Abstract
Introduction
Age-related hearing loss (ARHL) may affect working memory (WM), which impacts problem-solving, decision-making, language comprehension, and learning. Limited research exists on how ARHL affects WM using N-back tasks, but studying this is crucial for understanding neural markers and associated cognitive processes. Our study explores the impact of ARHL on WM using behavioral and electrophysiological measures and how it correlates with speech-in-noise scores in older individuals with ARHL.
Method
The study involved two groups, each with 20 participants aged 60–80. Group 1 had individuals with mild to moderate sensorineural hearing loss, while Group 2 had age- and education-matched controls with normal or near-normal hearing. Participants underwent audiological assessments and completed cognitive tests, including simple reaction time and N-back tests. During the performance of cognitive tasks, a simultaneous electroencephalography was recorded. Data analysis included behavioral and event-related potentials, source estimation, and functional connectivity analysis.
Results
The study revealed significantly poor accuracy, longer reaction time, and smaller P300 amplitude among individuals with ARHL, even after controlling for general slowing. Individuals with ARHL experience compromised neural activity, particularly in the temporal and parietal regions, which are vital for cognition and WM. Furthermore, individuals with ARHL exhibited poor communication between the superior temporal gyrus and insulae regions among the brain regions mediating WM during the 1-back task. Also, the study found a strong correlation between hearing measures and WM outcomes.
Conclusion
The study findings suggest that individuals with ARHL have impaired WM compared to those with normal hearing. This indicates a potential link between ARHL and cognitive decline, which could significantly affect daily life and quality of life. The widely used WM test with simultaneous EEG recording and source estimation analysis would further validate the usefulness of the study in assessing WM in this population.
Keywords: Age-related hearing loss, Event-related potentials, N-back task, Older individuals, Working memory
Introduction
Age-related hearing loss (ARHL) is a prevalent sensory impairment affecting almost one-third of the population in their 60s. This condition often goes untreated and is mistakenly considered a harmless consequence of natural aging, but it can have significant consequences. In addition, several studies have reported significant consequences of ARHL on various cognitive processes, including working memory (WM) [1–5].
WM is a crucial cognitive function that helps with problem-solving, decision-making, language comprehension, and learning [6]. There is a strong connection between WM and understanding speech in noisy environments [7, 8], making it relevant to study older individuals with ARHL. Several hypotheses explain the reduced cognition in individuals with ARHL, as these individuals may experience increased cognitive load [2, 4, 9], social isolation [10, 11], and cognitive fatigue [12–14] due to sensory deficits, thereby leading to WM deficits.
The N-back test [15] is a highly validated and widely used task to assess WM. The test requires individuals to remember a sequence of stimuli and indicate when the current stimulus matches the one presented N steps back in the sequence. The difficulty level can be adjusted by varying the value of N, which provides valuable insights into the cognitive resources involved in WM capacity. Literature on WM in older individuals using the N-back task shows poor accuracy and longer reaction time (RT) than healthy younger controls [16].
There is limited research on how ARHL affects WM. Some studies suggest a negative correlation between hearing ability and WM [17–25], but others using N-back tasks have had mixed results [22, 23, 26, 27]. Event-related potentials (ERPs) help understand sensory and cognitive processing. Earlier studies have reliably recorded ERPs to N-back tasks, with P300 as an indicator of WM capacity [28–30].
Overall, there is a dearth of research into the impact of ARHL on WM, especially using the N-back task. We have found no literature on neural markers of the N-back task in ARHL. Examining the impact of ARHL on WM through ERPs is crucial for several reasons. First, it broadens our understanding of the cognitive implications of hearing loss beyond audiological measures, revealing the broader effects on crucial cognitive processes for daily functioning. Furthermore, ERPs provide objective and reliable measures of neural activity linked to WM, enabling the development of diagnostic tools and interventions for those with ARHL. Therefore, the primary objective of the current study is to explore the impact of ARHL on WM during N-back tasks using both behavioral and ERP measures. The study also investigates the correlation between WM performance and speech-in-noise scores in individuals with ARHL.
Materials and Methods
Participants
The study involved two groups, with each group consisting of 20 participants between the ages of 60 and 80. Group 1 comprised individuals with mild to moderate hearing loss (26 dB–55 dB), while group 2 had individuals with normal or near-normal hearing (≤25 dB), with matched age and education. All participants had at least 10 years of formal education and scored above four on the MiniCog and below 26 on the Quick Neurological Screening Test (QNST). The MiniCog test and QNST ruled out cognitive and neurological conditions, respectively. Participants with psychological or neurological disorders, hearing aids, visual impairments, or ear pathologies were excluded from the study.
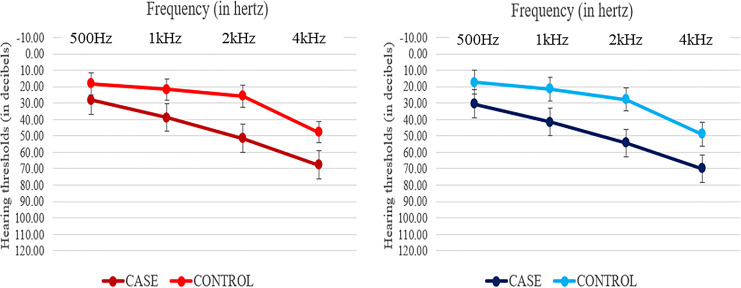
Participants underwent a comprehensive audiological assessment, including pure tone audiometry and the speech in noise test (Quick SIN) [31]. The Kasturba Medical College and Kasturba Hospital Institutional Ethics Committee (Registration No. ECR/146/Inst/KA/2013/RR-16) approved the study, approval number is IEC Project No. 465/2019, and it is registered with the Clinical Trials Registry of India (CTRI/2019/08/020784). All participants provided written informed consent (see Table 1 for participant characteristics and Figure 1 for mean pure tone thresholds with 1 SD).
