Abstract
Peritoneal carcinomatosis (PC) is a type of secondary cancer which is not sensitive to conventional intravenous chemotherapy. Treatment strategies for PC are usually palliative rather than curative. Recently, artificial intelligence (AI) has been widely used in the medical field, making the early diagnosis, individualized treatment, and accurate prognostic evaluation of various cancers, including mediastinal malignancies, colorectal cancer, lung cancer more feasible. As a branch of computer science, AI specializes in image recognition, speech recognition, automatic large-scale data extraction and output. AI technologies have also made breakthrough progress in the field of peritoneal carcinomatosis (PC) based on its powerful learning capacity and efficient computational power. AI has been successfully applied in various approaches in PC diagnosis, including imaging, blood tests, proteomics, and pathological diagnosis. Due to the automatic extraction function of the convolutional neural network and the learning model based on machine learning algorithms, AI-assisted diagnosis types are associated with a higher accuracy rate compared to conventional diagnosis methods. In addition, AI is also used in the treatment of peritoneal cancer, including surgical resection, intraperitoneal chemotherapy, systemic chemotherapy, which significantly improves the survival of patients with PC. In particular, the recurrence prediction and emotion evaluation of PC patients are also combined with AI technology, further improving the quality of life of patients. Here we have comprehensively reviewed and summarized the latest developments in the application of AI in PC, helping oncologists to comprehensively diagnose PC and provide more precise treatment strategies for patients with PC.
Keywords: Deep learning, Machine learning, Artificial intelligence, Peritoneal carcinomatosis
1. Introduction
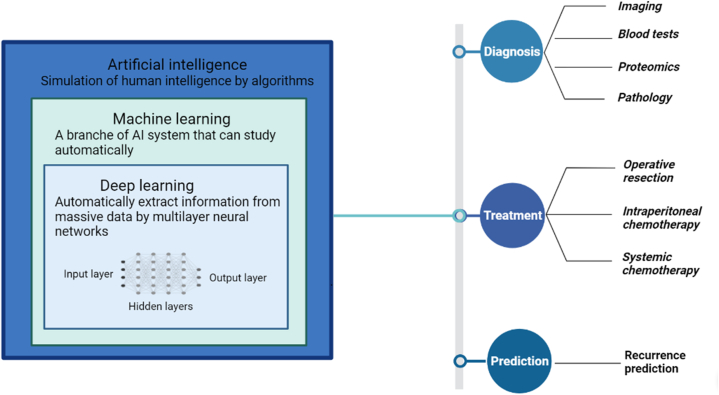
Peritoneal carcinomatosis (PC) is a type of secondary cancer caused by the metastasis of tumor cells from the primary tumor to the peritoneum via the bloodstream [1]. About 15 % of patients with colorectal cancer will eventually develop PC. The incidence even increases to 24 % and 45 % in patients with gastric cancer and those with ovarian cancer, respectively [2,3]. Unlike metastatic tumors in other sites, PC followed by malignant ascites is associated with impaired quality of life and poor prognosis, with a median overall survival (OS) of 2–5 months. And current treatment strategies for PC are mostly palliative and limited [4]. Due to the aggressive behavior of PC, early detection and reduction of recurrence are becoming increasingly important. Artificial intelligence (AI) is a new intellectual capability that can simulate and replicate human intelligence and judgement through data-driven algorithms [5,6]. Machine learning (ML), as one of the branches of AI, analyzes massive training data to teach machines, find out the rules and complete the learning process. Then, ML can automatically learn and continuously improve its performance accuracy to solve the desired data without human intervention [7]. Deep learning (DL) is an implementation technology of ML that is an effective approach for automatically extracting information from massive medical images and performing detection or classification tasks [7,8]. Convolutional Neural Networks (CNNs) are the most widely used DL architecture in the field of medical imaging. CNNs are sophisticated models with multiple hidden layers that can analyze various input data, extract, and classify internal features from massive data, and provide prediction outputs [5,9] (Fig. 1). In summary, based on the powerful learning ability and efficient computational power of AI, it has been widely applied in PC for diagnosis, treatment, and recurrence prediction. Therefore, we aimed to review and summarize the development of the application of AI in the field of PC based on current literature findings. In addition, the current evidence on the application of AI in peritoneal carcinomatosis over the past 6 years is summarized in Table 1.
Fig. 1.
The relationships among artificial intelligence (AI), machine learning (ML), with deep learning (DL) and the applications of AI technologies in the diagnosis, treatment, recurrence prediction of peritoneal carcinomatosis.
Table 1.
Summary of clinical studies for artificial intelligence in peritoneal carcinomatosis in recently 6 years.
