Abstract
Ticks inject serine protease inhibitors (serpins) into their feeding sites to evade serine protease-mediated host defenses against tick-feeding. This study describes two highly identitical (97%) but functionally different Amblyomma americanum tick saliva serpins (AAS41 and 46) that are secreted at the inception of tick-feeding. We show that AAS41, which encodes a leucine at the P1 site inhibits inflammation system proteases: chymase (SI = 3.23, Ka = 5.6 ± 3.7×103M−1 s−1) and α-chymotrypsin (SI = 3.18, Ka = 1.6 ± 4.1×104M−1 s−1), while AAS46, which encodes threonine has no inhibitory activity. Similary, rAAS41 inhibits rMCP-1 purified from rat peritonuem derived mast cells. Consistently, rAAS41 inhibits chymase-mediated inflammation induced by compound 48/80 in rat paw edema and vascular permeability models. Native AAS41/46 proteins are among tick saliva immunogens that provoke anti-tick immunity in repeatedly infested animals as revealed by specific reactivity with tick immune sera. Of significance, native AAS41/46 play critical tick-feeding functions in that RNAi-mediated silencing caused ticks to ingest significantly less blood. Importantly, monospecific antibodies to rAAS41 blocked inhibitory functions of rAAS41, suggesting potential for design of vaccine antigens that provokes immunity to neutralize functions of this protein at the tick-feeding site. We discuss our findings with reference to tick-feeding physiology and discovery of effective tick vaccine antigens.
Keywords: Tick feeding physiology and tick saliva serpins, Inflammation, RNAi-mediated silencing
1. Introduction
Ticks and tick-borne diseases (TBD) have enormous impacts on public and veterinary health. Amblyomma americanum is an important tick species that transmits both human and animal TBD agents. In public health, A. americanum is the principal vector of Ehrlichia chaffensis, the causative agent of human monocytic ehrlichiosis [1], and E. ewingii, which also causes ehrlichiosis in humans, referred to as human granulocytic ehrlichiosis [2–4]. This tick also transmits Francisella tularensis the causative agent of tularemia ([81],[5]), a yet to be described disease agent, suspected as Borrelia lonestari, which causes Lyme disease-like symptoms referred to as southern tick associated rash illness (STARI) [6,7] and also an E. ruminantium-like organism referred to as the Panola Mountain Ehrlichia (PME) [8,9]. There is also evidence that A. americanum may transmit Rickettsia amblyommii, R. rickettsia, and R. parkeri, the causative agents to rickettsiosis to humans [10,11]. This tick has also been reported to transmit the Heartland and Bourbon viruses to humans [12,13]. Most recently, this tick has been shown to be responsible for causing a α-gal allergy or mammalian meat allergy (MMA) in humans after a tick bite [14]. A. americanum appears to be the most dominant tick species that bite humans in the Southern USA as this tick was reported to be the cause in 83% of human tick infestations in the Southern states [15].
In veterinary health, A. americanum transmits Theileria cervi to deer [16], and E. ewingii to dogs [17]. A. americanum was also shown to transmit Cytauxzoon felis to cats [18]. There are reports of mortality in deer fawns that were attributed to a combination of heavy A. americanum infestation and T. cervi infections [19]. In livestock production, heavy infestations were thought to cause low productivity in cattle [20,21].
The disruptive feeding style of ticks; lacerating the host tissue and allowing blood to accumulate at the feeding site for ingestion triggers host defense pathways, the majority of which are serine protease mediated including blood clotting, inflammation, and complement activation, that are tightly controlled by serine protease inhibitors (serpins) [22–24]. On this basis, ticks were proposed to utilize serpins to evade host defense responses to tick feeding [25]. Tick serpin encoding cDNAs have since been cloned and expressed showing to be functional inhibitors of host defense system proteases [26–32]. There is also evidence that tick serpins might be effective targets for tick vaccine development [25,33–35]. When tick serpin transcripts were silenced by RNAi, tick feeding was disrupted and caused abnormal tick phenotypes [28,36,37].
In proposing that ticks use serpins to evade host defenses against tick feeding, the assumption is that ticks inject serpins into the host during feeding. Our laboratory and others have confirmed that different tick species including A. americanum, do indeed inject serpins into the host during feeding [27,31,38,39]. We have recently described up to 121 different serpin transcripts that are expressed by male and female A. americanum ticks [40]. We have shown that some of the 121 serpins are present in saliva of A. americaum that were stimulated to start feeding [41] and during tick feeding [42]. We previously described three A. americanum tick saliva serpins, AAS6 (formerly called Lospin6, [29]) and AAS19 [28] that have anti-blood clotting functions, and AAS27, an inhibitor of plasmin and trypsin, has anti-inflammatory functions [30]. The goal of this study was to define the functional roles of A. americanum tick saliva serpins, AAS 41 and 46. Data in this study demonstrate that although AAS41 and 46 are 97% identical with only two amino acid differences in the function domain reactive center loop (RCL), each one is functionally unique with the former having anti-inflammatory function, inhibiting chymase and chymotrypsin, while the latter shows moderate to no inhibitory activity. Findings in this study have been discussed in the context of the molecular basis of tick feeding and discovery of tick vaccine antigens.
2. Materials and methods
2.1. Ethics statement
All experiments were done according to the animal use protocol approved by Texas A&M University Institutional Animal Care and Use Committee (IACUC#2018–0001 (continued from 2014–0310 to 2014–0311) that meets all federal requirements, as defined in the Animal Welfare Act (AWA), the Public Health Service Policy (PHS), and the Humane Care and Use of Laboratory Animals.
2.2. Pairwise sequence alignment of Amblyomma americanum (AAS) 41 and 46
Coding sequences (CDS) of nucleotide sequences AAS 41 and 46 (NCBI accession #s GAYW01000021 and GAYW01000194, respectively) encoding the mature protein amino acid sequences were aligned using ClustalW in MacVector (MacVector, Apex, NC, USA) using default settings. Alignments were visualized using the Picture tab in MacVector.
2.3. Tick feeding, dissections, total RNA extractions, cDNA synthesis, and protein extractions
Amblyomma americanum ticks were purchased from the tick laboratory at Oklahoma State University (Stillwater, OK, USA). Routinely, ticks were fed on six female Oryctolagus cuniculus specific pathogen free New Zealand white rabbits as previously described [29,43]. Rabbits were housed in a temperature-controlled room (22–23 °C, on a 12 h light/dark cycle) and were given ad libitum access to water and food. A. americanum ticks were restricted to feed onto the outer part of the rabbit ear with orthopedic stockinet’s glued with Kamar adhesive (Kamar Products Inc., Zionsville, IN, USA). Six male ticks were pre-fed for three days prior to introducing 15 female ticks in each of the ear stockinette (total of 30 female ticks per rabbit).
Ticks were collected and dissected as previously described [29]. Ticks that were partially fed for 24 and 48 h (n = 45 ticks per time point), 72 and 96 h fed (n = 30 ticks per time point), and 120 h fed (n = 15 ticks) were manually detached. Within the first hour of detachment, tick mouthparts were inspected to remove remnant host tissues and washed in RNase inhibitor diethylpyrocarbonate (DEPC)-treated water to prepare for dissections. Dissected tick organs: salivary glands (SG), midguts (MG), ovary (OV), synganglion (SYN), Malpighian tubules (MT), and carcass (CA, the remnants after removal of other organs) were placed in RNA later (Thermo Fisher Scientific, Waltham, MA, USA) or 1 ml Trizol total RNA extraction reagent (Thermo Fisher Scientific) and stored at −80 °C until total RNA extraction.
Total RNA was extracted using the Trizol reagent according to manufacturer’s instructions (Thermo Fisher Scientific) and re-suspended in DEPC treated water. Total RNA was quantified using a microplate plate reader Synergy H1 (BioTek Instruments Inc., Winooski, VT, USA) and/or the Infinite M200 Pro plate reader (Tecan Group Ltd., Männedorf, Zürich, Switzerland). Up to 1 μg total RNA was used to synthesize cDNA using the Verso cDNA Synthesis Kit following the manufacturer’s instructions (Thermo Fisher Scientific).
To extract native proteins from specified tissues noted above, tissues were placed into sterile 1.5 ml tubes and sheared using sterile fine scissors in IP/Clean Blot Lysis buffer containing protease inhibitor cocktail (Thermo Fisher Scientific). Crude protein extracts from 22 days post-oviposition eggs, whole immatures (larvae and nymphs), and adult A. americanum ticks were flash frozen in liquid nitrogen and extracted in 1× PBS, 1 mM PMSF, 1 mM E-64 mM, 10 mM EDTA, pH 7.4 by crushing using a pestle or shearing using fine scissors. Protein extracts were stored in −80 °C until use for western blotting analysis.
2.4. Quantitative RT-PCR and western blotting analyses of AAS41/46 mRNA and protein in different life stages and tick organs
Quantitative (q) RT-PCR on Applied Biosystems 7300 Real Time PCR System (Thermo Fisher Scientific) or CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA) was used to determine transcription profiles during feeding as described [43]. In triplicate biological samples, template cDNA was synthesized from dissected SG, MG, and CA of unfed ticks and ticks that partially fed for 24 and 48 h fed (n = 15 ticks per pool), 72 and 96 h fed (n = 10 ticks per pool), and 120 h fed (n = 5 ticks per pool). Forward (5′GCAGCGACGACGAAGGCG3′) and reverse (5′GCCTGAAGATGTGCGTG-3′) qRT-PCR primers were designed using the Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/) for AAS 41 and 46, targeting an amplicon size of ~100 base pairs (bp). The Ct values and PCR efficiency of each reaction was determined using the LnReg program [44]. To verify if primer pairs are specific to these serpins, nucleotide sequences for each primer were subjected to Blastn (https://blast.ncbi.nlm.nih.gov/Blast.cgi) searches against the Ixodidae database. Cycling conditions were set to the following: stage one at 50 °C for 2 min, stage two at 95 °C for 10 min, and stage three contained two steps with 40 cycles of 95 °C for 15 s and 55 °C for 1 min. Reaction volumes in triplicate contained 10-fold diluted cDNAs that were originally synthesized from 1 μg total RNA, an optimized concentration of 350 nM of forward and reverse selected serpin primers each, and 2× SYBR Green Master Mix (Thermo Fisher Scientific). For internal reference control, a forward (5′GGCGCC GAGGTGAAGAA3′) and reverse (5′CCTTGCCGTCCACCTTGAT3′) primers targeting of A. americanum 40S ribosomal protein S4 (RPS4: NCBI accession # GAGD01011247.1) which is stably expressed in I. scapularis during feeding [82] was used. Relative quantification (RQ) of selected serpin transcripts was determined using the comparative CT (2−ΔΔCt) method [45] and adopted in [43]. The data was presented as percent mean (M) transcript abundance ± SEM per tissue.
To relate AAS41/46 protein expression to tick physiology, total protein extracts of tick eggs (22 days after being laid) and whole ticks: unfed larvae, nymph, male and female, and dissected organs (SG, MG, OV, SYN, MT and CA) of adult A. americanum ticks that were partially fed for 24, 72, and 120 h were subjected to western blotting analysis using a monospecific antibody to recombinant (r)AAS41/46 (detailed below). The monospecific antibody was purified from rabbit polyclonal antibody to cocktail mixture of recombinant tick saliva serpins using the Sepharose 4B CNBr-activated resin following manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO, USA). Approximately 2.3 mg of affinity purified rAAS41/46 (detailed below) that was coupled to 0.3 g of resin was reacted with rabbit polyclonal antibody that was diluted in phosphate-buffered saline, pH 7.4, (PBS) for 2 h at room temperature. The resin was washed with 30 ml of PBS and the bound monospecific antibody was eluted in ten 1 ml aliquots of 100 mM glycine-HCl, pH 2.4 and was immediately neutralized with 50 μl of 2 M Tris base. Eluted antibody fractions were dialyzed against PBS (pH 7.4). The polyclonal antibody to rAA41/46 that was used here was produced in a parallel study by immunizing rabbits twice biweekly with 50 μg of affinity purified cocktail mixture of serpins including rAAS41/46 that were mixed with TiterMax Gold adjuvant (Sigma-Aldrich).
2.5. RNAi-mediated silencing analysis to determine AAS41/46 importance in tick feeding
RNAi-mediated silencing of AAS41/46 mRNA was done as described [29,43]. Double stranded RNA (dsRNA) was synthesized using the Megascript RNAi kit (Thermo Fisher Scientific) according to manufacturer’s instructions. The dsRNA target sequence was searched against tick sequences in GenBank to verify specificity. Due to high nucleotide identities (N97%), dsRNA was synthesized targeting both serpins using 2 μg of purified PCR product as template, with forward (5′TAATACGACTCACTATAGGGCTGCACGAGACTCTGGGTTA3′) and reverse (5′TAATACGACTCACTATAGGGGGTAGACGTGGACATGATGC3′) primers with added T7 promoter sequence (bolded). PCR primers for an unrelated tick gene, enhanced green fluorescent protein coding cDNA (EGFP: NCBI accession # JQ064510.1) with added T7 promoter sequence [43] were used to synthesize the control EGFP-dsRNA. We performed five dsRNA synthesis reactions according to the manufacturer’s instructions and precipitated the combined fractions using sodium acetate precipitation methods to produce high amounts of dsRNA (3–5 μg/μl) that allow us to inject sufficient dose in unfed ticks in low volume. Briefly, 1:10 volume of 3 M sodium acetate pH 5.2 was mixed by vortexing to the dsRNA samples. Then four volumes of 100% ethanol were mixed and incubated overnight in −20 °C. The mixture was centrifuged at 14000 rpm for 30 min at 4 °C and the pellet was resuspended in water and purified using the dsRNA binding columns from the Megascript RNAi kit (Thermo Fisher Scientific).
Transcription analysis in this study and relative protein abundance was determined using published normalized spectral abundance factors (NSAF) of AAS 41 and 46 [41,42] indicated that AAS41/46 was highly expressed in unfed ticks, and the protein was injected at high abundance at start of feeding. Therefore, we first depleted AAS41/46 mRNA from unfed ticks before assessing the effects of RNAi-mediated silencing as published by our lab [36,46]. Subsequently RNAi-mediated silencing ticks were incubated for the empirically determined time frame that was required to deplete mRNA from unfed ticks before placing on animals to feed to assess the effects of RNAi-mediated silencing. This was to suppress the highly abundant AAS41/46 mRNA present in unfed ticks to prevent the translation of AAS41/46 protein at the start of feeding, which could compromise the effects of RNAi-mediated silencing. To determine time depletion of mRNA in unfed ticks, three female A. americanum ticks were injected with 0.5–1 μl (~3 μg/μl) EGFP-dsRNA or AAS41/46-dsRNA and incubated at 25 °C with >85% humidity alongside non-injected control ticks. Total RNA from individual ticks were extracted using Trizol method and 1 μg was used for cDNA synthesis to determine the levels of AAS41/46 transcripts at days 3, 7, 14 and 21 post-injection by qualitative PCR using the primers for recombinant protein expression (described above).
