Abstract
Background
Midwives are primary providers of care for childbearing women globally and there is a need to establish whether there are differences in effectiveness between midwife continuity of care models and other models of care. This is an update of a review published in 2016.
Objectives
To compare the effects of midwife continuity of care models with other models of care for childbearing women and their infants.
Search methods
We searched the Cochrane Pregnancy and Childbirth Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) (17 August 2022), as well as the reference lists of retrieved studies.
Selection criteria
All published and unpublished trials in which pregnant women are randomly allocated to midwife continuity of care models or other models of care during pregnancy and birth.
Data collection and analysis
Two authors independently assessed studies for inclusion criteria, scientific integrity, and risk of bias, and carried out data extraction and entry. Primary outcomes were spontaneous vaginal birth, caesarean section, regional anaesthesia, intact perineum, fetal loss after 24 weeks gestation, preterm birth, and neonatal death. We used GRADE to rate the certainty of evidence.
Main results
We included 17 studies involving 18,533 randomised women. We assessed all studies as being at low risk of scientific integrity/trustworthiness concerns. Studies were conducted in Australia, Canada, China, Ireland, and the United Kingdom. The majority of the included studies did not include women at high risk of complications. There are three ongoing studies targeting disadvantaged women.
Primary outcomes
Based on control group risks observed in the studies, midwife continuity of care models, as compared to other models of care, likely increase spontaneous vaginal birth from 66% to 70% (risk ratio (RR) 1.05, 95% confidence interval (CI) 1.03 to 1.07; 15 studies, 17,864 participants; moderate‐certainty evidence), likelyreduce caesarean sections from 16% to 15% (RR 0.91, 95% CI 0.84 to 0.99; 16 studies, 18,037 participants; moderate‐certainty evidence), and likely result in little to no difference in intact perineum (29% in other care models and 31% in midwife continuity of care models, average RR 1.05, 95% CI 0.98 to 1.12; 12 studies, 14,268 participants; moderate‐certainty evidence). There may belittle or no difference in preterm birth (< 37 weeks) (6% under both care models, average RR 0.95, 95% CI 0.78 to 1.16; 10 studies, 13,850 participants; low‐certainty evidence).
We are very uncertain about the effect of midwife continuity of care models on regional analgesia (average RR 0.85, 95% CI 0.79 to 0.92; 15 studies, 17,754 participants, very low‐certainty evidence), fetal loss at or after 24 weeks gestation (average RR 1.24, 95% CI 0.73 to 2.13; 12 studies, 16,122 participants; very low‐certainty evidence), and neonatal death (average RR 0.85, 95% CI 0.43 to 1.71; 10 studies, 14,718 participants; very low‐certainty evidence).
Secondary outcomes
When compared to other models of care, midwife continuity of care models likely reduce instrumental vaginal birth (forceps/vacuum) from 14% to 13% (average RR 0.89, 95% CI 0.83 to 0.96; 14 studies, 17,769 participants; moderate‐certainty evidence), and may reduceepisiotomy 23% to 19% (average RR 0.83, 95% CI 0.77 to 0.91; 15 studies, 17,839 participants; low‐certainty evidence).
When compared to other models of care, midwife continuity of care models likelyresult in little to no difference in postpartum haemorrhage (average RR 0.92, 95% CI 0.82 to 1.03; 11 studies, 14,407 participants; moderate‐certainty evidence) and admission to special care nursery/neonatal intensive care unit (average RR 0.89, 95% CI 0.77 to 1.03; 13 studies, 16,260 participants; moderate‐certainty evidence). There may be little or no difference in induction of labour (average RR 0.92, 95% CI 0.85 to 1.00; 14 studies, 17,666 participants; low‐certainty evidence), breastfeeding initiation (average RR 1.06, 95% CI 1.00 to 1.12; 8 studies, 8575 participants; low‐certainty evidence), and birth weight less than 2500 g (average RR 0.92, 95% CI 0.79 to 1.08; 9 studies, 12,420 participants; low‐certainty evidence).
We are very uncertain about the effect of midwife continuity of care models compared to other models of care on third or fourth‐degree tear (average RR 1.10, 95% CI 0.81 to 1.49; 7 studies, 9437 participants; very low‐certainty evidence), maternal readmission within 28 days (average RR 1.52, 95% CI 0.78 to 2.96; 1 study, 1195 participants; very low‐certainty evidence), attendance at birth by a known midwife (average RR 9.13, 95% CI 5.87 to 14.21; 11 studies, 9273 participants; very low‐certainty evidence), Apgar score less than or equal to seven at five minutes (average RR 0.95, 95% CI 0.72 to 1.24; 13 studies, 12,806 participants; very low‐certainty evidence) and fetal loss before 24 weeks gestation (average RR 0.82, 95% CI 0.67 to 1.01; 12 studies, 15,913 participants; very low‐certainty evidence). No maternal deaths were reported across three studies.
Although the observed risk of adverse events was similar between midwifery continuity of care models and other models, our confidence in the findings was limited. Our confidence in the findings was lowered by possible risks of bias, inconsistency, and imprecision of some estimates.
There were no available data for the outcomes: maternal health status, neonatal readmission within 28 days, infant health status, and birth weight of 4000 g or more.
Maternal experiences and cost implications are described narratively. Women receiving care from midwife continuity of care models, as opposed to other care models, generally reported more positive experiences during pregnancy, labour, and postpartum. Cost savings were noted in the antenatal and intrapartum periods in midwife continuity of care models.
Authors' conclusions
Women receiving midwife continuity of care models were less likely to experience a caesarean section and instrumental birth, and may be less likely to experience episiotomy. They were more likely to experience spontaneous vaginal birth and report a positive experience. The certainty of some findings varies due to possible risks of bias, inconsistencies, and imprecision of some estimates.
Future research should focus on the impact on women with social risk factors, and those at higher risk of complications, and implementation and scaling up of midwife continuity of care models, with emphasis on low‐ and middle‐income countries.
Plain language summary
Are midwife continuity of care models versus other models of care for childbearing women better for women and their babies?
Key messages
Women or their babies who received midwife continuity of care models were less likely to experience a caesarean section or instrumental birth with forceps or a ventouse suction cup, and may be less likely to experience an episiotomy (a cut made by a healthcare professional into the perineum and vaginal wall). They were more likely to experience spontaneous vaginal birth.
Women who experienced midwife continuity of care models reported more positive experiences during pregnancy, labour, and postpartum. Additionally, there were cost savings in the antenatal (care during pregnancy) and intrapartum (care during labour and birth) period.
Further evidence may change our results, and future research should focus on the impact on women with social risk factors, and those with medical complications, and understanding the implementation and scaling up of midwife continuity of care models, with emphasis on low‐ and middle‐income countries.
What are midwife continuity of care models?
Midwife continuity of care models provide care from the same midwife or team of midwives during pregnancy, birth, and the early parenting period in collaboration with obstetric and specialist teams when required.
What did we want to find out?
We wanted to find out how outcomes differed for women or their babies who received a midwife continuity of care model compared to other models of care.
Our main outcomes were: spontaneous vaginal birth, caesarean section, regional anaesthesia (spinal or epidural block to numb the lower part of the body), intact perineum (the area between the anus and the vulva), fetal loss after 24 weeks gestation, preterm birth, and neonatal death.
We also looked at a range of other outcomes, including women’s experience and cost.
What did we do?
We searched for studies that compared midwife continuity of care models with other models of care for pregnant women. We compared and summarised the results of the studies and rated our confidence in the evidence based on factors such as study methods and size.
What did we find?
We found 17 studies involving 18,533 women in Australia, Canada, China, Ireland, and the United Kingdom.
Many of these studies largely focused on women with a lower risk of complications at the start of pregnancy, or those drawn from a specific geographical location. Midwives continued to provide midwifery care in collaboration with specialist and obstetric teams if women developed complications in pregnancy, birth, and postpartum.
Our main results
Women or their babies who received midwife continuity of care models compared to those receiving other models of care were less likely to experience a caesarean section or instrumental vaginal delivery, and may be less likely to experience an episiotomy. They were more likely to experience a spontaneous vaginal birth.
Midwife continuity care models probably make little or no difference to the likelihood of having an intact perineum, and may have little or no impact on the likelihood of preterm birth.
We are uncertain about the effect of midwife continuity of care models on regional anaesthesia, fetal loss after 24 weeks' gestation, and neonatal death.
Women who experienced care from midwife continuity of care models reported more positive experiences during pregnancy, labour, and postpartum. Additionally, there were cost savings in the antenatal and intrapartum period.
What are the limitations of the evidence?
Our confidence in these findings varies and further evidence may change our results. For instance, it is not always clear if the people assessing the outcomes knew which type of care the women received. The evidence for fetal loss after 24 weeks' gestation and neonatal death is based on a very small number of cases and there are not enough studies to be certain about some results. We lack data on important aspects like maternal health status after birth, neonatal readmissions, or infant health status.
Few studies included a specific focus on women at high risk of complications, and none focused on women from disadvantaged backgrounds, indicating a need for future research in these areas. This highlights the need for more comprehensive and diverse studies to strengthen our understanding and confidence in these findings, particularly in varied populations and across different healthcare settings.
Future research should focus on the impact on women with social risk factors, and those with medical complications, and understanding the implementation and scaling up of midwife continuity of care models, with emphasis on low‐ and middle‐income countries.
How up‐to‐date is this evidence?
This is an update of our previous review. We included evidence up to 17 August 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Midwife continuity of care models compared to other models of care for childbearing women and their infants (all) (critical outcomes).
| Midwife continuity of care models compared to other models of care for childbearing women and their infants (all) (critical outcomes) | ||||||
| Patient or population: childbearing women and their infants (all) (critical outcomes) Setting: hospital and community‐based environments where midwife continuity of care and other care models are implemented for childbearing women and their infants Intervention: midwife continuity of care models Comparison: other models of care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other models of care | Risk with midwife continuity of care models | |||||
| Spontaneous vaginal birth (as defined by trial authors) assessed with: medical records (at the time of birth) | 663 per 1000 | 696 per 1000 (683 to 709) | RR 1.05 (1.03 to 1.07) | 17864 (15 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Caesarean birth assessed with: medical records (at the time of birth) | 161 per 1000 | 147 per 1000 (136 to 160) | RR 0.91 (0.84 to 0.99) | 18037 (16 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Regional analgesia (epidural/spinal) assessed with: medical records (during labour and delivery) | 285 per 1000 | 242 per 1000 (225 to 262) | RR 0.85 (0.79 to 0.92) | 17754 (15 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | |
| Intact perineum assessed with: clinical examination (immediately post‐delivery) | 291 per 1000 | 306 per 1000 (285 to 326) | RR 1.05 (0.98 to 1.12) | 14268 (12 RCTs) | ⊕⊕⊕⊝ Moderatef | |
| Fetal loss at or after 24 weeks gestation assessed with: medical records (from 24 weeks gestation to birth) | 3 per 1000 | 4 per 1000 (3 to 7) | RR 1.24 (0.73 to 2.13) | 16122 (12 RCTs) | ⊕⊝⊝⊝ Very lowg,h | |
| Preterm birth (< 37 weeks) assessed with: clinical records (gestational age at birth) (at the time of birth) | 59 per 1000 | 56 per 1000 (46 to 68) | RR 0.95 (0.78 to 1.16) | 13850 (10 RCTs) | ⊕⊕⊝⊝ Lowi,j | |
| Neonatal death (baby born alive at any gestation and dies within 28 days) assessed with: medical records (within 28 days post‐birth) | 3 per 1000 | 2 per 1000 (1 to 5) | RR 0.85 (0.43 to 1.71) | 14718 (10 RCTs) | ⊕⊝⊝⊝ Very lowk,l | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_440632394977539792. | ||||||
a For selection bias in random sequence generation, most studies (11 of 15) were low risk, none were high, and 4 were unclear. Similarly, for allocation concealment, most (11) were low risk, 1 was high, and 3 were unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mostly unclear (10 of 15), with 2 low and 3 high. Both attrition and reporting bias showed that most studies (12 of 15) were low risk, with 1 high and 2 unclear. For other bias, the majority (14 of 15) were low risk, with none high and 1 unclear. Downgraded by 1 level. b For selection bias in random sequence generation, most studies (12 of 16) were low risk, none were high, and 4 were unclear. In allocation concealment, most (12) were low risk, 1 was high, and 3 were unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mostly unclear (11 of 16), with 2 low and 3 high. Attrition bias showed that most studies (12 of 16) were low risk, with 2 high and 2 unclear. Reporting bias had the majority (13 of 16) as low risk, 1 high, and 2 unclear. For other bias, nearly all (15 of 16) were low risk, with none high and 1 unclear. Downgraded by 1 level. c Statistical tests of heterogeneity suggest moderate inconsistency (I2 = 51%, Chi2 P = 0.01). However, point estimates across studies appear relatively consistent and there is relatively good overlap of confidence intervals. Downgraded by 1 level. d Egger's test results indicate a statistically significant publication bias with a negative slope of −1.740 and a 2‐tailed P value of 0.026. The negative slope suggests that smaller studies are more likely to show fewer women in the experimental group receiving regional analgesia compared to larger studies. Downgraded by 1 level. e For selection bias in random sequence generation, most studies (11 of 15) were low risk, none were high, and 4 were unclear. In allocation concealment, most (11) were low risk, 1 was high, and 3 were unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (10 of 15), with 2 low and 3 high. In attrition bias, most studies (11 of 15) were low risk, 2 were high, and 2 were unclear. Reporting bias had the majority (13 of 15) as low risk, none high, and 2 unclear. For other bias, nearly all (14 of 15) were low risk, with none high and 1 unclear. Downgraded by 1 level. f For selection bias for both random sequence generation and allocation concealment, most studies (8 of 12) were low risk, with 1 high and 3‐4 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (9 of 12), with 1 low and 2 high. Attrition bias had most studies (9 of 12) as low risk, 1 high, and 2 unclear. Reporting bias showed a majority (10 of 12) as low risk, with none high and 2 unclear. For other bias, nearly all studies (11 of 12) were low risk, with none high and 1 unclear. Downgraded by 1 level. g Although the sample size is relatively large, the optimal information size criterion is not met because of a relatively small number of events in this population. We estimate a control event rate of 0.35%. Taking alpha as 0.05 and beta as 0.2, a sample size of > 800K is needed for a 10% relative risk reduction (RRR) and > 200K for a 20% RRR. Downgraded by 2 levels. h For both types of selection bias, random sequence generation and allocation concealment, most studies (9 of 12) were low risk, with none high and 3 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was primarily unclear (7 of 12), with 2 low and 3 high. For attrition bias, the majority (10 of 12) were low risk, with none high and 2 unclear. Reporting bias also had most studies (10 of 12) as low risk, none high, and 2 unclear. For other bias, nearly all (11 of 12) were low risk, with none high and 1 unclear. Downgraded by 1 level. i Statistical tests of heterogeneity suggest moderate inconsistency (I2 = 45%, Chi2 P = 0.06). There is some inconsistency in point estimates across studies. Relatively good overlap of confidence intervals. Downgraded by 1 level. j For both types of selection bias, the majority of studies (8 of 10) were low risk, with none high and 2 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mostly unclear (6 of 10), with 2 each in low and high categories. The majority of studies in attrition bias (8 of 10) were low risk, with none high and 2 unclear. Reporting bias had most studies (8 of 10) as low risk, none high, and 2 unclear. For other bias, the majority (9 of 10) were low risk, with none high and 1 unclear. Downgraded by 1 level. k Although the sample size is relatively large, the optimal information size criterion is not met because of a relatively small number of events in this population. We estimate a control event rate of 0.30%. Taking alpha as 0.05 and beta as 0.2, a sample size of > 900K is needed for a 10% relative risk reduction (RRR) and > 230K for a 20% RRR. Downgraded by 2 levels. l For both types of selection bias, most studies (8 of 10) were low risk, with none high and 2 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was fairly evenly distributed, with 2 low, 3 high, and 5 unclear. In attrition bias and reporting bias, most studies (8 of 10) were low risk, with none high and 2 unclear. For other bias, the majority (9 of 10) were low risk, with none high and 1 unclear. Downgraded by 1 level.
Summary of findings 2. Summary of findings table ‐ Midwife continuity models compared to other models of care for childbearing women and their infants (all) (important/secondary outcomes).
| Midwife continuity models compared to other models of care for childbearing women and their infants (all) (important/secondary outcomes) | ||||||
| Patient or population: childbearing women and their infants (all) (important/secondary outcomes) Setting: hospital and community‐based environments where midwife continuity of care and other care models are implemented for childbearing women and their infants Intervention: midwife continuity models Comparison: other models of care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other models of care | Risk with midwife continuity models | |||||
| Healthy mother assessed with: composite of various health metrics (see methods) (timing varies) | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | |
| Maternal death assessed with: medical records (while pregnant or within 42 days of the end of pregnancy) | Not pooled | Not pooled | Not pooled | 4282 (3 studies) | ‐ | No deaths were reported across the three studies |
| Induction of labour assessed with: medical records (at the time of labour initiation) | 223 per 1000 | 205 per 1000 (189 to 223) | RR 0.92 (0.85 to 1.00) | 17666 (14 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Instrumental vaginal birth (forceps/vacuum) assessed with: medical records (at the time of birth) | 144 per 1000 | 128 per 1000 (119 to 138) | RR 0.89 (0.83 to 0.96) | 17769 (14 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Episiotomy assessed with: medical records (at the time of birth) | 225 per 1000 | 187 per 1000 (174 to 205) | RR 0.83 (0.77 to 0.91) | 17839 (15 RCTs) | ⊕⊕⊝⊝ Lowc,d | |
| Third or fourth degree tear assessed with: medical records (at the time of birth) | 17 per 1000 | 19 per 1000 (14 to 26) | RR 1.10 (0.81 to 1.49) | 9437 (7 RCTs) | ⊕⊝⊝⊝ Very lowe,f | |
| Postpartum haemorrhage (as defined by trial authors) assessed with: as defined by trial authors (medical records) (typically within 24 hours of birth) | 85 per 1000 | 78 per 1000 (70 to 88) | RR 0.92 (0.82 to 1.03) | 14407 (11 RCTs) | ⊕⊕⊕⊝ Moderateg | |
| Breastfeeding initiation assessed with: self‐report or medical records (immediately post‐delivery to first few days postpartum) | 692 per 1000 | 733 per 1000 (692 to 775) | RR 1.06 (1.00 to 1.12) | 8575 (8 RCTs) | ⊕⊕⊝⊝ Lowh,i | |
| Maternal readmission within 28 days assessed with: medical records (within 28 days postpartum) | 23 per 1000 | 35 per 1000 (18 to 69) | RR 1.52 (0.78 to 2.96) | 1195 (1 RCT) | ⊕⊝⊝⊝ Very lowj,k | |
| Neonatal readmission within 28 days assessed with: medical records (within 28 days post birth) | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | |
| Attendance at birth by known midwife assessed with: self‐report or medical records (at the time of birth) | 96 per 1000 | 878 per 1000 (565 to 1000) | RR 9.13 (5.87 to 14.21) | 9273 (11 RCTs) | ⊕⊝⊝⊝ Very lowl,m,n | |
| Healthy baby assessed with: composite of various health metrics (see methods) (timing varies) | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | |
| Birth weight less than 2500 g assessed with: weighing at birth (at the time of birth) | 52 per 1000 | 47 per 1000 (41 to 56) | RR 0.92 (0.79 to 1.08) | 12420 (8 RCTs) | ⊕⊕⊝⊝ Lowo,p | |
| Birth weight equal to or more than 4000 g assessed with: weighing at birth (at the time of birth) | Not pooled | Not pooled | Not pooled | (0 studies) | ‐ | |
| Apgar score less than or equal to 7 at 5 minutes assessed with: medical records (at 5 minutes post‐birth) | 26 per 1000 | 25 per 1000 (19 to 32) | RR 0.95 (0.72 to 1.24) | 12806 (13 RCTs) | ⊕⊝⊝⊝ Very lowq,r | |
| Admission to special care nursery/neonatal intensive care unit assessed with: medical records (from birth to discharge from the unit) | 90 per 1000 | 80 per 1000 (69 to 92) | RR 0.89 (0.77 to 1.03) | 16260 (13 RCTs) | ⊕⊕⊕⊝ Moderates | |
| Fetal loss before 24 weeks gestation assessed with: medical records (from conception to 24 weeks gestation) | 27 per 1000 | 22 per 1000 (18 to 27) | RR 0.82 (0.67 to 1.01) | 15913 (12 RCTs) | ⊕⊝⊝⊝ Very lowt,u | |
| Maternal experience assessed with: surveys or interviews (typically postpartum period) | Not pooled | Not pooled | Not pooled | (16 RCTs) | ‐ | Women receiving care from midwife continuity of care models, as opposed to other care models, generally reported more positive experiences during pregnancy, labour, and postpartum (16 studies, 17,028 participants). |
| Cost assessed with: cost data (from start of care to a defined postpartum period) | Not pooled | Not pooled | Not pooled | (7 RCTs) | ‐ | Cost savings were noted in antenatal and intrapartum periods in midwife continuity of care models. 7 studies, 8244 participants. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_444754536843053174. | ||||||
a For selection bias, random sequence generation and allocation concealment, most studies (10 of 14) were low risk, with 1 high and 3‐4 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mostly unclear (9 of 14), with 2 low and 3 high. For attrition bias, the majority (11 of 14) were low risk, 1 was high, and 2 were unclear. Reporting bias had most studies (12 of 14) as low risk, none high, and 2 unclear. For other bias, nearly all (13 of 14) were low risk, with none high and 1 unclear. Downgraded by 1 level. b Statistical tests of heterogeneity suggest moderate inconsistency (I2 = 41%, Chi2 P = 0.05). There is some inconsistency in point estimates across studies. There is relatively good overlap of confidence intervals. Downgraded by 1 level. c For selection bias, random sequence generation and allocation concealment, most studies (11 of 15) were low risk, with 1 high and 3‐4 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (10 of 15), with 2 low and 3 high. For attrition bias, most studies (11 of 15) were low risk, 2 were high, and 2 were unclear. Reporting bias had the majority (13 of 15) as low risk, none high, and 2 unclear. For other bias, nearly all (14 of 15) were low risk, with none high and 1 unclear. Downgraded by 1 level. d Statistical tests of heterogeneity suggest moderate inconsistency (I2 = 44%, Chi2 P = 0.04). There is some inconsistency in point estimates across studies. There is relatively good overlap of confidence intervals, but some studies do not overlap fully. Downgraded by 1 level. e For both types of selection bias, the majority of studies were low risk (4‐5 out of 7), with none high and 2‐3 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (4 of 7), with 2 low and 1 high. In attrition bias, reporting bias, and other bias, most studies (6 of 7) were low risk, with none high and 1 unclear. Downgraded by 1 level. f The optimal information size criterion is not met because of a relatively small number of events in this population. We estimate a control event rate of 1.7%. Taking alpha as 0.05 and beta as 0.2, a sample size of > 53K is needed for a 20% RRR. Downgraded by 2 levels. g For selection bias for random sequence generation and allocation concealment, most studies (8‐9 of 11) were low risk, with none high and 2‐3 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mostly unclear (7 of 11), with 2 each in low and high categories. For attrition bias and other bias, the majority (10 of 11) were low risk, with none high and 1 unclear. Reporting bias had most studies (9 of 11) as low risk, none high, and 2 unclear. Downgraded by 1 level. h For both types of selection bias, the majority of studies (5‐6 out of 8) were low risk, with none high and 2‐3 unclear. Performance bias was judged to be low risk given objectivity of outcome. Detection bias was fairly evenly distributed, with 2 low, 3 high, and 3 unclear. For attrition bias and other bias, most studies (7 of 8) were low risk, with none high and 1 unclear. Reporting bias had a majority (6 of 8) as low risk, none high, and 2 unclear. In other bias nearly all were low risk (7 of 8) with none high and 1 unclear. Downgraded by 1 level. i Statistical tests of heterogeneity suggest considerable heterogeneity. Heterogeneity: Tau² = 0.00; Chi² = 53.93, df = 7 (P < 0.00001); I² = 87%. There is consistency though in the direction of point estimates. Downgraded by 1 level. j One study at low risk of selection, attrition, performance, reporting, and other bias. High risk for detection bias. Downgraded by 1 level. k The optimal information size criterion is not met. We estimate a control event rate of 2.3%. Taking alpha as 0.05 and beta as 0.2, a sample size of approx. 30K is needed for a 20% RRR. Downgraded by 2 levels. l For selection bias random sequence generation and allocation concealment, most studies (7‐8 of 11) were low risk, with 1 high and 3 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (6 of 11), with 2 low and 3 high. For attrition bias, most studies (8 of 11) were low risk, 2 were high, and 1 was unclear. Reporting bias and other bias had a majority (10 of 11) as low risk, none high, and 1 unclear. Downgraded by 1 level. m Statistical tests of heterogeneity suggest considerable heterogeneity. Heterogeneity: Tau² = 0.45; Chi² = 190.11, df = 10 (P < 0.00001); I² = 95%. There is consistency though in the direction of point estimates. Downgraded by 1 level n The Egger's regression‐based test indicates significant publication bias in the meta‐analysis on attendance at birth by a known midwife, with a significant intercept (P = 0.048) and a strong correlation between effect size and its standard error (P = 0.002), suggesting that smaller studies with less favourable outcomes are likely missing from the analysis. Downgraded by 1 level. o For both types of selection bias, the majority of studies (6‐7 out of 8) were low risk, with none high and 1‐2 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was fairly evenly distributed, with 2 low, 2 high, and 4 unclear. For attrition bias and reporting bias, most studies (6 of 8) were low risk, with none high and 2 unclear. For other bias, the majority (7 of 8) were low risk, with none high and 1 unclear. Downgraded by 1 level. p The optimal information size criterion is not met. We estimate a control event rate of 5.2%. Taking alpha as 0.05 and beta as 0.2, a sample size of approx. 14K is needed for a 20% RRR. Downgraded by 1 level. q For both types of selection bias, most studies (10 of 13) were low risk, with none high and 3 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (8 of 13), with 2 low and 3 high. For attrition bias and other bias, the vast majority (12 of 13) were low risk, with none high and 1 unclear. Reporting bias had most studies (10 of 13) as low risk, 1 high, and 2 unclear. Downgraded by 1 level. r The optimal information size criterion is not met. We estimate a control event rate of 2.6%. Taking alpha as 0.05 and beta as 0.2, a sample size of approx. 29K is needed for a 20% RRR. Downgraded by 2 levels s For both types of selection bias, most studies (10 of 13) were low risk, with none high and 3 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (8 of 13), with 2 low and 3 high. For attrition bias, reporting bias, and other bias, the majority (11‐12 of 13) were low risk, with none high and 1‐2 unclear. Downgraded by 1 level. t For both types of selection bias, most studies (10 of 12) were low risk, with none high and 2 unclear. Performance bias was judged to be low risk given the objectivity of the outcome. Detection bias was mainly unclear (7 of 12), with 2 low and 3 high. For attrition bias, reporting bias, and other bias, the majority (10‐11 of 12) were low risk, with none high and 1‐2 unclear. Downgraded by 1 level. u The optimal information size criterion is not met. We estimate a control event rate of 2.6%. Taking alpha as 0.05 and beta as 0.2, a sample size of approx. 26K to 27K is needed for a 20% RRR. Downgraded by 2 levels.
Background
The maternity care received by pregnant women, including how it is organised, who it is delivered by, and its quality and content, varies widely globally (De Vries 2001). Whether in high‐, middle‐, or low‐income countries, appropriate access to quality maternity care during pregnancy improves maternal and infant mortality and morbidity rates, promotes healthy behaviours, and addresses emotional and social issues (Koblinsky 2016; Victora 2016; World Health Organization 2016; World Health Organization 2018b; World Health Organization 2022). Evidence has shown that high‐quality care from a midwifery workforce is crucial to achieving international goals and targets in reproductive, maternal, newborn, and child health improvements (Nove 2021; Renfrew 2014; Renfrew 2021). In many parts of the world, midwives are the primary care providers for childbearing women (ten Hoope‐Bender 2014), however access to midwifery care in many low‐ and middle‐income countries, and in some high‐income countries, such as the USA, is markedly lower than in other high‐income countries, with midwives representing a small percentage of healthcare professionals (Bradford 2022; Lowe 2020; McFadden 2020; UNFPA 2021).
The World Health Organization recommends midwife continuity of care models, in which a known midwife licensed and educated to international standards (such as International Confederation of Midwives (ICM) global standards) (ICM 2017), or a small group of known midwives, supports a woman throughout the antenatal, intrapartum, and postnatal continuum, in settings with well‐functioning midwifery programmes (World Health Organization 2016; World Health Organization 2018b). In addition, there is increasing evidence that such models can mitigate inequity, social disadvantage, and structural determinants of poor maternal and newborn health outcomes in a range of populations and settings (Hadebe 2021; Homer 2017; Khan 2023; Kildea 2021; Rayment‐Jones 2023). However, there is debate about the clinical and cost‐effectiveness of these models (Ryan 2013), and how models are organised, implemented sustainably (Homer 2019), and evaluated (Bradford 2022).
Description of the condition
The concept of continuity of care more widely is rooted in primary care (Saultz 2003; Saultz 2004). It involves care over time by the same care provider/s to encompass informational, management, and relational continuity to improve personalised integrated care (Freeman 2007; World Health Organization 2018a). As defined by Haggerty 2003, informational continuity concerns the timely availability of relevant information; management continuity involves communicating facts and judgements across the team, institutional and professional boundaries, and between professionals and patients; and relationship continuity means an ongoing therapeutic relationship between the service user and one or more health professionals.
There is evidence that continuity of care between primary care physicians and their patients is associated with better patient outcomes, including diagnostic accuracy (Starfield 2009), improved patient satisfaction (Paddison 2015; Saultz 2005), fewer emergency department visits (Nyweide 2017), fewer hospital admissions (Barker 2017; Pourat 2015; Sandvik 2021), better care co‐ordination (O’Malley 2009), reduced mortality (Baker 2020; Pereira Gray 2018), lower healthcare costs, and lower or more appropriate use of services (Bazemore 2023; Sandvik 2021). Greater continuity of care is also independently associated with lower hospital utilisation for seniors with multiple chronic medical conditions in an integrated delivery system with high informational continuity (Bayliss 2015).
Midwife continuity of care models
Midwife continuity of care models aim to provide care in either community or hospital settings, usually to women with uncomplicated or low‐risk pregnancies for whom the midwife will be the lead professional. In some models, midwives provide continuity of midwife care to all women with social risk factors/living in deprivation, or from a defined geographical location, and continue to provide continuity of midwife care to women who experience complications, in partnership with obstetricians and other professionals. In other models, midwives may provide continuity of midwife care to women with medical or obstetric risk factors as part of a wider team. Midwife continuity of care models must include the provision of maternity care by one or a small team of midwives during the antepartum and intrapartum periods, and some models may extend to the postnatal period in the community in some settings.
Within midwife continuity of care models, women receive dedicated support from the same midwife or team of midwives as appropriate (NHSE 2021). Care may be provided in consultation and collaboration with other health and social care providers during pregnancy, birth, and the postpartum period. Around the world, midwife continuity of care has been implemented in different contexts with variation in the lead professional and degree of autonomous midwifery practice, the composition of the multidisciplinary team, and the target population. However, in all models, the aim is for women to develop relationships with their midwives throughout their pregnancy, birth, and the postnatal period, where offered in the health system. The midwife continuity of care model is based on the holistic premise that pregnancy, birth, and becoming a parent are transformative life events and includes: continuity of care; monitoring the physical, psychological, spiritual and social wellbeing of the woman and family throughout the childbearing cycle; providing the woman with individualised education, counselling and antenatal care; attendance during labour, birth and the immediate postpartum period by a known midwife; ongoing support during the postnatal period; minimising unnecessary technological interventions; and identifying, referring, and co‐ordinating care for women who require obstetric or other specialist attention.
Some midwife continuity of care models provide continuity of care to a defined group of women through a team of midwives sharing a caseload, often called 'team' midwifery. Thus, a woman will receive her care from a number of midwives in the team, the size of which can vary. Other models, often termed 'caseload midwifery', aim to offer greater relationship continuity by ensuring that childbearing women receive their ante‐, intra‐, and postnatal care from one midwife or their practice partner (Homer 2019; McCourt 2006).
Other models of care
Other models of care include:
Obstetrician‐provided care. Where obstetricians are the primary care provider for many childbearing women, an obstetrician (not necessarily the one who provides antenatal care) is present for the birth, and nurses (usually) provide intrapartum and postnatal care.
Family doctor‐provided care, with referral to specialist obstetric care as needed. Obstetric nurses or midwives provide intrapartum and immediate postnatal care, but not at a decision‐making level or throughout the entire care episode, and a medical doctor is present for the birth.
Shared models of care, where responsibility for the organisation and delivery of care is shared between different health professionals throughout the initial booking to the postnatal period. These models are similar in that they do not aim to provide midwife continuity of care. Other models of care must include the provision of maternity care during the antepartum and intrapartum periods, and some models may extend to the postnatal period in some settings.
How the intervention might work
The holistic concept of relational continuity refers to a continuous process of pregnancy, birth, and postnatal care and includes a "coordinated and smooth progression of care from the patient’s point of view" (Dahlberg 2013; Haggerty 2003), rather than isolated events. Continuity contributes to patient perceptions of having a trusted care provider who knows their social and medical history and harnesses an expectation that a known provider will care for them in the future, lessening stress and anxiety (Haggerty 2003; Kildea 2018; Parchman 2004; Rayment‐Jones 2022). This longitudinal aspect develops a trusting relationship between women and their midwives. It enables midwives to work to their full scope of practice across women’s care journeys, improving their ability to identify women’s individual needs and providing a safety net (Cook 2000; McInnes 2020; Rayment‐Jones 2020).
Relational continuity over time has been found to have a more significant effect on user experience and outcome in high‐income countries (Dahlberg 2013; Fernandez Turienzo 2016; Homer 2017; Kelly 2014; Rayment‐Jones 2015; Rayment‐Jones 2021; Saultz 2005). It has been argued that neither management nor informational continuity can compensate for the lack of an ongoing relationship over time (Guthrie 2008; Parchman 2004; World Health Organization 2018a).
Suggested mechanisms of effect in maternity and primary care literature include care providers taking greater responsibility, improved trust, confidence in the care provider, feeling safe to disclose concerns or risk factors, reduced stigma and discrimination, and improved engagement, access, and referral (Fernandez Turienzo 2021; McInnes 2020; Parchman 2004; Rayment‐Jones 2022; Rayment‐Jones 2023; Sidaway‐Lee 2021). Lower rates of interventions could be linked to the greater agency experienced by women and midwives within midwife continuity of care models, and these effects are mediated, in part, by the context of the settings (Walsh 2012). Continuity of care may also lead to enhanced co‐ordination or navigation of care, greater advocacy, timely follow‐up of test results, and greater adherence to treatments and multidisciplinary guidelines (Barker 2018; Fernandez Turienzo 2021; Rayment‐Jones 2020; Sidaway‐Lee 2021). It may also provide more opportunities for social support from multidisciplinary services, families, and the local community, timely care, earlier help‐seeking, opportunities for early prevention, escalation of concerns, and diagnosis of complications to facilitate management and intervention (Fernandez Turienzo 2021; Rayment‐Jones 2015; Sidaway‐Lee 2021). This literature has resulted in more recent interest in continuity of care models for those with multi‐morbidities and disproportionate risk of health inequalities and multiple morbidities (Chau 2021; Engamba 2019). The general literature on continuity notes that a lack of clarity in the definition and measurement of different types of continuity has been one of the limitations of research in this field and that there is a need for better specification between models of care and outcomes (Haggerty 2003; McInnes 2020).
Why it is important to do this review
This is an update of a Cochrane review first published in 2008 and last updated in 2016. The 2016 review is more than five years old, and new studies need to be incorporated. Midwife continuity of care is at the heart of maternal policy in some high‐income countries and is considered important globally by the World Health Organization (WHO). The applicability to low‐ and middle‐income settings is a key issue for local and national stakeholders. It is therefore important to explore whether the effects of midwife continuity of care models are influenced by variation in the model of care, maternal medical and obstetric risk status, social risk factors, and in low‐ to high‐income country settings.
This updated review aims to complement other systematic review work on models of maternity care (Bradford 2022; Homer 2016; Perriman 2018) and contribute to the knowledge base on the effects of midwife continuity of care models. This update includes a focus on how outcomes are influenced by variations in models of care, maternal medical and obstetric risk status, social risk factors, and low‐ to high‐income country settings. The definition of the intervention has changed from ‘midwife‐led continuity models’ to ‘midwife continuity of care models’ to include interventions for women with medical and obstetric risk who may receive collaborative specialist and obstetric‐led care and midwife continuity of care. This change aligns with current policy and recommendations for relational continuity and effective collaborative multidisciplinary networks of care (Carmone 2020; NHSE 2021; World Health Organization 2018a).
Objectives
The primary objective of this review is to compare the effects of midwife continuity of care models with other models of care for childbearing women and their infants. We also explore whether the effects of midwife continuity of care are influenced by: 1) variation in midwifery models of care; 2) obstetric and medical risk factors; 3) social risk factors; 4) country income level.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials using individual‐ or cluster‐randomisation methods and quasi‐randomised trials. The latter group refers to trials where allocation may not have been genuinely random, for example when the allocation was alternate or unclear.
Types of participants
Pregnant women from all demographics, regardless of age, ethnicity, socio‐economic status, education, place of residence, or level of deprivation. We also included pregnant women with various risk factors, including medical, obstetric, and social risks, and those receiving care in home, hospital, or community settings. The review also encompasses participants receiving care through either private or public healthcare systems and those residing in low‐ or high‐income countries. All women allocated to a midwife continuity of care model or another model of care were eligible for inclusion.
Types of interventions
Midwife continuity of care models ‐ intervention
For a model to be classified as a midwife continuity of care model, midwifery care is provided by a midwife and/or a small team of midwives throughout the antepartum and intrapartum periods, which may extend to the postpartum period in some settings.
In all models, care is provided in consultation with medical staff in pregnancy, birth, and the postpartum period as appropriate.
Midwife continuity of care models aim to provide care in either community or hospital settings. Normally, midwives are the lead professionals for healthy women with uncomplicated pregnancies. In some models, midwives provide continuity of midwifery care to all women with specialised needs, such as social risk factors, or from a defined geographical location, acting as lead professionals for women whose pregnancy and birth is uncomplicated and continuing to provide continuity of midwife care to women who experience medical and obstetric complications in partnership with other professionals. In some models, midwives may provide continuity of midwife care to women with obstetric risk factors as part of a wider team.
Some midwife continuity models provide continuity to a defined group of women through a team of midwives, often called 'team' midwifery. Thus, a woman will receive her midwifery care from a few midwives in the team, the size of which can vary (often between four and eight midwives). Other models, often termed 'caseload midwifery', aim to offer greater relationship continuity by ensuring that childbearing women receive their ante‐, intra‐, and postnatal care from one midwife or their practice partner backed up by a wider team or group practice.
Other models of care ‐ comparison
These models of care include:
Obstetrician‐provided care, where obstetricians are the primary antenatal care providers for childbearing women. An obstetrician (not necessarily the one who provides antenatal care) is present for the birth, and nurses offer intrapartum and postnatal care.
Family doctor‐provided care, with referral to specialist obstetric care as needed. Obstetric nurses or midwives provide intrapartum and immediate postnatal care but not at a decision‐making level or throughout the entire care episode, and a medical doctor is present for the birth.
Shared models of care, where health professionals share responsibility for the organisation and delivery of care throughout the initial booking to the postnatal period. Other models of care must include the provision of maternity care during the antepartum and intrapartum periods, and some models may extend to the postnatal period in some settings.
Types of outcome measures
Primary outcomes
Spontaneous vaginal birth (defined by trial authors)
Caesarean birth
Regional analgesia (epidural/spinal)
Intact perineum
Fetal loss at or after 24 weeks gestation
Preterm birth (< 37 weeks)
Neonatal death (baby born alive at any gestation and dies within 28 days)
Secondary outcomes
Healthy mother (defined as one who is alive at 28 days postpartum, without a Caesarean birth, postpartum haemorrhage (as defined by trial authors), third or fourth‐degree tear, or readmission within 28 days)
Maternal death
Induction of labour
Instrumental vaginal birth (forceps/vacuum)
Episiotomy
Third‐ or fourth‐degree tear
Postpartum haemorrhage (defined by trial authors)
Breastfeeding initiation (defined by trial authors)
Maternal readmission within 28 days
Maternal experience (defined by trial authors)
Attendance at birth by a known midwife who provided antenatal care
Cost (as defined by trial authors)
Healthy baby (defined as one born after 37 + 0 weeks gestation and alive at 28 days and without readmission within 28 days)
Birth weight less than 2500 g
Birth weight equal to or more than 4000 g
Apgar score less than or equal to seven at five minutes
Admission to special care nursery/neonatal intensive care unit
Fetal loss before 24 weeks gestation
Search methods for identification of studies
The following methods sections of this review are based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, in collaboration with the Cochrane Pregnancy and Childbirth Information Specialist, a search was conducted on 17 August 2022 of the Cochrane Pregnancy and Childbirth Trials Register, which contains over 34,000 reports of controlled trials related to pregnancy and childbirth and represents over 30 years of searching.
Full details of the current search methods used to populate the Register, including search strategies for CENTRAL, MEDLINE, Embase, and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, can be found in Appendix 1.
The Information Specialist maintains the Trials Register, which contains trials identified through monthly searches of CENTRAL, weekly searches of MEDLINE and Embase, monthly searches of CINAHL, handsearches of 30 journals and conference proceedings, and weekly current awareness alerts for an additional 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the search activities described above. Based on the intervention described, each trial report is assigned a number corresponding to a specific Pregnancy and Childbirth review topic and added to the Register.
The Information Specialist searches the Register for each review using this topic number for a more specific search set, fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification, or Ongoing).
Additionally, unpublished, planned, and ongoing trial reports were searched for on ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) using the search methods detailed in Appendix 2. This search was also conducted on 17 August 2022.
Searching other resources
We searched for further studies in the reference lists of the studies identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Sandall 2016.
This update was conducted using a standard template from Cochrane Pregnancy and Childbirth, as outlined in the following methods sections of this review.
Selection of studies
Three review authors (JS, CFT, HRJ) independently assessed for inclusion all potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted other review authors (DD, HS, LJ).
We created a study flow diagram to map out the number of records identified, included, excluded, or awaiting classification.
All studies meeting our inclusion criteria were evaluated by three review authors (LJ, CFT, HRJ) against the Cochrane Pregnancy and Childbirth Trustworthiness Screening tool (CPC‐TST). This screening tool is a set of predefined criteria to select studies that, based on available information, are deemed sufficiently trustworthy to be included in the analysis. The criteria are:
Research governance
Are any retraction notices or expressions of concern listed on the Retraction Watch Database relating to this study?
Was the study prospectively registered (for those studies published after 2010)? If not, was there a plausible reason?
Did the trial authors provide/share the protocol and/or ethics approval letter when requested?
Did the trial authors communicate with the Cochrane review authors within the agreed timelines?
Did the trial authors provide individual participant data (IPD) upon request? If not, was there a plausible reason?
Baseline characteristics
Is the study free from characteristics of the participants that appear too similar (e.g. distribution of the mean (SD) is excessively narrow or excessively wide, as noted by Carlise 2017).
Feasibility
Is the study free from characteristics that could be implausible? (e.g. large numbers of women with a rare condition (such as severe cholestasis in pregnancy) recruited within 12 months).
Is there a plausible explanation in cases with (close to) zero losses to follow‐up?
Results
Is the study free from results that could be implausible? (e.g. massive risk reduction for primary outcomes with a small sample size)?
Do the numbers randomised to each group suggest that adequate randomisation methods were used (e.g. is the study free from issues such as unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, if the authors say ‘no blocking was used’ but still end up with equal numbers, or if the authors say they used ‘blocks of 4’ but the final numbers differ by 6)?
The review did not include studies assessed as potentially ‘high‐risk’. Where a study was classified as ‘high‐risk’ for one or more of the above criteria, we attempted to contact the study authors to address any possible lack of information/concerns. Where adequate information was not obtained, the study remains ‘awaiting classification’, and the reasons and communications with the author (or lack of) are described in detail.
The process is described in its entirety in Figure 1.
1.
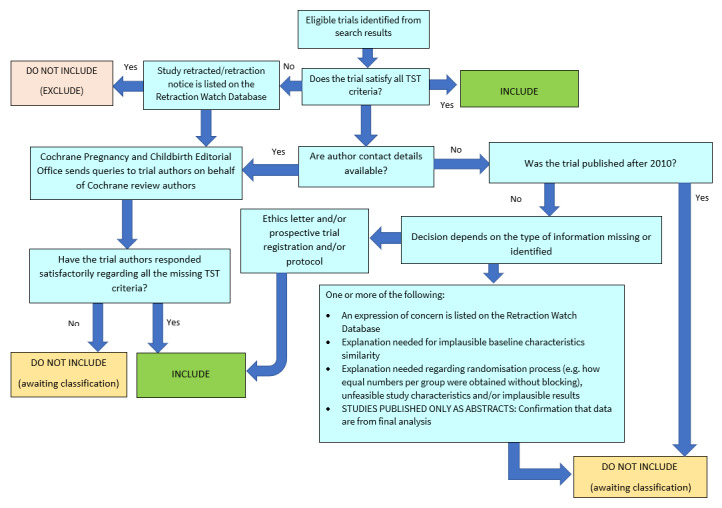
Applying the Cochrane Pregnancy and Childbirth Trustworthiness Screening Tool
Abstracts
Data from abstracts have only been included if, in addition to the trustworthiness assessment, the study authors have confirmed in writing that the data to be included in the review have come from the final analysis and will not change. Where such information is unavailable/not provided, the study remains ‘awaiting classification’ (as above).
Data extraction and management
We adapted the data extraction template from Cochrane Pregnancy and Childbirth to extract data. Six review authors (JS, CFT, DD, HS, LJ, HRJ) extracted the data for eligible studies using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the other review authors (AHS, SG, PG). Data were entered into Review Manager Web software (Review Manager Web 2023) and checked for accuracy.
When information regarding any of the above was unclear, we contacted the authors of the original reports to provide further details.
Review author D Devane is a co‐author of Begley 2011, and J Sandall, A Shennan, and C Fernandez Turienzo are co‐authors of Fernandez Turienzo 2020, so they were not involved in the data extraction or risk of bias assessment for the studies on which they were co‐authors.
Assessment of risk of bias in included studies
Six authors in groups of two (JS, CFT, DD, HS, LJ, HRJ) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving another assessor.
Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any genuinely random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions before assignment. We assessed whether intervention allocation could have been foreseen before, during recruitment, or changed after the assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded or if we judged that the lack of blinding would unlikely affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high, or unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
We described the completeness of data for each included study, and for each outcome or class of outcomes, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with the substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
Selective reporting (checking for reporting bias)
We described how we investigated the possibility of selective outcome reporting bias for each included study and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; the study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
Other bias (checking for bias due to problems not covered above)
For each included study, we described any important concerns we had about other possible sources of bias.
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio with 95% confidence intervals.
Continuous data
We planned to use the mean difference if outcomes were measured similarly between trials. In future updates, as appropriate, we will use the standardised mean difference to combine trials that measure the same outcome but use different methods.
Time‐to‐event data
No outcomes were expected using time‐to‐event data.
Cluster‐randomised trials
In addition to individually randomised trials, we included a cluster‐randomised trial in the analyses (North Stafford 2000). This trial found a negative ICC, so no adjustment was made for clustering. We considered it reasonable to combine the results from cluster‐randomised and individually randomised trials if there was little heterogeneity between the study designs and an interaction between the effect of the intervention and the choice of randomisation unit was considered to be unlikely. We also conducted a sensitivity analysis by excluding North Stafford 2000 from the meta‐analyses to which it contributed data (see Sensitivity analysis).
Other unit of analysis issues
Multiple pregnancies were included, and both infants were included in the denominator.
For any study with more than two intervention groups in a meta‐analysis, we planned to (i) omit groups that were not relevant to the comparison being made or (ii) combine multiple groups that were eligible as the experimental or comparator intervention to create a single pair‐wise comparison.
Dealing with missing data
For included studies, we noted levels of attrition. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We regarded heterogeneity as substantial if an I² value was greater than 30% and either a Tau² was greater than zero or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. We planned to explore several pre‐specified subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases using funnel plots. We assessed funnel plot asymmetry visually and using Egger's test with the software Comprehensive Meta‐Analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (Review Manager Web 2023).
Where clinical heterogeneity was sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary of whether an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. We would not have combined trials if the average treatment effect had been clinically meaningful. The results were presented as the average treatment effect with 95% confidence intervals and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful and, if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Caseload versus team models of midwifery care
Low‐risk versus mixed‐risk status
Women with social risk factors versus all women
Countries with a very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI
Subgroup analyses were restricted to the primary outcomes, which were:
Spontaneous vaginal birth (as defined by trial authors)
Caesarean birth
Regional analgesia (epidural/spinal)
Intact perineum
Fetal loss at or after 24 weeks gestation
Preterm birth (< 37 weeks)
Neonatal death (baby born alive at any gestation and dies within 28 days)
We assessed subgroup differences by interaction tests available within RevMan (Review Manager Web 2023). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We conducted sensitivity analyses to investigate the impact of the risk of bias on our findings. We repeated the analysis, retaining only the studies at low risk of bias for random sequence generation, allocation concealment, and incomplete outcome data, to evaluate whether this altered the overall results. We also conducted a sensitivity analysis by excluding North Stafford 2000 from the meta‐analyses to which it contributed data (see Sensitivity analysis).
Summary of findings and assessment of the certainty of the evidence
We employed the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach, as delineated in the GRADE Handbook, to assess the certainty of the evidence for all available outcomes in the primary comparisons between midwife continuity of care and all other models of care for childbearing women and their infants. This was undertaken to enable the use of GRADE‐recommended informative statements for communicating the results of systematic reviews, which necessitate a rating of the certainty of evidence. Critical outcomes (spontaneous vaginal birth, caesarean section, regional anaesthesia, intact perineum, fetal loss after 24 weeks gestation, preterm birth, and neonatal death) are presented in Table 1. Outcomes that we deemed to be less important are presented in Table 2.
We utilised the GRADEpro Guideline Development Tool to import data from Review Manager Web (Review Manager Web 2023) to create the summary of findings tables. Using the GRADE approach, we produced a summary of the intervention effect and a measure of certainty for each outcome, using five considerations to assess the certainty of the body of evidence (study limitations, consistency of effect, imprecision, indirectness, and publication bias). The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias. We explain any downgrading decisions in the footnotes in the summary of findings tables.
In this update, we present the results of the review and certainty ratings, focusing on both relative and absolute effects. For estimating the certainty of evidence, we used baseline risks observed in control groups across the included studies and multiplied them by the relative effects. We have applied the GRADE partially contextualised approach for interpreting findings, as suggested in the latest GRADE guidance on imprecision (Zeng 2022). This approach informed our interpretation of the size of the effects for the different outcomes. This was particularly since there are no known minimum clinically important differences (MCIDs) established for our critical outcomes. Our analysis and interpretation are aligned with GRADE's emphasis on absolute effects, particularly in the absence of MCIDs, thereby providing a clearer understanding of the practical significance of the results (Santesso 2020).
Results
Description of studies
This review update includes a total of 17 studies (77 study reports), 42 excluded studies (69 study reports), one study awaiting classification (one study report), and three ongoing studies (three study reports).
Results of the search
For this update, we identified a total of 221 records from electronic databases, and we found 17 potentially relevant studies from other sources. After the removal of duplicates, 218 records remained and we screened out 163 of these for either not being a trial or having a different scope. We assessed a total of 55 full trial reports for eligibility. Ten of these trial reports were found to be additional reports relating to three already included studies (Homer 2001; McLachlan 2012; Tracy 2013). One new trial (seven reports) has been included (Fernandez Turienzo 2020), two trials previously excluded in the last version of the review have now been included (Gu 2013; Marks 2003), and one trial previously included has now been excluded (Allen 2013), making the final number of included studies 17. Twenty‐one new studies (34 study reports) were excluded, with reasons. One study is awaiting further classification (Zhang 2016) (see Characteristics of studies awaiting classification). Three studies are ongoing (Cullinane 2021; Dickerson 2022; Xiaojiao 2020) (see Characteristics of ongoing studies). See Figure 2 for details of the most recent search results.
2.
Screening eligible studies for trustworthiness
We used the trustworthiness screening tool developed by Cochrane Pregnancy and Childbirth to assess the 17 studies meeting the review's inclusion criteria. These were screened by two review authors, and all assessments were discussed and agreed upon with the review team.
We had no concerns about trustworthiness for four studies and these were included (Begley 2011; Fernandez Turienzo 2020; McLachlan 2012; Tracy 2013). For the remaining studies, there were minor concerns relating to research governance and the results of two studies. In 13 studies, we sought clarification from trial authors on ethics approval and whether a protocol had been developed. None of these studies were prospectively registered, but we were not concerned about this because all but one of these studies were published before 2010, when trial registration was not a requirement.
Research governance
We sought clarification from the trial authors regarding ethics approval and the development of a protocol. In eight studies, although ethics approval was reported in the trial report, we also contacted the trial authors for further clarification and to obtain copies of any relevant paperwork. In two studies, the authors responded that all pertinent paperwork relating to ethics approval was no longer available due to the time‐lapse since the conduct of the study (MacVicar 1993; Turnbull 1996). In another study, the authors responded to confirm that there was no protocol or trial registration but that they had received ethics approval (North Stafford 2000). For Rowley 1995, the authors responded and provided copies of the protocol and ethics approval. For Waldenstrom 2001, the authors responded to confirm that they were almost sure that there had been no protocol before the commencement of the trial due to this not being a requirement of the time, but that ethics approval had been obtained. For Marks 2003, the author said there was no longer any paperwork available, but it went through the local ethics committee. In one study (Biro 2000), we could not get a response and received an email delivery failure. In one study (Gu 2013), we sought clarification from the author regarding the reason for retrospective trial registration and requested copies of both ethics approval and the protocol, which the authors provided. The authors responded that their trial was registered retrospectively because, at that time in China, it was not a requirement to pre‐register the trial. Hence, it was registered retrospectively after notification of this requirement from an international journal. In two studies, the authors responded to our enquiries and confirmed that a protocol was developed for each study and that ethics approval was also obtained (Flint 1989; Harvey 1996). In another study, the authors responded by saying local ethics approval was obtained (Hicks 2003). For Homer 2001, the authors confirmed there was no protocol, but a research proposal was developed prior to the study, and ethics approval was obtained. In the final study, the authors provided a copy of the ethics approval (Kenny 1994).
Results
In two studies, we had minor concerns relating to the domain of the results. In one study, the study flow of participants was not clear (North Stafford 2000). We contacted the trial authors, who clarified the study flow and confirmed no follow‐up loss in their cluster trial (North Stafford 2000). In the second study, the randomisation methods were unclear (Gu 2013). The trial authors responded to our email request to report that a simple randomisation scheme was conducted, using a computer‐generated computer random sequence from 1 to 110. The list of random numbers and group allocation were kept concealed in sealed, opaque envelopes. They also reported that, following informed consent, women were randomly allocated to one of the two groups. The allocation was not revealed until the clerical assistant recorded the woman’s details (Gu 2013).
All 17 eligible studies were assessed at low risk after screening for scientific integrity/trustworthiness.
Included studies
We included 17 studies involving 18,532 randomised women in total (Begley 2011; Biro 2000; Fernandez Turienzo 2020: Flint 1989; Gu 2013; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; Marks 2003; MacVicar 1993; McLachlan 2012; North Stafford 2000; Rowley 1995; Tracy 2013; Turnbull 1996; Waldenstrom 2001). See Characteristics of included studies.
Included studies were conducted in Australia, Canada, China, Ireland, and the United Kingdom, with variations in model of care, risk status of participating women, and practice settings. The Zelen method was used in three trials (Flint 1989; Homer 2001; MacVicar 1993), and one trial used cluster‐randomisation (North Stafford 2000).
Five studies offered a caseload model of care (Fernandez Turienzo 2020; McLachlan 2012; North Stafford 2000; Tracy 2013; Turnbull 1996) and 12 studies provided a team model of care (Begley 2011; Biro 2000; Flint 1989; Gu 2013: Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; Marks 2003; MacVicar 1993; Rowley 1995; Waldenstrom 2001). The composition and modus operandi of the teams varied amongst trials. Levels of continuity (measured by the percentage of women who were attended during birth by a known carer) ranged between 63% and 98% for midwife continuity models of care to between 0.3% and 21% in other models of care.
Ten studies compared a midwife continuity of care model with a shared model of care (Begley 2011; Biro 2000; Fernandez Turienzo 2020: Flint 1989; Hicks 2003; Homer 2001; Kenny 1994; Marks 2003; North Stafford 2000; Rowley 1995), four studies compared a midwife continuity of care model with medical‐led models of care (Gu 2013; Harvey 1996; MacVicar 1993; Turnbull 1996), and three studies compared a midwife continuity of care model with various options of standard care including shared, medical‐led, and shared care (McLachlan 2012; Tracy 2013; Waldenstrom 2001).
Participating women received ante‐, intra‐, and postpartum care in 15 studies (Begley 2011; Biro 2000; Fernandez Turienzo 2020; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; Marks 2003; McLachlan 2012; North Stafford 2000; Rowley 1995; Tracy 2013; Turnbull 1996; Waldenstrom 2001), and antenatal and intrapartum care only in two studies (Gu 2013; MacVicar 1993).
All midwife continuity of care models included visits to the obstetrician, family physicians (general practitioners, GPs), or both. The frequency of such visits varied. Such visits were dependent on women's risk status during pregnancy (Biro 2000), a routine for all women (one to three visits) (Flint 1989; Gu 2013; Harvey 1996; Kenny 1994; MacVicar 1993; McLachlan 2012; Rowley 1995; Waldenstrom 2001), or based on the development of complications (Fernandez Turienzo 2020; Hicks 2003; Homer 2001; Marks 2003; Tracy 2013; Turnbull 1996), antenatal care from midwives and, if desired by the woman, from the woman's general practitioner (Begley 2011), or not reported (North Stafford 2000).
Women were classified as being at low risk of complications in nine studies (Begley 2011; Flint 1989; Gu 2013: Harvey 1996; Hicks 2003; MacVicar 1993; McLachlan 2012; Turnbull 1996; Waldenstrom 2001), as 'low and high' in caseloads drawn from a defined geographical location in seven studies (Biro 2000; Homer 2001; Kenny 1994; Marks 2003; North Stafford 2000; Rowley 1995; Tracy 2013), and at high risk of preterm birth in one study (Fernandez Turienzo 2020).
No included studies specifically targeted women with social risk factors. Three studies offered midwifery continuity of care models in disadvantaged and ethnically diverse catchment areas (Fernandez Turienzo 2020; Homer 2001; Turnbull 1996), and one targeted women with major depressive disorder (Marks 2003). Two ongoing studies targeted 'vulnerable' or disadvantaged women (Cullinane 2021; Dickerson 2022).
There was wide variation in how socioeconomic status, ethnicity, and social risk factors were reported across all studies. Three studies reported area deprivation index measures (Fernandez Turienzo 2020; Tracy 2013; Turnbull 1996). Other socio‐economic indicators reported included employment (Gu 2013; Homer 2001; Kenny 1994; Marks 2003; McLachlan 2012; Rowley 1995), marital status (Flint 1989; Homer 2001; Marks 2003; North Stafford 2000; Rowley 1995; Turnbull 1996; Waldenstrom 2001), education (Biro 2000; Gu 2013; Harvey 1996; Homer 2001; Kenny 1994; McLachlan 2012; Waldenstrom 2001), income and home ownership (Flint 1989; McLachlan 2012; Waldenstrom 2001). Four studies did not report any measure of socio‐economic status (Begley 2011; Biro 2000; Hicks 2003; MacVicar 1993). Four studies reported ethnically diverse populations (Fernandez Turienzo 2020; Flint 1989; Homer 2001; Kenny 1994), five studies reported a majority white ethnic population (Begley 2011; Harvey 1996; Marks 2003; North Stafford 2000; Rowley 1995), and eight studies did not report the ethnicity of participants (Biro 2000; Gu 2013; Hicks 2003; MacVicar 1993; McLachlan 2012; Tracy 2013; Turnbull 1996; Waldenstrom 2001). Social risk factors were not reported in the majority of studies included, and those studies that did only reported specific risk factors, including migration status (Biro 2000; Kenny 1994), domestic violence and drug use (Fernandez Turienzo 2020), and the requirement for interpreter services (Kenny 1994). Three studies excluded women with specific social risk factors, including drug and alcohol use (Begley 2011; Rowley 1995), those who do not speak English (Biro 2000), or women who were receiving other specialist models of care due to social risk (Fernandez Turienzo 2020).
The midwife models of care were hospital‐based in five studies (Biro 2000; Gu 2013; MacVicar 1993; Rowley 1995; Waldenstrom 2001), or women were offered (i) antenatal care in an outreach community‐based clinic and intra‐ and postpartum care in a hospital (Homer 2001); (ii) ante‐ and postpartum community‐based care with intrapartum hospital‐based care (Hicks 2003; North Stafford 2000; Tracy 2013; Turnbull 1996); (iii) antenatal and postnatal care in the hospital and community settings with intrapartum hospital‐based care (Fernandez Turienzo 2020), or (iv) postnatal care in the community with hospital‐based ante‐ and intrapartum care (Flint 1989; Harvey 1996; Kenny 1994; Marks 2003; McLachlan 2012). Four studies offered intrapartum care in midwife birth centres in a maternity unit to all women in the trial (Waldenstrom 2001), or to women receiving midwife continuity of care only (Begley 2011; MacVicar 1993; Turnbull 1996). One study offered intrapartum care in a midwife birth centre in a maternity unit, and at home to all women in the trial according to local guidelines (Fernandez Turienzo 2020).
Excluded studies
We excluded 42 studies. Eleven studies were excluded as they were not a randomised trial (Bagheri 2021; Chapman 1986; Hailemeskel 2021; Hildingsson 2003; James 1988; Kildea 2021; Michel‐Schuldt 2021; Mortensen 2018; Qiu 2020; Runnerstrom 1969; Slome 1976). Two studies were excluded as the study closed before completion (Allen 2013; Kelly 1986). Twenty‐eight studies were excluded as the intervention was not a midwife continuity of care model or the comparator was not other models of care (Bergland 1998; Bergland 2007; Bernitz 2011; Brugha 2016; Byrne 2000; Chambliss 1991; de Wolff 2021; Famuyide 2014; Forster 2022; Giles 1992; Hans 2018; Heins 1990; Hundley 1994; Klein 1984; Law 1999; Lin 2020; Loy 2021; Mohammad‐Alizadeh‐Charandabi 2019; Morrison 2002; Nagle 2011; Ridgeway 2015; Stevens 1988; Tucker 1996; Waldenstrom 1997; Walker 2012; Wiggins 2020; Zelani 2011). One study was excluded as the population included additional data from outside the trial (Kildea 2017).
Risk of bias in included studies
See Figure 3 and Figure 4 for a summary of the risk of bias assessments.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirteen studies provided information on the methods used for generating randomisation sequences (Begley 2011; Biro 2000; Fernandez Turienzo 2020; Gu 2013; Harvey 1996; Hicks 2003; Homer 2001; MacVicar 1993; Marks 2003; McLachlan 2012; Rowley 1995; Tracy 2013; Turnbull 1996). Four studies did not offer enough information to make a clear assessment (Flint 1989; Kenny 1994; North Stafford 2000; Waldenstrom 2001).
Regarding allocation concealment, we considered 12 studies to have a low risk of bias (Begley 2011; Biro 2000; Fernandez Turienzo 2020; Gu 2013; Harvey 1996; Hicks 2003; Homer 2001; MacVicar 1993; McLachlan 2012; Tracy 2013; Turnbull 1996; Waldenstrom 2001). We judged four studies to have an unclear risk of bias: Kenny 1994, Marks 2003 and Rowley 1995 gave no information about the process of random allocation, while Flint 1989 used sealed, opaque envelopes without specifying any numbering. We considered the North Stafford 2000 trial, a cluster‐randomised trial, to have a high risk of bias for allocation concealment, as it was not possible to maintain concealment in this case.
Blinding
We judged 15 of the included studies as low risk for blinding of participants and personnel (Begley 2011; Biro 2000; Fernandez Turienzo 2020; Flint 1989; Gu 2013; Harvey 1996; Hicks 2003; Kenny 1994; MacVicar 1993; Marks 2003; McLachlan 2012; North Stafford 2000; Rowley 1995; Tracy 2013; Waldenstrom 2001), and two studies were at unclear risk of bias (Homer 2001; Turnbull 1996).
We judged two studies as low risk of bias for blinding of outcome assessment (Fernandez Turienzo 2020; McLachlan 2012). We considered three studies as high risk of bias (Homer 2001; Rowley 1995; Tracy 2013), and 12 at unclear risk of bias (Begley 2011; Biro 2000; Flint 1989; Gu 2013; Harvey 1996; Hicks 2003; Kenny 1994; MacVicar 1993; Marks 2003; North Stafford 2000; Turnbull 1996; Waldenstrom 2001).
Incomplete outcome data
We judged 13 of the included studies at low risk of bias for incomplete outcome data on the basis that the attrition rate was less than 20% for all outcomes (other than satisfaction), or missing outcome data were balanced across groups (Begley 2011; Biro 2000; Fernandez Turienzo 2020; Flint 1989; Gu 2013; Harvey 1996; Homer 2001; Kenny 1994; Marks 2003; McLachlan 2012; Tracy 2013; Turnbull 1996; Waldenstrom 2001). Two of the studies did not provide sufficient information on loss to follow‐up and we judged them as unclear (MacVicar 1993; Rowley 1995), and we judged two studies as at high risk of detection bias (Hicks 2003; North Stafford 2000).
Selective reporting
All outcomes stated in the methods section were adequately reported in the results of 14 studies (Biro 2000; Fernandez Turienzo 2020; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; Marks 2003; McLachlan 2012; North Stafford 2000; Rowley 1995; Turnbull 1996; Waldenstrom 2001). We judged two trials to be at unclear risk of bias due to selective reporting (Begley 2011; Tracy 2013), and one to be at high risk of reporting bias (Gu 2013).
Other potential sources of bias
In most included studies, no other potential sources of bias were identified. However, we considered the risk of bias in Tracy 2013 to be unclear, as a small number of women crossed over between the study arms.
Effects of interventions
Comparison 1 (main comparison): midwife continuity of care models versus other models of care for childbearing women and their infants ‐ all trials
The certainty of the evidence is reported for the seven outcomes specified in the summary of findings table (see Table 1). Pre‐specified subgroup analyses for each primary outcome (only) are presented separately under their respective outcomes.
Primary outcomes
1.1 Spontaneous vaginal birth
Midwife continuity of care models likely increase spontaneous vaginal birth compared to other models of care (average risk ratio (RR) 1.05, 95% confidence interval (CI) 1.03 to 1.07; I2 = 9%; 15 studies, 17,864 participants; Analysis 1.1). This translates to an absolute increase from 66% in those assigned to other models of care to 70% (68% to 71%) in those assigned to midwife continuity of care. We judged the certainty of evidence as moderate, downgrading for risk of bias (‐1).
1.1. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 1: Spontaneous vaginal birth (as defined by trial authors)
Subgroup: variation in midwife continuity of care models (caseload/one‐to‐one or team)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.01, df = 1 (P = 0.91), I² = 0%; Analysis 2.1).
2.1. Analysis.