| Reference | Study type | Number of cases | Tumor type | Methods | Results | Year |
|---|---|---|---|---|---|---|
| Diagnosis | ||||||
| Dong D et al. [10] | Retrospective | 554 advanced gastric cancer patients | GC with PC | Radiomic nomogram | AUC: 0.920 | 2019 |
| Zixing Huang et al. [11] | Retrospective | 554 patients | GC with PC | DCNN | AUC: 0.900 Sensitivity: 81.0 % Specificity: 87.5 %; |
2020 |
| Menglei Li et al. [12] | Retrospective | 779 patients | CRC with PC | Clinical-radiomics model | AUCs: up to 0.855 | 2020 |
| Zixing Huang et al. [13] | Retrospective | At least 109 cases | GC with PC | DCNN | NR | 2020 |
| Chengmao Zhou et al. [14] | Retrospective | 1080 patients with postoperative GC | GC with PC | ML | AUC: up to 0.938 Accuracy:up to 90.9 % |
2020 |
| Ruijiang Li et al. [15] | Multicenter | 1978 patients | GC | DCNN + PMetNet | AUC: 0.920–0.946 Sensitivity: 75.4%–87.5 % Specificity: 92%–98.2 % |
2021 |
| Bin Zheng et al. [16] | Retrospective | 159 patients | GC with and without PC | ML | AUC: 71.2 % Sensitivity: 43.10 % Specificity: 87.12 % |
2021 |
| Xinyu Jin et al. [17] | Retrospective | 11408 images from 131 patients | PC | Meta-learning-based DL | AUC: 0.877 Sensitivity: 73.4 % Specificity: 95.2 % |
2022 |
| Lili Wang et al. [18] | Retrospective | 810 patients | GC | ML | AUCs of clinical models: 0.902–0.969 AUCs of radiomics models: 0.896–0.975 |
2022 |
| Valentin Bejan et al. [19] | Retrospective | NR | CRC | ML | Optimal accuracy: 0.75. | 2022 |
| Zixu Yuan et al. [20] | Retrospective | 19,814 images from 130 patients | CRC with and without PC | DL | AUC: 0.922 Sensitivity: 93.75 % Specificity: 94.44 % Accuracy: 94.11 % |
2022 |
| Dailun Hou et al. [21] | Multicenter | 88 peritoneal tuberculosis and 90 PC patients | PC | ML | AUC: 0.914–0.971 | 2023 |
| Yanyan Chen et al. [22] | Retrospective | 25 patients | GC with and without PC | Proteomic analysis | NR | 2023 |
| Jihong Liu et al. [23] | Retrospective | 98 laboratory tests and clinical feature | Ovarian cancer | AI model | AUC of 0·949 | 2024 |
| Treatment | ||||||
| Milad Shamsi et al. [24] | Retrospective | NR | PC | Computational model | NR | 2018 |
| J M Bereder et al. [25] | Retrospective | 373 cases | PC | ML | Accuracy: close to 98 % | 2019 |
| Alexandros Laios et al. [26] | Retrospective | 154 cases | OC | ML | Accuracy: 66 % | 2020 |
| Nicholas et al. [27] | Retrospective | 60 cases | OC | ML | NR | 2021 |
| Mohsen et al. [28] | Retrospective | NR | PC | Mathematical model | MCDT increased penetration depth more than 13 times | 2022 |
| Mohamed A. et al. [29] | Retrospective | 1959 CRS-HIPEC procedures | PC | ML model | AUC: 0.74 | 2023 |
| Diederick De Jong et al. [30] | Retrospective | 508 patients with ovarian cancer | OC | ML and explainable AI | AUC: 0.91 | 2023 |
| Predicting recurrence | ||||||
| Ruijiang Li et al. [31] | Retrospective | 2320 patients with gastric cancer | GC | Multitask DL model | AUC: 0.843–0.857 C-index: 0.610–0.668 |
2022 |
| Sun, Zepang et al. [32] | Retrospective | 584 quantitative features from 2005 patients | GC | Radiomics | AUC: 0.721–0.732 | 2023 |
Abbreviation: PC: peritoneal carcinomatosis; AUC: area under curve; DCNN: deep convolutional neural network; ML: machine learning; DL: deep learning; NR: not reported; AI: artificial intelligence; GC: gastric cancer; CRC: colorectal cancer; OC: Ovarian cancer; CRS-HIPEC: cytoreductive surgery and hyperthermic intraperitoneal chemotherapy; MCDT: magnetically controlled drug targeting; PMetNet: peritoneal metastasis network.
2. Research methodology
Previous relevant articles using AI in cancer were identified from the literature. The core databases, including PubMed databases and Web of Science, were systematically searched from the inception dates to 2024, using the keywords "deep learning," "machine learning," “artificial intelligence”, “"peritoneal cancer”, and "peritoneal carcinomatosis". Studies that involved the relationship between artificial intelligence and peritoneal carcinomatosis were included.
3. Diagnosis of peritoneal carcinomatosis
3.1. Imaging diagnosis
The clinical manifestations of PC are atypical, making early diagnosis difficult. Traditionally, PC is often discovered incidentally during surgical exploration [33,34]. Specifically, PC can be observed in 15 % of patients with colorectal cancer during abdominal exploration. And in patients with gastric cancer, the proportion reaches almost 40 % [33]. Computed tomography (CT) is the most used imaging modality for the detection of peritoneal nodules. Some CT imaging features, including peritoneal thickening and massive ascites, suggest the presence of peritoneal metastasis. However, these classic imaging features usually appear at a late stage of the disease. The overall sensitivity of CT is only 28.3%–50.9 %. And it is difficult for CT to detect small tumor smaller than 5 mm [35,36]. Although clinical imaging has diagnostic value, it lacks the sensitivity to detect microscopic or small tumor. And the sensitivity for radiologists to detect PC is still insufficient [37]. Therefore, more accurate diagnostic methods for PC are urgently needed.
Due to the sensitive screening techniques of AI, it has been successfully applied in various approaches in the PC diagnosis process. DL and ML have shown great potential in image processing due to the automatic extraction function of the convolutional neural network (CNN) and the learning model based on ML algorithms [38,39]. The data consisting of massive medical images have been used to train the neural networks, which can further detect diseases using CT or MRI through pattern recognition [40]. Several studies have investigated the application of DL and AI in the detection of peritoneal metastasis [[10], [11], [15]]. J-F Ji et al. constructed a radiomic nomogram reflecting CT features of the primary tumor and adjacent peritoneum based on 266 quantitative image features from 554 patients with advanced gastric cancer (AGC). And they found the CT features with Lauren type can help in early detection of occult peritoneal metastasis with an area under the curve (AUC) of 0.920 (95%CI 0.862–0.978) [10]. In a multicenter retrospective cohort study, image data from 1225 surgically treated gastric cancer patients were included and a deep convolutional neural network (DCNN)-based peritoneal metastasis network (PMetNet) was developed. External data from 753 patients which were collected by other independent centers were used to validate the model with a sensitivity of 75.4 % and 87.5 % and an AUC of 0.946 and 0.920 in two external validation groups, respectively. According to the multivariable logistic regression analysis, PMetNet was an independent predictive factor for occult PC [15]. In another retrospective study [16], a radiomics-based ML model was developed using 315 image features from 159 patients. Then, five feature selection methods (including selection operator, recursive feature elimination, random projection algorithm, etc.) were used to optimize the ML model for predicting PC in gastric cancer patients from preoperative CT images. And the ML model which embedded with the random projection algorithm (71.2 %) was associated with significantly higher detection accuracy than that of other models (p < 0.05). Furthermore, a retrospective study described the preoperative CT imaging of colorectal cancer with synchronous PC and extracted the characteristics of the adjacent peritoneum to build a ResNet3D + SVM classifier for the detection of synchronous PC [20]. The ResNet3D + SVM classifier is associated with fast, non-invasive, and accurate features, which spent only 34 s for analyzing the images with AUC of 0.922 (0.912–0.944), specificity of 94.44 % and sensitivity of 93.75 %. The ResNet3D + SVM classifier was associated with a higher accuracy rate than routine CT identified by senior radiologist and junior radiologists (AUC: 0.922 vs 0.791 vs 0.780) [20]. Xinyu Jin et al. [17] proposed a novel model trained by meta-learning and extracted features using multimodal DCNN with enhanced CT to identify PC with an accuracy of 87.5 %. This model outperformed the routine PC prediction model based on DL method and logistic regression (AUC: 0.877 vs 0.827 vs 0.795). Some studies combine imaging and clinical characteristics to predict PC and achieve optimal efficacy. Menglei Li et al. [12] develop a radiomics signature, which included imaging characteristics of the primary site and the adjacent largest lymph node, and further used logistic regression analysis for selecting significant clinical factors to develop a clinical radiomics model. And the model is superior to the clinical-only and radiomics-only models (AUC: 0.855 vs 0.771 vs 0.764) for preoperative prediction of synchronous PC. These non-invasive AI strategies identify patients with occult PC, which may benefit the optimal treatment selection making and avoidance of unnecessary curative surgery.
