Abstract
For over a century, researchers have focused on how to optimize drug delivery. Systemic administration means that the drug becomes dilute and has the potential to diffuse to all tissues, which is only until the immune system steps in and rapidly clears it from blood circulation. Drug carriers are the solution for amplifying the intended effect and diminishing side effects. With drug carriers, tissue-specific drug delivery and controlled drug release is possible. Thus far, both synthetic and non-synthetic carriers exist. However, due to the numerous limitations of synthetic carriers, science has begun to concentrate on using live cells and cell-derivatives as drug carriers. The most problematic shortcomings of synthetic carriers are their limited biocompatibility and biodegradability. Most synthetic carriers are cytotoxic or induce immune responses. Moreover, synthetic carriers typically depend on passive diffusion and risk phagocytosis, further reducing their impact. On the other hand, live-cell carriers and their derivatives usually have a targeting mechanism and drug release is controlled, increasing the efficiency with which a drug accumulates and acts on a tissue. Still, both types of carriers face similar problems, including achieving high loading capacity, maintaining drug quality, efficiently accumulating in the target tissue, and minimizing side effects. This review aims to elucidate the advantages and disadvantages of each popular cell or cell-derived carrier and to spotlight novel solutions.
Keywords: Drug delivery, Immune cells, Red blood cells, Stem cells, Biomimetics, Exosomes
1. Introduction
As science and technology evolve, improving drug therapeutics, via the drug or the mechanism of delivery, has been a priority. Currently, existing drugs have the potential to effectively treat diseases but are unable to fulfill their function once they enter the patient. The drugs become degraded, cleared by the immune system, or are too dilute to be effective. Synthetic nanoparticles, such as micelles and liposomes, were the first type of effective drug carriers in which drug release could be controlled by the environment [1].
Micelles were–and despite their shortcomings, still are–attractive for hydrophobic drugs due to their ability to self-assemble in aqueous solutions into monolayer vesicles with hydrophobic cores. However, micelle formation requires a minimum concentration (critical micelle concentration; CMC) of amphiphilic polymers. When administered in vivo, the micelle concentration falls below the CMC and the micelles begin to disassemble, releasing their drug cargo. Therefore, researchers focused on using polymers that increase the stability of micelles–most of which are not biocompatible or biodegradable, negatively impacting healthy cells. This cytotoxic limitation [2–4], as well as potential immunological problems [2], can be overcome by using biocompatible and completely biodegradable materials [2,3,5,6]. Another shortcoming micelles have faced is their poor targeting ability [7] coupled with their tendency to be rapidly cleared from blood circulation via phagocytosis [8,9]. Their nanosize allows micelles to take advantage of the enhanced permeability and retention (EPR) effect to infiltrate and accumulate primarily in tumors, but this process is passive and micelles are still taken up by healthy cells. Different strategies have been applied to increase their targeting efficiency, such as relying on changing environments to activate micellar release of drugs [9] or adding specific cell-targeting ligands, namely mannose for cancer [8,10]. Even with the EPR effect and targeting ligands, clinical trials show nanoparticle accumulation in tumors is variable and, overall, disappointing [11,12].
On the other hand, liposomes are attractive for carrying hydrophilic drugs since they have bilayer membranes. Similar to micelles, liposomes face problems of cytotoxicity [4,13], inefficient delivery by the EPR effect [13,14], and rapid clearance by the immune system [5]. Unlike micelles, liposomes have in vivo stability, but liposome synthesis is typically expensive and complicated, and liposomes have poor storage stability [2,3]. Still, liposomes are widely used because their membranes can be modified with polymers, enzymes, and antibodies for targeting specificity, making them versatile structures [13,15–17]. They have been found to be effective in fighting general diseases through colonic delivery [16] and are used for cancers including breast [15] and colon [17] cancer. Polyethylene glycol (PEG) arose as a promising polymer capable of circumventing the immune system’s mononuclear phagocyte system (MPS) and increasing the duration of blood circulation [13,17,18]. However, PEG’s efficacy is limited since it is a foreign substance, resulting in the body eventually inducing an anti-PEG immune reaction after repeated dosing [8,13,18]. As a non-biodegradable polymer, PEG also poses a toxicity problem from potential tissue accumulation [8,13].
While synthetic vesicles were a good step towards developing effective drug delivery systems, their incompatibility produced as many problems as they solved. Therefore, researchers are investigating cells and their derivatives, discovering that the human body already provides delivery pathways waiting to be used to fulfill their potential. In this review, we explore each type of cell and cell-derived drug carrier, its benefits and risks, and how to mitigate the risks (Fig. 1).
Fig. 1.
