Abstract
Exercise has systemic health benefits through effects on multiple tissues, with inter-tissue communication. Recent studies indicate that exercise could be a strategy to improve breastmilk composition and reduce the intergenerational transmission of obesity. Even if breastmilk is considered optimal infant nutrition, there is evidence for variations in its composition between mothers who are normal weight, those with obesity, and those who are physically active. Nutrition early in life is important for later-life susceptibility to obesity and other metabolic diseases, and maternal exercise may provide protection against the development of metabolic disease. Here we summarise recent research on the influence of maternal obesity on breastmilk composition and discuss the potential role of exercise-induced adaptations to breastmilk as a kick-start to prevent childhood obesity.
Keywords: Lactation, postpartum, epigenetic modifications, aerobic exercise, diet, obesity
Can exercise affect breastmilk composition? (Why wouldn’t it?)
Exercise is a formidable regulator of overall systemic metabolism through both acute effects driven by individual exercise sessions and chronic adaptation. Exercise challenges whole-body homeostasis and affects multiple cells, tissues, and organs through the increased metabolic activity of contracting skeletal muscles. Moreover, the beneficial effects of exercise are not limited to adaptations within tissues, but instead stem from the integration of inter-tissue communication through various signalling molecules, hormones, and cytokines collectively known as ‘exerkines’ [1]. Dramatic shifts are observed for more than 80% of annotated metabolites in the circulating metabolome in response to a single endurance exercise session of just 12 minutes, with beneficial alterations for metabolites from key metabolic pathways for obesity, insulin resistance, and inflammation [2]. These alterations may partly explain the broad benefits of exercise for cardiometabolic health. Indeed, as little as five days of endurance training induced substantial changes in the serum metabolome, concomitant with improvements in aerobic fitness, glycaemic control, and circulating lipid levels in overweight/obese men [3].
Recent studies have focused on the effects of maternal exercise on maternal and fetal outcomes. In humans, most studies demonstrate that exercise during pregnancy is safe and beneficial to both the mother and the fetus [4,5]. Specific benefits of maternal exercise in humans include increased rates of full-term delivery, normalized birth measures, a reduced risk of macrosomia, and improved neurobehavioral abilities and cardiac autonomic health [5–9].
In rodents, numerous studies have identified the role of maternal exercise to improve metabolic health of adult offspring. Studies have shown that either maternal treadmill exercise or voluntary wheel running have a similar effect to reduce body weight, fat mass, and improve glucose metabolism and insulin sensitivity, even in the presence of a maternal high-fat diet [7,10–14]. The beneficial effects of maternal exercise on offspring metabolic health are not present in young animals, but instead in adult offspring. Importantly, these effects have been observed across species and strains of rodents (C57BL6 mice, ICR mice and Sprague-Dawley rats) [7,10,15,16]. The mechanisms underlying the beneficial effects of maternal exercise on offspring metabolism are only beginning to be elucidated, but we hypothesize that there are many factors involved. These include epigenetic modifications to metabolic tissues in the offspring, adaptations to the placenta, and changes to the offspring metabolome [7,11,17]. Little is known, however, about how exercise affects human breastmilk, and whether exercise-induced breastmilk modifications affect infant health (Figure 1).