To assess the effects of RNAi on tick feeding, EGFP-dsRNA or AAS41/46-dsRNA injected female ticks (n = 15) were incubated at 25 °C with >85% humidity for 21 days (time period required to deplete mRNA from unfed ticks). Subsequently ticks were fed on specific pathogen free New Zealand white rabbits to assess the effect(s) of RNAi-mediated silencing by several parameters: tick attachment (time when all ticks have mouthparts attached to the rabbit) and mortality rates (number of dead ticks), feeding period (FP-time to replete feeding and detachment after tick attachment), engorgement weight (EW- total weight [mg] of tick) as an index for amount of blood ingested by the tick, and egg mass conversion ratio (EMCR- egg weight/engorgement weight) as measure of utilizing blood meal to produce eggs. Tick phenotypes during feeding was documented daily using the Canon EOS Rebel XS camera attached to a Canon Ultrasonic EF 100 mm 1:2:8 USM Macro Lens (Canon USA Inc., Melville, NY, USA).
Prior to assessing the effects of RNAi-mediated silencing, disruption of AAS41/46 mRNA was verified in feeding ticks by quantitative (q) RT-PCR. Three ticks were manually detached from the rabbit ears 72 h post-attachment and individually processed for dissection of tick organs (SG, MG, and CA) as described above, mRNA extraction using the Dynabead mRNA Direct Kit (Thermo Fisher Scientific) and cDNA synthesized from ~200 ng of mRNA using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific). Relative quantification (RQ) of transcripts was determined using the formula, where S = mRNA suppression, RQT and RQC = RQ of tissues in selected serpin-dsRNA injected and EGFP-dsRNA injected ticks, respectively. Data was presented as the mean (M) of selected serpin mRNA suppression ± SEM.
2.6. Expression of recombinant AAS 41 and 46 in Pichia pastoris
Recombinants (r) AAS 41 and 46 serpin proteins were expressed using the Pichia pastoris and pPICZα plasmid expression system (Thermo Fisher Scientific) as described previously [29,43]. The mature protein open reading frames for AAS41 (NCBI accession # GAYW01000021) and AAS46 (NCBI accession # GAYW01000194) [40] was sub-cloned into pPICZα-C using forward (5′ATCGATGCAAGAGGAGGACAAGGTGAC3′) and reverse primers (5′GCGGCCGCTTAGTGGTG GTGGTGGTGGTGAAGATGGTTCACTTGACCCGGA5) with added respective ClaI and NotI restriction enzymes sites (in bold), an extra nucleotide guanine (in italics) in the forward primer to put in-frame with the expression vector, and addition of a hexahistidine-tag (underlined) in the reverse primer. The pPICZα C-rAAS41 or -rAAS46 expression plasmids were linearized with PmeI and electroporated into Pichia pastoris X-33 strain (Thermo Fisher Scientific) using ECM600 electroporator (BTX Harvard Apparatus Inc., Holliston, MA, USA) with parameters set to 1.5 kV, 25 μF, and 186 Ω. Transformed colonies were selected on Yeast Extract Peptone Dextrose Medium with Sorbitol (YPDS) agar plates with zeocin (100 μg/ml) incubated at 28 °C. Positive transformants were inoculated in buffered glycerol-complex medium (BMGY) and grown overnight at 28 °C with shaking (230–250 rpm). Subsequently the cells were used to inoculate buffered methanol-complex medium (BMMY) to A600 of 1, after which protein expression was induced by adding methanol (0.5% final concentration) every 24 h for five days. The pPICZα plasmid permits secretion of the recombinant protein into spent culture media, and was precipitated by ammonium sulfate saturation (75% saturation) with stirring overnight at 4 °C. The precipitate was pelleted at 10,000 rpm for 1 h at 4 °C and resuspended in and dialyzed against column binding buffer (20 mM sodium phosphate, 500 mM NaCl, 5 mM imidazole, pH 7.4) for affinity purification.
To verify expression of rAAS41 and 46, western blotting analysis was performed using the horseradish peroxidase (HRP)-labeled antibody to the C-terminus hexa-histidine tag (Thermo Fisher Scientific) diluted to 1:5000 in 5% blocking buffer (5% skim milk powder in PBS w/Tween-20). The positive signal was detected using Amersham ECL Prime Western Blotting Chemiluminescent Reagent (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). A negative control of pPICZα-C was included under same procedures. Subsequently, rAAS41 and 46 was affinity purified under native conditions using Hi-Trap Chelating HP Columns (GE Healthcare Bio-Sciences). Affinity purified putative rAAS41 and 46 were dialyzed against reaction buffer (20 mM Tris-HCl, 150 mM NaCl, pH 7.4) for downstream assays. To verify purity and background contamination, affinity purified rAAS41 and 46 were resolved on a 10% SDS-PAGE and silver stained. Samples with least background were selected and concentrated by centrifugation using 10 kDa molecular weight cut off Centrifugal Concentration Devices (Pall Corporation, Port Washington, NY, USA). Protein concentration were determined using the BCA quantification kit (Thermo Fisher Scientific).
2.7. Prediction and validation of post-translational glycosylation
Post translational glycosylation in eukaryotes is involved in promoting protein folding and improves protein stability thereby functional roles of proteins. Amino acid sequences of AAS 41 and 46 were scanned for potential N-linked and O-linked glycosylation sites using the software NetNGlyc 1.0 Server [47] and NetOGlyc 4.0 Server [48], respectively. Scanning AAS 41 and 46 amino acid sequences revealed three N-linked and six O-linked glycoslyation sites and thus to confirm if functional, affinity purified rAAS 41 and 46 were treated with protein deglycosylation enzyme mix according to manufacturer’s instructions (New England Biolabs, Ipswich, MA, USA). Deglycosylation was verified by western blotting analysis using an antibody against the C-terminus hexa histidine-tag (Thermo Fisher Scientific) and the positive signal detected using HRP chromogenic substrate (Thermo Fisher Scientific).
2.8. Determine if native AAS41/46 are part of tick salivary immunogens that provoke host immunity against tick feeding in repeatedly infested animals
Deglycosylated and non-deglycosylated affinity purified rAAS41 and 46 were subjected to western blotting analysis using tick-immune rabbit sera that were repeatedly infested with new cohorts of female and male A. americanum ticks every 24 h, 48 h, or until replete feeding that were produced in previous studies [29,49]. Due to the post-translational modifications in expressing recombinant proteins in P. pastoris, deglycosylated and non-deglycosylated rAAS 41 and 46 were used in western blots to confirm if antibody binding was directed at the protein backbone and not glycans that were attached onto the protein.
2.9. Inhibitor function profiling
Inhibitory activity profiles of rAAS 41 and 46 were tested against a panel of 19 mammalian serine proteases related to host defense pathways against tick feeding. Mammalian proteases (noted with molar concentration per reaction) tested were: pancreatic bovine α-chymotrypsin (2.7 nM), pancreatic porcine elastase (61.8 nM), human neutrophil proteinase-3 (280 nM), human chymase (83.3 nM), pancreatic bovine trypsin (0.2 nM), pancreatic porcine kallikrein (10 nM) (Sigma-Aldrich, St. Louis, MO, USA), human neutrophil cathepsin G (425.5 nM) (Athens Research & Technology), human plasmin (5.3 nM), human factor XIa (3.7 nM), bovine factor IXa (314.4 nM), human factor XIIa (15 nM), human thrombin (19.2 nM) (Enzyme Research Laboratories), human neutrophil elastase (14.9 nM), human t-PA (23.6 nM), human u-PA (29.6 nM) (Molecular Innovations, Inc., Novi, MI, USA), human factor Xa (10 nM) (New England Biolabs), and papain (427 nM) (Spectrum Chemical Manufacturing Corp., New Brunsick, NJ, USA). Mouse and rat chymase were obtained from peritoneal-derived mast cells described below.
Substrates were used at 0.20 mM final concentration, including N-succinyl-Ala-Ala-Pro-Phe-pNA for chymase, cathepsin G and chymotrypsin, N-benzoyl-Phe-Val-Arg-pNA for thrombin, and trypsin, N-succinyl-Ala-Ala-Ala-pNA for pancreatic elastase and pGlu-Phe-Leu-pNA was used for papain (Sigma-Aldrich, St. Louis, MO, USA). The substrate D-Pro-Phe-Arg-pNa was used for factor XIa, factor XIIa, and kallikrein, and substrate Bz-Ile-Glu (γ-OCH3)-Gly-Arg-pNA for factor Xa (Aniara Diagnostica, West Chester, OH, USA). Substrate H-D-Val-Leu-Lys-pNA was used for plasmin (Chromogenix, Philadelphia, PA, USA). The substrate CH3SO2-D-CHG-Gly-Arg-pNA was used for factor IXa, u-PA and t-PA (Molecular Innovations, Inc., Novi, MI, USA). The substrate N-methoxysuccinyl-Ala-Ala-Pro-Val-pNA was used for neutrophil elastase and proteinase-3 (Enzo Life Sciences, Farmingdale, NY, USA).
Reagents were mixed at room temperature and reactions performed in triplicate. Initially, 1 μM of rAAS 41 or 46 was pre-incubated with optimized amounts of the protease for 15 min at 37 °C in 20 mM Tris-HCl, 150 mM NaCl, BSA 0.1%, pH 7.4 buffer. The corresponding substrate (0.20 mM) for each enzyme was added in a 100 μl final reaction volume and substrate hydrolysis was measured at A405nm every 11 s for 30 min at 30 °C using the Synergy H1 (BioTek Instruments Inc.). Acquired A405nm data was subjected to one phase decay analysis in Prism 6 software (GraphPad Software, La Jolla, CA, USA) to determine plateau values as proxy for initial velocity of substrate hydrolysis (Vmax) or residual enzyme activity. The percent enzyme activity inhibition level was determined using the formula: where Vmax (Vi) = activity in presence of rSerpin, and Vmax (V0) = activity in absence of rSerpin. Data was presented as mean ± SEM of triplicate readings.
2.10. Stoichiometry of inhibition (SI) and Complex formation assay
The stoichiometry of inhibition (SI) for rAAS41 was determined against chymase and α-chymotrypsin as they were inhibited by >80% in the inhibitor profiling assay (above). At various molar ratios (0 to 10) of rAAS41:protease, various concentrations of rAAS41 were pre-incubated with constant concentration of chymase (13 nM) and chymotrypsin (8 nM) for an hour at 37 °C. The residual enzyme activity was measured using colorimetric substrates specific for each enzyme as described above. The data was plotted as the residual activity versus inhibitor to enzyme molar ratio. SI or the molar ratio of rAAS41:protease when enzyme activity is completely inhibited was determined by fitting data onto the linear regression line using Prism 6 software (GraphPad).
A typical inhibitory serpin forms a stable complex to the target protease [50,51]. Complex formation assay was done by incubating deglycosylated rAAS41 and human chymase (0.1 μg) or chymotrypsin (0.1 μg) at various molar ratios (0.625 to 10) in 20 mM Tris-HCl, 150 mM, NaCl, pH 7.4 for 1 h at 37 °C. We used deglycosylated rAAS41 to improve on visualization of rAAS41 and target protease complex. Following incubation, denaturing sample buffer was added to the reaction mix, and incubated at 95 °C for 5 min in thermal cycler. Samples were subjected to 12.5% SDS-PAGE and visualized by silver stain.
2.11. Rate of reaction (Ka) between rAAS41 and chymase or chymotrypsin
The rate of rAAS41 inhibiting chymase or chymotrypsin (Ka) was determined using the discontinuous method as described [52]. Constant amounts of chymase (33 nM) or chymotrypsin (8.3 nM) for different periods of time (0–15 min) were incubated with various amounts of rAAS41, 33–500 nM for the former and 8.3–100 nM for latter, at 37 °C followed by measurement of residual protease activity. The pseudo-first order constant, kobs, was determined from the slope of a semi-log plot of the residual protease activity against time. The second-rate constant (Ka) was determined by the slope of the best fit line of the kobs values that were plotted against rAAS41 concentration.
2.12. Effects of rAAS41/46 monospecific antibody on rAAS41 inhibitory activity
The purified monospecific antibody to rAAS41/46 (described above) was used to check if antibody-binding to serpin could block their protease inhibitory activity. Affinity purified rAAS41 (136 nM for chymase and 170 nM for chymotrypsin) was pre-incubated with various amounts of purified monospecific antibody to rAAS41 (varying from 0 to 2 μM for chymase and 0 to 1 uM for chymotrypsin) or purified rabbit IgG from pre-immune sera (2 μM for chymase and 1 μM for chymotrypsin) for 30 min at 37 °C prior to adding chymase (10.8 nM) or chymotrypsin (12.8 nM) and continuing to incubate for an additional 15 min at 37 °C. Subsequently N-succinyl-Ala-Ala-Pro-Phe-pNA substrate (0.20 mM) were added to a 100 μl final reaction volume and substrate hydrolysis was measured at OD405nm every 15 s for 15 min at 30 °C using the Synergy H1 microplate reader (Biotek, Winooski, Vermont, USA). The percent enzyme activity inhibition level was determined as previously described [30]. Data are representative of three independent replicate readings.
2.13. Anti-inflammatory function of rAAS41
To investigate the effect of rAAS41, chymase-mediated inflammation induced by compound 48/80 (reviewed in [53]), paw edema and vascular permeability rat models were used. These experiments were performed using adult male Wistar rats that were supplied by the Central Animal Facility (Universidade Federal do Rio Grande do Sul, Brazil). Rats were housed in a temperature-controlled room (22–23 °C, on a 12 h light/dark cycle) and were given ad libitum access to water and food. To induce inflammation, compound 48/80 (1 μg total in saline) with endotoxin free rAAS41(25 μg per paw) was injected in rat paws. Paw volume as an index of inflammatory edema was measured using a digital plethysmometer (Insight, Ribeirão Preto, SP, Brazil) to document inflammation. Before paw volume measurements, the paw was marked at the ankle in order to immerge always at the same extent into the plethysmometer. The left paw was used as a control and received the same volume of saline. As a control, each group of rats (n = 5 per group) received the same volume of saline (vehicle) in the presence of rAAS41. Paw volume (in ml) was measured at 0, 30, 60 and 120 min after compound 48/80 injection. Three measurements were performed per time point by the same operator. The increase in paw volume was calculated by subtracting average volume of the right paw by the average volume of the left paw at each time point.
