14284). H. F. pulvina (Niemelä 9281).
Basionym: Polyporus modestus Kuntze ex Fr., Linnaea 5: 519. 1830.
Typus: Surinam, [no locality], ‘in truncis, Weigelt’, [no collection date and collector] (holotype UPS F-174971!).
Synonyms:
Polyporus monochrous Mont., Ann. Sci. Nat., Bot. 2 (15): 109. 1841.
Typus: French Guiana, [no locality], 1838, Leprieur 536 (lectotype PC!) (selected by Ryvarden 1982: 79).
Polyporus albocervinus Berk., Hooker’s J. Bot. 8: 234. 1856.
Typus: Brazil, Panurè, dead trunks, Spruce 22 (holotype K, isotype PC!).
Daedalea rajchenbergiana Kossmann & Drechsler-Santos, Lilloa 59: 283. 2022.
Typus: Brazil, Bahia, Uruçuca, Parque Estadual da Serra do Condurú, 28 Nov. 2012, Drechsler-Santos 863* (holotype FLOR 70928).
Description: Basidiocarps short-living perennial, sessile, partly fusing together, often with a contracted base (fan-shaped), projecting up to 6 cm. Upper surface first cream coloured to beige, indistinctly zonate, later pinkish brownish, sometimes with numerous narrow concentric zones. Pileal edge sharp, concolourous with cap surface, first sterile, up to 2 mm wide, then fertile. Pore surface cream-coloured or beige to pale ochraceous, sometimes with a faint pinkish tint or vinaceous-reddish stains, flat or concave; pores roundish to angular, 6–8 per mm, with thin, entire dissepiments. Section: context corky, cream- to wood-coloured, normally less than 2 mm thick; tubes corky, one-layered, concolourous with hymenial surface, up to 2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline to brownish, densely interwoven or in subparallel bundles, occasionally branched, (3.0–)3.2–5.8(−6.0) μm diam (n = 20/1), lumen changing from wide to narrow, side branches 2–3 μm diam, generative hyphae infrequent, hyaline, slightly to distinctly thick-walled, 2.5–4 μm diam. Trama dimitic; skeletal hyphae dominating, pale ochraceous to brownish, densely interwoven, occasionally branched, (2.0–)2.1–3.6(−3.8) μm diam (n = 80/4), lumen mostly capillary, generative hyphae rather rare, thin- to slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Cystidioles abundant to rather rare, tapering, 10–14 × 3–6 μm. Basidia clavate, (9.8–)10.1–17.3(−17.8) × (4.2–)4.4–5.8(−6.0) μm (n = 38/4), occasionally pleural. Basidiospores with a distinct wall, broadly cylindrical to ellipsoid, longest spores subfusiform, (2.9–) 3.1–5.1(−5.2) × (2.0–)2.1–3.1(−3.2) μm (n = 120/4), L = 3.51–4.36, W = 2.23–2.69, Q = 1.57–1.86.
Specimens examined: Brazil, Bahia, Ituberá, hardwood, 2 Mar. 2006, Oinonen 60302003 (H). Costa Rica, Guanacaste, Rincón de la Vieja, hardwood, 28 Jan. 1997, Lindblad 2608A (O 19047); Villa La Paz, 21 Apr. 2015, Vlasák 1504/52* (JV, TUF); Limón, Lomas Bardubal Park, 30 Jul. 2014, Vlasák 1407/90* (JV, H); Puntarenas, Tarcoles, Carara Nat. Park, 18 Apr. 2015, Vlasák 1504/21* (JV, TUF). French Guiana, Montsinéry-Tonnegrande, Patawa, 31 Aug. 2018, Vlasák 1808/143 (JV, H); Remire-Montjoly: Rorota, wooden bridge, 24 Aug. 2018, Vlasák 1808/36 (JV, H); Roura, Amazon Lodge, boardwalk, 25 Aug. 2018, Vlasák 1808/58 (JV, H); Sentier Molokoï, Aug. 2018, Vlasák 1808/87* (JV, H). Guyana, Potaro-Siparuni, Paramakatoi, fallen log, 24 Feb. 1996, Ahti 53384 (H).
Notes: Here we re-introduce the species based on studies of type material and newly collected and sequenced specimens from the area in which it was originally found. Fomitopsis modesta is distributed in the eastern and southern parts of the Caribbean, as well as in South America. Northwards, it seems to be replaced by F. subectypa (see below). Sequences of F. modesta published by Cristaldo et al. (2022) indicate that they belong to another, probably yet unnamed neotropical species. See further remarks under F. atypa.
Fomitopsis monomitica (Yuan Y. Chen) Spirin & Viner, comb. nov. MycoBank MB 844937.
Basionym: Antrodia monomitica Yuan Y. Chen, Mycosphere 8 (7): 882. 2017.
Typus: China, Heilongjiang, Harbin, Morus sp., 6 Aug. 2016, Dai 16894* (holotype BJFC 22529).
Description: Chen & Wu (2017, as A. monomitica).
Specimen examined: Russia, Primorie, Khasan Dist., hardwood log, 25 Jul. 2016, Viner KUN2550* (H).
Note: See remarks to F. oleracea.
Fomitopsis morganii (Lloyd) Spirin & Vlasák, comb. nov. MycoBank MB 844938.
Basionym: Trametes morganii Lloyd, Mycol. Writ. 5 (Letter 69): 15. 1919.
Typus: USA, Ohio, Preston, [no collection date], Morgan (Lloyd Herb. #53852) (lectotype BPI 320499!) (selected by Stevenson & Cash 1936: 145).
Description and phylogenetic data: Spirin et al. (2017, as Antrodia morganii).
Fomitopsis moritziana (Lév.) Spirin & Miettinen, comb. nov. MycoBank MB 848363. Figs 23, 24.
Basionym: Polyporus moritzianus Lév., Ann. Sci. Nat., Bot. 3 (5): 130. 1846.
Typus: Indonesia, Java, ‘ad truncos’, [no collection date], Zollinger 2061 (syntype PC!).
Synonyms:
Trametes pseudodochmia Corner, Beih. Nova Hedwigia 97: 138. 1989.
Typus: Malaysia, Sabah, Kinabalu, on a living tree, 14 Jun. 1961, Corner (holotype E00430837).
Trametes fulvirubida Corner, Beih. Nova Hedwigia 97: 104. 1989.
Typus: Malaysia, Johore, Guning Panti, 31 Jan. 1930, Corner (holotype E00218200).
Description: Basidiocarps perennial, sessile, at first conchate, later ungulate, broadly attached, projecting up to 12 cm. Upper surface initially reddish brownish or greyish-brown, smooth, later almost black or fading to brownish grey, with distinct annual zones, occasionally cracking. Pileal edge sharp to rather blunt, concolourous with pore surface, up to 3 mm wide, sterile. Pore surface wood-coloured to brownish-grey, flat or slightly concave; pores angular, (2–)3–4 per mm, with rather thin or thick, entire dissepiments. Section: crust (present in old basidiocarps) exceptionally tough, greyish black, glancing or matt, up to 2 mm thick, context hard corky, brownish to reddish-brown, up to 3 mm thick, sharply delimited from tubes; tubes tough, clearly stratified, wood-coloured, brownish-grey or brown, in total up to 7.5 cm thick, each annual layer 2–6 mm thick, sterile tissue between tube layers absent. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, brown, tightly interwoven, occasionally branched, (3.1–)3.2–5.9(−6.2) μm diam (n = 40/2), lumen capillary to indistinct, generative hyphae very rare, hyaline, slightly or distinctly thick-walled, 1.5–2.5 μm diam. Trama dimitic; skeletal hyphae brownish to reddish-brown, tightly interwoven, occasionally branched, sometimes twisted, (2.9–)3.1–4.1(−4.2) μm diam (n = 20/1), lumen capillary to indistinct, generative hyphae hyaline, thin-walled, infrequent, 1.5–3 μm diam. Subhymenium indistinct. Skeletocystidia present as sharp or blunt (3–4 μm diam) apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles not seen; hyphidia present, simple or bi-trifurcate, 1.5–2 μm diam at the apex. Basidia clavate, pedunculate or occasionally pleural, 12–15 × 5–5.8 μm, rarely observed, scattered among hyphidia and skeletocystidia. Basidiospores with a distinct wall, broadly cylindrical, usually slightly concave at the ventral side, (3.8–)3.9–4.8(−5.0) × 2.0–2.3(−2.4) μm (n = 30/1), L = 4.27, W = 2.15, Q = 1.98.
Specimens examined: Indonesia, Riau, Indragiri Hulu, Bukit Tigapuluh NP, primary rainforest, fallen dicot tree, 22 Mar. 2002, Miettinen 4873 (BO, H). Malaysia, Penang, Pulau Pinang, Batu Feringgi, rainforest, on a dicot snag, 11 Feb. 2007, Miettinen 11662* (BORH, H).
Notes: Fomitopsis moritziana is a robust, perennial polypore developing a dark-coloured crust and having rather large pores. It is distributed in Southeast Asia (Indonesia, Malaysia) and is evidently rare. We were able to generate a single ITS sequence from one collection of F. moritziana (Miettinen 11662) which shows no close matches in the genus (Suppl. Fig. S2, as D. pseudodochmia). Based on morphological and DNA evidence, we concluded the earlier assumed conspecifity of F. moritziana (as T. pseudodochmia) and F. incana cannot be accepted (see notes to F. incana). Trametes fulvirubida evidently represents a juvenile form of F. moritziana and is placed in the synonymy of this species.
Fomitopsis neotropica (D.L. Lindner, Ryvarden & T.J. Baroni) Vlasák, comb. nov. MycoBank MB 844939.
Basionym: Daedalea neotropica D.L. Lindner, Ryvarden & T.J. Baroni, North American Fungi 6: 6. 2011.
Typus: Belize, Toledo, Cockscomb Ridge, Doyle’s Delight, Quercus sp., 12 Aug. 2004, Lindner 04-74* (holotype CFMR).
Description and phylogenetic data: Lindner et al. (2011, as D. neotropica); see also Fig. 5.
Fomitopsis nigra (Berk.) Spirin & Miettinen, comb. nov. MycoBank MB 844940.
Basionym: Polyporus niger Berk., London J. Bot. 4: 304. 1845.
Typus: USA, Ohio, ‘March, on rotten trunk’, [no collection date], Lea 112 (holotype K, isotype PC!).
Synonym: Melanoporia nigra (Berk.) Murrill, North American Flora 9: 15. 1907.
Description: Gilbertson & Ryvarden (1987, as M. nigra).
Specimen examined: USA, Pennsylvania, Montgomery Co., Schwenksville, Quercus sp., Oct 2014, Vlasák 1410/10J* (JV).
Note: This species is the generic type of Melanoporia and closely related to other species, M. castanea and M. condensa, formerly included in that genus (see Figs 1, 3).
Fomitopsis niveomarginata L.W. Zhou & Y.L. Wei, Mycol. Progr. 11: 437. 2012.
Typus: China, Jilin, Antu, Changbai, Tilia sp., 14 Sep. 2007, Dai 9175* (holotype IFP 15643).
Description and phylogenetic data: Zhou & Wei (2012); see also Fig. 3 and Suppl. Fig. S2.
Fomitopsis nivosella (Murrill) Spirin & Vlasák, comb. nov. MycoBank MB 844941. Fig 23.
Basionym: Tyromyces nivosellus Murrill, North American Flora 9: 32. 1907.
Typus: Cuba, Guantánamo, Baracoa, El Yunque, Roystonea regia, Mar. 1903, Underwood & Earle 1114 (holotype NY00776435!).
Synonyms:
Tyromyces palmarum Murrill, North American Flora 9: 32. 1907.
Typus: Cuba, Guantánamo: Baracoa, El Yunque, Roystonea regia, Mar. 1903, Underwood & Earle 1142 (holotype NY00776434!).
Polyporus durescens Overh. ex J. Lowe, Mycotaxon 2 (1): 65. 1975.
Typus: USA, Ohio, Preble Co., West Elkton, Fagus sp., 28 Jul. 1917, Overholts 4215* (holotype BPI, isotype PRM 807350!).
Description: Basidiocarps annual, sessile or effused-reflexed, occasionally fusing together in large groups, projecting up to 7 cm. Upper surface first cream coloured, azonate, smooth, later pale ochraceous, sometimes with greyish tints, smooth or irregularly wrinkled. Pileal edge sharp to rather blunt, concolourous with cap surface, occasionally with brownish stains, fertile, sometimes incurving during drying. Pore surface cream-coloured or pale ochraceous, in older basidiocarps often reddish brownish, as a rule concave; pores roundish to angular, very rarely sinuous, (4–)5–6(−7) per mm, with thin, even or only rarely serrate dissepiments. Section: context rather soft, white to cream-coloured, up to 20 mm thick; tubes soft, easily cut by a razor blade, one-layered, concolourous with hymenial surface, up to 10 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline or pale yellowish, densely interwoven or in subparallel bundles, occasionally branched, (2.6–)2.8–5.4(−6.2) μm diam (n = 100/5), lumen wide to almost invisible, side branches 1.5–3 μm diam, generative hyphae abundant to rare, hyaline, thin- or slightly thick-walled, 2–4.5 μm diam. Trama dimitic; skeletal hyphae hyaline, interwoven, occasionally branched, (2.0–)2.2–4.0(−4.2) μm diam (n = 120/6), lumen rather wide to capillary or indistinct, generative hyphae thin- or slightly thick-walled, 2–3.5 μm diam, in some parts dominating and producing distinct subhymenial layer up to 20–35 μm thick, dissepiment edges dimitic, consisting of thin-walled, flexuose generative hyphae and winding skeletals with a wide lumen. Cystidioles rare, tapering, 9–18 × 4–5 μm. Basidia clavate, often with a tapering basal part, (10.2–)10.9–17.0(−19.8) × (4.1–)4.2–6.8(−7.3) μm (n = 55/5), sometimes pleural. Basidiospores thin-walled, cylindrical to fusiform, longest spores sigmoid, (4.8–)5.0–8.0(−8.3) × 1.8–3.0(−3.1) μm (n = 160/6), L = 5.89–6.91, W = 1.96–2.65, Q = 2.49–3.02.
Specimens examined: Brazil, Amazonas, Manaus, Nov. 1983, de Jesus* (O F10833, SP 193509); Paraná, Antonina, Cacatu, Syagrus romanzoffiana, 12 Oct. 1997, de Meijer 3465* (O 16014). Puerto Rico, San Juan, Pinetas, palm tree, Jul. 2018, Kout 1807/19* (H). Venezuela, Aragua, Rancho Grande Res. Station, 14 Apr. 1999, Ryvarden 41410* (O 10796).
Notes: Fomitopsis nivosella is distributed in North and South America although it is evidently rarer than its look-alike, F. marianii. Morphological differences between the two species are discussed under F. marianii.
Fomitopsis oboensis (Decock, Amalfi & Ryvarden) Spirin, comb. nov. MycoBank MB 844942.
Basionym: Niveoporofomes oboensis Decock, Amalfi & Ryvarden, Mycol. Progr. 21 (2, no. 29): 7. 2022.
Typus: São Tomé and Príncipe, São Tomé, Ôbo de São Tomé National Park, Olea capensis, 12 Apr. 2011, Decock ST-11-04* (holotype O).
Description and phylogenetic data: Decock et al. (2022, as N. oboensis).
Fomitopsis ochracea Ryvarden & Stokland, Syn. Fung. 25: 46. 2008. Fig 24.
Typus: Canada, Alberta, Slave Lake, Populus tremuloides, 8 Jun. 2005, Stockland 223* (holotype O).
Description and phylogenetic data: Haight et al. (2019); see also Fig. 10 and Suppl. Fig. S5.
Specimens examined: Canada, Alberta, Yellowhead Co., William A. Switzer Provincial Park, P. tremuloides, 24 Jul. 2015, Spirin 8847* (H). USA, Idaho, Boundary Co., Upper Priest River, Tsuga heterophylla, 16 Oct. 2014, Miettinen 18865 (H); Washington, Clallam Co., Willoughby Creek, Picea sitchensis, 7 Oct. 2014, Spirin 8165* (H); Jefferson Co., Hoh River, P. sitchensis, 20 Oct. 2014, Miettinen 18984.1 (H).
Notes: This species belongs to the F. pinicola complex. Minor genetic differences, mating tests and a few morphological (mainly macroscopic) traits support its recognition as a separate species. See discussion under F. pinicola for more details.
Fomitopsis oleracea (R.W. Davidson & Lombard) Spirin & Vlasák, comb. nov. MycoBank MB 844943. Fig 16.
Basionym: Poria oleracea R.W. Davidson & Lombard, Mycologia 39: 317. 1947.
Typus: USA, Michigan, Detroit, unidentified wood, 15 Jul. 1942, Hirt* (holotype BPI 241465).
Synonym: Antrodia oleracea (R.W. Davidson & Lombard) Ryvarden, A preliminary polypore flora of East Africa: 252. 1980.
Description: Basidiocarps annual, effused, up to 10 cm in widest dimension. Margin adnate, compact, white to cream-coloured, up to 1 mm wide. Hymenial surface even or somewhat nodulose, cream-coloured to pale-ochraceous or greyish; pores angular, 4–6 per mm, dissepiments uneven to serrate. Section: subiculum compact, white, crumbling, up to 0.5 mm thick; tubes soft, fragile, easily crumbling in dry condition, cream-coloured to greyish or pale ochraceous, 2–5 mm thick. Hyphal structure monomitic; hyphae clamped. Subicular hyphae hyaline, thin- to moderately thick-walled, interwoven to subparallel, (2.4–)2.9–5.1(−5.2) μm (n = 20/1). Tramal hyphae hyaline, thin-walled, spaced, subparallel, (2.7–)2.8–4.1(−4.2) μm (n = 40/2), occasionally anastomosing. Subhymenium distinct, up to 20 μm thick. Cystidioles rather abundant to rare, tapering to hyphoid, (12.1–)12.2–18.1(−18.3) × (3.0–)3.3–5.0(−5.2) μm (n = 16/2). Basidia clavate, occasionally pleural, (11.4–)13.0–19.4(−20.1) × (5.0–)5.1–6.8(−7.2) μm (n = 20/2), in senescent hymenium with slightly thickened walls. Basidiospores thin-walled or with a distinct wall, cylindrical-subfusiform, (5.0–)5.1–8.4(−8.8) × (2.2–)2.3–3.1(−3.2) μm (n = 60/2), L = 6.11–6.47, W = 2.79–2.82, Q = 2.18–2.32.
Specimens examined: USA, Florida, Alachua Co., San Felasco Hammock Preserve, Quercus sp., 24 Nov. 2013, Miettinen 17902 (H); Gainesville, Quercus sp., 11 Aug. 1950, Lowe 4335 (H ex SYRF); Pennsylvania, Montgomery Co., Schwenksville, Goshenhoppen Creek, Quercus sp., Sep. 2010, Vlasák 1009/31* (JV, H).
Notes: Lowe (1966) and Gilbertson & Ryvarden (1986) described F. oleracea as dimitic. A culture obtained from the type specimen was sequenced (Vu et al. 2019) and the resulting ITS sequence is identical with that obtained from the specimen we studied (Vlasák 1009/31 from Pennsylvania, USA). This specimen is completely monomitic, as are two other collections from Florida we examined (Miettinen 17902 and Lowe 4335). Gilbertson and Ryvarden clearly used material of some other species when describing A. oleracea, possibly Fomitopsis dollingeri. Fomitopsis oleracea is phylogenetically closely related to F. monomitica from East Asia. Morphological differences between these species are subtle: F. oleracea has on average smaller pores (3–4 per mm in F. monomitica) and slightly wider basidiospores than F. monomitica (according to our measurements, spores in the latter species are (5.0–)5.1–6.8(−7.1) × (2.1–)2.2–3.1(−3.2) μm (n = 30/1), L = 5.74, W = 2.64, Q = 2.19). With so few specimens studied, it is eventually impossible to decide how constant these differences are. The species complex deserves a closer look with much wider specimen sampling.
Fomitopsis ostreiformis (Berk.) T. Hatt., Mycoscience 44 (4): 272. 2003. Figs 24, 25.
Fig 25.
Basidiospores of Fomitopsis spp. F. ostreiformis (isolectotype); F. pannucea (holotype); F. perhiemata (holotype); F. pinicola (Kotiranta 26782); F. pseudopetchii (Miettinen 14284); F. psilodermea (Vlasák 1504/34). Scale bar = 5 μm.
Basionym: Polyporus ostreiformis Berk., J. Linn. Soc., Bot. 16: 46. 1877.
Typus: Philippines, ‘Malamon Ids., 30 Jan.–4 Feb. 1875, Challenger’ (lectotype K, isolectotype NY 00730855!) (selected by Ryvarden 1984: 349).
Synonyms:
Polyporus griseodurus Lloyd, Mycol. Writ. 5 (68): 12. 1918.
Typus: Japan, Kyoto, Kasagi, Aug. 1910, Gono 6 (holotype BPI US0304291!).
Tyromyces perskeletalis Corner, Beih. Nova Hedwigia 96: 187. 1989.
Typus: Singapore, Jurong, ‘fallen and burnt trunk’, 25 Dec. 1932, Corner (holotype E00604926).
Fomitopsis cana B.K. Cui, Hai J. Li & M.L. Han, Mycol. Progr. 12: 710. 2013.
Typus: China, Hainan, Qiongzhong, Limushan, hardwood, 24 May 2008, Dai 9611* (holotype BJFC).
Description: Basidiocarps annual, sessile or effused-reflexed, often fusing together in large imbricate groups, projecting up to 2 cm, occasionally totally resupinate and then up to 4 cm in widest dimension. Upper surface felty or smooth, greyish-ochraceous to mouse-grey, occasionally with pinkish or violet hues disappearing in herbarium specimens, sometimes with a darker brownish tint after drying, azonate, occasionally indistinctly striate. Pileal edge sharp, concolourous with cap surface, usually fertile, sometimes incurving during drying, margin of resupinate parts white or cream-coloured, slightly elevated, adnate or partly detaching, sharply delimited. Pore surface pale ochraceous to dark grey, sometimes with brownish stains, as a rule concave; pores roundish to angular, 5–8 per mm, with thin, entire or serrate dissepiments. Section: context rather soft, white, pale cream-coloured or rarely greyish, up to 3 mm thick; tubes tough, one-layered, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae almost hyaline to brownish, densely interwoven or rarely in subparallel bundles, occasionally branched, (2.8–)3.0–6.3(−7.3) μm diam (n = 80/4), lumen varying from wide to narrow, side branches 2–3 μm diam, generative hyphae rather abundant to rare, hyaline, thin- or slightly thick-walled, 3–6 μm diam. Trama dimitic; skeletal hyphae pale ochraceous to brownish, interwoven, some flexuous, occasionally branched, (2.4–)2.8–4.3(−4.7) μm diam (n = 120/6), lumen capillary to invisible, generative hyphae thin- or slightly thick-walled, 2–3 μm diam, in some places forming subhymenial layer up to 10–15 μm thick, dissepiment edges dimitic, consisting of thin-walled generative hyphae and winding skeletals with a wide lumen. Rhomboid crystals occasionally present among tramal tissues, up to 15–20 μm in longest dimension. Cystidioles frequent to rare, gradually tapering to the apex, 10–14.5 × 4–6 μm, often collapsing at the very top. Basidia clavate, (9.7–)9.8–18.2(−18.4) × (4.3–)4.7–6.1(−6.2) μm (n = 50/5), partly glued together and covered by amorphous grainy substance. Basidiospores thin-walled, cylindrical to fusiform, (4.1–) 4.2–7.3(−7.6) × (2.0–)2.1–3.0(−3.1) μm (n = 180/6), L = 5.43–5.96, W = 2.47–2.59, Q = 2.14–2.38.
Specimens examined: Australia, Northern Territory, Buley Rockhole, Lichfield National Park, unidentified wood, 25 Jan. 2014, Barrett F118/14 (MEL 2382710A). China, Yunnan, Xishuangbanna Botanical Garden, Bambusoidae, 7 Aug. 2005, Miettinen 10071* (H). India, Gujarat, Bhavnagar, unidentified wood, 16 Jun. 1989, Yesodharan KY44 (O 12724). Indonesia, Papua Barat, Manokwari, Amban, Azadirachta indica, 5 Feb. 2007, Miettinen 11629* (MAN, H); Riau, Kampar, Balung, 24 Dec. 2006, Miettinen 11279.2 (BO, H); Pekanbaru, Sukajadi, Mangifera indica, 12 Nov. 2018, Miettinen 21986* (BO, H); Rokan Hilir, Sungai Majo, Koompassia malaccensis, 8 Jul. 2004, Miettinen 8854.1* (H); Teluk Nilap, Sapotaceae, 10 Jul. 2004, Miettinen 8879 (BO, H); Siak, Kampar Peninsula, Beruk S, 14 Apr. 2002, Miettinen 5912 (BO, H). Kenya, Western Province, Kakamega, Yala River, 21 Jan. 1970, Ryvarden 5556 (O 18054). Singapore, Windsor Nature Park, Hevea brasiliensis, 22 Mar. 2020, Miettinen 23661 (H); Yishun, Nee Soon pipeline, dicot, 17 Mar. 2020, Miettinen 23532 (SING, H). Sri Lanka, Beruwela, Plumeria obtusa (hanging branch), 15 Feb. 2003, Dämmrich (O 18269, H*).
Notes: Fomitopsis ostreiformis is a South Asian species, reaching East Africa (Kenya) as the westernmost point of its distribution, and northern Australia in the south. Morphologically, it is most similar to F. luzonensis; differences are listed under F. luzonensis. Basidiocarps of F. ostreiformis are macroscopically extremely variable, and pore size varies considerably between pileate and resupinate fruitbodies (6–8 vs. 5–6 pores per mm). See further remarks under F. meliae.
Fomitopsis palustris (Berk. & M.A. Curtis) Gilb. & Ryvarden, Mycotaxon 22 (2): 364. 1985.
Basionym: Polyporus palustris Berk. & M.A. Curtis, Hooker’s J. Bot. 1: 102. 1849.
Typus: USA, South Carolina, Santee River, Pinus palustris, [no collection date], Ravenel 1566 (lectotype K(M) 170428!) (selected by Ryvarden 1977: 223).