Figure 1.
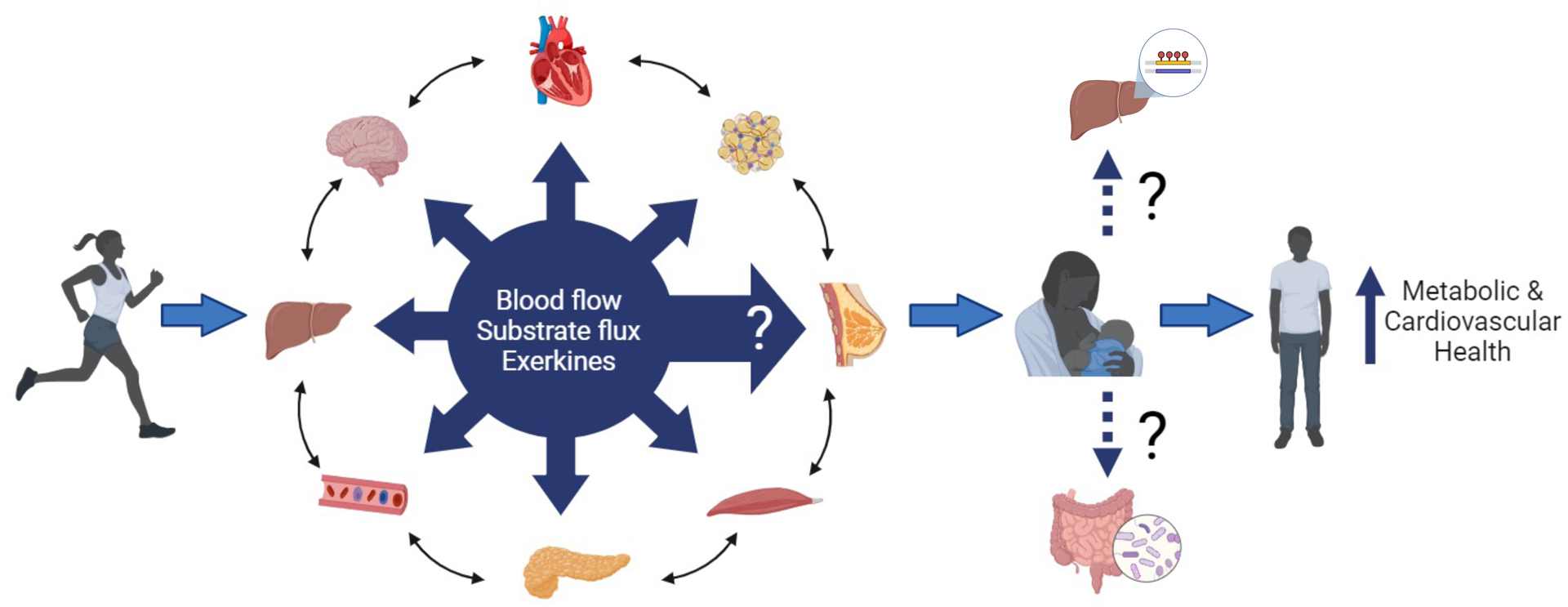
Exercise induces multiple molecular adaptations in the heart, adipose tissue, pancreas, skeletal muscle, circulation, liver, and brain, and via inter-organ crosstalk. Few data are currently available for exercise-induced adaptations in human breastmilk, but exercise may induce adaptations to breastmilk that can mediate whole-body metabolic and cardiovascular health in the offspring. The underlying mechanisms for such effects are unclear, but it is likely that breastmilk mediates improvements in the offspring liver and microbiome. Figure were created with biorender.com.
The interplay between maternal lifestyle, breastmilk composition, and infant health is an emerging field of research, with evidence of effects on breastmilk composition of maternal smoking, BMI, gestational diabetes, and diet [18–22]. Maternal diet can alter breastmilk composition, with demonstrated differences between a carbohydrate-rich and a high-fat diet, and between different types of carbohydrates [22]. However, there is currently no experimental evidence that lifestyle-induced breastmilk modifications affect infant obesity risk. One behavioural factor that has not been well studied in this context is exercise. Herein we review recent evidence for differences in breastmilk composition in response to maternal physical activity and metabolic health, the importance of breastmilk composition for infant obesity risk, and the potential role exercise can have in making the milk less obesogenic. This topic is timely, since recent advances in technology now allow much more detailed analyses of breastmilk and in light of the increasing prevalence of childhood obesity. We suggest that exercised breastmilk may be a kick-start to prevent childhood obesity (Figure 2, Key).
Figure 2.
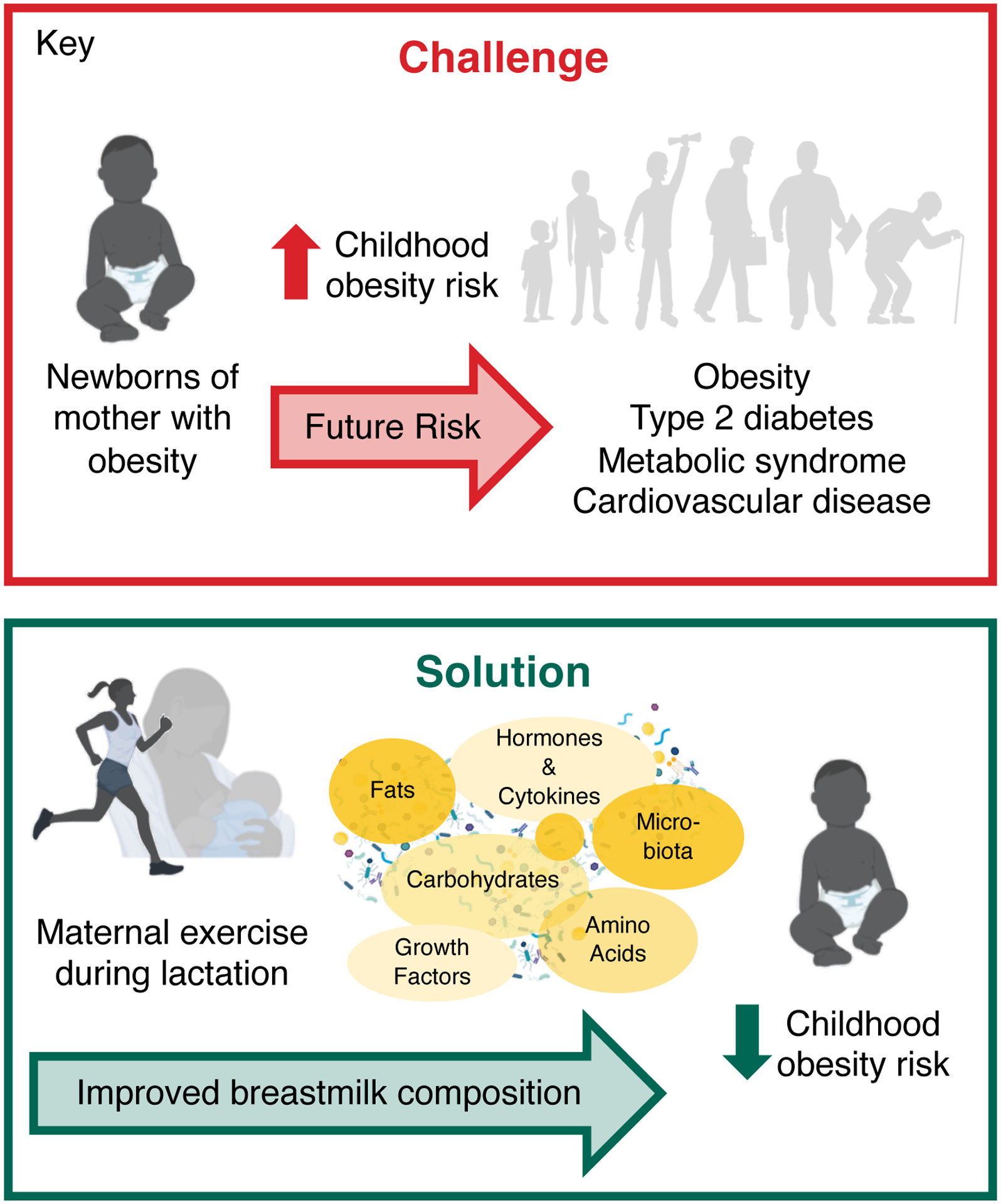
Maternal exercise during lactation may decrease childhood obesity risk, mediated via exercise-induced improvements in breastmilk composition. Parts of the figure were created with biorender.com
The origins of childhood obesity and early life nutrition
Childhood obesity is reaching alarming proportions in many countries and poses an urgent challenge to healthcare systems. Between 2006 and 2016, 18% of European children aged 2–7 years were overweight or obese [23], whereas 13% of US children aged 2–5 years were obese in 2013–2016 [24]. Obesity affects a child’s immediate health and quality of life, and children with obesity are also five times more likely to remain obese as adults compared with those without childhood obesity [25]. Maternal pre-pregnancy body mass index (BMI) is a strong risk factor for childhood obesity, accounting for up to 21% of the population attributable risk (see Glossary) [26], implying a strong mother-to-child transmission.