Table 1.
Characteristics of the study participants
| Hearing loss (n = 20) | Normal hearing (n = 20) | t | p value | |
|---|---|---|---|---|
| Age, years | 65.61±5.007 | 64.80±4.663 | 0.545 | 0.589 |
| Gender (male/female) | 11/09 | 14/06 | – | – |
| Handedness (right/left/mixed) | 20/0/0 | 20/0/0 | – | – |
| Education, years | 11.70±2.285 | 12±2.340 | −0.431 | 0.669 |
| MiniCog scores | 4.13±0.458 | 4.25±0.444 | −0.866 | 0.391 |
| Right ear – pure tone average* | 41.32±6.51 | 21.92±1.97 | 15.305 | <0.001 |
| Left ear – pure tone average* | 42.10±5.8 | 22.16±2.24 | 16.212 | <0.001 |
| SNR loss | 8.283±1.90 | 0.250±2.53 | 11.846 | <0.001 |
*Pure tone average across 500 Hz, 1 kHz, 2 kHz, and 4 kHz.
Fig. 1.
Mean pure tone thresholds for the right (red) and left (blue) ears of hearing loss and normal hearing group.
Test Procedure
Participants completed two cognitive tests, the simple reaction time (SRT) and N-back task, while simultaneous EEG was recorded. The tests evaluated processing speed and WM. The paradigms were created using E-Prime 3.0 software [32] and conducted in a sound-treated room. Participants were given practice trials and breaks between tasks. Feedback on RT and accuracy was provided during practice sessions.
SRT Task
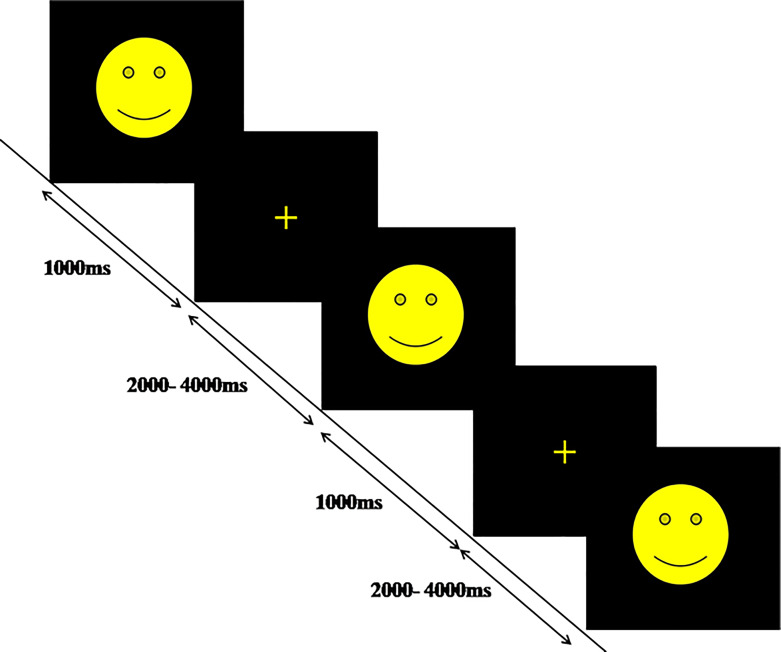
The experiment involved presenting a yellow smiley face with varied stimulus onset asynchrony (2–4 s) for 150 trials. Each trial had a fixation period followed by a 1,000 ms stimulus. A yellow cross was shown during fixation. The correct response was preloaded with Chronos as a response device. Figure 2 shows the schematic design. Participants were instructed to respond quickly to target stimuli, assessing basic RT without any task load to reflect general slowing.
Fig. 2.
Schematic design of SRT task.
N-Back Paradigm
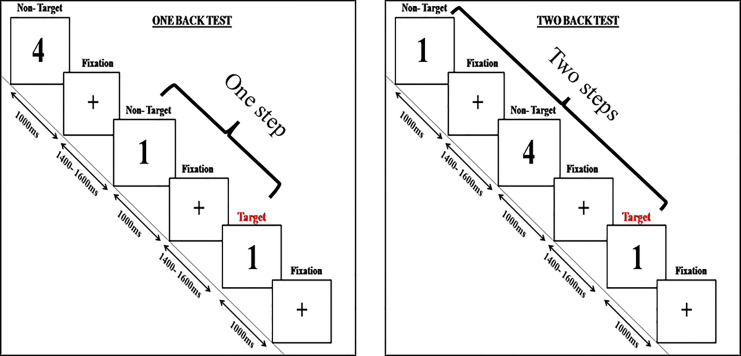
The paradigm used in the present study is depicted in Figure 3. The paradigm is similar to the one used in the study by Kuschpelet al. [33]. Participants completed a visual N-back task with two difficulty levels, 1-back and 2-back, presented as separate blocks. Stimuli were created in MATLAB and presented in black on a white background. Each block had 300 trials with varying fixation and presentation durations. Participants were instructed to press a button indicating whether the current stimulus matched the one presented 1 or 2 steps back. Appropriate rest periods were given.
Fig. 3.
Schematic depiction of N-back task.
EEG Recording
The behavioral and electrophysiological data were recorded simultaneously. The stimulus presentation and behavioral responses were controlled using E-Prime 3.0. EEG signals were recorded using a 32-channel EEG system (ActiChamp, Brain Vision v005 10/2017). The impedance was maintained below 50 kΩ throughout the testing and referenced to Cz. Furthermore, the raw EEG signals were digitized using a sampling frequency of 500 Hz and online filtering between 1 and 100 Hz.