Comparison 2: Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team), Outcome 1: Spontaneous vaginal birth (as defined by trial authors)
Subgroup: variation in risk status (low versus mixed)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.05, df = 1 (P = 0.82), I² = 0%; Analysis 3.1).
3.1. Analysis.

Comparison 3: Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed), Outcome 1: Spontaneous vaginal birth (as defined by trial authors)
Subgroup: variation in country setting (very High Human Development Index (HDI) > 0.8 versus high, medium, and low HDI)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 3.99, df = 1 (P = 0.05), I² = 75.0%; Analysis 5.1).
5.1. Analysis.

Comparison 5: Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), Outcome 1: Spontaneous vaginal birth (as defined by trial authors)
Subgroup: variation in social risk factors (women with social risk versus all women)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women with social risk factors, which precluded the assessment of subgroup differences.
1.2 Caesarean birth
Midwife continuity of care models likely reduce caesarean section birth compared to other models of care (average RR 0.91, 95% CI 0.84 to 0.99; I2 = 25%; 16 studies, 18,037 participants; Analysis 1.2). This translates to an absolute decrease from 16% in those assigned to other models of care to 15% (14% to 16%) in those assigned to midwife continuity of care. We judged the certainty of evidence as moderate, downgrading for risk of bias (‐1).
1.2. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 2: Caesarean birth
Subgroup: variation in midwife continuity of care models (caseload/one‐to‐one or team)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.02, df = 1 (P = 0.87), I² = 0%; Analysis 2.2).
2.2. Analysis.

Comparison 2: Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team), Outcome 2: Caesarean birth
Subgroup: variation in risk status (low versus mixed)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.47, df = 1 (P = 0.49), I² = 0%; Analysis 3.2).
3.2. Analysis.

Comparison 3: Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed), Outcome 2: Caesarean birth
Subgroup: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 3.49, df = 1 (P = 0.06), I² = 71.3%; Analysis 5.2).
5.2. Analysis.

Comparison 5: Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), Outcome 2: Caesarean birth
Subgroup: variation in social risk factors (women with social risk factors versus all women)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women with social risk factors, which precluded the assessment of subgroup differences.
1.3 Regional analgesia (epidural/spinal)
We are very uncertain about the effects of midwife continuity of care models compared to other models of care on the likelihood of regional analgesia (average RR 0.85, 95% CI 0.79 to 0.92; I2 = 51%; 15 studies, 17,754 participants; Analysis 1.3). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1), inconsistency (‐1), and publication bias (‐1).
1.3. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 3: Regional analgesia (epidural/spinal)
Subgroup: variation in midwife continuity of care models (caseload/one‐to‐one or team)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 1.94, df = 1 (P = 0.16), I² = 48.5%; Analysis 2.3).
2.3. Analysis.

Comparison 2: Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team), Outcome 3: Regional analgesia (epidural/spinal)
Subgroup: variation in risk status (low versus mixed)
Subgroup analysis for which data are available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 2.16, df = 1 (P = 0.14), I² = 53.8%; Analysis 3.3).
3.3. Analysis.

Comparison 3: Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed), Outcome 3: Regional analgesia (epidural/spinal)
Subgroup: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI)
In this subgroup analysis, there were no data available for one of the subgroups, specifically women from high, medium, and low HDI countries, which precluded the assessment of subgroup differences.
Subgroup: variation in social risk factors (women with social risk factors versus all women)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women with social risk factors, which precluded the assessment of subgroup differences.
1.4 Intact perineum
Midwife continuity of care models compared to other models of care likely result in little to no difference in intact perineum (average RR 1.05, 95% CI 0.98 to 1.12; I2 = 40%; 12 studies, 14,268 participants; Analysis 1.4), with the absolute rates being approximately 29% under other care models and about 31% (with a range of 29% to 33%) under midwife continuity of care. We judged the certainty of evidence as moderate, downgrading for risk of bias (‐1).
1.4. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 4: Intact perineum
Subgroup: variation in midwife continuity of care models (caseload/one‐to‐one or team)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.30, df = 1 (P = 0.58), I² = 0%; Analysis 2.4).
2.4. Analysis.

Comparison 2: Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team), Outcome 4: Intact perineum
Subgroup: variation in risk status (low versus mixed)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 2.16, df = 1 (P = 0.14), I² = 53.8%; Analysis 3.4).
3.4. Analysis.

Comparison 3: Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed), Outcome 4: Intact perineum
Subgroup: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI)
In this subgroup analysis, there were no data available for one of the subgroups, specifically women from high, medium, and low HDI countries, which precluded the assessment of subgroup differences.
Subgroup: variation in social risk factors (women with social risk factors versus all women)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women with social risk factors, which precluded the assessment of subgroup differences.
1.5 Fetal loss at or after 24 weeks gestation
We are very uncertain about the effect of midwife continuity of care models on fetal loss at or after 24 weeks gestation (average RR 1.24, 95% CI 0.73 to 2.13; I2 = 0%; 12 studies, 16,122 participants; Analysis 1.5). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1) and imprecision (‐2).
1.5. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 5: Fetal loss at or after 24 weeks gestation
Subgroup: variation in midwife continuity of care models (caseload/one‐to‐one or team)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 1.49, df = 1 (P = 0.22), I² = 33.0%; Analysis 2.5).
2.5. Analysis.

Comparison 2: Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team), Outcome 5: Fetal loss at or after 24 weeks gestation
Subgroup: variation in risk status (low versus mixed)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.13, df = 1 (P = 0.72), I² = 0%; Analysis 3.5).
3.5. Analysis.

Comparison 3: Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed), Outcome 5: Fetal loss at or after 24 weeks gestation
Subgroup: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI)
In this subgroup analysis, there were no available data for one of the subgroups, specifically fetal loss from women from high, medium, and low HDI countries, which precluded the assessment of subgroup differences.
Subgroup: variation in social risk factors (women with social risk factors versus all women)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women with social risk factors, which precluded the assessment of subgroup differences.
1.6 Preterm birth (< 37 weeks)
Midwife continuity of care models compared to other models of care may result in little to no difference in preterm birth (< 37 weeks) (average RR 0.95, 95% CI 0.78 to 1.16; I2 = 45%; 10 studies, 13,850 participants; Analysis 1.6). Accordingly, the absolute risk of preterm birth remains similar across both care models, estimated at around 6% for both groups, with a possible range of 5% to 7% for midwife continuity of care, reflecting the statistical uncertainty. We judged the certainty of evidence as low due to downgrading for risk of bias (‐1) and inconsistency (‐1).
1.6. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 6: Preterm birth (< 37 weeks)
Subgroup: variation in midwife continuity of care models (caseload/one‐to‐one or team)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.13, df = 1 (P = 0.72), I² = 0%; Analysis 2.6).
2.6. Analysis.

Comparison 2: Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team), Outcome 6: Preterm birth (< 37 weeks)
Subgroup: variation in risk status (low versus mixed)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.11, df = 1 (P = 0.74), I² = 0%; Analysis 3.6).
3.6. Analysis.

Comparison 3: Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed), Outcome 6: Preterm birth (< 37 weeks)
Subgroup: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women from high, medium, and low HDI countries, which precluded the assessment of subgroup differences.
Subgroup: variation in social risk factors (women with social risk factors versus all women)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women with social risk factors, which precluded the assessment of subgroup differences.
1.7 Neonatal death (baby born alive at any gestation and dies within 28 days)
We are very uncertain about the effect of midwife continuity of care models on neonatal death (baby born alive at any gestation and dies within 28 days) (average RR 0.85, 95% CI 0.43 to 1.71; I2 = 0%; 10 studies, 14,718 participants; Analysis 1.7). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1) and imprecision (‐2).
1.7. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 7: Neonatal death (baby born alive at any gestation and dies within 28 days)
Subgroup: variation in midwife continuity of care models (caseload/one‐to‐one or team)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.63, df = 1 (P = 0.43), I² = 0%; Analysis 2.7).
2.7. Analysis.

Comparison 2: Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team), Outcome 7: Neonatal death (baby born alive at any gestation and dies within 28 days)
Subgroup: variation in risk status (low versus mixed)
Subgroup analysis for which data were available reveals no significant subgroup differences or interactions, indicating a consistent effect across subgroups (test for subgroup differences: Chi² = 0.10, df = 1 (P = 0.76), I² = 0%; Analysis 3.7).
3.7. Analysis.

