The first long-term cultured haploid embryonic stem cell (ESC) line was established from medaka fish, which represented a breakthrough in vertebrates (Yi et al., 2009). Subsequently, haploid ESC lines were established from mammals and used for genetic analysis and complex genetic manipulation (Elling et al., 2011; Yang et al., 2012). The diploidization of haploid cells is an interesting biological phenomenon and has been widely studied, but the mechanism of diploidization has not yet been fully resolved (Sun et al., 2020). The zebrafish is an excellent vertebrate animal model used in biological studies (Huang et al., 2022). Researchers have isolated many cell lines from zebrafish, such as the zebrafish fibroblast line ZF4 (Driever and Rangini, 1993). However, there have been no reports regarding stable haploid cell lines of zebrafish, and whether diploidization occurs in zebrafish is unclear.
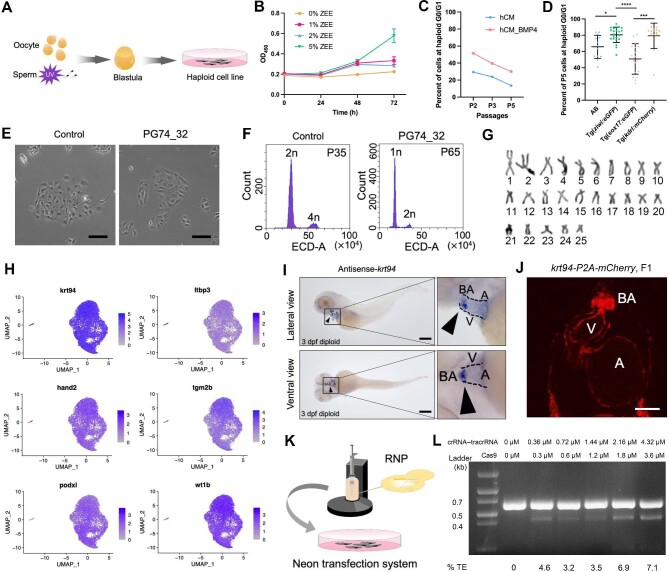
To derive zebrafish haploid cell lines, we generated parthenogenetic (PG) haploid embryos by ultraviolet (UV)-irradiating sperm used for the insemination and performing cell dissociation at the blastula stage followed by in vitro culture (Figure 1A). Ploidy analysis indicated that all haploid embryos maintained haploidy until death without any diploidization, while diploidization occurred in haploid cells in vitro (data not shown). We then examined several factors that may affect primary zebrafish cell culture. First, we found that a high concentration of zebrafish embryo extract increased the proliferation rate of cells (Figure 1B). Since the addition of BMP4 to a serum-free culture system was reported to improve the chromosomal integrity and proliferation of mouse ESCs (Wang et al., 2022), we added BMP4 to the haploid culture medium (hCM_BMP4), which elicited the increase in the proportion of haploid cells in the G0/G1 phase but did not affect diploidization (Figure 1C). The inbred strain is another important factor for establishing stable zebrafish haploid cell lines, as it contains fewer deleterious alleles (Yi et al., 2009). Single embryo-derived haploid cell lines of four strains, AB, Tg(ziwi:eGFP), Tg(sox17:eGFP), and Tg(kdrl:mCherry), were established and cultured in vitro, followed by flow cytometry analysis to determine the proportion of cells at haploid G0/G1 phase at passage 5, and the mean values were: AB, 65.77%; Tg(ziwi:eGFP), 80.58%; Tg(sox17:eGFP), 50.78%; and Tg(kdrl:mCherry), 79.33% (Figure 1D). Then, haploid embryonic cells of the Tg(ziwi:eGFP) and Tg(kdrl:mCherry) strains were isolated from zebrafish blastula at 3.3 h post fertilization (hpf) using hCM_BMP4 and seeded onto a Matrigel-coated dish. Cells formed dense clumps after 6 h, eventually grew, and spread by adhering to the dish after ∼24 h (Supplementary Figure S1A–H). Three stable zebrafish haploid cell lines, PG74_32, PG76_6, and PG72ziwi, were established and cultured for >50 passages without diploidization (Figure 1E and F; Supplementary Figure S1I–L). No proliferation stagnation was observed in PG74_32 during the culture process until passage 70. Karyotype analysis of PG74_32 revealed that 28% of the cells had a 25-chromosome karyotype and 51% of the cells had a 26-chromosome karyotype (Figure 1G; Supplementary Figure S1M). Karyotype analysis of PG76_6 at passage 20 showed that 87% of the cells had a normal 25-chromosome karyotype (Supplementary Figure S1L). Next, we performed genome sequencing to assess the genomic integrity of Tg(ziwi:eGFP) haploid embryonic cells at 24 hpf, ZF4 cells, PG74_32 cells at passage 15, and PG72ziwi cells at passage 47. PG74_32 showed the same pattern of copy number gain on chromosome 19 as the stable haploid cell line PG72ziwi, and all the haploid cell lines exhibited less copy number variation than ZF4 (Supplementary Figure S2).