For vascular permeability assay [54], male Wistar rats (weighing between 250 and 400 g) that were anesthetized by intraperitoneal injection of xylazin (10 mg/kg) and ketamine (75 mg/kg) were injected intravenously through the tail vein with 700 μl of Evans blue dye (50 mg/kg in saline). At 5 min post injection, animals (n = 6) were injected intradermally at two sites dorsally (n = 6; 12 spots per treatment) with (i) normal saline, (ii) compound 48/80, (iii) compound 48/80 with 25 μg of rAAS41 in saline, and (iv) 25 μg of rAAS41 diluted in saline in 100 μl volumes. At 60 min post treatment, animals were euthanized and an area of skin encompassing the entire injection region was removed and photographed to document leakage of the Evans blue dye. Subsequently, Evans blue dye spots were excised and the dye was extracted by incubating skin pieces in 2.5 mL of 50% formamide for 24 h at 55 °C. Subsequently after centrifugation at 4000 rpm for 10 min, absorbance of the supernatant was measured at 620 nm on the SpectraMax spectrophotometer (Molecular Devices, San Jose, CA, USA).
2.14. Rat and mouse peritoneum-derived chymase activity
Rat and mouse native chymase were extracted from peritoneum-derived mast cells (PDMC) extracts from adult Sprague Dawley rats and C57BL/6 mice provided by the Tissues Share Program at Texas A&M University, Comparative Medicine Program (CMP), Laboratory Animal Research and Resources (LARR) facility. To obtain the peritoneal cells, animals were first euthanized according to American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals, and sterilized by dipping into 70% ethanol. Using sterile scissors and forceps, the outer skin of the peritoneum was excised and gently pulled back to expose the inner skin lining (the peritoneal cavity). A 25 ml volume of ice-cold PBS pH 7.4 was injected using a 27-gauge needle and syringe into the peritoneal cavity and gently massaged the peritoneum to release any attached cells into the PBS solution. Using a 25-gauge needle and syringe, the peritoneal fluids were collected and centrifuged at 1300 rpm for 5 min at 4 °C to obtain the cells (pellet). To remove red blood cell (RBC) contamination, the pellet was resuspended in RBC lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4), incubated for 5 min at room temperature and centrifuged at 1300 rpm for 5 min at 4 °C. Washing with RBC lysis buffer was repeated twice. Subsequently, the cell pellet was resuspended in lysis buffer (20 mM Tris-HCl, 2 M NaCl, pH 7.4) and sonicated (3 cycles of 10 s ON and 30 s OFF, 50% amplitude) on ice. After lysis, PDMC extract was centrifuged at 12,000 rpm for 15 min at 4 °C and the supernatant containing PDMC proteases, including chymase was collected and stored at −80 °C upon use. Total protein concentrations of PDMC extracts were determined using the BCA quantification assay (Thermo Fisher Scientific).
For enzyme activity determination, an aliquot of the PDMC supernatant containing 1 U of chymase activity was incubated with rAAS41 (1 μM) at 37 °C for 15 min and triggered the reaction by adding the substrate N-Succinyl-Ala-Ala-Pro-Phe-pNA (0.2 mM - final concentration). Protease kinetics was monitored for 15 min at 30 °C with reads at every 11 s (in triplicate) using the Synergy H1 microplate reader (Biotek, Winooski, Vermont, USA). One unit of chymase is defined as the amount of protease necessary to achieve a velocity of 0.17 mOD405nm/s using experimental conditions described above.
To detect rat mast cell protease-1 (rMCP-1) proteolytic activity from rat PDMC extract, zymography was performed as previously described (Vandooren et al., 2013). Affinity purified rAAS41 (4 μg) was incubated at 37 °C with equal volume of rat PDMC extracts for various time points as described above and resolved using 10% SDS-PAGE containing 0.1% casein as the substrate. The enzymatic activity was visualized by Coomassie blue staining and destaining procedures.
To determine if rAAS41 forms an irreversible complex with native rat chymase, affinity purified rAAS41 (4 μg) was incubated with same volume of rat PDMC extract in 20 mM Tris-HCl, 150 mM, NaCl, pH 7.4 buffer for various time points: 5, 15, 30, 45, and 60 min. The samples were treated with deglycosylation enzyme mix under denaturing conditions according to manufacturer’s instructions (New England Biolabs). The rAAS41-native rat chymase complexes were resolved on 12% SDS-PAGE and visualized using Pierce Silver Stain for Mass Spectrometry kit (Thermo Fisher Scientific).
2.15. Sample preparation and LC-MS/MS
To confirm that rAAS41 was forming a complex with native rat chymase from the PDMC extracts, silver stained gel bands were excised, destained, and analyzed using LC-MS/MS. Gel bands from the silver stained SDS-PAGE were destained according to the manufacturer’s instructions (Thermo Fisher Scientific) and washed with 100 mM ammonium bicarbonate and dehydrated with acetonitrile. Gel pieces were then rehydrated in modified trypsin solution (20 ng/ml, Promega, Madison, WI, USA) in 50 mM ammonium bicarbonate and the digestion carried on overnight at 37 °C. Tryptic peptides were extracted from gel pieces with 50% acetonitrile/5% formic acid solution. Tryptic peptide extracts were dried and reconstituted in 20 μl 5% acetonitrile/0.1% formic acid solution for mass spectrometry analysis.
Reverse phase pre-columns were prepared to analyze peptide mixtures by nanoflow liquid chromatography mass spectrometry using an Easy NanoLC II and a Q Exactive mass spectrometer (Thermo Scientific) as previously described [41]. Peptides eluted from the analytical column were electrosprayed directly into the mass spectrometer. Buffer A consisted of 5% acetonitrile/0.1% formic acid, and buffer B was 80% acetonitrile/0.1% formic acid. Using a flow rate of 400 nl/min, a gradient of 45 min or 60 min was used depending on the gel band intensity. The gradients rapidly increased from 1 to 10% of buffer B, followed by a linear gradient up to 35% of buffer B, and then with another rapid increase up to 90% of buffer B. The column was held at 90% of buffer B for 1 min, reduced to 1% of buffer B and then re-equilibrated prior to next injection.
The mass spectrometer was operated in a data dependent mode, collecting a full MS scan from 400 to 1200 m/z at 70 K resolution and an AGC target of 1e6. The 10 most abundant ions per scan were selected for MS/MS at 17.5 K resolution and AGC target of 2e5 and intensity of 1000. Maximum fill times were 20 and 120 ms for respective MS and MS/MS scans, with dynamic exclusion of 15 s. Normalized collision energy was set to 25.
2.16. LC-MS/MS data analysis
Tandem mass spectra were extracted from raw files using RawExtract 1.9.9.2 [55] and searched with ProLuCID [56] against a combined database including AAS41 and rat proteins from GenBank using Integrated Proteomics Pipeline – IP2 (Integrated Proteomics Applications). The search space included all fully-tryptic and half-tryptic peptide candidates. Carbamidomethylation on cysteine was used and static modification. Data was searched with 50 ppm precursor ion tolerance and 20 ppm fragment ion tolerance. Identified proteins were filtered using DTASelect [57] and required a minimum of two peptides present, precursor delta mass cutoff of 10 ppm and <1% false-discovery rate (FDR).
2.17. Statistical analysis
Statistical software packages in PRISM version 6 (GraphPad Software Inc.) were used. Unpaired student t-test and Mann-Whitney analysis were used to determine if differences between AAS41/46-dsRNA and EGFP-dsRNA injected ticks are significant. Likewise, the unpaired student t-test was used to determine the statistical significance of paw volume and of the extracted Evans blue dye between treatments. Data is represented as mean of three biological replicates for RNAi-mediated silencing and five to six for paw edema assays. P ≤ .05 was considered statistically significant.
3. Results
3.1. Highly identical A. americanum serpin (AAS) 41 and 46 are highly expresses as glycoproteins in Pichia pastoris
Amblyomma americanum serpins (AAS) 41 and 46 (accession #s GAYW01000021 and GAYW01000194) are highly identical, showing 97% amino acid identity with differences of two amino acids within the functional reactive center loop (RCL) domain and six amino acids outside the RCL (Fig. 1, Supplementary Fig. 1). To begin understanding functional roles of these two proteins, we successfully expressed, and affinity purified both recombinant (r)AAS 41 and 46 in Pichia pastoris (Fig. 2 and Supplementary Fig. 2). The deduced molecular weight of both rAAS 41 and 46 with an added fusion tag was around 46 kDa. However in a SDS-PAGE analysis, both recombinant proteins migrated around 50 kDa. Amino acid sequence analysis predicted that both rAAS 41 and 46 have three N-linked and six O-linked glycosylation sites. When treated with glycosidases, rAAS 41 and 46 migrated lower than non-treated controls confirming the observed high molecular weight was due to glycosylation (Fig. 2).
Fig. 1.

Amblyomma americanum serpin (AAS) 41 and 46 are highly identical. Mature protein amino acid sequences of AAS 41 and 46 were aligned using ClustalW in MacVector. The reactive center loop (RCL) is boxed in blue; the P1 active P1 site in the RCL is indicated with an asterisks (*) showing AAS41 has a leucine “L” and AAS46 has threonine “T” at the P1 site.
Fig. 2.
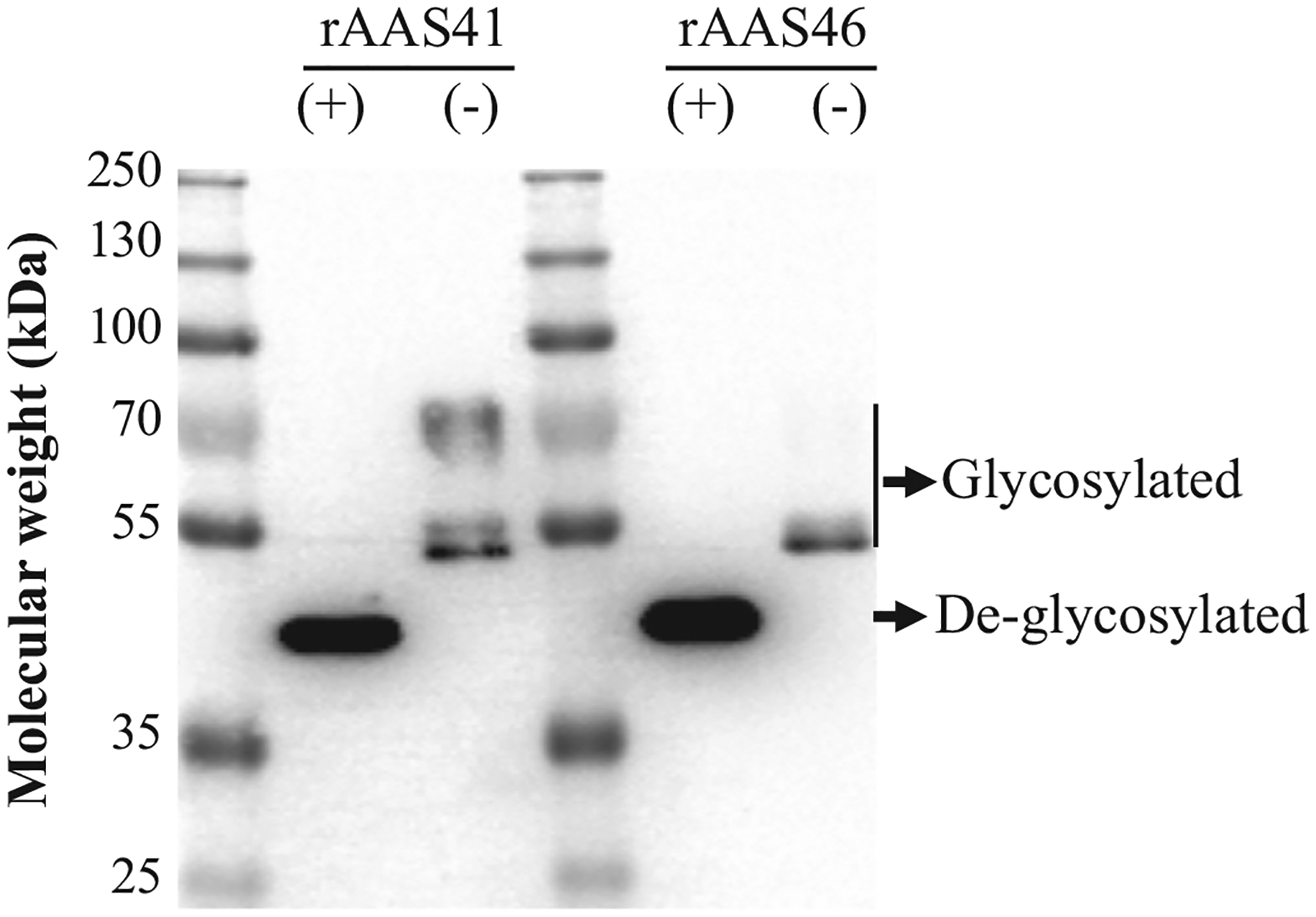
Recombinants Amblyomma americanum serpin (rAAS) 41 and 46 are expressed as glycoproteins in Pichia pastoris. P. pastoris transformed with AAS 41 and 46 recombinant plasmids were cultured at 28 °C and recombinant protein expressions were induced by inoculating 0.5% methanol (final concentration) daily through five days. Affinity purified rAAS 41 and 46 treated with deglycosylation enzyme mix (+) or without treatment (−) were resolved by 10% SDS-PAGE for western blot analysis using antibody to the hexa-histidine tag.
3.2. Amblyomma americanum serpin (AAS) 41 and 46 are highly expressed at unfed and early tick feeding stages in multiple tick tissues
Fig. 3 summarize AAS41 and 46 transcription and protein expression during tick feeding. Due to the high sequence identities, the transcription and protein secretion profiles in Fig. 3 represents both AAS 41 and 46. Quantitative RT-PCR analysis shows that AAS41/46 mRNA is highly transcribed in salivary glands (SG), midguts (MG), and carcass (CA, tissue remnant after removal of SG and MG) of unfed ticks and also present in fed ticks (Fig. 3 A1–A3). Transcription level of AAS41/46 mRNA is up to 30-, 10-, and 60- fold higher in respective SG, MG, and CA at the unfed stage compared to feeding stages. It is important to advise the reader to note that the 120 h time point was used as the calibrator, where the lowest transcript expression was detected.
Fig. 3.
