Abstract
Clay is naturally occurring and poses a low risk. It is distinguished by mineral composition and ability to adsorb plant colorants and phytochemicals effectively. This study aimed to enhance the stability of bio-clay by preparing body mud scrubs through a solid-state reaction, combining volcanic clay with herbal plants, including Bougainvillea spp., Pandanus amaryllifolius Roxb., and Curcuma longa L. (bio-clay). The characterization of purification clay revealed strong stability within its mineral composition. The optimum condition for sampling was 4 °C, which reserved the total phenolic content (TPC), 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. A high Trolox equivalent antioxidant capacity (TEAC; mg TEAC/g sample) and low half-maximal inhibitory concentration (IC50) indicated excellent antioxidant activity. Over a storage period of 28 d, the Bougainvillea spp., Curcuma longa L., purified clay + Bougainvillea spp., and purified clay + Curcuma longa L. samples retained their stability. Their TPC, % scavenging, TEAC, and IC50 showed dominant antioxidant activity, stable active phenolic compounds, and the maintenance of extensive amounts. This compound is widely applied as a unique cosmetic ingredient.
Keywords: Bio-clay, Antioxidant activity, Phenolic content, Stable, Body mud scrubs
1. Introduction
Natural volcanic clay is abundant, simple, and cost-effective. Its chemical composition comprises silicon, alumina, magnesium, potassium, calcium, and iron [[1], [2], [3]]. Clay contains essential elements beneficial for healthcare and cosmetic applications. Furthermore, clay possesses exceptional properties, such as cation exchange capacity (CEC), specific surface area, and clay minerals [4,5]. It is used in cosmetics for various purposes, including as a color additive [6], emulsion [7], abrasive [8], opacifying agent [9], and thickening agent [10]. Premium clay exhibits good adsorption [[11], [12], [13], [14]] because it is highly porous and has a large specific surface area, which adsorbs and protects the phytochemicals from plants [15]. However, recent research has revealed interesting alternative properties of clay, such as its ability to adsorb antioxidants derived from nature [[16], [17], [18]]. This exceptional interaction between clays and antioxidants indicates potential applications in the natural health field. Moreover, clay combines the antioxidants from plants and is hence suitable for cosmetic use because it is less toxic. Naturally occurring antioxidants in various plants and herbs have garnered significant attention in scientific research for their therapeutic properties and health benefits [19]. Polyphenols, primarily found in vegetables and fruits, exhibit antioxidant properties, serving as natural, economical, and oxidative-stable anti-aging agents [20].
Plants have primary phytochemicals and phenolic compounds that exhibit antioxidant activity, aiding in free radical scavenging. This mechanism activates oxidative stress in live cells, reducing highly oxidized reactive oxygen species and decreasing oxygen-free radicals [21]. The three plants selected for their antioxidant properties and sources are listed in Table 1. Research on clay-adsorbed antioxidants from plants (bio-clay) can develop methods to utilize and harness the powder of natural antioxidants. The preservation and controlled release of these valuable bioactive compounds through clay adsorption and stability presents exciting opportunities for enhancing their efficacy in promoting cosmetics. Bio-clay can be applied to body care products.
Table 1.
Plants and phytochemicals.
| Plants | Phytochemicals | Cosmetic properties | Ref. |
|---|---|---|---|
 Bougainvillea spp. Bougainvillea spp. |
Betacyanins | - color pigment (food) - antioxidant activity (anti-aging) |
[[22], [23], [24]] |
 Pandanus amaryllifolius Roxb. Pandanus amaryllifolius Roxb. |
Flavonoids and Saponins | - color pigment (food and medicinal herbs) - antioxidant activity (anti-aging) |
[25,26] |
 Curcuma longa L. Curcuma longa L. |
Curcuminoids | - color pigment (food and medicinal herbs) - antioxidant activity (anti-aging) - antibacterials |
[[27], [28], [29]] |
In this research, we developed a bio-clay by combining volcanic clay with plant antioxidants via a solid-state reaction. We characterized the physical and chemical properties of volcanic clay before and after decontamination. Additionally, we investigated storage conditions for the bio-clay (room temperature, 4 °C, and freezing temperature), assessing chemical properties and antioxidant activity (% scavenging, IC50, TEAC, TPC) and stability over storage times (0–28 d) to confirm the efficiency of the starting materials for cosmetics applications.
2. Materials and methods
2.1. Clay preparation
A volcanic clay sample was collected from Buriram Province, as shown in Fig. 1. The clay was washed with distilled water three times, and ferric oxide was removed using a magnet. The starting materials were dried in a well-ventilated environment. Subsequently, the clay was ground and sieved through a 325-mesh sieve before being stored in a desiccator. The volcanic clay sample was prepared after decontamination following the method in Ref. [3]. Subsequently, the physical properties, including pH, conductivity, percentage of organic matter, and CEC, were analyzed using a previously described method [3]. The characterization of clay was determined through specific surface area and porosity analysis by Brunauer–Emmett–Teller (BET: micromeritics, model TriStar II 3020), X-ray fluorescence (XRF: energy dispersive spectrometer model, XGT 5200), and X-ray diffraction (XRD: BRUKER, D2 Phaser). These were conducted under Cu Kα radiation (λ = 1.54060 Å) at 30 mA and 35 kV. The scanning step size was 0.02° s−1 in the range of 5–60°.
Fig. 1.
Area of sampling volcanic clay.
2.2. Preparation of plant materials
2.2.1. Chemicals and reagents
2, 2-diphenyl-1-picrylhydrazyl (DPPH), and gallic acid were purchased from Aldrich (Germany). 6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid (Trolox) was purchased from Aldrich (Switzerland). Ethanol was obtained from RCI Labscan, Ltd. (Thailand). Ferrous chloride and linoleic acid were purchased from Fluka (Germany). The Folin–Ciocalteu reagent was purchased from Fisher Chemical (Leicestershire, UK). All the chemicals used were of analytical or HPLC grade.
2.2.2. Plant material
The plants were collected from Buriram Province, Thailand, between March and August 2023. This study selected local plants, and the properties of the food pigments applied to them were examined, as shown in Table 1. The plants were washed, cleaned with tap water, and air-dried before being cut into small pieces.
2.2.3. Color clay preparation
Bio-clay was prepared via a solid-state reaction, a method known to be effective for enhancing properties such as clay and scrub [[30], [31], [32]]. Subsequently, a mixture of Bougainvillea spp., Pandanus amaryllifolius Roxb., and Curcuma longa L. were ground with volcanic clay in a 1:1 wt ratio. Then, a small amount of ethanol (95%) was added. The moisture was removed at 65 °C. Finally, the samples were dried and ground before the analysis, as shown in Fig. 5. The colored clay was characterized using the color space (polar CIE L* a* b* rectangular coordinate system) with HunterLab (ColorFlex EZ Spectrophotometer). The spectral range was 400–700 nm. The instrument was calibrated using a white tile, and the samples were analyzed in tetraplicate. The powdered mixture was examined using PerkinElmer Spectrum Two Fourier transform infrared spectroscopy (FTIR) in the 400–4000 cm−1 wavenumber range to prepare KBr pellets with colored clay.
Fig. 5.
Coloration of (A) purified clay, (B) purified clay + Bougainvillea spp., (C) purified clay + Pandanus amaryllifolius Roxb., and (D) purified clay + Curcuma longa L.
2.2.4. Extraction methods
Extraction was performed using a modified version of a previously reported method [33]. Subsequently, precisely 5 g of the sample was soaked in 50 ml of ethanol (95%). The bottle was then closed and vibrated in a shaking water bath (Thermo Precision SWB 15) at room temperature at 100 rpm for 2 h. This experiment was repeated three times. Finally, it was filtered using filter paper (Whatman No. 1) and refrigerated at 4 °C before studying its antioxidant activities.
2.3. Antioxidant activities
2.3.1. DPPH radical scavenging assay
This process was performed as described in Ref. [34]. The antioxidant activities of the plant extracts, clay-mixed plant extracts, and Trolox standard solution were reacted with a 0.1 mM solution of DPPH in ethanol. This solution was mixed at room temperature and stored in the dark for 30 min. The absorbance was determined at 517 nm by a Lambda 12 (PerkinElmer, USA) UV–Vis spectrometer instrument. The IC50 values were subsequently determined. The scavenging of the DPPH radical was investigated in triplicate, and the mean value ± SD was reported. The DPPH-radical scavenging activity was calculated using the following equation:
where Acontrol and Asample are the absorbance values of the controller (DPPH solution) and the sample and DPPH solution, respectively. The percentage antioxidant ability of the sample was compared to its ability to act as an antioxidant free radical in the standard Trolox solution (% scavenging). IC50, which is the concentration of the sample in the DPPH solution, decreased by 50%. The results were expressed as mg TEAC/g sample (TEAC). The experiment was repeated thrice, and the values obtained from the average of triplicate measurements were plotted.
