Abstract
Cellular sodium ion (Na+) homeostasis is integral to organism physiology. Our current understanding of Na+ homeostasis is largely limited to Na+ transport at the plasma membrane. Organelles may also contribute to Na+ homeostasis, however, the direction of Na+ flow across organelle membranes is unknown because organellar Na+ cannot be imaged. Here, we report a pH-independent, organelle-targetable, ratiometric probe that reports lumenal Na+. It is a DNA nanodevice containing a Na+-sensitive fluorophore, a reference dye and an organelle targeting domain. By measuring Na+ at single endosome resolution in mammalian cells and in C. elegans, we discovered that lumenal Na+ levels in each stage of the endolysosomal pathway exceed cytosolic levels and decrease as endosomes mature. Further, we find that lysosomal Na+ levels in nematodes are modulated by the Na+/H+ exchanger NHX-5 in response to salt stress. The ability to image sub-cellular Na+ will unveil mechanisms of Na+ homeostasis at an increased level of cellular detail.
Cells move Na+ across their plasma membrane and organelle membranes to regulate both cytosolic and organellar Na+ and thereby maintain cellular Na+ homeostasis. Organelle membranes account for ~95% of total membrane in the cell, yet most of our understanding of cellular Na+ homeostasis relates to its movement across only 2–5% that comprises the plasma membrane1. Few lines of evidence suggest that organelles could contribute to the mobilization and transport of Na+ in single cells. Many endosomal Na+/H+ exchangers (NHEs) were first identified in yeast due to the lethality they caused upon salt stress when they were knocked out2,3. In humans, there are thirteen NHE proteins (NHE1–9, NHA1–2, SLC9C1–2) encoded by the SLC9A-C gene family4,5. NHE9 resides in late endosomes and is genetically linked to autism6,7. Loss of function mutations in NHE6, which resides in early and recycling endosomes, causes the X-linked neurological disorder Christianson’s syndrome in humans8. In plants and fish, the loss of a vacuolar Na+/H+ exchanger (Nhx1) in Japanese morning glory or a lysosomal Na+/Ca2+/K+ transporter (slc24a5) in zebrafish cause stark pigmentation phenotypes9,10. Apart from transporters, organelles have voltage-gated Na+ channels11–13 that are likely functional since many endocytic organelles were recently found to harbor high membrane potential14. Although extracellular and cytosolic Na+ levels at the tissue and single cell levels are known15,16, those within organelles are not. Consequently, the direction of ion flow across an organelle membrane cannot be predicted if a given organelle-resident Na+ channel or transporter is activated and therefore we cannot predict how organelles might contribute, if at all, to cellular Na+ homeostasis in health and disease.
Results
Organellar Na+ levels have not been mapped because there are no probes that work in the acidic conditions of organelle lumens. All fluorescent Na+ probes are acid sensitive because they detect Na+ by coordination via protonatable groups17. Further, genetically encodable reporters of Na+ do not exist. Hence, previous estimates of lumenal Na+ relied on elemental analysis of isolated organelles or null point titration that averages the information from different organelles18,13. Given recent evidence of organelle subpopulations that differ either in lumenal ionic composition19, metabolite content20, or membrane potential14 population-averaged measurements may mask the precise contribution of organelles to cellular Na+ homeostasis13,18.
Here, we have developed a pH-insensitive Na+ reporter, denoted RatiNa, that can ratiometrically image intracellular Na+ in single organelles in intact cells. RatiNa is a 45-base pair DNA duplex comprising three single-stranded DNA (ssDNA) molecules: a 25-mer strand carrying a Na+ sensitive fluorophore for sensing (D1); a 45-mer strand bearing an ion-insensitive internal reference dye for ratiometry (D2), and a 20-mer strand harboring the targeting module that localizes RatiNa in the lumen of specific organelles (D3) (Fig. 1a).
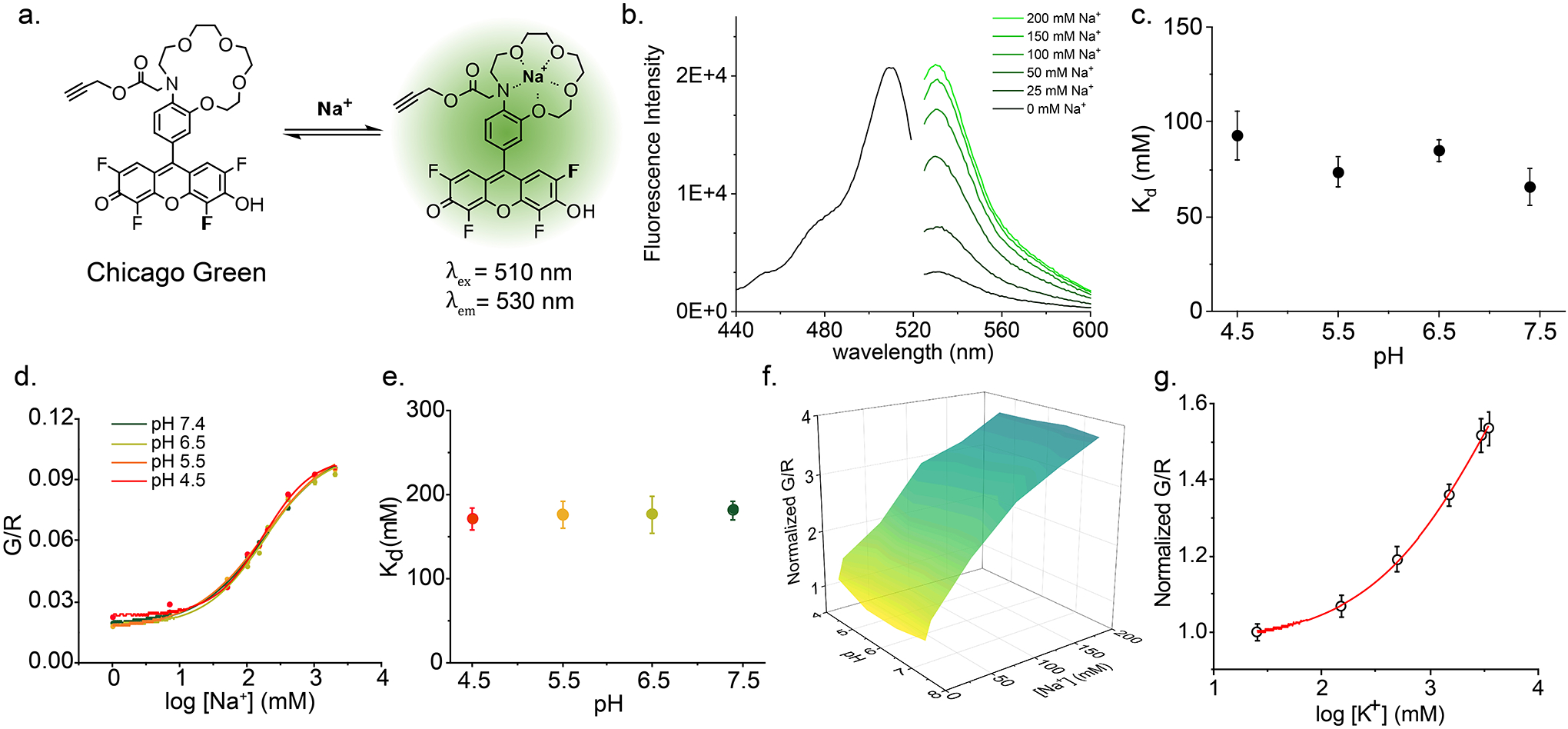
Figure 1 |. RatiNa is a ratiometric, pH-independent and specific reporter of Na+.
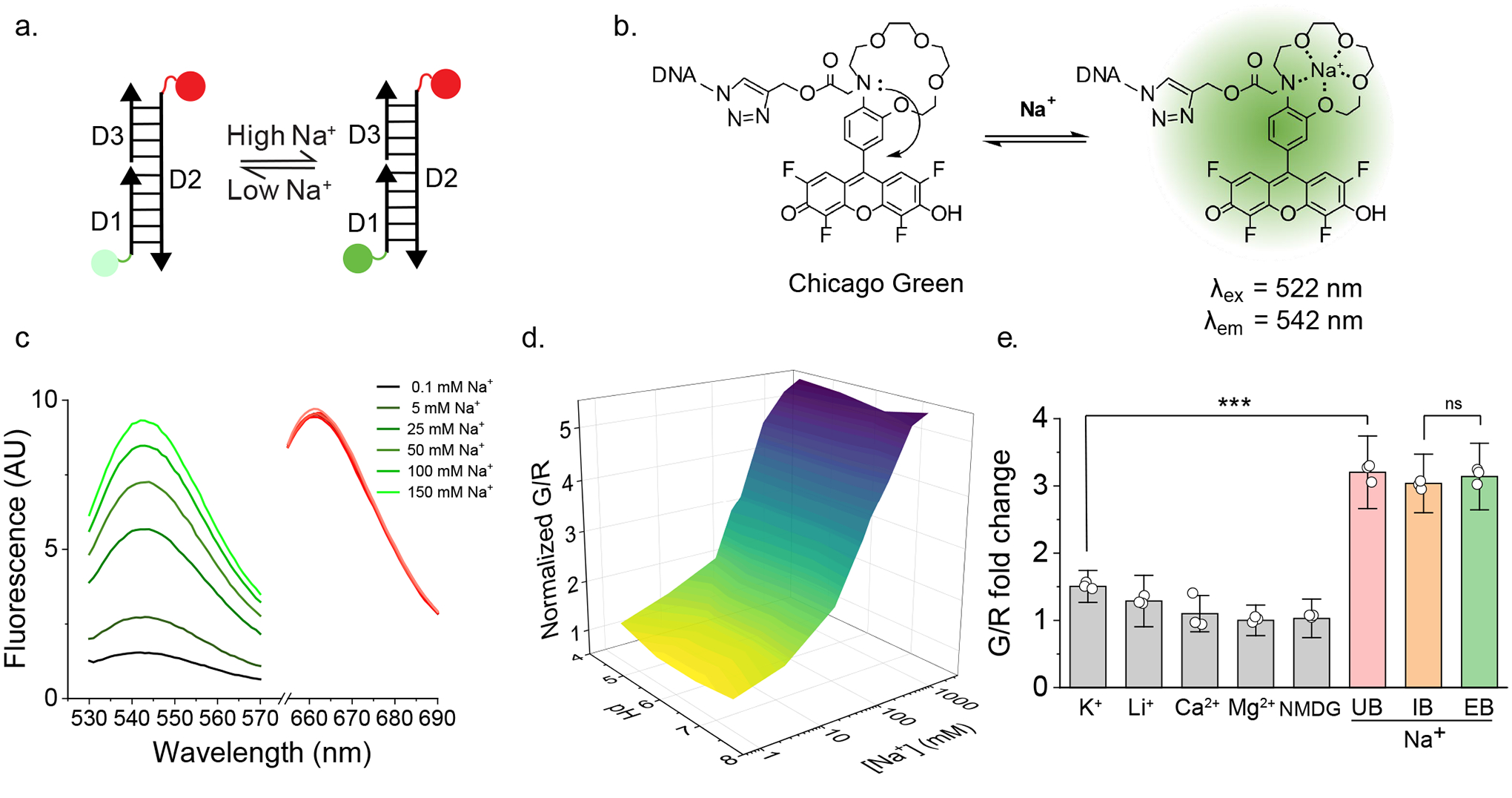
a, Schematic of the single stranded DNA molecules comprising RatiNa: D1 displays a Na+ sensing dye, Chicago Green (CG, green circle), D2 bears a reference ATTO647 fluorophore (red circle) and D3 harbors an organelle targeting motif. Na+ binding makes CG fluoresce (dark green circle). D1 and D3 are complementary to D2. b, Working principle of CG. CG is quenched by the N lone pair on the aza-crown ether via photoinduced electron transfer (PET). Na+ binding relieves PET and turns on CG fluorescence (right). c, RatiNa ratiometrically reports Na+. Increasing Na+ elevates fluorescence of CG (green traces) but not of ATTO647 (red traces). d, In vitro calibration profile of RatiNa on beads as a function of Na+ and pH levels. Na+ response of RatiNa is unaffected from pH 4.5 to 7.4. e, RatiNa responds specifically to Na+ in the presence of other physiologically relevant cations. RatiNa G/R fold change in universal buffer (UB, grey) with K+ = 145 mM; Li+, Ca2+, Mg2+ = 10 mM; NMDG = 0.3 M. G/R fold change with Na+ = 145 mM in UB (pink), intracellular buffer (IB, salmon) and extracellular buffer (EB, pistachio) n = 3 independent experiments. Data are presented as mean values ± standard deviation (SD). Two sample two-tailed t-test was used for statistical analysis with no multiple comparison correction, P = 3.18E-5.
