Abstract
Ferroptosis is an iron-dependent programmed cell death modality, which has showed great potential in anticancer treatment. Photodynamic therapy (PDT) is widely used in clinic as an anticancer therapy. PDT combined with ferroptosis-promoting therapy has been found to be a promising strategy to improve anti-cancer therapy efficacy. Fenton reaction in ferroptosis can provide oxygen for PDT, and PDT can produce reactive oxygen species for Fenton reaction to enhance ferroptosis. In this review, we briefly present the importance of ferroptosis in anticancer treatment, mechanism of ferroptosis, researches on PDT induced ferroptosis, and the mechanism of the synergistic effect of PDT and ferroptosis on cancer killing.
Keywords: Ferroptosis, Photodynamic therapy, Cancer, Anticancer treatment
Highlights
-
•
Ferroptosis, a recently discovered programmed cell death modality, is actually a result of imbalanced redox homeostasis.
-
•
Cancers have some characteristics that make them susceptible to ferroptosis.
-
•
Photodynamic therapy (PDT), a widely used anti-cancer treatment, has been recently found to be able to induce ferroptosis.
-
•
The combination of PDT and ferroptosis inducing was proved great synergistic effect on cancer cells killing.
Nomenclature
- ACSL:
long-chain acyl-CoA synthetase
- ALA
Aminolevulinic acid
- ALOXs
5-lipoxygenase
- AMPK
5‘-monophosphate-activated protein kinase
- BASHY
boronic acid-derived salicylidenehydrazone
- BAP1
BRCA1-Associated Protein 1
- BH4
tetrahydrobiopterin
- Ce6
Chlorin e6
- CI
Combination Index
- CoQ
reducing ubiquinone
- CoQH2
ubiquinol
- DFO
deferoxamine
- DHODH
dihydroorotate dehydrogenase
- Fer-1
ferrostatin-1
- FSP1
fibroblast specific protein 1
- GCH1
GTP cyclohydrolase 1
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- HYP
hypericin
- MB
methylene blue
- MMP2
matrix metalloproteinase 2
- OA
orotate
- PDT
Photodynamic therapy
- PLOOHs
phospholipid hydroperoxides
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SLC3A2
subunit solute carrier family 3 member 2
- SLC7A11
subunit solute carrier family 7 member 11
- System xc-
cystine/glutamate antiporters
In recent years, ferroptosis has been discovered as an important programmed cell death modality, which holds great potential for cancer therapy. Photodynamic therapy (PDT) has received more and more attention as an anti-cancer treatment for its advantages like small trauma, great selectivity towards tumor cells, few side effects and possibility of repeatable treatment. Since cancer has always been a difficult problem to be overcome in medicine, researches on cancer treatment increases constantly. Recently, it has been found that ferroptosis can be induced by PDT, and PDT combined with ferroptosis-promoting therapy is a promising strategy to improve anti-cancer therapy efficacy.
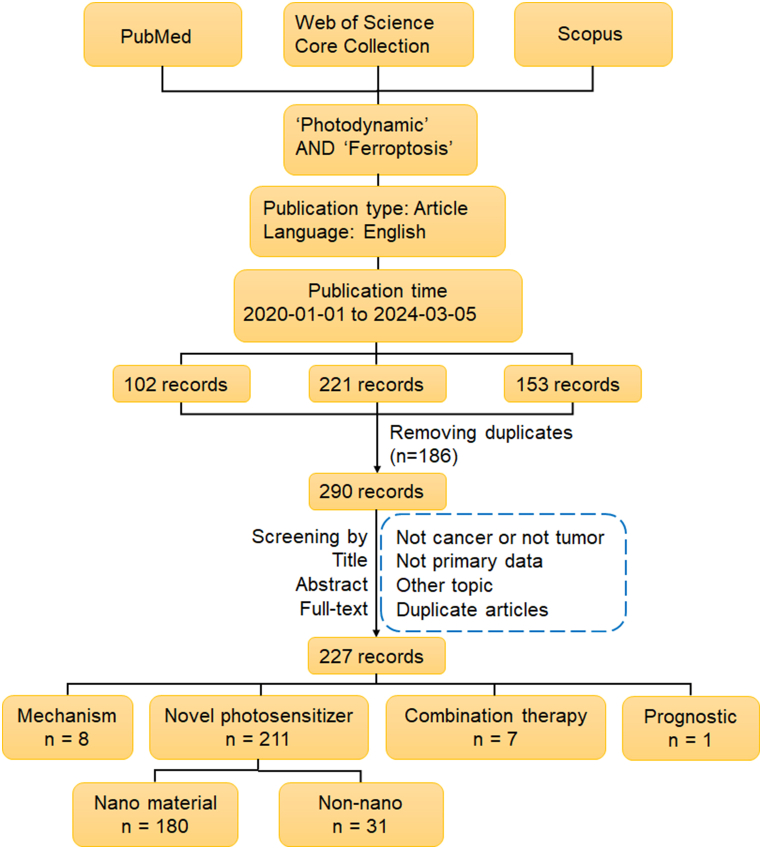
Therefore, we reviewed the known studies on PDT induced ferroptosis and studies on synergistic effect of PDT and ferroptosis-promoting therapy. In addition, we discussed the potential mechanisms of the synergistic effect of PDT and ferroptosis on cancer killing. With ‘Photodynamic’ and ‘Ferroptosis’ as key words, we searched Web of Science, PubMed and Scopus for related literatures limited to English-language from 2020 to the present. After removing duplicates, a manual review of these articles was performed by screening titles, abstracts and manuscripts. The detailed literature screening process is shown in Fig. 1, including search strategy and inclusion/exclusion criteria.
Fig. 1.
Literature search and outcome identification.
