Abstract
The aim of this study was to elucidate the nature of T cell abnormalities in bipolar disorder (BD). With the use of multicolor flow cytometry, we first quantified the composition of the different memory and pro-inflammatory immune subpopulations in samples of 58 patients with BD and compared them to 113 healthy controls. Second, to assess if cytomegalovirus infection was related to the resulted immune subpopulation compositions in the two groups, we measured cytomegalovirus-specific antibodies in serum. Thirdly, we assessed differences between the two groups in the serum levels of the immune cell differentiation factor interleukin-7. Compared to healthy controls, patients showed significantly higher T helper-17, T regulatory and T central memory cells (CD4+ and CD8+). Besides, patients showed significantly lower CD4+ T effector memory and CD4+ T effector memory re-expressing RA cells. Cytomegalovirus infection was not related to the observed abnormalities, with the exception of T helper-17 cells. This immune subpopulation was significantly higher only in patients seropositive to cytomegalovirus infection. Finally, interleukin-7 levels were significantly lower in BD compared to healthy controls. In conclusion, the aberrant levels of T memory cell populations in BD may suggest a T cell differentiation abnormality. The role of interleukin-7 in this putative abnormality should be further investigated.
Keywords: Bipolar disorder, T-lymphocytes, Memory T cells, T helper-17, Cytomegalovirus, Interleukin-7
Highlights
-
•
Abnormal apportioning between T central and effector memory cells in bipolar disorder.
-
•
Higher levels of T helper-17 and T regulatory cells in bipolar disorder.
-
•
T helper-17 abnormalities in patients are associated with cytomegalovirus infection.
-
•
T cell differentiation factor interleukin-7 is lower in bipolar disorder.
-
•
Bipolar disorder may intrinsically be linked to a T cell differentiation abnormality.
1. Introduction
Bipolar disorder (BD) is a severe psychiatric disorder characterized by (hypo)manic and depressive episodes, alternating with euthymic phases (McIntyre et al., 2020). BD is chronic in nature and shows a progressive pathophysiological deterioration (Berk et al., 2011).
There is ample evidence for an activation of the immune-inflammatory and compensatory immune-regulatory system in BD, the so-called signs of low-grade inflammation. Especially in the manic and depressive phases, increased levels of pro- and anti-inflammatory cytokines have been reported (Brietzke et al., 2009; Goldsmith et al., 2016). Moreover, an increased expression of pro-inflammatory genes has been found in circulating monocytes in the manic and depressed stages (Padmos et al., 2008), while no differences or even an anti-inflammatory state of monocytes were observed in the euthymic phase (Becking et al., 2015; Vogels et al., 2017).
Over the last decade, evidence has been accumulating on a disrupted function of the T cell system in BD. T lymphocytes are well known as regulators of inflammation, but also play an important role in key brain processes (Debnath et al., 2021). Previous studies have shown higher frequencies of circulating activated T cells as well as increased p-ERK signaling in T cells of patients with BD (Barbosa et al., 2014; Breunis et al., 2003; Do Prado et al., 2013). Mixed findings have been reported concerning the frequencies of pro-inflammatory T helper-1 (Th1) and T helper-17 (Th17) cells, and anti-inflammatory T helper-2 (Th2) cells and T regulatory cells (TREGS) (Barbosa et al., 2014; Do Prado et al., 2013; Drexhage et al., 2011).
Studies showing shortened telomere length in mononuclear cells of patients (Rizzo et al., 2013) and an increase in the age-related growth differentiation factors (Yang et al., 2018), support a concept of BD as a disorder of accelerated aging. Aging of the T cell compartment is characterized by accumulation of memory T cells, reduced generation of naïve T cells from the thymus and shrinkage of the T cell repertoire (Darrigues et al., 2018; Franceschi et al., 1999). Infection by cytomegalovirus (CMV) may play a role in the accelerated immune aging in BD, since anti-CMV IgG levels have been related to the number of late stage senescent CD8+ CD28− T cytotoxic cells in patients (Rizzo et al., 2013).
The aim of this study was to determine if patients with BD in a euthymic state show differences in the composition of the immune cell subpopulations involved in inflammation and those with senescent characteristics. Second, we aimed to investigate if these differences are due to an interaction between CMV infection and the diagnosis of BD. The last aim was to investigate if the serum level of interleukin-7 (IL-7), is different in patients compared to healthy controls.
2. Methods
2.1. Study participants
This study is part of the multicenter EU funded projects MOODSTRATIFICATION/MOODINFLAME (https://moodstratification.eu/). The study was conducted in accordance with the declaration of Helsinki and its subsequent revisions and approved by ethical committees of the participating universities (reference numbers: Groningen: 2009/019; Leuven: S51723; Munich: 291–09, Münster: 2009-019-f-S). Written informed consent was obtained from all participants.
The recruitment of patients and healthy controls took place at the University Medical Centre Groningen in the Netherlands and the University Hospitals Leuven in Belgium. Healthy controls were also recruited at the Ludwig Maximilian University Klinikum in Germany and the University Hospital Münster in Germany. Inclusion criteria for patients were a DSM-5 diagnosis of BD type I or BD type II (296. x), a euthymic state and no other primary major psychiatric diagnosis. The euthymic state was defined via a clinician-rated Inventory for Depressive Symptomatology (IDS-C) score<22 and a Young Mania Rating Scale (YMRS) score<12. Patients were in their regular medication scheme of lithium or other mood stabilizing medication. Healthy controls did not have any major axis I disorder and no current or previous psychiatric treatment/medication. All participants were between 18 and 65 years of age and did not have a current or recent severe infectious or inflammatory disease, an uncontrolled systemic (e.g. lupus erythematosus or rheumatoid arthritis), metabolic (e.g. diabetes, hyper- or hypothyroidism, Cushing disease of Addison disease), or any other significant somatic, organic, or neurological disorder. Additional exclusion criteria were current or recent use of somatic medication which may affect the immune system, current alcohol or other substance use disorder and pregnancy or recent delivery.
2.2. Clinical assessment
As described in section 2.1. severity and type of depression in patients was assessed with the clinician-rated version of the IDS-C. Severity of mania was assessed using the YMRS. Body mass index (BMI) was calculated using self-reported height and body weight. Information on medication use was obtained from clinical records.
2.3. Peripheral blood mononuclear cell (PBMC) collection
For the collection and characterization of PBMC, the same procedures as previously described were followed (Schiweck et al., 2020). In brief, PBMC suspensions of patients and controls were prepared from sodium-heparin blood tubes via Ficoll gradient centrifugation on the same day as the blood collection. Samples were frozen in 10% dimethylsulfoxide (DMSO) and stored in liquid nitrogen.
2.4. Fluorescence-activated cell sorting analysis
Fluorescence-activated cell sorting (FACS) was performed on defrosted PBMCs, once washed with complete culture medium (RPMI-1640plus 10% fetal calf serum plus 1% penicillin/streptomycin). Recovery and viability of cells were determined by Trypan blue staining.
