ABSTRACT
Human cytomegalovirus (CMV) is the leading cause of congenital infection worldwide and the most common cause of non-genetic sensorineural hearing loss. As there is no vaccine or other specific intervention to prevent congenital CMV infection, there is a need to identify maternal and congenital infections with sensitive and specific testing as early as possible. There is no widely accepted practice for screening during pregnancy or in all newborns for identification of possible cases of congenital CMV. Currently, screening during pregnancy is limited to those identified as at risk followed by fetal and/or neonatal testing when congenital infection is suspected. This review focuses primarily on the current status of laboratory testing for diagnosis of maternal and congenital CMV infections. Primary maternal infection is best diagnosed using serologic testing, including CMV IgM, IgG, and avidity testing, while fetal infection should be assessed by nucleic acid amplification testing (NAAT) of amniotic fluid. Urine and saliva NAATs are the mainstay for diagnosis of congenital CMV in the first 3 weeks of life. Testing of dried blood spots can be useful for diagnosis of congenital CMV outside of the newborn period. The gaps in knowledge such as the prognostic value of viral loads in various sample types are addressed.
KEYWORDS: congenital infection, cytomegalovirus, diagnosis, PCR, CMV serology, CMV avidity
INTRODUCTION
Human cytomegalovirus (CMV) is the leading cause of congenital infection worldwide and can result in serious clinical manifestations such as intellectual disability, learning difficulties, and other systemic findings, leading to permanent disability in infected children. It is also the most common cause of non-genetic sensorineural hearing loss (SNHL). The worldwide prevalence at birth is estimated to be 0.2%–2.4% (1). In the United States, one out of 200 babies is born with congenital cytomegalovirus (cCMV), and of those, one out of five will have symptoms or long-term sequela such as SNHL (Centers for Disease Control and Prevention; https://www.cdc.gov/cmv/congenital-infection.html, accessed 27 January 2024).
As there is no vaccine or other specific intervention to prevent cCMV infection, there is a need for screening strategies to identify maternal and congenital infections with sensitive and specific testing as early as possible. Prompt intervention can improve the outcomes of symptomatic infants, and therefore, prompt diagnosis is critical. There is no widely accepted practice for screening during pregnancy among all women. Currently, screening during pregnancy is limited to those identified at risk followed by fetal and/or neonatal testing when congenital infection is suspected. Thus, most infections are diagnosed at the time of birth or after.
This review article will focus primarily on the current status of laboratory testing for diagnosis of maternal and congenital CMV infections. The utility of testing for the virus using nucleic acid amplification testing (NAAT) and the appropriate sample types will be reviewed in detail. Serologic testing for detection of antibody response to CMV in pregnant women will also be covered.
TRANSMISSION AND CLINICAL PRESENTATION
Congenital CMV infection occurs through the transplacental passage of the virus to the fetus. Unlike infections with rubella and toxoplasma, which only occur after a primary maternal infection, congenital CMV can occur through not only primary infection but also non-primary infection (2). Pre-existing maternal antibody does not fully protect the fetus from infection. Both reinfection with a different strain of CMV and reactivation of latent CMV are thought to be modes of non-primary infection (3).
The highest risk for long-term, serious adverse outcomes appears to be in infants born to mothers with primary infection in the first half of pregnancy (1). It is not known if the timing of reactivation or reinfection during pregnancy has consequences for disease severity. It is estimated that one-third of cCMV infections result from mothers experiencing a primary infection, leaving two-thirds of cCMV infections in mothers who are already seropositive.
Maternal infection
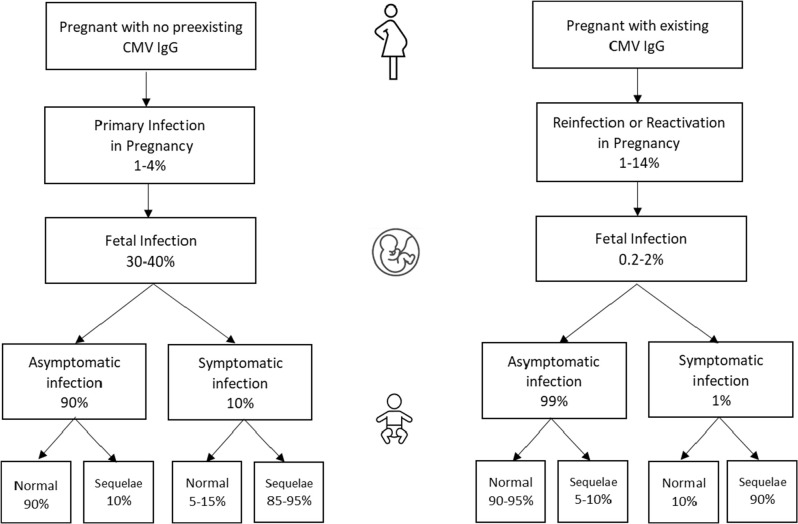
Primary CMV infection in women is often asymptomatic or with minor non-specific symptoms (fever, fatigue, and headache), so it is rarely diagnosed based on symptoms alone. It may also present with hepatitis-like symptoms (nausea, vomiting, abdominal pain, dark urine, joint pain, and jaundice). In seronegative women, approximately 1%–4% will experience a primary infection during pregnancy (4). The rate of non-primary infections during pregnancy is 1%–14%. Both can lead to transmission to the fetus albeit with primary infections transmitted more frequently (Fig. 1).
Fig 1.
Transmission of CMV from mother with risk of infection to fetus and sequela in infants. Asymptomatic denotes no signs or symptoms attributable to congenital CMV infection at birth,this catagory includes those with sensorineural hearing loss. Symptomatic denotes one or more manifestations attributable to congenital cytomegalovirus infection (see text). Normal denotes no signs or symptoms associated with congenital CMV infection, Sequela denotes demonstration of signs or symptoms associated with congenital CMV, including sensorineural hearing loss (3–5).
Fetal infection
The rate of transmission to the fetus during primary CMV infections is 30%–40% overall but varies by timing during pregnancy. In a study with 238 cases of maternal primary CMV infection, the rate of transmission to the fetus was 8.8%, 19.0%, 30.6%, 34.1%, and 40.0% in the pre-conception period, the peri-conception period, and the first, second, and third trimesters of pregnancy, respectively (6). Fetal infection may or may not result in abnormalities detectable by imaging studies. When present, findings on pre-natal ultrasound that may suggest CMV disease include peri-ventricular calcifications, ventriculomegaly, migrational abnormalities of the brain, microcephaly, hypergenic bowel, fetal growth restriction, ascites, pleural effusion, and hepatosplenomegaly (2). The sensitivity of ultrasound for detection of cCMV is approximately 15% (7).
Newborn infection
Approximately 90% of congenitally infected infants will be born with no symptoms. While these infants are less likely to have severe long-term effects, an estimated 10%–15% will go on to develop sequela such as SNHL (3). (Fig. 1). Among those that are born symptomatic, findings may include petechial rash, jaundice, hepatosplenomegaly, pneumonia, chorioretinitis, neurological manifestations such as encephalitis, microcephaly, and/or fetal growth restriction with low birth weight. Approximately 30% of severely infected infants will die. Of note, the definition of asymptomatic versus symptomatic cCMV infection can be confusing. Older literature reflects that the presence of SNHL alone at birth is still considered asymptomatic. Current consensus guidelines recommend using the designations of asymptomatic with or without hearing loss (8).
CLINICAL AND LABORATORY DIAGNOSIS OF MATERNAL AND CONGENITAL INFECTIONS
The use of both laboratory-based testing, along with imaging, is often necessary for the diagnosis of maternal and congenital CMV infections. The algorithms may vary by institution or with the unique clinical finding for a given patient; however, some general consensus exists about testing schema for both detection of primary CMV infection in pregnant women and diagnosis of cCMV in utero and in the newborn period (8, 9). The common practices will be reviewed, followed by a detailed discussion of both serologic and direct virus detections mainly by nucleic acid amplification. Older methods such as viral isolation are no longer widely available and are less sensitive than molecular methods. They will not be covered in full detail. The utility of different sample types to guide proper diagnosis will also be a focus.
Detection of maternal CMV infection during pregnancy
CMV serologic testing should be considered in pregnant women that develop an illness with influenza-like symptoms (fever, fatigue, and headache) not attributable to another pathogen or if imaging findings are suggestive of fetal cytomegalovirus infection. Given the increased risk of serious adverse outcomes resulting from primary infection, the goal of serologic testing in pregnancy is to diagnose primary infections early, allowing possible interventions and close monitoring.
The mainstay of diagnosis of primary infection includes detection of CMV IgM, CMV IgG, and CMV IgG avidity antibodies (8). Primary CMV infection can be identified serologically through the detection of CMV-specific IgM and IgG that is confirmed with low-avidity IgG. See Table 1. As will be detailed below, there are cases with individual patients that may not follow the usual patterns of antibody development having fast decay of CMV IgM (before 3 months) or accelerated development of IgG avidity, possibly leading to a misinterpretation of the timing of CMV infection. Because the results of serology may influence decisions to terminate pregnancy, it is imperative that this testing and other clinical factors be reviewed by a clinician with experience in congenital infections.
TABLE 1.
Sample types and testing for diagnosis of congenital and maternal CMV infectiona
| Sample type | Method | Timing of evaluation | Sens/Spec | Comments | References |
|---|---|---|---|---|---|
| Diagnosis of maternal primary CMV infection | |||||
| Serum | Serology: CMV IgG | <16 weeks’ gestation | 97%–100%/96%–100% |
|
(10) |
| CMV IgM | 54%–100%/62%–100% |
|
(10–17) | ||
| CMV IgG avidity | 85%/>90% |
|
(18, 19) | ||
| Diagnosis of congenital CMV in the fetus | |||||
| Amniotic fluid | NAAT | 6 weeks after maternal infection >21 weeks’ gestation |
90%–95%/99% |
|
(8, 20) |
| Chorionic villi, umbilical cord blood | NAAT | NA | ND |
|
(21–23) |
| Diagnosis of congenital CMV in infant or older child | |||||
| Urine | NAAT | <3 weeks old | 98.8%–100.0%/99% |
|
(24, 25) |
| Saliva | NAAT | <3 weeks old | 97.4%–100.0%/91.5%–99.9% |
|
(26, 27) |
| Dried blood spotb | NAAT | >3 weeks old | 34.4%–76.8%/99.9% |
|
(28, 29) |
| Blood, viral load | NAAT | <3 weeks old | ND |
|
(26, 30) |
| Serum | Serology CMV IgG CMV IgM |
NA | ND |
|
(31, 32) |
NA, not available; NAAT, nucleic acid amplification testing; ND, not detected; Sens, sensitivity; Spec, specificity.
Sample collected at birth.
Serologic testing
After primary infection, CMV IgM antibody is produced within 1–2 weeks post-infection. This is followed by IgG (1–2 weeks after IgM), which remains detectable for life. The primary response for IgG will mature over time post-primary infection reaching mature, high-avidity CMV IgG by 6 months (18).
