Abstract
Parkinson’s disease (PD) is a complicated neurodegenerative disease, characterized by the accumulation of α-synuclein (α-syn) in Lewy bodies and neurites, and massive loss of midbrain dopamine neurons. Increasing evidence suggests that gut microbiota and microbial metabolites are involved in the development of PD. Among these, short-chain fatty acids (SCFAs), the most abundant microbial metabolites, have been proven to play a key role in brain-gut communication. In this review, we analyze the role of SCFAs in the pathology of PD from multiple dimensions and summarize the alterations of SCFAs in PD patients as well as their correlation with motor and non-motor symptoms. Future research should focus on further elucidating the role of SCFAs in neuroinflammation, as well as developing novel strategies employing SCFAs and their derivatives to treat PD.
Keywords: Parkinson’s disease, Short-chain fatty acids, Microbial metabolites, Brain-gut-microbiota axis
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease in the world, causing huge health and economic burdens on society. According to estimates, the frequency of PD will increase as the population ages and the mortality risk for PD patients has risen by 1.5–2.2 times [1, 2]. PD is characterized by classical motor symptoms, including bradykinesia, resting tremor, rigidity, and postural instability. In addition, PD patients are often complicated with many non-motor symptoms, such as constipation, anosmia, cognitive impairment, and depression [3]. Since the first direct demonstration of the close relationship between PD and the gut microbiota using fecal transplantation, the role of intestinal microorganisms and metabolites in the development of PD has been gradually emphasized [4]. Short-chain fatty acids (SCFAs), produced in the colon by the fermentation of dietary fiber, not only play an essential role in maintaining intestinal homeostasis but also affect the function of the central nervous system (CNS) through the peripheral circulation and vagus nerve. A significant reduction in the synthesis of SCFAs has been found in the feces of PD patients, and many animal studies have demonstrated the protective effect of SCFAs against PD. Despite the rapid progress in recent years, there are still no reviews specifically summarizing and discussing these aspects. This review aims to explain the relationship between SCFAs and PD from the perspective of pathology and the clinic based on the existing evidence and put forward suggestions for future research directions.
Metabolism of SCFAs
SCFAs are organic fatty acids with fewer than six carbon atoms, and mainly include acetic acid (AA), propionic acid (PA), and butyric acid (BA), with a proportion of approximately 60:20:20. The primary source of SCFAs is microbial fermentation of dietary fiber. Microorganisms can also obtain carbohydrates from the colonic mucus layer [5]. After production, SCFAs are quickly absorbed by colon epithelial cells through the monocarboxylate transporter (MCT) and sodium-coupled monocarboxylate transporter (SMCT) [6]. In the colon epithelium, SCFAs can be used in mitochondria β-oxidation and the citric acid cycle to provide energy for epithelial cells [7]. Only 5–10% of SCFAs are excreted through feces, while the unmetabolized SCFAs are delivered to the portal vein circulation. To prevent high SCFAs concentrations in the blood, the liver metabolizes most of the propionate and butyrate in the portal circulation [8]. Finally, only a small amount of acetate, propionate, and butyrate from the colon reaches the systemic circulation and peripheral tissues. Due to the high-fat solubility of SCFAs and the high expression of transporters on endothelial cells, SCFAs entering the circulation can cross the blood-brain barrier (BBB) and reach the brain [9]. For the first time, 14 C-SCFA uptake in the brain was discovered after injection into the carotid artery of rats [10, 11]. A study also reported that the tissue concentration of butyrate in the human brain was 17.0 pmol/mg, and propionate was 18.8 pmol/g [12]. These results suggest a potential role of SCFAs in the brain.
Mechanism of SCFAs
Inhibition of Histone Deacetylase
In the nucleus, histone acetylation and histone deacetylation are in dynamic balance and are jointly regulated by histone acetyltransferase and histone deacetylase (HDAC). In neurodegenerative diseases, acetylation homeostasis is significantly disrupted, leading to a decrease in the histone acetylation level. HDACs inhibitors can increase histone acetylation and promote the expression of genes involved in cell survival and neuroprotection [13]. SCFAs can enter cells via transporters and inhibit HDACs activity, among which BA is one of the most effective inhibitors of Class I and Class IIa HDACs. According to the experimental results in various models, HDACs inhibitors have been proven to have neuroprotective effects on PD. In 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced PD mice, HDACs inhibitors, such as sodium valproate and sodium butyrate, increased the expression of glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) in astrocytes, while histone H3 acetylation was significantly increased [14–16]. In rotenone-induced PD drosophila, sodium butyrate effectively reduced dopaminergic neurodegeneration and increased dopamine levels in the brain. In addition, drosophila with HDAC gene knockout can resist rotenone-induced movement disorders and early death [17]. In lipopolysaccharide (LPS)-treated glial cells, HDACs inhibitors can provide neuroprotection by inhibiting the release of proinflammatory cytokines and chemokines from microglia [18, 19]. Therefore, SCFAs can increase the synthesis of neurotrophic factors and suppress the expression of inflammatory factors to inhibit neurodegeneration by inhibiting HDACs.
Combination with GPCR
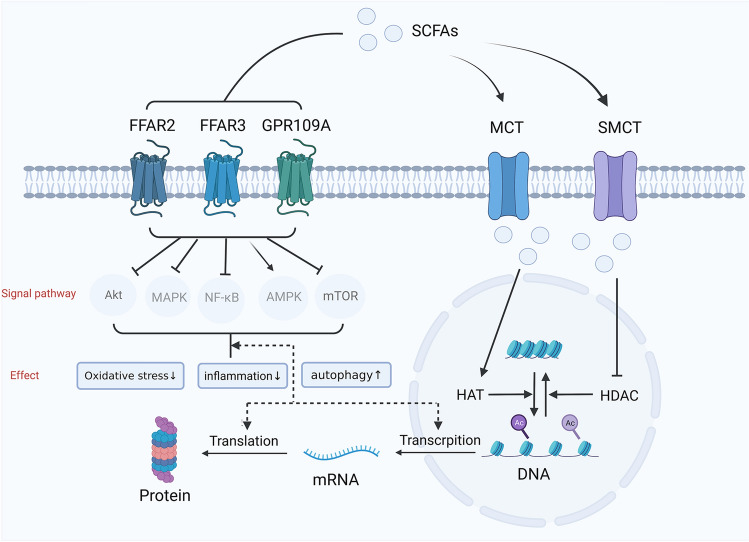
SCFAs can bind to G protein-coupled receptors (GPCRs) on the cell membrane, including GPR41/free fatty acid receptor 3 (FFAR3), GPR43/FFAR2, GPR42, and GPR109a [20]. After binding to these membrane receptors, SCFAs can inhibit downstream NF-κB and MAPK signaling to suppress inflammation, and enhance AMPK signaling while inhibiting mTOR signaling to enhance autophagy and enhance Nfr2 signaling to reduce oxidative stress [21–23]. These complicated signaling pathways regulate cellular immunity, metabolism, and other processes (Fig. 1). FFAR2 has a higher affinity for SCFAs with shorter chains, but FFAR3 has a higher affinity for SCFAs with longer chains, like butyrate. FFAR2 and FFAR3 are abundant in immune cells, adipose tissue, intestine, and bone marrow [24–26]. The expression of FFAR3 in sympathetic ganglia appears essential for controlling sympathetic nerve activity, as demonstrated by reduced activity in FFAR3 knockout mice [27]. Furthermore, the expression of FFAR3 and transporters of SCFAs have been confirmed in brain endothelial cells, indicating that SCFAs may affect the function of the BBB [28].
Fig. 1.
Mechanism of short-chain fatty acids. SCFAs primarily act on target cells through two mechanisms. First, SCFAs can enter the cell through MCT and SMCT transporters on the cell membrane, and then enter the cell nucleus to inhibit HDAC and to activate HAT, resulting in increased histone acetylation, the gradual loosening of dense chromosomes, and finally increased gene expression. Another mode of action is to combine GPCR on the cell membrane, such as FFAR2, FFAR3, or GPR109A, which can inhibit downstream NF-κB, Akt, MAPK, mTOR, and activate downstream AMPK signal pathways, thus regulating gene transcription and translation to alleviate inflammation, reduce oxidative stress and enhance the role of autophagy. NF-κB, nuclear factor-κB; AMPK, adenosine 5’-monophosphate (AMP)-activated protein kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin. Created with BioRender.com.
Although most research on FFAR2 and FFAR3 has focused on obesity, diabetes, and digestive system diseases, more and more scientists are exploring the role of these receptors in neurodegenerative diseases, especially PD. A cell experiment confirmed that sodium butyrate can protect dopaminergic cells from Salsolinol-induced neurotoxicity by activating FFAR3 [29]. FFAR2 was also proved to be involved in regulating the growth and maturation of microglia, which will be discussed later [30]. It can be seen that FFAR2 and FFAR3 may have a direct effect on the CNS, but it is controversial that the expression of FFAR2 and FFAR3 in nerve cells is very low and their function is unclear. Interestingly, studies have found that sodium butyrate can provide effective neuroprotection by acting on FFAR2 and FFAR3 expressed in peripheral tissues. SCFAs can combine FFAR2 and FFAR3 expressed in enteric endocrine cells to increase the secretion of glucagon-like peptide-1 (GLP-1), thus alleviating the motor symptoms and dopaminergic neurodegeneration induced by MPTP [31]. Additionally, osteocalcin could play a neuroprotective role in PD mice by increasing the production of propionate which acts as an FFAR3 agonist targeting the enteric nervous system (ENS) [32].
SCFAs and PD Pathology
It is known that PD is characterized by cerebral pathology. However, since Braak’s staging theory was proposed, people have gradually realized the essential role of the brain-gut axis in PD [33, 34]. As more evidence has emerged that gut microbiota might play a role in the interaction between the gut and brain, the concept of the “brain-gut-microbiota axis” has been proposed. SCFAs are critical for maintaining intestinal function and signal communication of the brain-gut-microbiota axis. Therefore, in this section, we analyzed the role of SCFAs in PD pathology from three dimensions: the gut, brain-gut-microbiota axis, and brain.
