Summary
Background
Alcohol use is common among people with HIV and is a risk factor for tuberculosis disease and non-adherence to isoniazid preventive therapy (IPT). Few interventions exist to reduce alcohol use and increase IPT adherence in sub-Saharan Africa. The aim of this study was to test the hypothesis that financial incentives conditional on point-of-care negative urine alcohol biomarker testing and positive urine isoniazid testing would reduce alcohol use and increase isoniazid adherence, respectively, in people with HIV who have latent tuberculosis infection and hazardous alcohol use.
Methods
We conducted an open-label, 2×2 factorial randomised controlled trial in Uganda. Eligible for the study were non-pregnant HIV-positive adults (aged ≥18 years) prescribed antiretroviral therapy for at least 6 months, with current heavy alcohol use confirmed by urine ethyl glucuronide (biomarker of recent alcohol use) and a positive Alcohol Use Disorders Identification Test—Consumption (AUDIT-C; ≥3 for women, ≥4 for men) for the past 3 months’ drinking, no history of active tuberculosis, tuberculosis treatment, or tuberculosis preventive therapy, and a positive tuberculin skin test. We randomly assigned participants (1:1:1:1) initiating 6 months of IPT to: no incentives (group 1); or incentives for recent alcohol abstinence (group 2), isoniazid adherence (group 3), or both (group 4). Escalating incentives were contingent on monthly point-of-care urine tests negative for ethyl glucuronide (groups 2 and 4), or positive on IsoScreen (biomarker of recent isoniazid use; groups 3 and 4). The primary alcohol outcome was non-hazardous use by self-report (AUDIT-C <3 for women, <4 for men) and phosphatidylethanol (PEth; past-month alcohol biomarker) <35 ng/mL at 3 months and 6 months. The primary isoniazid adherence outcome was more than 90% bottle opening of days prescribed. We performed intention-to-treat analyses. This trial is registered with ClinicalTrials.gov (NCT03492216), and is complete.
Findings
From April 16, 2018, to Aug 2, 2021, 5508 people were screened, of whom 680 were randomly assigned: 169 to group 1, 169 to group 2, 170 to group 3, and 172 to group 4. The median age of participants was 39 years (IQR 32–47), 470 (69%) were male, 598 (90%) of 663 had HIV RNA viral loads of less than 40 copies per mL, median AUDIT-C score was 6 (IQR 4–8), and median PEth was 252 ng/mL (IQR 87–579). Among 636 participants who completed the trial with alcohol use endpoint measures (group 1: 152, group 2: 159, group 3: 161, group 4: 164), non-hazardous alcohol use was more likely in the groups with incentives for alcohol abstinence (groups 2 and 4) versus no alcohol incentives (groups 1 and 3): 57 (17·6%) of 323 versus 31 (9·9%) of 313, respectively; adjusted risk difference (aRD) 7·6% (95% CI 2·7 to 12·5, p=0·0025). Among 656 participants who completed the trial with isoniazid adherence endpoint measures (group 1: 158, group 2: 163, group 3: 168, group 4: 167), incentives for isoniazid adherence did not increase adherence: 244 (72·8%) of 335 in the isoniazid incentive groups (groups 3 and 4) versus 234 (72·9%) of 321 in the no isoniazid incentive groups (groups 1 and 2); aRD −0·2% (95% CI −7·0 to 6·5, p=0·94). Overall, 53 (8%) of 680 participants discontinued isoniazid due to grade 3 or higher adverse events. There was no significant association between randomisation group and hepatotoxicity resulting in isoniazid discontinuation, after adjusting for sex and site.
Interpretation
Escalating financial incentives contingent on recent alcohol abstinence led to significantly lower biomarker-confirmed alcohol use versus control, but incentives for recent isoniazid adherence did not lead to changes in adherence. The alcohol intervention was efficacious despite less intensive frequency of incentives and clinic visits than traditional programmes for substance use, suggesting that pragmatic modifications of contingency management for resource-limited settings can have efficacy and that further evaluation of implementation is merited.
Introduction
Alcohol use is a major contributor to morbidity and mortality globally; sub-Saharan Africa has the highest burden of disease and injury attributable to alcohol use worldwide.1 In addition to direct effects of alcohol, such as liver disease, traumatic injury, and violence, alcohol consumption has been associated with negative health outcomes among people with HIV and people with tuberculosis—two leading causes of death worldwide, with disproportionate burdens of disease in sub-Saharan Africa. Uganda has one of the highest rates of alcohol use in sub-Saharan Africa with total alcohol per capita consumption of 9·5 L annually in adults, and heavy episodic alcohol use among 21% of adults.1 Uganda also has a high burden of tuberculosis and HIV, with a tuberculosis incidence of 196 per 100 000 person-years and an adult HIV prevalence of 5·2%.2,3 Hazardous alcohol use (ie, drinking at levels that increase the risk of health consequences) is common among people with HIV, and contributes to worse HIV and tuberculosis outcomes through decreased adherence to prevention and treatment for HIV4 and tuberculosis,5,6 among other causal pathways. Both hazardous alcohol use and HIV infection also greatly increase the risk of progression from latent tuberculosis infection to tuberculosis disease.7 Therefore, the public health consequences of hazardous alcohol use among people with HIV are substantial, fuelling onward transmission of HIV and tuberculosis and worsening health outcomes.
Tuberculosis preventive therapy is a crucial tool for reducing the risk of tuberculosis disease and mortality in people with HIV, and WHO recommends its use, in addition to antiretroviral therapy (ART), for all adults with HIV.8 However, due to concerns about alcohol-induced hepatotoxicity, WHO guidelines do not recommend isoniazid preventive therapy (IPT; a commonly available form of tuberculosis preventive therapy globally) in people with HIV who have “regular and heavy alcohol consumption”,8 despite increased risk of tuberculosis in this group. Given the high and overlapping prevalence of hazardous alcohol use, HIV, and tuberculosis, effective interventions to reduce alcohol use and increase IPT use and adherence among people with HIV with hazardous alcohol use in sub-Saharan Africa are urgently needed.
Contingency management is an incentive-based intervention designed to reinforce drug and alcohol abstinence that is associated with large reductions in drug and alcohol use.9,10 However, a typical contingency management model is not feasible in many resource-limited settings, given the high frequency of clinic visits required (eg, twice per week) in settings where direct (eg, travel) and indirect (eg, time away from work) costs of attending clinic are expensive. The effectiveness of pragmatic modifications to contingency management that use incentive-based approaches for alcohol use, but with less frequent visits and rewards to enhance feasibility, is unknown. Furthermore, contingency management relies on an objective biomarker to confirm abstinence, such as point-of-care drug testing. A point-of-care test to confirm alcohol abstinence only recently became available. To our knowledge, contingency management has not yet been evaluated to treat alcohol use in sub-Saharan Africa.
Although incentive-based interventions have been shown to promote a wide range of health behaviours, data supporting the use of incentives for medication adherence among people with HIV—including tuberculosis preventive therapy6—have been mixed.11,12 Like alcohol intervention studies, tuberculosis preventive therapy studies are subject to reporting biases, including overestimation of adherence when relying on self-report.13 Furthermore, a lack of a point-of-care biomarker test to confirm isoniazid pill-taking has, until recently, presented a challenge for offering incentives conditional on isoniazid adherence. Whether incentive-based interventions can improve IPT adherence is unknown.
To address these gaps, we conducted a trial to evaluate incentive-based interventions to reduce alcohol use and increase IPT adherence among adults with HIV and latent tuberculosis infection (hereafter HIV–tuberculosis co-infection) and hazardous alcohol use in Uganda. For objective trial endpoint monitoring, we used an alcohol biomarker in addition to self-report. For isoniazid adherence, we assessed pill-taking by electronic pill bottle opening and self-report. Our trial tested the hypothesis that incentives conditional on point-of-care negative urine alcohol biomarker testing and positive urine isoniazid testing would reduce alcohol use and increase isoniazid adherence, respectively, among adults with HIV–tuberculosis co-infection and hazardous alcohol use.
Methods
Study design and participants
The Drinkers’ Intervention to Prevent Tuberculosis trial is a 2 × 2 factorial randomised controlled trial among adults with HIV and latent tuberculosis infection, and hazardous alcohol use in southwestern Uganda. We conducted the trial at two rural and two urban clinics. We also screened participants at 15 lower-level satellite clinics, with referral to the four study clinics for enrolment and study visits. We previously published the study design, procedures, and outcome measures,14 and include the protocol in appendix 2 (p 16).