Description: Basidiocarps annual, pileate, sessile, often solitary, rarely fusing together in groups of 2–3, projecting up to 5.5 cm. Upper surface first cream coloured to pale ochraceous, azonate, finely velutinous, later greyish-ochraceous to brownish, smooth. Pileal edge sharp, concolourous with cap surface, fertile. Pore surface cream-coloured or pale ochraceous, sometimes with brownish stains, as a rule concave; pores roundish to angular, (4–)5–7(−8) per mm, with thin, entire or serrate dissepiments. Section: context soft corky, white to cream-coloured, up to 15 mm thick; tubes rather soft, easily cut by a razor blade, one-layered, concolourous with hymenial surface, up to 5 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline, mostly loosely interwoven, occasionally branched, (3.0–) 3.3–7.2(−8.1) μm diam (n = 60/3), with a variably pronounced lumen, generative hyphae thin- to moderately thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae hyaline, loosely interwoven, some flexuous, occasionally branched, (2.7–)2.8–4.8(−5.0) μm diam (n = 80/4), swelling in KOH and CB, lumen usually rather wide, generative hyphae thin- or slightly thick-walled, 2.5–4 μm diam, producing distinct subhymenial layer up to 35–40 μm thick. Rhomboid or cubical crystals occasionally present among context and tramal tissue, solitary or in large groups, up to 8 μm in widest dimension. Cystidioles infrequent, tapering, 14.5–18 × 3.5–6.0 μm. Basidia clavate, often with a tapering basal part, (13.4–)13.8–22.1(−22.2) × (5.6–)5.7–7.2(−7.4) μm (n = 44/4), sometimes pleural. Basidiospores thin-walled, cylindrical to fusiform, longest spores sigmoid, (6.1–)6.2–8.2(−9.2) × (2.3–)2.4–3.7(−3.9) μm (n = 120/4), L = 6.96–7.21, W = 2.80–3.11, Q = 2.34–2.50.
Specimens examined: Belize, Cayo, Douglas d’Silva, Pinus sp., 20 Nov. 2001, Ryvarden 44439* (O 17631); Five Sisters, Pinus caribaea, 2 Nov. 2002, Ryvarden 45413 (O 18279). USA, Florida, Manatee Co., Duette Preserve, Pinus ellitottii, 8 May 2016, Dollinger 782* (JV, H, PRM944378); Polk Co., Arbuckle Creek, Pinus clausa, Apr. 2019, Vlasák Jr. 1904/1J* (JV, H); Georgia, Laurens Co., Dublin, Pinus sp., 3 Aug. 1950, Lowe 4092* (PRM 559516); Louisiana, Shreveport, Pinus sp., 13 Oct. 1913, Bartholomew (Fungi Columbiani #4449, H).
Notes: Earlier sequences of F. palustris from USA and Japan are based on misidentified material and in fact belong to F. marianii (see above). Here we re-introduce the species based on type material and newly sequenced specimens. Fomitopsis palustris is restricted to warm-temperate or subtropical areas of the eastern part of North America, and it occurs exclusively on wood of Pinus spp. (P. palustris, P. caribaea and other southern pines). Morphologically, it is most similar to F. marianii and F. nivosella, and it differs from them mainly due to host specificity and rather small basidiocarps. Microscopically, long and wide basidia and basidiospores make F. palustris distinguishable from the two latter species. The single European record of F. palustris (Rivoire 2020) belongs to F. marianii.
Fomitopsis pannucea Runnel & Spirin, sp. nov. MycoBank MB 844944. Fig 25.
Typus: French Guiana, Roura, Patawa, 4.55410°–52.15245°, cut angiosperm log, 14 Nov. 2014, Runnel 824* (holotype TUF130274).
Etymology: Pannuceus (Lat., adj.) – shabby, frayed.
Description: Basidiocarps annual, sessile, dimidiate, projecting up to 1.5 cm, occasionally fusing together. Upper surface cream-coloured to greyish, smooth or finely pubescent, even. Pileal edge blunt, concolourous with pileal surface, sterile, up to 0.5 mm wide. Pore surface cream-coloured to greyish, flat or slightly concave; pores roundish to angular, 2–3 per mm, with rather thin, entire or serrate dissepiments. Section: context soft leathery, cream-coloured, up to 1 mm thick; tubes soft leathery, one-layered, concolourous with hymenial surface, up to 7 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline to brownish, loosely interwoven, occasionally branched, (3.2–)3.4–4.7(−5.2) μm diam (n = 20/1), lumen narrow to capillary, side branches 2–2.5 μm diam, generative hyphae rather rare, hyaline, slightly thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae dominating, hyaline to brownish, loosely interwoven, occasionally branched, (2.8–)2.9–4.1(−4.2) μm diam (n = 20/1), lumen mostly narrow to capillary, generative hyphae abundant, thin- to slightly thick-walled, 2–3.5 μm diam. Subhymenium distinct, up to 20 μm thick. Skeletocystidia present as slightly swollen (up to 4 μm diam) apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles abundant, tapering, 11–16 × 3–4 μm. Basidia clavate to long clavate, (13.1–)13.2–20.0(−21.8) × (4.2–) 4.3–5.3(−6.0) μm (n = 20/1), occasionally pleural, in senescent hymenium slightly thick-walled and encrusted by amorphous grainy matter. Basidiospores with a distinct wall, narrowly ellipsoid, longest spores subfusiform, (4.0–)4.1–5.2(−5.3) × (2.0–)2.1–3.1(−3.2) μm (n = 30/1), L = 4.49, W = 2.49, Q = 2.16.
Notes: Of the South American Fomitopsis species, F. pannucea is most similar to F. leioderma. It differs from the latter species in having slightly paler and softer basidiocarps with somewhat larger pores, rather abundant generative hyphae forming a distinct subhymenial layer, as well as shorter basidiospores. Despite their high outward similarity, these species are not closely related. Fomitopsis pannucea is so far known only from the type locality in French Guiana.
Fomitopsis perhiemata Viner & Spirin, sp. nov. MycoBank MB 844945. Figs 24, 25.
Typus: Russia, Krasnodar Reg., Sochi, Krasnaya Polyana, Melnichnyi, 43.69414° 40.20198°, Quercus petraea (base of living tree), 3 May 2021, Viner 2021/35* (holotype H7200198).
Etymology: Perhiematus (Lat., adj.) – overwintered.
Description: Basidiocarps perennial, effused-reflexed, projecting up to 2.5 cm. Upper surface greyishbrown, occasionally with a light pinkish tint, smooth, growing margin paler, light grey or pale ochraceous. Pileal edge blunt, concolourous with pileal margin, sterile, up to 2 mm wide. Pore surface grey to greyish brown, slightly concave; pores angular, 5–7 per mm, with rather thick, entire or serrate dissepiments. Section: context soft-corky, pinkish brown to brown, up to 5 mm thick; tubes corky, indistinctly stratified, concolourous with hymenial surface, up to 12 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, reddish-brown to brown, loosely interwoven, occasionally branched, (3.9–)4.0–5.0(−5.2) μm diam (n = 20/1), lumen mostly narrow to indistinct, side branches 2–3 μm diam, generative hyphae rare, hyaline, slightly thick-walled, 2–3.5 μm diam. Trama dimitic; skeletal hyphae dominating, brown, rather loosely interwoven, occasionally branched, (2.4–)2.8–3.8(−3.9) μm diam (n = 20/1), lumen mostly capillary to indistinct, side branches 2–2.5 μm diam, generative hyphae thin- or slightly thick-walled, 2–3 μm diam. Subhymenium indistinct. Cystidioles abundant, distinctly tapering, 12–18 × 3–6 μm. Basidia clavate, (12.1–)12.8–16.1(−16.3) × (4.3–)5.0–6.3(−6.4) μm (n = 20/1), senescent basidia slightly thick-walled and partly glued together. Basidiospores thin-walled or with a distinct wall, broadly cylindrical to narrowly ellipsoid, longest spores subfusiform, (4.0–)4.1–5.2(−5.3) × (2.0–)2.1–3.0(−3.1) μm (n = 30/1), L = 4.50, W = 2.40, Q = 1.89.
Notes: Fomitopsis perhiemata is introduced here as a member of the F. rosea complex (Fig. 12). So far, it was collected once in Caucasus from a living oak tree. Both macroscopically and anatomically, it is similar to the East Asian F. ussuriensis which also grows mainly on Quercus spp. The latter species differs from F. perhiemata in having more regular and slightly smaller pores, 6–7 per mm, and a clearly stratified tube layer (see Table 4).
Fomitopsis philippinensis (Murrill) Spirin & Vlasák, comb. nov. MycoBank MB 844946.
Basionym: Fomes philippinensis Murrill, Bull. Torr. Bot. Club 34: 477. 1907.
Typus: Philippines, Luzon, Bataan, Mt. Mariveles, dead trunk, 23 Mar. 1904, Williams (holotype NY00780679!).
Description: Basidiocarps perennial, sessile, conchate, solitary or in groups, projecting up to 10 cm. Upper surface first greyish-brown, then darkening to almost black, with several distinct annual zones and occasional radial fissures, in old specimens fading to ochraceous-brownish, densely longitudinally cracking, growing margin much paler, beige to pale ochraceous. Pileal edge sharp or rather blunt, concolourous with hymenial surface, first sterile, up to 1 mm wide, then fertile. Pore surface wood-coloured or beige, then ochraceous or brownish, slightly concave; pores roundish, 6–7 per mm, with thick, entire dissepiments. Section: crust dark brown to black, tough, up to 1 mm thick, context corky, pale ochraceous to brownish, up to 8 mm thick; tubes corky, stratified, concolourous with hymenial surface, up to 15 mm thick, sterile tissue often present between annual layers. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, almost hyaline to yellowish or brownish, densely interwoven, richly branched, (3.0–) 3.2–5.1(−5.2) μm diam (n = 40/2), lumen rather wide to indistinct, side branches 2–3 μm diam, generative hyphae rare, hyaline, thick-walled, 2–2.5 μm diam. Trama dimitic; skeletal hyphae dominating, yellowish to brownish, densely interwoven, occasionally branched, (2.2–)2.3–4.4(−5.0) μm diam (n = 60/3), lumen mostly capillary to indistinct, side branches 1.5–2.5 μm diam, generative hyphae rare, thin- or slightly thick-walled, 1.5–3 μm diam. Subhymenium indistinct. Skeletocystidia occasionally present as slightly swollen (up to 5.5 μm diam) apices of tramal skeletal hyphae, embedded or slightly projecting above hymenium. Cystidioles abundant, tapering, 8–12 × 3–4 μm. Basidia clavate, (8.0–)8.9–12.8(−13.2) × (3.9–)4.0–5.1(−5.3) μm (n = 20/2), senescent basidia slightly thick-walled and partly glued together. Basidiospores thin-walled, cylindrical to broadly cylindrical or narrowly ellipsoid, (3.1–)3.2–5.0(−5.2) × (1.9–)2.0–2.3(−2.8) μm (n = 48/3), L = 3.87–4.12, W = 2.11–2.15, Q = 1.83–1.92.
Specimens examined: India, Assam, 27 Aug. 1934, Parkinson (O 10853, H). Indonesia, Jambi, Bukit Perekas, 30 May 1995, Núñez 822 (O 10854, H). Malaysia, Sabah, Kinabalu, Sep. 1931, Clemens (O 10795, H). Thailand, Chiang Mai, Doi Inthanon, fallen log, 20 Feb. 1979, Ryvarden 17840* (O, H); Sukhotai, Khiri Mat, Ramkamhaeng, 12 Aug. 2014, Kout 1408/K2* (JV, H).
Notes: Fomitopsis philippinensis is a member of the F. dochmia complex distributed in Southeast Asia. It differs from F. elevata, another species from this group distributed in the same geographic region, in having a darker and clearly stratified tube layer, as well as distinctly rimose pileal surface strongly fading in senescent basidiocarps. Our data confirm the presence of F. philippinensis in Indonesia (Sumatra), Malaysian Borneo, Thailand and the easternmost part of India (Assam), in addition to the type locality in Philippines. ITS sequences labelled as F. dochmia in GenBank (DQ491401, MG719293) belong to F. philippinensis.
Fomitopsis pinicola (Sw.) P. Karst., Meddeland. Soc. Fauna Fl. Fenn. 6: 9. 1881. Figs 24, 25.
Basionym: Boletus pinicola Sw., K. Svenska Vetensk.-Akad. Handl. 2, 31: 88. 1810.
Typus: Plate 953 in Flora Danica 16, 1787 (iconotype) (selected by Ryvarden 1991: 149).
Description: Ryvarden et al. (2017).
Specimens examined: China, Jilin, Antu, Huangshongpu, Larix sp., 27 Jul. 1993, Dai 759 (H); Fushong, Shongjianghe, Larix sp., 4 Jun. 1993, Dai 261 (H). Czech Republic, South Bohemia, České Budějovice, Hluboká nad Vltavou, Picea abies, 19 May 2019, Vlasák 1905/1* (H). Finland, Uusimaa, Helsinki, Koskela, Alnus incana, 2 Oct. 2016, Miettinen 20458 (H); ibid., 6 Apr. 2008, Miettinen 12391* (H); Satakunta, Viljakkala, Niemelä, Betula sp., 12 Aug. 2019, Niemelä 9425 (H). Poland, Podlasie, Hajnówka, Białowieża, T. cordata, 12 Sep. 2009, Niemelä 8625 (H). Russia, Bashkortostan, Bashkirian Nat. Park, Nugush, Betula sp., 1 Aug. 2012, Kotiranta 25428 (H); Chukotka, Anadyr’ Dist., Beringovski, coniferous timber, 30 Aug. 2009, Kotiranta 27183* (H); Khabarovsk Reg., Solnechnyi Dist., Gorin, L. gmelinii, 13 Aug. 2011, Spirin 4111* (H); Tuva, Turgen, Larix sibirica, 23 Aug. 2014, Kotiranta 26782* (H). USA, Washington, Clark Co., Gifford Pinchot Nat. Forest, Tsuga heterophylla, 11 Oct. 2014, Spirin 8367* (H).
Notes: Ryvarden & Stokland (2008) were first to address species delimitation in the F. pinicola complex. Based on morphological and geographic evidence, they introduced F. ochracea, a species with a North American distribution. Haight et al. (2016, 2019) significantly expanded sampling in this group for their three-gene analyses. They found several lineages, which allowed them to introduce two more North American species, F. mounceae and F. schrenkii and exclude F. pinicola sensu stricto from the American mycota. Later Liu et al. (2021) further expanded sampling by a number of sequences from East Asia, an area which was poorly covered in the previous studies.
Our phylogenetic analyses constantly resolve F. ochracea as a separate clade. Fomitopsis schrenkii (including the genome strain FP-58527) receives decent support only in the three-gene analyses (BI pp = 0.99, ML bs = 96%; Fig. 10), but is nestled among the rest of F. pinicola sensu lato sequences in the ITS+TEF1 tree (Suppl. Fig. S5). As for all other North American sequences from Haight et al. (2016, 2019), our analyses do not resolve F. mounceae and the rest of F. pinicola as mutually exclusive clades – F. mounceae is not supported.
Why this different pattern? The first reason is the difference in genetic datasets. We detected only five parsimony informative sites across the three DNA loci (ITS, TEF1, RPB2) that distinguish F. mounceae unambiguously from European F. pinicola. These sites might have yielded support for the separate European F. pinicola and the American F. mounceae clades in analyses with Haight et al. (2016, 2019). However, adding more sequences from a wider geographic area to our analyses led to an attenuation of the phylogenetic signal and lack of support for separate lineages due to an increase in polymorphic sites. Consequently, after adding more sequences, the number of nucleotide positions that separate F. mounceae unambiguously from the Eurasian F. pinicola drops from five to two positions. Both of these positions are in ITS, and none remained in RPB2 and TEF1. Further sampling would likely reduce this number even more.
The second reason for the differences is that Haight et al. (2016, 2019) draw their conclusions based on a coalescent method. Haight et al. (2016) explained that they preferred this method due to its greater performance in phylogenetic resolution where reciprocal monophyly of the separate DNA loci has not been achieved, which was the case in their three-gene analyses. However, usefulness of coalescent models for the species recognition has been contested, especially for species with allopatric distribution, which is the case for North American and Eurasian populations of the F. pinicola species complex. Addressing this theoretical question, Sukumaran & Knowles (2017) and Leaché et al. (2019) came to the conclusion that such models cannot discern a true speciation event from geographically structured intraspecific variation. The application of coalescent models may still be useful for cases when analysed DNA loci are strongly incongruent due to such phenomena as incomplete lineage sorting or hybridization events (Degnan & Rosenberg 2009). This is not the case for F. pinicola complex, where trees of independent markers do not yield any mutually exclusive clades (Haight et al. 2016, our analysis – data not shown). Though we point out limitations of the coalescent approach, it is not the aim of this paper to judge between different analysis methods. Here we have consistently relied on phylogenetic analyses of concatenated datasets for taxonomic conclusions. Whatever the analysis method, there is no substitute for a high-quality dataset with enough informative characters and geographic coverage.
While the conclusions reached by Haight et al. (2016, 2019) may have been supported by their geographically limited dataset, the situation is different for the recently introduced Asian species. We see no obvious genetic pattern, which could justify the description of six more species from the F. pinicola complex by Liu et al. (2021). Re-running their phylogenetic analyses was not possible as the corresponding alignment was not traceable in TreeBase, but we see that it could suffer from a “gappy” alignment approach. The concatenated alignment assembled by Liu et al. contained a lot of missing data. A number of specimens were represented by just two loci while missing the third one; in the case of F. tianshanensis, all specimens lack the third DNA locus (RPB2). Such pattern of missing data could be problematic for the phylogenetic analysis (Hartmann & Vision 2008), but Liu et al. did not mention any statistical methods compensating for the missing data. Besides that, they excluded a number of Haight et al. (2016, 2019) specimens with all three loci sequenced, which could have been integrated in the analyses.
Based on the current genetic evidence, including our phylogenetic analyses, we question whether the F. pinicola complex consists of as many species as currently recognized based on our phylogenetic analyses. Our results do not preclude the existence of genetically isolated lineages within the F. pinicola group, especially considering the strong internal phylogenetic structure of the complex but the division into eight separate species seems unwarranted.
Previous mating tests between European, North American and East Asian material have identified two intersterility groups in the F. pinicola complex (Mounce & Macrae 1938, Högberg et al. 1999), with only one group present in Eurasia. Conveniently, Haight et al. (2019) used DNA data to connect four old cultures utilized in mating tests by Mounce & Macrae (1938) to four currently recognized species: F. ochracea, F. mounceae, F. schrenkii, and F. pinicola sensu stricto. They found that F. ochracea belongs to a group intersterile with the other three species, which were all compatible. This pattern corresponds well with the phylogenetic position of F. ochracea at the deepest split of the F. pinicola clade. Positive results of mating tests (i.e. interfertility) are not conclusive evidence that significant mating takes place in the natural environment, but clearly the bar for recognizing interfertile populations as species should be high.
Another line of evidence to distinguish species came from morphological data. In our opinion, only the North American F. ochracea can be separated from all the other representatives of the F. pinicola complex with some confidence. However, their morphological differences are not as obvious as presented by Ryvarden & Stockland (2008) and Haight et al. (2019). Inconveniently, most specimens of F. ochracea are sterile or contain juvenile spores only. These spores are often still attached to basidia and clearly thin-walled. We studied and measured two collections of F. ochracea with ripe basidiospores possessing slightly thickened wall (this feature is also characteristic for F. pinicola sensu stricto). They were clearly larger than described in the two aforementioned sources, i.e. (4.9–)5.0–6.2(−6.8) × (3.4–)3.6–5.2 μm (n = 60/2), L = 5.49–5.74, W = 3.91–4.13, Q = 1.40–1.41. Their average values are exactly between the East Asian specimens of the F. pinicola complex and the rest of the collections from Europe, North America and Siberia studied by us, severely restricting usability of spore size as a separating character. The easiest way to identify F. ochracea is to carefully check the macroscopic characters: the absence of a resinous substance in the crust (no signs of melting in a flame) and a pale-coloured, almost white growing margin, as well as rather small (5–6 per mm) pores, are the most stable morphological features of this species.
Basidiospore dimensions of the American F. mounceae and F. schrenkii, as reported by Haight et al. (2019) and corroborated by our own limited measurements, fall within the variation range of F. pinicola from Europe and Siberia. The Eurasian specimens excluding East Asian collections measure (5.9–)6.0–9.2(−10.2) × (3.0–)3.1–5.0(−5.1) μm (n = 150/5), L = 6.51–7.64, W = 3.58–4.06, Q = 1.61–2.15. All of them have a resinous pileal crust melting over a flame, and other morphological traits (pore size, basidiocarp colour, etc.) do not separate them either.
As for the East Asian taxa, we do not regard the morphological differences (pore and spore size) reported by Liu et al. (2021) as sufficiently strong evidence for maintaining them as separate species. While the protologues of F. abieticola and F. tianshanensis describe normal F. pinicola, F. subpinicola is different in several respects. We studied several (and sequenced two) specimens from temperate to boreal East Asia, which likely represent this taxon. They have smaller pores than F. pinicola from Europe and Siberia (5–7 vs. (2–)3–4 per mm) and their basidiospores are also smaller, (4.0–)4.2–6.1(−6.3) × (2.8–)3.0–4.1(−4.2) μm (n = 120/4), L = 4.97–5.23, W = 3.36–3.44, Q = 1.44–1.54. The same differences from F. pinicola are intrinsic to the other three species introduced in the same paper, F. hengduanensis, F. kesiyae, and F. massoniana and, besides them, to F. subungulata, described from Philippines over a hundred years ago. Nevertheless, small pore and spore size are characteristic of the East Asian F. pinicola sensu lato, regardless of how many lineages are recognized.
The fourth line of reasoning leading to the splitting of the F. pinicola complex into many species (in addition to DNA, mating tests and morphology) is that according to current information they all originate from different areas (except for the F. ochracea/F. mounceae pair separated by other traits). Considered together, the structure of genetic variation and morphological trends point to geographic differentiation within the F. pinicola complex. We argue that this variation is currently best interpreted as intraspecific variation, possibly warranting recognition of some subspecies such as F. schrenkii, rather than accepting ambiguous species of which three are found only in their type localities. Moreover, a proper revision of F. pinicola in East Asia is impossible without re-establishing the identity of Polyporus thomsonii Berk. from northern India and Fomes subungulatus Murrill from Philippines, both currently listed among the synonyms of F. pinicola (Ryvarden 1977, 1985).
In conclusion, current data do not support the division of F. pinicola into many species, with the exception of F. ochracea. At the same time, the observed pattern of geographic variation does not exclude the possibility of more than two species. Therefore, we treat F. abieticola, F. hengduanensis, F. kesiyae, F. massoniana, F. mounceae, F. schrenkii, F. subpinicola, F. tianshanensis and Fomes subungulatus as insufficiently known taxa. We call for solid evidence in the form of deeper genetic sampling based on a large number of spatially representative specimens and/or mating tests, before any of these names are accepted. Studies on Heterobasidion spp., conifer-dwelling polypores, offer an example of how to approach the question (Dai et al. 2003, Chen et al. 2015). Clearly, the F. pinicola complex will offer a highly interesting research subject on speciation and population genetics for years to come.
Fomitopsis primaeva (Renvall & Niemelä) Miettinen & Niemelä, comb. nov. MycoBank MB 844947.
Basionym: Antrodia primaeva Renvall & Niemelä, Karstenia 32: 30. 1992.
Typus: Finland, Sompion Lappi, Savukoski, Urho Kekkonen Nat. Park, Pinus sylvestris, 19 Sep. 1988, Renvall 1372 (holotype H!).
Description: Renvall & Niemelä (1992, as A. primaeva).
Note: For phylogenetic placement, see Spirin et al. (2013b, 2017).
Fomitopsis pseudopetchii (Lloyd) Ryvarden, Norw. J. Bot. 19: 231. 1972. Figs 24, 25.
Basionym: Fomes pseudopetchii Lloyd, Mycol. Writ. 7 (69): 1202. 1923.
Typus: Indonesia, Sumatra, Kisaran Asahan, 1923, Yates (lectotype K (M) 264878!) (selected by Ryvarden 1972: 231).
Synonym: Tyromyces singularis Corner, Beih. Nova Hedwigia 96: 194. 1989.
Typus: Solomon Ids., Guadalcanal, Mt. Gallego, 3 Jul. 1965, Corner (holotype E00604897).
Description: Basidiocarps perennial, sessile or pendant, ungulate, projecting up to 4 cm. Upper surface first greyish-white to brownish-grey, smooth, matt, then covered by brownish-black or black, sulcate, shining crust. Pileal edge blunt, greyish to brownish, sterile, up to 1 mm wide. Pore surface first greyish-white to cream-coloured, then brownish, concave or flat; pores roundish or angular, 7–10 per mm, with thin, entire dissepiments. Section: crust grey to brownish-black, 0.2–1 mm thick, context soft corky, easily crumbling, cream-coloured to pale ochraceous, up to 5 mm thick; tubes chalk-like, easily crumbling, indistinctly stratified, up to 15 mm thick, concolourous with hymenial surface. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline to brownish, interwoven, easily crumbling, occasionally branched, (2.0–)2.1–5.0(−5.1) μm diam (n = 40/2), lumen mostly capillary to indistinct, side branches 2–3 μm diam, generative hyphae rare, hyaline, slightly or distinctly thick-walled, 1.5–4 μm diam. Trama dimitic; skeletal hyphae sparse (and then trama seemingly monomitic) to abundant, hyaline to brownish, loosely interwoven, crumbling or sturdy, often covered by small angular crystals or sand-like deposits, occasionally branched, (1.8–)1.9–3.5(−3.8) μm diam (n = 60/3), lumen varying from rather wide to capillary or indistinct, generative hyphae rather abundant and in some parts dominating, thin- to slightly thick-walled, 2–3.5 μm diam. Subhymenium well-differentiated in younger part of tubes, up to 10 μm thick. Cystidioles rather frequent, tapering, 9–16 × 3–5 μm. Basidia clavate, (9.0–)9.4–11.8(−12.7) × (4.0–)4.1–6.2(−6.3) μm (n = 13/2), in senescent hymenium partly glued together and covered by sand-like deposits. Basidiospores with a distinct wall, ellipsoid to narrowly ellipsoid, more rarely broadly cylindrical, (2.6–)2.7–4.0(−4.1) × (1.6–)1.7–2.3(−2.4) μm (n = 71/3), L = 3.23–3.35, W = 1.98–2.16, Q = 1.56–1.60.
Specimens examined: Indonesia, Papua Barat, Manokwari, Amberbaken, Anjii, Pometia pinnata (fallen tree), 1 Nov. 2010, Miettinen 14284* (H); Minjanbiat, P. pinnata (felled tree), 3 Nov. 2010, Miettinen 14373.1* (H). Malaysia, Johor, Gunong Panti, 31 Jan. 1930, Corner (O 10856, H).
Notes: Perennial basidiocarps with multilayered, pale, soft tubes, spaced, easily crumbling skeletal hyphae and small basidiospores make F. pseudopetchii morphologically highly similar to F. caseosa and F. niveomarginata. In spite of their superficial similarity, these species are not closely related (Fig. 3, Suppl. Fig. S2). Morphological differences of F. pseudopetchii from F. caseosa are discussed under the latter species. In turn, F. niveomarginata possesses robust, often effused-reflexed basidiocarps with larger (5–6 per mm) pores than in F. pseudopetchii.