Comparison 3: Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed), Outcome 7: Neonatal death (baby born alive at any gestation and dies within 28 days)
Subgroup: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women from high, medium, and low HDI countries, which precluded the assessment of subgroup differences.
Subgroup: variation in social risk factors (women with social risk factors versus all women)
In this subgroup analysis, there were no available data for one of the subgroups, specifically women with social risk factors, which precluded the assessment of subgroup differences.
Secondary outcomes
When compared to other models of care, midwife continuity of care models likely reduce Instrumental vaginal birth (forceps/vacuum) (average RR 0.89, 95% CI 0.83 to 0.96; I2 = 0%; 14 studies, 17,769 participants; Analysis 1.11). This translates to an absolute decrease from 14% in those assigned to other models of care to 13% (12% to 14%) in those assigned to midwife continuity of care. We judged the certainty of evidence as moderate, downgrading for risk of bias (‐1).
1.11. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 11: Instrumental vaginal birth (forceps/vacuum)
Compared to other care models, midwife continuity of care models may reduce Episiotomy (average RR 0.83, 95% CI 0.77 to 0.91; I2 = 44%; 15 studies, 17,839 participants; Analysis 1.12). This translates to an absolute decrease from 23% in those assigned to other models of care to 19% (17% to 21%) in those assigned to midwife continuity of care. We judged the certainty of evidence as low, downgrading for risk of bias (‐1) and inconsistency (‐1).
1.12. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 12: Episiotomy
Midwife continuity of care models likely result in little to no difference in:
Induction of labour (average RR 0.92, 95% CI 0.85 to 1.00; I2 = 41%; 14 studies, 17,666 participants; Analysis 1.10), with the absolute rates being approximately 22% under other care models and about 21% (with a range of 19% to 22%) under midwife continuity of care. We judged the certainty of evidence as low, downgrading for risk of bias (‐1) and inconsistency (‐1).
Postpartum haemorrhage (average RR 0.92, 95% CI 0.82 to 1.03; I2 = 0%; 11 studies, 14,407 participants; Analysis 1.14), with the absolute rates being approximately 9% under other care models and about 8% (with a range of 7% to 9%) under midwife continuity of care. We judged the certainty of evidence as moderate, downgrading for risk of bias (‐1).
Breastfeeding initiation (average RR 1.06, 95% CI 1.00 to 1.12; I2 = 87%, 8 studies, 8575 participants; Analysis 1.15), with the absolute rates being approximately 69% under other care models and about 73% (with a range of 69% to 78%) under midwife continuity of care. We judged the certainty of evidence as low, downgrading for risk of bias (‐1) and inconsistency (‐1).
Birth weight less than 2500 g (average RR 0.92, 95% CI 0.79 to 1.08; I2 = 0%; 8 studies, 12,420 participants; Analysis 1.20), with the absolute rates being approximately 5% under other care models and the same (5% with a range of 4% to 6%) under midwife continuity of care. We judged the certainty of evidence as low, downgrading for risk of bias (‐1) and imprecision (‐1).
Admission to special care nursery/neonatal intensive care unit (average RR 0.89, 95% CI 0.77 to 1.03; I2 = 41%; 13 studies, 16,260 participants; Analysis 1.23), with the absolute rates being approximately 9% under other care models and about 8% (with a range of 7% to 9%) under midwife continuity of care. We judged the certainty of evidence as moderate, downgrading for risk of bias (‐1).
1.10. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 10: Induction of labour
1.14. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 14: Postpartum haemorrhage (as defined by trial authors)
1.15. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 15: Breastfeeding initiation
1.20. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 20: Birth weight less than 2500 g
1.23. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 23: Admission to special care nursery/neonatal intensive care unit
We are very uncertain about the effect of midwife continuity of care models compared to other models of care on:
Third or fourth‐degree tear (average RR 1.10, 95% CI 0.81 to 1.49; I2 = 0%; 7 studies, 9437 participants; Analysis 1.13). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1) and imprecision (‐2).
Maternal readmission within 28 days (average RR 1.52, 95% CI 0.78 to 2.96; I2 = NA, 1 study, 1195 participants; Analysis 1.16). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1) and imprecision (‐2).
Attendance at birth by a known midwife (average RR 9.13, 95% CI 5.87 to 14.21; I2 = 95%; 11 studies, 9273 participants; Analysis 1.18). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1), inconsistency (‐1), and publication bias (‐1).
Apgar score less than or equal to seven at five minutes (average RR 0.95, 95% CI 0.72 to 1.24; I2 = 22%; 13 studies, 12,806 participants; Analysis 1.22). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1) and imprecision (‐2).
Fetal loss before 24 weeks gestation (average RR 0.82, 95% CI 0.67 to 1.01; I2 = 0%; 12 studies, 15,913 participants; Analysis 1.24). We judged the certainty of evidence as very low, downgrading for risk of bias (‐1) and imprecision (‐2).
1.13. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 13: Third or fourth degree tear
1.16. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 16: Maternal readmission within 28 days
1.18. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 18: Attendance at birth by known midwife
1.22. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 22: Apgar score less than or equal to 7 at 5 minutes
1.24. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 24: Fetal loss before 24 weeks gestation
Three studies assessed maternal death (Begley 2011; Fernandez Turienzo 2020; McLachlan 2012). There were no reported cases in either group across all three studies (Analysis 1.9). The lack of events precludes calculating a risk ratio or assessing differences between midwife continuity of care and other care models.
1.9. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 9: Maternal death
No data were provided in any study for the outcomes of:
Healthy mother
Neonatal readmission within 28 days
Healthy baby
Birth weight equal to or more than 4000 g
Data were reported for the following outcomes:
Maternal experience
Cost
However, the data were too heterogeneous to be meta‐analysed. As a result, we report these findings narratively rather than as part of a quantitative comparison with other outcomes.
Maternal experience
Due to the lack of consistency in conceptualising and measuring women's experiences with their maternity care, a narrative synthesis of such data is presented. A total of 16 studies reported maternal experiences and/or satisfaction with various components of maternity care and childbirth (Biro 2000; Begley 2011; Fernandez Turienzo 2020; Flint 1989; Gu 2013; Harvey 1996; Homer 2001; Hicks 2003; Kenny 1994; MacVicar 1993; Marks 2003; McLachlan 2012; Rowley 1995; Turnbull 1996; Waldenstrom 2001). Ten studies reported satisfaction (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; MacVicar 1993; Kenny 1994; Tracy 2013; Turnbull 1996; Waldenstrom 2001), three reported women's experiences (Fernandez Turienzo 2020; Homer 2001; Tracy 2013), one reported women’s experiences within which satisfaction was a component (McLachlan 2012), and two reported women’s psychological state and satisfaction (Gu 2013; Marks 2003).
The concept of women’s experiences of their maternity care is complex (Beecher 2020), and concerns have been expressed about the methodological and psychometric quality of self‐report survey instruments to evaluate those experiences (Beecher 2021). It was not surprising to find inconsistency in instruments and adaptations, including variations in psychometric properties, timing of administration, and outcomes used to 'measure' women’s experiences across studies. Because of such heterogeneity and, as is common, survey response rates of lower than 80% for most studies, meta‐analysis for this outcome was considered inappropriate and was not conducted.
Satisfaction outcomes as reported in the included studies included maternal satisfaction with different domains of antenatal, intrapartum, and postnatal care, i.e. venue/location of care and number of visits; health care provider seen and relationship, continuity, and access; choice, information, and decision‐making; availability of interpreters; knowledge and preparation for labour and birth, fetal monitoring, pain management, control in childbirth; staff attitudes and behaviours; support and length of postnatal stay. One study used the Labour and Delivery Satisfaction Index (LDSI) and the Attitudes about Labour and Delivery Experience (ADLE) questionnaire at two weeks postpartum, including a Six Simple Questions (SSQ) questionnaire at 36 weeks gestation and 48 hours, two and six weeks postpartum, to measure fluctuations in satisfaction (Harvey 2002). Three studies assessed perceptions of control in labour using a three‐point scale (Flint 1989) and the Labour Agentry Scale between six and eight weeks and two and three years after birth (Begley 2011; Fernandez Turienzo 2021). Begley 2011 also used a performance, importance, and quality impact framework to measure satisfaction by assessing expectations against which women rate the importance of that expectation. Reports of two studies (Shields 1997; Waldenstrom 2000) included the Edinburgh Postnatal Depression Scale (EPDS) and Shields 1997 also examined women’s ratings of the structure of postnatal care, preparation for parenthood, postnatal depression, and support and advice with infant feeding. In brief, most of the included studies showed a higher level of satisfaction with most aspects of maternity care in midwife continuity compared to the other models of care.
Women’s experiences outcomes, as reported in included studies, included views and perceptions of care throughout the continuum, including their midwives and trust in them; safety and quality of care (i.e. access, clinic arrangements, continuity, communication, control); emotional, practical, and social support, and quality of life. Three reports from Tracy 2013 included postnatal surveys at six weeks and six months (including qualitative free‐text responses) to measure experiences of the quality of antenatal and early labour care (Allen 2019; Allen 2020) and to explore how women characterised their midwives (Allen 2017). Fernandez Turienzo 2021 used both qualitative interviews and postnatal surveys at six to eight weeks after birth that incorporated various scales (i.e. the Social Support Scale, Trust in Nurses Scale ‐ Adapted for Midwives, Perceptions of Safety Scale, Labour Agentry Scale, Mother‐Infant Bonding Scale, PROMIS‐10 global). Overall, continuity models of care in both studies showed higher levels and better experiences across measures of trust, safety, quality of care, support, bonding, and physical health postnatally. Two reports from Homer 2001 using adapted antenatal and postnatal questionnaires at eight to 10 weeks also found clear benefits for women who receive continuity of care (Homer 2002). They emphasised the importance of successful transfer services into community‐based settings (Homer 2000). Continuity midwives were described as empowering and going above and beyond such that women feel empowered, nurtured, and safe during their maternity journey (Allen 2017), yet regardless of the model of care, early labour care was primarily described negatively (Allen 2020).
McLachlan 2016 and Forster 2016 measured women's childbirth experience and satisfaction throughout the childbearing period using an adapted postal survey two months after birth based on previous studies of similar models of care in Australia. Women receiving caseload midwife continuity of care reported significantly higher overall satisfaction ratings with antenatal, intrapartum, hospital postnatal care, and home‐based postnatal care. They reported a more positive experience of pain overall and more often reported feeling very proud of themselves. Women also felt more in control and more able to cope physically and emotionally. Gu 2013 used the Chinese version State‐Trait Anxiety Inventory (C‐STAI) to measure women’s psychological state on admission to the labour and delivery room and a validated questionnaire to measure satisfaction with antenatal, intrapartum, and postnatal care at 42 days postpartum. Overall, women’s mean anxiety scores were not different between the midwife continuity model and other model, but at admission to the labour delivery room, the anxiety scores were lower in the continuity model. These women also reported greater satisfaction with maternity care. Similarly, Marks 2003 used both the EPDS at antenatal baseline and six to eight weeks after birth and the Maternity Services Questionnaire and found no differences in psychosocial outcomes, but midwife continuity was highly successful at engaging women with mental health problems in treatment.
Health economic analysis
No study included a definitive health economic evaluation to determine the cost‐effectiveness of the interventions under consideration ‐ rather the available health economics analysis comprised various forms of cost analysis. Seven trials included studies that reported costs for the compared models of maternity care (Flint 1987; Homer 2001; Kenny 1994; Kenny 2015; Rowley 1995; Tracy 2013; Young 1997). As findings from health economic analyses generally vary according to the structure of the healthcare system in a given country, the resource allocation, pricing and reimbursement mechanisms employed, the study perspective, design and the type of data collected, and the cost and outcome variables included in the analysis, considerable heterogeneity exists. Therefore, a narrative synthesis of the health economic data was undertaken and is presented below.
Flint 1987 (full report, providing cost analysis for Flint 1989) reported a cost analysis from the healthcare perspective to compare midwife continuity of care with standard care. The authors stated that it was impossible to accurately assess and compare the total care costs. Instead, they reported a limited cost comparison for epidural resource use. They demonstrated lower total costs for women in the midwife continuity of care group compared to the standard care group (88 x GBP 220 = GBP 19,360 versus 143 x GBP 220 = GBP 31,460 respectively), representing an overall epidural care total cost saving (GBP 12,100). In addition, in a subgroup analysis of the midwife continuity of care (n = 51) and standard care (n = 49) arms, the authors reported that antenatal consultation costs were 20% to 25% cheaper for the midwife continuity of care arm due to differences in staff costs. The authors did not report the results of a formal statistical analysis of the cost variables.
Kenny 1994 undertook a cost analysis, adopting a healthcare perspective, to compare the costs of the midwife continuity of care and other models of care groups. The cost analysis included healthcare resource usage during the antenatal, intrapartum, and postnatal periods. The cost in the antenatal period was assessed based on the number of antenatal visits, with average cost estimates of AUD 119 versus AUD 123 for low‐risk women and AUD 390 versus AUD 437 for high‐risk women, in the midwife continuity of care and other models of care groups, respectively. The cost of intrapartum care was assessed based on the midwife's attendance time and forceps delivery. This was estimated at costs of AUD 219 versus AUD 220 in the respective intervention and control groups. In the context of postnatal care, there was a shorter length of hospital stay in the midwife continuity of care arm, but they had more visits. The total postnatal care cost was estimated at AUD 745, on average, for the midwife continuity of care arm and AUD 833 for the other models of care arm. In terms of total cost, the study reported an average cost saving of AUD 98 or 8% per woman (i.e. AUD 1122 for midwife continuity of care versus AUD 1220 for standard care). The authors emphasised that incremental estimates only included the sum of those individual resource costs, with significant differences between the two programmes, and did not represent the full cost of intrapartum care or maternity care.
Rowley 1995 reported a cost analysis from the healthcare perspective to compare the continuity of care arm to the routine care arm. Hospital‐related costs were estimated directly using the Australian Diagnostic‐Related Groups (AN‐DRGs) methodology, a classification system that links groupings of acute episodes of care to resource usage and treatment costs. The cost analysis included a range of maternity care resources, including antenatal services, midwife salaries, mode of delivery, and neonatal intensive care. The study reported significant differences in related AN‐DRG resource requirements across treatment groups and hence in costs. The average cost per birth, estimated based on a combination of antenatal service, mode of birth, and neonatal intensive care costs, was AUD 3324 for the midwife continuity care arm and AUD 3475 for the routine care arm, resulting in a saving of AUD 151, or 4.5%. The authors did not report the results of a formal statistical analysis of the cost variables, but they did so for the AN‐DRG counts on which these cost estimates were based.
Young 1997 conducted a comprehensive cost analysis to compare the midwife‐managed versus standard shared care models evaluated Turnbull 1996 . The cost analysis was undertaken from the healthcare perspective (i.e. National Health Service (NHS)) and used an individual patient‐based costing approach. A key consideration in the cost analysis was the assumption made with respect to the number of caseloads per midwife, but the authors explicitly explored this assumption in sensitivity analysis. The cost analysis covered a wide range of resource use activities, including antenatal clinic visits, day care attendances, antenatal admissions, tests and drugs, mode of birth, postnatal stay in the hospital, and postnatal home visits. In addition, the authors included estimates of capital costs for continuous electronic fetal heart monitoring equipment, overhead costs related to hospital portering, administration, heat and light allocated to departments, and healthcare personnel costs, such as those for midwives, obstetricians, and general practitioners. Findings were reported for the subtotal cost categories for antenatal, intrapartum, and postpartum care. At the base‐case assumption of a median caseload of 29 women per midwife, the average cost of midwife‐managed care was not significantly different from the standard shared care group for antenatal care (GBP 288 versus GBP 296, P = 0.48) and intrapartum care (GBP 241 versus GBP 241, P = 0.40). However, the average cost of postpartum care for the midwife continuity of care was higher than the standard group (GBP 470 versus GBP 352, P < 0.001). The lack of differences in costs for antenatal care and intrapartum care between the midwife‐managed and standard groups and, in particular, the higher costs of the midwife continuity of care compared to the standard care in the postnatal period, were explained to be mainly due to differences in the organisation of care, including different grades of midwives, locations of care, and scale of the programmes for each arm. The authors employed an alternative assumption of 29 women per midwife caseload in the sensitivity analysis used. In this case, the average cost for antenatal care was lower for the midwife continuity of care group (GBP 275 versus GBP 296, P = 0.05) but was higher for postnatal care cost (GBP 444 versus GBP 397, P < 0.01). The authors recommended that a health economic evaluation should be conducted.
Homer 2001 conducted a comprehensive cost analysis alongside a randomised controlled trial to compare the STOMP continuity of care model versus standard care. The cost analysis was undertaken from the healthcare perspective. The cost analysis covered various resource‐use activities, including antenatal care, intrapartum care, and postnatal care. The individual service components included antenatal clinic visits and admissions, day assessment unit, labour and birth, hospital and domiciliary postnatal care, and neonatal admissions to the special care nursery. In addition, a 24‐hour on‐call cover service was provided for women in labour in the midwife continuity of care group and was costed accordingly. A total cost variable was generated by adding the individual resource costs for these components, and mean costs were estimated and analysed in statistical analysis. The mean cost per woman in the continuity of care arm was estimated at AUD 2579 (95% CI 2236 to 2974), compared to AUD 3483 (95% CI 2864 to 4188) for the control group. This represented a non‐statistically significant cost saving of AUD 904 or 25.9% per woman. The author concluded that the STOMP model could be implemented within current resources if organisations were firmly committed to change.
Tracy 2013 reported a cost analysis from the healthcare perspective to compare midwifery caseload care and standard maternity care based on data collected from a randomised controlled trial. Hospital‐related costs were estimated directly using the Australian Diagnostic‐Related Groups (AN‐DRGs) methodology, which is a classification system that links groupings of acute episodes of care to resource usage and treatment costs. The cost analysis included a range of maternity care resources, including the length of hospital stay, midwifery and obstetric clinical time, use of operating theatres, laboratory tests, imaging, wards, allied health, pharmacy, capital depreciation, and clinical overheads. Neonatal costs were not included. Total costs for each full episode of maternity care were estimated from the sum of the services provided to the woman for the duration of her stay. Overall, the median cost for the caseload midwifery arm was AUD 4628.27 (95% CI 2698.89 to 7164.96), compared to AUD 5903.67 (95% CI 3220.39 to 7541.55) for the standard care arm, resulting in a statistically significant cost saving of AUD 566.74 (95% 106.17 to 1027.30; P = 0.02) per woman. The authors suggest this saving was predominantly driven by the lower levels of unassisted vaginal birth in the midwifery arm. Further, they caution on the role of high‐cost outliers due to serious medical disorders, surgical complications, or accidental causes.
Kenny 2015 conducted a comprehensive costs analysis to compare the midwife‐led versus standard consultant‐led models of care evaluated in the Begley 2011 randomised controlled trial. The cost analysis was undertaken from the healthcare perspective. The cost analysis covered a wide range of resource use activities, including those related to antenatal visits, ultrasonography and cardiotocography, care in labour, provision of epidurals, antenatal, postnatal, and neonatal bed days, postnatal home visits, mode of delivery, administration, and overheads. A total cost variable was generated, and mean costs were estimated and analysed statistically. The mean cost per woman in the midwife continuity of care group was EUR 2598 (95% CI 2527 to 2670), compared to EUR 2780 (95% CI 2527 to 2670) in the standard consultant‐led group, resulting in an average cost saving of EUR 182 (95% CI 33 to 330) or 6.5% per woman.
In summary, seven studies employing various health economic methods and cost analysis presented cost data findings. Regardless of the cost analysis methods and the resource components included in the analyses, for those studies that reported overall total cost estimates (four studies), cost savings per woman (Rowley 1995: 4.3%, Begley 2011: 6.5%, Kenny 1994: 8.0%, and Homer 2001: 25.9%) were demonstrated for midwife continuity of care models relative to standard care. Overall cost savings were suggested to be influenced by multiple individual resource categories, including staff salaries, number, location and length of antenatal visits, length of hospital stay, use of interventions, mode of delivery, neonatal intensive care, and the caseload of women allocated to maternity care models.
Investigation of heterogeneity
The I² value was greater than 50% for three outcomes: regional analgesia (epidural/spinal) (I² = 51%) (Analysis 1.3), attendance at birth by a known midwife (I² = 95%) (Analysis 1.18), and breastfeeding initiation (I² = 87%) (Analysis 1.15). We downgraded the certainty of the evidence accordingly.
We note that outcomes with relatively low heterogeneity, such as caesarean section, instrumental vaginal birth, and postpartum haemorrhage, tend to be more consistent across studies. We suggest that this may be due to the influence of standardised clinical guidelines and protocols. These universally accepted practices may lead to more similar treatment effects within midwife continuity of care models and other models of maternity care. Conversely, outcomes with higher heterogeneity, as above, may be heavily impacted by factors that vary across different care models, populations, and study designs. These factors could include cultural norms, individual preferences (woman and clinician), clinical decision‐making processes, and the specific support provided in each care model. Ultimately, the degree of heterogeneity observed in some outcomes highlights the complex interplay of various factors that influence the effectiveness and treatment effects of different maternity care models.
Investigation of publication bias
When we assessed the study results using funnel plots and Egger's tests (performed with SPSS software) for groups of 10 or more studies, we found that most of the outcomes showed little evidence of publication bias. However, we suspected publication bias for regional analgesia (Analysis 1.3) and attendance at birth by a known midwife (Analysis 1.18).
Sensitivity analyses
We performed a sensitivity analysis excluding the cluster‐randomised North Staffordshire trial from all outcomes in the primary comparison (comparison 1) for which it had contributed data (North Stafford 2000). This exclusion did not alter the findings for any outcome, as the results remained consistent with the overall findings when all trials were included.
We also conducted sensitivity analyses to investigate the impact of risk of bias on our findings by repeating the analysis and retaining only those studies with low risk of bias for random sequence generation, allocation concealment, and incomplete outcome data to assess whether this would change the overall results. We discovered that the differences from the overall analyses were minor. The primary effect was that confidence intervals were slightly wider due to the reduced number of trials in the analysis. However, none of the conclusions drawn from the analysis were affected.
Discussion
Summary of main results
This review synthesises evidence from 17 studies involving 18,533 randomised women in five countries in a wide variety of settings and health systems. All studies involved midwife continuity of care models that included either team or caseload midwifery, women classified as at low, mixed, or high risk, and in high and middle‐income settings. All trials included qualified midwives, and none included lay or traditional midwives. There was wide variation in how socioeconomic status, ethnicity, and social risk factors were reported across all studies.
The studies compared midwife continuity of care throughout the antepartum and the intrapartum period (and postnatal period where offered) with other models of care. The latter involved obstetricians, family physicians, or both, collaborating with nurses and midwives in various organisational settings. Studies included models of care that offered intrapartum care in hospitals, midwife birth centres co‐located in a maternity unit, and home birth.
Midwife continuity of care models, as compared to other models of care, likely increase spontaneous vaginal birth, reduce caesarean sections and instrumental vaginal birth (forceps/vacuum), and may reduceepisiotomy.
It is likely that midwife continuity of care models, as compared to other models of care, result in little to no difference in intact perineum, postpartum haemorrhage, and admission to special care nursery/neonatal intensive care unit. There may be little or no difference in preterm birth (< 37 weeks), induction of labour, breastfeeding initiation, and birth weight less than 2500 g.
We are very uncertain about the effect of midwife continuity of care models, as compared to other models of care, on regional analgesia, fetal loss at or after 24 weeks gestation, neonatal death, third or fourth‐degree tear, maternal readmission within 28 days,attendance at birth by a known midwife, Apgar score less than or equal to seven at five minutes and fetal loss before 24 weeks gestation.
No maternal deaths were reported across three studies. Although the observed risk of adverse events was similar between midwifery continuity of care models and other models, our confidence in the findings was limited. Our confidence in the findings was lowered by possible risk of bias, inconsistency, and imprecision of some estimates.
No data were provided in any study for the outcomes of healthy mother, neonatal readmission within 28 days, healthy baby, and birth weight equal to or more than 4000 g.
Maternal experiences and cost implications were described narratively. Women receiving care from midwife continuity of care models, as opposed to other care models, generally reported more positive experiences during pregnancy, labour, and postpartum. Cost savings were noted in the antenatal and intrapartum periods in midwife continuity of care models.
Subgroup analyses were conducted based on variations in midwife continuity of care models (caseload/one‐to‐one or team), risk status (low versus mixed), country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), and maternal social risk factors (women with social risk factors versus all women). The subgroup analyses revealed no differences or interactions for any primary outcomes, indicating consistent effects across the subgroups. However, data for some subgroups, particularly women with social risk factors and those from countries with low, medium, and high HDI, were limited or absent, indicating that further research is needed to better understand these groups' outcomes and improve equity.
Overall completeness and applicability of evidence
This review supersedes the previous review published in 2016. See Differences between protocol and review for further details.
Informed by the latest Cochrane methodology and Cochrane Pregnancy and Childbirth trustworthiness assessments, data extraction and assessment of all studies in the review have been rechecked independently by two authors in this update and re‐entered. We made several outcome changes to ensure clinical and policy relevance. This resulted in changes to the certainty of outcomes from the 2016 review.
For preterm birth, the effect estimate moved from showing an effect to maybe little or no difference. We identified and corrected a transcription error in Begley 2011 and updated some data items following additional author correspondence. Other minor variations identified in comparison with the 2016 review were also addressed. This involved a change in both the number of events and denominators due to additional information being reported in Kenny 1994 and McLachlan 2012.
We found a decrease in caesarean birth in the midwife continuity of care models, a new finding in this review update. However, the mechanisms are undetermined.
Two subgroups were added: 'Women with social risk factors versus all women' and 'Countries with very high Human Development Index (HDI) > 0.8 versus high, medium, and low'.
Sixteen studies reported maternal experiences and/or satisfaction with various components of maternity care and childbirth. Due to the lack of consistency in conceptualising and measuring women's experiences with their maternity care, a narrative synthesis of such data is presented. The concept of women’s experiences of their maternity care is complex, and concerns have been expressed about the methodological and psychometric quality of self‐report survey instruments to evaluate those experiences. It was not surprising to find inconsistency in instruments, including their psychometric properties, timing of administration, and outcomes used to 'measure' women’s experiences across studies. Because of such heterogeneity and, as might be expected, response rates of lower than 80% for most studies, meta‐analysis for this outcome was considered inappropriate and was not conducted. Nonetheless, most of the included studies showed a more positive experience in various aspects of care in the midwife continuity models compared to the other models of care.
Findings from economic analyses vary according to the structure of health care in a given country, the type of data collected, and what factors are included in the modelling. Because of this heterogeneity, a narrative synthesis of such data is presented. Seven studies presented cost data using different economic evaluation methods. Most included studies suggest a cost‐saving effect in intrapartum and antenatal care associated with midwife continuity of care models. Regardless of the cost analysis methods and the components included in the analyses, for those who reported an overall cost estimation (four studies), cost savings per woman are demonstrated for midwife continuity of care models. Cost savings are suggested to be mainly influenced by staff salaries, number/location and length of antenatal visits, length of hospital stay, use of interventions, and the number of women allocated to maternity care models. More transparent and consistent approaches to cost analysis and health economic evaluation are required for robust cost‐effectiveness assessments and evidence synthesis in this important field.
Quality of the evidence
The quality of the evidence varied across the included studies, with some outcomes demonstrating high‐quality evidence and others presenting more uncertainty.
Most of the included studies had a low risk of bias for random sequence generation, allocation concealment, and incomplete outcome data, suggesting that the methodology used in these studies was generally rigorous. However, blinding of participants and personnel was a challenge in most studies, as it is often difficult to achieve complete blinding in trials comparing different models of care. This limitation was addressed by revising the approach for judging studies with clear evidence of a lack of blinding. This allowed for a more accurate assessment of the potential risk of performance bias and ultimately enhanced the quality and reliability of the review findings.
Regarding the blinding of outcome assessment, the risk of bias varied among the studies, with some demonstrating low risk, others high risk, and several presenting unclear risk due to insufficient information. The variability in the risk of bias for this aspect may have contributed to the heterogeneity observed in some outcomes.
Our investigation of heterogeneity revealed that some outcomes, such as caesarean section, instrumental vaginal birth, and postpartum haemorrhage, showed relatively low heterogeneity. These outcomes may have been more consistent across studies due to the influence of standardised clinical guidelines and protocols. On the other hand, outcomes with higher heterogeneity, like regional analgesia and breastfeeding initiation, might be heavily impacted by factors varying across different care models, populations, and study designs.
We assessed publication bias using funnel plots and Egger's tests, with most outcomes showing little evidence of publication bias. However, regional analgesia and postpartum haemorrhage had a higher degree of publication bias, which we considered when evaluating the certainty of the evidence for these outcomes using GRADE.
We conducted sensitivity analyses to explore the impact of the risk of bias on the findings. Our results demonstrated that the overall findings and conclusions were not affected significantly. Despite some limitations, the quality of the evidence in this review is generally robust, supporting the benefits of midwife continuity of care models for mothers and babies compared to other models of care.
Potential biases in the review process
In our review, we systematically searched for additional studies in the reference lists of the identified articles without imposing any language or date restrictions. We used the GRADE approach to make explicit judgements about the risk of bias in the included studies.
It is important to acknowledge that several review authors are also trial authors, which could potentially introduce bias. To mitigate this potential conflict of interest, we ensured that these review authors did not assess their own trials and their assessments, including data extraction and risk of bias assessment, were carried out by at least two other members of the review team.
We performed sensitivity and subgroup analyses to examine the effect of trial quality, including concealment of allocation, high attrition rates, or both. These analyses excluded poor‐quality studies to determine if their exclusion would significantly alter the overall results. We excluded the cluster‐randomised North Staffordshire trial from all outcomes in the primary comparison (comparison 1), where it contributed data (North Stafford 2000). This exclusion did not change the findings for any outcome, as the results remained consistent with the overall findings when all trials were included.
Additionally, we repeated the analysis while retaining only studies with a low risk of bias for random sequence generation, allocation concealment, and incomplete outcome data to evaluate whether this would impact the overall results. We found that the differences from the overall analyses were minor, primarily resulting in slightly wider confidence intervals due to the reduced number of trials in the analysis. However, these sensitivity analyses did not affect the conclusions drawn.
Subgroup analyses were conducted to explore potential differences in outcomes based on factors such as risk status, the type of midwife continuity of care models, variation in country setting, and variation in maternal social risk factors. Subgroup analyses for which there were available data revealed no significant subgroup differences or interactions, indicating a consistent effect across subgroups. Including these subgroup analyses in our review further strengthens our conclusions' robustness and helps address potential biases that may arise from the review process.
Agreements and disagreements with other studies or reviews
Systematic reviews of women's experiences are consistent with existing evidence. The Perriman 2018 meta‐synthesis found that the relationship between the childbearing woman and midwife is central and, through this, additional benefits are realised: trust, personalised care, and empowerment. The Cibralic 2022 narrative review found preliminary evidence showing that midwifery continuity of care is beneficial in reducing anxiety/worry and depression in pregnant women during the antenatal period.
A narrative review of reviews, which examined the impact of having midwife‐led maternity care for low‐risk women rather than from physicians, found that health and other benefits can result from having their maternity care led by midwives rather than physicians. Moreover, there appear to be no negative impacts on mothers and infants receiving midwife‐led care (Sutcliffe 2012). The Homer 2016 review included non‐randomised studies showing the midwifery continuity of care benefits for specific groups, such as Aboriginal and Torres Strait Islander women. Additionally, there are benefits for midwives, including high levels of job satisfaction and less occupational burnout, although these findings carry an inherently increased risk of bias.
The Donnellan‐Fernandez 2018 structured review appraised and summarised the evidence relating to the combined cost‐effectiveness, resource use, and clinical effectiveness of midwifery continuity models for women. Cost savings specific to women from high‐risk samples who received continuity of midwifery care compared with obstetric‐led standard care were stated for only one study in the review. Studies that measure the cost of continuity of midwifery care for women with complex pregnancies across the childbearing continuum are limited and apply inconsistent economic evaluation methods.
A review by Alderdice 2022 looked at the effectiveness of collaborative midwife continuity of care models in high‐income countries to improve pregnancy outcomes for women with medical and obstetric complexity, using any study design. Limited evidence was identified about using collaborative midwife continuity of care models for women with medical and obstetric complexity in high‐income countries. Fox 2023 also found limited evidence in this group with a need for further research.
A scoping review aimed to understand the global implementation of these models (Bradford 2022). In high‐income countries, the most dominant model was where small groups of midwives provided care for designated women across the antenatal, childbirth, and postnatal care continuum. In low‐income countries, there was more variation, with many implemented for women, newborns, and families from priority or vulnerable communities. With the exception of New Zealand, no countries have managed to scale up the continuity of midwifery care model nationally.
Wassen 2023 aimed to review the benefits and risks of caseload midwifery, compared with standard care comparable to the Swedish setting where the same midwife usually provides antenatal care and the check‐up postnatally but does not assist during birth and the first week postpartum. The risk of caesarean section may be reduced, with little difference found for several critical and important child and maternal outcomes, with low‐moderate certainty of evidence.
Authors' conclusions
Implications for practice.
Midwife continuity of care models offer important benefits. Women receiving care from midwife continuity of care models, as opposed to other care models, generally reported more positive experiences during pregnancy, labour, and postpartum. Cost savings were noted in the antenatal and intrapartum period in midwife continuity of care models.
Given the exclusion of women with significant maternal disease and substance abuse from some mixed‐risk trials, care should be taken in applying the findings of this review to women with considerable medical or obstetric complications.
Policymakers and healthcare providers should recognise that these benefits are associated when midwives provide relational continuity throughout pregnancy, childbirth, and into the postnatal period. In some parts of the world, health systems may not support the provision of midwife continuity of care models, and barriers to implementation could include societal and gendered norms, organisational traditions, and differences in power and authority between professions (Blomgren 2023), health system financing, and health system integration. Therefore, policymakers who wish to achieve meaningful improvements in maternal and newborn outcomes, particularly around humanising birth, should explore how the integration of midwife continuity of care models into health systems can be supported and financed.
Implications for research.
There are remaining questions about the best way to structure midwife continuity of care models in different contexts. Further research should investigate whether the observed benefits can be attributed to the continuity model, the philosophy of care, or the strength and quality of the relationship between the care provider and the woman. It is important to conduct more research on the recently developed midwife continuity of care models for women with social risk factors (Rayment‐Jones 2023), and medical and obstetric risk factors, with a focus on collaboration with obstetric and medical specialists (Alderdice 2022; Fernandez Turienzo 2020; Fox 2023).
Research in resource‐constrained countries is particularly needed. There was one study in resource‐constrained countries (Gu 2013), and additional trials are required in such settings.
The interface between midwife continuity of care models and the multidisciplinary support network is not well understood. Despite continuity of care being identified as a core component, its definition and measurement vary greatly, necessitating greater sophistication in future studies. Additional implementation studies should support countries transitioning to midwife continuity of care models, to determine optimal model types and strategies for sustainable national scale‐up. Acceptability to midwives of different models offering relational continuity should be assessed.
Future trials in this area could benefit from drawing on a framework for trials of complex interventions, and implementation science requiring theoretical modelling between processes and outcomes in the pre‐trial stage and a process evaluation of implementation outcomes in the trial (Skivington 2021). Trials should provide a detailed description of intervention and standard models of care being assessed (Hoffman 2014), include process evaluations of their implementation (Moore 2014), and use reporting guidelines for complex interventions. Future research in this area would benefit from exploring the theoretical underpinnings of these complex interventions and their associations with processes and outcomes, including implementation assessments that include the impact on staff and the organisation, and the consideration of hybrid‐effectiveness trials and systematic use of implementation measures (Curran 2022).
A core data set, such as that proposed by Devane 2007, would facilitate comparisons within and between trials and enable more effective meta‐analyses of similar studies. Future trials should also include measures of optimal outcomes for mothers, babies, and morbidity. There remains relatively little information about the effects of midwife continuity of care models on mothers' and babies' health and wellbeing in the longer postpartum period. Future research should pay particular attention to outcomes that have been under‐researched, such as infant feeding and the parent‐infant relationship, and causes of significant morbidity, including postpartum mental health, urinary and faecal incontinence, duration of caesarean incision pain, pain during intercourse, prolonged perineal pain, and birth injury (to the baby).
It is important to understand whether women feel involved in the decision‐making process, and their sense of control, self‐confidence, coping mechanisms post‐birth, and experiences of post‐traumatic stress disorder. There is wide variation in the instruments used to measure women's views of and experiences of care. There is a need to develop meaningful, robust, valid, and reliable methods to assess psychosocial outcomes and well‐being in pregnant and childbearing women. All trials should include an assessment of maternal and fetal well‐being.
There is a lack of consistency in estimating maternity care cost, and further research using standard cost estimation approaches is required, including the cost to women and families.
The inconsistency in measuring continuity (Reid 2002), the choice of routinely collected and reported outcome measures in evaluations of maternity care models, and the lack of consistency in estimating maternity care cost necessitate more robust, reliable methods to assess psychosocial outcomes and well‐being in pregnant and childbearing women. Future trials should include economic analyses of relative costs and benefits.
Future studies should employ health economic evaluation study designs, including trial‐based and decision analytic‐based methods, or both, to definitively assess cost‐effectiveness, cost benefit, and the associated uncertainty in this context. Local guidance for the conduct of health economic evaluation should be followed. Future studies should consider costs from both the healthcare and societal perspective, health outcomes in the form of quality‐adjusted life years (QALYs), and other important outcomes to patients and the public. Where possible, resource use and unit costs should be reported separately, and locally generated preference‐based utility index scores adopted. Studies should also consider alternative time horizons for analysis, ranging from a follow‐up of the pregnancy to the longer term, using decision analytical modelling techniques, where appropriate. Appropriate forms of sensitivity analysis, including deterministic and probabilistic methods, should be employed to report uncertainty transparently. With more transparent and consistent approaches to cost analysis and health economic evaluation, there may exist the possibility for more complex forms of evidence synthesis, although issues of heterogeneity are likely to maintain the need for narrative synthesis.
Feedback
Bacon, May 2004,
Summary
Are you planning to include intrapartum foetal death rates for women delivering in different types of unit, and with different levels of risk, as one of your outcome measures? We have been unable to find comparative data for a local review.
(Summary of comment from Sallie Bacon, May 2004)
Reply
We have not looked at intrapartum deaths specifically, but have addressed this issue in the 'Discussion'.
(Summary of response from Jane Sandall, November 2007)
Contributors
Sallie Bacon
Blake, 19 November 2013
Summary
The Society of Obstetricians and Gynaecologists of Canada (SOGC) is the longest established national organization for women’s reproductive care in North America, with membership made up of obstetricians, gynaecologists, nurses, midwives, family physicians and scientists. We have long supported a woman’s right to choose the care provider of her preference for obstetrical care, and we actively support and promote collaborative models of care. We were therefore very interested to read the review of midwifery‐led care that you published in August of this year. We were not surprised by the main findings cited in the abstract: less use of epidural or intra‐partum analgesia, fewer instrumental deliveries and, in consequence, fewer episiotomies, longer length of labour. These differences would be expected with the different model of care; for some women an unmediated delivery is a goal. However, for others, access to analgesia is a key consideration; we cannot conclude from this difference that the midwifery‐led model is better for all women. We were interested by the findings of fewer preterm births, fewer deaths <24weeks, findings which are unexplained, and for which it is unlikely that we could identify an explanation based on who was providing the care, given that there are few, if any, clinical interventions by any provider prior to 24 weeks which can affect these outcomes. Beyond these matters, however, we are primarily contacting you because the abstract failed to list the important outcomes which do not differ with provider: perineal trauma, induction of labour, oxytocin augmentation of labour, caesarean section, antenatal hospitalisation, post‐partum haemorrhage, length of hospital stay, initiation of breast feeding, neonatal Apgar score, admission to neonatal nursery, fetal loss or death >24 weeks. Our greatest concern is that, although the abstract failed to list or consider these fundamentally important clinical outcomes that were equivalent, the authors still asserted that “most women should be offered midwifery‐led continuity models of care and women should be encouraged to ask for this option…” We believe this conclusion received, and continues to receive, the bulk of media and lay attention. In fact, those who do not actually read the review but only the abstract will come away with an incorrect understanding that is not supported by the results, an outcome that appears to be self‐serving and misleading. We expect better from the Cochrane Collaboration. This was an opportunity to provide women with reassurance that they have healthful options for their pregnancy care, and that they can feel confident that, regardless of their choice, the outcomes will be similar with respect to a safe and healthy pregnancy and delivery. Instead, the way this issue has been positioned, and by the selective use of the data, the Cochrane appears to advocate for a particular model of care, a disservice to women and the many other health care professionals who care for them.
Comment received from Jennifer Blake, Society of Obstetricians and Gynaecologists of Canada, November 2013.
Reply
We are pleased to see the SOGC’s interest in our review and thank them for their comments.
We agree that findings of fewer preterm births and fewer deaths less than 24weeks are interesting. Midwife‐led continuity of care is a complex intervention, and it is impossible to unpick the relative importance of philosophy and continuity of care. We note in our review that questions remain about the mechanisms underlying these findings.
Our abstract is reported in original format in an effort to present information on multiple outcomes in as clear a manner as possible. Further to your comments, in the updated review, we have reformatted the presentation of outcomes in the abstract such that all primary outcomes are presented initially followed by all secondary outcomes. This will, we believe provide the reader with the totality of information on which to inform their health care decisions. Similarly, we have revised the conclusion to summarise the findings of the review and key areas for further research.
We trust this addresses your concerns.
Regards
Jane Sandall, August 2015
Contributors
Jane Sandall
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2024 | Amended | Correcting formatting in Abstract. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 16 April 2024 | Amended | Correcting formatting in Abstract. |
| 15 April 2024 | Amended | Formatting issue fixed |
| 10 April 2024 | New citation required and conclusions have changed | We have changed the title, and updated the inclusion and exclusion criteria, searches, and outcomes. We have complied with new methods guidance (i.e. Trustworthiness Screening), and re‐entered data for all studies in the review. New searches have been conducted, and we have reassessed all studies and re‐entered data for all included papers. We have updated the outcomes to ensure clinical and policy relevance. See Differences between protocol and review. One new trial has been included (Fernandez Turienzo 2020). Two trials previously excluded in the last version of the review have now been included (Gu 2013; Marks 2003), and one trial previously included has now been excluded (Allen 2013). We have added two new subgroup analyses (variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI) and variation in maternal social risk factors (women with social risk factors versus all women)). |
| 10 April 2024 | New search has been performed | Search updated. We assessed 55 new trial reports for eligibility. One new trial has been included in this update. The review now includes a total of 17 trials. |
| 25 January 2016 | New citation required but conclusions have not changed | For this update the results and conclusions of this review remain unchanged. |
| 25 January 2016 | New search has been performed | Search updated. Three new trial reports identified relating to three studies already included in the review (Begley 2011; McLachlan 2012; Tracy 2013). Additional data have been added from two of the new reports on cost (Begley 2011) and maternal satisfaction (McLachlan 2012). The primary neonatal outcome "Overall fetal loss and neonatal death (fetal loss was assessed by gestation using 24 weeks as the cut‐off for viability in many countries)" was changed to "All fetal loss before and after 24 weeks plus neonatal death." The secondary neonatal outcomes, "Fetal loss and neonatal death less than 24 weeks" and "Fetal loss and neonatal death equal to/after 24 weeks" were changed to "Fetal loss less than 24 weeks and neonatal death" and "Fetal loss equal to/after 24 weeks and neonatal death". |
| 23 September 2015 | Amended | Correction to abstract. Clarification of results for the outcomes "No intrapartum analgesia/anaesthesia" and "Attendance at birth by known midwife". |
| 31 May 2015 | New search has been performed | Search updated. A 'Summary of findings' table has been incorporated. |
| 31 May 2015 | New citation required but conclusions have not changed | Two new studies included (Allen 2013; Tracy 2013); two studies excluded (Famuyide 2014; Gu 2013). The conclusions remain the same. |
| 19 November 2013 | Feedback has been incorporated | Feedback 2 received from Jennifer Blake. |
| 2 May 2013 | New citation required and conclusions have changed | Two new studies included (Begley 2011; McLachlan 2012). In this update the evidence now suggests that women randomised to receive midwife‐led continuity models of care were less likely to experience preterm birth. There is now no evidence of a difference between different models of care in terms of antenatal hospitalisation and breastfeeding initiation. |
| 28 January 2013 | New search has been performed | Search updated. Methods updated. |
| 29 April 2009 | Amended | In response to feedback, we have clarified what is meant by midwife‐led care and have stressed the multidisciplinary network of care providers; have added information to the Abstract about the lack of effect on caesarean section; and revised the Abstract's conclusions from "All women" to "Most women should be offered midwife‐led models of care and women should be encouraged to ask for this option." |
| 9 November 2008 | Amended | Amended the graph labelling for control in childbirth (Analysis 1.32) and corrected a typographical error in the Results section. |
| 15 May 2008 | Amended | Converted to new review format. |
Acknowledgements
We are very grateful to the investigators who provided additional information: P Brodie, R Burr, H Cheyne, D Forster, M Foureur, C Gu, C Homer, H McLachlan, P Spurgeon, C Warwick, J Wyatt.
We thank Toby Lasserson, Deputy Editor‐in‐Chief of the Cochrane Library, for his support with our summary of findings and in particular for discussions around how to make the findings of this review most useful and practical.
We thank Nancy Medley for her support in the production of the summary of findings table in the 2015 update. Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
We also acknowledge previous authors of the review, including M Hatem and WD Fraser.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Jane Sandall is an NIHR Senior Investigator Emerita and, with Cristina Fernandez Turienzo and Hannah Rayment‐Jones, is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. Jane Sandall and Cristina Fernandez Turienzo are supported by a NIHR Global Health Research Group (NIHR133232) and Cristina Fernandez Turienzo by a NIHR DSE award in global clinical trials (NIHR301603). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Hora Soltani is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration Yorkshire and Humber at Sheffield Hallam University. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Robert Boyle, National Heart & Lung Institute, Section of Inflammation and Repair, Imperial College London
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Liz Bickerdike, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer reviewer comments and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Jo Platt, Central Editorial Information Specialist (search), Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods), Caroline Homer, Burnet Institute, Australia (clinical), Professor Alison McFadden, University of Dundee (clinical), and Jessica D'Urbano (consumer)
Appendices
Appendix 1. Detailed search methods for Cochrane Pregnancy and Childbirth
Detailed search methods used to maintain and update the Specialised Register
Note: The Search Methods section of each protocol or review will contain our standard search paragraph. This describes very briefly the Group’s broad searching activities. This document describes in detail the sources searched for the Specialised Register, how the search results are dealt with and how review authors receive the search results relevant to their reviews.
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents more than 30 years of searching.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described below is reviewed.
Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register.
The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that will be fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
Search strategies for the identification of studies
A. Electronic searches (none of these search strategies is meant to be a direct translation of another. They have been designed to complement each other but results do overlap).
(1) THE COCHRANE CENTRAL REGISTER OF CONTROLLED TRIALS (CENTRAL): The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library is searched using MeSH terms relevant to pregnancy and childbirth, together with free text terms. CENTRAL contains reports of randomised trials and quasi‐randomised trials mostly taken from PubMed and Embase but also ClincalTrials.gov, the Specialized Registers of groups within Cochrane and other sources. This search is run monthly. (See: Search strategy below)
(2) MEDLINE: This current search strategy (2018) is run weekly via OVID MEDLINE and uses the Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) published in Chapter 6, Section 6.4.11 of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (See: search strategy below)
(3) Embase: The search strategy is run weekly via NICE Healthcare Databases (provided by OVID) (See: search strategy below)
(4) CINAHL: NICE Healthcare Databases (provided by EBSCO) (See: search strategy below)
(5)Clinical Trials Registries: Each review has a topic specific search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports. The exact search methods are documented in the review.
B. Searching other resources
(1) Journal and conference proceedings screening: see below for the lists of journals and conference proceedings that have been searched for RCTs/quasi‐RCTs to add to the Specialised Register.
(2) Current Awareness: see below for the current awareness we use.
(1) CENTRAL search strategy
#1 MeSH descriptor: [Pregnancy] explode all trees
#2 MeSH descriptor: [Pregnancy Complications] explode all trees
#3 MeSH descriptor: [Infant, Newborn] explode all trees
#4 MeSH descriptor: [Fetus] explode all trees
#5 MeSH descriptor: [Fetal Development] explode all trees
#6 MeSH descriptor: [Prenatal Diagnosis] explode all trees
#7 MeSH descriptor: [Fetal Monitoring] explode all trees
#8 MeSH descriptor: [Fetal Therapies] explode all trees
#9 MeSH descriptor: [Heart Rate, Fetal] explode all trees
#10 MeSH descriptor: [Extraembryonic Membranes] explode all trees
#11 MeSH descriptor: [Placenta] explode all trees
#12 MeSH descriptor: [Placental Function Tests] explode all trees
#13 MeSH descriptor: [Uterine Monitoring] explode all trees
#14 MeSH descriptor: [Pelvimetry] explode all trees
#15 MeSH descriptor: [Oxytocics] explode all trees
#16 MeSH descriptor: [Tocolytic Agents] explode all trees
#17 MeSH descriptor: [Tocolysis] explode all trees
#18 MeSH descriptor: [Maternal Health Services] explode all trees
#19 MeSH descriptor: [Peripartum Period] explode all trees
#20 MeSH descriptor: [Parity] explode all trees
#21 MeSH descriptor: [Perinatal Care] explode all trees
#22 MeSH descriptor: [Postpartum Period] explode all trees
#23 MeSH descriptor: [Labor Pain] explode all trees
#24 MeSH descriptor: [Anesthesia, Obstetrical] explode all trees
#25 MeSH descriptor: [Obstetric Surgical Procedures] explode all trees
#26 MeSH descriptor: [Analgesia, Obstetrical] explode all trees
#27 MeSH descriptor: [Obstetric Nursing] explode all trees
#28 MeSH descriptor: [Maternal‐Child Nursing] explode all trees
#29 MeSH descriptor: [Midwifery] explode all trees
#30 MeSH descriptor: [Apgar Score] explode all trees
#31 MeSH descriptor: [Breast Feeding] explode all trees
#32 MeSH descriptor: [Bottle Feeding] explode all trees
#33 MeSH descriptor: [Milk, Human] explode all trees
#34 {OR #1‐#33}
#35 pregnan*
#36 fetus
#37 foetus
#38 fetal
#39 foetal
#40 newborn
#41 "new born"
#42 birth
#43 childbirth
#44 laboring
#45 labour*
#46 antepart*
#47 prenatal*
#48 antenatal*
#49 perinatal*
#50 postnatal*
#51 postpart*
#52 caesar*
#53 cesar*
#54 obstetric*
#55 tocoly*
#56 oxytoci*
#57 placent*
#58 parturi*
#59 preeclamp*
#60 eclamp*
#61 intrapart*
#62 puerper*
#63 episiotom*
#64 amnio*
#65 matern*
#66 gestation*
#67 lactati*
#68 breastfe*
#69 breast NEXT fe*
#70 preconcept*
#71 periconcept*
#72 interconcept*
#73 {OR #35‐#72}
#74 #34 OR #73
(2) MEDLINE search strategy
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp Pregnancy/ 11. exp Pregnancy Complications/ 12. exp Maternal Health Services/ 13. exp Fetus/ 14. exp Fetal Therapies/ 15. exp Fetal Monitoring/ 16. exp Prenatal Diagnosis/ 17. Perinatal Care/ 18. Labor pain/ 19. Analgesia, Obstetric/ 20. exp Obstetric Surgical Procedures/ 21. Infant, Newborn/ 22. exp Postpartum Period/ 23. Breastfeeding/ 24. or/10‐23 25. 9 and 24 26. exp animals/ not humans.sh. 27. 25 not 26
(3) Embase search strategy
CROSSOVER PROCEDURE/
DOUBLE BLIND PROCEDURE/
SINGLE BLIND PROCEDURE/
RANDOMIZED CONTROLLED TRIAL/
crossover$.ti,ab
(cross ADJ over$).ti,ab
placebo$.ti,ab
(doubl$ ADJ blind$).ti,ab
allocat$.ti,ab
random$.ti,ab
trial$.ti
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11
exp PREGNANCY/
exp PREGNANCY DISORDER/
exp OBSTETRIC PROCEDURE/
exp BREAST FEEDING/ OR exp BREAST FEEDING EDUCATION/
exp CHILDBIRTH/ OR exp CHILDBIRTH EDUCATION/
exp LABOR PAIN/
(antenatal* OR prenatal* OR puerper* OR postnatal* OR post‐natal* OR post ADJ natal* OR postpartum OR post‐partum OR post ADJ partum).ti,ab
(prepregnancy OR pre‐pregnancy OR pre ADJ pregnancy OR preconcept* OR pre‐concept* OR pre ADJ concept* OR periconcept* OR peri‐concept* OR peri ADJ concept*).ti,ab
((preterm OR premature) AND (labour OR labor)).ti,ab
(eclamp* OR preeclamp* OR pre ADJ eclamp*).ti,ab
amniocentes*.ti,ab
(chorion* ADJ vill*).ti,ab
(breastfe* OR breast‐fe* OR breast ADJ fe* OR lactation).ti,ab
(caesarean OR cesarean OR caesarian OR cesarian OR cesarien OR caesarien).ti,ab
(newborn OR new ADJ born).ti,ab
(pregnancy OR pregnant OR pregnancies).ti
episiotom*.ti,ab
13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29
12 AND 30
(4) CINAHL search strategy
exp CLINICAL TRIALS/
(clinic* ADJ trial*).ti,ab
(trebl* ADJ mask*).ti,ab
(tripl* ADJ blind*).ti,ab
(tripl* ADJ mask*).ti,ab
(doubl* ADJ blind*).ti,ab
(doubl* ADJ mask*).ti,ab
(singl* ADJ blind*).ti,ab
(singl* ADJ mask*).ti,ab
(randomi* ADJ control* ADJ trial*).ti,ab
RANDOM ASSIGNMENT/
(random* ADJ allocat*).ti,ab
placebo*.ti,ab
PLACEBOS/
QUANTITATIVE STUDIES/
(allocat* ADJ random*).ti,ab
breastfeeding.ti,ab
breastfed.ti,ab
exp BREAST FEEDING/
breast‐fe*.ti,ab
exp PREGNANCY/
exp PREGNANCY COMPLICATIONS/
(prenatal OR antenatal OR antepartum OR postpartum OR postnatal).ti,ab
(pregnant OR pregnancy).ti
((preterm OR premature) AND (labor OR labour)).ti,ab
(midwife OR midwifery).ti,ab
CHILDBIRTH EDUCATION/
exp PREGNANCY, MULTIPLE/ OR exp PREGNANCY TRIMESTERS/
exp MATERNAL‐CHILD CARE/
(prenatal* OR pre‐natal* OR perinatal* OR peripartum OR antenatal* OR postnatal* OR post‐natal* OR postpart* OR post‐part* OR puerper* OR prepregnancy OR pre‐pregnancy OR preconcept* OR pre‐concept* OR periconcept* OR peri‐concept*).ti,ab
OBSTETRIC EMERGENCIES/
OBSTETRIC NURSING/
exp SURGERY, OBSTETRICAL/
exp DIAGNOSIS, OBSTETRIC/
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16
17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34
Journal and conference proceedings screening (sometimes known as handsearching)
Journals
Currently, each issue (mainly the electronic version now) is scanned from start to end including supplements where available. Where a journal in the table below has stopped being searched, it is either because the journal is a more general journal; it was being searched by another Cochrane group; or that we no longer have access.
| Acta Anaethesiologica Scandinavica (and supplements) | 1950 and continuing |
| Acta Obstetricia et Gynecologica Scandinavica (and supplements) | 1950 and continuing |
| Acta Paediatrica Scandinavica | 1st issue to 1993 |
| American Journal of Clinical Nutrition | 1st issue and continuing |
| American Journal of Diseases of the Child | 1950 to 1993 |
| American Journal of Obstetrics and Gynecology | 1950 and continuing |
| Anaesthesia and Intensive Care | 1st issue and continuing |
| Anaesthesia | 1950 and continuing |
| Anesthesia and Analgesia | 1st issue and continuing |
| Anesthesiology | 1950 and continuing |
| Archives of Diseases of the Child | 1950 to 1993 |
| Australian and New Zealand Journal of Obstetrics and Gynaecology | 1st issue and continuing |
| Birth | 1st issue and continuing |
| British Medical Journal | 1950 to 1996 |
| British Journal of Anaesthesia | 1950 and continuing |
| British Journal of Obstetrics and Gynaecology | 1st issue and continuing |
| Canadian Journal of Anaesthesia | 1st issue and continuing |
| Canadian Medical Association Journal | 1950 to 1996 |
| Clinical Pharmacology and Therapeutics | 1st issue to 1998 |
| Current Medical Research and Opinion | 1st issue to 1993 |
| Developmental Medicine and Child Neurology | 1st issue to 1993 |
| Early Human Development | 1st issue to 1993 |
| European Journal of Obstetrics & Gynaecology and Reproductive Biology | 1st issue and continuing |
| Geburtshilfe und Frauenheilkunde | 1950 ‐ 2017 |
| Gynecologic and Obstetric Investigation | 1st issue to 1996, 2005 and continuing |
| Hypertension in Pregnancy | 2006 and continuing |
| Indian Journal of Anaesthesia | 2002 issue 3 to 2005 issue 5 |
| Infectious Diseases in Obstetrics and Gynecology | 1st issue and continuing |
| International Journal of Gynecology & Obstetrics | 1st issue to 2016 |
| International Journal of Obstetric Anaesthesia | Oct 94 to Oct 95, Jan 2003 and continuing |
| Journal of the American Medical Association | 1st issue to 1996 |
| Journal of the American College of Surgeons | 1950 to 2003 |
| Journal de Gynecologie, Obstetrique et Biologie de la Reproduction | 1st issue to 1998 |
| Journal of Human Lactation | 2001 and continuing |
| Journal of International Medical Research | 1st issue to 1993 |
| Journal of Midwifery and Womens Health | 1st issue and continuing |
| Journal of Obstetrics and Gynaecology | 1st issue and continuing |
| Journal of Obstetrics and Gynaecology Research | 2003 and continuing |
| Journal of Obstetric Gynecologic and Neonatal Nursing | 1st issue to 1993, 2001 to 2006 |
| Journal of Pediatrics | 1950 to 1993 |
| Journal of Pediatric Gastroenterology and Nutrition | 1st issue to 1993 |
| Journal of Perinatal Medicine | 1st issue to 1998 |
| Journal of Reproductive Medicine | 1st issue to 2003 |
| Lancet | 1950 to 1996 |
| Medical Journal of Australia | 1950 to 1996 |
| Midwifery | 1st issue and continuing |
| New England Journal of Medicine | 1950 to 1996 |
| Nurse Research | 1st issue to 1993 |
| New Zealand Medical Journal | 1950 to 1996 |
| Obstetrics & Gynecology | 1st issue and continuing |
| Pediatric Research | 1st issue to 1993 |
| Pediatrics | 1950 to 1993 |
| Practitioner | 1950 to 1996 |
| Prostaglandins | 1st issue to 1993 |
| Regional Anesthesia and Pain Medicine | 1st issue and continuing |
| Revista Brasileira de Anestesiologia | 2003 to 2006 |
| Revista Brasileira de Ginecologia e Obstetricia | 2001 to 2005 |
| South African Journal of Obstetrics and Gynaecology | 1st issue to 1993 |
| South African Medical Journal | 1950 ‐ 1993 |
| Surgery Gynecology and Obstetrics | 1950 to 1993 |
| Ugeskrift for Laeger | 1950 to 1993 |
| Ultrasound in Obstetrics and Gynecology | 2002 and continuing |
| Zeitschrift fur Geburtshilfe und Perinatologie | 1st issue to 1997 |
| Zentrablatt fur Gynakologie | 1st issue to 1997 |
(2) Conference proceedings (from conference abstract books, journal supplements and online sources)
We have searched other conference proceedings as and when the abstracts have been made available to us. The table below gives a list of all conference proceedings searched.
| All India Congress of Obstetrics and Gynaecology | 49th(2006), 54th (2011) |
| Allied Specialists in Maternal and Neonatal Care – European Congress | 4th (1989) |
| American College of Obstetricians and Gynecologists' Annual Meeting | 36th (1988), 37th(1989) 39th 1991), 40th (1992), 41st (1993), 47th (1999), 55th (2007), 58th (2010), 62nd (2014), 63rd (2015), 64th (2016), 65th (2017) |
| American Society of Anesthesiologists Annual Meeting | 2000, 2001, 2002, 2003, 2004, 2007, 2008, 2009 |
| American Society of Regional Anesthesia and Pain Medicine Annual Spring Meeting | 26th (2001), 27th (2002), 28th (2003) |
| American Society of Regional Anesthesia and Pain Medicine Annual Fall Meeting | 2002, 2003, 2007 |
| Argentinean Congress of Perinatology | 3rd |
| Asian & Oceanic Congress of Obstetrics & Gynaecology | 24th (2015) |
| Association of Anaesthetists of Great Britain and Ireland ‐ Annual Congress | 2007 |
| Australian Society of Anaesthetists National Scientific Congress | 58th (1999), 61st (2003) |
| Australian and New Zealand College of Anaesthetists Annual Meeting | 2013 |
| Birth Conference | 1st to 9th (1990) |
| British Congress of Obstetrics and Gynaecology | 23rd, 25th, 26th, 27th, 28th |
| British Maternal and Fetal Medicine Society | 6th (2001), 10th (2005), 17th, 18th |
| British Paediatric Association Annual Meeting | 14th, 15th, 27th, 60th, 61st, 62nd, 63rd, 65th |
| Controversies in Obstetrics, Gynecology & Infertility – World Congress | 4th (2003) |
| Endocrinology – European Congress | 17th (2015) |
| European Congress of Obstetrics and Gynaecology | 18th (2004) |
| European Society of Regional Anesthesia and Pain Medicine | 26th (2007), 29th (2010), 32nd (2013), 33rd, 34th |
| Federation of the Asia‐Oceania Perinatal Societies' Congress | 6th, 9th |
| FIGO African Regional Conference of Gynecology and Obstetrics | 1st |
| FIGO World Congress of Gynecology and Obstetrics | 11th, 12th (1988), 15th (1997)to 16th (2000),19th (2009) , 20th (2012) 21st (2015) |
| German Society of Gynaecology and Obstetrics (DGGG) ‐ Congress | 2016 |
| International Anesthesia Research Society Clinical and Scientific Congress | 76th (2002), 78th (2004), 80th (2006) |
| International Confederation of Midwives Triennial Congress | 24th, 30th (2014), 31st (2017) |
| International Conference of Maternity Care Researchers | 10th |
| International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists | 4th |
| International Society of Obstetric Medicine (ISOM) World Congress | 3rd (2006) |
| International Society for Research in Human Milk and Lactation Conference | 17th (2014), 18th (2016) |
| International Society for the Study of Hypertension in Pregnancy (ISSHP) European Branch | 1st (1978) |
| International Society for the Study of Hypertension in Pregnancy (ISSHP) World Branch | 1st (1978), 2nd (1980), 4th (1984), 5th (1986), 6th (1988), 7th (1990), 8th (1992), 9th (1994), 10th (1996), 11th (1998), 12th (2000), 13th 2002), 14th (2004), 15th (2006), 16th (2008), 18th (2012) |
| International Society of Psychosomatic Obstetrics and Gynaecology – International Congress | 26th (2010) |
| Japanese Society of Obstetrics and Gynecology | 54th (2002), 56th (2004), 68th, |
| Maternity Care Researchers International Conference | 10th (2004) |
| Nordic Federation of Societies of Obstetrics and Gynecology Congress | 34th (2004), 35th (2006), 38th (2012) |
| Obstetric Anaesthetists Association | 2005, 2009 |
| Obstetrical Anaesthesia and Analgesia – European Congress | 1st |
| Pediatric Academic Society Annual Meeting | 2004 to 2017 |
| Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting | 2014 |
| Perinatal Medicine – European Congress | 5th, 6th, 8th, 10th, 11th, 12th, 14th, 15th (1996), 16th (1998), 17th (2000), 21st (2008), 24th |
| Perinatal Medicine – World Congress | 1st, 2nd, 5th (2001), 10th (2011), 11th (2013) |
| Perinatal Society of Australia and New Zealand Annual Congress | 2nd, 4th (1998), 7th (2003) 8th, 10th, 11th (2007), 15th,17th (2013),18th , 20th (2016) |
| Perinatal Society of New Zealand Annual Scientific Meeting | 34th 35th (2015), |
| Priorities in Perinatal Care in South Africa | 2nd (1983), 4th, 7th, 9th, 10th, 11th, 12th (1993), 14th, 15th, 16th, 17th (1998) , 20th (2001), 21st (2002), 22nd (2003) |
| Prostaglandins in Reproduction ‐ European Congress | 1st, 2nd |
| Psychosomatic Medicine in Obstetrics and Gynaecology ‐ International Congress | 3rd, 5th |
| Regional Anesthesia and Pain Medicine ‐ European Society | 26th (2007), 29th (2010), 32nd (2013), 33rd, 34th |
| Royal College of Obstetricians and Gynaecologists International Meeting | 4th (1999), 7th (2008), 10th (2012) , 2015, 2016 |
| Society of Obstetricians and Gynaecologists of Canada Annual Meeting | 49th, 54th, 63rd (2007) |
| Society of Perinatal Obstetricians' (USA) Annual Meeting | 3rd 6th to 10th, 14th, 17th, 18th (1998) |
| Society for Gynecologic Investigation (USA) Annual Program | 31st, 34th, 37th, 39th, 40th |
| Society for Maternal‐Fetal Medicine | 19th (1999), 20th (2000), 21st (2001), 22nd (2002), 23rd (2003), 24th (2004), 25th (2005), 26th (2006), 27th (2007), 28th (2008), 29th (2009) 30th (2010), 31st (2011), 32nd (2012), 33rd (2013) 34th (2014), 35th (2015), 37th (2017) |
| Society for Obstetric Anesthesia and Perinatology (SOAP) Annual Meeting | 32nd (2000), 33rd (2001), 34th (2002), 37th (2005), , 38th (2006), 39th (2007), 46th (2014), 47th (2015), 48th (2016) |
| Sri Lanka College of Obstetricians & Gynaecologists Annual Scientific Congress | 48th (2015) |
| Swiss Society of Gynecology and Obstetrics | 19th (1996), 20th (1997), 21st 22nd |
| Twin Pregnancy – World Congress | 1st (2009) |
| Ultrasound in Medicine and Biology – European Congress | 6th |
| Ultrasound in Obstetrics and Gynecology ‐ World Congress | 13th (2003) 15th (2005) 16th (2006), 17th (2007), 18th (2008), 19th (2009), 20th (2010), 21st (2001) 22nd (2002), 23rd (2003) 24th (2004) |
Other strategies
(1) Current Awareness
a) ZETOC, The British Library's Electronic Table of Contents service sends the contents tables via e‐mail of the journals listed below. The contents are reviewed by the Trials Search Co‐ordinator. Hard copies of all possible reports of RCTs/CCTs relevant to the scope of the group are obtained, reviewed and added to the register by the Information Specialist if they meet the inclusion criteria.
African Journal of Reproductive Health
American Journal of Perinatology
Archives of Disease in Childhood
Archives of Disease in Childhood Fetal and Neonatal Edition
Archives of Gynecology and Obstetrics
Archives of Pediatrics and Adolescent Medicine
Archives of Women’s Mental Health
British Journal of Midwifery
Chinese Journal of Obstetrics and Gynecology
Clinica e Investigacion en Ginecologia y Obstetricia
Clinical and Experimental Obstetrics and Gynecology
Clinical Obstetrics and Gynecology
Contemporary Ob/GYN
Evidence Based Midwifery
Fetal Diagnosis and Therapy
Ginecologia y Obstetricia de Mexico
Giornale Italiano di Ostetricia e Ginecologia
Human Reproduction
Hypertension in Pregnancy
Italian Journal of Gynaecology and Obstetrics
JAMA Pediatrics
JOGC: Journal of Obstetrics and Gynecology Canada
Journal – Association of Chartered Physiotherapists in Womens Health
Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris)
Journal of Maternal Fetal and Neonatal Medicine
Journal of Obstetrics and Gynecology of India
Journal of Paediatrics Obstetrics and Gynaecology
Journal of Perinatal and Neonatal Nursing
Journal of Perinatology
Journal of Prenatal and Perinatal Psychology and Health
Journal of Psychosomatic Obstetrics and Gynaecology
Journal of Reproductive Medicine
Journal‐New Zealand College of Midwives
MCN, The American Journal of Maternal Child Nursing
MIDIRS Midwifery Digest
Midwifery Matters
Midwifery Today
Obstetrical and Gynecological Survey
Obstetrics, Gynaecology and Reproductive Medicine
Prenatal Diagnosis
Revue de Medecine Perinatale
Taiwanese Journal of Obstetrics and Gynecology
Women and Birth
Zeitschrift fur Geburtshilfe und Neonatologie
b)Biomed Central (http://www.biomedcentral.com/home/) sends an email alert every 30 days for the anything new published in the following:
BMC: Pregnancy and Childbirth Journal
International Breastfeeding Journal
Anything related to the subject areas of Pregnancy and Childbirth, Pediatrics or Women’s Health.
Specialised Register inclusion criteria
TOPIC SCOPE: Controlled trials comparing alternative forms of care used either during pregnancy (but not to terminate early pregnancy), or within 28 days of delivery.
STUDY DESIGN: A controlled trial has been defined as a trial involving humans in which allocation to the intervention has either been at random, or by some quasi‐random method, such as by alternation, or on the basis of the case record number or date of birth.
These criteria have been applied fairly liberally to avoid excluding potentially useful studies involving concurrent comparisons of alternative policies. In other words, the Register includes reports which, if necessary, can subsequently be rejected as methodologically inadequate by a member of the Group preparing a systematic review.
All search results are deduplicated, screened by two people at the editorial base, and the full text of all relevant trial reports identified through the searching activities described above is reviewed.
Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register.
The Information Specialist searches the Register for each review using this topic number rather than keywords and adds the results to the Studies Awaiting Classification section of the review for authors to assess. This results in a more specific search set that will be fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
No language or date restrictions are applied.
Appendix 2. Search methods for ICTRP and ClinicalTrials.gov
Each line was run separately
ClinicalTrials.gov
Advanced search
midwife | Interventional Studies | continuity
midwife | Interventional Studies | continuous
midwife | Interventional Studies | model
midwifery | Interventional Studies | support
midwife | Interventional Studies | support
continuity of care | pregnancy
midwifery‐led | Interventional Studies
midwife‐led | Interventional Studies
ICTRP (searched 'with synonyms')
midwifery AND models AND care
midwifery AND continuity
midwife‐led
midwifery‐led
Data and analyses
Comparison 1. Midwife continuity models versus other models of care for childbearing women and their infants (all).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Spontaneous vaginal birth (as defined by trial authors) | 15 | 17864 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 1.2 Caesarean birth | 16 | 18037 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 1.3 Regional analgesia (epidural/spinal) | 15 | 17754 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.79, 0.92] |
| 1.4 Intact perineum | 12 | 14268 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.12] |
| 1.5 Fetal loss at or after 24 weeks gestation | 12 | 16122 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.73, 2.13] |
| 1.6 Preterm birth (< 37 weeks) | 10 | 13850 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 1.7 Neonatal death (baby born alive at any gestation and dies within 28 days) | 10 | 14718 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.71] |
| 1.8 Healthy mother | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Maternal death | 3 | 4282 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.10 Induction of labour | 14 | 17666 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 1.00] |
| 1.11 Instrumental vaginal birth (forceps/vacuum) | 14 | 17769 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.83, 0.96] |
| 1.12 Episiotomy | 15 | 17839 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.77, 0.91] |
| 1.13 Third or fourth degree tear | 7 | 9437 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.81, 1.49] |
| 1.14 Postpartum haemorrhage (as defined by trial authors) | 11 | 14407 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.82, 1.03] |
| 1.15 Breastfeeding initiation | 8 | 8575 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.00, 1.12] |
| 1.16 Maternal readmission within 28 days | 1 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.78, 2.96] |
| 1.17 Neonatal readmission within 28 days | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.18 Attendance at birth by known midwife | 11 | 9273 | Risk Ratio (M‐H, Random, 95% CI) | 9.13 [5.87, 14.21] |
| 1.19 Healthy baby | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.20 Birth weight less than 2500 g | 8 | 12420 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.08] |
| 1.21 Birth weight equal to or more than 4000 g | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.22 Apgar score less than or equal to 7 at 5 minutes | 13 | 12806 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.24] |
| 1.23 Admission to special care nursery/neonatal intensive care unit | 13 | 16260 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.03] |
| 1.24 Fetal loss before 24 weeks gestation | 12 | 15913 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.01] |
| 1.25 Maternal experience | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | Not estimable |
| 1.26 Cost | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
1.8. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 8: Healthy mother
1.17. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 17: Neonatal readmission within 28 days
1.19. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 19: Healthy baby
1.21. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 21: Birth weight equal to or more than 4000 g
1.25. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 25: Maternal experience
1.26. Analysis.

