Abstract
To assess whether nitric oxide synthase 2 (NOS2) fulfills the criteria of an innate resistance locus against an acute viral infection, we inoculated genetically deficient NOS2−/− mice with virulent ectromelia virus (EV), the causative agent of mousepox. NOS2−/− mice proved highly susceptible to EV yet showed no diminution in other well-characterized anti-EV immune responses, i.e., gamma interferon secretion and NK cell and EV-specific cytotoxic T lymphocyte activities. Thus, the NOS2 locus can be considered a critical monogenic determinant of EV resistance that contributes to host survival.
Cytokine-inducible nitric oxide synthase 2 (NOS2) belongs to a multigene family of heme-containing flavoenzymes catalyzing the 5-electron oxidation of l-arginine to l-citrulline plus the cytotoxic radical gas nitric oxide (NO) (13). Rapid transcriptional expression of NOS2 in most nucleated cell types together with its sustained, high-output production of NO endows this enzyme with broad antimicrobial properties (13). The idea that NOS2 may subserve an important host protective function against viruses has recently been appreciated (8, 13, 19). Evidence was first obtained in experiments using ectromelia virus (EV), vaccinia virus (VV), and herpes simplex virus type 1 in permissive human and mouse cell lines rendered resistant by transfection with the NOS2 gene (11) or by pharmacologic provision of NO (3, 11). Since these initial observations were made, several other RNA and DNA viruses have been proven sensitive to the virustatic action of NO in vitro: Epstein-Barr virus, coxsackievirus type B3 (CVB3), Friend murine leukemia virus, vesicular stomatitis virus, mouse hepatitis virus, rhinovirus, Japanese encephalitis virus, and poliovirus (references 8, 13, and 19 and references therein). Some, however, like Sindbis and tick-borne encephalitis viruses, appear to be unaffected by NO, while others, such as human immunodeficiency virus type 1, may even benefit from cellular NO production (8, 13, 19).
Similarly disparate results have emerged in studies of the intact mammalian host (8, 13, 19). The differences in disease outcome could be explained in part by experimental design, where the results with nonspecific NOS inhibitors may also reflect the indirect antiviral contributions of NOS1 and/or NOS3 (1, 12). In an effort to distinguish the antiviral effects of NOS2 from those of other gene family members and establish its necessity or redundancy, we used NOS2-null (NOS2−/−) mice (14). Such mice express NOS1 and NOS3 genes normally (14). EV-induced mousepox was chosen as the infectious model for several reasons: (i) it is strictly subject to control by gamma interferon (IFN-γ) (10), with EV replicating in many of the same cells capable of expressing NOS2 after activation by this and other cytokines (e.g., fibroblasts, epithelial cells, and keratinocytes within the exanthematous lesions of the skin and resident macrophages and hepatocytes within infected viscera [11]); (ii) it was the first model in which IFN-γ-induced NOS2 was attributed a protective role against viruses (11); and (iii) it represents a natural host-virus relationship (10). These features appeared to make it well suited for evaluation of the contributions of the NOS2 gene toward virus eradication.
Adult (8- to 12-week-old) male and female NOS2−/− mice (H-2b; 129/SvEv × C57BL/6J F2) (14) and their wild-type littermates (NOS2+/+) were bred at the SPF Unit, John Curtin School of Medical Research, Australian National University. They were infected intravenously (i.v.) with virulent EV (Moscow strain) that had been propagated in BS-C-1 cells and purified on sucrose density gradients as outlined elsewhere (10). In some cases, both mutant and control mice were treated daily with 5 mg of the NOS inhibitor Nω-methyl-l-arginine (l-NMA; Sigma) or its inactive d-enantiomer (d-NMA) in 200 μl of phosphate-buffered saline administered intraperitoneally, a regimen previously shown to be efficacious (11). At selected times postinfection (p.i.), aseptically removed organs were homogenized in phosphate-buffered saline and serial dilutions were assayed for PFU on BS-C-1 cell monolayers (10). On days 0, 3, and 6 p.i., plasma NO3− and splenocyte NO2− levels were also determined via nitrate reductase-linked and -nonlinked diazotization assays (14, 15). The assay sensitivity was 4 μM for both NO3− and NO2−. At the same times, standard 