Abstract
Intravital multiphoton microscopy (MPM) allows the direct visualization of viral infections in real time as they occur in living animals. Here we describe the routes and considerations for murine infection with vaccinia virus (VACV) for imaging, and the preparation of the skin and inner lip (labial mucosa) of infected animals for MPM. Using different recombinant VACVs expressing fluorescent proteins in combination with transgenic fluorescent reporter mice, MPM imaging can be used to examine the movements, interactions, and functions of virus-infected cells or selected immune cell populations after infection.
Keywords: Virus, Vaccinia virus, Viral immunology, Multiphoton microscopy, Intravital microscopy, Skin immunology, Mucosal immunology
1. Introduction
While once considered a difficult and expensive technology, intravital multiphoton microscopy (MPM) has rapidly advanced in ease and availability, allowing the development of myriad approaches to image the immune response in the tissues and organs of living animals [1]. Intravital microscopy typically (but not always!) makes use of a powerful multiphoton laser that excites fluorophores with longer wavelengths of light, allowing deeper tissue penetration with less photodamage. Initially applied to increase our understanding of the basic principles of the immune response after administration of noninfectious immunogens, MPM is increasingly being employed to probe the immune response to pathogens [2–4]. We have routinely applied this technology to examine the anatomy of the antiviral immune response in VACV-infected skin [5–7], the route of infection employed for human vaccination with the smallpox vaccine. More recently, we have expanded this technology to understand antiviral immunity within the labial mucosa, a critical site for poxvirus pathogenesis. Here, we describe the experimental methods for MPM imaging of VACV-infected animals with an emphasis on the unique aspects of imaging a BSL-2 virus in live mice. Using recombinant viruses expressing a number of different fluorophores created using standard molecular biological approaches [8], we have successfully imaged the movement of both virus-infected cells and responding innate and adaptive lymphocytes in the skin and mucosa.
2. Materials
All solutions and reagents should be kept sterile and prepared in a biological safety cabinet. All reagents should be prewarmed to 37 °C and stored at 4 °C. VACV stocks are usually at a titer of ~2 × 108 pfu/mL [9].
2.1. VACV Infection
2.2. Intravital Microscopy of VACV-Infected Ear
Infected mice at the desired time postinfection (see Note 3).
Sterile phosphate-buffered saline (PBS) at 37 °C (see Note 4).
Cyanoacrylate (e.g., superglue).
Plastic 6-well plate and lid.
Office tape.
Athletic cloth tape.
Weatherstrip and caulking cord.
Cotton swab Q-tips.
Isoflurane or avertin for anesthesia (see Note 2).
27-gauge needle.
Butterfly needle with 1 mL syringe and extender if required.
Low-flow isoflurane machine with rodent facemask (Somnosuite, Kent Scientific); heart rate and oxygen saturation monitor are helpful. Other isoflurane vaporizers can also be used (such as SurgiVet) with mouse nose cone (Braintree Scientific). We find the Somnosuite the easiest and most consistent.
Biosafety Level 2-housed upright Leica intravital multiphoton microscope equipped with an environmental chamber and one or more multiphoton lasers, such as MaiTai DeepSee, Chameleon Coherent, or Spectra Physics Insight (see Notes 5 and 6); ultrasensitive hybrid detectors (HyD); and a 25× water-immersion objective.
External non-descanned detectors equipped with the following filters: a 495 nm dichroic mirror followed by emission filters of 460/50 nm bandpass and 525/50 nm bandpass; a longpass filter of 560 nm; and then a 610/60 nm bandpass, a 650 nm longpass, and a 685/50 bandpass (see Note 7).
Image-processing software: Imaris (Bitplane), ImageJ and Huygens (Scientific Volume Imaging) (see Note 8).
2.3. Intravital Microscopy of VACV-Infected Labial Mucosa
Infected mice at the desired time postinfection.
Water-based gel (e.g., KY jelly).
Cyanoacrylate (e.g., superglue).
Betadine (or other iodine-based surgical scrub) and 70% alcohol prep pads.
Surgical cloth tape.
Surgical scissors and dressing forceps.
Isoflurane for anesthesia.
Rodent warmer/heating pad (Braintree Scientific) (see Note 9).
Stainless steel mini-stage (see Note 10).