It is difficult for CT imaging alone to differentiate PC from peritoneal tuberculosis [41]. Although laparoscopic biopsies are considered as the most sensitive method to differentiate the two diseases. However, laparoscopy is expensive and invasive, which limits its application [42]. Therefore, it is necessary to develop a convenient and non-invasive tool for identification diagnosis. Dailun Hou et al. developed a ML model using CT to differentiate PC from peritoneal tuberculosis with an AUC of 0.971 and 0.923 in the training and test cohorts, respectively. There were significant differences between the two diseases in the following aspects, including massive ascites, calcified or ring enhancement of lymph nodes, irregular thickening or cake-like thickening of the peritoneum, scalloping sign and so on [21]. The diagnostic accuracy of PC will be improved and the physician's workload will be significantly reduced by the automatic learning ability and repeated assessment capabilities of AI.
3.2. Blood tests
Early prediction of PC through ML models using basic blood parameters is non-invasive and convenient, which may benefit patient management and reduce mortality and morbidity caused by aggressive surgery [14]. Valentin Bejan et al. [19] conducted a study to use different ML strategies, including random forests (RF), artificial neural networks (ANNs), and support vector machines (SVM), to verify the influence of routine blood tests (hematocrit, white blood cell count, platelet count, hemoglobin levels, neutrophil and lymphocyte counts) in the presence of PC. They found that the sensitivity analysis of the RF model can be applied to verify the influence of each parameter in the occurrence of PC with an optimal accuracy of 0.75. And the following parameters, including age, hemoglobin, and hematocrit, were associated with higher sensitivity. This study opens a promising study direction, more research is required to explore the predictive effect of basic blood parameters in the occurrence of PC.
3.3. Proteomics
Proteomics is the study of a large collection of proteins expressed by an organelle, cell type, tissue or even an organism at a given time [43]. Proteomic information plays an important role in identifying cancer stage, discovering tumorigenesis mechanisms, predicting recurrence, tumor aggressiveness, selecting individual treatment, reducing of cell resistance, in various tumors, such as breast cancer [44,45]. The proteomic features can also predict the presence of PC and further predict the characteristic of tumor immune microenvironment. Yanyan Chen et al. [22] developed a proteomic signature by whole-exome sequencing and proteome profiling in PC and PC-free of gastric cancer. They selected 10 proteins (MSRB3, TBC1D14, PLCB1, ITGA7, LIMS1, RAB6B, SMTN, TADA1, DUOXA2, SEMA3C) through the ML algorithms from the PC-related cohort for predicting the occurrence of PC and achieved an AUC of 0.83. They further established a PC risk score based on this 10-protein signature and divided gastric cancer patients into three cohorts: high-risk, moderate-risk, and low-risk groups according to the PC risk score. In contrast to the low-risk cohort, the high-risk cohort was associated with a higher percentage of regulatory T cells, M2 macrophages, and resting CD4+ memory T cells and a lower percentage of CD4+ naive T cells, plasma B cells, and eosinophils. This result showed that there is an association between PC risk score and tumor immune microenvironment and that PC risk score may be a predictive factor for immunotherapy response.
3.4. Pathological diagnosis
Histopathology through laparoscopic or laparotomy biopsy and cytopathology of malignant ascites remains the gold standard for the diagnosis of PC [46,47]. Recently, DL image analysis has used to identify morphology-based representations caused by molecular alterations in some tumors, including colorectal cancer, melanoma, breast cancer, prostate Cancer, and lung cancer [[48], [49], [50]]. Digital pathology has made significant progress due to the following advantages, including immediate availability of cases, remote diagnosis, and more convenient consultation with pathologists [51]. Faster slide scanning, cheaper data storage, and increased computing power have enabled the development of large-scale slide scanning as well as image analysis, and promoted the application of ML to address biological issues through combining tumor morphology with histology [48,52]. Machine learning is also associated with higher accuracy in error-prone tasks such as metastasis identification [51].
In cytopathology, whole slide scanners convert cytopathology into high resolution images [53]. In addition, DL-based models have been used in ascites cytology. Feng Su et al. [54] generated a novel database of peritoneal effusion cytopathology images and developed a DL model for ascites cytopathology. Based on Region-based CNN, they established Detection-Net for automatic cell detection and Classification-Net for cell classification. The average accuracy of the detection Net was 0.8316. And the Classification-Net showed outstanding performance in cell classification with an AUC of 0.8851, precision of 96.80 %. The DL model, which combined Detection-Net and Classification-Net, showed good performance in ascites cytopathology. With the application of AI in cytopathology, CNN can be used for pre-screening of ascites cytology, significantly reducing the burden on pathologists, and improving the accuracy.
