Key Points
Question
Does acetaminophen use during pregnancy increase children’s risk of neurodevelopmental disorders?
Findings
In this population-based study, models without sibling controls identified marginally increased risks of autism and attention-deficit/hyperactivity disorder (ADHD) associated with acetaminophen use during pregnancy. However, analyses of matched full sibling pairs found no evidence of increased risk of autism (hazard ratio, 0.98), ADHD (hazard ratio, 0.98), or intellectual disability (hazard ratio, 1.01) associated with acetaminophen use.
Meaning
Acetaminophen use during pregnancy was not associated with children’s risk of autism, ADHD, or intellectual disability in sibling control analyses. This suggests that associations observed in other models may have been attributable to confounding.
Abstract
Importance
Several studies suggest that acetaminophen (paracetamol) use during pregnancy may increase risk of neurodevelopmental disorders in children. If true, this would have substantial implications for management of pain and fever during pregnancy.
Objective
To examine the associations of acetaminophen use during pregnancy with children’s risk of autism, attention-deficit/hyperactivity disorder (ADHD), and intellectual disability.
Design, Setting, and Participants
This nationwide cohort study with sibling control analysis included a population-based sample of 2 480 797 children born in 1995 to 2019 in Sweden, with follow-up through December 31, 2021.
Exposure
Use of acetaminophen during pregnancy prospectively recorded from antenatal and prescription records.
Main Outcomes and Measures
Autism, ADHD, and intellectual disability based on International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes in health registers.
Results
In total, 185 909 children (7.49%) were exposed to acetaminophen during pregnancy. Crude absolute risks at 10 years of age for those not exposed vs those exposed to acetaminophen were 1.33% vs 1.53% for autism, 2.46% vs 2.87% for ADHD, and 0.70% vs 0.82% for intellectual disability. In models without sibling control, ever-use vs no use of acetaminophen during pregnancy was associated with marginally increased risk of autism (hazard ratio [HR], 1.05 [95% CI, 1.02-1.08]; risk difference [RD] at 10 years of age, 0.09% [95% CI, −0.01% to 0.20%]), ADHD (HR, 1.07 [95% CI, 1.05-1.10]; RD, 0.21% [95% CI, 0.08%-0.34%]), and intellectual disability (HR, 1.05 [95% CI, 1.00-1.10]; RD, 0.04% [95% CI, −0.04% to 0.12%]). To address unobserved confounding, matched full sibling pairs were also analyzed. Sibling control analyses found no evidence that acetaminophen use during pregnancy was associated with autism (HR, 0.98 [95% CI, 0.93-1.04]; RD, 0.02% [95% CI, −0.14% to 0.18%]), ADHD (HR, 0.98 [95% CI, 0.94-1.02]; RD, −0.02% [95% CI, −0.21% to 0.15%]), or intellectual disability (HR, 1.01 [95% CI, 0.92-1.10]; RD, 0% [95% CI, −0.10% to 0.13%]). Similarly, there was no evidence of a dose-response pattern in sibling control analyses. For example, for autism, compared with no use of acetaminophen, persons with low (<25th percentile), medium (25th-75th percentile), and high (>75th percentile) mean daily acetaminophen use had HRs of 0.85, 0.96, and 0.88, respectively.
Conclusions and Relevance
Acetaminophen use during pregnancy was not associated with children’s risk of autism, ADHD, or intellectual disability in sibling control analysis. This suggests that associations observed in other models may have been attributable to familial confounding.
This nationwide cohort study with sibling control analysis examines the association of acetaminophen use during pregnancy with children’s risk of autism, ADHD, and intellectual disability.
Introduction
Acetaminophen (paracetamol) is commonly used to manage pain and fever during pregnancy. The US Food and Drug Administration1 and the European Medicines Agency2 consider acetaminophen to pose minimal risk during pregnancy. However, a 2021 consensus statement by an international group of scientists and clinicians3 recommended that pregnant individuals “forego [acetaminophen] unless its use is medically indicated,” among other precautionary actions, due to potential risk of developmental disorders such as autism and attention-deficit/hyperactivity disorder (ADHD).
Multiple biases may explain the associations observed in previous studies between acetaminophen use and neurodevelopmental disorders. Confounding by indication may occur based on the reasons that acetaminophen was taken, eg, due to infection,4 fever,5 migraine,6 or pain from autoimmune disease.7 These indications for acetaminophen use may be risk factors for neurodevelopmental disorders and may thus result in spurious associations. Confounding by parental health and genetics is likely because neurodevelopmental disorders are highly heritable8,9,10 and those who used acetaminophen during pregnancy reported higher prevalence of multiple health conditions associated with neurodevelopmental disorders compared with nonusers.11 Confounding by other medications is possible given the potential for use of multiple medications during pregnancy.12 Previous studies have also been limited by small sample sizes, leading to inconsistent and imprecise estimates.13
The current study investigated use of acetaminophen during pregnancy and children’s risk of autism, ADHD, and intellectual disability among nearly 2.5 million children in Sweden. This analysis featured prospectively collected antenatal and prescription records to capture medication use in a nationwide cohort, clinical neurodevelopmental diagnoses, and sibling comparisons to account for unobserved familial confounding.
Methods
Study Population
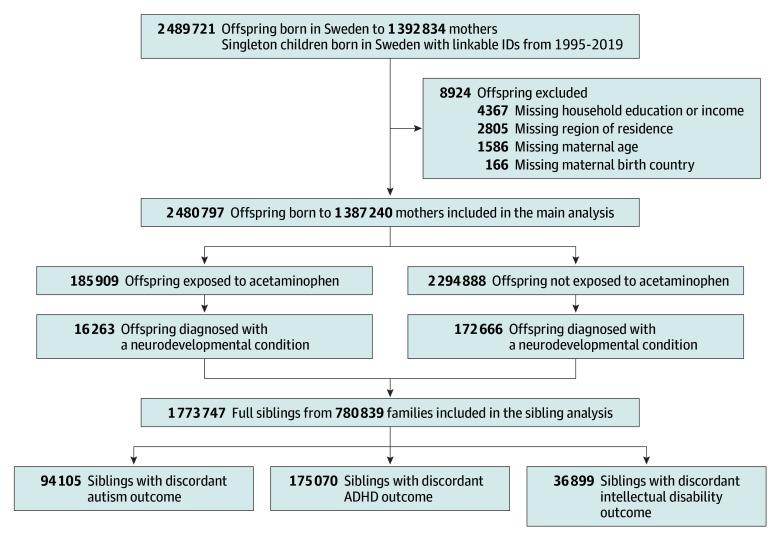
This study included all singleton liveborn children in Sweden from July 1, 1995, to December 31, 2019, with a linkable personal identifier (N = 2 489 721), with follow-up until December 31, 2021. Unique personal identifiers assigned to each resident at birth or emigration were used to link parents and children. The individual who gave birth is termed the birthing parent to use gender-inclusive terminology.14 Children were excluded if they had missing information on the birthing parent’s birth country (n = 166 [<0.01%]), age (n = 1586 [0.06%]), region of residence (n = 2805 [0.11%]), or household education or income (n = 4367 [0.18%]), resulting in a final analytical sample of 2 480 797 children (99.64% retained) (Figure 1). The Swedish Ethical Review Authority (2020-05516) approved the study and waiver for informed consent.
