Abstract
Anopheles stephensi, a malaria vector species previously only known from Asia, was first detected in Africa in Djibouti in 2012, has been subsequently collected in Ethiopia, Sudan, and Somalia, and may be spreading further. Countries may wish to implement mosquito surveys to determine if An. stephensi is present, or to determine the extent of its distribution, if present. Furthermore, mosquito surveys can provide data on the bionomics of An. stephensi and its adaptation to the local environment that can help plan and implement control activities. The present strategies provide suggestions on surveillance approaches for monitoring An. stephensi. The first step is to determine the aim of the study, as this will determine the specific activities conducted in each location. Challenges related to identification and detection of resistance and sporozoites are also discussed. Results should be communicated to relevant stakeholders in a timely manner, both in country and internationally, to help understand the introduction, distribution, and bionomics of An. stephensi in a given country and work towards cross-border and coordinated international response.
Keywords: Anopheles stephensi, Invasive vector, Mosquito, Urban malaria, Survey, Africa
1. Anopheles stephensi is spreading in Africa. Why is this important?
In its native range, An. stephensi predominantly breeds in urban settings with a preference for human-made water storage containers (Surendran et al. 2019) (Fig. 1). Recent reports indicate that An. stephensi is becoming more wide-spread, not only in Asia (Dharmasiri, Perera et al. 2017, Surendran et al. 2019) but in recent years also in the Horn of Africa, where it is an invasive species. In Africa, An. stephensi was first detected in Djibouti in 2012 (Faulde et al. 2014). In 2016, An. stephensi was detected in eastern Ethiopia (Carter et al. 2018) and the Republic of Sudan (Ahmed et al. 2021a). It was also detected in Somalia in 2019 (WHO 2022). Subsequent studies have found An. stephensi to be widespread throughout Ethiopia (Balkew et al. 2020; Balkew et al. 2021; Tadesse et al. 2021) and Sudan (Ahmed et al. 2021b; Abubakr et al. 2022) (Fig. 2). The recently growing reporting of the presence of An. stephensi in new areas in Africa indicates that An. stephensi may have been present for longer than previously thought (Ahmed et al. 2021c). In addition to its endemic existence in Asia, invasive spread of An. stephensi is increasingly growing globally, with reports from Sri Lanka in 2017 and Yemen in 2022 (Dharmasiri et al. 2017, WHO 2022).
Fig. 1.

Anopheles stephensi larval habitats
Anopheles stephensi generally inhabits containers such as wells, drums, and other water storage containers (Photos a, b, d, e) (Thomas et al. 2016, Balkew et al. 2020). But An. stephensi larvae can also be found in less “typical sites” such as ponds along rivers, sewage overflows, or flooded areas (Photo c, f). When conducting a rapid survey, typical larvae sites can be targeted, but for more thorough surveys, all sites should be inspected.
Fig. 2.
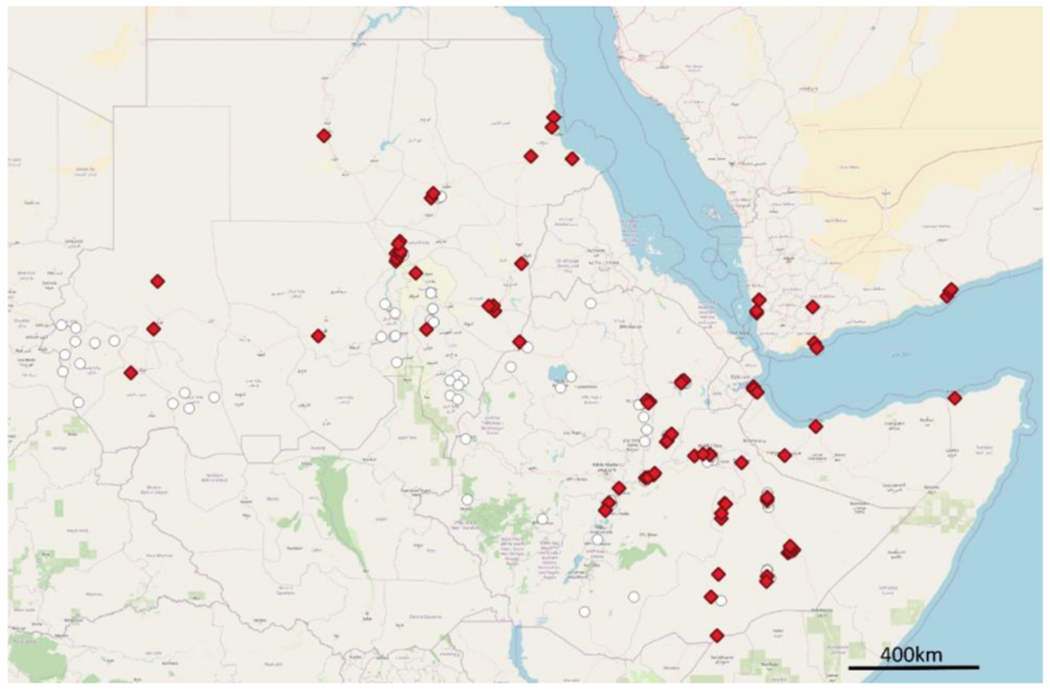
Sites where An. stephensi has been found (red diamonds) and sampled but not found (white circles) in the Republic of the Sudan, Ethiopia, and Djibouti, 2022.