Serologic testing can be approached in different ways; however, recent consensus documents suggest testing for CMV IgM, IgG, and IgG avidity if primary infection is suspected (8). This may be complicated by the need to send out some testing, particularly CMV avidity, which is not routinely done by local clinical labs. An alternative is to first test for CMV IgM and IgG. If both are negative, primary infection can be ruled out. As seronegative women are susceptible to primary infection, they should be counseled on prevention. If only IgG is positive, it is indicative of past infection “in most cases.” If both IgM and IgG are positive, additional testing with IgG avidity is needed to determine the timing of infection. When only CMV IgM is detected, a follow-up sample in 2–4 weeks is indicated to document if IgG seroconversion has taken place (see Table 1). Currently available serologic testing is not useful in women with non-primary infections.
The use of commercial testing for CMV IgM and IgG is widespread, and there are numerous assays available that have been cleared by the U.S. Food and Drug Administration (FDA) for testing on serum and, in some instances, plasma. They will differ in the antigens used [infected cell lysates versus viral proteins (33)], the assay detection system [i.e., enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassay, and enzyme linked fluorescent immunoassay], and the test equipment (manual versus automated) (5). The results are most often reported qualitatively as “detected” or “not detected” but may include a semi-quantitative unit of measure. Some complications may arise when comparing results from different manufacturers or when an atypical response patterns is encountered in an individual patient. For example, there are reported instances of rapidly declining CMV IgM or late developing IgG. CMV avidity assays are less widely available, and there are no FDA-cleared assays available in the United States.
CMV IgG
By definition, the detection of CMV-specific IgG in serum collected 2–4 weeks after a negative CMV IgG from a previous sample (i.e., documented seroconversion in paired sera) is considered diagnostic evidence of primary maternal infection. However, in most instances, a prior negative IgG test is not available. If there is stored sera from before the pregnancy, it can be used to help verify seroconversion. When unable to clearly define seroconversion, additional testing including CMV IgM and IgG avidity is needed for IgG-positive women.
The overall sensitivity and specificity for CMV IgG commercial testing range between 97% and 100% and 96% and 100%, respectively (10). There is concern for false-positive CMV IgG. In a study from Italy, 1,158 women were tested, among which 1.6% were found to have a false-positive IgG when confirmatory testing was performed. These were samples with “low” positivity by the immunoassay under study (34). Therefore, if assessing seroconversion, other markers may be considered (i.e., CMV IgM). Low levels of IgG could also be confirmed by testing with a different method such as Western blot analysis (35). The definition of a low positive is somewhat ambiguous as most assays are reported qualitatively. However, if available, semi-quantitative values near the cutoff value may be considered “low positive.” Most investigators suggest CMV IgG detection in cases of non-primary infection is not useful diagnostically. However, studies examining the antibody reactivity to polymorphic envelope proteins, such as gH and gB, may allow discrimination of strain-specific antibody responses and aid in the identification of non-primary infection with a new strain of CMV versus reactivation with the original infecting strain (36, 37). This testing is not currently available outside of research settings.
CMV IgM
The detection of CMV IgM in serum has variable sensitivity and poor specificity as a marker of primary CMV infection. In the majority of individuals, IgM will decline to undetectable levels within 4 months. However, in about 25%, it can persist for up to 9 months to 1 year following primary infection (38). Viral reactivation can also lead to production of CMV IgM (39). It is estimated that only 50% of all CMV IgM-positive individuals will have a true primary CMV infection (18); for pregnant women, it is estimated that only 10%–30% with a positive IgM are found to have primary infections (40).
There can be a significant variation in the results of different CMV IgM assays (11), with cited studies giving sensitivities and specificities ranging from 54% to 100% and from 62% to 100%, respectively (10–17). Understanding their performance characteristics is very important. In an evaluation of 369 IgG-positive sera from mostly females of child-bearing age, the performance of four different IgM assays was compared, and concordance of 83%–93% was found (41). An additional comparison to IgG avidity showed that among the 23 samples with low avidity, two to three samples were IgM-negative, depending on the IgM kit. Several other studies have demonstrated IgM-negative samples that have low avidity IgG (41, 42). Therefore, the IgM assay alone should not be used for diagnosis of primary CMV infections.
CMV IgG avidity
CMV IgG avidity testing is a critical assay to help define an infection as primary versus non-primary and to estimate the length of time that has elapsed since the infection has occurred (41). The importance of this finding relates to the risk of transmission to the fetus, which is significantly higher with a primary infection (see Fig. 1). The assay is based on the phenomenon of antibody response maturation. Early in infection, the aggregate strength of IgG binding to multiple epitopes of a given protein is relatively weak and thus has low avidity for up to 3–4 months. With time, the total IgG binding strength increases to an intermediate range (4–5 months after infection) before moving to high avidity by 6 months after primary infection (18). Those previously infected with CMV (>6 months post-infection) have only high-avidity IgG present.
The assay is performed by testing the serum with and without a denaturing agent such as urea, thiocyanate, or guanidine chloride in an ELISA-like format with a CMV antigen coating the solid phase. Results are calculated by dividing the value with denaturant by the value without (normal buffer). The results are expressed as a percentage of IgG still bound to CMV antigens following treatment. The higher the avidity, the higher the percentage of bound antibody after exposure to the denaturant. With the majority of assays, avidity indices are classified in three categories: low avidity (<30%–40%), which indicates a recent primary infection within the last 3–4 months; high avidity (>60%), which excludes the onset of a primary infection in the last 3–4 months; or intermediate avidity (40%–59%), which does not allow discrimination between recent or remote primary infection. The interpretive criteria and break points between categories must be determined and are unique for each assay (18, 43). There are multiple CMV avidity assays available outside the United States, and published studies have reported good concordance among the different commercial avidity assays (44–47). Within the United States, testing is limited to laboratory-developed assays or modification of FDA-cleared qualitative CMV IgG assays (43, 48). All these tests can differ in their performance characteristic, and until they can all be calibrated to a common standard, these differences are important to understand for proper interpretation (8).
Careful consideration should be given to the serologic algorithm used. Using CMV IgM positivity for reflex to CMV IgG avidity may miss some low avidity samples that are IgM-negative (41). Using avidity testing in all IgG-positive samples, without an IgM result, is not recommended as it leads to a large number of indeterminate results that are challenging to manage clinically (49). Testing both CMV IgG- and IgM-specific antibodies followed by avidity testing regardless of the presence of IgM antibody has been suggested to avoid missing a case of primary infection (20).
Detection of CMV IgM coupled with low-avidity CMV IgG, particularly within 12–16 weeks’ gestation, suggests a recent primary infection and thus a higher risk of symptomatic congenital infection (8, 50, 51). Testing in the second and third trimesters (after 20 weeks’ gestation) significantly lowers the predictive value for detection of primary infection (20). High avidity in a sample collected after 20 weeks cannot rule out primary infection in the first trimester; therefore, the pregnancy should be monitored for signs of congenital infection. Detection of low avidity, any time during pregnancy, indicates that fetal transmission is possible, and additional testing to monitor the fetus should be offered (18, 52).
It is estimated that approximately 30% of women with low-avidity IgG will transmit CMV to their fetus; in contrast, women with high avidity during the first trimester have very low risk (0%–2%) of transmission (18, 20). Those with intermediate avidity during the first trimester have a low risk of intrauterine transmission of around 5%–6% (19, 53, 54). Overall, low CMV avidity during the first trimester of pregnancy should detect most pregnant women (>85%) with primary infection, while high avidity will correctly identify most women (>90%) with past infection or reactivation (18, 19).
There are exceptions to the usual pattern of detection of CMV IgM-positive and low-avidity IgG during primary infection (20, 41). In an Italian study, 20 of 426 (4.7%) pregnant women with primary infections had no detectable IgM when tested within 1–3 months after a well-defined infection onset, demonstrating early clearance of IgM. For 26 of 418 (6.2%) of the women, high-avidity IgG was detected within 1–3 months of infection, showing early development of high-avidity IgG (55). These findings may or may not be specific to the testing used but point out that interpretation of results can be difficult with high stakes and require careful discussion with a clinician with expertise in diagnosing congenital infections.
Serologic testing is not useful in the diagnosis of cCMV in infants <3 weeks old. Maternal CMV IgG can cross the placenta and can be detected in fetal and neonatal blood. It does not necessarily reflect the immune response of the baby. With IgM antibody, there is no transplacental passage. While it represents the immune response of the fetus/newborn, it can lack sensitivity and specificity for diagnosis of cCMV (56). Congenital CMV infection can be excluded beyond the newborn period if CMV IgG antibody testing is negative (provided the infant has a normal immune system) (2).
CMV cell-mediated immunity
Measurement of cell-mediated immune responses to infectious agents has been proven to be useful in diseases like tuberculosis. In a similar fashion, investigators have published on monitoring T-cell responses to CMV as a way to assess the risk of transmission from an infected mother to her fetus. These assays commonly measure production of interferon-γ by cells stimulated with CMV-specific peptides. The results are somewhat contradictory, depending on the type of assay used (CMV enzyme-linked immunosorbent spot or QuantiFERON) with some showing an association with transmission and T-cell response and others not (57–59). Currently, there are no FDA-cleared assays, but there are research use-only products in the United States and some Conformité Européenne (CE)-marked products in Europe (e.g., T-SPOT.CMV kit; Oxford Immunotec, Abingdon, UK). More studies are needed to determine if immune-response monitoring can be used for clinical prognostic testing.
Direct detection of CMV in the fetus and newborn
Direct detection of the virus in samples can be done by both culture and nucleic acid amplification techniques. This testing is the mainstay of diagnosis of cCMV in the fetus and newborn as serology is not useful. The diagnostic value will vary based on the type of testing performed, the sample type used, and the individual being tested. It is important to understand the performance characteristic of the testing in a given situation.
Culture-based detection
Tissue culture for isolation and identification of CMV from fetal specimens has been used for cCMV diagnosis and was considered the gold standard prior to the introduction of NAAT. Cell lines, particularly human fibroblasts, that are permissive for growth and demonstrate a cytopathic effect typical of CMV. Definitive identification is often done using a virus-specific monoclonal antibody with fluorescent label to stain the infected cells. Faster culture-based techniques exist, such as shell vial cultures, in which the sample is centrifuged onto the cell monolayer, incubated from 12 to 72 h and then stained for detection of early proteins (60, 61).
Culture for CMV has, for all practical purposes, been supplanted by molecular techniques. Culture is labor intensive, requires specialized material and equipment, and takes up to 21 days for results. It is not offered widely outside of specialized settings. Growth of the virus has excellent specificity; however, its lower sensitivity in all sample types when compared to NAAT is a sub-optimal choice for diagnosis (24, 26). It can be useful for phenotypic evaluation of drug sensitivity (62); however, this, too, has been supplanted by molecular methods.
Nucleic acid amplification tests
PCR has been used since the 1980s for detection and diagnosis of CMV in many populations including congenitally infected infants (63). While there are several commercially available assays for the quantitative detection of CMV in blood or plasma that have been cleared by the FDA or CE marked in Europe, these are approved for monitoring viral load in immunocompromised adults or children. Only two FDA-cleared qualitative tests are approved for diagnosis of cCMV in neonates; these tests are the Alethia CMV Amplification Assay (Meridian Bioscience, Cincinnati, OH) and the Simplexa Congenital CMV Direct (Diasorin Molecular, Cypress, CA). Sample types approved for testing are limited to saliva and urine. Thus, the majority of clinical assays currently in use for detection of cCMV are laboratory-developed and have not undergone review by the FDA. With the advent of newer extraction methods and real-time chemistry for detection, the sensitivity and specificity of NAAT are greater than 90% in most instances, and performance is reliable. Many different conserved regions of the CMV genome have been used as targets, such as UL33 (64), UL83 (65), major immediate-early region protein (28), envelope glycoproteins (66, 67), and RNaseP (68), whether singly or in multiplex (69, 70).