Percentages of T cells (CD3+), T helper lymphocytes (CD3+CD4+), T cytotoxic lymphocytes (CD3+CD8+), natural killer cells (NK; CD3−CD56+), B cells (CD19+) and monocytes (CD14+) were assessed with staining A. 50.000 PBMCs were stained by an 8-color membrane staining. Cell determinations from staining A are presented as percentage of the total PBMCs.
Percentages of T helper cell subsets (Th1, Th2, Th17, TREGS) were determined by staining B. An 8-color membrane and intracellular staining was used on 1 × 106 of PBMCs after a 4-h stimulation at 37 °C in RPMI-1640 culture medium with 50 ng/ml phorbol 12-myristrate 13-acetate (PMA; Sigma Aldrich, St. Louis, MO, USA) and 1.0 μg/ml ionomycin (Sigma) in the presence of Golgistop (BD Biosciences). 200.000 events were collected on a BD FACS Canto II-3 laser instrument. The analysis was performed using FlowJo software. T helper cell subsets were identified by their secreting cytokines: Th1 (CD3+CD4+IFNγ+), Th2 (CD3+CD4+IL4+), Th17 (CD3+CD4+IL17A+). TREGS were identified by their transcription factor FOXP3 (CD3+CD4+CD25hiFOXP3+). T cell subsets of staining B are presented as percentages of total lymphocytes, which could be reliably detected as a clear population in scatter properties after the 4-h culture. When expressing T cell subsets as a percentage of total lymphocytes as determined in staining A (the non-cultured sample), the outcomes did not change.
For a more detailed determination of T helper and cytotoxic naïve/memory cell subsets staining C was used on a second vial of PBMCs. Recovery and viability of cells after thawing were on average 64% and 97%, respectively, as determined by Trypan blue staining. 1.5 × 106 of PBMCs were stained with a cocktail of CD45-V500, CD45RA-BB515, CD3-Alexa Fluor700, CD4-BUV805, CD8-BUV395, CD197-BV421 (BD Biosciences), CD28-BV711, CD27-APC and CD57-PE (BioLegend) for 15 min at room temperature, washed twice with PBS, pH 7.8 and subsequently stained with viability dye Zombie NIR (BioLegend). 500.000 events in a live/CD45 stopping gate were collected on an Aurora-5 laser instrument. The analysis was also performed using FlowJo software. Quadrant gating on CD45RA and CD197 (CCR7) was used to define the following subsets of the CD4+ and CD8+ populations: T naïve-like (TNL; CD45RA+CD197+), central memory (TCM; CD45RA+CD197+), effector memory (TEM; CD45RA+CD197+) and effector memory RA (TEMRA; CD45RA+CD197-). The total CD8+ and CD4+ T memory populations were calculated by adding the respective T memory subpopulations TCM, TEM, and TEMRA. The expression of the maturation markers CD27 and CD28 was assessed within each indicated T cell subset. The cell subsets of staining C are presented as percentages of total CD3+ T cells.
Details on the monoclonal antibodies used for each staining can be found at Supplementary Table S1. Gating strategies for staining A and B have been previously described (Counotte et al., 2018). The gating strategy for staining C is given in Supplementary Fig. S1.
2.5. Determination of antibodies against cytomegalovirus
Serum was separated on the same day as the blood collection and stored at −80 °C until the analysis. CMV-specific antibodies were measured with the cytomegalovirus IgG ELISA kit (Demeditec Diagnostics GmbH, Germany), according to manufacturer's instructions. Serum levels above 10 U/ml were considered an indication of chronic CMV infection.
2.6. Statistical analysis
Data analysis and visualization was performed in R version 4.1.2. Between-group comparison of categorical demographic variables was performed with the Chi2 test. All continuous variables are presented as mean and standard deviation (SD) when data follow a normal distribution and as median and the interquartile range (IQR) when they do not. Group comparisons between patients and controls for continuous variables were performed with an independent Student's t-test for normally distributed data, and with Wilcoxon test for non-normally distributed data. The differences in the levels of immune cells and IL-7 between patients and controls were calculated with the Wilcoxon test, with only exception CD3+CD4+ T cell where a t-test was used.
To evaluate if the observed differences in the levels of immune variables were not driven by confounders, we used multiple linear regression, with the percentage of the cell subset as the dependent variable, diagnosis as an independent variable and age, sex, and BMI as covariates. The effect of lithium on the level of immune subsets in patients with BD was evaluated in a multiple linear regression, with the percentage of the cell subset as the dependent variable, lithium as the independent variable and age, sex, and BMI as the covariates. When assumptions for linear regression were not met, the arcsine square root transformation of the percentage of the cell subset was used, thereafter referred to as arcsine transformation (Robert et al., 1995).
To evaluate if the observed differences in the levels of immune cell populations between patients and healthy controls were due to an interaction between BD and CMV infection, we first tested the interaction term in a multiple linear regression. In each model, the percentage of the cell subset was the dependent variable. The diagnosis, CMV status and the interaction of the two were used as independent variables. Age, sex, and BMI were used as covariates. When assumptions for linear regression were violated, the arcsine transformation of the cell percentage was used. In case the interaction term was statistically significant, we performed a stratified analysis. Patients and healthy controls were stratified in groups based on seropositivity to CMV-specific antibodies, and the levels of the immune subset among the groups was compared with Kruskal-Wallis test, followed by post hoc comparisons with Wilcoxon rank sum test and a Benjamini-Hochberg adjustment.
The significance level was set to alpha = 0.05 and was two-tailed.
3. Results
3.1. Demographic and clinical characteristics
The choice of participants for each of the analysis was based on availability of biomaterial. For the analysis of IL-7, characterization of immune cell subsets with staining A and B, and for staining C, samples of 309 participants (BD = 142, HC = 167), 171 participants (BD = 58, HC = 113), and 88 participants (BD = 38, HC = 50) have been used, respectively.
Table 1 shows the demographic and clinical characteristics of patients with BD and healthy controls per analysis. Below, only the significant differences between the group of patients and the healthy controls per analysis are listed. In staining A and B, the group of healthy controls included more females (χ2 = 5.14, p = 0.02). Healthy controls were significantly younger compared to patients for staining C (W = 708, p = 0.04) and IL-7 analysis (W = 9827, p = 0.02). Patients had a significantly higher BMI than healthy controls in staining A and B (W = 2355, p = 0.003), staining C (W = 483.5, p = 0.001) and IL-7 analysis (W = 5673.5, p < 0.001). 67.2% of patients in staining A and B, 54.3% of staining C and 58.1% of IL-7 analysis were lithium-users.
Table 1.
