Abstract
IgG4-related disease (IgG4-RD) is an increasingly recognized cause of fibroinflammatory lesions in patients of diverse racial and ethnic backgrounds and is associated with an increased risk of death. The aetiology of IgG4-RD is incompletely understood, but evidence to date suggests that B and T cells are important players in pathogenesis, both of which are key targets of ongoing drug development programmes. The diagnosis of IgG4-RD requires clinicopathological correlation because there is no highly specific or sensitive test. Glucocorticoids are highly effective, but their use is limited by toxicity, highlighting the need for studies investigating the efficacy of glucocorticoid-sparing agents. B cell-targeted therapies, particularly rituximab, have demonstrated benefit, but no randomized clinical trials have evaluated their efficacy. If untreated or under-treated, IgG4-RD can cause irreversible organ damage, hence close monitoring and consideration for long-term immunosuppression is warranted in certain cases.
Keywords: IgG4-related disease, epidemiology, outcomes, treatment, rituximab, glucocorticoids
Key messages.
IgG4-related disease (IgG4-RD) can affect patients of diverse racial and ethnic backgrounds and can lead to irreversible damage if not treated.
Nearly any organ can be affected by IgG4-RD, but common sites include the salivary glands, lacrimal glands, orbit, pancreatobiliary system, lung, kidney and retroperitoneum.
B and T cells are thought to be important in the pathogenesis, whereas the IgG4 molecule is often not considered a driver of disease.
The diagnosis requires clinicopathological correlation because there are no highly sensitive or specific tests, including serum IgG4 concentrations and IgG4+ plasma cell infiltrates.
Glucocorticoids are highly effective for IgG4-RD but are associated with toxicities, hence CS-sparing drugs are now being investigated as treatments.
Introduction
The condition that would become known as IgG4-related disease (IgG4-RD) was first described in 2001 [1]. Since this description of 20 patients in Japan with autoimmune pancreatitis (AIP) and elevated serum IgG4 concentrations, diverse organ involvement with and without elevated IgG4 levels has been described in cohorts worldwide. Prior to 2001, this was a disease known by various names, mostly eponyms, depending on the manifestation: Kuttner’s tumour (submandibular sialoadenitis), Riedel’s thyroiditis, Ormond’s disease (retroperitoneal fibrosis), Mikulicz syndrome (symmetric lacrimal and salivary gland disease) and AIP. These diverse manifestations share similar histopathological and immunohistochemical findings [2]. Recent advances have informed our understanding of the epidemiology of IgG4-RD, its pathogenesis, approaches to diagnosis and effective treatments.
Epidemiology of IgG4-RD
IgG4-RD tends to affect people in their fifth to seventh decades of life, but paediatric and older adult patients can also present with IgG4-RD. Most cohorts demonstrate a male predominance; however, this varies: pancreatobiliary disease and retroperitoneal disease more commonly affect males, whereas disease limited to the head and neck most commonly affects females. Population-based estimates of IgG4-RD incidence and prevalence are limited. Using a claims-based algorithm to identify cases in the USA, the estimated incidence was 0.78–1.39 per 100 000 person-years between 2015 and 2019, and the point prevalence as of 1 January 2019 was 5.3 per 100 000 persons [3, 4]. The incidence of pancreatic disease in Japan was estimated to be higher (3.1 per 100 000 persons) using different methods [5]. Patients with IgG4-RD may have an elevated risk of death compared with the general population [6], probably driven, in part, by irreversible organ damage from IgG4-RD in addition to treatment complications; the precise cause of excess mortality is unknown.
Patterns of presentation
The diverse manifestations of IgG4-RD can make the diagnosis difficult to establish [7], hence a high level of suspicion for IgG4-RD is needed to avoid diagnostic delays. Although nearly any organ can be affected, four typical presentations are most common (Table 1): head and neck disease, systemic disease, hepato-pancreatobiliary disease and retroperitoneal fibrosis/aortic disease [8].
Table 1.
Patterns of presentation
| Pattern | Pancreato-hepatobiliary disease | Retroperitoneum and aorta | Head- and neck-limited disease | Mikulicz and systemic disease |
|---|---|---|---|---|
| Typical manifestations | Autoimmune pancreatitis, sclerosing cholangitis | Retroperitoneal fibrosis, aortitis, large vessel disease | Salivary and/or lacrimal gland enlargement, adnexal orbital involvement | Classic symmetric lacrimal, salivary gland enlargement with involvement in the chest and/or abdomen |
| Male predominance | Yes | Yes | No | Yes |
| Age, mean, years | 63 | 58 | 55 | 63 |
| Serum IgG4 concentration | Elevated | Normal to mildly elevated | Elevated | Very high |
| Examples of potential mimics | Pancreatic cancer, autoimmune pancreatitis type 2, primary sclerosing cholangitis | Lymphoma, Erdheim–Chester disease, GCA | SS and other autoimmune CTD, granulomatosis with polyangiitis, lymphoma | |
Adapted from Wallace ZS, et al., Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis 2019; 78:406.
Head and neck disease with or without systemic involvement
Disease in the head and neck is often appreciable on physical examination. The most common manifestations include salivary gland and/or lacrimal gland enlargement, lymphadenopathy and orbital disease (e.g. orbital myositis, orbital pseudotumour). Salivary or lacrimal gland disease typically presents with painless swelling, often symmetrically. Orbital involvement often causes proptosis; it can cause pain and change in vision owing to involvement of extra-ocular muscles and/or optic nerve compression. Less common manifestations include pachymeningitis, thyroiditis and hypophysitis. Disease isolated to the head and neck tends to occur more often in females. Head and neck involvement in combination with systemic disease (e.g. pancreas, biliary tract, kidneys, where tubulointerstitial nephritis can occur) is associated with high serum IgG4 concentrations, frequent elevations in acute phase reactants and/or hypocomplementaemia. Many patients, especially those with head and neck involvement, have atopic disease (e.g. seasonal allergies) [9]; however, the significance of atopic disease in IgG4-RD in general is poorly understood. The symptoms of seasonal allergies are often distinct from IgG4-RD, except for sinusitis, which can be present in both seasonal allergic conditions and IgG4-RD. However, sinusitis from IgG4-RD is not seasonal and can lead to damage.
Retroperitoneum and large vessel involvement: a fibrotic phenotype
Some manifestations tend to present with a fibrotic phenotype, of which retroperitoneal fibrosis is prototypic. Biopsies of these lesions demonstrate prominent fibrosis and less inflammation. Retroperitoneal fibrosis typically presents as soft tissue surrounding the infrarenal aorta, extending distally to involve the iliac arteries and often laterally to encase and medialize the ureters. Patients can have groin or back pain but are often asymptomatic and present with renal failure from ureteral obstruction and hydronephrosis. Other fibrotic manifestations include orbital pseudotumours, thyroiditis and sclerosing mesenteritis and mediastinitis. Patients with these manifestations in isolation frequently have normal serum IgG4 concentrations. The diagnosis is often difficult to establish, especially when a biopsy reveals few features other than fibrosis.
Pancreatobiliary disease
Type 1 AIP (lymphoplasmacytic sclerosing pancreatitis) is one of the most common manifestations of IgG4-RD [10]. Of the patients with IgG4-related pancreatic disease, ≤20% also have biliary involvement manifesting as IgG4-related sclerosing cholangitis or biliary compression from pancreatitis. The most common presentation is painless jaundice, occurring in 70% of patients [11–14]. Imaging in AIP can present as diffuse or focal involvement (reviewed below). Less commonly, patients can present with acute pancreatitis. Type 2 AIP (idiopathic duct centric pancreatitis) is associated with IBD, not IgG4-RD, and will not be discussed here (Supplementary Table S1, available at Rheumatology Advances in Practice online) [15, 16].