Anopheles stephensi is an efficient and dominant vector for both Plasmodium vivax and P. falciparum in its native range (Sinka et al. 2011). Researchers at the Armauer Hansen Research Institute (AHRI) in Ethiopia demonstrated that the invading mosquito populations are more permissive to local Plasmodium strains than the native primary malaria vector, An. arabiensis (Tadesse et al. 2021). Anopheles stephensi emergence has been epidemiologically linked to an unusual resurgence in local malaria cases in Djibouti city (Seyfarth et al. 2019). Likewise, associated with the expansion of An. stephensi, increasing indigenous cases were also reported in Sri Lanka (Dharmasiri et al. 2017, Surendran et al. 2018, Surendran et al. 2019), a country that eliminated malaria in 2013 (Wijesundere and Ramasamy 2017). Malaria may therefore become an increasing problem in urban and heavily populated settings, particularly in non-endemic areas including Africa (Takken and Lindsay, 2019).
Malaria control programs in Africa traditionally focus on rural settings, which are where most infections occur due to the bionomics of native malaria vectors. Although malaria transmission is a health concern in some urban settings (Dash et al. 2008, Wilson et al. 2015), urban cases are often imported from (rural) areas of intense transmission due to mfovement of people at the urban-rural interface (Gómez et al. 2017, Fillinger & Lindsay 2011); as such, urban settings can be sinks of malaria transmission. With the adaptation of existing vectors to urban environments (Azrag & Mohammed 2018) and emergence of invasive vectors such as An. stephensi (Seyfarth et al. 2019), sustained malaria transmission in urban settings may be becoming more likely when An. stephensi is present. Urban areas can thereby form foci of active malaria transmission, challenging malaria control and elimination efforts (Chaparro et al. 2017).
A technical consultative meeting convened in 2019 at the World Health Organization (WHO) identified that there is potential for spread of An. stephensi across Africa (World Health Organization 2019). Anopheles stephensi is estimated to be capable of spreading in African towns and cities, putting approximately 126 million people at greater risk of malaria (Sinka et al. 2020). If An. stephensi spreads to all suitable areas in Ethiopia, it is estimated that P. falciparum malaria cases could increase by 50% (95% CI 14–90%), resulting in the need for substantial increases in financial investments just to maintain current levels of malaria cases (Hamlet et al. 2022). To draw attention to this situation, the WHO published a vector alert calling for active An. stephensi surveillance in the region and in response countries in the Horn of Africa intensified surveillance (WHO 2019). Data is particularly limited from other parts of the continent so the extent of invasive An. stephensi and therefore appropriate response strategies remain unclear. Surveillance of An. stephensi should be initiated to support immediate and appropriate action against this invasive vector before it spreads widely through the non-endemic areas worldwide, particularly in Africa, which has the highest malaria morbidity and mortality in the world.
2. Types of surveys
The first step in any survey is to determine the aim of the study (ECDC 2012). The aims will determine the activities that will be conducted as part of the survey. These aims may be to detect An. stephensi, determine its distribution, or to learn more about its bionomics.
There are four main types of surveys that can be conducted to detect and monitor An. stephensi. These include (1) exploratory surveys when An. stephensi has not yet been detected in a country, (2) post-detection surveys, (3) rapid surveys to further determine the distribution at finer scales, and (4) bionomic surveys to better understand biological and behavioral aspects of An. stephensi to inform future surveillance and control approaches. The logistical and financial needs must also be considered when organizing a study.
2.1. Exploratory surveys
When An. stephensi has not been detected in a country, but when there is concern that it may be present, an exploratory survey can be conducted. The aim of this survey should be to find An. stephensi if it is present. The survey should focus on the most likely locations An. stephensi might be detected. These may include:
Urban areas in regions neighboring countries where An. stephensi has been found
International and national points of entry including seaports, airports, dry ports, or other areas where movement of goods, animals, or people might permit the movement of An. stephensi adults or larvae
Urban areas along major transportation axes
Districts, cities, or other localities where increased malaria cases have been noted, particularly if there are no other explanations for the increase or if increased cases are occurring during the dry season
Once the locations to be sampled have been determined, a sampling protocol can be developed. Larvae are often more readily collected, and so the larval sites where An. stephensi larvae are often found, such as “wells, overhead or ground-level water tanks, cisterns, coolers, roof gutters, or other artificial containers” (Nagpal and Sharma, 1995), should be targeted and monitored. It should be noted that larvae can be found in other types of habitats (see Fig. 1), which can also be investigated. Adult female An. stephensi are primarily, but not exclusively, zoophilic, so monitoring larval sites around animal shelters/pens may be particularly productive. If Anopheles mosquitoes have been collected as part of other malaria entomological monitoring, these might also be examined with greater attention to ensure that no An. stephensi have been missed. Anopheles stephensi may be easily misidentified as An. gambiae s.l. if the morphological key being used does not include An. stephensi.
Teams of collectors (generally as teams of two) can examine the study area, and sample the potential larval sites as described below (Section 3.1). Each time a larval site is sampled, GPS coordinates should be recorded, along with other information about the habitat (habitat type, distance from human/animal habitation, source of water, shade, vegetation, presence of predators, depth, volume, presence of other larvae, etc). Data should be recorded for all sites sampled, including sites where no larvae were collected (negative sites), as this will provide information about the geographic extent of the survey and the total number of sites sampled. Whenever possible, information about the existence of other arthropods, particularly mosquito species, should be recorded.
