Abstract
Background
Dengue is a global health problem of high significance, with 3.9 billion people at risk of infection. The geographic expansion of dengue virus (DENV) infection has resulted in increased frequency and severity of the disease, and the number of deaths has increased in recent years. Wolbachia, an intracellular bacterial endosymbiont, has been under investigation for several years as a novel dengue‐control strategy. Some dengue vectors (Aedes mosquitoes) can be transinfected with specific strains of Wolbachia, which decreases their fitness (ability to survive and mate) and their ability to reproduce, inhibiting the replication of dengue. Both laboratory and field studies have demonstrated the potential effect of Wolbachia deployments on reducing dengue transmission, and modelling studies have suggested that this may be a self‐sustaining strategy for dengue prevention, although long‐term effects are yet to be elucidated.
Objectives
To assess the efficacy of Wolbachia‐carrying Aedes species deployments (specifically wMel‐, wMelPop‐, and wAlbB‐ strains of Wolbachia) for preventing dengue virus infection.
Search methods
We searched CENTRAL, MEDLINE, Embase, four other databases, and two trial registries up to 24 January 2024.
Selection criteria
Randomized controlled trials (RCTs), including cluster‐randomized controlled trials (cRCTs), conducted in dengue endemic or epidemic‐prone settings were eligible.
We sought studies that investigated the impact of Wolbachia‐carrying Aedes deployments on epidemiological or entomological dengue‐related outcomes, utilizing either the population replacement or population suppression strategy.
Data collection and analysis
Two review authors independently selected eligible studies, extracted data, and assessed the risk of bias using the Cochrane RoB 2 tool. We used odds ratios (OR) with the corresponding 95% confidence intervals (CI) as the effect measure for dichotomous outcomes. For count/rate outcomes, we planned to use the rate ratio with 95% CI as the effect measure. We used adjusted measures of effect for cRCTs. We assessed the certainty of evidence using GRADE.
Main results
One completed cRCT met our inclusion criteria, and we identified two further ongoing cRCTs. The included trial was conducted in an urban setting in Yogyakarta, Indonesia. It utilized a nested test‐negative study design, whereby all participants aged three to 45 years who presented at healthcare centres with a fever were enrolled in the study provided they had resided in the study area for the previous 10 nights.
The trial showed that wMel‐Wolbachia infected Ae aegypti deployments probably reduce the odds of contracting virologically confirmed dengue by 77% (OR 0.23, 95% CI 0.15 to 0.35; 1 trial, 6306 participants; moderate‐certainty evidence). The cluster‐level prevalence of wMel Wolbachia‐carrying mosquitoes remained high over two years in the intervention arm of the trial, reported as 95.8% (interquartile range 91.5 to 97.8) across 27 months in clusters receiving wMel‐Wolbachia Ae aegypti deployments, but there were no reliable comparative data for this outcome.
Other primary outcomes were the incidence of virologically confirmed dengue, the prevalence of dengue ribonucleic acid in the mosquito population, and mosquito density, but there were no data for these outcomes. Additionally, there were no data on adverse events.
Authors' conclusions
The included trial demonstrates the potential significant impact of wMel‐Wolbachia‐carrying Ae aegypti mosquitoes on preventing dengue infection in an endemic setting, and supports evidence reported in non‐randomized and uncontrolled studies. Further trials across a greater diversity of settings are required to confirm whether these findings apply to other locations and country settings, and greater reporting of acceptability and cost are important.
Keywords: Animals, Humans, Aedes, Aedes/microbiology, Dengue, Dengue/prevention & control, Dengue Virus, Mosquito Vectors, Mosquito Vectors/microbiology, Wolbachia
Plain language summary
Does releasing Wolbachia‐carrying mosquitoes prevent dengue infection?
Key messages
– People living in areas where Wolbachia‐carrying Aedes mosquitoes have been released are less likely to contract dengue than people living in areas with no release.
– People living in areas where Wolbachia‐carrying Aedes mosquitoes have been released are less likely to be admitted to hospital due to dengue than people living in areas with no release.
What is dengue?
Dengue is a viral disease transmitted by certain mosquitoes that is found in over 100 countries, with 3.9 billion people at risk of infection. Most dengue infections are mild (fever, headache, and muscle and joint pain), and some people do not have symptoms, but 1 in 20 people will develop a severe form of dengue. In the worst cases, this can cause organ failure and be life‐threatening. Therefore, preventing the spread of dengue virus is of high importance.
What is Wolbachia, and how could these bacteria prevent dengue?
Wolbachia is a bacterium that can infect mosquitoes and alter their ability to survive and mate. Mosquitoes carry viruses such as dengue, and infect people with this virus through biting. Some species of mosquitoes, known as Aedes, find it harder to carry and transmit the dengue virus when they have been infected with Wolbachia. Researchers have microinjected Wolbachia extracts into mosquitoes and released Wolbachia‐carrying mosquitoes into the wild population, where they have bred with other mosquitoes and passed on the Wolbachia infection. Once a large proportion of mosquitoes in an area become infected with Wolbachia, there is potential to reduce the mosquitoes' ability to spread dengue virus and prevent the frequency of dengue infections in the local human population. Wolbachia can only infect invertebrate organisms (that is, animals without a backbone), therefore, there is no risk that humans will become infected with the bacteria, and risks to humans and the environment associated with releasing Wolbachia‐carrying mosquitoes are believed to be minor.
What did we want to find out?
We wanted to determine whether releasing Wolbachia‐carrying Aedes mosquitoes into areas where dengue is regularly found could prevent dengue infection in the people who live there.
What did we do?
We searched for any randomized controlled trials (clinical trials where people are randomly put into one of two or more treatment groups) that investigated whether releasing Wolbachia‐carrying Aedes mosquitoes into an area where dengue is present prevented the spread or incidence of dengue, compared to areas with no releases.
What did we find?
We identified one completed trial that met our inclusion criteria, and two more that are ongoing. The completed trial was conducted in Yogyakarta, Indonesia, and included people aged three to 45 years. The trial involved releasing mosquito eggs that were infected with Wolbachia into half of the study area until more than 60% of the mosquitoes in that area were carrying Wolbachia. There were no releases in the other half of the study area.
The trial tested all people who presented at their local health facility with a fever to determine how many people had contracted dengue. They compared the results between people living in the areas where Wolbachia‐carrying mosquitoes were released and people living in the areas where no new mosquitoes were released over a 27‐month period.
The likelihood of people who lived within the area where Wolbachia‐carrying mosquitoes were released contracting dengue was probably reduced by 77% compared to people who lived in an area with a wild population of mosquitoes.
About 95.8% of mosquitoes in the population in the areas where Wolbachia‐carrying mosquitoes were released became infected with the bacteria. This demonstrates that the bacteria were being passed on to wild mosquitoes via mating after the Wolbachia‐infected mosquitoes were no longer being released. There were no reliable data for this in the group of the trial where no new mosquitoes were released.
We also wanted to find out whether releasing the bacteria‐carrying mosquitoes into the wild reduced the number of mosquitoes that were carrying the dengue virus, reduced the prevalence of dengue RNA (the genetic material of the virus) in the mosquito population, or caused any unwanted effects. Additionally, we were interested in the cost of this intervention, and the community's views towards it. We did not find any data that answered these questions.
What are the limitations of the evidence?
The effectiveness of the Wolbachia strategy will likely depend on factors that are specific to the location in which it is implemented, such as the climate, prevalence of dengue infection, existing vector control strategies, or community views towards the strategy. The ability to successfully achieve and sustain a high prevalence of Wolbachia‐carrying mosquitoes in the population is critical to the effectiveness of the intervention, and this may vary in different settings. Since we only identified one completed trial, we do not know if these results will apply to other settings and countries.
How up to date is this evidence?
This evidence is up to date to 24 January 2024.
Summary of findings
Summary of findings 1. wMel‐Wolbachia infected Aedes aegypti deployments compared to no deployments for preventing dengue virus infection.
|
Patient or population: children and adults at risk of dengue infection Setting: Indonesia, urban area Intervention:wMel‐Wolbachia infected Aedes aegypti deployments (population replacement strategy) Comparison: no deployments | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Wolbachia deployments | |||||
| Virologically confirmed dengue case incidence (any severity, any phenotype) | — | — | — | — | — | No data reported. |
|
Prevalence of DENV infection (any severity, any phenotype)a Assessed with: fever onset 1–4 days before presentation + positive laboratory assays (RT‐PCR or ELISA) Time period: 27 months |
94 per 1000b | 24 per 1000 (15 to 35)c |
OR 0.23 (0.15 to 0.35) | 6306 (1 cRCT) | ⊕⊕⊕⊝ Moderated |
wMel‐Wolbachia‐infected Ae aegypti deployments (via population replacement strategy) reduce DENV prevalence. |
| Prevalence of dengue RNA in the mosquito population | — | — | — | — | — | No data reported. |
|
Mosquito density (for population suppression strategy) |
— | — | — | — | — | No data reported. |
|
Prevalence of Wolbachia‐carrying mosquitoes (for population replacement strategy) |
— | — | — | — | — | No reliable comparative data were available. |
| Adverse events potentially related to Wolbachia‐carrying Aedes deployments | — | — | — | — | — | No data reported. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; cRCT: cluster‐randomized controlled trial; DENV: dengue virus; ELISA: enzyme‐linked immunosorbent assay; OR: odds ratio; RNA: ribonucleic acid; RT‐PCR: reverse transcription polymerase chain reaction. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
a Absolute risk in the control group was estimated by the prevalence amongst those who reported a fever during the study period, not the entire population residing in the study area. b We have assumed that all individuals recruited to the study were distinct, i.e. no participant was enrolled more than once for separate episodes of fever. c We calculated the absolute risk in the intervention group by converting the odds ratio to a risk ratio using the formula provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), and an assumed comparator risk of 0.094 (prevalence amongst those who reported a fever during the study period, not the entire population residing in the study area). d Downgraded one level due to indirectness as only one cRCT contributed to the result and evidence from non‐randomized studies suggests that the relative effect may vary in other settings due to confounding factors.