Removable microscope imaging stage with standard glass slide-sized insert.
Gold-seal coverslips No. 1 (Thermo Scientific) (see Note 11).
Low-flow isoflurane machine with rodent facemask (Somnosuite, Kent Scientific); heart rate and oxygen saturation monitor are helpful. Other isoflurane vaporizers can also be used (such as SurgiVet) with mouse nose cone (Braintree Scientific). We find the Somnosuite the easiest and most consistent.
Biosafety Level 2-housed Leica DMi8 inverted five-channel confocal microscope equipped with environmental chamber and dual-multiphoton lasers, such as MaiTai and InSight DeepSee (Spectra Physics); ultrasensitive hybrid detectors (HyD); and a 25× water-immersion objective (see Notes 5 and 6).
Four HyDs equipped with the following filters: a 495 nm dichroic mirror followed by emission filters of 460/50 nm bandpass and 525/50 nm bandpass for SHG and GFP; a longpass filter of 560 nm; and 610/60 nm bandpass, a 650 nm longpass, and a 685/50 bandpass for imaging RFP and far-red fluorophores (see Note 7).
Image-processing software: Imaris (Bitplane), Image J and Huygens (Scientific Volume Imaging) (see Note 8).
3. Methods
While working with VACV, follow all Biosafety Level 2 (BSL-2) procedures, including appropriate personal protective equipment (PPE) and proper animal handling as designated by your institute. A vaccine is available for VACV. All solutions are warmed to 37 °C unless otherwise stated.
3.1. Epicutaneous VACV Infection in the Ear Pinna and Labial Mucosa
Remove VACV stock solution, thaw on ice, and sonicate three times to break up aggregated virus.
Anesthetize mice for skin infection using isoflurane. Once mice are appropriately sedated, gently stretch the mouse ear on a solid support (such as the cap of 50 mL tube).
Remove sonicated VACV from vial with a 1 cc or insulin syringe and place a drop of virus (~10 μL) on the ventral or dorsal side of ear (depending on the experiment; see Note 12). Spread VACV droplet on ear using a bifurcated needle (saturating prongs of needle) and gently poke 5–15 times in the ear, taking care not to poke through the thin ear skin (see Note 13).
For mucosal infection use approved injectable anesthetic or isoflurane (see Note 14). Once mice are sedated, place a drop of virus inside the lower lip using an insulin syringe. Gently poke the mucosa five times with a bifurcated needle, making sure not to poke through too deeply and infect the skin (see Note 15).
Wipe ear/inner lip to remove the remaining VACV. Allow mice to recover from anesthesia. Mice may be kept in the vivarium until desired day postinfection (dpi).
Discard unused VACV appropriately—we typically do not freeze/thaw as this can affect viral titers.
3.2. Routes of VACV Infection for MPM Imaging Other Organs
For lymph node (LN) MPM imaging, mice may be infected subcutaneously or intradermally. To image the popliteal LN postinfection, we typically inject 105–108 pfu subcutaneously in the hind footpad in total volume of 20 μL. To image the inguinal LN, we achieve the best results with up to three injections subcutaneously in the flank skin immediately surrounding the node.
For imaging the spleen after infection, virus may be injected intravenously and intraperitoneally. High viral doses will result in mortality within a week, so the dose should be chosen carefully based on experimental requirements (see Note 16).
For imaging the lung, VACV is administered intranasally.
For imaging infected ovaries, VACV should be injected intraperitoneally.
3.3. Mouse Preparation for Intravital Microscopy of VACV-Infected Skin
MPM imaging of the mouse ear skin has been reviewed a number of times [10–13]. In this methods chapter, we focus on some of the unique aspects of imaging VACV-infected skin.
After epicutaneous viral inoculation in the mouse ear, viral replication proceeds as previously described [5, 6]. Select a day of imaging that allows visualization of the desired infected cell populations. For imaging motility of VACV-infected inflammatory monocytes, we recommend days 3–4 postinfection.
Remove mouse from vivarium at the selected time postinfection and anesthetize.
Following initial anesthesia, the mouse can be maintained with isoflurane using a facemask at a concentration of ~1% or with avertin using a butterfly needle into the peritoneum (see Note 17). The rate of anesthesia must be modulated in accordance with mouse weight and sex. Take special care to avoid overheating the mouse during anesthesia in the environmental chamber; temperature must be carefully monitored.
