Abstract
Acute normovolemic hemodilution (ANH) is a useful intraoperative blood conservation technique. However, the impact on long-term outcomes in pancreatic ductal adenocarcinoma (PDAC) remains unclear. The present study investigated the impact of ANH on long-term outcomes in patients with PDAC undergoing radical surgery. Data from 155 resectable PDAC cases were collected. Patients were categorized according to whether or not they had received intraoperative allogeneic blood transfusion (ABT) or ANH. Postoperative complications, recurrence-free survival (RFS) and disease-specific survival (DSS), before and after propensity score matching (PSM), were compared among patients who did and did not receive ANH. A total of 44 patients (28.4%) were included in the ANH group and 30 patients (19.4%) were included in the ABT group; 81 (52.3%) patients, comprising the standard management (STD) group, received neither ANH nor ABT. The ABT group had the worst prognosis among them. Before PSM, ANH was significantly associated with decreased RFS (P=0.043) and DSS (P=0.029) compared with the STD group before applying Bonferroni correction; however, no significant difference was observed after applying Bonferroni correction. Cox regression analysis identified ANH as an independent prognostic factor for RFS [relative risk (RR), 1.696; P=0.019] and DSS (RR, 1.876; P=0.009). After PSM, the ANH group exhibited less favorable RFS [median survival time (MST), 12.1 vs. 18.1 months; P=0.097] and DSS (MST, 32.1 vs. 50.5 months; P=0.097) compared with the STD group; however, these differences were not statistically significant. In conclusion, while ANH was not as harmful as ABT, it exhibited potentially more negative effects on long-term postoperative outcomes in PDAC than STD.
Keywords: ANH, blood transfusion, pancreatic neoplasms, prognosis, propensity score
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest solid cancers, worldwide (1,2). Surgery has long been considered a fundamental treatment option for this lethal disease (3–5). Despite ongoing improvements (6–11), pancreatic cancer surgery is often associated with significant intraoperative blood loss and the subsequent need for allogeneic blood transfusion (ABT) (12–14). The transfusion rate in patients who have undergone a pancreatic resection still falls in the 20 to 30% range, even when the procedure was performed by experienced surgeons in high-volume centers (13,15,16). Although ABT can be a lifesaving treatment during cancer surgery, it has been linked to a variety of negative outcomes from transfusion-related immunomodulation (TRIM) (14,17). Indeed, our previous study using propensity score matching analysis demonstrated the negative effects of intraoperative ABT on postoperative survival outcomes in patients with resectable PDAC (18). In order to minimize the use of ABT, the focus has been shifting to blood conservation strategies (19–21).
Acute normovolemic hemodilution (ANH) is an intraoperative blood conservation technique. ANH is performed immediately before the procedure and involves the removal of whole blood, while maintaining euvolemia with crystalloid and/or colloid solutions. ANH has been successfully performed in open-heart surgery since the 1970s (22,23). Subsequently, several studies, including those describing ANH use in various types of abdominal surgery, have shown that it is safe, inexpensive, and effectively reduces the need for ABT (24–27). ANH also offers a medical solution that respects religious and cultural beliefs about the use of ABT. These results seem to indicate that ANH can compensate for the disadvantages of ABT and improve the prognosis of patients who undergo pancreatic resection for PDAC.
However, in contrast to evaluations of short-term performance, few reports have examined the association between ANH and long-term outcomes in cancer patients (21,28). Furthermore, there is no recorded evidence linking use of ANH with PDAC patients. Therefore, this study aimed to assess the impact of ANH on long-term outcomes in PDAC patients undergoing radical surgery. We herein present the potentially negative impact of ANH on long-term oncological outcomes in patients with PDAC.
Materials and methods
Patients and study design
This single-center, retrospective cohort study was approved by the Committee of Medical Ethics of Hirosaki University Graduate School of Medicine (Aomori, Japan; reference no. 2022-032). Informed consent was obtained in the form of an opt-out system on our website (https://www.med.hirosaki-u.ac.jp/hospital/outline/resarch/resarch.html), which also had the approval of the Committee of Medical Ethics of Hirosaki University Graduate School of Medicine. Our study did not include minors. This study was designed and carried out in accordance with the Declaration of Helsinki. The study workflow is shown in Fig. 1. A total of 155 patients undergoing curative pancreatic surgery for resectable PDAC at our facility between January 2007 and May 2018 were included in the study. A portion of the subjects in this study had been included in our previous study (18,29). Resectability status was made based on National Comprehensive Cancer Network guidelines. All patients had a confirmed pathologic diagnosis based on the 8th edition of the Union for International Cancer Control staging system for PDAC (30). In this study, we excluded the following cases: patients who had received neoadjuvant chemotherapy or anyone with remnant pancreatic cancer. Baseline clinicopathologic data were obtained from the medical records.
Figure 1.
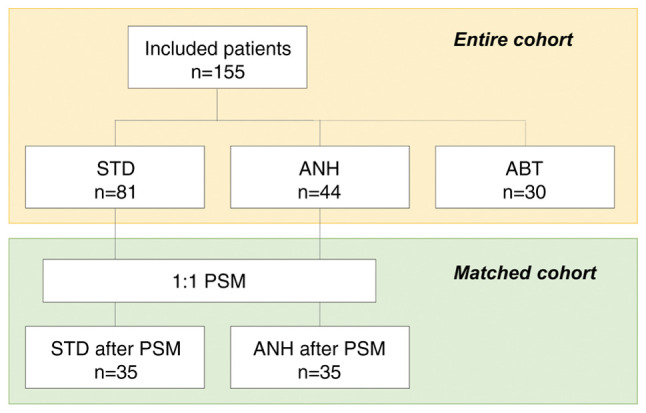
Study workflow. ABT, allogeneic blood transfusion; ANH, acute normovolemic hemodilution; PSM, propensity score matching; STD, standard management.
Patients were categorized according to whether or not they had received intraoperative ABT. Furthermore, patients who did not received intraoperative ABT were categorized according to whether or not they received ANH, and then compared. In this study, the patients who received both ANH and ABT were included in the ABT group. The patients who received neither ANH nor ABT were included in the standard management (STD) group. The primary analysis of this study was a comparison of the STD and the ANH group; a comparison of the ANH and the ABT group was performed as a sub-analysis. A comparison of the STD and the ABT group was not undertaken in this study because it had already been shown in previous studies (14,18).
Surgical procedures
We selected the type of pancreatic resection based on tumor location. Open pancreatoduodenectomy (PD) with lymph node dissection was usually conducted on cases of pancreatic head cancer during this study period. Reconstruction was typically done using a modified Child's method with an end-to-side pancreaticojejunostomy and an end-to-side choledochojejunostomy. All pancreaticojejunostomy anastomoses were conducted using a duct-to-mucosa technique. In cases of pancreatic body and tail cancer, an open or minimally invasive distal pancreatectomy (DP) was performed with lymph node dissection. If we detected swelling paraaortic lymph nodes, we generally performed paraaortic lymph node sampling during PD procedures whereas sampling was not routinely done during DP surgeries. After a PD, paraaortic lymph nodes were confirmed using standard histopathological assessment of corresponding paraffin-embedded, hematoxylin and eosin-stained material for surgical staging. Consequently, confirming whether or not the paraaortic lymph nodes were positive always occurred postoperatively. Regardless of whether there was paraaortic swelling or the paraaortic lymph nodes ended up testing positive, a pancreatectomy was performed in all cases. We performed a fresh frozen section analysis to confirm whether or not the pancreatic cut-end margin was clear of residual cancer. If residual cancer was present at the pancreatic cut end margin, we cut the pancreas further to reach negative margin status. If necessary, to achieve a curative resection, we performed a total pancreatectomy with lymph node dissection.
During this study, all surgical procedures were carried out by board certified surgeons. We classified surgeons into two groups in the same manner as a previous publication (31): junior surgeons, those whose surgical training experience was 10 years or less, and senior surgeons, those who had over 10 years of surgical training. All junior surgeons conducted surgeries with attending surgeons.
ANH protocol
Details of the ANH protocol are described in detail in other papers (32). Briefly, the principal indication for ANH at our institution is an estimated blood loss of more than 500 ml or a request from a surgeon for a patient with a hemoglobin (Hb) level of more than 10 g/dl. Patients with uncontrolled congenital heart failure including active ischemic heart disease, severe liver disease, or renal failure were excluded. After anesthetic induction, blood was withdrawn through the central venous line, and the withdrawn blood volume for ANH was selected to avoid a Hb level of less than 8 g/dl after hemodilution. The withdrawn blood volume was simultaneously replaced with an equal volume of 6% hydroxyethyl starch solution (130/4) (Volven; Fresenius Kabi, Bad Homburg, Germany). The blood collected was stored in a standard blood collection pack (JMS Blood Bag CPD400; JMS, Tokyo, Japan) at room temperature on a shaker in the operating room. The collected blood was then reinfused into the patients after specimen procurement.
Transfusion protocol
In the current study, intraoperative ABT was defined as the transfusion of red blood cell concentrate during the operation. At our institution, the intraoperative transfusion trigger was set at Hb <7 g/dl. Additionally, for cases involving an increased risk of ischemia, such as patients with preexisting concomitant pulmonary disease, coronary artery disease, or cerebral vascular disease, and those showing signs of cardiac ischemia based on new electrocardiographic information, the transfusion threshold was set at a Hb level of less than 9 g/dl. For the ANH group, if the trigger point was reached, autologous blood was given first. Allogeneic blood was used only after all autologous blood had been reinfused and the Hb remained at less than 7 g/dl.
