Abstract
A total of 576-day-old Ross 308 broilers chicks (male) were used to evaluate the effect of various levels of pistachio green hull aqueous extract (PHE) and Eimeria challenge on the growth performance, intestinal health and antioxidant capacity. During infection period (25–42 d), treatments included: 1) control + unchallenged (negative control, NC), 2) 200 ppm PHE + unchallenged, 3) 300 ppm PHE + unchallenged, 4) 400 ppm PHE + unchallenged, 5) control + challenged (positive control, PC), 6) 200 ppm PHE + challenged, 7) 300 ppm PHE + challenged and 8) 400 ppm PHE + challenged (with 6 replications for each treatment). The outcomes revealed that in the challenged birds, average body weight gain (ABW), daily weight gain (DWG), and feed conversion ratio (FCR) linearly improved with increasing the PHE levels (P < 0.05). Infected broilers had lower daily feed intake (DFI) compared to unchallenged birds (P < 0.05). Villus height (VH), villus height to crypt depth (VH: CD) ratio and villus surface area (VSA) reduced linearly (P < 0.05), while muscle layer thickness (MT) increased linearly in challenged birds (P < 0.05). The consumption of the PHE significantly reduced the excreta oocytes and duodenum and jejunum lesion scores in Eimeria-challenged broilers (P < 0.05). By increasing the PHE levels, total antioxidant capacity (TAC) and superoxide dismutase (SOD) levels increased (P < 0.05), while the Eimeria challenge reduced TAC, SOD, and glutathione peroxidase (GPx) levels (P <0.05). In general, the use of PHE in the broilers diet improved the antioxidant capacity, birds performance, but diminished the excreta oocytes and lesion scores with no negative effect on the intestinal morphology.
Key words: antioxidant capacity, broiler, Eimeria, intestinal morphology, performance
INTRODUCTION
One of the most important illness in the poultry industry is coccidiosis, which is caused by protozoan parasites Eimeria spp. It has been stated that this parasite has an adverse effect on the poultry digestive tract and decreased growth performance and increased mortality (Ali et al., 2019). Studies by Lin et al. (2022) and Teng et al. (2021) showed that Eimeria mild infection causes a significant decrease in growth performance-related traits. These parasites can reproduce in the mucous epithelium of different parts of the birds’ intestine and cause intestinal inflammation and bleeding, diarrhea, and death (Chapman, 2014). It has been reported that due to the reduced absorptive surface of the brush border in the small intestine, Eimeria-challenged broilers have less space to absorb nutrients from the intestinal tract. In addition to shorter villus in the jejunum, reduced nutrient transporters and enzymes in the brush border membrane contributed to reduced amino acid and energy digestibility in coccidiosis (Su et al., 2015; Rochell et al., 2016). Inflammatory reactions have been shown to contribute to the pathology of E. tenella infections. Evidence showed that E. tenella infection in chickens increased NO concentration, an effect also seen in E. acervulina infection (Allen, 1997). In order to prevent and control coccidiosis, many types of anti-coccidial drugs are applied, but their excessive use has caused concerns due to drug resistance Eimeria strains, tissue residue, and increasing breeding costs (Tewari and Maharana, 2011). Therefore, researchers are looking to identify different plant compounds and their by-products and investigate their active compounds, so that they can compensate for the damages caused by this disease (Abbas et al., 2006). These herbal compounds reduce the growth of pathogens through competitive elimination mechanisms and stimulation of the immune system, while they are also effective in increasing the digestibility of nutrients and reducing blood cholesterol (Lee et al., 2003).
Pistachio (Pistacia vera L.) a member of the Anacardiaceae family, is native to Asia and generally spread in the Mediterranean area (Ozcelik et al., 2005). One of the by-products of pistachio is its green hull, which makes up more than 60% of pistachio by-products (Arjeh et al., 2020). Pistachio green hull (PGH) contains high amounts of bioactive compounds, mainly phenolic compounds (Rafiee et al., 2018). It has been reported that the concentration of dry matter, crude protein, crude fiber, ash, crude fat, and nitrogen-free extract in PGH are 23.00, 11.00, 15.00, 12.00, 6.00, and 55.50%, respectively (Shakerardekani and Molaei, 2020). Various studies have investigated some of the functional attributes of the pistachio green hull extract (PHE) such as phenolic materials (Seifzadeh, et al., 2019), antimicrobial (Rajaei et al., 2010) and antioxidant (Abolhasani et al., 2018) activities and its biological profits on human health (Sila et al., 2014). Based on our research, few studies have been done on the effects of pistachio by-products in broilers (Hosseini-Vashan et al., 2020; Ahmadi Kohanali et al., 2022). Considering the widespread cultivation of pistachio in Iran and the easy access to its by-products, as well as taking into account the chemical compounds, the purpose of this study was to investigate the effect of PHE on the growth performance, intestinal morphology and antioxidant capacity of broilers that were infected by Eimeria.
MATERIALS AND METHODS
Birds, Diets, and Management
A total of 576 one-day-old Ross 308 broiler male chicks were obtained from a local commercial hatchery and reared in pens (1m*1m) for 42 d. Twelve chicks were allocated to each pen. During the starter and grower periods, treatments included 1) control, 2) 200 ppm PHE 3), 300 ppm PHE, and 4) 400 ppm PHE. Twelve replicates were used in each treatment. At the end of the grower phase (25 d), each treatment was divided into two groups (Eimeria-challenged and unchallenged broilers). Treatments included 1) control + unchallenged (negative control, NC), 2) 200 ppm PHE + unchallenged, 3) 300 ppm PHE + unchallenged, 4) 400 ppm PHE + unchallenged, 5) control + challenged (positive control, PC), 6) 200 ppm PHE + challenged, 7) 300 ppm PHE + challenged and 8) 400 ppm PHE + challenged. Six replicates were used for each treatment. At d 25 of age, half of the birds were orally gavaged with 20x dose of trivalent live attenuated vaccine (Livacox T, Biopharm Co, Prague, Czech Republic). One dose of vaccine (0.01 mL) contained 300 to 500 sporulated oocysts of each of Eimeria acervulina, Eimeria maxima, and Eimeria tenella. Before inoculation, the infectious dose of vaccine (0.5 mL) was diluted to 1 mL with distilled water. Control groups were inoculated with 1 mL of distilled water. Ingredients and nutrient composition of diets are shown in Table 1 (NRC, 1994) and diets were formulated to meet Ross 308 requirements (Aviagen, 2022). In the first 3 d of broilers life, rearing place temperature was fixed at 32 °C and then decreased by 3 °C each week to achieve 21 °C and remained stable till the termination of the study. Relative humidity was maintained at 50 to 60% within the rearing period. An 18:6 h light and darkness program was applied during the experiment. Birds had free access to feed and water.
Table 1.
