Abstract
Coxsackievirus B3 (CVB3), an enterovirus in the family Picornaviridae, induces cytopathic changes in cell culture systems and directly injures multiple susceptible organs and tissues in vivo, including the myocardium, early after infection. Biochemical analysis of the cell death pathway in CVB3-infected HeLa cells demonstrated that the 32-kDa proform of caspase 3 is cleaved subsequent to the degenerative morphological changes seen in infected HeLa cells. Caspase activation assays confirm that the cleaved caspase 3 is proteolytically active. The caspase 3 substrates poly(ADP-ribose) polymerase, a DNA repair enzyme, and DNA fragmentation factor, a cytoplasmic inhibitor of an endonuclease responsible for DNA fragmentation, were degraded at 9 h following infection, yielding their characteristic cleavage fragments. Inhibition of caspase activation by benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (ZVAD.fmk) did not inhibit the virus-induced cytopathic effect, while inhibition of caspase activation by ZVAD.fmk in control apoptotic cells induced by treatment with the porphyrin photosensitizer benzoporphyrin derivative monoacid ring A and visible light inhibited the apoptotic phenotype. Caspase activation and cleavage of substrates may not be responsible for the characteristic cytopathic effect produced by picornavirus infection yet may be related to late-stage alterations of cellular homeostatic processes and structural integrity.
Coxsackievirus B3 (CVB3) is an enterovirus in the family Picornaviridae. Following binding to the coxsackievirus and adenovirus receptor (6, 64), the viral RNA enters the cytoplasm, where it is translated into a single polyprotein by the host translational machinery. The polyprotein is then proteolytically processed by viral proteases to produce all of the viral structural and nonstructural proteins. The virus-encoded RNA-dependent RNA polymerase transcribes negative-strand viral RNAs, which serve as templates for the synthesis of multiple progeny genomes. Following viral packaging, the virus is released, potentially under the influence of the virus-encoded 2B protein (67). During the replicative process and viral progeny release, the cytopathic effect (CPE) occurs and the host cell is injured, with eventual loss of viability.
Multiple host cellular processes are altered during picornavirus parasitization. Virus protein 2Apro directly cleaves eukaryotic initiation factor 4 gamma (eIF4G). Cleavage of this translation initiation factor not only abolishes cap-dependent mRNA translation (19); the cleavage products are believed to stimulate translation of uncapped mRNA, such as the noncellular picornavirus genome (43), which uses a novel internal ribosome entry mechanism to begin protein translation (31, 76). Poliovirus proteins 2Apro and 3Cpro have been shown to cleave the TATA-binding protein, with 3Cpro also shutting off transcription of RNA polymerases I, II, and III (11, 72, 74). The transcription factors TFIIIC (10), CREB (73), and Oct-1 (75) are also cleaved by 3Cpro during picornavirus infection. CVB3 protein 2B has been shown to modify endoplasmic reticulum and plasma membrane permeability (14), causing an increase in the cytosolic free calcium concentration (28, 67) and membrane lesions which may facilitate viral progeny release. Ionic gradients collapse (40, 52), and the phospholipase activity is altered (24, 29). CVB3 infection of HeLa cells results in tyrosine phosphorylation of two cellular proteins at 4 h postinfection, and inhibition of these phosphorylations significantly reduces viral progeny production (27). It is clear that infection is a dynamic cellular process in which timely interactions between viral and host proteins determine the outcome for both the virus and the host cells.
It is now clear that cysteine proteases in the caspase family of enzymes are key effector molecules in apoptotic cell death. Once activated, caspases cleave specific substrates, including poly(ADP-ribose) polymerase (PARP) (35), DNA fragmentation factor (DFF) (37), gelsolin (34), lamin A (58), sterol regulatory element-binding proteins (68), α-fodrin (12, 66), focal adhesion kinase (71), and mdm2 (18), among many others. Such cleavage events result in important alterations to normal homeostatic cellular processes and corresponding cell morphological-structural changes.
Many viruses possess biochemical mechanisms to evade and/or induce apoptosis in cells in which they reside (for reviews, see references 49 and 60). Different viruses interact at different stages of the apoptotic death pathway. Viruses have evolved strategies targeting the Fas ligand-Fas or tumor necrosis factor alpha (TNF-α)–TNF receptor signalling complex, the Bcl-2 family of regulators, or the caspase family of executioners (49, 60). The mechanisms of death of CVB3-infected cells remain to be determined; however, there is limited morphological evidence regarding the induction of apoptosis in picornavirus-infected cells. Evidence obtained with Theiler’s murine encephalitis virus (32, 65) and poliovirus (63) has indicated that picornavirus-infected cells undergo apoptosis, based on morphological criteria including nuclear condensation and DNA fragmentation.
To determine if caspases are activated and responsible for the CPE observed following CVB3 infection, HeLa cells (American Type Culture Collection, Rockville, Md.) were either infected, at a multiplicity of infection (MOI) of 5, with CVB3 (generously provided by Charles Gauntt, University of Texas Health Sciences Center, San Antonio) or sham treated with minimum essential medium (MEM) lacking fetal bovine serum (FBS) for 45 min. Cells were washed with phosphate-buffered saline (PBS), and complete MEM containing 10% FBS was then substituted. A positive apoptosis control consisted of HeLa cells treated with the photosensitizer benzoporphyrin derivative monoacid ring A (BPD-MA) for 1 h and then exposed to visible light as previously described (22, 23). Cultures were examined and harvested at 0, 1, 3, 5, 6, 7, 8, 9, 10, and 12 h postinfection. Cells were washed two times in cold PBS and lysed in 1 ml of lysis buffer (20 mM Tris [pH 8], 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 0.15 U of aprotinin per ml) per 75-cm2 culture area. After a 20-min incubation on ice, supernatants were collected following centrifugation at 10,000 × g and stored at −20°C for further biochemical analyses.
