Abstract
We investigated the presence of human T-lymphotropic virus type 2 (HTLV-2) DNA in the peripheral blood mononuclear cell subsets obtained from 18 patients coinfected with human immunodeficiency virus type 1 and HTLV-2, 6 of whom also had predominantly sensory polyneuropathy (PSP). HTLV-2 DNA and RNA were found in CD8- and CD19-positive cells, and, for patients with PSP, in CD14-positive cells as well. Furthermore, the patients with PSP had higher proviral loads than those without PSP.
Previous studies have shown that human T-lymphotropic virus type 2 (HTLV-2) preferentially infects T lymphocytes expressing CD8+ molecules on their surfaces (12). Nevertheless, a recent paper reports the finding of HTLV-2 DNA in the B cells of patients with high proviral loads (4), thus suggesting the possibility that, under some conditions, it can infect cell targets other than T lymphocytes. HTLV-1 and human immunodeficiency virus type 1 (HIV-1) have been shown to interact in vitro by inducing a chimeric formation and a change in their cell tropism (18). Assuming that HTLV-2 behaves in a similar manner, it is possible to hypothesize that patients with HIV-1 and HTLV-2 coinfection might show a modification in the spectrum of cell targets regardless of viral burden. Furthermore, HIV-1 coinfection and the consequent impairment of host immune competence might allow HTLV-2 to act as an opportunistic causative agent of some disorders.
The pathogenetic role of HTLV-2 has not yet been elucidated, but the virus has been associated with such neurological diseases as tropical spastic paraparesis/HTLV-associated myelopathy (TSP/HAM) like syndrome (2, 3, 20). Moreover, HTLV-2 has also been found to be associated with peripheral neuropathies in intravenous drug users (9), and we recently reported a high prevalence of the infection in the predominantly sensory polyneuropathy (PSP) that frequently affects HIV-1-positive patients (26). This association might be the consequence of the opportunistic behavior of HTLV-2.
To investigate the presence of broader cell tropism in PSP, we studied the quantitative distribution of HTLV-2 DNA in peripheral blood mononuclear cell (PBMC) subpopulations obtained by means of cell sorting from patients dually infected with HIV-1 and HTLV-2.
Patients.
Eighteen patients coinfected with HIV-1 and HTLV-2 (14 males, 4 females; median age, 34.5 years; range, 23 to 45 years) were included in the study; 16 were drug addicts and 2 were homosexual men. All the patients had symptomatic HIV-1 infections, and eight had overt AIDS according to the 1987 Centers for Disease Control (CDC) classification (5). The mean CD4+ cell count at enrollment was 207 cells/μl (standard deviation [SD], ±131.9). Six of the patients were also affected by peripheral polyneuropathy diagnosed as PSP according to the clinical, physiological, and laboratory criteria of the American Academy of Neurology AIDS Task Force (23, 26).
Serology.
Antibodies to HTLV-2 were determined by means of a commercial enzyme-linked immunosorbent assay (ELISA) (Murex Diagnostic, Dartford, England) and Western blot analysis (Genelabs Diagnostics, Singapore) according to the criteria of the World Health Organization (24). Antibody titers were expressed as the reciprocal of the highest dilution reactive to ELISA.
Cell sorting.
The PBMC subpopulations were obtained by means of cell sorting using an EPICS Elite flow cytometer (Coulter Electronics, Hialeah, Fla.). One hundred microliters of whole blood collected in EDTA Vacutainer tubes were double stained with different monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE). In order to separate T cells (CD3+CD4+, CD3+CD8+), B cells (CD19+), and monocytes (CD14+), the following pairs of antibodies were used: CD3-FITC/CD19-PE, CD14-FITC/CD19-PE, and CD4-FITC/CD8-PE. The blood samples were incubated at 4°C for 30 min, then lysed and fixed by a commercial method (Immunoprep kit reagent and Q-Prep work station; Coulter Inc.). They were subsequently analyzed by means of flow cytometry, and the cells were sorted on the basis of the different fluorescence emissions. The purity of the sorted cells was tested by flow cytometry.
HTLV-2 nucleic acid detection.