Ingredients and nutrient composition of the experimental diets (as-fed basis).
| Ingredients (%) | Starter (1–10 d) | Grower (11–24 d) | Finisher (25–42 d) |
|---|---|---|---|
| Corn | 49.14 | 53.96 | 60.75 |
| Soybean meal (44%) | 42.23 | 38.08 | 32.12 |
| Soybean oil | 4.25 | 4.36 | 3.84 |
| Limestone | 0.97 | 0.72 | 0.67 |
| Dicalcium phosphate | 2.04 | 1.61 | 1.32 |
| Vitamin premix1 | 0.25 | 0.25 | 0.25 |
| Mineral premix2 | 0.25 | 0.25 | 0.25 |
| L-Lysine HCl | 0.07 | 0.02 | 0.07 |
| DL-Methionine | 0.29 | 0.24 | 0.23 |
| L-Threonine | 0.01 | - | - |
| Choline | 0.05 | 0.05 | 0.05 |
| Sodium Bicarbonate | 0.11 | 0.11 | 0.11 |
| Salt (NaCl) | 0.35 | 0.35 | 0.34 |
| Nutrient composition (%) | |||
| Metabolizable energy (kcal/kg) | 2975 | 3050 | 3100 |
| Crude protein | 23 | 21.50 | 19.50 |
| Lysine | 1.32 | 1.18 | 1.08 |
| Methionine + Cystine | 1.00 | 0.92 | 0.86 |
| Threonine | 0.88 | 0.80 | 0.72 |
| Calcium | 0.95 | 0.75 | 0.65 |
| Available Phosphorus | 0.50 | 0.42 | 0.36 |
| Sodium | 0.18 | 0.18 | 0.18 |
| Potassium | 0.99 | 0.92 | 0.82 |
| Chlorine | 0.26 | 0.25 | 0.26 |
Provided the followings per kg of diet: vitamin A (trans-retinyl acetate), 13,000 U; vitamin D3 (cholecalciferol), 5,000 U; vitamin E (D L-α tocopherol acetate), 80 U; vitamin K (menadione), 4 mg; riboflavin, 9 mg; pantothenic acid (D-Ca pantothenate), 25 mg; pyridoxine (pyridoxine-HCl), 5 mg; thiamine, 5 mg; vitamin B12 (cyanocobalamin), 0.02 mg; biotin, 0.35 mg; folic acid, 2.5 mg; nicotinic acid, 70 mg; ethoxyquin (antioxidant), 2.5 mg.
Provided the following per kg of diet: Fe, 20 mg; Zn, 120 mg; Mn, 120 mg; Cu, 16 mg; I, 1.25 mg; Se, 0.30 mg.
Preparation of Pistachio Green Hull Extract
Pistachio green hulls (Ahmadaghaei variety) were obtained from Fakhrabad, Iran. Hulls were dried, ground, and stored at -80C until analysis. Using a liquid-to-solid ratio of 15:1 (at 25 °C for 8 h), one gram of ground green hull was placed in the water, (Rajaei et al., 2010). Folin–Ciocalteu colorimetric method was applied to measure the total phenolic content (Waterhouse, 2002). By a calibration curve obtained with gallic acid, calculations were done. Total phenolic content was represented as milligram of gallic acid equivalents per gram of dry weight. The total tannin was measured by calculating the nontannin phenols and the precipitation of tannins using insoluble polyvinyl pyrrolidone. Difference between total phenol and nontannin phenols showed the total tannins content (Makkar et al., 1995). For standard, gallic acid was applied and the total flavonoid content was measured (Heimler et al., 2005). The dried pistachio green hull powder contained 3931.67 kcal/kg of gross energy, 941.64 g of dry matter/kg, 121.46 g of crude protein/kg, 58.13 g of crude fat/kg, 151.68 g of crude fiber/kg, and 119.83 g of crude ash/kg (AOAC, 2005). The chemical composition of the pistachio green hull extract is shown in Table 2.
Table 2.
Chemical composition of pistachio green hull extract (PHE).
| Characteristics | Amount |
|---|---|
| Total phenol (mg GAE/g DW pistachio hull) | 91.32 |
| Total tannin (mg GAE/g DW pistachio hull) | 32.03 |
| Flavonoid (mg CE/g DW pistachio hull) | 30.46 |
Abbreviations: GAE, gallic acid equivalents; DW, dry weight; CE, catechin equivalent.
Growth Indicators
Birds weight from each replicate was determined on 11, 25 and 42 d of age. Growth indicators including average body weight (ABW), weight gain (as daily, DWG), feed intake (as daily, DFI) and feed conversion ratio (FCR) were recorded (Noruzi et al., 2023).
Intestinal Morphology
All stages of the experiment from selection and slaughter of birds and sampling were done according to Noruzi et al. (2023). At the end of the experiment, the parameters including villus height (VH), villus width (VW), crypt depth (CD), and the thickness of muscle layer (MT) were recorded. Villus surface area (VSA) was measured by (2л)*(VW/2)*(VH). One bird from each replicate was randomly selected and euthanized by cervical dislocation at 42 d. The entire intestinal tract was separated, and 1 cm parts were taken from the mid-point of the jejunum. The sections were fixed in neutral buffered formalin solution (10%) and fixed in paraffin wax later. All histological analyses were done on 5 μm segments, stained with H&E. To assess the morphology of the samples, a computer-connected optical microscope (Olympus model B×51 microscope; magnification 100) was used.
Lesion Scoring
On d 7 post-Eimeria challenge (32 d of age), lesion scoring of the duodenum, jejunum, and cecum was done. One bird per pen was randomly selected and euthanized. The three mentioned parts were assessed according to method described by Johnson and Reid (1970) method with scores ranging from 0 (no gross lesions) to 4 (most severe lesions).
Number of Oocysts Per Gram of Excreta
The excreta were collected on d 5, 7, 9, and 11 postcoccidiosis challenge. Samples were kept in the refrigerator for measuring of oocysts per gram of excreta. A 10 % (w/v) fecal sample was suspended in a NaCl solution (151 g salt blended into 1 l of water). After shaking, 1 mL of the suspension was combined with 9 mL of a NaCl solution (311 g of NaCl mixed into 1 l of distal water). Then, the suspension was introduced into the McMaster chamber by a micropipette, and the number of oocysts was counted using a microscope (Ali et al., 2019).
Serum Antioxidant Capacity
On d 42, blood samples were obtained from the same birds that were slaughtered for intestinal morphology evaluation (each bird from each replicate). Assay kits for total antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GPx), and malondialdehyde (MDA) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All of the experiments followed the kit's instructions. The method of ferric reducing-antioxidant power assay was used to measure the TAC (Benzie and Strain, 1996) and detected at 520 nm. The concentration of SOD was determined via the xanthine oxidase method (Winterbourn et al., 1975). The amount of GPx was determined at 412 nm using a spectrophotometer (Hafeman et al., 1974). The MDA level was analyzed with 2-thiobarbituric acid, monitoring the change of absorbance at 532 nm (Placer et al., 1996). The concentration of nitric oxide (NO) was determined at 550 nm according to Wang et al. (2008a).
Statistical Analyses
This trial was performed based on a completely randomized design (CRD) during starter (1–10 d) and grower (11–24 d) phases with 4 treatments and 12 replicates for each of them. General Linear Model (GLM) procedure of SAS 9.4 (2012) software was used and linear and quadratic contrasts were reported. During the finisher (25–42 d) period, data (growth performance, jejunum morphology, antioxidant activity) was analyzed based on CRD in a factorial test of 4*2 (4, levels of PHE; 2, Eimeria challenged and unchallenged broilers) with 6 replications and GLM procedure of SAS 9.4 (2012) software was applied. Kruskal–Wallis test (nonparametric statistical method) followed by the DSCF1 post hoc test was used to analyze lesion score data. Results are shown as means ± standard error of the mean (SEM). The means were compared by Tukey's test (P < 0 .05).