Demographic and clinical characteristics of healthy controls and patients with bipolar disorder per analysis. All categorical variables are presented as a percentage over the total number of participants. All continuous variables are presented with the median and the interquartile range. Abbreviations: BMI Body Mass Index; CMV Cytomegalovirus; IDS-C The Inventory of Depressive Symptomatology, Clinician Rating; YMRS Young Mania Rating Scale.
| Healthy Controls | Bipolar Disorder | p-value | |
|---|---|---|---|
| Staining A and B | n = 113 | n = 58 | |
| Female (%) | 69.0% | 50.0% | 0.021 |
| Age | 38.5 (26.9, 50.4) | 43.0 (35.0, 53.5) | 0.12 |
| BMI (kg/m2) | 23.4 (20.7, 25.5) | 25.0 (22.4, 28.1) | 0.0032 |
| CMV seropositive (%) | 35.4% | 39.7% | 0.61 |
| Anti-CMV titers (U/ml) | 5.2 (3.7, 32.4) | 5.0 (3.1, 45.7) | 0.92 |
| IDS-C | – | 7.0 (4.0, 10.8) | – |
| YMRS | – | 0.0 (0.0, 2.0) | – |
| Lithium treated %) | – | 67.2% | – |
| Staining C | n = 38 | n = 50 | |
| Female (%) | 60.5% | 46.0% | 0.31 |
| Age | 37.2 (27.9, 46.0) | 44.2 (32.5, 55.5) | 0.042 |
| BMI (kg/m2) | 22.4 (20.7, 25.0) | 26.1 (22.8, 28.9) | 0.0012 |
| CMV seropositive (%) | 44.7% | 44.0% | 11 |
| Anti-CMV titers (U/ml) | 5.6 (3.8, 36.6) | 4.9 (3.5, 47.7) | 0.72 |
| IDS-C | – | 6.0 (3.2, 9.8) | – |
| YMRS | – | 0.0 (0.0, 2.0) | – |
| Lithium treated (%) | – | 54.3% | – |
| Interleukin-7 analysis | n = 167 | n = 142 | |
| Female (%) | 61.3%) | 56.3% | 0.41 |
| Age | 38.7 (26.8, 52.4) | 45.5 (34.7, 55.0) | 0.022 |
| BMI (kg/m2) | 23.6 (20.9, 25.7) | 24.9 (22.5, 27.9) | < 0.0012 |
| CMV seropositive (%) | 48.8% | 38.4% | 0.21 |
| Anti-CMV titers (U/ml) | 8.0 (3.8, 58.0) | 4.7 (3.5, 45.7) | 0.22 |
| IDS-C | – | 6.0 (4.0, 10.0) | – |
| YMRS | – | 0.0 (0.0, 1.0) | – |
| Lithium treated (%) | – | 58.1% | – |
| Interleukin-7 (pg/ml) | 12.3 (9.3, 16.7) | 10.8 (7.8, 13.7) | < 0.0012 |
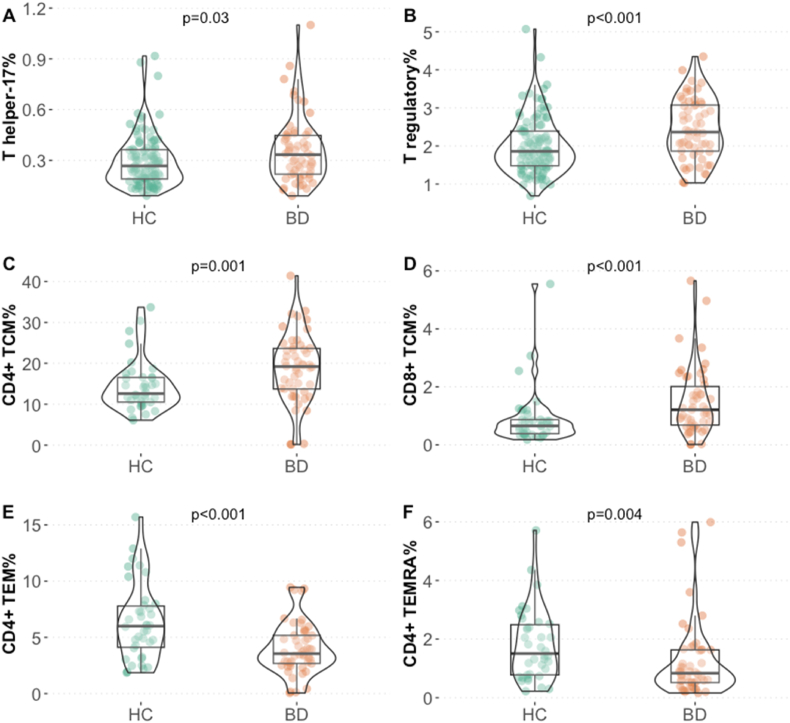
3.2. Higher Th17, TREGS, TCM and lower CD4+ TEM and TEMRA in BD
The results of staining A are presented in Table 2 (upper part). More information on the adjustment for confounders can be found in the materials and methods section 2.6. Patients with BD had lower percentages of CD3+ T cells when compared to healthy controls, but not when adjusted for confounders. The percentages of CD3+CD4+ T helper cells, CD3+CD8+ cytotoxic T cells, CD19+ B cells and CD56+ NK cells were not significantly different between patients and healthy controls.
Table 2.
Differences in immune subpopulations between patients and controls (Staining A & B). All immune subpopulations except for CD3+CD4+ T cell%, are presented as median and interquartile range. CD3+CD4+ T cell% is presented as mean and standard deviation. Abbreviations: BMI Body Mass Index; NK natural killer; Th1 T helper-1; Th2 T helper-2; Th17 T helper-17; TREGS T regulatory cells.
| Healthy Controls | Bipolar Disorder | p-value | p-value after age, sex, BMI adjustment | |
|---|---|---|---|---|
| CD3+ T cell%a | 59.6 (54.4, 65.1) | 56.7 (49.4, 62.2) | 0.03c | 0.4 |
| CD3+CD8+ T cytotoxic cell%a | 17.0 (13.3, 20.8) | 16.5 (12.0, 20.4) | 0.3c | 1 |
| CD19+ B cell%a | 7.3 (5.8, 8.9) | 7.0 (5.5, 9.3) | 0.98c | 0.4 |
| CD56+ NK cell%a | 8.3 (6.1, 11.6) | 8.6 (6.8, 11.2) | 0.5c | 0.5 |
| CD3+CD4+ T cell%b | 50.1 (±8.1) | 48.0 (±9.1) | 0.1d | 0.07 |
| 0.5 | ||||
| Th1 cell%b | 4.6 (3.0, 6.3) | 5.2 (3.6, 7.1) | 0.09c | 0.03 |
| Th2 cell%b | 0.44 (0.35, 0.62) | 0.42 (0.35, 0.64) | 0.7c | 0.8 |
| Th17 cell%b | 0.27 (0.19, 0.36) | 0.33 (0.22,0.45) | 0.02c | 0.04 |
| TREGS cell%b | 1.9 (1.5, 2.4) | 2.4 (1.9, 3.1) | <0.001c | 0.003 |
Percentage of cell subpopulation over the total peripheral blood mononuclear cells.
Percentage of cell subpopulation over the total lymphocyte population.
Wilcoxon rank sum test.
T-test.