Background
Description of the condition
Dengue is a mosquito‐borne viral infectious disease which is endemic in over 100 countries (CDC 2023). The dengue virus (DENV) is a virus of the Flaviviridae family containing a single‐stranded, positive‐sense ribonucleic acid (RNA) genome, which is spread through the bite of female Aedes aegypti (Ae aegypti) mosquitoes and, to a lesser extent, Aedes albopictus and Aedes polynesiensis. Aedes mosquitoes are vectors for several viruses as well as DENV, including yellow fever virus, chikungunya virus, West Nile virus, Japanese encephalitis virus, and Zika virus. The risk of infection is present in all areas inhabited by Aedes mosquitoes, particularly in tropical climates.
One study on the prevalence of dengue estimated that 3.9 billion people were at risk of infection in 2012, and 70% of the actual burden of disease was in Asia (Bhatt 2013). Since the 1960s, there has been a 30‐fold increase in global dengue incidence (WHO 2012). The geographic expansion of DENV infection has resulted in increased frequency and severity of the disease. While 80% of dengue infections are mild and asymptomatic, the number of reported deaths rose from 960 in 2000 to 4032 in 2015, mostly affecting younger age groups (WHO 2022a).
Dengue is caused by four distinct serotypes of DENV that are closely related (DENV‐1, DENV‐2, DENV‐3, and DENV‐4). Infection and recovery from a specific serotype provides lifelong immunity to that serotype; however, cross‐immunity to other serotypes is partial and temporary (Reich 2013). One in 20 people with dengue can go on to develop severe dengue (Alexander 2011). Those who develop severe dengue usually go through three phases: febrile, recovery, and critical. The critical phase is associated with clinically significant plasma leakage leading to shock; haemorrhage due to low platelet count and coagulopathy; and severe organ impairment such as hepatitis, encephalitis, or myocarditis (CDC 2021). Early recognition is crucial to clinical management as it allows for the identification of people who are likely to progress to severe dengue, and the adoption of timely and appropriate interventions (WHO 2009). Managing severe dengue effectively reduces mortality to less than 1% (WHO 2022a).
Description of the intervention
Background
Wolbachia is a genus of gram‐negative intracellular bacterial endosymbiont that is found in over 60% of all arthropod species (Hilgenboecker 2008). The bacterium is associated with phenotypic manipulations in host species, meaning it is able to modify characteristics of the host. Specifically, Wolbachia can decrease vectors' fitness (ability to survive and mate) and reproductive capacities, and it can also inhibit arbovirus replication (Rainey 2014). Wolbachia is maternally inherited, and its potential use in vector control is based on two strategies: population suppression and population replacement. Both strategies are driven by one of the most prominent features imposed by Wolbachia on their host: cytoplasmic incompatibility, a phenomenon that results in sperm and eggs being unable to form viable offspring (Yen 1971). This may be unidirectional (only one Wolbachia strain is involved during mating) or bidirectional (two different individuals carry different strains of Wolbachia). Unidirectional and bidirectional cytoplasmic incompatibility both drive the population suppression strategy (Werren 1997). Unidirectional cytoplasmic incompatibility may also drive the population replacement strategy when Wolbachia‐carrying females are present (Bourtzis 2014). Infected eggs can be fertilized by sperm from any male, whereas uninfected eggs can only be fertilized by sperm from uninfected males. Therefore, infected females will produce a greater number of offspring than uninfected females, and because Wolbachia is only inherited maternally, the frequency of infection increases with each generation.
Potential impact on dengue transmission
The introduction of Wolbachia‐carrying Aedes species presents a promising vector control strategy. Studies exploring the properties of different Wolbachia infections in insect hosts have identified both life‐shortening and antiviral effects of Wolbachia in Drosophila melanogaster (McMeniman 2009; Min 1997; Moreira 2009). By shortening the life‐span of the mosquito vector, viruses and parasites are unable to develop in the host due to their long extrinsic incubation period (Brownstein 2003; Moreira 2009; Rasgon 2003). These properties of Wolbachia constitute a potential vector control strategy for dengue, as well as for other vector‐borne diseases. They may reduce the ability of vectors to carry viruses that cause vector‐borne disease in humans or reduce the fitness of the vectors themselves; in both cases, the result is a reduction in viral transmission. To explore this possibility, experimental studies have investigated transinfection of vectors with strains of Wolbachia through embryo microinjection and adult microinjection (for details on the process of transinfection, see Hughes 2014).
In this review, we focused on strains of Wolbachia that have been demonstrated to stably transinfect vectors of DENV with a potential effect on dengue transmission; that is, strains of Wolbachia that can infect Aedes mosquitoes and be passed on to their progeny and cause a disadvantage in vector fitness or ability to be infected with DENV.
Stable transinfection of Aedes
Studies have shown that the Wolbachia strains wMelPop, wMel, and wAlbB can achieve stable transinfection of Aedes mosquitoes with the potential to reduce DENV transmission in humans.
Experimental transinfection of Ae aegypti with wMelPop demonstrated the life‐shortening effect of Wolbachia in the dengue vector, with around a 50% reduction in adult female lifespan (McMeniman 2009). To explore the hypothesis that this Wolbachia strain may alter vector competence for arboviruses, investigators transinfected Ae aegypti with wMelPop and exposed them to dengue and chikungunya viruses (Moreira 2009). This demonstrated a reduced ability for arboviruses to establish infection in wMelPop‐carrying Ae aegypti, potentially due to competition for host cell components and upregulation of immune effector genes (Moreira 2009).
Experimental investigations continued to explore the properties of Wolbachia‐carrying Aedes mosquitoes to identify the Wolbachia strain most suitable for a dengue prevention strategy. According to Walker 2011, wMel Wolbachia‐carrying Ae aegypti displays the reproductive phenotype cytoplasmic incompatibility with minimal apparent fitness costs and high maternal transmission, providing optimal phenotypic effects for invasion. The same study demonstrated the ability of wMel Wolbachia to provide protection against DENV in Ae aegypti.
Blagrove 2013 evidenced the infection of Aedes albopictus with wMel Wolbachia by bidirectional cytoplasmic incompatibility, demonstrating a promising new method to prevent or reduce dengue. Other studies have shown that the wAlbB strain of Wolbachia can induce a viral inhibitory effect against DENV in Ae aegypti and Aedes polynesiensis mosquitoes (Bian 2010; Bian 2013). Johnson 2015 provides a detailed summary of the effects of Wolbachia strains on vectors for mosquito‐borne disease. Table 2 summarizes evidence of the effect of Wolbachia on dengue vectors.
1. Evidence of stable transinfection of dengue vectors with Wolbachia.
| Mosquito species | Wolbachia strain |
| Aedes aegypti | wMelPop, wMel, wAlbB |
| Aedes albopictus | wMel |
| Aedes polynesiensis | wAlbB |
Table adapted from Johnson 2015
The deployment of Wolbachia‐carrying Aedes mosquitoes into dengue‐endemic areas is a potential strategy to prevent dengue transmission and infection in humans. Releases of female Wolbachia‐carrying mosquitoes would facilitate the spread of Wolbachia infection throughout the wild Aedes population by the mechanism of unidirectional cytoplasmic incompatibility, and reduce the ability of the wild vector population to carry DENV and transmit the infection.
Existing evidence of Wolbachia as a vector control strategy
Researchers have piloted vector control strategies utilizing Wolbachia in several global towns and cities that are inhabited by Aedes mosquitoes across many World Health Organization (WHO) regions including the Western Pacific, Americas, and Southeast Asia (CDC 2022; NEA 2022; Wellcome 2022).
Population suppression strategy
In Singapore, the National Environment Agency (NEA) has released male Wolbachia‐carrying Aedes mosquitoes and used the population suppression strategy for dengue prevention. Wolbachia‐carrying male mosquitoes mate with uninfected female mosquitoes, resulting in unhatched eggs and a reduced mosquito population (NEA 2022). In some towns, this has resulted in up to a 98% reduction in Ae aegypti populations, and sites with at least one year of releases have reported 88% fewer dengue cases than areas with no releases (NEA 2022).
Population replacement strategy
Experimental studies in Australia have demonstrated the effect of the population replacement strategy on Ae aegypti using the wMel strain of Wolbachia. The number and frequency of wMel Wolbachia‐carrying mosquito deployments varied from one to two releases per week for a duration of five to 23 weeks (Ryan 2019). Deployments were typically discontinued when the frequency of Wolbachia in field‐caught mosquitoes exceeded 50% for a period of more than two weeks, at which point it was expected that the frequency of infection would increase self‐sustainably without further deployments. However, some studies implement a higher Wolbachia infection threshold before discontinuation of Wolbachia‐carrying mosquito deployments. Across the experimental sites, short‐term releases of between five and 23 weeks with either eggs or adult mosquitoes resulted in the establishment of Wolbachia in mosquito populations (Ryan 2019). An analysis of case DENV notifications data prior to and after mosquito deployments indicated a 96% reduction in dengue incidence in Wolbachia‐treated populations (Ryan 2019). Ovitrapping data after the initial implementation of wMel Wolbachia‐carrying Aedes deployments showed that the frequency of Wolbachia infection in the Ae aegypti population was above 0.96 in all release areas, meaning infection was stable in the vector population (Ross 2022).
How the intervention might work
Experimental transinfection of Aedes mosquitoes with certain Wolbachia strains has demonstrated strong cytoplasmic incompatibility, shown no effect on egg viability (meaning the strain is more likely to persist in wild populations), and reduced vector competence to carry arbovirus infections (Bian 2010; Bian 2013; Blagrove 2013; Johnson 2015; Walker 2011). Wolbachia‐carrying Aedes mosquitoes can be periodically deployed into populated areas, either as adult mosquitoes or at the larval stage, where they mate with the wild population.
The population replacement strategy involves releasing both male and female Wolbachia‐carrying Aedes mosquitoes to pass Wolbachia on to Aedes offspring, meaning the prevalence of Wolbachia infection in the vector population continuously increases. As levels of Wolbachia transinfection increase, the capacity of the Aedes population to transmit arboviral infections such as DENV infection decreases, and the risk of disease outbreak also decreases. Conservative modelling estimates of wMel Wolbachia‐carrying Ae aegypti deployments in a large human population suggest that Wolbachia could lead to an immediate and long‐term reduction in dengue, nearing elimination (Dorigatti 2018). Currently, the World Mosquito Program facilitates dengue prevention programmes globally using the wMel Wolbachia‐carrying mosquito replacement strategy (www.worldmosquitoprogram.org).
