Abstract
Introduction
The COVID-19 pandemic had collateral effects on many health systems. Cancer screening and diagnostic tests were postponed, resulting in delays in diagnosis and treatment. This study assessed the impact of the pandemic on screening, diagnostics and incidence of breast, colorectal, lung, and prostate cancer; and whether rates returned to pre-pandemic levels by December, 2021.
Methods
This is a cohort study of electronic health records from the United Kingdom (UK) primary care Clinical Practice Research Datalink (CPRD) GOLD database. The study included individuals registered with CPRD GOLD between January, 2017 and December, 2021, with at least 365 days of clinical history. The study focused on screening, diagnostic tests, referrals and diagnoses of first-ever breast, colorectal, lung, and prostate cancer. Incidence rates (IR) were stratified by age, sex, and region, and incidence rate ratios (IRR) were calculated to compare rates during and after lockdown with rates before lockdown. Forecasted rates were estimated using negative binomial regression models.
Results
Among 5,191,650 eligible participants, the first lockdown resulted in reduced screening and diagnostic tests for all cancers, which remained dramatically reduced across the whole observation period for almost all tests investigated. There were significant IRR reductions in breast (0.69 [95% CI: 0.63-0.74]), colorectal (0.74 [95% CI: 0.67-0.81]), and prostate (0.71 [95% CI: 0.66-0.78]) cancer diagnoses. IRR reductions for lung cancer were non-significant (0.92 [95% CI: 0.84-1.01]). Extrapolating to the entire UK population, an estimated 18,000 breast, 13,000 colorectal, 10,000 lung, and 21,000 prostate cancer diagnoses were missed from March, 2020 to December, 2021.
Discussion
The UK COVID-19 lockdown had a substantial impact on cancer screening, diagnostic tests, referrals, and diagnoses. Incidence rates remained significantly lower than pre-pandemic levels for breast and prostate cancers and associated tests by December, 2021. Delays in diagnosis are likely to have adverse consequences on cancer stage, treatment initiation, mortality rates, and years of life lost. Urgent strategies are needed to identify undiagnosed cases and address the long-term implications of delayed diagnoses.
Keywords: breast cancer, colorectal cancer, lung cancer, prostate cancer, COVID-19, pandemic, cancer screening
1. Introduction
Breast, colorectal, lung and prostate cancer are the four most common causes of cancer death in the United Kingdom (UK) (1). Population screening programs (e.g. mammograms for breast cancer; faecal immunochemical tests (FIT) for colorectal cancer) aid early diagnosis, leading to better outcomes and prognosis (2). However, due to the COVID-19 pandemic, and the first UK national lockdown (23rd March, 2020), many health systems postponed cancer screening and diagnostic tests, to reduce spread of infection, and deployed staff towards critical COVID-19 patient care. ‘Stay at home’ advice, fear of contracting COVID-19, and social distancing measures introduced during the pandemic may also have altered health-seeking behaviour (3). Combined, these changes in clinical practice and patient behaviour resulted in delays in diagnosis and treatment initiation, impacting on prognosis, mortality rates and total years of life lost (4).
Data suggest that countries responded to the COVID-19 pandemic differently. A review of studies from various countries showed significant declines in breast and lung cancer screenings and diagnostic biopsies during the pandemic (4). In the USA, whilst there were initial reductions in breast, colorectal, prostate and cervical screening tests during the pandemic period (compared to 3 months prior and 3 months after the pandemic) (5), one report indicated that breast cancer screenings remained below expected levels even after one year (6). However, Canada saw a return to pre-pandemic screening levels for breast, cervical, and colorectal cancer by, 2021 (7). In the UK, urgent cancer referrals initially dropped by up to 80%, with routine referrals reduced as patients delayed appointments (8, 9). Referral rates for breast cancer mostly recovered by August, 2020 and remained stable during subsequent lockdowns (10). It remains unclear if this trend applies to other tests and cancer types. Reduced screening and referrals led to a decline in cancer rates globally (11–32). However, there is a lack of studies estimating cancer incidence specifically in the UK during the pandemic and post-lockdown periods.
Using data from routinely recorded primary care electronic health records, the present study aims to 1) examine the frequencies and incidence rates (IR) of all consultations, cancer screening/diagnostic tests/referrals and breast, colorectal, lung and prostate cancer diagnoses in the general population before (from January, 2017 to February, 2020), during (March, 2020 to June, 2020) and after (July, 2020 to December, 2021) the first lockdown; 2) characterise newly diagnosed cancer patients in terms of frequencies of all consultations, procedures, measurements, comorbidities and medication use before, during and after lockdown; and 3) use time-series analyses to model the discrepancy between the observed and expected cancer diagnosis rates using data from 3 years prior to the pandemic to estimate how many cancer diagnoses may have been missed due to the pandemic, and whether diagnosis rates have stabilised to pre-pandemic levels. We focus on these four cancers as they are the most common and those where we have rapid diagnostics/screening tools available in the UK.
2. Methods
2.1. Study participants
This study is a population-based cohort study using routinely collected electronic health records from UK Clinical Practice Research Datalink (CPRD) GOLD. CPRD GOLD contains anonymised patient-level information on demographics, lifestyle data, clinical diagnoses, prescriptions and preventive care contributed by general practitioners from the UK (33). The use of CPRD data for this study was approved by the Independent Scientific Advisory Committee (22_002331). This database has previously been mapped to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) (34). People were eligible if they were registered between January, 2017 and December, 2021 with at least one year of prior clinical history. Additional criteria for the incident cancer diagnosis cohorts were including individuals who had a diagnosis or record of cancer, specifically for breast, colorectal, lung, or prostate cancer; excluding individuals diagnosed with the same type of cancer at any point in their clinical history and excluding those with metastases. We did not specifically interrogate (or exclude on the basis of) whether individuals had intact organs.
2.2. Exposure
The ‘exposure’ was the date of the first UK national lockdown (23rd March, 2020), which was used to dissect the full study period into three distinct time-periods: pre-pandemic (January, 2017 to February, 2020), during lockdown (March, 2020 to June, 2020), and post-lockdown (July, 2020 to December, 2021). Additionally, we further dissected the extended post-lockdown periods distinguished by the changing social restrictions according to the specific dates shown in Figure 1, and lockdown periods categorized as follows: Lockdown (March, 2020 to June, 2020); post-first lockdown (July, 2020 to October, 2020); second lockdown (Nov, 2020 to Dec, 2020); third lockdown (Jan, 2021 to March, 2021); easing of restrictions (April, 2021 to June, 2021); and most legal restrictions removed (July, 2021 to December, 2021).
