Abstract
Aims
Pulmonary vein isolation (PVI) is increasingly performed in patients with atrial fibrillation (AF). Both AF phenotype and left atrial (LA) volume have been shown to influence ablation outcome. The inter-relationship of the two is incompletely understood. We aimed to investigate the impact of AF phenotype vs. LA volume on outcome after PVI.
Methods and results
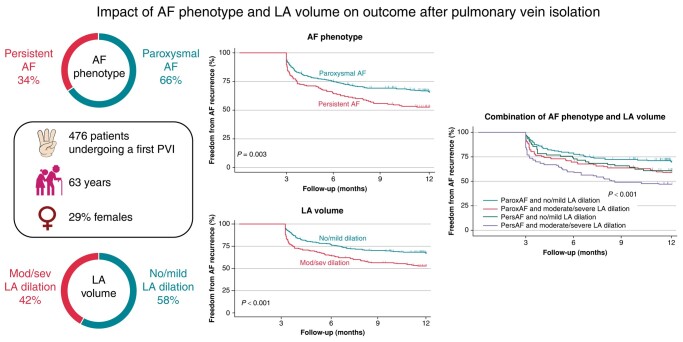
In a retrospective analysis of a prospective registry of patients undergoing a first PVI, the association of AF phenotype and LA volume index (LAVI) was assessed as well as their impact on AF recurrence during follow-up. Overall, 476 patients were enrolled (median age 63 years, 29% females, 65.8% paroxysmal AF). Obesity, hypertension, chronic kidney disease, and heart failure were all significantly more frequent in persistent AF. After 1 year, single-procedure, freedom from arrhythmia recurrence was 61.5%. Patients with paroxysmal AF had better outcomes compared with patients with persistent AF (65.6 vs. 52.7%, P = 0.003), as had patients with no/mild vs. moderate/severe LA dilation (LAVI <42 mL/m2 67.1% vs. LAVI ≥42 mL/m2 53%, P < 0.001). The combination of both parameters refined prediction of 1-year recurrence (P < 0.001). After adjustment for additional clinical risk factors in multivariable Cox proportional hazard analysis, both AF phenotype and LAVI ≥42 mL/m2 contributed significantly towards the prediction of 1-year recurrence.
Conclusion
Atrial fibrillation phenotype and LA volume are independent predictors of outcome after PVI. Persistent AF with no/mild LA dilation has a similar risk of recurrence as paroxysmal AF with a moderate/severe LA dilation and should be given similar priority for ablation.
Keywords: Atrial fibrillation (AF), Pulmonary vein isolation (PVI), Left atrial volume (LAV), Left atrial volume index (LAVI)
Graphical Abstract
Graphical Abstract.
What’s new?
Combination of atrial fibrillation (AF) phenotype and left atrial (LA) volume index has prognostic value for AF recurrence after pulmonary vein isolation.
Patients with persistent AF and no/mild LA dilation have outcomes comparable with patients with paroxysmal AF and moderate/severe LA dilation.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in adults and responsible for significant morbidity including recurrent hospitalizations. The current prevalence is 2–4% in the general population.1 Pulmonary vein isolation (PVI) is superior to anti-arrhythmic drugs with regard to both symptom control and prevention of arrhythmia recurrence.2,3 However, despite improved technologies, recurrence rates after PVI remain high.4,5
Several risk factors for AF recurrence after PVI have been identified including AF phenotype, left atrial (LA) dilation, AF duration, age, sleep apnoea, obesity, renal dysfunction, and advanced atrial cardiomyopathy on magnetic resonance imaging (MRI). Among those, an increased LA volume is one of the best predictors for both progression from paroxysmal AF (paroxAF) to persistent AF (persAF) and for worse outcomes after PVI. While PVI is recommended as a first-line therapy in patients with paroxAF in the current guidelines, the role of PVI in patients with persAF is less well established due to worse outcomes compared with patients with paroxAF.1,6,7
In clinical practice, patients may present with persAF and a non-dilated LA and have good ablation outcomes after PVI. Conversely, patients with paroxAF and a severely dilated LA may show poor outcomes after PVI. Accordingly, focusing on AF phenotype as the primary criterion for patient selection for catheter ablation of AF may be inadequate. The inclusion of LA volume in clinical decision-making for AF ablation may improve patient selection.1,8,9
This study therefore aimed to investigate the inter-relationship of AF phenotype and LA volume on outcomes after PVI in ablation naïve patients.
Methods
Study sample
This retrospective analysis of a prospective registry was conducted in a tertiary care ablation centre in Switzerland (Inselspital University Hospital Bern). Patients undergoing a first PVI between January 2017 and April 2020 were enrolled. Patients without a pre-procedural echocardiography enabling measurement of LA volume index (LAVI) and patients with LA lesion sets beyond PVI were excluded from the study. This study was approved by the local Ethics Committee and complies with the principles of the Declaration of Helsinki. The authors vouch for data integrity.