Place mouse on a solid support that allows transport between the surgical preparation area and the microscope. We typically use a disposable, 6-well polystyrene plate. Gently secure mouse to plate using tape (take care not to tape too tightly (breathing can be restricted)). The tape secures the mouse as well and also limits movement due to breathing.
Secure the inner ear to the 6-well plate using a small drop of cyanoacrylate glue. The ear should be very flat with no creases (see Fig. 1 and Note 18). Be careful not to disturb any scabs present on the VACV-infected skin. Though some protocols for MPM in the skin utilize a depilatory cream to remove hair, we do not do this on inflamed VACV-infected skin.
Create a well around the glued ear that will be imaged using caulking cord (see Fig. 1 and Note 19).
Fill the caulking cord well with 37 °C PBS. Virus may be released from the ear into the PBS—take care not to spill. Gloves should be exchanged after the mouse is prepared.
Use a cotton Q-tip to gently remove any bubbles from the well (see Note 20).
Transfer the mouse to the heated chamber of the MPM microscope and carefully lower the dipping objective into PBS. The microscope objective will likely come in contact with virus and should be properly decontaminated at the end of the experiment.
We typically euthanize the mouse at the end of the imaging session while still under anesthesia. However, as imaging the skin is minimally invasive, it is possible to allow the mouse to recover from anesthesia and image again at a later time.
Fig. 1.
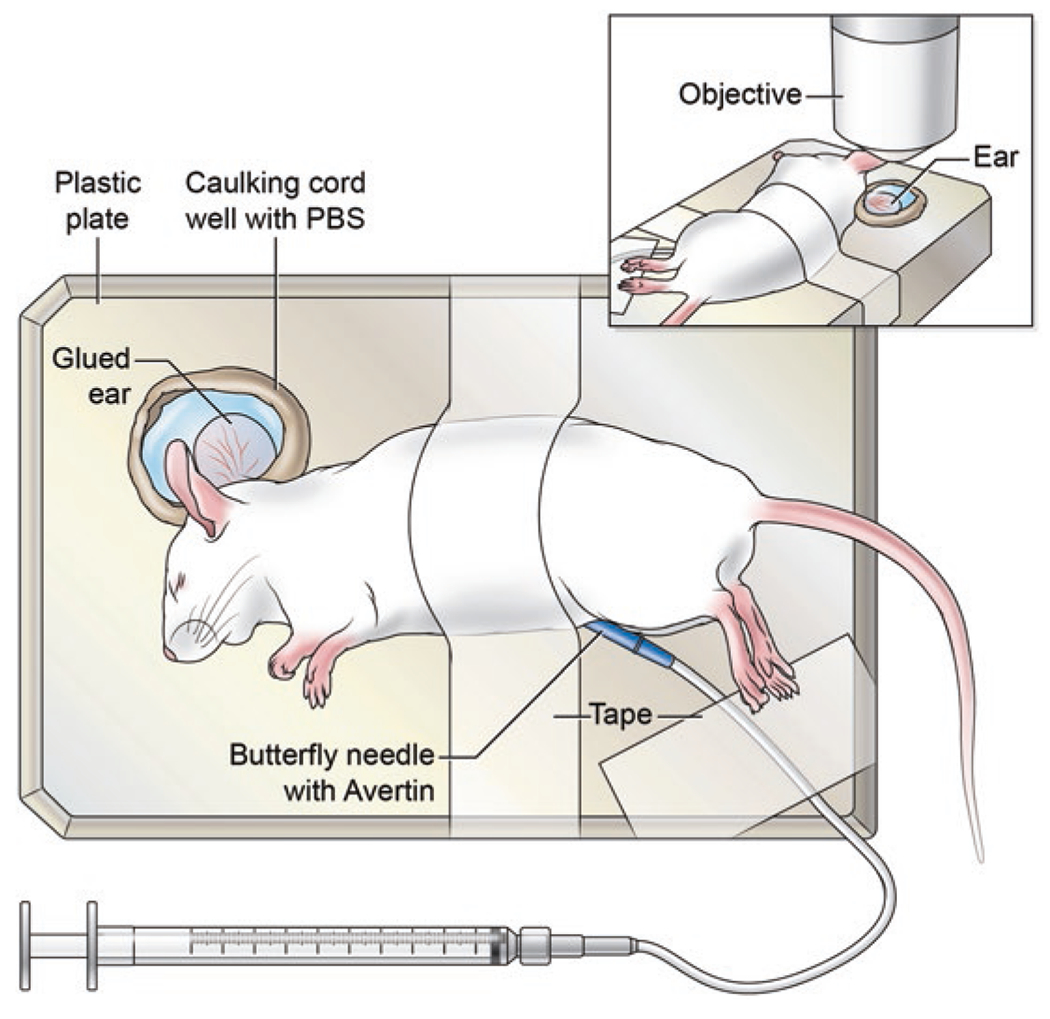
Mouse preparation for MPM imaging the VACV-infected ear skin. For imaging the ear skin, anesthetize the mouse with avertin and immobilize to a solid platform using cloth tape. The ear is also attached directly to the plate for added immobilization. The ear is then surrounded by a caulk well which is filled with prewarmed PBS. The prepared mouse is transferred to the environmental chamber of the microscope for MPM imaging
3.4. Mouse Preparation for Intravital Microscopy of VACV-Infected Labial Mucosa