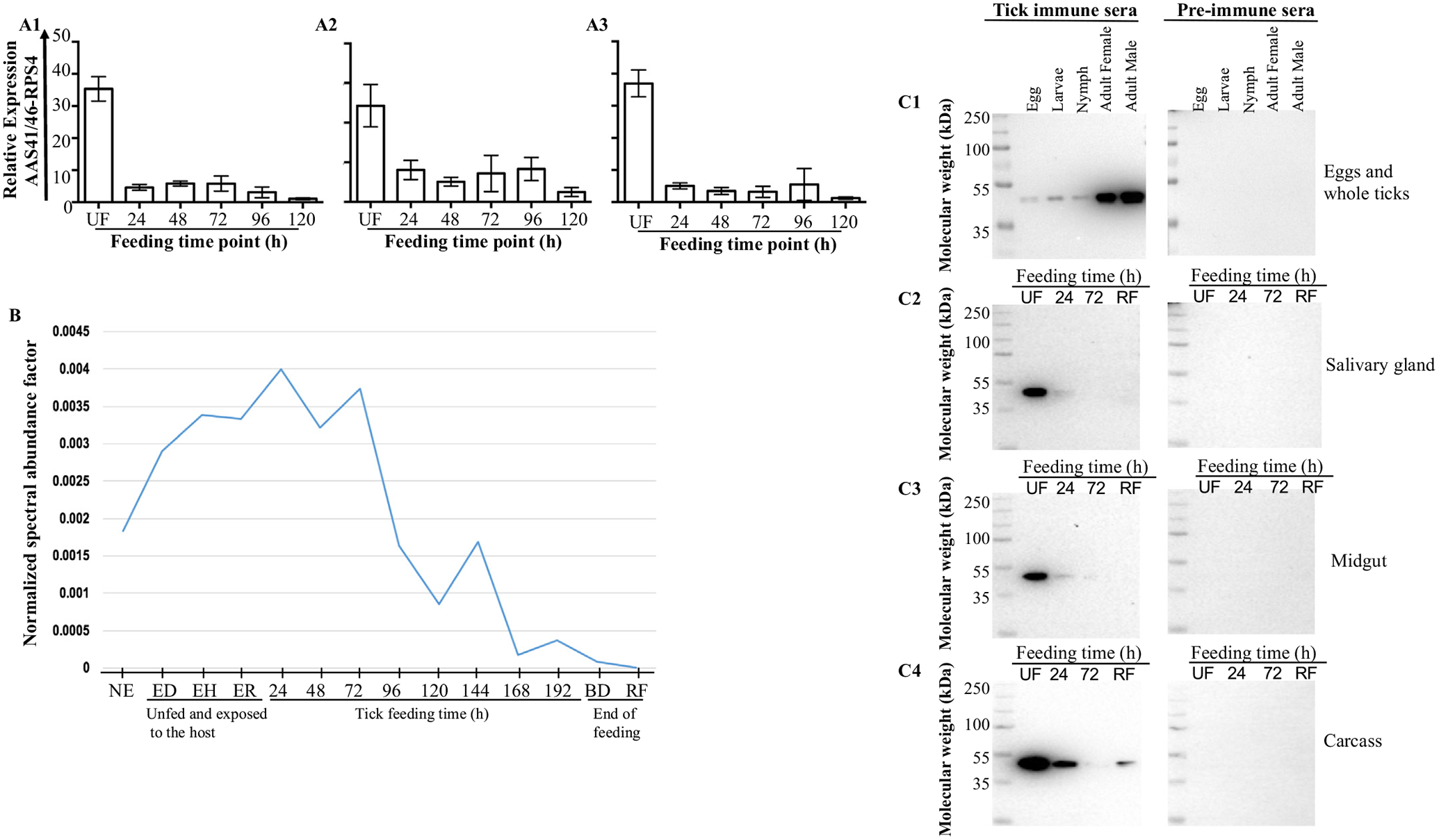
Amblyomma americanum (AAS) 41 and 46 transcript and protein are expressed in major tick organs and secreted saliva with identical profiles. (A) Temporal and spatial expression analysis of AAS41/46 mRNA was determined in dissected organs of unfed, 24, 48, 72, 96, and 120 h fed ticks: salivary glands (A1), midguts (A2) and carcass (A3; tick remnant after removal of SG and MG) were analyzed by qRT-PCR. (B) Normalized spectral abundance factor (NSAF) (y-axis) as index for relative AAS41/46 protein abundance extracted from published work [41,42] in saliva of unfed tick not exposed to the host (taken straight from incubator) and exposed to different hosts: dog-, human-, rabbit, and during feeding (24–192 h, BD, and SD; x-axis). BD = fully engorged but not detached; SD = fully engorged spontaneously detached. (C) Monospecific IgG to AAS41/46 (0.5 mg/ml, 1:500 dilution) that was purified from serum of rabbits that were repeatedly infested with A. americanum ticks was used to screen for native AAS41/46 in all life stages: egg, larvae, nymphs, female and male ticks (C1) and dissected adult tick tissues: salivary glands (C2), midguts (C3) and remnants as carcass (4). Purified IgG of non-infested rabbits were included as controls for respective western blots.
The secretion dynamics of native AAS41/46 (Fig. 3B) was determined based on previously published tick saliva proteomes of unfed ticks that were not stimulated to feed (taken from incubator), those that were stimulated to start feeding on rabbits, dogs, and humans [41] and those that had fed on rabbits [42]. It is interesting to note that similar to the transcription profile (Fig. 3B), native AAS41/46 protein was secreted at high levels in unfed ticks and during the 24–72 h of feeding as revealed by normalized spectral abundance factors (NSAF, index for relative protein abundance).
Finally, the immunoblots of native tick protein extracts of tick eggs and unfed whole ticks (larvae, nymph, adult female and male ticks) were probed using purified monospecific IgG antibodies to rAAS41/46 produced in rabbits (Fig. 3C). As shown in figure 3C1, native AAS41/S46 protein is expressed in all A. americanum tick life stages (eggs, larvae, nymphs, adult female and male). As equivalent amounts of total protein were electrophoresed, it is apparent that higher amounts of AAS41/46 are highly expressed in the adults. Fig. 3C shows the expression of AAS41/46 protein in dissected tick organs (salivary glands [SG], midgut [MG], and carcass [CA]), of unfed (UF) and those that fed for 24 and 72 h and replete fed (RF). Consistent with data in Fig. 3A and B, Fig. 3C show that native AAS41/46 were most abundantly present in tissues of unfed ticks and reduced during feeding (Figs. 3C2\\C4). The pre-immune sera did not bind native AAS41/46 confirming specificity of the test.
3.3. Native AAS41/46 protein is among tick saliva immunogens that provoke tick feeding resistance in repeatedly infested rabbits
Fig. 4 shows by western blot analysis that native rAAS 41 and 46 were among tick saliva immunogens that provoke immunity against tick feeding in repeatedly infested rabbits. Both rAAS 41 and 46 specifically bound antibodies to saliva of A. americanum female ticks that repeatedly infested rabbits for 24 h (Fig. 4A), 48 h (Fig. 4B), and were replete fed (Fig. 4C). Fig. 4D show that pre-immune sera did not bind rAAS 41 and 46 demonstrating specificity of the assay.
Fig. 4.
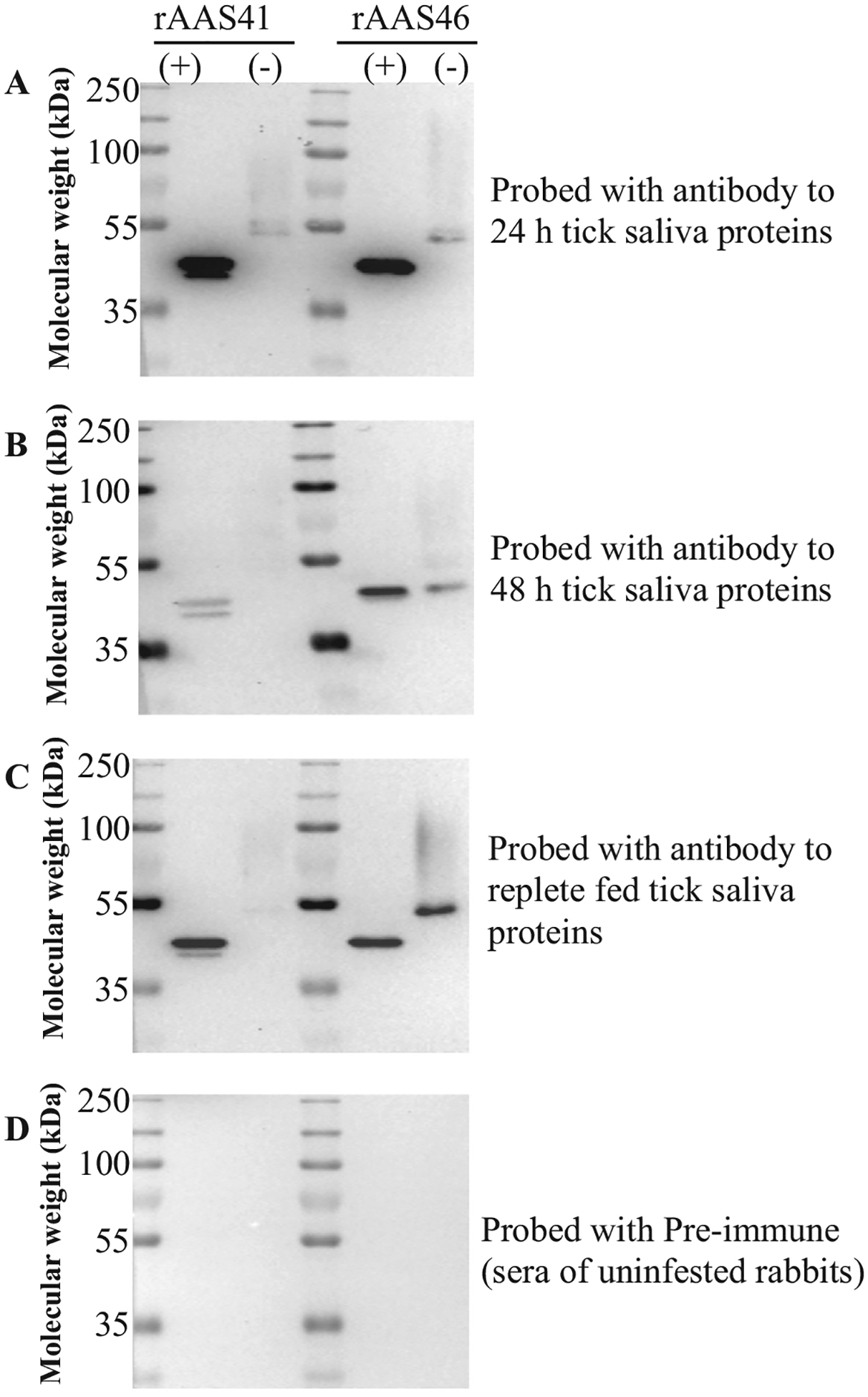
Amblyomma americanum serpins (AAS) 41 and 46 are immunogenic tick saliva proteins. Immunoblots of purified recombinant AAS 41 and 46 (deglycosylated and non-treated) were probed with antibodies to tick saliva proteins of A. americanum ticks that repeatedly fed on rabbits for 24 h (A), 48 h (B), and replete fed (C). Pre-immune serum was used for negative control (D). All antibodies were used at 1:500 dilution.
3.4. Functional roles of AAS41/46 are important to tick feeding success
To gain insight on the functional importance of AAS41/46 in tick feeding, we assessed the effects of RNAi-mediated silencing on A. americanum feeding summarized in Fig. 5. Given the fact that AAS41/46 were highly expressed in unfed ticks, it was important to first deplete the AAS41/46 transcript from unfed ticks before assessing the effects of RNAi-mediated silencing on tick feeding as described by our group [83],[84]. The rationale for depletion of target mRNA from unfed ticks is to prevent ticks from expressing the cognate protein when placed on rabbits to feed. In our experience, without depletion of mRNA from unfed ticks, the effects of RNAi-mediated silencing on constitutively expressed proteins such as AAS41/46 might be masked by proteins that were expressed before disruption of mRNA took effect.
Fig. 5.
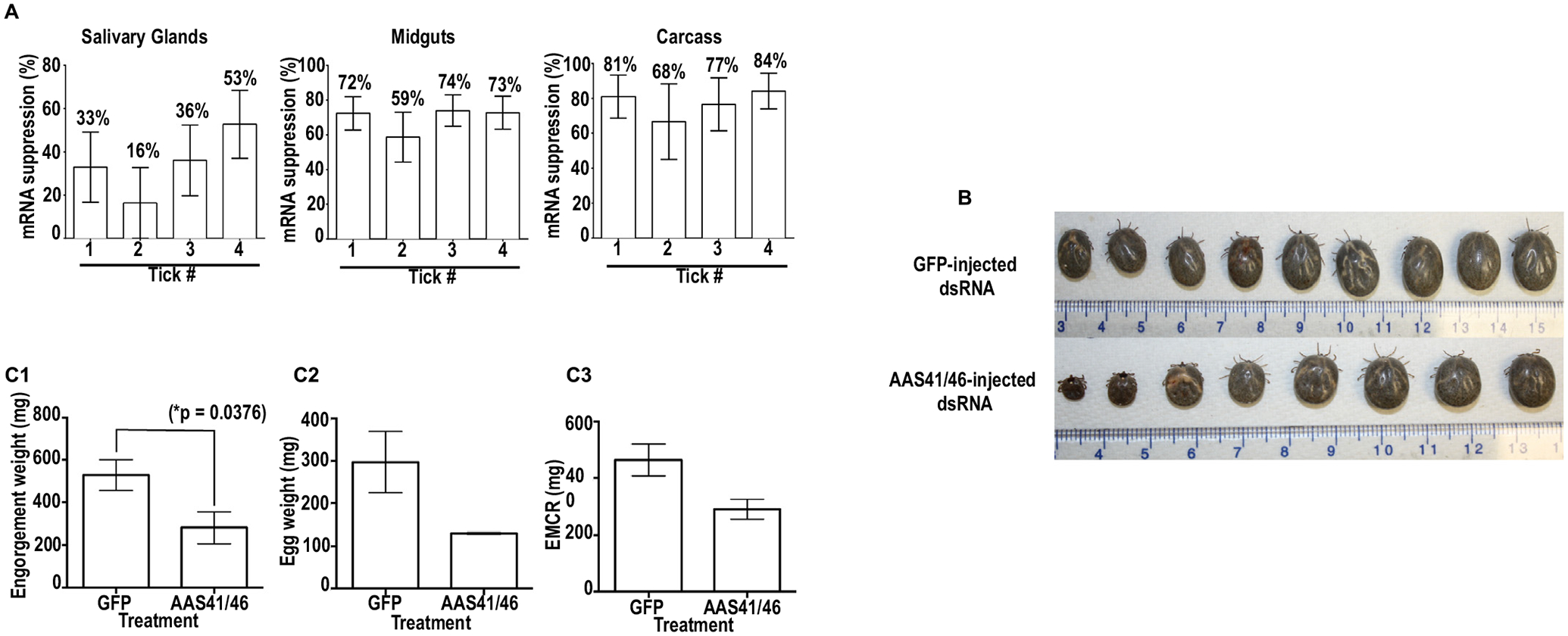
RNAi-mediated silencing of Amblyomma americanum serpins (AAS) 41/46 mRNA affects tick feeding. Double stranded (ds) RNA of AAS41/46 and GFP (control) was synthesized in vitro and microinjected in ticks to trigger disruption of AAS41/46 mRNA. (A) At 48 h post attachment AAS41/46-dsRNA and GFP-dsRNA injected ticks sampled disruption of cognate mRNA was validated by qRT-PCR in tissues that were dissected from individual ticks. (B) A. americanum adult female ticks were allowed to feed to repletion and observed phenotypes were documented upon detachment from the host. (C) Effects of RNA-mediated silencing on: (C1) amount of blood ingested, (C2) quantity of eggs laid, and (C3) ability to convert blood meal into eggs.
