Abstract
Objectives:
Papua New Guinea has among the highest prevalences of sexually transmissible infections (STIs) globally with no services able to accurately test for anorectal Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections. Here we prospectively evaluated the diagnostic performance of a molecular CT/NG assay used at the point-of-care (POC) with the aim of enhancing anorectal STI screening and same-day treatment.
Methods:
Men who have sex with men, transgender women and female sex workers taking part in Papua New Guinea’s first large-scale biobehavioural study were enrolled and asked to provide a self-collected anorectal swab for POC GeneXpert CT/NG testing. Same-day treatment was offered if positive. A convenience sample of 396 unique and randomly selected samples were transported to Australia for comparison using the Cobas 4800 CT/NG test (Roche Molecular Diagnostics, Pleasanton, CA, USA).
Results:
A total of 326 samples provided valid results by Cobas whereas 70 samples provided invalid results suggesting inhibition. The positive, negative and overall percentage agreements of GeneXpert CT/NG for the detection of C. trachomatis were 96.7% (95% CI 92.3%–98.9%), 95.5% (95% CI 91.3%–98.0%) and 96.0% (95% CI 93.3%–97.8%), and for N. gonorrhoeae were 93.0% (95% CI 86.1%–97.1%), 100.0% (95% CI 98.3%–100.0%) and 97.8% (95% CI 95.6%–99.1%), respectively.
Conclusions:
The overall rate of agreement between the GeneXpert and Cobas CT/NG assays was high with 96.0% for C. trachomatis and 97.8% for N. gonorrhoeae. Results from this study data suggest that the GeneXpert CT/NG assay is suitable for testing self-collected anorectal specimens at the POC and that same-day treatment was feasible.
Keywords: Female sex workers, GeneXpert, Men who have sex with men, Rectal, Self-collect
Introduction
Sexually transmissible infections (STIs) continue to cause a substantial burden of disease, particularly among disadvantaged populations [1,2]. Globally, there were 357 million new cases of curable STIs in 2012, of which 142 million were in the Asia Pacific Region [3]. The STIs Chlamydia trachomatis and Neisseria gonorrhoeae make up the most substantial proportion of new cases and are known to infect both the urogenital tract and anorectal canal. Anorectal infections are usually asymptomatic [4] and are considered a well-known risk factor for human immunodeficiency virus (HIV) transmission and acquisition associated with both ulcerative and non-ulcerative STIs [5].
Papua New Guinea is the largest and most populous Pacific island nation and has some of the highest prevalence of curable STIs in the world [6]. Among pregnant women, the prevalence of urogenital C. trachomatis ranges from 11.1% in Madang Province, to 23.6% in the Eastern Highlands Province [7,8]. Among women and girls engaged in the selling and exchanging of sex in the nation’s capital of Port Moresby, HIV and syphilis prevalence has been estimated to be 10.0% and 24.2% [9]. In all men, a large cross-sectional study showed the prevalence of C. trachomatis was 12.8% and that of N. gonorrhoeae was 14.2% from urogenital samples [10].
The prevalence of anorectal C. trachomatis/N. gonorrhoeae in women and men has not been determined in Papua New Guinea, but it is likely to be high based on bio-behavioural surveys. Papua New Guinea does not conduct routine STI screening because of a lack of laboratory infrastructure and relies on syndromic treatment algorithms instead [11,12]. Numerous studies have demonstrated the value of testing extra-genital sites. Testing only urogenital samples in the USA is likely to miss most C. trachomatis and N. gonorrhoeae infections in men who have sex with men (MSM) and up to 15% of women who report receptive anal intercourse [13].
Nucleic acid amplification tests (NAAT) are recommended by the US CDC for the detection of C. trachomatis and N. gonorrhoeae and have become the standard diagnostic method when testing genital and extra-genital samples [14]. The GeneXpert (Xpert) C. trachomatis/N. gonorrhoeae (CT/NG) Test (Cepheid, Sunnyvale, CA, USA) is a real-time PCR assay that is portable, easy to use and has demonstrated comparable sensitivity and specificity to established laboratory-based NAAT systems for urogenital C. trachomatis and N. gonorrhoeae detection [15].