Definition of intraoperative blood loss
Intraoperative blood loss was calculated based on the in/out balance of the operative field. At our institution, any fluid loss from the abdominal cavity including ascites, bile, and lymphatics is considered to be intraoperative bleeding. In this study, we estimated the circulating blood volume (CBV) using the following formula: CBV (ml)=70 × body weight (kg).
Definition of postoperative complications
In this study, postoperative complications were graded using the Clavien-Dindo classification system (33). Pancreatic fistula was defined and graded based on criteria outlined by the International Study Group of Pancreatic Fistula (ISGPF) (34), while Delayed Gastric Emptying was defined and graded according to criteria outlined by the International Study Group of Pancreatic Surgery (ISGPS) (35).
Statistical analysis
Continuous variables were expressed as medians (ranges) and analyzed using nonparametric methods for non-normally distributed data (Mann-Whitney U-test). Categorical variables were reported as numbers (percentages) and analyzed using the chi-squared test or Fisher's exact test, as appropriate. Additionally, in order to compare each group pairwise, Bonferroni correction was applied to the Mann-Whitney U-test/chi-squared test/Fisher's exact test (P-values were multiplied by three). Patients in the STD and the ANH groups were classified using the propensity score matching (PSM) method to minimize the impact of possible selective bias in the survival analysis. Propensity scores were based on the selected covariates, which were significantly associated with ANH in univariate analysis (P<0.1), including sex, age, body weight, C-reactive protein (CRP), and total bilirubin. In addition, based on the consensus reached at expert meetings during this study, the surgical procedure was also included in the covariate with which propensity scores were calculated. We did not include hemoglobin and hematocrit in the covariate. Nearest neighbor matching was performed in a one-to-one ratio without replacement. A caliper width of 0.08 was used to avoid bad matches. Recurrence-free survival (RFS) and Disease-specific survival (DSS) were calculated using the Kaplan-Meier method, and differences in the survival rates were compared using the log-rank test. We used Bonferroni correction for survival analysis. RFS was defined as the time from the operation to the date of disease recurrence. DSS was defined as the time from the operation to the time of death due to PDAC, or the last follow-up time. This study was planned with a maximum follow-up period of five years. Both univariate and multivariate analyses were conducted using Cox proportional hazards regression to identify independent predictors of RFS and DSS, with only significant variables from the univariate analysis included in the multivariate analysis. In this analysis, we divided continuous variables into two groups according to median values. A difference was considered to be significant for values of P<0.05. The statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp, Armonk, NY, USA).
Results
Comparison of the ABT rate between patients with/without ANH
We collected a dataset from 155 resectable cases of PDAC. First, we evaluated how much ANH reduced the need for ABT. Among the 109 patients who did not receive ANH, eight (7.3%) exhibited low hemoglobin levels (less than 10 g/dl) before surgery, and 28 (25.7%) required ABT. On the other hand, of the 46 patients who received ANH, only two patients (4.3%) needed ABT. The ABT rate in the ANH implementation group was significantly lower than in the non-implementation group (P=0.002). Of the total of 155 patients, 44 (28.4%) fell into the ANH group and 30 (19.4%) made up the ABT group. Eighty-one (52.3%) patients received neither ANH nor ABT.
Comparison of the clinicopathological characteristics across the groups
Next, we investigated the clinical characteristics across the groups (Table I). The ANH group was significantly associated with higher preoperative Hb and hematocrit levels compared to the other groups. The ANH group had a higher mean age and showed higher CRP levels than the STD group. Tumor biomarkers and pathological findings revealed minor differences between the STD and the ANH groups. These data indicated that the preoperative condition of the patients in the ANH group was no worse than that of the STD group.
Table I.
Comparison of clinicopathological characteristics for the entire cohort.
| Characteristics | All (n=155) | STH (n=81) | ANH (n=44) | ABT (n=30) | P-value (STH vs. ANH) | P-value (ANH vs. ABT) |
|---|---|---|---|---|---|---|
| Sex, male | 79 (51.0) | 34 (42.0) | 29 (65.9) | 16 (53.3) | 0.021 | 0.553 |
| Age, years | 70 (49–85) | 71 (50–85) | 67 (49–78) | 71 (50–80) | 0.026 | 0.835 |
| Body mass index, kg/m2 | 22.3 (14.1–36.3) | 22.0 (15.8–29.5) | 22.8 (14.1–33.3) | 24.2 (16.4–36.3) | 0.715 | 0.362 |
| ASA-PS | 0.354b | 0.415b | ||||
| PS2 | 126 (81.3) | 64 (79.0) | 39 (88.6) | 23 (76.7) | ||
| PS3 | 29 (18.7) | 17 (21.0) | 5 (11.4) | 7 (23.3) | ||
| Preoperative biliary drainage | 53 (34.2) | 22 (27.2) | 18 (40.9) | 13 (43.3) | 0.231 | >0.999 |
| Laboratory values | ||||||
| Hemoglobin, g/dl | 12.7 (7.2–16.5) | 12.7 (7.2–15.9) | 13.8 (10.8–16.3) | 11.6 (8.8–16.5) | 0.007 | <0.001 |
| CRP, mg/dl | 0.12 (0.02–9.59) | 0.10 (0.02–9.59) | 0.31 (0.02–6.50) | 0.23 (0.02–4.91) | 0.010 | 0.857 |
| Albumin, g/dl | 3.9 (2.0–5.7) | 3.9 (2.5–5.7) | 4.1 (2.4–4.9) | 3.8 (2.0–4.5) | 0.573 | 0.024 |
| Hemoglobin A1c, % | 6.1 (4.4–12.8) | 6.1 (4.4–12.8) | 6.4 (4.4–11.9) | 5.9 (4.7–8.7) | >0.999 | 0.292 |
| Creatinine, mg/dl | 0.67 (0.40–2.02) | 0.66 (0.43–2.02) | 0.70 (0.41–1.30) | 0.67 (0.40–1.43) | 0.636 | >0.999 |
| AST, U/l | 29 (11–406) | 27 (13–406) | 27 (11–241) | 52 (12–260) | >0.999 | 0.051 |
| ALT, U/l | 35 (9–627) | 25 (9–621) | 33 (12–616) | 68 (9–627) | 0.420 | 0.158 |
| Total bilirubin, mg/dl | 0.7 (0.2–32.7) | 0.6 (0.2–32.7) | 0.8 (0.2–24.0) | 2.6 (0.3–24.1) | 0.066 | 0.391 |
| CA19-9, U/ml | 92 (1–9,675) | 57 (1–3,199) | 118 (5–9,675) | 135 (1–6,370) | 0.345 | 0.969 |
| CEA, ng/ml | 2.7 (0.5–37.0) | 2.8 (0.6–37.0) | 2.5 (0.5–23.9) | 3.6 (0.5–10.5) | 0.776 | 0.238 |
| Operative variables | ||||||
| Procedure | 0.178b | 0.044b | ||||
| Pancreaticoduodenectomy | 95 (61.3) | 39 (48.1) | 30 (68.2) | 26 (86.7) | ||
| Distal pancreatectomy | 52 (33.5) | 37 (45.7) | 13 (29.5) | 2 (6.7) | ||
| Total pancreatectomy | 8 (5.2) | 5 (6.2) | 1 (2.3) | 2 (6.7) | ||
| Portal vein resection | 25 (16.1) | 8 (9.9) | 8 (18.2) | 9 (30.0) | 0.369 | 0.471 |
| Grouping of surgeons | >0.999b | 0.890b | ||||
| Junior surgeon | 10 (6.5) | 6 (7.4) | 1 (2.3) | 3 (10.0) | ||
| Senior surgeon | 145 (93.5) | 75 (92.6) | 43 (97.7) | 27 (90.0) | ||
| Pathology | ||||||
| Tumor size, mm | 30 (7–150) | 29 (7–150) | 33 (10–130) | 35 (15–57) | 0.753 | >0.999 |
| UICC 8th edition | ||||||
| T category | 0.726b | 0.620b | ||||
| T1 | 20 (12.9) | 10 (12.3) | 8 (18.2) | 2 (6.7) | ||
| T2 | 92 (59.4) | 51 (63.0) | 22 (50.0) | 19 (63.3) | ||
| T3 | 43 (27.7) | 20 (24.7) | 14 (31.8) | 9 (30.0) | ||
| T4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| N category | 0.473 | 0.749 | ||||
| N0 | 62 (40.0) | 38 (46.9) | 17 (38.6) | 7 (23.3) | ||
| N1 | 58 (37.4) | 31 (38.3) | 15 (34.1) | 12 (40.0) | ||
| N2 | 35 (22.6) | 12 (14.8) | 12 (27.3) | 11 (36.7) | ||
| M category | 0.065b | 0.263b | ||||
| M0 | 144 (92.9) | 78 (96.3) | 37 (84.1) | 29 (96.7) | ||
| M1a | 11 (7.1) | 3 (3.7) | 7 (15.9) | 1 (3.3) | ||
| UICC stage | 0.018b | 0.179b | ||||
| IA | 12 (7.7) | 6 (7.4) | 5 (11.4) | 1 (3.3) | ||
| IB | 24 (15.5) | 17 (21.0) | 3 (6.8) | 4 (13.3) | ||
| IIA | 12 (7.7) | 4 (4.9) | 7 (15.9) | 1 (3.3) | ||
| IIB | 72 (46.5) | 41 (50.6) | 15 (34.1) | 16 (53.3) | ||
| III | 24 (15.5) | 10 (12.3) | 7 (15.9) | 7 (23.3) | ||
| IV | 11 (87.1) | 3 (3.7) | 7 (15.9) | 1 (3.3) | ||
| R0 resection | 141 (91.0) | 75 (92.6) | 38 (86.4) | 28 (93.3) | 0.683 | 0.921 |
All of the patients were diagnosed with M1 due to positive lymph nodes other than the regional lymph nodes. Continuous variables are presented as the median (range) and were analyzed using the Mann-Whitney U-test. Categorical variables are reported as the number (percentage) and were analyzed using the χ2 test or Fisher's exact test, as appropriate.