As a general approximation, exploratory surveys should aim to investigate a minimum of 100 larval sites per urban area, and 20 larval sites per rural area. If the survey is at a national level, 10 localities might be investigated, and if at a sub-national level 5–10 localities may be investigated. If possible, a survey should be done during the dry season and during the rainy season. These are only strategies, and the number may vary considerably by the country, survey locality, resources available, and epidemiological change in malaria cases. Even if small numbers of localities or larval sites are sampled, this can provide essential information if An. stephensi is found, or valuable baseline data if it is not found.
2.2. Post-detection surveys
Once An. stephensi has been found in a country, the aim of the surveys shifts to determining the distribution of An. stephensi within the country. The distribution of An. stephensi in the country can be determined by conducting a post-detection survey in one or two localities per region (variable depending on size/population/geography of the region), followed by collections in increasing numbers of localities in regions where An. stephensi was detected. In some cases, An. stephensi may not be found in a region, but due to other factors, those organizing the survey may find it useful to conduct further sampling there. For example, if no An. stephensi were found in a region, but An. stephensi were found in all of the surrounding regions, it may be worth conducting further monitoring there. Those organizing the survey should engage local stakeholders and use their knowledge of the regions to guide them in the development of the post-detection survey.
The conduct of the post-detection survey can be quite similar to the sampling conducted in the exploratory survey. Teams can be organized to conduct larval sampling in urban or rural areas, targeting the most likely sites. Again, it is essential to record positive and negative sites using GPS coordinates and descriptions of the sites.
2.3. Rapid local distribution surveys
Rapid local distribution surveys can be conducted to quickly determine the distribution of An. stephensi within a region. These surveys can be conducted to determine where control interventions should be implemented. For these surveys, the primary question is whether An. stephensi is present or absent in a community. Teams of technicians should aim to conduct surveys in several villages every day, sampling a set number of larval sites in each village (i.e., 20), or sampling each village for a limited amount of time (i.e., one hour) before moving on to the next community. The number of dips taken in each larval site need not be recorded as the aim of this survey is not to assess larval density, rather to determine presence or absence. The principle behind this method of sampling is that even if some positive sites are missed, the rapid sampling will allow a higher number of sites to be sampled, and a better picture of the distribution of An. stephensi in an area.
The other benefit of this type of study is the reduced cost of sampling. Many localities can be sampled in a few days, providing information to health authorities in a short timeframe. This style of sampling can also be used in exploratory or post-detection surveys if costs or time are concerns.
2.4. Bionomic surveys
The aforementioned surveys focus on determining the presence, absence, and distribution of An. stephensi at different geographic scales. However, there is a severe lack of information about the bionomics of An. stephensi in Africa, which is needed to guide further surveillance and control activities. Bionomic surveys may assess the most common larval sites, determine the preferred hosts, evaluate the role of An. stephensi in malaria transmission, and determine insecticide susceptibility.
2.4.1. Determining the most common larval sites
Human activities such as construction, brick manufacturing, domestic water reservation, and well-digging play a major role in creating suitable larval habitats for An. stephensi mosquitoes. Anopheles stephensi larvae are typically found in containers or cisterns with clean water (including cryptic habitats such as deep wells) (World Health Organization 2019), overhead tanks (Thomas et al. 2016), curing water in construction sites, groundwater tanks, tires, barrels, jerrycans, and tins (Abubakr et al. 2022; Balkew et al. 2020; Kumar & Thavaselvam, 1992). Determining the most productive larval sites allows for better identification of potential larval sites in future surveys, as well as better targeting of the most productive larval sites for targeted vector control. This type of survey has been used for Aedes aegypti, and more information can be found in the Operational guide for assessing the productivity of Aedes aegypti breeding sites (WHO 2011).
2.4.2. Determining preferred hosts
Studies in Ethiopia confirmed that An. stephensi prefers to rest in animal shelters (>90% caught in animal shelters) and feed on animals (>50%) with a substantial proportion fed on multiple sources including humans (Tadesse et al. 2021). This is similar to findings in Asia that found most An. stephensi blood meals were taken from cattle (Mehravaran et al. 2012, Thomas et al 2017). In line with these findings, aspirator collections from animal shelters were the most efficient, followed by outdoor human landing-catches (HLC) (Tadesse et al. 2021). Centers for Disease Control (CDC) light traps and pyrethrum spray catches (PSC) caught low numbers of An. stephensi indoors (Balkew et al. 2020). It should be noted that collecting mosquitoes from animal shelters or human houses may result in a biased collection and this should be considered when making judgements about host preferences (WHO 1975).
2.4.3. Determining whether An. stephensi is involved in local malaria transmission
Collecting mosquitoes to identify infection status is challenging, especially in low transmission settings (Filler et al. 2006). Different adult mosquito-collection methods, including aspiration from resting sites, host-baited traps, or other methods, may be deployed in survey areas. Samples can be preserved in tubes with silica gel or with 70% ethanol to be transferred from the field to the laboratory (Faulde et al. 2014). The collected mosquitoes can then be analyzed in the laboratory using molecular and immunological methods described below (Sections 3.5, 3.7).
