Abstract
This study examined airborne emissions from cigarette butts for styrene, 2-methyl-2-cyclopenten-1-one, naphthalene, triacetin and nicotine. Ten experiments were conducted by placing butts in a stainless-steel chamber and measuring the chemical concentrations in chamber air. Emission rates were determined from the concentrations. Triacetin and nicotine concentrations were roughly 50 % of initial concentrations after 100 h, while concentrations of other chemicals decayed to less than 10 % of initial concentrations within 24 h. Initial emission rates per cigarette butt ranged from 200 ng h−1 to 3500 ng h-1. Triacetin and nicotine emission rates at 25 °C were 1.6 to 2.2 times higher than the rates at 20 °C, while the emission rates of other chemicals at 25 °C were 1.1 to 1.3 times higher than the rates at 20 °C only during the first sampling period. The chemical concentrations and emission rates at 30 °C were comparable or lower than the values at 25 °C, possibly due to different batches of cigarettes used. The 24 h emitted mass of nicotine from a cigarette butt at 25 °C could be up to 14 % of the literature reported nicotine masses emitted from a burning cigarette.
Keywords: cigarette, cigarette butt, emission, nicotine, air, exposure
1. Introduction
Globally, smokers produce over five trillion cigarette butts every year [1]. Cigarette butts are one of the most common forms of litter and are often discarded onto beaches, bus stops, roads, streets, parks and other public places. Over 75 % of smokers have been observed to litter cigarette butts in an urban environment, including 94 % of those who didn’t extinguish their cigarettes [2]. Just under 75 % of smokers admit to improperly disposing of cigarette butts into the outdoor environment at least once [3]. Removal of these butts in urban environments can cost millions of dollars [4]. Cigarettes are also likely to be disposed of in indoor environments, e.g., buildings and cars.
Typically, a cigarette butt includes three major components, i.e., ash, unburned tobacco and the filter [5]. Among those components, the cigarette filter was originally designed to reduce risks associated with smoking by partially removing some of the chemicals in mainstream smoke [5]. Even though the efficacy of filters in protecting smokers’ health has been questioned, a significant number of chemicals are expected to be captured by cigarette filters [6]. The ash and unburned tobacco may also adsorb chemicals emitted during smoking [7]. Overall, a wide range of chemicals in cigarette smoke have been detected in cigarette butts [8–14]. The presence of these chemicals in cigarette butts and the length of time of cigarette butts are located in potential exposure environments indicate that cigarette butts may extend the time period over which cigarettes may pose a risk to humans and wildlife [15]. Cigarette butts have been consumed by small children [16] and reported to alter wildlife reproduction habits and mortality rates [17–22].
With concerns for both environmental and human risks from cigarette butts, studies have been conducted to study leaching of chemicals from cigarette butts into water [15] and have shown that emissions from cigarette butts into water can be a fast process [23, 24]. However, little effort to date has been made to characterize airborne emissions from cigarette butts. Five studies have measured the air emissions from cigarette butts into the headspace of test vials [25–29], but emission rates were not quantified. To our knowledge, only one study has quantified the airborne emission rates from cigarette butts [29]. This study indicated that the 24 h emitted mass for nicotine from a non-smoldering cigarette butt could be up to 15 % of the emitted masses of nicotine from a burning cigarette reported in the literature. However, the Poppendieck, D and M Gong [29] study was only a single experiment at 25 °C. In addition, that study used chamber wall adsorption parameters for nicotine from the literature, potentially limiting the accuracy of the reported nicotine emission rates.
Due to the potential long duration of a cigarette butt’s presence in an indoor exposure environment and its potentially harmful chemical constituents similar to cigarette smoke, emission of chemicals into air from cigarette butts may be important in estimating aggregate chemical exposure due to cigarette consumption. To determine the potential human exposure from a cigarette butt and its relative contribution to aggregate exposure, the emission rates of chemicals into air from cigarette butts are needed. Hence, the objective of this study was to determine airborne emission rates for five target chemicals from cigarette butts in a walk-in chamber at different air temperatures.
2. Methods
To address the objective, a number of freshly smoked, non-smoldering cigarette butts were placed in the center of a 31 m3 walk-in, stainless-steel chamber. Concentrations of target chemicals were measured up to five days after placing the butts in the chamber.
2.1. Generation of cigarette butts
A leading cigarette brand consumed in the United States market [30], was chosen as the target cigarette in this study. Cigarette butts were generated using a smoking apparatus which was designed to burn two cigarettes at a time and simulate the puff sequence outlined in ISO 3308 [31]. Detailed descriptions of the smoking apparatus and smoking procedures are reported in Poppendieck, D and M Gong [29]. Briefly, each cigarette was smoked for 6 puffs, with a puff duration of 2.7 s, puff reoccurrence of 60 s and an airflow rate through the cigarette of 1.0 L min−1. After the sixth puff, each cigarette was immediately placed in a sand ashtray for 60 s to extinguish the butt. Then, each butt was sealed in a pre-weighed 20 mL glass headspace vial. The weight of the butt was determined by the difference of the weight of the bottle with the butt and the weight of the bottle without the butt. To ensure consistency, only butts with masses between 0.370 g and 0.442 g were used in the emission tests. Butts were typically placed in the emission chamber within three hours of generation.
2.2. Emission tests in the walk-in chamber
A total of 10 sets of experiments were conducted in a stainless-steel walk-in chamber. The 31 m3 insulated chamber has the ability to control temperature, relative humidity, air recirculation rate and outdoor air supply. Detailed descriptions of the walk-in chamber and measurement of air change rate are described in Section S.1 of the Supplementary Information (SI). Average chamber temperature, relative humidity, air change rate, number of butts, duration of the experiment and cigarette butt removal times are shown in Table 1. Cigarettes used for the 10 sets of experiments were purchased from a local supermarket, and three batches of cigarettes were purchased on different dates (Table 1).
Table 1.
Summary of experimental conditions
| # | Batcha | Temperature (°C) | Relative humidity (%) | Air change rate (h−1)b | Number of Butts | Duration, h | Butts removal time, h |
|---|---|---|---|---|---|---|---|
| 1 | B | 20.3 (±0.5) | 49 (±2) | 0.47 | 27 | 44 | 25.4 |
| 2 | A | 25.0 (±0.2) | 48 (±2) | 0.47 | 18 | 123 | Endc |
| 3 | A | 25.1 (±0.2) | 48 (±1) | 0.47 | 18 | 28 | End |
| 4 | A | 25.0 (±0.3) | 48 (±1) | 0.47 | 18 | 49 | End |
| 5 | A | 25.0 (±0.2) | 48 (±1) | 0.47 | 18 | 101 | End |
| 6 | A | 25.0 (±0.1) | 48 (±1.0) | 0.47 | 27 | 27 | End |
| 7 | A | 25.0 (±0.2) | 48 (±1.0) | 0.47 | 27 | 78 | End |
| 8 | A | 25.0 (±0.1) | 48 (±1.0) | 0.47 | 36 | 27 | End |
| 9 | B | 25.0 (±0.2) | 48 (±1.0) | 0.47 | 27 | 80 | 28.4 |
| 10 | C | 30.0 (±0.2) | 47 (±1.) | 0.47 | 27 | 57 | 27.6 |
Batch A was purchased on 11/16/2018; batch B was purchased on 05/09/2019; batch C was purchased on 06/18/19.
The air change rate in this study is presented to give context on the outdoor air dilution and the local air movement over the cigarette butts.
“End” indicates the experiment ended when the butts were removed.
Four sets of experiments using 18 cigarette butts (#2 through #5) and two sets of experiments using 27 cigarette butts (#6 through #7) were conducted to examine the repeatability of the experiments. Experiments #2 through #8 were conducted to investigate the influence of the number of cigarette butts (18, 27 or 36 butts) on the measured air concentrations and calculated emission rates. To study the influence of temperature on the emission rates, experiments were conducted at 20 °C (#1), 25 °C (#9), and 30 °C (#10). Experiments #1, #9 and #10 were conducted to understand the chemical sorption onto the chamber wall at the studied temperatures by taking chamber air samples after the cigarette butts were taken out from the chamber. Two sets of experiments were performed to investigate long-term emission (#2 and #5).