To deplete mRNA, unfed ticks that were injected with AAS41/46-double stranded RNA (dsRNA) were analyzed using qualitative RT-PCR for cognate mRNA expression at 3, 7, 14, and 21 days post dsRNA injection (Supplementary Fig. 3). As shown, most of AAS41/46 mRNA was depleted by day 21 after injecting dsRNA in two of the three ticks (Supplementary Fig. 3). Subsequently, dsRNA injected ticks were incubated for three weeks before assessing the effects of RNAi-mediated silencing on tick feeding. To validate target mRNA suppression in A. americanum ticks that had attached to feed, four ticks per treatment at 72 h of post-attachment were sampled and confirmed in individual ticks that AAS41/46 transcript was suppressed by 16–53%, 59–74%, and 68–84% in respective SG, MG and CA (Fig. 5A) when compared to the GFP-dsRNA injected control.
To assess the impact of disrupting AAS41/46 mRNA on feeding success, ticks were allowed to feed to repletion. Overall, RNAi-mediated silencing did not affect tick attachment and feeding rates as both control (GFP-dsRNA injected) and treatment ticks attached within 24 h of placement and completed feeding up to 10 days. Replete fed ticks were photographed to document morphological phenotypes due to RNAi (Fig. 5B). Subsequently engorgement mass of replete fed ticks, as index for amount of blood consumed by the tick, showed that RNAi-mediated silencing significantly (p = .0376) reduced the amount of blood ingested by ticks (Fig. 5C1). Replete fed ticks were incubated at 22 °C with >85% relative humidity to allow for egg laying. Our data show that, though not significant, RNAi-mediated silencing caused a reduction in total egg mass (Fig. 5C2), and the ability of fed ticks to convert the blood meal into eggs (Fig. 5C3). It is interesting to note that while 57% (4/7) of EGFP-dsRNA injected ticks laid eggs, AAS41/46-dsRNA injected ticks had only 33% (2/6) (not shown).
3.5. Recombinant (r)AAS41 is an inhibitor of chymase and chymotrypsin while rAAS46 is not inhibitory
To gain insight into probable functional roles of rAAS41 and 46 at the tick feeding site, their inhibitory activity was screened against 19 mammalian serine proteases related to host defense pathways as described [28,30]. This analysis revealed that rAAS41 (1 μM) inhibited activity of human chymase (83.3 nM) by 95%, rat and mouse chymase (PDMC extract) by a respective 100 and 96%, bovine pancreatic α-chymotrypsin (2.8 nM) by 99%, papain (4.3 μM) by 60%, cathepsin G (425.5 nM) by 23%, and pancreatic elastase (61.8 nM) by 19% (Fig. 6A). In contrast rAAS46 (1 μM) inhibited activity of human chymase (83.3 nM) by 11.6%, mouse chymase (PDMC extract) by 6%, bovine pancreatic α-chymotrypsin (2.8 nM) by 16%, papain (4.3 μM) by 62%, cathepsin G (425.5 nM) by 29%, human neutrophil proteinase 3 (280 nM) by 36%, and pancreatic elastase (61.8 nM) by 19% (Fig. 6B).
Fig. 6.
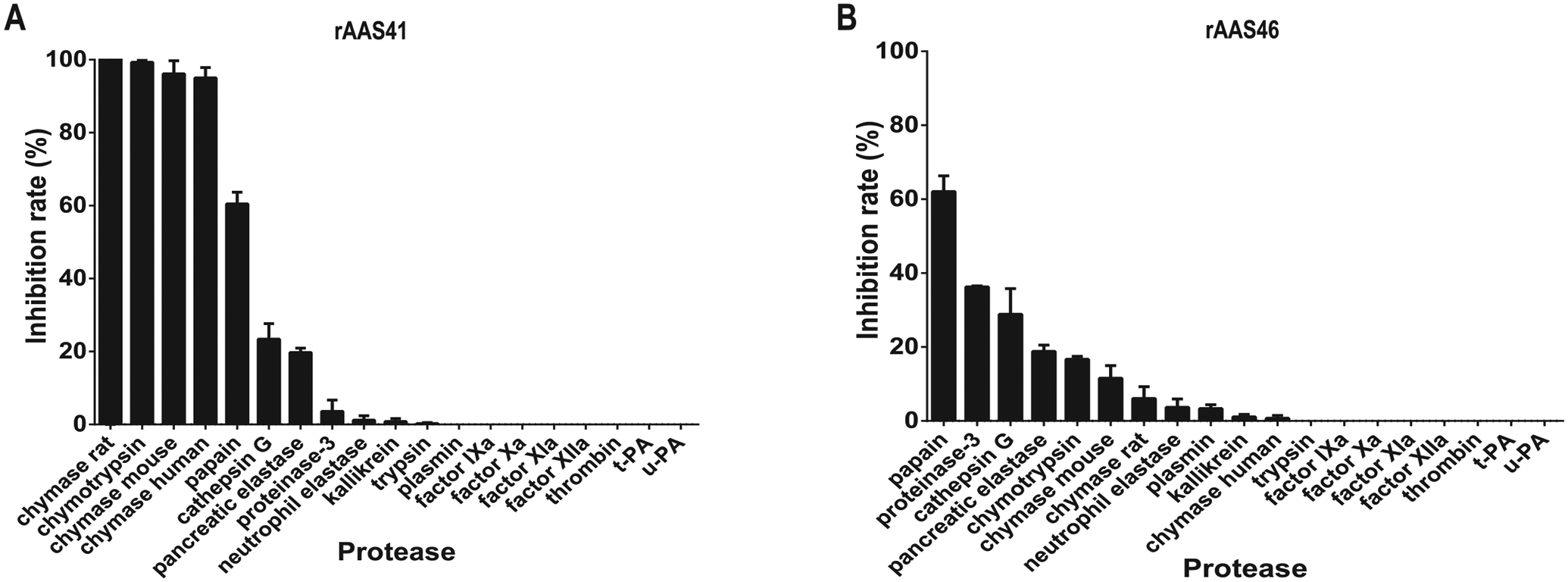
Protease inhibitor profiling for affinity purified recombinant Amblyomma americanum serpin (rAAS) 41 and 46. Indicated proteases (concentration indicated in materials and methods) were incubated with affinity purified (A) rAAS41 or (B) rAAS46 (1 μM) at 37 °C for 15 min followed by addition of appropriate substrate (0.2 mM). Protease kinetics was monitored every 11 s over 15 min in triplicate.
To check for inhibitory efficiency, the stoichiometery of inhibition (SI) for rAAS41 upon human chymase and bovine pancreatic α-chymotrypsin activities were determined. Further biochemical assays were not performed for rAAS46 as initial protease inhibition assays did not show inhibitory activity (>70%) against the 19 screened mammalian proteases. Accordingly, the SI index for rAAS41 was 3.18 against bovine pancreatic chymotrypsin (Fig. 7A) and 3.23 against human chymase (Fig. 7B). A typical inhibitory serpin will form a SDS and heat stable complex with its target protease [50,51]. Fig. 7C and D show that rAAS41 formed stable complexes with respective to human chymase and bovine α-chymotrypsin as revealed by high molecular weight complexes that were stable with SDS and heat treatments (indicated by arrow head). These irreversible complexes between rAAS41 and the target proteases were observed at similar molar ratios comparable to the SI.
Fig. 7.
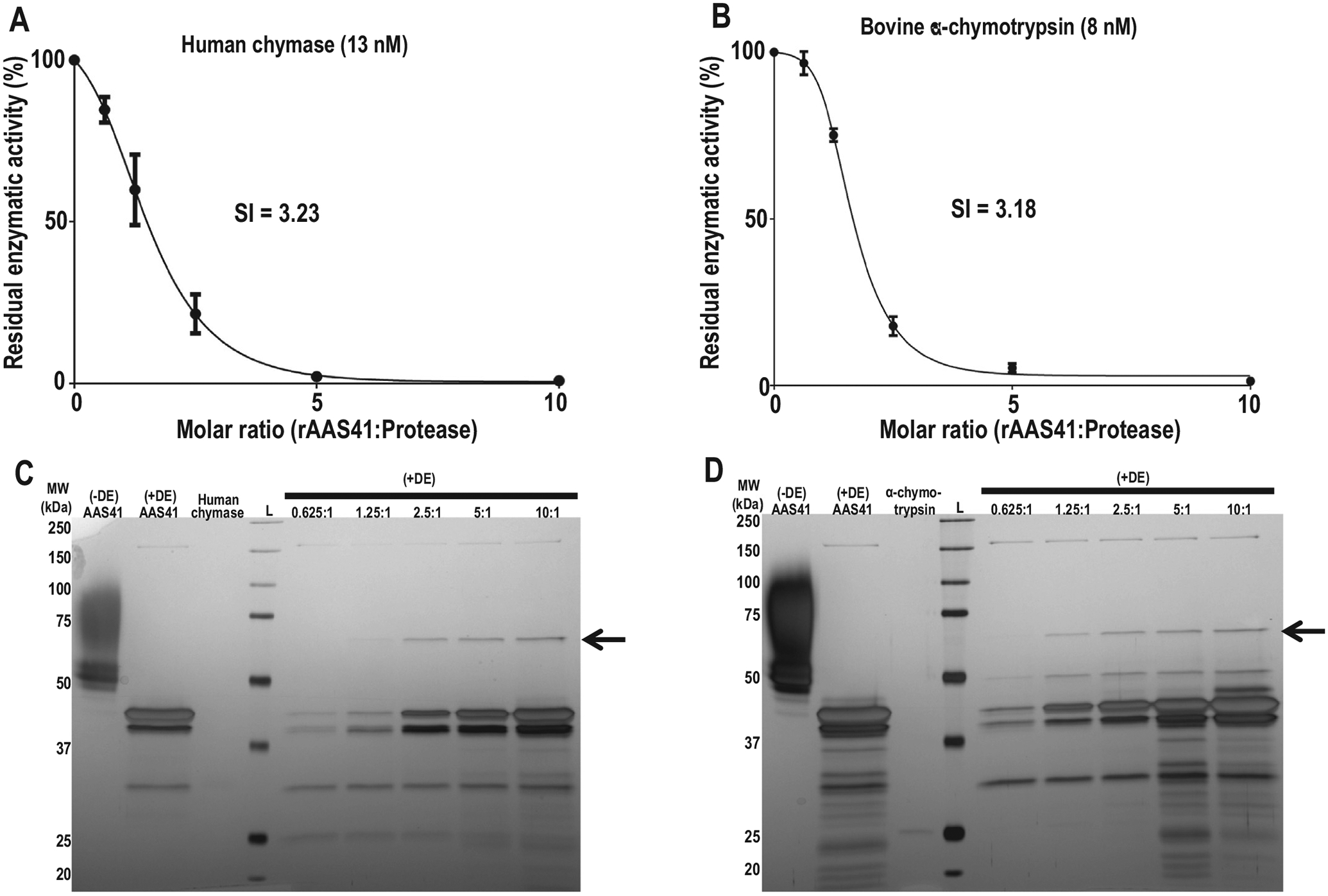
Affinity purified recombinant Amblyomma americanum serpin (rAAS) 41 forms stable heat- and SDS- stable complexes with target proteases. Stoichiometry inhibition (SI) was determined by measuring residual enzyme activity of (A) human chymase (13 nM) and (B) bovine α-chymotrypsin (8 nM) following incubation rAAS41 at variable molar ratios (rAAS41:protease) ranging from 0 to 10. The data were plotted as residual protease activity (Vi/V0) versus molar ratio (rAAS41:protease). Increasing amounts of rAAS41 were pre-incubated for 1 h at 37 °C with 0.1 μg of human chymase (C) or 0.1 μg of bovine α-chymotrypsin (D) in molar ratios varying from 0.625:1 to 10:1 (rAAS41:protease). Samples were treated with a deglycosylation enzyme (DE) mix (to remove glycans that were masking results) and resolved on a 10% SDS-PAGE and silver stained to visualize covalent complexes. The rAAS41:protease complex is denoted by the black arrow (←). The (+) or (−) denotes treatments with or without deglycosylation, respectively.
The association rate constant (ka) of rAAS41 with human chymase was determined under pseudo-first order conditions using a discontinuous method [52]. The ka for the interaction of rAAS41 and human chymase and chymotrypsin were determined as respective ka 5.6 ± 0.37 × 103 M−1 s−1 (Fig. 8A) and 1.6 ± 4.1 × 104 M−1 s−1 (Fig. 8B) demonstrating that rAAS41 might be an effective inhibitor of these proteases.
Fig. 8.
