Abstract
We have established cell-free replication for the human papillomavirus type 18 (HPV-18) origin of replication (ori)-containing DNA by using purified HPV-18 E1 and E2 gene products expressed as fusion proteins in Escherichia coli. The transcription factor YY1 has been shown to regulate RNA transcription by binding to a sequence overlapping the putative E1 protein binding site in the HPV-18 ori. We show that exogenously added YY1 fusion protein inhibited HPV-18 ori replication. Cotransfection of YY1 expression vectors also inhibited transient replication in 293 cells. However, inhibition did not appear to be mediated by binding to its cognate site in the ori as YY1 also inhibited the replication of the HPV-11 ori, which does not have a known or suspected YY1 binding site. Moreover, inhibition was not alleviated by the inclusion of YY1 binding oligonucleotides in the replication reaction mixtures. Rather, we demonstrated a direct interaction between purified fusion E2 protein and fusion YY1 protein by the pull-down assay and a partial restoration of replication activity by an elevated E2 protein concentration. These results suggest that YY1 can inhibit HPV ori replication by interfering with E2 protein functions.
The small double-stranded DNA tumor viruses simian virus 40 (SV40), polyomavirus, and papillomaviruses are model systems for studying eukaryotic DNA replication because they rely heavily on host proteins. Many cellular replication proteins have been identified and cloned based on cell-free replication initiated by the SV40 large T antigen (reviewed in reference 53). Human papillomaviruses (HPVs) are widespread pathogens that infect various epithelia, causing warts (reviewed in reference 65). Infections by high-risk HPVs, including HPV type 18 (HPV-18), can develop into dysplasias and cancers in which the viral DNA often becomes integrated into the host chromosomes, an event proposed to play an important role in disease progression (reviewed in reference 10). In productively infected benign lesions, two distinct modes of DNA replication make this virus family attractive as a possible paradigm for regulated host DNA replication. In these lesions, HPV DNA is maintained as low-copy-number, extrachromosomal plasmids in the basal and parabasal cells of the squamous epithelium. Only in the differentiated upper strata does vegetative amplification take place. Viral transcription also increases dramatically with cellular differentiation. These two aspects of the infection process are undoubtedly interconnected but are not yet fully understood.
Because cloned genomic HPV DNA replicates poorly, if at all, in transfected cells, two strategies have been used to examine the requirements for viral origin (ori)-specific replication (reviewed in references 9 and 52). One involves transient replication of ori-containing plasmids in transfected cells in the presence of vectors expressing viral replication proteins. E1 and E2 proteins from the same viral type and certain mixed pairs from distinct types can support replication from homologous and heterologous papillomavirus ori’s with varied efficiencies (8, 12, 18, 55, 56, 59, 64). The second assay is cell-free replication, which has been established for HPV-11 and bovine papillomavirus type 1 (BPV-1), using cellular extracts supplemented with viral proteins purified from insect Sf9 cells infected with recombinant baculoviruses (23, 61). These studies show that both the viral proteins and their ori’s are highly conserved among HPVs and animal papillomaviruses. Efficient ori replication requires both E1 and E2 proteins, with few exceptions (3, 18).
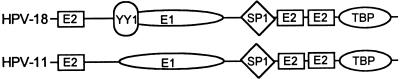
The functions of the papillomavirus replication proteins have been examined extensively. Briefly, the HPV-11 and the BPV-1 E1 protein binds to an imperfect palindrome (the E1 binding site [E1BS]), which is flanked by E2BSs (Fig. 1) (30, 47, 54, 60, 61). The E1 protein is an ATPase and helicase (5, 20, 31, 49, 62) and also binds to the 180-kDa catalytic subunit (4, 41) and p70 subunit (11) of the host DNA polymerase α. In cell-free replication, HPV-11 and BPV-1 E1 are required throughout initiation and elongation, as expected of a helicase at the replication fork (30). The E1 proteins also exhibit relatively high nonspecific DNA binding, which undoubtedly contributes to their ability to promote a low level of E2-independent as well as ori-independent replication when present at elevated concentrations in cell-free systems (23, 61, 62). The functions of the E2 proteins are severalfold. All E2 proteins are dimeric transcription factors and bind to a consensus sequence ACCN6GGT that exists in multiple copies in the upstream regulatory region (URR) of all papillomaviruses (9, 52). The binding of E1 protein to the E1BS is stabilized by interaction with the E2 proteins bound to nearby sites (17, 33, 36, 47, 48, 54). At low E1 concentrations in the cell-free system, the assembly of initiation complex on the ori requires both E1 and E2 proteins (30). These interactions provide specificity and increase the efficiency for ori replication (7, 23, 36, 46). However, the E2 protein appears to be dispensable for elongation (30).
FIG. 1.
Replication origins of HPV-18 and HPV-11. Indicated are the binding sites for viral proteins E1 and E2 and for cellular transcription factors, YY1, Sp1, and TBP. The YY1BS in HPV-18 ori overlaps the putative E1BS (1, 25).
As expected from the properties of the E1 and E2 proteins, the papillomavirus ori regions are highly conserved and consist of several copies of the E2BS and one proven or putative E1BS, overlapping the transcription enhancers and E6 promoter. One or more copies of the E2BS are critical to ori activity of genital HPVs, such as HPV-11 and HPV-18, whereas deletion or mutation of E1BS reduces but does not abolish the activity (7, 23, 31, 32, 43, 56). An efficient HPV-18 ori has been localized by transient-replication assays to a 210-bp fragment spanning nucleotides (nt) 7766 to 7857 and 1 to 119 (43, 55, 56). It contains three copies of the E2BS and one putative E1BS as inferred by analogy to the HPV-11 and BPV-1 E1 protein binding sequences (Fig. 1).
In the ori of SV40 or polyomavirus, the ori core binds the viral T antigen. In addition, transcription factor binding sites flanking the core are auxiliary elements that enhance ori activities. Enhancement of replication in vivo is attributed to the ability of bound transcription factors to prevent nucleosome formation around the ori (reviewed in reference 13). The BPV-1 E2 protein also functions in this capacity (29). In the short HPV-18 ori, there are several host transcription factor binding sites, including those for yin-yang 1 (YY1), which overlaps the EIBS, for Sp1, and for TATA-box binding protein (TBP) (Fig. 1). The Sp1 and TBP sites are positive transcriptional elements (see references 1, 14, 15, 42, and 57 and references therein), and mutations in these sites have a negative effect on transient replication (25, 45). YY1 protein interacts with diverse proteins, including TBP, transcription factor IIB, Sp1, c-Myc, and adenovirus E1A proteins (for a review, see reference 51). In some instances, YY1 and other transcription factors compete for binding to adjacent or overlapping sites. Thus, depending on the sequence context, YY1 either functions as a transcription repressor, an activator, or an initiator binding protein. It is of great interest to determine whether or how YY1 protein might regulate ori replication.