Figure 1.
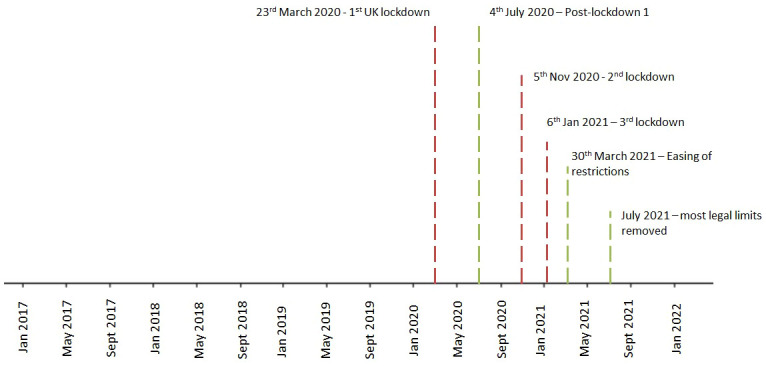
Dates of the observation period, dissected into periods distinguished by the changing social restrictions across the COVID-19 pandemic in the UK. None.
2.3. Outcomes
For aim 1, frequencies of screening/diagnostic tests and referrals relevant to each cancer were selected: they constitute the primary tools used in the cancer diagnostic pathways in the UK (see Supplementary Table S1, Additional File 1). For aim 1 and 3, IR of cancer diagnoses included first-ever (incident) diagnoses of breast, colorectal, lung and prostate cancer. For aim 2, cancer patients were characterised on all comorbidities and medication usage available within CPRD GOLD across the study periods. All diagnoses, observations, measurements, procedures and medications were defined based on SNOMED/Rx Norm/LOINC codes (as appropriate), in the OMOP-mapped data. A list of all codes used to define each outcome can be found in our associated shiny app: https://dpa-pde-oxford.shinyapps.io/CancerCovid_CohortDiagnosticsShiny_paper1/
2.4. Statistical analyses
2.4.1. Characterisations
Frequencies of screening, diagnostic tests, referrals, and interactions with the healthcare service, were calculated before, during and after the first lockdown in the general population and each cancer cohort. For cancer cohorts, counts were calculated in the 1-30 days; 31-180 days, and >180 days prior to index date (date of cancer diagnosis). Additionally, age at index date, sex, comorbidities, Charlson Comorbidity Index, Comorbidity Scale (CHADS2Vasc), Diabetes Complications and Severity Index (DCSI) and medication use were estimated for each cancer cohort using the FeatureExtraction R package (35). Continuous variables were summarised as means and standard deviation or variances; and categorical variables as counts and percentages. Significant differences in these variables across time-periods were estimated using standardised mean difference (SMD). Where frequency counts were less than five, data were censored to further enhance patient/practice confidentiality.
2.4.2. Incidence rates
Incidence rates (IR) with 95% confidence intervals (CI) were calculated for all outcomes and estimated annually, monthly, and within the pre-pandemic, lockdown, and extended post-lockdown periods across the entire study period (January 1st, 2017 to 1st December, 2021) using the IncidencePrevalence R package (36). Patients who entered the database within this time (also referred to as the denominator population) contributed time-at-risk up to their first screening/diagnostic test/referral/cancer diagnosis during the study period. Patients continued to contribute time-at-risk until the earliest of a record of screening/diagnostic test/referral/cancer diagnosis, transfer out of the database, end of the study period or death. Incidence rate ratios (IRR) with 95% CI were calculated to examine differences in incidence of the lockdown and extended post-lockdown periods compared to the 3 years prior to the pandemic. IR were stratified by age (in 20-year age bands) sex, and region in the UK (England, Northern Ireland, Scotland and Wales). Sensitivity analysis focussing on prevalent cancer diagnoses (removing the requirement of the diagnosis being the first in the person’s history) was performed. Incidence rate ratios (IRR) were calculated using the IR estimates across the post-lockdown periods divided by the reference period before lockdown.
2.4.3. Time series analyses
Negative binomial logistic regression models were used to predict cancer IR each month since the beginning of the pandemic and to use these predictions to compare with observed IR. To validate our method, models were trained on data from January, 2017 to February, 2019 and used to forecast IR from March, 2019 to March, 2020. To account for seasonality, month was fitted as a categorical variable, and time (in number of months since the beginning of the study) was fitted as a continuous variable, as has been used previously for forecasting diagnoses over the pandemic (37, 38). To validate our model fit, we examined whether the predicted versus observed counts and IR fell within 95% prediction intervals (PI). Results of our validation model can be visualised in Supplementary Figures S1, S2). Using this approach, we trained the model using pre-pandemic data from January, 2018 to February, 2020 to forecast expected counts and IR from March, 2020 onwards. Dates were chosen so that we had roughly equal number of months before vs. after lockdown. Number of ‘missing’ diagnoses were calculated as the difference between forecasted (observed) and expected number of incident cancer diagnoses during each time-period. The expected and observed counts were converted to IR by dividing the number of counts by the monthly observed person-month denominator population. The raw monthly cancer diagnosis counts were then extrapolated to the total population of the UK by multiplying the raw counts by a scalar representing the difference in population coverage of the CPRD database to the current UK population. All analyses were carried out using R Version 4.2.3.0
2.4.4. Patient and public involvement
No patients or members of the public were involved in the design, analysis or interpretation of this study or the reported data because the study aims to examine population-level trends and patterns rather than individual experiences or perspectives.