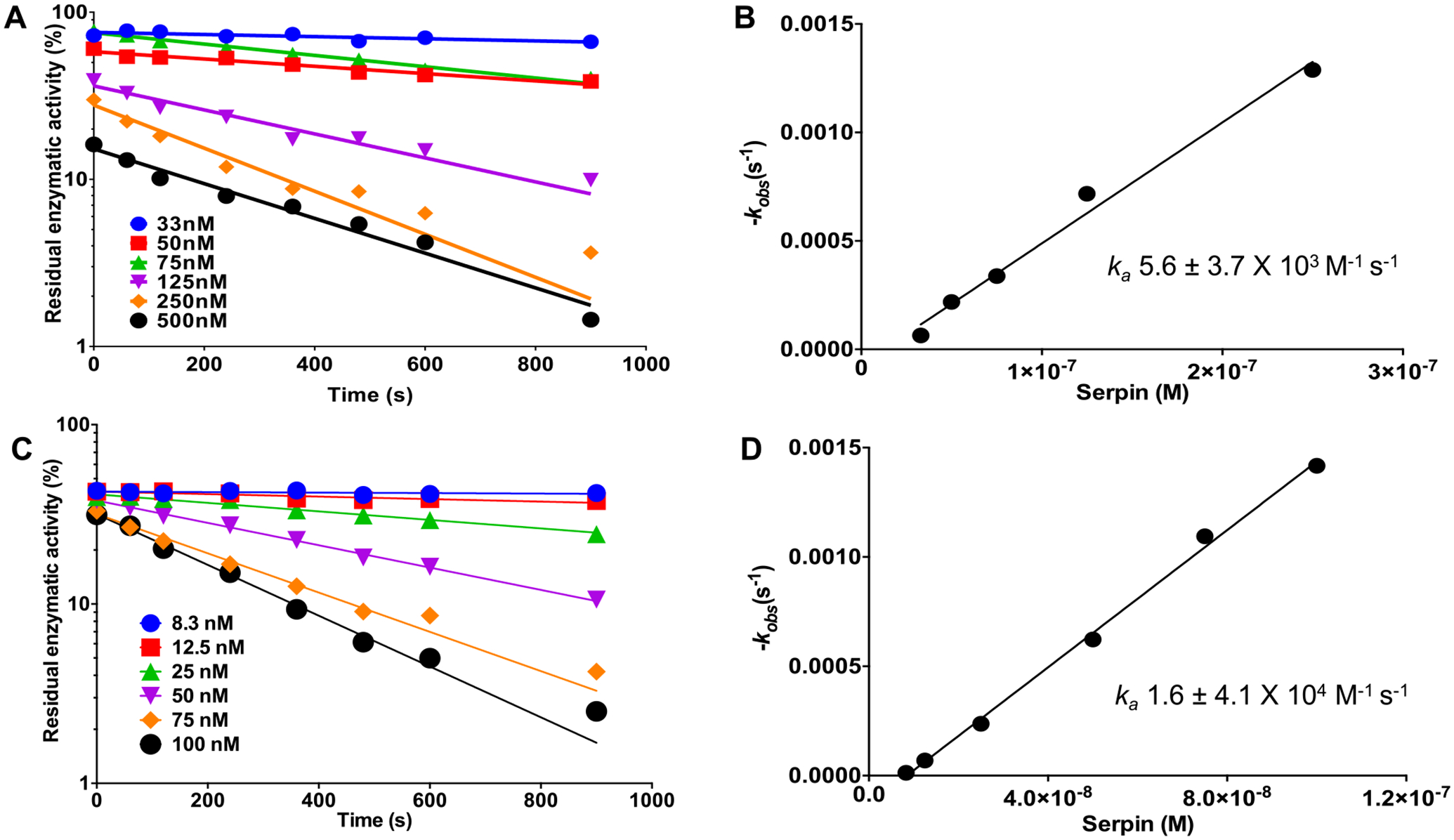
Recombinant Amblyomma americanum serpin (rAAS) 41 is an effective inhibitor of chymase and α-chymotrypsin. Discontinuous method inhibition of chymase or chymotrypsin by rAAS41 was conducted as described in materials and methods. Semilogarithmic plots of residual enzymatic activity of chymase (A) and α-chymotrypsin (C) versus time of incubation for reactions at various concentrations of rAAS41 (33–500 nM) for the former and rAAS41 (8.3–100 nM) for the latter. Plot of kobs as a function of rAAS41 concentration for chymase (B) and α-chymotrypsin (D). Linear regression of the slope represents the second-order rate constant (ka) for the inhibition of chymase and α-chymotrypsin by rAAS41.
Given that rAAS41 was immunogenic (Figs. 3 and 4) the ability of antibodies to rAAS41/46 to interfere with function of rAAS41 was tested. Fig. 9 shows that purified monospecific antibodies to rAAS41/46 dose responsively blocked inhibitory functions of rAAS41 against chymase and chymotrypsin by 17–84% and 5–60% respectively.
Fig. 9.
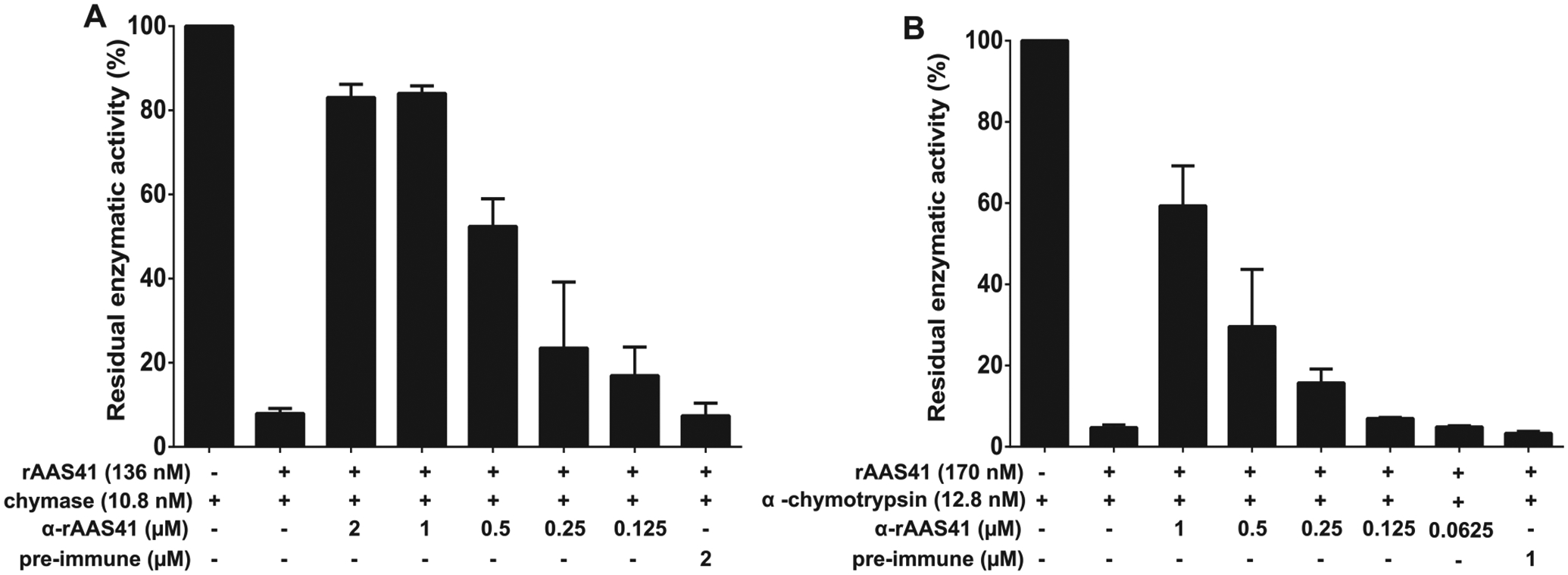
Recombinant Amblyomma americanum serpin (rAAS)41 inhibition of chymase and α-chymotrypsin is reduced using monospecific antibody to rAAS41. In triplicate reactions, purified monospecific IgG antibodies to rAAS41 or pre-immune (PI) serum were incubated with 136 nM or 170 nM of rAAS41 at 37 °C for 30 min before the addition of 10.8 nM of chymase (A) or 12.8 nM of α-chymotrypsin (B) following an additional 15 min incubation at 37 °C. Subsequently, the chymase or α-chymotrypsin substrate (0.2 mM) were added and protease kinetics was monitored for 15 min every 15 s. Data is reported as residual enzymatic activity.
3.6. rAAS41 inhibits compound 48/80 induced inflammation in a rat model
To investigate if rAAS41 was functional in vivo the rat paw edema model was used (Fig. 10). Injection of compound 48/80 (a mast cell degranulator) induced acute inflammation that was characterized by edema formation, as revealed by an increase in paw thickness reaching a maximum 30 min post injection. When co-injected with rAAS41 (25 μg/paw) edema formation was significantly reduced by 38% (p = .0142) at 30 min, 72% (p = .0009) at 60 min, and 55% (p = .0072) at 120 min post-injections compared to injection of compound 48/80 only (Fig. 10A). When injected intradermally, compound 48/80-induced mast cell degranulation also increases vascular permeability into the subcutaneous tissue and estimated using the Miles assay by measuring Evans blue dye fluid extravasation in rat skin [54]. When compared to saline injection, compound 48/80 significantly increased the vascular permeability into skin subcutaneous tissue. However, co-injection with rAAS41 (25 μg/spot) significantly reduced the amount of tissue extravasated Evans blue dye that was secreted when compared to injection of compound 48/80 only (p = .0033) (Fig. 10B and C).
Fig. 10.
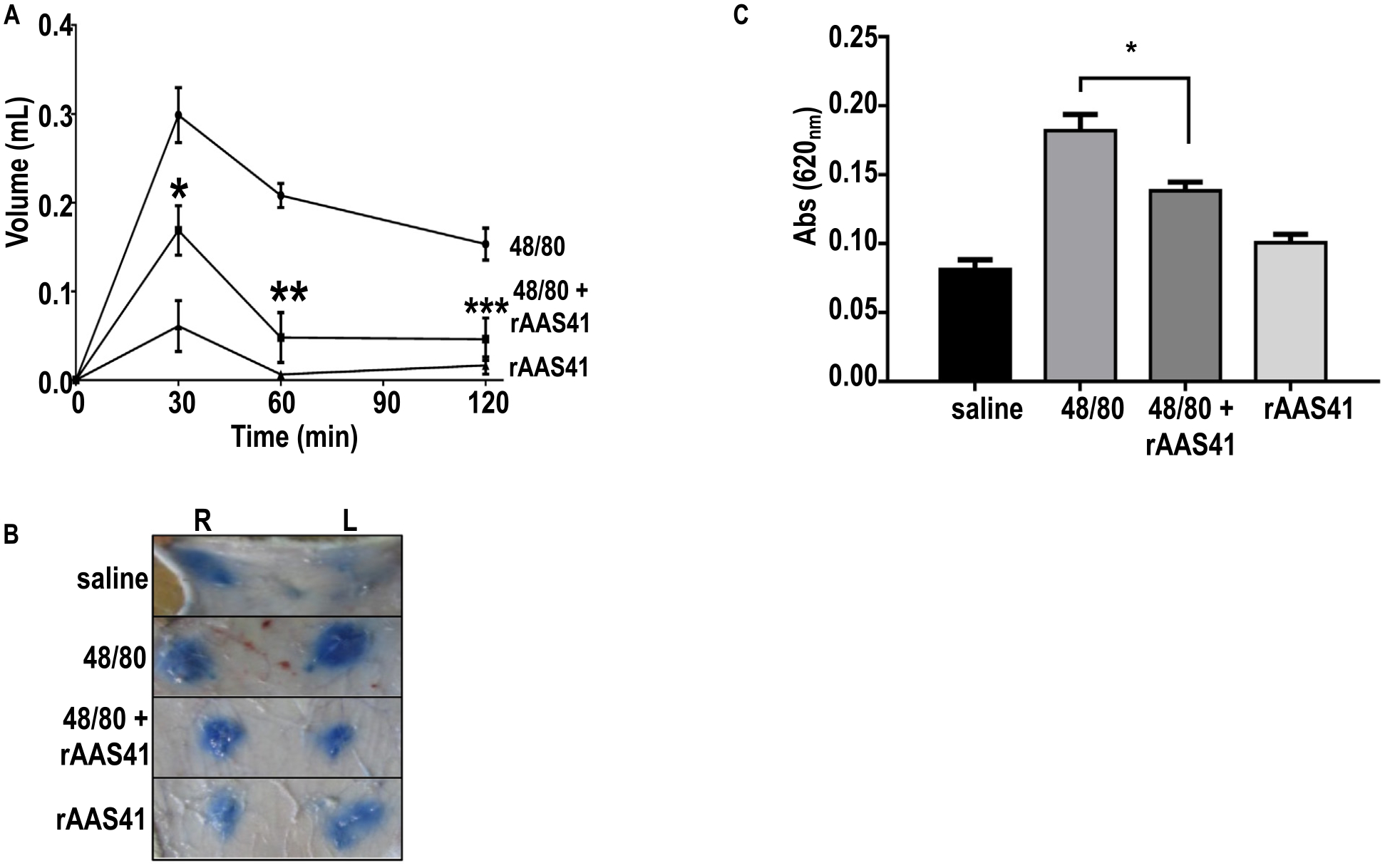
Recombinant Amblyomma americanum serpin (rAAS) 41 blocks compound 48/80-induced acute inflammation in rats. (A) Rat paw edema induced by intradermal injection of 100 μl of compound 48/80 (1 μg) in saline (circles), co-injected with 25 μg of rAAS41 (squares), or 100 μl of rAAS41 only (triangles). Edema formation was estimated using a digital plethysmometer at different intervals. Posterior paws from 5 animals were used for each data point. *p = .0142, **p = .009, and ***p = .0072 (t-test). (B) Vascular permeability (Miles assay) was performed by intravenous injection of 700 μl Evan’s blue dye into the tails of rats. Ten minutes later, 100 μl of (i) saline, (ii) compound 48/80 (1 μg) only, (iii) compound 48/80 (1 μg) co-injected with rAAS41 (25 μg), or (iv) only rAAS41 (25 μg) was injected intradermally on the dorsal right (R) and left (L) sides of the rat. After 60 min, animals were euthanized and skin removed to allow injection sites to be photographed. (C) Evans blue extravasation was estimated after extraction with formamide and reading at absorbance 620 nm. Results are the average of experiments obtained with 6 animals. *p = .0033 (t-test).
3.7. AAS41 is an inhibitor of mast cell native chymase from rat and mouse peritoneal fluids
Given that rAAS41 inhibited compound 48/80 induced inflammation that is chymase mediated (reviewed in [53]), Fig. 11 shows rAAS41 inhibition of chymase in a complex native solution. As shown in Fig. 11A, incubating rAAS41 (1 μM) at 37 °C for 15 min inhibited 100% chymase activity in rat peritoneum-derived mast cells (PDMC) extracts that contained 1 U of chymase activity. Likewise, under the same conditions, incubation of rAAS41 at various time points (5–60 min) progressively formed the rAAS41-rat chymase complex (Fig. 11C) as confirmed by LC-MS/MS analysis of the complex (Supplementary Table 1 and Supplementary Figure 4). Data in Supplementary Table 1 confirmed that rAAS41 formed an irreversible complex with native rMCP-1 as demonstrated by the 13 peptides that were identified in the complex mixture that matched to rMCP-1 (Uniprot #: P09650). Finally, Fig. 11C, confirms that chymase activity against casein was progressively inhibited by rAAS41 (Fig. 11C).
Fig. 11.
