ABSTRACT
Tongue dorsum swabbing is a potential alternative to sputum collection for tuberculosis (TB) testing. Previous studies showed that Cepheid Xpert MTB/RIF Ultra (Xpert Ultra) can detect Mycobacterium tuberculosis DNA on tongue swabs stored in buffer, with 72% sensitivity and 100% specificity relative to a sputum microbiological reference standard (sputum MRS). The present study evaluated a more convenient sample collection protocol (dry swab storage), combined with streamlined sample processing protocols, for evaluating two commercial TB diagnostic tests: Xpert Ultra and Molbio Truenat MTB Ultima (MTB Ultima). Copan FLOQSwabs were self-collected or collected by study workers from 321 participants in Western Cape, South Africa. All participants had symptoms suggestive of TB, and 245 of them had sputum MRS-confirmed TB (by sputum MGIT culture and/or Xpert Ultra). One tongue swab per participant was tested on Xpert Ultra, and another tongue swab was tested with MTB Ultima. Xpert Ultra was 75.5% sensitive and 100% specific relative to sputum MRS, similar to previous methods that used swabs stored in buffer. MTB Ultima was 71.6% sensitive and 96.9% specific relative to sputum MRS. When sample lysates that were false-negative or invalid by MTB Ultima were frozen, thawed, and re-tested, MTB Ultima sensitivity rose to 79.1%. Both tests were more sensitive with swabs from participants with higher sputum Xpert Ultra semi-quantitative results. Although additional development could improve diagnostic accuracy, these results further support tongue swabs as easy-to-collect samples for TB testing.
IMPORTANCE
Tongue dorsum swabbing is a promising alternative to sputum collection for tuberculosis (TB) testing. Our results lend further support for tongue swabs as exceptionally easy-to-collect samples for high-throughput TB testing.
KEYWORDS: tongue swab, non-sputum sampling, tuberculosis diagnosis, tuberculosis screening, oral swab, non-invasive
INTRODUCTION
Tuberculosis (TB) disease, caused by Mycobacterium tuberculosis (MTB), remains a major global cause of morbidity and mortality (1). The standard patient sample for microbiological diagnosis of TB is sputum, a viscous material expectorated from airways. The availability of alternative, non-invasive samples, which could be collected outside of a clinic, would greatly facilitate testing for active TB in community settings such as schools, workplaces, and institutions (2–4).
Researchers have sought sputum alternatives for years, but few materials have been identified that are both (i) easier than sputum to collect and (ii) reasonably accurate relative to sputum testing (2, 3). To address this need, we and others have found that MTB cells and/or DNA can be detected by oral swab (OS) testing (5–13). In TB OS testing, the dorsum of the tongue is gently brushed with a swab for at least 10 seconds. The material collected by the swab is then subjected to nucleic acid amplification testing (NAAT) targeting MTB DNA. Tongue swabbing is fast and painless and does not require specific infrastructure for privacy or infectious aerosol control. Everyone, including children and people living with HIV (PLWH), can be easily swabbed in any setting (14, 15).
As noted in a recent systematic review (16), the accuracy of the method can vary widely depending on methods used. Early evaluations of TB OS (8–10), as well as some more recent studies (6, 7, 13, 17), used laboratory-developed manual quantitative PCR (qPCR) tests to detect MTB DNA in tongue swab samples. Other evaluations used the commercial and the World Health Organization (WHO)-approved Cepheid Xpert MTB/RIF Ultra automated qPCR system (Xpert Ultra). Most evaluations of Xpert Ultra testing of tongue swabs used sample preparation protocols that mimicked those used for Xpert Ultra testing of sputum (12, 18–20). These approaches often yielded modestly accurate results when the sensitivity and specificity of OS were calculated relative to the sputum microbiological reference standard (sputum MRS). In a collaborative study conducted in Uganda, we evaluated new protocols that were designed explicitly for testing swabs in Xpert Ultra (5). These approaches resulted in a 72% sensitivity relative to sputum MRS (incorporating both sputum Xpert Ultra and sputum culture) and a 78% sensitivity relative to sputum Xpert Ultra alone. Specificity was 100% relative to these reference standards (5).
This study builds upon our previous work (5) in several ways. First, the previous study used swabs that were collected (either singly or in tandem) into 2-mL storage/transport tubes that were pre-filled with a sterile buffer, whereas the current study used single swabs that were collected, cold-stored, and transported dry in 2-mL tubes, without buffer. Dry collection and storage may be more user-friendly for patients and providers, especially in community (non-clinical) settings (15). Second, the current study used a streamlined protocol for swab sample processing (about 15 minutes from sample tube to start of test, compared to 45–60 minutes for the methods described previously (5)). Third, the current study evaluated an additional commercial TB diagnostic test, the Molbio Truenat MTB Ultima (MTB Ultima) (Molbio Diagnostics, LTD, Verna, Goa, India) (3), which is applied to additional tongue swabs collected from the same participants (although the study was not designed to compare the two automated sputum testing platforms to each other). Finally, with 245 participants with confirmed TB by sputum MRS (culture and/or Xpert Ultra), this is the largest sensitivity study of TB OS reported to date.