3. Results
3.1. Patient characteristics
Overall, there were 5,191,650 people eligible to be included in the denominator population from January, 2017. Total counts of patients excluded after applying the exclusion criteria for incidence estimates, are shown in Supplementary Table S2. The population structure of CPRD GOLD, in terms of age and sex was similar across the three time-periods (see Supplementary Tables S3, S4), but the proportion of practices from the different regions in the UK changed over time with fewer practices in England and greater proportion in Scotland during and after lockdown (Supplementary Figure S3). Demographics and total number of patients registered in CPRD in each of the time-periods, and diagnoses of first-ever breast, colorectal, lung and prostate cancer, are shown in Table 1. Mean age at date of diagnosis and sex distribution of cancer patients were largely the same across the three lockdown periods. Interactions with the healthcare system and routes to diagnosis were substantially reduced for patients receiving their cancer diagnosis during lockdown compared to those diagnosed pre-pandemic. Patients diagnosed after lockdown had fewer interactions with the healthcare system than pre-pandemic, though to a lesser extent than those diagnosed during lockdown (see Supplementary Tables S4–8). Across the lockdown periods there were no notable differences in comorbidities and medication prescriptions for those diagnosed with breast, colorectal, lung or prostate cancer (see Supplementary Tables S9–S12).
Table 1.
Counts, age and sex distribution in cancer cohorts diagnosed before, during and after lockdown.
| Pre-pandemic (Jan, 2017-Feb, 2020) | During lockdown (March, 2020-June, 2020) | After lockdown (July, 2020-Dec, 2021) | |
|---|---|---|---|
| Breast Cancer | |||
| (n) | 8815 | 459 | 2900 |
| Mean Age (variance) | 62.87 (188.2) | 62.07 (214.49)a | 62.98 (196.59)b |
| Sex (n, %) | |||
| Female | 8752 (99.3%) | 457 (~99%)c | 2884 (99.4%) |
| Male | 63 (0.7%) | <5 (~1%)c | 16 (0.6%) |
| Colorectal Cancer | |||
| (n) | 6025 | 349 | 2234 |
| Mean Age (variance) | 70.77 (149.15) | 69.99 (152.84) a | 70.19 (158.96) b |
| Sex (n, %) | |||
| Female | 2663 (44.2%) | 161 (46.1%) | 963 (43.1%) |
| Male | 3362 (55.8%) | 188 (53.9%) | 1271 (56.9%) |
| Lung Cancer | |||
| (n) | 5766 | 435 | 2102 |
| Mean Age (variance) | 72.23 (104.8) | 72.71 (106.96) a | 72.38 (100.42) b |
| Sex (n, %) | |||
| Female | 2898 (50.3%) | 215 (49.4%) | 1056 (50.2%) |
| Male | 2868 (49.7%) | 220 (50.6%) | 1046 (49.8%) |
| Prostate Cancer | |||
| (n) | 8103 | 427 | 2477 |
| Mean Age (variance) | 71.01 (82.72) | 70.83 (80.92) a | 71.58 (81.67) b |
| Sex (n, %) | |||
| Male | 8101 (100%) | 427 (100%) | 2476 (100%) |
a, Standardised Mean Difference between age at diagnosis before vs. during lockdown, >.1. b, Standardised Mean Difference between age at diagnosis before vs. after lockdown, >.1. c, proportions rounded to nearest 1% in order for frequencies <5 to remain masked. Cancer cohorts defined as first ever, incident cancer (excluding same cancer any time in history).
3.2. Incidence
3.2.1. Incidence of screening/diagnostic tests/referrals across lockdown periods
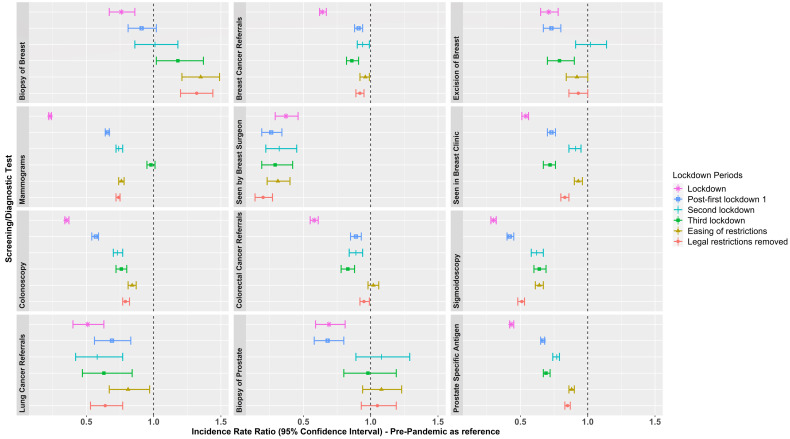
Figure 2 (and Supplementary Table S14) shows incidence rate ratios (IRR) of screening/diagnostic tests/referrals during the lockdown and extended post-lockdown periods compared to pre-pandemic rates. The number of routinely performed screening and diagnostic tests reduced during the first lockdown. Whilst rates of some screening/diagnostic tests increased across the extended post-lockdown periods (e.g. biopsy of breast IRR ranged from 0.76-1.35; and biopsy of prostate IRR ranged from 0.68-1.08), rates remained below those observed during the pre-pandemic era across nearly all extended post-lockdown periods, particularly so for colonoscopies (IRR ranged from 0.35-0.84), mammograms (IRR ranged from 0.23-0.98), and visits to breast surgeons (IRR ranged from 0.26-0.37). IR (per 100,000 person months or years as appropriate) of screening/diagnostic tests and referrals are shown in Supplementary Table S13, and Supplementary Figures S4-S7.
Figure 2.
Incidence Rate Ratios of screening/diagnostic tests and referrals in the extended post-lockdown periods compared to pre-pandemic rates. Lockdown periods defined as: Lockdown (March, 2020 to June, 2020); post-first lockdown (July, 2020 to October, 2020); second lockdown (Nov, 2020 to Dec, 2020); third lockdown (Jan, 2021 to March, 2021); easing of restrictions (April, 2021 to June, 2021); and most legal restrictions removed (July, 2021 to December, 2021).
