Abstract
Background
It has previously been reported that there are similar reoperation rates after elective colorectal surgery but higher failure-to-rescue (FTR) rates in low-volume hospitals (LVHs) versus high-volume hospitals (HVHs). This study assessed the effect of hospital volume on reoperation rate and FTR after reoperation following elective colorectal surgery in a matched cohort.
Methods
Population-based retrospective multicentre cohort study of adult patients undergoing reoperation for a complication after an elective, non-centralized colorectal operation between 2006 and 2017 in 11 hospitals. Hospitals were divided into either HVHs (3 hospitals, median ≥126 resections per year) or LVHs (8 hospitals, <126 resections per year). Patients were propensity score–matched (PSM) for baseline characteristics as well as indication and type of elective surgery. Primary outcome was FTR.
Results
A total of 6428 and 3020 elective colorectal resections were carried out in HVHs and LVHs, of which 217 (3.4%) and 165 (5.5%) underwent reoperation (P < 0.001), respectively. After PSM, 142 patients undergoing reoperation remained in both HVH and LVH groups for final analyses. FTR rate was 7.7% in HVHs and 10.6% in LVHs (P = 0.410). The median Comprehensive Complication Index was 21.8 in HVHs and 29.6 in LVHs (P = 0.045). There was no difference in median ICU-free days, length of stay, the risk for permanent ostomy or overall survival between the groups.
Conclusion
The reoperation rate and postoperative complication burden was higher in LVHs with no significant difference in FTR compared with HVHs.
This population-based retrospective multicentre cohort study compared results after reoperation for complications in colorectal surgery between high- and low-volume hospitals. We found the reoperation risk and cumulative burden of complications to be higher in low-volume hospitals, whereas failure to rescue remained similar.
Introduction
Colorectal surgery is associated with a risk of 20–37% postoperative complications, 6–8% emergency reoperation rate and 2–19% risk of mortality1–6. Previous studies have demonstrated improved short- and long-term outcomes for high-volume hospitals (HVHs) in complex oncological resections such as pancreatectomy, liver resections, urology, oesophagectomy7–11 and in colorectal cancer surgery12,13. Studies looking at differences in short-term outcomes have reported that postoperative complications and reoperations occur at a similar rate in HVHs versus low-volume hospitals (LVHs), and even in high- versus low-income countries14–17. The difference in short-term outcomes between HVHs and LVHs has been thought to arise mainly from mortality rate after a complication has occurred that is failure-to-rescue (FTR), which has been reported to be lower in HVHs15,18–21. However, there are also contradictory reports22,23. All studies are registry-based retrospective studies, and although they have used multivariable models to adjust outcomes, none have utilized matching of patients using propensity score. In order to improve quality of care, the mechanisms by which the outcomes are mediated are of the utmost importance for the healthcare district administration making decisions on the distribution (or centralization) of care.
The aim of this study was to assess the effect of hospital volume on reoperation rate and FTR after emergency reoperation following elective colorectal surgery in a defined district in Southern Finland. To diminish the effect of patient selection bias, propensity score matching (PSM) was performed.
Methods
Adult (over 18 years old) patients undergoing colorectal surgery between 1 January 2006 and 31 December 2017 in the Hospital District of Helsinki and Uusimaa (HUS) were screened. The HUS hospital district comprises 11 hospitals (3 university hospitals acting as both tertiary and secondary referral centres and 8 secondary referral centres) and serves a population of 1.7 million within a geographically defined area of 12,800 km2 in Southern Finland. All 11 hospitals are teaching hospitals supervising surgical residents. The hospitals work in close collaboration and patients may be transferred to another hospital for emergency operations if required, such as the need for specific surgical, anaesthetic or interventional radiological expertise. Patients were identified and the number of colorectal procedures performed annually in each hospital was obtained from the electronic patient records by interrogating the Nordic Medico-Statistical Committee (Surgical Procedural codes for elective colorectal resection (JFB20-JFB97, JFH00-JFH11, JFH96, JGB03-JGB11, JGB96-97)), which includes right- and left-sided hemicolectomies, subtotal colectomies and anterior rectal resections. Proctocolectomies and abdominoperineal resections were excluded because of centralization of these procedures. The first elective colorectal operation will be referred to as the index operation. Identified patients were assessed from the electronic records for a subsequent emergency reoperation within 30 days from the index operation, and these patients formed the final study cohort. Only operations that were due to a (suspected) complication and directly related to the index operation were considered as a reoperation. The patient records of these patients were assessed and data regarding pre-, peri- and postoperative characteristics were manually extracted. All hospitals used the same shared electronic patient record system during the study period.
Patients were divided into HVHs and LVHs depending on which hospital type the index elective operation was performed in. The cut-off used to define HVH and LVH in earlier reports has varied between five and 530 with a median of 126 operations per year24. In this study, 126 was hence used as the cut-off, yielding three hospitals classified as HVHs, also acting as university hospitals, and eight as LVHs.
The Charlson Comorbidity Index, which is a weighted index predicting 10-year survival based on patients’ co-morbidities, was calculated for all patients25. Primary outcome was FTR (defined as mortality for any cause within 90 days after a reoperation). Secondary outcomes included Comprehensive Complication Index (CCI) reflecting overall postoperative morbidity, length of stay (LOS)26, ICU-free days, permanent ostomy rate and overall survival.