We first screened people with HIV attending clinic appointments at study sites to identify those reporting any current (past 3 months) alcohol use. Once we identified patients with any alcohol use, study staff obtained written consent to participate in screening for trial eligibility. Participants were eligible if they met the following inclusion criteria at screening: adults (aged ≥18 years) with HIV prescribed ART for at least 6 months; current hazardous alcohol use confirmed by ethyl glucuronide (EtG), a metabolite of alcohol use that can be detected in urine for several days, using a commercial point-of-care dipstick test (Confirm Biosciences, San Diego, CA, USA) with a detection cutoff (indicating alcohol use in the past 48 h) of 300 ng/mL,15 and the Alcohol Use Disorders Identification Test—Consumption (AUDIT-C; ≥3 for women, ≥4 for men),16 modified for the past 3 months’ drinking; no history of active tuberculosis, tuberculosis disease treatment, or tuberculosis preventive therapy; fluent in Runyankole (the local dialect in southwestern Uganda) or English; residence within 2 h driving distance or 60 km of the study site; aspartate aminotransferase (AST) and alanine aminotransferase (ALT) less than two times the upper limit of normal; and positive tuberculin skin test (TST), defined as 5 mm induration or larger. TSTs were only performed on participants who met all other eligibility criteria. Participants were excluded if they had suspected or confirmed active tuberculosis (determined by symptom screening, followed by chest x-ray and sputum Xpert MTB/RIF assay [Cepheid, Sunnyvale, CA, USA] testing, if symptomatic); use of medications not recommended during IPT by Ugandan Ministry of Health guidelines, including nevirapine, anticonvulsants, or recent initiation (past 3 months) of dolutegravir; plans to move out of the study clinic catchment area within the next 6 months; pregnancy (by urine test) at the time of screening; or gross inebriation or inability to provide informed consent. Individuals with severe alcohol use disorder were not excluded, and with their consent, we notified their HIV providers (to whom we provided training in the management of alcohol use disorders before the start of the trial) about their level of alcohol use. Eligible individuals who provided written consent were enrolled in the trial.
The Mbarara University of Science and Technology Research and Ethics Committee, the Makerere University School of Medicine Research and Ethics Committee, the Uganda National Council for Science and Technology, and the University of California San Francisco Committee on Human Research approved the study protocol. Our institutional review board (IRB) applications noted that IPT is not recommended for individuals considered “regular and heavy drinkers” in resource-limited settings (such as Uganda) per WHO guidelines, and explicitly indicated that the trial would comprise individuals with heavy alcohol use. However, given that people who consume alcohol at hazardous levels are at increased risk of active tuberculosis and thus a high priority group for prevention, we received IRB approvals to provide IPT to participants with hazardous alcohol use with several specific precautions: (1) we excluded anyone with ALT or AST values of more than two times the upper limit of normal in pretreatment tests at baseline; (2) we implemented frequent monitoring of ALT and AST (every 2 weeks for the first month of IPT, and then monthly); and (3) we increased the frequency of monitoring for those with grade 2 ALT or AST elevations or symptoms, and discontinued IPT for grade 3 or higher adverse events attributed to isoniazid. The trial was conducted with oversight from a data safety monitoring board.
Randomisation and masking
We randomly assigned participants (1:1:1:1) initiating 6 months of IPT to: no incentives (group 1) or incentives contingent on no recent alcohol use (group 2), recent isoniazid adherence (group 3), or both (awarded independently, group 4). Randomisation was stratified by sex (female vs male) and study site. We used permuted blocks with random block sizes of 4 and 8 with the allocation sequence computer-generated before trial initiation. Participants selected a preprinted scratch card from eight cards presented by study staff that revealed randomisation assignment when scratched by the participant, with replacement of each card taken with the next card in the sequence, to ensure transparency and engagement in the randomisation process. Although participants and staff were not masked to study assignment given the nature of the intervention, the investigators, including the lead study statistician (SL), remained masked to participant randomisation assignment until trial completion.
Procedures
Before randomisation, participants completed a questionnaire and blood draw for HIV viral load (Xpert HIV-1 RNA assay; Cepheid, Sunnyvale, CA, USA), CD4+ cell count, and phosphatidylethanol (PEth; by dried blood spot) measurement. PEth assays were performed at the US Drug Testing Laboratory (Des Plaines, IL, USA) with a limit of quantification of 8 ng/mL for the 16:0/18:1 analog.17 Research assistants selected male or female on the research form. The baseline questionnaire included screening for self-reported other hazardous or illicit substance use in the past 3 months. In addition, we repeated these screening questions to evaluate emergent substance use at the 6-month study visit, midway through trial implementation (ie, for approximately half of participants). The study provided isoniazid 300 mg by mouth daily and pyridoxine 25 mg by mouth daily (with the option to increase to pyridoxine 50 mg by mouth daily, at study clinician discretion) for all participants at enrolment, with initial follow-up and refills at 2 weeks and 1 month post-randomisation, and monthly thereafter, for a 6-month course of IPT. In all groups, participants had liver function testing and screening for isoniazid side-effects and symptoms of active tuberculosis at each study visit. We allowed participants up to 9 months to complete a 6-month isoniazid course (180 tablets), to account for missed or late visits; incentives were only given over the first 6 months. During Uganda’s initial national COVID-19 lockdown, we halted study visits from April 1 to May 26, 2020, for 180 participants, as participants could not travel to study clinics. Participants resumed study visits, including intervention procedures, after the lockdown. All participants received brief (5-min) alcohol counselling at their baseline visit, and transport reimbursement (≤40 000 Uganda shillings [USh]; approximately US$11·40) to cover costs of travel for study visits.
Participants assigned to the incentive groups provided a urine sample for group-appropriate testing at each refill visit. At these visits, they were given escalating financial incentives contingent on point-of-care urine tests that were negative for EtG (groups 2 and 4), or positive on IsoScreen (GFC Diagnostics, Chipping Warden, UK), a biomarker of isoniazid use over the previous 1–3 days (groups 3 and 4; appendix 2 p 2).18 Participants assigned to alcohol reduction incentives who had a negative urine EtG test, and those assigned to isoniazid adherence incentives who had a positive urine isoniazid test, instantly won cash prizes, with prize value determined by drawing one or more lottery scratch cards. Scratch cards revealed low-value, medium-value, and high-value cash prizes, valued at 5000 USh (US$1·50; low), 10 000 USh (US$2·85; medium), and 50 000 USh (US$14·30; high), with each card having at least a low-value prize. Probabilities of winning were 90% for low-value, 7% for medium-value, and 3% for high-value prizes. The number of scratch cards awarded per participant increased by one card at each subsequent visit with a negative urine EtG or positive urine isoniazid test, thereby providing escalating prizes for sustained reductions in alcohol use or sustained isoniazid adherence. Escalating prizes were used based on previous studies demonstrating that escalating reinforcements maintain smoking abstinence longer than fixed reinforcements.19 If a participant had a positive urine EtG or negative urine isoniazid test, no cards were given, and the participant “reset” to drawing one card if meeting the incentive condition at the subsequent visit.
If participants discontinued isoniazid due to toxicity or pregnancy, they remained in the study for follow-up monitoring. However, these participants were no longer eligible to receive isoniazid adherence incentives if in groups 3 or 4, but were still eligible to receive alcohol reduction incentives (groups 2 and 4). In the isoniazid adherence group, all participants on isoniazid who returned to the clinic for their first visit after the 8-week COVID-19 lockdown (during which they could not obtain isoniazid refills) received incentives for adherence according to their escalating schedules, independent of urine isoniazid test results.