3.2.2. Incidence of breast, colorectal, lung and prostate cancer across different lockdown periods
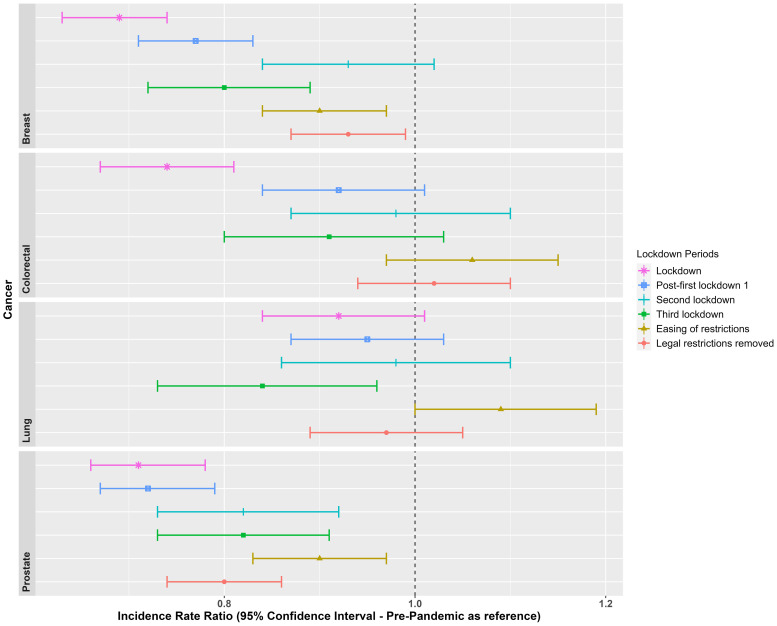
Figure 3 shows incidence rate ratios of the cancer diagnoses during the lockdown and extended post-lockdown periods compared to pre-pandemic rates. Diagnosis rates reduced during the initial lockdown for breast (IRR: 0.69 [95% CI: 0.63-0.74]), colorectal (IRR: 0.74 [95% CI: 0.67-0.81]); and prostate cancer (IRR: 0.71 [95% CI: 0.66-0.78]); but not for lung cancer (IRR: 0.92 [95% CI: 0.84-1.01]) (see Supplementary Table S15 for full results). Whilst diagnosis rates started to increase across the extended post-first lockdown periods (ranging from 0.72- 1.09), particularly during the second lockdown onwards for breast, colorectal and lung cancer, rates remained lower than the pre-pandemic era once legal restrictions were removed for breast (IRR: 0.93 [95% CI: 0.87-0.99]) and prostate cancer (IRR: 0.80 [95% CI: 0.74-0.86]).
Figure 3.
Incidence Rate Ratios for the cancer diagnoses, in each lockdown period, with the pre-pandemic era (Jan, 2017 to Feb, 2020) as a reference. Lockdown periods defined as: Lockdown (March, 2020 to June, 2020); post-first lockdown (July, 2020 to October, 2020); second lockdown (Nov, 2020 to Dec, 2020); third lockdown (Jan, 2021 to March, 2021); easing of restrictions (April, 2021 to June, 2021); and most legal restrictions removed (July, 2021 to December, 2021).
IR and IRR of cancer diagnoses overall and stratified by age and sex are included in Supplementary Figures S8–S10 and Supplementary Tables S16–S23. During the first lockdown, women aged 60-79 years were significantly underdiagnosed with breast cancer (IRR 0.65) compared to pre-pandemic levels, which improved once legal restrictions were lifted (IRR 0.9). The same age group was consistently underdiagnosed with colorectal cancer (IRR 0.66 during the first lockdown; IRR 0.6 during the third lockdown). Among men, those aged 80-150 years were most underdiagnosed with lung cancer during the third lockdown (IRR 0.66), while men aged 60-79 years consistently experienced underdiagnosis of lung cancer (IRR 0.84 during lockdown; 0.88 post-first lockdown). Men aged 40-59 years were consistently underdiagnosed with prostate cancer (IRR ranging from 0.49 to 0.83) across different lockdown periods. IR stratified by region across three lockdown periods showed slightly smaller IR in England for breast cancer post-lockdown, colorectal, lung and prostate cancer pre-pandemic, and prostate cancer post-lockdown, compared to the other UK regions (Supplementary Figure 11).
3.2.3. Forecasting expected cancer diagnosis rates after lockdown
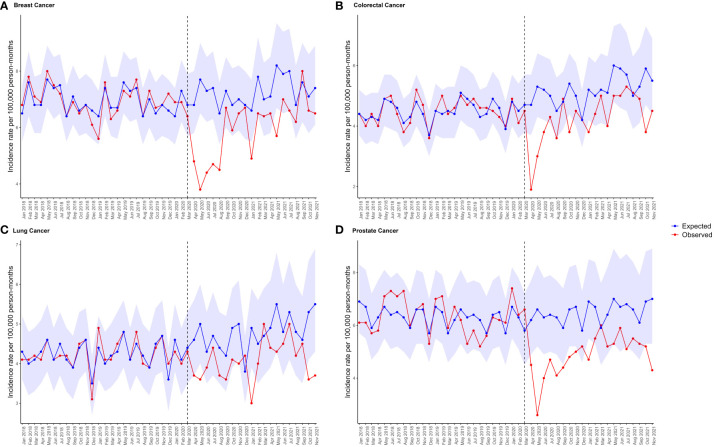
The forecasted cancer diagnosis rates after the lockdown were estimated using negative binomial regression models based on pre-pandemic data. Durbin-Watson statistics for all cancers were between 1.12-1.72, and plots of residuals show autocorrelation only for 22.5% of timepoints. Figure 4 shows that breast cancer incidence rates were significantly below expected levels for six months after the lockdown, and prostate cancer rates remained below expectations for a year (where points fall outside of the PI). The observed diagnosis rates during the first lockdown were much lower than expected for all four cancers, ranging from 15.4% to 33.9% reductions (Supplementary Table S24). Although the proportion of potential underdiagnoses decreased over time, diagnosis rates remained lower than expected in the last 2 months of follow-up for all bar breast cancer. Overall, the model estimated around 18,000 missed breast cancer diagnoses, 13,000 for colorectal cancer, 10,000 for lung cancer, and 21,000 for prostate cancer across the UK population from March, 2020 to December, 2021 (see Supplementary Table S25).
Figure 4.
Expected and observed IR per 100,000 person months of (A) breast cancer, (B) colorectal cancer, (C) lung cancer, and (D) prostate cancer in primary care records from CPRD GOLD UK. Points represent monthly IR. Expected rates (with 95% prediction intervals represented by the shaded areas) were calculated using negative binomial regression using observed data from January, 2017 to February, 2020 to estimate expected counts from March, 2020 to November, 2021. The vertical line indicates the start of lockdown in March, 2020.