Figure 1. Flow of Participants in a Study of Acetaminophen Use During Pregnancy and Risk of Neurodevelopmental Disorders.
Acetaminophen Use During Pregnancy
For an expanded discussion of assessment of acetaminophen use, see eAppendix 1 in Supplement 1. Briefly, acetaminophen use during pregnancy was identified between 1995 and 2019 from the Medical Birth Register.15 Early drug exposure information was prospectively collected during the first antenatal visit (typically at 8-10 weeks’ gestation), during which midwives conducted structured interviews and examinations and recorded use of over-the-counter (OTC) or prescription medications. Later medication use in pregnancy was also prospectively documented by the midwife and physician. These data were later translated into Anatomical Therapeutic Chemical (ATC) codes by the National Board of Health and Welfare. From July 1, 2005, onward, the Medical Birth Register was supplemented with Prescribed Drug Register data covering all prescription dispensations in Sweden.
The primary exposure metric was ever-use of acetaminophen during pregnancy, determined from Medical Birth Register and Prescribed Drug Register data. For the subsample of pregnancies with Prescribed Drug Register coverage (ie, birth cohorts from July 1, 2005, onward), a secondary exposure metric of dose was examined. Based on the cumulative prescribed dosage dispensed over the pregnancy (number of packs × pack size × dosage), the mean daily acetaminophen dose for each pregnancy was calculated and categorized into no use, low daily use (<166 mg/d; <25th percentile), medium daily use (<429 mg/d; 25th to 75th percentile), and high daily use (≥430 mg/d; 76th to 100th percentile).
Neurodevelopmental Disorders
The primary outcomes were autism, ADHD, and intellectual disability diagnoses identified using International Classification of Diseases (ICD) codes from the National Patient Register (see eTable 1 in Supplement 1 for ICD codes).16 The Prescribed Drug Register was also used to identify ADHD diagnoses based on the dispensation of the ADHD medications17 methylphenidate (ATC: N06BA04) or atomoxetine (ATC: N06BA09).
Covariates
Covariates were collected from the Medical Birth Register, the National Patient Register, and the longitudinal integrated database for health insurance and labor market studies.18 To capture common indications for analgesics, this analysis recorded any birthing parent’s inpatient or specialized outpatient (2005 onward) diagnosis of migraine, chronic pain, infection, fever, rheumatoid arthritis, and headaches from 1 year before pregnancy until the day of delivery. This analysis also examined the following covariates implicated in drug use and/or child neurodevelopmental disorders: birth year; calendar period of delivery; child sex; birthing parent’s parity; age at delivery (linear term and cube term); country of birth; residential region; parental cohabitation at delivery; body mass index at first antenatal visit; smoking status; autism, ADHD, and intellectual disability diagnosis; history of psychiatric conditions; prescription use of psycholeptic drugs, antidepressants, and antiseizure medications; health care visits in the year before pregnancy and number of antenatal visits; and education (highest of either of the parents) and disposable household income (income minus outgoing expenses, including taxes, with adjustment for family size).19 This study also considered use of the following other analgesics during pregnancy, using the same data sources as for acetaminophen: aspirin, nonaspirin nonsteroidal anti-inflammatory drugs (NSAIDs), opioid medications, and antimigraine medications. See eTable 1 in Supplement 1 for ICD and ATC codes.
Statistical Analysis
Population-Based Sample (Without Sibling Control)
This analysis used Cox proportional hazards models with cluster robust standard errors to estimate the hazard ratios (HRs) and 95% CIs while accounting for clustering of siblings by family. Children were followed up from birth, using the age of the child as the time scale, until the earliest date of diagnosis of a neurodevelopmental disorder, death, emigration, or end of follow-up. Models were adjusted for use of other analgesics and all covariates described above. Covariates with missing data were imputed using the dummy category approach. E-values, defined as the minimum association an unmeasured confounder would need to have with both acetaminophen use and the neurodevelopmental disorder to nullify observed associations,20 were reported for point estimates in the fully adjusted models. Differences in absolute risk at 10 years of age (ie, the marginal mean treatment effect) were calculated using Breslow baseline estimator and bootstrapped 95% CI with 1000 repetitions.21
Sibling Analysis
To account for unobserved genetic and environmental confounders shared between full siblings, sibling analysis of 1 773 747 full siblings (ie, siblings with the same biological parents as determined by the Multigenerational Register) was performed using stratified Cox regression with cluster robust standard errors, conditioned on the family (eAppendix 1 in Supplement 1). Characteristics of the sibling cohort are presented in eTable 2 in Supplement 1 and a comparison of pregnant individuals who had discordant vs concordant acetaminophen use across pregnancies is presented in eTable 3 in Supplement 1. The sibling analysis was adjusted for all putative confounders that differed among siblings (ie, excluding birthing parent’s birth country, psychiatric history, and diagnosis of autism, ADHD, and intellectual disability). Sample sizes for sibling analyses are reported in eTable 4 in Supplement 1.
Sensitivity Analyses
Several analyses were conducted to test the robustness of results. Sensitivity analyses examined whether results varied by source of medication data or across study period by stratifying by birth cohorts. Other analyses examined whether results differed by missing data approach by repeating total sample analyses using inverse probability weighting correction22 and complete case analysis. For dose analyses, sensitivity analyses examined whether OTC use of acetaminophen influenced estimates and repeated analyses using restricted cubic splines with knots at the fifth, 35th, 65th, and 95th percentiles. For sibling analyses, sensitivity analyses examined the generalizability of the full sibling cohort, whether carryover effects (in which one sibling’s exposure or outcome affects the other sibling’s exposure or outcome) were present, and whether exposure measurement error may have biased estimates (eAppendix in Supplement 1).
