Graphical abstract
Keywords: Soursop seeds, Protein isolate, Ultrasonication, Techno-functional properties, Protein fractionation, Structural properties
Abstract
The obtained seeds from fruit processing are considered by-products containing proteins that could be utilized as ingredients in food manufacturing. However, in the specific case of soursop seeds, their usage for the preparation of protein isolates is limited. In this investigation a protein isolate from soursop seeds (SSPI) was obtained by alkaline extraction and isoelectric precipitation methods. The SSPI was sonicated at 200, 400 and 600 W during 15 and 30 min and its effect on the physicochemical, functional, biochemical, and structural properties was evaluated. Ultrasound increased (p < 0.05) up to 5 % protein content, 261 % protein solubility, 60.7 % foaming capacity, 30.2 % foaming stability, 86 % emulsifying activity index, 4.1 % emulsifying stability index, 85.4 % in vitro protein digestibility, 423.4 % albumin content, 83 % total sulfhydryl content, 316 % free sulfhydryl content, 236 % α-helix, 46 % β-sheet, and 43 % β-turn of SSPI, in comparison with the control treatment without ultrasound. Furthermore, ultrasound decreased (p < 0.05) up to 50 % particle size, 37 % molecular flexibility, 68 % surface hydrophobicity, 41 % intrinsic florescence spectrum, and 60 % random coil content. Scanning electron microscopy analysis revealed smooth structures of the SSPI with molecular weights ranging from 12 kDa to 65 kDa. The increase of albumins content in the SSPI by ultrasound was highly correlated (r = 0.962; p < 0.01) with the protein solubility. Improving the physicochemical, functional, biochemical and structural properties of SSPI by ultrasound could contribute to its utilization as ingredient in food industry.
1. Introduction
Population growth has required the exploration of new sources of food ingredients, such as plant-based proteins [1]. In the case of fruits, world production rose from 591 to 899 million ton in the period 2001–2021, which meant an increase of 52.1 %, as well as in the generation of their waste by processing [2]. Currently, most of the by-products from fruits are discarded as waste, complicating the environment of the sites where they are dumped [3]. However, such by-products that include stem, pulp, peel/skin, seeds, and stones, contain bioactive compounds and other components that can be recovered to elaborate value-added products of importance for the food industry [4].
The soursop (Annona muricata L.) is an ovoid fruit with a white pulp containing around of 127 to 170 seeds [5]. Soursop is an exotic fruit belonging to the Annonaceae family native to tropical America, although it is currently distributed throughout the world, but Mexico is the main international producer [6]. During the production of pulp soursop, which is utilized to elaborate products like ice creams, juices, nectar, jam and yogurt, peel and seeds are discarded as waste [6], [7]. The valorization of the peel and seeds of the soursop fruit has been mainly proposed to produce bioactive compounds [8], [9] and oil [10], [11]. Soursop seeds represent 8.5 % of the weight of the fruit and contain 14.99 % proteins [12], [13], which has good functional properties [14].
Recent studies with jackfruit [15], passion fruit [16], mango [17], guamuchil [18], and orange [19] seeds from fruit processing, have been considered as good raw material in the production of protein concentrates or isolates. On the other hand, when the vegetable protein presents deficiencies in its functional or nutritional characteristics, which limits its application as a food ingredient, some physical, chemical, enzymatic treatments or their combination can be applied to improve its quality [20]. Within the physical treatments to improve the functionality of proteins, ultrasound stands out for being effective and friendly to the environment [21], [22], [23].
Ultrasound are sound waves with frequencies above the upper audible limit of the human ear (>16 kHz), which can be categorized into two types: high intensity or low frequency (16 to 100 kHz, power from 10 to 1000 W/cm2) and low intensity or high frequency (100 kHz to 1 MHz, power < 1 W/cm2). Proteins in solution exposed to high-intensity ultrasound alter their physicochemical, structural, and functional properties, mainly due to hydrodynamic shearing and high temperatures (up to 5000 K) and pressures (up to 1000 atm) produced by the formation and violent collapse of small bubbles of gases, a phenomenon known as cavitation [24], [25].
In the light of our knowledge, to date only two studies have considered the use of soursop seeds as a source of protein for food use. Chaparro et al. [14] prepared a protein concentrate (63.3 % protein) by saline extraction (1 % NaCl) and isoelectric precipitation (pH 4), which showed values of 43.2 % and 45.7 % of emulsifying activity and emulsifying stability, respectively. On the other hand, Villacís-Chiriboga et al. [10] evaluated the methods of pressurized water extraction (PWE) and alkaline water extraction (AWE), for the recovery of proteins from soursop seed flour de-oiled with n-hexane or cold pressed. The results of this study showed that at pH 8.1 and 40 °C, PWE resulted in a more protein-rich extract (48 %) compared to AWE (30 %), as well as better preservation of amino acid content, and higher solubility and in vitro protein digestibility.
However, the functional and nutritional properties of soursop proteins could be improved to make them more attractive as food ingredients [8], [15], suggesting that ultrasound could improve their utility in this industry [23].
In view of the scarce information on soursop seeds as a source of protein, additional research could generate new knowledge to provide other alternatives to produce protein materials for food use. Therefore, the objective of this study was to evaluate the impact of high-intensity ultrasound on the physicochemical, functional, biochemical and structural properties of a soursop seed protein isolate.
2. Materials and methods
2.1. Materials
The soursop seeds were recovered from waste generated during the pulp extraction process from fruits harvested in orchards of the municipality of Compostela, Nayarit, Mexico. All chemicals and solvents used were analytical grade and purchased from Sigma-Aldrich (St. Louis, Mo., USA), J.T. Baker and Bio-Rad Laboratories, Inc. (USA).
2.2. Preparation of soursop seed protein isolate (SSPI)
The soursop seeds were washed with running water, shelled manually, and dried in a Memmert model 30–1060 oven (Buchenbach, Germany) at 35 °C for 48 h. Subsequently, the dried seeds were processed in a Nutribullet model NB101B household food processor (Ningbo Bestwin Industrial Co., Ltd., China) for 30 s, to obtain the corresponding soursop seed meal (SSF). Later, samples of 100 g of SSF were de-oiled using 1000 mL of ethyl ether and magnetic stirring at 700 rpm and 25 °C by means 10 successive extractions of 1 h each, where the exhausted solvent was replaced by fresh solvent. The product obtained from the last de-oiling cycle was placed in a beaker for complete desolventization, in a fume hood overnight, and then pulverized in a Cyclotec 1093 mill (Foss Tecator, Sweden). The de-oiled soursop seed flour (DSSF) was stored in polyethylene bags at 25 °C for its subsequent characterization.
Next, a test was carried out to determine the pH values of maximum and minimum protein extraction of the DSSF [26], conditions that was used for the preparation of SSPI by alkaline extraction and isoelectric precipitation method [18], with slight modification. Fifty g of DSSF were added to 1000 mL of 0.5 % NaCl solution adjusting the pH with NaOH 0.1 N at the value previously determined for the maximum protein extraction. The slurry was subjected to magnetic stirring at 500 rpm and 25 °C for 30 min and then centrifugated at 8000 x g for 10 min at 4 °C. Next, the supernatant was adjusted to the pH value of minimal protein extraction with 0.1 N HCl, which was previously determined (isoelectric point), and the suspension obtained was stirred and centrifuged under the same conditions mentioned above. Finally, the protein precipitate was resuspended in distilled water in a 1:1.7 (w/v) ratio and adjusted to pH 7.0 with 1 N NaOH.
2.3. High-intensity ultrasound (HIUson) treatment
HIUson was applied to the resuspended protein precipitates (subsection 2.2) following the method of Resendiz-Vazquez et al. [15], with slight changes, using a Cole-Parmer Instruments model CPX750 ultrasound system (Vernon Hills, Illinois, U.S.A.) equipped with a titanium probe (2.54 diameter). Three different power levels of ultrasound (200 W, 400 W, and 600 W) and two times (15 and 30 min) were applied to the protein suspensions (pulse time: on time 5 s, off time 1 s), which generated six treatments: 200 W/15 min, 200 W/30 min, 400 W/15 min, 400 W/30 min, 600 W/15 min, and 600 W/30 min, where the numerator and denominator in each corresponded to the ultrasound power (W) and ultrasound exposure time (min), respectively.
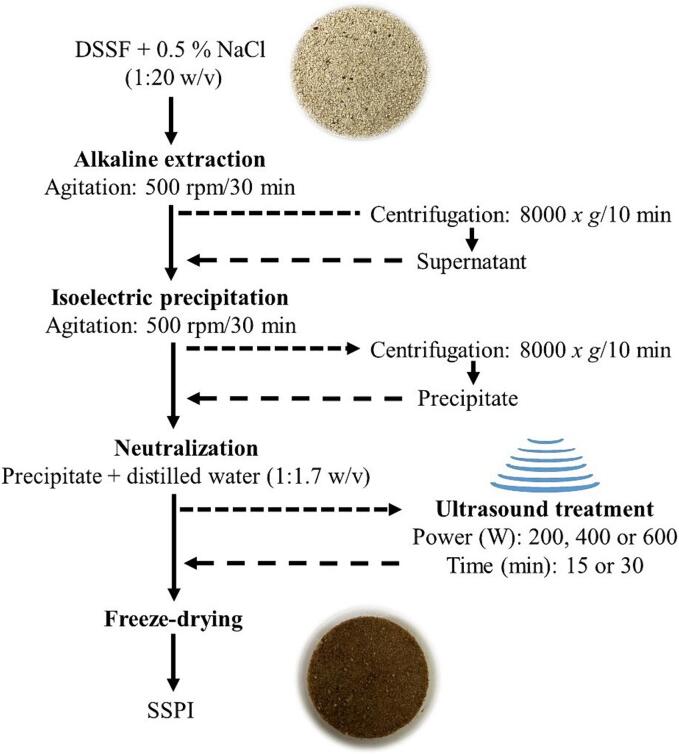
The samples were placed in a 500 mL beaker and the probe was inserted 2.5 cm into the protein suspension, using an ice-water bath to ensure that the system temperature was below 25 °C. After sonication, the samples were lyophilized in 50 mL plastic tubes in a FreeZone model 12 L unit (Labconco, USA). In addition, a control treatment without ultrasound was prepared. The products obtained after lyophilization were called SSPI, which were stored in hermetically closed glass bottles for further analysis. The production of SSPI and its treatment with ultrasound is shown in Fig. 1.
Fig. 1.
Process of preparation of soursop seed protein isolate (SSPI) from de-oiled soursop seed flour (DSSF).
2.4. Composition and physicochemical properties
2.4.1. Proximal chemical analysis
The crude protein (N x 6.25), lipids, moisture, and ash contents were determined by AOAC methods [27]. The percentage of nitrogen free extract was calculated by difference [25].
2.4.2. Water activity (Wa)
The Wa measurement was conducted using an AquaLab 4TEV equip (Decagon Devices Inc., Pullman, WA, USA).
2.4.3. Color
The L* (lightness), a* (+redness, -greenness), b* (+yellowness, -blueness) parameters were measured with a Minolta CR-400 (Minolta Ltd., Co., Tokyo, Japan) colorimeter as reported Ramani et al. [28]. The total color difference (ΔE) was obtained with equation (1), where Ls = 98.30, as = −0.18 and bs = 4.49 corresponded to the values of the white tile used as reference.
| (1) |
2.4.4. Bulk (ρb) and compacted (ρc) density
The ρb and ρc were determined using a 10 mL graduated cylinder and the results were obtained relating the weight and volume occupied by the samples. For ρc, samples were tapped 50 times to compact the powders [29].
2.5. Functional properties
2.5.1. Protein solubility (ProS)
ProS was performed as described by Zhang et al. [30] with modifications. 60 mg of SSPI were mixed in 10 mM sodium phosphate buffer (SPB) with pH 9 for 60 min with magnetic stirring. Then, the SSPI dispersions were centrifuged at 8000 x g for 20 min at 25 °C and the protein content of the supernatant was measured by the Bradford method [31], using bovine serum albumin (BSA) as a standard. ProS was quantified as the proportion of soluble protein present in the supernatant of HIUson-treated samples relative to the control treatment.
2.5.2. Water and oil absorption capacities
Water-absorption (WAc) and oil-absorption (OAc) capacities were determined by measuring 200 mg of protein from the SSPI. The sample was solubilized in 5 mL of water or oil, followed by stirring with a Vortex for 30 s at 25 °C. The suspension was then incubated for 30 min and centrifuged at 5000 g x for 20 min. The supernatant was removed, and the WAc and OAc were expressed as g of water or oil per g of protein [32].
2.5.3. Emulsifying properties
The emulsifying activity index (EAI) and emulsion stability index (ESI) were determined by Pearce and Kinsella [33] and Xu et al. [34] methods, with slights modifications. Protein suspensions of SSPI (0.1 % protein) were prepared with 10 mM SPB (pH 7.0). Then, 16 mL of each protein suspension of SSPI was mixed with 4 mL of canola oil and processed at 12000 rpm during 1 min with a T-25 Ultra-turrax homogenizer (IKA Instruments, Germany) at 25 °C. Then, 50 µL of each emulsion was diluted (1:100) with 0.1 % (w/v) sodium dodecyl sulphate (SDS) solution and the absorbance was measured at 0 min for EAI and 10 min after for ESI at 500 nm, calculating its values with the next equations:
| (2) |
| (3) |
where T = constant value (2.30), A0 and A10 = absorbance of the emulsion at 0 and 10 min, respectively, N = dilution factor (1 0 0), ϕ = volume fraction of oil (0.20), L = path length of the cuvette (1 cm), and C = protein weight per unit volume (g/mL).