Induce animal anesthesia in induction chamber using 2% isoflurane. Following initial anesthesia, maintain isoflurane influx using a nose cone at a continual concentration of ~1.5–1.75%. Adjust if necessary based on oxygen saturation.
For surgery and preparation, place the mouse and the microscope stage insert on a heated pad, and secure the mouse face up using surgical tape (see Note 9).
Disinfect the surgical site with three alternating scrubs of Betadine (or other iodine-based surgical scrub), and 70% ethanol.
Make 2-mm-long incision on both corners of the mouth, avoiding trauma to the anterior facial vein and other major blood vessels (see Fig. 2a.1 and Note 21).
Expose labial mucosa by flipping mouse lip inside out, and pulling it gently through the opening in stainless steel mini-stage, followed by gluing the external lip surface to the holder using superglue. Extra care should be taken so as not to allow superglue to enter the inner surface of the lip (see Fig. 2a.2, a.3).
After complete attachment of the lip to the mini-stage, immerse exposed labial mucosa in water-based gel.
For MPM imaging on an inverted microscope, flip the mouse face down over the coverslip of the microscope imaging stage, and immobilize the corners of the mini-stage using surgical tape (see Fig. 2a.4, b).
Carefully place a drop of water on the inverted objective and then transfer the secured mouse into the heated microscope chamber. Slowly raise the objective to come in contact with the coverslip (see Note 22 and Fig. 2b).
Imaging the mouse labial mucosa requires minor surgery; therefore mice are euthanized without recovery from anesthesia.
Fig. 2.
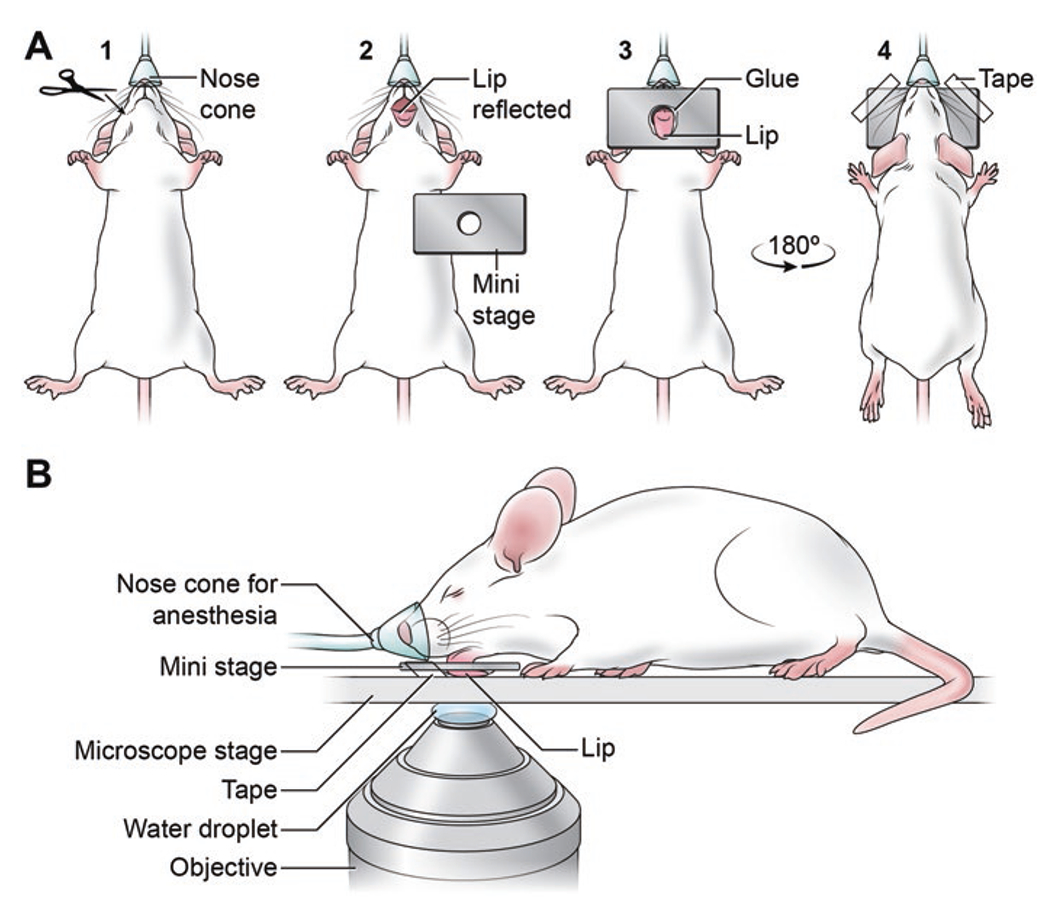
Mouse preparation for MPM imaging the labial mucosa. (a) Sequence of animal preparation for MPM imaging of the VACV-infected inner lip. (1) The anesthetized mouse is placed in the supine position and two 2 mm incisions are introduced at both oral angles. (2) The lower lip is gently pulled down to expose the inner mucosal tissue (the labial mucosa). (3) The lip is pulled through the opening of the stainless steel mini-stage taking care not to damage the tissue in the process. A drop of superglue is applied to the external lip surface in order to attach the lip to the mini-stage. (4) The mouse is now placed in prone position on the microscope platform, and the mini-stage is stabilized using surgical tape. (b) Side profile of a mouse prepared for MPM imaging of the labial mucosa. On an inverted microscope, the 25× water-immersion objective faces the exposed labial mucosa of the anesthetized mouse