The results of staining B are presented in Table 2 (lower part). The levels of Th1 were significantly higher in patients, but only after correcting for confounders (arcsine transformation, beta = 0.02, SE = 0.01, p = 0.03). Th2 cells did not differ significantly between the groups. Th17 cells (W = 2395.5, p = 0.02) (Fig. 1A) and TREGS (W = 2205, p < 0.001) (Fig. 1B) were higher in patients with BD, compared to healthy controls, also after adjusting for confounders.
Fig. 1.
Abnormal levels of T cell populations involved in inflammation and T memory cells in BD. Higher A) T helper-17, B) T regulatory, C) CD4+ T central memory, D) CD8+ T central memory and lower E) CD4+ T effector memory and F) CD4+ TEMRA cell% in patients with bipolar disorder compared to healthy controls. Abbreviations: HC Healthy controls; BD Bipolar disorder; TCM T central memory; TEMRA terminally differentiated effector memory; TEM effector memory.
The results of staining C are presented in Table 3. The percentages of naïve T cells in both the CD4+ T helper compartment and the CD8+ T cytotoxic compartment did not differ significantly between patients with BD and healthy controls. Patients with BD did show higher percentages of TCM cells in both the CD4+ T helper (W = 566, p = 0.001) (Fig. 1C) and CD8+ T cytotoxic cell compartment (W = 546, p < 0.001) (Fig. 1D). The association of BD with CD4+ TCM was borderline significant after adjustment for confounders and remained significant for CD8+ TCM. Patients with BD showed lower percentages of CD4+ TEM (W = 1354, p < 0.001) (Fig. 1E) and CD4+ TEMRA cells (W = 1295.5, p = 0.004) (Fig. 1F) compared to healthy controls, also after adjusting for confounders. The percentages of CD8+ cytotoxic TEM and TEMRA were not significantly different between patients with BD and healthy controls.
Table 3.
Differences in immune subpopulations between patients and controls (Staining C). All immune subpopulations are presented as the median and interquartile range of the percentage of cells over total lymphocyte subpopulation. Differences between the groups were assessed with Wilcoxon rank sum test. Abbreviations: BMI Body Mass Index; TNL naïve-like; TCM T central memory; TEMRA terminally differentiated effector memory; TEM effector memory.
| Healthy Controls | Bipolar Disorder | p-value | p-value after age, sex, BMI adjustment |
|
|---|---|---|---|---|
| CD4+ TNL cell% | 44.9 (36.3, 51.0) | 41.0 (33.3, 48.1) | 0.2 | 0.05 |
| CD4+ TCM cell% | 12.6 (10.5, 16.6) | 19.2 (13.7, 23.6) | 0.001 | 0.05 |
| CD4+ TEM cell% | 6.0 (4.1, 7.8) | 3.5 (2.7, 5.2) | <0.001 | 0.002 |
| CD4+ TEMRA cell% | 1.5 (0.8, 2.5) | 0.8 (0.5, 1.6) | 0.004 | 0.04 |
| CD8+ TNL cell% | 14.3 (10.6, 19.4) | 12.9 (7.9, 17.6) | 0.5 | 0.07 |
| CD8+ TCM cell% | 0.7 (0.4, 0.9) | 1.2 (0.7, 2.0) | <0.001 | 0.01 |
| CD8+ TEM cell% | 2.5 (1.6, 3.7) | 2.4 (1.2, 3.9) | 0.8 | 0.9 |
| CD8+ TEMRA cell% | 8.4 (7.4, 12.2) | 9.2 (6.1, 14.2) | 0.8 | 0.7 |
Lithium usage was not associated with the levels of Th17, TREGS, CD4+ TCM, TEM or TEMRA cells in patients with BD, but was positively associated with CD8+ TCM cell % (beta = 0.7, SE = 0.31, p = 0.03).
3.3. T cell abnormalities in BD and the role of CMV infection
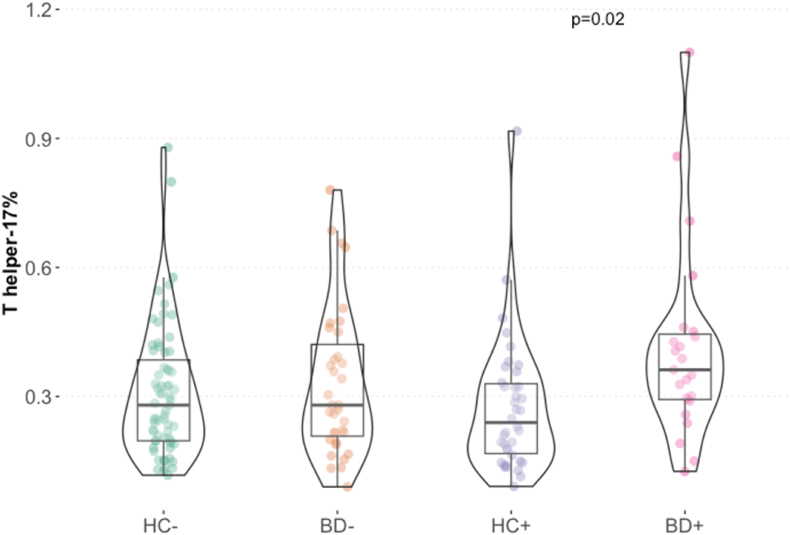
We then investigated if the observed differences between patients and healthy controls in the levels of Th17, TREGS, TCM TEM and TEMRA cells, as reported in section 3.2, were due to an interaction between BD and chronic CMV infection. The interaction between the diagnosis of BD and CMV did not have a significant effect on the levels of TREGS, TCM, CD4+ TEMRA nor on TEM. We only observed a trend when testing the effect of the interaction between BD and CMV on the Th17 (Supplementary Table S2). In a stratified analysis, the compared four groups (patients seropositive to CMV, patients seronegative to CMV, controls seropositive to CMV and controls seronegative to CMV) showed significantly different levels of Th17 cells (H (3) = 9.40, p = 0.02). Post-hoc analysis showed that patients with signs of chronic CMV infection had significantly higher percentages of Th17 cells compared to healthy controls with signs of CMV infection (pbh = 0.02) (Fig. 2).
Fig. 2.
T helper-17 cell% in patients with bipolar disorder and healthy controls stratified based on seropositivity to cytomegalovirus-specific IgG antibodies. Abbreviations: HC− Healthy controls without signs of cytomegalovirus infection; BD- Patients without signs of cytomegalovirus infection; HC+, Healthy controls with signs of cytomegalovirus infection; BD + Patients with signs of cytomegalovirus infection.
Next, we assessed if the interaction between CMV and BD is associated with the levels of late stage differentiated senescent TEM and TEMRA cells. Late stage differentiated TEM and TEMRA are additionally differentiated memory populations, characterized by a decreased expression of CD27 and CD28. CMV was significantly associated with the levels of TEM and TEMRA cells of both the CD4+ and CD8+ compartment, but the interaction between CMV and BD was not significant, suggesting that this effect is not specific to BD.