RESULTS
Growth Performance
During the preinfection phase (1–24 d of age), different levels of PHE did not significantly affect the growth performance data (Table 3). During the infection phase (25–42 d of age) (Table 4), in the absence of the Eimeria challenge, growth performance was not affected by different levels of PHE (0, 200, 300, and 400 ppm) (P < 0.05). However, in challenged birds, ABW, DWG, and FCR linearly increased and decreased with increasing the levels of PHE, respectively (P < 0.05). Infected broilers had lower DFI compared to the others (P < 0.05).
Table 3.
Effects of pistachio green hull aqueous extract on the performance of broilers during the starter (1–10 d) and grower phases (11–24 d).
| PHE levels (ppm) |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Starter phase | 0 | 200 | 300 | 400 | SEM | Model | Linear | Quadratic |
| ABW (g/bird) | 243.61 | 240.96 | 246.10 | 246.95 | 2.612 | 0.372 | 0.201 | 0.506 |
| DWG (g/bird/d) | 20.50 | 20.26 | 20.78 | 20.79 | 0.261 | 0.430 | 0.244 | 0.627 |
| DFI (g/bird/d) | 26.86 | 26.83 | 27.51 | 27.11 | 0.334 | 0.450 | 0.342 | 0.581 |
| FCR (g:g) | 1.32 | 1.34 | 1.33 | 1.32 | 0.009 | 0.414 | 0.652 | 0.130 |
| Grower phase | ||||||||
| ABW (g/bird) | 912.58 | 913.94 | 923.75 | 911.38 | 8.660 | 0.735 | 0.873 | 0.432 |
| DWG (g/bird/d) | 47.78 | 48.07 | 48.40 | 47.46 | 0.589 | 0.701 | 0.809 | 0.302 |
| DFI (g/bird/d) | 71.60 | 71.70 | 71.25 | 70.49 | 0.897 | 0.773 | 0.352 | 0.635 |
| FCR (g:g) | 1.50 | 1.49 | 1.48 | 1.50 | 0.008 | 0.322 | 0.443 | 0.114 |
Abbreviations: PHE, pistachio green hull aqueous extract; AWB, average body weight; DWG, daily weight gain; DFI, daily feed intake; FCR, feed conversion ratio; SEM, standard error of the mean.
Table 4.
Effects of pistachio green hull aqueous extract and Eimeria challenge on the performance of broilers during the finisher (25–42 d) and whole phases (1–42 d).
| Main effects | Finisher |
Whole phase |
|||||
|---|---|---|---|---|---|---|---|
| PHE levels (ppm) | ABW (g/bird) | DWG (g/bird/d) | DFI (g/bird/d) | FCR (g:g) | DWG (g/bird/d) | DFI (g/bird/d) | FCR (g:g) |
| 0 | 2,357.65b | 80.28b | 133.44 | 1.67 | 55.22b | 87.45 | 1.59a |
| 200 | 2,387.29ab | 81.85ab | 135.28 | 1.66 | 55.93ab | 88.26 | 1.58ab |
| 300 | 2,417.73a | 82.99a | 134.88 | 1.63 | 56.65a | 88.11 | 1.56bc |
| 400 | 2,417.67a | 83.68a | 134.72 | 1.63 | 56.63a | 87.69 | 1.55c |
| SEM | 12.803 | 0.579 | 0.648 | 0.012 | 0.305 | 0.374 | 0.007 |
| Eimeria challenge | |||||||
| (-) | 2,439.14a | 84.24a | 136.68a | 1.63b | 57.16a | 88.97a | 1.56b |
| (+) | 2,351.03b | 80.16b | 132.48b | 1.67a | 55.06b | 86.77b | 1.58a |
| SEM | 9.053 | 0.410 | 0.458 | 0.009 | 0.215 | 0.267 | 0.005 |
| Interaction effects | |||||||
| 0 × (-) | 2,448.88a | 84.89a | 136.26 | 1.61b | 57.39a | 88.82 | 1.55b |
| 200 × (-) | 2,419.14ab | 83.26ab | 137.25 | 1.64b | 56.68ab | 89.33 | 1.57b |
| 300 × (-) | 2,449.48a | 84.20a | 136.34 | 1.63b | 57.41a | 88.96 | 1.55b |
| 400 × (-) | 2,439.03a | 84.63a | 136.88 | 1.62b | 57.14a | 88.78 | 1.55b |
| 0 × (+) | 2,266.41c | 75.68c | 130.61 | 1.73a | 53.04c | 86.07 | 1.62a |
| 200 × (+) | 2,355.43b | 80.45b | 133.32 | 1.67ab | 55.17b | 87.20 | 1.58ab |
| 300 × (+) | 2,385.97ab | 81.80ab | 133.42 | 1.63b | 55.89ab | 87.25 | 1.56b |
| 400 × (+) | 2,396.31ab | 82.73ab | 132.57 | 1.63b | 56.12ab | 86.60 | 1.54b |
| SEM | 18.106 | 0.819 | 0.917 | 0.017 | 0.431 | 0.534 | 0.010 |
| P value | |||||||
| PHE levels | 0.005 | 0.001 | 0.223 | 0.050 | 0.005 | 0.408 | 0.002 |
| Eimeria challenge | <.0001 | <.0001 | <.0001 | 0.005 | <.0001 | <.0001 | 0.009 |
| PHE levels × Eimeria challenge | 0.002 | 0.0001 | 0.525 | 0.006 | 0.002 | 0.807 | 0.0009 |
| Linear (PHE levels at (-)) | 0.992 | 0.961 | 0.731 | 0.854 | 0.995 | 0.841 | 0.880 |
| Quadratic (PHE levels at (-)) | 0.587 | 0.224 | 0.715 | 0.256 | 0.603 | 0.536 | 0.311 |
| Linear (PHE levels at (+)) | <.0001 | <.0001 | 0.255 | 0.0002 | <.0001 | 0.493 | <.0001 |
| Quadratic (PHE levels at (+)) | 0.051 | 0.029 | 0.135 | 0.066 | 0.052 | 0.104 | 0.164 |
Abbreviations: PHE, pistachio green hull aqueous extract; AWB, average body weight; DWG, daily weight gain; DFI, daily feed intake; FCR, feed conversion ratio; (-), unchallenged groups; (+), challenged groups; SEM, Standard error of the mean.
Means within a column without a common superscript significantly differ (P < 0.05).
Intestinal Morphology
The main and compound effects of PHE levels and Eimeria challenge on the broiler's jejunum morphology are shown in Table 5. Villus height, VH: CD ratio, and VSA decreased linearly (P < 0.05) with the coccidiosis challenge. However, the MT increased (P < 0.05) in challenged birds.
Table 5.