Based on the sensing mechanism of the Na+ probe, CoroNa Green21, we synthesized a novel pH-insensitive fluorophore for D1, denoted Chicago Green (CG, = 510 nm, = 530 nm) (Extended Data Fig. 1a, Supplementary Fig. 1, Note 1). Like CoroNa Green, CG binds Na+ through a 1-aza-15-crown-5 ether moiety and uses photoinduced electron transfer (PET) to switch on or off fluorescence. Because fluoro substitutions generally lower the pKa of fluoresceins22, CG has a tetrafluoro fluorescein core to reduce its pH sensitivity. Hence CoroNa Green is pH sensitive because the aza-crown and hydroxyl groups are largely protonated at pH 4.5 while CG is not. CG is therefore suitable for organelles. We show the Na+ binding affinity (Kd) of CG is pH independent between pH 4.5–7 (Extended Data Fig. 1b–c). CG is further modified with a propargyl group for conjugation to a 5’-azido substituted D1 using copper-catalyzed azido-alkyne cycloaddition (Supplementary Fig. 2 – 3, Note 2)23,24. We chose ATTO647N ( = 646 nm, = 663 nm) as the reference dye on D2 because it is bright, photostable, and insensitive to pH, Na+ and other ions25. Because its emission spectrum does not overlap significantly with CG, the ratio of CG (G) to ATTO647N (R) fluorescence (G/R) in RatiNa corrects for CG intensity differences arising from inhomogenous probe distribution and/or uptake26. This correction gives a readout for Na+ concentration ([Na+]) alone. When D3 hybridizes to D1 and D2, it forms a 45-bp duplex DNA that targets RatiNa to endocytic organelles via receptor mediated endocytosis (Supplementary Fig. 4, Note 3, Table 1)27,28,29.
When attached to RatiNa, CG excitation and emission maxima were red shifted by ~12 nm but its intensity still increased with increasing [Na+], while that of ATTO647N was constant (Fig. 1b –c). We calibrated RatiNa immobilized on streptavidin-coated beads by fluorescence imaging in buffers of varying pH and [Na+] (Supplementary Fig. 5, Note 4). The three-dimensional surface plot of G/R values versus pH and [Na+] shows that the Na+ response of RatiNa is pH independent (Fig. 1d, Extended Data Fig.1d–f). Further, RatiNa response is specific to Na+ and barely affected by other physiological ions like K+, Li+, Ca2+ and N-methyl-D-glucamine (NMDG) (Fig. 1e, Extended Data Fig. 1g). RatiNa showed a similar Na+ response in buffers with a mixture of ions in proportions that matched either the extracellular or cytosolic milieu (Fig. 1e). Together, these data show RatiNa is pH-insensitive and specific enough to measure Na+ in acidic organelles.
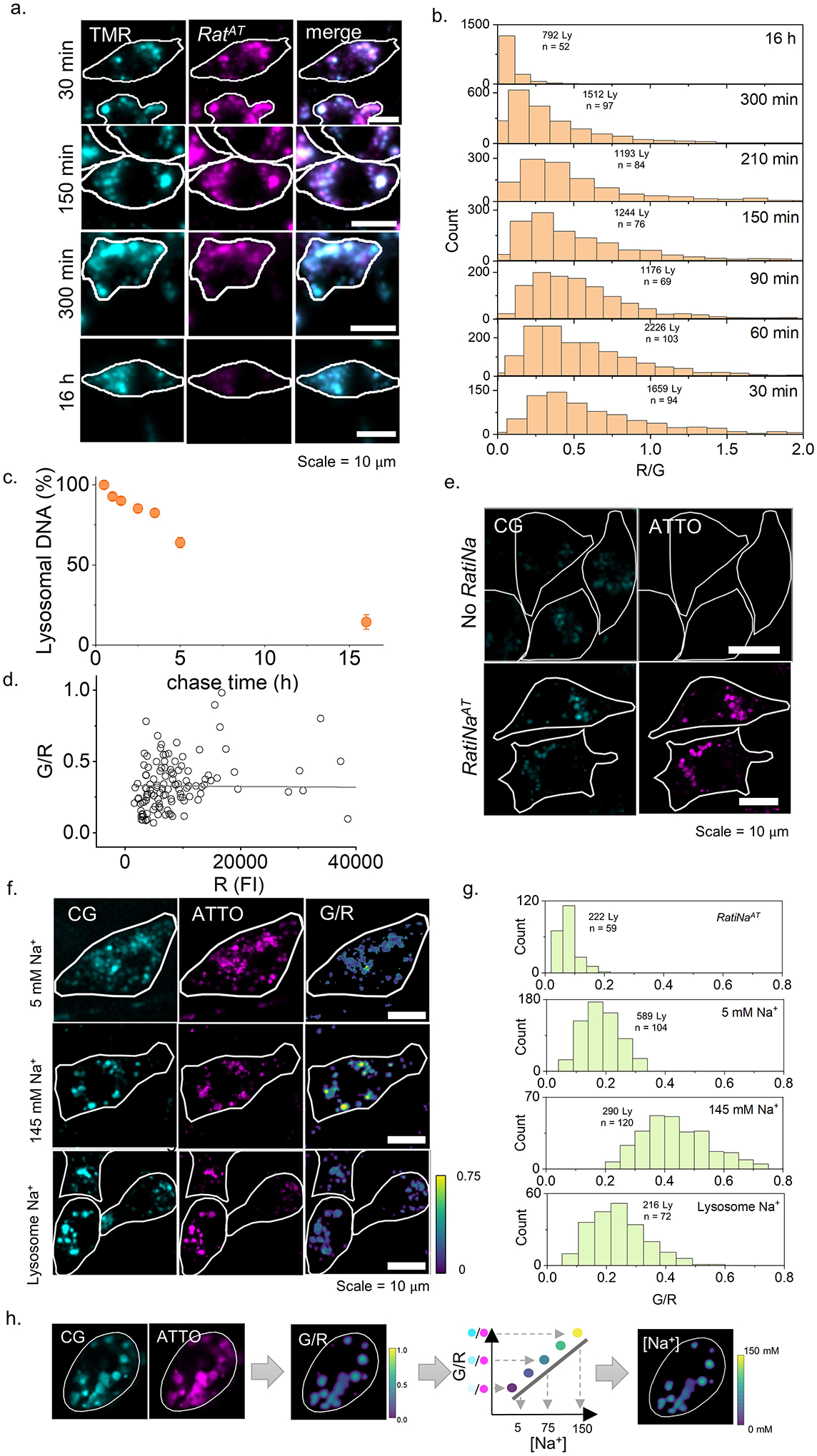
Given lysosomes are the only organelles with previous population averaged Na+ measurements that can be used for validation13,18, we used RatiNa to map lysosomal Na+ in cultured murine macrophages and in vivo in coelomocytes of the nematode C. elegans. Both these systems are amenable to analysis with DNA nanodevices because they express scavenger receptors abundantly, whose cognate ligand is duplex DNA28,29,30,. To track RatiNa trafficking and determine when it reaches lysosomes in both systems, we used a RatiNa lacking CG and carrying only the ATTO647N dye (RatiNaAT). We also used LMP1::GFP worms whose lysosomes are labeled with green fluorescence protein (GFP), and RAW 264.7 macrophages whose lysosomes are labeled with TMR dextran. In LMP1-GFP worms, RatiNaAT colocalized with GFP-labeled lysosomes 1 h post-injection (Fig. 2a). Pulsing RAW 264.7 macrophages with 500 nM RatiNaAT for 2 h followed by a 30 min chase led to robust colocalization with the TMR-labeled lysosomes. Because lysosomes of murine macrophages are more degradative than those in nematodes31, we tested the stability of RatiNaAT in macrophages and found that the fluorescence of RatiNaAT was stable up to 3 h (Extended Data Fig. 2a–c, Supplementary Note 5). Thereafter, fluorescence decreases as RatiNaAT progressively degrades thereby liberating the ATTO dye, which eventually leaches out of the lysosomes. Based on these results, we conducted all Na+ measurements within 30 min of RatiNa labeling lysosomes.
Figure 2 |. In cell and in vivo calibration of RatiNa to measure lysosomal Na+.
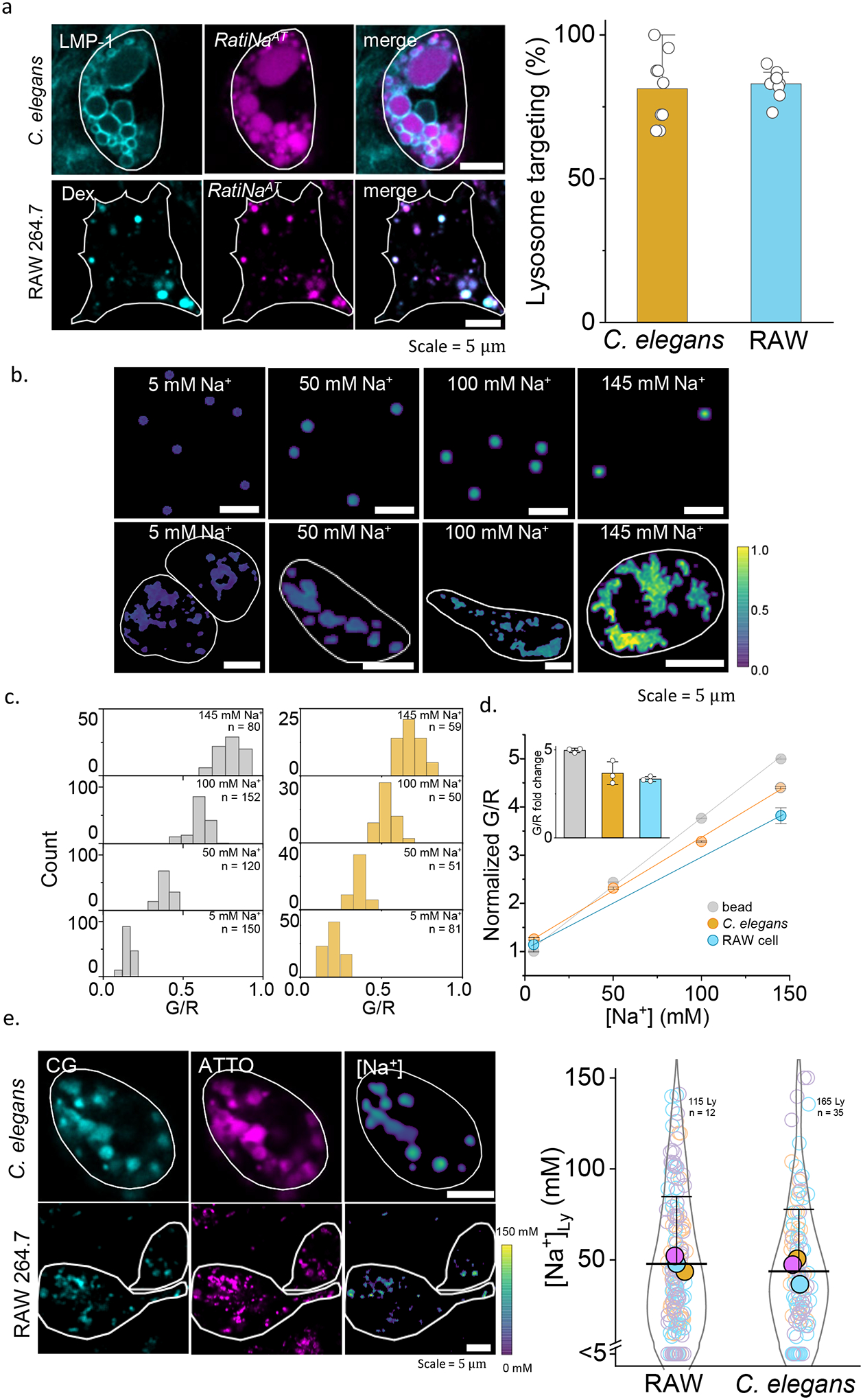
a, Fluorescence images (left) show RatiNaAT (DNA, magenta) colocalized with lysosome markers (cyan), LMP-1 in C. elegans and TMR-dextran (Dex) in RAW macrophages. Percentage colocalization of RatiNaAT in lysosomes (right) in n = 9 coelomocytes and n = 8 RAW cells in two independent experiments. b, Ratiometric images of RatiNabiotin on beads (upper panels) and RatiNa-labeled, Na+ clamped C. elegans lysosomes (lower panels) at the indicated Na+ levels. Cell outline shown in white. c, G/R values increase with increasing Na+ on beads (grey, n = 100–150) and in worm lysosomes (ochre, n = 50–75). d, Linear fits of normalized G/R values of RatiNa as a function of [Na+] on beads (grey), in C. elegans lysosomes (ochre) and in RAW macrophages (cyan). Inset shows RatiNa response from 5 to 145 mM Na+. All experiments performed in triplicate. Absolute Na+ heatmaps of single, native, RatiNa-labeled lysosomes in C. elegans and RAW macrophages imaged in the CG (cyan) and ATTO647 (magenta) channels. Na+ values in single lysosomes of RAW macrophages and C. elegans coelomocytes (n = 100–150 lysosomes from 35–50 cells). Experiments were performed in triplicate and data from each trial is colour coded. Mean value of each trial is given by a filled circle of the corresponding colour63. Lysosomes with Na+ values < 5 mM are shown below the break in the Y-axis (n = 21 and 17 for RAW cells and C. elegans). All error bar represents mean values ± SD.