When stratifying by age and sex, during the first lockdown women aged 60-79 years were most underdiagnosed for breast cancer (37.4%) and colorectal cancer (45.8%); whereas men aged 60-79 years were most underdiagnosed for lung cancer (31%); and men aged 40-59 years were most underdiagnosed for prostate cancer (35.3%).
Across the total observation period from March, 2020 to December, 2021, women aged 20 to 39 years had the greatest proportion of underdiagnoses of breast cancer (39.1%) (Supplementary Table S30). The greatest absolute number of potential missed breast cancer cases was for women aged 60 to 79 years (n=498, or n=10,751 extrapolated to the whole UK population). Women aged 60 to 79 years had the greatest proportion of underdiagnosed colorectal cancer (29.3%), with 260 estimated missed colorectal cancer cases (or n= 5,613 extrapolated to the whole UK population). For lung cancer, men aged 60 to 79 years had the greatest proportion of potential underdiagnoses (26.5%), reflecting potentially 310 missed lung cancer cases (or n= 6,693 extrapolated to the whole UK population). For prostate cancer, men aged 40 to 59 years suffered the greatest proportion of potential underdiagnoses (26.8%), reflecting potentially 104 missed prostate cancer cases (or n= 2,245 extrapolated to the whole UK population).
4. Discussion
4.1. Statement of principal findings
The findings of this study revealed a reduction in number of routinely performed screening, diagnostic tests, and referrals during the period from March, 2020 to December, 2021 compared to data from January, 2017 to February, 2020. Particularly during the first lockdown, there was a substantial decrease in mammograms, sigmoidoscopies, colonoscopies, and visits to breast surgeons by 77%, 70%, 65%, and 63% respectively, compared to pre-pandemic rates. Similar findings were reported in other countries, such as Slovenia (11) and Argentina (39).
Although some rates of screening, diagnostic tests, and referrals increased in the post-first lockdown period, they remained below pre-pandemic levels. For instance, mammograms, colonoscopies, and visits to breast surgeons were still reduced by 26%, 21%, and 80% respectively between July, 2021 and December, 2021. These findings contradict studies from Catalonia, Spain, and Canada, where mammograms and colonoscopies returned to expected levels by December, 2021 (18, 31). Similarly, data from Canada shows that breast cancer screening returned to pre-pandemic levels by December, 2020; and faecal occult blood tests for colorectal cancer by September, 2020 (7). In the UK, the data from CPRD GOLD did not show a recovery to pre-pandemic levels for screening and diagnostic tests. Possible explanations include the fact that the UK was the only European country to have additional lockdowns after the first, and that the NHS has experienced staff shortages and strikes over recent years impacting on its capacity to catch up.
Lockdown had varying effects on screening and referral procedures compared to diagnostic procedures in CPRD GOLD. Diagnostic procedures were not deprioritized after lockdown, except for visits to breast surgeons, indicating efforts to reduce the backlog. Screening, on the other hand, was more susceptible to postponement or lower prioritization, as it is used for asymptomatic individuals, as shown in the data.
4.2. Research in context
There are multiple explanations for the persistent reductions in screening, diagnostic tests, and referrals during extended lockdown periods. Variations in screening reductions may be related to the prevalence of COVID-19 restrictions/infections across countries (4). Reports indicate that the UK’s response to the pandemic was inadequate, resulting in a significant impact on the country and the need for subsequent lockdowns. The UK faced high infection rates, hospitalizations, and a substantial death toll (40). Healthcare resources were diverted from standard care, affecting cancer diagnostic pathways until December, 2021. Although rates showed some increase from March, 2020 to December, 2021, they were inconsistent. Data from a systematic review predicted a clearance of the screening backlog (specifically mammograms) in the USA within 12-24 weeks (4, 41), whereas our data suggest that even after 52-73 weeks, the queue was not cleared in the UK. That said, changes in screening methods (such as a switch from direct appointments to open invitations for routine mammograms) may have affected number of patients screened at least for breast cancer across this time period (42, 43).
Reduced screening and diagnostic tests lead to decreased cancer detection and diagnosis. Breast, colorectal, and prostate cancers were significantly underdiagnosed during lockdown and remained below expected levels until June, 2021 for breast cancer and until December, 2021 for prostate cancer. The expected effect of these reductions in rates is that diagnoses will be delayed, and prognosis worsened by these backlogs in diagnosis and treatment. These findings contradict a study from Catalonia, Spain, where breast cancer diagnoses recovered to pre-pandemic levels within this time-frame (18, 31). Belgium’s cancer registry data also showed recovery by June, 2020. Although incidence rates for colorectal and lung cancer returned to pre-pandemic levels, these rates likely represent missed diagnoses during lockdown, requiring substantial catch-up to compensate for the shortfall.
Our model predicts that prostate cancer had the highest number of missed cases, with an estimated 21,525 (25%) of expected cases missed from March, 2020 to December, 2021. Similar reductions in prostate cancer diagnosis were observed in other studies (18). Lung cancer was the least affected, with 14.2% of expected cases missed, which aligns with other reports (18, 31). It is possible this is because we have limited screening tools for lung cancer, leading to comparatively smaller diagnosis rates compared to other cancers. Though the increased use of chest radiography during COVID-19 infections may have inadvertently led to the identification of potential lung cancer symptoms and subsequent diagnosis (18). Stratification analyses revealed consistent underdiagnosis in specific age groups: women aged 60 to 79 for breast and colorectal cancer, men aged 60 to 79 for lung cancer, and men aged 40 to 59 years for prostate cancer. It is already known that we see a steep rise in risk for these cancers from these ages onwards (1, 44). These findings emphasize the urgency of prioritizing screening and diagnostics in these populations to detect the missed cases.
4.3. Strengths of the study
This study benefits from the strengths of CPRD GOLD, known for its extensive UK population coverage and comprehensive healthcare records (33), facilitating thorough phenotyping of screening, diagnostics, and cancer cases. The longitudinal nature of the database enabled an extended observation period beyond the typical one-year post-lockdown timeframe. Unlike most studies, our analysis covers screening and diagnostic rates up to December, 2021. In addition, our study uses innovative time-series forecasting to estimate the shortfall in cancer diagnosis rates, whilst the majority of research investigating the ramifications of COVID-19 on screening, diagnostics and incidence rates are descriptive in nature. Further research should explore additional data to assess if the UK has fully recovered from the rate shortfalls.