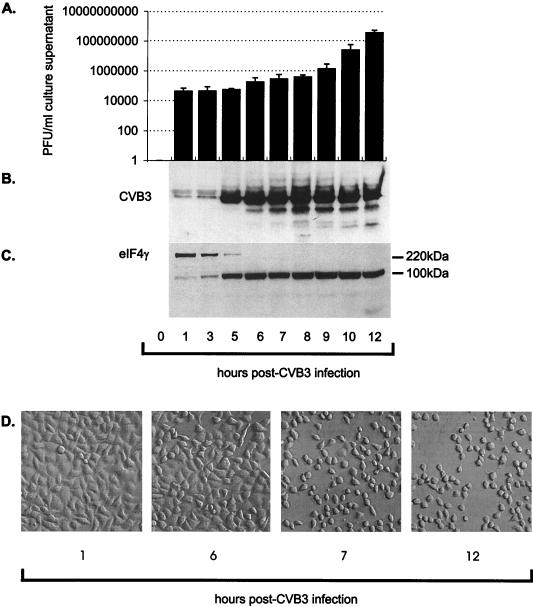
The temporal pattern of production of CVB3 viral proteins, progeny virus, and the evolution of HeLa cell degenerative morphological changes were considered in conjunction with an examination of host cell death proteins. Cell lysate samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose (Hybond ECL nitrocellulose membranes; Amersham). The membranes were incubated for 1 h at room temperature in blocking buffer (PBS with 0.1% Tween 20 and 5% powdered nonfat milk). Following two washings in wash buffer (PBS with 0.1% Tween 20), the membranes were incubated with antibody against CVB3 (rabbit polyclonal anti-CVB3, 1:1,000; Accurate Chemicals). The membranes were washed three times in wash buffer and incubated with a donkey anti-rabbit immunoglobulin secondary antibody (Amersham). The membranes were washed three times, and the horseradish peroxidase-conjugated secondary immunoglobulins were detected by the enhanced chemiluminescence method (ECL, Amersham) and exposed to Hyperfilm (Amersham) autoradiography film. Significant increases in viral protein synthesis could be detected between 3 and 5 h postinfection (Fig. 1B). The viral proteases cleave viral as well as host proteins early following infection. By immunoblot analysis with mouse monoclonal anti-eIF4G (1:1,000; Transduction Laboratories), it was found that eIF4G is cleaved by viral protease 2A beginning within 1 h postinfection, with further loss of detection of the 220-kDa protein by 5 h postinfection (Fig. 1C). The amount of CVB3 in the cell supernatant (released virus) was determined on monolayers of HeLa cells by the agar overlay plaque assay method as previously described (3). Briefly, sample supernatant was serially diluted 10-fold, the dilutions were overlaid on 90 to 95% confluent monolayers of HeLa cells in six-well plates (Costar), and the overlaid cells were incubated for 1 h (5% CO2, 37°C). Medium containing nonbound virus was removed, and warm complete MEM containing 0.75% agar was overlaid in each well. The plates were incubated 36 to 48 h (5% CO2, 37°C), fixed with Carnoy’s fixative (95% ethanol–acetic acid [3:1]), and stained with 1% crystal violet. Progeny virus was present in the supernatant at basal levels between 1 and 5 h. By 6 h postinfection there was a detectable increase in supernatant virus levels, and exponential virus production began at 9 h postinfection as determined by plaque assays (Fig. 1A). HeLa cells exhibited marked changes in morphology, including cellular condensation, rounding up, and release from the culture monolayer, between 6 and 7 h following infection, as noted by contrast microscopy (Fig. 1D).
FIG. 1.
Release of progeny CVB3 virus, host cell production of CVB3 viral protein, viral protease cleavage of host eIF4G, and cell morphology changes following infection with CVB3. (A) Culture medium was collected and assayed for infectious virus by the agar overlay plaque assay method. There was an increase in the amount of infectious virus (in PFU per milliliter) released over the 12-h experiment (B). Cellular lysate was collected from CVB3-infected HeLa cells, and immunoblot analysis with a CVB3 polyclonal antibody that recognizes major viral proteins was performed. (C) Cytosolic extract was then analyzed for the presence of the 220-kDa eIF4G component of the translation initiation complex. (D) Contrast microscopy of HeLa cells at 1, 6, 7, and 12 h postinfection was performed. Note the extensive cytopathic changes that occurred between 6 and 7 h postinfection.