Data Analysis
For the analysis, only the target trials were taken into account.
Behavioral Data
The RT and accuracy scores from E-Prime were extracted and analyzed in Excel within the interquartile range and excluding outliers. The analyzed data were then exported to SPSS for statistical analysis.
EEG Data
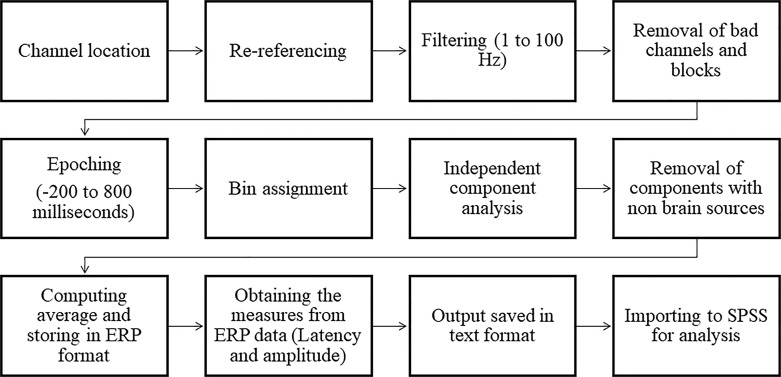
EEGLAB v2021.0 [34] was used to process electrophysiological data in MATLAB R2018b. The standard preprocessing pipeline (as shown in Fig. 4) was followed, and bad blocks were excluded. Channels were referenced to the average reference, and the EEG signals were filtered and epoched. The EEG data were filtered using ICA and a classifier called “ICLabels” to remove nonbrain activities. ERPs were computed and analyzed using the ERPLAB tool to measure two crucial components: N100 and P300.
Fig. 4.
Schematic representation of standard EEG preprocessing pipeline.
Source Estimation
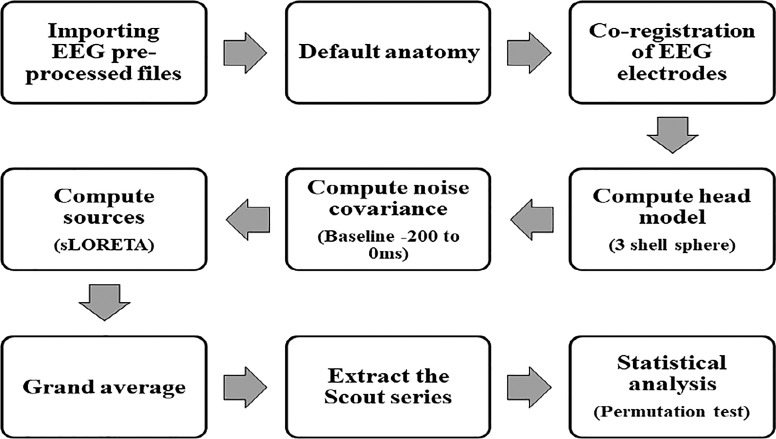
The source estimation analysis used Brainstorm version 3 (36) to estimate sources with sLORETA. Figure 5 shows the analysis pipeline. Source models were calculated for each trial and averaged across trials for each subject. Statistical comparisons of source density were performed between older individuals with and without ARHL using a nonparametric permutation test with default parameters.
Fig. 5.
Schematic representation of source estimation pipeline.
Functional Connectivity Analysis
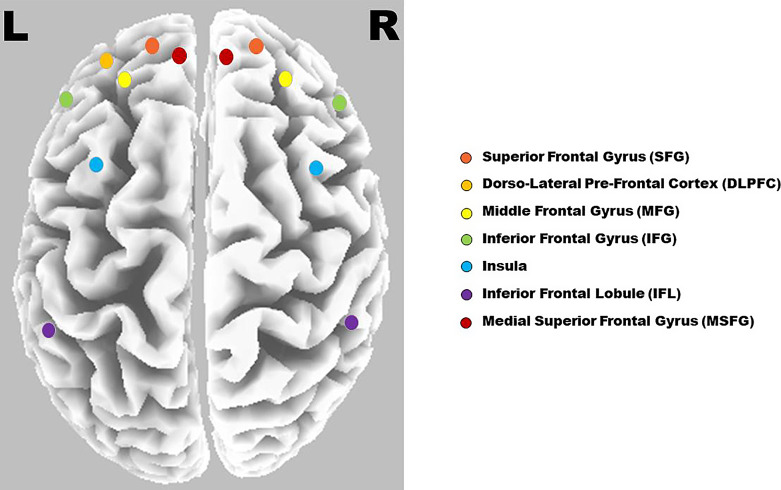
Functional connectivity was measured between the regions of interest (ROIs) using lagged linear connectivity measures. This is considered appropriate for ERP data and sufficiently immune to artifacts [35]. For functional connectivity analysis, 13 ROIs (shown in Fig. 6) were chosen based on the meta-analysis of the N-back task [36, 37]. During this, the “all voxels within the radius of 15 mm” option was chosen. Thus, the ROI consisted of all voxels within the predefined radius. These ROIs were compared statistically using an independent t test with unequal variance and 5,000 randomizations.
Fig. 6.
Fronto-parietal network with seed regions considered for WM process.