SCFAs and Gut
Intestinal Mucosal Barrier
A significant increase in 24-h urine excretion of sucralose was observed in PD patients compared to the control group, indicating an increase in colonic permeability [35]. Another method for assessing intestinal barrier function in vivo is to measure α1-antitrypsin and zonulin in feces. In a case-control study, the level of α1-antitrypsin and zonulin in PD patients increased significantly [36], indicating impaired intestinal barrier function in these patients. SCFAs contribute to the stability of the intestinal mucosal barrier in many ways. First, SCFAs provide energy for intestinal epithelial cells. SCFAs can also regulate the expression of tight junction proteins (TJPs) to enhance intestinal mucosal barrier function, through stabilization of hypoxia-inducible factor (HIF) and activation of AMP-Activated Protein Kinase (AMPK) [37, 38]. In addition, SCFAs can enhance the barrier by stimulating mucus production through the activation of FFAR3 [39]. In MPTP mice, propionate improved intestinal mucosal barrier damage and dyskinesia by inhibiting the AKT signaling pathway [40], and butyrate enhanced intestinal barrier stability by activating GPR109A and inhibiting the TLR4/NF-κB pathway [41]. By enhancing the intestinal barrier and reducing the penetration of inflammatory factors, bacterial products and α-synuclein (α-syn) into the systemic circulation, SCFAs will reduce systemic inflammation and neuroinflammation in PD.
Intestinal Inflammation
Intestinal inflammation is common in people with PD. Gene expression encoding proinflammatory cytokines in intestinal tissues is increased in PD patients [42, 43]. There were also more CD3+T cells and cells expressing Toll-like Receptor 4 (TLR4). After TLR4 was knocked out in PD mice, symptoms were significantly alleviated [35, 44, 45]. Another piece of evidence is the close association between PD and inflammatory bowel disease (IBD). Previous autopsy studies revealed aggregated α-syn in the ENS of PD patients [46, 47], whereas a recent study discovered α-syn aggregates in the ENS of IBD patients [48]. In addition, IBD patients have a higher risk of PD [49]. The long-term intestinal inflammatory state can induce abnormal α-syn aggregation and aggregation of α-syn can also aggravate intestinal inflammation.
The latest research found intestinal inflammation could trigger gut-to-brain propagation of α-syn and amyloid protein β (Aβ) in a C/EBPβ/AEP-dependent manner [50, 51]. Interestingly, SCFAs could inhibit the increased expression of C/EBPβ and thus further suppress intestinal inflammation [52]. Therefore, the role of SCFAs in regulating intestinal inflammation of PD and their effect on gut-brain transmission of α-syn should be a focus of research. GPR109A signals stimulated by BA can increase interleukin (IL)-18 secretion in intestinal epithelial cells and stimulate the production of Treg cells and IL-10-producing T-cells [53], which is essential for maintaining balance in the intestinal immune environment. In addition, butyrate can inhibit the TLR4/MyD88/NF-B signaling pathway, which is abnormally activated in the brain and gut of MPTP mice [54]. Thus, inhibition of intestinal inflammation by SCFAs is an important mechanism for resisting neuroinflammation in PD.
SCFAs and the Brain-Gut-Microbiota Axis
Gut Microbiota
Significant changes in gut microbiota were found in PD patients, and these changes occurred in the early stage, which may be a precursor biomarker of PD [55, 56]. In addition, dysbiosis of gut microbiota is likely to be a potential trigger factor for PD. Compared to germ-free (GF) mice, the presence of intestinal microorganisms can aggravate motor symptoms and central pathological changes in ASO (α-syn overexpressing) mice, while antibiotic treatment can alleviate these changes [4]. Transplantation of feces from MPTP mice to healthy mice could lead to motor function impairment and a decrease in striatal dopamine levels, whereas transplantation of feces from healthy mice to PD mice could relieve motor dysfunction and restore striatal dopamine levels [57]. Therefore, the gut microbiota is essential for the pathological progress of PD. Analyzing the composition of gut microbiota in PD patients, many studies have demonstrated a decrease in SCFAs-producing bacteria and an increase in proinflammatory bacteria [58, 59]. Interestingly, SCFAs can also affect the composition and structure of gut microbiota by modifying intestinal pH, mucosal permeability, mucin synthesis, and intestinal immunity. Supplementation of sodium butyrate effectively improved gut dysbiosis in rotenone and MPTP models and helped establish a new gut microbiota balance [54, 60]. Therefore, there may be a reciprocal regulatory relationship between microbial metabolites and gut microbiota.
Vagus Nerve
The vagus nerve is an important channel for signal transmission and material transport between the brain and intestine. Through activation of the vagus nerve, the changes in gut microbes can affect the brain in terms of behavior and mood. Vagal nerve stimulation is an effective treatment in a variety of PD models, displaying significant anti-inflammatory and neuroprotective effects, but chronic impairment of vagus nerve function led to inhibition of dopamine neurons [61–63]. FFAR3 is found in the afferent nervous system around the portal vein, as well as the intestinal plexus and autonomic nerve [64, 65]. SCFAs in the intestine can stimulate vagal afferent pathways, increasing parasympathetic output from various brain areas and altering the expression in specific brain regions [66]. The infusion of sodium butyrate (10 mM) into anesthetized rats caused vagal afferent discharge, which was eliminated after subphrenic vagotomy [67]. The mechanism of vagal activation by SCFAs in PD deserves further study.
Intestinal Endocrine System
The brain-gut interaction is also significantly influenced by the intestinal endocrine system. The intestine is not only a digestive organ, but also the largest endocrine organ. Changes in the intestinal endocrine system will significantly affect the activities of the CNS. After binding FFAR2 and FFAR3 expressed in intestinal endocrine cells, SCFAs will stimulate the secretion of peptide YY (PYY) and GLP-1 [68]. These peptides can be transmitted to the brain through vagal afferents and circulating blood, influencing appetite and eating behavior. Receptors of GLP-1 and PYY are expressed in different brain regions [69, 70]. In addition, these peptides can regulate cognitive and emotional processes, including anti-anxiety and anti-depression effects, as well as the improvement of memory and neural plasticity [70–73]. As described previously, sodium butyrate can increase the expression of GLP-1 in the intestine and GLP-1R in the brain to improve PD in animal models [31]. Therefore, SCFAs can mediate the brain-gut interaction by affecting the intestinal endocrine system, which may have positive implications for PD patients.
Systemic Circulation
Besides intestinal inflammation and neuroinflammation, proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1, and IL-6 increased in the peripheral blood of PD patients [74, 75]. The number of activated T cells and NK cells also increased, but naive T cells and IL-17-producing T cells decreased [76–78]. T-cell infiltration and α-syn were found in the CNS and peripheral blood of PD patients, and α-syn is the antigen target of some T cells [79–81]. Evidence suggests a subtle relationship between central inflammation and peripheral inflammation. SCFAs can prevent bacteria and bacterial inflammatory products from migrating into systemic circulation by enhancing the intestinal mucosal barrier. Furthermore, SCFAs can directly interact with immune cells to regulate peripheral immunity. As previously stated, FFAR2 and FFAR3 are abundantly expressed in various immune cells. It was reported that SCFAs could regulate the differentiation, recruitment, and activation of different immune cells [82]. For example, SCFAs can regulate the number and function of peripheral Treg cells and promote immune tolerance by inhibiting HDACs. Through the interaction between immune cells and SCFAs, NF-κB activity was inhibited, and inflammatory cytokines were reduced [83, 84]. These functions are crucial for maintaining balance in the immune environment.
SCFAs and the Brain
Blood-brain Barrier
The blood-brain barrier (BBB) is mainly composed of endothelial cells, astrocytes, and pericytes, embedded with various transmembrane proteins, such as the claudin protein family, occludins, and zonula occluden-1 (ZO-1) [85]. The BBB is highly selective and can prevent harmful toxins and pathogens from entering the brain. The integrity of the BBB is destroyed in PD patients, with decreased expression of tight junction proteins (TJPs) [86, 87]. α-syn aggregation can also increase BBB permeability and accelerate the pathological development of PD [88, 89]. Destruction of the BBB may allow harmful substances and inflammatory factors to enter the CNS, causing inflammation and neuronal damage. Currently, SCFAs have been proven to help maintain the integrity of the BBB. SCFAs can regulate the expression of TJPs through inhibition of NF-κB and activation of Nrf2, a transcription factor involved in the antioxidant pathway [90]. GF mice showed a decrease in the expression of TJPs, which led to an increase in the permeability of the BBB [91]. Interestingly, either GF mice monoclonal by SCFAs-producing strains or GF mice orally gavaged with sodium butyrate effectively reversed the increase in permeability through the increased expression of TJPs. A similar phenomenon has been verified in mice with MPTP-induced PD. The levels of occludins and ZO-1 in the brain were significantly reduced in PD mice but significantly increased in PD mice treated with intragastric sodium butyrate [31]. In addition, SCFAs can maintain the integrity of the BBB by interacting with FFAR3 receptors on brain endothelial cells. Studies have found that propionate can reduce the expression of CD14 on the cell surface and activate Nrf2, thereby reducing the oxidative stress injury of the BBB [28]. These results support the beneficial role of SCFAs in the protection of the BBB.
Neuroinflammation
Positron emission tomography revealed that activated microglia widely exist in different brain regions of PD patients, such as the substantia nigra, brainstem, and basal ganglia [92]. Microglia are associated with neuroinflammation during the development of PD due to the consistently elevated levels of proinflammatory cytokines in reactive microglia [93]. In normal conditions, microglia can clear up foreign pathogens and toxins, including aggregated α-syn, but long-term chronic microglial activation can increase neurotoxicity and induce abnormal aggregation of α-syn [94]. The gut microbiota and microbial metabolites appear to be crucial for the growth of the brain’s immune system. Erny et al. found that GF mice exhibited hypoplasia of microglia and impaired innate immune response, while the supplementation of a SCFAs mixture promoted the maturation of microglia [30]. Interestingly, this study also discovered that the microglia of FFAR2 knockout mice were severely malformed and showed immature morphology. However, in line with earlier studies, they did not detect FFAR2 mRNA expression in microglia and neurons; therefore, it is still unknown how SCFAs affect the development and maturation of microglia. One year later this question was raised, and the role of SCFAs in the neuroinflammation of PD triggered a new wave of controversy. Most studies have confirmed that SCFAs play an anti-inflammatory role in the periphery, but there are still different opinions on neuroinflammation. Similar to the previous research ideas, Sampson et al. expanded the ASO mouse model [4]. Compared with GF-ASO mice, SPF-ASO mice showed more severe motor defects and neuroinflammation. Surprisingly, the administration of a SCFAs mixture to GF-ASO mice promoted motor dysfunction, neuroinflammation, and α-syn aggregation, inducing similar alterations to SPF-ASO mice.