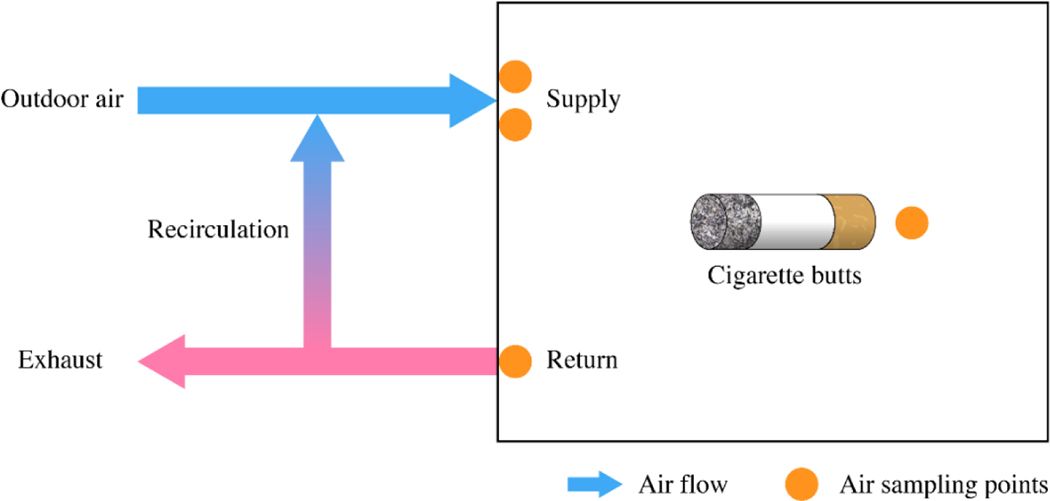
A diagram of the experimental setup is shown in Figure 1. For each set of emission tests, 18, 27 or 36 freshly smoked cigarette butts were removed from headspace vials and placed on an aluminum-foil-lined, stainless-steel mesh basket. The basket was hung in the center of the walk-in chamber to increase uniformity of the emitted chemicals with the chamber air (SI Figure S.3). The chamber was operated with an outdoor air supply of 14.6 m3 h−1 (equivalent to an air change rate of 0.47 h−1) and 111.4 m3 h−1 of recirculated air, at relative humidity of 50 % and at different temperature settings, i.e., 20 °C, 25 °C and 30 °C. Both outdoor makeup air and recirculated air passed through a prefilter, a high efficiency particulate air (HEPA) filter and a gas phase air cleaner containing a mixture of potassium permanganate and activated carbon.
Figure 1.
Diagram of experimental setup and sampling locations in the chamber
Initial sorption tube samples (0 h) were taken for a duration of 2 h immediately after the cigarette butts were placed in the chamber. For experiments #2 through #8, 3 h samples were then taken starting at approximately 2 h. For these experiments, an additional one to three 3 daily until the cigarette butts were removed from the chamber. Detailed sampling times are shown in SI Table S.1. In experiment #1, #9, and #10, cigarette butts were removed from the chamber between 25 h and 28 h (Table 1). Continuous 3 h samples were taken from the sample starting at 2 h until at least 18 h after cigarette butts were taken out of the chamber to quantify chemical adsorption and desorption to the chamber walls.
Except for experiment #8, four air samples were taken at each time point, two of which were from supply inlets, one was from a return outlet, and one was from the middle of the chamber. For experiment #8, three air samples were taken at each time point, with one from a supply inlet, one from the middle of the chamber and one from a return outlet. The 100 mL min−1 flow rates through the sampling tubes was maintained with mass flow controllers. Flow rates were confirmed with a calibrated bubble flow meter before and after each sample. Sampling lines ran from the locations of sorbent tubes to sample pumps and mass flow controllers located outside the chamber. The chamber was entered when samples were taken to change sorption tubes and measure flow rates. Given the short period of chamber door opening each time (roughly 15 s) compared to the duration of the sampling times (3 h), the influence of chamber door opening on the chemical concentrations in the chamber air was assumed to be negligible. Sorption tube air samples were analyzed by thermal desorption (TD) gas chromatograph (GC) mass spectrometer (MS). Details of the instrument operation, standard curves, internal standards and blanks can be found in the SI (Section S.4). Target chemicals were determined based on criteria including representativeness of the chemicals, response area abundance and repeatability, chamber background concentrations, and whether or not it is listed in the Food and Drug Administration (FDA) Harmful and Potentially Harmful Constituents (HPHC) list [32] for tobacco products and tobacco smoke [29]. Five chemicals (styrene, 2-methyl-2-cyclopenten-1-one, naphthalene, triacetin, and nicotine) were analyzed for this study.
2.3. Emission rate calculation
A chamber mass balance was used to calculate chemical emission rates. The mass balance accounted for emissions, inflow, outflow and deposition to and emissions from chamber walls [33]:
| (1) |
| (2) |
where:
is the volume of the chamber, 31 m3;
is the chemical concentration in the chamber air, ng m−3;
is the chemical emission rate of cigarette butts, ng h−1;
is the total flow rate of outdoor and recirculating air, 126 m3 h−1;
is the chemical concentration in the supply air, ng m−3;
is the surface area of the chamber surfaces, 59 m2;
is the surface concentration on the chamber walls, ng m−2;
is the deposition velocity to chamber walls, m h−1;
is the reemission rate from chamber walls, h−1;
The deposition velocity () and reemission rate constant () were used only for nicotine and determined from least squares fitting as described below. Unlike Van Loy, MD, VC Lee, LA Gundel, JM Daisey, RG Sextro and WW Nazaroff [33], chemical adsorption and desorption in this study was assumed to be linear with air concentration.
The chemical concentration at the midpoint of the sampling period was assumed to be equal to the measured average concentration during the period. For the more volatile chemicals (styrene, 2-methyl-2-cyclopenten-1-one, naphthalene, triacetin), adsorption to the stainless-steel chamber walls can be ignored (discussed in more detail in Section 3.2.4). For these chemicals, the emission rate at the middle of two sampling times can be calculated as follows:
| (3) |
where and are the concentrations in the chamber at two sequential time periods; and are the concentrations at the air inlet at two sequential time periods; and are the middle time point of the two sequential time periods.
Based on results from experiments #1, #9 and #10 that examined the chemical sorption to chamber wall (discussed in section 3.2.4), adsorption to the stainless-steel chamber walls is considered only for nicotine. Nicotine mass adsorbed to the chamber walls can be calculated by solving the following equation:
| (4) |
where and are the masses of chemical adsorbed onto the chamber wall at two sequential time periods. Once the adsorption mass is calculated, the emission rate at the middle of two sampling times can be calculated as follows:
| (5) |
The nicotine sorption parameters, and , were determined by comparing obtained by solving equation (4) and equation (5). Equation (4) was solved for over the duration of the entire experiment (cigarette butts present and removed) by using initial estimates of and determined from the literature [33], measured values for and and by assuming to be zero during the first sampling period. Equation (5) was solved for only during the time periods after the cigarette butts were removed from the chamber ( was zero) by using measured and , and taking the value calculated using equation (4) at the time point when the cigarette butts were removed from the chamber as the initial . For experiments #9 (25 °C) and #10 (30 °C), and were then derived by minimizing the difference of values calculated using equation (4) and equation (5) after the cigarettes were removed using the least-squares method in Microsoft Excel 2016. Because only five data points were available for the fitting after the removal of the cigarettes in experiment #1 (20 °C), at 20 °C was assumed to be the average of at 25 °C and 30 °C so that only was fitted.
2.4. Quality assurance/quality control
Instrument detection limits for each target chemical on sorbent tubes were determined by spiking the target chemicals in a 1μL methanol solution onto the sorbent tubes. The instrument detection limits were determined by multiplying three times the standard deviation of seven replicates at a concentration that was less than five times the determined method detection limit [34]. The method quantification limits were determined by multiplying the above determined standard deviation by ten and dividing the result by the sample volume of a three-hour sample. The instrument detection limits and method quantification limits are shown in Table S.2 in the SI. All concentration values below the quantification limits were assumed to be zero for data analysis.