FTR was defined as mortality for any cause within 90 days after a reoperation. CCI is the sum of all complications that are weighted on their severity based on the Clavien–Dindo classification (CCI=√(wC + wC2+….wCx)/2) and has values ranging from 0 to 10027. CCI was calculated for all patients within 30 days of the reoperation. CCI distribution was visualized with a boxplot. ICU-free days were defined as days alive within 30 days postoperative minus days spent in the ICU (range 0–30). LOS was defined as the time (days) between the (first) reoperation and the day of discharge. Permanent ostomy was defined as no stoma reversal within 2 years of reoperation, as nearly all reversals are performed within that time frame28. Overall survival was estimated using the Kaplan–Meier function from the reoperation until death for any reason and censored at the last day of follow-up.
PSM was carried out using variables clinically judged to impact patient selection and included age, BMI, sex, smoking, intake of oral corticosteroids, immunosuppression or anticoagulation, severe kidney disease, severe liver disease, congestive heart failure, ischaemic heart disease, chronic obstructive pulmonary disease, dementia, peripheral vascular disease, any cerebrovascular incident, diabetes mellitus, indication for surgery (diverticulosis, malignant or premalignant disease and inflammatory bowel disease) and type of resection (right- or left-sided, subtotal and rectal resection).
Match tolerance was defined as 0.2 s.d. of logit of the propensity score. Matching was done manually 1:1 using the ‘nearest neighbour’ method. For evaluating the effect of matching, the standardized mean difference (SMD) was defined before and after matching for pre- and intraoperative variables from the index operation. The groups were considered comparable if SMD was <0.10.
Categorical variables were compared using the chi-squared test, or Fisher’s exact test if expected cases in one cell were fewer than five. Normality of distribution of continuous variables was tested using the Kolmogorov–Smirnov test. As all continuous variables were non-normally distributed, they were analysed using the Mann–Whitney U test. Effect sizes for continuous non-normally distributed variables were calculated using R = Z/√(N), where <0.1 is a very small effect, 0.1–0.3 is a small effect, 0.3–0.5 is a medium effect and >0.5 is a large effect. Two-tailed P less than 0.05 was considered statistically significant. Statistical analyses were conducted using IBM SPSS software, version 27. Patients with missing values were excluded from analyses of that particular variable. The study is reported according to STROBE guidelines29.
Helsinki University Hospital Institutional Review Board approved the study. Ethical committee approval was not needed as this was a retrospective review of patient records.
Results
After excluding already centralized procedures, 9429 patients underwent an elective colorectal resection during the study period. Of these operations, 6428 (68.2%) were performed in HVHs and 3020 (32.0%) in LVHs. The exact number of operations in each hospital is reported in Table S1. A total of 382 (4.1%) patients had a reoperation within 30 days and these patients formed the final study cohort. Of these patients, 217 (56.8%) were primarily operated in HVHs and 165 (43.2%) in LVHs. The reoperation rate was 3.4% in HVHs and 5.5% in LVHs (P < 0.001). This difference in reoperation rate means a surplus of 62 reoperations in LVHs during the study period of 12 years and, on average, an excess of 0.6 reoperations per year per hospital in LVHs if compared to the rate in HVHs. In 28 patients (7.3%) the first reoperation was done in a different hospital type than the index operation (25 patients were referred from an LVH to an HVH and 3 from an HVH to an LVH. All patients were stratified to HVH or LVH based on hospital of index elective operation regardless of where the reoperation was carried out). The reasons for transfer from LVH to HVH were the need for computed tomography (n = 1), need for ICU care (n = 3) and lack of operating room resource or lack of gastrointestinal surgeon capable of dealing with the complication (n = 21).
After PSM, 284 patients, 142 in both HVHs and LVHs, remained. Patients operated in HVHs had more co-morbidities than patients in LVHs before PSM (Table 1). There were no significant differences in indications or procedures performed in the index operation. Rectal resections were slightly more common in HVHs (12.1% versus 7.1%) and protective ostomies were more frequently done in HVHs (7.0% versus 3.6%) before PSM (Table 1). After matching, SMD improved significantly for most of the variables, making the two groups comparable. This is visualized with a mirrored histogram showing the propensity score distribution and overlapping in unmatched and matched samples (Fig. 1).
Table 1.
