Abstract
This review aims to evaluate the therapeutic potential of green tea (GT), scientifically named Camellia sinensis, in treating eye diseases. We provide an overview of the ingredients and traditional use of Camellia sinensis, followed by a detailed discussion of its therapeutic uses in various eye diseases, including ocular surface diseases (allergic diseases, dry eye, pterygium, and infections), cataract, glaucoma, uveitis, retinal diseases, and optic nerve diseases. The pharmacologic activities related to ocular diseases, such as anti-vascular endothelial growth factor, aldose reductase inhibitor activity, anti-bacterial, anti-inflammatory, and antioxidant effects are also explored in this review. The dose and route of administration of GT in various studies are discussed. Safety issues related to the use of GT, such as the side effects associated with high doses and long-term use, are also addressed. The review highlights the potential of GT as a natural therapeutic agent for a variety of ocular diseases. Its various pharmacologic activities make it a promising treatment option. However, more well-designed studies are needed to determine the optimal dose and route of administration and to assess its long-term safety and efficacy. Overall, GT appears to be a promising adjunct therapy for various ocular diseases.
Keywords: Eye diseases, Ocular diseases, Green tea, Camellia sinensis, Catechin, Traditional Persian medicine, Integrative medicine
1. Introduction
Eye diseases are one of the leading causes of vision loss and blindness worldwide, and their prevalence is increasing due to the population aging and increased life expectancy [1]. According to the World Health Organization (WHO), around 253 million people are visually impaired, and 36 million people are blind globally [2,3]. Eye diseases include a variety of conditions such as age-related macular degeneration, diabetic retinopathy, glaucoma, cataract, and dry eye syndrome, among others [4]. These conditions impose a significant burden on patients' quality of life and the healthcare system, making it necessary to develop effective treatment options [5,6].
The historical use of medicinal plants for treating eye diseases spans traditional medicine, with ongoing exploration of their therapeutic potential in modern medicine [7]. Many medicinal plants have been shown to have beneficial effects on eye health due to their various bioactive compounds such as antioxidants, anti-inflammatory agents, phytochemicals, vitamins, minerals, and other health-promoting substances [7]. Among these medicinal plants, Green Tea, scientifically named Camellia sinensis, has gained attention due to its potential therapeutic effects on eye diseases.
Green Tea (GT)is a plant commonly used to make tea and is native to East Asia [8]. GT is made from unfermented leaves and is a rich source of polyphenols, particularly catechins, which are known for their antioxidant and anti-inflammatory effects [9]. The potential health benefits of GT have been extensively studied, and it has been shown to have beneficial effects on various health conditions, including cardiovascular diseases, cancer, and diabetes [10]. Despite multiple studies on the potential therapeutic effects of GT in eye diseases, no report has comprehensively reviewed these investigations.
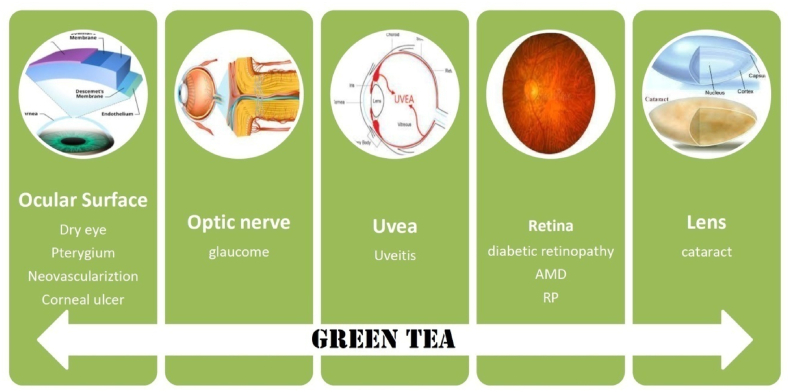
This review aims to provide an overview of the potential therapeutic effects of GT in eye diseases (Fig. 1). The review will summarize the current knowledge on the bioactive compounds of GT, the preclinical and clinical evidence of its therapeutic potential, and the mechanisms of action involved. This review will provide a comprehensive understanding of the potential benefits of GT in the management of eye diseases and will help in the development of new treatment options.
Fig. 1.
The potential beneficial effects of green tea in eye diseases. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The methodology of the study relied on a comprehensive search and analysis of available literature on the potential therapeutic effects of GT in various eye diseases. The search process involved querying electronic databases, namely PubMed, Scopus, Web of Science, and Google Scholar, covering the period from the inception of the databases to June 2023. The selected keywords for the search encompassed "Camellia sinensis" and "green tea".
The search process and data extraction were conducted by two independent reviewers to minimize bias and increase the reliability of the study. The selected literature was screened, and relevant data on the pharmacological activities, therapeutic uses, dose, route of administration, and safety issues associated with GT in various ocular diseases were extracted and synthesized. The final synthesis was based on a critical analysis of the selected literature, and the results were presented in a comprehensive narrative review.
2. Major constituents
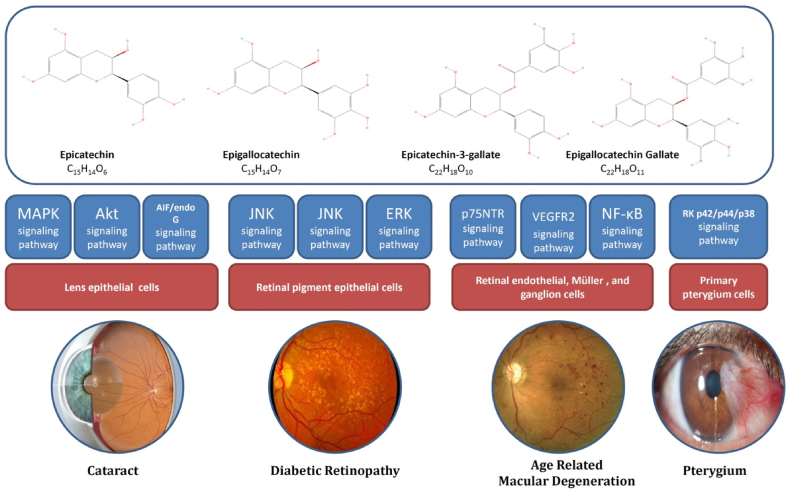
GT is composed of numerous compounds, primarily polyphenols. Among the polyphenols, catechin has been extensively investigateddue to its numerous pharmacological properties. The four main catechins in GT include epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epicatechin (EC) (Fig. 2).
Fig. 2.
The major constituents of GT and involved signaling pathways in their effects on eye diseases. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
EGCG is the most abundant and biologically active catechin found in GT, renowned for its potent antioxidant properties and different health benefits [11]. Its ability to scavenge free radicals and inhibit oxidative stress has garnered significant attention in various fields of research, including cancer prevention, cardiovascular health, and eye diseases. Numerous studies have highlighted EGCG's potential in modulating cellular signaling pathways, promoting apoptosis in cancer cells, and enhancing metabolic processes, making it a focal point in the exploration of GT's therapeutic effects [12]. With its potent antioxidant properties, EGCG has emerged as a promising candidate for combating various eye diseases, including age-related macular degeneration (AMD), diabetic retinopathy, and glaucoma. Additionally, EGCG's anti-inflammatory properties may help alleviate symptoms associated with ocular inflammation and contribute to overall ocular wellness [13].
Another important component of GT is caffeine, although in lower amounts compared to coffee. Caffeine acts as a stimulant that can improve alertness and concentration. Additionally, GT contains l-theanine, an amino acid that works synergistically with caffeine to provide a unique combination of focused energy and calming effects.
Beyond catechins, caffeine, and l-theanine, GT also contains various vitamins, minerals, and polyphenols, each contributing to its overall health-promoting properties. These include vitamins C and E, as well as minerals like manganese and zinc. The polyphenols found in GT are known for their potential to health support. The pharmacological effects of GT are not only determined by the percentage of compound abundance but also by environmental factors, such as plant growth conditions [14,15].
3. Traditional medicine applications
GT has been traditionally used in different formulations for its preventive and therapeutic effects on chronic diseases. In Traditional Chinese Medicine (TCM), it is believed to help prevent cardiovascular diseases, cancer (such as stomach, oral, and esophageal cancers), and metabolic syndrome [16,17]. In Ayurvedic medicine, GT has been used for gastrointestinal diseases [18].TCM has employed GT in medicinal applications for eye diseases such as glaucoma, diabetic retinopathy, and dry eye disease [[19], [20], [21]]. Traditional Persian medicine also recognized the therapeutic benefits of GT, using it for eye swelling and infection [22].
4. Pharmacologic activities related to ocular disease
4.1. Aldose reductase inhibitor activity
The polyol pathway is a significant contributor to the onset of diabetes, with Aldose reductase being the crucial enzyme involved in diabetic complications, including cataracts and retinopathy [23]. Inhibiting this enzyme can be an effective strategy for treating these conditions. GT has been shown in various studies to possess aldose reductase inhibitor activity, indicating its potential usefulness in treating retinopathy and cataracts [[24], [25], [26]].
In a 2020 report, researchers explored the inhibitory effects of GT extract (GTE) on aldose reductase by chromatographic fractionation. They showed that besides EGCG and gallic acid, other components may exhibit the differential inhibition of aldose reductase [24].Another study investigated the inhibitory activity of water extract from GT leaves on aldose reductase and identified the flavone-glycoside isoquercitrin as a potent inhibitor [25]. Isoquercitrin exhibited a mixed mode of uncompetitive and noncompetitive inhibitions with an IC50 of 1 x 10^(−6) M. In rat sciatic nerve tissue, isoquercitrin at 5 x 10^(−4) M inhibited sorbitol accumulation by 38% in the presence of high glucose concentrations (30 mM) [25].
4.2. Anti-VEGF activity
Angiogenesis and the growth of new blood vessels are involved in the development of certain eye diseases and blindness, with vascular endothelial growth factor (VEGF) being a significant factor in its pathology. VEGF plays a role in diseases such as diabetic retinopathy, age-related macular degeneration, and diabetic macula edema [[27], [28], [29]]. The introduction of anti-VEGF therapy, including drugs like Bevacizumab, has opened up new opportunities in the treatment of eye diseases [30]. Researchers have explored the effect of GT on angiogenesis signaling pathways, with GT being referred to as an anti-VEGF and a potential treatment for eye diseases [31,32].
A study investigated the impact of GTE and its components on VEGF-stimulated human endothelial cells and revealed that GTE induced apoptosis with a balanced metabolomics spectrum [31]. Another study showed that GTE exhibits anti-angiogenic properties attributed to its polyphenolic compound, EGCG, which influences cellular proteome and signalome, inhibiting the VEGF family and altering miRNA expression associated with angiogenesis in various cancer types [32].
4.3. Antibacterial activity
Bacterial infections can lead to eye infections, which can result from bacterial invasion following surgery or through the bloodstream [33]. Conjunctivitis and blepharitis are examples of diseases caused by bacteria [34,35]. Although antibiotics are a treatment option, it is important to consider bacterial resistance. To address this issue, compounds from medicinal plants with antibacterial activity are considered [36]. Multiple researches have been conducted in the field of medicinal plants, specifically on the antibacterial properties of GT against various types of bacteria, which can aid in treating bacterial eye infections and preventing biofilm formation [[37], [38], [39]]. Friedman discussed the antibacterial, antiviral, and antifungal activities of tea flavonoids, emphasizing their potential in combatting various pathogens [37]. Blanco and colleagues demonstrate EGCG's inhibitory effects on bacterial gelatinase activity and invasion, as well as its ability to prevent biofilm formation by ocular staphylococcal isolates, suggesting its potential as an antimicrobial agent [38,39].