Sample types for viral detection
Multiple sample types have been investigated for testing by PCR and culture to aid in the diagnosis of cCMV. Some are more useful than others based on suspected phase of infection at the time of sample collection. These sample types differ in their predictive value, and an understanding of the performance of each specimen is important and discussed below.
Detection of fetal CMV infection
Confirmation of infection of the fetus relies on non-invasive (imaging) and invasive sampling (amniocenteses) for viral testing. Imaging to examine the developing fetus can be used to detect abnormalities associated with cCMV. The most commonly used is ultrasound, and it may be the first test that uncovers a possible congenital infection. Abnormal findings related to CMV occur predominantly with primary infections in the first trimester. A normal ultrasound can be reassuring, but it does not rule out the possibility of asymptomatic neonatal infection, symptomatic infection, or the development of long-term sequela (21). In a study of 650 fetuses from mothers with primary CMV infection, there was a 48.1% probability that a normal ultrasound excluded the development of symptomatic infection. Conversely, abnormal ultrasound findings predict symptomatic infection in one-third of cases (7). Fetal magnetic resonance imaging has also been used in conjunction with ultrasound to better access infection. In review of studies including fetuses with confirmed cCMV infection, MRI found an anomaly in about 6% of cases that were negative on ultrasound (7, 71).
PCR testing on amniotic fluid is the reference method for diagnosis of fetal infection (8). Although viral culture in amniotic fluid has a near absolute specificity, it has a lower sensitivity than PCR and is no longer the reference method (20). The fetal kidneys are the major site for viral replication. Six to eight weeks following maternal primary infection, CMV excretion in the fetal urine is detectable in amniotic fluid. Amniocentesis should occur at least 6 weeks from primary infection and after 20 weeks’ gestation. With adherence to these two time points, the sensitivity is reported to be between 90% and 95% (20). There are, to-date, no FDA-cleared tests for amniotic fluid.
False-negative CMV PCR results on amniotic fluid have been reported. As mentioned above, samples collected earlier than 20 weeks’ gestation have a sensitivity as low as 45% when compared to viral culture (72). In a pooled meta-analysis of seven studies, the false-negative rate for CMV PCR from amniocentesis was 8% (73). The rate of severe symptoms at birth in those with a false-negative result was 0% as opposed to those with a true-positive result in which the rate for severe symptoms was 22%. Similarly, the rate of severe SNHL and/or neurodevelopmental delay was 0% in the false-negative group and 14.0% in the true-positive group. The authors conclude that a false-negative amniocentesis correlates well with a lack of fetal insult or long-term sequela.
There have also been reports of false-positive results for CMV PCR from amniotic fluid (74, 75). In a study of 68 pregnant women with documented primary infections, PCR on amniotic fluid was positive in 17 (33%) of 52 infants that were found to be CMV negative by urine culture after birth (76). The authors state that this demonstrates transfer of the virus to the amniotic fluid without infection of the fetus. However, it could represent contamination of the sample with mother’s blood, or with amplicon in the testing laboratory, or with a lack of sensitivity of culture used as a comparator method.
The use of other sample types for diagnosis of fetal infection has been studied albeit in a more limited fashion. Percutaneous umbilical cord blood sampling and chorionic villus sampling have been used to obtain samples for diagnosis of cCMV infection in the fetus (22, 23). In a study of mothers with primary infection, PCR testing of chorionic villi collected at a median of 12.7 weeks’ gestational age detected 3 CMV positives out of 37 women versus 6 detected by testing amniotic fluid collected at a median gestational age of 17.6 weeks (50% sensitivity). Collection of these sample types is considered more dangerous for the fetus than amniocentesis, so they are not routinely collected (21).
Detection of CMV in the placenta
Placental tissue collected after birth (live or stillborn) can be used to aid in the diagnosis of maternal and newborn CMV infections. Evidence of CMV infection is seen as one or more of the following on histology: chronic villitis with plasma cells, sclerosis of villous capillaries, chorionic vessel thrombosis, necrotizing villitis, or hemosiderin deposition in the villous stroma (21). Specific immunohistochemical staining can also be used for detection of CMV. These findings are more common in primary infections but have been seen in immunocompromised individuals with reactivation of the virus (77). PCR has also been used for testing on fresh (78) and formalin-fixed (79) placental tissue. Examination of the placenta can be used in cases where the newborn is asymptomatic at birth; however, placental tissue is not commonly saved, so it is it is not typically available for retrospective diagnosis.
Detection of cCMV in newborns
Testing for CMV should occur in infants born to mothers with known or suspected CMV infection during pregnancy, those with signs or symptoms consistent with cCMV disease, those with abnormal neuroimaging consistent with CMV, and those failing the newborn hearing screen (2). Diagnosis of cCMV is confirmed by detection of virus in body fluids such as saliva, urine, or blood if collected within the first 3 weeks of life.
Urine
Urine collected within the first 3 weeks of life is considered the specimen of choice for the diagnosis of cCMV in infants by some experts in the field (2, 9, 80). Infected newborns shed large quantities of virus in the urine. While saliva is easier to obtain than urine, CMV-positive saliva has a higher potential for false-positive PCR results thought to be due to human milk contamination through breast or bottle feeding (81). Urine does not have this specificity issue. A negative result in urine by PCR is considered sufficient in most cases to rule out cCMV (9). As with a positive saliva, it is recommended to confirm positive urine results using a new sample (urine or saliva) collected within the first 3 weeks of life (2, 8).
The sensitivity and specificity of PCR in urine range from 98.8% to 100% and 99%, respectively (24, 25). There is currently one FDA-cleared test for detection of CMV from urine samples in infants less than 21 days old. The Simplexa Congenital CMV Direct assay (Diasorin Molecular) is a moderate complexity test system using real-time PCR performed on the Liaison MDX instrument. It was cleared by the FDA in November 2022. The approved sample types are saliva (described below) and urine collected in bags, containers, or from a catheter. The positive percent agreement (PPA) and the negative percent agreement (NPA) for urine were 95.3 (41 of 43) and 100% (1,581 of 1,581), respectively, for prospectively collected samples when compared to a composite reference of two PCR assays and sequencing (82). The stated limits of detection are 400–800 copies/mL or 6,400 IU/mL, depending on the CMV strain tested. In a small retrospective study of stored samples, the performance on the Simplexa assay was evaluated in comparison to a laboratory-developed PCR (83). The authors found 100% clinical sensitivity in saliva swabs (22 of 22) and 91.2% for urine (21 of 24). The clinical specificity was 96% in saliva swabs (24 of 25) and 100% in urine (26 of 26). They note that if using a cutoff value for the PCR of Ct of ≤42 (as was used in the Investigational Use Only version of the kit) instead of a Ct value of 37.5 (which is what is directed in the FDA-cleared package insert), two falsely negative urine samples with Ct values of 38.3 and 40.3 would have been called positive in agreement with the comparator PCR assay. This suggests that negative samples with Ct values between 37.5 and 42 on the Simplexa platform warrant further interrogation (84).
Due to the difficulties of collecting urine using bags, sterile cotton balls have been proposed as a collection method and have proven satisfactory when using PCR but not viral culture (70). Filter paper for collection of urine samples for cCMV diagnosis has also been investigated (69, 85, 86). Koyano et al. screened 21,000 newborns using filter paper placed in the diaper, which was analyzed by quantitative PCR, and identified cCMV in 66 (0.31%) with a positive predictive value of 94% (87).
Saliva
Saliva is an easy sample to obtain, non-invasive, and relatively sensitive for diagnosis of cCMV. The cited sensitivity and specificity range from 97.4% to 100% and from 91.5% to 99.9%, respectively (26, 27). Saliva does have lower specificity than urine when testing by PCR (27, 80, 88–91). In a large study in Minnesota, screening over 12,000 births, 8 (13.3%) of the 60 CMV positive saliva samples were determined to be falsely positive (29). The false-positive rates from other studies have ranged from 7% to 48% (26, 29, 89, 92). In a study by Exler et al., saliva PCR demonstrated a positive predictive value of 73% with 10 false positives among the 133 newborns examined (80). They reported that the level of virus in the false positives was significantly lower (median Ct = 38.5) compared to true positives (median Ct = 20.3). The major contributor to these false-positive results is thought to be breast milk in the baby’s mouth after feeding (93). The virus is commonly excreted in breast milk, and studies have demonstrated viral excretion in milk from postpartum CMV-seropositive women at rates of >70% positive when tested by PCR (3). It is therefore key to wait at least 1–2 hours after breastfeeding to collect the saliva (8).
Due to its ease of collection, saliva can be used for initial testing in newborns and is listed as the preferred sample for screening for cCMV in a recent consensus recommendation (8). However, it should always be confirmed by collection of another sample (during the first 3 weeks of life), either urine or new salvia collection, with urine preferred for confirmatory testing.
Saliva can be collected by placing a swab in between the cheek and jaw then rotating for 5 seconds on both sides (29). Swabs can be put into viral transport media or air-dried before transport. In one study (26), the sensitivity of dried saliva swabs (97.4%) was lower than that of saliva in transport media (100%), while others have shown these two sample types perform similarly (94).
Currently, there are two FDA-cleared kits for qualitative detection of CMV in saliva for cases of suspected congenital infection: the Alethia CMV Amplification Assay and the Simplexa Congenital CMV Direct. Both are relatively simple to perform and provide results in about 1 h. The Alethia assay is a moderate-complexity test system using loop-mediated isothermal amplification technology performed on the Alethia platform. It was cleared by the FDA in November 2018. The approved sample type is saliva collected from infants less than 21 days old. The sample is collected on a flocked swab and can be transported dry or in 1 mL of transport media. Per the package insert, the PPA and NPA were 100% (5 of 5) and 99.8% (1,472 of 1,475) in prospectively collected samples compared to a composite reference of two PCR assays and sequencing (95). The stated limits of detection are 1,025 copies/mL for dry swabs and 15,686 copies/mL for swabs in transport. The false-positive rate of 0.3% as stated by the manufacturer may be higher in actual practice. A study by Atwood et al. demonstrated a false-positive rate of 4.5%–6.2% for 696 prospective saliva samples when compared to another NAAT (96).
For the Simplexa assay, saliva (from infants <21 days old) is collected on flocked swabs in transport media (1 or 3 mL). Per the package insert, the PPA and NPA for saliva swabs were 94.1% (16 of 17) and 99.9% (1,835 of 1,836), respectively, for prospectively collected samples when compared to a composite reference of two PCR assays and sequencing (82). The stated limits of detection are 19,250 copies/mL. As mentioned above, in a retrospective study, the performance on the Simplexa assay on saliva was evaluated in comparison to a laboratory-developed PCR and was found to have 100% and 96% sensitivity and specificity, respectively (83).