Comparison 1: Midwife continuity models versus other models of care for childbearing women and their infants (all), Outcome 26: Cost
Comparison 2. Midwife continuity models versus other models of care: variation in midwifery models of care (caseload or team).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Spontaneous vaginal birth (as defined by trial authors) | 15 | 17864 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 2.1.1 Caseload/one‐to‐one | 5 | 7101 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.00, 1.10] |
| 2.1.2 Team | 10 | 10763 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.08] |
| 2.2 Caesarean birth | 16 | 18037 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 2.2.1 Caseload/one‐to‐one | 5 | 7101 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 2.2.2 Team | 11 | 10936 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
| 2.3 Regional analgesia (epidural/spinal) | 15 | 17754 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.79, 0.92] |
| 2.3.1 Caseload/one‐to‐one | 5 | 6924 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 2.3.2 Team | 10 | 10830 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 2.4 Intact perineum | 12 | 14268 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.12] |
| 2.4.1 Caseload/one‐to‐one | 4 | 4806 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.95, 1.24] |
| 2.4.2 Team | 8 | 9462 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.13] |
| 2.5 Fetal loss at or after 24 weeks gestation | 12 | 16122 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.73, 2.13] |
| 2.5.1 Caseload/one‐to‐one | 4 | 5607 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.22, 2.06] |
| 2.5.2 Team | 8 | 10515 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.81, 2.76] |
| 2.6 Preterm birth (< 37 weeks) | 10 | 13850 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 2.6.1 Caseload/one‐to‐one | 4 | 5507 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.26] |
| 2.6.2 Team | 6 | 8343 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.32] |
| 2.7 Neonatal death (baby born alive at any gestation and dies within 28 days) | 10 | 14718 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.71] |
| 2.7.1 Caseload/one‐to‐one | 4 | 5607 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.78] |
| 2.7.2 Team | 6 | 9111 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.42, 2.77] |
Comparison 3. Midwife continuity models versus other models of care: variation in obstetric and medical risk factors (low versus mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Spontaneous vaginal birth (as defined by trial authors) | 15 | 17864 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 3.1.1 Low risk | 8 | 10983 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.02, 1.09] |
| 3.1.2 Mixed risk | 7 | 6881 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.02, 1.09] |
| 3.2 Caesarean birth | 16 | 18037 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 3.2.1 Low risk | 9 | 11156 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| 3.2.2 Mixed risk | 7 | 6881 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.85, 1.04] |
| 3.3 Regional analgesia (epidural/spinal) | 15 | 17754 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.79, 0.92] |
| 3.3.1 Low risk | 8 | 10873 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.72, 0.91] |
| 3.3.2 Mixed risk | 7 | 6881 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.83, 0.99] |
| 3.4 Intact perineum | 12 | 14268 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.12] |
| 3.4.1 Low risk | 6 | 8582 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.99, 1.25] |
| 3.4.2 Mixed risk | 6 | 5686 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.06] |
| 3.5 Fetal loss at or after 24 weeks gestation | 12 | 16122 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.73, 2.13] |
| 3.5.1 Low risk | 6 | 10692 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.58, 2.28] |
| 3.5.2 Mixed risk | 6 | 5430 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.59, 3.36] |
| 3.6 Preterm birth (< 37 weeks) | 10 | 13850 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 3.6.1 Low risk | 5 | 9625 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.36] |
| 3.6.2 Mixed risk | 5 | 4225 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.24] |
| 3.7 Neonatal death (baby born alive at any gestation and dies within 28 days) | 10 | 14718 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.71] |
| 3.7.1 Low risk | 6 | 10692 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.92] |
| 3.7.2 Mixed risk | 4 | 4026 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.09, 4.39] |
Comparison 5. Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Spontaneous vaginal birth (as defined by trial authors) | 15 | 17864 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 5.1.1 Very high HDI | 14 | 17758 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 5.1.2 High, medium, and low HDI | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.06, 2.19] |
| 5.2 Caesarean birth | 16 | 18037 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 5.2.1 Very high HDI | 15 | 17931 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 1.00] |
| 5.2.2 High, medium, and low HDI | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.39, 0.93] |
| 5.3 Regional analgesia (epidural/spinal) | 15 | 17754 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.79, 0.92] |
| 5.3.1 Very high HDI | 15 | 17754 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.79, 0.92] |
| 5.3.2 High, medium, and low HDI | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.4 Intact perineum | 12 | 14268 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.12] |
| 5.4.1 Very high HDI | 12 | 14268 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.12] |
| 5.4.2 High, medium, and low HDI | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.5 Fetal loss at or after 24 weeks gestation | 12 | 16122 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.73, 2.13] |
| 5.5.1 Very high HDI | 12 | 16122 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.73, 2.13] |
| 5.5.2 High, medium, and low HDI | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.6 Preterm birth (< 37 weeks) | 10 | 13850 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 5.6.1 Very high HDI | 10 | 13850 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 5.6.2 High, medium, and low HDI | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.7 Neonatal death (baby born alive at any gestation and dies within 28 days) | 10 | 14718 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.71] |
| 5.7.1 Very high HDI | 10 | 14718 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.71] |
| 5.7.2 High, medium, and low HDI | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
5.3. Analysis.

Comparison 5: Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), Outcome 3: Regional analgesia (epidural/spinal)
5.4. Analysis.

Comparison 5: Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), Outcome 4: Intact perineum
5.5. Analysis.

Comparison 5: Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), Outcome 5: Fetal loss at or after 24 weeks gestation
5.6. Analysis.

Comparison 5: Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), Outcome 6: Preterm birth (< 37 weeks)
5.7. Analysis.