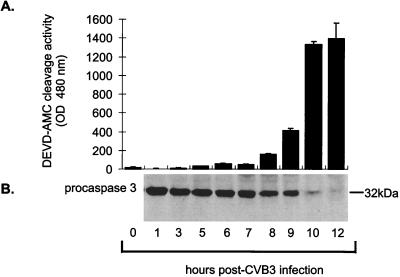
To determine whether the host cell death machinery is activated following CVB3 infection, immunoblot analysis of lysate collected at specific time points was performed. Caspase 3, which is present in cells as a precursor protein with a molecular mass of 32 kDa, is a primary molecule involved in the execution of cell death. Using mouse monoclonal anti-caspase 3 (1:1,000; Transduction Laboratories), it was determined that uninfected cells contained the 32-kDa precursor protein. Following CVB3 infection, the level of the 32-kDa precursor protein began to diminish between 7 and 8 h postinfection, and it was almost completely undetectable by 12 h postinfection (Fig. 2). To determine whether the depleted pro-caspase 3 had been proteolytically processed from a single-chain zymogen to its active two-chain enzyme, HeLa cell lysates were incubated with caspase 3 fluorescent substrates as previous described (23). Briefly, cellular lysates were incubated with reaction buffer (20 mM Tris [pH 7.5], 137 mM NaCl, 1% Nonidet P-40, 10% glycerol) containing 100 μM caspase 3 substrate acetyl-Asp-Glu-Val-Asp–7-amino-4-methylcoumarin (Ac-DEVD-AMC) (Calbiochem, Cambridge, Mass.) or Z-Asp-Glu-Val-Asp–7-amino-4-trifluoromethylcoumarin (Z-DEVD-AFC) (Enzyme Systems Products, Livermore, Calif.). The reaction mixture was incubated at 37°C for 2 h, and fluorescence excitation of AMC or AFC at 380 or 400 nm, respectively, was measured at 460 or 505 nm, respectively, with a CytoFluor 2350 cytofluorometer (Perseptive Biosystems, Burlington, Ontario, Canada). Using this approach, caspase 3 activity was evident by 5 h postinfection. The increase in caspase 3 activity from 7 to 10 h postinfection, when the maximum level of activation was reached, was maintained through to 12 h postinfection (Fig. 2). This protease assay demonstrated that caspase 3 was in an active form in infected cells and that it was capable of proteolytically processing other caspases and substrates.
FIG. 2.
Caspase 3 activation and cleavage of the 32-kDa proform following CVB3 infection of HeLa cells. (A) Ten micrograms of cell lysate was incubated in 150 μl of reaction buffer containing the caspase 3-specific substrate Ac-DEVD-AMC. After incubation at 37°C for 1 h, fluorescence levels were determined with an excitation wavelength of 380 nm and an emission wavelength of 460 nm. Note the increase in fluorescence, representing caspase activity, beginning after 7 h postinfection and increasing to maximum levels by 10 h postinfection. (B) HeLa cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Immunoblotting for the presence of the 32-kDa proform of caspase 3 demonstrates that this protein is processed between 7 and 12 h postinfection.
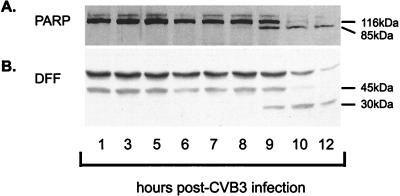
Caspase 3 cleaves specific substrates at aspartic acid residues (42). PARP, a nuclear protein involved in DNA repair (13, 69), has been shown to be a substrate for activated caspase 3 as well as other caspases (35). In apoptotic cells, PARP is cleaved from a 116-kDa protein, yielding fragments of 85 and 25 kDa, determined with antibodies for the amino and carboxyl termini of the protein, respectively. In CVB3-infected HeLa cells, PARP degradation, with the appearance of an 85-kDa fragment, was detectable by 9 h postinfection, with further reduction of levels of the 116-kDa peptide by 10 and 12 h postinfection, as determined by immunoblot analysis with mouse monoclonal anti-PARP (1:2,000; Biomol) (Fig. 3).
FIG. 3.
Specific cleavage of PARP and DFF substrates by caspase 3 following CVB3 infection. (A) Cellular lysate was collected from CVB3-infected HeLa cells, and immunoblot analysis was performed with an anti-PARP antibody which recognizes a 85-kDa cleavage fragment. Note that cleavage of PARP began at 9 h following infection, with a marked loss of the 116-kDa native protein occurring by 10 h postinfection. (B) Cellular lysate was similarly analyzed for DFF cleavage by immunoblotting following CVB3 infection. Note the change in DFF status beginning at 9 h postinfection, with the appearance of a 30-kDa fragment.
DFF is a cytosolic protein which can be cleaved by caspase 3 (37). Once cleaved, this protein releases an endonuclease that migrates to the nucleus, where it can cleave DNA at internucleosomal sites, resulting in DNA fragmentation (16). It has been demonstrated previously that internucleosomal DNA degradation is a cellular feature of picornavirus infection (32). As determined by using rabbit polyclonal anti-DFF (generously provided by Xiaodong Wang, University of Texas Southwestern Medical School, Dallas), DFF is cleaved from a 45-kDa protein, producing a 30-kDa fragment beginning at 9 h following infection, with continued processing and loss of the 45-kDa protein between 10 and 12 h postinfection (Fig. 3).