4.4. Limitations of the study
Although this study has many strengths it does have some limitations. First, as these data are derived from primary care and not linked to cancer registry data there were many screening and diagnostic tests of relevance to this study that were not captured in the database. This is common of studies using primary care data, as many diagnostic tests and procedures occur in hospital settings. Furthermore, cancer diagnoses may have shifted to hospital settings during the pandemic, and there may be a time-lag in recording cancer diagnoses in primary care records. Thus, it is likely that the estimated shortfall in screening/diagnostic tests, and cancer diagnosis rates in the present study, are underestimated. Relatedly, it would be informative to obtain information on cancer stage at time of diagnosis in order to determine the impact of the pandemic on disease severity. Linkage to cancer registry data would enable this analysis, but is beyond the scope of the current report. Similarly, it would be informative to investigate how the pandemic impacted on time to treatment. Not surprisingly, other reports have indicated that delayed treatment initiation was associated with increased mortality rates at 5- and 10-years after diagnosis, for all the cancers of focus here, particularly colorectal cancer (45). Linkage to cancer registry with treatment and cause of death data would allow us to replicate such analyses. That said, our own analyses have indicated that the pandemic impacted negatively on colorectal survival, with reductions in survivorship for patients diagnosed during the pandemic equivalent to returning to mortality seen in the first decade of the, 2000s (46). Second, the composition of patients and practices in the database have changed over time. Indeed, with the advent of the CPRD AURUM database, some practices were transferred out of GOLD and into AURUM, thus accounting for the reduced source population counts across time-points. Reassuringly, the IR of the cancers in the three broad time-periods across regions were largely similar, except for slightly smaller IR in England across some time-points, likely reflecting the change in population composition. Thirdly, the generalizability of findings is predominantly limited to Scotland and Wales, with less representation from England and Northern Ireland. Finally, as real-world evidence, causal inference is challenging, and other factors could have influenced the reduction in cancer diagnoses during lockdown, such as pre-existing trends in screening/diagnostic tests and cancer diagnoses, seasonality patterns, or COVID-19-related deaths. Whilst we did observe a pre-existing downward trend for visits to breast surgeons, there were no other observed pre-existing trends in our data, and our modelling statistically accounted for seasonal variability. However, data from Catalonia suggest a small proportion of missed diagnoses were attributed to COVID-19 deaths (47).
4.5. Conclusions: implications for clinicians and policymakers
Delays in diagnosis are likely to impact on cancer stage at time of diagnosis, treatment initiation, mortality rates and total years of life lost. To effectively tackle the existing backlog and potential long-term consequences on cancer survival, it may be necessary to implement strategies to identify those potential ~62,000 cancer cases missed. These could include raising public awareness through targeted campaigns aimed at particular age groups that have been most affected, encouraging participation in screening programs, and enhancing the coordination between primary care facilities and hospitals. Increases in screening and diagnostic testing may need to be increased in the months following December, 2021 to account for the observed shortfall in the UK. These measures are vital for effective public health intervention and reducing the impact of delayed diagnoses on cancer outcomes.
Data availability statement
Publicly available datasets were analysed in this study. This study is based in part on data from the Clinical Practice Research Datalink (CPRD) obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Patient- level data used in this study was obtained through an approved application to the CPRD (application number 22_002331) and is only available following an approval process to safeguard the confidentiality of patient data. Details on how to apply for data access can be found at https://cprd.com/data-access. Analytical code, and detailed definitions of algorithms for identifying the events are available in GitHub repositories (https://github.com/oxford-pharmacoepi/CancerCovid_CohortDiagnostics; https://github.com/oxford-pharmacoepi/CancerCovid_Characterisations; https://github.com/oxford-pharmacoepi/CancerCovid_IncidencePrevalence; https://github.com/oxford-pharmacoepi/CancerCovid_NegativeBinomialReg).
Ethics statement
The protocol for this research was approved by the independent scientific advisory committee for Medicine and Healthcare products Regulatory Agency database research (protocol number 22_002331). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because analyses were based on routinely collected electronic health records, contributed by general practitioners (GP) from the UK.
Author contributions
NB: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. MM: Conceptualization, Methodology, Supervision, Writing – review & editing. AJ: Writing – review & editing. DP-A: Conceptualization, Data curation, Funding acquisition, Supervision, Writing – review & editing. BR: Formal Analysis, Methodology, Writing – review & editing. DN: Conceptualization, Writing – review & editing. AD: Data curation, Writing – review & editing. WM: Data curation, Writing – review & editing. XC: Writing – review & editing. TC: Funding acquisition, Resources, Writing – review & editing. MC: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was partially funded by the European Health Data and Evidence Network (EHDEN) (grant number, 806968), the Optimal treatment for patients with solid tumours in Europe through Artificial Intelligence (OPTIMA) initiative (grant number, 101034347), and the Oxford NIHR Biomedical Research Centre. OPTIMA is funded through the IMI2 Joint Undertaking and is listed under grant agreement No. 101034347. IMI2 receives support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). IMI supports collaborative research projects and builds networks of industrial and academic experts in order to boost pharmaceutical innovation in Europe. The views communicated within are those of OPTIMA. Neither the IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained herein. The study funders had no role in the conceptualisation, design, data collection, analysis, decision to publish, or preparation of the manuscript. DPA receives funding from the UK National Institute for Health Research (NIHR) in the form of a senior research fellowship and the Oxford NIHR Biomedical Research Centre.
Conflict of interest
Author DP-A has performed paid consultancy services for the following commercial entities: European Medicines Agency; the Innovative Medicines Initiative; Amgen, Chiesi, and UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, and UCB Biopharma. Author NB has received paid consultancy fees for Theramex and Lindus Health; and provides research consultancy services for Sleep Universal Limited. All of these activities were independent from the research reported here.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1370862/full#supplementary-material
References
- 1. Cancer Research UK . Twenty most common causes of cancer death (2022). Available online at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality/common-cancers-compared#heading-Zero (Accessed 12th Oct 2022).