4. Treatment
4.1. Operative resection
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are regarded as the mainstay of treatment, but have limited efficacy in patients with PC [55]. In cytoreductive surgery, surgeons attempt to remove all macroscopic neoplasm (R0) from the peritoneal surface. Sometimes it may be necessary to remove heavily involved organs to achieve radical resection [56]. HIPEC is recommended only after complete CRS, and a survival benefit has been observed [57]. Recently, AI has been used in surgical procedures. Due to the improved accuracy and stability, high-definition 3D vision, and good ergonomic experience of Da Vinci robotic surgery, it is being used more widely in surgery [58].
Postoperative mortality and morbidity are also relatively high, making the selection of cytoreductive surgery a challenging issue. And accurate prediction of surgical effect and identification of R0 resection patients are important to improve patient prognosis and survival. ML and eXplainable Artificial Intelligence (XAI) are also used to predict R0 resection in ovarian cancer [[26], [30], [59]]. In a single institution study, two predictive models based on the eXtreme Gradient Boosting (XGBoost) and Deep Neural Network (DNN) algorithms were developed and compared to predict the probability of complete cytoreduction. Patients with a surgical complexity score greater than 5 were associated with a higher likelihood of incomplete cytoreduction on a receiver operator characteristic (ROC) curve with an AUC of 0.644, sensitivity of 34.1 %, and specificity of 86.5 %. And DNN scoring is inferior to XGBoost for predicting surgical efficacy (AUC: 0.739 vs 0.77) [59]. XAI technology is important for a more comprehensive understanding of the AI framework and to encourage the application of AI in the medical field. A retrospective study investigated the performance of the k-nearest neighbor (k-NN) classifier, an AI system, in predicting R0 resection in advanced ovarian cancer [26], and the k-NN algorithm relatively outperformed conventional logistic regression with a mean predictive accuracy of 66 %. More recently, Diederick De Jong et al. [30] developed a novel intraoperative score to capture anatomical fingerprints of disease (ANAFI score) and discovered that the presence of cancer dissemination in the following areas, including the colonic serosa, small bowel mesentery, and diaphragmatic peritoneum, was associated with higher predictive accuracy of CC0 than the existing scoring tools, including the intraoperative mapping for ovarian cancer (IMO) and the peritoneal carcinomatosis index (PCI). Early assessment of the above anatomical sites by ML and AI during surgery procedure can guide the selection of surgical approaches. Accurate identification of patients with complete resection can help in the selection of individualized treatment.
A previous study showed that patients undergoing CRS-HIPEC with major complications were associated with a higher 3-year OS rate than those with minor complications (62 % vs 28 %, p < 0.01) [60]. Mohamed A. Adam MD et al. established a risk score to predict the severity of 90-day complications of CRS with HIPEC using a data-driven ML model [29]. They used the comprehensive complication index (CCI) score, a continuous scale from 0 (no complication) to 100 (death), to identify the severe complications. And the CCI >61 was identified as severe complications with an AUC of 0.74. And the following factors, including PCI score, symptomatic status, and having undergone pancreatectomy, were positively associated with severe complications.
4.2. Intraperitoneal chemotherapy
Intraperitoneal (IP) chemotherapy delivers cytotoxic drugs directly into the peritoneal cavity, reducing the toxicity of systemic chemotherapy and enabling peritoneal lesions to be exposed to high concentrations of anticancer agents [61]. However, because of the poor penetration depth of cytotoxic drugs into the tumor tissue, and only tumor periphery cells can be in full contact with the high concentration of chemotherapy drugs, so IP chemotherapy is associated with limited efficacy [62]. It is necessary to develop new drug delivery systems to increase the depth of drug penetration and improve the efficacy of therapy. Recently, magnetically controlled drug targeting (MCDT) has been developed to improve the efficacy of IP therapy. In MCDT, drug-coated magnetic nanoparticles (MNPs) are targeted to tumor tissue through an external magnetic field [63,64]. Amir Sanati-Nezhad et al. established a computational model [24] for predicting the predictability and feasibility of magnetically assisted IP chemotherapy. The computer reconstructed drug delivery barriers, including a spatially heterogeneous construct of non-functional lymphatic vessels, increased interstitial fluid pressure, leaky vasculature, and denser extracellular matrix. Compared to IP-free cytotoxic drugs, the MCDT method increases agent penetration into large tumor tissue and significantly increases the final intra-tumoral concentration of cytotoxic drugs. Similarly, Kaamran Raahemifar et al. [28] also developed a mathematical modelling and confirmed the efficacy of MCDT. Compared with conventional IP injection, MCDT increased the drug penetration area by more than 1.4 times and the penetration depth by more than 13 times, and significantly enhanced the proportion of killed cancer cells (6.5 % vs 2.54 %).
Recently, some preclinical and clinical studies have investigated the efficacy of various IP immunotherapies in PC, including catumaxomab, chimeric antigen receptor (CAR)- T cells, immune stimulators, immune checkpoint inhibitors, cancer vaccines, and radioimmunotherapy [55]. More AI-based models are needed to predict the efficacy of different intraperitoneal immunotherapies and to guide drug selection in PC.
4.3. Systemic chemotherapy
Systemic chemotherapy may be considered for some patients who are unable to undergo surgery [65]. Chemotherapy drug resistance is a challenge issue in cancer treatment, caused by insufficient drug concentration reaching the tumor tissue through the blood circulation. AI can use its learning ability to predict the sensitivity of chemotherapeutic drugs, improve therapeutic decisions, and increase the effectiveness of therapy by individualizing therapy [58]. A retrospective study [27] generated a signature to predict cisplatin sensitivity using the Genomics of Drug Sensitivity in Cancer and The Cancer Genome Atlas databases to identify four biomarkers, including S100A14, CYTH3, ERI1, and GALNT3. Using ML, they found that ovarian cancer patients with lower expression of CYTH3 and S100A14 and negative lymph node status were associated with a low risk of platinum resistance. Close monitoring of response and relapse is required for tumors predicted to be chemo-resistant, and second-line chemotherapy should be considered if relapse is confirmed. PC arise from different primary tumor sites and are amenable to different chemotherapeutic agents. AI can help predict the therapeutic response to drugs and guide drug selection in PC. In addition, the AI technologies had been widely used in the various aspects of drug field, including new drug development, drug target identification and validation, and drug repurposing and so on [66].