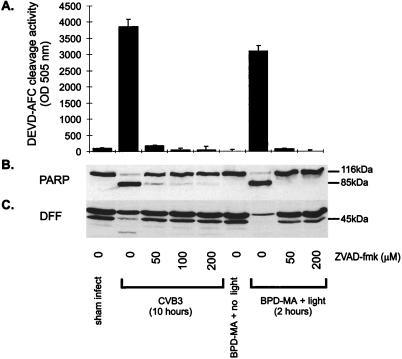
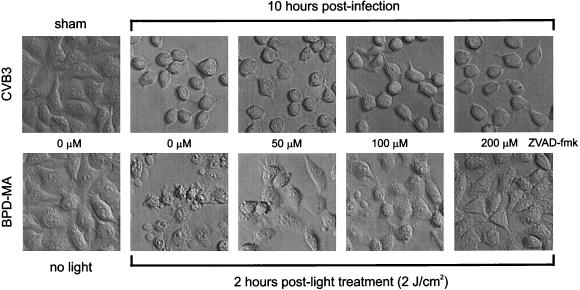
To determine whether caspases directly produce the characteristic CPE which occurs following picornavirus infection or are activated subsequent to the morphological alterations, cells were treated with the general caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (ZVAD.fmk). A stock solution (100 mM in dimethyl sulfoxide) of ZVAD.fmk (Bachem) was diluted in complete MEM to concentrations ranging from 0 to 200 μM, and the dilutions were incubated with cells for 30 min prior to infection or light treatment. After CVB3 infection, the cells were washed with PBS, and complete MEM containing 10% FBS and fresh ZVAD.fmk (0 to 200 μM) was then substituted. This peptide has been shown to inhibit the induction of morphological alterations by multiple apoptotic stimuli (46, 54). ZVAD.fmk at concentrations of 50, 100, and 200 μM blocked both caspase activity and cleavage of PARP and DFF in BPD-MA- and light-treated HeLa cells (positive control for apoptosis) (Fig. 4). In CVB3-infected HeLa cells, the cleavage of PARP and DFF was partially prevented by the inhibitor at concentrations of 50 and 100 μM (Fig. 4). At the highest concentration of the inhibitor (200 μM), there was no evidence of PARP or DFF cleavage fragments in CVB3-treated cells at 10 h following infection. Caspase cleavage of structural proteins such as actin, gelsolin, lamin B, and focal adhesion kinase is responsible for the morphological alterations observed following the induction of apoptosis (42, 45). At 2 h following photoactivation of BPD-MA, HeLa cells were condensed, had extensive membrane blebbing, and were releasing from the monolayer (Fig. 5). At increasing concentrations of ZVAD.fmk, this apoptotic phenotype was not apparent, with the cells maintaining a morphology similar to that of the control cells (Fig. 5). CVB3-infected HeLa cells were condensed and were releasing from the monolayer but exhibited no membrane blebbing at 10 h following infection (Fig. 5). Blockade of caspase activity by ZVAD.fmk at concentrations up to 200 μM did not alter the cytopathic phenotype even though cleavage of substrates (DFF and PARP) was inhibited.
FIG. 4.
ZVAD-fmk inhibits caspase activation as well as cleavage of PARP and DFF following CVB3 infection or induction of apoptosis by treatment of HeLa cells with BPD-MA and light. (A) Cell lysate was incubated in reaction buffer containing the caspase 3-specific substrate Ac-DEVD-AFC. After incubation at 37°C for 1 h, fluorescence levels were determined with an excitation wavelength of 400 nm and an emission wavelength of 505 nm. Note the lack of caspase activation in ZVAD-fmk (50 to 200 μM)-treated HeLa cells at 10 h following CVB3 infection and at 2 h following treatment with BPD-MA and light. (B) Cellular lysate was collected from treated HeLa cells, and immunoblot analysis was performed with an anti-PARP antibody. Note the equivalent cleavage of PARP in the CVB3-infected HeLa cells without ZVAD.fmk and the BPD-MA- and light-treated HeLa cells without ZVAD.fmk. ZVAD.fmk treatment (50 to 200 μM) of HeLa cells prevented PARP processing in the BPD-MA- and light-treated HeLa cells, while in CVB3-treated HeLa cells the PARP processing was limited, but not completely inhibited, by treatment with ZVAD.fmk at 50 or 100 μM. (C) Immunoblot analysis of DFF cleavage at 10 h following CVB3 infection and at 2 h following treatment with BPD-MA and light showed that the cleavage pattern was similar to that of PARP. Sham-infected cultures were treated the same as infected cultures, without virus.
FIG. 5.
Effects of ZVAD.fmk treatment on cell morphological changes following CVB3 infection or BPD-MA and light treatment of HeLa cells. HeLa cells were treated with ZVAD.fmk at 0 to 200 μM and then infected with CVB3 or treated with BPD-MA and light. At 10 h postinfection, caspases were processed and substrates were cleaved in virus-infected cells, and at 2 h post-light treatment, caspases were processed and substrates were cleaved in BPD-MA-treated cells. Note the difference in the morphological appearances of the CVB3-infected and photodynamically treated HeLa cells. CVB3-infected HeLa cells appeared rounded, with smooth cell surfaces, while the HeLa cells treated with BPD-MA and light displayed extensive membrane blebbing and shrinkage, with cellular heterogeneity. At higher concentrations of ZVAD-fmk, the morphological changes produced by BPD-MA and light treatment were inhibited, and the cell appearance was similar to that of control HeLa cells (BPD-MA treated plus no light). In CVB3-infected HeLa cells, the morphological changes were not inhibited with increasing concentrations of ZVAD.fmk, suggesting that the morphological alterations were independent of caspase processing and cleavage of substrates.
