Abstract
Pseudorabies virus (PRV; suid herpesvirus 1) infection causes heavy economic losses in the pig industry. Therefore, vaccination with live attenuated viruses is practiced in many countries. This vaccination was demonstrated to induce extrathymic virus-specific memory CD4+CD8+ T lymphocytes. Due to their major histocompatibility complex (MHC) class II-restricted proliferation, it is generally believed that these T lymphocytes function as memory T-helper cells. To directly prove this hypothesis, 15-amino-acid, overlapping peptides of the viral glycoprotein gC were used for screening in proliferation assays with peripheral blood mononuclear cells of vaccinated d/d haplotype inbred pigs. In these experiments, two naturally processed T-cell epitopes (T1 and T2) which are MHC class II restricted were identified. It was shown that extrathymic CD4+CD8+ T cells are the T-lymphocyte subpopulation that responds to epitope T2. In addition, we were able to show that cytokine secretion can be induced in these T cells through recall with inactivated PRV and demonstrated that activated PRV-primed CD4+CD8+ T cells are able to induce PRV-specific immunoglobulin synthesis by PRV-primed, resting B cells. Taken together, these results demonstrate that the glycoprotein gC takes part in the priming of humoral anti-PRV memory responses. The experiments identified the first T-cell epitopes so far known to induce the generation of virus-specific CD4+CD8+ memory T lymphocytes and showed that CD4+CD8+ T cells are memory T-helper cells. Therefore, this study describes the generation of virus-specific CD4+CD8+ T cells, which is observed during vaccination, as a part of the potent humoral anti-PRV memory response induced by the vaccine.
Pseudorabies virus (PRV), a member of the Alphaherpesvirinae, is the causative agent of Aujeszky’s disease. This disease is lethal to young pigs and causes important economic losses (52). Therefore, vaccination of pigs is practiced in many countries.
Several humoral immune system effector mechanisms are involved in the protection of pigs from PRV infection. Virus-neutralizing antibodies, antibodies mediating antibody-dependent cell-mediated cytotoxicity, and antibodies mediating complement-mediated lysis of PRV-infected target cells have been demonstrated (22, 23, 53, 54). The main targets of this humoral immune response were shown to be the viral glycoproteins (3, 45), and passive immunization with monoclonal antibodies (MAbs) against gB, gC, and gD protects pigs from a lethal challenge (20, 49).
The protection conferred through cell-mediated immunity is poorly understood. An increase in major histocompatibility complex (MHC)-unrestricted cell-mediated cytotoxicity against uninfected and PRV-infected cells has been detected after infection or vaccination of pigs with PRV (16, 53, 54), and specific cellular immune responses to PRV infections could be demonstrated by stimulation of proliferation and lymphokine secretion of porcine PRV-immune lymphocytes (10, 17, 42, 43, 51) as well as by the detection of PRV-specific cytotoxic lymphocytes (21, 56).
There are some difficulties in defining more precisely the impact of cell-mediated immune effector mechanisms to protection from PRV-infection and their interplay with the observed humoral immune response. Considerably fewer porcine than human or mouse differentiation markers are available (34). In addition, the immune system of swine differs considerably from that of humans and mice. The pig has a substantial number of CD4−CD8− T lymphocytes in the peripheral blood (4, 6, 12, 36, 39). In young animals, this subpopulation of T lymphocytes comprises up to 60% of the T lymphocytes and contains mainly γδ T lymphocytes. The pig is also the only species so far known to contain a substantial number of resting extrathymic CD4+CD8+ T lymphocytes (28, 36, 39). This T-lymphocyte population shows morphologically the phenotype of mature T lymphocytes (40) and increases with age to up to 60% of peripheral T lymphocytes (29, 35, 39, 55). Further, it was demonstrated that CD4+CD8+ T lymphocytes comprise memory T cells which proliferate upon stimulation with recall antigen (43, 55). Since the observed proliferative response was shown to be MHC class II-restricted, it was speculated that the porcine CD4+CD8+ T-cell subset contains memory T-helper lymphocytes (43). However, the ability of these T lymphocytes to secrete cytokines or to provide help to B cells has so far not been demonstrated.
To gain a better understanding of immune effector mechanisms conferring protection from PRV infection, the function of these unusual extrathymic T-lymphocyte subsets has to be elucidated. In the present study, we identified two T-cell epitopes on glycoprotein gC which are primed during vaccination of d/d haplotype inbred pigs (41) against PRV and demonstrated that MHC class II-restricted, peripheral CD4+CD8+ memory T lymphocytes are the responding T lymphocytes. We were further able to show that PRV-specific, extrathymic CD4+CD8+ T lymphocytes are able to secrete cytokines and have the capacity to stimulate the secretion of PRV-specific immunoglobulins (Ig) by PRV-primed B cells. These results demonstrate that porcine CD4+CD8+ T lymphocytes can function as memory T-helper cells and can direct humoral anti-PRV memory responses.
MATERIALS AND METHODS
Animals, immunizations, and challenge.
Three, 4- to 24-month-old d/d haplotype NIH miniature pigs (41) were immunized twice at an interval of 10 days with the PRV vaccine Nobi-Porvac live (Intervet, Boxmeer, The Netherlands) as recommended by the manufacturer. Blood samples were collected before and 2 to 24 months after immunization.
MAbs.
Murine MAbs against porcine CD4 (MAb 74-12-4 [27]), MHC class I (MAb 2.27.3a [13, 26]), and MHC class II DR (MAb MSA3 [11, 19]) were kindly provided by J. K. Lunney (USDA Agricultural Research Service, Beltsville, Md.). A murine MAb against porcine IgM (MAb 2E8 [2]) was kindly provided by M. Amadori (Instituto Zooprofilattico Sperimentale, Brescia, Italy). Murine MAbs against porcine IgG, IgG1, and IgG2 (47) were a generous gift of A. T. Bianchi (Central Veterinary Institute, Lelystad, The Netherlands). The murine MAbs against porcine CD8 (MAb 295/33 [14]) and SWC1 (MAb 8/1 [33, 37]) were established at the Bundesforschungsanstalt in Tübingen.
Cell lines and cell culture.