The population suppression strategy involves releasing non‐biting male Wolbachia‐carrying mosquitoes, resulting in incompatible mating with uninfected females and a reduction in the mosquito population. This is the strategy used in Singapore (NEA 2022).
Why it is important to do this review
Dengue is a rapidly spreading mosquito‐borne disease with 60 million cases of infection recorded in 2019, an increase of 30 million since 1990 (Yang 2021). Although the incidence of the disease is growing rapidly in middle‐high sociodemographic index (SDI) regions, dengue remains the most prevalent and most fatal in low‐ and middle‐income countries (Yang 2021).
Vector control is an important component of dengue prevention programmes, and the WHO recommends integrated vector control strategies, including targeted residual spraying, larval control, and personal protective measures (WHO 2009). Most approaches are expensive and need teams that understand the characteristics of the vector and people in the local area (Knerer 2020; Ritchie 2021; Soh 2021). Methods that do not rely on insecticides are becoming more important, as resistance to all four classes of insecticide has been reported in Aedes arbovirus vectors in the Americas, Asia, and Africa (Moyes 2017). Effective integrated vector control is difficult to achieve in resource‐limited endemic countries. In urban centres, vector control strategies are hampered by urbanization, building design, and inadequate water supply management (Jansen 2010). The WHO encourages city planners, environmentalists, and engineers to work together in urban environmental mosquito control, but in practice this is difficult to implement (WHO 2022b).
Vaccines for long‐lasting protection against all four DENVs are in development following the success of a live‐attenuated vaccine against closely related Japanese encephalitis virus (Monath 2002). Dengvaxia (CYD‐TDV), developed by Sanofi‐Pasteur, was the first approved vaccine for dengue, licenced in 2015 for use in individuals aged nine to 45 years living in endemic areas, and currently approved in 20 countries (WHO 2018). Analyses of the long‐term safety of this vaccine have demonstrated inconsistent efficacy and safety in seropositive and seronegative individuals, with lower vaccine efficacy and increased risk of hospitalization and severe dengue in seronegative individuals (Hadinegoro 2015). These results have led to considerable vaccine hesitancy, particularly in the Philippines, which was the first and only country to introduce Dengvaxia to their public vaccination programme: after 830,000 children had received at least one dose, Philippine policymakers suspended the vaccine (Wilder‐Smith 2019). In 2017, a Strategic Advisory Group of Experts (SAGE) working group on dengue vaccines recommended that countries considering introducing a dengue vaccination programme should implement a prevaccine screening strategy to determine the serostatus of individuals and ensure only seropositive individuals are included in the programme (WHO 2018). As a result, the use of vaccines for dengue is currently limited in favour of alternative dengue prevention methods.
Researchers are exploring the possibility of using the endosymbiotic bacteria Wolbachia as an innovative dengue prevention strategy (www.worldmosquitoprogram.org). One analysis of early observational studies on wMel‐Wolbachia‐carrying Ae aegypti deployments conducted in Australia demonstrated a protective efficacy of more than 95% (95% confidence interval (CI) 84 to 99; 2 studies) against cases of dengue fever (DF; Cochrane Response 2021). One controlled interrupted time series study conducted in Indonesia also demonstrated an adjusted protective efficacy of 73% (95% CI 49 to 89) for monthly incidence of dengue haemorrhagic fever (DHF; Cochrane Response 2021).
Objectives
To assess the efficacy of Wolbachia‐carrying Aedes species deployments (specifically wMel‐, wMelPop‐, and wAlbB‐ strains of Wolbachia) for preventing dengue virus infection.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), including cluster‐RCTs (cRCTs), as they have the best trial design for evaluating the efficacy of interventions (Higgins 2022).
Types of participants
Adults and children living in endemic and epidemic‐prone areas where DENV infection was prevalent.
Types of interventions
Intervention
wMel‐, wMelPop‐, and wAlbB‐carrying Aedes deployments plus any existing local mosquito‐control measures. Any co‐interventions should have been balanced between the control and intervention arms. Based on existing evidence on stable Wolbachia infections in transinfected hosts, we only included studies investigating specific combinations of Wolbachia and Aedes, as outlined in Table 2.
Control
Existing local mosquito‐control measures, including individual‐, household‐, and community‐level interventions. Such interventions may have included, but were not limited to, education programmes, reduction in larval source habitats, insecticide spraying, Abate temephos, and bed net use.
Types of outcome measures
We planned to assess the outcome measures at all time points up to the longest follow‐up. We planned to group the time points as short‐term (up to 12 months after final deployment) and long‐term (more than 12 months after final deployment). The outcomes listed are outcomes of interest and were not used as criteria for study inclusion.
Primary outcomes
Epidemiological outcomes
Virologically confirmed dengue (VCD) case incidence (local, imported, or both) confirmed by reverse transcription polymerase chain reaction (RT‐PCR) or enzyme‐linked immunosorbent assay (ELISA)
Prevalence of DENV infection
Entomological outcomes
Prevalence of dengue RNA in the mosquito population
Mosquito density (for population suppression strategy)
Prevalence of Wolbachia‐carrying mosquitoes (for population replacement strategy)
Secondary outcomes
Epidemiological outcomes
Notified DF or DHF cases (suspected or confirmed, based on self‐reporting or clinical examination)
Entomological outcomes
Spatial distribution of Wolbachia‐infected mosquitoes
Insecticide resistance phenotypes
Clinical outcomes
All‐cause mortality
Hospitalizations due to DF or DHF
Adverse events potentially related to Wolbachia‐carrying Aedes deployments
Other outcomes (narrative description)
Community acceptability
Cost and resources
Search methods for identification of studies
We identified all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). We included studies published from 2009 (as this was the year that wMel‐Wolbachia was first successfully transferred to Ae aegypti mosquitoes (Walker 2011)) up to 24 January 2024.
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL) published in the Cochrane Library, Issue 1, 2024
MEDLINE (Ovid, from 1946 to 23 January 2024)
Embase (Ovid, from 1947 to 24 January 2024)
Web of Science (Clarivate): Science Citation Index‐Expanded, 1900 to 24 January 2024; Conference proceedings citation index Science, 1990 to 24 January 2024; CAB Abstracts, 1910 to 24 January 2024
CINAHL (EBSCOhost, 1937 to 24 January 2024)
LILACS (BIREME, 1982 to 24 January 2024)
We also searched the WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch), and ClinicalTrials.gov (clinicaltrials.gov/ct2/home) for trials in progress, on 24 January 2024.
Searching other resources
We checked the reference lists of systematic reviews and included studies to identify additional references.
Conference proceedings
We searched the proceedings of the Global Sustainable Technological and Innovation (G‐STIC) conference from 2018 to 2023.
Data collection and analysis
Selection of studies
We used standard Cochrane methods for selecting studies (Higgins 2022). Two review authors (TF, YS) independently screened titles and abstracts of identified records, eliminating those considered clearly ineligible. We retrieved the full‐text articles of the remaining records and independently assessed them against predefined criteria. We resolved discrepancies by discussion or by involving a third review author, if necessary.
Data extraction and management
Two review authors (TF and WR or DD) independently extracted data using a standardized piloted data extraction form. We planned to contact the study authors to obtain missing data. At each step of data extraction, we resolved any discrepancies through discussion between the review authors.
We extracted the following information.
General information: author, title, publication date, country, study date(s), study location (urban/rural), baseline endemicity of dengue, funding details, conflicts of interest
Study characteristics: aim, unit of allocation, number of units, adjustment for clustering, length of follow‐up
Participants: number of participants, method of recruitment, withdrawal or loss to follow‐up, age, sex, socio‐economic status
Intervention: mosquito life stage (egg, larva, adult), number of deployments, timing/frequency of deployments, location of deployments, aimed percentage vector population replacement, achieved percentage vector population replacement, field monitoring strategies, co‐interventions (e.g. insecticide spraying, bed net use, larvicide control)
Comparator: description of local vector control strategies in place
Outcome(s): primary outcome(s), secondary outcome(s)
Assessment of risk of bias in included studies
We assessed the risk of bias of the included studies using the Cochrane RoB 2 tool (Higgins 2022; Sterne 2019). To assess individually randomized trials, we planned to use the RoB 2 Excel tool (available at www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2); for cRCTs, we used the modified tool with an additional domain for assessing bias arising from randomization of clusters (www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials). The effect of interest was the effect of assignment at baseline, regardless of whether the interventions were received as intended (the 'intention‐to‐treat effect'). We planned to assess risk of bias for all outcomes specified in the Primary outcomes section which contribute to the review's summary of findings table.
Two review authors (TF, IAR) independently assessed the risk of bias in all specified results. We resolved any disagreements through discussion with a third review author (GV).
The RoB 2 tool considers the following domains.
Bias arising from the randomization of clusters (for cRCTs only)
Bias arising from the randomization of participants
Bias due to deviations from the intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
We used the recommended signalling questions to assess the RoB 2 domains, responding 'yes', 'probably yes', 'probably no', 'no', or 'no information'. We used the RoB 2 algorithm to reach an overall risk of bias judgement ('low risk of bias', 'some concerns', or 'high risk of bias') for each domain.
We reached an overall risk of bias judgement for a specific outcome by combining the judgements for all domains. Any study with low risk of bias for all domains achieved an overall low risk of bias judgement; some overall concerns were assumed when at least one domain had some concerns, and studies with a high risk of bias for at least one domain obtained an overall high risk of bias judgement (Higgins 2022).
We stored the full RoB 2 data, which is available on request.
Measures of treatment effect
For dichotomous outcomes, we planned to use the risk ratio (RR) with the corresponding 95% CIs as the effect measure. The primary analyses of the included study reported odds ratios (OR) that were adjusted for clustering, so we used this instead. The authors did report an RR from a cluster‐level analysis, but we chose to use the ORs as these were available for all outcomes and subgroups, whereas the RR was only available for one outcome, and was from an additional analysis that was conducted to supplement the primary analysis. For count/rate outcomes, we planned to use the rate ratio with 95% CI as the effect measure.
