Abstract
Background:
Controlling tuberculosis (TB) determinant factors in Indonesia is one way to control TB in the community. A review is needed to explore risk factors for TB in Indonesia as the key strategies for accelerating the TB preventive program.
The purpose of this review was to determine modifiable and non-modifiable risk factors for TB among adults in Indonesia.
Materials and Methods:
A meta-analysis was undertaken to review current studies related to modifiable and non-modifiable risk factors for TB among adults in Indonesia. A search of PubMed, ProQuest, and Google Scholar for related articles published (January 2000 until December 2023). The Pooled Odds Ratio (POR) from the acquired data were calculated with a 95% CI. The fixed and random effects analysis was performed. The results were presented as forest plots, and Begg’s test and Egger’s test were used to examine study bias. Review Manager (RevMan) 5.4 and Stata 14.2 were used to process and analyze all of the data.
Results:
This study results revealed the POR of non-modifiable risk factor (family history of TB) for TB among adults in Indonesia was 6.08 (95% CI 2.99-12.34). Based on modifiable risk factors, it is known that household contact have the highest POR (6.01, 2.57-14.04), followed by malnutrition (5.86, 2.50-13.69), inappropriate ventilation (5.57, 1.74–17.86), diabetes mellitus (4.92, 3.04-7.96), smoking behavior (3.24, 2.22-4.72), and low-income level (2.34, 1.42-3.87).
Conclusion:
Based on significant factors that are related to TB incidence, the results of this review may be valuable to the government in identifying the optimal strategy for TB prevention among adults.
Keywords: Adults, Modifiable, Non-modifiable, Tuberculosis, Indonesia
Introduction
An important global health concern is tuberculosis (TB) (Adane et al., 2020). More than 1 million annual deaths are recorded from TB. TB illness continues to be a serious public health issue that affects people of all ages (Jiang et al., 2022). The action plans have already established a new aim to eliminate TB epidemics by 2030, in which endemic nations must make further efforts to stop or lessen the effect of TB (Jeremiah et al., 2021).
In a World Health Organization (WHO) report from 2020, Indonesia had the second-highest percentage of worldwide TB incidence (8.5%) (Jiang et al., 2022). A strategy was created by the Indonesian government to eradicate TB (Jeremiah et al., 2021). Controlling TB determinant factors is one way, thus keeping track of these elements in society is important for informing policymakers as they create a preventative program (Sulistyawati et al., 2021).
TB has multiple causes. There are currently a number of recognized TB risk factors (Sulistyawati et al., 2021). Low level of economic status, smoking, alcohol use, diabetes mellitus, HIV infection, malnutrition, contact history, exposure to silicosis, and ventilation condition are classified as modifiable risk factors (Jubulis et al., 2014). Age, gender, and family history are non-modifiable risk factors (Sadeghi et al., 2022; Bath et al., 2017).
Previous study found the modifiable risk factors contributed for TB were smoking and inappropriate ventilation condition (Sulistyawati et al., 2021). One study stated nutritional status, family history of TB and smoking as modifiable risk factors associated for TB (Nur et al., 2022). Other studies revealed malnutrition, diabetes mellitus, smoking, alcohol consumption, TB contact and poor families as modifiable risk factors for TB (Destiany et al., 2020; Fibriana et al., 2020; Stang et al., 2020). Non-modifiable risk factors, namely family history of TB and age were associated for TB (Fibriana et al., 2020; Nur et al., 2022). However, conflicting findings regarding the significance of age and sex as risk factors for TB exist (Destiany et al., 2020; Stang et al., 2020), likely due to variations in study populations and methodologies.
The novelty of this study lies in its focus on synthesizing existing knowledge of both modifiable and non-modifiable risk factors for TB specifically among adults in Indonesia. While previous studies have examined individual risk factors, there is a lack of a comprehensive review that consolidates and analyzes these findings within the context of Indonesia. By undertaking this review, the study aims to provide a more nuanced understanding of how these risk factors interact within the unique socio-cultural and environmental landscape of Indonesia. This approach is crucial for developing targeted and effective TB preventive programs tailored to the country’s specific needs. Furthermore, the study seeks to address discrepancies in previous research findings by critically evaluating the methodologies and populations involved, thereby contributing to the advancement of knowledge in the field of TB epidemiology and public health intervention.
Materials and Methods
Study design and research sample
A meta-analysis was undertaken to review current studies related to modifiable and non-modifiable risk factors for TB among adults in Indonesia. This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Page et al., 2021).
Eligibility criteria
Only original publications with a case-control or cohort study design, English language, and human participants as study subjects were included. Exclusion criteria for the study encompassed unavailability of a full-text version, inappropriate topics, and data from articles that could not be used for further examination.