MATERIALS AND METHODS
Study setting
The study was conducted in Worcester, located in the Cape Winelands of the Western Cape province of South Africa. TB burden in this area is high with a TB incidence rate of 700 per 100,000 (21).
Study participants
Participants were recruited at the local TB clinics in two cohorts with distinct study designs. Cohort 1 (April 2021–February 2022) was designed to study the impacts of patient oral hygiene behaviors and food/drink intake on OS testing results (22) and consisted solely of TB patients confirmed by sputum Xpert Ultra (N = 100). Sampling was split into three cases: Case 1 swabs were collected prior to eating/drinking or performing oral hygiene; Cases 2 and 3 swabs were collected after eating or performing oral hygiene. Only Case 1 samples were included in this analysis.
Cohort 2 (July 2021–March 2023) was designed to assess OS analytical methods. All participants were asked not to eat or drink or perform any oral hygiene for at least 30 minutes prior to providing samples. Cohort 2 had two phases. In the first phase, we enrolled patients with symptoms suggestive of TB, prior to confirmation of their disease. After enrollment of 96 participants, we observed that most (N = 79/96) did not have TB confirmed by MRS. Therefore, to meet the target numbers of enrollees with confirmed TB, the Cohort 2 protocol was amended to enroll only people with sputum Xpert-confirmed TB.
This project was approved by the University of Cape Town Human Research Ethics Committee (reference number 160/2020) and the University of Washington Human Subjects Division (STUDY00001840).
Sample collection
Swabs were Copan FLOQSwabs (520CS01; Copan Italia, Brescia, Italy), which are sterile, single-use, and individually packaged, with a break point at 30 mm. All swabs were collected within 3 days of initiation of TB treatment.
During their first visit, Cohort 1 participants were enrolled after informed consent and provided OSs if they met the oral hygiene criteria of the first case to which they were assigned. If they did not meet the criteria, their first OSs were collected during a subsequent visit to the participant’s home. During the participant’s Case 1 visit, six FLOQswabs were collected.
Cohort 2 participants were brought to the research site on Day 1 for informed consent, OSs, and sputum sample collection. On Day 1, five swabs were collected at the research site by the study team. On Day 2, two more swabs were self-collected by participants at their homes. The first two Day 1 swabs and the two Day 2 swabs were designated for assessment of OS analytical methods (including this study). Additional Day 1 swabs were saved for secondary analyses.
In both Cohorts, OSs were collected by study workers if the swabbing took place at the clinic or on site at the South African Tuberculosis Vaccine Initiative offices. Participants swabbed themselves under the supervision of study workers during home visits. The protocols for provider-collected swabs and supervised self-swabbing are provided elsewhere (15). After the swab was collected, either the study worker or the participant placed the swab into an empty 2-mL screw-cap tube (72.694.106; Sarstedt, Nümbrecht, Germany) held by the study worker. The worker then broke or cut off the head of the swab into the tube and discarded the shaft. The tube was closed and placed into storage at −80°C within 12 hours of collection. The samples were stored at −80°C and shipped on dry ice. Upon receipt in Seattle, they were immediately transferred to the −80°C freezer where they were stored until testing.
Sample analysis
Prior to sample analysis, swab samples were randomized by collection day and collection order, to minimize potential biases between the two platforms. Each of the samples collected per participant was assigned a number (e.g., Cohort 2, Day 1, Swab 1 was #1; Cohort 1, Day 1, Swab 2 was #2, etc). An online random number generator (randomlists.com) was used to make lists consisting of the assigned numbers, and swabs were selected for testing according to the number order from the list. Testing of Cohort 2 samples was blinded as to TB status.
The Molbio Truenat MTB Ultima (MTB Ultima; Molbio Diagnostics, LTD, Verna, Goa, India) is a new Molbio product that is still in development as of this report (https://www.molbiodiagnostics.com/uploads/certificate_analysisdoc/5676_certificate_ana_567620230906.164126.pdf). The MTB Ultima chip is run on the Molbio Truelab machine, executing an automated PCR targeting both IS6110 and IS1081. Prior to the PCR assay, the swab samples had to be processed to render them safe to handle and to release MTB DNA from bacilli. This was accomplished as follows. The wells of a heat block were filled with water, and it was pre-heated to 100°C. The water facilitated heat distribution. The samples were removed from the freezer and immediately placed into the heat block where they were heated for 10 minutes. Samples were removed from the heat block and 500-µL TE buffer (pH 8.0) was added to the tubes, followed by vortexing (Vortex-Genie 2, SI-0236; Scientific Industries, Inc, Bohemia, NY, USA) for 15 seconds. Samples were returned to the heat block and heated to 100°C for another 10 minutes to facilitate lysis and elution of cells/DNA off the swab. Samples were then removed, briefly centrifuged (2 seconds at 6,000 rpm), and allowed to cool for 5 minutes. Glass disruption beads (150 mg 0.1 mm) (9830; Research Products International, Mt. Prospect, IL, USA), pre-weighed and stored in individual tubes, were added, and samples were bead-beaten at max speed for 10 minutes on a vortexer using a vortex adapter (13000-V1-24; Mo Bio Laboratories, Inc, Carlsbad, CA, USA). After a brief spin (2 seconds at 6,000 rpm), the lysate was removed and transferred to a labeled 1.5-mL snap-cap tube. Six microliters of this lysate was run on the MTB Ultima chip, following the manufacturer’s instructions. Thus, 1.2% of the total sample volume (6/500 microliters) was analyzed by using this method. Two Truelab Duos were used to run the samples, with a capacity for running two samples each at a time. The lysis protocol for four samples took about 45 minutes, while the MTB Ultima assay took about 40 minutes.