In the context of Papua New Guinea’s first large-scale bio-behavioural study, here we report on the diagnostic performance characteristics of the Xpert CT/NG Test for the detection of anorectal infection in MSM, transgender women (TGW) and female sex workers (FSW) at point-of-care (POC), compared with the laboratory-based Cobas CT/NG 4800 assay [16] and the feasibility of providing same-day treatment based on Xpert results.
Materials and methods
Overview
This prospective study was conducted as part of a broader bio-behavioural survey, known locally as Kauntim mi tu, using the Xpert CT/NG method for POC diagnosis. Anorectal samples available from two study locations were subjected to additional testing using the Roche Cobas CT/NG 4800 platform to assess the diagnostic performance of the Xpert CT/NG assay (Roche Molecular Diagnostics, Pleasanton, CA, USA).
Biobehavioural survey study procedures
Using respondent-driven sampling, the Kauntim mi tu bio-behavioural survey and sample collection were undertaken among FSW, MSM and TGW in Port Moresby, National Capital District (June–November 2016) and Lae, Morobe Province (January–June 2017), Papua New Guinea [17]. To take part in this study, FSW had to be born a biological female, be 12 years of age or older, have sold or exchanged sex in the past 6 months, speak English or Tok Pisin (a form of Melanesian Pidgin English and one of three national languages in Papua New Guinea [18]). MSM and TGW had to be born a biological male, be 12 years of age or older, have had anal or oral sex with a male in the past 6 months and speak English or Tok Pisin. The age of consent was in accordance with Papua New Guinea law and staff were trained to identify sexually exploited children. If required, referral and a peer support escort were offered to partner organizations experienced in providing counselling, social and other protective services to this population.
Following informed verbal consent, and after instruction from a trained healthcare worker, each participant self-collected an anorectal swab using the privacy of a bathroom attached to the clinician’s office.
Participants were also asked to avoid cleaning the anal verge to limit a potential reduction in organism load and to insert the anorectal swab 2–3 cm inside the rectum. The swab was then to be rotated three times, removed and placed inside its packaging before being returned to the clinician.
Following collection, each swab was placed into an Xpert sample collection tube containing 2.3 mL of universal transport medium (UTM) by the clinician as per the manufacturer’s instructions (see Fig. 1).
Fig. 1.
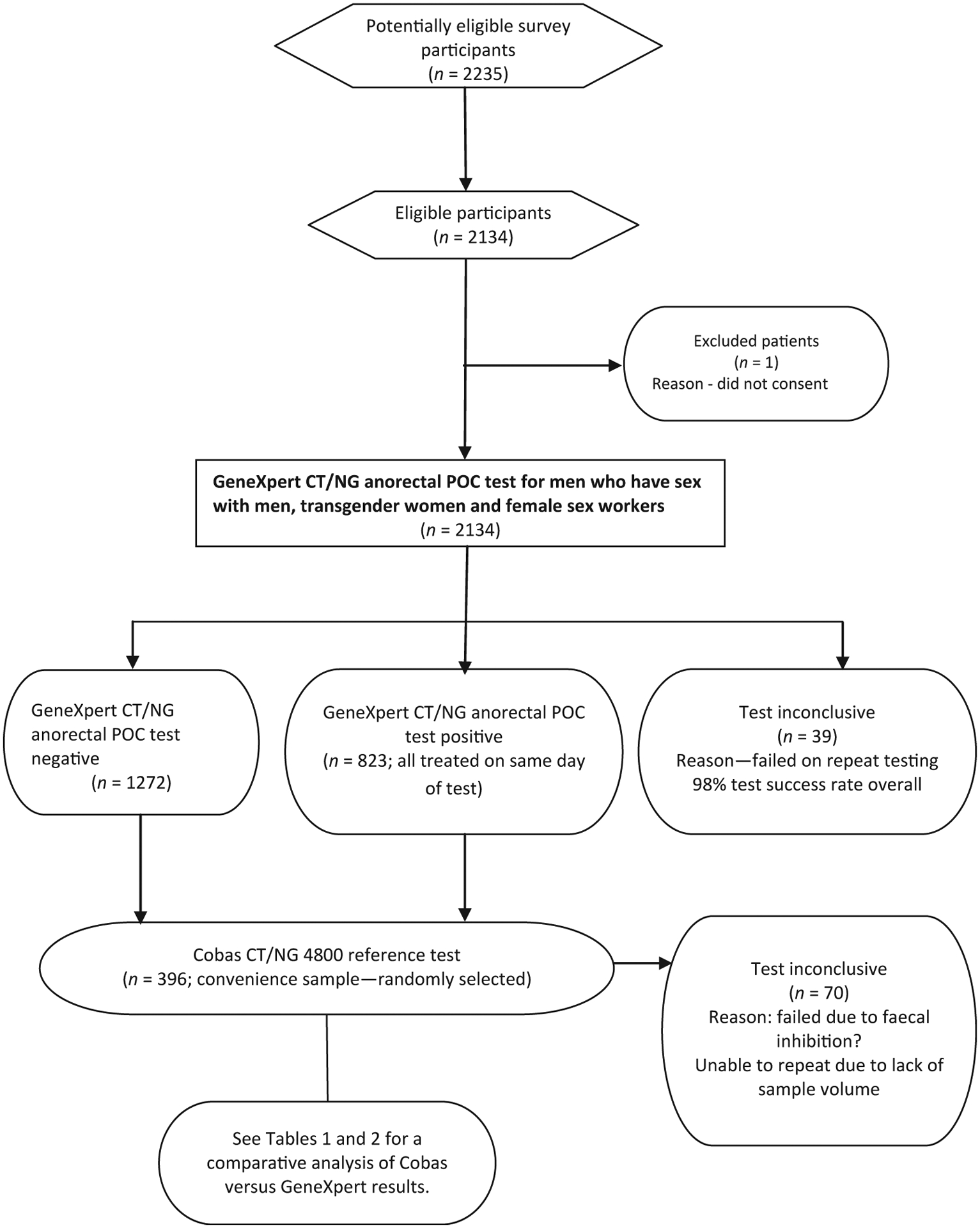
Flow of participants through the study.