Baseline evaluation
Each patient underwent a pre-procedural evaluation, including detailed assessment of clinical status, and standard blood tests. Patients were classified as paroxysmal if the duration of AF was <7 days and as persistent if AF lasted >7 days.1 A two-dimensional transthoracic echocardiography (TTE) was performed, with acquisition of images in parasternal and apical views according to the guidelines from the American Society of Echocardiography.10 The LA volume was measured from the temporal frame just prior to mitral valve opening on the four-chamber view and the two-chamber view. The resulting LA volume was calculated by the disk summation method of Simpson and adjusted for the patient’s body surface to calculate the LAVI.10
Peri-procedural examination, sedation, and left atrial access
Before the procedure, patients underwent transoesophageal echocardiography and/or computed tomography (CT) to exclude intra-cardiac thrombi and to obtain a detailed understanding of the left trial anatomy. Deep conscious sedation using midazolam, fentanyl, and propofol was used, guided by a physician-led, nurse-administered protocol.11 A small subset of patients with a high risk of sedation complications underwent general anaesthesia. Left atrial access was obtained by fluoroscopy-guided transseptal puncture using a standard transseptal sheath. Heparin was administered to maintain an activated clotting time above 350 s during the procedure.
Protocol for cryoballoon ablation
Cryoablation procedures were performed with a 28 mm cryoballoon catheter (Arctic Front Advance), a 20 mm circular mapping catheter (Achieve Advance), and a steerable sheath (FlexCath Advance; all Medtronic, Minneapolis, MN, USA). In case of an effective freeze (judged by the disappearance of all local pulmonary vein (PV) signals before 60s or reaching a temperature of −40°C), cryoablation was continued for 2 additional minutes after effect (‘time-to-effect plus 2 min strategy’).12 In case of an ineffective freeze, the ablation was stopped and the balloon repositioned, aiming for better occlusion of the PV. Pulmonary vein isolation was verified at the end of the procedure with the assessment of Entrance- and Exit-Block in all PVs using the circular mapping catheter.
Protocol for radiofrequency ablation
Radiofrequency procedures were performed using a three-dimensional (3D) mapping system (CARTO3, Biosense Webster, Irvine, CA, USA) in combination with a contact force-sensing ablation catheter (Smarttouch SF, Biosense Webster) and a high-density multipolar mapping catheter (Pentaray, Biosense Webster). A steerable sheath was used (Destino Reach, Oscor, Palm Harbor, FL, USA). Ablation was performed by adhering to the CLOSE protocol.13 Pulmonary vein isolation was verified by 3D mapping at the end of the procedure.
Follow-up
Patients were followed with 7-day Holter electrocardiograms after 3, 6, and 12 months. Cessation of anti-arrhythmic therapy was either recommend immediately after the procedure or the latest by 3 months after the procedure. The primary endpoint was the first recurrence of any atrial tachyarrhythmia (AF, atrial flutter, or atrial tachycardia) lasting longer than 30 s after a blanking period of 3 months.1 Repeat ablation during the blanking period was considered to be a primary endpoint event.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or as median and inter-quartile range (IQR) and as count and percentages, as appropriate. Comparisons between groups were made using the χ2 test (categorical variables) and Wilcoxon rank-sum test (continuous variables).
Univariate Cox regression analysis was used to identify hazard ratios (HRs) for individual predictors of arrhythmia-free survival. Multivariable Cox proportional hazards models were used to identify independent predictors of AF recurrence during 1 year of follow-up. Variables were included based on clinical relevance and literature review. Kaplan–Meier analyses were used to assess AF recurrence over a period of 365 days, across strata of AF phenotype, LA size, and the combination of AF phenotype and LA size. The log-rank and stratified log-rank test was used to assess the significant differences across strata. A P-value cut-off of <0.05 was used to indicate statistical significance. The statistical analyses were conducted using STATA 18 (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX, USA: StataCorp LLC).
Results
Patient and procedural characteristics
Between 2017 and 2019, 597 patients underwent a first PVI for AF in our centre. Eighteen patients were excluded from the analysis due to prior ablation in an external hospital, 24 patients for LA substrate ablation in addition to PVI, and 76 patients due to insufficient TTE image quality not allowing LAVI measurement (see Supplementary material online, Figure S1). Three patients were lost to follow-up, leaving 476 patients for analysis.
Baseline and procedural characteristics according to AF phenotype are summarized in Tables 1 and 2. The majority of patients had paroxAF (n = 313, 65.7%). The median age was 63 years (IQR 56–70), and 138 (29%) were female. When compared with patients with persAF, patients with paroxAF had lower body mass index and were less likely to have heart failure, chronic kidney disease, and previous cardioversion. They were more likely to have a left ventricular ejection fraction >50%. The CHA2DS2-VASc score, AF symptom severity, and symptom status graded by the European Heart Rhythm Association symptom scale were also different between the two groups (P < 0.001 and P = 0.04, respectively). Overall 64% of the patients underwent radiofrequency ablation and 36% underwent cryoablation.