HTLV-2 DNA and RNA were detected in the PBMCs and cell subsets of the patients by means of a nested-PCR technique for the amplification of tax-related sequences. Briefly, for HTLV-2 DNA detection, all the sorted cells and 2 × 106 PBMCs obtained by means of Ficoll centrifugation from 10-ml samples of EDTA-treated blood were resuspended at 103 to 104 cells/μl (depending on the starting number of cells) in a lysis buffer containing 50 mM KCl, 10 mM Tris (pH 8.3), 2.5 mM MgCl2, 0.5% Tween 20, and 200 μg of proteinase K/ml. After overnight digestion at 42°C and the inactivation of proteinase K at 95°C for 10 min, 10 μl of the lysate was amplified by using two pairs of primers recognizing a sequence included in the tax gene of the HTLV-2 provirus (sense outer primer, positions [pos.] 7219 to 7238; antisense outer primer, pos. 7483 to 7464; sense inner primer, pos. 7248 to 7267; antisense inner primer, pos. 7406 to 7386), as described elsewhere (25).
In order to verify the absence of contamination, a number of negative controls (DNA extracted from the PBMCs of known HTLV-free blood donors) and blank tubes (not containing DNA) were included in each PCR run; if a contaminated reagent was present, all the results obtained in that run were rejected.
Total RNA was extracted by the classic method of Chomczynski and Sacchi (6) from the whole number of available sorted cells and from 105 PBMCs; RNA underwent reverse transcription by using the antisense outer primer; nested PCR was then performed as previously described.
The specificity of the amplified sequences was evaluated by means of microplate hybridization with a probe recognizing HTLV-2 tax (pos. 7337 to 7376) (25).
The HTLV-2 provirus was molecularly characterized by means of the restriction endonuclease analysis of pol amplified sequences (sense primer, pos. 4735 to 4756; antisense primer, pos. 4920 to 4897) using HinfI (which recognizes a unique site in HTLV-2a) and MseI (which recognizes a unique site in HTLV-2b).
Semiquantitative PCR.
The amount of proviral HTLV-2 DNA and RNA was evaluated by means of limiting-dilution (LD-PCR). Tenfold dilutions of 10 μl of the crude lysate or 5 μl of cDNA were amplified by means of nested PCR for tax amplification as described previously. The dilutions around the end point were retested in a five-replicate amplification. For an analytical system giving the sensitivity of a single copy (such as nested PCR) and a solution containing only a few copies of a molecule, it is possible to use Poisson’s distribution to calculate the average copy number on the basis of the frequency of the negative results obtained during the replicate analysis (21). Application of the equation C = −ln(N0/NT), in which N0 is the number of negative results and NT is the number of replicate experiments involving a single dilution, makes it possible to calculate the exact number of PCR units (PU, the smallest unit yielding a PCR-positive signal) contained in the end point dilution (“C” in the equation) and then to extrapolate the number of PU present in the starting biological sample.
The titer of tax sequences was related to the number of cells counted by means of flow cytometry.
In order to verify the amount of DNA in each sample and the presence of any PCR-inhibitory substances, we titrated a common sequence enclosed in the beta-globin gene using primers PC04 and GH20 (respectively, sense, pos. −73 to −54 and antisense, pos. 195 to 176). Briefly, 10-fold dilutions of 10 μl of each cell lysate were submitted to 40 cycles of amplification at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min.
The efficiency of the RNA extraction was controlled by amplifying a ubiquitous mRNA coding for the beta-actin (sense primer, pos. 1196 to 1227; antisense primer, pos. 1415 to 1384) that is constantly expressed in human cells.
Evaluation of nested-PCR sensitivity and LD-PCR efficiency.
To evaluate the sensitivity of the nested-PCR protocol, we amplified different amounts of a plasmid containing the proviral genome of HTLV-2 (pMo4, kindly provided by G. Franchini of the National Cancer Institute, Bethesda, Md.) dispersed in 1 μg of HTLV-negative human DNA.
In order to verify the efficiency of the semiquantitative method, solutions containing known amounts of pMo4 dispersed in 1 μg of human DNA were titrated by means of LD-PCR, and the results were correlated with the number of plasmid copies.
Statistical analysis.
Statistical analysis was performed with CDC (Atlanta) Epi Info, version 6, and SPSS (SPSS Inc.) software. Differences were calculated by means of analysis of variance or chi-square tests, unless otherwise indicated.
Characteristics of the studied population.
All the subjects included in the study were seropositive for anti-HTLV-1 and -2 by ELISA. Seventeen of them showed reactivity against both gag (p19, p24) and env proteins (recombinant gp21 and K55) in Western blot analysis (Table 1). No difference was found between the anti-HTLV-2 antibody titers of PSP patients and those of the patients without PSP (mean values, 425 ± 775 versus 433 ± 739; P = 0.9 [Table 1]).
TABLE 1.