2.5.4. Foaming properties
The foaming capacity (FC) and the foaming stability (FS) were measured by Zhang et al. [35] method, with some changes. Protein suspensions of SSPI (4 % w/v) were prepared with SPB (pH 7.0). The initial volume of the SSPI protein suspensions of 20 mL (Vs) was mixed at 12000 rpm and 25 °C with a T-25 Ultra-turrax homogenizer (IKA Instruments, Germany) for 1 min. The foam initial volume (Vf0) and the produced foam after 20 min (Vf20) were registered to determine the foaming properties by the following equations:
| (4) |
| (5) |
2.5.5. Least gelation concentration (LGCo)
Protein suspensions of SSPI in the range of 2 % to 20 % were prepared with distilled water (w/v) in 15 mL tubes. The suspensions were heated in a bath water at 95 °C for 1 h and immediately cooled under running water and stored at 4 °C for 2 h. LGCo was identified as the concentration at which the tube content did not slip when inverted [36].
2.6. Biochemical properties
2.6.1. Protein fractional composition
Protein fractions were extracted using the Osborne method as reported by Kumar et al. [37], with some slight changes. A sample of each SSPI was mixed with distilled water (1:20 w/v) followed by centrifugation at 10000 x g for 15 min. Then, the obtained supernatant was considered as the albumins fraction. Sequentially, the generated precipitate was mixed in 0.5 N NaCl, 70 % ethanol, and 0.1 N NaOH to obtain the globulins, prolamins, and glutelins fractions, respectively, using the same centrifugation conditions. The quantification of the protein fractions was carried out by the Kjeldahl method (N x 6.25).
2.6.2. Molecular weight distribution
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the Laemmli method [38] as was described by Flores-Jiménez et al. [25], using a mini vertical gel electrophoresis apparatus, model MV-10DSYS (Major Science Co., Ltd, UK). Separating and stacking gels were prepared at 12 % and 4 % concentrations, respectively. Lanes were loaded with samples containing 20 µg protein. A molecular weight marker containing proteins of 10, 15, 20, 25, 37, 50, 75, 100, 150 and 250 kDa was used as reference (Bio-Rad Laboratories, Inc.). The electrophoresis process began with an initial voltage of 140 V with a duration of 10 min, followed by a voltage of 110 V for 45 min. Samples were run under both non-reducing (without 2-mercaptoethanol) and reducing conditions (with 2-mercaptoethanol). The gels were subsequently stained with Coomassie brilliant blue G-250 for over-night. To determine the molecular weight, the gels were decolorized until the bands became visible, and then scanned using GelAnalyzer 19.1 [39].
2.6.3. In vitro protein digestibility (IDig)
The IDig was performed by the multienzyme method of Bodwell et al. [40] with certain changes. A suspension of bovine trypsin (1.58 mg/mL), bovine pancreatic α-chymotrypsin (3.65 mg/mL) and bovine pancreatin (1 mg/mL) in distilled water at pH 8.0 was used for the enzymatic digestion, which was maintained to 37 °C. Subsequently, to 20 mL of each SSPI suspension (6.38 mg of protein/mL distilled water at pH 8.0 and 37 °C) 3 mL of the multienzyme suspension were added, maintaining the temperature at 37 °C in a water bath. After 10 min, the pH was measured and the IDig determined using the following equation:
| (6) |
where X = pH after 10 min of enzymatic digestion.
2.6.4. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (Act-DPPH)
The Act-DPPH was measured according to Estrada-Sierra et al. [41] method with some modifications. First, 8 µL of protein solution (1 mg/mL, SPB pH 9.0) was mixed with 292 µL of a DPPH solution (0.06 mM in methanol/water, 80:20 v/v) and incubated for 30 min in the dark at 25 °C. Absorbance of samples (abs sample) was measured at 517 nm using a Thermo Scientific Multiskan GO microplate reader (Waltham, Massachusetts, USA), with SPB as blank (abs blank) and Trolox (1 mg/mL) as control. The Act-DPPH was determined using the equation (7).
| (7) |
2.7. Structural characterization
2.7.1. Particle size (Ps)
The Ps of SSPI was determined with 0.20 mg/mL aliquots prepared with SPB (pH 9.0), following the methodology described by Flores-Jiménez et al. (2022) [28], using a Zetasizer ZEN 3600 equipment (Marvel Instrument Co., United Kingdom).
2.7.2. Surface hydrophobicity (Sh)
Sh was measured using 1-anilino-8-naphthalene-sulfonate (ANS) as a fluorescence probe, according to Li et al. [42], with some modifications. For this purpose, 2 mL aliquots of SSPI were prepared with concentrations of 0.05, 0.04, 0.03, 0.02, 0.01 and 0.001 mg/mL with SPB (pH 9.0). Immediately, 25 µL of ANS (8 mM, SPB pH 9.0) was added to each aliquot. Fluorescence intensity was recorded using a 200 Pro fluorescence spectrophotometer (Tecan Infinite, Grodig, Austria) at wavelengths of 364 nm (excitation) and 475 nm (emission). The initial slope of fluorescence intensity relative to protein concentration (mg/mL) was calculated by linear regression analysis and used as an indicator of Sh.
2.7.3. Intrinsic fluorescence spectrum (IFS)
IFS was obtained from 0.2 mg/mL (SPB pH 9.0) solutions of SSPI, using a 200 Pro fluorescence spectrophotometer (Tecan Infinite, Grodig, Austria) with an excitation wavelength of 290 nm, an emission wavelength between 320 and 450 nm and a slit width of 5 nm, as reported by Zhao et al. [43], with changes.
2.7.4. Molecular flexibility (MolFlex)
The MolFlex technique was carried out as described by Cui et al. [44], employing protein solutions from SSPI at a concentration of 1 mg/mL (SPB pH 9.0).
2.7.5. Free (SH-F) and total (SH-T) sulfhydryls
The determination of the content of SH-F and SH-T sulfhydryl groups of SSPI was carried out according to the method described by Ren et al. [45], with some adaptations. Tris-glycine buffer (0.086 M Tris, 0.09 M glycine, 0.04 M EDTA-Na2, pH 8.0) was prepared for the determination of SH-F. Subsequently, Ellman's reagent was prepared (4 mg of DTNB/mL of Tris-glycine buffer). Next, 100 µL of the SSPI suspension (1 mg/mL SPB, pH 9.0) was mixed with 500 µL of Tris-glycine buffer and 10 µL of Ellman's reagent, followed by incubation at 25 °C for 1 h. Samples were then centrifuged at 8000 rpm for 10 min using an Eppendorf Minispin centrifuge (Hamburg, Germany) and absorbances were measured at 412 nm with SPB as blank. For the analysis of the content of SH-T groups, the same process was followed, with the addition of 8 M urea to the Tris-glycine buffer. Equation (8) was used to calculate the content of SH-F and SH-T.
| (8) |
Where: Abs412 is the absorbance of the sample at 412 nm, for both SH-L and SH-T; C is the sample concentration (mg/mL), 73.53 is a constant, and D is the dilution factor (D = 1).
2.7.6. Scanning electron microscopy (SEM)
The microstructure of the SSPI samples was observed with a SNE-3200 M scanning electron microscope (SEC, South Korea), at an accelerating voltage of 30 kV. Prior to SEM analysis, the SSPI samples were coated for 120 s with a thin layer of gold using an MCM-100 ionization coater (SEC Co., LTD, Suwon, South Korea). Samples were witnessed at magnifications of 500x and 1000x [46].
2.7.7. Secondary structure
Secondary structure measurement of SSPI proteins was performed as reported by Resendiz-Vazquez et al. [47] with modifications. SSPI samples were scanned in the wavenumber range of 4000–515 cm−1 using a Perkin-Elmer FT-IR spectrometer (LR-64912C, PerkinElmer, Inc., Norwalk, CT, USA.). Spectra were averaged over 28 scans in total, and data transformation, deconvolution, and peak separation analysis of the amide I band (1700–1600 cm−1) were performed using Origin Pro 8 software [48] from OriginLab Corporation (Northampton, United States).
2.8. Statistical analysis
The results of the physicochemical, functional, biochemical, and structural properties were obtained from triplicates and presented as means ± standard deviations. Statistical analysis was performed using the one-way analysis of variance (ANOVA) with the Statgraphics Centurion Software version XV software (Statpoint Technologies, Inc. Virginia, USA). To evaluate significant differences between treatments, Tukey's test was applied considering at p < 0.05 value. A simple Pearson correlation analysis was performed to examine the relationships between all properties, considering a statistical significance of p < 0.01 and p < 0.05.
3. Results and discussion
3.1. Protein extraction profile of the DSSF
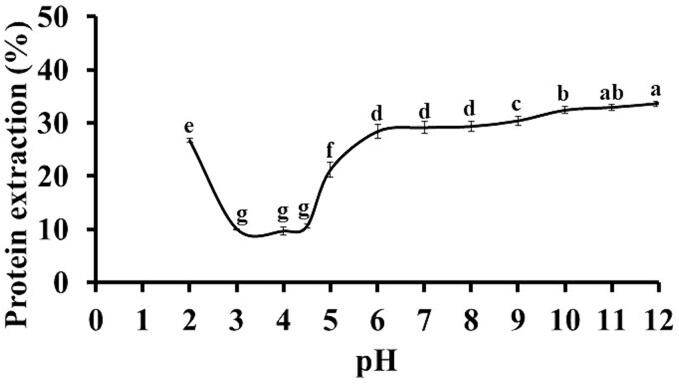
In the first instance, the method for the preparation of protein isolate by alkaline extraction and isoelectric precipitation is based on the identification of the pH values of maximum and minimum protein extraction. The minimum extraction pH value corresponds to the isoelectric point [49]. Fig. 2 shows the effect of pH on the protein extraction from DSSF. Maximum protein extraction of 36.6 % was at pH 12.0, while the minimum protein extraction of 9.7 % was at pH 4.0 (isoelectric point). Therefore, such pH values were chosen for the preparation of the SSPI by alkaline extraction and isoelectric precipitation. In previous studies, the pH values for protein extraction and isoelectric precipitation from fruit passion [16], mango [17], and orange [19] de-oiled seed flours were 12.0 and 4.5, 11.0 and 5.0, and 12.0 and 4.5, respectively, while that the maximal and minimal protein extractions were 49.5 % and 7.8 %, 53.4 % and 6.9 %, and 90.5 % and 30.7 %.
Fig. 2.
Profile of protein extraction from de-oiled soursop seed flour as a function of pH. The results are expressed as the mean of triplicates ± standard deviation. Different letters indicate significant differences (p < 0.05).
3.2. Composition and physicochemical properties
3.2.1. Proximal chemical composition
Table 1 shows the effect of HIUson on the proximal chemical composition of SPPI. In general, the main changes by effect of ultrasound were the decrease on the moisture and lipids, as well as the increase on the protein content. The highest augment on the protein content by effect ultrasound was 5.9 %, increasing from 78.3 % (0 W) to 82.9 % (600 W/30 min). On the other hands, reductions of 70.6 % (400 W/30 min) and 69.2 % (600 W/30 min) also were observed for lipids and moisture contents, respectively, with respect to 0 W. The reduction in the moisture content could be due to the structural modification of the proteins because of the ultrasonic cavitation, which promotes easier and greater water removal [16]. The decrease in moisture content by ultrasound could be the cause of a concentrating effect of the protein content in the SSPI [50]. This same behavior has been observed in a protein isolate obtained from jackfruit seeds [51]. The protein contents of the isolates from this study (78.3–82.9 %) fall in the range of those obtained for guamuchil [18], guava [52], plum [53] and lime seeds [54], whose values were 75.2 %, 94.2 %, 99.1 % and 81.4 %, respectively.
Table 1.
Influence of the high-intensity ultrasound on the proximal chemical composition of the soursop seed protein isolate.
| Components (%) | Ultrasound treatment |
||||||
|---|---|---|---|---|---|---|---|
| 0 W | 200 W/15 min | 200 W/30 min | 400 W/15 min | 400 W/30 min | 600 W/15 min | 600 W/30 min | |
| Crude protein (N x 6.25) | 78.31 ± 0.16b | 79.56 ± 0.42b | 79.29 ± 0.35b | 82.53 ± 0.35a | 82.53 ± 1.27a | 82.92 ± 0.36a | 82.92 ± 0.84a |
| Lipids | 5.52 ± 0.16a | 5.52 ± 0.54a | 2.41 ± 0.29b | 2.68 ± 0.40b | 1.62 ± 0.30c | 2.64 ± 0.35b | 2.40 ± 0.33b |
| Moisture | 7.49 ± 0.18a | 6.58 ± 0.33b | 5.69 ± 0.13c | 5.41 ± 0.08c | 5.41 ± 0.34d | 2.31 ± 0.19e | 2.30 ± 0.38e |
| Ash | 7.48 ± 0.08a | 6.70 ± 0.04a | 6.79 ± 0.13a | 6.86 ± 0.27a | 6.86 ± 0.64a | 6.81 ± 0.73a | 6.89 ± 0.30a |
| Nitrogen free extract | 1.71 ± 0.23d | 2.49 ± 0.15bcd | 4.92 ± 0.15abc | 2.52 ± 0.23 cd | 6.12 ± 2.69a | 5.72 ± 0.80a | 5.55 ± 1.56ab |
The data is presented as the average of three replicates ± standard deviation. Distinct superscripts in the identical row represent significant differences between treatments (p < 0.05). 0 W refers to the treatment where ultrasound was not applied. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
3.2.2. Water activity (Wa)
The Wa is a very important parameter for the preservation of food products, since it defines the balance of the free water content that participates in chemical reactions and the growth of microorganisms [55]. The HIUson significantly (p < 0.05) decreased the Wa iof the SSPI going from 0.502 for 0 W to 0.404 for 400 W/30 min (Table 2). The reduction in Wa iin sonicated protein isolates is due to structural modification of the proteins, which facilitates the removal of a greater amount of free water during freeze-drying [18]. Reductions in Wa by effect of ultrasound also have been reported for protein isolates from fruit passion [16], jackfruit [15] and guamuchil [18] seed fruits. The Wa values of SSPI of this study (0.404–0.502) were below of the limit value (0.660) considered as safe to prevent the microbiological decay in foods [56].