Current understanding of pathogenesis
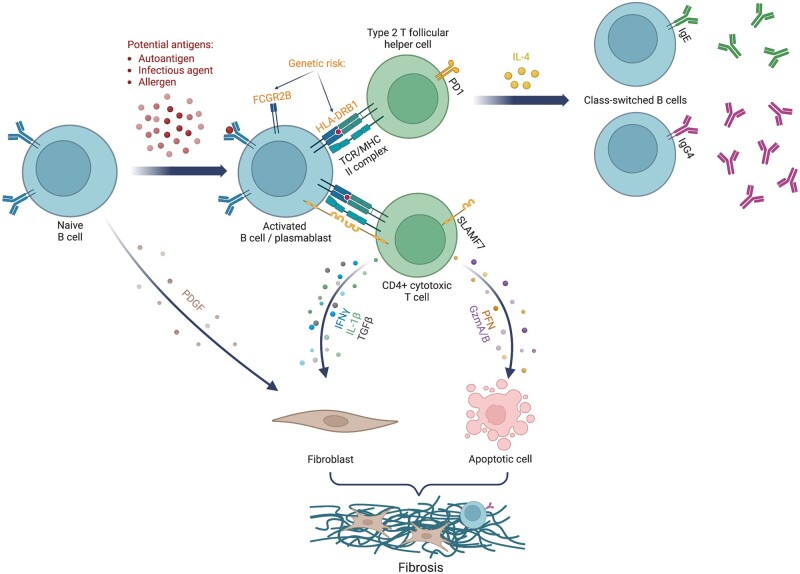
The aetiology of IgG4-RD and the role that IgG4 plays in its pathogenesis remain poorly understood. Environmental exposures and genetic factors might place certain individuals at an increased risk for IgG4-RD. Dysregulated immunity, characterized by an expansion of CD4+ cytotoxic T lymphocytes, is a key hallmark of the disease (Fig. 1) [15–17].
Figure 1.
IgG4-related disease pathogenesis
The role of the IgG4 molecule
Although not specific, most patients with IgG4-RD have an elevated serum IgG4 concentration. The degree of elevation is correlated with organ involvement and risk of relapse [18–20]. It remains unknown whether IgG4 is directly involved in pathogenesis, is a compensatory response to immune activation or is simply an epiphenomenon related to a misdirected immune response. IgG4 is considered an anti-inflammatory immunoglobulin because it undergoes Fab-arm exchange, which limits its ability to cross-link antigen effectively, it weakly fixes complement and it has a reduced capacity to bind activating Fc receptors but a preserved ability to bind inhibitory Fc receptors [21–23]. However, antigen-specific IgG4 antibodies can be pathogenic in certain conditions, as seen in muscle-specific tyrosine kinase myasthenia gravis, pemphigus foliaceus, pemphigus vulgaris, primary membranous nephropathy and chronic inflammatory demyelinated polyradiculoneuropathy [24].
The role of B cells
Several observations support the central role of B cells in IgG4-RD. First, the pathological hallmarks include a lymphoplasmacytic infiltrate rich in IgG4+ plasma cells, in addition to storiform fibrosis with or without obliterative phlebitis [2]. Second, elevations in serum immunoglobulin concentrations, including IgG4, are common [25, 26]. Third, patients have oligoclonally expanded plasmablasts, which decrease in remission [27]. Fourth, the resurgence of plasmablasts and memory B cells is correlated with an increase in disease activity [27, 28]. Despite these observations, the precise role of B cells remains uncertain and is an active area of investigation [29, 30].
The role of T cells
Two T cell subsets are thought to play key roles in IgG4-RD. Circulating type 2 T follicular helper cells expressing programmed cell death protein 1 are expanded in IgG4-RD. Their frequency is correlated with the number of organs involved, number of plasmablasts, IgG4 concentrations and IL-4 concentrations [30–32]. . Circulating type 2 T follicular helper cells produce IL-4, which is involved in class-switching of B cells to both IgG4 and IgE. T follicular helper cells expressing IL-4 are also expanded in affected tissue, where they promote B cell isotype switching, affinity maturation and oligoclonal expansion of IgG4+ B cells [33].
CD4+ effector memory T cells (defined as CD27− CD62L−) expressing SLAMF7 are also expanded in IgG4-RD and found in affected tissue, and they decline in number with treatment [17]. Also known as CD4+ cytotoxic T lymphocytes, these cells express perforin, granzymes, granulysin and other mediators of cytotoxicity and produce profibrotic cytokines (e.g. TGF-β, IFN-γ and IL-1β) [17]. They may also be involved in apoptosis.
Knowledge gaps in pathogenesis
The clonal expansion of both plasmablasts and CD4+ cytotoxic T lymphocytes seen in patients with IgG4-RD suggests that there might be a common antigen driving the disease, perhaps an autoantigen [27]. Proposed, but unconfirmed, potential autoantigens include carbonic anhydrase, plasminogen binding protein, lactoferrin, pancreatic secretory trypsin inhibitor, amylase alpha-2A, trypsinogen, annexin A11, laminin-511-E8, galectin-3 and IL-1 receptor antagonist [34–44]. The genetic contribution to IgG4-RD is also unclear. One genome-wide association study [45] found HLA-DRB1 and FCGR2B regions as susceptibility loci for IgG4-RD. Future work should continue to focus on autoantigen discovery, correlation of autoantibodies with specific organ involvement, the development of an animal model that recapitulates human disease and the identification of genetic risk factors. Understanding the inciting event that leads to disease onset and whether T cells and B cells are responding to the same antigen are key knowledge gaps that need to be addressed.
Approach to the diagnosis of IgG4-RD
Clinical practice
Although not meant for establishing a clinical diagnosis of IgG4-RD, the 2019 ACR/EULAR Classification Criteria for IgG4-RD provide a useful framework for evaluating a patient who might have IgG4-RD [46]. IgG4-RD should be suspected when a patient presents with a mass lesion (e.g. pancreatic mass or salivary gland enlargement) or wall thickening (e.g. biliary tract or aorta) in a characteristic organ, which includes the pancreas, salivary glands, bile ducts, orbits, kidneys, lungs, aorta, retroperitoneum, pachymeninges and/or thyroid gland [46]. Although nearly any organ can be affected, certain locations, such as the gut lumen, brain and bones, would be unusual. In most cases, the diagnosis is confirmed or supported by biopsy; however, this is not always possible because of the lesion location, procedure risk or patient preference. Regardless of biopsy findings, the diagnosis is established by clinicopathological correlation; no single finding by examination, pathology, imaging or laboratory tests is diagnostic. In classic presentations, a diagnosis can be made without a biopsy, assuming that mimicking conditions have been exonerated.
Imaging and laboratory tests are often useful when evaluating for IgG4-RD. In certain scenarios, classic radiographic findings (e.g. diffuse pancreatic enlargement with loss of lobulations and a halo sign around the pancreas, seen in 40% of patients with AIP) lend strong support to the diagnosis [47]. In other situations, such as a pancreatic mass, the diagnosis is challenging, and malignancy must be excluded. An elevated serum IgG4 concentration, especially at high levels, can support the diagnosis. However, IgG4 concentrations are neither highly sensitive nor specific to IgG4-RD, and elevations have been reported in pancreatic adenocarcinoma cases [25]. Additional laboratory findings, such as eosinophilia, elevated IgE, hypergammaglobulinemia and hypocomplementaemia can be observed in IgG4-RD [48–51].
Some laboratory tests can help to exclude IgG4-RD. Acute phase reactants, such as ESR and CRP, can be elevated but usually mildly so, particularly CRP; if either is very high (e.g. greater than several times the upper limit of normal), alternative diagnoses are likely. In addition, common mimickers of IgG4-RD, such as SS and ANCA-associated vasculitis, should be evaluated with anti-Ro, anti-La, anti-MPO and anti-PR3 antibodies, which are typically absent in IgG4-RD.
