Abstract
Acute hepatic porphyrias are inherited metabolic disorders of heme biosynthesis characterized by the accumulation of toxic intermediate metabolites responsible for disabling acute neurovisceral attacks. Givosiran is a newly approved siRNA-based treatment of acute hepatic porphyria targeting the first and rate-limiting δ-aminolevulinic acid synthase 1 (ALAS1) enzyme of heme biosynthetic pathway. We described a 72-year old patient who presented with severe inaugural neurological form of acute intermittent porphyria evolving for several years which made her eligible for givosiran administration. On initiation of treatment, the patient developed a major hyperhomocysteinemia (>400 μmol/L) which necessitated to discontinue the siRNA-based therapy. A thorough metabolic analysis in the patient suggests that hyperhomocysteinemia could be attributed to a functional deficiency of cystathionine β-synthase (CBS) enzyme induced by givosiran. Long-term treatment with vitamin B6, a cofactor of CBS, allowed to normalize homocysteinemia while givosiran treatment was maintained. We review the recently published cases of hyperhomocysteinemia in acute hepatic porphyria and its exacerbation under givosiran therapy. We also discuss the benefits of vitamin B6 supplementation in the light of hypothetic pathophysiological mechanisms responsible for hyperhomocysteinemia in these patients. Our results confirmed the importance of monitoring homocysteine metabolism and vitamin status in patients with acute intermittent porphyria in order to improve management by appropriate vitamin supplementation during givosiran treatment.
Keywords: Acute hepatic porphyria, Acute intermittent porphyria, Givosiran, Hyperhomocysteinemia, CBS deficiency, Vitamin B6
Highlights
-
•
Givosiran is a newly approved siRNA-based therapy for severe acute hepatic porphyria
-
•
Hyperhomocysteinemia is frequently observed in acute hepatic porphyria and increased after givosiran administration
-
•
Givosiran alters homocysteine metabolism, probably by inducing a functional deficit of CBS enzyme.
-
•
Supplementation with vitamin B6, a cofactor of CBS, allows to normalize homocysteine levels while maintaining treatment with givosiran. Supplementation with vitamin B6 allows to normalize homocysteine levels while maintaining treatment with givosiran.
1. Introduction
Acute hepatic porphyrias (AHP) are inherited metabolic diseases due to hepatic heme synthesis dysfunction [1]. Acute intermittent porphyria (AIP; OMIM 176000), the most common form of AHP, is an autosomal dominant disease caused by a partial deficiency of hydroxymethylbilane synthase (HMBS; EC 2.5.1.61), the third enzyme of heme biosynthesis pathway [2]. In patients carrying deleterious HMBS gene mutations, the enzymatic deficiency combined with triggering factors lead to upregulation of hepatic 5′-aminolevulenic acid (ALA) synthase 1 (ALAS1; EC 2.3.1.37), the first and rate-limiting step of heme synthesis, resulting to the accumulation of heme precursors ALA and PBG (porphobilinogen). The excess production of these neurotoxic intermediate products can precipitate acute neurovisceral attacks characterized by intense abdominal pain accompanied by digestive (e.g. nausea, vomiting, constipation, diarrhea) and cardiovascular (e.g. hypertension, tachycardia) disorders related to disturbance of the neurovegetative system. Severe crisis may be further complicated by central nervous system involvement (e.g. seizures, confusion, hallucinations) and hyponatremia [2,3]. Standard treatment of an acute attack relies on symptomatic measures, including analgesia, antihypertensive and anti-vomiting medication as well precipitating factors eviction [4]. Severe acute attacks are usually treated with intravenous hemin administration, which is also frequently used off-label as prophylaxis in patients with recurrent attacks [4]. However, repeated hemin administration can lead to chronic complications such as secondary hemochromatosis, tachyphylaxis and venous thrombosis [3,4]. Givosiran is a therapeutic small interfering RNA targeting hepatic ALAS1, which was recently approved in the United States and Europe for the treatment of AHP in adults and adolescents aged ≥12 years [5,6]. Results from a double-blind study followed by an open-label extension period in 94 patients with AHP and recurrent attacks, confirmed that monthly subcutaneous givosiran injection (2.5 mg/kg) leads to continuous and sustained reductions in annualized attack rate and use of hemin over time, as well as improved quality of life, with an acceptable safety profile [6]. Serious adverse effects (SAE) were reported in 39% of the patients and especially elevated blood homocysteine, retinal vein occlusion, injection-site reaction, pancreatitis, worsening of chronic renal failure, pulmonary embolism, right iliac thrombophlebitis, and worsening of liver function [6,7]. Moderate or intermediate hyperhomocysteinemia (HHcyt) has been previously reported in AHP with more marked trend in symptomatic patient or those with high excretion of ALA and PBG [[8], [9], [10], [11], [12]]. Homocysteine is an intermediate of sulfur amino acid metabolism, which is further metabolized via re-methylation or transsulfuration pathways (Fig. 1). Vitamins B6, B9 and B12 are involved in homocysteine metabolism as enzymatic cofactors [13]. HHcyt is widely considered as a risk factor of vascular diseases but suspect to be associated with neurodegeneration and neurocognitive impairment, inflammatory diseases, osteoporosis [13,14]. A variety of congenital and acquired conditions lead to HHcyt, including inherited metabolic diseases of Hcyt catabolism, cofactor deficiencies and kidney or liver failure [15]. In AHP patients, various factors may contribute to HHcyt [12]. Although vitamins B9 and B12 levels are within normal values, vitamin B6 deficiency has been reported in almost half of AHP patients, probably in relation with poor nutritional status and chronic hyperactivity of ALAS1 which could sequestrate cytosolic pyridoxal-phosphate (Fig. 2) [8,9,12,16,17]. Furthermore, Cystathionine β-synthase (CBS), a pivotal enzyme of the transsulfuration pathway, is dependent on heme and vitamin B6 as cofactors [18]. It was argued that reducing CBS activity by depleting intra-hepatic vitamin B6 or lowering heme availability might contribute to HHcyt in AHP patients [12]. Recently, several case series reported exacerbation of HHcyt in AHP treated with givosiran [10,11,[19], [20], [21]]. Final results of the ENVISION trial indicated that 16% of AHP had significant increase of plasmatic homocysteine after Givosiran [6]. The concomitant elevation of plasmatic homocysteine and methionine after treatment by givosiran probably reflects an impaired transsulfuration pathway catabolism by CBS (Fig. 2) [18,20,21].
