Abstract
Induction of mucosal and cell-mediated immunity is critical for development of an effective vaccine against human immunodeficiency virus (HIV). We compared intramuscular and intranasal immunizations with a DNA vaccine encoding env of HIV-1 and evaluated the QS-21 saponin adjuvant for augmentation of the systemic and mucosal immune responses to HIV-1 in a murine model. Vaccination via the two routes elicited comparable systemic immune responses, and QS-21 consistently enhanced antigen-specific serum immunoglobulin G2a (IgG2a) production, delayed-type hypersensitivity reaction, and cytolytic activity of splenocytes. Intestinal secretory IgA production and cytolytic activity of the mesenteric lymph node cells are preferentially elicited by intranasal immunization, and QS-21 augmented these activities as well. This adjuvant augmented production of interleukin-2 (IL-2) and gamma interferon (IFN-γ) associated with decrease in IL-4 synthesis by antigen-restimulated splenocytes. The serum immunoglobulin subtype profile showed a dominant IgG2a response and less strong IgG1 and IgE production in a QS-21 dose-dependent manner. As expected, enhancements of humoral and cell-mediated immune responses by QS-21 were abrogated by treatment with anti-IL-2 and anti-IFN-γ monoclonal antibodies. These results suggest that the intranasal route of DNA immunization is more efficient than the intramuscular route in inducing mucosal immunity mediated by sIgA and mesenteric lymphocytes. Furthermore, QS-21 is able to act as a mucosal adjuvant in DNA vaccination and demonstrates its immunomodulatory property via stimulation of the Th1 subset. This study emphasizes the importance of the route of immunization and the use of an adjuvant for effective DNA vaccination against HIV-1.
Vaccination by direct injection of plasmid DNA encoding viral antigens has been attempted in several animal models (9, 10, 24, 35, 47, 49). Furthermore, previous studies have demonstrated the potential value of DNA vaccination in protecting the host from exposure to live viruses (10, 18, 47). Boyer et al. showed a successful DNA vaccination against laboratory-isolated human immunodeficiency virus type 1 (HIV-1) in a nonhuman primate model (4). Hence, this novel vaccination approach may have some potential for controlling certain viral infections where conventional vaccines have failed.
In the history of vaccine development since Jenner’s innovation, a major goal has been enhancement of vaccine immunogenicity. An attractive approach to achieve this goal is the incorporation of immunologic adjuvants into a vaccine formulation. In fact, a number of adjuvants have been explored and have showed respectable facilitating effect on immune responses to polypeptide-based vaccine antigens (37, 48). Furthermore, we and others have attempted to boost the immune response to DNA vaccines using immunologic adjuvants (12, 20, 39–42), and such attempts have been successful.
Following up on previous studies (12, 20, 39–42), we intended to evaluate another potential adjuvant in DNA vaccination. The QS-21 saponin adjuvant (15) was chosen due to its capacity to induce interleukin-2 (IL-2) and gamma interferon (IFN-γ) (16), which are known to be crucial in enhancing DNA-derived cell-mediated immunity (8, 34, 46). In addition, this adjuvant has also been shown to augment the specific cytotoxic T-lymphocyte (CTL) response to ovalbumin and HIV-1 env subunit antigen vaccines in mice (33, 51) and to simian immunodeficiency virus env subunit vaccines in a rhesus macaque model (32). The current study was designed to evaluate whether QS-21 enhances humoral and cell-mediated immunity induced by DNA vaccination against HIV-1. T-helper type 1 (Th1) and Th2 cytokine function in QS-21-mediated DNA vaccination was also examined. We show that QS-21 acts as an effective adjuvant for an HIV-1 env-based DNA vaccine administered via both the intramuscular (i.m.) and the intranasal (i.n.) routes.
MATERIALS AND METHODS
Experimental animals.
Female BALB/c mice (8 to 10 weeks old) were purchased from Japan SLC Inc., Shizuoka, Japan, and were used for the vaccination studies. BALB/cAnNCrj-nu mice (Japan SLC) were used to produce anticytokine monoclonal antibodies (MAbs) by transplantation of the hybridoma cell lines which secrete objective MAbs. All mice were furnished with access to sterile food and water.
Plasmid DNA and MAbs.
Immunogenic DNAs, pCMV160IIIB and pcREV, which encode the env and rev genes of HIV-1IIIB, respectively, were described in our previous report (35). Although our DNA vaccine formulation was designed to elicit an env-specific immune response, the rev expression plasmid was included because a previous study (27) showed that expression of env protein is dependent on rev coexpression.
The hybridoma cell lines S4B6 (obtained from American Type Culture Collection, Rockville, Md.) and XMG1.2 (kindly provided by J. Miller, DNAX Research Institute, Palo Alto, Calif.), each of which secretes a rat MAb neutralizing mouse IL-2 and IFN-γ, were injected into the peritoneal cavity of the BALB/cAnNCrj-nu mice, and purified MAbs were obtained from the ascites by using an Ampure PA kit (Amersham Japan, Tokyo, Japan). As a control, GL113, a rat MAb to β-galactosidase (β-Gal), also provided by J. Miller, was prepared by the same procedure.
Vaccine formulations and animal treatment.
Purified QS-21 was prepared as described elsewhere (15). Plasmids pCMV160IIIB (IIIB) and pcREV (REV) were mixed with the indicated doses of QS-21. The DNA dose for immunization was 5 μg each of IIIB and REV (total, 10 μg) per mouse in both the i.m. application and the i.n. application. For i.m. immunization, the immunogen and adjuvant were diluted with sterile saline and 100 μl was injected into the biceps femoris muscle with the Williams needle (50). For i.n. immunization, mice were anesthetized with diethyl ether, and 30 μl of the prepared vaccine also diluted with sterile saline was dropped into the nostril gradually to prevent suffocation. The mice were therefore able to inhale the vaccine preparation in a natural manner. Inoculations via both the i.m. route and the i.n. route were performed twice at a 2 week interval. To determine whether QS-21 acted regionally at the administered site or systemically, some mice were separately inoculated with the immunogen and the adjuvant into a nostril and a leg, respectively, or into contralateral legs. To clarify the roles of IL-2 and IFN-γ in the mechanism of the adjuvant action, MAbs to mouse IFN-γ or IL-2 (derived from XMG1.2 and S4B6, respectively) were used to neutralize these cytokines in vivo. Experimental mice were injected intraperitoneally with 100 μg of anti-IFN-γ or anti-IL-2 MAb at 3- or 4-day intervals (twice per week) from the day of the initial immunization until the assay was performed. Control mice were treated with anti-β-Gal MAb (GL113) with the same protocol.