Patient demographics, comorbidities and index operation details
| Original | Matched | |||||
|---|---|---|---|---|---|---|
| High-volume hospital (n = 214) n (%) or median (i.q.r.) |
Low-volume hospital (n = 168) n (%) or median (i.q.r.) |
SMD before matching | High-volume hospital (n = 142) n (%) or median (i.q.r.) |
Low-volume hospital (n = 142) n (%) or median (i.q.r.) |
SMD after matching | |
| Sex | ||||||
| Male | 138 (63.6) | 109 (64.9) | 0.200 | 87 (61.3) | 90 (63.4) | 0.043 |
| Female | 78 (36.4) | 59 (35.1) | 55 (38.8) | 52 (36.7) | ||
| Age (years) | 68.4 (56.9–75.4) | 65.1 (57.1–74.3) | 0.052 | 67.2 (56.2–74.5) | 64.7 (57.1–74.9) | 0.021 |
| BMI (kg/m2) | 25.3 (22.4–29.0) | 26.8 (23.8–29.8) | 0.273 | 25.6 (23.0–30.0) | 26.7 (23.7–29.7) | 0.066 |
| Current smoker | 23 (10.7) | 19 (11.3) | 0.018 | 15 (10.6) | 18 (12.7) | 0.066 |
| Medication | ||||||
| Anticoagulation | 40 (18.7) | 23 (13.7) | 0.135 | 19 (13.4) | 22 (15.5) | 0.060 |
| Immunosuppressants | 13 (6.1) | 6 (3.6) | 0.115 | 4 (2.8) | 5 (3.5) | 0.040 |
| Corticosteroids | 12 (5.6) | 8 (4.8) | 0.153 | 8 (5.6) | 7 (4.9) | 0.031 |
| Previous abdominal operations | 95 (44.4) | 58 (34.5) | 0.202 | 60 (42.3) | 50 (35.2) | 0.152 |
| Charlson Comorbidity Index | 4 (0–6) | 4 (0–6) | 0.048 | 3 (0–6) | 4 (0–6) | 0.056 |
| Atrial fibrillation | 31 (14.5) | 18 (10.7) | 0.113 | 20 (14.1) | 18 (12.7) | 0.041 |
| Ischaemic heart disease | 34 (1.4) | 20 (11.9) | 0.114 | 17 (12.0) | 16 (11.3) | 0.022 |
| Myocardial infarct | 21 (9.8) | 9 (5.4) | 0.168 | 10 (7.0) | 5 (3.5) | 0.157 |
| Congestive heart failure | 31 (14.5) | 16 (9.5) | 0.151 | 13 (9.2) | 14 (5.6) | 0.024 |
| Peripheral vascular disease | 14 (6.5) | 5 (3.0) | 0.164 | 5 (3.5) | 5 (3.5) | 0.000 |
| Dementia | 7 (3.3) | 3 (1.8) | 0.093 | 2 (1.4) | 3 (2.1) | 0.053 |
| Cerebrovascular accident/transient ischaemic attack | 17 (7.9) | 9 (5.4) | 0.103 | 6 (4.2) | 8 (5.6) | 0.065 |
| Chronic obstructive pulmonary disease | 18 (8.4) | 6 (3.6) | 0.200 | 5 (3.5) | 6 (4.2) | 0.036 |
| Connective tissue disease | 9 (4.2) | 9 (5.4) | 0.054 | 6 (4.2) | 7 (4.9) | 0.034 |
| Diabetes mellitus | 34 (15.9) | 23 (13.7) | 0.070 | 19 (13.4) | 18 (12.7) | 0.021 |
| Peptic ulcer | 6 (2.8) | 3 (1.8) | 0.067 | 4 (2.8) | 3 (2.1) | 0.045 |
| Liver disease | 7 (3.3) | 2 (1.2) | 0.137 | 4 (2.8) | 2 (1.4) | 0.098 |
| Hemiplegia | 11 (5.1) | 3 (1.8) | 0.179 | 3 (2.1) | 2 (1.4) | 0.053 |
| Solid tumour, local | 95 (44.4) | 81 (48.2) | 0.077 | 62 (43.7) | 65 (45.8) | 0.042 |
| Solid tumour, metastatic | 19 (8.9) | 15 (8.9) | 0.002 | 13 (9.2) | 13 (9.2) | 0.000 |
| Leukaemia | 2 (0.9) | 1 (0.6) | 0.038 | 1 (0.7) | 1 (0.7) | 0.000 |
| Lymphoma | 2 (0.9) | 3 (1.9) | 0.075 | 1 (0.7) | 2 (1.4) | 0.069 |
| Acquired immune deficiency syndrome | 0 | 0 | – | 0 | 0 | |
| Chronic kidney disease | 12 (5.6) | 4 (2.4) | 0.161 | 5 (3.5) | 4 (2.8) | 0.040 |
| Previous thrombosis | 16 (7.5) | 6 (3.6) | 0.168 | 8 (5.6) | 5 (3.5) | 0.101 |
| Surgical approach | ||||||
| Laparoscopy | 112 (52.3) | 65 (38.7) | 0.275 | 79 (55.6) | 58 (40.8) | 0.298 |
| Open | 71 (33.2) | 74 (44.1)) | 0.225 | 43 (30.3) | 59 (41.5) | 0.236 |
| Converted | 31 (14.5) | 29 (17.3) | 0.076 | 20 (14.1) | 25 (17.6) | 0.096 |
| Operating time (min)* | 140 (112–189) | 154 (114.0–211.3) | 0.002 | 138.0 (112.0–189.0) | 154 (110–206) | 0.004 |
| Blood loss (ml)† | 150 (50–400) | 150 (75–350) | 0.001 | 100 (50–400) | 150 (50–350) | 0.026 |
| Reason for surgery | ||||||
| Colorectal cancer or premalignant lesion | 134 (62.6) | 111 (66.1) | 0.072 | 88 (62.0) | 91 (64.1) | 0.044 |
| Diverticulosis | 49 (22.9) | 41 (24.4) | 0.035 | 38 (26.8) | 35 (24.6) | 0.048 |
| Benign large bowel obstruction | 10 (4.7) | 4 (2.4) | 0.087 | 5 (3.5) | 5(3.5) | 0.000 |
| Inflammatory bowel disease | 13 (6.1) | 6 (3.6) | 0.115 | 5 (3.5) | 6 (4.2) | 0.036 |
| Other malignancy | 3 (1.4) | 3 (1.8) | 0.006 | 3 (2.1) | 3 (2.1) | 0.000 |
| Other resection ‡ | 5 (2.3) | 3 (1.8) | 0.054 | 3 (2.1) | 3 (2.1) | 0.053 |
| Right-sided colectomy | 79 (36.9) | 55 (32.7) | 0.087 | 48 (36.6) | 50 (35.2) | 0.030 |
| Left-sided colectomy | 104 (48.6) | 92 (54.8) | 0.111 | 77 (54.2) | 73 (51.4) | 0.042 |
| Rectal resection | 26 (12.1) | 12 (7.1) | 0.125 | 9 (6.3) | 10 (7.0) | 0.028 |
| Subtotal colectomy | 10 (4.7) | 12 (7.1) | 0.106 | 8 (5.6) | 9 (6.3) | 0.030 |
| Faecal contamination | 15 (7.0) | 12 (7.1) | 0.009 | 9 (6.3) | 8 (5.6) | 0.026 |
| Primary anastomosis | 211 (98.6) | 166 (98.8) | 0.019 | 141 (99.3) | 140 (98.6) | 0.069 |
| Protective diversion | 15 (7.0) | 6 (3.6) | 0.133 | 5 (3.5) | 5 (3.5) | 0.000 |
*Seventeen patients were missing data for operating time (10 in low-volume hospitals (LVHs) and 7 in high-volume hospitals (HVHs)). †Forty-five patients were missing data for blood loss (19 in LVHs and 26 in HVHs). ‡Other: iatrogenic lesion, n = 1; trauma, n = 1; endometriosis, n = 1; rectum prolapse, n = 1; hidradenitis, n = 1; polyposis, n = 2; other infection, n = 1; other benign lesion, n = 1. SMD, standardized mean difference.