The currently available FDA-cleared options are relatively low throughput and not amendable for use in universal screening. It seems likely that more FDA-cleared NAAT will become available for diagnosis of cCMV in the coming years, some suitable for high-volume testing. Our understanding of these tests’ performance is key if they are to be widely and effectively adopted.
Blood
Blood is not recommended as a primary sample type for diagnosis of cCMV as only 10%–20% of infants with cCMV have detectable viremia at birth (26, 30). When viral DNA is detectable, it is often lower than that detected in urine and saliva; the blood viral load is approximately two logs lower than that of urine or saliva (97). However, if blood is tested within the first 3 weeks of life and is positive for CMV DNA, it is considered diagnostic of cCMV.
Utility of quantitative viral load testing
The utility of quantitative testing for CMV DNA from various sample types has been studied quite extensively with conflicting results. Overall, there is not yet a standardized level or cutoff value for testing of newborns in blood, urine, saliva, or other sample types that has an absolute clinical correlation with the risk for disease development or worsening. Most infants with cCMV will have high levels of CMV DNA in their urine and saliva (98). If a low level is detected on CMV PCR, consideration should be given to confirming the results by culture or repeat PCR testing or collection of a new sample for testing (2). One of the issues with setting cutoffs for viral load is the significant variability in methods and materials used. The World Health Organization does have an international standard for use in construction of quantitative tests and thus provides for possible analytical harmonization across laboratories (99).
In an analysis of multiple studies, Cannon et al. found a strong and consistent association between higher viral load at birth in urine or blood and risk of developing SNHL (97). The risk of SNHL was, however, not zero among those with lower viral loads. Higher viral loads in blood were also seen in those infants who were symptomatic at birth. In a more recent report, as part of the of the CMV and Hearing Multicenter Screening (CHIMES) study, the authors similarly found higher viral loads in symptomatic infants (100). Conversely, they reported that viral load among asymptomatic children does not predict hearing loss; viral loads in urine and saliva were similar in asymptomatic infants that were born with or went on to develop SNHL and those that did not (100). In a large study in a Flemish population with 1,033 infants with cCMV infection, the researchers did find a lower viral load in blood from infants without hearing loss at birth, but it did not reach statistical significance (P value = 0.09)(101).
A collaborative group examining the utility of CMV antivirals in symptomatic infections provided guidance that viral load at baseline or during treatment should not be considered as a marker to assess the effectiveness of cCMV therapy and should not guide treatment decisions, including initiation or termination of therapy (16). Another study following 256 infected infants for up to 24 months after birth showed that viral loads in both blood and saliva fell over the 2-year period, but no threshold values were associated with a high risk of sequalae (102). In summary, until more research is done, it does not seem prudent to use viral load for prediction of outcomes in cCMV, for determination of treatment length, or for counseling of families.
Infants and children >3 weeks old
Often the diagnosis of cCMV needs to be made retrospectively, and appropriate samples are not available. Symptoms may not show up until months to years after birth. Most children who develop SNHL will do so outside of the newborn period. In those born asymptomatic, the median age of hearing loss is approximately 44 months of age (65). In these instances, retrospective diagnosis of cCMV (in infants or children >3 weeks old) is sometimes possible by testing dried blood spots (DBSs) collected within days of birth for newborn screening.
Dried blood spots
The utility of the DBS as a sample type for detection and diagnosis of cCMV has been recognized for nearly 30 years (103, 104). As part of newborn screening for metabolic and genetic disorders, these samples are collected on all newborns in the United States and in many other countries. The filter paper cards (Guthrie Cards) are stored after analysis for some period of time and can be obtained, usually through public health laboratories, for CMV PCR testing. The utility of these cards is that they provide a sample that clearly represents the congenital status of the baby so that detection of CMV can be ascribed to the pre-natal period.
Early studies showed promising sensitivities (71%–100%) for PCR-based detection of cCMV infection from DBS (69, 104). In 2010, results of the CHIMES study with DBS testing from over 20,000 enrolled infants were published. The DBS PCR result was compared to culture-based testing on saliva and was found to have a sensitivity of only 34.4% but good specificity at 99.9% (28). This low sensitivity was most likely due to inefficient DNA extraction or the testing methods employed as others have demonstrated better sensitivities (105, 106). In addition to the analytical performance, the lower clinical sensitivity with DBS testing may be due to low or negative viremia at the time of delivery in some cCMV cases.
Recent studies have reported improved analytical performance of DBS PCR (97, 104–106). Key aspects that improved sensitivity are the amount of the filter paper used for extraction (multiple punches from the DBS), the elution buffer used, the extraction method, and the target(s) used in the PCR reaction (68, 107). In a large-scale U.S. study, the performance of two DBS PCR assays were compared in a prospective newborn screening study of 12,554 infants with saliva PCR as the comparator. The sensitivities for the assays were 73.2% and 76.8%; the combined sensitivity for both assays was 85.7% (29). These sensitivities, generated in comparison to saliva PCR, may be higher if compared to urine PCR, which has lower rates of false positives.
Several retrospective studies have shown that DBS testing has been helpful to identify cCMV infection as a cause of SNHL (65, 108–113). In a meta-analysis of 15 studies of DBS PCR testing for cCMV, Wang et al. found that the pooled sensitivity was 84% with a specificity of >99% (114). While CMV detection in DBS is diagnostic, a negative result in a DBS sample does not rule out cCMV. Therefore, DBS testing is best suited for retrospective and not prospective screening. Several studies have demonstrated an increased risk of SNHL, and other findings have been associated with higher CMV viral loads detected from DBS (97, 104, 107, 110). However, in the CHIMEs study, such an association was not seen (28). The contrasting results may relate to the testing methodologies used, the population tested, and the overall study design. Further investigation is needed to fully understand the utility of DBS viral load as a prognostic indicator.
Dried umbilical cord (DUC) has been proposed as an alternative sample to DBS for confirmation of cCMV infection. While not routinely preserved in the United States, DUC is preserved by families in Japan as a symbol of mother-child bonding and has been studied as a sample type for diagnosis of cCMV in that population (115–117). In a study by Reyes et al. (118), researchers examined DUC in cases of 16 infants with cCMV and found three with detectable virus compared to five in DBS using CMV PCR. One infant with a negative DBS was positive in the DUC sample. More comprehensive studies are needed to determine the performance of this sample type for diagnosis of cCMV.
Direct detection of CMV in pregnant women
Detection of the virus through culture or NAAT in pregnant women’s blood has limited utility due to the lack of predictive value for primary infection and transmission to the fetus and is not routinely performed in the United States. While reports of prolonged maternal DNAemia in primary infection have been associated with risk of transmission to the fetus, CMV viral loads should only play a supporting role to serologic diagnosis (39). In non-primary infections, the role of detection of CMV in mothers’ blood is not defined. More research is needed to understand the nature of viremia during maternal infections and its utility in determination of primary versus non-primary infections or risk of transmission.
CMV can be detected in breast milk in seropositive women using PCR. This is not useful in the diagnosis of congenital infection but can aid in assessment of post-natal infections. Transmission to a full-term healthy infant is relatively rare and most often asymptomatic. There is concern for transmission in low and extremely low birthweight infants; testing of breast milk may be useful in these situations (119–121).
PREVENTION AND TREATMENT OF CONGENITAL AND MATERNAL CMV INFECTION
There is currently no licensed vaccine to prevent CMV infection in pregnant women. However, several intervention strategies have been studied to prevent maternal infection or transmission to the fetus. Hygiene measures are important; multiple studies have demonstrated that exposure to young children, particularly those between 1 and 2 years of age, presents a significant risk of infection (97). Thus, caregivers, particularly pregnant women, should be counseled to wash hands often to avoid exposure to body fluids from young children (8). In a study by Revello et al., they found that specific hygiene education among seronegative women resulted in fewer instance of seroconversion during pregnancy versus those that had no counseling (1.2% versus 7.6%, P value = 0.001)(122). This type of counseling would also be beneficial to women who are already seropositive for CMV as infection with a new strain is possible (123). The use of CMV hyperimmune immunoglobulin as a treatment for infected mothers to prevent transmission during pregnancy has been studied, but the results have been disappointing. In two randomized controlled trials, efficacy was not seen in comparison to placebo to prevent fetal infection (124, 125).
While there are no antivirals that are specifically licensed for the treatment of cCMV, the use of drugs, particularly ganciclovir, valganciclovir, and valacyclovir has been studied in both pregnant women and infants. An extensive review of treatment for maternal and congenital CMV is beyond the scope of this article, but readers are referred to other sources for more comprehensive review information (123, 126).
Treatment with antivirals during pregnancy is currently not recommended due to concerns for toxicity and a lack of high-quality evidence showing efficacy or cost-effectiveness (8, 127, 128). That being said, there have been several studies showing promising results with antiviral treatment during pregnancy. In a prospective, randomized controlled trial for valacyclovir looking at prevention of maternal-fetal transmission, among women with serologic evidence of primary CMV in the first trimester, it was found that the treatment group tested positive for CMV by amniotic fluid PCR in 11% versus 48% of the placebo group (129). Several other studies have demonstrated promising outcomes with valacyclovir used in the treatment of primary infection in the first trimester with less effect in infections acquired in the peri-conception period or third trimester (22, 127, 129, 130). More robust data are needed before recommendations for standard treatment of maternal CMV infection are proposed (123).
Current guidance limits treatment recommendation to infants with moderate or severe symptomatic CMV disease with or without hearing loss (8, 9). Intravenous ganciclovir and oral valganciclovir are the drugs of choice and have demonstrated a benefit when treatment is initiated within the first month of life and continued for 6 months. Such treatment has been shown to improve audiologic and neurodevelopmental outcomes at 2 years of age (131). During treatment, cell counts should be done weekly for 6 weeks, then at 8 weeks, and monthly thereafter to check for neutropenia along with monitoring transaminases monthly (8). For asymptomatic infants with only isolated hearing loss, expert opinion varies as to whether the benefits of antiviral therapy outweigh the risks (126). Currently, treatment in those infants greater than 30 days old is not recommended; however, there is ongoing research on treatment in this population. Of note, the ValEAR Randomized Controlled Trial (Valganciclovir for Cytomegalovirus Infected Hearing Impaired Infants) may shed more light on this issue when completed (https://clinicaltrials.gov/study/NCT03107871, accessed 27 January 2024). This multi-center, double-blind, randomized, placebo-controlled trial is designed to determine whether hearing-impaired infants with asymptomatic cCMV have better hearing and language outcomes if they receive valganciclovir antiviral treatment.
The appropriate follow-up in those with cCMV includes an ophthalmologic evaluation early in the treatment course, audiologic testing throughout childhood, and developmental assessments as appropriate on a case-by-case basis (8).
SCREENING FOR MATERNAL AND CONGENITAL CMV INFECTION
With increasing availability of testing to screen for infection in pregnant women and neonates, there are more calls for routine screening of both populations to prevent or treat cCMV infections. There are two distinct approaches to screening for cCMV among newborns: universal screening of all infants or targeted screening of those at higher risk. Currently, there are no public health organizations or medical societies recommending universal screening of all newborns for cCMV infection (8). Universal screening would involve testing saliva, urine, and/or dried blood spots on all newborns. Although the sensitivity of testing either saliva or urine is higher than DBS, DBSs are already collected on all babies born in the United States and many other countries, making it an easily accessible sample for universal screening. Targeted screening has been adopted using the newborn hearing screen as an initial test; infants who fail the hearing screen are then tested specifically for CMV.