Results
Among the 2 480 797 included children, 185 909 (7.49%) were exposed to acetaminophen during pregnancy (Table 1, Figure 2). Acetaminophen exposure was more common among children born to birthing parents with a lower socioeconomic position, a higher early pregnancy body mass index, those who smoked during pregnancy, and those with diagnoses of any psychiatric conditions, neurodevelopmental disorders, indications for acetaminophen, and co-prescription of related therapeutics.
Table 1. Patient Characteristics (2 480 797 Children and 1 387 240 Birthing Parents).
| Characteristic | No. (%) | |
|---|---|---|
| Exposed to acetaminophen (n = 185 909)a | Not exposed to acetaminophen (n = 2 294 888) | |
| Child’s characteristics | ||
| Birth sex | ||
| Female | 90 513 (48.7) | 1 114 030 (48.5) |
| Male | 95 396 (51.3) | 1 180 858 (51.5) |
| Neurodevelopmental diagnosesb | ||
| Autism | 5912 (3.2) | 62 672 (2.7) |
| ADHD | 12 834 (6.9) | 133 552 (5.8) |
| Intellectual disability | 2084 (1.1) | 22 470 (1.0) |
| Season of birth | ||
| December to February | 41 932 (22.6) | 533 332 (23.2) |
| March to May | 48 054 (25.8) | 614 690 (26.8) |
| June to August | 49 579 (26.7) | 607 166 (26.5) |
| September to November | 46 344 (24.9) | 539 700 (23.5) |
| Birthing parent’s characteristics | ||
| Age at delivery, y | ||
| <25 | 22 806 (12.3) | 285 975 (12.5) |
| 25-29 | 55 123 (29.7) | 688 245 (30.0) |
| 30-34 | 63 650 (34.2) | 807 129 (35.2) |
| 35-39 | 35 111 (18.9) | 415 644 (18.1) |
| >40 | 9219 (5.0) | 97 895 (4.3) |
| Swedish-born | ||
| Yes | 140 160 (75.4) | 1 789 206 (78.0) |
| Highest household education | ||
| Secondary schooling level (mandatory) | 13 012 (7.0) | 118 258 (5.2) |
| Upper secondary level | 82 504 (44.4) | 902 251 (39.3) |
| University level | 90 393 (48.6) | 1 274 379 (55.5) |
| Household disposable income, quintile | ||
| Q1 (lowest) | 36 593 (19.7) | 390 856 (17.0) |
| Q2 | 40 902 (22.0) | 440 275 (19.2) |
| Q3 | 38 982 (21.0) | 469 553 (20.5) |
| Q4 | 37 225 (20.0) | 486 196 (21.2) |
| Q5 (highest) | 32 207 (17.3) | 508 008 (22.1) |
| Residential region | ||
| Middle Sweden | 43 664 (23.5) | 441 481 (19.2) |
| Stockholm | 37 306 (20.1) | 585 786 (25.5) |
| West Sweden | 34 248 (18.4) | 465 287 (20.3) |
| Southern Sweden | 30 601 (16.5) | 377 450 (16.4) |
| Southeast Sweden | 22 856 (12.3) | 230 727 (10.1) |
| Northern Sweden | 17 234 (9.3) | 194 157 (8.5) |
| Parental cohabitation at delivery | ||
| Yes | 164 750 (88.6) | 2 067 690 (90.1) |
| Parity | ||
| Nulliparous | 74 902 (40.3) | 1 004 830 (43.8) |
| 1 | 64 873 (34.9) | 844 898 (36.8) |
| 2 | 29 544 (15.9) | 310 628 (13.5) |
| 3 | 10 112 (5.4) | 86 433 (3.8) |
| ≥4 | 6478 (3.5) | 48 099 (2.1) |
| Early pregnancy BMI category | ||
| Underweight | 3378 (1.8) | 53 191 (2.3) |
| Normal weight | 89 625 (48.2) | 1 265 747 (55.2) |
| Overweight | 49 856 (26.8) | 510 519 (22.2) |
| Obese | 30 804 (16.6) | 235 693 (10.3) |
| Missing category | 12 246 (6.6) | 229 738 (10.0) |
| Smoking status | ||
| No smoking | 148 659 (80.0) | 1 882 139 (82.0) |
| Smoked during pregnancy | 21 266 (11.4) | 189 115 (8.2) |
| Only smoked before pregnancy | 13 384 (7.2) | 131 662 (5.7) |
| Missing category | 2600 (1.4) | 91 972 (4.0) |
| Psychiatric conditionsb | ||
| Autismc | 1300 (0.7) | 10 226 (0.4) |
| ADHDc | 7273 (3.9) | 55 498 (2.4) |
| Intellectual disabilityc | 746 (0.4) | 5197 (0.2) |
| History of any psychiatric conditionsd | 26 639 (14.3) | 216 704 (9.4) |
| Diagnosesb,e | ||
| Infection | 12 544 (6.7) | 100 352 (4.4) |
| Diagnosed headache | 4915 (2.6) | 22 643 (1.0) |
| Chronic pain | 3223 (1.7) | 18 383 (0.8) |
| Asthma | 2573 (1.4) | 17 637 (0.8) |
| Rheumatoid arthritis | 1226 (0.7) | 2449 (0.1) |
| Migraine | 730 (0.4) | 3582 (0.2) |
| Fever | 704 (0.4) | 3993 (0.2) |
| Drug use in pregnancyb | ||
| Opioid medications | 27 845 (15.0) | 54 125 (2.4) |
| Nonaspirin NSAIDs | 19 634 (10.6) | 41 953 (1.8) |
| Aspirin | 5537 (3.0) | 34 274 (1.5) |
| Antimigraine medications | 4708 (2.5) | 14 979 (0.7) |
| Antidepressant medications | 10 655 (5.7) | 70 644 (3.1) |
| Psycholeptic medications | 7386 (4.0) | 22 413 (1.0) |
| Antiseizure medications | 1946 (1.0) | 10 314 (0.4) |
| Health care visits in the year before pregnancy | ||
| 0-3 | 162 096 (87.2) | 2 129 608 (92.8) |
| 4-10 | 20 371 (11.0) | 148 671 (6.5) |
| ≥11 | 3442 (1.9) | 16 609 (0.7) |
| No. of antenatal visits | ||
| ≥4 | 177 502 (95.5) | 2 055 729 (89.6) |
| <4 | 5148 (2.8) | 74 221 (3.2) |
| Missing category | 3259 (1.8) | 164 938 (7.2) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NSAID, nonsteroidal anti-inflammatory drug.