Fig. 1.
Mirrored histogram showing the propensity score distribution.
a before matching, b after matching.
The only medical history or index operation factors that did not meet the criteria of being comparable after matching (that is SMD < 0.1) were previous operations, myocardial infarction, previous thrombosis and operative technique (laparoscopy or laparotomy) (Table 1).
Details of reoperation are shown in Table 2. Intra-abdominal infection was the most common finding in reoperation in both HVHs (65.5%) and LVHs (62.0%), followed by intra-abdominal bleeding (20.4% in HVHs and 14.8% in LVHs) and fascial rupture (13.4% in LVHs and 11.3% in HVHs). Superficial wound infection was more common in LVHs than HVHs, 4.9% versus 0% (P = 0.007). There was no significant difference regarding surgical approach, blood loss or operating time. Procedures done in the reoperation were also similar for both LVHs and HVHs. Only resuturing of the anastomosis was performed more often in HVHs, 28 (19.7%) compared to 16 (11.3%) in LVHs. While time from index operation to reoperation was similar in HVHs and LVHs, more reoperations were carried out at night (between 22.00 and 8.00) in HVHs (Table 2). Although the distribution of different complications at reoperation was similar between HVHs and LVHs (shown in Table 2), the overall rate (complications per number of index elective operations) of intra-abdominal infection (93/6248 (1.4%) versus 88/3020 (2.9%), P < 0.001), bowel obstruction (8/6248 (0.1%) versus 17/3020 (0.6%), P = 0.001), wound infection (0/6248 (0%) versus 7/3020 (0.2%), P < 0.001) and fascial rupture (16/6248 (0.2%) versus 19/3020 (0.6%), P = 0.005) needing reoperation after elective colorectal surgery was higher in LVHs compared to HVHs. The rate of intra-abdominal bleeding (29/6248 (0.5%) versus 21/3020 (0.7%), P = 0.129) was similar.
Table 2.
Reoperation details in propensity score–matched cohorts
| High-volume hospital (n = 142) n (%) or median (i.q.r.) |
Low-volume hospital (n = 142) n (%) or median (i.q.r.) |
P | |
|---|---|---|---|
| Findings in reoperation* | |||
| Intra-abdominal infection | 93 (65.5) | 88 (62.0) | 0.537 |
| Intra-abdominal bleeding | 29 (20.4) | 21 (14.8) | 0.213 |
| Fascial rupture | 16 (11.3) | 19 (13.4) | 0.588 |
| Bowel obstruction | 8 (5.6) | 17 (12.0) | 0.059 |
| Wound infection (superficial) | 0 | 7 (4.9) | 0.007 |
| No findings (negative relaparotomy) | 4 (2.8) | 3 (2.1) | 0.211 |
| Surgical approach | |||
| Open | 127 (89.4) | 121 (85.2) | 0.285 |
| Laparoscopic | 5 (3.5) | 6 (4.2) | 0.758 |
| Conversion | 9 (6.4) | 11 (7.7) | 0.643 |
| Operating time (min)† | 91.0 (61.8–122.3) | 90.0 (58.0–128.0) | 0.912 |
| Blood loss (ml)‡ | 150 (10.0–300.0) | 100 (0.0–300.0) | 0.300 |
| Time to reoperation (days from index operation) | 5.2 (3.2–8.6) | 5.8 (3.2–9.2) | 0.425 |
| Time of day of reoperation | |||
| 8–16 h | 34 (23.9) | 35 (24.6) | 0.890 |
| 16–22 h | 55 (38.7) | 64 (45.1) | 0.279 |
| 22–6 h | 50 (35.2) | 34 (23.9) | 0.037 |
| Procedures | |||
| Diversion | |||
| Protective diversion | 33 (23.2) | 30 (21.1) | 0.645 |
| End ileostomy | 2 (1.4) | 7 (4.9) | 0.092 |
| End colostomy | 27 (19.0) | 27 (19.0) | 0.977 |
| Anastomotic stoma | 1 (0.7) | 2 (1.4) | 0.566 |
| Resection of anastomosis | 53 (37.3) | 52 (36.6) | 0.866 |
| New anastomosis | 29 (20.4) | 25 (17.6) | 0.526 |
| Resuturing of anastomosis | 28 (19.7) | 16 (11.3) | 0.046 |
| Fascial closure | 15 (10.6) | 20 (14.1) | 0.367 |
| Bowel resection (other than anastomosis) | 12 (8.5) | 15 (10.6) | 0.544 |
| Haemostasis and/or evacuation of haematoma* | 14 (9.9) | 9 (6.3) | 0.269 |
| Lysis of adhesions | 6 (4.2) | 11 (7.7) | 0.211 |
*Sum of percentages is over 100% as one patient may have several findings simultaneously. Bold values indicate significance (P < 0.05). †Thirteen patients were missing data for operating time (9 in LVHs and 4 in HVHs). ‡Forty-two patients were missing data for blood loss (24 in LVHs and 18 in HVHs).