Even if genetics partly accounts for the risk of common polygenic obesity (see Glossary) in both childhood and adulthood, the proportion of variation in BMI currently explained by sequence variations in genetic loci is only ~ 1–2% in children [27] and ~ 6% in adults [28]. A large portion of the heritability of obesity thus remains unexplained. Epigenetic modifications (see Glossary) are a key mechanism underlying this ‘missing heritability’ for obesity [29]. Early infant nutrition, and especially breastmilk, is thought to be a crucial factor influencing life-long health via epigenetic programming [30,31]. The period from conception to 2 years of age, known as ‘the first 1000 days’, is the most critical period for pathophysiological disorders leading up to obesity in childhood and later life (Figure 2) [32]. Indeed, data from human cohorts have shown that faster weight gain in early infancy is associated with a greater risk of subsequent obesity [33,34], with rapid weight gain in the first 3 months of life associated with a higher body fat percentage and a higher degree of central obesity in childhood [35] and later in life [36].
During early postnatal life, the role of breastfeeding is a recognised factor in discussions of the nutritional background of childhood and later-life obesity. Breastfeeding has well-established nutritional and immunological advantages [37]. Breastfed children have a 13% lower likelihood of becoming overweight or obese compared with bottle-fed children [38], but these observational data may be confounded by unadjusted or non-measured factors.
Maternal metabolism, exercise, and lactational programming of obesity
Human breastmilk contains diverse substances with potential mechanistic roles in metabolic health during early childhood, including macronutrients, micronutrients, metabolic hormones, adipokines, miRNAs (see Glossary), and inflammatory markers [39–41]. Our premise is that breastmilk is nutritionally optimal for infants, including for those born to women with overweight/obesity. However, recent evidence suggests differences in breastmilk composition between mothers with high and low BMI [19,42–48]. Maternal obesity is associated with changes in the breastmilk metabolome (see Glossary) reminiscent of the metabolic signature in the plasma of individuals with obesity and type 2 diabetes, with high concentrations of several acylcarnitines involved in branched-chain amino-acid metabolism [19]. For a subset of the metabolites differing between obese and normal-weight women, these differences are correlated with infant weight and fat percentage [19,42].
Maternal obesity is also associated with changes in the concentrations of human milk oligosaccharides (HMOs, see Glossary), which are associated with growth during the first 5 years of life [45–48]. Furthermore, Isganaitis and colleagues showed that breastmilk adenine was positively correlated with both maternal BMI and with infant weight at 1 month, whereas the metabolite 5-methylthioadenosine correlated with both maternal BMI and infant body fat percentage [19]. Another study showed that the three metabolites mannose, lyxitol, and shikimic acid, all which were increased in breastmilk from women with obesity, could predict higher infant adiposity over the first 6 months of life [42]. Collectively, these findings suggest that breastmilk compounds play a role in the mother-to-child transmission of obesity. This concept is supported by several studies in mice. For example, offspring born to lean dams and cross-fostered by obese dams have a profoundly dysmetabolic phenotype [49]. In humans, a study of infants born to mothers with type 2 diabetes who were fed either their own mothers’ milk or banked human milk from non-diabetic donors showed that consumption of the diabetic mothers’ milk was associated with a higher body weight at 2 years [50]. Maternal metabolic homeostasis during the lactation period may, therefore, influence the infant’s risk of childhood obesity.