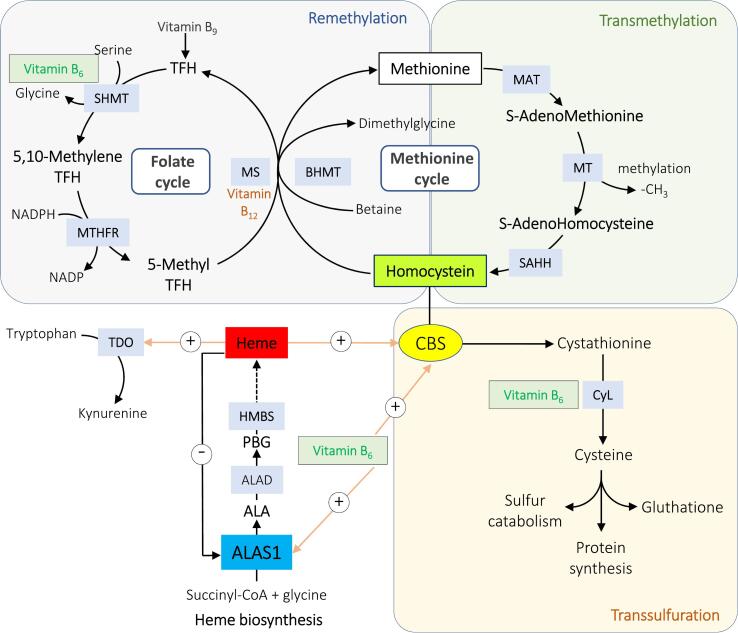
Fig. 1.
Pathways of homocysteine and heme metabolisms.
MAT, methionine adenosyltransferase; MT, methyltransferase; SAHH, S-adenosylhomocysteine hydrolase; BHMT, betaine-homocysteine methyltransferase; MS, methionine synthase; THF, tetrahydrofolate; SHMT, serine hydroxymethyltransferase; MTHFR, methylenetetrahydrofolate reductase; HMBS, hydroxymethylbilane synthase; TDO, tryptophane 2,3-dioxygenase; CBS, cystathionine β-synthase; CyL, cysthationine γ-lyase; ALAS1, aminolevulinate synthase 1; ALA, acid aminolevulinic; PBG, porphobilinogen. Putative links between heme and homocysteine metabolism pathways are heme and pyridoxal-5’phosphate (active form of vitamin B6). Heme exerts negative feedback on ALAS1 and may activate the CBS enzyme via binding to a heme-binding motif. Both ALAS1 and CBS use vitamin B6 as cofactor. TDO is a hemoprotein involved in tryptophan metabolism.
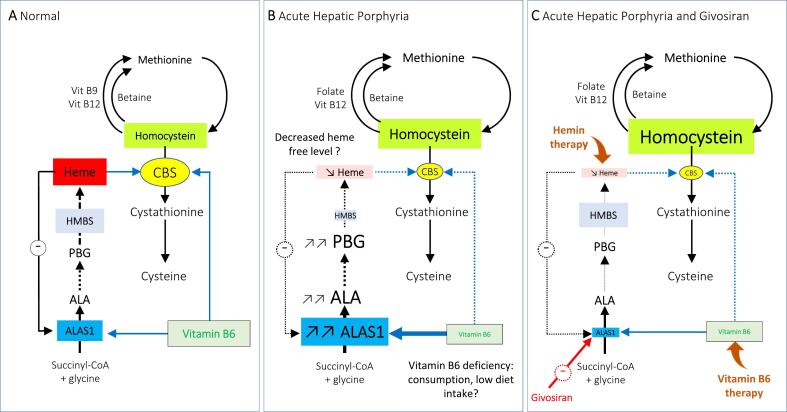
Fig. 2.
Mechanisms of homocysteine metabolism disorders in acute hepatic porphyria. (A) In healthy individuals, methionine is metabolized into homocysteine which is converted into cysteine by the enzyme CBS. ALAS1 is the first and regulating enzyme of the heme biosynthesis pathway. ALAS1 and CBS use vitamin B6 (pyridoxal-5′-phosphate) as cofactor. Heme exerts a negative feedback regulation on ALAS1 expression while it stimulates the CBS enzyme. (B) A moderate hyperhomocysteinemia (HHcyt) is frequently observed in acute hepatic porphyria, (here acute intermittent hepatic porphyria with HMBS deficiency as example) especially in symptomatic patients. Putative mechanisms of HHcyt are a decreased heme free level in hepatocytes due to triggering factors responsible for ALAS1 hyperactivation and heme biosynthesis pathway runaway. ALAS1 hyperactivity may participate to consume vitamin B6, which becomes unavailable for CBS activity. (C) Givosiran is responsible for a significant drop of ALAS1 enzymatic activity in the liver, which could restrict the availability of heme in hepatocytes, which impact severely CBS activity resulting in HHcyt. Treatment with hemin or vitamin B6 significantly improve CBS activity and reduce HHcyt in patients treated with givosiran.
