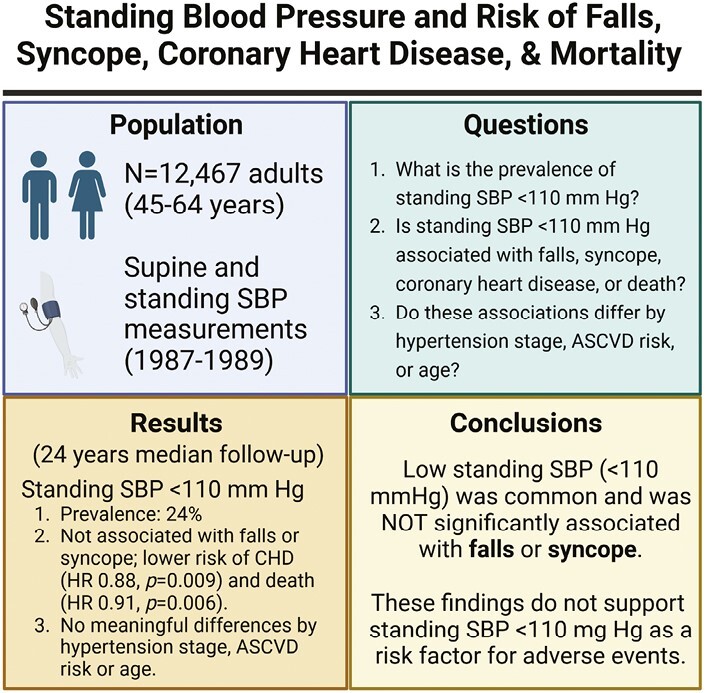
Abstract
BACKGROUND
ACC/AHA guidelines caution against the use of antihypertensive therapy in the setting of low standing systolic BP (SBP) < 110 mm Hg due to unclear benefits.
METHODS
The Atherosclerosis Risk in Communities (ARIC) Study measured supine and standing SBP in adults aged 45–64 years between 1987 and 1989. We used Cox regression to evaluate the associations of low standing SBP (<110 mm Hg) with risk of falls, syncope, coronary heart disease (CHD), and mortality through December 31, 2019. Falls and syncope were ascertained by hospitalization and outpatient claims; CHD events were adjudicated. Associations were examined overall and in strata of hypertension stage, 10-year atherosclerotic cardiovascular disease (ASCVD) risk, age, and sex.
RESULTS
Among 12,467 adults followed a median of 24 years (mean age at enrollment 54.1 ± 5.8 years, 55% women, 26% Black adults), 3,000 (24%) had a standing SBP < 110 mm Hg. A standing SBP < 110 mm Hg compared to standing SBP ≥ 110 mm Hg was not significantly associated with falls or syncope, and was associated with a lower risk of CHD events and mortality with HRs of 1.02 (95% CI 0.94, 1.11), 1.02 (0.93, 1.11), 0.88 (0.80, 0.97), and 0.91 (0.86, 0.97), respectively. There were no clinically meaningful differences when stratified by hypertension stage, 10-year ASCVD risk, age, and sex.
CONCLUSIONS
In this community-based population, low standing SBP was common and not significantly associated with falls or syncope, but was associated with a lower risk of CHD and mortality. These findings do not support screening for low standing BP as a risk factor for adverse events.
Keywords: blood pressure, coronary heart disease, falls, hypertension, mortality, standing blood pressure, syncope
Graphical Abstract
Blood pressure (BP) is an important modifiable risk factor for cardiovascular disease and mortality.1 The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that a lower systolic BP (SBP) treatment goal reduced the risk of coronary heart disease (CHD) and death from any cause without increasing risk of falls and only mildly increasing risk of syncope among adults with hypertension at higher risk for CHD.2 However, SPRINT excluded adults with a standing SBP < 110 mm Hg, raising questions about whether the risk of an intensive BP treatment goal may be underestimated for clinical populations that are not routinely screened for standing hypotension.3 Indeed, the 2017 ACC/AHA hypertension management guidelines caution against the initiation of antihypertensive medications for patients with low standing BP (<110 mm Hg).4 However, very little is even known about standing BP and clinical outcomes in the general adult population with hypertension and without hypertension.
The Atherosclerosis Risk in Communities (ARIC) Study was established to examine cardiovascular risk factors in community-dwelling, middle-aged U.S. adults. A significant number of ARIC participants further underwent standing BP measurement as part of an ancillary study focused on orthostatic hypotension. While prior ARIC studies demonstrated that orthostatic hypotension was an independent predictor of falls, syncope, CHD events, and all-cause mortality,5–7 standing hypotension (defined here as SBP < 110 mm Hg) and standing SBP have not been studied. These standing BP assessments afford a unique opportunity to characterize standing hypotension and standing SBP in an ambulatory population and determine its association with adverse events often attributed to hypertension treatment.
The objectives of the present study were to (i) quantify the prevalence of standing hypotension (i.e., standing SBP < 110 mm Hg), (ii) characterize the association of standing hypotension and standing SBP with adverse clinical events, that is, falls, syncope, CHD events, and mortality, and (iii) determine whether the association between standing hypotension or standing SBP with adverse outcomes differed by hypertension stage, 10-year estimated atherosclerotic cardiovascular disease (ASCVD) risk, or age among community-dwelling middle-aged adults. We hypothesized that while standing hypotension would be common, it would not be significantly associated with adverse clinical events regardless of hypertensive stage, 10-year ASCVD risk, age, sex, change in BP upon standing, hypertension status, or antihypertensive medication use at baseline.