Search approach and study collection
A search of PubMed, ProQuest, and Google Scholar for related articles published (January 2000 until December 2023) with four main keywords “adult” AND “risk factors” AND “tuberculosis” AND “Indonesia”. In this study, tuberculosis was the outcome variable, while the exposure variables comprised modifiable and non-modifiable risk factors. Two independent investigators conducted the literature search. After the initial search, duplicates were manually eliminated, and the titles/abstracts were screened for relevance. The full texts of potential articles were then assessed using the criteria.
Data extraction
Two different authors used structured extraction forms to obtain data. The processes of searching for research articles were depicted using PRISMA flowcharts (Figure 1).
Figure 1.
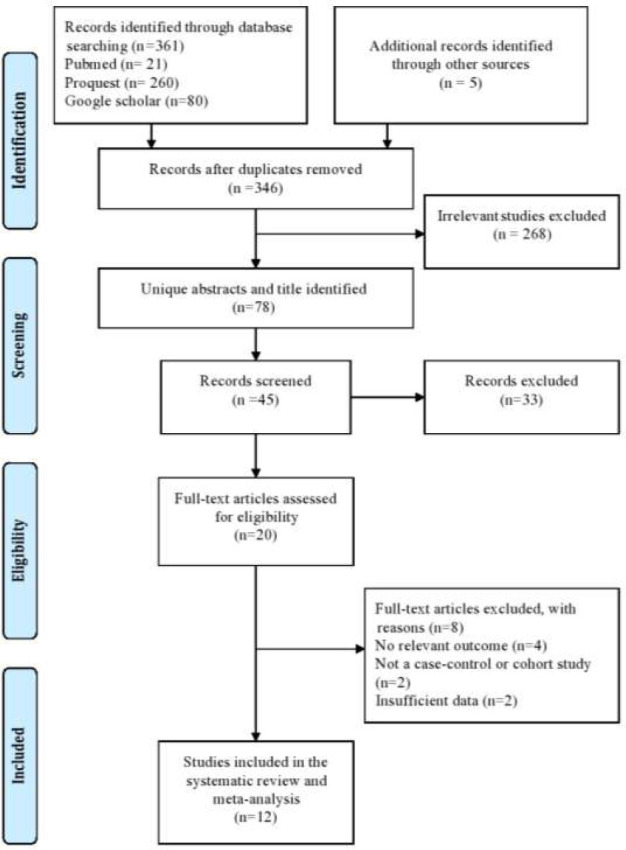
PRISMA flowchart.
The quality of the publications was evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS). The NOS assessed nine questions: 1) Is the case definition sufficient? 2) Are the cases representative? 3) Selection of controls; 4) Definition of controls; 5) Study controls for the most significant factor; 6) Study controls for any significant factor; 7) Exposure measurement; 8) Identical measurement procedure (cases and controls); 9) Non-response rate. Articles were categorized into low, medium, and high-quality groups using the numbers 0–3, 4–6, and 7–9 (Figure 2) (Gusnedi et al., 2023).
Figure 2.
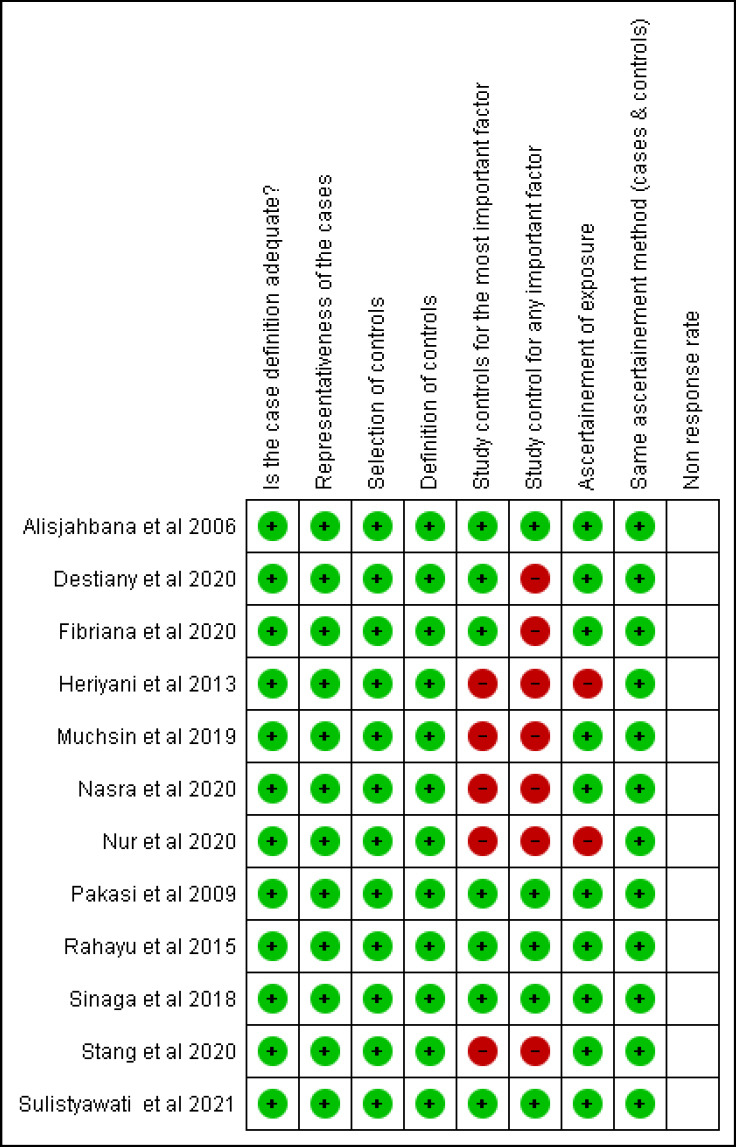
The quality of the publications using the Newcastle-Ottawa Quality Assessment Scale (NOS).