Figure 1.
Derivation of heart-related haploid cell lines from zebrafish embryos. (A) Schematic overview of the derivation of zebrafish parthenogenetic haploid embryonic cells. UV-irradiated sperm were used for the insemination of oocytes, and the zygote cells developing to the blastula stage were dissociated for culture. (B) CCK8 assays demonstrating a higher proliferation rate of the cells cultured with a high concentration (5%) of ZEE. (C) The haploid cells cultured in hCM_BMP4 exhibiting a higher proportion of cells at the G0/G1 stage compared to those cultured in hCM. (D) The proportion of cells at haploid G0/G1 stage in haploid cell lines derived from different strains at passage 5. (E) Phenotype of the haploid cell line PG74_32 derived from a single embryo. The control cell line was a haploid cell line with diploidization. Scale bar, 100 μm. (F) Ploidy analysis of the stable long-term cultured haploid cell line PG74_32 at passage 65. A diploid cell line at passage 35 was used as the control. (G) The 25-chromosome karyogram of PG74_32 at passage 70 arranged in decreasing lengths. (H) UMAP plot of heart-related marker genes expressed in the haploid cell line PG74_32 at passage 70. Two distinct cell clusters were identified, and most of the cells were in one cluster. (I) Whole-mount in situ hybridization was performed to determine krt94 expression in 3 dpf embryos. Black triangles indicate positive signals. Scale bar, 250 μm. (J) Phenotype of krt94-P2A-mCherry knock-in F1 at 3 dpf (the heart region is shown). The fluorescence can be observed at the bulbus arteriosus (BA), ventricular epicardium, ventricular endocardium, and atrial endocardium. V, ventricle; A, atrium. Scale bar, 50 μm. (K) Schematic of the Neon transfection system used to conduct gene editing by RNP. (L) The editing efficiency at the eef1a1l1 locus in PG72ziwi was assessed 3 days after transfection by the T7EI cleavage assay. crRNA–tracrRNA, the pairing of CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA). %TE, the indel efficiency tested by T7EI.
To identify the cell type of these derived haploid cells, we performed RNA sequencing (RNA-seq) of PG72ziwi cells. Pearson correlation analysis revealed that PG72ziwi cells were significantly different from ZF4 cells (Supplementary Figure S3A). Then, we compared the transcriptomic sequencing data of PG72ziwi with the published single-cell RNA-seq (scRNA-seq) data of zebrafish embryos during the first 24 hpf (Wagner et al., 2018) and found that the closest cluster was the 24 hpf heart marker genes and the gene with the highest expression level in PG72ziwi was krt94 (Supplementary Figure S3B). A heatmap analysis showed significantly higher expression level of krt94 in PG72ziwi than in ZF4, and reverse transcription–polymerase chain reaction analysis confirmed the high transcriptional level of krt94 in PG72ziwi (Supplementary Figure S3C and D). Furthermore, we performed scRNA-seq of PG74_32 at passage 70 and observed high expression levels of heart-related marker genes, including ltbp3, hand2, tgm2b, podxl, and wt1b, especially krt94 in almost all cells (Weinberger et al., 2020; Kemmler et al., 2021; Figure 1H).