2.3.2. Total phenolic content
This procedure was modified from a previous study [21] to obtain the TPC. Briefly, 200 μL of extracts were oxidized with 2500 μl of 10% v/v Folin–Ciocalteu reagent and added to 2000 μl of 7.5% w/v sodium carbonate. These solutions were incubated at room temperature for 30 min, and the absorbance was measured at 760 nm. The TPC was expressed in milligrams of gallic acid equivalent (GAE)/g sample. The results were calculated as the mean of three replicates.
2.3.3. Optimization of storage conditions
The mixture powders were dried and subsequently preserved in a zip-lock aluminum foil bag without moisture at various temperatures (room temperature, 4 °C, and freezing temperature) and storage times (0, 7, 14, 21, and 28 d). The stability and antioxidant activity of the dried plants and colored clay powders were studied.
2.3.4. Statistical analysis
Statistical analyses were performed in triplicate. The results were expressed as the mean ± SD. A significant one-way comparative analysis was accomplished at the 95% confidence level (p < 0.05).
3. Results and discussion
3.1. Clay characteristics
The physical properties of the clay samples are listed in Table 2. The clay was black before purification, demonstrating a high percentage of organic matter due to a high CEC value. However, the results showed that the conductivity and pH were 335.00 ± 4.00 and 5.35 ± 0.18, respectively. After purification, the color of the clay became lighter, indicating successful cleansing, which was attributed to a decrease in the percentage of organic matter and a low CEC. This analysis showed that the pH, conductivity, percentage of organic matter, and CEC values before and after purification were significantly different (p < 0.05). Furthermore, there was no conductivity, and the pH was low. The results of the clay CEC analysis showed that the pH and conductivity were significantly different. In particular, a significant amount of organic matter affects the CEC [35]. The CEC value influenced the pH of the clay and the percentage of organic matter in the clay. The negative surface charge results in the adsorption of positive ions and the exchange of negative ions. The high amount of organic matter in the clay translates into a high CEC because the organic matter is formed from the dissolution of compounds like carboxylic groups (COOH−) and phenolic groups (OH−) [36].
Table 2.
Physical properties of clay.
| Sample | Physical properties |
||||
|---|---|---|---|---|---|
| pH | Conductivity (dS/cm) | Organic matter (%) | CEC | Colors | |
| Clay before purification | 5.35 ± 0.18 | 335.00 ± 4.00 | 10.81 ± 0.50 | 84. 42 ± 1.54 |  |
| Clay after purification | 4.46 ± 0.06 | 0.00 ± 0.00 | 2.86 ± 0.20 | 6.07 ± 0.87 |  |
pHpzc of clay purification.
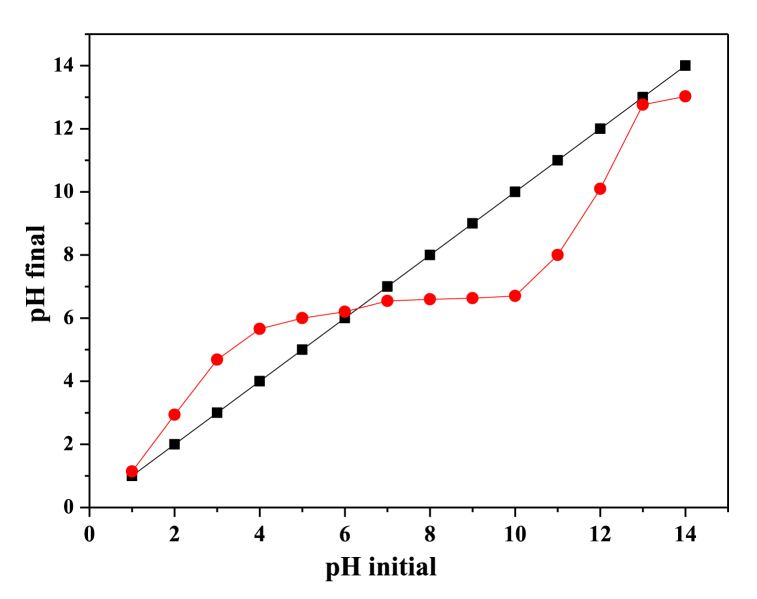
Consequently, before the purification of the clay and plant ground by the solid-state reaction, the purified clay's pH at the point of zero charge (pHpzc) is determined using the pH drift method [37,38]. pHpzc is the pH at which the sum of the surface charges of the clay is zero. When pH < pHpzc, the charge on the surface of the clay is positive; however, if pH > pHpzc, the surface charge of the adsorbent is negative [39]. The pHpzc of purified clay was found to be 6.36, as presented in Fig. 2.
Fig. 2.
pHpzc of purified clay.
3.1.1. Specific surface area
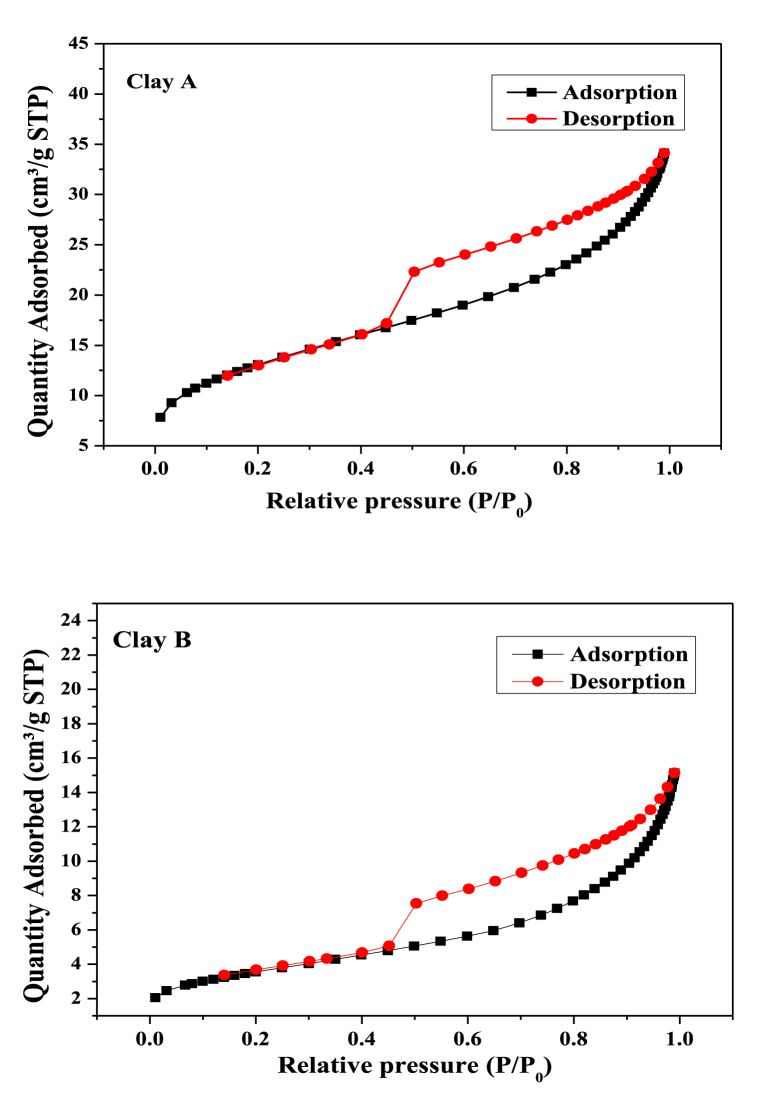
The specific surface area results showed that the purified clay had a lower specific surface area than that before purification. This was because the purified clay had a lower primary impurity due to removing a high percentage of organic matter and increased mineral components, as shown in Table 3, Table 4, respectively. This specific surface area was greater than cosmetic clay's [3]. The results of the study are consistent with those of [40]. The isotherms were classified using the Brunauer–Emmett–Teller (BET) technique, which involves the adsorption/desorption of N2. From the analysis results, it was found that Type IV isotherm. The adsorption isotherms were classified according to IUPAC standards and shown in Fig. 3 (A-B). These are porous materials based on the gas adsorption data and the indemnification of mesoporous (2–50 nm). From these results, purified clay was found. The average pore width after purification was larger than that before purification (Table 3). This is because the clay was decontaminated and burned by calcination at 550 °C, resulting in the decomposition of organic substances in the structure. This result is in accordance with that of a previous report [41].