Hattori (2003) re-described F. singularis with convincing details as similar to F. pseudopetchii. We studied one collection (Miettinen 14284) with a rudimentary, pale-coloured pileal crust and almost monomitic tubes, which were considered the main diagnostic features of F. singularis vs. F. pseudopetchii. However, ITS sequence obtained from this species showed only 1 bp difference from that one of F. pseudopetchii. Therefore, we tentatively treat F. singularis as a synonym of F. pseudopetchii. As interpreted here, F. pseudopetchii is a rare species distributed in South-East Asia and adjacent areas of Oceania.
Fomitopsis psilodermea (Berk. & Mont.) Spirin & Vlasák, comb. nov. MycoBank MB 848364. Figs 25, 26.
Fig 26.
Basidiocarps of Fomitopsis spp. A. F. quercina (Niemelä 4030). B. F. roseofusca (Vlasák 1909/82). C. F. scalaris (Vlasák 1909/66). D. F. sulcata (Miettinen 11275). E. F. psilodermea (Vlasák 1504/34). F. F. tristis (holotype). G. F. tunicata (holotype). H. F. uralensis (Spirin 10946).
Basionym: Polyporus psilodermeus Berk. & Mont., Ann. Sci. Nat., Bot. 3 (11): 239. 1849.
Typus: Brazil, Bahia, ‘ad truncos’, [no collection date], Blanchet (holotype PC!).
Synonym: Trametes supermodesta Ryvarden & Iturr., Mycologia 95 (6): 1074. 2003.
Typus: Venezuela, Bolívar, Las Nieves, hardwood log, 12 Jun. 1995, Ryvarden 37779 (isotype O!).
Description: Basidiocarps short-living perennial, sessile, dimidiate or with a contracted base (fan-shaped), projecting up to 3 cm. Upper surface first beige to pale ochraceous, smooth, indistinctly zonate, later ochraceous-brownish to dark brown. Pileal edge sharp to rather blunt, concolourous with hymenial surface, first sterile, up to 1 mm wide, then fertile. Pore surface cream-coloured or beige to pale ochraceous, sometimes with pinkish or greyish tint, flat or concave; pores angular to sinuous, occasionally fusing together, 3–4 per mm, with thin or rather thick, entire or serrate dissepiments. Section: context leathery, cream- to wood-coloured, normally less than 2 mm thick; tubes corky, one-layered, concolourous with hymenial surface, up to 2 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, interwoven, occasionally branched, (2.8–)2.9–4.3(−4.4) μm diam (n = 40/2), lumen narrow to indistinct, side branches 1.5–2.5 μm diam, generative hyphae infrequent, hyaline, slightly to distinctly thick-walled, 2–3.5 μm diam. Trama dimitic; skeletal hyphae dominating, brownish, interwoven, occasionally branched, (2.7–)2.8–4.1(−4.2) μm diam (n = 60/3), lumen mostly capillary to indistinct, side branches 1.5–2 μm diam, generative hyphae thin- to slightly thick-walled, 2–3.5 μm diam. Subhymenium distinct in some parts, up to 15–20 μm thick. Cystidioles infrequent, tapering, 12–15 × 3–4 μm. Skeletocystidia present as slightly swollen (up to 4 μm diam), acute or blunt apices of tramal skeletal hyphae, slightly projecting above hymenium. Basidia clavate, (12.4–)13.2–19.2(−20.0) × (4.4–)4.8–5.8(−6.0) μm (n = 20/2). Basidiospores with a distinct wall, broadly cylindrical to narrowly ellipsoid, longest spores subfusiform, (5.0–)5.1–8.1(−8.2) × (2.0–)2.1–3.1(−3.2) μm (n = 60/2), L = 5.55–6.42, W = 2.34–2.74, Q = 2.35–2.39.
Specimens examined: Brazil, Roraima, Alto Alegre, Maraca Res., 13 Mar. 1987, Edwards (O 17841 ex K). Costa Rica, Puntarenas, Golfito, Piedras Blancas Nat. Park, Peltogyne sp., 20 Apr. 2015, Vlasák 1504/34* (JV, TUF).
Notes: Fomitopsis psilodermea is a neotropical species from the Daedalea clade (Fig. 5). Morphologically, it is most similar to F. leioderma. It differs from the latter species in having a darker pileal surface and longer basidiospores. Another similar-looking species, F. subectypa, has distinctly smaller pores and basidiospores. We studied the types of P. psilodermeus and T. supermodesta, as well as two recent specimens from the neotropics, and concluded that they seem to be conspecific. However, small differences can be found in spore size, as well as in the shape and colour of basidiocarps. Therefore, we cannot preclude that F. psilodermea as interpreted here may turn out a species complex. More material is needed to solve this problem.
Fomitopsis pulverulenta (Rivoire) Rivoire, comb. nov. MycoBank MB 844949.
Basionym: Antrodia pulverulenta Rivoire, Bull. Mens. Soc. Linn. Lyon 79 (7–8): 185. 2010.
Typus: France, Savoie, Les Avanchers, Valmorel, Sorbus aucuparia, 20 Jul. 2008, Rivoire (holotype LY BR-3413!).
Description: Rivoire (2010, as A. pulverulenta).
Note: The species is a member of the Fomitopsis pulvinascens complex (Spirin et al. 2013b).
Fomitopsis pulvina (Pers.) Spirin & Vlasák, comb. nov. MycoBank MB 844950. Fig 24.
Basionym: Boletus pulvinus Pers., Obs. Mycol. 2: 7. 1799.
Typus: Czech Republic, Jihočeský kraj, České Budějovice, Hluboká nad Vltavou, Quercus robur, 16 Jun. 2014, Vlasák 1406/1* (neotype PRM 956226) (designated here, MycoBank MBT 10008292).
Synonyms:
Boletus quercinus Schrad., Spic. Fl. Germaniae 1: 157. 1794.
Typus: Plate 5, figs 3–5 in J.V. Krombholz, Naturgetreue Abbildungen und Beschreibungen der Schwämme 1, 1831 (iconotype) (selected by Ryvarden 1991: 119).
Buglossoporus quercinus (Schrad.) Kotlaba & Pouzar, Česká Mykol. 20: 82. 1966.
Description: Ryvarden et al. (2017, as Piptoporus quercinus).
Specimens examined: France, Seine-et-Marne, Fontainebleau, Quercus sp., 15 Sep. 2001, Rivoire (LY BR-2030, H). Poland, Podlasie, Hajnówka, Białowieża, Q. robur, 2 Sep. 2017, Niemelä 9281 (H). USA, Pennsylvania, Montgomery Co., Norristown, Valley Forge, Quercus sp., 28 Jun. 2009, Vlasák Jr. 0906/15-J* (JV).
Notes: Donk (1971) argued that Boletus pulvinus represents the same species as Boletus quercinus (= Buglossoporus quercinus). The latter name has priority over B. pulvinus. However, it is unavailable as a basionym if B. quercinus is being moved to Fomitopsis, due to the existence of Agaricus quercinus (= Daedalea quercina, see Fomitopsis quercina below). Therefore, we use Persoon’s epithet to recombine this species in Fomitopsis. No authentic material of B. pulvinus survived, and a neotype for this species is designated above.
Fomitopsis pulvinascens (Pilát ex Pilát) Niemelä & Miettinen, comb. nov. MycoBank MB 844951.
Basionym: Poria pulvinascens Pilát ex Pilát, Acta Mus. Nat. Pragae 9 (B), 2 (1): 106. 1953.
Typus: Sweden, Upland, Bondkyrka, Vårdsätra, Salix sp., 16 Oct. 1936, Lundell (holotype PRM 756485!).
Description: Niemelä (1978, as Antrodia plicata).
Note: See Figs 1 and 3 for phylogenetic placement.
Fomitopsis purpurea Spirin & Ryvarden, sp. nov. MycoBank MB 844952. Fig 27.
Fig 27.
Basidiospores of Fomitopsis spp. F. purpurea (holotype); F. quercina (Miettinen 12662); F. retorrida (holotype); F. roseofusca (Vlasák 1908/83J); F. sagraeana (O 14113); F. scalaris (Vlasák 1909/66). Scale bar = 5 μm.
Typus: Tanzania, Arusha, Arusha Nat. Park, Mt. Meru, −3.24° 36.753°, hardwood, 8 Feb. 1973, Ryvarden 10118* (holotype O F915520).
Etymology: Purpureus (Lat., adj.) – purple.
Description: Basidiocarps perennial, sessile, conchate to ungulate, broadly attached, projecting up to 7 cm. Upper surface blackish brown to totally black, with distinct annual zones, sometimes irregularly cracking. Pileal edge sharp to rather blunt, concolourous with pore surface, up to 2 mm wide, sterile. Pore surface pink to pinkish brown, usually concave; pores roundish, 6–7 per mm, with rather thick, entire dissepiments. Section: crust exceptionally tough, brownish black to black, matt, up to 2 mm thick, context corky, pinkish brown to brown, up to 1 cm thick, more or less clearly delimited from tubes; tubes corky, stratified, pinkish brown to brown, in total up to 2.5 cm thick, each annual layer 1–3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, brownish to reddish-brown, interwoven, occasionally branched, (3.0–)3.2–4.8(−5.1) μm diam (n = 20/1), lumen capillary to indistinct, generative hyphae rare, hyaline, variably thick-walled, 2–3.5 μm diam. Trama dimitic; skeletal hyphae brownish to reddish-brown, interwoven, occasionally branched, (2.4–)2.7–3.3(−3.7) μm diam (n = 40/2), lumen wide to capillary, side branches 2–3 μm diam, generative hyphae hyaline, thin-walled, 2–3 μm diam. Subhymenium distinct, up to 20 μm thick. Cystidioles rare, tapering, 9–14 × 3–5 μm. Basidia clavate, (10.1–)10.2–13.1(−14.3) × (4.9–)5.1–6.1(−6.2) μm (n = 20/2). Basidiospores with a distinct wall, long cylindrical to subfusiform, (4.8–)5.0–6.7(−7.1) × (1.9–) 2.0–2.2(−2.3) μm (n = 60/2), L = 5.60–5.98, W = 2.09–2.10, Q = 2.68–2.86.
Specimen examined: Tanzania, Arusha, Arusha Nat. Park, Ngurdoto, hardwood, 10 Aug. 1966, Cain et al. (O F915519*).
Notes: This species was treated as F. carnea by Ryvarden & Johansen (1980), Carranza-Morse & Gilbertson (1986) and Han et al. (2016). We argued above that the epithet F. carnea is not applicable to the African species so named and should be reserved for the member of the F. feei complex widely distributed in Southeast Asia. Therefore, the African species is formally introduced here as F. purpurea. Large, ungulate basidiocarps of F. purpurea are reminiscent of those of F. lilacinogilva. The latter species is distributed in Australia and differs from F. purpurea in having a hirsute pileal surface, larger pores and wider basidiospores (see description above). So far, F. purpurea has been recorded twice in Tanzania.
Fomitopsis quercina (L.) Spirin & Miettinen, comb. nov. MycoBank MB 844953. Figs 26, 27.
Basionym: Agaricus quercinus L., Syst. Pl.: 1176. 1753.
Typus: Plate 181 in Sowerby 1799 (iconotype) (selected by Ryvarden 1991: 135).
Synonym: Daedalea quercina (L.) Pers., Syn. Meth. Fung. 2: 500. 1801.
Description: Ryvarden et al. (2017, as Daedalea quercina).
Specimens examined: Czech Republic, Jihočeský Reg., České Budějovice, Hluboká nad Vltavou, Quercus robur, 1979, Vlasák 7913/63 (H). Finland, Varsinaissuomi, Parainen, Lenholm, Q. robur, 2 May 2008, Miettinen 12662* (H); Raasepori, Solböle, Q. robur, 11 Aug. 1987, Niemelä 4030 (H). USA, Georgia, Habersham Co., Tallulah, Quercus sp., IV.2004, Vlasák Jr. 0404/2-J* (JV, H).
Note: See remarks to F. derelicta.
Fomitopsis ramentacea (Berk. & Broome) Spirin & Vlasák, comb. nov. MycoBank MB 844954.
Basionym: Polyporus ramentaceus Berk. & Broome, Ann. Mag. Nat. Hist. 3: 210. 1879.
Typus: UK, Scotland, Angus, Glamis, ‘on dead sticks’ [Pinus sylvestris], Stevenson 284 (lectotype K(M) 192120!) (selected by Ryvarden 1984: 352).
Synonyms:
Polyporus cerebrinus Berk. & Broome, Ann. Mag. Nat. Hist. 3: 210. 1879.
Typus: UK, Scotland, Angus, Glamis, ‘fir’ [Pinus sylvestris], Stevenson w/n (lectotype K, isolectotype BPI US0205257!) (selected by Ryvarden 1984: 334).
Trametes subsinuosa Bres., Ann. Mycol. 1: 81. 1903.
Typus: Italy, Tyrol, Trento, Pinus sylvestris, 1897, Bresadola (lectotype BPI US0321236!) (selected by Ryvarden 1988a: 324).
Antrodia subramentacea A. David & Dequatre, Cryptog. Mycol. 5 (4): 299. 1985.
Typus: France, Var, Le Faron, Pinus halepensis, Mar. 1974, David 3182* (holotype LY AD-3182!).
Description: Basidiocarps effused-reflexed, often 3–4 cm diam, sometimes fusing together and then up to 7 cm in widest dimension. Reflexed part projecting up to 5 mm, upper surface pubescent or smooth, sometimes indistinctly zonate, almost white to cream-coloured; margin sharp, often incurved. Margin of resupinate part adnate or partly detaching, compact, white to cream-coloured, up to 2 mm wide. Hymenial surface even, white to cream-coloured or pale ochraceous; pores angular, 1–3 per mm, dissepiments uneven to serrate. Section: context soft, white, up to 0.5 mm thick; tubes soft, in older parts partly agglutinated, pale cream-coloured to pale ochraceous, 1–3 mm thick. Hyphal structure monomitic; hyphae clamped. Context hyphae hyaline, moderately thick-walled to subsolid, (3.0–)3.2–5.3(−5.6) μm diam (n = 30/2). Tramal hyphae hyaline, slightly to distinctly thick-walled, (2.6–)2.7–5.2(−5.3) μm diam (n = 180/9), with occasional inflations up to 6 μm diam, often distinctly twisted at dissepiment edges. Cystidioles absent. Basidia clavate, (13.3–)14.8–27.7(−28.4) × (4.2–)4.3–6.1(−6.7) μm (n = 31/3). Basidiospores with a distinct wall, cylindrical, occasionally slightly curved, longest spores subfusiform, (6.0–)6.1–10.3(−11.3) × (2.0–)2.1–3.2(−3.6) μm (n = 450/15), L = 6.70–8.40, W = 2.28–2.92, Q = 2.52–3.48.
Specimens examined: Czech Republic, South Bohemia, Tábor, Soběslav, Pinus uliginosa, 25 Jul. 1973, Kotlaba, Pouzar & Niemelä (H, TN); Plzeň, Plzeň Sever, Lesni Závod, Pinus sylvestris, Oct. 2001, Kout 1001/1* (H, JV). Finland, Ahvenanmaa, Vårdö, Vargata, P. sylvestris, 20 Nov. 1967, Olofsson (H); Varsinaissuomi, Korppoo, Hjortö, P. sylvestris, 17 Oct. 2010, Kunttu 7419 (H); Länsi-Turunmaa, Nauvo, P. sylvestris, 26 Jul. 2010, Kunttu 6172 (H); Uusimaa, Vantaa, Mustavuori, P. sylvestris, 27 Sep. 1987, Saarenoksa 34987 (H); Etelä-Häme, Hartola, Kirkkola, P. sylvestris, 24 Oct. 1991, Haikonen 13653 (H). Germany, Brandenburg, Triglitz, P. sylvestris, Jaap (H ex Fungi selecti exsiccati #943); Mecklenburg-Vorpommern, Rügen, P. sylvestris, 23 Nov. 1994, Doll (H). Italy, Friuli-Venezia Giulia, Trieste, Pinus nigra, 8 Oct. 1992, Bernicchia 5840 (H ex HUBO). Norway, Rogaland, Forsand, Skiftåsen, P. sylvestris, 18 Jan. 2004, Førland 6* (O F177697). Poland, Warmia-Masuria, Szczytno, P. sylvestris, 8 Jul. 1969, Niemelä & Rykowsky (H). Russia, Nizhny Novgorod Reg., Lukoyanov Dist., Panzelka, P. sylvestris, 15 Aug. 2006, Spirin 2552* (H); Razino, P. sylvestris, 13 Aug. 2006, Spirin 2540* (H).
Notes: Fomitopsis ramentacea is a rare European species occurring on dry branches or just fallen, still corticated logs or hanging branches of Pinus spp. It is easily recognizable due to almost white, flexible, effused-reflexed basidiocarps with large, irregular pores and a peculiar host. Two related species distributed in Europe, i.e. F. renehenticii and F. solaris, are microscopically nearly identical to F. ramentacea. Fomitopsis renehenticii has smaller pores than F. ramentacea, and it mainly inhabits angiosperm hosts (with a few records on conifers). In turn, F. solaris occurs on both coniferous and deciduous trees, and it has somewhat smaller pores than F. ramentacea (and larger than in F. renehenticii) showing a tendency to radial arrangement in pileate parts. The Asian F. uralensis has clearly smaller basidiospores than found in the three aforementioned species.
Fomitopsis renehenticii (Rivoire, Trichies & Vlasák) Rivoire & Vlasák, comb. nov. MycoBank MB 844955.
Basionym: Cartilosoma renehenticii Rivoire, Trichies & Vlasák, Bull. Mens. Soc. Linn. Lyon 84: 8. 2015.
Typus: France, Ain, Poncin, Corylus avellana, 7 Sep. 2011, Rivoire (holotype LY BR-4111*!).
Description: Basidiocarps effused-reflexed, often 3–5 cm diam, sometimes fusing together and then up to 10 cm in widest dimension. Reflexed part projecting up to 6 mm, upper surface finely pubescent or smooth, azonate, cream-coloured; margin sharp. Margin of resupinate part adnate or partly detaching, compact, white to cream-coloured, up to 1 mm wide. Hymenial surface even, cream-coloured to pale ochraceous; pores angular, 3–5 per mm, dissepiments uneven to serrate. Section: context soft, white, up to 0.5 mm thick; tubes soft, in older parts partly agglutinated, pale cream-coloured to pale ochraceous, 1–2 mm thick. Hyphal structure monomitic; hyphae clamped. Context hyphae hyaline, moderately thick-walled to subsolid, (3.4–)3.7–5.4(−5.7) μm diam (n = 20/1). Tramal hyphae hyaline, slightly to distinctly thick-walled, (2.4–)3.0–5.1(−5.2) μm diam (n = 40/2), more or less straight at dissepiment edges. Cystidioles absent. Basidia clavate, (18.7–)18.8–28.4(−29.2) × (5.0–)5.3–6.1(−6.3) μm (n = 20/2). Basidiospores with a distinct wall, cylindrical, occasionally slightly curved, longest spores subfusiform, (5.8–)6.2–9.3(−10.4) × (2.3–)2.4–3.0(−3.1) μm (n = 150/5), L = 6.94–7.88, W = 2.69–2.83, Q = 2.52–2.80.
Specimens examined: Fomitopsis renehenticii. Belgium, Namur, Philippeville, Viroinval, Fondry des Chiens, Corylus avellana, 11 Oct. 2019, Spirin 13498 (H); Salix caprea, 11 Oct. 2019, Spirin 13511, 13518 (H). France, Aveyron, Creissels, Rubus sp., 9 May 2022, Spirin 15353 (H); Bouches-du-Rhône: Cary le Rouet, Pinus halepensis, 20 Jan. 2007, Rivoire (LY-BR 3059*); Moselle, Tressange, Alnus glutinosa, 1 Nov. 2007, Trichies 07400 (H); Meuse, Ornes, S. caprea, 12 Jul. 2008, Trichies 08150 (H); Rhône, Orliénas, Cupressocyparis leylandis, 18 Dec. 2018, Rivoire (LY-BR 7347*); Seine-Maritime, Elbeuf sur Andelle, Salix sp., 19 Sep. 2015, Penz (LY BR-6274*). Germany, Baden-Württemberg, Hockenheim, Salix cinerea, 14 Dec. 1985, Winterhoff (H). Slovenia, Cerknica, Goričice, C. avellana, 30 Sep. 2023, Spirin 16857 (H). Fomitopsis renehenticii× solaris. France, Lot, Villesèque, Coumes, Juniperus communis, 13 Jan. 2016, Hannoire (LY-BR 6356*).
Notes: Fomitopsis renehenticii is a close relative of F. ramentacea and F. solaris; their differences are listed under the first species. The distribution of F. renehenticii is seemingly limited to the western and central parts of Europe (Belgium, Czech Republic, France, Germany, Italy, Slovenia).
Fomitopsis retorrida Spirin & Kotiranta, sp. nov. MycoBank MB 844956. Fig 27.
Typus: Russia, Sakhalin, Korsakov Dist., Bolshoe Vavaiskoe Lake, 46.637° 143.192°, Abies sp. (fallen corticated log), 23 Aug. 2007, Kotiranta 28979* (holotype H7200205).
Etymology: Retorridus (Lat., adj.) – burnt-up, sunburnt.
Description: Basidiocarps resupinate, first orbicular, then partly fusing together, up to 5 cm in widest dimension. Margin adnate, compact, white to cream-coloured, up to 2 mm broad. Hymenial surface even, pale ochraceous to bright ochraceous-brownish; pores angular to lacerate, 3–5 per mm, often fusing together, dissepiments uneven to serrate. Section: context soft, white or pale cream-coloured, up to 0.5 mm thick; tubes soft, in older parts partly agglutinated, pale cream-coloured to pale ochraceous-brownish, 0.2–0.5 mm thick. Hyphal structure monomitic; hyphae clamped. Context hyphae hyaline, slightly to distinctly thick-walled, (1.9–)2.0–3.4(−4.2) μm diam (n = 20/1). Tramal hyphae hyaline, thin- to distinctly thick-walled, interwoven, flexuous, partly glued together, (2.6–)2.8–4.2(−4.8) μm diam (n = 20/1), often twisted at dissepiment edges. Cystidioles rare, tapering, 15–20 × 3–4 μm. Basidia long clavate, (15.2–)16.0–25.3(−25.7) × (5.0–)5.1–6.3(−6.4) μm (n = 20/1). Basidiospores with a distinct wall, long cylindrical, occasionally slightly curved, longest spores subfusiform, (5.6–)5.8–7.3(−7.4) × (2.2–)2.3–3.1(−3.2) μm (n = 30/1), L = 6.49, W = 2.77, Q = 2.35.
Notes: The monomitic basidiocarps composed of predominantly thick-walled, flexuous generative hyphae with a variable lumen, rather long basidia and cylindrical basidiospores of F. retorrida are strongly reminiscent of the F. ramentacea complex. However, those taxa are phylogenetically not closely related. According to our data, F. retorrida is a sister species of F. mellita and F. pulvinascens (Figs 1, 3) although the latter ones show no certain morphological similarity to it. The aforementioned anatomical traits, in combination with totally resupinate basidiocarps and brightly coloured hymenial surface differentiate F. retorrida from all other Fomitopsis spp. treated here. The species is so far known only from the type locality in the Russian Far East.
Fomitopsis rosea (Alb. & Schwein.) P. Karst., Meddeland. Soc. Fauna Fl. Fenn. 6: 9. 1881.
Basionym: Boletus roseus Alb. & Schwein., Conspectus Fungorum in Lusatiae Superioris: 251. 1805.
Typus: Norway, Oslo, [no collection date], Blytt (herb. Fries) (lectotype UPS) (selected by Ryvarden 1991: 218).
Description: Carranza-Morse & Gilbertson (1986).
Notes: This species is the generic type of Rhodofomes. For phylogenetic affinity, see Figs 1, 3 and 12.
Fomitopsis roseoalba A.M. Soares, Ryvarden & Gibertoni, Fungal Diversity 83: 210. 2017.
Typus: Brazil, Amapá: Porto Grande, hardwood, Feb. 2014, Soares* (holotype URM 86923).
Description and phylogenetic data: Tibpromma et al. (2017).
Fomitopsis roseofusca (Romell) Spirin & Vlasák, comb. nov. MycoBank MB 844957. Figs 26, 27.
Basionym: Polyporus roseofuscus Romell, Bih. K. Svenska Vetensk.-Akad. Handl. 26 (16): 32. 1901.
Typus: Brazil, Mato Grosso, Serra da Chapada, Buriti, rotten log, 4 Jun. 1894, Malme 609 (holotype S F14845!).
Synonym: Nigroporus macroporus Ryvarden & Iturr., Mycologia 95 (6): 1070. 2004.
Typus: Venezuela, Bolívar, Canaima Nat. Park, 24 Feb. 2000, Ryvarden 42363* (isotype O!).
Description: Basidiocarps perennial, sessile, at first conchate or more rarely bracket-shaped, solitary, then fusing together in large imbricate groups, broadly attached, projecting up to 8 cm. Upper surface initially brownish black, indistinctly zonate, smooth, later totally black, with distinct annual zones, not cracking. Pileal edge sharp, concolourous with pore surface, up to 1 mm wide, sterile. Pore surface pinkish grey to brownish, flat or slightly concave; pores elongated-angular to sinuous, 1–2 per mm, with thick, entire dissepiments. Section: crust exceptionally tough, black, matt or glossy, up to 1 mm thick, context hard corky, dark brown, up to 2 mm thick, sharply delimited from tubes; tubes tough, clearly stratified, pinkish brown to brown, in total up to 2.5 cm thick, each annual layer 3–7 mm thick, separated from surrounding tube strata by a darker sterile tissue 0.2–0.5 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, dark brown, tightly interwoven, occasionally branched, sometimes encrusted by amorphous blackish matter, (2.8–)3.2–5.5(−5.6) μm diam (n = 20/1), lumen indistinct, generative hyphae brownish, slightly thick-walled, 2.5–3.5 μm diam. Trama dimitic; skeletal hyphae brownish to dark brown, tightly interwoven, occasionally branched, (2.4–)3.1–5.1(−5.2) μm diam (n = 40/2), lumen capillary to indistinct, generative hyphae hyaline, thin-walled, infrequent, (1.5–)2.0–3.0 μm diam. Skeletocystidia abundant, acute, not encrusted, distinctly thick-walled, 3–5 μm diam at the apex, slightly projecting above hymenium. Cystidioles rarely present in senescent hymenium, tapering, slightly thick-walled, 14.5–18.0 × 4.0–5.0 μm. Basidia clavate, (9.8–)10.7–16.2(−17.1) × (4.0–)4.2–6.0(−6.2) μm (n = 20/1), senescent basidia slightly thick-walled, often densely glued together. Basidiospores with a distinct wall, cylindrical to subfusiform, (4.6–)4.8–6.2(−6.8) × (1.7–)1.8–2.8(−3.0) μm (n = 36/2), L = 5.30–5.52, W = 2.24–2.55, Q = 2.12–2.51.