Table 2.
Influence of high-intensity ultrasound on the physicochemical properties of the soursop seed protein isolate.
| Properties | Ultrasound treatment |
||||||
|---|---|---|---|---|---|---|---|
| 0 W | 200 W/15 min | 200 W/30 min | 400 W/15 min | 400 W/30 min | 600 W/15 min | 600 W/30 min | |
| Water activity (Wa) | 0.502 ± 0.001a | 0.490 ± 0.001b | 0.481 ± 0.001c | 0.408 ± 0.001e | 0.404 ± 0.001f | 0.416 ± 0.001d | 0.405 ± 0.001ef |
| Color: | |||||||
| L* (lightness) | 48.69 ± 0.35e | 53.34 ± 0.27a | 49.39 ± 0.01d | 51.62 ± 0.29b | 48.73 ± 0.08e | 47.79 ± 0.46f | 49.85 ± 0.09c |
| a* (+redness, -greenness) | 7.07 ± 0.05a | 5.88 ± 0.01d | 7.05 ± 0.01a | 5.60 ± 0.17d | 6.29 ± 0.07b | 5.65 ± 0.13d | 6.12 ± 0.10c |
| b* (+yellowness, -blueness) | 14.81 ± 0.16a | 14.80 ± 0.10a | 14.42 ± 0.01b | 13.12 ± 0.11c | 12.78 ± 0.17d | 12.50 ± 0.32e | 13.22 ± 0.26c |
| ΔE (color difference) | 51.13 ± 0.31ab | 46.30 ± 0.16e | 50.44 ± 0.05bc | 47.58 ± 0.24d | 50.69 ± 0.02b | 51.31 ± 0.50a | 49.63 ± 0.01c |
| Density: | |||||||
| ρb (g/cm3) | 0.156 ± 0.01a | 0.128 ± 0.01c | 0.122 ± 0.01d | 0.155 ± 0.01a | 0.155 ± 0.01a | 0.133 ± 0.01b | 0.109 ± 0.01e |
| ρc (g/cm3) | 0.200 ± 0.01a | 0.179 ± 0.01b | 0.163 ± 0.01d | 0.205 ± 0.01a | 0.205 ± 0.01a | 0.174 ± 0.01c | 0.157 ± 0.01e |
The data is presented as the average of three replicates ± standard deviation. Distinct superscripts in the identical row represent significant differences between treatments (p < 0.05). 0 W refers to the treatment where ultrasound was not applied. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively. ρb = bulk density, ρc = compacted density.
3.2.3. Color
Color is an important characteristic of protein isolates that defines consumer acceptance of the products when such materials are used as food ingredients [54]. The impact of HIUson on the SSPI color parameters are shown in Table 2. In comparison with 0 W, the L* values of the sonicated SSPIs rose except for 400 W/30 min and 600 W/15 min. The largest increase in L* of 9.6 % was for 200 W/15 min, followed by 6 % for 400 W/15 min. On the contrary, the a* values of the ultrasonicated SSPIs were significantly reduced (p < 0.05) with respect to 0 W, except for 200 W/30 min. The range of decrease in a* values because of ultrasound was from 11 % (400 W/15 min) to 20.1 % (600 W/15 min). In the case of b*, ultrasonication also reduced this parameter in the SSPIs, except for 200 W/15 min, but the decrease ranged from 2.6 % (200 W/30 min) to 15.6 % (600 W/15 min). In general, ultrasound produced SSPIs more lightness as well as less redness and yellowness.
HIUson provoked significant (p < 0.05) differences in ΔE for 200 W/15 min (4.8), 400 W/15 min (3.5), 600 W/30 min (1.5) with respect to 0 W. ΔE is the measure of change in visual perception of two given colors. When ΔE = 2–10, the difference on color between two materials is perceptible immediately, as was the case for 200 W/15 min, 400 W/15 min, 600 W/30 min in comparison to 0 W.
Color changes by sonication have been reported for various protein concentrates or isolates from diverse sources, such as sunflower [57], canola [25] and peanut [58] meals, as well as pumpkin seeds [59]. Color modification by ultrasound could be a consequence of the alteration or destruction of pigments caused by the cavitation phenomenon, depending on factors like power and exposure time to sonication [60].
3.2.4. Bulk (ρb) and compacted (ρc) density
Density is an important characteristic of powdered foods with economic and functional implications. This property influences the behavior of dry mixes and defines the volume needed to package the powders [59]. HIUson significantly (p < 0.05) diminished the ρb for the SPPIs exposed at 200 W (17.9 %-21.8 %) and 600 W (14.7 %-30.1 %), in comparison with 0 W. No significant (p < 0.05) changes by effect of ultrasound between 0 W and 400 W/15 min and 400 W/30 min, respectively (Table 2). The same trend was observed for the ρc. The minimum and maximum reduction in ρc due to ultrasound compared to 0 W were for 200 W/15 min (10.5 %) and 600 W/30 min (21.5 %), respectively. The modification of the density of protein isolates treated with HIUson could be due to the decrease in the particle size of the proteins [61], the residual moisture content, as well as the shape and size of the particles after lyophilization [62]. The trend in the density of the SPPIs of this study by effect of ultrasound, also was observed in protein isolates from tamarind seeds [20], [22].
3.3. Functional properties
3.3.1. Protein solubility (ProS)
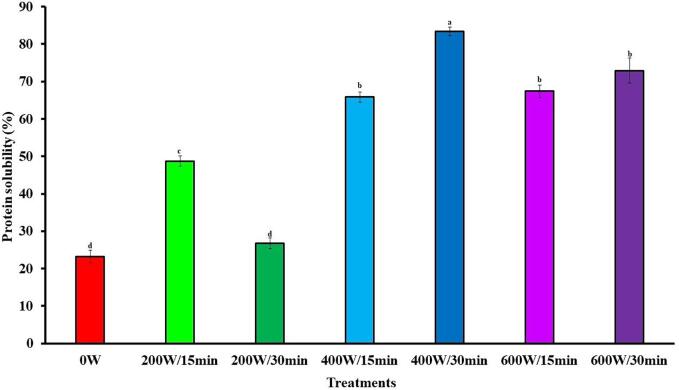
Solubility is a critical property in evaluating the possible uses of a protein as ingredient in food products [63]. Fig. 3 shows the effect of HIUson on ProS of SSPI. Compared to 0 W, sonication significantly (p < 0.05) increased ProS at all SSPIs except 200 W/30 min. The increase of ProS of the SSPIs ranged from 109 % (200 W/15 min) to 260 % (400 W/30 min) in comparison with 0 W. In other studies, ProS rises of 115.5 % and 1000 % by ultrasound were reported for protein isolates from guamuchil [18] and jackfruit [15] seeds, respectively, in consistency with the results of this study. According to Yan et al. [64], the increase in ProS by ultrasound may be related to the decrease in hydrophobic groups on the protein surface [15]. In addition, ultrasound reduces the size of protein particles by breaking intermolecular bonds, weakens protein–protein interactions, and facilitates protein-water interactions, which also rises ProS [65].
Fig. 3.
Effect of ultrasound on the protein solubility of soursop seed protein isolate. The results are expressed as the mean of triplicates ± standard deviation. Different letter on bars indicate significant differences between treatments (p < 0.05). 0 W is control without ultrasound treatment. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
3.3.2. Water and oil absorption capacities
The interactions of water and oil with proteins are very important, since they exert a marked influence on properties such as texture and flavor, which contribute to quality of foods. Such interactions can be estimated through water-absorption (WAc) and oil-absorption (OAc) capacities [66]. Table 3 shows the influence of HIUson on the WAc of the SSPI. Compared to 0 W, treatments at 200 W, 400 W and 600 W for 30 min showed significant increases (p < 0.05) in WAc of 20.1 %, 37.7.% and 38.4 %, respectively, in contrast to the observed decreases of 20.9 %, 18.5 % and 30.9 % for the treatments exposed to ultrasound exposure for 15 min. This may be due to the fact that ultrasound for 15 min caused greater exposure of hydrophobic groups on the surface of the proteins, while at 30 min the exposure of hydrophilic groups was higher [67].
Table 3.
Influence of the high-intensity ultrasound on the functional properties of the soursop seed protein isolate.
| Treatment | Functional property |
||
|---|---|---|---|
| WAc | OAc | LGCo | |
| 0 W | 5.08 ± 0.24bc | 5.93 ± 0.16b | 6.00 ± 0.00a |
| 200 W/15 min | 4.02 ± 0.08c | 7.10 ± 0.27a | 6.00 ± 0.00a |
| 200 W/30 min | 6.1 ± 1.63ab | 6.91 ± 0.23a | 6.00 ± 0.00a |
| 400 W/15 min | 4.14 ± 0.06c | 6.85 ± 0.35a | 6.00 ± 0.00a |
| 400 W/30 min | 6.69 ± 0.08a | 6.55 ± 0.17ab | 6.00 ± 0.00a |
| 600 W/15 min | 3.51 ± 0.21c | 7.19 ± 0.22a | 6.00 ± 0.00a |
| 600 W/30 min | 7.03 ± 0.42a | 7.02 ± 0.43a | 6.00 ± 0.00a |
The data is presented as the average of three replicates ± standard deviation. Distinct superscripts in the identical row represent significant differences between treatments (p < 0.05). 0 W refers to the treatment where ultrasound was not applied. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively. WAc: water absorption capacity; OAc: oil absorption capacity; LGCo: least gelation concentration.
The results demonstrated that the affinity of the oil measured as OAc for the SSPI was higher for all the ultrasonicated treatments than the SSPI unsonicated. Interestingly, contrary to what happened with WAc, the highest OAc values were for the treatments of 200 W, 400 W and 600 ultrasonicated for 15 min instead of 30 min (Table 3). The treatment with the greatest increase in OAc (21.2 %) was 600 W for 15, compared to the control. The cause of the increase in OAc of the protein isolates could be attributed to the conformational changes in the protein structure due to ultrasonic cavitation. These changes exposed the non-polar hydrophobic side chains of the amino acids of the protein molecule, which increase their interaction with the oil molecules [68].
In a study with ultrasound-treated orange seed proteins, a decrease in WAc was observed [19], consistent with the results obtained in this work for the SSPI ultrasonicated at 200 W, 400 W and 600 W at 15 min. On the other hand, Biswas and Sit [20] reported the improvement of the OAc of proteins obtained from tamarind seeds exposed to ultrasound, as observed in the SSPI proteins of this study.
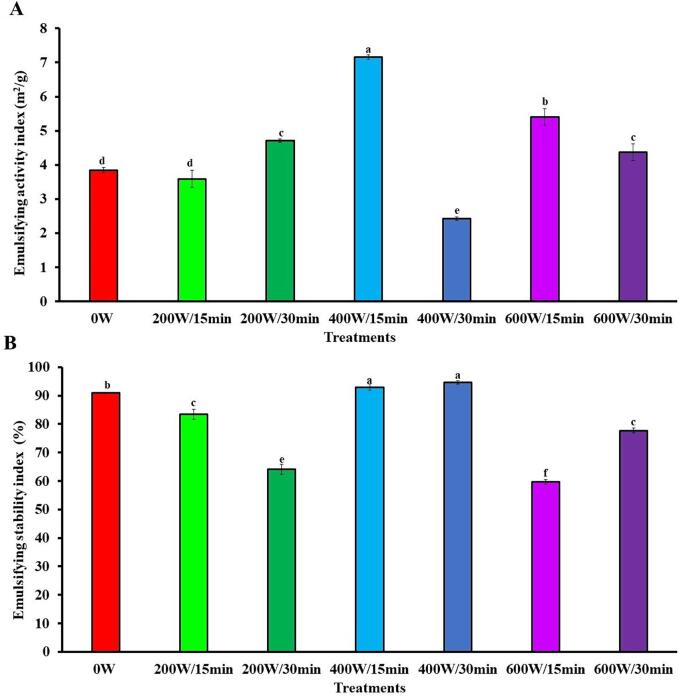
3.3.3. Emulsifying properties
The quality of proteins for emulsion formation is determined by the EAI and ESI, which measure the ability of proteins to be absorbed at the oil–water interface and stay there for a while, respectively [69]. The HIUson significantly (p < 0.05) improved the EAI of the treatments 200 W/30 min (22.3 %), 400 W/15 min (86 %), 600 W/15 min (40.2 %) and 600 W/30 min (13.8 %), in comparison with 0 W, but for 400 W/30 min a worsen of 34.4 % was observed (Fig. 4A). In the case of ESI, sonication provoked reductions in the range of 8.2 % (200 W/15 min) to 34.3 % (600 W/15 min), and a maximum increase of 5 % for 400 W/30 min (Fig. 4B).