Both universal and targeted newborn screening have been studied and found to be cost effective (132). Targeted screening programs are likely to detect far fewer infected infants than universal screening; it is estimated that targeted screening would miss over 43% of infants with cCMV-related hearing loss in infancy as they are asymptomatic at birth (133). The availability of FDA-cleared tests will hopefully lead to recommendations for screening on a larger scale (134–136). Minnesota was the first state to mandate universal newborn screening for cCMV using dried blood spots already collected for newborn screening. Connecticut is on track to become the second state to screen every baby beginning in 2025. Eight states (Illinois, Iowa, Kentucky, Maine, New York, Pennsylvania, Texas, and Utah) have passed legislation requiring both education of pregnant women and targeted CMV screening in infants with failed hearing tests [https://www.nationalcmv.org/about-us/advocacy, accessed 27 January 2024 (106)]. Also, individual hospitals have set up targeted screening strategies in their newborn nurseries. Barriers to adoption of universal newborn screening include a lack of consensus on the sample type to use, funding of the testing, and medical and administrative oversight. Also of concern is the psychosocial impact of screening, given the known issue of initial false-positive results that may not be confirmed, and the fact that not all babies with confirmed cCMV go on to develop symptoms.
Universal screening of all pregnant women for CMV IgG to identify those susceptible to primary infections is currently not recommended by any public health organizations (8). However, several European countries have adopted screening strategies (137). The utility of this approach has significant limitations. Identifying seronegative women could be useful for targeted education and to allow accurate documentation of seroconversion. However, the majority of cCMV infections occur in women with non-primary infection where CMV IgG is already present. In addition, there is no proven intervention that will prevent in-utero transmission. Screening might be useful if treatment with antivirals such as valacyclovir is convincingly shown to prevent vertical transmission (129). If this were the case, screening should be focus on detecting seroconversion during the first trimester (8–16 weeks gestation) when the risk of transmission and long-term sequela is highest (10).
CONCLUSIONS
The worldwide impact of cCMV is great, including the number of infants affected and the long-term burden of the adverse sequela seen with congenital infection. Advances in the laboratory diagnostics have helped to more quickly and accurately identify infected mothers and infants. This includes the recent availability of commercial FDA-cleared tests for testing infants. However, there are many issues still to be resolved. A vaccine to prevent CMV infection would be an effective means to prevent cCMV but does not seem to be eminent. Continued research is needed to better understand if antiviral treatment can be used to prevent fetal infection in cases of confirmed primary infection. If this is clearly demonstrated, the impetus for screening in pregnancy would be increased. Also, additional studies are needed on how to identify those women with non-primary infection who are likely to pass on the infection. The practice of universal or targeted screening of newborns seems likely to grow as the economic and public health benefits appear to be positive. Clinical laboratories have an important role in these efforts and must strive to understand current testing capabilities and innovations as they become available and to assist in interpretation of test results for both mother and baby.
Contributor Information
Amy L. Leber, Email: amy.leber@nationwidechildrens.org.
Romney M. Humphries, Vanderbilt University Medical Center, Nashville, Tennessee, USA
REFERENCES
- 1. Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. 2013. The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102. doi: 10.1128/CMR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demmler-Harrison G. 2023. Congenital cytomegalovirus infection: Clinnical features and diagnosis. UpToDate. [Google Scholar]
- 3. Wilson C, Nizet V, Maldonado Y, Remington J, Klein J. 2016. Remington and Klein's infectious disease of the fetus and newborn infant. 8th ed. Elsiver. [Google Scholar]
- 4. Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904–1908. [PubMed] [Google Scholar]
- 5. Iijima S. 2022. Pitfalls in the serological evaluation of maternal cytomegalovirus infection as a potential cause of fetal and neonatal involvements: a narrative literature review. J Clin Med 11:5006. doi: 10.3390/jcm11175006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Picone O, Vauloup-Fellous C, Cordier AG, Guitton S, Senat MV, Fuchs F, Ayoubi JM, Grangeot Keros L, Benachi A. 2013. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn 33:751–758. doi: 10.1002/pd.4118 [DOI] [PubMed] [Google Scholar]
- 7. Guerra B, Simonazzi G, Puccetti C, Lanari M, Farina A, Lazzarotto T, Rizzo N. 2008. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 198:380. doi: 10.1016/j.ajog.2007.09.052 [DOI] [PubMed] [Google Scholar]
- 8. Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, Daly K, Doutré S, Gibson L, Giles ML, Greenlee J, Hamilton ST, Harrison GJ, Hui L, Jones CA, Palasanthiran P, Schleiss MR, Shand AW, van Zuylen WJ. 2017. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 17:e177–e188. doi: 10.1016/S1473-3099(17)30143-3 [DOI] [PubMed] [Google Scholar]
- 9. Luck SE, Wieringa JW, Blázquez-Gamero D, Henneke P, Schuster K, Butler K, Capretti MG, Cilleruelo MJ, Curtis N, Garofoli F, et al. 2017. Congenital cytomegalovirus. Pediatr Infect Dis J 36:1205–1213. doi: 10.1097/INF.0000000000001763 [DOI] [PubMed] [Google Scholar]
- 10. Leruez-Ville M, Foulon I, Pass R, Ville Y. 2020. Cytomegalovirus infection during pregnancy: state of the science. Am J Obstet Gynecol 223:330–349. doi: 10.1016/j.ajog.2020.02.018 [DOI] [PubMed] [Google Scholar]
- 11. Genser B, Truschnig-Wilders M, Stünzner D, Landini MP, Halwachs-Baumann G. 2001. Evaluation of five commercial enzyme immunoassays for the detection of human cytomegalovirus-specific IgM antibodies in the absence of a commercially available gold standard. Clin Chem Lab Med 39:62–70. doi: 10.1515/CCLM.2001.014 [DOI] [PubMed] [Google Scholar]
- 12. Carlier P, Harika N, Bailly R, Vranken G. 2010. Laboratory evaluation of the new access cytomegalovirus immunoglobulin IgM and IgG assays. J Clin Virol 49:192–197. doi: 10.1016/j.jcv.2010.07.024 [DOI] [PubMed] [Google Scholar]
- 13. Lazzarotto T, Galli C, Pulvirenti R, Rescaldani R, Vezzo R, La Gioia A, Martinelli C, La Rocca S, Agresti G, Grillner L, Nordin M, van Ranst M, Combs B, Maine GT, Landini MP. 2001. Evaluation of the Abbott AxSYM cytomegalovirus (CMV) immunoglobulin M (IgM) assay in conjunction with other CMV IgM tests and a CMV IgG avidity assay. Clin Diagn Lab Immunol 8:196–198. doi: 10.1128/CDLI.8.1.196-198.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gentile M, Galli C, Pagnotti P, Di Marco P, Tzantzoglou S, Bellomi F, Ferreri ML, Selvaggi C, Antonelli G. 2009. Measurement of the sensitivity of different commercial assays in the diagnosis of CMV infection in pregnancy. Eur J Clin Microbiol Infect Dis 28:977–981. doi: 10.1007/s10096-009-0738-0 [DOI] [PubMed] [Google Scholar]
- 15. BaAlawi F, Robertson PW, Lahra M, Rawlinson WD. 2012. Comparison of five CMV IgM immunoassays with CMV IgG avidity for diagnosis of primary CMV infection. Pathology 44:381–383. doi: 10.1097/PAT.0b013e328353bec0 [DOI] [PubMed] [Google Scholar]
- 16. Binnicker MJ, Jespersen DJ, Harring JA. 2010. Multiplex detection of IgM and IgG class antibodies to Toxoplasma gondii, rubella virus, and cytomegalovirus using a novel multiplex flow immunoassay. Clin Vaccine Immunol 17:1734–1738. doi: 10.1128/CVI.00332-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kitamura A, Toriyabe K, Hagimoto-Akasaka M, Hamasaki-Shimada K, Ikejiri M, Minematsu T, Suga S, Kondo E, Kihira M, Morikawa F, Ikeda T. 2023. Revision of cytomegalovirus immunoglobulin M antibody titer cutoff in a maternal antibody screening program in Japan: a cohort comparison involving a total of 32,000 pregnant women. Viruses 15:962. doi: 10.3390/v15040962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prince HE, Lapé-Nixon M. 2014. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 21:1377–1384. doi: 10.1128/CVI.00487-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodéus M, Goubau P. 1999. Predictive value of maternal-IgG avidity for congenital human cytomegalovirus infection. J Clin Virol 12:3–8. doi: 10.1016/s1386-6532(98)00009-2 [DOI] [PubMed] [Google Scholar]
- 20. Leruez-Ville M, Ville Y. 2017. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol 38:97–107. doi: 10.1016/j.bpobgyn.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 21. Boppana S. 2023. Cytomegalovurs infection in pregnancy. UpToDate. [Google Scholar]
- 22. Faure-Bardon V, Fourgeaud J, Guilleminot T, Magny J-F, Salomon LJ, Bernard J-P, Leruez-Ville M, Ville Y. 2021. First-trimester diagnosis of congenital cytomegalovirus infection after maternal primary infection in early pregnancy: feasibility study of viral genome amplification by PCR on chorionic villi obtained by CVS. Ultrasound Obstet Gynecol 57:568–572. doi: 10.1002/uog.23608 [DOI] [PubMed] [Google Scholar]
- 23. Grazia Revello M, Zavattoni M, Sarasini A, Baldanti F, De Julio C, De-Giuli L, Nicolini U, Gerna G. 1999. Prenatal diagnostic and prognostic value of human cytomegalovirus load and IgM antibody response in blood of congenitally infected fetuses. J Infect Dis 180:1320–1323. doi: 10.1086/315036 [DOI] [PubMed] [Google Scholar]