In contrast to maternal obesity, maternal exercise may modify both the nutrients and non-nutrient bioactive agents in breastmilk. Breastmilk is rich in lipids, including ‘lipokines’, a crucial class of lipids that act as signalling molecules and influence systemic metabolism.[60,61] Some lipokines have been detected in human milk. A breastmilk-specific lipid group, alkylglycerols, may delay the transformation of the infant’s beige adipose tissue (which is more metabolically active) to lipid-storing white adipose tissue [62]. It is still unknown whether alkylglycerol abundance is influenced by maternal exercise. Another lipokine, 12,13-dyhydroxy-9Z-octadecenoic acid (12,13-diHOME), regulates brown adipose tissue fuel uptake and thermogenesis [63]. This lipokine was recently discovered in human milk, and its abundance is inversely correlated with infant adiposity [64], again suggesting that differences in breastmilk composition may be functionally related to early-life obesity risk. 12,13-diHOME increases fatty acid uptake in skeletal muscle and its plasma concentrations increase acutely after exercise [2,65]. However, others have reported no sustained increase in circulating 12,13-diHOME after daily exercise training [3], suggesting that these effects are transient. 12,13-diHOME concentrations in breastmilk increase acutely after exercise in most women [64], but whether such effect plays a causal role in limiting early rapid weight gain in the offspring has yet to be determined. Furthermore, the relative abundance of short-chain fatty acids in human breastmilk may affect weight gain and adiposity during infancy, with negative associations between the levels of short-chain fatty acids (butyrate, formic acid, and acetate) in breastmilk and infant adiposity between the ages of 3 and 12 months [66]. The abundance of several circulating lipid metabolites changes acutely after exercise, in the opposite direction to changes observed in cardiometabolic diseases [2].
Exercise training has been shown to increase the abundance in mouse milk of 3’sialyllactose (3’SL), an HMO crucial for mediating improvements to metabolic health in mouse offspring [12]. The same study also showed that levels of this HMO in human breastmilk 2 months postpartum were weakly, but significantly, correlated with the mean number of steps taken per day during pregnancy. In rodent studies, maternal exercise in 3’SL deficient mice (3’SL−/−) had no exercise-induced improvements in metabolic health, while cross-fostering offspring from trained 3’SL−/− to trained wild-type dams partially restored the benefits of maternal exercise on metabolic health. Supplementation of 3’-SL during the nursing period also improved metabolic and cardiovascular health of adult offspring. While the mechanisms by which maternal exercise changes the composition of milk and how 3’SL improves offspring metabolism are not known, the effects of maternal exercise on breast milk is an area ripe for investigation and an important topic to ultimately be translated to humans.
Epigenetic and inflammatory factors in breastmilk that can alter infant metabolism.
Breastmilk can act as an epigenetic regulator through various growth factors, immune factors, microbiota, appetite hormones, and miRNAs [51,52]. It is one of the richest sources of miRNA among the body fluids, and miRNAs packaged within extracellular vesicles in breastmilk are bioavailable to breastfeeding infants [53]. These small non-coding RNAs bind to regions of the messenger RNAs, modulating protein production, typically by degrading or repressing translation of the targeted RNA [52]. There is translational evidence for a role of breastmilk miRNA in the epigenetic programming of the offspring [51]. Indeed, miRNAs, particularly those in extracellular vesicles, have been identified as the most critical bioactive factors in human breastmilk for modifying postnatal epigenetic regulation [52], but little is known about the effect of human breastmilk miRNAs on infant body composition. Two studies have reported associations of selected breastmilk miRNAs with maternal BMI and infant body composition [43,44]. However, these studies investigated two completely different sets of miRNAs by targeted approaches, and the evidence concerning the effect of breastmilk miRNAs on infant body composition remains inconclusive. A recent systematic review reported associations between the circulating levels of some miRNAs and childhood obesity, the evidence being strongest for miR-122, miR-222, and miR-423 [54]. Both miR-222 and miR-423 are detected in breastmilk, and miR-222 levels are higher in breastmilk of mothers with obesity compared with that in breastmilk from normal-weight women [43].