Fig. 4.
Effect of ultrasound on the emulsifying activity index (A) and emulsifying stability index (B) of soursop seed protein isolate. The results are expressed as the mean of triplicates ± standard deviation. Different letter on bars indicate significant differences between treatments (p < 0.05). 0 W is control without ultrasound treatment. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
Ultrasound enhances emulsifying properties due to increased exposure of nonpolar protein residue side chains, which expands interaction with the hydrocarbon chains of acyl glyceride molecules [70], [67]. In addition, the decrease in particle size by ultrasound enlarges protein molecular flexibility and stretchability around oil droplets, resulting in better adsorption at the oil–water interface and the formation of denser coarse films [71]. As in this study with SSPI, ultrasound also improved emulsifying properties in protein isolates from hemp [72], grass pea [73], and potato [74].
3.3.4. Foaming properties
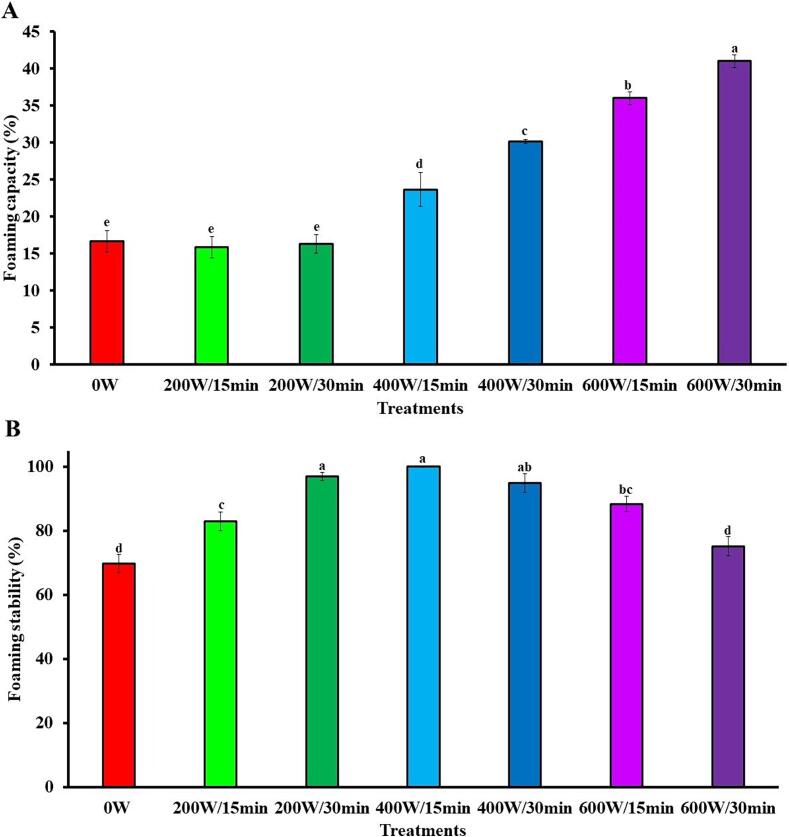
Foam is a colloidal system of air bubbles dispersed in a continuous aqueous phase. Foam formation is determined by the ability of surfactant components to absorb at the air–liquid interface and reduce surface tension [75]. Proteins from different sources have been used in food industry due to the ability as foaming agents [19]. HIUson significantly (p < 0.05) improved the foaming properties of SSPI (Fig. 5). The FC of 16.7 % for 0 W was augmented to 23.7 %, 30.2 %, 36 %, and 41 % for 400 W/15 min, 400 W/30 min, 600 W/15 min, and 600 W/30 min, respectively (Fig. 5A). In the case of FS, the improvement of this property by ultrasound was observed in all treatments, except 600 W/30 min. The FS of the SPPSs was in the range of 83 % (200 W/15 min) to 100 % (400 W/15 min), in comparison to 69.8 % for 0 W (Fig. 5B).
Fig. 5.
Effect ultrasound on the foaming capacity (A) and foaming stability (B) of soursop seed protein isolate. The results are expressed as the mean of triplicates ± standard deviation. Different letter on bars indicate significant differences between treatments (p < 0.05). 0 W is control without ultrasound treatment. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
The improvement of FC and FS by ultrasound is due a greater exposure of the hydrophobic groups caused by changes in the protein conformation, which accelerate absorption at the gas/water interface [23]. Studies with protein isolates from pea [76], chickpea [77], and sunnhemp [78] have demonstrated the beneficial effect of ultrasound on FC and FS.
3.3.5. Least gelation concentration (LGCo)
A protein gel is a three-dimensional network formed by the aggregation of individual protein molecules. Protein isolates are extensively used as gelling agents to enhance the texture and water-holding capacity of food as yogurts, cheese and meet products [68]. HIUson did not modify the LGCo of SPPI with respect to 0 W (Table 3). However, the LGCo of 6 % for all treatments places SSPI as a material with a medium gelling capacity, according to the classification that establishes that values <4 %, 4–8 % and >8 % correspond to the categories high, medium and low, respectively [47].
A lowering of LGCo from 4 % to 2 % and from 14 % to 2 % (at pH 7) has been obtained by ultrasound in protein isolates from passion fruit [16] and orange [19] seeds, contrary to the results of this study. The reduction in LGCo of protein isolates by sonication can be attributed to factors such as decreased particle size, partial denaturation, and increased exposure of protein hydrophobic and sulfhydryl groups [62]. The LGCo results of 6 % for the SPPI of this study were better than those obtained of 9 % and 12 % for protein isolates from jackfruit [51], and guamuchil [18] seeds.
3.4. Biochemical properties
3.4.1. Protein fractional composition
The fractional composition generates information about the solubility of the protein in different solvents. According to their solubility, proteins can be classified into albumins (soluble in water), globulins (soluble in saline solutions), prolamins (soluble in ethanol) and glutelins (soluble in alkaline solutions), and the presence and quantity of each protein fraction in protein isolates depend on the raw material and extraction method [79].
The effect of HIUson on the fractional composition of SSPI is shown in Table 4. The solubility pattern of the SSPI protein fractions was modified by the effect of ultrasound. The content of albumins, globulins and prolamins increased, while that of glutelins decreased. The greatest variation in the contents of the protein fractions with respect to 0 W was for 600 W/30 min. The contents of albumins, globulins and prolamins increased to 423.4 %, 230.8 % and 96.8 %, while that of glutelins decreased to 47.5 %. The contents of albumins and globulins also were risen by ultrasound in a protein isolate from orange seeds [19], conforming to the results observed in the SPPI of this study. The modification of solubility pattern of protein fractions could be due to the structural changes produced by ultrasound, which alter balance of hydrophobic-hydrophilic groups on the surface of the protein, as has been demonstrated in studies with proteins obtained from orange [19], passion fruits [16], guamuchil [18], and jackfruit [15] seeds.
Table 4.
Influence of the high-intensity ultrasound on the protein fractional composition of the soursop seed protein isolate.
| Treatment | Protein fraction (%) |
|||
|---|---|---|---|---|
| Albumins | Globulins | Prolamins | Glutelins | |
| 0 W | 7.55 ± 0.62f | 2.37 ± 0.15e | 4.63 ± 0.41d | 85.41 ± 1.17a |
| 200 W/15 min | 19.71 ± 0.75e | 5.39 ± 0.09c | 10.93 ± 0.49a | 63.90 ± 0.22c |
| 200 W/30 min | 14.81 ± 1.23d | 3.28 ± 0.87d | 6.63 ± 0.58c | 75.26 ± 1.20b |
| 400 W/15 min | 32.23 ± 0.09c | 5.62 ± 0.14b | 8.70 ± 0.21b | 53.44 ± 0.45d |
| 400 W/30 min | 39.53 ± 0.55a | 7.84 ± 0.64a | 6.63 ± 1.11b | 44.81 ± 0.34e |
| 600 W/15 min | 35.21 ± 0.53b | 5.56 ± 0.55c | 8.50 ± 0.77b | 50.40 ± 1.16d |
| 600 W/30 min | 39.52 ± 0.64a | 7.84 ± 1.11a | 9.11 ± 0.34b | 44.80 ± 0.34e |
The data is presented as the average of three replicates ± standard deviation. Distinct superscripts in the identical row represent significant differences between treatments (p < 0.05). 0 W refers to the treatment where ultrasound was not applied. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
3.4.2. Molecular weight distribution
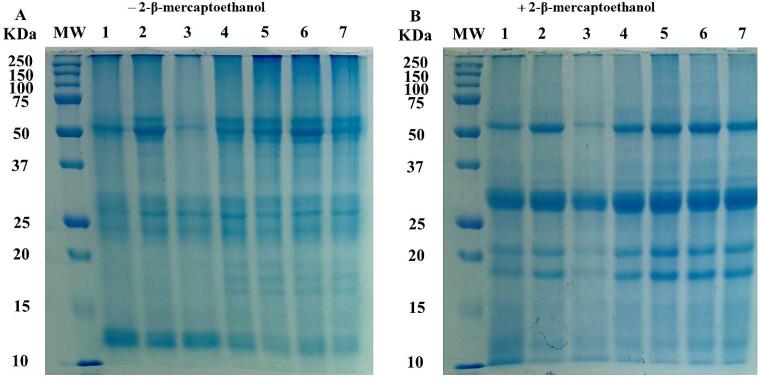
Generally, the proteins of the isolates are composed of several fractions of different molecular weights, which can undergo modifications such as hydrolysis when exposed to physical, chemical, or enzymatic treatments [15]. In such cases, the SDS-PAGE is a tool that allows to determine changes in the molecular weight pattern of proteins [80]. The impact of HIUson on the electrophoretic patterns of SPPI under non-reducing and reducing conditions is shown in Fig. 6.
Fig. 6.
Effect ultrasound on the electrophoretic profile of soursop seed protein isolate. Non-reducing (A) and reducing (B) SDS-PAGE electrophoretic profiles of SSPI: Lane MW, molecular weight marker; Lane 1, control (0 W); Lane 2, 200 W/15 min; Lane 3, 200 W/30 min; Lane 4, 400 W/15 min; Lane 5, 400 W/30 min; Lane 6, 600 W/15 min and Lane 7, 600 W/30 min. For each correspond treatment to the lines, the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
The same six fractions with molecular weights ∼ 65, 57, 27, 23, 13, and 12 kDa were observed for 0 W as well as for the sonicated SSPIs under non-reducing conditions, meaning that ultrasound did not affect the molecular weight profile (Fig. 6A). These results agree with those obtained for protein isolates from pumpkin [23], tamarind [20], and orange [19] seeds, in which ultrasound did not alter their electrophoretic profile either.
On the other hand, both 0 W and as well as the SSPIs treated with ultrasound also showed the same four fractions with molecular weights of ∼60, 30, 22, and 17 kDa under reducing conditions, which indicate that the sonication did not fracture any disulfide bonds (Fig. 6B). These results contrast with those observed for proteins isolates from jackfruit [15], and melon [62] seeds, where the protein fragmentation was detected by ultrasound due to the cavitation phenomena [81].
In a previous study with protein extracts from soursop seeds, protein fractions in the range of 20–35 kDa were identified [10]. The range of molecular weights of the protein fractions of the SPPIs of this study was in those corresponding to 10–35 kDa, 15–35 kDa, 25–48 kDa, 25–50 kDa, and 14–70 kDa for protein isolates from Persian lime [54], pomegranate [49], apple [82], plum [83], and kiwi [84] seeds.