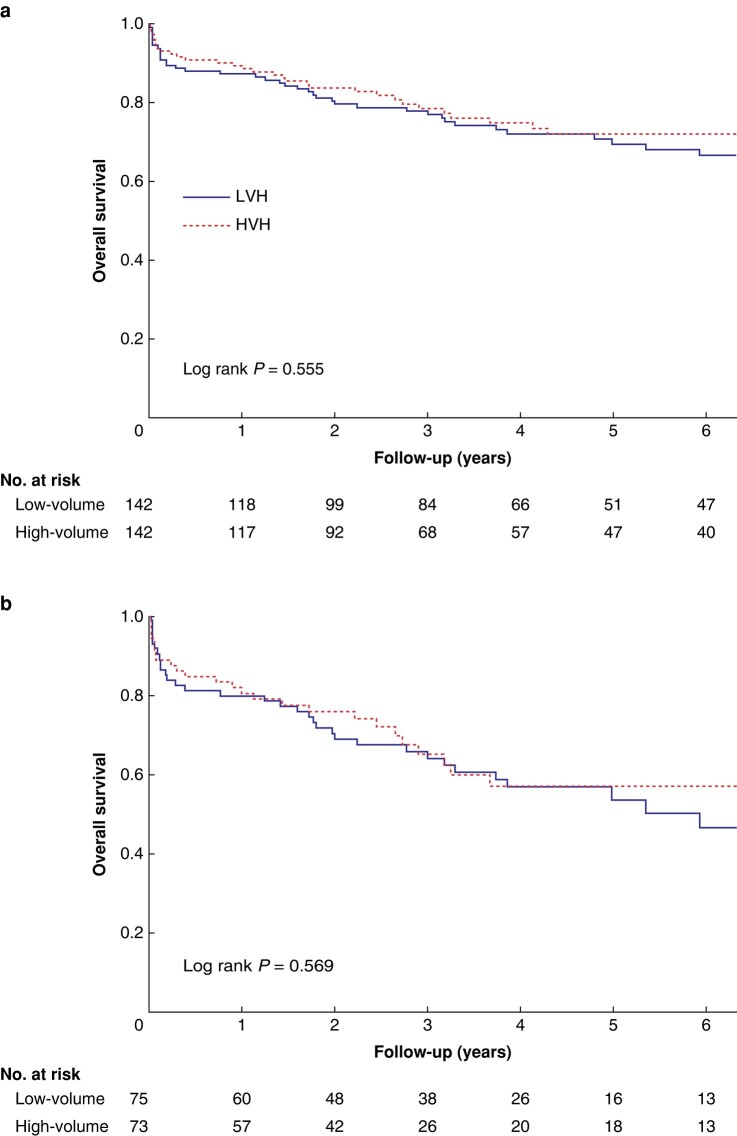
After PSM, FTR was 7.7% in HVHs and 10.6% in LVHs (P = 0.410; Table 3). Overall cumulative postoperative complications, assessed by CCI, were higher in LVHs (Table 3, Fig. S1) (median CCI 29.6 versus 21.8, P = 0.045). ICU-free days, length of hospital stay, and rate of permanent ostomy were similar for both groups (Table 3). The risk of permanent ostomy after anastomotic dehiscence was 14 of 82 patients (17.1%) for HVHs and 13 of 72 patients (18.1%) for LVHs. There was no difference in overall survival of all patients or patients with colorectal cancer within 10 years between HVHs and LVHs (Fig. 2). The median follow-up time was 40 (i.q.r. 19–82) months. Primary and secondary outcomes before matching are shown in Table S2.
Table 3.
Primary and secondary outcomes after reoperation for a complication after elective colorectal surgery in propensity score–matched cohorts
| High-volume hospital (n = 142) n (%) or median (i.q.r.) |
Low-volume hospital (n = 142) n (%) or median (i.q.r.) |
OR (95% c.i.) or effect size (r) | P | |
|---|---|---|---|---|
| FTR | 11 (7.7) | 15 (10.6) | 0.711 (0.315–1.607) | 0.410 |
| CCI | 21.8 (0.0–46.2) | 29.6 (8.7–54.1) | 0.119 | 0.045 |
| ICU-free days* | 30.0 (27.0–30.0) | 30.0 (25.0–30.0) | 0.080 | 0.179 |
| Length of stay (days) | 10.0 (6.0–17.0) | 12 (6.0–18.0) | 0.046 | 0.437 |
| Permanent stoma | 18 (12.7) | 20 (14.1) | 0.885 (0.447–1.755) | 0.727 |
*Days alive within 30 days postoperative minus all days spent in the ICU (range 0–30). Effect size for continuous non-normally distributed variables was calculated using r = Z/√(N), where <0.1 is a very small effect, 0.1–0.3 is a small effect, 0.3–0.5 is a medium effect and >0.5 is a large effect. Bold values indicate significance (P < 0.05). CCI, Comprehensive Complication Index; FTR, failure to rescue.