Results
SRT
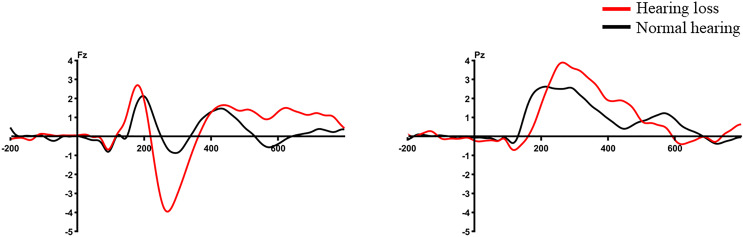
In electrophysiological measures, an independent-sample t test revealed no significant difference between the groups for P300 amplitude (t (24.906) = −1.433, p = 0.164), N100 amplitude (t (39) = 1.124, p = 0.268), and latencies (P300 latency: t (39) = 0.627, p = 0.534; N100 latency: t (39) = 1.270, p = 0.212). Similarly, the behavioral measures also showed no significant difference between the groups for RT (t (39) = 1.904, p = 0.64). However, as both groups had 100% accuracy, no statistical analysis was conducted. Figure 7 displays the averaged ERP waveform of the SRT test among both groups.
Fig. 7.
Averaged ERP waveform of SRT task at Fz and Pz electrode site.
N-Back Task
Behavioral Measures
Table 2 shows the mean RT in milliseconds and accuracy in the percentage for the behavioral measures among both groups. A 2 (groups) × 2 (memory load) repeated-measures ANOVA (RMANOVA) showed a significant main effect of group on RT (F(1, 39) = 31.723, p < 0.001, η2 = 0.449), where older individuals with hearing loss had longer RT than those with normal hearing. There was no main effect of memory load (F(1, 39) = 0.121, p = 0.730, η2 = 0.003) or interaction on RT (F(1, 39) = 0.103, p = 0.750, η2 = 0.003). Furthermore, an analysis of covariance was conducted while controlling the processing speed using simple RT. The results remained unchanged even after correcting for processing speed.
Table 2.
Mean RT (in milliseconds) and accuracy (in percentage) of behavioral measures
| Hearing loss | Normal hearing | |||
|---|---|---|---|---|
| RT, ms | accuracy, % | RT, ms | accuracy, % | |
| SRT | 276.99±23.9 | 100 | 261.75±27.2 | 100 |
| 1-Back | 687.19±157.7 | 93±14.4 | 503.49±6 | 91.85±9.3 |
| 2-Back | 686.96±148.6 | 46±14.5 | 517.89±83.3 | 76.06±8.2 |
Furthermore, similar to RT, 2 × 2 RMANOVA showed a significant main effect of memory load (F(1, 39) = 125.172, p < 0.001, η2 = 0.762) on accuracy, where the 1-back task had higher accuracy than the 2-back task. The significant main effect of group (F(1, 39) = 52.086, p < 0.001, η2 = 0.572) on accuracy revealed reduced accuracy in older individuals with hearing loss compared to their counterparts. In addition, there was also a significant interaction between the memory load and group (F(1, 39) = 14.298, p = 0.001, η2 = 0.268). Upon correction for processing speed, the significant main effect of the group (p < 0.001) and the interaction between memory load and the group remained unchanged (p = 0.002). However, the memory load showed no statistically significant difference (p = 0.185).
Electrophysiological Measures
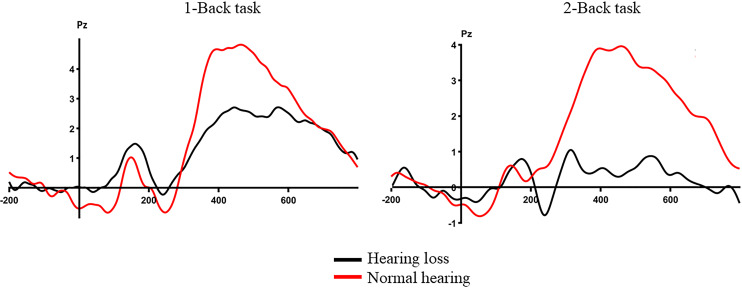
Table 3 displays the mean amplitude in microvolts and latency in milliseconds for the electrophysiological measures among both groups. A 2 (groups) × 2 (memory load) RMANOVA showed a significant main effect of group (F(1, 36) = 4.507, p < 0.001, η2 = 0.317) on amplitude, revealing a smaller P300 amplitude in the hearing loss group than in the normal hearing group. Similarly, a significant main effect of memory load (F(1, 36) = 4.857, p = 0.034, η2 = 0.119) on amplitude showed a larger P300 amplitude during the 1-back task than during the 2-back task. No significant interaction was reported between the group and memory load (F(1, 36) = 0.148, p = 0.703, η2 = 0.004). Furthermore, upon correcting for the processing speed, the significant main effect of the group remained unchanged (p = 0.001). However, the memory load showed no significance after correction. Figure 8 displays the averaged ERP waveform of the N-back task among both groups. In terms of P300 latency, 2 × 2 RMANOVA revealed no significant main effect of group (F(1, 36) = 0.255, p = 0.617, η2 = 0.007), memory load (F(1, 36) = 0.231, p = 0.634, η2 = 0.006), or group x memory load interaction (F(1, 36) = 0.784, p = 0.382, η2 = 0.021) on P300 latency.
Table 3.
Mean amplitude (in microvolts) and peak latency (in milliseconds) of electrophysiological measures
| Hearing loss | Normal hearing | |||
|---|---|---|---|---|
| amplitude, µV | latency, ms | amplitude, µV | latency, ms | |
| SRT N100 | −1.42±0.97 | 141.14±12.81 | −1.84±1.40 | 136.2±12.06 |
| SRT P300 | 3.56±2.07 | 455.52±97.44 | 2.99±1.86 | 436.6±95.69 |
| 1-Back P300 | 2.64±1.75 | 435.14±94.99 | 4.45±2.20 | 449.75±90.20 |
| 2-Back P300 | 1.74±0.86 | 448.33±117.32 | 3.60±2.13 | 441.15±84.74 |
Fig. 8.