Fisher's exact test was performed, while other comparisons for categorical variables were carried out using the χ2 test. All P-values presented were corrected using Bonferroni adjustments. ABT, allogeneic blood transfusion; ALT, alanine aminotransferase; ANH, acute normovolemic hemodilution; ASA-PS, American Society of Anesthesiologists physical status; AST, aspartate aminotransferase; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRP, C-reactive protein; STD, standard management; UICC, Union for International Cancer Control.
Comparison of the operative and postoperative outcomes across the groups
There was a trend toward a higher proportion of distal pancreatectomy in the STD group than in the ANH group (45.7% vs. 29.5%, P=0.157) (Table II). In comparison with the STD group, one of the key features of the ANH group was longer operation time (250 vs. 346 min, P<0.001) and anesthesia time (317 vs. 405 min, P<0.001), with more intraoperative blood loss (420 vs. 983 ml, P<0.001) and a higher volume of intraoperative fluids administered (2,850 vs. 4,975 ml, P<0.001). When we evaluated the intraoperative in-out balance by correcting for body weight and anesthesia time, we found no difference between the two groups.
Table II.
Comparison of operative and postoperative outcomes for the entire cohort.
| Outcomes | All (n=155) | STH (n=81) | ANH (n=44) | ABT (n=30) | P-value (STH vs. ANH) | P-value (ANH vs. ABT) |
|---|---|---|---|---|---|---|
| Operative results | ||||||
| Operation time, min | 307 (91–647) | 250 (91–619) | 346 (129–587) | 368 (127–647) | <0.001 | 0.206 |
| Anesthesia time, min | 372 (172–757) | 317 (172–676) | 405 (187–680) | 429 (209–757) | <0.001 | 0.327 |
| Intraoperative blood loss, ml | 750 (50–5,600) | 420 (50–2,400) | 983 (150–2,775) | 1,940 (540–5,600) | <0.001 | <0.001 |
| Intraoperative blood loss, ml/kg | 14.1 (0.8–77.6) | 7.9 (0.8–42.6) | 18.3 (2.3–39.0) | 29.5 (13.9–77.6) | <0.001 | <0.001 |
| IBL >20% in CBV | 78 (50.3) | 20 (24.7) | 29 (65.9) | 29 (96.7) | <0.001 | 0.003 |
| Blood volume removed, ml | 0 (0–800) | 0 (0–0) | 800 (400–800) | 0 (0–800) | <0.001 | <0.001 |
| Intraoperative fluid | 3,600 (200–9,000) | 2,850 | 4,150 | 4,688 | <0.001 | >0.999 |
| given, ml | (1,300–7,500) | (2,500–7,300) | (200–9,000) | |||
| Intraoperative albumin, ml | 0 (0–1,250) | 0 (0–500) | 0 (0–1,000) | 500 (0–1,250) | 0.001 | <0.001 |
| Intraoperative RBC, ml | 0 (0–1,400) | 0 (0–0) | 0 (0–0) | 560 (80–1,400) | - | <0.001 |
| Intraoperative FFP, ml | 0 (0–960) | 0 (0–480) | 0 (0–0) | 0 (0–960) | >0.999 | <0.001 |
| Intraoperative PC, ml | 0 (0–200) | 0 (0–0) | 0 (0–0) | 0 (0–200) | - | 0.169 |
| Total fluid volume | 3,900 | 2,850 | 4,975 | 5,885 | <0.001 | 0.411 |
| administered, ml | (1,300–11,510) | (1,300–8,000) | (2,950–8,850) | (2,530–11,510) | ||
| Intraoperative urine output, ml | 480 (20–3,800) | 420 (56–1,550) | 545 (20–1,750) | 598 (70–3,800) | 0.080 | >0.999 |
| Total in-out balance, ml | 2,300 | 1,880 | 2,735 | 3,072 | <0.001 | >0.999 |
| Total in-out balance, | (410–7,714) | (485–5,500) | (1,190–4,700) | (410–7,714) | ||
| ml/kg/ha | 6.7 (1.2–13.9) | 6.4 (1.8–12.4) | 6.7 (3.2–13.9) | 7.2 (1.2–13.7) | 0.627 | >0.999 |
| Postoperative results | ||||||
| Postoperative complications (Clavien-Dindo classification grade ≥3) | 27 (17.4) | 6 (7.4) | 11 (25.0) | 10 (33.3) | 0.012 | 0.870 |
| Pancreatic fistula | 24 (15.5) | 7 (8.6) | 9 (20.4) | 8 (26.7) | 0.118 | >0.999 |
| (ISGPF grade ≥B) | ||||||
| Delayed gastric emptying | 17 (11.0) | 7 (8.6) | 6 (13.6) | 4 (13.3) | 0.756 | >0.999b |
| (ISGPS grade ≥B) | ||||||
| Postoperative hospital | 19 (6–73) | 17 (7–73) | 22 (6–64) | 31 (10–57) | 0.007 | 0.585 |
| stay, days | ||||||
| 90-day mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - |
| Adjuvant chemotherapy | 122 (80.3) | 64 (80.0) | 34 (79.1) | 24 (82.8) | >0.999 | >0.999 |
Estimated using the following formula: Total in-out balance/body weight (kg)/anesthesia time (h). Continuous variables are presented as the median (range) and were analyzed using the Mann-Whitney U-test. Categorical variables are reported as the number (percentage) and were analyzed using the χ2 test or Fisher's exact test, as appropriate.
Fisher's exact test was performed, while other comparisons for categorical variables were carried out using the χ2 test. All P-values presented were corrected using Bonferroni adjustments. ABT, allogeneic blood transfusion; ANH, acute normovolemic hemodilution; CBV, circulating blood volume; FFP, fresh frozen plasma; IBL, intraoperative blood loss; ISGPF, International Study Group of Pancreatic Fistula; ISGPS, International Study Group of Pancreatic Surgery; PC, platelet concentration; RBC, red blood cells; STD, standard management.
Regarding the postoperative short-term outcomes, the ANH group displayed a higher frequency of postoperative complications (Clavien-Dindo grade ≥3, 3.4-fold, P=0.012) compared to the STD group. Specifically, there were more clinically relevant postoperative pancreatic fistulas in the ANH group (2.4-fold, P=0.118). Moreover, the ANH groups experienced longer postoperative hospital stays (P=0.007). There was no in-hospital or 90-day mortality for any patients in this study. In short, postoperative short-term outcomes in the ANH group were less favorable than those in the STD group, but not as poor as that of the ABT group.
Comparison of the survival outcomes of the entire cohort
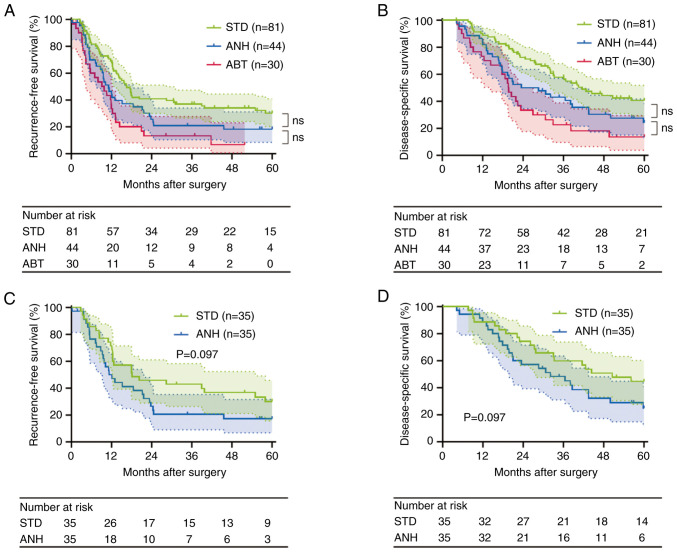
The groups were well matched in the proportion of adjuvant chemotherapy (Table II). The median follow-up period was 30.7 months (range: 4.2–60.0). A total of 117 patients (75.5%) had recurrences. The median RFS time was 13.0 months for the entire cohort. A total of 101 patients (65.2%) died due to the primary disease during the follow-up period. The median DSS time was 32.1 months for the entire cohort. The RFS and DSS curves for patients classified as requiring intraoperative blood management are shown in Fig. 2A and B. The RFS time was significantly shorter in the ANH group than in the STD group (median survival time (MST), 11.1 vs. 16.5 months, P=0.043 before correction). Likewise, the DSS was significantly shorter in the ANH group (MST, 28.6 vs. 41.6 months, P=0.029 before correction). However, these differences were not significant after Bonferroni correction (RFS, P=0.129; DSS, P=0.087). In the comparison between ANH and ABT, RFS was not significantly different between the two groups (MST, 11.1 vs. 9.5 months, P=0.143, before applying Bonferroni correction; P=0.429, after correction). The ANH group showed a longer DSS time than the ABT group, but it was not significant (MST, 28.6 vs. 19.7 months, P=0.136, before applying Bonferroni correction; P=0.408, after correction). Taken together, these data suggest that ANH has a negative impact on the postoperative long-term outcomes in PDAC, though not as severe as ABT.
Figure 2.