51chromium-release assays were enlisted for the activity measurements of splenic NK cells and cytotoxic T lymphocytes (CTL) (7, 9). YAC-1 cells were used to measure NK cell killing, while EV-infected and uninfected MC57G cells served as targets for the measurement of EV-specific, class I major histocompatibility complex (MHC)-restricted CTL activity. IFN-γ levels in plasma samples from infected mice and in supernatants from 2.5 × 106 EV-infected mouse splenocytes coincubated for 48 h with 5 × 105 EV-infected, mitomycin-treated (50 μg/ml) MC57G cells were measured by a sandwich enzyme-linked immunosorbent assay (10). Recombinant murine IFN-γ (Genzyme) served as the standard, with an assay detection limit of 3 U/ml. Concanavalin A (ConA) was added to positive control cultures at 4 μg/ml (Sigma, St. Louis, Mo.). For flow cytometry, the following fluorochrome-conjugated monoclonal antibodies (MAbs) were used for single-color analysis of 106 splenocytes: for T cells, biotinylated rat anti-CD3 (clone 145-2C11), anti-CD4 (clone GK1.5), and anti-CD8 (clone 53.6.7); for B cells, phycoerythrin-conjugated anti-CD45R/B220 (clone RA3-6B2) (Pharmingen, San Diego, Calif.); for macrophages, anti-F4/80 (clone F4/80). Biotinylated primary MAbs were detected with streptavidin-fluorescein isothiocyanate conjugate (Amersham International, Amersham, United Kingdom). After erythrocytes and dead cells were gated out, ≥2 × 104 events were collected via a FACScan flow cytometer by using LYSIS II software (Becton Dickinson, San Jose, Calif.).
Heightened susceptibility of NOS2−/− mice to EV infection.
At 104 or 105 PFU of EV, all NOS2−/− mice succumbed between days 5 and 10 p.i. (mean ± standard error of the mean, 8.1 ± 0.2 or 7.2 ± 0.7 days, respectively), while wild-type controls survived throughout the 21-day period of observation (Fig. 1a; n = 16 to 17 per group at each dose). Only at the highest inoculum tested (106 PFU) did the NOS2+/+ group become vulnerable, dying at around the same time as their NOS2-deficient counterparts (6.0 ± 0.3 and 6.2 ± 0.4 days, respectively). Markedly increased viral burdens were found within target organs such as the spleen, liver, and lungs of mutant mice just prior to death, similar to those of NOS2+/+ mice treated daily with the NOS inhibitor l-NMA (Fig. 1b). The latter treatment also led to early mortality at an i.v. dose of 104 PFU (7.4 ± 0.5 days, n = 5) (data not shown). In contrast, wild-type controls given the biologically inactive d-NMA effectively restricted virus replication, as illustrated by the 2,511-fold reduction in liver viral titers, 100-fold reduction in spleen viral titers, and 20-fold reduction in lung viral titers between days 3 and 6 p.i. (Fig. 1b).
FIG. 1.
Absence of NOS2 confers susceptibility to primary EV infection. (a) Mortality in female NOS2+/+ or NOS2−/− mice inoculated i.v. with EV and monitored for 21 days (n = 17 per group at each dose, except for 105 PFU, in which case 16 NOS2+/+ mice were used). Data represent three independent experiments. (b) Viral titers in liver, lung, and spleen of d-NMA- or l-NMA-treated NOS2+/+ and NOS2−/− mice given 104 PFU of EV i.v., determined at day 3 (n = 4 per group) and 6 (n = 9 per group) p.i. Data represent three experiments and are expressed as the mean log10 virus titer per gram of tissue ± standard error of the mean ∗, significantly different from value for d-NMA-treated group at P < 0.01 (unpaired t test).
C57BL/6 and 129/Sv parental inbred strains (obtained from the SPF Unit, John Curtin School of Medical Research) displayed identical survival patterns as those of otherwise-resistant NOS2+/+ (129/SvEv × C57BL/6J F2) hosts over a 102 to 106 dose range (n = 8 per group at each dose), except at 105 PFU of EV, at which dose 50% of the C57BL/6 mice died (Fig. 1a and data not shown). All three groups were rendered susceptible at the highest dose (106 PFU), with a mean time to death of 6.0 ± 0.9 and 6.6 ± 0.7 days for B6 and 129 strains, respectively, versus 6.0 ± 0.3 days seen earlier for NOS2+/+ 129/SvEv × C57BL/6J F2 mice (Fig. 1a). Further, the administration of anti-murine IFN-γ or anti-murine CD8 T-cell-depleting MAbs led to all three groups becoming susceptible, as previously established for the C57BL/6 strain (references 9 and 10 and data not shown). Hence, it appears unlikely that contaminating genes derived from either background account for the susceptibility of hybrid NOS2−/− hosts.