Unit of analysis issues
For cRCTs, we extracted measures of effect that were adjusted for clustering where possible. If the study authors had not performed any adjustments for clustering, we would have adjusted the raw data using an intraclass correlation coefficient (ICC) value. If the study had reported no ICC value, we would have requested this information from the study authors, obtained it from similar studies, or estimated it ourselves. If we had estimated the ICC, we would have performed sensitivity analyses to investigate the robustness of our results. We would not have presented results from cRCTs that were not adjusted for clustering.
If we had identified multi‐arm trials, we would have selected relevant arms for inclusion in our analyses. If more than two arms were relevant to this review, we would have either combined intervention arms so that there was one comparison, or split the control group between multiple comparisons to avoid double‐counting of participants in meta‐analyses.
Dealing with missing data
We planned to contact study authors to obtain missing study characteristics, outcomes, summary data, and individual data.
We assessed the risk of reporting bias due to missing studies and missing outcomes as described in the Assessment of reporting biases section.
If we had been unable to obtain missing summary data, we would have calculated or estimated the required data from other reported statistics using formulas specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
If we had been unable to obtain missing individual data, we would have taken this into account when assessing the risk of bias (Higgins 2022; Sterne 2019). In the first instance, we would have conducted a complete‐case analysis, and we may have performed sensitivity analyses to investigate the impact of missing data.
Assessment of heterogeneity
We planned to assess the extent of clinical and methodological heterogeneity by examining study characteristics (e.g. region, severity of clinical disease, insecticide resistance, dengue serotype, mosquito fitness, retention of cytoplasmic incompatibility).
We planned to present results of meta‐analyses in forest plots, which we would have inspected visually to assess statistical heterogeneity (non‐overlapping CIs generally signify statistical heterogeneity). We planned to use the Chi² test with a P value less than 0.1 to indicate statistical heterogeneity. We planned to quantify heterogeneity using the I² statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error. We planned to interpret this statistic using the following guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneitya
50% to 90%: may represent substantial heterogeneitya
75% to 100%: considerable heterogeneitya
aThe importance of the observed I² value depends on the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test, or a CI for the I² statistic: uncertainty in the value of the I² statistic is substantial when the number of studies is small).
Since we only included one study, we could not perform these assessments.
Assessment of reporting biases
We searched for ongoing trials that meet our eligibility criteria and classified them as 'ongoing' until they are published.
If we included 10 studies in a meta‐analysis, we planned to explore the possibility of small‐study biases (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) for the primary outcomes using funnel plots. In the case of asymmetry, we planned to consider various explanations such as publication bias, poor study design, and the effect of study size. Since only one study was included, we did not perform this analysis.
Data synthesis
We analyzed data using Review Manager (RevMan 2023), using random‐effects models in all cases. Where we considered meta‐analysis to be inappropriate due to important clinical, methodological, or statistical heterogeneity, we planned to summarize data in tables.
Subgroup analysis and investigation of heterogeneity
We intended to conduct subgroup analysis to explore whether the following characteristics constitute sources of heterogeneity in the meta‐analysis.
Endemicity (endemic versus epidemic‐prone)
Age (children under 18 years versus adults 18 years and older)
If there was still substantial unexplained heterogeneity (defined in Assessment of heterogeneity), we may have explored the following characteristics.
Region
Severity of clinical disease
Insecticide resistance
Dengue serotype
Mosquito fitness
Retention of cytoplasmic incompatibility
Sensitivity analysis
We planned to perform sensitivity analyses to investigate the impact of missing data; however, the included study reported data for all included participants. For example, we could have varied the event rate for missing participants from intervention and control groups within plausible limits, or we could have excluded studies thought to be at high risk of attrition bias from our meta‐analyses.
If we had estimated the ICC to adjust data from cRCTs for clustering, we would have performed sensitivity analyses to investigate the robustness of our results.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in a summary of findings table, rating the certainty of evidence according to the current GRADE guidance as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Two review authors (TF, GV) independently assessed the certainty of the evidence, considering the risk of bias, inconsistency, imprecision, indirectness, and publication bias.
The summary of findings table was planned to include the following outcomes.
VCD case incidence (local, imported, or both) confirmed by RT‐PCR or ELISA
Prevalence of DENV infection
Prevalence of dengue RNA in the mosquito population
Mosquito density (for population suppression strategy)
Prevalence of Wolbachia‐carrying mosquitoes (for population replacement strategy)
Adverse effects potentially related to Wolbachia‐carrying Aedes deployments
Results
Description of studies
Results of the search
We identified 451 potentially relevant articles through our comprehensive search strategy. After removing duplicates, we screened 159 articles at title and abstract level, of which we considered 31 for full‐text screening (Figure 1). Of these, 13 articles met our inclusion criteria, comprising three cRCTs (Collins 2022; Ong 2022; Utarini 2021), and 10 articles describing additional outcomes of interest relevant to these trials. One of these trials was complete with results (Utarini 2021), and the characteristics are described in the Characteristics of included studies table. The remaining two trials are ongoing, and the characteristics are found in Characteristics of ongoing studies table.
1.
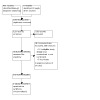
PRISMA diagram.
We excluded 12 articles due to ineligible study design, specifically not having randomized participants or not including a control group, and six articles were duplicates. We have listed the studies excluded at the full‐text stage in the Characteristics of excluded studies table.
Included studies
We included one study (Utarini 2021).
Trial design and location
Utarini 2021 is a cRCT conducted in Yogyakarta, Indonesia between 2018 and 2020. The trial adopted a test‐negative design.
Interventions
The trial investigated the efficacy of deployments of Ae aegypti mosquitoes infected with the wMel strain of Wolbachia using the population replacement strategy (Utarini 2021; Table 3).
2. Dengue control interventions.
| Trial | Mosquito species | Wolbachia strain | Dengue control strategy |
| Utarini 2021 | Ae aegypti | wMel | Population replacement |
| Collins 2022 (ongoing trial) | Ae aegypti | wMel | Population replacement |
| Ong 2022 (ongoing trial) | Ae aegypti | wAlbB | Population suppression |
Participants
Utarini 2021 included 6306 participants enrolled via primary care clinics, whereby cases and controls were identified by laboratory test results. All people presenting at the clinics were eligible, with a median age of 11.6 years (interquartile range (IQR) 7.0 to 21.1), and 48.7% of participants were female.
Outcomes
The primary outcome was symptomatic VCD of any severity caused by any DENV serotype. Other outcomes included symptomatic VCD caused by each of the four DENV serotypes (DENV‐1, DENV‐2, DENV‐3, and DENV‐4), prevalence of Wolbachia‐carrying mosquitoes, all‐cause mortality, hospitalizations due to DF or DHF, and insecticide resistance phenotypes of Ae aegypti.
Excluded studies
We excluded 18 articles at full‐text screening for the following reasons:
12 had ineligible study design;
6 were duplicates.
Ongoing studies
We identified two ongoing studies (Collins 2022; Ong 2022). The interventions are outlined in Table 3.
Risk of bias in included studies
Overall risk of bias
We assessed methodological and risk of bias for one cRCT contributing results to our outcomes using the RoB 2 for cRCTs tool. The study contributed results to two primary outcomes, 'Prevalence of DENV infection' and 'Prevalence of Wolbachia‐carrying mosquitoes'.
The study authors reported the median cluster‐level prevalence of wMel Wolbachia‐carrying mosquitoes across the 27‐month trial period in the intervention clusters, but did not report any numerical values for prevalence of wMel Wolbachia‐carrying mosquitoes in the control clusters. The authors did provide graphs which showed the prevalence of wMel Wolbachia‐carrying mosquitoes in intervention and control clusters across the 27‐month trial period, but we did not consider that the graphs were of sufficient resolution to allow for reliable data digitization. Therefore, we did not calculate an effect estimate for this outcome, or assess the risk of bias for this outcome.
However, we did access a digitized version of these data from an online repository by an independent researcher to allow us to calculate the median cluster‐level prevalence of wMel Wolbachia‐carrying mosquitoes across the 27‐month trial period in the control clusters (Cavany 2022). We present these in the review to allow an informal comparison of prevalence between the two arms of the study. We contacted study authors to obtain full prevalence data but received no response.
We assessed the outcome 'Prevalence of DENV infection' using RoB 2 for cRCTs (Higgins 2022). The risk of bias judgements are summarized below and presented in Table 8. Detailed risk of bias assessments are available on request.
Risk of bias for analysis 1.1 All serotypes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.1.1 All serotypes | ||||||||||||
| Utarini 2021 | Low risk of bias | Allocation sequence random and concealed. Imbalances in baseline characteristics appeared compatible with chance. | Low risk of bias | Unblinded study, but since participants were recruited upon presentation with symptoms, it is unlikely that this would affect the results. No deviations from intended interventions occurred. | Low risk of bias | No missing outcome data. | Low risk of bias | Appropriate measurement of the outcome performed with participants classified as having VCD if plasma sample obtained at enrollment was positive for dengue virus by reverse transcription polymerase chain reaction (RT‐PCR) or enzyme‐linked immunosorbent assay (ELISA). | Low risk of bias | Outcomes were reported as per the published protocol and prospective trial registry. | Low risk of bias | Domain 1b: risk of bias arising from the timing of identification or recruitment of participants was also judged to be at low risk of bias. Randomization was performed after community‐level consent to participate to minimize the likelihood that a cluster declines the deployments. The test‐negative design used means individual participants were not formally recruited. Overall low risk of bias. |
Overall risk of bias by outcome
Prevalence of dengue virus infection
We judged Utarini 2021 at overall low risk of bias for prevalence of DENV infection.
Effects of interventions
See: Table 1
Primary outcomes
We presented the primary outcomes in Table 1.
Epidemiological outcomes
Virologically confirmed dengue case incidence
The study did not report VCD case incidence (local, imported, or both) confirmed by RT‐PCR or ELISA.
Prevalence of dengue virus infection
Utarini 2021 reported the prevalence of VCD caused by any DENV serotype, confirmed by either RT‐PCR assay or ELISA for DENV non‐structural protein 1 (NS1) antigen. Across the 27‐month enrolment period, 6306 participants residing in trial clusters presented at 18 primary care clinics and were screened. Out of 2905 participants residing in the wMel‐carrying Ae aegypti deployment area there were 67 cases of DENV infection (2.3%), whilst in the control population of 3401 participants, there were 318 cases reported (9.4%) (OR 0.23, 95% CI 0.15 to 0.35; moderate‐certainty evidence; Analysis 1.1). There was a protective effect across all DENV serotypes (Analysis 2.1). The participant numbers reported in each subgroup were low, and wide CIs reflect this.
