Abstract
Objective:
This study was conducted to evaluate the association between neurodegenerative dementia and herpes zoster infection (HZI) using a national sample cohort.
Methods:
From the national cohort study conducted by the Korean National Health Insurance Service, we extracted data for patients with neurodegenerative dementia and for 1:4 matched control participants and searched the patient histories for HZI.
Results:
The adjusted odds ratio (OR) for HZI was 0.90 (95% CI = 0.84-0.97) in the dementia group. According to the subgroup analysis, the adjusted OR for HZI was 0.91 (95% confidence interval [CI] = 0.83 -1.00) in the < 80 years old group, 0.88 (95% CI = 0.78 -1.00) in the ≥ 80 years old group, 0.77 (95% CI = 0.66-0.89) in men and 0.96 (95% CI = 0.88 -1.05) in women.
Conclusions:
We concluded that HZI does not increase the risk of neurodegenerative dementia in individuals of any age or of either sex.
Keywords: cohort study, dementia, epidemiology, herpes zoster, nested case-control study
Introduction
Dementia is defined as a clinical syndrome that is characterized by progressive cognitive decline and interferes with cognition, function and behavior. 1 It is a devastating disease because of the significant physical, emotional and financial burden on patients, their families and society. Dementia is a neurodegenerative disease that is characterized by brain cell damage by abnormal deposits of amyloid-β and tau protein and is caused by various cardiovascular diseases (vascular dementia). Neurodegenerative dementia is 3 times more prevalent than vascular dementia. 2,3
Variable infectious pathogens, including human immunodeficiency virus, herpes virus, toxoplasmosis, cryptococcus, cytomegalovirus, and syphilis, are known to be causes of dementia. 4 Several population-based studies have reported that the Herpesviridae family, including herpes simplex virus (HSV), varicella zoster virus (VZV), Epstein-Barr virus (EBV), and human cytomegalovirus (HCMV), is associated with an increased risk of developing dementia. 5 -7 Herpes zoster infection (HZI) is a viral disease characterized by not only a painful skin rash with blisters but also postherpetic neuralgia, ophthalmicus, and variable diseases of the central nervous system (CNS), including meningitis, encephalitis, and cerebellitis. 8 These conditions with CNS involvement by herpes zoster virus lead to neurological sequelae, including long-term cognitive impairment. 9 The association between HZI and cognitive impairment was first introduced by Hokkanen et al. Compared to healthy controls, immunocompetent patients with acute herpes zoster encephalitis showed a decline in memory and cognitive processing speed Based on the above studies, several clinical studies have shown that HZI was associated with an increased risk of dementia. In a Taiwan population-based cohort study, herpes zoster was found to be associated with an increased risk of dementia (hazard ratio [HR] = 1.11 95% CI, 1.04 -1.17). 10 In another cohort study, the herpes zoster ophthalmicus group had a 2.97-fold higher risk of dementia than the control group. 11 The mechanism by which HZI causes dementia is thought to be inflammatory cerebral vasculopathy, but a cohort study on the relationship between HZI and neurodegenerative dementia has yet to be conducted.
The aim of this study was to investigate the risk of neurodegenerative dementia in patients with HZI in South Korea using a nationwide, population-based dataset obtained from the Korean National Health Insurance Service (NHIS).
Materials and Methods
Study Population and Data Collection
The ethics committee of Hallym University (2019-01-003) approved the use of these data. The need for written informed consent was exempted by the Institutional Review Board.