Table 3.
Specific surface area and total porosity of clay.
| Sample | Specific surface area (m2/g) | Total area in pores (cm3/g) | Average pore width (nm) |
|---|---|---|---|
| Clay before purification | 46.88 | 20.32 | 5.58 |
| Clay after purification | 12.66 | 6.75 | 8.51 |
Table 4.
Chemical composition of clay.
| Chemical composition (wt%) | Clay before purification | Clay after purification |
|---|---|---|
| SiO2 | 65.41 | 76.58 |
| Al2O3 | 13.06 | 9.10 |
| CaO | 0.67 | 0.38 |
| Na2O | – | – |
| K2O | 0.37 | 0.17 |
| MgO | 1.44 | 1.86 |
| TiO2 | 3.92 | 3.80 |
| MnO2 | 0.35 | 0.93 |
| Fe2O3 | 14.66 | 7.09 |
| Cr2O3 | 0.03 | 0.02 |
| CuO | 0.00 | 0.00 |
| ZnO | 0.01 | 0.00 |
| ZrO2 | 0.05 | 0.04 |
| Nb2O5 | 0.01 | 0.00 |
| NiO | 0.01 | 0.01 |
| LOI | 0.38 | 0.13 |
Fig. 3.
N2-adsorption/desorption isotherms before (A) and after (B) clay purification.
3.2. Chemical and mineralogical characterization
The chemical composition of the clay primarily experienced an increase in SiO2 and a reduction in Al2O3, K2O, Fe2O3, TiO2, Cr2O3, ZnO, Nb2O5, and NiO. Moreover, the MgO content increased, and the ferric oxide (Fe2O3) content decreased due to its removal by a magnet. These results of mass decomposition at 550 °C primarily reflect volatiles of moisture and free water on the surface of clay and decomposed aluminum and iron hydroxides [42]. However, carbon dioxide, sulfide gas, carbonates, and sulfates from the basic materials also have a volatile composition corresponding to the ignition loss. The loss on ignition (LOI) in purified clay is less than before purification, as shown in Table 4.
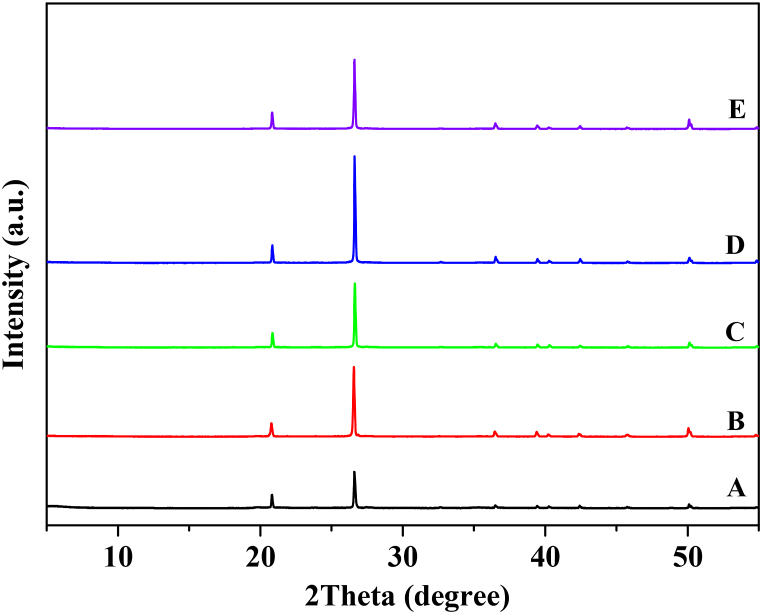
Fig. 4. (A-B) shows the primary mineral compositions of quartz (SiO2) and ferric oxide (Fe2O3) as JCPDS No. 33–1161 and 24–0072, respectively, and the minor compositions of montmorillonite ((Si,Al)4O10(OH)2·6H20) and kaolinite (Mg3(Si4O10)(OH)2) as JCPDS No. 007–330 and 006–221, respectively, which correspond to the XRF, as shown in Table 4. The observation of 2 at 11.89 showed that kaolinite was present before clay purification but disappeared after clay purification, as shown in Fig. 4 (A-B). Clay minerals, particularly kaolinite, are destroyed after heating at high temperatures. A slight shift was observed in the peak positions before and after clay purification. The purified clay showed 2 values of 20.82, 26.61, 27.44, 36.52, 39.45, and 50.13, indicating an increase in the intensity percentage, as shown in Fig. 4 (B). The XRD patterns present the non-degradation minerals from purified clay, which showed that the calcination at 550 °C decreased the organic matter, as presented in Table 1. This research is a pioneering report on XRD patterns [43]. The XRD pattern shows the stability of the minerals after the purification of the heated clay. These were predominantly clay minerals like quartz, hematite, and corundum, as shown in Fig. 4. (C-E). Therefore, Fig. 4. (C-E), the purified clay + Bougainvillea spp. (C), purified clay + Pandanus amaryllifolius Roxb. (D), and purified clay + Curcuma longa L. (E) demonstrated their constituent primary minerals. This result was conferring to the CIE color test.
Fig. 4.
XRD patterns of clay (A) before and (B) after purification, (C) purified clay + Bougainvillea spp., (D) purified clay + Pandanus amaryllifolius Roxb., and (E) purified clay + Curcuma longa L.
3.3. Color of bio-clay
The L*, a*, and b* values of the purified clay, purified clay + Bougainvillea spp., purified clay + Pandanus amaryllifolius Roxb., and purified clay + Curcuma longa L. are shown in Table 5. The L* values of purified clay + Bougainvillea spp., purified clay + Pandanus amaryllifolius Roxb., and purified clay + Curcuma longa L. were lower than those of purified clay. The a* values of purified clay + Bougainvillea spp. and purified clay + Pandanus amaryllifolius Roxb. decreased compared to the purified clay and were associated with a red color. However, the a* value of purified clay + Curcuma longa L. increased. The higher b* values were associated with the yellow color of the purified clay + Curcuma longa L. This performance substantiates the color change of the bio-clay, as observed in Fig. 5. (A-D).
Table 5.
Color test from CIE.
| Type of sample | Color CIE |
||
|---|---|---|---|
| L* | a* | b* | |
| Purified clay | 40.82 ± 0.09 | 9.56 ± 0.02 | 18.10 ± 0.06 |
| Purified clay + Bougainvillea spp. | 39.65 ± 0.04 | 5.33 ± 0.01 | 14.45 ± 0.04 |
| Purified clay + Pandanus amaryllifolius Roxb. | 37.75 ± 0.06 | 7.47 ± 0.04 | 18.31 ± 0.09 |
| Purified clay + Curcuma longa L. | 39.15 ± 0.03 | 13.09 ± 0.04 | 38.41 ± 0.20 |
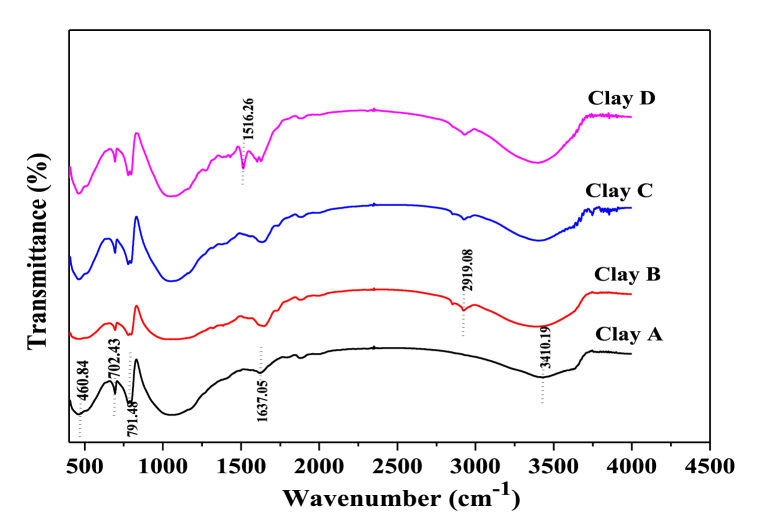
The FTIR spectra of purified clay, purified clay + Bougainvillea spp., purified clay + Pandanus amaryllifolius Roxb., and purified clay + Curcuma longa L. are shown in Fig. 6. The broad bands at 3410.19 cm−1 showed –OH groups, which are between 3000 and 3600 cm−1 [[44], [45], [46], [47]]. An aliphatic C–H vibration mode spectra were observed at 2919.08 cm−1. The bending vibration at 1637.05 cm−1 was coordinated to –C O. The C–H vibration was observed at 1516.26 cm−1 [[44], [45], [46], [47]]. The 791.48, 702.43, and 460.84 cm−1 spectra present symmetric stretching vibrations of Si–O–Si, Si–O–Al, and Si–O, respectively [48,49]. Finally, the FTIR spectra of the primary bio-clay were obtained. The characteristic peaks representing the C–H bonds at 1516.26 cm−1 confirm the presence of plant organic compounds.