The current controversy is mainly that supplementation with SCFAs under sterile conditions can activate the neuroimmune system and increase neuroinflammation, thereby exacerbating abnormal protein deposition in the brain [4, 95]. However, under SPF conditions, most studies still find that SCFAs play an anti-neuroinflammatory role. SCFAs can directly inhibit inflammatory signal pathways such as NF-κB and MAPK in the CNS, and can also inhibit neuroinflammation by reducing peripheral inflammation and increasing BBB stability. Toxic models of PD, such as MPTP and 6-OHDA, have significant neuroinflammation but are effectively suppressed by supplementation with SCFAs [23, 41, 54, 96]. A prebiotic diet effectively improved the symptoms and pathology of ASO mice by increasing the production of SCFAs to inhibit microglia activation and induce their conversion to a neuroprotective subtype [97]. Interestingly, this study found that the levels of SCFAs and their receptors were not increased in the brain and that histone acetylation levels were not significantly altered, consistent with the reports by Erny [30]. This evidence strongly supports the possibility that SCFAs may indirectly regulate the state of immune cells in the brain via the periphery. A chronic cerebral hypoperfusion model showed decreased fecal AA and PA but SCFAs replenishment exerted anti-neuroinflammatory effects by inhibiting microglial activation as well as switching microglial phenotype from M1 toward M2 [98]. Although the exact mechanism is unclear, it is certain that SCFAs have anti-neuroinflammation effects in PD by regulating microglia differentiation and maturation and inhibiting neuroinflammatory pathways through direct or indirect pathways.
Damage to Dopaminergic Neurons
The degeneration and loss of dopaminergic neurons is the most important pathological feature related to multiple factors, such as mitochondrial damage, oxidative stress, and cell apoptosis [99–101]. SCFAs can promote the synthesis of tyrosine hydroxylase (TH) to increase brain dopamine levels and reduce oxidative stress and cell apoptosis by inhibiting the NF-κB signaling pathway [102, 103]. SCFAs also increase the expression of GDNF and BDNF in astrocytes, which are important in regulating the growth, survival, and differentiation of neurons and synapses [104, 105]. In addition, SCFAs can increase the expression of DNA repair genes, thereby reducing α-syn-induced DNA damage [102]. Overall, SCFAs play an important protective role in dopaminergic neurons. We also suggest that attention should be paid to the effect of FFAR2 and FFAR3 expressed in neurons on PD. It was found that inhibition of FFAR2 increased Aβ-induced neurotoxicity, and the FFAR2 receptor agonist, Fenchol, could effectively prevent Aβ-induced neurodegeneration in a FFAR2-dependent manner [106]. The possible roles of FFAR2 and FFAR3 in α-syn-induced neurotoxicity also require future attention.
α-syn Aggregation and Transmission
Aggregation and propagation of α-syn is a key factor in the progression of PD pathology. SCFAs can affect α-syn aggregation by regulating α-syn expression and neuroinflammation. Early studies found that sodium butyrate could increase the α-syn protein level [107], and PA stimulated the transcriptional activation of α-syn in primary mesencephalic dopamine neurons treated with rotenone [103]. A recent study found that BA treatment increased α-syn transcription, but not the protein level [108]. Further exploration revealed that it was Atg5-mediated autophagy and activation of the PI3K/Akt/mTOR signaling pathway that led to the difference between mRNA and protein levels. Another study found that sodium butyrate could promote PGC-1 to activate the autophagy pathway and reduce α-syn expression by inhibiting HDACs [109]. Neuroinflammation is another important factor in promoting α-syn misfolding and aggregation. SCFAs can reduce the activation of microglia and the production of proinflammatory cytokines to mitigate α-syn-related pathology in the brain.
Targeted injection of PFF of α-syn provides an effective model for studying the transmission of α-syn. Pathological transmission of α-syn to the brain can be observed after PFF of α-syn was injected into the brain, olfactory bulb, and intestine [110–112]. Excitingly, the administration of sodium butyrate effectively reduced the phosphorylated α-syn content in the substantia nigra, after unilateral injection of PFF into the striatum [113]. SCFAs are involved in maintaining the integrity of the gut barrier, which might affect the transmission of α-syn between the gut and the brain; thus, it is necessary to verify this in the PD model with gastrointestinal injections of PFF. The mechanisms of SCFAs in PD are illustrated in Fig. 2 and relevant basic studies are summarized in Table 1.
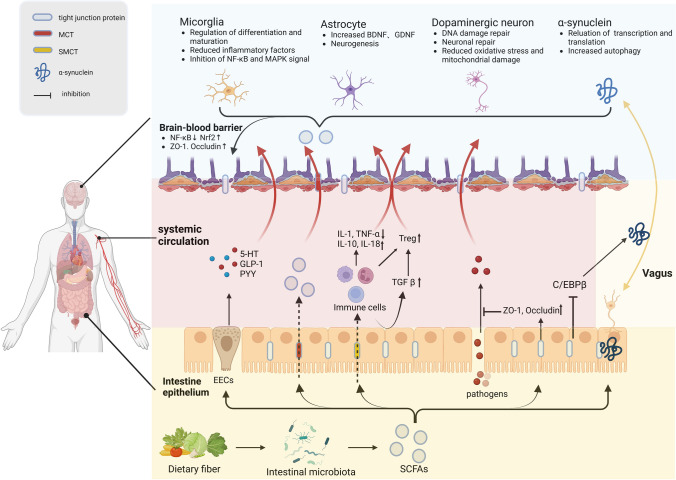
Fig. 2.
Role of SCFAs in the pathology of Parkinson’s disease. Dietary fiber is fermented by intestinal microbiota to produce SCFAs, which enter intestinal epithelial cells through MCT and SMCT transporters. SCFAs can enhance the integrity of the intestinal mucosal barrier by increasing the expression of tight junction proteins, which help reduce the penetration of pathogens. SCFAs can also inhibit the C/EBP pathway to reduce the spread of α-syn from the gut to the brain. In enteroendocrine cells, SCFAs will promote the secretion of 5-HT, GLP-1, and PYY, which are important neuroprotective factors. In the immune pathway, SCFAs regulate the differentiation and function of immune cells and the secretion of cytokines in the peripheral circulation, indirectly influencing the central immune environment. A small number of SCFAs can also directly cross the blood-brain barrier to play a central regulatory role. The SCFAs entering the brain can enhance the integrity of the blood-brain barrier, regulate the activation of microglia, increase the expression of BDNF and GDNF in glial cells, and modulate the expression and propagation of α-syn. Created with BioRender.com.
Table 1.
Effects of SCFAs in animal and cellular models of PD
| SCFAs | Treatment | PD model | Results | Mechanism |
|---|---|---|---|---|
| Butyrate | Oral gavage | Rat induced by PFF of α-syn | Motor defect↓, death of dopamine neurons↓, neuroinflammation↓[113] | Inhibition of HDACs–autophagy pathway↑- α-syn pathology↓ |
| Oral gavage | 6-OHDA mice pretreated with CFX | Motor defect↓, neuroinflammation↓, colon injury↓, peripheral inflammation↓, and dysbiosis↓ [114] | Anti-inflammatory and anti-apoptotic effects | |
| Oral gavage | MPTP mice | Motor defect↓, death of dopamine neurons↓ [31] | GLP-1 in the colon↑, GLP-1R↑ in the brain, blood-brain barrier↑ | |
| Oral gavage | MPTP mice | Motor defect↑, death of dopamine neurons↑, neuroinflammation↑[115] | Oxidative stress↑, intestinal barrier↓, pro-inflammatory effect↑ | |
| Oral gavage | MPTP mice | Motor defect↓, death of dopamine neurons↓, neuroinflammation↓ , α-syn accumulation↓[23] | NF-κB signaling pathway↓, MAPK signaling pathway↓ | |
| Oral gavage | MPTP mice | Motor defect↓, dopamine↑, dopamine neurons↑ , gut dysbiosis↓[54] | TLR4/MyD88/NF-κB pathway↓–intestinal inflammation and neuroinflammation↓ | |
| Oral gavage | MPTP mice | Motor defect↓, dopamine↑, neuroinflammation↓[41] | Activation of GPR109A–NF-κB pathway ↓– intestinal permeability↑ | |
| Oral gavage | Mice induced by rotenone | Motor defect↓, α-syn pathology↓, gut dysfunction↓ [60] | GLP-1↑, microbiota dysbiosis↓ | |
| Intraperitoneal injection | 6-OHDA rat | Motor defect↓, striatal dopamine level↑, neuroinflammation↓[96] | Inhibition of HDACs–histone H3 acetylation level↑– BDNF expression↑ | |
| Intraperitoneal injections | 6-OHDA rat | Cognitive impairment↓, attention concentration capacity↑[116] | None | |
| Addition in food | Drosophila induced by rotenone | Motor defect↓, lifespan↑[17] | Inhibition of HDAC–Dopamine↑ | |
| Addition in medium | SH-SY5Y cells treated with salsolinol | Cell viability↑[29] | Activation of FFAR3 | |
| Addition in medium | α-syn expressing LUHMES cells | DNA damage↓[102] | Inhibition of HDACs–histone H3 acetylation level↑– DNA repair gene expression↑ | |
| Addition in medium | PC12 cells treated with rotenone | Cell viability↑, α-syn↓, p-α-syn↓[109] | Inhibition of HDACs–PGC-1α↑– autophagy↑– α-syn expression↓ | |
| Propionate | Addition in medium | Primary dopaminergic neurons treated with rotenone | TH+ cells↑, neurite length↑[103] | STAT3 signaling pathway↑ |
| Addition to drinking water | 6-OHDA mice | Motor defect↓, death of dopamine neurons↓[32] | Activation of FFAR3 | |
| Oral gavage | MPTP mice | Motor defect↓, intestinal epithelial barrier↑[40] | AKT signaling pathway↓ | |
| Mixture | Addition to drinking water | ASO transgenic GF mice | Motor defect↑, neuroinflammation↑, α-syn aggregation↑[4] | Pro-inflammatory role |
LUHMES cells, Lund human mesencephalic cells; CFX, Ceftriaxone; PGC-1α, Peroxisome proliferator-activated receptor-γ coactivator-1α
SCFAs and Non-motor Symptoms of PD
The pathology discussed earlier relates primarily to motor symptoms, PD patients also show many non-motor symptoms, such as constipation, sleep disorders, and depression. Some non-motor symptoms often precede motor symptoms, causing a marked impact on the quality of life and treatment effect of PD patients. Therefore, early identification and treatment of non-motor symptoms will greatly improve the prognosis of PD patients. Some preclinical models also showed significant non-motor symptoms, such as depression and gastrointestinal dysfunction in the 6-OHDA model [117]. Recent evidence suggests a strong association between SCFAs and non-motor symptoms of PD, and SCFAs have potential therapeutic value for various non-motor symptoms.