METHODS
Study population
The ARIC Study is an ongoing prospective study of 15,792 middle-aged mostly White and Black adults, described in depth elsewhere.8–10 In brief, ARIC enrolled community-dwelling adults, aged 45–64 years, from four U.S. communities: Forsyth Country, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. Participants were enrolled between 1987 and 1989 (visit 1), and the original study protocol consisted of physical examinations, medical interviews, and laboratory tests. Participants have been followed for over three decades through active surveillance of community hospitals, annual or semiannual telephone interviews, and follow-up clinical examinations.10
The present analysis excluded participants who withdrew consent (N = 35), did not participate in standing BP measurements during visit 1 (N = 2,548), and those missing relevant covariate data at baseline (visit 1) (N = 742); the three most common missing covariates were relevant medication use data (N = 575), LDL-cholesterol (N = 82), and heart rate (N = 63). Our analytic sample included 12,467 participants.
All participants provided written informed consent and the study protocol was approved by institutional review boards from each study site. The Institutional Review Board at Beth Israel Deaconess Medical Center decided that this secondary analysis was human subjects exempt research.
Exposure: standing hypotension and standing SBP
During the baseline visit, after a 20-minute rest period, supine BP was measured up to five times following a standardized protocol using an automatic cuff (Dinamap 1846 SX oscillometric device) every 20–30 seconds for two minutes. Participants were then instructed to stand up from a supine position with their arm supported at heart level with a bedside table. They subsequently underwent up to five standing measurements (≥4 measurements obtained for 91% of participants) every 20–30 seconds within the first 2 minutes of standing, and the average of up to five measurements were used to determine standing BP. Measures occurred without a programmed pause between deflation and re-inflation. Further details for recording supine and standing BP have been described previously.11 In this study, we defined standing hypotension as a mean standing SBP < 110 mm Hg. Our reference group was standing SBP ≥ 110 mm Hg. We also examined standing SBP as a continuous variable (per 10 mm Hg).
Clinical outcomes: falls, syncope, CHD, and death
The clinical outcomes in this study were falls, syncope, incident CHD events, and all-cause mortality, after visit 1 through December 31, 2019 (for all clinical events; follow-up was not available for the Jackson site after December 31, 2017). Falls and syncope were defined as the first occurrence of any related hospitalization or claim for inpatient or outpatient services after the baseline visit. These outcomes were identified from active surveillance of all hospitalizations for all ARIC participants and linkage to Centers for Medicare and Medicaid Services (CMS) claims data from 1985 to 2018 for Medicare fee-for-service beneficiaries (see Supplementary Methods SM1).7,12 Fall and syncope claims corresponded to International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes (see Supplementary Methods SM1 for ICD codes). The sensitivity and specificity of this community surveillance approach has been described previously.10,12
Incident CHD events and death were adjudicated by an expert panel based on hospital discharge documents or death certificates through procedures described previously.7,10 Adjudicated CHD events were determined by a composite definition of fatal CHD, cardiac procedure, or silent myocardial infarction based on electrocardiogram (ECG) changes.10 Death was determined by surveillance of hospital discharge records, coroner reports, the National Death Index, and next-of-kin interviews.
Covariates of interest
Baseline data from visit 1 was collected by trained study personnel using standardized protocols with quality control measures. Covariates of interest were selected to examine the independent association between standing SBP and falls, syncope, CHD, and all-cause mortality (see Supplementary Methods SM2 for covariate definitions).
Statistical analyses
Baseline study population characteristics were described using means and proportions overall and according to hypertension stage.
We used Cox regression to evaluate the association between standing hypotension (standing SBP < 110 mm Hg vs. ≥110 mm Hg) or standing SBP (continuous variable) and falls, syncope, CHD, and all-cause mortality. In all models, we included covariates related to falls, syncope, and CHD disease, namely, age, sex, race-center, estimated glomerular filtration rate, body mass index, resting heart rate, high density lipoprotein cholesterol, total cholesterol, prevalent CHD, prior stroke, prior heart failure, diabetes, hypertension status, dizziness, alcohol use, education, leisure index, smoking status, and use of antihypertension medications in the last 2 weeks, and use of diuretics, cholesterol-lowering medications, antidepressants, sedatives, hypnotics, antipsychotics, or anticholinergics. We used log–log plots to assess the Cox proportionality assumption for standing hypotension. We also examined the occurrence of the four clinical outcomes according to standing hypotension (<110 mm Hg vs. ≥110 mm Hg) with cumulative incidence plots. To more flexibly model the continuous association of standing SBP with the four clinical outcomes, we also modeled standing SBP as a fully adjusted restricted cubic spline (4 knots, locations selected via Harrell’s method).13
In stratified analyses, we assessed for effect modification by baseline hypertension stage (3 strata: Normotension, stage 1, and stage 2 or treated hypertension), baseline 10-year atherosclerotic cardiovascular disease (ASCVD) risk calculated with the US-derived pooled cohort equations14 (2 strata: ASCVD < 10% and ASCVD ≥ 10% or prior CHD history), age (3 strata: <50 years, 50–59 years, and 60–66 years), sex (2 strata: male and female), change in BP upon standing (2 strata: postural change ≤ −20 mm Hg and postural change ≥ −20 mm Hg), baseline hypertension status (2 strata: no hypertension or any hypertension [stage 1, stage 2, or treated]), and antihypertensive medication use at baseline (2 strata: no antihypertensive medication use in the last two weeks and antihypertensive medication use in the last 2 weeks).