In this report, we describe our findings concerning the effect of YY1 in a cell-free replication system established with human 293 cell extracts supplemented with HPV-18 E1 and E2 proteins, each expressed as a fusion protein in Escherichia coli. In this system, efficient HPV-18 ori replication is dependent on both E1 and E2 proteins. We found that YY1 inhibited HPV-18 ori replication in cell-free and in transient replication. Unexpectedly, evidence suggests that inhibition is largely mediated via interference with the E2 protein functions and it does not appear to depend on binding to the ori. We show that YY1 protein interacts with E2 protein in vitro and inhibition can be relieved partially by elevated E2 protein concentrations or by an antibody to an intact YY1 protein.
MATERIALS AND METHODS
Plasmid construction.
The genomic HPV-18 clone was a gift of Harald zur Hausen. Prior to purifying E1 and E2 proteins from E. coli, we expressed both as native proteins from the eukaryotic vector pMTX, a derivative of pMT2 (22) with multiple-cloning sites (a gift from Jen-Sing Liu of our laboratory), and established their functionality by transient assays in human 293 cells as previously described (8). To generate pMTX-18E1, the TaqI-StuI fragment of HPV-18 (nt 838 to 3070) was blunt ended with the Klenow fragment of the E. coli DNA polymerase I and inserted into the SmaI site of pMTX. To generate pMTX-18E2, the NsiI fragment (nt 2707 to 3975) was cloned into the PstI site of pMTX. For expression in E. coli, the open reading frames (ORFs) were reconstituted from restriction fragments of cloned DNA and amino terminal fragments of the E1 and E2 ORFs generated by PCR amplification. The E1 ORF was fused in frame at the carboxyl terminus of the hexahistidine encoded by the vector pRSET (Invitrogen, Carlsbad, Calif.). A BamHI site (underlined) was introduced into the sense-strand primer 5′-TAAGGATCCATGGCTGATCCAG-3′, while the antisense-strand primer 5′-AATGATAGCCCATATGTGTC-3′ contains a native NdeI site (underlined). The BamHI-NdeI fragment of the PCR product (nt 905 to 1585) was ligated to the restriction fragment NdeI (in the E1 ORF)-EcoRI (in pMTX) (nt 1586 to 2885) and then cloned into the BamHI and EcoRI sites of pRSET to generate pRSET-H18E1. HPV-18 E2 was fused in frame at the carboxyl terminus of the glutathione S-transferase (GST) in pGEX2TM, a derivative of pGEX2T (Pharmacia, Uppsala, Sweden) with additional cloning sites (a gift from Jeffrey Kudlow) as follows. The sense-strand primer, 5′-AGAGGCATATGCAGACACCG-3′, contains an introduced NdeI site, while the antisense-strand primer, 5′-TTTGTGCAAGGCCTTGTAGG-3′, contains a native StuI site (underlined). The NdeI-StuI fragment of the PCR product (nt 2817 to 3070) spanning the amino terminus was ligated to the StuI-NdeI fragment (nt 3070 to 3920) and then inserted into the NdeI site of pRSET to yield pRSET-18E2. The NdeI fragment spanning the intact E2 ORF was then blunt ended and cloned into the SmaI site of pGEX2TM.
To prepare pBS-H18ori (nt 7766 to 7857 and 1 to 118), the BamHI fragment (nt 6929 to 118) of HPV-18 in pGEM-18URR (6) was cloned into pBluescript (Stratagene, La Jolla, Calif.). The fragment between the AccI site (in the vector) and the AvaI site (in HPV-18 URR) was then removed, and the vector was reclosed by blunt-end religation. The HPV-11 ori-containing plasmid pUC7874-99 has been described previously (23). The YY1 expression vector pCDNA-YY1 was constructed by insertion of an XbaI-BamHI fragment of the YY1 cDNA plasmid (44) into an XbaI-BamHI site of the expression vector pCDNA (Invitrogen). Additional YY1 expression vectors, pCMV-hYY1 and pCMV-hYY1DZnF, were kindly provided by K. Calame (44).
Antibodies and oligonucleotides.
Polyclonal rabbit anti-YY1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). C20 was raised against the C-terminal 20 residues, while H414 was raised against full-length YY1 protein. Oligonucleotides containing a strong YY1 binding site or a mutated YY1 binding site were also from Santa Cruz Biotechnology.
Transient replication assays.
Transient replication of HPV-18 ori plasmid (pBS-H18ori containing nt 7766 to 7857 and 1 to 118) in transfected 293 cells was conducted as described previously (8). Briefly, 5 million cells were transfected by electroporation (capacitance, 960 μF; potential difference, 170 V) in 250 μl of growth medium with 5 mM BES buffer (N,N-bis[2-hydroxyethyl]-2-aminoethane-sulfonic acid, pH 7.2) containing the ori plasmids, E1 and E2 expression vectors, and carrier salmon sperm DNA. The HPV proteins were expressed from pMTX-18E1 and pMTX-18E2. For some experiments YY1 expression vectors such as pCDNA-YY1, pCMV-hYY1, pCMV-hYY1DZnF, or the cloning vector pCDNA were also included.
Expression and purification of proteins from E. coli.