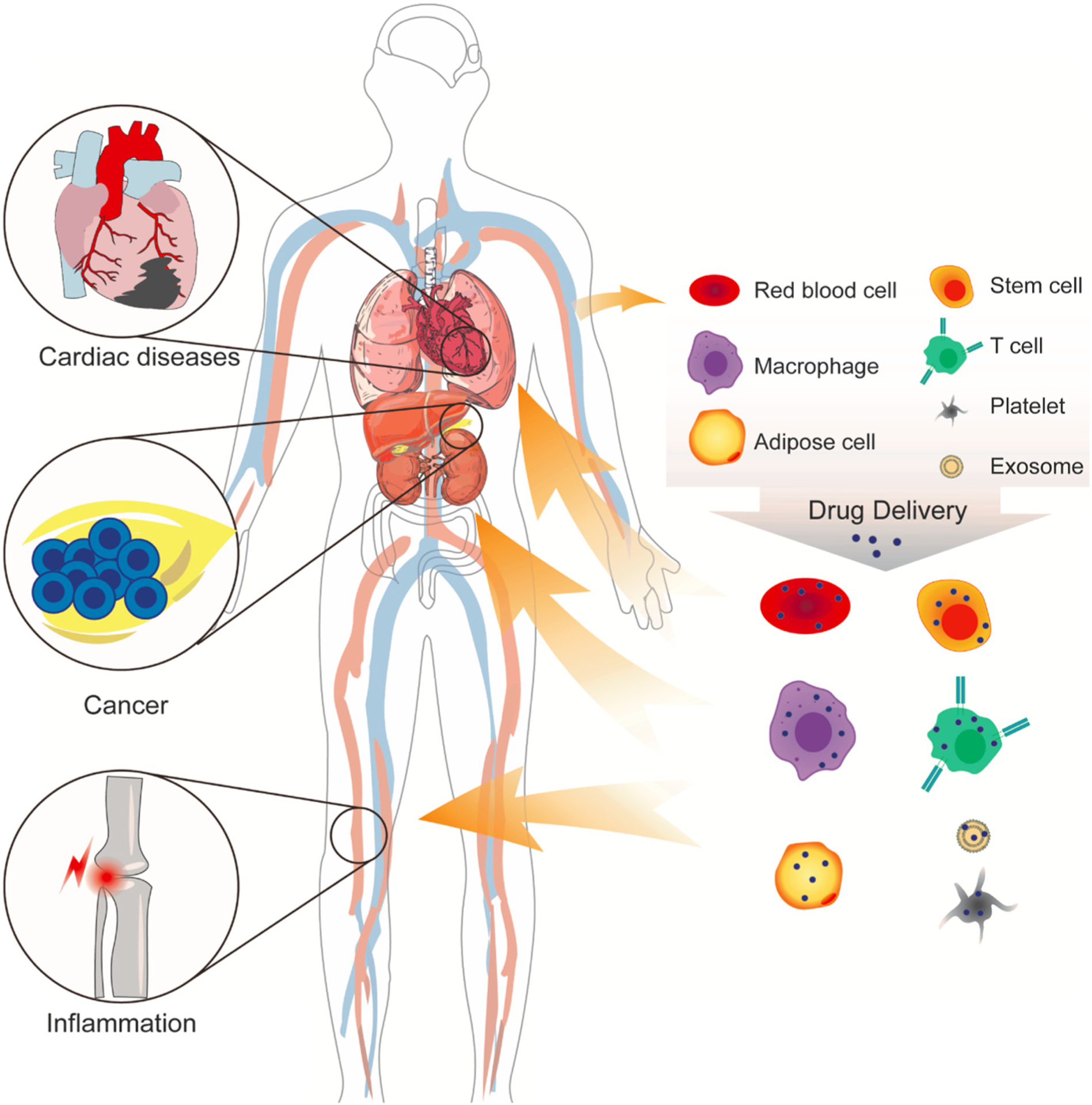
Currently, drug delivery focuses primarily on transporting cancer therapeutics. But each delivery mechanism is applicable to a variety of diseases, such as cardiovascular and inflammatory diseases.
2. Immune cells as drug carriers
For using live cells as drug carriers, immune cells were quickly identified as promising delivery systems. In order to effectively protect against pathogens, immune cells rely on intercellular signaling and fast migration. In addition, because immune cells compose the immune system, they will not induce an adverse immune response. Due to their attributes–superior biocompatibility [2], minimal interactions with normal cells [2], and the ability to actively target specific cells and sites [19]–immune cells are strong candidates as carriers.
2.1. Macrophages
Macrophages are a type of phagocytic white blood cells, most recognized for their role in traveling to and clearing an injury site of foreign debris and cells as part of the innate immune system. Their unique properties, including their ability to cross the blood-brain barrier (BBB) [20,21] and natural homing abilities [19], make them particularly attractive for targeting and treating neurodegenerative disorders [20], inflammatory diseases [20], and cancers [7,19]. Although, as a result of their dual ability to be both pro- and anti-inflammatory, in regards to cancer, macrophages pose the risk of supporting tumor proliferation and migration instead of eliminating tumor cells [14,22]. Still, natural phagocytosis and active migration in response to cell signaling signifies that drug uptake and drug transport would be greatly improved, especially in comparison to the passive diffusion by which micelles and liposomes are restricted. Macrophage status as immune cells reduces interactions with normal cells, thereby increasing the volume of and the rate at which the drug arrives at the target site.