Table 1.
Baseline characteristics of the patients overall and according to AF phenotype
| All patients N = 476 |
Paroxysmal AF N = 313 |
Persistent AF N = 163 |
P-value | |
|---|---|---|---|---|
| Age (years) | 63 (56–70) | 62 (55–69) | 64 (57–71) | 0.1 |
| Female gender | 138 (29) | 94 (30.0) | 44 (27) | 0.48 |
| BMI (kg/m2) | 27 (25–31) | 27 (24–30) | 29 (25–32) | <0.001 |
| Hypertension | 290 (61) | 179 (57) | 111 (68) | 0.02 |
| Coronary artery disease | 60 (13) | 34 (10) | 26 (15) | 0.11 |
| Heart failure | 97 (20) | 29 (09) | 68 (41) | <0.001 |
| Diabetes | 37 (8) | 21 (7) | 16 (10) | 0.22 |
| GFR < 60 mL/min | 82 (17) | 40 (13) | 42 (26) | <0.001 |
| CHA2DS2-VASc score | 2 (1–3) | 2 (1–3) | 2 (1–3) | <0.001 |
| EHRA score | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.04 |
| AF duration (months) | 16.5 (5–48) | 17 (4–49) | 16 (6–42) | 0.8 |
| Previous cardioversion | 158 (33) | 52 (16) | 106 (65) | <0.001 |
| Left ventricular EF (%) | 60 (50–60) | 60 (55–65) | 55 (43–60) | <0.001 |
| Left ventricular EF <50% | 86 (18) | 28 (32) | 58 (67) | <0.001 |
| Left atrial diameter (mm) | 44 (39–48) | 43 (37–47) | 47 (42–52) | <0.001 |
| Left atrial volume index (mL/m2) | 38 (29–49) | 35 (28–46) | 44 (32–55) | <0.001 |
| Moderate/severe LA dilation | 199 (42) | 105 (34) | 94 (58) | <0.001 |
| Radiofrequency ablation | 307 (64) | 214 (68.3) | 93 (57.0) | 0.014 |
| Cryoballoon ablation | 169 (35) | 99 (31.6) | 70 (42.9) | 0.014 |
| Number of patients with a complete 12 months follow-up, n (%) | 420 (88.3) | 269 (86) | 151 (92) | 0.03 |
Values are presented as median (IQR) or n (%). Moderate/severe LA dilatation if LAVI ≥42 mL/m2.
AF, atrial fibrillation; BMI, body mass index; EF, ejection fraction; EHRA, European Heart Rhythm Association; GFR, glomerular filtration rate; IQR, inter-quartile range; LA, left atrial; LAVI, LA volume index.
Table 2.
Cox regression analysis to predict recurrence during 1 year of follow-up
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Persistent AF | 1.53 (1.1–2.0) | 0.004 | 1.39 (1.03–1.88) | 0.03 |
| Age (years) | 1.10 (0.9–1.0) | 0.21 | 1.00 (0.98–1.01) | 0.7 |
| Female gender | 1.17 (0.9–1.6) | 0.30 | 1.20 (0.87–1.66) | 0.24 |
| BMI (≥30 kg/m2) | 1.26 (0.9–1.7) | 0.14 | ||
| Hypertension | 1.24 (0.9–1.7) | 0.15 | ||
| Coronary artery disease | 1.51 (1.03–2.2) | 0.03 | 1.39 (0.94–2.06) | 0.09 |
| Chronic kidney disease | 1.29 (0.9–1.8) | 0.16 | ||
| Heart failure | 1.00 (0.7–1.5) | 0.95 | ||
| Diabetes | 1.16 (0.7–1.9) | 0.56 | ||
| CHA2DS2-VASc score ≥2 | 1.1 (1.0–1.2) | 0.04 | ||
| AF duration (months) | 1.00 (0.99–1.00) | 0.06 | ||
| LVEF <50% | 1.08 (0.7–1.6) | 0.66 | ||
| Moderate/severe LA dilation | 1.82 (1.25–2.65) | 0.002 | 1.47 (1.08–1.99) | 0.01 |
Values are presented as median (IQR) or n (%). Moderate/severe LA dilatation if LAVI ≥42 mL/m2.
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; HR, hazard ratio; IQR, inter-quartile range; LVEF, left ventricular ejection fraction; LA, left atrial; LAVI, LA volume index.