Fig. 6.
FTIR spectra of (A) purified clay, (B) purified clay + Bougainvillea spp., (C) purified clay + Pandanus amaryllifolius Roxb., and (D) purified clay + Curcuma longa L.
3.4. Total phenolic content and antioxidant activity
3.4.1. Effect of storage conditions on antioxidant properties
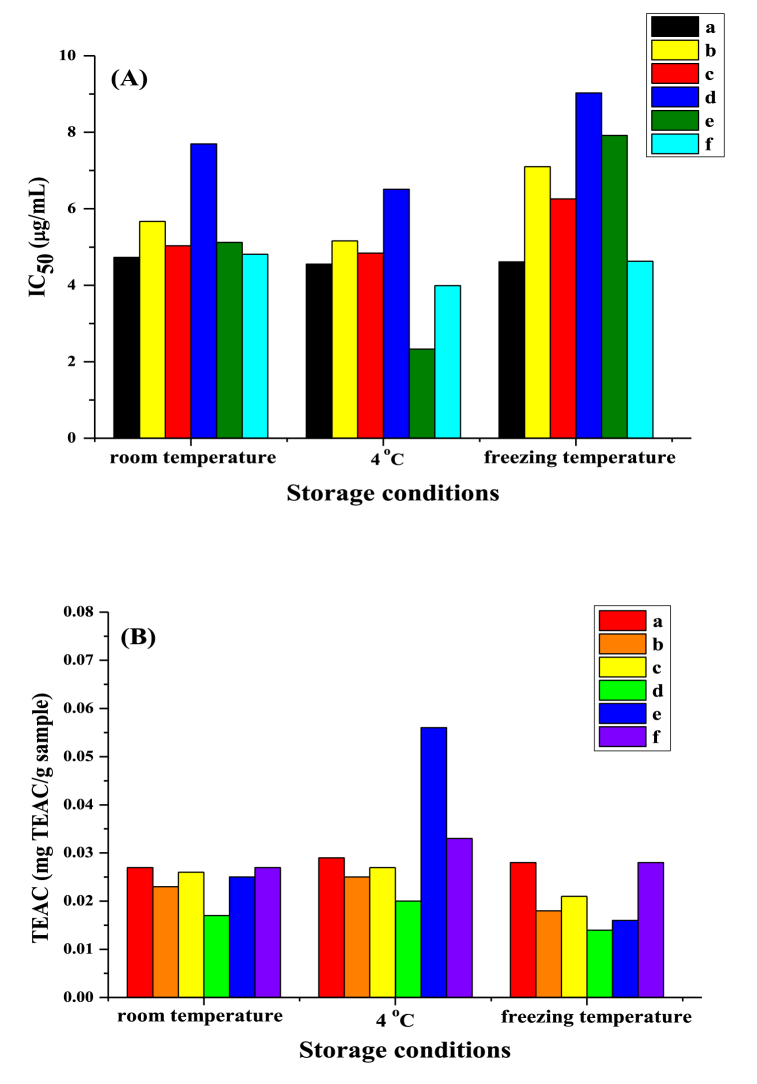
TPC, DPPH, TEAC, and IC50 analyses conducted under various storage conditions (room temperature, 4 °C, and freezing temperature) revealed optimal results at 4 °C. The findings indicate that storing the sample at 4 °C was appropriate for assessing TPC, antioxidant activity, and TEAC, as shown in Table 6 and Fig. 7. The IC50 value was low, indicating good antioxidant activity. Additionally, at 4 °C, the physical characteristics of the samples remained the same, unchanged from the beginning, and the stability of the essence group remained, as shown in Fig. 7. These results suggest that storage conditions at 4 °C affect the antioxidant properties of plants because they are sensitive to light and heat. If antioxidants were stored in areas with high temperatures and exposed to sunlight for a long time, it would decrease antioxidant efficiency. Particularly, phenolic hydroxyl groups have a remarkable ability to scavenge free radicals. Betalain, a phenolic compound found in Bougainvillea spp., gives color mainly to flowers and fruits. It is a structure containing nitrogen-heterocyclic compounds that affect the hydrophilic. The ability of antioxidant activity is betalamic acid process from conjugated double bonds to reduce agents by electrons sharing [50]. Colors obtained from betalain group substances will provide durable color when processed according to previous research, such as drying powder, freezing, and spray drying [[51], [52], [53]]. An ingredient that gives color includes amino/imino acids derivatives, which are attracted by the layer of betalain, consisting of balsamic acid, which is connected to cyclo-dopa and amino acids or amines. This is an ability to maintain stable results, which, regardless of the loss of electrons, still has superior antioxidant properties [50]. Additionally, flavonoids and curcuminoids, identified in Pandanus amaryllifolius Roxb. and Curcuma longa L., are biologically significant polyphenols known for their antioxidant, anticancer, and anti-inflammatory activities. This group of substances is unstable or less stable. When receiving temperature, light, and moisture, however, when purified clay + plants were found to have high TPC compared to plants alone. Accordingly, the previous research studied the zeolite of a mesoporous material and the negative charge of surface-adsorbed commercial curcumin, and the results showed that high adsorption of curcumin on the adsorbent [54]. These results corresponded to the purification clay's mesoporous and positive surface charge, as presented in Table 3 and Fig. 2, Fig. 3. As a result, purification clay is an inorganic substance found in abundance worldwide. These have a unique internal structure that can adsorb antioxidants and maintain the stability of the substance due to their high CEC and excellent medium properties. Then, clay is been used in cosmetic applications, which is low toxicity and inexpensive. As a result, purification clay minerals have successively attracted consideration for the loading and combination of phytochemicals and natural dyes. Bio-clay agrees with the silica-oxygen tetrahedra sheet and contains anionic charge, which is bonded to cations, as shown in Fig. 4.
Table 6.
Total phenolic content and antioxidant activity.
| Sample | Storage conditions | Antioxidant activity |
|
|---|---|---|---|
| TPC (mg GAE/g sample) | DPPH (% scavenging) | ||
| Bougainvillea spp. | room temperature | 45.44 ± 0.85c | 77.83 ± 1.71a |
| 4 °C | 64.13 ± 1.45a | 80.50 ± 0.68a | |
| freezing temperature | 56.32 ± 0.53b | 78.49 ± 0.71a | |
| Purified clay + Bougainvillea spp. | room temperature | 91.75 ± 0.80b | 66.39 ± 1.31a |
| 4 °C | 94.39 ± 0.61a | 69.20 ± 1.63a | |
| freezing temperature | 76.32 ± 1.58c | 62.82 ± 1.49b | |
| Pandanus amaryllifolius Roxb. | room temperature | 62.63 ± 0.46a | 50.74 ± 0.56ab |
| 4 °C | 63.60 ± 0.55a | 52.23 ± 2.23a | |
| freezing temperature | 61.06 ± 0.79b | 47.62 ± 1.70b | |
| Purified clay + Pandanus amaryllifolius Roxb. | room temperature | 119.30 ± 2.19a | 48.79 ± 0.50a |
| 4 °C | 119.47 ± 2.29a | 49.35 ± 0.52a | |
| freezing temperature | 112.81 ± 2.70b | 45.26 ± 0.79b | |
| Curcuma longa L. | room temperature | 63.86 ± 0.31a | 80.95 ± 0.68a |
| 4 °C | 64.39 ± 1.10a | 81.40 ± 0.46a | |
| freezing temperature | 62.28 ± 0.40b | 79.09 ± 0.93b | |
| Purified clay + Curcuma longa L. | room temperature | 131.93 ± 2.60b | 69.36 ± 2.19b |
| 4 °C | 142.98 ± 2.59a | 77.72 ± 0.65a | |
| freezing temperature | 128.95 ± 1.25b | 69.56 ± 1.32b | |
The experimental data were obtained in triplicate. The different letters in each column and the sample extract conditions indicate a significant difference (p < 0.05).