We confirmed RatiNa response was not obscured by autofluorescence in worms or live cells. In the physiologically relevant regime of Na+ (5 mM to 145 mM), the performance characteristics of RatiNa in both worms and live cells were similar to those on beads incubated in buffers of known pH and [Na+] (Fig. 2b–d, Extended Data Fig. 2d–g). We calibrated RatiNa response by imaging RatiNa-labeled beads in increasing [Na+], and RatiNa-labeled worm lysosomes whose lumenal pH and Na+ have been clamped using buffers of defined pH and [Na+] containing a cocktail of ionophores18,32 (Fig. 2c, Supplementary Note 6–7). Lysosomal G/R ratios increased linearly with [Na+] whether RatiNa was in C. elegans, RAW 264.7 macrophages, or on beads, demonstrating that degradation, if any, is negligible under these conditions (Fig. 2d,).
To obtain Na+ levels of single lysosomes, we generated heatmaps of Na+ from the G/R images of RatiNa-labeled lysosomes in resting cells and compared them to the in vivo or in cellulo calibration profile as relevant (Fig. 2e, Extended data Fig. 2h). In both worms and RAW 264.7 macrophages, lysosomes Na+ was surprisingly variable. Na+ in single lysosomes ranged between 5 mM to 145 mM, averaging ~43 mM in C. elegans and ~48 mM in RAW 264.7 macrophages. Since RatiNa labeling bypasses the need to permeabilize cells18 or purify lysosomes13, it enables lysosomal Na+ measurement under the most native conditions so far. Previous measures ranged from 20 mM18 in macrophages to 150 mM13 in HEK293T cells measured by different methods. Our measure of average lysosomal Na+ compares better with that in macrophages, but our single lysosome measurement shows that lysosomes can harbor high Na+, and lysosomal Na+ likely varies across different species and cell types.
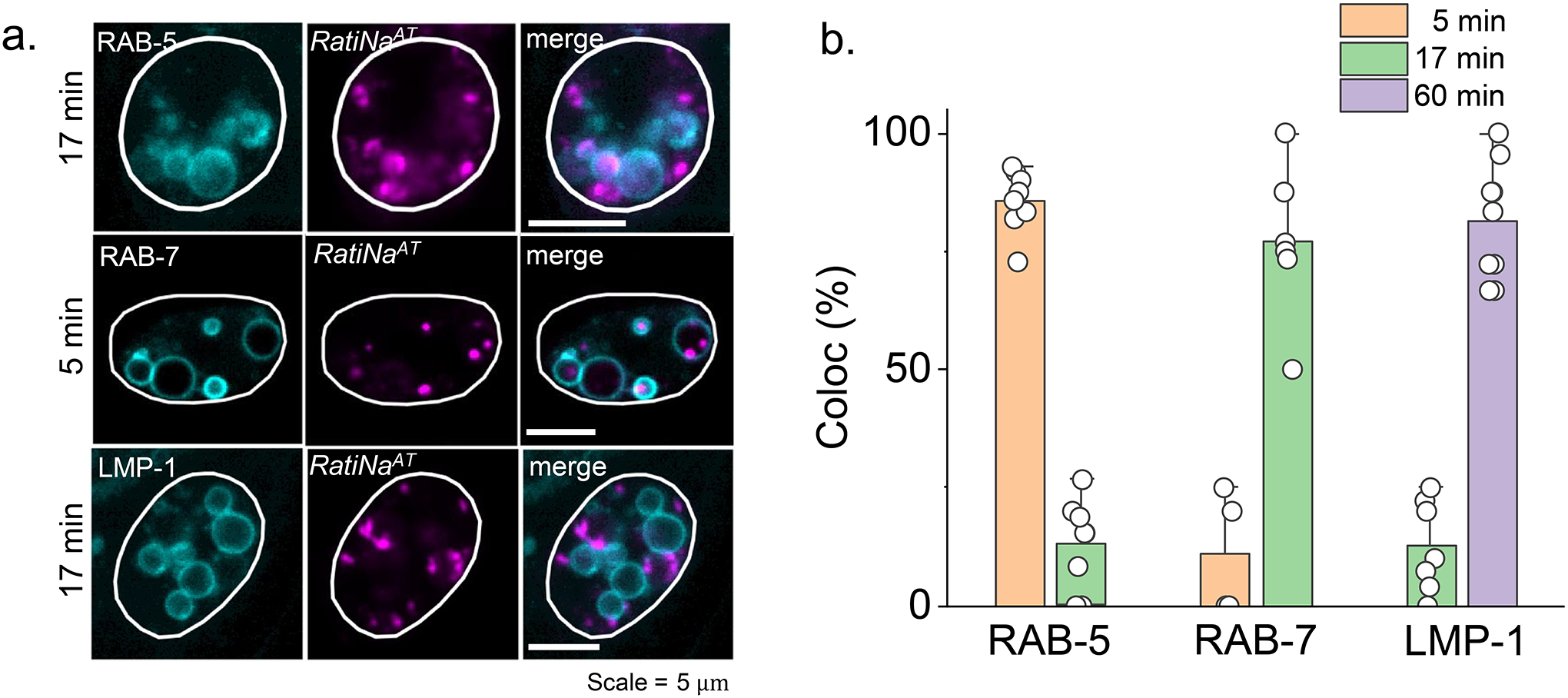
By mapping Na+ as a function of endosomal maturation, we show that RatiNa can capture physiological differences in organellar Na+. Because DNA nanodevices are internalized via scavenger receptors into early endosomes that mature to late endosomes and eventually lysosomes33, RatiNa acts as an endocytic tracer, labeling each stage of endosomal maturation as a function of chase time post-injection (Supplementary Note 8). We determined these chase times by injecting RatiNaAT into nematodes expressing either the early endosome marker, RAB-527, or the late endosome marker, RAB-727. RatiNaAT localized in early endosomes at 5 min and late endosomes at 17 min post-injection with negligible off-target labeling of other organelles on the endolysosomal pathway (Fig. 3a–b, Extended data Fig. 3a–b). When Na+ in early endosomes and late endosomes was measured using RatiNa as described above for lysosomes, we found that lumenal Na+ is highest in early endosomes (~74 mM) and drops to ~51 mM in late endosomes (Fig. 3c–d). The change in concentration was not due to overall differences in volume between EEs and LEs (Supplementary Fig. 6, Note 9). Notably, because every other ion mapped so far (i.e., H+, Cl− or Ca2+) increases progressively along the endolysosomal pathway27,34,35, our data reveals that uniquely, Na+ levels decrease as a function of endosomal maturation, implicating Na+ efflux mechanisms every endosomal stage.
Figure 3 |. RatiNa captures physiological changes in organellar Na+.
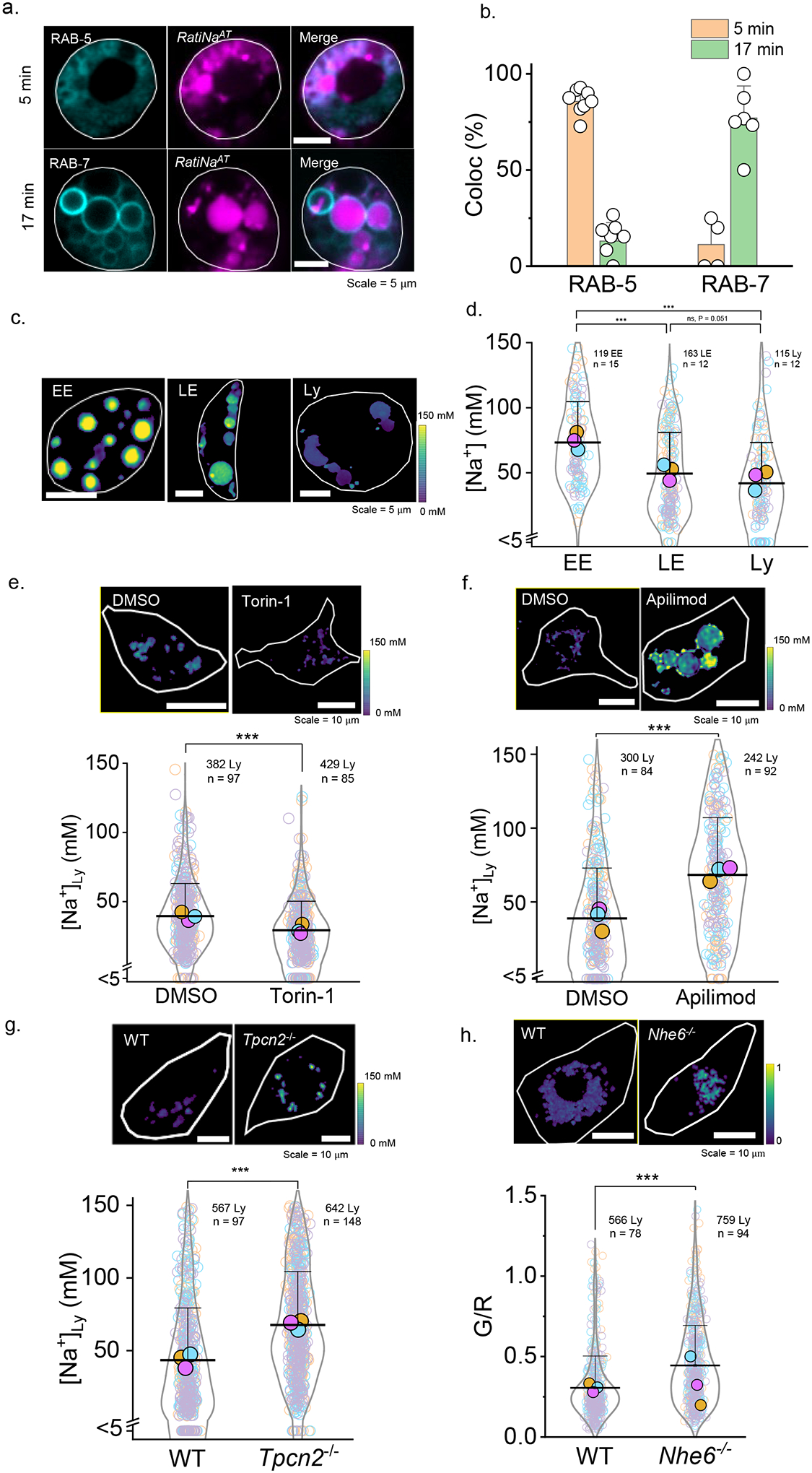
a, RatiNaAT (magenta) colocalizes with early endosome (EE) marker, RAB-5-GFP (cyan), and late endosome (LE) marker, RAB-7-GFP (cyan), time-dependently in C. elegans coelomocytes. b, Quantification of colocalization between RatiNaAT and the indicated markers at 5 min (n = 8, 4 coelomocytes) and 17 min (n = 7, 6 coelomocytes) respectively. Data are presented as mean values ± SD. c, RatiNa maps lumenal Na+ levels at each stage of endosomal maturation in coelomocytes of N2 C. elegans. Images are taken at 5 min, 7 min and 40 min post microinjection. d, Na+ levels decrease as endosomes mature with the biggest change from EE to LE. (n = 115–163 endosomes/lysosome from 12–15 worms). P = 2.6E-13 for EE to Ly, 7.7E-10 for EE to LE and 0.051 for LE to Ly. e, Pharmacological inhibition of mTOR with Torin-1 (1 mM) reduces lysosomal Na+. P = 9.3E-11. f, Pharmacological inhibition of TPCN2 by apilimod (100 nM) elevates lysosomal Na+ in RAW macrophages. Lysosomal Na+ is elevated in g, RAW264.7 macrophages where Tpcn2 is knocked out and h, in bone marrow derived macrophages from Nhe6−/− mice (n = 300–759 lysosomes from 84–148 cells). P = 1.3E-19, 7.5E-30, 5.5E-27 for f, g, h. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, no statistical significance. Data in d-f are presented as mean values, error bars represent SD and two sample two-tailed t-test was used for statistical analysis assuming equal variance.
RatiNa could also capture physiological differences in Na+ arising from the activity of a Na+ channel or transporter in a specific organelle. Two-pore channel type 2 (TPC2) is a lysosomal membrane protein that can function as an NAADP activated Ca2+ channel36 or a PI(3,5)P2 activated Na+ channel13. We first tested whether RatiNa could capture changes in lysosomal Na+ upon pharmacological perturbation of TPCN2. Torin-1 treatment is expected to activate TPCN2 because it inhibits mammalian target of rapamycin (mTOR), which in turn inhibits TPC212,37.. Indeed, treating RAW 264.7 macrophages with 1 μM Torin-1 for 1h lowers lysosomal Na+ to ~22 mM (Fig. 3e). In contrast, treating RAW 264.7 macrophages with apilimod, a specific PIKfyve inhibitor38 that depletes PI(3,5)P2 and blocks TPCN2, elevated lysosomal Na+ to ~70 mM (Fig. 3f). To specifically test the effect TPC2 activity on lysosomal Na+ levels, we knocked out the Tpcn2 gene in RAW 264.7 macrophages using CRISPR-Cas9 technology, confirmed by both genomic sequencing and qRT-PCR (Supplementary Fig. 7). Na+ levels in lysosomes of Tpcn2−/− RAW 264.7 macrophages increased to ~67 mM, compared to ~43 mM in WT macrophages (Fig. 3g). Thus, as expected, ablating an organellar Na+ channel elevates lumenal Na+.