We report a dramatic exacerbation of HHcyt (>400 μmol/L) after initiation of givosiran-based therapy in a patient affected by a severe form of acute intermittent porphyria (recurrent neurological form with late onset type of posterior reversible encephalopathy syndrome, i.a. PRES). As givosiran therapy was necessary to regulate heme metabolism and ALA/PBG excretion in the context of recurrent acute crisis, we treated the patient as a congenital CBS deficiency (congenital homocystinuria) with high doses of vitamin B6. We highlight the effectiveness of vitamin B6 therapy to normalize Hcyt, allowing the safe renewal givosiran therapy. We performed genetic screen of genes involved in homocysteine metabolism as well as blood amino-acid and vitamin profiling over time in the patient which sustained the hypothesis of an acquired CBS deficiency associated to givosiran therapy.
2. Material and methods
2.1. Biochemical studies
Biochemical data for the patient were retrieved since the diagnosis of AIP and presented in Supplementary Table 1. Urine and blood samples were collected for biochemical assessment in our French National Reference Laboratory for inherited metabolic diseases (amino acid and heme disorders) according to standard protocols. Creatinine was determined using a standardized enzymatic method and glomerular filtration rate was estimated with the CKD-EPI formula adjusted for 1,73m2. Vitamin B6 status was assayed in whole blood as total pyridoxal-5-phosphate levels by HPLC commercial kit (Chromsystem Instruments and Chemical GmBH, München, Germany). Vitamin B12, B9 and total L-homocysteine (Hcyt) were determined in plasma samples (and red blood cells for vitamin B9) by automated chemiluminescent microparticle immunoassay (Architect System, Abbott). Our laboratory's reference values for homocysteinemia are between 4.40 and 13.6 μmol/L in women. Classically, HHcyt is considered mild when the plasma Hcyt level is in the 16–30 μmol/L range, moderate between 30 and 50 μmol/L, intermediate between 50 and 100 μmol/L and severe above of 100 μmol/L [[13], [14], [15]]. Amino acids were determined in plasma samples by LC-MS/MS after AccQ-Tag derivatization using commercial kit (AccQ.Tag® Derivatization Kit Reagent, Waters™, Waters SAS, France). For ALA, PBG and urinary porphyrin profiling, samples were collected away from light and stored at −80 °C. Uroporphyrin, heptacarboxyporphyrin, hexacarboxyporphyrin, pentacarboxyporphyrin, coproporphyrin I and coproporphyrin III were separated and quantified by HPLC using commercial kit (Urinary porphyrin profiling, Chromsystem Instruments and Chemical GmBH, München, Germany). Total porphyrin was determined as the sum of porphyrin fractions. Urinary ALA and PBG were determined by ion-exchange chromatography followed by photometry assay using commercial kit (RECIPE Chemicals + Instruments GmbH, Germany).
2.2. Next-generation sequencing (NGS) of genes involved in homocysteine metabolism
Patient samples were analyzed after written informed consent by NGS using a panel of genes targeting homocysteine metabolism: ABCD4, AMN, CBLIF, CBS, CD320, CUBN, DHFR, HCFC1, LMBRD1, MMACHC, MMADHC, MTHFD1, MTHFR, MTR, MTRR, TCN2. Genomic DNA was extracted from peripheral blood using an automated method (TECAN Freedom EVO). The capture encompassed exons and flanking regions (±25 bp) following purification with a SureSelect XTHS2 kit from Agilent Technologies. Sequencing was performed on a Nextseq550 Illumina platform. The bioinformatic workflow employed Alissa Reporter and Alissa Interpret, both from Agilent Technologies. Biological interpretation was carried out according to the ACMG classification [22].
3. Clinical case
We report the case of a 72-year old woman with sporadic AIP as defined recently in international consensus study [23]. Her history includes high blood pressure treated with atenolol 50 mg/day, epilepsy and cerebral sylvian aneurysms. She was diagnosed with AIP at the age of 62-years following a severe acute inaugural attack with neurological involvement (seizure and posterior reversible encephalopathy syndrome, i.e. PRES) successfully treated with hemin [24]. She is heterozygous for a pathogenic c.849G > A (p.Trp283*) mutation in exon 14 of HMBS gene (transcript NM 000190.4). She relapsed four acute neurovisceral attacks in the eight years following diagnosis, clinically defined by abdominal pain, central nervous system involvement, chronic high blood pressure and increased urinary PBG/creatinine ratio (>10 times the upper limit of normal) (Supplementary Table 1). Infectious diseases (pyelonephritis and gastroenteritis) were identified as triggering factors for two acute attacks (Supplementary Table 1).
Givosiran is a siRNA-based drug approved for treatment of adult AHP patients with recurrent neurovisceral crisis [6]. Our patient was given serial subcutaneous injection with 2.5 mg givosiran and was enrolled in the ELEVATE study, a global observational longitudinal prospective registry of patients with AHP (Alnylam Pharmaceuticals, Inc.) which aims to characterize the natural history and real-world clinical management of patients with AHP as well as safety and effectiveness of givosiran. At treatment initiation, biological assessment showed persistent increase in urinary ALA (17 μmol/mmol creatinine, normal<3) and PBG levels (46 μmol/mmol of creatinine, normal<1) and slightly impairment of renal function with a glomerular filtration rate of 61.5 mL/min (CPK-EPI for 1.73m2) (Supplementary Table 1). As previously described in AIP patients, Hcyt was also increased (79 μmol/L, normal<16), vitamin B6, B9 and B12 status were not available (Fig. 3 and Supplementary Table 1).