- 24. de Vries JJC, van der Eijk AA, Wolthers KC, Rusman LG, Pas SD, Molenkamp R, Claas EC, Kroes ACM, Vossen A. 2012. Real-time PCR versus viral culture on urine as a gold standard in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 53:167–170. doi: 10.1016/j.jcv.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 25. Ross SA, Ahmed A, Palmer AL, Michaels MG, Sánchez PJ, Bernstein DI, Tolan RW, Novak Z, Chowdhury N, Fowler KB, Boppana SB, National Institute on Deafness and Other Communication Disorders CHIMES Study . 2014. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis 210:1415–1418. doi: 10.1093/infdis/jiu263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, Sánchez PJ, Bernstein DI, Tolan RW, Novak Z, Chowdhury N, Britt WJ, Fowler KB, National Institute on Deafness and Other Communication Disorders CHIMES Study . 2011. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med 364:2111–2118. doi: 10.1056/NEJMoa1006561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eventov-Friedman S, Manor H, Bar-Oz B, Averbuch D, Caplan O, Lifshitz A, Bdolah-Abram T, Wolf DG. 2019. Saliva real-time polymerase chain reaction for targeted screening of congenital cytomegalovirus infection. J Infect Dis 220:1790–1796. doi: 10.1093/infdis/jiz373 [DOI] [PubMed] [Google Scholar]
- 28. Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW Jr, Palmer AL, Ahmed A, Michaels MG, Sánchez PJ, Bernstein DI, Britt WJ, Fowler KB, National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study . 2010. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 303:1375–1382. doi: 10.1001/jama.2010.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dollard SC, Dreon M, Hernandez-Alvarado N, Amin MM, Wong P, Lanzieri TM, Osterholm EA, Sidebottom A, Rosendahl S, McCann MT, Schleiss MR. 2021. Sensitivity of dried blood spot testing for detection of congenital cytomegalovirus infection. JAMA Pediatr 175:e205441. doi: 10.1001/jamapediatrics.2020.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luck SE, Emery VC, Atkinson C, Sharland M, Griffiths PD. 2016. Compartmentalized dynamics of cytomegalovirus replication in treated congenital infection. J Clin Virol 82:152–158. doi: 10.1016/j.jcv.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 31. Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. 2008. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 41:192–197. doi: 10.1016/j.jcv.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 32. Bilavsky E, Watad S, Levy I, Linder N, Pardo J, Ben-Zvi H, Attias J, Amir J. 2017. Positive IgM in congenital CMV infection. Clin Pediatr (Phila) 56:371–375. doi: 10.1177/0009922816684596 [DOI] [PubMed] [Google Scholar]
- 33. Müller J, Flindt J, Pollmann M, Saschenbrecker S, Borchardt-Lohölter V, Warnecke JM. 2023. Efficiency of CMV serodiagnosis during pregnancy in daily laboratory routine. J Virol Methods 314:114685. doi: 10.1016/j.jviromet.2023.114685 [DOI] [PubMed] [Google Scholar]
- 34. Furione M, Sarasini A, Arossa A, Fornara C, Lilleri D, Perez L, Parea M, Zavattoni M, Spinillo A, Marone P, Baldanti F. 2018. False human cytomegalovirus IgG-positivity at prenatal screening. J Clin Virol 104:34–38. doi: 10.1016/j.jcv.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 35. Chiopris G, Veronese P, Cusenza F, Procaccianti M, Perrone S, Daccò V, Colombo C, Esposito S. 2020. Congenital cytomegalovirus infection: update on diagnosis and treatment. Microorganisms 8:1516. doi: 10.3390/microorganisms8101516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novak Z, Ross SA, Patro RK, Pati SK, Reddy MK, Purser M, Britt WJ, Boppana SB. 2009. Enzyme-linked immunosorbent assay method for detection of cytomegalovirus strain-specific antibody responses. Clin Vaccine Immunol 16:288–290. doi: 10.1128/CVI.00281-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zavaglio F, Fiorina L, Suárez NM, Fornara C, De Cicco M, Cirasola D, Davison AJ, Gerna G, Lilleri D. 2021. Detection of genotype-specific antibody responses to glycoproteins B and H in primary and non-primary human cytomegalovirus infections by peptide-based ELISA. Viruses 13:399. doi: 10.3390/v13030399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McVoy MA, Adler SP. 1989. Immunologic evidence for frequent age-related cytomegalovirus reactivation in seropositive immunocompetent individuals. J Infect Dis 160:1–10. doi: 10.1093/infdis/160.1.1 [DOI] [PubMed] [Google Scholar]
- 39. Gerna G, Fornara C, Furione M, Lilleri D. 2021. Congenital human cytomegalovirus infection: a narrative review of maternal immune response and diagnosis in view of the development of a vaccine and prevention of primary and non-primary infections in pregnancy. Microorganisms 9:1749. doi: 10.3390/microorganisms9081749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. American College of Obsteterics and Gynecology . 2015. Practice bulletine no. 151: cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 125:1510–1525. doi: 10.1097/01.AOG.0000466430.19823.53 [DOI] [PubMed] [Google Scholar]
- 41. Prince HE, Lapé-Nixon M, Brenner A, Pitstick N, Couturier MR. 2014. Potential impact of different cytomegalovirus (CMV) IgM assays on an algorithm requiring IgM reactivity as a criterion for measuring CMV IgG avidity. Clin Vaccine Immunol 21:813–816. doi: 10.1128/CVI.00106-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lazzarotto T, Spezzacatena P, Pradelli P, Abate DA, Varani S, Landini MP. 1997. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin Diagn Lab Immunol 4:469–473. doi: 10.1128/cdli.4.4.469-473.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prince HE, Leber AL. 2002. Validation of an in-house assay for cytomegalovirus immunoglobulin G (CMV IgG) avidity and relationship of avidity to CMV IgM levels. Clin Diagn Lab Immunol 9:824–827. doi: 10.1128/cdli.9.4.824-827.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vauloup-Fellous C, Lazzarotto T, Revello MG, Grangeot-Keros L. 2014. Clinical evaluation of the roche elecsys CMV IgG avidity assay. Eur J Clin Microbiol Infect Dis 33:1365–1369. doi: 10.1007/s10096-014-2080-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sellier Y, Guilleminot T, Ville Y, Leruez-Ville M. 2015. Comparison of the LIAISON CMV IgG avidity II and the VIDAS CMV IgG avidity II assays for the diagnosis of primary infection in pregnant women. J Clin Virol 72:46–48. doi: 10.1016/j.jcv.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 46. Delforge ML, Eykmans J, Steensels D, Costa E, Donner C, Montesinos I. 2019. Combination of line immunoassays Mikrogen recomLine CMV IgG and recomLine CMV IgG avidity helps to date the onset of CMV primary infection. Diagn Microbiol Infect Dis 93:208–212. doi: 10.1016/j.diagmicrobio.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 47. Revello MG, Genini E, Gorini G, Klersy C, Piralla A, Gerna G. 2010. Comparative evaluation of eight commercial human cytomegalovirus IgG avidity assays. J Clin Virol 48:255–259. doi: 10.1016/j.jcv.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 48. Prince HE, Lapé-Nixon M, Novak-Weekley SM. 2014. Performance of a cytomegalovirus IgG enzyme immunoassay kit modified to measure avidity. Clin Vaccine Immunol 21:808–812. doi: 10.1128/CVI.00105-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Macé M, Sissoeff L, Rudent A, Grangeot-Keros L. 2004. A serological testing algorithm for the diagnosis of primary CMV infection in pregnant women. Prenat Diagn 24:861–863. doi: 10.1002/pd.1001 [DOI] [PubMed] [Google Scholar]
- 50. Enders G, Daiminger A, Bäder U, Exler S, Schimpf Y, Enders M. 2013. The value of CMV IgG avidity and immunoblot for timing the onset of primary CMV infection in pregnancy. J Clin Virol 56:102–107. doi: 10.1016/j.jcv.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 51. Lazzarotto T, Varani S, Spezzacatena P, Gabrielli L, Pradelli P, Guerra B, Landini MP. 2000. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol 13:137–141. doi: 10.1089/vim.2000.13.137 [DOI] [PubMed] [Google Scholar]
- 52. Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, Landini MP. 2000. Prenatal indicators of congenital cytomegalovirus infection. J Pediatr 137:90–95. doi: 10.1067/mpd.2000.107110 [DOI] [PubMed] [Google Scholar]
- 53. Leruez-Ville M, Sellier Y, Salomon LJ, Stirnemann JJ, Jacquemard F, Ville Y. 2013. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: a retrospective cohort. Clin Infect Dis 56:1428–1435. doi: 10.1093/cid/cit059 [DOI] [PubMed] [Google Scholar]
- 54. Bodéus M, Hubinont C, Bernard P, Bouckaert A, Thomas K, Goubau P. 1999. Prenatal diagnosis of human cytomegalovirus by culture and polymerase chain reaction: 98 pregnancies leading to congenital infection. Prenat Diagn 19:314–317. doi: [DOI] [PubMed] [Google Scholar]
- 55. Sarasini A, Arossa A, Zavattoni M, Fornara C, Lilleri D, Spinillo A, Baldanti F, Furione M. 2021. Pitfalls in the serological diagnosis of primary human cytomegalovirus infection in pregnancy due to different kinetics of IgM clearance and IgG avidity index maturation. Diagnostics (Basel) 11:396. doi: 10.3390/diagnostics11030396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Demmler GJ. 1994. Congenital cytomegalovirus infection. Semin Pediatr Neurol 1:36–42. [PubMed] [Google Scholar]
- 57. Forner G, Saldan A, Mengoli C, Gussetti N, Palù G, Abate D. 2016. Cytomegalovirus (CMV) enzyme-linked immunosorbent spot assay but not CMV QuantiFERON assay is a novel biomarker to determine risk of congenital CMV infection in pregnant women. J Clin Microbiol 54:2149–2154. doi: 10.1128/JCM.00561-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eldar-Yedidia Y, Bar-Meir M, Hillel M, Abitbol G, Broide E, Falk R, Assous M, Schlesinger Y. 2016. Low interferon relative-response to cytomegalovirus is associated with low likelihood of intrauterine transmission of the virus. PLoS One 11:e0147883. doi: 10.1371/journal.pone.0147883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soriano-Ramos M, Esquivel-De la Fuente E, Albert Vicent E, de la Calle M, Baquero-Artigao F, Domínguez-Rodríguez S, Cabanes M, Gómez-Montes E, Goncé A, Valdés-Bango M, et al. 2023. The role of the T-cell mediated immune response to cytomegalovirus infection in intrauterine transmission. PLoS One 18:e0281341. doi: 10.1371/journal.pone.0281341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gleaves CA, Smith TF, Shuster EA, Pearson GR. 1985. Comparison of standard tube and shell vial cell culture techniques for the detection of cytomegalovirus in clinical specimens. J Clin Microbiol 21:217–221. doi: 10.1128/jcm.21.2.217-221.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thiele GM, Bicak MS, Young A, Kinsey J, White RJ, Purtilo DT. 1987. Rapid detection of cytomegalovirus by tissue culture, centrifugation, and immunofluorescence with a monoclonal antibody to an early nuclear antigen. J Virol Methods 16:327–338. doi: 10.1016/0166-0934(87)90018-8 [DOI] [PubMed] [Google Scholar]