Association of atrial fibrillation phenotype and left atrial dimensions
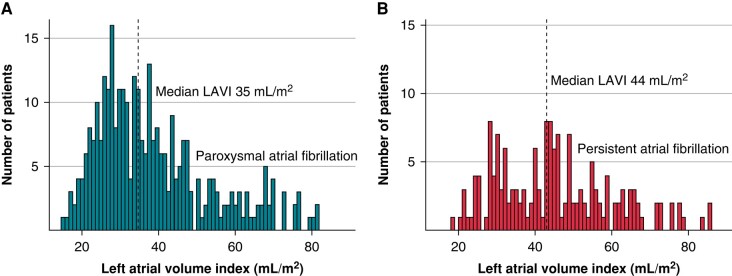
Overall, the median LAVI was 38 mL/m2 (IQR 29–49 mL/m2), and the median LA diameter was 44 mm (IQR 39–48 mm). Patients with persAF had higher LAVI [median 44 (32–55) vs. median 35 (28–46), P < 0.001] and higher LA diameter [median 47 (42–52) vs. median 43 (37–47), P < 0.001] compared with paroxAF patients. There was however a relevant overlap between the two groups (Figure 1).
Figure 1.
Medians for paroxysmal and persistent AF individually: (A) paroxysmal AF and (B) persistent AF. The dotted lines indicate the median LAVI values for paroxysmal and persistent AF. AF, atrial fibrillation; LAVI, left atrial volume index.
Overall, 41% of patients had a normal LAVI (<35 mL/m2), 17% a mildly dilated LAVI (35–41 mL/m2), 16% a moderately dilated LAVI (42–48 mL/m2), and 26% a severely dilated LAVI (>48 mL/m2). In patients with paroxAF, the distribution was 47, 19, 14, and 20%. In patients with persAF, the distribution was 29, 13, 20, and 37%.
Impact of atrial fibrillation phenotype vs. left atrial volume on outcome after pulmonary vein isolation
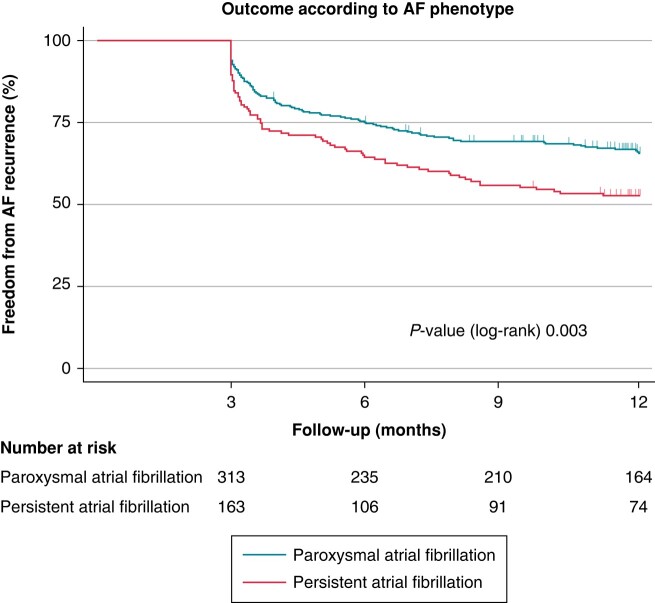
In Kaplan–Meier analysis, freedom from AF recurrence 1 year after ablation was 61.5%. Freedom from AF recurrence was higher in patients with parox AF compared with persAF (65.6 vs. 52.7%, P = 0.003, Figure 2).
Figure 2.
Freedom from AF recurrence after PVI according to AF phenotype. Kaplan–Meier estimates showing the recurrence of AF after PVI in patients with paroxysmal AF and persistent AF. Numbers at risk are indicated below the Kaplan–Meier curves. AF, atrial fibrillation; PVI, pulmonary vein isolation.
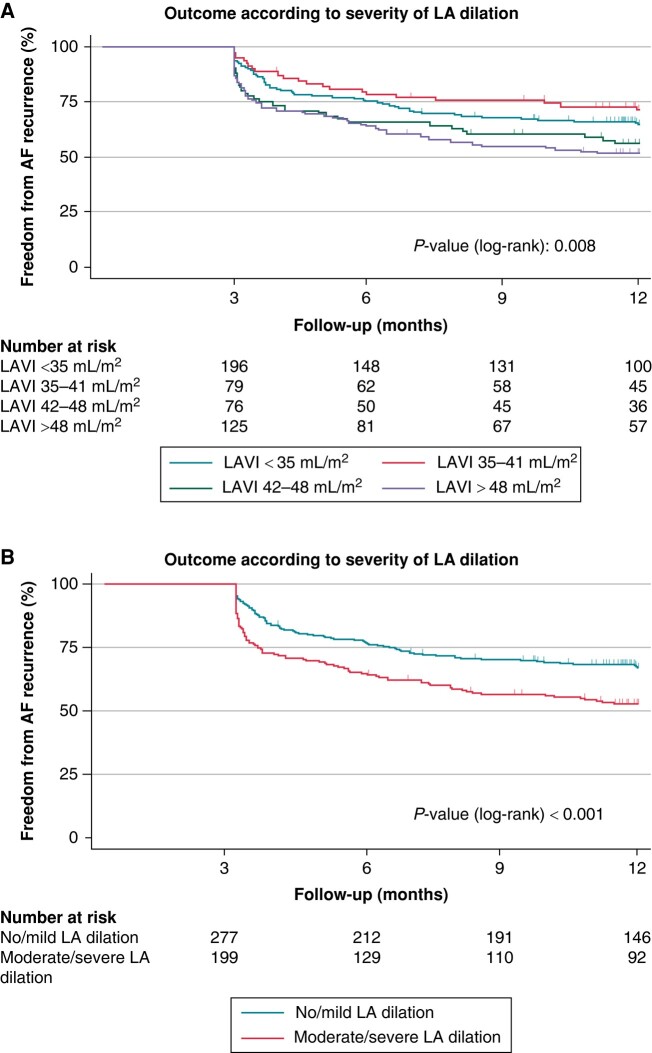
Freedom from AF recurrence according to the degree of LA dilation is shown in Figure 3A. Importantly, freedom from AF recurrence was higher in patients with no/mild LA dilation compared with patients with moderate/severe LA dilation (67.1 vs. 53%, P < 0.001, Figure 3B).