Samples were immediately transferred to the adjacent study field laboratory room and agitated by hand to ensure that the UTM in each tube was thoroughly mixed with the swab sample. A 1-mL aliquot of UTM was transferred by a laboratory technician into an Xpert CT/NG cartridge with a specific sample identification barcode label and tested onsite using Xpert.
If repeat sample testing was not required, the remaining 1.3 mL of UTM was placed in a 2-mL archive tube, labelled with a sample identification bar code and a unique study code number and frozen at −80°C.
Individuals with a positive anorectal Xpert CT/NG test result were provided with information to prevent further infection and given same-day treatment using national STI guidelines [19]. Drug therapy for men and women consisted of oral amoxil (2 g), pro-benecid (1 g), augmentin (1 g) and zithromax (1 g); although these recommendations are under review given that they do not comply with WHO guidelines.
A convenience sample of 396 unique samples was then selected for laboratory reference testing, as this was approximately 20% of all samples drawn, and as such, a proportion thought to be sufficiently representative of all Xpert CT/NG tests conducted at the POC.
Ethics
Ethics approval for this diagnostic accuracy study was received from the Papua New Guinea IRB (#1508), MRAC (# 15.12), RAC (# RES14004) and UNSW Sydney HREC (# HC/15355).
Diagnostic evaluation study procedures
A total of 175 positive C. trachomatis or N. gonorrhoeae samples and 221 negative samples from 101 MSM and TGW, and 295 FSW were chosen for comparative NAAT testing and by selecting every second sample with a minimum volume of 1 mL. All 396 samples were transported by air to the New South Wales State Reference Laboratory for HIV & Molecular Diagnostic Medicine (SydPath) in Sydney, Australia.
Transportation involved dry vapour liquid nitrogen to prevent thawing in transit. On arrival, all samples were reviewed to ensure that there had been no leakage in transit and were prepared for blind testing by laboratory staff using the Cobas 4800 CT/NG assay. This widely used method is approved for use with endocervical swabs, clinician-collected vaginal swabs, self-collected vaginal swabs, and male and female urine [20].The assay is not cleared for use with rectal specimens.
Following data quality checks; the entire data set was imported into Stata V14.0 (College Station, TX, USA) for comparative analysis. The positive percentage agreement (PPA), negative percentage agreement (NPA) and overall rate of agreement (ORA) were calculated using standard US Food and Drug Administration methods for diagnostic tests [21]. Confidence intervals were calculated using exact Clopper–Pearson methods. A comparison of Xpert Ct values was also conducted to determine if there was any difference in the mean point of C. trachomatis and N. gonorrhoeae organism detection between MSM, TGW and FSW.