Characteristics of the study populationa
| Patient no. | Sex | Neuropathy | AIDS | Anti-HTLV-2 titersb | WB reactivity patternc |
|---|---|---|---|---|---|
| 1 | M | Y | Y | 200 | K55, p24, gp21 |
| 2 | M | Y | Y | 200 | K55, p24, gp21 |
| 3 | M | Y | Y | 2,000 | K55, p24, p19, gp21 |
| 4 | M | Y | Y | 50 | p19, gp21 |
| 5 | M | Y | Y | 50 | gp21 |
| 6 | F | Y | Y | 50 | p24, gp21 |
| 7 | M | N | N | 200 | K55, p24, gp21, p19 |
| 8 | M | N | Y | 400 | K55, p24, gp21 |
| 9 | M | N | Y | 2,000 | K55, p24, p19, gp21 |
| 10 | M | N | N | 100 | K55, p24, gp21 |
| 11 | M | N | N | 2,000 | K55, p24, p19, gp21 |
| 12 | M | N | N | 200 | K55, p24, p19, gp21 |
| 13 | M | N | N | 50 | p24, p19, gp21 |
| 14 | M | N | N | 50 | K55, p24, gp21 |
| 15 | M | N | N | 50 | K55, p24, gp21 |
| 16 | F | N | N | 50 | p24, gp21 |
| 17 | F | N | N | 50 | K55, p24, p19, gp21 |
| 18 | F | N | N | 50 | K55, p24, gp21 |
M, male; F, female; Y, yes; N, no; WB, Western blot.
Reciprocal of highest dilution reactive in ELISA.
K55, recombinant gp-46-II.
The use of PCR led to the detection of HTLV-2 tax and pol specific sequences in the PBMCs of all of the patients included in the study. The HTLV-2b subtype was identified in 13 patients; 5 individuals remained untyped because of the small number of pol amplified sequences.
The subjects with PSP had a mean CD4+ cell count that was significantly lower than that for the subjects without PSP (113.3 ± 108 versus 254.3 ± 119.9 cells/μl; P = 0.03).
Sensitivity of nested PCR and LD-PCR efficiency.
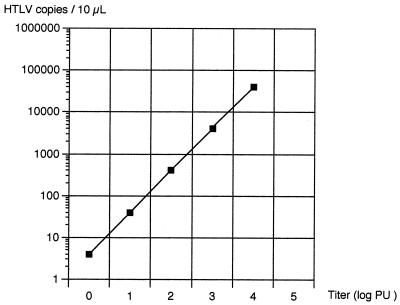
By means of nested PCR and solid-phase hybridization, we observed a signal from about 1 molecule of plasmid pMo4. The same result was obtained in three different experiments.
There was a correlation coefficient of 1 on a 5-log range between the titer and the number of amplified copies of plasmid pMo4 (Fig. 1).
FIG. 1.
Correlation between copies of HTLV-2 tax present in 10 μl of solution and LD-PCR titer.
HTLV-2 DNA titers in total PBMCs.
The semiquantitative results were interpretable in 13 patients because the small amount of provirus allowed only an approximate evaluation of the HTLV-2 DNA load in 5 cases (patients 14 to 18). The frequency of negative PCR results on these undiluted samples made it possible to estimate an HTLV-2 DNA concentration of less than 1 PU per 100,000 cells (<0.01 PU/103 cells). The median proviral titer in all 18 HIV-1-positive patients was 0.55 PU/103 PBMCs (range, 0.01 to 50 PU/103 cells) (Table 2).
TABLE 2.
HTLV-2 proviral titers in the different cell subsetsa
| Patient no. | PSP | Proviral titer (PU/103 cells) in:
|
||||
|---|---|---|---|---|---|---|
| Total PBMCs | CD3+ cells | CD8+ cells | CD19+ cells | CD14+ cells | ||
| 1 | Y | 20 | 20 | 1.5 | 2.2 | 0.2 |
| 2 | Y | 0.1 | 2 | 0.5 | 0 | 0 |
| 3 | Y | 50 | 100 | 25 | ND | 20 |
| 4 | Y | 10 | 10 | 100 | ND | 200 |
| 5 | Y | 1 | 0.4 | 0.3 | 0 | 0.5 |
| 6 | Y | 10 | ND | 250 | 645 | 408 |
| 7 | N | 20 | 20 | 10 | 200 | 0 |
| 8 | N | 0.1 | 2 | 2 | 0.5 | 0 |
| 9 | N | 0.1 | ND | 2 | 0.4 | 0 |
| 10 | N | 1 | ND | 25 | 1.7 | 0 |
| 11 | N | 1 | ND | 200 | 0.4 | 0 |
| 12 | N | 0.1 | ND | 2.8 | 0.6 | 0 |
| 13 | N | 1 | ND | 1.5 | 0 | 0 |
| 14 | N | 0.01 | 0 | 0 | 0 | 0 |
| 15 | N | 0.01 | 0 | 0 | 0 | 0 |
| 16 | N | 0.01 | 0 | 0 | 0 | 0 |
| 17 | N | 0.01 | 0 | 0 | 0 | 0 |
| 18 | N | 0.01 | 0 | 0 | 0 | 0 |
| Median | 0.55 | 1.2 | 1.75 | 0.2 | 0 | |
Y, yes; N, no; ND, not done. CD4+ cells were PCR negative in all the patients.