3.4.3. In vitro protein digestibility (IDig)
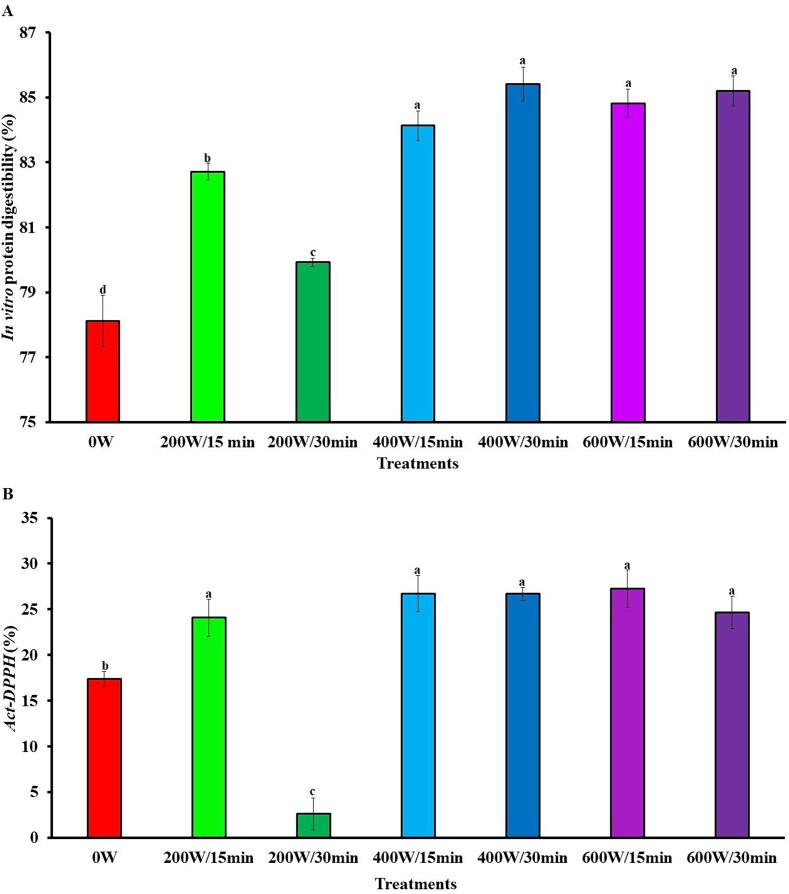
Digestibility is one of the main protein quality factors and a high value of this property is desirable for protein isolates [85]. HIUson significantly (p < 0.05) increased the IDig of SSPI (Fig. 7A). The IDig for 0 W of 78.1 % rose to the maximum value of 85.4 % for 400 W/15 min. The minimum beneficial in IDig was observed for 200 W/30 min, which had an additional 2.3 % to the 0 W value. The improvement of the IDig by ultrasound is due to modification of protein structure which facilitates the access of the digestive enzymes to the proteins [86]. The IDig of the SPPIs of this study were in the range of those obtained from 68.9 %, 76 % and 83.2 % for protein isolates from avocado [26], guamuchil [18], and watermelon [87] seeds, respectively.
Fig. 7.
Effect ultrasound on the in vitro protein digestibility (A) and DPPH radical scavenging activity (B) of soursop seed protein isolate. The results are expressed as the mean of triplicates ± standard deviation. Different letter on bars indicate significant differences between treatments (p < 0.05). 0 W is control without ultrasound treatment. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively. Act-DPPH: DPPH radical scavenging activity; DPPH: 2,2-diphenyl-1-picrylhydrazyl.
3.4.4. DPPH radical scavenging activity (Act-DPPH)
Proteins can be an important source of bioactive peptides with antioxidant capacity. These peptides can serve as potential radical scavengers by donating protons from their aromatic amino acid residues to free radicals [88]. Consumption of foods with a significant input of antioxidants could improve consumers' health [43].
Fig. 7B shows the effect of HIUson on Act-DPPH of the SSPI. In general, HIUson increased significantly (p < 0.05) the Act-DPPH with the augment of the power, in contrast with 0 W, except for 200 W/30 min. The increase of Act-DPPH ranged from 27.8 % (200 W/15 min) to 36.2 % (600 W/min), although no significant (p < 0.05) differences were detected between treatments. The increase in Act-DPPH of proteins because of ultrasound could be a consequence of the modification of their structure, causing the exposure of aromatic and sulfur amino acid residues, which can donate protons to reactive free radicals. and therefore, have antioxidant activity [89]. In studies with proteins from Dolichos lablab L. [43] and hemp seeds [90], ultrasound increased the Act-DPPH, in accordance with the results of this research.
3.5. Structural characteristics
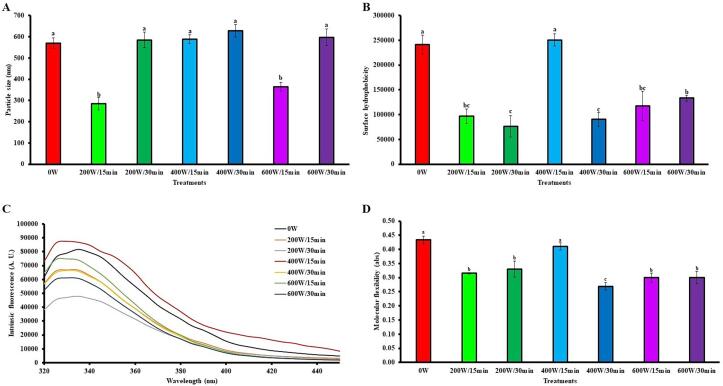
3.5.1. Particle size (Ps)
The Ps of proteins in the form of aggregates is an important characteristic that influences the functional properties of foaming and emulsification [82]. Fig. 8A shows the influence of the HIUson on the Ps of the SSPI. Overall, ultrasound did not significantly (p > 0.05) modify the Ps of the SSPI, except for the 200 W/15 min and 600 W/15 min treatments, in which it caused a decrease of 50 % and 36 %, respectively. A decrease in the Ps value can be attributed to microcurrents and turbulence due to the cavitational force generated during ultrasound treatment [80].
Fig. 8.
Effect of ultrasound on the structural properties of soursop seed protein isolate. The results are expressed as the mean of triplicates ± standard deviation. Different letter on bars indicate significant differences between treatments (p < 0.05). 0 W is control without ultrasound treatment. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
In a study by Sun et al. [91] with a protein isolate from peanut paste, HIUson decreased Ps by 34 %, while Gani et al. [82] reported that the reduction in the value of this property was 59 % for a protein isolate from apple seeds. The values in the decrease in Ps of the SSPIs in this study because of ultrasound were in the range of those previously reported for protein isolates from peanut paste and apple seeds. The dissociation of the aggregate particles of the protein isolates due to the effect of ultrasound could be due to the breaking of the disulfide bonds, thus increasing the exposure of the sulfhydryl groups on the surface and causing the fragmentation of the proteins [92].
3.5.2. Surface hydrophobicity (Sh)
Sh is a property that reflects the distribution of hydrophobic residues on the molecular surface [93], which is used as a key index of modification of the tertiary structure and hydrophobic interactions of proteins, with an impact on their functional properties [23]. Fig. 8B exhibits the effect of HIUson on the Sh of the SSPI. In general, Sh decreased significantly in the SSPI (p < 0.05) with the application of ultrasound, except for 400 W/30 min, where there was no significant difference compared to the control. The maximum decrease of Sh was 68 % 200 W/30 min, compared to the 0 W treatment. The application of HIUson induces a partial denaturation of the polypeptide chain, which leads to the formation of aggregates where hydrophobic groups become embedded within the protein molecules, causing the reduction of Sh [94].
In contrast to the results reported in this research, HIUson increased the Sh of protein isolates from plum [83] and jackfruit [15] seeds approximately 400 % and 50 %, respectively, compared to the control treatment. The increase in Sh can be explained by the effect of the ultrasonic cavitation phenomenon that induces a certain degree of molecular unfolding of the proteins, which causes an augment in the number of hydrophobic groups and regions on the surface of the proteins, which were originally found within the molecules of said polymers [95].
3.5.3. Intrinsic fluorescence spectrum (IFS)
The IFS is a tool that allows measuring conformational changes in the tertiary structure of proteins and is related to the intrinsic fluorescence emitted by aromatic amino acids such as tryptophan, tyrosine, and phenylalanine [96]. Fig. 8C shows the effect of HIUson on IFS of the SSPI. The wavelength corresponding to maximum fluorescence intensity of the control and the sonicated treatments was 335 nm and 325 nm, respectively, which implies that this small red shift by ultrasound can be associated to the structural changes in protein [97]. Except for 400 W/15 min, the intensity of fluorescence of all ultrasonicated treatments was lower than that of 0 W, and the minimal value was for 200 W/30 min. These changes occur due to the increase in exposure of chromogenic amino acid residues of proteins in a polar environment, because of the breakdown of hydrophobic bonds, which produces an extinction of fluorescence and a decrease in fluorescence intensity [98].
On the other hand, the maximum IFS length of the control was 335 nm, the same as in the treatments subjected to ultrasound for 30 min and contrary to those treated for 15 min which presented a maximum IFS of 325 nm. Generally, if the maximum IFS is recorded at a wavelength of 330 nm or less, it is assumed that tryptophan is located inside the protein (hydrophobic environment). On the other hand, when the maximum IFS is detected at a wavelength higher than 330 nm, it indicates that tryptophan is in a hydrophilic environment [99].
Results like those of this study regarding the effect of ultrasound on fluorescence intensity were reported for pea proteins [100]. However, other studies with guamuchil seed proteins [18] and whey [101] reported an increase in fluorescence intensity by ultrasound, which was also associated with the rupture of internal hydrophobic groups, causing the unfolding of protein molecules and the exposure of more chromophores on the surface of said polymers.
3.5.4. Molecular flexibility (MolFlex)
MolFlex is a very important property of proteins that is related to the function of these polymers, which depends on extrinsic factors including the interaction with other macromolecules, that can affect their spatial arrangement [44]. As observed in Fig. 8D, HIUson significantly decreased (p < 0.05) the MolFlex of the SSPI, except for 400 W/15 min. The most notable reduction in MolFlex was observed in 400 W/30 min (37 %) compared to the control. According to Wang et al. [102], ultrasound decreases the MolFlex of proteins due to the formation of disulfide bonds that lead to protein aggregation. In contrast to the results of this study, ultrasound increased the MolFlex of soybean proteins [44], [103] and guamuchil seed proteins [18], due to the alteration of the structure of their rigid region.
3.5.5. Free (SH-F) and total (SH-T) sulfhydryls
SH-F and SH-T groups are among the most active reactive groups of proteins and their exposure can affect the functional properties of proteins [104]. The impact of HIUson on the contents of SH-F and SH-T is shown in Table 5. The ultrasound significantly increased (p < 0.05) the contents of SH-F and SH-T in the SSPI, except at 200 W/30 min for SH-T. The increase in SH-T and SH-F contents depended on the ultrasound conditions and ranged between 13.0 % (600 W/30 min) and 83.1 % (200 W/15 min) and 133.3 % (600 W/15 min) and 316.7 % (200 W/15 min), respectively, in comparison with 0 W. The previous results can be explained by the effect of ultrasonic cavitation, which increases the exposure of the SH-F on the surface of the proteins, as well as that of the SH-F in the internal zone and those generated by the possible rupture of disulfide linkages, to achieve a higher SH-T value [105]. In other studies, HIUson also increased the SH-F content of pumpkin seed [23] and soybean [106] proteins, as observed in this research.
Table 5.
Influence of the high-intensity ultrasound on the structural properties of the soursop seed protein isolate.
| Structural property | Ultrasound treatment |
||||||
|---|---|---|---|---|---|---|---|
| 0 W | 200 W/15 min | 200 W/30 min | 400 W/15 min | 400 W/30 min | 600 W/15 min | 600 W/30 min | |
| Sulfhydryl group (µmol/g) | |||||||
| Total (SH-T) | 1.54 ± 0.16 cd | 2.82 ± 0.18a | 1.14 ± 0.03d | 1.99 ± 0.12bc | 1.84 ± 0.25c | 2.38 ± 0.27ab | 1.74 ± 0.15c |
| Free (SH-L) | 0.24 ± 0.06d | 1.00 ± 0.21a | 0.76 ± 0.04b | 0.83 ± 0.01ab | 0.81 ± 0.21ab | 0.56 ± 0.08c | 0.69 ± 0.25bc |
| Secondary structure (%) | |||||||
| β-sheet | 40.49 ± 0.91e | 45.09 ± 0.67d | 49.45 ± 0.64c | 59.06 ± 0.31a | 52.79 ± 0.36b | 30.82 ± 0.76f | 53.11 ± 0.64b |
| Random coil | 27.96 ± 3.18a | 21.21 ± 1.95c | 16.18 ± 0.01d | 26.19 ± 0.88b | 11.01 ± 0.35f | 22.36 ± 0.99c | 12.29 ± 0.23e |
| α-helix | 3.23 ± 1.60c | 2.17 ± 0.03d | 10.85 ± 1.68a | 0.31 ± 0.21d | 3.69 ± 0.16c | 6.39 ± 0.01b | 5.92 ± 0.05b |
| β-turn | 28.30 ± 0.67c | 30.98 ± 1.61b | 23.44 ± 1.25d | 14.50 ± 0.53e | 32.44 ± 0.69b | 40.41 ± 0.32a | 28.33 ± 0.30c |
The data is presented as the average of three replicates ± standard deviation. Distinct superscripts in the identical row represent significant differences between treatments (p < 0.05). 0 W refers to the treatment where ultrasound was not applied. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
3.5.6. Scanning electron microscopy (SEM)
The size, shape and the presence of pores or fissures on the surface of the particles are part of the microstructure of the protein isolates, which can be associated with some of their physicochemical, and functional properties [107]. These characteristics of the protein isolates can be observed by SEM [89]. The effect of ultrasound on the microstructure of SSPI is shown in Fig. 9. As can be seen, HIUson modified the microstructure of the SSPI proteins, especially those treated at 400 W and 600 W, producing larger and softer particles and with lamellar shape, compared to the control treatment (Fig. 9A). Furthermore, HIUson produced small cracks on the surface of the protein particles (Fig. 9B). The increase in the size of the SSPI particles could be due to the unfolding of the proteins as consequence of the ultrasonic waves, which causes greater exposure of the hydrophobic groups and SH-F on the surface of the molecules, promoting the interaction between them to form larger aggregates during freeze-drying [80], [71], [15]. The fractures on the surface of the protein particles can be due to the effect of the ultrasonic cavitation [108]. Previous studies on guamuchil [18], orange [19] and fruit passion [16] seed proteins showed the same effects provoked by ultrasound in the protein of SSPI of this study. Nonetheless, a study with proteins from apple seeds detected a reduction in the particle size by effect of ultrasound, as revealed by microstructural analysis using SEM [82].