To express the HPV-18 E1 protein, E. coli BL21(DE3) was transformed by pRSET-H18E1. The induction protocol and purification through a nickel-nitrilotriacetic acid column (Qiagen, Santa Clarita, Calif.) and a heparin column (Bio-Rad, Hercules, Calif.) will be described elsewhere (27). The fraction containing the E1 protein was identified by Western blotting with antibody against the hexahistidine-containing epitope. The E1 protein was dialyzed against buffer D (20 mM HEPES-KOH [pH 7.5], 1 mM dithiothreitol, 10% glycerol) and stored in aliquots at −80°C. To express HPV-18 E2 protein, DH5α cells freshly transformed by the pGEX2TM-18E2 plasmid were induced at mid-log phase by 0.5 mM of isopropylthio-β-galactoside (IPTG) for 20 h at 18°C. The bacteria were resuspended in buffer A (25 mM Tris [pH 7.5], 2 mM EDTA, 2 mM dithiothreitol), 10% glycerol, and 0.5 mM phenylmethylsulfonyl fluoride with 0.5 M NaCl and lysozyme (1 mg/ml). The lysates were sonicated and cleared of insoluble materials successively by centrifugation and ultrafiltration through a 0.45-μm-pore-size polysulfone filter. The filtrate was loaded onto a 0.5-ml GST column (Pharmacia) and washed with buffer A containing 0.8 M NaCl. The GST-E2 fusion protein was eluted with 0.5 ml of buffer A containing 0.3 M NaCl and 20 mM glutathione. The eluent was dialyzed against buffer D and stored in aliquots at −80°C.
The expression vectors for His-YY1 and GST-YY1 were gifts of Edward Seto and Yang Shi (26, 50). They were expressed in E. coli RR1 and DH5α, respectively, as soluble proteins by induction at 18°C with 0.5 mM IPTG. Both proteins were purified by chelation on a nickel-nitrilotriacetic acid column, as the YY1 protein naturally contains a polyhistidine domain. The fusion proteins were dialyzed and stored as described above. For the heat stability test, an aliquot of His-YY1 protein solution was overlaid with mineral oil and heated to 100°C for 10 min. The solution was then quickly cooled on ice. A polyhistidine-tagged chloramphenicol acetyltransferase (CAT) (pTrcHisCAT; Invitrogen) was purified similarly.
The purity and identity of the proteins were determined by Coomassie blue staining following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by Western blotting with antibodies directed against fusion tags of E1 or E2 proteins or with the anti-YY1 antibody (H414). The two YY1 proteins and the His-CAT protein were each more than 95% pure. The concentrations of proteins were determined by Bradford assay (Bio-Rad) and Coomassie blue staining following SDS-PAGE using known concentrations of bovine serum albumin as standards.
Cell-free DNA replication assays.
The cultures of human 293 cells in monolayer or in suspension, the preparation of whole-cell extracts, and the implementation of cell-free replication assays were as described previously (23). Briefly, the reaction mixture of 25 μl containing 40 ng of freshly prepared form I DNA template, 100 μg of cell extracts, viral proteins as specified for each experiment, an ATP-regenerating system, and eight unlabeled nucleotide substrates in reaction buffer was preincubated for 1 h at 37°C. [α-32P]dCTP (2.5 μCi; 3,000 Ci/mmol) was then added, and incubation continued for another hour at 37°C. As described previously (23), little or no replicative synthesis occurred in the first hour of incubation. Delayed addition of the labeled substrate reduced background repair synthesis which was independent of viral proteins and ori and approached a plateau during the 1-h preincubation (23; data not shown). In some assays, additional components were added prior to preincubation as described for each experiment. Reactions were terminated, and the products were analyzed by electrophoresis through 0.8% agarose gels as described previously (23). Autoradiograms were exposed for 3 to 4 h with an intensifying screen at −80°C. The relative intensities of replication products (the sum of signals from replication intermediates plus form I and form II DNA) were quantified by PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) after subtracting background obtained in the absence of E1 or E2 proteins, in the presence of the pBS cloning vector, or as described in the figure legends.
GST pull-down assays.
GST pull-down assays were performed as described previously (21, 24, 58). Aliquots (30 μg) of His-YY1 proteins were mixed with a constant amount of Sepharose-GST preloaded with increasing amounts of GST-H18 E2 protein or control Sepharose-GST. After extensive washing with a solution containing 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, 0.1 M NaCl, 0.5% Nonidet P-40, and 0.5% nonfat dry milk, the bound protein was recovered by centrifugation and solubilized by boiling in loading buffer. Twenty percent of the solubilized protein was separated by SDS–10% acrylamide gel electrophoresis and then detected after Western blotting by using an ECL kit (Amersham, Arlington Heights, Ill.) with anti-YY1 antibody (C20).
RESULTS
Bacterially expressed HPV-18 E1 and E2 fusion proteins support HPV-18 ori replication.
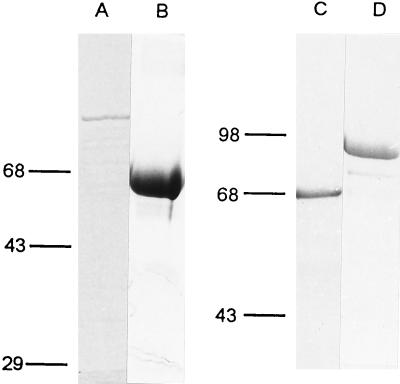
The HPV-18 E1 and E2 genes were individually cloned into a derivative of the mammalian expression vector described in Materials and Methods. After confirming their ability to support transient replication of an HPV-18 ori plasmid (pBS-H18ori containing nt 7766 to 7857 and 1 to 118) in transfected 293 cells, we expressed and purified E1 and E2 proteins from E. coli. E1 was generated as a fusion protein with a short polypeptide containing six histidine residues at the amino terminus. E2 protein was translated as a fusion protein with GST at the amino terminus. On the basis of Coomassie blue staining, the purity of E1 protein was over 70% (Fig. 2A) and that of E2 was over 90% (Fig. 2B).
FIG. 2.
SDS-PAGE profiles of proteins expressed in and purified from E. coli. Lanes: A, purified His-H18E1; B, GST-H18E2; C, His-YY1; and D, GST-YY1. Proteins were purified as described in Materials and Methods, electrophoresed in SDS-polyacrylamide gels, and stained with Coomassie brilliant blue. Western blots using antibodies against the fusion tag (E1 and E2) or YY1 confirmed the identities (data not shown).