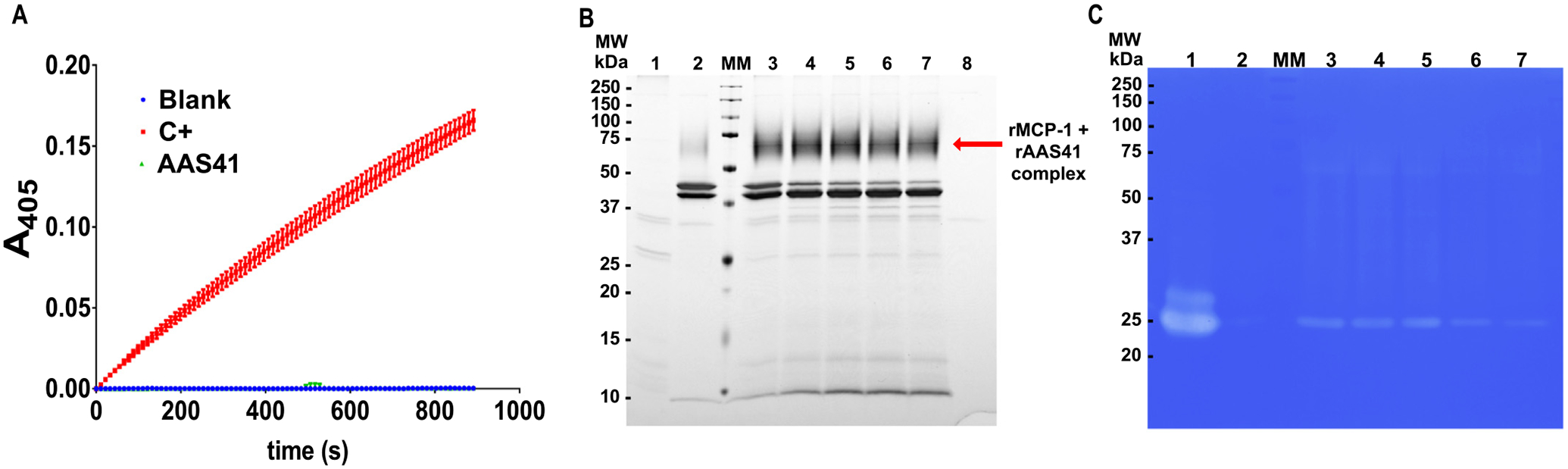
Recombinant Amblyomma americanum serpin (rAAS) 41 inhibits and forms covalent complex with rat mast cell protease 1 (rMCP-1). (A) Inhibition of rat mast cell protease-1 (rMCP-1) activity from peritoneum-derived mast cells (PDMC) protein extract (1 U of chymase activity) was incubated with rAAS41 (1 μM) at 37 °C for 15 min following addition of substrate N-Succinyl-Ala-Ala-Pro-Phe-pNA (0.2 mM). Protease kinetics was monitored for 15 min with reads at every 11 s in triplicate. MW = molecular weight, MM = molecular marker, C+ = positive control. (B) Purified rAAS41 and rMCP-1 complex was resolved by 12% SDS-PAGE and visualized by silver staining. As controls, the gel was loaded with a protein extract containing rat chymase isolated from PDMC (1) and A. americanum serpin AAS41 (2). Complex formation (in red arrow) was evaluated by incubating equal volume of PDMC with AAS41 (4 μg) at 37 °C for various time points: 5 min (3), 15 min (4), 30 min (5), 45 min (6) and 60 min (7) and treated with deglycosylation enzyme mix. The enzyme deglycosylation mix only was loaded as a control (8). (C) Zymography was performed using 10% SDS-PAGE containing 0.1% casein. Gel was loaded with a protein extract containing rMCP-1 activity isolated from PDMC only (1) and rAAS41 only (2) as controls. Inhibition of rMCP-1 was performed by incubation with equal volume of PDMC with rAAS41 (4 μg) at 37 °C for different times: 5 min (3), 15 min (4), 30 min (5), 45 min (6) and 60 min (7).
4. Discussion
This study demonstrates that two highly identical A. americanum tick saliva serpins, AAS 41 and 46 that are 97% identical have different biological functions. Host inflammation response to tick feeding is one of the cardinal host defenses that the tick must modulate to successfully feed and transmit TBD agents. While anti-inflammatory properties of tick saliva are well documented [58,59] the molecular identity of mediators in A. americanum saliva anti-inflammation effect are not fully known. This study provides evidence that AAS41 is likely among the saliva proteins that A. americanum utilizes to evade the host’s inflammation defense against tick feeding by targeting chymase and chymotrypsin. This study builds upon our recent study that described A. americanum tick saliva serpin (AAS) 27 as an inhibitor of inflammation that acts via trypsin and plasmin [30].
Given the high sequence identity (97%) between AAS 41 and 46, our transcription, RNAi-mediated silencing, and western blotting analyses is for both proteins. Our qRT-PCR and western blotting analyses data showing that AAS41/46 mRNA and protein are expressed in multiple tick organs suggested that one of these proteins or both played significant roles in tick feeding physiology. The finding that AAS41/46 proteins are highly abundant in unfed and early stage of tick feeding (24–72 h feeding time points) make these two proteins attractive candidates for anti-tick vaccine development. The 24–72 h tick feeding time points co-incides with key tick feeding processes when the tick creates its feeding lesion (through first 24–36 h of feeding [60,61]), and starts to transmit tick-borne disease agents (after first 48 h of feeding [62–64]). From this perspective it was encouraging to note that RNAi-mediated silencing of AAS41/46 mRNA significantly reduced the feeding efficiency resulting in reduced blood meal feeding and fecundity. These findings support the use of AAS41/46 as potential candidate vaccine antigens. Repeatedly infested animals can mount strong immunity against tick feeding that manifests in reduced tick feeding efficiency, high tick mortality [65–72], and most importantly those animals are protected against TBD infections [73–76]. Thus, it was encouraging by findings that both rAAS 41 and 46 specifically bound immune sera of repeatedly infested rabbits that had acquired protective immunity against tick feeding. These data suggest that native AAS41/46 is likely among tick salivary immunogens that provoked host immunity against tick feeding in repeatedly infested animals, and thus might be useful as a vaccine antigen target to prevent tick-borne disease infections. The finding that antibodies to rAAS41/46 blocked inhibitory functions of rAAS41 against both chymase and chymotrypsin is encouraging. These data, point to the possibility of designing an anti-tick vaccine that can immunologically block the functions of proteins important for the tick feeding process.
When ticks attach onto host skin to begin feeding, mast cells alongside neutrophils and macrophages are among pro-inflammatory immune cells that infiltrate the tick feeding site [77]. There is evidence that mast cells play critical roles in host response to tick feeding and comprised of 10% of the cutaneous cellular responses at the tick feeding site in cattle that were fed by A. americanum [69,70]. Two studies demonstrated that mast cell deficient mice could not develop resistance against Haemaphysalis longicornis larvae tick feeding [78], while wild type mice with intact mast cell response expressed tick feeding resistance by massive degranulation of these cells at the feeding site [79]. Although there is no empirical data, the observed mast cell degranulation at the tick feeding site is expected to result in release of chymase, which will lead to enhanced inflammation. On this basis, our findings that rAAS41 inhibited chymase and compound 48/80 induced inflammation, strongly suggest that A. americanum might utilize native AAS41 to inflammation in response to tick feeding by inhibiting chymase that is secreted by degranulating mast cells at the tick feeding site.
Ticks accomplish feeding by creating a lesion in the skin and ingesting blood that accumulates in the wound [60]. The expected host response is tissue repair, which if successful will prevent the tick from completing a blood meal. Chymotrypsin and trypsin play critical roles in tissue repair [24], such as might be required at the tick feeding site. Following injury, α1-antitrypsin and α2-macroglobulin, inhibitors of both trypsin and chymotrypsin are secreted in abundance, and if left uncontrolled could lead to delayed wound healing [24]. Given that rAAS41 is a strong inhibitor of chymotrypsin, it is conceivable that native AAS41 in A. americanum tick saliva might interfere with the host’s tissue repair response by inhibiting chymotrypsin at the tick feeding site. It is notable that native AAS41/46 are secreted at low levels from 168 h feeding when the tick is preparing to complete feeding, and the tick may no longer need to control inflammation.
It is important to note that a typical inhibitory serpin forms a complex with its target protease that is resistant to SDS and heat denaturation, has stoichiometry of inhibition (SI) of close to 1, and a rate of inhibition (Ka) of 105 M−1S−1 [50]. It is notable that rAAS41 formed an SDS and heat resistant complexes with chymase and chymotrypsin, had low SI values of under ~3, and Ka values of 103 and 104 for chymase and chymotrypsin, which is close to what has been observed for other tick inhibitory serpins [30,32,80]. One of the interesting lessons from this study is that function of tick serpins were not extrapolated from sequence homology. It is noteworthy that despite the high sequence similarity between AAS 41 and 46, the two proteins are functionally different. The function of serpins is strongly influenced by the amino acid residue at the P1 position [50,51]. The difference in function between rAAS 41 and 46 might be explained by the fact that the former has a leucine at its P1 site, while the latter has a threonine amino acid residue at this position.
In conclusion, this study brings a significant contribution toward understanding the molecular basis of tick feeding physiology and discovery of putative antigens for anti-tick vaccines. In order to improve the design of tick vaccines, it is imperative to understand the functions of candidate antigens in tick feeding regulation. The goal is to utilize such data to design effective tick vaccine antigens that will provoke host immunity neutralizing functions of those proteins at the tick feeding site.
Supplementary Material
Acknowledgements
This work supported by funding to AM from the National Institutes of Health, USA grants (AI081093, AI093858, AI074789, AI074789-01A1S1) and National Center for Research Resources (5P41RR011823) and National Institute of General Medical Sciences (8P41GM103533) to JRY. ISVJ and CT are receiver of CNPq, CAPES and INCT-EM grants. LT was a receiver of the CNPq (Brazil) “Ciência sem Fronteiras” doctoral fellowship program (PVE 211273/2013-9). MB is a receiver of grants from CNPq (Chamada Universal MCTI/CNPq No 01/2016, Grant 407041/2016-8) and Fundo de Incentivo à Pesquisa e Eventos (FIPEHCPA, GPPG Grants no 16-0054 and 19-0001) at Hospital de Clínicas de Porto Alegre. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijbiomac.2020.04.088.