EIA for antibody titration and cytokine measurement.
Enzyme immunoassay (EIA) was used for titration of the antigen-specific serum immunoglobulin G (IgG) and intestinal secretory IgA (sIgA) responses, determination of the specific immunoglobulin subtype, and quantification of the cytokines produced by in vitro-restimulated splenic mononuclear cells. Sample blood was collected by retro-orbital puncture at 2 weeks after the second immunization, and the assay was performed as follows. A gp160 protein of HIV-1IIIB (courtesy of B. Wharren, Karolinska Institute, Stockholm, Sweden) was employed as an antigen for detection of serum antibody responses to HIV-1IIIB env. It was coated on 96-well microtiter plates (Nunc, Roskilde, Denmark); after blocking with 3% bovine serum albumin in phosphate-buffered saline serially diluted antisera were added and incubated at 37°C for 2 h. Peroxidase-conjugated goat anti-mouse IgG (Organon Teknika Corp., West Chester, Pa.) was used as the secondary antibody; then, plates were stained with 3,3′,5,5′-tetramethylbenzidine (DAKO Corp., Carpinteria, Calif.). The antigen-specific intestinal sIgA response was measured in a fecal extract. Fecal samples were prepared as described elsewhere (34). For the estimation of the sIgA response, a peptide from the principal neutralizing determinant of HIV-1IIIB (NNTRKSIRIQRGPGRAFVTIGKIGN) was constructed with a multiple antigenic peptide system and used as a coating antigen. Secondary antibody was rabbit anti-rat secretory component antibody (provided generously by B. Underdown, McMaster University). Titers are expressed as the reciprocal log2 value of the final detectable dilution, which was defined as 2 standard deviations above the mean optical density at 450 nm of preimmune samples at the same titration point. The antigen-specific IgG1, IgG2a, and IgE responses were determined as relative amounts at the same time point. Horseradish peroxidase-coupled anti-mouse IgG1 or IgG2a (Organon Teknika) or IgE (Southern Biotechnology Associates, Inc., Birmingham, Ala.) was used as the secondary antibody, and the results are presented as optical density at 450 nm.
The cytokine profile of the mice inoculated by i.m. injection was also estimated with EIA. For quantification of IL-2, IFN-γ (representative Th1-type cytokine), and IL-4 (representative Th2-type cytokine), spleens were harvested at 2 weeks after the second immunization, and freshly isolated splenic mononuclear cells were cultured in the presence of the V3 peptide. This peptide, RGPGRAFVTIGK, contains both a helper epitope (22) and a CTL epitope (45) for HIV-1IIIB. Then culture media were collected at 48 h after the start of cell culture, and cell-free supernatants were stored at −80°C until assayed. Cytokine amounts in these samples were measured with a commercial EIA kit (Cytoscreen; BioSource International, Camarillo, Calif.) according to the manufacturer’s instructions.
DTH response.
The delayed-type hypersensitivity (DTH) reaction was assayed by a footpad swelling test. Two weeks after the second immunization, mice were injected with 5 μg of the V3 peptide into the right footpad. The same amount of a sperm whale myoglobin peptide, ALVEADVA (36), was injected into the left footpad as a control. After 24 h, the extent of the footpad swelling was measured with a dial thickness gauge (Ozaki Seisakusho, Tokyo, Japan) as the difference in footpad thickness between the pre- and postinjection measurements in units of 10−2 mm.
Determination of cytolytic activity.
Cytolytic activity was assayed in splenic mononuclear cells from i.m.- and i.n.-immunized animals and in mesenteric lymphoid cells from i.n.-immunized animals. Also 2 weeks after the second immunization, splenic or mesenteric lymphocytes were harvested and cultured in the presence of irradiated syngeneic spleen cells pulsed with the V3 peptide. The target cells were 51Cr-sodium chromate-labeled syngeneic cell lines (P815; H-2d) pulsed with or without the same peptide. The latter was prepared to evaluate nonspecific cytolytic activity. After being cultured for 5 days, bulk splenic mononuclear cells as effectors were cocultivated with the target cells pulsed with the V3 peptide at effector/target (E/T) ratios ranging from 5:1 to 80:1. Non-peptide-pulsed targets were mixed with the effectors at an E/T ratio of 80:1 only. Target cell lysis was measured by gamma ray counting of cell-free supernatants to determine the amount of 51Cr released. The percentage of chromium release was calculated as 100 × (Cr release in sample − spontaneous Cr release)/(maximum Cr release − spontaneous Cr release). Target cells incubated in the medium with or without 5% Triton X-100 were used to determine maximum or spontaneous 51Cr release, respectively.
Statistical analysis.
All values obtained from experiments were expressed as means ± standard errors of the means (SEM). Data were analyzed by one-way factorial analysis of variance, and significance was defined as P < 0.05.
RESULTS
QS-21 has an immunomodulatory effect on humoral immunity.