Specimens examined: Brazil, Amazonas, Yutajé, dead deciduous wood, 12 Jun. 1997, Ryvarden 40776 (O 10781). French Guiana, Camp Cayman, old fallen log, 31 Aug. 2019, Vlasák Jr. 1908/83-J* (JV, H).
Notes: Fomitopsis roseofusca is a Neotropical species morphologically most similar to F. cupreorosea (F. feei complex) which is distributed in the same geographic area. It produces thicker, tougher and darker basidiocarps than F. cupreorosea. Pores of F. roseofusca are on average larger than in F. cupreorosea. Microscopically, the species can be separated due to different skeletocystidia, acute in F. roseofusca and obtuse in F. cupreorosea. Phylogenetically, F. roseofusca seems to be isolated from other members of Fomitopsis (see Figs 1 and 3, as N. macroporus).
Fomitopsis sagraeana (Mont.) Vlasák & Spirin, comb. nov. MycoBank MB 844958. Fig 27.
Basionym: Polyporus sagraeanus Mont., Ann. Sci. Nat., Bot. 2 (17): 127. 1842.
Typus: Cuba, Sancti Spíritus, San Marcos, ‘ad truncos arborum’, [no collection date], de la Sagra (isotype BPI 248508!).
Description: Basidiocarps perennial, sessile, often with a contracted base (fan-shaped), projecting up to 7.5 cm. Upper surface first pinkish ochraceous to purplish-brownish, velvety, then occasionally fading to light greyish, smooth and indistinctly zonate. Pileal edge sharp to rather blunt, concolourous with hymenial surface, sterile, up to 1 mm wide, then fertile. Pore surface deep pink to pinkish-greyish, flat or concave; pores roundish to angular, 6–8 per mm, with thin or moderately thickened, entire or uneven dissepiments. Section: context corky, deep pink or pinkish brownish, up to 7 mm thick; tubes corky, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 5 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae almost hyaline to brownish, densely interwoven, occasionally branched, (3.1–)3.2–5.2(−5.8) μm diam (n = 40/2), lumen normally wide, side branches 2–3 μm diam, generative hyphae rare, hyaline, thin- to slightly thick-walled, 3–5 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to reddish-brown, densely interwoven to subparallel, occasionally branched, (2.2–)2.3–3.8(−4.0) μm diam (n = 100/5), lumen varying from rather wide to capillary, generative hyphae rare, thin- to slightly thick-walled, 2–4 μm diam. Subhymenium partly distinct, up to 10 μm thick. Cystidioles tapering, 11–28 × 4.0–6.0 μm. Basidia clavate, (11.3–)12.0–20.8(−23.2) × (4.8–)5.0–6.4(−6.8) μm (n = 38/3), occasionally pleural. Basidiospores with a distinct wall, cylindrical-subfusiform, (4.8–)4.9–7.3(−7.4) × (2.4–)2.6–3.9(−4.0) μm (n = 111/4), L = 5.75–5.89, W = 2.87–3.29, Q = 1.76–2.05.
Specimens examined: Costa Rica, Alajuela, San Carlos, Arenal, hardwood, 23 Dec. 2015, Vlasák Jr. 1512/2J* (JV, TUF); Guanacaste, Lomas Barbudal Park, hardwood, 24 Dec. 2014, Vlasák Jr. 1412/5J* (JV, TUF); Puntarenas: Las Tablas, 24 Apr. 2000, Navarro 1897 (O 14113, H). Dominican Republic, La Vega, Ebano Verde Nat. Res., angiosperm branch, 29 Dec. 2001, Perdomo 671 (O 16293, H). USA, Florida, Miami-Dade Co., Long Pine Key, hardwood, 19 Apr. 2009, Vlasák 0904/39* (JV, H); Monroe Co., Key Largo, John Pennekamp Coral Reef State Park, hardwood, 29 Aug. 2010, Vlasák Jr. 1008/76J* (JV, H).
Notes: Fomitopsis sagraeana is a member of the F. feei complex. It is distributed in the northern and western parts of the Caribbean. It differs from F. feei sensu stricto in having slightly smaller pores and larger basidiospores. As redefined here, F. feei occurs in tropical areas of South America (see above). Lowe (1957) accepted F. feei and F. sagraeana as two different species, but his description of the latter one refers to F. cupreorosea.
Fomitopsis sandaliae (Bernicchia & Ryvarden) Bernicchia & Vlasák, comb. nov. MycoBank MB 844960.
Basionym: Antrodia sandaliae Bernicchia & Ryvarden, Mycotaxon 79 (1): 58. 2001.
Typus: Italy, Sud Sardegna, Foresta Demaniale di Montarbu, Arbutus unedo, 4 Dec. 2000, Bernicchia & Ryvarden (holotype HUBO 7348!).
Description: Bernicchia & Ryvarden (2001), Bernicchia & Gorjón 2020 (as A. sandaliae).
Specimen examined: Italy, Sud Sardegna, Foresta Demaniale di Montarbu, A. unedo, 29 Nov. 2003, Bernicchia (HUBO7803*).
Note: This nearly monomitic species belongs to the Buglossoporus subclade (Suppl. Fig. S8).
Fomitopsis scalaris (Cooke) Ryvarden, Mycotaxon 20: 354. 1984. Figs 26, 27.
Basonym: Fomes scalaris Cooke, Grevillea 14 (69): 18. 1885.
Typus: Brazil, Panurè, dead trunks of trees, Feb. 1853, Spruce 62 (syntype K); ibid., Spruce 199 (syntype K, isosyntype PC!).
Synonym: Nigroporus rigidus Ryvarden, Mycotaxon 28: 532. 1987.
Typus: Venezuela, Amazonas, Cerro de la Neblima, on wood, 21 Apr. 1984, Samuels 1478 (isotype O 18149!).
Description: Basidiocarps perennial, sessile, at first bracket-shaped, later ungulate, broadly attached, projecting up to 5 cm. Upper surface initially brownish, indistinctly zonate, smooth or slightly felty, later blackish brown to totally black, with distinct annual zones, not cracking. Pileal edge sharp, concolourous with pore surface, up to 1 mm wide, sterile. Pore surface pinkish-grey to brownish-grey, flat or slightly concave, finally fading to mouse-grey; pores roundish, 6–8 per mm, with rather thin or thick, entire dissepiments. Section: crust (present in old basidiocarps) exceptionally tough, black, matt, up to 0.5 mm thick, context hard corky, brown, up to 1 mm thick, sharply delimited from tubes; tubes tough, clearly stratified, brownish grey to brown, in total up to 30 mm thick, each annual layer 1–3 mm thick, separated from surrounding tube strata by a darker sterile tissue 0.2–1 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, brown, tightly interwoven to subparallel, occasionally branched, (2.8–)3.2–4.3(−4.6) μm diam (n = 20/1), lumen capillary to indistinct, generative hyphae rare, hyaline, slightly thick-walled, 2–4 μm diam. Trama dimitic; skeletal hyphae brownish to dark brown, tightly interwoven, occasionally branched, sometimes covered by amorphous brownish encrustation, (2.1–)2.2–3.8(−4.2) μm diam (n = 60/3), lumen wide to capillary, some skeletals with frequent adventive septa, generative hyphae hyaline, thin- or slightly thick-walled, infrequent, (1.0–)1.5–2.5 μm diam. Subhymenium indistinct. Skeletocystidia present as swollen (up to 4.5 μm diam) apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles rare, tapering, 14–15 × 4–5 μm. Basidia short-clavate, (6.7–)8.4–11.1(−12.8) × (3.8–)3.9–4.9(−5.2) μm (n = 15/2), quickly collapsing. Basidiospores with a distinct wall, cylindrical, longest spores slightly tapering to the distal end, (3.0–) 3.1–5.0(−5.2) × (1.9–)2.0–2.3(−2.5) μm (n = 70/3), L = 3.39–3.90, W = 2.10–2.13, Q = 1.60–1.83, sometimes glued in groups of 3–4.
Specimens examined: Brazil, Paragominas, Mina da Hydro, angiosperm snag, 18 Nov. 2014, Runnel 1211 (MG211148, O). French Guiana, Kourou, Monkey Hill, old fallen log, 6 Sep. 2019, Vlasák 1909/66* (JV, H), Roura, Montagne Tresor, fence prop, 24 Aug. 2018, Vlasák 1808/50* (JV). Venezuela, Bolívar, Canaima, 10 Jan. 1972, Bresinsky (O 10869).
Notes: Ungulate basidiocarps with blackish crust and partly pinkish pore surface, as well as brown skeletal hyphae and rather small-sized basidiospores might suggest a close relationships of F. scalaris and members of the F. rosea or the F. feei complexes. Our phylogenetic analyses show that, contrary to morphological indications, it occupies an isolated position within the genus (Figs 1, 3). Fomitopsis scalaris is a Neotropical species, with confirmed records from the Amazon basin and adjacent areas. One of the authentic specimens (Spruce 199) is in perfect condition, fertile, and it is undoubtedly identical to our newly collected and sequenced material.
Fomitopsis sclerotina (Rodway) M.D. Barrett & Spirin, comb. nov. MycoBank MB 844961.
Basionym: Polyporus sclerotinus Rodway, Pap. Proc. R. Soc. Tasm. 1917: 108. 1918.
Typus: Australia, Tasmania, Mt. Field, ‘on gravelly earth’, Nov. 1917, Rodway (holotype HO 120637).
Description: Núñez & Ryvarden (1995, as Laccocephalum sclerotinum), Buchanan & Ryvarden (1993, as P. sclerotinus).
Specimen examined: Australia, Western Australia, Collis Rd, Walpole-Nornalup National Park, 7 May 2018, Syme 2967* (PERTH).
Notes: Fomitopsis sclerotina is an unusual species, with small, stipitate basidiocarps arising from a pebble-sized white sclerotium of the homogenous consistency. Skeletal hyphae are dominant in the stipe, but often almost absent in the pileus (Buchanan & Ryvarden 1993). See remarks under F. tumulosa for discussion of Laccocephalum.
Fomitopsis serialiformis (Kout & Vlasák) Vlasák, comb. nov. MycoBank MB 844962.
Basionym: Antrodia serialiformis Kout & Vlasák, Mycotaxon 108: 331. 2009.
Typus: USA, Pennsylvania, Philadelphia, Wissahickon Creek Park, Quercus sp., 31 Aug. 2008, Vlasák 0808/47* (isotype PRM 915459!).
Description and phylogenetic data: Kout & Vlasák (2009, as A. serialiformis); see also Fig. 3.
Fomitopsis serialis (Fr.) Spirin & Runnel, comb. nov. MycoBank MB 844963.
Basionym: Polyporus serialis Fr., Syst. Mycol. 1: 370. 1821.
Typus: Norway, Telemark, Nome, Mørkvasslia Nat. Res., Picea abies, 23 Sep. 2003, K.H. Larsson 12010* (neotype GB!) (designated by Spirin et al. 2017: 227).
Synonym: Antrodia serialis (Fr.) Donk, Persoonia 4: 340. 1966.
Description and phylogenetic data: Spirin et al. (2017, as Antrodia serialis); see also Figs 1 and 3.
Fomitopsis serrata (Vlasák & Spirin) Vlasák & Spirin, comb. nov. MycoBank MB 844964.
Basionym: Antrodia serrata Vlasák & Spirin, Mycologia 109: 228. 2017.
Typus: USA, New Hampshire, Carroll Co., Slippery Brook, Picea sp., Sep. 2008, Vlasák 0809/72* (holotype H!).
Description and phylogenetic data: Spirin et al. (2017, as A. serrata).
Fomitopsis solaris Rivoire, A.M. Ainsworth & Vlasák, sp. nov. MycoBank MB 844965. Fig 28.
Fig 28.
Basidiospores of Fomitopsis spp. F. solaris (holotype); F. subectypa (holotype); F. tristis (holotype); F. tunicata (holotype); F. uralensis (Spirin 5399); F. visenda (holotype). Scale bar = 5 μm.
Typus: France, Val de Marne, Vincennes, Bois de Vincennes, coniferous tree (Pinus?), 3 Dec. 2012, Hentic (holotype LY-BR 4732*, isotype H7200196).
Etymology: Solaris (Lat., adj.) – resistant to sunlight; in reference to open habitats where the species can sometimes occur.
Description: Basidiocarps annual or biennial, sessile, effused-reflexed or totally resupinate, often 2–4 cm diam, often not fusing together. Reflexed part projecting up to 10 mm, upper surface finely pubescent or smooth, azonate, uneven and sometimes with low concentric or radial ridges, cream-coloured, in persistent basidiocarps covered by epiphytic algae; margin sharp, incurved, or rather blunt. Margin of resupinate part adnate or partly detaching, compact, white to cream-coloured, up to 1 mm wide. Hymenial surface even, cream-coloured to pale ochraceous; pores angular, 2–3 per mm, in pileate parts showing a tendency to radial arrangement, dissepiments uneven to serrate. Section: context soft, white or pale cream-coloured, up to 1 mm thick; tubes soft, in older parts partly agglutinated, pale cream-coloured to pale ochraceous, 1–3 mm thick. Hyphal structure monomitic; hyphae clamped. Context hyphae hyaline, moderately thick-walled to subsolid, (3.0–) 3.2–5.2(−6.2) μm diam (n = 40/2). Tramal hyphae hyaline, slightly to distinctly thick-walled, (2.2–)2.3–4.3(−4.7) μm diam (n = 100/5), occasionally twisted at dissepiment edges. Cystidioles absent. Basidia (15.6–)15.7–24.0(−26.0) × (4.8–)5.0–7.1(−7.3) μm (n = 50/4). Basidiospores with a distinct wall, cylindrical, occasionally slightly curved, longest spores subfusiform, (5.8–)5.9–9.6(−10.4) × (2.0–)2.1–3.4(−3.6) μm (n = 210/7), L = 6.71–8.30, W = 2.35–2.99, Q = 2.78–3.15.
Specimens examined: Canada, Northwest Territories, Inuvik, Picea sp., 20 Sep. 1984, Niemelä 3058* (H); Salix sp., 14 Sep. 1984, Niemelä 2992 (H); Alnus crispa, 20 Sep. 1984, Niemelä 3061* (H). Denmark, Midtjylland, Herning, Stovbæk, Salix sp., 29 Mar. 2013, Boertmann 501446* (C, H); Syddanmark, Assens, Aarup, Salix cinerea, 26 Apr. 2016, Kaballe & Boertmann 737876 (C, H); Vejen, S. cinerea, 29 Dec. 2013 Boertmann 658643 (C, H). Germany, Niedersachsen, Hagenburg, Salix sp., 27 Mar. 1989, Wöldecke (H). Israel, [no collection locality], Cupressus sempervirens, 2008, Zmitrovich (LE, H*). Jersey, St. Martin, Rozel Woods, S. cinerea, 20 Jan. 2014, Legon (K(M) 191007*). Sweden, Skåne, Mörarp, Salix sp., 25 Dec. 1994, Hanson & Gustafsson (H ex S). United Kingdom, England, Berkshire vc22, Bisham Wood, Salix sp., 30 Jan. 2000, Ainsworth & Green (K(M) 85251*); Buckinghamshire vc24, Wraysbury, by Colne Brook, Salix sp., 24 Feb. 2006, Ainsworth (K(M) 137688*); North Hampshire vc12, Cricket Hill, Salix sp., 6 Feb. 2000, Ainsworth & Lucas (K(M) 84579*); South Somerset vc5, Yeovil, Salix sp., 16 Feb. 2011, Legon (K(M) 180117*); Surrey vc17, Langham Pond Salix fragilis, 29 Aug. 2007, Legon (K(M) 153625*); West Kent vc16, Lullingstone Park, Salix sp., 7 Feb. 2011, Pitt (K(M) 169193*).
Notes: Morphological differences between F. solaris and its close relatives are discussed under F. ramentacea. The species is widely distributed in Eurasia and it occurs in the north-western part of North America, as well as in the southern part of Argentina (reported as A. ramentacea by Rajchenberg et al. 2011). Fomitopsis solaris inhabits both gymnosperm and angiosperm wood. An “uncultured Antrodia” sequence (GenBank KT334658) from oak roots from Poland belongs to this species. In the UK, this species has thus far only been found on the wood of Salix spp. and, despite the chosen epithet, these have been in predominantly wet woodland habitats.
These British collections were previously referred to Antrodia ramentacea and photographs of the sequenced paratype collection K(M) 85251 can be found in Ainsworth (2001).
Fomitopsis spraguei (Berk. & M.A. Curtis) Gilb. & Ryvarden, Mycotaxon 22 (2): 364. 1985.
Basionym: Polyporus spraguei Berk. & M.A. Curtis, Grevillea 1 (4): 50. 1872.
Typus: USA, ‘New England, Murray’, [no collection date and collector] (lectotype K) (selected by Ryvarden 1984: 356).
Synonym: Polyporus castaneae Bourdot & Galzin, Bull. Soc. Mycol. France 41: 105 (1925).
Typus: France, Aveyron, Combette, Castanea sativa, 28 Aug. 1911, Galzin 9879 (lectotype PC!) (selected here, MycoBank MBT 10008293).
Description: Gilbertson & Ryvarden (1986).
Specimens examined: USA, New York, Oswego, Pulaski, Quercus rubra, Aug. 2016, Vlasák 1608/14* (JV); Tennessee, Great Smoky Mountains NP, dicot, 13 Dec. 2004, Ryvarden 46569* (O, H).
Note: See Figs 1, 3 and 15 for phylogenetic placement.
Fomitopsis squamosella (Bernicchia & Ryvarden) Bernicchia & Ryvarden, comb. nov. MycoBank MB 844966.
Basionym: Antrodia squamosella Bernicchia & Ryvarden, Cryptog. Mycol. 19 (4): 282. 1998.
Typus: Italy, Nuoro, Supramonte di Orgosolo, Juniperus oxycedrus, 9 Nov. 1994, Bernicchia 6650 & Curreli (isotype O 10239!).
Description: Bernicchia & Ryvarden (1998, as A. squamosella).
Specimen examined: Italy, Sardinia, Nuoro, Supramonte di Orgosolo, J. oxycedrus, 2 Dec. 2003, Bernicchia (HUBO 7690*).
Note: See Figs 1, 3 and Suppl. Fig. S8 for phylogenetic placement.
Fomitopsis stereoides (Fr.) Spirin, comb. nov. MycoBank MB 844967.
Basionym: Daedalea stereoides Fr., Nova Acta R. Soc. Scient. Upsal. 3, 1: 99. 1851.
Typus: Costa Rica, San José, ‘ad truncos’, [no collection date], Örsted (holotype UPS F-127299!).
Description: Ryvarden (2015, as D. stereoides).
Note: Lindner et al. (2011) showed that this species belongs to the Daedalea clade (see also Fig. 5).
Fomitopsis subectypa (Murrill) Spirin & Vlasák, comb. nov. Fig 25. MycoBank MB 844968. Fig 28.
Basionym: Coriolus subecTypus Murrill, North American Flora 9: 22. 1907.
Typus: USA, Florida, [no locality indicated], dead wood, Apr. 1885, Rau 254 (holotype NY00705010!).
Synonyms:
Coriolus hollickii Murrill, Mycologia 2: 187. 1910.
Typus: Jamaica, Moneague, Union Hill, on log in woods, 6–7 Apr. 1908, Britton & Hollick (holotype NY 00704966!).
Daedalea americana M.L. Han, Vlasák & B.K. Cui, Phytotaxa 204 (4): 280. 2015.
Typus: USA, Florida, Miami, Matheson Hammock, hardwood, 19 Apr. 2009, Vlasák 0904/20* (holotype BJFC 15575!).
Description: Basidiocarps short-living perennial, sessile, dimidiate or with a contracted base (fan-shaped), projecting up to 7 cm. Upper surface first wood-coloured to beige, velutinous, indistinctly zonate, later pale ochraceous to tan, smooth, with numerous narrow concentric zones, usually radially striate. Pileal edge sharp or rather blunt, concolourous with cap surface, first sterile, up to 1 mm wide, then fertile. Pore surface cream-coloured or beige to pale ochraceous, sometimes with a faint pinkish tint or vinaceous-reddish stains, flat or concave, later fading to greyish-white; pores roundish to angular, 5–7 per mm, with thin or rather thick, entire dissepiments. Section: context corky, cream- to wood-coloured, up to 7 mm thick; tubes soft corky, one-layered, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae brownish, interwoven or in subparallel bundles, occasionally branched, (2.6–)2.7–7.3(−7.8) μm diam (n = 60/3), lumen changing from wide to capillary, side branches 1.5–3 μm diam, generative hyphae infrequent, hyaline, thin- to slightly thick-walled, 2–4 μm diam. Trama dimitic; skeletal hyphae dominating, pale ochraceous to brownish, interwoven, occasionally branched, (2.0–)2.1–5.3(−5.8) μm diam (n = 60/3), in older basidiocarps sometimes inflated up 6–7 μm diam, lumen mostly narrow to capillary, generative hyphae thin- to slightly thick-walled, 2–3.5(−4) μm diam, occasionally forming distinct subhymenial layer up to 10 μm thick. Cystidioles rather rare, tapering, 11–16 × 3–5.5 μm. Skeletocystidia present as swollen (up to 6 μm diam) apices of tramal skeletal hyphae, slightly projecting above hymenium. Basidia clavate, (10.3–)10.8–17.4(−18.1) × (4.2–) 4.3–6.2(−6.4) μm (n = 41/3), occasionally pleural. Basidiospores thin-walled or with a distinct wall, ellipsoid-ovoid, longest spores subfusiform, (4.0–)4.1–6.0(−6.2) × (2.1–)2.2–3.2(−3.3) μm (n = 90/3), L = 4.52–5.02, W = 2.57–2.86, Q = 1.58–1.79.
Specimens examined: Costa Rica, Guanacaste: Rincón de la Vieja, hardwood, 1 Aug. 2014, Vlasák 1407/3* & 1407/23 (JV); Villa La Paz, hardwood, 22 Apr. 2017, Vlasák 1704/107J* (JV). USA, Florida, Miami, Matheson Hammock, Quercus sp., 19 Apr. 2009, Vlasák 0904/17 (JV, H).
Notes: Fomitopsis subectypa is a North and Central American species closely related to F. modesta (Fig. 5). It normally produces larger basidiocarps than those of F. modesta, and it has slightly wider pores and on average larger basidiospores than in the latter species. Fomitopsis subectypa has been found in Costa Rica, Jamaica, and the southern part of Florida (USA) although its distribution might be wider. Distribution areas of F. modesta and F. subectypa overlap at least in Costa Rica.
Fomitopsis substratosa (Malençon) Spirin & Miettinen, comb. nov. MycoBank MB 844970.
Basionym: Ungulina substratosa Malençon, Bull. Soc. Mycol. France 71: 311. 1956.
Typus: Morocco, Middle Atlas, Cedrus atlantica (burnt log), [no collection date indicated], Malençon 1297 (lectotype designated hereMPU!, MycoBank MBT 10008294).
Synonym: Fomitopsis subfeei B.K. Cui & M.L. Han, Mycoscience 56: 170. 2015.
Typus: China, Sichuan: Dujiangyan, Qingcheng, Cunninghamia sp., 13 Sep. 2010, Cui 9231* (holotype BJFC 8169).
Description and phylogenetic data: Han & Cui (2015, as F. subfeei); see also Fig. 11.
Specimens examined: China, Guangdong, Shixing, Chebaling Nat. Res., Pinus sp., 14 Sep. 2009, Cui 7448 (H); Hunan, Sangzhi, Badagongshan Nat. Res., 23 Sep. 2000, Härkönen K805 (H). Morocco, Middle Atlas, Cedrus atlantica, [no collection date indicated], Male inçon s/n, 1133, 1139, 1242, 1296, 2979 (MPU).
Notes: We studied the type material of U. substratosa from Morocco and found it morphologically indistinguishable from F. subfeei which was described as widely distributed in the southern part of China. Therefore, we place the latter species to the synonyms of F. substratosa, although sequenced specimens from North Africa are highly desirable for confirming this synonymy.
Fomitopsis sulcata (Berk.) Corner, Beih. Nova Hedwigia 96: 39. 1989. Fig 26.
Basionym: Hexagonia sulcata Berk., London J. Bot. 6: 510. 1847.
Typus: Sri Lanka, [no locality, collection date or collector], herb. E. Fries (lectotype designated here UPS F-126402!, MycoBank MBT 10008295).
Synonyms:
Favolus resinosus Murrill, Bull. Torrey Bot. Club 35: 398. 1908.
Typus: Philippines, Luzon, Rizal, Bosoboso, dead wood, Jul. 1906, Ramos 1214 (holotype NY00704732!).
Fomitopsis cystidiata B.K. Cui & M.L. Han, Mycol. Progr. 13: 908. 2014.
Typus: China, Hainan, Baoting, Qixianling, hardwood, 27 Nov. 2007, Cui 5481* (holotype BJFC).
Description and phylogenetic data: Han et al. (2014, as F. cystidiata); see also Fig. 3.
Specimens examined: China, Hainan, Guandong, rotten wood, 17 Apr. 1977, Han 701 & Zhao (H ex HMAS 40517). India, Uttarakhand, Dehradun, Lachhiwala Forest Range, Shorea robusta, 10 Oct. 1973, Dhanda 6844 (H ex O 10548). Indonesia, Riau, Kampar, Balung, 24 Dec. 2006, Miettinen 11275 (BO, H). Malaysia, Pahang, 9 Jun. 1931, Corner (H ex O 10556). Thailand, Sukhotai, Khiri Mat, hardwood, Aug. 2014, Kout 1408/K5* (JV, TUF).
Notes: Ryvarden (1977) stated he could not detect authentic material of H. sulcata in European herbaria. However, there is a specimen in Fries’s herbarium in Uppsala, evidently a part of the original collection sent to him by Berkeley. Here it is designated as a lectotype of H. sulcata. Corner (1989) combined H. sulcata in Fomitopsis and our data support his solution. The species can be easily identified in the field due to its robust but rather soft basidiocarps with a dark-coloured crust and exceptionally large pores. Fomitopsis sulcata seems to be widely distributed in Southeast Asia; here we confirm records from India, Indonesia, Malaysia, Sri Lanka, Thailand and the southern part of China (Hainan).
Fomitopsis tristis Miettinen & Spirin, sp. nov. MycoBank MB 844972. Figs 26, 28.
Typus: Indonesia, Papua Barat, Manokwari, Amberbaken, River Anjii/Wepai, −0.5771° 133.1634° (±300 m), seasonally dry riverbed, a piece of wood (dicot, decay stage 2, 27 cm in diameter), 1 Nov. 2010, Miettinen 14263* (holotype MAN, isotypes H7200140, BO).
Etymology: Tristis (Lat., adj.) – gloomy.