1.1. Analysis.

Comparison 1: Prevalence of dengue virus infection, Outcome 1: All serotypes
2.1. Analysis.

Comparison 2: Prevalence of dengue virus infection – subgroup analysis, Outcome 1: Subgroup investigations – dengue virus (DENV) serotype
Entomological outcomes
Prevalence of dengue ribonucleic acid in the mosquito population
The study did not report the prevalence of dengue RNA in the mosquito population.
Mosquito density (for population suppression strategy)
The study did not report mosquito density (for population suppression strategy).
Prevalence of Wolbachia‐carrying mosquitoes
Utarini 2021 recorded the median cluster‐level prevalence of wMel Wolbachia‐carrying mosquitoes across the 27‐month trial period in the intervention clusters, which was reported as 95.8% (interquartile range 91.5 to 97.8).
The study authors did not report any numerical values for prevalence in the control clusters, but did present graphical data for cluster‐level prevalence of wMel Wolbachia‐carrying mosquitoes for the control clusters in Figure 2 of their report. We did not receive a response to our request for the raw data for this outcome from the study authors. However, based on data extrapolated from Figure 2 in the study by an independent researcher (accessed via their repository, Cavany 2022), we calculated the median cluster‐level prevalence of wMel Wolbachia‐carrying mosquitoes in the control clusters. This prevalence was around 11%, compared with a monthly median cluster‐level wMel prevalence of 95.8% (IQR 91.5 to 97.8) during the 27 months of clinical surveillance in the intervention clusters.
Secondary outcomes
Epidemiological outcomes
Notified dengue fever or dengue haemorrhagic fever cases
The study did not report notified DF or DHF cases (suspected or confirmed, based on self‐reporting or clinical examination).
Entomological outcomes
Spatial distribution of Wolbachia‐infected mosquitoes
The study did not report spatial distribution of Wolbachia‐infected mosquitoes.
Insecticide resistance phenotypes
A sub‐study of Utarini 2021 (see Tantowijoyo 2022 under Utarini 2021 as primary reference) reported the insecticide resistance phenotypes of Ae aegypti in the first and second years after wMel‐deployments in the trial area. They reported no differences in the susceptibility of resistance to the following insecticides from the intervention clusters compared to the untreated clusters in either 2018 or 2019: cyfluthrin (0.15%) (2018: P = 0.16; 2019: P = 0.39), malathion (0.8%) (2018: P = 1.00; 2019: P = 0.77), or permethrin (1.25%) (2018: P = 0.71; 2019: P = 0.73).
Clinical outcomes
All‐cause mortality
Utarini 2021 reported no deaths within 21 days after enrolment in either group.
Hospitalizations due to dengue fever or dengue haemorrhagic fever
Utarini 2021 reported the number of hospitalizations associated with VCD in the population that presented with fever to local clinics, but did not specify if these were cases of DF or DHF. In the control population, there were 102 hospitalizations out of 3401 participants who presented with fever (3%), whilst in the Wolbachia deployment residing population there were 13 hospitalizations amongst 2905 participants who presented with fever (0.4%) (OR 0.14, 95% CI 0.06 to 0.34; Analysis 3.1).
3.1. Analysis.

Comparison 3: Hospitalizations due to dengue fever (DF) or dengue haemorrhagic fever (DHF), Outcome 1: Hospitalizations due to DF or DHF
Adverse events potentially related to Wolbachia‐carrying Aedes deployments
The study did not report adverse events potentially related to Wolbachia‐carrying Aedes deployments.
Other outcomes
Community acceptability
The study did not report community acceptability.
Cost and resources
The study did not report cost and resources.
Subgroup analyses
We could not perform planned subgroup analyses by endemicity and age since we identified only one study (see Subgroup analysis and investigation of heterogeneity).
Discussion
Summary of main results
The included trial investigated the effect of wMel‐carrying Ae aegypti egg deployments on preventing dengue infection, with the aim of replacing the uninfected mosquitoes with Wolbachia‐carrying mosquitoes and achieving greater than 60% population replacement. The results demonstrated that wMel‐Wolbachia‐infected Ae aegypti deployments probably result in a protective efficacy of 77% (95% CI 65 to 85) for VDC prevalence (OR 0.23, 95% CI 0.15 to 0.35; 1 trial, 6306 participants), which was consistent across all DENV serotypes.
The cluster‐level prevalence of wMel Wolbachia‐carrying mosquitoes remained high over a two‐year period, reported as 95.8% (IQR 91.5 to 97.8) across 27 months in clusters receiving wMel‐Ae aegypti deployments.
These deployments may also reduce the number of hospitalizations due to DF (protective efficacy 85%, 95% CI 73 to 99; 1 trial, 6306 participants).
Overall completeness and applicability of evidence
This Cochrane review summarizes evidence on the effects of wMel Wolbachia deployments on dengue prevalence from one cRCT conducted in the urban setting of Yogyakarta, Indonesia. Outcomes were assessed over a 27‐month period, evidencing the potential lasting impact of Wolbachia releases. The 6306 included participants were aged three to 45 years, with a median age of 11.6 years. The study utilized a nested test‐negative study design, with results that indicate a significant reduction in the prevalence of VCD and hospitalizations due to dengue in areas where wMel Wolbachia‐carrying Aedes have been deployed.
Due to the test‐negative design, these results are based on the population that presented with fever at local clinics, rather than the whole population residing in the study area or the population with confirmed dengue infection. As a result, it is possible that the results may not be comparable to future RCTs that do not adopt this study design, although the implications and benefits of this study design are discussed in the protocol for Utarini 2021 (included in the study Appendix). Nonetheless, this study demonstrated a significant reduction in VCD prevalence in areas with Wolbachia releases in this study setting.
An independent study investigated the potential effect of human movement, mosquito movement, and coupled transmission dynamics on the DENV case prevalence estimates in this trial using mathematical models (Cavany 2023). The report suggested biases arising from these three factors had not been appropriately mitigated in the trial, which may suggest inaccuracy in the reported result. In this regard, it is suggested that the number of DENV cases during the study period may have been underestimated.
Due to the lack of studies meeting our inclusion criteria, we were unable to conduct a pooled analysis. Our review aimed to assess evidence from all dengue vectors that have been evidenced to achieve stable transinfection with Wolbachia including Ae albopictus, Ae aegypti, and Ae polynesiensis, which we described in Description of the intervention. The included trial was limited to assessing Ae aegypti infected with wMel and utilized the population replacement strategy. We will be able to compare these results with one ongoing trial in Brazil in a future review update (Collins 2022). Another ongoing trial will allow us to investigate the effect of the population suppression strategy with wAlbB‐infected Ae aegypti on preventing dengue infection (Ong 2022). It will be important to assess between‐trial heterogeneity as new trials are published. Indeed, a non‐randomized study in Brazil reported a median wMel prevalence of 40% to 70% in some areas in which the infected mosquitoes had been released, which is lower than in the trial included in this report (Pinto 2021).
Since different approaches are applicable to different global settings, it is also important to assess the full range of approaches in order to elucidate the potential global impact of this dengue prevention strategy. Differences between the population replacement and population suppression strategies may be revealed in terms of levels of acceptability to participants, so it is important that qualitative outcomes are also captured in future trials. We planned to summarize data on cost and resources in this review, but did not identify any data on this outcome. It is important to know what costs are associated with this intervention, including those that may be required for acceptability campaigns surrounding the intervention. We identified limited data on potential harms related to the intervention, with only insecticide resistance reported. It will be important to identify other harms in terms of the human population (e.g. adverse events) or the mosquito population (e.g. genome evolution).
Certainty of the evidence
We assessed the certainty of evidence of the effect estimates for the primary outcomes in the included cRCT using the GRADE approach (Table 1).
wMel‐Wolbachia‐carrying Ae aegypti mosquitoes probably reduce the prevalence of VCD (moderate‐certainty evidence). The least optimistic effect of a 65% reduction in the odds of VCD is an important effect. The certainty of the evidence was downgraded due to indirectness, as the results seen in this study may not be applicable to other settings with different weather conditions and entomological contexts.
We were unable to extract reliable data on the prevalence of Wolbachia‐carrying mosquitoes in the control arm of the trial from the study report. Therefore, we did not calculate an effect estimate for this primary outcome, and we could not assess the risk of bias or the certainty of the evidence. Based on data obtained from an independent online repository, we calculated a median cluster‐level prevalence of Wolbachia‐carrying mosquitoes in the control areas which is reported in Effects of interventions, but due to the low resolution of graphs presented in the study report, we do not believe that these data are sufficiently reliable to calculate an effect estimate for this outcome. As a result, we have not reported these data within Table 1.
Potential biases in the review process
We used a comprehensive search strategy, and we assessed search results for eligibility irrespective of date of publication, publication status, or language. Two review authors independently screened search results, extracted data from included studies, and assessed the risk of bias. For this review, we focussed on strains of Wolbachia that have been demonstrated to stably transinfect vectors of DENV with a potential effect on dengue transmission; however, studies may be available investigating other strains of Wolbachia that are not included in this review. Our decision to restrict the inclusion criteria to RCTs in this review may limit our ability to identify rare adverse events associated with Wolbachia deployments.
Agreements and disagreements with other studies or reviews
We identified one systematic review via PROSPERO, which was registered in August 2021 and published in 2022 (Nordin 2022). This review included two studies from dengue‐endemic areas, and two from non‐endemic areas. Three of these studies did not meet our inclusion criteria as one was non‐randomized (Nazni 2019), and two were uncontrolled experimental studies (O'Neill 2018; Ryan 2019). The review reported long‐term establishment of Wolbachia in the mosquito population across all four studies, although it fell below 90% in a study from Kuala Lumpur (Nazni 2019). The Kuala Lumpur study also showed a 40% reduction in dengue cases in the intervention sites, compared to the 77% reduction in Utarini 2021. The review was consistent with our own in recognizing the limitations of the current evidence, including that we do not yet know how applicable these findings will be to other settings, or indeed on a larger scale of implementation (Nordin 2022).