Figure 3.
Freedom from AF recurrence after PVI according to LA volume. (A) Outcomes according to the degree of LA dilation and (B) outcomes comparing no/mild LA dilation with moderate/severe LA dilation. (A) Kaplan–Meier estimates showing the recurrence of AF after PVI in patients with normal left atrial volume (LAVI <35 mL/m2), mild LA dilation (LAVI 35–41 mL/m2), moderate LA dilation (LAVI 42–48 mL/m2), and severe LA dilation (LAVI >48 mL/m2). Numbers at risk are indicated below the Kaplan–Meier curves. AF, atrial fibrillation; LA, left atrial; LAVI, LA volume index; PVI, pulmonary vein isolation.
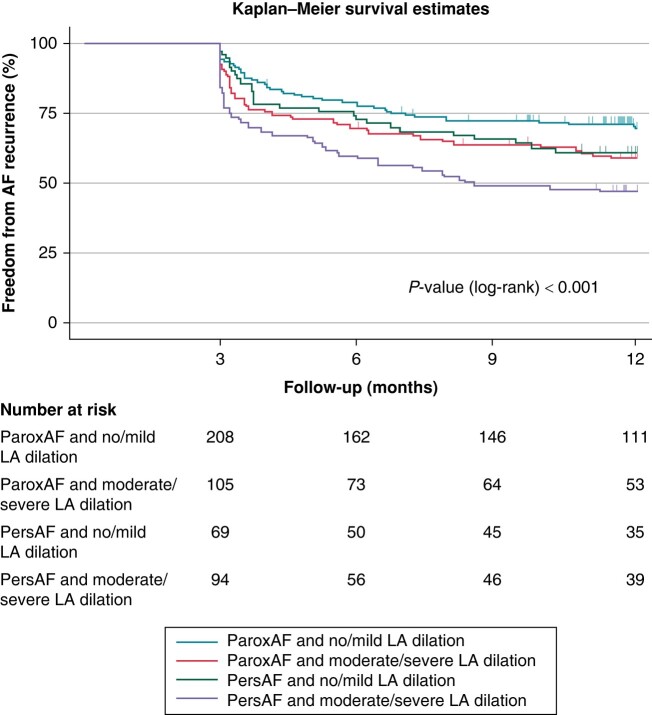
The combination of AF phenotype (paroxAF or persAF) and AF volume (no/mild vs. moderate/severe LA dilation) further refined the prediction of outcomes after PVI (P < 0.001, Figure 4). Freedom from AF after 1 year was highest in patients with paroxAF and no/mild LA dilation (69.1%) and lowest in patients with persAF and moderate/severe LA dilation (47%). No difference was found between patients with paroxAF and moderate/severe LA dilation and those with persAF and no/mild LA dilation (58.4 vs. 61%, P = 0.67).
Figure 4.
Freedom from AF recurrence after PVI according to the combination of AF phenotype and LA volume. Kaplan–Meier estimates showing the recurrence of AF after PVI in patients with paroxysmal AF and no/mild left atrial dilation, paroxysmal AF and moderate/severe LA dilation, persistent AF and no/mild LA dilation, and persistent AF and moderate/severe LA dilation. Numbers at risk are indicated below the Kaplan–Meier curves. AF, atrial fibrillation; LA, left atrial; PVI, pulmonary vein isolation.
A sensitivity analysis for the subgroups of patients undergoing cryoablation and radiofrequency ablation was performed. In both subgroups, a similar pattern of the impact of AF phenotype and LA volume was found (see Supplementary material online, Figures S2–S4).
Predictors of atrial fibrillation recurrence in multivariable analysis
Univariable Cox regression analysis showed persAF, moderate/severe LA dilation (LAVI ≥42 mL/m2), presence of coronary artery disease (CAD), and CHA2DS2-VASc score to be predictors of AF recurrence at 1 year (Table 2). In multivariable Cox proportional regression analysis, persAF [HR 1.39, 95% confidence interval (CI) 1.03–1.8, P = 0.03] and moderate/severe LA dilation (LAVI ≥42 mL/m2; HR 1.7, 95% CI 1.08–1.99, P = 0.01) remained independent predictors of AF recurrence (Table 2) after adjusting for age, sex, and CAD.