The porcine kidney cell line PSEK (American Type Culture Collection, Rockville, Md.) and peripheral blood mononuclear cells (PBMC), isolated from blood with a Ficoll gradient, were cultivated in RPMI 1640 medium supplemented with 10% (vol/vol) fetal calf serum, 10 mM HEPES [pH 7.0], 2 mM l-glutamine, 100 U of penicillin per ml, 0.1 mg of streptomycin per ml, and 5 × 10−5 M 2-mercaptoethanol. The medium for the murine interleukin-2 (IL-2)-dependent cell line HT-2 was additionally supplemented with 10 IU of recombinant human IL-2 (Boehringer, Mannheim, Germany) per ml. The bovine kidney cell line MDBK (American Type Culture Collection), used for virus titer determination, was cultivated as described previously (18). All cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Virus preparation.
The PRV strain Phylaxia was propagated on PSEK cells. The viral stocks were clarified and either subjected to titer determination by plaque assay and subsequently UV inactivated as described previously (43) or sedimented at 70,000 × g and resuspended in phosphate-buffered saline to prepare partially purified virions. The protein content of the virion suspension was subsequently determined with a protein quantitation kit (Bio-Rad, Munich, Germany) and a bovine serum albumin standard.
Synthesis and analysis of synthetic peptides.
Overlapping peptide amides of glycoprotein gC (31), 15 amino acids in length and overlapping by 10 amino acids, were synthesized by Fmoc (9-fluorenylmethoxycarbonyl) chemistry (multiple peptide synthesizer SMPS 350 A.; Zinsser, Frankfurt, Germany). The synthesized peptides were analyzed by amino acid analysis (ABI 420A; Applied Biosystems, Weiterstadt, Germany), analytical high-pressure liquid chromatography (System Gold, Beckman, San Ramon, Calif.) on a Nucleosil C18 column (Grom, Herrenberg, Germany), and ion-spray mass spectrometry (API III Triple-Quatrupol ion spray MS [Grom]).
PRV ELISA.
Partially purified PRV in phosphate-buffered saline was spread onto 96-well microtiter plates at a protein concentration of 5 μg/well, and a standard enzyme-linked immunosorbent assay (ELISA) was performed as described previously (21). Bound PRV-specific antibodies were either detected with rabbit anti-pig Ig heavy plus light chains (H+L) conjugated to horseradish peroxidase (HRP) or with isotype-specific murine MAbs against IgM, IgG, IgG1, and IgG2 followed by goat anti-mouse Ig (H+L)-HRP, which was preincubated with an equal volume of preimmune swine serum to eliminate cross-reactivity. The ELISA titer was determined as the highest dilution of the serum displaying more than 0.1 difference in optical density at 490 nm from the corresponding preimmune serum.
Virus neutralization assay.
The virus-neutralizing activity of the antisera was tested in a 50% plaque reduction assay (26) performed on MDBK cells in the absence or presence of 5% rabbit serum as a source of complement. The neutralization titer was expressed as the dilution of serum giving 50% plaque reduction.
Stimulation of PBMC.
To screen for T-cell epitopes with overlapping peptides, 105 PBMC were stimulated with different concentrations of peptide (ranging from 0.6 to 0.0005 mg/ml) for 4 days. Proliferation was subsequently measured by adding [3H]thymidine (1 μCi/well) for 18 h to the cultures followed by harvesting onto fiberglass filters, which were then analyzed in a scintillation counter as previously described (38). Virus-specific stimulation of PBMC was analyzed by adding UV-inactivated virus to the cell culture at multiplicity of infection of 10 (determined before inactivation). For stimulation of T lymphocytes or subpopulations of T lymphocytes, 30-Gy-irradiated autologous PBMC were added (105/well) to ensure antigen presentation. All experiments were performed in triplicate. For a better comparison between different experiments, the stimulation coefficient (ks) was calculated as follows: ks = (mean of peptide specific stimulation/mean of spontaneous proliferation) − 1.
Two-color flow cytometric analysis and cell sorting.
Two-color staining of cells for CD4 and CD8 was performed as described previously (43). For fluorescence-activated cell sorting of T lymphocytes, monocytes were depleted by plastic adherence and B lymphocytes were removed by passages over nylon wool columns (39). Cell separation was performed by setting electronic sort windows on the four CD4/CD8-defined T-lymphocyte subpopulations. The purity of the separated fractions was always higher than 98%.
Measurement of T-cell-dependent Ig synthesis of B lymphocytes.
PBMC of a PRV-vaccinated d/d haplotype inbred pig were stimulated for 4 days with UV-inactivated PRV-virus at an MOI of 2 and subsequently enriched for T lymphocytes on nylon wool. The activated T lymphocytes and B lymphocytes of a PRV-immunized d/d haplotype inbred pig, negatively selected by immunomagnetic cell separation with anti-SWC1, were cocultivated for 7 days at different T-cell/B-cell ratios (25 to 1.5 × 104 T cells/ml and 1 × 106 B cells/ml) in a final volume of 200 μl. Finally, the supernatants were tested for anti-PRV Ig production in a PRV ELISA.
Measurement of cytokine secretion.
Nylon wool-purified T lymphocytes (105 cells), together with 30-Gy-γ-irradiated autologous PBMC (105/well) to ensure antigen presentation were restimulated for 3 days, and the cell culture supernatant was collected. The supernatant (100 μl) was subsequently added to 5 × 103 HT-2 cells in a final volume of 150 μl of medium without IL-2 and incubated for 24 h. After addition of [3H]thymidine (1 μCi/well) and an 18-h incubation, the cells were harvested onto fiberglass filters, which were then analyzed in a scintillation counter as previously described (38).
RESULTS
A T-cell-dependent humoral immune response is induced by the live vaccine.