Authors' conclusions
Implications for practice.
The included trial provides evidence of the potential substantial impact of wMel‐carrying Ae aegypti mosquitoes on preventing dengue infection in an endemic setting, and supports the previous evidence reported in non‐randomized and uncontrolled studies.
Implications for research.
This dengue prevention strategy may present a sustainable, long‐term solution for dengue‐endemic settings, and future trials will facilitate further investigations of this strategy, allowing us to determine whether these results can be replicated across a variety of global settings. It will be important to elucidate community views on this intervention, and ascertain the costs involved with this strategy to determine the long‐term success. It is also important to compare the efficacy of different strains of Wolbachia in the real‐world setting to determine if results from field‐based experiments can be extended into dengue‐endemic communities.
What's new
| Date | Event | Description |
|---|---|---|
| 22 April 2024 | Amended | Small wording error in the PLS fixed |
History
Protocol first published: Issue 3, 2023 Review first published: Issue 4, 2024
| Date | Event | Description |
|---|---|---|
| 16 April 2024 | Amended | Formatting issue fixed |
| 15 April 2024 | Amended | Formatting issue fixed |
Risk of bias
Acknowledgements
TF and MC are supported by the Research, Evidence and Development Initiative (READ‐It) project. The Cochrane Infectious Diseases Group (CIDG) editorial base and READ‐It are funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK Government's official policies.
We are grateful to Vittoria Lutje, CIDG Information Specialist, for help with the literature search strategy and running the literature searches.
The CIDG supported the authors in the development of this Cochrane intervention review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Tari Turner, School of Public Health & Preventive Medicine, Cochrane Australia
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Samuel Hinsley, Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments and supported editorial team): Jacob Hester, Central Editorial Service
Copy Editor (copy editing and production): Anne Lawson, Cochrane Central Production Service
-
Peer‐reviewers (provided comments and recommended an editorial decision)
Kathryn Edenborough, Department of Microbiology, Biomedicine Discovery Institute, Monash University, Clayton, VIC 3800, Australia (clinical/content review)
Sean Cavany, University of Oxford (clinical/content review)
Dr Rakesh Mishra, Department of Neurosurgery, All India Institute of Medical Sciences, Bhopal, India (consumer review)
Jennifer Hilgart, Cochrane (methods review)
Yuan Chi, Beijing Yealth Technology Co, Ltd; McMaster University (search review)
Appendices
Appendix 1. Search strategies
Ovid MEDLINE(R) and In‐Process, In‐Data‐Review & Other Non‐Indexed Citations (1946 to 23 January 2024)
1 Dengue Virus/
2 exp Dengue/
3 dengue.tw, kf.
4 DENV*.tw,kf.
5 1 or 2 or 3 or 4
6 Aedes/
7 aedes.tw,kf.
8 mosquito*.tw,kf.
9 (dengue adj2 vector*).tw,kf.
10 6 or 7 or 8 or 9
11 5 and 10
12 Wolbachia/
13 wolbachia.tw,kf.
14 (Wmel or wMelPop or wAlbB ).tw.
15 12 or 13 or 14
16 11 and 15
17 Randomized Controlled Trial.pt.
18 controlled clinical trial.pt
19 (randomized or placebo or randomly or trial or groups).ab.
20 drug therapy.fs
21 17 or 18 or 19 or 20
22 exp animals/ not humans/
23 21 not 22
24 16 and 23
CENTRAL
Issue 1, 2024
#1 MeSH descriptor: [Dengue] explode all trees
#2 MeSH descriptor: [Dengue Virus] explode all trees
#3 (Dengue or DENV*):ti,ab,kw
#4 #1 or #2 or #3
#5 (aedes or mosquito* or vector*):ti,ab,kw
#6 #4 and #5
#7 Wolbachia or Wmel:ti,ab,kw
#8 #6 and #7
Embase (1947 to 24 January 2024), updated daily
1 exp dengue/
2 Dengue virus/
3 (Dengue or DENV*).mp.
4 1 or 2 or 3
5 (aedes or mosquito* or vector*).ti,ab.
6 Aedes/
7 5 or 6
8 4 and 7
9 Wolbachia/ or wolbachia.mp.
10 Wmel*.mp.
11 9 or 10
12 8 and 11
13 (random* or factorial* or placebo* or assign* or allocat* or crossover*).tw.
14 ((blind* or mask*) and (single or double or triple or treble)).tw.
15 crossover procedure/
16 double blind procedure/ or single blind procedure/
17 randomization/ or placebo/
18 parallel design/ or Latin square design/
19 randomized controlled trial/
20 controlled clinical trial/
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22 12 and 21
Science Citation Index‐Expanded; Conference Proceedings Citation Index; Emerging Sources Citation Index; CABI: CAB Abstracts; all from Web of science (Clarivate)
#3 Search: #1 AND #2
#2 Search: random* or "controlled trial" or double blind* or single blind* (Topic)
#1 Search: Dengue or DENV* (Topic) AND aedes or vector* or mosquito* (Topic) AND Wolbachia or Wmel (Topic) Editions: WOS.SCI,WOS.ISTP,WOS.ESCI
CINAHL EBSCOhost Research Databases
| # | Query |
| S3 | S1 AND S2 |
| S2 | TX random* or trial or compar* or evaluat* or double‐blind* or single‐blind* |
| S1 | TX ( dengue or DENV* ) AND TX ( aedes or mosquito* or vector* ) AND TX ( wolbachia or Wmel* ) |
ClinicalTrials.gov
wolbachia | Dengue
WHO ICTRP
Dengue and (wolbachia or Wmel)
LILACS
Search on: dengue or aedes [Words] and wolbachia or Wmel [Words] and randomized or controlled or trial [Words]
Data and analyses
Comparison 1. Prevalence of dengue virus infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All serotypes | 1 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 All serotypes | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.23 [0.15, 0.35] |
Comparison 2. Prevalence of dengue virus infection – subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Subgroup investigations – dengue virus (DENV) serotype | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.24 [0.18, 0.32] | |
| 2.1.1 DENV‐1 serotype | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.29 [0.10, 0.83] | |
| 2.1.2 DENV‐2 serotype | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.16 [0.09, 0.28] | |
| 2.1.3 DENV‐3 serotype | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.25 [0.06, 0.95] | |
| 2.1.4 DENV‐4 serotype | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.26 [0.17, 0.41] | |
| 2.1.5 Unknown serotype | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.29 [0.17, 0.49] |
Comparison 3. Hospitalizations due to dengue fever (DF) or dengue haemorrhagic fever (DHF).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Hospitalizations due to DF or DHF | 1 | 6306 | Odds Ratio (IV, Fixed, 95% CI) | 0.14 [0.06, 0.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Utarini 2021.
| Study characteristics | |
| Methods | Status: completed Aim: to assess the efficacy of deployments of Ae aegypti mosquitoes infected with the wMel strain of Wolbachia in reducing the prevalence of VCD in Yogyakarta, Indonesia. Study type: cRCT Study dates: 8 January 2018 to 5 May 2020 Country, location: Indonesia, urban Unit of allocation: cluster Number of units: 24 Length of follow‐up: 27 months |
| Participants | Number of participants: 6306; 2905 intervention, 3401 control Method of recruitment: screening at primary care clinics Loss to follow‐up: 222 intervention, 222 control (could not be contacted) Age (median): 11.6 years (IQR 7.0 to 21.1), 22% aged < 15 years Sex (% female): 48.7 Socio‐economic status: N/I |
| Interventions |
Wolbachia species: wMel Mosquito species: Ae aegypti Mosquito life stage at release: egg Strategy: population replacement Number of deployments: 9–14 rounds per cluster (mean 22,000–34,000 mosquitoes released per round) Timing of deployments: every 2 weeks between March and December 2017 Location of deployments: mosquito release containers placed outside houses, protected from sun and rain, at 1 or 2 randomly selected locations within each 50 × 50 m2 grid across the intervention area Aimed % vector population replacement: deployments stopped in a cluster when wMel prevalence was > 60% in the mosquito population for > 3 weeks Achieved % vector population replacement: monthly median cluster‐level wMel prevalence 95.8% (IQR 91.5 to 97.8) Field monitoring strategy: prevalence of wMel in Ae aegypti population measured weekly using BG Sentinel traps for adult mosquito collection, screened for wMel Wolbachia by qualitative PCR Taqman assay Co‐interventions: routine mosquito control measures, N/I |
| Outcomes | Primary outcome
Secondary outcomes
|
| Notes | Funding source: Tahija Foundation, Wellcome Trust, and the Bill and Melinda Gates Foundation. |
BG: Biogents; cRCT: cluster‐randomized controlled trial; DENV: dengue virus; IQR: interquartile range; N/I: no information; PCR: polymerase chain reaction; VCD: virologically confirmed dengue.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gesto 2021 | Ineligible study design – not randomized |
| Lozano 2022 | Ineligible study design – not randomized |
| Lwin 2022 | Ineligible study design – modelling the effect of public hesitancy on project success |
| Mains 2016 | Ineligible study design – not randomized |
| Nazni 2019 | Ineligible study design – not randomized |
| NCT03631719 | Ineligible study design – not randomized |
| Nguyen 2015 | Ineligible study design – not randomized or controlled |
| Ribeiro dos Santos 2022 | Ineligible study design – not randomized or controlled |
| Zeng 2022 | Ineligible study design – not randomized |
Characteristics of ongoing studies [ordered by study ID]
Collins 2022.