Next we tested whether RatiNa could be applied to study endosomal Na+ transporters. NHE6 is an endosomal Na+/H+ exchanger39 Mutations in NHE6 cause Christianson syndrome and these patients also show lysosomal disorder phenotypes8. Loss of NHE6 causes defects in endosome maturation and trafficking underlying lysosome deficiency8. We applied RatiNa in primary bone marrow derived macrophages from WT and Nhe6−/− mice. Because Nhe6−/− mice have defective endocytosis and trafficking, RatiNa required a 45 min chase to label lysosomes (Supplementary Fig. 8). We found that lysosomes in Nhe6−/−macrophages showed higher WT, lysosomal Na+ than in WT BMDMs (Fig. 3h) even though NHE6 is suggested to import Na+ into endosomes. Loss of NHE6 is linked to endosomal trafficking defect and impaired delivery of functional proteins such as Cathepsin D to lysosomes8. It is possible that Na+ could be elevated because the loss of NHE6 could impair the localization of lysosomal Na+ channels.
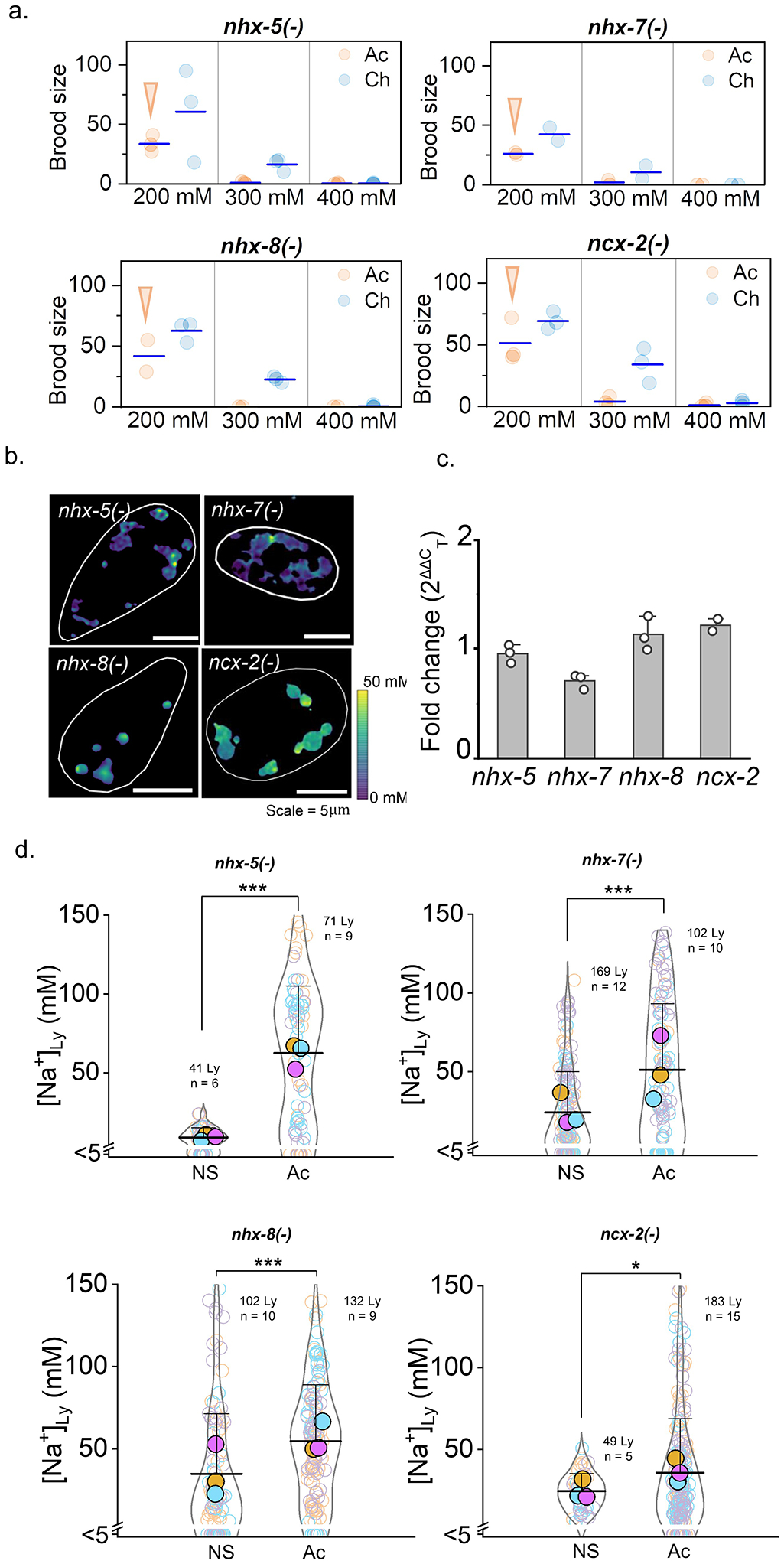
We then used RatiNa to map Na+ transport in lysosomes of C. elegans under salt stress. Excess salinity stresses many species, including C. elegans, by interfering with osmoregulation40,41. While salt stress has been studied at cellular resolution in whole animals42, it is not known whether salt stress can impact sub-cellular Na+ levels. C. elegans responds to salt stress by increasing glycerol and sorbitol synthesis and regulating its body volume41,42. We used a well-established assay41 to acutely (Ac) or chronically (Ch) stress C. elegans to high Na+ (Supplementary Fig. 9 and Supplementary Note 10). Briefly, worm eggs of the relevant genetic background were grown at either normal salt (50 mM NaCl, NS) or at elevated salinity (200 mM, Ac). Then, at the L4 stage larvae were transferred and grown in progressively higher levels of Na+ (Ch) up to a maximum of 400 mM Na+. Brood sizes at each salt concentration were measured and compared to those of unstressed worms (NS). At 400 mM Na+, only chronically stressed wild type (N2) worms produced progeny (Fig. 4a, Extended Fig. 4a).
Figure 4 |. Lysosomal Na+ transport is vital for salt adaptation in C. elegans.
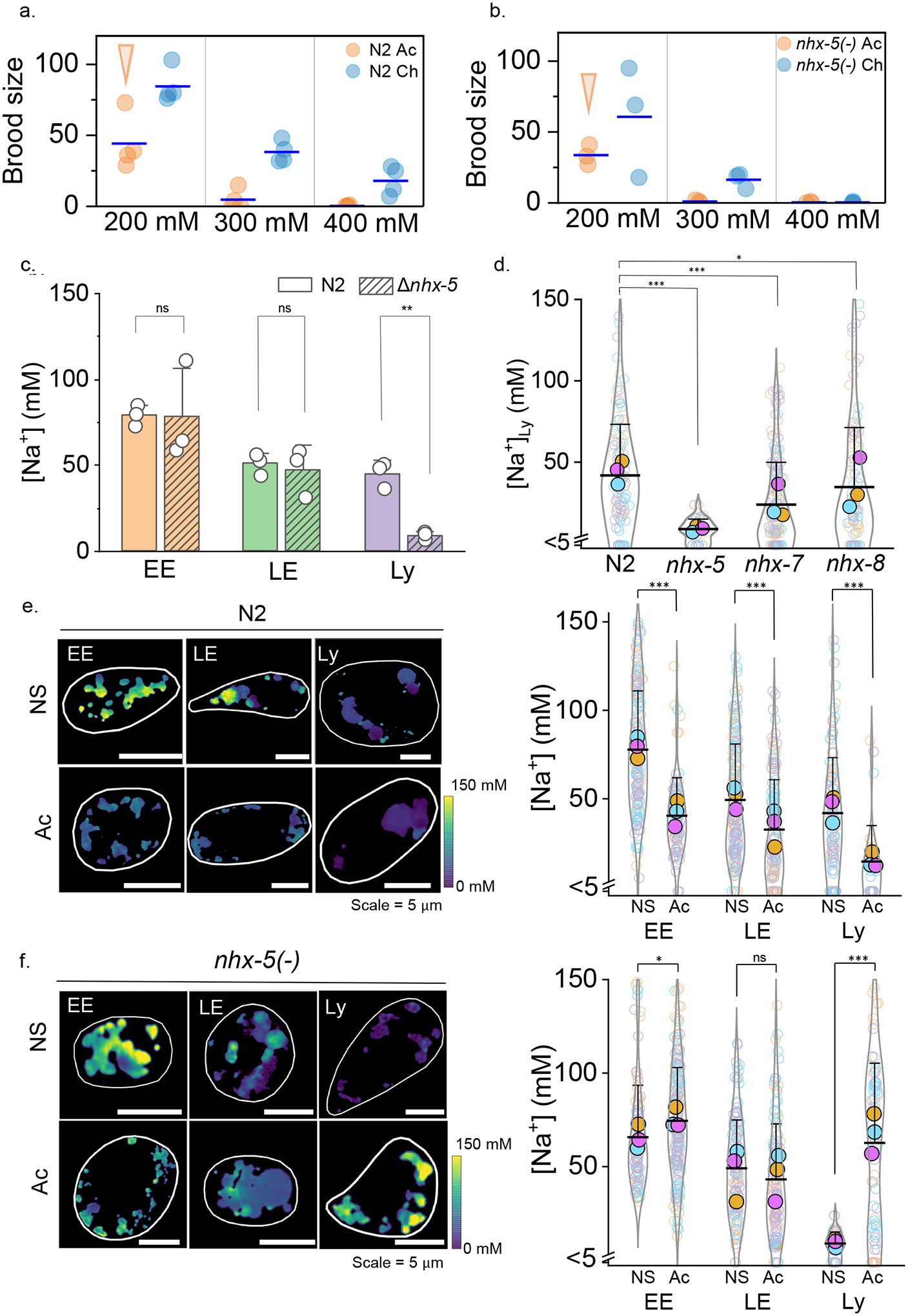
a,b, Brood size of acutely (Ac) and chronically salt stressed (Ch) N2 and nhx-5 (−) worms for the indicated salt levels. Arrows indicates condition used for lysosomal Na+ measurement in salt stressed worms is acute 200 mM c, Lumenal Na+ levels at each stage of the endolysosomal pathway in N2 and nhx-5(−) worms in normal salt (NS). Note that nhx-5(−) worms show lower Na+ levels only in lysosomes (Ly) and not in early (EE) or late endosomes (LE). P = 0.95, 0.71, 1.5E-3 for EE, LE, Ly d, In worms lacking the indicated nhx genes, Na+ levels in single lysosomes are affected specifically by loss of nhx-5. Levels in n = 100–150 lysosomes from 12–16 worms. For nhx-5 (−) alone, n=41 lysosomes, 6 worms. P = 1.4E-10, 2.2E-7, 0.11 for N2 to nhx-5 (−), nhx-7 (−), nhx-8 (−). e, f, Lysosomal Na+ reduces upon chronic salt stress in N2 worms but increases in nhx-5 (−) worms. Lumenal Na+ levels at each endosomal stage in normal salt (NS) and acutely (Ac) salt stressed N2 (e) and nhx-5(−) (f) worms. Cell outline is shown in white. All experiments were performed in triplicate and data from each trial is colour coded. Mean value of each trial given by a filled circle of the corresponding colour58. P = 8.9E-19, 2.7E-6, 2.2E-6 for EE, LE, Ly in N2 and P = 0.01, 0.09, 2.1E-13 for EE, LE, Ly in nhx-5(−) worms. For all trials, n = 130–160 organelles from 8–12 worms. Only for Ac N2 and NS nhx-5(−) worms, data is from n = 40 lysosomes from 6 worms. Data in c-f are presented as mean values, error bars represent SD and two sample two-tailed t-test was used for statistical analysis assuming equal variance.