All analyses were conducted using Stata 15.0 (StataCorp LP, College Station, TX). A two-tailed P-value < 0.05 was considered statistically significant.
RESULTS
Population characteristics
Overall, the mean age of participants was 54.1 ± 5.8 years (range 44–66 years); 55% were female, and 26% were Black. At baseline, 24% of participants had standing hypotension and 4% had orthostatic hypotension (Table 1). Standing hypotension was identified among 38% with normotension, 4% with stage 1 hypertension, and 14% of those with stage 2 or treated hypertension (Supplementary Table ST1).
Table 1.
Baseline study population characteristics by standing SBP < 110 mm Hg vs. standing SBP ≥ 110 mm Hg, mean (SD) or %
| Characteristics | Standing SBP < 110 mm Hg (N = 3,000) | Standing SBP ≥ 110 mm Hg (N = 9,467) |
Overall (N = 12,467) |
|---|---|---|---|
| Age, years | 53.4 (5.7) | 54.4 (5.7) | 54.1 (5.8) |
| Female, % | 69 | 51 | 55 |
| Race-study center, % | |||
| Washington County (White) | 27 | 24 | 25 |
| Jackson (Black) | 9 | 27 | 23 |
| Minneapolis (White) | 28 | 25 | 26 |
| Forsyth County (Black) | 2 | 4 | 3 |
| Forsyth County (White) | 34 | 20 | 23 |
| Body mass index, kg/m2 | 25.6 (4.5) | 28.3 (5.4) | 27.6 (5.3) |
| Seated SBP, mm Hg | 105.0 (11.3) | 126.2 (18.0) | 121.1 (18.9) |
| Seated DBP, mm Hg | 65.5 (8.1) | 75.9 (10.8) | 73.4 (11.2) |
| Supine SBP, mm Hg | 106.6 (9.3) | 130.9 (18.8) | 125.1 (19.9) |
| Supine DBP, mm Hg | 64.8 (6.9) | 74.8 (9.3) | 72.4 (9.8) |
| Hypertension stage, % | |||
| Normotension | 75 | 40 | 48 |
| Stage 1 hypertension | 3 | 16 | 13 |
| Stage 2 (or treated) hypertension | 22 | 44 | 39 |
| Resting heart rate, beats per minute | 65.2 (9.5) | 67.2 (10.4) | 66.7 (10.2) |
| Standing SBP < 110 mm Hg, % | 100 | 0 | 24 |
| Orthostatic hypotension, % | 6 | 4 | 4 |
| History of coronary heart disease, % | 5 | 5 | 5 |
| History of heart failure, % | 4 | 5 | 5 |
| History of stroke, % | 2 | 2 | 2 |
| Diabetes mellitus, % | 6 | 14 | 12 |
| eGFR, mL/min per 1.73 m2 | 102.7 (12.5) | 101.1 (13.4) | 101.5 (13.2) |
| HDL cholesterol, mg/dL | 54.3 (17.7) | 50.8 (17.0) | 51.6 (17.3) |
| Total cholesterol, mg/dL | 211.7 (40.1) | 215.2 (42.1) | 214.4 (41.6) |
| Baseline ASCVD risk ≥ 10% or prior CHD*, % | 13 | 32 | 27 |
| Leisure index, U | 2.4 (0.6) | 2.3 (0.6) | 2.4 (0.6) |
| Self-reported dizziness, % | 12 | 10 | 10 |
| Antihypertensive medication use**, % | 21 | 33 | 30 |
| Diuretic medication use, % | 12 | 19 | 17 |
| Cholesterol-lowering medication use, % | 2 | 2 | 2 |
| Antidepressant medication use, % | 3 | 3 | 3 |
| Sedative medication use, % | 2 | 1 | 2 |
| Hypnotic medication use, % | 2 | 2 | 2 |
| Antipsychotic medication use, % | 1 | 1 | 1 |
| Alcohol use, % | |||
| Never | 21 | 26 | 25 |
| Former | 18 | 19 | 19 |
| Current | 61 | 55 | 57 |
| Education attainment, % | |||
| Less than high school | 16 | 25 | 23 |
| High school degree or equivalent or vocational school | 43 | 41 | 41 |
| At least some college or professional school | 41 | 35 | 36 |
| Smoking status, % | |||
| Never | 40 | 42 | 41 |
| Former | 29 | 34 | 33 |
| Current | 31 | 25 | 26 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; SBP, systolic blood pressure.
*Sample size for ASCVD analysis is smaller (N = 12,463) due to missing low-density lipoprotein data.
**Antihypertensive medication use is within the last 2 weeks. Hypertension stages were defined as normotension SBP < 130 mm Hg/DBP < 80 mm Hg, Stage 1 hypertension SBP 130–139 mm Hg/DBP 80–89 mm Hg, and no antihypertension medication use, and stage 2 hypertension SBP ≥ 140 mm Hg/DBP ≥ 90 mm Hg, or antihypertensive medication use in the past 2 weeks (based on medication review).