Data analysis
The pooled stunting prevalence and the Pooled Odds Ratio (POR) from the acquired data were calculated with a 95% confidence interval (CI). I2 indicates that there was heterogeneity between publications if it was greater than 50%. If the outcome was heterogeneous, the random effect analysis was performed, and if it was homogeneous, the fixed-effect analysis was utilized. Furthermore, the findings were presented as forest plots, and Begg’s and Egger’s tests were used to identify study bias (Nindrea et al., 2018). There was no publication bias among the studies, according to the p > 0.05 findings of the two tests. Review Manager (RevMan) 5.4 and Stata 14.2 were used to process and analyze all of the data.
Results
Twelve current studies were considered in this systematic review research on modifiable and non-modifiable risk factors for TB among adults in Indonesia (Alisjahbana et al., 2006; Pakasi et al., 2009; Heriyani et al., 2013; Rahayu et al., 2015; Sinaga et al., 2018; Muchsin et al., 2019; Destiany et al., 2020; Fibriana et al., 2020; Nasra et al., 2020; Stang et al., 2020; Nur et al., 2020; Sulistyawati et al., 2021) (Table 1).
Table 1.
Systematic review of modifiable and non-modifiable risk factors for tuberculosis among adults in Indonesia.
| First author, year | Year of study | Region | Study design | Total samples | Characteristics | Risk factors |
|---|---|---|---|---|---|---|
| Alisjahbana et al | 2006 | Central Jakarta and Bandung | Case-control study | 1,010 | Age of cases and control (median 30 y); male, 52%; BMI of cases vs control (17.7 vs 21.5 kg/m2) | Diabetes mellitus and household contact |
| Pakasi et al | 2009 | Timor and Rote Islands | Case-control study | 492 | Mean age (30 y); Sex (M, 56.3%; F, 43.7%); BMI of cases vs control (16.1 vs 19.4 kg/m2) | Family history of TB and malnutrition |
| Heriyani et al | 2013 | Banjarmasin, Kalimantan | Case-control study | 154 | Sex (55.84% were male); age (35-<45 years were 25.97%) | Low income level, smoking behavior, inappropriate ventilation |
| Rahayu et al | 2015 | Semarang District | Case-control study | 212 | The mean age case and control was 41.2±15.3 and 35.7±11.7 years; Sex (M vs F were 50%) | Low income level |
| Sinaga et al | 2018 | Medan | Case-control study | 200 | Aged 16-55 years; male of cases vs control (70.0% vs 70.0%) | Smoking behavior |
| Muchsin et al | 2019 | Langsa, North Sumatera | Case-control study | 232 | Sex (M vs F were 50%) | Malnutrition, and inappropriate ventilation |
| Destiany et al | 2020 | Makassar | Case-control study | 90 | 45-54 years (cases, 37.78%; control, 35.56%); male (cases, 62.22%; control, 68.89%); high school educational background (cases, 37.78%; control, 37.78%) | Low income level and malnutrition |
| Fibriana et al | 2020 | Semarang | Case-control study | 75 | 15-54 years (cases, 86.7%; control, 88.9%); male (cases, 53.3%; control, 44.4%) | Diabetes mellitus and household contact |
| Nasra et al | 2020 | South Sulawesi | Case-control study | 102 | Productive age (cases, 79.4%; control, 79.4%); male (cases, 58.8%; control, 58.8%) | Family history, household contact, low income level, inappropriate ventilation |
| Stang et al | 2020 | Makassar | Case-control study | 120 | 26-33 years (cases, 24.5%; control, 22.9%); low level of education (cases, 83.7%; control, 70.8%) | Household contact |
| Nur et al | 2020 | Jeneponto District, South Sulawesi Province | Case-control study | 147 | Productive age (cases, 68.3%; control, 71.7%); male (cases, 71.7%; control, 55.0%) | Malnutrition, smoking behavior and household contact |
| Sulistyawati et al | 2021 | Yogyakarta | Case-control study | 69 | Male (cases, 48.0%; control, 39.0%); 17-45 years (cases, 74.0%; control, 70.0%) | Family history of TB, smoking behavior, inappropriate ventilation |
| Total samples | 2,903 | |||||
Abbreviation: BMI, body mass index; M, male; F, female; CI, confidence interval; NOS, Newcastle–Ottawa Quality Assessment Scale
The total sample from the included studies was 2,903 participants4,8-11,14-20. This study that revealed non-modifiable risk factors for TB among adults in Indonesia were a family history of TB, and modifiable risk factors (low income level, household contact, inappropriate ventilation, smoking behavior, diabetes mellitus, and malnutrition).