Outcomes
The primary alcohol outcome used to assess the alcohol intervention was non-hazardous use by AUDIT-C (<3 in women and <4 in men, in the past 3 months) and PEth below 35 ng/mL at 3 months and 6 months post-enrolmentr. The PEth cutoff was chosen a priori based on a previously recommended cutoff for no or low alcohol use.20 The primary isoniazid adherence outcome used to assess the adherence intervention was more than 90% electronic pill bottle cap opening (using medication event monitoring system [MEMS] caps [AARDEX Group, Seraing, Belgium]) of days isoniazid prescribed. Secondary outcomes included PEth and MEMS cap openings as continuous measures at 6 months, and isoniazid discontinuation due to grade 3 or higher hepatotoxicity. Hepatotoxicity was defined as elevation of AST or ALT to at least five times the upper limit of normal (grade 3) or at least ten times the upper limit of normal (grade 4) or based on symptoms that met prespecified grade 3 or 4 criteria for hepatotoxicity.
Statistical analysis
The trial sample size (n=680) was determined based on the a priori goal of detecting a 10–12% or greater difference in outcomes, comparing intervention to non-intervention groups (eg, groups 2 and 4 vs groups 1 and 3 for the alcohol intervention). We found that we needed to enroll 680 participants (170 per group, 340 per intervention), assuming 10% loss to follow-up for both 3-month and 6-month visits. For a two-sided χ2 test with continuity correction and alpha=0·05, this sample size would have given our study 80% power to detect a 10% absolute difference in the alcohol intervention versus no alcohol intervention groups, assuming proportions of participants with non-hazardous drinking of 25% and 15%, respectively. This sample size would have given our study 80% power to detect a 12% absolute difference in the isoniazid adherence versus no adherence intervention groups, assuming proportions with adherence of 62% and 50%, respectively.
For the alcohol outcome, we used a multivariable logistic regression model to estimate the effect of the alcohol intervention while adjusting for the adherence intervention and stratification randomisation factors (sex, study site). For the adherence outcome, we used a multivariable logistic regression model to estimate the effect of the isoniazid adherence intervention while adjusting for the alcohol intervention and stratification randomisation factors. For each model, we used the parameter estimates to predict the probability of the outcome under the intervention versus no intervention and obtain the adjusted absolute risk (proportions with outcome value=1) for intervention and no intervention, the risk difference (primary treatment effect measurement), and risk ratio, with 95% CIs and p values (two-sided, α=0·05; appendix 2 pp 5–15 [statistical analysis plan, also available online]). For both outcomes, we tested for effect modification by sex, very heavy alcohol use at baseline (AUDIT-C ≥6 or PEth ≥200 ng/mL [a PEth level that corresponds to approximately 4–5 drinks per day on a regular basis21]), and urban versus rural site, with p<0·10 being considered significant.
For the secondary continuous PEth outcome, we log-transformed PEth, and fit a linear regression model, with adjustment for baseline log10PEth, sex, site, and randomisation to isoniazid adherence incentives. When PEth was below the limit of quantification, we substituted 0 for log10PEth. For the secondary continuous MEMS cap opening outcome, we fit a negative binomial model for the number of MEMS cap opening days with an offset term defined as the natural logarithm of number of days of follow-up (given different follow-up times due to isoniazid discontinuation), adjusting for sex, site, and randomisation to alcohol reduction incentives.
For the hepatotoxicity outcome, we used a logistic regression model including study group and randomisation stratification factors (sex, study site). We conducted three pairwise comparisons between groups 2, 3, and 4 versus control (group 1).
Statistical analyses were conducted using Stata version 14.2. All analyses were performed as intention-to-treat analyses.
This trial is registered with ClinicalTrials.gov (NCT03492216).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
From April 16, 2018, to Aug 2, 2021, 5508 people with HIV underwent initial screening for eligibility: 1812 (33%) met eligibility for TST placement. Of individuals screened by TST, 712 (39%) tested TST positive, of whom 680 (96%) enrolled in the trial. Reasons for non-enrolment are shown in figure 1. At baseline, participant median age was 39 years (IQR 32–47), 470 (69%) were male, 598 (90%) of 663 had an HIV viral load below 40 copies per mL, median AUDIT-C was 6 (IQR 4–8), and median PEth was 252 ng/mL (IQR 87–579). At baseline, 181 (27%) of 680 participants reported smoking cigarettes, 22 (3%) used chewing tobacco, two (0·3%) used e-cigarettes, 46 (7%) used khat, 17 (3%) used marijuana, 11 (2%) used kuba, two (0·3%) sniffed petrol, and no participants reported use of cocaine, heroin, speed (methamphetamine), or hookah. Baseline characteristics of participants are shown in table 1. Participants with study visits impacted by the 2020 COVID-19 lockdown were evenly distributed by group (appendix 2 p 3).
Figure 1: Trial profile.
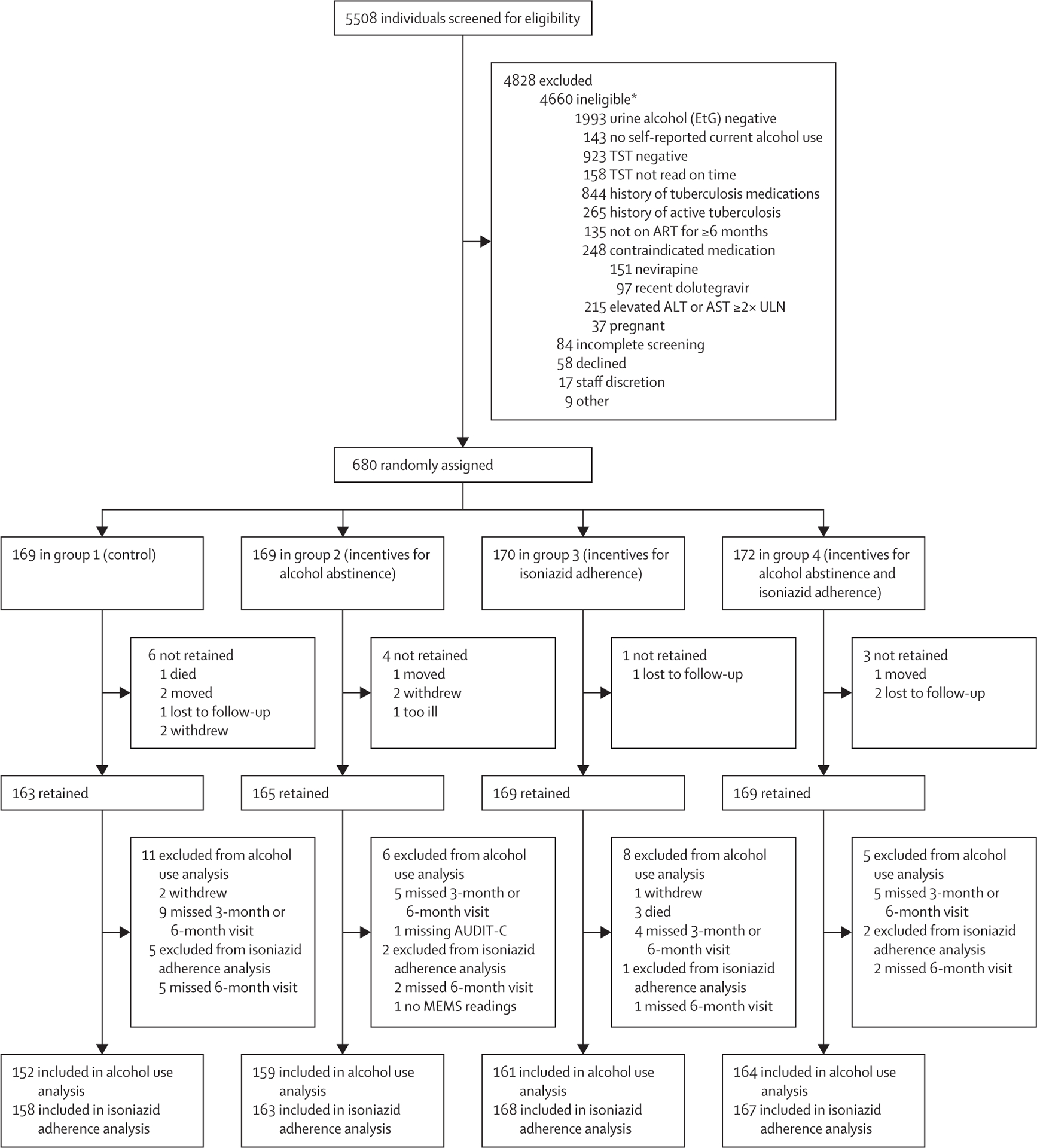
EtG=ethyl glucuronide. TST=tuberculin skin test. ART=antiretroviral therapy. ALT=alanine aminotransferase. AST=aspartate aminotransferase. ULN=upper limit of normal. AUDIT-C=Alcohol Use Disorders Identification Test—Consumption. MEMS=medication event monitoring system. *The individual numbers in this category do not add up to 4660 because the 4660 ineligible individuals could have more than one reason for inegibility.