This manual lysis protocol is considered off-label, as we did not use the Molbio Trueprep AUTO v2 cartridge-based sample prep device for sample lysis and concentration. The Trueprep cartridges contain an internal positive control (IPC) that is added to each sample during processing, so there was no IPC in our samples. Instead, we monitored the performance of the MTB Ultima chips and Truelab by running a designated positive control from the Truenat Positive Control Kit—Panel 1 after every 20–25 tests. If the positive control failed, the preceding tests were considered invalid.
The Xpert Ultra (Cepheid, Sunnyvale, CA, USA) processes samples internally in the test cartridge and then runs a hemi-nested qPCR for IS6110 and IS1081, as well as the rpoB gene for rifampin resistance. The sample preparation protocol for Xpert took an estimated 15 minutes per set of four samples. As in the Molbio MTB Ultima protocol, the swabs were heated at 100°C for 10 minutes in a water-filled heat block. Tubes were removed from the heat block and 1-mL TE buffer (pH 8.0) was added, followed by vortexing for 30 seconds. The 1-mL sample was transferred to the Xpert Ultra cartridge. Another 1 mL of TE was added to the original tube containing the swab. After another 30-second vortex, this additional 1-mL sample was added to the Xpert Ultra cartridge. The cartridge was then loaded into a four-module GeneXpert instrument for analysis. In contrast to MTB Ultima that tested 1.2% of total sample volume, this protocol analyzed 100% of the total sample volume.
RESULTS
Study population characteristics
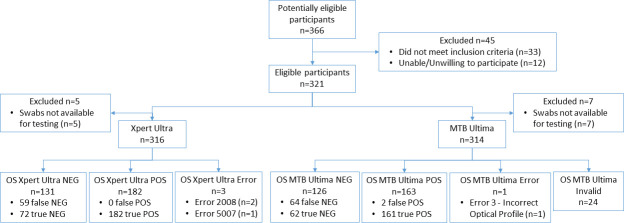
Three hundred and twenty-one participants (N = 321) were enrolled and included in the analysis. Cohort 1 had N = 100 participants, all of whom were TB-positive by sputum Xpert Ultra. Cohort 2 had N = 221, of whom N = 145 were TB-positive patients by sputum MRS (sputum MGIT culture and/or Xpert Ultra), and N = 76 were TB-negative patients. Among the TB-positive patients in Cohort 2, 84.8% (123/145) were positive by both sputum culture and Xpert Ultra, 5.5% (8/145) were positive by sputum culture only, and 9.7% (14/145) were positive by sputum Xpert Ultra only. One sputum sample per participant was tested by the reference method(s), and one swab per participant was tested by each index method. Table 1 shows their characteristics, with Cohort 2 divided between TB-positive and TB-negative participants as defined by sputum MRS. Figure 1 shows the combined flow of all Cohort 1 and Cohort 2 participants through the study, in accordance with Standards for Reporting of Diagnostic Accuracy Studies (STARD) (23).
TABLE 1.
Baseline characteristics of participants recruited for Cohorts 1 and 2
| Variables | Cohort 1 (N = 100) | Cohort 2 (N = 221) | |
|---|---|---|---|
| Sputum MRS-positive | Sputum MRS- positive (N = 145) |
Sputum MRS- negative (N = 76) |
|
| Age (median, interquartile range) | 34 (27–44) | 35 (25–45) | 38.5 (31.5–47.4) |
| Gender (male) | 63 (63.0%) | 86 (59.3%) | 31 (40.8%) |
| Mixed-race ancestry | 77 (77.0%) | 106 (73.1%) | 52 (68.4%) |
| Black | 23 (23.0%) | 38 (26.2%) | 22 (28.9%) |
| White | 0 | 1 (0.7%) | 1 (1.3%) |
| Asian | 0 | 0 | 1 (1.3%) |
| Employed | 31 (31.0%) | 47 (32.4%) | 36 (47.4%) |
| Previous TB | 37 (37.0%) | 54 (37.2%) | 42 (55.3%) |
| HIV-positive | 35 (35.0%) | 49 (34.0%) | 23 (30.3%) |
Fig 1.
STARD diagram showing flow of participants through the study and primary results. All Cohort 1 and Cohort 2 participants are shown in a single diagram. All participants in the diagram had valid reference test results (sputum MRS). Terms and abbreviations: false NEG, false-negative swab relative to sputum MRS; true NEG, true-negative swab relative to sputum MRS; false POS, false positive swab relative to sputum MRS; true POS, true-positive swab relative to sputum MRS; error, error messages reported by Xpert or Truenat instruments; invalid, swabs excluded due to failure of positive control. MTB Ultima had a relatively large number of invalid results because in our off-label protocol, a single positive control failure can invalidate up to 25 preceding sample results.