- 62. Landry ML, Stanat S, Biron K, Brambilla D, Britt W, Jokela J, Chou S, Drew WL, Erice A, Gilliam B, Lurain N, Manischewitz J, Miner R, Nokta M, Reichelderfer P, Spector S, Weinberg A, Yen-Lieberman B, Crumpacker C. 2000. A standardized plaque reduction assay for determination of drug susceptibilities of cytomegalovirus clinical isolates. Antimicrob Agents Chemother 44:688–692. doi: 10.1128/AAC.44.3.688-692.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Demmler GJ, Buffone GJ, Schimbor CM, May RA. 1988. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J Infect Dis 158:1177–1184. doi: 10.1093/infdis/158.6.1177 [DOI] [PubMed] [Google Scholar]
- 64. Gantt S, Goldfarb DM, Park A, Rawlinson W, Boppana SB, Lazzarotto T, Mertz LM. 2020. Performance of the Alethia CMV assay for detection of cytomegalovirus by use of neonatal saliva swabs. J Clin Microbiol 58:e01951-19. doi: 10.1128/JCM.01951-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meyer L, Sharon B, Huang TC, Meyer AC, Gravel KE, Schimmenti LA, Swanson EC, Herd HE, Hernandez-Alvarado N, Coverstone KR, McCann M, Schleiss MR. 2017. Analysis of archived newborn dried blood spots (DBS) identifies congenital cytomegalovirus as a major cause of unexplained pediatric sensorineural hearing loss. Am J Otolaryngol 38:565–570. doi: 10.1016/j.amjoto.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 66. Chou S. 1992. Molecular epidemiology of envelope glycoprotein H of human cytomegalovirus. J Infect Dis 166:604–607. doi: 10.1093/infdis/166.3.604 [DOI] [PubMed] [Google Scholar]
- 67. Chou SW, Dennison KM. 1991. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis 163:1229–1234. doi: 10.1093/infdis/163.6.1229 [DOI] [PubMed] [Google Scholar]
- 68. Koontz D, Baecher K, Amin M, Nikolova S, Gallagher M, Dollard S. 2015. Evaluation of DNA extraction methods for the detection of cytomegalovirus in dried blood spots. J Clin Virol 66:95–99. doi: 10.1016/j.jcv.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamamoto AY, Mussi-Pinhata MM, Pinto PCG, Figueiredo LTM, Jorge SM. 2001. Usefulness of blood and urine samples collected on filter paper in detecting cytomegalovirus by the polymerase chain reaction technique. J Virol Methods 97:159–164. doi: 10.1016/s0166-0934(01)00347-0 [DOI] [PubMed] [Google Scholar]
- 70. Ross SA, Ahmed A, Palmer AL, Michaels MG, Sánchez PJ, Stewart A, Bernstein DI, Feja K, Novak Z, Fowler KB, Boppana SB, National Institute on Deafness and Other Communication Disorders CHIMES Study . 2015. Urine collection method for the diagnosis of congenital cytomegalovirus infection. Pediatr Infect Dis J 34:903–905. doi: 10.1097/INF.0000000000000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Buca D, Di Mascio D, Rizzo G, Giancotti A, D’Amico A, Leombroni M, Makatsarya A, Familiari A, Liberati M, Nappi L, Flacco ME, Manzoli L, Salomon LJ, Scambia G, D’Antonio F. 2021. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol 57:551–559. doi: 10.1002/uog.23143 [DOI] [PubMed] [Google Scholar]
- 72. Donner C, Liesnard C, Brancart F, Rodesch F. 1994. Accuracy of amniotic fluid testing before 21 weeks' gestation in prenatal diagnosis of congenital cytomegalovirus infection. Prenat Diagn 14:1055–1059. doi: 10.1002/pd.1970141108 [DOI] [PubMed] [Google Scholar]
- 73. Chatzakis C, Ville Y, Makrydimas G, Dinas K, Zavlanos A, Sotiriadis A. 2020. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol 223:870–883. doi: 10.1016/j.ajog.2020.05.038 [DOI] [PubMed] [Google Scholar]
- 74. Enders G, Bäder U, Lindemann L, Schalasta G, Daiminger A. 2001. Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenat Diagn 21:362–377. doi: 10.1002/pd.59 [DOI] [PubMed] [Google Scholar]
- 75. Enders M, Daiminger A, Exler S, Ertan K, Enders G, Bald R. 2017. Prenatal diagnosis of congenital cytomegalovirus infection in 115 cases: a 5 years' single center experience. Prenat Diagn 37:389–398. doi: 10.1002/pd.5025 [DOI] [PubMed] [Google Scholar]
- 76. Guerra B, Lazzarotto T, Quarta S, Lanari M, Bovicelli L, Nicolosi A, Landini MP. 2000. Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 183:476–482. doi: 10.1067/mob.2000.106347 [DOI] [PubMed] [Google Scholar]
- 77. Schwebke K, Henry K, Balfour HH, Olson D, Crane RT, Jordan MC. 1995. Congenital cytomegalovirus infection as a result of nonprimary cytomegalovirus disease in a mother with acquired immunodeficiency syndrome. J Pediatr 126:293–295. doi: 10.1016/s0022-3476(95)70563-5 [DOI] [PubMed] [Google Scholar]
- 78. Ozono K, Mushiake S, Takeshima T, Nakayama M. 1997. Diagnosis of congenital cytomegalovirus infection by examination of placenta: application of polymerase chain reaction and in situ hybridization. Pediatr Pathol Lab Med 17:249–258. [PubMed] [Google Scholar]
- 79. Folkins AK, Chisholm KM, Guo FP, McDowell M, Aziz N, Pinsky BA. 2013. Diagnosis of congenital CMV using PCR performed on formalin-fixed, paraffin-embedded placental tissue. Am J Surg Pathol 37:1413–1420. doi: 10.1097/PAS.0b013e318290f171 [DOI] [PubMed] [Google Scholar]
- 80. Exler S, Daiminger A, Grothe M, Schalasta G, Enders G, Enders M. 2019. Primary cytomegalovirus (CMV) infection in pregnancy: diagnostic value of CMV PCR in saliva compared to urine at birth. J Clin Virol 117:33–36. doi: 10.1016/j.jcv.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 81. Razonable RR, Inoue N, Pinninti SG, Boppana SB, Lazzarotto T, Gabrielli L, Simonazzi G, Pellett PE, Schmid DS. 2020. Clinical diagnostic testing for human cytomegalovirus infections. J Infect Dis 221:S74–S85. doi: 10.1093/infdis/jiz601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Diasorin Molecular. 2022. Simplexa congenital CMV direct, Mol2255,Rev. 01. Package Insert. [Google Scholar]
- 83. Fernholz EC, Vidal-Folch N, Hasadsri L. 2023. Rapid and direct detection of congenital cytomegalovirus using a commercial real-time PCR assay. J Clin Microbiol 61:e0178122. doi: 10.1128/jcm.01781-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dunn JJ, Selvarangan R, Maggert K, Young S, Leber AL. 2023. Multicenter evaluation of the diasorin molecular simplexa congenital CMV direct PCR test on neonatal saliva and urine specimens. J Clin Microbiol 61:e0028323. doi: 10.1128/jcm.00283-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Forman M, Valsamakis A, Arav-Boger R. 2012. Dried urine spots for detection and quantification of cytomegalovirus in newborns. Diagn Microbiol Infect Dis 73:326–329. doi: 10.1016/j.diagmicrobio.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nozawa N, Koyano S, Yamamoto Y, Inami Y, Kurane I, Inoue N. 2007. Real-time PCR assay using specimens on filter disks as a template for detection of cytomegalovirus in urine. J Clin Microbiol 45:1305–1307. doi: 10.1128/JCM.02502-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Koyano S, Inoue N, Oka A, Moriuchi H, Asano K, Ito Y, Yamada H, Yoshikawa T, Suzutani T, Japanese Congenital Cytomegalovirus Study G. 2011. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: feasibility and outcomes from a multicentre study. BMJ Open 1:e000118. doi: 10.1136/bmjopen-2011-000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliveira PFC, Coelho TB. 2006. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol 36:228–230. doi: 10.1016/j.jcv.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 89. Nagel A, Dimitrakopoulou E, Teig N, Kern P, Lücke T, Michna D, Korn K, Steininger P, Shahada K, Neumann K, Überla K. 2020. Characterization of a universal screening approach for congenital CMV infection based on a highly-sensitive, quantitative, multiplex real-time PCR assay. PLoS One 15:e0227143. doi: 10.1371/journal.pone.0227143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Barkai G, Ari-Even Roth D, Barzilai A, Tepperberg-Oikawa M, Mendelson E, Hildesheimer M, Kuint J. 2014. Universal neonatal cytomegalovirus screening using saliva - report of clinical experience. J Clin Virol 60:361–366. doi: 10.1016/j.jcv.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 91. Cardoso E de C, Jesus B de, Gomes L da S, Sousa SMB, Gadelha SR, Marin LJ. 2015. The use of saliva as a practical and feasible alternative to urine in large-scale screening for congenital cytomegalovirus infection increases inclusion and detection rates. Rev Soc Bras Med Trop 48:206–207. doi: 10.1590/0037-8682-0200-2014 [DOI] [PubMed] [Google Scholar]
- 92. Ross SA, Michaels MG, Ahmed A, Palmer AL, Sánchez PJ, Bernstein DI, Feja K, Stewart A, Boppana SB, Fowler KB. 2018. Contribution of breastfeeding to false-positive saliva polymerase chain reaction for newborn congenital cytomegalovirus screening. J Infect Dis 217:1612–1615. doi: 10.1093/infdis/jiy057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Koyano S, Inoue N, Nagamori T, Moriuchi H, Azuma H. 2013. Newborn screening of congenital cytomegalovirus infection using saliva can be influenced by breast feeding. Arch Dis Child Fetal Neonatal Ed 98:F182. doi: 10.1136/archdischild-2012-302230 [DOI] [PubMed] [Google Scholar]
- 94. Xu A, Wang S, Zhang W, Wang X, Wang T, Liu X, Wang H, Ma W, Amin M, Dollard S, Wang C. 2018. Viral loads in congenital cytomegalovirus infection from a highly immune population. J Pediatric Infect Dis Soc 7:e160–e162. doi: 10.1093/jpids/piy048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Meridian BioSciences . 2018. Package insert, Alethia CMV DNA amplification assay. Rev 07/21.