When evaluating for IgG4-RD, a physical examination should be conducted to evaluate for common manifestations, such as lacrimal and/or salivary gland enlargement. Cross-sectional imaging of the chest, abdomen and pelvis is recommended to assess for other manifestations that might support the diagnosis and be amenable to biopsy. CT, MRI and fluoro-deoxyglucose PET can be useful in the work-up [52–56].
Histology of IgG4-RD
Tissue biopsy remains helpful in many cases to support a diagnosis of IgG4-RD and to rule out alternative diagnoses. Characteristic pathological features of IgG4-RD include a dense lymphoplasmacytic infiltrate rich in IgG4+ plasma cells and CD4+ T cells [2]. This infiltrate is often accompanied by fibrosis that has a storiform pattern. The word storiform derives from the Latin word storea, or woven mat, describing the irregular, whorled pattern of fibrosis observed. Obliterative phlebitis, a destruction of venous walls and obstruction of their lumen with immune cell infiltration and collagen deposition, might be observed. Obliterative arteritis is less commonly seen. These features, however, are distinct from necrotizing vasculitis; indeed, necrosis, microabscesses and prominent neutrophilic infiltrates are not expected in IgG4-RD.
IgG4+ plasma cells are increased in affected tissue. Although an infiltrate of ≥50 IgG4+ plasma cells per high-power field and/or an IgG4+:IgG+ plasma cell ratio of >40% strongly support the diagnosis, there are no universally accepted cut-offs. The histological and immunohistochemical findings (e.g. IgG4+:IgG+ cut-offs) might vary across organs affected by IgG4-RD. For instance, lacrimal gland biopsies might have less storiform fibrosis than biopsies from other organs [57, 58]. Of note, it is challenging to diagnosis IgG4-RD from a lymph node biopsy because, in part, the presence of IgG4+ plasma cells in lymph nodes is not considered specific to IgG4-RD [46, 59, 60].
Considerations for diagnosing IgG4-related pancreatic disease
In addition to the 2019 ACR/EULAR IgG4-RD Classification Criteria, patients with pancreatic disease can also be evaluated for IgG4-RD using the HISORt (histology, imaging, serology, other organ involvement, response to therapy) criteria [61]. AIP classically presents with either diffuse or focal involvement, and certain pancreatic imaging findings can be supportive of a diagnosis of IgG4-RD [47, 62]. Diffuse involvement will often have more classic imaging features, including diffuse enlargement with loss of lobulations, characteristics of a sausage-shaped pancreas, long pancreatic duct strictures traversing more than one-third of the pancreas without downstream dilatation, a hyperenhancing thin rim surrounding the pancreas (also known as the halo sign) and hyperenhancement on venous phase. Focal involvement usually presents as a mass in the head of the pancreas leading to common bile duct dilatation (see IgG4-RD sclerosing cholangitis below). Focal AIP does not typically cause compression and subsequent dilatation of the main pancreatic duct. Pancreatic duct dilatation or vascular involvement should prompt evaluation for adenocarcinoma.
There are no guidelines regarding when a pancreatic biopsy should be pursued, but exclusion of malignancy is required in all focal AIP via endoscopic US with fine needle biopsy to obtain a core biopsy [63, 64]. Although fine needle aspiration can establish the diagnosis of malignancy, it is insufficient to establish a diagnosis of AIP because sample architecture is lost.
Pitfalls of diagnosis
Establishing a diagnosis can be challenging when a biopsy is not feasible. Biopsy confirmation is especially important if there are unusual manifestations or other aspects of the history suggesting an alternative diagnosis. In general, one must not anchor on the sole finding of IgG4+ plasma cells in tissue to establish the diagnosis, because it is not a specific finding [59].
The serum IgG4 concentration is an important component when assessing IgG4-RD, but it is also not specific for IgG4-RD [65, 66]. Among patients with serum IgG4 testing in a health-care system, the positive predictive value of a level >135 mg/dl was 34% [25]. Among patients with pancreatic disease, the positive predictive value of a level >140 mg/dl was 36% [67]. Even when very high (more than five times the upper limit of normal), the positive predictive value was 73%; thus 27% of patients with an IgG4 concentration more than five times the upper limit of normal had an alternative diagnosis [68]. These data demonstrate the importance of considering a broad differential when evaluating patients with an elevated serum IgG4 concentration.
Treatment
The goal of treatment in IgG4-RD is to reduce disease activity and prevent irreversible damage. Without treatment, the natural history in some is to accrue new organ involvement over time; therefore, patients who are untreated (e.g. mild salivary gland disease or resected disease from a single site) should be monitored closely. The response to treatment can vary based on the organ(s) involved and the duration of disease, both of which can be associated with the amount or stage of fibrosis; ultimately, the degree of fibrosis can dictate responsiveness to current treatments. The treatment goal for most patients is to induce and then maintain remission, but this approach should be personalized according to individual patient characteristics, disease manifestations and preferences. When evaluating disease activity, it is important to consider whether a manifestation is highly fibrotic and unlikely to respond to therapy (i.e. has significant damage).
Inducing remission
Remission is the state in which the disease manifestations have either resolved or returned to a newly established baseline and is assessed using evaluations tailored to the specific organs involved. Although organs can have damage and fibrosis that is irreversible, most patients have significant reduction in the size of lesions and improvement in laboratory parameters with appropriate treatment.
Worldwide, glucocorticoids are first-line therapy for IgG4-RD [69]. The usual initial dose is 0.5–1.0 mg/kg of prednisone, based on the severity of the presentation. The optimal glucocorticoid regimen is unknown, but the initial dose is typically continued for 2–4 weeks and then tapered off over 2–3 months. Although effective, glucocorticoids have many toxicities (e.g. diabetes), especially in this older population that often has pancreatic damage and other co-morbidities [70]. Furthermore, glucocorticoids rarely provide a durable treatment response once stopped, and most patients flare within 3 years of glucocorticoid discontinuation [71].
Given the toxicities and brief response, conventional DMARDs are often combined with glucocorticoids for induction therapy. The decision to use a DMARD upfront is often guided by the patient’s manifestations (e.g. risk for damage with relapse), demographics and co-morbidities (e.g. high risk for CS toxicity), risk of future flare (e.g. very high serum IgG4 at baseline, multi-organ disease) and patient preference. Prospective studies (Table 2) have compared glucocorticoid monotherapy vs glucocorticoids plus either MMF, CYC or LEF [75–77]. In all cases, combination therapy improved both remission and relapse rates compared with glucocorticoids alone. Retrospective studies and case series have also reported benefit with other treatments, including MTX, AZA and iguratimod [84–86]. No studies have evaluated the comparative efficacy of CS-sparing agents. Larger randomized controlled trials of CS-sparing therapies are needed to understand their role.
Table 2.