Effects of pistachio green hull aqueous extract and Eimeria challenge on the jejunum morphology at 42 d of age.
| Main effects | Jejunum morphology Features |
|||||
|---|---|---|---|---|---|---|
| PHE levels (ppm) | VH (µm) | VW (µm) | CD (µm) | VH: CD | MT (µm) | VSA (mm2) |
| 0 | 1,176.65 | 148.63 | 261.36 | 4.50 | 269.50 | 0.55 |
| 200 | 1,185.52 | 147.00 | 259.27 | 4.57 | 268.65 | 0.55 |
| 300 | 1,175.36 | 149.18 | 258.72 | 4.55 | 269.42 | 0.55 |
| 400 | 1,166.07 | 148.25 | 257.91 | 4.52 | 266.41 | 0.54 |
| SEM | 15.691 | 1.313 | 1.529 | 0.072 | 1.263 | 0.009 |
| Eimeria challenge | ||||||
| (-) | 1,215.70a | 149.25 | 259.63 | 4.69a | 267.09b | 0.57a |
| (+) | 1,136.08b | 147.21 | 259.00 | 4.39b | 269.92a | 0.53b |
| SEM | 11.095 | 0.928 | 1.081 | 0.051 | 0.893 | 0.006 |
| Interaction effects | ||||||
| 0 × (-) | 1,221.00 | 149.32 | 260.15 | 4.69 | 266.50 | 0.57 |
| 200 × (-) | 1,242.84 | 147.50 | 259.17 | 4.80 | 267.33 | 0.58 |
| 300 × (-) | 1,200.15 | 150.50 | 260.15 | 4.62 | 268.67 | 0.57 |
| 400 × (-) | 1,198.80 | 149.67 | 259.00 | 4.63 | 265.84 | 0.56 |
| 0 × (+) | 1,132.33 | 148.00 | 262.50 | 4.31 | 272.50 | 0.53 |
| 200 × (+) | 1,128.16 | 146.51 | 259.35 | 4.35 | 270.00 | 0.52 |
| 300 × (+) | 1,150.51 | 147.49 | 257.32 | 4.48 | 270.16 | 0.53 |
| 400 × (+) | 1,133.36 | 146.83 | 256.80 | 4.42 | 267.00 | 0.52 |
| SEM | 22.190 | 1.857 | 2.162 | 0.102 | 1.786 | 0.012 |
| P value | ||||||
| PHE levels | 0.856 | 0.723 | 0.447 | 0.910 | 0.288 | 0.942 |
| Eimeria challenge | <.0001 | 0.128 | 0.685 | 0.0002 | 0.030 | <.0001 |
| PHE levels × Eimeria challenge | 0.493 | 0.928 | 0.623 | 0.419 | 0.521 | 0.844 |
| Linear (PHE levels at (-)) | 0.204 | 0.659 | 0.805 | 0.380 | 0.933 | 0.557 |
| Quadratic (PHE levels at (-)) | 0.540 | 0.804 | 0.971 | 0.638 | 0.306 | 0.809 |
| Linear (PHE levels at (+)) | 0.825 | 0.746 | 0.055 | 0.404 | 0.059 | 0.966 |
| Quadratic (PHE levels at (+)) | 0.799 | 0.810 | 0.530 | 0.660 | 0.857 | 0.923 |
Abbreviations: PHE, pistachio green hull aqueous extract; VH, villus height; VW, villus width; CD, crypt depth; VH: CD, villus height to crypt depth ratio; MT, muscle thickness; VSA, villus surface area; (-), unchallenged groups; (+), challenged groups; SEM, Standard error of the mean.
Means within a column without a common superscript significantly differ (P < 0.05).
Lesion Scoring
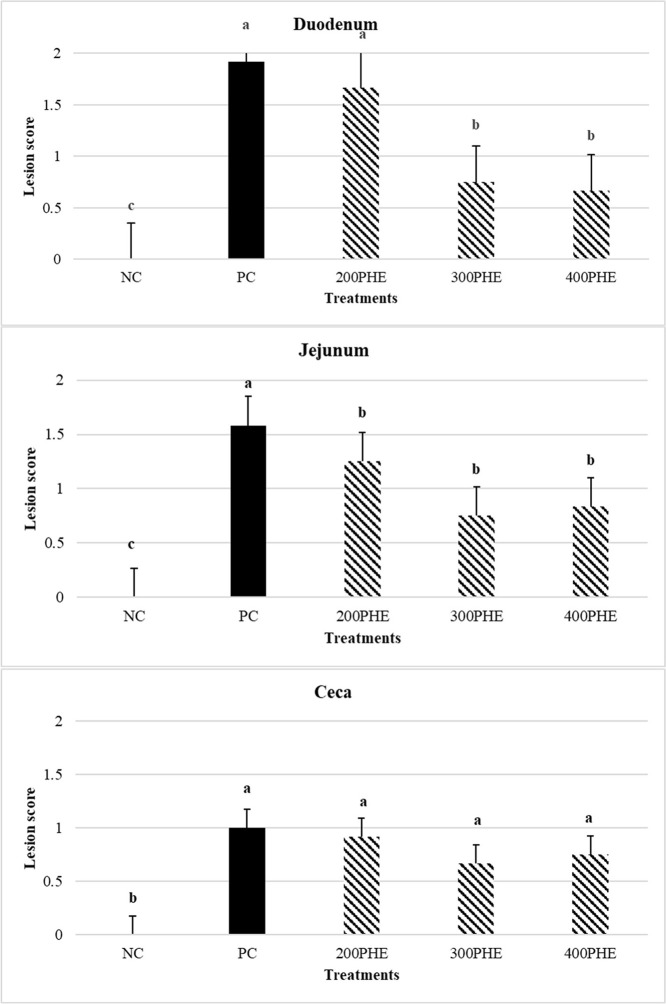
Figure 1 shows the main effects of PHE levels on the lesion score of duodenum, jejunum, and ceca on d 7 of post-infection. As the bar charts highlight, in the duodenum, 300 and 400 ppm of PHE considerably propitiated the lesions compared to PC, while for jejunum, in general, the use of PHE (200, 300, and 400 ppm) remarkably alleviated the lesions (P < 0.05). In ceca, no significant difference was observed between PHE treatments and PC (P > 0.05).
Figure 1.
Effect of dietary supplementation of pistachio green hull extract (PHE) on lesion score (d 7 postinfection) in feces of Ross 308 male broiler chicks challenged with Eimeria species on d 25. NC: negative control (no challenged-no supplementation group); PC: positive control (challenged-no supplementation group); 200, 300, and 400 PHE: challenged and supplemented with 200, 300, and 400 PHE in the diet respectively). Data are presented as means ±SEM.
Number of Oocysts Per Gram of Excreta
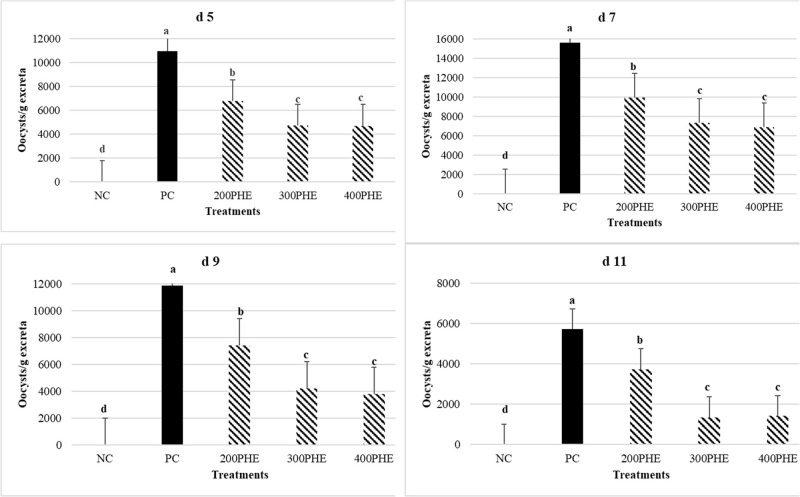
Figure 2 indicates the effects of dietary PHE on the excreta oocyst shedding (d 5, 7, 9, and 11 postinfection). In all measurement periods, consumption of 200, 300, and 400 ppm of PHE significantly reduced the amount of oocytes compared to PC (P < 0.05).