This national cohort study was conducted with data from the Korean Health Insurance Review and Assessment Service-National Sample Cohort (HIRA-NSC). The NHIS program started in Korea in 1989 and as of 2002, it has contracts with approximately 46 million beneficiaries, more than 98% of the total population. The Korean NHIS database contains outpatient and inpatient information about demographic characteristics, diagnosis, medication use, surgical and intervention procedures, outpatient prescription and patient outcomes. The diagnoses were coded according to the International Classification of Disease, Tenth Revision (ICD-10). 12 A detailed description of these data can be found in our previous studies. 13
Participant Selection
Out of 1,125,691 patients with 114,369,638 medical claim codes, we included participants who were diagnosed with dementia (n = 13,102). Neurodegenerative dementia was defined as the presence of Alzheimer’s disease (G30) or dementia with Alzheimer’s disease (F00). For the accuracy of diagnosis, we selected only the participants who were treated ≥ 2 times. We described the reliability of the diagnosis of dementia in the supplemental material (S1).
HZI was diagnosed with ICD-10 codes (B02). Among the patients with the appropriate codes, we included only the participants who were treated ≥ 2 times or who were treated with antiviral medication ≥ 1 time. From 2002 through 2013, 64,152 HZI participants were selected.
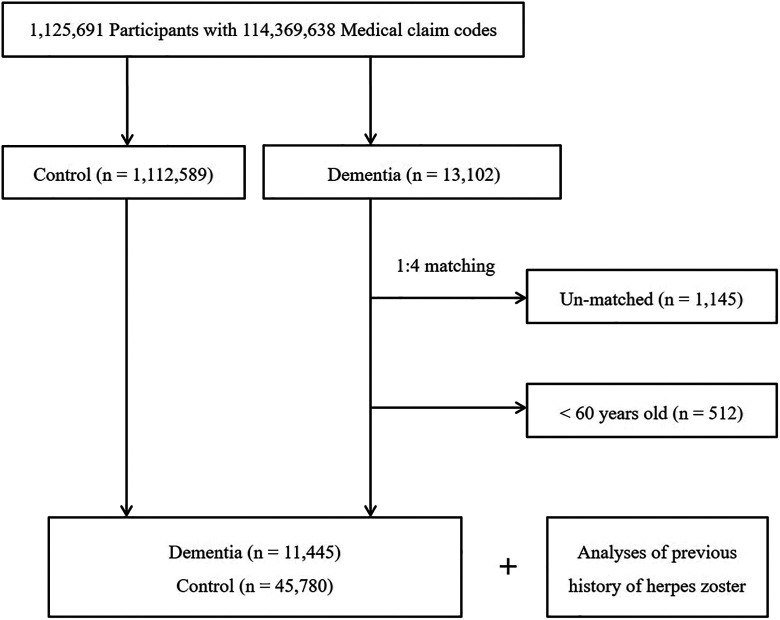
The dementia participants were matched at a 1:4 ratio with patients (control group) in this cohort who had never been treated for dementia from 2002 through 2013 (Figure 1). The control group was selected from the original population (n = 1,112,589). These subjects were matched for age, group, sex, income, region of residence, and medical histories (hypertension, diabetes mellitus (DM), and dyslipidemia). To prevent selection bias from occurring when the matched participants were selected, the control group participants were assigned random numbers, and they were then selected in numerical order. The matched control participants were assumed to be involved at the same time as each matched dementia participant (index date). Therefore, the control group subjects who died before the index date were excluded. Dementia participants for whom we could not identify enough matched participants were excluded (n = 1,145). We also excluded participants aged less than 60 years (n = 512). Finally, 1:4 matching resulted in the inclusion of 11,445 dementia participants and 45,780 control participants. However, the participants were not matched for ischemic heart disease, stroke, or depression because this type of strict matching increases the number of excluded study participants due to a lack of control participants. After we matched the participants, we searched the medical histories of both the dementia and control group participants for HZI.
Figure 1.
Schematic illustration of the participant selection process that was used in the present study. Of a total of 1,125,691 participants, 11,445 dementia participants were matched with 45,780 control participants with respect to age, group, sex, income, region of residence, and medical history.
Variables
The participants were grouped by age as follows using 5-year intervals: 60-64, 65-69, 70-74…, and 85+ years old. A total of 6 age groups were designated. The income groups were initially divided into 41 classes (one health aid class, 20 self-employed health insurance classes, and 20 employed health insurance classes). These groups were recategorized into 5 classes (class 1 [lowest income] − class 5 [highest income]). Region of residence was divided into 16 areas according to the administrative district. These regions were regrouped into urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju) areas.