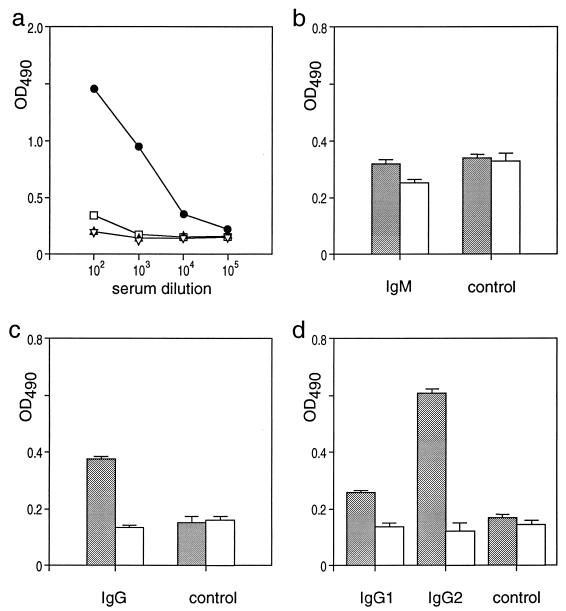
Three d/d haplotype inbred pigs were immunized twice with the live PRV vaccine Nobi-Porvac live. To monitor the humoral immune response, blood samples were collected before and at different times (starting at 2 months) after immunization and assayed in ELISA for anti-PRV reactivity. Figure 1 shows the representative analysis of the observed secondary humoral anti-PRV response. PRV-specific antibodies in immune but not in preimmune sera and antibodies to bovine serum albumin were detected up to a serum dilution of at least 1:10,000 (Fig. 1a). The induction of anti-PRV antibodies by the vaccine could also be confirmed by virus neutralization assays in the presence and absence of complement, which exhibited serum neutralization titers of 1:500 and 1:2,000, respectively (data not shown).
FIG. 1.
The PRV live vaccine induces anti-PRV antibodies of the IgG isotype. (a) Partially purified PRV (5 μg/well [circles and squares]) or bovine serum albumin (triangles) was coated and the reactivity of a PRV-immune (solid) and a preimmune (open) serum of a d/d haplotype inbred pig after the second vaccination was determined by an ELISA. (b to d) Detection of anti-PRV Igs of the IgM (b), IgG (c), and IgG1 and IgG2 (d) isotypes in an ELISA. Anti-PRV reactivity (5 μg of partially purified PRV per well) of PRV-immune (stippled bars) and preimmune (open bars) sera at a dilution of 1:2,000 (d) or 1:5,000 (b and c) detected by MAbs against the respective isotypes or an appropriate control antibody (control) is shown. The standard deviation of the single experiments is indicated by error bars. OD490, optical density at 490 nm.
Subsequently, we analyzed how the reactivity of the immune sera depends on the different Ig isotypes (Fig. 1b to d). When an anti-porcine IgM antibody was used for detection in an anti-PRV ELISA with immune and preimmune sera, PRV-specific antibodies of the IgM isotype could hardly be detected (Fig. 1b). In contrast, detection with an anti-IgG MAb showed a clear difference between preimmune and immune serum (Fig. 1c). Although IgG1 and IgG2 isotypes were present, anti-PRV-specific IgG2 antibodies showed the highest PRV-specific reactivity (Fig. 1d).
Taken together, the analysis of the anti-PRV-immune sera demonstrated that vaccination induced a potent humoral immune response in d/d haplotype inbred pigs. The observed isotype switch to IgG2 indicated that a T-cell-dependent anti-PRV memory response occurred during the course of vaccination.
Identification of the PRV-specific T-cell epitopes T1 and T2.
To identify T-cell epitopes which prime anti-PRV immune responses during vaccination, we used proliferation assays with PBMC of PRV-vaccinated d/d haplotype inbred pigs. With virus-derived, Escherichia coli-expressed fusion proteins for stimulation, preliminary results indicated the presence of epitopes on the viral glycoprotein gC (data not shown) which are naturally processed during vaccination. To delineate these epitopes, 94 overlapping peptides spanning the whole gC region were synthesized and tested for their ability to stimulate proliferation.
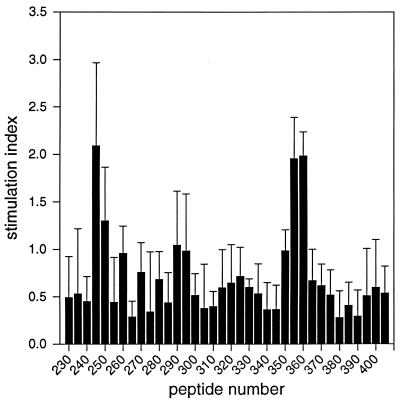
No specific proliferation could be detected for peptides covering amino acids 1 to 230 (peptides 1 to 46) and 420 to 479 (peptides 82 to 94) (data not shown). However, as shown for a representative experiment in Fig. 2, stimulation of PRV-primed PBMC with peptides 245, 355, and 360 identified two immunodominant T-cell epitopes (T1 and T2) between amino acids 230 and 420 of gC. All three peptides reproducibly showed stimulation coefficients (ks) of ca. 2, significantly higher than background (ks = 0 to 0.5). The observed stimulation was concentration dependent and reached its maximum (ks = ca. 3) at a peptide concentration of ca. 50 μg/ml (data not shown). PRV-primed PBMC did not proliferate in response to irrelevant peptides, and the identified PRV-specific peptides did not stimulate autologous nonimmune PBMC (data not shown). This demonstrated that the identified T-cell epitopes stimulate proliferation in a PRV-specific manner. Additional experiments showed, further, that both epitopes stimulated the proliferation of PBMC of all three vaccinated inbred pigs. Epitope T2 thereby elicited a stronger response than did epitope T1 at optimal peptide concentrations (data not shown).
FIG. 2.
Identification of two PRV-specific T-cell epitopes in proliferation assays with overlapping peptides of glycoprotein gC. The reactivity of 105 PBMC of a vaccinated d/d haplotype inbred pig toward synthetic, overlapping peptides of amino acid 230 by 420 of gC (final concentration, 5 μg/ml) is shown. The stimulation index of each peptide is plotted against the peptide number. The standard deviation of the single experiments is indicated by error bars.
The overlapping peptides therefore allowed us to map the core of the identified T-cell epitopes to amino acids 245 to 259 (T1; peptide 245, PVLFGEPFRAVCVVR) and 350 to 369 (T2; peptides 355 and 360; SVRFVEGFAVCDGLCVPPEA) of glycoprotein gC.
Epitope T2 stimulates PRV-specific MHC class II-restricted CD4+CD8+ memory T lymphocytes.