Figure 2.
Effect of dietary supplementation of pistachio green hull extract (PHE) on oocyst shedding (d 5, 7, 9, and 11 postinfection) in feces of Ross 308 male broiler chicks challenged with Eimeria species on d 25. NC: negative control (no challenged-no supplementation group); PC: positive control (challenged-no supplementation group); 200, 300, and 400 PHE: challenged and supplemented with 200, 300, and 400 PHE in the diet respectively). Data are presented as means ±SEM.
Serum antioxidant Activity
Table 6 illustrates the response of serum antioxidants to PHE levels and the Eimeria challenge. By increasing the levels of PHE in the diets, the amount of TAC and SOD in serum notably increased. However, birds that consumed diets containing 400 ppm PHE had the highest TAC and SOD (P < 0.05). The Eimeria challenge caused a notable reduction in TAC, SOD, and GPx levels (P < 0.05). Results showed that in the challenged birds, the levels of NO increased quadratically compared to healthy birds. The concentration of NO in the non-challenged and challenged birds consuming 300 and 400 ppm PHE was remarkably lower than others (P < 0.05).
Table 6.
Effects of pistachio green hull aqueous extract and Eimeria challenge on the serum antioxidants of broilers at 42 d of age.
| Main effects | Antioxidant capacity |
||||
|---|---|---|---|---|---|
| PHE levels (ppm) | TAC (U/mL) | NO (µmol/L) | MDA (nmol/mL) | SOD (U/mL) | GPx (U/mL) |
| 0 | 18.96b | 12.51a | 7.28 | 121.83b | 158.92 |
| 200 | 19.33ab | 11.52ab | 7.21 | 124.42b | 161.17 |
| 300 | 19.14ab | 10.32b | 7.19 | 128.67a | 163.67 |
| 400 | 19.93a | 10.42b | 7.24 | 131.20a | 163.20 |
| SEM | 0.229 | 0.465 | 0.147 | 1.062 | 1.291 |
| Eimeria challenge | |||||
| (-) | 20.10a | 9.27b | 7.11 | 129.42a | 164.83a |
| (+) | 18.58b | 13.11a | 7.35 | 123.64b | 158.64b |
| SEM | 0.162 | 0.336 | 0.097 | 0.769 | 0.935 |
| Interaction effects | |||||
| 0 × (-) | 19.65 | 9.71b | 7.13 | 123.83 | 163.50 |
| 200 × (-) | 20.14 | 8.29b | 7.03 | 127.17 | 165.83 |
| 300 × (-) | 19.77 | 9.44b | 7.11 | 132.67 | 166.00 |
| 400 × (-) | 20.84 | 9.66b | 7.15 | 134.00 | 164.00 |
| 0 × (+) | 18.28 | 15.31a | 7.43 | 119.84 | 154.32 |
| 200 × (+) | 18.52 | 14.75a | 7.39 | 121.67 | 156.50 |
| 300 × (+) | 18.51 | 11.21b | 7.26 | 124.66 | 161.35 |
| 400 × (+) | 19.03 | 11.19b | 7.32 | 128.40 | 162.40 |
| SEM | 0.324 | 0.690 | 0.190 | 1.502 | 1.825 |
| P value | |||||
| PHE levels | 0.027 | 0.005 | 0.969 | <.0001 | 0.053 |
| Eimeria challenge | <.0001 | <.0001 | 0.081 | <.0001 | <.0001 |
| PHE levels × Eimeria challenge | 0.835 | 0.005 | 0.939 | 0.615 | 0.129 |
| Linear (PHE levels at [-]) | 0.051 | 0.679 | 0.875 | <.0001 | 0.845 |
| Quadratic (PHE levels at [-]) | 0.401 | 0.129 | 0.704 | 0.474 | 0.263 |
| Linear (PHE levels at [+]) | 0.117 | 0.002 | 0.650 | 0.001 | 0.002 |
| Quadratic (PHE levels at [+]) | 0.655 | 0.731 | 0.804 | 0.576 | 0.764 |
Abbreviations: PHE, pistachio green hull aqueous extract; TAC, total antioxidant capacity; NO, nitric oxide; MDA, malonaldehyde; SOD, superoxide dismutase; GPx, glutathione peroxidase; (-), unchallenged groups; (+), challenged groups; SEM, Standard error of the mean.
Means within a column without a common superscript significantly differ (P < 0.05).
DISCUSSION
There are few studies regarding the effect of PHE in broilers. Thus, the objective of this trial was to investigate the impact of different concentrations of PHE on the performance of unchallenged and Eimeria-challenged broilers. Previous studies reported that plants rich in antioxidant compounds such as phenols, flavonoids, tannins, and saponins can be used as alternative factors to remedy birds’ coccidiosis and have anticoccidial impacts (Hussain et al., 2021). In the present experiment, different levels of PHE had no significant effect on the ABW (finisher phase), DWG (finisher and entire experiment period), and FCR (finisher and entire experiment period) of the unchallenged birds. Noruzi and Aziz-Aliabadi (2024) also reported that using different levels of garlic (Allium sativum) and mushroom (Agaricus bisporus) powder in the broilers diet did not have any considerable effect on the growth performance. However, in the current study, in the infected birds, performance parameters increased significantly with the increase in PHE dietary levels. On the other hand, infected broilers consumed less feed compared to uninfected birds. In accordance with our results, Xu et al. (2024) attributed the improvement in growth performance and reduction in the prevalence of E. coli in chickens consuming pomegranate peel to its antioxidant properties. In the case of the non-challenged period, treatments difference was not significant. It can be said that the use of PHE is more effective when the birds are struggling with the challenge. It has been reported that pistachio green hull has significant amounts of phenolic compounds, and the high level of these compounds indicates high antioxidant and antimicrobial capabilities (Goli et al., 2005). Therefore, due to the presence of polyphenols in PHE and its antibacterial effects, it provides the basis for efficient digestion and absorption of nutrients and improves performance traits by changing the digestive tract environment (Sharifian et al., 2019). Moreover, Upadhaya et al. (2019) also reported that the Eimeria-infected birds fed with dietary containing essential oils showed better BWG and FCR compared to un-infected broilers without essential oils. They attributed this improvement to increased nutrient digestibility resulting from greater secretion of digestive enzymes. Khorrami et al. (2022) observed that 100 and 200 ppm of pomegranate peel extract did not have significant effect on the weight gain of Eimeria-challenged birds. It has been reported that thymol- and carvacrol-based encapsulated essential oils in the diet of broiler chickens under coccidiosis challenge improved body weight gain compared to the PC (challenged control) (Lee et al., 2020).