Acetaminophen exposure is defined as ever-use of acetaminophen during pregnancy as determined by the Medical Birth Register and Prescribed Drug Register using Anatomical Therapeutic Chemical codes as described in Supplement 1.
Not mutually exclusive.
Diagnosed any time in life.
Diagnosed any time before pregnancy.
Diagnosed from 1 year before pregnancy until the day of delivery.
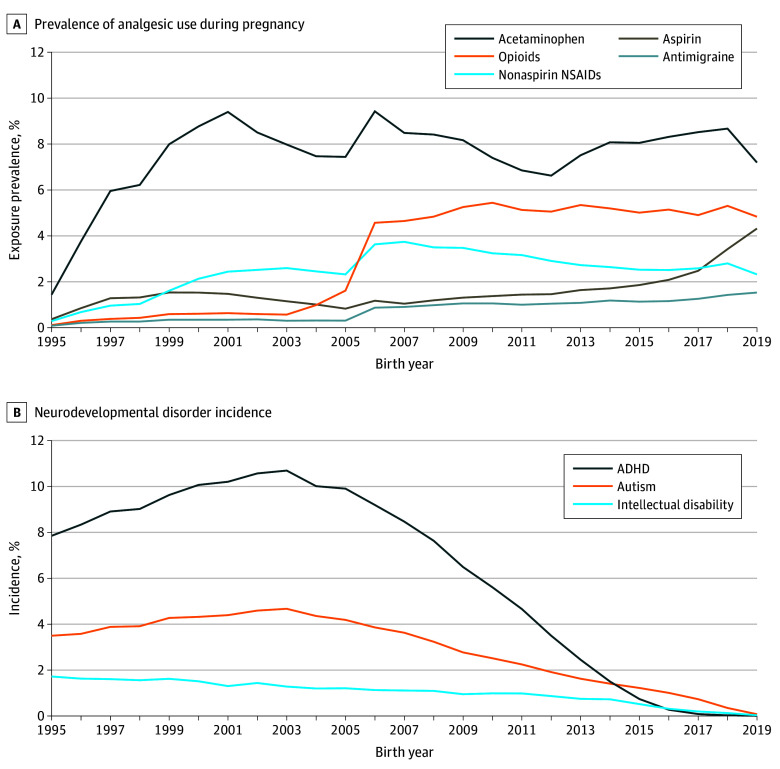
Figure 2. Prevalence of Analgesic Use During Pregnancy and Neurodevelopmental Disorder Incidence (N = 2 480 797 Children).
Prevalence of analgesic use during pregnancy was 185 909 (7.5%) for acetaminophen, 39 811 (1.6%) for aspirin, 61 597 (2.5%) for nonaspirin nonsteroidal anti-inflammatory drugs (NSAIDs), 81 970 (3.3%) for opioid medications, and 19 687 (0.8%) for antimigraine medications. Cumulative incidence of neurodevelopmental disorders was 68 584 (2.8%) for autism, 146 386 (5.9%) for attention-deficit/hyperactivity disorder (ADHD), and 24 554 (1.0%) for intellectual disorder.
During a median (IQR) follow-up of 13.4 (7.6-19.8) years, 188 929 children (7.62%) were diagnosed with at least 1 neurodevelopmental condition (ADHD, autism, or intellectual disability): 68 584 (2.76%) with autism, 146 386 (5.90%) with ADHD, and 24 554 (0.99%) with intellectual disability (Figure 2). Median (IQR) age of diagnosis was 11.6 (7.0-15.3) years for autism, 12.2 (9.2-15.6) years for ADHD, and 8.2 (5.3-12.7) years for intellectual disability.
Association Between Acetaminophen and Children’s Neurodevelopmental Disorders
Ever-Use vs No Use
Preliminary models are shown in eTable 5 in Supplement 1. In final adjusted models in the population-based sample, children exposed to acetaminophen were slightly more likely to be diagnosed with autism (HR, 1.05 [95% CI, 1.02-1.08]; e-value, 1.28), ADHD (HR, 1.07 [95% CI, 1.05-1.10]; e-value, 1.34), and intellectual disability (HR, 1.05 [95% CI, 1.00-1.10]; e-value, 1.28) compared with children not exposed to acetaminophen (Figure 3; eTable 6 in Supplement 1). Differences in absolute risk between acetaminophen users and nonusers were small. For example, for the population-based adjusted model of acetaminophen and autism, the absolute risk at 10 years was 0.09% higher with acetaminophen use (Figure 3; eTable 7 in Supplement 1). E-values indicated that an unobserved confounder would have to be only modestly associated with both the exposure and outcome to nullify the above associations. Sensitivity analysis using Cox models without sibling control in the full sibling cohort demonstrated that the sibling sample had the same apparent acetaminophen outcome associations as the total sample (eAppendix 2 and eTable 8 in Supplement 1).
Figure 3. Association Between Analgesic Use During Pregnancy and Children’s Risk of Autism, ADHD, and Intellectual Disability.