- 96. Atwood EN, Esper FP, Bailey K, Doud MK, Benton AM, Friesen J, Rodgers MJ, Humphries RM, Rhoads DD, Gaston DC, Wang H. 2023. False positive congenital cytomegalovirus saliva screening results with an FDA-approved assay at two institutions. J Clin Virol 166:105527. doi: 10.1016/j.jcv.2023.105527 [DOI] [PubMed] [Google Scholar]
- 97. Cannon MJ, Hyde TB, Schmid DS. 2011. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 21:240–255. doi: 10.1002/rmv.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nijman J, van Loon AM, de Vries LS, Koopman-Esseboom C, Groenendaal F, Uiterwaal C, Verboon-Maciolek MA. 2012. Urine viral load and correlation with disease severity in infants with congenital or postnatal cytomegalovirus infection. J Clin Virol 54:121–124. doi: 10.1016/j.jcv.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 99. Hayden RT, Sun Y, Tang L, Procop GW, Hillyard DR, Pinsky BA, Young SA, Caliendo AM. 2017. Progress in quantitative viral load testing: variability and impact of the WHO quantitative international standards. J Clin Microbiol 55:423–430. doi: 10.1128/JCM.02044-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kabani N, Pinninti S, Boppana S, Fowler K, Ross S. 2023. Urine and saliva viral load in children with congenital cytomegalovirus infection. J Pediatric Infect Dis Soc 12:230–233. doi: 10.1093/jpids/piad013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. De Cuyper E, Acke F, Keymeulen A, De Leenheer EMR, Van Hoecke H, Padalko E, Boudewyns A, Gilles A, Muylle M, Kuhweide R, Royackers L, Desloovere C, Verstreken M, Schatteman I, Dhooge I. 2023. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg 149:122–130. doi: 10.1001/jamaoto.2022.4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fourgeaud J, Magny J-F, Couderc S, Garcia P, Maillotte A-M, Benard M, Pinquier D, Minodier P, Astruc D, Patural H, Ugolin M, Parat S, Guillois B, Garenne A, Guilleminot T, Parodi M, Bussières L, Ville Y, Leruez-Ville M. 2023. Clinical value of serial quantitative analysis of cytomegalovirus DNA in blood and saliva over the first 24 months of life in congenital infection: the French Cymepedia cohort. J Pediatr 253:197–204. doi: 10.1016/j.jpeds.2022.09.040 [DOI] [PubMed] [Google Scholar]
- 103. Shibata M, Takano H, Hironaka T, Hirai K. 1994. Detection of human cytomegalovirus DNA in dried newborn blood filter paper. J Virol Methods 46:279–285. doi: 10.1016/0166-0934(94)90111-2 [DOI] [PubMed] [Google Scholar]
- 104. Barbi M, Binda S, Primache V, Caroppo S, Didò P, Guidotti P, Corbetta C, Melotti D. 2000. Cytomegalovirus DNA detection in Guthrie cards: a powerful tool for diagnosing congenital infection. J Clin Virol 17:159–165. doi: 10.1016/s1386-6532(00)00089-5 [DOI] [PubMed] [Google Scholar]
- 105. de Vries JJC, Claas ECJ, Kroes ACM, Vossen A. 2009. Evaluation of DNA extraction methods for dried blood spots in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 46 Suppl 4:S37–S42. doi: 10.1016/j.jcv.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 106. Kim JH, Robles V, Weimer KED, Gehtland LM, Kucera KS. 2023. Improved dried blood spot PCR assay for universal congenital cytomegalovirus screening in newborns. Microbiol Spectr 11:e0404122. doi: 10.1128/spectrum.04041-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vercauteren KOA, Keymeulen A, Mahieu L, Cossey V, Casaer A, Van Mol C, Smets K, Padalko E. 2020. Prospective multicenter comparison of urine culture with PCR on dried blood spots using 2 different extraction and PCR methods in neonates suspected for congenital cytomegalovirus infection. Diagn Microbiol Infect Dis 97:115051. doi: 10.1016/j.diagmicrobio.2020.115051 [DOI] [PubMed] [Google Scholar]
- 108. Misono S, Sie KCY, Weiss NS, Huang M-L, Boeckh M, Norton SJ, Yueh B. 2011. Congenital cytomegalovirus infection in pediatric hearing loss. Arch Otolaryngol Head Neck Surg 137:47–53. doi: 10.1001/archoto.2010.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barbi M, Binda S, Caroppo S, Ambrosetti U, Corbetta C, Sergi P. 2003. A wider role for congenital cytomegalovirus infection in sensorineural hearing loss. Pediatr Infect Dis J 22:39–42. doi: 10.1097/00006454-200301000-00012 [DOI] [PubMed] [Google Scholar]
- 110. Walter S, Atkinson C, Sharland M, Rice P, Raglan E, Emery VC, Griffiths PD. 2008. Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Arch Dis Child Fetal Neonatal Ed 93:F280–F285. doi: 10.1136/adc.2007.119230 [DOI] [PubMed] [Google Scholar]
- 111. Korver AMH, de Vries JJC, Konings S, de Jong JW, Dekker FW, Vossen ACTM, Frijns JHM, Oudesluys-Murphy AM, DECIBEL collaborative study group . 2009. DECIBEL study: congenital cytomegalovirus infection in young children with permanent bilateral hearing impairment in the Netherlands. J Clin Virol 46 Suppl 4:S27–S31. doi: 10.1016/j.jcv.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 112. Furutate S, Iwasaki S, Nishio S, Moteki H, Usami S. 2011. Clinical profile of hearing loss in children with congenital cytomegalovirus (CMV) infection: CMV DNA diagnosis using preserved umbilical cord. Acta Otolaryngol 131:976–982. doi: 10.3109/00016489.2011.583268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Del Valle Penella A, Miller J, Rochat R, Demmler-Harrison G. 2023. Utility of dried blood spots for the diagnosis of congenital cytomegaloviruses within the first 21 days of life in a single center. Int J Neonatal Screen 9:44. doi: 10.3390/ijns9030044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang L, Xu X, Zhang H, Qian J, Zhu J. 2015. Dried blood spots PCR assays to screen congenital cytomegalovirus infection: a meta-analysis. Virol J 12:60. doi: 10.1186/s12985-015-0281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ikeda S, Tsuru A, Moriuchi M, Moriuchi H. 2006. Retrospective diagnosis of congenital cytomegalovirus infection using umbilical cord. Pediatr Neurol 34:415–416. doi: 10.1016/j.pediatrneurol.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 116. Koyano S, Araki A, Hirano Y, Fujieda K, Suzutani T, Yagyu K, Murono K, Inoue N. 2004. Retrospective diagnosis of congenital cytomegalovirus infection using dried umbilical cords. Pediatr Infect Dis J 23:481–482. doi: 10.1097/00006454-200405000-00028 [DOI] [PubMed] [Google Scholar]
- 117. Uematsu M, Haginoya K, Kikuchi A, Hino-Fukuyo N, Ishii K, Shiihara T, Kato M, Kamei A, Kure S. 2016. Asymptomatic congenital cytomegalovirus infection with neurological sequelae: a retrospective study using umbilical cord. Brain Dev 38:819–826. doi: 10.1016/j.braindev.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 118. Reyes A, Taravillo I, Moral N, Moraleda C, Blázquez-Gamero D, Folgueira L. 2020. Feasible alternatives to DBS in the retrospective diagnosis of congenital cytomegalovirus infection. J Clin Virol 129:104504. doi: 10.1016/j.jcv.2020.104504 [DOI] [PubMed] [Google Scholar]
- 119. Garofoli F, Civardi E, Zanette S, Angelini M, Perotti G, Zecca M, Lombardi G. 2021. Literature review and an Italian hospital experience about post-natal CMV infection acquired by breast-feeding in very low and/or extremely low birth weight infants. Nutrients 13:660. doi: 10.3390/nu13020660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chung ML, Sung H, Jung E, Lee BS, Kim KS, Kim E-R. 2023. Prevention of human milk-acquired cytomegalovirus infection in very-low-birth-weight infants. BMC Pediatr 23:244. doi: 10.1186/s12887-023-04044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mukhopadhyay S, Itell HL, Hartman E, Woodford E, Dhudasia MB, Steppe JT, Valencia S, Roark H, Wade KC, Weimer KED, Permar SR, Puopolo KM. 2022. Breast milk and saliva for postnatal cytodaggermegalovirus screening among very low birth weight infants. Pediatr Infect Dis J 41:904–910. doi: 10.1097/INF.0000000000003671 [DOI] [PubMed] [Google Scholar]
- 122. Revello MG, Tibaldi C, Masuelli G, Frisina V, Sacchi A, Furione M, Arossa A, Spinillo A, Klersy C, Ceccarelli M, Gerna G, Todros T, Group CS. 2015. Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine 2:1205–1210. doi: 10.1016/j.ebiom.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pinninti S, Boppana S. 2023. Antiviral treatment of maternal and congenital cytomegalovirus (CMV) infections. Viruses 15:2116. doi: 10.3390/v15102116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hughes BL, Clifton RG, Rouse DJ, Saade GR, Dinsmoor MJ, Reddy UM, Pass R, Allard D, Mallett G, Fette LM, et al. 2021. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med 385:436–444. doi: 10.1056/NEJMoa1913569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G, Group CS. 2014. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214 [DOI] [PubMed] [Google Scholar]
- 126. Demmler-Harrison GJ. 2023. Congenital cytomegalovirus infection: management and outcome. UpToDate. [Google Scholar]
- 127. D’Antonio F, Marinceu D, Prasad S, Khalil A. 2023. Effectiveness and safety of prenatal valacyclovir for congenital cytomegalovirus infection: systematic review and meta-analysis. Ultrasound Obstet Gynecol 61:436–444. doi: 10.1002/uog.26136 [DOI] [PubMed] [Google Scholar]
- 128. Fisher SA, Miller ES, Yee LM, Grobman WA, Premkumar A. 2022. Universal first-trimester cytomegalovirus screening and valaciclovir prophylaxis in pregnant persons: a cost-effectiveness analysis. Am J Obstet Gynecol MFM 4:100676. doi: 10.1016/j.ajogmf.2022.100676 [DOI] [PubMed] [Google Scholar]
- 129. Shahar-Nissan K, Pardo J, Peled O, Krause I, Bilavsky E, Wiznitzer A, Hadar E, Amir J. 2020. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet 396:779–785. doi: 10.1016/S0140-6736(20)31868-7 [DOI] [PubMed] [Google Scholar]
- 130. Egloff C, Sibiude J, Vauloup-Fellous C, Benachi A, Bouthry E, Biquard F, Hawkins-Villarreal A, Houhou-Fidouh N, Mandelbrot L, Vivanti AJ, Picone O. 2023. New data on efficacy of valacyclovir in secondary prevention of maternal-fetal transmission of cytomegalovirus. Ultrasound Obstet Gynecol 61:59–66. doi: 10.1002/uog.26039 [DOI] [PubMed] [Google Scholar]
- 131. Cytomegalovivirus . Redbook: 2021-2024 report of the committee on infectious disease. AAp Publications. [Google Scholar]
- 132. Gantt S, Dionne F, Kozak FK, Goshen O, Goldfarb DM, Park AH, Boppana SB, Fowler K. 2016. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr 170:1173–1180. doi: 10.1001/jamapediatrics.2016.2016 [DOI] [PubMed] [Google Scholar]
- 133. Fowler KB, Ross SA, Shimamura M, Ahmed A, Palmer AL, Michaels MG, Bernstein DI, Sánchez PJ, Feja KN, Stewart A, Boppana S. 2018. Racial and ethnic differences in the prevalence of congenital cytomegalovirus infection. J Pediatr 200:196–201. doi: 10.1016/j.jpeds.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 134. Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. 2014. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol 24:291–307. doi: 10.1002/rmv.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schleiss MR, Panther L, Basnet S, Workneh M, Diaz-Decaro J. 2023. Comparison of overall sensitivity and specificity across different newborn screening algorithms for congenital cytomegalovirus. Int J Neonatal Screen 9:33. doi: 10.3390/ijns9020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sanchez-Durán MA, Maiz N, Liutsko L, Bielsa-Pascual J, García-Sierra R, Zientalska AM, Velasco I, Vazquez E, Gracia O, Ribas A, Sitja N, Nadales M, Martinez C, Gonce A, Frick MA, Guerrero-Martínez M, Violán C, Torán P, Falguera-Puig G, Gol R, CITEMB Group . 2023. Universal screening programme for cytomegalovirus infection in the first trimester of pregnancy: study protocol for an observational multicentre study in the area of Barcelona (CITEMB study). BMJ Open 13:e071997. doi: 10.1136/bmjopen-2023-071997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Saldan A, Forner G, Mengoli C, Gussetti N, Palù G, Abate D. 2017. Testing for cytomegalovirus in pregnancy. J Clin Microbiol 55:693–702. doi: 10.1128/JCM.01868-16 [DOI] [PMC free article] [PubMed] [Google Scholar]