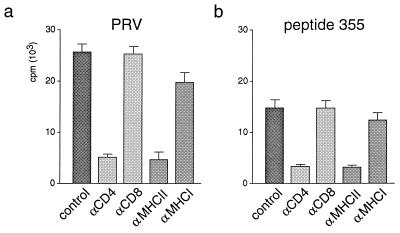
To obtain more information about the function of T lymphocytes which are primed by the identified T-cell epitope T2 during vaccination, we determined the MHC restriction of the proliferative response to peptide 355. After adding MAbs against CD4, CD8, MHC class I, and MHC class II molecules, we tested the inhibition of peptide-specific proliferation of PRV-primed T lymphocytes by the MAbs. As shown in Fig. 3b, only antibodies against the CD4 coreceptor and MHC class II molecules inhibited proliferation induced by the peptide by more than 80% in comparison to cultures without antibodies added. Antibodies to CD8 and MHC class I molecules, for which their blocking capacity was demonstrated previously (14, 24, 26), did not show a significant inhibition. Therefore, it could be concluded that presentation of the epitope T2 is MHC class II restricted and that only the CD4 coreceptor is essential for the recognition of the peptide. We further compared the inhibition of peptide-specific proliferation through MAbs against CD4 and MHC class II with the inhibition of PRV-specific proliferation observed with the same antibodies. As shown in Fig. 3a, both antibodies also inhibited stimulation with UV-inactivated PRV by more than 80% (Fig. 3a). This indicated that peptide 355 might stimulate the same T-lymphocyte population as UV-inactivated PRV does.
FIG. 3.
MHC restriction of the proliferative T-lymphocyte response specific for inactivated PRV and peptide 355 (T2). T lymphocytes (105 cells) of a PRV-vaccinated d/d haplotype inbred pig and irradiated autologous PBMC (105 cells) were stimulated with inactivated PRV (a) or peptide 355 (b) after addition of MAbs against molecules involved in antigen recognition (CD4, CD8, MHC class II, and MHC class I). The proliferative response of the microcultures was quantified by [3H]thymidine incorporation. T lymphocytes incubated without specific antibodies served as controls (control). The standard deviation of the single experiments is indicated by error bars. The spontaneous proliferative response of PBMC without adding PRV-specific antigens (medium control) was less than 1,500 cpm.
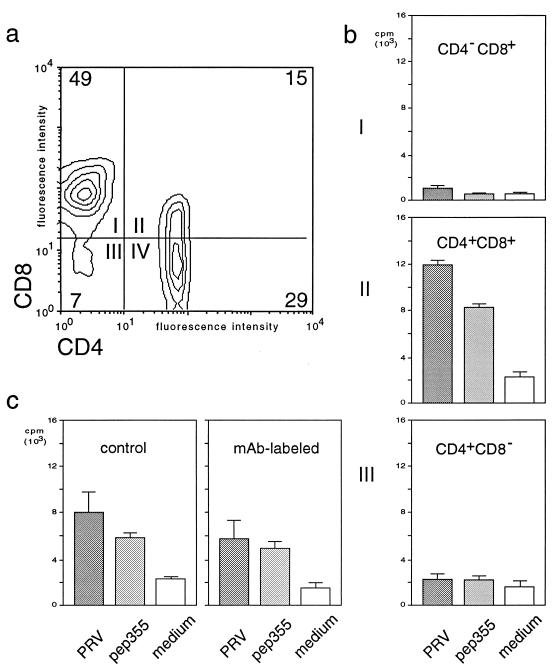
Several reports indicated that CD4+CD8+ T lymphocytes are involved in the proliferative memory response against PRV (17, 43, 55). Therefore, PRV-primed T lymphocytes were separated by flow cytometry into the four subpopulations CD4−CD8+, CD4+CD8−, CD4−CD8−, and CD4+CD8+ (Fig. 4a). The proliferative response of the different T-lymphocyte subsets to recall with peptide 355 was subsequently determined and compared to proliferation obtained upon stimulation with UV-inactivated PRV (Fig. 4b). To exclude a toxic effect of the MAbs used for separation, we labeled the whole T-lymphocyte fraction with anti-CD4 and anti-CD8 prior to antigenic stimulation and demonstrated that this did not influence the ability of the T lymphocytes to proliferate in response to the different stimuli (Fig. 4c). Subsequently, we demonstrated for the single T-lymphocyte subsets that neither CD4−CD8− (data not shown) nor CD4−CD8+ (Fig. 4b, panel I) T lymphocytes showed peptide-specific proliferation. Also, T lymphocytes with the phenotype of classical T-helper cells (CD4+CD8−) did not show a statistically significant increase in proliferation (Fig. 4b, panel III). A specific proliferative response to peptide 355 (T2) was detected only for the CD4+CD8+ T-lymphocyte subpopulation (Fig. 4b, panel II). In addition, only the CD4+CD8+ T-lymphocyte population showed a distinct enrichment of the responding cells in comparison to the nonseparated MAb-labeled T lymphocytes before sorting (Fig. 4c, MAb-labeled). The comparison of the peptide-induced proliferative response with the response induced by UV-inactivated PRV (Fig. 4b and c) demonstrated further that peptide 355 and inactivated PRV stimulated the same subset of T lymphocytes. Only CD4+CD8+ T lymphocytes showed a significant proliferative response, as well as an enrichment of responding cells upon sorting, with both stimuli (Fig. 4b, panel II). Since PRV-specific CD4+CD8+ T lymphocytes have been shown to be memory T lymphocytes (43, 55), these results demonstrate that epitope T2 primes CD4+CD8+ memory T lymphocytes during vaccination.
FIG. 4.
The T-cell epitope T2 selectively stimulates the CD4+CD8+ subset of T lymphocytes. T lymphocytes (105 cells) of a PRV-vaccinated d/d haplotype inbred pig and the respective CD4/CD8-defined, flow cytometry-separated T-lymphocyte subpopulations, together with autologous, irradiated PBMC (105 cells), were incubated with medium only (medium) or stimulated in vitro with peptide 355 (T2, pep72) or inactivated PRV (PRV). (a) CD4 (fluorescein isothiocyanate) versus CD8 (phycoerythrin) expression of nonseparated T lymphocytes. (b) Proliferative response of flow cytometry-separated T-lymphocyte subpopulations (representative of quadrants I, II, and IV) quantified by [3H]thymidine incorporation. (c) Proliferative response quantified by [3H]thymidine incorporation of nonseparated nonlabeled (control) and anti-CD4/CD8-labeled (MAb-labeled) T lymphocytes (105 cells). The standard deviation of the single experiments is indicated by error bars.
PRV-specific memory T-lymphocytes stimulate the secretion of cytokines and can provide help for Ig-secretion by B cells.