Clinical trials and comparative effectiveness studies in IgG4-related disease
| Study/trial | Study design a | Study arms | Primary outcome | Follow-up time | Results/status |
|---|---|---|---|---|---|
| Glucocorticoids | |||||
| Masaki et al. (2017) [72] | Single-arm open-label trial | PSL 0.6 mg/kg/day tapered to maintenance ≤10 mg (median 7 mg/day), n = 61 | CR | 12 months |
|
| Wu et al. (2017) [73] | Open-label RCT |
|
CR | 24 months |
|
| Masamune et al. (2017) [74] | Open-label RCT (AIP) |
|
Relapse | 36 months | Relapse rate 61% (group 1) vs 24% (group 2), P = 0.007 |
| Conventional synthetic DMARDs | |||||
| Yunyun et al. (2017) [75] | Non-randomized clinical trial |
|
Relapse rate | 12 months |
|
| Yunyun et al. (2019) [76] | Open-label RCT |
|
CR, PR | 12 months |
|
| Wang et al. (2020) [77] | Open-label RCT |
|
Relapse | 12 months | Hazard ratio for time to relapse 0.35 (0.13, 0.90, P = 0.23), favouring group 2 |
| Biologic DMARDs | |||||
| Carruthers et al. (2015) [78] | Single-arm open-label trial | RTX 1 g × 2 doses, either monotherapy (n = 26) or with concomitant glucocorticoids tapered off over 2 months (n = 4) | At 6 months: decline in IgG4-RD RI, no relapses, and no GC use after 2 months | 12 months |
|
| Ebbo et al. (2017) [79] | Retrospective cohort study |
|
Relapse | Mean 25 months |
|
| Majumder et al. (2018) [80] | Retrospective cohort study (pancreaticobiliary IgG4-RD) |
|
Relapse rate | Median 34 months (group 1), 27 months (group 2) |
|
| Campochiaro et al. (2020) [81] | Retrospective cohort study |
|
Relapse at 18 months | Median 26 months (group 1), 19 months (group 2a), 21 months (group 2b) | 71% relapse (group 1) vs 0% relapse (group 2), P = 0.006 |
| Matza et al. (2022) [82] | Single-arm open-label trial | Abatacept 125 mg s.c. weekly, n = 10 | CR | 6 months |
|
| Perugino et al. (2023) [83] | Single-arm, open-label trial | Obexelimab 5 mg/kg i.v. every 2 weeks with GCs discontinued within 2 months, n = 15 | Decline in IgG4-RD RI | 6 months |
|
| Other | |||||
| Zhang et al. (2019) [84] | Single-arm open-label trial (mild disease) | One i.m. injection of 5 mg betamethasone dipropionate with 2 mg betamethasone sodium phosphate + iguratimod 25 mg p.o. twice daily, n = 30 | CR, PR | 6 months |
|
Studies specific to AIP were limited to randomized controlled trials only.
AIP: autoimmune pancreatitis; CR: complete remission/response; GC: glucocorticoid; PDN: prednisone; PR: partial remission/response; PSL: prednisolone; RCT: randomized controlled trial; RI: responder index; RTX: rituximab; w/d: withdrawal.
Biologic DMARDs have also been studied in IgG4-RD (Table 2). Rituximab is an anti-CD20 mAb that depletes peripheral B cells. The clinical response to rituximab (and its biosimilar) in patients with IgG4-RD is often swift, leading to significant improvement in disease activity, as observed in two prospective, open-label single-arm trials. Patients are typically treated with 1 g twice over 14 days, and many patients achieve remission with no concomitant oral CS course. Although we frequently use rituximab, it has not been compared with CSs for remission induction, and the optimal dosing and use of concomitant glucocorticoids is unknown. Given the experience with rituximab, other B cell-targeted therapies are being investigated for IgG4-RD (Table 3).
Table 3.
Ongoing clinical trials in IgG4-related disease
| Trial | Design | Study arms | Primary outcome | Follow-up time | Results/status |
|---|---|---|---|---|---|
| Conventional synthetic DMARDs | |||||
| NCT05746689 | Open-label, single-arm trial | Sirolimus + PDN taper | Relapse rate | 3 months | Pre-enrolment |
| Biologic DMARDs | |||||
| NCT04918147 |
|
|
|
|
Part 1 b enrolling |
| NCT05662241 | Placebo-controlled RCT | Obexelimab + PDN taper vs placebo + PDN taper | Time to relapse | 12 months | Enrolling |
| NCT04660565 | RCT | Belimumab + GC vs GC monotherapy | Relapse rate | 12 months | Enrolling |
| NCT05728684 | Open-label single-arm trial | CM310 (anti-IL-4 receptor-α mAb) | Response rate | 3 months | Pre-enrolment |
| NCT04540497 | Placebo-controlled RCT | i.v. inebilizumab or placebo followed by optional 3-year open-label treatment period | Time to relapse | 12 months | Active, no longer enrolling |
| NCT02705638 | Open-label single-arm trial | Rituximab + lenalidomide | Remission | 24 months | Completed |
| Targeted synthetic DMARDs | |||||
| NCT05625581 | Non-randomized controlled trial | Tofacitinib + GC taper vs CYC + GC taper | Remission | 6 months | Enrolling |
| NCT04602598 | Open-label single-arm trial | Zanubrutinib | Submandibular and lacrimal gland volume | 6 months | Enrolling |
| NCT04520451 | Open-label two-arm trial |
|
Relapse | 12 months | Enrolling |
| NCT05781516 | RCT | Baricitinib + GC taper vs GC taper monotherapy | Relapse | 12 months | Enrolling |
GC: glucocorticoid; IgG4-RD RI: IgG4-related disease responder index; PDN: prednisone; RCT: randomized controlled trial.
T cell-targeted therapies are increasingly being studied in IgG4-RD. Abatacept, an inhibitor of T cell co-stimulation and activation, has been investigated but did not show promising results [82]. Several recent reports [87–89] have noted benefit in patients treated with dupilumab, a monoclonal anti-IL-4 receptor-α antibody, although this has not been studied in a prospective trial.
As of 2023, our usual practice is to induce remission using rituximab. Short course of glucocorticoids (up to 2–3 months) can be used for patients with severe or urgent disease (e.g. cholangitis, aortitis or vision-threatening orbital disease) in whom treatment is needed to prevent irreversible damage.
Approach to managing patients in remission
Some patients can benefit from maintenance therapy, although this remains poorly studied. First, patients with organ- and life-threatening manifestations (e.g. renal involvement with chronic kidney disease, pancreatic disease with insufficiency) can benefit from maintenance therapy given the risks imposed by disease flare. Second, patients with disease that can be detected only by imaging and in whom routine imaging might be difficult to obtain might benefit. Third, patients with multi-organ disease, elevated baseline serum IgG4 and/or IgE and/or peripheral eosinophilia are at the highest risk for relapse and might benefit from maintenance therapy [19, 90]. The risk of relapse can also vary based on the induction regimen; we routinely find that some patients, even with risk factors for relapse, have quiescent disease for ≥1 year after rituximab. Thus, the approach to maintain remission should be individualized to the specific manifestations of the patient, history of damage, co-morbidities and other factors.
Several maintenance regimens are used. In Asia, it is common to maintain remission with low-dose glucocorticoids (<10 mg/day of prednisone). In patients with IgG4-related pancreatitis, studies have shown a reduced risk of relapse when low-dose glucocorticoids are used compared with observation alone [71, 74], but relapse can occur even on low-dose glucocorticoids. CS-sparing medications have also been evaluated as maintenance therapies. A meta-analysis of 15 studies that included 1169 patients found a lower rate of relapse with a CS-sparing agent in combination with glucocorticoids compared with glucocorticoid monotherapy (odds ratio 0.39, 95% CI 0.20, 0.80) [91]. We and others frequently use rituximab to maintain remission, but this remains poorly studied [91]. When used as maintenance, 1 g of rituximab every 6 months is often used, but this can be spaced further apart depending on the individual patient history. Prospective studies to determine the optimal maintenance regimen are needed.
Monitoring disease activity and assessing damage in IgG4-RD
Close monitoring of disease activity is important to confirm successful induction of remission and to assess for disease relapses that require retreatment.
Biomarkers of disease activity and predictors of relapse
Laboratory tests used to monitor disease activity are often the same as those used to establish the diagnosis: serum IgG4 and IgE concentrations, complement levels (C3 and C4) and peripheral eosinophil counts (Table 4). The IgG4 concentration is most frequently used, particularly if it was previously elevated. When elevated at baseline, the IgG4 concentration typically decreases after the initiation of treatment with glucocorticoids, B cell depletion and other therapies [73, 78, 92, 93]. The serum IgG4 might not normalize and can remain elevated even in the absence of disease activity [78, 92, 93]. A rising level can indicate a brewing flare, but when or whether a flare will occur is not always clear [20, 92–96]. In those with hypocomplementaemia, elevated IgE concentrations or peripheral eosinophilia at baseline, recurrent abnormalities can herald a flare. Additionally, some baseline features can identify patients at high risk of relapse, including higher serum IgG4 concentrations, a greater extent of organ involvement, the presence of atopic features, peripheral eosinophilia and elevated serum IgE concentrations [19, 96–98]. Other organ-specific markers (e.g. urinary protein in tubulointerstitial nephritis, bilirubin and alkaline phosphatase in cholangitis) can be used to gauge disease activity at those sites.