Discussion
Our study aimed to assess the interplay of AF phenotype and LA volume on outcomes after PVI. We report the following major findings.
First, the median LA volume was higher in patients with persAF compared with paroxAF, linking AF volume with AF progression. There was however a significant overlap in LA volume between the two groups. Second, we found a better 1-year freedom from arrhythmia recurrence in patients with paroxAF as opposed to persAF (65.6 vs. 52.7%, P = 0.003) as well as in patients with no/mild vs. moderate/severe dilated left atria (67.1 vs. 53%, P < 0.001). Third, the combination of both parameters refined prediction of 1-year recurrence (P < 0.001). Last, both AF phenotype and dilated LA remained independent predictors of 1-year recurrence after adjustment for additional clinical risk factors in multivariable Cox proportional hazard analysis.
Our study corroborates and extends previous studies that have shown the impact of LA volume as predictors for AF recurrence. In a recent meta-analysis of 21 studies including 3822 subjects, significant group differences in LA volumes were found between patients with and without recurrence after PVI.14 These studies however did not assess the interplay between LA volume and AF phenotype. In this regard, Costa et al.15 showed that relapse rates in patients with paroxAF and dilated LA volume were higher compared with patients with non-paroxAF and small LA volume. In their analysis, LA volume emerged as an independent predictor of recurrence. They however used cardiac CT to assess LA volumes, which is more expensive than echocardiography and exposes patients to ionizing radiation. Our study used echocardiography, which is widely available, is less expensive, and does not expose patients to ionizing radiation. Despite the higher inter-observer variability in estimating LAVI from echocardiography than from CT, studies have shown good correlation to measurements by CT scan and consider it an independent risk factor for recurrence.7,14,16,17 In our study, LAVI was a predictor of AF recurrence independent from AF phenotype. Hence, our study expands the literature and may aid to better emphasize the role of LA dimensions in clinical decision-making and patient selection in ablation naïve patients.14,15 While current guidelines suggest PVI as a first-line treatment in patients with paroxAF but not persAF, our data indicate that patients with persAF and normal LA dimensions might also be good candidates for early AF ablation.1
Previous studies have evaluated clinical predictors for arrhythmia recurrence after PVI, both in isolation and in the aggregation of scores such as the CHA2DS2-VASc score or the APPLE score.18–23 Studies have also shown association between diabetes, hypertension, renal disease, heart failure, and obesity with LA sizes and that increased LA sizes predict recurrence after PVI.24–30 Most of these factors are linked to advanced atrial cardiomyopathy. Our data however indicate that the LA dimensions likely integrate many of these individual factors, making it an excellent single-parameter surrogate for AF substrate. This is reflected by its value in the Cox proportional hazard regression analysis, where the individual factors were non-significant.
A more comprehensive approach to phenotyping atrial remodelling in the future should incorporate advanced atrial parameters from MRI, CT, and echocardiography. It is imperative to move beyond the conventional reliance on ‘AP LA diameter’ as the primary metric, as it serves merely as a surrogate for structural changes and does not fully reflect the atrial volume and function. A more refined approach to assess atrial remodelling would entail the evaluation of true 3D volumes of the atria by CT or MRI. In addition to volumetric analysis, incorporating strain measurements from MRI or echocardiography would allow for the detailed assessment of deformation and contraction properties of the atrial walls. Magnetic resonance imaging, in particular, offers the advantage of evaluating tissue characteristics such as late gadolinium enhancement, enabling the direct visualization of atrial fibrosis. Incorporating these measurements into future studies and correlating them with outcomes will provide a deeper insight into atrial mechanics and structural alterations, thereby improving risk-stratifying patients.31–37
Our study also confirmed the well-known worse outcome of PVI in patients with persAF compared with patients with paroxAF. Randomized studies to date have failed to demonstrate that substrate modification beyond PVI is better than PVI alone in patients with persAF.38,39 We therefore excluded patients with LA substrate ablation beyond PVI from the current analysis. Recent observational studies using pulsed field ablation for substrate modification beyond PVI in patients with persAF have shown favourable early results.40,41 It seems worth to test substrate modification concepts that previously failed with radiofrequency ablation again in randomized studies using pulsed field ablation. Until those results are available, PVI should remain the standard for clinical routine in patients with persAF, and for this approach, our study results are valid.
Study limitations
Potential limitations of this study should be taken into consideration. First, this was a retrospective analysis of a prospective registry. Second, echocardiography rather than CT or MRI was used for estimation of the LAVI in our study. Left atrial volume index measurements from CT were available only in a small minority of patients. Accordingly, we were not able to compare the value of LAVI estimated from echocardiography vs. CT. Third, echocardiography recordings from the clinical routine were used in our study. Accordingly, we do not have additional LA markers such as LA ejection fraction or LA strain parameters, which might also reflect AF substrate and might be of value to predict AF recurrence.42,43 Another potential limitation of our study is that 3D electroanatomical mapping was not used in patients undergoing cryoablation. Accordingly, we cannot correlate LA voltage abnormalities with AF phenotype, LA volume, and outcomes after ablation. Lastly, while we derived our results from a relatively large sample size, larger multi-centre studies are needed to confirm our findings from a single-centre study.