The last decade has seen an exponential increase in evidence for a role of gut microbiota dysbiosis (see Glossary) in host obesity, in both adults and in children. Specifically, childhood obesity is associated with high levels of bacteria from phylum Firmicutes and low levels of bacteria from phylum Bacterioidetes [55]. HMOs have attracted particular attention as breastmilk bioactive compounds with effects on the infant gut microbiome, growth, and health [45–48,56–59]. The infant cannot digest HMOs, but these compounds are metabolised by some of the nursing child’s intestinal bacteria. Breastmilk concentrations of certain HMOs have been associated with growth rate in early infancy [45–48], but few data are available concerning the potential mechanisms by which these compounds modulate infant growth. HMOs have been identified as candidate breastmilk compounds linking maternal obesity to infant fat accretion and, thus, involved in maternal obesity-related postnatal nutritional programming [45–48]. The first 1000 days of life are central for constitution of the gut microbiota and provide a unique opportunity to modify this process via breastmilk. If maternal exercise can alter the composition of HMOs in breastmilk, mothers may impact their infant’s early gut microbiota via exercise training.
Chronic low-grade inflammation, with high circulating concentrations of pro-inflammatory cytokines (see Glossary), such as tumour necrosis factor-alpha (TNF-α), contributes to metabolic disorders, including insulin resistance and obesity. Regular exercise suppresses TNF-α-induced insulin resistance, providing a partial explanation for the protection against chronic diseases afforded by exercise [67]. There is some evidence for an anti-inflammatory effect of exercise during pregnancy on the first breastmilk produced after delivery (colostrum) [68], but the effect of maternal exercise during lactation on breastmilk inflammatory markers has been little studied. Only one previous study has investigated the relationship between exercise in lactating women and cytokines in breastmilk: an observational study reporting associations between exercise level and pro-inflammatory cytokine levels in breastmilk [69]. However, these correlational data provide no information about causality, and not any evidence for effects on the infants.
Concluding remarks and future perspectives
Current research clearly demonstrates the beneficial effects of breastmilk on metabolic health of the infant and into adulthood. Recent studies in both rodents and humans have highlighted the beneficial effects of exercise on breastmilk composition and identified some of the mechanisms that confer improved metabolism into the offspring. There are many critical areas for future investigation, and further studies are needed before we can comprehensively define the central mechanisms that regulate the beneficial effects of maternal exercise on breastmilk composition. For example, taking into consideration exercise-induced changes to epigenetic states like DNA methylation, histone modification, and non-coding RNAs that are altered in models of obesity could be critical to defining the effects of exercise. Identification of factors mediating the beneficial effects of exercise on offspring metabolism is essential for translation to humans, and given the constant rise in global obesity, this will become increasingly more important. Identification of exercise-regulated compounds in breastmilk with importance for prevention of childhood obesity could potentially be enriched in formula milk. One human milk oligosaccharide (2’Fucosyllactose) is already available in some commercial infant formulas and has been reported to have promising health benefits in infants.[70] Exercise is sometimes not an option during pregnancy and the potential for benefits to the infant induced by maternal exercise also after it is born is a future avenue for research.