2.4.4. Determining insecticide susceptibility of An. stephensi
Estimates suggested that insecticide-based vector control interventions had contributed more than 75% to the reduction of P. falciparum malaria in Africa between 2000 and 2015 (Bhatt et al., 2015). Continued use of insecticides results in the development of resistance in vectors and threatens the effectiveness of insecticide-based interventions, which led the WHO to launch the Global plan for insecticide resistance management in malaria vectors (GPIRM) (WHO, 2012). An important component of this resistance management approach is the monitoring of resistance in vector populations. This is generally done through a collection of larvae in the field (preferably from a variety of larval habitats), which are then reared in the laboratory before testing as adults. The methods for testing are further described below (Section 3.8).
3. Methods
3.1. Collection methods
Collection of larvae and pupae of An. stephensi can be made through dipping, netting or pipetting from aquatic habitats. The use of each sampling method depends on the nature, type, and volume of the aquatic site (O’Malley 1995). If the larval density is to be calculated, the number of dips and the number of larvae collected can be counted to determine the average number of larvae per dip. Larvae can be collected and stored in a well-labelled container and returned to the laboratory for preservation or rearing.
Anopheles stephensi are commonly found in water storage containers (particularly birka and construction water reservoirs), wells, stream margins, and can also be found in a variety of other water bodies (puddles, tires, plastic containers, barrels, etc). For rapid local distribution surveys, the most common sites can be targeted, whereas for the bionomics studies, a wide variety of potential sites should be sampled. Whenever possible, GPS points and larval site characteristics should be recorded for all sites sampled including the ones negative for the presence of An. stephensi aquatic stages.
The sampling of adult An. stephensi is more challenging (Balkew et al. 2020), with the most successful methods being aspiration of adult mosquitoes from animal shelters (Balkew et al. 2021). While standard methods such as HLC and trapping with CDC light traps have been only moderately successful in collecting An. stephensi, there is a need for a better method for collecting adult An. stephensi.
3.2. Data collection
When thinking about what data to record when conducting an An. stephensi survey, it can be valuable to develop a preliminary version of data recording tools (Appendix). This will ensure that the data categories and tools correspond to field conditions and changes to the data collection midway through the collection can be avoided. Furthermore, the standardization of data collection tools across different surveys in different areas can help in the collection of comparable data.
Another important aspect of data recording is the recording of negative sites. For example, in Fig. 3, only the positive sites (blue stars) were recorded. One might interpret from this display of the data that An. stephensi is widespread in the town. However, when positive and negative sites (white circles) are shown, as in Fig. 4, the interpretation of the data is more complete as the focal nature of the positive sites is much clearer. This can help to focus surveys on the positive area to understand why An. stephensi is present only in that area and target control measures.
Fig. 3.
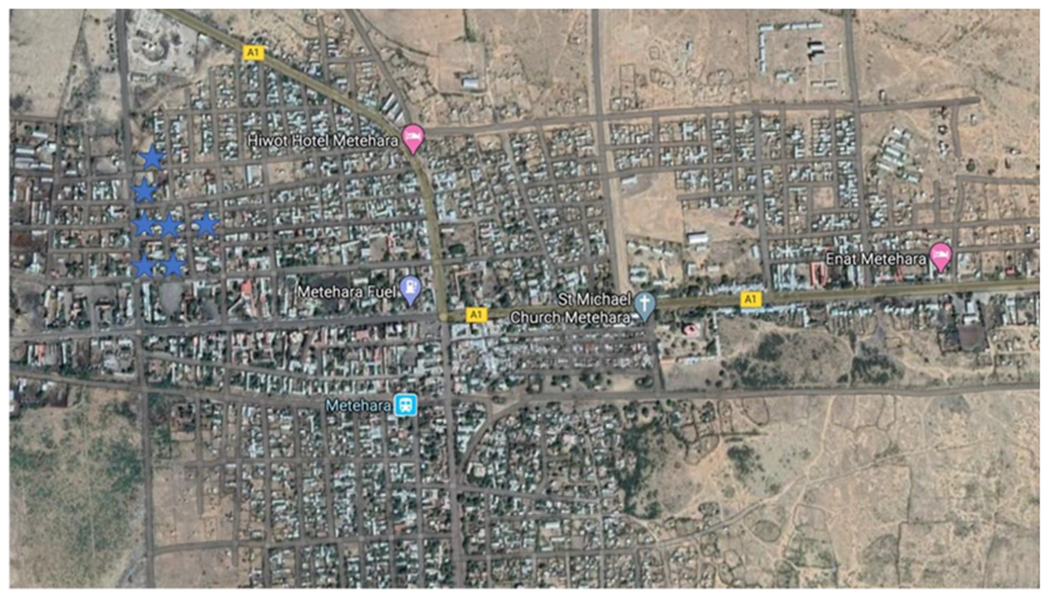
Survey results showing only positive larval sites (blue stars).
Fig. 4.