Sensitivity and specificity of TB testing platforms applied to tongue swab samples
Both Xpert Ultra and MTB Ultima exhibited better sensitivity in Cohort 2 than in Cohort 1 (Table 2), though the difference in cohort positivity for either platform was not statistically significant (P = 0.17 and P = 0.09, respectively, by two-tailed z score). When the results of both cohorts were combined, Xpert Ultra was 75.5% sensitive and 100% specific relative to sputum MRS. MTB Ultima was 71.6% sensitive and 96.9% specific relative to sputum MRS (Table 2).
TABLE 2.
Sensitivity and specificity of swab testing by Xpert Ultra and MTB Ultima, based on all samples tested
| Cohort | Sensitivity | Specificity | ||||||
|---|---|---|---|---|---|---|---|---|
| Xpert Ultra | MTB Ultima | Xpert Ultra | MTB Ultima | |||||
|
N swab-positive/ sputum-positive |
% (95% CI) |
N swab-positive/ sputum-positive |
% (95% CI) |
N swab-negative/ sputum-negative |
% (95% CI) |
N swab-negative/ sputum-negative |
% (95% CI) | |
| 1 | 71/100 | 71.0 (61.1, 79.6) | 58/89 | 65.2 (54.3, 75.0) | −a | −a | −a | −a |
| 2 | 111/141 | 78.7 (71.0–85.2) | 103/136 | 75.7 (67.7, 82.7) | 72/72 | 100 (95.0–100) | 62/64 | 96.9 (89.2, 99.6) |
| 1 + 2b | 182/241 | 75.5 (69.6–80.8) | 161/225 | 71.6 (65.2, 77.4) | 72/72 | 100 (95.0–100) | 62/64 | 96.9 (89.2, 99.6) |
Cohort 1 consisted entirely of TB-positive participants by sputum Xpert Ultra.
Totals fell below the total enrollment of 321 participants due to invalid results and errors, which differed between the two test platforms.
The STARD diagram (Fig. 1) shows where samples had to be excluded from either test due to lack of availability. It also shows the number of test errors and invalid tests (as defined by the positive control failure; see Methods). Due to the errors and invalids, the Xpert Ultra and MTB Ultima sample sizes in Table 2 do not precisely align. Table 3 shows a smaller sample set (N = 284) that consists entirely of samples from Cohort 1 and Cohort 2 participants whose swabs were tested by both methods. In this paired analysis set, Xpert Ultra appeared to have slightly higher sensitivity than MTB Ultima. However, the difference was not statistically significant (P = 0.33 by two-tailed z score).
TABLE 3.
Sensitivity and specificity of swab testing by Xpert Ultra and MTB Ultima, based on samples from participants whose swabs were tested by both methods
| Cohort | Sensitivity | Specificity | ||||||
|---|---|---|---|---|---|---|---|---|
| Xpert Ultra | MTB Ultima | Xpert Ultra | Xpert Ultra | |||||
| N swab-positive/ sputum-positive | % (95% CI) |
N swab-positive/ sputum-positive |
% (95% CI) |
N swab-negative/ sputum-negative |
% (95% CI) |
N swab-negative/ sputum-negative |
% (95% CI) | |
| 1 | 63/89 | 70.8 (60.2, 80.0) | 58/89 | 65.2 (54.3, 75.0) | −a | −a | −a | −a |
| 2 | 105/133 | 78.9 (71.0, 85.5) | 101/133 | 75.9 (67.8, 83.0) | 62/62 | 100 (94.2, 100) | 60/62 | 96.8 (88.8, 99.6) |
| 1 + 2 | 168/222 | 75.7 (69.5, 81.2) | 159/222 | 71.6 (65.2, 77.5) | 62/62 | 100 (94.2, 100) | 60/62 | 96.8 (88.8, 99.6) |
Cohort 1 consisted entirely of confirmed TB-positive participants by sputum Xpert Ultra.
Tongue swab sensitivity varied with sputum Xpert Ultra semi-quantitative results
Sputum Xpert semi-quantitative data were available for a subset of Cohort 2 participants with sputum MRS-confirmed TB (N = 91). By both Xpert Ultra and MTB Ultima, tongue swab positivity was more common among patients who had higher sputum semi-quantitative results (Table 4).
TABLE 4.
Sensitivity relative to sputum semi-quantitative resultsa
| Sputum Xpert Ultra semi-quantitative results | Sensitivity, Xpert Ultra | Sensitivity, MTB Ultima | ||
|---|---|---|---|---|
| N swab-positive/sputum MRS-positive | % (95% CI) |
N swab-positive/sputum MRS-positive |
% (95% CI) | |
| High | 43/43 | 100 (91.8, 100) | 38/40 | 95 (83.1, 99.4) |
| Medium | 16/16 | 100 (79.4, 100) | 13/15 | 86.7 (59.5, 98.3) |
| Low | 16/23 | 69.6 (47.1, 86.8) | 13/21 | 61.9 (38.4, 81.9) |
| Very low | 2/6 | 33.3 (4.33, 77.7) | 4/7 | 57.1 (18.4, 90.1) |
| Trace | 0/3 | 0 (0, 70.8) | 0/3 | 0 (0, 70.8) |
| Total | 77/91 | 84.6 (75.5, 91.3) | 68/86 | 79.1 (69.0, 87.1) |
Sputum semi-quantitative data were available for N = 91 participants with confirmed TB in Cohort 2.