Constipation
Constipation is the most common non-motor symptom in PD patients, with a prevalence of around 80% [118]. It is mainly due to impaired cholinergic transmission and α-syn aggregation of the underlying ENS. Compared to WT mice, ASO mice showed a robust expression of α-syn in the ENS and a typical syndrome of constipation characterized by a reduction in fecal water content, fecal pellet output, and prolonged gut transit time [119]. Similarly, human A53T α-syn transgenic mice displayed marked gastrointestinal dysfunction and α-syn pathology in the ENS that preceded motor abnormalities and central pathology by 6 months [120]. Clinical research found that the contents of SCFAs and butyrate-producing bacteria were negatively correlated with the severity of constipation in PD patients [121]. Butyrate could increase the colonic transport speed and the proportion of cholinergic neurons via the ENS. This neurochemical plasticity is related to the MCT2 expressed on intestinal neurons and HDACs inhibition [122]. As mentioned previously, SCFAs can enhance the integrity of the intestinal barrier, indicating a potential therapeutic effect on constipation. A recent study also found that sodium butyrate significantly improved the stool frequency and fecal water content of rotenone-induced PD mice [60]. Direct administration of SCFAs to patients is inefficient and impractical, but dietary adjustment, probiotics, and prebiotic fibers all represent potential strategies. When constipated mice were fed acylated starches derived from specific SCFAs for 1 month, it was found that these acylated starches resolved the issue of SCFAs absorption in the small intestine, and acetylated and butylated starch both effectively relieved constipation [123].
Sleep Disorders
Sleep disorders are very common in the setting of PD, with a prevalence of close to 90% [124]. PD patients exhibit various forms of sleep disorders, such as insomnia, daytime sleepiness, circadian rhythm disorder, and rapid eye movement (REM) sleep behavior disorder (RBD) [125]. Idiopathic RBD (iRBD) is the most dependable hallmark of prodromal PD and the likelihood ratio of iRBD developing into PD is greater than that for the general population [126, 127]. In a clinical study, 16S rRNA sequencing was used to analyze the difference in gut microbiota among healthy people, iRBD, and PD patients. It was found that the number of SCFAs-producing bacteria in iRBD patients was not significantly reduced, while recognized or putative SCFAs-producing genera Faecalibacterium, Roseburia, and the Lachnospiraceae ND3007 group were consistently decreased in PD patients. A decrease in SCFAs-producing bacteria may be a prerequisite for the development of PD [128].
Various studies have demonstrated that SCFAs ameliorate sleep disturbance through immune, neural, and endocrine pathways [129]. Significant alterations in the clock gene were observed both in animal models of PD and in patients [130]. Interestingly, significant rhythmicity was also detected in SCFAs and SCFAs oral gavage, and prebiotic supplementation can facilitate peripheral clock adjustment [131]. Due to the lack of microbial signals, GF mice had an obvious circadian disruption in the liver. Butyrate supplementation significantly increased the ratio of Per2: Bmal1 mRNA in the liver of GF mice [132]. In addition, SCFAs and related bacteria can stimulate the production of 5-HT and melatonin in the intestine [133]; therefore, SCFAs may be closely related to rhythm and sleep. Oral and portal vein injections of sodium butyrate can increase the time of NREM sleep and reduce body temperature by combining with FFAR2 and FFAR3 in the hepatoportal region, achieving the effect of improving sleep [134].
Depression
Depression is the most common neuropsychiatric disorder among people with PD and is related to the degeneration of monoaminergic neurotransmitter systems and fronto-cortical dysfunctions [135]. A case-control study confirmed that low fecal BA content and a decrease in the genera Roseburia, Romboutsia, and Prevotella were related to depressive symptoms in PD patients [136]. A deficiency of 5-HT in the CNS is an important mechanism in depression, but SCFAs can stimulate the production of 5-HT in enterochromaffin cells. Supplementation with SCFAs effectively improved depressive behavior in mice fed with high fructose by preventing the decline in hippocampal nerves and relieving neuroinflammation and BBB damage [137]. Another study also showed that sodium butyrate improved depressive behavior induced by LPS by inhibiting neuroinflammation and oxidative nitrosation stress [138]. In addition, BDNF plays an important role in the survival, maintenance, differentiation, and synaptic plasticity of nerve cells. A reduction in BDNF impaired neurogenesis, resulting in the onset of major depressive disorder, and SCFAs exerted a beneficial effect on depression by recovering the brain’s BDNF level [139] (Fig. 3).
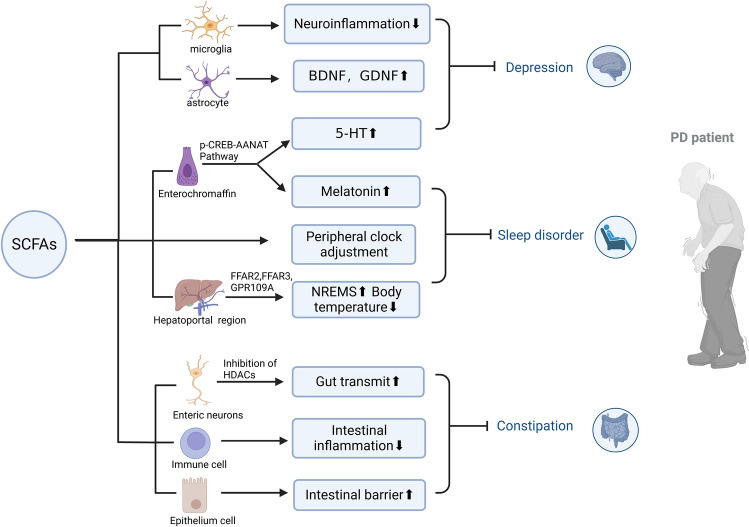
Fig. 3.
Role of short-chain fatty acids in non-motor symptoms of Parkinson’s disease. SCFAs have potential therapeutic value for non-motor symptoms of PD. SCFAs can promote enterochromaffin cells to secrete 5-HT through the p-CREB-AANAT signal pathway and combine with its role in promoting neurotrophic factors BDNF and GDNF and regulating neuroinflammation, which can effectively improve depressive symptoms. In addition, SCFAs can also promote the secretion of melatonin by intestinal chromaffin cells and regulate the peripheral biological clock. Also, SCFAs can combine with FFAR2 and FFAR3 in the hepatoportal region to release signals to the central nervous system, increase NREMS sleep and reduce body temperature to enhance sleep. Finally, SCFAs can act on intestinal neurons and increase intestinal motility by inhibiting HDACs. Combined with their functions of inhibiting intestinal inflammation and enhancing the intestinal mucosal barrier, they can improve constipation symptoms. Created with BioRender.com.
Clinical Relationship Between SCFAs and PD
Using 16s RNA sequencing and metagenomics, researchers found significant changes in the gut microbiota composition in PD patients. Although the results were not entirely consistent, the general trend showed that Akkermania, Lactobacillus, and Enterobacteriaceae increased in PD patients, while Prevotella, Blautia, Coprococcus, Roseburia, and Faecalbacterium decreased [140–144]. SCFAs-producing bacteria, especially BA-producing bacteria, were greatly reduced [145, 146]. Unger et al. were the first to measure the concentrations of SCFAs in fecal samples from PD patients, and they found that AA, PA, and BA in the feces of PD patients decreased [147]. After that, a series of studies were carried out, and similar conclusions were drawn on the alterations in fecal SCFAs in PD patients. Therefore, there is a consensus that the content of SCFAs in the feces of PD patients decreases.