4.4. Recurrence prediction of peritoneal carcinomatosis
Physicians need to predict the survival and recurrence rates of cancer patients by assessing their clinical characteristics. However, this prediction has great contingency and uncertainty, and often requires rich experience of doctors. With the application of AI in cancer recurrence prediction, the prediction accuracy will be improved. Peritoneal recurrence is the main site of relapse in gastric cancer patients who had undergone radical operation. Accurate prediction of peritoneal recurrence is important to identify patients who may benefit from local treatment or intensified systemic therapy [67,31]. DL models usually perform a single task, such as predicting survival endpoints. In contrast, multitask learning models perform multiple tasks simultaneously using a single model. Based on preoperative CT images, a retrospective study developed a multitask DL model that simultaneously predicts disease-free survival (DFS) and peritoneal recurrence for gastric cancer after radical operation. The AUC is 0.857 in the training cohort, 0.856 in the internal validation cohort, and 0.843 in the external validation cohort for predicting peritoneal recurrence. And the model performed well in predicting DFS with a C-index of 0.654, 0.668, and 0.610 in the training cohort, internal validation cohort, and external validation cohort, respectively [31]. The study also showed that the predictive accuracy of the multitask DL model was significantly higher than that of the traditional single-task DL-based model. In addition, the predictive models are also associated with response to adjuvant chemotherapy. Adjuvant chemotherapy can significantly improve DFS in stage II-III gastric cancer patients with a high risk of peritoneal recurrence. While chemotherapy had no effect on DFS in patients with a low predicted risk [31]. More recently, a multi-center study extracted 584 quantitative characteristics of the intra-tumoral and peritumoral regions on enhanced CT images and combined different algorithms, including penalized Cox regression algorithm, SVM-RFE, and LASSO, for generating a radiomics signature. This study enrolled 2005 patients with gastric cancer after surgical resection and developed an AI model to predict PC recurrence according to preoperative CT images with an AUC of 0.732 in the training cohort. Notably, this model helps doctors with 5–15 years of clinical experience to increase the PC diagnostic accuracy by 10.13 %–18.86 % [32]. AI can screen for features associated with peritoneal recurrence by comparing images with PC and cancer without PC, and integrate these features into a radiomic signature for clinical prediction, which will optimize the patient management and treatment.
4.5. Emotion analysis of patients with peritoneal carcinomatosis
The severity and lethality of PC has a profound impact on the mental health of patients, resulting in the production of depression, anxiety, and even suicidal psychology. Patients with these psychological problems are always resistant to cancer therapy, which seriously affects the therapeutic effect [[68], [69], [70]]. Therefore, accurate prediction of the patient's psychology and prompt counselling interventions are very vital for cancer treatment. Recently, the AI techniques have also been used to analyze patients' emotions. Compared with general verbal or pictural stimulation, virtual reality (VR) technology is more effective to induce emotions for diagnosis [71]. Li, ZB et al. [72] constructed a computational affection-based OCC-PAD-OCEAN federation cognitive modelling (OPO-FCM), which can capture expression features by training a deep neural network, map expression features to the PAD emotion space by the established expression–emotion space mapping connection, and complete the mapping of the average emotion during a period. Liu, TT et al. [73] found that the integration of AI in the research of emotions of loneliness, depression, and anxiety (EMO-LDA) research is a promising direction for addressing mental health. In general, AI technology is a powerful tool to diagnose and support mental health in cancer patients.
5. Discussion
Our analyses pooled data from the core database PubMed databases and Web of Science. We included studies that involved the association between AI and PC. We concluded that the application of AI technologies in different approaches in the field of PC. AI increases the diagnostic accuracy and treatment efficacy in PC. And recurrence prediction and emotion evaluation of PC patients are also combined with AI technology. However, our study also has limitations. Firstly, the literature on the use of AI in PC is not extensive, and some of the data in our review represent the conclusions of individual studies, which are not very rigorous. In addition, the studies we enrolled are almost retrospective datasets, the information of some clinical factors may be questionable, which may affect the accuracy. Future inclusion of more prospective studies could further strengthen the evidence. There are few studies on the application of AI in PC, and our center have conducted related study to expand the application prospect of AI in carcinoma.
6. Conclusions and perspectives
PC is a type of secondary cancer that is not sensitive to conventional intravenous chemotherapy. And treatment strategies are usually palliative and limited. Therefore, early diagnosis and sensitive treatment are needed for PC. Recently, AI has made rapid progress in the field of cancer, including PC. Due to the development of deep neural networks and machine learning algorithms of AI, it is generally applied in the diagnosis, recurrence assessment, and treatment of PC. However, the application of AI in PC still has some problems, such as the immature technology, the high cost, and the controversies in moral and ethical direction. And most of the current studies are retrospective studies. In the future, more prospective and rigorous studies are needed to enroll larger sample size to combine AI technology with different aspects of cancer, and apply AI in PC more systematically and normatively. Specifically, more radiomic nomograms based on imaging phenotypes were required to serve as a feasible approach for non-invasive prediction of suspicious PC. Researches using machine learning methods to select treatment strategies is needed, so that clinicians can better individualize treatment regimens to maximize survival benefits. And some of the models mentioned in our review need to be further validated by a larger prospective external center.
Ethics approval and consent to participate
Not applicable.
Data availability statement
No data was used for the research described in the article.
Funding
This study was supported by the Sichuan Science and Technology Department Key Research and Development Project (grant no. 2022YFS0209) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant no. ZYJC21017).