We also performed whole-mount in situ hybridization to determine krt94 expression in vivo and found that krt94 was specifically expressed at the bulbus arteriosus of diploid embryos at 3 days post fertilization (dpf) (Figure 1I). Moreover, we constructed a plasmid containing the last exon of krt94 and P2A-mCherry to generate the krt94-P2A-mCherry knock-in fish. Intense red fluorescence signals were detected at the bulbus arteriosus, ventricular epicardium, ventricular endocardium, and atrial endocardium in 3 dpf diploid embryos of krt94-P2A-mCherry knock-in fish (Figure 1J). Thus, the established haploid cells are zebrafish heart-related cells.
The haploid cell line is applicable for gene editing with the Neon transfection system (Figure 1K). In an experiment for gene knockout at the eef1a1l1 locus in PG72ziwi cells, various concentrations of the ribonucleoprotein (RNP) complex, consist of guide RNA and Cas9 protein, were tested. The highest efficiency of eef1a1l1 knockout reached 7.1%, as determined by the T7 endonuclease I (T7EI) cleavage assay (Figure 1L). In another experiment for gene knock-in at the krt94 locus in PG72ziwi cells, krt94-P2A-mCherry transgene was successfully knocked in, with an efficiency of up to 0.2% using various concentrations of RNP and the krt94-P2A-mCherry donor plasmid (Supplementary Figure S4).
Overall, we established the first stable long-term cultured haploid cell line from zebrafish, which expresses various heart-related marker genes and can be easily edited with the Neon transfection system, thus serving as a favorable tool for the genetic screening for heart research.
[We thank the Genome Tagging Project (GTP) Center and the Core Facilities for zebrafish, fruit fly, cell biology, and molecular biology for instrumental and technical supports. This work was partly supported by the National Key Research and Development Program of China (2019YFA0109900 and 2018YFA0801003), CAS Center for Excellence in Molecular Cell Science (2021DF06), and Shanghai Municipal Science and Technology Major Project.]
Supplementary Material
Contributor Information
Siqi Liu, State Key Laboratory of Cell Biology, Shanghai Key Laboratory of Molecular Andrology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Jia Xu, State Key Laboratory of Cell Biology, Shanghai Key Laboratory of Molecular Andrology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Yirui Ai, State Key Laboratory of Cell Biology, Shanghai Key Laboratory of Molecular Andrology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Yunbin Zhang, State Key Laboratory of Cell Biology, Shanghai Key Laboratory of Molecular Andrology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Shifeng Li, State Key Laboratory of Cell Biology, Shanghai Key Laboratory of Molecular Andrology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Jinsong Li, State Key Laboratory of Cell Biology, Shanghai Key Laboratory of Molecular Andrology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Yiping Li, State Key Laboratory of Cell Biology, Shanghai Key Laboratory of Molecular Andrology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
References
- Driever W., Rangini Z. (1993). Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell. Dev. Biol. Anim. 29, 749–754. [DOI] [PubMed] [Google Scholar]
- Elling U., Taubenschmid J., Wirnsberger G. et al. (2011). Forward and reverse genetics through derivation of haploid mouse embryonic stem cells. Cell Stem Cell 9, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Lin X., Liu F. et al. (2022). The rise of developmental biology in China. Dev. Growth Differ. 64, 106–115. [DOI] [PubMed] [Google Scholar]
- Kemmler C.L., Riemslagh F.W., Moran H.R. et al. (2021). From stripes to a beating heart: early cardiac development in zebrafish. J. Cardiovasc. Dev. Dis. 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.Y., Zhao Y.D., Shuai L. (2020). The milestone of genetic screening: mammalian haploid cells. Comput. Struct. Biotechnol. J. 18, 2471–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.E., Weinreb C., Collins Z.M. et al. (2018). Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science 360, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Zhao K., Liu M. et al. (2022). BMP4 preserves the developmental potential of mESCs through Ube2s- and Chmp4b-mediated chromosomal stability safeguarding. Protein Cell 13, 580–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M., Simoes F.C., Patient R. et al. (2020). Functional heterogeneity within the developing zebrafish epicardium. Dev. Cell 52, 574–590.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Shi L., Wang B.A. et al. (2012). Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell 149, 605–617. [DOI] [PubMed] [Google Scholar]
- Yi M., Hong N., Hong Y. (2009). Generation of medaka fish haploid embryonic stem cells. Science 326, 430–433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.