Fig. 9.
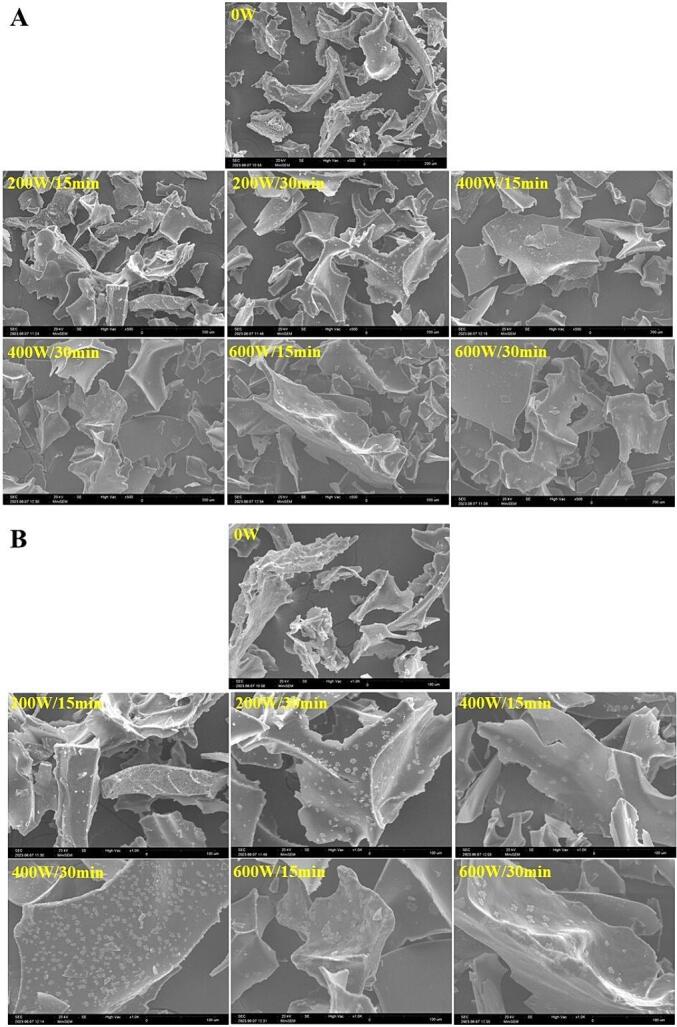
Effect of ultrasound on the microstructure of the soursop seed protein isolate, observed at 500x amplification (A) and 1000x amplification (B) by scanning electron microscopy. 0 W is control without ultrasound treatment. For each treatment the numerator and denominator represent the power (W) and time (min) of exposure to ultrasound, respectively.
3.5.7. Secondary structure
FT-IR is commonly used to evaluate the secondary structure of proteins, including β-turn, α-helix, random coil, and β-sheet. The amide I band (1700–1600 cm−1) of proteins primarily reflects the stretching vibration of carbonyl bonds (C = O) within the amide group (approximately 80 %), which can be utilized to analyze the secondary structure of proteins [109]. As observed in Table 5, HIUson modified the secondary structure of the SSPI. The significant (p < 0.05) modifications were the increases of 46 % in β-sheet (400 W/15 min), 236 % in α-helix (200 W/30 min), 43 % in β-turn (400 W/30 min), compared to the control treatment. Additionally, a 60 % decrease in coil random structure was observed (400 W/30 min).
According to Meng et al. [89], ultrasound alters the secondary structure of proteins by disrupting hydrogen bonds crucial for stabilizing these structures. In comparison to the results obtained in this study, ultrasound induced a 25 % decrease in β-turn in a plum seed isolate [83], while in a jackfruit seed protein isolate [47] there was an elevation of 11.4 % in the random coil and a decrease of 14.8 % in the β-sheet structures, in contrast to the control. Therefore, protein structural modification depends on the sonication conditions, protein source, and extraction method [68].
3.6. Pearson correlations analysis
Ultrasound is a physical treatment that has been used successfully to improve the functional properties of vegetable proteins [16], [17]. According to diverse studies, the improvement of the functional properties of proteins by ultrasound is a consequence of the changes that occur in their physicochemical, structural, and biochemical properties [18], [19], [20]. The Pearson correlation coefficients between the physicochemical, functional, biochemical, and structural properties of the SSPI and the SSPI exposed to HIUson are shown in Table 6. Particularly, the higher positive correlations were as follows: proteins/ProS (r = 0.948; p < 0.01), proteins/albumins (r = 0.976; p < 0.01), proteins/IDig (r = 0.936; p < 0.01), albumins/ProS (r = 0.977; p < 0.01), ProS/IDig (r = 0.971; p < 0.01), Sh/IFS (r = 0.838; p < 0.05), Sh/MolFlex (r = 0.799; p < 0.05), and MolFlex/random coil (r = 0.779; p < 0.05). Instead, the higher negative correlations were as follow: protein/glutelin (r = −0.953; p < 0.01), ProS/glutelins (r = −0.977; p < 0.01), albumins/glutelins (r = −0.990; p < 0.01), glutelins/IDig (r = −0.993; p < 0.01), Ps/SH-T (r = −0.833; p < 0.05), and IFS/α-helix (r = −0.814; p < 0.05). A study with soy proteins treated with ultrasound [106] reported positive correlations of MolFlex/EAI (r = 0.938; p < 0.01), MolFlex/ESI (r = 0.958; p < 0.01), Sh/EAI (r = 0.772; p < 0.05), and Sh/ESI (r = 0.883; p < 0.05). In another study with ultrasonicated soy proteins [110] were found the correlations of Sh/EAI (r = 0.572; p < 0.05), Sh/ESI (r = 0.629; p < 0.05), Ps/EAI (r = −0.769; p < 0.01) and Ps/ESI (r = −0.849; p < 0.01). The correlations between the properties of proteins treated with ultrasound could depend on their intrinsic characteristics, isolation method and ultrasound conditions.
Table 6.
Pearson correlations on the physicochemical, functionals, biochemicals, and structural properties a soursop seeds protein isolate (SSPI).
| CP | PSol | Albu | Glut | Ps | Sh | IFS | MolFlex | SH-T | α-H | RC | IDig | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP | 1 | |||||||||||
| PSol | 0.948** | 1 | ||||||||||
| Albu | 0.976** | 0.977** | 1 | |||||||||
| Glut | −0.953** | −0.977** | −0.990** | 1 | ||||||||
| Tp | 0.161 | 0.091 | 0.129 | −0.033 | 1 | |||||||
| Ps | −0.287 | −0.135 | −0.214 | 0.261 | 0.281 | 1 | ||||||
| IFS | −0.154 | 0.182 | 0.043 | −0.024 | −0.060 | 0.838* | 1 | |||||
| MolFlex | −0.653 | 0.647 | −0.685 | 0.724 | 0.247 | 0.799* | 0.469 | 1 | ||||
| SH-T | −0.058 | 0.363 | 0.264 | −0.363 | −0.833* | −0.105 | 0.343 | −0.374 | 1 | |||
| α-H | 0.205 | 0.308 | −0.133 | 0.174 | 0.109 | −0.622 | −0.814* | −0.234 | −0.534 | 1 | ||
| RC | −0.581 | −0.448 | −0.539 | 0.541 | −0.308 | 0.745 | 0.742 | 0.779* | 0.213 | −0.459 | 1 | |
| IDig | 0.936** | 0.971** | 0.972 | −0.993** | −0.067 | −0.270 | 0.056 | −0.729 | 0.459 | −0.219 | −0.487 | 1 |
Significant correlation (p < 0.05); **Highly significant correlation (p < 0.01); CP = crude protein; PSol = protein solubility; Albu = albumins; Glut = glutelins; Ps = particle size; Sh = surface hydrophobicity; ISF = intrinsic fluorescence spectrum; MolFlex = molecular flexibility; SHT = total sulfhydryls; α-H = α-helix; RC = random coil; and IDig = in vitro protein digestibility.
4. Conclusions
In general, the ultrasound modified the physicochemical, functional, biochemical, and structural properties of SSPI obtained by alkaline extraction and isoelectric precipitation. The main structural changes caused by ultrasonic cavitation were reflected in the modification of the indicators of the secondary (α-helix, β-sheet, β-turn and random coil) and tertiary (Ps, Sh, IFS, MolFlex, SH-F and SH-T) structures, as well as the pattern of protein fractions based on their solubility (albumins, globulins and prolamins, and glutelins). Such modifications increased significantly (p < 0.05) the functional properties and biochemical properties of ProS, WAc, OAc, EAI, ESI, FC, FS, IDig, and Act-DPPH. Therefore, HIUson can contribute to the utilization of SPPI as a new food ingredient. Further research on the nutritive and rheological properties of SPPI could contribute to expanding its uses in the food industry, specifically in amino acids composition and gel properties.
CRediT authorship contribution statement
Kevin Ulises López-Mártir: Conceptualization, Investigation, Methodology, Software, Writing – original draft. José Armando Ulloa: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Writing – original draft, Writing – review & editing. Judith Esmeralda Urías-Silvas: Investigation, Resources, Supervision. Petra Rosas-Ulloa: Investigation, Resources, Supervision. José Carmen Ramírez-Ramírez: Conceptualization, Supervision, Visualization. Juan Alberto Resendiz-Vazquez: Data curation, Software, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the National Council for Humanities, Sciences and Technology for the scholarship awarded (1182335) to QFB. Kevin Ulises López-Mártir and the Patronage of the Autonomous University of Nayarit for the funding of this study. Also, thanks to IBQ. Nancy Dinorah Ruelas Hernández for her invaluable help in preparing and capturing the SSPI scanning electron microscopy images included in this study.
Data availability
Data will be made available on request.
References
- 1.Morales R., Martínez K.D., Ruiz-Henestrosa V.M.P., Pilosof A.M.R. Modification of foaming properties of soy protein isolate by high ultrasound intensity: particle size effect. Ultrason. Sonochem. 2015;26:48–55. doi: 10.1016/j.ultsonch.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Lau K.Q., Sabran M.R., Shafie S.R. Utilization of vegetable and fruit by-products as functional ingredient and food. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.661693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FAOSTAT. Food and agriculture organization statistical database, (2023). https://www.fao.org/faostat/en/#data.