Results
A total of 2135 people consented and participated in this study across two study sites. All but one participant agreed to undergo Xpert CT/NG anorectal testing. Of those who tested (n = 2134), 144 samples (6.7%) were invalid at the first Xpert test, of which 105 (72.9%) generated a valid result on repeat testing, leaving 2095 (98%) valid results. Of the 1362 valid C. trachomatis and N. gonorrhoeae anorectal results in FSW, 427 (31.4%) were C. trachomatis positive, and 292 (21.4%) were N. gonorrhoeae positive. Among 733 valid test results in MSM and TGW, 61 were C. trachomatis positive (8.3%), and 43 (5.9%) were N. gonorrhoeae positive. All participants with a positive C. trachomatis/N. gonorrhoeae result received same-day treatment.
Of the 396 unique samples selected for the comparative NAAT testing, 326 (82.3%) produced a valid result on the Cobas CT/NG assay. A total of 70 samples (17.7%) generated invalid results. Repeat testing of those invalid tests was not possible because of insufficient sample volume. Test operators were blind to clinical information when conducting both the index and reference tests.
The PPA, NPA and ORA for the combined detection of the Xpert CT assay (Table 1) compared with Cobas CT were 96.7% (95% CI 92.3%–98.9%), 95.5% (95% CI 91.3%–98.0%) and 96.0% (95% CI 93.3%–97.8%), respectively; and for N. gonorrhoeae were 93.0% (95% CI 86.1%–97.1%), 100.0% (95% CI 98.3%–100.0%) and 97.8% (95% CI 95.6%–99.1%), respectively.
Table 1.
Combined test performance by assay type and pathogen
| Men who have sex with men, transgender women and female sex workers | Cobas CT | Xpert Test Performance % (95% CI) | ||
| Positive | Negative | |||
| Xpert CT (new test) | Positive | 144 | 8 | PPA 96.7% (92.3%–98.9%) |
| Negative | 5 | 169 | NPA 95.5% (91.3%–98.0%) | |
| ORA 96.0% (93.3%–97.8%) | ||||
| Men who have sex with men, transgender women and female sex workers | Cobas NG | |||
| Positive | Negative | |||
| PPA 93.0% (86.1%–97.1%) | ||||
| Xpert NG (new test) | Positive | 93 | 0 | NPA 100.0% (98.3%–100.0%) |
| Negative | 7 | 226 | ORA 97.8% (95.6%–99.1%) |
CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae; NPA, negative percentage agreement; ORA, overall rate of agreement; PPA, positive percentage agreement.
Table 2 provides a break down of test performance for FSW, MSM and TGW by assay types. In brief, the PPA by Xpert for C. trachomatis among anorectal samples for FWS was 96.6% (95% CI 91.7%–99.1%), NPA was 97.7% (95% CI 93.4%–99.5%) and ORA was 97.2% (95% CI 94.3%–98.9%). From MSM and TGW the PPA was 96.6% (95% CI 82.2%–99.9%), NPA was 89.4% (95% CI 76.9%–96.5%) and ORA was 92.1% (95% CI 83.6%–97.0%), respectively.
Table 2.
Test performance by assay type, pathogen and risk group
| Female sex workers | Cobas CT | Xpert Test Performance % (95% CI) | ||
| Positive | Negative | |||
| Xpert CT (new test) | Positive | 116 | 3 | PPA 96.6% (91.7%–99.1%) |
| Negative | 4 | 127 | NPA 97.7% (93.4%–99.5%) | |
| ORA 97.2% (94.3%–98.9%) | ||||
| Female sex workers | Cobas NG | |||
| Positive | Negative | |||
| Xpert NG (new test) | Positive | 72 | 0 | PPA 92.3% (84.0%–97.1%) |
| Negative | 6 | 172 | NPA 100.0% (97.9%–100.0%) | |
| ORA 97.6% (94.8%–99.1%) | ||||
| Men who have sex with men and transgender women | Cobas CT | Xpert Test Performance % | ||
| Positive | Negative | |||
| PPA 96.6% (82.2%–99.9%) | ||||
| Xpert CT (new test) | Positive | 28 | 5 | NPA 89.4% (76.9%–96.5%) |
| Negative | 1 | 42 | ORA 92.1% (83.6%–97.0%) | |
| Men who have sex with men and transgender women | Cobas NG | |||
| Positive | Negative | |||
| PPA 100.0% (84.6%–100.0%) | ||||
| Xpert NG (new test) | Positive | 22 | 0 | NPA 100.0% (93.4%–100.0%) |
| Negative | 0 | 54 | ORA 100.0% (95.3%–100.0%) |
CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae; NPA, negative percentage agreement; ORA, overall rate of agreement; PPA = positive percentage agreement.