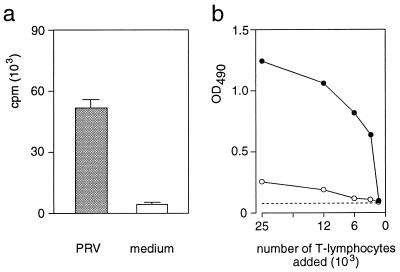
The restriction of the proliferative response against epitope T2 and UV-inactivated PRV (Fig. 3) indicated that PRV-specific CD4+CD8+ T cells are memory T-helper lymphocytes. One of the functions of T-helper lymphocytes is the secretion of cytokines (1, 44). Therefore, the supernatants of PRV-primed T lymphocytes after stimulation with inactivated PRV (only CD4+CD8+ T lymphocytes respond to this stimulus [Fig. 4b]) were tested in a bioassay with the murine IL-2-dependent cell line HT-2. As shown in Fig. 5a, HT-2 cells proliferated only in response to supernatants derived from PRV-primed T lymphocytes stimulated with inactivated PRV but not to supernatants of unstimulated T lymphocytes. In addition, no PRV-specific proliferation in response to supernatants derived from autologous nonimmune T lymphocytes stimulated with inactivated PRV was seen (data not shown).
FIG. 5.
Memory T lymphocytes activated by UV-inactivated PRV produce cytokines and provide help for the stimulation of PRV-specific Ig secretion by B cells. (a) PRV-primed T lymphocytes (105 cells) together with autologous, irradiated PBMC (105 cells) were cultivated for 3 days in medium only (medium) or in medium containing UV-inactivated PRV (PRV). The proliferation of the IL-2-dependent cell line HT-2 in response to 100 μl of the cell culture supernatant was subsequently determined by [3H]thymidine incorporation. (b) PBMC of a PRV-vaccinated d/d haplotype inbred pig were stimulated for 4 days with UV-inactivated PRV and subsequently enriched for T lymphocytes on nylon wool. The activated T lymphocytes and autologous B lymphocytes (5 × 104 cells) of a vaccinated d/d haplotype inbred pig were cultivated for 7 days at different T-cell/B-cell ratios. The supernatants were tested for anti-PRV Ig production in an anti-PRV ELISA, and the optical density at 490 nm (OD490) of T lymphocytes only (open circles) and B lymphocytes with different amounts of T lymphocytes added (solid circles) was determined and plotted against the number of T lymphocytes added. The dashed line shows the optical density obtained with supernatant of B lymphocytes (2 × 105 cells) without T lymphocytes added.
Another function of memory T-helper lymphocytes is to provide (upon activation) help for Ig secretion by B cells (5). Therefore, we examined the ability of PRV-primed memory T lymphocytes to stimulate the secretion of PRV-specific antibodies by PRV-primed B cells by using a modified version of the B-cell stimulation assay described by Croft and Swain (7). PBMC of a PRV-primed d/d haplotype inbred pig were isolated and stimulated with UV-inactivated PRV to activate the PRV-primed T lymphocytes (Fig. 4b). Different amounts of these activated T cells were subsequently cocultivated with autologous, PRV-primed B cells. The relative amount of secreted anti-PRV-antibodies in the supernatant, which is dependent on the number of T lymphocytes added, was subsequently determined in an anti-PRV ELISA. As shown in Fig. 5b, PRV-primed B cells secreted only marginal amounts of PRV-specific antibodies without T cells. However, after addition of activated, PRV-primed T lymphocytes, anti-PRV-specific antibodies could be detected in the supernatant of these cultures but not in cultures of activated T lymphocytes without B lymphocytes.
These results therefore demonstrated that activated extrathymic CD4+CD8+ memory T lymphocytes not only can be induced to secrete cytokines but also are able to provide T-cell help to B lymphocytes for the secretion of anti-PRV-specific antibodies.
DISCUSSION
Much progress has been made during the last few years in elucidating different immune mechanisms primed during PRV infection in pigs as well as in generating new anti-PRV vaccines. However, although currently available vaccines are able to protect pigs from a lethal infection, they still allow the establishment of a latent infection and the secretion of reactivated virus (32). The development of more efficient vaccines is hampered by a lack of precise knowledge of the different immune mechanisms which can confer protection. In this study, three d/d haplotype inbred pigs (41) were vaccinated with the commonly used PRV live vaccine Nobi-Porvac live (Tk−/gE− [48]) and used to study vaccine-induced protection against PRV on a defined genetic background.
The analysis of the humoral immune response in d/d haplotype inbred pigs demonstrated the presence of anti-PRV-specific antibodies with complement-dependent and independent virus-neutralizing activity. As demonstrated in outbred pig population with different live and killed PRV vaccines (15), in inbred pigs the main serum isotype of anti-PRV antibodies was IgG, and PRV-specific antibodies of the IgG1 and IgG2 isotypes could be detected. Thus, PRV vaccines generally seem to induce a long-term humoral memory response against PRV, which is characterized by efficient isotype switching to IgG. The observed isotype switch also indicated that a T-cell-dependent memory response was generated during vaccination (8).
In humans and mice, there are two functionally distinct classes of extrathymic memory T lymphocytes with the ability to proliferate upon contact with protein antigens. (i) CD4−CD8+ cytotoxic T lymphocytes are MHC class II restricted and mainly recognize replicating viral antigens. (ii) CD4+CD8− helper T-lymphocytes are MHC class II restricted and respond to nonreplicating protein antigens which have been processed by antigen-presenting cells (9, 46, 50). Conclusively, it was found that in pigs the proliferative response of PRV-primed T lymphocytes upon recall with UV-inactivated virus is MHC class II restricted and requires the CD4 coreceptor for recognition (28, 43).
So far, several studies implicated an involvement of glycoprotein gC in the induction of PRV-specific MHC class I- and class II-restricted memory responses. First, it was shown that vaccination of pigs with a recombinant gC subunit vaccine or gC vaccinia virus recombinants leads to a significant protection of pigs from PRV-infection (30, 31a). Second, cells expressing PRV gC could be used for in vitro restimulation of lymphocytes from PRV-immune inbred pigs of the same haplotype (17). Third, it was shown that PRV infection induces a significant amount of MHC class I-restricted and gC-specific cytotoxic activity by T cells against PRV-infected target cells in two Landrace pigs (56). In this study, we focused on the precise characterization of the MHC class II-restricted memory T-lymphocyte response and virus-specific components which are able to prime this response. Since it is well known that MHC class I and class II molecules present peptides of 8 to 9 and 11 to 30 amino acids, respectively, in humans and mice (46), we selected for MHC class II-restricted T-cell epitopes by using peptide screening with overlapping 15-amino-acid peptides of viral glycoprotein gC. This approach allowed us to map two T-cell epitopes (T1 and T2) on gC, which could subsequently be shown to be MHC class II restricted and to require the CD4 coreceptor for recognition.