Fig. 7.
Antioxidant activity of sample extracts at different storage conditions as determined by (A) IC50 (μg/mL) and (B) TEAC (mg TEAC/g sample). (a = Bougainvillea spp., b = purified clay + Bougainvillea spp., c = Pandanus amaryllifolius Roxb., d = purified clay + Pandanus amaryllifolius Roxb., e = Curcuma longa L., f = purified clay + Curcuma longa L.).
3.4.2. Antioxidant stability
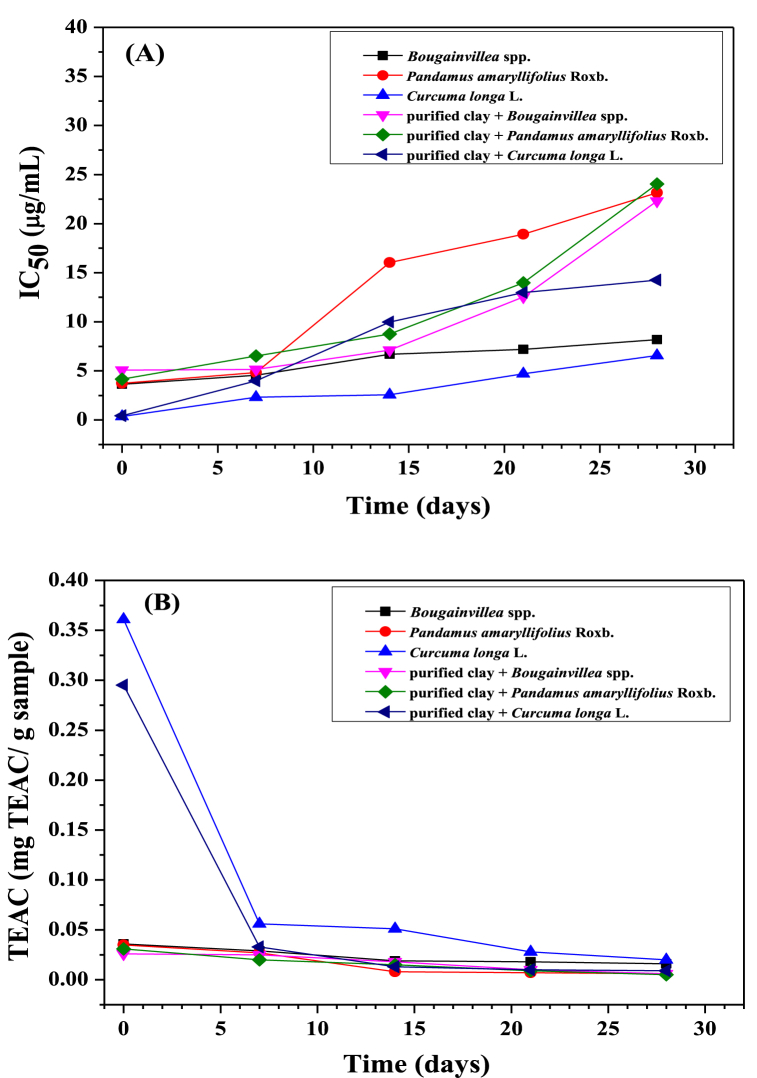
The antioxidant activity of Bougainvillea spp., purified clay + Bougainvillea spp., Pandanus amaryllifolius Roxb., purified clay + Pandanus amaryllifolius Roxb., Curcuma longa L., and purified clay + Curcuma longa L. extracts were evaluated using the DPPH free radical scavenging activity assay and TPC were presented in Table 7 and Fig. 8. (A-B), respectively. The sample's ability to donate hydrogen to DPPH free radicals can be assessed by the percentage of free radical scavenging activity [55]. The potential for eliminating free radicals can be seen from the IC50 value calculated from the regression equation as shown in Fig. 8 (A), with the lower IC50 value indicating the more stable the elimination of free radicals. However, the free radical scavenging activity estimation can also be calculated from TEAC in all of the samples, as shown in Fig. 8 (B). The TEAC value represented the ability to neutralize DPPH free radicals. One advantage of this method is its simplicity in evaluating the antioxidant potential of phenolic compounds in a sample. The TPC method is also straightforward and commonly employed, as it involves the transfer of electrons from the phenolic compounds to the Folin–Ciocalteu reagent in an alkaline solution [21]. Plant phenolic compounds are also essential because their groups have scavenging abilities [56]. The higher scavenging activity of the extract of Curcuma longa L. was 87.10 ± 0.72%, and purified clay + Curcuma longa L. was 86.48 ± 0.26%, which were found to be like that of the positive control Trolox (92.83 ± 0.40%). The IC50 value of Trolox (0.13 μg/mL) was low compared to other sample extracts. Nevertheless, the IC50 values of Bougainvillea spp. (3.64 μg/mL), Pandanus amaryllifolius Roxb. (3.74 μg/mL), Curcuma longa L. (0.36 μg/mL), purified clay + Bougainvillea spp. (5.07 μg/mL), purified clay + Pandanus amaryllifolius Roxb. (4.15 μg/mL) and purified clay + Curcuma longa L. (0.44 μg/mL). From Fig. 8 (B), the TEAC values of the samples were found to have superb free radical scavenging activity. The TEAC values were found that 0.005 to 0.361 mgTEAC/g sample. The TPC at 0 d condition of Bougainvillea spp. (78.57 ± 0.60 mgGAE/g sample), Pandanus amaryllifolius Roxb. (68.57 ± 2.61 mgGAE/g sample), Curcuma longa L. (86.45 ± 0.58 mgGAE/g sample)., purified clay + Bougainvillea spp. (155.88 ± 2.47 mgGAE/g sample), purified clay + Pandanus amaryllifolius Roxb. (131.58 ± 0.53 mgGAE/g sample) and purified clay + Curcuma longa L. (165.13 ± 1.04 mgGAE/g sample) was estimated in Table 7. The TPC value of the sample was predictable using the calibration curve (Y = 0.0048x + 0.0224; R2 = 0.995) that was prepared from standard solution (gallic acid) at various concentrations and reported as milligrams of gallic acid equivalent (GAE) per gram of sample. The TPC of the sample extracts was calculated using a regression equation and expressed regarding gallic acid equivalents. TPC are essential antioxidants, combatting free radicals such as superoxide, hydroxyl, nitric oxide, and peroxyl radicals, which are relatively stable free radicals and commonly found in the body. These radicals are neutralized by destroying or inhibiting free radicals [55,57]. Moreover, it was found that the antioxidant activities of the different sample extracts correlated significantly with their TPC. From the results of the sample analysis, it was found that the values were different. This occurs due to the cultivation period, geographic location, and sample extraction methods [55]. For example, the % scavenging, TEAC, and TPC of paper flower (Bougainvillea hybrid) was 62.44 ± 0.85%, 17.25 ± 0.19 mg/g sample, and 26.79 ± 0.24 mgGAE/g sample, respectively [58]. The % scavenging of paper flowers (Bougainvillea hybrid) was 59.21 ± 0.04% [59]. The TEAC and TPC of Bougainvillea glabra were 483.33 ± 0.00 mg TEAC/100 g dry basis and 232.64 ± 12.56 mgGAE/100g dry basis. Also, TEAC and TPC of Pandanus amaryllifolius were 324.56 ± 5.02 mgTEAC/100 g dry basis and 43.17 ± 7.41 mgGAE/100g dry basis [60]. In conclusion, the % scavenging, IC50, TEAC values, and TPC values of Bougainvillea spp., Pandanus amaryllifolius Roxb., Curcuma longa L., purified clay + Bougainvillea spp., purified clay + Pandanus amaryllifolius Roxb. and purified clay + Curcuma longa L. were evaluated. The results indicated that Curcuma longa L. and purified clay + Curcuma longa L. extracts had higher antioxidant activities than Trolox and GAE. Furthermore, the stability and antioxidant activity of the samples were studied by storing them at 4 °C for 28 d. TPC, DPPH, TEAC, and IC50 were analyzed for each sample 7 d, as shown in Table 7 and Fig. 8 (A-B). The antioxidant activity of sample extracts decreased with an increased sample period (0, 7, 14, 21, and 28 d). This indicates that the extract has the function of scavenging free radicals related to oxidative stability. However, the effect of the sampling duration showed that the TPC, % scavenging, and TEAC decreased with storage time, while the IC50 was elevated. However, at the sampling period of 28 d, The Bougainvillea spp., Curcuma longa L., purified clay + Bougainvillea spp., and purified clay + Curcuma longa L. samples retained their TPC, % scavenging, TEAC, and IC50 values indicating good antioxidant activity and stability of the main phenolic compounds were maintained in high volume. The results of this analysis are consistent with the research of Tereucan et al. [61], which found that TPC and antioxidant activity decreased with time in milk and yogurt using different methods. Moreover, storage conditions affected the levels of anthocyanins, hydroxycinnamic acids, and other antioxidants. Additionally, it was observed that after a 28 d storage period, the TPC was higher than that found in many fruits and vegetables, such as apples (58.12 ± 3.98 mgGAE/100g), avocados (21.86 ± 1.25 mgGAE/100g), and netted melons (28.72 ± 1.88 mgGAE/100g) [21], as well as Curcuma aromatica (37.90 ± 1.0 mgGAE/g extract), respectively [62].