Still, macrophages have shortcomings as delivery systems. Because macrophages are live cells, high loads of drugs or drugs in nanoparticles can interfere with cell survival, migration, and function, limiting the drug load [19,20]. Another drawback is that because macrophages are phagocytic, they do more than just uptake. Macrophages release enzymes and acid to kill and digest pathogens. Therefore, after taking up drugs and drug carriers, macrophages will quickly digest most drug carriers [19] and degrade drugs, reducing drug release and efficacy [20]. To counteract these characteristics, Klyachko et al. [20] created “cellular backpacks” that encapsulate drugs that adhere to macrophages. Although the drugs were successfully transported, backpacking covers the plasma membrane and functions such as signaling, adhesion, and migration are restricted [19]. Furthermore, there is a risk of the macrophages internalizing the backpacks. Taking advantage of macrophage endocytosis, Zhang et al. [19] created drug-loaded capsules designed to withstand macrophage enzymatic and oxidative degradation. Moreover, slow drug release from the capsules minimized adverse effects on macrophages, which allowed time for migration and drug delivery to the site of injury [19].
2.2. T cells
Part of the adaptive immune system, T cells naturally produce receptors for a large variety of antigens [23,24], with each T cell only presenting receptors to one type of antigen. In other words, each T cell is extremely specific, making T cells excellent targeting cells. When a cytotoxic T cell encounters its respective antigen, the T cell receptor (TCR) causes the cell surface reduction potential to increase [25], signaling the cell to secrete proteins that induce the antigen-presenting cell (APC) to die [23]. These combined features–cell specificity and induced apoptosis–make T cells very attractive for carrying drugs to target and eliminate tumors. As an immune cell, T cells are capable of crossing the BBB [20,26], giving them unrestricted access to diseases throughout the body, including HIV [23] and cancers such as melanoma [25] and glioblastoma [25,26].
Furthering their use, T cells can be reprogrammed and repurposed in order to modify antigen receptors [23,24] and secretions [25,26]. Cytotoxic T cells have been modified to display chimeric antigen receptors (CARs) on their membranes, which takes advantage of the T cell targeting and elimination pathways, but changes their original target to, for example, cancer cells evading the immune system [23,25–27]. Tang et al. [25] have designed “backpacking” nanogels that carry proteins on the tumor-targeting T cell membranes and only release the proteins when the CAR-T cell binds to the cancer cell and initiates T cell surface redox activity. They found that the release of the IL-15Sa protein from the nanogels promoted T cell expansion, resulting in faster tumor inhibition and clearance [25]. Jones et al. [23] had a similar backpacking design, but depended on a different step of the cytotoxic T cell mechanism: only when T cells secreted lytic granules would the backpacks release their cargo. CAR-T cells are an efficient system, but they can be too efficient, resulting in cytokine release syndrome or B cell aplasia–neither of which yet have sustainable solutions [27].
3. Blood cells as drug carriers
3.1. Platelets
Platelets are a type of red blood cell that prevents blood leakage from injured blood vessels by aggregating to form a clot. Therefore, platelets are able to precisely target specific sites and cells [2,3,28–30]. Coupled with their long lifespans, abundancy, high drug loading efficiencies, and immune system evasion, platelets are ideal drug carriers [2,3,5,31]. Moreover, a patient’s own platelets can be used for treatment [5]. Platelets have largely been used for wound healing [3,30,32], for hemostasis [2,3,33], to combat inflammation [2], and to treat vascular diseases such as lymphoma [2,3] and lung adenocarcinoma [5]. Xu et al. [2] demonstrated that platelets naturally release their cargo at faster rates in more acidic environments. Since cancerous tissues are more acidic than healthy tissues [9], drug release is controlled by the presence of tumor cells [6–8]. Metastatic cells naturally activate platelets such that platelets aggregate around tumor cells, helping them spread to new tissues through blood circulation [3,5,28]. Loading platelets with cancer therapeutics means that not only are tumors targeted, but also, tumors would not be able to metastasize. To further increase targeting efficiency, Xu et al. [3] conjugated platelets loaded with DOX to CD22 antibodies. CD22 is a marker for tumors, with the additional function of facilitating endocytosis [3]. In other words, platelets not only release DOX near tumor cells, but rather, platelets release DOX inside tumor cells, increasing potency [3].
3.2. Red blood cells and red blood cell mimics
Though carrying oxygen throughout the body is the most common attribute of red blood cells (RBCs), they have emerged as superior drug carriers. Exhibiting many of the same characteristics as immune cells – biocompatibility [2,3,34–38], biodegradability [2,18,36], and targeting ability [2,21]–RBCs have the advantage of already being identified as clinically safe for transfusions [34]. Furthermore, RBCs express CD47 on their cell surface, signaling to the immune system to avoid RBC uptake [35]. For the purpose of drug delivery, CD47 is beneficial for prolonging the circulation and efficacy of RBC drug cargo, which can be loaded onto the surface or inside RBCs [18,21,35–39]. The effects of loading RBCs with drugs is currently a matter of contention, with some studies indicating that regardless of the loading method, drug loading will unfavorably affect RBC durability [37] and others showing no effects at or below a nanoparticle-to-RBC ratio of 200:1 [39]. Still, RBCs have been shown to be effective carriers for alleviating symptoms of inflammation [34,35] and pulmonary embolism [34]. Brenner et al. [34] determined that red blood cells could become organ-targeted drug carriers based on the injection site.