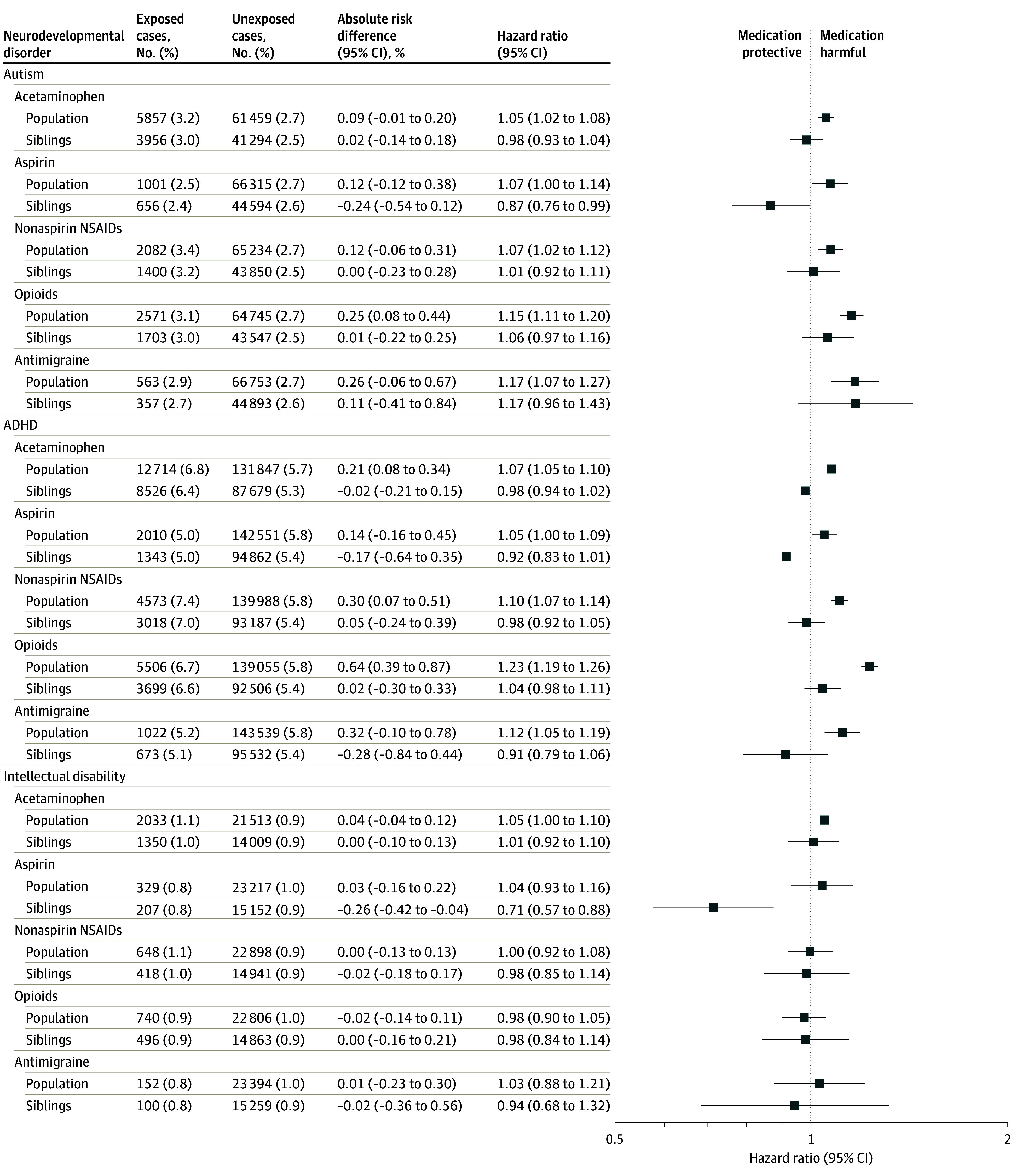
Absolute risk difference is the difference in absolute risk at 10 years of age between exposed and unexposed children, expressed as a percentage. For example, the 0.09% absolute difference for acetaminophen and autism can be interpreted as follows: the risk of child autism at 10 years of age is 0.09% higher with acetaminophen use compared with no acetaminophen use. The population-based model was adjusted for birth cohort; child sex; all other analgesics; birthing parent’s diagnoses of migraine, chronic pain, infections, fevers, rheumatoid arthritis, and headaches; calendar period of delivery; parity; age at delivery (linear and cubic term); country of birth; residential region; cohabitation at delivery; early pregnancy body mass index; smoking status; diagnosis of autism, attention-deficit/hyperactivity disorder (ADHD), and intellectual disability; history of psychiatric conditions and prescription use of psycholeptics, antidepressants, and antiseizure medication; health care visits in the year before pregnancy and an inadequate number of antenatal visits; and the highest household education and disposable income. The sibling control model was adjusted for all of the above excluding birthing parent’s birth country, psychiatric history, and diagnosis autism, ADHD, and intellectual disability. NSAID indicates nonsteroidal anti-inflammatory drug.
In contrast, in models with sibling control, acetaminophen was not associated with children’s risk autism (HR, 0.98 [95% CI, 0.93-1.04]), ADHD (HR, 0.98 [95% CI, 0.94-1.02]), or intellectual disability (HR, 1.01 [95% CI, 0.92-1.10]) (Figure 3; eTable 6 in Supplement 1). Carryover effects did not bias the autism and intellectual disability analyses among sibling controls, although the possibility of carryover effects for ADHD could not be ruled out (eAppendix 2 and eTable 9 in Supplement 1). Simulations indicated that even if acetaminophen use had been substantially underascertained, such measurement error would have been unlikely to result in the null associations observed with sibling control (eAppendix 2 and eTable 10 in Supplement 1). Additionally, sensitivity analyses suggested that results were consistent regardless of data source used for ascertainment of medication use (eTable 11 in Supplement 1), stratification by older vs younger birth cohorts (eTable 12 in Supplement 1), or method of handling missing data (eTable 13 in Supplement 1).
The magnitude of the association between acetaminophen and neurodevelopmental disorders was generally comparable to or lower than estimates for aspirin, nonaspirin NSAIDs, opioids, and antimigraine medications (Figure 3). As with acetaminophen, the association between nonaspirin NSAIDs, opioids, and antimigraine medications and neurodevelopmental disorders diminished to null in the sibling analysis. However, aspirin was inversely associated with neurodevelopmental disorders in the sibling analysis: HR of 0.87 (95% CI, 0.76-0.99) for autism, 0.92 (95% CI, 0.83-1.01) for ADHD, and 0.71 (95% CI, 0.57-0.88) for intellectual disability.