Mutant worms for various genes encoding Na+/H+ exchangers (NHX) proteins43 did not reproduce at 400 mM Na+ indicating intolerance to salt stress (Fig. 4b and Extended data Fig. 4a). We found that worms lacking nhx-5, which encodes the closest homolog to human NHE644, were the most severely affected, failing to produce progeny even at 300 mM Na+. To pinpoint organelle participation in tolerating salt stress, we measured lumenal Na+ in early endosomes (EE), late endosomes (LE), and lysosomes (LY) in nhx-5(−) mutants (Fig. 4c). While Na+ levels in EE and LE of NHX-5 mutants were comparable to those in N2 worms, those in lysosomes were significantly lower. Lysosomal Na+ in nhx-7(−) and nhx-8(−) mutants were not significantly altered, demonstrating that NHX-5 specifically facilitates lysosomal Na+ import (Fig. 4d and Extended data Fig. 4b). NHX-8 and NHX-7 are the closest worm homologs of mammalian NHE8 and NHE2 that reside on the Golgi and plasma membrane, respectively45,46. We found NHX-5::GFP localizes in lysosomes of coelomocytes (Supplementary Fig. 10, Note 11). Salt tolerance is compromised in worms lacking either plasma membrane or organellar Na+ transporters, revealing that even in metazoans, organelles participate in Na+ homeostasis (Supplementary Note 12).
When we mapped lumenal Na+ along the endolysosomal pathway in chronically stressed N2 worms, we found Na+ levels in EEs, LEs and LYs were all lowered. However, the effect was most pronounced in lysosomes, which showed ~67% decrease (Fig. 4e). Interestingly, in the few nhx-5 mutants that survived chronic salt stress, Na+ levels in EEs and LEs were similar to their unstressed counterparts. Yet, lysosomal Na+ in chronically stressed nhx-5(−) mutants increased ~8-fold, despite no change in the corresponding mRNA levels (Fig. 4f, Extended data Fig. 4c). Lysosomal Na+ mildly increases even in chronically stressed nhx-7(−), nhx-8(−) or ncx-2(−) single mutants (Extended data Fig. 4d, Supplementary Note 12). Our data show that organelles modulate their Na+ levels as worms are subjected to salt stress, with the biggest change occurring in lysosomes. These changes likely reflect both osmotic stress and Na+ stress47, which favour more inert osmolytes like glycerol over Na+ in the worm body41, and possibly also in the lysosome.
NHX family proteins are electroneutral transporters that work bi-directionally and can either import or export Na+ in a 1:1 ratio with H+ across membranes depending on cellular demand48. Therefore, to better understand the role of NHX-5 in lysosomes, we measured lysosomal pH in N2 and nhx-5(−) worms in unstressed and salt-stressed states (Extended data Fig 5a–d). Unstressed nhx-5(−) worms have less Na+ and lower pH compared to N2 worms, suggesting that NHX-5 imports Na+ using the pH gradient. Notably, the lysosomal pH change of 3 μM is eclipsed by the lysosomal Na+ change of ~30 mM suggesting that the pH changes due to NHX-5 activity are likely compensated by highly active lysosomal V-ATPase49. Interestingly, salt stress too, has no effect on lysosomal pH in either genetic background, indicating the importance of directly mapping organellar Na+ to study salt stress at the sub-cellular level.
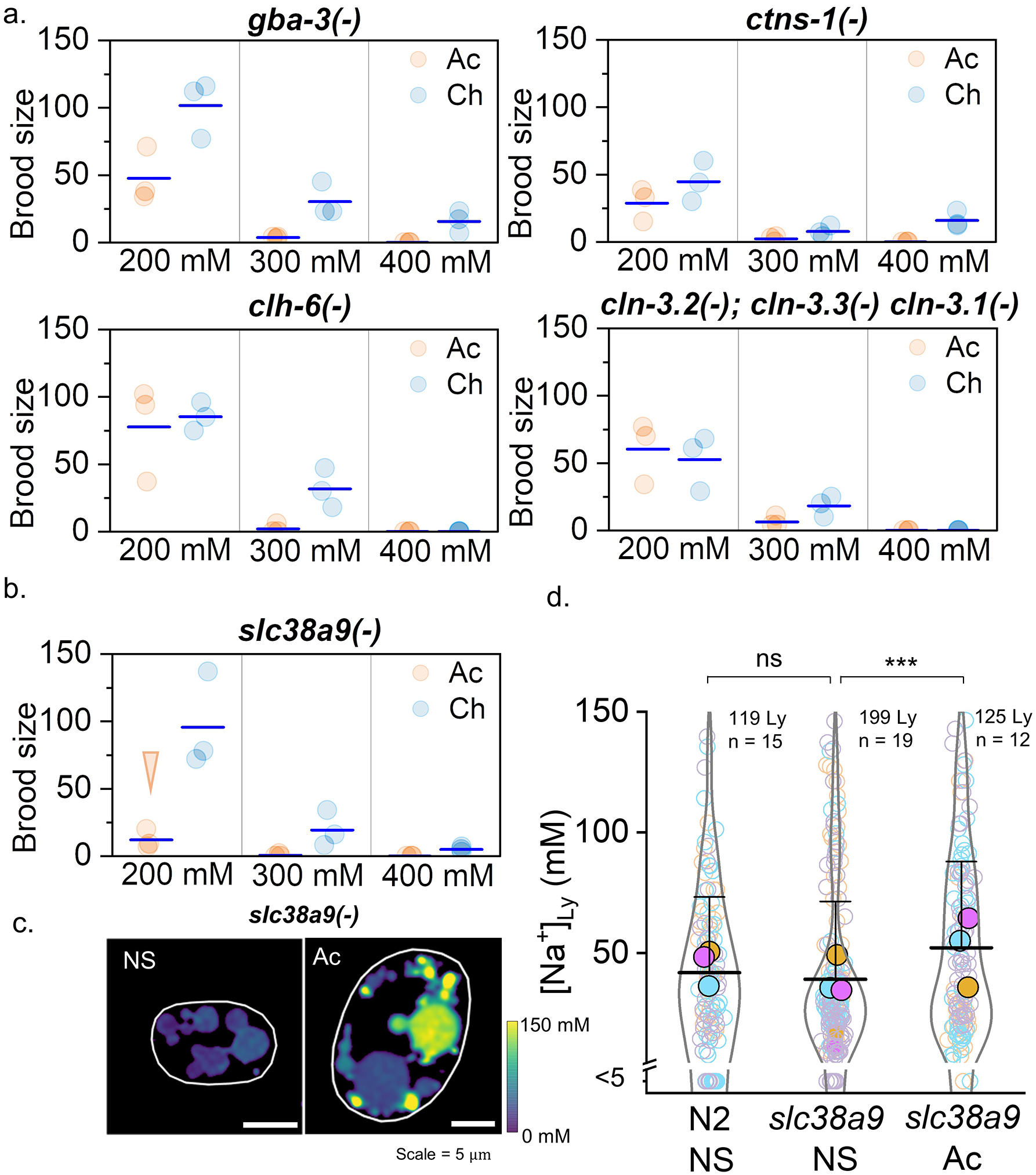
To test the role of lysosomes in counteracting salt stress, we subjected a range of mutant nematodes, each lacking a specific lysosomal enzyme or transporter, to salt stress. Each mutant is a genetic model of a given lysosomal storage disorder caused by a lysosomal defect50,51. Many mutants were hypersensitive to chronic salt stress (Extended data Fig. 6a). Interestingly, worms lacking slc38a9 were hypersensitive to acute salt stress (Extended data Fig. 6b). Human SLC38A9 is a lysosome-resident sodium-coupled neutral amino acid transporter which uses Na+ to regulate nutrient transport, lysosome functionality and metabolism52. Although lysosomal Na+ in slc38a9(−) worms and N2 worms is similar (~40 mM), acute salt stress elevates lysosomal Na+ in slc38a9(−) worms, just as seen in chronically salt-stressed nhx-5(−) worms (Extended data Fig. 6c–d). These findings underscore a role for the lysosome in regulating salt stress.
Discussion
In summary, we present a pH-independent, ratiometric fluorescent probe that reports absolute Na+ levels in acidic organelles with single organelle resolution. Using the probe, we found that unlike any other ion previously mapped on the endolysosomal pathway, Na+ levels decrease as endosomes mature. While average lysosomal Na+ is comparable in both C. elegans and mammalian macrophages, the levels in single lysosomes varied considerably. Even within in the same cell, lysosomes vary in their morphology, mobility and function19,53. This variability is often under-appreciated in terms of ion composition. For example, we consider that H+ levels are stringently regulated. Yet in an ensemble of lysosomes, pH routinely spans 4.4 – 4.6 i.e., 40 μM - 25 μM H+, nearly 60% difference28. Lysosomal Ca2+ can vary from 5 μM – 300 μM, nearly 60-fold difference28,35. Thus, the heterogeneity of lysosomal Na+ is commensurate with other ions. Our results show that at every stage of maturation, endosomes have both a sizeable transmembrane Na+ gradient in addition to high membrane potential14. The progressively decreasing Na+ level implicates the function of proteins that mediate Na+ efflux at each stage of the endolysosomal pathway. While we know of only TPC2 as a bona fide lysosomal Na+ channel13,36, our findings suggest the existence of more endosomal Na+ channels.
RatiNa can also capture physiological changes in lysosomal Na+ levels due to the activity of Na+ channels such as TPC2 or exchangers like NHE6. Given the ability to map organellar Na+ fluxes in vivo, we found that NHX-5, facilitates lysosomal Na+ import. When worms encounter salt stress, of all the organelles on the endolysosomal pathway, the biggest decrease in lumenal Na+ occurs in the lysosome. Worms lacking lysosomal Na+ transporters such as NHX-5 or SLC38A9 were highly susceptible to salt stress. The few mutants that survived salt stress showed abnormally high Na+ in their lysosomes. Further, worms with specific lysosomal defects were hypersensitive to salt stress, positioning lysosomes as a critical conduit for Na+ homeostasis in metazoans.
While vacuolar Na+/H+ exchangers regulate cytosolic pH and Na+ levels in yeast during salt stress3 and help counter hypotonic stress in cultured mammalian cells54,55, the direction of Na+ flux across organelle membranes was unknown. Our results indicate that in unstressed worms, NHX-5 imports Na+ into and extrudes H+ from lysosomes. However, the low level of lysosomal Na+ in nhx-5(−) worms under salt stress points to more complex mechanisms of lysosomal Na+ regulation. The minimal change in lysosomal pH under salt stress suggests that lysosomal Na+ is likely regulated by players other than NHX-5. A probe like RatiNa can potentially be used to identify more players.
To survive high Na+ stress, cells must undergo a metabolic shift56 to produce organic osmolytes such as sorbitol to increase the internal osmotic pressure57. Cells upregulate autophagy58 and lysosomal proteolysis to generate and recycle nutrients that are substrates for osmolyte production pathways. The metabolic shift is supported by numerous nutrient transporters that move these substrates across lysosomal and plasma membranes into the cytosol59. Many nutrient transporters move their substrates across membranes by co-transporting Na+ and thereby leveraging the transmembrane Na+ gradient52. Many nutrient transporters and their regulators, such as mTOR, reside on the lysosomal membrane60,61. Thus, lysosomes are a hub that supports this metabolic shift62. This provides a rationale for the large Na+ flux across lysosomes during salt stress. While in higher organisms we know of Na+ channels such as TPC2 that regulate organellar Na+ homeostasis, none are known in C. elegans. Our ability to map organellar Na+ in vivo can illuminate mechanisms of Na+ homeostasis at an increased level of cellular detail.
Methods
Reagents.
All oligonucleotides (Supplementary Table 1) were high performance liquid chromatography (HPLC) purified and purchased from Integrated DNA Technologies (USA). They were subjected to ethanol precipitation and quantified by UV absorbance. 1H NMR and 13C NMR spectra of the newly compounds were recorded on a Bruker AVANCE II+, 500 MHz NMR spectrophotometer in CDCl3 and tetramethylsilane was used as an internal standard. Mass spectra were recorded with an Agilent 6224 Accurate-Mass time-of-flight liquid chromatography–mass spectrometry. Streptavidin-coated microspheres were purchased from Bangs Laboratories, Inc., Gramicidin, nigericin, and monensin were purchased from Cayman Chemicals. All other reagents were purchased from Sigma-Aldrich (USA).
Conjugation of CG to DNA
5’-amine modified single strand DNA (IDT) was reacted overnight with 20 equivalents azide-(PEG)4-NHS ester (Click Chemistry Tool, AZ103) in 100 mM Na2HPO4 buffer with pH adjusted to ~8.5 by adding NaHCO3. DNA was ethanol precipitated for purification.
To test conversion of amine to azide, a small aliquot of azide-DNA was reacted with 2 equivalents 5kDa mPEG-DBCO (Nanocs, PG1-DB-5K) in 50 mM pH 7.0 phosphate buffer and run on 12% native PAGE. PEGylated DNA shows a gel shift and no azide-DNA band is seen.
CuAAC reaction was used to conjugate CG to azide-DNA. Copper catalyst was prepared by premixing CuSO4 and THPTA (100 mM, 10 eq.). Azide-DNA (1.3 mM, 1 eq.) was purged with nitrogen for 1 min, and added to propargyl-CG (2.6 mM in DMSO, 2 eq.), CuSO4/THPTA mix, and sodium ascorbate (1 M, 40 eq.) fully dissolved in 30% DMSO in buffer. The mixture was purged with N2, the tube was sealed and equilibrated for > 5 h. DNA was precipitated and analyzed by 12% native PAGE and UV-Vis. UVP VisionWorksLS v8.1.2 and Image Lab Software 6.0.0 was used for UV and fluorescence of gel imaging. Shimadzu UVProbe v2.43 software was used for UV-Vis absorbance aquisition.