Preliminary experiments were conducted to check if the target chemicals breakthrough the sampling tubes during a 3 h sample event. Standard solutions were spiked onto tubes, the tubes were connected to backup tubes and both were purged with nitrogen for 3 h. The results indicate that breakthrough of the target chemicals to the backup tubes was negligible (detailed information is presented in Section S.5 of the SI). In addition, for experiments #3, #4, #5, #7, all samples in the middle and outlet of the chamber were taken with a sampling tube connected to a back-up tube. The styrene, triacetin, and nicotine masses in all the backup tubes were below quantification limits for all samples, while 2-methyl-2-cyclopenten-1-one in less than 5 % of back up tubes were higher than quantification limits. In addition, the 2-methyl-2-cyclopenten-1-one masses in the backup tubes were less than 10 % of the masses in the front tubes. In 11 % of the backup tubes, naphthalene masses were above 10 % of the masses in the front tubes. In these cases, the naphthalene masses were not used for analysis. For experiments #1, #9, and #10, due to a limitation in the number of sampling tubes, backup tubes were only used for the initial samples with higher concentrations (i.e., 0 h, 2 h and 5 h) and all the chemical masses in backup tubes were less than 10 % of the masses in front tubes.
Before each set of experiments, the chamber was ventilated at 35 °C with increased airflow to clean the chamber and 3 h air samples at the experimental temperature were taken to measure the background concentrations in the chamber and inlet air. Chemical masses in background samples (SI Table S.4) were generally below quantification limits. Chemical masses in the inlet air samples were only higher than the instrument quantification limits for naphthalene in experiment #1. Nicotine background masses in the middle of the chamber and outlet air samples were higher than quantification limits for half of the experiments, but the masses were about ten times lower than the concentrations in the samples taken in the first 24 h. Other chemical background masses in the middle of the chamber and outlet air samples were generally below quantification limits except for styrene (#1), and naphthalene (#1, #8, and #9). However, these detected background masses were about ten times lower than the masses in the first air sample during each experiment.
3. Results and discussion
3.1. Air sample concentrations in the chamber
Over the ten sets of experiments, 114 pairs of air samples were analyzed. Each sample pair consisted of one or two inlet samples, one sample in the middle of the chamber and one at the outlet.
3.2.1. Air sample concentrations in inlets
Air entering the chamber went through a prefilter, a HEPA filter, and an activated carbon and potassium permanganate air cleaner (Section S.1 in SI). Even with 88 % recirculation air in the chamber supply, the concentrations in the inlet were much lower than the concentrations at the middle and outlet of the chamber, indicating the effectiveness of the filtration system. On average, the inlet concentrations were 12 %, 17 %, 20 %, 6 %, and 0.4 % of the average of middle and outlet samples for styrene, 2-methyl-2-cyclopenten-1-one, naphthalene, triacetin, and nicotine, respectively. Inlet concentrations were accounted for in the calculation of emission rates as shown in Equation (1).
3.2.2. Uniformity of chemicals in the chamber
The emission rate calculation discussed in Section 2.3 assumes the air in the chamber is well mixed. Over the course of the 25 °C experiments (#2 through #9), 81 sets of middle and outlet samples were taken. A comparison of these samples indicates the degree that chamber is well mixed. At 25 °C, for the target chemicals other than nicotine, the ratio of the concentration in the middle and outlet of the chamber averaged between 0.98 and 1.08 (Table 2). These data indicate that the concentrations of these four chemicals were fairly uniform in the chamber air at 25 °C. The nicotine concentrations at 25 °C in the middle of the chamber were on average 1.24 times higher than the concentrations in the outlet. For all five chemicals, the concentration ratio between the middle of the chamber and the outlet decreased with increasing