Participants’ past medical histories were assessed using ICD-10 codes. For the accuracy of diagnosis, hypertension (I10 and I15), DM (E10-E14), and dyslipidemia (E78) were examined if the participants were treated ≥ 2 times. Ischemic heart disease (I24 and I25) and stroke (I60-I66) were noted if the participants were treated ≥ 1 time. Depression was defined by the psychiatrist through the ICD-10 code from F31 (bipolar affective disorder) through F39 (unspecified mood disorder).
Statistical Analyses
Chi-square tests were used to compare the general characteristics of the dementia and control groups.
To analyze the OR for HZI with dementia, conditional logistic regression analysis was used. In this analysis, crude (simple) and adjusted (ischemic heart disease, stroke, and depression) models were used, and 95% CIs were calculated. The data were stratified by age, sex, income, region of residence, hypertension, DM, and dyslipidemia.
For the subgroup analyses, we divided the participants by age and sex (< 80 years old and ≥ 80 years old; men and women).
Two-tailed analyses were conducted, and P values < 0.05 were considered to indicate significance. The results were analyzed using SPSS v. 22.0 (IBM, Armonk, NY, USA).
Results
The general characteristics (age, sex, income, region of residence, hypertension, DM, and dyslipidemia) of the participants were exactly the same due to matching (P = 1.000). The rates of ischemic heart disease, stroke, and depression were higher in the dementia group than in the control group (all P < 0.05). There was no statistically significant difference in the incidence of HZI between the dementia and control groups (8.1% [928/11,445]) vs 8.6% [3,929/45,780]; P = 0.104, Table 1).
Table 1.
General Characteristics of Participants.
| Total participants | |||
|---|---|---|---|
| Characteristics | Dementia (n, %) | Control group (n, %) | P |
| Age (years old) | 1.000 | ||
| 60-64 | 580 (5.1) | 2,320 (5.1) | |
| 65-69 | 1,289 (11.3) | 5,156 (11.3) | |
| 70-74 | 2,325 (20.3) | 9,300 (20.3) | |
| 75-79 | 2,979 (26.0) | 11,916 (26.0) | |
| 80-84 | 2,706 (23.6) | 10,824 (23.6) | |
| 85+ | 1,566 (13.7) | 6,264 (13.7) | |
| Sex | 1.000 | ||
| Male | 3,666 (32.0) | 14,664 (32.0) | |
| Female | 7,779 (68.0) | 31,116 (68.0) | |
| Income | 1.000 | ||
| 1 (lowest) | 2,867 (25.1) | 11,468 (25.1) | |
| 2 | 1,031 (9.0) | 4,124 (9.0) | |
| 3 | 1,379 (12.0) | 5,516 (12.0) | |
| 4 | 1,886 (16.5) | 7,544 (16.5) | |
| 5 (highest) | 4,282 (37.4) | 17,128 (37.4) | |
| Region of residence | 1.000 | ||
| Urban | 4,620 (40.4) | 18,480 (40.4) | |
| Rural | 6,825 (59.6) | 27,300 (59.6) | |
| Hypertension | 1.000 | ||
| Yes | 8,311 (72.6) | 33,244 (72.6) | |
| No | 3,134 (27.4) | 12,536 (27.4) | |
| Diabetes mellitus | 1.000 | ||
| Yes | 4,065 (35.5) | 16,260 (35.5) | |
| No | 7,380 (64.5) | 29,520 (64.5) | |
| Dyslipidemia | 1.000 | ||
| Yes | 3,552 (31.0) | 14,208 (31.0) | |
| No | 7,893 (69.0) | 31,572 (69.0) | |
| Ischemic heart disease | <0.001* | ||
| Yes | 1,703 (14.9) | 6,004 (13.1) | |
| No | 9,742 (85.1) | 39,776 (86.9) | |
| Stroke | <0.001* | ||
| Yes | 5,517 (48.2) | 11,356 (24.8) | |
| No | 5,928 (51.8) | 34,424 (75.2) | |
| Depression | <0.001* | ||
| Yes | 3,237 (28.3) | 4,637 (10.1) | |
| No | 8,208 (71.7) | 41,143 (89.9) | |
| Herpes zoster infection | 0.104 | ||
| Yes | 928 (8.1) | 3,929 (8.6) | |
| No | 10,517 (91.9) | 41,851 (91.4) | |
*Chi-square test or Fisher’s exact test. Significance at P < 0.05.