Comparison 5: Midwife continuity models versus other models of care: variation in country setting (very high Human Development Index (HDI) > 0.8 versus high, medium, and low HDI), Outcome 7: Neonatal death (baby born alive at any gestation and dies within 28 days)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Begley 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 2004 to 2007 Study funding sources: Health Research Board (Health Information Infrastructure Grant‐EQ/2004/3) provided funding to support the introduction of the computerised Maternity Information System at two study sites, former North‐Eastern Health Board (NEHB), now Health Service Executive, Dublin North‐East (HSE‐DNE) provided funding for the study. Study authors’ declarations of interest: 1) support from the HSE‐DNE for the submitted work (travel expenses to travel to a research conference to present the literature review and methodology; PhD student stipend and travel expenses from the funding awarded; 2) awarded other grants by the HSE‐DNE, during the time of the MidU study, to conduct other studies; 3) one author at the time of the MidU study and at present, is an employee of the HSE‐DNE; all other authors, their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and 4) all authors have no non‐financial interests that may be relevant to the submitted work. Ethics approval obtained? yes – School of Nursing and Midwifery Research Ethics Committee, Trinity College Dublin approved the study on 28 March 2003. A Faculty of Health Sciences Research Ethics Committee was set up in Trinity College Dublin in 2005 and approved the study on 21 March 2005. An Ethics Committee was set up in the former NEHB in 2004 and confirmed approval of the study on 22 April 2004. Study prospectively registered? registered retrospectively on 7 September 2007 – the protocol for the MidU study was registered with the International Standard Randomised Controlled Trial Number Register (ISRCTN14973283, https://doi.org/10.1186/ISRCTN14973283) Clarification sought from authors regarding retrospective trial registration, the authors responded as follows: "Main paper notes ‘Recruitment to the main study took place from February 2005 to November 2006, with the last birth in June 2007 when the full sample size had been reached.’ TheICJMEnote the following: Do trials that began before July 1, 2005 need to be enrolled before September 13, 2005 in order to be eligible for consideration at an ICMJE journal? Trials that began before July 1, 2005: Investigators should register trials that began enrolling patients any time before July 1, 2005 as soon as possible if they wish to submit them to a journal that follows the ICMJE policy. While the ICMJE hoped that all such trials would be registered by September 13, 2005, the committee understands that the policy statement was not entirely clear. Thus, ICMJE journals will consider trials that began before July 1, 2005 that were not registered prior to September 13, 2005. However, beginning on September 13, 2005, ICMJE journals will consider such trials only if they were adequately registered before journal submission. The ICMJE journals will accept "retrospective registration" of trials that began before July 1, 2005 (retrospective meaning registration occurs after patient enrollment begins)." |
|
| Participants |
Setting: Health Service Executive, Dublin North‐East, Republic of Ireland Inclusion criteria: women were eligible for trial entry if they were: (a) healthy with an absence of risk factors for complications for labour and delivery as identified in the ‘Midwifery‐led Unit (Integrated) Guidelines for Practitioners’ (at http://www.nehb.ie/midu/guidelines.htm); (b) aged between 16 and 40 years of age; and (c) within 24 completed weeks of pregnancy Exclusion criteria: women with risk factors Participants randomised: 1101 midwife continuity model, 552 other model of care Participant demographics: Ethnicity: mixed race, with white Irish in the majority Socio‐economic indicators: not reported Social risk factors: excluded women who were current drug users or those women who smoked more than 20 cigarettes per day Parity: parity 0 = 565 midwife continuity model, 276 other model of care, parity > 0 = 536 midwife continuity model, 276 other model of care Maternal age: age (mean, SD): 29 (4.9) midwife continuity model, 28.7 (5.00) other model of care (excluded women ≥ 40 years of age and ≤ 16 years age at delivery) Smoking: not reported (excluded women that smoked more than 20 cigarettes per day) |
|
| Interventions |
Experimental: women randomised to the midwife continuity model (MLU), received antenatal care from midwives and, if desired, from their GPs for some visits. Where complications arose, women were transferred to a Consultant‐led unit (CLU) based on agreed criteria. Intrapartum care was provided by midwives in a Midwife‐led unit (MLU) with transfer to a Consultant‐led unit (CLU) if necessary. Postnatal care was by midwives in the MLU for up to 2 days, with transfer of women or neonates to CLU if necessary (and back, as appropriate). On discharge, MLU midwives visited at home, and/or provided telephone support, up to the 7th postpartum day. Target population: low risk (healthy with an absence of risk factors) Where is care provided: 2 maternity hospitals in Ireland – Our Lady of Lourdes Hospital (OLOL) in Drogheda (3200 births per year) and Cavan General Hospital (CGH), Cavan (1300 births per year), both located in large towns (28,000 and 4000 inhabitants respectively) serving a semi‐urban and rural population of mixed race, with white Irish in the majority. Both MLUs were housed within their parent hospital in re‐furbished existing accommodation, close to the main labour ward, and aimed to provide an integrated service using evidence‐based guidelines and procedural policies. Who provides care: the midwife continuity model on the MLU was provided by same small group of midwives throughout pregnancy, birth, and into the antenatal period. Antenatal care was provided by the midwives in the unit, or in an outreach clinic, and if desired by the woman’s GP. Organisation of team: care in MLU was provided by the full team of midwives (12 in OLOL and 7 in CGH) – women did not necessarily have the degree of continuity of care that they may get from caseload models of midwife led care. Role of midwife continuity model for women after they transfer due to complications in the antenatal and intrapartum period: where complications arose during the antenatal, intrapartum, or postpartum period, women could transfer to CLU, where they received usual care‐ but could be transferred back to MLU after obstetric assessment ‐ so there was no continuity of care for those who transferred out ‐ although if transferred back, would then receive COC again. Control: women randomised to consultant‐led care (CLU) received standard care Target population: low risk (healthy with an absence of risk factors) Where is care provided: 2 maternity hospitals in Ireland – OLOL and CGH, as above Who provides care: antenatal care provided by obstetricians and, if desired, the woman's GP and supported by the hospital medical team with assistance from the midwives; intrapartum care provided by midwives unless complications arose and postpartum care (2 to 3 days in hospital) provided by midwives, overseen by consultants. Women were discharged into the care of public health nurses. Organisation of team: antenatal care provided by obstetricians and GP with support from medical and midwifery team; intrapartum and postpartum care (2 to 3 days in hospital) provided by midwives, overseen by consultants. Women were discharged into the care of public health nurses. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | Women were randomised to MLU or CLU in a 2:1 ratio. There were 6 women lost to follow‐up from MLU (n = 5 moved house/country during pregnancy and n = 1 discontinued intervention – had home birth) – so we have outcome data for the 1 home birth, but not for 5 women who moved (1101 randomised to MLU, 1096 available data for analysis) There were 5 women lost to follow‐up from CLU (n = 3 moved house/country during pregnancy n = 2 discontinued intervention – had home birth) so no available data for 3 (552 randomised to CLU, 549 available data for analysis) Kenny 2015 reports an economic analysis ‐ a comparison of the cost of care of the 2 types of services. We have described these results above ‐ data added at the 2016 update. Any imbalance between baseline characteristics: baseline characteristics were similar. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random integers were obtained using a random number generator…" |
| Allocation concealment (selection bias) | Low risk | "…an independent telephone randomisation service." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible to blind. Access to MLU care was only through the study, so carers were aware that all women in the midwife‐led unit were included in the MidU study. Women allocated to CLU not masked either. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only some blinding of outcome assessment: "Assessors for certain outcomes, such as laboratory tests, were blinded to study group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 5 midwife‐led care, 3 CLC |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting: the trial was registered after the study had completed, although all expected outcomes as reported in the methods are presented. However, in the trial registration there are a number of outcomes that do not appear to have been reported in the published thesis or full paper, and for this reason we have assessed this domain as ‘unclear’, although we do not have any serious concerns. |
| Other bias | Low risk | No other bias identified |
Biro 2000.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 1996 to 1998 Study funding sources: the study was supported by a 3‐year programme grant (1994/95 to 1996/ 97) from the Australian Commonwealth Department of Health and Human Services, Canberra, Australian Capital Territory (A.C.T.) Study authors’ declarations of interest: not reported Ethics approval obtained? Monash Medical Centre’s Human Research and Ethics Committee gave approval to conduct the study in November 1995. Study prospectively registered? not reported; unable to contact authors, but trial pre‐dated requirement for prospective registration |
|
| Participants |
Setting: public tertiary hospital, Monash Medical Centre, Melbourne, Australia Inclusion criteria: participants included women at low and high risk of complications Exclusion criteria: women who requested shared obstetric care, needed care in the maternal‐fetal medicine unit, were > 24 weeks' gestation, did not speak English Participants randomised: 502 midwife continuity model, 498 to other model of care Participant demographics: Ethnicity: not reported but maternal (non) migration is reported (see below) Socio‐economic indicators: not reported but education is reported. Secondary school to year 12 (N (%)) 297 (61.6) midwife continuity model, 298 (61.3) other model of care. Social risk factors: indicator of migration status: born in Australia (N (%)): 253 (50.4) midwife continuity model, 261 (52.4) other model of care Parity: expecting first baby (N (%): 320 (63.7) midwife continuity model, 304 (61.0) other model of care Maternal age: (mean, SD): 28.2, 5.2 midwife continuity model, 28.3, 5.4 other model of care Smoking: not reported |
|
| Interventions |
Experimental: team of 7 full‐time midwives who provided antenatal, intrapartum, and some postnatal care in hospital in consultation with medical staff. Doctors and team midwife jointly saw women at 12 to 16, 28, 36, 41 weeks. Women at high risk of complications had individual care plans. Target population: this included both high‐risk and low‐risk women. Where is care provided: it seems to be hospital‐based. Team midwives were rostered to Monday/Tuesday clinics. Who provides care: a team of 7 full‐time midwives who provided antenatal, intrapartum, and postnatal care to the same group of women in consultation with medical staff 80% of women in team care compared with 0.3% of women in standard care met the midwife who cared for them in labour. A team midwife was present at 90% of all team care women’s labours. Organisation of team: team midwifery model was characterised by continuity of midwifery care from early pregnancy to the early postpartum period (p169, para 3). A team midwife saw low‐risk women at each visit, with 3 scheduled visits with the obstetric staff at 12 to 16, 28, and 36 weeks’ gestation. If a woman remained undelivered at 41 weeks, she had another obstetric consultation. High‐risk women had an individualised care plan developed in consultation with a senior consultant. The frequency of obstetric visits was determined by the woman’s high‐risk status. Those requiring visits with the obstetric staff also saw a team midwife at the visit. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: no information on transfer or continuity of midwifery care on/after transfer is provided. However, it has stated: “Those requiring visits with the obstetric staff also saw a team midwife at the visit”. Control: various options of care including shared care between GPs in the community and hospital obstetric staff, shared care between midwives in a community health centre and hospital obstetric staff, care by hospital obstetric staff only and, less commonly, care by hospital midwives in collaboration with obstetric staff. Women within these options experienced a variable level of continuity of care during their pregnancy, from seeing the same midwife or doctor at most visits to seeing several doctors and midwives. Target population: this included both high‐risk and low‐risk women Where is care provided: antenatally ‐ community and hospital settings Who provides care: several options were available within standard care. Antenatally: these included shared care between general practitioners in the community and hospital obstetric staff, shared care between midwives in a community health centre and hospital obstetric staff, care by hospital obstetric staff only and, less commonly, care by hospital midwives in collaboration with obstetric staff, similar to antenatal team care. Intrapartum: irrespective of the option of antenatal care within standard care, women were cared for by a variety of doctors and midwives during labour. A doctor they had met during pregnancy could care for them, but this was unusual. They had not met the midwives who provided their care during labour. After birth, women in standard care were transferred to one of two postnatal units where they were cared for by a variety of doctors and midwives. Organisation of team: antenatally: women within these options experienced a variable level of continuity of care during their pregnancy, from seeing the same midwife or doctor at most visits to seeing several doctors and midwives. Women had not met the midwives who provided their care during labour. Only 0.3% of women in standard care met the midwife who cared for them in labour. Postpartum: women were also cared for by a variety of professions. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | Any imbalance between baseline characteristics: 2 groups similar at baseline. 80% of experimental group and 0.3% of standard group had previously met midwife attending labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocations were computer generated..." |
| Allocation concealment (selection bias) | Low risk | "...the research team member telephoned the medical records staff and asked them to select an envelope with the randomized treatment allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There is no information available about whether blinding was carried out, but based on the intervention, it is improbable that blinding took place. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes other than maternal experience: 53/502 (11%) vs 59/498 (12%) loss to follow‐up for intervention and standard groups, respectively (no statistically significant differences between the groups in the participant characteristics were identified) Maternal experience: Follow‐up questionnaires were sent to 443 and 430 women, respectively. Reasons for not sending questionnaires were perinatal death (team = 5; standard = 4), and inadvertently not sent (team = 1; standard = 5). There was a statistically significant difference in the return rates between the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in the results. |
| Other bias | Low risk | No other bias identified |
Fernandez Turienzo 2020.
| Study characteristics | ||
| Methods |
Study design: a hybrid implementation‐effectiveness, randomised, controlled, unblinded, parallel‐group pilot trial at an inner‐city maternity service in London (UK) Duration of study: recruitment was between 9 May 2017 and 30 September 2018, with follow‐up to 31 May 2019 Study funding sources: the trial was funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research South London (NIHR CLAHRC South London), now recommissioned as NIHR Applied Research Collaboration (ARC) South London. Study authors’ declarations of interest: one author reports support from Mirvie and Vidya Health Limited outside the submitted work. One author reports grants from Hologic outside the submitted work. One author is partly funded by Tommy’s and NIHR ARC South London. All other authors declare no competing interests. Ethics approval obtained? yes, regulatory and ethical approvals were obtained from the Health Research Authority and the London South East National Health Service Research Ethics Committee (REC Ref 7/LO/0029; ID 214196). |
|
| Participants |
Setting: inner‐city maternity service in London, UK (Lewisham and Greenwich NHS Trust) Inclusion criteria: asymptomatic pregnant women attending antenatal care at less than 24 weeks’ gestation if they fulfilled one or more of the following: previous cervical surgery, cerclage, premature rupture of membranes, PTB, or late miscarriage; previous short cervix or short cervix this pregnancy; or uterine abnormality and/or current smoker Exclusion criteria: pregnant women aged less than 18 years at recruitment, those with multiple pregnancies, or those already receiving care from a specialist midwifery team (e.g. women with severe mental illness, alcohol, and substance misuse) Participants randomised: experimental POPPIE group n = 169 (outcome data available for 168); control standard group n = 165 (outcome data available for 163) Participant demographics: Ethnicity n (%): POPPIE Group ‐ white 98 (58.4), black 33 (19.6), Asian 13 (7.7), mixed 13 (7.7), other 11 (6.5), standard group ‐ white 108 (65.5), black 33 (20.0), Asian 7 (4.2), mixed 8 (4.8), other 9 (5.5) Socio‐economic indicators n (%): Deprivation Index quintiles 1‐2 (most deprived 40% of population) ‐ POPPIE group 113 (70.2); standard group 109 (67.7) Social risk factors n (%): past or present history of domestic violence ‐ POPPIE group 14 (8.6); standard group 8 (4.9)/past or present history of recreational drug use ‐ POPPIE group 8 (4.8); standard group 12 (7.3) Parity: nulliparous ‐ POPPIE group 49 (29.2); standard group 61 (37.0) Maternal age ‐ mean age in years (SD): POPPIE group 31.85 (5.55); standard group 31.78 (5.39) Smoking n (%): smokers at booking ‐ POPPIE group 51 (30.4); standard group 47 (28.5) Mean number of cigarettes per day POPPIE group 2.96 (1.44); standard group 2.74 (1.33) |
|
| Interventions |
Experimental: POPPIE group received continuity of antenatal, labour, birth, and postnatal care predominantly by a named (or primary) midwife, who was backed up by a partner midwife and other team colleagues Target population: high‐risk, third of local population were Black, Asian and Minority Ethnic (BAME) groups and overall the community had high levels of social deprivation and high levels of PTB (2 or more risk factors for PTB). More than half of the participants were overweight or obese, with nearly 30% having at least one pre‐existing medical condition. Where is care provided: the POPPIE group received continuity of antenatal, labour, birth, and postnatal care in the hospital, community, or at home. Who provides care: predominantly by a named (or primary) midwife, who was backed up by a partner midwife and other team colleagues Organisation of team: the POPPIE team consisted of 6 whole‐time equivalent midwives – this included a senior lead team midwife – they were hospital‐based. Caseload: each midwife was employed on an annual salary to work a flexible cycle of 162 hours per month to provide continuity of care to 35 births per year (team leader had caseload of 24). Some antenatal, intrapartum, and/or postpartum care was provided in consultation with medical staff and other services (e.g. GPs, haematologists, anaesthetists, physiotherapists, mental health specialists, interpreters, social services) and with rapid access within the hospital to a senior obstetrician with expertise in PTB. Care was provided throughout pregnancy, labour, birth and postnatal care. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: we contacted the author team who confirmed as per the POPPIE operating guidelines that the intention was too look after these high‐risk women even if they developed complications and transferred out: "Care is provided for all women however complex their medical or obstetric history is wherever they choose to birth their baby. It is acknowledged that women at increased risk of preterm birth or other complications may require additional obstetric, specialist and midwifery support. This extra support will be coordinated by the POPPIE team". Control: received standard maternity care in line with usual practice at the study site, during antenatal, labour, birth, and postnatal periods. The key difference was that women receiving standard care did not receive continuity of care during the childbearing continuum, and could potentially see a different midwife at each visit. Target population: high‐risk, third of local population were Black, Asian and Minority Ethnic (BAME) groups and overall the community had high levels of social deprivation and high levels of PTB (2 or more risk factors for PTB). More than half of the participants were overweight or obese, with nearly 30% having at least on pre‐existing medical condition. Where is care provided: community and hospital antenatal and postnatal clinics, labour ward and postnatal ward. Home visits also offered as part of postnatal care. Who provides care: antenatal care was provided by different midwives working in the community, children’s centres, and/or hospital. Some antenatal, intrapartum, and/or postpartum care was provided in consultation with hospital medical staff as required. Organisation of team: rostered midwifery and medical staff provided care during labour and birth on the labour ward and/or birthing centre and postnatal care on the postnatal ward. Women were also offered midwifery visits at home and in community postnatal clinics following discharge from hospital. Midwives in standard care group had a linked obstetrician, but not necessarily one who specialises in PTB. Midwives did not work directly with them – but could contact on‐call doctors/staff in other services to discuss any clinical concerns or issues and make referrals. Both groups: in line with hospital guidelines, women in both POPPIE and standard care groups being at increased risk of PTB followed the same obstetric care pathway:
|
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | 169 women assigned to POPPIE care:
165 assigned to standard care:
Main differences between intervention groups: the POPPIE team was hospital‐based and had rapid access to a senior consultant obstetrician with expertise in PTB. The key difference between the POPPIE and standard group was that women receiving standard care did not receive planned continuity of midwifery care along the childbearing continuum and midwives in the standard group had a linked obstetrician, but not necessarily one who specialised in PTB. Midwives did not work directly with them, relying on contacting on‐call doctors and staff in other services to discuss any clinical concerns, issues, or queries or to make referrals. Any imbalance between baseline characteristics: baseline characteristics were reported to be similar between the 2 groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment in a ratio of 1:1 via a secure computerised randomisation system (MedSciNet). They also used a minimisation algorithm with a random element to ensure balanced groups regarding previous PTB and smoking at booking. |
| Allocation concealment (selection bias) | Low risk | Central randomisation and use of a "secure system" ‐ contacted author team for clarification: "Research assistant and all midwives logged in to the database, added a participant and baseline information, confirmed eligibility and consent form, and then clicked the bottom 'randomise' ‐ then the MEdscinet program told us if the woman was allocated to standard care or poppie (there was no way we could know what the woman was going to be allocated to, neither the following women)". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and clinicians not possible due to the nature of the intervention. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study assignment was masked to the statistician and the researchers who analysed the data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for clearly in study flow diagram. Analyses reported to be by intention to treat – for all participants whose outcomes were known. |
| Selective reporting (reporting bias) | Low risk | All expected clinical primary and secondary maternal and neonatal outcomes have been reported – as per protocol and methods in trial report. Cost reported in trial registation and protocol, but not in published reports of trial; however, the authors clarified that cost is in a pending publication of an economic evaluation. |
| Other bias | Low risk | No other bias detected |
Flint 1989.
| Study characteristics | ||
| Methods |
Study design: RCT, Zelen design Duration of study: 1983 to 1985 Study funding sources: grant from South West Thames Regional Health Authority, a nursing research bursary from the Wellington Foundation Study authors’ declarations of interest: not reported Ethics approval obtained? not reported Study prospectively registered? not found |
|
| Participants |
Setting: tertiary hospital and community settings, St George's Hospital, London, UK Inclusion criteria: low risk of complications who booked at the study hospital and were likely to receive all their antenatal care at that hospital Exclusion criteria: under 5 feet tall, serious medical problems, previous uterine surgery, past obstetric history of > 2 miscarriages/TOP/SB/NND, Rh antibodies Participants randomised: 503 team midwifery, 498 to standard care (shared care) Participant demographics: Ethnicity: Caucasian: 73% team midwifery, 63% shared care; Asian: 10% team midwifery, 18% shared care; Afro‐Caribbean: 15% team midwifery, 15% shared care; other: 2% team midwifery, 4% shared care Socio‐economic indicators: Married: 76% team midwifery, 78% shared care *In paid employment at 37 weeks: 7% team midwifery, 7% shared care *Housing: own home 51% team midwifery, 51% shared care; rented 32% team midwifery, 32% shared care; other 17% team midwifery, 17% shared care Social risk factors: not specifically reported Parity: primiparous: 57% team midwifery, 58% caseload care Maternal age: mean ages (SD): 25.8 (5.1) team midwifery, 25.4 (5.0) in shared care Smoking: current smokers: 30% team midwifery, 22% shared care *Respondents to a 37‐week questionnaire (277 in team midwifery, 268 in shared care) |
|
| Interventions |
Experimental: team midwifery Target population: women at low risk of complications living in predefined geographic area Where is care provided: antenatal, intrapartum, and postnatal care in the hospital or the community, and postnatal care in the community. Option for place of birth: hospital labour ward Who provides care: a team of 4 midwives with the backup of hospital obstetricians Organisation of team: midwifery team give continuity of care during pregnancy, labour, and the puerperium. The midwives saw the women at every antenatal visit except for the first booking, and at 36 and 41 weeks of pregnancy when they saw either a consultant obstetrician or a senior registrar (obstetrician seen at any other time requested as appropriate). The midwives saw women in the antenatal clinic, but they could also visit at home antenatally, for example to check blood pressure or supervise women. When a woman under the team midwifery went into labour, she would bleep the midwife on call whom she would have got to know during pregnancy. Women were transferred to the postnatal ward by the team midwife and visited twice daily by midwives from the team. On return home, women who live within a reasonable distance from the hospital would be visited by a team midwife for the required length of time. No details of arrangements for out‐of‐hours care or level of continuity of care in team. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: "Each woman would see her Consultant Obstetrician at any other time during pregnancy if the midwives were concerned about any condition the women might develop". No further details provided. Control: standard care (shared care) Target population: women at low risk of complications Where is care provided: routine hospital care. Option for place of birth: not specified (likely also hospital labour ward). Who provides care: an assortment of midwives and obstetricians Organisation of team: conventional hospital care. No details provided. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | Any imbalance between baseline characteristics: at baseline, more Asian women in control group (18% vs 10%) and more smokers in experimental group (30% vs 22%). Sub‐analysis of case notes found that 98% of experimental group and 20% of standard group had previously met midwife attending labour. Discrepancy in instrumental birth data. Data taken from report and not published paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | "...randomly allocated, using sealed opaque envelopes, to one of two forms of hospital‐based care…" (Does not state who created the envelopes, whether the envelopes had other additional security measures like being sequentially numbered, or who opened the envelopes). "...randomised into two groups by pinning sealed envelopes on their notes containing either the motto KNOW YOUR MIDWIFE or CONTROL GROUP". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There is no information available about whether blinding was carried out, but based on the nature of the intervention, it is unlikely that blinding took place. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: all outcomes other than maternal experience: Loss to follow‐up = 15 team care, 19 standard care (moved away) Outcome group: maternal experience: Loss to follow‐up: 1st questionnaire: 8 team care, 6 standard care 2nd questionnaire: 4 team care and 6 standard care 3rd questionnaire: 26 team care and 34 standard care Similar proportions of missing outcome data in the experimental and control groups. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in the results. |
| Other bias | Low risk | No other bias identified |
Gu 2013.
| Study characteristics | ||
| Methods |
Study design: described as a "two‐group randomised controlled trial" Duration of study: September 2011 to December 2011 Study funding sources: The Nursing School of Fudan University Fund (No. FNF2011004) Study authors’ declarations of interest: none declared Ethics approval obtained? yes, Ethics Committee of Obstetrics and Gynaecology Hospital, Fudan University (see correspondence with authors – provided a copy of ethical approval – awaiting translation) |
|
| Participants |
Setting: Obstetrics and Gynaecology Hospital of Fudan University, Shanghai, China Inclusion criteria: primiparous women booking for care at antenatal clinics were eligible for the trial if they met the following inclusion criteria: Mandarin speaking: able to speak, read and write in Chinese; 29 to 30 weeks gestation at recruitment; low risk at recruitment in absence of medical or obstetric complications; singleton pregnancy Exclusion criteria: planned elective caesarean section and considered at increased medical or obstetric risk (based on criteria developed by midwives’ clinic team in consultation with obstetricians) Participants randomised: experimental group, total number randomised n = 55; control group, total number randomised n = 55
Participant demographics: Ethnicity: Chinese Socio‐economic indicators (education level, vocation): Education level: high school or below: 9.4% intervention group, 11.3% control group; college: 30.2% intervention group, 24.5% control group; bachelor: 47.2% intervention group, 47.2% control group; master or above: 13.2% intervention group, 16.9% control group Vocation: company employee: 33.9% intervention group, 35.8% control group; technician: 37.7% intervention group, 33.9% control group; liberal profession: 13.2% intervention group, 9.4% control group; unemployed: 15.1% intervention group, 20.7% control group Social risk factors: not reported Parity: mean gravida (SD): 1.40 (0.72) intervention group, 1.26 (0.66) control group Maternal age: mean ages (SD): 28.74 (2.42) intervention group, 29.28 (2.68) control group Smoking: not reported |
|
| Interventions |
Experimental: midwifery antenatal clinic service Target population: Chinese women at low risk of complications Where is care provided: antenatal and intrapartum care in the hospital (obstetric antenatal clinic and labour ward respectively). Immediate postnatal care in the hospital. Who provides care: a team of 10 full‐time midwives and obstetricians Organisation of team: the midwifery antenatal service was provided by a group of 10 midwives, trained to join the midwife‐led clinic. To be eligible to be part of the team, the midwives had at least 10 years’ clinical midwifery experience, delivered over 120 babies every year and had to be excellent communicators and have excellent midwifery skills. They were offered one‐to‐one training sessions by the research team. The training focussed on the research components of the trial and personal skills for each meeting with the woman and partner. Midwives in the new service were responsible for antenatal care for all women allocated to the intervention group (third trimester only, every 2 weeks from 28 to 24 weeks gestation, every week from 35 to 40; every 3 days after 40 weeks). Women saw the midwife at each attendance at obstetrician’s antenatal clinic in the outpatient department. The woman’s husband was also encouraged to join the midwives’ clinic. Midwife would take the time to listen to the women and for the women and partners to ask questions regarding information and support. The midwife usually focused on antenatal check‐ups, consultation, making birth plans, and parent education, and collaborated with other health professionals as necessary. A midwife would be on call for the woman’s labour and birth except in special circumstances such as annual leave, sick leave, having already worked more than 16 h in a 24‐h period and having more than one woman in labour – in which case care would be provided by an associate midwife. Each woman had the opportunity of having continuous one‐to‐one care from the onset of labour to 2 h postpartum. Onset of labour was defined as when the cervix was 2 cm dilated, with contractions occurring 5 to 6 min apart. No more details of arrangements for out‐of‐hours care, postnatal care, or level of continuity of care in team. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: unclear; not reported that any woman transferred from midwifery care Control: routine obstetrician‐led antenatal care Target population: Chinese women at low risk of complications Where is care provided: antenatal and intrapartum care in the hospital (obstetric antenatal clinic and labour ward respectively). Immediate postnatal care in the hospital. Who provides care: obstetrician at antenatal clinic and during labour by whichever midwife and obstetricians rostered for duty Organisation of team: women would line up for some time in order to register at the hospital clinics and then they would be seen by an obstetrician – this could be a different person at each visit. When women were in labour in the hospital, they would be cared for by whichever midwives and obstetricians were on duty. At the onset of labour, each woman had the opportunity of receiving one‐to‐one continuity of care by a duty midwife from the onset of labour to 2 h postpartum. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes |
Total number randomised to each group: n = 55
Any imbalance between baseline characteristics: baseline characteristics were reported to be similar between the 2 groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Referred to using a computer random number generator: "A randomisation series was computer generated". |
| Allocation concealment (selection bias) | Low risk | Reports that the randomisation scheme was independently prepared by a clerical assistant who was not involved in determining eligibility. The list was also reportedly kept concealed in sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Given the nature of the intervention, it would not have been possible to blind participants or personnel. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinical data were collected through retrospective review of medical records by a research team not involved in providing care. It was probably possible to tell which group from the records (even though it says there was no identifying mark on the control group records). However, the data were retrospectively collected – so unless the researchers altered the clinical outcome in the records, the outcome collection was probably not influenced by lack of blinding, but we cannot be certain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are available for 53 out of 55 women in each group. |
| Selective reporting (reporting bias) | High risk | Some outcomes specified in the translation of the protocol do not appear to have been reported in the trial report (instrumental or length of labour stage 1, 2, 3). |
| Other bias | Low risk | No other apparent bias |
Harvey 1996.
| Study characteristics | ||
| Methods |
Study design: parallel randomised controlled trial Duration of study: 1992 to 1994 Study funding sources: the research was supported by grants from the Alberta Foundation for Nursing Research and the Alberta Association of Registered Nurses. Funding for the Nurse‐Midwifery Programme was from the Job Enhancement Advisory Committee, Alberta Health. Study authors’ declarations of interest: not reported Ethics approval obtained? yes, approved by the conjoint medical ethics committee Study prospectively registered? no (pre‐2010) |
|
| Participants |
Setting: tertiary care hospital and community settings in Alberta, Canada Inclusion criteria: women at low risk of complications (according to the Alberta perinatal risk scoring system) who requested and qualified for nurse‐midwife care who requested and qualified for nurse‐midwife‐led care Exclusion criteria: past history of caesarean section, primigravidas < 17 or > 37, > 24 weeks' gestation at time of entry to study Participants randomised: 109 team midwife‐led care, 109 to standard care (physician care) Participant demographics: Ethnicity: Caucasian: 96.1% team midwife group, 97.8% physician group Asian: 2.8% team midwife group, 2.2% physician group Aboriginal: 1.1% team midwife group, 0.0% physician group Socio‐economic indicators: education (years, SD): 16.0 (2.49) team midwife group, 15.23 (2.32) physician group Social risk factors: (FTC1) not specifically reported Parity: nulliparas: 55.4% team midwife group, 47.3% physician group Maternal age: age (mean, SD): 30.26 (3.77) team midwife group, 30.9 (4.33) physician group Smoking: smokers at conception: 4.9% team midwife group, 9.7% physician group |
|
| Interventions |
Experimental: nurse‐midwifery clinic Target population: women at low risk of complications Where is care provided: antenatal and intrapartum care in the hospital and postnatal care in the community Who provides care: a team of 7 nurse‐midwives and linked obstetricians Organisation of team: women were seen for antenatal care in a nurse‐midwifery clinic with a rotation schedule designed to ensure that the women would meet as many of the nurse‐midwives as possible. Obstetrician was seen at booking and at 36 weeks (to confirm low‐risk status). Apart from these 2 routine obstetric visits, the nurse‐midwives made autonomous decisions on the care they provided, made referrals to, or consulted with, doctors and other health professionals when needed. A nurse‐midwife from the team provided care throughout the labour, delivery, and immediate postnatal period. Postnatal follow‐up occurred on the postnatal unit or at home by a member of the team, and a 6‐week follow‐up visit was conducted in the nurse‐midwifery clinic. The team of nurse‐midwives worked in collaboration with a group of obstetricians, one of whom was linked to the programme and saw most of the women for their routine visits. One obstetrician from the group was available on call in the hospital at all times for consultation or referral as required by the nurse‐midwives. No details of arrangements for out‐of‐hours care or level of continuity of care in team. Protocols and guidelines for the care were based on the midwifery philosophy and standards of practice developed by the Alberta Association of Midwives. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: not specified, but the paper does say that "two subjects, one in each group, were excluded after randomization at the first antenatal visit; one had anti‐kel antibodies, and the other showed a fetal anomaly on ultrasound examination and thus did not meet the inclusion criteria". Thus, it might be possible that women who developed complications in antenatal care were transferred to physician care. Comparison intervention: physician care Target population: women at low risk of complications Where is care provided: not specified (likely city hospitals) Who provides care: physician care (family practice or obstetrician), which women chose from the area following standard referral processes (all city hospitals were represented in the physician selections) Organisation of team: not specified |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes |
Any imbalance between baseline characteristics: at baseline, more women in the experimental group had a significantly longer period in education than women in the control group (16 years vs 15.23 years). It appears more women in the control group were smokers at conception too (9.7% vs 4.9 respectively, non‐significant?) Process of delegation of health care, approved by the hospital medical advisory committee, was used to facilitate the provision of primary care by nurse‐midwives in a country where licensing was not available. Level of continuity not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…envelopes containing a computer‐generated random allocation." |
| Allocation concealment (selection bias) | Low risk | "...using a series of consecutively numbered, sealed, opaque envelopes..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There is no information available about whether blinding was carried out, but based on the intervention, it is improbable that blinding took place. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: all outcomes other than maternal experience: Loss to follow‐up calculation: 218 women recruited and randomised; 24 attritions but not reported how many in each arm. 9 women (4 in each arm) experienced spontaneous abortion after randomisation but before 20 weeks’ gestation. After these attritions, the trial included in analysis: 101 in nurse‐midwife care; 93 in standard care. We include all women randomised women with outcome data (ITT: 105 (101 + 4) in nurse‐midwife care; 97 (93 + 4) in standard care). Outcome group: maternal experience: Overall 194/218 were retained to completion of questionnaires at 36 weeks’ gestation, and 2 h, 48 h, 2 and 6 weeks postpartum (101 in team midwifery care, 93 in physician care). |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in the results. |
| Other bias | Low risk | No other bias identified |
Hicks 2003.
| Study characteristics | ||
| Methods |
Type of study design: parallel, 2‐group, individual randomised trial Study dates: unclear Study funding sources: unclear Study authors’ declarations of interest: none stated Ethics approval obtained? yes; "Ethical approval was formally obtained from the Local Research Ethics Committee" Study prospectively registered? no |
|
| Participants |
Setting: tertiary hospital and community, the city not stated but UK Inclusion criteria: women at low risk of complications Exclusion criteria: not stated Participants randomised: 100 team midwife‐led care and 100 to standard care (shared care) Participant demographics: Ethnicity/socio‐economic indicators: there are no ethnicity/socio‐demographic details provided for women in the trial, however a preparatory stage of the study consisting of a questionnaire survey of 100 randomly selected women delivering in the hospital found that 75% were Caucasian, 25% were from ethnic minorities, 51% were employed, and 49% were unemployed. Social risk factors: see above Parity: mean number of previous births: 2.4 team care, 2.1 standard care Maternal age: mean ages: 29.9 team care, 28.2 standard care Smoking: not reported |
|
| Interventions |
Experimental intervention: Target population: women at “low risk” of complications Where is care provided: in both hospital and community Who provides care: a team of 8 midwives Organisation of team:
Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period:
Total number randomised: n = 100 Control/comparison intervention: Target population: women at “low risk” of complications Where is care provided: in both hospital and community Who provides care: a team of 8 midwives Organisation of team: shared care between community and hospital midwives and GPs and obstetricians when necessary. A mid‐trimester scan offered at 20 weeks, with another check‐up at 41 weeks if necessary. Antenatal care mainly in the community, either at the GP surgery or at a community clinic. Women delivered by hospital midwife or community midwife if under domino scheme (1 midwife provides care for a woman throughout pregnancy, accompanies her into hospital for birth and returns home with her and baby a few hours after the birth, and care in postnatal period). "Postnatal care is provided in hospital by hospital midwives and following discharge would be undertaken by community midwives. The midwives at each stage of care would not necessarily be known to the mothers." Total number randomised: n = 100 |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | Any imbalance between baseline characteristics: maternal experience measured by questionnaire with multiple items. Overall, "Women in the pilot group …. were generally more satisfied with their care…..felt that they had more choice over a variety of aspects of care". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes "...had been shuffled previously by an individual not involved in the recruitment process, and then numbered consecutively" (p620 and 621) |
| Allocation concealment (selection bias) | Low risk | "Allocation was undertaken by giving each woman a sealed envelope containing one of the care options." "While this process of allocation did not use the preferred approach of random number tables, the critical component of randomization, ie concealment of treatment allocation until after the woman had been entered into the trial, was achieved." (p620‐1) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There is no information available about whether blinding was carried out but, based on the intervention, it is improbable that blinding took place. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up = 19% (n = 19) team care and 8% (n = 8) standard care, due to non‐response to questionnaires Judged as high risk given the differences between the proportions of missing outcome data in the experimental and comparator groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported in the results. |
| Other bias | Low risk | No other bias identified |
Homer 2001.
| Study characteristics | ||
| Methods |
Study design: RCT, Zelen method Duration of study: 1997 to 1998 Study funding sources: National Health and Medical Research Council, the NSW Health Department and South East Health Study authors’ declarations of interest: none Ethics approval obtained? Evidence of ethical approval documented in the report "approved by the Australian National Health & Medical Research Council, and the New South Wales Health Dept." Study prospectively registered? N/A prior to 2010. Protocol not available due to age of paper. |
|
| Participants |