At present, most studies use fecal concentrations to represent the content of SCFAs produced in the colon. This is indeed a simple and effective alternative, but it should be noted that there are some influencing factors, such as intestinal transit speed, intestinal permeability, and sample handling. For example, faster colonic transit time may lead to reduced absorption of SCFAs and increased SCFAs content measured in feces [148]. In addition, the change in fecal SCFAs content cannot be used to evaluate the content of SCFAs in peripheral circulation or tissues. Therefore, the first case-control study on plasma SCFAs in PD patients has attracted the attention of scientists [149]. This study used gas chromatography to measure the plasma concentrations of SCFAs in 38 PD patients and 33 healthy controls. Before adjusting the covariates, no significant differences were found between the concentrations of AA, BA, and PA in PD patients and the control group, but after adjusting the covariates, the concentrations of AA in PD patients were significantly higher than those in the control group. This was speculated to be related to damage to the intestinal barrier and the leakage of intestinal SCFAs. These results indicate that the plasma SCFAs content may not change in parallel with the fecal SCFAs content. There are some limitations in this study, such as the small sample size and the absence of gut microbiological analysis. However, it is the first study to investigate the relationship between plasma SCFAs content and PD and it provided some new ideas. Based on this, Chen et al. investigated the association between the fecal and plasma levels of SCFAs in PD patients [146]. In addition, they analyzed the gut microbiota composition and evaluated its relationship with clinical severity in a large sample of 96 PD patients and 85 healthy controls, making up for the shortcomings of the previous study. Compared with the control group, the feces concentrations of acetate, propionate, and butyrate in PD patients were lower, but the plasma concentrations were higher. Later, Yang et al. reached a similar conclusion and found that the combination of fecal and plasma SCFAs could discriminate PD patients from healthy control subjects [121]. In addition, fecal AA and isobutyric acid in PD patients with constipation were lower compared to those without constipation, but plasma AA and PA were higher. Constipation may increase the permeability of the gut-blood barrier in patients with PD. However, different opinions can be found in other studies. Wu et al. found that serum PA and BA levels in PD patients were lower than those in the control group [150]. The serum PA level was negatively correlated with motor symptoms and Mini-mental State Examination scores and positively correlated with Hamilton Depression Scale scores. Interestingly, some studies also detected SCFAs in the urine and saliva of PD patients and found that BA in urine was elevated, while AA and PA in saliva were elevated [151, 152]. The relevant clinical research results are summarized in Table 2.
Table 2.
Alterations in short-chain fatty acids in Parkinson’s disease
| Studies | Participants | SCFAs in feces | SCFAs in plasma | Correlation |
|---|---|---|---|---|
| Unger et al. [147] | 34 PD, 34 HC | AA↓ PA↓ BA↓ | None | BA in feces (−) use of entacapone |
| Aho et al. [153] | 55 PD, 56 HC | PA↓ BA↓ | None |
SCFAs in feces (+) age of PD onset SCFAs in feces (-) total score of NMSS |
| Tan et al. [145] | 104 PD, 96 HC | BA↓ | None |
BA in feces (+) cognitive score BA in feces (−) severity of gait disorder and constipation |
| Huang et al. [40] | 17 PD, 17 HC | AA↓ PA↓ BA↓ | None | None |
| Pablo-Fernandez et al. [154] | 35 PD, 50 HC | AA↓ PA↓ BA↓ | None | None |
| Augustin et al. [155] | 77 PD, 113 HC | PA↓ BA↓ | None | BA in feces (−) colonic transit time |
| Shin et al. [149] | 38 PD, 33 HC | None | BA↑ |
AA in feces (+) age PA in feces (−) UPDRS Part III score and the use of entacapone BA in feces (+) use of monoamine oxidase inhibitors BA in feces (−) use of anticholinergic drugs |
| Wu et al. [150] | 50 PD, 50 HC | None | AA↓ PA↓ BA↓ |
PA in plasma (−) UPDRS Part III score and MMSE score PA in plasma (+) HAMD score |
| He et al. [156] | 46 PD, 46 HC | None | PA↑ | None |
| Voigt et al. [157] | 74 PD, 20 HC | None | LA↓ | BA in plasma (−) constipation |
| Toczylowska et al. [158] | 19 PD, 21 HC | None | AA↑ | None |
| Qi et al. [159] | 44 PD, 42 HC | None | AA↑ PA↓ | None |
| Chen et al. [146] | 96 PD, 85 HC | AA↓ PA↓ BA↓ | AA↑ PA↑ BA↑ |
AA, PA, BA in feces, and PA in plasma (-) UPDRS Part III score BA in plasma (−) MMSE score |
| Yang et al. [121] | 95 PD, 33 HC | AA↓ PA↓ BA↓ | AA↑ PA↑ |
Plasma/fecal ratio of SCFAs (+) α1-AT in feces SCFAs in plasma (+) constipation SCFAs in feces (−) constipation AA and BA in feces (−) disease severity AA and PA in plasma + disease severity |
AA, acetic acid; PA, propanoic acid; BA, butyric acid; LA, lactic acid; HC, healthy controls; NONE, not measured; UPDRS, Unified Parkinson’s disease rating scale; MMSE, Mini-mental state examination; HAMD, Hamilton depression scale; NMSS, Non-motor symptoms scale
(+) Positive correlation; (−) Negative correlation
In conclusion, the alterations and significance of plasma SCFAs content in PD patients are uncertain. Some other metabolic pathways can produce SCFAs, such as plasma acetate, which can be derived from endogenous products of fatty acid oxidation [160]. Furthermore, SCFAs in plasma cannot directly represent their role in the CNS but are closer to their role in peripheral tissues. Therefore, it is of little significance to simply analyze the content of SCFAs in peripheral blood. We need to jointly analyze the changes of SCFAs in feces and plasma. Most SCFAs originating from the intestine are used for intestinal energy supply and liver metabolism, and only a few SCFAs enter the peripheral circulation. If it is determined that the plasma SCFAs content in PD patients increases and the fecal SCFAs content decreases, it may reflect the increased permeability of the intestinal mucosal barrier or dysfunction of the liver, which causes the “leak” in SCFAs.
Discussion
Due to the significant changes in SCFAs that occur in PD and the protective effect of SCFAs on the nervous system, a number of scholars have proposed the view that alterations in SCFAs are responsible for the pathogenesis of PD. From existing evidence, however, this view is somewhat exaggerated. Changes in SCFAs and SCFAs-producing bacteria are not disease-specific. Reductions in fecal SCFAs can also be observed in IBS, chronic kidney disease, and other neurodegenerative diseases [161–163]. In addition, the distribution of SCFAs and their receptors in the brain is relatively low, and there is a lack of strong evidence to prove the direct regulatory effect of SCFAs on specific neurons. In particular, a clear explanation for the causal relationship between SCFAs and specific pathological features of PD such as the loss of dopaminergic neurons and the formation of a Lewy body, is still lacking. Alterations in SCFAs are more likely to be secondary to dysbiosis of gut microbiota in the early stages of PD. The reduction in SCFAs-producing bacteria and destruction of the intestinal barrier leads to a decrease in fecal SCFAs and an increase in plasma SCFAs. This secondary change, in turn, exacerbates intestinal inflammation and systemic immune disorders, as well as damage to the BBB, leading to the infiltration of peripheral immune cells, toxins, and cytokines. A reduction in SCFAs also leads to abnormal brain-gut interaction, indirectly affecting the immune status and neuronal function within the brain. We think it necessary to elucidate the relationship between SCFAs and the aggregation and spread of α-syn in the gut, as both SCFAs deficiency and intestinal α-syn aggregation are early alterations in PD. Their causal relationship is key in proving a direct link between SCFAs and PD.
In addition, it must be emphasized again that the relationship between SCFAs and neuroinflammation requires further clarification. Although we concluded that SCFAs remain predominantly neuroprotective in the presence of intestinal flora, there are studies with contradictory findings. In particular, one study found that oral administration of sodium butyrate to MPTP mice under SPF conditions surprisingly increased neuroinflammation and increased the loss of dopamine neurons [115]. Paradoxical results were also observed in models of Alzheimer’s disease, amyloidosis, and neuropathic pain [95, 164, 165]. Differences in the effect of SCFAs on neuroinflammation are related to many factors, such as microbiological control levels for laboratory animals, the method of administration, mixture or single drug administration, and the drug concentration. Future research should explore the effects of different types and concentrations of SCFAs on the immune function of the brain in both germ-free and conventional environments, and determine the optimal doses and types to promote brain health and immune function. Clarifying the role of SCFAs in the neuroimmune system under physiological conditions is a prerequisite for understanding their effects on neuroinflammation in PD.
To date, there is still no effective treatment for PD, and symptomatic treatment is still the main therapeutic option. Therefore, early diagnosis and treatment are crucial. Early changes in SCFAs combined with other more specific diagnostic aids, such as real-time vibration-induced protein amplification with high sensitivity and specificity for abnormally folded α-syn, can be used to identify fibrillar α-syn in biological fluids [166], as well as in conjunction with early detection of prodromal symptoms, such as RBD, to better diagnose PD at an early stage. Direct injection or oral administration of SCFAs as treatment is unreasonable and unrealistic in clinical practice. Therefore, SCFAs-producing probiotics and prebiotics are very promising alternatives, as they could effectively avoid the absorption of SCFAs in the upper digestive tract, which will better simulate the absorption and action mode of SCFAs in vivo. Prebiotics with specific chemical structures can be selected or designed to achieve an increase in the targeting of SCFAs and SCFAs-producing bacteria in the colon [167]. Many probiotics and prebiotics have been proven to be effective in improving PD by increasing the production of SCFAs. In MPTP mice, oral administration of Bifidobacterium breve CCFM1067 protected the blood-brain and intestinal barriers from damage by improving intestinal microecology and increasing the synthesis of SCFAs [168]. In ASO mice, a prebiotic diet modulated the activation of microglia and motor deficits by altering gut microbiome composition and the content of SCFAs [97]. Synbiotics are compound preparations of probiotics and prebiotics, which can more effectively exert the physiological activity of probiotics. Polymannuronic acid (PM) and Lacticaseibacillus rhamnosus GG (LGG) in combination were found to have much better neuroprotective effects on PD than PM or LGG alone [169], indicating the therapeutic potential of synbiotics in PD. Also worthy of attention are medicinal plants, such as Mucuna pruriens (Mp) and Withania somnifera (Ws), which are rich in crude protein, essential fatty acids, and starch. They not only promote the production of SCFAs, but also have many bioactive components, such as ursolic acid in Mp and chlorogenic acid in Ws, and both showed potent anti-Parkinsonian activity in a toxin-induced Parkinsonian mouse model [100, 170].