The crude and adjusted ORs for HZI were 0.95 (95% CI = 0.88-1.02, P = 0.120) and 0.90 (95% CI = 0.84-0.97, P = 0.008) in the dementia group, respectively (Table 2).
Table 2.
Crude and Adjusted Odds Ratios (95% Confidence Interval) of Dementia for Herpes Zoster Infection.
| Herpes zoster infection | ||||
|---|---|---|---|---|
| Characteristics | Crude | P | Adjusted† | P |
| Dementia | 0.95 (0.88-1.02) | 0.120 | 0.90 (0.84-0.97) | 0.008* |
| Control | 1.00 | 1.00 | ||
*Conditional logistic regression analyses stratified for age, sex, income, region of residence, hypertension, diabetes mellitus, and dyslipidemia. Significance at P < 0.05.
† Adjusted model for ischemic heart disease, stroke, and depression histories.
In the subgroup analyses by age, the adjusted ORs for HZI were 0.91 (95% CI = 0.83-1.00, P = 0.060) in the < 80 years old group and 0.88 (95% CI = 0.78-1.00, P = 0.057) in the ≥ 80 years old group (Table 3).
Table 3.
Subgroup Analyses of Crude and Adjusted Odds Ratios (95% Confidence Interval) of Dementia for Herpes Zoster Infection According to Age and Sex.
| Herpes zoster infection | ||||
|---|---|---|---|---|
| Characteristics | Crude | P | Adjusted† | P |
| Age < 80 years old (n = 35,865) | ||||
| Dementia | 0.95 (0.87-1.04) | 0.258 | 0.91 (0.83-1.00) | 0.060 |
| Control | 1.00 | 1.00 | ||
| Age ≥ 80 years old (n = 21,360) | ||||
| Dementia | 0.94 (0.83-1.06) | 0.277 | 0.88 (0.78-1.00) | 0.057 |
| Control | 1.00 | 1.00 | ||
| Men (n = 18,330) | ||||
| Dementia | 0.82 (0.71-0.94) | 0.005* | 0.77 (0.66-0.89) | 0.001* |
| Control | 1.00 | 1.00 | ||
| Women (n = 38,895) | ||||
| Dementia | 1.00 (0.92-1.09) | 0.972 | 0.96 (0.88-1.05) | 0.392 |
| Control | 1.00 | 1.00 | ||
*Conditional logistic regression analyses stratified for age, sex, income, region of residence, hypertension, diabetes mellitus, and dyslipidemia. Significance at P < 0.05.
†Adjusted model for ischemic heart disease, stroke, and depression histories.
In the subgroup analyses by sex, the adjusted ORs for HZI were 0.77 (95% CI = 0.66-0.89, P = 0.001) in men and 0.96 (95% CI = 0.88-1.05, P = 0.392) in women (Table 3).
Discussion
In our present nationwide cohort study, HZI did not increase the risk of neurodegenerative dementia in individuals of any age or sex after the models were adjusted for age, hypertension, DM, dyslipidemia, and a history of ischemic heart disease or stroke.