For N. gonorrhoeae among FSWs, the PPA was 92.3% (95% CI 84.0%–97.1%), NPA was 100.0% (95% CI 97.9%–100.0%) and ORA was 97.6% (95% CI 94.8%–99.1%). For MSM and TGW the PPA for N. gonorrhoeae was 100.0% (95% CI 84.6%–100.0%), NPA was 100.0% (95% CI 93.4%–100.0%) and ORA was 100.0% (95% CI 95.3%–100.0%).
Cobas detected five positive C. trachomatis results (1.5%) for which Xpert provided negative results and Xpert detected eight (2.4%) samples that were negative by Cobas. A review of the Ct values (a semi-quantitative measure of DNA load) for each assay, revealed that the Cobas-only C. trachomatis-positive samples (n = 5) had high Ct values ranging from 34.6 to 38.0 cycles (indicating low chlamydia organism loads in these samples). For the Xpert-only C. trachomatis-positive samples (n = 8), the Ct value range was slightly higher at 36.8 to 39.3 cycles. For N. gonorrhoeae, Cobas detected seven positive cases (2.1%), which Xpert did not, with Ct values ranging from 34.2 to 37.2 cycles. Xpert did not identify any additional N. gonorrhoeae cases over the Cobas test.
Low DNA loads also probably accounted for the seven N. gonorrhoeae-positive samples missed by Xpert, with the Cobas Ct results observed for these samples sitting in among the highest observed values and towards the outer limit of DNA detection (mean cycles 35.5).
A total of 64 dual infections (both C. trachomatis and N. gonorrhoeae) were detected by GeneXpert and 62 by Cobas 4800. Chlamydia trachomatis was not detected by Cobas in both cases.
The mean Xpert Ct value or point of C. trachomatis DNA detection between all positive FSW results (28.69 cycles) was significantly lower, indicating higher bacterial loads (p 0.009) present in samples compared with all positive C. trachomatis results for MSM and TGW (31.28 cycles). No significant difference in Ct values was observed for N. gonorrhoeae between the key population groups (p 0.086). Further comparison of Xpert positive results against matching Cobas results was not possible given differences in how the two platforms measure and report Ct values.
Discussion
To our knowledge, this is the first study evaluating the diagnostic performance of Xpert CT/NG where used for POC test and same-day treatment of anorectal specimens among FSW, MSM and TGW. The combined results for FSW, MSM and TGW show the Xpert assay had an overall rate of agreement of 96.0% for the detection of C. trachomatis and 97.8% for N. gonorrhoeae compared with established NAAT methods, indicating that few anorectal C. trachomatis and N. gonorrhoeae infections would be missed by this testing approach.
Furthermore, the combined negative percentage agreement for C. trachomatis was 95.5% and for N. gonorrhoeae it was 100%, minimizing the potential for misdiagnosis or adverse relationship events associated with false-positives [22].
The POC testing pathway enabled all 489 individuals with a C. trachomatis infection (23%) and 336 with an N. gonorrhoeae infection (16.1%) to be provided with information about the prevention of future STIs and treatment on the same day. A notable difference in C. trachomatis positivity was seen between MSM and TGW (8.3%), and FSW (31.4%).
Analysis of Ct value results suggests that FSWs also had higher C. trachomatis bacterial loads than anorectal samples from MSM and TGW. Reasons for this difference may include self-infection from vaginal fluids, transfer during sex or infection via the intestinal canal [23,24]. One other study has quantified anorectal bacterial loads in C. trachomatis samples from both MSM and women and found there was a similar mean log load between the two groups. This study noted that anorectal C. trachomatis infections among women were often concurrent with a urogenital infection and rectal bacterial loads were usually higher than vaginal loads [25].