- 2. Loud JT, Murphy J. Cancer screening and early detection in the 21st century. Semin Oncol Nurs. (2017) 33:121–8. doi: 10.1016/j.soncn.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abraham SA, Agyare DF, Yeboa NK, Owusu-Sarpong AA, Banulanzeki ES, Doku DT, et al. The influence of COVID-19 pandemic on the health seeking behaviors of adults living with chronic conditions: a view through the health belief model. J Prim Care Community Health. (2023) 14. doi: 10.1177/21501319231159459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alkatout I, Biebl M, Momenimovahed Z, Giovannucci E, Hadavandsiri F, Salehiniya H, et al. Has COVID-19 affected cancer screening programs? A systematic review. Front Oncol. (2021) 11:675038. doi: 10.3389/fonc.2021.675038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh Q-D. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. (2021) 7:458–60. doi: 10.1001/jamaoncol.2020.7600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milgrom ZZ, Milgrom DP, Han Y, Hui SL, Haggstrom DA, Fisher CS, et al. Breast cancer screening, diagnosis, and surgery during the pre- and peri-pandemic: Experience of patients in a statewide health information exchange. Ann Surg Oncol. (2023) 30:2883–94. doi: 10.1245/s10434-023-13119-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Decker KM, Feely A, Bucher O, Singh H, Turner D, Lambert P. Evaluating the impact of the COVID-19 pandemic on cancer screening in a central Canadian province. Prev Med. (2022) 155:106961. doi: 10.1016/j.ypmed.2022.106961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bodkin H. Cancer referrals down by the 80 per cent in some areas as coronavirus fears keep patients from hospitals. Telegraph. (2020). https://www.telegraph.co.uk/news/2020/04/15/cancer-referrals-80-per-cent-areas-coronavirus-fears-keep-patients/ [accessed 19th March 2024]. [Google Scholar]
- 9. Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. (2020) 21:1023–34. doi: 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gathani T, Dodwell D, Horgan K. The impact of the first 2 years of the COVID-19 pandemic on breast cancer diagnoses: a population-based study in England. Br J Cancer. (2022) 128(3):481–3. doi: 10.1038/s41416-022-02054-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zadnik V, Mihor A, Tomsic S, Zagar T, Bric N, Lokar K, et al. Impact of COVID-19 on cancer diagnosis and management in Slovenia - preliminary results. Radiol Oncol. (2020) 54:329–34. doi: 10.2478/raon-2020-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ward ZJ, Walbaum M, Walbaum B, Guzman MJ, Jimenez de la Jara J, Nervi B, et al. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: a simulation-based analysis. Lancet Oncol. (2021) 22:1427–37. doi: 10.1016/S1470-2045(21)00426-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vrdoljak E, Balja MP, Marušić Z, Avirović M, Blažičević V, Tomasović ČChecktae, et al. COVID-19 pandemic effects on breast cancer diagnosis in Croatia: A population- and registry-based study. Oncologist. (2021) 26:e1156–e60. doi: 10.1002/onco.13791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Wyk AC, De Jager LJ, Razack R, Van Wyk SS, Kleinhans W, Simonds HM, et al. The initial impact of the COVID-19 pandemic on the diagnosis of new cancers at a large pathology laboratory in the public health sector, Western Cape Province, South Africa. S Afr Med J. (2021) 111:570–4. https://hdl.handle.net/10520/ejc-m_samj-v111-n6-a20 [PubMed] [Google Scholar]
- 15. Uyl-de Groot CA, Schuurman MS, Huijgens PC, Praagman J. Fewer cancer diagnoses during the COVID-19 epidemic according to diagnosis, age and region. TSG-Tijdschrift voor gezondheidswetenschappen. (2021) 99:1–8. doi: 10.1007/s12508-020-00289-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quarello P, Ferrari A, Mascarin M, Milano GM, Tornesello A, Bertolotti M, et al. Diagnostic delay in adolescents with cancer during COVID-19 pandemic: A new price for our patients to pay. J Adolesc Young Adult Oncol. (2022) 11:316–9. doi: 10.1089/jayao.2021.0057 [DOI] [PubMed] [Google Scholar]
- 17. Peacock HM, Tambuyzer T, Verdoodt F, Calay F, Poirel HA, De Schutter H, et al. Decline and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium: a year-long, population-level analysis. ESMO Open. (2021) 6:100197. doi: 10.1016/j.esmoop.2021.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mora N, Guiriguet C, Cantenys R, Méndez-Boo L, Marzo-Castillejo M, Benítez M, et al. Cancer diagnosis in primary care after second pandemic year in Catalonia: a time-series analysis of primary care electronic health records covering about 5 million people. Fam Pract. (2023) 40:183–7. doi: 10.1093/fampra/cmac083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Longcroft-Wheaton G, Tolfree N, Gangi A, Beable R, Bhandari P. Data from a large Western centre exploring the impact of COVID-19 pandemic on endoscopy services and cancer diagnosis. Frontline Gastroenterol. (2021) 12:193–9. doi: 10.1136/flgastro-2020-101543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu C, Piao H, Zhang T, Yang D, Li X, Tang X. Delayed diagnosis and treatment of cancer patients during the COVID-19 pandemic in Henan, China: An interrupted time series analysis. Front Public Health. (2022) 10:881718. doi: 10.3389/fpubh.2022.881718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linck PA, Garnier C, Depetiteville MP, MacGrogan G, Mathoulin-Pélissier S, Quénel-Tueux N, et al. Impact of the COVID-19 lockdown in France on the diagnosis and staging of breast cancers in a tertiary cancer centre. Eur Radiol. (2022) 32:1644–51. doi: 10.1007/s00330-021-08264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knoll K, Reiser E, Leitner K, Kögl J, Ebner C, Marth C, et al. The impact of COVID-19 pandemic on the rate of newly diagnosed gynecological and breast cancers: a tertiary center perspective. Arch Gynecol Obstet. (2022) 305:945–53. doi: 10.1007/s00404-021-06259-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HY, Kim MG, Kang MR, Yang JH, Shin MH, Kweon SS. No evidence of delay in colorectal cancer diagnosis during the COVID-19 pandemic in Gwangju and Jeonnam, unclosed areas in Korea. Epidemiol Health. (2022) 44:e2022092. doi: 10.4178/epih.e2022092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kempf E, Lamé G, Layese R, Priou S, Chatellier G, Chaieb H, et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: A persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer. (2021) 150:260–7. doi: 10.1016/j.ejca.