Averaged ERP waveform of the N-back task at the Pz electrode site.
sLORETA Results
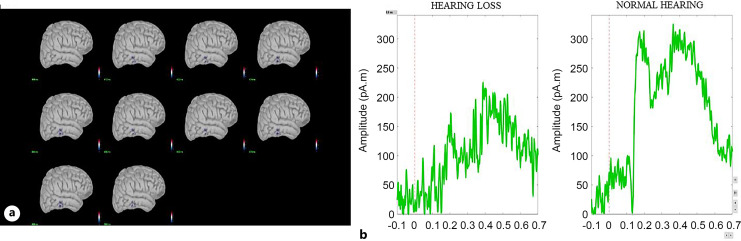
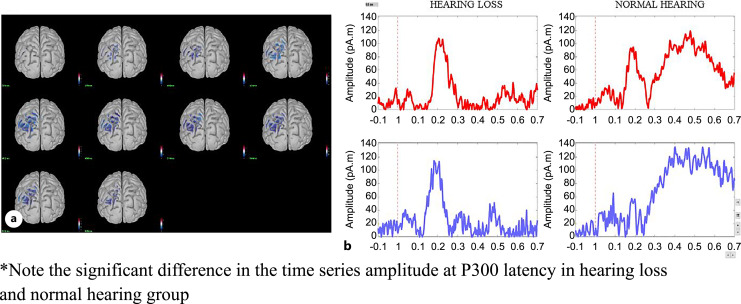
During the 1-back task, cortical activity was significantly reduced in the inferior and middle temporal lobes (p < 0.05). During the 2-back task, activity reduction was observed in the inferior and superior parietal regions (p < 0.05). Figures 9 and 10 show the cortical areas with significant differences and the corresponding time series for the 1-back and 2-back tasks, respectively.
Fig. 9.
a Cortical activity showing difference at the middle and inferior temporal lobe during the 1-back test. The activity shown is at the latency of the P300 peak in the right hemisphere. b Time series of the respective cortical activation of both hearing loss and normal hearing groups.
Fig. 10.
a Cortical activity showing difference at the superior and inferior parietal lobe during the 2-back test. The activity shown is at the latency of the P300 peak in the left hemisphere. b Time series of the respective cortical activation of both hearing loss and normal hearing groups.
Connectivity Analysis
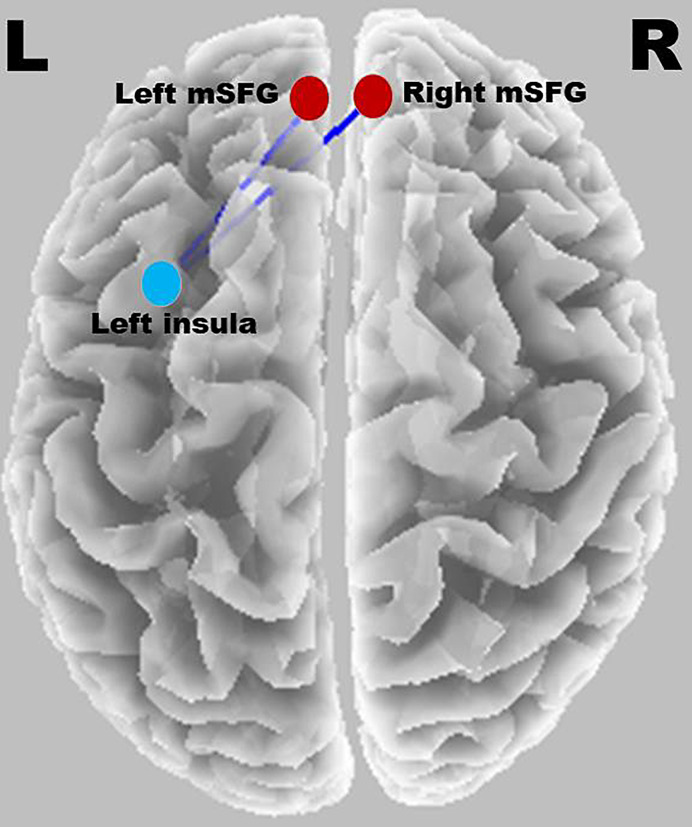
The threshold for significance was t = 4.473, corresponding to p < 0.05. In older individuals with hearing loss, significantly decreased lagged linear connectivity was observed in the gamma band between the superior frontal gyrus (SFG) (both right and left SFG) and left insula. Figure 11 shows a significant connection between the SFG and insula during the 1-back task. No significant differences were observed between other ROIs.
Fig. 11.
Significant connection between SFG and insula during the 1-back task.
Correlation Analysis
Table 4 shows the correlation between SNR loss, PTA, and N-back scores (both behavioral and electrophysiological measures) using Pearson’s correlation. The results revealed a significant positive correlation between RT, PTA, and SNR loss (p < 0.001) in the 1-back and 2-back tasks. SNR loss and PTA were also negatively correlated with accuracy and P300 amplitude in the 1-back and 2-back tasks.
Table 4.