Clinical outcomes: falls, syncope, CHD, and death
Participants were followed a median of about 24 years (median follow-up was 28 years for death) and there were a total of 3,526 (28%) incident falls, 3,161 (25%) incident syncopal events, 2,972 (24%) incident CHD events, and 6,713 (54%) deaths. After adjustment, standing hypotension (vs. SBP ≥ 110 mm Hg) was associated with a lower risk of CHD (HR 0.88; 95% CI: 0.80, 0.97) and death (HR 0.91; 95% CI: 0.86, 0.97), but was not significantly associated with falls or syncope (Table 2). Standing SBP (per 10 mm Hg) was associated with a higher risk of CHD events (HR 1.07; 95% CI: 1.05, 1.09) and mortality (HR 1.06; 95% CI: 1.04, 1.07), but not falls or syncope. Similar findings were seen with cumulative incidence plots (Supplementary Figure SF1). The associations between standing SBP and incident falls, syncope, CHD, and all-cause mortality were nonlinear, with a higher risk for CHD and all-cause mortality at greater standing SBP values, but not with falls or syncope (Figure 1).
Table 2.
Association of standing hypotension and standing systolic blood pressure with adverse events: The Atherosclerosis Risk in Communities (ARIC) Study (1987–2019)
| Outcomes | Standing SBP < 110 mm Hg (N = 3,000) vs. SBP ≥ 110 mm Hg (N = 9,467) |
Standing SBP per 10 mm Hg (N = 12,467) |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Fall | 1.02 (0.94, 1.11) | 0.66 | 1.00 (0.98, 1.02) | 0.83 |
| Syncope | 1.02 (0.93, 1.11) | 0.67 | 1.01 (0.99, 1.03) | 0.30 |
| CHD | 0.88 (0.80, 0.97) | 0.009 | 1.07 (1.05, 1.09) | <0.001 |
| Death | 0.91 (0.86, 0.97) | 0.006 | 1.06 (1.04, 1.07) | <0.001 |
Adjusted for age, sex, race-center, estimated glomerular filtration rate, body mass index, resting heart rate, high-density lipoprotein cholesterol, total cholesterol, prevalent CHD, prior stroke, prevalent heart failure, diabetes mellitus status, hypertension status, self-reported dizziness, alcohol consumption, education level, leisure index, smoking status, antihypertensive medication use in the last two weeks, and use of diuretics, antidepressants, sedatives, hypnotics, antipsychotics, and cholesterol-lowering medications. Participants were followed up through December 31, 2019, for a median of 24 years of follow-up. Abbreviations: CHD, coronary heart disease; SBP, systolic blood pressure.
Figure 1.
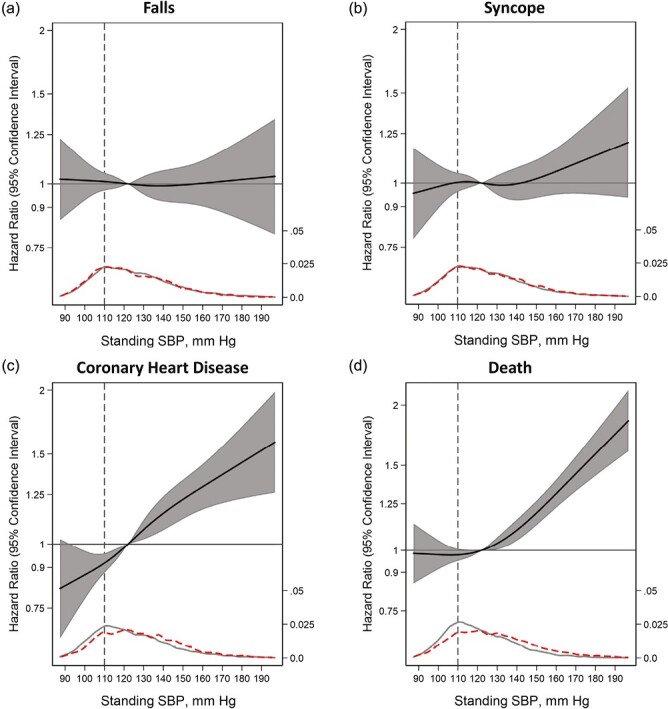
Adjusted association of standing SBP (mm Hg) modeled as a restricted cubic splines (solid line) with (a) fall (N = 2,366), (b) syncope (N = 2,634), (c) CHD (N = 2,767), and (d) death (N = 5,358). Hazards are presented relative to the 50th percentile of standing SBP with 4 knots via Harrell’s method (SBP < 110 mm Hg; vertical dotted line). All models used Cox proportional hazards models to determine hazard ratios shown on a natural log scale. Model included age, sex, race-center, estimated glomerular filtration rate, body mass index, resting heart rate, high-density lipoprotein cholesterol, total cholesterol, prevalent coronary heart disease, prior stroke, prevalent heart failure, diabetes mellitus status, hypertension status, self-reported dizziness, alcohol consumption, education level, leisure index, smoking status, antihypertensive medication use in the last 2 weeks, and use of diuretics, antidepressants, sedatives, hypnotics, antipsychotics, and cholesterol-lowering medications. The figure display was truncated at the 0.5th and 99.5th percentiles of standing SBP. Kernel density plots depict the distribution of standing SBP by participants who had the outcome of interest (dashed) vs. those who did not have the outcome of interest (gray solid). Vertical dash line represents SBP < 110 mm Hg. SBP indicates systolic blood pressure.
Clinical outcomes by subgroups: hypertension, 10-year ASCVD risk, age, and sex
Standing hypotension was associated with a higher risk of syncope (P-trend = 0.035) and CHD (P-trend = 0.0008) across hypertension strata (normotension, stage 1, stage 2, or treated), but there was no significant trend observed for falls or all-cause mortality (Table 3). Within each hypertension stage, however, standing hypotension was not significantly associated with risks of falls or syncope. Additionally, standing hypotension was not significantly associated with CHD or all-cause mortality in participants with stage 1 or stage 2/treated hypertension, but was associated with a lower risk of CHD (HR 0.83; 95% CI: 0.73, 0.95) and death (HR 0.90; 95% CI: 0.83, 0.98) in normotensive participants.