In three samples (patients 4, 8, and 13), we diluted the PBMCs before extracting DNA. The results were similar to those observed in the samples diluted after DNA extraction (correlation coefficient, 0.9; P = 0.01).
None of the patients with proviral loads of less than 0.01 PU/103 cells were affected by AIDS, and their mean CD4+ cell count was higher than that observed in the others (300 ± 125.1 versus 171.7 ± 120.4 cells/μl; P = 0.04 by the Kruskal-Wallis test). Furthermore, the patients with proviral loads of <0.01 PU/103 cells had a mean serum antibody titer significantly lower than that of the patients with higher proviral loads (mean, 50.0 ± 0 versus 576 ± 817; P = 0.01).
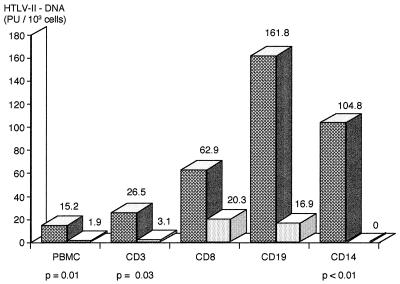
As shown in Fig. 2, the mean HTLV-2 DNA titer was higher in the patients with PSP than in those without PSP. In particular, assuming a value of 0.01 PU/103 cells for the five subjects with lower proviral loads, we calculated mean HTLV-2 DNA titers of 15.2 (SD, ±18.5; median, 10) PU/103 PBMCs in the patients with PSP and 1.9 (SD, ±5.7; median, 0.1) PU/103 PBMCs in those without PSP (P = 0.01 by the Kruskal-Wallis test).
FIG. 2.
Mean HTLV-2 DNA titers in different PBMC subsets in patients with PSP (dark bars) or without PSP (light bars).
HTLV-2 DNA in cell subsets.
By means of cell sorting, we obtained CD4+, CD8+, and CD14+ cells from all the patients; CD19+ cells were obtained from 16 patients, and CD3+ cells were obtained from 12. The median recoveries were 48,714 CD3+, 10,840 CD4+, 36,630 CD8+, 7,555 CD19+, and 31,820 CD14+ cells. The percent purity of each cell subset was always more than 98%.
The tax titer was normalized on the basis of the number of cells tested. Nevertheless, in order to evaluate the correspondence between the number of cells present in the lysate and the amount of amplified DNA, a volume of each cell lysate equal to that used for tax PCR was also titrated for the beta-globin gene. We observed a correlation coefficient of 0.8 (P < 0.01 by Spearman’s test).
None of the five patients with proviral loads of <0.01 PU/103 cells showed tax sequences by nested PCR in any of the tested PBMC subpopulations (Table 2).
CD3+ cells were PCR positive in 7 of the 12 available cases (58.3%). The median titer was 1.2 PU/103 cells (range, 0 to 100 PU/103 cells). CD8+ cells were positive in 13 of 18 cases (72.2%), with a median proviral titer of 1.75 PU/103 cells (range, 0 to 250 PU/103 cells), a higher value than that observed in the PBMCs of the same patients (Table 2). In contrast, CD4+ cells were negative in all 18 cases.
In 8 of 16 cases (50%), HTLV-2 DNA could be detected in CD19+ cells, with a median titer of 0.2 PU/103 cells (range, 0 to 645 PU/103 cells). Finally, HTLV-2 DNA was found in the CD14+ cells of 5 of the 18 patients analyzed (27.8%). The median titer was 0 PU/103 cells (range, 0 to 408 PU/103 cells).