temperature. A total of 91 % of the ratios were larger than one for experiments at 20 °C and 71 % of the ratios were smaller than one at 30 °C. Nicotine had the largest increase in the concentration ratios with the temperature decreasing. Chemical adsorption to chamber walls and temperature gradients in the chamber may impact the uniformity of chemicals in the chamber air. Temperature differences measured 50 cm from the top and bottom of the chamber were within 0.2 °C for the experiments at 20 °C and 25 °C. In contrast, there was a 0.5 °C gradient between the top and bottom of the chamber during the experiment at 30 °C. Nevertheless, nearly 75 % of the 322 concentration ratios were between 0.75 and 1.25. Hence, the average of the concentration in the middle of the chamber and at the outlet of the chamber are used as the air concentration in the chamber for all chemicals and all experiments in the remainder of this paper.
Table 2:
Ratio of chemical concentrations in the middle of chamber to the outlet at 25 °C
| Temperature (°C) | Value | Styrene | 2-methyl-2-cyclopenten-1-one | Naphthalene | Triacetin | Nicotine |
|---|---|---|---|---|---|---|
| 20 | Average | 1.13 | 1.30 | 1.28 | 1.34 | 1.48 |
| Stand. Dev. | 0.12 | 0.13 | 0.22 | 0.37 | 0.55 | |
| Count | 6 | 9 | 10 | 10 | 12 | |
| 25 | Average | 0.98 | 1.07 | 1.01 | 1.08 | 1.24 |
| Stand. Dev. | 0.19 | 0.20 | 0.15 | 0.20 | 0.31 | |
| Count | 31 | 45 | 55 | 72 | 78 | |
| 30 | Average | 0.88 | 0.85 | 0.94 | 0.90 | 0.86 |
| Stand. Dev. | 0.18 | 0.12 | 0.20 | 0.18 | 0.20 | |
| Count | 13 | 8 | 10 | 14 | 17 |
3.2.3. Repeatability of and influence of cigarette butt number on concentrations
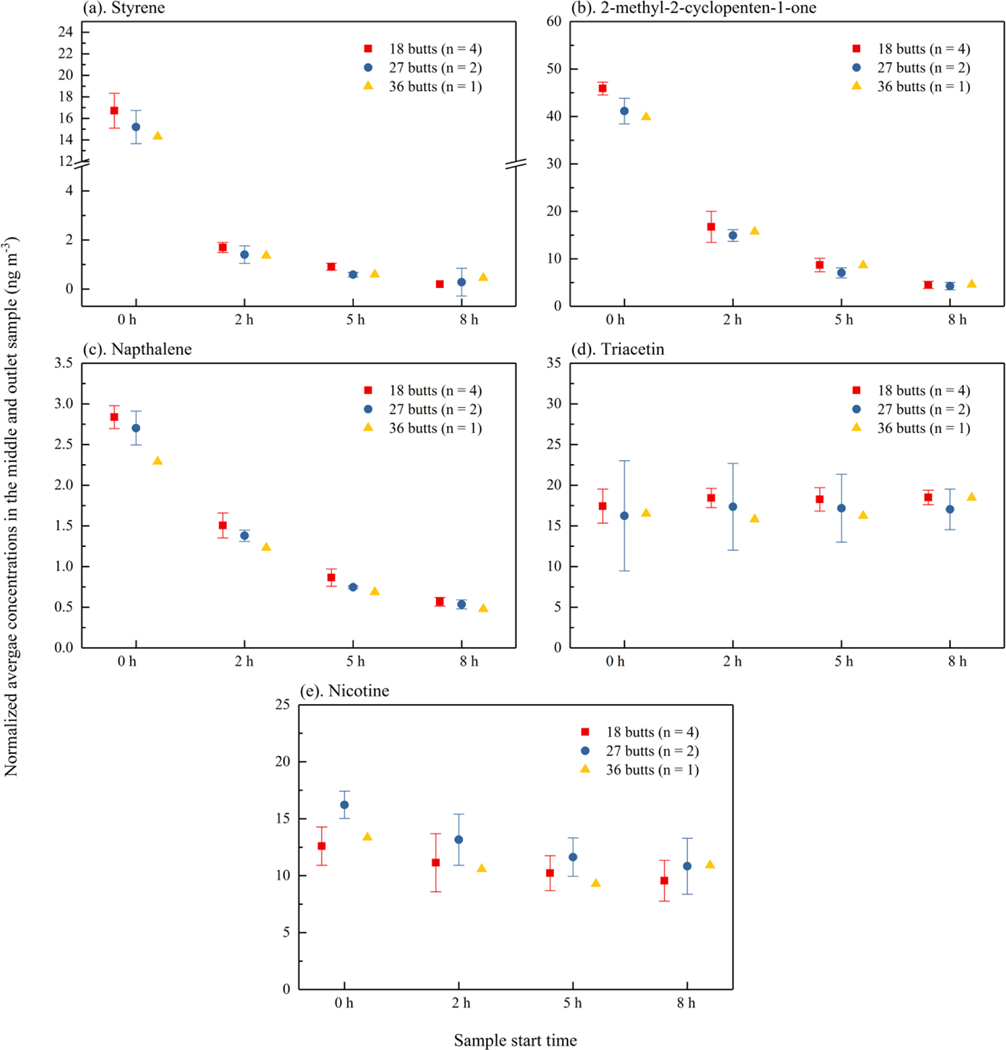
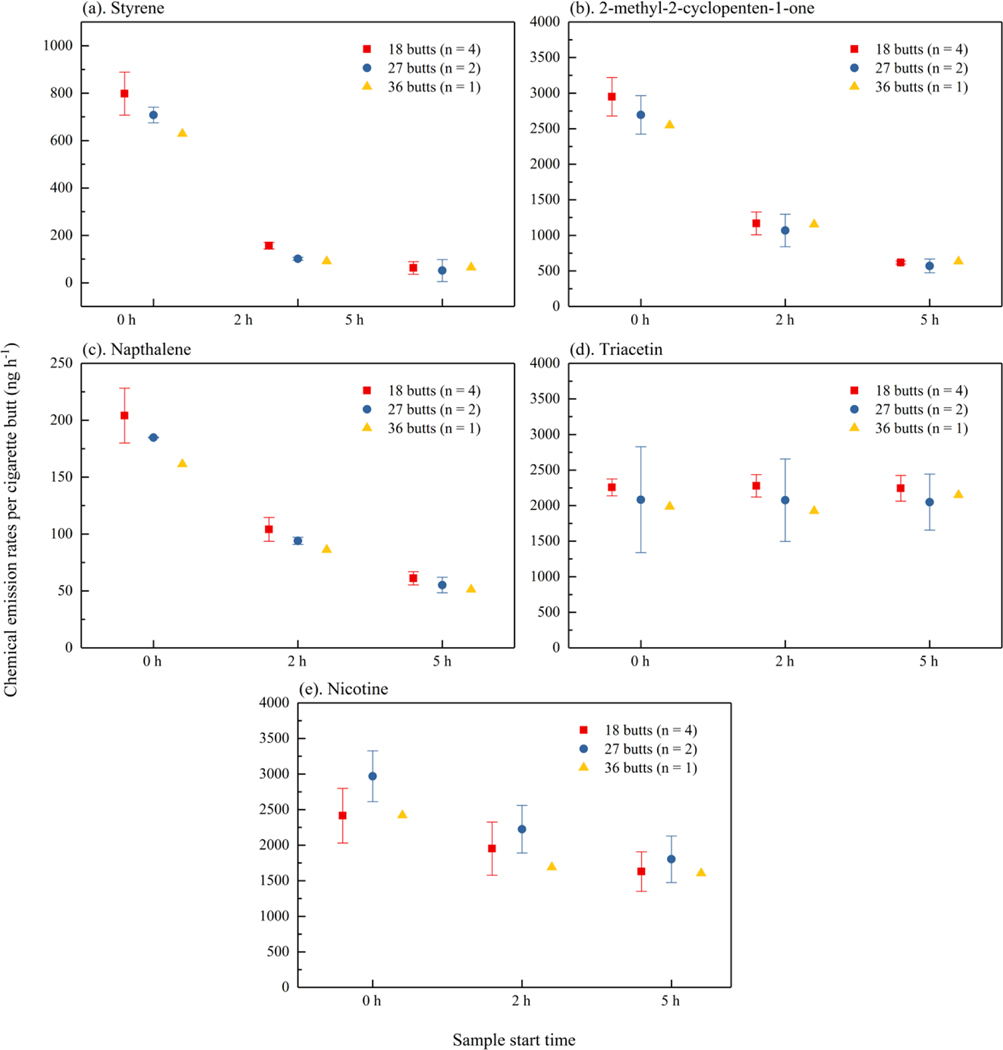
As described above, experiments #2 through #8 were analyzed for investigating the repeatability of the experiments and the influence of the number of cigarette butts. Chemical concentrations for experiments #2 through #8 are shown in SI Figure S4. Since the first four samples were started at about 0 h, 2 h, 5 h, and 8 h for all sets of experiments and the sampling times were not consistent after the fourth sample, only the first four samples were compared for investigating the repeatability and influence of number. For experiments with 18 butts at 25 oC (#2, #3, #4, and #5), the relative standard deviations (RSDs) of average chemical concentrations for the first four samples were mostly less than 20 % (SI Table S.5). For experiments with 27 butts at 25 oC (#6 and #7), relatively percent differences (RPDs) of concentrations were less than 31 %, except for styrene at 8h and triacetin at 2h. For styrene, the high RPD results from averaging quantified measurements with zero values for measurements below the quantification limit. Figure 2 shows the average chemical concentrations for the first four samples normalized by the number of cigarettes (18, 27 or 36). Error bars in Figure 2 and calculated RSDs or RPDs (SI Table S.6) highlight the variability in the normalized data. The RSDs of average chemical concentrations for different number of cigarette butts were mostly less than 13 %, excepting styrene. This indicates that the cigarette butt number placed in the chamber didn’t influence the normalized concentrations significantly.
Figure 2.
Normalized average concentrations in the middle and outlet sample (ng m−3) taken at 0h, 2h, 5h, and 8h. (a) styrene, (b) 2-methyl 2-cyclopenten-1-one, (c) naphthalene, (d) triacetin and (e) nicotine. Error bars show one standard deviation for data with 18 butts, and relative percentage difference for data with 27 butts.
3.2.4. Air sample concentrations at different temperatures
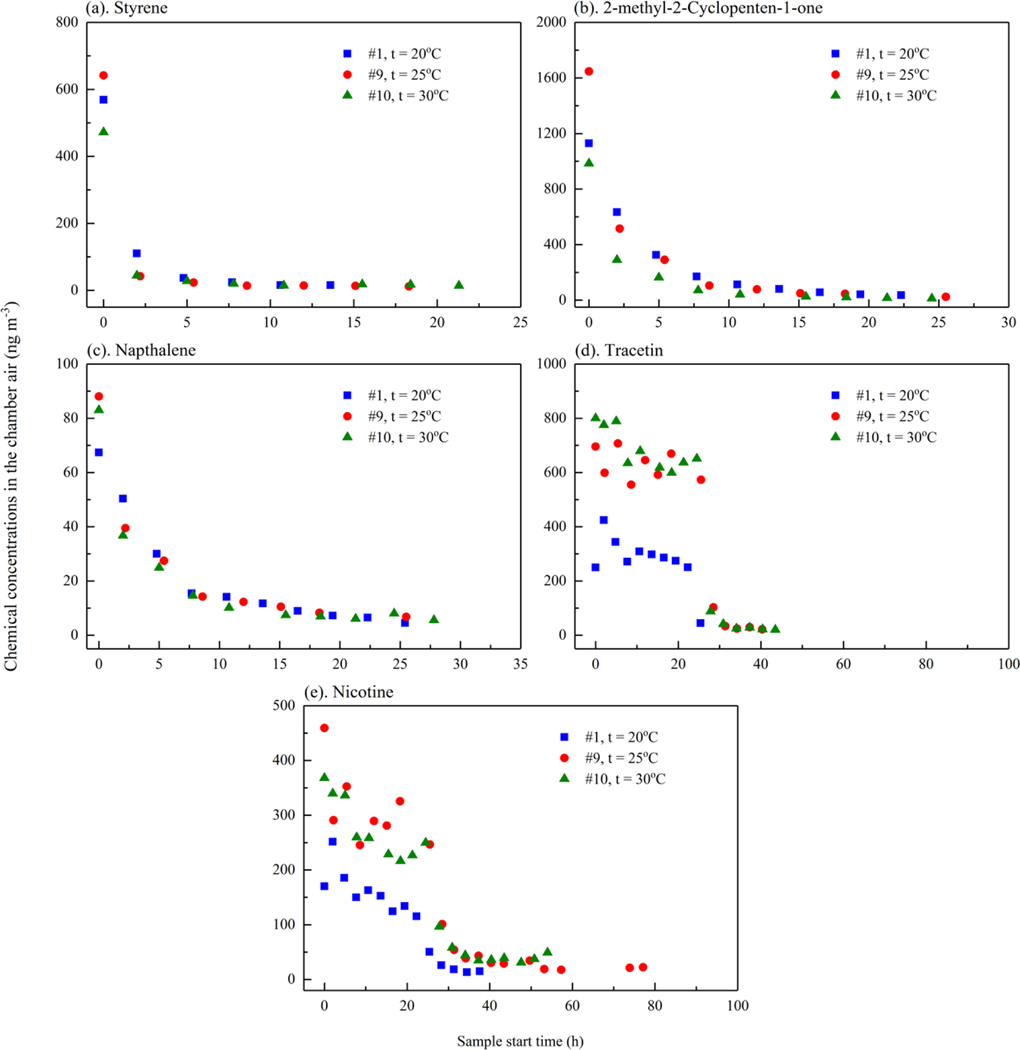
Experiments #1 (20 °C), #9 (25 °C) and #10 (30 °C) were conducted to understand the adsorption/desorption of the chemicals onto the chamber walls and the concentrations and emission rates at different temperatures. The average chemical concentrations from experiments #1, #9 and #10 are presented in Figure 3. Experiment #9 was chosen as representative of the experimental data at 25 °C, since it was the only experiment during which samples were taken every 2 h or 3 h continuously during the emission period and cigarettes used were from the same purchase batch as experiment #1 (Table 1).