Increased MTB Ultima sensitivity upon re-testing of swab sample lysates
Our swab sample processing protocol for MTB Ultima generated ~500 µL of crude lysate, of which only 6 µL was tested with the MTB Ultima chip. The remaining ~494 µL was returned to the −80°C freezer. We asked whether we might see improved overall sensitivity if the frozen material was thawed and re-tested by running another 6µL aliquot on MTB Ultima chips.
As shown in Table 2, the first-pass MTB Ultima analysis of 225 swab samples from participants with confirmed TB identified 161 swabs that were true-positive relative to sputum MRS and 60 that were false-negative. Fifty-nine of the false-negative lysates were thawed and re-tested in fresh MTB Ultima chips. One of these samples yielded an error (Error-3, Incorrect Optical Profile), and none were deemed invalid. Of the 58 samples with valid results, 41 remained false-negative, while 17 yielded true-positive results on the second try. Positive swab results on the second run included “very low” (N = 8), “low” (N = 6), “medium” (N = 2), and “high” (N = 1) MTB Ultima semi-quantitative values from swabs. When the 17 samples that became true-positive upon re-testing are added to the 161 samples that were true-positive in the first pass, the adjusted sensitivity of MTB Ultima relative to sputum MRS increases to 178/225, or 79.1% [95% confidence interval (CI) 73.2%, 84.2%].
In addition to false-negative samples, we also thawed and re-tested 28 true-negative samples (swabs from sputum MRS-negative participants that were negative by MTB Ultima on the first pass). All 28 of these samples (100%) yielded negative results upon re-testing.
DISCUSSION
As sputum is not always easy to collect, alternate sampling methods are expected to increase access to testing, thus possibly reducing diagnostic gap (4). This current study expanded on previous studies in three significant ways. It is among the first studies to evaluate dry collection/storage of tongue swab samples from TB patients. Dry swabs are likely to be easier to handle than buffer-filled tubes in the context of high-throughput community settings, which are often envisioned for non-sputum sampling (2–4, 14). Second, the current study used a streamlined protocol for swab sample processing for Xpert Ultra [about 15 minutes from sample tube to start of test, compared to 45–60 minutes for the methods described previously (5)]. For occupational safety, an initial heat inactivation step (10 min at 100°C) was conducted on the closed swab tube before it was opened for the first time. Similar, but not identical, 10-minute heat steps were reported by others to reduce viable MTB in pooled oral matrix by ≥104 fold (13); however, additional work is needed to confirm whether the protocol described here is fully mycobacteriocidal in the presence of diverse oral sample matrices. Third, we assessed an additional commercial TB diagnostic NAAT test, MTB Ultima, that was applied to replicate tongue swabs collected from the same participants. MTB Ultima is a next-generation version of the WHO-endorsed Molbio Truenat MTB Plus assay, which is in active use for TB testing in some parts of the world.
For Xpert Ultra testing of swabs, we found that the use of dry-stored swabs with a streamlined protocol resulted in a low error rate of 1%, with no loss of accuracy relative to previous methods that used wet-stored swabs and a lengthier protocol. The overall sensitivity of 75.5% observed here is similar to the previously reported sensitivity of 72% relative to sputum MRS and 78% relative to sputum Xpert Ultra (5). Specificity relative to sputum MRS was 100% in both studies. These similarities are notable given that the two studies were conducted in different settings and populations (Worcester, South Africa, and Kampala, Uganda, respectively). While higher sensitivity relative to sputum MRS would be desirable and appears feasible based on results from studies that used manual methods (7, 13), the consistency observed between these two studies shows promise for the biological reproducibility of tongue swab sampling. This consistency contrasts with the widely varying sensitivity of TB OS that is seen when diverse, non-uniform read-outs are used (16).
For MTB Ultima testing of swabs, we used a lengthier sample processing protocol that included bead-beating on a vortex adapter. This was deemed necessary because MTB Ultima does not have the automated sonic disruption step that is built into the Xpert Ultra process. The MTB Ultima process yielded an error rate of 0.8% and an initial sensitivity value that was similar to Xpert Ultra testing. Within the subset of participants (Table 3) for whom side-by-side comparison of replicate swabs was possible, sensitivities relative to sputum MRS were 75.7% and 71.6% for Xpert Ultra and MTB Ultima, respectively. Specificity of both methods was high at >95%.
Both molecular tests exhibited higher sensitivities in Cohort 2 than in Cohort 1, though the differences were not significant. The two cohorts had different, but overlapping, time periods. Most Cohort 1 participants were enrolled in 2021, while most Cohort 2 participants with confirmed TB were enrolled in 2022 and 2023. The evolving COVID-19 pandemic may have affected the timing of care-seeking behaviors.