There were several lines of evidence that resting peripheral CD4+CD8+ T lymphocytes play an important role in protection against PRV-infection. The CD4/CD8 depletion studies of Kimman et al. (17) indicated that CD4+CD8dull+ T lymphocytes might contribute to the lymphoproliferative response to UV-inactivated virus. Moreover, Summerfield et al. (43) and Zuckermann and Husmann (55) demonstrated through in vitro restimulation of the four different extrathymic T-lymphocyte subsets with UV-inactivated PRV that MHC class II-restricted memory T lymphocytes of the CD4+CD8+ phenotype are generated during PRV infection. These memory T lymphocytes were further shown to require only the CD4 (not the CD8) coreceptor for recognition (43). Since the restriction of the identified epitopes was in agreement with proliferating CD4+CD8− as well as with CD4+CD8+ T lymphocytes, we determined the responding T-lymphocyte subset to T2. We could demonstrate that only resting CD4+CD8+ T lymphocytes proliferated significantly in response to this epitope. Since PRV-specific CD4+CD8+ T lymphocytes were shown to be memory T lymphocytes (43, 55), these results not only identified the first T-cell epitope so far known to prime this unusual extrathymic T-lymphocyte subset but also indicated that a PRV live vaccine induces a long-lived MHC class II-restricted memory T-lymphocyte response to the glycoprotein gC. This interpretation was further supported by the observation that stimulation of proliferation through epitope T2 was seen with blood samples up to 12 months after the second administration of the vaccine.
We also compared the stimulation with the gC-derived peptide (T2) to the stimulation with inactivated PRV and demonstrated that both stimuli have the same restriction and activate the CD4+CD8+ T lymphocytes exclusively. This is in contrast to results described by Zuckermann and Husmann (55), where in addition to double-positive memory T lymphocytes a significant number of CD4+CD8− memory T-lymphocytes proliferated in response to UV-inactivated PRV. However, in several experiments we did not detect a statistically significant proliferation of the CD4+CD8− T-lymphocyte subset upon stimulation with gC-derived peptide or inactivated virus, nor did we observe an increase of proliferation upon cell sorting. Differences in the inbred haplotypes used for the experiments (c/c versus d/d haplotype animals), in the immunization protocol, and in the time points when blood samples were taken during the two studies might account for this discrepancy. In our study, we worked with d/d haplotype swine and used blood samples collected 2 to 12 months after the second immunization. These conditions should have favored the selective detection of a long-lasting memory response.
Although it could be shown that the CD4+CD8+ T-lymphocyte subset contains memory T lymphocytes (43, 55) and that the proliferation of these memory T cells is MHC class II restricted and can be inhibited by antibodies against CD4 (43), a direct demonstration of their helper activity was missing. A precondition of memory T-helper lymphocyte function is the ability to secrete cytokines and to provide help to B cells (1). Since only CD4+CD8+ memory T lymphocytes proliferated in response to stimulation with UV-inactivated virus in our system, by using PRV-primed T lymphocytes, we were able to demonstrate the virus-inducible production of cytokines.
Additionally, we established an assay for the detection of PRV-specific Ig-synthesis by resting PRV-primed B cells upon stimulation with activated, autologous, PRV-primed T cells. This assay allowed us to directly link the vaccine-induced generation of PRV-specific CD4+CD8+ memory T lymphocytes and the potent humoral anti-PRV memory response, which we detected in vaccinated d/d haplotype inbred pigs. Our demonstration of antibody synthesis in this assay, together with the MHC class II restriction of the proliferative response and the ability of PRV-primed CD4+CD8+ T cells to secrete cytokines, identified these T lymphocytes as memory T-helper cells. It indicates that they regulate the humoral memory response to PRV in vivo. The resting phenotype (39) of CD4+CD8+ T lymphocytes, the undetectable expression of IL-2 receptor on the cell surface (40a), as well as the high β1-integrin level of 75% (55) can therefore be interpreted as a prerequisite to fulfill their memory T-helper functions. However, in contrast to murine and human memory T-helper lymphocytes, resting porcine extrathymic CD4+CD8+ T lymphocytes also express significant levels of MHC class II molecules (40) and can act as antigen-presenting cells in mixed leukocyte culture (38). This indicates that the characterized resting, PRV-primed CD4+CD8+ T lymphocytes may not only act as memory T-helper cells but may also be able to act as antigen-presenting cells and may therefore display a higher regulatory potential than the CD4+CD8− memory T-helper lymphocytes described in humans and mice.
Taken together, this study demonstrated that vaccination against PRV induced a potent T-cell-dependent humoral memory response. It mapped two naturally processed T-cell epitopes to glycoprotein gC, which are able to prime MHC class II-restricted memory T lymphocytes of the CD4+CD8+ phenotype. Our demonstration that virus-specific CD4+CD8+ cells can function as memory T-helper cells allowed us to identify the generation of PRV-specific CD4+CD8+ memory T lymphocytes during vaccination as being part of the potent humoral immune response, which is induced by the vaccine.
ACKNOWLEDGMENTS
We thank S. Maurer for technical assistance and B. Teufel for help with peptide synthesis. We also thank W. Beck, M. Munari, and G.-J. Nicholson for acquiring the spectra for the peptide analysis.