| Study name | EVITA Dengue |
| Methods | Status: ongoing Aim: to determine the effectiveness of wMel mosquitoes in reducing the transmission of DENV, ZIKV, and CHIKV as indicated by the annual incidence of infection by these arboviruses Study type: cRCT Study dates: September 2020 to January 2025 Country, location: Brazil, urban Unit of allocation: community Number of units: 58 Length of follow‐up: ongoing |
| Participants | Number of participants: 3480 Method of recruitment: enrolled via eligible elementary schools Loss to follow‐up: N/I ongoing Age: 6–11 years at enrollment Sex: N/I ongoing Socio‐economic status: N/I ongoing |
| Interventions |
Wolbachia species: wMel Mosquito species: Ae aegypti Mosquito life stage at release: adult Strategy: population replacement Number of deployments: 5.5 mosquitoes/person/week Timing of deployments: 16‐week establishment phase, then 16‐week consolidation phase Location of deployments: N/I ongoing Aimed % vector population replacement: > 60% population replacement Achieved % vector population replacement: N/I ongoing Field monitoring strategy: N/I ongoing Co‐interventions: standard mosquito control for the area |
| Outcomes | Primary outcome: dengue infection incidence Secondary outcome: Wolbachia population replacement |
| Starting date | 28 July 2022 |
| Contact information | Lee Ching Ng E‐mail: NG_Lee_Ching@nea.gov.sg |
| Notes |
Ong 2022.
| Study name | Project Wolbachia – Singapore |
| Methods | Status: ongoing Aim: to determine whether large‐scale deployment of Wolbachia‐infected male Ae aegypti mosquitoes can reduce the incidence of dengue in individuals living in intervention clusters, compared to individuals living in non‐intervention clusters. Study type: cRCT Study dates: randomization February 2022, cRCT started July 2022, completion date December 2024 Country, location: Singapore Unit of allocation: community block Number of units: 15 Length of follow‐up: study duration expected to be 24 months |
| Participants | Number of participants: N/I ongoing Method of recruitment: N/I ongoing Loss to follow‐up: N/I ongoing Age: N/I ongoing Sex: N/I ongoing Socio‐economic status: N/I ongoing |
| Interventions |
Wolbachia species: wAlbB Mosquito species: Ae aegypti Mosquito life stage at release: adult Strategy: population suppression Number of deployments: N/I ongoing Timing of deployments: twice per week, weekdays between 06:30 and 11:00 hours and 13:00 to 18:00 hours Location of deployments: equally spaced release locations per apartment block, on the ground, middle (levels 5 or 6), and high floors (levels 10 or 11). Aimed % vector population suppression: N/I ongoing Achieved % vector population suppression: N/I ongoing Field monitoring strategy: gavitraps. 6–9 Gravitraps per high‐rise apartment block designed to lure and trap gravid female Aedes Co‐interventions: N/I ongoing |
| Outcomes | Primary outcomes
Secondary outcome
|
| Starting date | 9 September 2020 |
| Contact information | Srilatha Edupuganti E‐mail: sedupug@emory.edu |
| Notes |
CHIKV: chikungunya virus; cRCT: cluster‐randomized controlled trial; DENV: dengue virus; N/I: no information; ZIKV: zika virus.
Differences between protocol and review
In the protocol, we planned to assess the risk of bias and conduct a GRADE assessment on the following primary outcomes (Fox 2023).
Epidemiological outcomes
Virologically confirmed dengue (VCD) case incidence (local, imported, or both) confirmed by reverse transcription polymerase chain reaction (RT‐PCR) or enzyme‐linked immunosorbent assay (ELISA)
Prevalence of DENV infection
Entomological outcomes
Prevalence of dengue DNA in the mosquito population
Mosquito density (for population suppression strategy)
Prevalence of Wolbachia‐carrying mosquitoes (for population replacement strategy)
In the review, we determined that the entomological outcomes may reasonably not be assessed or reported in the control arm of included studies since entomological changes are not expected, and may be monitored via other methods. Since data on the prevalence of Wolbachia‐carrying mosquitoes in the control clusters of our included study could not be reliably digitized (Utarini 2021), we could not assess the risk of bias or perform the GRADE assessment for this outcome.
We did not plan to report the outcome of 'Insecticide resistance phenotypes' when planning the protocol. However, this is an important outcome that was reported in the included study; therefore, we have included it in this review.
Contributions of authors
TF and YS screened all references.
TF, WR, and DD extracted all data.
TF and IAR performed risk of bias assessments.
TF and GV performed GRADE assessments.
TF and MC analyzed the data.
All review authors contributed to the review design, including Background and Methods, and approved the final version.
Sources of support
Internal sources
-
Liverpool School of Tropical Medicine, UK
Host institute
External sources
-
Foreign, Commonwealth, and Development Office (FCDO), UK
Project number: 300342‐104
Declarations of interest
TF is a Cochrane Infectious Diseases Group (CIDG) Research Associate, and was not involved in the editorial process. She has no known conflicts of interest to declare.
YS works as a systematic reviewer for Cochrane Response, an evidence services unit operated by Cochrane, and was not involved in the editorial process. She has no known conflicts of interest to declare.
IAR has been an employee of the Cochrane Central Executive Team (Cochrane Response/Evidence, Production & Methods Directorate) since 2021, and was not involved in the editorial process. She has no known conflicts of interest to declare.
WR has no known conflicts of interest to declare.
MC is a CIDG Editor, and was not involved in the editorial process. She has no known conflicts of interest to declare.
DD has no known conflicts of interest to declare.
GV works as senior systematic reviewer for Cochrane Response, an evidence services unit operated by Cochrane, and was not involved in the editorial process. She has no known conflicts of interest to declare.
YS, IAR, and GV were contracted by the World Health Organization (WHO) to conduct a systematic review on wMel‐Wolbachia‐carrying mosquitoes for the biocontrol of dengue virus infection in 2023 via Cochrane Response. The WHO report was conducted independently of this review, which is not associated with the WHO.
Edited (no change to conclusions)
References
References to studies included in this review
Utarini 2021 {published data only}
- Anders K, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Andari B, et al. Update to the AWED (Applying Wolbachia to Eliminate Dengue) trial study protocol: a cluster randomized controlled trial in Yogyakarta, Indonesia. BMC Trials 2020;21:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders KL, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Andari B, et al. The AWED trial (Applying Wolbachia to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster-randomized controlled trial. BMC Trials 2018;19(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufault SM, Tanamas SK, Idriani C, Ahmad RA, Utarini A, Jewell NP, et al. Reanalysis of cluster randomised trial data to account for exposure misclassification using a per-protocol and complier-restricted approach. medRxiv (pre-print) 2023. [DOI] [PMC free article] [PubMed]
- Dufault SM, Tanamas SK, Indriani C, Utarini A, Ahmad RA, Jewell NP, et al. Disruption of spatiotemporal clustering in dengue cases by wMel Wolbachia in Yogyakarta, Indonesia. Nature Scientific Reports 2022;12(1):9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriani C, Ahmad RA, Wiratama BS, Arguni E, Supriyati E, Sasmono RT, et al. Dengue epidemiology in Yogyakarta City, Indonesia, before a randomized controlled trial of Wolbachia for arboviral disease control. American Journal of Tropical Medicine and Hygiene 2018;99(5):1299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03055585. Applying Wolbachia to Eliminate Dengue (AWED) [Applying Wolbach ia to Eliminate Dengue (AWED): a non-blinded cluster randomised controlled trial to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia]. classic.clinicaltrials.gov/ct2/show/NCT03055585 (first received 16 February 2017).
- Tantowijoyo W, Tanamas SK, Nurhayati I, Setyawan S, Budiwati N, Fitriana I, et al. Aedes aegypti abundance and insecticide resistance profiles in the Applying Wolbachia to Eliminate Dengue trial. PLOS Neglected Tropical Diseases 2022;16(4):e0010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. New England Journal of Medicine 2021;384(23):2177-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Gesto 2021 {published data only}
- Gesto J, Ribeiro G, Rocha M, Dias F, Peixoto J, Carvhalo F. Reduced competence to arboviruses following the sustainable invasion of Wolbachia into native Aedes aegypti from Southeastern Brazil. Scientific Reports 2021;11(1):10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozano 2022 {published data only}
- Lozano S, Pritts K, Duguma D, Fredregill C, Connelly R. Independent evaluation of Wolbachia infected male mosquito releases for control of Aedes aegypti in Harris County, Texas, using a Bayesian abundance estimator. PLOS Neglected Tropical Diseases 2022;16(11):e0010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lwin 2022 {published data only}
- Lwin MO, Ong Z, Panchapakesan C, Sheldenkar A, Soh LT, Chen I. Influence of public hesitancy and receptivity on reactive behaviours towards releases of male Wolbachia-Aedes mosquitoes for dengue control. PLOS Neglected Tropical Diseases 2022;16(11):e0010910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mains 2016 {published data only}
- Mains JW, Brelsfoard CL, Rose RI, Dobson SL. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Scientific Reports 2016;6:33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nazni 2019 {published data only}
- Nazni WA, Hoffmann AA, NoorAfizah A, Cheong YL, Mancini MV, Golding N, et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Current Biology 2019;29(24):4241-8.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03631719 {published data only}
- NCT03631719. Impact of Wolbachia deployment on arboviral disease incidence in Medellin and Bello, Colombia [World Mosquito Program – Colombia (WMP-COLOMBIA): the impact of city-wide deployment of Wolbachia-infected mosquitoes on arboviral disease incidence in Medellin and Bello, Colombia]. clinicaltrials.gov/study/NCT03631719 (first received 15 August 2018).
Nguyen 2015 {published data only}
- Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasites & Vectors 2015;8:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ribeiro dos Santos 2022 {published data only}
- Ribeiro dos Santos G, Durovni B, Saraceni V, Riback T, Pinto S, Anders K. Estimating the impact of the wMel release program on dengue and chikungunya incidence in Rio de Janeiro, Brazil. medRxiv 2022. [DOI: 10.1101/2022.03.29.22273035] [DOI] [PMC free article] [PubMed]
Zeng 2022 {published data only}
- Zeng Q, She L, Yuan H, Luo Y, Wang R, Mao W, et al. A standalone incompatible insect technique enables mosquito suppression in the urban subtropics. Communications Biology 2022;5(1):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Collins 2022 {published data only}
- Collins M, Potter G, Hitchings M, Butler E, Wiles M, Kennedy J, et al. EVITA dengue: a cluster-randomized controlled trial to EValuate the efficacy of Wolbachia-InfecTed Aedes aegypti mosquitoes in reducing the incidence of arboviral infection in Brazil. BMC Trials 2022;23(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04514107. A cluster-randomized trial to EValuate the Efficacy of Wolbachia-InfecTed Aedes aegypti mosquitoes in reducing the incidence of arboviral infection in Brazil (EVITA Dengue). clinicaltrials.gov/study/NCT04514107 (first received 14 August 2020).