Table 4.
Biomarkers for monitoring IgG4-related disease
| Test | Change may herald an IgG4-related disease flare |
|---|---|
| General laboratory tests | |
| Immunoglobulin G4 | ↑ |
| Immunoglobulin E | ↑ |
| Eosinophil count | ↑ |
| Complement components 3 and 4 | ↓ |
| ESR | ↑ |
| Plasmablast count | ↑ |
| Memory B cell count | ↑ |
| Organ-specific laboratory tests | |
| Lipase | ↑ |
| Alanine transaminase, aspartate transaminase | ↑ |
| Alkaline phosphatase, gamma-glutamyl transferase, bilirubin | ↑ |
| Creatinine | ↑ |
| Total urine protein to creatinine ratio | ↑ |
Monitoring disease activity
In patients with disease that is detectable by history and physical examination, this assessment, in combination with a laboratory evaluation, can be adequate to monitor disease activity. Many patients, however, have disease that is more apparent on imaging than on examination; in these cases, serial imaging is often necessary, and the preferred modality will vary by local practice and the organ affected. Disease relapses often affect previously involved organs, but new manifestations can occur, and clinicians should monitor for signs or symptoms thereof. Therefore, disease activity should be reassessed routinely every 3–6 months early on, even in the absence of symptoms, given the frequency of asymptomatic disease in IgG4-RD.
The management of serological relapse (e.g. rising IgG4 concentration) in the absence of a change in manifestations needs to be personalized. Patients with prior involvement of organs that are prone to damage and difficult to assess for active disease (e.g. pancreas) might benefit from treatment, even in the absence of other features of disease activity. In other cases, closer monitoring after a serological relapse is warranted to detect a flare early in its course.
Damage in IgG4-RD
IgG4-RD can cause irreversible organ damage because of untreated inflammation, the effect of a mass on neighbouring structures or iatrogenic damage related to diagnostic evaluation or treatment. In one series, 58% of patients had damage in at least one organ at diagnosis [99]. Examples of damage include sicca syndrome from resection of a salivary gland, proptosis from a fibrotic orbital pseudotumour, anosmia from sinonasal involvement [100–102], large vessel aneurysms or dissections [103, 104], and chronic ureteral obstruction or tubulointerstitial nephritis leading to chronic kidney disease, including end-stage kidney disease [99, 105–107].
The pancreas is among the most frequently damaged organ from IgG4-RD (Supplementary Table S2, available at Rheumatology Advances in Practice online); 60% of patients will have exocrine or endocrine damage at the time of diagnosis [15]. This reflects the indolent nature of pancreatic involvement such that patients will not generally exhibit symptoms until significant damage has occurred. In other forms of chronic pancreatitis, the risk of developing both diabetes and exocrine pancreatic insufficiency is high over the course of a patient’s lifetime with the disease. It is estimated that ≤65% of patients with type 1 AIP have diabetes. The majority of diabetes presents even before CS therapy is initiated [108, 109]. Exocrine pancreatic insufficiency with associated weight loss is seen in ≤50% of patients [110]. Patients with chronic pancreatitis are also at increased risk for osteopenia, osteoporosis and major micronutrient deficiencies [111]. All patients should be screened for diabetes with a haemoglobin A1c annually, for exocrine pancreatic insufficiency with a stool faecal elastase-1 and questions targeted at uncovering symptoms of maldigestion or malabsorption and micronutrient deficiencies, including vitamins A, E, D and K, zinc, selenium, iron, folate, vitamin B12 and magnesium at the time of diagnosis, annually and/or if new symptoms develop [112]. Exocrine pancreatic insufficiency should be managed with pancreatic enzyme replacement therapy at a dose of ≥1000 units/kg per meal and 500 units/kg per meal with snacks. Vitamins should be repleted and monitored annually.
Differentiating damage from active disease
Differentiating between active disease and damage in IgG4-RD can be challenging. Organ-specific markers, such as markers of cholestasis, proteinuria and glomerular filtration rate, almost always improve with treatment, but they frequently remain abnormal. Likewise, radiological findings (e.g. retroperitoneal fibrosis and mass lesions) often exhibit appreciable improvement after treatment, but in some cases a lack of progression can be a sign of effective treatment owing to the degree of damage that accrued before treatment. Two ways to differentiate damage from active disease include serial evaluations, with the expectation that damage will remain stable over time and active disease will worsen with time, and a trial of treatment with glucocorticoids, which would be expected to improve lesions that are attributable to active disease. Clinicians should be aware of any changes in activity that might suggest a possible malignancy developing at the site of prior IgG4-RD. A clue to this might be disease that was responsive to treatment in the past but now worsening despite resumption of previously effective treatment.
Future directions and knowledge gaps
Since the initial description of the disease that would become known as IgG4-RD in the early 2000s, knowledge of its epidemiology, pathogenesis and management has expanded dramatically (Table 5). Despite these advances, there are several important avenues for future investigation to improve the care of patients with IgG4-RD. First, further elucidating the pathogenesis of IgG4-RD, including the roles of IgG4 and complement, might identify new targets for therapeutics and approaches to management. Second, although there are now at least two phase 3 clinical trials investigating treatments for remission induction in IgG4-RD (Table 3), studies are also needed to define the risks and benefits of alternative strategies for managing the remission phase of the disease. Third, to inform the design of such studies, an improved understanding of the role of conventional and novel biomarkers of disease activity is needed, because these might guide decision-making. Fourth, although tools exist to measure disease activity objectively for research purposes, there are challenges with their implementation, and future efforts to revise these tools or identify new ones are needed. Fifth, many patients with IgG4-RD have highly fibrotic manifestations of the disease that do not improve substantially with our current treatments. The role of anti-fibrotic treatments is poorly understood and an important priority for future studies. Finally, IgG4-RD is a rare disease, and many patients express interest in connecting with other patients with this disease and in learning strategies to manage the uncertainty that comes with having IgG4-RD. At this time, there are no formal education or support resources for patients with IgG4-RD.
Table 5.
Future directions
Elucidating the pathogenesis of IgG4-related disease
|
Identifying safe and effective CS-sparing treatment strategies
|
Improving our understanding of the role of conventional and novel biomarkers of disease activity
|
| Refining instruments to measure disease activity from both the clinician and patient perspective |
Identifying ways to support patients through their journey with this IgG4-RD
|
Supplementary Material
Contributor Information
Zachary S Wallace, Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Harvard University, Boston, MA, USA.
Guy Katz, Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Harvard University, Boston, MA, USA.
Yasmin G Hernandez-Barco, Harvard Medical School, Harvard University, Boston, MA, USA; Division of Gastroenterology, Massachusetts General Hospital, Boston, MA, USA.
Matthew C Baker, Division of Immunology and Rheumatology, Stanford University, Palo Alto, CA, USA.
Supplementary material
Supplementary material is available at Rheumatology Advances in Practice online.
Data availability
No new data were generated or analysed in support of this article.
Funding
Y.G.H.-B. is supported by the Massachusetts General Hospital (MGH) Executive Committee on Research Physician and Scientist Development Award, MGH Department of Medicine Sanchez and Ferguson Research Faculty Award, and National Institutes of Health (NIH) RO1 CA215498-02. G.K. is supported by a Scientist Development Award from the Rheumatology Research Foundation and NIH T32 AR007258.
Disclosure statement: Z.S.W. reports research support from Zenas, Bristol-Myers Squibb, Principia, Amgen, and Sanofi and consulting/advisory board fees from Amgen, Viela Bio, Zenas BioPharma, PPD, Visterra, Novartis, BioCryst, Sanofi, Horizon and MedPace. G.K. reports research support from Sanofi. Y.G.H.-B. reports scientific advisory board fees from Nestle Health Science. M.C.B. reports research support from Zenas, Viela Bio, Beigene and Principia and consulting fees from Zenas and Viela Bio. The remaining authors have declared no conflicts of interest.