4.4. Anti-inflammatory activity
Eye diseases such as dry eye and retinopathy are associated with inflammation [40,41]. The first-line treatment for these diseases involves the use of corticosteroids [42]. Recently, there have been studies on targeting biological compounds that are involved in inflammation, including lymphocytes, cytokines, and TNF-α [42]. Traditional medicine offers several herbs that possess anti-inflammatory properties. In vitro and in vivo studies have demonstrated that the GT plant can help alleviate eye diseases by reducing inflammation [[43], [44], [45]].
Thichanpiang and Wongprasert revealed EGCG's ability to attenuate TNF-α-induced expression of intercellular adhesion molecule-1 and reduce monocyte adhesion to retinal pigment epithelial cells [43]. Ren and colleagues showcased the protective role of GTE against LPS-induced retinal inflammation in rats, emphasizing its potential to mitigate inflammatory responses [44]. Additionally, Zhang and co-authors highlighted EGCG's capacity to attenuate lipopolysaccharide-induced inflammation in human retinal endothelial cells, suggesting its therapeutic potential in retinal inflammatory conditions [45]. These studies demonstrated the anti-inflammatory effects of GT polyphenol EGCG in various retinal contexts.
4.5. Antioxidant activity
Oxidative stress is involved in the development of glaucoma, AMD, and diabetic retinopathy [46]. It causes damage to eye tissues, autophagy, and free radical production [47]. Antioxidant treatments, such as antioxidant vitamins and natural antioxidant compounds like curcumin and zeaxanthin, can be used to prevent oxidative stress [47]. Studies have shown that GT is also an effective natural antioxidant compound for treating eye diseases [[48], [49], [50]].
Gupta et al. showed that GT protects against selenite-induced oxidative stress in experimental cataractogenesis [48]. Yang and colleagues highlighted the potent antioxidant properties of GT catechins in ameliorating sodium iodate-induced retinal degeneration in rats [49]. Furthermore, Wu et al. emphasized the protective role of EGCGin promoting the survival of human lens epithelial cells against UVB irradiation through specific signaling pathways [50]. Overall, these findings suggested the potential of GT in preventing oxidative damage and associated ocular conditions.
5. Therapeutic uses in eye diseases
5.1. Clinical studies
Clinical trials investigating the efficacy of GTE in various ophthalmic diseases have provided valuable insights into its potential therapeutic benefits (Table 1). In a study by Doğan et al., it was observed that black tea consumption did not yield immediate effects on macular microcirculation in healthy individuals, suggesting limited impact on retinal vascular health [51]. However, other trials have shown more promising results. Gasiunas & Galgauskas conducted a trial involving individuals with elevated intraocular pressure or predisposition to glaucoma, indicating that moderate consumption of GT or its concentrated extracts could potentially mitigate these conditions [52]. This highlights the importance of further exploration into the mechanisms underlying the beneficial effects of GT components on ocular health, particularly in conditions like glaucoma where early intervention is crucial for preserving vision.
Table 1.
Clinical trials showing beneficial effects of GT and its ingredients on ocular diseases.
| ophthalmic disease | number of participants | intervention | final effect | reference |
|---|---|---|---|---|
| retinal microvasculature | 60 | black tea (2 mg/250 mL water) | Black tea consumption did not have any immediate effect on macular microcirculation in healthy individuals. | [51] |
| glaucoma | 43 | GTE and EGCG extract (capsule 400 mg) | Individuals with elevated intraocular pressure or risk factors for glaucoma development may benefit from moderate consumption of GT or its concentrated extracts. | [52] |
| dry eye and meibomian gland dysfunction (MGD) | 60 | topical GTE (1 mg/5 mL distilled water) | Topical application of GTE is a safe, effective, and well-tolerated treatment option for mild to moderate evaporative dry eyes and meibomian gland dysfunction. | [53] |
| diabetic retinopathy | 200 | Chinese GT (regularly drink every week for at least one year) | Regular consumption of Chinese GT for at least one year may decrease the risk of diabetic retinopathy by about 50% compared to those who do not consume it. This suggests that regular consumption of Chinese GT may be a new approach to prevent diabetic retinopathy. | [54] |
| ocular hypertension (OHT) and open-angle glaucoma (OAG) | 18 OHT patients and 18 OAG patients | EGCG oral treatment (200 mg/day) | Although this study did not provide evidence for the long-term benefits of EGCG supplementation in open-angle glaucoma, and the observed effect is small, the results suggest that EGCG may have a positive impact on inner retinal function in eyes with early to moderately advanced glaucomatous damage. | [55] |
EGCG: epigallocatechin-3-gallate.
Moreover, research on dry eye and meibomian gland dysfunction has demonstrated the efficacy of topical GTE as a safe and effective treatment option, as evidenced by Nejabat et al. [53]. Similarly, Ma et al. found that regular consumption of Chinese GT may significantly reduce the risk of diabetic retinopathy, offering a potential preventive strategy for individuals with diabetes [54]. Additionally, while the study by Falsini et al. did not establish long-term benefits of EGCG supplementation in open-angle glaucoma, it suggested a potential positive impact on inner retinal function in early to moderately advanced glaucomatous damage [55]. These findings collectively underscore the potential of GTE, including its constituents like EGCG, as adjunctive or preventive measures in managing various ophthalmic conditions, warranting further investigation and clinical exploration.
5.2. Preclinical studies
5.2.1. Ocular surface diseases (OSD)
Ocular surface diseases refer to various eye diseases that primarily affect the surface layers of the eye, such as the cornea, conjunctiva, and lacrimal glands [56]. These diseases can lead to vision loss or impairment [57]. Some of the most common ocular surface diseases are dry eye disease, pterygium, corneal ulcer, and corneal neovascularization.
5.2.1.1. Dry eye
Dry eye disease is a prevalent ocular surface disease that can result in vision impairment and tear film instability due to increased osmolarity [58]. The pathogenesis of the disease is associated with inflammation, which can activate inflammatory signaling pathways and result in the release of inflammatory cytokines [59]. There are available pharmaceutical treatments, including topical cyclosporine A 0.05% and lifitegrast 5% [60]. Traditional medicine suggests alternative treatments that should be explored through more robust studies [61]. Multiple studies support the potential benefits of the use of GT in dry eye.
A double-blind, randomized, controlled clinical trial involving 60 participants divided into control and intervention groups was conducted. All participants received standard treatment of artificial tear drops three times a day for a month. The intervention group received topical GTE in addition to the standard treatment. The study results suggest that the use of topical GTE, which has anti-inflammatory properties, can be a safe and effective treatment for dry eye and meibomian gland [53].
In an in-vivo study, mice with dry eye disease were treated with topical formulations of vehicle, EGCG 0.1%, and 0.01%. Both formulations showed anti-inflammatory effects by decreasing IL-1β and chemokine ligand 2 while being non-cytotoxic to corneal epithelial cells and reducing VEGF-A and VEGF-D levels in the cornea. Additionally, topical EGCG 0.1% was found to have a beneficial effect in reducing clinical symptoms of dry eye disease. These results suggest that topical EGCG is effective in treating dry eye disease in mice [62].
An investigation was conducted to examine the antioxidant and anti-inflammatory properties of EGCG in dry eye disease. Human corneal epithelial cells were subjected to interleukin-1β in a hyperosmolar culture medium during an in-vitro study. EGCG was applied to the culture medium, and a decrease in cytokines and chemokines produced in the cell culture medium was found, depending on the dose of EGCG. In contrast, EGCG did not affect the cellular metabolic activity. EGCG may function as a therapeutic agent for inflammations and oxidative stress [63].
5.2.1.2. Pterygium
Pterygium is a condition characterized by conjunctival degeneration and the triangular growth of conjunctiva towards the cornea [64]. The risk factors for this disease include age [65], male gender [66], and exposure to ultraviolet radiation [67]. Surgical intervention, chemotherapy agents (Thiothepa), and alkylating drugs (Mitomycin C) are among the available treatments [68,69]. Natural compounds, including curcumin, are also being investigated for this purpose and should be explored through more robust studies [70].
An in-vivo study examined the effects of catechin extract (16.25 μg/mL) and EGCG (25 μM) on human primary pterygium cells. The results showed improved migration and survival of these cells, while no effect was observed on cell apoptosis. These findings suggest that catechin extract and EGCG may serve as a potential treatment for pterygium [71].
5.2.1.3. Corneal ulcer
Corneal ulcer is a condition that results from infection caused by microorganisms such as bacteria, fungi, and parasites [72]. Factors that lead to corneal ulcers include the use of contact lenses, excessive use of topical antibiotics, and corneal trauma [73]. The available treatments for corneal ulcers differ depending on the type of infection and microorganism [74,75]. An in-vitro study has evaluated the effect of EGCG on corneal ulcer. Human corneal fibroblast cells were cultured in a three-dimensional (3D) collagen gel which was treated by EGCG. The study showed that EGCG can inhibit the inflammatory factor IL-1β, leading to the reduction of urokinase-type plasminogen activators in corneal fibroblasts [76].
5.2.2. Neovascularization
Eye neovascularization is a condition characterized by an imbalance between pro-angiogenic and anti-angiogenic factors, leading to an excess of the former [77]. This condition can occur in various parts of the eye, including the cornea, retina, and iris, and can cause irreversible damage, including blindness [78,79]. While there are major biological medicinal products available on the market, such as VEGF antibodies [77], herbal products have also been shown to inhibit neovascularization. EGCG, for instance, has been found to inhibit various angiogenic factors, including VEGF, COX2, matrix metalloproteinases, IL-1β, and NF-κB, which are known to play a critical role in corneal neovascularization [80]. Therefore, EGCG could serve as an alternative to biological drugs for the treatment of this condition.
5.2.3. Glaucoma
Glaucoma is a significant cause of irreversible blindness in the world. Aging, increased intraocular pressure, and genetics are among the risk factors associated with this disease [[81], [82], [83]]. Two types of glaucoma can be identified: open-angle and closed-angle glaucoma [84].
In glaucoma, various signaling pathways are implicated, including the inflammation-related NF κB pathway. An in vivo study investigated the effect of EGCG at a dose of 50 mg/kg/day for two periods of 14 and 28 days on a rat model of glaucoma. The results showed that EGCG decreased optic injury and improved inflammatory protein levels by suppressing the NF κB signaling pathway. In addition, EGCG restored the balance of T lymphocyte cell division rate [85].
Glaucoma is a leading cause of irreversible blindness that can damage optic nerve cells, including retinal ganglion cells, which play a critical role in vision [86]. Therapeutic methods can help reduce the destruction of these cells. In one study, the neuroprotective effect of EGCG on retinal ganglion cells was investigated in rats with high intraocular pressure. Rats that received EGCG showed a significant improvement in retinal ganglion cell survival [87]. Another study examined the effect of GTE on retinal ganglion cell damage caused by ischemic reperfusion in rats. The results showed that intragastric administration of GTE increased retinal ganglion cell survival by reducing apoptosis, inflammatory factors, and oxidative stress, as well as improving pupillary light reflex [88]. These findings suggest that GT may be an effective treatment for reducing neuron degeneration.
One of the most significant types of glaucoma is primary open-angle glaucoma, which can occur with or without increased intraocular pressure [89]. The trabecular meshwork/Schlemm's canal (TM/SC) pathway is one of the mechanisms that contribute to intraocular pressure. Oxidative stress and endoplasmic reticulum (ER) stress can disrupt this pathway and increase eye pressure. In an in vivo study, the therapeutic potential of EGCG was investigated on ER stress markers and HTM cell viability. Results indicate the efficacy of EGCG in treating primary open-angle glaucoma [90].