CRediT authorship contribution statement
Gui-Xia Wei: Writing – original draft. Yu-Wen Zhou: Writing – original draft. Zhi-Ping Li: Writing – review & editing. Meng Qiu: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., et al. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Ströhlein M.A., Heiss M.M., Jauch K.W. The current status of immunotherapy in peritoneal carcinomatosis. Expet Rev. Anticancer Ther. 2016;16(10):1019–1027. doi: 10.1080/14737140.2016.1224666. [DOI] [PubMed] [Google Scholar]
- 3.Ayantunde A.A., Parsons S.L. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann. Oncol. : official journal of the European Society for Medical Oncology. 2007;18(5):945–949. doi: 10.1093/annonc/mdl499. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed N., Stenvers K.L. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front. Oncol. 2013;3:256. doi: 10.3389/fonc.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z.H., Lin L., Wu C.F., Li C.F., Xu R.H., Sun Y. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun. 2021;41(11):1100–1115. doi: 10.1002/cac2.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X., Li W., Tu H. Big data and artificial intelligence in cancer research. Trends in cancer. 2024;10(2):147–160. doi: 10.1016/j.trecan.2023.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Choi R.Y., Coyner A.S., Kalpathy-Cramer J., Chiang M.F., Campbell J.P. Introduction to machine learning, neural networks, and deep learning. Translational vision science & technology. 2020;9(2):14. doi: 10.1167/tvst.9.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee P., Zhou M., Lee E., Schicht A., Balagurunathan Y., Napel S., et al. A Shallow convolutional neural network predicts prognosis of lung cancer patients in multi-institutional CT-image data. Nat. Mach. Intell. 2020;2(5):274–282. doi: 10.1038/s42256-020-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litjens G., Kooi T., Bejnordi B.E., Setio A.A.A., Ciompi F., Ghafoorian M., et al. A survey on deep learning in medical image analysis. Med. Image Anal. 2017;42:60–88. doi: 10.1016/j.media.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Dong D., Tang L., Li Z.Y., Fang M.J., Gao J.B., Shan X.H., et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol. : official journal of the European Society for Medical Oncology. 2019;30(3):431–438. doi: 10.1093/annonc/mdz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z., Liu D., Chen X., He D., Yu P., Liu B., et al. Deep convolutional neural network based on computed tomography images for the preoperative diagnosis of occult peritoneal metastasis in advanced gastric cancer. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M., Sun K., Dai W., Xiang W., Zhang Z., Zhang R., et al. Preoperative prediction of peritoneal metastasis in colorectal cancer using a clinical-radiomics model. Eur. J. Radiol. 2020;132 doi: 10.1016/j.ejrad.2020.109326. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z., Liu D., Chen X., Yu P., Wu J., Song B., et al. Retrospective imaging studies of gastric cancer: study protocol clinical trial (SPIRIT Compliant) Medicine. 2020;99(8) doi: 10.1097/MD.0000000000019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou C., Wang Y., Ji M.H., Tong J., Yang J.J., Xia H. Predicting peritoneal metastasis of gastric cancer patients based on machine learning. Cancer Control J. Moffitt Cancer Cent. 2020;27(1) doi: 10.1177/1073274820968900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y., Liang X., Wang W., Chen C., Yuan Q., Zhang X., et al. Noninvasive prediction of occult peritoneal metastasis in gastric cancer using deep learning. JAMA Netw. Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.32269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirniaharikandehei S., Heidari M., Danala G., Lakshmivarahan S., Zheng B. Applying a random projection algorithm to optimize machine learning model for predicting peritoneal metastasis in gastric cancer patients using CT images. Comput. Methods Progr. Biomed. 2021;200 doi: 10.1016/j.cmpb.2021.105937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Zhu X., Li B., Dai X., Bao X., Fu Q., et al. Development and validation of a meta-learning-based multi-modal deep learning algorithm for detection of peritoneal metastasis. Int. J. Comput. Assist. Radiol. Surg. 2022;17(10):1845–1853. doi: 10.1007/s11548-022-02698-w. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Lv P., Xue Z., Chen L., Zheng B., Lin G., et al. Novel CT based clinical nomogram comparable to radiomics model for identification of occult peritoneal metastasis in advanced gastric cancer. Eur. J. Surg. Oncol. : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2022;48(10):2166–2173. doi: 10.1016/j.ejso.2022.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Bejan V., Dragoi E.N., Curteanu S., Scripcariu V., Filip B. The prediction of peritoneal carcinomatosis in patients with colorectal cancer using machine learning. Healthcare (Basel, Switzerland) 2022;10(8) doi: 10.3390/healthcare10081425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Z., Xu T., Cai J., Zhao Y., Cao W., Fichera A., et al. Development and validation of an image-based deep learning algorithm for detection of synchronous peritoneal carcinomatosis in colorectal cancer. Ann. Surg. 2022;275(4):e645–e651. doi: 10.1097/SLA.0000000000004229. [DOI] [PubMed] [Google Scholar]
- 21.Pang Y., Li Y., Xu D., Sun X., Hou D. Differentiating peritoneal tuberculosis and peritoneal carcinomatosis based on a machine learning model with CT: a multicentre study. Abdominal radiology (New York) 2023;48(4):1545–1553. doi: 10.1007/s00261-022-03749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Cai G., Jiang J., He C., Chen Y., Ding Y., et al. Proteomic profiling of gastric cancer with peritoneal metastasis identifies a protein signature associated with immune microenvironment and patient outcome. Gastric Cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2023;26(4):504–516. doi: 10.1007/s10120-023-01379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai G., Huang F., Gao Y., Li X., Chi J., Xie J., et al. Artificial intelligence-based models enabling accurate diagnosis of ovarian cancer using laboratory tests in China: a multicentre, retrospective cohort study. The Lancet Digital health. 2024;6(3):e176–e186. doi: 10.1016/S2589-7500(23)00245-5. [DOI] [PubMed] [Google Scholar]