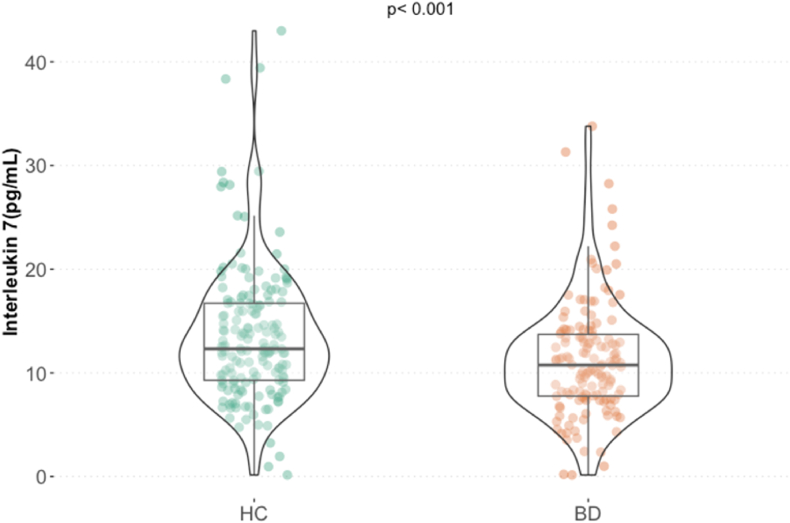
3.4. IL-7 serum levels
IL-7 levels were significantly lower in patients with BD compared to healthy controls (W = 14545, p < 0.001) (Fig. 3A), also after adjusting for age, sex, and BMI.
Fig. 3.
Lower interleukin 7 in patients with bipolar disorder compared to healthy controls. Abbreviations: HC Healthy Controls; BD Bipolar Disorder.
4. Discussion
4.1. Increased TCM and reduced TEM and TEMRA in BD
In this study we phenotypically determined different T cell subpopulations in patients with BD in euthymia. After controlling for age, sex and BMI, patients showed, compared to healthy controls, significantly higher Th17, TREGS and TCM cells. Besides, patients showed significantly lower CD4+ TEM and CD4+ TEMRA cells.
In a similar study by Foiselle et al. (2023), the authors measured naïve and memory CD4+ and CD8+ subpopulations in 152 mainly stabilized patients with BD, 118 patients with schizophrenia and 178 healthy controls. The objective of the study of Foiselle was to explore the role of childhood maltreatment and past infection exposure on these lymphocyte subpopulations in bipolar and psychotic disorders. The authors showed that patients with BD who had experienced childhood maltreatment exhibited higher levels of CD8+ cytotoxic T cells and anti-CMV antibodies, coupled with decreased levels of naïve CD8 T cells.
Although the objective in the study of Foiselle et al. (2023) was an investigation into the role of childhood maltreatment and past infection, it was possible to make a direct comparison of the above-mentioned T cell subpopulations between patients with BD and healthy controls of the Foiselle study, since the authors provided group means and standard deviations. The differences in immune subpopulations between patients and controls together with the relevant demographic variables can be found in Supplementary Table S3. Their findings showed reduced CD4+ TEM and increased CD4+ TEMRA cells and increased CD8+ TCM and CD8+TEM cells. Although these data are not corrected for age, they are congruent with a premature senescent profile of the T cell system, similar to the T cell aberrations found by us in MDD patients (Simon et al., 2023). Our study here, in contrast, indicates not a premature aging, but an aberrant development of the memory T cell populations from naive CD4+ and CD8+ T cells in patients with BD.
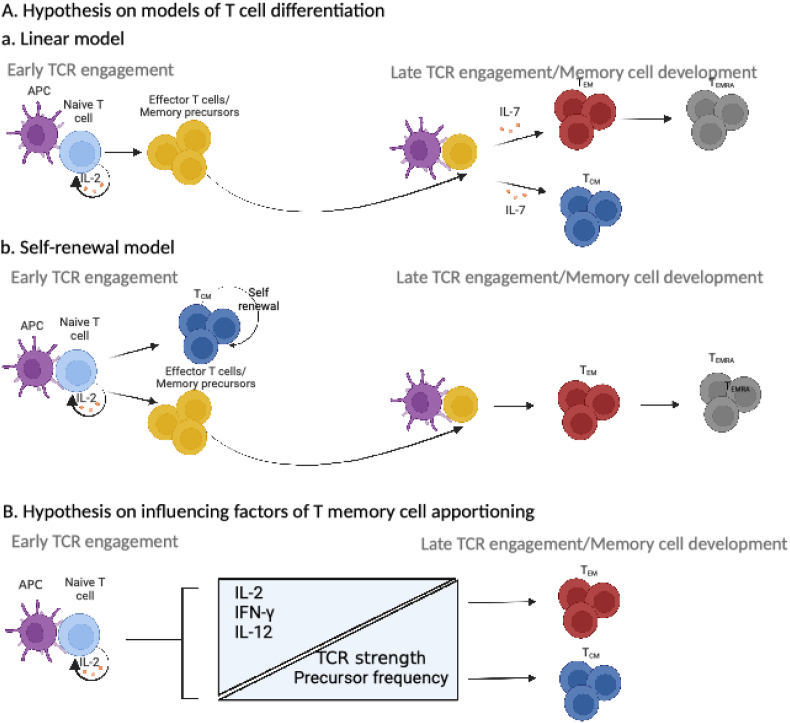
The developmental pathways of memory T cells from naïve T cells are still not fully understood. There are different suggestive models, for each of which there is supporting evidence as well as controversies (Ahmed et al., 2009). In Fig. 4A we summarize the linear and the self-renewal model, as the more relevant to the observations of this study. According to the linear model, our data implies a tendency of the effector/memory precursor cells to differentiate into TCM in patients with BD. In case of the self-renewal model our data would imply an excessive self-renewal of the TCM at the expense of the TEM in patients with BD.
Fig. 4.
A) Hypothesis on the models of T cell differentiation. a. Linear model: the data of this study would imply a tendency of the effector/memory precursor cells to differentiate into TCM. b. Self-renewal model: the data of this study would imply an excessive self-renewal of the TCM at the expense of the TEM. B) Hypothesis on influencing factors of T memory cell apportioning. High levels of IL-2, IFN-γ and IL-12 are critical for memory T cell development, during early TCR engagement. Increased levels of TCR affinity and T cell precursor frequencies favor TCM development, while decreased levels favor TEM. Abbreviations: APC Antigen Presenting Cell; TCR, T Cell Receptor; TCM, T central memory; TEMRA, terminally differentiated effector memory; TEM, effector memory; IL-2, Interleukin-2; IFN-γ, Interferon-γ; IL-12, Interleukin-12. Figure adjusted from (Raphael et al., 2020) in BioRender.com.
Multiple factors have been recognized to affect T cell developmental fate. In Fig. 4B we summarize the currently suggested influencing factors. Firstly, the nature of the interaction between the antigen-presenting cell and the T cell receptor (TCR) influences the fate of memory T cells. In more detail, the differentiation of the T memory cell populations takes place in the secondary lymphoid organs, such as the lymph nodes, the Peyer's patches, and the spleen. There, the MHC-antigen complex on the antigen-presenting cell and the TCR on the naïve T cell interact with each other. The strength of the interaction influences the memory T cell development. Increased levels of TCR affinity and T cell precursor frequencies favor TCM development while decreased levels favor TEM (Raphael et al., 2020). Secondly, high levels of interleukin (IL-2) are critical for memory T cell development, during early TCR engagement. There are numerous reports on abnormalities in the IL-2 network in patients with BD (Carvalho et al., 2016).