A classic feature of viruses of the family Picornaviridae is the cellular CPE following infection. Since the discovery of an extraneural cell culture technique for the multiplication of poliovirus (17), degenerative changes in cell morphology have been noted. First described by Robbins et al. (48) in 1950, these cytopathic changes include nuclear shrinkage, condensation of chromatin, cell rounding, and release from the monolayer, with eventual progression to acidophilic cytoplasm, nuclear pyknosis, and fragmentation of the nuclear chromatin (karyorrhexis) (47).
The recent understanding of cell death mechanisms sets the stage for examination of host cell death proteins and their possible role in the CPE of CVB3 infection. Many viruses inhibit or activate cell death, strategies that convey distinctive aspects of cell injury, inflammatory responses, or viral persistence. As noted above, previous picornavirus studies have revealed the morphological features of apoptotic cell death, including cell shrinkage, DNA fragmentation, and nuclear condensation (21, 32, 47).
Caspase 3, a cysteine protease with homology to the Caenorhabditis elegans protein Ced-3 (77), is considered one of the key proteins involved in the execution stage of cell death. Apoptosis induced by multiple stimuli, including Fas, TNF, etoposide, staurosporine, photodynamic therapy, ionizing radiation, and growth factor withdrawal, involves pro-caspase 3 processing and subsequent activation (15, 23, 30, 33, 51). Beginning at 7 to 8 h postinfection with CVB3, pro-caspase 3 is depleted, and caspase activation assays have demonstrated that this protein is cleaved into its active form. Several proteins can activate caspase 3, including caspase 8 (via signalling through TNF or Fas receptors) (55), granzyme B from cytotoxic lymphocytes (62), and caspase 9 (via release of mitochondrial cytochrome c and assembly of apoptotic protease activation factors) (36). Once activated, caspase 3 can degrade specific substrates, which in turn results in structural alterations and loss of homeostatic regulation of cellular processes. Numerous proteins have been shown to be cleaved by activated caspases. Consistent with the activation of caspase 3, both PARP and DFF are cleaved following CVB3 infection. Caspase activation and DNA fragmentation are directly linked through the cleavage of DFF (37). DFF is a human cytosolic factor consisting of two subunits of 45 and 40 kDa, the larger of which is degraded into smaller polypeptides by caspase 3. In recent studies by Enari et al. (16) using murine lymphoma cells, an endonuclease, caspase-activated DNase (CAD), was isolated. The murine equivalent of DFF protein was isolated and termed inhibitor of CAD (50). Caspase 3 cleavage of inhibitor of CAD (DFF) allows CAD nuclear translocation and DNA degradation. DFF is cleaved beginning at 9 h postinfection, resulting in a 30-kDa fragment (Fig. 3) which can be further processed to an 11-kDa fragment (37). PARP is located in the nucleus and is involved in DNA repair. Cleavage of PARP begins at 9 h following infection, suggesting that once caspases are activated in the cytosol, they are able to access nucleus-localized substrates.
Of note, caspase inhibition with the general caspase inhibitor ZVAD.fmk did not prevent the CPE induced by CVB3 following infection. At between 6 and 7 h postinfection, the CPE became apparent by contrast microscopy in our CVB3 infection model. The time between infection and appearance of the CPE, as observed by contrast microscopy, was consistent at ZVAD.fmk concentrations of from 50 to 200 μM. In addition to not affecting the time to CPE, ZVAD.fmk treatment resulted in cells with a morphological appearance similar to that of untreated, infected cells (Fig. 5). We used treatment with BPD-MA and light as an alternative method of inducing apoptosis in HeLa cells (22, 23). Inhibition of caspase activation with the inhibitor ZVAD.fmk prevented the apoptotic phenotype (Fig. 5). From these results, we conclude that caspase activity and cleavage of substrates do not account for the characteristic CPE associated with picornavirus infection but instead are activated subsequent to the morphological changes.
The point of intersection of the viral replicative cycle and activation of the host cell death pathway remains to be determined. Picornavirus infection soon results in inhibition of cellular RNA and protein synthesis (19, 74). Early studies of relationships between picornavirus-induced metabolic alterations and virus-induced CPE indicated that the inhibition of protein and RNA synthesis was not directly related to cell morphological changes (4). Protein and RNA synthesis inhibitors delayed cell death, but the cells displayed fewer morphological changes than did picornavirus-infected cells (5). Inhibition of protein and RNA synthesis with any one of multiple agents, such as actinomycin D, puromycin, and diphtheria toxin, results in apoptosis (39). Early studies done in poliovirus infection systems showed that puromycin, an inhibitor of the translation of viral as well as host proteins, delays the onset of cytopathic changes, suggesting that certain viral proteins may be directly cytotoxic (4). Increasing the MOI leads to a more rapid onset of CPE (unpublished observations), although almost all host protein translation is shut off within 3 h at a relatively low MOI (25) such as that used in this study (MOI = 5). Recently it has been shown that the 2B protein encoded by coxsackievirus and poliovirus associates with cellular membrane fractions, including the plasmalemma and endoplasmic reticulum, and disrupts ion movement, including the movement of Ca2+ to the cytosol (1, 67). Ca2+ influx occurs in apoptosis (7, 44), but it is not clear whether the influx occurs prior to or following caspase activation. By examination of the ionic requirements of caspases, it has been determined that the calcium ion concentration has little effect on caspase activity (56). An early calcium influx following coxsackievirus infection could result from the influence of the 2B protein on membrane permeability, and the large late calcium influx noted (>6 h postinfection) (67) could be a downstream effect of caspase activation.