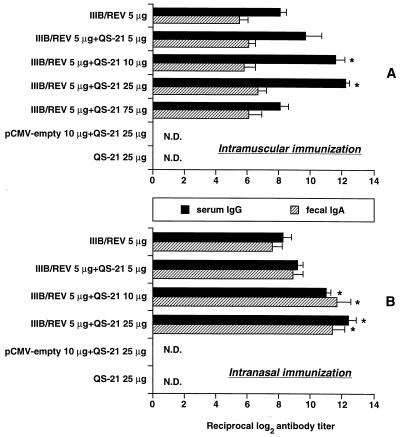
Figure 1 shows HIV-1IIIB-specific serum IgG and intestinal sIgA titers of mice inoculated with the cocktail of IIIB/REV and QS-21 by i.m. injection or i.n. inhalation. Samples were collected 2 weeks after the booster immunization. Both the 10- and the 25-μg doses of QS-21 significantly enhanced the antigen-specific serum IgG response elicited by the i.m. and i.n. immunizations. Serum antibody titers for i.m. and i.n. immunizations were almost the same. In contrast to the lower doses, the 75-μg dose of QS-21 did not enhance the IgG response to i.m. vaccination. Although i.m. vaccination could elicit a detectable fecal sIgA response, no facilitating effect of QS-21 on intestinal antibody production was noted. However, when 10 or 25 μg of QS-21 was administered via the i.n. route, significant enhancement of the antigen-specific sIgA titer was observed. Twenty-five micrograms of QS-21, either alone or with the empty vector, did not produce a detectable antibody response. These results indicate that the optimal dose of QS-21 to elicit an enhanced antibody response to IIIB/REV was 10 to 25 μg per mouse, and QS-21 certainly augmented the DNA-derived antibody response. In i.n. immunization, a 75-μg dose of QS-21 made mice sluggish and cyanotic, and some of them died due to probable respiratory failure. This suggests that a high dose of QS-21 has toxicity in mice when administered via the i.n. route; therefore, we discontinued the use of the 75-μg dose in i.n. immunization.
FIG. 1.
HIV-1-specific antibody responses induced by DNA vaccination via the i.m. (A) and i.n. (B) routes. BALB/c mice were immunized with 5 μg of IIIB/REV formulated with the indicated doses of QS-21. Inoculation was performed twice with a 2-week interval. The HIV-1 env-specific antibody titers were determined by EIA in duplicate on samples collected 2 weeks following the second immunization. The results are expressed as means ± SEM for six (experimental group) or four (control group) mice. ∗, significant enhancement of the antibody response compared with IIIB/REV alone (P < 0.05). Similar results were obtained in a repeat experiment (not shown). N.D., not detected.
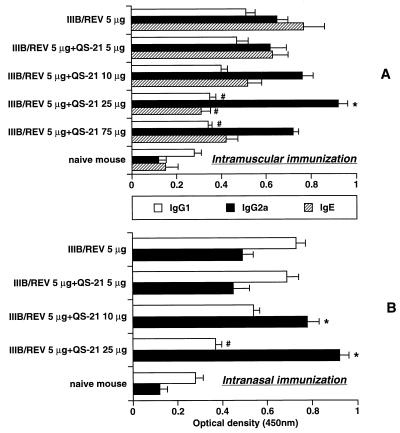
Figure 2 illustrates the relative amounts of antigen-specific IgG1, IgG2a, and IgE in the sera obtained from i.m.- and i.n.-immunized animals. Among the serum IgG subclasses, i.m. immunization without the adjuvant resulted in an IgG2a titer that was higher than the IgG1 titer, whereas i.n. immunization resulted in an IgG1 titer that was higher than the IgG2a titer. QS-21 increased the level of IgG2a elicited by the 25-μg dose administered by the i.m. route and the 10- and 25-μg doses by the i.n. route. The amount of IgG1 was decreased in a QS-21 dose-dependent manner for both routes. The IgE response in i.n.-immunized animals was similar to the IgG1 profile (data not shown). In i.m.-immunized animals, the IgG2a response was always greater than the IgG1 response irrespective of whether the adjuvant was used, and IgG1 and IgE levels were decreased with a dose escalation of QS-21. The relationship between the Th1-Th2 dichotomy and predominant immunoglobulin isotype has been established (31): IgG1 and IgG2a are classified as the Th2- and Th1-type responses, respectively. Our data therefore suggest that 25 μg of QS-21 elicited maximal activation of the Th1 subset via both immunization routes. The samples obtained from the i.m. group 2 weeks following the primary immunization showed similar IgG titers irrespective of QS-21 administration (antibody titer means ± SEM for IIIB/REV with and without 25 μg of QS-21, 10.8 ± 0.7 and 9.7 ± 0.8, respectively, for i.m. immunization). These results suggested that a booster immunization was required for enhancing the antibody response when QS-21 was used as adjuvant.
FIG. 2.
HIV-1-specific serum immunoglobulin subtype profiles induced by QS-21-mediated DNA vaccination via the i.m. (A) and i.n. (B) routes. The inoculation procedure and the time of blood collection were the same as those for Fig. 1. The subtype profile was determined by EIA and is presented as optical density at 450 nm. The results are expressed as means ± SEM for five or six (experimental group) or three or four (control group) mice. ∗ and #, significant enhancement or decrease in the antibody amount, respectively, compared with IIIB/REV alone (P < 0.05). Similar results were obtained in a repeat experiment (not shown).
Adjuvant activity of QS-21 for cell-mediated immune responses.
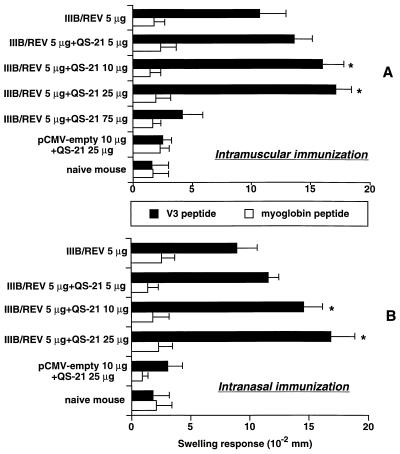
The DTH reaction was also assayed at 2 weeks after the booster immunization (Fig. 3). Similarly to the IgG response in antibody measurement, i.n. immunization could elicit a DTH reaction comparable to that produced by i.m. immunization. As the figure shows, a 25-μg dose of QS-21 was optimal for eliciting maximal footpad swelling via both immunization routes. In a separate experiment, the swelling response measured at 2 weeks after the initial i.m. immunization yielded results similar to those obtained after the booster immunization (Fig. 2A) (swelling response means ± SEM [in 10−2 mm] for IIIB/REV with and without 25 μg of QS-21, 16.1 ± 0.9 and 10.4 ± 1.2, respectively). These data suggest that the adjuvant-augmented DTH response did not require the booster immunization. Similarly to the results of antibody measurement, i.n. immunization could elicit a DTH reaction comparable to that produced by i.m. immunization.