Meta-estimate of modifiable and non-modifiable risk factors for TB among adults in Indonesia (Figure 3).
Figure 3.
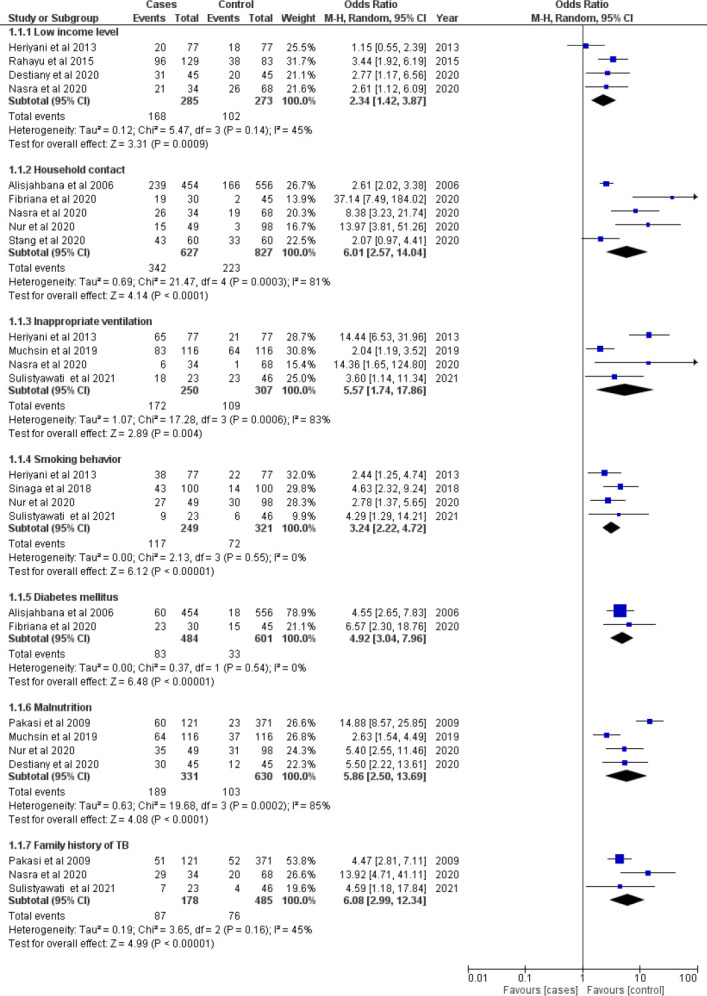
Meta-estimate of modifiable risk factors and non-modifiable risk factor (family history of TB) for TB among adults in Indonesia.
Figure 3 revealed modifiable risk factors, it is known that household contacts have the highest POR (95% CI) (6.01, 2.57-14.04), followed by malnutrition (5.86, 2.50-13.69), inappropriate ventilation (5.57, 1.74–17.86), diabetes mellitus (4.92, 3.04-7.96), smoking behavior (3.24, 2.22-4.72), and low-income level (2.34, 1.42-3.87). The heterogeneity analysis revealed homogenous in low-income level, smoking behavior, and diabetes mellitus for TB among adults in Indonesia (I2 ≤ 50%). However, heterogeneity in household contact, inappropriate ventilation, and malnutrition for TB among adults in Indonesia (I2 > 50%).
The non-modifiable risk factor (family history of TB) for TB among adults in Indonesia was 6.08 (95% CI 2.99-12.34). The heterogeneity analysis revealed homogeneity of family history of TB for TB among adults in Indonesia (I2 ≤ 50%).
The results of Egger’s test to assess bias among studies are included (Table 2).
Table 2.