1. Mechanism and regulation of ferroptosis
Ferroptosis, a term coined in 2012, refers to an iron-dependent programmed cell death modality, which is different from apoptosis, necrosis and pyroptosis [1]. Unique morphological changes can be found during ferroptosis, including reduced size of mitochondria and reduced mitochondrial cristae. Fe2+ ions play a vital role in the process of ferroptosis. Fe2+ ions react with hydrogen peroxide, and hydroxyl radical is produced, which is called Fenton reaction. Hydroxyl radical initiates free radical chain reactions, leading to lipid peroxide accumulation of cell membrane, finally resulting in plasma membrane rupture and subsequent cell death. One of the most important factors that determines the cell fate is how cells respond to oxidative stress, and ferroptosis is actually a result of imbalanced redox homeostasis and can be suppressed by inhibition of lipid peroxide or depletion of iron. A series of gene expression changes and biochemical reactions are involved.
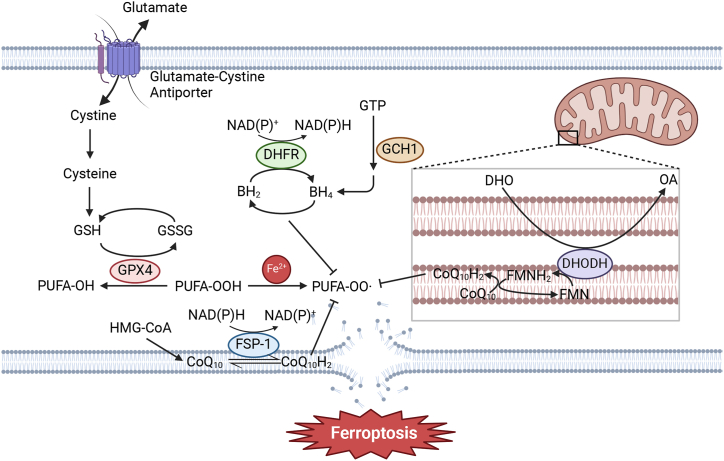
The understanding of ferroptotic mechanism has progressed rapidly in recent years. There are some systems that protect cells from ferroptosis (Fig. 2), among which the glutathione-glutathione peroxidase 4 (GSH–GPX4) system is the first to be found [1]. And GPX4-independent ferroptosis defense systems have been identified recently. Besides, cell metabolism is also found to be correlation with ferroptosis.
Fig. 2.
Ferroptosis defense systems, including GSH–GPX4 system, FSP1–CoQH2 system, GCH1–BH4 system and DHODH–CoQH2 system. (BH2: dihydrobiopterin, BH4: tetrahydrobiopterin, CoQ10: ubiquinone, CoQ10H2: Coenzyme Q reduced form (ubiquinol), DHO: deferoxamine, DHODH: dihydroorotate dehydrogenase, DHFR: dihydrofolate reductase, FMN: flavin mononucleotide, FSP-1: ferroptosis suppressor protein 1, GCH1: GTP cyclohydrolase 1, GSH: glutathione, GSSG: glutathione disulfide, GTP: guanosine triphosphate, HMG-CoA: 3-hydroxy-3-methylglutaryl-CoA, OA: orotate, PUFA: polyunsaturated fatty acid, GPX4: glutathione peroxidase 4).
Since accumulation of phospholipid hydroperoxides (PLOOHs) causes damage of cell membrane and finally leads to cell death in ferroptosis, PLOOHs play the key role in ferroptosis. GPX4 is the major enzyme that catalyzes the reduction of PLOOHs. In GSH-GPX4 system, there are cystine/glutamate antiporters (System xc−) on the cell membrane. The system xc− is composed of two components, subunit solute carrier family 7 member 11 (SLC7A11) and subunit solute carrier family 3 member 2 (SLC3A2), which can take in cystine and excrete glutamate. This system sustains the level of intracellular GSH, a master endogenous antioxidant and a key regulator of ferroptosis. GPX4 catalyzes the reduction of lipid peroxides by GSH, and lipid peroxides accumulation is reduced. The GPX4–GSH system was initially thought to be the only ferroptosis defense system, until 2019 when the Fibroblast Specific Protein 1- ubiquinol (FSP1–CoQH2) system was reported to defend against ferroptosis independently [2]. FSP1 functions as an NAD(P)H-dependent oxidoreductase capable of reducing ubiquinone (also known as coenzyme Q or CoQ) to CoQH2, and CoQH2 can trap lipid peroxyl radicals, thereby suppressing lipid peroxidation and ferroptosis. Subsequently, the GTP cyclohydrolase 1- tetrahydrobiopterin (GCH1–BH4) system was found to be another ferroptosis defense system [3]. GCH1 mediates the rate-limiting reaction in the BH4 biosynthesis pathway. BH4 is a radical-trapping antioxidant capable of trapping lipid peroxyl radicals, which can prevent lipid peroxides accumulation and inhibit ferroptosis. GCH1 also mediates the production of CoQH2 to suppress ferroptosis. The dihydroorotate dehydrogenase-CoQH2 (DHODH–CoQH2) system, a mitochondria-localized defense system, was revealed by a recent study [4]. DHODH is an enzyme in the inner mitochondrial membrane, catalyzing the oxidation of dihydroorotate (DHO) to orotate (OA). As this reaction occurs, CoQ in the inner membrane is reduced to CoQH2, thereby inhibiting the occurrence of ferroptosis.
Therefore, there is a balance between oxidative damage and antioxidant defense. Once the oxidation reaction is promoted or the antioxidant defense is inhibited, the lipid peroxides will accumulate, and then ferroptosis will occur. A sequence of molecules was found to be able to induce ferroptosis with different targets, such as erastin, sorafenib, RSL3 and sulphasalazine [5].