Dose-Response Patterns
Characteristics of the study subsample with prescription dispensation information for acetaminophen are displayed in eTable 14 in Supplement 1. Persons with higher acetaminophen use tended to have more diagnoses of psychiatric conditions, neurodevelopmental disorders, indications for acetaminophen, and co-prescriptions of related therapeutics, among other differences. Mean dispensed prescription acetaminophen dose per day across pregnancy is displayed in eFigure 1 in Supplement 1. The median (IQR) mean daily dose was 228.5 (165.6-429.0) mg/d. Table 2 shows that in population-based models, a dose-response pattern seen with partially adjusted models attenuated with covariate adjustment and fully disappeared in the sibling analysis. For example, in sibling control models for autism, compared with no use of acetaminophen, the HR was 0.85 (95% CI, 0.68-1.08) for low mean daily use (<166 mg/d), 0.96 (95% CI, 0.79-1.15) for medium (166-429 mg/d) mean daily use, and 0.88 (95% CI, 0.68-1.14) for high (≥430 mg/d) mean daily use. Spline analyses confirmed the lack of a dose-response association in sibling analyses (eFigure 2 in Supplement 1). Adjusting for OTC acetaminophen use or excluding persons who used only OTC, but not prescribed, acetaminophen did not alter results (eTable 15 in Supplement 1).
Table 2. Association of Mean Daily Dispensed Prescription Acetaminophen Dose During Pregnancy and Children’s Risk of Autism, ADHD, and Intellectual Disabilitya.
| Model 1b | Model 2c | Model 3d | Sibling analysise | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Autism | ||||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Low dose (<166 mg/d) | 1.33 (1.20-1.48) | <.001 | 1.14 (1.02-1.26) | .017 | 1.01 (0.91-1.12) | .89 | 0.85 (0.67-1.07) | .17 |
| Medium dose (166-429 mg/d) | 1.52 (1.41-1.64) | <.001 | 1.29 (1.19-1.39) | <.001 | 1.11 (1.03-1.20) | .007 | 0.96 (0.79-1.16) | .64 |
| High dose (≥430 mg/d) | 1.87 (1.71-2.06) | <.001 | 1.45 (1.32-1.60) | <.001 | 1.10 (1.00-1.22) | .06 | 0.88 (0.68-1.14) | .33 |
| ADHD | ||||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Low dose (<166 mg/d) | 1.46 (1.36-1.57) | <.001 | 1.17 (1.09-1.26) | <.001 | 1.14 (1.06-1.23) | <.001 | 1.01 (0.84-1.21) | .94 |
| Medium dose (166-429 mg/d) | 1.75 (1.65-1.85) | <.001 | 1.39 (1.31-1.47) | <.001 | 1.25 (1.18-1.33) | <.001 | 1.02 (0.88-1.18) | .81 |
| High dose (≥430 mg/d) | 1.97 (1.83-2.11) | <.001 | 1.41 (1.31-1.52) | <.001 | 1.17 (1.08-1.27) | <.001 | 0.98 (0.79-1.21) | .83 |
| Intellectual disability | ||||||||
| No use | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Low dose (<166 mg/d) | 1.34 (1.14-1.58) | .001 | 1.27 (1.07-1.50) | .006 | 0.98 (0.83-1.16) | .84 | 0.87 (0.60-1.24) | .43 |
| Medium dose (166-429 mg/d) | 1.47 (1.30-1.66) | <.001 | 1.38 (1.22-1.57) | <.001 | 1.06 (0.93-1.20) | .40 | 0.97 (0.74-1.27) | .82 |
| High dose (≥430 mg/d) | 1.92 (1.65-2.22) | <.001 | 1.75 (1.50-2.05) | <.001 | 1.12 (0.96-1.31) | .17 | 0.93 (0.63-1.38) | .73 |
Abbreviation: ADHD, attention-deficit/hyperactivity disorder; HR, hazard ratio; NSAID, nonsteroidal anti-inflammatory drugs.
Daily mean dose categories defined low (<25th percentile; <166 mg/d), medium (25th-75th percentile; 166-429 mg/d), and high (>75th percentile; ≥430 mg/d).
Model 1: Adjusted for birth cohort and child sex.
Model 2: Model 1 plus birthing parent’s diagnosis of migraine, chronic pain, infections, fevers, rheumatoid arthritis, and headaches, and any other analgesics (aspirin, nonaspirin NSAIDs, opioids, or anti-migraine medications).
Model 3: Model 2 plus calendar period of delivery, parity, birthing parent’s age at delivery, country of birth, residential region, cohabitation at delivery, early pregnancy body mass index, smoking status, and birthing parent’s autism, ADHD, intellectual disability, history of psychiatric conditions, and birthing parent’s prescription use of psycholeptic medications, antidepressants, and antiseizure medication, and health care visits in the year before pregnancy, and an inadequate number of antenatal visits, and the highest household education and disposable income.
Sibling analysis: Model 3 covariates, excluding those with perfect balance between full siblings (ie, birthing parent’s birth country, psychiatric history, and diagnosis of autism, ADHD, and intellectual disability).
Discussion
Sibling control analyses found no evidence that acetaminophen use during pregnancy was associated with children’s risk of autism, ADHD, or intellectual disability. This suggests that the small increase in children’s risk of neurodevelopmental disorders associated with acetaminophen use observed in statistical models without sibling control may have been due to unmeasured confounding. In addition, sibling control analyses did not identify a dose-response association between acetaminophen and neurodevelopmental disorders.
Results of this study indicate that the association between acetaminophen use during pregnancy and neurodevelopmental disorders is a noncausal association. Birthing parents with higher acetaminophen use differed in many aspects from those with lower use or no use. Results suggested that there was not one single “smoking gun” confounder, but rather that multiple birthing parents’ health and sociodemographic characteristics each explained at least part of the apparent association. The null results of the sibling control analyses indicate that shared familial confounders were involved, but do not identify the specific confounding factors.
Pregnant individuals’ genetics are likely candidates as unmeasured confounders, because certain genotypes or phenotypes increase both the probability of acetaminophen use and neurodevelopmental disorders. The Avon Longitudinal Study of Parents and Children found that birthing parent’s ADHD polygenic risk was associated with acetaminophen use in pregnancy.23 The Norwegian Mother, Father, and Child Cohort Study (MoBa) found that birthing parent’s ADHD polygenic risk was associated with pregnancy pain, migraine, and use of pain medications including acetaminophen, while birthing parent’s autism polygenic risk was associated with migraine.24 The Japan Environment and Children’s Study found that birthing parent’s autistic traits were associated with a higher degree of antenatal pain.25 These findings are consistent with the current study’s observation that higher acetaminophen use correlated with higher prevalence of birthing parent’s neurodevelopmental disorders. Time-varying indications for acetaminophen, use such as infection or fever, could also play a role, as indicated by previous findings that prenatal, but not postnatal, acetaminophen use was associated with increased risk of autism and ADHD.13
The present results demonstrated a dose-response pattern that was attenuated with increasing covariate control and nullified in sibling control. Although the dose analysis suggested that even the highest level of acetaminophen use was not associated with increased risk, the results should not be interpreted as benchmarks for safety. Dose in this study only reflected dispensed prescriptions and not actual use of those dispensations or OTC use.