Furthermore, a balanced availability of homeostatic cytokines has important implications in the differentiation and maintenance of T memory cell subsets, and so has the lack thereof. The γ-chain family of cytokines and in particular IL-7 and IL-15 are key regulators of survival and proliferation of T memory cell population (Boyman et al., 2009; Surh and Sprent, 2008). Geginat et al. (2001) for instance showed that upon IL-7 and IL-15 exposure TCM differentiate into TEM cells, even in an antigen-independent fashion. In this study we show lower levels of IL-7 in patients with BD. Unfortunately, the relationship between IL-7 and T memory cell subsets could not be further investigated due to the lack of sufficient power for this analysis. It is sensible that this relationship is clarified in further studies, especially since IL-7 in addition to its role in T cell development, has also been shown to be a neurotrophic and neuro-apoptotic factor (Michaelson et al., 1996; Nunnari et al., 2005), making it possible that an IL-7 deficiency acts as a double-edged sword in BD.
T memory cell differentiation is also pathogen guided. Accumulating evidence shows that infections with pathogens such as human CMV and dengue virus are associated with a terminal differentiation of TEM to TEMRA cells and to a further late-stage terminal differentiation of TEMRA cells characterized by a decreased expression of CD27 and CD28 and an increased expression of CD57 (Tian et al., 2017). In the present study, seropositivity to CMV-specific antibodies was associated with increased CD4+ TEMRA cells and late stage differentiated senescent cell populations (CD27−CD28−), in both patients and healthy controls, thus showing an equally strong aging effect in both groups. This indicates that the T cell differentiation abnormalities in patients with BD shown in this study, namely the increased TCM and reduced TEM, cannot be ascribed to an interaction effect between CMV infection and the diagnosis of BD.
In a recent study Aggio et al. (2023) the authors showed a negative association between CD8+ T memory cells with indexes of white matter integrity, while naïve CD8+ T cells (negative for granzyme and perforin) were positively associated with white matter microstructure. Given the here observed shifts in T memory cell populations among patients with BD, delving into the relationship between the different T memory cell populations and brain integrity promises to enhance our comprehension of their contribution to BD pathology.
4.2. Th17 cells in BD
This study shows that patients with BD in a euthymic state have increased levels of Th17 cells compared to healthy individuals. We made a similar observation before in a cohort of 97, largely euthymic patients with BD (Vogels et al., 2017), but did not in another cohort of 38 euthymic patients (Drexhage et al., 2011). Another study has shown higher levels of IL-17 in the circulation of patients with BD in remission (Keshri et al., 2018). We also report a trend towards increased Th17 levels in the presence of an interaction between CMV and BD. In the subsequent stratified analysis, patients with BD that were seropositive to CMV-specific antibodies had significantly higher Th17 cells compared to seropositive healthy controls. This may point towards a proneness of patients with BD to react to CMV with an increase in Th17 cells.
4.3. TREGS in BD
In this study euthymic patients with BD also show higher levels of CD4+CD25+FOXP3+ TREGS compared to healthy controls. The literature on BD and this cell population is contradictory. We have previously shown higher circulating TREGS in younger-than-40 years old patients with BD in an active disease state (Drexhage et al., 2011), but decreased levels in relatively old euthymic BD patients (Vogels et al., 2017). Other studies have also shown lower levels of TREGS in patients with BD in euthymia (Barbosa et al., 2014; Do Prado et al., 2013; Wieck et al., 2013). Lithium usage was not found to have an influence on this subpopulation in any of the above referenced studies.
If lithium usage and disease activity cannot explain the fact that some studies show increased while others decreased TREGS, which other influencing factors should be considered? First, abnormal setpoints in other parts of the patients’ immune or endocrine system could play a role. TREGS have been found to be reduced in patients with comorbid thyroid autoimmune disease (Drexhage et al., 2011) and in patients with higher levels of inflammatory cytokines (Do Prado et al., 2013). Second, the stage of the disease has been found to be related to the levels on TREGS. Maes et al. (2021) have shown that patients with a long disease duration (≥10 years) had depleted frequencies of CD4+CD25+FOXP3+CD152+ cells. Last, the presence of chronic infections seems to play a role. In the previously mentioned study, the authors also observed that CMV-specific IgG levels were associated with decreased levels of CD4+CD25+FOXP+GARP+ T regulator cells (Maes et al., 2021). In our here reported study, the interaction between CMV and BD was not significantly associated with the levels of “regular” CD4+CD25+FOXP3+ T regulator cells.
4.4. T cell differences in BD versus MDD
A previous study from our group has shown higher levels of Th17 cells in BD compared to MDD (Becking et al., 2018). In addition, the here reported discrepancy in the level of TCM cells is not evident in MDD (Simon et al., 2023). The two immune subsets seem to be linked, as there is literature suggesting that Th17 cells are predominantly TCM cells (Mehling et al., 2010). Other discriminating abnormalities in the T cell system of BD versus unipolar MDD should be further investigated, as they may open the way for the development of a panel of cellular immune tests with diagnostic value.
4.5. IL-7 in BD
In this study we also show that IL-7 is lower in patients with BD, compared to healthy individuals. The role of IL-7 and its therapeutic potential for the proper generation of TCM, TEM and TEMRA from naïve CD4+ T cells in BD warrants further investigation. There are two IL-7 preparations presently in experimental use for human treatment: a recombinant human IL-7 (rhIL7, CYT107) and a Fc-fused long-acting recombinant human IL-7 (hIL-7-hyFc, Efineptakin-α). Experimental trials with these drugs are ongoing in the field of tumor immunology, sepsis, chronic HIV infections and other CD4 cytopenia syndromes (Sheikh et al., 2016). Sheikh et al. (2016) showed that CYT107 therapy was well tolerated in patients with idiopathic CD4 lymphopenia (ICL), a disorder with T cell disturbances reminiscent of those found in this report in BD and those previously reported by us and others in MDD. ICL is a rare syndrome defined by low CD4 T-cell counts without evidence of HIV infection or other known cause of immunodeficiency. ICL confers an increased risk of opportunistic infections. The rhIL-7 treatment led to increases in CD4+ T cells in both peripheral blood and tissues. Regarding the newer drug Efineptakin-α, administration in healthy adults induced a sustained increase in the numbers of naïve, TCM, TEM and TEMRA CD4+ and CD8+ T cells without qualitative changes (Kim et al., 2022). The authors concluded that hIL-7-hyFc is a potential treatment for patients with compromised T-cell immunity.
4.6. Limitations
The presented findings should be viewed alongside the study's limitations. This is a cross-sectional study and therefore, no causality can be inferred, nor conclusions can be drawn about the temporal dynamics of the observed abnormalities. Secondly, the fact that the reported differences between patients and controls could be driven by unobserved confounders cannot be excluded until replicated. Moreover, as previously mentioned, the investigation of the relationship between low IL-7 levels and T memory cell subpopulations in BD was not possible due to lack of sufficient power.