Table 7.
Effect of time storage conditions on antioxidant properties.
| Sample | Time (days) | Antioxidant activity |
|
|---|---|---|---|
| TPC (mg GAE/g sample) | DPPH (% scavenging) | ||
| Bougainvillea spp. | 0 | 78.57 ± 0.60a | 81.28 ± 0.99a |
| 7 | 64.13 ± 1.45b | 80.80 ± 0.39a | |
| 14 | 58.69 ± 2.51c | 75.19 ± 0.36b | |
| 21 | 51.67 ± 0.99d | 70.95 ± 1.47c | |
| 28 | 44.48 ± 0.79e | 66.19 ± 0.42d | |
| Purified clay + Bougainvillea spp. | 0 | 155.88 ± 2.47a | 71.43 ± 0.27a |
| 7 | 94.39 ± 0.61b | 69.20 ± 1.63b | |
| 14 | 87.54 ± 2.90c | 64.29 ± 0.38c | |
| 21 | 77.89 ± 2.79d | 63.55 ± 0.35cd | |
| 28 | 66.49 ± 0.80e | 62.34 ± 0.62d | |
| Pandanus amaryllifolius Roxb. | 0 | 68.57 ± 2.61a | 67.45 ± 0.69a |
| 7 | 63.60 ± 0.55b | 52.23 ± 2.23b | |
| 14 | 37.90 ± 0.70c | 49.74 ± 0.18c | |
| 21 | 30.44 ± 0.15d | 46.57 ± 0.56d | |
| 28 | 27.81 ± 0.16e | 38.64 ± 1.55e | |
| Purified clay + Pandanus amaryllifolius Roxb. | 0 | 131.58 ± 1.08a | 53.98 + 0.08a |
| 7 | 119.47 ± 2.29b | 49.34 ± 0.52b | |
| 14 | 103.33 ± 1.99c | 45.62 ± 3.21c | |
| 21 | 92.81 ± 2.90d | 44.21 ± 0.41cd | |
| 28 | 87.54 ± 2.19e | 42.22 + 2.09d | |
| Curcuma longa L. | 0 | 86.45 ± 0.58a | 87.10 ± 0.72a |
| 7 | 64.39 ± 1.10b | 81.40 ± 0.46b | |
| 14 | 60.18 ± 0.40c | 79.39 ± 0.48c | |
| 21 | 54.13 ± 0.85d | 72.26 ± 0.48d | |
| 28 | 48.86 ± 0.85e | 67.10 ± 0.30e | |
| Purified clay + Curcuma longa L. | 0 | 165.13 ± 1.04a | 86.48 ± 0.26a |
| 7 | 142.98 ± 2.59b | 77.72 ± 0.65b | |
| 14 | 136.32 ± 0.53c | 73.21 ± 1.51c | |
| 21 | 122.28 ± 3.73d | 71.01 ± 1.42d | |
| 28 | 83.86 ± 4.97e | 68.32 ± 1.36e | |
The experimental data were obtained in triplicates. The different letters in each column and only sample extract indicate a significant difference (p < 0.05).
Fig. 8.
Antioxidant activity of the sample extracts at different storage times as determined by the (A) IC50 (μg/mL) and (B) TEAC (mg TEAC/g sample).
4. Conclusion
After purification, the clay exhibited a high CEC, large specific surface area, and an average pore width of 8.51 nm. This composition has attracted attention as an inorganic material. The bio-clay provided optimal storage conditions for maintaining high TPC, antioxidant activity, and low IC50 values, indicating strong antioxidant activity at 4 °C. Additionally, the physical properties revealed decreased L* values and significantly increased b* values. The FTIR spectra indicated the presence of C–H bonds at 1516.26 cm−1 in the residue of purification clay mixed with Curcuma longa L. Furthermore, both Curcuma longa L. and the combination of clay and Curcuma longa L. demonstrated strong antioxidant activities. Moreover, these extracts retained their antioxidant properties and stability over a 28 d storage period, making them suitable ingredients for various applications, particularly abrasives in cosmetics such as body mud scrubs and scrubbing soap. These compounds exhibit strong physicochemical properties for anti-aging purposes.
CRediT authorship contribution statement
Sarunya Maneetong: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Pattaranun Thuadaij: Writing – review & editing, Writing – original draft, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding: The authors thank the Thailand Science Research and Innovation, National Science Research and Innovation Fund, and Fundamental Fund (FF) 2023 for supporting this research at the Research and Development Institute, Buriram Rajabhat University. We thank Miss Chanadda Rattana, Faculty of Geo-Informatics, who helped draw the map of Buriram Province from where the sample clay was obtained. We thank Mr. Chatchai Phiakhantha, and Mrs. Phasita Daengchat was to help for sample preparation.