Correlation between SNR loss, PTA, and N-back task scores
| PTA | 1-Back task | 2-Back task | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P300 amplitude | P300 latency | RT | accuracy | P300 amplitude | P300 latency | RT | accuracy | ||
| SNR loss | 0.867** | −0.520** | −0.111 | 0.552** | −0.506** | −0.423** | −0.062 | 0.537** | −0.817** |
| PTA | 1 | −0.425** | −0.123 | 0.649** | −0.498** | −0.487** | 0.054 | 0.515** | −0.830** |
**Significant at the level of 0.001.
Overall, the study results revealed that individuals with ARHL had significantly lower accuracy, longer RTs, and smaller P300 amplitude compared to those who did not have ARHL, even after accounting for general slowing. Individuals with ARHL experience a compromised neural activity, mainly in the temporal and parietal regions, which are crucial for cognition and WM. Moreover, individuals with ARHL had poor communication between the superior temporal gyrus and insulae regions among the brain regions mediating WM during the 1-back task. Lastly, the study discovered a strong correlation between hearing measures and WM results.
Discussion
The current study investigates the effect of ARHL on WM using ERPs. Overall, the results of the current study show reduced WM in individuals with ARHL compared to their matched controls without hearing loss. This is evidenced by the ERP results that showed significantly smaller P300 amplitudes in ARHL, consistent with longer RTs and poorer accuracy in behavioral measures. These differences persisted even after controlling for age, education, and general slowing. These findings align with previous studies on WM deficits in ARHL [17–25], confirming the effect of ARHL on cognition.
The current study is the first attempt to explore the effect of ARHL on WM changes using ERPs during visual N-back tasks. The lower P3 amplitude in individuals with ARHL suggests that degraded auditory input may weaken neural activation [38, 39] and synchronization of cortical networks involved in higher-order stimulus processing [40]. Furthermore, these changes would result in difficulty in the brain allocating cognitive resources effectively. This reallocation of resources affects the encoding and maintenance of information in WM. As a result, tasks requiring WM may be compromised [41–43]. Although the stimulus encoding of the target in cognitive potentials occurs at a similar time, classifying them as targets takes longer. This explains the reason for longer RT and reduced cognitive potentials in individuals with hearing loss, with similar P300 latency across the groups.
Our study found no significant difference in WM performance between both groups across memory loads (1-back vs. 2-back). Increased memory load (i.e., 2-back test) trended toward poorer performance, but after correcting for processing speed, the effect of memory load was nullified. The literature suggests that elderly individuals show a reduced dynamic range of cognitive function due to limited resources [44]. Hence, it could be the saturation in terms of cognitive resources. This does not result in the effect of memory load.
In individuals with ARHL, there was a noticeable decrease in amplitude during the 350–600-millisecond latency range (P300 latency), as shown in Figures 9 and 10. This difference in amplitude was mainly observed in the parietal and temporal regions, depending on the task difficulty. Previous studies have indicated parietal and cingulate cortex engagement during N-back tasks, regardless of age [45].
To further understand the cognitive mechanisms involved, we used source estimation analysis. The sLORETA results showed that during the 1-back task, these individuals displayed less neural activity in the inferior and middle temporal lobes, associated with auditory processing and memory. Similarly, during the 2-back task, there was a decrease in neural activity in the inferior and superior parietal lobes, which are linked to attention and WM [46, 47]. The decreased activity in the temporal and parietal lobes supports previous research highlighting the significance of intact sensory input for optimal cognitive functioning [42]. These results also imply that hearing loss could lead to changes in neural activities, which can be attributed to altered resource allocation in individuals with hearing loss and may affect the cognitive abilities of older people.
Reduced activation in temporal and parietal regions suggests a link between ERP differences and poor cortical excitation. These regions are vital for cognition and WM. Various studies have shown that different regions mediate WM function as the difficulty changes [46, 48]. In ARHL, both 1-back and 2-back tasks had difficulty stemming from different brain regions.
Functional connectivity results suggest that this difference during WM tasks in ARHL was mainly due to gamma-band oscillations, which are highly linked to cognitive and memory deficits and neurodegenerative diseases such as Alzheimer’s disease [49, 50]. The current study showed that the gamma band oscillation deficit was mainly seen as poor communication between the superior temporal gyrus and insula regions among the brain regions mediating WM during the 1-back task.
The insula and SFG are vital for cognitive functions such as decision-making, attentional control, and sensory processing [51–53]. Their communication is crucial for higher-order processes and positively correlates with cognition. No significant differences in brain connectivity were found during the 2-back tasks. This could be due to a poor dynamic range, as seen in the ERP results.
The correlation analysis revealed a positive correlation between the hearing sensitivity measures (SNR loss and PTA) and behavioral RT. There was a negative correlation between hearing measures and P300 amplitude as well as accuracy. The literature shows poor WM scores in individuals with hearing loss [23–25]. Considering SNR loss and PTA across four frequencies would further provide more insights into the functional deficits individuals with ARHL face. The results of the study suggest poor WM in ARHL, attributing the consequences of neural alterations and poor synchronization. Thus, ERPs could serve as a neural marker for WM deficits in individuals with ARHL.
Additionally, the study included two groups that differed only in hearing sensitivity and controlled for their general processing speed, age, and education. This provides an added advantage to the study. By controlling for significant confounders, the noticeable difference observed between the two groups could be attributed solely to the impact of hearing loss in older individuals.
Overall, the study findings suggest that individuals with ARHL have impaired WM compared to those with normal hearing. This indicates a potential link between ARHL and cognitive decline, which could significantly affect daily life and quality of life. The widely used WM test with simultaneous EEG recording and source estimation analysis would further validate the study’s usefulness in assessing WM in this population.
The implications extend beyond audiology, emphasizing the importance of addressing hearing loss as a potential risk factor for cognitive decline in older adults. Early intervention using hearing aids or other assistive devices may hold promise in mitigating cognitive decline associated with presbycusis. In summary, the study sheds light on the negative consequences of hearing loss on WM in older individuals. Comprehensive assessments and interventions are necessary to address the sensory and cognitive aspects of ARHL. By better understanding the cognitive consequences of presbycusis, targeted interventions can enhance cognitive function and improve the overall well-being of older adults.