Table 3.
Association of standing hypotension (<110 mm Hg vs. ≥ 110 mm Hg) with adverse events stratified by hypertension category: The ARIC Study (1987–2019)
| Outcomes | Normotensive (N = 6,024) |
Stage 1 Hypertension (N = 1,600) |
Stage 2 (or treated) Hypertension (N = 4,843) |
P-trend |
|---|---|---|---|---|
| Fall | 1.00 (0.90, 1.11) | 1.22 (0.81, 1.83) | 1.03 (0.88, 1.21) | 0.42 |
| Syncope | 0.97 (0.87, 1.09) | 1.17 (0.76, 1.79) | 1.14 (0.97, 1.34) | 0.035 |
| CHD | 0.83 (0.73, 0.95) | 1.06 (0.65, 1.72) | 1.03 (0.88, 1.21) | 0.0008 |
| Death | 0.90 (0.83, 0.98) | 1.20 (0.89, 1.62) | 0.93 (0.83, 1.04) | 0.33 |
Hypertension stages were defined as normotension SBP < 130 mm Hg/DBP < 80 mm Hg, Stage 1 hypertension SBP 130–139 mm Hg/DBP 80–89 mm Hg, and no antihypertension medication use, and stage 2 hypertension SBP ≥ 140 mm Hg/DBP ≥ 90 mm Hg, or antihypertensive medication use in the past 2 weeks (based on medication review). Values are given as hazards ratio (95% confidence interval). Adjusted for age, sex, race-center, estimated glomerular filtration rate, body mass index, resting heart rate, high-density lipoprotein cholesterol, total cholesterol, prevalent CHD, prior stroke, prevalent heart failure, diabetes mellitus status, hypertension status, self-reported dizziness, alcohol consumption, education level, leisure index, smoking status, antihypertensive medication use in the last 2 weeks, and use of diuretics, antidepressants, sedatives, hypnotics, antipsychotics, and cholesterol-lowering medications. Participants were followed up through December 31, 2019, for a median of 24 years of follow-up. Abbreviations: CHD, coronary heart disease; SBP, systolic blood pressure.
The associations between standing SBP and outcomes when stratified by baseline hypertension status (stage 1 or stage 2/treated) were nonlinear, showing a higher risk for CHD and death at greater standing SBP values, but no association with falls or syncope (Supplementary Figure SF2).
Across baseline 10-year ASCVD risk strata (<10% vs. ≥10%), standing hypotension was differentially associated with higher risk of CHD events (P = 0.015) and all-cause mortality (P = 0.019), but not falls or syncope (Table 4). In stratified analyses, standing hypotension among participants with a baseline 10-year ASCVD risk < 10% was associated with lower rates of CHD (HR 0.84; 95% CI: 0.74, 0.95) and death (HR 0.89; 95% CI 0.82, 0.96), but was not significantly associated with fall or syncope. Standing hypotension among adults with higher baseline 10-year ASCVD risk or prior CHD history was not significantly associated with any outcomes.
Table 4.
Association of standing hypotension (<110 mm Hg vs. ≥ 110 mm Hg) with adverse events stratified by baseline 10-year atherosclerotic cardiovascular disease risk: The ARIC Study (1987–2019)
| Outcomes | ASCVD < 10% (N = 9,079) |
ASCVD ≥ 10% or prior CHD (N = 3,384) |
P-value |
|---|---|---|---|
| Fall | 1.01 (0.92, 1.11) | 1.05 (0.84, 1.31) | 0.79 |
| Syncope | 1.00 (0.90, 1.10) | 1.10 (0.88, 1.38) | 0.40 |
| CHD | 0.84 (0.74, 0.95) | 0.99 (0.84, 1.17) | 0.015 |
| Death | 0.89 (0.82, 0.96) | 1.03 (0.91, 1.17) | 0.019 |
Values are given as hazards ratio (95% confidence interval). Reduced sample size (N = 12,463) for ASCVD analysis due to missing low-density lipoprotein cholesterol data. Models are adjusted for age, sex, race-center, estimated glomerular filtration rate, body mass index, resting heart rate, high-density lipoprotein cholesterol, total cholesterol, prevalent CHD, prior stroke, prevalent heart failure, diabetes mellitus status, hypertension status, self-reported dizziness, alcohol consumption, education level, leisure index, smoking status, antihypertensive medication use in the last two weeks, and use of diuretics, antidepressants, sedatives, hypnotics, antipsychotics, and cholesterol-lowering medications. Participants were followed up through December 31, 2019, for a median of 24 years of follow-up. Abbreviations: ASCVD, baseline 10-year atherosclerotic cardiovascular disease risk; CHD, coronary heart disease; SBP, systolic blood pressure.
Across age strata (<50 years; 50–59 years; 60–66 years), standing hypotension was associated with a higher risk of syncope with increasing age category (P-trend = 0.043), but no trends across age categories were observed with other outcomes (Supplementary Table ST2). Within each age category, however, standing hypotension was not significantly associated with fall or syncope. Although the trend was not significant, in stratified analyses standing hypotension was associated with lower risk of CHD (HR 0.82; 95% CI: 0.71, 0.94) and death (HR 0.90; 95% CI: 0.83, 0.99) among participants aged 50–59 years, but not for other younger or older participants. Lastly, standing hypotension was associated with lower risk of death (HR 0.87; 95% CI: 0.78, 0.97) in patients aged ≥ 60 years, but not for younger patients.