In a clinical study, the effectiveness of GT and catechins in treating glaucoma was investigated. In a randomized, placebo-controlled trial with 43 healthy participants, the GTE and EGCG extract groups had a significant reduction in intraocular pressure compared to the control group. This suggests the potential of GT and catechins in treating glaucoma [91].
In another study, the short-term effect of EGCG on inner retinal function in individuals with ocular hypertension and glaucoma was investigated. The study included 18 individuals with glaucoma and 18 individuals with ocular hypertension who received a placebo or oral EGCG (200 mg/day) in addition to standard IOP reduction treatment for three months. Results suggest that EGCG has a positive effect on inner retinal function in people with glaucoma [55].
5.2.4. Uveitis
Uveitis, an inflammatory condition within the eye, is the third leading cause of blindness globally [92]. Risk factors for developing uveitis include age, race, genetic factors, environmental factors, and medication side effects [93,94]. Inflammatory cytokines are associated with the onset of uveitis and can be targeted for therapy. Corticosteroids are the main cornerstone of disease treatment. Anti-TNF agents and monoclonal antibodies, including Adalimumab, Infliximab, and Daclizumab, are often necessary for the treatment of chronic, non-infectious patients, especially in ones associated with systemic inflammatory diseases [95]. Herbal remedies may also be considered for treating uveitis [96].
In an in-vivo experiment, researchers investigated the potential effect of GTE at a concentration of 550 mg/kg on endotoxin-induced uveitis (EIU)in rats by injection of 1 mg/kg of lipopolysaccharide (LPS) into one footpad. LPS injection led to an increase in cytokine and chemokine production in the aqueous humor, edema, and severe hyperemia in the iris. Oral administration of GTE was found to down-regulate the LPS receptor, leading to a decrease in the production of inflammatory factors such as TNF-α, IL-6, and macrophages. These results suggest that GTE could be a therapeutic candidate for uveitis [97].
Another study investigated the effects of GTE on signaling pathways associated with EIU in rats. The study found that the extract led to a decrease in plasma phospholipids and an increase in membrane phospholipids and free antioxidants, as well as a reduction in inflammatory prostaglandins and corticosteroids and a decrease in fatty acid metabolites in the retina. These results indicate that GTE may have anti-inflammatory and antioxidant effects and could suppress inflammatory signaling pathways [98].
A study was conducted to investigate the impact of GTE and EGCG on mice with autoimmune uveoretinitis. The findings indicate a reduction in the expression of pro-inflammatory genes related to Th-17 such as IL-6, IL-17A, and TNF-α, which suggests that GTE and EGCG may be used as a treatment option [99].
5.2.5. Cataract
Cataract occurs when the normally transparent lens of the eye becomes cloudy, impeding the passage of light [100]. The risk factors for this disease include genetics, age, gender, skin disease, allergies, oxidative stress, corticosteroid use, and nutrition [101,102]. In addition, diabetes can increase the risk of developing cataract several times [103]. Diabetic cataract is thought to involve mechanisms such as the aldose reductase (AR) pathway, autoantibodies against insulin, and oxidative and osmotic stress [104]. Treatment options for diabetic cataract include surgery, antioxidants, aldose reductase inhibitors, and natural flavonoids [105,106]. In an animal study, green and black tea were investigated for their effects on cataracts in rats with type 1 diabetes. The results of the study suggested that both types of tea were equally effective in treating diabetic cataract, likely due to their hypoglycemic effects. Therefore, diabetics may benefit from consuming tea while managing their condition [107].
Crystallin is a structural protein that plays a crucial role in maintaining lens transparency, and its three types are α, β, and γ. The formation of fibrils from Crystallin can cause clouding of the lens and lead to cataract [108,109]. Various studies have examined the impact of EGCG on different types of crystallin. In vivo studies have demonstrated the suppressive effects of EGCG on the accumulation of αA (66–80) peptide and γB-crystallin under certain conditions [110,111].
A study was conducted to examine the effect of EGCG on the antioxidant properties of human lens epithelial (HLE) cells in cataract disease. When HLE cells were exposed to H2O2, it resulted in the creation of reactive oxygen species (ROS), oxidative stress, and apoptosis. The expression of caspase-9 and caspase-3 was increased, MAPKs and Akt pathways were activated, and the Bcl-2/Bax ratio was reduced. EGCG had an anti-apoptotic and antioxidant effect on these pathways [112].Table 2 has summarized the results of studies on the effects of GT and its active constituents on anterior segment ocular diseases.
Table 2.
Experimental studies showing beneficial effects of GT and its ingredients on anterior segmentocular diseases.
| type of study | cell type/animal model | target disease | intervention | results | reference |
|---|---|---|---|---|---|
| in-vivo | mice | dry eye disease | topical 0.01% and 0.1% EGCG | Administering EGCG topically can alleviate the clinical and inflammatory symptoms of DED by suppressing cytokine expression and CD11b + cell infiltration in the cornea. | [62] |
| in-vitro | human primary pterygium | pterygium | GTE(16.25 μg/mL) and EGCG (25 μM) | GTE and EGCG had a mitigating effect on the survival and movement of primary pterygium cells in vitro, without causing any harm to conjunctival cells. This discovery presents a new and innovative approach to treating primary pterygium. | [71] |
| in-vitro | human corneal fibroblasts | corneal ulcer | EGCG (10, 30, 100 and 300 μM) | EGCG suppresses the degradation of collagen by corneal fibroblasts that have been induced by IL-1b, possibly by impeding the upregulation of uPA, the conversion of plasminogen to plasmin through uPA, and the activation of pro-MMP1 by plasmin. As a result, EGCG requires additional research as a potential therapy for corneal ulcer. | [76] |
| in-vivo | rat | glaucoma | EGCG (50 mg/kg/day) | The NF-κB signaling pathway is involved in the anti-inflammatory impact of EGCG. | [85] |
| in-vivo | rat retinal ganglion cells | glaucoma | GTE (275 mg/kg) | To summarize, under ischemic conditions, GTE provides neuroprotection to retinal ganglion cells, which proposes a prospective therapeutic approach for the treatment of glaucoma and optic neuropathies. | [88] |
| in-vivo | mice | glaucomatous optic neuropathy (GON) | EGCG (50 mg/kg/day) | The results indicate that the intake of EGCG has a neuroprotective effect on retinal ganglion cells (RGCs) in a mouse model of increased intraocular pressure (IOP). | [87] |
| in-vitro | human trabecular meshwork (HTM) cell | primary open-angle glaucoma | EGCG (10, 20, 40, and 80 μM) | EGCG can shield TM cells from endoplasmic reticulum stress, indicating that it has the potential as a therapeutic choice for treating POAG. | [90] |
| in-vivo | rat | uveitis | GTE (550 mg/kg) | GTE is a powerful anti-inflammatory agent that effectively combats endotoxin-induced uveitis (EIU) inflammation, indicating its potential usefulness in treating acute uveitis. | [97] |
| in-vivo | murine | autoimmune uveitis | GTE (137.5 mg/kg and 275 mg/kg GTE suspension, 96.25 mg/kg and 192.5 mg/kg EGCG suspension in 0.1 mL distilled water) | Relief from intraocular inflammation can be achieved by suppressing the expression of pro-inflammatory genes associated with Th17 cells. | [99] |
| in-vivo | rat | uveitis | GTE (550 mg/kg) | The anti-inflammatory effects of a substance on ocular inflammation induced by endotoxin. | [98] |
| in-vivo | rat | uveitis | GTE (550 mg/kg), catechins mixtures, EGCG (375.2 mg/kg) | The combination of GTE and its catechins demonstrated a strong anti-inflammatory effect in a laboratory model of acute ocular inflammation. | [156] |
| in-vitro | αA-crystallin Peptide | cataract | EGCG (1, 5 and 50 mM) | EGCG effectively inhibits the concentration-dependent aggregation of αA (66–80) peptide. Additionally, it can also break up pre-existing aggregates of αA (66–80). This research indicates that EGCG may have the potential to prevent cataract formation and aid in the reversal of the disease. | [111] |
| in-vitro &docking study | human γB-crystallin | cataract | EGCG (4, 8, 10, 12, 16, 20, 30 and 40 μM) | EGCG has the capability to safeguard human γB-crystallin from photodamage induced by oxidative stress. | [157] |
| in-vitro & docking study | human γ-crystallin protein | cataract | EGCG (4, 8, 12 and 16 μM) | EGCG inhibits tryptophan oxidation of human γ-crystallin in cataractous ocular lenses in the presence of H2O2. | [158] |
| in-vitro &molecular dynamics study | human γB-crystallin protein | cataract | EGCG (2 mg/mL) | EGCG has inhibitory potency against the aggregation of human γB-crystallin at high temperatures and low pH. | [110] |
| in-vitro | human lens epithelial (HLE) cells | cataract | EGCG (10, 25, 50, 75, 100and 150 μM) | These discoveries imply that EGCG guards HLE cells against H2O2-induced apoptosis mediated by mitochondria, via the regulation of caspases, the Bcl-2 family, and the MAPK and Akt pathways. | [112] |
OSD - Ocular Surface Diseases.
DED - Dry Eye Disease.
EGCG - Epigallocatechin Gallate.
IL-1b - Interleukin-1 beta.
GTE - Green Tea Extract.
POAG - Primary Open-Angle Glaucoma.
TM - Trabecular Meshwork.
NF-κB - Nuclear Factor-kappa B.
RGCs - Retinal Ganglion Cells.
IOP - Intraocular Pressure.
H2O2 - Hydrogen Peroxide.
5.2.6. Retinal disease
The retina, which contains nerve and optical cells, is responsible for forming visual images of the environment. Retinal diseases are a significant cause of blindness in children. Research indicates that GT may provide beneficial effects in the treatment of various retinal diseases, such as AMD, diabetic retinopathy, and retinitis pigmentosa [[113], [114], [115], [116]].
5.2.6.1. Age-related macular degeneration
AMD is a leading cause of blindness worldwide. Several factors including environmental conditions, genetics, age, and oxidative stress affect the central retina, leading to AMD [117,118]. Depending on the type and mechanism of AMD, different treatment options including laser therapy, Anti-Vascular Endothelial Growth Factor Agents (intravitreal injection), and surgery are available for disease management [119]. In traditional medicine, research has been conducted on the use of saffron, carotenoids, ginkgo, and polyphenols in AMD [120].
Oxidative stress is a known factor in AMD. A study examined the potential antioxidant effect of GT polysaccharide at a concentration of 100 μg/mL on retinal pigment epithelium cells that were exposed to H2O2. The study demonstrated that GT polysaccharide may be a natural solution for AMD due to its inhibitory effect on apoptotic and oxidative stress pathways (Bax and caspase-3) [121]. Another study investigated the protective effect of EGCG on oxidative stress induced by H2O2 in rat retinal pigment epithelial cells. Cells were treated with different concentrations of EGCG (5–50 μM) before H2O2 exposure and cell viability was measured. The findings of the study suggest that EGCG has a dose-dependent protective effect against AMD as an antioxidant [122].
In another study, oxidative damage to retinal photoreceptors was induced by intraocular injection of sodium nitroprusside. Functionally and structurally changes related to RPE destruction were observed after the injection of sodium nitroprusside. Interestingly, EGCG co-injection with sodium nitroprusside significantly blunted these detrimental effects in the retina [123].
Choroidal neovascularization is a possible consequence of AMD, and it can occur through the HIF-1α/VEGF/VEGFR2 pathway. The secretion of IL-6 and TNF-α by M1 macrophages can contribute to the progression of the disease. A recent treatment method involves the use of EGCG prodrug, which has shown to be effective on cell viability factors. This treatment option exerts anti-inflammatory and anti-angiogenic effects and has potential as a new therapy for AMD [124].