Limitations
However, the participants were matched with major confounders like age and education and controlled for psychological neurological disorders. However, sample size and other uncontrolled factors like systematic diseases have not been controlled in the current study.
Statement of Ethics
The study was approved by the Institutional Ethics Committee of Kasturba Medical College and Hospital (Registration No. ECR/146/Inst/KA/2013/RR-16). The approval number is IEC Project No. 465/2019. It is registered with the Clinical Trials Registry of India, with registration number CTRI/2019/08/020784. All participants provided written informed consent prior to the recruitment.
Conflict of Interest Statement
No potential conflict of interest was reported by the author(s).
Funding Sources
The current research received no specific grants from funding agencies or other organizations.
Author Contributions
Sankalpa Madashetty: conceptualization, methodology, paradigm creation and validation, formal analysis, investigation, resources, and writing – original draft; Hari Prakash Palaniswamy: conceptualization, methodology, paradigm creation and validation, formal analysis, resources, writing – original draft, visualization, and supervision; and Bellur Rajashekhar: conceptualization, methodology, resources, writing – original draft, visualization, and supervision.
Funding Statement
The current research received no specific grants from funding agencies or other organizations.
Data Availability Statement
The data supporting the findings of the study are available from the corresponding author upon request due to privacy concerns. Further inquiries can be directed to the corresponding author.
References
- 1. Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore longitudinal study of aging. Neuropsychology. 2011;25(6):763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin FR, Yaffe K, Xia J, Xue Q-L, Harris TB, Purchase-Helzner E, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(2):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uchida Y, Sugiura S, Nishita Y, Saji N, Sone M, Ueda H. Age-related hearing loss and cognitive decline - the potential mechanisms linking the two. Auris Nasus Larynx. 2019;46(1):1–9. [DOI] [PubMed] [Google Scholar]
- 5. Powell DS, Oh ES, Reed NS, Lin FR, Deal JA. Hearing loss and cognition: what we know and where we need to go. Front Aging Neurosci. 2021;13:769405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kar BR, Kenderla PK. Working memory and executive attention: insights from developmental studies and implications for learning and education. J Indian Inst Sci. 2017;97(4):497–510. [Google Scholar]
- 7. Zekveld AA, Rudner M, Johnsrude IS, Ronnberg J. The effects of working memory capacity and semantic cues on the intelligibility of speech in noise. J Acoust Soc Am. 2013;134(3):2225–34. [DOI] [PubMed] [Google Scholar]
- 8. Lad M, Holmes E, Chu A, Griffiths TD. Speech-in-noise detection is related to auditory working memory precision for frequency. Sci Rep. 2020;10(1):13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ray J, Popli G, Fell G. Association of cognition and age-related hearing impairment in the English longitudinal study of ageing. JAMA Otolaryngol Head Neck Surg. 2018;144(10):876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swain SK. Age related hearing loss and cognitive impairment - a current perspective. Int J Res Med Sci. 2020;9(1):317. [Google Scholar]
- 11. Sharma RK, Chern A, Golub JS. Age-related hearing loss and the development of cognitive impairment and late-life depression: a scoping overview. Semin Hear. 2021;42(1):10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fellinger J, Holzinger D, Gerich J, Goldberg D. Mental distress and quality of life in the hard of hearing. Acta Psychiatr Scand. 2007;115(3):243–5. [DOI] [PubMed] [Google Scholar]
- 13. Mener DJ, Betz J, Genther DJ, Chen D, Lin FR. Hearing loss and depression in older adults. J Am Geriatr Soc. 2013;61(9):1627–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. [DOI] [PubMed] [Google Scholar]
- 15. Kirchner WKJJ. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352–8. [DOI] [PubMed] [Google Scholar]
- 16. Nowak K, Costa-Faidella J, Dacewicz A, Escera C, Szelag E. Altered event-related potentials and theta oscillations index auditory working memory deficits in healthy aging. Neurobiol Aging. 2021;108:1–15. [DOI] [PubMed] [Google Scholar]
- 17. Lunner T. Cognitive function in relation to hearing aid use. Int J Audiol. 2003;42(Suppl 1):S49–58. [DOI] [PubMed] [Google Scholar]
- 18. Foo C, Rudner M, Rönnberg J, Lunner T. Recognition of speech in noise with new hearing instrument compression release settings requires explicit cognitive storage and processing capacity. J Am Acad Audiol. 2007;18(7):618–31. [DOI] [PubMed] [Google Scholar]
- 19. Zekveld AA, Deijen JB, Goverts ST, Kramer SE. The relationship between nonverbal cognitive functions and hearing loss. 2007. [DOI] [PubMed] [Google Scholar]
- 20. Cervera TC, Soler MJ, Dasi C, Ruiz JC. Speech recognition and working memory capacity in young-elderly listeners: effects of hearing sensitivity. 2009. [DOI] [PubMed] [Google Scholar]
- 21. Janse E, Jesse A. Working memory affects older adults’ use of context in spoken-word recognition. Q J Exp Psychol. 2014;67(9):1842–62. [DOI] [PubMed] [Google Scholar]
- 22. Guerreiro MJ, Van Gerven PWM. Disregarding hearing loss leads to overestimation of age-related cognitive decline. Neurobiol Aging. 2017;56:180–9. [DOI] [PubMed] [Google Scholar]