Apparently, there are many metabolic reactions that can produce PLOOHs, and there are more and more researches suggesting that ferroptosis is closely related to cell metabolism. And autophagy also has complex correlation with ferroptosis. It was found that autophagy induced by amino acid starvation could induce ferroptosis, and iron-carrier transferrin and the amino acid glutamine were required in this process [6]. And the mechanism behind was that autophagic degradation of the iron-storage protein ferritin caused increased cellular labile iron content, which led to a higher sensitivity of cells to ferroptosis [7,8]. Apart from glutamate metabolism and iron metabolism, glucose can also affect ferroptosis. Glucose starvation of cells can suppress ferroptosis through activation of adenosine 5‘-monophosphate-activated protein kinase (AMPK) pathway. When AMPK is activated, the synthesis of polyunsaturated fatty acid (PUFA) is decreased, which leads to the suppression of ferroptosis [9,10].
2. Ferroptosis as an important cancer death modality
Ferroptosis holds great potential for cancer therapy. A series of compounds and drugs have been identified to be able to induce ferroptosis and suppress cancer. Actually, ferroptosis was correlation with cancers from the very beginning, since the discovery of ferroptosis is related to exploration of anticancer drugs. And it has been proved that some tumor suppressors can promote ferroptosis, such as p53 and BRCA1-Associated Protein 1 (BAP1), which can promote ferroptosis by downregulating SCL7A11 expression [11,12].
Inducing apoptosis in cancer cells with anti-cancer drugs is an important method for cancer treatment. However, acquired or intrinsic resistance to apoptosis of cancer cells leading to limited treatment effectiveness [13]. Some of these tumors are found to be sensitive to ferroptosis, which implicates alternative method to treat cancer and emphasizes the importance of ferroptosis [[14], [15], [16]]. Since ferroptosis is induced by oxidative stress and closely related to iron, cancer cells are reasonable to be susceptible to ferroptosis, for they have high reactive oxygen species (ROS) load and high iron contents.
Specific mutations of cancer cells may also affect their susceptibility to ferroptosis [17,18]. Mutations in RAS family are most common in human cancers, and there are compounds targeting RAS mutations to suppress cancers, although cancers has developed resistance to some of them [19]. Erastin and RSL3, two ferroptosis inducers, are found to selectively trigger cell death in cancer cells with RAS mutations [20,21]. TP53 is another common mutation in human cancer cells. As it has been mentioned before, p53 can downregulate SLC7A11 and thus promote ferroptosis. Some TP53 mutations retain the ability to suppress SLC7A11 expression and are able to induce ferroptosis, like p53 3KR (K117R, K161R, K162R) acetylation-defective mutant [11,22], but some are unable to induce ferroptosis anymore.
Furthermore, the ferroptosis defense systems are very active in some cancer cells to protect them from ferroptosis, such as increased expression of SLC7A11 on cell membrane [23]. Therefore, these cancer cells are more susceptible to ferroptosis, and disruption of those defenses would be fatal to such cancer cells while sparing normal cells. Some studies showed that ferroptosis is possibly a form of immunogenic cell death (ICD) [24]. The ferroptotic cancer cells can release several immunostimulatory signals to promote dendritic cells maturation, increase the efficiency of macrophages in the phagocytosis of ferroptotic cancer cells and further enhance the infiltration of CD8+ T cells into tumors.
3. Ferroptosis as a cell death modality induced by PDT
PDT, as a treatment for cancer, has received more and more attention. The approach combines photosensitizer and light with specific wavelength, generating ROS with the existence of oxygen, to damage the target tissue. PDT was found to be effective in the treatment of cancers for the first time in 1903. At present, PDT has been widely used in the treatment of cancers, such as oropharyngeal cancer, esophageal cancer and cutaneous carcinoma [25]. Compared with conventional anti-tumor therapies such as surgery, chemotherapy, and radiotherapy, PDT has many important advantages. PDT is a non-invasive therapy with high selectivity and few adverse effects, which leads to great preservation of structure and function of organs. Photocytotoxic reactions occur only in tissues with sufficient photosensitizers distribution, and photosensitizers accumulate in significantly higher concentrations in cancer cells than in regular cells, which enables selective destruction. This also makes it possible to eliminate some tiny cancer nests that are hard to be observed. PDT is a repeatable treatment for cancer patients. There is no resistance or increase toxicity observed with increasing number of treatments. Furthermore, PDT can lead to systemic anti-cancer response, which affects the vascular system of the tumor and stimulates the immune system.
PDT can induce different cell death modalities in cancer cells, including apoptosis, accidental necrosis, necroptosis and pyroptosis [26,27]. Recent studies suggest that PDT can also induce ferroptosis in cancer cells [[28], [29], [30], [31]]. Cell death in GL261 cells induced by photosens-PDT was found to be significantly blocked by ferrostatin-1 (Fer-1) and Deferoxamine (DFO), ferroptosis inhibitors, which implied that photosens-PDT induced ferroptosis in GL261 cells [24]. Ferroptosis induced by PDT has also been found in some triple-negative breast cancer cells (TNBCs) [28]. The increased lipid peroxidation and depletion of GPX4 was found in some TNBC cell lines after methylene blue-PDT (MB-PDT) treatment, which meant that ferroptosis occurred. Talaporfin sodium-PDT, which is commonly used in clinical practice in Japan, were also found to be able to induce ferroptosis in gastric cancer cells and esophageal cancer cells, with direct lipid peroxidation by the generated ROS and the inhibition of system xc− [31]. Aminolevulinic acid (ALA) is a widely used photosensitizer, and ferroptosis is also a potential cell death modality induced by ALA-PDT. Features of several cell death modalities were observed after ALA-PDT in esophageal Kyse 450 carcinoma cells by fluorescence-based live cell imaging, including apoptosis, necrosis and ferroptosis [30].