In one study, light-induced photoreceptor degeneration was reduced by intraperitoneal injection of EGCG. In these mousses' retina, cone b wave, and rod a and b waves were significantly improved after EGCG injection. The expression of the antioxidant gene Sod2 has also been rescued in EGCG-injected animals [125].
The retina can absorb a significant portion of UV rays, causing oxidative stress and increasing the risk of AMD, among other factors [126]. Several studies have focused on this relationship, demonstrating that long-term exposure to sunlight can be a risk factor for AMD [127]. Autophagy is a process within cells that reduces the accumulation of damaged proteins and improves the function of macular retinal pigment epithelial (RPE) cells. However, exposure to UVB can lead to autophagy in RPE cells, which results in a dose-dependent increase of LC3-II, a protein that marks autophagy. EGCG has been shown to reduce the formation of LC3-II and autophagy, thus acting as a protective agent against UV-induced RPE damage [128]. Other studies have examined the cellular mechanisms of the relationship between UV and AMD on adult human retinal pigment epithelial cells, and EGCG has been found to exert its therapeutic role in AMD by affecting oxidative stress and apoptosis pathways such as JNK1/c-Jun pathway, extracellular signal-regulated kinase (ERK), and c-jun-NH2 terminal kinase (JNK) [129,130].
5.2.6.2. Diabetic retinopathy
Diabetes mellitus has become more widespread in the last century, and one of its complications is damage to the retina, resulting in diabetic retinopathy. The severity and progression of this condition can be affected by the type of diabetes and its duration [131,132]. If left untreated, diabetic retinopathy can lead to vision loss and blurred vision [133]. The treatment options available depend on the type of diabetic retinopathy and include laser photocoagulation, vitrectomy, and drug therapy such as anti-VEGF and triamcinolone acetonide [134]. Medicinal plants such as Garlic (Allium sativum L.), Ginkgo biloba, Camellia nitidissima, and Litchi chinensis have been studied for their potential role in the treatment of diabetic retinopathy [135].
Various cellular pathways play a role in the development of diabetic retinopathy. In an animal model study on retinal Müller and ganglion cells, researchers examined the neurodegeneration pathways in the retina. This study found that increasing the level of pro-nerve growth factor by increasing p75NTR, increasing the phosphorylation of p38MAPK (which causes apoptosis), and decreasing the phosphorylation of tyrosine kinase A receptors all contribute to neurodegeneration. The use of EGCG in this study decreased retinal neurodegeneration and increased cell viability [136]. In another study, the impact of EGCG on the MAPK/ERK-VEGF pathway was explored in human retinal endothelial cells. This pathway is activated by high glucose. The study found that EGCG can increase cell viability and decrease apoptosis by influencing this pathway [137].
In the pathophysiology of diabetic retinopathy, mTOR is involved in maintaining the balance between the anabolism and catabolism of damaged proteins via autophagy. A study investigated the effect of EGCG on the apoptosis of retinal Müller cells in diabetic rats. The presence of high glucose disrupted the autophagy process, leading to cell apoptosis. However, EGCG played a protective role by increasing autophagy and inhibiting apoptosis in the cells [138].
In another study, retinal reduced glutathione (GSH) levels (antioxidant) were 1.5-fold lower in diabetic rats as compared to normal rats. However, in GT-treated rats, retinal GSH levels, as well as superoxide dismutase (SOD) and catalase (CAT), were restored close to those of the normal group. Also, expression of proinflammatory parameters and VEGF was significantly inhibited in GT-treated retina as compared to diabetic retina.Moreover, GT treatment prevented retinal capillary basement membrane thickness [139].
Another study examined the impact of GT on Rat Retinal Muller Cells and ARPE-19 cells in diabetic rats. This research investigated a new mechanism of oxidative stress, where GT may reduce glutamate toxicity in the retina by affecting the glutamate cycle and glutamate transporter, leading to its antioxidant effect [140].
A case-control study aimed to investigate the association between the consumption of Chinese GT and the risk of diabetic retinopathy in diabetic individuals. Participants were divided into two groups: diabetic individuals with diabetic retinopathy (100 people) and diabetic individuals without diabetic retinopathy (100 people). Results from the study revealed that regular consumption of Chinese GT on a weekly basis for at least one year could reduce the risk of diabetic retinopathy. Thus, Chinese GT may serve as an inexpensive treatment option for preventing diabetic retinopathy [54].
5.2.6.3. Retinitis pigmentosa
Retinitis pigmentosa (RP) refers to a set of genetic diseases, characterized by dysfunction in the retina's photoreceptors (cone and rod photoreceptors). Despite its name, inflammation does not play a significant role in its pathophysiology [141]. Given the strong genetic component in the development of the disease, new treatment trends have been studied, such as gene therapy and stem cell therapy [142]. Traditional Chinese medicine also considers treatments like herbal decoctions and acupuncture [143].
A study was conducted to investigate the effect of GTE on photoreceptors in a rat model of RP induced by N-methyl-N-nitrosourea injection. N-methyl-N-nitrosourea is known to cause apoptosis in photoreceptor cells. The study showed that administering GTE at a dose of 250 mg/kg/day before and during the study can reduce and inhibit apoptosis, suggesting that GTE may be used to prevent the progression of RP [144]. Another study used P23H rats as an animal model of RP and found that administering EGCG at a dose of 10 mg/kg/day had antioxidant effects and improved visual performance in the intervention group compared to the control group [145].Table 3 has summarized the results of studies on the effects of GT and its active constituents on retinal diseases.
Table 3.
Experimental studies showing beneficial effects of GT and its ingredients on posterior segment ocular diseases.
| type of study | cell type/animal model | target disease | intervention | results | reference |
|---|---|---|---|---|---|
| in-vitro | human retinal pigment epithelial cells (ARPE-19 cells) | AMD | GTWP (100 μg/mL) | Activated anti-apoptotic and endogenous antioxidant enzyme signaling pathways provide protection to RPE cells against oxidative injury. | [121] |
| in-vivo | mouse | AMD | pro-EGCG (100, 200 and 400 mg/kg/day) | The prodrug of EGCG (pro-EGCG) demonstrated a potential therapeutic application for AMD by reducing the area of mouse laser-induced CNV and alleviating leakage through down-regulating the HIF-1α/VEGF/VEGFR2 pathway, M1-type macrophage/microglia polarization, and by reducing endothelial cell viability, proliferation, migration, and tube formation. | [124] |
| in-vitro | human retinal pigment epithelial cells (ARPE-19 cells) | AMD | EGCG (5 μg/mL) | UVB irradiation can trigger apoptosis in ARPE19 cells via oxidative stress, but the administration of EGCG mitigates this harm. In this scenario, the JNK pathway has an anti-apoptotic function. The use of selective activators or antioxidants may be beneficial in reducing the oxidative damage that occurs in AMD. | [130] |
| in-vitro | human retinal pigment epithelial cells (ARPE-19 cells) | AMD | EGCG (0.1, 0.3, 1, 3 and 10 μM) | EGCG demonstrates efficacy in preventing UVA-induced damage in RPE cells, suggesting that it may be a suitable chemoprotective factor for the primary prevention of early AMD. | [129] |
| in-vitro | human retinal pigment epithelial cells (ARPE-19 cells) | AMD | EGCG (50 μM) | EGCG reduces the harmful effects of UVB irradiation on RPE cells in an autophagy-dependent manner. This study uncovers a new function of EGCG in RPE autophagy and suggests that it may have potential as a therapeutic agent for treating conditions related to irregular autophagy. | [128] |
| in-vitro | rat retinal pigment epithelial cells | ARMD | EGCG (5, 10, 25 and 50 μM) | Our research indicates that pretreatment with EGCG can safeguard primary rat RPE cells against death induced by H2O2. These findings suggest that EGCG may have the potential to prevent retinal diseases that arise from oxidative stress induced by H2O2. | [122] |
| in-vivo/in-vitro | rat/Müller & ARPE-19 cells | DR | GT (1, 10, 100 μg/mL and 5.7 g/kg/day) | GT was found to protect the retina against glutamate toxicity through an antioxidant mechanism. These results demonstrate a new way in which GT safeguards the retina against neurodegeneration in conditions like DR. | [140] |
| in-vitro | rat retinal Müller cell culture | DR | EGCG (10, 20, 30 and 50 μM) | High glucose levels decrease autophagy and result in the accumulation of P62 cargo due to lysosomal dysfunction, ultimately leading to an increase in apoptosis. However, treatment with EGCG can reverse these effects. These results could be beneficial for developing a new therapeutic approach to treating DR. | [138] |
| in-vivo | rat | retinopathy | GT fractions (0.01, 0.05 g/mL) | The results indicate that components of GT, other than catechins and caffeine, may inhibit neovascularization in a rat model of oxygen-induced retinopathy. | [159] |
| in-vitro | human retinal endothelial cell (HREC) | DR | EGCG (10, 20 and 40 μM) | Treating with EGCG improved cell viability and reduced the apoptotic rate of cells under high glucose conditions. The protective effects of EGCG in such conditions could be linked to its ability to regulate inflammatory cytokines and inhibit the MAPK/ERK-VEGF pathway. | [137] |
| in-vivo | rat | DR | Catechin (50, 100and 200 mg/kg/day) | Catechin can alleviate streptozotocin-induced DR by boosting HSP27 levels and suppressing the production of inflammatory factors linked to the disease. This finding may have potential implications for the clinical treatment of DR. | [160] |
| in-vivo/in-vitro | rat/retinal Müller and retinal ganglion cells | DR | Epicatechin(100 mg/kg/day and 100 μmol/l) | Diabetes-triggered peroxynitrite upregulates p75NTR and proNGF expression in Müller cells, while simultaneously impairing TrkA receptor phosphorylation and activating the p75NTR apoptotic pathway in RGCs, ultimately leading to neuronal cell death. However, these detrimental effects were prevented by epicatechin, a safe dietary supplement, suggesting its potential therapeutic value in treating diabetic patients. | [136] |
| in-vivo | rat | RP | GTE (250 mg/kg/day) | GTE was found to structurally and functionally inhibit photoreceptor cell apoptosis induced by MNU. These results suggest that GTE may have the potential to alleviate the development and advancement of human RP. | [144] |
| in-vivo | rat | RP | EGCG (10 mg/kg/day) | Treating P23H rats with EGCG enhances visual function and antioxidant status, but decreases antioxidant defenses in wild-type control animals and slightly impairs activity circadian rhythms. | [145] |
AMD - Age-Related Macular Degeneration.
GTWP - Green Tea Polysaccharide.
CNV - Choroidal Neovascularization.
HIF-1α - Hypoxia-Inducible Factor 1-alpha.
VEGF - Vascular Endothelial Growth Factor.
VEGFR2 - Vascular Endothelial Growth Factor Receptor 2.
UVB - Ultraviolet B.
JNK - c-Jun N-terminal Kinase.
MAPK - Mitogen-activated protein Kinase.
Akt - Protein Kinase B.
ARPE-19 - Human Retinal Pigment Epithelial Cells.
ARMD - Age-Related Macular Degeneration.
DR - Diabetic Retinopathy.
P62 - SQSTM1 (Sequestosome-1).
ERK - Extracellular Signal-Regulated Kinase.
HSP27 - Heat Shock Protein 27.
p75NTR - p75 Neurotrophin Receptor.
proNGF - Pro-nerve Growth Factor.
MNU - N-Methyl-N-Nitrosourea.