Compared to men, standing hypotension among women was associated with a lower risk of CHD events (P = 0.009) and death (P = 0.015), but not falls or syncope (Supplementary Table ST3). Standing hypotension among male adults was not significantly associated with any outcomes.
Sensitivity analyses stratified by degree of change in standing SBP, (≤−20 or > −20 mm Hg), no hypertension or any hypertension (stage 1 or 2 combined), or by anytihypertensive medication use in the past 2 weeks did not significantly alter our findings (Supplementary Tables ST4–ST6).
DISCUSSION
In this middle-aged, community-dwelling adult population, we found that the prevalence of standing hypotension was common overall (24%) and among adults with stage 2 (or treated) hypertension (14%). Standing hypotension was not significantly associated with falls or syncope. Furthermore, standing hypotension was associated with a lower risk for CHD and death, and higher standing SBP was associated with increased risk of these outcomes. While there was a nominally greater risk of syncope among higher hypertension stages and older age groups with standing hypotension, standing hypotension was not significantly associated with adverse events overall or in any hypertension stage. These findings do not provide compelling support to screen for standing hypotension among adults with stage 1 or stage 2 hypertension.
Clinical trials of hypertension treatment have repeatedly demonstrated reduced risk for CHD events and mortality from more intensive BP treatment goals.2,15–19 However, a critique of trials with more intensive BP goals, like SPRINT, has been their exclusion of adults with standing hypotension, causing many to question SPRINT’s generalizability in ambulatory populations where standing BP is not routinely assessed.3,4 Indeed, hypotensive events and syncope were among the most common complications of intensive BP treatment in SPRINT, generating speculation as to whether these complications could be greater in general populations of adults with standing hypotension.2 In response to these concerns, the 2017 ACC/AHA BP management guidelines caution that initiation of pharmacologic hypertension treatment or uptitration of existing therapy may portend adverse events for patients with low standing BP. However, it was unclear how common standing hypotension was in the general population or among adults with hypertension. Our study demonstrates that standing hypotension is common among a quarter of the general population and about 14% of the adults with stage 2 (or treated) hypertension.
The prognostic significance of standing hypotension has not been a focus of prior research. However, prior studies have demonstrated strong associations between orthostatic hypotension (a form of standing hypotension) and CHD events.7,20 Many have postulated that this is secondary to cumulative micro-ischemia and end-organ injury due to transient hypoperfusion injuries.7,21–23 Our study is among the first to characterize adverse clinical outcomes associated with standing hypotension among community-dwelling, middle-aged adults. Contrary to our hypothesis, we found that standing hypotension was inversely associated with risk of CHD and death. Although there was a higher risk of CHD with standing hypotension across hypertension strata, ASCVD risk, and antihypertensive medication use, standing hypotension was not itself significantly associated with increased risk of CHD. Rather, the associations across hypertension strata suggested that standing hypotension was a healthy phenotype among middle-aged, normotensive participants with no association with CHD even among participants with stage 2 (or treated) hypertension. Similarly, standing hypotension was inversely associated with CHD among participants at low risk for ASCVD and women with no association observed among those at higher risk of ASCVD or men.
Our study found no association between standing hypotension or standing SBP overall with falls or syncope. In the literature, there are mixed associations between standing SBP with falls or syncope in the setting of orthostatic hypotension. While previous observational studies demonstrated that orthostatic hypotension is a risk factor for falls5,24,25 and syncope,6,26,27 in SPRINT there was no association between orthostatic hypotension with falls or syncope among adults with a standing SBP > 110 mm Hg.28 Although the SPRINT population excluded adults with standing hypotension, our study suggests that neither standing SBP nor standing hypotension are significantly associated with falls or syncope. Nevertheless, there was nominally greater risk of syncope across higher hypertension and age strata. As standing hypotension was not significantly associated with increased syncope within any category of hypertension or age strata, the clinical implications of these trends are unclear. We can speculate that the higher risk of syncope from standing hypotension among adults with hypertension is due to orthostatic hypotension as drops in BP upon standing are thought to contribute to cerebral hypoperfusion and may be exacerbated by poor cardiac and vascular compliance or autonomic dysfunction.29,30 It is also possible that the trend toward higher syncope among this group is secondary to the effects of hypertension treatment, particularly among adults where the standing hypotension was not identified prior to treatment intensification. However, despite a trend towards increased risk of syncope among adults who used antihypertensive medications at baseline evaluation, confirmation of these mechanisms is beyond the scope of the current study, particularly given our lack of standing BP measurements longitudinally or at the time of clinically-driven medication changes.
It is important to note that our study included a large number of adults with BPs in a range that would not have been included in hypertension trials like SPRINT. Because of the SPRINT requirement to have an elevated seated BP ≥ 130 mm Hg, participants with a standing SBP < 110 mm Hg would have had orthostatic hypotension. However, sensitivity analyses by change in SBP upon standing did not demonstrate a significant difference between those with a change in SBP upon standing of > −20 mm Hg (no orthostatic hypotension) vs. ≤ −20 mm Hg (orthostatic hypotension). In contrast to SPRINT, about a fourth of adults were found to have baseline hypertension and standing hypotension in our study. Moreover, stratifying by hypertension stage allowed us to examine standing hypotension more thoroughly and how associations with outcomes differed by baseline hypertension status.