During the early phases of infection, it would be advantageous for the virus to inhibit host cell death, thereby allowing for maximal production of viral progeny. At late stages of the viral life cycle, it would also be beneficial for the virus to induce apoptosis rather than necrosis. Such a mechanism of death is a potential means of host immune system evasion by the virus during its release to the surrounding tissue. Apoptosis is characterized by the rapid phagocytosis of affected cells without the release of proinflammatory cytokines (53).
Viruses have been shown to interact at various levels of the apoptotic pathway. Several gammaherpesviruses (including Kaposi’s sarcoma-associated human herpesvirus 8) as well as the tumorigenic molluscum contagiosum virus contain FLICE-inhibitory proteins that interact with the Fas adapter protein FADD and compete to inhibit caspase 8 recruitment and subsequent activation (61). Expression of the cowpox virus serpin CrmA blocks the caspase 8-mediated activation of downstream caspases such as caspase 1 and caspase 3 (55). The IAP (for inhibitors of apoptosis) proteins constitute a family of proteins, expressed by baculoviruses, that block apoptosis induced by viral infection or by caspase 1 (78). Furthermore, several viral homologs of the Bcl-2 family of proteins have been discovered (2, 8, 20, 70). Adenovirus (9, 20, 57), African swine fever virus (41), and Epstein-Barr virus (26, 41, 59) encode proteins (E1B-19K, LMWS-HL, and BHRF1, respectively) that exhibit sequence homology to pro-survival genes of the Bcl-2 family.
The identification of viral proteins that directly induce apoptosis is not as extensively documented as that of viral proteins that inhibit cell death. The lentiviruses human immunodeficiency virus and human T-cell leukemia virus type 1 encode the transcription regulators Tat and Tax, which have been shown to increase expression of Fas ligand while decreasing the expression of Bcl-2 family members (79, 80). The human adenovirus-encoded E1A, E3, and E4 gene products cause cell death following expression in cell culture. The adenovirus death-inducing genes are expressed late in the infection cycle and ultimately overwhelm the virus-encoded death-inhibiting genes (38).
Our data demonstrate that caspase 3 activation follows, rather than precedes, CVB3-induced degenerative morphological changes in infected HeLa cells. Activated caspases process specific substrates, including PARP and DFF. However, inhibition of caspase activity does not eliminate the morphological appearance (CPE) of virus-infected cells, as determined by contrast microscopy. Caspase processing and cleavage of substrates may be important in the ultimate alteration of normal homeostatic processes in infected cells and may facilitate the final clearance of virus-infected cells. The viral proteases 2A, 3C, and 3CD may cleave specific structural proteins, resulting in morphological alterations consistent with CPE, in a fashion analogous to the action of caspases, which cleave separate substrates to achieve a distinct apoptotic phenotype.
Acknowledgments
We appreciate deeply the technical support of Lubos Bohunek. We thank Xiaodong Wang (University of Texas Southwestern Medical School, Dallas) for the generous gift of DFF antibody.
These studies have been supported by the Heart and Stroke Foundation of British Columbia and Yukon (B.M.M., C.M.C., D.J.G., and K.A.W.), the Medical Research Council of Canada (D.Y. and B.M.M.), and the B.C. Health Research Foundation (D.Y.)
REFERENCES
- 1.Aldabe R, Irurzun A, Carrasco L. Poliovirus protein 2BC increases cytosolic free calcium concentrations. J Virol. 1997;71:6214–6217. doi: 10.1128/jvi.71.8.6214-6217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosini G, Adida C, Altieri D C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D R, Wilson J E, Carthy C M, Yang D, Kandolf R, McManus B M. Direct interactions of coxsackievirus B3 with immune cells in the splenic compartment of mice susceptible or resistant to myocarditis. J Virol. 1996;70:4632–4645. doi: 10.1128/jvi.70.7.4632-4645.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bablanian R, Eggers H, Tamm I. Studies on the mechanism of poliovirus-induced cell damage. I. The relation between poliovirus-induced metabolic and morphological alterations in cultured cells. Virology. 1965;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- 5.Bablanian R, Eggers H J, Tamm I. Studies on the mechanism of poliovirus-induced cell damage. II. The relation between poliovirus growth and virus-induced morphological changes in cells. Virology. 1965;26:114–121. doi: 10.1016/0042-6822(65)90031-0. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 7.Bian X, Hughes F M, Jr, Huang Y, Cidlowski J A, Putney J W., Jr Roles of cytoplasmic Ca2+ and intracellular Ca2+ stores in induction and suppression of apoptosis in S49 cells. Am J Physiol. 1997;272:C1241–C1249. doi: 10.1152/ajpcell.1997.272.4.C1241. [DOI] [PubMed] [Google Scholar]
- 8.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou S-K, Tseng C-C, Rao L, White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark M E, Hammerle T, Wimmer E, Dasgupta A. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 1991;10:2941–2947. doi: 10.1002/j.1460-2075.1991.tb07844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark M E, Lieberman P M, Berk A J, Dasgupta A. Direct cleavage of human TATA-binding protein by poliovirus protease 3C in vivo and in vitro. Mol Cell Biol. 1993;13:1232–1237. doi: 10.1128/mcb.13.2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryns V L, Bergeron L, Zhu H, Li H, Yuan J. Specific cleavage of α-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1β-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J Biol Chem. 1996;271:31277–31282. doi: 10.1074/jbc.271.49.31277. [DOI] [PubMed] [Google Scholar]