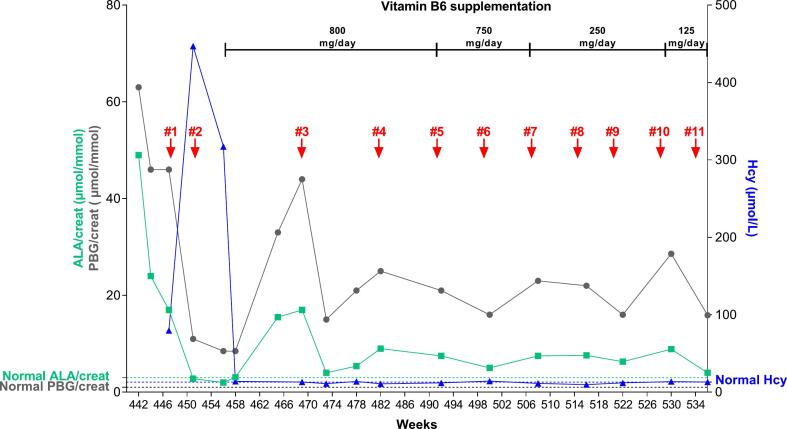
Fig. 3.
Urinary ALA and PBG, homocysteinemia in the AIP patient over the treatment with givosiran and vitamin B6 supplementation. The dates of givosiran injections are indicated by red arrows and given in weeks from the diagnosis of AIP.
One month after the first injection of givosiran, we observed a significant drop of urinary ALA (2.8 μmol/mmol of creatinine) and PBG (11.4 μmol/mmol of creatinine) (Fig. 3). However, Hcyt increased dramatically (447 μmol/L) to reach levels classified as very severe HHcyt. The analysis of plasmatic amino acids shows abnormal presence of homocystine (69 μmol/L, normally undetected) and low cystine levels (19 μmol/L, normal range 32–54) while methionine (156 μmol/L, normal range 21–36), and tryptophan (136 μmol/L, normal range 30–95) were increased. Vitamin B6 (46.7 nmol/L, normal range 35–110), vitamin B12 (234 pg/mL, normal range 187–885) and plasma methylmalonic acid (MA) (< 0.4 μmol/L, normal range < 0.4) were within the normal range while vitamin B9 were slightly decreased (2.5 ng/mL, normal range 3.1–20.5). Since HHcyt may be favored by intrinsic genetic factors affecting the transsulfuration or remethylation pathways, we analyzed 16 genes involved in homocysteine metabolism by next generation sequencing. We have identified two heterozygous variants classified as non-pathogenic in the CBS (c.832_833ins68, transcript NM 00071.3) and MTHFR (c.1286 A > C, transcript NM 005957.5) genes.
As reported in the phase III ENVISION clinical, givosiran injection (2.5 mg/kg) should be performed monthly in AHP patients [6]. Because ALA metabolite plays a key role in the pathophysiology, some authors suggested adapting the schedule of givosiran injection in patients by monitoring urinary ALA levels [25]. This personalized medicine approach may reduce the occurrence of serious adverse effects associated to recurrent injection of givosiran. Furthermore, vitamin B6 supplementation was successfully investigated to lower HHcyt in AHP treated by givosiran. Because urinary ALA and PBG progressively risen within three months of the second injection, givosiran injections were spaced approximately 2 to 3 months apart in combination with high-dose vitamin B6 therapy (800 mg/day). In total, the patient received 11 givosiran injections over a 22-months period (Fig. 3). A significant reduction of urinary ALA and PBG was obtained while plasmatic homocysteine progressively returns within normal range two months after the second injection of givosiran (Fig. 3). Urinary ALA maintained between 4 and 9 μmol/mmol creat, while PBG remained relatively high at around 10 to 30 times normal values (Fig. 3 and Supplementary Table 1). This has already been described in patients whose treatment with givosiran was initiated late in the disease course [25]. However, despite this partial biological response, our patient has not experienced new episodes of acute porphyria since starting givosiran. Despite the reduction in the dose of vitamin B6 administered to our patient (starting from 800 mg/day to 125 mg/day), we maintained a normal level of plasma homocysteine even though givosiran injections were repeated (Fig. 3). Plasma homocysteine and methionine levels were also reduced over time while we repeated givosiran administrations (Supplementary Table 1). Givosiran therapy was well tolerated with no reaction at the injection site.
4. Discussion
We report a patient with sporadic AIP who experienced severe acute neurovisceral attacks and chronic elevation of urinary ALA and PBG. Hyperhomocysteinemia was also identified concomitantly with initiation of givosiran therapy in this patient. Most subjects with symptomatic acute hepatic porphyria present HHcyt resulting from alterations in sulfur amino acid metabolism. To Figueras et al, first reported a group of twenty-four AHP patients with elevated urinary PBG (10 and 100 μmol/mmol of creatinine), of which 64% had HHcyt [8]. Similar data of HHcyt were subsequently reported in Italian (61% of 46 AHP patients [9]), Spanish (62% of non-recurrent and 82% of recurrent in 37 AIP patients [10] and 37% of 91 AIP patients [11]) and French (93% of 14 AIP patients [25]) porphyria groups. The relationship between HHcyt and urinary excretion of ALA/PBG or clinical status (recurrent acute attack, prophylactic hemin injection) remains unclear [12]. Our patient's vitamin status was unknown but an increased prevalence of vitamin B6 deficiency (< 25th percentile of reference values) associated with HHcyt has been described in symptomatic AHP patients or those with abnormalities in urinary precursors ALA and PBG [9]. When available, plasma methionine increased in most of these patients as a reflect of CBS deficiency suggesting that all patients to be treated with givosiran should be monitored for Hcyt and methionine levels.