Our study has some limitations. First, baseline assessments did not include fall history, thus we are unable to differentiate participants with falls prior to baseline vs. participants without any fall history. About 1% of middle-aged adults are estimated to have a fall each year.31 Second, although ICD injury codes are reportedly valid,32 falls and syncope are likely under-ascertained since only those reported to health care providers are recorded, missing the falls or syncopal events that do not result in serious injury. Further elaboration on the limitations from CMS claims data are discussed in the Supplementary Methods. Third, while we account for hypertension treatment at the time of standing BP measurement, we did not have standing BP measured over time or coupled with changes in BP management. Thus, while one might assume that participants with stage 1 or stage 2 hypertension were more likely to undergo hypertension treatment, our findings should not be used to infer causality related to BP treatment, but are more informative with respect to risks related to the identification of standing hypotension. Fourth, our study population focused on middle-aged adults with and without hypertension. These findings should be replicated in a population of older adults with a greater prevalence of stage 1 and stage 2 hypertension. Fifth, participants were not asked about hydration status, which may have implications for standing blood pressure measurements. Finally, residual confounding is always a concern with observational studies.
Our study also has several strengths. Our study population included a large sample of middle-aged, community-dwelling Black or White adults, who experienced a substantial number of events during follow-up. Blood pressure and other covariates were measured using a standardized protocol to enhance precision and accuracy. Hospitalization records were reviewed carefully by trained ARIC staff to adjudicate CHD outcomes using a rigorous protocol. Finally, falls and syncope were ascertained by ICD-9 and 10 codes, increasing the likelihood that these events were clinically relevant.
Our findings have clinical implications. Somewhat unexpectedly, we found that standing hypotension was present in about one of seven middle-aged adults with stage 2 hypertension. Thus, if patients are screened for low standing SBP, a sizeable number of patients may be considered inappropriate for hypertension treatment based on the standing hypotension threshold used in SPRINT. Alternatively, clinicians could initiate antihypertensive therapy after reviewing additional BP assessments (ambulatory or in clinic) or use lower doses of medications. However, our study also showed that standing hypotension was not significantly associated with non-cardiovascular or cardiovascular events and that only standing hypertension was associated with a higher risk of all-cause mortality. These findings suggest that standing BP assessments may not be an informative screening modality for determining middle-aged adults at risk for falls or syncope even among those with hypertension. While further work is needed, particularly research examining the impacts of BP treatment intensification on syncope among adults with standing hypotension, our study does not support current guideline recommendations that standing hypotension be viewed as a reason to avoid BP intensification.
In conclusion, in this middle-aged population with and without hypertension, low standing SBP < 110 mm Hg (i.e., standing hypotension) was not significantly associated with increased risk of falls, syncope, CHD events, or death. There was no clinically meaningful difference when stratified by hypertension stage, baseline 10-year ASCVD risk, age, or sex. These findings do not support screening for standing SBP < 110 mm Hg. Additional studies on treatment initiation are needed to determine if antihypertensive therapy intensification impacts syncope risk among hypertensive adults with standing hypotension.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions. For a full list of contributors to ARIC, please visit sites.cscc.unc.edu/aric/. An abstract of this work was presented at the AHA Hypertension Conference in San Diego, CA in September 2022.
Contributor Information
Jordan K Kondo, Harvard Medical School, Boston, Massachusetts, USA.
William B Earle, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Ruth-Alma N Turkson-Ocran, Harvard Medical School, Boston, Massachusetts, USA; Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Long H Ngo, Harvard Medical School, Boston, Massachusetts, USA; Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Jennifer L Cluett, Harvard Medical School, Boston, Massachusetts, USA; Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Lewis A Lipsitz, Harvard Medical School, Boston, Massachusetts, USA; Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Hinda and Arthur Marcus Institute for Aging Research, Boston, Massachusetts, USA.
Natalie R Daya, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Elizabeth Selvin, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Pamela L Lutsey, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota, USA.
Josef Coresh, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Beverly Gwen Windham, Department of Medicine, Division of Geriatric Medicine, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Karla N Kendrick, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Department of Medicine, Winchester Hospital, Beth Israel Lahey Health, Woburn, MA, USA.
Stephen P Juraschek, Harvard Medical School, Boston, Massachusetts, USA; Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
FUNDING
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). Kondo is supported by an Amazon Web Services (AWS) Cloud Credit for Research grant. Juraschek is supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant R01 HL153191. Selvin is supported by NIH/NHLBI grant K24 HL152440. Lutsey is supported by NIH/NHLBI grant K24 HL159246.
CONFLICT OF INTEREST
None.
DATA AVAILABILITY STATEMENT
The data underlying this article may be obtained with an approved ARIC proposal. Please visit sites.cscc.unc.edu/aric/.