Conclusion
In this review, we comprehensively summarize the relationship between SCFAs and PD from pathology to the clinic. Significant alterations in SCFAs are present in patients with PD and are closely associated with motor symptoms and non-motor symptoms. SCFAs affect the pathological progress of PD through multiple dimensions. In the future, more attention should be paid to the diagnostic and therapeutic value of SCFAs for PD. There are still some problems that need to be solved urgently. First, we need to clarify the regulatory mechanism of SCFAs on microglia. Direct central regulation or indirect peripheral regulation? Second, the decrease in fecal SCFAs in PD patients is now a consensus, but the significance and alteration of plasma SCFAs need to be further confirmed, including in other tissues. Third, it is necessary to identify a stable and highly recognized PD model with specific non-motor symptoms to verify the therapeutic potential of SCFAs in non-motor symptoms. Fourth, the value of SCFAs-producing probiotics, prebiotics, and synbiotics in PD deserves further development. Some shortcomings in this review should also be noted. The study of SCFAs-producing bacteria in PD has not been summarized. In addition, we did not focus on the interaction between SCFAs and other metabolites. It is hoped that future research and reviews will add to these aspects.
Acknowledgements
This review was supported by the Jiangsu Provincial Key R&D Program (BE2018658), Jiangsu Provincial Medical Key Discipline (ZDXK202217), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Jun-Yi Liu, Email: jyliu911@suda.edu.cn.
Chun-Feng Liu, Email: liuchunfeng@suda.edu.cn.
References
- 1.MacLeod AD, Taylor KSM, Counsell CE. Mortality in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord. 2014;29:1615–1622. doi: 10.1002/mds.25898. [DOI] [PubMed] [Google Scholar]
- 2.Li G, Ma J, Cui S, He Y, Xiao Q, Liu J, et al. Parkinson’s disease in China: A forty-year growing track of bedside work. Transl Neurodegener. 2019;8:22. doi: 10.1186/s40035-019-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Park Relat Disord. 2016;22:S119–S122. doi: 10.1016/j.parkreldis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-Kingdom intermediates. Nat Rev Microbiol. 2021;19:77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- 6.Bart, van der Hee, Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol 2021, 29: 700–712. [DOI] [PubMed]
- 7.Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J Lipid Res. 2016;57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halestrap AP, Meredith D. The SLC16 gene family—From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch - Eur J Physiol. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 10.Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldendorf WH. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol. 1973;224:1450–1453. doi: 10.1152/ajplegacy.1973.224.6.1450. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann C, Colombo JP, Berüter J. Short chain fatty acids in plasma and brain: Quantitative determination by gas chromatography. Clin Chim Acta. 1979;92:153–159. doi: 10.1016/0009-8981(79)90109-8. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Taliyan R. Targeting histone deacetylases: A novel approach in Parkinson’s disease. Parkinsons Dis. 2015;2015:303294. doi: 10.1155/2015/303294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 15.Zhu M, Li WW, Lu CZ. Histone decacetylase inhibitors prevent mitochondrial fragmentation and elicit early neuroprotection against MPP+ CNS Neurosci Ther. 2014;20:308–316. doi: 10.1111/cns.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11:1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent RS, O’Brien L, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng GS, Li G, Tzeng NS, Chen PS, Chuang DM, Hsu YD, et al. Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: Role of microglia. Brain Res Mol Brain Res. 2005;134:162–169. doi: 10.1016/j.molbrainres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Hao P, et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Bosch C, Boorman E, Zunszain PA, Mann GE. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021;47:102165. doi: 10.1016/j.redox.2021.102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou Y, Li X, Liu C, Zhang M, Zhang X, Ge S, et al. Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson’s disease. Exp Gerontol. 2021;150:111376. doi: 10.1016/j.exger.2021.111376. [DOI] [PubMed] [Google Scholar]
- 24.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 25.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson NE, Kotarsky K, Owman C, Olde BR. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/S0006-291X(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 27.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyles L, Snelling T, Umlai UK, Nicholson JK, Carding SR, Glen RC, et al. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome. 2018;6:55. doi: 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Getachew B, Csoka AB, Bhatti A, Copeland RL, Tizabi Y. Butyrate protects against salsolinol-induced toxicity in SH-SY5Y cells: Implication for parkinson’s disease. Neurotox Res. 2020;38:596–602. doi: 10.1007/s12640-020-00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015, 18: 965–977. [DOI] [PMC free article] [PubMed]
- 31.Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, et al. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. 2017;381:176–181. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- 32.Hou YF, Shan C, Zhuang SY, Zhuang QQ, Ghosh A, Zhu KC, et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome. 2021;9:34. doi: 10.1186/s40168-020-00988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 34.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut. 2019;68:829–843. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- 36.Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord. 2018;50:104–107. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Caleb J. Kelly, Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said H, Akiba Y, Narimatsu K, Maruta K, Kuri A, Iwamoto KI, et al. FFA3 activation stimulates duodenal bicarbonate secretion and prevents NSAID-induced enteropathy via the GLP-2 pathway in rats. Dig Dis Sci. 2017;62:1944–1952. doi: 10.1007/s10620-017-4600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang T, Shi H, Xu Y, Ji L. The gut microbiota metabolite propionate ameliorates intestinal epithelial barrier dysfunction-mediated Parkinson’s disease via the AKT signaling pathway. Neuroreport. 2021;32:244–251. doi: 10.1097/WNR.0000000000001585. [DOI] [PubMed] [Google Scholar]
- 41.Xu RC, Miao WT, Xu JY, Xu WX, Liu MR, Ding ST, et al. Neuroprotective effects of sodium butyrate and monomethyl fumarate treatment through GPR109A modulation and intestinal barrier restoration on PD mice. Nutrients. 2022;14:4163. doi: 10.3390/nu14194163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumitrescu L, Marta D, Dănău A, Lefter A, Tulbă D, Cozma L, et al. Serum and fecal markers of intestinal inflammation and intestinal barrier permeability are elevated in parkinson’s disease. Front Neurosci. 2021;15:689723. doi: 10.3389/fnins.2021.689723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulak A, Koszewicz M, Panek-Jeziorna M, Koziorowska-Gawron E, Budrewicz S. Fecal calprotectin as a marker of the gut immune system activation is elevated in parkinson’s disease. Front Neurosci. 2019;13:992. doi: 10.3389/fnins.2019.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Houser MC, Chang J, Factor SA, Molho ES, Zabetian CP, Hill-Burns EM, et al. Stool immune profiles evince gastrointestinal inflammation in parkinson’s disease. Mov Disord. 2018;33:793–804. doi: 10.1002/mds.27326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: The presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 48.Grathwhohl S, Quansah E, Maroof N, Steiner JA, Spycher L, Benmansour F, et al. Specific immune modulation of experimental colitis drives enteric alpha-synuclein accumulation and triggers age-related Parkinson-like brain pathology. Free Neuropathol 2021, 2: 2–13. 10.17879/freeneuropathology-2021-3326 [DOI] [PMC free article] [PubMed]
- 49.Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977–2014. Gut. 2019;68:18–24. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 50.Chen C, Zhou Y, Wang H, Alam A, Kang SS, Ahn EH, et al. Gut inflammation triggers C/EBPβ/δ-secretase-dependent gut-to-brain propagation of Aβ and Tau fibrils in Alzheimer’s disease. EMBO J. 2021;40:e106320. doi: 10.15252/embj.2020106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Chen G, Ahn EH, Xia Y, Kang SS, Liu X, et al. C/EBPβ/AEP is age-dependently activated in Parkinson’s disease and mediates α-synuclein in the gut and brain. NPJ Parkinsons Dis. 2023;9:1. doi: 10.1038/s41531-022-00430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q, Peng Z, Zhou L, Peng R, Li X, Zuo W, et al. Short-chain fatty acid decreases the expression of CEBPB to inhibit miR-145-mediated DUSP6 and thus further suppresses intestinal inflammation. Inflammation. 2022;45:372–386. doi: 10.1007/s10753-021-01552-6. [DOI] [PubMed] [Google Scholar]
- 53.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo TT, Zhang Z, Sun Y, Zhu RY, Wang FX, Ma LJ, et al. Neuroprotective effects of sodium butyrate by restoring gut microbiota and inhibiting TLR4 signaling in mice with MPTP-induced parkinson’s disease. Nutrients. 2023;15:930. doi: 10.3390/nu15040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heinzel S, Aho VTE, Suenkel U, von Thaler AK, Schulte C, Deuschle C, et al. Gut microbiome signatures of risk and prodromal markers of parkinson disease. Ann Neurol 2021, 90. [DOI] [PubMed]
- 57.Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Xue L, Wang Z, Xie A. Update to the treatment of parkinson’s disease based on the gut-brain axis mechanism. Front Neurosci. 2022;16:878239. doi: 10.3389/fnins.2022.878239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng SY, Li HX, Xu RC, Miao WT, Dai MY, Ding ST, et al. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Rev. 2021;69:101347. doi: 10.1016/j.arr.2021.101347. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Xu S, Qian Y, Mo C, Ai P, Yang X, et al. Sodium butyrate ameliorates gut dysfunction and motor deficits in a mouse model of Parkinson’s disease by regulating gut microbiota. Front Aging Neurosci. 2023;15:1099018. doi: 10.3389/fnagi.2023.1099018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziomber A, Thor P, Krygowska-Wajs A, Załęcki T, Moskała M, Romańska I, et al. Chronic impairment of the vagus nerve function leads to inhibition of dopamine but not serotonin neurons in rat brain structures. Pharmacol Rep. 2012;64:1359–1367. doi: 10.1016/S1734-1140(12)70933-7. [DOI] [PubMed] [Google Scholar]