Table 1:
Baseline participant characteristics
| Group 1—Control (n=169) | Group 2—Incentives for alcohol abstinence (n=169) | Group 3—Incentives for isoniazid adherence (n=170) | Group 4—Incentives for alcohol abstinence and isoniazid adherence (n=172) | Overall (n=680) | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 51 (30·2%) | 53 (31·4%) | 53 (31·2%) | 53 (30·8%) | 210 (30·9%) |
| Male | 118 (69·8%) | 116 (68·6%) | 117 (68·8%) | 119 (69·2%) | 470 (69·1%) |
| Age, years | 40 (33–46) | 38 (32–46) | 40 (32–48) | 39 (31–46) | 39 (32–47) |
| Educational attainment | |||||
| None | 26 (15·4%) | 27 (16·0%) | 26 (15·3%) | 28 (16·3%) | 107 (15·7%) |
| Any primary school | 85 (50·3%) | 77 (45·6%) | 57 (33·5%) | 78 (45·4%) | 297 (43·7%) |
| Completed primary school | 25 (14·8%) | 32 (18·9%) | 47 (27·7%) | 32 (18·6%) | 136 (20·0%) |
| Any secondary school | 33 (19·5%) | 33 (19·5%) | 40 (23·5%) | 34 (19·8%) | 140 (20·6%) |
| Marital status | |||||
| Married | 90 (53·3%) | 102 (60·4%) | 108 (63·5%) | 95 (55·2%) | 395 (58·1%) |
| Divorced, separated, or widowed | 66 (39·1%) | 52 (30·8%) | 50 (29·4%) | 62 (36·1%) | 230 (33·8%) |
| Never married and not living together | 13 (7·7%) | 15 (8·9%) | 12 (7·1%) | 15 (8·7%) | 55 (8·1%) |
| Occupation | |||||
| Business | 54 (32·0%) | 65 (38·5%) | 65 (38·2%) | 65 (37·8%) | 249 (36·6%) |
| Paid employment | 29 (17·2%) | 22 (13·0%) | 27 (15·9%) | 29 (16·9%) | 107 (15·7%) |
| Agricultural | 82 (48·5%) | 76 (45·0%) | 75 (44·1%) | 72 (41·9%) | 305 (44·9%) |
| Unemployed | 4 (2·4%) | 6 (3·6%) | 3 (1·8%) | 6 (3·5%) | 19 (2·8%) |
| Daily wage, Uganda shillings (n=673) | 5000 (3000–10 000) | 5000 (3000–10 000) | 5000 (3000–10 000) | 5000 (3000–10 000) | 5000 (3000–10 000) |
| PEth, ng/mL | 333·0 (96·0–673·0) | 243·0 (66·0–529·0) | 249·5 (95·0–597·0) | 219·0 (75·0–469·5) | 251·5 (86·5–578·5) |
| AUDIT-C score (past 3 months) | 6 (4–8) | 6 (4–8) | 6 (4–8) | 6 (4–8) | 6 (4–8) |
| High-risk alcohol use (PEth ≥200 ng/mL or AUDIT-C ≥6) | 133 (79·2%) | 126 (75·5%) | 127 (75·2%) | 130 (76·0%) | 516 (76·4%) |
| Timeline follow-back: number of drinking days over past 2 weeks (n=646) | 5 (3–9) | 5 (3–8) | 5 (2–8) | 5 (3–9) | 5 (3–8) |
| HIV virally suppressed (RNA <40 copies per mL) (n=663) | |||||
| No | 18 (11·0%) | 18 (10·8%) | 13 (7·8%) | 16 (9·6%) | 65 (9·8%) |
| Yes | 146 (89·0%) | 148 (89·2%) | 154 (92·2%) | 150 (90·4%) | 598 (90·2%) |
Data are n (%) or median (IQR). PEth=phosphatidylethanol. AUDIT-C=Alcohol Use Disorders Identification Test—Consumption.
There were 636 (94%) participants who had self-report and PEth data measured at 3 months and 6 months post-enrolment and for whom the primary alcohol use outcome (ie, reduction in drinking to non-hazardous use) could be evaluated (figure 1). The proportion of participants with non-hazardous alcohol use was 17·6% (57 of 323) in the alcohol reduction incentives groups (groups 2 and 4) and 9·9% (31 of 313) in the groups without alcohol reduction incentives (groups 1 and 3): adjusted risk difference (aRD) 7·6% (95% CI 2·7–12·5; p=0·0025, adjusting for sex, study site, and adherence intervention; figure 2). The number needed to treat with this intervention is 13·2 people for one person to reduce their alcohol use to non-hazardous levels. In a prespecified sensitivity analysis in which we assumed that the 44 participants with missing primary alcohol use endpoint data continued to have hazardous alcohol use (ie, did not meet the primary outcome), the effect of the alcohol intervention remained significant (aRD 7·1% [95% CI 2·5–11·8]; p=0·0027). Prespecified subgroup analyses evaluating the effect of the alcohol intervention on the primary alcohol outcome by sex, rural versus urban site, and very heavy alcohol use at baseline are shown in table 2. The proportions of participants with non-hazardous alcohol use were higher in the alcohol intervention versus no alcohol intervention groups in all subgroups, particularly for women, urban participants, and those with lower baseline PEth levels. In a post-hoc analysis that reanalysed the primary alcohol outcome while adjusting for log10PEth at baseline to account for higher baseline PEth level in group 1, non-hazardous alcohol use remained significantly more likely in the alcohol intervention versus no intervention groups.
Figure 2: Primary endpoints.
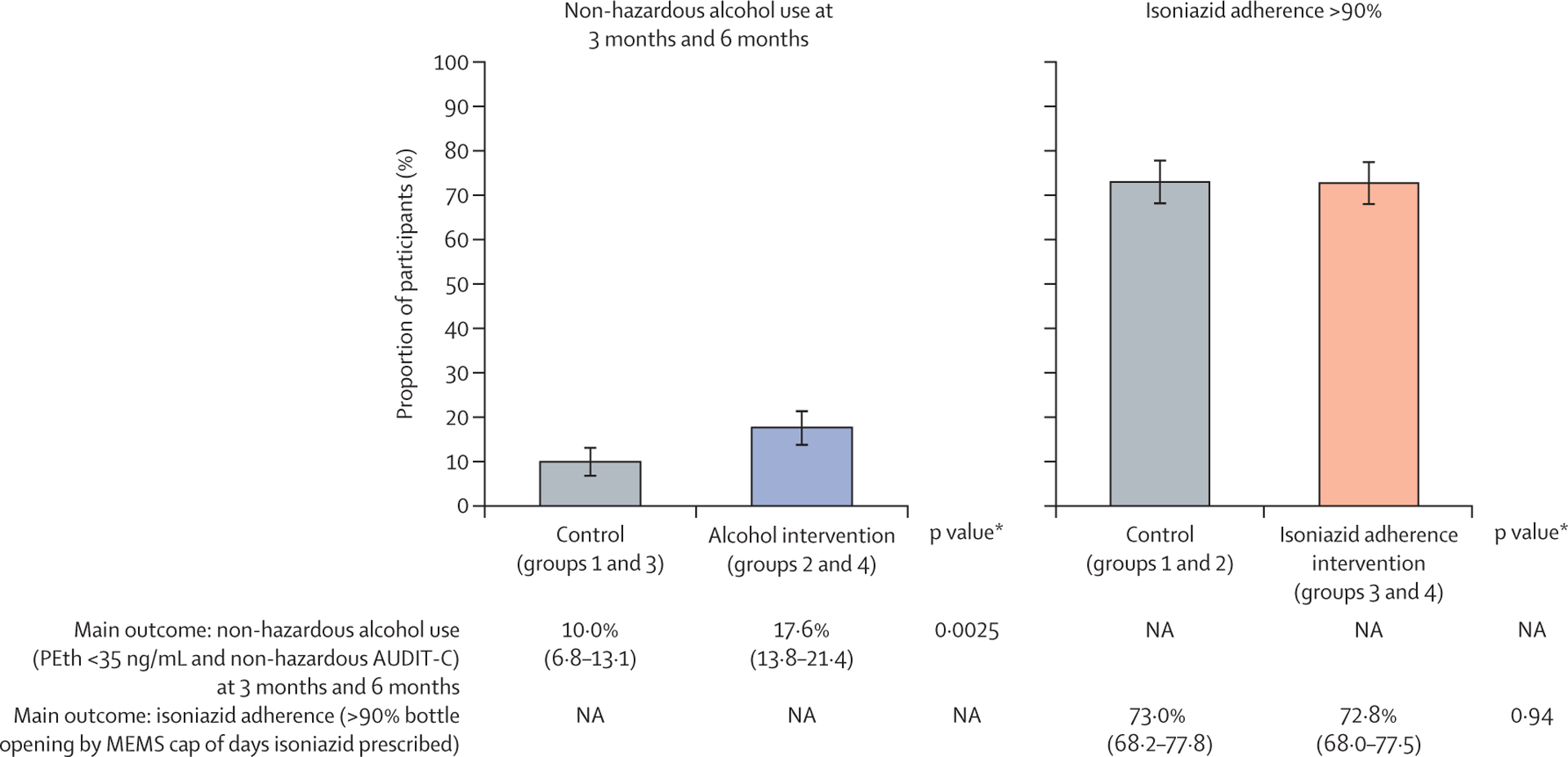
In the graphs, error bars show 95% CIs. Below the graphs, data in parentheses are 95% CIs. PEth=phosphatidylethanol. AUDIT-C=Alcohol Use Disorders Identification Test—Consumption. MEMS=medication event monitoring system. NA=not applicable. *p values based on multivariable models controlling for sex, site, and other intervention group.