In order to investigate the possibility that the PCR signals observed in CD14+ and CD19+ cells were due to contamination with CD8+ cells, we calculated the probable number of contaminating CD8+ cells in CD14+ and CD19+ subsets on the basis of the percentage of purification. As shown in Table 3, in all but one (patient 11) of the CD19+ cell samples and in all the CD14+ cell samples, the number of possible contaminant cells was lower than that needed to produce such a positive signal.
TABLE 3.
Possible CD8 contamination of PCR-positive CD14 or CD19 subsets
| Cell subset and patient no. | No. of cells needed for a PCR signal | % Purity | No. of possible contaminating cells | No. of CD8 cells needed for a PCR signal |
|---|---|---|---|---|
| CD14 | ||||
| 1 | 4,000 | 99.4 | 24 | 750 |
| 3 | 50 | 99.8 | 0.1 | 45 |
| 4 | 5 | 99 | 0.05 | 10 |
| 5 | 2,000 | 99.1 | 18 | 3,000 |
| 6 | 2.4 | 98.5 | 0.04 | 4 |
| CD19 | ||||
| 1 | 454 | 98.5 | 6.8 | 750 |
| 6 | 1.5 | 98.3 | 0.02 | 4 |
| 7 | 5 | 98.4 | 0.08 | 100 |
| 8 | 2,000 | 99 | 20 | 500 |
| 9 | 2,500 | 98.5 | 37.5 | 500 |
| 10 | 588 | 99 | 5.9 | 5,000 |
| 11 | 2,500 | 98.3 | 42.5 | 5 |
| 12 | 1,666 | 98.4 | 26.7 | 357 |
All of the five subjects with proviral sequences in monocytes were affected by PSP. As shown in Fig. 2, the patients with PSP had higher mean proviral loads in all the analyzed cell subpopulations, and this difference was significant for CD14+ cells, CD3+ cells, and total PBMCs. There were no other correlations between the proviral loads in the different cell subpopulations and the patients’ clinical characteristics or laboratory parameters, such as CD4+ cell counts or anti-HTLV-2 antibody titers.
HTLV-2 RNA in PBMCs and cell subsets.
HTLV-2 tax RNA was revealed in the PBMCs of 7 of the 12 patients without PSP (58.3%) and in those of 4 of the 6 patients with PSP (66.7%). All but one of the patients with proviral loads of <0.01 PU/103 were negative for HTLV RNA.
The median titer of HTLV-2 RNA in PBMCs was lower than that of HTLV-2 DNA (median titer, 0.01 PU/103 cells; range, 0 to 1 PU/103 cells). No significant correlation between HTLV-2 DNA and RNA titers was observed. No difference was found between the RNA titers of the subjects with and without PSP.
Reverse transcription-PCR could also be performed on the CD14+ and CD19+ cells of two patients each (patients 1 and 3 and patients 8 and 11, respectively) and on the CD3+ cells of six patients (patients 1, 2, 3, 7, 8, and 11). All the CD14+ cell samples, one CD19+ cell sample, and three of the six CD3+ cell samples showed HTLV-2 tax RNA sequences.
The titers of tax RNA in the CD14+ cells of patient 1 (3.7 PU/103 cells) and the CD19+ cells of patient 8 (5.9 PU/103 cells) were, respectively, 20 and 10 times higher than the proviral DNA titers.
The association of HTLV-2 with any human disease is still debated. Despite the evidence supporting the pathogenetic role of HTLV-2 in neurological diseases (1–3, 9, 20, 26), the mechanism involved has not yet been clarified. Some clues may be drawn from studies of HTLV-1, in which nervous-tissue damage has been attributed to the direct killing of infected glial cells by cytotoxic T lymphocytes or to the secretion of neurotoxic cytokines (such as tumor necrosis factor). Other authors have excluded the presence of the virus in glial cells (10) and suggested a possible breakdown in tolerance with an autoimmune reaction. Both of these hypotheses raise a crucial question concerning the cell tropism of the virus. In the case of HTLV-1, there are conflicting data as to whether the virus can infect cells other than T lymphocytes (8, 11, 14). Less is known about the ability of HTLV-2 to infect cell targets other than CD8+ T lymphocytes (12, 15) in vivo. Moreover, no data exist concerning the possible influence of HIV-1 coinfection on the cell tropism of HTLV-2.