In our previous evaluation of Xpert Ultra analysis of tongue swabs (5), there appeared to be a relationship between tongue swab positivity and sputum swab Xpert Ultra semi-quantitative results. Positivity ranged from 100% (21/21) in patients with “high” sputum semi-quantitative results, down to 0% (0/8) in patients with “very low” or “trace” sputum results (5). Similarly, when stratified by sputum Xpert Ultra semi-quantitative results, both platforms used here were very sensitive in participants with higher semi-quantitative results but less so in those with lower semi-quantitative results (Table 4). These findings suggest that tongue swab testing for pauci-bacillary TB, such as would be expected in PLWH, children, and mild subclinical disease, may need further optimization of sample collection and assay methods to improve performance relative to active TB with high bacillary burden.
Based on the results of our analysis of re-frozen and thawed lysates, there may be room for improvement in our MTB Ultima sample preparation protocol. In contrast to Xpert Ultra, which tests the entire swab sample eluate, MTB Ultima tests only a small portion of it (6 µL out of 500-µL total). This resulted in left-over sample suspensions that had been processed by the MTB Ultima method and were available for re-testing. Of the 58 samples that were false-negative by MTB Ultima, 17 became true-positive upon re-testing. The reasons for this are not known. Sample contamination during re-testing does not seem likely given the 100% specificity observed with 28 re-tested true-negative samples. One possibility is that the additional freeze-thaw cycle may have further disrupted MTB bacilli that were damaged, but not disintegrated, in our initial bead-beading protocol. Freeze-thaw cycles are reported to enhance disruption of other bacteria (24). An alternative explanation is stochastic. Because our bead-beating protocol does not fully solubilize the material in swab samples, lysates have particulate cellular debris that may associate with MTB DNA in ways that are not uniform throughout the 500-µL suspensions. If so, then 6-µL aliquots that are applied to the Truenat chip may not always have representative amounts of target MTB DNA. Consistent with this possibility, of the 17 false-negative samples that became positive upon re-testing, three had MTB Ultima semi-quantitative signals that were quite robust on the second pass (semi-quantitative “medium” or “high”). Thus, the effects of re-testing were non-uniform and greater than incremental in a few cases, consistent with a stochastic model. In view of these possibilities, improvements to the Truenat method could include enhanced mechanical disruptions (13, 25) and/or concentration of MTB DNA by methods such as magnetic particle hybridization capture (26, 27).
Despite potential limitations in our methodology, the sensitivity of MTB Ultima reported here was higher than that reported in a recent study of the Molbio Truenat MTB Plus assay applied to tongue swabs (28). In that study, sensitivity was 54% relative to sputum Xpert Ultra, compared to 72%–79% in the current study. This increase in sensitivity could be attributed to larger sample size, or to our use of MTB Ultima which detects two multi-copy genetic markers in the MTB genome (IS6110 and IS1081) versus MTB Plus which detects nrdZ and IS6110.
Strengths of our study included evaluating two molecular platforms for tongue swabs. Moreover, our participant population included 245 participants with sputum MRS-confirmed TB, a much larger sample size than our previous study of Xpert Ultra applied to tongue swabs (5). The previous study found that TB OS has high specificity and somewhat lower sensitivity, so the use of a relatively large sample of participants with confirmed TB is a strength.
There were several limitations. Whereas swabs were tested by both Xpert Ultra and MTB Ultima, the reference standard (sputum MRS) used Xpert Ultra but not MTB Ultima. This may have introduced bias in favor of Xpert Ultra as a swab read-out. Moreover, because swabs evaluated in this study were collected in South Africa and tested in the USA, all of them were frozen for storage and shipment and then thawed for analysis, a process that could have affected sensitivity in either direction. Sample preparation protocols for both tests required equipment (heat block, vortexer, and vortex adapter) which, although inexpensive, might not be available in all settings. A more powerful Biospec bead-beater was reported by Steadman et al. (13) to yield excellent results when paired with a manual MTB qPCR applied to swabs; however, the Biospec is expensive and not common outside of research laboratories. Finally, both commercial test platforms evaluated in this study were engineered to test sputum, not swabs, and there may be limitations to how well they can ever perform with swab samples.
Despite these limitations, our observations expand the evidence supporting tongue swabs as alternatives to sputum collection in settings where sputum collection is not possible. Tongue swabs are easier and faster to collect than any TB sample type that we are aware of, and their use could help increase access to TB testing and decrease occupational risks to healthcare workers. Further development of testing methods designed explicitly for TB tongue swabs, such as the high-volume qPCR approach described by Steadman et al. (13), may deliver further improvements in accuracy relative to sputum testing. As we learned when anterior nasal sampling was introduced for COVID-19 testing (2, 3, 29), sample types that are non-invasive and easy to self-collect (15) can be invaluable tools in the fight against transmissible diseases.
ACKNOWLEDGMENTS
We are grateful to Copan Italia for providing swabs. We are grateful to B.R. Sivakumar; Akron D’Souza; Winnie Gonsalves of Molbio Diagnostics, LTD.; and Gayatri Chilambi of Rutgers University, for facilitating access to Molbio tests and instruments.
This work was supported by the Bill and Melinda Gates Foundation (#INV-004527, OPP 1213054), by NIH grant R01AI139254, and by the Australian Government. K.A.L. was supported in part by a Mary Gates Undergraduate Research Fellowship. R.C. was supported in part by a University of Washington Department of Environmental and Occupational Health Sciences Top Scholar Award.