Setting: public tertiary hospital and community, Sydney, Australia Inclusion criteria: women at low and high risk of complications Exclusion criteria: women more than 24 weeks' gestation at their first visit to the hospital, women with an obstetric history of 2 previous caesareans or a previous classical caesarean and medical history of significant maternal disease Participants randomised: 639 team midwife‐led care, 643 standard care (shared care) Participants reported: 550 team midwife‐led care, 539 standard care Ethnicity n (%): defined as language spoken in country of birth. Team midwife‐led care: English‐speaking: 256 (46.5), Chinese‐speaking 90 (16.4), Arabic‐speaking 86 (15.6), other non‐English speaking 116 (21.1), unknown 2 (0.4). Standard care: English‐speaking: 256 (47.5), Chinese‐speaking 93 (17.3), Arabic‐speaking 87 (16.1), other non‐English speaking 98 (18.2), unknown 5 (0.9) Socio‐economic indicators n (%): reported as education level, married or de facto relationship, and employed outside home Education level: team midwife‐led care: none/primary school 16 (2.9), secondary school 206 (37.8), tertiary 154 (28.3), not reported 169 (31) Standard care: none/primary school 10 (1.9), secondary school 201 (37.5), tertiary 138 (25.6), not reported 187 (34.9) Married or de facto relationship: team midwife‐led care: 516 (95), standard care: 505 (94) Employed outside home: team midwife‐led care: 274 (50), standard care: 255 (48) Social risk factors n (%): not reported Parity: team midwife‐led care: nulliparity 253 (46), standard care: nulliparity 248 (46) Maternal age ‐ mean age in years (SD): Team midwife‐led care: 28.2, standard care:28 Smoking n (%): not reported |
|
| Interventions |
Experimental: community‐based model of continuity of care Target population: mixed risk (excluding significant maternal disease as defined below under 'Both groups' section), living in a metropolitan catchment area where 35% of the population were born overseas Where is care provided: 2 antenatal clinics in community centres. One clinic was based in an early childhood centre and the other in a family planning clinic, chosen due to the demographics of the areas, the suitability of the facilities, and the accessibility and parking arrangements. After the birth, women could either choose to remain in hospital for postnatal care with community‐based midwives or be discharged early and receive domiciliary care by the community‐based midwives. Who provides care: a team of 6 full‐time midwives provided care for 300 women per year in the community setting Organisation of team: 2 midwives and an obstetrician or obstetric registrar attended each clinic. This meant that the community‐based team continued to care for women who developed complications antenatally. Women generally saw 3 or 4 of the community‐based midwives during the antenatal period. An informal evening at which the women could meet all 6 midwives was held at each site every 2 months. One midwife from each community‐based team was always on call for women in labour and to provide advice and information. The on‐call community‐based team midwife provided care during the labour and birth in the delivery suite at the hospital. Caseload:a team of 6 full‐time midwives provided care for 300 women per year: that is, 50 births per midwife per year, or 25 births per month per team. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: "Women who developed medical complications during their pregnancy remained in the group to which they were randomised... Two midwives and an obstetrician or obstetric registrar attended each clinic. This meant that the community‐based team continued to care for women who developed complications antenatally and transfer to standard care was unnecessary... The on call community‐based team midwife provided care during the labour and birth in the delivery suite at the hospital. For women who underwent an elective or an emergency caesarean section, the community‐based midwife continued to provide midwifery care in the operating theatre." Control: standard care was provided in the hospital‐based antenatal clinic, the delivery suite, and the postnatal ward. Midwives and doctors saw women in the antenatal clinic. Women with risks were seen by an obstetrician or obstetric registrar. Low‐risk women were generally seen by midwives. Hospital‐based antenatal care could also include visits to the women's general practitioner in a system known as GP shared care. Midwives and doctors on duty at the time provided care in the delivery suite and the postnatal ward. Standard care was characterised by a lack of continuity of care across the antenatal, intrapartum, and postnatal periods as a large number of clinicians provided care. Target population: mixed risk (excluding significant maternal disease as defined below under 'Both groups' section), living in a metropolitan catchment area where 35% of the population were born overseas. Where is care provided: standard care was provided in the hospital‐based antenatal clinic, the delivery suite, and the postnatal ward. Who provides care: midwives and doctors saw women in the antenatal clinic. Women with risks were seen by an obstetrician or obstetric registrar. Low‐risk women were generally seen by midwives. Hospital‐based antenatal care could also include visits to the women's general practitioner in a system known as GP shared care. Midwives and doctors on duty at the time provided care in the delivery suite and the postnatal ward. Organisation of team: standard care was characterised by a lack of continuity of care across the antenatal, intrapartum, and postnatal periods as a large number of clinicians provided care. Both groups The trial was conducted in a New South Wales public hospital situated in a metropolitan area. Women were eligible for the trial if they were less than 24 weeks of gestation at their first visit, lived in the designated catchment area, and planned to have their baby in the delivery suite at the hospital. Exclusion criteria included the presence of significant maternal disease (for example, renal disease with impaired renal function, essential hypertension or insulin dependent diabetes), 2 previous caesarean sections or a previous classical caesarean section. Women who developed medical complications during their pregnancy remained in the group to which they were randomised. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes |
Any imbalance between baseline characteristics: baseline characteristics were reported to be similar between the 2 groups 639 women assigned to team midwifery care:
643 women assigned to standard care:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Referral letters from general practitioners provided the information on which to register women in the trial. A pre‐prepared list was generated using computer generated random numbers and women were stratified by parity." |
| Allocation concealment (selection bias) | Low risk | "Assignment occurred prior to the woman's first hospital visit. Women were randomised to either the community‐based group or the control group (standard care) prior to obtaining consent. A remote randomisation system was used to ensure allocation concealment. The allocation was not revealed until the woman's details were recorded on the list thus removing the chance of biasing the order in which women were registered." "The trial used the randomised consent design proposed by Zelen18. Women were randomised to either the community‐based group or the control group (standard care) prior to obtaining consent. Women who were selected to the community‐based model were then offered this option. These women were still able to reject the offer and receive standard care, however, they were still included in the analysis. Women in the control group were asked to participate in a satisfaction survey and received the standard hospital care. Records of women in the control group were not marked and their names were not available to the maternity staff. The randomised consent method was chosen to over‐come the potential bias that may exist when women become disappointed with their allocated group in the conventional consent±randomisation progression." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome group: all Participants: There is no information available about whether blinding was carried out but, based on the intervention, it is improbable that blinding took place. Personnel: "The research midwife who was registering women in the trial telephoned an administrative assistant, who was not associated with the study in any other way, to receive each allocation. Records of women in the control group were not marked, and their names were not available to the maternity staff". We judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome group: all "Data were collected from medical records by two experienced midwife researchers…An obstetrician, who did not work at the hospital and was unaware of the trial, the allocated groups or the ultimate aim of the review, 'blindly' assessed each perinatal death". "The trial was unblinded, and it was not possible to mask the data collectors to the woman's allocation. We attempted to reduce bias by blinding the woman's allocated group from the reviewer of the eight perinatal deaths". Therefore, it appears to be blinded for perinatal death outcome assessment only. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: all outcomes: "Women who had relocated to another hospital were removed as they had not attended the hospital for their first visit and were therefore unaware of their group allocation." "Eighty‐eight percent of women (483/550) in the community‐based group received their allocated model of care. The reasons for refusal included: anxiety about giving information (n5); rather come to the hospital (n39); wanted birth centre care (n11) and not interested (n12)." These women were included in the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Checked with authors: no protocol registered due to age of study. All outcomes from methods are reflected in the results section, but some outcomes (for example neonatal outcomes) are only reported in the text and not included in table format. |
| Other bias | Low risk | No other bias identified |
Kenny 1994.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 1992 to 1993 Study funding sources: New South Wales (NSW) National Women’s Health Program Study authors’ declarations of interest: not reported Ethics approval obtained? yes – authors provided a copy of the letter Study prospectively registered? no, not registered, but before trial registration became mandatory |
|
| Participants |
Setting: Westmead public hospital, NSW, Australia Inclusion criteria: women at low and high risk of complications Exclusion criteria: women requiring use of the 'Drug use in pregnancy service' or booked after 16 weeks' gestation Participants randomised: 213 team midwife‐led care, 233 standard care (shared care) Participant demographics: Ethnicity: non‐Australian born mothers (N (%)): 93 (48%) team versus 116 (55%) control Socio‐economic indicators: mothers unemployed (N (%)): 22 (11%) team versus 20 (10%) control; partner unemployed: 37 (19%) team versus 35 (17%) control; post‐secondary qualifications: 70 (37%) team versus 66 (37%) control Social risk factors:interpreter needed (n (%)): 20 (10%) team versus 35 (17%) control Parity: primiparous 83 (432%) team versus 91 (43%) control Maternal age (years, mean (SD)): 27.1 (5.3) team versus 27.6 (5.3) control Smoking: not reported |
|
| Interventions |
Experimental: team of 6.8 WTE midwives sharing a caseload. Provided antenatal and intrapartum care in hospital and postnatal care in hospital and community. Obstetrician saw all women at first visit and 32 weeks, and after 40 weeks, and as appropriate. Team midwife was on call for out‐of‐hours care. Target population: women with risk factors were not excluded There were no significant differences between the two groups in the study sample with the following characteristics in the intervention group: mean age (27.1 years), primiparous (43%), high‐risk at birth (27%), high‐risk at first visit (10%), partner unemployed (19%), no partner (1%), woman unemployed (11%), interpreter needed (10%), and not being Australian (48%) (p31, Table 5) Where is care provided: both hospital and domiciliary visits for antenatal and postnatal care (p33). The birth seemed to have been in hospital mainly (p38, Birth). Who provides care: the Team Midwifery Project (TMP) women saw a midwife at every clinic visit and saw a medical officer as well when deemed necessary by the medical officer (p1) During labour, each woman received the majority of care from a team midwife who she had previously met (one‐to‐one). Medical consultation/referral was made as/when needed. A team midwife was on call at all times for women in labour or women seeking care at any stages via phone or coming into delivery suite (p14). Postnatally, same midwife provided initial care and settling to postnatal ward with subsequent care consisting at least one daily visit according to the needs of woman and baby. Other postnatal care throughout stay was provided either by team midwifery or core midwifery/ward midwives (p15) Organisation of team: this included 6.8 WTE (8 personnel) providing care throughout antenatal, intrapartum, and early postnatal period for 240 women, over a 10‐month period (p1, Summary) The TMP aimed to provide continuity of care between phases of care rather than continuity of carer within the antenatal period (p38, end of 1st para) Almost all women in the TMP had met the midwives who cared for them in labour before being admitted to hospital (p38, Birth). 98% of women in the intervention group received postnatal care from a midwife that they had already met (p39, Postnatal). Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: during labour, each woman received the majority of care from a team midwife who they had previously met (one‐to‐one). Medical consultation/referral was made as/when needed. A team midwife was on call at all times for women in labour or women seeking care at any stages via phone or by coming into the delivery suite. Control: low‐risk women seen in midwives' hospital antenatal clinics, and all other women seen by medical staff. Women received intrapartum care from delivery suite midwives, and postnatal care from midwives on postnatal ward and community postnatal care. Target population: there were no significant differences between the two groups in the study sample with the following characteristics in the control group: mean age (27.6 years), primiparous (43%), high‐risk at birth (31%), high‐risk at first visit (14%), partner unemployed (17%), no partner (2%), woman unemployed (10%), interpreter needed (17%), and not being Australian (55%). Where is care provided: both hospital and domiciliary visits for antenatal and postnatal care (p33). The birth seemed to have been in hospital mainly (p38, Birth) Who provides care: women in the control group had all their antenatal clinic visits with a medical officer. With the exception of some low‐risk women who were attending the midwives’ clinic (this service was only provided for a minority of low‐risk women at the time of the study). During labour, women received care from different midwives whom usually they have not met before. Midwives cared for several women in various stages of labour and delivery changing from shift to shift (p15). Postnatally, women were cared for by a variety of midwives (p16). Organisation of team: 8% of women in the control group received care from a midwife that they had previously met Core research question evolved around the effects of continuity of care in the TMP (p7, para 1). |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes |
Any imbalance between baseline characteristics: 96% of experimental group and 13% of standard group had previously met midwife attending labour. Randomisation before consent to participate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible women were given information about the research study by the booking‐in midwife: "...allocated a numbered randomisation envelope (the number was recorded by the booking‐in midwife on a list of women booked in the session)." No information about how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | "Allocated a numbered randomisation envelope (the number was recorded by the booking‐in midwife on a list of women booked in the session). When each woman returned for her first visit to the doctor at the antenatal clinic she was approached in the waiting room by a program midwife, reminded about the research and asked to sign a consent form. If the woman agreed to join the study, the randomisation envelope was opened and the woman informed of the type of care she was to receive and the appropriate future appointments made." However, it is unclear where these envelopes were stored after allocation and who had access to them, so it is not clear if allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated but unlikely. Women and booking‐in midwife would have been aware of allocation. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: all outcomes other than maternal experience Loss to follow‐up = 19 team care and 22 standard care who either moved or had a miscarriage (p28) 19/213 (9%) team care vs 22/233 (9%) standard care attrition rate Outcome group: maternal experience: (p30, Table 4) Completed antenatal questionnaire: 184/194 (94%) vs 185/211 (88%) Completed postnatal questionnaire: 182/194 (94%) vs 168/211 (80%) For team and control group, respectively |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in the results. |
| Other bias | Low risk | No other bias identified |
MacVicar 1993.
| Study characteristics | ||
| Methods |
Study design: RCT, Zelen method Duration of study: 1989 to 1991 Study funding sources: Leicestershire District Research Committee for the award of a Locally Organised Research Grant (sustained jointly by the Leicestershire Health Authority and the Leicestershire Medical Research Foundation), which funded the Research Assistant’s salary and paid for collection of the data Study authors’ declarations of interest: not reported Ethics approval obtained? yes, reported to have been approved by the local ethics committee Study prospectively registered? no, but study published before 2010 when prospective registration started |
|
| Participants |
Setting: tertiary hospital and community in Leicester, UK Inclusion criteria: women at low risk of complications Exclusion criteria: women who had a previous caesarean section or difficult vaginal delivery, a complicating general medical condition, a previous stillbirth or neonatal death, or a previous small‐for‐gestational‐age baby, multiple pregnancy, Rhesus antibodies, and a raised level of serum alpha‐feto protein Participants randomised: 2304 team midwifery home from home (HFH), 1206 to standard care (shared care) (control) Participant demographics: Ethnicity: not reported Socio‐economic indicators:not reported Social risk factors:not reported Parity: n (%) primiparous 1040 (45%) HFH, 560 (46%) control; 1 + 2 1131 (49%) HFH, 570 (47%) control; ≥ 3 130 (6%) HFH, 76 (6%) control Maternal age: age (mean, SD): 25.3 (4.5) HFH, 25.4 (4.6) control Smoking: n (%): 554 (26%) HFH, 29.8 (326) control (the numbers do not look correct in table 1, page 318 of the main trial report ‐ the number in brackets looks like it should be the number and 29.8 the percentage, although 326 divided by 1206 = 27%) |
|
| Interventions |
Experimental: a team of 2 midwifery sisters assisted by 8 staff midwives provided hospital‐based antenatal, intrapartum (in hospital‐based 3 room home‐from‐home unit (no EFM or epidural)) and hospital postnatal care only. All the staff were volunteers with varying lengths of experience. Antenatal midwife‐led hospital clinic with scheduled visits at 26, 36, and 41 weeks' gestation. Intervening care shared with GPs and community midwives. Referral to obstetrician as appropriate. At 41 weeks, mandatory referral to consultant. Postnatal care in community provided by community midwife and GP. Target population: inclusion criteria not explicitly stated, but assumed to be women at low risk of complications Where is care provided: hospital ‐ home from home ‐ 3 rooms adjacent to the delivery suite in the Leicester Royal Infirmary Maternity Hospital were converted and furnished to appear like a normal household bedroom, with carpeted floors, patterned wall paper and matching curtains. Antenatal midwifery hospital clinic with scheduled visits at 26, 36, and 41 weeks’ gestation. Labour and delivery took place in 3 rooms adjacent to the delivery suite in the Leicester Royal Infirmary Maternity Hospital. Intervening care shared with GPs and community midwives. Referral to obstetrician as appropriate. At 41 weeks, mandatory referral to consultant. Postnatal care in community provided by community midwife and GP. Who provides care: 2 midwifery sisters assisted by 8 staff midwives provided care in the intervention. Antenatal care was undertaken by the midwives in the scheme at 26, 36, and 41 weeks and the intervening care was given by GP or community midwife. Care during labour was provided by the midwifery team, and after discharge home routine care was provided by a community midwife and health visitor – 6‐week postnatal visit provided by GP. Organisation of team: all the midwifery staff were volunteers with varying lengths of experience, but the sisters in charge had been active in midwifery for several years. All worked a 3 shift per day system and were not normally involved with women other than those on the scheme. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: 45% of women randomised to the home from home transferred to specialist care, 23% during antenatal period, 16% during first stage of labour, and 4% in second and third stage of labour or after delivery ‐ so no continuity if transferred out Control group: shared antenatal care with consultant and GP or midwife. Intrapartum care provided by hospital staff. Target population: inclusion criteria not explicitly stated, but assumed to be women at low risk of complications Where is care provided: antenatal care shared between a consultant and GP or community midwife and delivery of care within the hospital (consultant‐led) at Leicester Royal Infirmary Maternity Hospital Who provides care: consultant‐led, with antenatal care shared between consultant and GP or community midwife and all women booked for delivery in the hospital with care of consultant‐led hospital team Organisation of team: antenatal care was provided by the consultant shared with GP or community midwife, and delivery was within the specialist unit by hospital staff under the consultant |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | 2:1 randomisation ratio in favour of midwife‐led care 189/2304 (8%) women opted out of team midwife care post‐randomisation. Intention‐to‐treat analysis. Level of continuity not reported. Any imbalance between baseline characteristics: The groups were balanced for maternal age, height, gravidity and parity, however the control group was reported in the results section to have significantly more mothers that smoked ‐ although the numbers presented in the table for this do not look correct. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...by a random sequence..." |
| Allocation concealment (selection bias) | Low risk | "...sealed envelope...cards could not be read through the envelopes. Each envelope was numbered, and unused envelopes were not reallocated..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not reported, but not possible – home to home scheme delivery took place in rooms converted to appear like a normal household bedroom, and so all staff and women involved in their care would have been aware of group allocation, plus women in this group consented post randomisation and so were aware to which group they had been allocated. However, it is less clear about the control group women, as no consent was sought as they were receiving care as usual; it is therefore less clear if they ever knew they were in a trial and the same for the staff caring for them. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Data were completed after delivery for both cases including refusals and the controls from their case notes – women in the control group had no identifying mark on their case notes and staff were unaware whether a particular mother was a control". This implies that staff carrying out data analysis were blinded to controls, but it is not reported whether it would have been obvious for intervention group women from their case notes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up/dropouts not reported, although 1044 (45%) women transferred to specialist care and 1069 women were delivered by the midwife‐led team (46%). ITT principle adhered to: "the trial was a pragmatic one – those who refused to take part and those who subsequently transferred from home to home scheme were included in the Home from home group for analysis". |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in the results. |
| Other bias | Low risk | The proportion of smokers was reported to be higher in the control group mothers; however, the numbers in table 1 do not look correct, so this is not entirely clear. No other bias identified. |
Marks 2003.
| Study characteristics | ||
| Methods |
Type of study design: RCT Study dates: January 1997 to January 1999 Study funding sources: NHS National R&D Programme (Mother and Child Health) Study authors’ declarations of interest: not reported Ethics approval obtained: ethical approval for the study was obtained from the South London & Maudsley National Health Service Trust and from King’s College Hospital National Health Service Trust. Study prospectively registered? no |
|
| Participants |
Describe setting: tertiary hospital and community, UK Inclusion criteria: “women who had had at least one episode of major depressive disorder, defined according to DSM‐III‐R criteria, either in the past or during the current pregnancy.” Exclusion criteria: not stated Participants randomised: 51 continuous midwifery care; 47 standard maternity care Participant demographics: Ethnicity: white: 70% continuity group, 69% standard care (other ethnicities not stated) Socio‐economic indicators: "Marital status (% single)": 20% continuity group, 14% standard care "Social class (proportion manual)": 57% continuity group, 44% standard care Social risk factors: not specifically reported Parity: multiparous: 52% continuity group, 47% standard care Maternal age: mean age (SD): 31.7 (5.1) continuity group, 31.5 (4.3) standard care |
|
| Interventions |
Experimental intervention: caseload midwife‐led care Target population: “women who had had at least one episode of major depressive disorder, defined according to DSM‐III‐R criteria, either in the past or during the current pregnancy.” Where is care provided: antenatal care in the community/woman’s home, intrapartum and postnatal care in hospital and postnatal care (we assume given UK model in study period) in the community Who provides care: team of 6 midwives Organisation of team: all midwifery care of each participant was carried out by these midwives. Visits were either in the patient’s home or at the clinic or on the labour ward, according to the woman’s wishes about where she wanted to be seen and where she wanted to deliver her baby. Each woman was given a named midwife who, as far as possible, followed the patient through the pregnancy, delivery, and postnatally. However, other midwives in the team had also met her before the delivery, so that at delivery she would be sure of having a midwife that she knew. To facilitate women knowing all the midwives, a weekly ‘drop‐in’ group was also provided at which mothers could meet other mothers. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: no details provided Total number randomised: n = 51 Control: standard care (shared care) Target population: “women who had had at least one episode of major depressive disorder, defined according to DSM‐III‐R criteria, either in the past or during the current pregnancy.” Where is care provided: "women in the control group received a mixture of antenatal clinic visits, GP care or GP/ community midwife care". Does not specify location of intrapartum nor postnatal care, but a reasonable assumption is that intrapartum and postnatal care in hospital and postnatal care (we assume given UK model in study period) in the community. Who provides care: "Care of women in the control group was carried out by the regular King’s College Hospital maternity services… Thus, women in the control group received a mixture of antenatal clinic visits, GP care or GP/ community midwife care. However, none of these models of care included continuity of care". Organisation of team: not stated Total number randomised: n = 47 |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization schedule was determined independently from the research worker and was computer‐ generated. Complete randomization was conducted until 70 of the subjects had been allocated. Thereafter, subjects were allocated to a treatment condition using minimization methods10, with parity (0, 1+), social class (manual, non‐ manual) and marital status (single, married/co‐habiting) as the stratification variables, so that these factors became more balanced across treatment groups.” (p 120, Recruitment procedure) |
| Allocation concealment (selection bias) | Unclear risk | "Subsequently, women who agreed to take part in the research interviews were randomly allocated either to the specialized midwifery group or left free to choose whatever care there was available within the standard maternity service". Insufficient information to inform judgement (p 120, Recruitment procedure) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There is no information available about whether blinding was carried out but, based on the intervention, it is improbable that blinding took place. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: all outcomes other than maternal experience: 98 women randomised: 47 to control of which data included for 43 (91%); 51 to control of which data included for 44 (86%) Outcome group: maternal experience: As above |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: outcomes in the methods are reported in the results |
| Other bias | Low risk | Not stated |
McLachlan 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 2007 to 2010 Study funding sources: Australian National Health and Medical Research Council Study authors’ declarations of interest: none declared Ethics approval obtained? protocol published/ethical approval documented Study prospectively registered? yes |
|
| Participants |
Setting: Royal Women’s Hospital (RWH), Melbourne, Australia Inclusion criteria: low‐risk pregnant women; fewer than 24 completed weeks' gestation; a singleton pregnancy; and considered low obstetric risk at recruitment including an uncomplicated obstetric history Exclusion criteria: previous caesarean section, history of stillbirth or neonatal death, 3 or more consecutive miscarriages, previous fetal death in utero, previous preterm birth (< 32 weeks), previous midtrimester loss/cervical incompetence/cone biopsy/known uterine anomaly, previous early onset of pre‐eclampsia (< 32 weeks' gestation), or rhesus iso‐immunisation; complications during the current pregnancy (such as multiple pregnancy or fetal abnormality); medical conditions (such as cardiac disease, essential hypertension, renal disease, pre‐existing diabetes, previous gestational diabetes, epilepsy, severe asthma, substance use, significant psychiatric disorders and obesity (BMI > 35) or significantly underweight (BMI < 17)) Participants randomised: 1156 caseload, 1158 standard care Participant demographics: Ethnicity n (%): reported as "born in Australia" (1119/1118)* Caseload: 653 (58.4) Standard care: 645 (57.7) Socio‐economic indicators n (%): reported as total family income/year, highest education level, government benefit main family income, and employment Total family income/year (AUD) (1142/1134)*: Caseload: < AUD 33,800 per year: 123 (10.8), AUD 33,801 to 51,999 per year 201 (17.6), AUD 52,000 to 72,799 per year: 218 (19.1), AUD 72,800 t 103,999 per year: 311 (27.2), AUD 104,000 or more per year: 289 (25.3) Standard care: < AUD 33,800 per year: 137 (12.1), AUD 33,801 to 51,999 per year: 170 (15.0), AUD 52,000 to 72,799 per year 238 (21.0), AUD 72,800 to 103,999 per year: 298 (26.3), AUD 104,000 or more per year: 291 (25.7) Highest education level (1132/1125)*: Caseload: completed degree/diploma: 877 (77.5), completed secondary school: 187 (16.5), did not complete secondary school: 68 (6.0) Standard care: completed degree/diploma: 833 (74.0), completed secondary school: 210 (18.7), did not complete secondary school: 83 (7.3) Government benefit main family income (1146/1145)*: caseload: 42 (3.7), standard care: 67 (5.9) Employed (part‐time or full‐time) (1133/1130)*: caseload: 839 (74.1), standard care: 820 (72.6) Social risk factors n (%): not reported Parity: (nulliparous): caseload: 804 (70.0), standard care: 806 (69.7) Maternal age ‐ mean age in years (SD): caseload: 31.2 (4.7), standard care: 31.3 (4.7) Smoking n (%): Smoked before pregnancy (1147/1145)*: caseload: 199 (17.3), standard care: 208 (18.2) Smoking at recruitment (1132/1135)*: caseload: 44 (3.9), caseload: 36 (3.2) *Numbers in parentheses indicate number for whom this information was available (caseload/standard care). |
|
| Interventions |
Experimental: caseload care from a primary midwife in the hospital Target population: low‐risk pregnant women Where is care provided: majority of care from a ‘primary’ caseload midwife at the hospital Who provides care: the primary midwife collaborated with obstetricians and other health professionals and continued to provide caseload care if complications arose. Women saw an obstetrician at booking, at 36 weeks' gestation, and postdates if required, and usually had 1 or 2 visits with a ‘back‐up’ midwife. Organisation of team: Caseload: intrapartum care was provided in the hospital birthing suite. Where possible, primary midwife was on call for the woman’s labour and birth. The primary midwife (or a back‐up) attended the hospital on most days to provide some postnatal care and provided domiciliary care following discharge from hospital. Full‐time midwives had a caseload of 45 women per annum. During the trial there were 7.5 (at commencement) to 12 full‐time equivalent midwives employed in caseload care, equating to 10 to 14 midwives. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: if complications developed, the primary midwife collaborated with obstetricians and other health professionals and continued to provide caseload care. Control: standard care Target population: women identified as being at low obstetric risk Where is care provided: options included midwifery‐led care with varying levels of continuity, obstetric trainee care, and community‐based care ‘shared’ between a general medical practitioner (GP) and the RWH, where the GP provided the majority of antenatal care. Who provides care: in the midwife and GP‐led models, women saw an obstetrician at booking, 36 weeks gestation, and postdates if required, with other referral or consultation as necessary. Organisation of team: in all standard care options, women were cared for by whichever midwives and doctors were rostered for duty when they came into the hospital for labour, birth, and postnatal care. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes |
Any imbalance between baseline characteristics: baseline characteristics were reported to be similar between the 2 groups Denominator: Caseload: 1156 (‐6 who withdrew immediately or were randomised in error ‐ no outcome data) = 1150 Control: 1158 (‐1 withdrawal ‐ no outcome data) = 1157 This denominator includes fetal loss < 20/40 as included in outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using stratified permuted blocks of varying size." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was undertaken using an interactive voice response system activated by telephone..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There is no information available about whether blinding was carried out but, based on the intervention, it is improbable that blinding took place. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Obstetric and medical outcome data (including type of birth) were obtained directly from the electronic obstetric database, blinded to treatment allocation. Data not available this way (e.g. continuity of carer) were manually abstracted (unblinded) from the medical record." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 6 caseload and 1 standard care |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in the results. |
| Other bias | Low risk | No other bias identified |
North Stafford 2000.
| Study characteristics | ||
| Methods |
Study design: RCT, cluster‐randomisation Duration of study: not stated Study funding sources: not reported Study authors’ declarations of interest: not reported Ethics approval obtained? yes, the project was approved by North Staffordshire Ethical Committee Study prospectively registered? no (pre‐2010) |
|
| Participants |
Setting: tertiary hospital and community, UK Inclusion criteria: "all‐risks" Exclusion criteria: not stated Participants randomised: 770 midwife‐led caseload care, 735 standard care (shared care) Participant demographics: Ethnicity: white: 96.6% caseload care, 96.8% shared care Socio‐economic indicators: married, neighbourhood of residence Married: 63.8% caseload care, 65.5% shared care. Neighbourhood of residence: rural: 38.4% caseload care, 31.5% shared care; urban: 27.3% caseload care, 32.5% shared care; mixed urban and rural: 34.3% caseload care, 36% shared care Social risk factors: not specifically reported Parity: primiparous: 34% shared care, 32.4% caseload care Maternal age: mean age (SD): 27.8 (5.4) caseload care, 27.7 (5.3) in shared care Smoking: current smokers: 22.8% caseload care, 24.2% shared care |
|
| Interventions |
Experimental: caseload midwife‐led care Target population: women at low and high risk of complications living in 3 specific geographic areas Where is care provided: antenatal care in the community, intrapartum and postnatal care in the hospital, and postnatal care in the community Who provides care: 21 WTE midwives working in 3 practices offering a caseload model of care in collaboration with medical colleagues (within each of these 3 practices, midwives worked in pairs or threesomes to achieve high levels of continuity) Organisation of team: each midwife was attached to 2 to 3 GP practices and cared for 35 to 40 women. Midwives worked in pairs/threesomes in groups of 3 caseload areas (7, 9, and 10 respectively). Caseload midwives were existing community midwives, plus new midwives recruited from the community and hospital, resulting in a mix of senior and junior staff. During the antenatal clinics and parentcraft sessions, each woman met both her ‘named’ midwife and this midwife’s professional partner(s). "Three midwives withdrew prior to randomisation (one for family reasons and two for health reasons) but 16 were prepared to stay, offering valuable stability to the project". No details of arrangements for out‐of‐hours care or level of continuity of care in team. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: no details provided Control: standard care (shared care) Target population: women at low and high risk of complications in each of the 6 study areas Where is care provided: not specified Who provides care: shared care in the community between GPs, community midwives, and obstetricians Organisation of team: traditional caseload of 100/150 women within the current UK ‘shared‐care’ model, with approximately 10% of women expected to be delivered by a named midwife known to the women from her antenatal care. Options for place of birth: hospital (not specified if alongside midwife birth centre or hospital labour ward). |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was undertaken by one of the principal investigators...who had no prior knowledge of the area or medical and midwifery staff involved.... He was presented with three pairs, one of each randomised to receive caseload care and the other to traditional care." |
| Allocation concealment (selection bias) | High risk | No information given about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "It was not possible to mask allocation and both women and professionals were aware of the allocated type of midwifery care." However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: not reported Authors state "Demographic and outcome data are only presented for those women completing the study. In North Staffordshire a small number of women (approximately 1%) will have moved or chosen to give birth in another unit during the course of the pregnancy." Authors contacted but were unable to clarify loss to follow‐up. Therefore, we have used the denominators as reported and judged the study at high risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in the results. |
| Other bias | Low risk | No other bias identified |
Rowley 1995.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 1991 to 1992 Study funding sources: supported by a Human Services and Health Research Development Grant from the Commonwealth Department of Human Services and Health. Study authors’ declarations of interest: none given Ethics approval obtained? yes Study prospectively registered? no |
|
| Participants |
Setting: John Hunter Hospital, Newcastle, NSW, Australia Inclusion criteria: women booked for delivery at hospital of low and high risk Exclusion criteria: women who had chosen shared antenatal care with their GP or had a substance abuse problem Participants randomised: 405 team care, 409 standard care (shared care) Participant demographics: The two groups were similar in socio‐demographic, physical, and medical characteristics. Ethnicity: "Most women were white" Socio‐economic indicators: more than half were married and about 20% were employed in trade or skilled labour occupations Social risk factors: not reported Parity: team: 194 primiparous and 211 multiparous; routine: 202 primiparous and 207 multiparous Maternal age: for team and routine groups, respectively, mean ages were 26.5 and 26.3 years Smoking: more than half the women were non‐smokers |
|
| Interventions |
Experimental intervention: Target population: women at “low” and “high risk” of complications Where is care provided: in hospital setting Who provides care: a team of 6 midwives Organisation of team: a team of 6 experienced and newly graduated midwives provided antenatal care, intrapartum care, and postnatal care in the hospital Women at low risk had scheduled consultations with an obstetrician at 12 to 16, 36, 41 weeks, and additional consultations as needed. Women at high risk had consultations with an obstetrician at a frequency determined according to their needs. High‐risk women had an individualised care plan devised in consultation with a doctor; they were seen by a team midwife at each visit and by a doctor, at a frequency determined by their high‐risk status. Throughout labour, care was provided by a team midwife known to the mother. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: no details provided High‐risk women had an individualised care plan devised in consultation with a doctor; they were seen by a team midwife at each visit and by a doctor, at a frequency determined by their high‐risk status. Throughout labour, care was provided by a team midwife known to the mother. Total number randomised: n = 405 Control: Target population: women at “low” and “high risk” of complications Where is care provided: in hospital setting Who provides care: antenatal care from hospital physicians and intrapartum and postnatal care from midwives and doctors working in the delivery suite, and the postnatal ward. Control group midwives were also a mix of experienced and newly qualified midwives. Women were usually seen by a doctor at each visit. Organisation of team: no details additional to the above provided Total number randomised: n = 409 |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation to either team care or routine care was done by computer‐generated random assignments." (p290) |
| Allocation concealment (selection bias) | Unclear risk | "The women were allocated at random to team care or routine care...." (p290) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...the unblinded nature of the study could have led to differences in practice and measurement of outcomes..." (p293). However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "...the unblinded nature of the study could have led to differences in practice and measurement of outcomes..." (p293) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Notes that "...the Australian national cost weights for diagnosis‐related groups (AN‐ DRGs) were applied to the outcomes for 758 women for whom complete results were available by a medical records clerk blinded to the study." Note: does not differentiate this by group and that 814 women were randomised in total. Also notes that "Analysis was done on an "intention‐ to‐treat" basis; that is, women lost to follow‐up and those who had had a mis‐ carriage were included in the denominator and regarded as not having the outcome of interest." |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in the results. |
| Other bias | Low risk | No other bias identified |
Tracy 2013.
| Study characteristics | ||
| Methods | Study took place in 2 Australian centres (site 1: Royal Hospital for Women, Randwick and site 2: Mater Mother’s Hospital, Brisbane). The randomised trial compared caseload midwifery with standard care. Women were recruited to the study from site 1 between December 2008 and May 2011, and from site 2 between June 2010 and May 2011. Duration of study: 2008 to 2011 Study funding sources: National Health and Medical Research Council (Australia) Study authors’ declarations of interest: none Ethics approval obtained? yes, overall and site‐specific ethics approval was obtained from all relevant university and Area Health Service human research ethics committees Study prospectively registered? yes |
|
| Participants | Women were included if they were less than 24 weeks pregnant at the booking visit, and aged 18 years and older. Women were excluded if they had planned to have an elective caesarean section, had a multiple pregnancy, or were planning to book with another care provider (e.g. a GP, caseload midwife, or private obstetrician). Participants randomised: 871 caseload care; 877 standard care Participant demographics: Ethnicity: not reported Socio‐economic indicators: median SEIFA index: 10 (8 to 10) caseload care, 10 (8 to 10) standard care (Socio‐Economic Indexes For Areas, SEIFA, method provides a measure of social and economic wellbeing for Australian communities; a score of 1 is the lowest and 10 the highest) Social risk factors: 190 (22%) caseload care, 192 (22%) standard care (medical, obstetric, and social risk factors are categorised B or C (B = consult with a medical practitioner or other health‐care provider; C = refer a woman or her infant to a medical practitioner for secondary or tertiary care) Parity: nulliparas: 619 (71%) caseload care, 605 (69%) standard care Maternal age: age (mean, SD): 31.7 (4.8) caseload care, 30.9 (4.33) standard care Smoking: not reported |
|
| Interventions |
Experimental: caseload midwifery Target population: women at low and high risk of complications Where is care provided: antenatal, intrapartum, and postnatal care in the hospital and in the community. Option for place of birth: hospital labour ward. Who provides care: a named caseload midwife working in a small group of midwives known as a midwifery group practice (4 full‐time midwives) with the backup of hospital obstetricians Organisation of team: each midwife provides care to 40 women a year as named midwife. The named midwife was on call for labour and birth. The caseload midwives were backed up when necessary by other caseload colleagues and by hospital staff during women’s stay in the postnatal ward. The caseload midwives will attend the hospital on most days to provide some postnatal care until discharge. Community postnatal care was provided for up to 6 weeks. The caseload midwife is the woman’s lead professional, but one or more consultations with medical doctors may be part of routine practice. An obstetrician was allocated to each midwifery practice for consultation and referral using national guidelines. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: Women receive continuity of care from a named midwife or her small group practice of midwives for duration of pregnancy, labour, birth, and postnatal care, ensuring consistency of advice and information. Collaboration between medical staff and caseload midwives is guided by Australian national midwifery guidelines for consultation and referral. Midwifery care is offered simultaneously with medical care if required. Control: standard care (shared care) Target population: women at low and high risk of complications Where is care provided: routine hospital care. Option for place of birth: not specified (likely hospital labour ward too). Who provides care: GP, hospital midwives, and doctors Organisation of team: shared antenatal care from a GP and hospital midwives, labour and birth and postnatal hospital care from hospital midwives. Hospital/rostered midwives were paid on the basis of their years of service and whether they were full‐time (minimum 38 h per week) or part‐time; they were employed to provide a rostered service. Women attend routine antenatal clinics in accordance with hospital policies; antenatal classes were offered in the hospital or community. Women received postnatal care in hospital; a domiciliary follow‐up visit from a rostered community midwife might take place if the woman met the criteria for early discharge ‐ before 48 h for vaginal birth and 72 h for caesarean section (thus unclear whether community postnatal care was provided in standard care). Midwives had access to the national guidelines for consultation and referral. Options for place of birth: not specified. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: Primary outcomes:
Secondary outcomes:
|
|
| Notes | Forti 2015, an additional report of Tracy 2013 was identified in the 2016 update. This reports on a subset of publicly funded women randomised in the M@ngo trial (n = 420); women receiving caseload midwifery care saw fewer midwives and health professionals during their intrapartum care than did women in standard care. No additional data provided.
Any imbalance between baseline characteristics: Baseline characteristics were reported to be similar between the 2 groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned by a telephone‐based computer randomisation service provided by the ANHMRC clinical trials randomisation centre to each group. |
| Allocation concealment (selection bias) | Low risk | As above, centralised allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to the nature of the study, it is not possible to blind participants or clinicians. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to the nature of the study, it is not possible to blind participants or clinicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and losses outlined in a trial profile in Tracy 2013. 20/871 lost or withdrew from caseload care; 36 lost or withdrew from standard care. Pregnancies lost before 20 weeks and terminations of pregnancy have been added back in (see Notes above). |
| Selective reporting (reporting bias) | Unclear risk | Authors were emailed for length of neonatal stay and antepartum haemorrhage; these were mentioned in the protocol and were not included in publications. Authors asked to clarify if the length of stay outcome is for infants or women. Answer received 26 August 2022. No data available. Authors emailed for GA of the 2 terminations of pregnancy for lethal abnormalities. Reply received 25 August 2022. |
| Other bias | Unclear risk | 19 (2%) women crossed over from caseload to standard care and 65 (7%) crossed over from standard to caseload care. |
Turnbull 1996.
| Study characteristics | ||
| Methods |
Study design: RCT Duration of study: 1993 to 1994 Study funding sources: the Midwifery Development Unit is funded by a grant from the Scottish Office Home and Health Department Study authors’ declarations of interest: not reported Ethics approval obtained? yes Study prospectively registered? no (published in 1996) |
|
| Participants |
Setting: Glasgow Royal Maternity Hospital, Scotland, United Kingdom Inclusion criteria: women at low risk of complications Exclusion criteria: women booking after 16 weeks of pregnancy, not living in catchment area, or with medical/obstetric complications Participants randomised: 648 caseload, 651 standard care (shared care) Participant demographics: Ethnicity: not specifically reported Socio‐economic indicators: Married: 53.6% midwife care, 54.8% shared care Type of neighbourhood: 1 (most affluent) (3.0% midwife care, 2.5% shared care), 2 (10.3% midwife care, 9.5% shared care), 3 (7.5% midwife care, 8.5% shared care), 4 (8.9% midwife care, 6.8% shared care), 5 (12.5% midwife care, 12.6% shared care), 6 (18.8% midwife care, 20.1% shared care), least affluent (38.9% midwife care, 41.0% shared care) Social risk factors: not specifically reported Parity: nulliparas: 54.7% midwife care, 53.5% shared care Maternal age: age (mean, SD): 25.8 (5.0) midwife care, 25.8 (5.0) shared care Smoking: smokers at conception: 37.9% midwife care, 38.6% shared care |
|
| Interventions |
Experimental: a midwife‐managed programme of care for healthy women (also referred to as the Midwifery Development Unit (MDU)) Target population: women living in a relatively disadvantaged catchment area Where is care provided: the MDU was based in a major teaching hospital, in which is situated the largest and busiest of the Greater Glasgow Health Board’s maternity units, with around 5000 deliveries per year. The hospital serves a relatively disadvantaged community. Antenatal care was provided at home, community‐based clinics, or hospital clinics. Intrapartum care was in hospital (MDU ‐ 3 rooms with fewer monitors and homely surroundings) or main labour suite. Postnatal care was provided in a designated 8‐bed MDU ward and the community. Who provides care: care was provided by a group of 20 midwives, with obstetricians when appropriate Organisation of team: each pregnant woman had a named midwife whom she met at the first antenatal visit and who aimed to provide the majority of planned episodes of care from booking to discharge to the health visitor. When the named midwife was unavailable, the woman was cared for by an associate midwife from the MDU team. A medical visit was scheduled where there was a deviation from normal. Rather than being a form of ‘team midwifery’, the MDU provides a setting in which each midwife can practise primary midwifery, where she is the client’s lead care provider. The midwives do not practise in teams, but rather each midwife has an associate midwife with whom she alternates at antenatal clinics. Caseload: not reported Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: the programme of care allows for either permanent transfer, where management is transferred to the obstetrician (e.g. for major obstetric problems or caesarean section) or temporary transfer where the woman remains under the direct care of the MDU midwife but other members of the team are involved (e.g. the anaesthetist in the case of epidural analgesia or the obstetrician in the case of forceps delivery). Postnatal care for temporary transfers remains the remit of the MDU midwife, but with permanent transfers the integrated maternity care team assume responsibility for care. Control: Target population: women living in a relatively disadvantaged catchment area Where is care provided: intrapartum care on labour suite. Postnatal care on postnatal ward and in the community. Who provides care: all women seen by medical staff at booking. Shared antenatal care from midwives, hospital doctors, and GPs/family doctors. Intrapartum care from labour ward midwife. Postnatal care from postnatal ward midwife and community midwife. Organisation of team: not reported |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7
|
|
| Notes |
Any imbalance between baseline characteristics: baseline characteristics were reported to be similar between the 2 groups Women in the intervention group saw 7 fewer care providers across antenatal, labour and postnatal periods and 2 fewer providers during labour. Denominator: Caseload: total no. in group = 648 Denominator excluding loss to follow‐up = 643 Standard care: total no. in group = 651 Denominator excluding loss to follow‐up = 635 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...random number tables..." |
| Allocation concealment (selection bias) | Low risk | "The research team telephoned a clerical officer in a separate office for care allocation for each woman." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants: there is no information available about whether blinding was carried out but, based on the intervention, it is improbable that blinding took place. Personnel: "Women in the control group had no identifying mark on their records, and clinical staff were unaware whether a particular woman was in the control group or was not in the study." However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Clinical data were gathered through a retrospective review of records by the research team who were not involved in providing care." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 5 team care and 16 shared care |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in the results. |
| Other bias | Low risk | No other bias identified |
Waldenstrom 2001.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Duration of study: 1996 to 1997 Study funding sources: funding from State of Victoria, the Commonwealth Birthing Services programme Study authors’ declarations of interest: not stated Ethics approval obtained? yes, approved by the Research Ethics Committee at the Royal Women’s Hospital Study prospectively registered? no (pre‐2010) |
|
| Participants |
Setting: Royal Women's Hospital, Melbourne, Australia Inclusion criteria: women at low risk of complications Exclusion criteria: non‐English speaking women, women > 25 weeks' gestation at booking, women with high‐risk criteria including previous obstetric complications, preterm delivery, IUGR, PET, previous fetal loss, significant medical disease, > 3 abortions, substance addiction, infertility > 5 years Participants randomised: 495 team midwife care, 505 standard care (combination of different models of care) Participant demographics: Ethnicity: not reported Socio‐economic indicators: education, total family income per year, benefits, private health insurance Married/living with partner: 89.4% team care, 88.8% standard care Education: secondary school to year 12: 58.8% team care, 59.5% standard care; secondary school but not completed: 39.2% team care, 38.7% standard care; primary school only: 1.4% team care, 1.0% standard care; did not attend school: 0.6% team care, 0.8% standard care; further studies ‐ degree/diploma: 18.6% and 19.7% team care, 21.8% and 16.1% standard care Total family income per year: AUD 20,000 or less: 34.4% team care, 34.1% standard care; AUD 20,000 to 30,000: 23.1% team care, 27.4% standard care; AUD 30,000 to 40,000: 18.3% team care, 15.6% standard care; more than AUD 40,000: 24.2% team care, 25.5% standard care Pension/benefit main family income: 25.2% team care, 22.5% standard care Private health insurance: 2.9% team care, 2.2% standard care Social risk factors: not specifically reported Parity: primiparous: 59.1% team care, 60.7% standard care Maternal age: age at booking (mean, SD): 27.9 (5.2) team care, 27.9 (5.2) standard care Smoking: smoked prior to pregnancy: 39.3% team care, 35.7% standard care |
|
| Interventions |
Experimental: team midwife care Target population: women at low risk of complications Where is care provided: hospital‐based antenatal and intrapartum care (delivery suite or family birth centre) and some hospital postnatal care Who provides care: a team of 8 midwives in collaboration with medical staff Organisation of team: a member of the team was rostered on one of the hospital’s public labour wards 24 hours a day, and when no team woman was in labour she looked for other women outside the team. Each midwife did on average one shift per week in the hospital antenatal clinic where she saw only women enrolled in team care. The care provided by the team followed the same medical protocols as standard antenatal and intrapartum care. The midwives followed up new team mothers on the postnatal ward and, occasionally, "they worked entire postnatal shifts, but not enough to provide continuity of care". No details of arrangements for level of continuity of care in team. The team midwives were recruited from the midwifery staff of the hospital. Role of COC team for women after they transfer due to complications in the antenatal and intrapartum period: no details provided Control: standard care (combination of different models of care) included different options for care being provided mostly by doctors, care mainly by midwives in collaboration with doctors (midwives clinics), birth centres, and shared care between GPs and hospital doctors. Options for place of birth: hospital (delivery suite or family birth centre). Target population: women at low risk of complications Where is care provided: hospital (clinics, delivery suite, or family birth centre) Who provides care: different options of shared care being provided mostly by doctors, midwives in collaboration with doctors, birth centres, and shared care between GPs and hospital doctors Organisation of team: antenatal clinic care provided mostly by doctors; midwife clinic care provided by midwives in collaboration with the medical staff (midwives’ clinic); birth centre care (with antenatal and intrapartum care provided by a small team of midwives); and shared care between a local GP and doctors in the hospital. Shared care was an option encouraged by the hospital, and women who expressed any interest in shared care at the first booking visit were not approached by the research midwife. Intrapartum care took place in the hospital’s two public delivery suites with midwives and doctors, or in the hospital’s family birth centre, staffed predominantly with midwives. With the exception of birth centre care, no continuity of caregiver was available between the antenatal and intrapartum episodes in standard care. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study:
|
|
| Notes |
Any imbalance between baseline characteristics: none reported 65% and 9% of experimental (team) and control (standard) group participants had previously met midwife attending labour Birth centre care was considered as an option for women allocated to the control group since it was one of the established models of care provided by the hospital, and therefore part of standard care. By including this option, which emphasises continuity of midwifery care, it is possible that effects of the new team midwifery model may be diluted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Low risk | "The research midwife rang a clerk at the hospital's information desk who opened an opaque, numbered envelope that contained information about the allocated group." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There is no information available about whether blinding was carried out but, based on the intervention, it is improbable that blinding took place. However, we judged performance bias to be low risk given the objectivity of the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group ‐ all outcomes other than maternal experience: Lost to follow‐up: team care = 11, standard care = 9 Outcome group ‐ maternal experience: Follow‐up questionnaires were sent 2 months after birth to all women except to those who had perinatal death or serious medical problems (team care = 456; standard care = 461). Response rates: team care = 361 (79.2%), standard care = 323 (64.0%). |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in the results. |
| Other bias | Low risk | No other bias identified |
BMI: body mass index CLC: consultant‐led care CLU: consultant‐led unit COC: continuity of care EFM: electronic fetal monitoring GA: gestational age GP: general practitioner h: hour HFH: home from home scheme HDI: Human Development Index ITT: intention‐to‐treat IUGR: intrauterine growth restriction MDU: midwifery development unit MLU: midwife‐led unit N/A: not applicable NICU: neonatal intensive care unit PET: positron emission tomography PPH: postpartum haemorrhage PTB: preterm birth RCT: randomised controlled trial SCBU: special care baby unit SD: standard deviation SEIFA: Socio‐Economic Indexes For Areas US: ultrasound vs: versus WTE: whole time equivalent
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2013 | Excluded as only one participant recruited and full RCT therefore not feasible |
| Bagheri 2021 | Not an RCT |
| Bergland 1998 | Wrong design: not an RCT |
| Bergland 2007 | Wrong intervention: does not compare midwife continuity model with other models of care |
| Bernitz 2011 | Wrong intervention: does not compare midwife continuity model with other models of care |
| Brugha 2016 | Focuses on training vs no training rather than continuity of midwife care |
| Byrne 2000 | No continuity of care in the intervention arm |
| Chambliss 1991 | Wrong intervention |
| Chapman 1986 | Wrong design |
| de Wolff 2021 | No intrapartum care |
| Famuyide 2014 | Wrong intervention |
| Forster 2022 | Group antenatal care only |
| Giles 1992 | Wrong intervention |
| Hailemeskel 2021 | Non‐randomised, quasi‐experimental study |
| Hans 2018 | No midwife continuity of care |
| Heins 1990 | Wrong intervention |
| Hildingsson 2003 | Wrong design |
| Hundley 1994 | Wrong intervention |
| James 1988 | Wrong design |
| Kelly 1986 | Trial not finished |
| Kildea 2017 | Participants from Tracey et al's RCT (MANGO) not separated in results |
| Kildea 2021 | Non‐randomised study |
| Klein 1984 | Wrong intervention |
| Law 1999 | Wrong intervention |
| Li 2015 | Does not evaluate midwife continuity of care model |
| Lin 2020 | Does not evaluate midwife continuity of care model |
| Loy 2021 | Antenatal/postnatal continuity only; intervention does not include intrapartum continuity |
| Michel‐Schuldt 2021 | Not an RCT |
| Mohammad‐Alizadeh‐Charandabi 2019 | Intervention aimed at student midwives, not qualified midwives |
| Morrison 2002 | Compares two variations of continuity of midwife care models rather than providing a clear contrast between a midwife continuity of care model and a different model of care |
| Mortensen 2018 | Non‐randomised observational study |
| Nagle 2011 | Intervention includes antenatal continuity only |
| Qiu 2020 | Not an RCT and not evaluating midwife continuity model of care |
| Ridgeway 2015 | Intervention does not include intrapartum continuity |
| Runnerstrom 1969 | Wrong design |
| Slome 1976 | Wrong design |
| Stevens 1988 | Wrong intervention |
| Tucker 1996 | Wrong intervention |
| Waldenstrom 1997 | Wrong intervention |
| Walker 2012 | Wrong intervention |
| Wiggins 2020 | Group antenatal care |
| Zelani 2011 | Continuity only provided during antenatal period |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Zhang 2016.
| Methods | The included patients were divided randomly into observation group and control group. |
| Participants | Women in labour who had a history of previous caesarean section ‐ 96 participants, 48 in each group |
| Interventions | Continuing midwifery care, which provides personal support to women during labour and delivery through one‐to‐one care at the first and second stages of delivery vs standard care provided by a range of different midwives and obstetricians during pregnancy, birth, and the postnatal period |
| Outcomes | Duration of labour stage, rate of fetal distress, neonatal asphyxia, vaginal birth, postpartum bleeding |
| Notes | Unclear whether continuity of care was provided in the antenatal period. The paper states that the midwife provided care during the antenatal, labour and birth, and postnatal periods. However, it is unclear what the midwife provided in the antenatal period. |
Characteristics of ongoing studies [ordered by study ID]
Cullinane 2021.
| Study name | Exploring the impact of caseload midwifery on preterm birth among vulnerable and disadvantaged women: a multi‐centre randomised controlled trial |
| Methods | Multi‐centre randomised controlled trial |
| Participants | Not reported |
| Interventions | Caseload midwifery care throughout antenatal, intrapartum, and early postpartum care from a primary caseload midwife |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 6 September 2022 |
| Contact information | Dr Meabh Cullinane Judith Lumley Centre, George Singer Building, La Trobe University, Plenty Rd and Kingsbury Dr, Bundoora VIC 3086, Australia Telephone: +61 03 94798832 m.cullinane@latrobe.edu.au |
| Notes | — |
Dickerson 2022.
| Study name | Does the midwife‐led continuity of carer model improve birth outcomes and maternal mental health in vulnerable women? |
| Methods | Single‐centre, open‐labelled, individual, prospective randomised controlled trial with an internal pilot phase and qualitative process and economic evaluations |
| Participants | Target number of participants: 2740 |
| Interventions | The MCC (Midwife Continuity of Care) model of care provides women with a full continuity of carer service throughout the antenatal, intrapartum, and postnatal periods, delivered by a named midwife and support team. Compared to standard care, MCC midwives are given smaller caseloads, can offer longer appointment times, and prioritise discussion surrounding public health messages. |
| Outcomes | Primary outcome measure Internal pilot:
Effectiveness evaluation: Primary outcome measures: parent
Process evaluation: Midwifery teams:
Women:
Economic evaluation:
Secondary outcome measures Effectiveness evaluation: Secondary outcome measures: parent
Secondary outcome measure: child
|
| Starting date | 1 April 2021 |
| Contact information | Bradford Institute for Health Research Duckworth Lane Bradford BD9 6RJ United Kingdom +44 1274 383916 Josie.Dickerson@bthft.nhs.uk |
| Notes | — |
Xiaojiao 2020.
| Study name | Development and implementation of midwife‐based care model for urban women with uncomplicated pregnancies |
| Methods | Randomised controlled trial |
| Participants | Control group: 609 Intervention group: 609 |
| Interventions | Control group: obstetrician‐based maternal perinatal management programme Intervention group: midwife‐based maternal perinatal management programme |
| Outcomes |
|
| Starting date | Registered 1 June 2020 Recruitment status pending |
| Contact information | Wang Xiaojiao 419 Fangxie Road, Huangpu District, Shanghai, China 200011 +86 15021790424 Email: 805850995@qq.com Affiliation: Obstetrics and Gynecology Hospital affiliated to Fudan University |
| Notes | — |
Differences between protocol and review
2015 update
Breastfeeding on hospital discharge and maternal satisfaction were added as outcomes.
2016 update
Some of the primary and secondary outcomes were clarified. The primary neonatal outcome "Overall fetal loss and neonatal death (fetal loss was assessed by gestation using 24 weeks as the cut‐off for viability in many countries)" was changed to "All fetal loss before and after 24 weeks plus neonatal death."
The secondary neonatal outcomes, "Fetal loss and neonatal death less than 24 weeks" and "Fetal loss and neonatal death equal to/after 24 weeks" were changed to "Fetal loss less than 24 weeks and neonatal death" and "Fetal loss equal to/after 24 weeks and neonatal death".
2024 update
The title has changed for greater clarity of definition and relevance to women, families, decision makers, and the policy community (previous title "Midwife‐led continuity models versus other models of care for childbearing women").
Primary outcome changes: One primary outcome has been redefined as two separate primary outcomes. "All fetal loss before and after 24 weeks plus neonatal death" has been changed to "Fetal loss at or after 24 weeks gestation" and "Neonatal death" (baby born alive at any gestation and dies within 28 days). One primary outcome has been moved to a secondary outcome ("Instrumental vaginal birth (forceps/vacuum)").
Secondary outcome changes: Reduced from 35 to 18 outcomes. New secondary outcomes include: "Healthy mother (defined as one who is alive at 28 days postpartum, without a caesarean birth, postpartum haemorrhage (as defined by trial authors), third or fourth degree tear, or readmission within 28 days)"; "Healthy baby (defined as one who is born after 37 + 0 weeks gestation and is alive at 28 days and without readmission within 28 days)", and "Birth weight equal to or more than 4000 g". One outcome has been redefined: "Maternal satisfaction (not pre‐specified)" to "Maternal experience (defined by trial authors)".
New searches using the new definition in the review title identified three eligible studies. Gu 2013 (previously in excluded studies), Marks 2003, and Fernandez Turienzo 2020, broadening the scope of the review in terms of higher‐risk populations and middle‐income settings.
Data extraction and assessment of all studies in the review has been re‐entered and checked independently by two authors in this update. The methods have been informed by the latest Cochrane methodology and Cochrane Pregnancy and Childbirth trustworthiness assessments.
Two subgroups were added: "Women with social risk factors versus all women" and "Countries with very high Human Development Index (HDI) > 0.8 versus high, medium and low HDI".
Several outcome changes were made to ensure clinical and policy relevance:
Primary outcomes in the 2016 version
Spontaneous vaginal birth (as defined by trial authors)
Caesarean birth
Regional analgesia (epidural/spinal)
Instrumental vaginal birth (forceps/vacuum)
Intact perineum
All fetal loss before and after 24 weeks plus neonatal death
Preterm birth (less than 37 weeks)
Primary outcomes in the 2024 update have changed slightly to the following:
Spontaneous vaginal birth (as defined by trial authors)
Caesarean birth
Regional analgesia (epidural/spinal)
Intact perineum
Fetal loss at or after 24 weeks gestation
Preterm birth (< 37 weeks)
Neonatal death (baby born alive at any gestation and dies within 28 days)
Secondary outcomes in the 2016 version
Antenatal hospitalisation
Antepartum haemorrhage
Induction of labour
Amniotomy
Augmentation/artificial oxytocin during labour
No intrapartum analgesia/anaesthesia
Opiate analgesia
Attendance at birth by known midwife
Episiotomy
Perineal laceration requiring suturing
Mean labour length (hours)
Postpartum haemorrhage
Breastfeeding initiation
Duration of postnatal hospital stay (days)
Low birthweight (less than 2500 g)
Five‐minute Apgar score less than or equal to seven
Neonatal convulsions
Admission to special care nursery/neonatal intensive care unit
Mean length of neonatal hospital stay (days)
Fetal loss less than 24 weeks and neonatal death
Fetal loss equal to/after 24 weeks and neonatal death
Perceptions of control during labour and childbirth
Mean number of antenatal visits
Maternal death
Cord blood acidosis
Postpartum depression
Any breastfeeding at three months
Prolonged perineal pain
Pain during sexual intercourse
Urinary incontinence
Faecal incontinence
Prolonged backache
Breastfeeding on hospital discharge (not pre‐specified)
Maternal satisfaction (not pre‐specified)
Cost (not pre‐specified)
Secondary outcomes in the 2024 update
Healthy mother (defined as one who is alive at 28 days postpartum, without a Caesarean birth, postpartum haemorrhage (as defined by trial authors), third or fourth‐degree tear, or readmission within 28 days)
Maternal death
Induction of labour
Instrumental vaginal birth (forceps/vacuum)
Episiotomy
Third or fourth‐degree tear
Postpartum haemorrhage (defined by trial authors)
Breastfeeding initiation (defined by trial authors)
Maternal readmission within 28 days
Maternal experience (defined by trial authors)
Attendance at birth by a known midwife who provided antenatal care
Cost (as defined by trial authors)
Healthy baby (defined as one born after 37 + 0 weeks gestation and alive at 28 days and without readmission within 28 days)
Birth weight less than 2500 g
Birth weight equal to or more than 4000 g
Apgar score less than or equal to seven at five minutes
Admission to special care nursery/neonatal intensive care unit
Fetal loss before 24 weeks gestation
Contributions of authors
Jane Sandall (JS)
JS contributed to the protocol and protocol update in 2024 by contributing to the design and writing. JS contributed to the design, screened retrieved papers against inclusion criteria, and appraised the quality of papers.
JS has been the contact author for the review since July 2006 and is the first author of the review. Since 2006, she has co‐ordinated the review process, written to authors for additional information, managed data for the review, re‐extracted data from papers, re‐entered data into Review Manager, re‐entered data for the included studies section, analysed and interpreted data, and provided a clinical and policy perspective. She has rewritten the Plain language summary, Abstract, Background, Methods, Description of studies, Methodological quality, Results, Analysis, and Discussion, and edited the final draft of the review.
JS revised the review in response to feedback from referees and the editor.
JS is the guarantor for the review.
Cristina Fernandez Turienzo (CFT)
CFT contributed to the 2024 review by contributing to the design and writing of the protocol, assessing trials for inclusion and trustworthiness, contacting authors for clarifications, appraising the quality of and extracting data from selected papers, contributing to the interpretation of data, and writing and commenting on the review.
Declan Devane (DD)
DD contributed to the protocol by contributing to the design and writing.
DD contributed to the review by contributing to the design of the review, appraising the quality of and extracting data from selected papers, contributing to the interpretation of data, writing the review, and providing a methodological and clinical perspective.
Simon Gates (SG) SG provided methodological and statistical expertise in the development of the review, and assisted with analysis of data and interpretation of results.
Paddy Gillespie (PG)
PG provided health economic expertise, and assisted with the interpretation of results in the economic analysis section.
Leanne Jones (LJ)
LJ contributed to the 2024 review by assessing trials for inclusion and trustworthiness, extracting the data, and updating the methods sections of the review.
Andrew Shennan (AS)
AS provided specialist obstetric expertise, and assisted with the interpretation of results.
Hora Soltani (HS) HS contributed to the design and commented on the first draft of the protocol.
HS contributed to the development of the protocol and review by contributing to the design, evaluation of the quality of the articles against the inclusion/exclusion criteria, data extraction, writing to authors for clarification of original article information, data interpretation, commenting on as well as writing the review, and drafting the economic analysis section.
Hannah Rayment‐Jones (HRJ)
HRJ contributed to the 2024 review by contributing to the design and writing of the protocol, assessing trials for inclusion and trustworthiness, writing to authors for clarification of original article information, appraising the quality of and extracting data from selected papers, contributing to the interpretation of data by providing a clinical perspective, writing and commenting on the review, managing articles, and drafting the background.
Sources of support
Internal sources
-
Department of Women and Children's Health, School of Life Course Science and Population Health, Faculty of Life Science and Medicine, King's College, London, UK
Employer
-
Sheffield Hallam University, Sheffield, UK
Employer
-
Health Services Executive, Dublin North East, Ireland
Employer
-
Trinity College, Dublin, Ireland
Employer
External sources
-
National Institute for Health Research, UK
2013 update. NIHR Programme of centrally managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
-
2015 update. UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland
Support from WHO
-
National Institute for Health Research (NIHR) , UK
The research was supported by the National Institute for Health Research (NIHR) Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust and ARC Yorkshire and Humber at Sheffield Hallam University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Declarations of interest
Jane Sandall: Jane Sandall was Head of Maternity and Midwifery Research NHS England 2/21‐8/23. She is also a member of the WHO Strategic and Technical Advisory Group of Experts (STAGE) for maternal, newborn, child, adolescent health, and nutrition. JS was Chief Investigator on the study Fernandez Turienzo 2020, and had no involvement in the assessment of this trial for the review.
Cristina Fernandez Turienzo: CFT was lead author on Fernandez Turienzo 2020, and had no involvement in the assessment of this trial for the review.
Declan Devane: Declan Devane is Director of Evidence Synthesis Ireland, Scientific Director of Cochrane Ireland, but had no involvement in the editorial processing of this review. DD was an author on Begley 2011. He had no involvement in the assessment of this trial for the review.
Hora Soltani: no known conflict of interest.
Simon Gates: no known conflict of interest. Simon was the Statistical Editor for Cochrane Pregnancy and Childbirth but had no involvement in the editorial processing of this review.
Paddy Gillespie: no known conflict of interest.
Leanne Jones: Leanne Jones was the Acting Managing Editor of Cochrane Pregnancy and Childbirth but had no involvement in the editorial processing of this review. Leanne is currently a Managing Editor within the Evidence Production & Methods Directorate for the Cochrane Central Executive, but again had no involvement in the editorial or peer review processing of this review.
Andrew Shennan: Andrew Shennan is chair of the FIGO preterm birth committee and has advised WHO in this capacity. He leads a NIHR global health research group. AS was co‐investigator on Fernandez Turienzo 2020, and had no involvement in the assessment of this trial for the review.
Hannah Rayment‐Jones: no known conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Begley 2011 {published and unpublished data}
- Begley C, Devan D, Clarke M. An evaluation of midwifery-led care in the Heath Service Executive North Eastern Area: the report of the MiDU study. School of Nursing and Midwifery, Trinity College Dublin, HealthService Executive (HSE) http://hdl.handle.net/10147/299856 (accessed 3 January 2022).
- Begley C, Devane D, Clarke M, McCann C, Hughes P, Reilly M, et al. Comparison of midwife-led and consultant-led care of healthy women at low risk of childbirth complications in the Republic of Ireland: a randomised trial [Thesis]. Dublin: Trinity College, University of Dublin, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C, Devane D, Clarke M, McCann C, Hughes P, Reilly M, et al. Comparison of midwife-led and consultant-led care of healthy women at low risk of childbirth complications in the Republic of Ireland: a randomised trial. BMC Pregnancy and Childbirth 2011;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny C, Devane D, Normand C, Clarke M, Howard A, Begley C. A cost-comparison of midwife-led compared with consultant-led maternity care in Ireland (the MidU study). Midwifery 2015;31(11):1032-8. [DOI] [PubMed] [Google Scholar]
Biro 2000 {published data only}
- Biro MA, Waldenstrom U, Brown S, Pannifex JH. Satisfaction with team midwifery care for low- and high-risk women: a randomized controlled trial. Birth 2003;30(1):1-10. [DOI] [PubMed] [Google Scholar]
- Biro MA, Waldenstrom U, Pannifex JH. Team midwifery care in a tertiary level obstetric service: a randomized controlled trial. Birth 2000;27(3):168-73. [DOI] [PubMed] [Google Scholar]
Fernandez Turienzo 2020 {published data only}
- Brigante L, Sandall J. How is the implementation of a new continuity of care model for women at high risk of preterm birth (POPPIE) experienced by women? A qualitative thematic analysis. BJOG: An International Journal of Obstetrics and Gynaecology 2019;126:124. [CENTRAL: CN-01937133] [EMBASE: 627142613] [Google Scholar]
- Fernandez Turienzo C, Bick D, Bollard M, Brigante L, Briley A, Coxon K, et al. Poppie: protocol for a randomised controlled pilot trial of continuity of midwifery care for women at increased risk of preterm birth. Trials 2019;20(1):271. [CENTRAL: CN-01938207] [EMBASE: 627652423] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Turienzo C, Bick D, Briley AL, Bollard M, Coxon K, Cross P, et al. Midwifery continuity of care versus standard maternity care for women at increased risk of preterm birth: a hybrid implementation - effectiveness, randomised controlled pilot trial in the UK. PLOS Medicine 2020;17(10):e1003350. [CENTRAL: CN-02202771] [EMBASE: 2008387651] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Turienzo C, Hull LH, Coxon K, Bollard M, Cross P, Seed PT, Shennan AH, Sandall J. A continuity of care programme for women at risk of preterm birth in the UK: process evaluation of a hybrid randomised controlled pilot trial. PLOS One 2023;18(1):e0279695. [DOI: 10.1371/journal.pone.0279695] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Turienzo C, Silverio SA, Coxon K, Brigante L, Seed PT, Shennan AH, et al. Experiences of maternity care among women at increased risk of preterm birth receiving midwifery continuity of care compared to women receiving standard care: results from the POPPIE pilot trial. PLOS One 2021;16(4):e0248588. [CENTRAL: CN-02274637] [EMBASE: 2011800182] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN37733900. POPPIE: Pilot study Of midwifery Practice in Preterm birth Including women's Experiences. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN37733900 (first received 2017). [CENTRAL: CN-01887647]
- Sandall J, Fernandez-Turienzo C, Briley A, Shennan A, Bollard M, Cross P, et al. Pilot study of midwifery Practice in Preterm birth Including women's Experiences (POPPIE): development and implementation of a pilot randomised controlled trial of midwifery continuity of care and preterm birth clinic for women at higher risk of preterm birth in Lewisham. BJOG: an International Journal of Obstetrics and Gynaecology. Conference: 20th Annual Conference of the British Maternal and Fetal Medicine Society, BMFMS 2018. United Kingdom 2018;125(Suppl 2):107. [CENTRAL: CN-01607679] [EMBASE: 622019854] [Google Scholar]
Flint 1989 {published data only}
- Flint C, Poulengeris P, Grant AM. The 'Know your midwife' scheme - a randomised trial of continuity of care by a team of midwives. Midwifery 1989;5:11-6. [DOI] [PubMed] [Google Scholar]
- Flint C, Poulengeris, P. The 'Know your Midwife' Report. London: Heinemann, 1986. [Google Scholar]
- Flint C. Know your midwife. Nursing Times 1988;84:28-32. [PubMed] [Google Scholar]
Gu 2013 {published data only}
- Gu C, Wu X, Ding Y, Zhu X, Zhang Z. The effectiveness of a Chinese midwives' antenatal clinic service on childbirth outcomes for primipare: a randomised controlled trial. International Journal of Nursing Studies 2013;50(12):1689-97. [DOI] [PubMed] [Google Scholar]
Harvey 1996 {published data only}
- Harvey S, Jarrell J, Brant R, Stainton C, Rach D. A randomized, controlled trial of nurse-midwifery care. Birth 1996;23:128-35. [DOI] [PubMed] [Google Scholar]
- Harvey S, Rach D, Stainton MC, Jarrell J, Brant R. Evaluation of satisfaction with midwifery care. Midwifery 2002;18(4):260-7. [DOI] [PubMed] [Google Scholar]
Hicks 2003 {published data only}
- Hicks C, Spurgeon P, Barwell F. Changing childbirth: a pilot project. Journal of Advanced Nursing 2003;42(6):617-28. [DOI] [PubMed] [Google Scholar]
Homer 2001 {published data only}
- ACTRN12611001214921. A randomised controlled trial to determine whether continuity of care increases the rate of attempted vaginal birth after caesarean (VBAC). http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12611001214921 (first received 2011). [CENTRAL: CN-01810857]
- Homer C, Davis G, Brodie P, Sheehan A, Barclay L, Wills J, et al. Collaboration in maternity care: a randomised controlled trial comparing community-based continuity of care with standard hospital care. BJOG: an International Journal of Obstetrics and Gynaecology 2001;108:16-22. [DOI] [PubMed] [Google Scholar]
- Homer C. Incorporating cultural diversity in randomised controlled trials in midwifery. Midwifery 2000;16:252-9. [DOI] [PubMed] [Google Scholar]
- Homer CS, Besley K, Bell J, Davis D, Adams J, Porteous A, et al. Does continuity of care impact decision making in the next birth after a caesarean section (VBAC)? a randomised controlled trial. BMC Pregnancy and Childbirth 2013;13:140. [CENTRAL: CN-00963263] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer CS, Davis DL, Mollart L, Turkmani S, Smith RM, Bullard M, et al. Midwifery continuity of care and vaginal birth after caesarean section: a randomised controlled trial. Women and Birth 2021;35(3):e294-301. [CENTRAL: CN-02284366] [EMBASE: 2012854904] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Homer CS, Davis GK, Brodie PM. What do women feel about community-based antenatal care? Australian & New Zealand Journal of Public Health 2000;24:590-5. [DOI] [PubMed] [Google Scholar]
- Homer CS, Davis GK, Cooke M, Barclay LM. Women's experiences of continuity of midwifery care in a randomised controlled trial in Australia. Midwifery 2002;18(2):102-12. [DOI] [PubMed] [Google Scholar]
- Homer CS, Matha DV, Jordan LG, Wills J, Davis GK. Community-based continuity of midwifery care versus standard hospital care: a cost analysis. Australian Health Review 2001;24(1):85-93. [DOI] [PubMed] [Google Scholar]
Kenny 1994 {published data only}
- Kenny P, Brodie P, Eckerman S, Hall J. Final Report. Westmead Hospital Team Midwifery Project Evaluation. Sydney: University of Sydney, 1994. [Google Scholar]
MacVicar 1993 {published data only}
- MacVicar J, Dobbie G, Owen-Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1993;100:316-23. [DOI] [PubMed] [Google Scholar]
Marks 2003 {published data only}
- Marks MN, Siddle K, Warwick C. Can we prevent postnatal depression? A randomized controlled trial to assess the effect of continuity of midwifery care on rates of postnatal depression in high-risk women. Journal of Maternal-Fetal and Neonatal Medicine 2003;13:119-27. [DOI] [PubMed] [Google Scholar]
McLachlan 2012 {published data only}
- Davey M, McLachlan H, Forster D. Timing of admission and selected aspects of intrapartum care: relationship with caesarean section in the COSMOS (Caseload Midwifery) trial. Women & Birth 2013;26(Suppl 1):S3. [Google Scholar]
- Davey MA, McLachlan L, Forster D, Flood M. Influence of timing of admission in labour and management of labour on method of birth: results from a randomised controlled trial of caseload midwifery (COSMOS trial). Midwifery 2013;29(12):1297-302. [DOI] [PubMed] [Google Scholar]
- Flood M, Forster DA, Davey MA, McLachlan HL. Serious adverse event monitoring in a RCT of caseload midwifery (COSMOS). Journal of Paediatrics and Child Health 2012;48(Suppl 1):113. [Google Scholar]
- Forster DA, McLachlan HL, Davey MA, Biro MA, Farrell T, Gold L, et al. Continuity of care by a primary midwife (caseload midwifery) increases women's satisfaction with antenatal, intrapartum and postpartum care: results from the COSMOS randomised controlled trial. BMC Pregnancy and Childbirth 2016;16(1):28. [CENTRAL: CN-01200294] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster DA, McLachlan HL, Davey MA, Flood M. A randomised controlled trial of caseload midwifery for women at low risk of medical complications (cosmos): breastfeeding intentions, initiation and two month feeding outcomes. Journal of Paediatrics and Child Health 2015;51(Suppl 1):48. [CENTRAL: CN-01732070] [EMBASE: 71873897] [Google Scholar]
- McLachan H. A randomised trial comparing one-to-one midwifery care with standard hospital maternity care for women at low risk, in order to decrease operative birth and other interventions and increase the duration of breastfeeding and women's satisfaction with care, with no increase in costs of care. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 19 February 2008).
- McLachlan H, Forster D, Davey M-A, Gold L, Biro M-A, Flood M, et al. The effect of caseload midwifery on women's experience of labour and birth: Results from the COSMOS randomised controlled trial. Women & Birth 2013;26(Suppl 1):S13. [Google Scholar]
- McLachlan H, Forster D, Davey MA, Farrell T, Gold L, Oats J, et al. A randomised controlled trial of caseload midwifery for women at low risk of medical complications (COSMOS) - primary and secondary outcomes. Women and Birth 2011;24 Suppl 1:S13. [Google Scholar]
- McLachlan H, Forster D, Davey MA. The effect of caseload midwifery on women's experience of labour and birth: results from the COSMOS randomised controlled trial. In: International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1-4; Prague, Czech Republic. 2014:C085.
- McLachlan HL, Forster DA, Davey MA, Farrell T, Flood M, Shafiei T, et al. The effect of primary midwife-led care on women's experience of childbirth: results from the COSMOS randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2016;123(3):465-74. [DOI] [PubMed] [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Farrell T, Flood M, Shafiei, et al. The effect of primary midwife-led care on women's experiences of childbirth: results of the COSMOS randomised controlled trial. In: Perinatal Society of Australia and New Zealand 20th Annual Conference; 2016 May 22-25; Townsville, Australia. 2016.
- McLachlan HL, Forster DA, Davey MA, Farrell T, Gold L, Biro MA, et al. Effects of continuity of care by a primary midwife (caseload midwifery) on caesarean section rates in women of low obstetric risk: the COSMOS randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2012;119(12):1483-92. [DOI] [PubMed] [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Farrell T, Gold L, Oats J, et al. A randomised controlled trial of caseload midwifery for women at low risk of medical complications (COSMOS): maternal and infant outcomes. Journal of Paediatrics and Child Health 2011;47(Suppl 1):33. [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Farrell T, Gold L, Waldenstrom U, et al. A randomised controlled trial of caseload midwifery for women at low risk of medical complications (COSMOS): women's satisfaction with care. Journal of Paediatrics and Child Health 2012;48(Suppl 1):41-2. [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Flood M, Shafiei S, Farrell T, et al. The effect of primary midwife-led care on women's experiences of childbirth: results of the cosmos randomised controlled trial. Journal of Paediatrics and Child Health 2016;52:65-6. [CENTRAL: CN-01304545] [EMBASE: 614023893] [DOI] [PubMed] [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Lumley J, Farrell T, Oats J, et al. Cosmos: comparing standard maternity care with one-to-one midwifery support: a randomised controlled trial. BMC Pregnancy and Childbirth 2008;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
North Stafford 2000 {published data only}
- North Staffordshire Changing Childbirth Research Team. A randomised study of midwifery caseload care and traditional 'shared-care'. Midwifery 2000;16:295-302. [DOI] [PubMed] [Google Scholar]
Rowley 1995 {published data only}
- Rowley MJ, Hensley MJ, Brinsmead MW, Wlodarczyk JH. Continuity of care by a midwife team vs routine care during pregnancy and birth: a randomised trial. Medical Journal of Australia 1995;163:289-93. [DOI] [PubMed] [Google Scholar]
Tracy 2013 {published data only}
- Allen J, Jenkinson B, Tracy SK, Hartz DL, Tracy M, Kildea S. Women's unmet needs in early labour: qualitative analysis of free-text survey responses in the M@NGO trial of caseload midwifery. Midwifery 2020;88:102751. [CENTRAL: CN-02138832] [EMBASE: 632017720] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Allen J, Kildea S, Hartz DL, Tracy M, Tracy S. The motivation and capacity to go 'above and beyond': qualitative analysis of free-text survey responses in the M@NGO randomised controlled trial of caseload midwifery. Midwifery 2017;50:148-56. [CENTRAL: CN-01600476] [EMBASE: 621963008] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Allen J, Kildea S, Tracy MB, Hartz DL, Welsh AW, Tracy SK. The impact of caseload midwifery, compared with standard care, on women's perceptions of antenatal care quality: survey results from the M@NGO randomized controlled trial for women of any risk. Birth (Berkeley, Calif.) 2019;46(3):439-49. [CENTRAL: CN-02083893] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Forti A, Kildea S, Stapleton H. Intrapartum care for women: A sub-study of the M@NGO RCT. Women and Birth 2015;28 Suppl:S14-5. [Google Scholar]
- Hartz D, Hall B, Allen J, Lainchbury A, Forti A, Kildea S, et al. The M@NGO Trial: Does caseload midwifery reduce caesarean section operation rates? Women & Birth 2013;26(Suppl 1):S8-9. [Google Scholar]
- Hartz D, Tracy SK, Foureur M. Does caseload midwifery reduce casarean section rates. In: International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1-4; Prague, Czech Republic. 2014:C086.
- Hartz DL, Tracy S, Kildea S, Tracy M, Foureur M, Hall B, et al. Does caseload midwifery reduce caesarean section operation rates: The M@NGo trial. Journal of Paediatrics and Child Health 2012;48(Suppl 1):27. [Google Scholar]
- Kildea S, Simcock G, Liu A, Elgbeili G, Laplante DP, Kahler A, et al. Continuity of midwifery carer moderates the effects of prenatal maternal stress on postnatal maternal wellbeing: the Queensland flood study. Archives of Women's Mental Health 2018;21(2):203-14. [DOI] [PubMed] [Google Scholar]
- Tracy K, Hartz L, Tracy B, Allen J, Forti A, Hall B, et al. Caseload midwifery care versus standard maternity care for women of any risk: M@NGO, a randomised controlled trial. Lancet 2013;382(9906):1723-32. [DOI] [PubMed] [Google Scholar]