Survival analysis. (A and B) Survival cures of the STD, ANH and ABT groups for the entire cohort. (A) Recurrence-free survival (STD vs. ANH, P=0.043; ANH vs. ABT, P=0.143; log-rank test, before applying Bonferroni correction; after correction: STD vs. ANH, P=0.129; ANH vs. ABT, P=0.429). (B) Disease-specific survival (STD vs. ANH, P=0.029; ANH vs. ABT, P=0.136; log-rank test, before applying Bonferroni correction; after correction: STD vs. ANH, P=0.087; ANH vs. ABT, P=0.408). (C and D) Survival cures of the STD and ANH groups in the propensity score-matched cohort. (C) Recurrence-free survival (P=0.097; log-rank test). (D) Disease-specific survival (P=0.097; log-rank test). ABT, allogeneic blood transfusion; ANH, acute normovolemic hemodilution; ns, not significant; STD, standard management.
Clinicopathological characteristics influencing RFS and DSS of the STD and ANH groups
To assess whether ANH influences RFS and DSS in PDAC, we further performed Cox regression analysis. Since red blood cell transfusion has been shown to affect cancer prognosis negatively (18), we evaluated the clinicopathological factors influencing RFS and DSS in subjects, excluding the ABT group. In univariate analysis, significant predictors of decreased RFS were preoperative CRP, preoperative aspartate aminotransferase, preoperative carbohydrate antigen 19-9 (CA19-9), tumor size, Union for International Cancer Control (UICC) T category, UICC N category, ANH, and adjuvant chemotherapy. In multivariate analysis, preoperative CA19-9≥68 U/ml (relative risk (RR)=1.796 (95% confidence interval (CI), 1.124–2.871), P=0.014), UICC N1-2 (RR=2.207 (95% CI, 1.339–3.638), P=0.002), ANH (RR=1.696 (95% CI, 1.091–2.636), P=0.019), and adjuvant chemotherapy (RR=0.345 (95% CI, 0.204–0.584), were independent prognostic factors for RFS (Table III).
Table III.
Clinicopathological characteristics predicting RFS in the standard management and ANH groups.
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Characteristics | No. | 5-year RFS, % | MST, months | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Sex | 0.766 | 0.505–1.162 | 0.235 | ||||||
| Male | 63 | 24.9 | 12.4 | ||||||
| Female | 62 | 27.6 | 16.5 | ||||||
| Age, years | 1.210 | 0.800–1.831 | 0.365 | ||||||
| <70 | 65 | 28.1 | 17.9 | ||||||
| ≥70 | 60 | 23.7 | 13.0 | ||||||
| Body mass index, kg/m2 | 1.019 | 0.674–1.541 | 0.871 | ||||||
| <22.1 | 62 | 28.3 | 14.2 | ||||||
| ≥22.1 | 63 | 24.2 | 15.2 | ||||||
| Preoperative biliary drainage | 1.148 | 0.737–1.788 | 0.540 | ||||||
| No | 85 | 26.7 | 15.8 | ||||||
| Yes | 40 | 25.6 | 12.4 | ||||||
| Hemoglobin, g/dl | 1.088 | 0.719–1.647 | 0.689 | ||||||
| <12.9 | 65 | 25.0 | 15.8 | ||||||
| ≥12.9 | 60 | 28.8 | 12.4 | ||||||
| CRP, mg/dl | 1.613 | 1.050–2.478 | 0.022 | 0.982 | 0.619–1.556 | 0.937 | |||
| <0.11 | 60 | 35.5 | 21.6 | ||||||
| ≥0.11 | 65 | 17.4 | 12.7 | ||||||
| Albumin, g/dl | 1.013 | 0.670–1.534 | 0.933 | ||||||
| <3.9 | 64 | 22.2 | 15.7 | ||||||
| ≥3.9 | 61 | 30.2 | 14.2 | ||||||
| Hemoglobin A1c, % | 1.240 | 0.799–1.924 | 0.477 | ||||||
| <6.0 | 58 | 25.2 | 13.0 | ||||||
| ≥6.0 | 55 | 10.3 | 12.7 | ||||||
| Creatinine, mg/dl | 0.822 | 0.542–1.247 | 0.356 | ||||||
| <0.67 | 66 | 21.6 | 13.4 | ||||||
| ≥0.67 | 59 | 31.6 | 17.5 | ||||||
| AST, U/l | 1.775 | 1.168–2.700 | 0.006 | 1.108 | 0.669–1.834 | 0.691 | |||
| <27 | 64 | 36.3 | 21.2 | ||||||
| ≥27 | 61 | 15.4 | 12.1 | ||||||
| ALT, U/l | 1.420 | 0.937–2.152 | 0.096 | ||||||
| <28 | 63 | 32.4 | 17.7 | ||||||
| ≥28 | 62 | 19.6 | 12.4 | ||||||
| Total bilirubin, mg/dl | 1.135 | 0.749–1.720 | 0.551 | ||||||
| <0.7 | 67 | 24.8 | 17.5 | ||||||
| ≥0.7 | 58 | 27.1 | 12.3 | ||||||
| CA19-9, U/ml | 1.897 | 1.250–2.880 | 0.002 | 1.796 | 1.124–2.871 | 0.014 | |||
| <68 | 63 | 35.1 | 21.6 | ||||||
| ≥68 | 62 | 17.4 | 11.2 | ||||||
| CEA, ng/ml | 1.316 | 0.870–1.990 | 0.192 | ||||||
| <2.7 | 67 | 30.2 | 16.5 | ||||||
| ≥2.7 | 58 | 21.7 | 13.4 | ||||||
| Tumor size, mm | 1.807 | 1.193–2.737 | 0.005 | 1.229 | 0.777–1.944 | 0.379 | |||
| <30 | 71 | 33.5 | 17.9 | ||||||
| ≥30 | 54 | 16.2 | 9.8 | ||||||
| UICC T category | 3.300 | 1.522–7.154 | 0.001 | 2.026 | 0.864–4.750 | 0.104 | |||
| T1 | 18 | 58.3 | 42.8 | ||||||
| T2-3 | 107 | 20.7 | 12.4 | ||||||
| UICC N category | 2.552 | 1.635–3.983 | <0.001 | 2.207 | 1.339–3.638 | 0.002 | |||
| N0 | 55 | 43.9 | 39.8 | ||||||
| N1-2 | 70 | 12.1 | 12.3 | ||||||
| UICC M category | 1.804 | 0.903–3.607 | 0.090 | ||||||
| M0 | 115 | 27.5 | 15.2 | ||||||
| M1a | 10 | 10.0 | 8.3 | ||||||
| R0 resection | 0.787 | 0.380–1.628 | 0.831 | ||||||
| No | 12 | 33.3 | 13.0 | ||||||
| Yes | 113 | 25.4 | 14.5 | ||||||
| Procedure | 0.878 | 0.573–1.345 | 0.550 | ||||||
| PD, TP | 75 | 22.4 | 13.6 | ||||||
| DP | 50 | 31.7 | 15.7 | ||||||
| Portal vein resection | 1.235 | 0.686–2.225 | 0.480 | ||||||
| No | 109 | 28.2 | 14.5 | ||||||
| Yes | 16 | 0.0 | 13.6 | ||||||
| Operation time, min | 1.420 | 0.938–2.150 | 0.096 | ||||||
| <275 | 63 | 33.3 | 17.5 | ||||||
| ≥275 | 62 | 17.4 | 12.4 | ||||||
| Anesthesia time, min | 1.182 | 0.782–1.789 | 0.427 | ||||||
| <363 | 63 | 30.6 | 15.8 | ||||||
| ≥363 | 62 | 20.6 | 12.4 | ||||||
| Intraoperative blood loss, ml | 1.330 | 0.876–2.018 | 0.179 | ||||||
| <600 | 63 | 34.3 | 15.1 | ||||||
| ≥600 | 62 | 17.9 | 14.2 | ||||||
| ANH | 1.545 | 1.010–2.364 | 0.043 | 1.696 | 1.091–2.636 | 0.019 | |||
| No | 81 | 30.3 | 16.5 | ||||||
| Yes | 44 | 18.3 | 11.1 | ||||||
| Intraoperative fluid given, ml | 0.951 | 0.627–1.441 | 0.812 | ||||||
| <3,500 | 68 | 26.9 | 14.2 | ||||||
| ≥3,500 | 57 | 24.3 | 17.9 | ||||||
| Intraoperative urine output, ml | 1.262 | 0.834–1.910 | 0.269 | ||||||
| <465 | 64 | 29.6 | 16.5 | ||||||
| ≥465 | 61 | 22.2 | 12.7 | ||||||
| Total in-out balance, ml | 1.067 | 0.705–1.615 | 0.759 | ||||||
| <2,210 | 63 | 27.0 | 15.7 | ||||||
| ≥2,210 | 62 | 24.6 | 13.4 | ||||||
| Total in-out balance, ml/kg/h | 1.354 | 0.894–2.048 | 0.213 | ||||||
| <6.6 | 62 | 28.5 | 17.5 | ||||||
| ≥6.6 | 63 | 23.7 | 11.3 | ||||||
| Postoperative complications | 1.302 | 0.723–2.346 | 0.377 | ||||||
| Clavien-Dindo grade 0–2 | 108 | 26.5 | 15.7 | ||||||
| Clavien-Dindo grade ≥3 | 17 | 23.5 | 12.1 | ||||||
| Pancreatic fistula | 1.270 | 0.691–2.333 | 0.440 | ||||||
| ISGPF grade non-A | 109 | 26.2 | 15.1 | ||||||
| ISGPF grade B-C | 16 | 25.0 | 12.1 | ||||||
| Postoperative hospital stay, days | 1.104 | 0.730–1.669 | 0.640 | ||||||
| <17 | 64 | 26.6 | 15.7 | ||||||
| ≥17 | 61 | 25.2 | 13.0 | ||||||
| Adjuvant chemotherapy | 0.535 | 0.332–0.862 | 0.003 | 0.345 | 0.204–0.584 | <0.001 | |||
| No | 27 | 8.0 | 9.3 | ||||||
| Yes | 98 | 30.5 | 16.5 | ||||||
All of the patients were diagnosed with M1 due to positive lymph nodes other than the regional lymph nodes. Since red blood cell transfusion has been shown to affect cancer prognosis negatively (18), the clinicopathological factors influencing RFS in subjects, excluding the ABT group, were evaluated. P-values were obtained using Cox regression analysis. ALT, alanine aminotransferase; ANH, acute normovolemic hemodilution; AST, aspartate aminotransferase; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRP, C-reactive protein; DP, distal pancreatectomy; ISGPF, International Study Group of Pancreatic Fistula; MST, median survival time; PD, pancreatoduodenectomy; RFS, recurrence-free survival; RR, relative risk; TP, total pancreatectomy; UICC, Union for International Cancer Control.