Conclusions
Atrial fibrillation phenotype and LA volume are complementary in the prediction of outcome after PVI. Persistent AF with no/mild LA dilation has a similar risk of recurrence as paroxAF with a moderate/severe LA dilation and should be given similar priority for ablation.
Supplementary Material
Contributor Information
Laurève Chollet, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Salik ur Rehman Iqbal, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Severin Wittmer, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Gregor Thalmann, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Antonio Madaffari, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Nikola Kozhuharov, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Oskar Galuszka, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Thomas Küffer, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Christoph Gräni, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Nicolas Brugger, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Helge Servatius, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Fabian Noti, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Andreas Haeberlin, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Laurent Roten, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Hildegard Tanner, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Tobias Reichlin, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Supplementary material
Supplementary material is available at Europace online.
Funding
None declared.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692–700. [DOI] [PubMed] [Google Scholar]
- 4. Della Rocca DG, Marcon L, Magnocavallo M, Mene R, Pannone L, Mohanty S et al. Pulsed electric field, cryoballoon, and radiofrequency for paroxysmal atrial fibrillation ablation: a propensity score-matched comparison. Europace 2023;26:euae016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badertscher P, Weidlich S, Knecht S, Stauffer N, Krisai P, Voellmin G et al. Efficacy and safety of pulmonary vein isolation with pulsed field ablation vs. novel cryoballoon ablation system for atrial fibrillation. Europace 2023;25:euad329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerra JM, Moreno Weidmann Z, Perrotta L, Sultan A, Anic A, Metzner A et al. Current management of atrial fibrillation in routine practice according to the last ESC guidelines: an EHRA physician survey—how are we dealing with controversial approaches? Europace 2024;26:euae012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albano AJ, Bush J, Parker SL, Corner K, Lim HW, Brunner MP et al. Left atrial volume index predicts arrhythmia-free survival in patients with persistent atrial fibrillation undergoing cryoballoon ablation. J Atr Fibrillation 2019;12:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohari M, Zado E, Marchlinski FE, Callans DJ, Han Y. Left atrial volume best predicts recurrence after catheter ablation in patients with persistent and longstanding persistent atrial fibrillation. Pacing Clin Electrophysiol 2014;37:422–9. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida K, Rabbani AB, Oral H, Bach D, Morady F, Chugh A. Left atrial volume and dominant frequency of atrial fibrillation in patients undergoing catheter ablation of persistent atrial fibrillation. J Interv Card Electrophysiol 2011;32:155–61. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 11. Servatius H, Kuffer T, Baldinger SH, Asatryan B, Seiler J, Tanner H et al. Dexmedetomidine versus propofol for operator-directed nurse-administered procedural sedation during catheter ablation of atrial fibrillation: a randomized controlled study. Heart Rhythm 2022;19:691–700. [DOI] [PubMed] [Google Scholar]
- 12. Aryana A, Kenigsberg DN, Kowalski M, Koo CH, Lim HW, Neill O et al. Verification of a novel atrial fibrillation cryoablation dosing algorithm guided by time-to-pulmonary vein isolation: results from the Cryo-DOSING Study (Cryoballoon-ablation DOSING Based on the Assessment of Time-to-Effect and Pulmonary Vein Isolation Guidance). Heart Rhythm 2017;14:1319–25. [DOI] [PubMed] [Google Scholar]
- 13. Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Y et al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol 2018;4:99–108. [DOI] [PubMed] [Google Scholar]
- 14. Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace 2018;20:33–42. [DOI] [PubMed] [Google Scholar]
- 15. Costa FM, Ferreira AM, Oliveira S, Santos PG, Durazzo A, Carmo P et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. Int J Cardiol 2015;184:56–61. [DOI] [PubMed] [Google Scholar]
- 16. Alajaji W, Costantini O, Taigen TL, Iler MA. Left atrial volume by cardiac CTA prior to catheter ablation: comparison to echocardiography and association with recurrent atrial fibrillation. BMC Res Notes 2023;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soga F, Tanaka H, Mochizuki Y, Mukai J, Suto M, Takada H et al. Combined assessment of left atrial volume parameters for predicting recurrence of atrial fibrillation following pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Echocardiography 2019;36:862–9. [DOI] [PubMed] [Google Scholar]