In our study, HTLV-2 DNA was detected mainly in CD8+ and CD3+ cells, in which its concentrations were similar to that found in the total PBMCs of each patient. As CD8+ cells represent a high proportion of the circulating mononuclear cells in the majority of our HIV-1 patients, this result is in agreement with the observation that CD3+ and CD8+ cells are the preferential targets of HTLV-2 infection in patients coinfected with HIV-1, regardless of the presence of PSP. In contrast, the CD4+ T lymphocytes were always negative. This result appears to be discordant with a previous report of a low proviral load (1 proviral copy per 104 to 105 cells) in this cell type (15). The depletion of CD4+ cells in our HIV-1-positive patient population might be one of the reasons for this discordance.
We obtained comparable concentrations of HTLV-2 DNA whether the PBMCs were diluted before or after extraction. This finding suggests a provirus copy/infected cell ratio near 1:1. These data are different from those previously reported for HTLV-1 (17); more-accurate studies are needed to discover whether this difference is due to the different viral tropism of the two HTLVs or to the presence of HIV-1 coinfection in our study population.
Five subjects had the provirus in peripheral monocytes, and eight had it in B lymphocytes. This phenomenon seemed to be restricted to the subjects with the highest HTLV-2 proviral loads, who are frequently those with the lowest CD4+ cell counts. It is therefore possible to suggest that HIV-1 infection is the cause of a spread of HTLV-2 to different cell subpopulations. The patients with PSP had the highest proviral loads and always had a broader range of infected PBMC subsets.
Moreover, HTLV-2 DNA was present in the CD14+ cells of five of the six subjects affected by PSP but was absent in CD14+ cells obtained from the other patients. For two PSP patients, the proviral titer in these cells was at least 10 times higher than that in the PBMCs, suggesting a preferential concentration of HTLV-2 DNA in this subset. HTLV-2 RNA was detected in the CD14+ cells of the two PSP patients analyzed, and in one case, its level exceeded that of the provirus. This observation suggests active provirus expression in monocytes.
The presence of HTLV-2 in CD14+ cells prompts some further considerations. The way by which HTLV may enter the nervous system has not yet been clarified. The possible role of the monocyte/macrophage lineage as a “Trojan horse” carrying the virus into the nervous system has been proposed, and data relating to the ability of HTLV-1 to infect macrophages (8, 11) seem to support this hypothesis. Our findings suggest a common strategy of HTLVs to invade and/or damage the nervous system through monocytes/macrophages.
Information concerning the detectability of HTLV-2 in nervous tissues and cerebrospinal fluid is scanty and does not include the identification of the type of cell involved (16). In our experience, we have detected proviral DNA in tissue homogenates of PSP patients (26), but histological evaluation of the analyzed peripheral nervous tissues did not show any mononuclear cell infiltration. Further studies are therefore needed to verify the hypothesis that infected macrophages play a role in causing PSP.
As mentioned above, the patients with PSP had higher proviral loads than those without PSP. In the natural history of HTLV-1 infection, the proviral loads in subjects with TSP/HAM are commonly higher than in those without TSP/HAM. In particular, the presence of infected PBMCs in proportions as high as 1/5 vis-à-vis total PBMCs has been reported in TSP/HAM patients, versus means of 1/25 to 1/100 in asymptomatics (22). Nevertheless, the HTLV-2 proviral load in whole PBMCs observed in our study seems to be lower than that reported for HTLV-1-infected patients. However, our data do confirm the wide range of variation in proviral load previously reported by other authors (7). Unlike some other investigators studying both HTLV-1 and HTLV-2 (13, 19), we did not observe any significant correlations between antibody titers and proviral loads, although the patients with the lowest proviral loads also had the lowest antibody titers. Moreover, we did not observe any correlations between antibody titers and the presence of PSP, a finding that is in agreement with our recent observations of ELISA-negative patients with PSP, who probably have very low levels of circulating anti-HTLV-2 antibodies (25). A role of HIV-1 infection in reducing the specific humoral response cannot be excluded in this case.
Another finding of our study concerns the presence of significant HTLV-2 DNA titers in B lymphocytes. We have recently described a high frequency of anti-HTLV antibodies in the sera of HIV-1 patients affected by non-Hodgkin’s lymphoma (27). The possible broadening of viral tropism to B cells (probably facilitated by HIV-1 coinfection) should perhaps be taken into account in future studies of the role of HTLVs in AIDS-associated lymphomas.
In conclusion, our study shows that subjects affected by PSP have HTLV-2 proviral loads that are higher than those of patients without PSP.