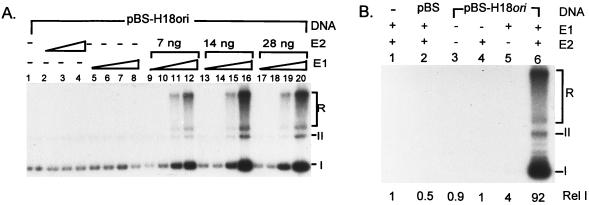
After preliminary tests that demonstrated the abilities of the E1 and E2 proteins to support cell-free replication of pBS-H18ori in the presence of 293 cell extracts, we optimized this system by reiterated titration of E1 and E2 proteins needed to promote ori-specific replication in the presence of 40 ng of pBS-H18ori and 100 μg of cell extracts. Representative titration experiments are shown in Fig. 3A. The optimal conditions included 180 ng of His-H18E1 protein and 14 ng of GST-H18E2 protein (Fig. 3A, lane 16). Under these conditions, replication is ori dependent and produced fast-migrating form I and form II and slow-migrating replication intermediates as previously described (23, 61). In the absence of E1 or E2 or in the presence of the vector pBS plasmid only repair synthesis was detected, which generated only labeled form I DNA in the absence of any slow-migrating replication intermediates (23). This background signal was less than 4.5% of that generated by pBS-H18ori, as quantified by a PhosphorImager (Fig. 3B).
FIG. 3.
HPV-18 ori replication in the presence of E1 and E2 fusion proteins. In this and subsequent figures, cell-free replication was conducted with 100 μg of 293 cell extracts and 0 or 40 ng of template DNA (pBS-H18ori or pBS). (A) Effects of E1 and E2 protein concentrations on HPV-18 ori replication. Cell-free replication reaction mixtures containing 40 ng of pBSH18ori (all lanes) were conducted in the absence of His-H18E1 and GST-H18E2 (lane 1), in the presence of GST-H18E2 alone (lanes 2 to 4), in the presence of His-H18E1 alone (lanes 5 to 8), or in the presence of both His-H18E1 and GST-H18E2 (lanes 9 to 20). The amount of E1 fusion protein in the reaction mixtures was 22.5 ng (lanes 5, 9, 13, and 17), 45 ng (lanes 6, 10, 14, and 18), 90 ng (lanes 7, 11, 15, and 19), or 180 ng (lanes 8, 12, 16, and 20). The amount of GST-H18E2 protein was 7 ng (lanes 2 and 9 to 12), 14 ng (lanes 3 and 13 to 16), or 28 ng (lanes 4 and 17 to 20). (B) Specificity of cell-free HPV-18 ori replication under optimal conditions as defined for panel A. Replication was conducted in the presence (+) or absence (−) of 180 ng of His-H18E1 and 14 ng of GST-H18E2 as indicated (lanes 2 to 6). form I (I), form II (II), and replication intermediates (R) are marked here and in Fig. 4 through 7. Incorporation in the absence of E1, E2, or both was due to repair synthesis, as evidenced by the lack of slow-migrating replication intermediates. The relative [α-32P]dCTP incorporation of each reaction was quantified by using a PhosphorImager. The relative intensity (Rel I) was calculated by comparing isotope incorporation to that in lane 1 (with no exogenous DNA template).
YY1 fusion proteins inhibit HPV-18 E2-dependent cell-free ori replication.
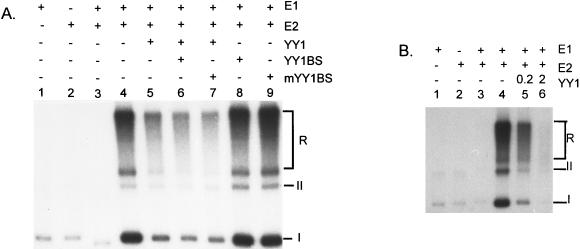
To examine whether YY1 might regulate HPV-18 ori replication, we purified both His-YY1 and GST-YY1 to near homogeneity (Fig. 2C and D). The purified His-YY1 protein was able to form specific and stable complexes with the ori fragment but not with a neighboring fragment in an electrophoretic mobility shift assay (27). When added to the cell-free replication reactions, both YY1 fusion proteins independently repressed the HPV-18 ori replication in a dose-dependent manner (Fig. 4A and data not shown). At 0.2 and 0.4 μg of His-YY1, ori replication was reduced to 29 and 21% of the control (compare lanes 1, 4, and 5). To eliminate the possibility that repression was due to contaminating proteins in the YY1 protein preparations, we performed the following control experiments. Repression was not observed after the addition of the same amounts of His-tagged CAT, which was purified similarly (lanes 2 and 3). The addition of anti-YY1 antibody partially restored ori activity (lane 7), whereas addition of anti-YY1 antibody to a reaction containing no added YY1 protein had a small positive effect on replication (lane 6). Since YY1 protein is heat resistant (63), we boiled His-YY1 protein for 10 min prior to addition to the replication mixture. It retained the repression activity (see Fig. 6B; compare lanes 2 to 6). These results and additional experiments described below strongly argue for a YY1-specific repression and against a nonspecific repression by contaminants.
FIG. 4.
Specificity of repression of HPV-18 ori replication by exogenous YY1 protein. (A) Lane 1 contains a standard reaction mixture; the rest of the lanes contain the standard reaction mixture with the following additions: His-CAT protein (lanes 2 and 3), His-YY1 protein (lanes 4 and 5), anti-YY1 antibody (H414) raised against the intact YY1 protein (lane 6), and both YY1 and the H414 anti-YY1 antibody (lane 7). (B) Replication of pBSH18ori in 293 cell extracts supplemented with His-H18E1 alone (lane 1), GST-H18E2 alone (lane 2), or with both E1 and E2 proteins (lanes 4 to 6). To lane 5 was added exogenous his-YY1. Lane 6 contains both His-YY1 and antibody (C20) raised against the DNA-binding domain of the YY1 protein. Lane 3 contains the product of a replication reaction conducted with pBS cloning vector in 293 cells supplemented with both E1 and E2 proteins. The relative intensity (Rel I) values were calculated as percentages by comparing isotope incorporation to that in lane 1 (A) or lane 4 (B) as measured by a PhosphorImager. For both panels, the amounts (in μg) of protein or antibody added were as indicated.
FIG. 6.
Mechanism of YY1 repression of HPV ori replication. (A) Interaction of YY1 protein with GST-H18E2 protein. A Western blot following SDS–10% PAGE was used to reveal His-YY1 retained on glutathione-beads loaded with increasing amounts of GST-H18E2 fusion protein but not on beads loaded with GST alone. Purified His-YY1 (3 μg, one half of input amount) was loaded in lane 4. A similar result was obtained when the binding assay was performed in the presence of ethidium bromide (30 μg/ml), which eliminates potential nonspecific binding caused by interactions with DNA (24). (B) Partial restoration of HPV-18 ori replication in the presence of exogenous YY1 by elevated E2 protein. Replication reactions of pBS-H18ori was conducted in the absence of the viral replication proteins (lane 1) or in the presence of 180 ng of His-H18E1 plus 14 ng of GST-H18E2 (lanes 2 to 6) or plus 140 ng of E2 (lanes 7 and 8). His-YY1 (lanes 3 and 4) or His-YY1, which was boiled for 10 min under mineral oil (lanes 5 and 6), was added as indicated (in micrograms). The relative intensity (Rel I) values were calculated in percentages by comparing isotope incorporation to that in lane 2, after subtracting the background incorporation in lane 1, as measured by a PhosphorImager.