REFERENCES
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet Lond Engl 2002; 360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2. Wright JTJ, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DCJ, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright JT, Whelton PK, Johnson KC, Snyder JK, Reboussin DM, Cushman WC, Williamson JD, Pajewski NM, Cheung AK, Lewis CE, Oparil S, Rocco MV, Beddhu S, Fine LJ, Cutler JA, Ambrosius WT, Rahman M, Still CH, Chen Z, Tatsuoka C; SPRINT Research Group. SPRINT revisited: updated results and implications. Hypertension 2021; 78:1701–1710. doi: 10.1161/HYPERTENSIONAHA.121.17682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 5. Juraschek SP, Daya N, Appel LJ, Miller ER 3rd, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens 2017; 30:188–195. doi: 10.1093/ajh/hpw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER 3rd, Windham BG, Griswold ME, Heiss G, Selvin E. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med 2017; 177:1316–1323. doi: 10.1001/jamainternmed.2017.2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Juraschek SP, Daya N, Appel LJ, Miller ER 3rd, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle-aged adults. J Am Heart Assoc 2018; 7:e008884. doi: 10.1161/JAHA.118.008884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atherosclerosis Risk in Communities Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702. [PubMed] [Google Scholar]
- 9. Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, Shahar E, Kalsbeek W. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. the Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol 1996; 49:1441–1446. doi: 10.1016/0895-4356(95)00047-x [DOI] [PubMed] [Google Scholar]
- 10. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol 1996; 49:223–233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 11. The National Heart, Lung, and Blood Institute. ARIC Manual 11: Sitting Blood Pressure and Postural Changes in Blood Pressure and Heart Rate. https://sites.cscc.unc.edu/aric/Cohort_Manuals/Sitting_Blood_Pressure_and_Postural_Changes_in_Blood_Pressure_and_Heart_Rate_11.PDF. Accessed 28 October 2022.
- 12. Kucharska-Newton AM, Heiss G, Ni H, Stearns SC, Puccinelli-Ortega N, Wruck LM, Chambless L. Identification of heart failure events in medicare claims: the Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail 2016; 22:48–55. doi: 10.1016/j.cardfail.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrell FE, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988; 80:1198–1202. doi: 10.1093/jnci/80.15.1198 [DOI] [PubMed] [Google Scholar]
- 14. Muntner P, Colantonio LD, Cushman M, Goff DCJ, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA 2014; 311:1406–1415. doi: 10.1001/jama.2014.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT, Pajewski NM; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ, HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 17. SHEP Cooperative Research Group. Prevention of Stroke by Antihypertensive Drug Treatment in Older Persons With Isolated Systolic Hypertension: Final Results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264. doi: 10.1001/jama.1991.03460240051027 [DOI] [PubMed] [Google Scholar]
- 18. Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet Lond Engl 1997; 350:757–764. doi: 10.1016/s0140-6736(97)05381-6 [DOI] [PubMed] [Google Scholar]
- 19. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet Lond Engl 2016; 387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 20. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. the Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 1992; 19:508–519. doi: 10.1161/01.hyp.19.6.508 [DOI] [PubMed] [Google Scholar]
- 21. Fan X-H, Wang Y, Sun K, Zhang W, Wang H, Wu H, Zhang H, Zhou X, Hui R. Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. Am J Hypertens 2010; 23:829–837. doi: 10.1038/ajh.2010.76 [DOI] [PubMed] [Google Scholar]
- 22. Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T, Shimada K. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol 2002; 40:133–141. doi: 10.1016/s0735-1097(02)01923-x [DOI] [PubMed] [Google Scholar]
- 23. Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 2007; 50:325–332. doi: 10.1161/HYPERTENSIONAHA.107.090084 [DOI] [PubMed] [Google Scholar]
- 24. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med 2000; 108:106–111. doi: 10.1016/s0002-9343(99)00425-8 [DOI] [PubMed] [Google Scholar]
- 25. Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol 1996; 143:1129–1136. doi: 10.1093/oxfordjournals.aje.a008690 [DOI] [PubMed] [Google Scholar]
- 26. Tan MP, Newton JL, Chadwick TJ, Parry SW. The relationship between carotid sinus hypersensitivity, orthostatic hypotension, and vasovagal syncope: a case-control study. Europace 2008; 10:1400–1405. doi: 10.1093/europace/eun278 [DOI] [PubMed] [Google Scholar]
- 27. O’Mahony D, Foote C. Prospective evaluation of unexplained syncope, dizziness, and falls among community-dwelling elderly adults. J Gerontol A Biol Sci Med Sci 1998; 53:M435–M440. doi: 10.1093/gerona/53a.6.m435 [DOI] [PubMed] [Google Scholar]
- 28. Juraschek SP, Taylor AA, Wright JTJ, Evans GW, Miller ER 3rd, Plante TB, Cushman WC, Gure TR, Haley WE, Moinuddin I, Nord J, Oparil S, Pedley C, Roumie CL, Whittle J, Wiggers A, Finucane C, Anne Kenny R, Appel LJ, Townsend RR; SPRINT Research Group. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension 2020; 75:660–667. doi: 10.1161/HYPERTENSIONAHA.119.14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hale GM, Valdes J, Brenner M. The treatment of primary orthostatic hypotension. Ann Pharmacother 2017; 51:417–428. doi: 10.1177/1060028016689264 [DOI] [PubMed] [Google Scholar]
- 30. Carthy ER. Autonomic dysfunction in essential hypertension: a systematic review. Ann Med Surg 2013; 3:2–7. doi: 10.1016/j.amsu.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verma SK, Willetts JL, Corns HL, Marucci-Wellman HR, Lombardi DA, Courtney TK. Falls and fall-related injuries among community-dwelling adults in the United States. PLoS One 2016; 11:e0150939. doi: 10.1371/journal.pone.0150939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKenzie K, Enraght-Moony EL, Walker SM, McClure RJ, Harrison JE. Accuracy of external cause-of-injury coding in hospital records. Inj Prev 2009; 15:60–64. doi: 10.1136/ip.2008.019935 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article may be obtained with an approved ARIC proposal. Please visit sites.cscc.unc.edu/aric/.