Contributor Information
Gerard A. Cangelosi, Email: gcang@uw.edu.
Christine Y. Turenne, University of Manitoba, Winnipeg, Manitoba, Canada
REFERENCES
- 1. World Health Organization . 2023. Global tuberculosis report 2023.
- 2. Pai M, Dewan PK, Swaminathan S. 2023. Transforming tuberculosis diagnosis. Nat Microbiol 8:756–759. doi: 10.1038/s41564-023-01365-3 [DOI] [PubMed] [Google Scholar]
- 3. Branigan D. 2023. Tuberculosis diagnostics pipeline report. Treatment Action Group (TAG). https://www.treatmentactiongroup.org/wp-content/uploads/2023/11/2023_pipeline_TB_diagnostics_final.pdf. [Google Scholar]
- 4. Reid M, Agbassi YJP, Arinaminpathy N, Bercasio A, Bhargava A, Bhargava M, Bloom A, Cattamanchi A, Chaisson R, Chin D, et al. 2023. Scientific advances and the end of tuberculosis: a report from the lancet commission on tuberculosis. The Lancet 402:1473–1498. doi: 10.1016/S0140-6736(23)01379-X [DOI] [PubMed] [Google Scholar]
- 5. Andama A, Whitman GR, Crowder R, Reza TF, Jaganath D, Mulondo J, Nalugwa TK, Semitala FC, Worodria W, Cook C, Wood RC, Weigel KM, Olson AM, Lohmiller Shaw J, Kato-Maeda M, Denkinger CM, Nahid P, Cangelosi GA, Cattamanchi A. 2022. Accuracy of tongue swab testing using Xpert MTB-RIF Ultra for tuberculosis diagnosis. J Clin Microbiol 60:e0042122. doi: 10.1128/jcm.00421-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shapiro AE, Olson AM, Kidoguchi L, Niu X, Ngcobo Z, Magcaba ZP, Ngwane MW, Whitman GR, Weigel KM, Wood RC, Wilson DPK, Drain PK, Cangelosi GA. 2022. Complementary nonsputum diagnostic testing for tuberculosis in people with HIV using oral swab PCR and urine lipoarabinomannan detection. J Clin Microbiol 60:e0043122. doi: 10.1128/jcm.00431-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wood RC, Andama A, Hermansky G, Burkot S, Asege L, Job M, Katumba D, Nakaye M, Mwebe SZ, Mulondo J, Bachman CM, Nichols KP, Le Ny A-L, Ortega C, Olson RN, Weigel KM, Olson AM, Madan D, Bell D, Cattamanchi A, Worodria W, Semitala FC, Somoskovi A, Cangelosi GA, Minch KJ. 2021. Characterization of oral swab samples for diagnosis of pulmonary tuberculosis. PLoS One 16:e0251422. doi: 10.1371/journal.pone.0251422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luabeya AK, Wood RC, Shenje J, Filander E, Ontong C, Mabwe S, Africa H, Nguyen FK, Olson A, Weigel KM, Jones-Engel L, Hatherill M, Cangelosi GA. 2019. Noninvasive detection of tuberculosis by oral swab analysis. J Clin Microbiol 57:e01847-18. doi: 10.1128/JCM.01847-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicol MP, Wood RC, Workman L, Prins M, Whitman C, Ghebrekristos Y, Mbhele S, Olson A, Jones-Engel LE, Zar HJ, Cangelosi GA. 2019. Microbiological diagnosis of pulmonary tuberculosis in children by oral swab polymerase chain reaction. Sci Rep 9:10789. doi: 10.1038/s41598-019-47302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood RC, Luabeya AK, Weigel KM, Wilbur AK, Jones-Engel L, Hatherill M, Cangelosi GA. 2015. Detection of Mycobacterium tuberculosis DNA on the oral mucosa of tuberculosis patients. Sci Rep 5:8668. doi: 10.1038/srep08668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song Y, Ma Y, Liu R, Shang Y, Ma L, Huo F, Li Y, Shu W, Wang Y, Gao M, Pang Y. 2021. Diagnostic yield of oral swab testing by TB-LAMP for diagnosis of pulmonary tuberculosis. Infect Drug Resist 14:89–95. doi: 10.2147/IDR.S284157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mesman AW, Calderon R, Soto M, Coit J, Aliaga J, Mendoza M, Franke MF. 2019. Mycobacterium tuberculosis detection from oral swabs with Xpert MTB/RIF ULTRA: a pilot study. BMC Res Notes 12:349. doi: 10.1186/s13104-019-4385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steadman A, Andama A, Ball A, Mukwatamundu J, Khimani K, Mochizuki T, Asege L, Bukirwa A, Kato JB, Katumba D, et al. 2024. New manual qPCR assay validated on tongue swabs collected and processed in Uganda shows sensitivity that rivals sputum-based molecular TB diagnostics. Clin Infect.Dis:ciae041. doi: 10.1093/cid/ciae041 [DOI] [PMC free article] [PubMed]