Figure 3.
Chemical concentrations in the chamber air (ng m−3) for different sets of experiments (a) styrene, (b) 2-methyl 2-cyclopenten-1-one, (c) naphthalene, (d) triacetin and (e) nicotine. For experiment #1, #9, and #10, cigarette butts were taken out at 25.4 h, 28.4 h, and 27.6 h, respectively
Triacetin and nicotine chamber concentrations at 25 °C (experiment #1) were 1.2 to 2.8 times higher than their concentrations at 20 °C (experiment #9) before the cigarette butts were taken out. Both triacetin and nicotine are initially present at high contents in the cigarette prior to burning [35], and emissions over 24 hours is unlikely to deplete the mass present [29]. In contrast, initial contents of styrene, 2-methyl 2-cyclopenten-1-one, and naphthalene in the butts were low as they are primarily a result of combustion and pyrolysis during the cigarette burning [29]. Hence, due to lower initial contents in the butts and higher vapor pressures, the concentrations of styrene, 2-methyl 2-cyclopenten-1-one, and naphthalene decayed much more quickly than nicotine and triacetin. For these chemicals, even though their concentrations at 25 °C during the first sampling period were 1.1 to 1.5 times higher than the concentrations at 20 °C, their concentrations tended to be lower at 25 °C than at 20 °C after the initial sampling period. Note that the emitted mass of these chemicals during the first sampling period were 45 % to 79 % of the mass emitted over 24 h at 25 °C and 37 % to 69 % of the mass emitted at 20 °C. Hence, due to the higher depletion of chemicals in the first sampling period at 25 °C, the masses left in butts after the first sampling period at 25 °C were less than the masses left at 20 °C, resulting in lower concentrations at 25 °C.
Surprisingly, chemical concentrations at 30 °C (experiment #10) were comparable or lower than the concentrations at 25 °C (experiment #9). This may be due to a difference in the initial components in the cigarettes before burning, given that the cigarettes used for experiment #10 (batch C) were from a different batch than used for experiment #1 and # 9 (batch B). The ratios of the concentrations in experiment #9 (batch B) and the average concentrations in experiments #6 and #7 (same temperature as #9, but cigarettes from batch A) ranged from 0.9 to 1.6. These concentration ratios between batches are similar to the ratios between the concentrations at 25 °C and at 20 °C for styrene, 2-methyl 2-cyclopenten-1-one, and naphthalene, which hinders comparisons of the concentrations at 25 °C and 30 °C. The differences in cigarettes from different batches may also partly contribute to the variations of the initial emitted masses (about two times) as seen in a previous study (Figure 2.21 in Poppendieck, D and M Gong [29]). Further studies are needed to examine the influence of temperature on cigarette butt emissions given the differences between different batches observed in this study.
During experiments #1, #9, and #10, the concentrations of the three target chemicals other than nicotine and triacetin were below quantification in the chamber less than 6 h after the cigarette butts were removed. This time period (6 h), equal to three times the inverse of the air change rate, represents the amount of time required for a non-sorbing/desorbing chemical to be removed from the chamber assuming clean supply air. Hence, there was no significant adsorption to the chamber surfaces when the cigarette butts were removed for these three chemicals. In a study by Singer, BC, AT Hodgson and WW Nazaroff [36], small differences in emission rates for styrene and naphthalene in a ventilated stainless steel chamber (with an air change rate of 2 h−1) and an unventilated chamber (with air change rate of less than 0.02 h−1) were seen, indicating that the adsorption of these chemicals to stainless steel surfaces was insignificant in that study. In experiments # 9 and # 10, triacetin in the chamber air was still above the quantification limit at 6 h after the cigarette butts were removed from the chamber. However, the differences between triacetin concentration in the inlet air and the chamber air were less than 10 %, which indicates that triacetin in the chamber may be from desorption from the gas phase air cleaner in the supply duct and not desorption from the chamber wall. In contrast, nicotine was the other chemical that was consistently above quantification limit in the chamber more than 6 h after the butts were removed. Since nicotine concentration in the inlet air was below the quantification limit, this indicates that nicotine adsorbed measurably to the chamber surfaces. As a result, sorption was accounted in the emission rate calculation for nicotine but not the other four chemicals, as noted in Section 2.3.
3.2.5. Air sample concentrations in long term experiments
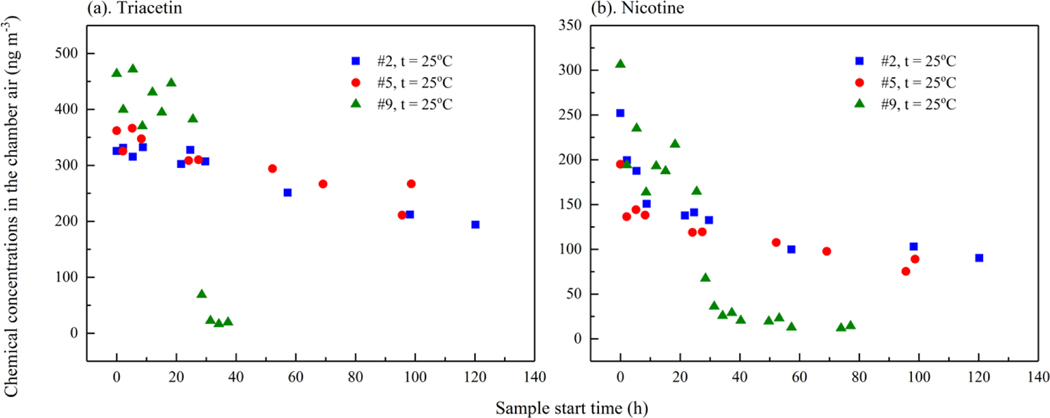
As shown in Figure 3, triacetin and nicotine concentrations decayed less than 50 % during the first 24 h, while concentrations of the other chemicals decreased quickly. Styrene concentrations typically dropped to 10 % of the initial concentration by the second sample period (22-methyl 2-cyclopenten-1-one dropped to 10 % of the initial concentration by the fourth sample period (8. In addition, naphthalene dropped to 10 % of the initial concentration by 24In experiment #2 and #5, cigarette butts were left in the chamber up to 120 h to examine the persistence of the nicotine and triacetin. Nicotine concentrations in the chamber were 45 % of initial values after 101 h (#5) and 36 % of initial values after 120 h (#2, Figure 4). Triacetin concentrations in the chamber were 60 % of initial values after 101 h (#5) and 74 % of initial values after 120 h (#2). As a comparison, concentrations in experiment #8 with cigarette butts taken out at 27.6 h were also included in Figure 4, which shows a much faster decay for both triacetin and nicotine than concentrations in experiment #2 and #5. Overall, the results in Figure 4 indicate that cigarette butts could be persistent sources of these chemicals for days after the cigarette is smoked.
Figure 4.
Triacetin and nicotine concentrations in the chamber air (ng m−3) (a) triacetin and (b) nicotine. Note that cigarette butts were taken out at 27.6 h in experiment # 9 and the concentrations in this figure were adjusted based on number of cigarette butts used.