An intestine with normal operation and structure is the basis of efficient digestion and absorption of nutrients and ultimately the growth of the animal. Villus height, CD, and the VH: CD ratio are vital factors in assessing intestinal digestion and absorption in birds. The longer villus illustrates the intestinal health improvement, which in addition to a higher capacity of nutrients absorption creates uniform and integrated mucosa (Aziz-Aliabadi et al., 2023). In the current study, PHE did not have significant effect on the jejunum morphology, although Eimeria decreased VH, VH: CD ratio, VSA, and increased MT. According to the VSA formula, it can be said that the reason for its decrease was the reduction in the VH. It has been stated that during pathogen challenge, lymphocytes will accumulate at the site of infection and cause swelling and inflammation of the tissue and as a result increase the MT (Kaya and Tuncer, 2009). In agreement with these results, Alagbe et al. (2023) observed that the coccidia challenge reduced VH and VH: CD ratio and increased CD in the jejunum. They reported that deeper crypts and reduced VH:CD ratios in the avian gut due to Eimeria led to inefficient nutrient absorption due to high rate of tissue turnover rates. Jelveh et al. (2023) also stated that the coccidiosis challenge reduced VH: CD ratio. Another study (Zhang et al., 2016) showed that the coccidiosis challenge had no significant effect on the length of intestinal villi. They attributed this result to the mild to moderate level of coccidiosis.
During coccidiosis, the intestinal lining cells are infected with sporozoites, resulting in intestinal mucosa and submucosa damage (Perez-Carbajal et al., 2010). Hence, the examination of the intestinal lesion is performed to determine lesion severity. Giannenas et al. (2003) reported that the lesion scores in the E. tenella-inoculated group supplemented with essential oils were considerably lower than in the inoculated control group. The antimicrobial properties of herbal compounds may reduce Eimeria oocysts and thus reduce the damage caused by these protozoans (Giannenas et al., 2003). In the present experiment, PHE reduced lesions in the duodenum and jejunum. Khorrami et al. (2022) reported that the use of pomegranate extract decreased intestinal lesions in a dose-dependent manner. Mohiti-Asli and Ghenaatparast-Rashti (2015) also stated that the use of oregano essential oil reduced the amount of lesions in the initial and middle parts of the intestine. It has been stated that the hydroxyl substances of phenolic compounds, transport ions and protons into and out of the cell membrane. They disrupt the lipid structure of the cell membrane and enable it to penetrate ions and lead to the inhibition of enzyme activity, followed by cell death (Ultee et al., 2002). In the current study, the consumption of different levels of PHE reduced the number of oocytes excreted in the feces. The birds that received 300 and 400 ppm of PHE had the lowest amount of oocytes. However, these data could be acquired in our experiment because we induced mild infection (lesion score below 2). In line with our findings, Zhang et al. (2020) reported anticoccidial impacts of green tea extract in broiler chickens. This extract reduced the Eimeria infection via decreasing the mortality, oocysts numbers, and lesion score in infected broiler. Another study showed that supplementation of broilers with green tea extract considerably reduced fecal oocyst output in chickens challenged with E. maxima. Overall, the higher amounts of green tea showed a more protective impact and reduced fecal oocyst shedding (Jang et al., 2007). Various studies have investigated the effect of medicinal plants containing phenolic compounds such as Aloe vera (Desalegn and Ahmed, 2020), Artemisia annua (Ferreira et al., 2010), Commiphora swynnertonii (Bakari et al., 2012), Curcuma longa (Sanchez-Hernandez et al., 2019), Emblica officinali (Sharma et al., 2021), Parkia biglobosa (Ugwuoke and Pewan, 2020) and Prunus domestica (Muthamilselvan et al., 2016) on the birds performance and number of Eimeria oocytes. Their results revealed that by penetrating the coccidia oocyst wall, tannins destroy its cytoplasm and probably deactivate the endogenous enzymes responsible for the sporulation cycle in chickens. As a result, oocysts numbers will decrease.
Redox equivalence is essential to maintain cell and animal life. It refers to the free radicals production and antioxidant capacity. Total antioxidant capacity shows the total activity of all antioxidants (enzymatic or nonenzymatic) (Mao et al., 2023). It has been shown that large amounts of NO are produced by chicken monocytes and macrophages upon exposure to bacteria such as Lactobacillus and Salmonella or after Eimeria infection (Lee et al., 2011). Since NO is produced by immune leukocytes in response to a variety of poultry infectious pathogens, we measured serum NO levels in different treatment groups. Our outcomes showed that the concentration of serum NO was significantly higher in birds challenged with Eimeria. However, NO levels in broilers that consumed 300 and 400 ppm PHE were considerably reduced. This result can indicate the strong antioxidant property of PHE and its effectiveness. This finding was in line with the results of Pirali Kheirabadi et al. (2011). They concluded that Eimeria infection stimulated NO production, which was significant at 10 and 14 d after the challenge. In the current study, PHE significantly increased TAC and SOD levels. However, Eimeria notably reduced TAC, SOD, and GPx levels in the serum.Wang et al (2008a) reported an increase in serum NO concentration following the coccidiosis challenge. They stated that an increase in plasma SOD and a decrease in MDA and NO concentrations after consumption of grape seed proanthocyanidin extract indicates its antioxidant properties (Wang et al., 2008a). According to their results, herbal compounds were able to restore the oxidant-antioxidant balance that was disrupted by parasite infection (through oxidative stress) through improving the antioxidant protection system or directly eliminating free radicals (Wang et al., 2008a). It has been stated that the use of Forsythia suspensa extract in the broilers diet increased TAC and SOD levels and decreased MDA levels, while it had no significant effect on the GPx levels (Wang et al., 2008b). In contrast to our results, Zhang et al. (2023) did not report any significant effect of essential oil and Eimeria on the antioxidant capacity in birds.
CONCLUSIONS
It can be concluded that the use of different levels of pistachio green hull aqueous extract in the broilers diets without any negative effect on the jejunum morphology, improved birds performance and antioxidant capacity. The results related to the performance, oocyte shedding and lesion scores emphasized that the pistachio green hull aqueous extract will perform more efficiently in the presence of Eimeria challenge. It is recommended to use 300 to 400 ppm of green hull aqueous in the broilers diet within coccidiosis infection.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Dwass-Steel-Critchlow-Fligner.