Since PDT can induce several cell death modalities, the major cell death pathway varies in different conditions. Although the researches on ferroptosis induced by PDT are still limited, it can be found that whether PDT induces ferroptosis as the major cell death pathway is affected by many factors. Cell death in GL261 cells induced by PS-PDT can be inhibited by ferroptosis inhibitors, but the cell death induced by PD-PDT was inhibited only by the apoptosis inhibitor zVAD-fmk but not by Fer-1 or DFO, which demonstrated that photosensitizer is one of the factors that affect major cell death pathway induced by PDT [24]. The susceptibility to ferroptosis of cells is different, which matters a lot. In TNBC cell lines, despite MB-PDT promoted depletion of GPX4 in all cell lines tested, lipid peroxidation after MB-PDT treatment was only increased in MCF-10A and MDA-MB-231 cells, and only MDA-MB-231 cells were cytoprotected by Fer-1 [28]. And the capacity to de novo GSH synthesis, abundance of PUFAs and basal levels of labile iron pool are possible factors influencing the susceptibility of cells to ferroptosis. In addition to the type of photosensitizers and cell type, the photosensitizer concentration, incubation time and light dose during treatment also matters the death modalities. Hypericin-PDT (HYP-PDT) was found to trigger apoptosis as the major cell death pathway at low HYP concentration, necrosis as the major pathway at high concentration and ferroptosis at medium concentration [27]. Major cell death pathway triggered by ALA-PDT may also changes, and pyroptosis can be major cell death modality at certain treatment parameters [32]. Therefore, adopting combination therapy based on the major cell death modality induced by PDT may achieve the best treatment efficacy.
In addition, whether the ferroptosis is induced by photodynamic reaction or the photosensitizer itself may also be a point that should be explored. 5-ALA, as we have mentioned before, is a natural amino acid widely used in cancer treatment, usually as photosensitizer for PDT. But a study showed that 5-ALA can induce ferroptosis in cancer cells even without the use of light. It was found that 5-ALA induces ferroptosis by suppressing GPX4 and increasing expression of HMOX1 and exerts antitumor effects in esophageal squamous cell carcinoma cell lines [33]. Similarly, verteporfin is a photosensitizer used for PDT, but it's also can function as a Yes-associated protein (YAP) inhibitor for treatment of several human cancers. Recent studies have showed that verteporfin can induce significant ferroptosis features independent of light activation, including accumulation of Fe2+ ions and malondialdehyde as well as reduction of GSH and GPX4 [34,35].
There were also many novel photosensitizers that were proved to induce ferroptosis [[36], [37], [38], [39], [40], [41]]. Many of them are metal complex, such as iridium (III), Pt (II) and Ru (II) complex [37,[42], [43], [44], [45]]. But there were also other types of novel photosensitizers that can induce ferroptosis [[46], [47], [48]]. Boronic acid-derived salicylidenehydrazone (BASHY) dyes were found to be able to induce ferroptosis in human glioblastoma multiform U87 cell line, and BASHY dyes accumulated in lipid droplets, which enhanced photodynamic reaction and the induction of ferroptosis [49]. Another novel photosensitizer, MBTB-PA, can target the endoplasmic reticulum and consume reducing substances, inducing ferroptosis [50].
4. Synergism between PDT and ferroptosis showed efficient anticancer effect
PDT is a non-invasive method that can preserve the integrity and function of tissues and organs to the greatest extent. However, there are still some disadvantages. Hypoxia in the tumor microenvironment limits the efficiency of PDT [[51], [52], [53], [54]]. The limited penetration depth of light also affects the application of PDT in clinic cancer treatment. Therefore, increasing number of attempts has been made to improve the efficiency of PDT in cancer treatment, and combining PDT with other anti-tumor therapies has been a research hotspot [55,56].
Recently, many researches have been focusing on combining PDT and ferroptosis-promoting therapies [[57], [58], [59], [60], [61], [62]], and mostly by designing nanoparticles [[63], [64], [65], [66]]. Chlorin e6 (Ce6) is the most commonly used photosensitizer among all these researches [[66], [67], [68], [69], [70]], and other photosensitizers include MB, porphyrin, protoporphyrin IX and 5,10,15,20-tetrakis (4-aminophenyl) porphyrin [[71], [72], [73], [74]]. As to the ferroptosis-promoting therapies, most researches chose ferroptosis inducers, among which erastin, sorafenib and iron compound are most commonly used [[75], [76], [77], [78], [79]]. Ce6 and erastin were found to be able to be self-assembled into nanodrug via hydrogen bonding and π-π interactions. Compared with Ce6-PDT or erastin treatment separately, this carried-free nanodrug named Ce6-erastin showed better anti-tumor efficacy towards oral tongue squamous cell carcinoma both in vitro and in vivo [23]. Sorafenib, Ce6, and Fe3+ were also found to be able to form self-assembly co-delivery nanoparticles, which showed excellent colloidal stability and water dispersity with good in vivo tumor-targeting ability [66]. In a DNA nanozyme, hemin, an iron-containing porphyrin cofactor, were inserted with Ce6 to enhance PDT [63]. There are also nanoparticles that stimulate ferroptosis by hijacking endogenous iron rather than containing a ferroptosis-inducer. A hypoxia responsive polymer bearing 18-crown-6 ring stimulates ferroptosis by releasing endogenous iron stored in the natural “iron pools" of cellular organelles, and was loaded with Ce6 [80]. Another nanomedicine called FP@MC also enhanced ferroptosis through endogenous iron de-hijacking [81]. And some novel photosensitizers, such as iron chlorophyll (Chl/Fe), contain iron, and can also be part of the nanoparticles. The cluster-structured Fe3O4@Chl/Fe was developed and showed great efficacy on killing bladder cancer by both PDT and inducing ferroptosis [64].
In order to further improve the anti-tumor efficacy, most nanoparticles contain not only a photosensitizer and a ferroptosis inducer, but also some other component parts. Since hypoxic tumor microenvironment is a bottleneck of PDT, as an oxygen-carrier, hemoglobin is a great choice for these nanoparticles [65,82,83]. In a nanoplatform named SRF@Hb-Ce6, hemoglobin was connected with the photosensitizer Ce6, resulting in a mutual benefit for both therapies in the supplementation of oxygen for PDT and replenishment of Fe for ferroptosis therapy [83]. And an amphiphilic matrix metalloproteinase 2 (MMP2, a protein highly expressed at tumor tissue)-responsive peptide was embedded into the skeleton of the micelles, ensuring the specific release of SRF at the tumor site via the decomposition of micelles in response to MMP2. In another nanodrug named BCFe@SRF, bovine serum albumin and ferritin were connected to Ce6 through the hypoxia-responsive azobenzene linker, which showed outstanding biocompatibility and excellent tumor targeting efficiency [53].