References
- 1. Hamano H, Kawa S, Horiuchi A. et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732–8. [DOI] [PubMed] [Google Scholar]
- 2. Deshpande V, Zen Y, Chan JK. et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–92. [DOI] [PubMed] [Google Scholar]
- 3. Wallace ZS, Fu X, Cook C. et al. Derivation and validation of algorithms to identify patients with immunoglobulin-G4-related disease using administrative claims data. ACR Open Rheumatol 2022;4:371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wallace ZS, Miles G, Smolkina E. et al. Incidence, prevalence and mortality of IgG4-related disease in the USA: a claims-based analysis of commercially insured adults. Ann Rheum Dis 2023;82:957–62. [DOI] [PubMed] [Google Scholar]
- 5. Masamune A, Kikuta K, Hamada S. et al. ; Collaborators. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol 2020;55:462–70. [DOI] [PubMed] [Google Scholar]
- 6. Huggett MT, Culver EL, Kumar M. et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol 2014;109:1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamisawa T, Zen Y, Pillai S, Stone JH.. IgG4-related disease. Lancet 2015;385:1460–71. [DOI] [PubMed] [Google Scholar]
- 8. Wallace ZS, Zhang Y, Perugino CA. et al. ; ACR/EULAR IgG4-RD Classification Criteria Committee. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis 2019;78:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanders S, Fu X, Zhang Y. et al. Lifetime allergy symptoms in IgG4-related disease: a case–control study. Arthritis Care Res (Hoboken) 2022;74:1188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hart PA, Kamisawa T, Brugge WR. et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013;62:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dillon J, Dart A, Sutherland T.. Imaging features of immunoglobulin G4-related disease. J Med Imaging Radiat Oncol 2016;60:707–13. [DOI] [PubMed] [Google Scholar]
- 12. Katabathina VS, Khalil S, Shin S. et al. Immunoglobulin G4-related disease: recent advances in pathogenesis and imaging findings. Radiol Clin North Am 2016;54:535–51. [DOI] [PubMed] [Google Scholar]
- 13. Huynh KN, Kong MJ, Nguyen BD.. Anatomic and functional imaging of immunoglobulin G4-related disease and its mimics. Radiographics 2023;43:e220097. [DOI] [PubMed] [Google Scholar]
- 14. Fujita A, Sakai O, Chapman MN, Sugimoto H.. IgG4-related disease of the head and neck: CT and MR imaging manifestations. Radiographics 2012;32:1945–58. [DOI] [PubMed] [Google Scholar]
- 15. Hart PA, Levy MJ, Smyrk TC. et al. Clinical profiles and outcomes in idiopathic duct-centric chronic pancreatitis (type 2 AIP): the Mayo Clinic experience. Gut 2016;65:1702–9. [DOI] [PubMed] [Google Scholar]
- 16. Shimosegawa T, Chari ST, Frulloni L. et al. ; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 2011;40:352–8. [DOI] [PubMed] [Google Scholar]
- 17. Mattoo H, Mahajan VS, Maehara T. et al. Clonal expansion of CD4+ cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol 2016;138:825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallace ZS, Mattoo H, Carruthers M. et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015;74:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallace ZS, Mattoo H, Mahajan VS. et al. Predictors of disease relapse in IgG4-related disease following rituximab. Rheumatology (Oxford) 2016;55:1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasaki T, Akiyama M, Kaneko Y. et al. Risk factors of relapse following glucocorticoid tapering in IgG4-related disease. Clin Exp Rheumatol 2018;36(Suppl 112):186–9. [PubMed] [Google Scholar]
- 21. Rispens T, Huijbers MG.. The unique properties of IgG4 and its roles in health and disease. Nat Rev Immunol 2023;23:763–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Neut Kolfschoten M, Schuurman J, Losen M. et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007;317:1554–7. [DOI] [PubMed] [Google Scholar]
- 23. Nirula A, Glaser SM, Kalled SL, Taylora FR.. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol 2011;23:119–24. [DOI] [PubMed] [Google Scholar]
- 24. Maslinska M, Dmowska-Chalaba J, Jakubaszek M.. The role of IgG4 in autoimmunity and rheumatic diseases. Front Immunol 2021;12:787422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH.. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14–8. [DOI] [PubMed] [Google Scholar]
- 26. Lian MJ, Liu S, Wu GY, Liu SY.. Serum IgG4 and IgG for the diagnosis of autoimmune pancreatitis: a systematic review with meta-analysis. Clin Res Hepatol Gastroenterol 2016;40:99–109. [DOI] [PubMed] [Google Scholar]
- 27. Mattoo H, Mahajan VS, Della-Torre E. et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014;134:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanzillotta M, Della-Torre E, Milani R. et al. Increase of circulating memory B cells after glucocorticoid-induced remission identifies patients at risk of IgG4-related disease relapse. Arthritis Res Ther 2018;20:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rastogi I, Jeon D, Moseman JE. et al. Role of B cells as antigen presenting cells. Front Immunol 2022;13:954936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Della-Torre E, Rigamonti E, Perugino C. et al. B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-related disease. J Allergy Clin Immunol 2020;145:968–81.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akiyama M, Yasuoka H, Yamaoka K. et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther 2016;18:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akiyama M, Suzuki K, Yamaoka K. et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol 2015;67:2476–81. [DOI] [PubMed] [Google Scholar]
- 33. Kamekura R, Takano K, Yamamoto M. et al. Cutting edge: a critical role of lesional T follicular helper cells in the pathogenesis of IgG4-related disease. J Immunol 2017;199:2624–9. [DOI] [PubMed] [Google Scholar]
- 34. Du H, Shi L, Chen P. et al. Prohibitin is involved in patients with IgG4 related disease. PLoS One 2015;10:e0125331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perugino CA, AlSalem SB, Mattoo H. et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol 2019;143:736–45.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hubers LM, Vos H, Schuurman AR. et al. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut 2018;67:728–35. [DOI] [PubMed] [Google Scholar]
- 37. Kino-Ohsaki J, Nishimori I, Morita M. et al. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjogren’s syndrome. Gastroenterology 1996;110:1579–86. [DOI] [PubMed] [Google Scholar]
- 38. Frulloni L, Lunardi C, Simone R. et al. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med 2009;361:2135–42. [DOI] [PubMed] [Google Scholar]
- 39. Okazaki K, Uchida K, Ohana M. et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology 2000;118:573–81. [DOI] [PubMed] [Google Scholar]
- 40. Asada M, Nishio A, Uchida K. et al. Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 2006;33:20–6. [DOI] [PubMed] [Google Scholar]
- 41. Endo T, Takizawa S, Tanaka S. et al. Amylase alpha-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes 2009;58:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lohr JM, Faissner R, Koczan D. et al. Autoantibodies against the exocrine pancreas in autoimmune pancreatitis: gene and protein expression profiling and immunoassays identify pancreatic enzymes as a major target of the inflammatory process. Am J Gastroenterol 2010;105:2060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shiokawa M, Kodama Y, Sekiguchi K. et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med 2018;10:eaaq0997. [DOI] [PubMed] [Google Scholar]