REFERENCES
- Abbas R.Z., Iqbal Z., Akhtar M.S., Khan M.N., Jabbar A., Sandhu Z.U. Anticoccidial screening of Azedarach Indica (Neem) in broilers. Pharmacol. Online. 2006;3:365–371. [Google Scholar]
- Abolhasani A., Barzegar M., Sahari M.A. Effect of gamma irradiation on the extraction yield, antioxidant, and anti tyrosinase activities of pistachio green hull extract. Radiat. Phys. Chem. 2018;144:373–378. [Google Scholar]
- Ahmadi Kohanali R., Hosseini-vashan S.J., Mojtahedi M., Sarir H. Effects of Kallequchi Pistachio Green Hull (Pistacia vera) and its processed with urea on performance, immune response, and blood biochemical indices and jejunal morphology in broiler chickens. IJASR. 2022;14:379–398. [Google Scholar]
- Alagbe E.O., Schulze H., Adeola O. Growth performance, nutrient digestibility, intestinal morphology, cecal mucosal cytokines and serum antioxidant responses of broiler chickens to dietary enzymatically treated yeast and coccidia challenge. J. Anim. Sci. Biotechnol. 2023;14:1–13. doi: 10.1186/s40104-023-00846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Chand N., Khan R.U., Naz S., Gul S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019;47:79–84. [Google Scholar]
- Allen P.C. Nitric oxide production during Eimeria tenella infections in chickens. Poult. Sci. 1997;76:810–813. doi: 10.1093/ps/76.6.810. [DOI] [PubMed] [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of AOAC International. 18th ed., 2005, Gaithersburg, MD
- Arjeh E., Akhavan H.R., Barzegar M., Carbonell-Barrachina A.A. Bioactive compounds and functional properties of pistachio hull: A review. Trends. Food Sci. Technol. 2020;97:55–64. [Google Scholar]
- Aviagen . Aviagen group; Huntsville (al): 2022. Ross Broiler: Nutrition Specifications. [Google Scholar]
- Aziz-Aliabadi F., Noruzi H., Hassanabadi A. Effect of different levels of green tea (Camellia sinensis) and mulberry (Morus alba) leaves powder on performance, carcass characteristics, immune response and intestinal morphology of broiler chickens. Vet. Med. Sci. 2023;9:1281–1291. doi: 10.1002/vms3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakari G.G., Max R.A., Mdegela R.H., Phiri E.C., Mtambo M.M. Effect of resinous extract from Commiphora swynnertonii (Burrt) on experimental coccidial infection in chickens. Trop. Anim. Health Prod. 2012;45:455–459. doi: 10.1007/s11250-012-0239-5. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”, the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Desalegn A.Y., Ahmed M.R. Anticoccidial activity of Aloe debrana and Aloe pulcherrima leaf gel against Eimeria oocysts. J. Parasitol. Res. 2020;2020 doi: 10.1155/2020/8524973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J.F., Luthria D.L., Sasaki T., Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannenas I., Florou-Paneri P., Papazahariadou M., Christaki E., Botsoglou N.A., Spais A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003;57:99–106. doi: 10.1080/0003942031000107299. [DOI] [PubMed] [Google Scholar]
- Goli A.H., Barzegar M., Sahari M.A. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005;92:521–525. [Google Scholar]
- Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rats. J. Nutr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- Heimler D., Vignolini P., Dini M.G., Romani A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005;53:3053–3056. doi: 10.1021/jf049001r. [DOI] [PubMed] [Google Scholar]
- Hosseini-Vashan S.J., Yousefi H., Ghiasi S.E., Namaei M.H. Two types of pistachio hull extract (Pistacia vera) on performance, blood indices and intestinal microbial population of broilers challenged with Staphylococcus aureus. J. Vet. Res. 2020;4:418–430. [Google Scholar]
- Hussain K., Abbas R.Z., Abbas A., Rehman M.A., Raza M.A., Rehman T., Hussain R., Mahmood M.S., Imran M., Zaman M.A., Sindhu Z.D. Anticoccidial and biochemical effects of Artemisia brevifolia extract in broiler chickens. Braz. J. Poult. Sci. 2021;23:1–6. [Google Scholar]
- Jang S.I., Jun M.H., Lillehoj H.S., Dalloul R.A., Kong I.K., Kim S., Min W. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet. Parasitol. 2007;144:172–175. doi: 10.1016/j.vetpar.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Jelveh K., Mottaghitalab M., Mohammadi M. Effects of green tea phytosome on growth performance and intestinal integrity under coccidiosis infection challenge in broilers. Poult. Sci. 2023;102:02627. doi: 10.1016/j.psj.2023.102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kaya C.A., Tuncer S.D. The effects of an organic acids and etheric oils mixture on fatting performance, carcass quality and some blood parameters of broilers. J. Anim. Vet. Adv. 2009;8:94–98. [Google Scholar]
- Khorrami P., Gholami-Ahangaran M., Moghtadaei-Khorasgani E. The efficacy of pomegranate peel extract on Eimeria shedding and growth indices in experimental coccidiosis in broiler chickens. Vet. Med. Sci. 2022;8:635–641. doi: 10.1002/vms3.714. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee J.W., Kim D.H., Kim Y.B., Jeong S.B., Oh S.T., Cho S.Y., Lee K.W. Dietary encapsulated essential oils improve production performance of coccidiosis-vaccine-challenged broiler chickens. Animals. 2020;10:481. doi: 10.3390/ani10030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jang S.I., Li G.X., Bautista D.A., Phillips K., Ritter D., Lillehoj E.P., Siragusa G.R. Effects of coccidiosis control programs on antibody levels against selected pathogens and serum nitric oxide levels in broiler chickens. J. Appl. Poult. Res. 2011;20:143–152. [Google Scholar]
- Lee K., Everts W., Kappert H., Frehner H.J., Losa M.R., Beynen A.C. Effects of dietary essential oil component on growth performance, digestive enzymes and lipid metabolism in female broiler chicken. Br. Poult. Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Lin Y., Teng P.Y., Olukosi O.A. The effects of xylo-oligosaccharides on regulating growth performance, nutrient utilization, gene expression of tight junctions, nutrient transporters, and cecal short chain fatty acids profile in Eimeria-challenged broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar H.P., Blummel M., Becker K. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br. J. Nutr. 1995;73:897–913. doi: 10.1079/bjn19950095. [DOI] [PubMed] [Google Scholar]
- Mao X., Dou Y., Fan X., Yu B., He J., Zheng P., Yu J., Luo J., Luo Y., Yan H., Wang J. The effect of dietary Yucca schidigera extract supplementation on productive performance, egg quality and gut health in laying hens with Clostridium perfringens and coccidia challenge. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev. Vet. Med. 2015;120:195–202. doi: 10.1016/j.prevetmed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Muthamilselvan T., Kuo T.F., Wu Y.C., Yang W.C. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Alternat. Med. 2016;2016 doi: 10.1155/2016/2657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) The National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry: Ninth Revised Edition, 1994. [Google Scholar]