This work was partially funded by grant DFG-Rz 2/1-5 from the Deutsche Forschungsgemeinschaft and by grant BRIDGE BIOT-CT91 from the European Community.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Archetti I L, Capucci L, Saalmüller A, Verardi R, König M, Amadori M. Production and characterization of monoclonal antibodies differentiating subpopulations of porcine B lymphocytes in blood and lymphoid tissues. J Vet Med. 1993;40:485–493. doi: 10.1111/j.1439-0450.1993.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Porat T, DeMarchi B, Lomniczi B, Kaplan A S. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology. 1986;154:325–334. doi: 10.1016/0042-6822(86)90458-7. [DOI] [PubMed] [Google Scholar]
- 4.Binns R M, Duncan I A, Powis S J, Hutchings A, Butcher G. Subsets of null and gamma delta T-cell receptor + T lymphocytes in the blood of young pigs identified by specific monoclonal antibodies. Immunology. 1992;177:219–227. [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley L M, Croft M, Swain S L. T-cell memory: new perspectives. Immunol Today. 1993;14:197–199. doi: 10.1016/0167-5699(93)90161-D. [DOI] [PubMed] [Google Scholar]
- 6.Carr M M, Howard C J, Sopp P, Manser J M, Parsons K R. Expression of porcine γ/δ T lymphocytes of a phylogenetically conserved surface antigen previously restricted in expression of ruminant γδ T lymphocytes. Immunology. 1994;81:36–40. [PMC free article] [PubMed] [Google Scholar]
- 7.Croft M, Swain S L. Analysis of CD4+ T cells that provide contact-dependent bystander help to B-cells. J Immunol. 1992;149:3157–3165. [PubMed] [Google Scholar]
- 8.DeFranco A L. B lymphocyte activation. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1997. pp. 505–529. [Google Scholar]
- 9.Doherty P C, Topham D J, Tripp R A. Establishment and persistance of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 10.Gutekunst D E, Pirtle E C. Humoral and cellular immune responses in swine after vaccination with inactivated pseudorabies virus. Am J Vet Res. 1979;40:1343–1347. [PubMed] [Google Scholar]
- 11.Hammerberg C, Schurig G G. Characterization of monoclonal antibodies directed against swine leukocytes. Vet Immunol Immunopathol. 1986;11:107–121. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- 12.Hirt W, Saalmüller A, Reddehase M J. Distinct γ/δ T cell receptors define two subsets of circulating porcine CD2−CD4−CD8− T-lymphocytes. Eur J Immunol. 1990;20:265–269. doi: 10.1002/eji.1830200206. [DOI] [PubMed] [Google Scholar]
- 13.Ivanoska D, Sun D C, Lunney J K. Production of monoclonal antibodies reactive with polymorphic and monomorphic determinants of SLA class I gene products. Immunogenetics. 1991;33:220–223. doi: 10.1007/BF01719247. [DOI] [PubMed] [Google Scholar]
- 14.Jonjic S, Koszinowski U H. Monoclonal antibodies reactive with swine lymphocytes. I. Antibodies to membrane structure that define the cytolytic T lymphocyte subset in the swine. J Immunol. 1984;133:647–652. [PubMed] [Google Scholar]
- 15.Kimman T G, Brouwers R A M, Daus F J, Van Oirschot J T, Van Zaane D. Measurement of isotype-specific antibody responses to Aujeszky’s disease virus in sera and mucosal secretions of pigs. Vet Immunol Immunopathol. 1992;31:95–113. doi: 10.1016/0165-2427(92)90089-9. [DOI] [PubMed] [Google Scholar]
- 16.Kimman T G, De Bruin T G M, Voermans J J M, Bianchi A T J. Cell-mediated immunity to pseudorabies virus: cytolytic effector cells with characteristics of lymphokine-activated killer cells lyse virus-infected and glycoprotein gB- and gC-transfected L14 cells. J Gen Virol. 1996;77:987–990. doi: 10.1099/0022-1317-77-5-987. [DOI] [PubMed] [Google Scholar]
- 17.Kimman T G, De Bruin T G M, Voermans J J M, Peeters B P H, Bianchi A T M. Development and antigen specificity of the lymphoproliferation response of pigs to pseudorabies virus: dichotomy between secondary B- and T-cell responses. Immunology. 1995;86:372–378. [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacs N, Thiel H-J, Mettenleiter T C, Rziha H-J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985;53:166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunney J K. Current status of the swine leukocyte antigen complex. Vet Immunol Immunopathol. 1994;43:19–28. doi: 10.1016/0165-2427(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 20.Marchioli C, Yancey R J, Timmins J G, Post L E, Young B R. Protection of mice and swine from pseudorabies virus induced mortality by administration of pseudorabies virus-specific mouse monoclonal antibodies. Am J Vet Res. 1988;49:860–864. [PubMed] [Google Scholar]
- 21.Martin S, Wardley R C. Local humoral and cellular responses in Aujeszky’s disease virus infection in pigs. Res Vet Sci. 1987;42:170–174. [PubMed] [Google Scholar]
- 22.Martin S, Wardley R C, Donaldson A I. Serological response of pigs infected with Aujeszky’s disease virus. Res Vet Sci. 1983;35:227–233. [PubMed] [Google Scholar]
- 23.Martin S, Wardley R C, Donaldson A I. Functional antibody responses in pigs vaccinated with live and inactivated Aujeszky’s disease virus. Res Vet Sci. 1986;41:331–335. [PubMed] [Google Scholar]
- 24.Martins C L V, Lawman M J P, Scholl T, Mebus C A, Lunney J K. African swine fever virus specific porcine cytotoxic T cell activity. Arch Virol. 1993;129:211–225. doi: 10.1007/BF01316896. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter T C, Schreurs C, Thiel H-J, Rziha H-J. Variability of pseudorabies virus glycoprotein gI expression. Virology. 1987;158:141–146. doi: 10.1016/0042-6822(87)90247-9. [DOI] [PubMed] [Google Scholar]
- 26.Pauly T, Elbers K, König M, Lengsfeld T, Saalmüller A, Thiel H-J. Classical swine fever virus-specific cytotoxic T lymphocytes and identification of a T cell epitope. J Gen Virol. 1995;76:3039–3049. doi: 10.1099/0022-1317-76-12-3039. [DOI] [PubMed] [Google Scholar]
- 27.Pescovitz M D, Lunney J K, Sachs D H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- 28.Pescovitz M D, Lunney J K, Sachs D H. Murine anti-swine T4 and T8 monoclonal antibodies reactive with porcine PBL. J Immunol. 1985;134:37–44. [PubMed] [Google Scholar]
- 29.Pescovitz M D, Sakopoulos A G, Gaddy J A, Husmann R J, Zuckermann F A. Porcine peripheral blood CD4+/CD8+ dual expressing T-cells. Vet Immunol Immunopathol. 1994;43:53–62. doi: 10.1016/0165-2427(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 30.Riviere M, Tartaglia J, Perkus M E, Norton E K, Molnar Bongermino C, Lacoste F, Duret C, Desmettre P, Paoletti E. Protection of mice and swine from pseudorabies virus conferred by vaccinia virus-based recombinants. J Virol. 1992;66:3424–3434. doi: 10.1128/jvi.66.6.3424-3434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins A K, Watson R J, Whealy M E, Hays W W, Enquist L W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986;58:339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Robbins, A. K., et al. 1985. European patent application 162,738.