Begg’s test and Egger’s test for small-study effects among modifiable and non-modifiable risk factors for tuberculosis among adults in Indonesia
| Variables | Test for small-study effects | |
|---|---|---|
|
| ||
| Begg’s test (p-value) | Egger’s test (p-value) | |
| Non-modifiable | ||
| Family history of TB | 0.450 | 0.590 |
| Modifiable | ||
| Low income level | 0.510 | 0.688 |
| Household contact | 0.055 | 0.085 |
| Inappropriate ventilation | 0.511 | 0.482 |
| Smoking behaviour | 0.602 | 0.807 |
| Diabetes mellitus | 0.411 | 0.500 |
| Malnutrition | 0.878 | 0.988 |
It revealed that non-modifiable factors (family history of TB), and modifiable factors (low-income level, household contact, inappropriate ventilation, smoking behavior, diabetes mellitus, and malnutrition) showed no study bias among the included publications (p>0.05).
Discussion
In this study, we found that modifiable risk factors for TB among adults in Indonesia, it was also revealed that household contacts have the highest POR (6.01). Household contacts were three times more likely to develop TB than individuals who lived in adequate-sized rooms. This is consistent with earlier studies conducted in Ethiopia, Pakistan, and sub-Saharan Africa (Hargreaves et al., 2011; Kirenga et al., 2015; Khaliq et al., 2015). This could be connected to cramped conditions and inadequate airflow, which can hasten the spread of TB (WHO, 2009). However, in a society with extended families, the contact network goes beyond the nuclear family despite the fact that the index case had many home contacts (WHO, 2009; Hargreaves et al., 2011). The balance of TB transmission, including whether it happens largely in homes or in the population as a whole, is unknown and may vary from region to region based on the frequency of the disease and the mixing patterns of infectious cases (Hargreaves et al., 2011). The location of TB transmission can have a significant impact on TB control strategies. In Indonesia, the limitations of the present diagnostic techniques for early or minimal disease make it difficult to evaluate interactions within households. Improved diagnostic techniques and assessments of the dangers associated with the household environment will be needed for new household contact tracing strategies.
According to our study, malnutrition increased the risk of TB by 5.86 times. Previous study stated dietary conditions and the prevalence of TB are related. Malnutrition will reduce the immune system’s ability to fight disease, making a person with poor nutritional status more prone to contracting TB. Another study found micronutrient deficits and protein-energy deficiency raise the risk of TB. Malnourished TB patients have been shown to have slower recovery times and greater fatality rates than well-nourished individuals (Gupta et al., 2009). In Ethiopia, the prevalence of undernutrition among adult TB patients was severe (Muse et al., 2021). The likelihood of malnutrition was 3.23 times higher in TB patients over the age of 25 years. This conclusion is consistent with one from Ghana (Dodor et al., 2008). This is as a result of the fact that co-morbid illnesses, such as chronic diseases, carry increasing risks as people age (Nunes et al., 2016). In Indonesia, a large percentage of patients with TB are malnourished. Especially among the indigenous populations who live in extended families, this undoubtedly plays a role in the development of TB. The local government is advised to create a Body Mass Index (BMI) based malnutrition screening program.
This study found that inappropriate ventilation also increased the risk of TB by 5.57 times. A common risk factor for TB is crowded living conditions, like shared housing (Lönnroth et al., 2009; Lienhardt et al., 2012). An earlier study found that TB exposure, incidence, and treatment adherence were all correlated with the affordability and quality features of substandard housing. Additionally, we discovered that practically all eight stages of TB development and its effects were connected to inadequate housing. The prevalence of homelessness, which was closely associated with TB infection, is rising globally (Lönnroth et al., 2009).
In this study diabetes mellitus was associated with TB among adults with POR = 4.92. Diabetes mellitus (DM) has been linked to TB in previous studies (Lönnroth et al., 2009; Fibriana et al., 2020). Another study found that 14.8% of the incidence of TB in India was caused by DM. According to a review of recently published reports, the odds ratio for DM as a risk factor for tuberculosis varied in different regions, ranging from 1.23 to 6 (Gupta et al., 2009). The high correlation found in our study between DM and TB may have a possible explanation. Indonesia has a young population and is still in development (Harahap et al., 2017). DM as a comorbidity with TB must therefore be disregarded. The discovery that DM is a significant risk factor for TB in our location has clear implications for TB control. High attention must be given to DM control programs in the population, especially young people and TB patients, due to the high prevalence of DM in TB patients.
We also found smoking behavior increased the risk of TB by 3.24 times. A higher risk of tuberculosis was also demonstrated by prior studies of cigarette use and chronic respiratory disease (Lönnroth et al., 2009; Nunes et al., 2016). There was no statistically significant difference in TB prevalence between cigarette smokers and those with chronic bronchitis. According to another study, cigarette use is linked to TB, and it is more common in men than in women. Smokers are more likely to develop TB than non-smokers (OR = 1.8). Additionally, the current study found a favorable relationship between tobacco usage and smoking for TB among adults (Khaliq et al., 2015). One of the highest smoking rates in the world is found in Indonesia (Jiang et al., 2022). Therefore, the task is not just to reduce the number of smokers but also to reduce the number of TB cases among smokers. To help a person stay smoke-free, smoking cessation messages must both prevent TB and continue after TB treatment. Healthcare professionals, providers, and family members are in an excellent position to support the doctor’s advice on quitting smoking and assist individuals who are smokers in doing so.