Research on the effects of herpesvirus infection on cognitive decline has been conducted since the mid-2000s. Pathogens such as HSV-1 and CMV have been linked to several neurological disorders, including cognitive impairment. 14,15 These pathogens can infect the CNS system, and viral replication within brain cells may lead to cell death, morphological changes, and subsequently, cell atrophy and the loss of brain cells. 16,17 Similar to HSV and CMV, VZV is a herpesvirus, and several studies have examined the association between VZV and cognitive impairment. Hokkanen et al. reported an association of cognitive impairment with frontal hypoperfusion by single photon emission computed tomography in 9 herpes zoster encephalitis patients. 18 In another study, the VZV group showed significantly worse scores on cognitive tests at a follow-up of 3 years than did the control group. 9 Since these original studies, several large-scale studies that have evaluated cognitive dysfunction in patients with HZI have been performed. In a nationwide study in Taiwan, herpes zoster ophthalmicus increased the risk for dementia during a 5-year follow-up period (HR = 2.83, 95% CI = 1.83-4.37). 11 In another nationwide study, herpes zoster was found to be associated with a slightly increased risk of dementia (HR = 1.11, 95% CI = 1.04-1.17). 10 However, 2 previous studies showed that there was no difference in the amount of VZV DNA detected in postmortem brain samples and serum antiviral antibody titers of VZV between the Alzheimer’s disease group and the control group. 19,20 The results of these studies could not be generalized due to the small number of participants.
The mechanisms by which HZI increases the risk of cognitive impairment are not well understood. Primary VZV infection can result in chickenpox, after which the virus becomes latent and reactivates to herpes zoster (shingles). 21 VZV reactivation is mainly T-cell mediated and occurs due to aging or immunosuppression. 22 These VZV reactivations can lead to vasculopathy, postherpetic neuralgia, retinal involvement, and CNS infections such as encephalitis or meningitis. 23 VZV-induced vasculopathy due to reactivation involving the intracranial artery can lead to several sequelae, including transient ischemic attack and ischemic or hemorrhagic stroke, and 4 cases of multiinfarct dementia after VZV infection of the brain have been reported. 24,25
In our study, HZI did not increase the risk of dementia irrespective of age and sex after the models were adjusted for DM, hypertension, hyperlipidemia, ischemic heart disease and stroke. The main pathogenesis of dementia is neuroinflammation caused by the abnormal accumulation of amyloid β (Aβ) peptides and tau protein, which destroy neuronal brain cells and synapses (neurodegenerative dementia), and impair cerebral blood flow due to atherosclerosis or vasculopathy (vascular dementia). 2,3 Viral infection-induced systemic inflammation triggers brain responses through microglial activation, exacerbates the accumulation of Aβ and tau protein, and promotes the progression of dementia. 26 Previous cell culture studies have shown that HSV induces abnormal Aβ accumulation in Alzheimer’s disease patients. 27,28 In a spinal astrocyte cell culture study, VZV-infected cells led to intracellular amyloid accumulation, but whether dementia occurs due to amyloid accumulation induced by HZI remains unclear. 29
Vascular dementia accounts for up to 20% of all cases of dementia and is caused by multiple cerebral infarctions from cerebrovascular or cardiovascular problems, including DM, hypertension, stroke and metabolic syndrome. 30 VZV invades the spinal cord or cerebral arteries directly or after reactivation. These conditions with CNS involvement by VZV lead to severe neurological sequelae, including vasculopathy involving small and large vessels of the brain, which leads to cerebral infarction. 31 Tsai et al. reported that herpes zoster ophthalmicus is associated with an increased risk of dementia due to an increase in cerebrovascular events. 11 The risk of dementia caused by HZI may be mainly associated with vascular dementia, and the development of dementia caused by HZI through neuroinflammation was not demonstrated in previous studies. In our study, we included a history of stroke as a covariation factor, and there was no difference in the incidence of dementia between the HZI and control groups.