Figure 3.
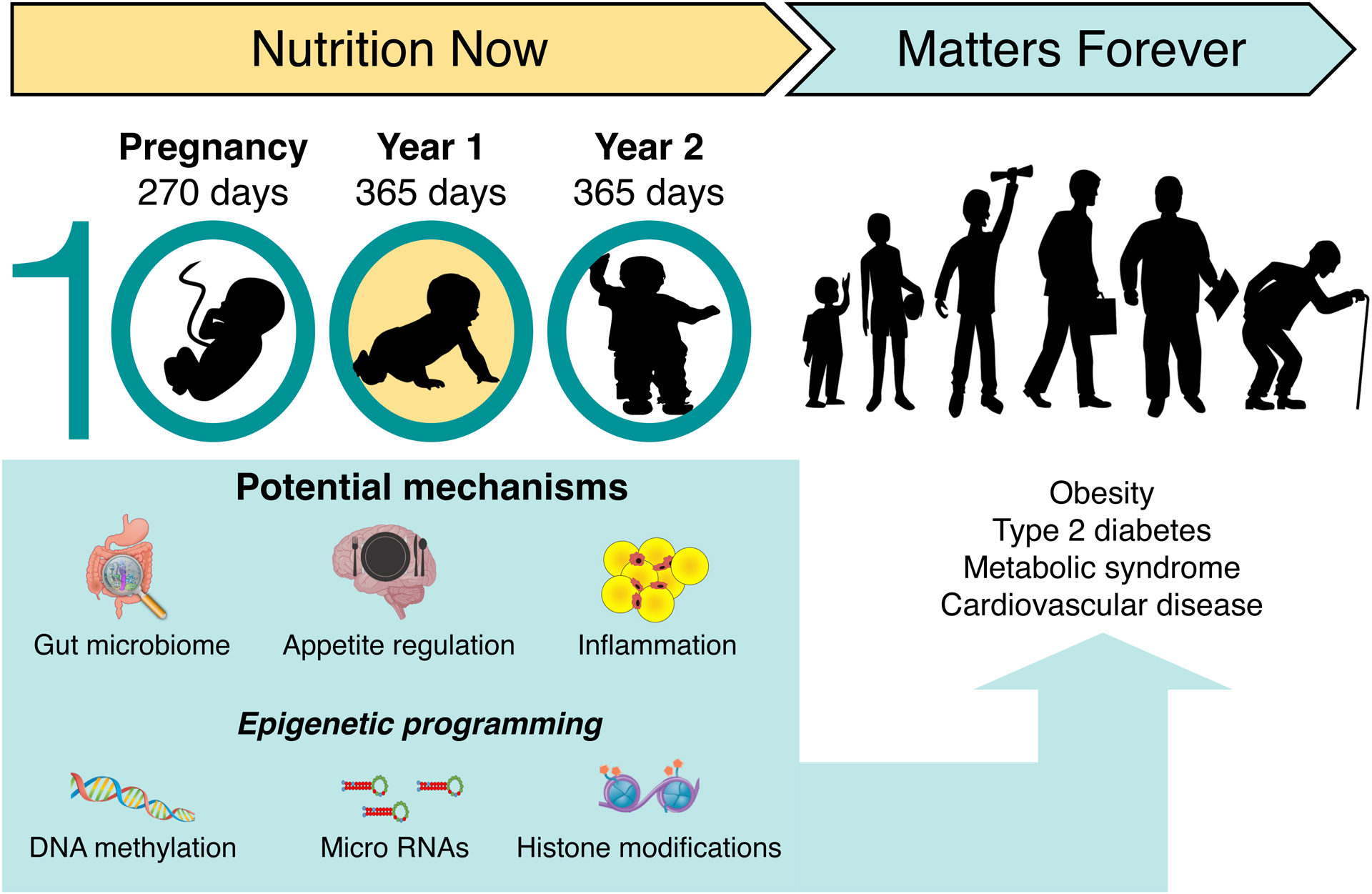
The first 1000 days include pregnancy and the first two years of life. Epigenetic DNA imprinting is particularly active during this period. Nutrition during this period, both via the placenta in utero and through breastmilk, formula milk and solid food after birth, plays a key role in epigenetic DNA imprinting, thus affecting individual susceptibility to the subsequent development of obesity, and other non-communicable diseases. Parts of the figure were created with biorender.com.
Acknowledgements:
TM has received funding from the European Research Council (ERC) under the European Union’s Horizon Europe research and innovation programme grant agreement No 101075421. KIS was supported by NIH R01DK133859 and AHA 23SFRNPCS1067042.
Glossary
- Common polygenic obesity
the results of hundreds of genetic variations that each have a small effect. The heritability of polygenic obesity follows a pattern that is similar to other complex traits and diseases. Polygenic obesity is classically considered as a different disease than monogenic obesity which is typically a rare, early-onset and severe type of obesity involving either chromosomal deletions or single-gene defects.
- Cytokines
is a broad category of small proteins that are important in cell signalling and that help control inflammation in the body.
- Epigenetic modifications
heritable changes in phenotype not involving changes to the genetic code itself. Epigenetic modifications include DNA methylation (the process by which methyl groups are added to the DNA molecule and thereby typically repress gene transcription), histone modifications (a post-translational modification to histone proteins that alters chromatin structure or recruits histone modifiers that can impact gene expression), and non-coding RNAs, such as miRNAs.
- Gut microbiota dysbiosis
an imbalance in bacterial composition, changes in bacterial metabolic activities, or changes in bacterial distribution within the gut. A dysbiotic microbiota can compromise the gut barrier, resulting in negative impact to on the host immune system and metabolism.
- Human Milk Oligosaccharides (HMOs)
comprise a group of structurally complex carbohydrate-based polymers, with around 200 different HMOs identified to date. HMOs are the third most abundant solid component in breast milk, after lactose and lipids.
- Metabolome
is the complete set of small-molecule chamicals in a biological sample (such as a cell, an organ, a tissue, a biofluid, or an entire orgaminsm).
- miRNA
is a type of non-coding RNA molecules, meaning that they are not translated into proteins. miRNAs are smaller than other types of RNA and can bind to messenger RNAs to block them from making protein. Also called microRNA.
- Population attributable risk
is the proportion of the incidence of a disease that is due to an exposure, and is the difference between the risk in the total population and that in unexposed individuals.