- 14. Valinetz ED, Cangelosi GA. 2021. A look inside: oral sampling for detection of non-oral infectious diseases. J Clin Microbiol 59:e0236020. doi: 10.1128/JCM.02360-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Codsi R, Errett NA, Luabeya AK, Van As D, Hatherill M, Shapiro AE, Lochner KA, Vingino AR, Kohn MJ, Cangelosi GA. 2023. Preferences of healthcare workers using tongue swabs for tuberculosis diagnosis during COVID-19. PLOS Glob Public Health 3:e0001430. doi: 10.1371/journal.pgph.0001430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Church EC, Steingart KR, Cangelosi GA, Ruhwald M, Kohli M, Shapiro AE. 2024. Oral swabs with a rapid molecular diagnostic test for pulmonary tuberculosis in adults and children: a systematic review. Lancet Glob Health 12:e45–e54. doi: 10.1016/S2214-109X(23)00469-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaCourse SM, Seko E, Wood R, Bundi W, Ouma GS, Agaya J, Richardson BA, John-Stewart G, Wandiga S, Cangelosi GA. 2022. Diagnostic performance of oral swabs for non-sputum based TB diagnosis in a TB/HIV endemic setting. PLoS One 17:e0262123. doi: 10.1371/journal.pone.0262123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flores JA, Calderón R, Mesman AW, Soto M, Coit J, Aliaga J, Mendoza M, Leon SR, Konda K, Mestanza FM, Mendoza CJ, Lecca L, Murray MB, Holmberg RC, Pollock NR, Franke MF. 2020. Detection of Mycobacterium tuberculosis DNA in buccal swab samples from children in Lima, Peru. J Pediatr Infect Dis 39:e376–e380. doi: 10.1097/INF.0000000000002828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lima F, Santos AS, Oliveira RD, Silva CCR, Gonçalves CCM, Andrews JR, Croda J. 2020. Oral swab testing by Xpert-« MTB/RIF Ultra for mass tuberculosis screening in prisons. J Clin Tuberc Other Mycobact Dis 19:100148. doi: 10.1016/j.jctube.2020.100148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox H, Workman L, Bateman L, Franckling-Smith Z, Prins M, Luiz J, Van Heerden J, Ah Tow Edries L, Africa S, Allen V, Baard C, Zemanay W, Nicol MP, Zar HJ. 2022. Oral swabs tested with Xpert MTB/RIF Ultra for diagnosis of pulmonary tuberculosis in children: a diagnostic accuracy study. Clin Infect Dis 75:2145–2152. doi: 10.1093/cid/ciac332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. du Toit T, Esterhuizen TM, Tiffin N, Abulfathi AA, Reuter H, Decloedt EH. 2020. Incident tuberculosis disease in patients receiving biologic therapies in the Western Cape, South Africa from 2007 to 2018. BMC Infect Dis 20:900. doi: 10.1186/s12879-020-05624-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luabeya AK, Olsen AM, As DV, Peterson C, Hadley K, Wood RC, Yan A, Yager P, Hatherill M, Cangelosi GA. 2023. Effects of oral hygiene, food intake and patient characteristics on oral swab quantitative polymerase chain reaction results in South African patients with TB. The Union World Conference on Lung Health 2023 Abstract OA18-340-16 [Google Scholar]
- 23. Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, Irwig L, Levine D, Reitsma JB, de Vet HCW, Bossuyt PMM. 2016. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 6:e012799. doi: 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xin Y, Xie J, Nan B, Tang C, Xiao Y, Wu Q, Lin Y, Zhang X, Shen H. 2021. Freeze-thaw pretreatment can improve efficiency of bacterial DNA extraction from meconium. Front Microbiol 12:753688. doi: 10.3389/fmicb.2021.753688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vandeventer PE, Weigel KM, Salazar J, Erwin B, Irvine B, Doebler R, Nadim A, Cangelosi GA, Niemz A. 2011. Mechanical disruption of lysis-resistant bacteria using a miniature, low power, disposable device. J Clin Microbiol 49:2533–2539. doi: 10.1128/JCM.02171-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reed JL, Basu D, Butzler MA, McFall SM. 2017. XtracTB assay, a Mycobacterium tuberculosis molecular screening test with sensitivity approaching culture. Sci Rep 7:3653. doi: 10.1038/s41598-017-03930-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oreskovic A, Panpradist N, Marangu D, Ngwane MW, Magcaba ZP, Ngcobo S, Ngcobo Z, Horne DJ, Wilson DPK, Shapiro AE, Drain PK, Lutz BR. 2021. Diagnosing pulmonary tuberculosis by using sequence-specific purification of urine cell-free DNA. J Clin Microbiol 59:e0007421. doi: 10.1128/JCM.00074-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahls CL, Emsweller D, Helfers SJ, Niu X, Wilson D, Padgett LR, Drain PK. 2024. No extraction? no problem. Direct to PCR processing of tongue swabs for diagnosis of tuberculosis disease as an alternative to sputum collection. Microbiol Spectr 12:e0310723. doi: 10.1128/spectrum.03107-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tu YP, Jennings R, Hart B, Cangelosi GA, Wood RC, Wehber K, Verma P, Vojta D, Berke EM. 2020. Swabs collected by patients or health care workers for SARS-CoV-2 testing. N Engl J Med 383:494–496. doi: 10.1056/NEJMc2016321 [DOI] [PMC free article] [PubMed] [Google Scholar]