This study also revealed low income level associated with TB among adults with POR = 2.34. According to a study from India (Bhat et al., 2017), poor people had a high frequency of incomplete TB knowledge. According to a study from Ethiopia (Dodor et al., 2008), those with low incomes tend to be less health-conscious. Economic status is related to access to health facilities, the ability to meet nutritional needs, and access to a good and standardized home environment (Khaliq et al., 2015). These factors all increase the likelihood of TB. Therefore, the government must take into consideration measures to raise income by encouraging the informal sector to engage in entrepreneurship and giving capital support to enhance their welfare. Such a gain in income will be consistent with an increase in the ability to purchase nutritious food, the availability of housing, and access to high-quality healthcare services.
This study found that family history of TB is a non-modifiable risk factor for TB among adults in Indonesia (POR = 6.08). According to a prior study in Uganda, 17.5% of TB patients reported having TB in their families. Family history is a well-known TB risk factor (Kirenga et al., 2015). Household contacts with a family history of TB were more likely to get the disease than those without such a history. This was similar to studies conducted in India and Ethiopia (Bhat et al., 2017; Nasra et al., 2020). People who have a prior history of TB in their families should have early screening and health examinations at medical facilities, such as hospitals or primary health care centers, on the risks they may have. In order to carry out successful preventative initiatives. In addition, there is a need for awareness, knowledge, and comprehension of other TB risk factors.
The Indonesian government developed a plan to end TB. Monitoring such factors in society is crucial for informing policymakers as they develop a preventative program since controlling TB determining factors is one method. Indonesia’s TB control policy is implemented through a health sector and multi-sector approach using a variety of risk factor management strategies, with a focus on improving individual health levels and reducing TB infection in public areas. To help Indonesia reach its 2030 TB elimination target, the central and local governments’ roles in TB control must be increased. One of them is the health reform, which highlights the value of sustainable implementation in Indonesia at the regional level (Nindrea et al., 2020; Jiang et al., 2022; Nindrea et al., 2023).
To the best of our knowledge, this study is the first meta-analysis of both modifiable and non-modifiable risk factors for TB among adults in Indonesia, providing a comprehensive overview of the current knowledge in this area. We revealed the POR for modifiable and non-modifiable risk factors with TB among adults identified from a diverse range of studies with large sample size and different demographic characteristics in the country. Thus, the analysis could provide a basis for concluding risk factors statistically associated with the incidence of TB among adults in Indonesia. However, risk factors were estimated by odds ratio and it may be affected by other confounding variables. The other limitation is that the search strategy restricted articles published in the selected databases and only in the English language. There might be articles published in other databases using another language that was not included. Furthermore, the study focuses specifically on adults in Indonesia, and the findings may not be generalizable to other age groups or populations in different geographic regions.
The implications of this study may be valuable to the government in identifying the optimal strategy for TB prevention among adults. Additionally, health promotion and education regarding the prevention of TB based on modifiable and non-modifiable risk factors must be carried out. Health professionals and capable health volunteers can provide community- or individual-based promotion and education from home to home. For the public to understand TB preventive initiatives, it is crucial to disseminate this information via the right promotional media, both online and offline.
Conclusion
This review revealed family history of TB as a non-modifiable risk factor for TB among adults in Indonesia and household contact, malnutrition, inappropriate ventilation, diabetes mellitus, smoking behavior, and low income level are all modifiable risk factors for TB. The findings of this study may be helpful to the government in determining the best plan for TB prevention among adults based on significant factors that are related to TB incidence. The prevention and control of TB based on modifiable and non-modifiable risk factors requires health promotion and education. From home to home, qualified health volunteers and health professionals can offer community- or individual-based promotion and education, for the general public to comprehend TB prevention efforts.
Acknowledgements
The authors would like to thank Riyani Betri Novialita, MA, for data coloection.
Conflict of interest statement
The authors declared that there is no conflict of interest associated with this review.