References
- [1].Anderson BE, Sims KG, Olson JG, Childs JE, Piesman JF, Happ CM, Maupin GO, Johnson BJ, Amblyomma americanum: a potential vector of human ehrlichiosis, Am. J. Trop. Med. Hyg 49 (1993) 239–244. [DOI] [PubMed] [Google Scholar]
- [2].Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA, Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis, N. Engl. J. Med 341 (1999) 148–155. [DOI] [PubMed] [Google Scholar]
- [3].Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA, A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma, Vet. Parasitol 79 (1998) 325–339. [DOI] [PubMed] [Google Scholar]
- [4].Wolf L, McPherson T, Harrison B, Engber B, Anderson A, Whitt P, Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina, J. Clin. Microbiol 38 (2000) 2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Taylor JP, Istre GR, McChesney TC, Satalowich FT, Parker RL, McFarland LM, Epidemiologic characteristics of human tularemia in the southwest-central states, 1981–1987, Am. J. Epidemiol 133 (1991) 1032–1038. [DOI] [PubMed] [Google Scholar]
- [6].Armstrong PM, Brunet LR, Spielman A, Telford SR 3rd, 2001. Risk of Lyme disease: perceptions of residents of a lone star tick-infested community. Bull. World Health Organ 79, 916–925. [PMC free article] [PubMed] [Google Scholar]
- [7].James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJ, Borrelia lonestari infection after a bite by an Amblyomma americanum tick, J. Infect. Dis 183 (2001) 1810–1814. [DOI] [PubMed] [Google Scholar]
- [8].Reeves WK, Loftis AD, Nicholson WL, Czarkowski AG, The first report of human illness associated with the Panola Mountain Ehrlichia species: a case report, J. Med. Case Rep 2 (2008) 13–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yabsley MJ, Loftis AD, Little SE, Natural and experimental infection of white-tailed deer (Odocoileus virginianus) from the United States with an Ehrlichia sp. closely related to Ehrlichia ruminantium, J. Wildl. Dis 44 (2008) 381–387. [DOI] [PubMed] [Google Scholar]
- [10].Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW, Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis 8 (2008) 597–606. [DOI] [PubMed] [Google Scholar]
- [11].Breitschwerdt EB, Hegarty BC, Maggi RG, Lantos PM, Aslett DM, Bradley JM, Rickettsia rickettsii transmission by a lone star tick, North Carolina, Emerg. Infect. Dis 17 (2011) 873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST, A new phlebovirus associated with severe febrile illness in Missouri, N. Engl. J. Med 367 (2012) 834–841. [DOI] [PubMed] [Google Scholar]
- [13].Savage HM, Godsey MS Jr., Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL, First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods, Am. J. Trop. Med. Hyg 89 (2013) 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Steinke JW, Platts-Mills TA, Commins SP, The alpha-gal story: lessons learned from connecting the dots, J. Allergy Clin. Immunol 135 (2015) 58–96 (quiz 597). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Felz MW, Durden LA, Oliver JHJ, Ticks parasitizing humans in Georgia and South Carolina, J. Parasitol 82 (3) (1996) 505–508. [PubMed] [Google Scholar]
- [16].Laird JS, Kocan AA, Kocan KM, Presley SM, Hair JA, Susceptibility of Amblyomma americanum to natural and experimental infections with Theileria cervi, J. Wildl. Dis 24 (1988) 679–683. [DOI] [PubMed] [Google Scholar]
- [17].Little SE, O’Connor TP, Hempstead J, Saucier J, Reichard MV, Meinkoth K, Meinkoth JH, Andrews B, Ullom S, Ewing SA, Chandrashekar R, Ehrlichia ewingii infection and exposure rates in dogs from the southcentral United States, Vet. Parasitol 172 (2010) 355–360. [DOI] [PubMed] [Google Scholar]
- [18].Allen KE, Thomas JE, Wohltjen ML, Reichard MV, Transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum nymphs, Parasit. Vectors 12 (2019) 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yabsley MJ, Quick TC, Little SE, Theileriosis in a white-tailed deer (Odocoileus virginianus) fawn, J. Wildl. Dis 41 (2005) 806–809. [DOI] [PubMed] [Google Scholar]
- [20].Barnard DR, Injury thresholds and production loss functions for the lone star tick, Amblyomma americanum (Acari: Ixodidae), on pastured, preweaner beef cattle, Bos taurus, J. Econ. Entomol 78 (1985) 852–855. [DOI] [PubMed] [Google Scholar]
- [21].Tolleson DR, Teel PD, Stuth JW, Strey OF, Welsh TH Jr, Carstens GE, Longnecker MT, Banik KK, Prince SD, 2010. Effects of a lone star tick (Amblyomma americanum) burden on performance and metabolic indicators in growing beef steers. Vet. Parasitol 173, 99–106. [DOI] [PubMed] [Google Scholar]
- [22].Gettins PG, Olson ST, Inhibitory serpins. New insights into their folding, polymerization, regulation and clearance, Biochem. J 473 (2016) 2273–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rau JC, Beaulieu LM, Huntington JA, Church FC, Serpins in thrombosis, hemostasis and fibrinolysis, J. Thromb. Haemost 5 (Suppl. 1) (2007) 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shah D, Mital K, The role of trypsin:chymotrypsin in tissue repair, Adv. Ther 35 (2018) 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mulenga A, Sugino M, Nakajim M, Sugimoto C, Onuma M, Tick-encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development, J. Vet. Med. Sci 63 (2001) 1063–1069. [DOI] [PubMed] [Google Scholar]
- [26].Chmelar J, Oliveira CJ, Rezacova P, Francischetti IM, Kovarova Z, Pejler G, Kopacek P, Ribeiro JM, Mares M, Kopecky J, Kotsyfakis M, A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation, Blood 117 (2011) 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ibelli AM, Kim TK, Hill CC, Lewis LA, Bakshi M, Miller S, Porter L, Mulenga A, A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting, Int. J. Parasitol 44 (2014) 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim TK, Tirloni L, Radulovic Z, Lewis L, Bakshi M, Hill C, da Silva Vaz I Jr, Logullo C, Termignoni C, Mulenga A, 2015. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol 45, 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mulenga A, Kim T, Ibelli AM, Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation, Insect Mol. Biol 22 (2013) 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tirloni L, Kim TK, Berger M, Termignoni C, da Silva Vaz I Jr, Mulenga A, 2019. Amblyomma americanum serpin 27 (AAS27) is a tick salivary anti-inflammatory protein secreted into the host during feeding. PLoS Negl. Trop. Dis 13, e0007660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tirloni L, Seixas A, Mulenga A, Vaz Ida S Jr, Termignoni C, 2014. A family of serine protease inhibitors (serpins) in the cattle tick Rhipicephalus (Boophilus) microplus. Exp. Parasitol 137, 25–34. [DOI] [PubMed] [Google Scholar]
- [32].Xu T, Lew-Tabor A, Rodriguez-Valle M, Effective inhibition of thrombin by Rhipicephalus microplus serpin-15 (RmS-15) obtained in the yeast Pichia pastoris, Ticks Tick Borne Dis 7 (2016) 180–187. [DOI] [PubMed] [Google Scholar]
- [33].Imamura S, da Silva Vaz I Junior, Sugino M, Ohashi K, Onuma M, 2005. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 23, 1301–1311. [DOI] [PubMed] [Google Scholar]
- [34].Imamura S, Konnai S, Vaz Ida S, Yamada S, Nakajima C, Ito Y, Tajima T, Yasuda J, Simuunza M, Onuma M, Ohashi K, Effects of anti-tick cocktail vaccine against Rhipicephalus appendiculatus, Jpn. J. Vet. Res 56 (2008) 85–98. [PubMed] [Google Scholar]
- [35].Sugino M, Imamura S, Mulenga A, Nakajima M, Tsuda A, Ohashi K, Onuma M, A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine, Vaccine 21 (2003) 2844–2851. [DOI] [PubMed] [Google Scholar]
- [36].Bakshi M, Kim TK, Mulenga A, Disruption of blood meal-responsive serpins prevents Ixodes scapularis from feeding to repletion, Ticks Tick Borne Dis 9 (2018) 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu Y, Cao J, Zhou Y, Zhang H, Zhou J, Isolation and characterization of two novel serpins from the tick Rhipicephalus haemaphysaloides, Ticks Tick Borne Dis 4 (2013) 297–303. [DOI] [PubMed] [Google Scholar]
- [38].Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A, Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 is secreted into tick saliva during tick feeding, J. Exp. Biol 214 (2011) 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim TK, Tirloni L, Pinto AF, Moresco J, Yates JR 3rd, da Silva Vaz I Jr, Mulenga A, 2016. Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS Negl. Trop. Dis 10, e0004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Porter L, Radulovic Z, Kim T, Braz GR, Da Silva Vaz I Jr, Mulenga A, 2015. Bioinformatic analyses of male and female Amblyomma americanum tick expressed serine protease inhibitors (serpins). Ticks Tick Borne Dis. 6, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tirloni L, Kim TK, Pinto AFM, Yates JR 3rd, da Silva Vaz I Jr, Mulenga A, 2017. Tick-host range adaptation: changes in protein profiles in unfed adult Ixodes scapularis and Amblyomma americanum saliva stimulated to feed on different hosts. Front. Cell. Infect. Microbiol 7, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kim TK, Tirloni L, Pinto AF, Moresco J, Yates JR 3rd, da Silva Vaz I Jr, Mulenga A, 2020. Time-resolved proteomic profile of Amblyomma americanum tick saliva during feeding. PLoS Negl. Trop. Dis (in press, Accepted 02/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim TK, Curran J, Mulenga A, Dual silencing of long and short Amblyomma americanum acidic chitinase forms weakens the tick cement cone stability, J. Exp. Biol 217 (2014) 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ramakers C, Ruijter JM, Deprez RH, Moorman AF, Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data, Neurosci. Lett 339 (2003) 62–66. [DOI] [PubMed] [Google Scholar]
- [45].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method, Methods 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [46].Hollmann T, Kim TK, Tirloni L, Radulovic ZM, Pinto AFM, Diedrich JK, Yates JR 3rd, da Silva Vaz I Jr, Mulenga A, 2018. Identification and characterization of proteins in the Amblyomma americanum tick cement cone. Int. J. Parasitol 48, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gupta R, Jung E, Brunak Søren, Prediction of N-glycosylation sites in human proteins, 46 (2004) 203–206. [Google Scholar]
- [48].Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Bennett EP, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H, Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology, EMBO J. 32 (2013) 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Radulovic ZM, Kim TK, Porter LM, Sze SH, Lewis L, Mulenga A, A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome, BMC Genomics 15 (2014) 51–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gettins PG, Serpin structure, mechanism, and function, Chem. Rev 102 (2002) 4751–4804. [DOI] [PubMed] [Google Scholar]
- [51].Huntington JA, Read RJ, Carrell RW, Structure of a serpin-protease complex shows inhibition by deformation, Nature 407 (2000) 923–926. [DOI] [PubMed] [Google Scholar]
- [52].Horvath AJ, Lu BG, Pike RN, Bottomley SP, Methods to measure the kinetics of protease inhibition by serpins, Methods Enzymol. 501 (2011) 223–235. [DOI] [PubMed] [Google Scholar]
- [53].Kubes P, Granger DN, Leukocyte-endothelial cell interactions evoked by mast cells, Cardiovasc. Res 32 (1996) 699–708. [PubMed] [Google Scholar]
- [54].Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T, Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo, Cell 139 (2009) 1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McDonald WH, Tabb DL, Sadygov RG, MacCoss MJ, Venable J, Graumann J, Johnson JR, Cociorva D, Yates JR 3rd, 2004. MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass Spectrom 18, 2162–2168. [DOI] [PubMed] [Google Scholar]
- [56].Xu T, Park SK, Venable JD, Wohlschlegel JA, Diedrich JK, Cociorva D, Lu B, Liao L, Hewel J, Han X, Wong CC, Fonslow B, Delahunty C, Gao Y, Shah H, Yates JR 3rd, 2015. ProLuCID: an improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J. Proteome [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cociorva D, L Tabb D, Yates JR, Validation of tandem mass spectrometry database search results using DTASelect, Curr. Protoc. Bioinformatics Chapter 13, Unit 13.4, 2007. [DOI] [PubMed] [Google Scholar]
- [58].Banajee KH, Verhoeve VI, Harris EK, Macaluso KR, Effect of Amblyomma maculatum (Acari: Ixodidae) saliva on the acute cutaneous immune response to Rickettsia parkeri infection in a murine model, J. Med. Entomol 53 (2016) 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rodrigues V, Fernandez B, Vercoutere A, Chamayou L, Andersen A, Vigy O, Demettre E, Seveno M, Aprelon R, Giraud-Girard K, Stachurski F, Loire E, Vachiery N, Holzmuller P, Immunomodulatory effects of Amblyomma variegatum saliva on bovine cells: characterization of cellular responses and identification of molecular determinants, Front. Cell. Infect. Microbiol 7 (2018) 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sonenshine DE, Biology of Ticks, Oxford University Press, Oxford, 1993. [Google Scholar]
- [61].Walade SM, Rice MJ, The sensory basis of tick feeding behavior, in: Obenchain FD, Glaun R (Eds.),Physiology of Ticks 1982, pp. 71–118. [Google Scholar]
- [62].des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC 3rd, Fish D, 2001. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J. Infect. Dis 183, 773–778. [DOI] [PubMed] [Google Scholar]
- [63].Ebel GD, Kramer LD, Short report: duration of tick attachment required for transmission of powassan virus by deer ticks, Am. J. Trop. Med. Hyg 71 (2004) 268–271. [PubMed] [Google Scholar]
- [64].Ribeiro JM, Mather TN, Piesman J, Spielman A, Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae), J. Med. Entomol 24 (1987) 201–205. [DOI] [PubMed] [Google Scholar]
- [65].Barriga OO, Andujar F, Andrzejewski WJ, Manifestations of immunity in sheep repeatedly infested with Amblyomma americanum ticks, J. Parasitol 77 (1991) 703–709. [PubMed] [Google Scholar]
- [66].Bowessidjaou J, Brossard M, Aeschlimann A, Effects and duration of resistance acquired by rabbits on feeding and egg laying in Ixodes ricinus L, Experientia 33 (1977) 528–530. [DOI] [PubMed] [Google Scholar]
- [67].Brossard M, Papatheodorou V, Immunity against female Ixodes ricinus L.: effect on feeding and haemoglobin digestion, Ann. Parasitol. Hum. Comp 65 (1990) 32–36. [DOI] [PubMed] [Google Scholar]
- [68].Brown SJ, Knapp FW, Response of hypersensitized Guinea pigs to the feeding of Amblyomma americanum ticks, Parasitology 83 (1981) 213–223. [DOI] [PubMed] [Google Scholar]
- [69].Brown SJ, Shapiro SZ, Askenase PW, Characterization of tick antigens inducing host immune resistance. I. Immunization of Guinea pigs with Amblyomma americanum-derived salivary gland extracts and identification of an important salivary gland protein antigen with Guinea pig anti-tick antibodies, J. Immunol 133 (1984) 3319–3325. [PubMed] [Google Scholar]
- [70].Brown SJ, Barker RW, Askenase PW, Bovine resistance to Amblyomma americanum ticks: an acquired immune response characterized by cutaneous basophil infiltrates, Vet. Parasitol 16 (1984) 147–165. [DOI] [PubMed] [Google Scholar]
- [71].Gebbia JA, Bosler EM, Evans RD, Schneider EM, Acquired resistance in dogs to repeated infestation with Ixodes scapularis (Acari: Ixodidae) reduces tick viability and reproductive success, Exp. Appl. Acarol 19 (1995) 593–605. [DOI] [PubMed] [Google Scholar]
- [72].Latif AA, Newson RM, Dhadialla TS, Feeding performance of Amblyomma variegatum (Acarina: Ixodidae) fed repeatedly on rabbits, Exp. Appl. Acarol 5 (1988) 83–92. [DOI] [PubMed] [Google Scholar]
- [73].Bell JF, Stewart SJ, Wikel SK, Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity, Am. J. Trop. Med. Hyg 28 (1979) 876–880. [PubMed] [Google Scholar]
- [74].Burke G, Wikel SK, Spielman A, Telford SR, McKay K, Krause PJ, Tick-borne Infection Study Group, Hypersensitivity to ticks and Lyme disease risk, Emerg. Infect. Dis 11 (2005) 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jones LD, Nuttall PA, 1990. The effect of host resistance to tick infestation on the transmission of Thogoto virus by ticks. J. Gen. Virol 71 ( Pt 5), 1039–1043. [DOI] [PubMed] [Google Scholar]
- [76].Nazario S, Das S, de Silva AM, Deponte K, Marcantonio N, Anderson JF, Fish D, Fikrig E, Kantor FS, Prevention of Borrelia burgdorferi transmission in Guinea pigs by tick immunity, Am. J. Trop. Med. Hyg 58 (1998) 780–785. [DOI] [PubMed] [Google Scholar]
- [77].Hermance ME, Thangamani S, Tick(−)virus(−)host interactions at the cutaneous Interface: the Nidus of Flavivirus transmission, Viruses 10 (2018) 10.3390/v10070362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Matsuda H, Fukui K, Kiso Y, Kitamura Y, Inability of genetically mast cell-deficient W/Wv mice to acquire resistance against larval Haemaphysalis longicornis ticks, J. Parasitol 71 (1985) 443–448. [PubMed] [Google Scholar]
- [79].Ushio H, Watanabe N, Kiso Y, Higuchi S, Matsuda H, Protective immunity and mast cell and eosinophil responses in mice infested with larval Haemaphysalis longicornis ticks, Parasite Immunol. 15 (1993) 209–214. [DOI] [PubMed] [Google Scholar]
- [80].Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, Brasseur R, Vanhaeverbeek M, Vanhamme L, Godfroid E, Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus, J. Biol. Chem 281 (2006) 26361–26369. [DOI] [PubMed] [Google Scholar]
- [81].Hopla CE D. C, The isolation of Bacterium tularense from the tick Amblyomma americanum, J. Kansas Entomol. Soc 26 (1953) 71–72. [Google Scholar]
- [82].Koci J, Simo L, Park Y, Validation of internal reference genes for real-time quantitative polymerase chain reaction studies in the tick, Ixodes scapularis (Acari: Ixodidae), J. Med. Entomol 50 (2013) 79–84, 10.1603/me12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hollmann T, Kim TK, Tirloni L, Radulovic ZM, Pinto AFM, Diedrich JK, Yates JR 3rd, da Silva Vaz I Jr, Mulenga A, Identification and characterization of proteins in the Amblyomma americanum tick cement cone, Int. J. Parasitol 48 (2018) 211–224, 10.1016/j.ijpara.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bakshi M, Kim TK, Mulenga A, Disruption of blood meal-responsive serpins prevents Ixodes scapularis from feeding to repletion, Ticks Tick Borne Dis. 9 (2018) 506–518, 10.1016/j.ttbdis.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