References
- 1.Alshameri A., He H., Xin C., Zhu Jianxi, Xinghu W., Zhu R., Wang H. Understanding the role of natural clay minerals as effective adsorbents and alternative source of rare earth elements: adsorption operative parameters. Hydrometallurgy. 2019;185:149–161. doi: 10.1016/j.hydromet.2019.02.016. [DOI] [Google Scholar]
- 2.Spinola D.N., Pi-Puig T., Solleiro-Rebolledo E., Egli M., Sudo M., Sedov S., Kühn P. Origin of clay minerals in early eocene volcanic paleosols on king george island, maritime Antarctica. Sci. Rep. 2017;7:6368. doi: 10.1038/s41598-017-06617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thuadaij P., Duangkham S., Hobanthad T. Characterization of Buriram volcanic clay for use in cosmetics. Mater. Res. Express. 2020;7:1–11. doi: 10.1088/2053-1591/abb6b5. [DOI] [Google Scholar]
- 4.Roselli C., Desideri D., Cantaluppi C., Mattioli M., Fasson A., Meli M.A. Essential and toxic elements in clays for pharmaceutical and cosmetic use. J. Toxicol. Environ. Health A. 2015;78:316–324. doi: 10.1080/15287394.2014.964430. [DOI] [PubMed] [Google Scholar]
- 5.JeanBaptistea BikeM., Daniele Benessoubo Kada, Charlène Eko Marie, Canuala Tekoumbo Tedontsa Larrissa, Antoine Elimbi, Richard Kamga. Adsorption mechanisms of pigments and free fatty acids in the discoloration of shea butter and palm oil by an acid-activated Cameroonian smectite. Sci. Afr. 2020;9 doi: 10.1016/j.sciaf.2020.e00498. 1–10. [DOI] [Google Scholar]
- 6.Gamoudi S., Srasra E. Green synthesis and characterization of colored Tunisian clays: cosmetic applications. Appl. Clay Sci. 2018;165:17–21. doi: 10.1016/j.clay.2018.07.042. [DOI] [Google Scholar]
- 7.Thiesen L.C., Bretzke P.E., Bittencourt C.M.D.S., Silva R.M.L., Bresolin T.M.B., Santin J.R., Couto A.G. Litchi chinensis leaf extract provides high in vitro photoprotection associated to a natural mineral clay. Photodermatol. Photoimmunol. Photomed. 2020;36:61–62. doi: 10.1111/phpp.12488. [DOI] [PubMed] [Google Scholar]
- 8.Rizo O.D., Muñoz M.S., Hernández P.G., Rudnikas A.G., Rodríguez K.D., Rodríguez C.M.M., Castillo J.R.F., Martínez-Villegas N.V., Zerquera J.T. Radioactivity levels in peloids used in main Cuban spas. J. Radioanal. Nucl. Chem. 2018;316:95–99. doi: 10.1007/s10967-018-5752-1. [DOI] [Google Scholar]
- 9.Cao L., Xie W., Cui H., Xiong Z., Tang Y., Zhang X., Feng Y. Fibrous clays in Dermopharmaceutical and cosmetic applications: traditional and emerging perspectives. Int. J. Pharm. 2022;625 doi: 10.1016/j.ijpharm.2022.122097. [DOI] [PubMed] [Google Scholar]
- 10.Daneluz J., Favero J., Santos Vd, Weiss-Angeli V., Bonan Gomes L., Mexias A.S., Bergmann C.P. The influence of different concentrations of a natural clay material as active principle in cosmetic formulations. Mater. Res. 2020;23 doi: 10.1590/1980-5373-mr-2019-0572. [DOI] [Google Scholar]
- 11.Kausar A., Iqbal M., Javed A., Aftab K., Nazli Zill-i-Huma, Nawaz Bhatti Haq, Nouren Shazia. Dyes adsorption using clay and modified clay: a review. J. Mol. Liq. 2018;256:395–407. doi: 10.1016/j.molliq.2018.02.034. [DOI] [Google Scholar]
- 12.Viseras C., Carazo E., Borrego-Sánchez A., García-Villén F., Sánchez-Espejo R., Cerezo P., Aguzzi C. Clay minerals in skin drug delivery. Clays Clay Miner. 2019;67:59–71. doi: 10.1007/s42860-018-0003-7. [DOI] [Google Scholar]
- 13.Lopes T.J., Gonçalves O.H., Quadri M.G.N., Machado R.A.F., Quadri M.B. Adsorption of anthocyanins using clay–polyethylene nanocomposite particles. Appl. Clay Sci. 2014;87:298–302. doi: 10.1016/j.clay.2013.11.038. [DOI] [Google Scholar]
- 14.Barakan S., Aghazadeh V. The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: a review. Environ. Sci. Pollut. Res. Int. 2021;28:2572–2599. doi: 10.1007/s11356-020-10985-9. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro H.L., de Oliveira A.V., de Brito E.S., Ribeiro P.R., Azeredo H.M. Stabilizing effect of montmorillonite on acerola juice anthocyanins. Food Chem. 2018;245:966–973. doi: 10.1016/j.foodchem.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 16.Lima L.C.B., Silva F.C., Silva-Filho E.C., Fonseca M.G., Zhuang Guanzheng, Jaber M. Saponite-anthocyanin derivatives: the role of organoclays in pigment photostability. Appl. Clay Sci. 2020;191 doi: 10.1016/j.clay.2020.105604. [DOI] [Google Scholar]
- 17.Deineka V.I., Doronin A.G., Oleinits E.Yu, Blinova I.P., Deineka L.A., Chulkov A.N. Sorption of anthocyanins on bentonite clay. Russ. J. Phys. Chem. A. 2020;94:1224–1229. doi: 10.1134/S0036024420060072. [DOI] [Google Scholar]
- 18.Kohno Y., Kinoshita R., Ikoma S., Yoda K., Shibata M., Matsushima R., Tomita Y., Maeda Y., Kobayashi K. Stabilization of natural anthocyanin by intercalation into montmorillonite. Appl. Clay Sci. 2009;42:519–523. doi: 10.1016/j.clay.2008.06.012. [DOI] [Google Scholar]
- 19.Mushtaq Z., Imran M., Hussain M., Saeed F., Imran A., Umar M., Abdelgawad M.A., El-Ghorab A.H., Ahmed A., Alsagaby S.A., Mahomoodally M.F., Jbawi E.A. Asiatic acid: a review on its polypharmacological properties and therapeutic potential against various Maladies. Int. J. Food Prop. 2023;26(1):1244–1263. doi: 10.1080/10942912.2023.2209702. [DOI] [Google Scholar]
- 20.Javed A., Ahmad A., Nouman M., Hameed A., Tahir A., Shabbir U. Turnip (Brassica Rapus L.): a natural health tonic. Braz. J. Food Technol. 2019;22 doi: 10.1590/1981-6723.25318. 1–9. [DOI] [Google Scholar]
- 21.Ahmad A., Mahmood N., Hussain M., Aiman U., Al-Mijalli S.H., Raza M.A., Al Jbawi E. Improvement in oxidative stability and quality characteristics of functional chicken meat product supplemented with aqueous coriander extract. Int. J. Food Prop. 2023;26:855–865. doi: 10.1080/10942912.2023.2189086. [DOI] [Google Scholar]
- 22.Wong Y.-M., Siow L.-F. Effects of heat, pH, antioxidant, agitation and light on betacyanin stability using red-fleshed dragon fruit (Hylocereus polarizes) juice and concentrate as models. J. Food Sci. Technol. 2015;52:3086–3092. doi: 10.1007/s13197-014-1362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karangutkar A.V., Ananthanarayan L. Evaluating the effect of additives on stability of betacyanin pigments from Basella rubra in a model beverage system during storage. J. Food Sci. Technol. 2021;58:1262–1273. doi: 10.1007/s13197-020-04635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calva-Estrada S.J., Jimenez-Fern M., Lugo-Cervantes E. Betalains and their applications in food: the current state of processing, stability and future opportunities in the industry. Food Chem.: Molecular Sciences. 2022;4:1–11. doi: 10.1016/j.fochms.2022.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quyen N.T.C., Quyen N.T.N., Nhan L.T.H., Toan T.Q. Antioxidant activity, total phenolics and flavonoids contents of Pandanus amaryllifolius (Roxb.), IOP Conf. S. Mater. Sci. Eng. 2020;991:1–8. doi: 10.1088/1757-899X/991/1/012019. [DOI] [Google Scholar]
- 26.Bhuyan R. Biman Sonowal, an overview of Pandanus amaryllifolius Roxb.exLindl. and its potential impact on health. Curr. Trends Pharm. Res. 2021;8:138–157. [Google Scholar]
- 27.Akter J.H., Amzad Takara Md, Islam K., Zahorul Hou Md. De Xing, Comp, Antioxidant activity of different species and varieties of turmeric (Curcuma spp): isolation of active compounds. Biochem. Physiol. C Toxicol. Pharmacol. 2019;215:9–17. doi: 10.1016/j.cbpc.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Tanvir E.M., Hossen Sakib Hossain Md, Fuad Afroz Md, Gan Rizwana, Khalil Siew Hua. Md Ibrahim Karim, Nurul, Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual. 2017:1–8. doi: 10.1155/2017/8471785. [DOI] [Google Scholar]
- 29.Kanda H., Zhu L., Zhu W., Wang T. Ethanol-free extraction of curcumin and antioxidant activity of components from wet Curcuma longa L. by liquefied dimethyl ether. Arab. J. Chem. 2023;16 doi: 10.1016/j.arabjc.2023.104585. [DOI] [Google Scholar]