References
- 1.Chow LS et al. (2022) Exerkines in health, resilience and disease. Nat Rev Endocrinol 18, 273–289. 10.1038/s41574-022-00641-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayor M et al. (2020) Metabolic Architecture of Acute Exercise Response in Middle-Aged Adults in the Community. Circulation 142, 1905–1924. 10.1161/circulationaha.120.050281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moholdt T et al. (2021) The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: a randomised trial. Diabetologia. 10.1007/s00125-021-05477-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinman SK et al. (2015) Exercise in Pregnancy: A Clinical Review. Sports Health 7, 527–531. 10.1177/1941738115599358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer C et al. (2016) The Influence of Prenatal Exercise on Offspring Health: A Review. Clin Med Insights Womens Health 9, 37–42. 10.4137/cmwh.S34670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barakat R et al. (2016) Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol 214, 649.e641–648. 10.1016/j.ajog.2015.11.039 [DOI] [PubMed] [Google Scholar]
- 7.Kusuyama J et al. (2020) Effects of maternal and paternal exercise on offspring metabolism. Nat Metab 2, 858–872. 10.1038/s42255-020-00274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May LE et al. (2014) Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Hum Dev 90, 33–38. 10.1016/j.earlhumdev.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 9.Clapp JF 3rd et al. (1999) Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. Am J Obstet Gynecol 180, 91–94. 10.1016/s0002-9378(99)70155-9 [DOI] [PubMed] [Google Scholar]
- 10.Harris JE et al. (2018) Maternal Exercise Improves the Metabolic Health of Adult Offspring. Trends in endocrinology and metabolism: TEM 29, 164–177. 10.1016/j.tem.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernández-Saavedra D et al. (2022) Maternal Exercise and Paternal Exercise Induce Distinct Metabolite Signatures in Offspring Tissues. Diabetes 71, 2094–2105. 10.2337/db22-0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris JE et al. (2020) Exercise-induced 3’-sialyllactose in breast milk is a critical mediator to improve metabolic health and cardiac function in mouse offspring. Nat Metab 2, 678–687. 10.1038/s42255-020-0223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanford KI et al. (2017) Maternal Exercise Improves Glucose Tolerance in Female Offspring. Diabetes 66, 2124–2136. 10.2337/db17-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford KI et al. (2015) Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 64, 427–433. 10.2337/db13-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laker RC et al. (2014) Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 63, 1605–1611. 10.2337/db13-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter LG et al. (2013) Maternal exercise improves insulin sensitivity in mature rat offspring. Med Sci Sports Exerc 45, 832–840. 10.1249/MSS.0b013e31827de953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusuyama J et al. (2021) Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metab 33, 939–956.e938. 10.1016/j.cmet.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuel TM et al. (2019) Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci Rep 9, 11767. 10.1038/s41598-019-48337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isganaitis E et al. (2019) Maternal obesity and the human milk metabolome: associations with infant body composition and postnatal weight gain. Am J Clin Nutr 110, 111–120. 10.1093/ajcn/nqy334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravi F et al. (2016) Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr 104, 646–662. 10.3945/ajcn.115.120881 [DOI] [PubMed] [Google Scholar]
- 21.Zhang L et al. (2021) Gestational Diabetes Mellitus-Induced Changes in Proteomes and Glycated/Glycosylated Proteomes of Human Colostrum. Journal of agricultural and food chemistry 69, 10749–10759. 10.1021/acs.jafc.1c03791 [DOI] [PubMed] [Google Scholar]
- 22.Seferovic MD et al. (2020) Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci Rep 10, 22092. 10.1038/s41598-020-79022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrido-Miguel M et al. (2019) Prevalence of Overweight and Obesity among European Preschool Children: A Systematic Review and Meta-Regression by Food Group Consumption. Nutrients 11. 10.3390/nu11071698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden CL et al. (2018) Differences in Obesity Prevalence by Demographics and Urbanization in US Children and Adolescents, 2013–2016. Jama 319, 2410–2418. 10.1001/jama.2018.5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmonds M et al. (2016) Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 17, 95–107. 10.1111/obr.12334 [DOI] [PubMed] [Google Scholar]
- 26.Voerman E et al. (2019) Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med 16, e1002744. 10.1371/journal.pmed.1002744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesi A and Grant SFA (2015) The Genetics of Pediatric Obesity. Trends in endocrinology and metabolism: TEM 26, 711–721. 10.1016/j.tem.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yengo L et al. (2018) Meta-analysis of genome-wide association studies for height and body mass index in ~700000 individuals of European ancestry. Human molecular genetics 27, 3641–3649. 10.1093/hmg/ddy271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao W et al. (2021) Epigenetic regulation of energy metabolism in obesity. J Mol Cell Biol 13, 480–499. 10.1093/jmcb/mjab043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indrio F et al. (2017) Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development. Front Pediatr 5, 178. 10.3389/fped.2017.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isganaitis E (2019) Developmental Programming of Body Composition: Update on Evidence and Mechanisms. Curr Diab Rep 19, 60. 10.1007/s11892-019-1170-1 [DOI] [PubMed] [Google Scholar]
- 32.Mameli C et al. (2016) Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int J Environ Res Public Health 13. 10.3390/ijerph13090838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baird J et al. (2005) Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ (Clinical research ed 331, 929. 10.1136/bmj.38586.411273.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng M et al. (2018) Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev 19, 321–332. 10.1111/obr.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chomtho S et al. (2008) Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr 87, 1776–1784. 10.1093/ajcn/87.6.1776 [DOI] [PubMed] [Google Scholar]