Likewise, in univariate analysis, significant predictors of decreased DSS were preoperative CRP, preoperative aspartate aminotransferase, UICC T category, UICC N category, ANH, and adjuvant chemotherapy. In multivariate analysis, UICC T2-3 (RR=3.045 (95% CI, 1.071–8.657), P=0.037), UICC N1-2 (RR=2.225 (95% CI, 1.275–3.883), P=0.005), ANH (RR=1.876 (95% CI, 1.174–2.998), P=0.009), and adjuvant chemotherapy (RR=0.268 (95% CI, 0.151–0.477), P<0.001) were independent prognostic factors for DSS (Table IV). These results provide us with a warning that ANH falls in the poor prognostic factor category with regard to the management of resectable PDAC.
Table IV.
Clinicopathological characteristics predicting DSS in the standard management and ANH groups.
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Characteristics | No. | 5-year DSS, % | MST, months | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Sex | 0.645 | 0.408–1.020 | 0.070 | ||||||
| Male | 63 | 28.8 | 32.1 | ||||||
| Female | 62 | 41.6 | 40.0 | ||||||
| Age, years | 1.427 | 0.908–2.242 | 0.121 | ||||||
| <70 | 65 | 42.0 | 42.6 | ||||||
| ≥70 | 60 | 27.1 | 32.2 | ||||||
| Body mass index, kg/m2 | 0.920 | 0.586–1.442 | 0.811 | ||||||
| <22.1 | 62 | 36.0 | 33.2 | ||||||
| ≥22.1 | 63 | 33.9 | 42.6 | ||||||
| Preoperative biliary drainage | 1.418 | 0.889–2.263 | 0.141 | ||||||
| No | 85 | 39.1 | 38.6 | ||||||
| Yes | 40 | 25.5 | 28.6 | ||||||
| Hemoglobin, g/dl | 0.957 | 0.609–1.505 | 0.850 | ||||||
| <12.9 | 65 | 33.7 | 36.5 | ||||||
| ≥12.9 | 60 | 35.9 | 38.6 | ||||||
| CRP, mg/dl | 1.617 | 1.013–2.581 | 0.025 | 0.931 | 0.566–1.532 | 0.779 | |||
| <0.11 | 60 | 44.8 | 50.0 | ||||||
| ≥0.11 | 65 | 24.2 | 32.2 | ||||||
| Albumin, g/dl | 1.063 | 0.677–1.668 | 0.913 | ||||||
| <3.9 | 64 | 32.7 | 38.6 | ||||||
| ≥3.9 | 61 | 37.2 | 36.5 | ||||||
| Hemoglobin A1c, % | 1.134 | 0.703–1.832 | 0.254 | ||||||
| <6.0 | 58 | 41.6 | 42.6 | ||||||
| ≥6.0 | 55 | 28.7 | 33.2 | ||||||
| Creatinine, mg/dl | 0.891 | 0.567–1.400 | 0.616 | ||||||
| <0.67 | 66 | 30.2 | 38.1 | ||||||
| ≥0.67 | 59 | 40.0 | 36.5 | ||||||
| AST, U/l | 1.609 | 1.023–2.531 | 0.038 | 1.422 | 0.831–2.434 | 0.119 | |||
| <27 | 64 | 42.0 | 43.5 | ||||||
| ≥27 | 61 | 26.9 | 29.4 | ||||||
| ALT, U/l | 1.349 | 0.859–2.119 | 0.192 | ||||||
| <28 | 63 | 40.0 | 41.6 | ||||||
| ≥28 | 62 | 29.5 | 32.2 | ||||||
| Total bilirubin, mg/dl | 1.236 | 0.788–1.939 | 0.355 | ||||||
| <0.7 | 67 | 35.7 | 40.0 | ||||||
| ≥0.7 | 58 | 33.1 | 32.2 | ||||||
| CA19-9, U/ml | 1.524 | 0.970–2.395 | 0.066 | ||||||
| <68 | 63 | 40.4 | 42.6 | ||||||
| ≥68 | 62 | 29.4 | 31.4 | ||||||
| CEA, ng/ml | 1.418 | 0.904–2.224 | 0.126 | ||||||
| <2.7 | 67 | 40.2 | 40.0 | ||||||
| ≥2.7 | 58 | 28.2 | 31.4 | ||||||
| Tumor size, mm | 1.499 | 0.955–2.352 | 0.076 | ||||||
| <30 | 71 | 41.6 | 38.1 | ||||||
| ≥30 | 54 | 26.0 | 36.5 | ||||||
| UICC T category | 4.570 | 1.667–12.530 | 0.001 | 3.045 | 1.071–8.657 | 0.037 | |||
| T1 | 18 | 73.8 | 53.0 | ||||||
| T2-3 | 107 | 28.4 | 32.1 | ||||||
| UICC N category | 2.245 | 1.387–3.634 | 0.001 | 2.225 | 1.275–3.883 | 0.005 | |||
| N0 | 55 | 52.1 | 42.8 | ||||||
| N1-2 | 70 | 20.8 | 28.6 | ||||||
| UICC M category | 1.521 | 0.758–3.051 | 0.235 | ||||||
| M0 | 115 | 37.8 | 38.1 | ||||||
| M1a | 10 | 0 | 30.8 | ||||||
| R0 resection | 1.239 | 0.595–2.578 | 0.278 | ||||||
| No | 12 | 31.3 | 21.0 | ||||||
| Yes | 113 | 35.3 | 38.3 | ||||||
| Procedure | 0.829 | 0.520–1.322 | 0.430 | ||||||
| PD, TP | 75 | 31.6 | 36.1 | ||||||
| DP | 50 | 39.6 | 38.6 | ||||||
| Portal vein resection | 1.015 | 0.487–2.112 | 0.969 | ||||||
| No | 109 | 34.0 | 38.1 | ||||||
| Yes | 16 | 44.4 | 33.0 | ||||||
| Operation time, min | 1.357 | 0.864–2.129 | 0.183 | ||||||
| <275 | 63 | 39.5 | 40.4 | ||||||
| ≥275 | 62 | 29.6 | 33.0 | ||||||
| Anesthesia time, min | 1.131 | 0.721–1.774 | 0.591 | ||||||
| <363 | 63 | 37.5 | 38.3 | ||||||
| ≥363 | 62 | 31.8 | 36.5 | ||||||
| Intraoperative blood loss, ml | 1.180 | 0.752–1.851 | 0.471 | ||||||
| <600 | 63 | 37.8 | 38.6 | ||||||
| ≥600 | 62 | 31.8 | 36.5 | ||||||
| ANH | 1.651 | 1.046–2.605 | 0.029 | 1.876 | 1.174–2.998 | 0.009 | |||
| No | 81 | 40.6 | 41.6 | ||||||
| Yes | 44 | 24.1 | 23.5 | ||||||
| Intraoperative fluid given, ml | 1.017 | 0.647–1.600 | 0.941 | ||||||
| <3,500 | 68 | 33.1 | 36.7 | ||||||
| ≥3,500 | 57 | 36.7 | 36.5 | ||||||
| Intraoperative urine output, ml | 1.461 | 0.930–2.295 | 0.098 | ||||||
| <465 | 64 | 40.8 | 42.6 | ||||||
| ≥465 | 61 | 27.9 | 33.2 | ||||||
| Total in-out balance, ml | 1.174 | 0.748–1.842 | 0.484 | ||||||
| <2,210 | 63 | 35.0 | 40.0 | ||||||
| ≥2,210 | 62 | 34.8 | 36.1 | ||||||
| Total in-out balance, ml/kg/h | 1.405 | 0.895–2.204 | 0.260 | ||||||
| <6.6 | 62 | 37.2 | 40.4 | ||||||
| ≥6.6 | 63 | 32.6 | 33.0 | ||||||
| Postoperative complications | 1.440 | 0.792–2.620 | 0.229 | ||||||
| Clavien-Dindo grade 0–2 | 108 | 37.7 | 36.7 | ||||||
| Clavien-Dindo grade ≥3 | 17 | 19.9 | 43.5 | ||||||
| Pancreatic fistula | 1.421 | 0.767–2.635 | 0.262 | ||||||
| ISGPF grade non-A | 109 | 37.2 | 36.7 | ||||||
| ISGPF grade B-C | 16 | 21.4 | 21.2 | ||||||
| Postoperative hospital stay, days | 1.201 | 0.765–1.885 | 0.425 | ||||||
| <17 | 64 | 39.8 | 38.1 | ||||||
| ≥17 | 61 | 29.9 | 36.5 | ||||||
| Adjuvant chemotherapy | 0.407 | 0.247–0.671 | <0.001 | 0.268 | 0.151–0.477 | <0.001 | |||
| No | 27 | 9.1 | 20.8 | ||||||
| Yes | 98 | 41.2 | 42.6 | ||||||
All of the patients were diagnosed with M1 due to positive lymph nodes other than the regional lymph nodes. Since red blood cell transfusion has been shown to affect cancer prognosis negatively (18), the clinicopathological factors influencing DSS in subjects, excluding the ABT group, were evaluated. P-values were obtained using Cox regression analysis. ALT, alanine aminotransferase; ANH, acute normovolemic hemodilution; AST, aspartate aminotransferase; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRP, C-reactive protein; DP, distal pancreatectomy; DSS, disease-specific survival; ISGPF, International Study Group of Pancreatic Fistula; MST, median survival time; PD, pancreatoduodenectomy; RR, relative risk; TP, total pancreatectomy; UICC, Union for International Cancer Control.