- 44. Jarrell JA, Baker MC, Perugino CA. et al. Neutralizing anti-IL-1 receptor antagonist autoantibodies induce inflammatory and fibrotic mediators in IgG4-related disease. J Allergy Clin Immunol 2022;149:358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Terao C, Ota M, Iwasaki T. et al. ; Japanese IgG4-Related Disease Working Consortium. IgG4-related disease in the Japanese population: a genome-wide association study. The Lancet Rheumatology 2019;1:e14–e22. [DOI] [PubMed] [Google Scholar]
- 46. Wallace ZS, Naden RP, Chari S. et al. ; Members of the ACR/EULAR IgG4-RD Classification Criteria Working Group. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis 2020;79:77–87. [DOI] [PubMed] [Google Scholar]
- 47. Sahani DV, Kalva SP, Farrell J. et al. Autoimmune pancreatitis: imaging features. Radiology 2004;233:345–52. [DOI] [PubMed] [Google Scholar]
- 48. Wallace ZS, Deshpande V, Mattoo H. et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol 2015;67:2466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sekiguchi H, Horie R, Kanai M. et al. IgG4-related disease: retrospective analysis of one hundred sixty-six patients. Arthritis Rheumatol 2016;68:2290–9. [DOI] [PubMed] [Google Scholar]
- 50. Della Torre E, Mattoo H, Mahajan VS. et al. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy 2014;69:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ebbo M, Daniel L, Pavic M. et al. IgG4-related systemic disease: features and treatment response in a French cohort: results of a multicenter registry. Medicine (Baltimore) 2012;91:49–56. [DOI] [PubMed] [Google Scholar]
- 52. Berti A, Della-Torre E, Gallivanone F. et al. Quantitative measurement of 18F-FDG PET/CT uptake reflects the expansion of circulating plasmablasts in IgG4-related disease. Rheumatology (Oxford) 2017;56:2084–92. [DOI] [PubMed] [Google Scholar]
- 53. Lee J, Hyun SH, Kim S. et al. Utility of FDG PET/CT for differential diagnosis of patients clinically suspected of IgG4-related disease. Clin Nucl Med 2016;41:e237–e243. [DOI] [PubMed] [Google Scholar]
- 54. Zhang J, Chen H, Ma Y. et al. Characterizing IgG4-related disease with 18F-FDG PET/CT: a prospective cohort study. Eur J Nucl Med Mol Imaging 2014;41:1624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ebbo M, Grados A, Guedj E. et al. Usefulness of 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography for staging and evaluation of treatment response in IgG4-related disease: a retrospective multicenter study. Arthritis Care Res (Hoboken) 2014;66:86–96. [DOI] [PubMed] [Google Scholar]
- 56. Takahashi H, Yamashita H, Morooka M. et al. The utility of FDG-PET/CT and other imaging techniques in the evaluation of IgG4-related disease. Joint Bone Spine 2014;81:331–6. [DOI] [PubMed] [Google Scholar]
- 57. Zen Y, Nakanuma Y.. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 2010;34:1812–9. [DOI] [PubMed] [Google Scholar]
- 58. Cheuk W, Yuen HK, Chan JK.. Chronic sclerosing dacryoadenitis: part of the spectrum of IgG4-related sclerosing disease? Am J Surg Pathol 2007;31:643–5. [DOI] [PubMed] [Google Scholar]
- 59. Strehl JD, Hartmann A, Agaimy A.. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol 2011;64:237–43. [DOI] [PubMed] [Google Scholar]
- 60. Rollins-Raval MA, Felgar RE, Krasinskas AM, Roth CG.. Increased numbers of IgG4-positive plasma cells may rarely be seen in lymph nodes of patients without IgG4-related sclerosing disease. Int J Surg Pathol 2012;20:47–53. [DOI] [PubMed] [Google Scholar]
- 61. Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic’s HISORt criteria. J Gastroenterol 2007;42(Suppl 18):39–41. [DOI] [PubMed] [Google Scholar]
- 62. Suzuki K, Itoh S, Nagasaka T. et al. CT findings in autoimmune pancreatitis: assessment using multiphase contrast-enhanced multisection CT. Clin Radiol 2010;65:735–43. [DOI] [PubMed] [Google Scholar]
- 63. Kanno A, Masamune A, Fujishima F. et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: a prospective multicenter study. Gastrointest Endosc 2016;84:797–804.e1. [DOI] [PubMed] [Google Scholar]
- 64. Iwashita T, Yasuda I, Doi S. et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol 2012;10:316–22. [DOI] [PubMed] [Google Scholar]
- 65. Culver EL, Sadler R, Simpson D. et al. Elevated serum IgG4 levels in diagnosis, treatment response, organ involvement, and relapse in a prospective IgG4-related disease UK cohort. Am J Gastroenterol 2016;111:733–43. [DOI] [PubMed] [Google Scholar]
- 66. Yu KH, Chan TM, Tsai PH, Chen CH, Chang PY.. Diagnostic performance of serum IgG4 levels in patients with IgG4-related disease. Medicine (Baltimore) 2015;94:e1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ghazale A, Chari ST, Smyrk TC. et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007;102:1646–53. [DOI] [PubMed] [Google Scholar]
- 68. Baker MC, Cook C, Fu X. et al. The positive predictive value of a very high serum IgG4 concentration for the diagnosis of IgG4-related disease. J Rheumatol 2023;50:408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Khosroshahi A, Wallace ZS, Crowe JL. et al. ; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 2015;67:1688–99. [DOI] [PubMed] [Google Scholar]
- 70. Da Silva JA, Jacobs JW, Kirwan JR. et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis 2006;65:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kamisawa T, Shimosegawa T, Okazaki K. et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009;58:1504–7. [DOI] [PubMed] [Google Scholar]
- 72. Masaki Y, Matsui S, Saeki T. et al. A multicenter phase II prospective clinical trial of glucocorticoid for patients with untreated IgG4-related disease. Mod Rheumatol 2017;27:849–54. [DOI] [PubMed] [Google Scholar]
- 73. Wu Q, Chang J, Chen H. et al. Efficacy between high and medium doses of glucocorticoid therapy in remission induction of IgG4-related diseases: a preliminary randomized controlled trial. Int J Rheum Dis 2017;20:639–46. [DOI] [PubMed] [Google Scholar]
- 74. Masamune A, Nishimori I, Kikuta K. et al. ; Research Committee of Intractable Pancreas Diseases in Japan. Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut 2017;66:487–94. [DOI] [PubMed] [Google Scholar]
- 75. Yunyun F, Yu C, Panpan Z. et al. Efficacy of cyclophosphamide treatment for immunoglobulin G4-related disease with addition of glucocorticoids. Sci Rep 2017;7:6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yunyun F, Yu P, Panpan Z. et al. Efficacy and safety of low dose mycophenolate mofetil treatment for immunoglobulin G4-related disease: a randomized clinical trial. Rheumatology (Oxford) 2019;58:52–60. [DOI] [PubMed] [Google Scholar]
- 77. Wang Y, Zhao Z, Gao D. et al. Additive effect of leflunomide and glucocorticoids compared with glucocorticoids monotherapy in preventing relapse of IgG4-related disease: a randomized clinical trial. Semin Arthritis Rheum 2020;50:1513–20. [DOI] [PubMed] [Google Scholar]
- 78. Carruthers MN, Topazian MD, Khosroshahi A. et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 2015;74:1171–7. [DOI] [PubMed] [Google Scholar]
- 79. Ebbo M, Grados A, Samson M. et al. Long-term efficacy and safety of rituximab in IgG4-related disease: data from a French nationwide study of thirty-three patients. PLoS One 2017;12:e0183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Majumder S, Mohapatra S, Lennon RJ. et al. Rituximab maintenance therapy reduces rate of relapse of pancreaticobiliary immunoglobulin g4-related disease. Clin Gastroenterol Hepatol 2018;16:1947–53. [DOI] [PubMed] [Google Scholar]