These analyses adjusted for use of other analgesics to address potential confounding. Examination of the associations of neurodevelopmental conditions with these other analgesics suggested that they are subject to the same confounding structures as acetaminophen. This study found an unexpected result in that aspirin use in the sibling control analyses, but not in the total population, was associated with reduced risk of neurodevelopmental disorders. The population of birthing parents who do not use aspirin during one pregnancy but use it in another pregnancy is likely a select subset of the population. Although routine aspirin use is not recommended during pregnancy, low-dose aspirin use for preeclampsia prevention is associated with lower risk of perinatal factors implicated in neurodevelopmental disorders, including preeclampsia, preterm birth, and intrauterine growth restriction.26,27,28,29,30 Thus, this finding appears to be biologically plausible and warrants further investigation.
A meta-analysis of 6 European cohorts (none of which used sibling controls) concluded that children prenatally exposed to acetaminophen had 19% and 21% higher odds of symptoms of autism and ADHD, respectively.13 However, results were driven by the largest cohort of 61 430 children, because none of the other cohorts found evidence of associations (all P > .05) with symptoms of autism or ADHD. The magnitudes of conventional risk estimates for acetaminophen in the present study were similar to those from other studies, indicating that the current study was not biased toward null findings. In the previous largest study of acetaminophen use during pregnancy and autism by Liew and colleagues, the crude HR for ever-use of acetaminophen and autism spectrum disorders was 1.22.31 In comparison, the crude HR for acetaminophen ever-use and autism was 1.26 in the present study.
Two studies, both using data from the MoBa Study, used sibling controls to examine acetaminophen use during pregnancy and neurodevelopmental outcomes. Brandlistuen and colleagues found that acetaminophen was associated with externalizing symptoms at 3 years of age.32 In contrast, Gustavson and colleagues found that although models without sibling control showed that long-term exposure (≥29 days) to acetaminophen was associated with higher risk (HR, 2.02 [95% CI, 1.17-3.25]) of ADHD diagnosis, the association was nullified (HR, 1.06 [95% CI, 0.51-2.05]) in sibling analyses.33 The differences in results between studies, even drawing from the same parent study, may be explained by several factors, including that clinical ADHD diagnoses differ from parental report of symptoms or the potential instability of symptoms at 3 years of age.33
Limitations
Although this study had several strengths (large, nationally representative sample; extensive confounding control; and systematic, prospective recording of both acetaminophen use and clinical diagnoses of neurodevelopmental disorders), there were also limitations. First, although a past medical record review confirmed Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) diagnosis of autism in 83 of 88 persons in this data set,16 the ADHD and intellectual disability diagnoses in the data analyzed have not been validated. In addition, the median ages of neurodevelopmental diagnoses in the present study were higher than in other studies, but this should not meaningfully affect generalizability. Median age of diagnosis is dependent on the age of children studied, so longer follow-up generally results in higher median ages of diagnoses.34
Second, exposure assessment was not perfect. Antenatal data in the Medical Birth Register only recorded whether a birthing parent used acetaminophen, without regard to dose, duration, or timing. Prescription dispensation records might not reflect OTC use of acetaminophen. Thus, more specific aspects of exposure could not be assessed, and one cannot definitively exclude the possibility that use of acetaminophen beyond a certain dose at a critical point might pose some risk. This study’s finding that 7.49% of birthing parents used acetaminophen during pregnancy is lower than in some studies, but is concordant with other studies. Self-reported medication use is subject to underreporting of actual use.35 However, even large amounts of exposure measurement error could not explain the nullification of associations seen with sibling control. Third, this study did not have data on conditions that did not require inpatient or outpatient medical care. Many indications for acetaminophen use, such as headache, infection, fever, and other pain, may not rise to a level that warrants seeking medical attention. Thus, capture of potential indications is incomplete.16
Conclusions
Acetaminophen use during pregnancy was not associated with children’s risk of autism, ADHD, or intellectual disability in sibling control analyses. This suggests that associations observed in models without sibling control may have been attributable to confounding.
Educational Objective: To identify the key insights or developments described in this article.
-
Which of the following was a feature of this analysis of acetaminophen use in pregnancy and children’s risk of autism and intellectual disability?
Artificial intelligence–assisted parental assessment of neurodevelopment.
Sibling comparisons as a control group.
Testing of acetaminophen levels in all 3 trimesters of pregnancy.
-
What association was found between acetaminophen use and children’s neurodevelopmental disorders?
Acetaminophen was similar to aspirin in demonstrating a protective effect.
In final adjusted models in the population-based sample, there was no difference in autism between children exposed and not exposed to acetaminophen.
In models with sibling control, acetaminophen was not associated with children’s risk of autism or intellectual disability.
-
How do the authors interpret the results?
Birthing parents with higher acetaminophen use were similar to those with low or no use in every measured way, making confounding by use patterns an unlikely explanation for the findings.
Shared familial confounders are unlikely to explain the results.
The association between acetaminophen use during pregnancy and neurodevelopmental disorders is a noncausal association.