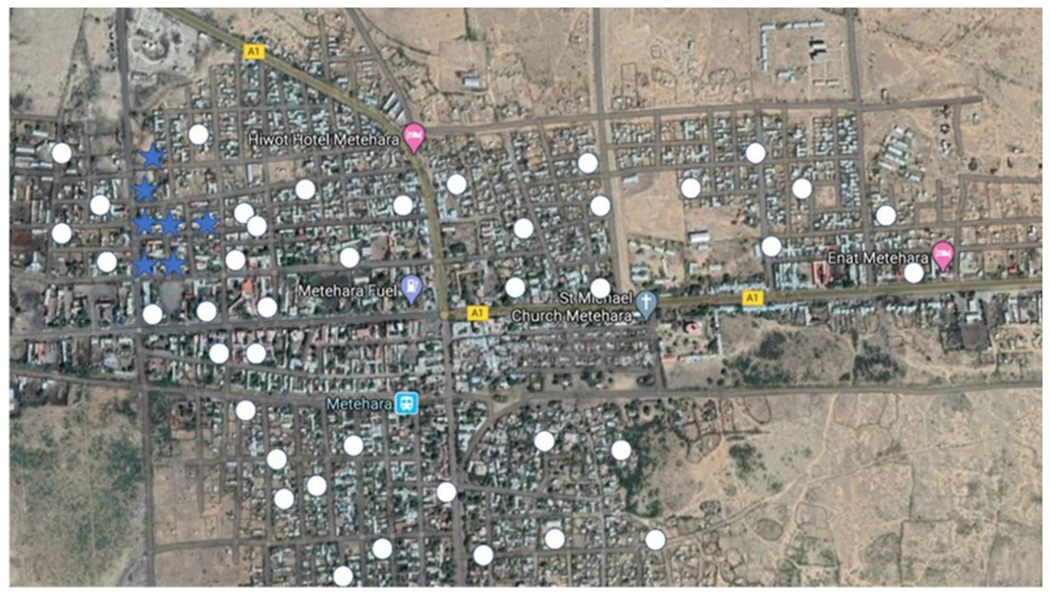
Survey results showing positive larval sites (blue stars) and negative larval sites (white circles).
3.3. Identification of An. stephensi eggs
There are three proposed ecological variants of An. stephensi: type form, mysorensis, and and intermediate form (Subbarao et al. 1987). The ridges on the floats of An. stephensi eggs can be used to determine the form of An. stephensi present, however, it is unclear exactly how this may affect malaria transmission, particularly in the African context.
3.4. Identification of An. stephensi larvae
Currently, there is no morphological identification key for African Anopheles larvae that includes An. stephensi. To examine any dead larvae to determine whether they are An. stephensi, reference can be made to both African identification keys (Gillies & De Meillon 1968; Gillies & Coetzee 1987) and Asian identification keys (Christophers 1933, Tyagi et al., 2015). But please note that use of any of these identification keys in isolation will not be sufficient to identify An. stephensi. If possible, rear the larvae to the adult stage to conduct the morphological identification.
3.5. Identification of An. stephensi adults
The identification of An. stephensi adult females is now possible as An. stephensi was included in the most recent African Anopheles identification key (Coetzee 2020). The key morphological characteristics of An. stephensi that distinguish it from An. gambiae are shown in the WHO vector alert (WHO 2019).
3.6. Molecular confirmation of An. stephensi
To confirm a morphological identification of An. stephensi, molecular laboratory analysis is needed. There is no species-specific PCR that can identify An. stephensi (as is available for other African Anopheles), so Sanger sequencing, most commonly of the ITS2 and COI regions, is required to confirm the identification. More information on the methods for sequencing is provided in the MR4 manual (MR4 2014).
3.7. Recording Aedes aegypti
One finding from surveys conducted in the Horn of Africa was the coexistence of Ae. aegypti larvae in the same larval sites as An. Stephensi (Balkew et al. 2021; Tadesse et al. 2021), as has been noted in India (Thomas et al. 2016). Aedes aegypti is a primary vector of dengue, chikungunya, Yellow fever, and other arboviruses. This sympatry provides an opportunity for integrated vector surveillance and control.
3.8. Detection of sporozoites in adult female An. stephensi
To determine whether An. stephensi is involved in local malaria transmission, sporozoite stages of Plasmodium parasites can be detected in field-collected An. stephensi through several methods. The primary method is detecting the circumsporozoite protein (CSPs) by enzyme linked immunosorbent assays-ELISA (Wirtz et al. 1987), or bead-based assays (Sutcliffe et al. 2021). The head and thorax are separated from the abdomen and are then processed according to published protocols (MR4 2014). As there is a risk of false positive results, particularly when mosquitoes have fed on animal blood (Durnez et al. 2011), any positive samples should be retested after boiling at 100°C for 10 minutes. Confirmation may also be conducted through PCR methods to detect Plasmodium DNA using a generic (Echeverry et al. 2017) or species-specific PCR (Singh et al. 1999) in settings where multiple Plasmodium species circulate.
3.9. Determination of insecticide susceptibility
There are two main test protocols for assessing mosquito susceptibility to insecticides: the WHO susceptibility (“tube”) test (WHO, 2016) and the CDC bottle bioassay (Brogdon & Chan 2010). Either or both tests may be used, but because of different methodologies and outcome measures, the results of the two test procedures are not directly comparable. Both protocols include procedures to measure intensity of resistance, which may be useful for detecting changes in resistance over time and for detecting metabolic-based mechanisms, through the use of synergists.
Larvae can be collected as described above, and reared to adults in laboratories or field insectaries (Khan, et al. 2013). Bioassays should be performed using 3-5 day-old female adult mosquitoes reared from field-collected larvae following the standard protocols (WHO, 2016).