The combination of PDT and ferroptosis showed great synergistic effect on cancer cells killing. Meng et al. studied whether photodynamic and ferroptosis have synergistic effects in the treatment of malignant tumors, and found that PDT combining with ferroptosis inducer caused more severe GSH depletion and lipid peroxide accumulation than either therapy alone [84]. Zhu et al. calculated the Combination Index (CI) of Ce6 and erastin as Ce6-erastin nanomedicines, and the results showed that there was a synergistic effect between the two (CI < 1) [23].
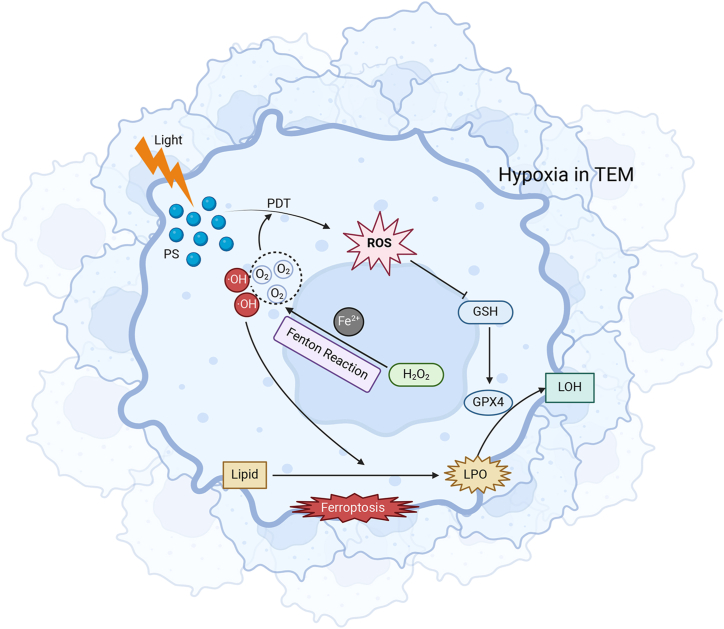
Although the mechanisms of this synergistic effect have not been elucidated, it can be partially understood based on the anti-tumor mechanisms of PDT and Ferroptosis. On the one hand, ferroptosis can promote the photodynamic reaction. As mentioned above, hypoxia in the tumor microenvironment is an important factor limiting the efficacy of PDT for malignant tumors, and the Fenton reaction in the process of ferroptosis can provide oxygen for the photodynamic reaction continuously and stably. On the other hand, GSH depletion is an important part in the process of ferroptosis, and the photodynamic reaction generates a large amount of reactive oxygen species, which can consume GSH, further aggravate the state of GSH depletion, and then promote ferroptosis (Fig. 3).
Fig. 3.
Synergistic effect of PDT and ferroptosis on cancer killing.
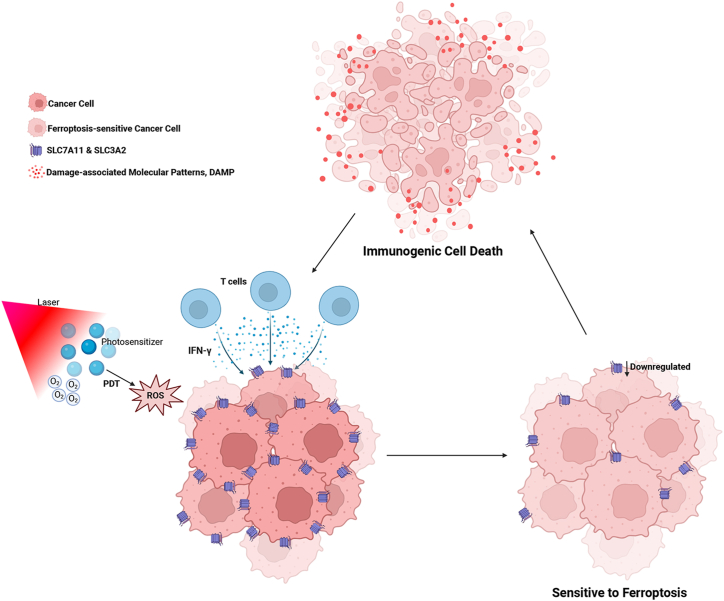
In addition to the direct mutual promotion between photodynamic reaction and ferroptosis, some studies have shown that PDT can cause high levels of lymphocyte infiltration in the local tumor and stimulate the secretion of interferon-γ, which leads to the downregulation of SLC7A11 and SLC3A2 on the cell membrane surface and makes tumor cells more sensitive to ferroptosis (Fig. 4) [83]. As a form of ICD, ferroptosis can further enhance the infiltration of CD8+ T cells into tumors. In addition, recent studies have shown that photodynamic therapy of malignant tumors can induce a new cell death pathway under specific conditions, which can directly trigger lipid peroxidation and is independent of 5-lipoxygenase (ALOXs) and long-chain acyl-CoA synthetase (ACSL4) enzymes in the classical ferroptosis pathway [27]. This pathway is significantly different from classical ferroptosis, but it can be speculated that the non-enzymatic lipid peroxidation directly triggered by PDT in this cell death pathway can deplete intracellular GSH, leading to a GSH-depleted state, which in turn promotes the occurrence of classical ferroptosis.
Fig. 4.
PDT induces ferroptosis-sensitive cancer cells, and ferroptosis further enhances infiltration of T cells.