Fig. 2.
Survival after reoperation for colorectal surgery complication for (a) all patients and (b) patients with cancer as indication for index operation.
Discussion
In this population-based, propensity score–matched study, the reoperation rate after elective colorectal surgery was higher in LVHs compared with HVHs (3.4% versus 5.5%). This indicates approximately less than one extra reoperation per LVH per year, compared to HVHs. Although the FTR was statistically similar between HVHs and LVHs (8% versus 11%, respectively), a larger burden on cumulative complications after the reoperation was noted in LVHs (median CCI score 21.8 versus 29.6). The difference in the median scores (approximately 8 points) can be considered clinically significant as a 10-point difference in the CCI score reflects 1-grade difference in the Clavien–Dindo30. There was no difference in LOS, ICU-free days, frequency of permanent ostomy or long-term survival between the groups.
Reported reoperation rates after elective colorectal resection vary from 4.8% to 12.8%31–33. The reoperation rate for HVHs in this study was 3.4%, which could, therefore, be considered low, whereas the reoperation risk for LVHs was 5.5%, which is similar to previous reports. Similarly, the FTR rate in this study can be considered low in HVHs (8%) and average in LVHs (11%) compared to the literature reporting FTR for only elective operations between 7.9% and 13.2%34. A large Dutch study reported a variance of 0–29% in the FTR for all postoperative complications (not just reoperations), depending on hospital status. This suggests that HVHs are better at managing complications even though they seem to occur at similar rates regardless of hospital volume15,21,35. The authors' results do not support these results, as there was a significantly higher reoperation rate in LVHs. Of patients needing reoperation in LVHs, 16% were transferred to HVHs for reoperation, which might have decreased the FTR rates of LVHs and could explain the difference with earlier reports of higher FTR in LVHs. The exact reasons for the higher reoperation rate in LVHs remain unclear. No single complication type could be found to contribute more than another and the rate of different types of complications (infection, fascial rupture, bowel obstruction) were increased in LVHs. The incidence of negative relaparotomy was similar for HVHs and LVHs. Even though the FTR rate was unaffected, the increased postoperative morbidity among LVHs remains a concern. The reasons for increased morbidity can only be speculated. While the median time from index operation to reoperation was similar in both HVHs and LVHs, reoperations occurred more frequently during the night (between 22.00 and 8.00) in HVHs. This might reflect the better night-time resources in HVHs, which could have contributed to the lower postoperative morbidity in HVHs. These results support some of the previous reports showing reduced overall morbidity in favour of HVHs12.
Many reports show an inverse correlation between LOS and hospital volume for all patients after colorectal surgery36,37. No clear correlation was found in our study. Median LOS for colorectal surgery in general, including also non-complicated cases, varies between five and 1438–40. However, the results are not comparable with this study, because this cohort only comprises patients with at least CD 3b complications (that is, needing a reoperation) and LOS is expected to be higher after colorectal surgery in general (including patients who do not need reoperation). The risk of permanent ostomy was similar for HVHs and LVHs (13–14%). Relaparotomy increases the risk of open abdomen and stomas especially after anastomotic leak with high rates (27–57%) of permanent stoma41. The rate of permanent stoma in this study can be considered low, but this cohort also included patients without anastomotic dehiscence.
This study has certain limitations. This is a retrospective study with an inherent risk of bias, such as patient selection bias and potential imbalance of prognostic factors. Although PSM was used to mitigate the bias, PSM does not balance the unmeasured and unknown confounders. However, this study was not registry-based like many other similar studies, and all patients’ records were screened and data extracted manually, which improves data quality. The study cohort included all patients undergoing elective colorectal resection for various indications, which can be considered both a limitation and a strength. Interpreting mixed data is difficult, increasing the risk for bias. However, by only focusing on cancer patients, 55% of patients undergoing colorectal surgery would have been left out of the analyses. A major strength of this study is the population-based approach including all patients and public hospitals within a defined geographical area.
The implications of the study are multidimensional. The reoperation rate was higher in LVHs compared to HVHs. In contrast, the FTR rate was similar, which suggests that LVHs have capabilities (including the option of patient transfer to HVHs) to deal with colorectal complications that require a reoperation. This study does not support further centralization within this national population even though the reoperation rate was lower in HVHs. Removing elective colorectal surgery from LVHs could have negative consequences for the healthcare service in the area, so other options in improving care should be sought. These could include closer collaboration, standardized perioperative care and joint multidisciplinary teamwork between LVHs and HVHs. The colorectal surgeon teams in a hospital should be large enough to support specialization and out-of-hours availability. Of note, the earlier studies’ findings of equal reoperation rate but increased FTR in LVHs14,18,19 was not supported by these data. This implies that the driving mechanisms behind differences in outcomes between LVHs and HVHs are not universal and likely differ from country to country. This reinforces the need for the national healthcare system to have knowledge regarding the outcomes of HVHs versus LVHs in order to improve the overall care for all patients.