As identification of An. stephensi larvae prior to testing is a challenge, all mosquitoes should be identified at the end of the test. Mosquitoes that are dead at the end of the test should be kept separately from those that survived, so that if there are a mix of species in the tests, the results for An. stephensi can still be tabulated.
3.10. Presenting results
After a survey is complete, it is important to present the results to the National Malaria Control Program and its stakeholders (research institutes, funding agencies, etc.) that are involved in vector control. As integrated control of An. stephensi may require collaboration with other ministries (Urban Affairs, Environment, Transportation, Veterinary Services), a whole government approach should be considered.
The key findings that can be shared with partners include GPS coordinates of all sites sampled, presence/absence status of each larval site, any larval site characteristics collected, and other bionomic findings (such as blood meal analysis, sporozoite rates, insecticide resistance, etc). These data can also be compared with other data from routine entomological monitoring to put the results into context.
The WHO maintains a map of the locations of endemic and invasive records of An. stephensi worldwide (https://apps.who.int/malaria/maps/threats/) and any confirmed positive sites should be shared with the WHO promptly to improve global understanding of the spread of this vector.
4. Concluding remarks
The spread of An. stephensi poses a threat to malaria control in non-endemic areas, particularly in Africa where malaria is a serious public health issue. Entomological surveys can determine the new introductions, current distributions, extent of spread over time, along with detecting the most common larval sites in a particular country context, and aid in understanding the overall bionomics of this invasive species in a given country context, in order to implement effective control measures. The sooner these surveys are initiated and conducted; the sooner countries can prepare and act with an appropriate prevention and control response.
5. Outstanding questions.
What is the best way to collect host-seeking adult An. stephensi?
How might malaria transmitted by An. stephensi be detected through epidemiological surveillance?
How long has An. stephensi been present in Africa, and how quickly is it spreading?
What vector control tools (existing or in development) are most effective in controlling An. stephensi?
How often are An. stephensi larvae found in the same larval sites as Ae. aegypti, and what might this mean for integrated vector control? What habitat characteristics are associated with co-habitation by both vectors?
Can An. stephensi widely inhabit rural settings? If so, how does An. stephensi compete with native rural Anopheles human malaria vectors?
Upon initial detection, how quickly should action be taken to implement interventions and should the control target be containment, elimination, or mitigation?
What are the routes of An. stephensi introductions and spread? Is this invasion facilitated by human or animal dynamics?
What is the role of climate change and anthropogenic factors in the invasion, spread, and establishment of An. stephensi into new environments?
What are the mechanisms of insecticide resistance in An. stephensi?
Acknowledgments
Financial support for three of the authors (SI, SZ, MY) was provided by the U.S. President’s Malaria Initiative.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or United States Agency for International Development (USAID).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Azrag RS, Mohammed BH, 2018. Anopheles arabiensis in Sudan: a noticeable tolerance to urban polluted larval habitats associated with resistance to Temephos. Malar. J 17, 204. 10.1186/s12936-018-2350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Pignatelli P, Elaagip A, Hamid MMA, Alrahman OF, Weetman D, 2021a. Invasive malaria vector Anopheles stephensi mosquitoes in Sudan, 2016-2018. Emerg. Infect. Dis 27, 2952–2954. https://wwwnc.cdc.gov/eid/article/27/11/21-0040_article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Khogali R, Elnour MAB, Nakao R, Salim B, 2021b. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum State, Central Sudan. Parasit. Vectors 14, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Abubakr M, Ali Y, Siddig EE, Mohamed NS, 2021c. Vector control strategy for Anopheles stephensi in Africa. The Lancet Microbe. 2022 Feb 25. https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(22)00039-8/fulltext. [DOI] [PubMed] [Google Scholar]
- Abubakr M, Sami H, Mahdi I, Altahir O, Abdelbagi H, Mohamed NS, Ahmed A, 2022. The phylodynamic and spread of the invasive Asian malaria vectors, Anopheles stephensi. in Sudan. Biol. 11, 409. https://www.mdpi.com/2079-7737/11/3/409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkew M, Mumba P, Dengela D, Yohannes G, Getachew D, Yared S, Chibsa S, Murphy M, George K, Lopez K, Janies D, Choi SH, Spear J, Irish SR, Carter TE, 2020. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit. Vectors 13, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkew M, Mumba P, Yohannes G, Abiy E, Getachew D, Yared S, Worku A, Gebresilassie A, Tadesse FG, Gadisa E, Esayas E, Ashine T, Ejeta D, Dugassa S, Yohannes M, Lemma W, Yewhalaw D, Chibsa S, Teka H, Murphy M, Yoshimizu M, Dengela D, Zohdy S, Irish S, 2021. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018-2020. Malar. J 20, 263. 10.1186/s12936-021-03801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Batde KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briët O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW, 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. https://www.nature.com/articles/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon WG, Chan A, 2010. Guidelines for evaluating insecticide resistance in vectors using the CDC bottle bioassay/methods in Anopheles research. CDC, CDC Atlanta USA. Technical Report. 28 pp. https://www.cdc.gov/malaria/resources/pdf/fsp/ir_manual/ir_cdc_bioassay_en.pdf. [Google Scholar]
- Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, Ibrahim M, Mohammed S, Janies S, 2018. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 188, 180–186. https://www.sciencedirect.com/science/article/pii/S0001706X18305618. [DOI] [PubMed] [Google Scholar]
- Chaparro PE, Molina K, Alzate A, Padilla J, Arévalo-Herrera M, Herrera S, 2017. Urban malaria transmission in a non-endemic area in the Andean region of Colombia. Mem. Inst. Oswaldo Cruz 112, 797–804. https://www.scielo.br/j/mioc/a/MGgBzfMFWwMjp47wSMpSk9m/?lang=en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers SR, 1933. The Fauna of British India including Ceylon and Burma. Diptera. Vol. IV. Family Culicidae. Tribe Anophelini. [Google Scholar]
- Coetzee M, 2020. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar. J 19, 1–20, 2020. 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash AP, Valecha N, Anvikar AR, Kumar A, 2008. Malaria in India: challenges and opportunities. J. Biosci 33, 583–592. 10.1007/s12038-008-0076-x. [DOI] [PubMed] [Google Scholar]
- Dharmasiri AGG, Perera AY, Harishchandra J, Herath H, Aravindan K, Jayasooriya HTR, Ranawaka GR, Hewavitharane M, 2017. First record of Anopheles stephensi in Sri Lanka: a potential challenge for prevention of malaria reintroduction. Malar. J 16, 326. 10.1186/s12936-017-1977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, Sochantha T, Coosemans M, 2011. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J 10, 195. 10.1186/1475-2875-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry DF, Deason NA, Makuru V, Davidson J, Xiao H, Niedbalski J, et al. , 2017. Fast and robust single PCR for Plasmodium sporozoite detection in mosquitoes using the cytochrome oxidase I gene. Malar. J 16, 230. 10.1186/s12936-017-1881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC, 2012. Guidelines for the surveillance of invasive mosquitoes in Europe. ECDC, Stockholm. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf. [PubMed] [Google Scholar]
- Faulde MK, Rueda LM, Khaireh BA, 2014. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop 139, 39–43. https://www.sciencedirect.com/science/article/pii/S0001706X14002216?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- Filler SJ, MacArthur JR, Parise M, Wirtz R, Eliades MJ, Dasilva A, Steketee R, 2006. Locally acquired mosquito-transmitted malaria; a guide for investigations in the United States. MMWR Recomm. Rep 55 (RR-13), 1–9. https://www.cdc.gov/mmwr/pdf/rr/rr5513.pdf. [PubMed] [Google Scholar]
- Fillinger U, Lindsay SW, 2011. Larval source management for malaria control in Africa: myths and reality. Malar. J 10, 353. 10.1186/1475-2875-10-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT, de Meillon B, 1968. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Publications of the South African Institute for. Med. Res 54, 1–343. [Google Scholar]
- Gillies MT, Coetzee M, 1987. A supplement to the Anophelinae of Africa South of the Sahara (Afro-Tropical region). Publications of the South African Institute for Medical Research, Johannesburg, p. 55. [Google Scholar]
- Gómez KM, Caicedo MA, Gaitán A, Herrera-Varela M, Arce MI, Vallejo AF, Padilla J, Chaparro P, Pacheco MA, Escalante AA, Arevalo-Herrera M, Herrera S, 2017. Characterizing the malaria rural-to-urban transmission interface: The importance of reactive case detection. PLoS Negl. Trop. Dis 11, e0005780. 10.1371/journal.pntd.0005780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlet A, Dengela D, Tongren JE, Tadesse FG, Bousema T, Sinka M, Seyoum A, Irish SR, Armistead JS, Churcher T, 2022. The potential impact of Anopheles stephensi establishment on the transmission of Plasmodium falciparum in Ethiopia and prospective control measures. BMC Med. 20, 135. 10.1186/s12916-022-02324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Farid A, Zeb A, 2013. Development of inexpensive and globally available larval diet for rearing Anopheles stephensi (Diptera: Culicidae) mosquitoes. Parasit. Vectors 6, 1–7. 10.1186/1756-3305-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thavaselvam D, 1992. Breeding habitats and their contribution to Anopheles stephensi in Panaji. Indian J. Malariol 29, 35–40. https://nimr.org.in/images/pdf/VOL_29_No.1March1992.pdf. [PubMed] [Google Scholar]
- Mehravaran A, Vatandoost H, Oshaghi MA, Abai MR, Edalat H, Javadian E, Mashayekhi M, Piazak N, Hanafi-Bojd AA, 2012. Ecology of Anopheles stephensi in a malarious area, southeast of Iran. Acta Med. Iranica 50, 61–65. https://acta.tums.ac.ir/index.php/acta/article/view/3860/3835. [PubMed] [Google Scholar]
- MR4. 2014. Methods in Anopheles Research. 419 pp. https://www.beiresources.org/portals/2/MR4/MR4_Publications/Methods%20in%20Anopheles%20Research%202014/2014MethodsinAnophelesResearchManualFullVersionv2tso.pdf. [Google Scholar]
- Nagpal BN, Sharma VP, 1995. Indian Anophelines. Science Publishers, New Hampshire, p. 416. [Google Scholar]
- O’Malley C, 1995. Seven Ways to a Successful Dipping Career. Wing Beats 6, 23–24. [Google Scholar]