Maintaining the advantage of the RBC membrane and its proteins, RBC mimics differ from parent cells only in their core, which is composed of a drug-encapsulated nanoparticle. So far, platelet mimics have been used in cancer [28], acute liver failure [40], and myocardial infarction [29] models, but it has the potential to be applied to other vascular diseases as well [29]. Su et al. [41] even captured cardiac stem cell secretome inside a platelet mimic for regenerative purposes. Having a platelet membrane makes the mimic biocompatible and has the natural homing ability of platelets [29,37,42,43]. The platelet membrane also allows for the flexibility to travel through capillaries smaller than the mimic [44] and for antibody conjugation to redirect their natural targeting [45]. The immunogenicity of the nanoparticle held within is minimized, which prolongs the blood circulation of the nanoparticles [28,31,40,42,46,]. The production of platelet mimics is fast, straightforward, and safe [29], and mimics have better stability in storage [41]. For now, there is not an identified delivery advantage of using mimics instead of the whole cell. Although, it would be noteworthy if high drug loading would become possible in the mimics, or if doing so would produce an adverse effect on the RBCs membranes.
4. Stem cells as drug carriers
Stem cells are undifferentiated cells with the potential to give rise to different types of cells and received attention as carriers due to their ability to survive in cancerous tissues and tolerate chemotherapeutic drugs [47]. As live cells, they are biodegradable and biocompatible [2,49]. Stem cells also have regenerative, immunomodulatory [4,47,50] and anti-inflammatory [47,51,52] properties. Furthermore, stem cells are able to target specific cells [2,14,47] based on chemotactic signals [53] and infiltrate specific tumor types [14]. In addition to being effective carriers, stem cells themselves are effective therapeutics due to the trophic factors they secrete [47,50,54]. Therefore, stem cells have been mainly used to regenerate disease-affected tissues and to combat cancer [47], including lung adenocarcinoma [14], glioblastoma [53], and leukemia [27].
However, using stem cells as therapeutic carriers introduces many problems from isolation to administration [40,50,51,55,56]. To prevent immunogenicity, autologous stem cells must be used [47,51,53]. The cells must be grown, stored, and survive transportation [40,50,56,57]. Administering stem cells by transplantation results in poor retention [29,41,45,52,58,59], and the transplantation process can cause infections [27]. Injecting stem cells intravenously means filtration by the lungs, reducing the amount that can travel to the target organ [40]. A risk posed by stem cells is tumorigenicity because they are actively proliferating cells [51,56,58].
Similar in concept to platelet mimics, biomimetic stem cells have nanoparticle cores covered by stem cell membranes and reflect the ability to evade the immune system [50,56] and the regenerative functions of stem cells [56]. Biomimetic stem cells are superior to stem cells in that they are more stable in vivo and in storage [56]. In addition, biomimetic stem cells are more standardized and do not pose the risk of tumorigenicity [50]. Tang et al. [56] tested the application of biomimetic stem cells in a myocardial infarction model.
5. Exosomes as drug carriers
Exosomes are extracellular vesicles, formed and released by cells, and were originally believed to merely be a cell’s waste disposal system. After the discovery that exosomes carry DNA, RNA, and proteins between cells, their role as intercellular messengers earned them the nickname of “natural nanoparticles” [4,57,59–63]. Created from a cell’s own membrane, exosomal membranes also contain the cell’s membrane proteins, along with some of the cell-specific functions [57,61,64]. As with cells, the exosomal membrane proteins can even be engineered to, for instance, display PD-1 receptors to interfere with cancer cell upregulation of PD-L1 and enable T cell infiltration into tumors [65]. The properties that bring attention to exosomes include their high availability [60], biocompatibility [4,60,63,66], cargo-protective membranes [50], and the ability to cross the BBB [60]. Exosomes overcome two major stem cell complications, tumorigenicity and graft-versus-host disease [4], while retaining all targeting and parent cell type advantages [61,67]. The variety and accessibility of exosomes allows for practically unlimited disease applications from cancer [4] to arthritis [55] to neurodegenerative disorders [60].
Additionally, even if allogenic, exosomes do not cause an adverse immune response [4,46,63,66], because exosomes are too small for the mononuclear phagocyte system (MPS) to remove [67]. This enables exosomes to act as a “cloaking” device for drugs [4]. One notable benefit of exosomes is that, compared to other drug carriers, exosomes have a relatively high loading capacity [4]. Also, Kim et al. [4] demonstrated that when paclitaxel-loaded exosomes were used to inhibit MDR tumors, the exosomes amplified the drug’s potency. Therefore, combining the high drug loading capacity with increased drug potency, exosomes are effective beyond simply just being drug carriers.