A limitation of this study was that 70 samples (17.7%) tested on the Cobas 4800 assay returned invalid results. Also, there was insufficient transport medium to conduct repeat tests or to further investigate the basis of the problem. We do not, however, believe this was due to sample transport or storage given the samples were stored at −80°C before shipping and transferred by air freight to the reference laboratory using liquid nitrogen, so the potential for a freeze–thaw effect does not seem likely to be an influencing factor. We also conducted pilot testing by diluting 36 separate positive and negative samples in Xpert transport medium and testing these in the Cobas 4800 instrument. A total of 19 anorectal clinical samples were included and no obvious compatibility issue between the Xpert medium and the Cobas 4800 test was identified despite the latter not being approved for C. trachomatis and N. gonorrhoeae testing of rectal samples. Results from this study indicate that is feasible to offer molecular anorectal C. trachomatis and N. gonorrhoeae testing at the POC in low-middle-income settings if test operator training is stringent and alternative power methods are used to prevent the loss of results due to fluctuating electricity supply. Frequent and dedicated molecular instrument maintenance is also required to prevent impact from dust and humidity. Finally, the C. trachomatis and N. gonorrhoeae positivity results among MSM, TGW and FSW in this study are based on unweighted data, and should not be interpreted as prevalence estimates, which need to be calculated factoring in the response-driven sampling design.
In summary, the overall rate of agreement between the Xpert and Cobas CT/NG assays was high with 96.0% detection for C. trachomatis and 97.8% for N. gonorrhoeae. Results from this study data suggest the Xpert CT/NG assay is suitable for testing self-collected anorectal specimens at the POC and that same-day treatment was feasible.
Acknowledgements
The authors wish to thank the members of the key populations who generously participated in our study, without whom we could not contribute to scientific knowledge of this kind. Our sincere thanks to Professor Mathew Law (Head of the Biostatistics and Databases Program, UNSW, Sydney) for advice regarding the selection of reference test samples. We would also like to express our appreciation to Leon McNally and Alex Carrera for processing study samples at the NSW State Reference Laboratory for HIV—Molecular Diagnostic Medicine Laboratory—SydPath, Sydney, Australia.
Funding
Kauntim mi tu is an initiative of the Government of Papua New Guinea with funding from the Government of Australia, The Global Fund to fight AIDS, Tuberculosis and Malaria, and the US Centers for Disease Control and Prevention through the US President’s Emergency Plan for AIDS Relief.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the funding agencies.
Footnotes
Transparency declaration
The authors have no conflicts of interest to declare.
Access to data
All data relating to the performance of the Xpert CT/NG assay when testing anorectal samples has been shown in this publication.
References
- [1].Fernandes FR, Zanini PB, Rezende GR, Castro LS, Bandeira LM, Puga MA, et al. Syphilis infection, sexual practices and bisexual behaviour among men who have sex with men and transgender women: a cross-sectional study. Sex Transm Infect 2015;91:142–9. [DOI] [PubMed] [Google Scholar]
- [2].Minichiello V, Rahman S, Hussain R. Epidemiology of sexually transmitted infections in global indigenous populations: data availability and gaps. Int J STD AIDS 2013;24:759–68. [DOI] [PubMed] [Google Scholar]
- [3].Gottlieb SL, Deal CD, Giersing B, Rees H, Bolan G, Johnston C, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine 2016;34:2939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Keshinro B, Crowell TA, Nowak RG, Adebajo S, Peel S, Gaydos CA, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc 2016;19:21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lucar J, Hart R, Rayeed N, Terzian A, Weintrob A, Siegel M, et al. Sexually transmitted infections among HIV-infected individuals in the District of Columbia and estimated HIV transmission risk: data from the DC cohort. Open Forum Infect Dis 2018;5:ofy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vallely AJ, MacLaren D, David M, Toliman P, Kelly-Hanku A, Toto B, et al. Dorsal longitudinal foreskin cut is associated with reduced risk of HIV, syphilis and genital herpes in men: a cross-sectional study in Papua New Guinea. J Int AIDS Soc 2017;20:21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vallely A, Page A, Dias S, Siba P, Lupiwa T, Law G, et al. The prevalence of sexually transmitted infections in Papua New Guinea: a systematic review and meta-analysis. PLoS One 2010;5:e15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vallely LM, Toliman P, Ryan C, Rai G, Wapling J, Tomado C, et al. Prevalence and risk factors of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis and other sexually transmissible infections among women attending antenatal clinics in three provinces in Papua New Guinea: a cross-sectional survey. Sex Health 2016;13:420–7. [DOI] [PubMed] [Google Scholar]