Specificity of the NOS2 defect: other anti-EV responses are intact and noncompensatory in mutant mice.
Aside from NOS2 (reference 11 and data from the preceding section), at least two additional components of host immunity are thought necessary to eliminate EV: IFN-γ (10) and class I MHC-restricted CD8+ CTL (9). Others belonging to either the innate (NK1.1 cells) or acquired (class II MHC-restricted CD4+ helper T lymphocytes) branches of the immune response also participate, although to a lesser extent (9). Partial protection is similarly conferred by tumor necrosis factor acting predominantly via TNF receptor 2 (22), a process that with IFN-γ may help signal NOS2 expression (13). Indeed, the NOS2 pathway could be detected within the plasma of wild-type hosts as early as day 3 p.i. following i.v. inoculation with 104 PFU (Fig. 2a). This was accompanied by a ∼50-fold elevation in circulating IFN-γ (Fig. 3c) and robust NK cell activity (Fig. 3a). Plasma NO3− levels further increased by day 6 p.i., coincident with the decline in viral titers, at which time NOS2 activity was readily evident in explanted splenocytes either without (control, ConA-treated, uninfected stimulators) or with further antigen-specific stimulation (Fig. 2b). This differed from results for both mutant animals and wild-type mice given l-NMA (Fig. 2); the former failed to exhibit inducible NO2− or NO3− levels, in agreement with earlier studies of infectious disease (14, 15), while NOS2 inhibition in l-NMA-treated mice ranged between 51 and 76% in plasma and 85 and 93% in splenocytes during the same period (Fig. 2). However, as in d-NMA-treated NOS2+/+ animals, NK cell activities together with systemic and splenic IFN-γ release were heightened in these two groups (Fig. 3a and c).
FIG. 2.
EV-induced expression of the immunologically responsive NOS2 pathway. (a) Plasma NO3− levels in d-NMA- or l-NMA-treated NOS2+/+ mice and NOS2−/− mice during i.v. infection with 104 PFU of EV. Symbols represent individual samples assayed in triplicate; horizontal bars represent group means. (b) Explanted splenocyte supernatant NO2− levels (mean ± standard error of the mean) in the same mice left untreated (control) or incubated with uninfected splenocytes, EV-infected stimulators, or ConA for 48 h. Data represent two independent experiments. NT, not tested.
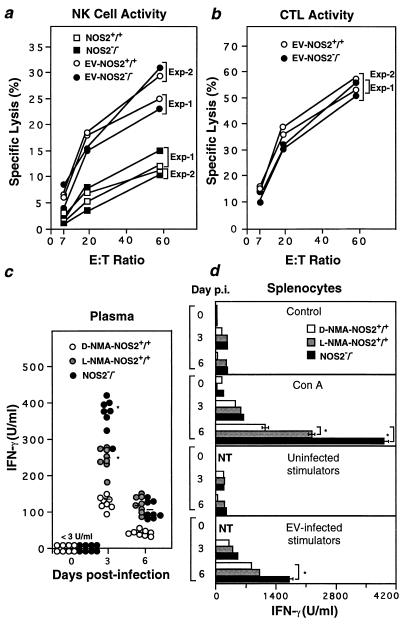
FIG. 3.
Other anti-EV immune components are intact in NOS2−/− mice. (a and b) Splenic NK cell activity (day 3 p.i.) (a) and EV-specific CTL activity (day 6 p.i.) (b) in d-NMA- or l-NMA-treated NOS2+/+ mice and NOS2−/− mice infected i.v. with 104 PFU of EV. Shown are group means (n = 4 for each of two independent experiments); standard errors of the means (SEMs) fall within the symbols. Splenocytes from uninfected mice served as controls. E:T ratio, effector/target cell ratio. (c) Plasma IFN-γ levels in the same mice as those described for panels a and b. Individual samples were assayed in triplicate; horizontal bars denote group means. (d) Explanted splenocyte supernatant IFN-γ levels (mean ± SEM) under the same conditions as those described in the legend to Fig. 2b. NT, not tested. ∗, significantly different from value for d-NMA-treated group at P < 0.01 (unpaired t test).