This study has several limitations. First, dementia and HZI were diagnosed according to the ICD codes from the administrative claims data and the number of visits patients made for dementia and HZI, which may not have been reflective of the actual number of dementia or HZI cases experienced by the patients. The use of ICD codes from large claim code data can potentially lead to misdiagnoses. Second, HZI includes varying severities of skin lesions, trigeminal neuralgia, ophthalmicus, meningitis, and encephalitis. However, the severity of HZI was not classified in this study. Third, although the control group was matched according to several previous conditions and demographic factors, some possible confounders were not considered, including alcohol intake, smoking, body mass index, and education level. Fourth, patients infected with HZV are more likely to receive outpatient or inpatient care, and because clinicians tend to take care of these patients more, they are more likely to be identified as dementia patients. This finding can reflect ascertainment bias.
Despite the limitations mentioned above, there were several strengths in this study. First, our study was the first to evaluate the association between HZI and dementia in a nationwide cohort study in South Korea. Second, we used a population-based dataset consisting of one million subjects with a 12-year follow-up period to assess the association between HZI and dementia. Studies with large sample sizes based on populations may have statistical power. Third, the control group was matched with the HZI group not only for basic characteristics, including age, sex, income, and region of residence but also for risk factors for dementia, such as hypertension, DM, and dyslipidemia. This detailed matching might provide valid evidence for the effect of the association between HZI and dementia.
Conclusion
We concluded that HZI does not increase the risk of dementia at any age or in either sex after adjusting for age and the history of hypertension, DM, dyslipidemia, ischemic heart disease, stroke, and depression.
Supplemental Material
Supplemental Material, sj-pdf-1-aja-10.1177_15333175211006504 for Herpes Zoster Does Not Increase the Risk of Neurodegenerative Dementia: A Case-Control Study by Hyo Geun Choi, Bum Jung Park, Jae Sung Lim, Song Yong Sim, Yoon Jung Jung and Suk Woo Lee in American Journal of Alzheimer's Disease & Other Dementias
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported in part by a research grant (NRF-2015-R1D1A1A01060860) from the National Research Foundation (NRF) of Korea.
ORCID iD: Suk Woo Lee  https://orcid.org/0000-0001-9200-8728
https://orcid.org/0000-0001-9200-8728
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Chertkow H, Feldman HH, Jacova C, Massoud F. Definitions of dementia and predementia states in Alzheimer’s disease and vascular cognitive impairment: consensus from the Canadian conference on diagnosis of dementia. Alzheimers Res Ther. 2013;5(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349(6248):1255555. [DOI] [PubMed] [Google Scholar]
- 3. Wanleenuwat P, Iwanowski P, Kozubski W. Alzheimer’s dementia: pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad Med. 2019;131(7):415–422. [DOI] [PubMed] [Google Scholar]
- 4. Almeida OP, Lautenschlager NT. Dementia associated with infectious diseases. Int Psychogeriatr. 2005;17(suppl 1):S65–S77. [DOI] [PubMed] [Google Scholar]
- 5. Tzeng NS, Chung CH, Lin FH, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections-a nationwide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15(2):417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torniainen-Holm M, Suvisaari J, Lindgren M, Harkanen T, Dickerson F, Yolken RH. Association of cytomegalovirus and Epstein-Barr virus with cognitive functioning and risk of dementia in the general population: 11-year follow-up study. Brain Behav Immun. 2018;69:480–485. [DOI] [PubMed] [Google Scholar]
- 7. Barnes LL, Capuano AW, Aiello AE, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis. 2015;211(2):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system—prognosis, diagnostics and treatment. J Infect. 2015;71(3):281–293. [DOI] [PubMed] [Google Scholar]