6. Adipocytes as drug carriers
Primarily known as fat cells, adipocytes store energy and secrete factors (adipokines) that regulate metabolic homeostasis [68,69]. Due to lipolysis and secretion of pro-inflammatory cytokines, adipocytes provide cancer cells leverage for developing into tumors and metastasizing [69,70]. Their abundance, biocompatibility, and close interactions with cancer cells makes adipocytes ideal for targeting cancers [69,70]. As adipocytes typically uptake lipids, drug-loading is largely limited to nonpolar drugs [69]. However, investigation into adipocytes for drug-loading and delivery is relatively new, with most research involving adipose tissue focused on targeting it, rather than using it to target. Wenet al. [70] have spearheaded the studies into using adipocytes as drug carriers, exploiting cancer cell-induced lipolysis for accelerated release chemotherapeutics rumenic acid (RA) and DOX prodrug [69]. Wenet al. [70] demonstrated that loading RA and DOX prodrug into adipocytes both increase the survival of B16F10 melanoma mice and reduce tumor recurrence in a tumor-resection model. Considering the prevalence of adipose tissue in the body and the far-reaching effects of adipocyte secretions, it would be worthwhile investigating the viability of adipocyte-mediated drug delivery as a treatment for other diseases.
7. Conclusion
Since the advent of therapeutic drugs, efficient carrier mechanisms have been examined to both improve drug delivery and reduce unwanted side effects. Each discovery and advance has far-reaching clinical impacts: although cancer and cardiovascular diseases are the most pertinent applications, drug delivery mechanisms to treat these diseases can be applied to a variety of diseases (Table 1). Already, exosomes have been shown effective as drug carriers for cancer [4], arthritis [55], and neurodegenerative diseases [60]. Live cells and cell derivatives have demonstrated superiority over synthetic nanocarriers [71,72]. They are naturally capable of unrestricted, actively targeted drug delivery with controlled drug release. Solely in terms of delivery, RBCs and immune cells hold the most promise as cell carriers, since they do not induce cytotoxicity or unfavorable immune system activity. The regenerative abilities of stem cells, though, cannot be ignored despite the risk of tumorigenicity. Exosomes and biomimetics have gained traction as optimized delivery systems, encapsulating the benefits of their parent cells without any of the associated hazards.
Table 1.
Publications using cells and cell derivatives as drug carriers.
| Reference | Cell carrier | Disease | Animal model |
|---|---|---|---|
| Brenner et al. 2018 [34] | Red blood cells | ARDS Pulmonary embolism |
C57BL/6 mouse; LPS i.t. C57BL/6 mouse; Plasma microemboli of fibrin clots i.v. |
| Wan et al. 2018 [35] | Red blood cells | Inflammatory diseases | C57BL/6 mouse |
| Klyachko et al. 2017 [20] | Macrophages | Encephalitis | C57/BL mouse; LPS intracranial |
| Zhang et al. 2018 [19] | Macrophages | Cancer (driven by inflammation) | U87MG tumor-bearing nude mouse |
| Xie et al. 2017 [7] | Macrophages | Melanoma | 1205Lu cell and WM35 cell |
| Xu et al. 2017 [2] | Platelets | B Cell lymphoma | Tumor-bearing BALB/c nude mouse |
| Xu et al. 2017 [3] | Platelets | B Cell lymphoma | Tumor-bearing BALB/c nude mouse |
| Sarkar et al. 2013 [5] | Platelets | Human lung adenocarcinoma | Swiss albino mouse; Ehrlich ascites carcinoma cells i.p. |
| Layek et al. 2018 [14] | Stem cells | Lung carcinoma | SCID beige mouse; A549 cell i.v.; C57BL/6 mouse; Lewis Lung Carcinoma cell i.v. |
| Bagó et al. 2017 [53] | Stem cells | Glioblastoma | Mouse; U87, GBM4, or GBM8 cells stereotactically implanted |
| Hu et al. 2018 [27] | Stem cells | Acute myeloid leukemia | C57BL/6 J mouse; C1498 or WEHI-3 cell i.v. |
| Kim et al. 2016 [4] | Exosomes | Lung carcinoma | C57BL/6 mouse; Lewis lung carcinoma cell i.v. |
| Wang et al. 2017 [32] | Exosomes | Osteoarthritis | C57BL/6 J mouse; Destabilization of medial meniscus surgery |
| Kojima et al. 2018 [60] | Exosomes | Parkinson’s | C57BL/6 J mouse; 6-OHDA solution intracerebral injection |
| Tang et al. 2018 [56] | T Cells | Solid cancers (melanoma and glioblastoma) | C57BL/6 mouse; B16F10 cell s.c. |
| Jones et al. 2017 [23] | T Cells | HIV | NSG mouse; CXCR4-tropic HIV molecular clone LAI i.p. |
| Pohl-Guimarães et al. 2019 [26] | T Cells | Brain tumors | C57BL/6 mouse; B16F10 OVA cell implant |
| Tang et al. 2017 [57] | Synthetic stem cells | Myocardial Infarction | SCID beige mouse; LAD artery ligation |
| Luo et al. 2017 [50] | Synthetic stem cells | Acute myocardial infarction | SCID beige mouse; LAD artery ligation |
| Hu et al. 2015 [28] | RBC mimics/nanovesicles |
Cancer (general) | Nude mouse; MDA-MB-231 cell s.c. |
| Tang et al. 2018 [52] | RBC mimics/nanovesicles |
Myocardial infarction | WKY rat; LAD artery ligation, followed by reperfusion; adult farm pig; LAD coronary balloon occlusion |
| Liang et al. 2018 [40] | RBC mimics/nanovesicles |
Acute liver failure | C57BL/6 mouse; Carbon tetrachloride i.p. |
i.p., intraperitoneal; i.t., intratracheal; i.v., intravenous; s.c., subcutaneous; LAD, left anterior descending.