4.7. Conclusion
The present study shows that BD is associated with higher levels of TCM cells in both the CD4+ and CD8+ compartment, and lower CD4+ TEM and CD4+ TEMRA cells. This may suggest an aberrant development of memory T cell populations from naïve CD4+ T cells in patients with BD. BD patients in addition showed increased Th17 and TREGS cells and deceased levels of the T cell differentiation factor IL-7.
Funding
This work was supported by the European Commission: EU 7th Framework program (grant number EU-FP7-CP-IP-2008-222963) and Horizon 2020 (grant number H2020– SC1-2016-2017/H2020-SC1-2017-Two-Stage-RTD). The grants were received by HAD, Erasmus Medical Center, Rotterdam. The funding source had no role in the study design, the data collection, the analysis and interpretation of data, the manuscript writing, and in the decision to submit the article for publication.
CRediT authorship contribution statement
Magdalini Ioannou: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Data curation. Maria S. Simon: Writing – review & editing, Methodology, Formal analysis, Data curation. Jenny Borkent: Writing – review & editing, Methodology. Annemarie Wijkhuijs: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization. Raf Berghmans: Writing – review & editing, Resources, Methodology. Bartholomeus C.M. Haarman: Writing – review & editing, Supervision, Resources, Methodology, Conceptualization. Hemmo A. Drexhage: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
MI, MSS, JB and AW and BH declare no potential conflict of interest. RB is an employee of Advanced Practical Diagnostics BVBA. HAD is the coordinator of the EU-MOODSTRATIFICATION project and declares no further potential conflict of interest.
Acknowledgments
We would like to thank Dr. Klaas Wardenaar and Georgios Papingiotis for their valuable advice on the statistical analysis. Moreover, we would like to thank the participants of the EU-MOODSTRATIFICATION study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100764.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Aggio V., Fabbella L., Poletti S., Lorenzi C., Finardi A., Colombo C., Zanardi R., Furlan R., Benedetti F. Circulating cytotoxic immune cell composition, activation status and toxins expression associate with white matter microstructure in bipolar disorder. Sci. Rep. 2023;13(1):1–11. doi: 10.1038/s41598-023-49146-6. 2023 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Bevan M.J., Reiner S.L., Fearon D.T. The precursors of memory: models and controversies. Nat. Rev. Immunol. 2009;9(9):662–668. doi: 10.1038/nri2619. 2009 9:9. [DOI] [PubMed] [Google Scholar]
- Barbosa I.G., Rocha N.P., Assis F., Vieira E.L.M., Soares J.C., Bauer M.E., Teixeira A.L. Monocyte and lymphocyte activation in bipolar disorder: a new piece in the puzzle of immune dysfunction in mood disorders. Int. J. Neuropsychopharmacol. 2014;18(1) doi: 10.1093/IJNP/PYU021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becking K., Haarman B.C.M., Grosse L., Nolen W.A., Claes S., Arolt V., Schoevers R.A., Drexhage H.A. The circulating levels of CD4+ t helper cells are higher in bipolar disorder as compared to major depressive disorder. J. Neuroimmunol. 2018;319:28–36. doi: 10.1016/J.JNEUROIM.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Becking K., Haarman B.C.M., van der Lek R.F.R., Grosse L., Nolen W.A., Claes S., Drexhage H.A., Schoevers R.A. Inflammatory monocyte gene expression: trait or state marker in bipolar disorder? Int. J. Behav. Dev. 2015;3(1):20. doi: 10.1186/S40345-015-0037-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M., Kapczinski F., Andreazza A.C., Dean O.M., Giorlando F., Maes M., Yücel M., Gama C.S., Dodd S., Dean B., Magalhães P.V.S., Amminger P., McGorry P., Malhi G.S. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011;35(3):804–817. doi: 10.1016/J.NEUBIOREV.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Boyman O., Létourneau S., Krieg C., Sprent J. Homeostatic proliferation and survival of naïve and memory T cells. Eur. J. Immunol. 2009;39(8):2088–2094. doi: 10.1002/EJI.200939444. [DOI] [PubMed] [Google Scholar]
- Breunis M.N., Kupka R.W., Nolen W.A., Suppes T., Denicoff K.D., Leverich G.S., Post R.M., Drexhage H.A. High numbers of circulating activated T cells and raised levels of serum IL-2 receptor in bipolar disorder. Biol. Psychiatr. 2003;53(2):157–165. doi: 10.1016/S0006-3223(02)01452-X. [DOI] [PubMed] [Google Scholar]
- Brietzke E., Stertz L., Fernandes B.S., Kauer-Sant’Anna M., Mascarenhas M., Escosteguy Vargas A., Chies J.A., Kapczinski F. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J. Affect. Disord. 2009;116(3):214–217. doi: 10.1016/J.JAD.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Carvalho A.F., Köhler C.A., Fernandes B.S., Quevedo J., Miskowiak K.W., Brunoni A.R., Machado-Vieira R., Maes M., Vieta E., Berk M. Bias in emerging biomarkers for bipolar disorder. Psychol. Med. 2016;46(11):2287–2297. doi: 10.1017/S0033291716000957. [DOI] [PubMed] [Google Scholar]
- Counotte J., Drexhage H.A., Wijkhuijs J.M., Pot-Kolder R., Bergink V., Hoek H.W., Veling W. Th17/T regulator cell balance and NK cell numbers in relation to psychosis liability and social stress reactivity. Brain Behav. Immun. 2018;69:408–417. doi: 10.1016/J.BBI.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Darrigues J., Van Meerwijk J.P.M., Romagnoli P. Age-dependent changes in regulatory T lymphocyte development and function: a mini-review. Gerontology. 2018;64(1):28–35. doi: 10.1159/000478044. [DOI] [PubMed] [Google Scholar]
- Debnath M., Raison C.L., Maes M., Berk M. Role of the T-cell network in psychiatric disorders. Immuno-Psychiatry: Facts and Prospects. 2021:109–132. doi: 10.1007/978-3-030-71229-7_7/COVER. [DOI] [Google Scholar]