YY1 inhibition of HPV ori replication is independent of a YY1 binding site in ori.
Because the YY1 site extensively overlaps the putative E1 binding site, it would be difficult to mutate the YY1 site without affecting E1 binding to ori and, hence, ori replication (25). To examine whether YY1 inhibition is mediated by binding to the cognate site, we used two alternative approaches. First, a synthetic double-stranded oligonucleotide containing a known strong YY1 binding site (YY1BS), GCCATCTTG, from the promoter in mouse ribosomal protein rPL30 and rPL32 genes (19) or a mutated oligonucleotide, mYY1BS, ATTATCTTG, which no longer bound YY1 was added to the cell-free replication reaction in the presence or in the absence of exogenous YY1 protein (Fig. 5A). The addition of mYY1BS had no effect on replication (compare lanes 4 and 9) or on repression (compare lanes 5 and 7), but to our surprise the YY1BS oligonucleotides did not affect replication (lane 8) or repression (lane 6), contrary to what one might expect if YY1 had repressed replication via binding to ori. The second approach is to test the effect of YY1 on HPV-11 ori replication by using the same HPV-18 E1 and E2 proteins. HPV-11 ori (Fig. 1) does not contain a known or suspected YY1 site, based on the consensus sequence of GACATNTT and VDCCATNWY (63). Replication repression was similarly observed (Fig. 5B). These results suggest that YY1 inhibition was mediated either through a cellular or a viral protein and that binding to ori did not significantly contribute to this repression.
FIG. 5.
YY1 inhibition of HPV ori replication is largely independent of binding to the ori. (A) Lack of discernible effects by the inclusion of YY1BS oligonucleotides in HPV-18 ori replication reaction mixtures containing exogenous YY1 protein. All the reactions were conducted under the optimal conditions described in the legend to Fig. 3B to replicate 40 ng of HPV-18 ori plasmid, which contains a YY1BS overlapping the E1BS, or HPV-11 ori plasmid, which does not have a known or suspected YY1BS (shown in panel B), except control reactions with a backbone plasmid (both panels, lanes 3). A double-stranded oligonucleotide containing YY1BS or mYY1BS was included in the cell-free replication reaction mixtures in the presence (+) or absence (−) of exogenous YY1. Proteins (180 ng of E1, 14 ng of E2, and 0.2 μg of YY1) or oligonucleotides (20 ng) were included as indicated. (B) All reactions were conducted under the optimal conditions described in the legend to Fig. 3B. The amounts of His-tagged YY1 added to the reactions were 0.2 μg (lane 5) and 2 μg (lane 6).
Partial relief of YY1 repression of HPV-18 ori replication by an elevated level of HPV-18 E2 protein.
Because YY1 interacts with a number of transcription factors, we examined the possibility that it also interacts with the E2 protein and that this interaction is part of the basis for repression. We conducted a pull-down assay using purified GST-H18E2 bound to GST-Sepharose. GST-H18E2 interacted with His-YY1 in the presence or in the absence of ethidium bromide (30 μg/ml) (24), as demonstrated by a Western blot of bound protein (Fig. 6A, lanes 2 and 3). Under the same condition, GST itself did not bind His-YY1 protein (lane 1). These results suggest that replication repression should be at least partially relieved by increasing amounts of E2 protein. We tested this prediction (Fig. 6B). In the absence of YY1, the presence of 140 ng (a 10-fold excess) of E2 had little effect on ori replication relative to that in the standard reaction mixture, which contained 14 ng of E2 protein (compare lanes 2 and 7). In comparison, addition of 140 ng of E2 protein to reaction mixtures containing 0.2 μg of His-YY1 protein restored activity from 25 to 70% (compare lanes 3 and 8). These results are consistent with an interpretation that YY1 repression is at least partly caused by interference with the HPV-18 E2 protein function.
YY1 expression vectors inhibit transient replication of HPV-18 ori.
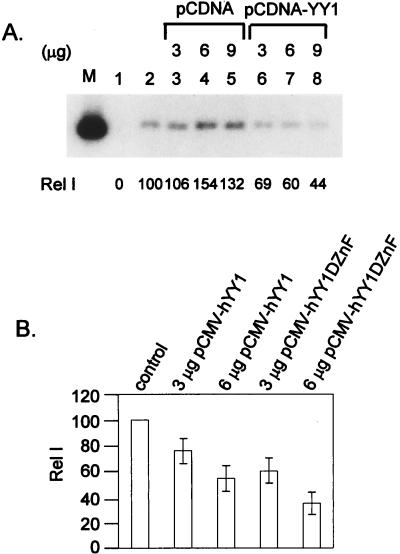
We note that the addition of anti-YY1 antibody to a reaction mixture containing no added YY1 protein slightly, but consistently, stimulated cell-free replication (Fig. 4A; compare lanes 6 and 1). This observation might be an indication that the endogenous YY1 has an inhibitory effect. To substantiate this hypothesis, we performed transient-replication assays in 293 cells after cotransfection of an expression vector of YY1. The results are reproducible and are presented in Fig. 7. Upon cotransfection of the empty cloning vector pCDNA, there was a slight stimulation of ori replication, whereas when pCDNA-YY1 was cotransfected, there was a dose-dependent repression (Fig. 7A). Furthermore, repression persisted when the YY1 lacking the DNA binding domain was cotransfected (Fig. 7B). Thus, in vivo, high levels of YY1 can also inhibit replication and the ability of YY1 to bind to ori is not essential. Consistent with the latter result and interpretation, we also observed that only the polyclonal antibody raised against the intact YY1, but not another polyclonal antibody raised against the DNA binding domain (Fig. 4B), can partially restore the replication activity.
FIG. 7.