Description: Basidiocarps annual, effused-reflexed, solitary or imbricate, projecting up to 0.5 cm, occasionally fusing together in large groups and then up to 5 cm in longest dimension. Upper surface ridged, greyish-brown to almost black, sometimes covered by incomplete pores. Pileal edge sharp to blunt, concolourous with cap surface, often covered by open pores, margin of resupinate parts greyish, compact, adnate, sharply delimited. Pore surface grey to greyish-brown; pores angular to elongated, 5–7 per mm, with thin, entire or uneven dissepiments. Section: context sturdy, dark grey to almost black, up to 0.5 mm thick; tubes sturdy, one-layered, concolourous with hymenial surface, up to 3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae greyish to brownish, interwoven or subparallel, sometimes glued together by amorphous brownish matter, occasionally branched, (2.7–)2.8–3.8(−4.2) μm diam (n = 20/1), lumen varying from narrow to capillary or indistinct, side branches 1.5–2.5 μm diam, generative hyphae hyaline, slightly thick-walled, 2–3 μm diam. Trama dimitic; skeletal hyphae greyish to brownish, rather loosely interwoven, some flexuous, occasionally branched, (2.8–)2.9–4.0(−4.1) μm diam (n = 20/1), lumen wide to capillary, generative hyphae thin- or slightly thick-walled, 2–3.5 μm diam. Subhymenium indistinct. Rhomboid or prismatic crystals abundant among tramal hyphae, up to 5 μm in longest dimension. Cystidioles abundant, gradually tapering to the apex, 12–15.5 × 4–6 μm. Basidia clavate, (11.8–)12.9–19.1(−19.7) × (5.1–)5.2–6.4(−6.8) μm (n = 20/1). Basidiospores with a distinct wall, broadly cylindrical to narrowly ellipsoid, sometimes slightly curved, (4.2–)4.4–5.8(−5.9) × (2.7–)2.8–3.1(−3.2) μm (n = 30/1), L = 5.05, W = 2.94, Q = 1.72.
Notes: Annual, small, half-resupinate, mouse-grey basidiocarps occurring in large groups are a unique macroscopic trait of F. tristis. Clearly coloured skeletal hyphae, non-encrusted cystidioles and rather small basidiospores of this species are reminiscent of F. nigra and its siblings (= Melanoporia spp.) but they are not closely related to F. tristis phylogenetically (Suppl. Fig. S2). To date, the species has been recorded only at the type locality in New Guinea.
Fomitopsis tropica (B.K. Cui) Spirin, comb. nov. MycoBank MB 844973.
Basionym: Antrodia tropica B.K. Cui, Mycol. Progr. 12: 226. 2013.
Typus: China, Hainan, Changjiang, Bawangling, Engelhardtia hainanensis, 9 May 2009, Cui 6471* (holotype BJFC).
Description and phylogenetic data: Cui (2013, as A. tropica); see also Suppl. Fig. S2.
Fomitopsis tumulosa (Cooke) M.D. Barrett & Spirin, comb. nov. MycoBank MB 844974.
Basionym: Polyporus tumulosus Cooke, Grevillea 17: 55. 1889.
Typus: Australia, Queensland, Brisbane, ‘on the ground’, [no collection date], Bailey 607 (lectotype K) (selected by Cunningham 1965: 78).
Description: Núñez & Ryvarden (1995, as Laccocephalum tumulosum).
Notes: This stipitate species belongs to the Buglossoporus subclade (see Fig. 3 and Suppl. Fig. S8). Fomitopsis tumulosa and F. hartmannii are unique in the genus in having sclerotia composed of sand bound within a mycelial mass, which in F. tumulosa can be very large and weighing up to 20 kg. The genus Laccocephalum was reinstated by Núñez & Ryvarden (1995) and expanded to contain five stipitate species arising from sclerotia, all Australian with L. hartmannii extending to East Asia. Laccocephalum sensu Núñez & Ryvarden (1995) is here considered polyphyletic, with only three of their five species belonging to the Fomitopsidaceae (L. hartmannii, L. sclerotinum and L. tumulosum), and even these do not form a monophyletic lineage, although all belong within the Buglossoporus subclade (Suppl. Fig. S8). The other two Laccocephalum species treated by Núñez & Ryvarden (1995), L. mylittae and the generic type L. basilapidoides, belong outside the Fomitopsidaceae, and will be treated elsewhere.
Fomitopsis tunicata Miettinen & Spirin, sp. nov. MycoBank MB 844975. Figs 26, 28.
Typus: Indonesia, Riau, Pekanbaru, Rumbai, Caltex Camp, 0.601° 101.442° (±500 m), selectively logged mosaic of freshwater swamp and mineral soil forest, fallen dicot tree, 17 Jun. 2004, Miettinen 8579* (holotype BO, isotype H7200147).
Etymology: Tunicatus (Lat., adj.) – coated, tunicate.
Description: Basidiocarps annual, effused, up to 13 cm in widest dimension. Margin adnate, compact, cream-coloured, up to 1 mm broad. Hymenial surface even, cream-coloured to ivory; pores roundish to angular, 7–9 per mm, dissepiments even. Section: subiculum compact, white to pale cream-coloured, compact, 0.2–0.5 mm thick; tubes leathery, concolourous with pore surface, 0.5–2 mm thick. Hyphal structure dimitic; hyphae clamped. Subiculum dimitic; skeletal hyphae hyaline or yellowish, interwoven to subparallel, occasionally branched, (3.7–)3.8–5.7(−6.3) μm diam (n = 20/1), lumen mostly capillary to indistinct, side branches 2–3 μm diam, generative hyphae rather abundant, hyaline, slightly thick-walled, 2–4 μm diam. Trama dimitic; skeletal hyphae hyaline or yellowish, occasionally branched, (2.8–)2.9–4.1(−4.2) μm diam (n = 20/1), lumen narrow to indistinct, generative hyphae abundant, hyaline, thin- to slightly thick-walled, 2–3.5 μm diam. Subhymenium distinct in some parts, up to 20 μm thick. Cystidioles abundant, tapering, 11–15 × 3–4 μm. Skeletocystidia present as somewhat swollen (up to 4.5 μm diam), blunt apices of tramal skeletal hyphae, slightly projecting above hymenium. Basidia broadly clavate, (10.8–)11.0–15.1(−16.0) × (5.2–)5.3–6.4(−7.2) μm (n = 20/1). Basidiospores slightly thick-walled, ellipsoid, (3.4–)3.5–4.3(−5.1) × (2.6–)2.7–3.0(−3.1) μm (n = 30/1), L =3.95, W = 2.87, Q = 1.38, slightly cyanophilous, often with a large central oil-drop.
Specimen examined: Indonesia, Sumatera Barat, Padang, Limau Manis, forest edge, fallen dicot branch, 14 Jul. 2008, Miettinen 13050* (ANDA, H).
Notes: Phylogenetically, Fomitopsis tunicata is most closely related to F. glabricystidia (Figs 1, 3). Both species possess slightly thick-walled and weakly cyanophilous basidiospores reminiscent those of Perenniporia spp. However, neither their spores nor skeletal hyphae are dextrinoid, and this feature certainly separates the two species from the representatives of Perenniporia. Fomitopsis tunicata differs from F. glabricystidia in having smaller pores (4–6 per mm in F. glabricystidia), as well as narrower skeletal hyphae in the tube trama (4–6 μm diam in the latter species).
Moreover, F. glabricystidiata bears more pronounced and abundant skeletocystidia, and its basidiospores are slightly larger than F. tunicata, (3.9–)4.0–4.7(−4.9) × (2.9–)3.0–3.3(−3.4) μm (n = 30/1), L =4.24, W = 3.13, Q = 1.35. Fomitopsis tunicata was found twice in Southeast Asia while F. glabricystidia has been so far detected in two localities in Central Africa (Uganda).
Fomitopsis tuvensis (Spirin, Vlasák & Kotir.) Spirin & Vlasák, comb. nov. MycoBank MB 844976.
Basionym: Antrodia tuvensis Spirin, Vlasák & Kotir., Mycol. Progr. 15 (51): 10. 2016.
Typus: Russia, Tuva, Kaa-Khem Dist., Malyi Yenissei, Populus sp., 21 Aug. 2014, Kotiranta 26735* (holotype H!).
Description and phylogenetic data: Spirin et al. (2016, as A. tuvensis).
Fomitopsis uralensis (Pilát) Spirin & Miettinen, comb. nov. MycoBank MB 844977. Figs 26, 28.
Basionym: Leptoporus uralensis Pilát, Bull. Soc. Mycol. France 48: 11. 1932.
Typus: Russia, Ural, Picea obovata, 13 Sep. 1930, Chomutsky 204 (holotype PRM 37776!).
Synonym: Antrodia huangshanensis Y.C. Dai & B.K. Cui, Mycotaxon 116: 16. 2011.
Typus: China, Anhui, Huangshan Co., Mt. Huangshan Nat. Park, Pinus massoniana, 11 Oct. 2004, Dai 6082* (holotype BJFC).
Description: Basidiocarps annual, effused-reflexed or totally resupinate, often 1–2 cm diam, sometimes fusing together and then up to 5 cm in widest dimension. Reflexed part projecting up to 7 mm, upper surface finely pubescent or smooth, azonate, cream-coloured; margin sharp, incurved. Margin of resupinate part adnate or partly detaching, compact, white to cream-coloured, up to 0.5 mm wide. Hymenial surface even, cream-coloured to pale ochraceous; pores angular, 2–4 per mm, dissepiments uneven to serrate. Section: context soft, white or pale cream-coloured, up to 0.5 mm thick; tubes soft, in older parts partly agglutinated, pale cream-coloured to pale ochraceous, 0.5–1.5 mm thick. Hyphal structure monomitic; hyphae clamped. Context hyphae hyaline, moderately thick-walled to subsolid, (2.8–)3.0–5.1(−5.2) μm diam (n = 45/2). Tramal hyphae hyaline, slightly to very thick-walled, (2.8–)2.9–4.9(−5.0) μm diam (n = 40/2), often distinctly twisted at dissepiment edges. Cystidioles absent. Basidia clavate, (13.4–)16.2–20.7(−21.0) × (3.8–)4.0–5.3(−5.4) μm (n = 25/2). Basidiospores with a distinct wall, cylindrical, occasionally slightly curved, (4.8–)4.9–6.8(−7.0) × (1.8–)1.9–2.3(−2.4) μm (n = 120/4), L = 5.16–6.23, W = 2.02–2.12, Q = 2.56–2.95.
Specimens examined: Russia, Khabarovsk Reg., Khabarovsk Dist., Malyi Kukachan, Picea ajanensis, 19 Aug. 2012, Spirin 5399* (H); Solnechnyi Dist., Igdomi, P. ajanensis, 4 Sep. 2016, Spirin 10946*, 10966 (H).
Notes: Kotlaba & Pouzar (1990) moved L. uralensis to Skeletocutis due to a superficial similarity of this species to S. amorpha, the type of the latter genus. While doing so, they also pointed out the absence of encrusted hyphal tips at the dissepiment edges, which are so characteristic of Skeletocutis spp. We studied the type of L. uralensis as well as three recent collections from the Russian Far East. The species is morphologically highly similar to F. ramentacea and its relatives, and it differs from them mainly due to distinctly smaller basidiospores. Like F. ramentacea, F. uralensis inhabits exposed, dry branches or recently fallen logs, and it seems to be restricted to spruce (Picea ajanensis, P. obovata) and pine (P. massoniana – Cui et al. 2011, as A. huangshanensis) in Asia.
Fomitopsis ussuriensis (Bondartsev & Ljub.) Spirin & Miettinen, comb. nov. MycoBank MB 844978.
Basionym: Phellinus ussuriensis Bondartsev & Ljub., Nov. Syst. Plant. non Vasc. 2: 143. 1965.
Typus: Russia, Primorie, Shkotovo Dist., Artyomovka, Kalopanax septemlobus, 3 Aug. 1946, Ljubarsky (holotype LE 22526!).
Synonym: Fomitopsis incarnatus K.M. Kim, J.S. Lee & H.S. Jung, Mycologia 99: 835. 2008.
Typus: Korea, Kangwon, Chiak, Fraxinus mandshurica, 25 Jul. 2005, Lee & Kim (holotype SNU m-05072501*).
Description: Basidiocarps perennial, sessile, at first bracket-shaped, later ungulate, broadly attached, projecting up to 4 cm, occasionally effused-reflexed or totally resupinate (effused part up to 10 cm in longest dimension). Upper surface initially pinkish brown, indistinctly zonate, smooth or slightly felty, later blackish brown to totally black, with distinct annual zones, not cracking. Pileal edge sharp to rather blunt, concolourous with pore surface, up to 2 mm wide, sterile; margin of resupinate parts pinkish-grey, sharply delimited. Pore surface pinkish-grey to brownish-grey, flat or concave, finally fading to mouse-grey; pores roundish to angular, 6–7 per mm, with thin or rather thick, entire dissepiments. Section: crust (present in old basidiocarps) exceptionally tough, black, matt, up to 0.5 mm thick, context hard corky, brown, up to 10 mm thick, clearly delimited from tubes; tubes corky, distinctly stratified, pinkish-grey to reddish-brown, in total up to 20 mm thick, each annual layer 2–3 mm thick, usually separated from surrounding tube strata by a darker sterile tissue 0.1–1 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, brownish to brown, tightly interwoven to subparallel, rarely branched, (1.8–)1.9–3.0(−3.4) μm diam (n = 20/1), lumen capillary to indistinct, side branches 1.8–2.2 μm diam, generative hyphae very rare, hyaline to brownish, slightly thick-walled, 1.5–2 μm diam. Trama dimitic; skeletal hyphae brownish to dark brown, tightly interwoven, sparsely branched, (2.2–)2.3–3.2(−3.3) μm diam (n = 40/2), lumen predominantly capillary to indistinct, side branches 1.8–2.2 μm diam, generative hyphae hyaline, thin- or slightly thick-walled, rare, 2–3 μm diam. Subhymenium indistinct. Cystidioles rather abundant, variably tapering, 9–17 × 3–5 μm. Basidia clavate, (8.6–)8.8–14.2(−14.3) × (3.8–)3.9–5.3(−5.8) μm (n = 20/2), in senescent hymenium partly glued together. Basidiospores thin-walled or with a distinct wall, cylindrical, sometimes slightly curved, (3.6–)3.7–4.7(−5.1) × (1.8–)1.9–2.2(−2.5) μm (n = 60/2), L = 4.04–4.09, W = 2.01–2.03, Q = 2.02.
Specimens examined: China, Hunan, Liuyang Co., Daweishan Nat. Park, angiosperm, 21 Dec. 2000, Dai 3269 (H); Sangzhi Co., Badagongshan Nat. Res., unidentified wood, 20 Sep. 2000, Härkönen K567 (H); ibid., 22 Sep. 2000, Härkönen K736 (H); ibid., 26–28 Sep. 1999, Härkönen K292, K401 (H); Prunus pseudocerasus, 26–28 Sep. 1999, Härkönen K353, 398 (H); Jilin, Antu Co., Dianzhan, Quercus sp., 20 Sep. 1998, Dai 3010, 3023* (H); Huadian Co., Dongxing, Acer sp., 19 Sep. 1993, Dai 1724 (H). Japan, Hokkaido, Takinoshita, hardwood, 25 Sep. 1994, Núñez 651 (O 10800, H).
Notes: Because of its dark-coloured basidiocarps, this species was first described as a member of Phellinus and then moved to Nigroporus (Dai & Niemelä 1995). Our data show, however, that P. ussuriensis belongs to the F. rosea complex (Fig. 12). Sequences of our specimens are identical to those of F. incarnata described from Korea; therefore, the latter species is treated here as a synonym of F. ussuriensis.
Fomitopsis variiformis (Peck) Vlasák & Spirin, comb. nov. MycoBank MB 844979.
Basionym: Polyporus variiformis Peck, Ann. Rep. N.Y. St. Mus. 42: 122. 1889.
Typus: USA, New York, Essex Co., North Elba and Cascadeville, Picea mariana, ‘July and September’ [year not indicated], Peck (syntypes NYSf3331, 3332).
Synonym: Antrodia variiformis (Peck) Donk, Persoonia 4: 340. 1966.
Description and phylogenetic data: Spirin et al. (2017, as A. variiformis); see also Figs 1 and 2.
Fomitopsis visenda Miettinen & Spirin, sp. nov. MycoBank MB 848365. Fig. 28.
Typus: Indonesia, Jambi, Sungai Penuh, Sungai Penuh-Muara Sako roadside, −2.059° 101.286° (±5 000 m), lower montane rainforest, on a snag (dicot, decay stage 2, 20 cm in diameter), 25 Apr. 2002, Miettinen 6223* (holotype BO, isotype H7200588).
Etymology: Visendus (Lat., adj.) – distinctive, remarkable.
Description: Basidiocarps perennial, conchate, broadly attached, partly fusing together, projecting up to 4 cm. Upper surface brown, smooth or slightly felty, sulcate, not cracking. Pileal edge sharp, brownish to brown, up to 1 mm wide, sterile. Pore surface light grey, with scattered brownish spots, concave; pores roundish, 8–10 per mm, with rather thin, entire dissepiments. Section: context corky, brown, up to 5 mm thick, sharply delimited from tubes; tubes tough, indistinctly or rather clearly stratified, brownish-grey to brown, in total up to 8 mm thick, each annual layer up to 1–3 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae dominating, brownish to brown, tightly interwoven, occasionally branched, (2.7–)2.8–4.0(−4.1) μm diam (n = 20/1), lumen capillary to indistinct, generative hyphae rare, hyaline, thin- or slightly thick-walled, 2–4 μm diam. Trama dimitic; skeletal hyphae dominating, brownish to brown, tightly interwoven, occasionally branched, (2.1–)2.2–3.1(−3.2) μm diam (n = 20/1), lumen mostly narrow to capillary, side branches 1.5–2.2 μm diam, generative hyphae hyaline, thin- or slightly thick-walled, not rare, 1.5–3 μm diam. Subhymenium indistinct. Skeletocystidia present as swollen (up to 4.5 μm diam) apices of tramal skeletal hyphae, slightly projecting above hymenium. Cystidioles rare, tapering or hyphoid, occasionally bifurcate, 9–13 × 3–4 μm. Basidia clavate, (8.2–)8.8–10.2(−10.3) × (4.2–)4.6–6.0(−6.2) μm (n = 10/1), in senescent hymenium slightly thick-walled and partly glued together. Basidiospores with a distinct wall, narrowly ellipsoid to cylindrical, longest spores slightly tapering to the distal end, (2.9–)3.0–4.2(−4.3) × (1.8–)1.9–2.3 μm (n = 30/1), L = 3.46, W = 2.07, Q = 1.67.
Notes: Fomitopsis visenda is morphologically similar and phylogenetically close to F. scalaris from the neotropics (Fig. 3). It differs from the latter species mainly due to rather thin, conchate basidiocarps with a downcurved margin, and it has no pileal crust so characteristic for F. scalaris. So far, F. visenda has been found only in the type locality.
Fomitopsis yunnanensis (M.L. Han & Q. An) Spirin, comb. nov. MycoBank MB 844980.
Basionym: Antrodia yunnanensis M.L. Han & Q. An, Phytotaxa 460: 6. 2020.
Typus: China, Yunnan, Jingdong, Wuliangshan Nat. Res., living angiosperm tree, 6 Oct. 2017, Han 1157* (holotype LFNC).
Description and phylogenetic data: Han et al. (2020, as A. yunnanensis); see also Suppl. Fig. S2.
Fomitopsis widdringtoniae Masuka & Ryvarden, Mycol. Helv. 5: 144. 1993.
Typus: Malawi, Southern Province, Thylo, Mulanje Mts., Widdringtonia nodiflora, 25 Jan. 1992, Ryvarden 31459 (holotype O).
Description: Masuka & Ryvarden (1993).
Notes: The species was discussed by Decock et al. (2022) as a relative of F. oboensis and F. spraguei.
Fomitopsis zuluensis (Wakef.) Ryvarden, Norw. J. Bot. 19: 231. 1972.
Basionym: Fomes zuluensis Wakef., Bothalia 4: 948. 1948.
Typus: South Africa, KwaZulu-Natal, King Cetshwayo, Nkandla, ‘ad lignum’, May 1935, Rump 374 (holotype K).
Description: Ryvarden & Johansen (1980).
Notes: A re-description of this species in Ryvarden and Johansen (1980) suggests F. zuluensis might be a close relative of F. pinicola. However, this morphological evidence should, if possible, be confirmed using newly collected and sequenced specimens.
A condensed key for the accepted Fomitopsis spp.
1. Hyphal structure monomitic throughout, basidiocarps annual, soft and fleshy ................................................................................... 2
(1). Skeletal hyphae present at least in tubes or in context, basidiocarps annual or perennial, of various consistency ........................... 3
2. Hyphae predominantly thin-walled, moderately thick-walled hyphae occasionally occurring in subiculum only ....................................................................................................................................................................... East Asia: F. monomitica; North America: F. oleracea; cf. also A. mappa, F. infirma, F. sandaliae, and Rhodonia placenta
(2). Hyphae with variably thickened walls, often distinctly swelling inwards in KOH ....................................................................................................................................... Asia: F. ramentacea, F. retorrida, F. uralensis; Europe: F. ramentacea, F. renehenticii, F. solaris; North America: F. fissa, F. solaris; South America: F. solaris
3. Basidiocarps stipitate or sessile (then usually with a contacted base), generative hyphae dominating in tubes ................................................................................................................................................................................. Asia: F. hartmannii; Europe: F. pulvina, F. squamosella; North America: F. amygdalina, F. pulvina; Oceania: F. hartmannii, F. maculatissima, F. sclerotina, F. tumulosa; South America: F. maculatissima
(3). Basidiocarps sessile (if funnel-shaped then tubes are clearly dimitic) to effused-reflexed or resupinate ........................................... 4
4. Basidiocarps soft, light-coloured, totally effused, generative hyphae dominating in tube trama ................................................................................................ Asia: F. maculosa, F. primaeva, F. tropica, F. tuvensis, F. yunnanensis; Europe: F. infirma, F. primaeva, F. pulverulenta, F. sandaliae; North America: F. dollingeri, F. flavimontis, F. madronae (note F. madronae and F. sandaliae occasionally produce effused-reflexed basidiocarps)
(4). Basidiocarps variable, if resupinate then rather tough, skeletal hyphae dominating .......................................................................... 5
5. Basidiocarps compound-imbiricate; hymenophore lamellate ......................................................................... Oceania (F. concentrica)
(5). Hymenophore poroid (occasionally irpicoid or incompletely poroid in F. albidoides) .......................................................................... 6
6. Pores 0.5–2 per mm, often irregular ................................................................................................................................................... 7
(6). Pores 2 and more per mm, sinuous to round ...................................................................................................................................... 8
7. Basidiocarps annual, soft to hard leathery .................................................................................................................................................................. Asia: F. juniperina, F. mellita; Europe: F. albidoides, F. algumicola, F. juniperina, F. labyrinthica, F. mellita; North America: F. alaskana, F. cellularis, F. juniperina, F. stereoides; South America: F. stereoides; cf. also F. gilvidula and Antrodia heteromorpha complex
(7). Basidiocarps perennial, as a rule corky .................................................................................................................................................... Asia: F. dickinsii, F. fragilis, F. sulcata; Europe: F. quercina; North America: F. derelicta, F. quercina; South America: F. roseofusca
8. Basidiocarps with rose or violet tints surviving in dry state ................................................................................................................. 9
(8). Basidiocarps lacking distinctive pink or violet colouration ................................................................................................................. 11
9. Pores 2–5 per mm .......................................................................................................................................................................... Europe: F. lilacinogilva; North America: F. cupreorosea, F. lilacinogilva, F. neotropica; South America: F. cupreorosea, F. flabellata, F. lilacinogilva; Oceania: F. lilacinogilva
(9). Pores smaller, usually more than 5 per mm ...................................................................................................................................... 10
10. Upper surface light-coloured, often fading in older individuals, no distinct crust; basidiocarps fan-shaped to effused-reflexed .................................................................................................................................................................... Asia: F. carnea, F. visenda; North America: F. sagraeana; Oceania: F. eucalypti, F. marchionica; South America: F. feei; cf. also F. atypa, F. gilvidula, F. modesta, F. roseoalba and F. subectypa
(10). Upper surface pinkish- or greyish-brown to black, often with a crust when old; basidiocarps of most species ungulate ...................................................................................................................................... Africa: F. africana, F. purpurea, F. substratosa; Asia: F. africana, F. cajanderi, F. rosea, F. substratosa, F. ussuriensis; Europe: F. cajanderi, F. perhiemata, F. rosea; North America: F. cajanderi, F. cupressicola, F. rosea; Oceania: F. foedata; South America: F. scalaris
11. Pores 2–5 per mm, regular, angular or sinuous ................................................................................................................................ 12
(11). Pores 5 and more per mm, usually regular ....................................................................................................................................... 14
12. Basidiocarps perennial, as a rule sessile, often covered by a crust ........................................................................................................................................................... Africa: F. avellanea, F. zuluensis; Asia: F. pinicola, F. moritziana; Europe: F. pinicola; North America: F. lignicolor, F. pinicola; Oceania: F. maire; cf. also F. circularis and Laricifomes officinalis
(12). Basidiocarps annual or biennial, sessile to totally resupinate, no crust on pileal surface ................................................................. 13
13. Basidiocarps sessile or effused-reflexed, often large, sappy in fresh condition, rather soft when dry, skeletal hyphae hyaline, richly branched ................................................................................................................................................................................. Africa: F. oboensis; Asia: F. betulina, F. hypoxantha, F. marianii; Europe: F. betulina, F. marianii, F. spraguei; North America: F. betulina, F. globispora, F. marianii, F. spraguei
(13). Basidiocarps sessile, effused-reflexed or resupinate, small or medium-sized, leathery, rather though when dry; skeletal hyphae occasionally or rarely branched, in some species brownish .................................................................................................................................................................................. Africa: F. malicola; Asia: F. aculeata, F. angusta, F. gilvidula, F. incana, F. kuzyana, F. leucaena, F. malicola, F. minutula; Europe: F. hyalina, F. kuzyana, F. leucaena, F. minutula, F. pulvinascens, F. serialis; North America: F. calcitrosa, F. malicola, F. morganii, F. serialiformis, F. serrata, F. psilodermea, F. variiformis; Oceania: F. cyclopis, F. eucalypticola; South America: F. aethalodes, F. leioderma, F. malicola, F. pannucea, F. roseoalba, F. psilodermea; cf. also F. glabricystidia and F. meliae
14. Basidiospores ellipsoid, slightly thick-walled and cyanophilous; prominent blunt non-encrusted skeletocystidia present. Basidiocarps completely resupinate, light-coloured .......................................................................................................................................................................... Africa: F. glabricystidia; Asia: F. tunicata
(14). Basidiospores cylindrical to narrowly ellipsoid, thin-walled or with a distinct wall, acyanophilous; skeletocystidia rather weakly differentiated. Basidiocarps of totally resupinate species dark-coloured .......................................................................................... 15
15. Basidiocarps perennial, sturdy; hymenophore mouse-grey to greyish-brown or almost black .................................................................................................................................................................................. Asia: F. castanea; North America: F. condensa, F. nigra; cf. also F. perhiemata and F. scalaris
(15). Basidiocarps annual or perennial; hymenophore light-coloured, if greyish then basidiocarps annual and leathery, not corky ......... 16
16. Basidiocarps annual, leathery to corky, tubes one-layered .......................................................................................................... Africa: F. atypa, F. caespitosa, F. ostreiformis, F. widdringtoniae; Asia: F. atypa, F. bambusae, F. caespitosa, F. luzonensis, F. micropora, F. microcarpa, F. ostreiformis; North America: F. meliae, F. modesta, F. nivosella, F. palustris, F. subectypa; Oceania: F. caespitosa, F. luzonensis, F. ostreiformis, F. tristis; South America: F. caespitosa, F. meliae, F. modesta, F. nivosella
(16). Basidiocarps perennial, corky, woody-hard or chalk-like, tubes indistinctly or clearly stratified ........................................................ 17
17. Hymenophore clearly stratified, tubes chalky, easily crumbling ............................................................................................................................................ Asia: F. niveomarginata, F. pseudopetchii; North America: F. caseosa; Oceania: F. pseudopetchii; South America: F. minutispora
(17). Hymenophore indistinctly or clearly stratified, tubes very tough ..................................................................................................... Asia: F. circularis, F. dochmia, F. elevata, F. ferrea,F. philippinensis; North America: F. ochracea, F. lignea; Oceania: F. lapidosa; cf. also F. pinicola
Excluded and insufficiently known taxa
Antrodia bambusicola Y.C. Dai & B.K. Cui, Mycotaxon 116: 14. 2011.