Na+ sensitivity by fluorescence spectroscopy
100 nM of RatiNa was taken in UB buffer (10 mM HEPES, MES and KOAc, 140 mM NaCl/KCl). pH adjusted by HCl or KOH) and fluorescence emission spectra recorded in a Fluoromax (Horiba). Horiba FluorEssence v3.5.8.63 software was used for acquisition with following collection parameters: For CG, = 522 nm, emission range 530 – 600 nm. For ATTO647N, = 645 nm, emission range 660 – 700 nm.
RatiNabiotin conjugation to streptavidin coated beads
1 μm streptavidin-coated polystyrene bead (Bangs Laboratories, CP01004) were washed twice with 50 mM pH 7.4 phosphate buffer by centrifuging at 4,000 xg and incubated with 10 μM of RatiNabiotin in 50 mM potassium phosphate buffer, pH 7.4 with agitation for good mixed. After 2 h the beads were recovered by centrifuging at 4,000 xg and stored in 50 mM potassium phosphate buffer, pH 7.4. with 0.1% of Tween-20 to prevent bead aggregation.
Na+ sensitivity by bead imaging
RatiNa beads were resuspended in clamping buffer (1 mM to 2 M NaCl, 10 mM HEPES, 10 mM MES, 10 mM KOAc) with varying concentrations of [Na+] (1 mM to 2 M) and pH (4.5–7.5). Beads were drop casted on poly-D-lysine coated glass bottom dishes (Cellvis D35–14) and imaged by wide field (Olympus IX83) or confocal microscopy (Leica Stellaris 8). Individual beads were analyzed from intensities in CG and ATTO channels. Average G/R for >100 beads were calculated and normalized to the lowest average G/R in all samples.
Na+ selectivity by bead imaging
To assay RatiNa ion selectivity, RatiNa-coated beads were resuspended in 5 mM Na+ buffer (5 mM NaCl, 10 mM HEPES, 10 mM MES, 10 mM KOAc, pH 7.4) and images acquired in both CG (G) and ATTO (R) channels in a widefield microscope. Then one of the extra cations or osmolyte (145 mM NaCl/ 145 mM KCl / 10 mM LiCl / 10 mM CaCl2 / 10 mM MgCl2 / 300 mM NMDG) was added to reach the indicated concentration. To assay RatiNa selectivity in a mixture of ions, RatiNa-coated beads were suspended in either 5 mM Na+ or 145 mM Na+ containing extracellular buffer (EB: 10 mM KCl, 0.1 mM MgCl2, 10 mM CaCl2, 10 mM HEPES, 10 mM MES, 5 mM glucose, pH = 7.4) or intracellular buffer (IB: 145 mM KCl, 10 mM MgCl2, 0.1 mM CaCl2, 10 mM HEPES, 10 mM MES, 5 mM glucose, pH = 7.4) respectively. Beads were imaged, and G/R values were calculated. The fold change in G/R was calculated from the G/R ratio after and before adding the cations/osmolytes. The values are represented as fold change of of 5 mM to 145 mM Na+.
Colocalization Analysis
Pixel based colocalization PCC is not suitable for C. elegans because endosomes are too large. Hence the percentage of DNA containing compartments that also have a membrane marker is used to quantify targeting specificity. In RAW macrophages, lysosomes are smaller and a pixel-based PCC analysis is used instead. ImageJ plugin Coloc-2 was used for PCC analysis.
Na+ clamping in C.elegans
For C. elegans, the Na+ clamping buffer has a pH of 5.5 and contains 150 mM Na+ and K+, 150 mM Cl−, 50 μM monensin, 50 μM nigericin, 10 μM gramicidin, 100 μM ouabain. 2 μM RatiNa is microinjected in C. elegans and after 30 min it labels endolysosomes in coelomocytes. A few perforations are introduced in worms, they are soaked in clamping buffer and imaged after 1 h.
Fluorescent microscopy of Imaging RatiNa
We used Olympus IX83 with metal halide lamp (X-cite 120Q), 60x objective (Olympus PlanApo N 60× 1.42 NA) and Photometric Evolve delta EMCCD camera. Acquisition was performed with Metamorph Premier v7.8.12.0 software. For CG channel we use 525/30 BP (Semrock FF01–525/30–25) as excitation filter, 532 nm dichroic mirror (Semrock Di02-R532–25×36) and 575/40 BP (Chroma ET575/40m) as emission filter. For ATTO647N channel we use 640/30 BP (Chroma ET640/30x) as excitation filter, tri-band dichroic mirror (Chroma 89016bs) and 705/72 BP (Chroma ET705/72m) as emission filter.
We used Leica Stellaris 8 for confocal microscopy. White laser was set to 85% and 63x objective (Leica HC PL APO CS2 63× 1.40NA) and Hybrid detectors (Leica HyD X) were used. Acquisition was performed with Leica LAS X 3.7.4.23463. Sequential scan is used, with 95.5 μm pinhole. For CG 522 nm laser was used (30% and 10% intensity for macrophages and C. elegans respectively). Emission was collected from 530 – 580 nm (HyD X2 Gain 100). For ATTO647N, 646 nm laser was used (2% intensity) and emission collected from 655 – 780 nm (HyD X4 Gain 10).
Image analysis
All image processing is performed with ImageJ4. For RatiNa beads, binary images were thresholded in the ATTO channel. Single beads in focus are automatically picked by “Analyze Particles” plugin in ImageJ with circularity >0.8. Rolling ball method was used for background subtraction. G/R value of each bead is obtained from the ratio of integrated intensity in CG and ATTO channels. For C. elegans, endosomes and lysosomes are selected manually in ATTO channel. Rolling ball method was used for background subtraction. G/R value of each endosome or lysosome is calculated as above. For RAW macrophages, bright puncta corresponding to lysosomes are selected as ROI in images. Rolling ball method was used for background subtraction. G/R value of each lysosome is calculated as above.
Na+ heatmap is generated from background subtracted images in CG and ATTO channels. Linear fit equation of G/R to Na+ is applied using both images. The resulting image was pseudo coloured with Vridis lookup table.
Cell culture
WT and Tpcn2−/− RAW 264.7 macrophages (ATCC, TIB-71) were grown in DMEM medium (Gibco, 11995) with 10% heat inactivated FBS (Gibco, 26140) and 100 U/mL Pen Strep (Gibco, 15140), maintained at 37 °C in a humidified chamber in 5% CO2. All the experiments were performed on cells >24 h post-passaging, at confluency ~50%.
RAW cell stability assay
Lysosomes are labeled with first with TMR-dextran (1 mg/mL TMR dextran with 1 h pulse and 16 h chase) and then with RatiNaAT (2h pulse of 500 nM) The ratio of ATTO647 to TMR (R/G) intensities indicates ATTO signal loss due to DNA degradation. Cells are imaged at indicated chase times by wide field microscopy and R/G values calculated at each time point5. DNA degradation is tracked from the average R/G at each time point normalized to the 30 min chase time where it is undegraded.
Na+ clamping in RAW 264.7 macrophages
Cells are pulsed with 1 μM RatiNa for 2 h and chased for 30 min to label lysosomes. Cells with RatiNa labeled lysosomes were treated with culture medium containing ionophores, to clamp organellar Na+, washed with clamping buffer twice, incubated in Na+ clamping buffer for 1h at RT and then imaged. Na+ clamping buffers (150 mM NaCl/KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 5 mM glucose, pH 5.5) recapitulate cell internal osmotic pressure (321 Osm) to prevent lysosome volume changes due to ion exchange. 50 μM monensin, 50 μM nigericin, 10 μM gramicidin are used as Na+ and K+ ionophores to clamp Na+ and K+ and 100 μM of ouabain is used to inhibit Na+/K+ ATPase and facilitate clamping.
Lysosome targeting in RAW 264.7 macrophages.
RAW 264.7 macrophage were pulsed with 0.5 mg/mL TMR dextran for 1 h in Opti-MEM (Gibco, 31985070) chased for 16 h in complete DMEM to label lysosomes. 1 μM RatiNaAT was pulsed for 2 h in Opti-MEM and chased for 30 min in complete DMEM. Live cells were then imaged in FluoroBrite (Gibco, A1896701) with confocal microscope (Leica Stellaris 8) in TMR and ATTO channels.
Pharmacological inhibition
RAW 264.7 macrophages were treated with either 100 nM apilimod for 1 h or 1 μM Torin-1. Lysosomes were labeled as mentioned with RatiNa containing 100 nM apilimod or 1 μM Torin-1 in the chase and imaging media.
CRISPR KO of Tpcn2 in RAW 264.7 macrophages
Tpcn2−/− RAW 264.7 macrophages were obtained from Creative Biogene (NY, USA). Briefly lentiviral transduction of Cas9 and sgRNA was used to specifically knock out mouse Tpcn2. The sgRNA sequence 5’-CATGGATGCTGGTTCATTGT-3’ targeted exon 3 of mouse Tpcn2. Single clones were picked and genomic sequence and qRT-PCR confirmed KO of Tpcn2 gene.
C. elegans strains and maintenance
Standard methods were followed for the maintenance of C. elegans. Wild type strain used was C. elegans isolated from Bristol (strain N2). Mutant strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
cdIs131 [pcc1::GFP::rab-5+unc-119(+)+myo-2p::GFP], a transgenic strain that expresses GFP-fused early endosomal marker RAB-5 inside coelomocytes.
cdIs66 [pcc1::GFP::rab-7+unc-119(+)+myo-2p::GFP], a transgenic strain that expresses GFP-fused late endosomal/lysosomal marker RAB-7 inside coelomocytes.
pwIs50 [lmp-1::GFP+Cbr-unc-119(+)], a transgenic strain expressing GFP-tagged lysosomal marker LMP-1.
nhx-5(ok661) is a deletion mutant with 1411 bp deletion in nhx-5 gene (F57C7.2) and referred to as nhx-5(−) elsewhere.
nhx-7(ok583) is a deletion mutant with 1702 bp deletion in nhx-7 gene (K09C8.1) and referred to as nhx-7(−) elsewhere.
nhx-8(ok549) is a deletion mutant with 1584 bp deletion in nhx-8 gene (Y18D10A.6) and referred to as nhx-8(−) elsewhere.
ncx-2(gk879849) is a substitution mutant of R634 to stop codon in ncx-2 gene (C10G8.5) and referred to as ncx-2(−) elsewhere.
gba-3(gk502826) is a substitution mutant of Q89 to stop ccodon in gba-3 gene (F11E6.1) and referred to as gba-3(−) elsewhere.
XT7 (cln-3.2(gk41) I; cln-3.3(gk118) cln-3.1(pk479) V) is a triple deletion mutant of all 3 cln-3 genes (C01G8.2, ZC190.1.1., F07B10.1), referred to as cln-3.2(−); cln-3.3(−) cln-3.1(−) elsewhere.
clh-6(ok791) is a deletion mutant for cln-6 gene (R07B7.1) and referred to as cln-6(−).
ctns-1(ok813) is a deletion mutant with 1942 bp deletion in ctns-1 gene (C41C4.7) and referred to as ctns-1(−) elsewhere.
slc38a9(syb6822) is a deletion mutant with 994 bp deletion in F13H10.3a gene generated with CRISPR-Cas9 at SunyBiotech. This mutant is referred to as slc38a9(−) elsewhere.
qRT-PCR
For C. elegans, total RNA was isolated from >50 young adult worms with Trizol. cDNA was synthesized with Maxima H Minus cDNA synthesis master mix (Thermo, M1661) according to manufacturer’s protocol. qPCR was performed with Roche LightCycler 96. ΔΔCT was used to calculate fold change difference of RNA level compared to control gene act-1. Following primers were used for qRT PCR:
nhx-5 fwd: CGT CAA CTG TAG CAG GTT CTA A
nhx-5 rev: GGA AAC GTA GGT GAG GAG TAT G
nhx-7 fwd: GGA GCT TTA CCA CAC GAC TTA T
nhx-7 rev: GTG CAT GAG CTG ACG AAT AGA
nhx-8 fwd: CCA TCG TTC AAC TCG TTA CCT
nhx-8 rev: GAG CAA TGC ACT CAA CAA TCC
ncx-2 fwd: GAT TGA TCG GAG GAG GAG ATA TTG
ncx-2 rev: GTA GTG AGC TGG ATC CAA GAA G
act-1 fwd: CGA GCG TGG TTA CTC TTT CA
act-1 rev: CTT CTG CAT ACG ATC AGC AAT TC
For RAW 264.7 macrophages, total RNA was isolated from cells from a single well from a 6 well plate. Trizol (Thermo) extraction, cDNA synthesis and qPCR was performed similar to worm. Gapdh was used as reference gene. Following primers were used for qRT PCR.