eResults
Data sharing statement
References
- 1.US Food and Drug Administration . FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. January 9, 2015. Accessed March 19, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy
- 2.Pharmacovigilance Risk Assessment Committee. PRAC recommendations on signals: adopted at the 12-15 March 2019 PRAC meeting. European Medicines Agency. April 8, 2019. Accessed March 19, 2024. https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-12-15-march-2019-prac-meeting_en.pdf
- 3.Bauer AZ, Swan SH, Kriebel D, et al. Paracetamol use during pregnancy: a call for precautionary action. Nat Rev Endocrinol. 2021;17(12):757-766. doi: 10.1038/s41574-021-00553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee BK, Magnusson C, Gardner RM, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100-105. doi: 10.1016/j.bbi.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornig M, Bresnahan MA, Che X, et al. Prenatal fever and autism risk. Mol Psychiatry. 2018;23(3):759-766. doi: 10.1038/mp.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie S, Karlsson H, Dalman C, et al. Family history of mental and neurological disorders and risk of autism. JAMA Netw Open. 2019;2(3):e190154. doi: 10.1001/jamanetworkopen.2019.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He H, Yu Y, Liew Z, et al. Association of maternal autoimmune diseases with risk of mental disorders in offspring in Denmark. JAMA Netw Open. 2022;5(4):e227503. doi: 10.1001/jamanetworkopen.2022.7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie S, Karlsson H, Dalman C, et al. The familial risk of autism spectrum disorder with and without intellectual disability. Autism Res. 2020;13(12):2242-2250. doi: 10.1002/aur.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein P, Tideman M, Sullivan PF, et al. Familial risk and heritability of intellectual disability: a population-based cohort study in Sweden. J Child Psychol Psychiatry. 2022;63(9):1092-1102. doi: 10.1111/jcpp.13560 [DOI] [PubMed] [Google Scholar]
- 10.Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med. 2014;44(10):2223-2229. doi: 10.1017/S0033291713002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandoli G, Palmsten K, Chambers C. Acetaminophen use in pregnancy: examining prevalence, timing, and indication of use in a prospective birth cohort. Paediatr Perinat Epidemiol. 2020;34(3):237-246. doi: 10.1111/ppe.12595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian A, Azcoaga-Lorenzo A, Anand A, et al. ; MuM-PreDiCT Group . Polypharmacy during pregnancy and associated risk factors: a retrospective analysis of 577 medication exposures among 1.5 million pregnancies in the UK, 2000-2019. BMC Med. 2023;21(1):21. doi: 10.1186/s12916-022-02722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alemany S, Avella-García C, Liew Z, et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: meta-analysis in six European population-based cohorts. Eur J Epidemiol. 2021;36(10):993-1004. doi: 10.1007/s10654-021-00754-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rioux C, Weedon S, London-Nadeau K, et al. Gender-inclusive writing for epidemiological research on pregnancy. J Epidemiol Community Health. 2022;76(9):823-827. doi: 10.1136/jech-2022-219172 [DOI] [PubMed] [Google Scholar]
- 15.National Board of Health Welfare . The Swedish Medical Birth Register: A Summary of Content and Quality. National Board of Health and Welfare Stockholm; 2003. [Google Scholar]
- 16.Ludvigsson JF, Reichenberg A, Hultman CM, Murray JA. A nationwide study of the association between celiac disease and the risk of autistic spectrum disorders. JAMA Psychiatry. 2013;70(11):1224-1230. doi: 10.1001/jamapsychiatry.2013.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosidou K, Dalman C, Widman L, et al. Maternal polycystic ovary syndrome and risk for attention-deficit/hyperactivity disorder in the offspring. Biol Psychiatry. 2017;82(9):651-659. doi: 10.1016/j.biopsych.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 18.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423-437. doi: 10.1007/s10654-019-00511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai D, Lewis G, Lundberg M, et al. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. J Am Acad Child Adolesc Psychiatry. 2012;51(5):467-476.e6. doi: 10.1016/j.jaac.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 20.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602-603. doi: 10.1001/jama.2018.21554 [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA. The Breslow estimator of the nonparametric baseline survivor function in Cox’s regression model: some heuristics. Epidemiology. 2008;19(1):101-102. doi: 10.1097/EDE.0b013e31815be045 [DOI] [PubMed] [Google Scholar]
- 22.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 23.Leppert B, Havdahl A, Riglin L, et al. Association of maternal neurodevelopmental risk alleles with early-life exposures. JAMA Psychiatry. 2019;76(8):834-842. doi: 10.1001/jamapsychiatry.2019.0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havdahl A, Wootton RE, Leppert B, et al. Associations between pregnancy-related predisposing factors for offspring neurodevelopmental conditions and parental genetic liability to attention-deficit/hyperactivity disorder, autism, and schizophrenia: the Norwegian mother, father and child cohort study (MoBa). JAMA Psychiatry. 2022;79(8):799-810. doi: 10.1001/jamapsychiatry.2022.1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Kimura T, Cui M, et al. Maternal autistic traits and antenatal pain by cross-sectional analysis of the Japan Environment and Children’s Study. Sci Rep. 2023;13(1):6068. doi: 10.1038/s41598-023-32945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326(12):1192-1206. doi: 10.1001/jama.2021.8551 [DOI] [PubMed] [Google Scholar]
- 27.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344-355. doi: 10.1542/peds.2010-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie S, Heuvelman H, Magnusson C, et al. Prevalence of autism spectrum disorders with and without intellectual disability by gestational age at birth in the Stockholm youth cohort: a register linkage study. Paediatr Perinat Epidemiol. 2017;31(6):586-594. doi: 10.1111/ppe.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel KM, Dalman C, Svensson AC, et al. Deviance in fetal growth and risk of autism spectrum disorder. Am J Psychiatry. 2013;170(4):391-398. doi: 10.1176/appi.ajp.2012.12040543 [DOI] [PubMed] [Google Scholar]
- 30.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7-14. doi: 10.1192/bjp.bp.108.051672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liew Z, Ritz B, Virk J, Olsen J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: a Danish national birth cohort study. Autism Res. 2016;9(9):951-958. doi: 10.1002/aur.1591 [DOI] [PubMed] [Google Scholar]
- 32.Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702-1713. doi: 10.1093/ije/dyt183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustavson K, Ystrom E, Ask H, et al. Acetaminophen use during pregnancy and offspring attention deficit hyperactivity disorder: a longitudinal sibling control study. JCPP Adv. 2021;1(2):e12020. doi: 10.1002/jcv2.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van ’t Hof M, Tisseur C, van Berckelear-Onnes I, et al. Age at autism spectrum disorder diagnosis: a systematic review and meta-analysis from 2012 to 2019. Autism. 2021;25(4):862-873. doi: 10.1177/1362361320971107 [DOI] [PubMed] [Google Scholar]
- 35.de Korte BAC, Smeets NJL, Colbers A, van den Bemt BJF, van Gelder MMHJ. Adherence to prescription medication during pregnancy: do pregnant women use pharmacological treatment as prescribed? Br J Clin Pharmacol. 2023;89(5):1521-1531. doi: 10.1111/bcp.15609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eResults
Data sharing statement