Typus: China, Anhui, Huangshan Co., Mt. Huangshan Nat. Park, Bambusa sp., 21 Oct. 2010, Dai 11901* (holotype BJFC).
Notes:Antrodia bambusicola was described based on two collections from the southern part of China (Cui et al. 2011). The presence of cystidioles with several side outgrowths rules out Fomitopsis in the current sense; Liu et al. (2022) published sequences of this species and re-confirmed its placement in Antrodia sensu stricto.
Antrodia conchata D.A. Reid, Bothalia 11: 222. 1974. (as ‘A. conchata (Lloyd) D.A. Reid’).
Basionym: Polyporus conchatus Lloyd, Mycol. Writ. 5: 700. 1917.
Typus: South Africa, Western Cape Province, Klapmuts, Populus sp., [Dec. 1916], van der Bijl 358 (lectotype PRE) (selected by Reid 1974: 222).
Notes: The species was illegitimately introduced as P. conchatus by Lloyd (1917) (a later homonym of P. conchatus (Pers.) Fr.) and then moved to Antrodia by Reid (1974). Combining it in Antrodia, Reid in fact correctly introduced it as a new species. Antrodia conchata is so far known from the type material only. Newly collected and sequenced specimens are highly desirable to assign it to Fomitopsis as outlined here.
Antrodia rupamii Virdi, Int. J. Mycol. Lichenol. 5: 307. 1992.
Typus: India, Arunachal Pradesh, West Kameng, Rupam, angiosperm log, 9 Sep. 1981, Virdi 21866 (holotype PAN).
Notes: The species was collected twice in the northeastern part of India (Virdi 1992). Antrodia rupamii was described as having large-pored, soft basidiocarps and medium-sized ellipsoid basidiospores. These features, as well as its occurrence on angiosperm hosts suggest it is likely related to the F. mellita – F. pulvinascens group. This problem should be addressed to a future DNA-based study of this species.
Antrodia uzbekistanica Yuan Yuan, Gafforov & F. Wu, Phytotaxa 303 (1): 51. 2017.
Typus: Uzbekistan, Jizzakh: Zomin Nat. Park, Juniperus seravschanica, 8 Sep. 2016, Dai 17104* (holotype BJFC 22509).
Notes: Antrodia uzbekistanica was described as a sibling species of A. juniperina (= Fomitopsis juniperina above) distributed in the Middle Asia (Yuan et al. 2017). We obtained five new sequences of F. juniperina and compared them with GenBank sequences labelled as A. juniperina. Only ITS sequences of ‘A. juniperina’ from Macedonia are surely different from the rest of the A. juniperina sequences, and therefore F. algumicola was described above to accommodate them. All other A. juniperina sequences from North America (eastern and western parts of USA), Europe (Spain) and Africa (Ethiopia and Tanzania) form a strongly supported clade including A. uzbekistanica (Suppl. Fig. S7). It is still possible that F. juniperina as accepted here is a collective species. Proving this hypothesis will require the use of non-ribosomal markers.
Buglossoporus americanus D.A. Reid, Mem. N.Y. Bot. Gdn. 28: 179. 1976.
Notes: Buglossoporus americanus was introduced as a relative of B. quercinus (= F. pulvina above) (Reid 1976). However, its small basidiospores and dimitic tube trama described in the protologue preclude an affinity with F. pulvina and satellite taxa. The species is unlikely to be a member of Fomitopsis as redefined in the present study.
Buglossoporus matangensis Corner, Beih. Nova Hedwigia 78: 172. 1984.
Typus: Malaysia, Sarawak, Gunong Matang, 20 Aug. 1972, Corner (holotype E00159665).
Notes: No sequenced collections are available for this species. The protologue (Corner 1984) suggests it could be a sister species of F. hartmannii. Hattori (2000) concluded it was a small-pored (5–6 per mm) form of B. malesianus, another species of an uncertain affiliation.
Coriolus perpusillus Murrill, Bull. Torrey Bot. Club 35: 396. 1908.
Typus: Philippines, Mindanao, Lake Lanao, Camp Keithley, dead wood, Jul. 1907, Clemens 6f (holotype NY 00704981!).
Notes: The species was listed among synonyms of F. modesta (Ryvarden 1985). We restudied the type material and concluded it belongs to Postia. Crumbling tubes and very thick-walled tramal hyphae are reminiscent of the core Postia spp., i.e. P. lactea (the generic type), P. grata and P. calcarea. Unfortunately, the type specimen is almost sterile and no clear conclusions about species identity can be drawn based on a morphological study only. Nevertheless, C. perpusillus should be taken into account in future studies of Postia spp. in Southeast Asia.
Coriolus substipitatus Murrill, North American Flora 9: 22. 1907.
Typus: Jamaica, Portland, Port Antonio, old log, 24 Jun. [no year indicated], Earle 629 (holotype NY00705001!).
Notes: The species was listed among synonyms of F. modesta (Ryvarden 1985). The type is completely sterile although the nearly white, leathery basidiocarps and trimitic hyphal structure clearly point to Trametes (Polyporaceae).
Daedaleopsis candicans (P. Karst.) Spirin, comb. nov. MycoBank MB 844981.
Basionym: Daedalea candicans P. Karst., Trudy Troitskogo Otdeleniya Russkogo Imperatorskogo Geograficheskogo Obshchestva 12: 110. 1911.
Typus: Russia, Zabaikalie, Chita, Bayan Darga, [1908 or 1909], Mikhno (lectotype H 7044541!) (selected by Lowe 1956: 105).
Synonym: Daedalea sinensis Lloyd, Mycol. Writ. 7 (66): 1112. 1922.
Notes: We re-studied the single authentic collection of D. candicans. It is richly fertile and undoubtedly represents Daedaleopsis sinensis widely distributed in East Asia. Since D. candicans has priority over Daedalea sinensis, we recombine it in Daedaleopsis.
Daedalea hydnoides I. Lindblad & Ryvarden, Mycotaxon 71: 339. 1999.
Typus: Costa Rica, Guanacaste, Guanacaste Nat. Park, hardwood, 3 Nov. 1997, Lindblad 3679 (isotype O 14083).
Notes: We restudied the authentic material of this species and concluded it is identical to Fuscocerrena portoricensis (Polyporaceae) or represents a closely related taxon. Han et al. (2016) claimed they sequenced the type of D. hydnoides and their sequences confirmed its placement within Daedalea. The ITS sequence of D. hydnoides from these authors (GenBank KP171203) is identical to the ITS sequences of D. sprucei and D. stevensonii that appeared in the same paper, as well as sequences of D. circularis published three years earlier (Li & Cui 2013). We consider this to be the result of a lab contamination and exclude D. hydnoides from Fomitopsis in the current sense.
Daedalea microsticta Cooke, Grevillea 10 (56): 122. 1882.
Typus: Brazil, Rio de Janeiro, [no collecting date], Glaziou 13381 (lectotype K) (selected by Fidalgo & Fidalgo 1967: 848).
Notes: Vlasák et al. (2011) proved this species is a member of Trametes. The correct name for the species is T. ochroflava.
Daedalea ryvardenica Drechsler-Santos & Robledo, Kurtziana 37: 66. 2012.
Typus: Brazil, Mato Grosso, Chapada dos Guimarães, Véu da Noiva, hardwood, 12 Jun. 2011, Ferreira-Lopes 001 (holotype FLOR 41052).
Notes: Daedalea ryvardenica was recently described from Brazil based on morphological evidence (Drechsler-Santos et al. 2012). Cristaldo et al. (2022) published DNA sequences of D. ryvardenica and proved that it belongs to the Daedalea clade of Fomitopsis (see also Fig. 5). We have not studied material of D. ryvardenica. We feel it is premature to combine it in Fomitopsis until the Neotropical species of the Daedalea clade have properly been revised and a dozen of the older names currently treated as synonyms of D. aethalodes and D. stereoides are sorted-out.
Daedalea sprucei Berk., Hooker’s J. Bot. 8: 236. 1856.
Typus: Brazil, Panurè, trunks of trees, Mar. 1853, Spruce 41 (lectotype K(M) 51997) (selected by Ryvarden 1984: 357)
Specimens examined: Cuba, Pinar del Rio, Sierra del Rosario, Guazuma tomentosa, 18 Feb. 1976, Ortiz (O F-910547). Taiwan, Keshun, Kuraru, Jan. 1909, Kusano (O F-910546, H7004131).
Notes: Figueroa and Decock (2007) studied this species by DNA methods and confirmed its placement in Trichaptum (Hymenochaetales). Han et al. (2016) sequenced two specimens of D. sprucei and accepted it as a member of Daedalea. We rechecked these collections (O F-910546, O F-910547) and could confirm they belong to Trichaptum. Two available ITS sequences (GenBank KP171211 and KR605794) are identical to those of D. circularis (= F. circularis above). This is evidently due to a lab contamination. Daedalea sprucei clearly does not belong to Fomitopsis.
Daedalea stevensonii Petr., Sydowia 13: 139. 1959.
Typus: Malaysia, Sabah, Kinabalu, 29 Apr. 1932, Stevenson (isotype O 10543!).
Notes: The species was described based on a single sterile collection (Petrak 1959). We have studied its isotype, which represents a typical member of Trametes although its species affiliation within the genus is vague. The ITS sequence from the type of D. stevensonii published by Han et al. (2016) is identical to D. circularis (= F. circularis above), morphologically a widely different species. We discarded it from our phylogenetic analyses as a lab contamination and excluded D. stevensonii from Fomitopsis.
Fomes subungulatus Murrill, Bull. Torrey Bot. Club 35: 410. 1908.
Typus: Philippines, Luzon, Benguet, Bagnio, Pinus insularis, Oct.–Nov.1905, Merrill 5005 (lectotype NY 00780696!) (selected by Ryvarden 1985: 176).
Notes: The species was treated among synonyms of F. pinicola (Ryvarden 1985); see notes under that species.
Fomitopsis abieticola B.K. Cui, M.L. Han & Shun Liu, Frontiers in Microbiology 12 (644979): 4. 2021.
Typus: China, Yunnan, Shangi-La Co., Pudacuo Nat. Park, Abies sp., 24 Sep. 2011, Cui 10532* (holotype BJFC 011427).
Note: See remarks to F. pinicola.
Fomitopsis hengduanensis B.K. Cui & Shun Liu, Frontiers in Microbiology 12 (644979): 7. 2021.
Typus: China, Yunnan, Lanping Co., Tongdian, Picea sp., 19 Sep. 2017, Cui 16259* (holotype BJFC 029558).
Note: See remarks to F. pinicola.
Fomitopsis kesiyae B.K. Cui & Shun Liu, Frontiers in Microbiology 12 (644979): 9. 2021.
Typus: Vietnam, Dam Dong, Da Lat, Bidoup Nui Ba Nat. Park, Pinus kesiya, 15 Oct. 2017, Cui 16437* (holotype BJFC 029736).
Note: See remarks to F. pinicola.
Fomitopsis massoniana B.K. Cui, M.L. Han & Shun Liu, Frontiers in Microbiology 12 (644979): 11. 2021.
Typus: China, Fujian, Wuping Co., Liangyeshan Nat. Reserve, Pinus massoniana, 25 Oct. 2013, Cui 11304* (holotype BJFC 015420).
Note: See remarks to F. pinicola.
Fomitopsis minuta Aime & Ryvarden, Syn. Fung. 23: 26. 2007.
Typus: Guyana, Potaro-Siparuni, Upper Potaro River, decorticated hardwood log, 15 Jun. 2002, Aime 2018 (isotype O).
Notes: Fomitopsis minuta was described as having trimitic hyphal structure and allantoid basidiospores 4 × 1 μm (Aime et al. 2007). These characters do not fit to the genus Fomitopsis as defined here. The identity and generic affiliation of this species should be reassessed with the use of DNA methods.
Fomitopsis mounceae Haight & Nakasone, Mycologia 111 (2): 344. 2019.
Typus: Canada, Alberta, Yellowhead Co., Edson, Populus tremuloides, 9 Oct. 2010, Haight 78* (holotype CFMR).
Note: See remarks to F. pinicola.
Fomitopsis rubida (Berk.) A. Roy & A.B. De, Mycotaxon 60: 317. 1996.
Basionym: Polyporus rubidus Berk., London J. Bot. 6: 500. 1847.
Typus: Sri Lanka, Southern Prov., Point de Galle, ‘on fallen trees’, Dec. 1844, Gardner 96 (holotype K(M) 189712!, isotype UPS F-175076!).
Note: See remarks to F. carnea.
Fomitopsis scortea (Corner) T. Hatt., Mycoscience 44: 275. 2003. Basionym: Tyromyces scorteus Corner, Beih. Nova Hedwigia 96: 195. 1989.
Typus: Solomon Islands, Guadalcanal, Tsuva, 8 Nov. 1965, Corner (holotype E00604914).
Notes: Hattori (2003) likened the type specimen to F. palustris. However, the unusually large pores are an aberrant character, and Hattori could not determine the type of rot. Its placement in Fomitopsis needs confirmation.
Fomitopsis schrenkii Haight & Nakasone, Mycologia 111 (2): 349. 2019.
Typus: USA, South Dakota, Custer Co., Black Hills, Pinus ponderosa, Jul. 2014, Haight 150* (holotype CFMR).
Note: See remarks to F. pinicola.
Fomitopsis subpinicola B.K. Cui, M.L. Han & Shun Liu, Frontiers in Microbiology 12 (644979): 12. 2021.
Typus: China, Heilongjiang, Yichun, Fenglin Nat. Reserve, Pinus koraiensis, 2 Aug. 2011, Cui 9836* (holotype BJFC 010729).
Note: See remarks to F. pinicola.
Fomitopsis subvinosa (Corner) T. Hatt. & Sotome, Mycoscience 54: 307. 2013.
Basionym: Trametes subvinosa Corner, Beih. Nova Hedwigia 97: 166. 1989.
Typus: Indonesia, Sumatra, Karo, Berastagi, 10 Sep. 1931, Corner (holotype E00159614).
Notes: Hattori and Sotome (2013) described this species as similar to F. scortea, differing in context colour and possibly spore length. The description leaves it open whether F. subvinosa might be a synonym of F. scortea and whether it belongs to Fomitopsis. Further analysis would be required to confirm its status and position.
Fomitopsis tianshanensis B.K. Cui & Shun Liu, Frontiers in Microbiology 12 (644979): 12. 2021.
Typus: China, Xinjiang, Fukang Co., Tianshan Tianchi Nat. Reserve, Picea schrenkiana, 4 Jul. 2018, Cui 16821* (holotype BJFC 030120).
Note: See remarks to F. pinicola.
Laricifomes officinalis (Vill.) Kotl. & Pouzar, Česká Mykol. 11 (3): 158. 1957.
Basionym: Boletus officinalis Vill., Histoire des Plantes du Dauphiné 3: 1041. 1783.
Typus: Plate 61, fig. 1 in Micheli 1727 (iconotype) (selected by Ryvarden 1991:171).
Specimens examined: Italy, Piedmont, Verbano-Cusio-Ossolo, Alpe Devero, Larix decidua, 2 Aug. 2019, Cartabia (H). Russia, Yakutia, Yakutsk, Larix cajanderi, 6 Aug. 2016, Kotiranta 27358* (H 7034504).
Notes: Based on ITS and nrLSU sequences, Ortiz-Santana et al. (2013) and Justo et al. (2017) suggested that L. officinalis should be excluded from Fomitopsis. Here we support their results (Figs 1, 2) and thus confirm Laricifomes as the correct genus for this species.
Megasporoporia eutelea (Har. & Pat.) Spirin & Viner, comb. nov. MycoBank MB 848366.
Basionym: Trametes eutelea Har. & Pat., Bull. Soc. Mycol. France 28: 144. 1912.
Typus: Mauritania, Adrar, Atar, Bat’a, Tamarix, 19 May 1911, Chudeau (lectotype FH!, isolectotype PC!) (selected by Ryvarden 1983: 18).
Description: Basidiocarps seasonal, effused, first orbicular, then fusing together and up to 5 cm in widest dimension. Margin adnate, densely floccose to compact, white to cream-coloured, up to 1 mm broad. Hymenial surface even, cream-coloured to pale ochraceous or greyish; pores roundish to angular, 5–7 per mm, dissepiments thin, uneven. Section: subiculum rather soft, white, up to 0.5 mm thick; tubes soft-corky, in older specimens often crumbling, concolourous with hymenial surface, 2–5 mm thick. Hyphal structure dimitic; hyphae clamped, skeletal hyphae moderately to distinctly dextrinoid, slightly or moderately cyanophilous. Subiculum dimitic; skeletal hyphae hyaline to brownish, interwoven or subparallel, occasionally branched, (2.9–)3.0–5.1(−5.2) μm diam (n = 30/2), lumen rather wide to capillary, terminal branches 1–2.5 μm diam, generative hyphae rather abundant, hyaline, thin- to slightly thick-walled, 3–6 μm diam. Trama dimitic; skeletal hyphae hyaline rather loosely interwoven, hyaline or brownish, occasionally dichotomously branched, (2.3–)2.7–4.4(−5.2) μm diam (n = 70/4), lumen rather wide to capillary, terminal branches 1–2 μm diam, generative hyphae rather abundant, hyaline, thin-walled, 2–3 μm diam. Subhymenium indistinct. Cystidioles rather rare, tapering to the apex, 11–17 × 4–6.5 μm. Hyphidia rarely present, bi- or trifurcate, often somewhat swollen at the base, apical ougrowths 1.5–2 μm diam. Basidia broadly clavate, (11.2–)12.3–18.3(−19.0) × (6.0–)6.1–10.2(−11.0) μm (n = 36/4), in senescent hymenium partly glued together. Basidiospores with a distinct wall, narrowly ellipsoid to cylindrical or subfusiform, (6.0–)6.3–9.2(−9.8) × (2.9–)3.0–4.3(−4.4) μm (n = 111/4), L =7.37–7.69, W = 3.56–3.79, Q = 1.99–2.10, often with a large central oil drop.
Specimens examined: Oman, Dhofar, Wadi Darbat, angiosperm, 2 Jan. 2022, Razmadze 2202/9* (TUF). Sudan, Wadi Halfa, Ashkeit, buried wood (under Acacia and Tamarix), 9 Oct. 1962, Ahti 16255 (H7045438).
Notes: Ryvarden (1983) combined T. eutelea in Antrodia. We studied the type material and a few recent collection of T. eutelea. The species has dextrinoid and cyanophilous, occasionally dichotomously branched skeletal hyphae and short, wide, barrelshaped basidia; these traits preclude Fomitopsidaceae and point to Polyporaceae. DNA data confirm this suggestion and show that T. eutelea belongs to Megasporoporia (as redefined by Wang et al. 2022). Megasporoporia eutelea is a sister species of M. minor differing from the latter species in 10 bp in ITS region. GenBank sequences MK157424, MW922713 (Egypt), KT153994, KF954704 (India), ON845731 (Saudi Arabia), JN630804 (Iran) and MN909156 (Pakistan) represent M. eutelea.
Melanoporia tropica B.K. Cui & Shun Liu, Fungal Diversity 118: 53. 2022.
Typus: Vietnam, Dam Dong, Da Lat, Bidoup Nui Ba National Park, living angiosperm tree, 15 Oct. 2017, Cui 16444* (holotype BJFC).
Notes: The species was described by Liu et al. (2022). We had no access to the type material. The name change is necessary for moving M. tropica to Fomitopsis.
Neofomitella hemitephra (Berk.) M.D. Barrett, comb. nov. MycoBank MB 844982.
Basionym: Polyporus hemitephrus Berk. in Hooker, The botany of Antarctic voyage II, Flora Novae Zealandiae 2: 179. 1855.
Typus: New Zealand, [no locality and collection date indicated], Colenso (lectotype K) (selected by Ryvarden 1977: 221).
Synonym: Neofomitella australiensis B.K. Cui & Xing Ji, Mycol. Progr. 18: 598. 2019.
Typus: Australia, Victoria, Yarra Ranges National Park, dead tree of Nothofagus, 10 May 2018, Cui 16571* (holotype BJFC; isotype stated to be in MEL but no collections annotated with this number exist).
Specimens examined: Australia, New South Wales, Mt. Boss State Forest, NW of Wanchope, 2 Feb. 1985, Coveny 3 (O 10806, H); Tasmania, Track to Philosopher Falls, near Waratah, 23 May 2016, S.J.M. McMullan-Fisher 2852 (MEL 2397595A). New Zealand, South Island, Golden Bay Dist., Cobb Valley, Asbestos track, dead wood, 23 Mar. 1996, Ryvarden 38462 (O 16449, H).
Notes: This species was described by Berkeley (in Hooker 1855) as having ungulate basidiocarps with a dark upper surface, small pores and stratified tubes. The species was described based on a New Zealand specimen, but it is also common in southeastern Australia. We studied specimens from Australia and New Zealand fitting well to the protologue and the later redescription of Heterobasidion hemitephrum by Cunningham (1965). Trimitic hyphal structure and distinctly coloured, strongly cyanophilous skeletal hyphae rule out Fomitopsis as a generic affiliation for this species and point to Polyporaceae as the correct family. The species has been shuffled between many different genera since its initial publication. Cunningham (1965) in his monograph of New Zealand polypores treated it under Heterobasidion, but earlier Cunningham (1948) had considered it a member of Fomitopsis. Based on culture studies, Rajchenberg (1995a) demonstrated that the species formed a white rot (associated with white heart rot, according to Cunningham 1965) and concluded it was best accommodated in the genus Fomes. More recently, Han et al. (2016) sequenced a single specimen purportedly of P. hemitephrus from New South Wales, and reinstated Cunningham’s combination in Fomitopsis. The conclusion of Han et al. (2016) was followed by Zmitrovich (2018) in creating the combination Pilatoporus hemitephrus. However, P. hemitephrus causes a white rot, so it cannot be placed in the exclusively brown-rot genus Fomitopsis.
Recently, a new species Neofomitella australiensis was described by Ji et al. (2019), for a novel genetic lineage of Neofomitella. The protologue of N. australiensis clearly matches all diagnostic features of P. hemitephrus, including large, tough perennial basidiocarps, a crustose pileus, a distinct orange layer beneath the pileus crust (‘tawny’ in the protologue of P. hemitephrus), variably cream- to dark-brown context, and pore and spore dimensions. Two sequences of F. hemitephra from New Zealand (GenBank MK404665 and MN007024) are identical to those of Neofomitella australiensis. However, ITS and TEF1 sequences of F. hemitephra published earlier by Han et al. (2016) are identical to those of F. marianii (Figs 4, 5). We did not study the specimen (O 10808) which was the source of these sequences, and therefore we cannot decide if the specimen was misidentified or there is a case of lab contamination, but neither case would affect the application of the epithet P. hemitephrus. It is likely that Ji et al. (2019) were persuaded to exclude P. hemitephrus as an earlier name for their N. australiensis due to the conclusions of Han et al. (2016) that it belonged in Fomitopsis. However, a broader reading of the literature for this species leaves no doubt that Polyporus hemitephrus is a white-rot species, and that Neofomitella australiensis is its later synonym.
Neolentiporus tropicus B.K. Cui & Shun Liu, Fungal Diversity 118: 55. 2022.
Typus: China, Hainan, Ledong, Jianfengling National Park, angiosperm, 19 Jun. 2016, Cui 13915* (holotype BJFC).
Notes: The species was described by Liu et al. (2022). We had no access to the type material. Phylogenetically, the species belongs to Buglossoporus clade (Fig. 2 and Suppl. Fig. S8). The identity of N. tropicus vs. Buglossoporus matangensis and B. malesianus should be verified before moving it to Fomitopsis.
Pilatoporus maroccanus Kotl. & Pouzar, Cryptog. Mycol. 14: 215. 1993.
Typus: Morocco, Middle Atlas, Ifrane, Cupressus sempervirens, 17 Apr. 1992, Kotlaba (holotype PRM 842893!*).
Notes: Kotlaba & Pouzar (1993) introduced this species as a member of Pilatoporus (= F. palustris complex). Vampola (1996) concluded that P. maroccanus is a genuine species of Trametes and suggested it could be identical to T. suaveolens. We restudied the type material with morphological and DNA methods, and our data confirm P. maroccanus is a later synonym of Trametes junipericola (Polyporaceae).
Polyporus fulvitinctus Berk. & M.A. Curtis, J. Linn. Soc., Bot. 10: 313. 1869.
Typus: Cuba, [no locality or collection date indicated], Wright 136 (holotype K(M) 264880!).
Notes: Ryvarden (1984) placed P. fulvitinctus to the synonyms of Fomitopsis nivosa (discussed below as Polyporus nivosus). However, this synonymy cannot be maintained because the type specimen represents a polypore with dichotomously branched, brown, strongly cyanophilous skeletal hyphae. These anatomical traits, in combination with sturdy, greyish-brownish, small-pored basidiocarps and rather long, cylindrical-subfusiform basidiospores indicate that P. fulvitinctus has affinities with Datronia (Polyporaceae).
Polyporus nivosus Berk., Hooker’s J. Bot. 8: 196. 1856.
Typus: Brazil, Panurè, dead trunks of trees, Feb. 1853, Spruce 192 (lectotype K(M) 257627!, isolectotype PC!) (selected by Ryvarden 1984: 348).