Funding
This study was supported by the U.S. National Institutes of Health (HL123920, HL137093, HL144002, HL146153, HL147357).
Footnotes
Declaration of competing interest
The authors declare that there are no conflicts of interest.
References
- [1].Rezaie HR, Esnaashary M, Arjmand AA, Öchsner A. The history of drug delivery systems. A review of biomaterials and their applications in drug delivery, SpringerBriefs in applied sciences and technology. Singapore: Springer; 2018. p. 1–8. [Google Scholar]
- [2].Xu P, Zuo H, Chen B, Wang R, Ahmed A, Hu Y, et al. Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci Rep 2017;7. 10.1038/srep42632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu P, Zuo H, Zhou R, Wang F, Liu X, Ouyang J, et al. Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: a novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget 2017;8:58322–37. 10.18632/oncotarget.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in Cancer cells. Nanomedicine 2016;12:655–64. 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sarkar S, Alam MA, Shaw J, Dasgupta AK. Drug delivery using platelet cancer cell interaction. Pharm Res 2013;30. 10.1007/s11095-013-1097-1. [DOI] [PubMed] [Google Scholar]
- [6].Shu Y, Yin H, Rajabi M, Li H, Viewegera M, Guo S, et al. RNA-based micelles: a novel platform for paclitaxel loading and delivery. J Control Release 2018;276:17–29. 10.1016/j.jconrel.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xie Z, Su Y, Kim GB, Selvi E, Ma C, Aragon-Sanabria V, et al. Immune cell-mediated biodegradable theranostic nanoparticles for melanoma targeting and drug delivery. Small 2017;13. 10.1002/smll.201603121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yin L, Chen Y, Zhang Z, Yin Q, Zheng N, Cheng J. Biodegradable micelles capable of mannose-mediated targeted drug delivery to cancer cells. Macromol Rapid Commun 2015;36:483–9. 10.1002/marc.201400650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guo X, Wang L, Duval K, Fan J, Zhou S, Chen Z. Dimeric drug polymeric micelles with acid-active tumor targeting and FRET-traceable drug release. Adv Mater 2018;30. 10.1002/adma.201705436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu DR, Guan QL, Gao MT, Jiang L, Kang HX. Mannose receptor as a potential biomarker for gastric cancer: a pilot study. Int J Biol Markers 2017;32:e278–83. 10.5301/jbm.5000244. [DOI] [PubMed] [Google Scholar]
- [11].Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv 2018;130:17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Natfji AA, Rabishankar D, Osborn HMI, Greco F. Parameters affecting the endhanced permeability and retention effect: the need for patient selection. J Pharm Sci 2017; 106:3179–87. [DOI] [PubMed] [Google Scholar]
- [13].Lane RS, Haller FM, Chavaroche AAE, Almond A, DeAngelis PL. Heparosan-coated liposomes for drug delivery. Glycobiology 2017;27:1062–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Layek B, Sadhukha T, Panyam J, Prabha S. Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Mol Cancer Ther 2018;17:1196–206. 10.1158/1535-7163.MCT-17-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim MW, Niidome T, Lee R. Glycol chitosan-docosahexaenoic acid liposomes for drug delivery: synergistic effect of doxorubicin-rapamycin in drug-resistant breast cancer. Mar Drugs 2019;17. 10.3390/md17100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Leo V, Milano F, Mancini E, Comparelli R, Giotta L, Nacci A, et al. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules 2018;23. 10.3390/molecules23040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao J, Li C, Wei X, Tu M, Zhang Y, Xu F, et al. Selective targeting and eradication of LGR5+ Cancer stem cells using RSPO-conjugated doxorubicin liposomes. Mol Cancer Ther 2018;17:1475–85. 10.1158/1535-7163.MCT-17-0694. [DOI] [PubMed] [Google Scholar]
- [18].Leuzzi V, Rossi L, Gabucci C, Nardecchia F, Magnani M. Erythrocyte-mediated delivery of recombinant enzymes. J Inherit Metab Dis 2016;39:519–30. 10.1007/s10545-016-9926-0. [DOI] [PubMed] [Google Scholar]
- [19].Zhang W, Wang M, Tang W, Wen R, Zhou S, Lee C, et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv Mater 2018;30:e1805557. 10.1002/adma.201805557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Klyachko NL, Polakd R, Haneya MJ, Zhao Y, Gomes Neto RJ, Hill MC, et al. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials 2017;140:79–87. 10.1016/j.biomaterials.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xia J, Wang Z, Yan Y, Cheng Z, Sun L, Li Y, et al. Catalase-laden microdevices for cell-mediated enzyme delivery. Langmuir 2016;32:13386–93. 10.1021/acs.langmuir.6b03160. [DOI] [PubMed] [Google Scholar]