Gapdh fwd: AAG CTC ATT TCC TGG TAT GAC A
Gapdh rev: CTT GCT CAG TGT CCT TGC TG
Tpcn2 fwd: GGA GAC TGG TAT TGG GGC TT
Tpcn2 rev: CAA TGC TGG CTG ATG AGT TC
C. elegans targeting and colocalization
For colocalization assays, worms with fluorescent marker for early endosome, late endosome or lysosome are microinjected with 1 μM of RatiNaAT.(8). Worms were transferred to fresh NGM plate after injection and imaged by confocal microscopy at 5 min, 17 min and 60 min to assess targeting to early endosomes, late endosomes and lysosomes respectively. Anti-colocalization was performed similarly. Targeting was evaluated as previously specified here.
NHX-5::GFP overexpression in C. elegans
NHX-5::GFP plasmid (pFH6) was gifted by Prof. Keith Nehrke from University of Rochester Medical Center9. It contains genomic sequences of nhx-5 and 1.5 Kb of upstream promoter sequences. EGFP with synthetic intron was fused to the C-terminus. Transgenic animals carrying an extrachromosomal Pnhx-5::NHX-5::GFP array were generated by microinjecting the plasmid (100 ng/μL) into the gonads of adult hermaphrodites with the myo-2::mCherry plasmid (2 ng/μL) as a co-injection marker at a final total DNA concentration of 100 ng/μL. The presence of the extrachromosomal array was confirmed by pharyngeal mCherry fluorescence and F1 progeny was picked for RatiNaAT colocalization.
C. elegans salt stress assay
C. elegans was stressed by salt using previously published protocol10. Briefly, high Na+ containing plates were made by supplementing NaCl to NGM plate with either 200 mM, 300 mM or 400 mM NaCl). High Na+ plates were sealed with parafilm and kept at 4 °C until needed. For chronic salt stress (Ch), gravid worms were transferred to 200 mM Na+ plate and hatched worms were allowed to grow on 200 mM Na+ plate for chronic salt stress. For acute salt stress (Ac), L4 worms were directly transferred from NGM plates to 200 mM, 300 mM or 400 mM NaCl plates.
To assay brood size, 5 Ch worms and Ac worms are transferred to 200 mM, 300 mM and 400 mM Na+ plate at L4 stage. Worms laid egg for 24 h, removed from the plate, but progeny allowed to grow for another 48 h. Plates were photographed and brood size was counted.
C. elegans coelomocyte lysosomal pH measurement
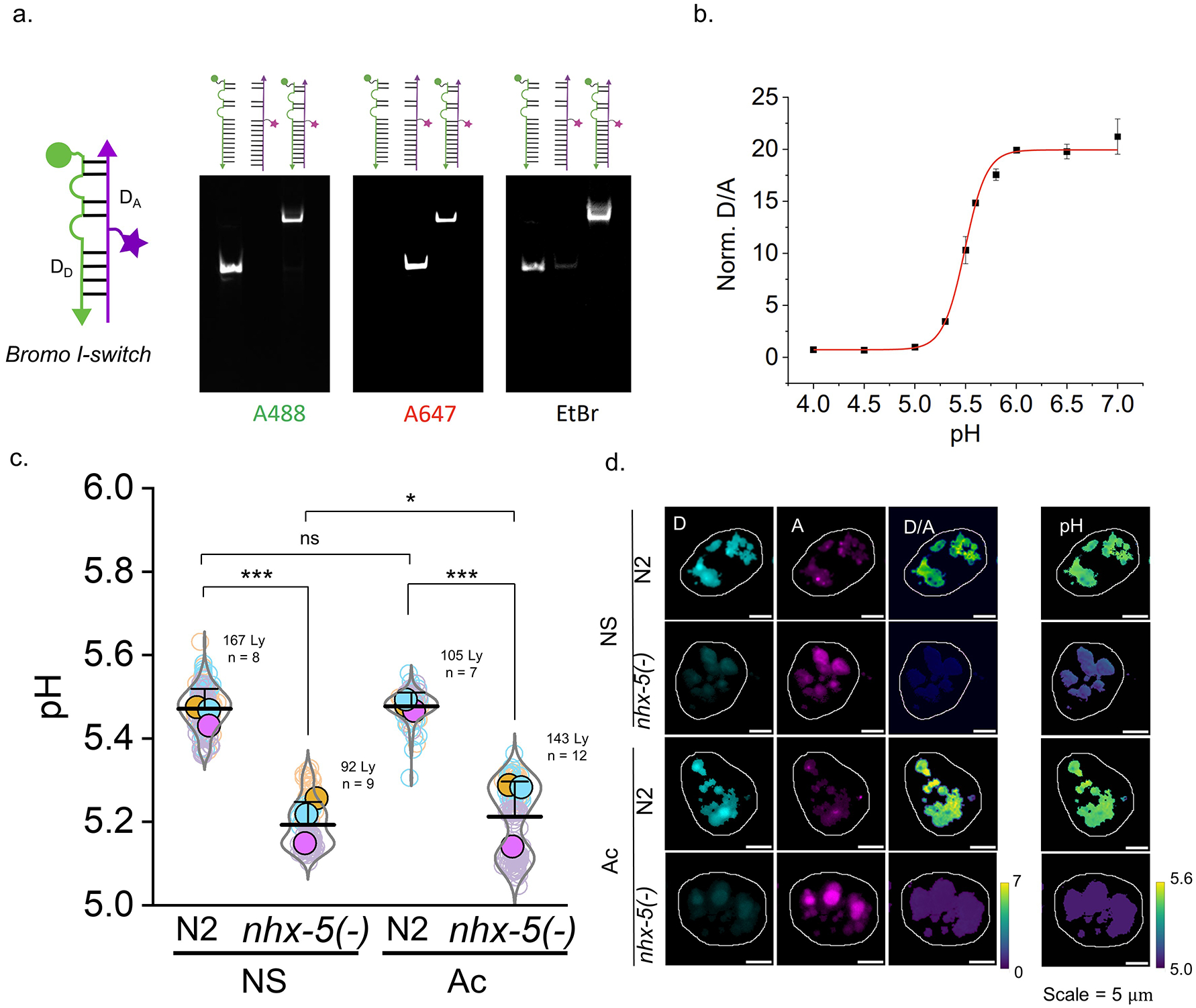
pH of C. elegans was measured with our previously published DNA-based sensor Bromo I-switch11. Briefly, Bromo I-switch was made by annealing component strands and calibrated from fluorescence intensity ratio of D/A signals from pH 4.0 to 7.0. Next in vivo pH clamping was performed by injecting Bromo I-switch and perforating worms with clamping buffers of desired pH (10 mM HEPES, 10 mM MES, 10 mM NaOAc, 140 mM KCl, 5 mM NaCl, 1 mM MgCl2, 2 mM CaCl2) contaning 50 μM nigericin and 50 μM monensin. The in vivo clamped D/A data and in vitro calibration curve was used to generate the in vivo calibration curve. pH was measured by injecting worms with Bromo I-switch, a chase for 30 min followed by imaging with widefield microscope.
Mouse BMDM isolation
Homozygous male and female Nhe6 (slc6a6) KO mice were obtained from Dr. Rajini Rao’s laboratory at Johns Hopkins University12, and rederived at the University of Chicago13. These mice were generated via insertion of the LacZ-Neo reporter gene encoding for β-galactosidase in the genomic locus of Slc6a6. The presence of the LacZ-Neo at this site added a stop codon and a polyadenylation termination signal which halted the transcription of the target gene resulting in the deficient mice. The tails clippings from the homozygous Nhe6 deficient male and female mice and the litters were used for genotyping as per procedure outlined by Strømme et al13. The mice were housed, fed a standard diet and maintained at the animal facility. For bone marrow derived macrophages14 the femur and tibia of the homozygous KO and WT female littermates were collected under sterile conditions and then differentiated for 7 days in culture media (1X DMEM supplemented with 10% fetal bovine serum, 1% Penicillin/Streptomycin and 0.002% prophylactic Plasmocin) containing recombinant murine M-CSF (10–20 ng/ml). The differentiated adherent macrophages were used for outlined assays. All procedures were conducted in compliance with Institutional Animal Care and Use Committee (IACUC) and Animal Resource Center (ARC) at University of Chicago.
Data analysis
Numerical data was processed and plotted with OriginPro 2023 10.0.0.154. For Na+ measurement of cultured cells and C. elegans, MATLAB R2018a and ImageJ 1.53t was used for endosomal ROI picking and analysis respectively.
Extended Data
Extended Data Fig 1 |. Chicago Green (CG) is pH insensitive and selective to Na+ prior to incorporation into RatiNa.
a, Na+ sensing mechanism of CG b, Excitation (black) and emission (green) spectra of free CG increases with increasing Na+. c, Dissociation constant (Kd) of CG for Na+ does not vary with pH from pH 4.5 – 7.4 d, Individual in vitro calibration profiles of RatiNa at different pH in Fig. 1d. e, Kd of RatiNa for Na+ at different pH values as calculated from d. Kd of RatiNa is higher than that of CG but is still pH invariant from pH 4.5 – 7.4. f, RatiNa response to K+ yields a Kd of 4.5 M and 27-fold selectivity for Na+ over K+. g, Magnified view of RatiNa Na+ calibration profile from 1 mM to 200 mM Na+.
Extended Data Fig 2 |. Calibration of RatiNa and its stability in lysosomes of RAW264.7 macrophages.
a, Lysosomes in RAW264.7 macrophages prelabeled with TMR-dextran (cyan) and imaged at different chase times of RatiNaAT (magenta). b, Histogram of single lysosome signal ratio of RatiNaAT/TMR-dextran (R/G) at different chase time. A decrease of R/G indicates DNA degradation in lysosome over time. c, DNA degradation as a function of chase time calculated from b. Note that for 30 min chase time DNA is intact and ratiometry is valid. Error bar represents standard deviation. d, Normalized RatiNa signal (G/R) of single lysosome in RAW macrophage are plotted against the normalizing dye signal (R). The PCC analysis shows no correlation between G/R and R, indicating RatiNa signal and Na+ measurement is independent of probe concentration. e, Fluorescence images of RatiNaAT labeled RAW264.7 macrophages in CG (G) and ATTO (R) channels. Low amount of autofluorescence can be detected in the CG channel. f, Images of RatiNa-labeled lysosomes clamped at high and low Na+ of 145 mM and 5 mM in native lysosomes. G/R heat maps show adequate change that Na+ can be measured in native lysosomes.g, Histogram of G/R values of RatiNa-labeled single lysosomes. Accounting for autofluorescence represented by G/R in RatiNaAT sample, the fold change in G/R signal of RatiNa in lysosomes of RAW264.7 macrophages is comparable to that in C. elegans and on beads. h, Schematic of workflow from raw images to Na+ heat maps of single organelles. Fluorescent images in the CG (G) and ATTO (R) channels are used to construct the G/R image. Then the Na+ heatmap was generated from the calibration curve of G/R to [Na+].
Extended Data Fig 3 |. Specificity of RatiNa targeting to endocytic organelles.
a, Representative images of C. elegans coelomocytes reveal negligible off-target labeling between RatiNaAT and indicated endocytic markers and chase times. b, RatiNa is targeted to a specific endocytic organelle at fixed chase time. Colocalization is calculated as percentage of organelles having both lumenal RatiNaAT and membrane marker fluorescence over all RatiNaAT containing organelles (n = 9 coelomocytes of 6 worms in 5 min chase of RAB-5::GFP, 7 coelomocytes of 6 worms in 17 min chase of RAB-5::GFP, 4 coelomocytes of 4 worms in 5 min chase of RAB-7::GFP, 6 coelomocytes in 5 worms of 17 min chase of RAB-7::GFP, 7 coelomocytes in 6 worms of 17 min chase of LMP-1::GFP, 9 coelomocytes in 6 worms for 60 min chase of LMP-1::GFP)
Extended Data Fig 4 |. RatiNa reports lysosomal Na+ changes with inhibition of TPC2 and mTOR.
a, Apilimod is a strong inhibitor of PIKfyve that phosphorylate PI3P to PI(3,5)P2, which activate TPC2 channel to export lysosomal Na+. Inhibiting PIKfyve causes less efflux of lysosomal Na+. Lysosomal Na+ increases from 39 mM in vehicle to 68 mM in 100 nM apilimod treated RAW macrophages. b, Torin-1 inhibits mTOR and induces autophagy. After acute 1 h treatment of 1 uM Torin-1, lower lysosomal Na+ of 22 mM is observed compared to 40 mM in vehicle. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, no statistical significance by two sample t-test.