RP - Retinitis Pigmentosa.
6. New formulations
Delivering drugs to the eye is a difficult task due to the presence of several barriers that limit the effectiveness of drug delivery [146]. These barriers include the tear film, cornea, blood-aqueous, and blood-retinal barriers. In order to enhance drug bioavailability, scientists are exploring various methods of ocular drug delivery, including the development of new formulations that are currently available in the market. Polymeric gels using pH-induced and osmotically induced gelation, as well as colloidal systems such as liposomes, niosomes, and nanoparticles, are among the new dosage forms being investigated to overcome these limitations [147] (Table 4).
Table 4.
Pharmaceutical formulations of GT ingredients investigated in different studies on eye diseases.
| Pharmaceutical formulation | Advantages of formulation | Reference |
|---|---|---|
| EGCG loaded in anionic liposome with Poloxamer-407 & magnesium | Enhance antioxidant activity and efficacy in the treatment of oxidative stress in ocular disease | [161] |
| PEG/catechin nanocomplex | Increase tear production, stabilize tear film, and better anti-inflammatory effect in dry eye disease | [162] |
| Catechin binding to PEG | Enhance solubility of catechin and show significant anti-inflammatory effect in dry eye disease | [163] |
| Gelatin–epigallocatechin gallate/hyaluronic acid nanoparticles | Better anti-inflammation effect in Dry-eye syndrome (DES) | [148] |
| GEH-RGD nanoparticle | Angiogenesis inhibitor in cornea neovascularization | (145) |
| EGCG loaded in CTAB & DDAB (cationic lipid) | Show Better Bioavailability, stability, biodegradability, and prolonged release of EGCG and safety for treatment of ocular disease | [164] |
| Epigallocatechin Gallate in Gelatin-g-Poly (N-Isopropylacrylamide) | Leading to extended-release formulation, and increased pharmacological efficacy in dry eye syndrome | [165] |
PEG: Poly Ethylene Glycol.
GEH: Gelatin-EGCG with Hyaluronic acid.
RGD: Arginine-Glycine-Aspartic.
CTAB: Cetyltrimethylammonium bromide.
DDAB: dimethyldioctadecylammoniumbromide
EGCG has various pharmacological effects that can be utilized for treating eye diseases such as dry eye syndrome. One study used EGCG nanoparticles and hyaluronic acid as an eye drop formulation in rabbits to overcome eye barriers and achieve the appropriate concentration at the site of action, resulting in improved eye inflammation [148]. Another study found that catechin, a flavonoid present in tea, has anti-inflammatory effects, but its poor solubility in water can be overcome by formulating PEG/catechin nanocomplexes for use in eye drops, which can increase the solubility and anti-inflammatory effect [149]. Additionally, gelatin-EGCG with hyaluronic acid nanoparticle formulation can prevent corneal neovascularization. This formulation increased the bioavailabilityof EGCG and inhibitedangiogenesis [150]. New drug delivery techniques, such as polymeric gels and colloidal systems, are being studied to overcome the barriers in ocular drug delivery and increase drug bioavailability.
7. Safety issues
According to the WHO Registry of Adverse Drug Events Reports, vigiaccess, some reported adverse events of consuming catechin and GT include transient blindness, ocular hyperemia, visual impairment, and periorbital edema [151].However, these reports are based on the pharmacovigilance systems, and the causality associated with the reported adverse events and the use of suspected agents (GT) are not well determined. It is reported that long-term use of GT can lead to decreased sensitivity of the cornea and a lack of stem cells in the corneal limbus, which may result in irreversible damage to the eye, such as corneal neovascularization [152]. In an in-vitro study, the use of black tea compress demonstrated that in the presence of an epithelial defect, the anterior part of the corneal stroma may turn brown [153].
However, the safety review by the US Pharmacopeia found that when used and formulated appropriately, GTE dietary supplements do not pose significant safety concerns warranting prohibition of monograph development, though cautionary labeling is recommended [154]. Based on the systematic review of published toxicology and human investigations GT and its extracts are considered safe in the recommended dosage range [155]. A recommended daily intake of 338 mg EGCG for adults was determined using safety information from both toxicology studies and human consumption of tea in solid form. Considering adverse effects observed in humans, a maximum safe level of 704 mg EGCG per day could be considered for tea consumed as a beverage [155].
8. Conclusion and future perspective
In conclusion, GT and its components have shown promising therapeutic effects in various eye diseases. The plant's bioactive components, including catechins, flavonoids, and alkaloids, have shown potent anti-inflammatory, antioxidant, antibacterial, and anti-VEGF activities. In addition, GTEs have been shown to improve ocular surface diseases, such as dry eye, allergic diseases, and infections, as well as retinal and optic nerve diseases. Moreover, GT has shown aldose reductase inhibitor activity, which could be beneficial in treating cataract.
Despite the promising therapeutic effects of GT, further research is needed to determine the optimal dose and route of administration to achieve maximum therapeutic effects in various eye diseases. The use of GT in combination with other medications could also be investigated to improve treatment outcomes. In addition, more preclinical and clinical studies are needed to evaluate the safety and long-term effects of GT in the eye.
In the future, GT and its components may become a valuable therapeutic option for the management of various eye diseases. The development of new formulations and drug delivery systems that improve the bioavailability and stability of GT components in the eye could enhance their therapeutic efficacy. Furthermore, the use of GT as a nutraceutical could also be explored as a preventive strategy in individuals at high risk of developing eye diseases. Overall, the potential therapeutic effects of GT in eye diseases provide a promising area of research for the future.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Data is available on request from the corresponding author.
Competing interests
No conflict of interest to declare.
Funding
No funding was received for this work.
CRediT authorship contribution statement
Mohadese Boroughani: Writing – original draft, Data curation, Conceptualization. Zahra Tahmasbi: Writing – review & editing, Investigation, Conceptualization. Mohamad Mahdi Heidari: Writing – review & editing, Data curation, Conceptualization. Mohammadkarim Johari: Writing – review & editing, Data curation. Mohammad Hashem Hashempur: Writing – review & editing, Data curation, Conceptualization. Mojtaba Heydari: Writing – review & editing, Writing – original draft, Supervision, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We want to thank the research consultation service for language editing of the manuscript.
References
- 1.Wang B., Congdon N., Bourne R., Li Y., Cao K., Zhao A., Yusufu M., Dong W., Zhou M., Wang N. Burden of vision loss associated with eye disease in China 1990-2020: findings from the Global Burden of Disease Study 2015. Br. J. Ophthalmol. 2018;102(2):220–224. doi: 10.1136/bjophthalmol-2017-310333. [DOI] [PubMed] [Google Scholar]
- 2.Keel S., Lingham G., Misra N., Block S., Bourne R., Calonge M., Cheng C.Y., Friedman D.S., Furtado J.M., Khanna R., et al. Toward Universal eye health coverage-key outcomes of the world health organization package of eye care interventions: a systematic review. JAMA Ophthalmol. 2022;140(12):1229–1238. doi: 10.1001/jamaophthalmol.2022.4716. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N., Kocur I. Chronic eye disease and the WHO universal eye health global action plan 2014-2019. Can. J. Ophthalmol. 2014;49(5):403–405. doi: 10.1016/j.jcjo.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Umesh M.L., Mrunalini M., Shinde D.S. Review of image processing and machine learning techniques for eye disease detection and classification. Int. Res. J. Eng. Technol. 2016;3(3):547–551. [Google Scholar]
- 5.Estcourt S., Quinn A.G., Vaidya B. Quality of life in thyroid eye disease: impact of quality of care. Eur. J. Endocrinol. 2011;164(5):649–655. doi: 10.1530/EJE-11-0055. [DOI] [PubMed] [Google Scholar]
- 6.Nutheti R., Shamanna B.R., Nirmalan P.K., Keeffe J.E., Krishnaiah S., Rao G.N., Thomas R. Impact of impaired vision and eye disease on quality of life in Andhra Pradesh. Invest. Ophthalmol. Vis. Sci. 2006;47(11):4742–4748. doi: 10.1167/iovs.06-0020. [DOI] [PubMed] [Google Scholar]
- 7.Amle V., Rathod D., E K., Kumar V., Kumar R., Saha P. Bioactive herbal medicine use for eye sight: a meta analysis. Journal for Research in Applied Sciences and Biotechnology. 2022;1:42–50. [Google Scholar]
- 8.Namita P., Mukesh R., Vijay K. Camellia sinensis (green tea): a review. Global J. Pharmacol. 2012;6 [Google Scholar]
- 9.Xu N., Chen Z-m. Green tea, black tea and semi-fermented tea. Tea: Bioactivity and therapeutic potential. 2002:35–57. [Google Scholar]
- 10.Basu A., Lucas E.A. Mechanisms and effects of green tea on cardiovascular health. Nutr. Rev. 2007;65(8 Pt 1):361–375. doi: 10.1301/nr.2007.aug.361-375. [DOI] [PubMed] [Google Scholar]
- 11.Zhong Y., Ma C.-M., Shahidi F. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. J. Funct.Foods. 2012;4(1):87–93. doi: 10.1016/j.jff.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du G.-J., Zhang Z., Wen X.-D., Yu C., Calway T., Yuan C.-S., Wang C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4(11):1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu K.O., Chan K.P., Yang Y.P., Qin Y.J., Li W.Y., Chan S.O., Wang C.C., Pang C.P. Effects of EGCG content in green tea extract on pharmacokinetics, oxidative status and expression of inflammatory and apoptotic genes in the rat ocular tissues. J. Nutr. Biochem. 2015;26(11):1357–1367. doi: 10.1016/j.jnutbio.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Du L., He J.N., Chu K.O., Guo C.L., Wong M.O.M., Pang C.P., Chu W.K. Anti-inflammatory effects of GTE in eye diseases. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.753955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reygaert W.C. Green tea catechins: their use in treating and preventing infectious diseases. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/9105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Xing J., Fei Y. Green tea (Camellia sinensis) and cancer prevention: a systematic review of randomized trials and epidemiological studies. Chin. Med. 2008;3(1):12. doi: 10.1186/1749-8546-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C.S., Chen G., Wu Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. Journal of Traditional and Complementary Medicine. 2014;4(1):17–23. doi: 10.4103/2225-4110.124326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumari V., Kaushal K. Ayurvedic herbs useful in gastrointestinal cancer. Journal of Medicinal Plants. 2017;5(1):26–28. [Google Scholar]
- 19.Qi S-m, Zhang J-t, Zhu H-y, Wang Z., Li W. Review on potential effects of traditional Chinese medicine on glaucoma. J. Ethnopharmacol. 2023;304 doi: 10.1016/j.jep.2022.116063. [DOI] [PubMed] [Google Scholar]