- [9].Kelly A, Kupul M, Man WYN, Nosi S, Lote N, Rawstorne P, et al. Askim na Save (Ask and understand): people who sell and/or exchange sex in Port Moresby. Key Quantitative Findings. Sydney, Australia: Papua New Guinea Institute of Medical Research and the University of New South Wales; 2011. [Google Scholar]
- [10].Vallely A, Ryan CE, Allen J, Sauk JC, Simbiken CS, Wapling J, et al. High prevalence and incidence of HIV, sexually transmissible infections and penile foreskin cutting among sexual health clinic attendees in Papua New Guinea. Sex Health 2014;11:58–66. [DOI] [PubMed] [Google Scholar]
- [11].Badman SG, Vallely LM, Toliman P, Kariwiga G, Lote B, Pomat W, et al. A novel point-of-care testing strategy for sexually transmitted infections among pregnant women in high-burden settings: results of a feasibility study in Papua New Guinea. BMC Infect Dis 2016;16:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Garrett NJ, McGrath N, Mindel A. Advancing STI care in low/middle-income countries: has STI syndromic management reached its use-by date? Sex Transm Infect 2017;93:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Danby CS, Cosentino LA, Rabe LK, Priest CL, Damare KC, Macio IS, et al. Patterns of extragenital chlamydia and gonorrhea in women and men who have sex with men reporting a history of receptive anal intercourse. Sex Transm Dis 2016;43:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb Mortal Wkly Rep 2010;59:1–109.20075837 [Google Scholar]
- [15].Tabrizi SN, Unemo M, Golparian D, Twin J, Limnios AE, Lahra M, et al. Analytical evaluation of GeneXpert CT/NG, the first genetic point-of-care assay for simultaneous detection of Neisseria gonorrhoeae and Chlamydia trachomatis. J Clin Microbiol 2013;51:1945–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marlowe EM, Hardy D, Krevolin M, Gohl P, Bertram A, Arcenas R, et al. High-throughput testing of urogenital and extragenital specimens for detection of Chlamydia trachomatis and Neisseria gonorrhoeae with Cobas® CT/NG. Eur J Microbiol Immunol 2017;7:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kelly-Hanku A, Willie B, Weikum DA, Boli Neo R, Kupul M, Coy K, et al. Kauntim mi tu: multi-site summary report from the key population integrated bio-behavioural survey, Papua New Guinea. Goroka, Papua New Guinea: Papua New Guinea Institute of Medical Research and Kirby Institute, UNSW Sydney; 2018. [Google Scholar]
- [18].English and Tok Pisin (New Guinea Pidgin English) in Papua New Guinea. World Englishes 1989;8:5–23. [Google Scholar]
- [19].National Department of Health, Papua New Guinea. Standard treatment guidelines for adults. 6th ed. 2012. Available from: http://www.adi.org.au/wp-content/uploads/2016/11/Standard-Treatment-Guidelines-for-Common-Illness-of-Adults-in-PNG.pdf.
- [20].Gaydos CA. Review of use of a new rapid real-time PCR, the Cepheid GeneXpert® (Xpert) CT/NG assay, for Chlamydia trachomatis and Neisseria gonorrhoeae: results for patients while in a clinical setting. Exp Rev Mol Diagn 2014;14:135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guidance for Industry and FDA Staff. Statistical guidance on reporting results from studies evaluating diagnostic tests. Washington DC: US Department of Health and Human Services—Food and Drug Administration; 2007. [Google Scholar]
- [22].Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ Qual Saf 2013;22: ii6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ding A, Challenor R. Rectal Chlamydia in heterosexual women: more questions than answers. Int J STD AIDS 2014;25:587–92. [DOI] [PubMed] [Google Scholar]
- [24].Rank RG, Yeruva L. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 2014;82:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Liere GA, Dirks JA, Hoebe CJ, Wolffs PF, Dukers-Muijrers NH. Anorectal Chlamydia trachomatis load is similar in men who have sex with men and women reporting anal sex. PLoS One 2015;10:e0134991. [DOI] [PMC free article] [PubMed] [Google Scholar]