Description: Basidiocarps annual or probably short-living perennial, sessile or effused-reflexed, projecting up to 3.5 cm. Upper surface first cream coloured to pale ochraceous, azonate, smooth. Pileal edge sharp, concolourous with cap surface, sterile, up to 0.5 mm wide. Pore surface cream-coloured or pale ochraceous, sometimes with a faint pinkish tint, concave; pores roundish to angular, (5–)6–8(−9) per mm, with thin, entire dissepiments. Section: context corky, cream- to wood-coloured, up to 5 mm thick; tubes sturdy, one-layered or indistinctly stratified, concolourous with hymenial surface, up to 7 mm thick. Hyphal structure dimitic; hyphae clamped. Context dimitic; skeletal hyphae hyaline to pale ochraceous, densely interwoven or in subparallel bundles, occasionally branched, (3.2–)3.7–5.8(−6.1) μm diam (n = 40/2), lumen changing from wide to almost invisible, side branches 2–3 μm diam, often subsolid, generative hyphae rare, hyaline, thin- or slightly thick-walled, 3–4 μm diam. Trama dimitic; skeletal hyphae dominating, hyaline to pale ochraceous, interwoven, some flexuous, accidentally branched, (3.0–)3.1–4.7(−4.8) μm diam (n = 40/2), lumen capillary to indistinct, generative hyphae rather rare, thin-walled, quickly collapsing, 2–3 μm diam, dissepiment edges dimitic, consisting of thin-walled, flexuose generative hyphae and winding skeletals with a wide lumen. Subhymenium indistinct. Cystidioles not seen. Basidia short-clavate, (8.2–)8.8–11.6(−12.3) × (4.0–)4.1–5.1(−5.2) μm (n = 14/2), rare; basidioles widely dominating in hymenium, broadly ovoid to subglobose, 6–9 × 5.5–7 μm. Basidiospores thin-walled, cylindrical-fusiform, (6.0–)6.1–8.0(−8.4) × (2.5–)2.6–3.2(−3.3) μm (n = 60/2), L = 6.91–7.07, W = 2.88–2.95, Q = 2.35–2.46, often with one or a few small guttulae.
Specimen examined: Brazil, Pernambuco, Paulista, hardwood, Jul. 2002, Barbosa 360* (O 17846).
Notes: Due to the light-coloured and seemingly annual basidiocarps, F. nivosa was considered a relative of F. palustris and its sibs (Gilbertson & Ryvarden 1986), and therefore transferred to the genus Pilatoporus (Kotlaba & Pouzar 1993). However, it differs from them in having rather small pores, short basidia and fusiform basidiospores, and it lacks sterile hymenial elements. Only two specimens of F. nivosa are known to us, i.e. the type specimen and a recent collection from Pernambuco (Brazil) which morphologically agrees with the type. From the latter specimen, we were able to generate a low-quality ITS sequence showing Antrodiella aurantilaeta from the residual polyporoid clade of the Polyporales as the closest hit in GenBank. Though the sequence is somewhat ambiguous (and was therefore not submitted to GenBank), it clearly excludes affinity to Fomitopsidaceae. For this reason, we exclude P. nivosus from Fomitopsis and leave the question about its identity and phylogenetic position for future studies.
Polyporus notopus Lév., Ann. Sci. Nat., Bot. 3 (2): 194. 1844.
Typus: Indonesia, Java, ‘ad truncos’, [no collection date and collector indicated] (holotype PC!).
Notes: Ryvarden (1981) suggested that P. notopus represents a juvenile individual of F. modesta. We studied the type of this species. It is a small pileate basidiocarp with resinous, tiny pores (9–12 per mm). It is sterile and infected by moulds. In our opinion, P. notopus does not belong to Fomitopsidaceae or Trametes. It gives the appearance of cottony, dimitic context and monomitic, agglutinated lower trama. Only simple septa were seen and they are not uncommon in the skeletal-like hyphae of context. No thick-walled or other cystidia could be seen. It reminds of Flaviporus liebmannii (Steccherinaceae), but the context structure, as well as the lack of cystidia and clamps suggest otherwise. In short, we cannot connect this low-quality specimen with any species or genus known to us.
Polyporus portentosus Berk., London J. Bot. 3: 188. 1844.
Typus: Australia, Western Australia, Swan River, [no collection date indicated], Drummond 125 (holotype K).
Notes: Based on morphological and cultural traits, Rajchenberg (1995) placed P. portentosus in Laetiporus. Blasting available ITS and LSU sequences of P. portentosus shows that it could be a member of Fomitopsis. Additional markers (in particular, RPB1) are highly desirable to re-establish its generic position.
Pseudoantrodia monomitica B.K. Cui, Y.Y. Chen & Shun Liu, Fungal Diversity 118: 56. 2022.
Typus: China, Fujian, Fuzhou, Gushan Forest Park, Pinus sp., 29 Nov. 2019, Dai 21129* (holotype BJFC).
Notes: The species was described by Liu et al. (2022). We had no access to the type material. According to the protologue, P. monomitica is morphologically very similar to F. monomitica and F. oleracea but phylogenetically quite distant. The name change is necessary for moving P. monimitica to Fomitopsis.
Pseudophaeolus soloniensis (Dubois) Spirin & Rivoire, comb. nov. MycoBank MB 844983.
Basionym: Agaricus soloniensis Dubois, Méthode Éprouvée (Orleans): 177. 1803.
Typus: France, Tarn, Montirac, Castanea sativa, 27 May 2014, Rivoire (neotype designated here LY BR-5263*, MycoBank MBT 10008296).
Synonym:
Polyporus irpex Schulz. ex E.H.L. Krause, Arch. Freunde Naturgesch. Mecklenb. 1: 129. 1925.
Typus: Slovenia, Črny gaj, Quercus sp., [no collection date and collector indicated] (syntypes W0132391!, W0132392!).
Notes: For a long time, this species was treated as a member of Piptoporus (see under P. soloniensis in Ryvarden et al. 2017). Han et al. (2016) discovered that P. soloniensis is phylogenetically distant from the generic type of Piptoporus, P. betulinus (= F. betulina above) and placed it in the separate genus, Piptoporellus. Based on DNA data, Tibuhwa et al. (2020) showed that Laetiporus baudonii is closely related to P. soloniensis and moved it to Piptoporellus. However, they overlooked that Polyporus baudonii is the generic type of Pseudophaeolus, a monotypic genus which was described 40 years earlier than Piptoporellus. Therefore, we recombine P. soloniensis in Pseudophaeolus and treat Piptoporellus as a synonym of the latter genus. Additionally, a neotype for A. soloniensis is designated above since authentic material of this species is lacking.
Han et al. (2016) introduced two more species, Piptoporellus hainanensis and P. triqueter, as close relatives of P. soloniensis. Unfortunately, they did not pay attention to six species (Polyporus appendiculatus, P. komatsuzakii, P. medullae, P. sambuceus, Tyromyces armeniacus and T. imbricatus) described from the southern and eastern parts of Asia and currently treated as synonyms of P. soloniensis. Therefore, we are unwilling to combine P. hainanensis and P. triqueter in Pseudophaeolus until the identity of these older taxa is fully resolved.
Pseudophaeolus trichrous (Berk. & M.A. Curtis) Vlasák & Spirin, comb. nov. MycoBank MB 844984.
Basionym: Polyporus trichrous Berk. & M.A. Curtis, Ann. Mag. Nat. Hist. 2 (12): 434. 1853.
Typus: USA, South Carolina, ‘old logs’, [no collection date indicated], Curtis 2844 & Ravenel 973 (syntypes K).
Specimen examined: Costa Rica, Puntarenas, Monteverde, Santa Elena, Quercus sp. (?), Mar. 2022, Vlasák 2203/5* (JV, TUF).
Notes: Pseudophaeolus trichrous is the North American relative of P. soloniensis. Morphologically, both species are highly similar and they were therefore considered conspecific (Gilbertson & Ryvarden 1987). However, DNA sequences clearly separate them. As redefined here, P. trichrous differs from P. soloniensis in having smaller pores (4–6 per mm vs. 2–3 per mm) and slightly narrower basidiospores, (4.1–)4.2–5.7(−6.2) × (2.1–)2.2–3.0 μm (n = 30/1), L = 4.88, W = 2.57, Q = 1.91 (vs. (4.1–)4.2–6.1(−6.2) × (2.6–)2.8–3.3(−3.6) μm (n = 30/1), L = 5.03, W = 3.05, Q = 1.65 in P. soloniensis).
Trametes fulvidochmia Corner, Beihefte zur Nova Hedwigia 97: 104. 1989.
Typus: Malaysia, Terengganu, Kemaman, fallen log, 24 Jun. 1932, Corner (holotype E00438040).
Notes: Hattori (2005) suggested this species is related to F. dochmia. No modern collections of T. fulvidochmia are available, and we have not studied the type, why we are uncertain if it is related to the F. dochmia complex or any other Fomitopsis spp.
DISCUSSION
In this study, we argued for retaining the largest clade of Fomitopsidaceae as one genus, Fomitopsis, encompassing 111 already existing and 17 newly introduced species. Based on a revision of over 500 herbarium specimens (including 133 types) from four continents and newly produced or already available DNA sequences, we reduced 26 recently described species to synonyms and resurrected 26 older species previously listed among synonyms of other taxa.
Being reassessed for 128 accepted species, Fomitopsis becomes one of the largest polypore genera. However, species diversity in the genus is likely much higher than presented in this study. Among newly described taxa, two conspicuous species (F. algumicola and F. perhiemata) came from Europe and two (F. cupressicola and F. derelicta) from North America, i.e. from areas studied by mycologists for over two centuries. It means that more extensive collecting, especially in tropical areas and the southern hemisphere, will likely result in further taxonomic novelties. To date, Fomitopsis is represented by approximately the same number of species in three regions: warm temperate to boreal northern hemisphere (with 29 species from Europe, 24 from Asia and 32 from North America), tropical Asia (32 species) and the Neotropics (31 species). At the same time, 18 species were detected in Oceania (including Australia) and only 13 are known from Africa. This is a clear indication of insufficient sampling in the latter regions. In addition to purely taxonomic and floristic purposes, discovery of new species accompanied by deeper sequencing will certainly facilitate a much better understanding of the inner structure and evolutionary trends in Fomitopsidaceae.
Numerous rearrangements of species names and identities highlight the primary challenge in all contemporary studies in fungal taxonomy. It lies in a choice between immediate publication of taxonomic novelties (mainly based on using of the ITS region as a universal barcoding marker; Schoch et al. 2012) and undertaking deeper study of a presumed new species, which requires critical reassessment of related taxa (including their type material), broader geographic sampling and the use of additional genetic markers, and exhaustive study of relevant type material. In general, the first alternative has certain advantages because it makes newly obtained data available for a broader audience in a reasonable time. Moreover, fungi are one of the largest groups with hidden and undersampled species diversity, which means that many quickly introduced species names will likely survive in the future. However, the fast way of doing taxonomy does carry several disadvantages.
It is particularly likely in well-studied conspicuous fungi such as polypores, that many potentially new species already have an older name. The only way to diminish the risk of introducing superfluous names is to investigate type material of all taxa, both accepted and considered as synonyms, which appear in phylogenetic analyses as close relatives of a newly detected species. Although it may appear self-evident, this important step is often neglected. This has caused an emergence of new unnecessary names making species delimitation in Fomitopsidaceae even more challenging than it was before the introduction of DNA methods. Alongside agarics, polypores are the most frequently collected basidiomycetes, both in temperate forests and tropical areas. Seventy-five Fomitopsis spp. treated above produce pileate, robust and in many cases perennial basidiocarps, which are frequently collected (and described as new). The Fomitopsis palustris complex revised in the present paper exemplifies this situation. Of five species described in this group in the last ten years (in addition to numerous names introduced in the pre-DNA era), only one will probably survive while we place four others to the synonymy of species described over a hundred years ago. The members of the F. palustris complex are primary wooddecomposers inhabiting various (including industrially important) plant species (Rungjindamai et al. 2009, Roccotelli et al. 2014), and therefore the introduction of ill-defined species has not only purely scientific but also practical consequences.
The next challenge facing taxonomists arises from the binomial nature of a species name. According to the Code (Art. 35.1), valid publication of a new species implies the species is going to be described as a member of an already existing or simultaneously introduced genus. Formally, indication of the single feature which, in opinion of the author, distinguishes a newly introduced taxon from other ones is enough for introducing a new species or a new genus (Code Art. 38.2). This, somehow ambiguous, provision in the Code opens a door for describing new genera based on arbitrarily selected traits, without critical evaluation of their constancy and taxonomic significance in a given group. As a consequence, many of these genera turn out to be monotypic (Parmasto 1994). Nowadays, it is not an exceptional situation that DNA data show a newly found species is equally distant from already described and sequenced members of other genera, and thus seemingly requiring its own genus. The next step often taken is using any features differentiating the new species from its phylogenetic neighbours for justifying a new genus for it. This ‘microgeneric approach’, i.e. a tendency to raise each particular clade in the phylogenetic tree to the generic rank, currently dominates fungal taxonomy.
The microgeneric approach to the taxonomy of Fomitopsis and allied taxa fits best with the ‘nomothetic paradigm’ as introduced by Sigwart et al. (2018). According to their view, plants and fungi are considered as groups containing large genera that should be further split to fulfil the criteria of monophyly. This conclusion was based on a comparison of genus-size distribution in six large groups of animals with simulated phylogenies constructed under various speciation/extinction rates, and it imposes a law that most genera across all eukaryotic organisms are naturally small or even monotypic, and the number of larger genera should decrease proportionally to their size.
[The term ‘nomothetic’ was first introduced by the German neo-Kantian philosopher Wilhelm Windelband (Windelband 1904). It refers to one of two possible ways of presenting scientific knowledge, namely in a form of a universal law or rule installed for a given group of objects. Its alternative is the so-called ‘idiographic’ method oriented to specific, non-repeatable aspects of things or events. While the first way of representing reality is typical for natural sciences, the second one is more characteristic for history and allied disciplines. However, Windelband emphasized these two methods are not mutually exclusive, and the same class of objects or even the single object could be studied by them both. Even more important is that these methods do not define the contents of scientific knowledge (empirical reality) but characterize its treatment or interpretation (“Behandlung”) in a given science.]
We argue that, in spite of being entitled nomothetic, the approach by Sigwart et al. (2018) has certain limitations and cannot serve as a theoretical framework for our study, at least at the present level of our knowledge about the target group. The reason is that primary data about species richness of genera used by Sigwart et al. in the generic size distribution analyses came from taxonomic experts in six selected groups of animals. This poses two problems. Firstly, the taxonomic experts are, to some degree, influenced by the ongoing taxonomic fashion. Sigwart et al. explicitly recognize and then reject this possibility: they suggest the analysed groups are so divergent and investigated by so different methods that the taxonomists studying them can hardly sway each other. Secondly, the groups that their analyses rely on are difficult to compare to fungi and plants. These groups (accepted genera of marine invertebrates, odonates, fish, reptiles, birds, and mammals) are much better structured organisms with (as a rule) more complex life cycle than plants and fungi, which possess plenty of morphological traits potentially significant for taxonomists. The situation in plants and especially in fungi is dramatically different: some sister genera or even similar-looking but not closely related genera can be distinguished through very few morphological characters whose relevance is strongly dependent on growing conditions and good preservation of collected samples (cf. Spirin et al. 2019a, 2021).
We exemplified that a single morphological character, however peculiar it is, cannot give a sufficient justification for erecting a new genus, even if this option has been supported by DNA data. In our case, the suprageneric unit we were looking at was the family Fomitopsidaceae as redefined in multigene analyses by Justo et al. (2017). Denser taxon sampling of Fomitopsidaceae initiated by Runnel et al. (2019) and continued in the present study revealed three strongly supported lineages none of which, however, could be separated from the others based on a single morphological trait. Nevertheless, they can be distinguished via sets or combinations of characters (see Results) and thus are morphologically definable. Further splitting of these clades is possible based on DNA data although attempts to define them in morphological terms mainly lead to evident overestimation of rather ephemeral features. Validity of these differences becomes vague when more extensive material is involved in the microscopic scrutiny. This conclusion is grounded in our critical study of 124 from 142 species currently accepted in the Fomitopsidaceae, i.e. 87% of the known species diversity. Our general conclusion is that searching for an appropriate genus for the species in hand should start with a critical reassessment of morphological traits taxonomically significant for a given suprageneric unit (a family or, in a case of a poorly established family division, an order) revealed in multigene phylogenetic analyses. Not the single character but only a stable combination of characters intrinsic to members of a well-supported clade in the family/order-level phylogeny can provide a good reason for (re) establishing it as a genus.
Several recent publications followed the same vein and argued in favour of a more conservative, ‘macrogeneric’ approach for fungal taxonomy, especially in the species-rich groups (e.g., Spirin et al. 2018, 2019b, 2019c, Kuo et al. 2020, Koukol & Delgado 2021, Meiras-Ottoni et al. 2021, Viner et al. 2023). However, merging smaller genera together is not an end in itself, and it should be undertaken only after thorough estimation of further changes the proposed solution would have for the neighbouring taxa. The present study also exemplified how lumping taxa together meets with a nomenclatural problem of choosing the most appropriate name for the re-established genus. Although the principle of priority is among the cornerstones of nomenclature for plants and fungi, the Code makes a provision for another solution – a conservation, i.e. retaining the younger generic name and suppressing the older one. The oldest available name is not always the most desirable, and consequences of possible nomenclatural and taxonomic actions, both for experts and for a broader audience, should be carefully weighed in each case.
ACKNOWLEDGEMENTS
We are deeply indebted to the curators of O, S, PC, K, PRM, TUF, LY, UPS, L, NY, BPI, MICH, FH, W and MCF who sent us types and important specimens. Neil Dollinger, Josef Vlasák Jr. (USA), Jiří Kout (Czech Republic), Heikki Kotiranta, Daria Razmadze (Finland), David Boertmann (Denmark), Evgeniy Dunayev (Russia), Katerina Rusevska and Mitko Karadelev (North Macedonia) provided us with valuable collections used in the present study. Kare Liimatainen (United Kingdom), Beatriz Ortiz-Santana (USA), Ellen Larsson and Elisabeth Sjökvist (Sweden) helped us with sequencing of some important specimens. Vera Malysheva (Russia) generously helped us with completing illustrations and provided us with sequence data of F. incana. The research of JV was financed by the institutional support of the Academy Sciences of the Czech Republic (RVO: 60077344). The research of IV has been supported by Societas pro Fauna et Flora Fennica. The contribution of TG was supported by research project J4-3098 “The unrevealed information on soil biodiversity in leached waters” and the Research Program in Forest Biology, Ecology and Technology (P4-0107), both of the Slovenian Research Agency.
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supplementary Material: https://studiesinmycology.org/
Phylogenetic placement of the Daedalea – Fomitopsis clade (box) within Polyporales based on Maximum Likelihood of the ITS + LSU + SSU + mtSSU + RPB1 + RPB2 + TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Daedalea – Fomitopsis clade based on Maximum Likelihood of the ITS + LSU dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The species represented with ITS only are marked with an asterisk (*). The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis marianii complex based on Maximum Likelihood of the TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis meliae complex based on Maximum Likelihood of the TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis pinicola complex based on Maximum Likelihood of the ITS + TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis (Antrodia) ramentacea clade based on Maximum Likelihood of the ITS dataset.
Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis (Antrodia) juniperina clade based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin. The tree is midpoint-rooted.
Phylogenetic relationships of species in the Buglossoporus clade based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
List of reference species used for comparisons in this study and their accession numbers.
Phylogenetic statistics for the different analyses performed in this study.