- 4.Selva-Ganesh K., Sridhar A., Vishali S. Utilization of fruit and vegetable waste to produce value-added products: conventional utilization and emerging opportunities-a review. Chemosphere. 2022;287(Part 3):e132221. doi: 10.1016/j.chemosphere.2021.132221. [DOI] [PubMed] [Google Scholar]
- 5.Sanusi S.B., Abu-Bakar M.F. Soursop (Annona muricata). Exotic Fruits. Academic Press; 2020. pp. 391–395. [DOI] [Google Scholar]
- 6.Santos I.L., De la Cruz-Rodrigues A.M., Amante E.R., Meller-da-Silva L.H. Soursop (Annona muricata) properties and perspectives for integral valorization. Foods. 2023;12:1448. doi: 10.3390/foods12071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordeiro-Dias D.D.R., Pimenta-Barros Z.M., Oliveira-de Carvalho C.B., Aráujo-Honorato F., Barbosa-Guerra N., Moreira-Azoubel P. Effect of sonication on soursop juice quality. LWT-Food Sci. Technol. 2015;62:883–889. doi: 10.1016/j.lwt.2014.09.043. [DOI] [Google Scholar]
- 8.Aguilar-Hernández G., Zepeda-Vallejo L.G., García-Magaña M.de L., Vivar-Vera M.de los Á., Pérez-Larios A., Girón-Pérez M.I., Coria-Téllez A.V., Rodríguez-Aguayo C., Montalvo-González E. Extraction of alkaloids using ultrasound from pulp and by-products of soursop fruit (Annona muricata L.) Appl. Sci. 2020;10:4869. doi: 10.3390/app10144869. [DOI] [Google Scholar]
- 9.Florez-Montes C., Rojas-Gonzalez A.F., Rodriguez-Barona S. Evaluation of extracts obtained from fruit wastes using different methods. Ingeniería. 2021;26:77–92. doi: 10.14483/23448393.16525. [DOI] [Google Scholar]
- 10.Villacís-Chiriboga J., Prandi B., Ruales J., Van Camp J., Sforza S., Elst K. Valorization of soursop (Annona muricata) seeds as alternative oil and protein source using novel de-oiling and protein extraction techniques. LWT- Food Sci. Technol. 2023;182 doi: 10.1016/j.lwt.2023.114777. [DOI] [Google Scholar]
- 11.Fonseca M., Ferreira L.M.B., Soares R.A.M., Kobelnik M., Fontanari G.G., Crespi M.S., Ribeiro C.A. Extraction of soursop oil (Annona muricata L.) by ultrasonic technique. J. Therm. Anal. Calorim. 2018;134:1893–1901. doi: 10.1007/s10973-018-7753-2. [DOI] [Google Scholar]
- 12.Aguilar-Hernández G., García-Magaña M., Vivar-Vera M., Sáyago-Ayerdi S., Sánchez-Burgos J., Morales-Castro J., Anaya-Esparza L.M., Montalvo-González E. Optimization of ultrasound-assisted extraction of phenolic compounds from Annona muricata by-products and pulp. Molecules. 2019;24:904. doi: 10.3390/molecules24050904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavares-Menezes E.G., Oliveira E.R., Carvalho G.R., Guimaraes I.C., Queiroz F. Assessment of chemical, nutritional and bioactive properties of Annona crassiflora and Annona muricata wastes. Food Sci. Technol. 2019;39:662–672. doi: 10.1590/fst.22918. [DOI] [Google Scholar]
- 14.Chaparro S., Tavera M., Martínez J., Gil J. Functional properties of flour and protein isolates from Annona muricata seeds. Revista Actualidad y Divulgación Científica. 2014;17:151–159. [Google Scholar]
- 15.Resendiz-Vazquez J.A., Ulloa J.A., Urías-Silvas J.E., Bautista-Rosales P.U., Ramírez- Ramírez J.C., Rosas-Ulloa P., González-Torres L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017;37:436–444. doi: 10.1016/j.ultsonch.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Espinosa-Murillo N.C., Ulloa J.A., Urías-Silvas J.E., Rosas-Ulloa P., Ramírez-Ramírez J.C., Gutiérrez-Leyva R., Ulloa-Rangel B.E. Impact of high-intensity ultrasound on the physicochemical and functional properties of a protein isolate from passion fruit (Passiflora edulis) seeds. Int. J. Food Eng. 2021;17:609–618. doi: 10.1515/ijfe-2021-0050. [DOI] [Google Scholar]
- 17.Pérez-Saucedo M.R., Ulloa J.A., Rosas-Ulloa P., Ramírez-Ramírez J.C., Silva-Carrillo Y., Ulloa-Rangel B.E. Caracterización tecno-funcional de un concentrado proteínico obtenido de la semilla de mango (Mangifera indica L.) Biotecnia. 2021;23:120–126. doi: 10.18633/biotecnia.v23i1.1306. [DOI] [Google Scholar]
- 18.Flores-Jiménez N.T., Ulloa J.A., Urías-Silvas J.E., Ramírez-Ramírez J.C., Bautista-Rosales P.U., Gutiérrez-Leyva R. Influence of high-intensity ultrasound on physicochemical and functional properties of a guamuchil Pithecellobium dulce (Roxb.) seed protein isolate. Ultrason. Sonochem. 2022;84:e105976. doi: 10.1016/j.ultsonch.2022.105976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas-Ulloa P., Ulloa J.A., Ulloa-Rangel B.E., López-Mártir K.U. Protein isolate from orange (Citrus sinensis L.) seeds: effect of high-intensity ultrasound on its physicochemical and functional properties. Food Bioproc. Tech. 2023;16:589–602. doi: 10.1007/s11947-022-02956-4. [DOI] [Google Scholar]
- 20.Biswas B., Sit N. Effect of ultrasonication on functional properties of tamarind seed protein isolates. J. Food Sci. Technol. 2020;57:2070–2078. doi: 10.1007/s13197-020-04241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonto A.P., Tiozon R.N., Jr, Sreenivasulu N., Camacho D.H. Impact of ultrasonic treatment on rice starch and grain functional properties: A review. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A., Sit N. Dual modification of manila tamarind protein isolate by ultrasonication and autoclaving and their characterization. Food Bioproc. Technol. 2023:1–14. doi: 10.1007/s11947-023-03100-6. [DOI] [Google Scholar]
- 23.Du H., Zhang J., Wang S., Manyande A., Wang J. Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata duch.)-seed protein isolates. LWT-Food Sci. Technol. 2022;155 doi: 10.1016/j.lwt.2021.112952. [DOI] [Google Scholar]
- 24.Akharume F.U., Aluko R.E., Adedeji A.A. Modification of plant proteins for improved functionality: A review. Compreh. Rev. Food Sci. Food Safety. 2021;20:198–224. doi: 10.1111/1541-4337.12688. [DOI] [PubMed] [Google Scholar]
- 25.Flores-Jiménez N.T., Ulloa J.A., Urías-Silvas J.E., Ramírez-Ramírez J.C., Rosas-Ulloa P., Bautista-Rosales P.U., Silva-Carrillo Y., Gutiérrez-Leyva R. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019;121:947–956. doi: 10.1016/j.foodres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Wang J.S., Wang A.B., Zang X.P., Tan L., Xu B.Y., Chen H.H., Jin Z.Q., Ma W.H. Physicochemical, functional and emulsion properties of edible protein from avocado (Persea americana Mill.) oil processing by-products. Food Chem. 2019;288:146–153. doi: 10.1016/j.foodchem.2019.02.098. [DOI] [PubMed] [Google Scholar]
- 27.AOAC . 21st ed. AOAC International; Gaithersburg, MD: 2019. Official Methods of Analysis of AOAC International. [Google Scholar]
- 28.Ramani A., Kushwaha R., Malaviya R., Kumar R., Yadav N. Molecular, functional and nutritional properties of chickpea (Cicer arietinum L.) protein isolates prepared by modified solubilization methods. J. Food Meas. Charact. 2021;15:2352–2368. doi: 10.1007/s11694-020-00778-6. [DOI] [Google Scholar]
- 29.Smita M., Bashir M., Haripriya S. Physicochemical and functional properties of peeled and unpeeled coconut haustorium flours. J. Food Meas. Charact. 2018;13:61–69. doi: 10.1007/s11694-018-9919-9. [DOI] [Google Scholar]
- 30.Zhang Z., Regenstein J.M., Zhou P., Yang Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017;34:960–967. doi: 10.1016/j.ultsonch.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Li M., Wen X., Peng Y., Wang Y., Wang K., Ni Y. Functional properties of protein isolates from bell pepper (Capsicum annuum L. var. annuum) seeds. LWT-Food Sci. Technol. 2018;97:802–810. doi: 10.1016/j.lwt.2018.07.069. [DOI] [Google Scholar]
- 33.Pearce K.N., Kinsella J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- 34.Xu J., Han D., Chen Z., Li M., Jin H. Effect of glucose glycosylation following limited enzymatic hydrolysis on functional and conformational properties of black bean protein isolate. Eur. Food Res. Technol. 2018;244:1111–1120. doi: 10.1007/s00217-018-3032-5. [DOI] [Google Scholar]
- 35.Zhang W., Zhao P., Li J., Wang X., Hou J., Jiang Z. Effects of ultrasound synergized with microwave on structure and functional properties of transglutaminase-crosslinked whey protein isolate. Ultrason. Sonochem. 2022;83 doi: 10.1016/j.ultsonch.2022.105935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso-Miravalles L., Jeske S., Bez J., Detzel A., Busch M., Krueger M., Wriessnegger C.L., O’Mahony J.A., Zannini E., Arendt E.K. Membrane filtration and isoelectric precipitation technological approaches for the preparation of novel, functional and sustainable protein isolate from lentils. Eur. Food Res. Technol. 2019;245:1855–1869. doi: 10.1007/s00217-019-03296-y. [DOI] [Google Scholar]
- 37.Kumar A., Nayak R., Purohit S.R., Rao P.S. Impact of UV-C irradiation on solubility of Osborne protein fractions in wheat flour. Food Hydrocoll. 2021;110 doi: 10.1016/j.foodhyd.2020.105845. [DOI] [Google Scholar]
- 38.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.I. Lazar Jr., E. Horvath-Lazar, I. Lazar Sr. (2021). GelAnalyzer 19.1 software. Download December 5, 2021, from:www.gelanalyzer.com.
- 40.Bodwell C.E., Satterlee L.D., Hackler L.R. Protein digestibility of the same protein preparations by human and rat assays and by in vitro enzymic digestion methods. Am. J. Clin. Nutr. 1980;33:677–686. doi: 10.1093/ajcn/33.3.677. [DOI] [PubMed] [Google Scholar]
- 41.Estrada-Sierra N.A., Rincon-Enriquez G., Urías-Silvas J.E., Bravo S.D., Villanueva-Rodríguez S.J. Impact of ripening, harvest season, and the nature of solvents on antioxidant capacity, flavonoid, and p-synephrine concentrations in Citrus aurantium extracts from residue. Future Foods. 2022;6 doi: 10.1016/j.fufo.2022.100153. [DOI] [Google Scholar]
- 42.Li H., Hu Y., Zhao X., Wan W., Du X., Kong B., Xia X. Effects of different ultrasound powers on the structure and stability of protein from sea cucumber gonad. LWT-Food Sci. Technol. 2021;137 doi: 10.1016/j.lwt.2020.110403. [DOI] [Google Scholar]
- 43.Zhao Y., Wen C., Feng Y., Zhang J., He Y., Duan Y., Zhang H., Ma H. Effects of ultrasound-assisted extraction on the structural, functional and antioxidant properties of Dolichos lablab L. protein. Process Biochem. 2021;101:274–284. doi: 10.1016/j.procbio.2020.11.027. [DOI] [Google Scholar]
- 44.Cui Q., Zhang A., Li R., Wang X., Sun L., Jiang L. Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates. Food Biosci. 2020;38 doi: 10.1016/j.fbio.2020.100747. [DOI] [Google Scholar]
- 45.Ren X., Li C., Yang F., Huang Y., Huang C., Zhang K., Yan L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2020;265 doi: 10.1016/j.jfoodeng.2019.109697. [DOI] [Google Scholar]
- 46.Xu L., Xia Q., Cao J., He J., Zhou C., Guo Y., Pan D. Ultrasonic effects on the headspace volatilome and protein isolate microstructure of duck liver, as well as their potential correlation mechanism. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Resendiz-Vazquez J.A., Urías-Silvas J.E., Ulloa J.A., Bautista-Rosales P.U., Ramírez-Ramírez J.C. Effect of ultrasound-assisted enzymolysis on jackfruit (Artocarpus heterophyllus) seed proteins: structural characteristics, technofunctional properties and the correlation to enzymolysis. J. Food Process. Technol. 2019;10 doi: 10.4172/2157-7110.1000796. [DOI] [Google Scholar]
- 48.May R.A., Keith J.S. Software review of Origin 8. J. Am. Chem. Soc. 2009;131:872. doi: 10.1021/ja809638x. [DOI] [Google Scholar]
- 49.Coşkun Ö., Gülseren İ. Aqueous extraction and functionality of protein concentrates manufactured from cold press meals of pumpkin, pomegranate, and grape seeds. Nutrire. 2020;45 doi: 10.1186/s41110-020-00114-4. [DOI] [Google Scholar]
- 50.Olvera-Ríos Y.A., Armando-Ulloa J., Rosas-Ulloa P., Bautista-Rosales P.U., Ramírez-Ramírez J.C., Gutiérrez Leyva R., Silva Carrillo Y. Effect of ultrasound treatment on dehydration kinetics and physicochemical, microbiological, structural and rehydration characteristics of tilapia. CyTA-J. Food. 2020;18:31–42. doi: 10.1080/19476337.2019.1702106. [DOI] [Google Scholar]
- 51.Ulloa J.A., Villalobos-Barbosa M.C., Resendiz-Vazquez J.A., Rosas-Ulloa P., Ramírez-Ramírez J.C., Silva-Carrillo Y., González-Torres L. Production, physico-chemical and functional characterization of a protein isolate from jackfruit (Artocarpus heterophyllus) seeds. CyTA – J. Food. 2017;15:497–507. doi: 10.1080/19476337.2017.1301554. [DOI] [Google Scholar]
- 52.Fontanari G., Batistuti J., Bannach G., Pastre I., Ionashiro E., Fertonani F. Thermal study and physico-chemical characterization of some functional properties of guava seeds protein isolate (Psidium guajava) J. Therm. Anal. Calorim. 2006;83:709–713. doi: 10.1007/s10973-005-6802-9. [DOI] [Google Scholar]
- 53.Čakarević J.C., Vidović S.S., Vladić J.Z., Jokić S.D., Pavlović N.S., Popović L.M. Plum oil cake protein isolate: A potential source of bioactive peptides. Food Feed Res. 2019;46:171–178. doi: 10.5937/FFR1902171C. [DOI] [Google Scholar]
- 54.Fathollahy I., Farmani J., Kasaai M.R., Hamishehkar H. Characteristics and functional properties of Persian lime (Citrus latifolia) seed protein isolate and enzymatic hydrolysates. LWT-Food Sci. Technol. 2021;140 doi: 10.1016/j.lwt.2020.110765. [DOI] [Google Scholar]
- 55.Al-Jassar S.A., Mikajiri S., Roos Y.H. Rehydration of whey protein isolate: effect of temperature, water activity and storage time. LWT-Food Sci. Technol. 2020;133 doi: 10.1016/j.lwt.2020.110099. [DOI] [Google Scholar]
- 56.Lafarga T., Álvarez C., Bobo G., Aguiló-Aguayo I. Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. Food Sci. Technol. 2018;98:106–112. doi: 10.1016/j.lwt.2018.08.033. [DOI] [Google Scholar]