- 24.Shamsi M., Sedaghatkish A., Dejam M., Saghafian M., Mohammadi M., Sanati-Nezhad A. Magnetically assisted intraperitoneal drug delivery for cancer chemotherapy. Drug Deliv. 2018;25(1):846–861. doi: 10.1080/10717544.2018.1455764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maubert A., Birtwisle L., Bernard J.L., Benizri E., Bereder J.M. Can machine learning predict resecability of a peritoneal carcinomatosis? Surgical oncology. 2019;29:120–125. doi: 10.1016/j.suronc.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Laios A., Gryparis A., DeJong D., Hutson R., Theophilou G., Leach C. Predicting complete cytoreduction for advanced ovarian cancer patients using nearest-neighbor models. J. Ovarian Res. 2020;13(1):117. doi: 10.1186/s13048-020-00700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon N.B., Tan L.L.Y., Tan Q.X., Tan J.W., Hendrikson J., Ng W.H., et al. A machine learning approach to identify predictive molecular markers for cisplatin chemosensitivity following surgical resection in ovarian cancer. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-96072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaeian M., Soltani M., Naseri Karimvand A., Raahemifar K. Mathematical modeling of targeted drug delivery using magnetic nanoparticles during intraperitoneal chemotherapy. Pharmaceutics. 2022;14(2) doi: 10.3390/pharmaceutics14020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam M.A., Zhou H., Byrd J., Greenberg A.L., Kelly Y.M., Hall L., et al. Predicting severe complications from cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a data-driven, machine learning approach to Augment clinical judgment. Ann. Surg Oncol. 2023;30(9):5433–5442. doi: 10.1245/s10434-023-13657-3. [DOI] [PubMed] [Google Scholar]
- 30.Laios A., Kalampokis E., Johnson R., Munot S., Thangavelu A., Hutson R., et al. Development of a novel intra-operative score to record diseases' anatomic fingerprints (ANAFI score) for the prediction of complete cytoreduction in advanced-stage ovarian cancer by using machine learning and explainable artificial intelligence. Cancers. 2023;15(3) doi: 10.3390/cancers15030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y., Zhang Z., Yuan Q., Wang W., Wang H., Li T., et al. Predicting peritoneal recurrence and disease-free survival from CT images in gastric cancer with multitask deep learning: a retrospective study. The Lancet Digital health. 2022;4(5):e340–e350. doi: 10.1016/S2589-7500(22)00040-1. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z., Wang W., Huang W., Zhang T., Chen C., Yuan Q., et al. Noninvasive imaging evaluation of peritoneal recurrence and chemotherapy benefit in gastric cancer after gastrectomy: a multicenter study. Int. J. Surg. 2023;109(7):2010–2024. doi: 10.1097/JS9.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coccolini F., Gheza F., Lotti M., Virzì S., Iusco D., Ghermandi C., et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013;19(41):6979–6994. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vassos N., Piso P. Metastatic colorectal cancer to the peritoneum: current treatment options. Curr. Treat. Options Oncol. 2018;19(10):49. doi: 10.1007/s11864-018-0563-8. [DOI] [PubMed] [Google Scholar]
- 35.Burbidge S., Mahady K., Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin. Radiol. 2013;68(3):251–255. doi: 10.1016/j.crad.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Kim S.J., Kim H.H., Kim Y.H., Hwang S.H., Lee H.S., Park D.J., et al. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology. 2009;253(2):407–415. doi: 10.1148/radiol.2532082272. [DOI] [PubMed] [Google Scholar]
- 37.Xu S., Bulin A.L., Hurbin A., Elleaume H., Coll J.L., Broekgaarden M. Photodynamic diagnosis and therapy for peritoneal carcinomatosis: emerging perspectives. Cancers. 2020;12(9) doi: 10.3390/cancers12092491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulshan V., Peng L., Coram M., Stumpe M.C., Wu D., Narayanaswamy A., et al. Development and validation of a deep learning algorithm for detection of diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316(22):2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 39.Raman A.G., Fisher D., Yap F., Oberai A., Duddalwar V.A. Radiomics and artificial intelligence: Renal cell carcinoma. Urol. Clin. 2024;51(1):35–45. doi: 10.1016/j.ucl.2023.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Shan H., Padole A., Homayounieh F., Kruger U., Khera R.D., Nitiwarangkul C., et al. Competitive performance of a modularized deep neural network compared to commercial algorithms for low-dose CT image reconstruction. Nat. Mach. Intell. 2019;1(6):269–276. doi: 10.1038/s42256-019-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshpande S.S., Joshi A.R., Deshpande S.S., Phajlani S.A. Computed tomographic features of abdominal tuberculosis: unmask the impersonator. Abdominal radiology (New York) 2019;44(1):11–21. doi: 10.1007/s00261-018-1700-3. [DOI] [PubMed] [Google Scholar]
- 42.Talat N., Afzal M., Ahmad S., Rasool N., Wasti A.R., Saleem M. Role of diagnostic laparoscopy in evaluation and treatment of chronic abdominal pain in children. J. Ayub Med. Coll. Abbottabad : JAMC. 2016;28(1):35–38. [PubMed] [Google Scholar]
- 43.Chandramouli K., Qian P.Y. Proteomics: challenges, techniques and possibilities to overcome biological sample complexity. Hum. Genom. Proteonomics : HGP. 2009;2009 doi: 10.4061/2009/239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neagu A.N., Whitham D., Buonanno E., Jenkins A., Alexa-Stratulat T., Tamba B.I., et al. Proteomics and its applications in breast cancer. Am. J. Cancer Res. 2021;11(9):4006–4049. [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung C.H.Y., Juan H.F. Quantitative proteomics in lung cancer. J. Biomed. Sci. 2017;24(1):37. doi: 10.1186/s12929-017-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer J.A., Swift S.E., Wilkinson N., Boon A.P., Lane G., Perren T.J. Peritoneal carcinomatosis: image-guided peritoneal core biopsy for tumor type and patient care. Radiology. 2001;221(1):173–177. doi: 10.1148/radiol.2203010070. [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Ji J. Application of laparoscopy in the diagnosis and treatment of gastric cancer. Ann. Transl. Med. 2015;3(9):126. doi: 10.3978/j.issn.2305-5839.2015.03.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laury A.R., Blom S., Ropponen T., Virtanen A., Carpén O.M. Artificial intelligence-based image analysis can predict outcome in high-grade serous carcinoma via histology alone. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-98480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azadi Moghadam P., Bashashati A., Goldenberg S.L. Artificial intelligence and pathomics: prostate cancer. Urol. Clin. 2024;51(1):15–26. doi: 10.1016/j.ucl.2023.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Soliman A., Li Z., Parwani A.V. Artificial intelligence's impact on breast cancer pathology: a literature review. Diagn. Pathol. 2024;19(1):38. doi: 10.1186/s13000-024-01453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehteshami Bejnordi B., Veta M., Johannes van Diest P., van Ginneken B., Karssemeijer N., Litjens G., et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in Women with breast cancer. JAMA. 2017;318(22):2199–2210. doi: 10.1001/jama.2017.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hekler A., Utikal J.S., Enk A.H., Solass W., Schmitt M., Klode J., et al. Deep learning outperformed 11 pathologists in the classification of histopathological melanoma images. European journal of cancer (Oxford, England : 1990) 2019;118:91–96. doi: 10.1016/j.ejca.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Cucoranu I.C., Parwani A.V., Pantanowitz L. Digital whole slide imaging in cytology. Arch. Pathol. Lab Med. 2014;138(3):300. doi: 10.5858/arpa.2013-0270-LE. [DOI] [PubMed] [Google Scholar]
- 54.Su F., Sun Y., Hu Y., Yuan P., Wang X., Wang Q., et al. Development and validation of a deep learning system for ascites cytopathology interpretation. Gastric Cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2020;23(6):1041–1050. doi: 10.1007/s10120-020-01093-1. [DOI] [PubMed] [Google Scholar]
- 55.Wei G.X., Du Y., Zhou Y.W., Li L.J., Qiu M. Peritoneal carcinomatosis with intraperitoneal immunotherapy: current treatment options and perspectives. Expet Rev. Gastroenterol. Hepatol. 2022;16(9):851–861. doi: 10.1080/17474124.2022.2125866. [DOI] [PubMed] [Google Scholar]
- 56.Sugarbaker P.H. Peritonectomy procedures. Ann. Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Bree E., Koops W., Kröger R., van Ruth S., Verwaal V.J., Zoetmulder F.A. Preoperative computed tomography and selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2006;32(1):65–71. doi: 10.1016/j.ejso.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Pang J., Xiu W., Ma X. Application of artificial intelligence in the diagnosis, treatment, and prognostic evaluation of mediastinal malignant tumors. J. Clin. Med. 2023;12(8) doi: 10.3390/jcm12082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laios A., Kalampokis E., Johnson R., Munot S., Thangavelu A., Hutson R., et al. Factors predicting surgical effort using explainable artificial intelligence in advanced stage Epithelial ovarian cancer. Cancers. 2022;14(14) doi: 10.3390/cancers14143447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graziosi L., Marino E., De Angelis V., Rebonato A., Donini A. Survival prognostic factors in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy treatment: analysis from a single oncological center. World J. Surg. Oncol. 2016;14:97. doi: 10.1186/s12957-016-0856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambert L.A. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA A Cancer J. Clin. 2015;65(4):284–298. doi: 10.3322/caac.21277. [DOI] [PubMed] [Google Scholar]
- 62.Minchinton A.I., Tannock I.F. Drug penetration in solid tumours. Nat. Rev. Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 63.Nacev A., Beni C., Bruno O., Shapiro B. Magnetic nanoparticle transport within flowing blood and into surrounding tissue. Nanomedicine (London, England) 2010;5(9):1459–1466. doi: 10.2217/nnm.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nacev A., Kim S.H., Rodriguez-Canales J., Tangrea M.A., Shapiro B., Emmert-Buck M.R. A dynamic magnetic shift method to increase nanoparticle concentration in cancer metastases: a feasibility study using simulations on autopsy specimens. Int. J. Nanomed. 2011;6:2907–2923. doi: 10.2147/IJN.S23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klaver Y.L., Lemmens V.E., Nienhuijs S.W., Luyer M.D., de Hingh I.H. Peritoneal carcinomatosis of colorectal origin: incidence, prognosis and treatment options. World J. Gastroenterol. 2012;18(39):5489–5494. doi: 10.3748/wjg.v18.i39.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mak K.K., Pichika M.R. Artificial intelligence in drug development: present status and future prospects. Drug Discov. Today. 2019;24(3):773–780. doi: 10.1016/j.drudis.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Ikoma N., Chen H.C., Wang X., Blum M., Estrella J.S., Fournier K., et al. Patterns of initial recurrence in gastric Adenocarcinoma in the era of preoperative therapy. Ann. Surg Oncol. 2017;24(9):2679–2687. doi: 10.1245/s10434-017-5838-y. [DOI] [PubMed] [Google Scholar]
- 68.Podina I.R., Todea D., Fodor L.A. Fear of cancer recurrence and mental health: a comprehensive meta-analysis. Psycho Oncol. 2023;32(10):1503–1513. doi: 10.1002/pon.6205. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Flannery M., Zhang Z., Underhill-Blazey M., Bobry M., Leblanc N., et al. Digital health Psychosocial intervention in Adult patients with cancer and their families: systematic review and meta-analysis. JMIR cancer. 2024;10 doi: 10.2196/46116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pini S., Buck C., Lally P., Beeken R., Fisher A. The impact of the COVID-19 pandemic on mental health and quality of life in people living with and beyond breast, prostate and colorectal cancer - a qualitative study. BMC psychology. 2024;12(1):25. doi: 10.1186/s40359-023-01471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng W.H., Cai H.S., Yao Z.J., Zhang X.W., Li X.W., Hu B., editors. Modelling Mental States via Computational Psychophysiology: Benefits and Challenges. 5th International Conference on Human Centered Computing (HCC) 2019. Aug 05-07; Cacak, SERBIA2019. [Google Scholar]
- 72.Liu F., Wang H.Y., Shen S.Y., Jia X., Hu J.Y., Zhang J.H., et al. OPO-FCM: a computational affection based OCC-PAD-OCEAN federation cognitive modeling approach. Ieee Transactions on Computational Social Systems. 2023;10(4):1813–1825. [Google Scholar]
- 73.Zheng Q.J., Liu F., Xu S.Y., Hu J.Y., Lu H.X., Liu T.T. Artificial intelligence empowering research on loneliness, depression and anxiety - using Covid-19 as an opportunity. Journal of Safety Science and Resilience. 2023;4(4):396–409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.