- 20.Ai X., Yu P., Hou Y., Song X., Luo J., Li N., Lai X., Wang X., Meng X. A review of traditional Chinese medicine on treatment of diabetic retinopathy and involved mechanisms. Biomed. Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110852. [DOI] [PubMed] [Google Scholar]
- 21.Ling J., Chan B.C., Tsang M.S., Gao X., Leung P.C., Lam C.W., Hu J.M., Wong C.K. Current advances in mechanisms and treatment of dry eye disease: toward anti-inflammatory and immunomodulatory therapy and traditional Chinese medicine. Front. Med. 2021;8 doi: 10.3389/fmed.2021.815075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamedi A., Zarshenas M.M., Sohrabpour M., Zargaran A. Herbal medicinal oils in traditional Persian medicine. Pharm. Biol. 2013;51(9):1208–1218. doi: 10.3109/13880209.2013.777462. [DOI] [PubMed] [Google Scholar]
- 23.Snow A., Shieh B., Chang K.C., Pal A., Lenhart P., Ammar D., Ruzycki P., Palla S., Reddy G.B., Petrash J.M. Aldose reductase expression as a risk factor for cataract. Chem. Biol. Interact. 2015;234:247–253. doi: 10.1016/j.cbi.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balestri F., Poli G., Pineschi C., Moschini R., Cappiello M., Mura U., Tuccinardi T., Del Corso A. Aldose reductase differential inhibitors in green tea. Biomolecules. 2020;10(7) doi: 10.3390/biom10071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata M., Irie J., Homma S. Aldose reductase inhibitors from green tea. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 1994;27(5):401–405. [Google Scholar]
- 26.Sakai I., Izumi S.I., Murano T., Okuwaki S., Makino T., Suzuki T. Presence of aldose reductase inhibitors in tea leaves. Jpn. J. Pharmacol. 2001;85(3):322–326. doi: 10.1254/jjp.85.322. [DOI] [PubMed] [Google Scholar]
- 27.Tah V., Orlans H.O., Hyer J., Casswell E., Din N., Sri Shanmuganathan V., Ramskold L., Pasu S. Anti-VEGF therapy and the retina: an update. Journal of Ophthalmology. 2015;2015 doi: 10.1155/2015/627674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahrami B., Hong T., Gilles M.C., Chang A. Anti-VEGF therapy for diabetic eye diseases. The Asia-Pacific Journal of Ophthalmology. 2017;6(6) doi: 10.22608/APO.2017350. [DOI] [PubMed] [Google Scholar]
- 29.Witmer A.N., Vrensen G.F.J.M., Van Noorden C.J.F., Schlingemann R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003;22(1):1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 30.Cornel S., Adriana I.D., Mihaela T.C., Speranta S., Algerino S., Mehdi B., Jalaladin H.R. Anti-vascular endothelial growth factor indications in ocular disease. Rom J Ophthalmol. 2015;59(4):235–242. [PMC free article] [PubMed] [Google Scholar]
- 31.Chu K.O., Chan K.P., Chan S.O., Ng T.K., Jhanji V., Wang C.C. Pang CP: metabolomics of green-tea catechins on vascular-endothelial-growth-factor-stimulated human-endothelial-cell survival. J. Agric. Food Chem. 2018;66(48):12866–12875. doi: 10.1021/acs.jafc.8b05998. [DOI] [PubMed] [Google Scholar]
- 32.Rashidi B., Malekzadeh M., Goodarzi M., Masoudifar A., Mirzaei H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017;89:949–956. doi: 10.1016/j.biopha.2017.01.161. [DOI] [PubMed] [Google Scholar]
- 33.Jackson T.L., Paraskevopoulos T., Georgalas I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv. Ophthalmol. 2014;59(6):627–635. doi: 10.1016/j.survophthal.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5–17. doi: 10.1111/j.1600-0420.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 35.Pflugfelder S.C., Karpecki P.M., Perez V.L. Treatment of blepharitis: recent clinical trials. Ocul. Surf. 2014;12(4):273–284. doi: 10.1016/j.jtos.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Gupta P.D., Birdi T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017;8(4):266–275. doi: 10.1016/j.jaim.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007;51(1):116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco A.R., Mulè S.L.T., Babini G., Garbisa S., Enea V., Rusciano D. (−) Epigallocatechin-3-gallate inhibits gelatinase activity of some bacterial isolates from ocular infection, and limits their invasion through gelatine. Biochim. Biophys. Acta Gen. Subj. 2003;1620(1–3):273–281. doi: 10.1016/s0304-4165(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 39.Blanco A.R., Sudano-Roccaro A., Spoto G.C., Nostro A., Rusciano D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob. Agents Chemother. 2005;49(10):4339–4343. doi: 10.1128/AAC.49.10.4339-4343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hessen M., Akpek E.K. Dry eye: an inflammatory ocular disease. J. Ophthalmic Vis. Res. 2014;9(2):240–250. [PMC free article] [PubMed] [Google Scholar]
- 41.Tang J., Kern T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim L., Suhler E.B., Smith J.R. Biologic therapies for inflammatory eye disease. Clin. Exp. Ophthalmol. 2006;34(4):365–374. doi: 10.1111/j.1442-9071.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 43.Thichanpiang P., Wongprasert K. Green tea polyphenol epigallocatechin-3-gallate attenuates TNF-α-induced intercellular adhesion molecule-1 expression and monocyte adhesion to retinal pigment epithelial cells. Am. J. Chin. Med. 2015;43(1):103–119. doi: 10.1142/S0192415X1550007X. [DOI] [PubMed] [Google Scholar]
- 44.Ren J.L., Yu Q.X., Liang W.C., Leung P.Y., Ng T.K., Chu W.K., Pang C.P., Chan S.O. Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci. Rep. 2018;8(1):1–10. doi: 10.1038/s41598-017-18888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H.-Y., Wang J.-Y., Yao H.-P. Epigallocatechin-3-gallate attenuates lipopolysaccharide-induced inflammation in human retinal endothelial cells. Int. J. Ophthalmol. 2014;7(3):408. doi: 10.3980/j.issn.2222-3959.2014.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masuda T., Shimazawa M., Hara H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (edaravone) Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/9208489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsueh Y.-J., Chen Y.-N., Tsao Y.-T., Cheng C.-M., Wu W.-C., Chen H.-C. The pathomechanism, antioxidant biomarkers, and treatment of oxidative stress-related eye diseases. Int. J. Mol. Sci. 2022;23(3):1255. doi: 10.3390/ijms23031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S., Halder N., Srivastava S., Trivedi D., Joshi S., Varma S. Green tea (Camellia sinensis) protects against selenite-induced oxidative stress in experimental cataractogenesis. Ophthalmic Res. 2002;34(4):258–263. doi: 10.1159/000063881. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., Qin Y.J., Yip Y.W., Chan K.P., Chu K.O., Chu W.K., Ng T.K., Pang C.P., Chan S.O. Green tea catechins are potent anti-oxidants that ameliorate sodium iodate-induced retinal degeneration in rats. Sci. Rep. 2016;6 doi: 10.1038/srep29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Q., Li Z., Lu X., Song J., Wang H., Liu D., Guo D., Bi H. Epigallocatechin gallate protects the human lens epithelial cell survival against UVB irradiation through AIF/endo G signalling pathways in vitro. Cutan. Ocul. Toxicol. 2021;40(3):187–197. doi: 10.1080/15569527.2021.1879112. [DOI] [PubMed] [Google Scholar]
- 51.Doğan M., Akdoğan M., Alizada A., Eroğul Ö., Sabaner M.C., Gobeka H.H., Gülyeşil F.F., Seylan M.A. Impacts of Camellia sinensis fermentation end-product (black tea) on retinal microvasculature: an updated OCTA analysis. J. Sci. Food Agric. 2021;101(15):6265–6270. doi: 10.1002/jsfa.11294. [DOI] [PubMed] [Google Scholar]
- 52.Gasiunas K., Galgauskas S. Green tea-a new perspective of glaucoma prevention. Int. J. Ophthalmol. 2022;15(5):747–752. doi: 10.18240/ijo.2022.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nejabat M., Reza S.A., Zadmehr M., Yasemi M., Sobhani Z. Efficacy of green tea extract for treatment of dry eye and meibomian gland dysfunction; A double-blind randomized controlled clinical trial study. J. Clin. Diagn. Res. 2017;11(2):Nc05–nc08. doi: 10.7860/JCDR/2017/23336.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Q., Chen D., Sun H.P., Yan N., Xu Y., Pan C.W. Regular Chinese green tea consumption is protective for diabetic retinopathy: a clinic-based case-control study. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/231570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falsini B., Marangoni D., Salgarello T., Stifano G., Montrone L., Di Landro S., Guccione L., Balestrazzi E., Colotto A. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247(9):1223–1233. doi: 10.1007/s00417-009-1064-z. [DOI] [PubMed] [Google Scholar]
- 56.Schmidl D., Schlatter A., Chua J., Tan B., Garhöfer G., Schmetterer L. Novel approaches for imaging-based diagnosis of ocular surface disease. Diagnostics. 2020;10(8):589. doi: 10.3390/diagnostics10080589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tatlipinar S., Akpek E.K. Topical ciclosporin in the treatment of ocular surface disorders. Br. J. Ophthalmol. 2005;89(10):1363–1367. doi: 10.1136/bjo.2005.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gayton J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pflugfelder S.C., de Paiva C.S. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11, Supplement):S4–S13. doi: 10.1016/j.ophtha.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Neil E.C., Henderson M., Massaro-Giordano M., Bunya V.Y. Advances in dry eye disease treatment. Curr. Opin. Ophthalmol. 2019;30(3):166–178. doi: 10.1097/ICU.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Majtánová N., Cernák M., Nekorancová J., Cernák A., Majtán J. The potential use of honey in ophthalmology. Ceska a Slov. Oftalmol.: Casopis Ceske Oftalmologicke Spolecnosti a Slovenske Oftalmologicke Spolecnosti. 2013;69(3):128–132. [PubMed] [Google Scholar]
- 62.Lee H.S., Chauhan S.K., Okanobo A., Nallasamy N., Dana R. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea. 2011;30(12):1465–1472. doi: 10.1097/ICO.0b013e31821c9b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cavet M.E., Harrington K.L., Vollmer T.R., Ward K.W., Zhang J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011;17:533–542. [PMC free article] [PubMed] [Google Scholar]
- 64.Chu W.K., Choi H.L., Bhat A.K., Jhanji V. Pterygium: new insights. Eye. 2020;34(6):1047–1050. doi: 10.1038/s41433-020-0786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shahraki T., Arabi A., Feizi S. Pterygium: an update on pathophysiology, clinical features, and management. Therapeutic Advances in Ophthalmology. 2021;13 doi: 10.1177/25158414211020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh S.K. Pterygium: epidemiology prevention and treatment. Community Eye Health. 2017;30(99):S5. [PMC free article] [PubMed] [Google Scholar]
- 67.Fonseca E.C., Rocha E.M., Arruda G.V. Comparison among adjuvant treatments for primary pterygium: a network meta-analysis. Br. J. Ophthalmol. 2018;102(6):748–756. doi: 10.1136/bjophthalmol-2017-310288. [DOI] [PubMed] [Google Scholar]
- 68.Baheran S.S., Alany R.G., Schwikkard S., Muen W., Salman L.N., Freestone N., Al-Kinani A.A. Pharmacological treatment strategies of pterygium: drugs, biologics, and novel natural products. Drug Discov. Today. 2023;28(1) doi: 10.1016/j.drudis.2022.103416. [DOI] [PubMed] [Google Scholar]