Generation of splenic EV-specific CTL activity was unaltered in the absence of NOS2 at day 6 p.i. (Fig. 3b), the time at which peak cytolytic effector function is normally observed (9). The proportions of CD8+ T cells, macrophages, and other lymphoid populations found within the infected spleen at this time also did not differ between genotypes (Table 1). IFN-γ secretion was, however, increased in NOS2-null hosts by 2.2- to 2.8-fold in plasma and 1.5- to 6.2-fold in splenocyte cultures relative to those of NOS2+/+ controls (Fig. 3c and d), perhaps as a consequence of the increased viral load in these animals (Fig. 1b).
TABLE 1.
Composition of EV-infected spleen cells from NOS2+/+ and NOS2−/− mice at day 6 p.i.
| Genotype (no. of mice) | % Composition by FACSa analysis (mean ± SD)
|
||||
|---|---|---|---|---|---|
| CD3+ | CD4+ | CD8+ | CD45R/ B220+ | F4/80+ | |
| NOS2+/+ (8) | 38.3 ± 2.5 | 23.4 ± 1.1 | 13.5 ± 0.5 | 53.6 ± 3.1 | 8.1 ± 0.5 |
| NOS2−/− (8) | 40.0 ± 2.1 | 23.0 ± 0.8 | 13.3 ± 0.5 | 52.5 ± 3.4 | 7.2 ± 0.3 |
FACS, fluorescence-activated cell sorter.
Based on our results, NOS2 could be added to a list of resistance loci considered important for recovery from EV infection and disease: H-2Db (termed rmp-3, resistance to mousepox, on chromosome 17) (17, 18), the C5 genes (rmp-2, on chromosome 2), and Rmp-1 (23), recently localized to a region on chromosome 6 encoding the NK cell receptor NKR-P1 alloantigens (2). Two of these loci, rmp-1 and rmp-3, appear functionally unaltered by NOS2 deficiency, as shown by the ability of mutant mice to mount robust NK cell and EV-specific CTL responses. In the case of rmp-1, which acts before an acquired response can be detected (17), its protective effects may largely be mediated by two distinct mechanisms: (i) direct NK cell cytolysis (18) of infected targets (e.g., hepatocytes) (23); and (ii) indirectly, via elicitation of NOS2 through NK cell secretion of IFN-γ.
A requirement for NOS2 in the maturation of NK cell cytotoxicity and innate IFN-γ release has been recently posited in studies of Leishmania major infection in mutant mice (4); however, the effect was apparent only within the first 24 h. Thereafter, IFN-γ release reached or slightly exceeded that of NOS2+/+ controls at later time points (4). We too, found that NOS2−/− mice had heightened IFN-γ levels by day 3 in plasma and day 6 in spleen as EV infection continued to progress unabated. Moreover, the inability to control EV replication did not appear to be due to defects in lymphocyte or monocyte accumulation within infected areas as shown by fluorescence-activated cell sorter analyses of spleen (Table 1). On the contrary, more precise measurements of leukocyte recruitment by using intravital microscopy have shown that the absence of NOS2 may, if anything, enhance this immigration (5).
For each of the immunologic parameters tested, as well as overall susceptibility, the responses of wild-type mice given l-NMA quite closely resembled those of mice in which NOS2 was genetically ablated. This contrasts with reports on the related orthopoxvirus, VV, in which results of genetic manipulation differed from those of phamacologic inhibition. In those studies, transfection of the NOS2 gene restricted VV replication in vitro (11, 20) and attenuated replication of NOS2 encoding recombinant VV in vivo (20). Yet administration of l-NMA failed to render B6, CBA/H, or nude mice more susceptible (20, 21). In the latter mouse strains, other IFN-γ-dependent pathways may have compensated for the loss of NO, as suggested by experiments in which healthy NOS2-deficient animals infected with VV become vulnerable once they are treated with anti-IFN-γ antibodies (11a). No such compensation is observed for EV, however, against which NOS2 appears to be a critical, nonredundant effector mechanism. A recent study of CVB3 infection in NOS2-deficient mice also showed increased viral burdens and severe myocarditis (24), suggesting that the NOS2 locus is important for controlling CVB3 replication as well. However, the heightened virus susceptibility in NOS2−/− mice is not universal, pulmonary clearance of influenza A (A/PR/8/34) virus is in fact more effective in mutant than in wild-type animals (6). This indicates a spectrum of antimicrobial activity for NOS2, one which mirrors that now starting to emerge for other pathogens examined in a host selectively and completely deficient for this enzyme (13, 16).