Propensity score matching analysis
To reduce confounding biases and confirm the influence of ANH, we further performed PSM analysis between the STD and the ANH groups. After one-to-one PSM, 35 pairs of patients were included in further analysis. The comparison of the clinicopathological characteristics between the STD group and the ANH group, after matching, is shown in Table V. After PSM, the ANH group showed a longer operation time (327 vs. 261 min, P=0.042), with more intraoperative blood loss (970 vs. 570 ml, P<0.001) compared to the STD group. The ANH group was also administered a higher volume of intraoperative fluids (4,850 vs. 3,200 ml, P<0.001) and showed more intraoperative in-out balance than the STD group. However, after correcting the balance by body weight and anesthesia time, there was no difference between the two groups (Table VI).
Table V.
Comparison of clinicopathological characteristics after propensity score matching.
| Characteristics | Post-match all (n=70) | STH (n=35) | ANH (n=35) | P-value | ASD |
|---|---|---|---|---|---|
| Sex, male | 40 (57.1) | 19 (54.3) | 21 (60.0) | 0.629 | 0.116 |
| Age, years | 69 (49–85) | 69 (52–85) | 69 (49–78) | 0.668 | 0.146 |
| Body mass index, kg/m2 | 22.3 (14.1–33.3) | 22.0 (18.8–28.4) | 22.5 (14.1–33.3) | 0.729 | 0.074 |
| ASA-PS | 0.324b | 0.237 | |||
| PS2 | 59 (84.3) | 28 (80.0) | 31 (88.6) | ||
| PS3 | 11 (15.7) | 7 (20.0) | 4 (11.4) | ||
| Preoperative biliary drainage | 32 (45.7) | 17 (48.6) | 15 (42.9) | 0.631 | 0.115 |
| Laboratory values | |||||
| Hemoglobin, g/dl | 12.9 (8.8–16.3) | 12.5 (8.8–15.6) | 13.6 (10.8–16.3) | 0.011 | 0.675 |
| CRP, mg/dl | 0.20 (0.02–9.59) | 0.16 (0.02–9.59) | 0.27 (0.02–5.45) | 0.375 | 0.068 |
| Albumin, g/dl | 3.9 (2.4–5.0) | 3.8 (2.5–5.0) | 4.0 (2.4–4.9) | 0.110 | 0.193 |
| Hemoglobin A1c, % | 5.8 (4.4–11.8) | 5.8 (4.4–11.8) | 6.2 (4.4–9.0) | 0.273 | 0.227 |
| Creatinine, mg/dl | 0.67 (0.41–1.30) | 0.67 (0.43–1.21) | 0.66 (0.41–1.30) | 0.934 | 0.073 |
| AST, U/l | 32 (14–406) | 36 (14–406) | 27 (16–241) | 0.526 | 0.131 |
| ALT, U/l | 45 (12–621) | 51 (12–621) | 35 (12–616) | 0.991 | 0.105 |
| Total bilirubin, mg/dl | 0.8 (0.2–32.7) | 0.8 (0.3–32.7) | 0.9 (0.2–24.0) | 0.684 | 0.093 |
| CA19-9, U/ml | 73 (1–9,675) | 60 (1–3,199) | 112 (5–9,675) | 0.277 | 0.279 |
| CEA, ng/ml | 2.5 (0.5–37.0) | 2.7 (0.7–37.0) | 2.4 (0.5–23.9) | 0.577 | 0.002 |
| Operative variables | |||||
| Procedure | 0.568b | 0.256 | |||
| Pancreaticoduodenectomy | 50 (71.4) | 26 (74.3) | 24 (68.6) | ||
| Distal pancreatectomy | 19 (27.1) | 9 (25.7) | 10 (28.6) | ||
| Total pancreatectomy | 1 (1.4) | 0 (0.0) | 1 (2.9) | ||
| Portal vein resection | 9 (12.9) | 4 (11.4) | 5 (14.3) | >0.999b | 0.085 |
| Grouping of surgeons | |||||
| Junior surgeon | 4 (5.7) | 3 (8.6) | 1 (2.9) | 0.614b | 0.248 |
| Senior surgeon | 66 (94.3) | 32 (91.4) | 34 (97.1) | ||
| Pathology | |||||
| Tumor size, mm | 28 (7–130) | 26 (7–56) | 30 (10–130) | 0.331 | 0.344 |
| UICC 8th edition | |||||
| T category | 0.228b | 0.420 | |||
| T1 | 11 (15.7) | 4 (11.4) | 7 (20.0) | ||
| T2 | 43 (61.4) | 25 (71.4) | 18 (51.4) | ||
| T3 | 16 (22.9) | 6 (17.1) | 10 (28.6) | ||
| T4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| N category | 0.679 | 0.211 | |||
| N0 | 30 (42.9) | 16 (45.7) | 14 (40.0) | ||
| N1 | 25 (35.7) | 13 (37.1) | 12 (34.3) | ||
| N2 | 15 (21.4) | 6 (17.1) | 9 (25.7) | ||
| M category | 0.284b | 0.258 | |||
| M0 | 61 (87.1) | 32 (91.4) | 29 (82.9) | ||
| M1a | 9 (12.9) | 3 (8.6) | 6 (17.1) | ||
| UICC stage | 0.061b | 0.842 | |||
| IA | 8 (11.4) | 3 (8.6) | 5 (14.3) | ||
| IB | 13 (18.6) | 11 (31.4) | 2 (5.7) | ||
| IIA | 6 (8.6) | 1 (2.9) | 5 (14.3) | ||
| IIB | 25 (35.7) | 13 (37.1) | 12 (34.3) | ||
| III | 9 (12.9) | 4 (11.4) | 5 (14.3) | ||
| IV | 9 (12.9) | 3 (8.6) | 6 (17.1) | ||
| R0 resection | 63 (90.0) | 31 (88.6) | 32 (91.4) | >0.999b | 0.093 |
All of the patients were diagnosed with M1 due to positive lymph nodes other than the regional lymph nodes. Continuous variables are presented as the median (range) and were analyzed using the Mann-Whitney U-test. Categorical variables are reported as the number (percentage) and were analyzed using the χ2 test or Fisher's exact test, as appropriate.
Fisher's exact test was performed, while other comparisons for categorical variables were carried out using the χ2 test. ALT, alanine aminotransferase; ANH, acute normovolemic hemodilution; ASA-PS, American Society of Anesthesiologists physical status; ASD, absolute standardized difference; AST, aspartate aminotransferase; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRP, C-reactive protein; STD, standard management; UICC, Union for International Cancer Control.
Table VI.
Comparison of operative and postoperative outcomes after propensity score matching.
| Outcomes | Post-match all (n=70) | STH (n=35) | ANH (n=35) | P-value |
|---|---|---|---|---|
| Operative results | ||||
| Procedure | 0.568b | |||
| Pancreaticoduodenectomy | 50 (71.4) | 26 (74.3) | 24 (68.6) | |
| Distal pancreatectomy | 19 (27.1) | 9 (25.7) | 10 (28.6) | |
| Total pancreatectomy | 1 (1.4) | 0 (0.0) | 1 (2.9) | |
| Portal vein resection | 9 (12.9) | 4 (11.4) | 5 (14.3) | >0.999b |
| Operation time, min | 310 (129–619) | 261 (139–619) | 327 (129–587) | 0.042 |
| Anesthesia time, min | 374 (187–680) | 368 (226–676) | 392 (187–680) | 0.086 |
| Intraoperative blood loss, ml | 728 (130–2,200) | 570 (130–2,000) | 970 (150–2,200) | <0.001 |
| IBL >20% in CBV | 33 (47.1) | 10 (28.6) | 23 (65.7) | 0.002 |
| Blood volume removed, ml | 0 (0–800) | 0 (0–0) | 800 (400–800) | <0.001 |
| Intraoperative fluid given, ml | 3,700 (1,300–7,500) | 3,200 (1,300-7.500) | 4,000 (2,550–6,300) | <0.001 |
| Intraoperative albumin, ml | 0 (0–750) | 0 (0–500) | 0 (0–750) | 0.138 |
| Intraoperative FFP, ml | 0 (0–0) | 0 (0–0) | 0 (0–0) | - |
| Intraoperative PC, ml | 0 (0–0) | 0 (0–0) | 0 (0–0) | - |
| Total fluid volume administered, ml | 4,075 (1,300–8,000) | 3,200 (1,300–8,000) | 4,850 (2,950–7,600) | <0.001 |
| Intraoperative urine output, ml | 500 (20–1,550) | 430 (188–1,550) | 530 (20–1,550) | 0.332 |
| Total in-out balance, ml | 2,368 (485–5,500) | 1,935 (485–5,500) | 2,670 (1,190–4,555) | 0.003 |
| Total in-out balance, ml/kg/ha | 6.5 (1.8–13.9) | 6.3 (1.8–11.2) | 6.6 (3.2–13.9) | 0.104 |
| Postoperative results | ||||
| Postoperative complications (Clavien- | 13 (18.6) | 4 (11.4) | 9 (25.7) | 0.124b |
| Dindo classification grade ≥3) | ||||
| Pancreatic fistula (ISGPF grade ≥B) | 11 (15.7) | 4 (11.4) | 7 (20.0) | 0.324b |
| Delayed gastric emptying (ISGPS grade ≥B) | 9 (12.9) | 3 (8.6) | 6 (17.1) | 0.477b |
| Postoperative hospital stay, days | 17 (6–64) | 17 (8–61) | 19 (6–64) | 0.101 |
| Adjuvant chemotherapy | 54 (78.3) | 29 (82.9) | 25 (73.5) | 0.348 |
Estimated using the following formula: Total in-out balance/body weight (kg)/anesthesia time (h). Continuous variables are presented as the median (range) and were analyzed using the Mann-Whitney U-test. Categorical variables are reported as the number (percentage) and were analyzed using the χ2 test or Fisher's exact test, as appropriate.