Cotransfection of YY1 expression vectors represses HPV-18 ori replication in transient replication. (A) Repression of HPV-18 ori replication by YY1 in transfected 293 cells. Increasing amounts of pCDNA (lanes 3 to 5) or pCDNA-hYY1 (lanes 6 to 8) were cotransfected into 293 cells with expression vectors of viral E1 and E2 proteins (pMTX-H18E1 and pMTXH18E2), and the origin-containing vector (pBS-H18ori). Replicated DNA was revealed by Southern blotting after digestion with DpnI and BamHI as described in Materials and Methods. Cotransfection with pBS without viral ori (lane 1) was used as a negative control for transient replication assays. Lane M, 200 pg of linearized pBS-H18ori. (B) Repression of HPV-18 ori replication by YY1 mutated in the DNA-binding domain. An expression vector of the full-length YY1 protein (pCMV-hYY1) or of a truncated YY1 lacking the zinc finger DNA-binding domain (pCMV-hYY1DZnF) was cotransfected into 293 cells. The intensity of replication in each transfection containing YY1 expression vectors was compared to that of a control transfection. Data were collected from three independent experiments. Open bars indicate averages, and error bars indicate standard deviations.
DISCUSSION
Replication-competent epitope-tagged BPV-1 and HPV-11 E1 proteins have been expressed previously in insect Sf9 cells from a recombinant baculovirus system (23, 61). In addition, after enzymatic cleavage to remove the GST amino-terminal tag, bacterially expressed BPV-1 E1 protein supported ori replication in the presence of native E2 protein purified from Sf9 cells or from E. coli (35, 37). This report demonstrates that bacterially expressed HPV E1 and E2 fusion proteins function in cell-free replication assays without first removing the fusion protein moiety (Fig. 3). The replication properties of the bacterially expressed HPV-18 fusion proteins are similar to those of HPV-11 and BPV-1 expressed in Sf9 cells. Efficient ori replication depends on both E1 and E2 proteins.
YY1 protein binds to many transcription factors and displays diverse functions in regulating transcription (51). Transcription factors that function as auxiliary replication factors in vivo normally do not affect ori replication in cell-free systems due to a paucity of histones. In contrast, the addition of YY1 significantly inhibited HPV-18 ori replication, a novel observation in the control of ori replication (Fig. 4). Repression is specific, as it was heat resistant (Fig. 6), a property of YY1, and was partially relieved by a polyclonal antibody against the intact YY1 protein (Fig. 4A). Unexpectedly, despite the overlapping nature of the YY1BS and putative E1BS, our data strongly suggest that binding to the YY1 site is not the primary cause for the repression based on several pieces of evidence. (i) First, the inclusion of an oligonucleotide containing a known strong YY1BS or mutated oligonucleotides did not restore ori replication in the presence of exogenous YY1 protein (Fig. 5A). (ii) YY1 also inhibited HPV-11 ori replication which is not known to have a YY1BS (Fig. 5B). (iii) The addition of an antibody raised against the DNA-binding domain of YY1 failed to restore activity (Fig. 4B). Rather, our data showed that interaction with E2 protein was at least largely responsible for the ori repression. We demonstrated that E2 interacted with YY1 in a pull-down assay and repression was partially alleviated by E2 protein at 10-fold excess concentrations (Fig. 6). Since E2 plays important roles during the assembly of the initiation complex (30) by interacting with both the E1 protein and host proteins, such as RP-A (28), we suggest that the E2-YY1 interaction might adversely affect these E2 protein functions. That YY1 is indeed capable of inhibiting HPV ori replication in vivo by mechanisms independent of binding to the ori is substantiated by transient replication assays in cells transfected with an expression vector of either the intact YY1 or a YY1 mutant with the DNA binding zinc finger domain truncated (Fig. 7).
YY1 is thought to be a ubiquitous protein (16, 19, 40, 50). Although the distribution of YY1 in differentiating epithelium has not been determined, the existence of multiple YY1BSs in HPV-8, HPV-16, and HPV-18 (34, 38, 39) regulatory regions suggest that this factor may play some role in modulating viral activities. Indeed, in epithelial cell lines, YY1 negatively regulates the URR promoter of HPV-16. It has been postulated that deletion or other mutations of YY1 sites in the HPV-16 URR observed in several cases of carcinomas might have contributed to the enhanced expression of viral oncogenes, leading to the development of the carcinomas. In HPV-18, YY1 switched from being a transcription repressor to an activator when the transcription factor CREB was bound to a switch site (1, 2). E2 protein also regulates the viral enhancer-promoter, either positively or negatively in epithelial cells and cell lines, depending on the amounts of E2 protein and interactions with host factors bound to the URR (see references 14, 15, and 57 and references therein). The E2-YY1 interaction and YY1 repression of the HPV-18 ori described in this report add another level of intricate cross-talk between the viral and the host proteins in the regulation of viral transcription and replication during epithelial cell growth and differentiation.
In conclusion, we describe the establishment of a cell-free replication system for the HPV-18 ori by using HPV-18 E1 and E2 fusion proteins purified from E. coli and report the novel E2-mediated repression of HPV-18 ori replication by YY1. The functionality of bacterially expressed fusion viral proteins should facilitate future studies of the mechanisms and regulation of papillomavirus DNA replication and should also be useful for drug discovery assays.
ACKNOWLEDGMENTS
This work is supported by USPHS grant CA36200. We thank Jen-Sing Liu and Shu-Ru Kuo for many helpful discussions, Harald zur Hausen for HPV-18 DNA, Edward Seto and Yang Shi for the YY1 expression vectors, Kathryn Calame for pCMV-hYY1 and pCMV-hYY1DZnF, and Jeffrey Kudlow for vector pGEX2TM.