The spread of the virus to different types of cells might represent the trigger points of the pathologic processes, although the role of HIV-1 coinfection in this broadening of the cell tropism of HTLV-2 remains to be defined. Studies including HTLV-2-positive, HIV-1-negative individuals are needed in order to clarify whether the HTLV-2-associated conditions in HIV-1-positive patients, such as PSP, are the consequence of opportunism on the part of an otherwise nonpathogenetic virus in immunodeficient patients or are due to an intrinsic pathogenicity that may also emerge in immunocompetent hosts.
Acknowledgments
We thank M. Osio for neurological consultancy.
This work was supported by grant 920465/95 from the Istituto Superiore di Sanità, Rome, Italy.
REFERENCES
- 1.Beilke M A, Greenspan D L, Impey A, Thompson J, Didier P J. Laboratory study of HIV-1 and HTLV-I/II coinfection. J Med Virol. 1994;44:132–143. doi: 10.1002/jmv.1890440205. [DOI] [PubMed] [Google Scholar]
- 2.Berger J R, Raffanti S, Svenningsson A, McCarthy M, Snodgrass S, Resnick L. The role of HTLV in HIV neurologic diseases. Neurology. 1991;41:197–202. doi: 10.1212/wnl.41.2_part_1.197. [DOI] [PubMed] [Google Scholar]
- 3.Berger J R, Svenningsson A, Raffanti S, Resnick L. Tropical spastic paraparesis-like illness occurring in a patient dually infected with HIV-1 and HTLV-II. Neurology. 1991;41:85–87. doi: 10.1212/wnl.41.1.85. [DOI] [PubMed] [Google Scholar]
- 4.Casoli C, Cimarelli A, Bertazzoni U. Cellular tropism of human T-cell leukemia virus type II is enlarged to B lymphocytes in patients with high proviral load. Virology. 1995;206:1126–1128. doi: 10.1006/viro.1995.1036. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 1987. Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Morbid. Mortal. Weekly Rep. 36(Suppl. 1):S1–S15.
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Cimarelli A, Angelin Duclos C, Gessain A, Cattaneo E, Casoli C, Biglione M, Mauclère P, Bertazzoni U. Quantification of HTLV-II proviral copies by competitive polymerase chain reaction in peripheral blood mononuclear cells of Italian injection drug users, Central Africans, and Amerindians. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:198–204. doi: 10.1097/00042560-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 8.De Revel T, Mabondzo A, Gras G, Delord B, Roques P, Boussin F, Neveux Y, Bahuau M, Fleury H, Dormont D. In vitro infection of human macrophages with human T-cell leukemia virus type 1. Blood. 1993;81:1598–1606. [PubMed] [Google Scholar]
- 9.Dooneief G, Marlink R, Bell K, Marder K, Renjifo B, Stern Y, Mayeux R. Neurologic consequences of HTLV-II infection in injection drug users. Neurology. 1996;46:1556–1560. doi: 10.1212/wnl.46.6.1556. [DOI] [PubMed] [Google Scholar]
- 10.Hara H, Morita M, Iwaki T, Hatae T, Itoyama Y, Kitamoto T, Akizuki S, Goto I, Watanabe T. Detection of human T lymphotropic virus type I (HTLV-I) proviral DNA and analysis of T cell receptor Vb CDR3 sequences in spinal cord lesions of HTLV-I-associated myelopathy/tropical spastic paraparesis. J Exp Med. 1994;180:831–839. doi: 10.1084/jem.180.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman P M, Dhib-Jalbut S, Mikovits J A, Robbins D S, Wolf A L, Bergey G K, Lohrey N C, Weislow O S, Ruscetti F W. Human T-cell leukemia virus type I infection of monocytes and microglial cells in primary human cultures. Proc Natl Acad Sci USA. 1992;89:11784–11788. doi: 10.1073/pnas.89.24.11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ijichi S, Ramundo M B, Takahashi H, Hall W W. In vivo cellular tropism of human T-cell leukemia virus type II (HTLV-II) J Exp Med. 1992;176:293–296. doi: 10.1084/jem.176.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan J E, Khabbaz R F, Murphy E L, Hermansen S, Roberts C, Lal R, Heneine W, Wright D, Matijas L, Thomson R, Rudolph D, Switzer W M, Kleinman S, Busch M, Schreiber G B the Retrovirus Epidemiology Donor Study Group. Male-to-female transmission of human T-cell lymphotropic virus type I and II: association with viral load. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:193–201. doi: 10.1097/00042560-199606010-00014. [DOI] [PubMed] [Google Scholar]