Supplementary Material
Acknowledgements
The authors thank biostatistician Olavi Koivisto from the University of Helsinki for help in statistical analyses and propensity score matching.
Contributor Information
Marie T Grönroos-Korhonen, Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland; Gastroenterological Surgery, Päijät-Häme Central Hospital, Lahti, Finland.
Laura E Koskenvuo, Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Panu J Mentula, Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Taina P Nykänen, Gastroenterological Surgery, Hyvinkää Hospital, Helsinki, Finland.
Selja K Koskensalo, Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Ari K Leppäniemi, Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Ville J Sallinen, Gastroenterological Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland; Transplantation and Liver Surgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Funding
This study was financially supported by Finnish Government Research Funds. Dr Grönroos-Korhonen received a grant from M I Turunen Relief Association. Dr Koskenvuo reports grants from Mary and Georg Ehrnrooth’s Foundation and grants from the Cancer Foundation Finland (Syöpäsäätiö), outside the submitted work. Dr Sallinen reports grants from Mary and Georg Ehrnrooth’s Foundation, Vatsatautien tutkimussäätiö Foundation, Cancer Foundation Finland (Syöpäsäätiö) and Academy of Finland.
Disclosure
All authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
Study permissions do not allow sharing of individual patient data.
Author contributions
Marie Grönroos-Korhonen (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Visualization, Writing—original draft, Writing—review & editing), Laura Koskenvuo (Investigation, Methodology, Project administration, Supervision, Writing—review & editing), Panu Mentula (Formal analysis, Methodology, Project administration, Supervision, Writing—review & editing), Taina Nykänen (Conceptualization, Validation, Writing—review & editing), Selja Koskensalo (Data curation, Validation, Writing—review & editing), Ari Leppäniemi (Funding acquisition, Project administration, Writing—review & editing), and Ville Sallinen (Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing—original draft, Writing—review & editing)
References
- 1. Cone MM, Herzig DO, Diggs BS, Dolan JP, Rea JD, Deveney KE et al. Dramatic decreases in mortality from laparoscopic colon resections based on data from the Nationwide Inpatient Sample. Arch Surg 2011;146:594–599 [DOI] [PubMed] [Google Scholar]
- 2. Longo WE, Virgo KS, Johnson FE, Oprian CA, Vernava AM, Wade TP et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum 2000;43:83–91 [DOI] [PubMed] [Google Scholar]
- 3. Ragg JL, Watters DA, Guest GD. Preoperative risk stratification for mortality and major morbidity in major colorectal surgery. Dis Colon Rectum 2009;52:1296–1303 [DOI] [PubMed] [Google Scholar]
- 4. Michaels AD, Mullen MG, Guidry CA, Krebs ED, Turrentine FE, Hedrick TL et al. Unplanned reoperation following colorectal surgery: indications and operations. J Gastrointest Surg 2017;21:1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bokey EL, Chapuis PH, Fung C, Hughes WJ, Koorey SG, Brewer D et al. Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum 1995;38:480–486, discussion 486–487 [DOI] [PubMed] [Google Scholar]
- 6. Ricciardi R, Roberts PL, Read TE, Marcello PW, Hall JF, Schoetz DJ. How often do patients return to the operating room after colorectal resections? Colorectal Dis 2012;14:515–521 [DOI] [PubMed] [Google Scholar]
- 7. Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–1137 [DOI] [PubMed] [Google Scholar]
- 8. Grande P, Campi R, Rouprêt M. Relationship of surgeon/hospital volume with outcomes in uro-oncology surgery. Curr Opin Urol 2018;28:251–259 [DOI] [PubMed] [Google Scholar]
- 9. Abdelsattar ZM, Habermann E, Borah BJ, Moriarty JP, Rojas RL, Blackmon SH. Understanding failure to rescue after esophagectomy in the United States. Ann Thorac Surg 2020;109:865–871 [DOI] [PubMed] [Google Scholar]
- 10. El Amrani M, Clement G, Lenne X, Farges O, Delpero JR, Theis D et al. Failure-to-rescue in patients undergoing pancreatectomy: is hospital volume a standard for quality improvement programs? Nationwide analysis of 12,333 patients. Ann Surg 2018;268:799–807 [DOI] [PubMed] [Google Scholar]
- 11. Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg 2003;138:721–725, discussion 726 [DOI] [PubMed] [Google Scholar]
- 12. Huo YR, Phan K, Morris DL, Liauw W. Systematic review and a meta-analysis of hospital and surgeon volume/outcome relationships in colorectal cancer surgery. J Gastrointest Oncol 2017;8:534–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeo HL, Abelson JS, Mao J, O’Mahoney PR, Milsom JW, Sedrakyan A. Surgeon annual and cumulative volumes predict early postoperative outcomes after rectal cancer resection. Ann Surg 2017;265:151–157 [DOI] [PubMed] [Google Scholar]
- 14. GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery . Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet 2021;397:387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care 2011;49:1076–1081 [DOI] [PubMed] [Google Scholar]
- 16. van Erning FN, van Steenbergen LN, van den Broek WT, Rutten HJ, Lemmens VE. No difference between lowest and highest volume hospitals in outcome after colorectal cancer surgery in the southern Netherlands. Eur J Surg Oncol 2013;39:1199–1206 [DOI] [PubMed] [Google Scholar]