- 69.Hacıoğlu D., Erdöl H. Developments and current approaches in the treatment of pterygium. Int. Ophthalmol. 2017;37:1073–1081. doi: 10.1007/s10792-016-0358-5. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M., Bian F., Wen C., Hao N. Inhibitory effect of curcumin on proliferation of human pterygium fibroblasts. J. Huazhong Univ. Sci. Technol. 2007;27(3):339–342. doi: 10.1007/s11596-007-0332-6. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y., Chen S.-L., Xu Y., Yao Y., Liang J.-J., Wang L., Jhanji V., Sun X., Ma D., Ng T.K. Green tea catechins attenuate human primary pterygium cell survival and migration via modulation of ERK p42/p44 and p38 pathways. J. Agric. Food Chem. 2021;69(41):12209–12218. doi: 10.1021/acs.jafc.1c04422. [DOI] [PubMed] [Google Scholar]
- 72.Garg P., Rao G.N. Corneal ulcer: diagnosis and management. Community Eye Health. 1999;12(30):21–23. [PMC free article] [PubMed] [Google Scholar]
- 73.Ibrahim Y.W., Boase D.L., Cree I.A. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br. J. Ophthalmol. 2009;93(10):1319–1324. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 74.Loh A.R., Hong K., Lee S., Mannis M., Acharya N.R. Practice patterns in the management of fungal corneal ulcers. Cornea. 2009;28(8):856–859. doi: 10.1097/ICO.0b013e318199fa77. [DOI] [PubMed] [Google Scholar]
- 75.Gangopadhyay N., Daniell M., Weih L., Taylor H.R. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. Br. J. Ophthalmol. 2000;84(4):378–384. doi: 10.1136/bjo.84.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugioka K., Yoshida K., Murakami J., Itahashi M., Mishima H., Nishida T., Kusaka S. Inhibition by epigallocatechin gallate of IL-1–induced urokinase-type plasminogen activator expression and collagen degradation by corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2019;60(8):2895–2903. doi: 10.1167/iovs.19-27306. [DOI] [PubMed] [Google Scholar]
- 77.Chang J.-H., Garg N.K., Lunde E., Han K.-Y., Jain S., Azar D.T. Corneal neovascularization: an anti-VEGF therapy review. Surv. Ophthalmol. 2012;57(5):415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee P., Wang C.C., Adamis A.P. Ocular neovascularization: an epidemiologic review. Surv. Ophthalmol. 1998;43(3):245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 79.Voiculescu O.B., Voinea L.M., Alexandrescu C. Corneal neovascularization and biological therapy. J Med Life. 2015;8(4):444–448. [PMC free article] [PubMed] [Google Scholar]
- 80.Sánchez-Huerta V., Gutiérrez-Sánchez L., Flores-Estrada J. (-)-Epigallocatechin 3-gallate (EGCG) at the ocular surface inhibits corneal neovascularization. Med. Hypotheses. 2011;76(3):311–313. doi: 10.1016/j.mehy.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 81.Schuster A.K., Erb C., Hoffmann E.M., Dietlein T., Pfeiffer N. The diagnosis and treatment of glaucoma. Dtsch Arztebl Int. 2020;117(13):225–234. doi: 10.3238/arztebl.2020.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casson R.J., Chidlow G., Wood J.P., Crowston J.G., Goldberg I. Definition of glaucoma: clinical and experimental concepts. Clin. Exp. Ophthalmol. 2012;40(4):341–349. doi: 10.1111/j.1442-9071.2012.02773.x. [DOI] [PubMed] [Google Scholar]
- 83.Wiggs J.L., Pasquale L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017;26(R1):R21–R27. doi: 10.1093/hmg/ddx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee D.A., Higginbotham E.J. Glaucoma and its treatment: a review. Am. J. Health Syst. Pharm. 2005;62(7):691–699. doi: 10.1093/ajhp/62.7.691. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W.-H., Chen Y., Gao L.-M., Cao Y.-N. Neuroprotective role of epigallocatechin-3-gallate in acute glaucoma via the nuclear factor-κB signalling pathway. Exp. Ther. Med. 2021;22(5):1–9. doi: 10.3892/etm.2021.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almasieh M., Wilson A.M., Morquette B., Cueva Vargas J.L., Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Shen C., Chen L., Jiang L., Lai T.Y. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci. Lett. 2015;600:132–136. doi: 10.1016/j.neulet.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y., Xu C., Chen Y., Liang J.-J., Xu Y., Chen S.-L., Huang S., Yang Q., Cen L.-P., Pang C.P. Green tea extract ameliorates ischemia-induced retinal ganglion cell degeneration in rats. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/8407206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon Y.H., Fingert J.H., Kuehn M.H., Alward W.L.M. Primary open-angle glaucoma. N. Engl. J. Med. 2009;360(11):1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou L., He J.N., Du L., Ho B.M., Ng D.S., Chan P.P., Tham C.C., Pang C.P., Chu W.K. Epigallocatechin-3-Gallate protects trabecular meshwork cells from endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/7435754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gasiunas K., Galgauskas S. Green tea—a new perspective of glaucoma prevention. Int. J. Ophthalmol. 2022;15(5):747. doi: 10.18240/ijo.2022.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trivedi A., Katelaris C. The use of biologic agents in the management of uveitis. Intern. Med. J. 2019;49(11):1352–1363. doi: 10.1111/imj.14215. [DOI] [PubMed] [Google Scholar]
- 93.Tsirouki T., Dastiridou A., Symeonidis C., Tounakaki O., Brazitikou I., Kalogeropoulos C., Androudi S. A focus on the epidemiology of uveitis. Ocul. Immunol. Inflamm. 2018;26(1):2–16. doi: 10.1080/09273948.2016.1196713. [DOI] [PubMed] [Google Scholar]
- 94.Agarwal M., Majumder P.D., Babu K., Konana V.K., Goyal M., Touhami S., Stanescu-Segall D., Bodaghi B. Drug-induced uveitis: a review. Indian J. Ophthalmol. 2020;68(9):1799. doi: 10.4103/ijo.IJO_816_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwartzman S. Advancements in the management of uveitis. Best Pract. Res. Clin. Rheumatol. 2016;30(2):304–315. doi: 10.1016/j.berh.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Li S., Liu F., Zhang K., Tong Y., Liu X. Research progress on the mechanism of natural product ingredients in the treatment of uveitis. Journal of Immunology Research. 2021;2021 doi: 10.1155/2021/6683411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin Y.J., Chu K.O., Yip Y.W.Y., Li W.Y., Yang Y.P., Chan K.P., Ren J.L., Chan S.O., Pang C.P. Green tea extract treatment alleviates ocular inflammation in a rat model of endotoxin-induced uveitis. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chu K.O., Chan K.P., Yip Y.W.Y., Chu W.K., Wang C.C., Pang C.P. Systemic and ocular anti-inflammatory mechanisms of green tea extract on endotoxin-induced ocular inflammation. Front. Endocrinol. 2022:1566. doi: 10.3389/fendo.2022.899271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J., Yip Y.W.Y., Ren J., Hui W.K., He J.N., Yu Q.X., Chu K.O., Ng T.K., Chan S.O., Pang C.P., et al. Green tea catechins alleviate autoimmune symptoms and visual impairment in a murine model for human chronic intraocular inflammation by inhibiting Th17-associated pro-inflammatory gene expression. Sci. Rep. 2019;9(1):2301. doi: 10.1038/s41598-019-38868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alshamrani A.Z. Cataracts pathophysiology and managements. The Egyptian Journal of Hospital Medicine. 2018;70(1):151–154. [Google Scholar]
- 101.Gupta V.B., Rajagopala M., Ravishankar B. Etiopathogenesis of cataract: an appraisal. Indian J. Ophthalmol. 2014;62(2):103. doi: 10.4103/0301-4738.121141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thiagarajan R., Manikandan R. Antioxidants and cataract. Free Radic. Res. 2013;47(5):337–345. doi: 10.3109/10715762.2013.777155. [DOI] [PubMed] [Google Scholar]
- 103.Obrosova I.G., Chung S.S., Kador P.F. Diabetic cataracts: mechanisms and management. Diabetes/metabolism research and reviews. 2010;26(3):172–180. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 104.Kiziltoprak H., Tekin K., Inanc M., Goker Y.S. Cataract in diabetes mellitus. World J. Diabetes. 2019;10(3):140–153. doi: 10.4239/wjd.v10.i3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pollreisz A., Schmidt-Erfurth U. Diabetic cataract—pathogenesis, epidemiology and treatment. Journal of ophthalmology. 2010;2010 doi: 10.1155/2010/608751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stefek M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscipl. Toxicol. 2011;4(2):69–77. doi: 10.2478/v10102-011-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vinson J.A., Zhang J. Black and green teas equally inhibit diabetic cataracts in a streptozotocin-induced rat model of diabetes. J. Agric. Food Chem. 2005;53(9):3710–3713. doi: 10.1021/jf048052l. [DOI] [PubMed] [Google Scholar]
- 108.Meehan S., Berry Y., Luisi B., Dobson C.M., Carver J.A., MacPhee C.E. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J. Biol. Chem. 2004;279(5):3413–3419. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- 109.Graw J. Genetics of crystallins: cataract and beyond. Exp. Eye Res. 2009;88(2):173–189. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 110.Chaudhury S., Dutta A., Bag S., Biswas P., Das A.K., Dasgupta S. Probing the inhibitory potency of epigallocatechin gallate against human γB-crystallin aggregation: spectroscopic, microscopic and simulation studies. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018;192:318–327. doi: 10.1016/j.saa.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 111.Kumar V., Gour S., Peter O.S., Gandhi S., Goyal P., Pandey J., Harsolia R.S., Yadav J.K. Effect of green tea polyphenol epigallocatechin-3-gallate on the aggregation of αa(66-80) peptide, a major fragment of αA-crystallin involved in cataract development. Curr. Eye Res. 2017;42(10):1368–1377. doi: 10.1080/02713683.2017.1324628. [DOI] [PubMed] [Google Scholar]
- 112.Yao K., Ye P., Zhang L., Tan J., Tang X., Zhang Y. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol. Vis. 2008;14:217–223. [PMC free article] [PubMed] [Google Scholar]
- 113.Lee S.S., Robinson M.R. Novel drug delivery systems for retinal diseases. Ophthalmic Res. 2009;41(3):124–135. doi: 10.1159/000209665. [DOI] [PubMed] [Google Scholar]
- 114.Yorston D. Retinal diseases and vision 2020. Community Eye Health. 2003;16(46):19–20. [PMC free article] [PubMed] [Google Scholar]
- 115.Masland R.H. The fundamental plan of the retina. Nat. Neurosci. 2001;4(9):877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 116.Sancho-Pelluz J., Arango-Gonzalez B., Kustermann S., Romero F.J., van Veen T., Zrenner E., Ekström P., Paquet-Durand F. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol. Neurobiol. 2008;38(3):253–269. doi: 10.1007/s12035-008-8045-9. [DOI] [PubMed] [Google Scholar]
- 117.Fleckenstein M., Keenan T.D.L., Guymer R.H., Chakravarthy U., Schmitz-Valckenberg S., Klaver C.C., Wong W.T., Chew E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021;7(1):31. doi: 10.1038/s41572-021-00265-2. [DOI] [PubMed] [Google Scholar]
- 118.van Lookeren Campagne M., LeCouter J., Yaspan B.L., Ye W. Mechanisms of age‐related macular degeneration and therapeutic opportunities. J. Pathol. 2014;232(2):151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 119.Thomas C.J., Mirza R.G., Gill M.K. Age-related macular degeneration. Med. Clin. 2021;105(3):473–491. doi: 10.1016/j.mcna.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Bosch-Morell F., Villagrasa V., Ortega T., Acero N., Muñoz-Mingarro D., González-Rosende M.E., Castillo E., Sanahuja M.A., Soriano P., Martínez-Solís I. Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration. Neural Regen Res. 2020;15(12):2207–2216. doi: 10.4103/1673-5374.284978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yan Y., Ren Y., Li X., Zhang X., Guo H., Han Y., Hu J. A polysaccharide from green tea (Camellia sinensis L.) protects human retinal endothelial cells against hydrogen peroxide-induced oxidative injury and apoptosis. Int. J. Biol. Macromol. 2018;115:600–607. doi: 10.1016/j.ijbiomac.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 122.Cia D., Vergnaud-Gauduchon J., Jacquemot N., Doly M. Epigallocatechin gallate (EGCG) prevents H2O2-induced oxidative stress in primary rat retinal pigment epithelial cells. Curr. Eye Res. 2014;39(9):944–952. doi: 10.3109/02713683.2014.885532. [DOI] [PubMed] [Google Scholar]