Table 2:
Prespecified subgroup analyses of primary alcohol use outcome (non-hazardous alcohol use) by alcohol incentive group
| Unadjusted proportion with non-hazardous alcohol use (PEth <35 ng/mL and non-hazardous AUDIT-C) at 3 months and 6 months | Adjusted risk difference (95% CI) | p value | ||
|---|---|---|---|---|
| Intervention (groups 2 and 4) | Control (groups 1 and 3) | |||
| Sex | ||||
| Female | 33/99 (33·3%; 24·6 to 43·3) | 22/98 (22·4%; 15·2 to 31·9) | 10·0% (−1·5 to 21·6) | 0·089 |
| Male | 24/224 (10·7%; 7·3 to 15·5) | 9/215 (4·2%; 2·2 to 7·9) | 6·4% (1·7 to 11·2) | 0·0084 |
| Site | ||||
| Rural | 46/166 (27·7%; 21·4 to 35·1) | 26/156 (16·7%; 11·6 to 23·4) | 11·1% (2·7 to 19·5) | 0·0094 |
| Urban | 11/157 (7·0%; 3·9 to 12·3) | 5/157 (3·2%; 1·3 to 7·5) | 3·9% (−1·0 to 8·7) | 0·12 |
| Baseline alcohol use | ||||
| PEth <200 ng/mL and AUDIT-C <6 | 34/78 (43·6%; 32·8 to 55·0) | 20/70 (28·6%; 19·0 to 40·5) | 18·4% (4·1 to 32·6) | 0·011 |
| PEth ≥200 ng/mL or AUDIT-C ≥6 | 22/242 (9·1%; 6·0 to 13·5) | 10/241 (4·1%; 2·2 to 7·6) | 4·8% (0·5 to 9·2) | 0·030 |
For the unadjusted proportion columns, data are n/N (%; 95% CI). PEth=phosphatidylethanol. AUDIT-C=Alcohol Use Disorders Identification Test—Consumption.
Median PEth measured at 6 months post-enrolment (n=645 participants) was 134·0 ng/mL (IQR 25·0–379·0) in the alcohol intervention versus 170·5 ng/mL (58·5–483·5) in the no alcohol intervention groups. In a linear regression model evaluating PEth (log10 transformed) at 6 months post-enrolment, the alcohol intervention was associated with lower PEth levels (β=–0·08 [95% CI −0·18 to 0·01]; p=0·084) after adjusting for sex, site, randomisation to incentives for isoniazid adherence, and log10PEth at baseline, but this finding did not meet statistical significance. Among the subset of 286 participants who underwent repeat screening for other substance use at 6 months post-randomisation, 57 (20%) reported smoking cigarettes, one (0·3%) used chewing tobacco, five (2%) used khat, six (2%) used marijuana, one (0·3%) used kuba, and no participants reported use of cocaine, heroin, speed (methamphetamine), hookah, sniffing petrol, or e-cigarettes.
For the primary isoniazid adherence outcome, 656 (96%) participants had data on isoniazid adherence by MEMS cap opening. The proportion of participants with more than 90% adherence by MEMS cap openings during IPT was 72·8% (244 of 335) in the isoniazid incentive groups (groups 3 and 4) and 72·9% (234 of 321) in the no isoniazid incentive groups (groups 1 and 2): aRD −0·2% (95% CI −7·0 to 6·5; p=0·94, adjusting for sex, site, and participation in the alcohol intervention; figure 2). There was no independent effect of the alcohol incentives intervention on the isoniazid adherence endpoint. In a prespecified sensitivity analysis in which we assumed that the 24 participants with missing isoniazid adherence endpoint data did not achieve more than 90% adherence (ie, did not meet the primary adherence outcome), the effect of the isoniazid adherence intervention remained non-significant (aRD 0·2% [95% CI −4·8 to 8·9]; p=0·56). In subgroup analyses of the primary isoniazid adherence outcome by sex, baseline alcohol use, and rural versus urban site, there were no significant differences in proportion of participants with more than 90% adherence by MEMS cap opening in the isoniazid incentive versus no incentive groups.
Median MEMS cap openings out of days prescribed isoniazid was 96·7% (IQR 89·4–99·4) in the isoniazid incentive groups and 96·7% (89·4–99·4) in the no isoniazid incentive groups. In a negative binomial regression model evaluating adherence as a continuous measure, there was no significant association between the isoniazid intervention and isoniazid adherence (p=0·15), after adjusting for sex, site, and randomisation to incentives for reduced alcohol use.
Isoniazid discontinuation due to a grade 3 or higher adverse event occurred in 53 (8%) of 680 participants. Hepatotoxicity resulting in isoniazid discontinuation occurred in 47 (7%) participants. There was no significant association between randomisation group and hepatotoxicity resulting in isoniazid discontinuation, after adjusting for sex and site (table 3). There was significant heterogeneity in hepatotoxicity by site: one rural site had significantly greater hepatotoxicity rates than the urban site used as a reference (appendix 2 p 4). Grade 3 or higher adverse events and serious adverse events by group are shown in table 3.
Table 3:
Hepatotoxicity resulting in isoniazid discontinuation, and serious adverse events
| Group 1 (n=169) | Group 2 (n=169) | Group 3 (n=170) | Group 4 (n=172) | Total (n=680) | |
|---|---|---|---|---|---|
| Adverse events | |||||
| All grade 3 and higher adverse events | 18 (10·7%) | 14 (8·3%) | 25 (14·7%) | 13 (7·6%) | 70 (10·3%) |
| Hepatotoxicity resulting in isoniazid discontinuation (grade 3 or higher hepatotoxicity)* | 13 (7·7%) | 10 (5·9%) | 15 (8·8%) | 9 (5·2%) | 47 (6·9%) |
| Other grade 3 or higher adverse events resulting in isoniazid discontinuation (ie, not due to hepatotoxicity) | 1 (0·6%) | 2 (1·2%) | 3 (1·8%) | 0 | 6 (0·1%) |
| Serious adverse events | |||||
| Severe hepatotoxicity (grade 4) | 3 (1·8%) | 2 (1·2%) | 3 (1·8%) | 3† (1·7%) | 11 (1·6%) |
| Hospitalisation | |||||
| Unrelated to isoniazid | 1 (0·6%) | 2 (1·2%) | 2 (1·2%) | 1 (0·6%) | 6 (0·9%) |
| Possibly related to isoniazid | 0 | 1‡ (0·6%) | 0 | 0 | 1 (0·1%) |
| Death§ | 1 (0·6%) | 0 | 4 (2·4%) | 0 | 5 (0·7%) |
| Total serious adverse events | 5 (3·0%) | 5 (3·0%) | 9 (5·3%) | 4 (2·3%) | 23 (3·4%) |
Grade 3 hepatotoxicity defined by laboratory or clinical criteria as alanine aminotransferase or aspartate aminotransferase elevation ≥5 (but <10) times the upper limit of normal or symptoms consistent with hepatotoxicity; and grade 4 as ≥10 times the upper limit of normal or potentially life-threatening symptoms.