FIG. 3.
DTH response induced by the V3 peptide (helper epitope of HIV-1IIIB). BALB/c mice were inoculated as described in the legend for Fig. 1. (A and B) Dose-related DTH reactions induced by the i.m. and i.n. immunizations, respectively. The DTH response was assayed by the footpad swelling test 2 weeks following the second immunization. For the test, mice were injected with V3 and the myoglobin peptide into the right and left footpads, respectively, and the swelling responses 24 h after the peptide injection were measured with a dial thickness gauge. The mean swelling responses ± SEM for five or six (experimental group) or four (control group) mice are given in 10−2 mm. ∗, significant enhancement of the swelling response compared with IIIB/REV alone (P < 0.05). Similar results were obtained in a separate experiment.
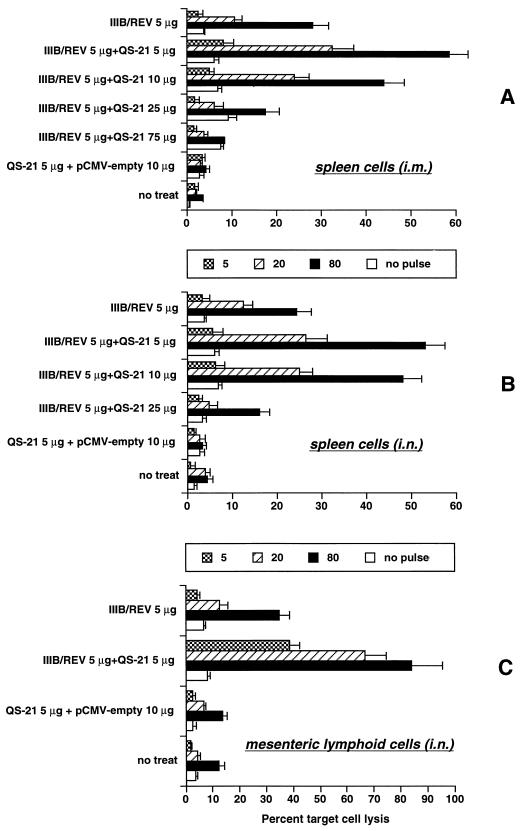
Cytolytic activities of the splenocytes and mesenteric lymphocytes were also tested at the same time point. As Fig. 4 shows, a strongly enhanced specific cytolysis was attained with 5 μg of QS-21 via both immunization routes. Unexpectedly, the 25-μg dose diminished the cytolytic activity in spite of a consistent enhancement of the antibody production and DTH reaction. The optimal QS-21 doses for activation of CD8+ CTL and CD4+ helper T cells, which are responsible for cytolytic and DTH responses, respectively, may be different in DNA vaccination. i.n. immunization preferentially induced cytolytic activity of mesenteric lymphoid cells, whereas i.m. immunization did not elicit detectable cytolysis by these cells (data not shown). Furthermore, a 5-μg dose of QS-21 prominently enhanced cytolytic activity of mesenteric lymphoid cells by i.n. immunization.
FIG. 4.
Cytolytic activity of the immune lymphoid cells harvested from animals receiving QS-21-mediated DNA vaccination as described in the legend to Fig. 1. (A to C) Cytolytic activities of splenic mononuclear cells from i.m.-immunized mice, splenic mononuclear cells from i.n.-immunized mice, and mesenteric lymphoid cells from i.n.-immunized mice, respectively. Splenocytes were harvested and cultured with the V3 peptide (a CTL epitope of HIV-1IIIB) for 5 days. Syngeneic cells (P815 cells; H-2d) pulsed with or without the same peptide were used as targets, and percent target cell lysis was determined by a 5-h 51Cr-releasing assay. The activity was titrated at E/T ratios of 5, 20, and 80. Nonspecific cytolytic activity (open bars) was measured at an E/T ratio of 80. The results were determined by duplicate assays and are presented as means ± SEM for three to six mice for each group.
We also evaluated whether the adjuvant could be utilized systemically. When the immunogen and adjuvant were separately injected into contralateral legs, no enhancement of the DTH reaction or cytolytic response was observed (data not shown). Similarly, mice receiving DNA i.n. and adjuvant i.m. did not show enhanced CTL activity. Hence, the adjuvanticity of QS-21 is apparently exerted locally at the site of DNA administration but not systemically.
Influence of QS-21 upon cytokine secretion by splenocytes from animals receiving an i.m. DNA immunization.
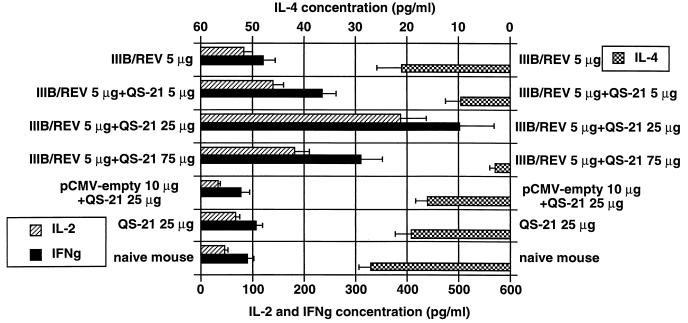
Th1- and Th2-type cytokines produced by the restimulated bulk spleen cells from i.m.-immunized mice were measured by EIA. As Fig. 5 shows, the 25-μg dose of QS-21 elicited maximal production of IL-2 and IFN-γ and a decrease in IL-4 synthesis, suggesting that the Th1 subset was maximally activated with this dose. This correlates with the immunoglobulin subtype profile shown in Fig. 2A.