Fisher's exact test was performed, while other comparisons for categorical variables were carried out using the χ2 test. ANH, acute normovolemic hemodilution; CBV, circulating blood volume; FFP, fresh frozen plasma; IBL, intraoperative blood loss; ISGPF, the International Study Group of Pancreatic Fistula; ISGPS, the International Study Group of Pancreatic Surgery; PC, platelet concentration; STD, standard management.
Next, we evaluated the postoperative complications in the matched cohort. As a result, there were no significant differences in the incidences of postoperative complications between the two groups after PSM (Table VI). Furthermore, we performed a survival analysis of the matched cohort. RFS time was slightly but not significantly poorer in the ANH group compared with the STD group (MST, 12.1 vs. 18.1 months, P=0.097; Fig. 2C). In addition, a similar trend was noted in the DSS rate (MST, 32.1 vs. 50.5 months, P=0.097; Fig. 2D). After PSM, in contrast with short-term outcomes, postoperative long-term outcomes in the ANH group were less favorable than those in the STD group.
To assess whether ANH influences RFS and DSS in the matched cohort, we performed a Cox regression analysis. As a result, we identified some factors that had a greater effect on poor prognosis than ANH (Table SI).
Discussion
This report represents the first study to examine the effect of ANH on PDAC prognosis longitudinally. This study demonstrated that ANH has a negative impact on long-term outcomes in PDAC compared to standard management, though not as negative as ABT. Similar results were confirmed even after propensity score matching analysis. These results elucidate the potential negative effects of ANH compared to management without transfusion in resectable PDAC.
Despite the proven short-term outcomes (36,37), there are surprisingly few studies evaluating the long-term effects of ANH on cancer patients. A recent post-hoc analysis from a prospective trial demonstrated that ANH did not have any detrimental effects on long-term oncologic outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer (21). In the field of gastroenterology, an RCT evaluating ANH during major hepatectomy procedures for metastatic colorectal cancer showed no detrimental impact of ANH on survival outcomes (28). These findings are the opposite of our results. However, the studies were originally conducted to determine if ANH reduced the need for ABT. In short, these post-hoc analyses comparing the long-term outcomes between the STD and ANH groups included patients who received ABT. This heterogeneity may have affected the survival outcomes, because ABT can cause an immunomodulatory effect leading to worse oncologic outcomes (17). Thus, we excluded patients who received ABT and then directly compared the ANH and STD groups. As a result, our study figured out the potential differences in prognosis between the STD and ANH groups.
How does ANH affect the prognosis in PDAC patients who have undergone pancreatic resection? Direct and indirect effects can be assumed. One of the possible direct effects is the immunosuppressive effect of ANH (38). Additionally, compared to standard management, ANH is logically associated with circulatory overload. The only prospective RCT, in which every assessed ANH inpatient underwent PD, determined that ANH was related to greater intraoperative fluid management, and resulted in more pancreatic anastomotic complications (19). A similar trend was observed in this study as well. Postoperative complications may have a negative effect on survival outcomes in cancer patients (28,39,40), including PDAC (41,42). It has been suggested that postoperative complications could have immunosuppressive effects (39,40,43). Therefore, in terms of postoperative complications after ANH, immunosuppressive effects may be an additional consideration.
In our cohort, after PSM, the ANH group was associated with great intraoperative blood loss compared to the STD group. This trend was observed in a previous RCT of ANH in patients undergoing PD (19). Conversely, this trend was not observed in another liver surgery RCT (27). Several lines of evidence have shown an association between increased blood loss and poor outcomes in PDAC surgery (15,16,44). Our previous study also demonstrated those relationships (29). These data suggest that more intraoperative blood loss may negatively influence the prognosis of ANH. At the same time, we must be deliberate and critically consider that ANH can potentially cause increased intraoperative blood loss.
The present study has several limitations. First, this is a retrospective single-institution cohort study and not a randomized control trial. The patient population was not large. In this study, we performed propensity score matching using caliper matching, achieving a satisfactory balance of pre-ANH variables between the STD and ANH groups. However, despite our best efforts, there are instances where standardized difference scores exceed 0.2 for certain variables. One possible explanation for this is that large variations in certain variables, such as CA19-9, may contribute to such an imbalance. In addition, not having a large sample size may have created a non-ideal balance after PSM. These results speak to the desirability of a larger sample size for achieving optimal balance. However, if we had added the borderline resectable PDAC cohort to the current resectable PDAC cohort, the borderline cases would have greatly increased the ANH group due to the estimated increased blood loss associated with vascular resection. Adding borderline cases, however, would make it difficult to validate the true impact of ANH on long-term outcomes in PDAC patients. Moreover, typical study biases, such as fluid overloading, more intraoperative blood loss, longer operation time, etc., were not excluded from this study. These biases make drawing definitive conclusions difficult. Nonetheless, there have been no previous studies examining the effect of ANH on the long-term prognosis of PDAC. Accordingly, the suggestions from this study should not be ignored.
In conclusion, the present study, using PSM analysis, showed that ANH could be associated with poor postoperative long-term outcomes in resectable PDAC patients compared to STD. Various biases make it difficult to conclude whether or not ANH is inherently harmful. However, the one thing we can say without hesitation is that management without transfusion is the best course of action. Furthermore, ABT has the worst negative impact. Thus, we should make every effort to avoid ABT, and ANH is certainly a valuable approach to achieve this goal. In general, at least until a definitive conclusion is reached, it is better to limit the use of ANH in certain specific PDAC cases.
Supplementary Material
Acknowledgements
The authors would like to thank Professor Shari Joy Berman (Hirosaki University Graduate School of Medicine, Hirosaki, Japan) for professionally editing the English draft of this manuscript.
Glossary
Abbreviations
- ABT
allogeneic blood transfusion
- ANH
acute normovolemic hemodilution
- DP
distal pancreatectomy
- DSS
disease-specific survival
- MST
median survival time
- RFS
recurrence-free survival
- RR
relative risk
- PD
pancreatoduodenectomy
- PDAC
pancreatic ductal adenocarcinoma
- PSM
propensity score matching
- STD
standard management
- UICC
Union for International Cancer Control
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
TW, KI, NK and KHa contributed to the study conception and design. TW, KI, NK, HN and KHa performed surgical resection. TW, KI, NK, HN, TK, SK, HF, YT, TY, KC, JS, KHi and KHa collected the clinical data. TW, KI, NK, HN, TK, SK, HF, YT, TY, KC, JS, KHi and KHa analyzed and interpreted the data. TW wrote the first draft of the manuscript. KI, NK, HN, TK, SK, HF, YT, TY, KC, JS, KHi and KHa contributed to the review and/or critical revision of the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TW and KI confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Committee of Medical Ethics of Hirosaki University Graduate School of Medicine (reference no. 2022-032; Aomori, Japan). Informed consent was obtained in the form of an opt-out feature on our website, with the approval of the Committee of Medical Ethics of Hirosaki University Graduate School of Medicine. The present study did not include minors. The present study was designed and carried out in accordance with the Declaration of Helsinki.
Patient consent for publication
Informed consent for publication was obtained in the form of an opt-out feature on our website, with the approval of the Committee of Medical Ethics of Hirosaki University Graduate School of Medicine (Aomori, Japan).
Competing interests
The authors declare that they have no competing interests.