- 32.Rziha H-J, Ohlinger V F, Lüttiken D, Visser N. Latency characteristics of pseudorabies vaccine virus. In: Van Oirschot J T, editor. Vaccination and control of Aujeszky’s disease. Boston, Mass: Kluwer Academic Publisher; 1989. pp. 79–85. [Google Scholar]
- 33.Saalmüller A, Aasted B, Canals A, Dominguez J, Goldman T, Lunney J K, Pauly T, Pescovitz M D, Pospisil R, Salmon H, Sinkora J, Summerfield A, Valpotic I, Viscaino J S, Zuckermann F. Analysis of mAb reactive with the porcine SWC1. Vet Immunol Immunopathol. 1994;43:249–254. doi: 10.1016/0165-2427(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 34.Saalmüller A. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1996;17:352–354. doi: 10.1016/S0167-5699(96)90273-X. [DOI] [PubMed] [Google Scholar]
- 35.Saalmüller A, Bryant J. Characteristics of porcine T lymphocytes and T-cell lines. Vet Immunol Immunopathol. 1994;43:45–52. doi: 10.1016/0165-2427(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 36.Saalmüller A, Hirt W, Reddehase M J. Porcine γ/δ T lymphocyte subsets differing in their propensity to home to the lymphoid tissue. Eur J Immunol. 1990;20:2343–2346. doi: 10.1002/eji.1830201026. [DOI] [PubMed] [Google Scholar]
- 37.Saalmüller A, Jonjic S, Bühring H J, Reddehase M J, Koszinowski U H. Monoclonal antibodies reactive with swine leukocytes. II. Detection of a antigen on resting T cells down-regulated after activation. J Immunol. 1987;138:1852–1857. [PubMed] [Google Scholar]
- 38.Saalmüller A, Maurer S. Major histocompatibility antigen class II expressing resting porcine T lymphocytes are potent antigen presenting cells in mixed lymphocyte culture. Immunobiology. 1994;190:23–34. doi: 10.1016/S0171-2985(11)80281-0. [DOI] [PubMed] [Google Scholar]
- 39.Saalmüller A, Reddehase M J, Bühring H J, Jonjic S, Koszinowski U H. Simultaneous expression of CD4 and CD8 antigens on a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987;17:1297–1301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- 40.Saalmüller A, Weiland F, Reddehase M J. Resting porcine T lymphocytes expressing class II major histocompatibility antigen. Immunobiology. 1991;183:102–111. doi: 10.1016/S0171-2985(11)80190-7. [DOI] [PubMed] [Google Scholar]
- 40a.Saalmüller, A. Unpublished observation.
- 41.Sachs D H, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Smith P C, Mengelin W L. A skin test for pseudorabies virus infection in swine. Can J Comp Med. 1977;41:364–367. [PMC free article] [PubMed] [Google Scholar]
- 43.Summerfield A, Rziha H-J, Saalmüller A. Functional characterization of porcine CD4+CD8+ extrathymic T lymphocytes. Cell Immunol. 1996;168:291–296. doi: 10.1006/cimm.1996.0078. [DOI] [PubMed] [Google Scholar]
- 44.Swain S L, Croft M, Dubey C, Hayes L, Rogers P, Zhang X, Bradley L M. From naive to memory T cells. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 45.Todd D, Hull J, McNair J. Antigenically important proteins Aujeszky’s disease (pseudorabies) virus identified by immunoblotting. Arch Virol. 1987;96:215–224. doi: 10.1007/BF01320961. [DOI] [PubMed] [Google Scholar]
- 46.Unanue E R. Macrophages, antigen-presenting cells, and the phenomena of antigen handling and presentation. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1993. pp. 111–144. [Google Scholar]
- 47.Van Zaane D, Hulst M M. Monoclonal antibodies against porcine immunoglobulin isotypes. Vet Immunol Immunopathol. 1987;16:23–36. doi: 10.1016/0165-2427(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 48.Visser N, Lütticken D. Experiences with a gI-/TK-modified live pseudorabies vaccine: strain Begonia. In: Van Oirschot J T, editor. CEC seminar on vaccination control of Aujeszky’s disease. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1989. pp. 37–44. [Google Scholar]
- 49.Wathen L M K, Platt K B, Wathen M W. Production and characterization of monoclonal antibodies directed against PRV. Virus Res. 1985;4:19–29. doi: 10.1016/0168-1702(85)90017-6. [DOI] [PubMed] [Google Scholar]
- 50.Weiss A. T lymphocyte activation. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1997. pp. 467–504. [Google Scholar]
- 51.Wittman G, Bartenbach G, Jakubik J. Cell-mediated immunity in Aujeszky’s disease virus-infected pigs. 1. Lymphocyte stimulation. Arch Virol. 1976;50:215–222. doi: 10.1007/BF01320575. [DOI] [PubMed] [Google Scholar]
- 52.Wittman G, Rziha H-J. Aujeszky’s disease (pseudorabies) In: Wagner E, editor. Herpesvirus diseases of cattle, horses and pigs. Boston, Mass: Kluwer Academic Publisher; 1987. pp. 230–327. [Google Scholar]
- 53.Wittmann G, Leitzke I, Hohn U. Cell-mediated cytotoxicity stimulation with Aujeszky’s disease. II. After vaccination of pigs followed by infection. Zentralbl Veterinaermed Reihe B. 1985;32:181–191. [PubMed] [Google Scholar]
- 54.Wittmann G, Leitzke I, Hohn U. Cell-mediated cytotoxicity and lymphocyte stimulation with Aujeszky’s disease. I. In experimentally infected pigs. Zentralbl Veterinaermed Reihe B. 1985;32:101–115. [PubMed] [Google Scholar]
- 55.Zuckermann F A, Husmann R J. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double positive T cells. Immunology. 1996;87:500–512. [PMC free article] [PubMed] [Google Scholar]
- 56.Zuckermann F A, Zsak L, Mettenleiter T C, Ben-Porat T. Pseudorabies virus glycoprotein gIII is a major target antigen for murine and swine virus-specific cytotoxic T lymphocytes. J Virol. 1990;64:802–812. doi: 10.1128/jvi.64.2.802-812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]