FIG. 5.
Cytokine amounts in the culture media of the splenocytes harvested from the mice inoculated with the vaccine formulated as shown. Mice were treated as specified in the legend to Fig. 1, and the cells were also harvested at 2 weeks after the second immunization. These cells were cultured in the presence of the V3 peptide, and 48 h later, cell-free supernatants were collected and subjected to the cytokine enzyme-linked immunosorbent assay by using appropriate assay kits. The results were determined by a duplicate assay and are presented as means ± SEM for three and six mice for control and experimental groups, respectively.
Role of Th1-type cytokines in immune responses produced by an i.m. DNA immunization with the QS-21 adjuvant.
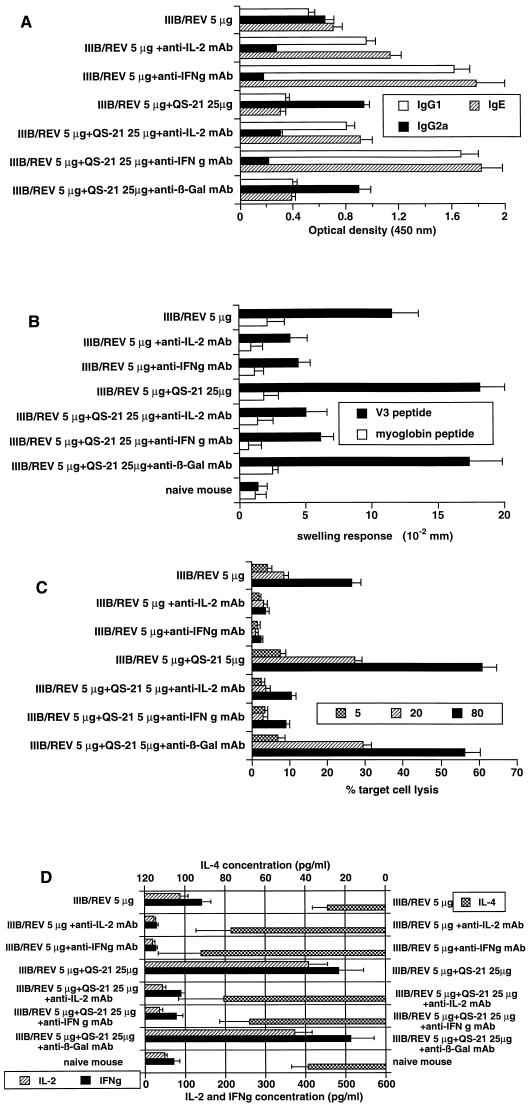
To clarify the roles of IL-2 and IFN-γ in augmentation of the humoral and cell-mediated immunity induced by the QS-21 adjuvant DNA vaccination, mice receiving two i.m. immunizations with IIIB/REV alone or formulated with QS-21 were treated with an intraperitoneal injection of anti-mouse IL-2 and IFN-γ MAbs. As shown in Fig. 6A, injection of these MAbs notably altered the immunoglobulin subtype profile, with IgG1 and IgE being markedly increased and IgG2a being decreased. These findings indicate that the Th2-type response was dominant and the Th1-type response was suppressed in animals treated with the MAbs to these Th1-type cytokines. Enhancement of IgG2a production by QS-21 is therefore suggested to be mediated through IL-2 and IFN-γ. As for the DTH reaction, a significant drop in the swelling response was observed when mice were treated with these MAbs whereas administration of an anti-β-Gal MAb (GL113) did not affect the enhanced response (Fig. 6B). In the CTL assay, injection of anticytokine MAbs abrogated the cytolytic response irrespective of the presence or absence of QS-21; the control MAb GL113 had no effect on the response (Fig. 6C). These results show the pivotal roles of IL-2 and IFN-γ in generation and enhancement of the DTH reaction and the CTL response induced by the QS-21 adjuvant DNA vaccination.
FIG. 6.
Inhibition of DNA-derived humoral and cell-mediated immunity by treatment with anticytokine MAbs. (A to C) Influences of the antibodies on the serum antibody profile, DTH reaction, and cytolytic response, respectively. MAbs were intraperitoneally injected at 3- or 4-day intervals (twice per week) from the day of primary immunization until the day of assay. An anti-β-Gal MAb was used as a control antibody. (D) Inhibition of cytokine production by in vivo treatment with anticytokine MAbs. The procedure for determination of each response and mode of data presentation are identical to those for Fig. 2 to 5.
The in vivo neutralizing capability of the MAbs to IL-2 and IFN-γ (S4B6 and XMG1.2) in our assay system was verified also by cytokine EIA on restimulated splenocytes from i.m.-immunized mice treated with these MAbs (Fig. 6D). As expected, the predominant synthesis of IL-4 associated with a notable drop in IL-2 and IFN-γ production was observed in the splenocytes from groups injected with these antibodies whereas the negative-control anti-β-Gal MAb (GL113) did not affect the cytokine production.
DISCUSSION
We undertook the present study to evaluate the immunomodulatory effect of the QS-21 adjuvant on the systemic and mucosal immunity induced by i.m. and i.n. DNA vaccinations for HIV-1. This adjuvant showed substantial facilitating effects on the antigen-specific serum and intestinal antibody responses (Fig. 1 and 2) and cell-mediated immune responses (Fig. 3 and 4). Th1- and Th2-type cytokine profiles were examined in i.m.-immunized animals, and the profile showed induction of Th1-type cytokines by QS-21 (Fig. 5). QS-21-mediated immune enhancement was nullified by anti-Th1-type cytokine MAbs administered in vivo (Fig. 6). Although QS-21 has been examined in various vaccination models (13, 23, 32, 51), to our knowledge this is the first published study using QS-21 together with DNA vaccines.