- 36.Leunissen RW et al. (2009) Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. Jama 301, 2234–2242. 10.1001/jama.2009.761 [DOI] [PubMed] [Google Scholar]
- 37.Victora CG et al. (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490. 10.1016/s0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- 38.Horta BL et al. (2015) Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr 104, 30–37. 10.1111/apa.13133 [DOI] [PubMed] [Google Scholar]
- 39.de la Garza Puentes A et al. (2019) The Effect of Maternal Obesity on Breast Milk Fatty Acids and Its Association with Infant Growth and Cognition-The PREOBE Follow-Up. Nutrients 11. 10.3390/nu11092154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fields DA et al. (2017) Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes 12 Suppl 1, 78–85. 10.1111/ijpo.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galante L et al. (2020) Growth Factor Concentrations in Human Milk Are Associated With Infant Weight and BMI From Birth to 5 Years. Front Nutr 7, 110. 10.3389/fnut.2020.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saben JL et al. (2020) Maternal adiposity alters the human milk metabolome: associations between nonglucose monosaccharides and infant adiposity. Am J Clin Nutr 112, 1228–1239. 10.1093/ajcn/nqaa216 [DOI] [PubMed] [Google Scholar]
- 43.Zamanillo R et al. (2019) Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 11. 10.3390/nu11112589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah KB et al. (2021) Human Milk Exosomal MicroRNA: Associations with Maternal Overweight/Obesity and Infant Body Composition at 1 Month of Life. Nutrients 13. 10.3390/nu13041091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saben JL et al. (2021) Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Maternal Overweight and Obesity and Predict Infant Growth. Nutrients 13. 10.3390/nu13020446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagström H et al. (2020) Associations between human milk oligosaccharides and growth in infancy and early childhood. Am J Clin Nutr 111, 769–778. 10.1093/ajcn/nqaa010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alderete TL et al. (2015) Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr 102, 1381–1388. 10.3945/ajcn.115.115451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson MW et al. (2019) Human Milk Oligosaccharide Composition Is Associated With Excessive Weight Gain During Exclusive Breastfeeding-An Explorative Study. Front Pediatr 7, 297. 10.3389/fped.2019.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oben JA et al. (2010) Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 52, 913–920. 10.1016/j.jhep.2009.12.042 [DOI] [PubMed] [Google Scholar]
- 50.Plagemann A et al. (2002) Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care 25, 16–22. 10.2337/diacare.25.1.16 [DOI] [PubMed] [Google Scholar]
- 51.Ozkan H et al. (2020) Epigenetic Programming Through Breast Milk and Its Impact on Milk-Siblings Mating. Frontiers in genetics 11, 569232. 10.3389/fgene.2020.569232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melnik BC et al. (2021) Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 11. 10.3390/biom11060851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alsaweed M et al. (2015) MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int J Environ Res Public Health 12, 13981–14020. 10.3390/ijerph121113981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alfano R et al. (2021) Perspectives and challenges of epigenetic determinants of childhood obesity: A systematic review. Obes Rev, e13389. 10.1111/obr.13389 [DOI] [PubMed] [Google Scholar]
- 55.Riva A et al. (2017) Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol 19, 95–105. 10.1111/1462-2920.13463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doherty AM et al. (2018) Human Milk Oligosaccharides and Associations With Immune-Mediated Disease and Infection in Childhood: A Systematic Review. Front Pediatr 6, 91. 10.3389/fped.2018.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira ALL et al. (2021) Associations Between Human Milk Oligosaccharides at 1 Month and Infant Development Throughout the First Year of Life in a Brazilian Cohort. J Nutr 151, 3543–3554. 10.1093/jn/nxab271 [DOI] [PubMed] [Google Scholar]
- 58.Pace RM et al. (2021) Variation in Human Milk Composition Is Related to Differences in Milk and Infant Fecal Microbial Communities. Microorganisms 9. 10.3390/microorganisms9061153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borewicz K et al. (2020) The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age. Sci Rep 10, 4270. 10.1038/s41598-020-61024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao H et al. (2008) Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944. 10.1016/j.cell.2008.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yore MM et al. (2014) Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332. 10.1016/j.cell.2014.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu H et al. (2019) Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J Clin Invest 129, 2485–2499. 10.1172/jci125646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lynes MD et al. (2017) The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nature medicine 23, 631–637. 10.1038/nm.4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolfs D et al. (2021) Brown Fat-Activating Lipokine 12,13-diHOME in Human Milk Is Associated With Infant Adiposity. J Clin Endocrinol Metab 106, e943–e956. 10.1210/clinem/dgaa799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanford KI et al. (2018) 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab 27, 1357. 10.1016/j.cmet.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prentice PM et al. (2019) Human Milk Short-Chain Fatty Acid Composition is Associated with Adiposity Outcomes in Infants. J Nutr 149, 716–722. 10.1093/jn/nxy320 [DOI] [PubMed] [Google Scholar]
- 67.Petersen AM and Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol (1985) 98, 1154–1162. 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- 68.Aparicio VA et al. (2018) Influence of a Concurrent Exercise Training Program During Pregnancy on Colostrum and Mature Human Milk Inflammatory Markers: Findings From the GESTAFIT Project. J Hum Lact 34, 789–798. 10.1177/0890334418759261 [DOI] [PubMed] [Google Scholar]
- 69.Groër MW and Shelton MM (2009) Exercise is associated with elevated proinflammatory cytokines in human milk. J Obstet Gynecol Neonatal Nurs 38, 35–41. 10.1111/j.1552-6909.2008.00303.x [DOI] [PubMed] [Google Scholar]
- 70.Reverri EJ et al. (2018) Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2’-Fucosyllactose. Nutrients 10. 10.3390/nu10101346 [DOI] [PMC free article] [PubMed] [Google Scholar]