- 81. Campochiaro C, Della-Torre E, Lanzillotta M. et al. Long-term efficacy of maintenance therapy with rituximab for IgG4-related disease. Eur J Intern Med 2020;74:92–8. [DOI] [PubMed] [Google Scholar]
- 82. Matza MA, Perugino CA, Harvey L. et al. Abatacept in IgG4-related disease: a prospective, open-label, single-arm, single-centre, proof-of-concept study. Lancet Rheumatol 2022;4:e105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Perugino CA, Wallace ZS, Zack DJ. et al. Evaluation of the safety, efficacy, and mechanism of action of obexelimab for the treatment of patients with IgG4-related disease: an open-label, single-arm, single centre, phase 2 pilot trial. Lancet Rheumatol 2023;5:e442–e50. [DOI] [PubMed] [Google Scholar]
- 84. Zhang P, Gong Y, Liu Z. et al. Efficacy and safety of iguratimod plus corticosteroid as bridge therapy in treating mild IgG4-related diseases: a prospective clinical trial. Int J Rheum Dis 2019;22:1479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Della-Torre E, Campochiaro C, Bozzolo EP. et al. Methotrexate for maintenance of remission in IgG4-related disease. Rheumatology (Oxford) 2015;54:1934–6. [DOI] [PubMed] [Google Scholar]
- 86. de Pretis N, Amodio A, Bernardoni L. et al. Azathioprine maintenance therapy to prevent relapses in autoimmune pancreatitis. Clin Transl Gastroenterol 2017;8:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Otani T, Iwamoto H, Yoshida Y. et al. Dupilumab as an adjunct treatment for a patient with steroid-dependent immunoglobulin G4-related disease complicated by asthma: a case report. J Asthma 2022;59:2395–401. [DOI] [PubMed] [Google Scholar]
- 88. Kanda M, Kamekura R, Sugawara M. et al. IgG4-related disease administered dupilumab: case series and review of the literature. RMD Open 2023;9:e003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Simpson RS, Lau SKC, Lee JK.. Dupilumab as a novel steroid-sparing treatment for IgG4-related disease. Ann Rheum Dis 2020;79:549–50. [DOI] [PubMed] [Google Scholar]
- 90. Culver EL, Sadler R, Bateman AC. et al. Increases in IgE, eosinophils, and mast cells can be used in diagnosis and to predict relapse of IgG4-related disease. Clin Gastroenterol Hepatol 2017;15:1444–52.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Omar D, Chen Y, Cong Y, Dong L.. Glucocorticoids and steroid sparing medications monotherapies or in combination for IgG4-RD: a systematic review and network meta-analysis. Rheumatology (Oxford) 2020;59:718–26. [DOI] [PubMed] [Google Scholar]
- 92. Tabata T, Kamisawa T, Takuma K. et al. Serial changes of elevated serum IgG4 levels in IgG4-related systemic disease. Intern Med 2011;50:69–75. [DOI] [PubMed] [Google Scholar]
- 93. Gan L, Luo X, Fei Y. et al. Long-term outcomes of IgG4-related ophthalmic disease in a Chinese IgG4-related disease cohort. Front Med (Lausanne) 2021;8:784520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu Y, Zeng Q, Zhu L. et al. Relapse predictors and serologically unstable condition of IgG4-related disease: a large Chinese cohort. Rheumatology (Oxford) 2020;59:2115–23. [DOI] [PubMed] [Google Scholar]
- 95. Suzuki D, Shimizu K, Tokushige K.. Relative rise of serum IgG4 levels after steroid therapy for autoimmune pancreatitis predicts the likelihood of relapse. Pancreas 2018;47:412–7. [DOI] [PubMed] [Google Scholar]
- 96. Peng Y, Li JQ, Zhang PP. et al. Clinical outcomes and predictive relapse factors of IgG4-related disease following treatment: a long-term cohort study. J Intern Med 2019;286:542–52. [DOI] [PubMed] [Google Scholar]
- 97. Zhou J, Peng Y, Peng L. et al. Serum IgE in the clinical features and disease outcomes of IgG4-related disease: a large retrospective cohort study. Arthritis Res Ther 2020;22:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhao Z, Liu Y, Bai M. et al. Clinical profiles differ in IgG4-related disease with and without allergy: a large case-control study in China. Clin Exp Rheumatol 2023;41:1808–14. [DOI] [PubMed] [Google Scholar]
- 99. Martin-Nares E, Hernandez-Molina G, Rodriguez-Ramirez S. et al. IgG4-related kidney disease: experience from a Mexican cohort. Clin Rheumatol 2020;39:3401–8. [DOI] [PubMed] [Google Scholar]
- 100. Yagi-Nakanishi S, Kondo S, Kaneda M. et al. Olfactory dysfunction in IgG4-related disease. Chem Senses 2016;41:721–5. [DOI] [PubMed] [Google Scholar]
- 101. Wallace ZS, Deshpande V, Stone JH.. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum 2014;43:806–17. [DOI] [PubMed] [Google Scholar]
- 102. Han X, Zhang P, Li J. et al. Clinical features and treatment efficacy for IgG4-related thyroiditis. Orphanet J Rare Dis 2021;16:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Perugino CA, Wallace ZS, Meyersohn N. et al. Large vessel involvement by IgG4-related disease. Medicine (Baltimore) 2016;95:e3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Katz G, Hedgire SH, Stone JR. et al. IgG4-related disease as a variable-vessel vasculitis: a case series of 13 patients with medium-sized coronary artery involvement. Semin Arthritis Rheum 2023;60:152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liao S, Wang Y, Li K. et al. Idiopathic retroperitoneal fibrosis: a cross-sectional study of 142 Chinese patients. Scand J Rheumatol 2018;47:198–205. [DOI] [PubMed] [Google Scholar]
- 106. Peng L, Zhang P, Li J. et al. IgG4-related aortitis/periaortitis and periarteritis: a distinct spectrum of IgG4-related disease. Arthritis Res Ther 2020;22:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Su T, Wang H, Wang S, Yang L.. Clinicopathological patterns and predictors of the functional restoration of immunoglobulin G4-related kidney disease: a Chinese single-center cohort study. Front Med (Lausanne) 2021;8:736098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ito T, Nakamura T, Fujimori N. et al. Characteristics of pancreatic diabetes in patients with autoimmune pancreatitis. J Dig Dis 2011;12:210–6. [DOI] [PubMed] [Google Scholar]
- 109. Kamisawa T, Egawa N, Inokuma S. et al. Pancreatic endocrine and exocrine function and salivary gland function in autoimmune pancreatitis before and after steroid therapy. Pancreas 2003;27:235–8. [DOI] [PubMed] [Google Scholar]
- 110. Deshpande V, Gupta R, Sainani N. et al. Subclassification of autoimmune pancreatitis: a histologic classification with clinical significance. Am J Surg Pathol 2011;35:26–35. [DOI] [PubMed] [Google Scholar]
- 111. Vujasinovic M, Nikolic S, Gordon Achour A, Lohr JM.. Autoimmune pancreatitis and micronutrients. Dig Liver Dis 2023;55:1375–81. [DOI] [PubMed] [Google Scholar]
- 112. Whitcomb DC, Duggan SN, Martindale R. et al. AGA-PancreasFest joint symposium on exocrine pancreatic insufficiency. Gastro Hep Adv 2023;2:395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sah RP, Chari ST, Pannala R. et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology 2010;139:140–8. quiz e12–3. [DOI] [PubMed] [Google Scholar]
- 114. Kamisawa T, Takuma K, Tabata T. et al. Serum IgG4-negative autoimmune pancreatitis. J Gastroenterol 2011;46:108–16. [DOI] [PubMed] [Google Scholar]
- 115. Song TJ, Kim JH, Kim MH. et al. Comparison of clinical findings between histologically confirmed type 1 and type 2 autoimmune pancreatitis. J Gastroenterol Hepatol 2012;27:700–8. [DOI] [PubMed] [Google Scholar]
- 116. Milosavljevic T, Kostic-Milosavljevic M, Jovanovic I, Krstic M.. Extraintestinal manifestations of autoimmune pancreatitis. Dig Dis 2012;30:220–3. [DOI] [PubMed] [Google Scholar]
- 117. Notohara K, Nishimori I, Mizuno N. et al. Clinicopathological features of type 2 autoimmune pancreatitis in Japan: results of a multicenter survey. Pancreas 2015;44:1072–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this article.