- [22].Aizik G, Grad E, Golomb G. Monocyte-mediated drug delivery systems for the treatment of cardiovascular diseases. Drug Deliv Transl Res 2018;8:868–82. 10.1007/s13346-017-0431-2. [DOI] [PubMed] [Google Scholar]
- [23].Jones RB, Mueller S, Kumari S, Vrbanac V, Genel S, Tager A, et al. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials 2017;117:44–53. 10.1016/j.biomaterials.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kooreman NG, Kim Y, de Almeida PE, Termglinchan V, Diecke S, Shao NY, et al. Autologous iPSC-based vaccines elicit anti-tumor responses in vivo. Cell Stem Cell 2018;22: 501–13. 10.1016/j.stem.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tang L, Zheng Y, Melo MB, Mabardi L, Castaño AP, Xie YQ, et al. Enhancing T cell therapy through TCR signaling-responsive nanoparticle drug delivery. Nat Biotechnol 2018; 36:707–16. 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pohl-Guimarães F, Yang C, Dyson KA, Wildes TJ, Drake J, Huang J, et al. RNA-modified T cells mediate effective delivery of immunomodulatory cytokines to brain tumors. Mol Ther 2019;27:837–49. 10.1016/j.ymthe.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hu Q, Sun W, Wang J, Ruan H, Zhang X, Ye Y, et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat Biomed Eng 2018;2:831–40. 10.1038/s41551-018-0310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater 2015;27:7043–50. 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tang J, Su T, Huang K, Dinh PU, Wang Z, Vandergriff A, et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng 2018;2:17–26. 10.1038/s41551-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang X, Wang J, Chen Z, Hu Q, Wang C, Yan J, et al. Engineering PD-1-presenting platelets for cancer immunotherapy. Nano Lett 2018;18:5716–25. [DOI] [PubMed] [Google Scholar]
- [31].Lu Y, Hu Q, Jiang C, Gu Z. Platelet for drug delivery. Curr Opin Biotechnol 2019;58: 81–91. [DOI] [PubMed] [Google Scholar]
- [32].Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat Biomed Eng 2017;1:1–10. [Google Scholar]
- [33].Li Z, Hu S, Cheng K. Platelets and their biomimetics for regenerative medicine and cancer therapies. J Mater Chem B 2018;6:7354–65. 10.1039/C8TB02301H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P, et al. Nat Commun 2018;9. 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wan X, Zhang S, Wang F, Fan W, Wu C, Mao K, et al. Red blood cell-derived nanovesicles for safe and efficient macrophage-targeted drug delivery in vivo. Biomater Sci 2018;7:187–95. 10.1039/c8bm01258j. [DOI] [PubMed] [Google Scholar]
- [36].Biagiotti S, Paoletti MF, Fraternale A, Rossi L, Magnani M. Drug delivery by red blood cells. IUBMB Life 2011;63:621–31. 10.1002/iub.478. [DOI] [PubMed] [Google Scholar]
- [37].Villa CH, Cines DB, Siegel DL, Muzykantov V. Erythrocytes as carriers for drug delivery in blood transfusion and beyond. Transfus Med Rev 2017;31:26–35. 10.1016/j.tmrv.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang X, Qiu M, Guo P, Lian Y, Xu E, Su J. Autologous red blood cell delivery of betamethasone phosphate sodium for long anti-inflammation. Pharmaceutics 2018; 10. 10.3390/pharmaceutics10040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pan D, Vargas-Morales O, Zern B, Anselmo AC, Gupta V, Zakrewsky M, et al. The effect of polymeric nanoparticles on biocompatibility of carrier red blood cells. PLoS One 2016;11. 10.1371/journal.pone.0152074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liang H, Huang K, Su T, Li Z, Hu S, Dinh PU, et al. Mesenchymal stem cell/red blood cell-inspired nanoparticle therapy in mice with carbon tetrachloride-induced acute liver failure. ACS Nano 2018;12:6536–44. 10.1021/acsnano.8b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Su T, Huang K, Ma H, Liang H, Dinh PU, Chen J, et al. Platelet-inspired nanocells for targeted heart repair after ischemia/reperfusion injury, advanced functional. Mater 2018;29. 10.1002/adfm.201803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release 2015;220:600–7. 10.1016/j.jconrel.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Luk BT, Jiang Y, Copp JA, Hu CJ, Krishnan N, Gao W, et al. Biomimetic targeting of nanoparticles to immune cell subsets via cognate antigen interactions. Mol Pharm 2018;15:3723–8. 10.1021/acs.molpharmaceut.8b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S. Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci U S A 2009;106:21495–9. 10.1073/pnas.0907127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shen D, Li Z, Hu S, Huang K, Su T, Liang H, et al. Antibody-armed platelets for the regenerative targeting of endogenous stem cells. Nano Lett 2019;19:1883–91. 10.1021/acs.nanolett.8b04970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li B, Wang F, Gui L, He Q, Yao Y, Chen H. The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine (Lond) 2018;13:2099–118. 10.2217/nnm-2018-0017. [DOI] [PubMed] [Google Scholar]