Two participants with severe hepatotoxicity diagnosed upon or after completing course of isoniazid.
Acute psychosis attributed to alcohol withdrawal-associated delirium.
Causes of death: group 1—one participant with oesophageal cancer; group 3—one participant with haemorrhagic pancreatitis with pneumonia; one participant with active tuberculosis disease; one participant with sepsis; one participant died of unknown cause (participant had completed isoniazid).
The distributions of participants receiving incentives for negative point-of-care urine EtG tests and positive isoniazid tests at each visit are shown in figure 3. The proportion of participants meeting incentive criteria for all visits was 31·4% (107 of 341) in the alcohol intervention and 42·4% (145 of 342) in the isoniazid intervention groups. The proportion meeting none of the incentive criteria was 3·8% (13 of 341) in the alcohol intervention and 1·2% (four of 342) in the isoniazid intervention groups. Overall, 90% of prizes earned were low-value prizes in both intervention groups. Median value of prizes received overall was US$25·35 (IQR 8·85–44·70) in group 2, US$43·35 (22·20–54·80) in group 3, and US$68·13 (45·18–92·65) in group 4. There were no instances in which a participant met point-of-care urine testing criteria but did not receive incentives.
Figure 3: Proportion of participants receiving incentives for negative point-of-care urine EtG tests and positive point-of-care urine isoniazid tests at each visit.
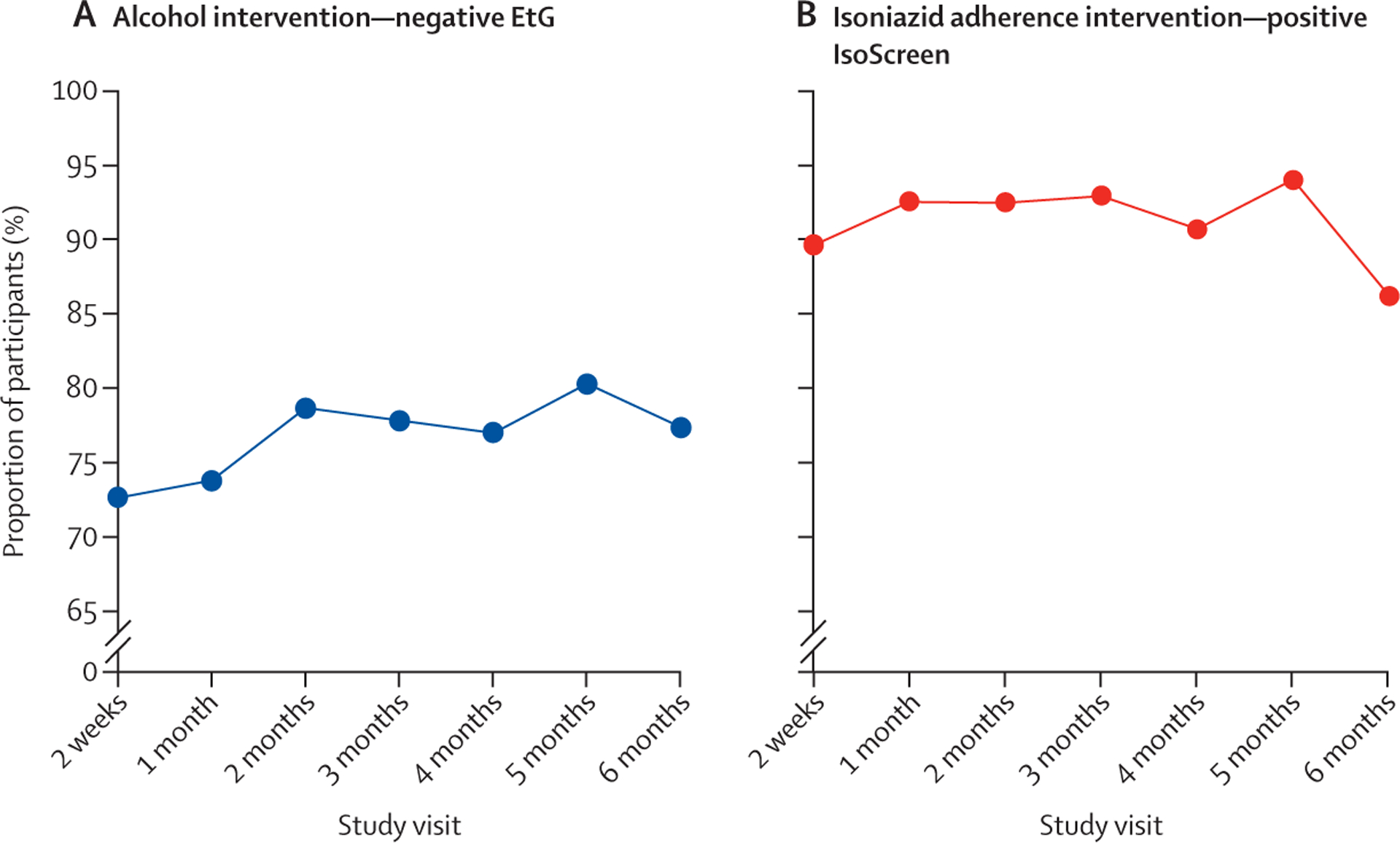
(A) Proportion of participants assigned to the alcohol intervention (groups 2 and 4) with negative urine EtGs at each study visit (indicative of no recent drinking). (B) Proportion of participants assigned to the isoniazid adherence intervention (groups 3 and 4) with positive urine IsoScreen at each study visit (indicative of recent isoniazid use). EtG=ethyl glucuronide.
Discussion
In this factorial randomised trial, escalating financial incentives contingent on recent alcohol abstinence led to significantly lower biomarker-confirmed alcohol use compared with no incentives over 6 months among adults with HIV–latent tuberculosis co-infection and hazardous alcohol use in Uganda. In contrast, escalating incentives contingent on adherence to isoniazid did not lead to significant increases in isoniazid use. The alcohol reduction intervention was efficacious despite less intensive frequency of incentives and clinic visits than traditional contingency management programmes for substance use, suggesting that pragmatic modifications of contingency management for resource-limited settings can have efficacy. Despite an intervention effect, a large proportion of participants continued to drink at hazardous levels. The lack of isoniazid adherence intervention effect adds to a mixed literature on the effectiveness of incentives in promoting daily medication adherence, suggesting other interventions are needed to support adherence to tuberculosis preventive therapy among people with HIV at high tuberculosis risk (with >25% of participants not achieving >90% daily IPT adherence), including those with hazardous alcohol use.
To our knowledge, this study is the first to demonstrate efficacy of incentives for reducing alcohol use in sub-Saharan Africa. To date, strategies to reduce hazardous alcohol use in sub-Saharan Africa have relied on counselling-based interventions ranging from brief (one session) to lengthy (12 sessions) counselling, with mixed success.22 Some studies among people with HIV in sub-Saharan Africa have shown efficacy for counselling-based interventions;23,24 others have not.25–27 However, alcohol use is frequently under-reported, which can lead to spurious results; only a few of these previous studies included an objective alcohol use measure, such as PEth, as part of their main alcohol outcomes,25,27 which might explain, in part, the mixed findings. Our study is one of the first to show a biomarker-confirmed effect of an intervention on alcohol use among people with HIV in sub-Saharan Africa.