REFERENCES
- 1.Bauknecht T, Jundt F, Herr I, Oehler T, Delius H, Shi Y, Angel P, zur Hausen H. A switch region determines the cell type-specific positive or negative action of YY1 on the activity of the human papillomavirus type 18 promoter. J Virol. 1995;69:1–12. doi: 10.1128/jvi.69.1.1-12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauknecht T, See R H, Shi Y. A novel C/EBP β-YY1 complex controls the cell-type-specific activity of the human papillomavirus type 18 upstream regulatory region. J Virol. 1996;70:7695–7705. doi: 10.1128/jvi.70.11.7695-7705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonne-Andrea C, Santucci S, Clertant P. Bovine papillomavirus E1 protein can, by itself, efficiently drive multiple rounds of DNA synthesis in vitro. J Virol. 1995;69:3201–3205. doi: 10.1128/jvi.69.5.3201-3205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonne-Andrea C, Santucci S, Clertant P, Tillier F. Bovine papillomavirus E1 protein binds specifically DNA polymerase α but not replication protein A. J Virol. 1995;69:2341–2350. doi: 10.1128/jvi.69.4.2341-2350.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bream G L, Ohmstede C A, Phelps W C. Characterization of human papillomavirus type 11 E1 and E2 proteins expressed in insect cells. J Virol. 1993;67:2655–2663. doi: 10.1128/jvi.67.5.2655-2663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S, Schmidt-Grimminger D-C, Murant T, Broker T R, Chow L T. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9:2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- 7.Chiang C-M, Dong G, Broker T R, Chow L T. Control of human papillomavirus type 11 origin of replication by the E2 family of transcription regulatory proteins. J Virol. 1992;66:5224–5231. doi: 10.1128/jvi.66.9.5224-5231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang C M, Ustav M, Stenlund A, Ho T F, Broker T R, Chow L T. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc Natl Acad Sci USA. 1992;89:5799–5803. doi: 10.1073/pnas.89.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow L T, Broker T R. Papillomavirus DNA replication. Intervirology. 1994;37:150–158. doi: 10.1159/000150373. [DOI] [PubMed] [Google Scholar]
- 10.Chow L T, Broker T R. Small DNA tumor viruses. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 267–302. [Google Scholar]
- 11.Conger, K. L., J.-S. Liu, S.-R. Kuo, L. T. Chow, and T. S.-F. Wang. Submitted for publication.
- 12.Del Vecchio A M, Romanczuk H, Howley P M, Baker C C. Transient replication of human papillomavirus DNAs. J Virol. 1992;66:5949–5958. doi: 10.1128/jvi.66.10.5949-5958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DePamphilis M L. Eukaryotic DNA replication: anatomy of an origin. Annu Rev Biochem. 1993;62:29–63. doi: 10.1146/annurev.bi.62.070193.000333. [DOI] [PubMed] [Google Scholar]
- 14.Dong G, Broker T R, Chow L T. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J Virol. 1994;68:1115–1127. doi: 10.1128/jvi.68.2.1115-1127.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dostatni N, Lambert P F, Sousa R, Ham J, Howley P M, Yaniv M. The functional BPV-1 E2 trans-activating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 1991;5:1657–1671. doi: 10.1101/gad.5.9.1657. [DOI] [PubMed] [Google Scholar]
- 16.Flanagan J R, Becker K G, Ennist D L, Gleason S L, Driggers P H, Levi B Z, Appella E, Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992;12:38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frattini M G, Laimins L A. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. Proc Natl Acad Sci USA. 1994;91:12398–12402. doi: 10.1073/pnas.91.26.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopalakrishnan V, Khan S A. E1 protein of human papillomavirus type 1a is sufficient for initiation of viral DNA replication. Proc Natl Acad Sci USA. 1994;91:9597–9601. doi: 10.1073/pnas.91.20.9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hariharan N, Kelley D E, Perry R P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci USA. 1991;88:9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins O, Earnshaw D, Sarginson G, Del Vecchio A M, Tsai J, Kallender H, Amegadzie B, Browne M. Characterization of the helicase and ATPase activity of human papillomavirus type 6b E1 protein. J Gen Virol. 1996;77:1805–1809. doi: 10.1099/0022-1317-77-8-1805. [DOI] [PubMed] [Google Scholar]
- 21.Kaelin W J, Pallas D C, DeCaprio J A, Kaye F J, Livingston D M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman R J, Davies M V, Pathak V K, Hershey J W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989;9:946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo S-R, Liu J-S, Broker T R, Chow L T. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J Biol Chem. 1994;269:24058–24065. [PubMed] [Google Scholar]
- 24.Lai J S, Herr W. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci USA. 1992;89:6958–6962. doi: 10.1073/pnas.89.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee D, Kim H, Lee Y, Choe J. Identification of sequence requirement for the origin of DNA replication in human papillomavirus type 18. Virus Res. 1997;52:97–108. doi: 10.1016/s0168-1702(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee J S, Galvin K M, Shi Y. Evidence for physical interaction between the zinc-finger transcription factors YY1 and Sp1. Proc Natl Acad Sci USA. 1993;90:6145–6149. doi: 10.1073/pnas.90.13.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, K.-Y., T. R. Broker, and L. T. Chow. Unpublished results.
- 28.Li R, Botchan M R. The acidic transcription activation domains of VP16 and p53 bind the cellular replication protein A and stimulate in vitro BPV-1 DNA replication. Cell. 1993;73:1207–1221. doi: 10.1016/0092-8674(93)90649-b. [DOI] [PubMed] [Google Scholar]
- 29.Li R, Botchan M R. Acidic transcription factors alleviate nucleosome-mediated repression of DNA replication of bovine papillomavirus type 1. Proc Natl Acad Sci USA. 1994;91:7051–7055. doi: 10.1073/pnas.91.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J-S, Kuo S-R, Broker T R, Chow L T. The functions of human papillomavirus type 11 E1, E2, and E2C proteins in cell-free DNA replication. J Biol Chem. 1995;270:27283–27291. doi: 10.1074/jbc.270.45.27283. [DOI] [PubMed] [Google Scholar]
- 31.Liu, J.-S., S.-R. Kuo, T. R. Broker, and L. T. Chow. Unpublished results.