3.5. MPM Image Acquisition
Set the multiphoton laser wavelength to 900 nm for simultaneous eGFP and dsRed excitation (if using a single MaiTai laser to excite these two fluorophores). For far-red fluorophores tune InSight DS laser to 1150 nm. Set to other wavelengths as appropriate [14]. We have imaged VACV expressing tagBFP, eGFP, tdTomato, mCherry, and turboRFP [5–7, 15].
Allow the mouse to warm to the temperature of the chamber before acquiring images for analysis of motility. Small changes in body temperature can drastically impact cellular movement.
Determine and set the imaging parameters as needed. Image acquisition must balance the area scanned (x-y) and the image depth (z) versus the time it will take to acquire each 3-dimensional set (stack) of images. If using an inverted scope with motorized stage, tiled scans of multiple regions of interests over time can be acquired (up to 2 mm2). The interval between stacks must be adjusted depending on the parameter being analyzed. Rapidly moving cells (such as T cells and neutrophils) will require shorter time intervals between stacks.
Acquire z-stacks over time for the desired time range. To determine cellular movement, we recommended tracking cells for at least 1 h. Multiple datasets (containing many hours of data) may be concatenated in order to keep individual file sizes down.
Monitor the depth of anesthesia of the mouse during the imaging period (see Note 17).
Analyze datasets using the appropriate software. Typically, we make maximum intensity projections of each stack over time and use these to quantitate cellular speed and directionality using software such as Imaris, which contains several automated spot-counting detection and tracking algorithms. Data image analysis depends largely on experimental design. For previous reviews, see refs. 16–18.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Ethan Tyler (NIH Medical Arts) created illustrations.