- Seyfarth M, Khaireh BA, Abdi AA, Bouh SM, Faulde MK, 2019. Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: populations established-malaria emerging. Parasit. Res 118, 725–732. 10.1007/s00436-019-06213-0. [DOI] [PubMed] [Google Scholar]
- Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA, 1999. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg 60, 687–692. [DOI] [PubMed] [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IRF, Kabaria CW, Harbach RH, Hay SI, 2011. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit. Vectors 4, 89. 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, Willis KJ, et al. , 2020. A new malaria vector in Africa: Predicting the expansion range of An. stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. U. S. A 117, 24900–24908. 10.1073/pnas.2003976117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao SK, Vasantha K, Adak T, Sharma VP, Curtis CF, 1987. Egg-float ridge number in Anopheles stephensi: ecological variation and genetic analysis. Med. Vet. Entomol 1, 265–271. [DOI] [PubMed] [Google Scholar]
- Surendran SN, Sivabalakrishnan K, Gajapathy K, Arthiyan, Jayadas TTP, Karvannan K, Raveendran S, Karanaratne SHPP, Ramasamy R, 2018. Genotype and biotype of invasive Anopheles stephensi in Mannar Island of Sri Lanka. Parasit. Vectors 11, 3. 10.1186/s13071-017-2601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran SN, Sivabalakrishnan K, Sivasingham A, Jayadas TTP, Karvannan K, Santhirasegaram S, Gajapathy, et al. , 2019. Anthropogenic factors driving recent range expansion of the malaria vector Anopheles stephensi. Front. Public Health 7, 53. 10.3389/fpubh.2019.00053/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe AC, Irish SR, Rogier E, Finney M, Zohdy S, Dotson EM, 2021. Adaptation of ELISA detection of Plasmodium falciparum and Plasmodium vivax circumsporozoite proteins in mosquitoes to a multiplex bead-based immunoassay. Malar. J 20, 377. 10.1186/s12936-021-03910z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse FG, Ashine T, Teka H, Esayas E, Messenger LA, Chali W, Meerstein-Kessel L, Walker T, Behaksra SW, Lanke K, Heutink R, Jeffries CL, Mekonnen DA, Hailemeskel E, Tebeje SK, Tafesse T, Gashaw A, Tsegaye T, Emiru T, Simon K, Bogale EA, Yohannes G, Kedir S, Shumie G, Sabir SA, Mumba P, Dengela D, Kolaczinski JH, Wilson A, Churcher TS, Chibsa S, Murphy M, Balkew M, Irish S, Drakely C, Gadisa E, Bousema T, 2021. Anopheles stephensi Mosquitoes as Vectors of Plasmodium vivax and falciparum, Horn of Africa. Emerg. Infect. Dis 27, 603–607. https://wwwnc.cdc.gov/eid/article/27/2/20-0019_article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, Lindsay S, 2019. Increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerg. Infect. Dis 25, 1431. https://wwwnc.cdc.gov/eid/article/25/7/19-0301_article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Ravishankaran S, Justin JA, Asokan A, Mathai MT, Valecha N, Thomas MB, Eapen A, 2016. Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai, India. Malar. J 15, 274. 10.1186/s12936-016-1321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Ravishankaran S, Justin NA, Asokan A, Mathai MT, Valecha N, Montgomery J, Thomas MB, Eapen A, 2017. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar. J 16, 1–7. 10.1186/s1293601717645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi BK, Munirathinam A, Venkatesh A, 2015. A catalogue of Indian mosquitoes. Int. J. Mosq. Res 2, 50–97. https://www.dipterajournal.com/pdf/2015/vol2issue2/PartB/1-4-3-308.pdf. [Google Scholar]
- Wijesundere DA, Ramasamy R, 2017. Analysis of Historical Trends and Recent Elimination of Malaria from Sri Lanka and Its Applicability for Malaria Control in Other Countries. Front Public Health 5, 212. 10.3389/fpubh.2017.00212/iull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Krogstad DJ, Arinaitwe E, Arevalo-Herrera M, Chery L, Ferreira MU, Ndiaye D, Mathanga DP, Eapen A, 2015. Urban Malaria: Understanding its Epidemiology, Ecology, and Transmission across seven diverse ICEMR network sites. Am. J. Trop. Med. Hyg 93 (3 Suppl), 110–123. https://www.ajtmh.org/view/journals/tpmd/93/3_Suppl/article-p110.xml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, Esser KM, Beaudoin RL, Andre RG, 1987. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull. World Health Organ 65, 39–45. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2490858/. [PMC free article] [PubMed] [Google Scholar]
- WHO. 1975. Manual on practical entomology in malaria. Part II Method and Techniques. 196 pp. [Google Scholar]
- WHO. 2011. Operational guide for assessing the productivity of Aedes aegypti breeding sites. 30 pp. https://tdr.who.int/publications/m/item/2011-10-31-operational-guide-for-assessing-the-productivity-of-aedes-aegypti-breeding-sites. [Google Scholar]
- WHO. (2012) Global plan for insecticide resistance management in malaria vectors (GPIRM). 24 pp. https://apps.who.int/iris/bitstream/handle/10665/44846/9789241564472_eng.pdf?sequence=1&isAllowed=y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2016. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Second edition. 54 pp. https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf. [Google Scholar]
- WHO. 2019. Vector alert: Anopheles stephensi invasion and spread. WHO/HTM/GMP/2019.09. 4 pp. https://www.who.int/publications/i/item/WHO-HTM-GMP-2019.09. [Google Scholar]
- WHO. 2022. Malaria threats map. Accessed March 23, 2022. https://apps.who.int/malaria/maps/threats/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