- [47].Salehi H, Al-Arag S, Middendorp E, Gergely C, Cuisinier F, Orti V. Dental pulp stem cells used to deliver the anticancer drug paclitaxel. Stem Cell Res Ther 2018;9:103. 10.1186/s13287-018-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhao J, Vykoukal J, Abdelsalam M, Recio-Boiles A, Huang Q, Qiao Y, et al. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology 2014;25:405101. 10.1088/0957-4484/25/40/405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Luo L, Tang J, Nishi K, Yan C, Dinh PU, Cores J, et al. Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ Res 2017; 120:1768–75. 10.1161/CIRCRESAHA.116.310374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Huang K, Li Z, Su T, Shen D, Hu S, Cheng K. Bispecific antibody therapy for effective cardiac repair through redirection of endogenous stem cells. Advanced Therapeutics 2019; 2. 10.1002/adtp.201900009. [DOI] [Google Scholar]
- [52].Tang J, Wang J, Huang K, Ye Y, Su T, Qiao L, et al. Cardiac cell-integrated microneedle patch for treating myocardial infarction. Sci Adv 2018;4 (eaat 9365). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bagó JR, Okolie O, Dumitru R, Ewend MG, Parker JS, Werff RV, et al. Tumor-homing cytotoxic human induced neural stem cells for cancer therapy. Sci Transl Med 2017;9. 10.1126/scitranslmed.aah6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xia J, Tsai AC, Cheng W, Yuan X, Ma T, Guan J. Development of a microdevice-based human mesenchymal stem cell-mediated drug delivery system. Biomater Sci 2019;7: 2348–57. 10.1039/c8bm01634h. [DOI] [PubMed] [Google Scholar]
- [55].Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther 2017;8:189. 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tang J, Shen D, Caranasos TG, Wang Z, Vandergriff AC, Allen TA, et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun 2017;8:13724. 10.1038/ncomms13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Qiao L, Hu S, Liu S, Zhang H, Ma H, Huang K, et al. microRNA-21–5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest 2019;129:2237–50. 10.1172/JCI123135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu F, Hu S, Yang H, Li Z, Huang K, Su T, et al. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of asherman’s syndrome. Adv Healthc Mater 2019;8. 10.1002/adhm.201900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 2018;8:1869–78. 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun 2018;9:1305. 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano 2019. 10.1021/acsnano.9b04384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res 2019. 10.1093/cvr/cvz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang D, Lee H, Wang X, Rai A, Groot M, Jin Y. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther 2018;26: 2119–30. 10.1016/j.ymthe.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sabu C, Rejo C, Kotta S, Pramod K. Bioinspired and biomimetic systems for advanced drug and gene delivery. J Control Release 2018;287:142–55. 10.1016/j.jconrel.2018.08.033. [DOI] [PubMed] [Google Scholar]
- [65].Zhang X, Wang C, Wang J, Hu Q, Langworthy B, Ye Y, et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv Mater 2018;30. [DOI] [PubMed] [Google Scholar]
- [66].Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun 2018;9:2359. 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shahabipour F, Barati N, Johnston TP, Derosa G, Maffioli P, Sahebkar A. Exosomes: Nanoparticulate tools for RNA interference and drug delivery. J Cell Physiol 2017; 232:1660–8. 10.1002/jcp.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kim S, Moustaid-Moussa N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr 2000;130:3110S–5S. 10.1093/jn/130.12.3110S. [DOI] [PubMed] [Google Scholar]
- [69].Ye M, Gong S. Drug loaded adipocytes: sugar-coated bullets for cancer. Matter 2019;1: 1104–5. 10.1016/j.matt.2019.09.023. [DOI] [Google Scholar]
- [70].Wen D, Wang J, Driessche GVD, Chen Q, Zhang Y, Chen G, et al. Adipocytes as anticancer drug delivery depot. Matter 2019;1:1203–14. 10.1016/j.matt.2019.08.007. [DOI] [Google Scholar]
- [71].Li Z, Hu S, Cheng K. Chemical engineering of cell therapy for heart diseases. Acc Chem Res 2019;52:1687–96. 10.1021/acs.accounts.9b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hu S, Ogle BM, Cheng K. Body builder: from synthetic cells to engineered tissues. Curr Opin Cell Bio 2018;54:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