- 13.de Murcia J M, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver F J, Masson M, Dierich A, LeMeur M, Walztinger C, Chambon P, de Murcia G. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doedens J R, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1994;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 16.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 17.Enders J F, Weller T H, Robbins R C. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85–87. doi: 10.1126/science.109.2822.85. [DOI] [PubMed] [Google Scholar]
- 18.Erhardt P, Tomaselli K J, Cooper G M. Identification of the MDM2 oncoprotein as a substrate for CPP32-like apoptotic proteases. J Biol Chem. 1997;272:15049–15052. doi: 10.1074/jbc.272.24.15049. [DOI] [PubMed] [Google Scholar]
- 19.Etchison D, Milburn S C, Edery I, Sonenberg N, Hershey J W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982;257:14806–14810. [PubMed] [Google Scholar]
- 20.Farrow S N, White J H, Martinou I, Raven T, Pun K T, Grinham C J, Martinou J C, Brown R. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 21.Godman G C. The cytopathology of enteroviral infection. In: Richter G W, Epstein M A, editors. International review of experimental pathology. Vol. 5. New York, N.Y: Academic Press; 1966. pp. 67–110. [PubMed] [Google Scholar]
- 22.Granville D J, Jiang H, An M T, Levy J G, McManus B M, Hunt D W. Overexpression of Bcl-X(L) prevents caspase-3-mediated activation of DNA fragmentation factor (DFF) produced by treatment with the photochemotherapeutic agent BPD-MA. FEBS Lett. 1998;422:151–154. doi: 10.1016/s0014-5793(97)01616-5. [DOI] [PubMed] [Google Scholar]
- 23.Granville D J, Levy J G, Hunt D W. Photodynamic therapy induces caspase-3 activation in HL-60 cells. Cell Death Differ. 1997;4:623–629. doi: 10.1038/sj.cdd.4400286. [DOI] [PubMed] [Google Scholar]
- 24.Guinea R, Lopez-Rivas A, Carrasco L. Modification of phospholipase C and phospholipase A2 activities during poliovirus infection. J Biol Chem. 1989;264:21923–21927. [PubMed] [Google Scholar]
- 25.Helentjaris T, Ehrenfeld E. Inhibition of host cell protein synthesis by UV-inactivated poliovirus. J Virol. 1977;21:259–267. doi: 10.1128/jvi.21.1.259-267.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber M, Selinka H-C, Kandolf R. Tyrosine phosphorylation events during coxsackievirus B3 replication. J Virol. 1997;71:595–600. doi: 10.1128/jvi.71.1.595-600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irurzun A, Arroyo J, Alvarez A, Carrasco L. Enhanced intracellular calcium concentration during poliovirus infection. J Virol. 1995;69:5142–5146. doi: 10.1128/jvi.69.8.5142-5146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irurzun A, Perez L, Carrasco L. Enhancement of phospholipase activity during poliovirus infection. J Gen Virol. 1993;74:1063–1071. doi: 10.1099/0022-1317-74-6-1063. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen M D, Weil M, Raff M C. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang S K, Davies M V, Kaufman R J, Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5′ nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989;63:1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jelachich M L, Lipton H L. Theiler’s murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J Virol. 1996;70:6856–6861. doi: 10.1128/jvi.70.10.6856-6861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann S H, Desnoyers S, Ottaviano Y, Davidson N E, Poirier G G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- 34.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry T J, Kirschner M W, Koths K, Kwiatkowski D J, Williams L T. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 35.Lazebnik Y A, Kaufmann S H, Desnoyers S, Poirier G G, Earnshaw W C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates and apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Kitsis R N. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J Cell Biol. 1996;133:325–334. doi: 10.1083/jcb.133.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin S J, Lennon S V, Bonham A M, Cotter T G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990;145:1859–1867. [PubMed] [Google Scholar]
- 40.Nair C N. Monovalent cation metabolism and cytopathic effects of poliovirus-infected HeLa cells. J Virol. 1981;37:268–273. doi: 10.1128/jvi.37.1.268-273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neilan J G, Lu Z, Afonso C L, Kutish G F, Sussman M D, Rock D L. An African swine fever virus gene with similarity to the proto-oncogene bcl-2 and the Epstein-Barr virus gene BHRF1. J Virol. 1993;67:4391–4394. doi: 10.1128/jvi.67.7.4391-4394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholson D W, Thornberry N A. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 43.Ohlmann T, Rau M, Pain V, Morley S. The C-terminal domain of eukaryotic protein synthesis initiation factor (eIF) 4G is sufficient to support cap-independent translation in the absence of eIF4E. EMBO J. 1996;15:1371–1382. [PMC free article] [PubMed] [Google Scholar]
- 44.Orrenius S, Nicotera P. The calcium ion and cell death. J Neural Transm Suppl. 1994;43:1–11. [PubMed] [Google Scholar]
- 45.Porter A G, Ng P, Janicke R U. Death substrates come alive. Bioessays. 1997;19:501–507. doi: 10.1002/bies.950190609. [DOI] [PubMed] [Google Scholar]
- 46.Pronk G J, Ramer K, Amiri P, Williams L T. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science. 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 47.Reissig M, Howes D W, Melnick J L. Sequence of morphological changes in epithelial cell cultures infected with poliovirus. J Exp Med. 1956;104:289–309. doi: 10.1084/jem.104.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins F C, Enders J F, Weller T H. Cytopathogenic effect of poliomyelitis viruses “in vitro” on human embryonic tissues. Proc Soc Exp Biol Med. 1950;75:370. doi: 10.3181/00379727-75-18202. [DOI] [PubMed] [Google Scholar]