In a strategy for AIDS vaccine development, induction of a strong mucosal immunity and CTL activity to HIV-1 are pivotal tasks since the major route of HIV transmission is through mucosal tissues and CTLs can lyse infected cells directly. For this reason, we believe that consideration of both the use of adjuvant and optimal immunization route is quite important, as suggested in earlier reports (2, 34, 41). Because some adjuvants show adjuvanticity in DNA vaccination (12, 20, 39–42) as well as in peptide-based vaccination, systemic immunization routes do not generally induce substantial mucosal immunity (25, 29). In the present study, QS-21 demonstrated its immunomodulatory effect on systemic immunity and mucosal immunity, with the latter being enhanced by QS-21 via the i.n. immunization route but not via the i.m. immunization route (Fig. 1 and 4).
As for humoral immunity in mucosal tissues, we and others found that HIV-1-specific intestinal sIgA induced by a peptide (5, 43) or a DNA vaccine (34) is capable of neutralizing HIV-1 in vitro. HIV-1-specific sIgA in mucosal tissues therefore may act effectively in helping to block HIV penetration of the mucosal membrane. When the immunogenic DNA was formulated with QS-21 and administered via the i.n. route, intestinal sIgA production was significantly augmented in a QS-21 dose-dependent manner (Fig. 1). Accordingly, the i.n. route of DNA vaccination together with QS-21 may be more suitable than the i.m. route in view of the stimulation of sIgA antibody production; the elicitation of mucosal immunity observed in this study was consistent with that in earlier studies (2, 41).
Induction of HIV-specific CTL activity in the gastrointestinal or urogenital tract and associated lymphoid tissues is also considered to be important (6, 17), as is the systemic CTL response, for prevention of mucosa-associated HIV transmission. DNA vaccination via the i.n. route elicited cytolytic responses by both splenic and mesenteric lymphoid cells, and a 5-μg dose of QS-21 remarkably enhanced these CTL activities (Fig. 4). In the case of HIV infection via mucosal tissues, CTLs in the regional lymph nodes may be a barrier against HIV-infected cells. If the infected cells break through this barrier and HIV spreads into the bloodstream, systemic CTLs can disturb viral replication (1). Since a two-step barrier against HIV can be prepared with i.n. DNA–QS-21 vaccination, the i.n. route has advantages over the i.m. route in a murine model.
Most nasal- or oral-vaccination studies targeting mucosal immunity have used the cholera toxin (CT) adjuvant (5, 20, 43, 52). CT is classical but reliable as a mucosal adjuvant; however, it is classified as a Th2-type adjuvant (48) and is reported to induce a Th2-biased immunity to both polypeptide vaccination (43, 52) and DNA vaccination (20). Hence, CT is not considered suitable for inducing systemic Th1-type cell-mediated immunity, which is important to combat fresh HIV infection and to control disease progression of AIDS (1, 38).
QS-21 is a highly purified triterpene glycoside saponin isolated from the bark of the Quillaja saponaria Molina tree (15). This adjuvant has demonstrated consistent adjuvant activity in previous vaccination studies (13, 23, 32, 51) and preferentially elicits Th1-type immune responses (16, 26, 32). These observations suggest that QS-21 exhibits its adjuvancy through stimulation of the Th1 subset (30, 44). The current results indicate that this adjuvant did elicit Th1-type immune responses to the DNA vaccine (Fig. 2, 3, and 5) and enhancement of the humoral and cell-mediated immunity is mediated by IL-2 and IFN-γ (Fig. 5 and 6). Therefore, in the case of i.n. DNA vaccination, immunogenic DNA formulated with QS-21 may be more useful than the conventional CT adjuvant-mucosal vaccines because the former can induce a CTL activity as well as a mucosal sIgA response, both of which are important in controlling HIV infection.
Antigen-specific cytolytic activity was also enhanced by QS-21 (Fig. 4), but the optimal QS-21 dose was different from that for IgG2a production (Fig. 2), the DTH reaction (Fig. 3), and the cytokine secretion profile (Fig. 5). Five micrograms was optimal for the CTL response, whereas other immunological parameters (antibody and DTH) were maximally augmented by a 25-μg dose. Although a clear explanation for this discrepancy is impossible at present, it may be due to different QS-21 dose effects on activation of CD8+ CTL and CD4+ T-helper cells. Emphasis is placed on the fact that the cytolytic response is a downstream event of CD8+ T-lymphocyte activation whereas the IgG2a and DTH response or cytokine secretion itself directly reflects CD4+ T-helper-cell activity. The optimal conditions for inducing CD8+ CTL and CD4+ helper T cells could be different, as reported previously (3, 14), although Th1-type cytokines are necessary for CTL priming (28). In fact, repeated inoculation was necessary for QS-21-mediated CTL enhancement, whereas the DTH reaction was enhanced with a single immunization.
HIV-specific CTL activity is useful in preventing disease progression of AIDS and for protecting an individual from fresh HIV infection (1), whereas neutralizing antibody is effective at least for the prophylaxis of the infection or early treatment of AIDS (7). This suggests that, in a murine model, the optimal QS-21 dose for a therapeutic DNA AIDS vaccine formulation is 5 μg (for maximal CTL induction) and is 10 μg for a prophylactic vaccine (enhanced antibody titer and CTL activity).
The mechanism for DNA-derived immunity elicited by i.n. DNA administration, although important, remains an inadequately explored topic. It is known, however, that the surface of the nasal mucosa contains lymphoid tissues which are histologically similar in structure to Peyer’s patches (21). DNA-transfected antigen-presenting cells in nasal lymphoid tissue can migrate through lymph vessels and/or the bloodstream, which may result in their dissemination to remote lymphoid tissues such as those of the gut and spleen. Hypotheses similar to this have been advocated by other groups (10, 11). We believe that i.n. immunization is more likely to cause lymphoid cell dissemination to remote sites than i.m. immunization.