REFERENCES
- Aime L, Ryvarden L, Henkel W. (2007). Studies in neotropical polypores 22. Additional new and rare species from Guyana. Synopsis Fungorum 23: 15–31. [Google Scholar]
- Ainsworth AM. (2001). Antrodia ramentacea on Salix in S.E. England. Field Mycology 2 (2): 46–49. [Google Scholar]
- Audet S. (2017). New genera and new combinations in Antrodia sensu lato. In: Mushrooms nomenclatural novelties nos. 1–9. https://sergeaudetmyco.com/antrodia/
- Berkeley MJ, Curtis MA. (1853). Centuries of North American fungi. The Annals and Magazine of Natural History 2: 417–435. [Google Scholar]
- Berkeley MJ. (1854). Decades of fungi. Decades XLI – XLIII. Hooker’s Journal of Botany 6: 129–143. [Google Scholar]
- Bernicchia A. (2005). Polyporaceae sensu lato Fungi Europaei 10: 1–808. [Google Scholar]
- Bernicchia A, Gorjón S. (2020) Polypores of the Mediterranean region. Romar, Gessate. [Google Scholar]
- Bernicchia A, Ryvarden L. (1998). Two new brown-rot polypores from Italy. Mycologia Helvetica 8: 3–10. [Google Scholar]
- Bernicchia A, Ryvarden L. (2001). A new Antrodia species (Coriolaceae, Basidiomycetes). Mycotaxon 79: 57–66. [Google Scholar]
- Blume C, Nees von Esenbeck TFL. (1826). Fungi Javanici, editi conjunctis studiis et opera. Nova Acta Physico-Medica Academiae Caesareae Leopoldino-Carolinae Naturae Curiosum 13: 10–22. [Google Scholar]
- Buchanan PK, Ryvarden L. (1988). Type studies in the Polyporaceae. 18. Species described by G.H. Cunningham. Mycotaxon 31: 1–38. [Google Scholar]
- Buchanan PK, Ryvarden L. (1993). Type studies in the Polyporaceae 24. Species described by Cleland, Rodway and Cheel. Australian Systematic Botany 6: 215–235. [Google Scholar]
- Carranza-Morse J, Gilbertson RL. (1986). Taxonomy of the Fomitopsis rosea complex. Mycotaxon 25: 469–486. [Google Scholar]
- Castresana J. (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17: 540–552. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Cui BK, Zhou LW. et al. (2015). Phylogeny, divergence time estimation, and biogeography of the genus Heterobasidion (Basidiomycota, Russulales). Fungal Diversity 71: 185–200. [Google Scholar]
- Chen YY, Wu F. (2017). A new species of Antrodia (Basidiomycota, polypores) from China. Mycosphere 8: 878–885. [Google Scholar]
- Cooke MC. (1886). Praecursores ad Monographia Polypororum. Grevillea 14: 77–87. [Google Scholar]
- Corner EJH. (1935). The fruit-body of Polystictus xanthopus Fr. Transactions of the British Mycological Society 46: 71–111. [Google Scholar]
- Corner EJH. (1953). The construction of polypores 1. Introduction: Polyporus sulphureus, P. squamosus, P. betulinus and Polystictus microcyclus. Phytomorphology 3: 152–167. [Google Scholar]
- Corner EJH. (1984). Ad Polyporaceaes II & III. Beihefte zur Nova Hedwigia 78: 1–222. [Google Scholar]
- Corner EJH. (1989). Ad Polyporaceaes VI. Beihefte zur Nova Hedwigia 97: 1–197. [Google Scholar]
- Cristaldo E, Kossmann T, Campi M. et al. (2022). Neotropical Daedalea (Basidiomycota, Fomitopsidaceae) revisited: Daedalea rajchenbergiana sp. nov. from Brazil. Lilloa 59: 273–289. [Google Scholar]
- Cui BK. (2013). Antrodia tropica sp. nov. from southern China inferred from morphological characters and molecular data. Mycological Progress 12: 223–230. [Google Scholar]
- Cui BK, Li HJ, Dai YC. (2011). Wood-rotting fungi in eastern China 6. Mycotaxon 116: 13–20. [Google Scholar]
- Cunningham GH. (1948). New Zealand Polyporaceae. 5. The genus Fomitopsis. Bulletin of the New Zealand Department of Scientific and Industrial Research 76: 1–8. [Google Scholar]
- Cunningham GH. (1954). Hyphal systems as aids in identification of species and genera of the Polyporaceae. Transactions of the British Mycological Society 37: 44–50. [Google Scholar]
- Cunningham GH. (1965). Polyporaceae of New Zealand. Bulletin of the New Zealand Department of Scientific and Industrial Research 164: 1–304. [Google Scholar]
- Dai YC, Niemelä T. (1995). Changbai wood-rotting fungi 4. Annales Botanici Fennici 32: 211–226. [Google Scholar]
- Dai YC, Vainio E, Hantula J. et al. (2003). Investigations on Heterobasidion annosum s.lat. in central and eastern Asia with the aid of mating tests and DNA fingerprinting. Forest Pathology 33: 269–286. [Google Scholar]
- Darriba D, Posada D, Kozlov AM. et al. (2020). ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution 37: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. (1980). Étude du genre Tyromyces sensu lato: répartition dans les genres Leptoporus, Spongiporus et Tyromyces sensu stricto. Bulletin Mensuel de la Société Linnéenne de Lyon 49: 6–56. [Google Scholar]
- David A, Dequatre B. (1985). Deux ultraspecies: Antrodia malicola (Berk. et M.A. Curtis) Donk et A. ramentacea (Berk. et Br.) Donk. Cryptogamie, Mycologie 5: 293–300. [Google Scholar]
- Decock CA, Ryvarden L, Amalfi M. (2022). Niveoporofomes (Basidiomycota, Fomitopsidaceae) in tropical Africa. Mycological Progress 21: 29. [Google Scholar]
- Degnan JH, Rosenberg NA. (2009). Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends in Ecology and Evolution 24: 332–340. [DOI] [PubMed] [Google Scholar]
- Donk MA. (1966). Notes on European polypores – I. Persoonia 4: 337–343. [Google Scholar]
- Donk MA. (1971). Notes on European polypores – VI (A). Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen, Section C 74: 1–14. [PubMed] [Google Scholar]
- Drechsler-Santos ER, Cavalcanti MAQ, Loguercio-Leite C. et al. (2012). On neotropical Daedalea species: Daedalea ryvardenica sp. nov. Kurtziana 37: 65–72. [Google Scholar]
- Fidalgo O, Fidalgo MEPK. (1967). Polyporaceae from Trinidad and Tobago. 2. Mycologia 59: 833–869. [Google Scholar]
- Figueroa SH, Decock C. (2007). On Trichaptum sprucei and the genus Phaeodaedalea (Basidiomycota, polypores). Cryptogamie, Mycologie 28: 189–201. [Google Scholar]
- Garbelotto MM, Lee HK, Slaughter G. et al. (1997). Heterokaryosis is not required for virulence of Heterobasidion annosum. Mycologia 89: 92–102. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Gilbertson RL. (1980). Wood-rotting fungi of North America. Mycologia 72: 1–49. [Google Scholar]
- Gilbertson RL, Ryvarden L. (1986). North American polypores. 1. Fungiflora, Oslo. [Google Scholar]
- Gilbertson RL, Ryvarden L. (1987). North American polypores. 2. Fungiflora, Oslo. [Google Scholar]
- Haight JE, Laursen GA, Glaeser JA. et al. (2016) Phylogeny of Fomitopsis pinicola: a species complex. Mycologia 108: 925–938. [DOI] [PubMed] [Google Scholar]
- Haight JE, Nakasone K, Laursen GA. et al. (2019). Fomitopsis mounceae and F. schrenkii – two new species from North America in the F. pinicola complex. Mycologia 111: 339–357. [DOI] [PubMed] [Google Scholar]
- Hallenberg N. (1990). Culture studies in the Corticiaceae (Basidiomycetes). Windahlia 18: 25–30. [Google Scholar]
- Han ML, Song J, Cui BK. (2014). Morphology and molecular phylogeny for two new species of Fomitopsis (Basidiomycota) from South China. Mycological Progress 13: 905–914. [Google Scholar]
- Han ML, Cui BK. (2015). Morphological characters and molecular data reveal a new species of Fomitopsis (Polyporales) from southern China. Mycoscience 56: 168–176. [Google Scholar]
- Han ML, Chen YY, Shen LL. et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Diversity 80: 343–373. [Google Scholar]
- Han ML, An Q, Fu WX. et al. (2020). Morphological characteristics and phylogenetic analyses reveal Antrodia yunnanensis sp. nov. from China. Phytotaxa 460: 1–11. [Google Scholar]
- Hartmann S, Vision T. (2008). Using ESTs for phylogenomics: can one accurately infer a phylogenetic tree from a gappy alignment? BMC Evolutionary Biology 8: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T. (2000). Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. I. Mycoscience 41: 339–349. [Google Scholar]
- Hattori T. (2003). Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. V. Mycoscience 44: 265–276. [Google Scholar]
- Hattori T. (2005). Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. VII. Mycoscience 46: 303–312. [Google Scholar]
- Hattori T, Ryvarden L. (1994). Type studies in the Polyporaceae 25. Species described from Japan by R. Imazeki and A. Yasuda. Mycotaxon 50: 27–46. [Google Scholar]
- Hattori T, Sotome K. (2013). Type studies of the polypores described by E.J.H. Corner from Asia and West Pacific Areas. VIII. Mycoscience 54: 297–308. [Google Scholar]
- Hjortstam K, Ryvarden L. (1984). Some new and noteworthy Basidiomycetes (Aphyllophorales) from Nepal. Mycotaxon 20: 133–151. [Google Scholar]
- Hooker JD. (1855). Flora Novae-Zelandiae. Part 2. Flowerless plants. The Botany of the Antarctic Voyage of H.M. Discovery Ships Erebus and Terror, in the years 1839 – 1843. 2: 1–378. [Google Scholar]
- Högberg N, Holdenreider O, Stenlid J. (1999). Population structure of the wood decay fungus Fomitopsis pinicola. Heredity 83: 354–360. [DOI] [PubMed] [Google Scholar]
- Ipulet P, Ryvarden L. (2005). New and interesting polypores from Uganda. Synopsis Fungorum 20: 87–99. [Google Scholar]
- Ji W, Wu DM, Song CG. et al. (2019). Two new Neofomitella species (Polyporaceae, Basidiomycota) based on morphological and molecular evidence. Mycological Progress 18: 593–602. [Google Scholar]
- Justo A, Miettinen O, Floudas D. et al. (2017). A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biology 121: 798–824. [DOI] [PubMed] [Google Scholar]
- Karasiński D, Niemelä T. (2016). Anthoporia, a new polypore genus in the Polyporales (Agaricomycetes). Polish Botanical Journal 61: 7–14. [Google Scholar]
- Karsten PA. (1881a). Enumeratio Boletinearum et Polyporearum Fennicarum, systemate novo dispositarum. Revue Mycologique Toulouse 3: 16–19. [Google Scholar]
- Karsten PA. (1881b). Symbolae ad mycologiam Fennicam. Meddelanden af Societas pro Fauna et Flora Fennica 6: 7–13. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Yoon YG, Jung HS. (2005). Evaluation of the monophyly of Fomitopsis using parsimony and MCMC methods. Mycologia 97: 812–822. [DOI] [PubMed] [Google Scholar]
- Kim KM, Lee JS, Jung HS. (2008). Fomitopsis incarnatus sp. nov. based on generic evaluation of Fomitopsis and Rhodofomes. Mycologia 99: 833–841. [DOI] [PubMed] [Google Scholar]
- Kim CS, Jo JW, Kwang YN. et al. (2015). Mushroom flora of Ulleunggun and a newly recorded Bovista species in the Republic of Korea. Mycobiology 43: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AM, Darriba D, Flouri T. et al. (2019). RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35: 4453–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlaba F, Pouzar Z. (1957). Notes on classification of European pore fungi. Česká Mykologie 11: 152–170. [Google Scholar]
- Kotlaba F, Pouzar Z. (1958). Polypori novi vel minus cogniti Cechoslovakiae 3. Česká Mykologie 12: 95–104. [Google Scholar]
- Kotlaba F, Pouzar Z. (1990). Type studies of polypores described by A. Pilát 3. Česká Mykologie 44: 228–237. [Google Scholar]
- Kotlaba F, Pouzar Z. (1993). Pilatoporus maroccanus sp. nov., a new polypore of the Polyporus palustris group. Cryptogamie, Mycologie 14: 215–218. [Google Scholar]
- Kotlaba F, Pouzar Z. (1998). Notes on the division of the genus Fomitopsis (Polyporales). Folia Cryptogamica Estonica 33: 49–52. [Google Scholar]
- Koukol O, Delgado G. (2021). Why morphology matters: the negative consequences of hasty descriptions of putative novelties in asexual ascomycetes. SIM 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kout J, Vlasák J. (2009). Antrodia serialiformis from the eastern USA, a new and abundant polypore similar to A. serialis. Mycotaxon 108: 329–335. [Google Scholar]
- Kout J, Vlasák J, Vlasák J., Jr (2017). Antrodia multiformis and A. tenerifensis spp. nov. (Fomitopsidaceae, Basidiomycota): new brown-rot polypores. Mycological Progress 16: 737–742. [Google Scholar]
- Kuo M, Ortiz-Santana B. (2020). Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia 112: 197–211. [DOI] [PubMed] [Google Scholar]
- Larsson A. (2014). AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30: 3276–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaché AD, Zhu T, Rannala B, Yang Z. (2019). The spectre of too many species. Systematic Biology 68: 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillé JH. (1844). Champignons exotiques. Annales des Sciences Naturelles 3: 167–221. [Google Scholar]
- Léveillé JH. (1846). Descriptions des champignons de l’herbier du Múseum de Paris. Annales des Sciences Naturelles 3: 111–167. [Google Scholar]
- Li HJ, Cui BK. (2013). Two new Daedalea species (Polyporales, Basidiomycota) from South China. Mycoscience 54: 62–68. [Google Scholar]
- Li HJ, Han ML, Cui BK. (2013). Two new Fomitopsis species from southern China based on morphological and molecular characters. Mycological Progress 12: 709–718. [Google Scholar]
- Liimatainen K, Ainsworth AM. (2018). Fifteen Cortinarius species associated with Helianthemum in Great Britain: results of a DNA-based analysis. Field Mycology 19: 119–135. [Google Scholar]
- Lindblad I, Ryvarden L. (1999). Studies in neotropical polypores 3. Mycotaxon 71: 335–359. [Google Scholar]
- Lindner DL, Ryvarden L, Baroni TJ. (2011). A new species of Daedalea (Basidiomycota) and a synopsis of core species in Daedalea sensu stricto. North American Fungi 6: 1–12. [Google Scholar]
- Liu S, Song CG, Cui BK. (2019). Morphological characters and molecular data reveal three new species of Fomitopsis (Basidiomycota). Mycological Progress 18: 1317–1327. [Google Scholar]
- Liu S, Han ML, Xu TM. et al. (2021). Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from East Asia. Frontiers in Microbiology 12: 644979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chen YY, Sun YF. et al. (2022). Systematic classification and phylogenetic relationships of the brown-rot fungi within the Polyporales. Fungal Diversity 118: 1–94. [Google Scholar]
- Lloyd CG. (1915). Synopsis of the genus Fomes. Mycological Writings 4: 209–288. [Google Scholar]
- Lloyd CG. (1917). Mycological notes 49. Mycological Writings 5: 685–700. [Google Scholar]
- Lowe JL. (1957). Polyporaceae of North America: the genus Fomes. Technical Publication of the State University College of Forestry at Syracuse University 80: 1–96. [Google Scholar]
- Lowe JL. (1966). Polyporaceae of North America: the genus Poria. Technical Publication of the State University College of Forestry at Syracuse University 90: 1–183. [Google Scholar]
- Lowe JL. (1975). Polyporaceae of North America: the genus Tyromyces. Mycotaxon 2: 1–82. [Google Scholar]
- Lowe JL, Pegler D. (1973). Polyporus amygdalinus and P. pseudosulphureus. Mycologia 65: 208–211. [Google Scholar]
- Löytynoja A, Goldman N. (2010). webPRANK: a phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC Bioinformatics 11: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KJ, Rygiewicz PT. (2005). Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiology 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuka A, Ryvarden L. (1993). Two new polypores from Malawi. Mycologia Helvetica 5: 143–148. [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF, Hall BD. (2002). Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Wang Z, Binder M. et al. (2007). Contributions of RPB2 and TEF1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Molecular Phylogenetics and Evolution 43: 430–451. [DOI] [PubMed] [Google Scholar]
- de Meiras-Ottoni A, Larsson KH, Gibertoni TB. (2021). Additions to Trechispora and the status of Scytinopogon (Trechisporales, Basidiomycota). Mycological Progress 20: 203–222. [Google Scholar]
- Miettinen O, Vlasák J, Rivoire B. et al. (2018). Postia caesia complex (Polyporales, Basidiomycota) in temperate Northern Hemisphere. Fungal Systematics and Evolution 1: 101–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne I, Lindner D, Bayer M. et al. (2008). TOPALi v2: a rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics 25: 126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi S, Kiss E, Kuo A. et al. (2020). Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nature Communications 11: 5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne JPFC. (1840). Sécond centurie de plantes cellulaires exotiques nouvelles, Décades I et II. Annales des Sciences Naturelles 2: 193–207. [Google Scholar]
- Montagne JPFC. (1854). Cryptogamia Guyanensis. Annales des Sciences Naturelles 4: 91–144. [Google Scholar]
- Mossebo DC, Ryvarden L. (1997). Fomitopsis africana nov. sp. (Polyporaceae, Basidiomycotina). Sydowia 49: 147–149. [Google Scholar]
- Mounce I, Macrae R. (1938). Interfertility phenomena in Fomes pinicola. Canadian Journal of Research 16: 354–376. [Google Scholar]
- Müller J, Müller K, Quandt D. (2010). PhyDE – Phylogenetic Data Editor, version 0.997. http://phyde.de.
- Nguyen LT, Schmidt HA, von Haeseler A. et al. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä T. (1978). On Fennoscandian polypores 6. Antrodia plicata n. sp. Karstenia 18: 43–48. [Google Scholar]
- Niemelä T. (2005). Polypores, lignicolous fungi. Norrlinia 13: 1–320. [Google Scholar]
- Niemelä T, Penttilä R. (1992). Antrodia mellita (Basidiomycetes), a new large-pored polypore species with a continental distribution. Annales Botanici Fennici 29: 55–65. [Google Scholar]
- Niemelä T, Ryvarden L. (1975). Studies in the Aphyllophorales of Africa 4. Antrodia juniperina, new to Africa. Transactions of the British Mycological Society 65: 427–432. [Google Scholar]
- Nobles MK. (1958). Cultural characters as a guide to the taxonomy and the phylogeny of the Polyporaceae. Canadian Journal of Botany 36: 883–926. [Google Scholar]
- Nobles MK. (1971). Cultural characters as a guide to the taxonomy of the Polyporaceae. In: Evolution in the higher basidiomycetes (Petersen R. ed.). University of Tennessee Press: 169–196. [Google Scholar]
- Núñez M, Ryvarden L. (1995). Polyporus (Basidiomycotina) and related genera. Synopsis Fungorum 10: 1–85. [Google Scholar]
- Núñez M, Ryvarden L. (2001). East Asian polypores 2. Synopsis Fungorum 14: 170–522. [Google Scholar]
- Ortiz-Santana B, Lindner DL, Miettinen O. et al. (2013). A phylogenetic overview of the Antrodia clade (Basidiomycota, Polyporales). Mycologia 105: 391–1411. [DOI] [PubMed] [Google Scholar]
- Parmasto E. (1994). Limits of splitting (on schizotaxia). Mycologia Helvetica 6: 8–34. [Google Scholar]
- Persoon CH. (1801). Synopsis methodica fungorum. 2. Heinrich Dieterich, Göttingen. [Google Scholar]
- Petrak F. (1959). Über eine neue Daedalea-Art aus Borneo. Sydowia 13: 139–142. [Google Scholar]
- Rajchenberg M. (1986). On Trametes aethalodes and other species of Daedalea (Polyporaceae). Canadian Journal of Botany 64: 2130–2135. [Google Scholar]
- Rajchenberg M. (1994). A taxonomic study of the subantarctic Piptoporus (Polyporaceae, Basidiomycota) 12. Nordic Journal of Botany 14: 435–449. [Google Scholar]
- Rajchenberg M. (1995a). A taxonomic study of the subantarctic Piptoporus (Polyporaceae, Basidiomycota) 2. Nordic Journal of Botany 15: 105–119. [Google Scholar]
- Rajchenberg M. (1995b). New polypores from the Nothofagus forests of Argentina. Mycotaxon 54: 427–453. [Google Scholar]
- Rajchenberg M, Gorjón SP, Pildain MB. (2011). The phylogenetic disposition of Antrodia sensu lato taxa (Polyporales, Basidiomycota) from Patagonia, Argentina. Australian Systematic Botany 24: 111–120. [Google Scholar]
- Rambaut A, Drummond AJ, Xie D. et al. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DA. (1974). A reappraisal of type and authentic material of the larger Basidiomycetes in the Pretoria herbarium. Bothalia 11: 221–230. [Google Scholar]
- Reid DA. (1976). Notes on polypores 2. Memoirs of the New York Botanical Garden 28: 179–198. [Google Scholar]
- Renvall P, Niemelä T. (1992). Basidiomycetes at the timberline in Lapland 3. Karstenia 32: 29–42. [Google Scholar]
- Rivoire B. (2010). Antrodia pulverulenta (Basidiomycetes, polypore), une espèce nouvelle produisant des amas de mitospores blanches. Bulletin Mensuel de la Société Linnéenne de Lyon 79: 185–190. [Google Scholar]
- Rivoire B, Trichies G, Vlasák J. (2015). Cartilosoma rene-hentic (Basidiomycota, Polyporales), une espèce nouvelle dans le groupe d’Antrodia ramentacea. Bulletin Mensuel de la Société Linnéenne de Lyon 84: 5–18. [Google Scholar]
- Rivoire B. (2020). Polypores de France et d’Europe. Mycopolydev, Orliénas. [Google Scholar]
- Roccotelli A, Schena A, Sanzani SM. et al. (2014). Characterization of basidiomycetes associated with wood rot of Citrus in southern Italy. Phytopathology 104: 851–858. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P. et al. (2012). MrBayes 32: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungjindamai N, Pinruan U, Choeyklin R. et al. (2009). Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Diversity 33: 139–161. [Google Scholar]
- Runnel K, Spirin V, Miettinen O. et al. (2019). Morphological plasticity in brown-rot fungi: Antrodia is redefined to encompass both poroid and corticioid species. Mycologia 111: 871–883. [DOI] [PubMed] [Google Scholar]
- Ryvarden L. (1972). A critical checklist of the Polyporaceae in tropical east Africa. Norwegian Journal of Botany 19: 229–238. [Google Scholar]
- Ryvarden L. (1976). Type studies in the Polyporaceae 7. Kew Bulletin 31: 83–103. [Google Scholar]
- Ryvarden L. (1977). Type studies in the Polyporaceae 10. Norwegian Journal of Botany 24: 213–230. [Google Scholar]
- Ryvarden L. (1981). Type studies in the Polyporaceae 13. Mycotaxon 13: 175–186. [Google Scholar]
- Ryvarden L. (1982). Type studies in the Polyporaceae 11. Nordic Journal of Botany 2: 75–84. [Google Scholar]
- Ryvarden L. (1983). Type studies in the Polyporaceae 14. Occasional Papers of the Farlow Herbarium of Cryptogamic Botany 18: 1–39. [Google Scholar]
- Ryvarden L. (1984). Type studies in the Polyporaceae 16. Mycotaxon 20: 329–363. [Google Scholar]
- Ryvarden L. (1985). Type studies in the Polyporaceae 17. Mycotaxon 23: 169–198. [Google Scholar]
- Ryvarden L. (1988a). Type studies in the Polyporaceae 20. Mycotaxon 33: 303–327. [Google Scholar]
- Ryvarden L. (1988b). Type studies in the Polyporaceae 19. Mycotaxon 31: 45–58. [Google Scholar]
- Ryvarden L. (1990). Type studies in the Polyporaceae 22. Mycotaxon 38: 83–102. [Google Scholar]
- Ryvarden L. (1991). Genera of polypores: nomenclature and taxonomy. Synopsis Fungorum 5: 1–363. [Google Scholar]
- Ryvarden L. (2015). Neotropical polypores 2. Synopsis Fungorum 34: 232–443. [Google Scholar]
- Ryvarden L. (2016). Neotropical polypores 3. Synopsis Fungorum 36: 445–613. [Google Scholar]
- Ryvarden L, Aime MC, Baroni TJ. (2009). Studies in neotropical polypores 26. Synopsis Fungorum 26: 24–26. [Google Scholar]
- Ryvarden L, Johansen I. (1980). A preliminary polypore flora of East Africa. Fungiflora, Oslo. [Google Scholar]
- Ryvarden L, Gilbertson RL. (1993). European polypores 1. Synopsis Fungorum 5: 1–387. [Google Scholar]
- Ryvarden L, Melo I, Niemelä T. (2017). Poroid fungi of Europe. Synopsis Fungorum 37: 1–431. [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S. et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the United States of America 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigwart JD, Sutton MD, Bennett KD. (2018). How big is a genus? Towards a nomothetic systematics. Zoological Journal of the Linnean Society 183: 237–252. [Google Scholar]
- Soares AM, Nogueira-Melo G, Plautz L. et al. (2017). A new species, two new combinations and notes on Fomitopsidaceae (Agaricomycetes, Polyporales). Phytotaxa 33: 75–83. [Google Scholar]
- Spirin V, Zmitrovich I. (2003). Notes on some rare polypores found in Russia 1. Karstenia 43: 67–82. [Google Scholar]
- Spirin V, Zmitrovich I, Wasser S. (2006). Oligoporus balsameus – rare Eurasian species plus notes on some related taxa. Mycotaxon 97: 73–82. [Google Scholar]
- Spirin V, Vlasák J, Niemelä T. et al. (2013a). What is Antrodia sensu stricto? Mycologia 105: 1555–1576. [DOI] [PubMed] [Google Scholar]
- Spirin V, Miettinen O, Pennanen J. et al. (2013b). Antrodia hyalina, a new polypore from Russia, and A. leucaena, new to Europe. Mycological Progress 12: 53–61. [Google Scholar]
- Spirin V, Runnel K, Vlasák J. et al. (2015a). Species diversity in the Antrodia crassa group (Polyporales, Basidiomycota). Fungal Diversity 119: 1291–1310. [DOI] [PubMed] [Google Scholar]
- Spirin V, Vlasák J, Milakovsky B. et al. (2015b). Searching for indicator species of old-growth spruce forests: studies in the genus Jahnoporus (Polyporales, Basidiomycota). Cryptogamie, Mycologie 36: 1–10. [Google Scholar]
- Spirin V, Vlasák J, Rivoire B. et al. (2016). Hidden diversity in the Antrodia malicola group (Polyporales, Basidiomycota). Mycological Progress 15: 1–13. [Google Scholar]
- Spirin V, Vlasák J, Miettinen O. (2017). Studies in the Antrodia serialis group (Polyporales, Basidiomycota). Mycologia 109: 217–230. [DOI] [PubMed] [Google Scholar]
- Spirin V, Malysheva V, Trichies G. et al. (2018). A preliminary overview of the corticioid Atractiellomycetes (Pucciniomycotina, Basidiomycota). Fungal Systematics and Evolution 2: 311–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin V, Malysheva V, Haelewaters D. et al. (2019a). Studies in the Stypella vermiformis group (Auriculariales, Basidiomycota). Antonie van Leeuwenhoek 112: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin V, Malysheva V, Roberts P. et al. (2019b). A convolute diversity of the Auriculariales (Agaricomycetes, Basidiomycota) with sphaeropedunculate basidia. Nordic Journal of Botany 37: e02394. [Google Scholar]
- Spirin V, Malysheva V, Miettinen O. et al. (2019c). On Protomerulius and Heterochaetella (Auriculariales, Basidiomycota). Mycological Progress 18: 1079–1099. [Google Scholar]
- Spirin V, Volobuev S, Viner I. et al. (2021). On Sistotremastrum and similar-looking taxa (Trechisporales, Basidiomycota). Mycological Progress 20: 453–476. [Google Scholar]
- Stevenson JA, Cash EK. (1936). The new fungus names proposed by C. G. Lloyd. Bulletin of the Lloyd Library and Museum 35: 1–209. [Google Scholar]
- Stiller JW, Hall BD. (1997). The origin of red algae: implications for plastid evolution. Proceedings of the National Academy of Sciences of the United States of America 94: 4520–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockland J, Ryvarden L. (2008). Fomitopsis ochracea species nova. Synopsis Fungorum 25: 44–47. [Google Scholar]
- Sukumaran J, Knowles LL. (2017). Multispecies coalescent delimits structure, not species. Proceedings of the National Academy of Sciences of the United States of America 114: 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm H, Põldmaa K. (2013). Diversity, host associations and phylogeography of temperate aurofusarin-producing Hypomyces/Cladobotryum including causal agents of cobweb disease of cultivated mushrooms. Fungal Biology 117: 348–367. [DOI] [PubMed] [Google Scholar]
- Thiers B. (2021). Index Herbariorum: a global directory of public herbaria and associated staff New York Botanical Garden’s Virtual Herbarium. http://sweetgumnybgorg/science/ih/
- Tibpromma S, Hyde K, Jeewon R. et al. (2017). Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 83: 1–261. [Google Scholar]
- Tibuhwa DD, Hussein JM, Ryvarden L. et al. (2020). A phylogeny for the plant pathogen Piptoporellus baudonii using a multigene data set. Mycologia 112: 1017–1025. [DOI] [PubMed] [Google Scholar]
- von Thümen F. (1875). Symbolae ad floram mycologicam Australiae. Grevillea 4: 70–76. [Google Scholar]
- Vampola P. (1996). New localities of Pilatoporus ibericus in Europe and Asia. Czech Mycology 49: 85–90. [Google Scholar]
- Vellinga EC, Kuyper TW, Ammirati J. et al. (2015). Six simple guidelines for introducing new genera of fungi. SIM 6: 65–68. [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner I, Kokaeva L, Spirin V. et al. (2021). Significance of incongruent DNA loci in the taxonomy of wood-decaying Basidioradulum radula. Mycologia 113: 995–1008. [DOI] [PubMed] [Google Scholar]
- Viner I, Spirin V, Larsson KH. et al. (2023). Systematic placement of Lagarobasidium cymosum and description of two new species. Mycologia 115: 122–134. [DOI] [PubMed] [Google Scholar]
- Virdi SS. (1992). Two Antrodia species from Arunachal Pradesh (India). International Journal of Mycology and Lichenology 5: 307–311. [Google Scholar]
- Vlasák J, Kout J, Vlasák J., Jr et al. (2011). New records of polypores from southern Florida. Mycotaxon 118: 159–176. [Google Scholar]
- Vlasák J, Vlasák J, Jr, Ryvarden L. (2012). Four new polypore species from the western United States. Mycotaxon 119: 217–231. [Google Scholar]
- Vlasák J, Vlasák J, Jr, Cui BK. (2013). Antrodia kmetii, a new European polypore similar to Antrodia variiformis. Cryptogamie, Mycologie 34: 203–209. [Google Scholar]
- Vlasák J, Vlasák J, Jr, Ryvarden L. (2016). Studies in neotropical polypores 42. Synopsis Fungorum 35: 9–33. [Google Scholar]
- Wang YR, Dai YC, Liu HG. et al. (2022). A new contribution to Megasporoporia sensu lato: six new species and three new combinations. Frontiers in Microbiology 13: 1046777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S. et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand H, Sninsky JS. et al. eds). Academic Press: 315–322. [Google Scholar]
- Windelband W. (1904). Geschichte und Naturwissenschaft. Heitz & Mündel, Straßburg. [Google Scholar]
- Wu SH, Yu ZH, Dai YC. et al. (2004). Taiwanofungus, a polypore new genus. Fungal Science 19: 109–116. [Google Scholar]
- Yuan Y, Gafforov Y, Chen YY. et al. (2017). A new species of Antrodia (Basidiomycota, Polyporales) from juniper forest of Uzbekistan. Phytotaxa 303: 47–55. [Google Scholar]
- Yuan HS, Lu X, Dai YC. et al. (2020). Fungal diversity notes 1277–1386: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 104: 1–266. [Google Scholar]
- Vu D, Groenewald M, de Vries M. et al. (2019). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LW, Wei YL. (2012). Changbai wood-rotting fungi 16. Mycological Progress 11: 435–441. [Google Scholar]
- Zhou M, Wang CG, Wu YD. et al. (2021). Two new brown rot polypores from tropical China. MycoKeys 82: 173–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmitrovich IV. (2018). Conspectus systematis Polyporacearum v. 1.0. Folia Cryptogamica Petropolitana 6: 1–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic placement of the Daedalea – Fomitopsis clade (box) within Polyporales based on Maximum Likelihood of the ITS + LSU + SSU + mtSSU + RPB1 + RPB2 + TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Daedalea – Fomitopsis clade based on Maximum Likelihood of the ITS + LSU dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The species represented with ITS only are marked with an asterisk (*). The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis marianii complex based on Maximum Likelihood of the TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis meliae complex based on Maximum Likelihood of the TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis pinicola complex based on Maximum Likelihood of the ITS + TEF1 dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
Phylogenetic relationships of species in the Fomitopsis (Antrodia) ramentacea clade based on Maximum Likelihood of the ITS dataset.
Phylogenetic relationships of species in the Fomitopsis (Antrodia) juniperina clade based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin. The tree is midpoint-rooted.
Phylogenetic relationships of species in the Buglossoporus clade based on Maximum Likelihood of the ITS dataset. Numbers on nodes represent bootstrap values > 70% and Bayesian Inference posterior probabilities > 0.85. The scale bar indicates the number of expected substitutions per site. Two-letter codes in the parentheses denote the country of origin.
List of reference species used for comparisons in this study and their accession numbers.
Phylogenetic statistics for the different analyses performed in this study.