5. Concluding remarks
Combination of PDT and ferroptosis-promoting therapy is a promising strategy for increasing efficacy of anti-cancer therapy. There have been a few nanodrugs designed for this combination therapy, but successful translation of these promising concepts into pre-clinical and clinical studies presents great challenges. Although there are increasing number of researches on mechanism of ferroptosis, researches on PDT induced ferroptosis are still limited and the mechanism remained unknown. PDT induced ferroptosis are regulated by many possible factors, including the susceptibility to ferroptosis of cells, type of photosensitizers, and treatment parameters of PDT. Since PDT can induce several cell death modalities, the anti-cancer mechanisms of PDT are very complex and still unclear. Further studies should be focusing on the key factors influencing the major cell death pathway induced by PDT. And further understanding of the interplay and synergism between PDT and ferroptosis is necessary. In conclusion, ferroptosis is an alternative cell death modality in cancer cells, and PDT combined with ferroptosis-promoting therapy is a promising strategy to improve anti-cancer therapy efficacy, which requires more attention to developing nanodrugs and studying the mechanism of PDT induced ferroptosis.
Funding
This work was supported by the National Natural Science Foundation of China (82073013).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
CRediT authorship contribution statement
Qihang Chang: Writing – original draft. Peiru Wang: Supervision. Qingyu Zeng: Writing – review & editing. Xiuli Wang: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Qingyu Zeng, Email: xiaojinyuhen@126.com.
Xiuli Wang, Email: wangxiuli_1400023@tongji.edu.cn.
References
- 1.Dixon S.J., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doll S., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 3.Kraft V.A.N., et al. GTP cyclohydrolase 1/tetrahydrobiopterin Counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 2020;6(1):41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao C., et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu T., et al. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell Mol. Med. 2019;23(8):4900–4912. doi: 10.1111/jcmm.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao M., et al. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao M., et al. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou W., et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H., et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020;22(2):225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C., et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct. Targeted Ther. 2020;5(1):187. doi: 10.1038/s41392-020-00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang L., et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018;20(10):1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammad R.M., et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015;35(Suppl):S78–S103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., et al. Frizzled-7 identifies Platinum-tolerant ovarian cancer cells susceptible to ferroptosis. Cancer Res. 2021;81(2):384–399. doi: 10.1158/0008-5472.CAN-20-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J., et al. Discovery of a novel stilbene derivative as a microtubule targeting agent capable of inducing cell ferroptosis. J. Med. Chem. 2022;65(6):4687–4708. doi: 10.1021/acs.jmedchem.1c01775. [DOI] [PubMed] [Google Scholar]
- 16.Balihodzic A., et al. Non-coding RNAs and ferroptosis: potential implications for cancer therapy. Cell Death Differ. 2022;29(6):1094–1106. doi: 10.1038/s41418-022-00998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J., et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769):402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou Y., et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585(7826):603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue J.Y., et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577(7790):421–425. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolma S., et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 21.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D.S., et al. Inhibiting the system x(C)(-)/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat. Commun. 2017;8 doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu T., et al. Ferroptosis promotes photodynamic therapy: supramolecular photosensitizer-inducer nanodrug for enhanced cancer treatment. Theranostics. 2019;9(11):3293–3307. doi: 10.7150/thno.32867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turubanova V.D., et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer. 2019;7(1):350. doi: 10.1186/s40425-019-0826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., et al. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 26.Li L., et al. Photodynamic therapy induces human esophageal carcinoma cell pyroptosis by targeting the PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 2021;520:143–159. doi: 10.1016/j.canlet.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Shui S., et al. Non-enzymatic lipid peroxidation initiated by photodynamic therapy drives a distinct ferroptosis-like cell death pathway. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dos Santos A.F., et al. Distinct photo-oxidation-induced cell death pathways lead to selective killing of human breast cancer cells. Cell Death Dis. 2020;11(12):1070. doi: 10.1038/s41419-020-03275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojima Y., et al. Photodynamic therapy with Talaporfin sodium (TS-PDT) induces Ferroptosis and synergizes with Ferroptosis inducer. Cancer Sci. 2023;114:1341. 1341. [Google Scholar]
- 30.Čunderlíková B., et al. Modifications of DAMPs levels in extracellular environment induced by aminolevulinic acid-based photodynamic therapy of esophageal cancer cells. Int. J. Radiat. Biol. 2024:1–15. doi: 10.1080/09553002.2024.2310002. [DOI] [PubMed] [Google Scholar]