- 62.Kin I, Sasaki T, Yasuhara T, Kameda M, Agari T, Okazaki M, et al. Vagus nerve stimulation with mild stimulation intensity exerts anti-inflammatory and neuroprotective effects in Parkinson’s disease model rats. Biomedicines. 2021;9:789. doi: 10.3390/biomedicines9070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang C, Su T, Xiao L, Wang Y, Huo X, Li W, et al. Right vagus nerve stimulation improves motor behavior by exerting neuroprotective effects in Parkinson’s disease rats. Ann Transl Med. 2022;10:1314. doi: 10.21037/atm-22-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 65.Nøhr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, et al. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory Ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 66.Bruning J, Chapp A, Kaurala GA, Wang R, Techtmann S, Chen QH. Gut microbiota and short chain fatty acids: Influence on the autonomic nervous system. Neurosci Bull. 2020;36:91–95. doi: 10.1007/s12264-019-00410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 68.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morimoto R, Satoh F, Murakami O, Totsune K, Saruta M, Suzuki T, et al. Expression of peptide YY in human brain and pituitary tissues. Nutrition. 2008;24:878–884. doi: 10.1016/j.nut.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 70.Katsurada K, Yada T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J Diabetes Investig. 2016;7:64–69. doi: 10.1111/jdi.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isacson R, Nielsen E, Dannaeus K, Bertilsson G, Patrone C, Zachrisson O, et al. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol. 2011;650:249–255. doi: 10.1016/j.ejphar.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Painsipp E, Herzog H, Holzer P. The gut-mood axis: A novel role of the gut hormone peptide YY on emotional-affective behaviour in mice. BMC Pharmacol. 2009;9:A13. doi: 10.1186/1471-2210-9-S2-A13. [DOI] [Google Scholar]
- 73.Rozita H. Anderberg, GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology. 2016;65:54–66. doi: 10.1016/j.psyneuen.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 74.Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in peripheral inflammatory cytokine levels in parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016;73:1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 75.Rocha NP, Teixeira AL, Scalzo PL, Barbosa IG, de Sousa MS, Morato IB, et al. Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson’s disease. Mov Disord. 2014;29:527–531. doi: 10.1002/mds.25752. [DOI] [PubMed] [Google Scholar]
- 76.Sommer A, Marxreiter F, Krach F, Fadler T, Grosch J, Maroni M, et al. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell. 2019;24:1006. doi: 10.1016/j.stem.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 77.Yan Z, Yang W, Wei H, Dean MN, Standaert DG, Cutter GR, et al. Dysregulation of the adaptive immune system in patients with early-stage parkinson disease. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1036. doi: 10.1212/NXI.0000000000001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mihara T, Nakashima M, Kuroiwa A, Akitake Y, Ono K, Hosokawa M, et al. Natural killer cells of Parkinson’s disease patients are set up for activation: A possible role for innate immunity in the pathogenesis of this disease. Parkinsonism Relat Disord. 2008;14:46–51. doi: 10.1016/j.parkreldis.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546:656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindestam Arlehamn CS, Dhanwani R, Pham J, Kuan R, Frazier A, Rezende Dutra J, et al. α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat Commun. 1875;2020:11. doi: 10.1038/s41467-020-15626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang P, Yao L, Luo M, Zhou W, Jin X, Xu Z, et al. Single-cell transcriptome and TCR profiling reveal activated and expanded T cell populations in Parkinson’s disease. Cell Discov. 2021;7:52. doi: 10.1038/s41421-021-00280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28:321–328. doi: 10.1016/j.nutres.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 84.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costea L, Mészáros Á, Bauer H, Bauer HC, Traweger A, Wilhelm I, et al. The blood-brain barrier and its intercellular junctions in age-related brain disorders. Int J Mol Sci. 2019;20:5472. doi: 10.3390/ijms20215472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knox EG, Aburto MR, Clarke G, Cryan JF, O’Driscoll CM. The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry. 2022;27:2659–2673. doi: 10.1038/s41380-022-01511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Bachari S, Naish JH, Parker GJM, Emsley HCA, Parkes LM. Blood-brain barrier leakage is increased in parkinson’s disease. Front Physiol. 2020;11:593026. doi: 10.3389/fphys.2020.593026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jangula A, Murphy EJ. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci Lett. 2013;551:23–27. doi: 10.1016/j.neulet.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fock E, Parnova R. Mechanisms of blood-brain barrier protection by microbiota-derived short-chain fatty acids. Cells. 2023;12:657. doi: 10.3390/cells12040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with[11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Ho MS. Microglia in Parkinson’s Disease. In: Neuroglia in Neurodegenerative Diseases. 1st ed. Singapore: Springer, 2019, 1175: 335–353. [DOI] [PubMed]
- 94.Zhu B, Yin D, Zhao H, Zhang L. The immunology of Parkinson’s disease. Semin Immunopathol. 2022;44:659–672. doi: 10.1007/s00281-022-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seo DO, O’Donnell D, Jain N, Ulrich JD, Herz J, Li Y, et al. ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science. 2023;379:eadd1236. doi: 10.1126/science.add1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharma S, Taliyan R, Singh S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: Modulation of histone deacetylase activity. Behav Brain Res. 2015;291:306–314. doi: 10.1016/j.bbr.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 97.Abdel-Haq R, Schlachetzki JCM, Boktor JC, Cantu-Jungles TM, Thron T, Zhang M, et al. A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. Elife. 2022;11:e81453. doi: 10.7554/eLife.81453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su SH, Chen M, Wu YF, Lin Q, Wang DP, Sun J, et al. Fecal microbiota transplantation and short-chain fatty acids protected against cognitive dysfunction in a rat model of chronic cerebral hypoperfusion. CNS Neurosci Ther. 2023;29(Suppl 1):98–114. doi: 10.1111/cns.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, Singh SP. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res. 2014;39:2527–2536. doi: 10.1007/s11064-014-1443-7. [DOI] [PubMed] [Google Scholar]
- 100.Yadav SK, Rai SN, Singh SP. Mucuna pruriens reduces inducible nitric oxide synthase expression in Parkinsonian mice model. J Chem Neuroanat. 2017;80:1–10. doi: 10.1016/j.jchemneu.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 101.Zhang X, Xu S, Hu Y, Liu Q, Liu C, Chai H, et al. Irisin exhibits neuroprotection by preventing mitochondrial damage in Parkinson’s disease. NPJ Parkinsons Dis. 2023;9:13. doi: 10.1038/s41531-023-00453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paiva I, Pinho R, Pavlou MA, Hennion M, Wales P, Schütz AL, et al. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum Mol Genet. 2017;26:2231–2246. doi: 10.1093/hmg/ddx114. [DOI] [PubMed] [Google Scholar]
- 103.Ostendorf F, Metzdorf J, Gold R, Haghikia A, Tönges L. Propionic acid and fasudil as treatment against rotenone toxicity in an in vitro model of parkinson’s disease. Molecules. 2020;25:2502. doi: 10.3390/molecules25112502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, et al. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res. 2015;61:114–121. doi: 10.1016/j.jpsychires.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38:2027–2034. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Razazan A, Karunakar P, Mishra SP, Sharma S, Miller B, Jain S, et al. Activation of microbiota sensing - free fatty acid receptor 2 signaling ameliorates amyloid-β induced neurotoxicity by modulating proteolysis-senescence axis. Front Aging Neurosci. 2021;13:735933. doi: 10.3389/fnagi.2021.735933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qiao CM, Sun MF, Jia XB, Shi Y, Zhang BP, Zhou ZL, et al. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res. 2020;387:111772. doi: 10.1016/j.yexcr.2019.111772. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Xu S, Qian Y, He X, Mo C, Yang X, et al. Sodium butyrate attenuates rotenone-induced toxicity by activation of autophagy through epigenetically regulating PGC-1α expression in PC12 cells. Brain Res. 2022;1776:147749. doi: 10.1016/j.brainres.2021.147749. [DOI] [PubMed] [Google Scholar]
- 110.Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, et al. Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med. 2016;213:1759–1778. doi: 10.1084/jem.20160368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models parkinson’s disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kakoty V, K C S, Dubey SK, Yang CH, Taliyan R. Neuroprotective effects of trehalose and sodium butyrate on preformed fibrillar form of α-synuclein-induced rat model of parkinson’s disease. ACS Chem Neurosci 2021, 12: 2643–2660. [DOI] [PubMed]
- 114.Avagliano C, Coretti L, Lama A, Pirozzi C, De Caro C, De Biase D, et al. Dual-hit model of parkinson’s disease: Impact of dysbiosis on 6-hydroxydopamine-insulted mice-neuroprotective and anti-inflammatory effects of butyrate. Int J Mol Sci. 2022;23:6367. doi: 10.3390/ijms23126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiao CM, Sun MF, Jia XB, Li Y, Zhang BP, Zhao LP, et al. Sodium butyrate exacerbates parkinson’s disease by aggravating neuroinflammation and colonic inflammation in MPTP-induced mice model. Neurochem Res. 2020;45:2128–2142. doi: 10.1007/s11064-020-03074-3. [DOI] [PubMed] [Google Scholar]
- 116.Pallavi Rane. The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacology. 2012;62:2409–2412. doi: 10.1016/j.neuropharm.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 117.Rai SN, Singh P. Advancement in the modelling and therapeutics of Parkinson’s disease. J Chem Neuroanat. 2020;104:101752. doi: 10.1016/j.jchemneu.2020.101752. [DOI] [PubMed] [Google Scholar]