Ong 2022 {published data only}
- Liew C, Soh LT, Chen I, Ng LC. Public sentiments towards the use of Wolbachia-Aedes technology in Singapore. BMC Public Health 2021;21:1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT05505682. Impact of Project Wolbachia – Singapore on dengue incidence [Assessing the efficacy of male Wolbachia-infected mosquito deployments to reduce dengue incidence in Singapore: a cluster randomized controlled trial]. clinicaltrials.gov/study/NCT05505682 (first received 18 August 2022). [DOI] [PMC free article] [PubMed]
- Ong J, Ho SH, Soh SX, Wong Y, Ng Y, Vasquez K, et al. Assessing the efficacy of male Wolbachia-infected mosquito deployments to reduce dengue incidence in Singapore: study protocol for a cluster-randomized controlled trial. BMC 2022;23:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alexander 2011
- Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, et al. Multicentre prospective study on dengue classification in four South-east Asian and three Latin American countries. Tropical Medicine & International Health 2011;16(8):936-48. [DOI] [PubMed] [Google Scholar]
Bhatt 2013
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature 2013;496(7446):504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bian 2010
- Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLOS Pathogens 2010;6(4):e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bian 2013
- Bian G, Zhou G, Lu P, Xi Z. Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to dengue virus in a mosquito vector. PLOS Neglected Tropical Diseases 2013;7(6):e2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blagrove 2013
- Blagrove M, Arias-Goeta C, Di Genua C, Failloux A, Sinkins SP. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits chikungunya virus. PLOS Neglected Tropical Diseases 2013;7(3):e2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourtzis 2014
- Bourtzis K, Dobson S, Xi Z, Rasgon J, Calvitti M, Moreira L, et al. Harnessing mosquito–Wolbachia symbiosis for vector and disease control. Acta Tropica 2014;132:S150-63. [DOI] [PubMed] [Google Scholar]
Brownstein 2003
- Brownstein JS, Hett E, O'Neill SL. The potential of virulent Wolbachia to modulate disease transmission by insects. Journal of Invertebrate Pathology 2003;84:24-9. [DOI] [PubMed] [Google Scholar]
Cavany 2022
- Cavany S. AWED trial modelling. github.com/scavany/awed_trial_modeling/ (accessed 12 January 2024).
Cavany 2023
- Cavany S, Huber JH, Wieler A, Minh Tran Q, Alkuzweny M, Elliott M, et al. Does ignoring transmission dynamics lead to underestimation of the impact of interventions against mosquitoborne disease? BMJ Global Health 2023;8:e012169. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2021
- Centers for Disease Control and Prevention. Dengue: symptoms and treatment (updated September 2021). www.cdc.gov/dengue/symptoms/index.html (accessed 25 August 2022).
CDC 2022
- Centers for Disease Control and Prevention. Mosquitoes with Wolbachia for reducing numbers of Aedes aegypti mosquitoes (updated July 2022). www.cdc.gov/mosquitoes/mosquito-control/community/emerging-methods/wolbachia.html (accessed 17 January 2023).
CDC 2023
- Centers for Disease Control and Prevention. Dengue around the world. www.cdc.gov/dengue/areaswithrisk/around-the-world.html (accessed 26 March 2024).
Cochrane Response 2021
- Henschke N, Villanueva G, Arévalo Rodriguez I, Cogo E, Hamel C, Petkovic J, et al. Systematic review on wMel Wolbachia-carrying mosquitoes for the biocontrol of dengue virus infection. Cochrane Response 2021.
Dorigatti 2018
- Dorigatti I, McCormack C, Nedjati-Gilani G, Ferguson NM. Using Wolbachia for dengue control: insights from modelling. Trends in Parasitology 2018;34(2):102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadinegoro 2015
- Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. New England Journal of Medicine 2015;373(13):1195-206. [DOI] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Hilgenboecker 2008
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? – a statistical analysis of current data. FEMS Microbiology Letters 2008;281(2):215-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2014
- Hughes GL, Rasgon JL. Transinfection: a method to investigate Wolbachia-host interactions and control arthropod-borne disease. Insect Molecular Biology 2014;23(2):141-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jansen 2010
- Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next. Microbes and Infection 2010;12(4):272-9. [DOI] [PubMed] [Google Scholar]
Johnson 2015
- Johnson KN. The impact of Wolbachia on virus infection in mosquitoes. Viruses 2015;7(11):5705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knerer 2020
- Knerer G, Currie CS, Brailsford SC. The economic impact and cost-effectiveness of combined vector-control and dengue vaccination strategies in Thailand: results from a dynamic transmission model. PLOS Neglected Tropical Diseases 2020;14(10):e0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMeniman 2009
- McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009;323(5910):141-4. [DOI] [PubMed] [Google Scholar]
Min 1997
- Min K, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proceedings of the National Academy of Sciences of the United States of America 1997;94(20):10792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monath 2002
- Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002;15(20):7-8. [DOI] [PubMed] [Google Scholar]
Moreira 2009
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 2009;139(7):1268-78. [DOI] [PubMed] [Google Scholar]
Moyes 2017
- Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLOS Neglected Tropical Diseases 2017;15(1):e0009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
NEA 2022
- National Environment Agency. Wolbachia-Aedes mosquito suppression strategy (updated November 2022). www.nea.gov.sg/corporate-functions/resources/research/wolbachia-aedes-mosquito-suppression-strategy (accessed 17 January 2023).
Nordin 2022
- Nordin NR, Arsad FS, Mahmud MH, Kamaruddin PS, Amir SM, Bahari NI, et al. Wolbachia in dengue control: a systematic review. Open Access Macedonian Journal of Medical Sciences 2022;10(F):501-12. [Google Scholar]
O'Neill 2018
- O’ Neill SL, Ryan PA, Turley AP, Wilson G, Retzki K, Iturbe-Ormaetxe I, et al. Scaled deployment of Wolbachia to protect the community from dengue and other aedes transmitted arboviruses. Gates Open Research 2018;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2021
- Pinto S, Riback T, Sylvestre G, Costa G, Peixoto J Dias F, et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: a quasi-experimental study. PLOS Neglected Tropical Diseases 2021;15(7):e0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rainey 2014
- Rainey S, Shah P, Kohl A, Dietrich I. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. Journal of General Virology 2014;95(3):517-30. [DOI] [PubMed] [Google Scholar]
Rasgon 2003
- Rasgon JL, Styer LM, Scott TW. Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. Journal of Medical Entomology 2003;40:125-32. [DOI] [PubMed] [Google Scholar]
Reich 2013
- Reich NG, Shrestha S, King AA, Rohani P, Lessler J, Kalayanarooj S, et al. Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity. Journal of the Royal Society, Interface 2013;10(86):20130414. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2023 [Computer program]
- Review Manager (RevMan). Version 5.6.0. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Ritchie 2021
- Ritchie SA, Devine GJ, Vazquez-Prokopec GM, Lenhart AE, Manrique-Saide P, Scott TW. Insecticide-based approaches for dengue vector control. In: Koenraadt CJ, Spitzen J, Takken W, editors(s). Innovative Strategies for Vector Control. Wageningen (the Netherlands): Wageningen Academic Publishers, 2021:59-89. [Google Scholar]
Ross 2022
- Ross PA, Robinson KL, Yang Q, Callahan AG, Schmidt TL, Axford JK, et al. A decade of stability for wMel Wolbachia in natural Aedes aegypti populations. PLOS Pathogens 2022;18(2):e1010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2019
- Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Research 2019;3:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soh 2021
- Soh S, Ho SH, Seah A, Ong J, Dickens BS, Tan KW, et al. Economic impact of dengue in Singapore from 2010 to 2020 and the cost-effectiveness of Wolbachia interventions. PLOS Global Public Health 2021;1(10):e0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Walker 2011
- Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011;476(7361):450-3. [DOI] [PubMed] [Google Scholar]
Wellcome 2022
- Wellcome. Trials of Wolbachia-carrying mosquitoes scaled up in South America. wellcome.org/news/trials-wolbachia-carrying-mosquitoes-scaled-south-america (accessed 17 January 2023).
Werren 1997
- Werren J, O'Neill S. The evolution of heritable symbionts. In: O'Neill SL, Hoffmann AA, Werren JH, editors(s). Influential Passengers. Oxford (UK): Oxford University Press, 1997. [Google Scholar]
WHO 2009
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. apps.who.int/iris/handle/10665/44188 (accessed 1 September 2022).
WHO 2012
- World Health Organization. Global strategy for dengue prevention and control 2012–2020. apps.who.int/iris/bitstream/handle/10665/75303/9789241504034_eng.pdf (accessed 1 September 2022).
WHO 2018
- World Health Organization. Vaccine and immunization: dengue. who.int/news-room/questions-and-answers/item/dengue-vaccines (accessed 15 July 2022).
WHO 2022a
- World Health Organization. Dengue and severe dengue. who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed 1 September 2022).
WHO 2022b
- World Health Organization. Global Vector Control Response Panel. who.int/news/item/11-04-2022-genuine-intersectoral-collaboration-is-needed-to-achieve-better-progress-in-vector-control (accessed 13 July 2022).
Wilder‐Smith 2019
- Wilder-Smith A, Flasche S, Smith PG. Vaccine-attributable severe dengue in the Philippines. Lancet Correspondence 2019;394(10215):2151-2. [DOI] [PubMed] [Google Scholar]
Yang 2021
- Yang X, Quam MB, Zhang T, Sang S. Global burden for dengue and the evolving pattern in the past 30 years. Journal of Travel Medicine 2021;28(8):146. [DOI] [PubMed] [Google Scholar]
Yen 1971
- Yen J, Barr R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens. Nature 1971;232:657-58. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fox 2023
- Fox T, Sguassero Y, Chaplin M, Rose W, Doum D, Arevalo-Rodriguez I, et al. Wolbachia-carrying Aedes mosquitoes for preventing dengue infection. Cochrane Database of Systematic Reviews 2023, Issue 3. Art. No: CD015636. [DOI: 10.1002/14651858.CD015636] [DOI] [PMC free article] [PubMed] [Google Scholar]