3.2. Calculated emission rates
3.2.1. Curve fitting for desorption study
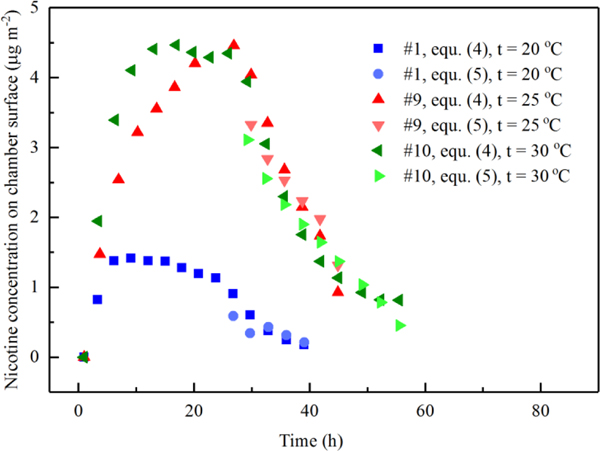
To derive the deposition velocity to the chamber walls () and reemission rate from the chamber walls () for nicotine, the mass of nicotine sorbed to the chamber walls was determined. Figure 5 shows the calculated sorbed masses to the chamber walls over the course of the experiment before and after the cigarette butts’ removal (equation (5)), and after the butts were removed (equation (4)). The maximum sorbed nicotine mass at 20 °C was roughly three times lower than at 25 °C and 30 °C, which may be the result of the combination of the difference of air concentrations and different partition between chamber and air at different temperatures. The maximum nicotine mass calculated to be sorbed to the chamber walls equated to between 15 % and 24 % of the total nicotine mass emitted into the chamber during experiments #1, #9, and #10.
Figure 5.
Deriving desorption parameters by minimizing the difference of nicotine surface concentration calculated by equation (4) and equation (5) after the cigarette butts were taken out.
Table 3 shows the derived deposition velocity to the chamber walls () and reemission rate from the chamber walls () through least squares fitting. Since only one data set was used for the curve fitting at each temperature, trends in and should be viewed with caution.
Table 3.
Calculated parameters for nicotine sorption to stainless steel chamber walls.
| Parameter | Temperature (°C) | ||
|---|---|---|---|
| 20 | 25 | 30 | |
| (m h−1) | 2.1 | 1.7 | 2.5 |
| (h−1) | 0.24 | 0.10 | 0.14 |
3.2.2. Repeatability of and influence of cigarette butt number on emission rate
Calculated emission rates per cigarette butt for experiments #2 through #8 for sample periods starting at 0 h, 2 h, and 5 h are shown in Figure 6. Similarly, for experiments with 18 butts at 25 °C (#2, #3, #4, #5), RSDs of calculated emission rates at the first three time periods are mostly less than 20 % (SI Table S.5). For experiments with 27 butts at 25 °C (#6, #7), RPDs of calculated emission rates were mostly less than 31 % (SI Table S.5). Styrene is an exception with higher RSDs resulting from the low and inconsistent air concentrations at 8 h. Likewise, RSDs or RPDs of the average emission rate per cigarette butt in experiments using 18, 27, or 36 cigarette butts were less than 14 % except for styrene (SI Table S.6).
Figure 6.
Chemical emission rates per cigarette butt (ng h−1) at 0h, 2h, 5h. (a) styrene, (b) 2-methyl 2-cyclopenten-1-one, (c) naphthalene, (d) triacetin and (e) nicotine. Error bars show one standard deviation for data with 18 butts, and relative percentage difference for data with 27 butts.
3.2.3. Air emission rates at different temperatures
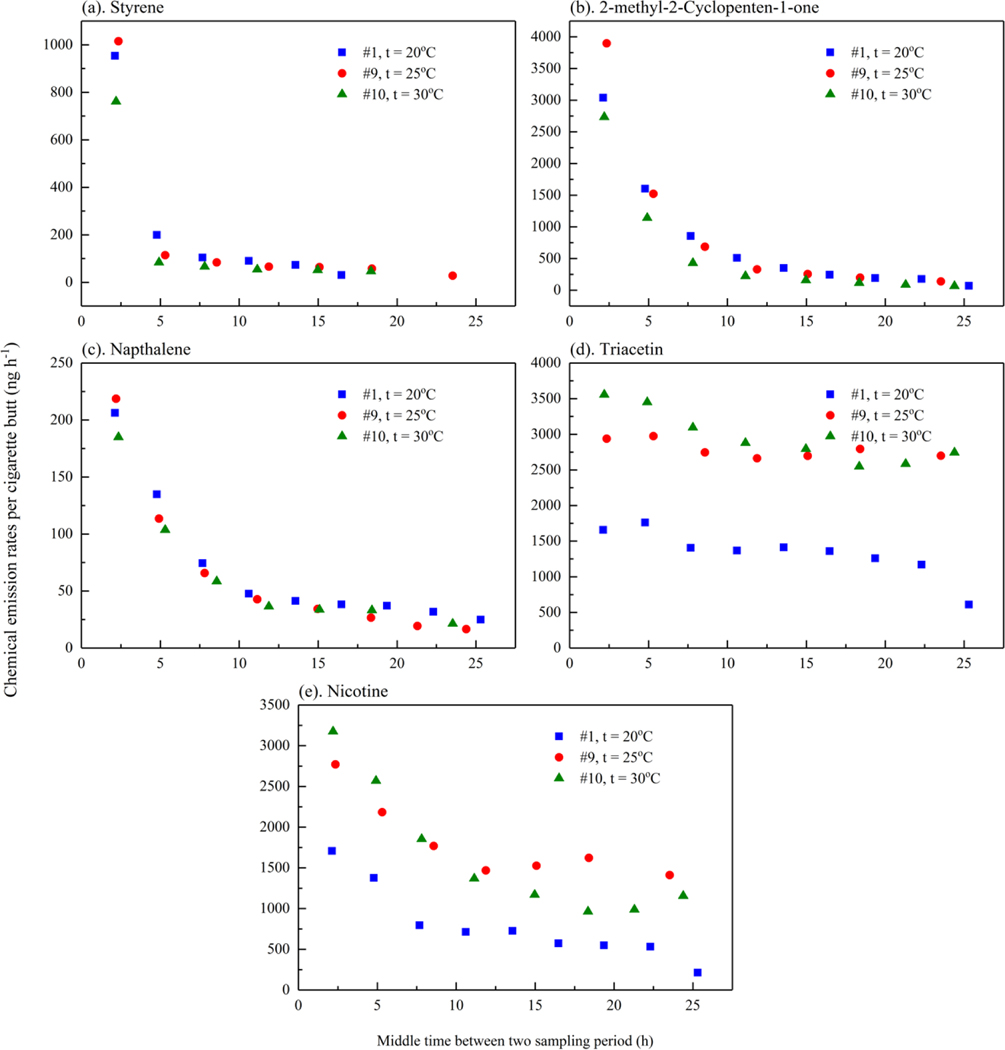
The emission rate profiles for the three experiments at different temperatures (#1, #9, and #10) over 24 h (Figure 7) were similar to the concentration profiles. Styrene emission rates dropped to 10 % of the initial emission rate by the third sample period (52-methyl 2-cyclopenten-1-one dropped to 10 % of the initial emission rate by the fifth sample period (11 and naphthalene dropped to 10 % of the initial emission rate by 24Nicotine emission rates decreased on average 60 % over the first 24 h, while triacetin emission rates were still 80 % of the initial emission rates during the first 24 h.
Figure 7.
Chemical emission rates per cigarette butt (ng h−1) for different sets of experiments (a) styrene, (b) 2-methyl 2-cyclopenten-1-one, (c) naphthalene, (d) triacetin and (e) nicotine
Similar to the concentration results, the emission rates of triacetin and nicotine at 25 °C were 1.6 to 2.2 times higher than their emission rates at 20 °C. For styrene, 2-methyl 2-cyclopenten-1-one, and naphthalene, the emission rates at 25 °C during the first sampling period were 1.1 to 1.3 times higher than the emission rates at 20 °C, but their emission rates tended to be lower at 25 °C than at 20 °C after the initial sampling period. In addition, chemical emission rates at 30 °C were comparable to or lower than the concentrations at 25 °C, which may be because different batches of cigarettes were used.