- 17. Burns EM, Bottle A, Aylin P, Darzi A, Nicholls RJ, Faiz O. Variation in reoperation after colorectal surgery in England as an indicator of surgical performance: retrospective analysis of Hospital Episode Statistics. BMJ 2011;343:d4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diers J, Wagner J, Baum P, Lichthardt S, Kastner C, Matthes N et al. Nationwide in-hospital mortality rate following rectal resection for rectal cancer according to annual hospital volume in Germany. BJS Open 2020;4:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diers J, Baum P, Matthes H, Germer CT, Wiegering A. Mortality and complication management after surgery for colorectal cancer depending on the DKG minimum amounts for hospital volume. Eur J Surg Oncol 2021;47:850–857 [DOI] [PubMed] [Google Scholar]
- 20. Spolverato G, Gennaro N, Zorzi M, Rugge M, Mescoli C, Saugo M et al. Failure to rescue as a source of variation in hospital mortality after rectal surgery: the Italian experience. Eur J Surg Oncol 2019;45:1219–1224 [DOI] [PubMed] [Google Scholar]
- 21. Henneman D, van Leersum NJ, Ten Berge M, Snijders HS, Fiocco M, Wiggers T et al. Failure-to-rescue after colorectal cancer surgery and the association with three structural hospital factors. Ann Surg Oncol 2013;20:3370–3376 [DOI] [PubMed] [Google Scholar]
- 22. Lillo‐Felipe M, Ahl Hulme R, Forssten MP, Bass GA, Cao Y, Matthiessen P et al. Center‐level procedure volume does not predict failure‐to‐rescue after severe complications of oncologic colon and rectal surgery. World J Surg 2021;45:3695–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Almoudaris AM, Burns EM, Mamidanna R, Bottle A, Aylin P, Vincent C et al. Value of failure to rescue as a marker of the standard of care following reoperation for complications after colorectal resection. Br J Surg 2011;98:1775–1783 [DOI] [PubMed] [Google Scholar]
- 24. van Gijn W, Gooiker GA, Wouters MW, Post PN, Tollenaar RA, van de Velde CJ. Volume and outcome in colorectal cancer surgery. Eur J Surg Oncol 2010;36:S55–S63 [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: a critical review of clinimetric properties. Psychother Psychosom 2022;91:8–35 [DOI] [PubMed] [Google Scholar]
- 26. Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 2010;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7 [DOI] [PubMed] [Google Scholar]
- 28. Salusjärvi JM, Koskenvuo LE, Mali JP, Mentula PJ, Leppäniemi AK, Sallinen VJ. Stoma reversal after Hartmann's procedure for acute diverticulitis. Surgery 2022;173:920–926 [DOI] [PubMed] [Google Scholar]
- 29. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BPL, Breitenstein S et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–762, discussion 762–763 [DOI] [PubMed] [Google Scholar]
- 31. Visser BC, Keegan H, Martin M, Wren SM. Death after colectomy: it’s later than we think. Arch Surg 2009;144:1021–1027 [DOI] [PubMed] [Google Scholar]
- 32. Adam MA, Turner MC, Sun Z, Kim J, Ezekian B, Migaly J et al. The appropriateness of 30-day mortality as a quality metric in colorectal cancer surgery. Am J Surg 2018;215:66–70 [DOI] [PubMed] [Google Scholar]
- 33. Vogelsang RP, Bojesen RD, Hoelmich ER, Orhan A, Buzquurz F, Cai L et al. Prediction of 90-day mortality after surgery for colorectal cancer using standardized nationwide quality-assurance data. BJS Open 2021;5:zrab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mik M, Magdzinska J, Dziki L, Tchorzewski M, Trzcinski R, Dziki A. Relaparotomy in colorectal cancer surgery–do any factors influence the risk of mortality? A case controlled study. Int J Surg 2014;12:1192–1197 [DOI] [PubMed] [Google Scholar]
- 35. Johnston MJ, Arora S, King D, Bouras G, Almoudaris AM, Davis R et al. A systematic review to identify the factors that affect failure to rescue and escalation of care in surgery. Surgery 2015;157:752–763 [DOI] [PubMed] [Google Scholar]
- 36. Lee JA, Kim SY, Park K, Park EC, Park JH. Analysis of hospital volume and factors influencing economic outcomes in cancer surgery: results from a population-based study in Korea. Osong Public Health Res Perspect 2017;8:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siragusa L, Sensi B, Vinci D, Franceschilli M, Pathirannehalage Don C, Bagaglini G et al. Volume–outcome relationship in rectal cancer surgery. Discov Oncol 2021;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aravani A, Samy EF, Thomas JD, Quirke P, Morris EJA, Finan PJ. A retrospective observational study of length of stay in hospital after colorectal cancer surgery in England (1998–2010). Medicine (Baltimore) 2016;95:e5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kelly M, Sharp L, Dwane F, Kelleher T, Comber H. Factors predicting hospital length-of-stay and readmission after colorectal resection: a population-based study of elective and emergency admissions. BMC Health Serv Res 2012;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forsmo HM, Pfeffer F, Rasdal A, Østgaard G, Mohn AC, Körner H et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis 2016;18:603–611 [DOI] [PubMed] [Google Scholar]
- 41. Thornton M, Joshi H, Vimalachandran C, Heath R, Carter P, Gur U et al. Management and outcome of colorectal anastomotic leaks. Int J Colorectal Dis 2011;26:313–320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study permissions do not allow sharing of individual patient data.