List of Abbreviations:
- BMI:
Body mass index
- CI:
Confidence interval
- NOS:
Newcastle–Ottawa quality assessment scale
- POR:
Pooled odds ratio
- PRISMA:
The preferred reporting items for systematic reviews and meta-analysis
- TB:
Tuberculosis
References
- 1.Adane A, Damena M, Weldegebreal F, Mohammed H. Prevalence and associated factors of tuberculosis among adult household contacts of smear positive pulmonary tuberculosis patients treated in public health facilities of Haramaya District, Oromia Region, Eastern Ethiopia. Tuberculosis Research and Treatment. 20202020:6738532. doi: 10.1155/2020/6738532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alisjahbana B, van Crevel R, Sahiratmadja E, den Heijer M, Maya A, Istriana E. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. The International Journal of Tuberculosis and Lung Disease. 2006;10(6):696–700. [PubMed] [Google Scholar]
- 3.Bhat J, Rao V G, Sharma R. K, Muniyandi M, Yadav R, Bhondley M. K. Investigation of the risk factors for pulmonary tuberculosis:A case-control study among Saharia tribe in Gwalior district, Madhya Pradesh, India. Indian Journal of Medical Research. 2017;146(1):97–104. doi: 10.4103/ijmr.IJMR_1029_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Destiany C, Zulkifli A, Thaha R M, Mallongi A, Astuti R. D. P. Risk factor of pulmonary tuberculosis among people with diabetes mellitus in Makassar. Enfermería Clínica. 2020;30(4):269–272. [Google Scholar]
- 5.Dodor E. Evaluation of nutritional status of new tuberculosis patients at the EffiaNkwanta regional hospital. Ghana Medical Journal. 2008;42:22–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Fibriana A I, Azam M, Maryuni S, Indrawati F, Windraswara R, Turnbull N. Risk factors of pulmonary tuberculosis among diabetes mellitus patients:a case-control study in Dr. Kariadi General Hospital, Semarang, Indonesia. Malaysian Journal of Public Health Medicine. 2020;20(2):101–107. [Google Scholar]
- 7.Harahap W A, Ramadhan R, Khambri D, Haryono S, Dana Nindrea R. Outcomes of trastuzumab therapy for 6 and 12 months in Indonesian national health insurance system clients with operable HER2-positive breast cancer. Asian Pacific Journal of Cancer Prevention. 2017;18(4):1151–1156. doi: 10.22034/APJCP.2017.18.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hargreaves J R, Boccia D, Evans C. A, Adato M, Petticrew M, Porter J. D. The social determinants of tuberculosis:from evidence to action. The American Journal of Public Health. 2011;101(4):654–62. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heriyani F, Sutomo A H, Saleh Y. D. Risk factors of the incidence of pulmonary tuberculosis in Banjarmasin City, Kalimantan, Indonesia. International Journal of Public Health Science. 2013;2(1):1–6. [Google Scholar]
- 10.Gupta K B, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India. 2009;26(1):9–16. doi: 10.4103/0970-2113.45198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gusnedi G, Nindrea R D, Purnakarya I, Umar H. B, Andrafikar A, Syafrawati S. Risk factors associated with childhood stunting in Indonesia:A systematic review and meta-analysis. Asia Pacific Journal of Clinical Nutrition. 2023;32(2):184–195. doi: 10.6133/apjcn.202306_32(2).0001. [DOI] [PubMed] [Google Scholar]
- 12.Jeremiah C, Petersen E, Nantanda R, Mungai B N, Migliori G. B, Amanullah F, et al. The WHO Global Tuberculosis 2021 Report - not so good news and turning the tide back to End TB. The International Journal of Infectious Diseases. 2022;S1201-9712(22):00149–7. doi: 10.1016/j.ijid.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, Trimawartinah, Rahman F M, Wibowo A, Sanjaya A, Silitonga P. I. I. The co-management of tuberculosis-diabetes co-morbidities in Indonesia under the National Tuberculosis Control Program:results from a cross-sectional study from 2017 to 2019. BMC Public Health. 2022;22(1):689. doi: 10.1186/s12889-022-13017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jubulis J, Kinikar A, Ithape M, Khandave M, Dixit S, Hotalkar S. Modifiable risk factors associated with tuberculosis disease in children in Pune, India. The International Journal of Tuberculosis and Lung Disease. 2014;18(2):198–204. doi: 10.5588/ijtld.13.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaliq A, Khan I H, Akhtar M. W, Chaudhry M. N. Environmental risk factors and social determinants of pulmonary tuberculosis in Pakistan. Epidemiology (Sunnyvale) 2015;2015(5):3. [Google Scholar]
- 16.Kirenga B J, Ssengooba W, Muwonge C, Nakiyingi L, Kyaligonza S, Kasozi S. Tuberculosis risk factors among tuberculosis patients in Kampala, Uganda:implications for tuberculosis control. BMC Public Health. 2015;15:13. doi: 10.1186/s12889-015-1376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lienhardt C, Glaziou P, Uplekar M, Lönnroth K, Getahun H, Raviglione M. Global tuberculosis control:lessons learnt and future prospects. Nature Reviews Microbiology. 2012;10(6):407–16. doi: 10.1038/nrmicro2797. [DOI] [PubMed] [Google Scholar]