- 31.Kojima Y., et al. Induction of ferroptosis by photodynamic therapy and enhancement of antitumor effect with ferroptosis inducers. J. Gastroenterol. 2024;59(2):81–94. doi: 10.1007/s00535-023-02054-y. [DOI] [PubMed] [Google Scholar]
- 32.Chen D., et al. Modified 5-aminolevulinic acid photodynamic therapy induces cutaneous squamous cell carcinoma cell pyroptosis via the JNK signaling pathway. Biochim. Biophys. Acta Mol. Cell Res. 2024;1871(1) doi: 10.1016/j.bbamcr.2023.119603. [DOI] [PubMed] [Google Scholar]
- 33.Shishido Y., et al. Antitumor effect of 5-aminolevulinic acid through ferroptosis in esophageal squamous cell carcinoma. Ann. Surg Oncol. 2021;28(7):3996–4006. doi: 10.1245/s10434-020-09334-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang X.-W., et al. Verteporfin exerts anticancer effects and reverses resistance to paclitaxel via inducing ferroptosis in esophageal squamous cell cancer cells. Mol. Biotechnol. Advance online publication. 2023 doi: 10.1007/s12033-023-00891-z. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W., et al. Verteporfin induces lipid peroxidation and ferroptosis in pancreatic cancer cells. Free Radic. Biol. Med. 2024;212:493–504. doi: 10.1016/j.freeradbiomed.2024.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng X., et al. An AIE-based monofunctional Pt(ii) complex for photodynamic therapy through synergism of necroptosis-ferroptosis. RSC Chem Biol. 2024;5(2):141–147. doi: 10.1039/d3cb00113j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y., et al. An electron-accepting half-sandwich iridium(III) complex for the treatment of hypoxic tumors via synergetic chemo- and phototherapy. Inorg. Chem. Front. 2024;11(2):436–450. [Google Scholar]
- 38.Yang X., et al. "Two-Stage rocket-propelled" strategy boosting theranostic efficacy with mitochondria-specific type I-II photosensitizers. ACS applied materials & interfaces. 2024;16(8):9816–9825. doi: 10.1021/acsami.3c17723. [DOI] [PubMed] [Google Scholar]
- 39.Fang L., et al. Single component organic photosensitizer with NIR-I emission realizing type-I photodynamic and GSH-depletion caused ferroptosis synergistic theranostics. Adv. Healthcare Mater. 2023;12(21) doi: 10.1002/adhm.202300134. [DOI] [PubMed] [Google Scholar]
- 40.Hu Q., et al. A GPX4-targeted photosensitizer to reverse hypoxia-induced inhibition of ferroptosis for non-small cell lung cancer therapy. Chem. Sci. 2023;14(34):9095–9100. doi: 10.1039/d3sc01597a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu N., et al. An osmium-peroxo complex for photoactive therapy of hypoxic tumors. Nat. Commun. 2022;13(1):2245. doi: 10.1038/s41467-022-29969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y., et al. An electron-accepting half-sandwich iridium(iii) complex for the treatment of hypoxic tumors via synergetic chemo- and phototherapy. Inorg. Chem. Front. 2023;11(2):436–450. [Google Scholar]
- 43.Zheng X., et al. An AIE-based monofunctional Pt(ii) complex for photodynamic therapy through synergism of necroptosis-ferroptosis. RSC Chemical Biology. 2023;5(2):141–147. doi: 10.1039/d3cb00113j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling Y.-Y., et al. Self-amplifying iridium(III) photosensitizer for ferroptosis-mediated immunotherapy against transferrin receptor-overexpressing cancer. Small. 2022;18(49) doi: 10.1002/smll.202203659. [DOI] [PubMed] [Google Scholar]
- 45.Qi F., et al. Type I photoreaction and photoinduced ferroptosis by a Ru(II) complex to overcome tumor hypoxia in photodynamic therapy. CCS Chem. 2023;5(7):1583–1591. [Google Scholar]
- 46.Guo X., et al. Synthesis and biological evaluation of NO-donor containing photosensitizers to induce ferroptosis of cancer cells. Bioorg. Chem. 2021;116 doi: 10.1016/j.bioorg.2021.105355. [DOI] [PubMed] [Google Scholar]
- 47.Tseng H.-C., et al. Indocyanine green as a near-infrared theranostic agent for ferroptosis and apoptosis-based, photothermal, and photodynamic cancer therapy. Front. Mol. Biosci. 2022;9 doi: 10.3389/fmolb.2022.1045885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., et al. Activation of ALOX12 by a multi-organelle-orienting photosensitizer drives ACSL4-independent cell ferroptosis. Cell Death Dis. 2022;13(12):1040. doi: 10.1038/s41419-022-05462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva M., et al. BASHY dyes are highly efficient lipid droplet-targeting photosensitizers that induce ferroptosis through lipid peroxidation. Bioconjugate Chem. 2023;34(12):2337–2344. doi: 10.1021/acs.bioconjchem.3c00449. [DOI] [PubMed] [Google Scholar]
- 50.Wang B., et al. Bioconjugation and reaction-induced tumor therapy via alkynamide-based thiol-yne click reaction. Small. 2023 doi: 10.1002/smll.202307309. [DOI] [PubMed] [Google Scholar]
- 51.Henderson B.W., Fingar V.H. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987;47(12):3110–3114. [PubMed] [Google Scholar]
- 52.Jing X., et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer. 2019;18(1):157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X., et al. Hypoxia-responsive nanoreactors based on self-enhanced photodynamic sensitization and triggered ferroptosis for cancer synergistic therapy. J. Nanobiotechnol. 2021;19(1):204. doi: 10.1186/s12951-021-00952-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dang J., et al. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT) Biomater. Sci. 2017;5(8):1500–1511. doi: 10.1039/c7bm00392g. [DOI] [PubMed] [Google Scholar]
- 55.Zeng Q., et al. PD-L1 blockade potentiates the antitumor effects of ALA-PDT and optimizes the tumor microenvironment in cutaneous squamous cell carcinoma. OncoImmunology. 2022;11(1) doi: 10.1080/2162402X.2022.2061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez N., Sevilla A. Current advances in photodynamic therapy (PDT) and the future potential of PDT-combinatorial cancer therapies. Int. J. Mol. Sci. 2024;25(2) doi: 10.3390/ijms25021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J., et al. Cell membrane-targeting type I/II photodynamic therapy combination with FSP1 inhibition for ferroptosis-enhanced photodynamic immunotherapy. Adv. Healthcare Mater. 2024 doi: 10.1002/adhm.202304436. [DOI] [PubMed] [Google Scholar]