- 9. Grahn A, Nilsson S, Nordlund A, Linden T, Studahl M. Cognitive impairment 3 years after neurological Varicella-zoster virus infection: a long-term case control study. J Neurol. 2013;260(11):2761–2769. [DOI] [PubMed] [Google Scholar]
- 10. Chen VC, Wu SI, Huang KY, et al. Herpes zoster and dementia: a nationwide population-based cohort study. J Clin Psychiatry. 2018;79(1):16m11312. [DOI] [PubMed] [Google Scholar]
- 11. Tsai MC, Cheng WL, Sheu JJ, et al. Increased risk of dementia following herpes zoster ophthalmicus. PLoS One. 2017;12(11):e0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. [DOI] [PubMed] [Google Scholar]
- 13. Kim SY, Lim JS, Kong IG, Choi HG. Hearing impairment and the risk of neurodegenerative dementia: a longitudinal follow-up study using a national sample cohort. Sci Rep. 2018;8(1):15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer’s disease: the enemy within. J Alzheimers Dis. 2008;13(4):393–405. [DOI] [PubMed] [Google Scholar]
- 15. Lurain NS, Hanson BA, Martinson J, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013;208(4):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kapur N, Barker S, Burrows EH, et al. Herpes simplex encephalitis: long term magnetic resonance imaging and neuropsychological profile. J Neurol Neurosurg Psychiatry. 1994;57(11):1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeBiasi RL, Kleinschmidt-DeMasters BK, Richardson-Burns S, Tyler KL. Central nervous system apoptosis in human herpes simplex virus and cytomegalovirus encephalitis. J Infect Dis. 2002;186(11):1547–1557. [DOI] [PubMed] [Google Scholar]
- 18. Hokkanen L, Launes J, Poutiainen E, et al. Subcortical type cognitive impairment in herpes zoster encephalitis. J Neurol. 1997;244(4):239–245. [DOI] [PubMed] [Google Scholar]
- 19. Hemling N, Roytta M, Rinne J, et al. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann Neurol. 2003;54(2):267–271. [DOI] [PubMed] [Google Scholar]
- 20. Ounanian A, Guilbert B, Renversez JC, Seigneurin JM, Avrameas S. Antibodies to viral antigens, xenoantigens, and autoantigens in Alzheimer’s disease. J Clin Lab Anal. 1990;4(5):367–375. [DOI] [PubMed] [Google Scholar]
- 21. Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88(5):2704–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skripuletz T, Pars K, Schulte A, et al. Varicella zoster virus infections in neurological patients: a clinical study. BMC Infect Dis. 2018;18(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagel MA, Bubak AN. Varicella zoster virus vasculopathy. J Infect Dis. 2018;218(suppl_2):S107–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silver B, Nagel MA, Mahalingam R, Cohrs R, Schmid DS, Gilden D. Varicella zoster virus vasculopathy: a treatable form of rapidly progressive multi-infarct dementia after 2 years’ duration. J Neurol Sci. 2012;323(1-2):245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giridharan VV, Masud F, Petronilho F, Dal-Pizzol F, Barichello T. Infection-induced systemic inflammation is a potential driver of Alzheimer’s disease progression. Front Aging Neurosci. 2019;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett. 2007;429(2-3):95–100. [DOI] [PubMed] [Google Scholar]
- 28. De Chiara G, Marcocci ME, Civitelli L, et al. APP processing induced by herpes simplex virus type 1 (HSV-1) yields several APP fragments in human and rat neuronal cells. PLoS One. 2010;5(11):e13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bubak AN, Como CN, Coughlan CM, et al. Varicella-zoster virus infection of primary human spinal astrocytes produces intracellular amylin, amyloid-beta, and an amyloidogenic extracellular environment. J Infect Dis. 2020;221(7):1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE. Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement. 2013;9(1):76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiang F, Panyaping T, Tedesqui G, Sossa D, Costa Leite C, Castillo M. Varicella zoster CNS vascular complications. A report of four cases and literature review. Neuroradiol J. 2014;27(3):327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-aja-10.1177_15333175211006504 for Herpes Zoster Does Not Increase the Risk of Neurodegenerative Dementia: A Case-Control Study by Hyo Geun Choi, Bum Jung Park, Jae Sung Lim, Song Yong Sim, Yoon Jung Jung and Suk Woo Lee in American Journal of Alzheimer's Disease & Other Dementias