- Do Prado C.H., Rizzo L.B., Wieck A., Lopes R.P., Teixeira A.L., Grassi-Oliveira R., Bauer M.E. Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology. 2013;38(5):667–676. doi: 10.1016/J.PSYNEUEN.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Drexhage R.C., Hoogenboezem T.H., Versnel M.A., Berghout A., Nolen W.A., Drexhage H.A. The activation of monocyte and T cell networks in patients with bipolar disorder. Brain Behav. Immun. 2011;25(6):1206–1213. doi: 10.1016/J.BBI.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Foiselle M., Lajnef M., Hamdani N., Boukouaci W., Wu C.L., Naamoune S., Chami L., Mezoued E., Richard J.R., Bouassida J., Sugunasabesan S., Le Corvoisier P., Barrau C., Yolken R., Leboyer M., Tamouza R. Immune cell subsets in patients with bipolar disorder or schizophrenia with history of childhood maltreatment. Brain Behav. Immun. 2023;112:42–50. doi: 10.1016/J.BBI.2023.05.015. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Valensin S., Fagnoni F., Barbi C., Bonafè M. Biomarkers of immunosenescence within an evolutionary perspective: the challenge of heterogeneity and the role of antigenic load. Exp. Gerontol. 1999;34(8):911–921. doi: 10.1016/S0531-5565(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Geginat J., Sallusto F., Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J. Exp. Med. 2001;194(12):1711–1719. doi: 10.1084/JEM.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatr. 2016;21(12):1696. doi: 10.1038/MP.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshri N., Nandeesha H., Kattimani S. Elevated interleukin-17 and reduced testosterone in bipolar disorder. Relation with suicidal behaviour. Asian Journal of Psychiatry. 2018;36:66–68. doi: 10.1016/J.AJP.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Kim S., Lee S.W., Koh J.Y., Choi D., Heo M., Chung J.Y., Lee B.H., Yang S.H., Sung Y.C., Lee H., Shin E.C., Park S.H. A single administration of hIL-7-hyFc induces long-lasting T-cell expansion with maintained effector functions. Blood Advances. 2022;6(23):6093–6107. doi: 10.1182/BLOODADVANCES.2021006591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Nani J.V., Noto C., Rizzo L., Hayashi M.A.F., Brietzke E. Impairments in peripheral blood T effector and T regulatory lymphocytes in bipolar disorder are associated with staging of Illness and anti-cytomegalovirus IgG levels. Mol. Neurobiol. 2021;58(1):229–242. doi: 10.1007/S12035-020-02110-1. [DOI] [PubMed] [Google Scholar]
- McIntyre R.S., Berk M., Brietzke E., Goldstein B.I., López-Jaramillo C., Kessing L.V., Malhi G.S., Nierenberg A.A., Rosenblat J.D., Majeed A., Vieta E., Vinberg M., Young A.H., Mansur R.B. Bipolar disorders. Lancet. 2020;396(10265):1841–1856. doi: 10.1016/S0140-6736(20)31544-0. [DOI] [PubMed] [Google Scholar]
- Mehling M., Lindberg R., Raulf F., Kuhle J., Hess C., Kappos L., Brinkmann V. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology. 2010;75(5):403–410. doi: 10.1212/WNL.0B013E3181EBDD64. [DOI] [PubMed] [Google Scholar]
- Michaelson M.D., Mehler M.F., Xu H., Gross R.E., Kessler J.A. Interleukin-7 is trophic for embryonic neurons and is expressed in developing brain. Dev. Biol. 1996;179(1):251–263. doi: 10.1006/DBIO.1996.0255. [DOI] [PubMed] [Google Scholar]
- Nunnari G., Xu Y., Acheampong E.A., Fang J., Daniel R., Zhang C., Zhang H., Mukhtar M., Pomerantz R.J. Exogenous IL-7 induces Fas-mediated human neuronal apoptosis: potential effects during human immunodeficiency virus type 1 infection. J. Neurovirol. 2005;11(4):319–328. doi: 10.1080/13550280500187005. [DOI] [PubMed] [Google Scholar]
- Padmos R.C., Hillegers M.H.J., Knijff E.M., Vonk R., Bouvy A., Staal F.J.T., De Ridder D., Kupka R.W., Nolen W.A., Drexhage H.A. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch. Gen. Psychiatr. 2008;65(4):395–407. doi: 10.1001/ARCHPSYC.65.4.395. [DOI] [PubMed] [Google Scholar]
- Raphael I., Joern R.R., Forsthuber T.G. Memory CD4+ T cells in immunity and autoimmune diseases. Cells. 2020;9(3) doi: 10.3390/CELLS9030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo L.B., Do Prado C.H., Grassi-Oliveira R., Wieck A., Correa B.L., Teixeira A.L., Bauer M.E. Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013;15(8):832–838. doi: 10.1111/BDI.12121. [DOI] [PubMed] [Google Scholar]
- Sokal R., James Rohlf F. Biometry : the principles and practice of statistics in biological research. 1995. https://search.worldcat.org/title/1028729125 [Google Scholar]
- Schiweck C., Valles-Colomer M., Arolt V., Müller N., Raes J., Wijkhuijs A., Claes S., Drexhage H., Vrieze E. Depression and suicidality: a link to premature T helper cell aging and increased Th17 cells. Brain Behav. Immun. 2020;87:603–609. doi: 10.1016/J.BBI.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Sheikh V., Porter B.O., DerSimonian R., Kovacs S.B., Thompson W.L., Perez-Diez A., Freeman A.F., Roby G., Mican J., Pau A., Rupert A., Adelsberger J., Higgins J., Bourgeois J.S., Jensen S.M.R., Morcock D.R., Burbelo P.D., Osnos L., Maric I., et al. Administration of interleukin-7 increases CD4 T cells in idiopathic CD4 lymphocytopenia. Blood. 2016;127(8):977–988. doi: 10.1182/BLOOD-2015-05-645077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M.S., Ioannou M., Arteaga-Henríquez G., Wijkhuijs A., Berghmans R., Musil R., Müller N., Drexhage H.A. Premature T cell aging in major depression: a double hit by the state of disease and cytomegalovirus infection. Brain, Behavior, & Immunity - Health. 2023;29 doi: 10.1016/J.BBIH.2023.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C.D., Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/J.IMMUNI.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tian Y., Babor M., Lane J., Schulten V., Patil V.S., Seumois G., Rosales S.L., Fu Z., Picarda G., Burel J., Zapardiel-Gonzalo J., Tennekoon R.N., De Silva A.D., Premawansa S., Premawansa G., Wijewickrama A., Greenbaum J.A., Vijayanand P., Weiskopf D., et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 2017;8(1):1–13. doi: 10.1038/s41467-017-01728-5. 2017 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R.J., Koenders M.A., van Rossum E.F.C., Spijker A.T., Drexhage H.A. T cell deficits and overexpression of hepatocyte growth factor in anti-inflammatory circulating monocytes of middle-aged patients with bipolar disorder characterized by a high prevalence of the metabolic syndrome. Front. Psychiatr. 2017;8(MAR):34. doi: 10.3389/FPSYT.2017.00034/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieck A., Grassi-Oliveira R., do Prado C.H., Rizzo L.B., de Oliveira A.S., Kommers-Molina J., Viola T.W., Teixeira A.Ô.L., Bauer M.E. Differential neuroendocrine and immune responses to acute psychosocial stress in women with type 1 bipolar disorder. Brain Behav. Immun. 2013;34:47–55. doi: 10.1016/J.BBI.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Yang F., Barbosa I.G., Vieira E.L., Bauer M.E., Rocha N.P., Teixeira A.L. Further evidence of accelerated aging in bipolar disorder: focus on GDF-15. Transl. Neurosci. 2018;9(1):17–21. doi: 10.1515/TNSCI-2018-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.