- 118.Mukherjee A, Biswas A, Das SK. Gut dysfunction in Parkinson’s disease. World J Gastroenterol. 2016;22:5742–5752. doi: 10.3748/wjg.v22.i25.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O. α-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis. 2012;47:258–267. doi: 10.1016/j.nbd.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rota L, Pellegrini C, Benvenuti L, Antonioli L, Fornai M, Blandizzi C, et al. Constipation, deficit in colon contractions and alpha-synuclein inclusions within the colon precede motor abnormalities and neurodegeneration in the central nervous system in a mouse model of alpha-synucleinopathy. Transl Neurodegener. 2019;8:5. doi: 10.1186/s40035-019-0146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang X, Ai P, He X, Mo C, Zhang Y, Xu S, et al. Parkinson’s disease is associated with impaired gut-blood barrier for short-chain fatty acids. Mov Disord. 2022;37:1634–1643. doi: 10.1002/mds.29063. [DOI] [PubMed] [Google Scholar]
- 122.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 123.Wang L, Cen S, Wang G, Lee YK, Zhao J, Zhang H, et al. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J Funct Foods. 2020;69:103953. doi: 10.1016/j.jff.2020.103953. [DOI] [Google Scholar]
- 124.Kurtis MM, Rodriguez-Blazquez C, Martinez-Martin P, ELEP Group. Relationship between sleep disorders and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2013, 19: 1152–1155. [DOI] [PubMed]
- 125.Shen Y, Lv QK, Xie WY, Gong SY, Zhuang S, Liu JY, et al. Circadian disruption and sleep disorders in neurodegeneration. Transl Neurodegener. 2023;12:8. doi: 10.1186/s40035-023-00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30:1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 127.Postuma RB, Berg D. Advances in markers of prodromal Parkinson disease. Nat Rev Neurol. 2016;12:622–634. doi: 10.1038/nrneurol.2016.152. [DOI] [PubMed] [Google Scholar]
- 128.Nishiwaki H, Hamaguchi T, Ito M, Ishida T, Maeda T, Kashihara K, et al. Short-chain fatty acid-producing gut microbiota is decreased in parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. mSystems. 2020;5:e00797–e1720. doi: 10.1128/mSystems.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang M, Song X, Zhong W, Xie Y, Liu Y, Zhang X. Dietary fiber ameliorates sleep disturbance connected to the gut-brain axis. Food Funct. 2022;13:12011–12020. doi: 10.1039/D2FO01178F. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y, Niu L, Liu X, Cheng C, Le W. Recent progress in non-motor features of parkinson’s disease with a focus on circadian rhythm dysregulation. Neurosci Bull. 2021;37:1010–1024. doi: 10.1007/s12264-021-00711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep. 2018;8:1395. doi: 10.1038/s41598-018-19836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song L, He M, Sun Q, Wang Y, Zhang J, Fang Y, et al. Roseburia hominis increases intestinal melatonin level by activating p-CREB-AANAT pathway. Nutrients. 2021;14:117. doi: 10.3390/nu14010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szentirmai É, Millican NS, Massie AR, Kapás L. Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci Rep. 2019;9:7035. doi: 10.1038/s41598-019-43502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chikatimalla R, Dasaradhan T, Koneti J, Cherukuri SP, Kalluru R, Gadde S. Depression in parkinson’s disease: A narrative review. Cureus. 2022;14:e27750. doi: 10.7759/cureus.27750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie A, Ensink E, Li P, Gordevičius J, Marshall LL, George S, et al. Bacterial butyrate in parkinson’s disease is linked to epigenetic changes and depressive symptoms. Mov Disord. 2022;37:1644–1653. doi: 10.1002/mds.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang CF, Wang CY, Wang JH, Wang QN, Li SJ, Wang HO, et al. Short-chain fatty acids ameliorate depressive-like behaviors of high fructose-fed mice by rescuing hippocampal neurogenesis decline and blood-brain barrier damage. Nutrients. 1882;2022:14. doi: 10.3390/nu14091882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qiu J, Liu R, Ma Y, Li Y, Chen Z, He H, et al. Lipopolysaccharide-induced depression-like behaviors is ameliorated by sodium butyrate via inhibiting neuroinflammation and oxido-nitrosative stress. Pharmacology. 2020;105:550–560. doi: 10.1159/000505132. [DOI] [PubMed] [Google Scholar]
- 139.Suda K, Matsuda K. How microbes affect depression: Underlying mechanisms via the gut-brain axis and the modulating role of probiotics. Int J Mol Sci. 2022;23:1172. doi: 10.3390/ijms23031172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nishiwaki H, Ito M, Ishida T, Hamaguchi T, Maeda T, Kashihara K, et al. Meta-analysis of gut dysbiosis in parkinson’s disease. Mov Disord. 2020;35:1626–1635. doi: 10.1002/mds.28119. [DOI] [PubMed] [Google Scholar]
- 141.Toh TS, Chong CW, Lim SY, Bowman J, Cirstea M, Lin CH, et al. Gut microbiome in parkinson’s disease: New insights from meta-analysis. Parkinsonism Relat Disord. 2022;94:1–9. doi: 10.1016/j.parkreldis.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 142.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7:27. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nishiwaki H, Ito M, Hamaguchi T, Maeda T, Kashihara K, Tsuboi Y, et al. Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:65. doi: 10.1038/s41531-022-00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tan AH, Chong CW, Lim SY, Yap IKS, Teh CSJ, Loke MF, et al. Gut microbial ecosystem in parkinson disease: New clinicobiological insights from multi-omics. Ann Neurol. 2021;89:546–559. doi: 10.1002/ana.25982. [DOI] [PubMed] [Google Scholar]
- 146.Chen SJ, Chen CC, Liao HY, Lin YT, Wu YW, Liou JM, et al. Association of fecal and plasma levels of short-chain fatty acids with gut microbiota and clinical severity in patients with parkinson disease. Neurology. 2022;98:e848–e858. doi: 10.1212/WNL.0000000000013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 148.Primec M, Mičetić-Turk D, Langerholc T. Analysis of short-chain fatty acids in human feces: A scoping review. Anal Biochem. 2017;526:9–21. doi: 10.1016/j.ab.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 149.Shin C, Lim Y, Lim H, Ahn TB. Plasma short-chain fatty acids in patients with parkinson’s disease. Mov Disord. 2020;35:1021–1027. doi: 10.1002/mds.28016. [DOI] [PubMed] [Google Scholar]
- 150.Wu G, Jiang Z, Pu Y, Chen S, Wang T, Wang Y, et al. Serum short-chain fatty acids and its correlation with motor and non-motor symptoms in Parkinson’s disease patients. BMC Neurol. 2022;22:13. doi: 10.1186/s12883-021-02544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumari S, Goyal V, Kumaran SS, Dwivedi SN, Srivastava A, Jagannathan NR. Quantitative metabolomics of saliva using proton NMR spectroscopy in patients with Parkinson’s disease and healthy controls. Neurol Sci. 2020;41:1201–1210. doi: 10.1007/s10072-019-04143-4. [DOI] [PubMed] [Google Scholar]
- 152.Kumari S, Kumaran SS, Goyal V, Sharma RK, Sinha N, Dwivedi SN, et al. Identification of potential urine biomarkers in idiopathic parkinson’s disease using NMR. Clin Chim Acta. 2020;510:442–449. doi: 10.1016/j.cca.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 153.Aho VTE, Houser MC, Pereira PAB, Chang J, Rudi K, Paulin L, et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol Neurodegener. 2021;16:6. doi: 10.1186/s13024-021-00427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.De Pablo-Fernandez E, Gebeyehu GG, Flain L, Slater R, Frau A, Ijaz UZ, et al. The faecal metabolome and mycobiome in Parkinson’s disease. Parkinsonism Relat Disord. 2022;95:65–69. doi: 10.1016/j.parkreldis.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 155.Augustin A, Guennec AL, Umamahesan C, Kendler-Rhodes A, Tucker RM, Chekmeneva E, et al. Faecal metabolite deficit, gut inflammation and diet in Parkinson’s disease: Integrative analysis indicates inflammatory response syndrome. Clin Transl Med. 2023;13:e1152. doi: 10.1002/ctm2.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He X, Qian Y, Xu S, Zhang Y, Mo C, Guo W, et al. Plasma short-chain fatty acids differences in multiple system atrophy from parkinson’s disease. J Parkinsons Dis. 2021;11:1167–1176. doi: 10.3233/JPD-212604. [DOI] [PubMed] [Google Scholar]
- 157.Voigt RM, Wang Z, Brown JM, Engen PA, Naqib A, Goetz CG, et al. Gut microbial metabolites in Parkinson’s disease: Association with lifestyle, disease characteristics, and treatment status. Neurobiol Dis. 2022;170:105780. doi: 10.1016/j.nbd.2022.105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Toczylowska B, Zieminska E, Michałowska M, Chalimoniuk M, Fiszer U. Changes in the metabolic profiles of the serum and putamen in Parkinson’s disease patients - in vitro and in vivo NMR spectroscopy studies. Brain Res. 2020;1748:147118. doi: 10.1016/j.brainres.2020.147118. [DOI] [PubMed] [Google Scholar]
- 159.Qi A, Liu L, Zhang J, Chen S, Xu S, Chen Y, et al. Plasma metabolic analysis reveals the dysregulation of short-chain fatty acid metabolism in parkinson’s disease. Mol Neurobiol. 2023;60:2619–2631. doi: 10.1007/s12035-022-03157-y. [DOI] [PubMed] [Google Scholar]
- 160.Dalile B, van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 161.Li HB, Xu ML, Xu XD, Tang YY, Jiang HL, Li L, et al. Faecalibacterium prausnitzii attenuates CKD via butyrate-renal GPR43 axis. Circ Res. 2022;131:e120–e134. doi: 10.1161/CIRCRESAHA.122.320184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 163.Qian XH, Xie RY, Liu XL, Chen SD, Tang HD. Mechanisms of short-chain fatty acids derived from gut microbiota in alzheimer’s disease. Aging Dis. 2022;13:1252–1266. doi: 10.14336/AD.2021.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Colombo AV, Sadler RK, Llovera G, Singh V, Roth S, Heindl S, et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. elife. 2021;10:e59826. doi: 10.7554/eLife.59826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou F, Wang X, Han B, Tang X, Liu R, Ji Q, et al. Short-chain fatty acids contribute to neuropathic pain via regulating microglia activation and polarization. Mol Pain. 2021;17:1744806921996520. doi: 10.1177/1744806921996520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016;3:812–818. doi: 10.1002/acn3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cantu-Jungles TM, Rasmussen HE, Hamaker BR. Potential of prebiotic butyrogenic fibers in parkinson’s disease. Front Neurol. 2019;10:663. doi: 10.3389/fneur.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li T, Chu C, Yu L, Zhai Q, Wang S, Zhao J, et al. Neuroprotective effects of Bifidobacterium breve CCFM1067 in MPTP-induced mouse models of parkinson’s disease. Nutrients. 2022;14:4678. doi: 10.3390/nu14214678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu X, Du ZR, Wang X, Sun XR, Zhao Q, Zhao F, et al. Polymannuronic acid prebiotic plus Lacticaseibacillus rhamnosus GG probiotic as a novel synbiotic promoted their separate neuroprotection against Parkinson’s disease. Food Res Int. 2022;155:111067. doi: 10.1016/j.foodres.2022.111067. [DOI] [PubMed] [Google Scholar]
- 170.Singh SS, Rai SN, Birla H, Zahra W, Kumar G, Gedda MR, et al. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front Pharmacol. 2018;9:757. doi: 10.3389/fphar.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]