Givosiran is approved for the treatment of AHP in adults allowing significant reductions in annualized attack rate (AAR), hemin use, urinary ALA and PBG levels, and improvements in daily worst pain [6]. We observed a significant reduction in urinary ALA and PBG in our patient after two injections of givosiran one months apart. However, it was accompanied by a huge increase in HHcyt associated with high methionine and low cystine in plasma while circulating vitamin B9 and B12 were normal, suggesting an alteration in transsulfuration rather than remethylation pathway of homocysteine metabolism. In addition, the increase in plasma tryptophan, which is exceptional, would be consistent with a deficiency in the hemoprotein tryptophan pyrrolase. Plasma HHcyt was reported in other cases series of AHP patients treated with givosiran [8,10,11,[19], [20], [21],25] and reviewed by Ventura et al [12]. In cases where homocysteine values were available before and after treatment with givosiran, HHcyt increased in 3 /4 AIP patients (>100 μmol/L in two patients) in the report of Fontanellas et al [11], in 9/9 AHP patients (>100 μmol/L in five patients) in the case series of Ricci et al [20], in 4/4 AHP patients (>100 μmol/L in two patients) in the study by Vassiliou et al [21], and finally in 14/14 AIP patients reported by Poli et al [25]. Exploratory and final analysis of the ENVISION trial data demonstrated that at a population level, givosiran increased HHcyt without being able to correlate it with a change in efficacy or safety [6,12]. To our knowledge, an HHcyt level of 447 μmol/L observed in our patient from the first injection of givosiran represents the highest level described in the literature. This rate of extreme severity is similar to those that are observed during genetic enzymatic deficiencies either by classical CBS deficiency or enzymatic alterations of the remethylation pathway of homocysteine metabolism. After analysis of 16 genes involved in homocysteine metabolism in our patient, we have identified two heterozygous variants classified as non-pathogenic in the CBS (c.832_833ins68, transcript NM 00071.3) and MTHFR (c.1286 A > C, transcript NM 005957.5) genes. The MTHFR variant c.1286 A > C (p.Glu429Ala), considered as polymorphism, is found in 40–50% of individuals of Northern European ancestry and may be responsible for a mild to moderate HHcyt in homozygous subjects due to reduced enzymatic activity. The CBS variant c.832_833ins68 is associated with higher levels of CBS enzyme activity and is not responsible for HHcyt [26]. In previously published cases series of HHcyt under givosiran therapy, analyses of genes involved in homocysteine metabolism revealed only MTHFR polymorphism (homozygous or heterozygous c.677C > T and one case of homozygous c.665C > T), which is associated with mild to moderate HHcyt [8,11,19,20,27]. Overall, these results suggest that HHcyt may be attributed to a secondary cause affecting homocysteine metabolism and linked to the mechanism of action of givosiran. These results suggest that siRNA-targeting of the regulatory enzyme ALAS1 by givosiran may chronically affect heme status and hemoprotein function in the liver (Fig. 2). CBS is a key enzyme of homocysteine metabolism which contains heme-binding motif [18]. Although heme does not participate directly in the catalytic process, it helps binding of the cofactor pyridoxal-phosphate involved in enzyme stability [18]. This hypothesis needs to be confirmed since heme-dependent hepatic cytochrome and kynurenine pathway enzymes do not appear to be affected in patients treated with givosiran [10,20,28].
Pyridoxal-phosphate is a cofactor of CBS enzyme which is able to reduce plasma Hcyt in vitamin B6 responsive patients with congenital homocystinuria depending on their genotype [29]. The role of vitamin B6 status in AIP patients has already been mentioned and the prevalence of vitamin B6 deficiency is higher in symptomatic AHP patients or those with abnormal ALA and PBG urinary excretion [9]. Oral vitamin B6 treatment (80 mg/day) for 8 weeks normalized or significantly decreased Hcyt levels in nine AIP patients with Hcyt >50 μmol/L, as methionine levels decreased in most patients [21]. Vitamin B6 supplementation (250 mg/day) in 4 AIP patients with homocysteine levels >100 μM significantly decreased in three of them and normalized in one patient [25]. Authors obtained a reduction of Hcyt levels after administration of vitamin B9 [11] or supplementation with a daily mixture of B-vitamin during 30–45 days (vitamin B9 400μg, vitamin B6 3mg, vitamin B12 5 μg, vitamin B2 2,4 mg, betain 250 mg and zinc 12,5 mg) [20]. In all these studies, it is not clear whether the observed reduction in Hcyt under vitamin supplementation was obtained under continuous treatment with givosiran. In our study, it was surprising to find that the observed reduction in ALA, but even more so in PBG, was not as great after starting vitamin supplementation (Fig. 3). As vitamin B6 is also the cofactor of the ALAS1 enzyme, it is possible that vitamin supplementation can stimulate residual ALA-synthase enzymatic activity and limits the effect of givosiran on the production of ALA and PBG precursors. The vitamin B6 dose generally administered for congenital CBS deficiency varies from 200 to 500 mg/day, but never exceeds 900 mg/day due to the iatrogenic risk of sensory neuropathy [30,31]. Owing to the severe HHcyt, our patient was maintained under high regimen vitamin B6 (800 mg vitamin B6/day) simultaneously to the second injection of givosiran. The potentially deleterious effects of long-term administration of very high doses of vitamin B6 need to be assessed. Insufficiency dietary intake of vitamin B6 may be responsible for a peripheral sensory polyneuropathy [32]. The upper limit of acceptable intake is not clear but the long-term administration of high doses (>900 mg/day) is considered to be neurotoxic [29,31,32]. High levels of vitamin B6 may inhibits pyridoxal kinase (PDXK) enzyme and even though the mechanism behind PDXK-induced neuropathy is unknown it may be related to γ-aminobutyric acid (GABA) neurotransmission [33]. In our patient, we were able to reduce HHcyt with low doses of vitamin B6 (125mg/day) while maintaining givosiran injections. Twenty-two months after the start of givosiran therapy in our patient and 11 injections, no sign was reported in favor of a sensory neurological impairment in absence of acute neurovisceral attack.