4 Notes
Sonicate VACV prior to infection (either in vitro or in vivo) to dissociate aggregated virus.
Depending on the experiment, anesthesia can be administered either as an injectable (avertin or ketamine/xylazine) or inhaled (isoflurane). Each anesthetic has advantages and disadvantages for imaging. While isoflurane anesthesia typically yields the most consistent results, the requirement for delivery through a nose cone can impede imaging of some areas.
Mouse strains should be chosen carefully for imaging the skin with selection of nonpigmented mice whenever available. Pigmented mice (such as C57Bl/6) have melanocytes and melanin-containing dermal cells that can absorb light resulting in heat and photodamage [13].
PBS should be warmed to 37 °C prior to application to the mouse. The addition of cold buffers can alter the movement of cells within the tissue.
Anesthetized mice do not maintain body temperature, so an appropriate heat source must be provided during imaging. A heated blanket with a feedback thermometer will work in most cases; however an environmental chamber that keeps the entire microscope stage and anesthetized mouse at a constant temperature provides the most accuracy.
Many options exist for turnkey MP microscopes. The first selection criterion is the use of an upright (objective comes from the top) versus inverted (objective comes from underneath) microscope. Upright scopes do not have to image through a cover glass like inverted scopes; however the addition of a cover glass can act as a stabilizer for some tissues with high water content. Multiple MP laser options now exist as well; here price can be a major factor. Many scopes are equipped with more than one MP laser, allowing fluorophore excitation at multiple wavelengths simultaneously.
Filters should be optimized for the fluorophores being used; our most common set is listed.
Multiple choices exist for image-processing software.
Imaging the labial mucosa requires a minor surgery, which increases the preparation time. Since mice are unable to regulate body temperature under sedation it is important to maintain physiological temperature during this time.
This is a custom-built 2 cm × 5 cm stainless steel plate with a circular window in the middle. After animal preparation, the window will face water-immersion lens on the inverted microscope. The mini-stage was designed to stabilize a small region of interest within a larger organ (here the labial mucosa) by gluing the organ to the stage and allowing exposure of the region of interest through the opening.
It is essential to use the proper coverslip thickness based on a given objective’s numerical aperture (NA). Most objectives are designed to use #1 coverslips (0.13–0.17 mm thickness). Using an incorrect coverslip thickness may result in decreased signal intensity and resolution.
We typically infect on the dorsal ear pinna containing hair follicles. If imaging pigmented (such as C57Bl/6) mice, it can be helpful to switch to the ventral ear to avoid some of the autofluorescence (as this side has less pigment).
Infections are performed in areas that maximize MPM imaging ability. Avoid areas too close to the mouse’s head, as this will impede imaging on an upright scope due to the objective. Likewise, avoid the ear edges, as these areas have dense hair and can prove more difficult to image.
Infection of the labial mucosa is difficult using an isoflurane nose cone. Injectable avertin can be used if approved by institutional committee. Intraperitoneal injection can result in inflammation over time, which should be considered for immunological studies. Avertin injection volumes are based on weight. For most consistent results, make fresh stocks of avertin. Avertin can be stored at 4 °C in the dark for ~14 days.
For MPM imaging of mucosal VACV infection, viral inoculation between the inside floor of the lip and outer skin maximizes the ability to image; however it is essential to prevent penetration of the outer lip or infection of the skin (unless analysis of skin infection is desired).
When selecting virus dose, one must balance the ability to visualize virus-infected cells and physiology of infection. Typically, a rather high viral dose is required to find fluorescent protein-expressing VACV-infected cells, particularly when animals are imaged soon after injection (such as in the LN). In the skin and labial mucosa, lower virus doses allow the visualization of infected cells after VACV replication.
Attach the facemask delivering isoflurane to the mouse securely using athletic cloth tape. We advise the use of a heart rate/oxygen saturation monitor. This allows noninvasive determination of the level of anesthesia during imaging. We have found that an animal with a heart rate of 300–450 bpm and an oxygen saturation of ~90% can be imaged for >4 h [19].
We find it helpful to gently press the entire ear with your finger. The glue will fluoresce if accidentally transferred to the skin so avoid contamination from glue adhered to gloves.