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaltofen T, Hagemann F, Harbeck N, Wuerstlein R, Kost BP, Burges A, et al. Changes in gynecologic and breast cancer diagnoses during the first wave of the COVID-19 pandemic: analysis from a tertiary academic gyneco-oncological center in Germany. Arch Gynecol Obstet. (2022) 305:713–8. doi: 10.1007/s00404-021-06211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacob L, Kalder M, Kostev K. Decrease in the number of patients diagnosed with cancer during the COVID-19 pandemic in Germany. J Cancer Res Clin Oncol. (2022) 148:3117–23. doi: 10.1007/s00432-022-03922-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrara G, De Vincentiis L, Ambrosini-Spaltro A, Barbareschi M, Bertolini V, Contato E, et al. Cancer diagnostic delay in northern and central Italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic. Am J Clin Pathol. (2021) 155:64–8. doi: 10.1093/ajcp/aqaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erdmann F, Wellbrock M, Trübenbach C, Spix C, Schrappe M, Schüz J, et al. Impact of the COVID-19 pandemic on incidence, time of diagnosis and delivery of healthcare among paediatric oncology patients in Germany in 2020: Evidence from the German Childhood Cancer Registry and a qualitative survey. Lancet Reg Health Eur. (2021) 9:100188. doi: 10.1016/j.lanepe.2021.100188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dinmohamed AG, Visser O, Verhoeven RHA, Louwman MWJ, van Nederveen FH, Willems SM, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. (2020) 21:750–1. doi: 10.1016/S1470-2045(20)30265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Vincentiis L, Carr RA, Mariani MP, Ferrara G. Cancer diagnostic rates during the 2020 'lockdown', due to COVID-19 pandemic, compared with the 2018-2019: an audit study from cellular pathology. J Clin Pathol. (2021) 74:187–9. doi: 10.1136/jclinpath-2020-206833 [DOI] [PubMed] [Google Scholar]
- 31. Coma E, Guiriguet C, Mora N, Marzo-Castillejo M, Benitez M, Mendez-Boo L, et al. Impact of the COVID-19 pandemic and related control measures on cancer diagnosis in Catalonia: a time-series analysis of primary care electronic health records covering about five million people. BMJ Open. (2021) 11:e047567. doi: 10.1136/bmjopen-2020-047567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cantini L, Mentrasti G, Russo GL, Signorelli D, Pasello G, Rijavec E, et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): fewer cases and higher stages from a real-world scenario. ESMO Open. (2022) 7:100406. doi: 10.1016/j.esmoop.2022.100406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, Van Staa T, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. (2015) 44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voss EA, Makadia R, Matcho A, Ma Q, Knoll C, Schuemie M, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc. (2015) 22:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schuemie M, Suchard M, Ryan P, Reps J, Sena A. Feature extraction. R Package Version 3.2.0 [online]. (2021). Available at: https://ohdsi.github.io/FeatureExtraction/ (Accessed 23rd June 2023).
- 36. Burn E, Raventos B, Catala M, Du M, Guo Y, Black A, et al. Incidence prevalence: estimate incidence and prevalence using the OMOP Common Data Model. R Package Verion 0.4.0 [online]. (2023). Available at: https://cran.r-project.org/web/packages/IncidencePrevalence/index.html (Accessed 23rd June 2023).
- 37. Raventós B, Pistillo A, Reyes C, Fernández-Bertolín S, Aragón M, Berenguera A, et al. Impact of the COVID-19 pandemic on diagnoses of common mental health disorders in adults in Catalonia, Spain: a population-based cohort study. BMJ Open. (2022) 12:e057866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams R, Jenkins DA, Ashcroft DM, Brown B, Campbell S, Carr MJ, et al. Diagnosis of physical and mental health conditions in primary care during the COVID-19 pandemic: a retrospective cohort study. Lancet Public Health. (2020) 5:e543-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bozovich GE, Alves De Lima A, Fosco M, Burgos LM, Martinez R, Dupuy De Lome R, et al. Collateral damage of COVID-19 pandemic in private healthcare centres of Argentina. Medicina (B Aires). (2020) 80(Suppl 3):37–41. [PubMed] [Google Scholar]
- 40. World Health Organization (WHO) . Coronavirus disease (COVID-19) pandemic [online]. (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed 23rd June 2023). [Google Scholar]
- 41. Song H, Bergman A, Chen AT, Ellis D, David G, Friedman AB, et al. Disruptions in preventive care: Mammograms during the COVID‐19 pandemic. Health Services Res. (2021) 56:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. NHS . Breast Cancer Screening Recovery Services [online]. (2020). Available at: https://selondonccg.nhs.uk/wp-content/uploads/2020/11/Breast-screening-new-tel-no.pdf (Accessed 26th June 2023).
- 43. Rossi PG, Giordano L. Mammography screening: please don't be vague, tell me when I should come! Lancet Oncol. (2017) 18:848–9. [DOI] [PubMed] [Google Scholar]
- 44. Cancer Research UK . Statistics by cancer type [online]. (2021). Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type (Accessed 26th June 2023).
- 45. Cone EB, Marchese M, Paciotti M, Nguyen D-D, Nabi J, Cole AP, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. (2020) 3:e2030072-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barclay NL, Burkard T, Burn E, Delmestri A, Dominguez AM, Golozar A, et al. The impact of the COVID-19 pandemic on short-term cancer survival in the United Kingdom: a cohort analysis. medRxiv. (2023) 2023.09. 14.23295563. [Google Scholar]
- 47. Pifarre IAH, Vidal-Alaball J, Gil J, Lopez F, Nicodemo C, Saez M. Missing Diagnoses during the COVID-19 Pandemic: A Year in Review. Int J Environ Res Public Health. (2021) 18:5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analysed in this study. This study is based in part on data from the Clinical Practice Research Datalink (CPRD) obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Patient- level data used in this study was obtained through an approved application to the CPRD (application number 22_002331) and is only available following an approval process to safeguard the confidentiality of patient data. Details on how to apply for data access can be found at https://cprd.com/data-access. Analytical code, and detailed definitions of algorithms for identifying the events are available in GitHub repositories (https://github.com/oxford-pharmacoepi/CancerCovid_CohortDiagnostics; https://github.com/oxford-pharmacoepi/CancerCovid_Characterisations; https://github.com/oxford-pharmacoepi/CancerCovid_IncidencePrevalence; https://github.com/oxford-pharmacoepi/CancerCovid_NegativeBinomialReg).