References
- 1.GBD 2017 Pancreatic Cancer Collaborator, corp-author. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–947. doi: 10.1016/S2468-1253(19)30347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 4.Doi R, Imamura M, Hosotani R, Imaizumi T, Hatori T, Takasaki K, Funakoshi A, Wakasugi H, Asano T, Hishinuma S, et al. Surgery versus radiochemotherapy for resectable locally invasive pancreatic cancer: Final results of a randomized multi-institutional trial. Surg Today. 2008;38:1021–1028. doi: 10.1007/s00595-007-3745-8. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahola R, Sand J, Laukkarinen J. Pancreatic resections are not only safest but also most cost-effective when performed in a high-volume centre: A Finnish register study. Pancreatology. 2019;19:769–774. doi: 10.1016/j.pan.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: A new tool to assess sarcopenia. J Gastrointest Surg. 2015;19:1593–1602. doi: 10.1007/s11605-015-2835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, Porte RJ, Gouma DJ, Busch OR, Molenaar IQ, Dutch Pancreatic Cancer Group Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- 9.Gooiker GA, Lemmens VE, Besselink MG, Busch OR, Bonsing BA, Molenaar IQ, Tollenaar RA, de Hingh IH, Wouters MW. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg. 2014;101:1000–1005. doi: 10.1002/bjs.9468. [DOI] [PubMed] [Google Scholar]
- 10.Onete VG, Besselink MG, Salsbach CM, Van Eijck CH, Busch OR, Gouma DJ, de Hingh IH, Sieders E, Dejong CH, Offerhaus JG, et al. Impact of centralization of pancreatoduodenectomy on reported radical resections rates in a nationwide pathology database. HPB (Oxford) 2015;17:736–742. doi: 10.1111/hpb.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hachey K, Morgan R, Rosen A, Rao SR, McAneny D, Tseng J, Doherty G, Sachs T. Quality comes with the (Anatomic) territory: Evaluating the impact of surgeon operative mix on patient outcomes after pancreaticoduodenectomy. Ann Surg Oncol. 2018;25:3795–3803. doi: 10.1245/s10434-018-6732-y. [DOI] [PubMed] [Google Scholar]
- 12.Snyder RA, Prakash LR, Nogueras-Gonzalez GM, Kim MP, Aloia TA, Vauthey JN, Lee JE, Fleming JB, Katz MHG, Tzeng CD. Perioperative blood transfusions for vein resection during pancreaticoduodenectomy for pancreatic adenocarcinoma: Identification of clinical targets for optimization. HPB (Oxford) 2019;21:841–848. doi: 10.1016/j.hpb.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Ecker BL, Simmons KD, Zaheer S, Poe SL, Bartlett EK, Drebin JA, Fraker DL, Kelz RR, Roses RE, Karakousis GC. Blood transfusion in major abdominal surgery for malignant tumors: A trend analysis using the national surgical quality improvement program. JAMA Surg. 2016;151:518–525. doi: 10.1001/jamasurg.2015.5094. [DOI] [PubMed] [Google Scholar]
- 14.Kneuertz PJ, Patel SH, Chu CK, Maithel SK, Sarmiento JM, Delman KA, Staley CA, III, Kooby DA. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18:1327–1334. doi: 10.1245/s10434-010-1476-3. [DOI] [PubMed] [Google Scholar]
- 15.Seykora TF, Ecker BL, McMillan MT, Maggino L, Beane JD, Fong ZV, Hollis RH, Jamieson NB, Javed AA, Kowalsky SJ, et al. The beneficial effects of minimizing blood loss in pancreatoduodenectomy. Ann Surg. 2019;270:147–157. doi: 10.1097/SLA.0000000000002714. [DOI] [PubMed] [Google Scholar]
- 16.Rystedt J, Tingstedt B, Ansorge C, Nilsson J, Andersson B. Major intraoperative bleeding during pancreatoduodenectomy-preoperative biliary drainage is the only modifiable risk factor. HPB (Oxford) 2019;21:268–274. doi: 10.1016/j.hpb.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Remy KE, Hall MW, Cholette J, Juffermans NP, Nicol K, Doctor A, Blumberg N, Spinella PC, Norris PJ, Dahmer MK, et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion. 2018;58:804–815. doi: 10.1111/trf.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda T, Wakiya T, Ishido K, Brierley, Kimura N, Nagase H, Kubota S, Fujita H, Hagiwara Y, Hakamada K. Intraoperative allogeneic red blood cell transfusion negatively influences prognosis after radical surgery for pancreatic cancer: A propensity score matching analysis. Pancreas. 2021;50:1314–1325. doi: 10.1097/MPA.0000000000001913. [DOI] [PubMed] [Google Scholar]
- 19.Fischer M, Matsuo K, Gonen M, Grant F, Dematteo RP, D'Angelica MI, Mascarenhas J, Brennan MF, Allen PJ, Blumgart LH, Jarnagin WR. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: Results of a prospective randomized trial of acute normovolemic hemodilution compared with standard intraoperative management. Ann Surg. 2010;252:952–958. doi: 10.1097/SLA.0b013e3181ff36b1. [DOI] [PubMed] [Google Scholar]
- 20.Tanner EJ, Filippova OT, Gardner GJ, Long Roche KC, Sonoda Y, Zivanovic O, Fischer M, Chi DS. A prospective trial of acute normovolemic hemodilution in patients undergoing primary cytoreductive surgery for advanced ovarian cancer. Gynecol Oncol. 2018;151:433–437. doi: 10.1016/j.ygyno.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boerner T, Tanner E, Filippova O, Zhou QC, Iasonos A, Tew WP, O'Cearbhaill RE, Grisham RN, Gardner GJ, Sonoda Y, et al. Survival outcomes of acute normovolemic hemodilution in patients undergoing primary debulking surgery for advanced ovarian cancer: A Memorial Sloan Kettering Cancer Center Team Ovary study. Gynecol Oncol. 2021;160:51–55. doi: 10.1016/j.ygyno.2020.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson NW, Ochsner JL, Mills NL, Leonard GL. The use of hemodilution and fresh autologous blood in open-heart surgery. Anesth Analg. 1974;53:672–683. doi: 10.1213/00000539-197409000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Grant MC, Resar LM, Frank SM. The efficacy and utility of acute normovolemic hemodilution. Anesth Analg. 2015;121:1412–1414. doi: 10.1213/ANE.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 24.Spahn DR, Casutt M. Eliminating blood transfusions: New aspects and perspectives. Anesthesiology. 2000;93:242–255. doi: 10.1097/00000542-200007000-00035. [DOI] [PubMed] [Google Scholar]
- 25.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies, corp-author. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American society of anesthesiologists task force on perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Barile L, Fominskiy E, Di Tomasso N, Alpìzar Castro LE, Landoni G, De Luca M, Bignami E, Sala A, Zangrillo A, Monaco F. Acute normovolemic hemodilution reduces allogeneic red blood cell transfusion in cardiac surgery: A systematic review and meta-analysis of randomized trials. Anesth Analg. 2017;124:743–752. doi: 10.1213/ANE.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 27.Jarnagin WR, Gonen M, Maithel SK, Fong Y, D'Angelica MI, Dematteo RP, Grant F, Wuest D, Kundu K, Blumgart LH, Fischer M. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248:360–369. doi: 10.1097/SLA.0b013e318184db08. [DOI] [PubMed] [Google Scholar]
- 28.Correa-Gallego C, Gonen M, Fischer M, Grant F, Kemeny NE, Arslan-Carlon V, Kingham TP, Dematteo RP, Fong Y, Allen PJ, et al. Perioperative complications influence recurrence and survival after resection of hepatic colorectal metastases. Ann Surg Oncol. 2013;20:2477–2484. doi: 10.1245/s10434-013-2975-9. [DOI] [PubMed] [Google Scholar]
- 29.Wakiya T, Ishido K, Kimura N, Nagase H, Kubota S, Fujita H, Hagiwara Y, Kanda T, Matsuzaka M, Sasaki Y, Hakamada K. Prediction of massive bleeding in pancreatic surgery based on preoperative patient characteristics using a decision tree. PLoS One. 2021;16:e0259682. doi: 10.1371/journal.pone.0259682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. John Wiley & Sons; Hoboken, NJ, USA: 2017. pp. 93–95. [Google Scholar]
- 31.Shirai Y, Shiba H, Horiuchi T, Saito N, Furukawa K, Sakamoto T, Gocho T, Ishida Y, Yanaga K. Assessment of outcome after pancreaticoduodenectomy by junior surgeons. Anticancer Res. 2016;36:3505–3510. [PubMed] [Google Scholar]
- 32.Takekawa D, Saito J, Kinoshita H, Hashiba EI, Hirai N, Yamazaki Y, Kushikata T, Hirota K. Acute normovolemic hemodilution reduced allogeneic blood transfusion without increasing perioperative complications in patients undergoing free-flap reconstruction of the head and neck. J Anesth. 2020;34:187–194. doi: 10.1007/s00540-019-02714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Ni Y, Ding XH, Xu ZJ, Zhang ZF, Zhang Y, Gui B. Association of acute normovolemic hemodilution with decreased length of hospital stay in rhesus-negative patients undergoing major cancer surgeries: A retrospective study. Ann Palliat Med. 2021;10:1815–1824. doi: 10.21037/apm-20-1327. [DOI] [PubMed] [Google Scholar]
- 37.Ni Y, Xu ZJ, Zhang ZF, Yang C, Liu CM, Gui B. Acute normovolemic hemodilution for major cancer surgeries during the COVID-19 pandemic: A beacon of hope. J Clin Anesth. 2020;65:109871. doi: 10.1016/j.jclinane.2020.109871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vamvakas EC. Meta-analysis of randomized controlled trials comparing the risk of postoperative infection between recipients of allogeneic and autologous blood transfusion. Vox Sang. 2002;83:339–346. doi: 10.1046/j.1423-0410.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 39.Pucher PH, Aggarwal R, Qurashi M, Darzi A. Meta-analysis of the effect of postoperative in-hospital morbidity on long-term patient survival. Br J Surg. 2014;101:1499–1508. doi: 10.1002/bjs.9615. [DOI] [PubMed] [Google Scholar]
- 40.Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann Surg. 2015;261:497–505. doi: 10.1097/SLA.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 41.Aahlin EK, Olsen F, Uleberg B, Jacobsen BK, Lassen K. Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery: A complete national cohort study. BMC Surg. 2016;16:32. doi: 10.1186/s12893-016-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubrano J, Bachelier P, Paye F, Le Treut YP, Chiche L, Sa-Cunha A, Turrini O, Menahem B, Launoy G, Delpero JR. Severe postoperative complications decrease overall and disease free survival in pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Eur J Surg Oncol. 2018;44:1078–1082. doi: 10.1016/j.ejso.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 44.Kazanjian KK, Hines OJ, Duffy JP, Yoon DY, Cortina G, Reber HA. Improved survival following pancreaticoduodenectomy to treat adenocarcinoma of the pancreas: The influence of operative blood loss. Arch Surg. 2008;143:1166–1171. doi: 10.1001/archsurg.143.12.1166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.