- 32.Lu Z-J, Sun Y-N, Rose R C, Bonnez W, McCance D J. Two E2 binding sites (E2BS) alone or one E2BS plus an A/T-rich region are minimal requirements for the replication of the human papillomavirus type 11 origin. J Virol. 1993;67:7131–7139. doi: 10.1128/jvi.67.12.7131-7139.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lusky M, Hurwitz J, Seo Y S. The bovine papillomavirus E2 protein modulates the assembly of but is not stably maintained in a replication-competent multimeric E1-replication origin complex. Proc Natl Acad Sci USA. 1994;91:8895–8899. doi: 10.1073/pnas.91.19.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May M, Dong X P, Beyer-Finkler E, Stubenrauch F, Fuchs P G, Pfister H. The E6/E7 promoter of extrachromosomal HPV16 DNA in cervical cancers escapes from cellular repression by mutation of target sequences for YY1. EMBO J. 1994;13:1460–1466. doi: 10.1002/j.1460-2075.1994.tb06400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melendy T, Sedman J, Stenlund A. Cellular factors required for papillomavirus DNA replication. J Virol. 1995;69:7857–7867. doi: 10.1128/jvi.69.12.7857-7867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohr I J, Clark R, Sun S, Androphy E J, MacPherson P, Botchan M R. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- 37.Müller F, Seo Y S, Hurwitz J. Replication of bovine papillomavirus type 1 origin-containing DNA in crude extracts and with purified proteins. J Biol Chem. 1994;269:17086–17094. [PubMed] [Google Scholar]
- 38.O’Connor M J, Tan S H, Tan C H, Bernard H-U. YY1 represses human papillomavirus type 16 transcription by quenching AP-1 activity. J Virol. 1996;70:6529–6539. doi: 10.1128/jvi.70.10.6529-6539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pajunk H S, May C, Pfister H, Fuchs P G. Regulatory interactions of transcription factor YY1 with control sequences of the E6 promoter of human papillomavirus type 8. J Gen Virol. 1997;78:3287–3295. doi: 10.1099/0022-1317-78-12-3287. [DOI] [PubMed] [Google Scholar]
- 40.Park K, Atchison M L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3′ enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci USA. 1991;88:9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park P, Copeland W, Yang L, Wang T, Botchan M R, Mohr I J. The cellular DNA polymerase alpha-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc Natl Acad Sci USA. 1994;91:8700–8704. doi: 10.1073/pnas.91.18.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker J N, Zhao W, Askins K J, Broker T R, Chow L T. Mutational analyses of differentiation-dependent human papillomavirus type 18 enhancer elements in epithelial raft cultures of neonatal foreskin keratinocytes. Cell Growth Differ. 1997;8:751–762. [PubMed] [Google Scholar]
- 43.Remm M, Brain R, Jenkins J R. The E2 binding sites determine the efficiency of replication for the origin of human papillomavirus type 18. Nucleic Acids Res. 1992;20:6015–6021. doi: 10.1093/nar/20.22.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riggs K J, Saleque S, Wong K-K, Merrell K T, Lee J-S, Shi Y, Calame K. Yin-yang 1 activates the c-myc promoter. Mol Cell Biol. 1993;13:7487–7495. doi: 10.1128/mcb.13.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell J, Botchan M. cis-Acting components of human papillomavirus (HPV) DNA replication: linker substitution analysis of the HPV type 11 origin. J Virol. 1995;69:651–660. doi: 10.1128/jvi.69.2.651-660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedman J, Stenlund A. Co-operative interaction between the initiator E1 and the transcriptional activator E2 is required for replicator specific DNA replication of bovine papillomavirus in vivo and in vitro. EMBO J. 1995;14:6218–6228. doi: 10.1002/j.1460-2075.1995.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sedman T, Sedman J, Stenlund A. Binding of the E1 and E2 proteins to the origin of replication of bovine papillomavirus. J Virol. 1997;71:2887–2896. doi: 10.1128/jvi.71.4.2887-2896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo Y S, Müller F, Lusky M, Gibbs E, Kim H Y, Phillips B, Hurwitz J. Bovine papilloma virus (BPV)-encoded E2 protein enhances binding of E1 protein to the BPV replication origin. Proc Natl Acad Sci USA. 1993;90:2865–2869. doi: 10.1073/pnas.90.7.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo Y S, Müller F, Lusky M, Hurwitz J. Bovine papilloma virus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc Natl Acad Sci USA. 1993;90:702–706. doi: 10.1073/pnas.90.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y, Seto E, Chang L S, Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991;67:377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- 51.Shrivastava A, Calame K. An analysis of genes regulated by the multi-functional transcriptional regulator Yin Yang-1. Nucleic Acids Res. 1994;22:5151–5155. doi: 10.1093/nar/22.24.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenlund A. Papillomavirus DNA replication. Cold Spring Harbor Monogr Ser. 1996;31:679–697. [Google Scholar]
- 53.Stillman B. Smart machines at the DNA replication fork. Cell. 1994;78:725–728. doi: 10.1016/s0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y N, Lu J Z, McCance D J. Mapping of HPV-11 E1 binding site and determination of other important cis elements for replication of the origin. Virology. 1996;216:219–222. doi: 10.1006/viro.1996.0050. [DOI] [PubMed] [Google Scholar]
- 55.Sverdrup F, Khan S A. Replication of human papillomavirus (HPV) DNAs supported by the HPV type 18 E1 and E2 proteins. J Virol. 1994;68:505–509. doi: 10.1128/jvi.68.1.505-509.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sverdrup F, Khan S A. Two E2 binding sites alone are sufficient to function as the minimal origin of replication of human papillomavirus type 18 DNA. J Virol. 1995;69:1319–1323. doi: 10.1128/jvi.69.2.1319-1323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan S H, Leong L E, Walker P A, Bernard H-U. The human papillomavirus type 16 E2 transcription factor binds with low cooperativity to two flanking sites and represses the E6 promoter through displacement of Sp1 and TFIID. J Virol. 1994;68:6411–6420. doi: 10.1128/jvi.68.10.6411-6420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usheva A, Shenk T. YY1 transcription initiator: protein interactions and association with a DNA site containing unpaired strands. Proc Natl Acad Sci USA. 1996;93:13571–13576. doi: 10.1073/pnas.93.24.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ustav M, Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson V G, Ludes-Meyers J. A bovine papillomavirus E1-related protein binds specifically to bovine papillomavirus DNA. J Virol. 1991;65:5314–5322. doi: 10.1128/jvi.65.10.5314-5322.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L, Li R, Mohr I J, Clark R, Botchan M R. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature. 1991;353:628–632. doi: 10.1038/353628a0. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Mohr I, Fouts E, Lim D A, Nohaile M, Botchan M. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA. 1993;90:5086–5090. doi: 10.1073/pnas.90.11.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yant S R, Zhu W, Millinoff D, Slightom J L, Goodman M, Gumucio D L. High affinity YY1 binding motifs: identification of two core types (ACAT and CCAT) and distribution of potential binding sites within the human beta globin cluster. Nucleic Acids Res. 1995;23:4353–4362. doi: 10.1093/nar/23.21.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou N, Liu J-S, Kuo S-R, Broker T R, Chow L T. The carboxyl-terminal domain of the human papillomavirus type 16 protein determines the E2 protein specificity during DNA replication. J Virol. 1998;72:3436–3441. doi: 10.1128/jvi.72.4.3436-3441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.zur Hausen H, de Villiers E-M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]