- 58.Zou P., et al. A ferroptosis microneedle integrated wireless implanted photodynamic therapy pellet for cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 2023;117(2):E280. E280. [Google Scholar]
- 59.Zou P., et al. Implanted, wireless, self-powered photodynamic therapeutic tablet synergizes with ferroptosis inducer for effective cancer treatment. Adv. Sci. 2023;10(36) doi: 10.1002/advs.202302731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delcanale P., et al. A photoactive supramolecular complex targeting PD-L1 reveals a weak correlation between photoactivation efficiency and receptor expression levels in non-small-cell lung cancer tumor models. Pharmaceutics. 2023;15(12) doi: 10.3390/pharmaceutics15122776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhan F., et al. YAP knockdown in combination with ferroptosis induction increases the sensitivity of HOS human osteosarcoma cells to pyropheophorbide-α methyl ester-mediated photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022;39 doi: 10.1016/j.pdpdt.2022.102964. [DOI] [PubMed] [Google Scholar]
- 62.Su X., et al. Localized disruption of redox homeostasis boosting ferroptosis of tumor by hydrogel delivery system. Materials Today Bio. 2021;12 doi: 10.1016/j.mtbio.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao X., et al. Hemin-incorporating DNA nanozyme enabling catalytic oxygenation and GSH depletion for enhanced photodynamic therapy and synergistic tumor ferroptosis. J. Nanobiotechnol. 2022;20(1):410. doi: 10.1186/s12951-022-01617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chin Y.C., et al. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J. Nanobiotechnol. 2022;20(1) doi: 10.1186/s12951-022-01575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X., et al. A metal-phenolic network-assembled nanotrigger evokes lethal ferroptosis via self-supply loop-based cytotoxic reactions. Chem. Eng. J. 2024:479. [Google Scholar]
- 66.Xu Y., et al. Ferroptosis boosted oral cancer photodynamic therapy by carrier-free Sorafenib-Ce6 self-assembly nanoparticles. J Control Release. 2024;366:798–811. doi: 10.1016/j.jconrel.2023.12.056. [DOI] [PubMed] [Google Scholar]
- 67.Wang M., et al. Photodynamic and ferroptotic Ce6@ZIF-8@ssPDA for head and neck cancer treatment. Mater. Des. 2022:224. [Google Scholar]
- 68.Zhang P., et al. Triapine/Ce6-loaded and lactose-decorated nanomicelles provide an effective chemo-photodynamic therapy for hepatocellular carcinoma through a reactive oxygen species-boosting and ferroptosis-inducing mechanism. Chem. Eng. J. 2021:425. [Google Scholar]
- 69.Zhou Y., et al. Rosmarinic acid-crosslinked supramolecular nanoassembly with self-regulated photodynamic and anti-metastasis properties for synergistic photoimmunotherapy. Small. 2023;19(23) doi: 10.1002/smll.202300594. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z.-j., et al. Magnetic resonance and fluorescence imaging superparamagnetic nanoparticles induce apoptosis and ferroptosis through photodynamic therapy to treat colorectal cancer. Materials Today Physics. 2023;36 [Google Scholar]
- 71.Liu J.-Y., et al. NaErF4-Based nanocarrier for enhanced ferroptosis therapy of cancer. ACS Appl. Nano Mater. 2023;6(1):270–282. [Google Scholar]
- 72.Zhao Z., et al. Sonodynamic therapy of NRP2 monoclonal antibody-guided MOFs@COF targeted disruption of mitochondrial and endoplasmic reticulum homeostasis to induce autophagy-dependent ferroptosis. Adv. Sci. 2023;10(30) doi: 10.1002/advs.202303872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z.-Y., et al. Synergistic photochemo effects based on light-activatable dual prodrug nanoparticles for effective cancer therapy. Adv. Healthcare Mater. 2023;12(27) doi: 10.1002/adhm.202301133. [DOI] [PubMed] [Google Scholar]
- 74.Li J., et al. PDT-enhanced ferroptosis by a polymer nanoparticle with pH-activated singlet oxygen generation and superb biocompatibility for cancer therapy. Biomacromolecules. 2021;22(3):1167–1176. doi: 10.1021/acs.biomac.0c01679. [DOI] [PubMed] [Google Scholar]
- 75.Chen L., et al. Combined photothermal and photodynamic therapy enhances ferroptosis to prevent cancer recurrence after surgery using nanoparticle-hydrogel composite. Chem. Eng. J. 2023:468. [Google Scholar]
- 76.Luo S., et al. Iridium photosensitizer constructed liposomes with hypoxia-activated prodrug to destrust hepatocellular carcinoma. Chin. Chem. Lett. 2023;34(4) [Google Scholar]
- 77.Li Y., et al. Coenzyme-depleting nanocarriers for enhanced redox cancer therapy under hypoxia. J. Colloid Interface Sci. 2023;641:135–145. doi: 10.1016/j.jcis.2023.03.057. [DOI] [PubMed] [Google Scholar]
- 78.Fang M., et al. Asymmetric mesoporous nanoformulation for combination treatment of soft tissue sarcoma. ACS Mater. Lett. 2023;5(3):811–821. [Google Scholar]
- 79.Wu G.L., et al. Tumor microenvironment-responsive one-for-all molecular-engineered nanoplatform enables NIR-II fluorescence imaging-guided combinational cancer therapy. Anal. Chem. 2023;95(47):17372–17383. doi: 10.1021/acs.analchem.3c03827. [DOI] [PubMed] [Google Scholar]
- 80.Li Y., et al. Hijacking endogenous iron and GSH via a polyvalent ferroptosis agonist to enhance tumor immunotherapy. Adv. Funct. Mater. 2023;33(44) [Google Scholar]
- 81.Yang S., et al. GSH/pH dual activatable cross-linked and fluorinated PEI for cancer gene therapy through endogenous iron de-hijacking and in situ ROS amplification. Adv. Mater. 2023;36(2) doi: 10.1002/adma.202304098. [DOI] [PubMed] [Google Scholar]
- 82.Liu N., et al. Carbonic anhydrase IX-targeted nanovesicles potentiated ferroptosis by remodeling the intracellular environment for synergetic cancer therapy. Nanoscale Horizons. 2023;8(6):783–793. doi: 10.1039/d2nh00494a. [DOI] [PubMed] [Google Scholar]
- 83.Xu T., et al. Enhanced ferroptosis by oxygen-boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano. 2020;14(3):3414–3425. doi: 10.1021/acsnano.9b09426. [DOI] [PubMed] [Google Scholar]
- 84.Meng X., et al. Triggered all-active metal organic framework: ferroptosis machinery contributes to the apoptotic photodynamic antitumor therapy. Nano Lett. 2019;19(11):7866–7876. doi: 10.1021/acs.nanolett.9b02904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.