Many observational studies have shown that HHcyt is associated with cardiovascular risk, cardiovascular mortality, ischemic heart disease, peripheral arterial disease, and arterial and venous thromboses [34]. The meta-analyses of prospective and retrospective studies concluded that HHcyt is a modest independent predictor of CHD and stroke events and all-cause mortality in healthy populations and acute coronary syndrome patients [[34], [35], [36]]. However, severe HHcyt (>100 μmol/L) is known to be associated with acute arterial and venous thromboembolic events. Therapeutic management of HHcyt varies but vitamin supplementation (usually vitamin B9 and B12) is recommended in case of deficiency, and depending on the mechanism involved or suspected (vitamin B6 in case of B6-responsive CBS deficiency as in our case of AIP). In CBS deficiency, current evidence suggests that patients are unlikely to develop acute vascular events if Hcyt is maintained below 100 μmol/L and a threshold of 50 μmol/L is recommended as therapeutic target for B6-responsive CBS patients [31]. Given the potential vascular risks, it seems reasonable to try to normalize Hcyt level using vitamin treatment. Interestingly in our patient, although a huge HHcyt, 125 mg/day pyridoxine was sufficient to maintain norm Hcyt levels. The clinical relevance of HHcyt induced by givosiran remains to be clarified in order to estimate the risk of renal and vascular involvement in AHP patients. In our patient treated for high blood pressure, we observed a slightly disturbed renal assessment which remains stable throughout the treatment course; the GFR dropped from a baseline value of approximately 70 ml/min to 45 ml/min after 22 months of givosiran (Supplementary Table 1). It has been shown that HHcyt may alter renal function in hypertensive patients [37]. Transient creatinine elevation was also observed in AIP patients treated by givosiran [38]. In the phase III ENVISION trial, a small decrease in estimated GFR was observed and remained stable in most patients [6]. Acute pancreatitis was reported in two patients treated by givosiran (one with HHcyt>100 μmol/L) and pulmonary embolism in one patient [19,25]. Although in AIP patients there is no clear evidence of a relationship between HHcyt and these adverse effects, in untreated patients with congenital CBS deficiency, thromboembolism is associated with severe HHcyt (>100 μmol/L) [31]. No signs of vascular disease were detected in our patient, liver function tests were normal, as were αFP and liver ultrasound (not shown).
In addition, the answer to the question concerning the use of an antiplatelet agent during HHcyt remains debated. The prothrombotic effects of plasma homocysteine are known. Indeed, Hcyt promotes blood clotting and reduces fibrinolysis [38]. HHcyt produced by methionine loading is associated with increased platelet aggregation in rats [39] and platelet-dependent thrombin formation in humans [40]. Hcyt is also the cause of endothelial dysfunction leading to a release of inflammatory cytokines (IL-6, IL-8 and TNFα) in mice [41]. In the current absence of suitable studies and official recommendations and based on cases report it may be possible to consider an antiplatelet agent associated with appropriate vitamin treatment in cases of major HHcyt (> 100 μmol/L) until normalization of the homocysteine level [42] especially since therapeutic approach is recommended in European guidelines for congenital homocystinuria [31].
5. Conclusion
We report a case of extremely severe HHcyt arriving from the first injection of givosiran administered as part of the management of potentially uncontrolled AIP. Thanks to the introduction of long-term vitamin B6 supplementation with decreasing doses it was possible to normalize the Hcyt level and to maintain it below the normality threshold, even during repeated givosiran injections over a 22-months period. Metabolic analysis confirms that a secondary functional CBS deficiency induced by givosiran is the most likely mechanism of HHcyt which can be counteracted by administering moderate doses of vitamin B6. This study confirms previously published results that Hcyt should be carefully monitored in AHP patients treated with givosiran, and shows that Hcyt monitoring should begin from the first injection. We suggest that the vitaminic nutritional status (vitamin B6, B9, B12 and/or methylmalonic acid) should be monitored in AHP treated with givosiran (pre and post-treatment) and that vitamin B6 supplementation should be started early in case of Hcyt elevation during the treatment. The use of antiplatelet remains debated but must be considered in cases of major elevation of Hcyt (>100 μmol/L). Subsequent studies on a larger number of patients are necessary to define more precisely vitamin B6 dosage which should be considered at treatment initiation and during long-term maintenance.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Human subject study
This work has been carried out in accordance with the Code of ethics of the World Medical Association (declaration of Helsinki) for experiments involving humans. The patient gave informed consent to participate in the study and authorization to the publication of related data.
CRediT authorship contribution statement
Isabelle Redonnet-Vernhet: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis, Conceptualization. Patrick Mercié: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Investigation, Formal analysis, Conceptualization. Louis Lebreton: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis. Jean-Marc Blouin: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis. Didier Bronnimann: Visualization, Validation, Investigation. Samir Mesli: Validation, Investigation. Claire Guibet: Visualization, Investigation. Noémie Gensous: Visualization, Validation, Investigation. Pierre Duffau: Visualization, Investigation. Laurent Gouya: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis. Emmanuel Richard: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
Pr. Patrick Mercie received fees for his participation in an advisory board for Alnylam Pharmaceuticals (France).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2024.101076.