- 18. Rordorf R, Iacopino S, Verlato R, Arena G, Tondo C, Molon G et al. Role of CHA(2)DS(2)-VASc score in predicting atrial fibrillation recurrence in patients undergoing pulmonary vein isolation with cryoballoon ablation. J Interv Card Electrophysiol 2023;66:1193–200. [DOI] [PubMed] [Google Scholar]
- 19. Kornej J, Hindricks G, Arya A, Sommer P, Husser D, Bollmann A. The APPLE score—a novel score for the prediction of rhythm outcomes after repeat catheter ablation of atrial fibrillation. PLoS One 2017;12:e0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang A, Truong T, Black-Maier E, Green C, Campbell KB, Barnett AS et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus. Heart Rhythm O2 2020;1:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Creta A, Providencia R, Adragao P, de Asmundis C, Chun J, Chierchia G et al. Impact of type-2 diabetes mellitus on the outcomes of catheter ablation of atrial fibrillation (European Observational Multicentre Study). Am J Cardiol 2020;125:901–6. [DOI] [PubMed] [Google Scholar]
- 22. Bahnson TD, Giczewska A, Mark DB, Russo AM, Monahan KH, Al-Khalidi HR et al. Association between age and outcomes of catheter ablation versus medical therapy for atrial fibrillation: results from the CABANA trial. Circulation 2022;145:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naruse Y, Tada H, Sekiguchi Y, Machino T, Ozawa M, Yamasaki H et al. Concomitant chronic kidney disease increases the recurrence of atrial fibrillation after catheter ablation of atrial fibrillation: a mid-term follow-up. Heart Rhythm 2011;8:335–41. [DOI] [PubMed] [Google Scholar]
- 24. Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension 1995;25:1155–60. [DOI] [PubMed] [Google Scholar]
- 25. Parikh SS, Jons C, McNitt S, Daubert JP, Schwarz KQ, Hall B. Predictive capability of left atrial size measured by CT, TEE, and TTE for recurrence of atrial fibrillation following radiofrequency catheter ablation. Pacing Clin Electrophysiol 2010;33:532–40. [DOI] [PubMed] [Google Scholar]
- 26. Zhuang J, Wang Y, Tang K, Li X, Peng W, Liang C et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace 2012;14:638–45. [DOI] [PubMed] [Google Scholar]
- 27. McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation 2010;121:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47:2357–63. [DOI] [PubMed] [Google Scholar]
- 29. Paoletti E, Zoccali C. A look at the upper heart chamber: the left atrium in chronic kidney disease. Nephrol Dial Transplant 2014;29:1847–53. [DOI] [PubMed] [Google Scholar]
- 30. Li T, Li G, Guo X, Li Z, Yang J, Sun Y. The influence of diabetes and prediabetes on left heart remodeling: a population-based study. J Diabetes Complications 2021;35:107771. [DOI] [PubMed] [Google Scholar]
- 31. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 32. Mor-Avi V, Yodwut C, Jenkins C, Kuhl H, Nesser HJ, Marwick TH et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging 2012;5:769–77. [DOI] [PubMed] [Google Scholar]
- 33. den Uijl DW, Bax JJ. Left atrial size as a predictor of successful radiofrequency catheter ablation for atrial fibrillation. Europace 2009;11:1255–6. [DOI] [PubMed] [Google Scholar]
- 34. Abecasis J, Dourado R, Ferreira A, Saraiva C, Cavaco D, Santos KR et al. Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 2009;11:1289–94. [DOI] [PubMed] [Google Scholar]
- 35. Kojima T, Kawasaki M, Tanaka R, Ono K, Hirose T, Iwama M et al. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging 2012;13:227–34. [DOI] [PubMed] [Google Scholar]
- 36. Blyalova D, Abdrakhmanov AS, Baydurin SA, Nuralinov О, Balmukhamedova ZHA. Left atrial strain as a predictor of atrial fibrillation recurrence after catheter ablation. Europace 2023;25:euad122.100. [Google Scholar]
- 37. Gawalko M, Adriaans B, Habibi Z, Weerts J, Posea P, Hubers S et al. Left atrial strain is associated with atrial fibrillation recurrence after catheter ablation: data from the ISOLATION registry. Europace 2023;25:euad122-731. [Google Scholar]
- 38. Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi S et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the CAPLA randomized clinical trial. JAMA 2023;329:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 40. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami K et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 41. Reddy VY, Peichl P, Anter E, Rackauskas G, Petru J, Funasako M et al. A focal ablation catheter toggling between radiofrequency and pulsed field energy to treat atrial fibrillation. JACC Clin Electrophysiol 2023;9:1786–801. [DOI] [PubMed] [Google Scholar]
- 42. Kaufmann R, Rezar R, Strohmer B, Wernly B, Lichtenauer M, Hitzl W et al. Left atrial ejection fraction assessed by prior cardiac CT predicts recurrence of atrial fibrillation after pulmonary vein isolation. J Clin Med 2021;10:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Li Y, Sun L, Ye X, Cai Q, Zhu W et al. Left atrial strain for predicting recurrence in patients with non-valvular atrial fibrillation after catheter ablation: a single-center two-dimensional speckle tracking retrospective study. BMC Cardiovasc Disord 2022;22:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.