Acknowledgments
This work was supported by the National Centre for HIV Research (G.K. and J.-H.C.) and National Institutes of Health grants HL51967 and AI34543 (C.F.N.).
REFERENCES
- 1.Barna M, Komatsu T, Reiss C S. Activation of type III nitric oxide synthase in astrocytes following a neurotropic viral infection. Virology. 1996;15:332–343. doi: 10.1006/viro.1996.0484. [DOI] [PubMed] [Google Scholar]
- 2.Brownstein D G, Gras L. Differential pathogenesis of lethal mousepox in congenic DBA/2 mice implicates natural killer cell receptor NKR-P1 in necrotizing hepatitis and the fifth component of complement in recruitment of circulating leukocytes to spleen. Am J Pathol. 1997;150:1407–1420. [PMC free article] [PubMed] [Google Scholar]
- 3.Croen K. Evidence for an antiviral effect of nitric oxide. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Röllinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFN) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;7:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 5.Hickey M J, Sharkey K A, Sihota E G, Reinhardt P H, MacMicking J D, Nathan C, Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997;11:955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- 6.Karupiah, G., J.-H. Chen, S. Mahalingam, C. F. Nathan, and J. D. MacMicking. Rapid IFN-γ-dependent clearance of influenza virus and protection from consolidating pneumonitis in NOS2-deficient mice. J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 7.Karupiah G, Couper B E H, Andrew M E, Boyle D B, Phillips S M, Müllbacher A, Blanden R V, Ramshaw I A. Elevated natural killer cell responses in mice infected with recombinant vaccinia virus encoding murine IL-2. J Immunol. 1990;144:290–298. [PubMed] [Google Scholar]
- 8.Karupiah G, Harris N. Gamma interferon-induced nitric oxide in antiviral defense and immunopathology. In: Karupiah G, editor. Gamma interferon in antiviral defense. R. G. Austin, Tex: Landes; 1997. pp. 119–143. [Google Scholar]
- 9.Karupiah G, Buller R M L, Van Rooijen N, Duarte C, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karupiah G, Fredrickson T N, Holmes K L, Khairallah L H, Buller R M L. Importance of interferons in recovery from mousepox. J Virol. 1993;67:4214–4226. doi: 10.1128/jvi.67.7.4214-4226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karupiah G, Xie Q-W, Buller R M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 11a.Karupiah, G. Unpublished data.
- 12.Komatsu T, Bi Z, Reiss C S. Interferon-γ induced type 1 nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 13.MacMicking J, Xie Q-w, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 14.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q-w, Sokol K, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 15.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neill H C, Blanden R V. Mechanisms of determining innate resistance to ectromelia virus infection in C57BL/6 mice. Infect Immun. 1983;41:1391–1394. doi: 10.1128/iai.41.3.1391-1394.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neill H C, Blanden R V, O’Neill T J. H-2-linked control of resistance to ectromelia virus infection in B10 congenic mice. Immunogenetics. 1983;18:255–265. doi: 10.1007/BF00952964. [DOI] [PubMed] [Google Scholar]
- 19.Reiss C S, Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolph M S, Ramshaw I A, Rockett K A, Ruby J, Cowden W B. Nitric oxide production is increased during murine vaccinia virus infection but may not be essential for virus clearance. Virology. 1996;217:470–477. doi: 10.1006/viro.1996.0141. [DOI] [PubMed] [Google Scholar]
- 21.Rolph M S, Cowden W B, Medveczky C J, Ramshaw I A. A recombinant vaccinia virus encoding inducible nitric oxide synthase is attenuated in vivo. J Virol. 1996;70:7678–7685. doi: 10.1128/jvi.70.11.7678-7685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruby J, Bluethmann H, Peschon J J. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J Exp Med. 1997;186:1591–1596. doi: 10.1084/jem.186.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace G D, Buller R M L, Morse H C., III Genetic determinants of resistance to ectromelia (mousepox) virus-induced mortality. J Virol. 1985;55:890–891. doi: 10.1128/jvi.55.3.890-891.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zargoza C, Ocampo C, Suara M, Leppo M, Wei X-Q, Quick R, Moncada S, Liew F Y, Lowenstein C J. The role of inducible nitric oxide synthase in the host response to coxsackievirus myocarditis. Proc Natl Acad Sci USA. 1998;95:2469–2474. doi: 10.1073/pnas.95.5.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]