Appendix A. Supplementary data
Supplementary Table 1
Data availability
Data will be made available on request.
References
- 1.Phillips J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019;128:164–177. doi: 10.1016/j.ymgme.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonkovsky H.L., Dixon N., Rudnick S. Pathogenesis and clinical features of acute hepatic porphyrias (AHPs) Mol. Genet. Metab. 2019;128:213–218. doi: 10.1016/j.ymgme.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouya L., Ventura P., Balwani M., Bissell D.M., Rees D.C., Stölzel U., et al. EXPLORE: a prospective, multinational, natural history study of patients with acute hepatic porphyria with recurrent attacks. Hepatology. 2020;71:1546–1558. doi: 10.1002/hep.30936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stölzel U., Doss M.O., Schuppan D. Clinical guide and update on Porphyrias. Gastroenterology. 2019;157:365–381.e4. doi: 10.1053/j.gastro.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 5.Balwani M., Sardh E., Ventura P., Peiró P.A., Rees D.C., Stölzel U., et al. Phase 3 trial of RNAi therapeutic Givosiran for acute intermittent Porphyria. N. Engl. J. Med. 2020;382:2289–2301. doi: 10.1056/NEJMoa1913147. [DOI] [PubMed] [Google Scholar]
- 6.Kuter D.J., Bonkovsky H.L., Monroy S., Ross G., Guillén-Navarro E., Cappellini M.D., et al. Efficacy and safety of givosiran for acute hepatic porphyria: final results of the randomized phase III ENVISION trial. J. Hepatol. 2023 Jul 19;S0168-8278(23):04933–04934. doi: 10.1016/j.jhep.2023.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Lazareth H., Poli A., Bignon Y., Mirmiran A., Rabant M., Cohen R., et al. Renal function decline with small interfering RNA silencing Aminolevulinic acid synthase 1 (ALAS1) Kidney Int. Rep. 2021;6:1904–1911. doi: 10.1016/j.ekir.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J. To-Figueras, Lopez R.M., Deulofeu R., Herrero C. Preliminary report: hyperhomocysteinemia in patients with acute intermittent porphyria. Metab. Clin. Exp. 2010;59:1809–1810. doi: 10.1016/j.metabol.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Ventura P., Corradini E., Di Pierro E., Marchini S., Marcacci M., Cuoghi C., et al. Hyperhomocysteinemia in patients with acute porphyrias : a potentially dangerous metabolic crossroad? Eur. J. Intern. Med. 2020;79:101–107. doi: 10.1016/j.ejim.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 10.J. To-Figueras, Wijngaard R., García-Villoria J., Aarsand A.K., Aguilera P., Deulofeu R., et al. Dysregulation of homocysteine homeostasis in acute intermittent porphyria patients receiving heme arginate or givosiran. J. Inherit. Metab. Dis. 2021;44:961–971. doi: 10.1002/jimd.12391. [DOI] [PubMed] [Google Scholar]
- 11.Fontanellas A.A., Avila M.A., Arranz E., de Salamanca R. Enríquez, Montserrat M.C. Acute intermittent porphyria, givosiran, and homocysteine. J. Inherit. Metab. Dis. 2021;44:790–791. doi: 10.1002/jimd.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ventura P., Sardh E., Longo N., Balwani M., Plutzky J., Gouya L., et al. Hyperhomocysteinemia in acute hepatic porphyria (AHP) and implications for treatment with givosiran. Expert Rev. Gastroenterol. Hepatol. 2022;16:879–894. doi: 10.1080/17474124.2022.2110469. [DOI] [PubMed] [Google Scholar]
- 13.Wald D.S., Law M., Morris J.K. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Mutairi F. Hyperhomocysteinemia: clinical insights. J. Cent. Nerv. Syst. Dis. 2020;12 doi: 10.1177/1179573520962230. 1179573520962230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaddon A., Miller J.W. Homocysteine-a retrospective and prospective appraisal. Front. Nutr. 2023;10:1179807. doi: 10.3389/fnut.2023.1179807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Diz L., Murcia M.A., Gris J.L., Pons A., Monteagudo C., Martínez-Tomé M., et al. Assessing nutritional status of acute intermittent porphyria patients. Eur. J. Clin. Investig. 2012;42:943–952. doi: 10.1111/j.1365-2362.2012.02673.x. [DOI] [PubMed] [Google Scholar]
- 17.Di Pierro E., Granata F. Nutrients and Porphyria: an intriguing crosstalk. Int. J. Mol. Sci. 2020;21:3462. doi: 10.3390/ijms21103462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badawy A.A. Multiple roles of haem in cystathionine β-synthase activity: implications for hemin and other therapies of acute hepatic porphyria. Biosci. Rep. 2021;41 doi: 10.1042/BSR20210935. BSR20210935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrides K.E., Klein M., Schuhmann E., et al. Severe homocysteinemia in two-givosiran-treated porphyria patients: is free heme deficiency the culprit? Ann. Hematol. 2021;100:1685–1693. doi: 10.1007/s00277-021-04547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricci A., Marcacci M., Cuoghi C., Pietrangelo A., Ventura P. Hyperhomocysteinemia in patients with acute porphyrias: a possible effect of ALAS1 modulation by siRNAm therapy and its control by vitamin supplementation. Eur. J. Intern. Med. 2021;92:121–123. doi: 10.1016/j.ejim.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Vassiliou D., Sardh E. Homocysteine elevation in givosiran treatment: suggested ALAS1 siRNA effect on cystathionine beta-synthase. J. Intern. Med. 2021;290:928–930. doi: 10.1111/joim.13341. [DOI] [PubMed] [Google Scholar]