- 23. Völter C, Götze L, Falkenstein M, Dazert S, Thomas JPJC. Application of a computer-based neurocognitive assessment battery in the elderly with and without hearing loss. 2017; p. 1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jayakody DM, Friedland PL, Eikelboom RH, Martins RN, Sohrabi HR. A novel study on association between untreated hearing loss and cognitive functions of older adults: baseline non-verbal cognitive assessment results. Clin Otolaryngol. 2018;43(1):182–91. [DOI] [PubMed] [Google Scholar]
- 25. Humes LE, Language RH. Longitudinal changes in auditory and cognitive function in middle-aged and older adults. J Speech Lang Hear Res. 2021;64(1):230–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bruce H, Aponte D, St-Onge N, Phillips N, Gagné JP, Li KZH. The effects of age and hearing loss on dual-task balance and listening. J Gerontol B Psychol Sci Soc Sci. 2019;74(2):275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nixon G, Sarant JZ, Tomlin D, Dowell R. The relationship between peripheral hearing loss and higher order listening function on cognition in older Australians. Int J Audiol. 2019;58(12):933–44. [DOI] [PubMed] [Google Scholar]
- 28. Lubitz AF, Niedeggen M, Feser M. Aging and working memory performance: electrophysiological correlates of high and low performing elderly. Neuropsychologia. 2017;106:42–51. [DOI] [PubMed] [Google Scholar]
- 29. Spironelli C, Carbone E, Borella E. Electrophysiological correlates of the categorization working memory span task in older adults. Behav Brain Res. 2020;393:112809. [DOI] [PubMed] [Google Scholar]
- 30. Pobric G, Taylor JR, Ramalingam HM, Pye E, Robinson L, Vassallo G, et al. Cognitive and electrophysiological correlates of working memory impairments in neurofibromatosis type 1. J Autism Dev Disord. 2022;52(4):1478–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Avinash M, Meti R, Kumar U. Development of sentences for quick speech-in-noise (QuickSIN) test in Kannada. J Indian Speech Hearing Assoc. 2010;24:59–65. [Google Scholar]
- 32. Psychology software tools, Inc. E-Prime 3.0. 2016. Available from: https://support.pstnet.com/. [Google Scholar]
- 33. Kuschpel MS, Liu S, Schad DJ, Heinzel S, Heinz A, Rapp MA. Differential effects of wakeful rest, music and video game playing on working memory performance in the n-back task. Front Psychol. 2015;6:1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 35. Shreekantiah Umesh D, Tikka SK, Goyal N, Nizamie SH, Sinha VK. Resting state theta band source distribution and functional connectivity in remitted schizophrenia. Neurosci Lett. 2016;630:199–202. [DOI] [PubMed] [Google Scholar]
- 36. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mencarelli L, Neri F, Momi D, Menardi A, Rossi S, Rossi A, et al. Stimuli, presentation modality, and load-specific brain activity patterns during n-back task. Hum Brain Mapp. 2019;40(13):3810–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Profant O, Balogova Z, Dezortova M, Wagnerova D, Hajek M, Syka J. Metabolic changes in the auditory cortex in presbycusis demonstrated by MR spectroscopy. Exp Gerontol. 2013;48(8):795–800. [DOI] [PubMed] [Google Scholar]
- 39. Profant O, Tintera J, Balogova Z, Ibrahim I, Jilek M, Syka J. Functional changes in the human auditory cortex in ageing. PLoS One. 2015;10(3):e0116692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cardin V. Effects of aging and adult-onset hearing loss on cortical auditory regions. Front Neurosci. 2016;10:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell J, Sharma A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci. 2013;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(Pt B):154–66. [DOI] [PubMed] [Google Scholar]
- 44. Rieck JR, DeSouza B, Baracchini G, Grady CL. Reduced modulation of BOLD variability as a function of cognitive load in healthy aging. Neurobiol Aging. 2022;112:215–30. [DOI] [PubMed] [Google Scholar]
- 45. Yaple ZA, Stevens WD, Arsalidou M. Meta-analyses of the n-back working memory task: fMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. Neuroimage. 2019;196:16–31. [DOI] [PubMed] [Google Scholar]
- 46. Cairo TA, Liddle PF, Woodward TS, Ngan ETC. The influence of working memory load on phase specific patterns of cortical activity. Brain Res Cogn Brain Res. 2004;21(3):377–87. [DOI] [PubMed] [Google Scholar]
- 47. Alain C, Shen D, Yu H, Grady C. Dissociable memory-and response-related activity in parietal cortex during auditory spatial working memory. Front Psychol. 2010;1:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Honey G, Fu CH, Kim J, Brammer MJ, Croudace T, Suckling J, et al. Effects of verbal working memory load on corticocortical connectivity modeled by path analysis of functional magnetic resonance imaging data. Neuroimage. 2002;17(2):573–82. [PubMed] [Google Scholar]
- 49. Nimmrich V, Draguhn A, Axmacher N. Neuronal network oscillations in neurodegenerative diseases. Neuromolecular Med. 2015;17(3):270–84. [DOI] [PubMed] [Google Scholar]
- 50. Sahu PP, Tseng P. Gamma sensory entrainment for cognitive improvement in neurodegenerative diseases: opportunities and challenges ahead. Front Integr Neurosci. 2023;17:1146687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5–6):435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. [DOI] [PubMed] [Google Scholar]
- 53. Qi J, Li BZ, Zhang Y, Pan B, Gao YH, Zhan H, et al. Altered insula-prefrontal functional connectivity correlates to decreased vigilant attention after total sleep deprivation. Sleep Med. 2021;84:187–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the study are available from the corresponding author upon request due to privacy concerns. Further inquiries can be directed to the corresponding author.