The implication from the present study is that QS-21 stimulates Th1-biased immunomodulation in DNA vaccination by both i.m. and i.n. DNA applications. The i.n. route of immunization is more efficient than the i.m. route in inducing mucosal immunity mediated by sIgA and mesenteric lymphocytes. These results may provide insight for the design of AIDS DNA vaccine formulations containing immunologic adjuvants. An essential follow-up to the present study is the evaluation of QS-21 adjuvant DNA vaccines in nonhuman primate models as an intermediate step between the murine model and phase I clinical trials. Direct DNA administration to urogenital or rectal mucosa is also necessary in these studies since suggestive data have been obtained for DNA inoculation into mucosal tissues in macaques (17) and humans (19). Finally, since major routes of QS-21 administration have been i.m. or subcutaneous (13, 23, 32, 51), investigation into the safety of i.n.-administered QS-21 should be also important, although QS-21 is reported to be safer than other products purified from bulk saponin (15).
ACKNOWLEDGMENTS
We express gratitude to B. Wahren for providing gp160 protein, J. Miller for hybridoma cell lines XMG1.2 and GL113, and B. Underdown for rabbit anti-rat secretory component antibody. We also thank T. Kaneko, A. Honsho, and K. Niikura for skillful technical assistance.
This work was partially supported by a grant-in-aid from the Yokohama Foundation of Medical Science Promotion.
REFERENCES
- 1.Ada G L, McElrath M J. HIV type 1 vaccine-induced cytotoxic T cell responses: potential role in vaccine efficacy. AIDS Res Hum Retroviruses. 1997;13:205–210. doi: 10.1089/aid.1997.13.205. [DOI] [PubMed] [Google Scholar]
- 2.Asakura Y, Hinkula J, Leandersson A C, Fukushima J, Okuda K, Wahren B. Induction of HIV-1 specific mucosal immune responses by DNA vaccination. Scand J Immunol. 1997;46:326–330. doi: 10.1046/j.1365-3083.1997.d01-146.x. [DOI] [PubMed] [Google Scholar]
- 3.Azuma M, Cayabyab M, Buck D, Phillips J H, Lanier L L. CD28 interaction with B7 costimulates primary allogenic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 5.Bukawa H, Sekigawa K, Hamajima K, Fukushima J, Yamada Y, Kiyono H, Okuda K. Neutralization of HIV-1 by secretory IgA induced by oral immunization with a new macromolecular multicomponent peptide vaccine candidate. Nat Med. 1995;1:681–685. doi: 10.1038/nm0795-681. [DOI] [PubMed] [Google Scholar]
- 6.Caley I J, Betts M R, Irlbeck D M, Davis N L, Swanstrom R, Frelinger J A, Johnston R E. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J Virol. 1997;71:3031–3038. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cease K B, Berzofsky J A. Toward a vaccine for AIDS: the emergence of immunobiology-based vaccine development. Annu Rev Immunol. 1994;12:923–989. doi: 10.1146/annurev.iy.12.040194.004423. [DOI] [PubMed] [Google Scholar]
- 8.Chow Y-H, Huang W-L, Chi W-K, Chu Y-D, Tao M-H. Improvement of hepatitis B virus DNA vaccine by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis H L, Mccluskie M J, Gerin J L, Purcell R H. DNA vaccine for hepatitis B—evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213–7218. doi: 10.1073/pnas.93.14.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines—protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harokopakis E, Hajishengallis G, Greenway T E, Russell M W, Michalek S M. Mucosal immunogenicity of a recombinant Salmonella typhimurium-cloned heterologous antigen in the absence or presence of coexpressed cholera toxin a2 and b subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii N, Fukushima J, Kaneko T, Okada E, Tani K, Tanaka S-I, Hamajima K, Xin K-Q, Kawamoto S, Koff W, Nishioka K, Yasuda T, Okuda K. Cationic liposomes are a strong adjuvant of DNA vaccine for a human immunodeficiency virus type-1 (HIV-1) AIDS Res Hum Retroviruses. 1997;13:1421–1428. doi: 10.1089/aid.1997.13.1421. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez de Baqüés M P, Elzer P H, Blasco J M, Marin C M, Gamazo C, Winter A J. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect Immun. 1994;62:632–638. doi: 10.1128/iai.62.2.632-638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keene J, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kensil C R, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 16.Kensil C R, Wu J Y, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. In: Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 525–541. [DOI] [PubMed] [Google Scholar]
- 17.Klavinskis L S, Bergmeier L A, Gao L, Mitchell E, Ward R G, Layton G, Brookes R, Meyers N J, Lehner T. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J Immunol. 1996;157:2521–2527. [PubMed] [Google Scholar]
- 18.Kodihalli S, Haynes J R, Robinson H L, Webster R G. Cross-protection among lethal H5N2 influenza viruses induced by DNA vaccine to the hemagglutinin. J Virol. 1997;71:3391–3396. doi: 10.1128/jvi.71.5.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozlowski P A, Cu U S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuklin N, Daheshia M, Karem K, Manickan E, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuper C F, Koornstra P J, Hameleers D M, Biewenga J, Spit B J, Duijvestijn A M, van Breda V P, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 22.Layton G T, Harris S J, Gearing A J, Hill P M, Cole J S, Griffiths J C, Burns N R, Kingsman A J, Adams S E. Induction of HIV-specific cytotoxic T lymphocytes in vivo with hybrid HIV-1 V3:Ty-virus-like particles. J Immunol. 1993;151:1097–1107. [PubMed] [Google Scholar]
- 23.Livingston P O, Adluri S, Helling F, Yao T J, Kensil C R, Newman M J, Marciani D. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine. 1994;12:1275–1280. doi: 10.1016/s0264-410x(94)80052-2. [DOI] [PubMed] [Google Scholar]