3.2.4. Comparison of emitted mass from cigarette butts to emitted mass from active cigarette smoking
To understand the relative contribution of exposure from cigarette butts to the aggregate exposure from cigarettes, it is helpful to compare the emissions from extinguished cigarette butts with the emissions during active cigarette smoking, including mainstream and sidestream smoke. Chemical masses emitted from active cigarette smoking have been quantified for the duration of the smoking event [36–41]. In contrast, no literature is available that reports durations of cigarette butts left in indoor environments, e.g., cars, buildings. To make a reasonable comparison between emissions from actively smoked cigarettes and cigarette butts, a 24 h emitted mass was calculated for cigarette butt emissions at different temperatures.
For styrene, 2-methyl-2-cyclopenten-1-one, and naphthalene, the relative percentage differences between the 24 h emitted masses at 20 °C and 25 °C are within 12 % (Table 4). This again indicates that these three chemicals were mostly depleted in 24 h. In contrast, the 24 h emitted masses for triacetin and nicotine at 25 °C are about two times the masses at 20 °C. The 24 h emitted mass at 25 °C from a previous study [29] is similar to the emitted mass in this study.
Table 4.
Mass of chemical emitted from a cigarette butt (this study, μg/cigarette butt, first row) compared to mass of chemicals emitted during active cigarette burning (μg/cigarette)
| Reference | Sampling description | Styrene | 2-methyl-2-cyclopenten-1-one | Naphthalene | Triacetin | Nicotine |
|---|---|---|---|---|---|---|
| This studya | Air sampling from a 31 m3 stainless steel chamber with air change rate of 0.5 h−1 and 27 cigarette butts in the middle of the chamber at 20 °C | 4.6 | 22 | 1.9 | 34 | 18 |
| This studya | Air sampling from a 31 m3 stainless steel chamber with air change rate of 0.5 h−1 and 27 cigarette butts in the middle of the chamber at 25 °C | 5.1 | 25 | 1.8 | 67 | 34 |
| This studya | Air sampling from a 31 m3 stainless steel chamber with air change rate of 0.5 h−1 and 27 cigarette butts in the middle of the chamber at 30 °C | 3.8 | 16 | 1.6 | 71 | 31 |
| Poppendieck and Gong (2019) | Air sampling from a 31 m3 stainless steel chamber with air change rate of 0.54 h−1 and 18 cigarette butts in the middle of the chamber at 25 °C | 6.7 | 40 | 2.5 | 48 | 36 |
| Charles et al. [37] | Mainstream and sidestream smoke sampling from a smoking machine | 134 | —b | 13 | — | 241 |
| Bi et al. [38] | Environmental tobacco smoke sampling in a 75.5 m3 unoccupied office | 146 | — | — | — | 323 |
| Baek and Jenkins [39] | Environmental tobacco smoke sampling in a 30 m3 stainless steel chamber | 107 | — | — | — | 552 |
| Singer, BC, AT Hodgson and WW Nazaroff [36] | Environmental tobacco smoke sampling in a 45 m3 stainless steel chamber with air change rate of 2 h−1 | 186 | — | 39 | — | 3370 |
| Daisey et al. [40] | Environmental tobacco smoke sampling in an unventilated 20 m3 stainless steel chamber with air filtration rate of 0.03 h−1 | 147 | — | — | — | 919 |
| Martin et al. [41] | Environmental tobacco smoke sampling in an 18 m3 unventilated stainless steel chamber | 94 | — | — | — | 1580 |
24 h emitted mass per cigarette butt calculated by integrating the emission rate at different times;
Dashed indicate not reported.
As far as we know, no emission rates for 2-methyl-2-cyclopenten-1-one and triacetin for active cigarette smoking have been previously reported. Table 4 shows that for styrene, naphthalene and nicotine, the 24 h emitted masses per cigarette butt are about 1 % to 15 % of the emitted mass per active cigarette burning. It is noteworthy that the 24 h emitted mass for nicotine from a cigarette butt at 25 °C could be up to 14 % of the emitted masses from a burning cigarette. In addition, as stated in section 3.2.4 and 3.2.5, nicotine emissions could increase as temperature increases, and nicotine concentrations were close to half of the initial concentrations even after 5 days of emissions. This indicates that if a butt is left in an indoor environment for more than 5 days or at higher temperature than 25 °C, the emitted mass of nicotine may approach the mass emitted during active smoking, depending on the cigarette butt components and environment conditions, i.e., temperature, relative humidity and ventilation rate.
4. Conclusions
Emissions of styrene, 2-methyl-2-cyclopenten-1-one, naphthalene, triacetin and nicotine from cigarette butts were measured at different temperatures, i.e., 20 °C, 25 °C and 30 °C. The air concentrations during the first sampling period ranged from 80 ng m−3 for naphthalene to 1500 ng m−3 for 2-methyl-2-cyclopenten-1-one. Concentrations of styrene, 2-methyl-2-cyclopenten-1-one, and naphthalene decayed to less than 10 % of the initial concentrations within 24 h. However, triacetin and nicotine concentrations were about 50 % of the initial concentrations after over 100 h. For triacetin and nicotine, the emission rates at 25 °C were 1.6 to 2.2 times higher than their emission rates at 20 °C. For styrene, 2-methyl 2-cyclopenten-1-one, and naphthalene, emission rates at 25 °C during the first sampling period were 1.1 to 1.3 times higher than the emission rates at 20 °C, but their emission rates tended to be lower at 25 °C than at 20 °C after the initial sampling period. The chemical concentrations and emission rates at 30 °C were comparable to or lower than the concentrations at 25 °C, which may due to different batches of cigarettes being used. The study showed that the total emitted mass over 24 h for nicotine from a cigarette butt in this study could be up to 14 % of the literature reported emitted mass from a burning cigarette. Hence, the emitted nicotine mass from a butt over five days could be comparable to the nicotine mass emitted from mainstream and sidestream smoke, especially at higher temperatures.
This study has several limitations and further research is needed to understand the airborne emissions from cigarette butts. The average of the concentration in the middle of the chamber and at the outlet of the chamber was used as the air concentration in the chamber by assuming the concentrations in the chamber were uniform. But, under some conditions in this study, the concentrations in the middle and outlet of the chamber varied by more than 30 %, especially for nicotine, which may decrease the accuracy of the calculated emission rates. Further emission studies that increase air mixing in the chamber and reduce the chemical levels in the supply air would be valuable. The use of cigarettes from different batches limited the ability to compare emission rates at 25 °C and 30 °C. More studies are therefore needed to examine the variation of emissions from cigarette butts produced from different batches of cigarettes. Furthermore, the influence of temperature on cigarette butt emissions warrants further investigation by considering the variations among cigarettes from different batches.
Supplementary Material
Practical implications.
Globally, over five trillion cigarette butts are generated every year, in which many chemicals are associated with human health risks. Disposed cigarette butts in indoor and outdoor environments may prolong human exposure to chemicals produced during cigarette burning or present in cigarettes before burning. However, little attention has been paid to airborne emissions from cigarette butts. Experiments in this study indicate that nicotine emission from cigarette butts may contribute substantially to aggregate nicotine exposure due to cigarette consumption. Exposure scientists, risk assessors, and public health officials should be mindful of indoor exposure related to airborne emission from cigarette butts.
Acknowledgement
The authors would like to acknowledge preliminary experiments for this research that were funded by the United States Food and Drug Administration under the Interagency Agreement #224-15-9012. This publication represents the views of the author(s) and does not represent the U.S. Food and Drug Administration/Center for Tobacco Products position or policy.