For our primary alcohol use outcome, we used a stringent definition of non-hazardous use, defined by self-report and PEth below 35 ng/mL at both 3-month and 6-month visits: only 18% of participants in the intervention group and 10% in the control group met this definition. We chose this outcome with the goal of promoting very low levels of alcohol use to reduce risk of hepatotoxicity during IPT. Baseline severity of alcohol use was high, with a mean PEth level of 252 ng/mL, above the suggested cutoff of 200 ng/mL (akin to approximately four drinks per day) for chronic high-risk use.21 Therefore, our secondary analysis that showed greater declines in PEth levels in intervention versus control (p=0·084) suggests intervention efficacy in reducing alcohol use, even without abstinence. Our subgroup analyses found that people with HIV with lower baseline alcohol use (PEth <200 ng/mL and AUDIT-C <6) experienced a greater intervention effect compared with those with higher baseline alcohol use. More work is needed to confirm these findings and their impacts on other health and psychosocial outcomes, including implementation considerations and combining economic incentives with effective elements of counselling interventions. Moreover, pharmacotherapy should be considered as a potential adjuvant, or stepped intervention, in future studies. Additionally, several previous studies of incentive interventions have found that incentivised behaviours might decrease after incentives are withdrawn.9 Long-term, post-intervention effects of our alcohol intervention merit evaluation and are ongoing for this study at time of publication.
The isoniazid adherence intervention did not result in increased isoniazid pill bottle opening, with overall high adherence. One explanation for this finding is that we enrolled people with HIV engaged in care and with high baseline viral suppression. As such, we might have selected for participants who were largely adherent to medications despite alcohol use, limiting our ability to detect an intervention effect. We also included reactive TST as an inclusion criterion to identify people with HIV at very high risk of active tuberculosis. Global guidelines recommend tuberculosis preventive therapy for people with HIV in high tuberculosis prevalence settings without TST, due to benefits of IPT in TST-negative individuals and the logistical challenges of TST interpretation.8 Anecdotally, study staff noted that some participants reported motivation to take IPT after seeing their reactive TSTs at baseline, considering it evidence of tuberculosis infection in need of preventive therapy. Our findings add to the literature of interventions to promote adherence to tuberculosis preventive therapy. In a systematic review of such interventions, incentives had mixed results in increasing tuberculosis preventive therapy adherence, whereas use of short-course preventive therapy had positive effects.6 Increasing availability of short-course therapy for people with HIV in sub-Saharan Africa, including weekly isoniazid and rifapentine for 12 weeks or daily isoniazid and rifapentine for 4 weeks, might improve adherence to tuberculosis preventive therapy among people with HIV with hazardous alcohol use. Our results suggest incentives for isoniazid use are unlikely to impact IPT adherence in this population.
We did not find significant differences in grade 3 or higher hepatotoxicity by study group, although point estimates of hepatotoxicity were lower in the alcohol intervention group and closer to expected hepatotoxicity rates among non-drinkers (around 3–5%).28 We excluded individuals with abnormal transaminase levels (more than two times the upper limit of normal) and conducted monthly liver function testing during IPT, limiting generalisability of our findings to people with abnormal baseline transaminase levels or settings where no liver function testing is available. Our observed hepatotoxicity levels are consistent with other recent studies from sub-Saharan Africa,29,30 suggesting isoniazid might not increase the risk for serious toxicity under these circumstances.
Our study has limitations. First, bottle opening might not reflect pill ingestion. Long-term biomarkers of adherence, such as hair isoniazid measures, could provide a more accurate adherence measure, and analyses of hair measures in this trial are underway. Second, we did not conduct cost-effectiveness analyses. Our goal was to evaluate efficacy of the interventions. Based on our findings, cost-effectiveness modelling of the alcohol reduction intervention is merited. Third, our results might not be generalisable to people with HIV with hazardous alcohol use offered short-course tuberculosis preventive therapy or isoniazid for active tuberculosis treatment. However, there are substantial health benefits to reduced alcohol use, and incentives to reduce alcohol use merit further evaluation for other health outcomes where periods of low (or no) alcohol use are needed, such as active tuberculosis treatment or pregnancy. Fourth, intervention masking was not possible. This could have discouraged control (group 1) participants from decreasing alcohol use or increasing isoniazid adherence. We think this was unlikely, given the high retention across groups. Furthermore, the relatively high attention provided to group 1, with surveys, liver function testing, MEMS cap measures, and counselling, compared with standard care, would probably bias the results towards a null effect (ie, greater reduction in alcohol use in our control group than in an unobserved control receiving standard care), suggesting that we might have observed a conservative estimate of our alcohol intervention. Lastly, Uganda’s national COVID-19 lockdown resulted in an 8-week period during which study activities were paused for a subset of participants. However, the lockdown did not result in participants missing study procedures, apart from delayed refills of isoniazid. Our study also has strengths, including a randomised controlled trial design, high trial retention, objective measures of alcohol use and isoniazid pill bottle opening, and measures of fidelity to intervention implementation.
In conclusion, escalating financial incentives contingent on reduced alcohol use led to significantly lower biomarker-confirmed alcohol use, whereas incentives based on recent isoniazid adherence did not lead to changes in isoniazid adherence, among people with HIV with latent tuberculosis infection and hazardous alcohol use receiving IPT. Given the high prevalence of heavy alcohol use among people with HIV in this setting, the positive effect of our intervention is promising and merits further study and implementation.
Supplementary Material
Research in context.
Evidence before this study
Alcohol use, a major contributor to morbidity and mortality globally, is common among people living with HIV and is a risk factor for tuberculosis. Sub-Saharan Africa has the highest burden of disease and injury attributable to alcohol use, as well as the highest prevalence of HIV and tuberculosis co-infection, worldwide. Hazardous alcohol use contributes to worse HIV and tuberculosis outcomes due to non-adherence to isoniazid preventive therapy (IPT) and antiretroviral therapy (both of which significantly reduce the risk of tuberculosis disease among people with HIV) and hepatotoxicity during IPT. Interventions are urgently needed to reduce hazardous alcohol use and improve IPT adherence among people with HIV. We searched PubMed on June 14, 2023, for literature on interventions to reduce hazardous alcohol use in sub-Saharan Africa, using the search terms (alcohol AND intervention AND Africa AND HIV), as well as (alcohol AND [contingency management OR incentive] AND Africa AND HIV), without language or date restrictions. We also searched PubMed on June 14, 2023, for literature on interventions to increase adherence to tuberculosis preventive therapy, using the search terms ([tuberculosis preventive therapy OR isoniazid preventive therapy] AND adherence AND intervention), without language or date restrictions. To date, strategies to reduce hazardous alcohol use in sub-Saharan Africa have relied on counselling-based interventions with mixed success. In addition, alcohol use is frequently under-reported which can lead to spurious results: only a few of these previous alcohol intervention studies included an objective alcohol use measure as part of their main alcohol outcomes, which possibly explains the mixed findings. To our knowledge, incentive-based strategies to treat alcohol use have not previously been evaluated in sub-Saharan Africa. Similarly, data supporting incentives to promote tuberculosis preventive therapy adherence are limited, despite evidence that adherence to IPT is a challenge. In a 2016 systematic review, mixed results were found for the use of incentives to promote tuberculosis preventive therapy. Like alcohol use, tuberculosis preventive therapy studies are subject to reporting biases, including overestimation of adherence when relying on self-report. Whether incentive-based interventions can improve IPT adherence remains unknown.
Added value of this study
In this 2 × 2 factorial randomised trial that enrolled adults with HIV–tuberculosis co-infection and hazardous alcohol use initiating a 6-month course of IPT in Uganda, escalating financial incentives contingent on recent alcohol abstinence led to significantly lower biomarker-confirmed alcohol use compared with no incentives. In contrast, escalating incentives contingent on adherence to isoniazid did not lead to significant increases in isoniazid use, as measured by electronic pill bottle opening monitoring. The alcohol reduction intervention was efficacious despite less intensive frequency of incentives and clinic visits than traditional contingency management programmes for substance use, suggesting that pragmatic modifications of contingency management for resource-limited settings can have efficacy. Our study is one of the first studies to show a biomarker-confirmed effect of an intervention on alcohol use among people with HIV in sub-Saharan Africa.