- 57.Dabbour M., He R., Ma H., Musa A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018;41:e12799. doi: 10.1111/jfpe.12799. [DOI] [Google Scholar]
- 58.Li C., Huang X., Peng Q., Shan Y., Xue F. Physicochemical properties of peanut protein isolate–glucomannan conjugates prepared by ultrasonic treatment. Ultrason. Sonochem. 2014;21:1722–1727. doi: 10.1016/j.ultsonch.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 59.Vinayashree S., Vasu P. Chemical, nutritional and functional properties of the protein isolate and pumpkin seed fractions (Cucurbita moschata var. Kashi Harit) Food Chem. 2021;340 doi: 10.1016/j.foodchem.2020.128177. [DOI] [PubMed] [Google Scholar]
- 60.Mir N.A., Riar C.S., Singh S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019;96:433–441. doi: 10.1016/j.foodhyd.2019.05.052. [DOI] [Google Scholar]
- 61.Golly M.K., Ma H., Yuqing D., Dandan L., Quaisie J., Tuli J.A., Mintah B.K., Dzah C.S., Agordoh P.D. Effect of multi-frequency countercurrent ultrasound treatment on extraction optimization, functional and structural properties of protein isolates from walnut (Juglans regia L.) meal. J. Food Biochem. 2020;44:e13210. doi: 10.1111/jfbc.13210. [DOI] [PubMed] [Google Scholar]
- 62.Naik M., Natarajan V., Modupalli N., Thangaraj S., Rawson A. Pulsed ultrasound assisted extraction of protein from defatted bitter melon seeds (Momardica charantia L.) meal: Kinetics and quality measurements. LWT-Food Sci. Technol. 2022 doi: 10.1016/j.lwt.2021.112997. [DOI] [Google Scholar]
- 63.Li M., Wen X., Peng Y., Wang Y., Wang K., Ni Y. Functional properties of protein isolates from bell pepper (Capsicum annuum L. var. annuum) seeds. LWT- Food Sci. Technol. 2018;97:802–810. doi: 10.1016/j.lwt.2018.07.069. [DOI] [Google Scholar]
- 64.Yang J., Liu G., Zeng H., Chen L. Effects of high-pressure homogenization on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocoll. 2018;83:275–286. doi: 10.1016/j.foodhyd.2018.05.020. [DOI] [Google Scholar]
- 65.Chen W., Ma H., Wang Y.Y. Recent advances in modified food proteins by high intensity ultrasound for enhancing functionality: potential mechanisms, combination with other methods, equipment innovations and future directions. Ultrason. Sonochem. 2022;85 doi: 10.1016/j.ultsonch.2022.105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu A., Li L. Effects of ultrasound pretreatment on functional property, antioxidant activity, and digestibility of soy protein isolate nanofibrils. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jahan K., Ashfaq A., Younis K., Yousuf O., Islam R.U. A review of the effects of ultrasound-assisted extraction factors on plant protein yield and functional properties. J. Food Measure. Charact. 2022;16:2875–2883. doi: 10.1007/s11694-022-01390-6. [DOI] [Google Scholar]
- 68.Rahman M.M., Lamsal B.P. Ultrasound-assisted extraction and modification of plant-based proteins: impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021;20:1457–1480. doi: 10.1111/1541-4337.12709. [DOI] [PubMed] [Google Scholar]
- 69.Shen X., Fang T., Gao F., Guo M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocoll. 2017;63:668–676. doi: 10.1016/j.foodhyd.2016.10.003. [DOI] [Google Scholar]
- 70.Yücetepe A., Saroğlu Ö., Özçelik B. Response surface optimization of ultrasound-assisted protein extraction from Spirulina platensis: investigation of the effect of extraction conditions on techno-functional properties of protein concentrates. J. Food Sci. Technol. 2019;56:3282–3292. doi: 10.1007/s13197-019-03796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y., Zeng Q.-H., Liu G., Peng Z., Wang Y., Zhu Y., Liu H., Zhao Y., Jing-Wang J. Effects of ultrasound-assisted basic electrolyzed water (BEW) extraction on structural and functional properties of Antarctic krill (Euphausia superba) proteins. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X., Wang M., Xue F., Adhikari B. Application of ultrasound treatment to improve the technofunctional properties of hemp protein isolate. Future Foods. 2022;6 doi: 10.1016/j.fufo.2022.100176. [DOI] [Google Scholar]
- 73.Mozafarpour R., Koocheki A., Nicolai T. Modification of grass pea protein isolate (Lathyrus sativus L.) using high intensity ultrasound treatment: structure and functional properties. Food Res. Int. 2022;158 doi: 10.1016/j.foodres.2022.111520. [DOI] [PubMed] [Google Scholar]
- 74.Zhao R., Liu X., Liu W., Liu Q., Zhang L., Hu H. Effect of high-intensity ultrasound on the structural, rheological, emulsifying and gelling properties of insoluble potato protein isolates. Ultrason. Sonochem. 2022;85 doi: 10.1016/j.ultsonch.2022.105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Omura M.H., Hanke-de Oliveira A.P., de Souza-Soares L., dos Reis-Coimbra J.S., Ribeiro-de Barros F.A., Ribeiro-Vidigal M.C.T., Baracat-Pereira M.C., Basílio-de Oliveira E. Effects of protein concentration during ultrasonic processing on physicochemical properties and techno-functionality of plant food proteins. Food Hydrocoll. 2021;113 doi: 10.1016/j.foodhyd.2020.106457. [DOI] [Google Scholar]
- 76.Xiong T., Xiong W., Ge M., Xia J., Li B., Chen Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018;109:260–267. doi: 10.1016/j.foodres.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y., Wang S., Li R., Wang Y., Xiang Q., Li K., Bai Y. Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocoll. 2022;124 doi: 10.1016/j.foodhyd.2021.107351. [DOI] [Google Scholar]
- 78.Rawat R., Saini C.S. High-intensity ultrasound (HIUS) treatment of sunnhemp protein isolate (Crotalaria juncea L.): Modification of functional, structural, and microstructural properties. Food Bioprocess Technol. 2023;16:1–14. doi: 10.1007/s11947-023-03011-6. [DOI] [Google Scholar]
- 79.Sachdev N., Goomer S., Singh L.R., Pathak V.M., Aggarwal D., Chowhan R.K. Current status of millet seed proteins and its applications: a comprehensive review. Appl. Food Res. 2023;3 doi: 10.1016/j.afres.2023.100288. [DOI] [Google Scholar]
- 80.Malik M.A., Sharma H.K., Saini C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: effect on physicochemical and functional properties. UltrasonicsSonochemistry. 2017;39:511–519. doi: 10.1016/j.ultsonch.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 81.Jambrak A.R., Mason T.J., Lelas V., Paniwnyk L., Herceg Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014;121:15–23. doi: 10.1016/j.jfoodeng.2013.08.012. [DOI] [Google Scholar]
- 82.Gani A., ul Ashraf Z., Noor N., Wani I.A. Ultrasonication as an innovative approach to tailor the apple seed proteins into nanosize: Effect on protein structural and functional properties. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue F., Zhu C., Liu F., Wang S., Liu H., Li C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J. Sci. Food Agric. 2018;98:5690–5699. doi: 10.1002/jsfa.9116. [DOI] [PubMed] [Google Scholar]
- 84.Deng J., Sun T., Cao W., Fan D., Cheng N., Wang B., Gao H., Yang H. Extraction optimization and functional properties of proteins from kiwi fruit (Actinidia chinensis Planch.) seeds. Int. J. Food Prop. 2014;17:1612–1625. doi: 10.1080/10942912.2013.772197. [DOI] [Google Scholar]
- 85.T. Benhammouche, A. Melo, Z. Martins, M. A. Faria, S. C. M. Pinho, I. M. L. P. V. O-Ferreira, F. Zaidi. Nutritional quality of protein concentrates from Moringa Oleifera leaves and In Vitro digestibility. Food Chem. 348 (2021), 128858. https://doi.org/10.1016/j.foodchem.2020.128858. [DOI] [PubMed]
- 86.Bhat Z.F., Morton J.D., Bekhit A.E.D.A., Kumar S., Bhat H.F. Emerging processing technologies for improved digestibility of muscle proteins. Trends Food Sci. Technol. 2021;110:226–239. doi: 10.1016/j.tifs.2021.02.010. [DOI] [Google Scholar]
- 87.Gadalkar S.M., Rathod V.K. Extraction of watermelon seed proteins with enhanced functional properties using ultrasound. Prep. Biochem. Biotech. 2020;50:133–140. doi: 10.1080/10826068.2019.1679173. [DOI] [PubMed] [Google Scholar]
- 88.Horax R., Vallecios M.S., Hettiarachchy N., Osorio L.F., Chen P. Solubility, functional properties, ACE-I inhibitory and DPPH scavenging activities of alcalase hydrolysed soy protein hydrolysates. Int. J. Food Sci. Technol. 2016;52:196–204. doi: 10.1111/ijfs.13267. [DOI] [Google Scholar]
- 89.Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P., Li A., Shao H., Li C., Liu L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT – Food Sci. Technol. 2021;152 doi: 10.1016/j.lwt.2021.112272. [DOI] [Google Scholar]
- 90.Karabulut G., Feng H., Yemiş O. Physicochemical and antioxidant properties of industrial hemp seed. Plant Foods Hum. Nutr. 2022;77:577–583. doi: 10.1007/s11130-022-01017-7. [DOI] [PubMed] [Google Scholar]
- 91.Sun X., Zhang W., Zhang L., Tian S., Chen F. Effect of ultrasound-assisted extraction on the structure and emulsifying properties of peanut protein isolate. J. Sci. Food Agric. 2021;101:1150–1160. doi: 10.1002/jsfa.10726. [DOI] [PubMed] [Google Scholar]
- 92.Zheng T., Li X., Taha A., Wei Y., Hu T., Fatamorgana P.B., Hu H. Effect of high intensity ultrasound on the structure and physicochemical properties of soy protein isolates produced by different denaturation methods. Food Hydrocoll. 2019;97 doi: 10.1016/j.foodhyd.2019.105216. [DOI] [Google Scholar]
- 93.Yolandani Y., Ma H., Li Y., Liu D., Zhou H., Wan Y., Liu X. Ultrasound-assisted limited enzymatic hydrolysis of high concentrated soy protein isolate: alterations on the functional properties and its relation with hydrophobicity and molecular weight. Ultrason. Sonochem. 2023;95 doi: 10.1016/j.ultsonch.2023.106414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vargas S.A., Delgado-Macuil R.J., Ruiz-Espinosa H., Rojas-López M., Amador-Espejo G.G. High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: evaluation of selected functional properties. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin J., Okagu O.D., Yagoub A.E.A., Udenigwe C.C. Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian R., Zhu G., Feng J., Tian B., Zhang Y., Jiang L., Sui X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.10520. [DOI] [PubMed] [Google Scholar]
- 97.Yu Y., Wang T., Huang X., Lian Y., Yang F., Yu D. Hemp seed protein and chlorogenic acid complex: effect of ultrasound modification on its structure and functional properties. Int. J. Biol. Macromol. 2023;233 doi: 10.1016/j.ijbiomac.2023.123521. [DOI] [PubMed] [Google Scholar]
- 98.Gao K., Zha F., Yang Z., Rao J., Chen B. Structure characteristics and functionality of water-soluble fraction from high-intensity ultrasound treated pea protein isolate. Food Hydrocoll. 2022;125 doi: 10.1016/j.foodhyd.2021.107409. [DOI] [Google Scholar]
- 99.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. doi: 10.1016/j.foodres.2014.04.022. [DOI] [Google Scholar]
- 100.Cheng J., Cui L. Effects of high-intensity ultrasound on the structural, optical, mechanical and physicochemical properties of pea protein isolate-based edible film. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z., Zhao H., Tao H., Yu B., Cui B., Wang Y. Ultrasound improves the physicochemical and foam properties of whey protein microgel. Front. Nutr. 2023;10:1140737. doi: 10.3389/fnut.2023.1140737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y., Li B., Guo Y., Liu C., Liu J., Tan B., Guo Z., Wang Z., Jiang L. Effects of ultrasound on the structural and emulsifying properties and interfacial properties of oxidized soybean protein aggregates. Ultrason. Sonochem. 2022;87 doi: 10.1016/j.ultsonch.2022.106046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu L., Zeng J., Sun B., Zhang N., He Y., Shi Y., Zhu X. Ultrasound-assisted mild heating treatment improves the emulsifying properties of 11S globulins. Molecules. 2020;25:875. doi: 10.3390/molecules25040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J., Xu Z., Jiang L., Zhang Y., Sui X. Further evaluation on structural and antioxidant capacities of soy protein isolate under multiple freeze–thaw cycles. Food Chem. 2023;X, 17 doi: 10.1016/j.fochx.2023.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amiri A., Sharifian P., Soltanizadeh N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018;111:139–147. doi: 10.1016/j.ijbiomac.2017.12.167. [DOI] [PubMed] [Google Scholar]
- 106.Yan S., Xu J., Zhang S., Li Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT-Food Sci. Technol. 2021;142 doi: 10.1016/j.lwt.2021.110881. [DOI] [Google Scholar]
- 107.Zhao Q., Xie T., Hong X., Zhou Y., Fan L., Liu Y., Li J. Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of O/W emulsion. Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130848. [DOI] [PubMed] [Google Scholar]
- 108.Bi A.Q., Xu X.B., Guo Y., Du M., Yu C.P., Wu C. Ultrasound pre-fractured casein and in-situ formation of high internal phase emulsions. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2019.104916. [DOI] [PubMed] [Google Scholar]
- 109.Kang S., Zhang J., Guo X., Lei Y., Yang M. Effects of ultrasonic treatment on the structure, functional properties of chickpea protein isolate and its digestibility in vitro. Foods. 2022;11:880. doi: 10.3390/foods11060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang F., Liu X., Huang Y., Huang C., Zhang K. Swirling cavitation improves the emulsifying properties of commercial soy protein isolate. Ultrason. Sonochem. 2018;42:471–481. doi: 10.1016/j.ultsonch.2017.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.