- 24.Lu S, Santoro J C, Fuller D H, Haynes J R, Robinson H L. Use of DNAs expressing HIV-1 Env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology. 1995;209:147–154. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 25.Lue C, van den Wall B A, Prince S J, Julian B A, Tseng M L, Radl J, Elson C O, Mestecky J. Intraperitoneal immunization of human subjects with tetanus toxoid induces specific antibody-secreting cells in the peritoneal cavity and in the circulation, but fails to elicit a secretory IgA response. Clin Exp Immunol. 1994;96:356–363. doi: 10.1111/j.1365-2249.1994.tb06567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Bulger P A, Davis D R, Perilli P B, Bedore D A, Kensil C R, Young E M, Hung C H, Seals J R, Pavia C S. Impact of the saponin adjuvant QS-21 and aluminium hydroxide on the immunogenicity of recombinant OspA and OspB of Borrelia. Vaccine. 1994;12:925–932. doi: 10.1016/0264-410x(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 27.Malim M H, Hauber J, Fenrick R, Cullen B R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988;335:181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- 28.Maraskovsky E, Chen W-F, Shortman K. IL-2 and IFN-γ are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989;143:1210–1214. [PubMed] [Google Scholar]
- 29.Moldoveanu Z, Clements M L, Prince S J, Murphy B R, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. 1. Definition according to the profiles of lymphokine activities and secreted protein. J Immunol. 1986;138:2348–2357. [PubMed] [Google Scholar]
- 31.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 32.Newman M J, Munroe K J, Anderson C A, Murphy C I, Panicali D L, Seals J R, Wu J Y, Wyand M S, Kensil C R. Induction of antigen-specific killer T lymphocyte responses using subunit SIVmac251 gag and env vaccines containing QS-21 saponin adjuvant. AIDS Res Hum Retroviruses. 1994;10:853–861. doi: 10.1089/aid.1994.10.853. [DOI] [PubMed] [Google Scholar]
- 33.Newman M J, Wu J Y, Gardner B H, Munroe K J, Leombruno D, Recchia J, Kensil C R, Coughlin R T. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T lymphocyte responses. J Immunol. 1992;148:2357–2362. [PubMed] [Google Scholar]
- 34.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with interleukin 12 and granulocyte macrophage colony stimulating factor (GM-CSF) expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigen. J Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 35.Okuda K, Bukawa H, Hamajima K, Kawamoto S, Sekigawa K, Yamada Y, Tanaka S, Ishi N, Aoki I, Nakamura M, Yamamoto H, Cullen B R, Fukushima J. Induction of potent humoral and cell-mediated immune responses following direct injection of DNA encoding the HIV type 1 env and rev gene products. AIDS Res Hum Retroviruses. 1995;11:933–943. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 36.Okuda K, Twining S S, David C S, Atassi M Z. Genetic control of immune response to sperm whale myoglobin in mice. II. T lymphocyte proliferative response to the synthetic antigenic sites. J Immunol. 1979;123:182–188. [PubMed] [Google Scholar]
- 37.Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. [Google Scholar]
- 38.Romagnani S, Maggi E. Th1 versus Th2 responses in AIDS. Curr Opin Immunol. 1994;4:616–622. doi: 10.1016/0952-7915(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki S, Fukushima J, Arai H, Kusakabe K, Hamajima K, Ishii N, Okuda K, Hirahara F, Kawamoto S, Ruysschaert J-M, Vandenbranden M, Wahren B, Okuda K. Human immunodeficiency virus type 1 specific immune responses induced by DNA vaccination are greatly enhanced by mannan-coated diC14-amidine. Eur J Immunol. 1997;27:3121–3129. doi: 10.1002/eji.1830271207. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki S, Fukushima J, Hamajima K, Tsuji T, Xin K-Q, Ishii N, Mohri H, Okuda K. Adjuvant effect of Ubenimex on a DNA vaccine for HIV-1. Clin Exp Immunol. 1998;111:30–36. doi: 10.1046/j.1365-2249.1998.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki S, Hamajima K, Fukushima J, Ihata A, Ishii N, Gorai I, Hirahara H, Mohri H, Okuda K. Comparison of intranasal and intramuscular immunization against human immunodeficiency virus type 1 with a DNA-monophosphoryl lipid A adjuvant vaccine. Infect Immun. 1998;66:823–826. doi: 10.1128/iai.66.2.823-826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki S, Tsuji T, Hamajima K, Fukushima J, Ishii N, Kaneko T, Xin K-Q, Mohri H, Aoki I, Okubo T, Nishioka K, Okuda K. Monophosphoryl lipid A enhances both humoral and cell-mediated immune responses to DNA vaccination against human immunodeficiency virus type 1. Infect Immun. 1997;65:3520–3528. doi: 10.1128/iai.65.9.3520-3528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staats H F, Nichols W G, Palker T J. Mucosal immunity to HIV-1—systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN (A) J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 44.Stevens T L, Bossie A, Sanders V M, Fernandez-Botran R, Coffmann R L, Mossman T R, Vitetta E S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi H, Germain R N, Moss B, Berzofsky J A. An immunodominant class I-restricted cytotoxic T lymphocyte determinant of human immunodeficiency virus type 1 induces CD4 II-restricted help for itself. J Exp Med. 1990;171:571–576. doi: 10.1084/jem.171.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuji T, Hamajima K, Fukushima J, Xin K-Q, Ishii N, Aoki I, Ishigatsubo Y, Tani K, Kawamoto S, Nitta Y, Miyazaki J, Koff W C, Okubo T, Okuda K. Enhancement of cell-mediated immunity against HIV-1 induced by coinoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J Immunol. 1997;158:4008–4014. [PubMed] [Google Scholar]
- 47.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 48.Vogel F R. Immunologic adjuvants for modern vaccine formulations. Ann N Y Acad Sci. 1995;754:153–160. doi: 10.1111/j.1749-6632.1995.tb44448.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Rafaeli Y, Sato A I, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolff J A, Williams P, Acsadi G, Jiao S, Jani A, Chong W. Conditions affecting direct gene transfer into rodent muscle in vivo. BioTechniques. 1991;11:474–485. [PubMed] [Google Scholar]
- 51.Wu J Y, Gardner B H, Murphy C I, Seals J R, Kensil C R, Recchia J, Beltz G A, Newman G W, Newman M J. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992;148:1519–1525. [PubMed] [Google Scholar]
- 52.Yamamoto M, Vancott J L, Okahashi N, Marinaro M, Kiyono H, Fujihashi K, Jackson R J, Chatfield S N, Bluethmann H, McGhee J R. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]