Implications of all the available evidence
To date, published interventions to reduce hazardous alcohol use in sub-Saharan Africa have focused on counselling-based interventions, with mixed results, due in part to limited use of objective alcohol use measures. Our study demonstrates that escalating financial incentives contingent on recent alcohol abstinence (a pragmatic modification to traditional, more intensive, contingency management interventions) are efficacious in lowering biomarker-confirmed alcohol use, adding a novel alcohol reduction intervention for use in resource-limited settings to the literature. Despite an intervention effect, a large proportion of participants continued to drink at hazardous levels, and more work is needed, including evaluation of combination intervention approaches, to reduce hazardous alcohol use. In addition, our study adds to mixed evidence in the literature on the efficacy of incentive interventions to promote adherence to tuberculosis preventive therapy. Our results suggest that incentives are not efficacious in increasing adherence to IPT, and other interventions are needed to support adherence to tuberculosis preventive therapy among people with HIV at high risk of tuberculosis, including those with hazardous alcohol use.
Acknowledgments
We acknowledge the participants involved in the trial, as well as our research staff, data safety monitoring board members, and the communities involved in this study. This study was supported by grants from the National Institute of Alcohol Abuse and Alcoholism at the US National Institutes of Health: grants U01AA026223 (to JAH), U01AA026221 (to GC), and K24 AA022586 (to JAH). The content is solely the responsibility of the authors and does not represent the views of the National Institute of Alcohol Abuse and Alcoholism.
Footnotes
Equitable partnership declaration
The authors of this paper have submitted an equitable partnership declaration (appendix 3). This statement allows researchers to describe how their work engages with researchers, communities, and environments in the countries of study. This statement is part of The Lancet Global Health’s broader goal to decolonise global health.
Declaration of interests
We declare no competing interests.
For the Runyankole translation of the abstract see Online for appendix 1
See Online for appendix 2
For the statistical analysis plan see https://arxiv.org/abs/2208.08527
See Online for appendix 3
Data sharing
De-identified participant data that were collected for this study can be obtained by contacting the corresponding author. Data will be made available, beginning with publication, after approval of a short proposal summarising the analyses to be done.
References
- 1.WHO. Global status report on alcohol and health. Geneva: World Health Organization, 2018. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Uganda country profile https://www.cdc.gov/globalhivtb/where-we-work/uganda/uganda.html (accessed Feb 2, 2023).
- 3.UNAIDS. Country: Uganda. https://www.unaids.org/en/regionscountries/countries/uganda (accessed April 4, 2023).
- 4.Velloza J, Kemp CG, Aunon FM, Ramaiya MK, Creegan E, Simoni JM. Alcohol use and antiretroviral therapy non-adherence among adults living with HIV/AIDS in sub-Saharan Africa: a systematic review and meta-analysis. AIDS Behav 2020; 24: 1727–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muyindike WR, Fatch R, Cheng DM, et al. Unhealthy alcohol use is associated with suboptimal adherence to isoniazid preventive therapy in persons with HIV in Southwestern Uganda. J Acquir Immune Defic Syndr 2022; 91: 460–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuurman AL, Vonk Noordegraaf-Schouten M, van Kessel F, Oordt-Speets AM, Sandgren A, van der Werf MJ. Interventions for improving adherence to treatment for latent tuberculosis infection: a systematic review. BMC Infect Dis 2016; 16: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imtiaz S, Shield KD, Roerecke M, Samokhvalov AV, Lönnroth K, Rehm J. Alcohol consumption as a risk factor for tuberculosis: meta-analyses and burden of disease. Eur Respir J 2017; 50: 1700216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. WHO consolidated guidelines on tuberculosis: module 1: prevention—tuberculosis preventive treatment. Geneva: World Health Organization, 2020. [PubMed] [Google Scholar]
- 9.Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prev Med 2016; 92: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonell MG, Hirchak KA, Herron J, et al. Effect of incentives for alcohol abstinence in partnership with 3 American Indian and Alaska Native communities: a randomized clinical trial. JAMA Psychiatry 2021; 78: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thirumurthy H, Ndyabakira A, Marson K, et al. Financial incentives for achieving and maintaining viral suppression among HIV-positive adults in Uganda: a randomised controlled trial. Lancet HIV 2019; 6: e155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petry NM, Rash CJ, Byrne S, Ashraf S, White WB. Financial reinforcers for improving medication adherence: findings from a meta-analysis. Am J Med 2012; 125: 888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendall EA, Durovni B, Martinson NA, et al. Adherence to tuberculosis preventive therapy measured by urine metabolite testing among people with HIV. AIDS 2020; 34: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodi S, Emenyonu NI, Marson K, et al. The Drinkers’ Intervention to Prevent Tuberculosis (DIPT) trial among heavy drinkers living with HIV in Uganda: study protocol of a 2×2 factorial trial. Trials 2021; 22: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe JM, McDonell MG, Leickly E, et al. Determining ethyl glucuronide cutoffs when detecting self-reported alcohol use in addiction treatment patients. Alcohol Clin Exp Res 2015; 39: 905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007; 31: 1208–17. [DOI] [PubMed] [Google Scholar]
- 17.Jones J, Jones M, Plate C, Lewis D. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Anal Methods 2011; 3: 1101. [Google Scholar]
- 18.Guerra RL, Conde MB, Efron A, et al. Point-of-care Arkansas method for measuring adherence to treatment with isoniazid. Respir Med 2010; 104: 754–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romanowich P, Lamb RJ. The effects of fixed versus escalating reinforcement schedules on smoking abstinence. J Appl Behav Anal 2015; 48: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helander A, Hansson T. [National harmonization of the alcohol biomarker PEth]. Lakartidningen 2013; 110: 1747–48. [PubMed] [Google Scholar]
- 21.Luginbühl M, Wurst FM, Stöth F, Weinmann W, Stove CP, Van Uytfanghe K. Consensus for the use of the alcohol biomarker phosphatidylethanol (PEth) for the assessment of abstinence and alcohol consumption in clinical and forensic practice (2022 Consensus of Basel). Drug Test Anal 2022; 14: 1800–02. [DOI] [PubMed] [Google Scholar]
- 22.Francis JM, Cook S, Morojele NK, Swahn MH. Rarity and limited geographical coverage of individual level alcohol interventions in sub Saharan Africa: findings from a scoping review. J Subst Use 2020; 25: 11–19. [Google Scholar]
- 23.Madhombiro M, Kidd M, Dube B, et al. Effectiveness of a psychological intervention delivered by general nurses for alcohol use disorders in people living with HIV in Zimbabwe: a cluster randomized controlled trial. J Int AIDS Soc 2020; 23: e25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papas RK, Gakinya BN, Mwaniki MM, et al. A randomized clinical trial of a group cognitive-behavioral therapy to reduce alcohol use among human immunodeficiency virus-infected outpatients in western Kenya. Addiction 2021; 116: 305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn JA, Fatch R, Emenyonu NI, et al. Effect of two counseling interventions on self-reported alcohol consumption, alcohol biomarker phosphatidylethanol (PEth), and viral suppression among persons living with HIV (PWH) with unhealthy alcohol use in Uganda: a randomized controlled trial. Drug Alcohol Depend 2023; 244: 109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huis In ‘t Veld D, Ensoy-Musoro C, Pengpid S, Peltzer K, Colebunders R. The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa, a randomized clinical trial. PLoS One 2019; 14: e0220799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magidson JF, Joska JA, Belus JM, et al. Project Khanya: results from a pilot randomized type 1 hybrid effectiveness-implementation trial of a peer-delivered behavioural intervention for ART adherence and substance use in HIV care in South Africa. J Int AIDS Soc 2021; 24 (suppl 2): e25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute of Diabetes and Digestive and Kidney Diseases. Isoniazid. In: LiverTox: clinical and research information on drug-induced liver injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2012. https://www.ncbi.nlm.nih.gov/books/NBK548754/ (accessed April 4, 2023). [Google Scholar]
- 29.Nanyonga SM, Kitutu FE, Kalyango J, Frank M, Kiguba R. High burden of adverse drug reactions to isoniazid preventive therapy in people living with HIV at 3 tertiary hospitals in Uganda: associated factors. J Acquir Immune Defic Syndr 2022; 89: 215–21. [DOI] [PubMed] [Google Scholar]
- 30.Ngongondo M, Miyahara S, Hughes MD, et al. Hepatotoxicity during isoniazid preventive therapy and antiretroviral therapy in people living with HIV with severe immunosuppression: a secondary analysis of a multi-country open-label randomized controlled clinical trial. J Acquir Immune Defic Syndr 2018; 78: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data that were collected for this study can be obtained by contacting the corresponding author. Data will be made available, beginning with publication, after approval of a short proposal summarising the analyses to be done.