- 30.de Freitas Marques M.B., Almeida O.P., da Silva F.L.O., Araújo B.C.R., Ardisson J.D., Sebastião R. de C. de O., Mussel W. da N., Yoshida M.I., Carneiro G. Solid-state properties of pink clay from Jequitinhonha Valley in Brazil for pre-formulation study. Braz. J. Pharm. Sci. 2023;59:e21460 1–e2146013. doi: 10.1590/s2175-97902023e21460. [DOI] [Google Scholar]
- 31.Amraoui A., Gamoudi S., Baenas N., Periago M.J., Srasra E. Eco-friendly hybrid materials of Tunisian clay/natural flower. Clay Miner. 2022;57(3–4):1–34. doi: 10.1180/clm.2022.31. [DOI] [Google Scholar]
- 32.Putri D.E., Djamil R., Faizatun F. Body scrub containing virgin coconut oil, coffee grounds (coffea arabica linn) and carbon active coconut shell (activated carbon cocos nucifera L) as a moisturiser and a skin brightener. Scr. Med. (Brno) 2021;52(1):76–81. doi: 10.5937/scriptamed52-30814. [DOI] [Google Scholar]
- 33.Hobanthad T., Maneetong S. Simple extraction for the scanning of antioxidant activity of vegetables and fruits in Buriram, Thailand by DPPH, ABTS and FRAP assays. SNRU J. Sci. Technol. 2019;11:114–121. [Google Scholar]
- 34.Judprasong K., Charoenkiatkul S., Thiyajai P., Sukprasansap M. Nutrients and bioactive compounds of Thai indigenous fruits. Food Chem. 2013;140:507–512. doi: 10.1016/j.foodchem.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 35.Harada Y., Inoko A. Cation-exchange properties of soil organic matter. Soil Sci. Plant Nutr. 1975;21:361–369. doi: 10.1080/00380768.1975.10432651. [DOI] [Google Scholar]
- 36.Idowu E.O., Johnson T.F., Adefemi S.O. Modeling cation exchange capacity and soil water holding capacity from basic soil properties. Eurasian. 2016;5:266–274. doi: 10.18393/ejss.2016.4.266-274. [DOI] [Google Scholar]
- 37.Nasiruddin Khan M., Sarwar A. Determination of points of zero charge of natural and treated adsorbents. Surf. Rev. Lett. 2007;14:461–469. doi: 10.1142/S0218625X07009517. [DOI] [Google Scholar]
- 38.Kumar T.K.M.P., Mandlimath T.R., Sangeetha P., Sakthivel P., Revathi S.K., Kumar S.K.A., Sahoo S.K. Highly efficient performance of activated carbon impregnated with Ag, ZnO and Ag/ZnO nanoparticles as antimicrobial materials. RSC Adv. 2015;5 doi: 10.1039/C5RA19945J. [DOI] [Google Scholar]
- 39.Nabil B., Malek O.H. Characterization and purification of Algerian natural bentonite for pharmaceutical and cosmetic applications. BMC Chem. 2021;15:1–11. doi: 10.1186/s13065-021-00776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komadel P. Acid activated clays: materials in continuous demand. Appl. Clay Sci. 2016;131:84–99. doi: 10.1016/j.clay.2016.05.001. [DOI] [Google Scholar]
- 41.Tahmasebi Yamchelou M.T., Law D., Brkljača R., Gunasekara C., Li J., Patnaikuni I. Geopolymer synthesis using low-grade clays. Constr. Build. Mater. 2021;268 doi: 10.1016/j.conbuildmat.2020.121066. [DOI] [Google Scholar]
- 42.Qiao Z., Liu Zejuan, Zhang S., Yang Y., Wu Yingke, Liu L., Liu Qinfu. Purification of montmorillonite and the influence of the purification method on textural properties. Appl. Clay Sci. 2020;187:1–8. doi: 10.1016/j.clay.2020.105491. [DOI] [Google Scholar]
- 43.Gueu S. Physicochemical characterization of three natural clays used as adsorbent for the humic acid removal from aqueous solution. Adsorpt. Sci. Technol. 2019;37:77–94. doi: 10.1177/0263617418811469. [DOI] [Google Scholar]
- 44.Zafar F., Sharmin E., Zafar H., Shah M.Y., Nishat N., Ahmad S. Facile microwave-assisted preparation of waterborne polyesteramide/OMMT clay bio-nanocomposites for protective coatings. Ind. Crops Prod. 2015;67:484–491. doi: 10.1016/j.indcrop.2015.01.057. [DOI] [Google Scholar]
- 45.Heidarian M., Shishesaz M.R., Kassiriha S.M., Nematollahi M. Characterization of structure and corrosion resistivity of polyurethane/organoclay nanocomposite coatings prepared through an ultrasonication assisted process. Prog. Org. Coat. 2010;68:180–188. doi: 10.1016/j.porgcoat.2010.02.006. [DOI] [Google Scholar]
- 46.Hamd A., Rady D., Shaban M., Elsayed K.N.M., Al Mohamadi H., Elzanaty A.M., Ahmed S.A., El-Sayed R., Soliman N.K. Application of Nano bio-clay composite in a scaling-up study for wastewater treatment. Biointerface Res. Appl. Chem. 2022;12:6393–6414. doi: 10.33263/BRIAC125.63936414. [DOI] [Google Scholar]
- 47.Zayed M., Ahmed A.M., Shaban M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrog. Energ. 2019;44:17630–17648. doi: 10.1016/j.ijhydene.2019.05.117. [DOI] [Google Scholar]
- 48.Nana A., Singla R., Alomayri T., Epey N., Kassem N.N., Sakue E.N., Kaze R.C., Kamseu E., Kumar S., Leonelli C. Design and characterization of Cameroonian pegmatite-calcined clay binary mortars via geopolymerization. J. Build. Eng. 2023;76 doi: 10.1016/j.jobe.2023.107078. [DOI] [Google Scholar]
- 49.Hajimohammadi A., Ngo T., Kashani A. Glass waste versus sand as aggregates: the characteristics of the evolving geopolymer binders. J. Clean. Prod. 2018;193:593–603. doi: 10.1016/j.jclepro.2018.05.086. [DOI] [Google Scholar]
- 50.Sadowska-Bartosz I., Bartosz G. Biological properties and applications of betalains. Molecules. 2021;26:2520. doi: 10.3390/molecules26092520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trishitman D., Negi P.S., Rastogi N.K. Concentration of beetroot juice colorant (betalains) by forward osmosis and its comparison with thermal processing. LWT. 2021;145 doi: 10.1016/j.lwt.2021.111522. [DOI] [Google Scholar]
- 52.Kerr W.L., Varner A. Chemical and physical properties of vacuum-dried red beetroot (Beta vulgaris) powders compared to other drying methods. Drying Technol. 2020;38:1165–1174. doi: 10.1080/07373937.2019.1619573. [DOI] [Google Scholar]
- 53.Gouws C.A., D'Cunha N.M.D., Georgousopoulou E.N., Mellor D.D., Naumovski N. The effect of different drying techniques on phytochemical content and in vitro antioxidant properties of Australian grown prickly pears (Opuntia ficus indica) J. Food Process. Preserv. 2019;43 doi: 10.1111/jfpp.13900. [DOI] [Google Scholar]
- 54.Musielak E., Feliczak-Guzik A., Jaroniec M., Nowak I. Modification and functionalization of zeolites for curcumin uptake. Materials. 2022;15:1–19. doi: 10.3390/ma15186316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kedir W.M., Geletu A.K., Weldegirum G.S., Sima M.F. Antioxidant activity of selected plants extract for palm oil stability via accelerated and deep frying study. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siddiqui N., Rauf A., Latif A., Mahmood Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth) J. Taibah Univ. Med. Sci. 2017;12:360–363. doi: 10.1016/j.jtumed.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatoui K., Harhar H., El Kamli T., Tabyaoui M. Chemical composition and antioxidant capacity of Lepidium sativum seeds from four regions of Morocco. Evid. Based Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/7302727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ounamornmas P., Sommano S. Analyses of bioactive ingredients and antioxidant activities of some edible flowers. J. Agric. 2016;32:435–445. doi: 10.1016/j.jff.2011.03.002. [DOI] [Google Scholar]
- 59.Yommarat S., Jamjan K. Rice admixed with the antioxidant compounds production from 5 types of edible flowers. 3. 3rd Kamphaeng Phet Rajabhat University Conference. 2016:382–390. [Google Scholar]
- 60.Sae-Oung W., Chalermchaiwat P., Limsuwan T. The 58th Kasetsart University Annual Conference. 2018. Content of main pigment, total phenolic acid and antioxidant activity of freeze-dried coloring plants and its application in Khao Niew Moon; pp. 625–634. [Google Scholar]
- 61.Tereucan G., Ercoli S., Cornejo P., Winterhalter P., Contreras B., Ruiz A. Stability of antioxidant compounds and activities of a natural dye from coloured-flesh potatoes in dairy foods. LWT Food Sci. Technol. 2021;144 doi: 10.1016/j.lwt.2021.111252. [DOI] [Google Scholar]
- 62.Akter J., Hossain M.A., Takara K., Islam M.Z., Hou D.X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp.): isolation of active compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;215:9–17. doi: 10.1016/j.cbpc.2018.09.002. [DOI] [PubMed] [Google Scholar]