- 49.Rudin C M, Thompson C B. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–281. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 50.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 51.Sarin A, Wu M L, Henkart P A. Different interleukin-1 beta converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaefer A, Kühne J, Zibirre R, Koch G. Poliovirus-induced alterations in HeLa cell membrane functions. J Virol. 1982;44:444–449. doi: 10.1128/jvi.44.2.445-449.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartzman R A, Cidlowski J A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993;14:133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- 54.Slee E A, Zhu H, Chow S C, MacFarlane M, Nicholson D W, Cohen G M. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stennicke H R, Salvesen G S. Biochemical characteristics of caspase-3, -6, -7, and -8. J Biol Chem. 1997;272:25719–15723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 57.Subramanian T, Tarodi B, Chinnadurai G. Functional similarity between adenovirus E1B 19-kDa protein and proteins encoded by Bcl-2 proto-oncogene and Epstein-Barr virus BHRF1 gene. Curr Top Microbiol Immunol. 1995;199:153–161. doi: 10.1007/978-3-642-79496-4_9. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi A, Alnemri E S, Lazebnik Y A, Fernandes-Alnemri T, Litwack G, Moir R D, Goldman R D, Poirier G G, Kaufmann S H, Earnshaw W C. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci USA. 1996;93:8395–8400. doi: 10.1073/pnas.93.16.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarodi B, Subramanian T, Chinnadurai G. Epstein-Barr virus BHRF1 protein protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology. 1994;201:404–407. doi: 10.1006/viro.1994.1309. [DOI] [PubMed] [Google Scholar]
- 60.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J, Schroter M, Scaffidi C, Krammer P, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 62.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the capsase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 63.Tolskaya E A, Romanova L I, Kolesnikova M S, Ivannikova T A, Smirnova E A, Raikhlin N T, Agol V I. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J Virol. 1995;69:1181–1189. doi: 10.1128/jvi.69.2.1181-1189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsunoda I, Kurtz C I, Fujinami R S. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- 66.Vanags D M, Pron-Ares M I, Coppola S, Burgess D H, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 67.van Kuppeveld F J, Hoenderop J G, Smeets R L, Willems P H, Dijkman H B, Galama J M, Melchers W J. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Zelenski N G, Yang J, Sakai J, Brown M S, Goldstein J L. Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J. 1996;15:1012–1020. [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z Q, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K, Wagner E F. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11:2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wattre P, Bert V, Hober D. Apoptosis and human viral infections. Ann Biol Clin. 1996;54:189–197. [PubMed] [Google Scholar]
- 71.Wen L P, Fahrni J A, Troie S, Guan J L, Orth K, Rosen G D. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056–26061. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- 72.Yalamanchili P, Banerjee R, Dasgupta A. Poliovirus-encoded protease 2APro cleaves the TATA-binding protein but does not inhibit host cell RNA polymerase II transcription in vitro. J Virol. 1997;71:6881–6886. doi: 10.1128/jvi.71.9.6881-6886.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yalamanchili P, Datta U, Dasgupta A. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J Virol. 1997;71:1220–1226. doi: 10.1128/jvi.71.2.1220-1226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yalamanchili P, Harris K, Wimmer E, Dasgupta A. Inhibition of basal transcription by poliovirus: a virus-encoded protease (3Cpro) inhibits formation of TBP-TATA box complex in vitro. J Virol. 1996;70:2922–2929. doi: 10.1128/jvi.70.5.2922-2929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yalamanchili P, Weidman K, Dasgupta A. Cleavage of transcriptional activator Oct-1 by poliovirus encoded protease 3Cpro. Virology. 1997;239:176–185. doi: 10.1006/viro.1997.8862. [DOI] [PubMed] [Google Scholar]
- 76.Yang D, Wilson J E, Anderson D R, Bohunek L, Cordeiro C, Kandolf R, McManus B M. In vitro mutational and inhibitory analysis of the cis-acting translational elements within the 5′ untranslated region of coxsackievirus B3: potential targets for antiviral action of antigens oligomers. Virology. 1997;228:63–73. doi: 10.1006/viro.1996.8366. [DOI] [PubMed] [Google Scholar]
- 77.Yuan J. Evolutionary conservation of a genetic pathway of a programmed cell death. J Cell Biochem. 1996;60:4–11. doi: 10.1002/(sici)1097-4644(19960101)60:1<4::aid-jcb2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 78.Yuan J. Transducing signals of life and death. Curr Opin Cell Biol. 1997;9:247–251. doi: 10.1016/s0955-0674(97)80069-5. [DOI] [PubMed] [Google Scholar]
- 79.Zauli G, Gibellini D. The human immunodeficiency virus type-1 (HIV-1) Tat protein and Bcl-2 gene expression. Leuk Lymphoma. 1996;23:551–560. doi: 10.3109/10428199609054864. [DOI] [PubMed] [Google Scholar]
- 80.Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, Mazzoni M, Capitani S. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood. 1995;86:3823–3834. [PubMed] [Google Scholar]