- 18.Lönnroth K, Jaramillo E, Williams B G, Dye C, Raviglione M. Drivers of tuberculosis epidemics:the role of risk factors and social determinants. Social Science and Medicine. 2009;68(12):2240–6. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Muchsin M, Siregar F A, Sudaryati E. The Influence of Nutritional Status and Ventilation on the Incidence of Pulmonary Tuberculosis at Langsa. Open Access Macedonian Journal of Medical Sciences. 2019;7(20):3421–3424. doi: 10.3889/oamjms.2019.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muse A I, Osman M. O, Ibrahim A. M, Wedajo G. T, Daud F. I, Abate K. H. Undernutrition and Associated Factors Among Adult Tuberculosis Patients in Jigjiga Public Health Facilities, Somali Region, East, Ethiopia. Research and Reports in Tropical Medicine. 2021;12:123–133. doi: 10.2147/RRTM.S311476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasra N, Arsunan A A, Thamrin Y, Wahiduddin W, Maria I. L, Nurhaedar J. Analysis of risk factors for tuberculosis in the lake of coastal area, Towuti District, East Luwu Regency, Indonesia. European Journal of Molecular and Clinical Medicine. 2020;7(6):67–73. [Google Scholar]
- 22.Nindrea R D, Sari N. P, Harahap W. A, Haryono S. J, Kusnanto H, Dwiprahasto I. Survey data of multidrug-resistant tuberculosis, Tuberculosis patients characteristics and stress resilience during COVID-19 pandemic in West Sumatera Province, Indonesia. Data in Brief. 2020;32:106293. doi: 10.1016/j.dib.2020.106293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nindrea R D. Impact of Telehealth on the Environment During the COVID-19 Pandemic in Indonesia. Asia Pacific Journal of Public Health. 2023;35(2-3):227. doi: 10.1177/10105395231152580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nindrea R D, Harahap W. A, Aryandono T, Lazuardi L. Association of BRCA1 promoter methylation with breast cancer in Asia:a meta- analysis. Asian Pacific Journal of Cancer Prevention. 2018;25;19(4):885–889. doi: 10.22034/APJCP.2018.19.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunes H E, Goncalves E. C, Vieira J. A, Silva D. A. Clustering of risk factors for non-communicable diseases among adolescents from southern Brazil. PLoS One. 2016;11(7):e0159037. doi: 10.1371/journal.pone.0159037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nur I, Noor N N, Salmah A. U, Mallongi A, Amqam H. Risk factors analysis and mapping of pulmonary tuberculosis in community health centre Tamalatea of Jeneponto District. Open Access Macedonian Journal of Medical Sciences. 2020;8(T2):59–62. [Google Scholar]
- 27.Page M J, McKenzie J. E, Bossuyt P. M, Boutron I, Hoffmann T. C, Mulrow C. D. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. The BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pakasi T A, Karyadi E, Dolmans W. M, van der Meer J. W, van der Velden K. Malnutrition and socio-demographic factors associated with pulmonary tuberculosis in Timor and Rote Islands, Indonesia. The International Journal of Tuberculosis and Lung Disease. 2009;13(6):755–9. [PubMed] [Google Scholar]
- 29.Rahayu S R, Katsuyama H, Demura M, Katsuyama M, Ota Y, Tanii H. Factors associated with tuberculosis cases in Semarang District, Indonesia:case-control study performed in the area where case detection rate was extremely low. Environmental Health and Preventive Medicine. 2015;20(4):253–61. doi: 10.1007/s12199-015-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadeghi K, Poorolajal J, Doosti-Irani A. Prevalence of modifiable risk factors of tuberculosis and their population attributable fraction in Iran:A cross-sectional study. PLoS One. 2022;17(8):e0271511. doi: 10.1371/journal.pone.0271511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinaga B. Y. M, Siregar Y, Amin M, Sarumpaet S. Effect of smoking and alcohol consumption on pulmonary tuberculosis among Batak ethnic population in Medan, Indonesia. IOP Conference Series:Earth and Environmental Science. 2018;125:1–5. [Google Scholar]
- 32.Sulistyawati S, Ramadhan A. W. Risk Factors for Tuberculosis in an Urban Setting in Indonesia:A Case-control Study in Umbulharjo I, Yogyakarta. Journal of UOEH. 2021;43(2):165–171. doi: 10.7888/juoeh.43.165. [DOI] [PubMed] [Google Scholar]
- 33.Stang S, Mallongi A, Dwinata I, Sumarni S. Risk factors of lung tuberculosis occurrence in the working area of Kaluku Bodoa Health Center Makassar City. Medico Legal Update. 2020;20(3):714–719. [Google Scholar]
- 34.WHO. WHO Policy on TB Infection Control in Health-Care Facilities, Congregate Settings and Households. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]