Extended Data Fig 5 |. Na+ transporter mutants are susceptible to salt stress.
a, Brood sizes of Na+ transporter mutants upon high salt stress. Na+ transporter deletion mutants cannot survive 400 mM NaCl compared to WT worms. Arrows indicates condition used for lysosomal Na+ measurement in salt stress worms is acute 200 mM (Ac worm in d.) b, Representative Na+ heatmaps of Na+ transporter deletion mutant worms. c, qRT-PCR shows that mRNA expression level of Na+ transporters do not change appreciably upon high salt stress in N2 worms. Fold change of between Ac and Ch condition N2 worms is plotted for mRNA level of Na+ transporters. act-1 was used as reference gene. d, Lysosomal Na+ levels of Na+ transporter mutant worms under NS and Ac condition. Higher lysosomal Na+ is observed in all investigated Na+ transporter mutant worms: 9 mM in NS and 63 mM in Ac for nhx-5(−) worms. 24 mM in NS and 51 mM in Ac for nhx-7(−) worms. 34 mM in NS and 55 mM in Ac for nhx-8(−) worms. 25 mM in NS and 36 mM in Ac for ncx-2(−) worms. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, no statistical significance by two sample t-test.
Extended Data Fig 6 |. Lysosomal pH of salt stressed worms.
a. PAGE analysis of the I-switch-based pH reporter module denoted Br-I-switch59. DD strand has Alexa488N as a donor dye and DA strand has Alexa647N as acceptor dye. b. pH calibration curve of Br-I-switch shows ~20 fold change of D/A signal from pH 5.0 to 6.0, with highest sensitivity near pH 5.5 which is the pH of coelomocyte lysosome38. c. pH measurement of single lysosome of N2 and nhx-5(−) worms in normal salt (NS) and acutely salt stressed (Ac) condition. nhx-5(−) has lower pH in both NS and Ac conditions. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, no statistical significance by two sample t-test. d. Representative images of Br- I-switch in Donor (D), acceptor (A) FRET (D/A) channels and calculated pH heatmaps.
Supplementary Material
Acknowledgements
We thank Eduardo Perozo, Gary Ruvkun, Axel Concepcion and Ai Lin Chun for valuable discussions and input on the manuscript. We thank Christine Labno at the integrated light microscopy facilities at the University of Chicago for technical help and Tong Wu for assistance with qRT-PCR. We thank Keith Nehrke for sharing NHX-5::GFP plasmid. YK acknowledges funding from NIH grants DP1GM149751, 1R01NS112139-01A1, 1R21NS114428-01, R21HL161825-01A1, 1R01GM147197-01 (YK and RR), FA9550-19-0003 from the AFOSR, HFSP grant no: RGP0032/2022, and the Ono Pharma Foundation.
Footnotes
Competing interests
The authors declare no competing interests. YK is a co-founder of Esya Inc and MacroLogic Inc that use DNA nanodevices to develop diagnostics and therapeutics respectively.
Data Availability
The raw data supporting Figures 1–4 are available for public access at Figshare: https://doi.org/10.6084/m9.figshare.23938503
References
- 1.Shapovalov G et al. Organelle membrane derived patches: reshaping classical methods for new targets. Sci. Rep 7, 14082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nass R, Cunningham KW & Rao R Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J. Biol. Chem 272, 26145–26152 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Nass R & Rao R Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem 273, 21054–21060 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Orlowski J & Grinstein S Na+/H+ exchangers. Compr. Physiol 1, 2083–2100 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Pedersen SF & Counillon L The SLC9A-C Mammalian Na+/H+ Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol. Rev 99, 2015–2113 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Kondapalli KC et al. Functional evaluation of autism-associated mutations in NHE9. Nat. Commun 4, 2510 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow EM et al. Identifying autism loci and genes by tracing recent shared ancestry. Science 321, 218–223 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pescosolido MF, Ouyang Q, Liu JS & Morrow EM Loss of christianson syndrome na+/h+ exchanger 6 (NHE6) causes abnormal endosome maturation and trafficking underlying lysosome dysfunction in neurons. J. Neurosci 41, 9235–9256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukada-Tanaka S, Inagaki Y, Yamaguchi T, Saito N & Iida S Colour-enhancing protein in blue petals. Nature 407, 581 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Lamason RL et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310, 1782–1786 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Cang C, Bekele B & Ren D The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol 10, 463–469 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Cang C et al. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 152, 778–790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 151, 372–383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saminathan A et al. A DNA-based voltmeter for organelles. Nat. Nanotechnol 16, 96–103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erecińska M & Silver IA Ions and energy in mammalian brain. Prog. Neurobiol 43, 37–71 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Harootunian AT, Kao JP, Eckert BK & Tsien RY Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J. Biol. Chem 264, 19458–19467 (1989). [PubMed] [Google Scholar]
- 17.Minta A & Tsien RY Fluorescent indicators for cytosolic sodium. J. Biol. Chem 264, 19449–19457 (1989). [PubMed] [Google Scholar]
- 18.Steinberg BE et al. A cation counterflux supports lysosomal acidification. J. Cell Biol 189, 1171–1186 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung K, Chakraborty K, Saminathan A & Krishnan Y A DNA nanomachine chemically resolves lysosomes in live cells. Nat. Nanotechnol 14, 176–183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H et al. Metabolomic profiling of single enlarged lysosomes. Nat. Methods 18, 788–798 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Martin VV, Rothe A & Gee KR Fluorescent metal ion indicators based on benzoannelated crown systems: a green fluorescent indicator for intracellular sodium ions. Bioorg. Med. Chem. Lett 15, 1851–1855 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Holmehave J, Pedersen SK, Jensen H & Ogilby PR Aarhus green: a tetrafluoro-substituted derivative of fluorescein. Arkivoc 2015, 52 (2015). [Google Scholar]
- 23.Rostovtsev VV, Green LG, Fokin VV & Sharpless KB A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed 41, 2596–2599 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Chang PV et al. Copper-free click chemistry in living animals. Proc Natl Acad Sci USA 107, 1821–1826 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veetil AT et al. DNA-based fluorescent probes of NOS2 activity in live brains. Proc Natl Acad Sci USA 117, 14694–14702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan Y, Zou J & Jani MS Quantitative imaging of biochemistry in situ and at the nanoscale. ACS Cent. Sci 6, 1938–1954 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surana S, Bhat JM, Koushika SP & Krishnan Y An autonomous DNA nanomachine maps spatiotemporal pH changes in a multicellular living organism. Nat. Commun 2, 340 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Suresh B et al. Tubular lysosomes harbor active ion gradients and poise macrophages for phagocytosis. Proc Natl Acad Sci USA 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui C et al. A lysosome-targeted DNA nanodevice selectively targets macrophages to attenuate tumours. Nat. Nanotechnol 16, 1394–1402 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Dan K, Veetil AT, Chakraborty K & Krishnan Y DNA nanodevices map enzymatic activity in organelles. Nat. Nanotechnol 14, 252–259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surana S, Bhatia D & Krishnan Y A method to study in vivo stability of DNA nanostructures. Methods 64, 94–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiguro H, Steward MC, Lindsay AR & Case RM Accumulation of intracellular HCO3- by Na(+)-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol (Lond) 495 (Pt 1), 169–178 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saminathan A, Zajac M, Anees P & Krishnan Y Organelle-level precision with next-generation targeting technologies. Nat. Rev. Mater (2021) doi: 10.1038/s41578-021-00396-8. [DOI] [Google Scholar]
- 34.Saha S, Prakash V, Halder S, Chakraborty K & Krishnan Y A pH-independent DNA nanodevice for quantifying chloride transport in organelles of living cells. Nat. Nanotechnol 10, 645–651 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Narayanaswamy N et al. A pH-correctable, DNA-based fluorescent reporter for organellar calcium. Nat. Methods 16, 95–102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calcraft PJ et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogunbayo OA et al. mTORC1 controls lysosomal Ca2+ release through the two-pore channel TPC2. Sci. Signal 11, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang Y-L et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc Natl Acad Sci USA 117, 20803–20813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad H & Rao R The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. J. Biol. Chem 290, 5311–5327 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y & Guo Y Unraveling salt stress signaling in plants. J. Integr. Plant Biol 60, 796–804 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Lamitina ST, Morrison R, Moeckel GW & Strange K Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol, Cell Physiol 286, C785–91 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Urso SJ & Lamitina T The C. elegans Hypertonic Stress Response: Big Insights from Shrinking Worms. Cell. Physiol. Biochem 55, 89–105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nehrke K & Melvin JE The NHX family of Na+-H+ exchangers in Caenorhabditis elegans. J. Biol. Chem 277, 29036–29044 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Kim J et al. NHX-5, an Endosomal Na+/H+ Exchanger, Is Associated with Metformin Action. J. Biol. Chem 291, 18591–18599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins JF et al. Molecular cloning, sequencing, tissue distribution, and functional expression of a Na+/H+ exchanger (NHE-2). Proc Natl Acad Sci USA 90, 3938–3942 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberheide K, Puchkov D & Jentsch TJ Loss of the Na+/H+ exchanger NHE8 causes male infertility in mice by disrupting acrosome formation. J. Biol. Chem 292, 10845–10854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R & Yaish MW The role of na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol 8, 509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mager T, Rimon A, Padan E & Fendler K Transport mechanism and pH regulation of the Na+/H+ antiporter NhaA from Escherichia coli: an electrophysiological study. J. Biol. Chem 286, 23570–23581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mindell JA Lysosomal acidification mechanisms. Annu. Rev. Physiol 74, 69–86 (2012). [DOI] [PubMed] [Google Scholar]
- 50.de Voer G, Peters D & Taschner PEM Caenorhabditis elegans as a model for lysosomal storage disorders. Biochim. Biophys. Acta 1782, 433–446 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Chakraborty K, Leung K & Krishnan Y High lumenal chloride in the lysosome is critical for lysosome function. eLife 6, e28862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rebsamen M et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ba Q, Raghavan G, Kiselyov K & Yang G Whole-Cell Scale Dynamic Organization of Lysosomes Revealed by Spatial Statistical Analysis. Cell Rep 23, 3591–3606 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platt CD et al. Leucine-rich repeat containing 8A (LRRC8A)-dependent volume-regulated anion channel activity is dispensable for T-cell development and function. J. Allergy Clin. Immunol 140, 1651–1659.e1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.López-Hernández T, Puchkov D, Krause E, Maritzen T & Haucke V Endocytic regulation of cellular ion homeostasis controls lysosome biogenesis. Nat. Cell Biol 22, 815–827 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Jeffery J & Jörnvall H Enzyme relationships in a sorbitol pathway that bypasses glycolysis and pentose phosphates in glucose metabolism. Proc Natl Acad Sci USA 80, 901–905 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burg MB, Ferraris JD & Dmitrieva NI Cellular response to hyperosmotic stresses. Physiol. Rev 87, 1441–1474 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Galluzzi L, Pietrocola F, Levine B & Kroemer G Metabolic control of autophagy. Cell 159, 1263–1276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kandasamy P, Gyimesi G, Kanai Y & Hediger MA Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci 43, 752–789 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Wang S et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347, 188–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Efeyan A, Comb WC & Sabatini DM Nutrient-sensing mechanisms and pathways. Nature 517, 302–310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamming DW & Bar-Peled L Lysosome: The metabolic signaling hub. Traffic 20, 27–38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lord SJ, Velle KB, Mullins RD & Fritz-Laylin LK SuperPlots: Communicating reproducibility and variability in cell biology. J. Cell Biol 219, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods only references
- 1.Ellison DH & Welling P Insights into Salt Handling and Blood Pressure. N. Engl. J. Med 385, 1981–1993 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Müller DN, Wilck N, Haase S, Kleinewietfeld M & Linker RA Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat. Rev. Immunol 19, 243–254 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Kitada K et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J. Clin. Invest 127, 1944–1959 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schindelin J et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suresh B et al. Tubular lysosomes harbor active ion gradients and poise macrophages for phagocytosis. Proc. Natl. Acad. Sci 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty K, Leung K & Krishnan Y High lumenal chloride in the lysosome is critical for lysosome function. eLife 6, e28862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gholami Yarahmadi S, Sarlaki F & Morovvati S Cystinosis and two rare mutations in CTNS gene: two case reports. J. Med. Case Reports 16, 181 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surana S, Bhat JM, Koushika SP & Krishnan Y An autonomous DNA nanomachine maps spatiotemporal pH changes in a multicellular living organism. Nat. Commun 2, 340 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Nehrke K & Melvin JE The NHX Family of Na+-H+ Exchangers in Caenorhabditis elegans *. J. Biol. Chem 277, 29036–29044 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Lamitina ST, Morrison R, Moeckel GW & Strange K Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am. J. Physiol.-Cell Physiol 286, C785–C791 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Leung K, Chakraborty K, Saminathan A & Krishnan Y A DNA nanomachine chemically resolves lysosomes in live cells. Nat. Nanotechnol 14, 176–183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad H & Rao R Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc. Natl. Acad. Sci 115, E6640–E6649 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strømme P et al. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain J. Neurol 134, 3369–3383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasser H et al. Establishment of bone marrow-derived M-CSF receptor-dependent self-renewing macrophages. Cell Death Discov 6, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting Figures 1–4 are available for public access at Figshare: https://doi.org/10.6084/m9.figshare.23938503