- 123.Zhang B., Osborne N. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006;1124(1):176–187. doi: 10.1016/j.brainres.2006.09.067. [DOI] [PubMed] [Google Scholar]
- 124.Xu J., Tu Y., Wang Y., Xu X., Sun X., Xie L., Zhao Q., Guo Y., Gu Y., Du J., et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109606. [DOI] [PubMed] [Google Scholar]
- 125.Qi S., Wang C., Song D., Song Y., Dunaief J.L. Intraperitoneal injection of (−)-Epigallocatechin-3-gallate protects against light-induced photoreceptor degeneration in the mouse retina. Mol. Vis. 2017;23:171. [PMC free article] [PubMed] [Google Scholar]
- 126.Bellezza I. Oxidative stress in age-related macular degeneration: nrf2 as therapeutic target. Front. Pharmacol. 2018;9:1280. doi: 10.3389/fphar.2018.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Plestina-Borjan I., Klinger-Lasić M. Long-term exposure to solar ultraviolet radiation as a risk factor for age-related macular degeneration. Coll. Antropol. 2007;31(Suppl 1):33–38. [PubMed] [Google Scholar]
- 128.Li C.P., Yao J., Tao Z.F., Li X.M., Jiang Q., Yan B. Epigallocatechin-gallate (EGCG) regulates autophagy in human retinal pigment epithelial cells: a potential role for reducing UVB light-induced retinal damage. Biochem. Biophys. Res. Commun. 2013;438(4):739–745. doi: 10.1016/j.bbrc.2013.07.097. [DOI] [PubMed] [Google Scholar]
- 129.Chan C.M., Huang J.H., Lin H.H., Chiang H.S., Chen B.H., Hong J.Y., Hung C.F. Protective effects of (-)-epigallocatechin gallate on UVA-induced damage in ARPE19 cells. Mol. Vis. 2008;14:2528–2534. [PMC free article] [PubMed] [Google Scholar]
- 130.Cao G., Chen M., Song Q., Liu Y., Xie L., Han Y., Liu Z., Ji Y., Jiang Q. EGCG protects against UVB-induced apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19 cells. Mol. Med. Rep. 2012;5(1):54–59. doi: 10.3892/mmr.2011.582. [DOI] [PubMed] [Google Scholar]
- 131.Tarr J.M., Kaul K., Chopra M., Kohner E.M., Chibber R. Pathophysiology of diabetic retinopathy. Int. Sch. Res. Notices. 2013;2013 doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zimmet P.Z., Magliano D.J., Herman W.H., Shaw J.E. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 133.Whitehead M., Wickremasinghe S., Osborne A., Van Wijngaarden P., Martin K.R. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expet Opin. Biol. Ther. 2018;18(12):1257–1270. doi: 10.1080/14712598.2018.1545836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kollias A.N., Ulbig M.W. Diabetic retinopathy: early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107(5):75–83. doi: 10.3238/arztebl.2010.0075. quiz 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parveen A., Kim J.H., Oh B.G., Subedi L., Khan Z., Kim S.Y. Phytochemicals: target-based therapeutic strategies for diabetic retinopathy. Molecules. 2018;23(7):1519. doi: 10.3390/molecules23071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Al-Gayyar M.M., Matragoon S., Pillai B.A., Ali T.K., Abdelsaid M.A., El-Remessy A.B. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor expression in a rat model of diabetes [corrected] Diabetologia. 2011;54(3):669–680. doi: 10.1007/s00125-010-1994-3. [DOI] [PubMed] [Google Scholar]
- 137.Zhang L., Zhang Z.K., Liang S. Epigallocatechin-3-gallate protects retinal vascular endothelial cells from high glucose stress in vitro via the MAPK/ERK-VEGF pathway. Genet. Mol. Res. 2016;15(2) doi: 10.4238/gmr.15027874. [DOI] [PubMed] [Google Scholar]
- 138.Wang L., Sun X., Zhu M., Du J., Xu J., Qin X., Xu X., Song E. Epigallocatechin-3-gallate stimulates autophagy and reduces apoptosis levels in retinal Müller cells under high-glucose conditions. Exp. Cell Res. 2019;380(2):149–158. doi: 10.1016/j.yexcr.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 139.Kumar B., Gupta S.K., Nag T.C., Srivastava S., Saxena R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 2012;47(2):103–108. doi: 10.1159/000330051. [DOI] [PubMed] [Google Scholar]
- 140.Silva K.C., Rosales M.A., Hamassaki D.E., Saito K.C., Faria A.M., Ribeiro P.A., Faria J.B., Faria J.M. Green tea is neuroprotective in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013;54(2):1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- 141.Shintani K., Shechtman D.L., Gurwood A.S. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry - Journal of the American Optometric Association. 2009;80(7):384–401. doi: 10.1016/j.optm.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Q. Retinitis pigmentosa: progress and perspective. The Asia-Pacific Journal of Ophthalmology. 2016;5(4):265–271. doi: 10.1097/APO.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 143.Xu J., Peng Q. Retinitis pigmentosa treatment with western medicine and traditional Chinese medicine therapies. J Ophthalmol. 2015;2015 doi: 10.1155/2015/421269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Emoto Y., Yoshizawa K., Kinoshita Y., Yuri T., Yuki M., Sayama K., Shikata N., Tsubura A. Green tea extract suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252(9):1377–1384. doi: 10.1007/s00417-014-2702-7. [DOI] [PubMed] [Google Scholar]
- 145.Perdices L., Fuentes-Broto L., Segura F., Cuenca N., Orduna-Hospital E., Pinilla I. Epigallocatechin gallate slows retinal degeneration, reduces oxidative damage, and modifies circadian rhythms in P23H rats. Antioxidants. 2020;9(8) doi: 10.3390/antiox9080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Razavi M.S., Ebrahimnejad P., Fatahi Y., D'Emanuele A., Dinarvand R. Recent developments of nanostructures for the ocular delivery of natural compounds. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.850757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Achouri D., Alhanout K., Piccerelle P., Andrieu V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013;39(11):1599–1617. doi: 10.3109/03639045.2012.736515. [DOI] [PubMed] [Google Scholar]
- 148.Huang H.Y., Wang M.C., Chen Z.Y., Chiu W.Y., Chen K.H., Lin I.C., Yang W.V., Wu C.C., Tseng C.L. Gelatin-epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018;13:7251–7273. doi: 10.2147/IJN.S173198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shim W., Kim C.E., Lee M., Lee S.H., Park J., Do M., Yang J., Lee H. Catechin solubilization by spontaneous hydrogen bonding with poly(ethylene glycol) for dry eye therapeutics. J. Contr. Release. 2019;307:413–422. doi: 10.1016/j.jconrel.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 150.Miyagawa T., Chen Z.Y., Chang C.Y., Chen K.H., Wang Y.K., Liu G.S., Tseng C.L. Topical application of hyaluronic acid-RGD peptide-coated gelatin/epigallocatechin-3 gallate (EGCG) nanoparticles inhibits corneal neovascularization via inhibition of VEGF production. Pharmaceutics. 2020;12(5) doi: 10.3390/pharmaceutics12050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shankar P.R. VigiAccess: promoting public access to VigiBase. Indian J. Pharmacol. 2016;48(5):606. doi: 10.4103/0253-7613.190766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kuo I.C., Falco M., Olmedo A., Misani L., O'Brien T.P., Reviglio V.E. Corneal tattoo with tea infusion. Food Chem. Toxicol. 2008;46(6):2303–2305. doi: 10.1016/j.fct.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 153.Achiron A., Birger Y., Karmona L., Avizemer H., Bartov E., Rahamim Y., Burgansky-Eliash Z.Z. Corneal staining and hot black tea compresses. Isr. Med. Assoc. J. 2017;19(3):152–155. [PubMed] [Google Scholar]
- 154.Sarma D.N., Barrett M.L., Chavez M.L., Gardiner P., Ko R., Mahady G.B., Marles R.J., Pellicore L.S., Giancaspro G.I., Low Dog T. Safety of green tea extracts : a systematic review by the US Pharmacopeia. Drug Saf. 2008;31(6):469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- 155.Hu J., Webster D., Cao J., Shao A. The safety of green tea and green tea extract consumption in adults - results of a systematic review. Regul. Toxicol. Pharmacol. : RTP (Regul. Toxicol. Pharmacol.) 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 156.Qin Y., Chu K.O., Yip Y.W.Y., Li W.Y., Chan S.O., Pang C.P. Green tea extract as a multiple compound is the most potent anti-inflammatory agents in endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 2014;55(13) 2879-2879. [Google Scholar]
- 157.Chaudhury S., Bag S., Bose M., Das A.K., Ghosh A.K., Dasgupta S. Protection of human γB-crystallin from UV-induced damage by epigallocatechin gallate: spectroscopic and docking studies. Mol. Biosyst. 2016;12(9):2901–2909. doi: 10.1039/c6mb00256k. [DOI] [PubMed] [Google Scholar]
- 158.Chaudhury S., Ghosh I., Saha G., Dasgupta S. EGCG prevents tryptophan oxidation of cataractous ocular lens human γ-crystallin in presence of H2O2. Int. J. Biol. Macromol. 2015;77:287–292. doi: 10.1016/j.ijbiomac.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 159.Saito Y., Hasebe-Takenaka Y., Ueda T., Nakanishi-Ueda T., Kosuge S., Aburada M., Shimada T., Ikeya Y., Onda H., Ogura H., et al. Effects of green tea fractions on oxygen-induced retinal neovascularization in the neonatal rat. J. Clin. Biochem. Nutr. 2007;41(1):43–49. doi: 10.3164/jcbn.2007006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang W., Zhang Y., Jin W., Xing Y., Yang A. Catechin weakens diabetic retinopathy by inhibiting the expression of NF-κB signaling pathway-mediated inflammatory factors. Ann. Clin. Lab. Sci. 2018;48(5):594–600. [PubMed] [Google Scholar]
- 161.Minnelli C., Moretti P., Fulgenzi G., Mariani P., Laudadio E., Armeni T., Galeazzi R., Mobbili G. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: characterization and protective effect against oxidative damage. Int. J. Pharm. 2018;552(1–2):225–234. doi: 10.1016/j.ijpharm.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 162.Lee H., Shim W., Kim C.E., Choi S.Y., Lee H., Yang J. Therapeutic efficacy of nanocomplex of poly(ethylene glycol) and catechin for dry eye disease in a mouse model. Invest. Ophthalmol. Vis. Sci. 2017;58(3):1682–1691. doi: 10.1167/iovs.16-20843. [DOI] [PubMed] [Google Scholar]
- 163.Shim W., Kim C.E., Lee M., Lee S.H., Park J., Do M., Yang J., Lee H. Catechin solubilization by spontaneous hydrogen bonding with poly(ethylene glycol) for dry eye therapeutics. J. Contr. Release. 2019;307:413–422. doi: 10.1016/j.jconrel.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 164.Fangueiro J.F., Andreani T., Fernandes L., Garcia M.L., Egea M.A., Silva A.M., Souto E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces. 2014;123:452–460. doi: 10.1016/j.colsurfb.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 165.Luo L.J., Lai J.Y. Epigallocatechin gallate-loaded gelatin-g-poly(N-isopropylacrylamide) as a new ophthalmic pharmaceutical formulation for topical use in the treatment of dry eye syndrome. Sci. Rep. 2017;7(1):9380. doi: 10.1038/s41598-017-09913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request from the corresponding author.