- Noruzi H., Aziz-Aliabadi F. Garlic (Allium sativum) and mushroom (Agaricus bisporus) powder: Investigation of performance, immune organs and humoural and cellular immune response in broilers. Vet. Med. Sci. 2024;10:31367. doi: 10.1002/vms3.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noruzi H., Hassanabadi A., Golian A., Aziz-Aliabadi F. Effects of dietary calcium and phosphorus restrictions on growth performance, intestinal morphology, nutrient retention, and tibia characteristics in broiler chickens. Br. Poult. Sci. 2023;64:231–241. doi: 10.1080/00071668.2022.2136510. [DOI] [PubMed] [Google Scholar]
- Ozcelik B., Aslan M., Orhan I., Karaoglu T. Antibacterial, antifungal, and antiviral activities of the lipophylic extracts of Pistacia vera. Microbiol. Res. 2005;160:159–164. doi: 10.1016/j.micres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Perez-Carbajal C., Caldwell D., Farnell M., Stringfellow K., Pohl S., Casco G., Pro-Martinez A., Ruiz-Feria C.A. Immune response of broiler chickens fed different levels of arginine and vitamin E to a coccidiosis vaccine and Eimeria challenge. Poult. Sci. 2010;89:1870–1877. doi: 10.3382/ps.2010-00753. [DOI] [PubMed] [Google Scholar]
- Pirali Kheirabadi K.H., Hassanpour H., Nourani H., Farahmand E., Bashi M.C., Hossainpour Jaghdani F. Increasing of serum nitric oxide metabolites in chicken infection Eimeria. Int. J. Vet. Res. 2011;5:99–103. [Google Scholar]
- Placer Z.A., Cushman L.L., Johnson B.C. Estimation of lipid peroxidation, malindialdehyde in biochemical system. Anal. Biochem. 1996;16:359–367. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Rafiee Z., Barzegar M., Sahari M.A., Maherani B. Nano liposomes containing pistachio green hull's phenolic compounds as natural bio-preservatives for mayonnaise. Eur. J. Lipid Sci. Technol. 2018;120 [Google Scholar]
- Rajaei A., Barzegar M., Mobarez A.M., Sahari M.A., Esfahani Z.H. Antioxidant, anti-microbial and anti-mutagenicity activities of pistachio (Pistachia vera) green hull extract. Food. Chem. Toxicol. 2010;48:107–112. doi: 10.1016/j.fct.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Rochell S.J., Parsons C.M., Dilger R.N. Effects of Eimeria acervulina infection severity on growth performance, apparent ileal amino acid digestibility, and plasma concentrations of amino acids, carotenoids, and alpha1-acid glycoprotein in broilers. Poult. Sci. 2016;95:1573–1581. doi: 10.3382/ps/pew035. [DOI] [PubMed] [Google Scholar]
- Sanchez-Hernandez C., Castaneda-Gomez del Campo J.A., Trejo-Castro L., Mendoza-Martínez G.D., Gloria-Trujillo A. Evaluation of a feed plant additive for coocidiosis control in broilers herbals for coccidiosis control. Braz. J. Poult. Sci. 2019;21:1–6. [Google Scholar]
- Seifzadeh N., Sahari M.A., Barzegar M., Gavlighi H.A., Calani L., Del Rio D., Galaverna G. Evaluation of polyphenolic compounds in membrane concentrated pistachio hull extract. Food Chem. 2019;277:398–406. doi: 10.1016/j.foodchem.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Shakerardekani A., Molaei M. Post-harvest pistachio waste: methods of its reduction and conversion. PHJ. 2020;3:40–51. [Google Scholar]
- Sharifian M., Hosseini-Vashan S.J., Nasri M.F., Perai A.H. Pomegranate peel extract for broiler chickens under heat stress: Its influence on growth performance, carcass traits, blood metabolites, immunity, jejunal morphology, and meat quality. Livest. Sci. 2019;227:22–28. [Google Scholar]
- Sharma U.N.S., Fernando D.D., Wijesundara K.K., Manawadu A., Pathirana I., Rajapakse R.P.V. Anticoccidial effects of Phyllanthus emblica (Indian gooseberry) extracts: potential for controlling avian coccidiosis. Vet. Parasitol. Reg. Stud. Rep. 2021;25 doi: 10.1016/j.vprsr.2021.100592. [DOI] [PubMed] [Google Scholar]
- Sila A., Bayar N., Ghazala I., Bougatef A., Ellouz-Ghorbel R., Ellouz-Chaabouni S. Water-soluble polysaccharides from agro-industrial by-products: functional and biological properties. Int. J. Biol. Macromol. 2014;69:236–243. doi: 10.1016/j.ijbiomac.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Statistical Analyses System (SAS) SAS Institute Inc; Cary (NC): 2012. SAS/STAT Software, Version 9.4. [Google Scholar]
- Su S., Miska K.B., Fetterer R.H., Jenkins M.C., Wong E.A. Expression of digestive enzymes and nutrient transporters in Eimeria-challenged broilers. Exp. Parasitol. 2015;150:13–21. doi: 10.1016/j.exppara.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Teng P.Y., Choi J., Tompkins Y., Lillehoj H., Kim W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021;52:81. doi: 10.1186/s13567-021-00949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari A.K., Maharana B. Control of poultry coccidiosis: changing trends. J. Parasit. Dis. 2011;35:10–17. doi: 10.1007/s12639-011-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwuoke M.U., Pewan S. Effect of methanol extract of Parkia biglobosa root bark on organ and carcass weight and histopathological changes in Eimeria tenella infected broiler chickens. Anim. Res. Int. 2020;17:3587–3595. [Google Scholar]
- Ultee A., Bennik M.H.J., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S.D., Cho S.H., Chung T.K., Kim I.H. Anti-coccidial effect of essential oil blends and vitamin D on broiler chickens vaccinated with purified mixture of coccidian oocyst from Eimeria tenella and Eimeria maxima. Poult. Sci. 2019;98:2919–2926. doi: 10.3382/ps/pez040. [DOI] [PubMed] [Google Scholar]
- Wang L., Piao X.L., Kim S.W., Piao X.S., Shen Y.B., Lee H.S. Effects of Forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult. Sci. 2008;87:1287–1294. doi: 10.3382/ps.2008-00023. [DOI] [PubMed] [Google Scholar]
- Wang M.L., Suo X., Gu J.H., Zhang W.W., Fang Q., Wang X. Influence of grape seed proanthocyanidin extract in broiler chickens: effect on chicken coccidiosis and antioxidant status. Poult. Sci. 2008;87:2273–2280. doi: 10.3382/ps.2008-00077. [DOI] [PubMed] [Google Scholar]
- Waterhouse A.L. John Wiley and Sons; New York: 2002. Current Protocols in Food Analytical Chemistry: Determination of Total Phenolics. [Google Scholar]
- Winterbourn C.C., Hawkins R.E., Brain M., Carrell R. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975;85:337–341. [PubMed] [Google Scholar]
- Xu P., Wang J., Chen P., Ding H., Wang X., Li S., Fan X., Zhou Z., Shi D., Li Z., Cao S. Effects of pomegranate (Punica granatum L.) peel on the growth performance and intestinal microbiota of broilers challenged with Escherichia coli. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Li X., Na C., Abbas A., Abbas R.Z., Zaman M.A. Anticoccidial effects of Camellia sinensis (green tea) extract and its effect on Blood and Serum chemistry of broiler chickens. Pak. Vet. J. 2020;40:77–80. [Google Scholar]
- Zhang Q., Chen X., Eicher S.D., Ajuwon K.M., Applegate T.J. Effect of threonine deficiency on intestinal integrity and immune response to feed withdrawal combined with coccidial vaccine challenge in broiler chicks. Br. J. Nutr. 2016;116:2030–2043. doi: 10.1017/S0007114516003238. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang X., Huang S., Huang Y., Shi H., Bai X. Effects of dietary essential oil supplementation on growth performance, carcass yield, meat quality, and intestinal tight junctions of broilers with or without Eimeria challenge. Poult. Sci. 2023;102:102874. doi: 10.1016/j.psj.2023.102874. [DOI] [PMC free article] [PubMed] [Google Scholar]