- 14.Koyanagi Y, Itoyama Y, Nakamura N, Takamatsu K, Kira S, Iwamasa T, Goto I, Yamamoto N. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology. 1993;196:25–33. doi: 10.1006/viro.1993.1451. [DOI] [PubMed] [Google Scholar]
- 15.Lal R B, Owen S M, Rudolph D L, Dawson C, Prince H. In vivo cellular tropism of human T-lymphotropic virus type II is not restricted to CD8+ cells. Virology. 1995;210:441–447. doi: 10.1006/viro.1995.1360. [DOI] [PubMed] [Google Scholar]
- 16.Lehky T J, Flerlage N, Katz D, Houff S, Hall W H, Ishii K, Monken C, Dhib-Jalbut S, McFarland H F, Jacobson S. Human T-cell lymphotropic virus type II-associated myelopathy: clinical and immunologic profiles. Ann Neurol. 1996;40:714–723. doi: 10.1002/ana.410400507. [DOI] [PubMed] [Google Scholar]
- 17.Levin M C, Fox R J, Lehky T, Walter M, Fox C H, Flerlage N, Bamford R, Jacobson S. PCR-in situ hybridization detection of human T-cell lymphotropic virus type 1 (HTLV-1) tax proviral DNA in peripheral blood lymphocytes of patients with HTLV-1-associated neurologic disease. J Virol. 1996;70:924–933. doi: 10.1128/jvi.70.2.924-933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusso P, Lori F, Gallo R C. CD4-independent infection by human immunodeficiency virus type 1 after phenotypic mixing with human T-cell leukemia viruses. J Virol. 1990;64:6341–6344. doi: 10.1128/jvi.64.12.6341-6344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyata H, Kamahora T, Iha S, Katamine S, Miyamoto T, Hino S. Dependency of antibody titer on provirus load in human T-lymphotropic virus type I carriers: an interpretation for the minor population of seronegative carriers. J Infect Dis. 1995;171:1455–1460. doi: 10.1093/infdis/171.6.1455. [DOI] [PubMed] [Google Scholar]
- 20.Murphy E L, Fridey J, Smith J W, Engstrom J, Sacher R A, Miller K, Gibble J, Stevens J, Thomson R, Hansma D, Kaplan J, Khabbaz R, Nemo G the REDS investigators. HTLV-associated myelopathy in a cohort of HTLV-I and HTLV-II-infected blood donors. Neurology. 1997;48:315–320. doi: 10.1212/wnl.48.2.315. [DOI] [PubMed] [Google Scholar]
- 21.Troutt A B. PCR-limiting dilution analysis. In: Griffin H G, Griffin A M, editors. PCR technology—current innovations. Boca Raton, Fla: CRC Press; 1994. pp. 147–150. [Google Scholar]
- 22.Wattel, E., M. Carvois, A. Gessain, and S. Wain-Hobson. 1996. Clonal expansion of infected cells: a way of life for HTLV-I. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl.1):S92–S99. [DOI] [PubMed]
- 23.Working Group of the American Academy of Neurology AIDS Task Force. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus type 1 (HIV-1) infection. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Proposed WHO criteria for interpreting results from Western blot assay for HIV-1, HIV-2, and HTLV-I/II. Weekly Epidemiol Rec. 1996;65:282–288. [Google Scholar]
- 25.Zehender G, De Maddalena C, Gianotto M, Cavalli B, Santambrogio S, Orso M, Moroni M, Galli M. High prevalence of false-negative anti-HTLV type I/II enzyme-linked immunosorbent assay results in HIV type 1-positive patients. AIDS Res Hum Retroviruses. 1997;13:1141–1146. doi: 10.1089/aid.1997.13.1141. [DOI] [PubMed] [Google Scholar]
- 26.Zehender G, De Maddalena C, Osio M, Cavalli B, Parravicini C, Moroni M, Galli M. High prevalence of HTLV-II infection in patients affected by HIV-1 associated predominantly sensory polyneuropathy (PSP) J Infect Dis. 1995;172:1595–1598. doi: 10.1093/infdis/172.6.1595. [DOI] [PubMed] [Google Scholar]
- 27.Zehender G, Meroni L, Piconi S, De Maddalena C, Parravicini C, Clerici M, Ridolfo A L, Moroni M, Galli M. Frequent detection of antibodies directed against HTLV antigens in patients affected by AIDS-related non-Hodgkin lymphoma. AIDS Res Hum Retroviruses. 1995;11:823–827. doi: 10.1089/aid.1995.11.823. [DOI] [PubMed] [Google Scholar]