We find window caulk to be the easiest material to make a well holding PBS around the skin, but other material can also be used.
The hair will trap air bubbles after addition of PBS. Bubbles will interfere with imaging.
Small diagonal cuts at the oral angles of the mouth result in increased exposure of the labial mucosa for imaging with minimal bleeding.
While using water-immersion lens, the water drop between objective and the MPM imaging stage must be reapplied every hour. Sequential 1-h time-lapse videos can be combined later into continuous 6-h time-lapse during post-acquisition data processing using Huygens (SVI) software.
References
- 1.Secklehner J, Lo Celso C, Carlin LM (2017) Intravital microscopy in historic and contemporary immunology. Immunol Cell Biol 95(6):506–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halin Cornelia MJR, Cenk S, von Andrian Ulrich H (2005) In vivo imagining of lymphocyte trafficking. Annu Rev Cell Dev Biol 21:581–603 [DOI] [PubMed] [Google Scholar]
- 3.Qi H, Kastenmuller W, Germain RN (2014) Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol 30:141–167 [DOI] [PubMed] [Google Scholar]
- 4.Hickman HD (2017) New insights into antiviral immunity gained through intravital imaging. Curr Opin Virol 22:59–63 [DOI] [PubMed] [Google Scholar]
- 5.Hickman HD, Reynoso GV, Ngudiankama BF, Cush SS, Gibbs J, Bennink JR et al. (2015) CXCR3 chemokine receptor enables local CD8(+) T cell migration for the destruction of virus-infected cells. Immunity 42(3):524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickman HD, Reynoso GV, Ngudiankama BF, Rubin EJ, Magadan JG, Cush SS et al. (2013) Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin. Cell Host Microbe 13(2):155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cush SS, Reynoso GV, Kamenyeva O, Bennink JR, Yewdell JW, Hickman HD (2016) Locally produced IL-10 limits cutaneous vaccinia virus spread. PLoS Pathog 12(3):e1005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyatt LS, Earl PL, Moss B (2017) Generation of recombinant vaccinia viruses. Curr Protoc Protein Sci 89:5.13.1–5.13.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynoso GV, Shannon JP, Americo JL, Gibbs J, Hickman HD (2018) Growth and purification of vaccinia virus stock. Methods Mol Biol 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh CC, Li JL, Becker D, Weninger W, Angeli V, Ng LG (2016) Inducing ischemia-reperfusion injury in the mouse ear skin for intravital multiphoton imaging of immune responses. J Vis Exp (118). 10.3791/54956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaylo A, Overstreet MG, Fowell DJ (2016) Imaging CD4 T cell interstitial migration in the inflamed dermis. J Vis Exp (109):e53585. 10.3791/53585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egawa G, Kabashima K (2016) In vivo imaging of cutaneous DC’s in mice. Methods Mol Biol 1423:269–274 [DOI] [PubMed] [Google Scholar]
- 13.Li JL, Goh CC, Keeble JL, Qin JS, Roediger B, Jain R et al. (2012) Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat Protoc 7(2):221–234 [DOI] [PubMed] [Google Scholar]
- 14.Drobizhev M, Makarov NS, Tillo SE, Hughes TE, Rebane A (2011) Two-photon absorption properties of fluorescent proteins. Nat Methods 8(5):393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman HD, Li L, Reynoso GV, Rubin EJ, Skon CN, Mays JW et al. (2011) Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J Exp Med 208(12):2511–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltman JB, Maree AF, de Boer RJ (2009) Analysing immune cell migration. Nat Rev Immunol 9(11):789–798 [DOI] [PubMed] [Google Scholar]
- 17.Benson RA, Brewer JM, Garside P (2017) Visualizing and tracking t cell motility in vivo. Methods Mol Biol 1591:27–41 [DOI] [PubMed] [Google Scholar]
- 18.Sharaf R, Mempel TR, Murooka TT (2016) Visualizing the behavior of HIV-infected T cells in vivo using multiphoton intravital microscopy. Methods Mol Biol 1354:189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewald AJ, Werb Z, Egeblad M (2011) Monitoring of vital signs for long-term survival of mice under anesthesia. Cold Spring Harb Protoc 2011(2):pdb prot5563. [DOI] [PMC free article] [PubMed] [Google Scholar]