- Tracy SK, Hartz D, Hall B, Allen J, Forti A, Lainchbury A, et al. A randomised controlled trial of caseload midwifery care: M@NGO (Midwives @ New Group practice Options). BMC Pregnancy and Childbirth 2011;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy SK, Welsh A, Hall B, Hartz D, Lainchbury A, Bisits A, et al. Caseload midwifery compared to standard or private obstetric care for first time mothers in a public teaching hospital in Australia: a cross sectional study of cost and birth outcomes. BMC Pregnancy and Childbirth 2014;14(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy SK. A randomised controlled trial of caseload midwifery care. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 31 July 2009) 2009.
Turnbull 1996 {published data only}
- Cheyne H, McGinley M, Turnbull D, Holmes A, Shields N, Greer I, et al. Midwife managed care: results of a randomised controlled trial of 1299 women. Prenatal and Neonatal Medicine 1996;1(Suppl 1):129. [Google Scholar]
- Holmes A, McGinley M, Turnbull D, Shields N, Hillan E. A consumer driven quality assurance model for midwifery. British Journal of Midwifery 1996;4(10):512-8. [Google Scholar]
- McGinley M, Turnbull D, Fyvie H, Johnstone I, MacLennan B. Midwifery development unit at Glasgow Royal Maternity Hospital. British Journal of Midwifery 1995;3(7):362-71. [Google Scholar]
- Shields N, Holmes A, Cheyne H. Knowing your midwife in labour. British Journal of Midwifery 1995;7(8):504-10. [Google Scholar]
- Shields N, Reid M, Cheyne H, Holmes A, McGinley M, Turnbull D, et al. Impact of midwife-managed care in the postnatal period: an exploration of psychosocial outcomes. Journal of Reproductive and Infant Psychology 1997;15:91-108. [Google Scholar]
- Shields N, Turnbull D, Reid M, Holmes A, Cheyne H, McGinley M, et al. Satisfaction with midwife-managed care in different time periods: a randomised controlled trial of 1299 women. Midwifery 1998;14:85-93. [DOI] [PubMed] [Google Scholar]
- Shields N, Turnbull D, Reid M, Holmes A, Cheyne H, McGinley M, et al. Women's satisfaction and continuity of care with midwife managed care. Prenatal and Neonatal Medicine 1996;1(1):320. [Google Scholar]
- Turnbull D, Holmes A, Cheyne H, Shields N, McGinley M, McIlwaine G, et al. Does midwife-led care work? The results of a randomised controlled trial of 1299 women. In: 27th British Congress of Obstetrics and Gynaecology; 1995 July 4-7; Dublin, Ireland. 1995:527.
- Turnbull D, Holmes A, Shields N, Cheyne H, Twaddle S, Harper Gilmour W, et al. Randomised, controlled trial of efficacy of midwife-managed care. Lancet 1996;348:213-8. [DOI] [PubMed] [Google Scholar]
- Turnbull D, McGinley M, Fyvie M, Johnstone IEA. Implementation and evaluation of a midwifery development unit. British Journal of Midwifery 1995;3(9):465-8. [Google Scholar]
- Turnbull D, Reid M, McGinley M, Shields N. Changes in midwife attitudes to their professional role following implementation of the midwifery development unit. Midwifery 1995;11(3):110-9. [DOI] [PubMed] [Google Scholar]
- Turnbull D, Shields N, McGinley M, Holmes A, Cheyne H, Reid M, et al. Professional issues: can midwife-managed units improve continuity of care? British Journal of Midwifery 1999;7(8):499-503. [Google Scholar]
- Young D, Lees A, Twaddle S. The costs to the NHS of maternity care: midwife-managed vs shared. British Journal of Midwifery 1997;5(8):465-72. [Google Scholar]
- Young D, Shields N, Holmes A, Turnbull D, Twaddle S. Aspects of antenatal care. A new style of midwife-managed antenatal care: costs and satisfaction. British Journal of Midwifery 1997;5:540-5. [Google Scholar]
Waldenstrom 2001 {published data only}
- Waldenstrom U, Brown S, McLachlan H, Forster D, Brennecke S. Does team midwife care increase satisfaction with antenatal, intrapartum, and postpartum care? A randomized controlled trial. Birth 2000;27(3):156-67. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, McLachlan H, Forster D, Brennecke S, Brown S. Team midwife care: maternal and infant outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(3):257-64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 2013 {published data only}
- Allen J, Stapleton H, Tracy S, Kildea S. Is a randomised controlled trial of a maternity care intervention for pregnant adolescents possible? An Australian feasibility study. BMC Medical Research Methodology 2013;13:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bagheri 2021 {published data only}
- Bagheri A, Simbar M, Samimi M, Nahidi F, Alavimajd H, Sadat Z. Comparing the Implications of midwifery-led care and standard model on maternal and neonatal outcomes during pregnancy, childbirth and postpartum. Journal of Midwifery & Reproductive Health 2021;9(3):1-10. [CENTRAL: CN-02309182] [Google Scholar]
- IRCT201408318801N9. Development and evaluating a model of continuous midwifery care during pregnancy, labor and postpartum, an action research study [Evaluation the effect of continuous midwifery care model during pregnancy, labor and postpartum on maternal and neonatal]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201408318801N9 (first received 2016). [CENTRAL: CN-01850113]
Bergland 1998 {published data only}
- Berglund A, Lindmark G. Midwife managed care - impact on use of health services: and area-based randomised controlled trial. In: XVI FIGO World Congress of Obstetrics & Gynecology . Vol. Book 4. Washington DC USA, 2000:116.
- Berglund A, Lindmark GC. Health services effects of a reduced routine programme for antenatal care. European Journal of Obstetrics & Gynecology and Reproductive Biology 1998;77(2):193-9. [DOI] [PubMed] [Google Scholar]
Bergland 2007 {published data only}
- Berglund A, Lindberg M, Nystrom L, Lindmark G. Combining the perspectives of midwives and doctors improves risk assessment in early pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2007;86(2):177-84. [DOI] [PubMed] [Google Scholar]
Bernitz 2011 {published data only}
- Bernitz S, Aas E, Oian P. Economic evaluation of birth care in low-risk women. A comparison between a midwife-led birth unit and a standard obstetric unit within the same hospital in Norway. A randomised controlled trial. Midwifery 2012;28(5):591-9. [DOI] [PubMed] [Google Scholar]
- Bernitz S, Rolland R, Blix E, Jacobsen M, Sjoborg K, Oian P. Is the operative delivery rate in low-risk women dependent on the level of birth care? A randomised controlled trial. BJOG: International journal of obstetrics and gynaecology 2011;118(11):1357-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernitz S, Rolland R, Blix E, Jacobsen M, Sjoborg K, Oian PL. Is the operative delivery rate in low-risk women dependent on birth care level? A randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2012;91(159):45-6. [Google Scholar]
Brugha 2016 {published data only}ISRCTN72346869
- Brugha TS, Smith J, Austin J, Bankart J, Patterson M, Lovett C, et al. Can community midwives prevent antenatal depression? An external pilot study to test the feasibility of a cluster randomized controlled universal prevention trial. Psychological Medicine 2016;46(2):345-56. [CENTRAL: CN-01200735] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byrne 2000 {published data only}
- Byrne JP, Crowther CA, Moss JR. A randomised controlled trial comparing birthing centre care with delivery suite care in Adelaide, Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology 2000;40(3):268-74. [DOI: 10.1111/j.1479-828x.2000.tb03333.x] [DOI] [PubMed] [Google Scholar]
Chambliss 1991 {published data only}
- Chambliss L, Daly C, Medearis AL, Ames M, Turnquist R, Kayne M, et al. Significant differences in cesarean birth rates for resident physician and nurse midwife services are the result of selection criteria. American Journal of Obstetrics and Gynecology 1991;164:313. [Google Scholar]
- Chambliss LR, Daly C, Medearis AL, Ames M, Kayne M, Paul R. The role of selection bias in comparing cesarean birth rates between physician and midwifery management. Obstetrics & Gynecology 1992;80(2):161-5. [PubMed] [Google Scholar]
Chapman 1986 {published data only}
- Chapman MG, Jones M, Spring JE, De Swiet M, Chamberlain GVP. The use of a birthroom: a randomized controlled trial comparing delivery with that in the labour ward. British Journal of Obstetrics and Gynaecology 1986;93:182-7. [DOI] [PubMed] [Google Scholar]
de Wolff 2021 {published data only}
- De Wolff M, Midtgaard J, Johansen M, Rom L, Tabor A, Hegaard H. A midwife-coordinated maternity care intervention (ChroPreg) vs. standard care for pregnant women with chronic medical conditions: results from a randomized controlled trial. European Journal of Obstetrics and Gynecology and Reproductive Biology 2022;270:e84. [CENTRAL: CN-02421972] [EMBASE: 2017204835] [Google Scholar]
- De Wolff MG, Johansen M, Ersboll AS, Rosthoj S, Brunsgaard A, Midtgaard J, et al. Efficacy of a midwife-coordinated, individualized, and specialized maternity care intervention (ChroPreg) in addition to standard care in pregnant women with chronic disease: protocol for a parallel randomized controlled trial. Trials 2019;20(1):291. [CENTRAL: CN-01956269] [EMBASE: 627902223] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegaard HK. Pregnancy and chronic disease: the effect of a midwife-coordinated maternity care Intervention [Efficacy of a midwife-coordinated, individualized, and specialized maternity care intervention (ChroPreg) in addition to standard care in pregnant women with chronic disease: a parallel randomized controlled trial]. https://clinicaltrials.gov/show/NCT03511508 2018. [CENTRAL: CN-02047550] [DOI] [PMC free article] [PubMed]
- Wolff MG, Midtgaard J, Johansen M, Rom AL, Rosthoj S, Tabor A, et al. Effects of a midwife-coordinated maternity care intervention (Chropreg) vs. standard care in pregnant women with chronic medical conditions: results from a randomized controlled trial. International Journal of Environmental Research and Public Health 2021;18(15):7875. [CENTRAL: CN-02291979] [EMBASE: 2007881315] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Famuyide 2014 {published data only}
- Famuyide A. OB Nest; redefining continuity of care for expectant mothers. http://clinicaltrials.gov/ 2014.
Forster 2022 {published data only}
- Forster. Exploring the impact of midwife-led group antenatal care on caesarean section rates and infant health: a multi-site randomised controlled trial. ACTRN12622000607774. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12622000607774 2022. [CENTRAL: CN-02424850]
Giles 1992 {published data only}
- Giles W, Collins J, Ong F, MacDonald R. Antenatal care of low risk obstetric patients by midwives. Medical Journal of Australia 1992;157:158-61. [PubMed] [Google Scholar]
Hailemeskel 2021 {published data only}
- Hailemeskel S, Alemu K, Christensson K, Tesfahun E, Lindgren H. Health care providers’ perceptions and experiences related to midwife-led continuity of care–a qualitative study. PLOS One 2021;16(10):e0258248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailemeskel S, Alemu K, Christensson K, Tesfahun E, Lindgren H. Midwife-led continuity of care improved maternal and neonatal health outcomes in North Shoazone, Amhararegional state, Ethiopia: a quasi-experimental study. Women and Birth 2021;3:340-8. [DOI: 10.1016/j.wombi.2022.01.005] [DOI] [PubMed] [Google Scholar]
- Hailemeskel S, Alemu K, Christensson K, Tesfahun E, Lindgren H. Midwife-led continuity of care increases women’s satisfaction with antenatal, intrapartum, and postpartum care: North Shoa, Amhara regional state, Ethiopia: a quasi-experimental study. Women and Birth 2022;20(35):553-62. [DOI] [PubMed] [Google Scholar]
Hans 2018 {published data only}
- Hans SL, Edwards RC, Zhang Y. Randomized controlled trial of doula-home-visiting services: impact on maternal and infant health. Maternal and Child Health Journal 2018;22(Suppl 1):105-13. [CENTRAL: CN-01650829] [EMBASE: 624237872] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heins 1990 {published data only}
- Heins HC, Nance NW, McCarthy BJ, Efird CM. A randomized trial of nurse-midwifery prenatal care to reduce low birth weight. Obstetrics & Gynecology 1990;75:341-5. [PubMed] [Google Scholar]
Hildingsson 2003 {published data only}
- Hildingsson I, Waldenstrom U, Radestad I. Swedish women's interest in home birth and in-hospital birth center care. Birth 2003;30(1):11-22. [DOI] [PubMed] [Google Scholar]
Hundley 1994 {published data only}
- Hundley VA, Cruickshank FM, Lang GD, Glazener CMA, Milne JM, Turner M, et al. Midwife managed delivery unit: a randomised controlled comparison with consultant led care. BMJ 1994;309:1400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley VA, Cruickshank FM, Milne JM, Glazener CMA, Lang GD, Turner M, et al. Satisfaction and continuity of care: staff views of care in a midwife‐managed delivery unit. Midwifery 1995;11:163-73. [DOI] [PubMed] [Google Scholar]
- Hundley VA, Donaldson C, Lang GD, Cruickshank FM, Glazener CMA, Milne JM, et al. Costs of intrapartum care in a midwife managed delivery unit and a consultant led labour ward. Midwifery 1995;11:103-9. [DOI] [PubMed] [Google Scholar]
James 1988 {published data only}
- James DK. A comparison of a schematic approach to antenatal care and conventional shared care. Personal communication 1988.
Kelly 1986 {published data only}
- Kelly J. Comparison of two different methods of delivering antenatal care, one with components provided by an obstetrician, the other by a midwife. Personal communication 1986.
Kildea 2017 {published data only}
- Kildea S, Simcock G, Liu A, Elgbeili G, Laplante DP, Kahler A, et al. Continuity of midwifery carer moderates the effects of prenatal maternal stress on postnatal maternal wellbeing: the Queensland flood study. Archives of Women's Mental Health 2018;21(2):203-14. [CENTRAL: CN-01656702] [EMBASE: 618503182] [DOI] [PubMed] [Google Scholar]
Kildea 2021 {published data only}
- Kildea S, Gao Y, Hickey S, Nelson C, KruskeS, Carson A, et al. Effect of a Birthing on Country service redesign on maternal and neonatal health outcomes for First Nations Australians: a prospective, non-randomised, interventional trial. Lancet Global Health 2021;1(9):e651-9. [DOI] [PubMed] [Google Scholar]
Klein 1984 {published data only}
- Klein M, Papageorgiou AN, Westreich R, Spector-Dunsky L, Elkins V, Kramer MS, et al. Care in a birth room vs a conventional setting: a controlled trial. Canadian Medical Association Journal 1984;131:1461-6. [PMC free article] [PubMed] [Google Scholar]
Law 1999 {published data only}
- Law YY, Lam KY. A randomized controlled trial comparing midwife-managed care and obstetrician-managed care for women assessed to be at low risk in the initial intrapartum period. Journal of Obstetrics & Gynaecology Research 1999;25:107-12. [DOI] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li YP, Yeh CH, Lin SY, Chen TC, Yang YL, Lee CN, et al. A proposed mother-friendly childbirth model for taiwanese women, the implementation and satisfaction survey. Taiwanese Journal of Obstetrics & Gynecology 2015;54(6):731-6. [CENTRAL: CN-01200475] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2020 {published data only}
- Lin X, Yang T, Zhang X, Wei W. Lifestyle intervention to prevent gestational diabetes mellitus and adverse maternal outcomes among pregnant women at high risk for gestational diabetes mellitus. Journal of International Medical Research 2020;48(12):300060520979130. [CENTRAL: CN-02268776] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loy 2021 {published data only}
- Loy SL, Thilagamangai, Teo J, Chan SW, Razak NKA, Chay OM, et al. A Community-enabled Readiness for first 1000 Days Learning Ecosystem (CRADLE) for first-time families: study protocol of a three-arm randomised controlled trial. Trials 2021;22(1):1-10. [CENTRAL: CN-02265376] [EMBASE: 2010705084] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04275765. Community enabled readiness for first 1000 days learning ecosystem. https://clinicaltrials.gov/show/NCT04275765 (first received 19 February 2020). [CENTRAL: CN-02088295]
Michel‐Schuldt 2021 {published data only}
- Michel-Schuldt M. Midwife-Led Care in Low-and Middle-Income Countries with a Focus on Implementation in Bangladesh. Doctoral dissertation 2021.
Mohammad‐Alizadeh‐Charandabi 2019 {published data only}
- Mohammad-Alizadeh-Charandabi S. Effect of continuous care model from pregnancy to postpartum by midwifery students [The effect of implementing the continuous care model by midwifery students during pregnancy, childbirth, and postpartum on childbirth experience, fear of childbirth, and postpartum depression]. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20100414003706N41 2019. [CENTRAL: CN-02410556]
Morrison 2002 {published data only}
- Morrison J, Neale L, Taylor R, McCowan L. Caring for pregnant women with diabetes. British Journal of Midwifery 2002;10(7):434-9. [DOI: 10.12968/bjom.2002.10.7.10587] [DOI] [Google Scholar]
Mortensen 2018 {published data only}
- Mortensen B, Diep LM, Lukasse M, Lieng M, Dwekat I, Elias D, et al. Women’s satisfaction with midwife-led continuity of care: an observational study in Palestine. BMJ Open 2019;9(11):e030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen B, Lieng M, Diep LM, Lukasse M, AtiehK, Fosse E. Improving maternal and neonatal health by a midwife-led continuity model of care–an observational study in one governmental hospital in Palestine. EClinicalMedicine 2019;1(10):84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen B, Lukasse M, Diep LM, Lieng M, Abu-Awad A, Suleiman M, et al. Can a midwife-led continuity model improve maternal services in a low-resource setting? A non-randomised cluster intervention study in Palestine. BMJ Open 2018;3(8):e019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen B. Making midwifery matter -the introduction of a midwife-led continuity model of care in occupied Palestine. Doctoral thesis 2020.
Nagle 2011 {published data only}
- Nagle C, Skouteris H, Hotchin A, Bruce L, Patterson D, Teale G. Continuity of midwifery care and gestational weight gain in obese women: a randomised controlled trial. BMC Public Health 2011;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle C. The impact of continuity of care on weight gain in obese pregnant women. ACTRN12610001078044 8 December 2010.
Qiu 2020 {published data only}
- Qiu J, Liu Y, Zhu W, Zhang C. Comparison of effectiveness of routine antenatal care with a midwife-managed clinic service in prevention of gestational diabetes mellitus in early pregnancy at a hospital in China. Medical Science Monitor 2020;26:e925991. [CENTRAL: CN-02277357] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ridgeway 2015 {published data only}
- Baron AM, Ridgeway JL, Stirn SL, Morris MA, Branda ME, Inselman JW, et al. Increasing the connectivity and autonomy of RNs with low-risk obstetric patients. American Journal of Nursing 2018;1(118):48-55. [DOI] [PubMed] [Google Scholar]
- Ridgeway JL, LeBlanc A, Branda M, Harms RW, Morris MA, Nesbitt K, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy and Childbirth 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobah YS, LeBlanc A, Branda ME, Inselman JW, Morris MA, Ridgeway JL, et al. Randomized comparison of a reduced-visitprenatal care model enhanced with remote monitoring. American Journal of Obstetrics and Gynecology 2019;1(221):638. [DOI] [PubMed] [Google Scholar]
- Mooij MJ, Hodny RL, O'Neil DA, Gardner MR, Beaver M, Brown AT, et al. OB Nest: reimagining low-risk prenatal care. Mayo Clinic Proceedings 2018;93(4):458-66. [DOI] [PubMed] [Google Scholar]
Runnerstrom 1969 {published data only}
- Runnerstrom L. The effectiveness of nurse-midwifery in a supervised hospital environment. Bulletin of the American College of Midwives 1969;14:40-52. [DOI] [PubMed] [Google Scholar]
Slome 1976 {published data only}
- Slome C, Wetherbee H, Daly M, Christensen K, Meglen M, Thiede H. Effectiveness of certified nurse-midwives. A prospective evaluation study. American Journal of Obstetrics and Gynecology 1976;124:177-82. [DOI] [PubMed] [Google Scholar]
Stevens 1988 {published data only}
- Stevens A. A randomised trial of community antenatal care in Birmingham. Oxford Database of Perinatal Trials Registration 1988.
Tucker 1996 {published data only}
- Ratcliffe J, Ryan M, Tucker J. The costs of alternative types of routine antenatal care for low-risk women: shared care vs care by general practitioners and community midwives. Journal of Health Services & Research Policy 1996;1:135-40. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Hall MH, Howie PW, Reid ME, Barbour RS, Florey CD, et al. Should obstetricians see women with normal pregnancies? A multicentre randomised controlled trial of routine antenatal care by general practitioners and midwives compared with shared care led by obstetricians. BMJ 1996;312:554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waldenstrom 1997 {published data only}
- Gottvall K, Waldenstrom U. Does birth center care during a woman's first pregnancy have any impact on her future reproduction? Birth 2002;29(3):177-81. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA, Winbladh B. The Stockholm birth centre trial: maternal and infant outcome. British Journal of Obstetrics and Gynaecology 1997;104:410-8. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. A randomized controlled study of birth center care versus standard maternity care: effects on women's health. Birth 1997;24:17-26. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. Experience of childbirth in birth center care: a randomized controlled study. Acta Obstetricia et Gynecologica Scandinavica 1994;73:547-54. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. No effect of birth centre care on either duration or experience of breast feeding, but more complications: findings from a randomised controlled trial. Midwifery 1994;10:8-17. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U. Effects of birth centre care on fathers' satisfaction with care, experience of the birth, and adaptation to fatherhood. Journal of Reproductive and Infant Psychology 1999;17(4):357-68. [Google Scholar]
Walker 2012 {published data only}
- Walker D, Demaria L, Gonzalez-Hernandez D, Padron-Salas A, Romero-Alvarez M, Suarez L. Are all skilled birth attendants created equal? A cluster randomised controlled study of non-physician based obstetric care in primary health care clinics in Mexico. Midwifery 2013;29(10):1199-205. [DOI] [PubMed] [Google Scholar]
- Walker DM, DeMaria L, Suarez L, Gonzales D, Romero M, Padron A. Are all skilled birth attendants created equal? Evidence from Mexico? International Journal of Gynecology and Obstetrics 2012;119(3):S516-7. [Google Scholar]
Wiggins 2020 {published data only}
- Wiggins M, Sawtell M, Wiseman O, McCourt C, Eldridge S, Hunter R, et al. Group antenatal care (Pregnancy Circles) for diverse and disadvantaged women: study protocol for a randomised controlled trial with integral process and economic evaluations. BMC Health Services Research 2020;20(1):919. [CENTRAL: CN-02191692] [EMBASE: 633136011] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelani 2011 {published data only}
- Zelani BD. Effectiveness of a midwife led continuity of antenatal care caseload model on preterm birth and maternal satisfaction among women in Malawi. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12621000008820 2021. [CENTRAL: CN-02238621]
References to studies awaiting assessment
Zhang 2016 {published data only}
- Zhang T, Liu C. Comparison between continuing midwifery care and standard maternity care in vaginal birth after cesarean. Pakistan Journal of Medical Sciences 2016;32(3):711-4. [DOI: 10.12669/pjms.323.9546] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Cullinane 2021 {published data only}
- Cullinane D. Exploring the impact of caseload midwifery on preterm birth among vulnerable and disadvantaged women: a multi-centre randomised controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12621001211853 2021. [CENTRAL: CN-02327273]
Dickerson 2022 {published data only}
- Dickerson. Does the midwife-led continuity of carer model improve birth outcomes and maternal mental health in vulnerable women? [Effectiveness of a midwife-led continuity of carer model on birth outcomes and maternal mental health in vulnerable women: study protocol for a randomised controlled trial with an internal pilot and process and economic evaluations]. https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN31836167 2022. [CENTRAL: CN-02429872] [DOI] [PMC free article] [PubMed]
Xiaojiao 2020 {published data only}
- Xiaojiao. Development and implementation of midwife-based care model for urban women with uncomplicated pregnancies: a randomized controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000033459 2020. [CENTRAL: CN-02166314]
Additional references
Alderdice 2022
- Alderdice F, Malouf R, McLeish J, Roberts N, Rowe R. What evidence is available about the use and effectiveness of collaborative midwife continuity of care models to improve pregnancy outcomes for women with medical and obstetric complexity? A scoping review. Oxford: NIHR PRU-MNHC, National Perinatal Epidemiology Unit, University of Oxford 2022. [ISBN: 978-1-7392619-0-0]
Allen 2017
- Allen J, Kildea S, Hartz DL, Tracy M, Tracy S. The motivation and capacity to go ‘above and beyond’: qualitative analysis of free-text survey responses in the M@ NGO randomised controlled trial of caseload midwifery. Midwifery 2017;1(50):148-56. [DOI: 10.1016/j.midw.2017.03.012] [DOI] [PubMed] [Google Scholar]
Allen 2019
- Allen J, Kildea S, Tracy MB, Hartz DL, Welsh AW, Tracy SK. The impact of caseload midwifery, compared with standard care, on women's perceptions of antenatal care quality: survey results from the M@ NGO randomized controlled trial for women of any risk. Birth 2019;46(3):439-49. [DOI: 10.1111/birt.12436] [DOI] [PubMed] [Google Scholar]
Allen 2020
- Allen J, Jenkinson B, Tracy SK, Hartz DL, Tracy M, Kildea S. Women's unmet needs in early labour: qualitative analysis of free-text survey responses in the M@ NGO trial of caseload midwifery. Midwifery 2020;1(88):102751. [DOI: 10.1016/j.midw.2020.102751 Get rights and content] [DOI] [PubMed] [Google Scholar]
Baker 2020
- Baker R, Freeman GK, Haggerty JL, Bankart MJ, Nockels KH. Primary medical care continuity and patient mortality: a systematic review. British Journal of General Practice 2020;70(698):e600-11. [DOI: 10.3399/bjgp20X712289] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2017
- Barker I, Steventon A, Deeny SR. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ 2017;356:j84. [DOI: 10.1136/bmj.j84] [DOI] [PubMed] [Google Scholar]
Barker 2018
- Barker I, Steventon A, Williamson R, Deeny SR. Self-management capability in patients with long-term conditions is associated with reduced healthcare utilisation across a whole health economy: cross-sectional analysis of electronic health records. BMJ Quality & Safety 2018;27(12):989-99. [DOI: 10.1136/bmjqs-2017-007635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayliss 2015
- Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Annals of Family Medicine 2015;13(2):123-9. [DOI: 10.1370/afm.1739] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bazemore 2023
- Bazemore A, Merenstein Z, Handler L, Saultz JW. The impact of interpersonal continuity of primary care on health care costs and use: a critical review. Annals of Family Medicine 2023;21(3):274-9. [DOI: 10.1370/afm.2961] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beecher 2020
- Beecher C, Devane D, White M, Greene R, Dowling M. Women’s experiences of their maternity care: a principle-based concept analysis. Women and Birth 2020;33(5):419-25. [DOI] [PubMed] [Google Scholar]
Beecher 2021
- Beecher C, Greene R, O’Dwyer L, Ryan E, White M, Beattie M, et al. Measuring women's experiences of maternity care: a systematic review of self-report survey instruments. Women and Birth 2021;34(3):231-41. [DOI] [PubMed] [Google Scholar]
Blomgren 2023
- Blomgren J, Gabrielsson S, Erlandsson K, Wagoro MC, Namutebi M, Chimala, et al. Maternal health leaders’ perceptions of barriers to midwife-led care in Ethiopia, Kenya, Malawi, Somalia, and Uganda.. Midwifery 2023;124:103734. [DOI: 10.1016/j.midw.2023.103734] [DOI] [PubMed] [Google Scholar]
Bradford 2022
- Bradford, BF, Wilson AN, Portela A, McConville F, Fernandez Turienzo C, Homer CS. Midwifery continuity of care: a scoping review of where, how, by whom and for whom? PLOS Global Public Health 2022;2(10):e0000935. [DOI: 10.1371/journal.pgph.0000935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlise 2017
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72(8):944-52. [DOI] [PubMed] [Google Scholar]
Carmone 2020
- Carmone AE, Kalaris K, Leydon N, Sirivansanti N, Smith JM, Storey A, et al. Developing a common understanding of Networks of Care through a scoping study. Health Systems & Reform 2020;6(2):e1810921. [DOI: 10.1080/23288604.2020.1810921] [DOI] [PubMed] [Google Scholar]
Chau 2021
- Chau E, Rosella LC, Mondor L, Wodchis WP. Association between continuity of care and subsequent diagnosis of multimorbidity in Ontario, Canada from 2001-2015: a retrospective cohort study. PLOS One 2021;16(3):e0245193. [DOI: 10.1371/journal.pone.0245193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cibralic 2022
- Cibralic S, Pickup W, Diaz AM, Kohlhoff J, Karlov L, Stylianakis A, et al. The impact of midwifery continuity of care on maternal mental health: a narrative systematic review. Midwifery 2022;7:103546. [DOI: 10.1016/j.midw.2022.103546] [DOI] [PubMed] [Google Scholar]
Cook 2000
- Cook RI, Render M, Woods DD. Gaps in the continuity of care and progress on patient safety. BMJ 2000;320:791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curran 2022
- Curran GM, Landes SJ, McBain SA, Pyne JM, Smith JD, Fernandez ME, et al. Reflections on 10 years of effectiveness-implementation hybrid studies. Frontiers in Health Services 2022;2:105349. [DOI: 10.3389/frhs.2022.1053496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahlberg 2013
- Dahlberg U, Aune I. The woman’s birth experience-the effect of interpersonal relationships and continuity of care. Midwifery 2013;29:407-15. [DOI: 10.1016/j.midw.2012.09.006] [DOI] [PubMed] [Google Scholar]
De Vries 2001
- De Vries R, Benoit C, Van Teijlingen E, Wrede S. Birth by design: pregnancy, maternity care and midwifery in North America and Northern Europe. New York: Routledge, 2001. [Google Scholar]
Devane 2007
- Devane D, Begley CM, Clarke M, Horey D, OBoyle C. Evaluating maternity care: a core set of outcome measures. Birth 2007;34(2):164-72. [DOI] [PubMed] [Google Scholar]
Donnellan‐Fernandez 2018
- Donnellan-Fernandez RE, Creedy DK, Callander EJ. Cost-effectiveness of continuity of midwifery care for women with complex pregnancy: a structured review of the literature. Health Economics Review 2018;8(1):1-6. [DOI: 10.1186/s13561-018-0217-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engamba 2019
- Engamba SA, Steel N, Howe A, Bachman M. Tackling multimorbidity in primary care: is relational continuity the missing ingredient? British Journal of General Practice 2019;69(679):92-3. [DOI: 10.3399/bjgp19X701201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez Turienzo 2016
- Fernandez Turienzo C, Sandall J, Peacock JL. Models of antenatal care to reduce and prevent preterm birth: a systematic review and meta-analysis. BMJ Open 2016;6:e009044. [DOI: 10.1136/ bmjopen-2015-009044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez Turienzo 2021
- Fernandez Turienzo C, Rayment-Jones H, Roe Y, Silverio SA, Coxon K, Shennan AH, Sandall J. A realist review to explore how midwifery continuity of care may influence preterm birth in pregnant women. Birth 2021;48(3):375-88. [DOI: 10.1111/birt.12547] [DOI] [PubMed] [Google Scholar]
Flint 1987
- Flint C, Poulengeris P. The 'Know your Midwife' Report. London: Heinemann, 1987. [Google Scholar]
Forster 2016
- Forster DA, McLachlan HL, Davey MA, Biro MA, Farrell T, Gold L, et al. Continuity of care by a primary midwife (caseload midwifery) increases women’s satisfaction with antenatal, intrapartum and postpartum care: results from the COSMOS randomised controlled trial. BMC Pregnancy and Childbirth 2016;16:1-13. [DOI: 10.1186/s12884-016-0798-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2023
- Fox D, Scarf V, Turkmani S, Rossiter C, Coddington R, Sheehy A, et al. Midwifery continuity of care for women with complex pregnancies in Australia: an integrative review. Women and Birth 2023;36(2):e187-94. [DOI: 10.1016/j.wombi.2022.07.001] [DOI] [PubMed] [Google Scholar]
Freeman 2007
- Freeman GK, Woloshynowych M, Baker R, Boulton M, Guthrie B, Car J, et al. Continuity of care 2006: what have we learned since 2000 and what are policy imperatives now? Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation (NCCSDO). London: NCCSDO, 2007. [Google Scholar]
Guthrie 2008
- Guthrie B, Saultz JW, Freeman GK, Haggerty JL. Continuity of care matters. BMJ 2008;337:a867. [DOI] [PubMed] [Google Scholar]
Hadebe 2021
- Hadebe R, Seed PT, Essien D, Headen K, Mahmud S, Owasil S, et al. Can birth outcome inequality be reduced using targeted caseload midwifery in a deprived diverse inner city population? A retrospective cohort study, London, UK. BMJ Open 2021;11(11):e049991. [DOI: 10.1136/bmjopen-2021-049991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haggerty 2003
- Haggerty JL, Reid RJ. Continuity of care: a multidisciplinary review. BMJ 2003;327(7425):1219-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvey 2002
- Harvey S, Rach D, Stainton MC, Jarrell J, Brant R. Evaluation of satisfaction with midwifery care. Midwifery 2002;18(4):260-67. [DOI: 10.1054/midw.2002.0317] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). CCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hoffman 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Homer 2000
- Homer C. Incorporating cultural diversity in randomised controlled trials in midwifery. Midwifery 2000;16:252-9. [DOI: 10.1054/midw.2000.0230] [DOI] [PubMed] [Google Scholar]
Homer 2002
- Homer CS, Davis GK, Cooke M, Barclay LM. Women's experiences of continuity of midwifery care in a randomised controlled trial in Australia. Midwifery 2002;18(2):102-12. [DOI: 10.1054/midw.2002.0298] [DOI] [PubMed] [Google Scholar]
Homer 2016
- Homer CS. Models of maternity care: evidence for midwifery continuity of care. Medical Journal of Australia 2016;205(8):370-4. [DOI: 10.5694/mja16.00844] [DOI] [PubMed] [Google Scholar]
Homer 2017
- Homer C S, Leap N, Edwards N, Sandall J. Midwifery continuity of carer in an area of high socio-economic disadvantage in London: A retrospective analysis of Albany Midwifery Practice outcomes using routine data (1997-2009). Midwifery 2017;48:1-10. [DOI: 10.1016/j.midw.2017.02.009] [DOI] [PubMed] [Google Scholar]
Homer 2019
- Homer C, Brodie P, Sandall J, Leap N. Midwifery continuity of care: a practical guide. Chatswood: Elsevier Australia, 2019. [Google Scholar]
ICM 2017
- International Confederation of Midwives. International definition of the midwife. ICM core document 2017;CD2005_001 V2017 ENG.
Kelly 2014
- Kelly J, West R, Gamble J, Sidebotham M, Carson V, Duffy E. 'She knows how we feel': Australian Aboriginal and Torres Strait Islander childbearing women's experience of Continuity of Care with an Australian Aboriginal and Torres Strait Islander midwifery student. Women and Birth 2014;27(3):157-62. [DOI: 10.1016/j.wombi.2014.06.002] [DOI] [PubMed] [Google Scholar]
Kenny 2015
- Kenny C, Devane D, Normand C, Clarke M, Howard A, Begley C. A cost-comparison of midwife-led compared with consultant-led maternity care in Ireland (the MidU study). Midwifery 2015;31:1032-8. [DOI: 10.1016/j.midw.2015.06.012] [DOI] [PubMed] [Google Scholar]
Khan 2023
- Khan Z, Vowles Z, Fernandez Turienzo C, Barry Z, Brigante L, Downe S, et al. Targeted health and social care interventions for women and infants who are disproportionately impacted by health inequalities in high-income countries: a systematic review. International Journal for Equity in Health 2023;22:1-19. [DOI: 10.1186/s12939-023-01948-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kildea 2018
- Kildea S, Simcock G, Liu A, Elgbeili G, Laplante DP, Kahler A, et al. Continuity of midwifery carer moderates the effects of prenatal maternal stress on postnatal maternal wellbeing: the Queensland flood study. Archive of Women's Mental Health 2018;21(2):203-14. [DOI: 10.1007/s00737-017-0781-2] [DOI] [PubMed] [Google Scholar]
Kildea 2021
- Kildea S, Gao Y, Hickey S, Nelson C, Kruske S, Carson A, et al. Effect of a Birthing on Country service redesign on maternal and neonatal health outcomes for First Nations Australians: a prospective, non-randomised, interventional trial. Lancet Global Health 2021;9(5):e651-9. [DOI: 10.1016/ S2214-109X(21)00061-9] [DOI] [PubMed] [Google Scholar]
Koblinsky 2016
- Koblinsky M, Moyer CA, Calver C, Campbell J, Campbell OM, Feigl AB, et al. Quality maternity care for every woman, everywhere: a call to action. Lancet 2016;388(10057):2307-20. [DOI: 10.1016/S0140-6736(16)31333-2] [DOI] [PubMed] [Google Scholar]
Lowe 2020
- Lowe NK. Birth settings in America: outcomes, quality, access, and choice. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2020;49(4):e651-9. [DOI: 10.1016/j.jogn.2020.06.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCourt 2006
- McCourt C, Stevens T. Continuity of carer–what does it mean and does it matter to midwives and birthing women? Canadian Journal of Midwifery Research and Practice 2006;4(3):10-20. [ISSN: 1703-2121] [Google Scholar]
McFadden 2020
- McFadden A, Gupta S, Marshall JL, Shinwell S, Sharma B, McConville F, et al. Systematic review of barriers to, and facilitators of, the provision of high-quality midwifery services in India. Birth 2020;47(4):304-21. [DOI: 10.1111/birt.12498] [DOI] [PubMed] [Google Scholar]
McInnes 2020
- McInnes RJ, Aitken-Arbuckle A, Lake S, et al. Implementing continuity of midwife carer – just a friendly face? A realist evaluation. BMC Health Services Research 2020;20:304. [DOI: 10.1186/s12913-020-05159-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLachlan 2016
- McLachlan HL, Forster DA, Davey MA, Farrell T, Flood M, Shafiei T, et al. The effect of primary midwife‐led care on women's experience of childbirth: results from the COSMOS randomised controlled trial. BJOG: An International Journal of Obstetrics & Gynaecology 2016;123(3):465-74. [DOI: 10.1111/1471-0528.13835] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Cooper C, et al. Process evaluation in complex public health intervention studies: the need for guidance. Journal of Epidemiology and Community Health 2014;68(2):101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHSE 2021
- NHS England and NHS Improvement. Delivering midwifery continuity of carer at full scale: guidance on planning, implementation and monitoring. NHS England and Improvement 2021.
Nove 2021
- Nove A, Friberg IK, Bernis L, McConville F, Moran AC, Najjemba M, et al. Potential impact of midwives in preventing and reducing maternal and neonatal mortality and stillbirths: a lives saved tool modelling study.. Lancet Global Health 2021;9(1):e24-w32. [DOI: 10.1016/S2214-109X(20)30397-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyweide 2017
- Nyweide DJ, Bynum JPW. Relationship between continuity of ambulatory care and risk of emergency department episodes among older adults. Annals of Emergency Medicine 2017;69(4):407-15. [DOI: 10.1016/j.annemergmed.2016.06.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
O’Malley 2009
- O’Malley AS, Cunningham PJ. Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. Journal of General Internal Medicine 2009;24:170-7. [DOI: 10.1007/s11606-008-0885-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paddison 2015
- Paddison CA, Abel GA, Roland MO, Elliott MN, Lyratzopoulos G, Campbell JL. Drivers of overall satisfaction with primary care: evidence from the English General Practice Patient Survey. Health Expectations 2015;18(5):1081-92. [DOI: doi: 10.1111/hex.12081] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parchman 2004
- Parchman ML, Burge SK. The patient-physician relationship, primary care attributes, and preventive services. Family Medicine 2004;36(1):22-7. [PMID: ] [PubMed] [Google Scholar]
Pereira Gray 2018
- Pereira Gray DJ, Sidaway-Lee K, White E, Thorne A, Evans PH. Continuity of care with doctors-a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open 2018;28(8):e021161. [DOI: 10.1136/bmjopen-2017-021161] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perriman 2018
- Perriman N, Davis DL, Ferguson S. What women value in the midwifery continuity of care model: a systematic review with meta-synthesis. Midwifery 2018;62:220-9. [DOI] [PubMed] [Google Scholar]
Pourat 2015
- Pourat N, Davis AC, Chen X, Vrungos S, Kominski GF. In California, primary care continuity was associated with reduced emergency department use And fewer hospitalizations. Health Affairs 2015;34(7):1113-20. [DOI: 10.1377/hlthaff.2014.1165] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayment‐Jones 2015
- Rayment-Jones H, Murrells T, Sandall J. An investigation of the relationship between the caseload model of midwifery for socially disadvantaged women and childbirth outcomes using routine data--a retrospective, observational study. Midwifery 2015;31(4):409-17. [DOI] [PubMed] [Google Scholar]
Rayment‐Jones 2020
- Rayment-Jones H, Silverio SA, Harris J, Harden A, Sandall J. Project 20: Midwives' insight into continuity of care models for women with social risk factors: what works, for whom, in what circumstances, and how. Midwifery 2020;8:102654. [DOI: 10.1016/j.midw.2020.102654] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayment‐Jones 2021
- Rayment-Jones H, Dalrymple K, Harris J, Harden A, Parslow E, et al. Project20: Does continuity of care and community-based antenatal care improve maternal and neonatal birth outcomes for women with social risk factors? A prospective, observational study. PLOS One 2021;4(16):e0250947. [DOI: 10.1371/journal.pone.0250947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayment‐Jones 2022
- Rayment-Jones H, Harris J, Harden A, Fernandez Turienzo C, Sandall J. Project20: maternity care mechanisms that improve (or exacerbate) health inequalities. A realist evaluation. Women and Birth 2022;36:e314-27. [DOI: 10.1016/j.wombi.2022.11.006] [DOI] [PubMed] [Google Scholar]
Rayment‐Jones 2023
- Rayment-Jones H, Dalrymple K, Harris JM, Harden A, Parslow E, Georgi T, et al. Project20: maternity care mechanisms that improve access and engagement for women with social risk factors in the UK - a mixed-methods, realist evaluation. BMJ Open 2023;13(2):e064291. [DOI: 10.1136/bmjopen-2022-064291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2002
- Reid RJ, Haggerty JL, McKendry R. Defusing the confusion: concepts and measures of continuity of health care. Report to the Canadian Health Services Research Foundation 2002.
Renfrew 2014
- Renfrew MJ, Homer C, Downe S, McFadden A, Muir N, Prentice, et al. Midwifery: an executive summary for The Lancet’s series. Lancet 2014;384(1):8. [Google Scholar]
Renfrew 2021
- Renfrew MJ. Scaling up care by midwives must now be a global priority [Lancet Global Health]. 9 2021;1:e2-3. [DOI: 10.1016/S2214-109X(20)30478-2] [DOI] [PubMed] [Google Scholar]
Review Manager Web 2023 [Computer program]
- Review Manager Web (RevMan Web). Version 4.28.1. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Ryan 2013
- Ryan P, Revill P, Devane D, Normand C. An assessment of the cost-effectiveness of midwife-led care in the United Kingdom. Midwifery 2013;29(4):368-76. [DOI] [PubMed] [Google Scholar]
Sandvik 2021
- Sandvik H, Hetlevik Ø, Blinkenberg J, Hunskaar S. Continuity in general practice as predictor of mortality, acute hospitalisation, and use of out-of-hours care: a registry-based observational study in Norway. British Journal of General Practice 2021;72(715):e84-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI] [PubMed] [Google Scholar]
Saultz 2003
- Saultz JW. Defining and measuring interpersonal continuity of care. Annals of Family Medicine 2003;1(3):134-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saultz 2004
- Saultz JW, Albedaiwi W. Interpersonal continuity of care and patient satisfaction: a critical review. Annals of Family Medicine 2004;2(5):445-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saultz 2005
- Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Annals of Family Medicine 2005;3(2):159-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 1997
- Shields N, Reid M, Cheyne H, Holmes A, McGinley M, Turnbull D, et al. Impact of midwife-managed care in the postnatal period: an exploration of psychosocial outcomes. Journal of Reproductive and Infant Psychology 1997;15(2):91-108. [DOI: 10.1080/02646839708404537] [DOI] [Google Scholar]
Sidaway‐Lee 2021
- Sidaway-Lee K, Pereira Gray OBE D, Harding A, Evans P. What mechanisms could link GP relational continuity to patient outcomes? British Journal of General Practice 2021;71(707):278-81. [DOI: 10.3399/bjgp21X716093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skivington 2021
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. British Medical Journal 2021;30(374):n2061. [DOI: 10.1136/bmj.n2061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Starfield 2009
- Starfield B. Primary care and equity in health: the importance to effectiveness and equity of responsiveness to peoples' needs. Humanity & Society 2009;33(1-2):56-73. [DOI: 10.1177/016059760903300105] [DOI] [Google Scholar]
Sutcliffe 2012
- Sutcliffe K, Caird J, Kavanagh J, Rees R, Oliver K, Dickson K, et al. Comparing midwife-led and doctor-led maternity care: a systematic review of reviews. Journal of Advanced Nursing 2012;68(11):2376-86. [DOI] [PubMed] [Google Scholar]
ten Hoope‐Bender 2014
- ten Hoope-Bender P, Bernis L, Campbell J, Downe S, Fauveau V, Fogstad H, et al. Improvement of maternal and newborn health through midwifery. The Lancet 2014;384(9949):1226-35. [DOI] [PubMed] [Google Scholar]
UNFPA 2021
- Bar-Zeev S, Bernis L, Boyce M, Chhugani M, Homer C, Hughes K et al. The State of the World’s Midwifery 2021. United Nations Population Fund 2021.
Victora 2016
- Victora C, Requejo J, Boerma T, Amouzou A, Bhutta ZA, Black RE et al. Countdown to 2030 for reproductive, maternal, newborn, child, and adolescent health and nutrition. The Lancet Global Health 2016;4(11):e775-6. [DOI: 10.1016/S2214-109X(16)30204-2] [DOI] [PubMed] [Google Scholar]
Waldenstrom 2000
- Waldenstrom U, Brown S, McLachlan H, Forster D, Brennecke S. Does team midwife care increase satisfaction with antenatal, intrapartum, and postpartum care? A randomized controlled trial. Birth 2000;27(3):156-67. [DOI: 10.1046/j.1523-536x.2000.00156.x] [DOI] [PubMed] [Google Scholar]
Walsh 2012
- Walsh D, Devane D. A metasynthesis of midwife-led care. Qualitative Health Research 2012;22(7):897-910. [DOI] [PubMed] [Google Scholar]
Wassen 2023
- Wassén L, Borgström Bolmsjö B, Frantz S, Hagman A, Lindroth M, Rubertsson C, et al. Child and maternal benefits and risks of caseload midwifery–a systematic review and meta-analysis. BMC Pregnancy and Childbirth 2023;23(1):663. [DOI: 10.1186/s12884-023-05967-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Health Organization 2016
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. https://www.who.int/publications/i/item/9789241549912 2016. [IBSN: 9789241549912] [PubMed]
World Health Organization 2018a
- World Health Organization. Continuity and coordination of care: a practice brief to support implementation of the WHO Framework on integrated people-centred health services. Geneva 2018. [LICENCE: CC BY-NC-SA 3.0 IGO]
World Health Organization 2018b
- World Health Organization. Intrapartum care for a positive childbirth experience WHO recommendations. https://www.who.int/publications/i/item/9789241550215 2018. [ISBN: 978-92-4-155021-5] [PubMed]
World Health Organization 2022
- World Health Organization. WHO recommendations on maternal and newborn care for a positive postnatal experience. https://www.who.int/publications/i/item/9789240045989 2022. [ISBN: 978 92 4 004598 9] [PubMed]
Young 1997
- Young D, Lees A, Twaddle S. The costs to the NHS of maternity care: midwife-managed vs shared. British Journal of Midwifery 1997;5(8):465-72. [Google Scholar]
Zeng 2022
- Zeng L, Brignardello-Petersen R, Hultcrantz M, Mustafa RA, Murad MH, Iorio A, et al. GRADE Guidance 34: update on rating imprecision using a minimally contextualized approach. Journal of Clinical Epidemiology 2022;150:216-24. [DOI: 10.1016/j.jclinepi.2022.07.014] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hatem 2008
- Hatem M, Sandall J, Devane D, Soltani H, Gates S. Midwife-led versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD004667. [DOI: 10.1002/14651858.CD004667.pub2] [DOI] [PubMed] [Google Scholar]
Sandall 2013
- Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004667. [DOI: 10.1002/14651858.CD004667.pub3] [DOI] [PubMed] [Google Scholar]
Sandall 2015
- Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD004667. [DOI: 10.1002/14651858.CD004667.pub4] [DOI] [PubMed] [Google Scholar]
Sandall 2016
- Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD004667. [DOI: 10.1002/14651858.CD004667.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