- 22.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein P.E., Edel Y., Mansour R., Mustafa R.A., Sandberg S. Members of the acute Porphyria expert panel. . Key terms and definitions in acute porphyrias: Results of an international Delphi consensus led by the European porphyria network. J. Inherit. Metab. Dis. 2023;46:662–674. doi: 10.1002/jimd.12612. [DOI] [PubMed] [Google Scholar]
- 24.Fourgeaud M., Vidal T., Schmitt C., Blouin J.M., Ged C., Richard E. Recurrent posterior reversible encephalopathy syndrome in a patient with acute intermittent porphyria. Rev. Neurol. (Paris) 2020;176:118–120. doi: 10.1016/j.neurol.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Poli A., Schmitt C., Moulouel B., Mirmiran A., Talbi N., Rivière S., et al. Givosiran in acute intermittent porphyria: A personalized medicine approach. Med. Genet. Metab. 2022;135:206–214. doi: 10.1016/j.ymgme.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Tsai M.Y., Yang F., Bignell M., Aras O., Hanson N.Q. Relation between plasma homocysteine concentration, the 844ins68 variant of the cystathionine beta-synthase gene, and pyridoxal-5′-phosphate concentration. Mol. Genet. Metab. 1999;67:352–356. doi: 10.1006/mgme.1999.2874. [DOI] [PubMed] [Google Scholar]
- 27.Moll S., Varga E.A. Homocysteinemia and MTHFR mutations. Circulation. 2015;132:e6–e9. doi: 10.1161/CIRCULATIONAHA.114.013311. [DOI] [PubMed] [Google Scholar]
- 28.Vassiliou D., Sardh E., Harper P., Simon A.R., Clausen V.A., Najafian N., et al. A drug-drug interaction study evaluating the effect of Givosiran, a small interfering ribonucleic acid, on cytochrome P450 activity in the liver. Clin. Pharmacol. Ther. 2021;110:1250–1260. doi: 10.1002/cpt.2419. [DOI] [PubMed] [Google Scholar]
- 29.Kumar T., Sharma G.S., Singh L.R. Homocystinuria: therapeutic approach. Clin. Chim. Acta. 2016;458:55–62. doi: 10.1016/j.cca.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Clayton P.T. B6-responsive disorders: a model of vitamin dependency. J. Inherit. Metab. Dis. 2006;29:317–326. doi: 10.1007/s10545-005-0243-2. [DOI] [PubMed] [Google Scholar]
- 31.Morris A.A., Kožich V., Santra S., Andria G., Ben-Omran T.I., Chakrapani A.B., et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J. Inherit. Metab. Dis. 2017;40:49–74. doi: 10.1007/s10545-016-9979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammond N., Wang Y., Dimachkie N.M., Barohn R.J. Nutritional neuropathies. Beurol. Clin. 2013;31:477–489. doi: 10.1016/j.ncl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadtstein F., Vrolijk M. Vitamin-B-6-induced neuropathy: exploring the mechanisms of pyridoxin toxicity. Adv. Nutr. 2021;12:1911–1929. doi: 10.1093/advances/nmab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guéant J.L., Guéant-Rodriguez R.M., Oussalah A., Zuily S., Rosenberg I. Hyperhomocysteinemia in cardiovascular diseases: revisiting observational studies and clinical trials. Thromb. Haemost. 2023;123:270–282. doi: 10.1055/a-1952-1946. [DOI] [PubMed] [Google Scholar]
- 35.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 36.Zhu M., Mao M., Lou X. Elevated homocysteine level and prognosis in patients with acute coronary syndrome: a meta-analysis. Biomarkers. 2019;24:309–316. doi: 10.1080/1354750X.2019.1589577. [DOI] [PubMed] [Google Scholar]
- 37.Liu C., Lin L., Xu R. Elevated homocysteine and differential risks of the renal function decline in hypertensive patients. Clin. Exp. Hypertens. 2020;42:565–570. doi: 10.1080/10641963.2020.1739698. [DOI] [PubMed] [Google Scholar]
- 38.Undas A., Brozek J., Szczeklik A. Homocysteine and thrombnosis: from basic science to clinical evidence. Thomb. Haemost. 2005;94:907–915. doi: 10.1160/TH05-05-0313. [DOI] [PubMed] [Google Scholar]
- 39.Durand P., Lussier-Cacan S., Blache D. Acute methionine load-induced Hyperhomocysteinemia enhances platelet agregation, thromboxane biosynthesis, and macrophage-derived tissue factor activity in rats. FASEB J. 1997;11:1157–1168. [PubMed] [Google Scholar]
- 40.Domagala T.B., Undas A., Sydor W.J., Szczeklik A. Thrombin formation in platelet-rich plasma after oral methionine loading: preliminary report. Thromb. Res. 2002;105:503–506. doi: 10.1016/s0049-3848(02)00048-8. [DOI] [PubMed] [Google Scholar]
- 41.Balint B., Jepchumba V.K., Guéant J.L., Guéant-Rodriguez R.M. Mechanisms of homocysteine-induced damage to the endothelial, medial, and adventitial layers of the arterial wall. Biochimie. 2020;173:100–106. doi: 10.1016/j.biochi.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Sato K., Morofuji Y., Horie N., Izumo T., Anda T., Matsuo T. Hyperhomocysteinemia causes severe intraoperative thrombotic tendency in superficial temporal artery-middle cerebral artery bypass. J. Stroke Cardiovasc. Dis. 2020;5 doi: 10.1016/j.jstrokecerebrovasdis.2019.104633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Data Availability Statement
Data will be made available on request.