5. Disclaimer
Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- 1.Torkashvand J, Farzadkia M. A systematic review on cigarette butt management as a hazardous waste and prevalent litter: control and recycling. Environ Sci Pollut Res Int 2019; 26: 11618–11630. [DOI] [PubMed] [Google Scholar]
- 2.Patel V, Thomson GW, Wilson N. Cigarette butt littering in city streets: a new methodology for studying and results. Tob Control 2013; 22: 59–62. [DOI] [PubMed] [Google Scholar]
- 3.Rath JM, Rubenstein RA, Curry LE, Shank SE, Cartwright JC. Cigarette litter: smokers’ attitudes and behaviors. Int J Environ Res Public Health 2012; 9: 2189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider JE, Peterson NA, Kiss N, Ebeid O, Doyle AS. Tobacco litter costs and public policy: a framework and methodology for considering the use of fees to offset abatement costs. Tob Control 2011; 20 Suppl 1: i36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiss Otmar, Kotzias D, Tobacco, cigarettes and cigarette smoke: an overview, Institute for Health and Consumer Protection, Editor. 2007. [Google Scholar]
- 6.Harris B. The intractable cigarette ‘filter problem’. Tob Control 2011; 20 Suppl 1: i10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertz-Schunemann R, Ehlert S, Streibel T, Liu C, McAdam K, Baker RR, et al. High-resolution time and spatial imaging of tobacco and its pyrolysis products during a cigarette puff by microprobe sampling photoionisation mass spectrometry. Agric Biol Chem 2015; 407: 2293–2299. [DOI] [PubMed] [Google Scholar]
- 8.Wu D, Landsberger S, Larson SM. Determination of the elemental distribution in cigarette components and smoke by instrumental neutron activation analysis. J Radioanal Nucl Ch 1997; 217: 77–82. [Google Scholar]
- 9.Ding YS, Ward J, Hammond D, Watson CH. Mouth-level intake of benzo[a]pyrene from reduced nicotine cigarettes. Int J Environ Res Public Health 2014; 11: 11898–11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirci A. Factorial design optimization of solid-phase microextraction for high-performance liquid chromatography-ultraviolet spectrometry analysis of polycyclic aromatic hydrocarbons in cigarette filter tar. Polycycl Aromat Comp 2014; 34: 115–134. [Google Scholar]
- 11.Watson C, McCraw J, Polzin G, Ashley D. Development of a method to assess cigarette smoke intake. Environ Sci Technol 2004; 38: 248–253. [DOI] [PubMed] [Google Scholar]
- 12.Hu N, Du W, Dai Y. Determination of major phenolic compounds retained by cigarette filter with high performance liquid chromatography. Tob Sci Tech 2015; 48: 49–55. [Google Scholar]
- 13.Verdolotti L, Salerno A, Lamanna R, Nunziata A, Netti P, Iannace S. A novel hybrid PU-alumina flexible foam with superior hydrophilicity and adsorption of carcinogenic compounds from tobacco smoke. Micropor Mesopor Mat 2012; 151: 79–87. [Google Scholar]
- 14.Yu J, Wang S, Wang B, Zhao X, Cai J, Yan Q, et al. Determination of eight volatile carbonyl compounds in cigarette filter by LC-ESI-MS/MS. Tob Sci Tech 2013: 39–46. [Google Scholar]
- 15.Kadir AA, Sarani NA. Cigarette butts pollution and environmental impact - a review. Applied Mechanics and Materials 2015; 773–774: 1106–10. [Google Scholar]
- 16.Novotny TE, Hardin SN, Hovda LR, Novotny DJ, McLean MK, Khan S. Tobacco and cigarette butt consumption in humans and animals. Tob Control 2011; 20 Suppl 1: i17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieng H, Rajasaygar S, Ahmad AH, Ahmad H, Rawi CSM, Zuharah WF, et al. Turning cigarette butt waste into an alternative control tool against an insecticide-resistant mosquito vector. Acta Tropica 2013; 128: 584–590. [DOI] [PubMed] [Google Scholar]
- 18.Dieng H, Rajasaygar S, Ahmad AH, Rawi CSM, Ahmad H, Satho T, et al. Indirect effects of cigarette butt waste on the dengue vector Aedes aegypti (Diptera: Culicidae). Acta Tropica 2014; 130: 123–130. [DOI] [PubMed] [Google Scholar]
- 19.Suarez-Rodriguez M, Lopez-Rull I, Garcia CM. Incorporation of cigarette butts into nests reduces nest ectoparasite load in urban birds: new ingredients for an old recipe? Biology Letters 2013; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters H. Bird butts, used cigarette filters in nests may protect hatchlings. Sci Am 2013; 308: 24–24.23469424 [Google Scholar]
- 21.Wright SL, Rowe D, Reid MJ, Thomas KV, Galloway TS. Bioaccumulation and biological effects of cigarette litter in marine worms. Sci Rep 2015; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booth DJ, Gribben P, Parkinson K. Impact of cigarette butt leachate on tidepool snails. Mar Pollut Bull 2015; 95: 362–364. [DOI] [PubMed] [Google Scholar]
- 23.Green ALR, Putschew A, Nehls T. Littered cigarette butts as a source of nicotine in urban waters. J Hydrol 2014; 519: 3466–3474. [Google Scholar]
- 24.Moerman JW, Potts GE. Analysis of metals leached from smoked cigarette litter. Tob Control 2011; 20: I30–I35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuhara K, Sakaki T, Sakuma H, Sugawara S. Odor analysis of cigarette butts by a headspace technique. Agric Biol Chem 1985; 49: 2177–2179. [Google Scholar]
- 26.Huang H, Liang H, Liu X, Yu Y. Determination of VOCs in cigarette tipping paper by HS-GC/MS. Acta Tabacaria Sinica 2014; 20: 15–20. [Google Scholar]
- 27.You J, Zhu G, Zhang Y, Ni J. Determination of menthol in mentholated cigarettes by headspace gas chromatography. Tob Sci Tech 2014: 51–4. [Google Scholar]
- 28.Ji H, Man J, Liu J, Liu N, Wang F, Han W. Determination of benzene compounds in mainstream cigarette smoke entrapped by filter using static headspace GC/MS method. Acta Tabacaria Sinica 2015; 21: 23–28. [Google Scholar]
- 29.Poppendieck D, Gong M, Measurement of airborne emissions from extinguished cigarettes: Final report, Engineering Laboratory, Editor. 2019, National Institute of Standards and Technology Gaithersburg, MD. [Google Scholar]
- 30.United States Center for Disease Control and Prevention, Tobacco brand preferences. 2017. [Google Scholar]
- 31.ISO3308. Routine analytical cigarette-smoking machine - Definitions and standard conditions. International Standard 2012. [Google Scholar]
- 32.United States Food and Drug Administration, Harmfu and potentially harmful constituents in tobacco products and tobacco smoke: Established list. 2012. [Google Scholar]
- 33.Van Loy MD, Lee VC, Gundel LA, Daisey JM, Sextro RG, Nazaroff WW. Dynamic behavior of semivolatile organic compounds in indoor air. 1. Nicotine in a stainless steel chamber. Environ Sci Technol 1997; 31: 2554–2561. [Google Scholar]
- 34.Code of Federal Regulations, Definition and procedure for the determination of the method detection limit – Revision 1.11. 2003. [Google Scholar]
- 35.Poppendieck DG, Khurshid SS, Emmerich SJ, Measuring airborne emissions from cigarette butts: literature review and experimental plan final report to U.S. Food and Drug Administration. 2016, Department of Commerce: National Institute of Standards and Technology [Google Scholar]
- 36.Singer BC, Hodgson AT, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos Environ 2003; 37: 5551–5561. [Google Scholar]
- 37.Charles SM, Jia C, Batterman SA, Godwin C. VOC and particulate emissions from commercial cigarettes: analysis of 2, 5-DMF as an ETS tracer. Environ Sci Technol 2008; 42: 1324–1331. [DOI] [PubMed] [Google Scholar]
- 38.Bi X, Sheng G, Feng Y, Fu J, Xie J. Gas-and particulate-phase specific tracer and toxic organic compounds in environmental tobacco smoke. Chemosphere 2005; 61: 1512–1522. [DOI] [PubMed] [Google Scholar]
- 39.Baek S-O, Jenkins RA. Characterization of trace organic compounds associated with aged and diluted sidestream tobacco smoke in a controlled atmosphere—volatile organic compounds and polycyclic aromatic hydrocarbons. Atmos Environ 2004; 38: 6583–6599. [Google Scholar]
- 40.Daisey J, Mahanama K, Hodgson A. Toxic volatile organic compounds in simulated environmental tobacco smoke: emission factors for exposure assessment. J Expo Anal Environ Epidemiol 1998; 8: 313–334. [PubMed] [Google Scholar]
- 41.Martin P, Heavner DL, Nelson PR, Maiolo KC, Risner CH, Simmons PS, et al. Environmental tobacco smoke (ETS): a market cigarette study. Environ Int 1997; 23: 75–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.